Note: Descriptions are shown in the official language in which they were submitted.
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
OXAZOLIDINONE ANTIBACTERIAL
AGEI'TTS HAVING A THIOCARBONYL FUNCTIONALITY
BACKGROUND OF THE INVENTION
The present invention relates to new and useful oxazolidinone compounds
and their preparations, and more particularly to oxazolidinone compounds in
which
the carbonyl functionality of -NH-C(O)-R is converted to a thiocarbonyl
functionality,
such as a thiourea -NH-C(S)-NH_" an alkyl thiourea -NH-C(S)-NH-(C,.4 alkyl),
thioamide -NH-C(S)-(C,_; alkyl) or -NH-C(S)-H.
Replacement of the oxygen atom with a sulfur atom has unexpectedly
improved the antimicrobial properties of the compounds. The compounds are
useful
antimicrobial agents, effective against a number of human and veterinary
pathogens, including Gram-positive aerobic bacteria such as multiply-resistant
staphylococci and streptococci, Gram-negative organisms such as H. influenzae
and
M. catarrahlis as well as anaerobic organisms such as bacteroides and
clostridia
species, and acid-fast organisms such as Mycobacterium tuberculosis and
Mycobacterium avium. The compounds are particularly useful because they are
effective against the latter organisms which are known to be responsible for
infection in persons with AIDS.
SUMMARY OF THE INVENTION
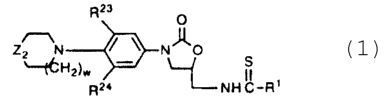
In one aspect the subject invention is a compound of the Formula I
A
SC_R t
i
N
H
I
or pharmaceutical acceptable salts thereof wherein:
G is o 0
i 'o ~N~o ~o
-1-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Rl is
a) H,
b) NHz,
c) NH-CI_4 alkyl,
d) Ct_4 alkyl,
e) -OC,_4 alkyl,
f) -S C,_4 alkyl,
g) C,~ alkyl substituted with 1-3 F, 1-2 Cl, CN or -COOC~_, alkyl,
h) C3_6 cycloalkyl,
i) N(C,~ alkyl)z or
j) N(CHz)z_5;
A is
a) R
R
3
R2s
b)
R2a
R~
c) R~
Ras R~
d) a 5-membered heteroaromatic moiety having one to three atoms
selected from the group consisting of S, N, and O,
wherein the 5-membered heteroaromatic moiety is bonded via a carbon
atom,
wherein the 5-membered heteroaromatic moiety can additionally have
a fused-on benzene or naphthyl ring,
wherein the heteroaromatic moiety is optionally substituted with one
to three R~~,
-2-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
e) a 6-membered heteroaromatic moiety having at least one nitrogen
atom,
wherein the heteroaromatic moiety is bonded via a carbon
atom,
wherein the 6-membered heteroaromatic moiety can additionally have
a fused-on benzene or naphthyl ring,
wherein the heteroaromatic moiety is optionally substituted with one
to three Rss,
f7 a (i-carbolin-3-yl, or indolizinyl bonded via the 6-membered ring,
optionally substituted with one to three Rss~
g)
Rya R~s R~s ~ or
R~3 K
R"
h)
R~5 R~s
N
R ao~
~T ~ '
R"
wherein R.1 is
a) H,
b) F,
c) C1,
d) Br,
e) C1_~ alkyl,
f7 N02, or
g) R,~ and R;, taken together are -O-(CHL)h-O-;
R~ is
a) -S(=O); R~,
b> -S(=O)2-N=S(O)~RSRs,
c) -SC(=O)R~,
d) -C(=O)R&,
e) -C(=O)Ry,
fl -C(=O)NR,oR",
-3-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
g) -C(=NR,~)R~,
h) -C(Re)(R")-OR,~,
i) -C(R9)(R")-OR,3,
j) -C(Re)(R")-OC(=O)R,3,
k) -C(Rg)(R")-OC(=O)R,3,
1) -NR,oRii,
m) -N(Rlo)-C(=O)R->>
n) -N(R,o)-S(=O);RT,
o) -C(OR,4)(ORI~)R8,
p) -C(Rg)(R,o)-NR,oR,I, or
q) C,.e alkyl substituted with one or more =O
other than at alpha
position, -S(=O);R1T, -NR,oR", CZ_S alkenyl,
or CZ_~ alkynyl;
R4 is
a) C1.4 alkyl optionally substituted with one or more halos, OH, CN,
NRIOR", or -COzR,3,
b) Cz_4 alkenyl,
c) -NRisRia,
d) -N3,
e) -NHC(=O)R,,,
f7 -NR2oC(=O)R,,
g) -N(R,9)z
h) -NR,sR,s, or
i) -NR,9R~,
R5 and Rb
at each
occurrence
are the
same or
different
and are
a) C,_2 alkyl, or
b) RS and Rs taken together are -(CH
)
;
l
k
R., is C1.~
alkyl optionally
substituted
with one
or more
halos;
R.e is
a) H, or
b) C,.R alkyl optionally substituted with one or
more halos, or C;,_8
cycloalkyl;
Rg is C,~
alkyl substituted
with one
or more
a) -S(=O)R",
b) _OR,s,
c) -OC(=O)R,3,
d) -NR,oR", or
-4-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
e) C,_5 alkenyl optionally substituted
with CHO;
Rlo and
R" at
each
occurrence
are the
same
or different
and are
a) H,
b) C,.~ alkyl, or
c) C3_$ cycloalkyl;
RIZ is
a) -NR,oR",
b) -OR,o; or
c> -NHC(=O)R,o;
I0 R,3 1S
a) H, or
b) C,_4 alkyl;
R,4 and
R,5 at
each
occurrence
are the
same
or different
and are
a) C,.4 alkyl, or
I5 b) R,4 and R,5 taken together are -(CH)
-;
,
R,s is
a) H,
b) C,_4 alkyl, or
c) C3,$ cycloalkyi;
20 R,~ is
a) C,_4 alkyl, or
b) C3_~ cycloalkyl;
R,8 is
a ) H,
25 b) C,_4 alkyl,
c) Cz_4 alkenyl,
d) C;,_4 cycloalkyl,
e) -OR,3 or
fl -NR.z,R..ll,
30 R,9 is
a) Cl,
b) Br, or
c) I;
RZO is
a physiologically
acceptable
cation;
35 R.r, and at each occurrence are the same or different
R.,.~ and are
a) H,
-5-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
b) C,.4 alkyl, or
c) -NR.z,R.,.~ taken together are -(CHz)
-;
m
wherein R,,~and R.la at each occurrence are the same or different
and are
a) H,
b) F,
c) Cl,
d) C1.2 alkyl,
e) CN
f) OH,
g) C1-z alkoxy,
h) nitro, or
i) amino;
Q is
a) Y
x
Ni
~Y
c) w x
Y~_-\~
Z
d ) Y IV X
M
e) ,
Y
/N
Z
-6-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
X ~N~ '
,N
N
M
N '
X i
/N
N
I
M
h)
'
x
N
I Y
M
x
w
i) ~~ ~ '
N Y
g\V~X
(~ ~Z
k)
OR3o
O
1)
'
(CH2)~~R38
-7-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
m> a diazinyl group optionally substituted with X and Y,
n) a triazinyI group optionally substituted with X and Y,
o) a quinolinyl group optionally substituted with X and Y,
p) a quinoxalinyl group optionally substituted with X and Y,
q) a naphthyridinyl group optionally substituted with X and Y,
r) A2 ,
At (CHZ)n
Z1
8)
,N
(CH2)w
t)
Z3~
N
u)
R ~o~
~ N
N
V)
Y--~N-
X
W) N
Y ~ ,N-
x
_g_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
x) Y ,
N~-
X
N
X ~ ~~ N
i
N
Y I
z) Y
~N
N-'N\
X
aa) N
X~ N-
Y N
bb) X or,
/'\
Y J
N
Q and Rz~ taken together are
R'os
N
wherein Z' is
a > -CH,~-,
b) -CH(R'°~)-CHZ-,
c) -C(O)-, or
-g_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
d) -CH.~CHZCH.~-;
wherein Z2
is
a) -OZS-,
b) -O-,
c) -N(R~o~)-
d) -OS-,
or
e) -S-;
wherein Z3
is
a) -OzS-,
b ) -O-,
c) -OS-,
or
d ) -S-;
wherein A'
is
a) H-,
or
b) CH3;
wherein AZ is
a) H-,
b) HO-,
c) CHa-,
d) CH30-,
e) RlozO-CH2-C(O)-NH-
f) R,'o'~O-C(O)-NH-,
g) (C,-Cl)alkyl-O-C(O)-,
h) HO-CHz-,
i) CH30-NH-,
j) (C,-C;~)alkyl-02C-
k) CH3-C(O)-,
1) CH.,-C(O)-CH.~-,
m) , or
o~~C~
U
-10-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
n)
O
~o
A' and Al taken together are:
a) R"2
~o
o-~- .~r~
b) o= , or
R "o
C) ~ ~c ~ ;
wherein R'z
is
a) H-,
b) CH;~-,
c) phenyl-CH2-,
or
d) CH3C(O)-;
wherein R'3 is
a) (C'-C;,)alkyl-,
or
b) phenyl-;
wherein is
R'4
a) H-, or
b) HO-;
wherein R'5
is
a ) H-,
b) (C'-C;,)alkyl-,
c) CH2 = CH-CH.~-,
or
d) CHI-O-(CH~)Z-;
wherein R'o~ s
i
a) CH,,-C(O)-,
b) H-C(O)-,
c) CI,,CH-C(O)-,
-11-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
d> HOCH"-C(O>-,
e) CH~SOz-,
115 S
f7
g) FzCHC(O>-,
h) NV _p(p)_ ,
i) HOC-C(O)-O-CH1-C(O)-,
j) H-C(O)-O-CHz-C(O)-,
k) ~~ C(O)-
1) HC--_C-CH,~O-CH.,-C(O)-,
or
m) phenyl-CH2-O-CHz-C(O)-;
wherein R' ' is
a) R'o2p-C(R'io)(R'm)-C(O)-
b) Rl3O-C(O)-,
c) Rloa-C(O)-
p
d)
0
e) o
H
fl H3C-C(O)-(CHl)2-C(O)-,
g) Rtos-SOZ-,
0
0
<o ~ I
h)
i) HO-CH.~-C(O)-,
j) Rms-(CH2)1-,
k) R"3-C(O)-O-CH.~-C(O)-,
1) (CH3>.,N-CH.,-C(O)-NH-,
-12-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
m) NC-CH.~-,
n) FZ-CH-CH.~-, or
o) RlsoR's'NSOZ
wherein
R'~ is
a) H-,
b) (C,-C4>alkyl,
c) aryl -(CHZ)P,
d) CIHIC-,
e) C12HC-,
fl FHZC-,
g) FZHC-,
h) (C;~-C6)cycloalkyl,
or
i> CNCHz-.
wherein
R'9 is
a) alkylC,-C4,
b) -CHzCI
c) -CHZCH=CH2,
d) aryl, or
e) -CH2CN;
wherein and R"' are independently
R"
a ) H-,
b) CH;,-; or
wherein is
R''1
a) H-,
b) CH30-CHzO-CH2-,
or
c> HOCH1-;
wherein is
R"3
a) CH;,-,
b) HOCHL-,
c) (CH.;)zN-phenyl,
or
d> (CH;,>zN-CH.~-;
wherein
R"; is
a ) HO-,
b> CH;,O-,
c) H.~N-,
d) CH,,O-C(O>-O-,
-13-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
e) CH3-C(O)-O-CH"-C(O)-O-,
phenyl-CH.,-O-CHl-C(O)-O-,
g) HO-(CH,,)2-O-,
h) CH;,O-CH,~-O-(CH.>)1-O-, or
i) CH30-CH.,-O-;wherein R"3 is
a) CH3-,
b) HOCH1-,
c) (CH.;)zN-phenyl, or
d) (CH3)zN-CH2-;
wherein
R"5 is
a ) H-, or
b) Cl-;
wherein R"6 is
a) HO-
b) CH30-, or
c) F;
wherein R'SOand R's' are each H or alkyl C,-C4 or R'S and R'S'
taken together with
the nitrogenatom to which each is attached form a monocyclic
heterocyclic ring
having from
3 to 6 carbon
atoms;
B is an
unsaturated
4-atom linker
having one
nitrogen
and three
carbons;
M is
a) H,
b) C,_e alkyl,
c> C;,_8 cycloalkyl,
d) -(CH1)mOR,~, or
e) -(CH1),,-NR.l,R.zl,
Z is
a) O,
b> S, or
c> NM;
W is
a) CH,
b) N, or
c) S or O when Z is NM;
Y is
a) H,
-14-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
b) F,
c) Cl,
d) Br,
e) C1_3 alkyl, or
f) N02;
X is
a) H,
b) -CN,
c) ORZ~,
d) halo,
e) NOz,
f) tetrazoyl,
g) -SH,
h) -S(=O);R4,
i) -S(=O)2-N=S(O)~RSRs,
j) -SC(=O)R,,
k) -C(=O)R.ZS,
1) -C(=O)NR.l~RzB,
m) -C(=NR29)RZS,
n) -C(R25)(RzA)-OR,3,
o) -C(R25)(R.za)-OC(=O)R,a,
p) -C(RZa)(OR,~)-(CH.,)h-NR,z.,Rza~
-N~z~Rzs~
r) -N(R27)C(=O)R.,,
s) -N(R.z~)-S(=O);R7,
t) -C(OR,,,)(OR,S)Ria,
u) -C(R25)(R,~)-NR.~~R.16, or
v) C,_a alkyl substituted with one or more halos,
OH, =O other than at
alpha position, -S(=O);R,7, -NR.~.,R,.la, Cl.s
alkenyl, Ci_5 alkynyl, or
C;,,a cycloalkyl;
R4, R5, R~,
R~, R,,3,
R,~, R,S,
R,b, and
R" are the
same as
defined
above;
R25 is
a) H,
b) C,_a alkyl optionally substituted with one or more halos, C3_e
cycloalkyl, C,_, alkyl substituted with one or more of -S(=O);R1T,
-OR,s, or OC!=O)R,;" NR.=~Rza, or
-15-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
c) C1_5 alkenyl optionally substituted with CHO, or CO.~R,;,;
R26 is
a ) R.28, or
b) NR.,~NIS;
R.,~ and R.ze at each occurrence are the same or different and are
a) H,
b> C,_e alkyl,
c) C3_g cycloalkyl,
d) -(CH1)mOR,3,
e) -(CHl),,-NR.zIR22, or
f7 R.l~ and R.18 taken together are -(CHz)l0(CHz)1-, -(CHZ),,CH(COR.,)-,
or -(CHZ)ZN(CH.,)2(R.,);
R29 1S
a) -NR,~7Rza~
b) -OR2~, or
c) -NHC(=O)R.ze;
wherein R3o is
a) H,
b) C,_e alkyl optionally substituted with one or more halos, or
c) C,_8 alkyl optionally substituted with one or more OH, or C1_6 alkoxy;
wherein E is
a) NR3s,
b) -S(=O);, or
c) O;
R38 is
a) H,
b) C,_s alkyl,
c) -(CHz)q-aryl, or
d ) halo;
R;ss is
a) H,
b) C,_s alkyl optionally substituted with one or more OH, halo, or -CN,
c) -(CHt)q-aryl,
d) -C02R~o,
e) -CORD,,
f) -C(=O)-(CH~>~-C(=O>R;o,
-16-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
g) -S(=O).,-C,_s alkyl,
h) -S(=O).,-(CH1)q-aryl, or
i) -(C=O)~-Het;
Rao is
a ) H,
b) C,_~ alkyl optionally substituted with one or more
OH, halo, or -CN,
c) -(CH.~)q-aryl, or
d> -(CHz)~-OR,z;
R,,1 is
a) C,.s alkyl optionally substituted with one or more
OH, halo, or -CN,
b) -(CH1)q-aryl, or
C) -(CHZ)q-OR42;
R~1 is
a) H,
b) C1_6 alkyl,
c) -(CH1)q-aryl, or
d) -C(=O)-C,_s alkyl;
aryl is
a) phenyl,
b) pyridyl, or
c) napthyl; a to c optionally substituted with one or
more halo, -CN, OH,
SH, C,_~ alkyl, C,_~ alkoxy, or C,_~ alkylthio;
wherein R4,,is
a) H,
b) C,_ j alkyl,
c) F, or
d) OH;
Rq4 1S
a) H,
b> CF3,
c) C,.~ alkyl optionally substituted with one or more
halo,
d) phenyl optionally substituted with one or more halo,
e) R~4 and R~~ taken together are a 5-, 6-, or 7-membered
ring of the
formula,
-17-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
or
H
~C ~ C ~
C =~ (C H 2)h
U
f7 R44 and R~5 taken together are -(CHl)k-, when Ras is an electron-
withdrawing group;
R45 and R46 at each occurrence are the same or different and are
a) an electron-withdrawing group,
b) H,
c) CF3,
d) C,_3 alkyl optionally substituted with one halo,
e) phenyl, provided at least one of R~,, or R;b is an electron-withdrawing
group, or
f) R4,, and R,,s taken together are a 5-, 6-, 7-membered ring of the formula
0
c -
(CH2)r
U is
a) CH2,
b) O,
c) S, or
d) NR4,;
R4., is
a) H, or
b> C,.S alkyl;
wherein
R~8 is
a) carboxyl,
b) halo,
c> -CN,
d) mercapto,
e) formyl,
f) CF,,,
g) -NO.~,
-18-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
h ) C, _s alkoxy,
i) C,_s alkoxycarbonyl,
j) C,_s alkythio,
k) C,.6 acyl,
1) -NRa9 Rso,
m) C,_6 alkyl optionally substituted with OH, C,_~
alkoxy, C,.S acyl, or
-NRasRso~
n) C2_e alkenylphenyl optionally substituted with
one or two RS,,
o) phenyl optionally substituted with one or two R
S"
p) a 5-, or 6-membered (un)saturated heterocyclic
moiety having one to
three atoms selected from the group consisting
of S, N, and O,
optionally substituted with one or two RS,, or
0
loH2)'
R49 and Rso
at each
occurrence
are the
same or
different
and are
a) H,
b) C,_4 alkyl,
c) C5_s cycloalkyl, or
d) R49 and R5o taken together with the nitrogen atom
is a 5-, 6-
membered saturated heterocyclic moiety which optionally
has a
further hetero atom selected from the group consisting
of S, N, and O,
and can in turn be optionally substituted with, including
on the
further nitrogen atom, C,_;, alkyl, or C,_a acyl;
R5, is
a) carboxyl,
b) halo,
c) -CN,
d) mercapto,
e) formyl,
f) CF3,
g) -N01,
h) C,,6 alkoxy,
i) C,.6 alkoxycarbonyl,
j) C,_6 alkythio,
k ) C, _~ acyl,
-19-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
C,_s alkyl optionally substituted with OH, C,_~ alkoxy, C,_~ acyl, or
-NR49Rso~
m) phenyl,
n) -C(=O)NR52 Rsa,
0) -NR,,9RSO,
P) -N(Rsz)(-SO,,R54),
q) -SOS NR5zR53, or
r) -S(=O);R54;
R52 and R53 at each occurrence are the same or different
and are
a) H,
b) C,_s alkyl, or
c) phenyl;
Rg4 1S
a) C,_,, alkyl, or
b) phenyl optionally substituted with Ci~, alkyl;
wherein is
R55
a) carboxyl,
b) halo,
c) -CN,
d) mercapto,
e) formyl,
CF3,
g) -NO2.
h) C,_s alkoxy,
i) C,_s alkoxycarbonyl,
j) C1_6 alkythio
k) C,_s acyl,
1 ) -NR56 RS~,
m) C,_s alkyl optionally substituted with OH, C,_,,
alkoxy, C,_~ acyl, or
-NR56R5,,
n) C1_fl alkenylphenyl optionally substituted with
one or two RSA,
o) phenyl optionally substituted with one or two
RSa,
p) a 5- or 6-membered (un)saturated heterocyclic
moiety having one to
three atoms selected from the group consisting
of S, N, and O,
optionally substituted with one or two RSp, or
-20-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Q) O
(C H2)~
Rss and Rs,
at each occurrence
are the same
or different
and are
a) H,
b) formyl,
c) C,_,, alkyl,
d) C,~, acyl,
e) phenyl,
f) C3_s cycloalkyl, or
g) Rss and Rs, taken together with the nitrogen atom
is a 5-, 6-
membered saturated heterocyclic moiety which optionally
has a
further hetero atom selected from the group consisting
of S, N, and O,
and can in turn be optionally substituted with, including
on the
further nitrogen atom, phenyl, pyrimidyl, C,_3 alkyl,
or C,.a acyl;
R58 is
a) carboxyl,
b) halo,
c) -CN,
d) mercapto,
e) formyl,
CFA,
g) -NOa~
h) C,_s alkoxy,
i) C,-s alkoxycarbonyl,,
j) C,_s alkythio,
k) C,_s acyl,
1) phenyl,
m) C,_6 alkyl optionally substituted with OH, azido,
C,_s alkoxy, Ci_s
acyl, -NRsSRss, -SRs;, -O-SO.~Rsx, or
NH-CO-O- '
n) -C(=O)NRs9 Rso,
0) -NRssRs~~
p) -N(Rso)(-SO.,Rsa),
-21-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
q) -SO.~-NR59Rso,
r) -S(=O);R54,
s) -CH=N-Rsl, or
t) -CH(OH)-S03Rs4;
R54 is the same as defined above;
RS~ and Rso at each occurrence are the same or different and are
a) H,
b) Cl,s alkyl,
c) phenyl, or
d) tolyl;
Rsl is
a) OH,
b) benzyloxy,
c) -NH-C(=O)-NHz,
d) -NH-C(=S)-NHI, or
e) -NH-C(=NH)-NRs2Rs3;
Rs2 and Rs3
at each
occurrence
are the
same or
different
and are
a) H, or
b) CI_4 alkyl optionally substituted with phenyl or
pyridyl;
Rs4 is
a) H, or
b) a sodium ion;
Rss and Rss at each occurrence are the same or different and
are
a) H
b) formyl,
c) C,~ alkyl,
d) C,_4 acyl,
e) phenyl,
C;;_s cycloalkyl,
g) Rss and Rss taken together are a 5-, 6-membered saturated
heterocyclic
moiety having one to three atoms selected from the
group consisting of
S, N, and O, optionally substituted with, including
on the nitrogen
atom, phenyl, pyrimidyI, CI_3 alkyl, or C,_;, acyl,
h) -P(O)(OR,o)(OR"), or
i) -SOz-R7z;
-22-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Rs, is
N -N _
N N
(C H 3)3C S
CH3
10
N
I
or
CH3
CH3
R68 is C,.3 alkyl;
Rs9 is
a) C1_6 alkoxycarbonyl, or
b) carboxyl;
R,a and R" at each occurrence are the same or different and are
a) H, or
b) C~_3 alkyl;
R7z is
a) methyl,
b) phenyl, or
c) tolyl;
wherein K
is
a ) O, or
b) S;
R.,3, R",
R~" RTS,
and R." at
each occurrence
are the same
or different
and are
a) H,
b) carboxyl,
c> halo,
d) -CN,
e) mercapto,
f) formyl,
g> CF;,,
-23-
CA 02351062 2001-05-10
WO 00132599 PCTNS9$/25308
h) -N01,
i) C,.s alkoxy,
J) Ci-s alkoxycarbonyl,
k) C,_s alkythio,
1) C,_s acyl,
m) -NR.rs R~s
n) C1_s alkyl optionally substituted with OH, C,.s
alkoxy, C,_5 acyl,
-NR,gR~9, -N(phenyl)(CH2-CH.,-OH), -O-CH(CH;,)(OCHZCH3),
or
-O-phenyl-[para-NHC(=O)CH3],
0) CZ_e alkenylphenyl optionally substituted with R
s,,
p) phenyl optionally substituted with Rs" or
q) a 5-, or 6-membered (un)saturated heterocyclic moiety
having one to
three atoms selected from the group consisting of
S, N, and O,
optionally substituted with Rs,;
Rsl is
the same
as defined
above;
R,a and R.,s
at each occurrence
are the same
or different
and are
a) H,
b) C,_4 alkyl,
c) phenyl, or
d) R,e and R..,s taken together with the nitrogen atom
is a 5-, 6-
membered saturated heterocyclic moiety which optionally
has a
further hetero atom selected from the group consisting
of S, N, and O,
and can in turn be optionally substituted with,
including on the
further nitrogen atom, C,_;~ alkyl, or C1_~ acyl;
wherein
T is
a) O,
b ) S, or
c) SO2;
~s~ R-rs~ R.,~ are the same as defined above;
and
Reo is
a) H,
b) formyl,
c) carboxyl,
d) C,_s alkoxycarbonyl,
e) C,_fl alkyl,
f) C2_s alkenyl,
-24-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
wherein the substituents (e) and (f) can be optionally substituted with
OH, halo, C,_6 alkoxy, C,.s acyl, C,.6 alkylthio or C,_s
alkoxycarbonyl, or phenyl optionally substituted with halo,
g) an aromatic moiety having 6 to 10 carbon atoms optionally substituted
with carboxyl, halo, -CN, formyl, CF3, -NO.,, C,.~ alkyl, C,.6 alkoxy,
C1.6 acyl, C,_6 alkylthio, or C1.6 alkoxycarbonyl;
h> -NRA,R~2,
i) -ORgo,
j) -S(=O);-R~,,
k) -S02-N(R~2)(R,~3), or
1) a radical of the following formulas:
RR1 and R8z at each occurrence are the same or different and are
a) H,
b) C3~ cycloalkyl,
c) phenyl,
d) C,_6 acyl,
e) C,.e alkyl optionally substituted with OH, C,_s alkoxy which can be
substituted with OH, a 5-, or 6-membered aromatic heterocyclic
moiety having one to three atoms selected from the group consisting of
S, N, and O, phenyl optionally substituted with OH, CF3, halo, -NO2,
C,_4 alkoxy, -NR83Rg4, or
o ~ ;
0
O
or
Ras- C H-
g) ~N-(CH2)~-- '
V is
a) O,
-25-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
b) CH.~, or
c) NRA,;
R83 and Rx~ at each occurrence are the same or different and are
a) H, or
b) C,_~ alkyl;
RA5 is
a) OH,
b) C,_4 alkoxy, or
C) -NR88 R89,
Rgs is
a) H, or
b) C1_., alkyl optionally substituted with indolyl, OH, mercaptyl,
imidazoly, methylthio, amino, phenyl optionally substituted with OH,
-C(=O)-NH.,, -CO.,H, or -C(=NH)-NHz;
ftR~ is
a) H,
b) phenyl, or
c) C1_s alkyl optionally substituted by
OH;
R88 and at each occurrence are the same or
Re9 different and are
a) H,
b> C,_5 alkyl
c) C;,.s cycloalky, or
d) phenyl;
Rio is
a) C,.B alkyl optionally substituted with C,.~ alkoxy or C,_s hydroxy,
C.3_~ cycloalkyl, a 6-membered aromatic optionally benzo-fused
heterocyclic moiety having one to three nitrogen atoms, which can in
turn be substituted with one or two -NOz, CF.,, halo, -CN, OH, C,.S
alkyl, C,", alkoxy, or C,.~ acyl;
b> ,
c) phenyl, or
d) pyridyl;
-26-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Rg, is
a) C,_,s alkyl,
b) C,,_,s alkenyl,
wherein the substituents (a) and (b) can be optionally substituted with
C,.s alkoxycarbonyl, or a 5-, 6-, 7-membered aromatic heterocyclic
moiety having one to three atoms selected from the group consisting of
S, N, and O,
c) an aromatic moiety having 6 to 10 carbon atoms, or
d) a 5-, 6-, 7-membered aromatic heterocyclic moiety having one to three
atoms selected from the group consisting of S, N, and O,
wherein the substituents (c) and (d) can be optionally substituted with
carboxyl, halo, -CN, formyl, CF.,, -NO.~, C,.s alkyl, C,.s alkoxy, C,.s
acyl, C,_s alkylthio, or C,_6 alkoxycarbonyl;
R,~z and R,,;, at each occurrence are the same or different and are
a) H,
b> phenyl,
c) C,_s alkyl, or
d) benzyl;
Rg4 and R<,S at each occurrence are the same or different and are
a) H,
b) OH,
c) C,_s alkyl optionally substituted with -NRs3 Re4, or
d ) R,,n and R,~,s taken together are =O;
Rgs is
a) an aromatic moiety having 6 to 10 carbon atoms,
b) a 5-, or 6-membered aromatic optionally benzo-fused
heterocyclic moiety having one to three atoms selected from the group
consisting of S, N, and O,
wherein the substituents (a) and (b) which can in turn be substituted
with one or three -NO.>, CF." halo, -CN, OH, phenyl, C,_~ alkyl, C,_.,
alkoxy, or C,.S acyl,
c) morpholinyl,
d ) OH,
e) C,_s alkoxy,
f7 -NRR3RH;,
g> -C(=O)-R<", or
-27-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
h) < ~ ;
O
Rg~ is
a? morpholinyl,
b) OH, or
c) C,.~ alkoxy;
h is 1, 2, or 3;
i is 0, 1, or 2;
jis0orl;
k is 3, 4, or 5;
lis2or3;
mis4or5;
n is 0, 1, 2, 3, 4, or 5;
p is 0, 1, 2, 3, 4, or 5; with the proviso that n and p together are 1, 2, 3,
4, or 5;
q is 1, 2, 3, or 4;
r is 2, 3, or 4;
t is 0, i, 2, 3, 4, 5, or 6;
a is 1 or 2;
w is 0, 1, 2, or 3.
DETAILED DESCRIPTION OF THE INVENTION
The new compounds of the invention can be prepared using known
compounds and intermediates of oxzolidinones, isoxazolines and butyolactones
as
intermediates and synthetic methods known in the art. Thioamides of the
invention
can typically be prepared by the reaction of the corresponding amide with
Lawesson's reagent.
Compounds disclosed in the following publications are suitable intermediates
for preparation of the compounds of this invention and are hereby incorporated
by
reference for their disclosure of suitable compounds that can be converted to
the
subject thiocarbonyl derivatives.
U.S. Patents 5,225,565; 5,182,403; 5,164,510; 5,247,090; 5,231,188; 5,565,571;
5,547,950; and 5,523,403.
PCT Application and publications PCT/LTS93/04850, W094/01110;
PCT/US94/08904, W095/07271; PCT/US95/02972, W095/25106; PCT/US95/I0992,
W096/13502; PCT/US96/05202, W096/35691; PCT/US96/12766; PCT/LJS96/13726;
-28-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
PCT/US96/14135; PCT/US96/17120; PCT/US96/191-19; PCT/US97/01970;
PCT/US95/12751, W096/15130; and PCT/US96/00718, W096/23788.
Chemical conversion techniques for converting various intermediates having
a CH~,NH., on the oxazolidinone ring to CH.,NH-C(S)-CH.s is disclosed by
Hartke, K.,
Barrmeyer, S., J. prakt. Chem. 1996, 338, 251-6. Similarly, conversion of
CH.,NHC(=0>CH., to CH.,NHC(S>NHCH.f is reported by Cava, M.P.; Levinson, M.L,
Thionation Reactions of Lawesson's Reagents, Tetrahedron 1985, 41, 5061-87.
For the purpose of the present invention, the carbon content of various
hydrocarbon containing moieties is indicated by a prefix designating the
minimum
and maximum number of carbon atoms in the moiety, i.e., the prefix C;_~
defines the
number of carbon atoms present from the integer "i" to the integer "j",
inclusive.
Thus, C,_a alkyl refers to alkyl of 1-4 carbon atoms, inclusive, or methyl,
ethyl,
propyl, butyl and isomeric forms thereof.
The terms "C,_z alkyl", "C,,3 alkyl", "C,_~ alkyl", "C,.,, alkyl", "C,_s
alkyl", "C,_s
alkyl", and "C,.,s alkyl" refer to an alkyl group having one to two, one to
three, one
to four, one to five, one to six, one to eight, or one to sixteen carbon atoms
respectively such as, for example, methyl, ethyl, propyl, butyl, pentyl,
hexyl, heptyl,
octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl and their isomeric
forms
thereof.
The terms "Cl_4 alkenyl", "Cz.S alkenyl", "C2.8 alkenyl", "C2.,4 alkenyl" and
"C2_,s
alkenyl" refer to at least one double bond alkenyl group having two to four,
two to
five, two to eight, two to fourteen, or two to sixteen carbon atoms,
respectively such
as, for example, ethenyl, propenyl, butenyl, pentenyl, pentdienyl, hexenyl,
hexdienyl,
heptenyl, heptdienyl, octenyl, octdienyl, octatrienyl, nonenyl, nonedienyl,
nonatrienyl, undecenyl, undecdienyl, dodecenyl, tridecenyl, tetradecenyl and
their
isomeric forms thereof.
The terms "C.,_5 alkynyl", "Cz_8 alkynyl", and_ . "Cz.,o alkynyl" refer to at
least
one triple bond alkynyl group having two to five, two to eight, or two to ten
carbon
atoms respectively such as, for example, ethynyl, propynyl, butynyl, pentynyl,
pentdiynyl, hexynyl, hexdiynyl, heptynyl, heptdiynyl, octynyl, octdiynyl,
octatriynyl,
nonynyl, nonediynyl, nonatriynyl and their isomeric forms thereof.
The terms "C:,_~ cycloalkyl", "C:;_s cycloalkyl", "C~.s cycloalkyl", and
"C.,.e
cycloalkyl" refer to a cycloalkyl having three to four, three to six, five to
six, or three
to eight carbon atoms respectively such as, for example, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, and their isomeric forms
thereof.
The terms "C,__, alkoxy", "C,,s alkoxy", and "C,_H alkoxy" refer to an alkyl
group
-29-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/Z5308
having one to four, one to six, or one to eight carbon atoms respectively
attached to
an oxygen atom such as, for example, methoxy, ethoxy, propyloxy, butyloxy,
pentyloxy, hexyloxy, heptyloxy, or octyloxy and their isomeric forms thereof.
The terms "C~.6 alkylamino", and "C,_H alkylamino" refer to an alkyl group
having one to six, or one to eight carbon atoms respectively attached to an
amino
moiety such as, for example, methylamino, ethylamino, propylamino, butylamino,
pentylamino, hexyiamino, heptylamino, or octoylamino and their isomeric forms
thereof.
The terms "C,_6 dialkylamino", and "C1_~ dialkylamino" refer to two alkyl
groups having one to six, or one to eight carbon atoms respectively attached
to an
amino moiety such as, for example, dimethylamino, methylethylamino,
diethylamino, dipropylamino, methypropylamino, ethylpropylamino, dibutylamino,
dipentylamino, dihexylamino, methylhecylamino, diheptylamino, or dioctoylamino
and their isomeric forms thereof.
The terms "C,_3 acyl", "C1_4 acyl", "C,.S acyl", "C,_s acyl", "C1_e acyl", and
"C2_A acyl" refer to a carbonyl group having an alkyl group of one to three,
one to
four, one to five, one to six, one to eight, or two to eight carbon atoms.
The terms "C1_4 alkoxycarbonyl", "C1_6 alkoxycarbonyl", and "C1-e
alkoxycarbonyl" refer to an ester group having an alkyl group of one to four,
one to
six, or one to eight carbon atoms. -
The term "C,_g alkyl phenyl" refers to an alkyl group having one to eight
carbon atoms and isomeric forms thereof which is substituted with at least one
phenyl radical.
The term "C1_H alkenyl phenyl" refers to a at least one double bond alkenyl
group having one to eight carbon atoms and isomeric forms thereof which is
substituted with at least one phenyl radical.
The term "C,_R alkyl pyridyl" refers to an alkyl group having one to eight
carbon atoms and isomeric forms thereof which is substituted with at least one
pyridyl radical.
The term "C,_~ hydroxyl" refers to an alkyl group having one to eight carbon
atoms and isomeric forms thereof attached to a hydroxy group.
The term "C,_a alkylsulfonyl" refers to an alkyl group having one to eight
carbon atoms and isomeric forms thereof attached to a SO., moiety.
The term "C,_~ alkylthio" refers to an alkyl group having one to six carbon
atoms and isomeric forms thereof attached to a sulfur atom.
The term "Het" refers to 5 to 10 membered saturated, unsaturated or
-30-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
aromatic heterocyclic rings containing one or more oxygen, nitrogen, and
sulfur
forming such groups as, for example, pyridine, thiophene, furan, pyrazoline,
pyrimi-
dine, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-
pyrimidinyl, 3-
pyridazinyl, 4-pyridazinyl, 3-pyrazinyl, 2-quinolyl, 3-quinolyl, 1-
isoquinolyl, 3-
isoquinolyl, 4-isoquinolyl, 2-quinazolinyl, 4-quinazolinyl, 2-quinoxalinyl, 1-
phthalazinyl, 4-oxo-2-imidazolyl, 2-imidazolyl, 4-imidazolyl, 3-isoxazolyl, 4-
isoxazolyi,
5-isoxazolyl, 3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl, 2-oxazolyl, 4-oxazolyl, 4-
oxo-2-
oxazolyl, 5-oxazolyl, 4,5,-dihydrooxazole, 1,2,3-oxathiole, 1,2,3-oxadiazole,
1,2,4-
oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 2-thiazolyl, 4-thiazolyl, 5-
thiazolyl, 3-
isothiazole, 4-isothiazole, 5-isothiazole, 2-indolyl, 3-indolyl, 3-indazolyl,
2-
benzoxazolyl, 2-benzothiazolyl, 2-benzimidazolyl, 2-benzofuranyl, 3-
benzofuranyl,
benzoisothiazole, benzisoxazole, 2-furanyl, 3-furanyl, 2-thienyl, 3-thienyl, 2-
pyrrolyl,
3-pyrrolyl, 3-isopyrrolyl, 4-isopyrrolyl, 5-isopyrrolyl, 1,2,3,-oxathiazole-1-
oxide, 1,2,4-
oxadiazol-3-yl, 1,2,4-oxadiazol-5-yl, 5-oxo-1,2,4-oxadiazol-3-yl, 1,2,4-
thiadiazol-3-yl,
1,2,4-thiadiazol-5-yl, 3-oxo-1,2,4-thiadiazol-5-yl, 1,3,4-thiadiazol-5-yl, 2-
oxo-1,3,4-
thiadiazol-5-yl, 1,2,4-triazol-3-yl, 1,2,4-triazol-5-yl, 1,2,3,4-tetrazol-5-
yl, 5-oxazolyl, 1-
pyrrolyl, 1-pyrazolyl, 1,2,3-triazol-1-yl, 1,2,4-triazol-1-yl, 1-tetrazolyl, 1-
indolyl, 1-
indazolyl, 2-isoindolyl, ?-oxo-2-isoindolyl, l-purinyl, 3-isothiazolyl, 4-
isothiazolyl and
5-isothiazolyl, 1,3,4,-oxadiazole, 4-oxo-2-thiazolinyl, or 5-methyl-1,3,4-
thiadiazol-2-yl,
thiazoledione, 1,2,3,4-thiatriazole, 1,2,4-dithiazolone. Each of these
moieties may be
substituted as appropriate.
The term halo refers to fluoro, chloro, bromo, or iodo.
The compounds of the present invention can be converted to their salts,
where appropriate, according to conventional methods.
The term "pharmaceutically acceptable salts" refers to acid addition salts
useful for administering the compounds of this invention and include
hydrochloride,
hydrobromide, hydroiodide, sulfate, phosphate, acetate, propionate, lactate,
mesylate, maleate, malate, succinate, tartrate, citric acid, 2-hydroxyethyl
sulfonate,
fumarate and the like. These salts may be in hydrated form.
When Q is the structure of
(CH2)n ,R3s
E
~ (CH2)p
the dotted line in the heterocyclic ring means that this bond can be either
single or
double. In the case where the dotted line is a double bond, the R.,~ group
will not be
present.
-31-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
The compounds of Formula I of this invention contain a chiral center at C5 of
the isoxazoline ring, and as such there exist two enantiomers or a racemic
mixture
of both. This invention relates to both the enantiomers, as well as mixtures
containing both the isomers. In addition, depending on substituents,
additional
chiral centers and other isomeric forms may be present in any of A or R,
group, and
this invention embraces all possible stereoisomers and geometric forms in
these
groups.
The compounds of this invention are useful for treatment of microbial
infections in humans and other warm blooded animals, under both parenteral and
oral administration.
The pharmaceutical compositions of this invention may be prepared by
combining the compounds of this invention with a solid or liquid
pharmaceutically
acceptable carrier and, optionally, with pharmaceutically acceptable adjuvants
and
excipients employing standard and conventional techniques. Solid form composi-
tions include powders, tablets, dispersible granules, capsules, cachets and
suppositories. A solid carrier can be at least one substance which may also
function
as a diluent, flavoring agent, solubilizer, lubricant, suspending agent,
binder, tablet
disintegrating agent, and encapsulating agent. Inert solid carriers include
magnesium carbonate, magnesium stearate, talc, sugar, lactose, pectin,
dextrin,
starch, gelatin, cellulosic materials, low melting wax, cocoa butter, and the
like.
Liquid form compositions include solutions, suspensions and emulsions. For
example, there may be provided solutions of the compounds of this invention
dissolved in water and water-propylene glycol and water-polyethylene glycol
systems, optionally containing suitable conventional coloring agents,
flavoring
agents, stabilizers and thickening agents.
Preferably, the pharmaceutical composition is provided employing
conventional techniques in unit dosage form containing effective or
appropriate
amounts of the active component, that is, the compound according to this
invention.
The quantity of active component, that is the compound according to this
invention, in the pharmaceutical composition and unit dosage form thereof may
be
varied or adjusted widely depending upon the particular application, the
potency of
the particular compound, the desired concentration. Generally, the quantity of
active component will range between 0.5oJo to 90010 by weight of the
composition.
In therapeutic use for treating, or combatting, bacterial infections in warm-
blooded animals, the compounds or pharmaceutical compositions thereof will be
administered orally, parenterally and/or topically at a dosage to obtain and
maintain
-32-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
a concentration, that is, an amount, or blood-level of active component in the
animal
undergoing treatment which will be antibacterially effective. Generally, such
antibacterially effective amount of dosage of active component will be in the
range of
about 0.1 to about 100, more preferably about 3.0 to about 50 mg/kg of body
weight/day. It is to be understood that the dosages may vary depending upon
the
requirements of the patient, the severity of the bacterial infection being
treated, and
the particular compound being used. Also, it is to be understood that the
initial
dosage administered may be increased beyond the above upper level in order to
rapidly achieve the desired blood-level or the initial dosage may be smaller
than the
optimum and the daily dosage may be progressively increased during the course
of
treatment depending on the particular situation. If desired, the daily dose
may also
be divided into multiple doses for administration, e.g., 2-4 four times per
day.
When the compounds according to this invention are administered
parenteralIy, i.e., by injection, for example, by intravenous injection or by
other
parenteral routes of administration. Pharmaceutical compositions for
parenteral
administration will generally contain a pharmaceutically acceptable amount of
the
compound or a soluble salt (acid addition salt or base salt) dissolved in a
pharmaceutically acceptable liquid carrier such as, for example, water-for-
injection
and a buffer to provide a suitably buffered isotonic solution, for example,
having a
pH of about 3.5-6. Suitable buffering agents include, for example, trisodium
orthophosphate, sodium bicarbonate, sodium citrate, N-methylglucamine, L(+)-
lysine
and L(+)-arginine to name but a few representative buffering agents. The
compound
of this invention generally will be dissolved in the carrier in an amount
sufficient to
provide a pharmaceutically acceptable injectable concentration in the range of
about
1 mg/mL to about 400 mg/mL of solution. The resulting liquid pharmaceutical
composition will be administered so as to obtain the above-mentioned
antibacterially
effective amount of dosage. The compounds according to this invention are
advantageously administered orally in solid and liquid dosage forms.
As a topical treatment an effective amount of Formula I is admixed in a
pharmaceutically acceptable gel or cream vehicle that can be applied to the
patient's
skin at the area of treatment. Preparation of such creams and gels is well
known in
the art and can include penetration enhancers.
MIC Test Method
The in vitro MICs of test compounds were determined by a standard agar
dilution method. A stock drug solution of each analog is prepared in the
preferred
solvent, usually DMSO:H_,O ( 1:3). Serial 2-fold dilutions of each sample are
made
-33-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98125308
using I.0 ml aliquots of sterile distilled water. To each 1.0 ml aliquot of
drug is
added 9 ml of molten Mueller Hinton agar medium. The drug-supplemented agar is
mixed, poured into 15 x 100 mm petri dishes, and allowed to solidify and dry
prior
to inoculation.
Vials of each of the test organisms are maintained frozen in the vapor phase
of a liquid nitrogen freezer. Test cultures are grown overnight at 35°C
on the
medium appropriate for the organism. Colonies are harvested with a sterile
swab,
and cell suspensions are prepared in Trypticase Soy broth (TSB) to equal the
turbidity of a 0.5 McFarland standard. A 1:20 dilution of each suspension is
made in
TSB. The plates containing the drug supplemented agar are inoculated with a
0.001
ml drop of the cell suspension using a Steers replicator, yielding
approximately 10"
to I05 cells per spot. The plates are incubated overnight at 35°C.
Following incubation the Minimum Inhibitory Concentration (MIC ug/ml), the
lowest concentration of drug that inhibits visible growth of the organism, is
read and
recorded. The data is shown in Tables I and II.
-34-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
0. ~
p ~' ~ N
U
.~ ..
N
i
C v W ~
:3
N r.r
w O ~ '''
~ co .h
=~rf
=Z
O ~ O
O-1 ~ O~ C
2
.,.
~7
i
O O
I-2
O
a
0
a
-35-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
0. ~ ~ ~! N ..r
V7 1%
7
U
N b ~ N
G
,~ v
b
_
N N v.r
~ ~r
C4
N N r1 H N
~ C1
w
v
o~=c) a~a~ z-:
Z= _= Z= e:c;
z:
O
O ~ O~ O~ o
v t 1!f Z
z
tI1 ~ / / ~ / /
v ~) ~ w
W
z z z
Co) C) C) C~ °
O o 0
0
a
-36-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/Z5308
O N
w N
N
~
U
..' .a'
W
... ~..,
."
..
a' o
W N N
=C
C
v
z
~V
a
O
a
.Q
t ~ Z
< <~
~(' 3~s~x
-' a
z~ z~ ~ ~ 4..
a r ~ 4 °
v~ ai ~i v~ vi a
8.
0
a
' W ~ a~
..
~W~a~
V? f/) W V~ U~
-37-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
a
LL H H
h H H V1 N N H N vp N H h
N
O' O atN O N O ~ O ~ O O N ~ e'1~ V N th o~~ O O
a
up
~ ~
en --/~etN o0 ~ N O ~ ~ O of~ ~ ~ '~~"'~ N N N N
a
w~
Z
""
~_o
~ ~ N ~
~1 00/1~ ~?~ ~ ttN N N ~ A eo t ~t~ N
~1
U
H Y1H ~ 41H ~ H H
~~ e o o o o e o o e ~ p ~ ~ N ~ ~ .~o o e o
~ ~
H
~ N ~ ~ N
rN N Nr .N.n.N.v~ .. H H ~
G .
.. H .
~'~ E O e o ~ ~ e ~ e e o o . N ~ e ~ N E e e o
N
U
r
<
N
_
H H
h H H H H N H N N ~ r1
N
~ ,~ ~ N f Ip~ GiO ~
~' O ao~-O N O O O O O O N ~
a
H H H H H
H
~
.-.O N O ~ O ~ O O ~ O ~ ~ N ~ ~ O O O O
u
_
CG
~
<
<~ H H ~ H H
H
~
a ~ op~ ~ N O N O N O ~ ~ e'~aoN N O ~ O ~
O
H
V O ...N er1~ ~ r0h COOv N N N N N
N f~1V11~ h 00~ '. r ~ r ~ ~ r
E ~, n
-38-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
~U
a~
V1N
O p ~ d ~ N ~
U
_
F-
~
a
UO
N ~ O N ~ N N ~ N
V
rw
~
O
erf ~ N N 00~ 00'?_
H
_V
O O N
~
N N N N N N N h
E
O c c o c c ~ c
-'
a
~
E" ~ ~ N ~ N
~ ~
N N N Y1N
~'~ E o E ~ E o a o o ~
.~ h
y r
3 .~ v
..
~
t h
v
:
r' ~r,u,r, ~n d = ~ ~
N ~
~ ~ ~ a t~
O O O O ~ N N ~ ,
V 1r Q V V
3 00
a o c >,
V 3 a ~ C C a o
ri. vi vi tzl vi vi
~ ~ ~ W
h
v1N N
e01 O O O ~ N N - O
U ~ e:i i~i ~; o
~j
'~' ~ V'f f'~ ~ N
~. cn e~ O~
V1 V'1Y1N ,p
N
O~ O O O ~ N ~ ~ N ~
O f.
~0.azz~a
N N W :,~ ~ '~~,~ a t~ ~. a. a. -
u.
~ f/~ ~ T
~1
N r
.
GIG V~
a
Y
-39-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
As shown in Scheme 1, the intermediates II for the compounds of this
invention are also intermediates disclosed in the oxazolidinone patents and
published applications hereinabove incorporated by reference. The
intermediates
IV for this invention are final products (Examples) from the oxazolidinone
patents
and published applications hereinabove incorporated by reference.
As shown in Scheme 1, Step 1, and illustrated in Example 5, the
isothiocyanates III can be conveniently prepared by allowing the amine
intermediates (II) to react with 1,1'-thiocarbonyldi-2(1H)-pyridone in
solvents such
as methylene chloride at 0 to 25°C. The thioureas (Ia, R' = H,
alkyI,_,,) can then be
prepared as shown in Step 2 by the reaction of III with ammonia or the
appropriate
primary amines in solvents such as 1,4-dioxane or tetrahydrofuran at 0-
50°C.
Alternatively, as illustrated in Example 6 and shown in Step 3, the thioureas
can be
prepared by allowing II to react with an appropriate isothiocyanate (R' - N =
C = S)
in solvents such as tetrahydrofuran at 0-50°C. Thioamides (Ib, R" = H,
alkyl~~) are
prepared by allowing II to react with an appropriate dithioester (R"' S-C(=S)-
R",
Step 4 as illustrated in Example 4. This reaction is carried out in aqueous-
alcoholic
solvents at 0-50°C in the presence of an equivalent of an alkali metal
hydroxide.
This reaction, especially when R"' is methyl or ethyl, can be catalyzed by an
alkali
metal fluoride.
The reaction of II with R"'-S-C(S)-R"' (R"'=CH;~, C1H5) to give Ib (Step 4)
can
also be carried out in the presence of a tertiary amine base such as
triethylamine in
solvents such as THF, dioxane or methylene chloride at 10-50°C for 3-48
hr.
When the reaction conditions are tolerated by the substituents on R lsee, for
example, Examples 1-3) the thioamides (Ib, R" - H, alkyl,_,,) can also be
conveniently
prepared (Step 5) by allowing the appropriate amide intermediates (IV) to
react with
reagents such as 2,4-bis(p-methoxyphenyl)-1,3-dithiadiphosphetane-2,4-
disulfide
(Lawesson's Reagent) in 1,4-dioxane, benzene, toluene or tetrahydrofuran at 60-
110°C; phosphorus decasulfide and sodium carbonate in tetrahydrofuran
at 20-50°C
[Brillon, D., Synthetic Communications, 20, 3085 (1990)) or phosphorus
decasulfide
and sodium fluoride in 1,2-dimethoxyethane at 20-50°C (Hartke, K.,
Gerber, H.-D.,
J. Prakt. Chem., 338, 763 (1996)).
Compounds Ic are prepared (Step 6) by allowing II to react first with carbon
disulfide and a tertiary amine base such as triethylamine in solvent mixtures
containing water and methanol, ethanol or isopropanol at 10-50°C for 5-
24 hours.
The resulting intermediate is treated with an alkylating agent (R"" X where X
represents bromo, iodo, alkylsulfonyloxy or arylsulfonyloxy) at 0-30°C
to give
-40-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
compounds Ic. In Step 7, compounds Ic are allowed to react with alkali metal
alkoxide such as sodium methoxide or potassium ethoxide in the corresponding
alkanol as solvent. This reaction is conveniently carried out at the reflux
temperature of the aIkanol for 1-24 hr.
-41-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
SCHEME 1
i
N N
O ~ O
R-NH2 -,. p-N-C=S
II STEP 1
III
S
R'-NH2 R-NH-C-NH-R' (R' = H, alkyl-4)
STEP 2 la
II R'-N=C=S la (R' = H, alkyl~_4)
STEP 3
S S
II R~"-S-C-R" R-NH_C~ -R" (R~~ = H, alkyl~.4)
STEP 4 Ib
(R"' = CH3, C2H5, HOOC._CH2)
O
R-NH-C-R" Ib (R" = H, alkyl~_4)
--
IV STEP 5
S
II 1) CS2/Et3N (~ (R""= C~_4 alkyl, X=Br, I,
"" ~ RNH-C-SR"" OS02 alkyl, OS02 aryl)
2)R X
STEP 6 Ic
Ic MOR"'. S
""
HOR"" RNH-C-OR (M=L_i,+ Na,+K+)
STEP 7 Id
In order to more fully illustrate the nature of the invention and the manner
of practicing the same, the following experimental examples are presented.
-42-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
EXAMPLE 1: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]thioacetamide (I)
s
Me0 / \ PI\S~p / \ ~H~
oII s II~ oII
~N / \ N~p O S O N / \ N~p
~NH-C-CH 1.4-dioxane '--~ F H S
NH-C-CH3
II
C ~ sH2oFNa0sS
I
A stirred mixture of II (PCT/US94/08904, 3.37 g, 10.0 mmol) in dry dioxane
(100
mL), under nitrogen was treated with Lawesson's Reagent (4.04g, 10.0 mml),
warmed to reflux during 1 h and refluxed for 1.5 h. The reaction was complete
by
TLC on silica gel with lO~Jo MeOH-CHCI.~. It was kept at ambient temperature
for
18 h and concentrated in vacuo. Chromatography of the residue on silica gel
with
mixtures of acetone-methylene chloride containing 10-l5~lo acetone gave the
product
which was crystallized from acetone-hexane to give 1: mp 157.5-158.5
°C; HRMS
theory for C,sHioFN3O3S (M'): 353.1209; found: 353.1212. Anal. calcd for
C,6H2oFN30~S: C, 54.38; H, 5.38; N, 11.89; S, 9.07. Found: C, 54.21; H, 5.58;
N,
11.78; S, 8.93.
EXAMPLE 2: (S)-N-([3-[3-Fluoro-4-[4-(5-methyl-1,3,4-thiadiazol-2-yl)-1-
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide (2)
s
Me /_\ ~~S'P ~/ \--~
N-N /.~ O ~ ~S~S~ N-N O
~5~ U / \ ~pH ~~~N / \ ~O
V ...H S
~NHAc ~~''''~ II
~NH-Cra-i~
21
2
According to Example 1, for the preparation of 1, 21 (PCT/US97/01970) was
allowed
to react with Lawesson's Reagent in refluxing dioxane to give 2: mp 222-223
°C;
HRMS theory for C,~HzaFNfiO.,S., (M+H'): 451.1386; found 451.1381.
EXAMPLE 3: (S)-N-[[3-[3-Fluoro-4-[2',5'-dioxospiro[piperidine-4,4'-
imidazolidine]-
1-yl]phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide (3).
-43-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
STEP A: (S)-N-([3-[3-Fluoro-4-[2',5'-dioxospiro[piperidine-4,4'-imidazolidine]-
1-yl]phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide (32).
0 0 0
/ \ ~ T KCN
p -----.~ ~N~ / \ N~O
..,H (NF,~pp~ C N ,..H
H
~NHAc ~NHAc
31 C'
32
A stirred suspension of 31 (W095/25106, 0.349 g, 1.00 mmol) in 1:1 EtOH:HzO (5
mL), under nitrogen, was treated with potassium cyanide (0.130 g, 2.00 mmol)
and
ammonium carbonate (0.701 g, 7.30 mmol), warmed at 55-60 °C for 5 h 15
min and
kept at ambient temperature for 17 h 15 min. It was then chromatographed on
silica gel with mixtures of MeOH-NH40H-CHC13 containing 5-20% MeOH and 0.5%
NH40H to give 0.280 g of 32: HRMS calcd for C,9HlzFNsOs: 419.1605 (M'); found
419.1613; Anal. calcd for C,9H22FN505 ~ 1 HiO: C; 52.17; H, 5.53; N, 16.01.
Found:
C, 52.44; H, 5.30; N, 16.11.
STEP B: (S)-N-[[3-[3-Fluoro-4-[2',5'-dioxospiro[piperidine-4,4'-imidazolidine]-
1-yl]phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide (3).
s
HN~ O/ ~ Me0 ~ ~ PI~S~SI / \ oMe O
'~ O
x N ~ ~ N O HN-~/~ ''
~/ J X N ~ ~ N~O
~../N
o H ~ H ~N ...H S
NHAc O H F II
32 NH-C-CHI
C,eHz2FN50~S
3
A stirred suspension of 32 (0.210 g, 0.500 mmol) in dioxane (5.0 mL), under
nitrogen
was treated with Lawesson's Reagent (0.202 g, 0.500 mmol), refluxed for 4 h
and
concentrated in vacuo. The residue was chromatographed on silica gel with
mixtures of MeOH-NHaOH-CHCI.; containing 1-IO% MeOH and 0.1-0.5% NH40H
and the resulting product was crystallized from MeOH-CHCI.;-EtOAc to give
0.0491
g of 3: mp 218.5 °C; HR FAB MS theory for C,9H.~ZFN;,O.,S (M'):
435.1376; found
435.1370. Anal. calcd for C,~H.,.~FN50aS ~ 0.5 H.~O: C, 51.34; H, 5.21; N,
15.76.
Found: C, 51.69; H, 5.00; N, 15.25.
-44-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
EXAMPLE 4: (S)-N-([3-[3-Fluoro-4-(4-morpholinyl)phenyl~-2-oxo-5-
oxazolidinyl]methyl)thioacetamide (4).
s
0
O~N I ~ N~O HOC I~ O N I ~
N O
F H KOH / NaF V ~ ~H S
NHZ F NH-C-CHI
41
CvsHzoFNa03S
4
A solution of 41 ( 148 mg, 0.500 mmol) and 0.97 M KOH (0.515 mL) in absolute
EtOH (5 mL) was added to a solution of ethyl dithioacetate (57 ~tL, 0.50 mmol)
and
sodium fluoride (20 mg, 0.47 mmol) in absolute EtOH (5 mL) and the mixture was
kept
at ambient temperature for 3 h 40 min. Additional ethyl dithioacetate (5 ~L)
was added
after I h 55 min and additional 0.97 M KOH (40 mL) and sodium fluoride (6 mg)
were
added to the mixture after 3h 5 min. The reaction was followed by TLC on
silica gel
with 10% MeOH-CHCI, and 30% acetone-CH,Ch. The major product had an Rf on
TLC that was the same as that of 4.
EXAMPLE 5: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methylJthiourea (5).
STEP A:
s
a
O'I ~NiCwN~ _ OII
~N F ~ ~ N~O O O VN F ~ ~ Nn0
~NHp ~N=C=S
51 C,SH~sFN~03S
A solution of 51 (PCT/US94/08904, 2.07 g, 7.00 mmol) in CH,CI, was added,
dropwise
during 30 min, under nitrogen to an ice cold, stirred solution of 1,1'-
thiocarbonyldi-
2(1H)-pyridone (1.95 g, 8.40 mmol) in CH,CI, (70 mL). The mixture was warmed
slowly to ambient temperature and kept for 18 h. It was then diluted with
CH,CI,,
washed with water and aqueous NaCI, dried (Na,SO,) and concentrated.
Chromatography of the residue on silica gel with 10% acetonitrile-CH,CI, gave
1.60 g
of the isothiocyanate: HRMS theory for C,SH,~FN,O,S (M'): 337.0896: found
-45-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
337.0888.
STEP B:
0'I 0
~N ~ ~ N~O - N--3 -w O N ~ ~ N~O
~H ~.--~ ~ S
F N=C=S F NH_C-NHZ
C,SH,yFN,03S
5
Anhydrous ammonia was bubbled for 7 min through a stirred solution of the
product
from Step I ( 1.00 g, 2.96 mmol) in THF ( 10 mL) and the mixture was kept at
ambient
temperature for 3 h 25 min and concentrated in vacuo. Crystallization of the
residue
from acetone-hexane gave 0.861 g of 5: mp 199-199.5 °C: MS m/z 354
(M'). Anal.
calcd for C,SH,9FN~O,S: C, 50.84; H, 5.40; N, 15.81. Found: C, 50.87; H, 5.39;
N,
15.72.
EXAMPLE 6: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl)-2-oxo-S-
oxazolidinyl]methyl]-N'-methylthiourea (6).
0 0
/'1 ~ CH~NCS
VN / ~ N O ~ O N / ~ N O
F ~H ~l ~ H S
NHZ F ~NH-C-NHCH~
C,6Hy,FN~O~S
6
A stirred solution of methyl isothiocyanate (93 mg, 1.27 mmol) in THF, was
treated
with 6i (295 mg, 1.00 mmol), kept at ambient temperature for 18 h and
concentrated in
vacuo. The residue was recrystallized from EtOAc-hexane to give 246 mg of 6:
mp
158-160 °C: MS »r/.: 368 (M'). Anal. calcd for C,bH,,FN~O,S: C, 52.16;
H, 5.74; N,
15.21. Found: C. 52.20; H. 5.85: N. I S. I 7.
-46-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
EXAMPLE 7 (S)-cis-N-([3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-thiopyran-4-
yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]ethanethioamide
Step 1: A mixture of (S)-(-)-N-[[3-[3-fluoro-4-(3,6-dihydro-2H-thiopyran-4-
yl)phenyl]-2-nxo-5-oxazolidinyl]methyl]acetamide S-oxide (4.50 g, can be
obtained
according to the procedures disclosed in International Publication No. WO
97/09328)
and platinum oxide (697 mg) in methanol (164 mL) is shaken on the Pan
apparatus
under a hydrogen atmosphere at 40 psi for 18 hours. The catalyst is then
removed
by filtration through Celite, and the filtrate is concentrated under reduced
pressure
and the residue chromatographed on silica gel (230 - 400 mesh, 350 g), eluting
with
a gradient of methanol/methylene chloride (3/97 - 7/93). Pooling and
concentration
of those fractions with an R~ = 0.44 by TLC (methanol/chloroform, 10/90) gives
(S)-
cis-(-)-N-[[3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-thiopyran-4-yl)phenyl]-2-oxo-
5-
oxazolidinyl]methyl]acetamide, mp 203 - 204°C.
Step 2: A mixture of the compound prepared in Step 1 (2.50 g) and
hydroxylamine hydrochloride (2.36 g) in pyridine (30.6 mL) and ethanol (3.4
mL) is
stirred in a screw-cap vial at 100°C for 22 hrs and at ambient
temperature for 16
hrs, during which additional hydroxylamine hydrochloride (944 mg) and pyridine
(4
mL) is added. The reaction mixture is then concentrated under reduced
pressure,
diluted with saturated aqueous sodium bicarbonate (100 mL) and saline (50 mL),
adjusted to pH 11 with solid sodium carbonate and extracted with
methanol/methylene chloride ( 10/90, 5 x 100 mL). The combined organic phase
is
concentrated under reduced pressure, and the crude product is chromatographed
on
silica gel (230 - 400 mesh, 150 g), eluting with a gradient of
methanol/methylene
chloride (6/94 - 10/90). Pooling and concentration of those fractions with an
R~ _
0.14 by TLC (methanol/chloroform, 10/90) gives (S)-cis-3-[3-fluoro-4-
(tetrahydro-1-
oxido-2H-thiopyran-4-yl)phenyl]-5-aminomethyl-2-oxazolidinone, mp I59 -
161°C.
Step 3: A solution of ethyl dithioacetate ( 105 mL, 0.919 mmoi) and sodium
fluoride (39 mg, 0.919 mmol) in ethanol (9.2 mL) under a nitrogen atmosphere
was
treated with a mixture of (S)-cis-3-(3-fluoro-4-(tetrahydro-1-oxido-2H-
thiopyran-4-
-47-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
yl)phenyl]-5-aminomethyl-2-oxazolidinone, as prepared in Step 2,(300 mg, 0.919
mmol) and aqueous potassium hydroxide (1M, 0.92 mL) in ethanol (46 mL). The
resulting solution was stirred at ambient temperature for 4 hours and was then
diluted with methylene chloride (150 mL) and washed with water (50 mL),
aqueous
potassium hydrogen sulfate (1M, 50 mL) and brine (25 mL). The organic phase
was
dried over anhydrous sodium sulfate and concentrated in uacuo, and the crude
product was triturated with methylene chloride/diethyl ether and filtered to
give the
title compound, mp 176 - 177oC (dec. ).
EXAMPLE 8 (S)-cis-[[3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-thiopyran-4-
yl )phenyl]-2-oxo-5-oxazolidinyl] methyl] thiourea
Step 1: A solution of 1,1'-thiocarbonyldi-2(1H)-pyridone (235 mg, 1.01
mmol) in anhydrous methylene chloride (10 mL) at OoC under a nitrogen
atmosphere was treated with a solution of (S)-cis-3-[3-fluoro-4-(tetrahydro-1-
oxido-
2H-thiopyran-4-yl)phenyl]-5-aminomethyl-2-oxazolidinone, as prepared in
Example
7, Step 2, (275 mg, 0.843 mmol) in anhydrous methylene chloride (34 mL> over
30
minutes. The resulting mixture was stirred at OoC for 30 minutes and at
ambient
temperature for 1 hour and was then diluted with methylene chloride (40 mL),
washed with water (25 mL) and brine (25 mL), dried over anhydrous sodium
sulfate
and concentrated in uacuo. The crude product was chromatographed on silica gel
(70 - 230 mesh, 20 g), eluting with acetonitrile/methylene chloride (40/60),
and those
fractions with an R~ = 0.0? by TLC (acetonitrile/methylene chloride, 30/70)
were
pooled and concentrated to give (S)-cis-3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-
thiopyran-4-yl)phenyl]-5-isothiocyanatomethyl-2-oxazolidinone, mp 187 - 190oC
( dec. ).
Step 2: A solution of (S)-cis-3-[3-fluoro-4-(tetrahydro-1-oxido-2H-thiopyran-4-
yl)phenyl]-5-isothiocyanatomethyl-2-oxazolidinone (Step 1, 290 mg, 0.787 mmol)
in
anhydrous tetrahydrofuran (39 mL) at OoC under a nitrogen atmosphere was
treated
-48-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
(bubbled) with a stream of ammonia gas for 5 minutes. The reaction pot was
sealed,
and the resulting mixture was stirred at OoC for 1 hour. The excess ammonia
was
then removed under a stream of nitrogen, and the reaction mixture was
concentrated in vacuo to give the crude product. Recrystallization from
methanol/methylene chloride/diethyl ether gave the title compound, mp 206 -
208oC
(dec. ).
EXAMPLE 9 (S)-traps-N-[[3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-thiopyran-4-
yl)phenyl)-2-oxo-5-oxazolidinyl)methyl)ethanethioamide
o,,,
Step 1: (S)-(-)-N-[[3-[3-fluoro-4-(3,6-dihydro-2H-thiopyran-4-yl)phenyl)-2-oxo-
5-ox azolidinyl)methyl)acetamide S-oxide (disclosed in International
Publication No.
WO 97/09328) may be reduced to the corresponding cis- and traps-sulfoxides by
catalytic hydrogenation in the presence of a catalyst and solvent.
Alternatively, the
sulfide by product of this reduction reaction can be oxidized with an
oxidizing agent
such NaIOa or meta-chloroperoxybenzoic acid in solvent to provide the cis- and
traps-sulfoxides. Alternatively, the sulfide byproduct acn be oxidized
selectively to
the traps isomer using t-butyl hydroperoxide and a catalyst such as Ti(OiPr)4
and
D-diisopropyl tartrate in a suitable solvent. The isomeric mixture can then be
separated by chromatography to isolate the traps-sulfoxide, mp 211 -
212°C (dec.).
A mixture of the traps-sulfoxide, (S)-traps-(-)-N-[[3-(3-fluoro-4-(tetrahydro-
1-oxido-
2H-thiopyran-4-yl)phenyl)-2-oxo-5-oxazolidinyl)methyl)acetamide, (0.90 g) and
hydroxylamine hydrochloride (0.85 g) in pyridine ( 11.0 mL) and ethanol ( 1.2
mL) is
stirred in a screw-cap vial at 100°C for 23 hrs and at ambient
temperature for 19
hrs, during which additional hydroxylamine hydrochloride (340 mg) and pyridine
(1
mL) is added. The reaction mixture is then concentrated under reduced
pressure,
diluted with saturated aqueous sodium carbonate (50 mL) and saline (50 mL) and
extracted with methanol/methylene chloride ( i0/90, 6 x 100 mL). The combined
organic phase is concentrated under reduced pressure, and the crude product is
-49-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
chromatographed on silica gel (230 - 400 mesh, 45 g), eluting with a gradient
of
methanol/methylene chloride (7.5/92.5 - 10/90). Pooling and concentration of
those
fractions with an Rf = 0.14 by TLC (methanol/chloroform, 10/90) gives (S)-
trans-3-[3-
fluoro-4-( tetrahydro-1-oxido-2H-thiopyran-4-yl )phenyl)-5-aminomethyl-2-
oxazolidinone, mp 138 - 140°C.
Step 2: A solution of ethyl dithioacetate (105 mL, 0.919 mmol) and sodium
fluoride (39 mg, 0.919 mmol) in ethanol (9.2 mL) under a nitrogen atmosphere
was
treated with a mixture of (S)-trans-3-[3-fluoro-4-(tetrahydro-1-oxido-2H-
thiopyran-4-
yl)phenyl]-5-aminomethyl-2-oxazolidinone, as prepare in Step 1, (300 mg, 0.919
mmol) and aqueous potassium hydroxide (1M, 0.92 mL) in ethanol (46 mL). The
resulting solution was stirred at ambient temperature for 17 hours and was
then
diluted with methylene chloride (150 mL), washed with water (2 x 50 mL) and
brine
(25 mL), dried over anhydrous sodium sulfate and concentrated ire vacuo. The
crude
product was chromatographed on silica gel (230 - 400 mesh, 35 g), eluting with
methanol/methylene chloride (3/97), and those fractions with an R~ = 0.56 by
TLC
(methanol/chloroform, 10/90) were pooled and concentrated and the residue
recrystallized from methylene chloride/diethyl ether to give the title
compound, mp
193 - 194°C (dec.).
EXAMPLE 10 (S)-trans-[[3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-
thiopyran-4-yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]thiourea
O;,
Step 1: A solution of 1,1'-thiocarbonyldi-2( 1H)-pyridone ( 192 mg,
0.827 mmol) in anhydrous methylene chloride (8.3 mL) at OoC under a nitrogen
atmosphere was treated with a solution of (S)-trans-3-[3-fluoro-4-(tetrahydro-
1-oxido-
2H-thiopyran-4-yl)phenyl]-5-aminomethyl-2-oxazolidinone, as prepared in
Example
9, Step 1, (225 mg, 0.689 mmol) in anhydrous methylene chloride (28 mL) over
30
minutes. The resulting mixture was stirred at OoC for 30 minutes and at
ambient
temperature for 40 minutes and was then diluted with methylene chloride (20
mL),
-50-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
washed with water (15 mL> and brine (15 mL), dried over anhydrous sodium
sulfate
and concentrated in vacuo. The crude product was chromatographed on silica gel
(32 - 63 mm, 40 g), eluting with a gradient of acetonitrile/methylene chloride
(30/70 -
60/40) under 15 psi. Nz, and those fractions with an R~= 0.12 by TLC
(acetonitrile/methylene chloride, 30/70) were pooled and concentrated to give
(S)-
trans-3-[3-Fluoro-4-(tetrahydro-1-oxido-2H-thiopyran-4- yl)phenyl]-5-
isothiocyanatomethyl-2-oxazolidinone, mp 165 - 167°C.
Step 2: A solution of (S)-trans-3-[3-fluoro-4-(tetrahydro-1-oxido-2H-
thiopyran-4-yl)phenyl]-5-isothiocyanatomethyl-2-oxazolidinone (Step 1, 230 mg,
0.624
mmol) in anhydrous tetrahydrofuran (31.2 mL) at OoC under a nitrogen
atmosphere
was treated (bubbled) with a stream of ammonia gas for 5 minutes. The reaction
pot was sealed, and the resulting mixture was stirred at OoC for i hour. The
excess
ammonia was then removed under a stream of nitrogen, and the reaction mixture
was concentrated in uacuo to give the crude product. Trituration with
methanol/methylene chloride/diethyl ether gave the title compound, mp 209 -
210°C
(dec.).
EXAMPLE 11 (S)-N-([3-[3-Fluoro-4-(tetrahydro-1,1-dioxido-2H-
thiopyran-4-yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]ethanethioamide
oz
Step i: Starting with (S)-cis-(-)-N-[[3-(3-Fluoro-4-(tetrahydro-1-oxido-
2H-thiopyran-4-yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide as prepared in
Example ?, Step 1, and following the general procedure of Step 2, and making
non-
critical variations by substituting (S)-(-)-N-[[3-[3-fluoro-4-(tetrahydro-2H-
thiopyran-4-
yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide S,S-dioxide (disclosed in
International Publication No. WO 97/09328) for (S)-cis-(-)-N-[[3-[3-fluoro-4-
(tetrahydro-1-oxido-2H-thiopyran-4-yl )phenyl] -2-oxo-5-oxazolidinyl] methyl]
acetamide,
the product (S)-(-)-3-[3-Fluoro-4-(tetrahydro-1,1-dioxido-2H-thiopyran-4-
yl)phenyl]-5-
aminomethyl-2-oxazolidinone is obtained, mp 194°C (dec. ).
-51-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Step 2: A solution of ethyl dithioacetate (100 mL, 0.$76 mmol) and sodium
fluoride (37 mg, 0.876 mmol) in ethanol (8.8 mL) under a nitrogen atmosphere
was
treated with a mixture of (S)-(-)-3-[3-fluoro-4-(tetrahydro-1,1-dioxido-2H-
thiopyran-4-
yl)phenyl]-5-aminomethyl-2-oxazolidinone, as prepared in Step 1, (300 mg,
0.876
mmol) and aqueous potassium hydroxide (1M, 0.88 mL) in ethanol (43.8 mL). The
resulting mixture was stirred at ambient temperature for 26 hours, during
which
additional ethyl dithioacetate (50 mL, 0.438 mmol), sodium fluoride (19 mg,
0.438
mmol), aqueous potassium hydroxide (IM, 0.44 mL) and ethanol (3.0 mL> was
added, and was then diluted with methylene chloride (150 mL), washed with
water
(50 mL), aqueous potassium hydrogen sulfate (IM, 50 mL) and brine (25 mL),
dried
over anhydrous sodium sulfate and concentrated in aacuo. The crude product was
recrystallized from methylene chloride/diethyl ether to give the title
compound, mp
186 - 187oC (dec.).
EXAMPLE 12 (S)-N-[[3-[3-Fluoro-4-(tetrahydro-1,1-dioxido-2H-
thiopyran-4-yl)phenyl]-2-oxo-5-oxazolidinyl] methyl] thiourea
S
li
,C
~NH2
H
H
Step 1: A solution of 1,1'-thiocarbonyldi-2(1H)-pyridone (304 mg, 1.31 mmol)
in anhydrous methylene chloride (13 mL) at OoC under a nitrogen atmosphere was
treated with a solution of (S)-(-)-3-[3-fluoro-4-(tetrahydro-1,1-dioxido-2H-
thiopyran-4-
yl)phenyl]-5-aminomethyl-2-oxazolidinone, as prepared in Example 11, Step 1,
(375
mg, 1.09 mmol) in anhydrous methylene chloride (88 mL) over 30 minutes. The
resulting mixture was stirred at OoC for 30 minutes and at ambient temperature
for
30 minutes and was then diluted with methylene chloride (40 mL), washed with
water (25 mL) and brine (25 mL), dried over anhydrous sodium sulfate and
concentrated in vacuo. The crude product was chromatographed on silica gel
(230
400 mesh, 45 g), eluting with acetonitrile/methylene chloride (7.5/92.5), and
those
fractions with an Rf= 0.64 by TLC (acetonitrile/methylene chloride, 20/80)
were
pooled and concentrated to give (S)-3-[3-fluoro-4-(tetrahydro-1,1-dioxido-2H
-52-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
thiopyran-4-yl)phenyl]-5-isothiocyanatomethyl-2-oxazolidinone, mp 158 - 162oC
(dec. ).
Step 2: A solution of (S)-3-[3-fluoro-4-(tetrahydro-1,1-dioxido-2H-
thiopyran-4-yl)phenyl]-5-isothiocyanatomethyl-2-oxazolidinone (Step 1, 380 mg,
0.988
mmol) in anhydrous tetrahydrofuran (49 mL) at OoC under a nitrogen atmosphere
was treated (bubbled) with a stream of ammonia gas for 5 minutes. The reaction
pot was sealed, and the resulting mixture was stirred at OoC for 1 hour. The
excess
ammonia was then removed under a stream of nitrogen, and the reaction mixture
was concentrated in uacuo to give the crude product. Recrystallization from
methanol/methylene chloride/diethyl ether gave the title compound, mp 196 -
198oC
(dec. ).
EXAMPLE 13: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl)-2-oxo-5-
oxazolidinyl)methyl)-thioformamide ('7).
O O
1~ ~ N / ~ N~O HCOOH
F ...H Ac20 ~N / ~ N~O'H
~NH2 F
NHCHO
39 6
A stirred mixture of acetic anhydride (0.23 mL, 0.0024 mol) and 95-97~Jo
formic acid
(0.10 mL, 0.0027 mL) was warmed, under nitrogen at 50-55 °C for 2 h,
cooled to
ambient temperature and treated, portionwise during 2 min, with 398 (0.45 g,
0.0015
mol). The suspension was kept at ambient temperature for 4 h and the resulting
solution was treated with EtlO ( 1 mL) and kept at ambient temperature for 18
h.
The mixture was diluted with additional EtzO ( 10 mL) and the solid was
collected by
filtration, washed with Et.~O and dried to give 0.38 g of 69: MS (ES) m /z 324
(M+H'), 346 (M+Na'); 'H NMR (300 mHz, CDCI.j) d 3.08 (m, 4H), 3.72 (m, 2H),
3.77
(d,d, 1H), 3.89 (m, 4H), 4.04 (t, 1H), 4.80 (m, 1H), 6.33 (s, 1H), 7.05 (m,
2H), 7.45
(d,d, 1H), 8.27 (s, 1H).
-53-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
2.
CH~O / ~ p~S~p / ~ OCH
N O 4 /SI
O 1 / ~ N O
F ~H ~ ",H
~NHCHO dioxane F
~NH-CHS
A stirred mixture of 6 (0.38 g, 0.00118 mol) in dioxane (20 mL), under
nitrogen was
treated with 4 (0.51 g, 0.00126 mol), warmed to reflux during 30 min and kept
at
this temperature for 90 min. It was then evaporated under a stream of
nitrogen.
The residue was chromatographed on silica gel with 1.25% MeOH-CHICK and the
slightly impure product was rechromatographed on silica gel with 25% EtOAc-
CHZCIz. The resulting product was crystallized from EtOAc-methyl tent-butyl
ether
to give 0.114 g of 7: mp 150-155 °C (dec); IR (DRIFT) 3322, 1752 cm-';
MS(ES) m/z
340 (M+H+), 362 (M+Na'); 'HNMR (300 MHz, (CD~)iS0] d 2.94 (m, 4H), 3.72 (m,
4H), 3.77 (d,d, 1H), 3.94 (t, 2H), 4.12 (t, 1H), 4.93 (m, 1H), 7.05 (t, 1H),
7.16 (d,d,
1H), 7.47 (d,d, 1H), 9.33 (d, 1H), 10.59 (s, 1H). Anal. calcd for
C'5H,8FN303S: C,
53.08; H, 5.35; N, 12.38. Found: C, 53.02; H, 5.44; N, 12.36.
EXAMPLE 14: (S)-N-[[3-[3-FTuoro-4-(4-morpholinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl)thiopropion-amide (9).
1. O O
O N / ~ ~ CH3CH2COC1
N O -------~ O N / ~ N O
",H Et3N ~--~ H O
F ~ F ~ ii
NH2 NH-C-CH2CH3
39
An ice cold, stirred solution of 39 (0.395 g, 0.00134 mol) and triethyl amine
(0.186
mL, 0.002? mol) in CHzCl1 (20 mL), under nitrogen was treated, dropwise during
2
min, with a solution of propionyl chloride (0.128 mL, 0.0014? mol> in CHIClz
(3 mL).
The mixture was kept in the ice bath for 20 min and at ambient temperature for
1
h. It was then diluted with CH.~CIz, washed with saturated NaHCO;~, water and
brine, dried (MgSOa) and concentrated. The residue (8) was used without
further
purification in the next reaction.
-54-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
2. S
/ \ ~~~ / \
O ~~I~ O
O / \ ~O S n
/\
Nt+C- V ~H
8 Iw-~y
9
A stirred mixture of the product (8) from the previous reaction and dioxane
(20 mL),
under nitrogen, was treated, portionwise during 1 min, with Lawesson's reagent
(0.58 g, 0.0014 mol) and refluxed for 2 h; it was then concentrated. The
residue was
chromatographed on silica gel with 2% MeOH-CHCl3 and the product was
crystallized from methyl tert-butyl ether to give 0.259 g of 9: mp 138-139
°C;
MS(ES) m /z 368 (M+H'), 390 (M+Na'); IR (DRIFT) 3284, 3266, 1748, 1744 cm-';
[a)1'p +20° (MeOH); 1H NMR[300 MHz, (CD3)1S0) d 1.12 (t, 3H), 2.56 (q,
2H), 2.94
(m, 4H), 3.72 (m, 4H), 3.78 (d,d, 1H), 3.90 (t, 2H), 4.11 (t, 1H), 4.93 (m,
1H), 7.05 (t,
1H), 7.16 (d,d, 1H), 7.47 (d,d, 1H), 10.30 (broad s, 1H). Anal. calcd for
Cl7HzzFNsOsS: C, 55.57; H, 6.03; N, 11.44. Found: C, 55.68; H, 6.21; N, 11.37.
EXAMPLE 15: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-6-
oxazolidinyl]methyl]-2-chlorothioacetamide (11).
1. O O
O N / ~ N~O CICH2C~ O N
L--~ ,..H Et3N ~ ~N O
~ ~H O
~NH2 ~NH-C-CH CI
39
25 A stirred solution of 39 (1.54 g, 5.2 mmol) and triethylamine (750 mg, 7.5
mmol) in
CHZCh (50 mL), under nitrogen, was treated, dropwise, during 15 min with a
solution of chloroacetyl chloride (465 mL, 5.8 mmol) in CHlCIz (30 mL) and
kept at
ambient temperature for 18 h. It was then washed with saturated NaHCO~, and
dilute NaCl, dried (Na2S04) and concentrated. The residue was flash
30 chromatographed on silica gel with 20-30% acetone-CH2Clz to give 1.49 g of
109
which was used in the next reaction without further purification.
-55-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
2. S
O / \ ~~~ / \
III
O / \ NCO S O / \ O
t--(...H O ~--i
~H S
NH-C Ct+~Q
11
A stirred mixture of 10 (0.371 g, 1.0 mmol) and Lawesson's reagent (0.420 mg,
1.04
mmol) in dioxane (10 mL) was refluxed, under nitrogen for 2 h and concentrated
under reduced pressure. The residue was chromatographed on silica gel with 3-
10%
10 acetone-CHlCl1 to give 0.143 g of I1: MS (CI) mlz 388 (M+H'); 'H NMR (300
MHz,
CDCh) d 3.07 (m, 4H), 3.77 (d,d, 1H), 3.88 (m, 4H), 4.04 (m, 1H), 4.12 (t,
1H), 4.35
(m, 1H), 4.61 (s, 2H), 4.98 (m, 1H), 6.96 (t, 1H), 7.08 (d,d, 1H), 7.44 (d,d,
1H), 8.69 (s,
1H). Anal. calcd for C,~H,3CIFN:,O~S: C, 49.55; H, 4.94; N, 10.83. Found: C,
49.38;
H, 5.20; N, 10.27.
EXAMPLE 16: (S)-N-[[3-[3-Fluoro-4-(4-moropholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-a,a,a-trifluorothioacetamide (13).
1.
O O
VN / ~ N~O (CF3C0~ O N
N O
F ~H Et3N ~ F ~H O
'NH2 ~NH-C-CF3
39 12
An ice cold stirred solution of 39 (0.590 g, 2.0 mmol) and triethylamine (640
mL, 4.6
mmol) in CHzCIz (10 mL) was treated with trifluoroacetic anhydride (325 mL,
2.3
mmol) and kept in the ice bath for 10 min and then at ambient temperature. The
reaction was followed by TLC on silica gel with 30% acetone-CH2Clz. Additional
trifluoroacetic anhydride and triethylamine were added after 3 d (64 mL / 125
mL),
4 d (I00 mL / 220 mL) and 6 d (325 mL / 1.0 mL). The reaction was complete 1 h
after the last addition; it was mixed with CH.~C1.,, washed with water and
dilute
NaCI, dried (Na.,SOa) and concentrated. The solid residue was- recrystallized
from
acetone-heptane to give 0.566 g of 12: mp 161-164 °C (dec); MS(EI) m/z
391 (M').
Anal. calcd for C1~H"F,N,jO,: C, 49.11; H, 4.38; N, 10.74. Found: C, 48.99; H,
4.56;
N, 10.73.
-56-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
S
2. O / \ ~p~~ / \
O / \ ~O ~S p / \ ~O
~H O a
~H S
N+GCF3 ~N+~GCF3
12 13
A stirred mixture of i2 (0.391 g, 1.0 mmol) and Lawesson's reagent (0.422 g,
1.1
mmol) in dioxane (10 mL) was refluxed, under nitrogen for 2 h, cooled slowly
to
ambient temperature and concentrated in vacuo. The residue was flash
chromatographed on silica gel with 5-15% acetone-CH.~C11 and the product was
crystallized from acetone-heptane to give 0.249 g of 13: mp 151-152 °C;
MS(EI) m /z
407 (M'), 363, 209, 151, 95; 'H NMR (300 MHz, CDC13) d 3.05 (m, 4H), 3.75
(d,d,
1H), 3.87 (m, 4H), 3.95 (m, 1H), 4.14 (t, 1H), 4.32 (m, 1H), 5.01 (m, 1H);
6.92 (t, 1H),
?.05 (d,d, 1H), 7.38 (d,d, 1H), 9.03 (s, 1H). Anal. calcd for C,~H"F~,N;~03S:
C, 47.17;
H, 4.21; N, 10.31. Found: C, 47.09; H, 4.35; N, 10.27.
EXAMPLE 17: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl)-a-fluorothioacetamide (16).
O O
1. ~N ~ ~ N~0 FCEiC~ ~N ~ ~ N~O
F ~H 3 F ~H O
1~NH2 'NH-C-CH2F
39 14
A stirred, ice cold solution of 39 (0.590 g, 2.0 mmol) and triethylamine (611
mL, 4.4
mmol) in CHICK (10 mL), under nitrogen, was treated, dropwise, with a solution
of
fluoroacetyl chloride (220 mL, 2.2 mmol) in CH2C11 (5 mL), kept in the ice
bath for
10 min and at ambient temperature for 2 h. It was then diluted with CHlCl2,
washed with water and dilute NaCI, dried (NalSO~) and concentrated. The
residue
was chromatographed on silica gel with IO-30% acetone-CH.~CIz to give 0.180 g
of 14:
MS(ES) m/z 356 (M+H'), 378 (M+Na').
2.
O CH~O / ~ P~S~P / ~ pCH~
_ S ~~
F/\N~H O S VN/~N O
~NH-G-CH F F ~H S
1d 2 NH-C-CHEF
-57-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
A solution of 14 (O.I80 g, 0.507 mmol) in dioxane, under nitrogen, was treated
with
Lawesson's reagent (0.206 g, 0.51 mmol), warmed at 90-100 °C for 1
h and
concentrated in vacuo. The residue was chromatographed on silica gel with 15%
acetone-CH.,CIz to give 0.161 g of 15: MS(EI) m/z 371 (M'); 'H NMR (300 MHz,
CDCI;,) d 3.05 (m, 4H), 3.78 (d,d, 1H), 3.87 (m, 4H), 4.03 (m, 1H), 4.11 (t,
1H), 4.38
(m, 1H), 4.98 (m, 1H), 5.07 (s, 1H), 5.23 (s, 1H), 6.93 (t, 1H), 7.08 (dd,
1H), 7.42 (d,d,
1H), 8.42 (s, 1H). Anal. calcd for C,6H,9F.,N~O;'S: C, 51.74; H, 5.16; N,
11.31.
Found: C, 51.79; H, 5.31; N, 11.02.
EXAMPLE 18: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-a,a-difluorothioacetamide (1?).
1. o O
O N / ~ N~O + F2CHCOOH EDC~~ O 1 / ~ N~O
~ ,"H HOBT U ~ ",H O
F F ~ II
~NH2 NH-C-CHF2
39 16
A stirred, ice cold mixture of 39 (0.590 g, 2.0 mmol), difluroacetic acid (I90
mL, 2.0
mmol), and 1-hydroxybenzotriazole (0.297 g, 2.2 mmol) in DMF (5 mL) under
nitrogen, was treated with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (0.843 g, 4.4 mmol) and kept at ambient temperature for 18 h. It
was
diluted with CH.~CIz, washed with water and dilute NaCI, dried (NazSO~) and
concentrated. The solid residue was crystallized form EtOAc-heptane to give
0.617 g
of 16: mp 149-150 °C; 1H NMR (300 MHz, CDC13) d 3.05 (m, 4H), 3.66 (m,
2H),
3.85 (m, 5H), 4.08 (t, 1H), 4.80 (m, 1H), 5.93 (t, J = 53.9 Hz, 1H), 6.92 (t,
1H), 7.06
(m, 2H), 7.39 (d,d, 1H); MS(EI) m/z 373 (Mi). Anal. calcd for C,~H,HF.,N.,O~:
C,
51.48; H, 4.86; N, 11.26. Found: C, 51.59; H, 4.91; N, 11.29.
2.
p O
~N / ~ N~O ----r O N / \ N~O.
F ,"H O U ~ ~ H S
F ~ II
~NH-C-CHF2 NH-C-CHF
2
16
17
A stirred solution of 16 (0.373 g, 1.00 mmol) in dioxane (10 mL), under
nitrogen was
treated with Lawesson's reagent (0.404 g, 1.00 mmol), warmed at about 95
°C for 1
-58-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
h and concentrated in vacuo. Chromatography of the residue on silica gel with
1090
acetone-CH=Ch and cyrstallization of the product from EtOAc-heptane gave 0.276
g
of 17: mp 125-127 °C; MS(EI) m/z 389 (M'), 345, 305, 247, 209, 195,
151, 138, 123,
109, 95; 'H NMR (300 MHz, CDCh) d 3.05 (m, 4H), 3.76 (d,d, 1H), 3.86 (m, 4H),
4.01
(m, 1H>, 4.12 (t, 1H), 4.30 (m, 1H), 4.99 (m, 1H), 6.20 (t, J = 55.9 Hz, 1H),
6.92 (t,
1H), 7.06 (d,d, 1H), 7.38 (d,d, 1H), 8.78 (broad s, 1H). Anal. calcd for
C,6H,8F3N3O3S:
C, 49.35; H, 4.66; N, 10.79. Found: C, 49.37; H, 4.71; N, 10.83.
EXA_~IPLE 19: (S)-N-[[3-[3-Fiuoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyI]methyl]-a-cyanothioacetamide (19).
1.
0 0
O N ' \ ~ EDC.HCI /-
N O + N=CCHpCOOH ----~ O N ~ \ N O
~H HOBT U ~H O
F n
NHp NH-C-CNpCN
39 1A
An ice cold, stirred mixture of 39 (0.646 g, 2.19 mmoI), cyanoacetic acid
(0.179 g, 2.1
mmol) and 1-hydroxybenzotriazole (0.351 g, 2.6 mmol) in DMF (5 mL), under
nitrogen, was treated with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (0.997 g, 5.2 mmol) and kept at ambient temperature for 24 h. It
was
diluted with CH1C12, washed with water and dilute NaCI, dried (Na2S0,) and
concentrated. The solid residue was crystallized from EtOAc-heptane to give
0.546 g
of 18: mp 172-174 °C: IR (DRIFT) 3316, 2256, 1754, 1684 cm~'; MS(EI)
m/z 362
(M'). Anal. calcd for C"H,9FN,0;: C, 56.35; H, 5.28; N, 15.46. Found: C,
56.33; H,
5.30; N, 15.36.
2.
a
a cK,o ~ v P ~ ~n i v oc",
k s.~ ~l
OvN N 0 0~N ~ ~ H O
1 a ~.".G,'+f 6
a ~~il ~ CiI~CH ~ » 'NH-C-CH~N
A stirred solution of 18 (0.453 mg, 1.25 mmol) in dioxane ( 10 mL), under
nitrogen,
was treated v~zth Lawesson's reagent (0.505 g, 1.25 mmol) and warmed at about
100
°C. ~L'hen the reaction was over (TLC with 30~c acetone-CH.,CI.,) the
mixture was
-59-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
cooled and concentrated in vacuo. Chromatography of the residue on silica gel
with
10-20010 acetone-CH.~CIl and crystallization of the product from EtOAc-heptane
gave
0.110 g of 19: mp I86-187 °C (dec); MS(ES) m /z 379 (M+H'), 402
(M+Na'); 'H NMR
(300 MHz, CDCI;,) d 3.05 (m, 4H), 3.81 (d,d, 1H), 3.86 (m, 4H), 3.89 (s, 2H),
4.09 (t,
1H), 4.14 (m, 2H), 5.01 (m, 1H), 6.92 (t, 1H), 7.05 (d,d, 1H), 7.34 (d,d, 1H),
9.15 (s,
1H); IR (DRIFT) 3244, 2260, 1754 cm~'. Anal. calcd for C"H,~FN;O.;S: C, 53.96;
H,
5.06; N, 14.81. Found: C, 53.88; H, 5.39; N, 14.61.
EXAMPLE 20: (S)-N-[j3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-a,a-dichlorothioacetamide (21).
1.
0 0
O N ~ ~ N~O (CI2CHC0)20 O N
N O
...H Et3N '-.J ~ ..,H O
~NH2 F ~NH-C-CHCIZ
39 20
A stirred, ice cold solution of 39 (0.885 g, 3.00 mmol) and triethylamine (975
mL, ?
mmol) in CHzCl1 ( 15 mL), under nitrogen was treated, dropwise with a solution
of
dichloroacetic anhydride (555 mL, 3.5 mmol) in CHZC12 (5 mL) and kept in the
ice
bath for 15 min and at ambient temperature for 18 h. It was diluted with
CH2Cl2,
washed with water, saturated NaHC03 and dilute NaCI, dried (NalSOa) and
concentrated. Chromatography of the residue on silica gel with 10% acetone-
CH2Clz
and crystallization of the product from acetone-heptane gave 0.463 g of 20: mp
197-
198 °C (dec); MS(ES) m/z 406 (M+H'), 428 (M+Na'); 'H NMR (300 MHz,
CDC13) d
3.05 (m, 4H), 3.75 (m, 3H), 3.86 (m, 4H), 4.07 (t, 1H), 4.83 (m, 1H), 5.94 (s,
1H), 6.92
(t, 1H), ?.06 (m, 2H), 7.41 (d,d, 1H).
2. o O
O N ~ ~ N~O ----r O I ~ ~ N~O
'--J F ...H O U ~ ...H S
I I
~NH-C-CHCI2 NH-C-CHCI
20 2~ 2
A stirred solution of 20 (0.305g, 0.75 mmol) in dioxane (5 ml), under
nitrogen, was
treated with Lawesson's reagent (0.202g, 0.5 mmoi), warmed at about
90°C for 1
hour, cooled and concentrated in vacuo. Chromatography of the residue on
silica gel
first with 30 ~'o acetone-heptane and then with 10~o acetone-methylene
chloride and
-60-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
crystallization of rh product form methylene chloride - heptane gave 0.203g
with 21:
mp 143-144°cd.; HR17S (EI) calculated for C,6H,bcl.~ F N1 03 S(M)
421.0431. Anal.
calcd for C,6H,acl., F N~ 0.3 S, C, 45.51; H, 4.30; N, 9.95. Found: C, 45.47;
H, 4.24;
H, 9.88.
EXAMPLE 21: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-a-(methoxycarbonyl)thioacetamide (23).
1. p O
CH OCOCH COCI
O N ~ ~ N O 3 2 O N ~ ~ N O
~H Et3N F ~H O
39 NH2 22 NH-C-CH2COOCH3
A stirred solution of 39 (0.955 g, 3.2 mmol) and triethylamine (650 mL, 4.5
mmol) in
CH2C12 (50 mL), under nitrogen, was treated, dropwise during 15-20 min with a
solution of methyl malonyl chloride (475 mL, 4.3 mmol) in CHZCIz (10 mL) and
kept
at ambient temperature for 3 days. It was then washed with water and dilute
NaCI,
dried and concentrated. The residue was flash chromatographed on silica gel
with
15-30% acetone-CHZCh and the product was crystallized form acetone-hexane to
give
0.873 g of 22: mp 150-151 °C; 'H NMR (300 MHz, CDCl3) d 3.03 (m, 4H),
3.34 (s,
2H), 3.67 (s, 3H), 3.69 (m, 2H), 3.76 (d,d, 1H), 3.85 (m, 4H), 4.00 (t, 1H),
4.78 (m,
1H), 6.90 (t, 1H), 7.06 (d,d, 1H), 7.41 (d,d, 1H), 7.57 (t, 1H); MS(ES) m /z
396
(M+H'), 418 (M+Na'); HRMS (FAB) calcd for C,~Hz;3FN.~0~ (M+H') 396.1571, found
396.15?9. Anal. calcd for CI~HzIFN.306: C, 54.68; H, 5.61; N, 10.63. Found: C,
54.69; H, 5.68; N, 10.58.
2. O CH~O ~ ~ P~S~P I ~ OCH~
_ I' S I~ O
O N ~ N~O S 0~N ~ ~ N O
V F H ~ ~ ~ l--(~~'H S
NH-C-CH2COOCH~ 23 NH-C-CHZCOOCH3
2Z
A stirred solution of 22 t0.395 g, 1.0 mmol) in dioxane (10 mL), under
nitrogen, was
treated with Lawesson's reagent (0.202 g, 0.5 mmol) and kept at ambient
temperature for 4 h 10 min and at 80-90 °C for 1.5 h. The reaction was
followed by
TLC on silica gel with 107o MeOH-CHCI.,. At this time a new, less polar
product
-61-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
had begun to form. It was kept at ambient temperature for 18 h and at 80
°C for 2
h; additional Laewsson's reagent (40 mg, 0.099 mmol) was added and warming at
80
°C was continued for 2 h; some starting material still remained. The
mixture was
concentrated and the residue was chromatographed on silica gel with 15%
acetone-
CH.,CI., to give 0.348 g of 23: 'H NMR (300 MHz, CDCI~,) d 3.05 (m, 4H), 3.71
(s,
3H), 3.81 (d,d, 1H), 3.86 (m, 4H), 3.88 (s, 2H), 4.07 (t, 1H), 4.19 (m, 2H),
4.99 (m,
1H), 6.91 (t, 1H), 7.07 (d,d, 1H), 7.42 (d,d, 1H), 9.52 (s, 1H); IR (DRIFT)
3269, 1743
cm~i; MS(EI) m/z 411 (M'). Anal. calcd for C,~HIlFN;,OsS: C, 52.54; H, 5.39;
N,
10.21. Found: C, 52.58; H, 5.43; N, 10.14.
EXAMPLE 22: (S)-N-[[3-[4-[1-[1,2,4]Triazolyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]thioacetamide (25).
0'I
N~N ~ ~ Nx0 + Me0 ~ ~ p~S~p ~ ~ OMe -i. NON ~ ~ N O
~N H II\S~ ~N ~H S
F S F
24 ~NHCOCH~ ZS NH-C-CHI
A stirred mixture of 24 (0.150 g, 0.470 mmol) and dioxane (12.5 mL), under
nitrogen, was treated with Lawesson's reagent (0.20 g, 0.50 mmol), refluxed
for 1.5
h, kept at ambient temperature for 18 h and concentrated in vacuo. Flash
chromatography of the residue on silica gel with 5% MeOH-CHC13 gave the
product
which was crystallized from MeOH to give 0.100 g (63.4%) of 25: mp 161-163
°C; 'H
NMR [300 MHz, (CD:j)1S0) d 2.43 (s, 3H), 3.87 (m, 3H), 4.22 (t, 1H), 4.99 (m,
1H),
7.51 (d, 1H), 7.77 (m, 2H), 8.26 (s, 1H), 8.97 (d, 1H), 10.35 (broad s, 1H);
IR (mull)
3259, 3226, 3044, 1752 cm''; MS(ES) m/z 336 (M+H'), 358 (M+Na'). Anal. calcd
for
C,~H,4FNSOzS: C, 50.14; H, 4.21; N, 20.88. Found: C, 50.18; H, 4.26; N, 20.94.
EXAMPLE 23: (S)-N-[[3-[4-[1-[1,2,4]Triazolyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]thioacetamide (25).
S
O I I O
N ~ CH3-C-SEt
---~ ~ Nv
~N N O KOH/NaF ~ ~ N / \ N O
....H EtOH/H20 N~ ~~~~ H S
F 277.27 NHz F 335.36 NH-C-CH3
26 C~aHiaFNsOzS
25
-62-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
A stirred mixture of 26 (0.26 g, 0.938 mmol), ethyl dithioacetate (0.12 g,
0.998
mmol), sodium fluoride (0.040 g, 0.953 mmol) and absolute EtOH (10 mL), under
nitrogen, was treated during 5 min with a solution of 0.97 M KOH (1.03 mL) in
EtOH and kept at ambient temperature for 2 h. It was then diluted with CHZCLz
(75mL), washed with water, 1M KHS04, water and brine and evaporated. The
residue was flash chromatographed on silica gel with 5~1o MeOH-CHCh and the
product was crystallized from MeOH to give 0.118 g, mp 164-165°C (dec)
and 0.026
g, mp 162-163°C (dec) of 25.
EXAMPLE 24: (S)-N-((3-(1-(Hydroxyacetyl)-5-indolinyl]-2-oxo-5-
oxazolidinyl]methyl]thioacetamide (28).
1.
Boc
~N / 1. TFA
NHCbz CHZCI2 ~ I NHCbz
2. NaHC03
52 53
A stirred, ice cold solution of 52 (8.80 g, 0.0240 mol) in CHzCh (25 mL) was
treated
during 20 min with a solution of trifluoroacetic acid (25 mL) in CHzCl2 ( 10
mL). The
mixture was kept in the ice bath for 2 h 15 min and concentrated under reduced
pressure. A solution of the residue in CH1C11 was washed with saturated NaHC03
and dilute NaCI, dried (Na.~S04) and concentrated. The residue was used in the
next
reaction without further purification. A sample of this material (53) had: 'H
NMR
(300 MHz, CDCI.s) d 3.00 (t, 2H), 3.54 (t, 2H), 3.85 (broad s, 1H), 5.17 (s,
2H), 6.59
(d, 1H), 6.66 (broad s, 1H), 6.91 (d, 1H), ?.23 (s, 1H), 7.36 (m, 5H); MS m/z
269
(M+H'>.
2.
Bz0-CH2-C=O
BzOCH2COCl N
Na O
NHCbz (CHa)2C0/H20 ~ NHCbz
268.22 416.48
C~ sH ~ sN202 C25H2aN20a
53 54
-63-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
An ice cold, stirred mixture of 53 (crude product from the previous reaction),
acetone
(200 mL>, saturated NaHCO., (200 mL) and water (30 mL) was treated, dropwise
during 20 min, with a solution of benzyloxyacetyl chloride (4.70 mL, 0.030
mol) in
acetone (55 mL), warmed slowly to ambient temperature and kept for 18 h.
Additional benzyloxytacetyl chloride (1.0 mL) in acetone 35 mL) was added
dropwise
and the mixture was kept at ambient temperature for an additional 3 h and
diluted
with EtOAc and water. A solid was collected by filtration and dried to give
4.00 g of
crude product. The EtOAc solution was dried (Na2SOa) and concentrated to give
5.36 g of additional crude product. Crystallization of the product from EtOAc
gave a
total of 6.35 g of 54", mp 157-159.5°C. The analytical sample had: mp
158-159.5°C;
'H NMR (300 MHz, CDCI~) 8 3,16 (t,2H), 4.01(t,2H), 4.21 (s, 2H), 4.69 (s, 2H),
5.19
(s, 2H), 6.67 (s, 1H), 6.97 (d, 1H), 7.36 (m, lOH), ?.50 (brand s, 1H), 8.15
(d, 1H);
MS(EI) m/z (relative intensity) 416 (M', 9), 310 (8), 202 (10), 133 (8), 92
(8), 91 (99),
79 (7), 77 (9), 65 (12), 51 (6); IR (mull) 2381, 1722, 1659, 1608, 1558 crri'.
Anal.
calcd for C.>5Hi4Nz0,: C, 72.10; H, 5.81; N, 6.73. Found: C, 72.05; H, 5.86;
N, 6.68.
3.
Bz0-CH2-C=O Bz0-CH2-C=O
t . nBuLi
N / I ~ N /
NHCbz 2. ~ ~ ~ N- _O
416.48 ~O'C~ 382.42 ~~,,,H
54 =H C2~ H22N205 OH
25 A stirred suspension of 54 (1.16 g, 2.78 mmol) in THF (42 mL) was cooled,
under
nitrogen, to -?8°C and treated, dropwise, during 5 min with 1.6 M n-
BuLi in hexane
(1.83 mL). It was kept at -78°C for 50 min, treated, dropwise, during 5
min with a
solution of (R)-(-)-glycidyl butyrate (0.500 g, 3.47 mmol) in THF (2 mL),
allowed to
warm to ambient temperature during 3 h and kpet for 18 h. It was then diluted
30 with EtOAc, washed with saturated NH;CI, water and brine, dried (MgSO~) and
concentrated. Chromatography of the residue on silica gel with 3% MeOH-0.2%
NHaOH-CHCI;, gave 0.60 g (56%) of 55'4: 'H NMR [300 MHz, (CD;,).,SOJ 8 3.14
(t,
2H), 3.59 cm, 2H), 3.79 (d,d, 1H), 4.03 (m, 3H), 4.29 (s, 2H), 4.58 (s, 2H),
4.65 (m,
1H), 5.20 (t, 1H), 7.31 (m, 6H), 7.55 (s, 1H), 8.03 (d, 1H); MS(ES> m/z 383
(M+H'),
35 405 (M+Nat).
-64-
CA 02351062 2001-05-10
WO 00/32599 PCTNS9$/2530$
4.
Bz0-C H2-C=O N02 Bz0-C H2-C=O
N , O ~ ~ S02C1 N / O''
w I N~O w I N~O
~--~ H EI3N H
~OH 56 ~O-Nos
An ice cold, stirred mixture of 55 (0.60 g, 1.57 mmol), triethylamine (2.2
mL), and
CHZCh (12 mL), under nitrogen, was treated with 3-nitrobenzenesulfonyl
chloride
(0.44 g, 1.99 mmoI) and kept in the ice bath for 30 min and at ambient
temperature
10 for 60 min. It was then diluted with CH~CIl, washed with water and brine,
dried
(Na~SO~) and concentrated. Chromatography of the residue on silica gel with
15%
CH3CN-CH2C11 gave 0.70 g of 56: 'H NMR (300 MHz, CDCI.') d 3.19 (t, J = 8.3
Hz,
2H), 3.88 (d,d, 1H), 4.04 (t, J = 8.4 Hz, 2H), 4.14 (t, 1H), 4.23 (s, 2H),
4.42 (m, 2H),
4.70 (s, 2H), 4.84 (m, 1H), 6.97 (m, 1H), 7.34 (m, 5H), 7.58 (s, 1H), 7.81 (t,
1H), 8.22
15 (m, 2H), 8.53 (m, 1H), 8.73 (m, 1H); MS(ES) m /z 568 (M+H'), 590 (M+Na').
5.
Bz0-CH2-C=O Bz0-CH2-C=O
I
N / I O
20 ~ I N O C
~N~O
~/ H H
56 -ONos 57 ~NH2
A stirred mixture of 56 (crude product from 0.00314 mol of 55), acetonitride
(70 mL),
isopropanol (70 mL) and 29% ammonium hydroxide (70 mL) was warmed at 40-44
25 °C for 7h and kept at ambient temperature for 18 h. it was
concentrated in vacuo to
an aqueous residue with was extracted with CHZCIz. The extract was washed with
water and brine, dried (NalSO~) and concentrated. Chromatography of the
residue
on silica gel with 8% MeOH-0.5% NH40H-CHC1~, gave 1.05 g of 57: 'H NMR [300
MHz, (CD3)zS0] d 2.78 (m, 2H), 3.13 (t, 2H), 3.82 (d,d, 1H), 4.01 (m, 3H),
4.29 (s,
30 2H), 4.58 (s, 2H), 4.58 (m, 1H), ?.31 (m, 6H), 7.54 (broad s, 1H), 8.03 (d,
1H);
MS(ES) m/z 382 (M+H'), 404 (M+Na').
-65-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
6.
HO CH2-C=O
Bz0-CH2-C=O
I O N O HCI
N / H2/Pd /
\ ~ ~ MeOH/HCI \
N O N O
H H
381.42 ~~~, 327.77
C H CIN O ~NH
57 NH2 14 18 3 4 2
27
A mixture of 57 (0.46 g, 1.21 mmol), MeOH (150 mL), 1 M HC1 (1.2 mL) and 5%
palladium-on-carbon catalyst (250 mg) was hydrogenated at an initial pressure
of 49
psi for 5 h. Additional 1M HC1 (0.5 mL) and catalyst (100 mg) were added and
hydrogenation was continued for 18 h. The catalyst was removed by filtration
and
the filtrate was concentrated to give 0.34 g of 27: 'H NMR [300 MHz, (CD3)2S0]
S
3.15 (t, 2H), 3.22 (broad s, 2H), 3.84 (d,d, 1H), 4.00 (t, 2H), 4.15 (s, 2H),
4.15 (m,
1H), 4.92 (m, 1H), 7.24 (q, 1H), 7.50 (d, 1H), 8.03 (d, 1H), 8.37 (broad s,
3H); MS(ES)
m/z 2.92 (M+H').
7.
HO CH2-C=O HO CH2-C=O
I S N
N \ I N~O ~HCI CH3_~_SEt
H NaF. KOH ~H S
27 NH2 28 NH-C-CH3
A suspension of 2? (0.10 g, 0.34 mmol) in a mixture of EtOH (15 mL) and 0.97 M
KOH (0.7 mL) was added, under nitrogen to a stirred mixture of ethyl
dithioacetate
(0.0412 g, 0.343 mmol) and sodium fluoride (0.0137 g, 0.326 mmol) in EtOH (5
mL)
and the mixture was kept at ambient temperature for 2h 15 min. Additional 0.97
M
KOH (0.2 mL), sodium iodide (6 mg) and ethyl dithioacetate (20 mg) were added
and
the mixture was stirred for 2 h, mixed with CH.~CI., ( 150 mL), washed with
water,
1M KHSO, and brine, dried (NazSO~) and concentrated. The residue was
crystallized from acetone to give 0.0404 g of 28: mp 175-176 °C (dec);
MS (FAB)
m/z 350 (M+H"), 349 (M'>, 331, 316, 205, 73; HR MS (FAB) calcd for
C,6HzoN.jOaS
(M+H') 350.1174, found 350.1183; 'H NMR [300 MHz, (CD.~).,SO] d 2.42 (s, 3H),
3.14
-66-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
(t, 2H), 3.79 (d,d, 1H), 3.89 (t, 2H), 4.00 (t, 2H), 4.12 (m, 3H), 4.83 (t,
1H), 4.90 (m,
1H), 7.25 (d, 1H), 7.50 (s, IH), 8.03 (d, 1H), 10.35 (s, 1H); IR (DRIFT) 3255,
3223,
3068, 1747, 1639, 1614 ciri'.
EXAMPLE 25: (S)-N-([3-[3-Fluoro-4-[4-(hydroxyacetyl)-1-
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide (30).
1.
O1' O
Cbz-NON ~ ~ N~O' H Hz / Pd H NON ~ ~ N~01"H
EtOH / THF
F OH F ~OH
58 59
A mixture of 58 (3.00 g, 7.00 mmol), THF (60 mL), absolute EtOH (100 mL) and
10%
palladium-on-carbon catalyst (415 mg) was hydrogenated at an initial pressure
of 58
psi for 2 h 50 min. The catalyst was removed by filtration and the filtrate
was
concentrated in vacuo to give 2.67 g of 59 which was used without further
purification in the next reaction: 'H NMR (300 MHz, CDCl3) d 2.16 (broad s),
3.02
(m, 8H), 3.73 (d,d, J = 3.9, 12.6 Hz; 1H), 3.96 (m, 3H), 4.72 (m, 1H), 6.92
(t, J = 9.2
Hz, 1H), ?.11 (m, 1H), 7.43 (d,d, J = 2.6, 14.3 Hz, 1H); MS(ES) m lz 296
(M+H').
2. o o O
I' PhCH20CH2COCl p- fI
H VN ~ ~ N~O",H BzOCH2-C-N N ~ ~ N~O
~--~ NaHC03 ~--~ ~H
F 'OH (CH3)2C0 / H20 F OH
59
A stirred, ice cold mixture of 59 (2.67 g from the previous reaction), acetone
(190
mL) and saturated NaHC0.3 (70 mL) was treated, dropwise during 2-3 min with a
30 solution of benzyloxyacetyl chloride (1.34 mL, 8.61 mmol) in acetone (25
mL), kept in
the ice bath for 1 h and diluted with EtOAc. The aqueous layer was extracted
with
EtOAc and the combined organic solution was washed with dilute NaCI, dried and
concentrated. Chromatography of the residue on silica gel with 3090 acetone-
CHzCIz
gave 2.64 g of 60: 'H NMR (300 NIHz, CDCI;,) d 2.28 (broad s, 1H), 3.00 (m,
4H),
35 3.66 (m, 2H), 3.77 (m, 3H), 3.96 (m, 3H), 4.22 (s, 2H), 4.61 (s, 2H), 4.74
(m, 1H), 6.88
(t, J = 9.2 Hz, 1H), 7.12 (m, 1H), 7.35 (s, 5H), 7.46 (d,d, J = 2.6, 14.2 Hz,
1H); IR
-6?-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
(mull) 3406, 1748, 164? cm''; HRMS(EI) calcd for C,~.~H1~FN.,05 (M') 443.1856,
found
443.1842.
3.
0 0 0 0
II /-1 ~ SOZCI Et N it /'--~
820CH?-C-NVN ~ ~ N O H .t. \ ~ .~ BZOCHz-C-NVN ~ ~ N p H
CHZCIZ
OH NO F ONos
60 T
n
A stirred, ice cold mixture of 60 (2.64 g, 6.00 mmol) and triethylamine (1.14
mL,
8.16 mmol) in CHiCh (200 mL), under nitrogen, was treated with 3-
nitrobenzenesulfonyl chloride (1.78 g, 8.04 mmol), warmed to ambient
temperature
and kept for 5 h 20 min. Additional 3-nitrobenzenesulfonyl chlroide (180 mg)
and
triethylamine (0.20 mL) were added and the mixture was kept at ambient
temperature for 18 h, diluted with CHiCl1 and washed with water and dilute
NaCI,
dried (NazS04) and concentrated. Chromatography of the residue on silica gel
with
40-60°!o acetone-hexane gave 3.36 g of T7: 'H NMR (300 MHz, CDC13) d
3.02 (broad
s, 4H), 3.66 (broad s, 2H), 3.78 (broad s, 2H), 3.87 (d,d, J = 5.9, 9.1 Hz,
1H), 4.09 (t,
J = 9.2 Hz, 1H), 4.22 (s, 2H), 4.41 (m, 2H), 4.61 (s, 2H), 4.84 (m, 1H), 6.88
(t, J = 9.1
Hz, 1H), 7.02 (m, 1H), 7.35 (m, 6H), 7.82 (t, J = 8.0 Hz, 1H), 8.23 (m, 1H),
8.53 (m,
1H), 8.73 (m, 1H); MS(ES) m/z 629 (M+H').
4.
0 0
O NH O
BzOCHz-C-NVN ~ ~ N~O, H --~ BZOCHZ-C-NON ~ ~ N~O
' ' U ~H
~ONos F ~NHZ
s~
A solution of ?7 (3.36 g, 5.34 mmol) in a mixture of acetonitrile (90 mL),
isopropanol
(90 mL) and concentrated ammonium hydroxide (90 mL) was warmed at 40-45
°C
for 18 h, treated with additional ammonium hydroxide (30 mL), warmed at 40-45
°C
for 8 h, treated with additional ammonium hydroxide (25 mL) and warmed at 45
°C
for 18 h. It was then mixed with water and extracted with CH.~CI.,. The
extract was
washed with dilute NaCl, dried (Na.,SOa) and concentrated. Chromatography of
the
residue on silica gel with 5oJo MeOH-0.5~o NHaOH-CHCh gave 2.44 g of 61: 'H
NMR
(300 MHz, CDCI.,) d 1.50 (broad s), 3.04 (m, 6H), 3.65 (broad s, 2H), 3.81 (m,
3H),
3.99 (t, 1H), 4.21 (s, 2H), 4.61 (s, 2H), 4.66 (m. 1H), 6.88 (t, 1H), 7.12 (m,
1H), 7.33
-68-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/Z5308
(m, 5H), 7.47 (d,d, 1H); MS(ES) m/z 443 (M+H')
5.
0 0
101 /"1 Hp. 5% Pd / C I I /"~ O
BzOCHz-C-N ~N ~ ~ N~H Et~ HOCHz-C-N ~N ~ ~ N~O..,H ~HCf
F NHz HCI F ~NH
z
61 pg
A solution of 61 (1.45 g, 3.3 mmol) and 1.0 N HCl (3.65 mL) in 95% EtOH (150
mL)
was treated with 5~1o palladium-on-carbon catalyst (500 mg) and hydrogenated
at an
initial pressure of 54 psi for 20 h 15 min. Additional 1.0 N HCl (0.5 mL) and
catalyst ( 100 mg) were added and hydrogenation was continued for 20 h 30 min
at
an initial pressure of 60 psi. The reaction was compete by TLC; it was
neutralized
with concentrated NHaOH, filtered and concentrated in vacuo to give 1:18 g of
29:
'H NMR [300 MHz, (CD3)1S0) d 2.94 (broad s, 4H), 3.i9 (m, 2H), 3.48 (broad s,
2H),
3.60 (broad s, 2H), 3.84 (m, 1H), 4.14 (m, 3H), 4.66 (broad s, 1H), 4.93 (m,
1H), 7.07
(t, 1H), 7.16 (d,d, 1H), ?.48 (d,d, 1H), 8.04 (broad s); IR (mull) 3420, 3099,
3040,
3008, 1755, 1641 cm~l; MS(ES) m/z 353 (M+H'). Anal. calcd for C,sH22C1FN,04:
C,
49.42; H, 5.70; Ci, 9.12; N, 14.41. Found: C, 48.16; H, 5.82; Cl, 10.00; N,
14.28.
6.
o s
HOCH -O-N N ~ ~ ~ CHI-C-SEt
z V ~H ~HCI ~ HOCHz-C-N N ~ ~ N~O
F KOH, NaF ~/ ~.-( H S
NHz F ~NH-C-CH3
29 30
A stirred mixture of ethyl dithioacetate (180 mL, 1.56 mmol), sodium fluoride
(72
mg, 1.7 mmol), 29 (500 mg, 1.29 mmol) and EtOH (70 mL) under nitrogen, was
treated with 0.97M KOH ( 1.46 mL, 1.42 mmol) and the resulting solution was
kept
at ambient temperature for 3 h 35 min, diluted with CHCl.3, washed with water
and
dilute NaCl, dried (NazSO,) and concentrated. Chromatography of the residue on
silica gel with 5% MeOH-0.5~1o NH~OH-CHCI:, and crystallization of the
resuiting
product from absolute EtOH gave 0.238 mg (44.910) 30: mp 163-165 °C; 'H
NMR
(300 MHz, CDCI;;) d 2.60 (s, 3H), 3.06 (m, 4H), 3.45 (m, 2H), 3.61 (m, 1H),
3.82 (m,
3H), 4.07 (m, 2H), 4.25 (m, 3H), 4.97 (m, 1H), 6.91 (t, 1H), 7.07 (m, 1H),
?.45 (d,d,
1H), 7.91 (broad s, 1H); MS(FAB) m /z (relative intensity) 411 (M+H', 100),
410 (M',
66.5), 266 (3.1); IR 3292, 1733, 1653 cm-'. Anal. calcd for C,~Hz~FNaO~,S: C,
52.67;
H, 5.65; N, 13.65. Found: C, 52.76; H, 5.58; N, 13.64.
-69-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
EXAMPLE 26: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]thio-acetamide (32).
s
O It O
SAN ~ ~ N~O CH3-C-SEt
~H Et3N S\..JN~N~-- ~O",H
~NHZ F 'NH-C-CH3
3~ 32
An ice cold, stirred mixture of 31 (0.38 g, 0.0012 mol) and triethylamine
(0.38 mL,
0.0027 mol) in THF (12 mL), under nitrogen, was treated with ethyl
dithioacetate
(0.16 mL, 0.0014 mol) and then kept at ambient temperature for 24.5 h and
concentrated in vacuo. A solution of the residue in CHzCl2 was washed with
saturated NaHCO.,, water and brine, dried (MgSOa) and concentrated.
Crystallization of the residue from EtOAc-hexane gave 0.355 g of 32: mp 155-
156
°C; MS(ES) m /z 370 (M+H'), 392 (M+Na'); IR (DRIFT) 3206, 3042, 1759,
1738 cm~l;
'H NMR (300 MHz, CDC13) d 2.60 (s, 3H), 2.95 (s, 4H), 3.43 (m, 4H), 3.82 (d,
d, 1H),
4.08 (m, 2H), 4.27 (m, 1H), 4.98 (m, 1H), 7.06 (m, 1H), 7.33 (broad s, 1H),
7.51 (d,
1H), 8.03 (broad s, 1H). Anal. calcd for C,6H2oFN30zS2: C, 52.01; H, 5.46; N,
11.37.
Found: C, 51.86; H, 5.43; N, 11.20.
EXAMPLE 27: (S)-N-([3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]thio-acetamide, thiomorpholine S-oxide (34).
1.
O O
S/~N ~ ~ ~ Na104
~N O",H MeOH / H20 O=SVN ~ ~ N O",H
~OH
s2
63
An ice cold, stirred mixture of sodium metaperiodate (1.08 g, 5.05 mmol) and
water
(12 mL), under nitrogen, was treated with 62 (1.5 g, 4.8 mmol) and MeOH (17
mL)
and kept at 6 °C for 18 h and at 4 °C for 3 h. It was then
treated with additional
sodium metaperiodate (0.1 g), kept at 4°C for 3 h and extracted with
CHCI.,. The
extract was dried (MgSOa) and concntrated to give 1.4 g of 63: 'H NMR (300
MHz,
(CD3)"SO] d 2.84 (m, 2H), 3.01 (m, 2H), 3.16 (m, 2H), 3.50 (m, 3H), 3.65 (m,
1H),
3.77 (d,d, 1H), 4.03 (t, 1H), 4.66 (m, 1H), 5.18 (t, 1H), 7.16 (m, 2H), 7.52
(m, 1H);
-70-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
MS(ES) m/z 329 (M+H'), 351 (M+Na').
N02
2. O / \
S02C1 O
O=SAN / \ N~O...H O=S~N / \ N~O
F ~ Et3N '.-/ ~~--~ ...H
OH CH2Ct2 F ~ONos
63
An ice cold, stirred mixture of 63 (1.27 g, 3.87 mmol) and triethylamine
(0.732 mL,
5.25 mmol) in CHlCl2 (130 mL), under nitrogen, was treated with m-
nitrobenzenesulfonyl chloride (1.15 g, 5.19 mmol) and kept at ambient
temperature
for about 24 h. It was diluted with CH2Ch, washed with water and brine, dried
(NaZSO,~) and concentrated to give 78 which was used in the next reaction
without
purification.
O
II _ OII
3. O=SVN / \ N~O.,.H CH4ON O SVN / \ N~~.,.H
F ~--~
ONos (CH3)2CHOH F ~NH2
33
A stirred mixture of the product (78) from the previous reaction, acetonitrile
(?0 mL)
and isopropanol (70 mL) was treated with concentrated ammonium hydroxide (?0
mL, 29.9% NH.;) and kept at 40 °C for 2 h, at ambient temperature for
18 h and at
40-45 °C for 4 h; it was concentrated to about 50 mL, diluted with
water and
extracted with CH~C1L. The extracts were washed with water and brine, dried
(MgSO,,) and concentrated. Chromatography of the residue on silica gel with 5%
MeOH-CHCl3 gave 0.58 g of 33: MS(ES) m /z 328 (M+H'), 350 (M+Na'); 'H NM8
[300 MHz, (CD,3)1S0] d 2.81 (m, 4H), 3.01 (m, 2H), 3.16 (m, 2H), 3.30 (broad
s), 3.49
(m, 2H), 3.80 (d,d, 1H), 4.01 (t, 1H), 4.58 (m, 1H), ?.19 (m, 2H), 7.51 (m,
1H).
4. S
o I I o
O=SAN / ~ N~O CHs-C-SEt ~ /
~--~ H Et N O=S~N~N O, ,H S
F ~.J ~--~
~NH2 F '-NH-C-CH3
33 34
-71-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
A stirred suspension of 33 (3.7 g, 0.011 mol) and triethylamine (3.5 mL, 0.025
mol)
in THF ( 120 mL) was cooled, in an ice bath, under nitrogen, treated, dropwise
during 2 min, with a solution of ethyl dithioacetate (1.47 mL, 0.0128 mol) in
THF (2
mL) and kept at ambient temperature for 22 h. The resulting solution was
concentrated and the residue crystallized from acetonitrile to give 3.61 g of
34: mp
176-177 °C ; 'H NMR (300 MHz, (CD.,)2S0] d 2.42 (s, 3H), 2.85 (m, 2H),
3.01 (m,
2H), 3.18 (m, 3H), 3.50 (m, 2H), 3.78 (d,d, 1H), 3.89 (broad s, 2H), 4.12 (t,
1H), 4.92
(m, 1H), 7.18 (m, 2H), 7.49 (m, 1H), 10.33 (s, 1H); IR (DRIFT) 3186, 3102,
1741 cm'';
MS(ES) m / z 386 (M+H'), 408 (M+Na'). Anal. calcd for C,sH2oFN.iO3Szo0.5 HiO:
C,
48.71; H, 5.37; N, 10.65; S, 16.26; HIO, 2.38. Found: C, 48.75; H, 5.17; N,
10.72; S,
16.07; H10, 1.72.
EXAMPLE 28: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]thio-acetamide, thiomorpholine S, S-dioxide (38).
1.
O O
/'~ ~ Os04 I'
SAN ~ ~ N O,.,H NMO ~2=SAN ~ ~ N~O",H .
~OH (CH3)2C0 / H20 F ~OH
62 64
A stirred mixture of 62 (0.399 g, 0.00128 mol) in 25% water/acetone (12 mL),
under
nitrogen was treated with N-methylmorpholine, N-oxide (0.45 g, 0.00384 mol)
and
0.1 mL of a 2.5 wt°lo solution of osmium tetroxide in tert-butanol. It
was kept at
ambient temperature for 18 h, mixed with saturated NaHSO.~ (50 mL) and
extracted
with CHICK. The extract was washed with saturated NaHSO~, and brine, dried
(Na2S0,,) and concentrated. The residue was mixed with 3.5% MeOH-CH~C11 and
filtered; the solid was dissolved in 15% MeOH-CHzCl1 and concentrated to give
0.29
g of 64. The filtrate was chromatographed on silica gel with 3.5% MeOH-CH2C12
to
give 0.1 of additional 64: MS(ES) m/z 345 (M+H*), 367 (M+Na'); 'H NMR [300
MHz, (CD.3).,SO] d 3.26 (m, 4H), 3.44 (m, 4H), 3.60 (m, 2H), 3.80 (d,d, 1H),
4.05 (t,
1H), 4.69 (m, 1H), 7.22 (m, 2H), 7.54 (d, 1H).
-72-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
2.
p t. ~ ~ So2C~ p
p2=SAN ~ ~ N~~".H p2N Et3N p2-SUN / \ N~~.,.H
~pH 2. NH40H F "-NH2
64 35
A stirred mixture of 64 (0.39 g, 0.00113 mol) and triethylamine (0.214 mL,
0.00154
mol) in CH1C11 (37 mL) was cooled, under nitrogen, in an ice bath and treated,
portionwise during 5 min, with 3-nitrobenzenesulfonyl chloride (0.335 g,
0.00151
mol). The mixture was kept in the ice bath for 20 min and at ambient
temperature
for 18 h and concentrated in vacuo. A stirred solution of the residue in 2-
propanol
(25 mL) and acetonitrile (25 mL), under nitrogen, was treated with 30% NH;OH
(25
mL), warmed at 50-55 °C for 6 h and kept at ambient temperature for 48
h. It was
concentrated to remove the organic solvents, diluted with water and extracted
with
CH2Ch. The extract was washed with water and brine, dried (MgS04) and
concentrated. Flash chromatography of the residue on silica gel with 6% MeOH-
0.4% NH40H-CHC13 gave 0.29 g of 35: 'H NMR [300 MHz, (CD~)lS0] d 1.59 (broad
s, 2H), 2.78 (m, 2H), 3.24 (m, 4H), 3.43 (m, 4H), 3.81 (d,d, 1H), 4.01 (t,
1H), 4.57 (m,
1H), 7.18 (m, 2H), 7.52 (rn, 1H); MS(ES) m/z 344 (M+H'), 366 (M+Na').
3.
o I I o
II CH3-C-SEt I'
Op=SAN ~ ~ N~O O =S~N ~ ~ N~O
~J ~H 2 ~/ ~H S
H2 Et3N F NH-C-CH3
35 36
A stirred, ice cold suspension of 35 (0.28 g, 0.85 mmol) in a mixture of Et.3N
(0.26
mL, 1.9 mmmol) and THF (10 mL) was treated with ethyl dithioacetate (0.11 mL,
about 6 drops) and kept in the ice bath for 20 min and then at ambient
temperature;
the reaction was followed by TLC. After 20 h there was still a suspension and
only
partial reaction; additional THF (10 mL) and ethyl dithioacetate (3 drops)
were
added. After an additional 48 h the reaction was still incomplete; the
suspension
was treated with CH.,CIz (10 mL) and kept for ?2 h. At this time almost
complete
solution and an almost complete conversion to product had been obtained. An
additional drop of ethyl dithioacetate was added and the mixture was kept at
ambient temperature for 5 d and concentrated in vacuo. The residue was mixed
with EtOAc, washed with saturated NaHC03, water and brine, dried (MgSOa) and
-?3-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
concentrated. Crystallization of the residue from IVIeOH-EtOAc gave 0.209 g of
36:
mp 197-198 °C; 'H NMR (300 MHz, (CD~)2S0] d 2.42 (s, 3H), 3.24 (m, 4H),
3.43 (m,
4H), 3.78 (d,d, 1H), 3.88 (m, 2H), 4.12 (t, 1H), 4.92 (m, 1H), 7.18 (m, 2H),
7.50 (m,
1H), 10.37 (broad s, 1H); IR (mull) 3300, 3267, 1743 cm''; MS(ES) m/z 424
(M+Na').
Anal. calcd for C'~H.,oFN:,O~,S1: C, 47.87; H, 5.02; N, 10.47. Found: C,
47.84; H,
5.23; N, 10.28.
EXAMPLE 29: (S)-N-[[3-[3,5-Difluoro-4-[4-(hydroxyacetyl)-1-
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl]methyl]thioscetamide (38).
1.
F O F O
/-'1 ~ ~ ~ NH20H . HCI
Boc-NON _ ~ --.w Boc-N N ~ ~ N~O
F Py / EtOH
NHAc
65 F NH2
ss
A stirred mixture of 65 ( 1.8 g, 0.00396 moll, pyridine (30 mL) and absolute
EtOH (3
mL), under nitrogen, was treated with hydroxylamine hydrochloride ( 1.44 g,
0.0207
moll, warmed to the reflux temperature during 2 h, refluxed for 3.5 h, kept at
ambient temperature for 18 h and at reflux for 4 h. It was concentrated in
vacuo
and the residue was mixed with water, adjusted to pH 11 with saturated NaHC03
and extracted with EtzO. The extracts were washed with brine, dried (Na2S04)
and
concentrated. Chromatography of the residue on silica gel with 5~1o MeOH-0.35%
NH40H-CHCl3 gave 0.75 g of recovered 65 and 0.72 g of 66: 'H NMR [300 MHz,
(CD3)lS0] d 1.40 (s, 9H), 1.72 (broad s, 2H), 2.78 (m, 2H), 2.97 (m, 4H), 3.40
(m, 4H),
3.80 (d,d, 1H), 4.00 (t, 1H), 4.59 (m, 1H), 7.27 (d, 2H); MS(ES) m /z 413
(M+H+), 435
(M+Na~).
2.
F O F O
PhCH20COCl /-'~
Boc-N N ~ ~ N O Boc-N N ~ ~ N O
F ~ Et3N / THF ~--~ F
NH2 NHCbz
66
An ice cold, stirred mixture of 66 (0.75 g, 0.0018 moll and triethylamine
(0.315 mL,
0.00225 moll in THF (12 mL), under nitrogen, was treated, dropwise with benzyl
chloroformate (0.29 mL, 0.0020 moll, kept in the ice bath for 15 min and at
ambient
-74-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
temperature for 2 h and concentrated in vacuo. The residue was mixed with
CH2Cl1
and washed with saturated NaHC03, water and brine, dried (NalSO~) and
concentrated. This residue was mixed with EtlO and filtered to give 0.939 g of
67:
mp 116-118 °C; 'H NMR (300 MHz, CDC13) d 1.48 (s, 9H), 3.08 (m, 4H),
3.53 (m,
4H), 3.60 (m, 2H), 3.73 (m, 1H), 3.96 (t, 1H), 4.76 (m, 1H), 5.10 (s, 2H),
5.21 (m,
1H),7.07 (d, 2H), 7.31 (s, 5H); MS(ES) m /z 547 (M+H'), 569 (M+Na').
3.
F O F O
goc-N~ ~ ~ N~01,H ~ ~ TFA H N N ~ ~ N~O
2. NaHC03 ~/
F ~NHCbz F NHCbz
67 68
Compound 67 (0.805 g, 0.00147 mol) was added with stirring, portionwise during
5
min, under nitrogen, to ice cold trifluoroacetic acid (9 mL). The resulting
solution
was kept in the ice bath for 1 h and then concentrated under a stream of
nitrogen.
The residue was mixed with ice and saturated NaHC03 and extracted with CHzCl2;
the extract was washed with water and brine, dried (Na2S04) and concentrated
to
give 0.63 g of product. The combined aqueous layer was reextracted with EtOAc;
the extracts were washed with water and brine, dried (NaZS04) and concntrated
to
give additional product. The combined product amounted to 0.68 g of 68 which
was
used in the next reaction without further purification.
4.
F o o F o
/-'1 ~ PhCH20CH2COC1 ~~ n
H N N ~ ~ N O BzOCHz-C-N N ~ ~ N O
~J
NaHC03
NHCbz (CH3)zC0 ~ HZO F NHCbz
68
69
An ice cold, stirred mixture of 68 (0.68 g, 0.00152 moI), saturated NaHCO~
(15.2 mL)
and acetone (40 mL), under nitrogen was treated, dropwise during 15 min, with
a
solution of benzyloxyacetyl chloride (0.29 mL, 0.0019 mol) in acetone (5 mL),
kept at
ambient temperature for 6 h, diluted with EtOAc and washed with water and
brine.
The extract was dried (MgSO,) and concentrated in vacuo to give 0.72 g of 69:
MS(ES) m/z 395 (M+H'), 617 (M+Na'); 'H NMR (300 MHz, CDCh) d 3.12 (m, 4H),
3.59 (m, 4H), 3.74 (m, 3H), 3.96 (t, 1H), 4.22 (s, 2H), 4.62 (s, 2H), 4.75
(broad s, 1H),
-75-
CA 02351062 2001-05-10
WO 00/32599 PCT/U598/25308
5.10 (s, 2H), 5.22 (m, 1H), 7.08 (d, 2H), 7.33 (m, lOH).
5.
O F O O F O
BzOCHz-C-NVN ~ ~ N~O."H H--.-~ HOCHz-C-N~N ~ ~ N~O
' ' HCI J MeOH U
F ~NHCbz F NHz
69 37
A mixture of 69 (0.72 g, 0.0012 mol), MeOH and 5% palladium-on-carbon catalyst
(0.4 g) was hydrogenated at an initial pressure of 45 psi for 4 h. By TLC (8%
MeOH-0.5% NH40H-CHCl3) the starting material had been reduced and two
products formed. 1M Hydrochloric acid (1.34 mL) was added and hydrogenation
was
continued at an initial pressure of 40 psi for 21 h. By TLC only the more
polar
product remained. The catalyst was removed by filtration and the filtrate was
concentrated to give 0.40 g of 37: MS(ES) m/z 371 (M+H'), 393 (M+Na'); 'H NMR
[300 MHz, (CD3)iS0] d 3.02 (s, 4H), 3.20 (m, 2H), 3.43 (s, 2H), 3.56 (s, 2H),
3.84 (m,
1H), 3.84 (broad s), 4.10 (s, 2H), 4.14 (t, 1H), 4.96 (m, 1H), 7.26 (d, 2H),
8.41 (broad
s, 3H).
6.
HOCH -C-N N ~ ~ ~ CHa-C-SEt ~ /--~ F
z ~ Et~ HOCHz-C-N N ~ ~ N O
U ~ S
F NHz F NH-C-CHI
37
x HCI
38
A stirred suspension of 37 (0.38 g) in a solution of Et3N (0.31 mL) and THF
(10 mL),
under nitrogen, was treated with ethyl dithioacetate (0.13 mL, about 7 drops)
and
kept at ambient temperature for 7 d; the reaction was followed by TLC (8% MeOH-
0.501o NH40H-CHCI,;). Additional ethyl dithioacetate (2 drops) was added after
24 h;
after 30 h CH1C11 ( 10 mL) and ethyl dithioacetate (3 drops) were added; after
48 h
additional triethylamine (0.3 mL) was added. The mixture was concentrated in
vacuo and the residue was mixed with ice and saturated NaHCO., an extracted
with
CH_,Clz. The extract was washed with water and brine, dried (MgSO~) and
concentrated. The residue was chromatographed on silica gel with 2.5% MeOH-
CH.,C1.~ and the product was crystallized from MeOH to give 0.182- g of 38: mp
110-
I11 °C (dec); MS(ES) m/z 429 (M+H'), 451 (M+Na'); HRMS (FAB) calcd
for
-?6-
CA 02351062 2001-05-10
WO 00/32599
PCT/US98/25308
C,eHl~F,~N~OaS (M+H') 429.1408, found 429.1415; IR (DRIFT) 1760, 1652, 1639 cm-
';
[a24p 8° (MeOH).
EXAMPLE 30: (S)-N-[[3-[4-[1-[1,2,4]Triazolyl]phenyl]-2-oxo-5.
oxazolidinyl]methyl)thiourea (44).
1.
o
IV~
vN ~/.~~/ N~O...H + ~ N N I "'-'~ ANN / ~ N~O
ld F ~ ~ ....H
NH2 p S O F ~N;C=S
26 79
A solution of 26 (0.190 g, 0.685 mmol) in CH2C12 (20 mL) was added, dropwise
during 20 min, under nitrogen, to an ice cold, stirred solution of 1,1~-
thiocarbonyldi-
2(1H)-pyridone (0.193 g, 0.831 mmol) in CHlCl2 (7 mL). The mixture was kept in
the ice bath for 20 min and at ambient temperature for 2 h, diluted with
CHZC12,
washed with water and brine, dried (MgS04) and concentrated. Chromatography of
the residue on silica gel with 10-15% CH3CN-CHzCIz gave O.I1 g of ?9 which was
used in the next reaction without further purification: MS(ES) m /z 320
(M+H'), 342
(M+Na').
2.
0
ANN / \ N~O...H N-"~ N NN / ~ N~C
THF v ~ ...H S
F N=C=g F ~ II
NH-C-NH2
T9
A stirred, ice cold solution of 79 (0.10 g, 0.31 mmol) in THF (15 mL) was
treated
with excess anhydrous ammonia and kept in the ice bath for 90 min. It was then
evaporated under a stream of nitrogen to a volume of about 5 mL to give a
solid
which was collected by filtration and washed with cold THF to give 0.105 g of
44:
mp 214-215 °C; 'H NMR [300 MHz, (CD~)1S0) d 3.82 (m, 3H), 4.18 (t, 1H),
4.89
(broad s, 1H), 7.20 (broad s, 2H), 7.50 (d, 1H), 7.79 (m, 2H), 7.93 (t, 1H),
8.26 (s, 1H),
8.97 (s, 1H); MS(ES) m /z 337 (M+H'), 359 (M+Na'). Anal. calcd for
C1;,H,3FN~02S:
C, 46.42; H, 3.90; N, 24.99. Found: C, 46.22; H, 3.98; N, 24.55.
-??-
CA 02351062 2001-05-10
WO 00/32599 PCT/tJS98/25308
EXAMPLE 31: (S)-N-([3-[3-Fluoro-4-[4-(hydroxyacetyl)-1-
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]-methyl]thiourea (45).
1.
O O N N
II /~ ~ O O
HOCHZ-C-N N / \ N~O O S O il /"'~
~.l H ~ HOCHz-C-N N / \ N~O
Et3N / CHZCIZ '--~ ~ ~--~"..H
NHZ F ~N~S
~HCI
29 80
An ice cold, stirred solution of 1,1~-thiocarbonyl-2(1H)-dipyridone (0.123 g,
0.530
mmol) in CHICIz (5 mL), under nitrogen, was treated with a suspension of 29
(0.17
g, 0.4 mmol) in CHzCl2 (20 mL) and then during 10 min with a solution of
triethylamine (0.111 mL, 0.8 mmol> in CHzCIz (10 mL). It was kept in the ice
bath
for 30 min, at ambient temperature for 2 h and at < 0 °C for 18 h. It
was then
diluted with CHzCIz, washed with water and brine, dried (MgS04) and
concentrated.
The residue (80) was used without further purification in the next reaction. A
sample of 80 that was purified by flash chromatography on silica gel with 10-
20%
acetonitrile-CHICIz had: 'H NMR (300 MHz, CDCl3) d 1.60 (broad s), 3.0? (m,
4H),
3.45 (m, 2H), 3.85 (m, 4H), 3.97 (d,d, 1H), 4.16 (t, 1H), 4.21 (s, 2H), 4.82
(m, 1H),
6.95 (t, 1H), 7.13 (d,d, 1H), 7.4? (d,d, 1H); MS m l a 395 (M+H+); 417
(M+Na').
2.
0 0
HOCH2-C-N~N / \ N~O N-~ HOCHZ-O-N~N / \ O
U ~ N~O H S
F N=C=S F ~NH-C-NH2
80 45
Excess anhydrous ammonia was bubbled into a stirred, ice cold solution of 80
(crude
product from the previous reaction) in THF (25 mL) and the mixture was kept in
the
ice bath for 90 min and concentrated under a stream of nitrogen. The residue
was
chromatographed on silica gel with 5010 MeOH-0.4% NH,OH-CHC13 and the product
was crystallized from acetonitrile to give 0.0544 g of 45: mp 209-210
°C; 'H NMR
[300 MHz, (CD3)lS0] d 294 (broad s, 4H), 3.4? (broad s, 2H), 3.60 (broad s,
2H), 3.78
(broad s, 3H), 4.07 (t, 1H), 4.10 (d, J = 5.5 Hz, 2H), 4.63 (t, J = 5.5 Hz,
1H), 4.81
(broad s, 1H), 7.05 (t, 1H), ?.16 (d,d, 1H), 7.15 (broad s, 2H), 7.49 (d,d,
1H), 7.91 (t,
1H); IR (mull) 3443, 3403, 3321, 3202, 3081, 1753, 1655, 1648 cm~'; HRMS (FAB)
-78-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
calcd for Cl;HIaFN50;S (M+H') 412.1454, found 412.1447. Anal. calcd for
C,.,H.~2FNSO,,S: C, 49.63; H, 5.39; N, 17.02. Found: C, 49.63; H, 5.48; N,
16.99.
EXAMPLE 32: (S)-N-E[3-[1-(Hydroxyacetyl)-5-indolinyl]-2-oxo-5-
oxazolidinyl]methyl]thiourea (46).
1.
\ i
HOCH2-C=O ~N N~ HOCH2-C=O
lO ~N ~I \ OI' .HCI O S O ~N I \ O''
~N~O Et3N ~N~O
~NHZ 81 ~N~=S
27
An ice cold, stirred solution of l,ld-thiocarbonyldi-2(1H)-pyridone (0.096 g,
0.41
mmol) in CH2C12 (5 mL) was treated with a suspension of 27 (0.10 g, 0.34 mmol)
in
CHZC12 ( 15 mL) and then with 0.05 mL (0.36 mmol) of triethylamine. It was
kept in
the ice bath for 30 min and at ambient temperature for 2 h, diluted with
CH2C12,
washed with water and brine, dried (MgSO,) and concentrated. Chromatography
ofthe residue on silica gel with 20-40~1o CH~CN-CHzCIz gave 0.04 g of 81.
2.
HOCHi-C=0 HOCH=-C=O
t
NH3 N ~ 0
I ~ ~ .~- I ~ ~ 0
N 0
H ~H S
~N=C=S ~~-C-~t
Excess anhydrous ammonia was bubbled into an ice cold solution of 81 (0.04 g)
in
THF (30 mL) and the mixture was kept in the ice bath for 80 min and
concentrated
under a stream of nitrogen. The residue was crystallized from CH3CN to give
0.0151 g of 46: mp 214-215 °C (dec); MS (FAB) mla 351 (M+H'), 350 (M'),
319, 304,
147; HRbIS (FAB) calcd for C15H,9N,O~S (M+H') 351.1127, found 351.1130; IR
(DRIFT) 3329, 3296, 3196, 1746, 1655, 1626 cm''.
EXAMPLE 33: (S)-N-[[3-[3~Fluoro-4-(4-thiomorpholinyl)phenyl]~2-oxo-5-
-79-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
oxazolidinyl]methyl)thiourea, thiomorpholine S-oxide (47).
1.
n i
O ~N N
'I TT11 O
OSUN / \ N~O",H O S O OS~N / \ N~O
~.-( CH C~ ~/ ..,H
F ~NH 2 2
2 F ~NCS
33 82
A suspension of 33 (0.30 g, 0.92 mmol) in CHlCIZ (7 mL) was added, during 20
min,
to an ice cold, stirred mixture of 1,1 ~-thiocarbonyldi-2( 1H)-pyridone (0.258
g, 1.11
mmol) and CHzCIz (20 mL). The mixture was kept in the ice bath for 20 min and
at
ambient temperature for 2 h, mixed with CH jCl1 (50 mL), washed with water and
brine, dried (MgSOa) and concentrated. Chromatography of the product on silica
gel
with 20-50% CH3CN-CH.~C12 gave 0.27 g of 82 which was used in the next
reaction:
MS(ES) m/z 370 (M+H'), 392 (M+Na').
2.
0 0
OS~N /_\ N~~O,..H NH3 i~ OS ~ / \ N~O
"~ THF \---~ ,."H S
F ~N= _ ~''~ II
C-S F ~NH-C-NH2
82 4~
A stirred, ice cold solution of 82 (0.27g , 0.73 mmol) in THF (15 mL), under
nitrogen,
was treated with excess anhydrous ammonia, kept in the ice bath for 1 h and
concentrated; crystallization of the residue from MeOH gave 0.1?5 g of 47; mp
212-
213 °C; 'H NMR [300 MHz, (CD3)1S0] d 2.83 (m, 2H), 3.01 (m, 2H), 3.17
(m, 2H),
3.50 (t, 2H), 3.78 (broad s, 3H), 4.08 (t, 1H), 4.80 (broad s, 1H), ?.17 (m,
2H), 7.17
(broad s, 2H), ?.50 (d, 1H), 7.90 (t, 1H); MS(ES) m/z 409 (M+Na'); IR (mull)
3335,
3284, 3211, 3175, 3097, 1750, 1630 cm-'. Anal. calcd for C15H1sFN~03Sz: C,
46.62; H,
4.95; N, 14.50. Found: C, 46.50; H, 4.95; N, 14.40.
EXAMPLE 34: (S)-N-[[3-(3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl-S-methyldithiocarbamate (48).
O O
~ N / \ ~ 1. CS2 / Et3N
~N O,..H O N / \
~/ 2. Mel L--~ ~N O...H S
F ~NHZ F ~NH-C-SCH
3
48
39 -80-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
An ice cold, stirred mixture of 39 (0.59 g, 0.0020 mol), EtOH (1.5 mL), water
(2
drops) and triethylamine {0.613 mL, 0.00440 mol), under nitrogen, was treated
with
carbon disulfide (0.066 mL, 0.0011 mol) and kept in the ice bath for 2 h and
at
ambient temperature for 18 h. (A solution was obtained after the addition of
carbon
disulfide; a white precipitate began to form soon after the mixture was warmed
to
ambient temperature. ) The thick suspension was treated, dropwise during 2
min,
with a solution of methyl iodide (0.137 mL; 0.00220 mol) in EtOH (2 mL) and
the
mixture was kept at ambient temperature for 1.5 h and concentrated in vacuo. A
solution of the residue in EtOAc was washed with saturated NaHC03, water and
brine, dried (MgS04) and concentrated. The residue was chromatographed on
silica
gel with 1.8% MeOH-CHlCl2 and the product was crystallized from EtOAc to give
0.197 g of 48: mp 154-155 °C; IR (mull) 3354, 3346, 1726 cm-'. Anal.
calcd for
C'sHzoFN:,OsS.~: C, 49.85; H, 5.23; N, 10.90. Found: C, 49.73; H, 5.25; N,
10.82.
EXAMPLE 35: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl-O-methylthiocarbamate (50).
O NaOCH3 O
2O ~ ~ ~ N o s Meo~ ~ ~ ~ N o s
NH-C-SCH3 F NH-C -~H3
48 50
A stirred mixture of 48 (0.200 g, 0.518 mmol), sodium methoxide (0.003 g, 0.06
mmol) and MeOH (5 mL), under.nitrogen, was refluxed for 4 h and kept at
ambient
temperature for 18 h. It was found that the starting material and product had
similar mobilities on TLC. the reaction was therefore followed by MS(ES).
Starting
material was still present. The mixture was refluxed for 3 h, additional
sodium
methoxide (0.005 g) was added and reflux was continued for 2 h. It was kept at
ambient temperature for 18 h, refluxed for 1 h, kept at ambient temperature
1.5 h
and concentrated in vacuo. The residue was mixed with ice, the pH was adjusted
to
9-10 with 1M KHS04 and saturated NaHCO~, and the mixture was extracted with
CH2C1.~. The extract was washed with water and brine, dried (MgSO;) and
concentrated. The residue was chromatographed on silica gel with 5% acetone-
CH2C1.~ and the product was crystallized from EtOAc-hexane to give 0.10? g of
50:
mp 128-129 °C; MS(ES) m/z 3?0 (M+H'), 392 (M+Na'); IR (DRIFT) 3282,
3251,
-81-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
1753, 1735 cm~'; 'H NMR (300 MHz, (CD3)zS0] d 2.94 (m, 4H), 3.47, 374 (m,m,
7H),
3.86, 3.91 (s,s, 3H), 4.10 (m, 1H), 4.73, 4.86 (m,m, 1H), 7.05 (t, 1H), 7.I6
(d,d, 1H),
?.4? (d,d, 1H), 9.41, 9.50 (s,s, 1H). Anal. calcd for C'sH2oFN30~,S: C, 52.02;
H, 5.46;
N, 11.38. Found: C, 51.97; H, 5.49; N, 11.35.
-82-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
When in the procedure of Example 35 an appropriate amount of sodium ethoxide
was substituted for sodium methoxide, the compound of Example 36 below in
Table A was
obtained.
When in the procedure of Example 1 an appropriate amount of (S)-N-[[3-[3-
fluoro-
4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]isopropylcarboxamide, (S)-N-
[[3-[3-
fluoro-4-morpholinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]cyclopropylcarboxamide, or
(S)-N-[[3-[3,5-difluoro-4-morpholinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]acetamide was
substituted for (S)-N-[[3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-S-
oxazolidinyl]-
methyl]acetamide (Compound 11 ) and the general procedures of Example 1 was
followed,
t0 the compounds of Examples 37, 38 and 39 respectively as set forth below in
Table A were
obtained. The isopropylcarboxamide and the cyclopropylcarboxamide are obtained
by
following the procedure in Example 5 of U.S. Patent No. 5,688,792 only
substituting
isobutyric anhydride and cyclopropane carbonyl chloride respectively for
acetic anhydride
in step 7. The acetamide is obtained as described in U.S. Patent No. 5,688,792
at Example
t5 4.
When in the procedure of Example 5, step B, an excess amount of dimethylamine
in
THF is substituted for anhydrous ammonia, the compound of Example 40 set forth
below in
Table A is obtained.
TABLE A
R oI'
O N ~ ~ N~O
U
NH-C-R'
Example No. Compound R, R'
36 (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)- R = H, R' = OOHS
phenyl)-2-oxo-5- oxazolidinyl]methyl)-O-
ethylthiocarbamate; mp 120 °C. MS(ES)
m/z 384 (M+H+). Anal. calcd for
C,~H»FNtO:,S: C. 53.25; H, 5.78; N, 10.96.
Found: C. 53.23; H, 5.82; N, 10.92.
-83-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example No. Compound R, R'
37 (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)- R = H. R' = CH(CH~),
phenyl]-?-oxo=5-oxazolidinyl]methyl]-~-
methylpropanethioamide: mp 152-153 °C
(dec.); Anal. calcd for C,~H~~FN~O;S: C,
56.67; H. 6.34; N, I 1.02. Found: C, 56.58:
H. 6.41: N, 10.81
38 (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)- R = H, R' _ -
phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropane-carbothioamide: mp 155-156
°C; Anal. calcd for C,~H,~FN;O;S: C,
56.98; H, 5.84; N, I 1.07. Found: C, 56.98:
H, 5.85; N, 10.97
39 (S)-N-[[3-[3,5-Difluoro-4-(4-morpholinyl)- R = F. R' = CHI
phenyl]-2-oxo-5-oxazolidinyl]methyl]-
thioacetamide
40 (S)-N-[[3-[3-Fluoro-~.-(4-morpholinyl)- R = H. R' = N(CHj)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-N',N'-
dimethylthiourea
PREPARATION Z Methyl Dithiopronionate
1) CH3CH2MgBr S
CS2 CH3CH2-C-SCH3
2) CH31
(a)
A stirred mixture of magnesium turnings (I?.6 g, 0.5?0 g atom) and THF (100
mL)
under nitrogen is treated with a crystal of iodine and about S~lo of a
solution of bromoethane
(30.0 mL. 0.-10 mol) in THF (200 mL). When the reaction starts, the remainder
of the
bromoethane solution is added. dropwise at a rate sufficient to maintain a
gentle reflux.
After the addition, stirring is continued for 1 hour: the resulting solution
is cooled to -20 °C
and treated, during 10 minutes with carbon disulfide (?4.0 mL. 0.=10 mol). The
mixture is
warmed to I~ °C, treated with methyl iodide (28.0 mL, 0.45 mol) and
kept at 60 °C for I
hour. It is then cooled in an ice bath, treated with ice and extracted with
Et,O. The extract
is washed with brine, dried (M~SO.~) and concentrated. Distillation of the
residue gives
34.0 g of the titled product. by 48-5? °C ( 12 mmHg).
-84-
CA 02351062 2001-05-10
WO 00132599 PCTNS98/25308
The following methyl dithio compounds were obtained when the appropriate alkyl
magnesium bromide was substituted for ethyl magnesium bromide in the above
procedure:
TABLE B
S
I I
Rs-C-SCH3
Rs =
(b) (CH~)~CH- (h)
(c) ~ (i)
(d) CH~CH~CH~- (j)
(e) CH3 (k) ~--CH -
CH3CH-CH2- 2
CH3 (!) ~--CH -
2
CH3CH2CH-
(g) (CHI);C-CHI- (m)
~CH2-
When following the general procedure of Example 27, step 4, an appropriate
to amount of the amine listed below is reacted with the dithio compound listed
below the
respective compounds. Examples 41 to 61 of Table C are obtained.
When following the general procedure of Example 25, step 6, an appropriate
amount of the amine listed below is reacted with the dithio compound listed
below, the
respective compounds, Examples 62 to 67, of Table C are obtained.
-ss-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
rABI.E c
Example Comeound Amine Dithio Compound
No. (from Preparation Z)
4I (S)-N-[[3-[3-Fluoro-4- ~ ° Z (a)
(4-thiomorpholinyl)- o=sVN ! ~ N~o
phenyl)-2-oxo-5- F ~ "
'-N"2
oxazolidinyl)methyl]-
propanethioamide,
thiomorpholine S-oxide:
mp 196-197°C;
Anal. calcd for
C,~H>?FN~O;S~: C,
S 1.11: H, 5.55; N, 10.52;
S, 16.05. Found: C.
50.99: H, 5.60: N, 10.55:
S, I5.75
42 S)-N-[[3-[3-Fluoro-4-(4- Same as above Z (b)
thiomorpholinyl )-
phenyl)-2-oxo-5-
oxazolidinyl]methyl)-2-
methylpropanethio-
amide, thiomorpholine
S-oxide; mp 195-196°C;
Anal. calcd for
C,gH~.~FN;O~S~: C,
52.28; H, 5.85; N, 10.16:
S, 15.51. Found: C,
52.24; H, 5.97: N, 10.16:
S, 15.28
43 (S)-N-([3-[3-Fluoro-4- Same as above Z (c)
(4-thiomorpholinyl)-
phenyl)-2-oxo-5-
oxazolidinyl)methyl)-
cyclopropanecarbothio-
amide, thiomorpholine
S-oxide; mp 109-I 10°C:
Anal. calcd for
C,RH»FN,O~S~_: C,
52.54: H, 5.39; N, 10.21:
S, 15.58. Found: C,
52.48; H, x.51: N, 10.?8:
S, 15.29
- 86 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
No. (from Preparation Z)
44 (S)-N-[[3-[3-Fluoro-4- Same as above Z (d)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
butanethioamide,
thiomorpholine S-oxide
45 (S)-N-[[3-[3-Fluoro-4- Same as above Z (e)
(4-thiomorphoIinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-3-
methylbutanethioamide,
thiomorpholine S-oxide
46 (S)-N-[[3-[3-Fluoro-4- Same as above Z (~
(4-thiomorpholinyl )-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylbutanethioamide,
thiomorpholine S-oxide
47 (S)-N-[[3-[3-Fluoro-4- Same as above Z (g)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
3,3-dimethylbutanethio-
amide, thiomorpholine
S-oxide
48 (S)-N-[[3-[3-Fluoro-4- Same as above Z (h)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclobutanecarbothio-
amide, thiomorpholine
S-oxide
49 (S)-N-[[3-[3-Fluoro-4- Same as above Z (i)
(:~-thiomorpholinyl)-
phenyl]-2-oxo-~-
oxazolidinyl]methyl]-1-
cyclopentanecarbothio-
amide, thiomorpholine
S-oxide
_87_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
No. (from Preparation Z)
50 (S)-N-[[3-[3-Fluoro-:~- Same as above Z ~)
(4-thiomorpholinyl )-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclohexanecarbothio-
amide, thiomorpholine
S-oxide
S I (S)-N-[[3-[3-Fluoro-.~- Same as above Z (k)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclopropylethanethio-
amide, thiomorpholine
S-oxide
52 (S)-N-[[3-[3-Fluoro-4- Same as above Z (1)
(4-thiomoipholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl)-2-
cyclobutylethanethio-
amide, thiomorpholine
S-oxide
53 (S)-N-([3-(3-Fluoro-4- Same as above Z (m)
(4-thiomorpholinyl)-
phenyi]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclopentylethanethio-
amide, thiomorpholine
S-oxide
54 (S)-N-[[3-[3,5-Difluoro- F ~ Ethyl dithioacetate
4-(4-thiomorpholinyl)- o=s~N ~ ~ rv o
phenyl]-2-oxo-5- U
-H
oxazolidinyl]methyl]- F NH
2
thioacetamide,
thiomorpholine S-oxide
55 (S)-N-[[3-[3.5-Difluoro- Same as above
Z (a)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-~-
oxazolidinyl]methyl]-
propanethioamide,
thiomorpholine S-oxide
_~s_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
No. (from Preparation Z)
56 (S)-N-[[3-[3.5-Difluoro- Same as above Z (b)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-S-
oxazolidinyl]methyl]-2-
methylpropanethio-
amide, thiomorpholine
S-oxide
57 (S)-N-[[3-[3.5-Difluoro- Same as above Z (c)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide, thiomorpholine
S-oxide
58 (S)-N-[[3-[4-(4- ~ Ethyl dithioacetate
thiomorpholinyl)- o-SAN ~ ~ N o
phenyl]-2-oxo-S- U
oxazolidinyl)methyl]-
thioacetamide,
thiomorpholine S-oxide
59 (S)-N-[[3-[4-(4- Same as above Z (a)
thiomorpholinyl)-
phenyl)-2-oxo-5-
oxazolidinyl]methyl]-
propanethioamide,
thiomorpholine S-oxide
60 (S)-N-[[3-[4-(4- Same as above Z (b)
thiomorpholinyl )-
phenyl]-2-oxo-5-
oxazolidinyl)methyl]-2-
methylpropanethio-
amide, thiomorpholine
S-oxide
61 (S)-N-([3-[4-(4- Same as above Z (c)
thiomorphoIinyl )-
phenyl]-2-oxo-5-
oxazolidinyl)methyl)-
cyclopropanecarbothio-
amide, thiomorpholine
S-oxide
-89-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
No.
62 (S)-N-[[3-[3.5-Difluoro- o F o (from Preparation Z)
4-(4-hydroxyacetyl)-1- H~H2'C-N~N ~ ~ N~o Z (a)
~H
piperazinyl]phenyl]-2- F ~NHZ
oxo-5-oxazolidinylJ-
methyl]propanethio-
amide
63 (S)-N-[[3-[3.5-Difluoro- Same as above Z (b)
4-(4-hydroxyacetyl)- I -
piperazinyl ]phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]-2-methyl-
propanethioamide
64 (S)-N-[[3-[3.5-Difluoro- Same as above Z (c)
4-(4-hydroxyacetyl)- I -
piperazinyl]phenyl]-2-
oxo-5-oxazolidinyl]-
methyl)cyclopropane-
thioamide
65 (S)-N-[[3-[3-[4- o ~ a Z (a)
(hydroxyacetyl)-1- HOCH2-C-N N ~ ~ N~o
-H
piperazinyl]phenyl]-2- ~NHq
oxo-5-oxazolidinyl]-
methyl]propanethio-
amide
66 (S)-N-[[3-[3-[4- Same as above Z (b)
(hydroxyacety!)-I-
piperazinyl]phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]-2-methyl-
propanethioamide
67 (S)-N-[[3-[3-[4- Same as above Z (c)
(hydroxyacetyl )- I -
piperazinyl]phenyl]-2-
oxo-S-oxazolidinyl]-
methyl]cyclopropane-
carbothioamide
When following the procedure of Example 28, step 3, an appropriate amount of
the
amine listed below is reacted with the dithio compound listed below, the
respective
compounds, Examples 68 to 78 of Table D are obtained.
-90-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TAB LE D
Example Compound Amine Dithio Compound
No.
(see Preparation Z)
6$ (S)-N-[[3-[3-Fluoro-a- ~ Z (a)
(4-thiomorpholinyl)- o2s~N ~ ~ N o
phenyl]-2-oxo-5- U l~-~
oxazolidinyl]methyl]- F NH
z
propanethioamide,
thiomorpholine S,S-
dioxide
69 (S)-N-[(3-[3-Fluoro-4- Same as above
(4-thiomorpholinyl)- Z (b)
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylpropanethio-
amide, thiomorpholine
S,S-dioxide
70 (S)-N-([3-[3-Fluoro-4- Same as above Z (c)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide, thiomorpholine
S.S-dioxide
71 (S)-N-[[3-[3,5- Difluoro- F o Ethyl dithioacetate
4-(4-thiomorpholinyl)- o2s~N ~ ~ N~o
phenyl]-2-oxo-5- U
~-H
oxazolidinyl)- ~NH
2
methyl]thioacetamide,
thiomorpholine S,S-
dioxide
72 (S)-N-[[3-[3,5- Difluoro- Same as above Z (a)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
propanethioamide,
thiomorpholine S,S-
dioxide
-91 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
(see Preparation Z)
73 (S)-N-[[3-[3.5- Difluoro- Same as above Z (b)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylpropanethio-
amide. thiomorpholine
S,S-dioxide
74 (S)-N-[[3-[3,5- Difluoro- Same as above
Z (c)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidiny(]methyl]-
cyclopropanecarbothio-
amide, thiomorpholine
S.S-dioxide
75 (S)-N-[[3-[4-(4-thio- o Ethyl dithioacetate
morpholinyl)phenyl]-2- o2s~N ~
oxo-5-oxazolidinyl]- V _H
methyl]thioacetamide, ~NH2
thiomorpholine S,S-
dioxide
76 (S)-N-[[3-[4-(4-thio- Same as above Z (a)
morpholinyl)phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]propanethio-
amide, thiomorpholine
S.S-dioxide
77 (S)-N-[[3-[4-(4-thio- Same as above Z (b)
morpholinyl)phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]-2-methyl-
propanethioamide,
thiomorpholine S,S-
dioxide
78 (S)-N-[[3-[4-(4-thio- Same as above Z (c)
morpholinyl)phenyl]-2-
oxo-S-oxazolidinyl]-
methyl ]cyclopropane-
carbothioamide,
thiomorpholine S.S-
dioxide
_92_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
When following the procedure of Example 26, an appropriate amount of the amine
listed below is reacted with the dithio compound listed below the respective
compounds.
Examples 79 to 99 of Table E are obtained.
TABLE E
Example Compound Amine Dithio Compound
No. (See Preparation Z)
79 (S)-N-[[3-[3-Fluoro-4- ~ Z (a)
(4-thiomorpholinyl)- n ~~ \
phenyl]-2-oxo-5- UN \-/ N O---H
oxazolidinyl)methyl]- ~'''F
~NH
propanethioamide
80 (S)-N-[[3-[3-Fluoro-4- Same as above
Z (b)
(4-thiomorphoiinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylpropanethioamide
81 (S)-N-[[3-[3-Fluoro-4- Same as above
Z (c)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide
82 (S)-N-[[3-[3-Fluoro-4- Same as above Z (d)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
butanethioamide
83 (S)-N-[[3-[3-Fluoro-4- Same as above Z (e)
(4-thiomorpholinyl)-
phenyl]-2-oxo-S-
oxazolidinyl]methyl]-3-
methylbutanethioamide
84 (S)-N-[[3-(3-Fluoro-~- Same as above Z {~
(~-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl)-2-
methylbutanethioamide
-93-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Amine Dithio Compound
Compound
No.
( See Preparation
Z)
85 (S)-N-[(3-[3-Fluoro-4-Same as above Z (g)
(4-thiomorpholinyl)-
phenyl]-2-oxo-S-
oxazolidinyl]methyl]-
3.3-dimethylbutanethio-
amide
86 (S)-N-([3-[3-Fluoro-4-Same as above Z (h)
(4-thiomorpholinyl)-
phenyl)-2-oxo-5-
oxazolidinyl)methyl]-
cyclobutanecarbothio-
amide
87 (S)-N-[[3-[3-Fluoro-~-Same as above Z (i)
(4-thiomorpholinyl)-
phenyl]-2-oxo-S-
oxazolidinyl]methyl]-
cyclopentanecarbothio-
amide
88 (S)-N-[[3-(3-Fluoro-4-Same as above Z (j)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclohexanecarbothio-
amide
89 (S)-N-[[3-[3-5 Fluoro-4-Same as above Z (k)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclopropylethanethio-
amide
90 (S)-N-[[3-[3-Fluoro-4-Same as above Z (1)
(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclobutylethanethio-
amide
-94-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
No. (See Preparation Z)
91 (S)-N-[[3-[3-Fluoro-4- Same as above Z (m)
(4-thiomorpholinyl)-
phenyl]-2-oxo-~-
oxazolidinyl ]methyl]-2-
cyclopentylethanethio-
amide
92 (S)-N-[[3-[3,5-Difluoro- F o Ethyl dithioacetate
4-(4-thiomorpholinyl)- SAN ~ ~ N~O
phenyl]-2-oxo-5- U
oxazolidinyl]methyl]- F H
thioacetamide NH2
93 (S)-N-[[3-[3,5-Difluoro- Same as above Z (a)
-l-(4-thiomorpholinyl )-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
propanethioamide
94 (S)-N-[[3-[3,5-Difluoro- Same as above Z (b)
4-(4-thiomorpholinyl)-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylpropanethioamide
95 (S)-N-[[3-[3,5-Difluoro- Same as above Z (c)
4-(4-thiomorphoiinyl)-
phenyl]-2-oxo-~-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide
96 (S)-N-[[3-[4-(4-thio- O
morpholinyl)phenyl]-2- SAN ~ ~ N~o Ethyl dithioacetate
oxo-5-oxazolidinyl.]- ~/ ~ ---H
methyl]thtoacetamide
~NH
2
97 IS)-N-[(3-[4-(~-thio- Same as above Z (a)
morpholinyl )phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]propanethio-
amide
-95-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithio Compound
No. (See Preparation Z)
98 (S)-N-([3-[4-(4-thio- Same as above Z (b)
morpholinyl)phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]-2-methyl-
propanethioamide
99 (S)-N-j[3-[4-(4-thio- Same as above Z (c)
morpholinyl)phenyl]-2-
oxo-5-oxazolidinyl]-
methyl]cyclopropane-
carbothioamide
The amine utilized in Examples 41 to 53 is prepared as described in Example
27,
Step 3. The amine utilized in Examples 54 to 57 is prepared by the procedure
of Example
27, steps 1 to 3 by substituting the appropriate (S)-N-[(3-[3.5-difluoro-:~-(4-
thio-
morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methanol for compound 62 in step 1 of
Example
27.
The amine utilized in Examples 58 to 61 is prepared by the procedure of
Example
27, steps 1 to 3 by substituting the appropriate (S)-N-[[3-[4-(4-
thiomorpholinyl)phenyl]-2-
oxo-5-oxazolidinyl]methanol for compound 62 in Example 27, step 1. The
appropriate
oxazolidinyl methanol compound is obtained by following the procedure of
Example 1 in
U.S. Patent 5.688,792, steps 1 through 3, only substituting 4-
fluoronitrobenzene for 3,4-
difluoronitrobenzene in step 1 thereof.
The amine utilized in Examples 62 to 64 is prepared as compound 37 in Example
29
from the amide, 65, which is prepared as described in Example 32 of U.S.
Patent No.
5,700,799. The amine utilized in Examples 65 to 67 is prepared by the general
procedure of
Example 29 from the following amide, the preparation of which is decribed in
Example 3 of
U.S. Patent x.700,799:
O
Boc -NON ~ ~ N~O
~NHAc
-96-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
The amine utilized in Examples 68 to 70 is prepared as described in step 2 of
Example 28 above.
The amine utilized in Examples 71 to 74 is prepared as described in Example 28
by
substituting (S)-N-[[3-[3.5-difluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-~-
oxazolidinyl]methanol for compound 62 in step 1 and following the procedure of
steps 1
and 2. The appropriate oxazolidinyl methanol compound is prepared by following
the
general procedure of Example 4 of U.S. Patent No. 5,688,79?, steps I through
4, only
substituting thiomorpholine for morpholine in step I thereof.
The amine utilized in Examples 75 to 78 is prepared as described in Example
28,
to step 1, above by substituting (S)-N-[3-[4-(4-thiomorpholinyl)phenyl]-2-oxo-
S-
oxazolidinyl]methanol for compound 62 in step 1. The appropriate oxazolidinyl
methanol
is obtained by following the procedure of Example 1 in U.S. Patent No.
5,688,792, steps I
through 3, only substituting 4-fluoronitrobenzene for 3,4-difluoronitrobenzene
in step 1
thereof.
~5 The amine utilized in Examples 79 to 91 is prepared as described in Example
1, step
4, of U.S. Patent No. 5,688,792. The amine utilized in Examples 92 to 95 is
prepared as
described in Example 4 of U.S. Patent No. 5,688,792 only substituting
thiomorpholine for
morpholine in step 1 thereof. The amine utilized in Examples 96 to 99 is
prepared by the
procedure of Example I of U.S. Patent No. 5,688,792, only substituting 4-
fluoronitro-
2o benzene for 3,4-difluoronitrobenzene in step I thereof.
EXAMPLE 100 (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate, thiomorpholine S-oxide
O
O=SAN ~ ~ N~O
~H S
~NHC-OCH3
A solution of ?01 mg {0.55=~ mmol) of (S)-N-[[3-[3-fluoro-4-(4-
thiomorpholinyl)-
35 phenyl]-2-oxo-5-oxazolidinyl]methyl]isothiocyanate, thiomorpholine s-oxide
compound 82
from Example 33, step 1, in methanol ( 10 mL) is refluxed, under nitrogen for
18 hours and
cooled. The solid is collected by filtration to give 0.138 g of the titled
product. m.p. 208-
209°C: Anal. calcd for C,~H~,~FNzO~S~: C, 47.87; H, 5.02: N, 10.47.
Found: C. 47.81; H,
5.04: N, 10.49.
_97_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
When in the procedure of Example 100 the thioisocyanate listed below is
substituted
for compound 82 the products listed below as Examples 101 to 109 are obtained.
TABLE F
Isothiocyanate
Ra p
n II
Rc N ~ ~ N~O
Rh [ =I H -
~N-C=S
Rc Ra Rb Example Compound
No.
OS F F 101 (S)-N-[[3-[3.5-Difluoro-4-(4-
thiomorpholinyl)phenyl)-2-oxo-5-
oxazolidinyl)methyl]-O-methylthio-
carbamate, thiomorpholine S-oxide
OS H H 102 (S)-N-[[3-[4-(4-thiomorpholinyl)phenyl)-2-
oxo-5-oxazolidinyl]methyl)-O-methyithio-
carbamate, thiomorphoIine S-oxide
O~S H F 103 (S)-N-[[3-[3-Fluoro-4-(4-thiomoipholinyl)-
phenylj-2-oxo-5-oxazolidinyl]methyl)-O-
methylthiocarbamate, thiomorpholine S,S-
dioxide
O~S F F 104 (S)-N-[(3-[3,5-Difluoro-4-(4-
thiomorpholinyf)phenyl]-2-oxo-5-
oxazolidinyl ] methyl]-O-methylthio-
carbamate, thiomorpholine S,S-dioxide
O~S H H 105 (S)-N-[[3-(4-(4-thiomorpholinyl)phenyl]-2-
oxo-5-oxazolidinyl]methyl]-O-methylthio-
carbamate, thiomorpholine S,S-dioxide
S H F 106 (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)-
phenyl)-2-oxo-~-oxazolidinyl]methyl]-O-
methylthiocarbamate
S F F 107 (S)-N-[[3-[3,5-Difluoro-4-(4-thiomorph-
olinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
_98_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
12c lZa ltb Example Compound
No.
S H H 108 (S)-N-[[3-[4-(4-thiomorpholinyl)phenyl]-2-
oxo-S-oxazolidinyl]methyl]-O-methylthio-
carbamate
H H 109 (S)-N-[[3-[3-Fluoro-~-(4-(hydroxyacetyl)-1-
HOCHZCN piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]-
methyl]-O-methylthiocarbamate
When in the procedure of Example 100 an appropriate amount of ethanol and
isopropyl alcohol were substituted for methanol, the following respective
compounds were
obtained:
S EXAMPLE 110: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyi)phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-ethylthiocarbamate, thiomorpholine S-oxide. m.p. 198-
199°C;
Anal. calcd for C,~H~,_FN~04S~: C, 49.14; H, 5.34; N, 10.11. Found: C, 49.06;
H, 5.27; N,
10.10.
EXAMPLE 1 I I: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-S-
0 oxazoiidinyl)methyl]-O-isopropylthiocarbamate, thiomorpholine S-oxide. m.p.
180-181°C;
Anal. calcd for C,sH~,~FN~O.~S,: C, 50.33; H, 5.63: N, 9.78. Found: C, 50.29:
H, 5.69; N,
9.82.
When in the procedure of Example 1 14 an appropriate amount of (S)-N-[(3-[3-
fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-S-oxazolidinylJisothiocyanate is
substituted for
1S compound 82 and ethanol or isopropyl alcohol is substituted for methanol,
the following
respective products are obtained:
EXAMPLE 112: (S)-N-[(3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-ethylthiocarbamate;
EXAMPLE 113: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-S-
20 oxazolidinyl]methyl]-O-iso-propylthiocarbamate;
EXAMPLE 114: (S)-N-[(3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl)-2-oxo-S-
oxazolidinyl]methyl]-N-methylthiourea, thiomorpholine S-oxide.
A stirred suspension of 240 mg (0.650 mmol ) of compound 82 from Example 33,
step 1 in THF (S mL) at 0 °C is treated with a 2M solution of
methylamine in THF (0.42
2S mL. 0.845 mmol) and kepi at ambient temperature for I8 hours. The solid is
collected by
filtration to dive 0.221 g of the titled product.
-99-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98l25308
Following the procedure of Example 1 14, only substituting an appropriate
amount
of dimethylamine and azetidine for methylamine, the following compounds are
obtained:
EXAMPLE 115: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-S-
oxazolidinyl]methyl]-N',N'-dimethylthiourea, thiomorpholine S-oxide: Anal.
Calcd for
C,~H~~FN:~O,S~, C. 49.26: H. x.59; N, 13.52. Found C, 49.1 l: H, 5.57; N.
13.40; mp 180-
182°C.
EXAMPLE 116: (S)-N-[(3-(3-Fluoro-4-(4-thiomorpholinyl)phenyl)-2-oxo-S-
oxazolidinyl]methyl]-l-azetidinecarbothioamide, thiomorpholine S-oxide; Anal.
Calcd for
C,BH,~FN~,OzS~, C, 50.69; H. x.43; N, 13.14. Found: C, 50.79; H, 5.45; N,
12.82; mp 2I3-
to 214°C.
When in the procedure of Example 114 an appropriate amount of (S)-N-[[3-[3-
fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-S-oxazolidinyl)methyl]isothiocyanate
is
substituted for compound 82. the following compound is obtained:
EXAMPLE 117: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-
is oxazolidinyl]methyl]methyl-N'-methylthiourea.
When in the procedure of Example I 17 an appropriate amount of dimethylamine
and azetidine are substituted for methylamine, the following respective
products are
obtained:
EXAMPLE 118: (S)-N-[(3-(3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-
20 oxazolidinyl]methyl]-N',N'-dimethylthiourea;
EXAMPLE 119: (S)-N-[(3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-1-azetidinecarbothioamide.
When in the procedure of Example 33 an appropriate amount of compound 31 from
Example 26 is substituted for compound 33 and the general procedure of steps 1
and 2 of
25 Example 33 are followed. the following compound is obtained.
EXAMPLE 120: (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]thiourea.
EXAMPLE 121: (S)-N-[[3-[3-Fluoro-4-[4-(hydroxyacetyl)-1-piperazinyl)phenyl-2-
30 oxo-5-oxazolidinyl]methyl]propanethioamide
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
S
il
O O CH3CH2CSCH3 O O
-- II
HOCH2CiN~N ~ ~ N~O Et3N HOCH2C N N ~ ~ N~O
~.J F - ~H ~./ F -_ ~H S
~'~-NH2 NH-IC
2g CH2
CH3
A stirred mixture of 200 mg (0.514 mmol) of 29 methyl dithiopropionate (247
mg,
2.06 mmol), triethylamine (0.58 mL, 4.11 mmol), THF (5.4 mL) and methylene
chloride
(5.4 mi.,) is kept, under nitrogen, for 3 days, diluted with water and
extracted with
methylene chloride. The extracts are dried (MgSO.~) and concentrated.
Chromatography of
the residue on silica gel and crystallization of the product from methanol
gives 0.132 g of
the titled product. m.p. 190-191°C: Anal. calcd for C,yH~SFN.~O.,S: C.
53.76; H, 5.94; N,
13.20; S, 7.55. Found: C, 53.66; H, 5.94; N, 13.20; S, 7.37.
Following the procedure of Example 121 only substituting dithio compounds Z
(b)
to Z (m) from Preparation Z above for methyl dithiopropionate, the following
compounds
are obtained.
TABLE G
O _ O
HOCH2IGI VAN ~ ~ N~O --H
S
~NH-C-R
Example No. Compound
122 (S)-N-[(3-[3-Fluoro-4-[4-(hydroxy- R = CH(CH~)
acetyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-methylpropane-
thioamide: Anal. calcd for
C~~,H~~FNaO.,S: C, 54.78; H, 6.21; N,
12.78; S, 7.31. Found: C, 54.67; H,
6.34; N, 12.41: S, 7.15
CA 02351062 2001-05-10
WO 00/32599 PCT/US9$/Z5308
Exam-ple No. Compound
123 (S)-N-[[3-j3-Fluoro-4-[4-(hydroxy-
acetyl )- I-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]cyclopropane-
carbothioamide: mp 179-181 °C: Anal.
calcd for C~,~H~SFiV,O:,S: C, 55.03: H,
5.77: N, 12.84; S, 7.34. Found: C,
55.15: H, 5.72; N, 12.76: S, 7.09
124 (S)-N-[[3-[3-Fluoro-4-[4-(hydroxy- R = CH_~-CHI-CH,
acetyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl)butanethioamide
125 (S)-N-[[3-[3-Fluoro-4-[4-(hydroxy- CH3
acetyl)-1-piperazinyl)phenyl]-2-oxo-S- R - CH2-cH-CH3
oxazolidinyl ] methyl ]-3-methyibutane-
thioamide
126 (S)-N-[[3-[3-Fluoro-4-[4-(hydroxy- CH3
acetyl)-1-piperazinyl]phenyl]-2-oxo-5- R = CH-CH2-CH3
oxazolidinyl]methyl]-2-methyibutane-
thioamide
127 (S)-N-([3-[3-Fluoro-4-[4-(hydroxy- R = CH,-C(CH~)
acetyl)-1-piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]-3,3-dimethyl-
butanethioamide
128 (S)-N-[[3-[3-Fluoro-4-[4-(hydroxy-
acetyl)-1-piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]cyclobutane-
carbothioamide
129 (S)-N-[[3-[3-Fluoro-4-(4-(hydroxy- R =
acetyl)-I-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]cyclopentane-
carbothioamide
130 (S)-N-[[3-[3-Fluoro-4-[4-(hydroxy-
acetyl)- i-piperazinyl)phenyl]-2-oxo-5-
oxazolidinyi]methyl]cyclohexane-
carbothioamide
131 (S)-N-[[3-[3-Fluoro-4-[4-(hydroxy-
2
acetyl)-1-piperazinyl]phenyl]-2-oxo-~-
oxazolidinyl]methyl]-2-cyclopropyl-
ethanethioamide
_~p?_
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example No. Compound
132 (S)-N-j[3-[3-Fluoro-4-[4-(hydroxy- R = CH2--O
acetyl)-1-piperazinyl]phenyl]-Z-oxo-5-
oxazolidinyl ]methyl]-2-cyclobutyl-
ethanethioamide
133 (S)-N-((3-[3-Fluoro-4-(4-(hydroxy- R = CH2-
acetyl )- l -piperazinyl]phenyl ]-2-oxo-5 ~-
oxazoiidinyl]methyl]-2-cyclopentyl-
ethanethioamide
When in the procedure of Example 100 an appropriate amount of compound 80
from Example 31 is substituted for compound 82, and ethanol or isopropyl
alcohol is
substituted for methanol, the following respective compounds are obtained:
EXAMPLE 134: (S)-N-[(3-[3-Fluoro-4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl]-2-
oxo-5-oxazolidinyl)methyl]-O-ethylthiocarbamate:
EXAMPLE 135: (S)-N-[[3-[3-Fiuoro-4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl)-2-
oxo-5-oxazolidinyl]methyl]-O-iso-propylthiocarbamate;
EXAMPLE 136: (S)-N-[[3-[3-Fluoro-4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl)-2-
to oxo-5-oxazolidinyl]methyl)-N'-methylthiourea.
When in the procedure of Example 114 an appropriate amount of compound 80
from Example 31 is substituted for compound 82, the title compound is
obtained.
Following the procedure of Example 1 14 only substituting an appropriate
amount of
compound 80 from Example 31 for compund 82 and substituting an appropriate
amount of
~5 dimethylamine and azetidine for methylamine, the following compounds.
Examples 137
and 138, are obtained:
EXAMPLE 137: (S)-N-[[3-(3-Fluoro-4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl)-2-
oxo-5-oxazolidinyl)methyl]-N'.N'-dimethylthiourea:
EXAMPLE 138: (S)-N-[[3-[3-Fluoro-4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl]-2-
20 oxo-5-oxazolidinylJmethyl]-I-azetidinecarbothioamide.
EXAMPLE 139: (S)-N-[(3-[3.5-Dil7uoro-4-[-1-(hydroxyacetyl)-I-
piperazinyl)phenyl]-
2-oxo-~-oxazolidinyl]methyl]-O-tnethylthiocarbamate.
Part A: Following the procedure of Example 33, step 1, only substituting an
35 appropriate amount of compound 37 from Example ?9, step 5, for compound 33.
(S)-N-
- 103 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
[[3,5-[3-difluoro-~-[4-(hydroxyacetyl )-1-piperazinyl]phenyl]-2-oxo-~-
oxazolidinyl]-
methyl]isothiocyanate is obtained.
Pan B: Upon substitution of an appropriate amount of (S)-N-[[3-[3,5-difluoro-4-
[4-
(hydroxyacetyl)-1-piperazinyl]phenyl]-2-oxo-~-
oxazolidinyl]methyl~sothiocyanate for
compound 8? in the general procedure of Example 100, the title compound is
obtained.
EXAMPLE 140: (S)-N-[[3-[4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
Part A: Following the procedure of Example 33, step I, only substituting an
to appropriate amount of (S)-N-[[3-[4-[4-(hydroxyacetyl)-1-piperazinyl]phenyl]-
2-oxo-5-
oxazolidinyl]methyl]amine for compound 33, (S)-N-[[3-[4-[4-(hydroxyacetyl)-I-
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]isothiocyanate is obtained.
- f 04 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Part B: Upon substituting an appropriate amount of (S)-~'V-[[3-[4-(:~-Ihydroxy-
acetyl)-I-piperazinyl)phenyl)-2-oxo-5-oxazolidinyl)methylbsothiocyanate for
compound 82
in the general procedure of Example 100, the title compound is obtained.
s EXAMPLE 141: (S)-N-[[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]thioacetamide
Step 1
N02
O O / ~ O
II ~"~ ~ ~ S02C1 ''
PhCH20C NON / ~ N p Cbz-N N / ~ N~O
~t3N U ~~H
OH F ONos
58
l0 An ice cold, stirred solution of 30.4 g (70.8 mmol) of starting material 58
from
Example 25, step 1 ), and triethylamine ( 15.4 mL, 110 mmol) in methylene
chloride (2570
mL) is treated with ~»-nitrobenzenesulfonyl chloride ( 18.8 g, 84.9 mmol) and
kept, under
nitrogen, at ambient temperature (24 °C) for 24 hours. Additional m-
nitrobenzenesulfonyl
chloride ( I .88 g) and triethylamine ( 1.54 mL) are added and the mixture is
kept for one
is additional day at ambient temperature, washed with water, saturated sodium
bicarbonate
and brine, dried (Na~SO,~) and concentrated to give an oily product, 85. The
alcohol, 58 is
prepared according to the procedures of Brickner (J. Med. Chem. 1996, 39, 673-
679), see
compound Sa therein.
Step 2
0 0
Cbz N~N / ~ N~O NH- 4--~ Cbz N~N / ~ N~O
U .,.,H U ~--~
~ONos F ~NI-12
20 85 86
A stirred mixture of 85, acetonitrile ( 1270 mL), isopropanol ( 1270 mL) and
ammonium hydroxide ( I 270 mL) is kept at ambient temperature for 3 days and
concentrated i» vaccro. Chromatography of the residue on silica gel with 0.590
NHL~OH-I%
25 MeOH-CH~CI~ gives 2?.4 g of the amine. 86.
Step 3
- l05 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
O O
CbzN~N ~ ~ N~01"H Bob Cbz ~N ~ ~ N~O,
-~- ..H
NH2 F ~NHBoc
86 8~
An ice cold. stirred solution of the amine 86 in THF (6S0 mL) is treated,
during 20
mintues with a solution of di-tert-butyl dicarbonate ( 12.0 g, 55.2 mmol) in
THF (90 mL).
The mixture is kept at ambient temperature for 18 hours and concentrated in
vacuo. The
residue, dissolved in methylene chloride, is washed with dilute sodium
bicarbonate, dried
(MgSO.~) and concentrated. Crystallization of the residue from methanol-ethyl
acetate gives
20.0 g of the Boc protected amine. Additional product (4.1 g) is obtained by
chromatographing the mother liquors on silica gel with I-2% methanol-methylene
chloride.
Step 4
0 0
CbzN~N ~ ~ N~O --? -~ HN N ~ ~ N~O'.
~H Pd/C U ~ " H
NHBoc F
~NHBoc
t0 8~ 88
A solution of the protected amine, 87, (5.00 g, 9.46 mmol) in ethanol ( 1 SO
mL) is
treated with 10~'o palladium-on-carbon catalyst ( 1.0 g) and hydrogenated at
an initial
pressure of 30 psi for 3 hours. The catalyst is removed by filtration through
Celite and the
filtrate was concentrated to give 3.66 g of compound 88.
a5 Step S
0I' 0 0
HNVN ~ ~ N~O A- ~-~ CH CNN ~ ~ N~O
~--~ .."H Py 3 ~/ ~ .".H
~NHBoc F ~NHBoc
88 89
A stirred solution of compound 88 ( 1.10 g, 2.79 mmol) in pyridine ( 10 mL) is
treated with acetic anhydride (289 ~tL. 3.07 mmol), kept at ambient
temperature for 2 hours
and concentrated ui vacuo. A solution of the residue in methylene chloride is
washed with
2o dilute hydrochloric acid, dried (MgSO.~) and concentrated to give 1.23 g of
compound 89:
MS m/z 436 (M+)
Step 6
- I 06 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
O O
O
CH3 CNN ~ ~ N~O...H HC~ CH3 ON~N ~ ~ N~O
dioxane ~ ~ .,..H
F NHBoc F ~NHz
89 P-90 ~ HCI
An ice cold, stirred 4N solution of HCl in dioxane ( l0 mL) is treated with
compound 89 (1.10 g. 2.52 mmol). The mixture is kept in the ice bath for 30
minutes and
at ambient temperature for 1 hour. It was then mixed with methylene chloride
and
concentrated. The residue is triturated with methylene chloride to give 1.03 g
of the amine
hydrochloride.
Step 7
o 0II
CH3G~ ~N / ~ N~Ot CH3~C~ CH3ICOI.N~N ~
"H Et3N ~../ N ~...H S
F ~ II
NH2 F ~NH-C-CH3
~HCI
P-90
A stirred mixture of compound P-90 (250 mg), triethylamine (0.75 mL, 5.36
mmol),
to ethyl dithioacetate (307 p.L, 2.68 mmol), methylene chloride (7.4 mL) and
THF (7.4 mL) is
kept at ambient temperature for 1 day, concentrated and chromatographed on
silica gel with
mixtures of methanol-methylene chloride containing 1-2% methanol.
Crystallization of the
product from ethyl acetate-heptane gives 0.160 g of the titled product: Anal.
calcd for
C,$H~3FNaO~S: C, 54.81; H, 5.88; N, 14.20; S, 8.13. Found: C, 54.92; H, 5.95;
N, 14.08;
t5 S. 7.94; mp 158°C.
When in the general procedure of Example 1-t 1 an appropriate amount of
F O O O
O
II n If
PhCH20C NON ~ ~ N~O111H Or PhCH20C ~N ~ ~ N~O'.
F ~--~ H
~OH y ~,OH
X
is substituted for compound 58 and the procedure of steps 1 through 6 are
followed, the
respective amine compounds P-91 and P-92 listed below are obtained:
- t o7 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
O F O
CH3CIN~N ~ ~ N~O
~/ ~H P-91
F NH2
O O
CHgC- ~N ~ ~ N~O -,H P-92
~NH
2
The alcohols above designated as x and y are prepared according to the
procedures
of Brickner (J. Med. Chem., 1996, 39, 673-679), by substituting an appropriate
amount of
2,6-difluoro-4-nitrobenzene (trifluoromethane) sulfonate and 4-
fluoronitrobenzene
respectively for 3,4-difluoronitrobenzene in the preparation of 2a therein.
When in the procedure of Example 141 an appropriate amount of x or y is
substituted for compound 58 and the procedures of steps 1 through 4 are
followed, the
following Boc protected compounds listed below are obtained.
F O
H ~N ~ ~ N~O x-b
-H
F
~NH
Boc
O
HN~N ~ ~ N~O
H Y-b
~NHB c
0
to When in the procedure of Example 141, step 5, an appropriate amount of
compound
88, compound x-b or compound y-b is treated with the reagent listed below and
the general
procedures of step 5 and step 6 are followed, the amines listed below as
Preparation P-93
through P-128 are obtained.
The amine compound set forth below as P-129 is obtained by refluxing for 6
days a
IS solution of compound 88 ( 1.00 g, 2.54 mmol), sulfamide (305 mg, 3.I8 mmol)
and 1,2-
dimethyoxyethane (6 mL). The solid which precipitates is collected by
filtration and
chromatographed on silica gel with 59c methanol-methylene chloride.
Crystallization of the
product from methanol-methylene chloride gives 0.551 g of the sulfamoyl
derivative, which
is used in step 6 of Example 141 to Give P-129. When compounds x-b and y-b are
- I o8 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
substituted for compound 88 and this general procedure is followed.
Preparations P-130 and
P-13 I respectively set forth below are obtained.
Following the general procedures of steps ~ and 6 of Example 141 only in step
5
substiwting chloroacetonitrile or ?-fluoroethyI bromide respectively for
acetic anhydride
and using potassium carbonate in acetonitrile, and using either compound 88,
compound x-
b or compound y-b, the respective amines set forth below as Preparations P-132
to P-137
are obtained.
The amine compound set forth below as Preparation P-138 is obtained by
combining compound 88 (I.10 g, 2.75 mmol) set forth in step 5 of Example 141
with N
to formylbenzotriazole (493 mg, 3.35 mmol) in THF (30 mL) and the mixture is
kept at
ambient temperature for 18 hours. The mixture is concentrated and the residue
in
methylene chloride is washed with 1 N sodium hydroxide and dilute sodium
chloride, dried
(MgSO.,), concentrated, and chromatographed on silica gel with mixtures of
methanol and
methylene chloride containing I-2% methanol to give 1.09 g of the N-formyl
derivative
which is utilized in the general procedure of step 6 of Example 141 to give
Preparation P-
138. When in this foregoing procedure compound x-b or compound y-b is
substituted for
compound 88, Preparations P- i 39 and and P-140 as set forth below are
obtained.
R"
O
R- ~N ~ ~ N~O
-H
R~ ~NH
2
Reasent Boc R R" R' Preparation
Compound No.
methoxyacetylchloride 88 O H F P-93
x-b CH30CH2IC- F F P-94
Y-b H H P-95
cyanoacetylchloride 88 O H F P-96
x-b NCCH2C F F P-97
Y-b H H P-98
acetoxyacetyl chloride 88 O O H F P-99
x-b CH3C-O-CH2i!- F F P-100
y-b H H P-101
- I 09 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Rea ent Boc R R" R' Preparation
Compound No.
benzyloxyacetyl chloride88 O H F P-102
x-b PnCH20CH2~- F F P-103
Y-b H H P-I04
methyl chloroformate88 O H F P-105
x-b C~30~.- F F P-106
Y-b H H P-107
methanesulfonyl chloride88 CH3S02- H F P-108
x-b F F P-109
Y-b H H P-110
ethanesulfonylchloride88 H F P-111
x-b CH~CH~SO~- F F P-112
Y-b H H P-113
chloromethanesulfonyl88 H F P-114
chloride x-b C1CH~S0~- F F P-115
Y-b H H P-116
cyanomethanesulfonyl88 H F P-117
chloride x-b NCCH~SO~- F F P-118
Y-b H H P-119
N-methylsulfamoyl 88 H F P-120
chloride x-b CH~NHSO~- F F P-121
Y-b H H P- I 22
N,N-dimethylsulfamoyl88 H F P-123
chloride x-b
(CHz)~NSO,- F F P-124
Y-b H H P-125
ethyl chloroformate 88 O H F P-126
x-b CH3CH2OC- F F P-127
Y-b H H P-128
sulfamide 88 H F P-129
x-b H~NSO~- F F P-130
Y-b H H P-131
chloroacetonitrile 88 H F P-132
x-b V'CCH~- F F P-I33
Y-b H H P-134
- ito-
CA 02351062 2001-05-10
WO 00/32599 PCT1US98/25308
Reagent Boc R R" R' Preparation
Compound No.
2-fluoroethyl bromide 88 H F P-135
x-b FCH~CH~- F F P-136
Y-b H H P-137
N-formylbenzotriazole 88 O H F P-138
x-b H ~-. F F P- I 39
Y-b H H P-140
EXAMPLES 142-161:
When following the general procedures of Example 141, step 7, an appropriate
amount of the amine listed below and the dithio compound from Preparation Z
listed below
are utilized, the respective products designated as Examples 142 to 400 in
Table H are
obtained.
TABLE H
Example Dithio
No. Product Amine Compound
142 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1-piperazinyl)phenyl]- P-90 Z (a)
2-oxo-5-oxazolidinyl]methyl]propanethioamide; mp
161-I62°C; Anal. calcd for Ci9H?SFN.~O;S: C, 55.87;
H, 6.17; N, 13.72; S, 7.85. Found: C, 55.79; H, 6.26;
N, 13.60; S, 7.71
143 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl]- P-90 Z (b)
?-oxo-5-oxazolidinyl]methyl]-2-methylpropane-
thioamide
144 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl]- P-90 Z (c)
2-oxo-5-oxazolidinyl]methyl]cyclopropanecarbo-
thioamide: mp I 59-160°C; Anal. calcd for
C,oH~SFN.,O~S: C. 57.13: H, 5.99: N, 13.32: S, 7.62.
Found: C, 57.05: H, 6.01; N, I 3. I 5; S, 7.45.
145 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1-piperazinyl)phenyl]- P-90 Z (d)
2-oxo-5-oxazolidinyl]methyl]butanethioamide
146 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl]- P-90 Z (e)
?-oxo-5-oxazolidinyl]methyl]-3-methylbutane-
thioamide
CA 02351062 2001-05-10
WO 00132599 PCT/US98/25308
Example
Dithio
N-o~ Product Amine Compound
147 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1-piperazinyl)phenyl]- p-90 Z (~
2-oxo-~-oxazolidinyl]methyl]-2-methylbutane-
thioamide
148 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl)- P-90 Z (g)
2-oxo-5-oxazolidinyl]methyl]-3,3-dimethylbutane-
thioamide
149 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1-piperazinyl)phenyl)- P-90 Z (h)
2-oxo-5-oxazolidinyl]methyl)cyclobutanecarbo-
thioamide
150 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl)- P-90 Z (i)
2-oxo-5-oxazolidinyl)methyl)cyclopentanecarbo-
thioamide
151 (S)-N-([3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl]- P-90 Z (j)
2-oxo-5-oxazolidinyl]methyl)cyclohexanecarbo-
thioamide
152 (S)-N-[(3-(3-Fluoro-4-(4-acetyl-1-piperazinyl)phenyl)- P-90 Z (k)
2-oxo-5-oxazolidinyl]methyl]-2-cyclopropylethane-
thioamide
153 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1-piperazinyl)phenyl)- P-90 Z (1)
2-oxo-5-oxazolidinyl]methyl]-2-cyclobutylethane-
thioamide
I54 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-I-piperazinyl)phenyl)- P-90 Z (m)
2-oxo-5-oxazolidinyl]methyl]-2-cyclopentylethane-
thioamide
I55 (S)-N-[[3-[3,5-Difluoro-4-(4-acetyl-1-piperazinyl)- P-91 Ethyl
phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide dithio-
acetate
156 (S)-N-[[3-[3.5-Difluoro-4-(4-acetyl-1-piperazinyl )- P-91 Z (a)
phenyl]-2-oxo-S-oxazolidinyl)methyl)propane-
thioamide
157 (S)-N-[[3-[3.S-Difluoro-4-(4-acetyl-1-piperazinyl)- P-91 Z {b)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-methyl-
propanethioamide
- li2-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product
Amine Compound
158 (S)-N-[(3-(3,5-Difluoro-4-(4-acetyl-1-piperazinyi)-P-91 Z (c)
phenyl]-2-oxo-5-oxazolidinyl]methyl)cyclopropane-
carbothioamide
159 (S)-N-[[3-j4-(4-Acetyl-1-piperazinyl)phenyl]-?-oxo-5-P-92 Ethyl
oxazolidinyl)methyl]thioacetamide
dithio-
acetate
160 (S)-N-([3-(4-(4-Acetyl-I-piperazinyl)phenyl)-2-oxo-5-P-92 Z (a)
oxazoiidinyl]methyl]propanethioamide
161 (,~-N-[[3-[4-(4-Acetyl-1-piperazinyl)phenyl]-2-oxo-5-P-92 Z (b)
oxazolidinyl]methyl]-2-methylpropanethioamide
162 (S)-N-[[3-[4-(4-Acetyl-I-piperazinyi)phenyl]-2-oxo-5-P-92 Z (c)
oxazolidinyl]methyl]cyclopropanecarbothioamide
163 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1-P-93 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide
acetate
164 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1-p-93 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl)-
propanethioamide
165 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1-P-93 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
16b (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-i-P-93 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
167 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1-P-93 Z (d)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-
butanethioamide
168 (S)-N-([3-[3-Fluoro-4-[4-(methoxyacetyl)-1-P-93 Z (e)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-3-
methylbutanethioamide
169 (S)-N-[[3-[3-Fluoro-4-[:1-(methoxyacetyl)-1-P-93 Z (~
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylbutanethioamide
-1i3-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98125308
Example Dithio
No. Product Amine Compound
170 (S)-N-[[3-[3-Fluoro-~i-[4-(methoxyacetyl)-I- P-93 Z (g)
piperazinyl]phenyl]-?-oxo-5-oxazolidinyl]methylJ-3,3-
dimethylbutanethioamide
171 (S)-N-[[3-[3-Fluoro--4-[4-(methoxyacetyl)-I- P-93 Z (h)
piperazinyl]phenyl )-2-oxo-5-oxazolidinyl]methyl]-
cyclobutanecarbothioamide
172 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-I- P-93 Z (i)
piperazinyl]phenyl]-?-oxo-5-oxazolidinyl]methyl)-
cyclopentanecarbothioamide
173 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-I- P-93 Z (j)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclohexanecarbothioamide
174 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-I-P-93 Z (k)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclopropylethanethioamide
175 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1-P-93 Z (1)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclobutylethanethioamide
176 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-I-P-93 Z (m)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclopentylethanethioamide
177 (S)-N-([3-[3,5-Difluoro-[4-[4-(methoxyacetyl)-I-P-94 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide
acetate
178 (S)-N-[[3-[3,5-Difluoro-[4-[4-(methoxyacetyl)-I- P-94 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
179 (S)-N-[[3-[3,5-Difluoro-[4-(4-(methoxyacetyl)-1- P-94 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-?-
methylpropanethioamide
1g0 (S)-N-[(3-[3,5-Difluoro-[4-[4-(methoxyacetyl)-I- P-94 Z (c)
piperazinyl]phenyl]-?-oxo-5-oxazolidinyl]methyl]-
eyclopropanecarbothioamide
- 114-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product Amine Compound
181 (S)-N-[[3-[4-[4-(methoxyacetyl)-1-piperazinyl]phenyl)- P-95 Ethyl
2-oxo-5-oxazolidinyl]methyl]thioacetamide dithio-
acetate
182 (S)-N-[[3-[4-[4-(methoxyacetyl)-1-piperazinyl]phenyl)- P-95 Z (a)
2-oxo-~-oxazolidinyl)methyl]propanethioamide
183 (S)-N-[[3-[4-[4-(methoxyacetyl)-1-piperazinyl]phenyl)- P-95 Z (b)
2-oxo-5-oxazolidinyl]methyl]-2-methylpropane-
thioamide
184 (S)-N-[[3-[4-[4-(methoxyacetyl)-1-piperazinyl]phenyl)- P-95 Z (c)
2-oxo-5-
oxazolidinyl)methyl)cyclopropanecarbothioamide
185 (S)-N-[[3-[3-Fluoro-4-[4-(cyanoacetyl)-I-piperazinyl)- P-96 Ethyl
phenyl]-2-oxo-5-oxazolidinyl]methyl)thioacetamide dithio-
acetate
186 (S)-N-[[3-[3-Fluoro-4-[4-(cyanoacetyl)-I-piperazinyl)- P-96 Z (a)
phenyl]-2-oxo-5-oxazolidinyl]methyl)propanethio-
amide
187 (S)-N-((3-[3-Fluoro-4-[4-(cyanoacetyl)-I-piperazinyl]- P-96 Z (b)
phenyl)-2-oxo-5-oxazolidinyl)methyl)-2-methyl-
propanethioamide
188 (S)-N-[[3-[3-Fiuoro-4-[4-(cyanoacetyl)-1-piperazinyl]- P-96 Z (c)
phenyl]-2-oxo-5-oxazolidinyl]methyl]cyclopropane-
carbothioamide
189 (S)-N-[[3-[3,5-Difluoro-4-[4-(cyanoacetyl)-1- P-97 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)- dithio-
methyl]thioacetamide acetate
190 (S)-N-[[3-[3,5-Difluoro-4-[4-(cyanoacetyl)-1- P-97 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)-
methyl)propanethioamide
191 (S)-N-[[3-[3,5-Dif7uoro-4-[4-(cyanoacetyl)-I- P-97 Z (b)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl)methyl)-~-
methylpropanethioamide
192 (S)-N-([3-[3.5-Difluoro-4-[4-(cyanoacetyl)-1- P-97 Z (c)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]-
methyl )cyc lopropanecarbothioamide
- I is -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product Amine Compound
193 (S)-N-[(3-[4-[4-(Cyanoacetyl)-I-piperazinyi]phenyl]-2-Ethyl
P-98
oxo-5-oxazolidinyl]methyl)thioacetamide dithio-
acetate
194 (S)-N-[[3-[4-[4-{Cyanoacetyl)-I-piperazinyl]phenyl]-2-Z (a)
P-98
oxo-5-oxazolidinyl]methyl]propanethioamide
195 (S)-N-[[3-[4-[4-{Cyanoacetyl)-I-piperazinyi]phenyl]-2-Z (b)
P-98
oxo-5-oxazolidinyl] methyl ]-2-methylpropanethioamide
196 (S)-N-[[3-[4-[4-(Cyanoacetyl)-I-piperazinyl]phenyl]-2-Z (c)
P-98
oxo-5-oxazolidinyl]methyl)cycopropanecarbothio-
amide
I97 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]- dithio-
thioacetamide acetate
198 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-I- P-99 Z (a)
piperazinyl)phenyl]-2-oxo-S-oxazolidinyl]methyl]-
propanethioamide
199 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
200 (S)-N-([3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
201 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 Z (d)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-
butanethioamide
202 (S)-N-[[3-[3-Fluoro-4-j4-(acetoxyacetyl)-1- P-99 Z (e)
piperazinyl]phenyl )-2-oxo-5-oxazolidinyl]methyl)-3-
methylbutanethioarnide
203 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyi)-1- P-99 Z (~
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-2-
methylbutanethioamide
204 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-I- P-99 Z (g)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyi]methyl)-3.3-
dimethylbutanethioamide
- 116-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Compound
205 (S)-N-[(3-[3-Fluoro-4-[4-(acetoxyacetyl)-l- P-99 Z (h)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclobutanecarbothioamide
206 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 Z (i)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopentanecarbothioamide
207 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 Z (j)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclohexanecarbothioamide
208 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1-P-99 Z (k)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclopropylethanethioamide
209 (S)-N-([3-[3-Fluoro-4-[4-(acetoxyacetyl)-1-P-99 Z 1
()
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclobutylethanethioamide
210 (S)-N-j[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1-P-99 Z (m)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclopentylethanethioamide
211 (S)-N-[[3-[3,S-Difluoro-4-[4-(acetoxyacetyl)-1-P-100 Ethyl
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
212 (S)-N-[[3-[3,5-Difluoro-4-(4-(acetoxyacetyl)-1-P-100 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
213 (S)-N-[[3-[3,5-Difluoro-4-[4-(acetoxyacetyl)-1-P-100 Z (b)
piperazinyl]phenylJ-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
214 (S)-N-[[3-(3,5-Difluoro-4-(4-(acetoxyacetyl)-1- P-100 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
215 (S)-N-[[3-[4-[4-(Acetoxyacetyl)-l-piperazinyl]phenyl]- P-101 Ethyl
2-oxo-5-oxazolidinyl]methyl]thioacetamide dithio-
acetate
216 (S)-N-[[3-[4-[4-(Acetoxyacetyl)-I-piperazinyl]phenyl]- P-101 Z (a)
?-oxo-~-oxazolidinyl)methyl)propanethioamide
- tW-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
Product Amine Compound
217 {S)-N-[[3-(4-[4-(Acetoxyacetyl)-1-piperazinyl]phenyl]- P-10l Z (b)
2-oxo-5-oxazolidinyl]methyl]-2-methylpropane-
thioamide
218 (S)-N-[[3-[4-[4-(Acetoxyacetyl)-I-piperazinyl]phenyl]- P-101 Z (c)
2-oxo-5-oxazolidiny(]methyl]cyclopropanecarbo-
thioamide
219 (S)-N-[[3-[3-Fluoro--~-[4-(benzyloxyacetyl)-1- P-102 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl)- dithio-
thioacetamide
acetate
220 (S)-N-[[3-[3-Fluoro-4-[4-(benzyloxyacetyl)-1- P-102 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
221 (S)-N-[[3-[3-Fluoro-~-[4-(benzyloxyacetyl)-1- P-102 Z (b)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
222 (S)-N-[[3-(3-Fluoro-4-[4-(benzyloxyacetyl)-1- P-102 Z (c)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyciopropanecarbothioamide
223 (S)-N-[[3-[3,5-Difluoro-4-[4-(benzyloxyacetyl)-1- P-103 Ethyl
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]methyl)- dithio-
thioacetamide acetate
224 (S)-N-([3-[3.5-Dilluoro-~-[4-(benzyloxyacetyl)-1-P-103 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
225 (S)-N-[(3-[3,5-Difluoro-~-[4-(benzyloxyacetyl)-I-P-103 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
226 (S)-N-[[3-[3,5-Difluoro-4-[4-(benzyloxyacetyl)-1-P-103 Z (c)
piperazinyl]phenyl]-?-oxo-5-oxazofidinyl]methyl]-
cyclopropanecarbothioamide
227 (S)-N-[[3-[3-Fluoro-=1-[.~-(methoxycarbonyl)-I-P-105 Ethyl
piperazinyl)phenyl]-2-oxo-5- dithio-
oxazolidinyl)methyl]thioacetamide
acetate
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Example Dithio
No. Product Amine Compound
228 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)-1- P-105 Z (a)
piperazinyl]phenyl]-2-oxo-~-oxazolidinylJmethyt)-
propanethioamide
229 (S)-N-([3-[3-Fluoro-4-[:~-(methoxycarbonyl)-1- P-105 Z (b)
piperazinyl)phenyl]-2-oxo-~-oxazolidinyl]methyl]-2-
methylpropanethioamide
230 (S)-N-[[3-[3-Fluoro-4-(4-(methoxycarbonyl)-I- P-105 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
231 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)-I- P-105 Z (d)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl)-
butanethioamide
232 (S)-N-[[3-[3-Fluoro-4-(4-(methoxycarbonyl)-I- P-105 Z (e)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-3-
methylbutanethioamide
233 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)-I- P-105 Z (~
piperazinylJphenyl]-2-oxo-5-oxazolidinyl)methyl]-2-
methylbutanethioamide
234 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)-I- P-105 Z (g)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-3,3-
dimethylbutanethioamide
235 (S)-N-[[3-(3-Fluoro-4-[4-(methoxycarbonyl)-1- P-105 Z (h)
piperazinyl]phenylJ-2-oxo-~-oxazolidinylJmethyl]-
cyclobutanecarbothioamide
236 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)-I- P-105 Z (i)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl]methyl)-
cyclopentanecarbothioanude
237 (S)-N-[(3-[3-Fluoro-4-[4-(methoxycarbonyl)-1- P-10~ Z (j)
piperazinylJphenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclohexanecarbothioamide
238 (S)-N-[(3-[3-Fluoro-4-[4-(methoxycarbonyl)-1- P-10> Z (k)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-2-
cyclopropylethanethioamide
- 119-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product Amine Compound
239 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)-1- P-105 Z (1)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-2-
cyciobutylethanethioamide
240 (S)-N-[[3-j3-Fluoro-4-[4-(methoxycarbonyl)-1- P-105 Z (m)
piperazinyl]phenyl]-?-oxo-5-oxazolidinyl]methyl]-2-
cyclopentylethanethioamide
241 (S)-N-([3-[3,5-Difluoro-4-[4-(methoxycarbonyl)-1- P-106 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]- dithio-
thioacetamide acetate
242 (S)-N-[[3-[3.5-Difluoro-4-[4-(methoxycarbonyl)-1- P-106 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazoiidinyl]methylJ-
propanethioamide
243 (S)-N-[[3-[3.S-Difluoro-4-[4-(methoxycarbonyl)-1- P-106 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
244 (S)-N-[[3-[3,5-Difluoro-4-[4-(methoxycarbonyl)-1- P-106 Z (c)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methylJ-
cyelopropanecarbothioamide
245 (S)-N-[[3-[4-[4-(methoxycarbonyl)-1- P-107 Ethyl
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]- dithio-
thioacetamide acetate
246 (S)-N-[[3-(4-[4-(methoxycarbonyl)-1- P-107 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyi]methylJ-
propanethioamide
247 (S)-N-[[3-(4-[:~-(methoxycarbonyl)-1- P-107 Z (b)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-2-
methylpropanethioamide
248 (S)-N-[[3-[4-[:i-(methoxycarbonyl)-I- P-107 Z (c)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
249 (S)-N-[[3-[3-Fluoro-4-[4-(methanesulfonyl)-1- P-108 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyt]methyl)- dithio-
thioacetamide: mp 197-198°C: Anal. calcd for acetate
C,~H~,FN.,O.,S~: C, :~7..~3: H, 5.39: N, 13.01; S. 14.89.
Found: C. -X7.25; H. 5.40; N, 12.82; S. 14.56.
- ~20-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Example Dithio
No. Product Amine Compound
250 (,S~-N-[[3-[3-Fluoro-4-[4-(methanesulfonyl)-i-P-108 Z (a)
piperazinyl]phenylJ-?-oxo-5-oxazolidinylJmethyl]-
propanethioamide: mp 207-208C: Anal.
calcd for
C,gH~sFN.~O.~S~: C, 48.63: H, 5.67:
N, 12.60; S, 14.42.
Found: C, 48.51: H, 5.59; N, 12.52;
S, 14.09.
251 (S)-N-[[3-[3-Fluoro-4-[4-(methanesulfonyl)-I-P-108 Z (b)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide; mp 204-206C;
Anal. calcd
for C,9H~~FNaO.,S~: C, 49.76; H, 5.93;
N, 12.22: S,
13.98. Found: C, 49.63; H, 5.92; N,
14.14; S, 13.91.
252 (S)-N-[[3-[3-Fluoro-4-[4-(methanesulfonyl)-l-P-108 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide; Anal. calcd
for
C,9HzsFN.~O.~Sa: C, 49.98: H, 5.52;
N. 12.27; S, 14.04.
Found: C, 49.42; H, 5.50; N, 12.08;
S, 13.80.
253 (S)-N-[[3-[3,5-Difluoro-4-[4-(methanesulfonyl)-I-P-109 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl)-dithio-
thioacetamide acetate
254 (S)-N-[[3-[3,5-Difluoro-4-[4-(methanesulfonyl)-1-P-109 Z (a)
piperazinyi]phenyl)-2-oxo-5-oxazolidinyl]methyl)-
propanethioamide
255 (S)-N-[[3-[3,5-Difluoro-4-[4-(methanesulfonyl)-1-P-109 Z (b)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
256 (S)-N-[[3-[3,5-Difluoro-4-[4-(methanesulfonyl)-1-P-109 Z (c)
piperazinyl ]phenyl ]-2-oxo-5-oxazolidinyl
J methyl ]-
cyclopropanecarbothioamide
257 (S)-N-[[3-[4-[4-(methanesulfonyl)-1- P-110 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
258 (S)-N-[[3-[4-[4-(rnethanesulfonyl)-1- P-110 Z (a}
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl)-
propanethioamide
259 (S)-N-[[3-[4-[4-(methanesulfonyl)-1- P-110 Z (b)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]methyl]-~-
methylpropanethioamide
- ~2~ -
CA 02351062 2001-05-10
WO 00/3ZS99 PCTNS98/25308
Example
Dithio
No. Product Amine Compound
260 (S)-N-[[3-[4-[4-(methanesulfonyl)-I- P-110 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-
cyclopropanecarbothioamide
261 (S)-N-[[3-[3-Fluoro-~I-[4-(ethanesulfonyi)-I- P-111 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinylJmethyi]- dithio-
thioacetamide
acetate
262 (S)-N-[[3-[3-Fluoro-4-(4-(ethanesulfonyl)-1- P-111 Z (a)
piperazinyl)phenyl]-2-oxo-S-oxazolidinylJmethyl)-
propanethioamide
263 (S)-N-((3-[3-Fluoro-4-[4-(ethanesulfonyl)-1- P-111 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinylJmethyl)-2-
methylpropanethioamide
(S)-N-[[3-[3-Fluoro-4-[4-(ethanesulfonyl)-1- P-111 Z (c)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl)-
cyclopropanecarbothioamide
265 (S)-N-[[3-[3,5-Difluoro-4-[4-(ethanesulfonyl)-1- P-112 Ethyl
piperazinyl)phenylJ-2-oxo-5-oxazolidinyl)methyl)- dithio-
thioacetamide
acetate
266 (S)-N-[[3-[3,5-Difluoro-4-[4-(ethanesulfonyl)-1-
P-112
Z (a)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]methyl)-
propanethioamide
(S)-N-[[3-[3,5-Difluoro-4-[4-(ethanesulfonyl)-1-
P
112
- Z (b)
piperazinyl Jpheny 1 J-2-oxo-5-oxazolidinyl ]methyl]-2-
methylpropanethioamide
268 (S)-N-[[3-[3,5-Difluoro-4-[4-(ethanesulfonyl)-1-
P
112
- Z (c)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl)methyl]-
cyclopropanecarbothioamide
269 (S)-N-[[3-(4-[4-(ethanesulfonyl)-1-piperazinyl]phenyl]-Ethyl
P-113
?-oxo-5-oxazoiidinyl]methyl]thioacetamide dithio-
acetate
(S)-N-[[3-[4-[4-(ethanesulfonyl)-1-piperazinyl]phenyl]- P-113 Z (a)
?-oxo-~-oxazolidinyl]methyl]propanethioamide
?~1 (S)-N-[[3-[4-(4-(ethanesulfonyl)-1-piperazinylJphenylJ- P-113 Z (b)
2-oxo-~-oxazolidinyl]methyl]-2-methylpropane-
thioamide
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Comvound
272 (S)-N-[[3-(4-[4-(ethanesulfonyl)-1-piperazinyl]phenyl]- P-113 Z (c)
2-oxo-~-oxazolidinyl]methyl]cyclopropanecarbothio-
amide
273 (S)-N-[[3-[3-Fluoro-4-[4-(chloromethanesulfonyl)-1-P-114 Ethyl
piperazinylJphenyl)-2-oxo-5-oxazolidinyl]methyl)-dithio-
thioacetamide acetate
274 (S)-N-[[3-[3-Fluoro-4-[4-(chloromethanesulfonyl)-1-P-114 Z (a)
piperazinylJphenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
275 (S)-N-[[3-[3-Fluoro-4-[4-(chloromethanesulfonyl)-1-P-114 Z (b)
piperazinyl]phenylJ-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
276 (S)-N-[[3-[3-Fluoro-4-[4-(chloromethanesulfonyl)-1-P-114 Z (c)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl)methyl]-
cyclopropanecarbothioamide
277 (S)-N-[[3-[3,5-Difluoro-4-[4-(chloromethanesulfonyl)-P-115 Ethyl
1-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
278 (S)-N-[[3-(3,5-Difluoro-4-[4-(chloromethanesulfonyl)-P-115 Z (a)
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
279 (S)-N-[[3-[3.5-Difluoro-4-[4-(chloromethanesulfonyl)-P-115 Z (b)
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
280 (S)-N-[[3-[3.5-Difluoro-4-(4-(chloromethanesulfonyl)-P-115 Z (c)
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-
cyclopropanecarbothioamide
281 (S)-N-[(3-[4-[4-(chloromethanesulfonyl)-1-P-116 Ethyl
piperazinylJpheny!)-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
282 (S)-N-[[3-[4-[4-(chloromethanesulfonyl)-1-P-116 Z (a)
piperazinyl]phenyl]-2-oxo-~-oxazolidinyl]methyl]-
propanethioamide
- 123 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product Amine Compound
283 (S)-N-[[3-[4-[4-(chloromethanesulfonyl)-1- P-116 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
284 (S)-N-[[3-[4-(4-(chloromethanesulfonyl)-1- P-116 Z (c)
piperazinyl]phenyl]-2-oxo-~-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
285 (S)-N-[[3-[3-Fluoro-4-[4-(cyanomethane-sulfonyl)-1- P-117 Ethyl
piperazinyl]phenyl]-2-oxo-5- dithio-
oxazoIidinyl]methyl]thioacetamide acetate
286 (S)-N-[(3-[3-Fluoro-4-[4-(cyanomethane-sulfonyl)-1- P-117 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
287 (S)-N-[[3-[3-Fluoro-4-[4-{cyanomethane-sulfonyl)-I- P-117 Z (b)
piperazinyi)phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
288 (S)-N-[[3-[3-Fluoro-4-(4-(cyanomethane-sulfonyl)-1-P-117 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
289 (S)-N-([3-[3,5-Difluoro-4-[4-(cyanomethane-sulfonyl)-P-118 Ethyl
1-piperazinyl]phenyl]-2-oxo-5- dithio-
oxazolidinyl ] methyl ]thioacetamide acetate
290 (S)-N-[[3-[3,5-Difluoro-4-[4-(cyanomethane-sulfonyl)-P-118 Z (a)
1-piperazinyl ]phenyl ]-2-oxo-5-oxazolidinyl]-
methyl]propanethioamide
291 (S)-N-[[3-[3,5-Difluoro-4-[4-(cyanomethane-suifonyl)-P-118 Z (b)
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
292 (S)-N-[[3-(3,5-Difluoro-4-[4-(cyanomethane-sulfonyl)-P-118 Z (c)
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methylJ-
cyclopropanecarbothioamide
293 (S)-N-[[3-[4-[4-(Cyanomethanesulfonyl)-I-P-119 Ethyl
piperazinyl]phenyl]-2-oxo-5-
dithio-
oxazolidinyl]methyl]thioacetamide acetate
- 124 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product
flmine Compound
294 (S)-N-[[3-[4-[4-(Cyanomethanesulfonyl)-I-P
119
- Z (a)
piperazinyl]phenyl]-2-oxo-S-
oxazolidinyl)methyl]propanethioamide
29S (S)-N-[[3-[4-[4-(Cyanomethanesulfonyl)-1-P
I 19
- Z (b)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-2-
methylpropanethioamide
296 (S)-N-[[3-[4-[4-(Cyanomethanesulfonyl)-1-P-119
Z (c)
piperazinyl)phenyl]-2-oxo-S-
oxazolidinyl]methyl ]cyclopropanecarbothioamide
297 (S)-N-[[3-[3-Fluoro-4-[4-(N-methylsulfamoyl)-1-P-120
Ethyl
piperazinyl]phenyl]-2-oxo-S-
dithio-
oxazolidinyl ]methyl ]thioacetamide
acetate
298 (S)-N-((3-[3-Fluoro-4-[4-(N-methylsuIfamoyl)-1- P-120 Z (a)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-
propanethioamide
299 (S)-N-[[3-[3-Fluoro-4-[4-(N-methylsulfamoyl)-1- P-120 Z (b)
piperazinylJphenyl]-2-oxo-S-oxazolidinyl]methyl]-2-
methylpropanethioamide
300 (S)-N-[[3-[3-Fluoro-4-[4-(N-methylsulfamoyl)-1- P-120 Z (c)
piperazinyi]phenyl]-2-oxo-S-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
301 (S)-N-[[3-[3,S-Difluoro-4-[4-{N-methylsulfamoyl)-I- P-12I Ethyl
piperazinyl]phenyl]-2-oxo-S- dithio-
oxazolidinyl]methyl]thioacetamide acetate
302 (S)-N-[[3-[3,S-Difluoro-4-[4-(N-methyIsulfamoyl)-1- P-121 Z (a)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-
propanethioamide
303 (S)-N-((3-[3.S-Difluoro-4-[4-(N-methylsulfamoyl)-1- P-121 Z (b)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-2-
methylpropanethioamide
304 (S)-N-[[3-[3.S-Difluoro-4-[4-(N-methylsulfamoyl)-1- P-121 Z (c)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
- 125 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product Amine Compound
305 (S)-N-[[3-[4-[4-(N-methylsulfamoyl)-I- P-122 Ethyl
piperazinyl ]phenyl ]-2-oxo-5-oxazolidinyl ] methyl )- dithio-
thioacetamide acetate
306 (S)-N-[[3-(4-[4-(N-methyisulfarnoyl)-I- P-122 Z (a)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyI)methyl]-
propanethioamide
307 (S)-N-[[3-[4-[4-(N-methylsulfamoyl)-1- P-122 Z (b)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-2-
methylpropanethioamide
308 (S)-N-[[3-[4-[4-(N-methylsulfamoyl)-I- P-122 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)methyl]-
cyclopropanecarbothioamide
309 (S)-N-[[3-[3-Fluoro-4-(4-(N,N-dimethylsulfamoyl)-1- P-123 Ethyl
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl)methyl]- dithio-
thioacetamide acetate
310 (S)-N-([3-[3-Fluoro-4-[4-(N,N-dimethylsulfamoyl)-I-Z (a)
P-123
piperazinyl )phenyl )-2-oxo-5-oxazolidinyl )methyl]-
propanethioamide
31 I (S)-N-[[3-[3-Fluoro-4-[4-(N,N-dimethylsulfamoyl)-1-Z (b)
P-123
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
31? (S)-N-[[3-[3-Fluoro-4-[4-(N,N-dimethylsulfamoyl)-1-Z (c)
P-123
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl)methyl]-
cyclopropanecarbothioamide
313 (S)-N-[[3-[3,5-Difluoro-4-[4-(N,N-dimethylsulfamoyl)-Ethyl
P-124
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide
acetate
314 (S)-N-[[3-[3,5-Difluoro-4-[4-(N,N-dimethylsulfamoyl)- P-124 Z (a)
1-piperazinyl]phenyl]-2-oxo-~-oxazolidinyl)methyl]-
propanethioamide
31~ (S)-N-[[3-[3,5-Difluoro-4-[4-(N,N-dimethylsulfamoyl)- P-124 Z (b)
!-piperazinyl)phenyl]-2-oxo-~-oxazolidinyl]methyl]-2-
methylpropanethioamide
_ ~?6 _
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Example Dithio
No. Product Amine Compound
316 (S)-N-([3-[3,5-Difluoro-4-[4-(N.N-dimethylsulfamoyl)-P-I24 Z (c)
1-piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
317 (S)-N-[[3-[4-[4-(N,N-dimethylsulfamoyl)-1-P-125 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
318 (S)-N-[[3-[4-[4-(N,N-dimethylsulfamoyl)-I-P-125 Z (a)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
319 (S)-N-[[3-[4-[4-(N,N-dimethylsulfamoyl)-I-P-125 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
320 (S)-N-[[3-[4-{4-(N,N-dimethylsulfamoyl)-1-P-125 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
321 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
322 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (a)
piperazinyI)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
323 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (b)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-2-
methylpropanethioamide
324 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-l-P-126 Z (c)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
325 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (d)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
butanethioamide
326 (S)-N-{[3-(3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (e)
piperazinyl]phenyl]-2-oxo-S-oxazolidinyl]methyl]-3-
methylbutanethioamide
- 127 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Compound
327 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-(- P-126 Z (~
piperazinyl]phenyl]-2-oxo-~-oxazolidinyl]methyl]-2-
methylbutanethioamide
328 (S)-N-[(3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1- P-126 Z (g)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl)methyl]-3,3-
dimethylbutanethioamide
329 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1- P-126 Z (h)
piperazinyl]phenyl]-2-oxo-5-oxazolidinylJmethyl]-
cyclobutanecarbothioamide
330 (,S~-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (i)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl)-
cyclopentanecarbothioamide
331 (S)-N-[[3-(3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (j)
piperazinyl]phenylJ-2-oxo-5-oxazolidinyl]methyl)-
cyclohexanecarbothioamide
332 (S)-N-[[3-(3-FIuoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (k)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methylJ-2-
cyclopropylethanethioamide
333 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (1)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclobutylethanethioamide
334 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1-P-126 Z (m)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
cyclopentylethanethioamide
335 (S)-N-[[3-[3,5-Difluoro-4-[4-(ethoxycarbonyl)-1-P-127 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methylJ-dithio-
thioacetmide
acetate
336 (S)-N-[[3-[3.5-Difluoro-4-[4-(ethoxycarbonyl)-1-P-127 Z (a)
piperazinyiJphenyl)-2-oxo-5-oxazolidinylJmethyl]-
propanethioamide
337 (S)-N-[[3-[3.5-Difluoro-4-[4-(ethoxycarbonyl)-1- P-127 Z (b)
piperazinyl)phenyl]-2-oxo-5-oxazolidinylJmethyl]-2-
methylpropanethioamide
- 128 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Compound
338 (S)-N-[[3-[3.5-Difluoro-4-[4-(ethoxycarbonyl)-l-P-127 Z (c)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
339 (S)-N-[[3-[4-[4-(ethoxycarbonyl)-I-piperazinyl)-P-128 Ethyl
phenyl]-2-oxo-5-oxazolidinyl]methyl)thioacetamidedithio-
acetate
340 (S)-N-[[3-[4-[4-{ethoxycarbonyl)-1-piperazinyl]-P-128 Z (a)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
341 (S)-N-([3-[4-[4-(ethoxycarbonyl)-I-piperazinyl]-P-128 Z (b)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
342 (S)-N-[[3-[4-[4-(ethoxycarbonyl)-1-piperazinyl]-P-128 Z (c)
phenyl)-2-oxo-5-oxazolidinyl]methyl]cyclopropane-
carbothioamide
343 (S)-N-([3-[3-Fluoro-4-(4-sulfamoyl-1-piperazinyl)-P-129 Ethyl
phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamidedithio-
acetate
344 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1-piperazinyl)-P-129 Z (a)
phenyl]-2-oxo-5-oxazolidinyl]methyl)-
propanethioamide
345 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-I-piperazinyl)-P-129 Z (b)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
346 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-i-piperazinyl)-P-129 Z (c)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioanude
347 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1-P-129 Z (d)
piperazinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]butanethioamide
348 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1-P-129 Z (e)
piperazinyl)phenyl]-2-oxo-S-oxazolidinyl]methyl]-3-
methylbutanethioamide
- 129 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Compound
349 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyi-1- P-129 Z (f)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl)methylj-2-
methylbutanethioamide
350 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 Z (g)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl)methyl)-3,3-
dimethylbutanethioamide
351 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 Z (h)
piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl)methyl)cyclobutanecarbothioamide
352 (S)-N-[[3-[3-Fluoro-4-{4-sulfamoyl-1- P-129 Z (i)
piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl)cyclopentanecarbothioamide
353 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 Z (j)
piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl)cyclohexanecarbothioamide
354 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 Z (k)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]methyl]-2-
cyclopropylethanethioamide
355 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 Z (1)
piperazinyi)phenyl]-2-oxo-5-oxazolidinyl)methyl)-2-
cyclobutylethanethioamide
356 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 Z (m)
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]methyl)-2-
cyclopentylethanethioamide
357 (S)-N-[[3-[3.5-Difluoro-4-(4-sulfamoyl-1- P-130 Ethyl
piperazinyl )phenyl)-2-oxo-5- dithio-
oxazolidinyl )methyl]thioacetamide acetate
358 (S)-N-([3-[3.~-Difluoro-4-(4-sulfamoyl-1- P-130 Z (a)
piperazinyl )phenyl )-2-oxo-5-
oxazolidinyl)methyl)propanethioamide
359 (S)-N-[[3-[3.5-Dit7uoro-4-(4-sulfamoyl-1- P-130 Z (b)
piperazinyl )phenyl)-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
- 130 -
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Example Dithio
No. Product Amine Compound
360 (S)-N-[[3-[3.5-Difluoro-4-(4-sulfamoyl-I-P-I30 Z (c)
piperazinyl)phenyl]-2-oxo-5-
oxazolidinyl ]methyl]cyclopropanecarbothioamide
361 (S)-N-[[3-[4-(4-sulfamoyl-I-piperazinyl)phenyl]-2-oxo-P-131 Ethyl
5-oxazolidinyl ]methyl]thioacetamide dithio-
acetate
362 (S)-N-[[3-[4-(4-sulfamoyl-I-piperazinyl)phenyl]-2-oxo-P-131 Z (a)
5-oxazolidinyl]methyl]propanethioamide
363 (S)-N-[[3-[4-(4-sulfamoyl-I-piperazinyl)phenyl]-2-oxo-P-131 Z (b)
5-oxazolidinyl]methyl]-2-methylpropanethioamide
364 (S)-N-[[3-[4-(4-sulfamoyl-I-piperazinyl)phenylJ-2-oxo-P-131 Z (c)
v
S-oxazolidinyl]methyl]cyclopropanecarbothioamide
365 (S)-N-[(3-[3-Fluoro-4-(4-(cyanomethyl)-1-P-132 Ethyl
piperazinyl]phenyl]-2-oxo-5- dithio-
oxazolidinyl]methyl]thioacetamide acetate
366 (S)-N-[[3-[3-Fluoro-4-[4-(cyanomethyl)-1-P-132 Z (a)
piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]propanethioamide
367 (S)-N-[[3-[3-Fluoro-4-[4-{cyanomethyl)-I-P-132 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
368 (S)-N-[[3-[3-Fluoro-4-[4-(cyanomethyl)-1-P-132 Z (c)
piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl)cyclopropanecarbothioamide
369 (S)-N-[[3-(3,5-Difluoro-4-[4-( cyanomethyl)-1-P-133 Ethyl
piperazinyl]phenyl]-2-oxo-5- dithio-
oxazolidinyl )methyl]thioacetamide acetate
370 (S)-N-[[3-[3,5-Difluoro-4-[4-( cyanomethyl)-1-P-I33 Z (a)
piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]propanethioamide
371 (S)-N-[[3-[3.5-Difluoro-4-{4 cyanomethyl)-1-P-133 Z (b)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
- 131 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example
Dithio
No. Product Amine Compound
372 (S)-N-([3-[3,5-Difluoro-4-[4-(cyanomethyl)-1-P-133 Z (c)
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
373 (,S~-N-[[3-[4-[4-(cyanomethyl)-1-piperazinyl)phenyl]-2-P-134 Ethyl
oxo-5-oxazolidinyl]methyl]thioacetamidedithio-
acetate
374 (S)-N-([3-[4-[4-(cyanomethyl)-I-piperazinyl]phenyl]-2-P-134 Z (a)
oxo-5-oxazolidinyl]methyl]propanethioamide
375 (S)-N-[[3-[4-[4-(cyanomethyl)-I-piperazinyl]phenyl]-2-P-134 Z (b)
oxo-5-oxazolidinyl]methyl]-2-methylpropanethioamide
376 (S)-N-[[3-[4-[4-( cyanomethyl)-I-piperazinyl]phenyl]-P-134 Z (c)
2-oxo-S-oxazolidinyl]methyl]cyclopropane-
carbothioamide
377 (S)-N-[[3-[3-Fluoro-4-(4-(2-fluoroethyl)-I-P-135 Ethyl
piperazinyl]phenyl]-2-oxo-5- dithio-
oxazolidinyl]methyl]thioacetamide acetate
378 (S)-N-[(3-[3-Fluoro-4-[4-(2-fluoroethyl)-I-P-135 Z (a)
piperazinyl]phenyl)-2-oxo-5-
oxazolidinyl]methyl]propanethioamide
379 (S)-N-[[3-[3-Fluoro-4-[4-(2-fluoroethyl)-1-P-135 Z (b)
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl)methyl]-2-
methylpropanethioamide
380 (S)-N-[[3-[3-Fluoro-4-[4-(2-fluoroethyl)-1-P-135 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
381 (S)-N-[[3-[3,5-Difluoro-4-[4-(2-fluoroethyl)-I-P-136 Ethyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-dithio-
thioacetamide acetate
382 (S)-N-[[3-[3,5-Difluoro-4-[4-(2-fluoroethyl)-1-P-136 Z (a)
piperazinyl]phenyl]-2-oxo-~-oxazolidinyl]methyl]-
propanethioamide
383 (S)-N-[[3-[3.5-Difluoro-4-[4-(2-fluoroethyl)-1-P-136 Z (b)
piperazinyl ]phenyl ]-2-oxo-~-ox azolidinyl
] methyl]-2-
methylpropanethioamide
- 132 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Compound
384 (S)-N-[[3-(3.5-Difluoro-4-(4-(2-fluoroethyl)-1- P-136 Z (c)
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-
cyclopropanecarbothioamide
385 (S)-N-[[3-[4-[4-(2-fluoroethyl)-I-piperazinyl]phenyl]- P-137 Ethyl
2-oxo-5-oxazolidinyl]methyl]thioacetamide dithio-
acetate
386 (S)-N-[[3-[4-[4-(2-fluoroethyl)-I-piperazinyl]phenyl]- P-137 Z (a)
2-oxo-5-oxazolidinyl]methyl)propanethioamide
387 (S)-N-[[3-[4-(4-(2-fluoroethyl)-I-piperazinyl]phenyl]- P-137 Z (b)
2-oxo-5-oxazolidinyl]methyl]-2-
methylpropanethioamide
388 (S)-N-[[3-[4-[4-(2-fluoroethyl)-1-piperazinyl]phenyl]- P-137 Z (c)
2-oxo-5-oxazolidinyl]methyl)cyclopropane-
carbothioamide
389 (S)-N-[[3-[3-Fluoro-4-(4-formyl-I-piperazinyl)phenyl]- P-138 Ethyl
2-oxo-5-oxazolidinyl]methyl]thioacetamide; Anal calcd dithio-
for C,~H~,FN40~S: C, 53.67; H, 5.56; N, 14.73; S, acetate
8.43. Found: C, 53.14; H, 5.42; N, 14.25; S, 8.18.
390 (S)-N-[[3-[3-Fluoro-4-(4-formyl-1-piperazinyl)phenylJ- P-138 Z (a)
2-oxo-5-oxazolidinyl]methyl]propanethioamide; mp
166-167°C: Anal. calcd for C,~H~~FN.~O~S: C, 54.81;
H. 5.88; N, 14.20: S, 8.13. Found: C, 54.83; H, 6.00;
N, 14.12; S, 7.96.
391 (S)-N-[[3-[3-Fluoro-4-(4-formyl-I-piperazinyl)phenyl]- P-138 Z (b)
2-oxo-5-oxazolidinyl)methyl]-2-methylpropane-
thioamide: mp I57-158°C: Anal. calcd for
Ci9H25FNdO;S: C, 55.87, H, 6.17; N, 13.72; S, 7.85.
Found: C, 55.67; H, 6.19; N, 13.50; S, 7.70.
392 fS)-N-[(3-[3-Fluoro-4-(4-formyl-1-piperazinyl)phenyl]- P-138 Z (c)
2-oxo-5-oxazolidinyl]methyl]cyclopropane-
carbothioamide: mp 178-179°C: Anal. calcd for
C,9H~;FN,O;S: C, 56.14; H, 5.70; N, 13.78: S, 7.89.
Found: C. 56.13: H, 5.64; N, 13.64; S. 7.75.
393 fS)-N-[[3-(3,5-Difluro-4-(4-formyl-1-piperazinyl)- P-139 Ethyl
phenyl]-2-oxo-5-oxazolidinyl]methyl)thioacetamide dithio-
acetate
- 133 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Dithio
No. Product Amine Compound
394 (S)-N-[[3-[3,5-Difluro-4-(4-formyl-1-piperazinyl)-P-139 Z (a)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-
propanethioamide
395 (S)-N-[[3-[3,5-Difluro-4-(4-formyl-1-piperazinyi)-P-139 Z (b)
phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-methyl-
propanethioamide
396 (S)-N-[[3-[3,5-Difluro-4-(4-formyl-I-piperazinyl)-P-139 Z (c)
phenyl]-2-oxo-5-oxazolidinyl]methyl]cyclo-
propanecarbothioamide
397 (S)-N-[[3-[4-(4-formyl-I-piperazinyl)phenyl]-2-oxo-5-P-140 Ethyl
oxazolidinyl]methyl]thioacetamide dithio-
acetate
398 (S)-N-[[3-[4-(4-formyl-1-piperazinyl)phenyl]-2-oxo-5- P-140 Z (a)
oxazolidinyI]methyl)propanethioamide
399 (S)-N-[(3-[4-(4-formyl-I-piperazinyl)phenyl]-2-oxo-5- P-140 Z (b)
oxazolidinyl]methyl]-2-methylpropanethioamide
400 (S)-N-[[3-[4-(4-formyl-I-piperazinyl)phenyl]-2-oxo-5- P-140 Z (c)
oxazolidinyl ]methyl]cyclopropane-carbothioamide
When in the general procedure of Example 3 I , step I , an appropriate amount
of the
amine listed below is substituted for compound 33, the isothiocyanate
corresponding to the
amines P-90, P-93, P-99, P-105, P-126 and P-129 are obtained.
When in the general procedure of Example 114 an appropriate amount of the
isothiocyanate and the amine listed below are substituted for compound 82 and
methylamine, the respective products listed below are obtained.
TABLEI
Isothiocyanate
Example Corresponding
No. Product to Amine No. Amine
401 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-l- P-90 methylamine
piperazinyl )phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N'-methylthiourea
- 134 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Isothiocyanate
Example Corresponding
No. Product to Amine No. Amine
402 (.S~-N-[[3-[3-Fluoro-4-(4-acetyl-1-P-90 dimethylamine
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl
J-
methyl]-N',N'-dimethylthiourea
403 (.S~-N-[[3-[3-Fluoro-4-(4-acetyl-1-P-90 azetidine
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl)-1-azetidinecarbothioamide
404 (S)-N-[[3-[3-Fluoro-4-(4-thiomorpholinyl)-P-90 anhydrous
phenyl]-2-oxo-5-oxazolidinyl]methyl]- i
ammon
thiourea a
405 (S)-N-[[3-[3-Fluoro-4-(4-(methoxyacetyl)-1- P-93 methylamine
piperazinyl]phenyl]-2-oxo-5-oxazolidinylJ-
methyl]-N'-methylthiourea
406 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1- P-93 dimethylamine
piperazinyi]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N',N'-dimethylthiourea
407 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1- P-93 azetidine
piperazinyI]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-1-
azetidinecarbothioamide
408 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1-P-99 methylamine
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N'-methylthiourea
409 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-I-P-99 dimethylamine
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N',N'-dimethylthiourea
410 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1-P-99 azetidine
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)-
methyl]-1-azetidinecarbothioamide
411 (S)-N-([3-[3-Fluoro-4-[4-(methoxycarbonyl)-P-105 methylamine
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N'-methylthiourea
412 (S)-N-[[3-(3-Fluoro-4-[4-(methoxycarbonyl)-P-105 dimethylamine
1-piperazinyl]phenyl]-2-oxo-~-oxazolidinyl]-
methyl]-N',N'-dimethylthiourea
- 135 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Isothiocyanate
Example Corresponding
No. Product to Amine No. Amine
413 (S)-N-[[3-[3-Fluoro-4-(4-{methoxycarbonyl)- P-105 azetidine
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-1-azetidinecarbothioamide
414 (S)-N-[[3-(3-Fluoro-4-(4-(ethoxycarbonyl)-1- P-l26 methylamine
piperazinyl )phenyl ]-2-oxo-~-oxazolidinyl )-
methyl)-N'-methylthiourea
415 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1- P-126 dimethylamine
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N',N'-dimethylthiourea
416 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-1- P-126 azetidine
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyI]-1-azetidinecarbothioamide
417 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-I- P-129 methylamine
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-N'-methylthiourea
418 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1- P-129 dimethylamine
piperazinyl)phenyl]-2-oxo-5-oxazolidinyi]-
methyl]-N'.N'-dimethylthiourea
419 (S)-N-([3-(3-Fluoro-4-(4-sulfamoyl-1- P-129 azetidine
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl]- l-azetidinecarbothioamide
When in the general procedure of Example 100 an appropriate amount of the
isothiocyanate and alcohol listed below are utilized in the same manner as
Compound 82
and methanol are utilized, the respective products listed below are obtained.
TABLEJ
Isothiocyanate
Example Corresponding
No. Product to Amine No. Amine
420 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1- P-90 methanol
piperazinyl)phenyl)-2-oxo-~-oxazolidinyl]-
methyl]-O-methylthiocarbamate
- 136 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/Z5308
Isothiocvanate
Example Corresponding
No. Product to Amine No. Amine
421 (S)-N-[(3-[3-Fluoro-4-(4-acetyl-1- P-90 ethanol
piperazinyl )phenyl]-2-oxo-5-oxazolidinyl]-
methyI)-O-ethylthiocarbamate
422 (S)-N-[[3-[3-Fluoro-4-(4-acetyl-1- P-90 isopropyl
piperazinyl)phenyl)-2-oxo-5-oxazolidinylJ- alcohol
methyl]-O-iso-propylthiocarbainate
423 (S)-N-[[3-[3,5-Difluoro-4-(4-acetyl-1- P-91 methanol
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
424 (S)-N-((3-[4-(4-Acetyl-I- P-92 methanol
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
425 (S)-N-[[3-(3-Fluoro-4-[4-(methoxyacetyl)-1- P-93 methanol
piperazinyl]phenyl)-2-oxo-5-oxazolidinyl]-
methyl)-O-methylthiocarbamate
426 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1- P-93 ethanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl)-O-ethylthiocarbamate
427 (S)-N-[[3-[3-Fluoro-4-[4-(methoxyacetyl)-1- P-93 isopropyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]- alcohol
methyl]-O-i.sn-propylthiocarbamate
428 (,S~-N-[[3-[3.5-Difluoro-[4-(4-(methoxy- P-94 methylaminel
acetyl)-1-piperazinyl]phenyl)-2-oxo-S-
oxazolidinyl]methyl)-O-methylthiocarbamate
429 (S)-N-[[3-[4-[4-(methoxyacetyl)-1- P-95 methylamine
piperazinyl]phenyl]-2-oxo-S-
oxazolidinyl)methyl]-O-methylthioearbamate
430 (S)-N-[3-(3-Fluoro-4-[4-(cyanoacetyl)-1- P-96 methanol
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
431 (S)-N-[[3-[3,5-Difluoro-4-[4-(cyanoacetyl)- P-97 methanol
1-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl)-O-methylthiocarbamate
- 137 -
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Isothiocvanate
fixarnple Corresponding
No. Product to Amine No. Amine
432 (S)-N-([3-[4-[4-(Cyanoacetyl)-1- P-98 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
433 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-1- P-99 methanol
piperazinyl]phenyl]-2-oxo-5-oxazoiidinyl]-
methyl]-O-methylthiocarbamate
434 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-I- P-99 ethanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyi]-
methyl]-O-ethyithiocarbamate
435 (S)-N-[[3-[3-Fluoro-4-[4-(acetoxyacetyl)-I- P-99 isopropyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]- alcohol
methyl]-O-iso-propylthiocarbamate
436 (S)-N-[[3-[3,5-Difluoro-4-[4-(acetoxyacetyl)- P-100 methanol
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
437 (S)-N-[[3-[4-[4-(Acetoxyacetyl)-1- P-101 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
438 (S)-N-[[3-[3-Fluoro-4-[4-(benzyloxyacetyl)- P-102 methanol
i-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
439 (S)-N-[[3-[3.5-Difluoro-4-[4-(benzyloxy- P-103 methanol
acetyi)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
440 (S)-N-((3-[3-Fluoro-4-[4-(methoxycarbonyl)- P-105 methanol
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
441 (S)-N-[[3-[3-Fluoro-4-[4-(methoxycarbonyl)- P-i05 ethanol
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-ethylthiocarbamate
442 (S)-N-[[3-[3-Fluoro-4-[4-{methoxycarbonyl)- P-105 isopropyl
I-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]- alcohol
methyl]-O-i.so-propylthiocarbamate
- 138 -
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Isothiocvanate
Example Corresponding
No. Product to Amine No. Amine
443 (S)-N-[[3-[3,5-Difluoro-4-[4-(methoxy- P-106 methanol
carbonyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
444 (S)-N-[[3-[4-[4-(methoxycarbonyl)-1-P-107 methanol
piperazinyl]phenyl]-2-oxo-~-oxazolidinyl]-
methyl]-O-methylthiocarbamate
445 (S)-N-[[3-[3-Fluoro-4-[4-(methanesulfonyl)-P-108 methanol
1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
446 (S)-N-[[3-[3,5-Difluoro-4-[4-(methane-P-109 methanol
sulfonyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
447 (S)-N-[[3-[4-[4-(methanesulfonyl)-1-P-110 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
448 (S)-N-[[3-(3-Fluoro-4-j4-(ethanesulfonyl)-1-P-111 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
449 (S)-N-[[3-[3,S-Difluoro-4-[4-(ethane-P-112 methanol
sulfonyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]cyclopropane-
carbothioamide
450 (S)-N-[[3-[4-[4-(ethanesulfonyl)-1-P-113 methanol
piperazinyl]phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-methylthiocarbamate
451 (S)-N-[[3-[3-Fluoro-4-[4-(chloromethane-P-114 methanol
sulfonyl )- I -piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
452 (S)-N-[[3-[3,5-Difluoro-4-[4-(chloro-P-115 methanol
methanesulfonyl )-1-piperazinyl]phenyl]-2-
oxo-5-oxazolidinyl]methyl]-O-methyl-
thiocarbamate
453 (S)-N-([3-[4-[4-(chloromethanesulfonyl)-I-P-116 methanol
piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
- 139 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Isothiocyanate
Example Corresponding
No. Product to Amine No. Amine
454 (S)-N-[[3-[3-Fluoro-4-[4-(cyanomethane- P-117 methanol
sulfonyl)-1-piperazinyl]phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-methylthiocarbamate
455 (S)-N-([3-[3,5-Difluoro-4-[4-(cyanomethane- P-118 methanol
sulfonyl)-1-piperazinyl]phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-methylthiocarbamate
456 (S)-N-[[3-(4-(4-(Cyanomethanesulfonyl)-1- P-119 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
457 (S)-N-([3-[3-Fluoro-4-[4-(N-methyl- P-120 methanol
sulfamoyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyi]methyl]-O-methylthiocarbamate
458 (S)-N-[[3-[3,5-Difluoro-4-[4-(N-methyl- P-121 methanol
sulfamoyl)- I -piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
459 (S)-N-[[3-[4-[4-(N-methylsulfamoyl)-1- P-122 methanol
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
460 (S)-N-[[3-[3-Fluoro-4-[4-(N,N-dimethyl- P-123 methanol
sulfamoyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-meihylthiocarbamate
461 (S)-N-[[3-[3,5-Difluoro-4-[4-(N,N-dimethyl- P-124 methanol
sulfamoyl)-1-piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
462 (S)-N-[[3-[4-(4-(N,N-dimethylsulfamoyl)-1- P-125 methanol
piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
463 (S)-N-[[3-(3-Fluoro-4-[4-(ethoxycarbonyl)-1- P-126 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl)-O-methylthiocarbamate
464 (S)-N-[[3-[3-Fluoro-4-[4-(ethoxycarbonyl)-t- P-126 ethanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-ethylthiocarbamate
- 140 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Isothiocyanate
Example Corresponding
No. Product to Amine No. Amine
465 (S)-N-[[3-[3-Fluoro-4-(4-(ethoxycarbonyl)-I-P-126 isopropyl
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]-alcohol
methyl]-O-iso-propylthiocarbamate
466 (,S~-N-[(3-[3,5-Difluoro-4-[4-(ethoxy-P-127 methanol
carbonyl )- I -piperazinyl]phenyl]-2-oxo-5-
oxazolidinyl]methyl)-O-methylthiocarbamate
467 (S)-N-[[3-[4-[4-(ethoxycarbonyl)-I-P-128 methanol
piperazinyl]phenyl]-2-oxo-5-
oxazolidiny))methyl]-O-methylthiocarbamate
468 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1-P-129 methanol
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate,
469 (S)-N-[[3-[3-Fluoro-4-(4-sulfamoyl-1-P-129 ethanol
piperazinyl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]-O-ethylthiocarbamate
470 (S)-N-[[3-[3-Fiuoro-4-(4-sulfamoyl-I-P-129 isopropyl
piperazinyl)phenyl]-2-oxo-S-oxazolidinyl]-alcohol
methyl]-O-iSO-propylthiocarbamate
471 (.S~-N-[[3-[3,5-Difluoro-4-(4-sulfamoyl-1-P-130 methanol
piperazinyl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-methylthiocarbamate
472 (S)-N-[[3-[4-(4-sulfamoyl-1-piperazinyl)-P-131 methanol
phenyl)-2-oxo-5-oxazolidinyl]methyl)-O-
methylthiocarbamate
473 (S)-N-[[3-[3-Fluoro-4-[4-(cyanomethyl)-I-P-132 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
474 (S)-N-[j3-[3,5-Difluoro-4-[4-(cyanomethyi)- P-133 methanol
i-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]-
methyl)-O-methylthiocarbamate
475 (S)-N-[[3-[4-[4-( cyanomethyl)- I - P-134 methanol)
piperazinyl ]phenyl ]-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
-141-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Isothiocvanate
Example Corresponding
No. Product to Amine No. Amine
476 (S)-N-[[3-[3-Fluoro-4-[4-(2-fluoroethyl}-1-P-135 methanol
piperazinyl]phenyl]-2-oxo-5-oxazolidinyl)-
methyl)-O-methylthiocarbamate
477 (S)-N-[[3-[3,5-Difluoro-4-[4-(2-fluoroethyl)-P-136 methanol
1-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl)-
methyl)-O-methylthiocarbamate
478 (S)-N-[[3-[4-(4-(2-fluoroethyl)-I-P-137 methanol
piperazinyl)phenyl)-2-oxo-5-oxazolidinyl]-
methyl)-O-methylthiocarbamate
479 (S)-N-[[3-[3-Fluoro-4-(4-formyl-I-P-138 methanol
piperazinyl)phenyl]-2-oxo-5-oxazolidinyl)-
methyl]-O-methylthiocarbamate
480 (S)-N-([3-[3,5-Difluro-4-(4-formyl-1-P-139 methanol
piperazinyl}phenyl)-2-oxo-5-oxazolidinyl]-
methyl]-O-methylthiocarbamate
481 (S)-N-[[3-[4-(4-formyl-1-piperazinyl)-P-140 methanol
phenyl]-2-oxo-5-oxazolidinyl]methyl]-O-
methylthiocarbamate
- 142 -
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
EXAMPLE -182. (SS)-N-([3-[3-Fluoro-4-(tetrahydro-1.-1-thiazepin-:1(SI~-
yt)phenyl)-2-oxo-~-oxazolidinyl)methyl]thioacetamide, thiazepine S-oxide
~s~
IN ~ ( o
F~N~O
H
~N~CH3
I IS
Step 1. Hexahydro-5-oxo-1,4-thiazepine is prepared according to the procedure
described by Gallego (J. Org. Cherry. 1993. 58, 3905-3911 ).
Step 2. Lithium aluminum hydride (5.5 mL of a 1 M solution in THF) is added
dropwise to a stirred solution of hexahydro-S-oxo-1.4-thiazepine (721.5 mg) in
dry THF
(21 mL) cooled to 0 °C. The reaction mixture is stirred at 0 °C
for 10 min, then at room
temperature for 4 h. The reaction mixture is quenched by careful successive
addition of
water (0.2 mL), 5 N aqueous NaOH (0.2 mL) and water (0.74 mL). The reaction
nnixture becomes very thick and gel-like. The reaction mixture is diluted with
ether {50
mL) and filtered through a pad of celite. The filter cake is washed with ether
( 100 mL).
The filtrate is concentrated to afford 616.6 mg of l ,4-hexahydrothiazepine
which is used
immediately in the next step.
Step 3. To a stirred solution of 1.4-hexahydrothiazepine (596.0 mg) and 3,4-
difluoronitrobenzene (0.51 mL) in acetonitrile ( 14 mL) is added
diisopropylethylamine
( 1.0 mL). The yeilow solution is heated at ret7ux for 18 h, then cooled and
concentrated.
2o The residue is diluted with CH,CI, ( 100 mL) and washed with saturated
aqueous NH~CI
(35 mL). The phases are separated and the organics are dried (MgSO~), filtered
and
concentrated. The residue is purified by flash chromatography urine 209c.
EtOAc in
hexane as the eluent to afford 830.2 mg of the nitrobenzene. Mp I 15-116
°C; Anal.
Calcd for C~1H~;FN,O,S: C, 51.55; H. 5.11; N, 10.93: S, 12.51. Found: C,
51.47; H,
5.1?; N. 10.79; S, 12.42.
Step 4. To a stirred suspension of the nitrobenzene prepared in Step 3 (5.5 g)
in
EtOH (260 mL) is added a solution of ? M aqueous CuSO~ ( 1 1.9 mL). The
mixture is
cooled to 0 °C and NaBH; (=t.1 g) is added in portions. The reaction
mixture turns very
- 143 -
CA 02351062 2001-05-10
WO 00/32599 PCT/lJS98/25308
dark and is Stirred at 0 °C for 10 min. at room temperature for 30 min,
and then heated at
reflux for 3 h. The cooled reaction mixture is diluted with EtOAc (500 ml) and
washed
with water (?00 mL). The aqueous mixture is extracted with EtOAc (3 x 200 mL).
The
combined organics are dried (VIgSO~). filtered and concentrated to afford the
aniline
intermediate.
Step ~. The dark residue from Step 4 is dissolved in 2:1 acetone/water (255
mL)
and cooled to 0 °C. To this stirred mixture is added solid NaHCO~ (~.4
g) followed by
benzylchloroformate (7.7 mL). The reaction mixture is stirred at 0 °C
for 10 min, then
at room temperature for 24 h. The reaction mixture is quenched with 10%
aqueous
to NaHSO~ (200 mL) and then poured into EtOAc (300 mL). The phases are
separated and
the aqueous phase is extracted with EtOAc (2 x 250 mL). The combined organics
are
dried (MgSO~), filtered and concentrated. The residue is purified by MPLC
using 20%
EtOAc in hexane to afford 6.03 g of the benzylcarbamate as a yellow solid. mp
72-74
°C; Anal. Calcd for C~9H,~FN,O,S: C, 63.31; H, 5.87; N, 7.77; S, 8.89.
Found: C,
t5 63.31; H, 5.97; N, 7.69; S, 8.79.
Step 6. To a stirred solution of the carbamate from Step 5 (3.0 g) in dry THF
(33
mL) under N, cooled to -78 °C, is added dropwise via syringe a 1.6 M
solution of nBuLi
in hexane (5.=~ mL). The reaction mixture was stirred at -78 °C for 35
min, then R-
glycidyl butyrate ( 1.2 mL) is added. The reaction mixture is stirred at -78
°C for 30 min,
2o then at room temperature overnight during which time a precipitate forms.
The reaction
mixture is quenched with saturated aqueous NH~CI (33 mL) and poured into EtOAc
( 100 mL). The phases are separated. The organic phase is washed with
saturated
aqueous NaHCO~ (50 mL), brine ( 50 mL), dried (MgSO~), filtered and
concentrated.
The residue i5 purified by flash chromatography using EtOAc as the eluent to
afford 2.5
~5 g of a hydroxymethyl oxazolidinone. Mp 100-102 °C. Anal. Calcd for
CiSH~yFN,O~S:
C, 55.20; H. x.87; N, 8.58; S. 9.82. Found: C, 55.09; H, 5.91; N, 8.36; S,
9.57.
Step 7. To a stirred solution of the alcohol prepared in Step 6 ( 1.7 g) in
CH,CI,
(35 mL) cooled to 0 °C, is added triethylamine ( 1.1 mL) followed by
methanesulfonyl
chloride (0.~ mL). The reaction mixture is stirred at 0 °C for 10 min,
then at room
3o temperature for 1 h. The reaction mixture is treated with water (35 mL).
The phases are
separated and the aqueous phase is extracted with CH,CI, (35 mL). The combined
- I 44 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
organic phases are dried (MgSO~), filtered and concentrated. The residue is
purified by
flash chromatography using 80% EtOAc in hexane as the eluent to afford 2.1 g
of the
mesylate. Mp 132-142 °C. Anal. Calcd for C~6H,~FN,OSS,: C, 47.51; H,
5.?3: N, 6.93:
S. 15.85. Found: C. 47.18: H, 5.28; N, 6.84: S, 15.60.
Step 8. Ammonia gas is bubbled into a stirred suspension of the mesylate
prepared in Step 7 (941.7 mg) in I: I THF/CHzOH (40 mL) until saturated
(approx. 5
min). The reaction mixture is heated in a sealed tube at 100 °C for 72
h. The cooled
reaction mixture is concentrated to give the crude amine, which is immediately
suspended in CH,CI~ (35 mL) and cooled to 0 °C. To this stirred
suspension is added
triethylamine (0.97 mL, 6.9 mmol) followed by di-tert-butyl dicarbonate (759.5
mg, 3.5
mmol). The reaction mixture becomes homogeneous and is stirred at RT for 18 h.
The
reaction mixture is poured into CH~CI~ (75 mL) and washed with HBO ( 1 x 50
mL). The
organic phase is dried (MgSOs), filtered and concentrated. The resulting
residue is
purified on a Biotage 40 S column using 30-35 % ethyl acetate in CH~OH as the
eluent
to afford 867.4 mg of the protected amine. mp 74-75 °C. Anal Cald: C,
56.45; H, 6.63;
N, 9.88. Found: C, 56.95; H, 6.85; N, 9.55.
Step 9. To a stirred suspension of the protected amine prepared in Step 8
(205.2
mg) in 1:1 CH;OH/H,O (6 mL) cooled to 0°C is added sodium meta
periodate ( 113.5
mg). The resulting suspension is stirred at RT for 18 h. The reaction mixture
is filtered
2o and the solid is washed with CH~CI~ (2 x 20 mL). The filtrate is extracted
with HBO ( 1
x 10 mL). The phases are separated. The aqueous phase is extracted with CHaCI~
( 1 x
mL). The combined organic phases are dried (MgSO.~), filtered and
concentrated.
The white solid residue is purified on a Biotage 12 M column using 5% CH;OH in
CH~CI~ as the eluent to afford 187.3 mg of the sulfoxide. mp 78-81
°C.
25 Step 10. Dry HCl gas is passed over the surface of a stirred solution of
the
sulfoxide prepared in Step 9 ( 179.3 mg) in CHaOH (2 mL) cooled to 0 °C
for 1 minute.
The reaction mixture is stirred at 0 °C for 10 min, then at room
temperature for 15 min,
then concentrated. The resulting yellow residue is suspended in THF (5 mL) and
CH~CI~ (5 mL) and cooled to 0°C. To this stirred suspension is added
triethylamine
3o {0.46 mL) followed by ethyldithioacetate (0. I8 mL). The dark reaction
mixture is stirred
at RT overnight then concentrated. The dark residue is diluted with CH~CI~ (30
mL)
and washed with H,O (? x 15 mL). The organic phases are dried (MgSO.~),
filtered and
- 145 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
concentrated. The dark residue is purified on a Biotage 12 M column using ~9c
CHzOH
in CH~CI~ as the eluent to afford 71.5 mg of the title compound as a tan
solid. mp 85-89
°C.
Following the general procedure outlined in Step 10 of Example 482, but
substituting the dithioesters listed below, the compounds of Examples 483 to
495 of
Table K can be obtained.
TABLE K
Example Compound Amine Dithioester
No. (from Preparation
Z)
483 (SS)-N-[[3-[3-Fluoro-4-o~~ Z {a)
(tetrahydro-1,4-
~
thiazepin-4(SH)-yl))-~
~ o
phenyl]-2-oxo-S-
F ~ N~o
oxazolidinyl]methylJ-~NH
propanethioamide, z
thiazepine S-oxide
484 S)-N-[[3-[3-Fluoro-4-Same as above Z (b)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl)-2-
methylpropanethio-
amide, thiazepine
S-
oxide.
485 (SS)-N-[[3-[3-Fluoro-4-Same as above Z (c)
( tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenylJ-2-oxo-5-
oxazolidinyl]methylJ-
cyclopropanecarbothio-
amide, thiazepine
S-
oxide.
- 1.t6 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/Z5308
Example Compound Amine Dithioester
N' (from Preparation
Z)
486 (SS)-N-[(3-[3-Fluoro--t-Same as above
Z (d)
(tetrahydro-1,.~-
thiazepin-~l(SH)-yl
) )-
phenyl]-2-oxo-~-
oxazolidinyl]mcthylj-
butanethioamide,
thiazepine S-oxide
487 (SS)-N-[[3-[3-Fluoro-4-Same as above
Z (e)
(tetrahydro-1.4-
thiazepin-4(5H)-yl))-
phenylj-2-oxo-S-
oxazolidinyljmethyl]-3-
methylbutanethioamide.
thiazepine S-oxide
488 (SS)-N-[[3-[3-Fluoro-4-
Same as above Z (~
(tetrahydro- I
,4-
thiazepin-4(SH)-yI))-
phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylbutanethioamide.
thiazepine S-oxide
489 (SS)-N-[[3-(3-Fluoro-4-Same as above Z (g)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl
j-
3.3-dimethylbutanethio-
amide, thiazepine
S-
oxide
490 (SS)-N-[[3-[3-Fluoro--~-Same as above Z (h)
( tetrahydro-1.4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl)methyl]-
cyclobutanecarbothio-
amide, thiazepine
S-
oxide
- 147 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithioester
No. (from Preparation
Z)
491 (SS)-N-[(3-[3-Fluoro-4-Same as above Z (i)
(tetrahydro-1.4-
thiazepin-4(SH)-yl))-
phenyi]-2-oxo-5-
oxazolidinyl]methyl]-1-
cyclopentanecarbothio-
amide, thiazepine
S-
oxide
492 (SS)-N-[[3-[3-Fluoro-4-Same as above
Z U)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclohexanecarbothio-
amide, thiazepine
S-
oxide
493 (SS)-N-[[3-(3-Fluoro-4-Same as above
Z (k)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2--
cyclopropylethanethio-
amide, thiazepine
S-
oxide
494 (SS)-N-[[3-[3-Fluoro-4-Same as above Z (l)
(tetrahydro- I
,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl)methyl]-2-
cyclobutylethanethio-
amide. thiazepine
S-
oxide
- 148 -
CA 02351062 2001-05-10
WO 00/32599 PCT/IJS98/25308
Example Compound Amine D ester
No. (from Preparation
Z)
495 (SS)-N-[(3-(3-Fluoro-4-Same as above
Z (m)
(tetrahydro-1,4-
thiazepin-=l(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclopentylethanethio-
amide, thiazepine
S-
oxide
Example 496. (5S)-N-([3-[3,S-Difluoro-4-(tetrahydro-1,4-thiazepin-4(SFn-
yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide, thiazepine S-oxide
0
~s~
F
N i ~ O
F ~ N~O
H
~N~CH3
S
The title compound can be prepared by the procedure of Example 482, by
substituting an appropriate quantity of 2,6-difluoro-4-nitrobenzene
(trifluoromethane)
sulfonate for 3,4-dil7uoronitrobenzene in Step 1.
to Utilizing the amine prepared in Example 496, but substituting the
dithioester
listed below for ethyl dithioacetate in the final step, the compounds of
Examples 497 to
499 of Table L are obtained.
- 149 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TABLE L
Example Compound Amine Dithioester
No. (from Preparation Z)
497 (SS)-N-([3-[3.5- o~~ Z (a)
Difiuoro-4-(tetrahydro-
1,4-thiazepin-4(SH)-yl))- ~N ~ o
phenyl]-2-oxo-5-
oxazolidinyl)methyl)- F NCO
propanethioamide, ~NHZ
thiazepine S-oxide
498 (SS)-N-[[3-[3,5- Same as above Z (b)
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(SH)-yl ))-
phenyl)-2-oxo-S-
oxazolidinyl]methyl)-2-
methylpropanethio-
amide, thiazepine S-
oxide
499 (SS)-N-[[3-[3,5- Same as above Z (c)
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(SH)-yl))-
phenyt)-2-oxo-5-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide,thiazepine S-
oxide
Example 500. (SS)-N-[[3-[4-(Tetrahydro-1,4-thiazepin-4(SH)-yl)phenyl)-2-oxo-5-
oxazolidinyl)methyl)thioacetamide, thiazepine S-oxide.
~s~
~NI ~ ~ o
~N~O
H
~N~CH3
S
- 150 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
The title compound can be prepared by the procedure of Example -182, by
substituting an appropriate quantity of 4-fluoronitrobenzene for 3,4-
difluoronitro-
benzene in Step 1.
Utilizing the amine prepared in Example 500. but substituting the dithioester
listed below for ethyl dithioacetate in the final step, the compounds of
Examples SOI to
503 of Table M are obtained
TABLE M
to
Example Compound Amine Dithioester
No.
( from Preparation
Z)
501 (SS)-N-[[3-[4- o\~ Z a
( )
(Tetrahydro-1,4-
s-,.1
thiazepin-4(SH)-yltv
))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
propanethioamide, ~NHZ
thiazepine S-oxide
502 (SS)-N-[[3-[4- Same as above
Z (b)
(Tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylpropanethio-
amide, thiazepine
S-
oxide
503 (SS)-N-[[3-[4- Same as above
Z (c)
(Tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide, thiazepine
S-
oxide
Example 50-t. (SS)-N-[[3-[3-Fluoro-a-(tetrahydro-1,.1;-thiazepin-4(5f~-
yl)phenyl]-2-
oxo-5-oxazolidinyl]methyl]thioacetamide, thiazepine S,S-dioxide
-I51-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
O
O'1S
~N i I O
F~N~O
H
~N~CH3
I IS
Step 1. To a stirred solution of the thiazepine prepared in Step 8 of Example
482
(243.7 mg) in 25 9o H~O/acetone (8 mL) is added 4-methylmorpholine N-oxide
(201.5
mg) followed by a solution of osmium tetroxide in 2-methyl-2-propanol (2.5 wt
%, 30
~tL). The reaction mixture is stirred at room temperature for 18 h. The
reaction mixture
is treated with saturated sodium bisulfate (8 mL), then poured into CH~CI~ (50
mL).
The phases are separated. The aqueous phase is extracted with CH~CI~ (2 x 25
mL).
The combined organic phases are washed with brine ( 1 x 25 mL), dried
(MgSO.~),
l0 filtered and concentrated. The residue is purified on a Biotage 40 S column
using 1 %
CH30H in CH~CI, as the eluent to afford 216.1 mg (0.47 mmol, 83%) of the
thiazepine
S,S-dioxide as a white solid. mp 144-146 °C.
Step 2. Dry HCI gas is passed over the surface of a stirred solution of the
thiazepine S,S-dioxide prepared in Step l ( 108.2 mg) in CH~OH(3 mL) at
0°C for 1
is minute. The reaction mixture is stirred at 0 °C for 10 min and then
at room temperature
for 15 min. The reaction is concentrated and the yellow residue is suspended
in CH~CIa
(2 mL) and THF (2 mL). This stirred suspension is cooled to 0°C and
triethylamine
(0.27 mL) is added followed by a solution of ethyldithioacetate (0.11 mL) in
THF (0.5
mL) with 0.25 mL rinse. The yellowish-green solution is stirred at 0°C
for l0 min then
2o at room temperature for 18 h. The reaction mixture is poured into CH~CI~
(~0 mL) and
washed with HBO (? x 10 mL). The organic phase was dried (MgSO~), filtered and
concentrated. The residue is purified on a Biotage 12 M column using ~ % CHzOH
in
CH~CI~ as the eluent to afford 77.3 mg of the title compound as a white solid.
mp 88-90
°C.
25 Following the general procedure outlined in Step ? of Example 504, but
substituting the dithioester listed below for ethyl dithioacetate, the
compounds of
Examples 50~ to X07 of Table N are obtained.
- 152 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TABLE N
Example Compound Amine Dithioester
No. (from Preparation
Z)
505 (5S)-N-[(3-[3-Fluoro-~.-o Z (a)
(4-thiomorpholinyl]-o
h
l
2
p
eny N
)-
-oxo-5-
oxazolidinyl]methyl]-~
o
I
propanethioamide, 'I
F ~ N~O
thiazepine S,S-dioxidel-( NH2
506 (5S)-N-[(3-[3-Fluoro-4-Same as above Z {b)
(4-thiomorpholinyl]-
phenyl)-2-oxo-5-
oxazolidinyl]methyl)-2-
methylpropanethio-
amide, thiazepine
S,S-
dioxide
507 (SS)-N-[[3-[3-Fluoro-4-Same as above Z (c)
{4-thiomorpholinyl]-
phenyl)-2-oxo-5-
oxazolidinyl)methyl]-
cyclopropanecarbothio-
amide, thiazepine
S,S-
dioxide
Example 508. (5S)-N-[[3-[3,5-Difluoro-4-(tetrahydro-1,4-thiazepin-4(5Fn-
yl)phenyl]-2-oxo-5-oxazolidinyl]methyl)thioacetamide, thiazepine S,S-dioxide
0
o Is~
F
N i ~ O
F W N~O
H
~N~CH3
I IS
- 153 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
The title compound can be prepared by the procedures of Examples 504 and 48?.
by substituting an appropriate quantity of ?.6-difluoro-4-nitrobenzene
(trifluoromethane)
sulfonate for 3,4-difluoronitrobenzene in Step 1 of Example 482.
Utilizing the amine prepared in Example 508, but substituting the dithioester
listed below for ethyl dithioacetate in the final step, the compounds of
Examples 509 to
511 of Table O are obtained.
TABLE O
Example Compound Amine Dithioester
No. (from Preparation
Z)
509 (SS)-N-[[3-[3,5- o Z (a)
Difluoro-4-(4- o.= s
thiomorpholinyl]-
phenyl]-2-oxo-5- ~
o
~
oxazolidinyl]methyl]-I'
F ~ N~O
propanethioamide, L-.( NH
2
thiazepine S,S-dioxid~
e
510 (SS)-N-((3-[3,5- Same as above Z (b)
Difl uoro-4-(4-
thiomorpholinyl]-
phenyl]-2-oxo-5-
oxazoIidinyl]methyl]-2-
methylpropanethio-
amide, thiazepine
S,S-
dioxide
511 (SS)-N-[[3-[3,5- Same as above Z (c)
Difluoro-4-(4-
thiomorpholinyl]-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclopropanecarbothio-
amide, thiazepine
S,S-
dioxide
to Example 512. (5S)-N-[[3-[4-(Tetrahydro-1,.1-thiazepin-d(SH)-yl)phenyl]-2-
oxo-S-
oxazolidinyl]methyl]thioacetamide, thiazepine S,S-dioxide
- 154 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/Z5308
O
olClS
~N i I O
~N~O
H
~N~CH3
S
The title compound can be prepared by the procedure of Examples 504 and 482,
by substituting an appropriate quantity of 4-fluoronitrobenzene for 3,4-
difluoronitro-
benzene in Step I of Example 482.
Utilizing the amine prepared in Example 512, but substituting the dithioester
listed below for ethyl dithioacetate in the final step, the compounds of
Examples 513 to
I S of Table P are obtained.
TABLE P
l0
Example Compound Amine Dithioester
No. (From Preparation
Z)
513 (SS)-N-[[3-[4- Z (a)
(tetrahydro-1,4- 11
o=s
thiazepin-4(SH)-
yl))phenyl]-2-oxo-5-N ~ o
oxazolidinyl]- ~ I N~o
methyl]propanethio-L--( NH
2
amide, thiazepine ~
S.S
-
dioxide
514 (SS)-N-[[3-[4- Same as above Z (b)
(tetrahydro- l,4-
thiazepin-4(SH)-
yl))phenyl]-?-oxo-5-
oxazolidinylJmethyl]-2-
methylpropanethio-
amide, thiazepine
S.S-
dioxide
- I55 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithioester
No. (From Preparation
Z)
515 (SS}-N-[[3-[4- Same as above
Z (c)
(tetrahydro-1,4-
thiazepin-4(SH)-
yl))phenyl)-2-oxo-5-
oxazolidinylJ-
methyl Jcyclopropane-
carbothioamide,
thiazepine S.S-dioxide
EXAMPLE 516. (SS)-N-[[3-[3-Fluoro-4-(tetrahydro-1,4-thiazepin-4(5f~-
yt)phenyl]-2-oxo-5-oxazolidinyljmethyljthioacetamide.
s~
~N i ~ O'I
F~N~O
H
~N~CH3
S
This compound is prepared according to the procedure of Step 8 in Example 482,
but stubstituting an appropriate quantity of ethyl dithioacetate for di-tert-
butyl
dicarbonate; mp 129-13 I °C.
Utilizing the amine prepared in Step 8 of Example 482. but substituting an
appropriate quantity of the dithioester listed below for di-tert-butyl
dicarbonate, the
compounds of Examples 517 to 529 of Table Q are obtained.
TABLE Q
Example Compound Amine Dithioester
No. (From Preparation
Z)
517 (SS)-N-[(3-[3-Fluoro-4-s
Z (a)
(tetrahydro-1,4- ~N
thiazepin-4(SH)-yl
))- ~'
h F ~
l
p N
eny O
J-2-oxo-S-
oxazolidinyl)methylJ-~NH2
propanethioamide
- 156 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
ExampleCompound Amine Dithioester
No. (From Preparation
Z)
518 (SS)-N-[[3-[3-Fluoro-4-Same as above
Z (b)
(tetrahydro-1,4-
thiazepin--~(SH)-yl))-
phenyl)-2-oxo-5-
oxazolidinyl)methyl]-2-
methylpropanethioamide
519 (SS)-N-[[3-[3-Fluoro-4-Same as above
Z (c)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl)-
cyclopropanecarbothio-
amide
520 (SS)-N-[[3-[3-Fluoro-4- Same as above Z (d)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-
butanethioamide
521 (SS)-N-[[3-[3-Fiuoro-4- Same as above
(tetrahydro-1,4- Z (e)
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinylJmethyl]-3-
methylbutanethioamide
522 (SS)-N-[[3-[3-Fluoro-4- Same as above
(tetrahydro-1,4- Z (t~
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl)methyl]-2-
methylbutanethioamide
523 (SS)-N-[[3-[3-Fluoro--4- Same a~ above
(tetrahydro- I ,4- Z (g)
thiazepin-4(SH)-yl))-
phenyl)-2-oxo-5-
oxazolidinyl)methyl]-
3,3-dimethylbutanethio-
amide
t57 -
CA 02351062 2001-05-10
WO 00/32599
PCT/US98/25308
ExampleCompound Amine
Dithioester
'V'' (From Preparation
Z)
524 (5S)-N-[(3-[3-Fluoro-:1-Same as above
Z (h)
(tetrahydro-1,4-
thiazepin--1(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl)-
cyclobutanecarbothioami
de
525 (SS)-N-[[3-[3-Fluoro-=l-Same as above
Z (i)
(tetrahydro-1.4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclopentanecarbothio-
amide
526 (SS)-N-([3-[3-Fluoro-4-Same as above
Z ~ )
(tetrahydro-1,4-
thiazepin-4(SH)-yl
))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
cyclohexanecarbothio-
amide
527 (SS)-N-[[3-[3-5 Same as above
Fluoro- Z (k)
4-(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclopropylethanethio-
amide
528 (SS)-N-[[3-[3-Fluoro-d-Same as above Z (I)
(tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl)methyl]-2-
cyclobutylethanethio-
amide
- ~5g-
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Amine Dithioester
No. (From Preparation Z)
529 (SS)-N-[[3-[3-Fluoro-4- Same as above Z (m)
(tetrahydro- l ,.l-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl]-2-
cyclopentylethanethioam
ide
EXAMPLE X30. (SS)-N-[(3-[3,5-Difluoro-4-(tetrahydro-1,4-thiazepin-4(5F>n-
yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]thioacetamide.
S~ F
~N i I O
F ~ N~O
H
~,N~CH3
S
This compound can be prepared according to the procedures of Example 482 and
Example 516, but substituting an appropriate quantity of 2,6-difluoro-4-
nitrophenyl
trifluoromethane sulfonate for 3,4-difluoronitrobenzene in Step 1 of Example
482.
i o Utilizing the amine prepared in Example 530, but substituting an
appropriate
quantity of the dithioester listed below for di-tert-butyl dicarbonate. the
compounds of
Examples 531 to 533 of Table R can be prepared.
TABLE R
ExampleCompound Amine Dithio Compound
No. (from Preparation
Z)
531 (SS)-N-[[3-[3,5- S~ F Z (a)
Difluoro-=~-(tetrahydro-~N
~
1,4-thiazepin-=1(SH)-yl))-
~
~
phenyl]-2-oxo-5- F
N
O
oxazolidinyl]methyl]-~""'NH2
propanethioamide
- t59-
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
Example Compound Amine Dithio Compound
No. (from Preparation
Z)
532 (SS)-N-[[3-[3,5- Same as above Z (b)
Difluoro-=t-(tetrahydro-
1,4-thiazepin-4(SH)-yl ))-
phenyl]-2-oxo-5-
oxazolidinyl]methylJ-2-
methylpropanethioamide
533 (SS)-N-[[3-[3,5- Same as above Z (c)
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl]methyl)-
cyclopropanecarbothio-
amide
EXAMPLE 534. (SS)-N-[[3-[4-(Tetrahydro-1,4-thiazepin-4(SF~-yl)phenyt)-2-oxo-S-
oxazolidinyl]methyl]thioacetamide; mp I29-131°C
s~
N~ i I O''
~N~O
H
~N~CH3
S
This compound can be prepared according to the procedures of Example 482 and
Example S 16, but substituting an appropriate quantity of 4-fluoronitrobenzene
for 3,4-
difluoronitrobenzene in Step 1 of Example 482.
to Utilizing the amine prepared in Example 534, but substituting an
appropriate
quantity of the dithioester listed below for di-tart-butyl Bicarbonate, the
compounds of
Examples 535 to 537 of Table S can be prepared.
- I 60 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TABLE S
Example Compound Amine Dithio Compound
No. (from Preparation
Z)
535 (SS)-N-[[3-[-1- s~ Z (a)
(Tetrahydro-1.4- ~N
/ I o
thiazepin-.1(SH)-yl))-~
~
~
phenyl]-2-oxo-5- N
o
oxazolidinyl]- ~NHz
methyl]propanethio-
amide
536 (SS)-N-[[3-[4- Same as above Z (b)
(Tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-2-
methylpropanethioamide
537 (SS)-N-[[3-[4- Same as above
Z (c)
(Tetrahydro-1,4-
thiazepin-4(SH)-yl))-
phenyl]-2-oxo-5-
oxazolidinyl)-
methyl]cyclopropane-
carbothioamide
EXAMPLE 538. (SS)-N-[[3-j3-Fluoro-4-(tetrahydro-1,4-thiazepin-4(SFn-
yl)phenyl]-2-oxo-5-oxazolidinyl]methylJthiourea, thiazepine S-oxide
~s~
'--i N / I O
F~N~O
H
~N~NH2
I IS
This compound can be prepared by the procedure described in Example 33, but
to substituting the amine prepared in Example 48? foi the amine 33.
By reaction of the isothiocyanate prepared in Example 538 with the amines and
alcohols listed in Table T, the compounds of Examples 539 to 54-f can be
prepared.
- 161 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TABLE T
Example Compound Isothioc~~anate Amine or
No. Alcohol
539 (SS)-N-[[3-[3-Fluoro-~4-
~ CH
NH
( tetrahydro-1.4- ~ ~
5~ ~
thiazepin-4(SFn- ~~N ~
o
yl)phenyl)-?-oxo-5-
oxazolidinyl]methyl]-N'-F ~ N O
methylthiourea, ~N=c=S
thiazepine S-oxide
540 (SS}-N-[[3-[3-Fluoro-4-Same as above
(CH3)~NH
(tetrahydro-1,4-
thiazepin-4(SFn-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
N',N'-dimethylthiourea,
thiazepine S-oxide
541 (SS)-N-[[3-[3-Fluoro-4-Same as above Azetidine
(tetrahydro-1,4-
thiazepin-4(Sf~-
yl)phenylJ-2-oxo-5-
oxazolidinyl]methyl)-I-
azetidinecarbothioamide,
thiazepine S-oxide
542 (SS)-N-[[3-[3-Fluoro-4-Same as above CHzOH
(tetrahydro- I
.4-
thiazepin-4(SF~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
methylthiocarbamate.
thiazepine S-oxide
543 (SS)-N-[[3-[3-Fluoro-4-Same as above CH;CH,OH
{tetrahydro-1.4- -
thiazepin-4(SH}-
yl)phenylJ-2-oxo-S-
oxazolidinyl)methyl)-O-
ethylthiocarbamate,
thiazepine S-oxide
- 162 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
ExampleCompound Isothiocyanate Amine or
No. Alcohol
544 (SS)-N-[[3-[3-Fluoro-4-
(CH;)~CHOH
(tetrahydro-1.4- Same as above
thiazepin-4(Sl~-
yl )phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-O-
isopropyithiocarbamate,
thiaze ine S-oxide
EXAMPLE 545. (SS)-N-[[3-[3,5-Ditluoro-4-(tetrahydro-1,4-thiazepin-4(SFn-
yl)phenyl]-2-oxo-~-oxazolidinyl]methyl]thiourea, thiazepine S-oxide
0
~s~
I F
N i ~ O
F ~ N~O
H
~N~NH2
S
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example 496 for the amine 33.
By reaction of the isothiocyanate prepared in Example 545 with the amines and
to alcohois listed in Table U, the compounds of Examples 546 to 5~1 can be
prepared.
TABLE U
ExampleCompound I sothiocyanate Amine or
No. Alcohol
546 (SS)-N-[[3-[3,5- o~ CH;NHS
Difluoro-4-(tetrahydro-s~ F
1,4-thiazepin-4(Sl~-~
N
yl)phenyl]-2-oxo-5-~ ~ o
' \
oxazolidinyl]methyl]-NF
- N- -O
methylthiourea, ~
N=C=S
thiazepine S-oxide
- 163 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Isothiocyanate Amine or
No. Alcohol
547 (5S)-N-[[3-[3,5- Same as above (CH3),NH
Difluoro=4-(tetrahydro-
1.4-thiazepin-4(5f~-
yl)phenyl]-2-oxo-5-
oxazolidinyl Jmethyl ]-
N'.N'-dimethylthiourea,
thiazepine S-oxide
548 (5S)-N-[[3-[3,5- Same as above Azetidine
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(5f~-
yl)phenyl)-2-oxo-5-
oxazolidinyl]methyl]-1-
azetidinecarbothioamide.
thiazepine S-oxide
549 (5S)-N-[[3-[3,5- Same as above CH;OH
Difluoro=4-(tetrahydro-
1,4-thiazepin-4(5f~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl J-O-
methylthiocarbamate,
thiazepine S-oxide
550 (5S)-N-[[3-[3,5- Same as above CH~CH~OH
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(5F~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
ethylthiocarbamate,
thiaze ine S-oxide
551 (5S)-N-[[3-[3,5- (CH;),CHOH
Difluoro=4-(tetrahydro- Same as above
1,4-thiazepin-4(5l~-
yl )phenyl )-2-oxo-S-
oxazolidinyl]methyl]-O-
isopropylthiocarbamate,
thiazepine S-oxide
EXAMPLE SS2. (SS)-N-[[3-[4-(Tetrahydro-1,4-thiazepin-4(SI~-yl)phenyi]-2-oxo-5-
oxazolidinyl]methyl]thiourea, thiazepine S-oxide
_ 1 G4 _
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
O~
~S~
~N i I OII
~N~O
H
~N~NH2
' IS
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example 500 for the amine 33.
By reaction of the isothiocyanate prepared in Example 552 with the amines and
alcohols listed in Table V, the compounds of Examples 553 to 558 can be
prepared.
TABLE V
Example Compound Isothiocyanate Amine or
No. Alcohol
553 (5S)-N ([3-[4- o
.~S~ CH~NH
(Tetrahydro-1,4 - 1-
thiazepin-4{51~- ~N
yl)phenylJ-2-oxo-5- ~ ~ O
oxazolidinyl]methyl]-N'- ~ N~O
methylthiourea, ~"~N=C=S
thiazepine S-oxide
554 (5S)-N-[[3-[4- Same as above
(Tetrahydro-1,4- (CH;)~NH
thiazepin-4(SI~-
yl )phenyl )-2-oxo-5-
oxazolidinyl]methyl]-
N',N'-dimethylthiourea,
thiazepine S-oxide
555 (SS)-N-[[3-[4- Same as above
(Tetrahydro-1,4- Azetidine
thiazepin-4(5F~-
yl)phenylJ-2-oxo-5-
oxazolidinyl]methyl]-1-
azetidinecarbothioamide,
thiazepine S-oxide
- 165 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Isothiocvanate Amine or
N-'' Alcohol
556 (SS)-N-[[3-[4- Same as above CH~OH
(Tetrahydro- I
,4-
thiazepin-=l(5~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
methylthiocarbamate.
thiazepine S-oxide
557 (SS)-N-[[3-[4- Same as above CH3CH~OH
(Tetrahydro-1,4-
thiazepin-4(Sf~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
ethylthiocarbamate,
thiaze ine S-oxide
558 (SS)-N-[[3-[4-
(CH~)~CHOH
(Tetrahydro-1,4- Same as above
thiazepin-4(SF~-
yl)phenyl)-2-oxo-5-
oxazolidinyl)methyl]-O-
isopropylthiocarbamate,
thiaze ine S-oxide
EXAMPLE 559. (SS)-N-[[3-[3-Fluoro-4-(tetrahydro-1,4-thiazepin-4(SI~-
yl)phenyl)-2-oxo-5-oxazolidinyl)methyl)thiourea, thiazepine S,S-dioxide
0
o Is~
'..i N ~ I O
F~N~O
H
~N~NH2
S
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example 504 for the amine 33.
By reaction of the isothiocyanate prepared in Example 559 with the amines and
Io alcohols listed in Table W, the compounds of Examples 560 to 565 can be
prepared.
- 166 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TABLE W
Example Comvound Isothiocyanate Amine or
No.
Alcohol
560 (SS)-N-[[3-[3-Fluoro-4- o
CH~NH
(tetrahydro-1,4- o ~s~
thiazepin-4(Sl~- ~ IN
yl)phenyl]-2-oxo-5- ~ o
oxazolidinyl]methyl]-N'- F iv o
methylthiourea, ~N=c=s
thiazepine S,S-dioxide
561 (SS)-N-[[3-[3-Fluoro-4- Same as above (CH3)~NH
(tetrahydro- I ,4-
thiazepin-4(Sf~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
N',N'-dimethylthiourea,
thiazepine S.S-dioxide
562 (SS)-N-[[3-[3-Fluoro-4- Same as above Azetidine
(tetrahydro-1,4-
thiazepin-4(Sl~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-1-
azetidinecarbothioamide,
thiazepine S,S-dioxide
563 (SS)-N-[[3-[3-Fluoro-4- Same as above
(tetrahydro-1,4- CH~OH
thiazepin-4(Sf~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
methylthiocarbamate,
thiazepine S.S-dioxide
564 (SS)-N-[[3-[3-Fluoro-4- Same as above CH;CH~OH
(tetrahydro-1,4-
thiazepin-~1(51~-
yl )phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-
ethylthiocarbamate.
thiazepine S,S-dioxide
- 167 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Examt~leCompound Isothiocyanate Amine or
No. Alcohol
S6S (SS)-N-[[3-[3-Fluoro-4-
(CH~),CHOH
(tetrahydro-1.4- Same as above
thiazepin-4(Sl~-
yl)phenyl]-2-oxo-S-
oxazolidinyl)methyl]-O-
isopropylthiocarbamate,
thiaze ine S.S-dioxide
EXAMPLE 566. (5S)-N-[[3-[3,5-Difluoro-4-(tetrahydro-1,4-thiazepin-4(51~-
yl)phenylJ-2-oxo-5-oxazolidinylJmethylJthiourea, thiazepine S,S-dioxide
0
0
N ~~ o
F ~ N~O
H
~N~NH2
' IS
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example S08 for the amine 33.
By reaction of the isothiocyanate prepared in Example S66 with the amines and
1 o alcohols listed in Table X, the compounds of Examples S61 to S72 can be
prepared.
TABLE X
ExampleCompound I sothiocyanate Amine or
No. Alcohol
S67 (SS)-N-[[3-(3,S- o CH;NHS
Difluoro-4-(tetrahydro-
I ,4-thiazepin-4(Sl~-
yl)phenyl]-2-oxo-S-o
N ~
II
oxazolidinyl]methyl]-N'-~
F ~ N~O
methylthiourea, ~
N=c=s
thiazepine S.S-dioxide
- 168 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Exam-pleCompound Isothiocvanate Amine or
N-'' Alcohol
568 (SS)-N-[[3-[3,5- Same as above
(CH~)~NH
Difluoro-.~-(tetrahydro-
1,4-thiazepin-=1(St~-
yl)phenyl]-2-oxo-5-
oxazolidinyl)methyl
]-
N',N'-dimethylthiourea,
thiazepine S.S-dioxide
569 (SS)-N-((3-[3,5- Same as above Azetidine
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(51~-
yl)phenyl]-2-oxo-5-
oxazolidinyl)methyl]-1-
azetidinecarbothioamide.
thiazepine S,S-dioxide
570 (SS)-N-[[3-[3,5- Same as above CH30H
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(SFn-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl)-O-
methylthiocarbamate,
thiazepine S,S-dioxide
571 (SS)-N-[[3-[3,5- Same as above CH;CH~OH
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(51~-
yl)phenyl]-2-oxo-5-
oxazolidinyl)methyl]-O-
ethylthiocarbamate,
thiaze ine S.S-dioxide
572 (SS)-N-[[3-[3,5-
(CHz)~CHOH
Difluoro-4-(tetrahydro-Same as above
1,4-thiazepin-4(Sl~-
yl)phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-O-
isopropylthiocarbamate,
thiaze ine S.S-dioxide
EXAMPLE 573. (SS)-N-[[3-[4-(Tetrahydro-1,4-thiazepin-~l(Shi7-y!)phenyl]-2-oxo-
S-
oxazolidinyl]methyl)thioarea, thiazepine S,S-dioxide
- 169 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
O
O IS
~N i I O
~N~O
H
~N~NH2
' fS
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example 512 for the amine 33.
By reaction of the isothiocyanate prepared in Example 573 with the amines and
alcohols listed in Table Y, the compounds of Examples 574 to 579 can be
prepared.
TABLE Y
Example Compound Isothiocvanate Amine or
No. Alcohol
574 (SS)-N-[[3-[4- o
CH3NH
~
(Tetrahydro-1,4- o,lS
thiazepin-4(51~-
~
yl)phenyl]-2-oxo-5-N i o
oxazolidinyl]methyl]-N'-~ , N~O
methylthiourea, 1"
~N=c=S
thiazepine S,S-dioxide
575 (SS)-N-[[3-[4- Same as above
(CH~)~NH
(Tetrahydro-1.4-
thiazepin-4(5~-
yl)phenyl]-2-oxo-S-
oxazolidinyl]methyl]-
N';N'-dimethylthiourea,
thiazepine S.S-dioxide
576 (SS)-N-[[3-[4- Same as above
Azetidine
(Tetrahydro- t
,4-
thiazepin-4(Sl~-
yI )phenyl J-2-oxo-5-
oxazolidinylJmethyl]-1-
azetidinecarbothioamide,
thiazepine S,S-dioxide
- ~ 7o
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Isothiocvanate Amine or
No. Alcohol
577 (SS)-N-[[3-(4- Same as above
CH~OH
(Tetrahydro- I
.4-
thiazepin-4(51~-
yl)phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-O-
methylthiocarbamate,
thiazepine S,S-dioxide
578 (SS)-N-[[3-[4- Same as above CH~CH~OH
(Tetrahydro-1,4-
thiazepin-4(Sl~-
yl)phenyl)-2-oxo-5-
oxazolidinyi]methyl]-O-
ethylthiocarbamate,
thiaze ine S.S-dioxide
579 (SS)-N-([3-[4-
(CHi)~CHOH
(Tetrahydro-1,4- Same as above
thiazepin-4(51~-
yl)phenyl]-2-oxo-5-
oxazolidinyl)methyl]-O-
isopropylthiocarbamate,
thiaze ine S.S-dioxide
EXAMPLE 580. (5S)-N-[[3-[3-Fluoro-4-(tetrahydro-1,4-thiazepin-4(SI~-
yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]thiourea
s
Nl / ( O
F~N~O
H
~N~NH2
I IS
This compound can be prepared by the procedure described in Example 33. but
substituting the amine prepared in Step 8 of Example 482 for the amine 33.
By reaction of the isothiocyanate prepared in Example 580 with the amines and
alcohols listed in Table Z, the compounds of Examples 581 to X86 can be
prepared.
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
TAB LE Z
Example Compound Isothiocvanate Amine or
No. Alcohol
581 (SS)-N-[[3-[3-Fluoro-=l-s~ CH~NH
(tetrahydro-1,4- ~N ~ o
thiazepin-4(Sl~- ,'
~
~
yl)phenyl ]-2-oxo-5-F
N
O
oxazolidinyl]methyl]-N'-~N=c=s
methylthiourea
582 (SS)-N-[[3-[3-Fluoro-4-Same as above
(CH~)-
~NH
(tetrahydro-1,4-
thiazepin-4(51~-
yl)phenyl ]-2-oxo-5-
oxazolidinyl]methyl]-
N',N'-dimethylthiourea
583 {SS)-N-[[3-[3-Fluoro-4-Same as above Azetidine
(tetrahydro-1,4-
thiazepin-4(Sl~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-1-
azetidinecarbothioamide
584 (SS)-N-[[3-[3-Fluoro-4-Same as above CH~OH
(tetrahydro-1,4-
thiazepin-4(51~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
methylthiocarbamate
585 (SS)-N-[[3-[3-Fluoro-4-Same as above CH~CH~OH
(tetrahydro-1,4-
thiazepin-4(Sf~-
yl)phenyl]-2-oxo-S-
oxazolidinyl]methyl]-O-
ethylthiocarbamate
586 (SS)-N-[[3-[3-Fluoro-4-
(CH;)~CHOH
(tetrahydro-1.4- Same as above
thiazepin-~l(Sl~-
yl )phenyl ]-?-oxo-5-
oxazolidinyl ]methyl)-O-
i.sn ro vlthiocarbamate
- ~ 72 -
CA 02351062 2001-05-10
WO 00/32599 PCTNS98/25308
EXAMPLE X87. (~S)-N-[[3-[3,S-Ditluoro-.t-(tetrahydro-1.-1-thiazepin-d(51~-
yl)phenyl]-2-oxo-~-oxazolidinyl]methyl]thiourea
S~ F
~N i I O
F ~ N~O
H
~N,~NH2
S
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example 530 for the amine 33.
By reaction of the isothiocyanate prepared in Example 587 with the amines and
alcohols listed in Table AA, the compounds of Examples 588 to 593 can be
prepared.
i0
TABLE AA
Example Compound Isothiocyanate Amine or
No. Alcohol
588 (SS)-N [[3-[3,5- S~ F CH3NH~
Difluoro-4-(tetrahydro-~
N
1,4-thiazepin-4(51-~ ~ oI'
l \
h ~
l
2
y F
)p N
eny O
]-
-oxo-5-
oxazolidinyl]methyl]-N'-'"~N=c=s
methylthiourea
589 (SS)-N-[[3-[3,5- Same as above
(CH3)~NH
Difluoro-4-(tetrahydro-
1,4-thiazepin-4(Sl~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-
N',N'-dimethylthiourea
590 (SS)-N-[[3-[3,5- Same as above Azetidine
Difluoro-4-( tetrahydro-
1,4-thiazepin-4(Sl~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-I-
azetidinecarbothioamide
- 173 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
Example Compound Isothiocyanate Amine or
No. Alcohol
591 (SS)-N-[[3-[3.5- Same as above CH30H
Difluoro-4-(tetrahydro-
1.4-thiazepin-4(Sf~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
methylthiocarbamate
592 (SS)-N-[[3-[3,5- Same as above CH
;CH~
OH
Difluoro-4-(tetrahydro- _
_
1,4-thiazepin-4(Sl~-
yl)phenyl]-2-oxo-5-
oxazolidinyl)methyl]-O-
ethylthiocarbamate
593 (SS)-N-[[3-[3,5-
(CHI)'-CHOH
Difluoro-4-(tetrahydro-Same as above
1,4-thiazepin-4(Sf~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
iso ro lthiocarbamate
EXAMPLE 594. (SS)-N-([3-(4-(Tetrahydro-1,4-thiazepin-4(5H)-yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]thiourea
s~
NI / I O
~N~O
H
~N~NH2
S
This compound can be prepared by the procedure described in Example 33, but
substituting the amine prepared in Example 534 for the amine 33.
By reaction of the isothiocyanate prepared in Example 594 with the amines and
to alcohols listed in Table BB, the compounds of Examples 595 to 600 can be
prepared.
- 174 -
CA 02351062 2001-05-10
WO 00/32599 PCT/US98/25308
TABLE BB
Example Compound Isothioc ay nate Amine or
No. Alcohol
595 (SS)-N [[3-(4- s~
(Tetrahydro-1,4- CH~NH
0
thiazepin-4(51~-
yl)phenyiJ-2-oxo-5-
oxazolidinyl]methylJ-N'- ~N=c=s
methylthiourea
596 (SS)-N-[[3-[4- Same as above (CH3)~NH
(Tetrahydro-1,4-
thiazepin-4(SF~-
yl)phenyl]-2-oxo-S-
oxazolidinyl]methyl]-
N',N'-dimethylthiourea
597 (SS)-N-[[3-[4- Same as above
Azetidine
(Tetrahydro-1,4-
thiazepin-4(51~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl)-1-
azetidinecarbothioamide
598 (SS)-N-[[3-[4- Same as above
(Tetrahydro-1,4- CH30H
thiazepin-4(51~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl J-O-
methylthiocarbamate
599 (SS)-N-[[3-[4- Same as above
CH~CH~OH
(Tetrahydro-1,4-
thiazepin-4( Sf~-
yl)phenyl]-2-oxo-5-
oxazolidinyl]methyl]-O-
ethylthiocarbamate
600 (SS)-N-[(3-[4-
(Tetrahydro-1,4- Same as above (CH;)zCHOH
thiazepin-4(Sl~-
yl )phenyl]-2-oxo-5-
oxazolidinyl]methyl)-O-
iso ro lthiocarbamate
- 175 -