Note: Descriptions are shown in the official language in which they were submitted.
CA 02351075 2001-05-11
WO 01/25209 PCTIIB99/01687
PYRIDI1VIUM DERIVATIVES FOR THE TREATMENT OF DIABETIC
AND AGING-RELATED VASCULAR COMPLICATIONS
s
to FIELD OF THE INVENTION
The present invention relates to a class of compounds of pyridinium series
and to their use in treatment of diabetes and related illnesses. More
particularly
is the invention relates to compounds of this series, methods for their
preparation,
pharmaceutical composition containing these compounds and their use in the
treatment of complications of diabetes mellitus. The compounds of this series
exhibit AGE breaking activity, which is essential for the treatment of
diabetic and
aging-related complications including kidney disease, nerve damage,
2o atherosclerosis, retinopathy and dermatological conditions. The invention
also
extends to the method of reversing the discoloration of teeth resulting from
nonenzymatic browning in the oral cavity which comprises administration of an
amount effective to reverse pre-formed advanced glycosylation crosslinks.
BACKGROUND OF THE INVENTION
2s
Maillard in 1912 found that reducing sugars, such as glucose and ribose
react with proteins to form brown pigments. Further studies have shown that
this
is an irreversible non-enzymatic reaction, which occurs irl several natural
systems
including stored foodstuff. Maillard reaction occurs in two stages, early and
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s advanced. Initially, proteins react with glucose to form stable Amadori
products,
which subsequently cross-links to form advanced glycation end products (AGE).
In most cases, the formation of AGE also accompanies browning of the proteins
and increase in the fluorescence.
In diabetes, where blood glucose level is significantly higher than normal,
to the reaction of glucose with several proteins such as haemoglobin, lens
crystallin
and collagen, gives rise to the formation of AGE, which in turn, is
responsible for
the complications associated with diabetes, such as nephropathy,
microangiopathy, endothelial dysfunction and other organ dysfunctions. In
addition, the activity of several growth factors, such as basic fibroblast
growth
is factor, is also impaired. AGE products, unlike normal proteins in tissue,
have a
slower rate of turnover and replenishment. It has been reported that AGE
products may in fact elicit a complex immunological reaction involving RAGE
(Receptor for Advanced Glycation End Products) receptors and activation of
several incompletely defined immunological processes. It has been documented
2o that diabetes with evidence of microangiopathy and macroangiopathy also
show
evidence of oxidative stress, the mechanism of which has not been elucidated.
in vitro AGE formation can be studied in the laboratory by incubating
reducing sugars, such as ribose or glucose with bovine serum albumin. AGE
formation can be detected by increase in the fluorescence or increased cross
2
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s reactivity with anti-AGE antibodies. The increase in fluorescence seems to
precede formation of AGE specific antigenic epitopes. This increase in
fluorescence is used to monitor the increased AGE formation in vitro (Brownlee
M et al, Science 1986; 232:1629-1632). In addition to the increase in the
fluorescence, one of the most important features of in vitro AGE formation is
the
to formation of antigenic epitopes that are specific to AGE and not to the
native
proteins. Therefore, it is possible to raise antibodies against advanced
glycation
end products of one protein and use them to detect AGE formation in other
proteins. This has served as an important analytical tool in AGE research.
Due to the clinical significance of AGE formation, many approaches are
is being used to diagnose, prevent, or revert AGE formation in the body. The
formation of AGE could be inhibited by reacting with an early glycosylation
product that results from the original reaction between the target protein and
glucose. The inhibition was believed to take place as the reaction between the
inhibitor and the early glycosylation product appeared to interrupt the
subsequent
ao reaction of the glycosylated protein with additional protein material to
form the
cross linked late stage product. Compounds Iike aminoguanidine act to inhibit
AGE formation by such mechanism.
The formation of AGE on long-lived proteins is also associated with cross-
linking of these proteins. The AGE derived protein cross-links have been shown
3
CA 02351075 2001-05-11
WO O1/Z5Z09 PCT/IB99101687
s to be cleaved by compounds like N- phenacyl thiazolium bromide (PTB), which
reacts with and cleaves covalent, AGE derived protein cross links (Vasan et
al.
Nature 1996; 382: 275-278; US 5,853,703, Date of Patent : Dec. 29, 1998). The
mechanism of reducing the AGE content in tissues is expected to take place
relatively rapidly, in contrast to aminoguanidine, which acts slowly by its
very
la nature of mechanism of action. This current specification is related to
compounds
of pyridinium class, which break pre-formed AGE, like PTB, and in some cases
even more effectively than PTB.
SUMMARY OF THE INVENTION
is The main objective of the present invention is to provide a class of
compounds of the pyridinium series which are useful for the management of
diabetes and aging-related vascular complications, and particularly in the
treatment of complications of diabetes mellitus and other aging-related
conditions
including kidney disease, nerve damage, atherosclerosis, retinopathy anal
ao dermatological conditions. The invention also extends the method to reverse
the
discoloration of teeth resulting from nonenzymatic browning in the oral cavity
which comprises administration of an amount effective to reverse the pre-
formed
advanced glycosylation crosslinks etc.
Another object of the present invention is to provide compounds of the
Zs pyridinium series, which exhibit AGE breaking activities.
4
CA 02351075 2001-05-11
WO 01125209 PCT/IB99/01687
s
Yet another object of the present invention is to provide a method of
preparation of compounds of the pyridinium series which exhibit AGE breaking
activities.
Still another object of the invention is to provide pharmaceutical
to compositions with a new class of compounds of the pyridinium series
according
to the invention and their pharmaceutically acceptable salts in combination
with
suitable carriers, solvents, excepients, diluents and other media normally
employed in preparing such compositions.
Still another object of the invention is to provide a method of treatment of a
is diabetic patient by administration of the compounds of the invention,
either singly
or in combination with drugs for anti-diabetic therapy, or pharmaceutically
acceptable salts thereof in required dosage in admixture with pharmaceutically
acceptable diluent, solvent, excepients, carriers or other media as may be
appropriate for the purpose.
DETAILED DESCRIPTION OF THE INVENTION
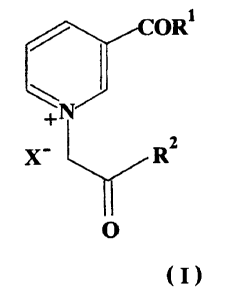
The present invention provides for a new class of AGE breakers, of general
2s formula I,
5
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
R1
s
a
O
(I)
wherein
is Rl is -Y-R3
Y is selected from oxygen or NH.
.R3 is selected from hydrogen, alkyl, aryl
RZ is selected from group consisting of alkyl, -Oalkyl, aryl, -Oaryl, NHalkyl
and
-NHaryl
2o X is selected from group consisting of a halide ion, acetate ion,
perchlorate ion,
sulfonate ion, oxalate ion, citrate ion, tosylate ion, maleate ion, mesylate
ion,
carbonate ion, sulfite ion, phosphoric hydrogen ion, phosphonate ion,
phosphate
ion, BF4 , PF6 , etc.
with proviso that,
2s (a) when Rl is phenyl, then R2 is also phenyl, and
(b) when R2 is phenyl and X is halide ion, then Rl is other than -OCH3.
As used herein "alkyl" refers to an optionally substituted, saturated
hydrocarbon group joined by single carbon-carbon bonds and having 1-4 carbon
6
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99101687
s atoms joined together. The saturated alkyl hydrocarbon group may be linear
or
branched. The substituent is -OH.
As used herein "aryl" refers to an optionally substituted aromatic group
with atleast one ring having a conjugated pi- electron system, containing upto
two
conjugated or fused ring systems. Aryl includes carbocyclic aryl and
heterocyclic
to aryl all of which may be optionally substituted with halogen, sulfonamido,
thio,
amino, cyano, -Oalkyl, -NHalkyl, hydroxy, folmyl, -Oaryl and -NHaryl.
The representative compounds of general formula I which are novel are
listed in Table IA. These compounds with the following chemical names are
suggested by way of example alone and in no way restrict the invention.
is 3-Carbonylamino-1-(2-(2,4-dichlorophenyl)-2-oxoethyl)) pyridinium bromide
(compound 1 ).
3-(Tetrahydrobenzothiazol-2-yl)aminocarbonyl-1-(2-(2,4-dichlorophenyl)-2-
oxoethyl)pyridinium bromide (compound 2 ).
1-(2-Phenyl-2-oxoethyl)-3-((2-hydroxyethyl)aminocarbonyl) pyridinium bromide
20 (compound 3).
3-Carbonylamino-1-(2-thien-2'-yl-2-oxoethyl~yridinium bromide(compound 4 ).
1-(2-Phenyl-2-oxoethyl)-3-((p-sulfonamidophenylene)aminocarbonyl) pyridinium
bromide (compound 5 ).
7
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s 1-(2-Ethoxy-2-oxoethyl)-3-((2-hydroxyethyl)aminocarbonyl)pyridinium bromide.
(compound 6 ).
1-(2-Phenyl -2- oxoethyl) -3-(isopropyloxycarbonyl) pyridinium bromide
(compound 7).
1-(2-Methyl-2-oxoethyl~3-((2-hydroxyethyl)aminocarbonyl) pyridinium chloride
to (compound 8).
1-(2-Thien-2'-yl-2-oxoethyl}-3-((2-hydroxyethyl)aminocarbonyl)pyridinium
bromide (compound 9 ).
1-(2-(2,4 - Dichlorophenyl-2-oxoethyl) -3- (isopropyloxycarbonyl) pyridinium
bromide (compound 10 ).
is 1- (2-Phenyl - 2- oxoethyl) - 3- ((4-methylthiazol - 2 -yl) aminocarbonyl)
pyridinium bromide (compound 11 ).
1-(2-Phenylamino-2-oxoethyl)-3-(n-butyloxycarbonyl)pyridinium chloride
(compound 12 ).
25
I -(2- Phenylamino - 2- oxoethyl ) - 3 - (n-butylaminocarbonyl ) pyridinium
chloride (compound 13 ).
1- (2- Phenylamino - 2- oxoethyl )- 3- ((2-hydroxyethyl)aminocarbonyl)
pyridinium chloride (compound 14 ).
8
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s 1- (2- (2,4 - Dichlorophenyl ) - 2-oxoethyl) - 3- (n - butoxycarbonyl)
pyridinium
bromide (compound 15 )
1-(2 - (2, 4 - Dichlorophenyl) - 2-oxoethyl) - 3- (n-butylamino-carbonyl )
pyridinium bromide (compound 16 ).
Further, some known pyridine derivatives have also been studied for their AGE
to breaking activity, which was not known earlier for these molecules, and are
as
listed in Table IB, with their chemical names as given below:
1- (2-Phenyl-2-oxoethyl) -3- (1-phenyl-1-oxomethyl) pyridinium bromide
(compound 17 : (Ref-. Chemical Abstracts: 91, P47378Y, (1979))
1- (2 Phenyl - 2-oxoethyl ) - 3- (methoxycarbonyl ) pyridinium bromide
is (compound 18 : (Ref Chemical Abstracts: 91, P47378Y, (1979))
TahlP 1A - Pvridinium derivatives (Novell
Compound -Rl - 2 -X
No.
1 NH2 2,4-dichloro Br
hen 1
2 tetrahydrobenzo-thiazol-2-yl-2,4-dichlorophenylBr
amino
3 NHCH2CH20H Phen 1 Br
4 NHz 2 thien 1 Br
-sulfonamido- hen lease Phen 1 Br
amino
6 NHCHZCHZOH OEt Br
7 OCH CH3 2 Phen 1 Br
g NHCH2CH20H CH3 Cl
9 NHCH2CH20H 2-thiea 1 Br
OCH CH3 2 2,4-dichloro Br
hen 1
11 4-meth lthiazol-2- 1 aminoPhen 1 Br
12 OCH2CH2CH2CH3 NHPh Cl
13 NHCH2CH2CH2CH3 NHPh Cl
9
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99101687
14 N_HCH2CH20H NHPh Cl
15 OCH2CH2CH2CH3 2,4-dichloro hen Br
1
16 NHCH2CH2CH2CH3 2,4-dichloro hen Br
1
s
Table IB - Pyridinium Derivatives (Known)
Compound -Rl -R2 -X
No.
17 Phen 1 Phen 1 Br
18 OCH3 Phen 1 Br
to According to one embodiment of the present invention, the present
compounds are used for the treatment of diabetic complications, aging-related
complications including kidney disease, nerve damage, atherosclerosis,
retinopathy, dermatological conditions and colouration of teeth occurring due
to
the higher levels of preformed AGE. The increased levels of preformed AGE can
is be brought under control by breaking the AGE products using compounds
mentioned in the invention.
The invention also provides a process for the preparation of compounds of
the pyridinium series.
The said process for the preparation of compound 1, comprises, adding a
2o solution of 2,4- dichlorophenacyl bromide in toluene to nicotinamide
dissolved in
refluxing toluene, refluxing for seven and half hours, cooling, filtering the
precipitated solid, dissolving in methanol, treating with activated charcoal,
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s concentrating under vacuum, cooling in ice-salt mixture, filtering the
precipitated
solid and washing with methanol to obtain the desired compound.
Siml~arly, the other compounds of general formula I, are prepared from
properly substituted pyridine derivatives followed by quarternization with
appropriate reagent by refluxing in alcoholic solvents like methanol, ethanol,
to propanol, etc., and high boiling solvents like, toluene or xylene for 6 -
48 hrs. to
give the desired compounds.
The in vitro AGE formation, studied in the laboratory, by incubating
reducing sugar n'bose, with protein bovine serum albumin, resulted in browning
of
solution and increase in the fluorescence. Fluorescence was used as the
criteria to
is monitor the increased AGE formation.
Example 1
AGE breaker activity has been confirmed by the screenine_prrocedure as
mentioned below:
Materials:
Zo Bovine serum albumin (fraction V) (BSA)
Ribose, analytical grade
Phosphate buffered saline (PBS)
Equipment:
Microplate ELISA Reader - Spectramax Plus (Molecular Devices, USA)
11
CA 02351075 2001-05-11
WO OI/25209 PCTIIB99/01687
s Microplate washer, (Bio -Tec Instruments, USA)
pH meter
Methods of experiment:
160 mg/ml of protein, bovine serum albumin, BSA and 1.6M glucose sugar
were dissolved in phosphate buffered saline, PBS. Sodium azide was added at
l0 0.02% concentration as a preservative. The solution was filtered
asceptically
through a 0.22 E,iM filter and kept for aging at 37°C for 16 weeks.
After 16 weeks
the solution was dialyzed against PBS, aliquoted and stored at -20°C.
To determine the AGE breaking activity, lOp,g/ml and 100~.g/ml of the 16
weeks AGE-BSA was incubated with different concentrations of the test
is compounds at 37°C for 24 hours and AGE breaking activity of the test
compounds by ELISA was determined.
ELISA was performed as follows:
1. Different concentrations of 16 weeks AGE-BSA were coated on a microtitre
plate as standard. Each concentration is coated in triplicates.
20 2. The test samples were coated on microtitre plate at a concentration of 5
ng. to
20 ng per well in triplicates.
3. The plate was incubated at 37°C for one hour.
4. After incubation the plate was washed with PBST (PBS with 0.05% Tween
20).
12
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s 5. Blocking with S% slummed milk in PBS at 37°C for one hour was
done.
6. The plate was washed with PBST.
7. Primary antibody against AGE-BSA was added and the plate is incubated at
37°C for one hour.
8. The plate was washed with PBST
l0 9. Secondary antibody anti rabbit HRPO (Horse-Radish Per Oxidase) conjugate
was added and the plate is incubated at 37°C for one hour.
10. The plate was washed with PBST.
11. Colour development with OPD (orthophenylenediamine dihydrochloride) and
hydrogen peroxide was done.
is 12. OD (optical density) at (450nm reading - 620nm reading) was measured
after
incubation at 37°C for 15 minutes with Microplate ELISA Reader.
The breaker activity of the compounds were detenmined by the following
formula:
Breaker activity = OD4~ZOControl - OD45o.62oTest
Zo _~_~~~_~_____~~______ _ x 100
OD 450.20 Control
OD4so.~zoControl=Absorbance of 20ng AGE-BSA after incubation at 37°C
for 24
hours without test compound
i3
CA 02351075 2001-05-11
WO OI/25209 PCT/IB99/01687
s OD4so.~2oTest=Absorbance of 20ng AGE-BSA after incubation at 37°C for
24
hours with required concentration of test compound
Using specific examples, the % AGE breaking activity was calculated and
recorded in Table 2.
Table 2
Sample Concentration %Breakage
PTB 10 mM 27
20 mM 47
Compound 3 10 mM 5
Compound 4 10 mM 3
Compound 6 10 mM 43
Compound 9 T 10 mM 50
Compound 12 10 mM 57
Compound 13 10 mM 27
Compound 14 20 mM 48
Compound 17 5 mM 45
Compound 18 20 mM 36
to
14
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s Hence, for example, compound 12 has significant AGE - breaking activity i.e.
a
comparatively much superior potency vis - a - vis PTB.
The following examples give method for preparation of the specific
compounds of the invention as given in Table 1. The following compounds
suggested are by way of example alone and in no way restrict the invention.
to Example 2
Preparation of 3- carbonvlamino -1- (2- (2,4-dichlorouhenyl) -Z- oxoethvl)
pyridinium bromide (comuound 1)
Nicotinamide (1.228, O.Oi mol) was dissolved in refluxing toluene (40 ml)
and a solution of 2,4-dichlorophenacyl bromide (3.Og, 0.012moI) in 14m1 of
is toluene was added. The reaction mixtwre was refluxed for 7.5 hours and
cooled.
The precipitated solid was filtered and dissolved in methanol, decolourized
with
activated charcoal and concentrated under vacuum to one-fourth volume. It was
cooled in ice - salt mixture and the precipitated solid was filtered and
washed
with methanol (3x10m1) to afford a pure solid.
2o Yield : 39%
m.p. : 237-239°C
IR(KBr,clri 1) : 3331, 3133, 1706, i6?8
1H NMR (DMS4d6, 400 MHz) 8 : 9.54(lH,s), 9.18-9.11(ZH,m), 8.67(lH,s),
8.40(lH,t), 8.42-8.38(2H,m), 7.88(lH,s), 7.75 -7.72(lH,m), 6.49(2H,s)
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s Mass (m/z): 309,310,311,312,187,159
According to the above mentioned procedure the following compounds are
synthesized by reacting the corresponding pyridine derivatives with
appropriate
reagents by refluxing in alcoholic solvents like methanol, ethanol, propanol,
etc.
and high boiling solvents like toluene or xylene for 6-48 hours to give the
desired
to compounds:
Eaamule 3
3-(Tetrahvdrobenzothiazol-2-vl)aminocarbonylY 1-(2-(2,4-dichloropheny,~,l~2-
ogoethyl) uyridinium bromide (comuound 2):
Yield : 48%
is m.p. : 165 -167 °C(decomp.)
IR(KBr,cni 1) : 3333, 1714, 1684, 1635
1H NMR(CD30D, 400MHz) 8: 9.45(IH,s), 9.27-9.24(lH,m), 8.92-8.91(1H, m),
8.24 - 8.21(1H, m), 8.01 - 7.99(1H, m), 7.72 - 7.71(1H, m) 7.57-7.54(lH,m),
2.59-2.57 (4H,m), 1.85(4H,m)
ao Mass (m/z) : 446, 447, 448, 449, 416, 307 and 266
Ezamule 4
1-(2- Phenyl -2- ozoethyl) -3- ((2- hydrogyethyl) aminocarbonvl) pyridinium
bromide (comuound 3):
Yield : 98%
16
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s m.p. : 182 - 184°C(decomp.)
IR(KBr,clii 1) : 3289, 3241, 1690 and 1660
1H NMR(DMSOd6, 400MHz) 8: 9.47(lH,s), 9.21(lH,t), 9.09(2H,t), 8.41-
8.37(lH,m), 8.08-8.04(2H,m), 7.82-7.78(lH,m), 7.69-7.65(2H,m), 6.52(2H,s),
4.86(lH,t), 3.58-3.54(2H,m), 3.42-3.38(2H,m)
to Mass (m/z) : 285,242,149,1 I 9,91
Example 5
3-Carbonylamino-1- (2-thien-2'-yl-2-oxoethvl) nyridinium bromide
compound 4 ).
Yield : 35%
is m.p. : 212 - 215°C (decomp.)
IR(KBr,cni 1) : 3295, 3126, 1680, 1671, 1640
1H NMR (DMSOd6, 400 MHz) 8: 9.49(IH,s), 9.13-9.11(lH,d), 9.07-9.05(lH,d),
8.60(lH,bs), 8.40-8.38(lH,m), 8.25-8.19(3H,m),7.43-7.40,(lH,t), 6.44(2H,s)
Mass(m/z) : 247,248,249,193
2o Examine 6
1- (2-Phenyl -2- oxoethyl) -3- ((u-sulfonamidophenylene) aminocarbonYl)
pyridinium bromide (comuound 5):
Yield : 44%
m.p. : 188-190°C
17
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s IR(KBr,ctri') : 3296, 1700, 1679.
1H NMR (DMSOd~, 400MHz) S: 11.25 (lH,s), 9.58 (lH,s), 925 (lH,d), 9.16
(1H, d), 8.45 (lH,t), 8.10 (2H,d), 7.94 (2H,d), 7.86 (2H,d), 7.82(lH,t),
7.68(2H,t), 7.36(2H,s), 6.5(2H,s)
Mass (m/z) : 396, 277
to Example 7
1- (2- Ethoay -2- oxoethyl) -3- ((2- hydroayethvhocarbonyl) uvrid, i~n~'um
bromide compound 6):
Yield : 87%
m.p. : 138-140°C
is IR(KBr, cni ~) : 1748,1669
~H NMR {CD30D, 400 MHz) 8: 9.43 (lH,s), 9.09-9.02 (2H,ln), 8.26 (lH,m),
5.64 (2H,s), 4.31 (2H,~, 3.73 (2H,t), 3.54 {2H,t}, 1.32 (3H,t)
Mass (m/z) : 251, 252, 165, 166
Example 8
Zo 1- (2- Phenyl -2- oxoethvl) -3- (isoprouyloxycarbonvl) uvridinium bromide
(compound 7):
Yield : 46%
m.p. : 172-174°C
IR(KBr, cm 1) : 1726, 1692
18
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s 'H NMR (DMSOd6, 400MHz) 8: 9.55 (lH,s), 9.16 (lH,d), 9.08(lH,d), 8.39-8.36
(lH,m), 8.04 (2H,d), 7.77 (lH,t), 7.64 (2H,t), 6.53 (2H,s), 5.25-5.19 (lH,m),
1.34 (6H,d)
Mass (m/z) : 284, 285, 242
Example 9
l0 1- (2- Methyl-2-oxoethyl) -3- ((2- hydroxyethvl) aminocarbonvl) pyridinium
chloride (compound 8):
Yield : 47%
m.p. : 178-180°C
IR(KBr, clri') : 1727, 1660
is 1H NMR (DMSOdb, 400 MHz) S: 9.33 (lH,t), 9.30 (iH,s), 9.06(lH,d), 8.90
(lH,d), 8.25-8.21 (lH,m), 5.75 (2H,s), 4.84(lH,bs), 3.47 (2H,t), 3.30 (2H,t),
2.23
(3H,s)
Mass (m/z) : 223, 224, 225
Example 10
20 1- (2- Thien -2'-yl -2- oxoethyl) -3- ((2- hvdroxyethvly aminocarbonyl~
pyridinium bromide (compound 9):
Yield : 60%
m.p. : 207-209°C
IR(KBr, czri ~) : 1673, 1656
19
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s 1H NMR (DMSOd6, 400MHz} 8: 9.47 (lH,s}, 9.18-9.05 (3H,m),8.38-8.34
(lH,m), 8.23-8.19 (2H,m), 7.39 (lH,t), 6.44 (2H,s),3.55-3.50 (2H,m), 3.40-3.37
(2H,m)
Mass (m/z) : 291, 292, 293
Eaample 11
l0 1- (2- (2,4- Dichloronhenyly -2- ozoethyl) -3- (isouropyloaycarbonvl)
~yridinium bromide (comuound 101:
Yield : 26%
m.p. : 160-162°C
is IR (KBr, cm 1) : 1726, 1705
1H NMR (DMSOdb, 400 MHz) 8: 9.55 (lH,s}, 9.15 (lH,d), 9.08(lH,d), 8.40-
8.36 (lH,m), 8.11 (lH,d), 7.89 (lH,bs), 7.75-7.72 (lH,m), 6.44 (2H,s), 5.26-
5.20
(lH,m), 1.34 (6H,d).
Mass (m/z) : 352, 353, 354, 310
2o Eaamule 12
1- (2- Phenyl -2- oaoethyl) -3- ((4- methylthiazol -2- yl) aminocarb
pvridinium bromide (compound 11):
Yield : 30%
m.p. : 165-167°C
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s IR (KBr, clri I) : 3409, 3319 and 1698
1H NMR (DMSOd6, 400 MHz) 8: 9.58 (lH,s), 9.22 (lH,d), 9.11(lH,d), 8.42-
8.38 (lH,m), 8.07 (2H,d), 7.81 (lH,t), 7.68(2H,t), 6.86 (lH,bs), 6.56 (2H,s),
2.30
(3H,s)
Mass (m/z) : 337, 338, 232, 105.
to Example 13
1- (2- Phenylamino -2- oxoethyl) -3- (n- butoxycarbonvl) pyridinium chloride
(compound 1
Yield : 10%
m.p. : 150-152°C
is IR (KBr, crri') : 3228, 1742, 1678 (bs)
'H NMR (DMSOd6, 400 MHz) 8: 10.96 (lH,s), 9.65 (lH,s), 9.28(lH,t), 9.09
(lH,d), 8.37 - 8.34 (lH,m), 7.b2 - 7.59 (2H,m),7.37 - 7.33 (2H,m), 7.11
(lH,t),
5.79 (2H,s), 4.41(2H,t), 1.76-1.72(2H,m), 1.48-1.43 (2H,m), 0.94 (3H,t)
Mass (m/z) : 314, 3I5
2o Example 14
1- (2- Phenylamino -2- ozoethyl) -3- (n- butvlaminocarbonyl) pyridinium
chloride (compound 13):
Yield : 37%
m.p. : 182-185°C
21
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s IR (KBr, clri 1) : 3245, 1742, 1679
'H NMR (DMSOd~, 400MHz) 8: 10.97 (lH,s), 9.50(IH,s), 9.24(lH,t), 9.13
(lH,d), 9.02 (lH,d), 8.28-8.25 (lH,m), 7.57 (2H,d), 7.30(2H,t), 7.05 (lH,t),
5.70
(2H,s), 3.30 - 3.26 (2H,m), 1.52 -1.48 (2H,m), 1.34 -1.30 (2H,m), 0.86 (3H,t)
Mass (m/z) : 312, 313
to Example 15
1- (2- Phenylamino -2- oxoethyl) -3- ((2- hvdroavethyl) aminocarbonyl
pvridinium chloride (comuound l14
Yield : 58%
m.p. : 225-227°C
is IR (KBr, clri 1) : 3448, 3271, 1702 and 1663
1H NMR (DMSOd6,400 MHz) 8: 11.07 (lH,s), 9.58 (lH,s) 9.35(lH,t), 9.I7
(lH,d), 9.11 (lH,d), 8.33-8.29 (lH,m), 7.60(2H,d), 7.32 (2H,t), 7.08 (lH,t),
5.75
(2H,sj, 4.90 (lH,t), 3.57-3.53 (2H,m), 3.40-3.36 (2H,m)
Mass (m!z) : 300, 301, 302
zo Exam lu a 16
1- (2- (2,4- Dichlorophenyl) -2- oxoethvl) -3- (n- butoxycarbonyl) pvridinium
bromide (com~round 15):
Yield : 38%
m.p. : 154-156°C
22
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s IR(KBr, clri 1) : 3435, 3389, 1731 and 1704
1H IVMR (DMSOd6, 400 MHz) 8: 9.60 (lH,s), 9.21 (lH,d), 9.14(lH,d), 8.43
(lH,t), 8.16 (lH,d), 7.92 (lH,s), 7.78-7.76(lH,m), 6.51 (2H,s), 4.42 (2H,t),
1.76-
1.72 (2H,m), 1.48-1.42 (2H,m), 0.94 (3H,t)
Mass (m/z) : 366, 367, 368, 369, 370
to Example 17
1- (2- (2,4- Dichlorophenyl) -2- oxoethyl) -3- (n-butylaminocarbonyl)
pyridinium bromide (compound 16):
Yield : 35%
m.p. : 142-144°C
is IR(KBr, clri') : 3382, 1698, 1672
1H NMR (DMSOd6, 400 MHz) 8: 9.37 (lH,s), 9.07 (lH,t), 8.99(2H,t), 8.31-8.28
(lH,m), 8.04 (lH,d) 7.82-7.81 (lH,d), 7.68-7.65 (lH,m), 6.34(2H,s), 3.27-3.24
(2H,m), 1.47-1.43 (2H,m),1.29-1.24 (2H,m), 0.81 (3H,t)
Mass (m/z) : 365, 366, 367, 368, 369
2o Pharmaceutical Compositions
Pharmaceutical compositions may be prepared with a pharmaceutically
effective quantity of compounds of general formula I, individually or in
combination. The following pharmaceutical formulations suggested are by way of
example alone and in no way restrict the forms in which they can be used.
23
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s Oral formulations
Oral formulations may be administered as solid dosage forms for example
pellets, powders, sachets or discreet units such as tablets or capsules and
like.
Other orally administered pharmaceutical preparations include monophasic and
biphasic liquid dosage forms either in ready to use form or forms suitable for
to reconstitution such as mixtures, syrups, suspensions or emulsions. The
preparations in addition may contain diluents, dispersing agents, buffers,
stabilizers, solubilizers, surfactants, preservatives, chelating agents and/
or other
pharmaceutical additives as are used. Aqueous or non-aqueous vehicle or their
combination may be used and if desired may contain suitable sweetener,
flavoring
Is agent or similar substances. In case of suspension or emulsion a suitable
thickening agent or suspending agent or emulsifying agent may be present in
addition. Alternatively, the compounds may be administered as such in their
pure
form unassociated with other additives for example as capsules or sachets. It
may
also be administered with a vehicle. Pharmaceutical preparations can have a
slow,
2o delayed or controlled release of active ingredients as is provided by a
matrix or
diffusion controlled system.
When the present invention or its salts or suitable complexes is presented
as a discreet unit dosage form like a tablet, it may contain in addition
medically
inert excipients as are used in the art. Diluents such as starch, lactose,
dicalcium
24
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s phosphate, talc, magnesium stearate, polymeric substances like methyl
cellulose,
fatty acids and derivatives, sodium starch glycollate, etc. may also be used.
Example 18
Preparation of oral dosage form:
A typical tablet has the following composition:
to Active ingredient of formula I as given above
Lactose 13 5 mg
Starch 7b mg
is Polyvinyl pyrolidone (K-30) 2 mg
Talc ~ 1. S mg
Magnesium Stearate 1.0 mg
Parenteral Formulations
For parenteral administration, the compounds or their salts or suitable
complexes thereof may be present in a sterile vehicle which may be an aqueous
or non aqueous vehicle or a combination thereof. The examples of vehicles are
as water, ethyl oleate, oils and derivatives of polyols, glycols and their
derivatives. It
may contain additives common in injectable preparations like stabilizers,
solubilizers, pH modifiers, buffers, antioxidants, cosolvents, complexing
agents,
tonicity modifiers, etc.
CA 02351075 2001-05-11
WO 01/25209 PCT/IB99/01687
s Some suitable additives are for example tartrate, citrate or similar
buffers,
alcohol, sodium chloride, dextrose and high molecular weight polymers. Another
alternative is sterile powder reconstitution. The compound may be administered
in the form of injection for more than once daily administration, or
intravenous
infusion/ drip or suitable depot preparation.
to Eaamule 19
Preparation suitable for narenteral administration has the following
comuosition:
Actlve mgredlent of formula I as given above
Polyethylene glycol (400) 0.7 S ml
is Sodium metabisulphite 0.01%
Isotonic saline/ WFI q.s.
Other Formnlarions.
For the dermatological application and for the discoloration of teeth, the
recommended formulations are lotions, oral rinse and toothpaste containing
2o appropriate amount of the compounds of general formula I.
The above examples are presented by way of illustration alone and in no
way limit the scope of the invention.
2b