Note: Descriptions are shown in the official language in which they were submitted.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 1 -
Substituted beazo[de~isoquinoliae-1,3-diones
This application is a continuation-in-part of
Serial No. 09/199,409, the entirety of which is
incorporated by reference herein.
The invention relates to substituted benzo[de]-
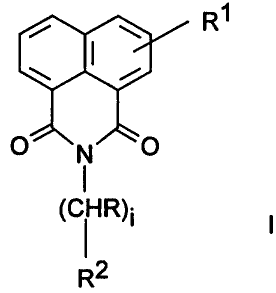
isoquinoline-1,3-diones of the formula I
i ~ R1
I
i
O~N/''~O [
(CI R)i
R2
in which
R is H, A or CH2-Ph,
Rl i s -Het, -N- [ ( CH2 ) s-OH ] 2, -N- [ ( CH2 ) g-OA] 2,
-NA- ( CH2 ) s-Ar, -NA- ( CH2 ) ,n R5, -Y- ( CH2 ) m-R5,
-Y- ( CH2 ) 2-NHA, -Y- ( CH2 ) 2-NH- ( CH2 ) s-OH, -Y- ( CH2 ) 2-NA2,
-Y- ( CH2 ) m OH, -Y- ( CHz ) n- ( CHR4 ) -R3, -Y- ( CH2 ) n-R9 r R4 ,
-Y- ( CH2 ) n-Het- ( CHZ ) o-R6 r -Y- ( CH2 ) n-Ar' - ( CH2 ) o-R6 r
-Het- ( CHZ ) n-Ar, -Het-Het, -Y- ( CH2 ) g-Ar' - ( CHZ ) o-Rll,
-Y- [ X-O ] t- [ X1-0 ] u-XZ-RS or -Y- ( CH2 ) n-NA- ( CH2 ) o-R5 r
R2 is H, OH, OA, COOH, COOA, CH (Ph) -Ph, Ar, Hetl, R5,
R' or Re
R3 is CH3,
R9 is -CH=CH2, -Ar, COOA, COOH
O HaC
H H N H H 'N
-N-C ~S or -N-C i ,
p N ~ ~Ar
RS is NH2, NHA, NA2, NHAr, -NH- (CH2) n-OH or
-NH- ( CH2 ) n-OA,
R6 is H or R5
R' i s -Ar' - ( CH2 ) n-RB or -Ar' - ( CH2 ) n-R5
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 2 -
R$ is CONH2, CONHA, CONA2, CONH- (CH2) o-Ar,
CONH- ( CHz ) o-Het , CONH- ( CHZ ) o-R5, CONH- ( CH2 ) o-CH (Arl ) -
Ar2, CONH- (CH2) o-CH (A) -Ph, CONH- (CHZ) o-Ar' -NH-CO-Ar,
CONH-Ar'-Het or CHA-CONHZ,
R11 i s -NH- ( C=NH ) -NH2, -NH- ( C=NH ) -NHA, -NH- ( C=NH ) -NA2,
-NA- ( C=NH ) -NHZ, -NA- ( C=NH ) -NHA er -NA- ( C=NH ) -NAZ,
Ar' is phenylene, cycloalkylene or biphenylene, which is
unsubstituted or mono- or disubstituted by A, OH, OA,
Hal, CN, NH2, NHA, NA2, N02, CF3, CO-A, S02NH2, S02NAH,
S02NA2,
Ar is phenyl, cycloalkyl, naphthyl, cyclohex-1-enyl,
biphenyl, bicyclohexyl, 4-cyclohexyl-phenyl,
benzo[1,3]dioxol-5-yl, or indanyl, which is
unsubstituted or mono-, di- or trisubstituted by
A, OH, OA, 0-Ph, O-CH2-Ph, O-Ph-CH3, 0 (cycloalkyl) ,
Hal, CN, NH2, NHA, NA2, NH-C (0) A, (CHZ) n-NH2, (CHZ) n-
NHA, (CH2)n-NA2, N02, CF3, C(0)A, SOZ-Ph, SOzNHz,
SOZNAH, SOzNA2 or SOZNA-Ph,
Arl and Ar2
are each independently phenyl,
Het is a saturated, partially or complete ly unsatura-
ted mono- or bicyclic heterocyclic ra dical having
5 to 10 ring members, where 1 or 2 N and/or 1
or
2 S or O atoms can be present and the heterocyclic
radical can be mono- or disubstituted by CN, Hal,
OH, OA, CF3, A, NO2, C0, CO-A or R5,
Hetl is an unsaturated mono- or bicyclic heterocyclic
radical having 5 to 10 ring members, where 1 or
2 N and/or 1 or 2 S or 0 atoms can be present
and/or can be mono- or disubstituted by Hal, OH,
OA,
A is unbranched or branched alkyl h aving 1-6
C
atoms,
Hal is F, C1, Br or I,
X,
X1,X2 in each case independently of one another are
alkylene having 1 to 12 C atoms,
Y is 0, S or NH
i is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11
or 12,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99108560
- 3 -
m is 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12,
n,o in each case independently of one another are 0,
l, 2, 3 or 4,
s is 1, 2, 3 or 9,
t is 0, 1 or 2,
a is 1 or 2,
where if Rz is Ar or H, R1 is not
H
~C~
-N~C~ (CH2)1-5
Hi
and their pharmaceutically tolerable salts and
solvates.
Similar compounds having a benzo[de]iso-
quinoline-1,3-dione parent structure as dyes are
disclosed in US 4,200,752, FR 2 271 216 A and Chemical
Abstracts, Vol. 111, No. 20, November 1989.
US 3,821,383 describes benzo[de]isoquinoline-
acetic acid derivatives as active compounds in a
pharmaceutical preparation against diabetes mellitus.
Similar benzo[de]isoquinolines are also disclosed in
EP 0 243 841 A and DE 37 07 652 A, which carry
alkylamino groups mainly in the 2-position. These
derivatives are suitable for the treatment of solid
tumours and certain forms of leukemia, as well as for
the control of viral disorders. The benzo[de]iso-
quinoline derivatives from US 5,235,045 are mainly
present alkylated or alternatively fluoroalkylated in
the 2-position and cause a chemical change to the lipid
membrane of viruses or other target cells.
The invention is based on the object of finding
novel compounds having valuable properties, in parti
cular those which can be used for the production of
medicaments.
It has been found that the compounds of the
formula I and their salts or solvates have very
valuable pharmacological properties together with good
tolerability. They act especially as GPIbIX inhibitors,
in particular inhibiting the interaction of this
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 4 -
receptor with the ligand von Willebrand factor (vWF).
This action can be demonstrated, for example, by a
method which is described by S. Meyer et al. in
J. Biol. Chem. 1993, 268, 20555-20562. The GPIbIX
alpha-thrombin receptor (N. J. Greco, Biochemistry 1996,
35, 915-921) can also be blocked by the compounds
mentioned.
The significance of GPIbIX as an adhesion receptor on
platelets, which mediates the primary interaction of
platelets with an arteriosclerotically modified
vascular wall via binding to the vWF expressed there,
has been described by many authors (e. g. Z.M. Ruggeri
in Thromb. Hemost. 1997, 78, 611-616). The activation
of another platelet adhesion receptor, GPIIbIIIa,
following the GPIbIX-vWF interaction, leads to platelet
aggregation and thus to thrombotic vascular occlusion.
A GPIbIX antagonist can thus prevent the start
of thrombus formation and thus also release of active
substances from the platelets which, for example,
promote thrombus growth and have an additional trophic
action on the vascular wall. This has been shown with
inhibitory peptides or antibodies in various experi-
mental models (e. g. H Yamamoto et al., Thromb. Hemost.
1998, 79, 202-210).
In the case of higher shear forces, the block-
ing action of GPIbIX inhibitors exerts its maximum
effect, as described by J.J. Sixma et al. in
Arteriosclerosis, Thrombosis, and Vascular Biology
1996, 16, 64-71. According to the flow chamber method
used there, the compounds of the formula I can be
characterized as GPIbIX inhibitors in whole blood.
The inhibition of thrombus formation of the
GPIbIX inhibitors can be measured by a modified Born
method (Nature 1962, Q832, 927-929) using botrocetin or
ristocetin as an aggregation stimulant.
The compounds of the formula I according to the
invention can therefore be employed as pharmaceutical
active compounds in human and veterinary medicine. They
act as adhesion receptor antagonists, in particular as
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 5 -
glycoprotein IbIX antagonists, and are suitable for the
prophylaxis and/or therapy of thrombotic disorders and
sequelae deriving therefrom. The preferentially best
action is to be expected in the case of thrombotic
disorders in the arterial vascular system, but GPIbIX
inhibitors also have an effect in the case of
thrombotic disorders in the venous vascular bed. The
disorders are acute coronary syndromes, angina
pectoris, myocardial infarct, peripheral circulatory
disorders, stroke, transient ischaemic attacks,
arteriosclerosis, reocclusion/restenosis after angio-
plasty/stent implantation. The compounds can further-
more be employed as anti-adhesive substances where the
body comes into contact with foreign surfaces such as
implants, catheters or cardiac pacemakers.
Comparison medication introduced onto the
market which may be mentioned are aspirin and GPIIbIIIa
antagonists.
The invention relates to the compounds of the
formula I and their salts or solvates, and to a process
for the preparation of these compounds and their salts
or solvates, characterized in that
a) a compound of the formula I is liberated from one
of its functional derivatives by treating with a
solvolysing or hydrogenolysing agent,
or
b) a compound of the formula II
R9
i
w ~ II
OwOiwO
in which
R9 is C1, Br, N02 or R1, and
R1 has the meaning indicated in Claim 1
is reacted with a compound of the formula III
H2N-(CHR) i RZ III
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 6 -
in which R, RZ and i have the meanings indicated in
Claim 1,
and, if necessary, the radical R9 is converted into a
radical R1,
or
(c) a compound of the formula IV
R9
IV
O NCO
I
H
in which
R9 is C1, Br, NOZ or R1, where
R1 has the meaning indicated in Claim 1
is reacted with a compound of the formula V
L (CHR)~ RZ V
in which L is C1, Br, or I, OH or a reactive esterified
OH group and R, R2 and i have the meanings indicated in
Claim 1
and, if appropriate, the radical R9 is converted into a
radical Rl,
or
(d) a radical R and/or R2 and/or R9 is converted into
another radical R and/or R2 and/or Rg by, for
example
- converting an amino group into a guanidino group
by reaction with an amidinating agent,
- reacting an aryl bromide or iodide to give the
corresponding coupling products by means of a
Suzuki coupling with boronic acids,
- reducing a nitro group, sulfonyl group or
sulfoxyl group,
- etherifying an OH group or subjecting an OA
group to ether cleavage,
- alkylating a primary or secondary amino group,
- partially or completely hydrolysing a CN group,
- cleaving an ester group or esterifying a
carboxylic acid radical,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
-
- or carrying out a nucleophilic or electrophilic
substitution,
and/or
(e) a base or acid of the formula I is converted intc
one of its salts or solvates.
The compounds of the formula I can have a
chiral centre and therefore occur in a number of
stereoisomeric forms. All these forms (e.g. R and S
forms) and their mixtures (e.g. the RS forms) are
included in the formula I.
The compounds according to the invention also include
so-called prodrug derivatives, i.e. compounds of the
formula I modified with, for example, alkyl or acyl
groups, sugars or oligopeptides and which are rapidly
cleaved in the, body to give the active compounds
according to the invention.
Furthermore, free amino groups as substituents of
compounds of the formula I can be provided with appro-
priate conventional protective groups.
Solvates of the compounds of the formula I are
understood as meaning adducts of inert solvent
molecules to the compounds of the formula I which are
formed on account of their mutual power of attraction.
Solvates are, for example, mono- or dehydrates or
alcoholates.
The abbreviations used have the following
meanings:
BOC tert-butoxycarbonyl
CBZ benzyloxycarbonyl
DCC dicyclohexylcarbodiimide
DMF dimethylformamide
Et ethyl
Fmoc fluorenylmethoxycarbonyl
Me methyl
Mtr 4-methoxy-2,3,6-trimethylphenylsulfonyl
OBut tert-butyl ester
OMe methyl ester
OEt ethyl ester
POA phenoxyacetyl
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ g -
Ph phenyl
TFA trifluoroacetic acid
In the above formulae, A is alkyl and has 1 to
6, preferably 1, 2, 3 or 4 C atoms. Alkyl is preferably
methyl, furthermore ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl or tert-butyl, additionally also
pentyl, 1-, 2- or 3-methylbutyl, 1,1-, 1,2- or
2,2-dimethylpropyl, 1-ethylpropyl, hexyl, 1-, 2-, 3- or
4-methylpentyl, 1,1-, 1,2-, 1,3-, 2,2-, 2,3- or
3,3-dimethylbutyl, 1- or 2-ethylbutyl, 1-ethyl-
1-methylpropyl, 1-ethyl-2-methylpropyl, 1,1,2- or
1,2,2-trimethylpropyl,.
Ar is preferentially phenyl, preferably
- as indicated - monosubstituted phenyl, specifically
preferentially phenyl, o-, m- or p-methylphenyl, o-, m
or p-ethylphenyl, o-, m- or p-propylphenyl, o-, m- or
p-isopropylphenyl, o-, m- or p-tert-butylphenyl, o-, m-
or p-aminophenyl, o-, m- or
p-(N,N-dimethylamino)phenyl, o-, m- or
p-sulfonamoylphenyl, o-, m- or p-nitrophenyl, o-, m- or
p-hydroxyphenyl, o-, m- or p-methoxyphenyl, o-, m- or
p-ethoxyphenyl, o-, m- or p-phenoxyphenyl, o-, m- or
p-(phenylmethox)yphenyl, o-, m- or p-
(trifluoromethyl)phenyl, o-, m- or
p-(trifluoromethoxy)phenyl, o-, m- or p-fluorophenyl,
o-, m- or p-chlorophenyl, o-, m- or p-bromophenyl, o-,
m- or p-iodophenyl, 9-benzenesulfonyl-phenyl, 4-(4-
chloro-phenoxy)-phenyl, furthermore preferentially
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dimethylphenyl,
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dimethoxyphenyl,
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dihydroxyphenyl,
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-difluorophenyl,
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dichlorophenyl,
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dibromophenyl,
2-chloro-3-methyl-, 2-chloro-4-methyl-, 2-chloro-5-
methyl-, 2-chloro-6-methyl-, 3-chloro-2-methyl-, 4-
chloro-2-methyl-, 5-chloro-2-methyl-, 3-chloro-4-
methyl-, 3-chloro-5-methyl-, 4-chloro-3-methylphenyl,
2-bromo-3-methyl-, 2-bromo-4-methyl-, 2-bromo-5-
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- g _
methyl-, 2-bromo-6-methyl-, 3-bromo-2-methyl-, 4-bromo-
2-methyl-, 5-bromo-2-methyl-, 3-bromo-4-methyl-,
3-bromo-5-methyl-, 4-bromo-3-methylphenyl, 2-iodo-3-
methyl-, 2-iodo-4-methyl-, 2-iodo-5-methyl-, 2-iodo-6-
methyl-, 3-iodo-2-methyl-, 4-iodo-2-methyl-, 5-iodo-
2-methyl-, 3-iodo-4-methyl-, 3-iodo-5-methyl-, 4-iodo-
3-methylphenyl, 2-chloro-3-methoxy-, 2-chloro-4-
methoxy-, 2-chloro-5-methoxy-, 2-chloro-6-methoxy-, 3-
chloro-2-methoxy-, 4-chloro-2-methoxy-, 5-
chloro-2-methoxy-, 3-chloro-4-methoxy-, 3-chloro-5-
methoxy-, 4-chloro-3-methoxyphenyl, 2-chloro-3-
hydroxy-, 2-chloro-4-hydroxy-, 2-chloro-5-hydroxy-,
2-chloro-6-hydroxy-, 3-chloro-2-hydroxy-, 4-
chloro-2-hydroxy-, 5-chloro-2-hydroxy-, 3-chloro-4-
hydroxy-, 3-chloro-5-hydroxy-, 4-chloro-3-hydroxy-
phenyl, 3-fluoro-4-methoxy, 4-fluoro-3-methoxyphenyl,
2-chloro-3-fluoro-, 2-chloro-4-fluoro-, 2-chloro-5-
fluoro-, 2-chloro-6-fluoro-, 3-chloro-2-fluoro-, 4-
chloro-2-fluoro-, 5-chloro-2-fluoro-, 3-chloro-4-
fluoro-, 3-chloro-5-fluoro-, 4-chloro-3-fluorophenyl,
2-fluoro-3-methyl-, 2-fluoro-4-methyl-, 2-fluoro-5-
methyl-, 2-fluoro-6-methyl-, 3-fluoro-2-methyl-, 4-
fluoro-2-methyl-, 5-fluoro-2-methyl-, 3-fluoro-9-
methyl-, 3-fluoro-5-methyl-, 4-fluoro-3-methylphenyl,
2,5- or 3,4-dimethoxyphenyl, 3-diethylaminomethyl-4-
hydroxy-phenyl, 1-dimethylamino-2-(toluene-4-sulfonyl)-
phenyl-4-yl, N-ethyl-2-methyl-N-phenyl-5-yl-
benzenesulfonamoyl, 3-cyclopentyloxy-4-methoxy-phenyl,
3,4-bis-benzyloxy-phenyl, 2-cyano-4,5-dimethoxyphenyl,
5-chloro-2,4-dimethoxy-phenyl, 2-cyano-3,9-
dimethoxyphenyl or 3,4,5-trimethoxy-phenyl,
furthermore, however, also preferentially unsubstituted
naphthyl, cyclohex-1-enyl, benzo[1,3]-dioxol-5-yl, 4-
cyclohexyl-phenyl, bicyclohexyl or indanyl.
Furthermore, however, Ar is also preferentially
unsubstituted biphenyl - as indicated - or
alternatively monosubstituted biphenyl, specifically
preferentially biphenyl-4-yl or biphenyl-3-yl,
2'-methylbiphenyl-4-yl, 3'-methylbiphenyl-4-yl,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 10 -
4'-methylbiphenyl-4-yl, 2'-methylbiphenyl-3-yl,
3'-methylbiphenyl-3-yl, 4'-methylbiphenyl-3-yl,
2-methylbiphenyl-4-yl, 3-methylbiphenyl-4-yl,
2-methylbiphenyl-3-yl, 4-methylbiphenyl-3-yl,
2'-tert-
butylbiphenyl-4-yl, 3'-tert-butylbiphenyl-4-yl,
4'-tert-butylbiphenyl-4-yl, 2'-tert-butylbiphenyl-3-yl,
3'-tert-butylbiphenyl-3-yl, 4'-tert-butylbiphenyl-3-yl,
2-tert-butylbiphenyl-4-yl, 3-tert-butylbiphenyl-4-yl,
2-tertbutylbiphenyl-3-yl, 4-tert-butylbiphenyl-3-yl,
2'-isopropylbiphenyl-4-yl, 3'-isopropylbiphenyl-4-yl,
4'-isopropylbiphenyl-4-yl, 2'-isopropylbiphenyl-3-yl,
3'-isopropylbiphenyl-3-yl, 4'-isopropylbiphenyl-3-yl,
2-isopropylbiphenyl-4-yl, 3-isopropylbiphenyl-4-yl,
2-isopropylbiphenyl), 4-isopropylbiphenyl-3-yl,
2'-fluorobiphenyl-4-yl, 3'-fluorobiphenyl-4-yl,
9'-fluorobiphenyl-4-yl, 2'-fluorobiphenyl-3-yl,
3'-fluorobiphenyl-3-yl, 4'-fluorobiphenyl-3-yl,
2-fluorobiphenyl-4-yl, 3-fluorobiphenyl-4-yl,
2-fluorobiphenyl-3-yl, 4-fluorobiphenyl-3-yl,
2'-methoxybiphenyl-4-yl, 3'-methoxybiphenyl-4-yl,
4'-methoxybiphenyl-4-yl, 2'-methoxybiphenyl-3-yl,
3'-methoxybiphenyl-3-yl, '-methoxybiphenyl-3-yl, 2-
4
methoxybiphenyl-4-yl, 3-methoxybiphenyl-4-yl,
2-methoxybiphenyl-3-yl, 4-methoxybiphenyl-3-yl,
2'-nitrobiphenyl-4-yl, 3'-nitrobiphenyl-4-yl,
4'-nitrobiphenyl-4-yl, 2'-nitrobiphenyl-3-yl,
3'-nitrobiphenyl-3-yl, 4'-nitrobiphenyl-3-yl,
2-nitrobiphenyl-4-yl, 3-nitrobiphenyl-9-yl,
2-nitro-
biphenyl-3-yl, 4-nitrobiphenyl-3-yl,
2'-trifluoromethylbiphenyl- 9-yl,
3'-trifluoromethylbiphenyl- 4-yl, 4'-trifluoromethyl-
biphenyl-4-yl, 2'-trifluoromethylbiphenyl-3-yl,
3'-trifluoromethylbiphenyl- 3-yl, 4'-trifluoromethyl-
biphenyl-3-yl, 2-trifluoromethylbiphenyl-4-yl,
3-tri-
fluoromethylbiphenyl-4-yl, 2-trifluoromethylbiphenyl-3-
yl, 4-trifluoromethylbiphenyl-3-yl,
2'-trifluoromethoxybiphenyl -4-yl, 3'-trifluoromethoxy-
biphenyl-4-yl, 4'-trifluoromethoxybiphenyl-4-yl,
2'-trifluoromethoxybiphenyl -3-yl,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 11 -
3'-trifluoromethoxybiphenyl-3-yl, 4'-tri-
fluoromethoxybiphenyl-3-yl, 2-trifluoromethoxybiphenyl-
4-yl, 3-trifluoromethoxybiphenyl-4-yl,
2-trifluoromethoxybiphenyl-3-yl,
4-trifluoromethoxybiphenyl-3-yl, furthermore
preferentially disubstituted biphenyls, such as
2'-methyl-3'-nitrobiphenyl-4-yl, 2'-methyl-4'-nitro-
biphenyl-4-yl, 2'-methyl-5'-nitrobiphenyl-4-yl, 2'-
methyl-6'-nitrobiphenyl-4-yl, 3'-methyl-2'-
nitrobiphenyl-4-yl, 3'-methyl-4'-nitrobiphenyl-4-yl,
3'-methyl-5'-nitrobiphenyl-9-yl, 3'-methyl-6'-
nitrobiphenyl-4-yl, 4'-methyl-2'-nitrobiphenyl-4-yl,
4'-methyl-3'-nitrobiphenyl-4-yl, 2'-methyl-3'-
nitrobiphenyl-3-yl, 2'-methyl-4'-nitrobiphenyl-3-yl,
2'-methyl-5'-nitrobiphenyl-3-yl, 2'-methyl-6'-nitro-
biphenyl-3-yl, 3'-methyl-2'-nitrobiphenyl-3-yl,
3'-methyl-9'-nitrobiphenyl-3-yl, 3'-methyl-5'-
nitrobiphenyl-3-yl, 3'-methyl-6'-nitrobiphenyl-3-yl,
4'-methyl-2'-nitrobiphenyl-3-yl, 4'-methyl-3'-
nitrobiphenyl-3-yl, 2'-methoxy-2-methylbiphenyl-4-yl,
3'-methoxy-2-methylbiphenyl-4-yl, 4'-methoxy-2-
methylbiphenyl-4-yl, 4'-methoxy-3-nitrobiphenyl-4-yl,
2'-chloro-3'-fluorobiphenyl-4-yl, 2'-chloro-4'-
fluorobiphenyl-4-yl, 2'-chloro-5'-fluorobiphenyl-9-yl,
2'-chloro-6'-fluorobiphenyl-4-yl, 3'-chloro-2'-
fluorobiphenyl-4-yl, 3'-chloro-4'-fluorobiphenyl-4-yl,
3'-chloro-5'-fluorobiphenyl-4-yl, 3'-chloro-6'-
fluorobiphenyl-4-yl, 4'-chloro-2'-fluorobiphenyl-4-yl,
4'-chloro-3'-fluorobiphenyl-4-yl, 2'-chloro-3'-
fluorobiphenyl-3-yl, 2'-chloro-4'-fluorobiphenyl-3-yl,
2'-chloro-5'-fluorobiphenyl-3-yl, 2'-chloro-6'-
fluorobiphenyl-3-yl, 3'-chloro-2'-fluorobiphenyl-3-yl,
3'-chloro-4'-fluorobiphenyl-3-yl, 3'-chloro-5'-
fluorobiphenyl-3-yl, 3'-chloro-6'-fluorobiphenyl-3-yl,
9'-chloro-2'-fluorobiphenyl-3-yl, 4'-chloro-3'-
fluorobiphenyl-3-yl, (2',3'-dimethoxy)biphenyl-4-yl,
2',4'-dimethoxy)biphenyl-4-yl,
(2',5'-dimethoxy)biphenyl-4-yl, (2',6'-dimethoxy)-
biphenyl-4-yl, (3',4'-dimethoxy)biphenyl-9-yl,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/085b0
- 12 -
(3',5'-dimethoxy)biphenyl-4-yl, (2',3'-dimethoxy)-
biphenyl-3-yl, (2',4'-dimethoxy)biphenyl-3-yl,
(2',5'-dimethoxy)biphenyl-3-yl, (2',6'-dimethoxy)-
biphenyl-3-yl, (3',4'-dimethoxy)biphenyl)-3-yl,
(3',5'-dimethoxy)biphenyl-3-yl,
(2',3'-di(trifluoromethyl))biphenyl-4-yl,
(2',4'-di(trifluoromethyl))biphenyl-4-yl,
(2',5'-di(trifluoromethyl))biphenyl-4-yl,
(2',6'-di(trifluoromethyl))biphenyl-4-yl,
(3',4'-di(trifluoromethyl))biphenyl-4-yl, (3',5'-
di(trifluoromethyl))biphenyl-4-yl,
(2',3'-di(trifluoromethyl))biphenyl-3-yl,
(2',4'-di(trifluoromethyl))biphenyl-3-yl, (2',5'-
di(trifluoromethyl))biphenyl-3-yl,
(2',6'-di(trifluoromethyl))biphenyl-3-yl,
(3',4'-di(trifluoromethyl))biphenyl-3-yl, (3',5'-
di(trifluoromethyl)biphenyl-3-yl,
(2,2'-dimethyl)biphenyl-4-yl, (2,'3-dimethyl)biphenyl-
4-yl, (2,4'-dimethyl)biphenyl-4-yl,
(2,2'-dimethyl)biphenyl-3-yl, (2,3'-dimethyl)biphenyl-
3-yl or (2,4'-dimethyl)biphenyl-3-yl.
Furthermore, Ar is preferably cycloalkyl,
particularly preferred cyclohexyl or cyclopentyl.
Arl and Ar2 are each independently phenyl.
Ar' preferentially is phenylene, cyclohexylene
or biphenylene, which is unsubstituted or
monosubstituted by Hal or CN.
In -Ar' - (CH2) n_R5, Ar' is preferentially
unsubstituted or substituted phenylene or
cycloalkylene, where RS is preferentially an amino,
alkylamino or dialkylamino group and where n can be 0,
1, 2, 3 or 4.
CH2NH2 CH2NH2
CH2NH2
or
is particularly preferred for -Ar' - (CH2) n-R5.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 13 -
In -Ar' - (CHZ) n-R8, Ar' is preferably
unsubstituted or substituted phenylene or
cycloalkylene, where R8 is preferentially an amido, an
alkylamido or dialkylamido group or -CONH-(CH2)o-Ar,
-CONH- ( CH2 ) o-Het , -CONH- ( CH2 ) a-CH (Arl ) -Ar2, -CONH- ( CH2 ) o-
CH (A) -Ph or -CONH- (CHZ) o-R5 and where n is 0, 1, 2, 3 or
4.
CONH2 CONH2
CONH2 ,
CONH(C3H~) , CONH(C3H~)
CONH-(CH2)2-C(CH3)s
,
CONH(C5H~~) or
CONH(C5H»)
is particularly preferred for -Ar' - (CHZ) n-R8.
CONH-CH2
CONH
CONH (CH2)z
CONH
CONH-CH2
CONH-(CH2)2
,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 14 -
CONH-(CH2)3
CONH-(CH2)a
CONH
Br
r
CONH
CN
CONH-(CH2)2 ~ ~ N02
,
CONH-CH2
N02
r
- CH
CONH-CH2 ~ ~ N~ 3
CH3
r
- CH
CONH ~ ~ N~ CH r
3
~ C2H5
CONH ~ ~ N~
C2Hs
CONH-CH2 ~ ~ S02-NHZ
CONH-(CH2)2 ~ ~ CH3
CONH ~ ~ CH3
CH3
CONH
CH3
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 15 -
CONH-CHZ
CF3
CONH
CF3
,
CF3
CONH
CF3
C2H5 ,
CONH
.
CONH ~ \ CH
3
CONH
CH3
CONH CH3
CH3
.
CONH-(CHz)2
CI
CONH
CI
CONH-CH2
l0 CI
CONH-(CH2)2
CI
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 16 -
CONH ~ ~ CI
,
CONH-(CH2)2 ~ ~ CI
CONH CH2 ~ ~ CI
C!
CONH-CH2
CI
CI CI
CONH-CH2
,
CI
CONH-CH2 ~ ~ CI
CI
CONH-(CH2)2 ~ ~ CI
Ci
CONH-CH2
CI
CI
CONH
CI
CI
CONH ~ ~ CI
,
CONH-CH2 ~ ~ F
CI
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 17 -
/ CONH / ~ F
CI
/ CONH-CH2 / ~ F
CI
.
CONH-(CH2)2 / ~ F
/
/ CONH-CHZ
F
/ CONH
F,
CON
/ ~ ~._/
F
.
CONH-(CH2)z
/
OCH3
/ CON / ~
OCH3
,
/ CONH-CH2 /
OCH3
/ CONH / ~ OCH3
,
/ CONH-(CH2)2 / ~ OCH3
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 18 -
CONH-CH2 ~ ~ OCF3
F
CONH ~ ~ OCH3
F
CONH ~ ~ H3
CF3
CONH
OCH3
tert-butyl
CONH
tert-butyl
CH3
CONH-(CH2)2
OCH3
OC
CONH
OC
H3
H3
OCH3
CONH-(CHZ)2 ~ ~ OCH3
OPh
CONH
CONH ~ ~ OPh
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 19 -
CONH ~ ~ O ~ ~ CH3
CONH
O
~--Ph
CONH
CONH-CHz
CONH /
,
O\
CONH-CH2 ~ ~ O
CONH ~ ~ OCH3
CI
CONH ~ ~ CI
CF3
.
CONH-(CH2)2
CONH-CH2
CONH-(CH2)z
CI
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 20 -
CONH-CH2 ~ ~ C!
.
CONH ~ ~ CI
CI
CONH-CH2
CI
CI CI
CONH-CH2
CONH-(CHZ)2 ~ ~ F
,
CONH-(CHz)Z ~ ~ OCH3
C2H5
CONH
CONH
CONH ~ ~ OPh
CONH CH2
CH3
CONH ~ ~ (~CH
3
CH3
CONH-CH2 ~ ~ f~CH
3
CONH-CH2
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99108560
- 21 -
O\
CONH-CH2 ~ ~ ,O
CONH- ~
or
CONH-(CH2)s
are furthermore particularly preferred for
-Ar' - ( CH2 ) n-R8 where R8 is -CONH- ( CHZ ) o-Ar .
CONH-CH2
-N
CONH
-N
,
CONH-CH2 ~ ~N
CONH ~ ~N
N
CONH-(CH2)2
CONH-(CH2)2-N
CONH-(CH2)2-N O
O
CONH-(CH2)3-N
,
CONH-(CH2)2-N'
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 22 -
CONH-(CH2)3 -N
=N
S
CONH-CHz
.
O
CONH-CH2
CONH-(CH2)2-N O
O
CONH-CH2
CONH-(CH2)3 -N
=N
CONH-CH2 ~ ~N
CONH ~ ~N
CONH
-N
or
CONH-CH2
-N
is particularly preferred for -Ar' - (CHZ) "-R8 where R$ is
-CONH- ( CH2 ) o-Het .
~I
CONH-(CH2)2-CH /
\I
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 23 -
CONH-(CH2)2-CH /
or
CONH-CH2-CH
is particularly preferred for -Ar'-(CH2)n-Re where Re is
-CONH- ( CH2 ) o-CH (Arl ) -Ar2 .
,CH3
CONH-CH2-CH /
is particularly preferred for -Ar' - (CH2) n-R8 where RB is
-CONH- (CH2) o-CH (A) -Ph.
Furthermore,
CONH-(CHZ)4 -NHZ ,
CONH (CH2)a - NH2
CONH-(CH2)2-NH2
or / \ CONH (CH2)2 -NH2
is particularly preferred for -Ar' - (CH2) n-R8 where R8 is
-CONH- ( CHZ ) o-RS .
Cycloalkyl having 3 to 8 carbon atoms is
preferentially cyclobutyl, cyclopentyl, cyclohexyl or
cycloheptyl; particularly preferred cyclohexyl or
cyclopentyl.
Cycloalkylene having 3 to 8 carbon atoms is
preferentially cyclobutylene, cyclopentylene,
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 24 -
cyclohexylene, or cycloheptylene; particularly
preferred cyclohexylene.
0(cycloalkyl) having 3 to 8 carbon atoms is
preferentially cyclobutyloxy, cyclopentyloxy,
cyclohexyloxy or cycloheptyloxy; particularly preferred
cyclopentyloxy.
Hal is preferably F, C1, Br or iodine.
Hetl is preferentially substituted or
unsubstituted 2-, 3-, or 4-pyridyl, 2- or
3-benzo[b]thiophenyl, 1H-indol-2-yl, 1H-indol-3-yl, 4-,
5-, 6-, 7-fluoro-1H-indol-2-yl, 4-, 5-, 6-, 7-fluoro-
1H-indol-3-yl, 4-, 5-, 6-, 7-methyl-1H-indo-2-yl, 4-,
5-, 6-, 7-methyl-IH-indol-3-yl, 4-, 5-, 6-, methoxy-1H-
indol-2-yl or 4-, 5-, 6-, 7-methoxy-1H-indol-3-yl,
4-Pyridyl, 3-benzo[b]thiophenyl, 1H-indol-3-yl, 5-
fluoro-1H-indol-3-yl, 5-methyl-1H-indol-3-yl, 5- or 6-
methoxy-1H-indol-3-yl are furthermore particularly
preferred.
Het is preferably substituted or unsubstituted
2- or 3-furyl, 2- or 3-thienyl, 2-chloro-thienyl-5-yl,
2-acetyl-thienyl-5-yl, 1-, 2- or 3-pyrrolyl, 1-, 2-, 4
or 5-imidazolyl, 1-, 3-, 9- or 5-pyrazolyl, 2-, 4- or
5-oxazolyl, 3-, 4- or 5-isoxazolyl, 2-, 4- or
5-thiazolyl, 3-, 4- or 5-isothiazolyl, 2-, 3- or
4-pyridyl, 2-, 4-, 5- or 6-pyrimidinyl, furthermore
preferably 1,2,3-triazol-1-, -4- or -5-yl,
1,2,9-triazol-1-, -4- or -5-yl, 1- or 5-tetrazolyl,
1,2,3-oxadiazol-4- or -5-yl, 1,2,4-oxadiazol-3- or
-5-yl, 1,3,4-thiadiazol-2- or -5-yl,
1,2,4-thiadiazol-3- or -5-yl, 1,2,3-thiadiazol-9- or
-5-yl, 2-, 3-, 4-, 5 - or 6-2H-thiopyranyl, 2-, 3- or
4-4H-thiopyranyl, 3- or 9-pyridazinyl, pyrazinyl, 2-,
3-, 4-, 5-, 6- or 7-benzofuryl, 2-, 3-, 4-, 5-, 6- or
7-benzothienyl, 1-, 2-, 3-, 4-, 5-, 6- or 7-1H-indolyl,
1-, 2-, 4- or 5-benzimidazolyl, 1-, 3-, 4-, 5-, 6- or
7-benzopyrazolyl, 2-, 4-, 5-, 6- or 7-benzoxazolyl, 3-,
4-, 5-, 6- or 7-benzisoxazolyl, 2-, 4-, 5-, 6- or
7-benzothiazolyl, 2-, 4-, 5-, 6- or
7-benzisothiazolyl, 4-, 5-, 6- or 7-benz-2,1,3-
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 25 -
oxadiazolyl, 1-, 2-, 3-, 4-, 5-, 6-, 7- or
8-quinolinyl, 1-, 3-, 4-, 5-, 6-, 7- or
8-isoquinolinyl, 1-, 2-, 3-, 4- or 9-carbazolyl, 1-,
2-, 3-, 4-, 5-, 6-, 7-, 8- or 9-acridinyl, 3-, 4-, 5-,
6-, 7- or 8-cinnolinyl, 2-, 4-, 5-, 6-, 7- or
8-quinazolinyl, 1,1-dioxo-1H-1~,6-benzo[b]thiophen-5-yl
or 2,~-dioxo-2,3-dihydro-1H-2~,6-benzo[c]thiophen-5-yl.
The heterocyclic radicals can also be partially or
completely hydrogenated. Het can thus also be
2,3-dihydro-2-, -3-, -4- or -5-furyl, 2,5-dihydro-2-,
-3-, -4- or -5-furyl, tetrahydro-2- or -3-furyl,
1,3-dioxolan-4-yl, tetrahydro-2- or -3-thienyl,
2,3-dihydro-1-, -2-, -3-, -4- or -5-pyrrolyl,
2, 5-dihydro-1-, -2-, -3-, -4- or -5-pyrrolyl, 1-, 2- or
3-pyrrolidinyl, pyrrolidine-2-on-1-yl, tetrahydro-1-,
-2- or -3-pyrrolyl, tetrahydro-1-, -2- or 4-imidazolyl,
2, 3-dihydro-1-, -2-, -3-, -4-, -5-, -6-, -7-1H-indolyl,
2,3-dihydro-1-, -2-, -3-, -4- or -5-pyrazolyl,
tetrahydro-1-, -3- or -4-pyrazolyl, 1,4-dihydro-1-,
-2-, -3- or -4-pyridyl, 1,2,3,4-tetrahydro-1-, -2-,
-3-, -4-, -5- or -6-pyridyl, 1,2,3,6-tetrahydro-1-,
-2-, -3-, -4-, -5- or -6-pyridyl, 1-, 2-, 3- or
4-piperidinyl, 1-, 2-, 3- or 4-azepanyl, 2-, 3- or
4-morpholinyl, tetrahydro-2-, -3- or -4-pyranyl,
1,4-dioxanyl, 1,3-dioxan-2-, -4- or -5-yl, hexahydro-
1-, -3- or -4-pyridazinyl, hexahydro-1-, -2-, -4- or
-5-pyrimidinyl, 1-, 2- or 3-piperazinyl,
1,2,3,4-tetrahydro-1-, -2-, -3-, -4-, -5-, -6-, -7- or
-8-quinolinyl, 1,2,3,4-tetrahydro-1-, -2-, -3-, -4-,
-5-, -6-, -7- or -8-isoquinolinyl.
2-, 3- or 4-pyridyl, 1-imidazolyl,
2-methyl-1-imidazolyl, 2-pyrimidinyl, 5-fluoro-1H-
indol-2-yl, 2, 3-dihydro-1-, -2-, -3-, -4-, -5-,
-6-, -7-1H-indolyl, 1-quinolinyl, 1-isoquinolinyl,
1,2,3,4-tetrahydroisoquinoline-1-yl, tetrahydro-
1-pyrrolyl, 1-piperidinyl, 2,6-tetramethyl-
piperidine-4-yl, 1-azepanyl, 4-morpholinyl, 1-
piperazinyl, 4-methyl-piperazin-1-yl, 4-phenyl-
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 26 -
piperazin-1-yl or 4-phenylmethylpiperazin-1-yl is
particularly preferred.
In -Het-(CHz)"-Ar, Het and Ar have one of the
preferred meanings indicated above, where Het is
preferably piperidine-1,4-diyl or piperazine-1,4-diyl
and n can be 0, 1, 2, 3, or 4.
01
-NON-CH2 ~ ~ O
U
or -NON-CH2
is particularly preferred for -Het-(CHZ)n-Ar.
In -Het-Het, Het has one of the preferred
meanings indicated above, where in -Het-Het the first
heterocycle is preferably piperidine-1,4-diyl or
piperazine-1,4-diyl.
N-
- NON --~~
U N
~--\ N -
or - N N
U
is particularly preferred for -Het-Het.
In -Y- (CHz) n-Het- (CHZ) o-R6, Y is preferably O, S
or NH, where Het has one of the preferred meanings
indicated above and R6 is preferentially H, amino or
alkylamino. Furthermore, n and o independently of one
another are preferably 0, 1, 2, 3 or 4.
-NH-(CH2)2-N . -NH-(CH2)3-N N-CH3 ,
2 0 ~ ~--/
N~ N-
-NH-(CH2)2 \ / . -NH-CH2 \ / '
-NH-(CH2)2 \ > , -NH-(CH2)3-N~N
N
-NH-(CH2)3- ~ -(CH2)3 NHZ
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 27 _
or _ NH N
are particularly preferred for -Y- (CH2) n-Het (CH2) o-R6.
Furthermore, in -Y- (CH2) n-Ar' - (CHZ) o-R6, Y is
preferentially 0, S, or NH, where Ar' has a preferred
meaning indicated beforehand and R6 is preferably H,
amino or alkylamino and n and o independently of one
another are 0, 1, 2, 3 or 4.
-NH~ , -NH , -NH
- NH-(CH2)2 ~ ~ OH . - NH-CH2
-NH-CH2 ~ ~ 02-NH2 .
-NH-CH2 ~ ~ NH2 . -NH_(CH2)2 ~ ~ .
_ NH-CHZ ~ ~ CH2- NH2
_NH-CH2 CH2- NH2
CH2- NH2
_ NH-CH2
CH2- NH2
or _ NH-CH2
is particularly preferred for -Y- (CH2) n-Ar' - (CHZ) o-R6.
Furthermore, in -Y- (CHZ) s-Ar' - (CHZ) o-Rll, Y is
preferentially 0, S, or NH, where Ar' has a preferred
meaning indicated beforehand and R11 is preferably -NH
(C=NH) -NH2 and s and o independently of one another are
0, 1, 2, 3 or 4.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 28 -
H
CHZ ~ ~NH2
_ NH-CH2
is particularly preferred for -Y- (CHz) s-Ar' - (CH2) o-R11.
X and/or X1 and/or X2 is alkylene and is
preferably methylene, ethylene, propylene, butylene,
furthermore also pentylene or hexylene.
In -Y- [X-0] t- [X1-0] u-XZ-R5, Y is preferentially
0, S or NH, where X, X1 and X2 have a preferred meaning
indicated beforehand. Furthermore, R5 is preferably
amino, alkylamino or dialkylamino, t is 0, 1 or 2 and a
i s 1 or 2 . -NH- ( CH2 ) 3-0- ( CHZ ) 4-O- ( CH2 ) 3-NHZ i s
particularly preferred for -Y- [X-0] t- [X1-O] "-XZ-R5.
Y is preferentially O, S or NH, particularly
preferentially NH.
R is preferably H, A, or CH2-Ph, where A has a
preferred meaning indicated above. H, sec-butyl or
CHZ-Ph is particularly preferred.
R1 is preferably -Het, -N- [ (CH2) S-OH] 2.
-N- [ ( CH2 ) s-OA ] 2 i -NA- ( CHZ ) s-Ar, -NA- ( CH2 ) m-R5 i
2 0 -Y- ( CH2 ) m-R5 r -Y- ( CH2 ) z-NHA, -Y- ( CH2 ) 2-NA2 i
-Y- ( CH2 ) 2-NH- ( CH2 ) s-OH, -Y- ( CHz ) m-OH, -Y- ( CH2 ) n- ( CHR4 ) -R3.
-Y- ( CH2 ) n-R4 i R4 ~ -Y- ( CHZ ) n-Het- ( CH2 ) o-R6 i
-Y- (CH2) n-Ar' - (CH2) o-R6, -Het- (CHZ) n-Ar, -Het-Het,
-Y- [ X-0 ] t- [ X1-0 ] "-X2-RS or -Y- ( CH2 ) n-NA- ( CHZ ) o-R5. where
2 5 -Het , -Y- ( CH2 ) n-Het- ( CH2 ) o-R6, -Y- ( CH2 ) n-Ar' - ( CH2 ) o-R6,
-Y- ( CH2 ) s-Ar' - ( CH2 ) o-R11, -Het- ( CH2 ) n-Ar, -Het-Het and
-Y- [X-O] t- [X1-O] "-XZ-R5 in particular have the preferred
meanings indicated beforehand. Furthermore, s in
-N- [ (CH2) s-OH] 2 and -N- [ (CH2) S-OA] 2 is preferably 1, 2, 3
30 or 4 and A has the preferred meaning indicated
beforehand. -N-[(CHZ)2-OH]2 is particularly preferred
for -N- [ (CH2) s-OH] 2.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 29 -
In -NA-(CH2)s-Ar, A and Ar have a preferred
meaning indicated beforehand and s is preferably l, 2,
3 or 4.
OH
-N(CH3)-(CH )2 / ~ OH
is particularly preferred for -NA-(CH2)g-Ar.
Furthermore, A in -NA- (CH2) S-RS as a substituent
for R1 has a preferred meaning indicated beforehand,
where R5 is preferably amino, alkylamino or dialkylamino
and s is 1, 2, 3 or 4 . -N (CH3) - (CH2) 3-NH (CH3) and
-N (CH3) - (CH2) 2-N (C2H5) 2 are particularly preferred for
-NA- ( CH2 ) s-RS .
In -Y- (CHZ) m-R5 Y is preferentially 0, S or NH, where RS
is preferably amino, alkylamino or dialkylamino and m
is 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12. -NH- (CHZ) 3-NHZ,
-NH- ( CH2 ) 3-NH ( CH3 ) , -NH- ( CHZ ) 3-N ( CH3 ) 2.
-NH- ( CHZ ) 3-NH [ CH ( CH3 ) 2 ] . -NH- ( CH2 ) 3-NH ( C6Hli ) ,
-NH- ( CHZ ) 4-NH2, -NH- ( CH2 ) 5-NH2, -NH- ( CH2 ) ~-NH2 and
-NH-(CH2)e-NHZ are particularly preferred for
-Y- ( CHZ ) m-R5 .
In -Y- (CH2) Z-NHA and -Y- (CH2) 2-NA2, Y is preferably O, S
or NH, where A has a preferred meaning indicated
beforehand. -NH- (CH2) 2-NH (CZHS) , -NH- (CHZ) 2-NH (C3H~) and
-NH- (CHZ) 2-NH [CH (CH3) 2] are particularly preferred for
-Y- ( CH2 ) 2-NHA .
In -Y- (CH2) 2-NH- (CH2) g-OH, Y is preferably 0, S or NH,
where s is 1, 2, 3 or 4 . -NH- (CH2) 2-NH (CH2) 2-OH is
particularly preferred for -Y- (CHZ) 2-NH- (CHZ) S-OH.
In -Y-(CHZ)m-OH, Y is preferably O, S or NH, where m is
3, 4, 5, 6, 7, 8, 9, 10, 11 or 12. -NH-(CH2)5-OH is
particularly preferred for -Y-(CHZ)m-OH.
In -Y- (CHZ) n- (CHR9) -R3, Y is preferably 0, S or NH, where
R3 is preferably methyl, R4 is preferentially Ar and n
is 0, l, 2, 3 or 4. -NH-(CHPh)-CH3 is particularly
preferred for -Y- (CH2) n- (CHR4) -R3.
In -Y- (CH2) ~-R4, Y is preferentially 0, S or NH, where
in this formula R4 is preferably COOH or CODA, A has a
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 30 -
preferred meaning indicated beforehand and n is 0, 1,
2, 3 or 4. -NH-(CH2)z-COOMe is particularly preferred .
In -Y- (CH2) n_NA- (CH2) o-R5, Y is preferentially 0, S, NH,
where A has a preferred meaning indicated beforehand, R'
is preferably amino, alkylamino or dialkylamino and n
and o in each case independently of one another are 0,
1, 2, 3 or 4 . -NH- (CHZ) 3-N (CH3) - (CH2) 3-NH2 is
particularly preferred for -Y- (CH2) n-NA- (CH2) o-R5.
i is preferentially 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11 or 12, particularly preferentially l, 2, 3, 5,
7, 10 or 11.
Rz is preferably H, OH, OA, COOH, CODA, Ar,
CH (Ph) -Ph, Hetl, R5, R' or Re, where A, Ar and Hetl have
a preferred meaning indicated beforehand.
R3 is preferentially CH3.
R4 is preferably -CH=CH2, Ar, COOA, COOH
p H3C
H H N H H \N
-N-C ~S or -N-C i
'Ar
O O
where Ar and A have a preferred meaning indicated
beforehand.
H3C
H H
~N
-N C i
O ~ \
2 0 H3C CH3
is particularly preferred for
H3C
H H
i i ~N
-N C i
O 'Ar
R5 is preferentially amino, alkylamino,
dialkylamino, NHAr, -NH-(CH2)n-OH or -NH-(CH2)n-OA, where
A and Ar have a preferred meaning indicated beforehand
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 31 -
and n is 0, 1, 2, 3, or 4. -NH-(CHZ)2-OH is particularly
preferred for -NH- (CHZ) n-OH.
R6 ~is H or R5, where R5 has a preferred meaning
indicated beforehand.
R' is preferentially -Ar' - (CH2) n-RB or
-Ar' - ( CH2 ) n-R5, where -Ar' - ( CHZ ) n-Re and -Ar' - ( CH2 ) "-R5
have a preferred or particularly preferred meaning
indicated beforehand.
R8 is preferably CONHZ, CONHA, CONA2,
CONH- (CH2) o-Ar, CONH- (CH2) o-Het, CONH- (CHZ) o-CH (Arl) -Ar2,
CONH-Ar'-NH-CO-Ar, CONH-Ar'-Het, CONH-(CHZ)o-RS or
CHA-CONH2, where A, CONH- (CHZ) o-Ar, CONH- (CHZ) o-Het,
CONH- ( CHZ ) o-CH (Arl ) -Ar2, and CONH- ( CH2 ) o-R5 have a
preferred or particularly preferred meaning indicated
beforehand in -Ar' - (CHz) n-Re. CH (CH3) -CONH2 is
particularly preferred for CHA-CONHz.
~CH3
O
O
CONH ~ ~ N
O-CH3
is particularly preferred for CONH-Ar'-NH-CO-Ar.
CONH ~ N
CI
CONH ~ ~ N,
Ji
CN
CONH
S
CONH
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 32 -
-._
CONH
S
CONH ~ \ CH3
S
O
CONH
S CI
or
-N
CONH
is particularly preferred for CONH-Ar'-Het.
R11 i s -NH- ( C=NH ) -NHZ, -NH- ( C=NH ) -NHA,
-NH- ( C=NH ) -NA2, -NA- ( C=NH ) -NH2, -NA- ( C=NH ) -NHA or
-NA-(C=NH)-NA2, where A has a meaning indicated
beforehand. -NH-(C=NH)-NHZ is particularly preferred for
Ril.
i is preferably 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
11 or 12, particularly preferred 1, 2, 3, 4, 5 or G.
m is preferably 3, 4, 5, 6, 7, 8, 9, 10, 11 or
12, particularly preffered 3, 4, 5, 6, 7 or 8.
n and o are in each case independently of one
another preferably 0, 1, 2, 3 or 9.
s is preferably 1, 2, 3 or 4.
t is preferably 0, 1 or 2.
a is preferably 1 or 2.
Some preferred groups of compounds can be
expressed by the following subformulae Ia to Iv, which
correspond to the formula I
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 33 -
R1
i
i
O~N/'O I
(CI R)i
R2
and in which the radicals not designated in greater
detail have the meanings indicated in formula I, but in
which:
in Ia R is H,
RZ is H,
Rl is -Het, -N- [ (CH2) s-OHJ 2, -Y- (CH2) n-R4 or R4
and
i is 1, 2, 3, 9, 5, 6, 7, 8, 9, 10, 11 or 12;
in Ib R is H,
RZ i s OH
R1 is -Het or -Y- (CHZ) n- (CHR4) -R3 and
i is 2;
in Ic R is H,
R2 is COOA,
R1 1 s -Het, -Y- ( CH2 ) n- ( CHR4 ) -R3,
-Y- ( CH2 ) n-Ar' - ( CHZ ) o-R6, -Het- ( CH2 ) n-Ar or
-NA- ( CHZ ) $-Ar and
i is 2;
in Id R is H,
RZ i s Ar,
Rl i s -Het, -NA- ( CH2 ) S-Ar, -Y- ( CH2 ) m-Rs,
-Y- ( CH2 ) m-OH, -Y- ( CH2 ) n- ( CHR4 ) -R3 r
3 0 -Y- ( CH2 ) n-R9 r -Y- ( CH2 ) n-Het- ( CH2 ) o-R6 r
-Y- ( CH2 ) n-Ar' ( CH2 ) o-R6 or -Het- ( CH2 ) n-Ar and
i is 1 or 2;
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 34 -
in Ie R is H,
R2 is Hetl,
Rl i s -Het , -NA- ( CH2 ) s-Ar, -Y- ( CH2 ) m-R5,
-Y- ( CH2 ) m-OH, -Y- ( CH2 ) n- ( CHR4 ) -R3.
-Y- ( CH2 ) n-R4. -Y- ( CH2 ) n-Ar' ( CHZ ) o-R6 or
-Het- ( CHZ ) n-Ar and
i is 1 or 2;
in If R is H,
R2 i s R5 ,
R1 i S -Het, -Y- ( CH2 ) m-R5, -Y- ( CH2 ) m-OH,
-Y- ( CHZ ) n- ( CHR4 ) -R3, -Y- ( CHZ ) n-R4.
-Y- ( CHZ ) n-Het- ( CHZ ) o-R6,
-Y- ( CH2 ) n-Ar' - ( CH2 ) o-R6 or -Het- ( CHZ ) n-Ar and
i is 3 or 5;
in Ig R is H,
R2 is Re,
R1 1 s -Het, -NA- ( CH2 ) m-R5, -Y- ( CHZ ) m-R5,
2 O -Y- ( CH2 ) 2-NH- ( CHZ ) S-OH, -Y- ( CHZ ) m-OH,
-Y- ( CH2 ) n- ( CHR4 ) -R3, -Y- ( CH2 ) n-R4.
-Y- ( CHZ ) n-Het- ( CHZ ) o-R6.
-Y- ( CH2 ) n-Ar' - ( CHz ) o-R6. -Het- ( CHZ ) n-Ar,
-Het-Het, -Y- [X-0] t- [X1-O] "-XZ-R5 or
2 5 -Y- ( CHZ ) n-NA- ( CH2 ) o-RS and
i is 1, 2, 7, 10 or 11;
in Ih R is H,
R2 is R',
30 Rl is -Het, -NA- ( CH2 ) m R5, -Y- ( CH2 ) m-R5,
-Y- ( CH2 ) 2-NHA, -Y- ( CH2 ) 2-NH- ( CHz ) S-OH,
-Y- ( CHZ ) m-OH, -Y- ( CH2 ) n- ( CHR4 ) -R3.
-Y- ( CHZ ) n-R4. -Y- ( CH2 ) n-Het- ( CH2 ) o-R6 r
-Y- ( CH2 ) n-Ar' - ( CH2 ) o-R6.
35 -Y- (CH2) s-Ar' - (CH2) o-Rll, -Het- (CH2) n-Ar,
-Het-Het, -Y- [X-O] t- [X1-O] "-X2-R5 or
-Y- ( CHZ ) n-NA- ( CH2 ) o-R5 and
i is 1 or 2:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 35 -
in I k R is A or CH2-Ph,
R2 is Re,
R1 1 s -Het, -Y- ( CHZ ) m-R5, -Y- ( CH2 ) n-Het- ( CHz ) o-R6
or -Y- ( CHZ ) n-Ar' - ( CH2 ) o-R6 and
i is 1;
in Im R is H,
RZ is CONH2,
R1 1 S -Het, -NA- ( CH2 ) m-R5, -Y- ( CH2 ) m-R5 r
1 O -Y- ( CH2 ) 2-NH- ( CH2 ) s-OH, -Y- ( CHZ ) m-OH,
-Y- ( CH2 ) n- ( CHR4 ) -R3, -Y- ( CH2 ) n-R4 r
-Y- ( CHZ ) n-Het- ( CH2 ) o-R6,
-Y- ( CHZ ) n-Ar' - ( CHZ ) o-R6 i -Het- ( CH2 ) n-Ar ,
-Het-Het, -Y- [X-O] t- [X1-O] u-X2-RS Or
-Y- ( CHZ ) n-NA- ( CHZ ) o-RS and
i is 1, 2, 7, 10 or 11;
in In R is H,
R2 is CONHA,
2 0 R1 i s -Y- ( CH2 ) m RS or -Y- ( CH2 ) n-Ar' - ( CH2 ) o-R6 and
i is 2;
in Io R is H,
RZ is CONH- (CHZ) o-Ar,
Rl 1 s -Y- ( CHZ ) m-Rs r -Y- ( CH2 ) n-Het- ( CHZ ) o-R6 or
-Y- ( CHZ ) n-Ar' - ( CHz ) o-R6 and
i is 1 or 2;
in Ip R is H,
Rz is CONH- (CH2) o-Het,
R1 i s -Y- ( CH2 ) m-R5, or -Y- ( CHZ ) n-Ar' - ( CH2 ) o-R6 and
i is 2;
in Iq R is H,
RZ is CONH- (CH2) o-CH (Arl) -Ar2,
R1 i s -Y- ( CH2 ) n-Ar' - ( CHZ ) o-R6 and
i is 2;
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 36 -
in Ir R is H,
R2 is CONH-Ar'-NH-CO-Ar,
R1 i s -Y- ( CHZ ) n-Ar' - ( CHZ ) o-R6 and
i is 2;
in Is R is H,
R2 is CONH-Ar'-Het,
R1 i s -Y- ( CHZ ) n-Ar' - ( CHz ) o-R6 and
i is 2;
in It R is H,
R2 is CH (Ph) -Ph,
R1 i s -Y- ( CH2 ) m-R5. or -Y- ( CHz ) n-Ar' - ( CH2 ) o-R6 and
i is 2;
in Iu R is H,
RZ is Ar' - (CHZ) "-Re,
R1 i s -Y- ( CH2 ) m-R5. -Y- ( CHz ) a-Ar' - ( CHZ ) o-R6 or
-Y- ( CH2 ) n-Ar' - ( CH2 ) o-Ri1 and
i is 1 or 2;
in Iv R is H,
R2 is Ar, Re or Ar' - (CHZ) n-Re,
R1 i s -Y- ( CH2 ) m-R5. or -Y- ( CHZ ) ~-Ar' - ( CH2 ) o-R6 and
i is 1 or 2.
The compounds of the formula I and also the
starting substances for their preparation are otherwise
prepared by methods known per se, such as are described
in the literature (e.g. in the standard works such as
Houben-Weyl, Methoden der organischen Chemie [Methods
of Organic Chemistry), Georg-Thieme-Verlag, Stuttgart),
namely under reaction conditions which are known and
suitable for the reactions mentioned. In this case, use
can also be made of variants which are known per se,
but not mentioned here in greater detail.
The starting substances, if desired, can also
be formed in situ such that they are not isolated from
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 37 -
the reaction mixture, but immediately reacted further
to give the compounds of the formula I.
The compounds of the formula I can be obtained
by liberating them from their functional derivatives by
solvolysis, in particular hydrolysis or by hydrogen
olysis.
Preferred starting substances for the
solvolysis or hydrogenolysis axe those which otherwise
correspond to the formula I, but instead of one or more
free amino and/or hydroxyl groups contain corresponding
protected amino and/or hydroxyl groups, in particular
those which instead of an H-N- group carry an R'-N-
group, in which R' is an amino protective group and/or
those which instead of the H atom of a hydroxyl group
carry a hydroxyl protective group, e.g. those which
correspond to the formula I, but instead of a group -
COOH carry a group -COOK", in which R" is a hydroxyl
protective group.
A number of - identical or different
protected amino and/or hydroxyl groups can also be
present in the molecule of the starting substance. If
the protective groups present are different from one
another, in many cases they can be removed selectively.
The expression "amino protective group" is
generally known and relates to groups which are
suitable for protecting (for blocking) an amino group
against chemical reactions, but which are easily
removable after the desired chemical reaction has been
carried out at other positions in the molecule. Typical
groups of this type are, in particular, unsubstituted
or substituted acyl, aryl, aralkoxymethyl or aralkyl
groups. Since the amino protective groups are removed
after the desired reaction (or reaction sequence),
their nature and size is otherwise not critical;
however, those having I-20, in particular 1-8, C atoms
are preferred. The expression "acyl group" is to be
interpreted in the widest sense in connection with the
present process. It includes acyl groups derived from
aliphatic, araliphatic, aromatic or heterocyclic
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 38 -
carboxylic acids or sulfonic acids and, in particular,
alkoxycarbonyl groups, aryloxycarbonyl groups and
especially aralkoxycarbonyl groups. Examples of acyl
groups of this type are alkanoyl such as acetyl,
propionyl, butyryl; aralkanoyl such as phenylacetyl;
aroyl such as benzoyl or toluyl; aryloxyalkanoyl such
as POA; alkoxycarbonyl such as methoxycarbonyl,
ethoxycarbonyl, 2,2,2-trichloroethoxycarbonyl, BOC,
2-iodoethoxycarbonyl; aralkyloxycarbonyl such as CBZ
("carbobenzoxy"), 9-methoxybenzyloxycarbonyl, Fmoc;
arylsulfonyl such as Mtr. Preferred amino protective
groups are BOC, furthermore CBZ, Fmoc, benzyl and
acetyl.
The expression "hydroxyl protective group" is
also generally known and relates to groups which are
suitable for protecting a hydroxyl group against
chemical reactions, but which are easily removable
after the desired chemical reaction has been carried
out at other positions in the molecule. Typical groups
of this type are the abovementioned unsubstituted or
substituted aryl, aralkyl or acyl groups, furthermore
also alkyl groups . The nature and size of the hydroxyl
protective groups is not critical, since they are
removed again after the desired chemical reaction or
reaction sequence; groups having 1-20, in particular
1-10 C atoms, are preferred. Examples of hydroxyl
protective groups are, inter alia, benzyl,
p-nitrobenzoyl, p-toluolsulfonyl, tert-butyl and
acetyl, benzyl and tert-butyl being particularly
preferred.
The liberation of the compounds of the
formula I from their functional derivatives is carried
out - depending on the protective group used - for
example using strong acids, expediently using TFA or
perchloric acid, but also using other strong inorganic
acids such as hydrochloric acid or sulfuric acid,
strong organic carboxylic acids such as trichloroacetic
acid or sulfonic acids such as benzene- or p-toluene-
sulfonic acid. The presence of an additional inert
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 39 -
solvent is possible, but not always necessary. Suitable
inert solvents axe preferably organic, for example
carboxylic acids such as acetic acid, ethers such as
tetrahydrofuran or dioxane, amides such as DMF,
halogenated hydrocarbons such as dichloromethane,
furthermore also alcohols such as methanol, ethanol or
isopropanol, and also water. Furthermore, mixtures of
the abovementioned solvents are possible. TFA is
preferably used in an excess without addition of a
further solvent, perchloric acid in the form of a
mixture of acetic acid and 70% perchloric acid in the
ratio 9:1. The reaction temperatures for the cleavage
are expediently between approximately 0 and
approximately 50°C; the reaction is preferably carried
out between 15 and 30°C (room temperature).
The groups BOC and Obutyl can preferably be
removed, for example, using TFA in dichloromethane or
using approximately 3 to 5N HC1 in dioxane at 15-30°C,
the Fmoc group using an approximately 5 to 50% solution
of dimethylamine, diethylamine or piperidine in DMF at
15-30°C.
Hydrogenolytically removable protective groups
(e.g. CBZ or benzyl) can be removed, for example, by
treating with hydrogen in the presence of a catalyst
(e. g. of a noble metal catalyst such as palladium,
expediently on a support such as carbon). Suitable
solvents in this case are those indicated above, in
particular, for example, alcohols such as methanol or
ethanol or amides such as DMF. As a rule, the
hydrogenolysis is carried out at temperatures between
approximately 0 and 100°C and pressures between
approximately 1 and 200 bar, preferentially at 20-30°C
and 1-10 bar. Hydrogenolysis of the CBZ group takes
place readily, for example, on 5 to 10% Pd/C in
methanol or ammonium formate (instead of hydrogen) on
Pd/C in methanol/DMF at 20-30°C.
Compounds of the formula I can also preferably
be obtained by reacting compounds of the formula II
with compounds of the formula III. As a rule, the
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 40 -
starting compounds of the formulae II and III are known
or commercially available. The unknown compounds,
however, can be prepared by methods known per se. The
compounds of the formula II are naphthalene-
1,8-dicarboxylic anhydride derivatives. They can be
prepared in a conventional manner from appropriately
substituted 1,8-naphthalenedicarboxylic acids or
corresponding derivatives. It is furthermore possible
to introduce appropriate substituents into the aromatic
by conventional electrophilic or alternatively
nucleophilic substitutions.
The compounds of the formula III are primary
amines, which, as a rule, are also commercially
available. Furthermore, syntheses for the preparation
of primary amines, such as, for example, the Gabriel
synthesis, can be used.
As a rule, the reaction is carried out in an
inert solvent. Depending on the conditions used, the
reaction time is between a few minutes and a number of
days, the reaction temperature between approximately 0°
and 150°C, normally between 20° and 130°C. The
reactions can be carried out in analogy to the methods
indicated in Eur. J. Chem. Chim. Ther. 1981, 16, 207-
212 and in J. Med. Chem. 1982, 25, 714-719.
Suitable inert solvents are, for example,
hydrocarbons such as hexane, petroleum ether, benzene,
toluene or xylene; chlorinated hydrocarbons such as
trichloroethylene, 1,2-dichloroethane, carbon
tetrachloride, chloroform or dichloromethane; alcohols
such as methanol, ethanol, isopropanol, n-propanol,
n-butanol or tert-butanol; ethers such as diethyl
ether, diisopropyl ether, tetrahydrofuran (THF) or
dioxane; glycol ethers such as ethylene glycol
monomethyl or monoethyl ether (methyl glycol or ethyl
glycol), ethylene glycol dimethyl ether (diglyme);
ketones such as acetone or butanone; amides such as
acetamide, N-methylpyrrolidone (NMP), dimethylacetamide
or dimethylformamide (DMF); nitriles such as
acetonitrile; sulfoxides such as dimethyl sulfoxide
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 41 -
(DMSO); carbon disulfide; carboxylic acids such as
formic acid or acetic acid; nitro compounds such as
nitromethane or nitrobenzene; esters such as ethyl
acetate or mixtures of the solvents mentioned.
For the preparation of compounds of the
formula I in which R11 is H2N-C(=NH)-NH-, an appropriate
amino-substituted compound can be treated with an
amidinating agent. The preferred amidinating agent is
1-amidino-3,5-dimethylpyrazole (DPFN), which is
employed, in particular, in the form of its nitrate, or
pyrazole-1-carboxamidine. The reaction is expediently
carried out with addition of a base such as
triethylamine or ethyldiisopropylamine in an inert
solvent or solvent mixture, e.g. DMF at temperatures
between 0° and 150°C, preferably between 60° and
120°C.
For the preparation of compounds of the
formula I in which RZ is unsubstituted or substituted
biphenyl, -Ar'-Het and/or -CONH-(CHZ)o-Ar, an
appropriate compound of the formula I in which RZ is
aryl bromide or aryl iodide can be reacted with the
appropriate boronic acid derivatives in a Suzuki
reaction. The Suzuki reaction is expediently carried
out in palladium-mediated form, preferably by addition
of Pd ( PPh3 ) 4, in the presence of a . base such as
potassium carbonate in an inert solvent or solvent
mixture, e.g. DMF at temperatures between 0° and 150°,
preferably between 60° and 120°. Depending on the
conditions used, the reaction time is between a few
minutes and a number of days. The boronic acid
derivatives can be prepared by conventional methods or
are commercially available. The reactions can be
carried out in analogy to the methods indicated in
Suzuki et al., J. Am. Chem. Soc. 1989, 111, 314ff. and
Suzuki et al., Chem. Rev. 1995, 95, 2457ff.
The compounds of the formula IV are derived
from the anhydrides of the formula II and can be
prepared from these by reaction with ammonia under
conditions known per se. As a rule, the imides of the
formula IV, however, are known and commercially
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 42 -
available. The substituent R1 can preferably be
introduced into the aromatic by substitution. In many
cases, it is expedient to introduce this substituent
before the reaction with ammonia.
As a rule, the alcohols, activated alcohols or
halides of the formula V are known and their
preparation is familiar to the person skilled in the
art, so that a description of the syntheses is
unnecessary here.
The reaction of the compounds of the formula IV
with compounds of the formula V is preferably carried
out in an inert solvent, with addition of a base and at
temperatures and with reaction times as indicated
beforehand. Suitable acid-binding agents are preferably
alkali metal or alkaline earth metal hydroxides,
carbonates or bicarbonates or other salts of a weak
acid of the alkali or alkaline earth metals, preferably
of potassium, sodium, calcium or caesium. The addition
of an organic base such as triethylamine,
dimethylaniline, pyridine or quinoline or an excess of
the amine component of the formula IV may also be
favourable.
Derivatives having a free primary or an
additional secondary amino group are expediently
employed in protected form. Possible protective groups
are those mentioned beforehand.
For the esterification, an acid of the
formula I (R1 - COOH or -Y- (CHZ) n-COOH and/or Rz - COOH)
can be treated with an excess of an alcohol,
expediently in the presence of a strong acid such as
hydrochloric acid or sulfuric acid at temperatures
between 0° and 100°C, preferably between 20° and
50°C.
Conversely, an ester of the formula I (R1 - COOA or
-Y-(CHZ)n-CODA and/or R2 - COOA) can be converted into
the corresponding acid of th,e formula I, expediently by
solvolysis according to one of the methods indicated
above, e.g. using NaOH or KOH in water-dioxane at
temperatures between 0° and 40°C, preferably between
10° and 30°C.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 43 -
Furthermore, free amino groups can be acylated
in a customary manner using an acid chloride or
anhydride, expediently in an inert solvent such as
dichloromethane or THF and/or in the presence of a base
such as triethylamine or pyridine at temperatures
between -60°C and +30°C.
A base of the formula I can be converted into
the associated acid addition salt using an acid, for
example by reaction of equivalent amounts of the base
and of the acid in an inert solvent such as ethanol and
subsequent evaporation. Acids which give physiolo-
gically acceptable salts are particularly suitable for
this reaction. Thus inorganic acids can be used, e.g.
sulfuric acid, nitric acid, hydrohalic acids such as
hydrochloric acid or hydrobromic acid, phosphoric acids
such as orthophosphoric acid, sulfamic acid, further-
more organic acids, in particular aliphatic, alicyclic,
araliphatic, aromatic or heterocyclic mono- or
polybasic carboxylic, sulfonic or sulfuric acids, e.g.
formic acid, acetic acid, propionic acid, pivalic acid,
diethylacetic acid, malonic acid, succinic acid,
pimelic acid, fumaric acid, malefic acid, lactic acid,
tartaric acid, malic acid, citric acid, gluconic acid,
ascorbic acid, nicotinic acid, isonicotinic acid,
methane- or ethanesulfonic acid, p-toluenesulfonic
acid, naphthalenemono- and disulfonic acids or
laurylsulfuric acid. Salts with physiologically
unacceptable acids, e.g. picrates, can be used for the
isolation and/or purification of the compounds of the
formula I.
On the other hand, compounds of the formula I
with bases (e.g sodium or potassium hydroxide or
carbonate) can be converted into the corresponding
metal salts, in particular alkali metal or alkaline
earth metal salts, or into the corresponding ammonium
salts.
All synthesis methods indicated here and all
other suitable processes for the preparation of
compounds of the formula I can also be carried out by
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 44 -
means of the novel methods of combinatorial chemistry,
i.e. by robot- and computer-assisted syntheses, and
subjected to mass screening (for this see: US
5,463,564; M. A. Gallop et al., J. Med. Chem. 1994, 37,
1233-1251 and 1385-1401 and M.J. Sofia, Drugs Discovery
Today 1996, 1, 27-34).
The invention furthermore relates to
pharmaceutical preparations comprising at least one
compound of the formula I and/or one of its
physiologically acceptable salts, which are prepared,
in particular, in an non-chemical way. In this case,
the compounds of the formula I can be brought into a
suitable dose form together with at least one solid,
liquid and/or semi-liquid excipient or auxiliary and,
if appropriate, in combination with one or more other
active compounds.
These preparations can be used as medicaments
in human or veterinary medicine. Possible excipients
are organic or inorganic substances which are suitable
for enteral (e.g. oral) or parenteral administration or
topical application and do not react with the novel
compounds, for example water, vegetable oils, benzyl
alcohols, alkylene glycols, polyethylene glycols,
glyceryl triacetate, gelatin, carbohydrates such as
lactose or starch, magnesium stearate, talc and
petroleum jelly. Tablets, pills, coated tablets,
capsules, powders, granules, syrups, juices or drops
are used, in particular, for oral administration,
suppositories are used for rectal administration,
solutions, preferably oily or aqueous solutions,
furthermore suspensions, emulsions or implants, are
used for parenteral administration, and ointments,
creams or powders are used for topical application. The
novel compounds can also be lyophilized and the
lyophilizates obtained used, for example, for the
production of injection preparations. The preparations
indicated can be sterilized and/or can contain
auxiliaries such as lubricants, preservatives,
stabilizers and/or wetting agents, emulsifiers, salts
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 45 -
for affecting the osmotic pressure, buffer substances,
colourants, flavourings and/or one or more other active
compounds, e.g. one or more vitamins.
The compounds of the formula I and their
physiologically acceptable salts act as adhesion
receptor antagonists, in particular glycoprotein IbIX
antagonists, and can be employed for the prophylaxis
and/or therapy of thrombotic disorders and sequelae
deriving therefrom. The disorders are acute coronary
syndromes, angina pectoris, myocardial infarct,
peripheral circulatory disorders, stroke, transient
ischaemic attacks, arteriosclerosis and
reocclusion/restenosis after angioplasty/stent
implantation.
In this case, the substances according to the
invention are as a rule administered in the dose of the
glycoprotein IIbIIIa antagonist ReoPro~ of preferably
between approximately 1 and 500 mg, in particular
between 5 and 100 mg, per dose unit. The daily dose is
preferably between approximately 0.02 and 10 mg/kg of
body weight. The specific dose for each patient
depends, however, on all sorts of factors, for example
on the efficacy of the specific compound employed, on
the age, body weight, general state of health and sex,
on the diet, on the time and route of administration,
and on the excretion rate, pharmaceutical combination
and severity of the particular disorder to which the
therapy applies. Oral administration is preferred.
Above and below, all temperatures are indicated
in °C. In the following examples, "customary working
up" means: if necessary, water is added, if necessary,
depending on the constitution of the final product, the
mixture is adjusted to pHs between 2 and 10 and
extracted with ethyl acetate or dichloromethane, the
organic phase is separated off, dried over sodium
sulfate and evaporated, and the residue is purified by
chromatography on silica gel and/or by crystallization.
Mass spectrometry (MS) apparatuses Kratos Maldi III and
Finnigan LCQ. (M+H)+ values are determined.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 46 -
In the foregoing and in the following examples,
all temperatures are set forth uncorrected in degrees
Celsius; and, unless otherwise indicated, all parts and
percentages are by weight.
The entire disclosure of all applications,
patents and publications, cited above and below, is
hereby incorporated by reference.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 47 -
EXAMpI,ES
Example 1
A suspension of 4 g of 6-chlorobenzo[de]iso
chromene-1,3-dione in 100 ml of toluene is treated with
0.8 g of methylamine and heated under reflux. After
reaction is complete, the reaction mixture is allowed
to cool and is worked up as is customary. 6-Chloro-
2-methylbenzo[de]isoquinoline-1,3-dione is obtained.
This compound is then heated in morpholine until
conversion is complete. After cooling the reaction
mixture, it is worked up as is customary and 2-methyl-
6-morpholin-4-ylbenzo[de]isoquinoline-1,3-dione is
obtained.
Analogously, by reaction of 6-chloro-2
methylbenzo[de]isoquinoline-1,3-dione with R1-H, the
following compounds of the formula Ia are obtained:
R1
i
i
~ la
O ' 'O
N
CH3
R in R -H and Ia
-N [ (CH2) 2-OH] Z
N
O
H H N
-N C ~S
N
O
H3C
H H
~N
-N C i
O ~ \
HsC CHs
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 48 -
Example 2
A suspension of 4 g of 6-chlorobenzo[de)iso
chromene-1,3-dione in 100 ml of dichloromethane is
treated with 3.2 g of 2-aminoethanol and heated under
reflux. After reaction is complete, the reaction
mixture is allowed to cool and is worked up as is
customary. 6-Chloro-2-(2-hydroxyethyl)benzo[de]iso-
quinoline-1,3-dione is obtained. This compound is then
heated in morpholine until conversion is complete.
After cooling the reaction mixture, it is worked up as
is customary and 2-(2-hydroxyethyl)-6-morpholin-9-yl-
benzo[de]isoquinoline-1,3-dione is obtained.
Analogously, by reaction of 6-chloro
2-(2-hydroxyethyl)benzo[de]isoquinoline-1,3-dione with
R1-H, the following compounds of the formula Ib are
obtained
R1
i
Ib
O ~O
N
OH
R1 in R -H and in Ib
-N N
U
-N
-N
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
R in R -H and in Ib
- G~ -
-N~N
U
-N
i
H
Example 3
Analogously to Example 2, 6-chloro
benzo[de]isochromene-1,3-dione is reacted with methyl
3-aminopropionate and then with R1-H. The following
compounds of the formula Ic are obtained:
R1
i
i
~ is
O ' '-O
N
COOMe
R~ in R -H and in Ic MS
calculated found
-N O 368 369
U
-N 366 367
-N, I 352 353
- N ~ ~ 443 444
U
CA 02351348 2001-05-23
WO 00/31039 PCT1EP99/08560
~n
R in R -H and in Ic MS
calculated found
Ph
-N~CH3
H
-N CHZ / \ 388 389
H
-N (CH2)2 / \ 402 403
H
-N-CH2 / \ Sp2NH2
H
--N (CH )2 / \ OH
H
H3 OH-
-N (CH )2 / \ pH
-N 380 381
H
- 366 367
H
i
/ \ 419 415
-N
Example 4
Analogously to Example 2, 6-chloro
benzo[de]isochromene-1,3-dione is reacted with
benzylamine and then with R1-H. The following compounds
of the formula Ida are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 51 -
R'
i
i
Ida
O ~O
N
~Ph
Rl in R1-H and in Ida MS
calculated found
- 372 373
Q
~
-N 370 371
-N I 356 357
,.
-
447 448
/
Ph 406 407
H CH3
-NH- (CHa) 5-OH 38$ 389
-NH- ( CH2 ) Z-COOMe 3 8 8 3 g g
-NH- ( CH2 ) 3-N ( CH3 ) 2
/ ~
-N CH2 392 393
H
/ ~
-N (CH2)2 406 407
H
-. N -- CH2 / ~ g02NH2
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ ~~ _
R in R -H and in Ida MS
calculated found
-N -(CH2)2 / \ pH 422
423
H
-N - CH2 / \ NHZ
H
CH2- 2
421 422
-N - CH2
H
N
-N-(CH2)2 / \ 407 408
H
-N (CH2)2 ~ \ 407 408
H
N
-N CH2 ~ \ 393 394
H
-N (CH2)2-N p 415 416
i U
H
-N (CH2)3-N N-CH3
H \--/
-N (CH2)3-N~N 410 411
H
i H ~h
-N -(CH2)2 / \ pH
-N 384 385
H
- 370 371
i
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 53 -
R in R -H and in Ida i MS
calculated found
418 419
I
H
418 419
-N
Example 5
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with
phenylethylamine and then with R1-H. The following
compounds of the formula Idb are obtained:
R1
i w
i
~ Idb
O ~O
N
Ph
R in R -H and in Idb MS
calculated found
-N 384 385
-N~ 370 371
- 461 462
~ ~ ~
Ph 420 421
-N ~
CH3
H
-NH- ( CHZ ) 5-OH 4 0 2 4 0 3
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 54 -
Ra in R''-H and in Idb MS
calculated found
-NH- (CH2) 2-COOMe 402 403
-NH- (CH2) 3-N (CH3) 2 401 402
-N CH2 / \ 406 407
H
-N (CH2)2 / \ 420 421
H
-N - CH2 / \ Sp2NH2
H
-N (CH2)2 / \ pH
H
CH2 / \ NH2 421 422
H
CH2- 2
435 436
-N - CH2 / \
H
N
-N -(CH2)2 / ~ 421 422
H
N
-N (CH2)2 / \ 421 422
H
-N CHZ / \ 407 408
H
-N (CH2)2-N p 429 430
U
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 55 -
R1 in R1-H and in Idb MS
calculated found
-N -(CH2)3-N N-CH3
I ~J
H
-N -(CH2)3- ~N 424
425
H
H3 vn
-N (CH2)2 / \ pH
-N 398 3gg
H
- 384 385
I
H
i
H
/ \
-N
Example 6
A suspension of 4 g of 6-chlorobenzo[de]iso
quinoline-1,3-dione and 7 g of KZC03 in 100 ml of DMF is
heated under reflux with 6 g of phenylethyl chloride.
After conversion is complete, the mixture is allowed to
cool, and is filtered and worked up as is customary.
6-Chloro-2-(2-phenylethyl)benzo[de]isoquinoline-1,3-dione
is then heated in morpholine until conversion is
complete. After cooling of the reaction mixture, it is
worked up as is customary and 2-(2-phenylethyl-
6-morpholin-4-ylbenzo[de]isoquinoline-1,3-dione is
obtained. MS: calculated: 386; found: 387.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 56 -
Example 7
Analogously to Example 2, 6-chlorobenzo-
[de]isochromene-1,3-dione is reacted with C-
cyclohexylmethylamine and then with R1-H. The following
compounds of the formula Idc are obtained:
R1
i
I
i
Idc
O ' 'O
N
R in R -H and in Idc MS
calculated found
- ~O 378 379
-N 37 6 377
-N~ 362 363
453 454
/
-N CH 330 331
3
H
-NH- (CH2) 5-OH 394 395
-NH- ( CH2 ) 2-COOMe 3 94 3 95
-NH- (CH2) 3-N (CH3) 2 393 394
/ ~
-N CH2 398 399
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 57 _
R in R -H and in Idc ~ MS
calculated found
-N -(CH2)2 / \ 412 913
H
-N -CH / \ SOZNH2
H
-N (CH2)2 / \ OH 428 429
H
-N CH2 / \ NH2
H
CH2- N 2
-N-CH / \
H
-N
-N (CH2)2 / ~ 413 414
H
N-
-N (CHZ)2 ~ \ 413 414
H
-N CH2 ~ \ 399 400
H
-N (CH2)2-N p 421 422
I
H
-N -(CH2)3-N N-CH3
I U
H
-N
I (CH2)3- ~ 416 417
H
H3 vn
-N (CH2)2 / ~ OH
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 58 -
R in R -H and in Idc MS
calculated found
-N 390 391
H
'- 376 377
H
i
424 425
I
H
424 425
-N
Example 8
Analogously to Example 2, 6-chlorobenzo-
[de]isochromene-1,3-dione is reacted with (3-
chlorophenyl)methylamine and then with R1-H. The
following compounds of the formula Idd are obtained:
R1
i
i
Idd
O ' 'O
N
~ CI
Rl in R1-H and in Idd MS
calculated found
-NH- ( CH2 ) 3-N ( CH3 ) 2 4 21 4 22
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 5 c~ _
R1 in R -H~ and in Idd MS
calculated found
-N (CH2)2 ~ ~ 441 442
H
N
-N-(CH2)2 ~ ~ 441 442
H
-N CH2 ~ ~ 427 428
H
-N-(CH2)2-N p 449 450
H ~--/
-N -(CH2)3- N-CH3 476 -- 477
i
H
-N - CH2)3 ~N 444 445
H
-NH- (CHZ) 5-OH 422 423
CH2- NH2
-N-CH / \
H
Example 9
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with 4-(2
aminoethyl)benzenesulfonamide and then with R1-H. The
following compounds of the formula Ide are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 60 -
R1
i
i
Ide
O " O
N
2
R1 in R -H and in Ide MS
calculated found
-NH- (CHz) 7-NH2 508 509
-NH- ( CHZ ) S-NHZ 4 8 0 . 4 81
-NH- ( CH2 ) 3-NHZ 4 52 4 53
CHZ- NH2
514 515
-N - CH2
H
Example 10
Analogously to Example 2, 6-chlorobenzo-
[de]isochromene-1,3-dione is reacted with C-pyridin-4-
ylmethylamine and then with R1-H. The following
compounds of the formula Iea are obtained:
R1
i
i
~ lea
O ' '_O
N
i
~ N
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 61 -
R1 in Rr-H and in Iea MS
calculated found
-N p 373 374
U
-N 371 372
-N, I 357 358
\ / 448 449
-N CH 407 408
H 3
-NH- ( CH2 ) 2-COOMe
-N CH2 / \ 393 394
H
-N (CH )2 / \ 407 408
H
-N,CHZ / \ S02NH2 472 473
H
--N (CH )2 / \ OH
H
i H3 OI-f
--N (CH )2 / \ OH
-N 385 386
I
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
-
R1 in Rl-H and in Iea MS
calculated found
371 372
H
i
4I9 420
I
H
/ \
-N
Example 11
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with C
benzo[b]thiophen-3-ylmethylamine and then with R1-H. The
following compounds of the formula Ieb are obtained:
R1
i
i
leb
O ~O
N
~ ~S
\ /
R in R -H and in Ieb MS
calculated found
-NH- ( CH2 ) 5-NHZ 4 4 3 9 4 4
-NH- (CHZ) ~-NH2 471 472
CH2- NH2
477 478
_N,CH / \
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 63 -
Example 12
ml of TFA are added at room temperature to a
5 solution of 2 g of tert-butyl [3-(2-benzo[b]thiophen-
3-ylmethyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]iso-
quinolin-6-ylamino)propyl]carbamate in 40 ml of
dichloromethane [obtainable by reaction of
6-chlorobenzo[de]isochromene-1,3-dione with C-benzo[b]-
10 thiophen-3-ylmethylamine and H2N-(CHZ)3-NH-BOC] and the
reaction mixture is stirred until removal is complete.
After customary work-up 6-(3-aminopropylamino)-
2-benzo[b]thiophen-3-ylmethylbenzo[de]isoquinoline-
1,3-dione is obtained. MS: calculated: 915; found: 416.
Example 13
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1,3-dione is reacted with H2N-(CH2)2-Hetl and
then with R1-H. The following compounds of the formula
Iec are obtained:
R1
i
i
~ lec
O "O
N
Het1
Het R in R -H and in Iec
-NH- ( CH2 ) s-NH2
/ -NH- ( CH2 ) s-NHZ
/ ' 1 -NH- ( CHZ ) ~-NHZ
N CHZ- NH2
-N-CH2 /-\
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
Fd -
Hetl Rl in R -H and in Iec
-NH- ( CH2 ) 3-NHZ
-NH- ( CH2 ) s-NHZ
-NH- ( CH2 ) ~-NH2
CHZ NH2
-N-CH2 /-\
H
-NH- ( CH2 ) 3-NH2
CH3 -NH- ( CH2 ) s-NH2
-NH- ( CH2 ) ~-NHz
CH2- NH2
-N-CHZ /-\
H
-NH- ( CHz ) 3-NH2
CH3 -NH- ( CHz ) s-NHZ
/ ' -NH- ( CHz ) ~-NH2
CH2- NH2
-N - CH2
H
-NH- ( CH2 ) 3-NH2
-NH- ( CH2 ) s-NH2
OCH -NH- ( CH2 ) ~-NH2
N 3 CHZ- NH2
-N-CH2 /-\
H
Example 14
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1,3-dione is reacted with 1,3-propanediamine
and then with R1-H. The following compounds of the
formula If are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 65 -
R'
i
i
O~N~O
NH2
R in R -H and in If
- ~O
-N
N _
- \-/N \ /
Ph
-N ~CH3
H
-NH- ( CHZ ) 2-COOMe
-N CH2 / \
H
( / \
-N CH2)2
H
-N-CH2 / \ Sp2NH2
H
-N -(CH2)2 / \ pH
H
-NH- ( CHZ ) s-OH _
-NH- ( CH2 ) 3-N ( CH3 ) 2 _
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 66 -
Rl in Rl-H and in If
-N CH2 ~ ~ NH2
H
CH2- NH2
-N - CH2
H
-N (CH2)2
H
-N -(CH2)2
i
H
-N CH2
i
H
-N -(CH2)2-N p
H
-N -(CH2)3-N N-CH3
i U
H
-N (CH2)3-
H
-NH- ( CH2 ) 5-NHZ
-NH- C CH2 ) ~-NH2
Example 15
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1,3-dione is reacted with 3-amino-2
methylpropionamide and then with R1-H. The following
compounds of the formula Iga are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 67 -
R1
i
i
~ Iga
O ' 'O
N
O
HsC NH~
R1 in R -H and in Iga -
-NH- ( CHZ ) 3-N~( CHs ) 2
-N-(CH2)2-N--C
H H
w
-N - (CH2)2 / \
i
H
-N -(CH2)3- ~N
i
H
-NH- ( CH2 ) 9-NH2
-NH- ( CH2 ) ~-NH2
-NH- ( CH2 ) e-NHZ
CH2- NH2
--N -. CH / \
H
Example 16
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1,3-dione is reacted with 2-aminoacetamide and
then with R1-H. The following compounds of the formula
Igb are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 68 -
R1
i
i
Igb
p ' 'O
N
O
NHS
R1 in R -H and in Igb MS
calculated found
-N O
U
-N 337 338
-N~ 323 324
~N \ /
.-N~CH3
'H
-NH- ( CHZ ) 2-COOMe
CH2 / \
H
-N -(CH2)2 / \
i
H
-N - CH / \ S02NH2
H
-N '(CH2)2 / \ OH
H
-NH- (CH2) 5-OH
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 69 -
R1 in R -H and i MS
calculated found
-NH- (CH2) 3-N (CH3) 2 354 355
-N CH2 / \ NH2
H
CH2- NHZ
-N -- CH2 / \
H
/ ~\
-N (CH2)z
t
H
N
-N (CH2)2 / \ 374 375
H
N
-N CH2
H
N (CH2)2 Nip
i U
H
-N -(CH2)3-N N-CH3
i
H
N (CH2)3-N~ N 37 9
380
-NH- ( CH2 ) s-NHZ
-NH- ( CH2 ) ~-NHZ
Example 17
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1,3-dione is reacted with 3-aminopropionamide
and then with R1-H. The following compounds of the
formula Igc are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 70 -
R'
i
i
~ Igc
O ~O
N
H2N O
R1 in R1-H and in Igc MS
calculated found
-' ~O
-N 351 352
-N~ 337 338
~N \ /
Ph
-N~CH3
H
-NH- ( CH2 ) Z-COOMe
-N CH2 / \
H
-N -(CH2)2 / \
i
H
,N -CH2 / \ g02NH2
H
--N (CH )2 / \ OH
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 71 -
R~ in R -H and in Igc MS
calculated found
-NH- (CHZ) 5-OH 369 370
-NH- ( CHZ ) 3-N ( CH3 ) 2
-N-CH / \ HZ
H
CHZ- N 2
-N - CH2 / \
H
-N -(CH2)2 / N.
i
H
-N (CH2)2 \
H
-N CH2
H
-N (CH2)2-N O
H
-N -(CH2)3- N-CH3
U
H
-N (CH2)3 N~
i
H
-NH- ( CHZ ) s-NH2
-NH- (CHZ) ~-NHZ 396 397
Example 18
Analogously to Example 2, 6-chlorobenzo[de]iso-
chromene-1,3-dione is reacted with 6-amino-hexanoamide
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99l08560
- 72 -
and then with R1-H. The following compounds of the
formula Igd are obtained:
R1
i
I
w i
~ Igd
O ~O
N
t IH2)5
CONH2
R1 in R -H and in Ms R in R -H and in Igd Ms
Igd talc. talc
fnd.
fnd.
- ~ -NH- (CHZ) 3-NH (CH3) 396
397
-N -N (CH3) - (CHZ) 3-NH 410
(CH3)
411
-N 379 -N (CH3) - (CH2) 2-N 938
(CZHS) 2
380 439
- N - 470 -N-(CH2)2-N---~ 910
U \ / 971 H H 411
Ph
N~
CH3 -N N-CH
H 2
\~
-NH- ( CH2 ) Z-COOMe / \ 4 8
4
_ _
H2
U 485
-N -CH / \ -N N- 471
H
./ \ ~ 472
N-
-N -(CH2)2 / \ -N N--~\
H ~--/ N
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 73 -
R in R -H and in MS R in Rr-H and in Igd Ms
Igd calc. calc
fnd.
fnd.
-N - CH2 ~-~ SOZNH2 -NH-(CH2)3- ~ -(CH2)3--NH2 5 0 $
H
509
-N (CH2) / \
H -NH N
-NH- { CHZ ) 5-OH -NH- ( CHZ ) 2-NH- ( CH2 ) 2-OH 412
413
-NH- ( CH2 ) 3-N ( CH3 ) 2 -NH- ( CHZ ) Z-NH ( C3H~ ) 410
411
-N - CH2 ~-~ NH2 -NH- ( CHz ) 3-O- ( CHz ) a-O- ( CHz ) 3-NHz 512
H
513
CH2- NH2
-tV-CH2~ -NH-(CH2)3 N 464
" H 465
CHzNH2
-N -(CH2)2 --~~ 450
-N H-C H2
451
-N -(CH2)2 ~ ~ 430 -NH- (CHz) 3-N (CH3) - (CHZ) 3-NHz 453
H 431 454
-N CH2 N \ 416 -NH- 396
( CH2 ) 2-NH ( CZHS )
H 417 3g7
-H -(CH2)2- ~O -NH- (CHZ) s-NHZ
-N -(CH2)3- N-CH3 4 62
H ~ -NH- ( CHZ ) ~-NHZ
4 63
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 7a -
R in R -H and in Ms R in R -H and in Igd Ms
Igd calc. calc
fnd.
fnd.
(CH2)3- -N-CHZ /
\ CH2._NH2
~ -
H H
Exam lp a 19
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1, 3-dione is reacted with HZN- (CH2) i-CONHZ and
then with R1-H. The following compounds of the formula
Ige are obtained:
R1
i
i
Ige
O ' 'O
N
~ ~H2~i
CONH2
R~ in R -H and in Ige i MS
calculated found
-N-(CH2)2 ~ \ 7 458 _ 459
H 10 500 501
11
'H--(CH2)2-H-C 7 4 52 4 53
10 494 495
11 508 509
7
-NH- (CH2) 4-NH2
10 466 467
11
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ ~5 _
R in R -H and in Ige i MS
calculated found
7 438 439
-NH- ( CHZ ) 3-N ( CH3
) 2
10
11 4 94 4 95
7
-NH- { CHZ ) ~-NHz
10
11
7 480 481
-NH- (CH2) 8-NH2
10 522 523
11 536 537
7 472 473
CHZ- NH2
10
H 11 528 529
-N -(CH2)3- ~ N 7 9 63 4 64
H
10 505 506
11 519 520
Example 20
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1, 3-dione is reacted with H2N- (CH2) 2-CONH (Rlo)
and then with R1-H. The following compounds of the
formula Igf are obtained:
R1
i
i
Igf
O ' '-O
N
( ~H2~2
CONH(R10)
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
76
Rj in Rl-H and in Igf R MS
calculated found
CsH~ 438 439
-NH- ( CH2 ) ~-NH2 C5H11 4 6 6 4 67
CH2-NHZ C3H~ 444 445
-N -CH
CsHii 472 473
Example 21
Analogously to Example 2, 6-chlorobenzo[deJiso
chromene-1, 3-dione is reacted with HZN- (CHZ) 2-CONH
(CH2)o-Ar and then with R1-H. The following compounds of
the formula Iha are obtained:
R1
i
i
Iha
O ' 'O
N
CONH-(CH2)o Ar
CONH- (CH2) o-Ar R in R -H and in Iha MS
calc. fnd.
-NH- ( CH2 ) ~-NH2
CONH-CH2----( ) / \ CH2-NH2 499 500
~J -N - CH2
H
-N-(CH2)3-N~N 489 490
H
Example 20
Analogo
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 77 -
CONH- (CH2) o-Ar R in R -H and in Iha MS
calc. fnd.
-NH- ( CH2 ) ~-NH2 515 516
- /CH3 CH2- NH2
CONH 521 522
~CH3 -N - / \
CH2
H
-N -(CH2)3-N
i
H
-NH- ( CH2 ) ~-NHZ 52 0 521
CONH-CH2 ~ ~ C~ CH2- NH2 52 6 52 7
-N-CHZ / \
H
-N (CH2)3-NON
i
H
-NH- (CH2) ~-NHZ 548 549
CONH ~ ~ ~ CH2- NHZ 517 518
-N-CH2 / \
H
-NH- ( CH2 ) ~-NH2 514 515
CH2- NH2
CONH--(CH2)3
-N - CH2 / \
H
-N -(CHZ)3-N~N 511 512
H
Example 22
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1, 3-dione is reacted with HZN- (CHZ) Z-CONH
(CH2) o-R5 and then with Rl-H. The following compounds of
the formula Ihb are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 78 _
R1
I
i
Ihb
O ' 'O
N
CONH-(CH2)o-R5
CONH- (CH2) o-R R in R -H and in Ihb MS
calc. fnd.
-NH- ( CH2 ) ~-NH2 4 67 4 68
CONH- ( CH2 ) 4-NHz CHZ- NH2
473 474
-N -CH2
H
-NH- ( CH2 ) ~-NH2 5 3 9 5 4 0
CONH- (CH2) 2-NHZ CH2-NH2
445 446
-N - CH2
H
Example 23
Analogously to Example 2, 6-chlorobenzo[de]iso-
chromene-1,3-dione is reacted with H2N-(CHZ)2-CONH-
(CH2)o-Het and then with R1-H. The following compounds
of the formula Ihc are obtained:
R1
i
(
i
Ihc
O " O
N
CONH-(CH2)o Het
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 79 -
CONH- (CH2) o-Het R in R -H and in Ihc MS
calc. fnd.
-NH- ( CH2 ) ~-NH2 4 8 7 4 g g
CH2-NI
CONH-CH2 ~ ~ -N-CH2 ~ ~ 493 494
H
Example 24
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1,3-dione is reacted with 3
aminomethylbenzylamine and then with R1-H. The following
compounds of the formula Iia are obtained:
R1
i
w i
lia
O N O
NH2
i
R1 in R -H and in Iia
'- ~O
-N
N
\ /
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 80 -
R in R -H and in Iia
h
-N ~CH3
H
-NH- ( CH2 ) Z-COOMe
-N CH2 / \
H
-N (CHZ)2 / \
i
H
-N - CH2 / \ Sp2NH2
H
-N (CH2)2 / \ pH
H
-NH- ( CHz ) 5-OH
-NH- ( CH2 ) 3-N ( CH3 ) z
-N - CH / \ NH
H
CH2- NH2
-N -CH2 / \
H
~\
-N (CH2)2
i
H
-N (CH2)2 ~ \
H
-N CH2
H
-N (CH2)2-N O
WJ
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- R1 -
Rl in R -H and in Iia
-N -(CH2)3-N N-CH
3
N (CH )3 N~
i
H
-NH- ( CHZ ) s-NH2
-NH- ( CH2 ) ~-NH2
Example 25
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1, 3-dione is reacted with H2N-CHZ-Ar' - (CH2) n
CONH2 and then with Rl-H. The following compounds of the
formula Iib are obtained:
R1
i
l
i
~ lib
O "_O
N
~H2
Ar'-(CH2)n-CONH2
Ar' - (CH2) n-CONHZ R in R -H and in Iib MS
calc. fnd.
CONH2 - p 415 416
CONH2
CO - NH2
/ \
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- R7
Ar' - (CH2) n-CONHZ R in R -H and in Iib MS
calc. fnd.
CONH2 -N,
CONH2 419 420
CO - NH2
/ \ 413 414
CONH2 -NJ 399 400
CONH2 405 406
CO - NH2
/ \ 399 400
CONH2 -NN N
\ /
CONH2
CO - NH2
/ \ 490 491
CONH2 Ph 449 450
-N~CH3
H
CONH2
CO - NH2
/ \
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 83 -
Ar' - (CHZ) n-CONH2 R in R -H and in Iib MS
calc. fnd.
CONH2 -NH- (CHZ) 2-COOMe 431 432
CONH2
CO - NH2
/ \
CONH2 -N - CH2 / \
H
CONH2 4 41 4 4 2
CO - NH2
/ \
CONH2 -N-(CH2)2 / \ 449 450
H
CONH2 455 456
CO - NH2
/ \
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- R4 -
Ar'-(CH2)n-CONH2 Ri in R -H and in Iib MS
calc. fnd.
CONH2 -N '-CH2 /-\ SO2NH~
H
CONH2 520 521
CO - NH2
/ \
CONH2 -N -(CH2)2 / \ O~ 9 65 4 66
H
CONH2 4 71 4 72
CO - NHz
/ \
CONH2 -N-(CH2)2 / f~ 450 451
H
CONH2 457 458
CO - NH2
/ \ 450 451
CONHZ -N-(CH2)2 ~ \ 450
H 4s1
CONH2
456
CO- NH2
457
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 85 -
Ar' - (CH2) n-CONH2 R in R -H and in Iib MS
calc. fnd.
CONH2 -N -CH2 / \ 436 437
H
CONH2 442 443
CO - NH2
/ \ 436
437
CONH2 -N (CH2)2-N p 458 459
H
CONH2 469 465
CO - NH2
/ \ 458 459
CONH2 /~ 485 986
-H -(CH2)3- ~N-CH;
CONH2 4 91 4 92
CO - NH2
/ \ 485 486
CONH2 -N-(CH2)3-N~N 455 456
H
CONH2 4 61 4 62
CO - NH2
/ \
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 86 -
Ar' - (CH2) n-CONH2 R in R -H and in Iib MS
calc. fnd.
CONH2 -NH- ( CH2 ) 5-NH2
CONH2 436 437
CO - NH2
/ \ 430 431
CONH2 -NH- ( CHZ ) ~-NH2 4 5 8 4 5 9
CONH2 464 465
CO - NH2
/ \
CONH2 -NH- ( CH2 ) 3-N ( CH3 ) z 9 3 0 4 31
CONH2 436 437
CO NH2
/ \ 930 931
CONH2 -NH- ( CH2 ) 5-OH 4 31 4 32
CONH2 437 438
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 87 _
Ar' - (CH2) n-CONH2 R in R -H and in Iib MS
calc. fnd.
CO - NH2 -NH- ( CHZ ) 5-OH
CONH2 -N -CH2 ~ ~ NH2
H
CONH2
CO ' NH2
/ \
CONH2 CH2-NH2 4 64 4 65
-N -CH2 ~ \
H
CONH2
CO - NH2
/ \
CONH2 -NH- ( CH2 ) 3-NH ( CH3 ) 416 417
CONH2 422 423
CONH2 -N (CH3) - (CH2) 3-NH (CH3) 430 431
CONH2 436 437
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- RR -
Ar' - (CH2) n-CONHZ R in R -H and in Iib MS
calc. fnd.
CONHZ -N (CH3) - (CHZ) z-N (C2H5) 2 458 459
CONH2 4 64 4 65
CONH2 N-(CH2)2-N--~ 4 30 4 31
H H
CONH2 436 437
CONH2 -N N-CH ~ ~ p 548 549
U z
CONH2 554 555
CONH2 - ~ -CHZ ~-~ 504 505
CONH2
CONH2 -N N ~ ~ 4 91 4 92
U
CONH2
CONHZ -N N--C~ ~ 4 92 4 93
N
CONH2
CONH 'NH-(CH2)3- ~ -(CH2)3--NH2 528 529
2
CONH2
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 8g _
Ar' - (CH2 ) n-CONHZ R in R -H and in Iib MS
calc. fnd.
CONH2 484 485
-NH N
CONH2 4 90 4 91
CONH2 -NH- ( CH2 ) 2-NH- ( CHZ ) z-OH 4 32 4 3 3
CONH2 438 439
CONH2 -NH- ( CHZ ) 2-NH ( C3H~ ) 9 3 0 4 31
CONH2 436 437
CONHZ -NH- (CHz) 3-0- (CHz) a-0- (CH2) 3-
NHZ
CONH2 538 539
CONH2 -NH-(CH2)3 N 484 485
H
CONH2 4 90 4 91
CONH2 CH2NH2
-NH-CH2
CONH2 476 477
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- An -
Ar' - (CHZ) "-CONHZR in R -H and in Iib MS
calc. fnd.
CONH2 473 474
-NH- (CH
)
-N (CH
2
3
3) - (CH2) 3-
NH2
CONH 479 480
2
CONH2 416 417
-NH- ( CH
)
-NH ( C
H
2
2
Z
S )
CONH2 422 423
CONH N-'CH2 ~-~ CHZ NHS
2 H
CONH2
Example 26
Analogously to Example 2, 6-nitrobenzo[de]iso
chromene-1, 3-dione is reacted with H2N-CH2-Ar' - (CH2) n
CONH- (CH2) o-NH2 and then with R1-H. The following
compounds of the formula Iic are obtained:
R1
i
i
lic
O ' 'O
N
~H2
Ar'-(CHZ)~-CONH-(CH2)o-NH2
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
a~
Ar' - (CH2) n-CONH- R in R -H and Iic MS
(CH2) o-NH2 calc. fnd.
~CONH-(CHZ)4 NHZ -NH- ( CHZ ) ~-NHZ
CH2- NH2
-N-CH2 / 541 542
\
_
H
CONH-(CH2)4-NHZ
-NH- (CHZ) ~-NH2 529 530
CH2- NHz
-N-CHZ / 535 536
\
_
H
'-~-CONH-(CH2)Z NHZ -NH- ( CH2 ) ~-NH2 5 0 7 5
0
8
CH2-NH2
-N-CH2 / 513 514
\
-
H
~ CONH-(CHZ)z-NHi -NH- (CHz) ~-NH2 501 502
CH2- NH2
\
-N-CH2 /
-
H
Example 27
Analogously to Example 2, 6-chlorobenzo[de]iso-
chromene-1, 3-dione is reacted with HZN-CHZ-Ar' - (CHZ) n-
CONH- (CH2) o-Het and then with R1-H. The following
compounds of the formula Iid are obtained:
i
i
lid
O ' 'O
N
~H2
Ar'-(CH2)n-CONH-(CH2)o Het
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
92
Ar' - (CHz) n-CONH- R in R -H and Iid MS
(CHz) o-Het calc. fnd.
--~-CONH-CH ~ -NH- ( CHz ) 3-N ( CH3 ) z
2~
1d -NH- (CHz) 7-NHz 555 556
CH2 NH2
-N-oHZ /-\ 561 562
H
~CONH-CH2~ -NH- (CHz) 3-N (CH3) z 521 522
-NH- ( CHz ) ~-NHz
CH2- NH2
-N-CH2 ~_~ 555 556
H
Example 28
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1, 3-dione is reacted with H2N-CHz-Ar' - (CHz) n
CONHA and then with Rl-H. The following compounds of
the formula Iie are obtained:
R1
i
i
~ lie
O ' \-O
N
~HZ
Ar'-{CH2)n-CONHA
Ar' - (CHz) n-CONHA R in R -H and Iie MS
calc. fnd.
-NH- ( CHz ) 7-NHz
CONH(C3H~)
CHp- NH2
-N-CH2 ~-~ 512 513
H
- -NH- (CHz) 3-N (CH3) z 472 473
CONH(C3H7) -NH- ( CHz ) ~-NHz
CH2- NH2
-N---CH2 ~_~ 506 507
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
93
Ar'-(CHZ)n-CONHA R in R -H and Iie MS
calc. fnd.
-NH- ( CHZ ) ~-NHZ
CONH(CSH~ ~ )
CH2- NHZ
-N-CH2 /_\ 540 541
H
-NH- (CH2) 3-N (CH3) 2 500 50
CONH(C5H~ ~ )
-NH- ( CHZ ) ~-NH2
CH2- NH2
-N ~ CH2 /-\
H
Example 29
Analogously to Example 2, 6-chlorobenzo[de]iso
chromene-1, 3-dione is reacted with H2N-CH2-Ar' - (CH2) n
CONH- (CH2) o-Ar and then with R1-H. The following
compounds of the formula Iif are obtained:
R1
i
i
lif
O ~O
N
~H2
Ar'-(CH2)n-CONH-(CH2)o Ar
Ar' - ( CH2 ) n-CONH- R in R -H and I i f MS
(CH2}o-Ar calc. fnd.
~CONH-CH2--( ) -NH- ( CH2 ) 3-N ( CH3 ) z
-NH- ( CH2 ) ~-NH2
CH2 NHZ
-H-CH2 ~_~ 566 567
--\ r-CONH-CH2-( ) -NH- (CH2) ~-NHZ 554 555
~l CHZ NHZ
-H-CHZ ~_~ 560 561
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
94
Ar'-(CHZ)n-CONH- R in R -H and Iif MS
(CHZ) o-Ar calc. fnd.
-NH- ( CH2 ) 3-N
\-/ ( CH3 ) 2
CONH ~
-NH- (CHZ) ~-NH2 583 584
CH2-NH2
\ 589 590
-H-CH2 /
-
\ / CONH \-/ ~ -NH- (CH2) ~-NH2 577 578
CH2- NHZ
\
-H -. CH2 /
-
/ \ -NH- ( CH2 ) ~-NH2
~CONH
\ /
CH2-NH2
-H -CH2 /
\
-
/ -NH- ( CH2 ) 3-N
\ ( CH3 ) 2
CONH
-
\ /
-NH- ( CH2 ) 7-NH2
CH2- NHZ
-CH / \
-
H
~CONH-CH2 ~ / CI -NH- (CHZ) 3-N (CH3}560 561
Z
-NH- (CHz) ~-NH2 588 589
CH2-NH2
-H-CH2 / 594 595
\
_
~CONH-CH2 \ / CI WNH- (CHZ) ~-NHZ 582 583
CH2- NH2
--N-CHZ / 5 8 8 5 8
\ 9
_
H
~CONH-(CH2)3 \ / -NH- (CH2) 3-N (CH3)554 555
2
-NH- (CH2) ~-NH2 582 583
CH2-NH2
- -CHZ / \
CONH-(CHZ)3 \~/ -NH- ( CHZ ) ~-NHZ 57 6 577
CH2- NH2
\
_N-CH2 /
-
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
Example 30
Analogously to Example 2, 6-chlorobenzo[de)iso
chromene-1,3-dione is reacted with HZN-CH(R)-CONH2 and
then with R1-H. The following compounds of the formula
5 Ik are obtained:
R1
i
i
Ik
O N' ' O
~H(R)
CONH2
R in R -H and Ik R MS
calc. fnd.
CH2-NH2 CH2-Ph
~N-eH2 /-\ CH2-CH (CH3) 2
H
-NH- (CH2) 3-N (CH3) CH2-Ph
2
CHZ-CH ( CH3 } 2
-NH- ( CHZ ) 4-NH2 CH2-Ph
CH2-CH ( CH3 ) 2
-NH- ( CHZ ) ~-NHZ CHZ-Ph
CHZ-CH (CH3) 2
-NH- ( CH2 ) g-NH2 CH2-Ph
CH2-CH ( CH3 ) z
-N -(CH -N~N CHz-Ph 4 69 4 70
CH2-CH ( CH3 ) 2 4 3 5 4 3 6
CH2-Ph
CH2-CH ( CH3 ) z
-N-(CH2)2-N--C CH2-Ph
H H CH2-CH ( CH3 ) 2
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 96 -
Example 31:
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with H2N-Ar and
then with R1-H. The following compounds of the formula
I1 are obtained:
R~
11
C N ~O
~CH2);
IAr
Ar R1 in R1-H and in I1
2
O-CHz-Ph -NH- ( CH2 ) s-NH2
_
/ O CHz_Ph -NH- ( CH2 ) s-NHz
-NH- ( CHz ) 7-NHZ
CH2- NH2
\
-N - CH2 /
-
H
CHz - NHz
-N-CHz--( )
HH
-N-CHz-( r-CHz-NHz
~
/H
.
-NH- ( CH2 ) 3-NHZ
/ O-Ph
-NH- ( CHz ) s-NHZ
-NH- ( CH2 ) ~-NHz
CH2- NHZ
\
-N-CH2 /
-
H
CHz - NHz
-N-CHz--( )
~J
H
-N-.CHz~CHz-NHz
H
1~
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 97 -
Ar R1 in R1-H and in I1
1 -NH- ( CH2 ) 3-NH2
-NH- ( CHZ ) s-NHZ
-NH- (CHZ) ~-NHZ
CH2- NHZ
\
-N-CH2 /
-
H
CHz - NHZ
-N-CH2-( )
~/
H
-N-CHZ-( r--CHz-NHZ
\
/H
~
1 ""' -NH- ( CHZ ) 3-NH2
oCH, -NH- ( CH2 ) 5-NH2
oCH -NH- ( CHz ) ~-NH2
3
CHZ- NH2
\
-N-CH2 /
-
H
CHi - NHZ
-N-CHz--( )
~
/
..
H
-N-CHZ--( r-CHz-NHz
\
/H
~
Example 32:
Equimolar amounts of 6-(3-amino-propylamino)-2-(3,9,5-
trimethoxy-benzyl)-benzo[de]isoquinoline-1,3-dione
(according to example 31, page 96, table line 4) and
methanesulfonic acid are reacted according to known
procedures to give the acid addition salt 6-(3-amino-
propylamino)-2-(3,4,5-trimethoxy-benzyl)-
benzo[de]isoquinoline-1,3-dione, methane sulfonate.
1H-nmr (DMSO-d6)
8.74 (dd, J - 0.8 and J - 8.5 Hz, 1H), 8.46 (dd, J -
0.$ and J = 7.3 Hz, 1H), 8.29 (d, J = 8.5 Hz, 1H), 7.81
(t, J = 5. 5 Hz, 1H (NH) ) , 7 . 77-7 . 68 (m, 4H ( 3xNH) ) , 6. 86
(d, J = 8.7 Hz, 1H), 6.66 (s, 2H), 3.75 (s, 3H), 3.74
(s, 3H) , 3. 53-3. 47 (m, 2H) , 3. 02-2. 94 (m, 2H) , 2.31 (s,
3H), 2.04-1.96 (m, 2H).
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 98 -
Abbreviations of the nmr-signals (nmr - nuclear
magnetic resonance):
s singlet,
d doublet,
dd double doublet,
t triplet,
sbr broad singlet,
m multiplet,
q quadruplet,
J coupling constant J in Hz
Example 33:
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with H2N-CHZ-Ar'
(CH2) n-CONH- (CH2) o-Ar and then with R1-H. The following
compounds of the formula Iif are obtained:
R~
I if
O N" O
CH2
Ar'-(CH2)~-CONH-(CH2)o Ar
Ar' - ( CHZ ) "-CONH- R1 1n R1-H and I
( CHZ ) o-Ar i. f
H
CONH-CH2 ~ N
~
NHi
-N -~H~
H
CH2-NH2
CONH ~ -N-CH
2
H
NH
N
NHZ
-N_CH=
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
_ 99 _
Ar' - (CHZ ) "-CONH- (CH2 ) o-Ar Rl in Rl-H and I i f
~ CH =
~ CONH-(CH2)Z-(' ) 2 2
~.J -N-CHZ ~-~ '
H
NH
N NHs
-N -CHZ
H
CH2-NH2 -
CONH ~ ~ -N-CH2
H
jJ H
N NH=
-N -CHZ
H
CH2- NHZ
~ CONH-CHZ ~ ~ -N-CHz
H
JJH
N NHi
-N-CHZ
H
CONH-(CHz)2 ~ ~ / \ CH2- 2
-N-CH2
H
JJH
~//N
NHz
-N -CHZ
H
H
CONH-(CHZ)~ ~ ~
NHz
-N -CHz
H
CH2 NH2
CONH-(CHZ)a _
N CH2
H
JJH
N NHz
-N -CHZ
H
- CHZ- NH2 -
CONH ~ ~ -N-CH
H 2
JJH
N NHa
-N-CH=
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 100 -
Ar~-(CHz)~-CONH-(CHZ)o-Ar R1 in R1-H and Iif
CH2 2
CONH ~ ~ -N-CH2 /-\
H
CN H
N NHz
-N -CHz
H
CH -
CONH-(CH2)z ~ ~ NOz / \ 2 2
-N CH2 '-/
H
NH
N NHz
-N -CHz
H
- CH2-
CONH-CHZ ~ ~ -N-CH2 /-\
O H
f,IH
N NHz
-N -CHi
H
~CH~ CH2-
CONH-CHz ~ ~ N~CH~ -N-CH2 / \
H
~H
N NHz
-N -CHz
H
~CH3 H
CONH
CHI ~ N NHz
'_ ~ -CHz
H
CONH ~ / N\ CZHS C -
C2H3 ~N-CH2 /-\
H
NH
N NHz
-N -CHZ
H
CONH-CHz ~ ~ SOz-NHz CH2 2
-N-CH2 /-\
H
JJH
N NHz
-N -CHz
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 101 -
Ar~-(CHZ)"-CONH-(CHZ)o-Ar R1 in R1-H and Iif
/ CONH-(CHz)z / ~ CH3 CH2- 2
-N-CH2 /-\
H
/JH
N NH:
-N -CHZ
H
CH2- 2
CONH ~ ~ CH3 -N-CH2 /-\
H
~H
N NHS
-N -CH2
H
CH3 CH2- ~
CONH ~ ~ -N-cH2 /-\
H
CH3 ~H
N NHz
- N - CHI
H
CH2- NH2 _
~ CONH-CHZ / \
-~ -CH2
CF3 H
fIH
N NHz
-N -CHZ
H
CHZ-NH2
CONH -N-CH / \
i 2
H
CF3 NH
NJ~NH:
-N -CH2
H
Lh3 CHZ- NH2-
CONH ~ ~ -N-CH2 /-\
H
CF3 rH
N NHS
-N -CHz
H
1:2r15 CHZ-NH2
-N-CH2 /-\
~ CONH
TI H
N NHi
-N-CHz
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 102 -
Ar ~- (CH2) ~-CONH- (CHZ)R1 in R1-H arid
o-Ar Iif
CH2= 2
CONH / ~ CH / \
I -N-CH
2
H
flH
N
NH=
-N-CHz
H
CH2 N z
CONH -N- / \
CH2
i
H
r1H
N
NHS
-N-CHZ
H
CONH / ~ CH3 / \ CH2- 2
-N-
CH3 CH
i 2
H
JJH
N
NHz
-N -CHI
H
CH2-NHz
CONH-(CHz)2 -N-CHZ / \
H
CI H
N~
NH~
-N -~HZ
H
CHZ-NHZ
CONH ~ ~ -N-cH /
\
2
-
H
CI H
N
NHi
-N -CHI
H
CH2- NH2
CONH-CH2 -N-CH / \
2
i
H
CI H
N~
NH~
-N -CHZ
H
CH2-NH2
CONH-(CHz)Z / \
' --N - CHZ
H
CI H
N~
NH~
-N -CHi
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 103 -
Ar' - (CHz) "-CONH- (CHZ) o-Ar R1 lri R1-H drid Ilf
n
CHZ- 2
CONH ~ ~ CI -N-CH
H 2
JJH
N NHz
-N -CHz
H
CH2- NH2
CONH-(CHz)z / ~ CI / \
-~ - CH2
H
TlH
N NHz
-N -CHz
H
CONH -CHz ~ ~ CI N H
~NHz
- N - CHz
H
CI CHZ- 2
CONH-CHz ~ ~ -N-CHZ
H
CI H
N NH=
-N -CHz
H
CI CI CH2 2
CONH-CH2 ~ ~ -N-CHZ
H
Tl H
N NHZ
-N -CHz
H
CI CHy- 2
CONH-CHz / ~ CI -N-CHz
H
H
N~NHx
- N -CHz
H
CI CH2- 2
CONH-(CHz)z ~ ~ CI -N-CH2
H
H
N~NH=
-N-CHz
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 104 -
Ar ~ - ( CHZ ) ~-CONH- ( CHZ ) o-Ar R1 in R1-H and T i f
CI CH2-
CONH-CHZ ~ ~ -N-CH2
H
CI H
N
NHi
-N -CHZ
H
CI CH2- 2
CONH ~ ~ -N-cH2
H
CI
N NHz
-N -CHI
H
CI CH2=R
CONH ~ ~ CI N-cH2
H
NH
N
NHz
-N -CHI
H
CH2- NH2
CONH-CHZ ~ ~ F
N CH2
CI H
rIH
N NH?
-N-CHZ
H
CH2-N 2
~ CONH ~ \ F -N-CH
H 2
CI ,,,H
N NH:
-N -CHz
H
H -
CH2-
CONH-CH2 ~ ~ F
-N -CH2
CI H
H
N NHZ
-N -CH2
H
CHZ- NH2
CONH-(CHZ)2 ~ ~ F
-N - CH2
H
JJ H
N NHz
-N-CHi
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 105 -
Ar'- (CHz) "-CONH- (CHZ) o-Ar R1 lri R1-H and Ilf
C 2 2
~ CONH-CH2 -N-CH / \
i 2~
F H
f1H
N NHz
-N -CHz
H
CHZ- NH2
/ CONH / ~ -N-CH2 /-\
H
F H
N NHz
-N -CHs
H
CHZ- NHZ -
/ CON / ~ -N-CHZ /-\
H
F H
N NHz
-N -CHZ
H
CHZ- NH2
CONH-(CH2)z / \ -N-CH2 / \
OCH~ H
JJ H
N NHi
-N -CHz
H
CHZ-NH2 -
CON ~ ~ -N-CHZ / \
H
OCH3 ~H
N NHZ
-N-CHz
H
CH2--NHZ
CONH-CH2 / \
-N -CH2
OCH3 H
NH
N NH=
-N -CHz
H
CH2-NH2
CON ~ ~ OCH3 / \
-N -CHZ
H
JJ H
N NHz
-N -CH2
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 106 -
Ar~-(CHZ)n-CONH-(CHZ)o-Ar R1 in_ R1-H and Iif
CH2- 2
CONH-(CHZ)i ~_~ OCH~ / \
-N -CH2
H
JJH
N NH=
-N -CHZ
H
-N-CH2 /-\
CH2= 2
CONH-CHi ~ ~ OCF~
H
rI H
N N H:
- N - CHZ
H
F -
CONH ~ ~ OCH3 -N-CHZ /-\
H
fJH
N NHz
-N -CHj
H
F CH2- 2
CONH ~ ~ H3 -N-CH2 /-\
H
NH
N NHz
-N-CHs
H
CF3 C -
CONH ~ ~ -N-CH2 /-\
H
NH
OCH3 ''//\N
NH2
-N-CHz
H
tert-butyl CH2- 2
-N-CHZ /-\
CONH
H
tert-butyl H
N NHz
- N - CHz
H
CH3 CHZ- 2
-N-CH /-\
CONH-(CHz)z ~ p
H
OCH~ H
N NHS
-N-CHz
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 107 -
Ar'-(CHz)"-CONH-(CHz)o-Ar R1 in R1-H and Iif
C~tig CH2- NH2
CONH ~ ~ -N -CH2 /-\
H
jJH
OCH3 ~//N
NHi
-N -CHz
H
v.n~ CH2-N 2
CONH-(CHI Z ~ ~ OCH~ -N-CHZ /-\
H
NH
N NHz
-N -CHi
H
OP CH2- 2
-N-CH2 /-\ ,
~ CONH H
fIH
N NH=
-N -CHz
H
CH2- NH2
CONH ~ ~ OPh -N-CH / \
2~
H
JJH
N~l/ NH:
-N -CH2
H
/ \ ~ CH2-NH2 _
\ / CONH O ~ ~ CHI
-N-CH2 /-\
H
H
N' \NHI
-N -CHZ
H
CHZ- NHZ
CONH /-\
-H - CH2
O
~--Ph H
N NHz
-N -CHi
H
CH2- NH2 _
CONH-CHZ -N- H~ / \
C z \.-/
H
TIH
~//N
NHS
-N -CHZ
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 108 -
Ar'-(CHZ)~-CONH-(CHZ)o-ArR1 i.ri R1-H arid
Iif
CH2 H2
~ \
~
-C
-
/ -
CONH H2
N
H
NH
N
NHi
-N -CH2
H
O CH2- 2
O -N-CH2
CONH-CH
2 ~ ~ H
H
N~
NH~
-N-MHz I ~
H
CH2- NHZ -
CONH ~ ~ OCH3
-N -CH2
CI H
~H
N NHZ
-N -CHZ
H
CH2-NHZ _ _
CONH ~ ~ CI -N-CHZ
H
CF3 ~H
N NHs
-N -CHZ
H
CHp- NH2
CONH-(CH2)2 -N -cH
2
H
CH2 NH2 -
CONH-CH2 -N -CHZ
H
CONH-(CHz)z ~ ~ ,.
CH2- 2
-N -CH
CI H
CHZ- NHZ _
CONH ~ ~ CI \
-N -cH2 ~
-
H
CI _ _ _ cH - 2
CONH-CH2 ~ ~ -N-CH2 ~-\
H
CI
CI CI cH2- 2
CONH-CHZ ~ ~ -N -cH2 ~-\
H
/~
---( r-CONH-(CHz)2 /-\ ,.
F CH2- y
~/ ~ \
N
CH2
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 109 -
Ar~-(CHZ)~-CONH-(CHZ)o-Ar R1 in R1-H and Iif
CONH-(CHz)z ~ ~ OCH~ C 2 2
-N -CH2
H
C2H5 CH2-
CONH ~ ~ -N-cH2 /~\ ~
H
CONH ~ ~ cH2 2
-N-CH2 /-\
H
CHZ- 2
CONH ~ ~ OPh / \
-N -CH2
H
CH3
CONH ~ ~ t~ , -N-CH? / \ CHz-NHz
CHI
CHI CH
CONH-CHI
~CH3 -N -CH2 /-\
H
CH2- NH2
-N-CH2 /-\
H
CONH-CHZ
~~ CH2 2
1~f1
CONH-CH2 ~ ~ 0 -N -CH2 /_\
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 110 -
Example 34:
Analogously to Example 2, 6-chlorobenzo-
[de]isochromene-1,3-dione is reacted with HZN-CHZ-Ar'-
(CH2) n-CONH- (CH2) o-Het and then with R1-H. The following
compounds of the formula Iid are obtained:
R~
/ \
\ I /
lid
O ~O
N
CH2
Ar'-(CH2)~ CONH-{CH2)o Het
Ar' - ( CHZ ) ~-CONH- (CHZ ) o-Het R1 in R1-_H and I id
H
CONH-CH2 ~ ~ N 'NHz
N
-N -CHZ
H
CH2- NH2
CONH ~-~ -N-CHZ
N H
rIH
N NHS
-N -CHz
H
CH2- NH2
CONH-CH2 ~ \ -N-CH2
H
TI H
N NHi
-N -CH2
H
CH2-NH2 -
CONH ~ ~N -N-CH ~-~
i 2
H
H
N~NHz
-N -CHZ
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 111 -
Ar'-(CHZ)n-CONH-(CH2),o-HetR1 iri R1-H arid
Iid
CH2- 2
CONH-(CHZ)Z ~-~ -N-CH / \
2~ ,
i
H
f,IH
N
NH=
-N -CHZ
H
CH2-NHZ
CONH-(CH2)Z -N-CH / \
i 2
H
NH
N'//\
NH~
-N -CHz
H
CHZ- NH2
CONH-(CH2)2-N~ / \
CH2
H
O CH2-
/ \
CONH-(CHZ)3-N -N-CHZ--d
H
CH2- NH2.
CONH-(CHZ)z N / \
CH
2
H
JJH
N
NHz
-N-CHI
H
CH2- NH2
CONH-(CHZ)3 -N~ / \
~ H-CH
N 2
H
S
H
CONH-CH2 \ I CHZ-
-N-CH / \
2~
H
f! H
N
NHz
-N -CHz
H
- CHZ- 2
CONH-CHz \ ( --N-CH / \
2
i
H
fiH
N
NHZ
-N-CHI
H
/~ /~ CH2-NHZ -
--C r--CONH-(CHZ)2-~O -N-cH /
~/ \
~/ -
H 2
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 112 -
Ar'- (CHZ) ~-CONH- (CHz) o-Het R1 in R1-H and Iid
/~ O ~ CH2- 2
--( r--CONH-CH2 ~ I -N-cH ~-~ H
~/ H
CH2-NH2
CONH-(CHZ)3 -~~ -N -cH2
N '
H
/'~ CH2-NHZ -
~CONH-CH2 ~ ~N -N-CH
H
CHZ NHZ
CONH ~ ~N -N-cH2 i-\
H
CHZ NH2
CONH ~ ~ -H -cH2
-N
Example 35:
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with HZN-CH2-Ar'
(CH2) n-CONH- (CHZ) o-CH (Arl) -Ar2 and then with R1-H. The
following compounds of the formula I2 are obtained:
R~
/
12
O " O
N
CH2
Ar'-(CH2)~-CONH-(CH2)o CH(Ar~)-Ar2
Ar ~ - (CHZ ) "-CONH- R1 in R1-H and
(cH2 ) o- I2
CH;ArI ) -Arz
CHZ- NH2
-N - CH
2
CONH=
(CHZ)z-CH H
J H
\ J
N
NHz
-N -CHi
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 113 -
Ar' - (CHZ ) ~-CONH- (CHz ) o- R1 in R1-H and I2
CH (Arl ) -Arz
CH2-NHZ
-N-CH2 / \
CONH- CH -CH
( z)z / H
NH
NN
NHS
-N-CHz
H
/ CH2 NH2
-N -CH2 / \ -
CONH-CHz-CH , H
N'\H
NH=
~N-CHz
H
Example 36:
Analogously to Example 2, 6-chlorobenzo
[de]isochromene-1,3-dione is reacted with H2N-CH2-Ar'
(CH2) n-CONH- (CH2) o-CH (A) -Ph and then with R1-H. The
following compounds of the formula I3 are obtained:
R~
~ 13
O " O
N
I
CH2
Ar'-(CH2)~-CONH-(CHZ)o CH(A).Ph
Ar ~ - ( CHz ) n-CONH- (CHZ ) o- R1 in R1-H and I 3
CH(A)-Ph
/CH3 CH2- 2
h
CONH-CHz-CH , _N-CH2 / \
H
,CH3 H
CONH-CHz-CH / N~NH
-N-CHz
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 114 -
Example 37:
Analogously to Example 2, 6-chlorobenzo-
[deJisochromene-1,3-dione is reacted with HZN-CH2-Ar'-
(CH2)n-CONHA and then with R1-H. The following compounds
of the formula Iie are obtained:
R~
/ \
\
~ lie
O " O
N
CH2
Ar'-(CH2)~-CONHA
Ar' - (CHZ) "-CONHA R1 in R1-H and Iie
CH2- NH2
CONH-(CH2)Z C(CH
)
3 ,N-CH
3 i 2
H
H
N"tNHx
-N -CHZ
H
_ NH
N~
CONH-C3H~ NH,
~ ~
-N-CHZ
H
Example 38:
Analogously to Example 2, 6-nitrobenzo-
[de]isochromene-1,3-dione is reacted with HZN-(CH2)z-
CONH- (CH2) o-Ar and then with R1-H. The following
compounds of the formula Iha are obtained:
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 115 -
R~
iha
O N"O
CONH-(CH2)o Ar
CONH- (CHZ) o-Ar R1 in R1-H and in IHa
- CH2-
CONH-(CH2)2~ / \
-N -CH2
_ H
CI GH2- 2
-N-CHZ /-\ ~
CONH-(CH2)z H
CH2- NH2
CONH-(CHZ)z ~ ~ F / \
-N -CH2
H
CHZ-N 2
CONH-(CHz)z ~ ~ OCH3
-N -CH2
H
CH2- NH2
CONH-CHz -N-GHZ /-\
H
CI CH2- 2
/-\ ~
CONH-CH2 ~ ~ -H-CH2
CI
CH2- NH2
CONH-CH2 -N-CHZ /-\
H
CI CI
CH CHZ- NN2
CONH-CHZ ~ ~ N, '
CH3 -N -, GH2 /-\
H
CH2 N 2
-N-CHZ /-\
CONH-CH2
C - 2
/ \
CONH-CH ~ ~ O -N-GH2--
H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 116 -
CONH- (CHz) o-Ar R1 in R1-H and in IHa
C2H5 CH2- 2
\
CONH ~ ~ -N-cH2
H
CHZ-NH2
CONH ~ ~ -N-CH
H 2
CH2-
CONH ~ ~ OPh -N-CH2
H
CHZ- NH2 -
CONH ~ ~ C~ -N-CH2
H
CHZ NH2 -
CONH ~ ~ -N-cH
H
CHs
CHZ- NH2 -
CONH ~ ~ -N -cH
i 2
H
N02
CHs _ cH -
CONH ~ \ CHs -N-CH
CHs
N02
CHZ- NH2
CONH ~ ~ OH -N -cH2
H
N
CZHS ~C2H5
CHZ-NH2
CONH ~ ~ CH3
-N - CH2
SOz-N, H
CzHS
\ ,CH3 CH2- 2
CONH N'CH
SOz \ / CH3 H CHZ
CH2- NH2 _
O -N - CH2
CONH ~ ~ OCH3
CH2- NH2 _
CONH-CHZ ~ ~ B~ -N-cH
i 2
H
CH2 NHZ
CONH ~ ~ -N-CH
H 2
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 117 -
CONH- (CH2 ) o-Ar R1 in R1-H and in IHa
CH2- NHZ
CONH
-N - CH2
H
Example 39:
A suspension of 4 g of 6-nitrobenzo[de]iso
chromene-1,3-dione in 100 ml of toluene is treated with
3.1 g of 2-amino-N-(4-iodo-phenyl)-acetamide and the
mixture is heated under reflux. After reaction is
complete, the reaction mixture is allowed to cool and
is worked up as is customary. N-(4-Iodo-phenyl)-2-(6-
nitro-1,3-dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-
acetamide is obtained. 1.2 Equivalents of KZC03, 1.2
equivalents of Ph-B-(OH)2 and 10 mol% of Pd((PPh)3)4 are
added to a solution of this compound in 80 ml of DMF
and it is heated at 80°C until conversion is complete.
After filtering off the catalyst and customary working
up, N-biphenyl-4-yl-2-(6-nitro-1,3-dioxo-1H,3H-
benzo[de]isoquinolin-2-yl)-acetamide is heated with 3-
aminomethyl-benzylamine until conversion is complete.
After.cooling the reaction mixture, it is worked up as
is customary and 2-[6-(3-aminomethyl-benzylamino)-1,3-
dioxo-1H,3H-benzo[de]isoquinolin-2-yl]-N-biphenyl-4-yl-
acetamide is obtained.
Analogously, by reaction of N-(4-iodo-3-
methoxyphenyl)-2-(6-nitro-1,3-dioxo-1H,3H-
benzo[de]isoquinolin-2-yl)-propionamide with Ph-B-(OH)Z
and 3-aminomethyl-benzylamine, 3-[6-(3-aminomethyl-
benzylamino)-1,3-dioxo-1H,3H-benzo[de]isoquinolin-2-
yl]-N-(2-methoxy-biphenyl-4-yl)-propionamide is
obtained.
Analogously, by reaction of N-(4-iodo-benzyl)-
3-(6-nitro-1,3-dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-
propionamide with Ph-B-(OH)2 and 3-aminomethyl-
benzylamine, 3-[6-(3-aminomethyl-benzylamino)-1,3-
dioxo-1H,3H-benzo[de]isoquinolin-2-yl]-N-biphenyl-4-
ylmethyl-propionamide is obtained.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 118 -
Example 40:
Analogously to example 39, by reaction of 6-
nitro-2-{4-iodophenyl)benzo[de]isoquinoline-1,3-dione
with R12-B-(OH)2 and 3-aminomethyl-benzylamine, the
following compounds of the formula I4 are obtained:
N ~ / ~NHZ
O N" O
14
CONH ~ ~ R~z
R12 in I4
F
OCH3
CF3
CF3
H3C
CF3
CF3
CH3
N02
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 119 -
R12 in I4
CH3
CH'
CH3
O
CH3
OCF3
CH3
,_O
-~N
CH3
Example 41:
Analogously to Example 2, 6-nitrobenzo
[de]isochromene-1,3-dione is reacted with H2N-(CH2)2
CONH-(CHZ)o-Het and then with R1-H. The following
compounds of the formula Ihc are obtained:
R~
/ \
Ihc
O ' 'O
N
CONH-(CH2)o Het
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 120 -
CONH- (CH2) o-Het R1 in R1-H and in Ihca
CH2 N 2
CONH-(CH2)2-N ~
\
~ -N-CH2
_
H
/~ CH2-NH2_
CONH-(CH2)s -N ~ \
-N-CH
2 L.J
H
N C 2- 2
CONH-CHZ ~ \ -N-cH ~
\
2
_
H
CH2-NHz
CONH-CH2 ~ \N \
~
-N-CH
-
2
H
_
O CH2- 2
CONH-CH
\
-N-CH2 ~
-
H
N CH2- 2
CONH ~ \ \
-N-cH ~
2
-
i
H
\ CH2- NH2 _
CONH~N -N-CH ~
\
_
H
CH2- NH2
,O \
~
-N - CH
CONH -
Z
/O CH - 2
-N-CH2 ~
\
~ ~ -
H
CONH
Example 42:
Analogously to example 2, by reaction of 6
nitro-benzo[de]isochromene-1,3-dione with 3-amino-N
(3,3-diphenyl-propyl)-propionamide and 3-aminomethyl
benzylamine, 3-[6-(3-aminomethyl-benzylamino)-1,3-
dioxo-1H,3H-benzo[de]isoquinolin-2-yl]-N-(3,3-diphenyl-
propyl)-propionamide is obtained.
Example 43:
Analogously to example 2, by reaction of 6-
nitro-benzo[de]isochromene-1,3-dione with N-[4-(3-
amino-propionylamino)-2-methoxy-phenyl]-2-methoxy-
benzamide and 3-aminomethyl-benzylamine, N-(4-(3-[6-(3-
Aminomethyl-benzylamino)-1,3-dioxo-1H,3H-
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 121 -
benzo[de]isoquinolin-2-yl]-propionylamino}-2-methoxy-
phenyl)-2-methoxy-benzamide is obtained.
Example 44:
Analogously to example 2, 6-nitrobenzo-
[de]isochromene-1,3-dione is reacted with H2N-(CH2)z-
CONH-Ar'-Het and then with 3-aminomethyl-benzylamine.
The following compounds of the formula I5 are obtained:
N ~ / \NH2
\ I
O ' 'O
N
CONH-Ar'-Het
CONH-Ar'-Het in I5
CONH ~ ~ p
U
CI
CONH ~ ~ N
CN
CONH
S
CONH
\ S
1
CONH
S
CONH / \ CHs
S
O
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 122 -
CONH-Ar'-Het in I5
CONH
CI
CONH
Example 45:
Analogously to example 2, 6-nitrobenzo
[de]isochromene-1,3-dione is reacted with 3,3-diphenyl
propylamine and then with R1-H. The following compounds
of the formula I6 are obtained:
R~
~ 16
O " O
N
R1 in R1-H and I6
-NH- ( CH2 ) 3-NH2
-NH- ( CH2 ) 5-NHZ _
-NH- ( CHz ) ~-NHZ
CH2- NHZ
-N-CHZ ~
H I
CHZ-NHi
-N-CHz-( j
~H
-N-CHZ~CHZ-NHZ
~/H
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 123 -
The following examples relate to pharmaceutical
preparations:
Example A: Injection vials
A solution of 100 g of an active compound of
the formula I and 5 g of disodium hydrogenphosphate is
adjusted to pH 6.5 in 3 1 of double-distilled water
using 2N hydrochloric acid, sterile-filtered, dispensed
into injection vials, lyophilized under sterile
conditions and aseptically sealed. Each injection vial
contains 5 mg of active compound.
Example 8: Suppositories
A mixture of 20 g of an active compound of the
formula I is melted with 100 g of Soya lecithin and
1400 g of cocoa butter, poured into moulds and allowed
to cool. Each suppository contains 20 mg of active
compound.
Example C: Solution
A solution is prepared from 1 g of an active
compound of the formula I, 9.38 g of NaH2P04.2H20,
28.48 g of Na2HP04.12Hz0 and 0.1 g of benzalkonium
chloride in 940 ml of double-distilled water. The
mixture is adjusted to pH 6.8, made up to 1 1 and
sterilized by irradiation. This solution can be used in
the form of eye drops.
Example D: Ointment
500 mg of an active compound of the formula I
is mixed with 99.5 g of petroleum jelly under aseptic
conditions.
Example E: Tablets
A mixture of 1 kg of active compound of the
formula I, 4 kg of lactose, 1.2 kg of potato starch,
0.2 g of talc and 0.1 kg of magnesium stearate is
compressed in a customary manner to give tablets such
that each tablet contains 10 mg of active compound.
CA 02351348 2001-05-23
WO 00/31039 PCT/EP99/08560
- 124 -
Example F: Coated tablets
Analogously to Example E, tablets are pressed
which are then coated with a coating of sucrose, potato
starch, talc, tragacanth and colourant in a customary
manner.
Example G: Capsules
2 kg of active compound of the formula I are
dispensed into hard gelatin capsules in a customary
manner such that each capsule contains 20 mg of the
active compound.
Example H: Ampoules
A solution of 1 kg of active compound of the
formula I in 60 ml of double-distilled water is
sterile-filtered, dispensed into ampoules, lyophilized
under sterile conditions and aseptically sealed. Each
ampoule contains 10 mg of active compound.
The preceding examples can be repeated with
similar success by substituting the generically or
specifically described reactants and/or operating
conditions of this invention for those used in the
preceding examples.
From the foregoing description, one skilled in
the art can easily ascertain the essential
characteristics of this invention and, without
departing from the spirit and scope thereof, can make
various changes and modifications of the invention to
adapt it to various usages and conditions.