Note: Descriptions are shown in the official language in which they were submitted.
WO 00/31750 PCT/IB99/01947
1
ELECTRICALLY CONDUCTIVE ELECTROACTIVE FUNCTIONALIZED
CONJUGATED POLYMERS, AND USES THEREOF
Conjugated polymers, such as polypyrroles,
polythiophenes, polyanilines, polyphenylenes and
derivatives thereof are known for their electroactive
nature, which is widely described in review works such as
the "Handbook of Organic Conducting Polymers" (T. J.
Skotheim Editor, Marcel Dekker, New York, 1986). These
polymers are obtained in the form of a film on an
electrode, in the form of self-supporting films or
alternatively in the form of a composite when combined
with a polycationic or polyanionic polymer and behave like
organic electrodes, which charge up according to an anodic
oxidation process, by insertion of ions from the
electrolytic medium. This electrochemica l process is
reversible, the reduction leading to the expulsion of the
ions from this conjugated polymer or from the
electroactive composite.
A second generation of conjugated polymers was
then described in the literature, obtained by the covalent
grafting, on to the monomer units of the polymers, of
functional groups-capable of providing these electroactive
conjugated polymers with an additional function. By way of
example, electrocatalytic metal complexes were grafted on
to the monomer units of the polypyrrole, specific
complexing macrocycles were grafted on to the polypyrrole
or polythiophene chains for the recognition of rations in
an electrolytic medium, and chiral groups were grafted on
to polythiophenes for the recognition of optically active
anions. All of these routes of functionalization have also
formed the subject of development procedures detailed in
the literature (F. Garnier, Angew. Chemie, 1989, 101, 529;
A. Deronzier, J.C. Moutet, Acc. Chem. Res. 1989, 22, 249;
J. Roncali, Chem Rev., 1992, 92, 713.).
CA 02351479 2001-05-17
wo oor~mso fCTIIB99/o1947
2
In the last few years authors have become
interested in the use of functionalized conductive
polymers for the development of analyte scavengers, in
particular for diagnostic purposes. However, as indicated
in Patent Application EP0,324,009, it was commonly
accepted by the scientific community that pyrrole polymers
substituted either on the nitrogen atom or directly on the
carbon atoms of the pyrrole ring were not good candidates
for the development of analyte scavengers, in particular
on account of the loss of conductivity of said polymers
when functional groups are introduced on to the
heteroatomic ring. In order to overcome this problem, the
authors of this patent application thus envisaged the use
of 2,5-di(2-thienylpyrrole) polymers which were grafted in
the 3-position of the pyrrole ring with a reactive moiety
with which an organic molecule could become covalently
bonded. It should, however, be noted that on account of
the hydrophobicity of the thiophene rings, the polymers
described cannot be conductive and electroactive in
aqueous media and consequently do not appear to be
suitable for the detection and/or characterization of an
analyte in a biological sample (see J. Roncali et al.,
Chem. Comm., 1986, page 783 and G. Tourillon et al.,
Electronal. Chem., 161, 407, 1984).
It has now been discovered, entirely surprisingly
and contrary to what was hitherto accepted by specialists,
that the conductivity and electroactivity of polypyrroles
are retained provided that a functional group is grafted
in the 3- or 4-position on the pyrrole ring using a
functionalizing agent which allows the intended function
to be distanced from the pyrrole ring. An antiligand is
covalently bonded to the free end of the functional group,
without the abovementioned properties of the polymer being
modified. Such functionalized polymers have to date never
been described and have shown themselves to be entirely
suitable as scavengers for a biological ligand. Moreover,
CA 02351479 2001-05-17
PE11tB99101947
~~ ;,
~'~'~$~~~U~ _ ~ ~? '~T~tB~9~lQ~~94~t S~ ~ESC~fi a
:~.._.~,.:.~;~.
3
polypyrroles prove to be advantageous polymers on. account
of their biocompatibility. Lastly, the polypyrroles thus
functionalized make it possible to prepare electroactive
and conductive polymers of considerable thickness (up to
several millimeters thick), which thereby allows a great
density of functional sites and proportionately improves
the sensitivity.
The subject of the invention is thus an
electrically conductive electroactive functionalized
polymer which corresponds to the formula (I):
R 1 R i Rn
N ...
in which:
n is a non-zero integer and i is an integer
ranging from 2 to n-1, and
R2, Ri and Rn, which may be identical or
different, each represent H or a functional group capable
of covalently bonding With a first biological molecule or
antiligand, and in that said polymer has a conductivity
and an electroactivity which are substantially of the same
order as that of the corresponding non-functionalized
conjugated polymer, that is to say of the corresponding
polymer of formula I, in which R1, Ri and Rn each
represent H.
More particularly, the functional groups) is(are)
independently chosen from the following set of functional
groups:
Yp-C-X where X represents H, OH, a substituted or
.35 unsubstituted lower O-alkyl radical, or a halogen, in
particular C1; Yp-NHZ, Z representing H or an alkyl
SU$STITUTE SHEET (RULE ~6) .
*~ ~enCA 02351479 2001-05-17
~~~~~~~ ~,.4 VL G,V 1/~ f
,.. . ,-w w<. " ~ , .~ .;., ,.~. , .".. ..~ ~;.....~ ;=.ri,..~,t
_ rv~rm~mv~a~rr
~~~~_~~aao~ ~~T~~~~s~~~~~~~ ~~~~c~s
_ R f :;
.~ ~~r.w. _~.~
4
radical; Yp-NH-CO-CF3; YP-X where X corresponds to the
above definition, p being an integer preferably equal to
0, 1 or 2; -Si{alkyl)s, -Si{alkoxyl)a or an activated ester
group such as COON-hydroxysuccinimide.
Y preferably represents a group chosen from alkyls
having from 1 to 5 carbon atoms, alkoxyls having from 1 to
5 carbon atoms and polyethers corresponding to the general
formula (CH2-CH2-O)m-(CH2)m'-, m representing an integer
ranging from I to 3 and m' an integer equal to 1 or 2.
l0 The invention also concerns an electrically
conductive, electroactive functionali2ed~ conjugated
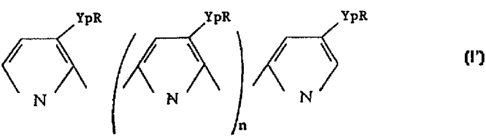
polymer of formula (I')
YpR YpR YpR
N ' \ N \ \ N
n
wherein
n is an integer or zero,
each R, which may be identical or different from
one monomer unit to one another, is selected from the
group consisting of H and functional groups capable of
covaiently bonding with a first biological molecule or
antiligand with the proviso that (a) at least one said R
of formula (I') represents said functional group or (by if
each YpR in formula (I' ) is identical, they are different
from CH2-COON,
each Yp, which may be identical or different from
one monomer unit to one another, is a coupling arm wherein
p is zero or an integer,
wherein said polymer has a conductivity and an
electroactivity which are substantially of the same order
SUBSTITUTE SHEET (fZULE 26)
s>
~y .~.. ACA 02351479 2001-05-17
~r~~~~~~r~ Lv v~ c_vv E=; ,g~
."~ , _ > .. ... " ... . "_.~ ~~~f~'
WO 00/31750 PCT/IB99/01947
as a conductivity and an electroactivity of a
corresponding polymer of formula (III), in which each said
R represents H.
Preferably, p is 0, 1 or 2
5 Preferred polymers of formula (I') are the
following .
- polymers wherein R is selected from the group
consisting of COX, where X represents H, OH, a substituted
or unsubstituted lower O-alkyl radical or an halogen ;
activated esters ; NHZ where Z represents H, an alkyl
radical or CO-CFs ; Si(alkyl)3 ; Si(alkoxyl)s ;
electrochemical probes ; electrochemical probes bound to
an activated ester ; electrochemical probes are preferably
selected from the group consisting of ferrocene and
quinone and/or activated esters are selected from the
group consisting of COON-hydroxysuccinimide, COON-
hydroxyphtalimide, and COOpentafluorophenol ; the halogen
is preferably chlorine ;
- polymers wherein p is at least one and R is
selected from the group consisting of H, OH, a substituted
lower O-alkyl radical, and a halogen ;
polymers wherein Y is selected from the group
consisting of alkylene groups having from 1 to 5 carbon
atoms ; oxy-alkylene groups having from 1 to 5 carbon
atoms ; polyethers having the formula
[ (CHz-CHz-O) m (CH2) m' ] where m is an integer ranging from 1
to 3 and m' is an integer equal to 1 or 2
(CH2)m CONH (CHz)m" where each of m and m" identical or
different is. an integer ranging from 1 to 3 ;
(CH2jm CON (CH2)~~' where m is an integer ranging from 1 to 3
and m"' is 2 or 3 ;
Said antiligand is able to form an
antiiigand/target molecule complex. Preferably, said
complex is selected from the group consisting of
peptide/antibody, antibody/haptene, hormone/receptor,
CA 02351479 2001-05-17
WO 00131750 PCT/IB99/01947
6
polynucleotide hybrids/polynucleotide and
polynucleotide/nucleic acid couple.
The target molecule may comprise a histidine tag.
More preferred polymers are the following .
- polymers (A) wherein p is 1, Y is selected from
the group consisting of CHz, CHz-CHz and CHz-CHz-CHz, and at
least one said R is COOH ; this substituent YPR is able to
show a reactivity toward amino functions of a biological
probe (for example, oligonucleotide, oligonucleotide
derivatized with an amino function, amino acid, peptide);
allowing the further grafting of this biological probe on
the homo- or co-polymer ; such substituent is also very
useful for increasing the hydrophilicity of the homo- or
co-polymer, while it is essential for the electrochemical
behavior in aqueous solution
- polymers (B) wherein p is 1, Y is selected from
the group consisting of CHz, CHz-CHz and CHz-CHz-CHz, and at
least one said R is an activated ester ; a preferred
activated ester is selected from the group consisting of
COON-hydroxysuccinimide, COON-hydroxyphtalimide, and
COOpentafluorophenol ; this substituent Y~R has a high
reactivity with amino functions of amino acids or peptides
for example, allowing the further grafting of such a
biological probe on the homo- or co-polymer ;
- polymers (C) wherein p is 1, Y is
(CHzjm CONH (CH2jm~~ where each of m and m" identical or
different is an integer ranging from 1 to 3, and at least
one said R is an electrochemical probe ; a preferred
electrochemical probe is selected from the group
consisting of ferrocene and quinone ; the electrochemical
probe is selected for its sharp electrochemical signal,
which improves the electrochemical detection sensivity of
the biological sensor ;
- polymers (D) wherein p is l, Y is
(CH2jm CONH (CH2jm~~ where each of m and m'~ identical or
different is an integer ranging from 1 to 3 , and at Ieast
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99101947
7
one said R is an electrochemical probe bound to an
activated ester ; preferred electrochemical probes and
preferred activated esters are as set forth above ; the
objective of this substituent YpR is to allow, through the
activated ester, the further grafting of a biological
probe ;
- polymers (E) wherein p is 1, Y is
(CHz) m CONH (CHz) m" where each of m and m" identical or
different is an integer ranging from 1 to 3, and at least
one said R is NHz or any substituent possessing a terminal
amino function NH2 ; the amino function is dedicated to the
further grafting of a biological probe through an acidic
function (COOH or P(OH)r,) present in the biological probe
(for example, amino acid, peptide, oligonucleotide,
antigen) ;
- polymers (F} wherein p is 1, Y is
(CHz}m CoN (CHz)m~~~ where m is an integer ranging from 1 to 3
and m"' is 2 or 3 , and at least one said R is COOH ; this
substituent YpR having the formula
(CHz) m CON (CH2COOH) m"' can complex transition metal ions
(Ni, Co, Fe, Cu, ...) on which a further complexation of
biological probe is possible, awing to the complexing
properties of these transition metal ions with some
derivatives such as histidine ; for instance, a histidine
tag can be grafted on a biological probe, allowing the
probe to be complexed to the metal ion, and thus anchored
to the functionalized poly(pyrrole).
Those preferred mono- or co-polymers may be
obtained by electropolymerization of the corresponding
monomer units, in various solvents such as acetonitrile or
propylene carbonate, in presence of an electrolyte such as
LiC104, with a concentration of about 0.1 M/l, at a
constant potential of about 0.9 V/SCE, or a constant
curreint density of some mA/cmZ, or by cyclic voltammetry.
Typical concentration of monomer units is ranging from
10-Z to IO'~ M/I. They can be polymerized alone leading to
CA 02351479 2001-05-17
~_ ~.'~~a~k~'~~ ~~9~~~~ P CTyi R4ct ~~ 1~~=a?
~.~~~Ya:a~,.."..~~~a,a.,:- ~v;p.~r'~s.&,5' ~~'szF~,.zz'.;.i:,.,.M._:'. ,~.
,,~, .~. r,r.
., .. WY..
homopolymers. Mixtures of monomers will result in co-
polymers, in which one can associate various properties ;
for instance, a copolymer of poly (A, B,C} allows to
associate the hydrophilic property of A, required for a
sensing in aqueous solution, with the sharp signal of the
electrochemical probe C together with the recognition
property exerted by the biological probe of H with the
biological target in solution.
Another subject of the invention is an
electrically conductive electroactive conjugated polymer
comprising at least one functional group which is
covalently bonded to a first biological molecule or
antiligand corresponding to the formula (II}:
RT1 R'i Rn
_ ' \ ~... \
N ... N N
in which:
n is a non-zero integer and i is an integer
ranging from 2 to n-1, and
R'1, R'i and R'n, Which may be identical or
different, each represent H or a functional group capable
of covalently bonding with, or covalently bonded to, a
first biological molecule or antiligand.
The functional groups bonded to a biological
molecule or ligand are advantageously chosen, before
reaction With the latter, from the following set of
functional groups:
YP-C-X where X represents H, OH, a substituted or
unsubstituted lower O-alkyl radical, or a halogen, in
particular Cl; Yp-NHZ, Z representing H or an alkyl
radical; Yp-NH-CO-CF3; Yp-X where X corresponds to the
above definition, p being an integer preferably equal to
SUBSTITUTE SHEET (RULE 26}
~ , CA 02351479r2001-05-17
~rEn~ed 2~ W~.Y~WV ~,,
.... f:
_ rw s imaam m~r~
',~,-""~ ~v'°%'°'..$ ~s~, ,t~ o n~ rgz~s ~; -~ ~ ~ r~.a"~ ~ a
,.. r ~_ ~. ~..:~
9
0, 1 or 2; -Si(alkyl)3, -Si(alkoxyl)s or an activated ester
group such as COON-hydroxysuccinimide.
Tn particular, the functional groups bonded to a
biological molecule are identical and consist, before
reaction with the first biological molecule(s), of -(CHz)
COOH, said first biological molecules or antiligands being
chosen from peptides or peptide derivatives, in particular
Gly-Phe, Phe-Pro and Phe-HEA-Pro, and from polynucleotides
such as the oligonucleotide of sequence: CCTAAGAGGGAGTG.
The invention further concerns an electrically
conductive, electroactive conjugated polymer of formula
(IT' )
YPRf YPRr YPR.
~~ ~ ~ ~ N
. N N
n
wherein
n is an integer or zero,
each R', which may be identical or different from
one monomer unit to one another, is selected from the
group consisting of H and functional groups capable of
covalently bonding with, or covalently bonded to, a first
biological molecule or antiligand With the proviso that at
least one said R' of formula (TI') represents one said
functional group covalently bounded to one said first
biological molecule or antiligand,
each YP, which may be identical or different from
one monomer unit to one another, is a coupling arm wherein
p is zero or an integer,
wherein said first biological molecule comprises a
polynucleotide or peptide sequence.
SUBSTITUTE SHEET (RULE 26)
CA 02351479 2001-05-17
~d sy ~,~,vr.w r .;
--. a. ......r.~-., .-, ... ,.... ".,. r.~ ~~'x~'-~
WO 00/31750 PCT/IB99/01947
Preferred polymers of formula (II') are the
following .
- polymers wherein p is 1, Y is selected from the
group consisting of CHz, CHz-CH2 and CHz-CHz-CHz, and at
5 least ane said functional group, before being bonded to a
first biological molecule or antiligand, is COOH ;
- polymers wherein p is 1, Y is selected from the
group consisting of CHz, CHz-CHz and CHz-CHz-CHz, and at
least ane said functional group, before being bonded to a
IO first biological molecule or antiligand, is an activated
ester ; a preferred activated ester is selected from the
group consisting of COON-hydroxysuccinimide, COON-
hydroxyphtalimide, and COOpentafluorophenol ;
- polymers wherein p is 1, Y is
(CHz) m CONH (CHz) m" where each of m and m" identical or
different is an integer ranging from 1 to 3, and at least
one said functional group, before being bonded to a first
biological molecule or antiligand, is an electrochemical
probe ; a preferred electrochemical probe is selected from
the group consisting of ferrocene and quinone ;
- polymers wherein p is 1, Y is
(CHz)m CONH (CHz)m~~ where each of m and m" identical or
different is an integer ranging from 1 to 3, and at least
one said functional group, before being bonded to a first
biological molecule or antiligand, is an electrochemical
probe bound to an activated ester ; preferred
electrochemical probes and preferred activated esters are
as set forth above ;
- polymers wherein p is 1., Y is
(CHz)m CONH (CHz)m~~ where each of m and m" identical or
different is an integer ranging from 1 to 3, and at least
one said functional group, before being bonded to a first
biological molecule or antiligand, is NH2 or any
substituent possessing a terminal amino function NHz ;
- polymers wherein p is 1, Y is
(CHz)m CON (CHz)m~« where m is an integer ranging from 1 to 3
CA 02351479 2001-05-17
WO 00!31750 PCT/IB99l01947
11
and m"' is 2 or 3, and at least one said functional group,
before being bonded to a first biological molecule or
antiligand, is COOH.
The first biological molecule or antiligand is
preferably selected from the group consisting of amino
acids, peptides, oligonucleotides, antigens.
Another subject of the invention is the use of a
conjugated polymer as defined above for detecting or
assaying, in vitro or in vivo, a second biological
IO molecule. or ligand, which is different from the antiligand
and which interacts specifically with the latter, said
ligand being detected and/or assayed by observation and/or
measurement of a potential difference or of a variation in
current between the conjugated polymer not bonded to the
ligand and the conjugated polymer bonded to the ligand.
In particular, the polymers of the invention are
used to detect and/or assay an enzyme, such as a
proteolytic enzyme and in particular carboxypeptidase A,
or a polynucleotide, or to extract, in vitro or in vivo, a
second biological molecule or ligand, which is different
from the antiligand and which interacts specifically with
the latter.
In one embodiment of the invention, the conjugated
polymer is deposited on a conductive substrate, such as
metal or a carbon derivative, or in the form of a self
supporting film.
Lastly, the invention relates to an electrode and
to a self-supporting film which consists of a conductive
substrate such as a metal or a carbon derivative and of a
3o polymer as defined above.
In one embodiment, the antiligand is specific for
the ligand or target molecule. The antiligand is chosen in
particular in order to form an antiligand/target molecule
complex, By way of example, the complex may be represented
in particular by any peptide/antibody, antibody/haptene,
CA 02351479 2001-05-17
WO 00/31750 PCTIIB99J01947
12
hormone/receptor, palynucleotidehybrids/polynucleotide,
polynucleotide/ nucleic acid couple or the like.
The term "polynucleotide" as used in the present
invention denotes a sequence of at least five
deoxyribonucleotides or ribonucleotides optionally
comprising at least one modified nucleotide, for example a
nucleotide containing a modified base such as inosine, 5-
methyldeoxycitidine, 5-dimethylamino-deoxyuridine,
deoxyuridine, 2,6-diaminopurine, 5-bromodeoxyuridine or
any other modified base which allows hybridation. This
polynucleotide may also be modified at the internucleotide
band (for example such as phosphorothioate, H-phosphonate
and alkylphosphonate bonds), or on the skeleton, for
example alpha-oligonucleotides (FR 2,607,507} or PNAs (M.
Egholm et al., J. Am. Chem. Soc., (1992), 114, 1895-1897).
Each of these modifications may be taken in combination.
The term "peptide" as used in the present
invention refers in particular to any peptide of at least
two amino acids, in particular a protein, protein fragment
or oligapeptide, which is extracted, separated or
substantially isolated or synthesized, in particular those
obtained by chemical synthesis or by expression in a
recombinant organism; any peptide in whose sequence one or
more amino acids from the L-series are replaced by an
amino acid from the D-series, and vice versa; any peptide
in which at Ieast one of the CO-NH bonds, and
advantageously all of the CO-NH bonds, of the peptide
chain is{are) replaced by one (or more) NH-CO bonds; any
peptide in which at least one of the CO-NH bonds, and
advantageously alI of the CO-NH bonds, is or are replaced
by one or more NH-CO bond{s), the chirality of each
aminoacyl residue, whether or not this is involved in one
or more abovementioned CO-NH bonds, being either conserved
or inverted with respect to the aminoacyl residues
constituting a reference peptide, these compounds also
being referred to as immunoretroids, a mimotope.
CA 02351479 2001-05-17
WO OOI31750 PCT/IB99/01947
13
Many classes of peptides may be grafted, as shown
by the non-exhaustive list below: adrenocorticotropic
hormones or fragments thereof; angiotensin analogs or
inhibitors thereof (components of the renin-angiotensin
system which regulate renal hypertension); natriuretic
peptides; bradykinin and peptide derivatives thereof;
chemotactic peptides; dynorphin and derivatives thereof;
endorphins or the like; encephaiins or derivatives
thereof; inhibitors of enzymes (such as proteases);
fragments of fibronectin and derivatives; gastrointestinal
peptides; peptides associated with the release of growth
hormones; neurotensins and the like; opioid peptides;
oxytocin, vasopressin, vasotocin and derivatives; kinase
proteins.
Peptides and polynucleotides have high biological
activity, and are known to control many biological
functions (A. S. Dutta, Advances in Drug Research, B. Testa
Editor, Academic Press, New York, 1991, 21, 145). Fox
example, peptides show very considerable therapeutic
potential as agonist or antagonist receptors, and as very
powerful inhibitors which bind strongly to enzymes, this
being the principle upon which affinity chromatography is
based. Moreover, by a selective hybridization reaction
with other target nucleic acid fragments or nucleotides,
polynucleotides may give rise to advantageous recognition
phenomena, allowing in particular the development of novel
gene scavengers. Thus, in order to detect and/or assay a
target nucleic acid or nucleic acid fragment, a
functionalized polymer which is at least partially bonded
to an antiligand palynucleotide is placed in contact with
a sample liable to contain the target, and the
hybridization reaction is then detected if it takes place,
either directly by measuring a potential difference or a
variation in current between the non-bonded polymer and
the bonded polymer which has reacted with the target, or
indirectly by the same measurement as above, but using an
CA 02351479 2001-05-17
WO 00/31750 PCTIIB99/01947
14
additional detection polynucleotide which is capable of
reacting with the target, said additional polynucleotide
preferably adjoining the antiligand polynucleotide and
being labeled with an electroactive molecule.
The term "antibody" as used in the present
application refers to any monoclonal or polyclonal
antibody, any fragment of a said antibody such as an Fab,
Fab'2 or Fc fragment, as well as any antibody obtained by
genetic modification or recombination.
Functionalization of the polypyrrole in the 3- or
4-position of the pyrrole ring may be carried out either
on the monomer units with a subsequent polymerization
step, or on the monomer units of a presynthesized polymer.
Any suitable functionalizing agent may be used, provided
that it comprises at least one reactive function capable
of reacting with atoms 3 and/or 4 of the pyrrole ring. The
functionalizing agent may thus be a monofunctional agent,
provided that after the step of grafting on to the pyrrole
ring the novel reactive function is introduced for
subsequent reaction with the antiligand, and that this
reactive function is multifunctional, such as bifunctional
agents arid in particular homo- or heterobifunctional
agents. By way of example, the functionalizing agent is
chosen from substituted or unsubstituted alkyl or alkoxyl
or poiyether chains ending with a group bearing a reactive
function. The reactive function is represented in
particular by a functional group such as a carboxylic,
hydrazide, amine, nitrile, aldehyde, thiol, disulfide,
iodoacetyl, ester, anhydride, tosyl, mesyl, trityl or
silyl group or the like.
The formation of a conjugate resulting from the
covalent coupling of an antiligand, for example a
polynucleotide, with a functionalized polypyrrole
according to the invention may be carried out according to
the known, so-called direct or indirect methods.
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99/01947
For example, in the case of a polynucleotide,
according to the direct method, a polynucleotide is
synthesized having a reactive function on any site of the
nucleotide chain such as, for example, the 5' end or the
5 3' end, or on a base or on an internucleotide phosphate,
or on the 2' position of a sugar. The polynucleotide is
then coupled with the polymer, which is prepared
beforehand and contains a reactive function complementary
to the above, that is to say one which allows the
10 formation of a covalent bond by reaction between the two
complementary reactive functions, one borne by the
polynucleotide and the other by the functionalized
polymer. For example, in a known manner, primary amines
may be coupled with an activated carboxylic acid or an
15 aldehyde or alternatively a thiol function may be coupled
with a haloalkyl. Preferably, the reactive function of the
polynucleotide for the coupling to the polymer is at the
5' or 3' end.
In the indirect coupling method, the
polynucleotide and the polymer each bear a reactive
function, it being possible for these reactive functions
to be identical to or different from each other, these two
functions not being complementary but being capable of
reacting with an intermediate coupling agent which is a
bifunctional reagent (homobifunctional if the two
functions are identical or heterobifunctional if the two
functions are different)., Among the homobifunctional
coupling agents which may be mentioned are DITC (1,4-
phenylene diisothiocyanate), DSS (disuccinimidyl suberate)
or analogs thereof. Among the heterobifunctional coupling
agents which may be mentioned are SMCC (succinimidyl-4-(N-
maleimidomethyl) cyclohexane-1-carboxylate) or SMPB
(succinimidyl-4-(p-maleimidophenyl) butyrate), which are
capable of reacting with a primary amine, on the one hand,
and with a thiol, on the other hand.
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99l01947
16
The invention will be better understood on reading
the detailed description which follows, made with
reference to the attached figures- in which,
Figure 1 represents examples of conjugated
polymers such as 1) polyacetylene; 2) polypyrrole; 3)
polythiophene; 4) polyphenylene; 5) polyaniline;
Figure 2A represents a polypyrrole substituted in
the 3-position with acetic acid (1) and Figures 2B, 2C,
2D, 2E and 2F represent polypyrroles substituted in the 3-
_10 position with various peptides (2 to 6 respectively);
Figure 3 represents the voltammograms of four
functionalized polymers, identified below, in 0.5 M H20-
NaCI medium
poly(pyrrole-acetic acid) in {1),
poly{pyrrole{Gly-DPhe)) in (2), poly(pyrrole(Val)) in (3)
and poly(pyrrale(Phe)) in (4);
Figure 4 represents the voltamrnogram of poly(2),
in 0.5M Hz0-NaCI medium, in the presence of
carboxypeptidase A at concentrations respectively of O.Omg
in 5 cm3 of electrolyte {a), 1.2 mg in 5 cm3 of electrolyte
(b), 2.4 mg in 5 cm3 of electrolyte (c) and 5.0 mg in 5 cm3
of electrolyte (d);
Figure~5~corresponds to the amperometric response
of an electrode, poly(2), as a function of the amount of
enzyme, carboxypeptidase A, in nanomoles, present in the
medium. The linear relationship between the current
observed and the amount of enzyme, at a potential of 0.3
V, is given with reference to a saturated calomel
electrode,
Figure 6 is a theoretical diagram of a field
effect microelectrochemical transistor for the (amplified)
detection of the presence of a biological species
recognized by a functionalized conductive polymer. The
abbreviations which follow have the respective meanings:
P, polymer. Sub, substrate. S and D, source and drain
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99/01947
17
electrodes respectively. CE, counterelectrode acting as a
grille G. R, reference electrode. Potent., potentiostat,
Figure 7 is a theoretical diagram of a 2
compartment electrochemical cell containing a
functionalized polypyrrole membrane, for the extraction of
biological species recognized by a substituent grafted on
to an electroactive conjugated polymer chain,
Figure 8 represents the voltammogram of poly[N-3-
hydroxysuccinimidepyrrole], in 0.1 M LiC104- acetonitrile
medium with a saturated calomel reference electrode,
showing high electroactivity and high electrochemical
reversibility,
Figure 9 represents the voltammogram of a
poly{pyrrole-ODN][pyrrole-COOH]) copolymer electrode, with
ODN of sequence CCTAAGAGGGAGTG as polynucleotide or
aligonucleotide. No modification is observed after
incubation of this polymer with a non-target sequence,
GGTGATAGAAGTATC, and
Figure 10 represents the voltammogram of a
poly([pyrrole-ODN][pyrrole-COOH]) copolymer electrode,
with the sequence: CCTAAGAGGGAGTG as oligonucleotide ODN.
This electrode was incubated in the presence of a target,
335 nmol CACTCCCTCTTAGG, at 37°C for 2 h. This electrode
was then rinsed and analysed in electrochemical medium. A
potential shift is observed with respect to the previous
voltammogram.
Figure 11 represents the cyclic voltammogram of a
film P[Py-NHR], poy(B), (0.9 V/SCE with grown charge 40
mCcm-s, in 0.1 M La.ClOa acetonitrile solution, scan rate 20
3 0 mVs-~ .
Figure I2 represents the cyclic voltammograms of a
thin film of P[Py-FeCp2], poly(C) (thickness, a = 1nm) in
CH3CN/0.1 M LiCIOa at scan rates in the range of
5-130 mVs'~ .
CA 02351479 2001-05-17
WO 00131750 PCTliB99/01947
18
Figure 13 represents the voltammogram of
copoly[pyNHFe(Cp)2-pyNHP], copoly[(B)(C)] in an aqueous
solution of 0.5M NaCl.
Figure 14 represents an electrochemical
caracterisation of films of PEG and of poly[pyrrole-ODN,
pyrrole-COOH] in aqueous medium, before (1) or after
hybridization with ODN for different target ODN
proportions [(2) 66 nmoles, (3) 165 nmoles, (4) 500
nmoles)].
20 . Figure 15 represents voltammograms of
copoly[pyNHFe(Cp)z,PyODN], in aqueous solution of 0.5M
NaCl. Initial voltammogram and after incubation with non-
target ODN (-) and after incubation with complementary
target ODN (0.02 nmole/5 ml) (--):
The polymers according to the invention may be
used in particular for the detection of biologically
active species which may be present in a sample and which
may react with the antiligand or grafted antiligands.
Indeed, as shown above, it is observed that the conjugated
polymers functionalized in the 3-position of their
heterocycle and on to which are grafted one or more
antiligands, after reaction with one or more ligands,
exhibit a modification of the electrochemical response
with respect to a reference polymer which has not reacted
with the iigand or ligands of a biological medium, this
being visualized by a change in the oxidation potential.
This variation in the oxidoreduction of the polymer in the
electrochemical voltammogram inparts a scavenger-type
function and may thus be used for a quantitative
measurement of the biologically active species, either by
variation of the potential, at fixed current, or by
variation of the current at fixed potential, or
alternatively by the production of field effect
microelectrochemical transistors.
Moreover, the polymers of the invention may also
be used for the extraction of biologically active species
CA 02351479 2001-05-17
WO 0013175U PCT/IB99101947
19
in solution. In many cases, the biologically active
species in solution combines strongly with the antiligand
grafted on to the polymer chain, such as a bioactive
peptide or a polynucleotide, thereby making it possible to
extract the biologically active species selectively from a
medium. This type of extraction may be performed in vitro
or even in vivo when the support polymer is biocompatibie,
such as polypyrrole for example.
Lastly, the polymers of the invention may be a
source of release, from one medium into another medium, of
biologically active species (enzymes or the like).
The functionalization of the electroactive
conductive polymers of the invention, such as
polypyrroles, by groups showing recognition with respect
to compounds of biological interest may be extended to
recognition of nucleic acids (NAs). Thus, the grafting of
polynucleotides or oligonucleotides, ODN, along the
conjugated chain of polymers should allow the
discrimination of corresponding NAs or NA fragments within
a biological medium. This recognition will be performed by
selective hybridization between the ODN, grafted on to the
polymer, and the corresponding NA present in the external
medium, in which the film of functionalized polymer is
immersed, just like the "peptide/enzyme" recognition
described later. The "ODN/NA" complexation results in a
modification of the physicochemical properties of the
conjugated polymer, characterization of which will make it
possible to confirm the presence of the desired NA.
The essential point relates to the nature of the
physicochemical properties of the polymer destined for
modification during the "ODN/NA'! recognition. Indeed, in
order to develop a rapid, sensitive and quantitative
method for measuring the presence of NA, the aim of the
present invention relates to the development of
electroactive materials whose electrochemical response
will be modified after "ODN/NA" hybridization. The
CA 02351479 2001-05-17
WO 00!31750 PCT/IB99l01947
modification will relate to a potentiometric-type
variation, such as variation of the oxidation potential of
the polymer, or an amperometric-type variation, by
variation of the oxidation (or reduction) current observed
5 at a given potential. These variations in electrochemical
response may be measured quantitatively, the
functionalized polymer films being used either as
electrochemical scavengers of amperometric or
potentiometric type, or alternatively in a field effect
10 microelectrochemical transistor structure, as has been
described above in the case of enzymatic recognition
starting with peptides grafted on to polypyrrole. The
advantages of measurements of this type are the speed, the
sensitivity and the possibility of readily producing
15 matrix cards of 2n measuring elements, containing n target
and nontarget ODNs, which are thus capable of rapidly
discriminating between the presence and absence of genes
in a medium.
Just as in the case of the enzymatic recognition
20 described above, a second essential point relates to the
fact that in order to obtain an electrochemical response
to a recognition phenomenon, the functionalization in the
3-position of a heterocyclic (pyrrole) ring is essential.
In order to ensure a precise response of
electrochemical type for these polymers, it is necessary
for the functionaiization of the conjugated chains to be
compatible with considerable electroactivity of the
functionalized ~ polymer. The need for such an
electroactivity requires, in the case of hydrophilic
polyheterocycles such as polypyrrole, the
functionalization to be performed in the 3-position of the
pyrrole ring. The polymers of the invention are
electroactive polymers in which either all of the monomer
units are functionalized with an antiligand such as an
oligonucleotide., or only some of the monomer units are
thus funetionalized. It is clearly understood that the
CA 02351479 2001-05-17
WO 00!31750 PCT/IB99/01947
21
monomer units may be functionalized with identical or
different antiligands, and in the latter case the polymers
of the invention may be used for the detection of several
target ligands within the same sample. The polymers of the
invention may be prepared by the following different
routes:
a) Totally functionalized polymers.
Tn this route, the first step relates to the
funetionalization of the monomer, such as pyrrole, by an
antiligand such as a given oligonucleotide. The second
step then relates to the polymerization of this monomer,
resulting in a film of polymer in which alI the monomer
units are functionalized.
b) Partially functionalized copolymers.
In the particular case of target nucleic acids,
and bearing in mind their generally large size, the
functionalization of all the monomer units, of small
sizes, in the polymer is not necessary, and one of the
routes thus relates to the production of a copolymer which
involves, on the one hand, the functionalized monomer
units described in a), and also pyrrole units which are
not functionalized with the antiligand oligonucleotide.
c) Functionalization of a precursor polymer
The partial functionalization of a polymer film
may also be performed starting with a conjugated polymer
film, into which chemical groups compatible with the
grafting of an antiligand such as an oligonucleotide are
introduced beforehand. In this route, a monomer containing
a grafting synthon is first produced, such as [N-3
hydroxysuccinimidepyrrole]. The synthon [N-
hydroxysuccinimide] is known to allow the subsequent
grafting of an oligonucieotide. This monomer is
subsequently polymerized or copolymerized with another
pyrrole derivative. The polymer film obtained is then
immersed into the reaction medium containing an
oligonucleotide, and the reaction to graft this
CA 02351479 2001-05-17
WD 00131750 PCTIIB99l01947
22
oligonucleotide to the pyrrole monomers is then carried
out. This grafting in fact only involves some of the
pyrrole monomer units constituting the polymer.
Example 1: Synthesis of the monomers
In the example described below, the polypyrrole
{1) was chosen as conjugated polymer support on account of
its biocompatibility (H. Naarmann, personnel
communication). An acetyl spacer arm A is grafted between
carbon atom 3 of the pyrrole ring and the peptide
substituent in order to preserve the conductivity and the
electroactivity of the corresponding functionalized
polypyrrole. Various peptides, with their carboxylic end
function unprotected or protected in methyl ester form,
were chosen for their biological pertinence and were
grafted on to a pyrrole-acetic acid monomer, PyA (1).
Several mono- and dipeptides were grafted, and led to the
following pyrrole derivatives, represented in Figure 2:
pyrrole-acetic acid, PyA (1), pyrrole(Glycine-
dPhenylalanine), Py(Gly-DPhe) (2), for its capacity for
complexation with proteolytic enzymes such as
carboxypeptidase A {Sigma) and trypsin (Sigma) (J. R. Uren,
Biochim. Acta, 1971, 236, 67), pyrrole(valine), Py(Vai)
(3), pyrrole(phenylalanine), Py(Phe) (4);
pyrrole(phenylalanine-proline), Py(Phe-Pro) (5). Bulkier
dipeptide derivatives may also be grafted, such as
phenylalanine-hydroxyethylamine-proline, Py(Phe[HEA]Pro)
(6), which is known to be an advantageous potential
inhibitor for the protease associated with the HIV-1 virus
of AIDS. These monomers were synthesized according to a
described chemical route (D. Delabouglise, F. Garnier,
Synth. Met. , 1990, 39, 117) . These monomers were purified
and characterized by NMR, microanalysis and mass
spectrometry.
Example 2: Polymerization
CA 02351479 2001-05-17
WO 00!31750 PCT/fB99/01947
23
These monomers were polymerized electrochemically
on a 0.7 cm2 platinum electrode, as well as on a platinum
grille of surface area 10 cmz in propylene carbonate
medium with 0.5 M NaCI, at a constant potential of 0.8
V/SCE. Thick polymer films are obtained, in thicknesses of
up to 10 ~cm. As shown in Figure 3 , the electroactivity of
these polymers was confirmed by cyclic voltammetry in 0.5
M H20-NaCI medium, at a neutral pH of 7. The oxidation
potential values, of the order of 0.30 V/SCE, close to
that of unsubstituted polypyrrole, confirm the
electroactivity of these polypyrroles functionalized with
dipeptides.
Example 3: Recognition of carboxypeptidase A
The specific properties of complexation of these
polypyrroles with respect to proteolytic enzymes were
analysed with carboxypeptidase A, with which (Gly-DPhe) is
known to form stable complexes at neutral pH. Solutions of
increasing concentration of carboxypeptidase A, ranging
from 1 mg to 5 mg in 5 cm3 of 0.5 M H20-NaCl, were
analysed. When nonspecific electrodes such as
unsubstituted polypyrrole, or poly(3, 4, 5 or 6) are
immersed~in this solution, a voltammogram identical to
that obtained in Example 2 is observed, without any
modification. However, when poly(pyrrole(G1y-DPhe)), poly
(2), is used, the voltammogram shows a shift towards
higher potentials, of 0.340 V/SCE for an initial zero
amount of carboxypeptidase A up to a limit value of 0.500
mV/SCE for an amount of 5 mg of carboxypeptidase A in the
solution. This result is given in Figure 4, which shows
the potential shift, attributed to static hindrance and
rigidification of the polypyrrole chain, produced during
the complexation of the enzyme with the dipeptide borne by
the polymer chain. The formation of this complex between
enzyme and paly(pyrrole-dipeptide) was confirmed by the
release of the enzyme into acidic medium, at pH = 3, as is
CA 02351479 2001-05-17
WO 00131750 PCT/IB99/01947
24
performed conventionally in affinity chromatography (I. M.
Chaiken, M. Wilchek, I. Parikh, Affinity Chromatography
and Biological Recognition, Academic Press; New York,
1983). The released enzyme was characterized
conventionally by the Bradford test, involving measurement
of the enzymatic activity with Coomassie brilliant blue,
and by the use of a standard bovine serum albumin. When a
poly(Gly-DPhe) film is used containing 5 x 10-6 monomer
units, corresponding to a polymerization charge of 1
ZO coulomb, a significant amount, 400 micrograms, of
carboxypeptidase A was obtained after release into an
acidic medium. Considering the size of this enzyme, 307
amino acid units, this amount of released enzyme shows
that about 1 molecule of enzyme is complexed per 200
monomer units of (pyrrole-dipeptide), which appears to be
reasonable given the difference in size (of about a factor
of 100). This result also shows that the enzyme must be
distributed inside the polymer film, thereby demanstrating
the permeability of this film with respect to the enzyme.
Comparable results were obtained when trypsin was used as
the enzyme.
At a given electrode potential, as seen in Figure
4, a variation in current is observed as a function of the
enzyme concentration. This result corresponds to the
amperometric response of the electrode, and one of the
advantageous characteristics of this respanse relates to
its linearity with the concentration of enzyme, as seen in
Figure 5. Such a linear relationship makes it possible to
propose a quantitative assay of the biological species in
solution, either by the use of a scavenger of
electrochemical type, or by the deveiapment of a field
effect transistor, represented schematically in Figure 6.
The operating principle is as follows. The polymer is made
on the source and drain electrodes, which are arranged on
a substrate. This assembly is immersed into the solution
to be analysed, and a counterelectrode in this same medium
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99/OI947
represents the grille of this transistor. When this grille
electrode is placed at a potential at which the variation
of the voltammogram a~. a function of the enzyme
concentration' is at a maximum, at about 0.2 V in the case
5 represented in Figure 4, tt.e conductivity of the polymer
varies considerably with the enzyme concentration. A
potential then applied between source and drain electrodes
makes it possible to obtain an amplified signal, this
transistor operating according to the principle of a field
10 effect transistor.
Example 4: Controlled release of an enzyme
An additional advantageous characteristic of these
15 electrodes relates to the fact that it has been shown in
the literature that poly(pyrrole-acetic acid), or poly(1),
releases protons into the electrolytic medium when it is
subjected to electrochemical oxidation (P. Baerle et al.
Adv. Mater. , 1990, 2, 490) . Large variations in pH may be
20 observed in a small electrolytic volume, up to values of
the order of 3. This release of protons is also observed
when the carboxylic function is distanced from the pyrrole
ring, such as is the case for an unprotected amino acid or
peptide grafted on to the pyrrole. Two routes exist for
25 the controlled release of protons; either the use of a
peptide whose carboxylic end function is not protected,
COOH, or the use of a copolymer between pyrrole-acetic
acid and pyrrole-protected peptide. An example of this
second route is given. A copolymer between Py(A}, (1), and
Py(Gly-DPhe}, (2}, was prepared electrochemically, under
the same conditions as above. When subjected to
electrooxidation at 0.3 V/SCE, this poly(1, 2) copolymer
leads to a large variation in pH of up to pH - 4 in the
region of the electrode.
Starting with this poly(1, 2) copolymer or
starting with the poly(2} polymer whose carboxylic
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99101947
26
function is free, two processes may be carried out for the
extraction; a batchwise process and a continuous process.
a) Batchwise process
This process consists in using a membrane or
electrode based on the abovementioned materials, and in
placing it in contact with the medium in which the desired
biologically, active species coexists with other species.
The selective affinity provided by the grafted peptide
with respect to this desired species will cause
complexation _of the latter with the peptide grafted on to
the polymer of the electrode or of the membrane. This
membrane or electrode is then removed from the analysis
medium and.intraduced into another medium, referred to as
the recovery medium. In this recovery medium, containing a
support salt of NaCl type, the electrode or membrane is
subjected to an electrochemical oxidation, which causes
the expulsion of protons, by means of the carboxylic acid
groups, up to a pH which is sufficient for the
dissociation and release of the desired species.
b) Continuous process
The poly(1, 2) copolymer, or the poly(2) polymer
with its carboxylic function free, is prepared in membrane
or electrode form, optionally using a polycationic or
polyanionic support polymer of the polystyrene sulfonate
type or alternatively perfluoro membrane type such as
NAFI~N. This possibly composite electrode or membrane
constitutes the junction between two compartments A and B,
as represented schematically in Figure 7. An example of
the continuous extraction of carboxypeptidase A by this
process is described below.
In the case outlined in Figure 7, the bioselective
element consists of a poly(1, 2) copolymer described
above, polymerized in a NAFION (Aldrich) membrane,
obtained by evaporation of 10 ael of a 5 % solution of
NAFION in a mixture of linear higher alcohols (Aldrich) on
a very fine platinum grille. The film of NAFI~N resulting
CA 02351479 2001-05-17
WO 00/31750 PCT/IB99/01947
27
therefrom, which has a thickness of about 5 aem, then
serves as a support for the electropolymerization of the
poly(1, 2)copolymer, resulting in a poly(1, 2)-NAFION
electroactive composite membrane.
In a first step l, 10 grams of carboxypeptidase A
are introduced into the compartment A, in 0.5 M H20-NaCI
medium. A complexation on the [poly(1,2)]-NAFION composite
membrane then occurs immediately on the peptide units of
(2), as described above in Example 3. An oxidation is
subsequently performed, using a counter-electrode and a
reference electrode which are introduced into the
compartment B. In a second step 2, the electrochemical
oxidation leads to the release of protons in this
compartment B, up to a pH of about 4, and to the immediate
release of carboxypeptidase A. 1.2 grams of
carboxypeptidase A ware then recovered in the compartment
A over one cycle of this continuous process, as was
determined by assaying the enzyme activity.
These results thus confirm the phenomenon of
recognition of these electrodes with respect to enzymes,
as well as their capacity to extract these enzymes. This
behavior is linked in a one-to-one manner with the
chemical nature of the dipeptide grafted on to the
polypyrrole chain.
Example 5: Synthesis of a polypyrrole
functionalized with a polynucleotide or oligonucleotide
This synthesis is carried out according to the
following 3 steps.
a) Synthesis of the [N-3-hydroxy-
succinimidepyrrole] monomer
0.4 mol of pyrrole-acetic acid, (I), 30 ml of
chloroform and 0.4 mol of N-hydroxysuccinimide, NHS; (II)
are added to a 3-necked flask. The mixture is set stirring
and a solution of 0.4 mol of dicyclohexylcarbodiimide,
DCC, in 20 ml of chloroform is then added dropwise. After
CA 02351479 2001-05-17
~~-,.. ~ ~. .~ ~ PCT
~~.'CI~B~~~~~9'~'~~ ~ DESK ~_ -~~'
a,;~ ~>~a~~,-~ ~ .-~_0.~c~';~.,
.. ..e..q.a-"~._"",a
2$' ~.~u.~
stirring for 2 hours, a white solid is formed, which is
filtered off and washed with chloroform. After evaporation
and washing with acetonitrile in order to get rid of
unnecessary reaction products, the product is
recrystallized from chloroform. The desired compound, [3-
(acetate-N-hydroxysuccinimido)pyrrole] (III), is thus
obtained in the form of a white powder.
IO 0 Q o 0
~ OH DCC
g0 ..._ N ---~- 0 - N
N CHC13 N
H 0 H 0 I
(II') (III)
IS (I ~ + DcH
Melting point, m.p. - 135°C. 1H NMR (ppm): (N~i,
1H, 9, s) ; pyrrole (2H, 6. 7, m) : pyrrole (1H, 6.1, s) ; CH2
20 (2H, 3_8, s); hydroxysuccinimide (4H, 2.8, s).
b) Synthesis of the polymer by
electropolymerization
The electropolymerization solution contains 0.5 M
LiC104 and 0.1 M monomer (III) in acetonitrile. The
25 electropolymerization is performed on a platinum electrode
of surface area 0.7 cm2, at a potential of 0.9 V relative
to a saturated calomel electrode, SCE, using an
electropolymerization charge of 30 mC. A black film
appears on the electrode, corresponding to poly(III), the
30 thickness of which is about 200 nm.
o 0
0
0
n N ~ 0 N ---.--f-~,
N
N
of t
H pI
~~'~ T ~~ CAS 02351479 2001-05-17
~~r~~~ea:L AMENDED SHEET
..~ ~~~ . Raa 4. ~~~..M.
1 V ! / fyVVI V 1 V'TI
~~,~-~~~ ~_~~~~~B~~I~k'~~~7 ~S'~ESG2~'
~.~~ ~._,. 4.~w~~a,a.
29
The electroactivity of this polymer was analyzed
in a 0.5 M LiC104-acetonitrile medium, showing an oxidation
peak at 0.28 V/SCE and a reduction peak at 0.24 VJSCE, as
seen in Figure 8. The small separation between these
oxidation and reduction peaks, 40 mV, confirms the very
great electroactivity and reversibility of this polymer.
c) Grafting of an oligonucleotide ODN
The above electrode bearing the polymer film is
immersed in a reaction medium formed of a mixture of
dimethylformamide with 10 0 0.1 M borate buffer at a pH of
9.3 and 26.2 nanomol of the 14-base oligonucleotide, oDN,
containing the sequence CCTAAGAGGGAGTG as well as a 5/
amine function on which the coupling reaction will be
performed with the N-hydroxysuccinimide group borne by the
pyrrole units of the polymer. This coupling reaction takes
place over 2 hours. The grafting is also accompanied by
hydrolysis of the succinimide groups, which are not
substituted with the oligonucleotide, in acetic acid. The
. polymer obtained on the electrode thus corresponds to a
poly([pyrrole-ODN][pyrrole-COOH]) copolymer.
0 0
A ~0-NH-ODN
~~ N n N
E
H H
Example 6: Characterization of the recognition
phenomenon
The recognition phenomenon was confirmed by
electrochemical characterization of the poly([pyrrole-
ODN][pyrrole-COOH]) polymer film obtained in Example 5c.
The electrochemical analysis was first carried out
directly on the electrode obtained after synthesis, and
then after this electrode had been placed in the presence
of the target ODN and the non-target ODN. The
hybridization reaction of this polymer was thus performed
SUBSTITUTE SHEET (RULE 26)
y~ ~~~~:~~CA 02351479s 2001-05-17
~...,s..~ ... ._... ,.,."i...::~.,..
.<x.;~
WO 00/3750 PCT/IB99/01947
in the presence of complementary ODN (target} and nori-
complementary (ODN) {non-target}, in an aqueous solution
buffered with PEG. Incubation is carried ouir at 37oC for 2
hours, either in the presence of target ODN
5 CACTCCCTCTTAGG, at a concentration of 335 nanomol, or in
the presence of non-target ODN GGTGATAGAAGTATC, at a
concentration of 272 nanomol. After reaction, the films
were rinsed with the PEG buffer and analyzed by cyclic
voltammetry. The voltammograms obtained are represented in
10 Figures 9 and 10. The results show firstly, in Figure 9,
that after synthesis and before being placed in the
presence of the target ar non-target sequence, the polymer
shows a reversible oxidation peak at 0.34 V/SCE,
confirming the electroactivity of this functionalized
15 polymer. After the incubation reaction in the presence of
the non-target sequence, and after rinsing, the
voltammogram obtained shows no modification. On the other
hand, when this polymer is incubated in the presence of
the target, the voltammogram obtained, Figure 10,- is
20 different, with an increase of the oxidation potential to
0.40 V/SCE, equivalent to an increase of 60 mV. This
increase is entirely indicative of a hybridization which
has taken place between the ODN and the corresponding
target. This increase in the oxidation potential is
25 attributed to a phenomenon of complexation of the arm
hanging along the polypyrrole chains, thus accompanied by
an increase in the energy required to oxidize this
polymer.
This result confirms that this novel class of
30 electroactive materials, of conjugated polyheterocycles
substituted in the 3-position with an oligonucleotide,
effectively gives rise to a phenomenon of recognition of
complementary DNA, and, moreover, that these materials
allow electrochemical reading of this selective
hybridization. This electrochemical reading opens the way
to gene scavengers of electrochemical, amperometric or
CA 02351479 2001-05-17
~ ~.~~~Oa ~~ S~E~~9~f~'~9'~.'~ ~~ ~E5~2~;~~
C~,emrya..~' a e!a.~..,ms,~.~"4~"4,.F3 ae.Y'~~ ,',~
",.~.ys~A"3e,~.,~,.,.::.;c~; % ",.v~...~:...........~~~~,sa~,zx3a~1
31
potentiometric type, and, moreover, to gene scavengers of
field effect microelectrochemical transistor type, based
on an amperometric response.
Example 7 . Synthesis of preferred polymer (A).
1) Synthesis of 1-tosyl pyrrole
c~
N'
o s o
,- ~ ~ NaOH ~ THF 0 S 0
Na013 - 1 1
CH3
Z5
CH3
Method
8 ml of pyrrole that has been previously distilled
in 100 ml of THF is put into a 500 ml twin necked flask
fitted with a magnetic stirrer and a condenser. After a
quarter of an hour, when the mixture is homogeneous, 6 ml
of tetrabutyl ammonium hydroxide (the reaction catalyst)
is added. The colourless solution becomes orange. While
the mixture homogenises, a 50% solution of sodium
hydroxide is prepared (62.5 g in 125 ml of water), which
is then added drop by drop to the mixture. This operation
causes a proton to leave the nitrogen on the pyrrole so
that substitution by the tosyl group is optimised.
The mixture is stirred for 15 minutes and the
tosyl chloride solution is added in excess (32 g in 100 ml
of THF) and drop by drop so as to have an excess of
radicals and to encourage the reaction. Vigorous stirring
is maintained during the addition and then for 1 hour. The
product changes from an orange colour to a brown colour.
SUBSTITUTE SHEET (RULE 26)
P~x~~~~~'CA 02351479 2001-05-17
y.x..~
<W ~" ,:~ ~, .. ,_ < ..,_, ~.,.
~~~~,~~~ , ~~~~ ~~ x~.~~. ~ ~fh'1 ~tlfl
~'~~~~dt7(? t~'~'9~7
c~u ,i.. - ~,....asS. _..~;<,.ea r ~""~ a_ .,.o8.a~
~afash... a ., _.,.,.....,~
32
When the stirring is stopped, 2 phases are seen to
form. After adding 300 ml of water, they are allowed to
separate. The aqueous phase is extracted with ethyl
acetate. The first organic phase is evaporated to remove
the THF, the second organic phase is added to it and the
ethyl acetate is evaporated.
The crystals are dissolved in dichloromethane and
this organic phase is washed with water until the washing
water is neutral. The organic phase is dried over
anhydrous MgS04, _ filtered and the dichloromethane
evaporated.
Recrystallisation is carried out to purify the
crystals. The 1-tosyl pyrrole is dissolved in 300 m.l of
heptane by heating under reflux for 30 minutes. On
z5 filtering at a Buchner, the impurities (coloured brown)
remain on the filter and a pale yellow product is obtained
which gives white crystals once the solution has cooled.
22.3 g of crystals are obtained which is a yield
of 87.5%.
2) Synthesis of 3-acetyl 1-tosyl pyrrole
~0
0 - S 0 CH3 - C ~ ~C13 CH3
'~'- 0
CH2C12 N ~
CH3 - C ~ Ts
3 0 CH3 0
Method
94.6 g of aluminium chloride (the reaction
catalyst) and 600 ml of dichioromethane are mixed in a
three-necked flask fitted with a magnetic stirrer and a
condenser with a CaCl2 moisture guard. The mixture is
~~.~~, ~,~~.~" , ~~~x~ SUBSTITUTE SHEET (RULE 26)
~~~~~~~~ ~CA 02351479 2001-05-17 ~~
. v'~. :,.~ .~.t~. c 4 ~. a . , ., .,, _.. .... . rte:.
t~~~.~~ '~' ~ kl~~g ~.~a ~~ PCTI~ ~T ~~~'
'3 ! ~' ~ y ~~ ? ~. '
''.. ... ..H:r'..~1.....,:F.,.....i' ;
fi~ ,~~a~ Ize.. ,D>,YYua au~F..". ...s~a:.'a''~.2fr"'
a .,zM~ s~- s. ,.s.,ae.a....,,",.,
33
stirred until homogenous and then 37.5 m1 of acetic
anhydride (reactant in excess) is added drop by drop.
After a quarter of an hour, a brown transparent
solution is obtained. A solution of 1-tosyl pyrrole (31.4
g in 80 ml of dichloromethane) is added drop by drop.
The reaction is allowed to proceed for an hour
under agitation and at ambient temperature. The entire
mixture is then poured into 500 ml of iced water. After
allowing it to separate, the organic phase is extracted
20 aid the aqueous phase is washed with dichloromethane. This
operation is repeated several times. The organic phases
are washed with 1M NaOH to make the A13+ soluble and remove
it with the water washings. At the end of the washing
process, the neutral pH of the aqueous phase is checked.
The organic phases are dried over anhydrous
magnesium sulphate and the dichloromethane is evaporated.
A recrystallisation is then carried_ out to purify
the crystals obtained: the 3-acetyl 1-tosyl pyrrole is
dissolved in heptane (500 ml) . It is heated under reflux,
filtered at a Buchner and the filtrate placed in a
freezer. The material in the bottom of the flask is
crystallised several times. The heptane is evaporated and
violet crystals are obtained.
19.61 g of crystals are obtained, a yield of
52.4%.
3j Synthesis of methyl 1-tosylpyrrole-3-acetate
O
~ 0
cH may
\ 3 TI(N0~).~,3H~0 ~ ~ \ OCH3
MeOH
trimethylorthoformatel N
MeOH
Ts Ts
"~*~~~' ~ ~-A ~ ~~ ~ SUBSTITUTE SHEET (RULE 26)
x~~# e~"~CA 02351479 2001-05-17
?;~; 3~.,. ,a»~ ..".,. " k ......,..
PCT~~~~C-' ~ ~~~~,
39
Method
In a first step, the esterification catalyst is
prepared. This consists of depositing Tl(N03)3.3HZ0 on
clay. In a single necked flask, 55 ml of trimethyl
orthoformate, is mixed with 45 ml of methanol and 22 g of
T1 (N03) 3. 3H2o: This is stirred for 5 minutes and then 45 g
of clay is added in such a way that a greyish' beige
suspension is obtained. This is stirred for a further 15
minutes and the solvents evaporated using a rotary
vaporiser. Once it is well dried, the catalyst on clay is
recovered in the form of a beige powder.
One can then progress to the synthesis of the
ester. Z1 g of acetyl tosyl pyrrole (dissolved in 400 ml
L5 of methanol) is mixed with the thallium catalyst on clay
in a single necked flask. Taking the precaution of fitting
a condenser, it is left to stir overnight.
The stirring is stopped and two phases can then be
seen (an orange phase above and a thick phase with a
greyish beige colour below) The organic phase is recovered
after filtration. The methanol is evaporated using a
rotary vaporiser and then CH2C12 and water are added. The
aqueous phase is washed with CHZC12 and the organic phase
(a blood red colour) with water in order to remove the
thallium salts. This organic phase is dried over Mgso4 and
evaporated using a rotary vaporiser.
As the reaction can give rise to several by-
products such as
0 0 0
fl j! r! 1! l!
C -.., CH2 r", C -.,. CH3
CH2 CH2 CH3
N l
Ts Ts
methoxy-3-acetyl 1-tosyle pyrrole uimethoxy 3-acety? 1-tosyle pyrrole
,~P~~~'~~('~'~CA 02351479 2001-05-17 AMENDED SHEET
:. ;>~
~_:ro .~e ... ... _ . .. ,. Y
~.~'~ ~ ~c~
~~5~-C~ ~E~~~6~~
~x., ~ ~a~w
the desired ester will be isolated on a silica gel
column. Having made a chromatographic plate it may be
5 noted that this ester is already isolated but nevertheless
a column is used to remove excess clay (which remains at
the head of the column). For the column, it is necessary
to use a mixture of two solvents of different polarity as
eluent since using a suitable composition of the two
10 solvents, better separation is obtained. Therefore a
mixture made up of 30% ethyl acetate (polar solvent) and
70% heptane (non-polar solvent) is used. The solvent is
evaporated and a yellow oil is collected.
9.23 g of ester is obtained being a yield of
15 75.6%.
4) Synthesis of pyrrole-3-acetic acid
o 0
2 O NaOH
O~H3 MeOH ~ ~ ~OH
_ - -- N
H
Ts
25 3-~G~~71( t~msyte pyrrole
Method
30 7.65 g of 1-tosyl pyrrole-3-acetate, 100 ml of
methanol and 300 ml of 5M sodium hydroxide are mixed in a
single necked flask fitted with a condenser. The medium is
stirred and heated under reflux for 2 hours 30 minutes. It
is left to cool to ambient temperature. The sodium
35 carboxylate is obtained. The methanol is evaporated and
100 ml of ether and water are added. Two phases separate,
,., SUBSTITUTE SHEET (RULE 26) r.
~_~ v
~~'~~~ ACA 02351479 2001-05-17 ~ E
;~~~ ~a~°i~~,;
~?2~~' ~~~~.~ ~-~~~ ~.x PCTI~,t~~~r~~ ~4t
~,-...~,.,".r_r~.....~,.:a.x~~ .a~~c,..~,~~.~.ad,~.~:~...R,x.' ,.< ~,
~.~e..~_,z ~._" ,
36
with the aqueous phase containing the carboxylate. This
phase is washed several times with ether. Then, very
slowly, concentrated HC1 is added, taking care to control
the temperature of the reaction medium using an ice bath
in order to prevent polymerisation. The product is then to
be found, in the form of an acid, in the organic phase.
The aqueous phase is extracted with ether and the organic
phase is dried over sodium sulphate.
A rotary vaporiser is then used and a silica gel
20 column is used to separate the- acid and the clay. The
solvent is evaporated and a yellow liquid is obtained that
crystallises rapidly. Tt is advisable to store it in ether
in the freezer in order to restrict the risk of
polymerisation.
2.7 g of product is obtained representing a yield
of 82.60.
Example 7 . Synthesis of preferred polymer (Bj
ij Synthesis of 3-acetate N-hydroxyphthalimide
OOH 0 0
DCC
I ~ ~. OH-N / _~ ~ ~ \0-N
CHClg N ~
H 0 H 0
Method
1.38 g of pyrrole-3-acetic acid, 1.98 g of N-
hydroxyphthalimide and 50 ml of chloroform are mixed in a
three necked flask. Using an addition ampoule, a solution
of DCC (2.67 g of DCC in 30 ml of CHCl3j is slowly added.
,~- ~ ~ .
~~t~~t~et sCAt02351479y 2ooi-o5-i7 SUBSTITUTE SHEET {RULE 26)
r w v m o ~ m a mr.t~
M ,~1 '"s'~1'~a.q;
'G ~'"~°~3~a"Hk"ts~sx""R x ~~~"e t
~~ ~$~ '~~ -
~'~a~ar3Y.~..e."t~#.,v,~,.:.~ °.~s;:sS..'~,' ,'.~.,4~.~n~~ar.d-
ir."«:.~.~a...,:..,~"-:;i ~"~y z%~
~ 3.. ~,~, n~ ~~ .~,..N ,~,~,:.c ;
37
During the addition of the DCC, the mixture forms
a solution.
It is left to stir overnight. DCU forms. It is
filtered and the CHC13 evaporated. A new white precipitate
of DCU forms. It is placed in the refrigerator for 1 hour,
filtered and evaporated. This operation is repeated until
the DCU is completely removed.
Plate chromatography is carried out using a
dichloromethane/methanol 2/.98 solvent. A mark
-10- corresponding to NHP is seen to appear. To remove it, a
silica gel column is used with the same eluent as that
used for the plate. The product is recrystallised in
chloroform.
A product of mass 1.04 g is obtained representing
a yield of 35%.
Example 8 . Synthesis of preferred polymer (C)
1) Synthesis of (3-ferrocene ethylamine
HZN
Et20 anhyd
Fe - CH2 - CN + A1C13 + Li:AlH4
H2504,Na0H Fe
30
Method
1.36 g of A1C13 in 12 ml of anhydrous Etzo is put
into a three necked flask. 0.388 g of LiAlH4 is added
slowly. Once the addition is complete, 1.36 g of
ferrocene-1-cyanomethyl (diluted in 12 ml of anhydrous
SUSSTiTUTE S~-tEET {RU~.~ 2fi)
~~~ , s ~~ ~~ x~
nrY nr~a r~nn.s:
"~p~~~~~,sCA 02351479 2001-05-17
'ra~a~s'~~ ".'~:~ ~. ~",~,~, ~.~s a ~- r ur ~ ' .~ . a v ~ . w v ~ !!rA A I1
fl,.-
..,... .r_.
38
Et20) is added. The solution is stirred for 2z hours under
ref lux .
Then the reaction medium is acidified with 15 ml
of 6N sulphuric acid. The two phases are separated and the
aqueous phase is washed with anhydrous Et20. The product
is then in the NH3~ form and is therefore to be found in
the aqueous phase. KOH is added until the pH - 9. The
product is then in the NH2 form and therefore in the
organic phase and is recovered, dried over potassium
carbonate and evaporated. -
0.45 g of a viscous orange liquid is obtained
representing a yield of 15%.
2) NHP-ferrocene ethylamine coupling
0 0 H2 0
0_~ ' CH3CrI /
~ +
~/' Fe
~ 0 H
~p F
Method
160 g of NHP-pyrrole, 150 mg of ferrocene
ethylamine and 10 ml of CH3CN are mixed in a three necked
flask. The solution is stirred for 48 hours. The CH3CN is
evaporated and the residue is dissolved in CHC13. The
solution is washed twice with a 10 % solution of NaHC03
then with water until pH7 is obtained. The organic phase
is dried over MgSOa and the NaHC03 is evaporated.
120 mg of product are obtained representing a
yield of 60%.
~~~..~~~ ~ ~ ~ SUBSTITUTE SHEET (RULE 2fi)
~~~~~(~~CA 02351479 2001-05-17
~~~ ~E'~~26~~'
39
Example 9 . Synthesis of preferred polymer (D)
_ 1) Synthesis of
NHP
Fe
NHP
NHP
COOH 0
DCC
Fe
Fe ~- 2 HO-N
i5
CHC1
HOOC ~ 3 NHP
0
2) Coupling
NHP
H CH2 CH2 NH2 .;- Fe
N
H NHP
H
CH3CN
in excess
NHP
0 Fe
N-CH2-CH2-N-C-
N H
H 0
H
~°~.
~'~~'~ '~'~ '' ' ''wr~2 "' SUBSTITUTE SHEET (RULE 26)
~~~~'~~(~ CA 02351479 2001-05-17 a
.Tw , _." ~ ., ." ,_.
,, ~' '~'Z" , g~ ,~ss~~ ~~~'.~'a~~'~~~~ a~ ~°~ PCTI~,~.w.w (n.~ w a.~
~~~~ES~r
,
r.~ . ». x.. ,..a
Example 10 . Synthesis of preferred polymer (E)
0
N-CH2-CH2-NH2
N ~ H
H
0
COON C - NHP
1 ) ~ ~ -,: ~ ---
N N N
I I f
H H
2)
0
~! 0
C-NHP acetonitrile ~~ N-CH2-CH2-NHBoc
-+- HZN-CH2-NH2-N -------
N , H
I N'
H i
H
3)
0 triflaoro
acetic acid 0
-N-CH2-CHZ-NHBoc CH2C12T
. ~ N-CHZ-CH2-NHZ
H N l
N i H
H H
~'~'° ~ ~' f ~ ~ '~'~ ' ' SUBSTITUTE SHEET (RULE 26)
~P~~~~~~CA 02351479' 2001-05-17
~ Vita f11 tltaz
~~~'~~,k~ ~k~~~'~~ ~~~D~SC26~ as
.. ......
~ ~.,~,~~ ~:
Example 22 . Synthesis of preferred polymer (F)
p o
~ N(CH2-COOH)2 or /_.~ ~IDA
N N
i a
H H
1)
0
Q
0- N
N
I
H 0
2)
NH(COC1)2 + 2 C(Me)3 OH ---~~ HN(COOtBu)2
30
SUBST1TUT~ S~IEET (RULE 26)
~~k_; _-~
CA 02351479 2001-05-17
~" .nY
'fis ~~.x'~.eY~,Y.n. r, ". "xi~e3 a i.a..tY...~ e~
7- .~ 1.,G~'
tf 2
3)
0 0
N ~ 2 - N, N(COOTBu) 2
NHP -~-. NH(COOtBu) ----
t
H H
4)
0
0
"' ~ N(COOH)2
~ N ( COOtBu) 2 Ba (0H) 2 8HZ0 N
N 1
H
H
Example 12 . Electropolymerisation
Homo- and co-polymers are obtained by
electropolymerisation of corresponding monomers or monomer
mixtures, in the following conditions .
solvent . acetonitrile or propylene carbonate
electrolyte . LiC104 O.1M
potential . 0.9 V/SCE
or current . 2-5 mA.cm-2
or potential scanning between 0-0.9 V/SCE
electrode : Pt, Ag, Pd, Sn02, Fe, A1
concentration of monomer (s) . 10'x-2.10' M.1'~
,~f~,~ . SUBSTITUTE SHEET {RULE 26)
~~"~~'~(~' (z~~ aCA 02351479 2001-05-17
~..~ . .. v% .~~ . ., . ... , ..,...
a~ .~