Note: Descriptions are shown in the official language in which they were submitted.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/091 i 5
QUINOLINE-4-CARBOXAMIDE DERIYATIYES AS NK-3 AND NK-2 RECEPTOR ANTAGONISTS
The present invention relates to novel compounds, in particular to novel
quinoline
derivatives, to processes for the preparation of such compounds, to
pharmaceutical
compositions containing such compounds and to the use of such compounds in
medicine.
The mammalian peptide Neurokinin B (NKB) belongs to the Tachykinin (TK)
peptide family which also include Substance P (SP) and Neurokinin A (NKA).
Pharmacological and molecular biological evidence has shown the existence of
three
subtypes of TK receptor (NK1, NK2 and NK3) and NKB binds preferentially to the
NK3
receptor although it also recognises the other two receptors with lower
affinity (Maggi et
al, 1993, J. Auton. Pharmacol., l3, 23-93).
Selective peptidic NK3 receptor antagonists are known (Drapeau, 1990 Regul.
Pept., 3 I, 125-135), and findings with peptidic NK3 receptor agonists suggest
that NKB,
by activating the NK3 receptor, has a key role in the modulation of neural
input in
airways, skin, spinal cord and nigro-striatal pathways (Myers and Undem, 1993,
.I. Physiol., 470, 665-679; Counture et al., 1993, Regul. Peptides, 46, 426-
429; Mccarson
and Krause, 1994, J. Neurosci., 14 {2), ?12-720; Arenas et al.
1991,,LNeurosci., 11,
2332-8). However, the peptide-like nature of the known antagonists makes them
likely to
be too labile from a metabolic point of view to serve as practical therapeutic
agents.
Copending International Patent Application number PCT/EP98/03014 discloses
certain compounds stated to be non-peptide NK-3 antagonists and also to have
NK-2
antagonist activity. These compounds are therefore considered to be of
potential use in
the prevention and treatment of a wide variety of clinical conditions which
are
characterized by overstimulation of the tachykinin receptors, in particular NK-
3 and NK-
2.
We have now discovered a further novel class of non-peptide NK-3 antagonists
which are far more stable from a metabolic point of view than the known
peptidic NK-3
receptor antagonists and are of potential therapeutic utility. These compounds
also have
NK-2 antagonist activity and are therefore considered to be of potential use
in the
prevention and treatment of a wide variety of clinical conditions which are
characterized
by overstimulation of the tachykinin receptors, in particular NK-3 and NK-2.
These conditions include respiratory diseases, such as chronic obstructive
pulmonary disease (COPD), asthma, airway hyperreactivity, cough; inflammatory
diseases such as inflammatory bowel disease, psoriasis, fibrositis,
osteoarthritis,
rheumatoid arthritis and inflammatory pain; neurogenic inflammation or
peripheral
neuropathy, allergies such as eczema and rhinitis; ophthalmic diseases such as
ocular
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
2
inflammation, conjunctivitis, vernal conjuctivitis and the like; cutaneous
diseases, skin
disorders and itch, such as cutaneous wheat and flare, contact dermatitis,
atopic
dermatitis, urticaria and other eczematoid dermatitis; adverse immunological
reactions
such as rejection of transplanted tissues and disorders related to immune
enhancement or
suppression such as systhemic lupus erythematosis; gastrointestinal (GI)
disorders and
diseases of the GI tract such as disorders associated with the neuronal
control of viscera
such as ulcerative colitis, Crohn's disease, irritable bowel syndrome (IBS),
gastro-
exophageous reflex disease (GERD}; urinary incontinence and disorders of the
bladder
function; renal disorders (hereinafter referred to as the 'Primary
Conditions').
Certain of these compounds also show CNS activity and hence are considered to
be of particular use in the treatment of disorders of the central nervous
system such as
anxiety, depression, psychosis and schizophrenia; neurodegenerative disorders
such as
AIDS related dementia, senile dementia of the Alzheimer type, Alzheimer's
disease,
Down's syndrome, Huntington's disease, Parkinson's disease, movement disorders
and
convulsive disorders (for example epilepsy); demyelinating diseases such as
multiple
sclerosis and amyotrophic lateral sclerosis and other neuropathological
disorders such as
diabetic neuropathy, AIDS related neuropathy, chemotherapy-induced neuropathy
and
neuralgia; addiction disorders such as alcoholism; stress related somatic
disorders; reflex
sympathetic dystrophy such as shoulder/hand syndrome; dysthymic disorders;
eating
disorders (such as food intake disease); fibrosing and collagen diseases such
as
scleroderma and eosinophilic fascioliasis; disorders of the blood flow caused
by
vasodilation and vasospastic diseases such as angina, migraine and Reynaud's
disease and
pain or nociception, for example, that is attributable to or associated with
any of the
foregoing conditions especially the transmission of pain in migraine,
(hereinafter referred
to as the 'Secondary Conditions').
The compounds of formula (I) are also considered to be useful as diagnostic
tools
for assessing the degree to which neurokinin-3 and neurokinin-2 receptor
activity
(normal, overactivity or underactivity) is implicated in a patient's symptoms.
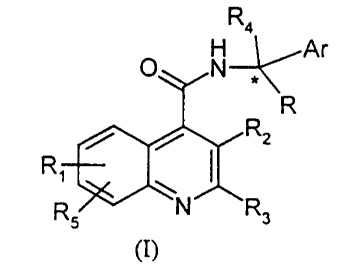
According to the present invention there is provided a compound, or a solvate
or a
salt thereof, of formula (I):
Ra
O N~Ar
R
R~
Rs
(I)
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
wherein, Ar is an optionally substituted aryl or a CS_7 cycloalkdienyl group,
or an
optionally substituted CS_~ cycloalkyl group, , or an optionally substituted
single or fused
ring aromatic heterocyclic group;
R is hydrogen, linear or branched Cl_6 alkyl, C3_~ cycloalkyl, C3_~
cycloalkylalkyl;
Rl represents hydrogen or up to three optional substituents selected from the
list
consisting of: C 1 _6 alkyl, C 1 _6 alkenyl, aryl, C 1 _6 alkoxy, hydroxy,
halogen, vitro,
cyano, carboxy, carboxamido, sulphonamido, C l _6 alkoxycarbonyl,
trifluoromethyl,
acyloxy, amino or mono- and di-C 1 _6 alkylamino;
R2 represents a moiety -(CH2)"-NYlY2 wherein n is an integer in the range of
from 1 to 9, Y 1 and Y2 are independently selected from C 1 _6-alkyl; C 1 _6
alkyl
substituted with hydroxy, alkoxy, C l _6 alkylamino or bis (C l _6 alkyl)
amino; C3-6
cycloalkyl; C4-6 azacycloalkyl; Cl_6-alkenyl; aryl or aryl-Cl_6-alkyl or Yl
and Y2
together with the nitrogen atom to which they are attached represent an
optionally
substituted N-linked single or fused ring heterocyclic group;
R3 is branched or linear C1_6 alkyl, C3_~ cycloalkyl, C4_~ cycloalkylalkyl~,
optionally substituted aryl, or an optionally substituted single or fused ring
aromatic
heterocyclic group; and
R4 represents hydrogen or C 1 _6 alkyl.
RS represents hydrogen or halogen.
Preferably RS represents hydrogen. In another preferred aspect RS is chloro or
bromo.
Suitably, Ar represents optionally substituted phenyl,unsubstituted phenyl or
cyclohexyl.
Suitably, Ar represents cyclohexyl.
Preferably Ar is phenyl or cyclohexyl.
Suitably, R represents C,.6 alkyl, for example methyl or ethyl or iso-propyl.
In one preferred aspect, R is ethyl. In another preferred aspect, R is methyl
or
isopropyl.
Suitably R 1 represents hydrogen, C 1 _6 alkoxy, for example methoxy, or
hydroxy.
Preferably, R1 represents hydrogen. In another preferred aspect, Rl is methoxy
or
hydroxy.
Suitably, NYlY2 represents an optionally substituted N-linked single or fused
ring heterocyclic group.
CA 02351865 2001-05-18
WO 00/31037 4 PCT/EP99/09115
Suitable N-linked single or fused heterocyclic groups, include groups in which
any single or fused ring is saturated or unsaturated and consists of 5- or 6-
ring atoms,
said ring atoms optionally comprising 1 or 2 additional heteroatoms selected
from 0 or N
and wherein one or two ring atoms are optionally substituted with one or two
oxo groups
or one or two of hydroxy, carboxy, carboxy C1-6 alkyl, CI_6 alkoxycarbonyl,
aminocarbonyl, Cl-6 alkylcarbonyl optionally substituted with an aromatic
heterocyclic
group, arylcarbonyl, aryl C1-6 alkylcarbonyl, carboxy C1-6 alklycarbonyl,
carboxyarylcarbonyl, amino, C 1-6 alkylcarbonylamino, C 1-6 alkyl, C 1-6
hydroxyalkyl,
aryl. aryl, C1-6 alkyl, C3_~ cycloalkyl, optionally substituted C4-7
cycloalkenyl,
optionally substituted C4-7 azacycloalkyl, optionally substituted C4-7
diazacycloalkyl,
optionally substituted C4-7 oxaazacycloalkyl, optionally substituted C4-7
thiazacycloalkyl, optionally substituted C4-7 thiazacycloalkenyl, C3_~
cycloalkylalkyl,
hydroxy C 1-6 alkoxy C 1-6 alkyl, C 1-b alkoxy C 1-6 alkyl, di C 1-6
alkylaminocarbonyl,
di C1-6 alkylamino CI-6 alkylcarbonyl, optionally substituted C4-7
azacycloalkyl C1-6
alkylcarbonyl, optionally substituted C4-7 diazacycloaklyl CI-6 alkylcarbonyl,
optionally
substituted C4-7oxaazacycloalkyl CI-6 alkylcarbonyl, optionally susbtituted
carboxamidine, C1-6 alkylaminothiocarbonyl, optionally substituted nitrovinyl,
aminosulphonyl, di Cl-6 alklyaminosulphonyl, or an optionally substituted
spiroheterocyclic ring or a single or fused ring aromatic heterocyclic group,
or the
substituents on adjacent ring atoms form a carbocyclic ring; said aryl or
aromatic
heterocyclic groups being optionally substituted with one or two C 1 _6 alkyl,
alkoxy,
hydroxy, halogen or halogenalkyl groups; wherein, unless otherwise defined
optionally
substituted means substituted with up to three substituents selected from the
list
consisting of: amino, alkylamino, alkyl, aryl, heterocyclyi, alkylaryl,
aralkyl, oxo,
hydroxy and nitrite.
Preferably, the additional heteroatom is N.
Favoured optional substituents for the N-linked single or fused heterocyclic
groups are selected from carboxy C1-6 alkyl, aminocarbonyl, C1-6 alkylcarbonyl
optionally substituted with an aromatic heterocyclic group, arylcarbonyl, aryl
C1-6
alkylcarbonyl, carboxy CI-6 alklycarbonyl, carboxyarylcarbonyl, amino, C1-6
alkylcarbonylamino, C 1 _6 alkyl, C 1 _6 hydroxyalkyl, aryl, aryl C 1-6 alkyl,
C3-~
cycloalkyl, optionally substituted C4-7 cycloalkenyl, optionally substituted
C4-7
azacycloalkyl, optionally substituted C4-7 diazacycloalkyl, optionally
substituted C4-7
oxaazacycloalkyl, optionally substituted C4-7 thiazacycloalkyl, optionally
substituted
C4-7 thiazacycloalkenyl, C;-~ cycloalkylalkyl, hydroxy Cl-6 alkoxy C1-b alkyl,
C1-6
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
alkoxy CL-6 alkyl, di CL-6 alkylaminocarbonyl, di C1-6 alkylamino C1-6
alkylcarbonyi,
optionally substituted C4-7 azacycloalkyl CL-6 alkylcarbonyl, optionally
substituted C4-
7 diazacycloaklyl C1-6 alkylcarbonyl, optionally substituted C4-
7oxaazacycloalkyl C1-6
alkylcarbonyl, optionally susbtituted carboxamidine, C1-6
alkylaminothiocarbonyl,
optionally substituted nitrovinyl, aminosulphonyl, di C1-6
alklyaminosulphonyl, or an
optionally substituted spiroheterocyclic ring; wherein, unless otherwise
defined
optionally substituted means substituted with up to three substituents
selected from the
list consisting of: amino, alkylamino, alkyl, aryl, heterocyclyl, alkylaryl,
aralkyl, oxo,
hydroxy, nitrite. Preferred optional substituents for the N-linked single or
fused
heterocyclic groups include isopropylcarbonyl, hydroxyethyl, cyclohexyl,
phenyl, benzyl,
isopropyl, phenethyl, 1-piperidinyl, hydroxyethoxyethyl, (4-hydroxy)-1-
piperidinyl, 4-
piperidinyl, (I-methyl)-4-piperidinyl, dimethylaminomethylcarbonyl,
diethylaminoethylcarbonyl, (4-methyl)-1-piperazinylmethylcarbonyl, 4-
morpholinylethylcarbonyl, amino, (4-methyl)-1-piperazinyl, I-piperazinyl, N-
methyl-N'-
cyanocarboxamidine, 2-thiazolinyl, pyrrolidinyl-N-cyanomethyleneimine,
pyrrolidinyl-
N-methylmethyleneimine, I-pyrrolidinyl-2-nitrovinyl, carboxamidine,
carboxyethylcarbonyl, pyrrolidinyl-N-methylsulphonylmethyleneimine, (2-
carboxy)-
phenylcarbonyl, aminosulphonyl, dimethylaminosulphonyl, carboxymethyl.
When present oxo substituents are preferably alpha to the point of linkage of
the
N-linked single or fused heterocyclic group.
When a hetero atom of the N-linked single or fused heterocyclic group is
substituted, preferred substituents are selected from C1-6 alkyl, hydroxy C1-6
alkyl for
example hydroxyethyl, C3_~ cycloalkyl, C3-~ cycloalkylalkyl, aryl and
arylalkyl, for
example methyl, ethyl, isopropyl, phenyi,phenethyl, or benzyl, optionally
substituted C4-
7 azacycloalkyl for example 4-piperidinyl or (I-methyl)-4-piperidinyl,
dialkylaminoalkylcarbonyl for example dimethylaminomethylcarbonyl or
diethylaminoethylcarbonyl, hydroxy CI-b alkoxy CI-6 alkyl for example
hydroxyethoxyethyl, optionally substituted C4-7 diazacycloalkyl CI-6
alkylcarbonyl or
C4-7 oxaazacycloalkyl C1-6 aIkylcarbonyl for example , (4-methyl)-I-
piperazinylmethylcarbonyl, 4-morpholinylethylcarbonyl, optionally substituted
carboxamidine for example carboxamidine or N-methyl-N'-cyanocarboxamidine, or
pyrrolidinyl-N-cyanomethyleneimine or pyrrolidinyl-N-methylmethyleneimine or
pyrrolidinyl-N-methylsulphonylmethyleneimine, optionally substituted
nitrovinyl for
example 1-pyrrolidinyl-2-nitrovinyl, optionally substituted C4-7
thiazacycloalkenyl for
example 2-thiazolinyl, carboxy C 1-6 alklycarbonyl for example
carboxyethylcarbonyl,
CA 02351865 2001-05-18
WO 00/31037 6 PCT/EP99/09115
carboxyarylcarbonyl for example (2-carboxy)-phenylcarbonyl, aminosulphonyl, di
C l -6
alklyaminosulphonyl for example dimethylaminosulphonyl, carboxy CI-6 alkyl for
example carboxymethyl.
Fused heterocyclic groups include groups having one or more rings which share
one or more atoms, such as spiro fused rings, or one or more bonds.
A suitable N-linked single ring heterocyclic group comprising a 5- membered
saturated heterocyclic ring is a pyrrolidin -1- yi group.
A suitable N-linked single ring heterocyclic group comprising a 6- membered
saturated heterocyclic ring is an optionally substituted piperidin-1-yl group,
for example a
4-(piperidin-1-yl)piperidin-1-yl group or 4-aminopiperidin-1-yl group.
A suitable N-linked single ring 6- membered saturated heterocyclic group
comprising an additional heteroatom is an optionally substituted piperazin-1
yl group, for
example an optionally substituted 4-alkylpiperazin-1-yl group.
A suitable N-linked fused ring heterocyclic group includes a 5-or 6- membered
saturated or unsaturated heterocyclic ring fused to a benzene ring.
A suitable N-linked fused ring heterocyclic group comprising a 6- membered
saturated heterocyclic ring fused to a benzene ring is a 2-(l, 2 ,3 ,4-
tetrahydro)isoquinolinyl group.
Suitable, N-linked fused heterocyclic groups include spiro fused groups, for
example 1,4-dioxa-8-azaspiro[4.5]dec-8-yl group or 3-oxo-2,8-
diazaspiro(4.5]dec-8-yl or
2,4-dioxo-1,3,8-triazaspiro[4.5]dec-8-yl or 2,7-diazaspiro[4.4]non-2-yl or 2,3-
dioxa-1,8-
diazaspiro[4.5]dec-8-yl.
One preferred value of -NYlY2 is a piperazin-1-yl group, especially a 4-
hydroxyalkylpiperazin-1-yl, or 4-(dialkylaminoalkylcarbonyl)piperazin-1-yl, or
4-
(azacycloalkyl)piperazin-1-yl, which piperazinyl group may be substituted or
unsubstituted
A particularly preferred value of -NYlY2 is a group of formula (a), (b) (c) or
(d):
_~ _
(a) (b)
wherein Tl represents isopropylcarbonyl, hydroxyethyl, cyclohexyl, phenyl,
benzyl,
isopropyl, phenethyl, 1-piperidinyl, hydroxyethoxyethyl, (4-hydroxy)-1-
piperidinyl, 4-
piperidinyl, (1-methyl)-4-piperidinyl, dimethylaminomethylcarbonyl,
CA 02351865 2001-05-18
WO 00/31037 ~ PCT/EP99/09115
diethylaminoethylcarbonyl, (4-methyl)-1-piperazinylmethylcarbonyl, 4-
morpholinylethylcarbonyl, amino, (4-methyl)-1-piperazinyl, 1-piperazinyl, N-
methyl-N'-
cyanocarboxamidine, 2-thiazolinyl, pyrrolidinyl-N-cyanomethyleneimine,
pyrrolidinyl-
N-methylmethyleneimine, 1-pyrrolidinyl-2-nitrovinyl, carboxamidine,
carboxyethylcarbonyl, pyrrolidinyl-N-methylsulphonylmethyleneimine, (2-
carboxy)-
phenylcarbonyl, aminosulphonyl, dimethylaminosulphonyl, carboxymethyl.
or
Ti
T4
-N N-T~ -N
T3
(c) (d)
wherein T1 together with T2 and the atoms to which each is attached form an
optionally
substituted single or fused ring heterocyclic group and either T3 together
with T4 form an
optionally substituted single or fused ring heterocyclic group;
Suitably T, represents one of the following groups:
O R8
wN~N~R6 m N Q~
R7
O O
wherein Rb represents H or a lower alkyl, preferably H or methyl,
m is an integer from 1 to 5 and R~ and Rg represent a lower alkyl, preferably
methyl or
ethyl or together form an heterocycle, for example a piperidine, morpholine or
optionally
substituted piperazine.
Q, represents 2-phthalic acid, a saturated or unsaturated C1-6 carboxylic acid
or
an heterocycle for example 2-imidazolyl or thiazolyl.
In a group of formula (a), suitably T, represents also an heterocycle for
example
imidazolyl, thiazolyl, pyridyi, pyrimidyl, tetrazolyl or T, represents an
optionally
substituted carboxamidine or a corresponding quaternary carboxamidine
derivative.
In a group of formula (a) suitable T, represents also one of the chemical
entities
below:
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
8
R9~N~R10
- ~~ -SOZR~~ / NOZ
O
N-R10 N-R10 N-R10
R9 R9 R9 O
wherein R9 and R,a represent hydrogen, alkyl or together form a 5 to 7
membered
ring with the N atom to which they are attached, preferably a pyrrolidin or
piperidin ring
and R, i represents C, _6 linear or branched alkyl or optionally substituted
aryl
wherein Qz is hydrogen, alkyl, aralkyl, aryl, cyano.
In a group of formula (a) suitable T1 represents also a sulphonamide of
formula:
SOzNRiZR~3
wherein R1z and R,3 are independently selected from hydrogen; C,_6 alkyl;
optionally
substituted aryl or R,2 and Ri3 together with the nitrogen atom to which they
are attached
represent an optionally substituted N-linked single or fused ring heterocyclic
group.
In one particular aspect -NYlY2 is a moiety of formula (a).
In one particular aspect -NYlY2 is a moiety of formula (b).
In one particular aspect -NY1 Y2 is a moiety of formula (c).
In one particular aspect -NY1 Y2 is a moiety of formula (d).
Suitably, R3 is optionally substituted aryl, preferably an unsubstituted aryl
group
such as a phenyl group.
Suitably, Rq, is hydrogen.
Suitably, n is an integer from 1 to 6, favourably 1 to 4 and most preferably
1, 2 or
3.
Favourably, n' represents 1.
Favourably, n' represents 2.
Favourably, n' represents 3.
Preferred compounds of formula (I) are those wherein:
Ar is phenyl or cyclohexyl, R is methyl, ethyl, or isopropyl, R1 is hydrogen
or methoxy
or hydroxy, R2 is a moiety (CH2)n wherein n is 1, 2, 3 or 4, R3 is phenyl and
R4 is
hydrogen and NY 1 Y2 is~
(i) an optionally substituted piperazinyl group, especially a moiety of the
above
defined formula (a);
(ii) a moiety of the above defined formula (b); or
CA 02351865 2001-05-18
WO 00/31037 9 PCT/EP99/09115
(iii) a moiety of the above defined formula (c); or
(iv) a moiety of the above defined formula (d).
Further preferred compounds of formula (I) are those wherein: Ar is phenyl or
cyclohexyl, R is methyl, ethyl or isopropyl, R1 is hydrogen, methoxy or
hydroxy R2 is a
moiety -(CH2)n-NYlY2 wherein n is 1,R3 is phenyl and R4 is hydrogen and NYlY2
is~
(i) an optionally substituted piperazinyl group, especially a moiety of the
above
defined formula (a); or
(ii) a moiety of the above defined formula (b).
In particular should be mentioned the compounds of examples 20, 29, 32, 33,
34,
46, 47, 48, 53, 55, 62, 67, 78, 79, 80, 81 and 95.
The compounds of formula (I) may have at least one asymmetric centre - for
example the carbon atom labelled with an asterisk (*) in the compound of
formula {I) -
and therefore may exist in more than one stereoisomeric form. The invention
extends to
all such stereoisomeric forms and to mixtures thereof, including racemates. In
particular,
the invention includes compounds wherein the asterisked carbon atom in formula
(I) has
the stereochemistry shown in formula (Ia):
Ar
0;... ~ N ~ :,.
R
R' ~,//. ' \~.// \ '~~. Z
.. ,,- . N . _. R3
Rs
wherein Ar, R, R1, R2, R3, R4 and RS are as defined in relation to formula
(I).
The compounds of formula (I) or their salts or solvates are preferably in
pharmaceutically acceptable or substantially pure form. By pharmaceutically
acceptable
form is meant, inter alia, having a pharmaceutically acceptable level of
purity excluding
normal pharmaceutical additives such as diluents and carriers, and including
no material
considered toxic at normal dosage levels.
A substantially pure form will generally contain at least 50% (excluding
normal
pharmaceutical additives), preferably 75%, more preferably 90% and still more
preferably 95% of the compound of formula (I) or its salt or solvate.
One preferred pharmaceutically acceptable form is the crystalline form,
including
such form in pharmaceutical composition. In the case of salts and solvates the
additional
ionic and solvent moieties must also be non-toxic.
CA 02351865 2001-05-18
WO 00/31037 1 O PCT/EP99/09115
Suitable salts are pharmaceutically acceptable salts.
Suitable pharmaceutically acceptable salts include the acid addition salts
with the
conventional pharmaceutical acids, for example malefic, hydrochloric,
hydrobromic,
phosphoric, acetic, fumaric, salicylic, citric, lactic, mandelic, tartaric,
succinic, benzoic,
ascorbic and methanesulphonic.
Suitable pharmaceutically acceptable salts include salts of acidic moieties of
the
compounds of formula (I) when they are present, for example salts of carboxy
groups or
phenolic hydroxy groups.
Suitable salts of acidic moieties include metal salts, such as for example
aluminium, alkali metal salts such as lithium, sodium or potassium, alkaline
earth metal
salts such as calcium or magnesium and ammonium or substituted ammonium salts,
for
example those with lower alkylamines such as triethylamine, hydroxy
alkylamines such
as 2-hydroxyethylamine, bis-(2-hydroxyethyl)-amine or tri-(2-hydroxyethyl)-
amine,
cycloalkylamines such as bicyclohexylamine, or with procaine,
dibenzylpiperidine,
N-benzyl-(3-phenethylamine, dehydroabietylamine, N,N'-bisdehydroabietylamine,
glucamine, N-methylglucamine or bases of the pyridine type such as pyridine,
collidine,
quinine or quinoline.
Suitable solvates are pharmaceutically acceptable solvates.
Suitable pharmaceutically acceptable solvates include hydrates.
The term 'alkyl' (unless specified to the contrary) when used alone or when
forming part of other groups (such as the 'alkoxy' group) includes straight-
or
branched-chain alkyl groups containing 1 to 12 carbon atoms, suitably 1 to 6
carbon
atoms, examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl
or tert-butyl
group.
The term 'carbocylic' refers to cycloalkyl and aryl rings.
The term 'cycloalkyf includes groups having 3 to 12, suitably 4 to 6 ring
carbon
atoms.
The term'aryf includes phenyl and naphthyl, preferably phenyl which unless
specified to the contrary optionally comprise up to five, preferably up to
three
substituents selected from halogen, alkyl, phenyl, alkoxy, haloalkyl,
hydroxyalkyl,
hydroxy, amino, nitro, cyano, carboxy, alkoxycarbonyl, alkoxycarbonylalkyl,
alkylcarbonyloxy, or alkylcarbonyl groups.
The term 'aromatic heterocyclic group' includes groups comprising aromatic
heterocyclic rings containing from 5 to 12 ring atoms, suitably 5 or 6, and
comprising up
to four hetero-atoms in the or each ring selected from S, O or N.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
11
Unless specified to the contrary, suitable substituents for any heterocyclic
group
includes up to 4 substituents selected from the group consisting of: alkyl,
alkoxy, aryl and
halogen or any two substituents on adjacent carbon atoms, together with the
carbon atoms
to which they are attached, may form an aryl group, preferably a benzene ring,
and
wherein the carbon atoms of the aryl group represented by the said two
substituents may
themselves be substituted or unsubstituted.
When used herein the term "halogen" refers to fluorine, chlorine, bromine and
iodine, preferably fluorine, chlorine or bromine.
When used herein the term "acyl" includes residues of acids, in particular a
residue of a carboxylic acid such as an alkyl- or aryl- carbonyl group.
The invention also provides a process for the preparation of a compound of
formula (I), or a salt thereof and/or a solvate thereof, which process
comprises reacting a
compound of formula (II) or an active derivative thereof:
O OH
/ / ! R's
R'~
~N~R,a
R~s
(II)
wherein R'1, R'2, R'3 and R'S are R1, R2, R3 and RS respectively as defined in
relation to formula (I) or a group convertible to R1, R2, R3 and RS
respectively; with a
compound of formula (III):
Ar
~N~R~
'H
Rs
(III)
wherein R', R4' and Ar' are R, R4 and Ar as defined for formula (I) or a group
or
atom convertible to R, R4 and Ar respectively; to form a compound of formula
(Ib):
H A~'
O N --~ R'
R'a
/ / , R'Z
R'~
R N~R~s
(Ib)
CA 02351865 2001-05-18
WO 00/31037 12 PCT/EP99/09115
wherein Ar', R', R'l, R'2, R'3, R'4 and R'S are as defined above, and
thereafter carrying
out one or more of the following optional steps:
(i) converting arty one of Ar', R', R'l, R'2, R'3, R'4 and R'S to Ar, R, R1,
R2, R3, R4
or RS respectively as required, to obtain a compound of formula (I);
(ii) converting a compound of formula (I) into another compound of formula
(I); and
(iii) preparing a salt of the compound of formula (I) and/or a solvate
thereof.
Suitable groups convertible into other groups include protected forms of said
groups.
Suitably Ar', R', R'l, R'2, R'3, R'4 or R'S each represents Ar, R, Rl, R2, R;,
R4 or
R~ respectively or a protected form thereof.
It is favoured if the compound of formula (II) is present as an active
derivative.
A suitable active derivative of a compound of formula (II) is a transient
activated
form of the compound of formula (II) or a derivative wherein the carboxy group
of the
compound of formula (II) has been replaced by a different group or atom, for
example by
an acyl halide, preferably a chloride, or an acylazide or a carboxylic acid
anhydride.
Other suitable active derivatives include: a mixed anhydride formed between
the
carboxyl moiety of the compound of formula (II) and an alkyl chloroformate; an
activated ester, such as a cyanomethyl ester, thiophenyl ester, p-nitrophenyl
ester, p-
nitrothiophenyl ester, 2,4,6-trichlorophenyl ester, pentachlorophenyl ester,
pentafluorophenyl ester, N-hydroxy-phtalimido ester, N-hydroxypiperidine
ester, N-
hydroxysuccinimide ester, N-hydroxy benzotriazole ester; alternatively, the
carboxy
group of the compound of formula (II) may be activated using a carbodiimide or
N,N'-
carbonyldiimidazole.
The reaction between the compound of formula (II) or the active derivative
thereof and the compound of formula (III) is carried out under the appropriate
conventional conditions for the particular compounds chosen. Generally, when
the
compound of formula (II) is present as an active derivative the reaction is
carried out
using the same solvent and conditions as used to prepare the active
derivative, preferably
the active derivative is prepared in situ prior to forming the compound of
formula (Ib)
and thereafter the compound of formula (I) or a salt thereof and/or a solvate
thereof is
prepared.
For example, the reaction between an active derivative of the compound of
formula (II) and the compound of formula (III) may be carried out:
(a) by first preparing an acid chloride and then coupling said chloride with
the
compound of formula (III) in the presence of an inorganic or organic base in a
suitable
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
13
aprotic solvent such as dimethylformamide (DMF) at a temperature in a range
from -70
to 50°C (preferably in a range from -10 to 20°C); or
(b) by treating the compound of formula (II) with a compound of formula {III)
in
the presence of a suitable condensing agent, such as for example N,N'-carbonyl
diimidazole (CDI) or a carbodiimide such as dicyclohexylcarbodiimide {DCC) or
N-
dimethylaminopropyl-N'-ethylcarbodiimide, preferably in the presence of N-
hydroxybenzotriazole (HOBT) to maximise yields and avoid racemization
processes (see
Synthesis, 453, 1972), or O-benzotriazol-1-yl-N,N,N',N'-
tetramethyluroniumhexalluorophosphate {HBTU), in an aprotic solvent, such as a
mixture of acetonitrile (MeCN) and tetrahydrofuran (THF), for example a
mixture in a
volume ratio of from 1:9 to 7:3 (MeCN:THF), at any temperature providing a
suitable
rate of formation of the required product, such as a temperature in the range
of from -70
to SO°C, preferably in a range of from -10 to 25°C, for example
at 0°C.
A preferred reaction is set out in Scheme 1 shown below:
Scheme 1
H Ar'
O OH O N -~- R'
R'
R,, i i I R'Z + ' N Ar' DCC and HOST or HBTU~ R , , I R,2
' ~ H ~ R TEA _ '' '
R's N R~3 R'4 o'C. 2h, r.t. 4-6 h. R'S N R~3
THF/CHzCN 80/20
(II) (III) (Ib)
wherein Ar', R', R'1, R'2, R'3, R'4 and R'g are as defined above.
It will be appreciated that a compound of formula (Ib) may be converted to a
compound of formula (I), or one compound of formula (I) may be convened to
another
compound of formula (I) by interconversion of suitable substituents. Thus,
certain
compounds of formula (I) and (Ib) are useful intermediates in forming other
compounds
of the present invention.
Accordingly, in a further aspect the invention provides a process for
preparing a
compound of formula (I), or a salt thereof and/or a solvate thereof, which
process
comprises converting a compound of the above defined formula (Ib) wherein at
least one
of Ar', R', R' 1, R'~, R'3, R'4 or R'S is not Ar, R, R~, R2 , R3, R4 or R~
respectively,
thereby to provide a compound of formula (I); and thereafter, as required,
carrying out
one or more of the following optional steps:
(i) converting a compound of formula (I) into another compound of formula (I);
and
CA 02351865 2001-05-18
WO 00/31037 14 PCT/EP99/09115
(ii) preparing a salt of the compound of formula (I) and/or a solvate thereof.
Suitably, in the compound of formula (Ib) the variables Ar', R', R' 1, R'2,
R'3, R'4
and R'S are Ar, R, Ra, R2, R3, R4 or RS respectively or they are protected
forms thereof.
The above mentioned conversions, protections and deprotections are carried out
using the appropriate conventional reagents and conditions and are further
discussed
below.
A compound of formula (II) or the corresponding alkyl (such as methyl or
ethyl)
ester wherein n is an integer 1, is prepared by reacting a compound of formula
(IV) or the
corresponding alkyl (such as methyl or ethyl) ester:
O OH
CH2L~
Ft''
N
(IV)
wherein R' 1, R'3 and R'g are as defined above and L 1 represents a halogen
atom such as
a bromine atom, with a compound of formula (V):
HNY'1 Y'2 (V)
wherein Y'1 and Y'2 are respectively Y1 and YZ as defined in relation to
formula (I) or
protected forms thereof.
Suitably, Y'1 and Y'2 are Y1 and Y2.
Suitably, reaction between the compounds of formulae (IV) or the corresponding
alkyl (such as methyl or ethyl) ester and (V) is carried out under
conventional amination
conditions, for example when L 1 is a bromine atom then the reaction is
conveniently
carried out in an aprotic solvent, such as tetrahydrofuran or
dimethylformamide at any
temperature providing a suitable rate of formation of the reduired product,
usually at
ambient temperature; preferably the reaction is carried out in the presence of
triethylamine (TEA) or K2C03.
A compound of formula (IV) or the corresponding alkyl (such as methyl or
ethyl)
ester is prepared by appropriate halogenation of a compound of formula (VI) or
the
corresponding alkyl (such as methyl or ethyl) ester:
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
O OH
~ C
R'~
R~a
(VI)
wherein R'1, R'3 and R'S are as defined above in relation to formula (II).
Suitable halogenation reagents are conventional reagents depending upon the
nature of the halogen atom required, for example when L 1 is bromine a
preferred
halogenation reagent is N-bromosuccinimide (NBS).
The halogenation of the compound of formula (VI) or the corresponding alkyl
(such as methyl or ethyl) ester is carried out under conventional conditions,
for example
bromination is carried out by treatment with NBS in an inert solvent, such as
1,2-
dichloroethane or CH3CN, at any temperature providing a suitable rate of
formation of
the required product, suitably at an elevated temperature such as a
temperature in the
range of 60°C to 100°C, for example 80°C; preferably the
reaction is carried out in the
presence of a catalytic amount on benzoyl peroxide.
In the case in which the corresponding alkyl (such as methyl or ethyl) ester
of
compounds (VI), (IV) and (II) are utilised, an hydrolysis to compound (II) is
required
before conversion to compound (Ib) in Scheme 1. Such hydrolysis can be carned
out
under acidic conditions, such 10-36% hydrochloric acid at a temperature in the
range
between 30 and 100 °C. A compound of formula (II) wherein R'2
represents -
(CH2)2-9-NYlY2~ is conveniently prepared by reacting a compound of formula
(VII):
O
R'~
N~O
Rs
(VII)
wherein R' 1 and R'S are as defined in relation to formula (II), with a
compound of
formula (VIII):
R3 - CO - CHZ - (CHZ)p -Ts (VIII)
wherein R'3 is as defined in relation to formula (II), and TS is a group -
NYlY2 as
defined in relation to formula (I) or a protected form thereof or a group
convertible
thereto, and p is an integer in the range of 2 to 9; and thereafter as
required removing any
protecting group and/or converting any group TS to NY 1 Y2.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
16
The reaction between the compounds of formula (VII) and (VIII) is conveniently
carried out using Pfitzinger reaction conditions (see for example J. Prakt.
Chem. 33, 100
{I886), J. Prakt. Chum. 38, 582 (1888), J. Chem. Soc. 106 (1948) and Chem.
Rev. 3~,
152 ( 1944)), for example in an alkanolic solvent such as ethanol, at any
temperature
providing a suitable rate of formation of the required product, but generally
at an elevated
temperature, such as the reflux temperature of the solvent, and preferably in
the presence
of a base such as potassium hydroxide or potassium tert-butoxide.
Protected forms of -NY1 Y2 will vary according to the particular nature of the
group being protected but will be chosen in accordance with normal chemical
practice.
Groups convertible to -NY 1 Y2 include groups dictated by conventional
chemical
practice to be required and to be appropriate, depending upon the specific
nature of the -
NY 1 Y2 consideration.
Suitable deprotection methods for deprotecting protected forms of NY 1 Y2 and
conversion methods for converting TS to NYlY2 will be those used
conventionally in the
art depending upon the particular groups under consideration with reference to
standard
texts such as Greene, T.W. and Wuts, P.G.M. Protective Groups in Organic
Synthesis,
John Wiley & Sons Inc. New York, 1991 (Second Edt.) or in Kocienski, P.J.
Protecting
groups. George Thieme Verlag, New York, 1994 and Chemistry of the Amino Group,
Patais (Ed.), Interscience, New York 1968; or Advanced Organic Chemistry,
March J,
John Wiley & Sons, New York, 1992.
A compound of formula (VIII) is prepared from a compound of formula (IX):
R3 -CO -CHZ-(CHZ)P-OH (IX)
wherein R'3 is as defined in relation to formula (II) and p is as defined in
relation to
formula (VIII), by first halogenating, preferably brominating, or mesylating
the
compound of formula (IX) and thereafter reacting the halogenation or
mesylation product
so formed with a compound capable of forming a group Tg so as to provide the
required
comound of formula (VII).
When TS is a group -NY 1 Y2, a compound capable of forming a group T~, is a
compound of the above defined formula (V).
The halogenation of the compound of formula (IX) is suitably carried out using
a
conventional halogenation reagent. Mesylation is conveniently carried out
using mesyl
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
17
chloride in an inert solvent such as methylene dichloride, at a temperature
below room
temperature, such as 0°C, preferably in the presence of triethylamine.
The reaction conditions between the compound of formula (IX) and the compound
capable of forming a group TS will be those conventional conditions dictated
by the
specific nature of the reactants, for example when the TS required is a group
NY1 Y2 and
the required compound capable of forming a group TS is a compound of the above
defined formula {V), then the reaction between the halogenation or mesylation
product of
the compound of formula (IX) and the compound of formula (V) is carned out
under
analogous conditions to those described for the reaction between the compounds
of
formulae (IV) and (V).
Other compounds capable of forming a group TS will depend upon the particular
nature
of T5, but will be those appropriate compounds dictated by conventional
chemical
practice with reference to standard texts such asChemistry of the Amino Group,
Patais
(Ed.), Interscience, New York 1968; and Advanced Organic Chemistry, March J,
John
Wiley & Sons, New York, 1992.
A compound of formula (IX) may be prepared by reacting a compound of formula
°~°
'-(CH ) _
2P1 (X)
wherein p is as defined in relation to formula (VIII), with a lithium salt of
formula (XI):
(XI)
wherein R'3 is as defined in relation to formula (II).
The reaction between the compounds of formulae (X) and (XI) can be carried out
in an aprotic solvent, such as diethyl-ether at any temperature providing a
suitable rate of
formation of the required product, usually at a low temperature such as in the
range of -
10°C to -30°C, for example -20°C.
The compounds of formula (III) are known commercially available compounds or
they can be prepared from known compounds by known methods, or methods
analogous
to those used to prepare known compounds, for example the methods described in
Liebigs Ann. der Chemie, (1936), 523, 199.
Chiral compound of formula (III) wherein Ar is a C; or C~ cycloalkyl group, R
is
methyl and R4 is H are described in J. Org. Chem. (1996), 61 (12), 4130-4135.
A chiral
compound of formula (III) wherein Ar is phenyl, R is isopropyl and Ra is H is
a known
compound described in for example Tetrahedron Lett. (1994), 35(22), 374-6.
CA 02351865 2001-05-18
WO 00/31037 PCT/lJP99/09115
18
The compounds of formula (V) are known, commercially available compounds or
they can be prepared using methods analogous to those used to prepare known
compounds; for example the methods described in the Chemistry of the Amino
Group,
Patais (Ed.), Interscience, New York 1968; Advanced Organic Chemistry, March
J, John
Wiley & Sons, New York, 1992 ; J. Heterocyclic Chem. (1990), 27, 1559;
Synthesis
(1975), 13~, Bioorg. Med. Chem. Lett. (1997), 7, 555, or Protective Groups in
Organic
Synthesis (second edition), Wiley Interscience, (1991) or other methods
mentioned
herein.
4-amino substituted piperidines are generally prepared by reductive amination
of
4-oxo-piperidine, or a 4-oxo-piperidine N-substituted with an appropriated
protecting
group, with an appropriate amine. Typical examples can be found in J. Org.
Chem.
(1990), 5~ (8), 2552-4 or ibid. (1995), 60 (1~), 4928-9.
Certain diazaspirononane intermediates used herein are known compounds, for
example that used to prepare example 68 is described in J. Med. Chem. (1990),
33 (8),
2270-227.
The condensation of succinic and phthalic anhydrides used to generate examples
83 and 85-87 is described in J. Indian Chem. Soc. (1979), 56 (2), 171-2.
4-Heterocyclic substituted piperidine as used for the preparation of example
77 are
described in US 4329348 A 19820511.
The compounds of formula (VII) are known compounds or they are prepared
according to methods used to prepare known compounds for example those
disclosed in
J. Org. Chem. 21, 171 (1955); J. Org. Chem. 21, 169 (1955).
The compounds of formula (X) and (XI) are known compounds or they are
prepared according to methods used to prepare known compounds for example
those
disclosed by Krow G. R. in Organic Reactions, Vol 43, page 251, John Wiley &
Sons
Inc.1994 (for the compounds of formula (X)) and Organometallics in Synthesis,
Schlosser M.(Ed), John Wiley & Sons Inc.1994 (for the compounds of formula
(XI)).
Compounds of formula (I) wherein RZ represents a moiety -(CHZ)"-NYtY2 and -
NY, Y2 is a piperazinyl group of formula (a) can suitably be prepared by
reacting a
compound of formula XII
R'
Ar' 1 'R',
O ~NH
N~ H
R',
i
R~y ~ N R'z
(XII)
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
19
wherein Ar', R', R' l, R'2, R'3, R'4 and R'S are as defined above, with
reactive
species of formula (XIII), for example:
L2 L'2
~2~L 2 ~2 ~ z
~z~~~2
O O N~CN N02 N~S02R"
Xllla Xllib Xilic Xllld
wherein LZ and L'2 represent leaving groups such as -SAlkyl or -OAlkyl,
preferably -SCH3 and -OButyl and R11 is as defined above.
Mono substitution of compounds of formula (XIII) by a compound of formula
(XII) generates news structures bearing still one leaving group, L'Z, which
can then be
reacted with compounds of formula:
~9RIo
wherein R9 and Rlo are as defined above to give the final compounds of formula
(I).
Substituted carboxamidinopiperazines are best prepared by reacting compounds
of formula (XII) with substituted isothiocyanates following scheme 2
Scheme 2
R' , R.
Ar'~R', A~ R, Ar R'~
O 'N~'H ~ S-N-R12 O NH N_R12 ~H3 O NH N-R12
N NH ---~ ~ ~ N~N-"C --~- NON---~~
Ry / ~ . ~---~ R', / ~ . ~--~ S R.~ ~ ~ U SCH'
R s N R' R s N R' R' / N Rn
s
(XII)
(Xlln
(XV)
wherein RIZ represents lower alkyl, optionally substituted aryl or aralkyl,
followed by the
substitution of the group -SCH3, which takes place of the leaving group LZ,
with a
compound of formula
HNR9RIo
as mentioned above.
Unsubstituted carboxamidinopiperazines of formula (XVI)
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
20
R'
Ar' ~ R',
0 NH
NH
N N-
R'~ ~ NHz
R,s N R s
(XVI)
are prepared by reacting a compound of formula (XII) with the benzotriazole
derivative
of formula (XVII).
N (XVIi)
~NHZ+
H2N
(Dimethylaminolethylene)dimethylammonium piperazines of formula (XVIII)
R'
At'~R',
O 'N~'H
~N-
N N-
R'~ ~ N-
Rs / N~R,'. /
(XVill)
are prepared by heating a compound of formula (XII) with HBTU in the presence
of a
base, for example TEA, in an appropriate solvent, usually one, or a mixture,
of those used
in peptide coupling reactions.Compounds of formula (I) wherein R2 represents a
moiety -
(CH2)"-NYlY2 and -NY~Y2 is a piperazinyl group of formula (a) wherein T1
represents
carboxy, alkoxycarbonyl, optionally substituted alkyl, optionally substituted
aryl, aralkyl,
cycloalkyl, can suitably be prepared by reacting a compound of formula XII
with a
compound of formula
T ,L3
Wherein T, represents one of the radicals defined as above and L3 a leaving
N\
N
group for example halogen or sulfonate, preferably chlorine, bromine or
mesylate.
Compounds of formula (XII) are prepared by removing the protective group of a
compound of formula (XIX)
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
21
(XIX}
wherein Ar', R', R'I, R'2, R'3, R'q, and R'S are as defined above and P is an
amine
protective group, for example fmoc or benzyl, preferably fmoc. The protective
group is
removed by standard methods described in the literature, for example the fmoc
residue is
splitted by action of piperidine at room temperature in a solvent like
acetonitrile.As
hereinbefore mentioned, the compounds of formula (I) may exist in more than
one
stereoisomeric form - and the process of the invention may produce racemates
as well as
enantiomerically pure forms. Accordingly, a pure enantiomer of a compound of
formula
(I) is obtained by reacting a compound of the above defined formula (II) with
an
appropriate enantiomerically pure primary amine of formula (IIIa) or (IIIc):
Ar' H Ar'
H . N R; R, H . N R; R,4
4
(IIIa)
(IIIc)
wherein R', R'4 and Ar' are as defined above, to obtain a compound of formula
(I'a) or (I'c):
H Ar, H Ar'
O N --C~~ R~ O
R~a R, a
R~z
R,5 N _ _ R,3 R,5 N , , R,
(I'a) (I~c)
wherein Ar', R', R' ~, R'2, R'3, R'4 and R'S are as defined above.
Compounds of formula (fa) or (fc) may subsequently be converted to compounds
of formula (Ia) or (Ic) by the methods of conversion mentioned before:
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
22
H Ar H Ar
O N -~~,a R O N R
~~ 4
RZ R4 R
R w ~ R~ ~ / I RZ
R5 N R3 w
R5 N ~ R3
(Ia)
(Ic)
wherein Ar, R, RI R2 , R3, R4 and RS are as defined above.
Suitably, in the above mentioned compounds of formulae (Ia), (Ic), (I'a),
(fc),
(IIIa) and (IIIc) R4 represents hydrogen.
An alternative method for separating optical isomers is to use conventional,
fractional separation methods in particular fractional crystallization
methods. Thus, a
pure enantiomer of a compound of formula (I) is obtained by fractional
crystallisation of
a diastereomeric salt formed by reaction of the racemic compound of formula
(I) with an
optically active strong acid resolving agent, such as camphosulphonic acid, in
an
appropriate alcoholic solvent, such as ethanol or methanol, or in a ketonic
solvent, such
as acetone. The salt formation process should be conducted at a temperature
between
20°C and 80°C, preferably at 50°C.
In the case in which other basic functionalities, such as primary, secondary
or
tertiary amine, are present in the molecule, a wider range of optically active
acid
resolving agents become available, including tartaric acid, O,O'-di-p-
toluoyltartaric acid
and mandelic acid.
A suitable conversion of one compound of formula (I) into a further compound
of
formula (I) involves converting one group R2 into another group R2 by for
example:
(i) converting a ketal into a ketone, by such as mild acidic hydrolysis, using
for example
dilute hydrochloric acid;
(ii) reducing a ketone to a hydroxyl group by use of a borohydride reducing
agent;
(iii) converting a carboxylic ester group into a carboxyl group using basic
hydrolysis;
and/or
(iv) reducing a carboxylic ester group to a hydroxymethyl group, by use of a
borohydride
reducing agent.
As indicated above, where necessary, the conversion of any group Ar', R', R' 1
R'2,
R';, R'4 and R'S into Ar, R, R1, R2, R3, R4 or RS which as stated above are
usually
protected forms of Ar, R, R1, R2, R3, R4 or RS may be carried out using
appropriate
conventional conditions such as the appropriate deprotection procedure.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
23
It will be appreciated that in any of the above mentioned reactions any
reactive
group in the substrate molecule may be protected and deprotected according to
conventional chemical practice, for example as described by Greener T.W. and
Wuts,
P.G.M. Protective Groups in Organic Synthesis, John Wiley & Sons Inc. New
York,
1991 (Second Edt.) or in Kocienski, P.J. Protecting groups. George Thieme
Verlag, New
York, 1994.
Suitable protecting groups in any of the above mentioned reactions are those
used
conventionally in the art. Thus, for example suitable hydroxyl protecting
groups include
benzyl or trialkylsilyl groups.
The methods of formation and removal of such protecting groups are those
conventional methods appropriate to the molecule being protected. Thus for
example a
benzyloxy group may be prepared by treatment of the appropriate compound with
a
benzyl halide, such as benzyl bromide, and thereafter, if required, the benzyl
group may
be conveniently removed using catalytic hydrogenation or a mild ether cleavage
reagent
such as trimethylsilyl iodide or boron tribromide.
As indicated above, the compounds of formula (I) have useful pharmaceutical
properties.
Accordingly the present invention also provides a compound of formula (I), or
a
pharmaceutically acceptable salt or solvate thereof, for use as an active
therapeutic
substance.
In particular, the present invention also provides a compound of formula (I),
or a
pharmaceutically acceptable salt or solvate thereof, for the treatment or
prophylaxis of
the Primary and Secondary Conditions.
The present invention further provides a pharmaceutical composition comprising
a compound of formula (I), or a pharmaceutically acceptable salt or solvate
thereof, and a
pharmaceutically acceptable carrier.
The present invention also provides the use of a compound of formula (I), or a
pharmaceutically acceptable salt or solvate thereof, in the manufacture of a
medicament
for the treatment of the Primary and Secondary Conditions.
As mentioned abvove the Primary conditions include respiratory diseases, such
as
chronic obstructive pulmonary disease (COPD), asthma, airway hyperreactivity,
cough;
inflammatory diseases such as inflammatory bowel disease, psoriasis,
fibrositis,
osteoarthritis, rheumatoid arthritis and inflammatory pain; neurogenic
inflammation or
peripheral neuropathy, allergies such as eczema and rhinitis; ophthalmic
diseases such as
ocular inflammation, conjunctivitis, vernal conjuctivitis and the like;
cutaneous diseases,
CA 02351865 2001-05-18
WO 00/31037 24 PCT/EP99/09115
skin disorders and itch, such as cutaneous wheat and flare, contact
dermatitis, atopic
dermatitis, urticaria and other eczematoid dermatitis; adverse immunological
reactions
such as rejection of transplanted tissues and disorders related to immune
enhancement or
suppression such as systhemic lupus erythematosis; gastrointestinal (GI)
disorders and
diseases of the GI tract such as disorders associated with the neuronal
control of viscera
such as ulcerative colitis, Crohn's disease, irritable bowel syndrome (IBS),
gastro-
exophageous reflex disease (GERD); urinary incontinence and disorders of the
bladder
function; renal disorders..
As mentioned abvove, the Secondary conditions include disorders of the central
nervous system such as anxiety, depression, psychosis and schizophrenia;
neurodegenerative disorders such as AIDS related dementia, senile dementia of
the
Alzheimer type, Alzheimer's disease, Down's syndrome, Huntington's disease,
Parkinson's disease, movement disorders and convulsive disorders (for example
epilepsy); demyelinating diseases such as multiple sclerosis and amyotrophic
lateral
sclerosis and other neuropathological disorders such as diabetic neuropathy,
AIDS related
neuropathy, chemotherapy-induced neuropathy and neuralgia; addiction disorders
such as
alcoholism; stress related somatic disorders; reflex sympathetic dystrophy
such as
shoulder/hand syndrome; dysthymic disorders; eating disorders (such as food
intake
disease); fibrosing and collagen diseases such as scleroderma and eosinophilic
fascioliasis; disorders of the blood flow caused by vasodilation and
vasospastic diseases
such as angina, migraine and Reynaud's disease and pain or nociception, for
example,
that is attributable to or associated with any of the foregoing conditions
especially the
transmission of pain in migraine.
Such a medicament, and a composition of this invention, may be prepared by
admixture of a compound of the invention with an appropriate carrier. It may
contain a
diluent, binder, filler, disintegrant, flavouring agent, colouring agent,
lubricant or
preservative in conventional manner.
These conventional excipients may be employed for example as in the
preparation of compositions of known agents for treating the conditions.
Preferably, a pharmaceutical composition of the invention is in unit dosage
form
and in a form adapted for use in the medical or veterinarial fields. For
example, such
preparations may be in a pack form accompanied by written or printed
instructions for
use as an agent in the treatment of the conditions.
The suitable dosage range for the compounds of the invention depends on the
compound to be employed and on the condition of the patient. It will also
depend, inter
alia, upon the relation of potency to absorbability and the frequency and
route of
administration.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
The compound or composition of the invention may be formulated for
administration by any route, and is preferably in unit dosage form or in a
form that a
human patient may administer to himself in a single dosage. Advantageously,
the
composition is suitable for oral, rectal, topical, parenteral, intravenous or
intramuscular
administration. Preparations may be designed to give slow release of the
active
ingredient.
Compositions may, for example, be in the form of tablets, capsules, sachets,
vials, powders, granules, lozenges, reconstitutable powders, or liquid.
preparations, for
example solutions or suspensions, or suppositories.
The compositions, for example those suitable for oral administration, may
contain conventional excipients such as binding agents, for example syrup,
acacia,
gelatin, sorbitol, tragacanth, or polyvinylpyrrolidone; fillers, for example
lactose, sugar,
maize-starch, calcium phosphate, sorbitol or glycine; tabletting lubricants,
for example
magnesium stearate; disintegrants, for example starch, polyvinyl-pyrrolidone,
sodium
starch glycollate or microcrystalline cellulose; or pharmaceutically
acceptable setting
agents such as sodium lauryl sulphate.
Solid compositions may be obtained by conventional methods of blending,
filling, tabletting or the like. Repeated blending operations may be used to
distribute the
active agent throughout those compositions employing large quantities of
fillers. When
the composition is in the form of a tablet, powder, or lozenge, any carrier
suitable for
formulating solid pharmaceutical compositions may be used, examples being
magnesium
stearate, starch, glucose, lactose, sucrose, rice flour and chalk. Tablets may
be coated
according to methods well known in normal pharmaceutical practice, in
particular with
an enteric coating. The composition may also be in the form of an ingestible
capsule, for
example of gelatin containing the compound, if desired with a carrier or other
excipients.
Compositions for oral administration as liquids may be in the form of, for
example, emulsions, syrups, or elixirs, or may be presented as a dry product
for
reconstitution with water or other suitable vehicle before use. Such liquid
compositions
may contain conventional additives such as suspending agents, for example
sorbitol,
syrup, methyl cellulose, gelatin, hydroxyethylcellulose,
carboxymethylcellulose,
aluminium stearate gel, hydrogenated edible fats; emulsifying agents, for
example
lecithin, sorbitan monooleate, or acacia; aqueous or non-aqueous vehicles,
which include
edible oils, for example almond oil, fractionated coconut oil, oily esters,
for example
esters of glycerine, or propylene glycol, or ethyl alcohol, glycerine, water
or normal
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
26
saline; preservatives, for example methyl or propyl p-hydroxybenzoate or
sorbic acid;
and if desired conventional flavouring or colouring agents.
The compounds of this invention may also be administered by a non-oral route.
In accordance with routine pharmaceutical procedure, the compositions may be
formulated, for example for rectal administration as a suppository. They may
also be
formulated for presentation in an injectable form in an aqueous or non-aqueous
solution,
suspension or emulsion in a pharmaceutically acceptable liquid, e.g. sterile
pyrogen-free
water or a parenterally acceptable oil or a mixture of liquids. The liquid may
contain
bacteriostatic agents, anti-oxidants or other preservatives, buffers or
solutes to render the
solution isotonic with the blood, thickening agents, suspending agents or
other
pharmaceutically acceptable additives. Such fortes will be presented in unit
dose form
such as ampoules or disposable injection devices or in mufti- dose forms such
as a bottle
from which the appropriate dose may be withdrawn or a solid form or
concentrate which
can be used to prepare an injectable formulation.
The compounds of this invention may also be administered by inhalation, via
the
nasal or oral routes. Such administration can be carried out with a spray
formulation
comprising a compound of the invention and a suitable carrier, optionally
suspended in,
for example, a hydrocarbon propellant.
Preferred spray formulations comprise micronised compound particles in
combination with a surfactant, solvent or a dispersing agent to prevent the
sedimentation
of suspended particles. Preferably, the compound particle size is from about 2
to 10
microns.
A further mode of administration of the compounds of the invention comprises
transdermal delivery utilising a skin-patch formulation. A preferred
formulation
comprises a compound of the invention dispersed in a pressure sensitive
adhesive which
adheres to the skin, thereby permitting the compound to diffuse from the
adhesive
through the skin for delivery to the patient. For a constant rate of
percutaneous
absorption, pressure sensitive adhesives known in the art such as natural
rubber or
silicone can be used.
As mentioned above, the effective dose of compound depends on the particular
compound employed, the condition of the patient and on the frequency and route
of
administration. A unit dose will generally contain from 20 to 1000 mg and
preferably
will contain from 30 to 500 mg, in particular 50, 100, 150, 200, 250, 300,
350, 400, 450,
or X00 mg. The composition may be administered once or more times a day for
example
2, 3 or 4 times daily, and the total daily dose for a 70 kg adult will
normally be in the
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
27
range 100 to 3000 mg. Alternatively the unit dose will contain from 2 to 20 mg
of active
ingredient and be administered in multiples, if desired, to give the preceding
daily dose.
No unacceptable toxicological effects are expected with compounds of the
invention when administered in accordance with the invention.
The present invention also provides a method for the treatment and/or
prophylaxis of the Primary and Secondary Conditions in mammals, particularly
humans,
which comprises administering to the mammal in need of such treatment and/or
prophylaxis an effective, non-toxic pharmaceutically acceptable amount of a
compound
of formula (I) or a pharmaceutically acceptable salt or solvate thereof.
The activity of the compounds of the present invention, as NK3 ligands, is
determined by their ability to inhibit the binding of the radiolabelled NK3
ligands, [1251]-
[Me-Phe7]-NKB or [3H]-Senktide, to guinea-pig and human NK3 receptors
(Renzetti et
a1,1991, Neuropeptide, 18, 104-114; Buell et al, 1992, FEBS, 299(1), 90-95;
Chung et al,
1994, Biochem. Biophys. Res. Commun., 198(3), 967-972).
The binding assays utilized allow the determination of the concentration of
the
individual compound required to reduce by SO% the [1251]_[Me-Phe7]-NKB and
[3H]-
Senktide specific binding to NK3 receptor in equilibrium conditions (IC50).
Binding assays provide for each compound tested a mean IC50 value of 2-5
separate experiments performed in duplicate or triplicate. The most potent
compounds of
the present invention show IC50 values in the range 0.1-1000 nM. The NK3-
antagonist
activity of the compounds of the present invention is determined by their
ability to inhibit
senktide-induced contraction of the guinea-pig ileum (Maggi et al, 1990, Br.
J.
Pharmacol., 101, 996-1000) and rabbit isolated iris sphincter muscle (Hall et
al.,1991,
Eur. J. Pharmacol.,199, 9-14) and human NK3 receptors-mediated Ca'~'~'
mobilization
(Mochizuki et al, 1994, J. Biol. Chem., 269, 9651-9658). Guinea-pig and rabbit
in-vitro
functional assays provide for each compound tested a mean KB value of 3-8
separate
experiments, where KB is the concentration of the individual compound required
to
produce a 2-fold rightward shift in the concentration-response curve of
senktide. Human
receptor functional assay allows the determination of the concentration of the
individual
compound required to reduce by SO% (IC50 values} the Ca'~"i' mobilization
induced by
the agonist NKB. In this assay, the compounds of the present invention behave
as
antagonists.
The activity of the compounds of the present invention, as NK-2 ligands, is
determined by their ability to inhibit the binding of the radiolabelled NK-2
ligands,
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
28
[125IJ_NKA or [3H]-NKA, to human NK-2 receptors (Aharony et al, 1992,
Neuropeptide, 23, 121-130).
The binding assays utilized allow the determination of the concentration of
the
individual compound required to reduce by 50% the [1251J_NKA and [3HJ-NKA
specific
binding to NK2 receptor in equilibrium conditions (IC50).
Binding assays provide for each compound tested a mean IC50 value of 2-S
separate experiments performed in duplicate or triplicate. The most potent
compounds of
the present invention show IC50 values in the range 0.5-1000 nM, such as I-
1000 nM.
The NK-2-antagonist activity of the compounds of the present invention is
determined by
their ability to inhibit human NK-2 receptor-mediated Ca'~' mobilization
(Mochizuki et
al, 1994, J. Biol. Chem., 269, 9651-9658). Human receptor functional assay
allows the
determination of the concentration of the individual compound required to
reduce by 50%
(IC50 values) the Ca~"~' mobilization induced by the agonist NKA. In this
assay, the
compounds of the present invention behave as antagonists.
The therapeutic potential of the compounds of the present invention in
treating the
conditions can be assessed using rodent disease models.
As stated above, the compounds of formula (I) are also considered to be useful
as
diagnostic tool. Accordingly, the invention includes a compound of formula (I)
for use as
diagnostic tools for assessing the degree to which neurokinin-3 and neurokinin-
2 receptor
activity (normal, overactivity or underactivity) is implicated in a patient's
symptoms.
Such use comprises the use of a compound of formula (I) as an antagonist of
said
activity, for example including but not restricted to tachykinin agonist-
induced inositol
phosphate turnover or electrophysiological activation, of a cell sample
obtained from a
patient. Comparison of such activity in the presence or absence of a compound
of
formula (I), will disclose the degree of NK-3 and NK-2 receptor involvement in
the
mediation of agonist effects in that tissue.
The following Descriptions illustrate the preparation of the intermediates,
whereas
the following Examples illustrate the preparation of the compounds of the
invention.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
29
Descriptions and Examples
DESCRIPTION A: 3-Methyl-2-phenyl-quinoline-4-carboxylic acid methyl ester
30 g (114 mmol) of3-methyl-2-phenyl-quinoline-4-carboxylic acid (CAS [43071-45-
O])
were suspended in 250 ml of dry CHZCIz; 20 mI (230 mmol) of oxalyl chloride
dissolved
in 120 ml of CH2Clz were added dropwise and the reaction mixture was stirred
at room
temperature for 30 min. Two drops of N,N-dimethylformamide (DMF) were added
and
the reaction was stirred for additional 30 min. The solvent was evaporated in
vacuo to
dryness, the residue was taken up with 100 ml of CH2Clz and 100 ml of MeOH,
dissolved
in 400 ml of CHZCIz, were added dropwise. After stirring for 18 h, the solvent
was
evaporated in vacuo to dryness, the residue was taken up with CHZCIz and
washed with
1 % NaHC03; the organic layer was dried over NazS04, filtered and evaporated
in vacuo
to dryness to yield 31.6 g of the title compound as a solid, which was used in
the
following reaction without further purification.
C i aH~ sNOz
MW 277.31
MP = 73-75°C
IR (KBr) 3441, 3051, 2954, 1731, 1582, 1556 cm's.
DESCRIPTION B : 3-Bromomethyl-2-phenyl-quinoline-4-carboxylic acid methyl
ester
10 g (36 mmol) of 3-methyl-2-phenyl-quinoiine-4-carboxylic acid methyl ester
(compound of Description A) were dissolved in 500 ml of CH3CN; I3 g (72 mmol)
of N-
bromosuccinimide were added and the reaction mixture was heated to reflux.
After
adding 1 g (4.1 mmol) of dibenzoylperoxide, the reaction was refluxed for 24
h; then
additional 4 g (22.5 mmol) of N-bromosuccinimide and 0.5 g (2.0 mmol) of
dibenzoylperoxide were added and the reaction was refluxed for 4 h. The
solvent was
evaporated in vacuo to dryness to yield 26.1 g of crude methyl 3-bromomethyl-2-
phenylquinoline-4-carboxylate (theorical amount, 12.8 g) which was used in the
following reaction without further purification.
C, gH i4BrNOz
MW = 356.23
DESCRIPTION 1: 3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-
carboxylic
acid methyl ester
5 g ( 14 mmol) of 3-bromomethyl-2-phenyl-quinoline-4-carboxylic acid methyl
ester
(compound of Description B), 2.9 g, (15.4 mmol) of 90% 4-piperidinopiperidine
(Aldrich), 2.7 ml (15.4 mmol) diisopropylethyl amine were dissolved in 100 ml
of dry
THF and the mixture was stirred for one night at 50°C. The solvent was
concentrated, the
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
residue was dissolved in methylene chloride, washed with water, and the
organic phase
was dried over MgS04. After concentration of the solvent the residue was
purified by
flash chromatography over 160 g of silicagel (eluent CHZCIz/MeOH/NH40H :
9515/0.5)
affording 3.S g (yield 56%) of the title compound as a white solid.
C28H33N3~2
MW = 443.59
8 (CDC13) : 1.29-2.02(12H); 2.25(1H); 2.47(4H); 2.78(2H); 3.66(2H); 4.05(3H);
7.38-
7.SS(SHar); 7.58(lHar); 7.72(lHar); 7.88(lHar); 8.17(lHar)ppm.
DESCRIPTION 2 : 3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-
carboxylic
acid dihydrochloride
3.5 g (7.9 mmol) of 3-[1,4']bipiperidinyl-1'-yfmethyl-2-phenyl-quinoline-4-
carboxylic
acid methyl ester (compound of Description 1 ) and SO ml 6N HCl are refluxed
for 1.5 h.
then concentrated to dryness. The residue is triturated in acetone. This
process is re-
applied twice to the solid thus obtained affording, after drying in vacuo 4.5
g of the title
compound as a crude dihydrochloride used without further purification in the
next step.
C2~H3N302.2HC1
MW = 502.56
8 (DMSOd6): 1.16-2.29(lOH); 2.62-3.38(8H); 4.46(2H); 5.77(lHexch with D20);
7.45-
8.30(9Har); 11.12 (lHexch with DZO)ppm.
DESCRIPTION 3: 2-Phenyl-3-(4-phenyl-piperidin-1-ylmethyi)-quinoline-4-
carboxylic acid methyl ester
5.4 g of crude 3-bromomethyl-2-phenyl-quinoline-4-carboxylic acid methyl ester
(compound of Description B) were dissolved, under nitrogen atmosphere, in 30
ml of dry
THF. The solution was cooled to 10 °C and 4.0 g (24.8 mmol) of 4-
phenylpiperidine,
dissolved in 5 ml of THF, were added dropwise. The reaction mixture was
allowed to
warm to room temperature and stirred overnight. Salts were filtered off and
the filtrate
was evaporated in vacuo to dryness, taken up with 2 N HCl and washed with
EtOAc; the
aqueous Layer was basified with 10% NaOH and extracted With CHZCl2. The
organic
layer was dried over NazS04, filtered and evaporated in vacuo to dryness to
obtain a
crude material which was purified by gradient flash column chromatography on
230-400
mesh silica gel, utilising a mixture of EtOAc/hexane 10:90 containing 0.5 %
NH.~OH
(28%) as starting eluent and a mixture of EtOAc/hexane 15:85 containing 0.5 %
NH.~OH
(28%) as final eluent. 3.0 g of the title compound were recovered as an off
white solid.
C29HzsN20z
MW = 436.56
IR: (KBr) 3440, 3062, 2945, 1731, 1577, 1555 cm's.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
31
DESCRIPTION 4: 2-Phenyl-3-(4-phenyl-piperidin-1-ylmethyl)-quinoline-4-
carboxylic acid hydrochloride
3.0 g (6.87 mmol) of 2-phenyl-3-(4-phenyl-piperidin-1-ylmethyl)-quinoline-4-
carboxylic
acid methyl ester (compound of Description 3) were dissolved in 100 ml of 6 N
HCI and
refluxed for 1 h. Evaporation to dryness afforded 3.5 g of crude title
compound, which
was used in the following reaction without further purification.
C28H26N2~2.HC1
MW = 459.00
MP = 175-178°C
IR: (KBr) 3385, 3062, 2495, 1973, 1718, 1630 cm's.
DESCRIPTION 5: 3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic acid methyl ester
7.8 g of crude 3-bromomethyl-2-phenyl-quinoline-4-carboxylic acid methyl ester
(compound of Description B) were dissolved, under nitrogen atmosphere, in 130
ml of
dry THF. The solution was cooled to 10 °C and 2.8 g (21.6 mmol) of 1-
isopropylpiperazine, dissolved in 20 ml of THF, were added dropwise. The
reaction
mixture was allowed to warm to room temperature and stirred overnight. Salts
were
filtered off and the filtrate was evaporated in vacuo to dryness, taken up
with 2 N HCl
and washed with EtOAc; the aqueous layer was basified with 10% NaOH and
extracted
with CH2Cl2. The organic layer was dried over Na2S04, filtered and evaporated
in vacuo
to dryness to obtain a crude material which was purified by flash column
chromatography
on 230-400 mesh silica gel, utilising a mixture of Et20/iPr20 70:30 containing
0.3
NH40H (28%). 3.8 g of the title compound were recovered as a yellow solid.
C25H29N3~2
MW = 403.54
IR: (KBr) 3441, 3065, 2946, 1731, 1580, 1555 cm's.
DESCRIPTION 6: 3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quinoiine-4-
carboxyiic acid dihydrochloride
3.8 g (9.42 mmol) of 3-(4-isopropyl-piperazin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic acid methyl ester (compound of Description 5) were dissolved in 100
ml of 6
N HCI and refluxed for 4 h. Evaporation to dryness afforded 4.0 g of crude
title
compound, which was used in the following reaction without further
purification.
CzaHnN302.2HC1 .
MW = 389.50
MP = 177-180°C
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
32
IR: (KBr) 3408, 2928, 2666, 1716, 1632 cm's.
DESCRIPTION 7: (S)-1-Cyclohexyl-propylamine hydrochloride
2.0 g (14.8 mmol) of (S)-1-phenyl-propylamine were dissolved in 250 ml of a 4%
solution of citric acid in HZO. 0.6 g of 20% Pd(OH)z /C were added and the
reaction
mixture was hydrogenated in a steel autoclave at 50 bar and 60 °C for
24 h. The catalyst
was filtered off, the filtrate was evaporated and the residue was taken up
with 40% NaOH
and extracted several times with H20. The combined organic layers were dried
over
NazS04 and acidified with HCl/EtzO. Evaporation to dryness afforded 0.3 g of
the title
compound as a solid.
C9H,9N.HCl
MW = 389.50
DESCRIPTION 8 : 3-(4-Fmoc-piperazin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic acid methyl ester
6.6 g ( 18.5 mmol) of 3-bromomethyl-2-phenyl-quinoline-4-carboxylic acid
methyl ester
{compound of Description B) were reacted with 6.8 g (20 mmol) of Fmoc
piperazine in
150 ml of THF following the procedure used in Description 3 and afforded 7.5 g
(yield
69%) of the title compound.
Cs~H33NsOa
MW = 583.68
~H NMR b(DMSOd6) : 1.99(4H); 3.10(4H); 3.62(2H); 3.97(3H); 4.20(1H); 4.42(2H);
7.18-7.40(4Har); 7.45-7.92(l2Har); 8.09(lHar)ppm.
DESCRIPTION 9 : 3-(4-Fmoc-piperazin-I-ylmethyl)-2-phenyl-quinoline-4-
carboxylic acid hydrochloride
7.5 g (13 mmol) of the ester of Description 8 are hydrolysed with 6 N aqueous
hydrochloric acid following the procedure used in Description 4 affording 9.5
g of crude
title compound which was used without purification in the next step.
C36H3,N304.HC1
MW = 606.12
~H NMR 8(DMSOdb) : 2.50(4H); 3.32(4H); 4.22(2H); 4.23(1H); 4.35(2H);
6.50(lHexch
with DZO); 7.22-7.88(l4Har); 7.98(lHar); 8.17(2Har)ppm.
DESCRIPTION 10 : 3-(4-Fmoc-piperazin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic acid ((S)-1-phenyl-propyl)-amide
5.35 g (8.3 mmol) of crude acid of Description 9 were condensed on 1.7 ml (
12.5 mmol)
of (S)-1-phenyl-propylamine following the procedure of Example 2 affording,
after flash
chromatography on silica gel, 3.2 g (56%) of the title compound.
CasHazNa03
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
33
MW = 686.86
1H NMR 8(DMSOdb) : 0.94(3H); 1.40-2.18(6H); 2.57-3.13(4H); 3.50(2H); 4.2I(1H);
4.34(2H); 5.08(IH); 7.09-7.98(2IHar); 8.03(lHar): 9.12(lHexch with DZO)ppm.
DESCRIPTION 11 : 3-(4-Fmoc-piperazin-1-ylmethyl)-2-phenyl-quinoiine-4-
carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
4.75 g (8.3 mmol} of crude acid of Description 9 were condensed on 1.65 ml (11
mmol)
of (S)-1-cyclohexyl-ethylamine following the procedure of Example 2 affording,
after
flash chromatography on silica gel, 2.2 g (yield 43.9%) of the title compound.
C44H4sN403
MW = 678.87
1H NMR S(DMSOd6) : 0.95(3H); I.68-4.00(2IH); 2.60(3H); 5.08(1H); 7.22-
8.24(l3Har);
8.11 ( 1 Har); 9.32( 1 Hexch with D20); 10.82(2Hexch with DZO)ppm.
DESCRIPTION 12 : 3-(4-Fmoc-piperazin-1-ylmethyl)-2-phenyl-quinoiine-4-
carboxylic acid ((S)-2-methyl-1-phenyl-propyl)-amide
6.95 g ( 10.8 mmol) of crude acid of Description 9 were condensed on 2 g (
13.5 mmol) of
(S)-2-methyl-1-phenyl propylamine following the procedure of Example 2
affording,
after flash chromatography on silica gel, S.4 g (yield 7I%) of the title
compound.
C46H44N4O3
MW = 700.86
'H NMR 8(CDCl3) : 0.96(3H); 1.18(3H); 1.56-2.98(4H); 2.28(IH); 3.04(4H);
3.53{2H);
4.20(IH); 4.35(2H); S.I7(IH); 7.18-7.63(lBHar); 7.74(3Har); 7.97(IHexch with
D20);
8.14( 1 Har)ppm.
DESCRIPTION 13 : 2-Phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic acid
((S)-2-methyl-1-phenyl-propyl)-amide
S.4 g (7.7 mmol) of the Fmoc derivative of Description 12 was reacted with
1.25 ml of
piperidine in 200 ml acetonitrile, at room temperature for one night. The
reaction mixture
is concentrated to dryness and the residue was purified by flash
chromatography on
silicagel (eluant: CH2Cl2/CH30H/HH40H ; 90/10/2), affording 2.SS g (yield
69.3%) of
the title compound.
C3 i H3aN4O
MW = 478.64
~H NMR 8{DMSOdb) : 0.79(3H); 1.06(3H); 1.49-2.SS(9H); 3.45(2H and lHexch with
D20); 4.88( 1 H); 7.12-8.10( l4Har); 9.16( 1 Hexch with D20)ppm.
DESCRIPTION 14 : 2-Phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic acid
((S)-1-phenyl-propyl)-amide
2.75 g (41 mmol) of the Fmoc protected derivative of Description 12 afforded
by
applying the procedure of Description 13, 1.14 g (yield 60%) of the title
compound.
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
34
C30H32N4~
MW = 464.61
'H NMR 8(DMSOdb) : 0.94(3H); 1.57-2.08(6H); 2.31(4H); 3.36(2H and lHexch with
Dz0); 5.07( 1 H); 7.13-7.94( 13Har); 8.01 ( 1 Har); 9.17( 1 Hexch with
D20)ppm.
DESCRIPTION 1 S : 3-[4-(1-Cyanoimino-1-methylsulfanyl-methyl)-piperazin-1-
ylmethylj-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
O.S g (1.1 mmol) of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid ((S)-1-
cyclohexyl-ethyl)-amide (compound of Example 34) and 0.16 g ( 1.1 mmol) of
dimethy!
N-cyanodithioiminocarbonate (Aldrich) were heated at reflux for 6 h in a
mixture of 2.2
ml of DMF and 8.8 ml of EtOH.
The solvent was concentrated and the residue purified by flash chromatography
on
silicagel (CH2C12/MeOH : 98/2) affording O.S6g (yield 91.8%) of the title
compound
which was used without purification in the following step.
C33H34N6~s
MW = 562.74
~H NMR 8(CDCl3) : 1.00-1.39(SH); 1.24(3H); 1.48(1H); 1.63-1.96(SH); 2.25(4H);
2.69(3H); 3.57(4H); 3.72(2H); 4.25(1H); 6.42(lHexch with DZO; 7.38-7.SS(SHar);
7.60(lHar); 7.75(lHar); 7.95(lHar); 8.14(lHar)ppm.
DESCRIPTION 16 : 3-[4-(1-Methanesulfonylimino-1-methylsulfanyi-methyl)-
piperazin-1-ylmethylj-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyi-
ethyl)-
amide
0.48 g (1.OS mmol) of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid ((S)-
1-cyclohexyl-ethyl)-amide (compound of Example 34) and 0.21 g (1.OS mmol) of
carbonimidodithioic acid, (methylsulfonyl)-dimethyl ester (RN 13068-10-S) were
heated
at reflux for S h in a mixture of 2 ml of DMF and 8 ml of EtOH.
The solvent was concentrated and the residue purified by flash chromatography
on
silicagel (CH2Cl2/MeOH : 97/3) affording O.S2g of crude title compound which
was used
without purification in the following step.
C33H37N4~3s2
MW = 615.82
~H NMR S(CDCl3) : 0.95-1.38(SH); 1.28(3H); 1.48(1H); 1.62-1.94(SH); 2.28(4H);
2.47(3H); 3.01(3H); 3.54(4H); 3.59(2H); 4.25(1H); 6.52(lHexch with D20); 7.36-
7.53(SHar); 7.59(lHar); 7.75(lHar); 7.95(IHar); 8.14{lHar)ppm.
DESCRIPTION 17 : 4-[4-((S)-1-Cyclohexyl-ethylcarbamoyl)-2-phenyl-quinolin-3-
ylmethylj-N-methyl-piperazine-1-carboximidothioic acid methyl ester
O.OS g (0.95 mmol) of 3-(4-methylthiocarbamoyl-piperazin-1-ylmethyl)-2-phenyl-
quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide (compound of
Example 7S)
was suspended in S ml acetone and 0.41 g (2.85 mmol) methyl iodide was added.
After
4h stirring at room temperature the mixture became clear. The solvent was
concentrated
and the residue triturated with di-ethyl ether affording, after f ltration and
drying, 0.63 g
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
35
of the hydroiodide salt of the title compound. This compound was used without
further
purification in the next step.
C33H37NS~S
MW = SSI.76
IH NMR S(DMSOd6) : 0.92-1.36(SH); 1.75(3H); 1.47(1H); I.58-1.92(SH); 2.24(4H);
2.46(3H); 3.05(3H); 3.36(4H}; 3.63(2H); 4.02(1H); 7.36-7.91(BHar); 8.04(lHar);
8.55(iHexch with D20)ppm.
DESCRIPTION 18 : 3-(4-Oxo-piperidin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic
acid ((S)-1-phenyl-propyl)-amide
Starting from 3-bromomethyl-2-phenylquinoline-4-carboxylic acid methyl ester
(compound of Description B) and 4-oxopiperidine, following the procedure of
description 1, then applying procedures analogous to those described in
description 2 and
example 2 afforded the title compound after purification on silicagel
(EtOAc/Heptane
I/1).
C3 tH3 tN3~2
Mw = 477.60
IH NMR S(DMSOd6) : 0.83(3H); I.57-2.30(8H); 2.45(2H); 3.34-3.98(2H); 5.08(1H);
7.12-8.18( l4Har); 9.21 ( 1 Hexch with D20)ppm.
DESCRIPTION 19 : 4-tert-Butylsulfamoyl-piperazine-1-carboxylic acid tert-butyl
ester
6.1 g (32.62mmo1} of piperazine-I-carboxylic acid tert-butyl ester (RN 76535-
74-5) were
dissolved in 150 ml of CHZCIz and 4.5 g (32.6 mmol) of K2C03 were added. The
mixture
was cooled to 0°C and 5.6 g (32.62 mmol) of tert-butyl-
sulfamoylchloride (prepared
according to Catt, J.D. JOC, 1974, 39, 566-8) dissolved in 50 ml of CH2Clz
were added
dropwise and the reaction mixture was stirred at room temperature for 2 h. 50
ml of water
were added, the two phase separated in a separatory funnel and the aqueous
phase
extracted with CHzCIz. The organic phases were collected, dried over NazS04
and
evaporated in vacuo to dryness to yield 4.6 g of the title compound as a
yellow solid
C I3Hz7N3OaS
MW = 321.43
IR: (KBr) 3273, 2971, 1701, 1364, 1137, 1023, 934, 768 czri t.
DESCRIPTION 20 : 4-Dimethylsulfamoyl-piperazine-1-carboxylic acid tert-butyl
ester
Prepared as described in Description 19 from 10.38 g (55.7 mmol) of piperazine-
1-
carboxylic acid tent-butyl ester (RN 76535-74-5), 7.7 g (55.7 mmol) of KZC03
and 8g
(55.7 mmol) of dimethyl-sulfamoylchloride 15.2 g of the title compound were
obtained
as a yellow solid
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
36
C I I H23N304S
MW = 293.39
IR: (KBr) 2979, 286, 1687, 1142, 952, 752 cm I
DESCRIPTION 21 : Piperazine-1-sulfonic acid tert butylamide
4.6 g (14.3 mmol) of 4-tert-butylsulfamoyl-piperazine-1-carboxylic acid tert-
butyl ester
(compound of Description 19) were dissolved in 10 ml of CH2Cl2 and 50 ml of
30%
ethereal HCI were added. The solution was stirred at room temperature for 2 h.
The
solvent was evaporated in vacuo to dryness yielding 1.5 g of the title
compound as a
white solid
CgH19N3O2s
MW = 221.32
IR: (KBr) 3207, 2730, 1591, 1326, 1143, 1001, 917, 720, 631 crri l
DESCRIPTION 22 : Piperazine-1-sulfonic acid dimethylamide
13 g (44.31 mmol) of 4-dimethylsulfamoyl-piperazine-1-carboxylic acid tert-
butyl ester
(compound of Description 20) were dissolved in 100 ml of CH2C12 and 20 mI of
30%
ethereal HCl were added. The solution was stirred at room temperature for 2 h.
The
solvent was evaporated in vacuo to dryness yielding 9.2 g of the title
compound as a
white solid
C6HISNs02S
MW = 193.27
IR: (KBr) 2786, 1688, 1356, 1152, 1037, 942, 867, 737,677 crri l
DESCRIPTION 23 : 3-(4-tert Butyisulfamoyl-piperazin-1-ylmethyl)-Z-phenyl-
quinoline-4-carboxylic acid methyl ester
1.5 g (6.78 mmol) of piperazine-1-sulfonic acid tert-butylamide (compound of
Description 21) and 0.94 g (6.78 mmol) of K2C03 were suspended in 70 ml of
CH3CN.
2.42 g (6.78 mmol) of 3-bromomethyl-2-phenylquinoline-4-carboxylic acid methyl
ester
(compound of Description B) were dissolved in 30 ml of CH3CN and the solution
was
added to the previous suspension. The resulting mixture was stirred at room
temperature
for 4h. The solvent was evaporated in vacuo to dryness, the residue was taken
up with 6N
HCl and washed with EtOAc. The aqueous phase was basified with 1 N NaOH and
extracted with EtOAc. The organic phase was dried over Na2S04 and evaporated
to
dryness to yield a 3.0 of crude title compound used without further
purification
C26H32N4O4S
MW = 496.63
CA 02351865 2001-05-18
WO 00/3103? 37 PCT/EP99/09115
IR: (KBr) 3280, 2974, 1734, 1575, 1555, 1444, 1220, 1146, 940 764 cm's
DESCRIPTION 24 : 3-(4-Dimethylsulfamoyl-piperazin-I-ylmethyl)-2-phenyl-
quinoline-4-carboxylic acid methyl ester
1.6 g (8.24 mmol) of piperazine-1-sulfonic acid dimethylamide (compound of
Description 22) and 1.16 g (8.42 mmol) of K2C03 were suspended in 70 ml of
CH3CN.
3.0 g (8.42 mmol) of 3-bromomethyl-2-phenylquinoline-4-carboxylic acid methyl
ester
(compound of Description B) were dissolved in 30 ml of CH3CN and the solution
was
added to the previous suspension. The resulting mixture was stirred at room
temperature
for 4h. The solvent was evaporated in vacuo to dryness, the residue was taken
up with 6N
HCl and washed with EtOAc. The aqueous phase was basified with 1 N NaOH and
extracted with EtOAc. The organic phase was dried over Na2S04 and evaporated
to
dryness to yield a crude material which was purified by flash column
chromatography on
230-400 mesh silica gel, utilising a mixture of EtOAc/hexane 3:7 as eluent.
After
evaporation of the solvent, 3.0 g of the title compound as a yellow solid were
obtained.
C24H28N404S
MW = 468.58
IR: (KBr) 2938, 1736, 1574, 1552, 1452, 1244, 1156, 942 748 cm's
DESCRIPTION 25 : 2-Phenyl-3-(4-sulfamoyl-piperazin-1-ylmethyl)-quinoline-4-
carboxylic acid
3.0 g (6.04 mmol) of 3-(4-tert-butylsulfamoyl-piperazin-1-ylmethyl)-2-phenyl-
quinoline-
4-carboxylic acid methyl ester (compound of Description 23) were suspended in
50 ml of
6N HC1 and the mixture was refluxed for 4h. The solvent was evaporated in
vacuo to
dryness. For three times the residue was treated with Et20 and the solvent was
evaporated
to dryness to yield 3.0 of crude title compound used without further
purification
C2~H22N404S
MW = 426.44
IR: (KBr) 3281, 2974, 1734, 1556, 1221, 1146, 1056, 941, 765 cm's
DESCRIPTION 26 : 3-(4-DimethylsuIfamoyl-piperazin-I-ylmethyl)-2-phenyl-
quinoline-4-carboxylic acid
3.0 g (6.40 mmol) of 3-(4-dimethylsulfamoyl-piperazin-1-ylmethyl)-2-phenyl-
quinoline-
4-carboxylic acid methyl ester (compound of Description 24) were suspended in
50 ml of
6N HCl and the mixture was refluxed for 4h. The solvent was evaporated in
vacuo to
dryness. After trituration of the residue with Me2C0, 1.4 g of the title
compound were
recovered as a pale yellow solid used without further purification.
C23H26N404s
CA 02351865 2001-05-18
WO 00/31037 38 PCT/EP99/09115
MW = 454.55
IR: (KBr) 3427, 2658, 1726, 1632, 1581, 1452, 1344, 1151, 932 745 cm ~
DESCRIPTION 27 '7-Methovy-3-methyl-2-phenyl-quinoline-4-carboxylic acid
methyl ester
16 g (54.5 mmol) of 7-methoxy-3-methyl-2-phenyl-quinoline-4-carboxylic acid
(prepared
analogously to starting material of Description A) were suspended in 400 ml of
dry
CH2C12 and 9.52 ml (126.93 mmol) of oxalyl chloride were added dropwise. Two
drops
of N,N-dimethylformamide (DMF) were added and the reaction mixture was stirred
for
3h at room temperature. The solvent was evaporated in vacuo to dryness, the
residue was
taken up with 150 ml of CH2C12 and quickly dropped in a solution of 200 ml of
MeOH
and 200 ml of CH2C12. After stirring for 1 h, the solvent was evaporated in
vacuo to
dryness, the residue was taken up with EtOAc and washed with I % NaHC03; the
organic
layer was dried over Na2S04, filtered and evaporated in vacuo to dryness.
After
trituration of the residue with Et20, 19 g of the title compound were
recovered as a dark
powder used without further purification.
C,9H,~N03
MW = 307.35
IR (KBr) 3067, 2947, 1918, 1729, 1634, 1581, 1246, 846 cm's.
DESCRIPTION 28 : 3-[1,4']Bipiperidinyl-1'-ylmethyl-8-bromo-7-methoxy-2-phenyl-
quinoline-4-carboxylic acid methyl ester
Prepared as described in Description B and Description I from 4.7 g (15.3
mmol) of 7-
methoxy-3-methyl-2-phenyl-quinoline-4-carboxylic acid methyl ester (compound
of
Description 27), 5.5 g (30.6 mmol) of N-bromosuccinimide, 0.5 g (2.05 mmol) of
dibenzoylperoxide, 3.85 g (23 mmol) of 4-piperidinopiperidine and 3.18 g (
23.0 mmol)
of K2C03, by stirnng in CH3CN at room temperature for 4h. 6.2 g of the title
compound
were obtained.
C29H34BrN3O3
MW = 552.51
IR (KBr) 3370, 2938, 1712, 1612, 1352, 1268, 1 I74, 704 cm's
DESCRIPTION 29 : 3-(1,4']Bipiperidinyl-1'-ylmethyl-8-bromo-7-methoxy-2-phenyl-
quinoline-4-carboxylic acid hydrochloride
Prepared as described in Description 4 from 6.0 g (10.9 mmol) of 3-
[1,4']bipiperidinyl-1'-
ylmethyl-8-bromo-7-methoxy-2-phenyl-quinoline-4-carboxylic acid methyl ester
(compound of Description 28) and 50 ml of 6 N HCl yielding 4.7 g of a slightly
brown
powder.
CA 02351865 2001-05-18
WO 00/31037 39 PCT/EP99/09115
C2gH32BrN303.HC1
MW = 574.94
IR: (KBr) 3453, 2939, 2532, 1714, 1607, 1598, I271, 1072, 960, 779, 705, cm ~
DESCRIPTION 30 : 3-[1,4']Bipiperidinyl-1'-ylmethyl-8-chloro-7-methoxy-2-phenyl-
quinoline-4-carboxylic acid methyl ester
Prepared as described in Description B and Description 1 from 9.0 g (29.3
mmol) of 7-
methoxy-3-methyl-2-phenyl-quinoline-4-carboxylic acid methyl ester
hydrochloride
(compound of Description 27 ~ HCI), 10.4 g (58.6 mmol) of N-bromosuccinimide,
1.0 g
(4.10 mmol) of dibenzoylperoxide 9.9 g (58.6 mmol) of 4-piperidinopiperidine
and 3.I 8
g ( 23.0 mmol) of K2C03. Purified by flash column chromatography on 230-400
mesh
silica gel, utilising a mixture of EtOAc/MeOH 9: I containing 0.1 % NH40H
(28%)
affording 1.7 g of the title compound.
C29H34CIN3O3
MW = 508.06
IR (KBr) 2934, 1730, 1610, 1501, 1238, 1079, 774, 706 cni i.
DESCRIPTION 31 : 3-[1,4']Bipiperidinyl-1'-ylmethyl-8-chloro-7-hydroxy-2-phenyl-
quinoline-4-carboxylic acid dihydrobromide
1.S g (3.0 mmol) of 3-[1,4']bipiperidinyl-1'-ylmethyl-8-chloro-7-methoxy-2-
phenyl-
quinoline-4-carboxylic acid methyl ester (compound of Description 30) were
dissolved in
SO ml of 48% HBr and the solution was refluxed for 8h. The solvent was
evaporated in
vacuo to dryness yielding 2.2 g of the crude title compound as a dark powder
used
without further purification.
C27H3,C1N303.2HBI
MW = 736.76
IR: {KBr) 2948, 1725, 1624, 1226, 959, 705 cni t
The following Examples illustrate the invention; Table 1 summarizes all the
compounds
of the Examples 1-9S and their analytical data; Table 2 describes NMR
spectroscopic
data of Examples 1-9S and Table 3 illustrates chemical names of compounds of
Examples 1-9S.
EXAMPLE 2: 2-Phenyl-3-(4-phenyl-piperidin-1-ylmethyl)-quinoline-4-carboxylic
acid ((S)-1-phenyl-propyl)-amide
2.S g (S.0 mmol) of crude 2-phenyl-3-(4-phenyl-piperidin-I-ylmethyl)-quinoline-
4-
carboxylic acid hydrochloride (compound of Description 4) were dissolved in SO
ml of
dry THF; 1.1 ml (7.8 mmol) of triethylamine (TEA) and 2.4 g (6.S mmol) of O-
benzotriazol-1-yl-N,N,N',N'-tetramethyluroniumhexafluoro-phosphate (HBTU) were
CA 02351865 2001-05-18
WO 00/31037 4o PCT/EP99/09115
added and the reaction mixture was cooled at 0 °C. 0.72 ml (5 mmol) of
(S)-1-phenyl-
propylamine, dissolved in 20 ml of dry CHZCl2, were added dropwise and the
reaction
mixture was stirred at room temperature for 24 h and at 50 °C for 2 h.
The solvent was
evaporated in vacuo to dryness and the residue was taken up with EtOAc and
washed
with H20, 1 N NaOH and brine, dried over Na2S04 and evaporated to dryness to
yield a
crude material which was purified by gradient flash column chromatography on
230-400
mesh silica gel, utilising a mixture of EtOAc/hexane 3:7 as starting eluent,
and a mixture
of EtOAc/hexane 4:6 as final eluent. After trituration with iPr20, 1.0 g of
the title
compound were recovered as a pale yellow solid used without further
purification.
C37H37N3~
MW = 539.72
IR: (KBr) 3279, 3060, 3028, 2931, 1633, 1536, 1494, 757, 699 cm's.
EXAMPLE 4 : 3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic
acid ((S)-1-cyclohexyl-ethyl)-amide dihydrochloride
2.3 g (5.0 mmol) of crude 3-(4-isopropyl-piperazin-1-ylmethyl)-2-phenyl-
quinoline-4-
carboxylic acid dihydrochloride (compound of Description 6) were dissolved in
200 ml
of a 1:1 mixture of CHZCi2/CH3CN; 2.0 ml (15 mmol) of triethylamine (TEA) and
2.5 g
(6.5 mmol) of O-benzotriazol-1-yl-N,N,N',N'-
tetramethyluroniumhexafluorophosphate
(HBTU) were added and the reaction mixture was cooled at 0 °C. 0.74 ml
(5 mmol) of
{S)-1-cyclohexyl-ethylamine, dissolved in 10 ml of dry CH2Cl2, were added
dropwise
and the reaction mixture was stirred at room temperature for 24 h. The solvent
was
evaporated in vacuo to dryness and the residue was taken up with EtOAc and
washed
with HZO, 1 N NaOH and brine, dried over Na2S04 and evaporated to dryness to
yield a
crude material which was purified by flash column chromatography on 230-400
mesh
silica gel, utilising a mixture of CHZC12/MeOH 95:5 containing 0.5 % NH40H
(28%).
The residue was dissolved in acetone and acidified with HC1/Et20; the
precipitate so
formed was recovered by suction filtration to yield 0.9 g of the title
compound as a
yellow solid.
C32HaaNa0.2HC1
MW = 571.64
IR: (KBr) 3411; 2927; 2851; 2667; 1650; 1546 cm's.
EXAMPLE 13: 3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-carboxylic
acid ((S)-1-cyclohexyl-ethyl)-amide dihydrochloride
4.0 g (8.0 mmol) of crude 3-[1,4']bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-
4-
carboxylic acid dihydrochloride (compound of Description 2) were dissolved in
300 ml
of a 1:1 mixture of CHZCl2/CH3CN; 3.4 ml (24.6 mmol) of triethylamine (TEA)
and 4.0 g
(10.7 mmol) of O-benzotriazol-1-yl-N,N,N',N'-
tetramethyluroniumhexafluorophosphate
CA 02351865 2001-05-18
WO 00/31037 41 PCT/EP99/09115
(HBTU) were added and the reaction mixture was cooled of 0 °C. 1.22 mI
(8.2 mmol) of
(S) 1-cyclohexylethylamine, dissolved in 10 ml of dry CH2C12, were added
dropwise and
the reaction mixture was stirred at room temperature for 24 h. Additional 2.0
g (5.3
mmol) of HBTU and 2.0 ml (13.4 mmol) of (S)-1-cyclohexyl-ethylamine were added
and
the reaction mixture was heated to 40 °C for 8 h. The solvent was
evaporated in vacuo to
dryness and the residue was taken up with EtOAc and washed with H20, 1 N NaOH
and
brine, dried over Na2S04 and evaporated to dryness to yield a crude material
which was
purified by flash column cromatography on 230-400 mesh silica gei, utilising a
mixture
of EtOAc/MeOH 95:5 containing 0.5 % NH40H (28%). The residue was dissolved in
acetone and acidified with HCl/Et20; the precipitate so formed was recovered
by suction
filtration to yield 3.2 g of title compound as a pale yellow solid.
C3sHa6Na0-2HC1
MW =611.70
IR: (KBr) 3422, 2928, 2852, 2659, 1647, 1546 cm ~.
EXAMPLE 34 : 2-Phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic acid ((S)-
1-
cyclohexyl-ethyl)-amide
Synthesised starting from the compound of Description 11 and following the
procedure
of Description 13.
C29H36N4~
MW = 456.63
EXAMPLE 47 : 3-(4-(3-Diethylamino-propanoyl)-piperazin-1-ylmethyl]-2-phenyl-
quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
0.4 g (0.88 mmol} of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid ((S)-
1-cyclohexyl-ethyl)-amide (compound of Example 34}, 0.5 g (1.3 mmol) of HBTU,
360
microliters (2.5 mmol) of triethyl amine and 240 mg of 3-diethylaminopropionic
acid
were dissolved in 10 ml of anhydrous THF and were stirred 16 hours at room
temperature. The solvent was concentrated to dryness and the residue was
dissolved in 20
ml of EtOAc and washed with water then with 0.5 N aqueous NaOH and again with
water. The organic phase was dried over MgS04, concentrated to dryness. The
residue
was purified by flash chromatography on silicagel (CHZCl2/MeOH : 90/10). The
fractions
containing the desired compound were concentrated and the residue was
crystallised from
di-isopropyl ether affording 250 mg (yield 48.7%) of the title compound as
white
crystals.
C36H49Ns~2
MW = 583.82
EXAMPLE 53 : ({4-[4-((S)-1-Cyclohexyl-ethylcarbamoyl)-2-phenyl-quinolin-3-
ylmethyl)-piperazin-1-yl}-dimethylamino-methylene)-dimethyl-ammonium
herafluorophosphate
CA 02351865 2001-05-18
WO 00/31037 42 PCT/EP99/09115
50 mg (0.11 mmol) of the piperazine of Example 34 were reacted with 62 mg
(0.16
mmol) of HBTU and 18 mg (0.17 mmol) of triethylamine in a mixture of 1.2 ml of
anhydrous THF and 1 ml of CH2C12. This mixture was stirred 48h at room
temperature,
then concentrated to.dryness. The residue was dissolved in 1 ml of water and 1
ml of
ethyl acetate. The aqueous phase was extracted twice with EtOAc, washed twice
with
water, dried over MgS04, concentrated to dryness. The residue was purified by
flash
chromatography on silicagel (CHZCI2/MeOH : 95/5) to afford 43 mg of the title
compound as a white solid (yield 55.8%).
C34Ha7N60.PF6
MW = 700.75
EXAMPLE 55 : 3-(4-Amino-piperidin-1-ylmethyl)-2-phenyl-quinoline-4-carboxylic
acid ((S)-1-phenyl-propyl)-amide
0.477 g {1 mmol) of 3-(4-oxo-piperidin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic
acid ((S)-1-phenyl-propyl)-amide (compound of Description 18), 0.462 g (6
mmol) of
ammonium acetate were dissolved in I O ml of methanol and stirred at room
temperature
for 1 h. Then 0.08 g of sodium cyanoborohydride were added and the mixture was
stirred
one night at room temperature. The reaction mixture was poured in 50 ml of
water and
the formed precipitate was filtered off. The aqueous phase was extracted with
methylene
chloride. The collected solid was dissolved in methylene chloride, both
organic phases
merged, washed with water, dried over MgS04, concentrated to dryness. The
residue was
purified by micro flash chromatography on silicagel (CH2C12/MeOH/NH40H :
90/10/1)
to afford 47 mg of the title compound as a white solid (yield ca 10 %).
C3l H34N40
MW = 478.64
EXAMPLE 66 : 3-[1,4'JBipiperidinyl-1'-ylmethyl-8-bromo-7-methoxy-2-phenyl-
quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide
Prepared as described in Exemple 2 from 2 g (3.7 mmol) of 3-
[1,4']bipiperidinyl-1'-
ylmethyl-8-bromo-7-methoxy-2-phenyl-quinoline-4-carboxylic acid (compound of
Description 29), 1.55 ml (11.1 mmol) of triethylamine (TEA) 1.82 g (4.8 mmol)
of O-
benzotriazol-1-yl-N,N,N',N'-tetramethyluroniumhexa-fluorophosphate (HBTLn and
0.75
g (5.5 mmol) of (S)-1-phenyl-propylamine The crude material was purified by
flash
column chromatography on 230-400 mesh silica gel, utilising a mixture of
EtOAc/MeOH
95:5 containing 0.05 % NH40H (28%) affording 0.4 g of title compound.After
trituration
with iPr20, 0.3 g of the title compound were recovered as a pale yellow solid.
C37I"143BrN402
MW = 65,68
IR: (KBr) 3278, 2936, 1641, 1276, 1073, 845, 702cni ~.
CA 02351865 2001-05-18
WO 00/31037 43 PCT/EP99/09115
EXAMPLE 67 : 3-[1,4'jBipiperidinyl-1'-ylmethyl-7-methoxy-2-phenyl-quinoline-4-
carboxylic acid ((S)-1-phenyl-propyl)-amide hydrochloride
0.2 g (0.31 mmol) of 3-[1,4']Bipiperidinyl-1'-ylmethyl-8-bromo-7-methoxy-2-
phenyl-
quinoIine-4-carboxylic acid ((S)-1-phenyl-propyl)-amide (compound of Example
66) and
0.43 ml (0.31 mmol) of TEA were dissolved in 100 ml of EtOH. 20 mg of 10%
Palladium on charcoal were added under nitrogen atmosphere and the mixture was
hydrogenated at 1 psi for 3h. The catalyst was filtered off, the solvent was
evaporated in
vacuo to dryness and the residue was purified by gradient flash column
chromatography
on 230-400 mesh silica gel, utilising EtOAc containing 0.05 % NH40H (28%) as
starting
eluent, and a mixture of EtOAc/MeOH 95:5 containing 0.05 % NH40H (28%) as
final
eluent. The residue was dissolved in acetone and acidified with HCl/Et20; the
precipitate
so formed was recovered by suction filtration to yield 0.1 g of the title
compound as a
pale yellow solid.
C37H44N4O2
MW = 576.78
IR: (KBr) 3239, 2943, 2530, 1619, 1534, 1222, 1027, 844, 703 cm's.
EXAMPLE 72 : 3-[4-{3,4-Dioxo-2-pyrrolidin-1-yl-cyclobut-1-enyl)-piperazin-1-
ylmethyl]-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
0.25 g (0.55 mmol) of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid ((S)-
1-cyclohexyl-ethyl)-amide (compound of Example 34) and 0.124 g (0.55 mmoi) of
3,4-
di-N-butoxy-3-cyclobuten-1,2-dione (Aldrich) were stirred in 3 ml of ethanol
at room
temperature for 7 h. Then, 0.15 g (2.2 mmol) of pyrrolidine was added and
stirnng was
continued for one night. The mixture was concentrated in vacuo and the residue
was
purified by flash chromatography on silicagel (CH2C12/MeOH : 98/2). After
concentration of the desired fractions, the residue was crystallised from di-
isopropyl
ether. The solid obtained was purified again by chromatography on silicagel
(EtOAc as
eluent). After concentration of the desired fractions the residue was re-
crystallised from
di-isopropyl ether to afford 0.180 g (yield 54%) of the title compound as
white crystals.
C37H43N503
MW = 605.78
EXAMPLE 75 : 3-(4-Methytthiocarbamoyl-piperazin-1-ylmethyl)-2-phenyl-
quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
0.4 g (0.87 mmol) of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid ((S)-
1-cyclohexyl-ethyl)-amide (compound of Example 34) were dissolved in 10 ml of
methylene chloride and 0.09 g methylisothiocyanate were added. The mixture was
stirred
for 5 h at room temperature and the solvent was concentrated in vacuo and the
residue
was purified by flash chromatography on silicagel (EtOAc/heptane : 95/5) to
afford 0.43
g (yield 92%) of the title compound as white crystals.
C3 i H39N;OS
MW = 529.75
CA 02351865 2001-05-18
WO 00/31037 44 PCT/EP99/09115
EXAMPLE 76 : 3-[4-(1-Cyanoimino-1-pyrrolidin-1-yt-methyl)-piperazin-1-
ylmethylj-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
0.2~ g (0.45 mmol) of 3-[4-(1-cyanoimino-1-methylsulfanyl-methyl)-piperazin-1-
ylmethyl]-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
(compound of Description 15) and 1.5 ml of pyrrolidine were heated to reflux
for 2 h.
The excess of pyrrolidine was removed in vacuo and the residue was purified by
flash
chromatography on silicagel (EtOAc/CH2Cl2 : 80/20). After concentration of the
desired
fractions the residue was crystallised from di-isopropyl ether to afford 0.195
g (yield
75%) of the title compound as white crystals.
C3iH43N7~
MW = 577.77
EXAMPLE 78 : 3-[4-(1-Methylimino-1-pyrrolidin-1-yl-methyl)-piperazin-1-
ylmethylJ-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide
0.2 g (ca 0.3 mmol) of the crude salt of Description 17 was dissolved in 10 ml
of
acetonitrile and 1 g of pyrrolidine and 1.5 g of ICF were added. The mixture
was refluxed
for one night. After prolonged concentration under vacuum, the residue was
dissolved in
methylene chloride and the solid filtered off and discarded. The solution was
concentrated and the residue purified by flash chromatography on silicagel
(EtOAc/MeOH/NH40H : 90/10//). After concentration of the desired fractions,
the
residue was crystallised from diethyl ether to afford 0.120 g (yield 71 %) of
the title
compound as a white amorphous solid.
C3 SH46N6~
MW = 566.79
EXAMPLE 82.: 3-(4-Carbamitnidoyl-piperazin-1-ylmethyl)-2-phenyl-quinoline-4-
carboxylic acid ((S)-1-cyclohexyl-ethyl)-amide sesqui p-toluenesulphonate
0.3 g (0.66 mmol) of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid ((S)-
I-cyclohexyl-ethyl)-amide (compound of Example 34), p.313 g (0.94 mmol) of
benzotriazole-1-carboxamidiniump-toluenesulphonate (reagent described in
Synthetic
Communications, 1995, 25 (8), 1 I73-I 186), 0.167 ml (0.94 mmol) of
diisopropylethylamine were stirred for 3 days. Addition of diethyl ether led
to the
formation of a precipitate which was further triturated with ethyl ether. The
white solid
was purified by two successive flash chromatographies on silicagel, eluting
first with
CH2Cl2/MeOH : 90/10, then with CH2C12/MeOH/NH40H : 90/10/1. Concentration of
the
desired fractions gave a solid which was triturated with diethyl ether to
afford 0.225 g of
the title compound as a salt ofp-toluenesulfonic acid. Analysis of the NMR
spectra
suggested the occurrence of 1.6 equivalent of acid for one molecule of parent
compound
(in Table 2, the NMR refers to the parent compound).
C30H38N6~' I .SC~Hg03S
MW = 757.00
CA 02351865 2001-05-18
WO 00/31037 45 PCT/EP99/09115
EXAMPLE 84 : 3-[4-(1-Methanesulfonylimino-1-pyrrolidin-1-yl-methyl)-piperazin-
1-ylmethyl]-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-ethy 1)-
amide
0.46 g (0.76 mmol) of crude 3-[4-(1-methanesulfonylimino-1-methylsulfanyl-
methyl)-
piperazin-1-ylmethyl]-2-phenyl-quinoline-4-carboxylic acid ((S)-1-cyclohexyl-
ethyl)-
amide (compound of Description 16) and S ml of pyrrolidine were heated to
reflux for S
h. The excess of pyrrolidine was removed in vacuo and the residue was purified
by flash
chromatography on silicagel (CHzCl2/MeOH : 97/3). After concentration of the
desired
fractions, the residue was crystallised in di-isopropyl ether to afford 0.310
g (yield 6S%)
of the title compound as white crystals.
C35H46N6~3s
MW = 630.85
EXAMPLE 8S : 4-{4-[4-((S)-2-Methyt-1-phenyl-propylcarbamoyl)-2-phenyl-
quinolin-3-ylmethylJ-piperazin-1-yl}-4-oxo-butyric acid
200 mg (0.42 mmol) of 2-phenyl-3-piperazin-1-ylmethyl-quinoline-4-carboxylic
acid
((S)-1-phenyl-propyl)-amide (compound of Description 13) were dissolved in S
ml
acetone and 42 mg of succinic anhydride were added. The mixture was then
refluxed for
10 hours. After cooling the mixture was diluted with SO ml of CH2C12, washed
three
times with 30 ml water, dried over MgS04, concentrated to dryness. The residue
was
purified by flash chromatography on silicagel (CHzCI2/MeOH : 90/10) to afford
130 mg
of the title compound as white crystals (yield S4%).
C35H38N4O4
MW = 578.71
EXAMPLE 90 : 2-Phenyl-3-(4-sulfamoyl-piperazin-1-ylmethyl)-quinoline-4-
carboxylic acid ((S)-1-phenyl-propyl)-amide
3.0 g (5.78 mmol) of crude 2-phenyl-3-(4-sulfamoyl-piperazin-1-ylmethyl)-
quinoline-4-
carboxylic acid (compound of Description 2S) were dissolved in 1 SO ml of 1:1
mixture of
CHZCI2 and dry THF; 2.41 ml (17.34 mmol) of triethylamine (TEA) and 4.38 g
(11.56
mmol) of O-benzotriazol-1-yl-N,N,N',N'-tetramethyl-uroniumhexafluorophosphate
(HBTU) were added and the reaction mixture was cooled at 0 °C. 1.56 g
(11.56 mmol) of
(S)-1-phenyl-propylamine, dissolved in 20 ml of dry CH2C12, were added
dropwise and
the reaction mixture was stirred at room temperature for 24 h and at SO
°C for 4 h. The
solvent was evaporated in vacuo to dryness and the residue was taken up with
EtOAc,
washed with H20 and 1 N NaOH, dried over Na2S04 and evaporated to dryness. The
crude material was purified by flash column chromatography on 230-400 mesh
silica gei,
utilising a mixture of EtOAc/hexane 8:2. After trituration with iPrZO, 1.OS g
of the title
compound were recovered as a pale yellow solid.
C30H33N5~3S
MW = 543.69
CA 02351865 2001-05-18
WO 00/31037 46 PCT/EP99/09115
IR: (KBr) 3270, 3060, 2967, 1959, 1644, 1537, 1492, 1455, 1354, 1163, 949,
764, 702
cm's.
EXAMPLE 91 : 3-(.~-Dimethylsulfamoyl-piperazin-1-ylmethyl)-2-phenyl-quinoiine-
4-carboxylic acid ((S)-1-phenyl-propyl)-amide
1.4 g (2.85 mmol) of crude 3-(4-Dimethylsulfamoyl-piperazin-1-ylmethyl)-2-
phenyl-
quinoline-4-carboxylic acid (compound of Description 26) were dissolved in 100
ml of
1:1 mixture of CH2Clz and dry THF; 1.19 ml (8.55 mmol) of triethylamine (TEA)
and
2.16 g (5.70 mmol) of O-benzotriazol-1-yl-N,N,N',N'-
tetramethyluroniumhexafluorophosphate (HBTU) were added and the reaction
mixture
was cooled at 0 °C. 0.77 g (5.70 mmol) of (S)-1-phenyl-propylamine,
dissolved in 1~ ml
of dry CHzCIz, were added dropwise and the reaction mixture was stirred at
room
temperature for 24 h and at SO °C for 4 h. The solvent was evaporated
in vacuo to dryness
and the residue was taken up with EtOAc and washed with H20 and 1 N NaOH,
dried
over NazS04 and evaporated to dryness to yield a crude material which was
purified by
flash column chromatography on 230-400 mesh silica gel, utilising a mixture of
EtOAc/hexane 8:2. After trituration with iPrzO, 0.3 g of title compound were
recovered
as a white powder.
C3zHs~Ns03S
MW = 571.74
IR: (KBr) 3315, 3059, 2965, 2813, 1955, 1661, 1638, 1533, 1491, 1455, 1349,
1152, 947,
748, 702 crri t.
CA 02351865 2001-05-18
WO 00/31037 4,~ PCT/EP99/09115
.. _
'° ~ V~,,
O o
O h I~ O O
yr ~. w.r 'r
..r V'7 O
O~ v0
~- -~ y
aro U M ,cue ~.,, ,...
G v ~O v1 ('~ 00 I~
i: N G1 .--~ .-,
L' ~ O ~O O 00
C Q1 00 ~D
0. N .... ..
s.
o c~ oo ~ .-.
on ~ ~ ~o ~o
,~ ~ ~ ~ O~
03
~. x o x x x
N N N
o z o 0 0
c~
00 ~ x z z z
v .~ ~w x ~ x x x
U U U U
II
~i
i
)
Z CZ)
CZ)
~x x x x x
1 v, v~ , ~,~
N M ~ tn
CA 02351865 2001-05-18
WO 00/31037 4g PCT/EP99/09115
V7 N n ~ °~ pp
V~' M ~ i
i i , , ~ 'ct'
.C ~ O TS ..C b .~ 'O O TI 'C N
O ~ O ~ O H O ~~ O ~ O N
c~C ~ ~ ~ ~ N
n
M ~ ~ ~ ~ O °~
N N N
0 0
x ~ ~ M z z z
v»o ~. ~ x x w
U U U U U ~j U
'' ~-i
_ ~ ~ z / \ z
'o
C2~ Cz~ CZ~ C ~ Cz~ z ° . z
x x x x x x
w
U ,',
~O r. op ~ O N
CA 02351865 2001-05-18
WO 00/31037 49 PCT/EP99I09115
1
+ i
rn
ono ono ~ ,tea. ~ oo v~ o0
O N 00 00 M ~O t~ \p
00 00 N M ~~ ~O V1 N
r. ..r ...r .-. .-. .-. .r ....
c~ ca
~O lW C t~ n I~ ~ ~ 00
~~ N N ~ ~O et ~ oNo
i \p ~O t/1 l!1 Vl U1 lfi ~!1 N U1
,.
U U
N N O O O O O O ~ O
0 o z z~ z° z z z z z~
? T O o0 N N O Q N
z z x° x x x x x x x
x ~ '~ r~ N .~ ~ ~ V U
U U U U U U
U U
x x
0
~~ w0 ~O ~. _O ~~ w ~~ w
C1 0
I ~.Z~ 'Z' I z z
C ) ~ ~., ~' ~
'' Z z~ J~' r~ C ~
z z ~. ~ z ' z ~' J ~ z
.,z, '~ ~, ~-z -z -z
x ~ x x x ~ x x x x
~, 1 ! ! .1 ! 1
i ' f
c~5 ef ~1 ~O t~ 00 G~ O ~ N
CA 02351865 2001-05-18
WO 00/31037 5~ PCT/EP99/09115
0
II
'.
M
:
~
H
n ~ ~ 'd o p
....~ .--~ r. ..~ ....
_
f~ O O~ l~ l~ N ' o0 O
.- v1 et O~ ~ ~ ~ O o0
~..... ~. ... N ~..
c~
~O ~ n y 0 ~O vD ~p tt' n
1~ ~ VN'1 ~ V~1 N ~ ~C ~D N
U
O O O 0 ~ O O N p O
z z z z z z z o z x z
M H1
M ~ U x x M ~ z x ~ U
U U U U U U x ~
U
U
o. . '~~, r~~ f'~'1 0 0 f-~ z
-:;-w ~\~.\ ~ o~ z .z z_ ,z r~ ~~ ~ o~: ~c.
z ~r ~1 =
(; z ( z-, z r.z, rz, z Z. .z
C> I > ' > C~ ~ y
I,r~ 'z ~ l ~ z ~~z' z z
'z
l
x x x x x x x x x x
1 ~~ ~ t ! t
~~
r ~ r - ~ z
~ U " " ,~
t'1 ~f v1 sC t~ o0 pv p -.. N
CA 02351865 2001-05-18
WO 00/31037 51 PCT/EP99/09115
N
O
U
O
.C 'b .~ ;b ,t 'b N ~ Cv O ,~ ,'C
C. . ~ ~ ~ N
VJ O H O 'fOIJ O pip ~ ~ O~
~ ~. ... N
N td
n
n ~ oNO, vp vp n n
V N ~ h ~ 0~0
et h vo v~
U
z z z ~ N o" o 0
a ~ ~, a
a
x x x x
x
U U U ~j U U U
U
I
Z 2 Z
0~ ~ ; o ,_ -CJ
,.Z,L J=,; ~ ~_~ _\
Z Z. _ lrl
-Z
z ~~' ~ ,~ ~'' ~z .z. l~s.,
~x x x x x x x x
v ~~ r, w\
U
M ~ V1 ~D (~ 00 C~ O
M M M M M M M et
CA 02351865 2001-05-18
WO 00/31037 52 PCT/EP99/09115
N
O O
.~ .O .C ~C ~ O ~D
O V1 O ~' O
N ~ ..~... N N ~ O
.~.
00 ~ (~~, ~ ~ ~t N .-.
n 00 00
O
~t ~ v~'1 N M ~G
H h
0 ~ ~ ~ ~ 0
z° z~ z z z z z
N M ~ p
Q Q <
x x x
x x x x
V U U U
U U U U
_.O ~ ~ ~ /Z' / z
D
.. _ _ _ \ w~ _
p ~~a ~' , _ ..
C=
x x x x x x x
s
'~
/_\
't 'r v °' ~* v v
CA 02351865 2001-05-18
WO 00/31037 53 PCT/EP99/09115
N
M
O O
~r
N
V'1
p ~ p p N ~ O
C - V ~ TJ o0 p ~ .G
O ;O
_ N . ~
.. 0 ~ ~
O
o G ~ p
n " m
G
N y ~ ~ Ov
wr
~
cG eC cC
Q M O M V1
I~ 00 l~ ~ ~ ~O ~D ~O
~ ~
I~ N Ov O '1 ~ d'
~
v~ ~D v~ V1 n et d' v~ ~n
N1 N N
N N O O O O
,~ ",
z z z
z
0 o z z z
z z x ~
~ x x ~ ~ x
U U U U U U U
U U
x
J /-' z
n . Z ~, Z ~ I
,Z~Z
Z = y ~ 1 -~~l ~ y
l
z ,Z z, ,
J . n
C ~. I
Z .~. ~,
~.
L~.
Ix x x x x x x ac x
t i
w
O~ C ~ N M ~' ~1 ~D n
V'1 V1 V1 V'1 N ~1 Vl ~!1
CA 02351865 2001-05-18
WO 00/31037 54 PCT/EP99/09115
M
C
U
~r
M
O
M
:r
,... .--~ N 00 ~ M r.
.-. .... ...
N ~ '~t C~ ~ O ~ O ~O
.... v1 00 ~ N O m O N
r. ... ... .... ~ ~ .-.
cC cG
n ~ ~ n ~ ~ n
~
M M N M_ fV 1~ r1 Vi
~D M t!' M
H N ~ v1 h U1 t~ v1 ~O
N
0
0 0 0 0 0 0 0 0
z
z z z z z z z
x x x x x x x x m
Q N N ~O ~ M a
V x
V V V V V V V
U
I :1 x ~o z o~~ o.~~ .z.
I l
O ~~ ~.z ~ CZ) .\~ xz_,~o z
Z l_ ~ ~I ._ ..,, .. .
z ( J z
~. ,. .z ~ l r ~ l o.z ~ z
x x x x x x x x
1 1 .! ! t
w w w w ~' ') w
C~ O ~ N M et v_1 y0
CA 02351865 2001-05-18
WO 00/31037 55 PCT/EP99/09115
N
O
il
~r
M
O
V1 u1 N N
a a a a
b ~ b ~ p ~ .~ 000 000 ~ O
N O H O ~ O ~ O ~ ~ n
~ ~ ~ ~ ~ N
c~i cC c~ cd
n ~ ~ n ~ ~ ~ n n
~C o_o ~t .-. d w n n p~
U1 N N W 00 vM1 H
N ~ ~ N fy1 f~1
a
z z z z z z ~ z z
x x x x
x ~ ~ x x x x N
a
U U U U U U U
U U
0
_ ~ ~~° o~\r° Cz ~ o =Z..,~Z =~ uYH
C~
CZO
:~°
o x x x x x x x x
'~ ! 1
/ \ f / \ r ~ / \ r / \ t '
~O ~O ~O ~ n ~ n l~ n
CA 02351865 2001-05-18
WO 00/31037 56 PCT/EP99/09115
N V1
M_ V1 V1 ~ Ov O
N .~ _.~.-. ~ ~ .~ .C N
v1 O ~ ~ Wit' O V' ~ O o0 O
Wit' ~ N ~ N v0 ~ N O M
N ~ .-~ .-~ .-. ~ N
td ca
n oNO n O
N
N O O N e1 Cn d' CI~
z z 0 0 0 0
z O
z a ~ z z z~ x zQ z
x
x x x x x x v x x
H I~
fv1 ~ , t~1 M ~ ~ er1 ~ M U
U U U U U U U .-.U
a i-1 ~ ~ ~ ~ z ~" ,~ o ~ o
<: _. .:z o~. . ~z z ~ ~Z ~~ n i
1' Z ( o ~ ~' o ~_. ,.Z
y
i _ ~ ~ y CZ~ ~ C.Z ~ ~Z-
.;i.. ;'~ ~ I~
x x x x x x x x x
~.
~, / C~ U U
n o°o o~o oNO oMO o'to
CA 02351865 2001-05-18
WO 00/31037 5~ PCT/EP99/09115
N N n
N ~. N M
O
O ~ O O
~
pv M pp
-f- + V1 U1
n o ~
v
~' ~ ~ _ ~ -- ... o, o
.-.
O
N C~ N t~ TJ .-, r-. ,
U ..C
a1 ~ ~ ' ~o p..
~ ~
~
l~ 00 O p M ~ p
~ 00 v
7
N ~ N
td
n n ~ ~ ~ (' n
n ~ ~C h ~O I~.
~ ~ ~
v v0 v0 v0 h v~ v1 v
1 1
C)
z z zo z x ~ x z z z z
x x x N ~ N x x
x
x x ~
U U U U x ~ N U
U U U
U
$ ~ ~ x
~ i
0 0 ~ o ~ I I v_
-Z z o CZJ
0 0 ( , o ~ ~ z z o_~=o o=~= 10
C~ Cz~ C=J CZ~
C=) C=~ CZ~ z z z z z
x x x o o ~ x x x x
~~ y ' ! t t
,. ;
w~% i
Two t~ ~ ~ p ,...
00 00 00 ov
CA 02351865 2001-05-18
WO 00/31037 58 PCT/EP99/09115
N
N
O
Ii
U
wr
00
00
'
b
_
00
O m n
r.
cC
~
0 N
0
.
O N
z o
z
U U
h
v v
x
x o
r~
et N
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
59
c ~ =
_ o :: T c c
c N o o n o "l ";
c' 00 W 00 ~ ~ o00
ri vi ~ n = ~ r S M
a~w v .:~ .., . ~ ::?
.i. CV ~"? n N ,~~, ~ .~.~ .~.. ~ ~~..
_ I r _ ""' r ~ pp
O ~ ,_r~, ~ ~ N Ovo oVO OvO ~t ~ A~p
V1 M N n O ~ t~ I~ l~- ~ J Vj
~'UW C ~ ~ .. . ._ ~ , ..
T ' ' _. ~ ~ ..~. N
O V O_ w N 'a:
O .., ~' _ ~ ,..~.. _
~n " ~ N p .r ~ Y~1 r
~n ~ ~_- p O o0 M
M (~7 _ 'r 00 v1 N ~!1 l~ .
yr O M N t~ .. O ..
~ ~ C n . . ~
,
.. ,~ ... ~ i~ ~,. M
. ., M ~, D C W ~ ~ v _ ~J
u ~ _ ~ i v 00 ~
M O O O N ~ N
G..-. C~Iw N ~ ~f ~ n a M
C, M M V1 M ~ M
T r ~ r.~w vN.r ~ ~ ~ wp. n~w r ~.. ~ _
f'l C'l o0pp ..v. ....
O . O " Ov V'1y r vl N y ~ ~
L", v1 00 I~ ~ ~ ~ O ~ CV
NG - o ~ ~o M ~ Q ~ - o0
fV fV M M M yrN N M O ~ - N
r.~.O O ..~..~ ~ O' h ., N ~ ~
C3 O 00 ' ~ p' ._.O ~ ~ ~ ~T" O op
C~N - ~ et ~ !V ~ ono oxo pOO .Nr N ~
~ ~ n N M .%-s.~w~ G1 CT
(;~ ~ ~ M ~. ~ .L, O O ~ M M _
~ v ~ v1 ~ N si N N rT.. .., ~
w O n ~ ~ ~ ~., ~ i ,.-v ~ 'r
V~o ooo.-T.. cy ~ o o ~ _ %: co 'r
p m o
,~ ~ 0 0 ~ M N "'
O O ~ , ~ O N N ~ o0
C Li1 w ~ .- ~r n N
~ o'o = ~ x x ~n O ._..
~
O z ~ ~__ N v.~,.r O ~ v _ N ~f ~_ N N N v_r
T ~ 'X.~ ~ ~j ap o~0~'~. O O n 000 ~ ~~.O
o .Vlr~ ~ ~ N p ~ wr N N ~ N N
~ oo~ Z o v, o ~ ,~; o o ~ :.: ~.", o
V"~ ,~~ ""~C'! N ~ ~ S,00 nL00 O ~'~' et O
O ., ~rN ~ ~ ~ .-. ~
O ..~..M ~ :~ '~ , ~ .~: ~ N N iv
V1~ ~, M ~ .L I~,y_ ~ _ ~ ,~N v'1 O
....~ _ V N t~N x Z o ~"!2 M o0
~/ O ~ N v ~ ~, ...
.r. ~ ~ '~ 0~0~O .~..N 00'~ G1'~ ~' n N -~.
00. = 00N ~ 1~ ~ fV C~\D n I~00 v~..L N
,~~" ...~. N v1 ~ p ~ n ~ ,.,~~'i~00~ Cr~1 ~
a .~ I~~'00~ V'1l~M ~ .a..~ .r., 00 ..~. yrC1 M ~O
~ N n i~~ ~ ~ v a V ~ v ~ ~ ~M_ _ _
O ~ N .i..~r,'~,~ ., ~O~ O ~ O ~ ~_ p~ v1 N d.
~ Q_~~ =_ ~ O ~ p ~ O yrZ ~O G~
"~'M N O p O 00 ._.~..~ ~ ;.~.,~.,~ O N :-:... .r~ ~~y
M_ !f v~1 .L.4~ .a.~ O v ,T"~ ~ M
~ M_ ~ _ ~ ~. M W ~ON N 'T'00~ r v
~O~ rn ..~~O ~ M G_ r yr~ C ~ ~ p Nvp ~ ~ M_
Q1~ ,n "" .Tr.~ n ~ 00~ 00 ~ 00T ~ ~~ .Tr. ~ M
O O ~ N ~ O ~ ~ O ~ O l O ~ O ~O ~ O 00Q
'J _ 00- O ..n . - ..~'.. i~.. O
'9I'~ ~ 00 ~ '.O a M
'J ~ t~.CO~O~J .Cn ~ _00'O 00 ,-~,~"
f~ C/Jy ~".9 00~ 000 = ~ ~O O J M
~ V7 ~'V1~ fl1p~C~~ C/J~N ' O
c0r y r ~ ~C~ ~ ,~n ,~ ~ ~'Ov0~"' ~ O N
tA n ~ V w ~ ~ ~!1~ -y~ I~ sr
c0n c0 00 n c0n s0 Q ~.Oh c0 !~~O I~c000c0 n
c0 t'
d
G
..
~ N M ~' v~ ~p ~ pp ~ O N
i!
W
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
' ' a _
. N ~
~
"' , V V1 M _ N U
. ~~
'~ '~ ..,
h
O 1 d n f'I .., ~ = _
G '
O v'1 ' ~
~1 ~
00 l~ ~ ~ R '~ - N n
I~ n
n V y _ n f3 _ O M M
~ r U
c .. -' ~ _ '~ ; w X cs n
6
_ cC _ y r N ~ ~.-" y 0 M
~ n
~ ~r nl ~r . .:.
'r N ~1' ..
_
".R.. ~r W O _ O ~ ~. y .
~ ~ O 1 n _ _R
t~ fuel O I~ ~ r .W. M yr C . ~
'
- . v O N . ~ r
,. f .,
_ _ _ ~ ~
O
"' ~ tC a n ~ i-s M T
~ r
a I l~ O ~ ~ M N V1 ~O
G1 i .~. _ ~ ~
~ O M ;~ C' ri
_ v ~ ~
~ r; M N p M v ~ ~ T
(v .. ... M ~ i~ _ v V1 [~ y
~ ~ ' ('I ~ ~
~p
f'~l ~ V1 ~ ..~ 00 I !V n..N
r
_ T ~ Q _ v ~ l' M M "
'
C i~ M yr
)
v1 n ~ I~ ,..1 n T f'C1 ~ N ~ ,.,~.,
_ N .., .. V1 ~ Q. et ~ _
~ v't ~ .~. M I~ M W N n i~ W r ~ ~ N
~O R ~ O ~ 00 h v
~ .
' O ..~. ~ ~ M ~ T ~.f~)-~ ... ~ R pp
~
00 M ~ _ ~ O _ wr0 U1 ~ M CV T M
f' I
M Q = 00 G1 O y ~ R
M _
N
rj ..~. ~ N OVO ~ ~ N 3 n ~ si ~ O C N
~
n v r . y~ ' n i~n T i_-v N . 00
l . ~ ' r
O G1
V ~ ~.,~ ~ R .i.U _--. ~ i-y
1
M 'T yr N ~ U T ~ U V W i M C~l~ ~"~00
M ~O ~ ,~D O X v -.,T1~ G1 n R n
n in ~ M O n N T U pp Qv, ~.,~ V1 ~ ~ .L,
Z T N ~ .~
. T ~ N ~_ 0 " ~ O ,_,~ , M ~ ,~ ~ ~ R
. ~
~
'r T N M 0 3 ' ~ 00 ;1~ ..~. N ~''~
~r
~ v .
~ ~ ~ C ~ 1 1
O O M N ; ~ CV %~ .i. ~ ' N ~ ~ t~N M
O _
N M - 3 x 3 R O ~ n pp ~ N s
fy ;~ ~ i~N
n M ~ U ~ ,'L",N O R C: C i~ M ~- 00
O
'"' ~ ~ ~ U r.T~X ~'~ ~ ~ .~'~...M .Y
N v ~ ~
~~ v .~ = M ~ L p'N ~ y ~ ~ v O
1
Q ~ p i~ N
O ~ 3 '~'~
v N ~ N .. ~ 00T, ~ G~L7
..
M ~ ~ v N ~ s jj ~ N ~ ~ M ~ i~ t~-.C
N [ ~ , " (~ v .
v ' M ,~" ~ .
...
3 M ~ '~~ '* 3 ' ~ ~ ~ N ~ ~o v ~ 3
~
~ ~ . ~ . . ~ ;
~
c~ a C = ~ v a _ x ~ ~ :-: N 'r -' Z ...s
~
O O y L ~l~ ..~ ~O ftfN X ~ Wr ~ V ','t,' i-; i1~ n ,n c)
.
'C' ~ v ".' N ~ N ~ ~ ~ ~'N ~ c0.'~ .T.O ,."'.. y y
'
3 , T O Z ~
~
M N " M .-N N O wrtn V ~ , y r t~v ~!1~
Q ~ _ Q V Q: ~ I~v ~ , O 00O ~ M v1. O yr_
U
N N ; X t~etO ...N ~ p~ Q ' ~..~r'N yr
N
c3 O a~~ ~ pp~ ~O.~~O ~ ~ ~.. cC ~ ~
_
~ _ _ ... 00
Q ~. pp _ ~ Opn = pp I~ R ... Z M ~
~ T ~ ~ ~ ~ ~ _ ~
_
_ _ .
R ~ ~ ~ ~ ~ ~ T ~ ' ,
00v ~ M M sr C r~~ W
N ~ V ~'" yrR 00 ~ v ~ ~
0 v1~ OO l M 'rN tl l M cC
N M ~ ~ T ~O' N v1 0 O
i~ M N o0N sr ~ . . T 0 ~ v1 ~ .L
. ( ' N
S fV N ~ v N O~~ ~ ~ N N O O
N ~ M ' O ..~ .Q O u N _ n ~, y r
O . y
_ v "~ I~N R y t~ p~fV_ ~ N ~ C V t .'
l~ C r
~O M ~ l i~ i-~~
~ h x ~ ~ t' ~ ~
~
~ .~ ,n C :-:_ - . oyoo. T
N . ,
W i _ ~ v
00~ L ~ ~pN cUy~ ~ ~,~~v ~ ~Ov.r' v n ~ V
_ ~" M X
T ~ ~ x M ~ T O O w ! vp M .
M ~ 00 ~r
O Q~ v ._. N M y .
'r ' O v eT N v ' ~ ~ "' ~
r
~ O O v Z Q . ~ ~ i _ ~ ~ ~ ' T
~ ~ i ~ N
O "~~ O ~ ~ ~ O Q~~ ~ ~ n N O 1 O V1O
~
h
O 00 !~ ~~ T ~ N vV'1G1~O
~,o I~ .. _ a0O n p p h
'
,~00 ~ . ' . , . . ~r.
. ~ . . . .
O O ~ is~ ~-~ ~ ~ fC~ ~.~. ~ ~ ~ %'~
~ n
~ O ,.,eC~ R = ~'= ~ R = ~,., ~.w H n _'~,
U ~ '
V Q 0 ~ Z Q v U U - U y U - U ~ U U ~ U = U
. ?
~ , v~
~ ~r0 = O v 0 0 !~ ~
"' ~ ~ U _ U U U U U ~r ~ U ~ V o
...
~ '~? ~ ~ ~ ~ ~i~ = U N ~ = r vrV1
~o co~ ~ yr W N
0o
o ~oc~co~ co ~ co~ oo~ ~ovi~03 co O 3 c .
~0 ~o ~o
' W a t~ oo ~1 p
~
N N N N N N (
J
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
61
N
.
'
_ ' s.
x . : O
M _ W ~ ~r oo
~ h ~
. M
' ' .. ~.' cV ' N .
;b T v v ~ ~ ~' n ~ y ~ h
"' ' a
. i v1 y -~~ ~ ~ h ' N
N
W r . . G1 h r ' ... v... .. i. wr
r . ~n y
V1 - "~",
O V1 t~ W v _
'
. r h O
h ~ W . N I~ c9
M h N , y r
h cJ h 00 h ~'
. N ' M h i~ h ~ f4
_ _ ~
rl v _ cJ n . ~ ~ h ..,
.
N C _ .. . Q
h Q ~D y . T ~ ". ~ ~
_
O r .. ~O ~
.. ~' "
_ 00 v,. ~ ..., GO
h .:. yr :
00 V1 C1 ~r ~ h ~ l
M ,~
v ~ ~~.. ' h ~ Q ~ ~O ~ N ~ M
1
h N ~ h Q'
Gl s ~_' ~ ' _ h O
~
O ~ X N 00 ..R.,. ~ ~ ' ~ ~
-
~ _ M
O O L~.. ~ V1 (Q Z' O
o ~ c ~ = " ~ ~ _ o
~
_ y = ~ c'O_ O v .n.
'
V V1 ' = ~ O
1
a ~_ n h ~ ~ h
ro N ~ ~ - N 3 M ~ N ~ ~ ' f~J
"
CV M ~ ~ ~
~~
~t O _. ~ M ~ I\ X ~ ~ Q ~ O
~G N ~ 6) 00N h '
00.,~.. ~ ~ = ~ N .., ,;"~ a N Q
_ Q v T _ v.h T_ O o
O
'n N O ~ O sr yr C ~ N N ~ M ~ ~O
~
00 ~ M r;~ N O tn M l~. cV J Q ,~00W r ~ N
M O O ' Q n N ~ . =" , t!1O M Q .T.3 i-v
' O .
j N ~ N Z M i ~ ~ '' v " N M l'l N ~ U ~"
-"
y N .r . O pp Q ~ ..
Q V Q ~ . n n ~ .3 Q Q ~ ..
f ~ " V ~ N .~ 0 ~
, 0
v L M 3 r. O ~ T N V1 .C ~ ~ ~ .~M .~~M
i ~ '
. w v 00 N N N n V p :-'y~,. N - O
. .
i i N U ~ M Q ~ N O ~ y 3 r ~ i ~ N
~ N ~ i~
~O ~ ~ N i-: ~ ~ ~ N ..T.L ~ ~ h ...~ C\..,
~ N Q V
V V ~ M M ~. ~ r U O U 00'"'
N O ~ ~ x ~ n n ~ ~ n ~ N ~ N ~
~ ~ O~ ,., ~ n
" ~ - , "
....:-Q w N N ~ M .i ~ 3 M M ~ O ..~ "",~N ~ O
v ~ ~ ~r N v V ~ f~ v M .~~.~ fV
'
M ~ r N h ~ M 00 O ~ ~ ~ ~'N v v ~ O
~O V
~ . ~ Ov n X O n ~ ' p O OOi-v~
O ' O ~
~ ~ _. M ~ ~ ~ M ~ ~ ~ N
~ ~ i, ' %~ ~ 00~~ (~
= N N = M ~ 00N _,~ L
M "~ f~ A ..,iv ~
v k v _ _ O v ~ ~ i C' ~ '~n ~ ~ ~ ~ si'w
n N = .i, .~ ~ . O Cd0 t.n 0 ~'?
. ~ . ~ .
. W
~ Y ~ T Q ~ Q N V ~ .L ~ v ~ _ vptox N M v n
. ....f~i n .~:_ i _ tJ
. .
N ~ .-.n N N N ~ 'r ~ U
~ V r v1~ ~ y~ ~
1
N C ~- 3 y N N ~. v N M p ~ ~ ~ '?~. v T y
~ ~ ~ ' : oo"' N ''"" p
:
' . s N . ~ N ~,..\ pp N .'
. -:
W ~ ~' T x _ ~ ~ U ~ 00_
h
~ O t . ' v N O~ O M O .T,wr O ~ i~.. ", ~'~
. . ' ., r,
y ~ ..~.~ ~ v1 ~, ~ CVv N M v1 V ~ C ~ G1d M ~ V
_ '
n O ~ O~ ~ V = p Q N Q ~ h ~ Q N Q .r wr' 'r O c~v
'
M ~ N v1._, :-v M ~ r"~~ C O ~ o0~ V (~Jit'
N V'N h p "~ - _ t S . .CN ~ C
,... ~ _. ~ O r' N ~ ~ ~ C r'
~"~ o.% ~ 3 ~ ~ T 3 ' R
~
~ ~ ~ ~ ~ N ~ ~ . ~. ~ ~ ~ ~ ~ ' ~ z
N s ~ ,~ L
O _ " ~ V O O
= = '~ ~ fV~ i a v N h O ~ y y ' R -a
v O
O "'~1 N . .T~ . ~ .T.M ~ ~ .ZO ~ ~ c x C~ X ~ 0
.''" _ . w w. .. C
M M ~ ...V1..~ U M v _ h ~ N ~ ~ "., y U 0
N , ~
~
r ~ I'_ _ p M O 0 ~ h n 'rT ~ M ~ M ~ ~ W
Y
O O O 0 ' O O O 0 ~ 0 C O 0 N O N ~ O
0 o 0 0
00h v M ~ 0 C~T C~ R h C h G~G'v,h _ O 00
1
_ _ O M M ~ -.G1O ~ O T O ~ O h O h . 60_ _
~ . . . r . . . R ' M
. . . - ..
= ~ ~ ~ O ~ O ""\~ ~ . y~. . _ _ . _.. o
A ~ ~ ~ N . . ' . . .
~ '
? ; _ T = R = ~ ~
U ~ U ~ U ~ ~ ~ U T.,U x V '~U .T.V
r Q ~ ~ ~~; D " D ~ D '.O ...D ' ~ '-p ~ p
, ~ v
h ~ ~n~ ~ ~ ~ V ; V ~ v '~ '
cv , ~ _ V c~~ ~ V o ~ o ~
~ ~ , o
.o c ~o co v co~ ~.o ~.000~.000~.oc~co00~o , ~.oh ~.o0oco c~toh
D r.
f~ ~ ~ V'1 ~O I~ OO G1 0
N J N M M M M M M M M M
Image
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
63
: '
a :;
, ~
R , ~ R ~
L ~ ~, s ~ o :;
O = = ~ y ~
v j ~ ~ ~l o .
L
C v ~ : n s ~ v l
:
. . . v ~ , :
o ,
v s ~ ~ 0 .~ '" ~ ,~ ~ , ~ 3 N
l h
~ ~ D .,
ll
l
lV ~ ~ ~ ~ ~ h - ~ ~. .= 00
l' h L R - ~ 00 ~ ~ X i-v
. V cO O h ~ '7 ~ . 'r h
~ ~
'~' h ~ _ h '~ ~ h v G
i~ 3 e ~ ~ x
r3 i~ h ~ C. . fJ
., _
_
.r. .~' y r S ~- Q ' ~ ~." ~!1 .:. V v
h ~O
f'I ~ V .~ ~ _ O _ _.. _~' M
' '
O G N N ~ t~ M c T ~ h
I
C h _ h . C
O . ~
,n vp R N ~ ~ O N '_' _ h v :-s
et h
R M '' h O .r ~.l
h ... ~ ~ C h r .%r G
o
o_ o ' ~ R ~ ~..: oo ~ M _
.
_
N v. ~ ~. ~ O M yr ~D
h O vv1 ~~ . ~r ~~ X ~ _ O
r
"
h '~ _ . h ~ ~ _ O O ~O
.
.
...
N
~ ~ M ~ ~ M i C ~ V
~
".. h _ = V1 n
.,
r~ ~ lp V W !1 ~ y ~ h ~' ' L
'~~
_ _ P M
~D ~O h .. v G1 ~ M J
~r .
O 00 h ~ 0 I~ V ~O ' W . N
~
i1 0 1 v O h ~ M
_ ~ ~
M ~D h U? Q ~O G
,.,~." n _~ N1 . h . M ~~, V1 M ~!1
., ~
N ~ M O ~ .. ~~, .., ~ O N C~l ~ h Q.
~ ~
_ N G1 ~' ., vl M V1 ~j n N
~
h ~ N ~ V O p ~ ~ 'C
V v 0 ~ f~ ~ v1 ~
l 0 1
. '~ ~ ~ N o0 N v ~ N '~ N h ~
M
C . ~ h ~ ~p M N x
N
v ~l N ~ ~ i~ N v
~
~ o in :: = ~ .. N ~ x c .a.
. ~- _
~ ~ N T , v
,
_ ". d. M N M
.,
et 'r M M O ~ (~ N C M v ~ O
M i n o0 Ov 00 i.~ ' ~ ~ ~ .
v ~.. v1 ~
_ _ , ~
.L ~ N ~ r M ~ r .
.~ M M .. ,. ~
. .. .."
N
T O v1 x .r D .r. N N ~ x ~ . N
.r
N ~ ~ iv ~ ~ ~ N
M N ~ 00 S C~j n
~ ~; ~ c o ~3~ ~ ~ ~ a ~ ~ oo M ..,
_ ~,
y ; ~n - ~ - Z
_ M . ~ ,..~ ~ , ~
O O ' n ~ ..~ N ~ n % M
" ~
0 X ~ .rM y - M
"~l~r C'
Q c _, OvR G1 ~ ' h 5~,~
0 Q IVT - ~ ~ R W r ~jp O ~ ~ ~ _ N
R ' ~ f ~ n
~
y r M M M ~ N N C .~: O ~ 00 vp 'n N N
i N ~ .~..N N ~ ~ f4 ~ ' ~ ~ R .j N
~
M M 3 ~ _
_
~ ~ ~ N ~ ~ ~ ~
V V ~~, M n ~ N '.~ W r .., ,~~. ~ ~
X M - oo ~ -.. ~ r , ., ~ O '~ T
X ~ ;.\x , p , X v ~, .
" .
~ V ~ W O ~ ~ ~ 3 ~ r
o ~ ~ r ~ r ,C~O ~ ~
o i
v
' v N ~ ' s N - N pp~ ~ N N C' ...V 00~ N 0 ~ ~ ~ N cn
~ 0 x O ~
_
C O N . N r _ .. ~ _ O ~ ~ X oolctN ...-.' .,. n
fV ~ .~ .
R ~ ~ 3 o x N o ~ ~ .;y ' ~ ~ ~ ~ _ '_' 3 " '~
_ oo, N :.~--v,N .., . . ~.' - ~ 3 ~ s
- .
~ T'~ (1Q pp 00N _ . 00O ...y r i.U ~ ~" x
' .~r
N ~ ~ . _
X , v O~O ~ O ' ~Dn ,~~, ...n ~ V ~ y _ O~ N
v1 R ~ ~ ~ . r. ~ W r L _, v X ..
R h
N ~r eT 3 ...-.. X ~ R M C~~..~ NM M
' ~ =
'
:':~ r..O x ,--~ ~ . o..
V _
~ ~ '-'v ~ i O ~ ~ ~ ~ l ~ ~ ~ i~r1~ y ~ ~ ~.'~ ~'T
~1
- ~ c N
00 . O
.
~ N O X h ~ V1..~O M A N ~ .a~
O
M O ~ G1 3~
p 00.~00p h p ~ ~ p 00j ~ ~ N M ~ O
~ ~ C O ~ d = ~ '~~ " O ~ -
..... .. - ..vS..- ~,r ~
,o T O 3 ~,
~ ~ O
U " U -"U ._ U -cU U x U U , v~ ~ U ~ V ,..., r.,~ -
. ...
O D :
.r .
~ . ~ ' ~ ' ' U
' "
a r ~ r V o ~ ~ - ~;~ ~ ~ -a
t.Q00W~00GO(~ t0~~~Oh cp h t0DOt000t0 D _ c,",
' ~
60( GO 00t0h CO 00f.QGO
l
w n 00 G1 Q
V'1
V1 V~ ~!1 v1 ~O ~O ~D ~ ~D ~C ~D V~' ~O ~O
CA 02351865 2001-05-18
WO 00/31037 PC'T/EP99/09115
64
:: ~
! ~ = _.c
C ~ ~
a n s ;' - O
fuel
I~ n 00 ~
~ v ~ ~ ~ i~ fV 'O' .~.~~..M
r _ ~. ~ M
N U1 ~ C~ N v r
~ 1
_
U ~ ~p C' ~' V1 o n ~ M p i O
~ ~ C'l N
L
N = '.r J
'~ ~ ~ .' O h ~ X O
~
d CSI ~ . .~. ~ N C l'
..
r r ... C L ' ~ ..~..
.
_ _ _ _ c~I M
~' V1 '-' ~ N ~ siv i
G1 ~ -y f'! O ' ' : ~...O ~ I~
O N s N ~ .. ~ ~p .-. O ~
Q. f~l . ~T M 'J' M r I~ M ~ U
T .~ ~ ~ , T M ~ X
~ , ~
..~
_ i- ~ .= U t~ M _ f~ . yJ
~
3. L V X 00 00M _
~ ~ Wi vi _ ~ N n ~ I~I M ' .~..~
yr 3 ~ c ~ ~N '
m _.. r n M . vi ~ c
O ~ I~ ~ v ~ ~ a!~ " ~ ' O N ... ,JO
M M M M M O ~ N _. M M ~ _ M
~ M ~ C'M ~ .
~
n ~ ~ .....~ ~ 00i~ ~O ~ C~I? T ~..~~ 00_
r .r. .
.
. ~ M C y ~ ~
N ~ yr Z ~ ~ ~ a W ~ Q I~n PI d 00.~' ~f'
-
z '~ ~ Rv ' c ' W n v = o
vp ' ~p ~ ~ N v1 ~M _ V' ~ = c ~ , ' t ? D
" _ oo - R w
...
~ '~fR M ~ M M ! ~ r ~
~ ,
M ~.,~ ... vM O N _ V! f'!3 M . v N
~ ..
T = . . . r --.
~ ~ ~ '~ 0 1'f\I . OO v N
r ~ r ~ ~ a ~ G, ~ f'I~ V1 ~_~ CT 00~ ~ Q
' 1~~., ~
_ N 00M C'~ f'!~'~.~~ (V
.
00'~~ G1 ~ N v p ~.00 ~ O ~ .~~ ~ 00~ ~ '''v
0
L ~ M . (Cr ' v y ~ T ' ~ ~ .i.
~ M M , N M M " f'1
R .
M ' ~. ~ ~M l0~ 'S M 00 (VL '~'~
.
_ yr w r N ,yr
M h v '700 '
r~ ~ M 1 ~ .., " ~
O I
(~l M ~ ~~ v ,i ~ v1 _,~.M ~ ~ N N
N Q N ...~ v ~ W a ~ 1 N N (V' N Q ~ N
w r .
l ~ yrv M E nC ~ O~ 00 O O ~O..00 ~ fi~ 00
N O r ~ f'! ~
.. .
O O M ~ N ~ N " N ~N 00~ 00 1 ' !~r
" '~
r (rG C J n O
M oocVR N N M N ~N r ._. ~.jn rj r'!~ N
~ '
' " '- ~
O .... oo..~.--. ~ .~p ' ~ - ~sx ,
,.
~ fV~ ~ ~ v ~ n ~_ ~ N v '..
0
~ l ~ y,, _ V sr~ W r
O O O D 00 .r G h D V N
O
~ y~M R ~ ~D!y I~ M M yrG1 O ~ v't
~
~ lp~ l~ _ 00 y o0 N M Gy 0 ~ f~Oy
~ ~ ._' r,
,r,3 ' :-:~ %-~~ .:.~ ~; 3 ~ ~' r ~ v ~ O
'
R'M y 0 t vp R Y M ~D RM ~ N N v ~ ~~.. ~ ~
C 1
U ~ . ~ .L~D cC ~ ~ (~~O ~ ~ V1 R ~Dl ~O
"
'
~ X _.T r _. " v~ ~ ~ ~- N r .a.O r .....C?..,
V n ...
v ' _ ~ _..~ X ~ ~ ~ yrQ is ~ %~
1 N U O ~D '
CV..Z, r2" M ~ N ~ ~ Z ~i-v~ N ~ " C'l00 ~ , c te
. f " ~ C
N ~ N ~ ~ ,n ~ ~ ~ ",~,~ . . y ,~.~ .T...
r
[v~ I~~ v ~ ~ 00yr ~ O ~ -r
0
~ ~ v ~ v !
cln ~ ~ y r M~ ~'t! ~ ' '." ~ ' '
' ' " "
T 1 V _ . ' ~ n ~ ~t
' n ' M 00 ~ n ~"!. . r
R ~ ~.:~ ~ ~ ~!1r f~.~ ~ ..~.~ N ...3 ..r M .~'..
~ . ~~..r ,~.I .~rh ~ ~n ..~'..V1L ~ ~ '~U ~ V ~ W r ,%~T
.a,~ . n ~ X
L
R v .:. ~ O~' ~ ,..~ ~ X " " ~ N O r
,n
~
_ .. = O ~ _ M C'!M
. . '
"'%: ~ ~ ~ p o ~ v O = o i ~. " 0 0 ~
" . ~ .o .l~ , p o0
~ ~ ..
I~y M N I N N Q = =N t~~ N t~!,r1~ v
C r O .~.I~M l~~OR3-
V
v
L ~ ~ ' ~ 3 ...~n 3 n O 00n M ~ O v i
cC ~ ~
.. R ~ Cr1 pp= i~ n
..: = ~ ~ - ~ s~ : ~ o ~ 3 N s
3
_ . ~ .~
N O ~ X U V X 'u" '~w.r _ yr X wr
~'
V ~ ..O~M (~ U (~ ~ " ' r V ~
,. ~
Q Z ~ ,~ .~M v1M M ~ M wM V1v ~ C: ~ '~r ~ ,~M ~ .~..'.M
r _
~ ' ~ ~ , T ('~Ov1~~..vp~ v v1,~
I ~ ,!1 . ~~ r G~
~
~ 00~ l~G~M G1 '~L1 O G1Q O ~ ~ ~ C O
, ~ ~ ~ ~ ~ ~ ~ M N
C
~ - ~ C n C ~ C C O ~C,_ ~ ~ ~ p M p o0p N O '~~ ~ C
~
O O "",~ . ~ ~.~ ..~ ...O
O _ _ A
~
_
C C C ~ ~ ~ W
r ~ d ~ ~ W r W r r i
0 ~ ~ G ~ 0
w
V N V U - V = V ~ V ;=V 0 U '~U '
. , ~ - o U ~ c~i~
~ -
00,,ov,~o ~ co~ ,.03 ,.on ~.o:~0 3 , ~ ; 0 i o ~
o
. ~.or co0 0ov rA co ,.o
O ~ fuel t~1 ~ v1 ~O (~ 00 ! O ~ N M
1
n , 00 00 00 00
n
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
o
h ~
.'. h
R ~ v x 0 ..
_ _ i 00 cC N ~ v
_
yr N 00 t~ ~ O ~ i
'r
v~ 0~0 M ri ~ O ~
N, ~ ~ w.. N ~ 00 d. ~ ..r
M ~ C ~f r'1 V 00 ~ O
_ oo h h ~ X oo ~ vy
00 v.O i~ i-s ~_ 61
f'7 N R O "" c~3 ~ _ N ~ ~
i~~
=
~ z :
_ o :. ~ = - h c'~ ~ ~ :
''"i.'? M ~ " ~ o o '~'~ h R ~ V O
~!1 ... M M
~ N 00 '
~
1 ~ i-v00yr ~ ~ ~ . S h M
fr' p h vo ~
o,~
~ ~ v ~!
~
Ca= ~' ... C ~ ~? o O y N
M f'!N
~ N
C Q
x v0 ~ ~ ~T Q O N ~ ....st
.3 x = s ' c~i:
-
00~ ~ ~ .~ ~ ~ ~ - .-
~ t ~ N %-: ~ h o 3 3 "'
~ cC X ~ M ' r ~ L1 L S h
h ",y ~ ~ n ~ ,.", x iJ ~' N X
.. , ~O X
~
n ~"~a ~ ~ ~ V
c~0N ~ v .~ M O ~ ~ Y T c~ M
~ M '
x ~ O v v ' ~ ~~.. f'I
N 00 ~ C M M
O
~~~ O h M Q x ~ O C M V1 M
R
~ s N
p c' O ~ ~ ~ N ~ ~Q V1 N ....00~
x
h tt...M ., M . V1 . . i-~ ~Oy ~ ~
., ,r ., ~ fT
C' ~ ~
'
M O ~ ' a O '~ N ~' x ~ R M .r00
.jO ; O N M N V V v v'f~ h ' Qv
,
~ ~ ~ _ M n O 0 ~ ~O N ~ O G1
~ 00
~!100 v1M ~ ~ ~
_
~' b n ~ "~~ ~ t~j U1 M h! a ~
N R (V w r
~ O n %'~ :-~ ~ v1 N
~ c3N N ~ "' x Z ~ O .~~ M ~ Q
~ =
~ ~ ~i ~ ~ A W ~
M v C N ~ . ~ ~ ~ x n .
n N x : .~.
: '
- ~ ~ ~ ~ _ v _ '~3
~
:-; x N ~, ~ ~; _ M N o o -= , _..
~
' v :: ~b T :.: ' 3 ' " V
R N _ ~ _ ~? U . r.
n
O ,a N ~", = Z ~
N %'~ ~ " v ~ _.'X M ~ M x
_ ._ N h h N N ~ ~ ~ ~ ~ ~ ~'R ~ ~
n ~ n M O O x
x h ' N ~ N '~ ~y."'~., O
' ._' ' .n O
iv~ ~ N N ~ ~ ~ ~ OO
h ~?' G1 0 V y -"'
O _ ~ r ..~ ..r O
~ i1 '
r n M N ~.j ~ O't ~ ~, ~ ~!1, Q V1
, j
O ~ = a;'3~ ~ y ~ ~ ooa~~ M ~ R
~
.-. ~ n U O ~ ~ V 00 .~~~ '_'4~,~_ N1..
Y ..
V1~ M ~ r ~ ~ X ~OO .-..X ..... '~"w ~ fC.
..~ ~ N ~ ~ N ~ ~ V _ ~ x =
c "
a.i.;::" o w o _ o .o ~ V = -.v,
::N ~ x ~ M ~ ; ;-;~ N . N _ ~ ~-oo
_ M V x ~ w ~.r ~._ . ~. ~.:o M N G1u
O~N G1
- a0 ~O 1
h N '~3 ~ 00~ ~ ~
_ p . ,~ ~ ~ ~ N
~Dx ~ " 00p S -. 00.. ~.00~
p M
~.;. y M ~
'r
v ~ V C O ~ O ~ O
O j~ Qv ~ O~ 00 l'~ '~'
0 Q
.~~,~ ~ M ~ C'n ~ Q ~ x x O x O ~. p ~ ~ ~ ~ x
Q
O 'rM v W -~~ G1 ..N v1_
~ v O N ...~ p ~ h ~ ~ r ~ ' O~Oh ~ ~ =,~ p N
0
~ 0 3 ~ i
f~_ ~ O ~ ~ ~ 3 ~ h O ~ M ~ M ~ N ~ _
~r v _
Q
i~n y n ~ O ~ V 1.~ ~. ' X ~o h ~ V1~
O V
X O R O ~oO cvO ~ ~ ~;~ ;~M O ~e
U ~ U -'V V ~ N
=
- ~ W n y n v~ .i,v~x .r.._ x x cn_
C7- Q v, N U
~ N ~ .-. _
V ' ~ ~ ' ~ ~ ~ ~ N Q N V
~. c ~ r 0 0 Ca O U v v O
C o ',v1
M c0v0 c0M c0N cp N cph c0h c0 h c000V Q'c0N c0 V
op 00
oph
00 00 00 OO OO 00 C~ G1 C1 G1 G1 CT
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
66
I
-a~~ ~ >, ,
~
~
a
L7 C C ~, O G ~ .= = G
cCC) ~'c ~ 1 ~ ~ . ~' _ ' . L
S ~ .
;
.~. ~ .~ _~. s- _ _ G G I ~ O ~ G
~ a >, ~ ' ~ ~ ,\ '
C ~
O ~ ~ ~ ?,~ . ~ > C ~ T3
s '
~ , ~, v~
o c'cs~ " > ~ c ~
. ~ , _ _ = _
c , C. ~,~ ' '.' ;T~ ~ cc
_~ ?> >,, U
~ G C ~ C ~ C G
N C U v . >''
..
_
O 7, ~ " j ~ .~ cr U ~ ~.,V i
- "
G X .C yr ~ ~ G U O T ~
. ~ , N O O ~ '-~ C/J ~ ~,.~>, ~,c. V:
.
~ ~ ~ = G x
a C 0 >,~,' U N " ~C O N y
.'
vj ~ ~ ~ r ,.~~; ~ C -~ Q >,s..
U C
d ~ x U 'O U ~ U ~ U U U
~ , U " I ~ '~ C3 , U U I C
~ .. ~ _.-
~ V X ~ U U ~ ~ U G ~ U
.O_, O _ ~ ~
~
~ V7 'r U C _i_i~ ,
U ~ ~ b X . . .
c ' U r c3 b O 7 C O V7C/1O C
C '~
i. U 'p. cC~ O U ~ , _ C ~.~r~ C
~ U y~ O t
~ -
U U V .U~ ~'. cCcC O C ~ 'GO C, cr
U ~ ~ U L ~ G' U U 'i U
O U , X I C d- r G- ' r cs v
.
~ U
c k X .O~ N O x C ~. ~, ~ C
C
U U O O r..,c3. ~ .G_ ' C ~ >,>,~ _
~ y
C ~ ~..a.~ v Y _ _ c~CC C ~ t3. O O G' O
~
_ U V U CtV O C Y Q C.. N ~ .a
C
C ~ I d , ~ ~ ~ d'C C N ~ cpsc3"""
U C'
U . N :~ ~ V
M y C T . ~
~
E..., ~ ~ .~~ ~ C G G U ~ U N 'NU
w, ~ C"O O _ U N ~ .._~.~ y ~ C C
~
C1~ _ .~ C ~'. ~ C ~ ~' O O ~'
U
m ~ ~ , G. N .~ N
' > ~ ~ >' 00 C C -'
C C j.,, N _ ~ G _ ~ ,
C ~ ' : .C
.
G .~iw..:.C .~. ~ - U . C
G"d
"
C ~'~ C .~~ ~ : ~ :. '= C .Q ' ,
N .ZN Gl.,G~ ~ ~ a) j~ ~p ~ >,?.
G N N ~ ~' p,~ ~ ' ~ ~ ~ ~ E
z.
~+. >,~ , ~ ~ ~ (V~ U ~ a .C.C,ll.
N _ T ~ ; ~ 4 G1,G.~. ,
>
~n ~ ~,'~ ?,, ~ N , " _ ~ N N ~ it
~ ..
~ I
~' .~. . ~ >,,C >, ~., cn _ _'..~ C
C,1. .C ~ >,
.
U
C j'G '~ ~ ..~~. U N N I C ~ ~ ~''r _
N
. ~ ~ rl'N G ~ ,~,rC ~ N .....C~
G L
C., . ',r~, ~ , c ~~,y ~ ' a Gli ~ ~ ~
. 3 N .
, L,
C C N ~ .... a~ ~ ?,G r ~ ~ ~ C >, G
a ~ y C ~ . j ~ "O' ~ ~ pp 7,7.
C ~
y ~ . , , ~
N
_ _
CC1 C ~ . v ~~"~ ~ N .~ ,~'~'~ ~. ~ ! N
C '~ N
, ~ ~,NSy a y, , ~ ~ ~ cs . _ ~ ~,
a ' ~ ~,~,a3
c ' s ~ .c. ~ ~ " ~ ~, >,~ c x~ a~
. c
. j.G p" G n a _.. tY ~ ~ .~-- :.J"~cC >,
c... ~
, > ~ ~ ~ a'x ~ a~ > i i ~ c
~ ~ ~
U , ~. , _..__.~ ~ ~ ~ a , G a y =
G ~ G' G ~'
T7 G
, _ , _
c~N O o C~ ~ J O '= = ' . , N
~ O
O
~ c ~ , .~V ~ ; G y O
U M L ~ ~
c3 c3 "'
O
C
C 7,~ C~ O , f~~~"
r p ~ G I
- U C ca N ..M M~ ..~'U ~ ~,~~,X n r,~ ~
~ c.. a~ a~ ~ O : ~
a~ c
~ ~ ~ >, ~ ~.~, a~
U ~' ~ t .~ ~,
G
et ~ ~' .~. ~ ~. ~f' ~ ~' .r._.d'
. G ' .? .a G ~ C
r w . ~ ~ ~I ~ .~ ~ ~....~ .,...,.-.r,..,_,
,., I , , , O , ~ r- O . I , ei I
~ 1 , , m : .
>... C , ~
M N M M M M M M M M . G M M M M
c3 G cC CC M M G cr
G ,
V _U
G
N M ~ v1vp (~ op~ O N M ~ v'W p
x
w
CA 02351865 2001-05-18
WO 00/31037 67 PCT/EP99/09115
~ ,
_ _ _. _.
._, o -, .r ~ ~ ~ ~ c
O ~ X
~'
X Q C1. ~ Q. ~ ~O p ~ ~ G,
,
C ~ , .._. ~ ..
~
O T f='.. n i~ ca>, ~ ~G L~ ~ O
V C , = C/~ C!1 ;l ~ _ = v
O C V ~ ~ . a) ~
~ . ~
~
v e ~,~ , _O , c3
G O S 'C S ~ .. C G U ~
C V ~ T Q, C/~ V ~ ~ [/7
iw ~ " T U U O 'J ~ U G
,~ cCf fti ~
X U c~ ~ .'O -C . ._. N ~ y 'O
~..
~ /; ~ _ U
~
.71 ~ C h, ~ _ .
~ ~ 'r a3 rr
0 0 ~ c? v '=.c'~
' ~ ~ ~ '
V U U ~ c' c X x ' ~ V U C.k
3 C j
C3 U U U ~ ~ p
i ~ ~ r ~ j
~ ~ T _i.tr
~ U
0 j~ O ~ dj a V C '~ O O C/7
O V !
'
G C .a~ ?~ 7, ,.a .J w_r.
.
X X ,.. v
p O U d. ~. O O
i u., i C C ~ U U .~ Jp ~ , C~
U
U _ _ _ ~ .C C c. v d' tt c'._
? C r ~ C ~
, C"' C~. p U V V
C C V' U , , X ,~ C ~ ~l" ~ ~ j
. _ ' ~ ...
~
~ ~ .....
o ~ ~ c c '~ ~ c o x
C C N n~ a~ ~ C C C C ~ C O C
.C _ ~ _ _ " _
U
O.. C ,~ CL G. ~ >, O O ~ G' c~C?
,
i _. .. ~, N N V~= C C C , ~ U ~~.
_, ~ .... .....
G ~ ~"~ "'" ~ C d' O" C ~ ~
j ~ . ,
~ ~ C
c >, C ~ s -~?~ N >, ~ ~ ~ ~ >,
C =, o ~ > ..
~
. " >, a~ v C .r ~ : , c. a, o
.: ~ ~
.
p - E E C ' ~' y G N N C
, r=, , Q". .~ ~ y --m -, y
=,
N _ ~ N ~ lV
-C ~ ~ N C C ~,,~ ~ ~' ~ ~ ~ ~ v
U ,. ~ ':- C _, ._. .~ ~ j >,U
?, O
C
C .C G cCG ~ ~ ' ~ C C C ~ C G.
'
_ _ _
~ . ~ = !?'Q' ;' ~ ~
' N
>, - , 4 N .~, >,
r" ~ ~ ~ ~ . f . v ~
~ V N
_ ~ .. s.
' .. N
~
C _.' > 7, a i = O ~ p, .. C C O
C L f
~
?. _ C
N " E E _Ccs 'N ~N
'L1. cr
~ = ~ ~ '~' ~ es ~ ~
~ .
b ,,. ~ ~ r1 .T1 . .. _
. ?, ." C i y
J~
~ k ~ ~ N N C' O. '~-"
' '
G. L, O ~ ~ ~ p C C. G.
~ O
G ' .b .~ ~ ~ = ~
~
" ~ C C 3 TJ - .-..
~C C ~ C
~
' -C C. E ~ .. r
N
Gl ~ V ~ ~ _ _ ~ QJ C .
L1 L ~.. C.
i
O . ~ ~ Cr ~!1 .71 ~ T .~
" ~
l..n
X . ~ Or C., Q,'r i Q 0., iC k'
C n ~
..
, >, W . ~ ~ G '
~ C 'Q
'C Q ~ - N N C .._ ~ ~ ~ '~ ~
~ .~ '' C ~
,~
i, . N ~ ~ ~ ' ' M M ~ >, . M
~. 7 C M ~ ... C.C
.
~ p , cs X a~~ k .~ ~ ~ ... d ~
" C1a C~ ~ ~ ~-.
> ~ ~
C
~3 , ~ ~ O U , ~ ~ 7~ i c'
p ~ ~ S
~:. .:. ~ o ~ o Q ~ v
c =
,n ~ ~ ~. ~ ~ . .~ ~ .~ L1
; '~ = '
~
a ~ ~ ~ ~ .. ~ w ~ ~ ~ ~..
.C ~ C U p U d ~ ~
~
M M M M M M M N M N N M M _ N
N cC ~ U G. U N N c~C3 ~ ~ W M C.
I~ 00 G1 O -. N M Ct U1 ~O I~~ 00 G1 O -.
N fuel N N N N N N N ('V M M
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
68
r
~ r
.-,
~' ~ : > %
c ~ , ~ ~ .-.
.,
?, ~ C~ G
v v G ~ a
, > >-, >, ~ ~ .;
r r >, ~ ~ j x ~ X '
, >, .:. X C. r
X x , o >, ~ ~ , ~ ~
i-. .-. ~ ~ ~ O O ~ U X O ~ j, wr C/~
G ~ ~ ~ ~ ~, ~ O ,-
w r U ~ X , cC of ~'' v O ? U ~ '_~ ..O
~
'O .~, U ~ U V ~ ~ V , U _ U U
' U G1
U U ~ .~ _,~. ~ U ~ ?~ . ~ ~ r fCS
~ y O ' ~.
cC r r ?,~ ~ ~ ~ V ~ V V
r ~ _
U U . ~ C C
~ C . t_3 ~ v ~_ v_.rk
X k C U - O C ~ X ~
~ c . U . 't7 ~Q '~ .C O
S . V . O ~3 .r ~ O
, ~. '. -
_U ~ ~ ~' _. ~ U . r ~ ~ r ..
C
~ C G" U ' a V U
U j ~ X ~ U U U U '
_.~.~ ~ ~ C C ~' p U ~ A ~ U N
' ' O y,..U C C> C~ J ~ X k ,
/1 7G
_ ~ O
U J ,~G. C. _ V X .~ .~ O
.
U ~ N cal O ' O ~ cs r ~ C
U
C C ~ k c ~ ~ C ~ ~ V U U ~ 'C
C
'_" J C O U ~ ?, G, C V ~ Q' ~ ~ C"
v ~ . ~ . ' ' '
~, >, 'rC U k ~ ~ ~ O ~ ~ C C
' .... ...
C .~. ;G'~ , Q C C C C ~ O p p ~ U
.
U C.. y ~, " C ~ C C. O
'
c ~ ~C.~ _., C O .'. .
O G C c . r ~C N
N . U ~ C U ~ , C ~ . G
N ' N ' , C
C ~ C C G ~ ~
k s ~ ~W a ~~~
' ~ '
O . ~ C ~ ~. ~ C .c
ss. ~ ~ w
~, . a~ ~ ~ _~ a~
N a..N r _ C' a' e N ' ~
O L
. ~ N , C1. G. ~
~ C V T ~ a D. ~ ~~ 0. N N _
_ _ _ N
,
= ,
U > ~
~ ,
, ~ ~ ~ ~ ~ c c
. ~ .N
~ N _y . .. ~ U U N cC N
N N O ~ U O O r .C '. r- . e
cs C O ~ ~ N N ' ~ . ~ m C
C
cs . _ ~ U
. C ~ C N ~, N
C
0. O ~ . N , . ~, C _ >, >, G .
. ~ C3 C
_ .
Cl. C ~ R ~ iw~ ~ V ~ ?' _ ~ n
' '
. ~ _ _ ~ ~ ~ ; ~, ~ ~ 7,
~ ~ O ~
O N
N ~, ~, "~N ~ _ ~ C C "~ '~ ~ O
,~ ..O
V T ~ ~ ~ . '~ U U
~ ~ ~ ' ~ J~. ~ CJ ~.. ~ ~ ~C
CC ~.. G L.,' a,
'L7~'~~ O~ ~4 O Gl. a C.. C ~U O
's:. 'C7 '~ ' _C ~ O C.
O ' >_, ., ? ~ :p
O
a~ a~ C ~ C >' ~ ?~ ~ C L~ , O ' O
~ . ~ - O ~..'
~
. , . ~ ~ ~t ~ _ r-. O
p C
. . v >, O N ~CS "O rJ _c ~ C ~ c~i
d d ~ "
.~..
_, _, <v~ _. r.' ' r'
~ CL ? .,~ ~ ~ ~ .~ ~ ~ CC
~ U M M
C k
7, ~, ~ ~ ~ ~ N ~ C ~ r _
U - O CV ...
- _
.C ~~. Gi. . ~ ~ U ~ cr ,.C ~.,
~ y ~ ~C N U U a~ ~
.Q
a~ y ~.:~~ . O O ' "C ~ x ~ _
~:. C O ~ .~ ~
k ~ ~ ~ j.~ X T _ _ ~ ~ ~ U
~ U ~ N ~
~
N ~ , O O ' N >,
x ~
.~. n C G' U y N O j~
i~ ~ c3
_ ~'
~
o ~' c"C~ c' v ~i' ~ ~ d ~
~ ~ ~. ~ a. ~ -v
'.
. ~
M M , , ~/ ~ a .r ....~.r.r , a.y r ~ yr
C U N C y ~ O ~. ~ ~ r
. M M M M M M c1 M
c3 ~ ~ C1. ~ ~
M M M M ~ t~00 ~- (V M ~ ,!1 lp l~
M M M M
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
69
~
-u o -v ~ ~ '
C >, ,~.
U c
o w ' >, ~ o.
... ,~ ;~ C = ~ ~ = C G.
v ~, ~: ..-, r ~ ~ c~ y c ~ o
, . ~ X
>, >, ~,
~ v " ~ ~ '' ~
'
~. - Q c. x C
e a '~., ~ a~ ~ a~ o '
o
~ ~ G . _ x G _ V1 a~
. O ~ -
~'
c3 ~ r r ' ~ >,~ C y , >, v ,
J ~ G
U ~ U U z ,...C ~ ~ ~ ~ V ,~
C O O
_ X C C.
~
~ v ~, . G ~ .y U ~ .~
'1 J
>, ~ n i~ U ~ v V a
C3 y, ~ .t'r ._.,.C/:
C
cs r ~ ' ~ ~ ~ v ~t7
~ J U U N ~ ~ ~ _ ~ c3 x
C y '~?'.r ~ ~ O U
C ~, cf' ~' ~ ~ ~'.r ~ ~U 'r .
~ .. ~ . U
U
p , rN U ~ ~ ~ er c3 .p ~ U ~ C~
U - G
= .. _ 'C_ U . V ~j x ~ i~
-' L''. CI7 V p
o _~ o _..~cs r x ~ a
_.~.~ C = ~, >,~ x x ~ ~ .a ~ -c
G ~
.' ct 'C~ =, ~ U _ _ O G _ U s~
C~I ' " C ~ . >' -O .~ >, ~ cd ~ f5
U
, U U , i y c3X ~.. s-. x ~' U Q J
C U ~ j, ~ ~ v O V V O N ~' C ~.
1 V r
~ ' .~.
,~., n ~ ~ ~ 1 ~$ ~ ~ ~ C
' o ~
'
x a. ~ ~ -a ~. c ~ ~. c o >, o
"
C G ~ N N C ~ O y ~ C C ~
R ~ r-' O U U ~ C C C C
i N U , ~ ~h _ _ ? C
~ C :
. C ~, O r C , ', >,
N ~ ~ . a- x ~ c a~ ~' ~ ~
~
. ~ . _ ~ , c
' ~ C ~
cs _G~ C ~ ~ ~ ~ >, N
U i, p J, ~ y cLC~ ~ ~ N ~ N 0. C
a ~ U . .~ .: ~ ~ y
C. G ~ ' d.C S a. G. ~ .~~ ( ~ f
~1 ~1
. ~ ~ C , ' ti N '_'>' ~ ~
"' C ' "
, f'7 ~, ~
N C . N U U i i 4~ ~ . .~, ..
'
?. ~ ~ ~ ~ C ~ ~ .-..
~
C ~, ~~~ GL.~ ?, p N ~ ~ ~ ?, ~ ~ ~ N
c3 G~G1. . O G
C.
C
. ~ i
: 4 ~" ~ ~ '
N : N c ~ C E C C .. 7, v.. >,
3
N ~ ~ ..:.n N n n N C 00 L' 00
N
e ?, c O O C
C C c
>, ~ C C .~ ~ ~~N C C ~ ~ O. N
C
~ ~ TC C ~ ~ ~ C C' ~ ~
y " ~., U ,
cc .-~ ~ 0. C. . r ~ G ~n ?w n
~ cs ~
G :~ ~ N ~ ~ ~ ~ j G ~
, _ , , , i
~ ~ ~ ~' C - ' ~' ~ O C O
;C ~ ,=, _ - ~ a ?,. N ~ .~ N
G. p 'p ~' X C . .
cC c
'
-C ' ~ y ' C C G> C~ C_ LL N C..
C. O ~ ~T' ~ ~ ; ~ ~ G~ 7 n
~ "
~,
~ ~ ,~ v ~
' 4 C i ~ ~~ ~ ~ ' X ~ ~
N7
.. _ C , C x ~ .~...
~ v gy U . N
~ ~ ~
j.,x ~ '~~ - 7, ?,N ~ O O j~ s ~s a a
. ' p E . a a : ~
o c,
>,
, ~, U ~ _ ._ a, Vl . ,
J
N O L (w~ ~ .~, ' ~'~.~ ~ ~ ~' ~ ~1
~ ~ ,71 ~ ~ ~
_ C O ~ i1 '~O ~ ~ .. 'r M X ~_ 00
~ ' x U ~.. U 'O
~'
k '~-'j3 . ~ "" C/7 O .CM N N ~; ~ p ~ f~t
~ ~, ;: C .~ ~
c
t , k p ~ o ~ ~ ~, N ~ '~ _
~ : : O
~: U 'CT, N n ~ Q ~ ' y ~ '
" ~ ,. ~
~
O n a ~ L , ~ ~ ,~~ ~y a C?
~ ~ N ", i1 j~ v
U N
' yr v..~ ~ '. ~ ~ r' a ~ M
C O U V U G .~ .~ '
j
i _ ~ a r ~ ,~ ~r ~ ~../a a
~ ~ r~ ~ O
U M fr1 M M M ~ M M N M M N M M M V
L. U i C3 cS C3 U G
~
. M
c
00 C\ O -- fuel M '~'U1V' I~ 00 '\ O - N M
'~' V~V'1 '
U1 ~/ U1V1N V1 V1 V1 Vr ~O ~O V'
1
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
' ~, U
1 -. ~
7, ~ ~ 1 1 r '
X ~
G ~ ~ C X =
C
a~ E ~ O ~ O O v ~ ~ ~
~G s. ~ ~ ~ -C ~ C.
C O k X
>'1 G. y c~ G U ~ _:.
U
~ 7, ~ r , ' O .-. >, O ~ =.
~ ~ U 'fl
~J~ C 7w V ~ , .O Cl~
j ~ c; V ~ '
1 ..., _ C ~ J
C. c~ _ v .~ ~
1 .. ' j V'
'O U U ~ ? O O G ~ ~ ~ J J
J r '~ ; ~ ""'-C U ' C' ~ v C'
', ~
U ,U ~ _:r C c3 r ~ ~ U ,'C _' U
_ -
.
J ~ ~ U G, '~ U G" C ~ U C
. ....r . 1 .
>
>, ~ ~ ~, X ~ = .
~ . v ~ _~.
'
x o U _ ~ x ~ - o ~
r
cr >, V ~ ~ ~ C -O ~ c ,
v v x 1 ., ~ ' ~ x ~ r >,
U > O r.
U '(Y'd' 7 .~ i C U 1 , , ,r J ;J
, v ~"~' 1 f C ~ ~
V
V ' , , cC -' ~ ,_, N 1 ~ ;? -~- .-
U X
c o ~,.~~ c ~ ~ U a.
_ - ~ > c. 1 ,
,
= _ ~ ~ ~ ~ _ __ cn
, . f ~' .
V
O ~ cG N U O r=, O ~_
O r U :J O ~ -=. C N
._.. C ~ , _ . _
C ~ ~ G _r O L.
. ? -r
c c; ' o U , -. , c
'~ ' s ~'
' ~ ~ C ~ f~! C ~ ~ ' C N _7
'
C J 7G ~-. 1 ~ - ~ ~ _r
v O c O 7, C C '~ C
~ ~
C.7 ..C C ~ ~ N N ~' .C ~
C ~
C., ~ ' c3 y ~ ~ .~ L1, ?, ; ~ i
~ " .. C > ' ~'
C N d ; ~ C1. p ' C p , _-
C .
..C ~' " N C7. ~ ~ N (V
'
~ k C '~ ~ ~ ~ O cC ~ ~ L ~ t~
U '
.r >,~ C~ N ~ . 7, , j . N U ~ U
>, .~ ~ = G
.-~. _, ,r~ _ C .C ~ _ _ ~ G
O ~ G, O . C1. v ~ " a~ a.
.
C ~ N ~ C '~ ~ ~ G' C >'
1 1 C ' ~ ~ ~ ~~.
~ ' ~.
>, ~ ~ ~ 'U C, 7, !. G
1 O O ' C . 1 E ..
0o G. oo
C ~ ~_ >. C ~ C~ _C ~ C
U C
~
N R ~ U N _~ ~' C C N ... I '~ '
J
r-. s_'~ C Q. ~ n ~ O r_' 1 _
O C T
v'1 a,00 t~ O N ; V1 U C ~ U U
, p . O N G.
1
~
_.'.C ~ C . ~ . _
_ _ ' ~
o ~ ~' x ~: r ~ o ' .~ i
~
' ?'v ' " ~ ~ ' ' ~ ~ c' : ~
n c = ~
, _ ., . ; , , _~
~' ' _ '
C , n , C7 N C ~
., C ~ ~
>'
cc = >, c. r ~ ~ >, ~, ~ >,
~' . cs ~ .C
'v:., a ~ ' ~ o ~ o o Q, c.
_ : ~ y ~ .-,
' N
ce _ y ~ ,....c c ._. zs ._.
~-, a >, '
:
_
>, ~, ~ j~ j ~ 4 O .C ~ W ~ ~
Q ~ ~ ~ ~ >,
~ N
00 . C C :~ C _ ~ 1 C_ ~ v ~ N
.... ~ c3 N
.~.'
M ~.~, -p _ ~ i N ~ O V ' .O ~
~ i ~ ~
: '
~ O , O ~ .
y r O X '~ C ~ .
>. O
~ X F
' ~ C C N~ O ~ p~ > ~ >,: ' 'pa
~ X C
O ; , Y, , _ ~ ~ ...N 7,
O N' k .~ O e~ ~ -r C '
' ~ U a
~ ~ _ _ . 1 J U ~ ~_
O ~ C3. .~,.G X 'r L7 ~ - 'O R O U
C ~ O ' ~ M ? O '.
_
_'cC CS ,~ ,~ ~' OC3 '~ UU . , ~U x
C '~ ~?~ .r
:. ~ y~ 7 , , ~ ~ ~., ~ U O
~ , .-. ~, x >,
a
, ,-, ~ ~ : o ~ , s
~ a ~ Z ~
, r ~ . ' ~ ~ .;. ~
v , .
O
G ~ ~ n ,~, N ~' 1
0. ; ~ C, '~ 1 O 1 ' .~. et
a C'
'r ~ ~~.
~ ~ a ~ a a
~
M M M M M M M M U M ~ U
C. G C3 G C. G M
cC
, ~ M M M M M
U ~ C. c
C
~!1V' I~ 00 ~ ~
~D ~O~ ~D ~O ' ~ n
J n
CA 02351865 2001-05-18
WO 00/31037 PCT/EP99/09115
71
'
- ~
I
. U ' , _ ~.. ?,
i ~ ' ~ > x ~ ?~ >, U G
~~
_ , O . i.
~ .
~' N v ~ ~ ~' O ' C
~r
O ~ . j 'G ~ , ,- S ~, G c~
V' ~
'"' . ~ ~ O ~ , ~' ~ k , ~ ?, U
V7 7G ' ,~... ~,
k p >, , ~ ~ , T C r
c
~
7, 'O O ~ C : C , O C/~ G C J I
_ ~ ,. _ _ . _
U -' U ~, ~, ., N U ~ ~ ~',~'U ?,
'
cs ~, >, r... ~ C cs ?. >, G ., U
' 'O , .'.'
G U K U , N ~ " U _ _vI ~
~, ~
. O . >, ~ G ~ ..-. U >, _,..r,
: > 'n ~. ~ C W ' C ~ ~
, , C .
' x ~ n 1 U G C. N ~ U N wr ~ .
'
C'~I
O U ~ O ..C C '~ ~ ~ j ~ ~ '
' s L' n
~ I ~ N p, . ~ .. 'r ~ ~ . ~
' .
'
cr ~ .'~ ~ N ~ ~" G -~ O
;~ rU U ~ r=, ~ ~ C. ~ -O ~ U ~ '
-
~, vJ
cs C = ~ f~
y p U G ~' ~ r V V ~ ~, ~ U
,
_r, C_ _r~ y~ O ~,'' ~ ? er ~'O
O X ~ ~ ~ M _ , ~ . ~ ~ U
C X U
O ' ' C O ~ c'r >,
M ~ ~
..
~ ~ ~ _ ~ ~ U U ~ SG
U ~ .-. C O -'' _
- ~ U O '
C3 r J .=. O ~,d C!
' ~ ~ Q M ' >
U 1 ,~ y _. k ~ _.
~ C C
7, C ~ t ~ ,C C ~ G" ~ , ~ U
I .
~ ~ C ~, O C ~ asO .:V'
p p" ~ - G ' C C _ _ ~?_ ~ c~
. ~ ~
'
_ ' O O 7, ~ O .., C C
'L O ~ C ~ ~ '~ .....
' '
c N ~ C ' , O . C. _ _ , a, _
S . ' ~ G a~,
V ,. J ~ ~ ~ . ' ~ G _ O O
,
V,' ~ C ~' ~ ~ _ N _ N _C~ _ C
G1 >, C . .
.
N _ , ~. C ' O ~ O .r
y G'
C ~ N ~' p ~ X G d ~
... s ..C C j. O ~ ,~ ~ C..W '
1 ' 1
?, C 0. 7 , ~ a. ~ C
O .
' . . N , ~ .a .=~,N . .~~~=. r:"''
N N U ~ a U
:L
7
C3 I c3 ~ _ c ~ >, > ~ , >, , F
_ = , s .c '
. ~; ~ c ~ O , ~ ,~ >
E
C ~ C. ~, ~ ajcs~ ~ ~ p p ~ a~ ~ ,
U ~.. . r U G ~ ~ ~ ~
C.
~ . O ~,'
O O -a
>,
Q. 7 O ~ .C D C ~ ~ E '
G , ~ O , U C3
V
~ . ~ U A~ G ~ ~ I~ 00 C O .ny
j
' 7, i ~ ~-' j~ ? ~ ~ C~'CG
X N
>, O .: O ~ .. ~ ~ , ~ U .
~ ~ G
_ s.
rU r ~ O Q j~ N N ~ . _ '
N ' N G N ?~.
"
.! G , O I r, ,~ ~ r r G : A
. O
j, C ~ ~ j, ~ C. ~ ~ ~ ~ G _ a~:
U G
_' ~ ~ C_ X j~ ,.~ ~ ~ ~, ~, --~._. ..
N C ~ b
U
' , G, ~ O _~ ~ O - ' ~ >, ~,O
~ Q ~ '
> r= s-O ' O ~ 7, y ~ _ .r~ ~ O U N
~ ~ ' ~ cC 1 '
, C.7 ~ ~ r. , c
. ~ ..
:o ~ ~%. .~ >, N~ ~ 'o U c ~ ~=w o v
~, U ~ ~ N a _ ~ .
_.' " N C.
?, " ~ = 1 O
~ ~.., ' 'L7 U
~
G " o ~ ' ~ N ~~ ~ a~ a~ ~ >, -~
o ~ v ~
_ ~ _ ~ V . W J ~ '/
G =~ CJ C!1 U (I~ ~ ..~ ~ 1 "'J
C. ~ ,
~
_ ~ ~ 1 ~, C
~ '~~''x .y ~r ~' a ,~ y M ''~"'M G i1
~ ~. U , U ~
V ~
. , . a r r ,
, c' ' j ~ I C L'~ ~i ~ v
U ~ O 3 ~
1
~ ~ T
M N'C U V' '~t'et ~f.v~ ~
y U U C
'~ ..~r~0 v " "p ~.i.~ ~ ~ ~ ~ ~ ' N
-a ~ O U ?, v
'
_
'. y. ..~.r~ ".~ ~1' d' '.~..N .r .....~ ' ~ G
'r G U ' ~ ~ ~ ; G ~
'O ~
1 I I ~.. , a yr ~ ~ a V ~ ~~./ ....,
~'.. I U V L. ~ ~ ~. ~ p
~ ~.'
M M M M '~' M '~' N N L
. - c3 c U ~ ~ G .
S
. M M N M ~....~1
cs G ~ G
0~0 00 00 OMO ~ ~ ~ ~ ~ '~ S
'
~ O o O o O ~ C~ C G1
O O 0 O 0 O T
CA 02351865 2001-05-18
WO 00/31037 ~2 PCT/EP99/09115
°~ >,
:~ c
O
c~ G
C U
v t
.'.. d.
1
V
1
/1
O
U ~_
~U
U r
U
~ _
'r O
~_ ~
U c3
cC U
U
C
x
.°c
d1 >,
C_ C
U
~O
C 4
~~ N
1 X
O
U "~?
L1. .C
N l~
1
_~
_ _'
C C
.b "O
L ~.
G
'a. 'c
p ~
~r a
1 1
M r'1 c3
C' G~