Note: Descriptions are shown in the official language in which they were submitted.
CA 02352502 2001-07-06
r
Express Mail No. EL502556792 US
METHOD OF MANUFACTURE OF
ABSORBENT PARTICLES
1, Field of the Invention:
io This invention relates to processes for manufacturing moisture absorbent
materials. Specifically, this invention relates to the manufacture of a
clumping, odor
controlling cat litter.
2. Background of the Invention:
Moisture absorbent particles have many uses, both industrial and domestic. One
particularly large domestic use is in the pet products industry as cat litter.
Cat litter is a
broad term for waste and odor absorbent products useful for many different
types of
animals, however, cats being the most plentiful due to their popularity as
pets and their
ease in house-breaking. It is desirous to provide a cat litter material which
is capable of
absorbing urine and odors associated with animal waste.
2o Swelling and binding of the litter particles, commonly known as clumping,
is
particularly desirable in a cat litter product. This is because the clumped
mass including
the liquid waste contained therein, could be easily and integrally removed and
discarded.
Thus, the source of odor in the litter box is conveniently removed without the
necessity of
changing out the entire box. Initially, it was determined that attapulgite and
sepiolite
clay particles, upon absorbing moisture, swell and bind together as a mass.
These were
the initial clumping litter products.
CA 02352502 2001-07-06
2
Materials traditionally used as clumping cat litter due to their moisture
absorbent
characteristics include bentonite (montmorillonite) clays and attapulgite and
sepiolite.
Such bentonite clays include sodium montmorillonite, calcium montmorillonite,
potassium montmorillonite, lithium montmorillonite (hectorite) magnesium
montmorillonite .(saponite), or some combination of those clays. However, the
moisture
1o absorbing characteristics of these clays are not equal. Sodium bentonite
was found to
have good clumping properties and was less expensive than the other clays.
Accordingly, sodium bentonite is the product used in most cat litter products
from the
least expensive generic product to the more expensive premium brands.
Calcium montmorillonite, in comparison, typically has poor swelling and
binding (clumping) properties and is considered of less value. As a result,
calcium
montmorillonite is commonly used in non-clumping traditional and less
expensive,
economy cat litter products.
The industry found that sodium bentonite worked in this application as well
forming a better and harder clumping product. Patents assigned to American
Colloid
2o Company; RE33,983; 5,129,365; 5,317,990; 5,386,803; 5,503,111; 5,452,684;
and,
5,577,463 are examples. The result of this, however, was to create a new
market for
sodium bentonite for cat litter purposes, although there are still limitations
to the
clumping litters based on sodium bentonites. They help control odor by
assisting in the
removal of the odorous products but have no independent odor controlling
properties. A
need, therefore, exists for products with inherent odor control properties,
which possess
CA 02352502 2001-07-06
3
enhanced moisture absorbing (swelling) and clumping characteristics comparable
to
sodium bentonite clay and a lower bulk density.
Using present manufacturing processes, bentonite clay is selectively mined
from
large deposits using open pit mining methods. After mining the bentonite clay
is dried
then sized by crashing and screening. Sizes beriveen 3400 to 300 microns are
to considered acceptable for cat litter. A problem with sodium bentonite
clumping cat
litter products is their high weight/volume ratio typically 60-70 lbs/cuft. A
significant
amount of cat litter product is necessary to fill a litter box, and cat litter
boxes are
changed every one to two weeks, depending upon the number of cats, to avoid
odor
problems. As a result, it is necessary to purchase a sufficient volume of cat
litter in order
is to avoid frequent trips to the pet store or pet aisle of a grocery store.
Moreover, as with
most products, there are certain economics to be gained through the purchase
of larger
volumes. The problem is that the container for sufficient volume and economy
of litter
product is generally heavy, approximately 60 lbs. per cubic feet. Such weights
are
awkward and, in some cases, impossible for people to handle. A need,
therefore, exists
2o for an effective moisture swellable cat litter product with a reduced
weight/volume ratio
similar to conventional litters, typically 42-46 lbs/cuft.
CA 02352502 2001-07-06
SUM1VIARY OF THE INVENTION
The present invention is a process for manufacturing a moisture absorbent
particle
having usages such as for cat litter. In the process, calcium montmorillonite
clay is
intimately mixed with a solution of sodium carbonate. This mixing process
forces the
exchange of calcium canons in the montmorillonite with the sodium canons in
the
to sodium carbonate solution and the formation of an agglomerated particle.
It has been found that the cat litter product manufactured according to the
present
process includes inherent odor control characteristics not found in
traditional, naturally
occurring sodium bentonite clay. The product manufactured by the present
process
swells and clumps when subjected to animal liquid waste (urine) but also
absorbs (or
15 neutralizes) the odor of this waste. Moreover, the product absorbs odor
associated with
animal solid waste (feces) in the same manner.
As a result of the process of the present invention, non-swelling calcium
montmorillonite is converted to the sodium form becoming a swelling, clumping
bentonite with the additional benefit of having ammonia neutralizing
properties.
2o Accordingly, although manufacturing costs may arguably be slightly greater
than
processing the naturally occurring product, the value added to the raw
material are a
result of the process far exceeds any increase in cost of manufacture.
The manufactured agglomerated particle is very porous with internal air spaces
resulting in a final product which includes a lower weight/volume ratio than
the naturally
25 occurring mined product. The final product also has a lower weight than the
additive
weight of constituent components.
CA 02352502 2001-07-06
5 A lower weight/volume ratio is desirable for a variety of reasons: (1) less
weight
for the consumer to carry for the same volume; (?) less shipping costs from
manufacturer to retailer; and, (3) a higher cost per pound for the
manufacturer yet still
providing the retailer the ability to sell the same volume for the same price
as heavier
scoopable cat litter products. The weight/volume ratios of the manufactured
final product -
to is approximately 40 lbs./cubic feet, while the naturally occurring product
weight/volume
ratio is approximately 60 lbs./cubic feet.
As a result, more volume is obtained per pound. This allows for lighter
containers
containing the same volume as the natural occurring product. Alternatively,
for the same
weight, the customer receives a greater volume. This allows the product
manufactured by
1 s the process of the present invention the possibility of being sold for a
higher price per
pound.
An additional benefit of the process of the present invention is that almost
100%
of the raw material can be processed to produce a useable product. However,
with the
present process, the small particles (fines) can be reprocessed into a usable
size particle.
2o This means that all of the raw material is processed to a usable form
subject to de
minimus waste associated with manufacturing (lost through spills, dust,
residue, etc.).
Additional additives such as zeolites or activated carbon powder may also be
introduced in the process for enhanced odor control. It is also contemplated
that
bacteriostatic properties could be provided to the manufactured particle by
the addition of
25 a bacteriostat to the soda ash solution. Other additions are also
contemplated as needed
by a particular application.
CA 02352502 2001-07-06
It is thus an object of the present invention to chemically modify calcium
montmorillonite to make a clumping litter product.
An additional object of the present invention is to provide a process for
manufacturing a chemically modified calcium montmorillonite product that
possesses
inherent odor controlling properties.
1o A still further abject of the present invention is to manufacture a
moisture
absorbent, moisture swellable product.
Another additional object of the present invention is to manufacture a
moisture
absorbent sodium bentonite particle which has a lower weight/volume ratio than
naturally
occurring sodium bentonite.
15 A yet further object of the present invention is to provide a process for
manufacture of a moisture absorbent particle which is capable of utilizing all
of the raw
material.
A better understanding of the invention and its objects and advantages as well
as
further objects will become apparent to those skilled in this art from the
following
2o detailed description, taken in conjunction with the attached drawings,
whether is shown
and described only the preferred embodiment of the invention, simply by way of
illustration of the best mode contemplated for carrying out the invention. As
will be
realized, the invention is capable of modifications and various obvious
respects, all
without departing from the scope of the invention. Accordingly, the
description should
25 be regarded as illustrative in nature and not as restrictive.
CA 02352502 2001-07-06
7
BRIEF DESCRIPTION OF THE DRAWINGS
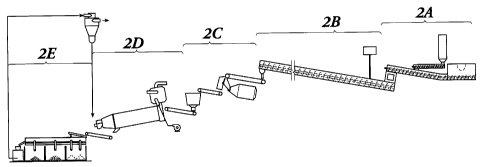
FIG. 1 is an overall process sketch of the method of manufacture of an
absorbent
particle of the present invention identified with its component substeps.
FIG. 2A depicts the reactant stream introduction substep ?A of FIG. 1.
FIG. 2B depicts the particle manufacture substep 2B of FIG. 1.
1o FIG. 2C shows the particle shaping substep 2C of FIG. 1.
FIG. 2D depicts the particle drying substep 2D of FIG. 1.
FIG. 2E shows the particle separation substep 2E of FIG. 1.
FIG. 3 is a view taken along line 3-3 of FIG. 2A.
FIG. 4 is a detail view of the double ribbon flighting of FIG. 2B.
FIG. 5 is a detail view of the cut and fold auger flighting of FIG. 2B.
FIG. 6 is a cross sectional view taken along line 6-6 of FIG. 4 showing the
cross
section of the double ribbon auger flighting of the present invention.
FIG. 7 is a cross section view taken along line 7-7 of FIG. 5 depicting the
cut and
fold auger flighting of the present invention.
2o FIG. 8 is a back view of the shaking conveyer detailing the apparatus for
reducing
large particles in size and reprocessing those particles through the shaking
conveyer.
CA 02352502 2001-07-06
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The process of the present invention can be seen, generally, in FIG. 1. 'Vith
reference to FIG. 1, the process can be broken down for the purpose of
illustration into
five main substeps: (1) reactant stream introduction (2A); (2) particle
manufacture
(2B); (3) particle shaping (2C); (4) particle drying (2D); and (5) particle
separation
(2E). This general description is for the purpose of illustration herein and
shall not be
considered limiting. According to this process, a useful clumping litter with
odor control
properties, and a lowered bulk density may be manufactured from calcium
montmorillonite using an ion exchange reaction. An incremental increase in the
product
to utility (and thereby value) is thus achieved.
Throughout this specification, the preferred equipment is referenced. It
should be
understood, however, that this equipment is provided to illustrate the best
mode known at
the time for carrying out the invention. The use of equivalent equipment or
equipment
such as extruders or pin mills that achieve the same end product should be
understood to
fall within the scope of this invention.
Reference will now be made to FIGS. 2A and 3 in the preferred process of
manufacturing an absorbent particle. In the process, calcium montmorillonite
clay in
powder form (approximately -10 mesh) is deposited and stored in a dump hopper
10. At
the bottom of the dump hopper 10, there is an auger 12 for conveying the
calcium
2o montmorillonite to the upstream end of a mix/auger 14. By varying the size
or speed of
rotation of the auger 12, the amount of clay processed into mix/auger 14 can
be
CA 02352502 2001-07-06
9
controlled. A predetermined amount of calcium montmorillonite in powder form
is
delivered by auger 12 into the mix/auger 14 to create a reactant stream.
The mix auger 14 functions to transport the reactant stream from the dump
hopper
to a reaction mix unit 16. In the event that additional additives to the clay
are desired,
5 such additives are introduced into mix auger 14 such as through bag silo 18
to mix with
the calcium montmorillonite (reactant stream) and thereby conveyed to the
reaction mix
unit 16.
In the preferred embodiment, a zeolite, preferably one with a high surface
area
and good odor control and sorption characteristics like chabazite is contained
within bag
10 silo 18 and discharged into the reactant stream by a metering conveyer 20.
The chabazite
particle size is preferably minus 60 mesh and is added in an amount so as to
provide an
end product that is 1-15% by weight.. Natural zeolites are hydrated
aluminumino silicate
minerals available commercially. Natural zeolites are frequently used in water
treatment, and sorption applications. In the present process, however, the
zeolite is
introduced to provide its odor elimination properties.
Another additive contemplated in the present process activated carbon is added
in
powder form of SO-150 micron particles also for the purpose of odor
control/elimination. The powder activated carbon becomes incorporated into the
litter
as the particles are manufactured according to the present process and
functions to absorb
gas molecules in the final product providing a superior odor adsorbing
product.
The zeolite and activated carbon are useful for drawing odors from the
surrounding air. The manufactured product of the present invention itself has
odor
CA 02352502 2001-07-06
control properties which eliminate the odor of the animal waste itself. The
odor control
additives thereby enhance the inherent odor control properties of the
manufactured
product.
Once the additives are introduced (if any) into mix auger 14, the reactant
stream is
conveyed by mix auger 14 and deposited into reaction mixer unit 16. It is
within reaction
mixer unit 16 that ion exchange takes place and the manufacture of the desired
particle.
Reference is next made to FIG. 2B for a discussion of reaction mixer unit 16.
Reaction mixer unit 16 is a 60' long reaction chamber in the preferred
embodiment. The
length of reaction mixer unit 16 is divided into two major phases. The first
phase
10 includes subjecting the reactant stream to a thorough mixin~~agitation
process, and the
second phase includes mixing/agitationishear of the particles comprising the
reactant
stream and also includes ion exchange. A resultant particle is agglomerated.
The length of the reaction mixer unit 16 is set at a 10 degree incline and
powered
by motor 17. The total 60' length is divided into auger flighting, each flight
being 12' in
length. The mixing/agitation/action phase within reaction mixer unit 16 is
accomplished
by a 12' double ribbon auger flighting 22. The shear/mixer/agitation/ion
exchange and
particle agglomeration phase is carried out by cut and fold auger flighting
24. In the
preferred embodiment, there are four (4) cut and fold auger flighting
segments, each 12'
in length. Reaction mixer unit 16 comprised of its two phases operates at a
speed of 60
rpm in order to convey a desired discharge of 19 cubic tons per hour weighing
approximately 45-50 lbs./cubic feet.
CA 02352502 2001-07-06
11
The solution for providing ion exchange is introduced into the reactant stream
in
reaction mixer unit 16 from a reservoir 26 through a conduit and sprayed onto
the
reactant stream (discussed further below). A soda ash (sodium carbonate)
solution is the
preferred solution for carrying out the ion exchange in the present process.
Soda ash is
readily available commercially and known for use in ion exchange in water
softening
systems. The soda ash solution is obtained by dissolving dry sodium carbonate
(Na,~,C03)
in water. A 15% solution is acceptable for the present purpose. It has been
found that
sodium carbonate ranges of 0.1-10% by weight of the final product dry are
acceptable in
the present process where 1.0- 2% is believed to be an optimal addition. It
has been
to found that the lower the sodium content in the final product, the better
odor control
properties it possesses. It is, however, preferred that enough sodium be
present in the
modified final product to provide the desired clumping properties.
If desired, a bacteriostat may be added to the soda ash solution to be
introduced
into the manufactured particle. By way of example, it has been determined that
a
bacteriostat manufactured by Stepan Company, Northbrook, Illinois, marked BTC-
1010
is suitable for this purpose. However, it is understood that other
bacteriostat solutions
from other manufacturers could be substituted. The desired concentration of
bacteriostat
in the soda ash solution is 1,200 ppm.
In the preferred embodiment, for the purpose of exemplification only, the
capacity
of soda ash reservoir 26 is 1,300 gallons. Accordingly, in order to obtain the
desired
concentration of bacteriostat, 1.56 gallons of BTC-1010 is added to the 1,300
gallon
charge of soda ash solution.
CA 02352502 2001-07-06
12
FIG. 4 is a detailed view of double ribbon auger flighting 22 of FIG. 2B. As
can
be seen, double ribbon auger flighting 22 includes an outer ribbon 28 and an
inner ribbon
30. The double ribbon auger flighting 22 acts to convey the reactant stream
while
providing thorough mixing and agitation of the particles conveyed
therethrough.
Agitation of the reactant stream causes heat which facilitates the cationic
exchange
occurring within the cut and fold auger segment.
Outer ribbon 28 includes a 2" wide blade with a 12" inner diameter, while
inner
ribbon 30 includes a 1" blade and 9" outer diameter in the preferred
embodiment. Such
double ribbon auger configurations are available commercially. Outer ribbon 26
and
to inner ribbon 28 are mounted on and supported from a ~" diameter central
pipe 32. The
soda ash solution for ion exchange is conveyed within conduit 34 above the
reactant
stream. A series of spray nozzles, collectively 36, direct a spray of soda ash
solution onto
the reactant stream as it is conveyed along double ribbon auger flighting 22.
The soda
ash is then mixed throughout the reactant stream by double ribbon auger
flighting 22.
FIG. 6 depicts double ribbon auger flighting 22 from a cross section showing
outer ribbon 28 and inner ribbon 30 supported from central pipe 32. FIG. 6
also shows a
second soda ash conduit 38 and representative spray nozzle 40 which is
positioned behind
and not shown in FIG. 4. The purpose of two sets of nozzles directed toward
both sides
of double ribbon auger flighting 22 along its length is to apply the soda ash
solution
evenly onto the reactant stream.
Referring back to FIG. 2B, cut and fold auger flighting 24 provides intimate
mixing, shearing, and agitation of the particles of the reactant stream
saturated with soda
CA 02352502 2001-07-06
13
ash solution to facilitate displacement of the calcium canons out of the
bentonite clay to
be replaced with sodium canons from the soda ash solution as a result of an
ion exchange
reaction.
FIG. 5 is a detail of cut and fold auger flighting 24 of FIG. 2B. Cut and fold
auger flighting is known commercially to provide intimate mixing, agitation,
and shear.
Cut and fold auger flighting 24 includes blade 38 on a 5" center shaft 40.
Screw blade 38
is 16" in diameter and is notched along its outer circumference. The reactant
stream
covers a 45% area of screw blade 38 such that 70°,% of the reactant
stream is conveyed
and 30% is dropped so as to be worked back into the reactant stream thereby
providing
the intimate mixing/agitation/shear described above. The mixing/agitation in
the
presence of the soda ash solution causes agglomeration of the powder particles
together
to form a manufactured particle.
Soda ash conduit 34 of FIG. 4 is in fluid communication with reservoir 26
(FIG.
?B) and extends through both double ribbon auger flighting 22 and cut and fold
auger
flighting 24. Spray nozzles 36 extend from soda ash conduit 34 so as to
introduce the
soda ash solution along the entire length of reaction mixer unit 16.
FIG. 7 depicts cut and fold auger flighting 24 in cross section. Notches 46 in
blade 42 allow material in the reactant stream to be dropped and folded back
into the
reactant stream. Soda ash conduit 38 and spray nozzles 40 aid in the even
application of
2o soda ash solution into the reactant stream.
As the reactant stream moves through reaction mixer unit 16, in particularly
cut
and fold auger flighting 24, and upon addition of the soda ash solution, the
powder of the
CA 02352502 2001-07-06
1~
cationic exchanged calcium montmorillonite agglomerates together to form a
porous
sodium bentonite particle. The addition of the soda ash solution, after ion
exchange,
causes the agglomerated particle to swell due to the hydrophilic
characteristic of sodium.
As a result of this process, a sodium bentonite agglomerated particle is
manufactured.
The water in the soda ash solution serves three purposes in the agglomeration
process: (1) the water puts the sodium carbonate in solution; (2) serves as a
transport
means to infuse the sodium into the calcium montmorillonite powder to
facilitate ion
exchange; and (3) serves an agglomeration and lubricity function thereby
cementing the
powder particles together. The agglomerated sodium bentonite particle now
1o manufactured is very porous containing internal air pockets.
Once the agglomerated particles are manufactured, the reactant stream is
conveyed for further processing. A belt conveyer 48 transports the reactant
stream to a
shaper/mixer 50. The shaper/mixer 50 aids in shaping the agglomerated
particles into
generally spheroidal agglomerations. In the preferred embodiment, shaper/mixer
50 is
15 comprised of a truck mixer, such as a standard cement mixer, mounted on a
skid and
capable of rotation by rollers 52. Once the reactant stream reaches the
shaper/mixer 50,
the process becomes a batch process in that a batch of agglomerated particles
is supplied
to shaperimixer 50 and then rolled therein.
Once the step of shaping/mixing is completed, the batch reactant stream is
2o transferred from shaper/mixer 50 through a transfer point 54 and supplied
to a conveyor
56 such that the shaped agglomerated particle may be conveyed to surge hopper
58.
Surge hopper 58 acts to shake the agglomerated particles thereby separating
adjacent
CA 02352502 2001-07-06
particles to form a granulated mixture of individual agglomerated particles.
Surge hopper
58 also acts to control the volume of granulated mixture processed through the
dryer as
shall be next described. As a result, the moisture content of the manufactured
particles is
controlled. The desired moisture content of the agglomerated particles before
drying is
5 approximately 2~-40 % by weight with approximately 34% believed to be
optimal.
From surge hopper 58, the granulated mixture is transferred via a conveyor 60
to a
dryer 62. Reference is next made to FIG. ZD. Dryer 62 is in a declined
orientation so as
to assist the flow of the granulated mixture along its length. Dryer 62 may be
a rotating
sand dryer, a fluid bed dryer, or a straight air dryer. The dryer 62
illustrated in the
10 drawings is a rotating dryer having a firing cone 64 at which the
temperature is
approximately 1600-1700 F. Dryer 62 is rotated by a plurality of rollers,
collectively 66.
The heated rotation of the particles in the dryer acts to remove moisture and
form the
agglomerated particle. It is in the heated, mixing action of the dryer that
most of the
particle shaping takes place.
15 The granulated mixture is conveyed through dryer 62 and has a residency
time of
approximately 8 minutes. The exit temperature of the granulated mixture is
approximately 250 F. The rolling and heating action of dryer 62 accomplishes
the
purpose of removing moisture from the manufactured particles comprising the
granulated
mixture. When the manufactured particle dries, it shrinks and cements
together. Also,
2o the zeolite and other additives are cemented within the particle.
Fresh air is input into dryer 62 through several air intakes 68. An air pump
70 is
ducted to a heat exchanger 72 to provide fresh air and evacuate dead air. The
dead air is
CA 02352502 2001-07-06
16
taken off through a heat exchanger/steam vent 74 which is exhausted into an
emissions
control device (not shown) such as a cyclone for the recovery of aerosolized
materials.
Thus, all emissions, including vaporized water and any aerosolized small
powder (dust)
are recovered. Heat exchanger 72 is also ducted to fresh air ducts via conduit
76. A dried
granule mixture 78 is thus output from dryer 62 and deposited on a conveyer
80.
Reference is next made to FIG. 2E wherein the dry granule mixture 78 is
conveyed by conveyer 80 onto a shaking conveyor 82. The shaking conveyor 82
functions as a separator for receiving the dried particles of the granulized
mixture 78 for
separation based upon particle size to form piles of segregated product
particles. The dry
1o granule mixture is passed over screens of various mesh sizes to segregate
the product
which is then dispensed into piles through chutes 84, 86, 88, and 90.
Particles larger than one-half inch are conveyed from shaking conveyer 82
through chute 90 and deposited into a roll mill 92. Roll mills such as roll
mill 92 are
available commercially. Roll mill 92 mechanically reduces the size of the
manufactured
15 particle which exits chute 90 which is then evacuated to a cyclone
separator and is
deposited on conveyer 80 for reprocessing through shaking conveyer 82.
Reference is made to FIG. 8 which shows shaking conveyer 82 from a back view.
Oversized particles exit through chute 90 and into roll mill 92 and are
mechanically
reduced in particle size therein. Once reduced, the particles are conveyed via
duct 94 by a
20 vacuum created by cyclone separator 96 which follows the schematic dotted
path shown
on FIG. 8. From the duct 94 the particles are received into the suction inlet
98 of cyclone
separator 96 wherein the particles are rotated within cyclone separator 96 and
fall out
CA 02352502 2001-07-06
17
through exit 100 onto conveyer 80. Air is exhausted through exhaust 102.
Exhaust 102
may contain particle dust and is therefore ducted to the emission recovery
system.
The particles which are mechanically reduced in size by roll mill 92 and
processed through cyclone separator 96 and deposited on conveyer 80 are then
processed
through shaking conveyer 82 in the manner described above.
Referring back to FIG. 2E, the particles exiting through chutes 84 pass
through a
20 mesh sieve screen and are collected in a first bin 104. These particles are
usually
considered too fine and are simply reprocessed as described above and thereby
remanufactured into a useable particle size. Thus, substantially all raw
material becomes
to useful.
The remaining particles are preferably divided into t~~o or more groups. The
screen sizes utilized to divide these particles may vary, but are generally
between 40
mesh up to 6 mesh. As an example, particles passing through a 12 mesh sieve
screen
may be exited through chute 88 into a bin 106. Larger particles may be passed
through
an 8 mesh sieve screen and exited though chute 86 into a bin 108. According to
this
example, particles which are too large to pass through the 8 mesh sieve screen
are
processed through chute 90 into roll mill 92 as described above.
The manufactured particles according to the present process are useful and
suitable for use as a waste absorbent product such as cat litter. Thus a
highly useful
2o product is obtained.
While the invention has been described with a certain degree of particularity,
it is
manifest that many changes may be made in the details of construction without
departing
CA 02352502 2001-07-06
I8
from the spirit and scope of this disclosure. It is understood that the
invention is not
limited to the embodiment set forth herein for purposes of exemplification,
but is to be
limited only by the scope of the attached claim or claims, including the full
range of
equivalency to which each element thereof is entitled.