Note: Descriptions are shown in the official language in which they were submitted.
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
PRODUCTION OF LOW SULFUR/LOW AROMATICS DISTILLATES
BACKGROUND OF THE DISCI.O 1L .RF.
FIELD OF THE INVENTION
The present invention relates to a process for hydroprocessing a
hydrotreated liquid distillate stream to produce a stream exceptionally low in
sulfur as well as aromatics. A hydrotreated distillate stream is further
hydrotreated in a co-current reaction zone wherein the reaction product is
passed
to a separation drum wherein a vapor product is collected overhead and a
liquid
product is passed to an aromatics saturation zone countercurrent to the flow
of
hydrogen treat gas.
BACKGRQUND OF THE INVENTION
Environmental and regulatory initiatives are requiring ever lower
levels of both sulfur and aromatics in distillate fuels. For example, proposed
sulfur limits for distillate fuels marketed in the European Union for the year
2005
is 50 wppm or less. There are also regulations that will require lower levels
of
total aromatics in hydrocarbons and, more specifically, the multiring
aromatics
found in distillate fuels and heavier hydrocarbon products. Further, the
maximum allowable aromatics level for U.S. on-road diesel, CARB reference
diesel and Swedish Class I diesel are 35, 10 and 5 vol.%, respectively.
Further,
the CARB and Swedish Class I diesel fuels allow no more than 1.4 and 0.02
vol.% polyaromatics, respectively.
Much work is presently being done in hydrotreating because of greater
demands for the removal of heteroatoms, most notably sulfur, from
transportation and heating fuel streams. Hydrotreating, or in the case of
sulfur
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
-2-
removal. hydrodesulfurization, is well known in the art and usually requires
treating the petroleum streams with hydrogen in the presence of a supported
catalyst at hydrotreating conditions. The catalyst is typically comprised ot'a
Group
VI metal with one or more Group VIII metals as promoters on a refractory
support.
Hydrotreating catalysts which are particularly suitable for
hydrodesulfurization and
hydrodenitrogenation generally contain molybdenum or tungsten on alumina
promoted with a metal such as cobalt, nickel, iron, or a combination thereot:
Cobalt promoted molybdenum on alumina, and nickel promoted molybdenum on
alumina catalysts are most widely used when the limiting specifications are
hydrodesulfurization, while nickel promoted molybdenum on alumina catalysts
are
the most widely used for hydrodenitrogenation and partial aromatic saturation.
Much work is being done to develop more active catalysts and
improved reaction vessel designs in order to meet the demand for more
effective
hydroprocessing processes. Various improved hardware configurations have
been suggested. One such configuration is a countercurrent design wherein the
feedstock flows downward through successive catalyst beds counter to
upflowing treat gas, which is typically a hydrogen containing treat-gas. The
downstream catalyst beds, relative to the flow of feed can contain high
performance, but otherwise more sulfur sensitive catalysts because the
upflowing
treat gas carries away heteroatom components such as H2S and NH3 that are
deleterious to the sulfur and nitrogen sensitive catalysts. While such
countercurrent reactors have commercial potential, they never-the-less are
susceptible to flooding. That is, where upflowing treat gas and gaseous
products
impede the downward flow of feed. Such flooding tendency is increased with
increases in treat gas rate.
Other process configurations include the use of multiple reaction
stages, either in a single reaction vessel, or in separate reaction vessels.
More
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
-3-
sulfur sensitive catalysts can be used in downstream stages as the level of
heteroatom components becomes successively lower. European Patent
Application 93200165.4 teaches a two-stage hydrotreating process performed in
a single reaction vessel, but there is no suggestion of a unique stripping
arrangement for the liquid reaction stream from each reaction stage.
Two types of process schemes are commonly employed to achieve
substantial hydrodesulfurization (HDS)/ aromatics saturation (ASAT) of
distillate fuels and both are operated at relatively high pressures. One is a
single
stage process using Ni/Mo or Ni/W sulfide catalysts operating at pressures in
excess of 800 psig. To achieve high levels of saturation pressures in excess
of
2,000 psig are required. The other is a two stage process in which the feed is
first processed over Co/Mo, Ni/Mo or Ni/W sulfide catalyst at moderate
pressure
to reduce heteroatom levels while little aromatics saturation is observed.
After
the first stage the product is stripped to remove H2S, NH3 and light
hydrocarbons. The first stage product is then reacted over a Group VIII metal
hydrogenation catalyst at elevated pressure to achieve aromatics saturation.
The
two stage processes are typically operated between 600 and 1,500 psig.
In light of the above, there is a need for improved
desulfurization/aromatic saturation process for treating feedstreams so that
they
can meet the ever stricter environmental regulations.
SUMMARY OF THE INVENTION
In accordance with the present invention there is provided a two
stage process for hydroprocessing a hydrotreated distillate feedstock which
process comprises:
a) reacting said feedstock in a first reaction stage in the presence of a
hydrogen-containing treat gas cascaded from, or partially cascaded from, the
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
-4-
second stage herein, said first reaction stage containing one or more reaction
zones operated at hydrodesulfurization conditions wherein each reaction zone
contains a bed of hydrotreating catalyst;
b) passing the resulting reactant to a separation zone wherein a vapor
phase stream and a liquid phase stream are produced;
c) collecting said vapor phase stream overhead; and
d) passing said liquid phase stream to a second reaction stage in the
presence of a hydrogen-containing treat gas, said reaction stage containing
one or
more reaction zones operated at aromatics saturation conditions wherein each
reaction zone contains a bed of aromatics saturation catalyst, and wherein
said
hydrogen-containing treat gas is passed through said reaction stage
countercurrent to the flow of said liquid phase stream.
In a prefered embodiment of the present invention, the liquid phase
stream, before it passes through said second reaction stage is contacted with
a
vapor to strip dissolved gases from the liquid phase.
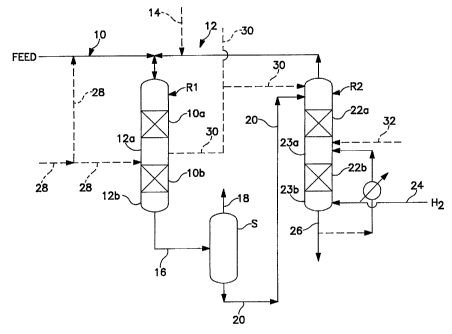
BRIEF DESCRIPTION OF THE FIGURE
The figure hereof shows multiple reaction vessels of the present
invention showing separation of the liquid phase product from the vapor phase
product and further processing of the liquid phase product stream in an
aromatics
saturation stage.
DETAILED DESCRIPTION OF THE INVENTION
Feedstocks suitable for being treated by the present invention are
those petroleum based feedstocks boiling in the distillate range and above and
which have previously been hydrotreated to reduce the sulfur and nitrogen
levels.
Typical sulfur levels in such hydrotreated distillates are in the range of
less than
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
-5-
about 3,000 wppm, more preferably to less than about 1,000 wppm, most
preferably to less than about 500 wppm sulfur, ideally to less than about 350
wppm. Non-limiting examples of such feeds include diesel fuels, jet fuels,
heating oils, and lubes. Such feeds typically have a boiling range from about
150
to about 600 C, preferably from about 175 to about 400 C. It is highly
desirable
for the refiner to upgrade these types of feedstocks by removing as much of
the
sulfur as possible, as well as to saturate aromatic compounds.
The process of the present invention can be better understood by a
description of a preferred embodiment illustrated by Figure 1 hereof. The
current invention offers an improvement over the prior art by using only once
through hydrogen treat gas. For purposes of discussion, the first reaction
stage
RI is a hydrotreating stage to further reduce the level of sulfur and
nitrogen, and
the second reaction stage R2 is an aromatics saturation stage. The hydrogen
reacts with the impurities to convert them to H2S, NHJ, and water vapor, which
are removed as part of the vapor effluent, and it also saturates olefins and
aromatics. Miscellaneous reaction vessel internals, valves, pumps,
thermocouples, and heat transfer devices etc. are not shown for simplicity.
Figure 1 shows reaction vessel Rlwhich contains reaction zonesl0a and 1Ob,
each of which is comprised of a bed of hydrotreating catalyst, although only a
single or more than two reaction zones can be employed. It is preferred that
the
catalyst be in the reactor as a fixed bed, although other types of catalyst
arrangements can be used, such as slurry or ebullating beds. Downstream of
each reaction zone is a non-reaction zone 12 a and 12b. The non-reaction zone
is
typically void of catalyst, that is, it will be an empty section in the vessel
with
respect to catalyst. Although not shown, there may also be provided a liquid
distribution means upstream of each reaction stage. The type of liquid
distribution means is believed not to limit the practice of the present
invention,
but a tray arrangement is preferred, such as sieve trays, bubble cap trays, or
trays
CA 02352887 2001-05-29
WO 00134416 PCT/US99128790
-6-
with spray nozzles, chimneys, tubes, etc. A vapor-liquid mixing device (not
shown) can also be employed in non-reaction zone 12a for the purpose of
introducing a quench fluid (liquid or vapor) for temperature control.
The feedstream is fed to reaction vessel R1 via line 10 along with a
hydrogen-containing treat gas via line 12. The hydrogen-containing treat gas
is
cascaded from reaction stage R2. Make up hydrogen-containing treat gas can
also be added via line 14. It is preferred that the rate of intoduction of
treat gas
be less than or equal to 3 times the chemical hydrogen consumption of the
reactions in both stages, more preferably less than about 2 times, and most
preferably less than about 1.5 times. The feedstream and hydrogen-containing
treat gas pass, cocurrently, through the one or more reaction zones of
reaction
vessel R1, which represents the first reaction stage wherein the feedstream is
further hydrotreated to remove substantially all of the heteroatoms from the
feedstream. It is preferred that the first reaction stage contain a Co-Mo, or
Ni-
Mo, on refractory support catalyst, and a downstream reaction stage contain a
Ni-Mo on refractory support catalyst.
The term "hydrotreating" as used herein refers to processes
wherein a hydrogen-containing treat gas is used in the presence of a suitable
catalyst which is primarily active for the removal of heteroatoms, such as
sulfur,
and nitrogen, and for some hydrogenation of aromatics. Suitable hydrotreating
catalysts for use in the present invention are any conventional hydrotreating
catalyst and includes those which are comprised of at least one Group VIII
metal,
preferably Fe, Co and Ni, more preferably Co and/or Ni, and most preferably
Co;
and at least one Group VI metal, preferably Mo and W, more preferably Mo, on
a high surface area support material, preferably alumina. Other suitable
hydrotreating catalyst supports include zeolites, amorphous silica-alumina,
and
titania-alumina Noble metal catalysts can also be employed, preferably when
CA 02352887 2004-12-03
-7-
the noble metal is selected from Pd and Pt. It is within the scope of the
present
invention that more than one type of hydrotreating catalyst he used in the
same
reaction vessel. The Group VII1 metal is typically present in an amount
ranging
from about 2 to 20 wt.%, preferably from about 4 to 12%. The Group Vl metal
will typically be present in an amount ranging from about 5 to 50 wt.%,
preferably from about 10 to 40 wt.%, and more preferably from about 20 to 30
wt.%. All metals weight percents are on support. By "on support" we niean that
the percents are based on the weight of the support. For example. if the
support
were to weigh 100 g. then 20 wt.% Group VIII metal would mean that 20 g. of
Group VIII metal was on the support. Typical hydrotreating temperatures range
from about 100 C to about 400 C with pressures from about 50 psig to about
3,000 psig, preferably from about 50 psig to about 2,500 psig.
A combined liquid phase and vapor phase product stream exit
reaction vessel R1 via line 16 and into separation zone S wherein a liquid
phase
product stream is separated from a vapor phase product stream. The liquid
phase
product stream will typically be one that has components boiling in the range
from about 150 C to about 650 C, but will not have a boiling range greater
than
the feedstream. The vapor phase product stream is collected overhead via line
18.
The liquid reaction product from separation zone S is passed to
reaction vessel R2 via line 20 and is passed downwardly through the reaction
zones 22a and 22b of reaction stage R2. Prior to being passed downwardly
through reaction stage R2, said liquid reaction product stream can first be
contacted in a stripping zone to remove entrapped vapor components from the
liquid stream. For example, as the liquid product stream tiows through the
stripping zone, it is contacted by uptlowing hydrogen-containing treat gas
under
conditions effective for transferring at least a portion of the feed
impurities in the
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
-8-
vapor into the liquid. The contacting means comprises any known vapor- liquid
contacting means, such as rashig rings, berl saddles, wire mesh, ribbon. open
honeycomb, gas-liquid contacting trays, such as bubble cap trays and other
devices, etc.
Fresh hydrogen-containing treat gas is introduced into reaction
stage R2 via line 24 and is is passed in an upward direction counter to the
flow of
liquid reaction product. The introduction of clean treat gas (gas
substantially free
of H2S and NH3) allows reaction stage R2 to be operated more efficiently owing
to a reduction in the activity suppression effects on the catalyst exerted by
H,S
and NH3 and an increase in H2 partial pressure. This type of two stage
operation
is particularly attractive for very deep removal of sulfur and nitrogen or
when a
more sensitive catalvst (i.e., hydrocracking, aromatic saturation. etc) is
used in
the second reactor. Another advantage of the present invention is that the
treat
gas rate is relatively low compared with more conventional processes. The use
of
relatively low treat gas rates is primarily due to the use of previously
hydrotreated distillate feedstocks. Further efficiencies are gained by not
requiring recycle of treat gas.
The liquid/vapor separation step (S) may be a simple flash or may
involve the addition of stripping steam or gas to improve the removal of H,S
and
NH3. The liquid stream and treat gas are passed countercurrent to each other
through one or more catalyst beds, or reaction zones, 22a and 22b. The
reulting
liquid product stream exits reaction stage R2 via line 26, and a hydrogen-
containing vapor product stream exits reaction stage R2 and is cascaded to
reaction stage Rl. Reaction stage R2 also contains non reaction zones 23a and
23b following each reaction zones. The catalyst in this second reaction stage
is
an aromatic saturation catalyst.
CA 02352887 2009-07-17
-9-
"1'he figure also shows several options. For example. lines 30 and
32 can carry kerosene which can be used as a quench fluid. Also. a unsaturated
feedstock can also be introduced into the first reaction staa.e via line 28.
The
deeree of unsaturation can be up to about 50 wt. ' .
The reaction stages used in the practice ot'the present invention are
operated at suitable temperatures and pressures for the desired reaction. For
example, typical hydroprocessing temperatures will range from about 40 C to
about 450 C at pressures from about 50 psig to about 3.000 psig, preferably 50
to 2,500 psig.
For purposes of hvdroprocessing and in the context of the
invention. the terms "hvdrogen" and "hydrogen-containing treat gas" are
synonymous and may be either pure hydrogen or a hydrogen-containin2 treat gas
which is a treat gas stream containing hydrogen in an amount at least
sufficient
for the intended reaction, plus other gas or gasses (e.g., nitrogen and light
hydrocarbons such as methane) which will not adversely interfere with or
affect
either the reactions or the products. Impurities, such as H,7S and NH3 are
undesirable and, if present in significant amounts. will normally be removed
from the treat gas. before it is fed into the reactor. The treat gas stream
introduced into a reaction stage will preferably contain at least about 50
vol. %.
more preferably at least about 75 vol. % hydrogen, and most preterablv at
least
95 vol. %. in operations in which unreacted hydrogen in the vapor effluent of
any particular stage is used for hydroprocessing in any stage, there must be
sufficient hvdrogen present in the fresh treat gas introduced into that stage,
for
the vapor effluent of that stage to contain sufficient hydrogen for the
subsequent
stage or stages. It is preferred in the practice of the invention, that all or
a
portion of the hvdroQen required for the first stage hvdroprocessing be
contained
in the second stage vapor effluent fed up into the first stage. The first
stage
CA 02352887 2001-05-29
WO 00/34416 PCT/US99/28790
-10-
vapor effluent will be cooled to condense and recover the hvdrotreated and
relatively ciean, heavier (e.g., C4-C5+ ) hydrocarbons.
Non-limiting examples of aromatic hydrogenation catalysts include
nickel, cobalt-molybdenum, nickel-molybdenum, and nickel-tungsten. Noble
metal containing catalysts can also be used. Non-limiting exatnples of noble
metal catalysts include those based on platinum and/or palladium, which is
preferably supported on a suitable support material, typically a refractory
oxide
material such as alumina, silica, alumina-silica, kieselguhr, diatomaceous
earth,
magnesia, and zirconia. Zeolitic supports can also be used. Such catalysts are
typically susceptible to sulfur and nitrogen inhibition or poisoning. The
aromatic
saturation stage is preferably operated at a temperature from about 40 C to
about
400 C, more preferably from about 200 C to about 350 C, at a pressure from
about 100 psig to about 3,000 psig, preferably from about 200 psig to about
1,200 psig, and at a liquid hourly space velocity (LHSV) of from about 0.3
V/V/Hr. to about 10 V/V/Hr, preferably from about 1 to 5 V/V/Hr.
The liquid phase in the reaction vessels used in the present
invention will typically consist primarily of the higher boiling point
components
of the feed. The vapor phase will typically be a mixture of hydrogen-
containing
treat gas, heteroatom impurities like H,S and NH3, and vaporized lower-boiling
components in the fresh feed, as well as light products of hydroprocessing
reactions. If the vapor phase effluent still requires further hydroprocessing,
it
can be passed to a vapor phase reaction stage containing additional
hydroprocessing catalyst and subjected to suitable hydroprocessing conditions
for further reaction. Alternatively, the hydrocarbons in the vapor phase
products
can be condensed via cooling of the vapors, with the resulting condensate
liquid
being recycled to either of the reaction stages, if necessary. It is also
within the
scope of the present invention that a feedstock which alreadv contains
adequately
CA 02352887 2004-12-03
-11-
low levels ot'heteroatoms be fed directlv into the reaction stage for aromatic
saturation and/or crackint;
The present invention can be better understood by ret'erence to the
foilowing example that is present lor illustrative purposes only and is not to
be
taken as limiting the invention in any way.