Note: Descriptions are shown in the official language in which they were submitted.
F 7 5 51 cp 1 ( V ) CA 02354475 2001-08-O1
1
BLENDS OF ISOFLAVONES AND FLAVONES
Isoflavones are known as health components that can be
applied to prevent or treat many health deficiencies or to
achieve certain health effects not directly related with a
health deficiency. E.g. these compounds are known to
achieve benefits in the women's health area in particular
for postmenopausal women. These effects are disclosed in
e.g. US 5 498 631; WO 98/ 56373; WO 98/08503; US 5 733 926;
US 5 952 374 and many other references. Health effects that
are also attributed to isoflavones include skin effects and
anti-inflammatory effects.
Although for a few of these effects some experimental
support can be found in the literature the majority of the
pretended effects are mere statements in the prior art
without any experimental support. We found on basis of a
number of tests specifically developed in order to find
experimental support to confirm the pretended effects that
indeed some of the pretended effects exist however only to
a low or medium extend.
Although WO 00/07607 discloses that a cancer-protective and
cancer therapeutic composition is obtained by combining
1) a plant extract with antioxidant effect with
2) a neovascular regulator that inhibits angiogenesis and
3) with absorbable zinc
whereas in the text the possibility of synergy between one
or more of the components is suggested, there is no clear
teaching that a synergy could be achieved by combining the
components from which we found that they gave a synergy
with respect to anti-inflammatory effects or with respect
to skin benefits in particular to antiageing effects. In
fact the preferred antioxidants are in this WO'607
F 7 551 cpl ( V ) CA 02354475 2001-08-O1
2
bioflavanoids such as proanthocyanidins. The neovascular
regulator can be genistein, daidzein or a soy isolate.
We therefore studied whether we could find ways wherein the
existing effects in particular with respect to anti-
inflammatory and to antiageing of the skin of the
isoflavones could be enhanced. As a result of this study we
found that this can be done in a synergistic way by
combining the effects from a number of selected isoflavones
with the effect of a specific flavone. In fact we found
that by combining one or more of the isoflavones selected
from the group consisting of genistein, daidzein and
glycetin with quercetin (= a specific flavone) synergistic
effects could be achieved that were far higher than could
be expected on basis of the components present in the
combination. Therefore our invention concerns in the first
instance a blend of a synergistic mix of a natural flavone
and natural isoflavones, wherein the flavone is quercetin
and the isoflavones are selected from at least one of the
isoflavones from the group consisting of genestein,
daidzein and glycetin either in the glucon or in the
aglucon form.
In these blends the weight ratios quercetin to isoflavone
can vary over a wide range, however we found that the best
results were obtained when weight ratios of 1:50 to 50:1,
preferably ratios of 1:20 to 20:1, more preferably ratios
of 1:6 to 6:1 and even more preferably from 1:4 to 4:1 and
most preferably from 1:2 to 2:1, calculated as aglucon,
were applied. Even better results were obtained by using
weight ratios of 1:2 to 1:1.
The most preferred isoflavones in these compositions are
genestein and daidzein (or as glucons the genistin and
daidzin). Although these components can be applied in a
F 7 5 51 Cpl ( V ) CA 02354475 2001-08-O1
3
wide range of ratios the best results were obtained, when
the genistein and daidzein were used in weight ratios of
2:1 to 1:2.
The isoflavones and the quercetin that can be applied
according to the invention are suitably derived from
natural sources. Quercetin e.g. is present in onions,
garlic and tomatoes and concentrates wherein this component
is present in relatively high levels can be obtained from
these sources. Very suitable sources for the isoflavones
are soy flour or clover and in particular extracts from soy
or clover with an increased content of isoflavones, these
concentrates are available as commercial products.
The application of above teaching might result in a blend
wherein the quercetin is present in amounts of lOmg to 200
mg per RDI (recommended daily intake) while the isoflavones
can be present in amounts of lOmg to 200mg per RDI. In this
way the health components can be delivered as part of the
daily servings of the food product.
According to another embodiment of our invention the
invention also concerns food products containing a health
component (= Functional food) wherein the food product
comprises an amount of the blend of quercetin and at least
one of the isoflavones genestein, daidzein and glycitin
according to the invention, so that the total recommended
daily intake (= RDI ) of the health components is delivered
by one to 5 servings per day of the food product.
Typically the food products can be selected from the group
consisting of spreads, margarines, creams, sauces,
dressings, mayonnaises, ice creams, fillings,
confectioneries, health bars, cereals, health drinks.
F 7 551 cpl ( V ) CA 02354475 2001-08-O1
4
In these food products 20 to 400 mg recommended daily
intake of the synergistic blends according to the invention
can be present. Because of the occurence of the synergy the
food product can contain less of the individual flavone and
iso-flavones, than otherwise would be required to achieve
similar effects. In this way the performance of the food
product is not negatively affected by the presence of the
synergistic blend of health components while the health
benefits are obtained.
In addition to the above components the blends and the food
products can contain other micronutrients, examples thereof
being anti oxidants (Vitamin C or Vitamin E), other
vitamins in particular Vitamin B1, B6 and B12, Vitamin K,
folic acid, minerals like calcium, magnesium, iron, copper,
or zinc, however, emulsifiers also can be present as well
as minor amounts of polyunsaturated fatty acids in
particular DHA and EPA and in particular (deodorised) fish
oils or concentrates thereof.
According to a last embodiment of our novel finding we also
found that the blends or food products containing them can
be used to achieve certain health effects, in particular
certain cosmetical effects. Therefore our invention also
concerns the use of a health composition comprising natural
flavones and isoflavones wherein the health composition is
the blend according to the invention or the food
composition according to the invention and wherein the
blend or food product is applied to achieve cosmetical
effects, in particular skin benefits and skin related
effects such as anti-ageing effects or for promoting the
formation of collagen or for promoting the decorin
formation in the skin. Further the invention concerns the
use of a health composition comprising natural flavones and
F 7551 Cpl (v) CA 02354475 2001-08-O1
isoflavones wherein the health composition is the blend
according to the invention and wherein the blend is applied
for the production of a functional food with anti-
inflammatory properties with a synergistic effect on the
5 anti-inflammatory and related health properties.
This use can also result in a method for the treatment
respectively the prevention of inflammations and related
health deficiencies in animals or humans by administering
to the animal or human in one or more servings in total an
effective amount of the synergistic blend according to the
invention or of the food product containing this blend
according to the invention.
However the method can also comprise a method to achieve
skin benefits or skin related effects, respectively to
achieve the promotion of collagen formation or decorin
formation in the skin by administering to an animal or
human in one or more servings per day in total an effective
amount of the synergistic blend according to the invention
or the food product according to the invention.
In these methods for administering the amount to be
administered should be the effective amount of the health
component corresponding with the recommended daily intake
of the isoflavones cq the flavone.
Procedure For Measuring Procollaqen-I and Decorin Synthesis
In Human Dermal Fibroblasts
Preparation of Dermal Fibroblast Conditioned Medium Primary
human foreskin fibroblasts at passage 2 (P2) were seeded
into 12-well plates at 10,000 cells/cm2 and maintained for
24 hours in atmospheric oxygen in Dulbecco's Modified Eagle
Medium (DMEM) supplemented with 10~ foetal calf serum.
F 7 551 Cpl ( V ) CA 02354475 2001-08-O1
6
After this time the cells were washed with serum free DMEM
and then incubated in fresh serum free DMEM for a further
60 hours. Novasoy 40R containing 20 mM isoflavones,
genistein, daidzein and glycetin (in a ratio of 1:1.3:0.3)
and 5mM quercetin were, either independently or in
combination added to the cells in triplicate in a final
volume of 0.4m1/well fresh serum free DMEM and incubated
for a further 24 hours. 1% ethanol was used as the vehicle
control. This fibroblast conditioned medium was either
analysed immediately or snap frozen in liquid nitrogen and
stored at -70°C for future analysis. The cells were then
counted and data from the dot-blot analysis subsequently
standardised to cell number.
Dot Blot Assay for ProcollaQen-I and Decorin Protein in
Dermal Fibroblast Conditioned Medium
Samples of conditioned medium from dermal fibroblasts
treated with actives as listed above were supplemented with
20mM dithiothreitol (1:10 dilution of 200mM stock solution)
and 0.1~ sodium dodecylsulphate (1:100 dilution of 10°s
stock solution), mixed well and then incubated at 75°C for
2 minutes. A standard for the assay was generated by serial
dilution of neat fibroblast conditioned medium from
fibroblasts seeded at 10,000 cells/cm2 in a 175 cm2 flask
and maintained in serum free DMEM as described above.
Assay samples were subsequently applied in triplicate to a
pre-wetted sheet of Immobilon-P transfer membrane using the
96-well Bio-Dot Apparatus from Bio-Rad as described in the
manufacturers guidelines. Approximately 200u1 of medium was
applied per well. The medium was allowed to filter through
the membrane under gravity (30 minutes) after which the
~ 5 51 Cpl ( V ) CA 02354475 2001-08-O1
7
membrane was washed twice with PBS (200u1). These PBS
washes were allowed to filter through the membrane under
gravity (2x15 minutes). The Bio-Dot apparatus was then
attached to a vacuum manifold and a third and final PBS
wash carried out under suction. The apparatus was
disassembled, the membrane removed and quickly cut as
required before being placed in blocking buffer overnight
at 4°C.
Membranes prepared for procollagen-I and decorin analysis
were both blocked with 5% (w/v) non fat dried milk powder/
0.05s Tween 20 in PBS. The following day, the membranes
were probed with 1:10000 dilution of primary antibodies to
either human procollagen-I (MAB1912; rat monoclonal;
Chemicon Int. Inc., Temecula, CA) or human decorin (mouse
polyclonal; AMS, UK) for 2 hours at room temperature. The
membranes were subsequently washed with TBS/ 0.05°s Tween 20
(3 x 5 minutes) and then incubated with 1:1000 dilution of
125I-conjugated anti-rat or anti-mouse F(ab')2 fragments
(ICN, Amersham respectively) as required for 1 hour at room
temperature.
Following this the Immobilon strips were again washed with
TBS/Tween 20 (3 x 5 minutes) before being allowed to dry in
air at room temperature. The dried membranes were wrapped
in cellophane and exposed to a Molecular Dynamics storage
phosphor screen for 16-18 hours. At the end of this time
the exposed screen was scanned by a phosphorimager
(Molecular Dynamics Phophorimager SF) using ImageQuantTM
software. Dot intensity was assessed by computer-assisted
image analysis using quantification tools in ImageQuantTM,
standardised to cell number and the effects of various test
reagents on decorin and procollagen-I synthesis were
/ 551 Cpl ~ V ~ CA 02354475 2001-08-O1
8
determined relative to a vehicle treated control value of
100 arbitrary units.
Results
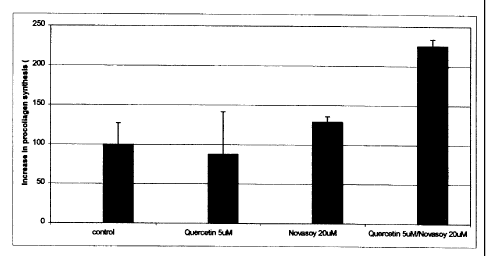
When quercetin and Novasoy 40 are added to cells at sub-
optimal concentrations (i.e. those concentrations just
below the concentrations that produce maximal upregulation
of procollagen-1) the synthesis of procollagen-1 can be
enhanced when quercetin and Novasoy 40 are combined at
concentrations of 5uM and 20uM (isoflavones), respectively.
A synergistic interaction was observed between Novasoy 40
and quercetin when they were combined at the above
concentrations. (cf. fig. l)
Fibroblasts PGE2 Assay
PGE2 production by human skin fibroblasts can be induced by
the inflammatory stimulus PMA (phorbal myristate acetate).
PMA represents and external stressor which induces
oxidative stress and inflammatory responses in cells. This
model is used to model inflammation in vivo.
Primary human foreskin fibroblasts at passage 2 (P2) were
seeded into 96-well plates at 35,000 cells/well and
maintained for 24 hours in an atmosphere of 5% carbon
dioxide in Dulbecco's Modified Eagle Medium (DMEM)
supplemented with 10% foetal calf serum. Novasoy 40 R
containing 20 mM isoflavones, genistein, daidzein and
glycetin (in a ratio of 1:1.3:0.3) and 5mM quercetin were ,
either independently or in combination added to the cells
(DMEM, supplemented with 10% foetal calf serum) in
dimethylsulphoxide (ethanol, final concentration 1%) in
r~ / 551 Cpl ~ V ~ CA 02354475 2001-08-O1
9
triplicate and incubated for a further 24 hours. Phorbal
myristate acetate (PMA) (Sigma) was added to the media and
the cells incubated for a further 24 hours. The control did
not contain any test compounds nor any PMA. The
fibroblasts/media were then analysed immediately or snap
frozen in liquid nitrogen and stored at -70°C for future
analysis. The cells were then counted and data from the
dot-blot analysis subsequently standardised to cell number.
Prostaglandin E2 (PGE2) assay: Volumes of 50u1 culture
medium were taken for PGE2 assay after gently shaking the
culture plate. PGE2 levels in the medium were determined
with a Biotrak PGEZ immunoassay kit (Amersham, UK). The
assay is based on the competition between unlabelled PGE2
in the sample and a fixed quantity of horseradish
peroxidase labeled PGEZ for a limited amount of fixed PGE2
specific antibody. Concentrations of unlabeled sample PGEZ
are determined according to a standard curve, which was
obtained at the same time.
When quercetin and Novasoy 40 are added to cells at sub-
optimal concentrations (i.e. those concentrations just
below the concentrations that produce maximal down-
regulation of PGE2) the down-regulation of PGE2 can be
enhanced when quercetin and Novasoy 40 are combined at
concentrations of lOuM and 20uM (isoflavones),
respectively. A synergistic interaction was observed
between Novasoy 40 and quercetin when they were combined at
the above concentrations. (cf. fig. 2)
/ 551 Cpl- ~ V ~ CA 02354475 2001-08-O1
Example
RECIPE
5
3.4 grams of vegetable fat
0.5 g of modified egg yolk } together named "creamer"
6.0 g of maltodextrin
0.025 grams of Novosoy 40 R containing 40 wt of the
o
10isoflavones genistin / daidzin and glycitin in a weight
ratio of 1.3 . 1 . 0.3
0.010 grams of quercitin
0.6 grams of maize starch croutons
16.1 .grams of dried potato starch
151Øgrams of salt
0.3 grams of onion solids
0.7 grams of onions
0.2 grams of parsley and herb extract
3.2 grams of flavouring agents
The creamer and the other components are mixed in mixer
The
blend obtained is a dried instant oninon soup hat can be
t
used for making a soup by mixing it with 200 of boiling
ml
water under stirring. The soup that is made thisway tastes
25very well.