Note: Descriptions are shown in the official language in which they were submitted.
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
DEVICES, METHODS AND SYSTEMS FOR COLLECTING
MATERIAL FROM A BREAST DUCT
BACKGROUND OF THE IIWENTION
Field of the Invention
The field of this invention is devices, methods and systems for collecting
breast duct fluid from humans.
2. Description of the Background Art
For several decades significant members of the medical community
dedicated to studying breast cancer have believed and shown that the
cytological analysis
of cells retrieved from nipple discharge from the breast milk ducts can
provide valuable
information leading to a identifying patients at risk for breast cancer.
Indeed
Papanicolaou himself contributed to the genesis of such a possibility of a
"Pap" smear for
breast cancer by analyzing the cells contained in nipple discharge. See
Papanicolaou et
al, "Exfoliative Cytology of the Human Mammary Gland and Its Value in the
Diagnosis
of Cancer and Other Diseases of the Breast" Cancer (1958) March/April 377-409.
See
also Petrakis, "Physiological, biochemical, and cytological aspects of nipple
aspirate
fluid", Breast Cancer Research and Treatment 1986; 8:7-19; Petrakis, "Studies
on the
epidemiology and natural history of benign breast disease and breast cancer
using nipple
aspirate fluid" Cancer Epidemiology, Biomarkers and Prevention (Jan/Feb 1993)
2:3-10;
Petrakis, "Nipple Aspirate Fluid in epidemiological studies of breast
disease",
Epidemiologic Reviews (1993) 15:188-195. More recently, markers have also been
detected in nipple fluid. See Sauter et al, "Nipple aspirate fluid: a
promising non-invasive
method to identify cellular markers of breast cancer risk", British Journal of
Cancer
76(4):494-501 (1997). The detection of CEA in fluids obtained by a nipple blot
is
described in Imayama et al. (1996) Cancer 78: 1229-1234.
Breast cancer is believed to originate in the lining of a single breast milk
duct in the breast; and additionally human breasts are believed to contain
from 6 to 8 of
these ducts. See Sartorius , JAMA 224 (6): 823-827 (1973). Sartorious
describes use of
hair-like single lumen catheters that are inserted into breast ducts using an
operating
microscope and the ducts were flushed with saline solution as described in
Cassels, D
March 20'h, 1973, The Medical Post, article entitled "New tests may speed
breast cancer
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
detection". Sartorius et al, Contrast ductography for recognition and
localization of
benign and malignant breast lesions: an improved technique. pp. 281-300. In:
Logan
WW, ed. BREAST CARCINOMA New York, Wiley, 1977. After the fluid was infused,
the
catheter was removed because it was too small to collect the fluid, the breast
was
squeezed and fluid that oozed onto the nipple surface was removed from the
surface by a
capillary tube. Similarly, Love and Barsky, "Breast-duct endoscopy to study
stages of
cancerous breast disease", Lancet 348(9033):997-999, 1996 describes
cannulating breast
ducts with a single lumen catheter and infusing a small amount of saline,
removing the
catheter and squeezing to collect the fluid that returns on the nipple
surface. The use of a
rigid 1.2 mm ductscope to identify intraductal papillomas in women with nipple
discharge
is described in Makita et al (1991 ) Breast Cancer Res Treat 18: 179-188. It
would be
advantageous to develop methods and devices to collect the ductal fluid from
within the
duct.
Galactography, or contrast ductography has for years located breast ducts
I 5 based on spontaneous nipple discharge, infused the ducts (using cannulas
for this
purpose) with contrast dye solutions, and taken x-ray pictures to determine
the source of
the discharge within the duct. See generally, The Breast: Comprehensive
Management of
Benign and Malignant Breast Diseases, Bland and Copeland eds. W.B. Saunders
Co.
Philadelphia PA 1991 pages 61-67.
Method and kits for obtaining fluid and cellular material from breast ducts
09/067,661 filed April 28, 1998, and its CIP 09/301,058 filed April 28, 1999
describe and
claim infusing a small amount of fluid into the duct and collecting the fluid
using a
catheter. It would be beneficial to optimize the cells and fluid collected
from this
procedure.
USSN 60/143,359 filed July 12, 1999 describes and claims a multilumen
catheter for collection of infused fluid. USSN 60/143,476 filed July 12, 1999
describes
and claims devices and methods for accessing the lactiferous sinus of a breast
duct.
USSN 60/122,076 filed March I, 1999 describes devices, methods and kits for
accessing
more than one breast duct at a time for delivering and/or retrieving agents or
materials to
and from more than one breast duct at the safe time. Related applications are
USSN
60/143,476 and 60/143,359 both filed July 12, 1999 and 60/134,613 filed May
18, 1999,
and 60/114,048 filed December 28, 1998, all of which are herein incorporated
by
reference in their entirety.
2
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
Osmotic agents including sugars that are poorly absorbed, for example
lactulose or sorbitol, have been used as laxatives. See THE MERCK MANUAL OF
MEDICAL INFORMATION, Berkow, Beers and Fletcher Eds, 1997 Merck Res. Lab.,
Whitehouse Station, N.J. pp. 522-523. The osmotic agent mannitol is available
as an
injectable, 25% (Physicians Desk Reference 1996) for a variety of indications
(e.g. renal
insufficiency, congestive heart failure). A mixture of sorbitol and mannitol
is compared
to distilled water as an in-igant during transurethral prostatectomy in Sargin
et al, (1997)
Int Urol Nephrol 29:575-80. Intracranial pressure therapy has been provided by
solutions of mannitol, sorbitol or glycerol as described in Treib et al,
(1998) Eur Neurol
40: 212-219. Osmotherapy for increased intracranial pressure comparing the use
of
mannitol and glycerol is discussed in Biestro et al, (1997) Acta Neurochir
(alien) 138:
725-32; discussion 732-3. Mannitol therapy for renal conditions is described
generally in
Better et al, (1997) Kidney Int 52:886-894, and use of the osmotic diuretic
mannitol for
renal protection is analyzed in Visweswaran et al, (1997) JAm Soc Nephrol 8:
1028-33.
1 S Use of mannitol during cardiac catheterization is described in Willerson
et a1, ( 1975)
Circulation 51:1095-1100 and Kurnick et al, ( 1991 ) Am J Kidney Dis I 7: 62-
8. The
osmotic effects of polyethylene glycol are discussed in Schiller et al, (1988)
Gastroenterology 94: 933-41. Raffinose is used for peritoneal dialysis as
described in
Kohan et al (1998) JLab Clin Med 131: 71-6.
Relevant Literature
Hou et al, "A simple method of Duct Cannulation and Localization for
Galactography before Excision in Patients with Nipple Discharge." Radiology
1995; 195;
568-569 describes injecting a "small volume of sterile, water soluble contrast
material...(O.SmI - 2.Om1)...the catheter was taped on the breast or
nipple...the contrast
material was aspirated with the same syringe and gentle manual pressure was
exerted on
the breast to expel the opaque medium."
The use of a 0.4 mm flexible scope to investigate nipple discharge is
described in Okazaki et al (I991) Jpn J. Clin. Oncol. 21:188-193 in which
before the
fiberoptic ductoscopy "a Iacrimal cannula was inserted (into the duct] for
ductal washing
by infusing 0.2 to 0.5 ml physiological saline twice or three times, citing
also Okazaki et
al Nyugan No Ringsho 4:587-594 (1989) (in Japanese).
A company called Diagnostics, Inc. formed in 1968, produced devices to
obtain breast ductal fluid for cytological evaluation. The devices included a
hair-like
3
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
single lumen breast duct catheter to infuse fluid into a breast duct and the
procedure
dictated that after removal of the catheter oozing fluid was collected from
the nipple
surface with a capillary tube. The devices were sold prior to May 28, 1976 for
the
propose of collecting breast ductal fluid for cytological evaluation.
A lacrimal irrigating cannula is described in USPN 5,593,393 to inventors
Trudell and Prouty. The cannula is graduated and used for insertion, dilation,
probing
and irrigating of the lacrimal drainage system of the eye. Lacrimal probes
have been used
to access breast ducts as depicted in The Breast: Comprehensive Management of
Benign
and Malignant Diseases (1991) vol 2, Bland & Kirby eds. W.B. Saunders Co,
Philadelphia, PA pp. 63, figure 3-26.
Patents and applications that describe use of a fixed support wire or
support generally to reinforce the catheter include PCT publication WO
97/44084, PCT
publication WO 97/44082, USPN 5,221,255, JP 6-154334 (unexamined patent
publication), USPN 3,792,703 to Moorehead, USPN 4,596,564 to Spetzler et al,
USPN
1 S 5,209,734 to Hurley et al, USPN 5,456,674 to Bos et al, PCT publication WO
97/31677,
PCT publication WO 94/07549, PCT publication WO 94/02197, European patent
application EP 630 657 A1, European patent application EP 800 842 Al, Japanese
unexamined patent publications JP 4-226675, JP 6-277289, and JP 6-277294,
Japanese
examined patent publication JP 3-4232, and PCT publication WO 97/47230.
Patents and publications that describe use of a very small atraumatic tip
include USPN 4,652,255 to Martinez, USPN 5,246,430 to MacFarlane, PCT
publication
WO 97/37699, PCT publication WO 97/1001 S, PCT publication WO 94/07549,
European
patent application EP 729 766 A1, European patent application EP 643 979 A1,
Japanese
examined utility model publication JP 4-4730, and Japanese examined patent
publications
2S JP 1-14794, JP 61-24022, and JP 61-24023.
Patents and publications that describe and claim fluid collection catheters
having a narrow distal portion and a larger diameter proximal portion with a
shoulder
therebetween include PCT publication WO 97/44084, PCT publication WO 97/44082,
USPN 5,221,255 to Mahurkar, JP 2,519,873 (LJSPN 5,470,318), USPN 4,553,957 to
Williains et al, USPN 4,652,255 to Martin~~; USPN 4,709,705 to Truglio, USPN
5,451,208 to Goldrath, USPN 5,246,430 to MacFarlane, PCT publication WO
97/48435,
PCT publication WO 97/31677, PCT publication WO 95/20983, PCT publication WO
94/02197, European patent application EP 682 954 A2, European patent
application EP
4
CA 02356963 2001-06-27
WO 00139557 PCT/US99/31086
631 791 A1, Japanese examined patent publication JP 4-45186, Japanese
unexamined
utility model publication 6-77709, PCT publication WO 98/39046, and WO
97/47230.
Other patents or publications related in the art include the following: JP 5-
184664 assigned to Terumo Corp. describes a catheter with a distal tip formed
by heating;
JP 2.631,320 Moriuchi et aI, assigned to Terumo Corp. showing vascular
catheter with
multiple axial wire supports extending the length of the catheter; JP 3-264045
to Sato;
assignee Terumo Corp. has a central reinforcement wire extending the length of
intravascular catheter body; JP 61-268266 (WO 89/09079) to Hurley et aI,
assignee
Sumitomo Bakelite (abandoned) depicting another wire reinforcement but in a
uterine
catheter; JP 6-502314 to Hurley et aI, assignee Brigham & Women's Hospital
shows a
spinal catheter with spinal wire reinforcement; JP 8-112354 to Takane depicts
probe with
isolate Lumens and distal side ports; JP S-237I91 (EP 542246) to Pearsall,
assignee
Becton Dickinson shows rounded tips softer than the body of the catheter; JP 3-
36363 (JP
4-S 16C) to Kamogawa, assignee Terumo Corp. is expired but has atraumatic tip
with side
1 S ports and a single lumen; JP 2,531,583 to Onishi, assigned to Mitsubishi
shows a catheter
having a soft tip formed from polymer having a glass transition temperature at
body
temperature; JP 2,681,345 to Inoue, assignee Kitasato Supply shows
insemination device
with syringe; JP 5-184664 to Takeoka, assignee Terumo Corp. shows a single
lumen
rounded tip catheter with side ports; JP 58-46337 (JP 59-2345) to Fujimoto
depicts a
slidable stop on rectal catheter having side ports; and JP 58-146356 to Harris
depicts an
intrauterine catheter with shoulder stop. Patents and publications that
describe breast
access for purposes other than lavage include USPN 5,800,534 to Jeter et al.
SLIrvIMARY OF THE INVENTION
According to the present invention, a method for obtaining cellular
material frorri a human breast milk duct comprises introducing a wash fluid to
the breast
milk duct, using a volume of at least 2 ml that is present within the duct for
a preselected
time, and collecting at least a portion of the introduced wash fluid from
within the duct,
with the portion of wash fluid carrying the cellular material. The preselected
time is
preferably less than one second, but will usually be in the range from one
second to one
hour. The wash fluid is preferably introduced to a volume of at least 2 ml,
often at least
5 ml, and typically in the range between S ml and 25 ml, prior to collecting
any of the
wash fluid from the duct. The wash fluid is preferably introduced to a single
breast milk
duct and collected from the same breast milk duct without mixing with
materials from
5
CA 02356963 2001-06-27
WO 00/39557 PGT/US99I31086
other breast milk ducts. The method may further comprise separating cellular
material
from the collected fluid. The method may still further comprise examining the
separated
cellular material. The cellular material usually includes a substance selected
from the
group consisting of whole cells, cellular debris, proteins, nucleic acids,
polypeptides,
glycoproteins, lipids, fats, glycoproteins, small organic molecules,
metabolites, and
macromolecules.
Another aspect of the invention comprises a method for obtaining cellular
material from a human breast milk duct including introducing a ductal access
device
having at least one lumen into a duct, introducing a wash fluid through the
access device
lumen into the milk duct, providing a volume of at least 2 ml to be present
within the duct
for a preselected time, and then collecting at least a portion of the wash
fluid from the
duct through the lumen of the access device. The method preferably further
comprises
massaging and squeezing the breast tissue after introducing the wash fluid but
prior to
and/or during collecting a portion of the wash fluid. Introducing the ductal
access device
typically comprises positioning a distal end of the device distal to the
ductal sphincter in
the breast duct. The access device preferably includes only a single lumen
that extends
into the duct. The wash fluid is preferably introduced to a volume of at /east
2 ml prior to
collecting any of wash fluid from the duct. The preselected time can be less
than one
second, but will usually be in the range from one second to one hour. The wash
fluid can
be introduced to a single breast milk duct and collected from the same breast
milk duct
without mixing with materials from other breast milk ducts. The method may
still further
comprise separating cellular material from the collected fluid. The method may
still
further comprise examining the separated cellular material. The cellular
material is
usually a substance selected from the group consisting of whole cells,
cellular debris,
proteins, nucleic acids, polypeptides, glycoproteins, lipids, fats,
glycoproteins, small
organic molecules, metabolites, and macromolecules.
Another aspect of the invention is a method for obtaining cellular material
from a human breast milk duct comprising introducing a wash fluid to the
breast milk
duct, providing that the wash fluid is present within the duct for a
preselected time, and
collectiiig~ at /east a portion of the introduced wash fluid from within the
duct, where the
portion carries the cellular material; the wash fluid is introduced to a
single breast milk
duct and collected from the same breast milk duct without mixing with
materials from
other breast milk ducts. The volume of wash fluid can be at least 2 ml. The
preselected
time can be less than one second or can be in a range from one second to one
hour. The
6
CA 02356963 2001-06-27
WO OOf39557 PCT/US99I31086
method can further comprise separating cellular material from the collected
fluid. The
method can also further comprise examining the separated cellular material.
The cellular
material can be a substance selected from the group consisting of whole cells,
cellular
debris, nucleic acids, lipids, protein metabolites, small organic molecules,
and
macromolecules.
An aspect of the invention is another method for obtaining cellular
material from a human breast milk duct comprising introducing a ductal access
device
having at least one lumen into a duct, introducing a wash fluid through the
access device
lumen into the milk duct, where the wash fluid is present within the duct for
a preselected
time, and collecting at least a portion of the wash fluid from the duct
through the lumen of
the access device; the wash fluid is introduced to a single breast milk duct
and collected
from the same breast milk duct without mixing with materials from other breast
milk
ducts. The volume of wash fluid can be at least 2 ml. The preselected time can
be less
than one second or in a range from one second to one hour. The method can
further
comprise separating cellular material from the collected fluid, and the
separated material
can be examined. The cellular material can be a substance selected from the
group
consisting of whole cells, cellular debris, nucleic acids, lipids, protein
metabolites, small
organic molecules, and macromolecules.
An aspect of the invention is a kit comprising a ductal access device; and
instructions for use setting forth a method provided above comprising
introducing a
ductal access device having at least one Iumen into a duct.
An aspect of the invention is a ductal access device comprising an access
tube having a distal end, at least one lumen, and dimensions which permit
introduction of
the distal end through a ductal orifice and positioning a distal end distal to
the ductal
sphincter of a human breast. The device can further comprise means on the
access tube
for positioning the distal end distal to the ductal sphincter. The positioning
means can
comprise length indicia on the tube which permit a user to determine the depth
to which
the distal end of the tube has been introduced. The positioning means can
comprise a
stop element formed or attached to the tube; the stop will have dimensions
which prevent
further insertion of the tube into the duct and-the stop is positioned on the
tube so that the
distal tip will be located distal to the ductal sphincter when the device is
fully inserted up
to the stop. The stop element can comprise a collar affixed to or formed on an
exterior
surface of the tube. The device can comprise means for anchoring the device to
the
breast. The device can include a receiving portion comprising a water tight
seal for
7
CA 02356963 2001-06-27
WO 00/39557 PC'T/US99/31086
receiving the dilator. The stop element can comprise a hub attached to a
proximal end of
the tube, where the hub has a width which is greater than the diameter of the
tube so that a
shoulder is formed at a junction between the tube and the hub. The positioning
means
can comprise a rob on the access tube having an increase diameter for
anchoring the tube
in the lactiferous sinus once the rob has passed the sphincter and rests in
the sinus. The
access tube can have an outer diameter of 0.05 inches (or I.27 mm) or less.
The access
tube can have an outer diameter of 0.010 inches (or 0.254 mm) or greater. The
outer
diameter can be in the range from 0.010 inches (or 0.254 mm) to 0.050 inches
(or
1.27mm). The access tube can have a lumen diameter 0.007 inches (or 0.178 mm)
or
greater. The access tube can have a lumen diameter in the range from 0.007
inches (or
0.178 nun) to 0.047 inches (or 1.19 mm). The access device can further
comprise an
infusion connector providing a fluid flow path into the lumen of the tube; and
a collection
connector providing a fluid outlet path from the lumen of the tube; the
infusion and
collection connectors are isolated from each other so that the fluid may be
infused
1 S through the infusion connector and simultaneously removed through the
collection
connector. The device can further comprise a dilator removably received in the
access
tube and having a distal tip which is positionable through the access tube to
extend from
the distal end of the device. The dilator can have an outer diameter of 0.024
inches (or
0.61 mm) or less. The dilator can be tapered. A receiving portion of the
device for
receiving the dilator can comprise a water-tight seal.
An aspect of the invention is a ductal access system comprising a ductal
access device as described and a container holding a premeasured volume of
ductal wash
fluid. The container can comprises a syringe for connection to the first side
port. The
pre-measured volume can be in the range from 2 ml to 100 ml. The ductal access
fluid is
can be selected from the group consisting of saline, phosphate buffered
saline, a
nonabsorbable fluid, an isotonic solution, an osmotic solution, a hypotonic
solution, and a
hypertonic solution.
A further aspect of the invention is a ductal access device comprising an
access tube having a distal end, a single lumen, and dimensions which permit
introduction
of the distal end through a ductal orifice and~positioning a distal end of the
device distal
to the ductal sphincter, an infusion connector providing a fluid flow path
into the lumen
of the access tube, and a collection connector providing a fluid outlet path
from the lumen
of the access tube; the infusion and collection connectors being isolated from
each other
so that fluid may be infused through the infusion connector and simultaneously
removed
8
CA 02356963 2001-06-27
WO 00/39557 PC'T/US99/31086
through the collection connector. The tube has an outer diameter of 0.010
inches (or
0.254 mm) or greater or the tube has an outer diameter of 0.050 inches (or
1.27 mm) or
less, or the outer diameter can be in the range from 0.010 inches (or 0.254
mm) to 0.50
inches (or 1.27 mm).
S The access tube has a lumen diameter 0.007 inches (or 0.178 mm) or
greater, or a lumen diameter in the range from 0.007 inches (or 0.178 mm) to
0.047
inches (or 1.19 mm). The device can fiu~ther comprise means on the access tube
positioning a distal end of the device distal to the ductal sphincter. The
positioning means
can comprise length indicia on the tube which permit a user to determine the
depth to
which the distal end of the tube has been introduced. The positioning means
comprises a
stop element formed or attached to the tube, and the stop has dimensions which
prevent
further insertion of the tube into the duct; the stop is positioned on the
tube so that a distal
end of the distal tip is positioned distal to the ductal sphincter. The stop
element an
comprise a collar affixed to or formed on an exterior surface of the tube. The
stop
element can comprise a hub attached to a proximal end of the tube, where the
hub has a
width which is greater than the diameter of the tube so that a shoulder is
formed at a
junction between the tube and the hub. The positioning means can comprise a
nob on the
access tube having an increased diameter for anchoring the distal portion of
the tube distal
to the sphincter once the nob has passed the sphincter. The device can
comprise means
for anchoring the device to the breast. The device can additionally comprise a
dilator
removably received in the access tube and having a distal tip which is
positionable
through the access tube to extend from the distal end of access device. The
dilator can
have an outer diameter of 0.024 inches (or 0.61 mm) or less. The dilator can
be tapered.
A receiving portion of the device for receiving the dilator an comprise a
water-tight seal.
_An aspect of the invention is a ductal access system comprising a ductal
access device as just described and a container holding a premeasured volume
of ductal
wash fluid. The container can comprise a syringe for connection to the first
side port.
The premeasured volume can be in the range from 2 ml to 100 ml. The ductal
access
fluid can be selected from the group consisting of saline, phosphate buffered
saline, a
nonabsorbab~le~fluid, an isotonic solution, an-osmotic solution, a hypotonic
solution, and a
hypertonic solution.
An aspect of the invention is a ductal access device comprising a hub
having an internal elongate manifold, a lower port at a bottom of the
manifold, and first
and second side ports spaced above the lower port; and an access tube having a
distal end,
9
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
a proximal end, a lumen, and dimensions which permit introduction of the
distal end
through a ductal orifice and a positioning a distal end of the device distal
to the ductal
sphincter of the human breast, provided also that the proximal end of the tube
is attached
to the lower port of the hub. The first and second side ports can be at the
same level
relative to the lower port. The first side port can be below the second side
port. The
access tube can have an outer diameter of 0.010 inches (or 0.254 mm) or
greater. The
access tube can have an outer diameter of 0.50 inches (or 1.27 mm) or less.
The outer
diameter can be in the range from 0.010 inches (or 0.245 mm) to 0.50 inches
(or 1.27
mm). The access tube can have a lumen diameter 0.007 inches (0.178 mm) or
greater, or
a lumen diameter in a range from 0.007 inches (0.178 mm) to 0.047 inches (1.19
mm).
The device can have an infusion tube connected to the first port of the hub;
and a
collection tube connected to the second port of the hub. The device can
further comprise
a means for controlling a flow of fluid through the infusion tube, a means for
controlling
a flow of fluid through the collection tube, or both a means for controlling a
fluid flow
through the infusion lumen and a means for controlling a fluid flow through
the
collection lumen. The fluid control means can comprise compressible lumens or
the
fluid control means can comprise stopcocks on each lumen. The hub or manifold
can
have a volume in the range from 0.01 ml to 1.0 ml. The first side port can be
spaced
above the lower port by a distance less than 5 mm and the second side port can
be spaced
above the first side port by a distance in the range from 0.10 mm to 5 mm. The
device
can further comprise a dilator removably received in the hub and having a
distal tip which
is positionable through the access tube to extend from the distal end of the
device. The
dilator can have an outer diameter of 0.024 inches (or 0.61 mm) or less. The
dilator can
be positionable through the hub manifold and into the lumen of the access
tube. The
dilator can be tapered. A receiving portion of the hub for receiving the
dilator can
comprise a water-tight seal. The device can further comprise a means on the
access tube
for positioning the distal end of the access tube distal to the ductal
sphincter. The
positioning means can comprise length indicia on the tube which permit a user
to
determine the depth to which the distal end of the tube has been introduced.
The
positioning means can comprise a stop element formed or attached to the tube;
the stop
has dimensions which prevent further insertion of the tube into the duct and
the stop is
positioned on the tube so that the distal tip will be located distal to the
ductal sphincter
when the device is fully inserted up to the stop. The stop element can
comprises a collar
affixed to or formed on an exterior surface of the tube. The stop element can
comprise a
CA 02356963 2001-06-27
WO 00!39557 PCT/US99l31086
hub attached to a proximal end of the tube, where the hub has a width which is
greater
than the diameter of the tube so that a shoulder is formed at a junction
between the tube
and the hub. The device can further comprise a means for anchoring the device
to the
breast. The positioning means can comprise a nob on the access tube having an
increased
diameter for anchoring the tube distal to the ductal sphincter once the nob
has passed the
sphincter and rests distal to it.
An aspect of the invention is a ductal access system comprising a ductal
access device as just described and a container holding a premeasured volume
of ductal
wash fluid. The container can comprise a syringe for connection to the first
side port.
The pre-measured volume can be in the range from 2 ml to 100 ml. The ductal
access
fluid can be selected from the group consisting of saline, phosphate buffered
saline, a
nonabsorbable fluid, an isotonic solution, an osmotic solution, a hypotonic
solution, and a
hypertonic solution.
An aspect of the invention provides a ductal access catheter comprising a
1 S catheter body having a distal end and a proximal end and including at
least a distal
portion and a proximal portion wherein the distal portion.has a cross-
sectional geometry
which can be inserted through a ductal orifice into a ductal lumen of a human
breast;
wherein the proximal portion has a cross-sectional geometry which inhibits
insertion
through the ductal orifice and into the ductal lumen; and wherein the catheter
body has at
least an infusion lumen and an collection lumen each of which has a distal
port near a
distal end of the distal portion and a proximal connector near a proximal end
of the
proximal portion. The device can further comprise a means for controlling a
flow of
fluid through the infusion lumen, a means for controlling a flow of fluid
through the
collection lumen, or both a means for controlling a fluid flow through the
infusion lumen
and a means for controlling a fluid flow through the collection lumen. The
fluid control
means can comprise compressable lumens, or the fluid control means can
comprise
stopcocks on each lumen.
The distal portion of the catheter body can be stiffened over at least a part
of its length to facilitate insertion through the ductal orifice and into the
ductal lumen.
The stifferi~d~distal portion of the catheter body has an average bending
stiffness in the
range from about 0.010 inch-lbs to about 0.5 inch-lbs. The stiffening member
is disposed
in the distal portion of the catheter body.
The distal portion of the catheter body has a maximum width in the range
from 0.016 inches to 0.022 inches (0.56 mm) and the proximal portion of the
catheter
11
CA 02356963 2001-06-27
WO OOI39557 PCT/US99I31086
body has a minimum width in the range from 0.023 inches (0.58 mm) to 0.028
inches
(0.71mm). The distal portion of the catheter body has a generally tubular
structure with a
diameter in the range from 0.010 inches (0.254mm) to 0.020 inches (0.51 mm)
and the
proximal portion of the catheter body has a generally tubular structure with a
diameter in
the range from 0.030 inches (0.762 mm) to 0.10 inches (0.254 mm) and wherein
the
proximal diameter is greater than the distal diameter by at least 0.010 inches
(or .254
mm). At least one of the distal collection port and the distal infusion
portion can be
disposed on a side of the distal portion of the catheter body. The distal
collection port
and the distal infusion port can both be located on the side of the distal
portion of the
catheter body. The distal collection port and the distal infusion port can be
axially
aligned. The distal collection port and the distal infusion port can be
axially spaced apart.
The catheter body can include an atraumatic distal tip. The tip can be
composed of a soft
polymeric material, have a diameter in the range from about 0.008 inches (0.20
mm) to
about 0.035 inches (0.89mm), and a length in the range from about 0.25 cm to
about to
2.5 cm.
The invention further provides a ductal access catheter comprising a
catheter body having a distal end and a proximal end and including at least a
distal
portion and a proximal portion; wherein the distal portion has a cross-
sectional geometry
which can be inserted through a ductal orifice into a ductal lumen of a human
breast;
wherein the distal portion of the catheter body is stiffened over at least a
part of its length
to facilitate insertion through the ductal orifice and into the ductal lumen;
and wherein the
catheter body has at least an infusion lumen and an collection lurrlen each of
which has a
distal port near a distal end of the distal portion and a proximal connector
near a proximal
end of the proximal connector. The stiffened distal portion of the catheter
body can have
an average bending stiffness in the range from about 0.010 inch-Ibs to about
0.5 inch-lbs.
The proximal portion can have a cross-sectional geometry that inhibits
insertion through
the ductal orifice and into the ductal lumen.
The invention also provides a ductal access catheter comprising a catheter
body having a distal end and a proximal end and including at least a distal
portion and a
proximal portion; wherein the distal portion-Itxs a cross-sectional geometry
which can be
inserted through a ductal orifice into a ductal Lumen of a human breast; and
wherein the
catheter body has at least an infusion lumen and an collection lumen each of
which has a
distal port near a distal end of the distal portion and a proximal connector
near a proximal
end of the proximal connector; and wherein the distal collection port and the
distal
12
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
infusion port are both located on the side of the distal portion of the
catheter body. The
distal collection port and the distal infusion port can be axially aligned.
The distal
collection port and the distal infusion port can be axially spaced apart. The
proximal
portion can have a cross-sectional geometry that inhibits insertion through
the ductal
S orifice and into the ductal lumen.
Another aspect of the invention is a method for lavage of a ductal network
in a human breast comprising providing a multi-lumen catheter as just
described and
inserting the distal portion of the catheter through a ductal orifice and into
a distal lumen
of the ductal network; introducing a wash fluid through the infusion lumen
into the ductal
network; and withdrawing the wash fluid and substances borne by the wash fluid
from
the ductal network through the collection lumen.
Another aspect of the invention is a system comprising a mufti-lumen
catheter as just described and instructions for use setting forth a method for
lavage of a
ductal network in a human breast including introducing a wash fluid through
the infusion
lumen into the ductal network and withdrawing the wash fluid and substances
borne by
the wash fluid from the ductal network through the collection lumen.
The agent infused into the duct can comprise a non-absorbable fluid and/or
an oncotic agent and/or an osmotic agent. The agent can be soluble. The agent
can
comprise a molecule that is a protein, a colloid, a sugar, or a polymer. The
agent can be
mannitol, sorbitol, glucose, glycerol, sucrose, raffinose, fivctose,
lactulose, sodium
chloride, polyethyleneglycol (PEG), maltodextrin, dextran (e.g. dextran 70),
hydroxyethyl starch, fluid gelatin, or a synthetic colloid. The agent can
comprise a
protein and the protein can be a binding protein or an antibody. The binding
protein can
be albumin. Administering can comprise administering locally, and local
administration
can comprise administering intraductally. A system for increasing or
standardizing an
amount of fluid collectable from a milk duct of a breast can comprise infusing
a
nonabsorbable fluid and/or an osmotic agent and/or an oncotic agent into the
ductal
lumen, a medical tool for delivering the agent to the ductal lumen, and
instructions for
use.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. l shows a single lumen catheter with a stop and external infusion and
collection tubes.
13
CA 02356963 2001-06-27
WO 00/39557 PCT/US99I31086
Fig. 2 is a detailed view of a calibrated ductal access portion of a single
lumen catheter. The calibration serves to identify a depth of penetration.
Fig. 3 is a single lumen ductal access catheter having a hub and infusion
and collection lumens and a retractable dilator.
Fig. 3A is a cross section of the device in Fig. 3.
Fig. 4A illustrates access of a breast duct and penetration to at least a
region distal to the ductal sphincter.
Fig. 4B illustrates filling a duct with infusion fluid.
Fig. 4C illustrates bidirectional flow of infused fluid in the duct through
the access lumen to be collected.
Fig. 4D illustrates a single lumen catheter accessing a breast duct having
the capacity to infuse and collect fluid outside the accessed breast duct.
Fig. 5 depicts a kit comprising a single lumen catheter having infusion and
collection lumens outside the ductal access portion of the catheter, a
premeasured
1 S solution to infuse into the duct and instructions for use of the catheter
and wash fluid to
access a breast duct and retrieve cellular material.
Fig. 6 depicts a single or double lumen catheter having an infusion and
collection lumen outside the catheter with stopcocks on each external lumen to
control
fluid flow into or out of each lumen.
Fig. 7 illustrates a breast duct accessed by a single or double lumen
catheter having separate infusion and collection lumens outside the ductal
access portion
of the catheter, having also stop cocks on each external lumen for controlling
fluid flow
in the lumen, and having an infusion receptacle on the infusion lumen and a
collection
receptacle on the collection lumen.
Fig. 8 illustrates an alternative embodiment of the breast duct access
device of the present invention.
Figs. 8A and 8B are cross sectional views taken along lines 8A-8A and
8B-8B of Fig. 8, respectively.
Fig. 8C is a detailed view of the distal end of the device of Fig. 8.
Figs. 9A and 9B depict alternative transition zones in a ductal access
catheter. Fig. 9A is a stepped transition zone.
14
CA 02356963 2001-06-27
WO 00/39557 PCT/US99131086
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
OF THE INVENTION
The following preferred embodiments and examples are offered by way of
illustration and not by way of limitation.
The invention provides methods for obtaining cellular material from a
human breast duct. A wash fluid is introduced and a volume of at least 2 ml is
allowed to
remain in the duct for a preselected time that can range from less than or
about one
second to about an hour, including any length of time in between. During the
time that
the wash fluid remains in the breast duct, it may mix with the ductal fluid
already present
in the duct, and it may accumulate cellular material either from the ductal
lumen walls or
that already present in the existing resident ductal fluid. The breast duct
may be filled
with wash fluid before the wash fluid mixed with ductal fluid and comprising
cellular
material is collected. For example, a wash fluid may be infused into the duct
until a point
of resistance to infusion, a which point it may be considered that the breast
duct may is
filled with wash fluid, and the just infused fluid can be allowed to reside in
the duct for a
preselected time. Once the time has elapsed, the infused fluid and the
contents of the duct
with which it has mixed is collected. If a ductal access tool is used to
access the duct and
infuse the fluid into the duct, the in-dwelling tool can obtain or collect the
infused fluid
either through the same lumen that was used to infuse the wash fluid into the
duct
originally, or through a separate second lumen adjacent or coaxial to the
infusion lumen.
In any event the access tool remains in place in the duct during the infusion,
filing,
preselected waiting time (e.g. less than one second or about one second to one
hour), and
collection of the wash fluid mixed with ductal fluid and cellular material
from the breast
duct.
Methods of the invention include accessing a single breast duct and
obtaining cellular material from that duct without allowing the cellular
material or ductal
fluid from the accessed duct to contact the cellular material or ductal fluid
of any other
duct, or cellular material or ductal fluid that happens to be residing on the
nipple surface.
Thus is provided the opportunity to analyze a single individual breast duct
separate from
other breast ducts of the patient. The washwflvid can be introduced into the
duct by
accessing the breast duct with a ductal access device having at least one
lumen. Infusion
of wash fluid into the duct is provided through the lumen accessing the duct.
Collection
of the wash fluid mixed with ductal fluid and comprising cellular material can
also be
provided through the Lumen accessing the duct. Access of a single breast duct
provides
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
also the opportunity to collect ductal fluid and cellular material from the
accessed breast
duct separate from other breast ducts on the breast, without mixing or
contacting the
collected fluids and cellular material with that of the other ducts, and so
providing the
opportunity to analyze the condition of the accessed duct separately.
During the procedure the breast may be massaged and squeezed.
Massaging and squeezing the breast may facilitate collection of the infused
fluid and
mixed ductal fluid and cellular material. The actions of massaging and
squeezing the
breast may also provide some disruption of the cells on the lumen walls,
thereby
increasing a yield of cellular material from the procedure. Collection from a
collection
lumen (either the same lumen as was used to infuse or a separate lumen) can be
further
facilitated in some cases with aspiration applied into the lumen. Preferably,
where an
indwelling tool is used, a single lumen accesses the breast duct, and external
to the breast
and breast duct the tool branches into ar< infusion lumen and a collection
lumen. From
this collection lumen, during the period when the fluid is being collected
from the duct,
aspiration may be applied.
Additionally, where a manifold hub is present in the design of the access
tool, once the wash fluid mixed with ductal fluid and cellular material is
passed out of the
duct and into the hub, collection may be facilitated from the collection lumen
without risk
of collapsing the ductal wall, but providing an aspiration pressure in the
collection lumen
(e.g. using a syringe and pulling back to collect material into the syringe).
Additionally,
or alternatively, the hub filled with collected material may be flushed into
the collection
lumen using an infusion of wash fluid from the infusion lumen. The fluid flow
into and
out of the infusion and collection lumens may be facilitated with means on the
device
lumens to stop or open the fluid flow into or out of the lumens.
Additionally, when a ductal access device is used to access a breast duct,
the distal end of the device comprising an infusion and/or collection port or
ports is
placed distal to the ductal sphincter to provide an optimal position for
infusion and
collection of fluid and/or other agents or materials to and from the breast
duct. Means to
assure placement of the distal tip of the device distal to the ductal
sphincter can be
provided on the device as further discussed~t~elow in the ductal access device
design.
The wash fluid that is introduced into the duct can comprise any
biocompatable agent or solution. Thus, the wash fluid can comprise e.g.
saline,
phosphate buffered saline. Additionally or alternatively, the wash fluid can
comprise an
agent or agents or solution that reduces the ability of the~fluid or agent to
diffuse through
16
CA 02356963 2001-06-27
WO 00/39557 PC'T/US99/31086
the ductal wall or otherwise leave the duct and enter other parts of the body.
Accordingly, the wash fluid may comprise a nonabsorbable fluid, an isotonic
solution, an
osmotic solution, a hypotonic solution or a hypertonic solution. Fluid or
agents may be
administered to the breast duct in order to facilitate, increase, and/or
optimize the amount
of material obtained or obtainable from the breast duct during the procedure.
Agents or
solutions that may comprise the infused wash fluid can include, e.g. protein,
colloid,
sugar, polymer, mannitol, sorbitol, glucose, glycerol, sucrose, raffinose,
fructose,
lactulose, sodium chloride, polyethyleneglycol (PEG), maltodextrin, dextran
(e.g. dextran
70), hydroxyethyl starch, fluid gelatin, albumin, a synthetic colloid, an
antibody or part of
an antibody, or a binding protein.
Once the wash fluid had been infused in the duct and the wash fluid and
ductal fluid is collected from a breast duct, the cellular material can be
separated and can
be examined. The cellular material can include, e.g. substances selected from
the group
consisting of whole cells, cellular debris, proteins, nucleic acids,
polypeptides,
glycoproteins, lipids, fats, glycoproteins, small organic molecules,
metabolites, and
macromolecules. Whole cells can be examined by cytology, or any other suitable
method
for analyzing the condition of the cells. Other markers present in the
cellular material,
ductal fluid generally, or other material obtained from the breast duct can be
analyzed as
is appropriate for the marker being sought, including e.g. binding assays,
immunohistochemistry, or using other analytical technology for distinguishing
and
identifying biological molecules obtained from biological material.
Chromosomal abnormalities in ductal epithelial cells can also provide
information and act as a marker to identify cancer or precancer as described
in Mark et al
(1999)-Cancer Genet Cytogenet 108:26-31; Lundlin and Mertens (1998) Breast
Cancer
Res Treat 51:1-15; Newsham (1998) Am JPathol 153:5-9; Larson et al (1998) Am J
Pathol 152:1591-8; Adelaide et al (1998) Genes Chromosomes Cancer 22:186-99;
Fejzo
et al (1998) Gene Chromosome Cancer 22:105-1 I3; Dietrich et al (1998} Hum
Pathol 12:
1379-82; Cavalli et al (1997) Hereditas 126:261-8; Adeyinka et al (1997)
Cancer Genet
Cytogenet 97:119-21; Afify and Mark (1997) Cancer Genet Cytogenet 97:101-5;
Brenner
and Aldaz (1997) Prog Clin Biol Res 396: 63'=82; Mark et al (1997) Ann Clin
Lab Sci
27:47-56; and Fabian et al 1993 J. Cellular Biochemistry 176:153-16.
In addition, exemplary markers are described in Masood S., (Prediction of
recurrence for advanced breast cancer. Traditional and contemporary pathologic
and
molecular markers) Surgical Oncology Clinics of North America. 4(4):601-32,
1995;
17
CA 02356963 2001-06-27
WO 00/39557 PCTlUS99/31086
Lopez-Guerrero et al (1999} JHematother 8(1):53-61; Marjumdar and Diamandis
(1999)
Br J Cancer 79(9-10):1594-602; Balleine et al (1999) Br J Cancer 79 (9-
10):1564-71;
Houston et al (1999) Br J Cancer 79(7-8):1220-6; Nikolic-Vukosavljevic et al (
1998)
Tumori 84(6):691-4; Maguire et al (1998) Int JBiol Markers 13(3):139-44;
Steams et al
(1998) Breast Cancer Res Treat 52(1-3):239-59; Eiriksdottir et al (1998) Eur J
Cancer
34(13):2076-81, and USPN 5,169,774. Many known breast cancer markers are
discussed
and described in readily available medical text books on breast cancer. In
addition,
several markers can be identified and analyzed in the same sample, e.g. Fabian
et al 1993
J. Cellular Biochemistry 176:153-16 and Fabian et al 1994 Breast Cancer Res
Treat
30(3):263-74 looking at estrogen receptor (ER}, epidermal growth factor
receptor
{EGFR), mutant p53, HER-2 neu by immunohistochemistry and aneuploidy by image
analysis in fine needle aspirates.
Cytological assays that can be performed on the cells retrieved from a duct
or from nipple aspirate can include e.g. assays described in King et al, J.
Nat'1 Cancer
Inst (1983) 71:1115-21, Wrensch et al. (1992) Am. J. Epidem. 135: 130-141,
Papanicolaou et al, (1958) Cancer, 11:377-409 and Goodson WH & King EB,
Chapter
4: Discharges and Secretions of the Nipple , THE BREAST: COMPREHENSIVE
MANAGEMENT OF BENIGN AND MALIGNANT DISEASES (1998) 2"d Ed. VOI 2, Bland &
Kirby eds. W.B. Saunders Co, Philadelphia, PA pp. 51-74. For example, as
described in
Goodson and King (page 60) atypical hypeiplasia presents as having cellular
abnormalities, increased coarseness of the chromatin, and tendency for more
single cells
as well as groups of cells. With regard to carcinoma in situ, Papanicolaou et
al, described
cellular abnormalities, e.g. nuclear abnormalities diagnosed by cytology of
fluid from
nipple -secretions containing ductal cells. The cytology of abnormal cells can
also be
conducted as described in Sartorius et al (1977) J. Natl Cancer Inst 59: 1073-
1080. and
King et al, (1983) JNC171(6) 1115-1121. Atypia and carcinoma in situ are
widely
characterized pathologically, as described in Page et al, (1998) Mod Pathol
11(2): 120-8.
The ductal fluid can be analyzed by cytological techniques by placing some of
the fluid
on a slide with a standard cytological stain using a light microscope. The
cells can be
studied for atypical growth patterns in individual cells and clusters of cells
using
published methods, including Mouriquand J, ( 1993) S Karger Pub, "Diagnosis of
Non-
Palpable Breast Lesions: UltrasonographicalIy Controlled Fine-Needle
Aspiration:
Diagnostic and Prognostic Implications of Cytology" (ISBN 3805557477); Kline
TS and
IK, Pub Igaku-Shoin Medical ""Breast: Guides to Clinical Aspiration Biopsy"
(LSBN
I8
CA 02356963 2001-06-27
WO OOI39557 PCT/US99131086
0896401596; Masood, American Society of Clinical Pathology: Nov. 199S,
"Cytopathology of the Breast" ISBN 0891893806; and Feldman PS, American
Society of
Clinical Pathology, Nov. 1984, "Fine Needle Aspiration Cytology and Its
Clinical
Applications: Breast and Lung" ISBN 0891891846.
Other references that discuss cytological analysis and which give guidance
to an analysis of ductal epithelial cells derived from ductal fluid include
Silverman et al,
(Can FNA biopsy separate atypical hyperplasia, carcinoma in situ, and invasive
carcinoma of the breast?: Cytomorphologic criteria and limitations in
diagnosis,
Diagnostic Cytopathology) 9(6):713-28, 1993; Masood et al,
(Immunohistochemical
differentiation of atypical hyperplasia vs. carcinoma in situ of the breast)
Cancer
Detection & Prevention. 16(4):225-35, 1992; Masood et al, (Cytologic
differentiation
between proliferative and nonproliferative breast disease in mammographically
guided
fine-needle aspirates) Diagnostic Cytopathology.7(6):581-90, 1991; Masood S.,
(Occult
breast lesions and aspiration biopsy: a new challenge) Diagnostic
Cytopathology.
9(6):613-4, 1993; Masood S., (Prognostic factors in breast cancer: use of
cytologic
preparations) Diagnostic Cytopathology. 13(5):388-95, 1995; Novak and Masood,
(Nuclear grooves in fme-needle aspiration biopsies of breast lesions: do they
have any
significance?) Diagnostic Cytopathology. 18(5):333-7, 1998; Sidawy et al,
(Interobserver
variability in the classification of proliferative breast lesions by fine-
needle aspiration:
results of the Papanicolaou Society of Cytopathology Study) Diagnostic
Cytopathology.
18(2):150-65, 1998; Masood et al, (Automation in cytology: a survey conducted
by the
New Technology Task Force, Papanicolaou Society of Cytopathology) Diagnostic
Cytopathology. 18(1):47-55, 1998; and Frykberg and Masood Copeland EM 3d.
Bland
KL, (Ductal carcinoma in situ of the breast) Surgery, Gynecology & Obstetrics
177(4):425-40, 1993.
Appropriate animal models for breast cancer therapies have been
described, e.g. McKenzie and Sukumar, (Molecular mechanisms of chemical
carcinogenesis in rodent models) Cancer Treatment & Research 71:313-29, 1994;
Chen
et al, (Midkine in the progression of rat N-nitroso-N-methylurea-induced
mammary
tumors) Molecular Carcinogenesis. 17(3):1'2-6, 1996; and Sukumar et al,
(Animal
models for breast cancer) Mutation Research 333(1-2):37-44, 1995.
In addition to some markers discussed and/or articles or books cited on
breast cancer and breast precancer markers, the following cancer markers are
listed here
as exemplary and may be used as well as other markers to analyze the condition
of a
19
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
breast duct. Standard assay procedures for identifying the markers can be
used, including
antibodies or other binding partners, labels, stains, pattern analysis (for
cells and cell
components), and in general any other chemical or visual identification
techniques. The
following are exemplary potential markers for such identification and
analysis:
cathepsins (including cathepsin D); maspin, fas, fas ligand, tissue inhibitor
of matrix
metalloproteinas-1 (TIMP-1); chemokines (both C-C and C-X-C type chemokines);
collagenases, metalloproteinases, TIMP's, cathepsins, disrupted basement
membrane
epitopes, stromolysin-3; cytokeratins (e.g. keratin 14, B1, KAI, KA4 and 31X8-
1);
estrogen and progesterone receptors (or any androgen or other steroid
receptor); growth
factor receptors for members of the fibroblast growth family (FGF) including
FGF1-18,
vascular endothelial growth factor (VEGF), insulin-like growth factor -1 (IGF-
I), IGF-II,
platelet-derived growth factor (PDGF), keratinocyte growth factor (KGF), and
epithelial
growth factor (EGF); placental growth factor (PLGF), hepatocyte growth factor
(HGF),
tumor necrosis factor (TNF), transforming growth factor (TGF) both alpha and
beta
forms, and angiopoietin, for example; growth factors and cytokines including
e.g. FGF1-
I8, VEGF, IGF-I, IGF-II, PDGF, KGF, EGF, PLGF, HGF, TNF, TGF alpha and beta,
angiopoietin; heat shock proteins (HSP) (e.g. HSP27) 27 (HSP27); ErB type 1
tyrosine
kinase receptors (e.g. Her2 (an EGF receptor) or any ligand or receptor of the
ErbB
family of ligands and receptors); integrins, selectins, cadherins, for example
(i.e. alpha
and beta 3 integrin); keratin-14; known cancer antigens including, for example
Ki-67, Ki-
S 1, p53, nm23, bcl-2, p21 ras, cyclins, and pS2; thrombin receptor activating
peptide;
urokinase, urokinase-type plasminogen activator (UPA), plasmin antiplasmin;
UPA
receptor (UPAR), fibrinogen, plasmin activator inhibitor-1 and 2 (PAI-1 and
2);
telomerase; antibodies to tumor associated antigen-72 (TAG-72) (e.g. B72.3,
B6.2, and
TKH2); carcinoembryonic antigen (CEA) (see e.g. EP 319,686); prostate specific
antigen
(PSA); gross cystic disease fluid protein - 15 (GCDFP-15); lactose
dehydrogenase
(LDH); chromosomal abnormalities (e.g. aneuploidy or other abnormalities); S 1
protein;
alkaline phosphatase; myosin; siaIyl Tn (STn) glycopeptide (e.g. TAG-72); Tn
glycopeptide; and nuclear matrix proteins (as described in provisional patent
application
filed 11-17-99 docket no. PDH 99-029, hereirr-incorporated by reference in its
entirety).
In general, markers can be categorized nonexclusively, and often in
overlapping categories as follows: 1. Markers that are detected or detectable
by virtue of
protein expression or overexpression (detection may occur, e.g. by
immunohistochemistry
or in situ hybridization); 2. Markers that are detected or detectable by
virtue of mRNA
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
expression or overexpression (detection may occur, e.g. by differential
display
techniques); 3. Markers that are detected or detectable by virtue of a post
translational
change in a protein, e.g. a phosphorylation of the protein, a ubiquitination,
a
farnesylation, methylation, or other modification to the protein that can be
detected, e.g.
S by antibodies specific to the post translational modification.
Accordingly, markers such as the following can sought in ductal fluid, e.g.
proteins that are overexpressed, mRNA transcripts that are over expressed, and
proteins
comprising post translational modifications. For example, the following
markers can be
identified to distinguish a cancer or precancer cell from a normal cell.
Proteins that are
overexpressed can include e.g. Stromelysin-3, Membrane Type 1 Matrix
Metalloproteinase (MT1-MMP), Matrix Metalloproteinase-3 (MMP-3), Placental
Isoferrintin (p43) , Nuclear Matrix Protein (NMP22), NM-200.4 specific
antigen,
Vascular Endothelial Growth Factor (VEGF), Endoglin (CDI05), Telomerase, ErbB-
2,
ErbB-3, Carcinoembryonic Antigen (CEA), Heat Shock protein-27 (HSP-27), Breast
IS Cancer-specific Gene (BCSG), Plasminogen Activator Inhibitor (PAI-I),
Urokinase
Plasminogene Activator (uPA), Urokinase Plasminogene Activator Receptor
(uPAR),
Colony Stimulating Factor-1 (CSF-1), Colony Stimulating Factor-I receptor
(fins),
Annexin I, Vasopressin, the CC Chemokine Regulated on Activation Normal T cell
Expressed and Secreted (RANTES), 44-3A6 specific antigen, A-80 specific
antigen,
MIJC-1, H23 specific antigen, 83 D4 specific antigen, SP-2 specific antigen,
323/A3
specific antigen, tumor associated antigen-72 (TAG-72) , and MBE6 specific
antigen.
Other breast cancer markers detected by any means including e.g. protein
expression, mRNA expression, or post translational modification can include
e.g. (listed
alphabetically) alanine aminopeptidase, alpha 6 integrin, alpha-IactaIbumin,
AN43, p53,
Bcl2-antagonist of cell death (Bad), Bcl2-associated athanogene (BAG-1), Bcl2-
antagonist/killer I (Bak), Bcl2-associated X protein (Bax), Breast cancer
antigen 225
(BCA225), B-cell CLL/lymphoma 2 (Bcl-2), Bcl2-like 1 (Bcl-x), beta 1-6
branched
oligosaccharides, beta-2 microglobulin (BMG), Bcl2 related protein A1 (Bfl-1),
bone
sialoprotein (BSP), CCAAT/enhancer-binding protein liver-enriched inhibitory
protein
(C/EBPbeta-LIP), Carcinoma Antigen 1 (Cavil), Carcinoma Antigen 27.29 (CA
27.29),
Carcinoma Antigen M26 (CA M26), Carcinoma Antigen M29 (CA M29), Carcinoma
Antigen 125 (CA125), Carcinoma Antigen 15.3 (CA15.3), Carcinoma Antigen 195
(CA195), Carcinoma Antigen 19-9 (CA19-9), Carcinoma Antigen 50 (CA50),
Carcinoma
Antigen 549 (CA549), Cadherin-I 1, calcitonin receptor (CTR), cathepsin B,
cathepsin L,
21
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
Endoglin (CD105), CD24, CD34 (pan-endothelial marker), CD44, c-met/hepatocyte
growth factor receptor, c-myc, cyclooxygenase-1 (Cox-1 ), cyclooxygenase-2
(Cox-2),
caspase-3 (CPP32), Cyclic nucleotide phosphodiesterase, cycline E, DNA
topoisomerase
II-alpha, DNA topoisomerase II-beta, EGF, EGF receptor, E-selectin, fast
homoarginine-
sensitive alkaline phosphatase (FHAP), fatty acid synthase, ferritin, gross
cystic disease
fluid protein (GCDFP-15BRST-2), metastasis-associated h-mtsl (S100A4), heat
shock
cognate protein-73 (hsc73), heat shock protein-70 (hsp70), heat shock protein-
90 alpha
(hsp90alpha), heat shock protein-90 beta (hsp90beta), inhibitors of
differentiation-1
(IDI), inhibitors of differentiation-3 (ID3), interleukin-1 beta, Keratin 8,
Keratin 18,
Keratin 19, Laminin, Laminin receptor (MLuCS), Leucine Aminopeptidase (LAP),
lipid-
bound sialic acid (LSA), Melanoma antigen-1 IMAGE-1), Melanoma antigen-2 (MAGE-
2}, Melanoma antigen-3 IMAGE-3), Man6-P glycoproteins, Mucin-like carcinoma
associated antigen (MCA), myeloid cell leukemia-1 (Mcl-1), metallothionein
(MT),
mitogen-activated protein kinase phosphatase-1 (MKP-1), Matrix
Metalloproteinase-2
1 S (MMP-2), Matrix Metalloproteinase-9 (MMP-9), mammary serum antigen (MSA),
breast
cancer mucin-2 (MUC-2), breast cancer mucin-3 (MUC-3), breast cancer mucin-6
(MUC-
6), Nm23 nucleoside diphosphate kinase, ornithine decarboxylase (ODC),
osteopontin
(OPN), P114 (MAR binding protein), P120 (a nucleolar proliferation antigen),
focal
adhesion kinase p125FAK, nuclear autoantigen p330d/CENP-F, plasminogen
activator
inhibitor-2 (PAI-2), Pepsinogen C, placental alkaline phosphatase (FLAP),
Platelet factor
4 (angiogenic marker), protein kinase C (PKC), prostate specific antigen
(PSA),
pyrimidine nucleoside phosphorylase, ras p21, reduced glutathione (GSH),
retinoid X
receptor alpha, ribosomal S2 protein, siaIyltransferase, Stromelysin-1 (MMP-
3),
surfactant proteins A, surfactant proteins B, tumor associated antigen-12 (TAG-
12),
trefoil gene TFF1, trefoil gene TFF3/ITF/hPI.B, Thrombin, Thrombomodulin,
thymidine
phosphorylase (TP), thymosin beta 1 S, tissue cytosol fen-itins, tissue
polypeptide antigen
(TPA), tissue polypeptide specific antigen (TPS), Vascular Endothelial Growth
Factor -B
(VEGF-B), Vascular Endothelial Growth Factor-C (VEGF-C), Vascular Endothelial
Growth Factor receptor-1(VEGFRl), Vascular Endothelial Growth Factor receptor-
2
(VEGFR2), arid Vascular Endothelial GrowfIi Factor receptor-3 (VEGFR3).
Some Genes are overexpressed and can be found by differential display,
including e.g. Claudin-7, Zinc-alpha-2-glycoprotein, Apolipoprotein B, B94,
EST
(R08988),Thrombospondin (THBS1), FGF-1, NGAL/Lipocalin 2, EST (N77731), BS247
[Abbott Labs WO 9922027J, AIB-1. Post translational modifications can be
identified in
22
CA 02356963 2001-06-27
WO 00/39557 PCTIUS99/31086
proteins, including e.g. Tyrosine phosphorylation, ErbB-2, and EGFR. Absence
of key
tumor suppression markers include e.g. mammastatin and maspin.
Turning now to the figures, Fig. 1 provides a ductal access device
comprising 10 an access tube 12 having a distal end 14, at least one lumen
therethrough,
and dimensions which permit introduction of the distal end through the ductal
orifice and
positioning a distal end thereof distal to the ductal sphincter of a human
breast,
e.g., typically having an outer access tube diameter in the range from 0.5 mrn
to 1 mm,
preferably being tapered within this range over a length from 2 to 3 mm. The
device can
also comprise means on the access tool for positioning the distal end distal
to the ductal
sphincter. The device can have a stop 16 or other means to prevent the device
from
penetrating the duct too deeply. Alternatively, the tube could have a shoulder
or other
enlargement to block penetration at a point at which it is desirable to stop
the penetration
of the tool; or, alternatively, a collar can be placed or built onto the
external portion of the
access tube to prevent penetration beyond the collar.
The means provided to position the device distal to the ductal sphincter
can comprise marks 18 on the access portion of the device to indicate a
penetration depth
as indicated in Fig. 2. Additionally, it may be desired that the device is
anchored just
distal to the ductal sphincter once the distal tip has passed through the
ductal sphincter.
This may be facilated by any number of means, including, e.g. placing a small
nob or
hub 20 on the tube 12 which acts a stop to resist removing the distal tip once
the nob has
passed by the ductal sphincter and resides distal to it. Anchoring the distal
tip of the
ductal access device distal to the ductal sphincter may also be accomplished
by placing
the distal tip to a depth beyond the ductal sphincter and inflating a balloon
(not shown) to
anchor the device below the ductal sphincter during the infusion and
collection procedure.
The device may also comprise a stop or hub or other means for keeping the
tube accessing the duct from penetrating too far, and for positioning the
access tube distal
to the ductal sphincter. Thus, the device may include a positioning means
comprising a
stop element formed or attached to the tube. The stop element has dimensions
which
prevent further insertion of the tube into the duct, and the stop is
positioned on the tube so
that the distal tip will be located distal to the-ductal sphincter when the
device is fully
inserted up to the stop, thus ensuring correct positioning of the tube in the
duct relative to
the ductal sphincter. The access lumen will terminate in at least one port for
fluid
infusion and/or collection, and the port is preferably placed at the end of
the distal tip of
the device so that it opens in a distal (axial) direction relative to the
access tube 12, and
23
CA 02356963 2001-06-27
WO 00/39557 PCT/US99131086
the port is preferably located relative to the stop element so that the port
resides distal to'
the ductal sphincter when the stop element engages the nipple. The stop
element can
comprise a hub attached to a proximal end of the tube, wherein the hub has a
width which
is greater than the diameter of the tube so that a shoulder is formed at a
junction between
the tube and the hub.
The access device can also be anchored to the external portions of the
accessed breast by any means capable of accomplishing the anchoring. During
the
procedure it is important that the access device not slip out of the duct.
Portions of the
device that are external to the access breast duct can be affixed, strapped,
tethered, taped,
or otherwise anchored to the breast during the procedure in order to ensure
that the device
does not slip out of the duct. Such anchoring also provides the practitioner
with better
control of the device parts if part or all of the device is anchored, and
therefore does not
need to be held by the practitioner or an assistant.
In a preferred aspect of the catheter design, the access tube 12 will branch
1 S into an infusion arm 22 and collection arm 24. The infusion arm 22
terminates in a
connector 26 which removably connects to a syringe 28 or other pressurized
source of
wash fluid. The collection arm 24 will preferably include a valve 30 and an
end
connector 32 for removable attachment to a collection apparatus, such as a
vial, tube, tray,
microliter plate, another syringe, or the like. As discussed below, the
collection arm will
usually be closed, e.g., with valve 30, during infusion of the wash fluid.
Preferably, both
the infusion arm lumen and collection arm lumen will be connected to a single
lumen
within the access tube 12.
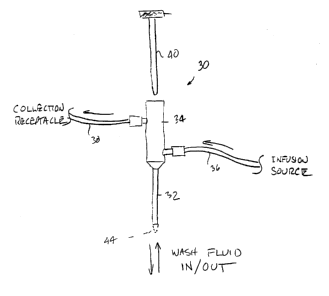
Turning now to Fig. 3, a preferred embodiment of the device is shown in a
single lumen ductal access device 30 having a tube 32 that accesses the duct
and through
which fluid is infused, and from which fluid is collected or drawn up out of
the duct. A
hub 34 is connected to an infusion tube 36 from which fluid is infused into
the access
tube 32 and a collection tube 38 from which fluid is collected from the access
tube. The
collection tube 38 is preferably attached to the hub 34 at a position no
closer to the access
tube 32 than the infusion tube 36. Preferably, the collection tube 38 is
located fiuther
away from the~access tube 32 than is the infrtsion tube 36. A stylet 40 is
optionally
provided to facilitate introduction of the access tube through a ductal
orifice into a ductal
lumen. The stylet 40 will pass through a pneumostatic seal at a proximal end
42 of the
hub 34 so that the stylet can be removed after positioning of the access tube
32 and prior
to the infusion/collection of the wash fluid.
24
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
Fluid is infused into the hub 34 and into the duct until resistance is met
during the infusion. At this time, it is assumed that the duct is filled. The
infusion lumen
can be closed and the fluid allowed to remain in the duct for a preselected
time. During
this preselected time, the breast may be massaged and squeezed to stimulate
mixing of the
wash fluid and ductal fluid, and also ultimately to encourage the fluid to
leave the duct
and enter the manifold hub. The collection lumen is opened and the breast
squeezed to
urge the fluid to progress through the access tube in the hub. If desired,
when cloudy
return fluid is seen in the hub (which is preferably transparent or includes a
transparent
window), the infusion lumen can be opened and fluid infused to push the fluid
that has
I O collected in the hub into the collection lumen and a waiting collection
receptacle.
Alternatively, and possibly additionally, aspiration pressure can be applied
at the
collection lumen to aspirate any fluid remaining in the hub into the
collection receptacle.
The process is repeated either following another infusion of fluid into the
duct or by
another round of squeezing to encourage return and collection of the infused
fluid.
1 S The stylet 40 can be made of metal or hard plastic and may have a tapered
and/or an atraumatic tip for gently probing and accessing a breast duct.
Preferably, a
tapered tip 44 will extend distally of the access tube 32 as the tube is
introduced. After
access of the duct is complete, the stylet 40 can be withdrawn and the access
tube
positioned so that its distal end is distal to the ductal sphincter. The
dilator receiving
20 portion at the proximal end of the device can be a water tight membrane or
sheath to
provide a sterile environment in the hub even with penetration and withdrawal
of the
dilator, and to provide an appropriate amount of resistance so that the probe
can be
manipulated into the out of the duct and the access tube. The dilator stated
in Fig. 3 is
removably received in the access tube and has a distal tip which is
positionable through
25 the access tube to extend from the distal end of the access tube. In
addition to providing
tapered access, the stylet 40 selectively stiffens the access tube to further
ease
introduction into and through the ductal orifice. The access tube 32 may have
an outer
diameter in a range from about 0.25 mm to 1.25 mm with an inner lumen diameter
in the
range from 0.2 mm to I.2 mm.
30 ~ wAs illustrated in Fig. 3A, thewhub 34 cari have an infusion connector 46
providing a fluid outlet path into the lumen of the tube 32, and a collection
connector 48
providing a fluid outlet path from the lumen of the tube. These infusion and
collection
connectors are preferably isolated from each other so that the fluid may be
infused
through the infusion connector and simultaneously removed through the
collection
CA 02356963 2001-06-27
WO OOI39557 PC'T/US99/31086
connector. The distance e~ between the infusion port 46 and access tube 32 is
preferably
minimized, usually being 1 cm or less, while the distance ~2 between the
infusion port 46
and collection port 48 may be from 0 mm to 2 cm, preferably being 1 cm or
less. While
illustrated on opposite sides of the hub 34, the infusion port 46 and
collection port 48 may
have any relative radial orientation, with an alignment of both ports on the
same side of
the hub being presently preferred.
Fig. 4A depicts a single lumen access tube 50 accessing the breast duct D
and positioned with its distal end 14 distal to the ductal sphincter S of the
breast duct.
Fig. 4B depicts filling the breast duct D and allowing the fluid to remain in
the duct for a
preselected time. Fig. 4C depicts removing the infused fluid mixed with ductal
fluid
through the access tube that remains in the duct during the filling of the
duct and
collecting of the fluid. Fig. 4D depicts a catheter 52 having infusion and
collection anms
54 and 56 exterior to the accessed duct for separately infusing fluid into the
duct and
collecting fluid from the duct. The invention provides a device having an
access tube, a
distal end, a single lumen, dimensions to permit insertion of the device
distal to the ductal
sphincter. The device also has an infusion connector, a collection connector,
and that the
infusion and collection connectors are isolated.
Fig. 6 depicts an alternative ductal access device 72 with stop cocks 74 and
76 to control the fluid flow into and out from an accessed duct. A syringe 78
for infusing
fluid into the duct is connected to one arm 80 of an access tube 82. A
collection tube 86
is connected to another arm 84 connected to the access tube 82. The access
tube may
have single, dual, or multiple lumens as described elsewhere herein. Fig. 7
depicts the
device of Fig. 6 accessing a breast duct BD. Fluid may be infused from syringe
78 with
stopcock 76 open and stopcock 74 closed. After infusing a desired volume as
set forth
above, and optionally massaging the breast, stopcock 74 may be opened and
fluid
collected in passive receptacle 86 (Fig. 6) or actively withdrawn using a
second syringe
88 to apply a vacuum through the collection artn 84.
Figs. 8 and 8A-8C illustrate a dual lumen catheter 100 having lumens 102
and 104 that access the breast duct. A reduced diameter region 106 accesses
the breast
duct and the catheter resides in the duct at a depth of about 3.5 cm. The
reduced diameter
portion 106 of the catheter has three lumens 103, 105, and a third central
lumen which
receives a fixed stiffening wire 108 (Fig. 8B). The proximal part of the
catheter 100 that
does not access the breast duct is depicted in cross section in Fig. 8A having
interior
26
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
lumen 104 and an annular lumen 102 both which provide fluid flow (either
collection or
infusion) during use of the catheter to retrieve cellular material from the
breast duct.
Proximal connector 110 is near a proximal end of the proximal portion and
branches into
an infusion arm 112 and aspiration arm 114, each connected to one of the
lumens 102 and
S 109. The distal portion 106 and proximal portion meet at shoulder 116 where
the lumens
102 and 104 make a transition to annular lumens 103 and 105 (Fig. 8B). Distal
tip I20
may be atraumatic for entry into a ductal orifice and ductal lumen. Usually,
side ports
122 will be formed on the distal section 106 to permit fluid inflow and
outflow from the
lumens 103 and 105, respectively.
Figs. 9A and 9B depict two formats of transition between a distal end of
an access tube 130 which accesses the breast duct and the proximal end which
resides
outside the breast duct. Shoulder 132 in Fig. 9A has a graduated transition,
and shoulder
134 in Fig. 9B has a stepped transition.
The invention also provides systems and kits 60 for collecting cellular
1 S material from a breast duct. Fig. 5 depicts a system comprising an access
device 62, such
as a single lumen catheter having infusion and collection an-ns 64 and 66
outside the
ductal access portion of the catheter, optionally a premeasured solution to
infuse into the
duct and instructions for use of the catheter and wash fluid to access a
breast duct and
retrieve cellular material, e.g., contained in syringe 70. The system 60
includes
instructions setting forth any of the methods described herein, such as the
method for
obtaining cellular material from a human breast milk duct comprising
introducing a ductal
access device having at least one lumen therethrough into a duct, introducing
a wash fluid
through the access device lumen into the milk duct, wherein a volume of at
least 2 ml is
present within the duct for a preselected time; and collecting at least a
portion of the wash
fluid from the duct through the lumen of the access device. Wash fluid can be
included in
the kit and can comprise for example, saline, phosphate buffered saline, a
nonabsorbable
fluid, an isotonic solution, an osmotic solution, a hypotonic solution, a
hypertonic
solution, a protein, a colloid, a sugar, a polymer, mannitol, sorbitol,
glucose, glycerol,
sucrose, raffinose, fructose, lactulose, sodium chloride, polyethyleneglycol
(PEG),
maltodextrin;wdextran (e.g: dextran 70), hydroxyethyl starch, fluid gelatin, a
synthetic
colloid, an antibody, a binding protein, or albumin.
Other ductal access systems available comprise a ductal access device as a
container holding a premeasured volume of ductal wash fluid. The ductal access
device
comprises an access tube having a distal end, at least one lumen therethrough,
and
27
CA 02356963 2001-06-27
WO 00/39557 PCT/US99131086
dimensions which permit introduction of the distal end through a ductal
orifice and
positioning a distal end thereof distal to the ductal sphincter of a human
breast. The
device may also comprise a means on the access tube for positioning the distal
end distal
to the ductal sphincter. The container can comprise a syringe for connection
to the first
side port. The pre-measured volume is in the range from 2 ml to 100 ml_. The
wash
fluid can comprise for example, saline, phosphate buffered saline, a
nonabsorbable fluid,
an isotonic solution, an osmotic solution, a hypotonic solution, a hypertonic
solution.a
protein, a colloid, a sugar, a polymer, mannitol, sorbitol, glucose, glycerol,
sucrose,
raffinose, fructose, lactulose, sodium chloride, polyethyIeneglycol (PEG),
maltodextrin,
dextran (e.g. dextran 70), hydroxyethyl starch, fluid gelatin, a synthetic
colloid, an
antibody, a binding protein, or albumin.
Another ductal access system comprises a ductal access device comprising
an access tube having a distal end, a single lumen therethrough, and
dimensions which
permit introduction of the distal end through a ductal orifice and positioning
a distal end
thereof distal to the ductal sphincter, an infusion connector providing a
fluid flow path
into the lumen of the access tube; and a collection connector providing a
fluid outlet path
from the lumen of the access tube, said infusion and collection connectors
being isolated
from each other so that fluid may be infused through the infusion connector
and
simultaneously removed through the collection connector, the system also
including a
container holding a premeasured volume of ductal wash fluid. The container can
comprise a syringe for connection to the first side port, and the premeasured
volume can
be in the range from 2 ml to 100 ml. The fluid can comprise saline, phosphate
buffered
saline, a nonabsorbable fluid, an isotonic solution, an osmotic solution, a
hypotonic
solution; a hypertonic solution, a protein, a colloid, a sugar, a polymer,
mannitol, sorbitol,
glucose, glycerol, sucrose, raffinose, fiuctose, lactulose, sodium chloride,
polyethyleneglycol (PEG), maltodextrin, dextran (e.g. dextran 70),
hydroxyethyl starch,
fluid gelatin, a synthetic colloid, an antibody, a binding protein, or
albumin.
Another ductal access system can comprise a ductal access device
comprising a hub having an internal elongate manifold, a lower port at a
bottom of the
manifold, and first and second side ports spaced above the lower port; and an
access tube
having a distal end, a proximal end, a lumen therethrough, and dimensions
which permit
introduction of the distal end through a ductal orifice and a positioning a
distal end
thereof distal to the ductal sphincter of the human breast, wherein the
proximal end of the
tube is attached to the lower port of the hub, the ductal access system
comprising also a
28
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
container holding a premeasured volume of ductal wash fluid. The container can
comprise a syringe for connection to the first side port. The pre-measured
volume is in
the range from 2 ml to 100 ml. The ductal access fluid can comprise, e.g.,
saline,
phosphate buffered saline, a nonabsorbable fluid, an isotonic solution, an
osmotic
S solution, a hypotonic solution, a hypertonic solution, a protein, a colloid,
a sugar, a
polymer, mannitol, sorbitol, glucose, glycerol, sucrose, raffinose, fructose,
lactulose,
sodium chloride, polyethyleneglycol (PEG), maltodextrin, dextran (e.g. dextran
70),
hydroxyethyl starch, fluid gelatin, a synthetic colloid, an antibody, a
binding protein, or
albumin.
Additionally, a ductal access system is provided comprising a ductal
access catheter comprising a catheter body having a distal end and a proximal
end and
including at least a distal portion and a proximal portion, wherein the distal
portion has a
cross-sectional geometry which can be inserted through a ductal orifice into a
ductal
lumen of a human breast, wherein the proximal portion has a cross-sectional
geometry
1 S which inhibits insertion through the ductal orifice and into the ductal
lumen; and wherein
the catheter body has at least an infusion lumen and an collection lumen each
of which
has a distal port near a distal end of the distal portion and a proximal
connector near a
proximal end of the proximal portion, the ductal access system comprising also
instructions for use setting forth a method for lavage of a ductal network in
a human
breast including introducing a wash fluid through the infusion lumen into the
ductal
network and withdrawing the wash fluid and substances borne by the wash fluid
from the
ductal network through the collection lumen.
At least one of the lumens of the breast duct access device can have a
means to control the fluid flow through that lumen. The inflow lumen can be
connected to
a syringe or other infusion mechanism for infusing lavage fluid into the
breast duct. The
outflow lumen can be connected to a collection tube, a collection syringe, or
other
collection means for collecting the lavage fluid after it has mixed with the
ductal fluid in
the breast duct. The means to control the fluid flow in a lumen can be, e.g. a
stopcock,
valve or other control unit that is capable of closing or opening a port of
the lumen. The
lumens themselves may be compressible or pinchable with fingers or clamps or
other
pinching or compressing mechanism. A stopcock may be attached at a lumen of a
dual
lumen catheter to control the fluid flow through that lumen. An inflow lumen
may have
an inflow stop cock to control fluid flow through an inflow port. An outflow
lumen may
have an outflow stop cock to control fluid flow through an outflow port. The
device can
29
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
have stopcocks (or other means to control fluid flow) on both an inflow and an
outflow
lumen. These control units, valves or stopcocks are capable of operating
separately, e.g.
so that when an inflow port is opened, an outflow port can be closed, etc.
Thus, patterns
of control of the fluid flow in a lavage procedure of a breast duct can
include, e.g. an open
inflow when an outflow is closed, an open inflow when an outflow is opened, a
closed
inflow when an outflow is opened, and a closed inflow when an outflow is
closed. The
catheter may have the graduated duct probe attached to it at the distal end
for accessing
the duct. Where the probe is unattached to the catheter, and is used for
dilating the orifice,
the catheter can have a tip appropriate for accessing the dilated duct upon
removal of the
probe.
Lavage fluid can be a saline solution, e.g. normal saline, or phosphate
buffered saline (PBS), or other fluid capable and suitable for washing a body
duct. The
lavage fluid will generally be biocompatible and nontoxic to the patient. The
lavage fluid
can further comprise additives, e.g. gas, particles or other fluids. These
additives to the
lavage fluid may have various proposes, however, during a lavage procedure,
the
preeminent purpose will generally be to increase a recovery of fluid and/or
cellular
material, and/or molecular species from the ducts. Thus, such gas may provide
a
cleansing action on the ductal walls for example, encouraging ductal
epithelial cells
located e.g. in a lesion in the duct to shed and be retrievable during the
lavage procedure.
Similarly, particle additives may serve to encourage fluids, cellular material
and/or
molecular species to follow the particles in the flow of lavage fluid through
the ducts and
be retrieved in the lavage procedure. Such additives as detergents, e.g.
agents tending to
form micelles for collecting ductal contents including cells and molecular
species may
provide additional yields of cells, molecular species and fluids in a lavage
procedure.
The gas can be ambient air or a related product, and the lavage fluid can
comprise the air
mixed in with the fluid for delivery into the duct. The presence of air or
other gas may
serve to increase the retrieval of cells and fluid as compared to a procedure
conducted
using lavage fluid alone. The air can be bubbled into the fluid, or introduced
into the
fluid mixture by other standard means. The air may also be mixed into the
lavage fluid as
the lavage fluid is deliveredwinto the duct, e.g: where the infusion port
allows for delivery
of both air and lavage fluid into the inflow lumens where the two mix and both
are
delivered to the accessed ducts.
The lavage fluid can further contain other agents that may aid in the
retrieval of fluid or cells or both from the duct or may serve some other
useful purpose in
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
the procedure. For example, the lavage fluid may include or be preceded by or
followed
by such other agents that may aid in the retrieval of fluid or cells or both
from the duct, or
may serve some other useful purpose in the procedure. Such other agents can
be, for
example, an oncotic and/or osmotic agent capable of increasing the amount of
collectable
fluid in the ductal lumen, or a detergent that can help wash out more cells,
or an agent
that may help detach more cells from the duct wall into the ductal lumen (e.g.
trypsin,
collagenase, or EDTA}. The agent can be an oncotic agent and/or an osmotic
agent or
both. Oncotic and osmotic agents are agents that retain fluid around them or
draw fluid
to them. The agent can be soluble, e.g. soluble in a suitable solvent,
including e.g. water,
buffered water, or a saline solution. Preferably the solvent is biologically
compatible
with mammals. Suitable solvents will be those that both effectively dissolve
the agent
and are not toxic to a mammal. The agent can be a molecular species including
e.g. a
protein, colloid, sugar, or polymer. The agent can be mannitol, sorbitol,
glucose,
glycerol, sucrose, raff nose, fructose, lactulose, sodium chloride, albumin,
polyethyleneglycol (PEG), maltodextrin, dextran (e.g. dextran 70),
hydroxyethyl starch,
fluid gelatin, or a synthetic colloid. Agents including e.g. mannitol,
sorbitol, PEG,
glycerol are described in THE MERCK INDEX, 12'" ed. 1996, Whitehouse Station,
NJ.
Others, including maltodextrin, dextran and others are available from Aldrich
Chemical
Co. in Milwaukee, WI or Sigma Chemical Co. in St. Louis, MO. The molecular
weight
of a suitable oncotic agent can be determined as optimally within the range of
the
molecular weights of suitable oncotic agents available.
Where the agent in the Iavage fluid is a protein, the protein can be a
binding protein or an antibody. The binding protein can be albumin. The
antibody can
be capable of binding an epitope found in a breast duct, e.g. an epithelial
cell surface
marker or cancer cell marker, etc. Where the agent is a protein, the protein
is of a
molecular weight in the neighborhood of albumin or higher, so that it is
capable of acting
as an oncotic agent iri the lumen of the milk duct. Suitable antibodies are
commercially
available. Also the agent can be a mixture of osmotic and/or an oncotic
agents. The
oncotic agent and/or osmotic agent can comprise a mixture of any two or more
osmotic
and/or oncotic agents, e.g. mannitol, sorbitol, glucose, glycerol, sucrose,
raffinose,
fructose, lactulose, sodium chloride, albumin, polyethyleneglycol (PEG),
maltodextrin,
dextran (e.g. dextran 70), hydroxyethyl starch, fluid gelatin, an antibody or
a synthetic
colloid. Preferably the agent is not toxic to a mammal, particularly not toxic
to a human.
The agent can be an agent not capable of freely diffusing into or beyond the
cells that line
31
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
the milk ducts of the breast. The agent can also be an agent not capable of
absorption
into the cells within the duct. For example, the agent can have a molecular
weight large
enough to make absorption or diffusion into the breast duct lining, cells or
interstitial
space beyond the lining improbable.
The method provides that the catheter is used to access the breast duct
after being primed (i.e. filled) with lavage fluid with both outflow and
inflow ports
closed. The outflow stop cock can be opened and the fluid allowed to infuse
into the duct
from the outflow to flush the outflow port at the catheter tip and make it
ready to receive
the ductal fluid and wash fluid into the outflow lumen during the Iavage
procedure. The
outflow port at the stopcock is then closed by closing the outflow stopcock.
The inflow
port is opened by opening the inflow stopcock. Wash fluid is infused into the
breast duct
until resistance is met. The amount of this first infusion bolus will vary
depending on the
size of the breast duct being infused. The inflow port is then closed by
closing the inflow
stopcock. The breast is massaged by applying manual external pressure on the
breast
tissue. The outflow lumen is opened and the breast is massage and squeezed and
fluid is
collected in the collection receptacle attached to the outflow lumen. The
process can be
repeated several times. Subsequent to the first larger bolus of wash fluid,
lesser amounts
of wash fluid can be infused into the duct and collected in the outflow
collection
receptacle as just described.
A practitioner desirous of increasing a yield of fluid and cells from a
lavage of a patient's breast ducts, and/or desirous of retrieving fluid and
cells from distal
regions of the ductal architecture, can massage the breast of the patient once
the fluid has
been infused into the duct. The fluid is infused into the duct (with the
outflow port
closed)~to a point of resistance and then the inflow port is closed. The
breast can be
massaged at this point to effect a mixing of the ductal fluid with the lavage
fluid, and to
generally provide some gentle disruption of ductal cells in the duct and allow
them to
enter the fluid mix. The outflow port can be opened at this point, and allow
the
massaging can continue, it can be supplemented with a squeezing or compressing
of the
breast, i.e. from the base of the breast upwards towards the nipple in order
to encourage
as much fluid to escape via the outflow lumen and into the collection
receptacle.
Modifications to the method of lavage can include that the patient is seated
during the lavage procedure, rather than the standard or classic supine (face
up) position.
In addition, the patient may be lavaged in a prone position, face down, with
nipples and
breast down. The prone face down position takes advantage of gravity and
allows the
32
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
breast ducts to drain into the collection receptacle during the procedure when
the outflow
port is open. Thus, the lavaging procedure can include infusing the breast
duct with a
wash fluid through an open inflow lumen while an outflow lumen is closed;
closing the
inflow lumen when the duct is filled; squeezing or massaging the breast or
both; and
opening the outflow lumen to collect the wash fluid.
The cells collected can comprise ductal epithelial cells; the ductal fluid
collected can comprise molecular and cellular material. Analysis of the ductal
epithelial
cells and/or the molecular and cellular material in the ductal fluid can
proceed as
described below discussing the diagnostic methods possible of these collected
materials.
The collected cells and fluid and fluid components can be analyzed, e.g. as
described or
suggested herein. The lavage fluid including the ductal cells can be analyzed
for
diagnostic purposes. Conditions in a breast milk duct that are desirable to
diagnose
include a cancer or precancer condition. The precancer condition can include
atypical
ductal hypetplasia (ADH) or low grade ductal carcinoma in situ (LG-DCIS). The
diagnostic agent may also have the ability to diagnose other breast related
conditions,
including, e.g. fibrotic, cystic or conditions relating to lactation.
Diagnostic agents can be
mixed with the ductal fluid (either in the lavage procedure, or after the
fluid is collected).
The diagnostic agents can include tags for detecting lesions or other
abnormalities or characteristic anatomical or molecular identities in the
breast ducts,
including e.g. chemical tags or antibodies. The tags may provide the capacity
for
visualizing the location of a lesion, including, e.g. fluorescent tags, or
biotinylated tags.
Antibodies can also be tagged so that the binding antibody is identifiable.
Antibodies can
be whole antibodies, or parts of antibodies including, e.g. Fab fragments,
heavy and/or
light chain fragments, single chain antibodies and other modified antibodies
commonly
known about and used in the field of antibody-assisted diagnosis. Diagnostic
antibodies
or other tags can be to a number of markers, including e.g. the following
cancer markers
that are exemplary and may be used to analyze the breast duct condition.
Standard assay
procedures for identifying the markers can be used. analyzed for the presence
of soluble
factors or other components that might indicate the presence of cancerous or
precancerotts-~ductal epithelial cells in the duct. The epithelial cells
retrieved from the
breast duct can be analyzed for protein markers, nucleic acid markers,
chromosomal
abnormalities, or other characteristic changes that would signal the presence
of cancerous
or precancerous cells. In addition, other cells found in the duct can also be
analyzed, e.g.
for an increase or decrease in these cells as compared to normal ductal fluid,
or for
33
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
qualities of these cells themselves. Thus, the condition of the breast duct
can be analyzed
e.g. for soluble protein content or presence of other ductal fluid components,
including
also secreted products of ductal epithelial cells) or the ductal epithelial
cells themselves
can be analyzed, for example, for cell morphology, for protein markers, for
nucleic acid
markers, and for biochemical markers.
In addition, any of the cells of the duct can be analyzed for morphological
abnormalities in cell components, including, e.g. morphological abnormalities
of the
nucleus, cytoplasm, Golgi apparatus or other parts of a cell. The cells can be
analyzed for
whether they do or don't aggregate (e.g. in clumps) or by making comparisons
of the
ductal epithelial cells with other cell types retrieved in the ductal fluid
(e.g. macrophages,
lymphocytes, foam cells and other possible components of ductal fluid). The
ductal
epithelial cells can be analyzed for their molecular contents or the
morphology of the
ductal epithelial cells, including, e.g. protein markers, nucleic acid
markers, biochemical
markers in the cells or on the cell surfaces or for any evidence of neoplasia.
In addition to some markers discussed and/or articles or books cited on
breast cancer and breast precancer markers, including markers listed in Porter-
Jordan and
Lippman, "Overview of the biological markers of breast cancer",
Hematology/Oncology
Clinics of North America vol. 8 (1 ):73-100, 1994), the following cancer
markers are
listed here as exemplary and may be used as well as other markers to analyze
the
condition of a breast duct, including analysis of the ductal contents
(including fluid and
cells). Standard assay procedures for identifying the maskers can be used,
including
antibodies or other binding partners, labels, stains, pattern analysis (for
cells and cell
components), and in general any other chemical or visual identification
techniques.
Markers that are presently being studied by researchers presently include,
carcinoma embryonic antigen (CEA), prostate specific antigen (PSA) Erb B2
antigen,
gross cystic disease fluid protein -15 (GCDFP-15), and lactose dehydrogenase
(LDH).
For CEA see Imayama et al, Cancer 1996, 78(6):1229-34; Inaji et al, Cancer
1987,60(I2):3008-13; Mori Int Conger Seer 1989, 807:211-8; Inaji, et al, An To
Kagaku
Ryoho 1991, 18(2):313-7; Yayoi, et al Gan To Kagaku Ryoho 1994, 21 Suppl 2:133-
9;
Mori, et ~al Jpn J Clin Oncol 1989,19(4):373j9; Foretova, et al Proc Annu Meet
Am Soc
Clin Oncol 1995,14:A101; and Nishiguchi, et al Rinsho Byori 1992,40(1):67-72.
For
PSA see Foretova, Garter Lancet 1996,347(9015):1631; Sauter et al, Cancer
Epidemiology, Biomarkers & Prevention. 5(12):967-70, 1996; Sauter and Daly
(1996)
Proc Annu Meet Am Assoc Cancer Res 37:A1458; and Foretova and Garter (1996)
Proc
34
CA 02356963 2001-06-27
WO 00/39557 PC'T/US99/31086
Annu Meet Am Assoc Cancer Res 37:A1446. For Erb B2 see Motomura (1995) Breast
Cancer Res and Treat 33:89-92; and Inaji et al ( 1993) Tumour Biol 14: 271-8.
For
GCDFP-I 5 see Petrakis et al ( 1994) Proc Annu Meet Am Assoc Cancer Res 35:A
1698.
For LDH see Mannello et al (1995) Cancer 76:152-4; and Kawamoto (1994) Cancer
S 73:1836-4.1.
Chromosomal abnormalities in ductal epithelial cells can also provide
information and act as a marker to identify cancer or precancer as described
in Mark et al
(1999) Cancer Genet Cytogenet 108:26-31; Lundlin and Mertens (1998) Breast
Cancer
Res Treat 51:1-15; Newsham (1998) Am JPathol 153:5-9; Larson et al (1998) Am J
Pathol 152:1591-8; Adelaide et al (1998) Genes Chromosomes Cancer 22:186-99;
Fejzo
et al (1998) Gene Chromosome Cancer 22:105-113; Dietrich et al (1998) Hum
Pathol 12:
1379-82; Cavalli et al (1997) Hereditas 126:261-8; Adeyinka et al (1997}
Cancer Genet
Cytogenet 97:119-21; Afify and Mark (1997) Cancer Genet Cytogenet 97:101-5;
Brenner
and Aldaz (1997) Prog Clin Biol Res 396: 63-82; Mark et al (1997) Ann Clin Lab
Sci
27:47-56; and Fabian et al 1993 J. Cellular Biochemistry 176:153-16.
In addition, exemplary markers are described in Masood, (Prediction of
recurrence for advanced breast cancer. Traditional and contemporary pathologic
and
molecular markers) Surgical Oncology Clinics of North America. 4(4):601-32,
1995;
Lopez-Guerrero et al (1999) JHematother 8(1):53-61; Marjumdar and Diamandis
(1999)
BrJCancer 79(9-10):1594-602; Balleine et al (1999) BrJCancer79 (9-10):1564-71;
Houston et al (1999) Br J Cancer 79(7-8):1220-6; Nikolic-Vukosavlj evic et al
( 1998)
Tumori 84(6):691-4; Maguire et al (1998) Int JBiol Markers 13(3):139-44;
Steams et al
(1998) Breast Cancer Res Treat 52(1-3):239-59; Eiriksdottir et.al (1998) Eur J
Cancer
34(13):2076-81, and USPN 5,169,774. Many Iaiown breast cancer markers are
discussed
and described in readily available medical textbooks on breast cancer. Other
markers are
also listed herein.
The morphology of the cells or cellular contents retrieved in the ductal
fluid and wash fluid may also be examined. The cellular contents can include,
e.g.
protein, nucleic acid, or other molecular markers in the cells. Cell
morphology can serve
to establish whether the ductal epithelial cells~are normal (i.e. not
precancerous or
cancerous or having another noncancerous abnormality), precancerous (i.e.
comprising
hyperplasia, atypical ductal hyperplasia (ADH) or low grade ductal carcinoma
in situ
(LG-DCIS)) or cancerous (i.e. comprising high grade ductal carcinoma in situ
(HG-
DCIS), or invasive carcinoma). Analysis of cell contents may serve to
establish similar
CA 02356963 2001-06-27
WO 00/39557 PC'T/US99l31086
staging as established by morphology, capturing generally a progression of a
precancerous or cancerous condition in the cells.
Administering fluid to the ductal lumen for the purpose of collecting that
fluid mixed with the fluid from the duct is complicated by the fact that
absorbable wash
fluids are partly absorbed into the breast from the duct. Thus, the fluid
retrieved is less
than that infused, even considering that it includes the ductal fluid that was
residing in the
duct. Administering an agent in the wash fluid that is capable of increasing
or
maintaining the fluid volume in the duct is a great advantage to the process.
Thus, the
invention provides administering a nonabsorbable fluid or a fluid that
actually draws fluid
to it, e.g. an oncotic or osmotic fluid in the process of collecting fluid
from the duct.
Administering the nonabsorbable fluid has the advantage also of providing the
practitioner with a way to monitor or standardize the ductal fluid and
cellular return in
any given volume of fluid infused and retrieved. For example 10 ml of the
nonabsorbable
fluid is administered to the duct, and 9.5 ml of that fluid is collected.
Maybe 100
epithelial clusters are contained in the fluid collected. This information can
be noted, and
during future procedures on that same duct can be compared. The advantage of
using a
nonabsorbable is that the ductal fluid yield may be increased with the
retrieval of most or
all of the infused fluid, and the practioner will be able to keep track of the
amount infused
versus the amount collected.
A nonabsorbable fluid can be used in order to provide a standardization to
the process so that the amount infused can be correlated with the amount
collected,
knowing that since the fluid cannot be absorbed in the duct, and collecting of
all or most
of the fluid that is infused is possible.
Identification of the location of the ducts prior to accessing them can be
made as described in PCT application to the Regents of the University of
California at
Los Angeles filed September 15, 1998 to Barsky et al entitled "Methods and
Kits for
Identifying Ductal Orifices in a Nipple", or USSN 09/153,564 filed September
15, 1998
to Barsky et al.
The agent is an agent capable of in effect increasing the amount of
collectable fluid in the ductal lumen. Thus-the agent can be a nonabsorbable
agent or
fluid or an oncotic agent and/or an osmotic agent or a combination of two or
all three.
Oncotic and osmotic agents are agents that retain fluid around them or draw
fluid to
them. The agent can be soluble, e.g. soluble in a suitable solvent, including
e.g. water,
buffered water, or a saline solution. Preferably the solvent is biologically
compatible
36
CA 02356963 2001-06-27
WO 00/39557 PCT/US99l31086
with mammals. Suitable solvents will be those that both effectively dissolve
the agent
and are not toxic to a mammal.
The agent can be a molecule including e.g. a protein, colloid, sugar, or
polymer. The agent can be mannitol, sorbitol, glucose, glycerol, sucrose,
raffinose,
S fructose, lactulose, sodium chloride, albumin, polyethyleneglycol (PEG),
maltodextrin,
dextran (e.g. dextran 70), hydroxyethyl starch, fluid gelatin, or a synthetic
colloid. Agents
including e.g. mannitol, sorbitol, PEG, glycerol are described in THE MERCK
INDEX,
12'h ed. 1996, Whitehouse Station, NJ. Others, including maltodextrin, dextran
and
others are available from Aldrich Chemical Co. in Milwaukee, WI or Sigma
Chemical
Co. in St. Louis, MO. The molecular weight of a suitable oncotic agent can be
determined as optimally within the range of the molecular weights of suitable
oncotic
agents available.
Where the agent is a protein, the protein can be a binding protein or an
antibody. The binding protein can be albumin. The antibody can be capable of
binding
IS an epitope found in a breast duct, e.g. an epithelial cell surface marker
or cancer cell
marker, etc. Where the agent is a protein, the protein is of a molecular
weight in the
neighborhood of albumin or higher, so that it is capable of acting as an
oncotic agent in
the lumen of the milk duct. Suitable antibodies are commercially available.
Also the agent can be a mixture of osmotic and/or an oncotic agents. The
oncotic agent and/or osmotic agent can comprise a mixture of any two or more
osmotic
and/or oncotic agents, e.g. mannitol, sorbitol, glucose, glycerol, sucrose,
raffinose,
fructose, lactulose, sodium chloride, albumin, polyethyleneglycol (PEG),
maltodextrin,
dextran (e.g. dextran 70), hydroxyethyl starch, fluid gelatin, an antibody or
a synthetic
colloid:
The agent can be an agent not capable of freely diffusing into or beyond
the cells that line the milk ducts of the breast. The agent can also be an
agent not capable
of absorption into the cells within the duct. For example, the agent can have
a molecular
weight large enough to make absorption or diffusion into the breast duct
lining, cells or
interstitial space beyond the lining improbable.
~ Whether an agent is capable r apable of increasing or at least maintaining
the amount of collectable fluid (with relation to the amount of fluid infused)
in the ductal
lumen can be determined by experimentation to identify whether collectable
fluid in the
duct is increased upon administration of an agent as compared to
administration of a
control isotonic solution to a neighboring control duct. Likewise the best
volume and
37
CA 02356963 2001-06-27
WO 00139557 PCT/US99/31086
concentration of the agent can be determined by a comparison of the amount of
collectable fluid yielded with a change in a variable such as a volume or
concentration of
agent administered. The agents including nonabsorbable fluid and/or oncotic
and/or
osmotic agents to be tested can be delivered to the duct of a human, rat,
rabbit, pig or
other appropriate mammal, and the ductal fluid can be collected. Where the
fluid yield is
greater than control fluid collected from a neighboring duct (after injection
of a control
solution, preferably of equal volume as the tested solution), that agent is
suitable for use
in the method. The increased fluid amount should be at least 50% and more
preferably
close to 100% of an increase of fluid collectable from the ducts that are
compared. In the
case where the practitioner seeks to increase the amount of fluid collected
from the
amount infused, the fluid yield from the duct administered with.the agent
being tested can
be several fold that of the control fluid yield. Where the goal is merely to
provide for a
collection fluid amount that is close to the amount infused, the parameters
for success are
that the amount of fluid collected from the duct after infusion of a set
aliquot of fluid is
closer to the amount infused that would have been possible if the infusion
fluid had been
an absorbable fluid such as saline. Such a comparison can be tested by doing a
control
infusion and collection in a duct using e.g. saline and then repeating the
procedure in the
same duct using a nonabsorbable fluid, e.g. a PEG containing fluid or the
like.
The appropriate concentration and volume of oncotic agent and/or osmotic
agent in solution injected into a duct can be determined by routine
experimentation
including cannuladon or catheterization of mammalian nipples (e.g. rat,
rabbit, pig or
human nipples) to determine at which concentration and volume the agent in
solution
yields the most volume of fluid collectable from the duct as compared to the
fluid
collectable from a control duct. Experiments can be designed for testing a
variety of
oncotic and/or osmotic agents, concentrations, volumes, and mixtures of agents
in all
varieties of mammals having breast ducts.
Fluid collected from the milk ducts, can include constituents of biological
fluids, e.g. those typically found in breast duct fluid, e.g. water, cells,
cellular markers,
molecular markers, nucleic acids, proteins, cellular debris, salts, or organic
molecules.
.These constituents can be analyzed by any appropriate method depending on the
practitioner's purposes in obtaining the fluid.
The fluid can comprise cells including e.g. epithelial cells and abnormal
cells. The cells can be analyzed for cellular, protein, nucleic acid, or other
molecular
markers or for shape or other abnormalities. Analysis of the cells can provide
diagnostic
38
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
or prognostic information for an evaluation of the condition of the breast or
breast ducts.
Removal of cells can be conducted in the presence of the agent, and preferably
the action
of the osmotic and/or oncotic agent provides for removing cells that can be
analyzed.
The invention includes a kit for increasing the amount of fluid collectable
from a milk duct of a breast comprising an nonabsorbable agent andlor an
osmotic agent
and/or an oncotic agent, a medical tool for delivering the agent to the ductal
lumen, and
instructions for use. The nonabsorbable agent and/or oncotic andlor osmotic
agent can be
those described herein or other comprising like properties and/or functions in
a breast
duct. The medical tool can be any tool that enables delivery of such agent.
The
instructions can direct a protocol for administration including how to
administer the
agent, how much time to wait before collecting the fluid, how to collect the
fluid, and
how to analyze the fluid collected.
The retrieved fluid can comprise constituents of the breast milk duct fluid,
e.g. including water, cells, cellular markers, molecular markers, nucleic
acids, proteins,
1 S cellular debris, salts, or organic molecules. Analyses can be made that
identify molecular
or cellular markers, cellular characteristics, e.g. by cytology, and for
making any other
assessment of any of the constituents of the fluid. Cells that are retrieved
and analyzed
can be epithelial cells or abnormal cells.
Multiple lumen ductal access catheters, having more than one lumen in the
access portion of the catheter, according to the present invention will
comprise a catheter
body having a distal end and a proximal end and including at least a distal
portion and a
proximal portion. The catheter will have at least two continuous lumens
extending
through both the proximal and distal portions. The lumens can be fluid
carrying, and
fluid can pass from the proximal portion to the distal portion of one lumen
and from the
distal portion to the proximal portion of a second lumen. The distal portion
has a cross-
sectional geometry which can be inserted through a ductal orifice into a
ductal Lumen of a
human breast for the purpose of lavaging the breast duct. The proximal portion
has a
cross-sectional geometry which inhibits insertion through the ductal orifice
and into the
ductal lumen thereby placing limits on the extent that the catheter penetrates
the breast
duct during the Iavage procedure. The lumens of the catheter are an infusion
lumen and
an aspiration or collection lumen. Each lumen has a distal port near the
distal end of the
distal portion of the catheter. One distal port is for infusing Liquid into
the duct (a port on
the infusion lumen). The other distal port is for aspirating or collecting
fluid from the
duct (a port on the aspiration lumen). The catheters also have a proximal
connector near
39
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
a proximal end of the proximal portion for connecting e.g. to a fluid
receptacle that holds
fluid for infusion into the duct (e.g. a wash or lavage fluid) and for
connecting to a
collection receptacle for collecting the contents of the duct that are
aspirated in the
aspiration lumen (e.g. a syringe that can both aspirate and collect the ductal
fluid and/or
ductal contents).
The ductal access catheter will have a total length in the range of from
about 2-cm to about 60-cm, usually about 30 cm to about 45-cm. The length of
the
proximal portion will typically be in the range of about 15-cm to about 50 cm,
more
typically in the range from about 30 cm to about 40 cm. The distal portion
will typically
be in the range from about 2.5-cm to about 8 cm, more typically in the range
from about
3.0 cm to about S.5 cm.
The proximal and distal portions will preferably be joined to each other
with an intermediate zone between them to accommodate the difference in cross-
sectional
geometry between the proximal and distal portions. The intermediate zone can
be a
1 S stepped decrease in cross-sectional geometry from proximal to distal
portions, or may be
a gradual decrease in cross-sectional geometry from proximal to distal
portions. The
body segments can be joined in any conventional manner to each other (and/or
to the
either end of the element creating the intermediate zone) including methods
such as heat
fusion, adhesive bonding, coextrusion, or the like. In the exemplary
embodiment, the
distal and proximal portions will be coextruded and the coextrusion process
will generate
the intermediate zone in accommodating the differential cross-sectional
geometry of the
proximal to the distal portions.
The catheter can have the distal portion of the catheter body stiffened over
at least-a part of its length to facilitate insertion through the ductal
orifice and into the
ductal Lumen. The stiffening effect can be created by insertion of a third
lumen in the
distal portion, the third lumen comprising a wire. The wire can be made of
some
relatively stiff metal, e.g. tungsten or steel. The stiffened distal portion
of the catheter
body can have an average bending stiffi~ess in the range from about 0.010 inch-
Ibs to
about 0.5 inch-lbs. Typically the bending stiffness of the distal portion will
be about
0.105 inch-lbs:
The catheter may be composed of any biologically compatible polymeric
resins or metal having suitable characteristics when formed into the tubular
catheter
portions. Exemplary materials include polyvinyl chloride, polyethers,
polyamides,
polyethylenes, polycarbonate, polyurethanes, copolymers thereof and the like.
The distal
CA 02356963 2001-06-27
WO 00/39557 PCT/US99l31086
portion may be formed of the same or different material as the proximal
portion.
Although a stiffening wire may be placed in the distal portion, if the
stiffening wire is not
present, the distal portion may be composed of materials that are slightly
more stiff than
the materials that compose the proximal portion. Optionally, the distal body
portion may
S be reinforced with a metal or polymeric braid or other conventional
reinforcing layering.
The distal portion will be sufficiently rigid to permit axial positioning of
the distal tip in a ductal orifice with the distal portion extending either
partly or wholly
into the breast ductal lumen. The distal portion will typically have a
hardness in a
durometer range at least greater than that of the proximal portion, and thus
generally
greater than 75D. The hardness of the distal portion thus may be a range from
about 70D
to about 90D. The proximal portion will be more flexible and less stiff and
also less hard
than the distal portion. The durometer of the proximal portion outer tubing
can be in a
range from about 45A to about 100A, and typically about 80A. The inner tubing
of the
proximal portion can have a durometer in the range from about SOD to about
75D, and
typically about 63D. The flexibility of the proximal portion provides the
catheter with
the advantages that the distal portion (which is stiffer) can be inserted into
the breast duct,
meanwhile the proximal portion can connect at its hubs with infusion or
collection
apparatus and not kink during the placement of the distal portion in the
breast duct.
Additionally, the flexibility of the proximal portion provides the advantage
that once the
distal portion is placed in the breast duct the catheter will have less
tendency to pull out
of the duct. The stiffness of the distal portion benefits the procedure by
allowing access
into the orifice of the duct and the duct itself, an action that requires a
probe-like quality
of the distal portion and distal tip in order the duct to be accessed
successfully.
The catheter body may further comprise other components, such as
radiopaque fillers; colorants; reinforcing materials; reinforcement layers,
such as braids
and helical reinforcement elements; or the like. In particular it would be
possible to
reinforce the distal portion in order to enhance its duct penetration or probe-
like
capabilities while optionally limiting its wall thickness and outside diameter
so that the
catheter can easily access even ducts with small ductal orifices.
- The cross-sectional geometry of the distal portion of the catheter body will
be smaller than the cross-sectional geometry of the proximal portion. The
cross-sectional
geometry of the distal portion provides that the distal portion can be
inserted into a breast
duct orifice and through the orifice into the breast duct lumen. The distal
portion of the
catheter body has a maximum width in the range from 0.008 inches to 0.050
inches. The
41
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
distal portion of the catheter body has a generally tubular structure with a
diameter in the
range from about 0.008 inches to about 0.035 inches. The proximal portion has
a cross-
sectional geometry which inhibits insertion of the proximal portion into the
ductal orifice
and the ductal lumen. Thus, the proximal portion of the catheter body has a
minimum
width in the range from about 0.023 inches to about 0.028 inches. The proximal
portion
of the catheter body has a generally tubular structure with a diameter in the
range from
about 0.030 inches to about 0.10 inches. The proximal diameter is greater than
the distal
diameter by at least about 0.010 inches.
The region between the proximal and distal portions of the catheter body
provides for the reduced diameter moving from the proximal to the distal
portions. The
transition preserves the fluid flow capability and communication in the lumens
between
the proximal and distal portions and can provide a place to anchor or lodge a
wire or
stiffening lumen that extends longitudinally in the distal portion. The
transition may be
stepped, abrupt, or somewhat gradual, provided it allows the proximal portion
to retain its
I 5 function of inhibiting insertion of the catheter into the duct beyond the
length of the distal
portion.
The ductal access catheter body can comprise at least an infusion lumen
and an aspiration lumen each of which has a distal port near a distal end of
the distal
portion. At least one of the distal aspiration port and the distal infusion
portion can be
disposed on a side of the distal portion of the catheter body. Thus one port
can be a side
port and one port can be and end port. The distal aspiration port and the
distal infusion
port can both be located on the side of the distal portion of the catheter
body. Thus, both
ports for both lumens can be side ports. The distal aspiration port and the
distal infusion
port can be axially aligned. Thus, the side ports can be located e.g. opposite
each other
on the on the distal portion of the catheter body at the longitudinal
position. For example,
both side ports can be located about 2.5 cm from the distal tip. The distal
aspiration port
and the distal infusion port can be axially spaced apart. Thus, the side ports
can be
located at different longitudinal positions on the distal portion, for example
one port can
be located about 2.0 cm from the distal tip and one port can be located about
2.5 cm from
the distal tip. The side ports themselves maybe round or oval or any other
geometric
shape conducive to fluid flow either into the duct or out from the duct. The
diameter of
the ports can be that diameter which is suitable to achieve a desired flow
rate into the duct
or aspiration or collection rate out from the duct. Thus, the diameters of the
ports can be
in a range from about 0.015 inches (.038 mm) to about 0.022 inches (0.056 mm),
most
42
CA 02356963 2001-06-27
WO 00/39557 PC?/US99/31086
typically in a range from about 0.016 inches (.041 nun) to about 0.020 inches
(0.051 mm).
One side port can be larger or smaller than the other, especially where such
differential
port size provides a desired flow rate into or out from one of the lumens, or
an overall
lavage efficiency of infusion and aspiration or collection of lavage and
ductal fluid.
The catheter body can include an atraumatic distal tip. The tip can be
contoured and/or rounded to reduce or eliminate trauma to the duct upon entry
through
the ductal orifice and penetration into the ductal lumen. The tip may also be
fashioned to
reduce or eliminate trauma upon withdrawal of the tool from the duct after the
lavage
procedure is completed. The tip can be composed of a soft polymeric material,
e.g.
including polyvinyl chloride, polyethers, polyamides, polyethylenes,
polyurethanes,
copolymers thereof and the like. The tip can have a diameter in the range from
about
0.012 inches (0.031mm) to about 0.020 inches (0.05Imm), more typically a
diameter in
the range from about 0.014 inches (0.036mm) to about 0.018 inches (0.046mm).
The
length of the tip (extending from the distal end of the distal portion of the
catheter) can be
in a range from about 0.25cm to about 2.Scm, more typically in the range from
about
O.SOcm to about l.8cm.
The invention also provides a method for lavage of a ductal network in a
human breast comprising providing a catheter as any described above for
performing the
lavage procedure. The distal portion of the catheter is inserted through a
ductal orifice
and into a distal lumen of the ductal network. A wash fluid is introduced
through the
infusion lumen into the ductal network. The wash fluid can be, e.g. saline or
phosphate
buffered saline, or any biocompatable fluid suitable for washing a breast duct
lumen. The
wash fluid and substances borne by the wash fluid are withdrawn from the
ductal network
through the aspiration lumen. The various features of the catheters described
above can
serve to facilitate the practice of the lavage procedure. For example, the
narrow distal tip
provides the catheter the ability to penetrate the ductal orifice and move the
catheter into
the ductal lumen for performing the lavage procedure; the larger diameter of
the proximal
portion inhibits the catheter from passing too deeply into the duct, and stops
the
penetration of the catheter at the place where the distal portion ends and the
proximal
portion begins; the atraumatic tip provides the catheter the ability to
penetrate the duct
without trauma to the tissue walls of the ductal lumen; the stiffening
material placed in at
least a part of the distal portion of the catheter (e.g. a stiffening wire or
a supporting braid
or the like) provides the practitioner with stiffness to better control the
entry and further
penetration of the catheter into the ductal lumen; the ports on the lumens
provide the
43
CA 02356963 2001-06-27
WO 00/39557 PCTNS99/31086
catheter the ability to infuse liquid into the duct from the infusion lumen
and the ability to
aspirate or collect fluid from the duct into the aspiration lumen; and where
the ports are
side ports, the presence of side ports may better facilitate the function of
the ports for
infusing and collecting to and from the duct.
The invention further provides a ductal access system comprising any of
the catheters describe herein and instructions for use setting forth a method
for lavage of a
ductal network in a human breast including introducing a wash fluid through
the infusion
lumen into the ductal network and withdrawing the wash fluid and substances
borne by
the wash fluid from the ductal network through the aspiration lumen, e.g. as
described for
the method above.
EXAMPLES
1. Collecting Cells and Cellular Material Using Single Lumen Ductal Access
Device
Device as depicted in FIG 3 was used to access breast ducts of patients A,
B, C, D, E, F, G, and H. Before ductal access patient's nipple was cleaned
with alcohol,
and dekeratinized with cerumetix. An aspiration cup was placed on the nipple
and areola
and the patient's nipple was aspirated to identify the breast duct and to
collect fluid for a
comparison with the fluid retrieved from inside the duct. A small quantity of
fluid was
observed on the nipple surface after aspiration and this fluid was collected
with one or
more capillary tubes placed in contact with the fluid. The aspiration fluid
was preserved
in a preservative solution for cells for analysis later.
Ducts that yield fluid were accessed using a dilator that extended from the
device depicted in Fig. 3, and once the duct was accessed by the access tube,
the dilator
was withdrawn. The collection tube was closed, and the system including the
infusion
tube and manifold were primed with fluid. A total of from 10 ml to about 25 mI
of saline
infusion fluid was infused into the duct until resistance was felt in the
infusion syringe.
The assumption made at that point was that the duct was filled with the
infusion fluid.
The infusion tube was closed and the collection tube opened. The breast was
massaged
and then squeezed and cloudy fluid was caused to enter the hub and begin to
exit the
collection tube. To encourage the fluid to exit, the infusion tube was opened
and
additional irifiision fluid was pushed into th~~hub, causing more cloudy fluid
to exit the
collection tube. The following fluid amounts refer to the procedure with
Patient A. When
a volume of about 11.5 ml of fluid was collected, the collection tube was
closed and more
fluid infused until a resistance was felt in the duct. More fluid was infused
to refill the
duct. The collection tube was opened, and infusion tube was closed and the
breast was
44
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
massaged and squeezed to encourage more fluid to enter the hub and exit the
collection
tube. Additionally, the fluid was encouraged to leave the hub with an
injection of fluid
from the infusion lumen. About 6 ml was collected from the second filling.
The results of the nipple aspiration (NAF), first filling and second filling
S are reported below for patient A in the Table I. Patient's B, C, and D also
have NAF
results compared to results using the single lumen catheter, as depicted in
Table I.
Patients E, F, G and H have yields solely with respect to access and retrieval
using the
single lumen catheter. Epithelial cell clusters are defined as clusters of
cells having
greater than 10 epithelial cells per cluster.
Table I
Sample Collection Total Epithelial Clusters
volume ( > 10 cells /
collectedcluster)
Patient A; NAF (nipple aspiration> 0.1 I epithelial cluster
duct R2 fluid) ml
lavage with single 17.5 23 epithelial clusters
lumen ml
Patient B; NAF ------- 0 epithelial clusters
duct L6
lavage with single --- 31 epithelial clusters
lumen
Patient C; NAF 0.2 ml 0 epithelial clusters
duct RI
lavage with single 6 ml 27 epithelial clusters
lumen
Patient D; NAF < 0.1 0 epithelial clusters
duct on left ml
nipple
lavage with single 7 ml 101 epithelial
lumen clusters
Patient E; lavage with single 11 ml 3 epithelial clusters
duct L6 lumen
Patient F; lavage with single I0.5 12 epithelial clusters
duct L6 lumen ml
duct L7 lavage with single 7 ml 7 epithelial clusters
lumen
duct Rl lavage with single 21 ml 6 epithelial clusters
lumen
duct R2 lavage with single 7 ml S epithelial clusters
lumen
Patient G; lavage with single 6 ml 400 epithelial
duct L6 lumen clusters
duct Rl lavage with single 8.5 ml 350 epithelial
lumen clusters
Patient H; layage with single 11 ml 154 epithelial
duct L6 Lumen clusters
duct R1 lavage with single 7 ml 131 epithelial
lumen clusters
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
2. Comuarative Study: Pig Pelt Lava~e with Stopcock Catheter vs. Catheter
without
Stopcocks
In order to test the efficiency of the stop cock catheter, a comparison was
run comparing the amount of fluid and cells retrieved from a catheter which
did not have
stop cocks (or on which both stop cocks were kept open during the entire
procedure), and
a catheter having a stop cock on the inflow lumen and a stop cock on the
outflow lumen
that are opened and closed alternately during the procedure (in the manner
specified
below). Two experiments (experiment I and experiment II) were conducted using
modified procedures A and B to test modifications to the basic principles of
the
comparison. The combined results show the increased efficiency of using the
stop cock
catheter (in the manner described below in the procedure A portion of
experiments I & II)
to retrieve a larger volume of infused wash fluid and a larger number of
ductal cells from
the lavaged breast milk duct.
A dual lumen catheter having a support wire is used for the tests. The
catheter also has a stopcock on the inflow lumen and a stopcock on the outflow
lumen.
Frozen pig pelts were purchased from Yosemite Meats, located in Menlo Park,
CA.
Procedure A was conducted using the stopcocks controlling the opening and
closing of
the inflow and outflow lumens at ports located in the stopcocks. Procedure B
was
conducted with both the inflow and outflow lumens open during the entire
procedure.
Experiments I and II were conducted as follows:
Procedure A included the following steps:
1. Catheter was inserted into a duct. Both inflow and outflow lumens were
primed with wash fluid, and the ports closed (by placing the respective
stopcocks in a
closed position). The catheter was placed in the duct.
2. The outflow port was opened, and lml of phosphate buffered saline (PBS)
was infused into the duct to flush out the outflow lumen. The outflow port was
closed.
3. The inflow port was opened and PBS was infused into the duct until
resistance to infusion was met. The inflow port was closed.
4. The outflow port was opened.' Tlie breast was massaged and squeezed.
The outflow fluid was collected.
46
CA 02356963 2001-06-27
WO 00/39557
PCT/US99/31086
5. The outflow port remained opened, the inflow port was opened, and 0.5 ml
of fluid was infused into the inflow to flush out the outflow lumen. The
inflow port was
closed and the fluid collected in the collection syringe attached to the
outflow lumen.
6. The outflow port was closed and about 1 ml of PBS was infused. The
inflow port was closed. The outflow port was opened. The breast was massaged
and
squeezed, and the fluid collected. Steps S and 6 were repeated until about 3
ml of fluid
was collected.
Procedure B included the following steps:
1. A dual lumen catheter (with both inflow and outflow ports open) was
inserted into the duct.
2. The duct was infused with PBS until the fluid flow into the duct met
resistance.
3. The duct was lavaged using massaging and squeezing technique as the
fluid was collected in the collection receptacle (located at the end of the
outflow lumen).
4. More PBS was infused (each time about 1 ml) and the massaging,
squeezing and collecting proceeded. The procedure was repeated until about 3
ml was
collected.
Procedure A (one-way flow procedure) included the following steps:
1. Both the inflow and outflow lumens were primed with PBS. The inflow
and outflow ports were closed and the catheter inserted into the pig duct to a
depth of
about 1.5 cm.
2. The outflow port was opened and 1 ml of PBS was infused into the duct to
flush out the outflow lumen.
3. The outflow port was closed. The inflow port was opened. Fluid was
infused in the inflow port (about 4-5 ml) until resistance was felt. The
inflow port was
closed.
4. The breast was massaged with both ports closed.
5. The outflow port was opened and the breast squeezed to collect fluid in the
collection receptacle until no more fluid comes out.
6. The inflow port was opened and about 0.5 ml of fluid was infused to flush
out the outflow lumen.
47
CA 02356963 2001-06-27
WO 00/39557 PCTIUS99131086
7. The outflow port was closed and about 1 ml of PBS was infused into the
duct in the inflow lumen.
8. The inflow port was closed. The outflow port was opened, and the breast
massaged and squeezed to collect the outflow fluid. Steps 6, 7, and 8 were
repeated until
S the collection volume totaled 3 ml.
Procedure B included the following steps:
1. Both the inflow and outflow lumens of a dual lumen catheter were primed
with PBS. The catheter tip was inserted into a breast duct of a pig pelt to a
depth of about
1.5 cm. About 1 ml of PBS was infused into the duct through the outflow lumen
to flush
out the outflow lumen.
2. PBS was infused into the inflow port until resistance was felt.
3. The breast was massaged and squeezed as the duct was lavaged with PBS.
Fluid flowing to the outflow lumen was collected.
4. More PBS was infused into the duct (each time about 1 ml) and the duct
lavaged, (using massaging and squeezing) and the fluid collected in the
collection
receptacle. The procedure was repeated until about 3 ml of fluid was
collected.
The fluid was used to prepare Cytospin~ slides by taking 10 ul of
collected fluid (plus 90 ul of PBS), using a cytospin machine to place the
fluid on a slide.
The slides are air-dried and Diff Quik~ stained. The results are shown below
in Table II.
In all cases 3 ml of fluid was collected, but the infusion volume varied as
shown in the
table.
Table II
Procedure A Procedure B
experiment
I
nipple infusion cell densitynipple infusion cell density
vol vol
X2 15 ml 55% Z2 13 ml 45%
X3 18 ml 65% X1 10 ml 35%
Z3 13 ml 40% Z1 13 ml 45%
ZS 12 ml 55%
experiment
II
nipple infusion cell densitynipple infusion cell density
vol vol
X1 . 14 ml 60% Zl 7 ml - 50%
'
X2 12 ml 65% Z2 6 ml 50%
X3 13 ml 65% Z3 6 ml 40%
X4 11 ml 55% Z4 6 ml 35%
average 13.5 ml 58% 8.7 ml 43%
48
CA 02356963 2001-06-27
WO OOI39557 PCT/I1S99l31086
The results indicated a 35% increased cell yield using procedure A over
procedure B.
3. Optimal Stop Cock Catheter Usaee for Retrieving Cells
A procedure was developed that appeared to optimize the potential yield of
cells from the ductal fluid retrieved was a catheter-based lavage procedure of
a breast
duct. Using pig pelts the following technique resulted in maximized cell yield
from a set
volume of collected fluid.
1. Inflow and outflow lumens of a dual lumen catheter having stop cocks on
both lumens were primed with PBS. The inflow and outflow ports were closed and
the
catheter inserted into the duct to a depth of about 1.5 cm.
2. The outflow port was opened and 1 ml of PBS was infused into the duct
from the outflow lumen to flush out the outflow Lumen.
3. The outflow port was closed (using the stopcock) and the inflow port was
opened. PBS was infused into the duct until resistance was felt (about 4-5 ml
of PBS).
The inflow port was closed.
4. The breast was massaged while both the inflow and outflow ports were
closed (using the stopcock controls).
5. The outflow port was opened and the breast was massaged and squeezed,
and the outflow fluid was collected until no more fluid came out.
6. The outflow port was closed and the inflow opened, and more fluid (about
1 ml) was allowed to infuse into the duct.
7. The inflow port was closed and the outflow port was opened. The breast
was massaged and squeezed to collect the fluid.
8. Steps 6 and 7 were repeated until a total of 3 ml of fluid was collected.
This procedure was found to generate the best cell density collected in the 3
ml of fluid, and also eliminates one step from previous procedure A.
4. Mannitol Solution Introduced into Breast Ducts of Live Rabbit Results In
Increased Ductal Fluid Collection
The objective of these expe~irnents was to test the effects of the
introduction of a solution containing mannitol on the secretion of fluid from
the breast
ducts of live rabbits. New Zealand rabbit #3242, female, from Kraelik Farm in
CA
weighing 4.1 kg was used. The rabbit was anesthetized by injection of 200 mg
of
49
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
ketamine and 40 mg of Zylazine. A second injection of 100 mg of ketamine and
20 mg
of xylazine was made 2 hours later to maintain the rabbit in a deep plane of
anesthesia.
The thorax and abdomen of the rabbit was shaved to expose the breasts and
nipples.
A single lumen blue color catheter (O.D. 0.23" m 0.017; O.D. at the tip
0.011" - 0.012") was inserted into a duct in each nipple. Three nipples were
tested, and 2
ducts per nipple were accessed with a catheter. The nipples were identified A,
B, and C.
A duct on nipple A was injected with 0.20 ml of a 12.5% solution of D-
Mannitol in H20 (available from Sigma Chemicals, St. Louis, MO cat# M-9546 lot
6710402: C6H,4O6 FW 182.2) with a single catheter. The control duct on nipple
A was
injected with 0.20 ml of phosphate buffered saline (PBS). A microfuge tube was
attached
to the end of each catheter to collect out flow liquid. Ten minutes later 0.2
ml of a 12.5%
solution of D-Mannitol in H20 was injected into the first duct and the second
duct was
injected with 0.20 ml of phosphate buffered saline, for a total volume
injected in each
duct of 0.40m1.
A duct on nipple B was injected with 0.5 mI of a I2.5% solution ofD-
Mannitol in HZO with a single catheter. The control duct on nipple B was
injected with
0.50 ml of phosphate buffered saline (PBS). A microfuge tube was attached to
the end of
each catheter to collect out-flow liquid.
A duct on nipple C was injected with 0.7 ml of a 12.5% solution of D-
Mannitol in H20 with a single catheter. The control duct on nipple C was
injected with
0.70 ml of phosphate buffered saline (PBS). A microfuge tube was attached to
the end of
each catheter to collect out flow liquid.
About an hour after the fluid containing mannitol or PBS was injected into
the ducts via the catheters, the microfuge tubes were checked for whether any
fluid was
returned. The results are summarized in the following Table III:
Table III
nippleduct solution recovery notes
A AI 0.4m1 mannitol 12.5%310 ul liquidfluid was a milky
color
A A2 0.4m1 PBS none -------
B B1 O.SmI mannitol 12.5%4JU ul liquidfluid was a millcy
color
B B2 O.SmI PBS 240 ul liquidfluid was a milky
color
C CI 0.7m1 mannitol 12.5%280 ul liquidfluid was a milky
color
C CZ 0.7m1 PBS none ----
SO
CA 02356963 2001-06-27
WO 00/39557 PCT/US99/31086
Davidson green dye (1 ul) was added to each microfuge tube containing
fluid for the purpose of taking a picture. The rabbit was euthanized by IV
injection of
supersaturated KCI. PBS (1.5 ul) was added to each collection. The cells were
spun onto
Shandon coated slide using megafunnel and cytospin-3 machine (Shandon, Inc.
located in
S Pittsburgh, PA) at a speed of 1500/per minute for 1 S minutes. The cells
were fixed on the
slide in 95% ethanol for 10 minutes. The cells were stained using Hematoxylin
and
Eosin (1-lE) method of cytology of collected fluid. The results of the
cellular analysis are
in Table IV:
Table IV
Nipple A Nipple B / Nipple B /ductNipple C /
/ duct A1 duct Bl B2 duct Cl
A few ductalA few ductai A few ductal A few ductal
cell cell cell cell
clusters clusters and clusters and clusters and
and
scattered scattered scattered scattered
histocytes histocytes histocytes histocytes
and apocrineand apocrine and apocrine and apocrine
metaplastic metaplastic metaplastic metaplastic
cells cells cells cells
The observations made from this experiment are that fluid can be collected
from three out of three ducts injected with mannitol solution; that fluid
could be collected
from 1 out of 3 ducts injected with PBS solution, and with approximately 50%
less
volume in the ducts where fluid was collected. There were cells detected from
the fluid
collected from each duct. The cell morphology looked similar between the
mannitol and
1 S the PBS injected ducts.
All publications and patent applications cited in this specification are
herein incorporated by reference as if each individual publication or patent
application
were specifically and individually indicated to be incorporated by reference.
Although
the foregoing invention has been described in some detail by way of
illustration and
example for purposes of clarity of understanding, it will be readily apparent
to those of
ordinary skill in the art in light of the teachings of this invention that
certain changes and
modifications may be made thereto without departing from the spirit or scope
of the
appended claims.
51