Note: Descriptions are shown in the official language in which they were submitted.
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-1-
TITLE OF THE INVENTION
ELECTRO-PNEUMATIC DISTRIBUTOR FOR MULTIPLEXED
u-TAS DEVICES
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims the priority of U.S.
Provisional Patent Application No. 60/115,167 filed,
January 8, 1999 entitled ELECTRO-PNEUMATIC DISTRIBUTOR
FOR MICROFABRICATED u-TAS DEVICES, the whole of which is
hereby incorporated by reference herein.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
N/A
BACKGROUND OF THE INVENTION
Microfabricated systems, or microdevices,
particularly multiplexed systems, with integrated
channels for performing chemical analyses on a micro-
scale level are an integral part of modern analytical
methods. Such systems, frequently called Micro-Total-
Analytical-Systems (u-TAS), are expected to play a
significant role in analytical and bioanalytical
chemistry as well as in modern chemistry in general.
Simultaneously, highly parallel structures are being
developed for high throughput analyses. Although many
structures can be completely integrated on the same
microdevice, it is always necessary to use supporting
CA 02357623 2001-07-06
WO 00/41214 PCT/C1S00/00470
-2-
devices to communicate with the "macro-world."
Additional supporting devices suitable for high
throughput analyses would be highly desirable.
BRIEF SUMMARY OF THE INVENTION
The invention is directed to a universal electro-
pneumatic distributor for supplying electric current and
pressurized gas where needed, e.g., to microfabricated
devices, and to methods for its use. The distributor of
the invention is suitable for simultaneous, selective
application of pressure and electric current, e.g., to
individual channels of a microdevice, in a
microfabricated u-TAS system, so as to cause a fluid
sample in an individual well in the surface of the device
to flow in the associated individual channel and an
electric current to flow across the channel. The
function of the distributor of the invention is described
here as a distributor assembly in conjunction with a
microdevice for electrospray mass spectrometry, e.g.,
according to U.S. Patent No. 5,872,010, the whole of
which is~hereby incorporated by reference herein.
An electro-pneumatic distributor assembly for
electrospray mass spectrometry can be attached to a
linear computer controlled translation stage. When the
system is in use, an individual channel exit port is
aligned with the mass spectrometer sampling orifice, and
gas pressure, e.g., is applied sequentially through a
switching board coupled with the system. The switching
board can also be used to connect the high voltage power
supply to induce electrospray sample ionization. High
throughput ESI/MS is achieved by application of both
electrospray voltage and pressure sequentially to the
CA 02357623 2001-07-06
WO 00/41214 PCT/LTS00/00470
-3-
samples loaded in the individual sample wells in the
microdevice. Sample throughput is maximized since a
subsequent sample can be analyzed immediately after
sufficient information is acquired from the previous one.
There are barely any delays between the analysis of
individual samples since no injection or washing steps
are involved.
Alternatively, the system of the invention is for
matrix assisted laser desorption ionization mass
spectrometry. Such a system includes an interface having
multiple deposition tips in conjunction with the electro-
pneumatic distributor of the invention.
In another embodiment of the system of the
invention, a liquid sample handling microdevice
comprising an array of electrodes embedded in the device
is associated with a pneumatic distributor that includes
a microfabricated structure comprising an array of
channels for gas transport. Preferably, the liquid
sample handling microdevice is an electrospray interface
having multiple electrospray tips, said electrospray
interface further comprising an array of electrodes
embedded in said interface, wherein individual electrodes
in said array connect with individual said electrospray
tips, and the microfabricated structure includes an array
of channels for gas transport, said channels being
oriented to permit application of pressure to selected
individual electrospray tips of said interface.
The acceleration of drug discovery in recent years
has presented significant analytical challenges. The
number of compounds to be analyzed has increased
dramatically since the introduction of combinatorial
chemistry with automated parallel synthesis. High
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-4-
throughput analytical techniques have become critical for
determining the identity and purity of synthesized
substances, as well as for clinical screening,
pharmacokinetics and proteome related research.
Most of the current protocols for high throughput
analysis are based on 96 (or larger) microtiter well
plate technology where a large number of samples can be
processed in parallel. The electro-pneumatic distributor
assembly of the invention can be made compatible with
the standard microtiter well plate technology format so
that currently used sample processing procedures, such
as solid phase extraction/desalting or enzyme digestion,
can be combined on-line for complete, high throughput
sample analysis.
BRIEF DESCRIPTION OF THE DRAWINGS
Other features and advantages of the invention will
be apparent from the following description of the
preferred embodiments thereof and from the claims, taken
in conjunction with the accompanying drawings, in which:
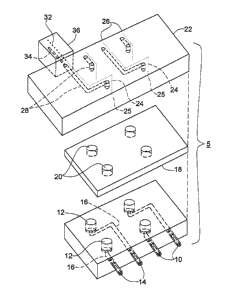
Fig. 1 is an exploded view of an electro-pneumatic
distributor assembly of the invention;
Figs. 2A-2B show high throughput ESI-MS analysis
using a plastic distributor system of the invention
having 96 electrospray tips. (A) Cytochrome c and
myoglobin solutions (5 uL) were alternately loaded into
consecutive sample wells, and each well was analyzed
every 5 seconds over a 40 sec time period. The
concentrations for both proteins were 0.1 mg/mL. (B)
Angiotensin II and angiotensin III solutions (5 uL) were
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-5-
alternately loaded into the sample wells, and all 96
samples were analyzed as in (A). Concentrations of both
peptides were 10 ug/mL;
Figs. 3A-3B show MS determination of HIV-1 protease
inhibition using the system of the invention. (A)
Relative signals of selected ion monitoring (SIM) spectra
of the product tripeptide (Pro-Ile-Val; m/z =328 +/- 4)
and the internal standard (Glu-Ile-Val; m/z =360 +/- 4)
after incubation with increasing concentrations of
pepstatin A (0-5uM). (B) Plot of data extracted from Fig.
3A; the ICSO was determined to be 0 . 7 5 uM with an RSD of
1.30;
Figs. 4A-4B shows fabrication of a 96 ESI channel,
96 well microdevice for use in the system of Fig. l,
wherein Fig. 4A shows preparation of a silicone rubber
negative imprint used for epoxy casting and Fig. 4B is a
flow chart for device fabrication;
Fig. 5A is a micrograph of a microdevice for the
system of the invention;
Fig. 5B is a detail of the microdevice of Fig. 5A
showing sample wells connected to 300 um wide
semicircular distribution channels;
Fig. 5C is a detail of the microdevice of Fig. 5A
showing an array of embedded electrodes for sequential
connection of the electrospray high voltage; and
Fig. 6 is an exploded view of the system of the
invention in position on a translation stage.
DESCRIPTION OF THE PREFERRED EMBODIMENT OF THE INVENTION
Mass spectrometry (MS) has become an indispensable
tool for pharmaceutical research because of its
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-6-
capability of sample identification, structure
elucidation, quantitation and sensitivity. Electrospray
ionization (ESI) and atmospheric pressure chemical
ionization (APCI) are the most frequently used sample
ionization techniques for automated high throughput MS
analysis and are often coupled on-line with liquid
chromatography (LC) or capillary electrophoresis (CE).
Nevertheless, a significant portion of ESI-MS
applications are also performed in the direct infusion
mode. Typically, infusion ESI-MS is carried out with a
flow injection (FIA) system equipped with an autosampler.
Since every sample in such a system flows through the
same conduit from the sampling probe through the
injection valve to the ESI tip, the sampling probe must
be carefully washed, and the flow conduit appropriately
flushed to minimize sample cross contamination. Thus,
useful mass spectrometric information can be observed
only during a fraction of the total analysis time,
leading to a low duty cycle. The electro-pneumatic
distributor assembly of the invention is a qualitatively
different approach to sample injection, permitting a
significant improvement in performance with maximization
of sample throughput.
Considering the wide acceptance of the microtiter
well plate format in automated analysis and the
potentially low cost of plastic devices, a disposable
microdevice system equipped with an independent
electrospray exit port for each sample well represents an
attractive alternative to FIA. A microdevice with sample
reservoirs positioned in the format of a standard
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
_7_
microtiter well plate can be used as the final receiving
plate in a parallel sample processing scheme, such as
selective enrichment, affinity capture, desalting, etc.
The advantages of such a device compared to the standard
FIA method include significantly simplified
instrumentation, fast switching times for analysis of
consecutive samples (high duty cycle) and elimination of
sample cross contamination, The latter advantage,
especially, leads to a significantly decreased number of
runs required to validate that sample cross contamination
did not occur.
Disclosed herein is a prototype plastic electro-
pneumatic distributor, multisprayer device interfaced
with a mass spectrometer for ESI-MS. Each of the sample
wells was connected by an independent microchannel to a
separate electrospray tip. All samples loaded onto the
well plate could be analyzed in rapid sequence without
the need for injection or washing. When coupled to a
quadrupole ion trap mass spectrometer, all 96 sample
wells could be scanned in 8 min, corresponding to a
throughput as high as 720 samples/hr (5 sec per sample).
Even shorter analysis times could, in principle, be
obtained with a fast mass spectrometer, such as a time of
flight instrument. It is important to note that, unlike
in the case of flow injection, in the examples reported
herein, a useful signal could be observed practically
immediately and could be maintained as long as was needed
(e. g., MS/MS) before advancing to the next sample.
The configuration of the electro-pneumatic
distributor of the invention and its use in an electro
pneumatic distributor assembly for electrospray mass
spectrometry will now be presented. Referring to Fig. 1,
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
_g_
an electro-pneumatic distributor assembly 5 includes an
electro-pneumatic distributor 22, a gasket 18 and an
electrospray microdevice 10. Electrospray microdevice 10
contains an array of individual sample wells 12 set in
the device surface and an array 13 of electrospray tips
14 protruding from the side of the device. Each well 12
is connected through an independent channel 16 to an
independent electrospray tip 14. A gasket 18, having an
array of holes 20, is sandwiched between device 10 and
electro-pneumatic distributor 22. Both the number of
holes 20 in gasket 18 and the pattern of the holes are
the same as those of wells 12 on microdevice 10. Gas
flow channels 28, for supplying pressurized gas, and
electrodes 24 are integrated within distributor 22.
Electrodes 24, having opposite ends 25, 26, are arranged
so that ends 25 of each electrode protrude from the
undersurface of distributor 22 according to the format of
the wells on microdevice 10. Electrode ends 25 are
positioned so as to be in direct contact with the sample
solutions in individual wells of device 10 when electro-
pneumatic distributor assembly 5 is in use. Gas flow
channels 28 have outlets 29 on the underside of
distributor 22, which are also positioned according to
the format of wells 12 on microdevice 10. The inlets 30
to gas flow channels 28, along with electrode contact
ends 26, are positioned in separate linear arrays on the
side of distributor 22, each array having the same
spacing as that of electrospray tip array 13 on
microdevice 10.
Electric current and pressurized gas are supplied to
distributor 22 through electric conductor 32 and gas
supply channel 34, respectively, situated in supply block
CA 02357623 2001-07-06
WO 00/41214 PCT/L1S00/00470
_g_
36, which is positioned against the side of electro-
pneumatic distributor 22 and accessible to gas flow
channel inlets 30 and electrode contact ends 26. Supply
channel 34 is connected to a pressurized gas, e.g.,
nitrogen, and aligned with a gas flow channel inlet 30 on
distributor 22. At the same time, electric conductor 32,
to which high voltage is connected, is in communication
with an electrode contact end 26 in distributor 22.
Distributor 22 and microdevice 10 are brought together
with gasket 18 sandwiched in between and then mounted on
a translation stage (not shown).
The diameter of channels 16 connecting sample wells
12 with their respective electrospray tips is
significantly larger (e.g., 300 um) than the ESI tip
inner diameter, e.g., at 26 um. Therefore, the channel
length, e.g., (1-8 cm) has an insignificant effect on the
sample flow rate. Practically all flow resistance is due
to the electrospray tip. After application of gas
pressure and high voltage, the electrospray stabilizes in
1 sec, as can be observed by monitoring the total ion
current. At the beginning of a run, the first of the 96
tips was aligned with the mass spectrometer sampling
orifice, with the remaining tips being sequentially
positioned at the orifice automatically by means of the
fixed step movement of the stage controlled by the
computer.
The system of the invention was first tested with an
aqueous solution of 10 ug/mL angiotensin II at various
pressures (3-40 psi) and voltages (2.5-7 kV), as well as
at various distances between the ESI tip and the MS
sampling orifice (1-8 mm). Based on the observed signal
intensity and stability, settings of 5 psi, 4.5 kV and 3
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-10-
mm were chosen for all further experiments. Under these
conditions, the samples were electrosprayed at a flow
rate of 200 nL/min, i.e., within the optimum range for
the capillary electrospray tip. With the motor and the
motor driver used, the minimum time required to move from
one channel to the next was 1 sec; however, much faster
stages would be commercially available, if necessary.
The electro-pneumatic distributor system for EST/MS
analysis can be viewed as a logical extension of the
microtiter well plate technology. All 96 (384, 1536 ....)
samples deposited in a microtiter well plate can, in
principle, be automatically processed (e. g., incubation,
desalting, solid phase extraction, affinity capture,
etc.) in parallel and finally deposited into the
microfabricated device with electrospray tips, for rapid
sequential MS analysis. Kinetics studies and multi-step
analysis can be performed periodically for an individual
sample in the well plate. During the interval of the
analysis, the well plate can be taken away from the stage
for further appropriate treatment of the samples. By
combining parallel off-line SPE sample preparation with
the multichannel device of the invention, sensitive and
high throughput quantitation using ESI-MS can be realized
(low ng/uL, sample/5 sec, RSD 130).
The system of the invention is a disposable
counterpart to standard microtiter well plate technology
and should be useful in situations where throughput is a
key factor, such as compound confirmation and purity
estimation of combinatorial libraries, pharmacokinetics
studies, substance aging testing, etc. Arranging the
electrospray tips, electrodes or gas channels in
2-dimensional (or even 3-dimensional) arrays can further
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-11-
increase density without increasing the size of the
device.
Although the system of the invention can be made
compatible with standard well plates, the dimension,
density, geometry and pattern of the wells can be varied,
as well as the orientation of channels connecting the
wells to individual electrospray tips. Miniaturized,
microfabricated devices may provide higher throughput for
analysis, as appropriate. The number of wells in a
microdevice is, in theory, unlimited. The volume of a
well can range anywhere from, e.g., 0.1-2000 ul, and the
channel diameter of an individual gas channel can be,
e.g., 50-500 um.
Using a computer controlled on the basis of the
information from the mass spectrometer, an operator can
continue mass spectrometer analysis for an individual
channel as long as the sample in the well lasts. During
this analysis period, the operation mode of the MS system
can be varied (e.g., from full scan to single ion
monitoring to MS/MS) to achieve the goal of the analysis.
For example, if the sample is a synthetic library and the
quality of the library is to be determined, the first
determination would be MW. If there is no ambiguity, then
another sample would be tested. If the structure in not
clear from MW determination, a fragmentation would be
carried out, with this decision being under computer
control.
Thus, it can be seen that the system of the
invention is suitable for any type of high throughput
ESI-MS analysis. For example, after sample preparation or
any other procedures are carried out on other systems,
the samples can be transferred to the system of the
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-12-
invention for ESI-MS analysis. As described in the
Examples section, below, this system has been employed in
HIV inhibitor studies using a synthesized peptide
library. After reaction of a mixture of the peptides,
substrate and the HIV protease, salts were removed
through a solid phase extraction (SPE) process performed
on a commercially available cartridge array in standard
well plate format. Then, the sample was transferred to
the system of the invention for high throughput analysis
of the substrate and cleavage products.
The following examples are presented to illustrate
the advantages of the present invention and to assist one
of ordinary skill in making and using the same. These
examples are not intended in any way otherwise to limit
the scope of the disclosure.
EXAMPLE I
High throughput ESI/MS infusion analysis
In order to demonstrate the high throughput
capability of the system, several sample solutions were
alternately deposited in the wells and then analyzed
sequentially and automatically. The spectra of cytochrome
c and myoglobin from 8 consecutive channels are shown in
Fig. 2A. Strong signals with well defined envelopes of
the multiply charged protein ions were obtained every 5
seconds for each consecutive sample. Since fine
electrospray capillary tips were used, the electrospray
stabilized practically instantly, and no sample cross
contamination was observed. If required, even smaller
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-13-
diameter ESI tips (nanospray) could be used without
modification of the basic device.
In a similar experiment shown in Fig. 2B,
angiotensins II and III were electrosprayed in 8 minutes
from all 96 wells, with singly charged ions of the two
peptides being observed. The data demonstrate the
validity of the approach to high throughput infusion
analysis where all the samples loaded on the plate can be
analyzed in a rapid sequence without risk of cross-
contamination. Although several channels were blocked
during the manual gluing of the device, it can be
expected that this would be completely eliminated, if
produced commercially. It is also worth noting that even
higher throughput could be achieved with the use of a
time of flight, instead of an ion trap mass spectrometer.
Although, a detection level test was not included in this
study, it is reasonable to expect the sensitivity to be
equal to that achieved with single sprayer under the same
conditions (tip dimension, sample flow rate, ESI
voltage). Of course, the analysis may be programmed in
such a way that the next sample is analyzed only after
sufficient signal (information) is obtained. At a flow
rate of 200 nL/min the sample consumption will be minimal
even after extended data accumulation (minutes or more)
and the unused samples may be used for additional
studies, e.g. enzymatic digestion. Further improvements
may also be expected by using a microfabricated array of
electrospray tips instead of individual capillaries.
Besides higher throughput, the current device has
additional advantages compared to ESI-MS analysis
performed in the FIA mode. In the latter mode, the MS
signal can be observed for only a limited time, as a
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-14-
result of the fixed injected sample volume and flow rate.
In the present system, the signal can be observed almost
immediately and as long as desired, allowing a short time
to acquire strong signals or a longer time to acquire
weak signals of lower concentration samples. Switching to
the next sample is not accompanied by any delays related
to the system washing and sample injection. Furthermore,
the sample amount consumed can be maintained as small as
possible (e.g., ~15 nL or 150 fmol). Moreover, if
necessary, practically all the sample deposited in the
sample wells can reach the ESI tip and generate useful
signal. This would be important with very low
concentrated samples or when MS/MS analysis was
necessary.
EXAMPLE II
HIV-1 Protease Inhibition Assay and IC50 Determination
The in vitro inhibition of HIV-1 protease was used
as an illustration of the functionality of the high
throughput system of the invention. The preparation of a
series of samples with increasing concentration of the
HIV-1 inhibitor (pepstatin A) is described in detail in
Materials and Methods. Prior to ESI/MS analysis, 25 uL
sample aliquots were desalted on a 96 well C1$ solid phase
extraction (SPE) plate. The substrate and standard, with
no HIV-1 protease added, were also analyzed by direct
infusion ESI-MS. No side product formation was observed,
except Ser-Gln-Asn-Tyr(t-butyl)-Pro-Ile-Val (MW 875),
which was expected from the substrate synthesis. This
side product, however, had no influence in the present
study since the m/z value was far removed from the
internal standard (MW 359) and the enzymatically formed
CA 02357623 2001-07-06
WO 00/41214 PCT/11500/00470
-15-
tripeptide Pro-Lle-Val (MW 327). Fig. 3A presents
selected ion monitoring (SIM) mass spectra with
increasing amounts of inhibitor (pepstatin A), and the
corresponding data are plotted in Fig. 3B. Inhibition by
another peptidomimetic inhibitor N-Acetyl-Thr-Ile-Nle-~r-
[Ch2N]-Nle-Gln-Arg amine, MVT 101) and some other small
organic molecules were also studied and the ICSO obtained
are listed in Table 1. The experimental ICSO value of
pepstatin A and the Ki value of MVT 101 were in agreement
with those found in the literature within the
experimental error, typical for this type of analysis
20% or more).
Table 1. ICso values of investigated HIV-1-protease inhibitorsa
Inhibitor IC50 IC50
Concentration (this work) (refs. ...)
Inhibitor Range (uM) (uM) (uM)
Pepstatin A 0-5 0.75+/-0.1 0.55 ~.zM
MVT 101 0-10 0.65 (Ki:~0.5uM) K;: 0.8 uM
Compound 0-12.5 9.5 -
Compound 0-40 6 -
Compound 0-30 24 -
a Assay conditions: 5 uL of 1 mg/mL HIV- 1 protease in a 100 uL total assay
volume; incubation for 90 min at 37° C.
MATERIALS AMD METHODS
Fabrication of the Multi-Sprayer Microdevice
The 96 channel device was fabricated by casting from
a solvent resistant polymer resin (EpoFix, EMS, Ft.
Washington, PA), as shown in Figs. 4A-4B. The required
patterns of channels and wells (master plates) were first
created on rectangular plastic sheets (Lucite S-A-R,
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-16-
Small Parts Inc., Miami Lakes, FL) using a digital
milling machine. Second, the master plates were placed
in a plastic box and silicone polymer (Silastic L-RTV
silicone rubber kit, Dow Coming Corp., Midland, MI) was
cast over the plates. Fig. 4A shows the fabrication of
the silicone rubber negative with recessed channels of
semicircular shape with diameter ~ 300 um. Fig. 4B shows
the complete flow diagram of the fabrication of the
microdevice (only one of the 96 sample wells is
depicted). The silicone negative imprints (c and d in
Fig. 4B) of the Lucite master plates (a and b) were
created, as described above. Master plate (a) contained
96 channels with starting points distributed in the
standard 96 well plate pattern and ending in an array
arrangement at the edge of the plate. The master plate
(b) contained 96 wells with 5 mm diameter, 5 mm deep,
connected to a 0.5 mm diameter 0.5 mm deep hole in the
bottom. In the next step, both rubber imprints (c and d)
were aligned to form a cavity, which was then filled with
the liquid EpoFix resin. Two other polymeric resins were
also tested: Acrylic-polyester based Casolite AP (AIN
Plastics, Mt. Vernon, NY) and epoxy based Araldite
(Fluka, Buchs, Switzerland); however, the EpoFix resin
exhibited the best mechanical and chemical resistance
properties. After hardening, the EpoFix part (e) was
recovered and glued together with a bottom plate (f). The
bottom plate, also prepared by casting, had 96 embedded
electrodes (0.5 mm in diameter, 1.125 mm center to center
distance). The electrodes were prepared from
electrically conductive epoxy (Epo-Tek 4156, Epoxy
Technology, Billerica, MA).
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-17-
Finally, fused silica capillaries (2.5 cm in length,
26 um i. d. , 140 um o. d. ) were inserted into the exits of
the channels to a depth of 1.5 cm and glued in place.
About 1 mm of the polyimide coating at the capillary tips
was removed by heat. This procedure produced a 96 well
plate with closed channels and embedded electrodes
connecting each well with a separate capillary
electrospray tip, as can be seen in the micrograph of
Fig. 5A.. The detail of Fig. 5B, at higher
magnification, shows individual wells with their
connected channels, and the detail of Fig. 5C shows an
array of electrodes embedded into the channels just prior
the attachment point of the electrospray tips.
An exploded view of the completed system in position
on a translation stage is given in Fig. 6. The dimensions
of the assembled electrospray were 16 cm x 10 cm x 0.9
cm.
Mass Spectrometry
An ion trap mass spectrometer (LCQ, Finnigan MAT,
San Jose, CA), operated in the positive ion mode was
used throughout this study. Since the sampling orifice
of the instrument was located in a small hemispherical
indentation, which cannot accommodate the size of the
microdevice, an orifice extension was used to overcome
the space restriction around the mass spectrometer
inlet. The orifice extension was machined from an
aluminum rod (2.5 cm long, 8 mm o.d.) with a 0.35 mm
i.d. channel drilled axially. The extension was
connected to the sampling orifice by a 2 cm long piece
of silicone rubber tubing.
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-18-
System Design and Operation
The exploded schematic diagram in Fig. 6 shows the
total system design. During operation, the 96 well/96 ESI
tips plate (sample plate) was positioned on a computer
controlled translation stage so that the ESI tips were
aligned with the MS sampling orifice extension. The
sample plate was then closed by a pressure distribution
plate. A thin sheet of silicone rubber with 96 properly
positioned holes was placed between the two plates to
seal the connection (not shown in Fig. 6).
Sequential sample flow through the ESI tips was
initiated with the aid of a stationary gas pressure
nozzle (200 um i.d., 1 mm. o.d. Teflon tube) connected to
a nitrogen tank. The nozzle contacted the surface of the
pressure distribution cover plate so that channels were
individually pressurized during the movement of the
translation stage. The pressure distribution cover plate,
with well and channel patterns as a mirror image of the
sample well plate, was made by the same casting procedure
as the sample plate. The stationary high voltage
electrode (1 mm diameter stainless steel wire) was
positioned so that the high voltage was connected only to
the pressurized channel. The high voltage and nitrogen
supply were applied during the course of analysis; as the
translation stage moved the device to the next position,
pressurized gas and high voltage were automatically
connected to the respective sample well and channel. An
aluminum plate was placed on top of the gas distributor
to ensure gas tight sealing of all the wells. The linear
translation stage (LS3-6-B 10, Del-Tron Precision, Inc.,
Bethel, CT) was driven by a NEMA 23 step motor controlled
by a computer through a motor driver (6006-DB, American
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-19-
Scientific Instrument Corp., Smithtown, NY). A simple
computer routine (written in Basic) was used to control
the translation stage.
r1-, ...., ; ,. -, , r
Myoglobin, cytochrome c and angiotensins II, III,
purchased from Sigma (St. Louis, MO), were each prepared
at a concentration of 1 mg/mL and then diluted to the
desired concentration with 0.20 (v/v) acetic acid in 50%
(v/v) methanol. Fmoc-amino acids and H- val- 2-
chlorotrityl resin were purchased from Anaspec (San Jose,
CA). 1-hydroxybenzotriazol(HOBt), 2-(1H-benzotriazol-
1,1,3,3 -tetramethyluronium) hexafluorophosphate (BBTU),
diisopropylethylamine (DIEA), dimethylformamide (DMF),
dichloromethane (DCM)], potassium cyanide, phenol,
ninhydrin, pyridine and piperidine were obtained from
Fluka (Ronkonkoma, NY). BPLC- grade acetonitrile (ACN)
and methanol were also from Fluka. HIV- 1 protease was
obtained from Pharmacia and Upjohn (Kalamazoo, MI) and
pepstatin A and N-acetyl-Thr-Ile-Nle-~r-[CH2N]-Nle-Gln-Arg
amine (MVT 101) from Sigma. The organic compounds,
158393, 117027, 32180, were kindly donated by the Drug
Synthesis & Chemistry Branch, Development Therapeutics
Program, Division of Cancer Treatment, National Cancer
Institute (Bethseda, MD). Hack's balanced salt solution
(HBSS) was obtained by Parker-Davis. Milli-Q water
(Millipore, Medford, NL4,) was used throughout.
Sample Preparation for HIV-1 Protease Inhibition Assay
An 8-mer peptide substrate (Ser-Gln-Asn-Tyr-Pro-Ile
-Val) and a 3-mer peptide internal standard (Glu-Ile-Val)
were prepared, following procedure described in the
Anaspec solid phase synthesis catalog (San Jose, CA).
Peptide synthesis was begun from 0.5 mmol of
CA 02357623 2001-07-06
WO 00/41214 PCT/LJS00/00470
-20-
H-val-2-chlorotrityl resin, and coupling was performed by
adding 1 mmol of FMOC amino acid in 1 mmol HBTU/HOBT, 2
mmol DIEA. The final peptide was then cleaved from the
resin with a mixture of acetic acid/trifluoroacetic acid
in dichloromethane and precipitated in ice cold ether.
HIV-1 protease inhibition was measured by monitoring the
concentration of the enzymatic degradation product - Pro-
Ile-Val. The total assay volume was 100 uL, containing 50
ug/mL of HIV-1 protease, 1 mM substrate and a defined
amount of inhibitor (pepstatin A or MVT 101) in a
MES-buffer (100 mM MES, 300mM KCI, 5mM EDTA, 4.5% (v/v)
DMSO, pH 5.5). The solution was incubated at 37° C for 90
min and then quenched by addition of 10 uL TFA. Finally,
the solution was spiked with 600 uM of Glu-Val-Ile, the
internal standard.
Aliquots of sample reaction products of 25-50 uL
were taken and desalted on a 96 well C18 solid phase
extraction (SPE) plate (Varian, Harbor City, CA). The
plate was washed with 3x200 uL of methanol followed by
3x200 uL of water. The sample was introduced on the resin
and washed extensively (4x 300 uL acidified water (l00
(v/v) formic acid)). The sample was then eluted from the
SPE resin with 3x 20 uL 1% (v/v) formic acid in 500 (v/v)
ACN/H20. The eluate solutions were used for direct
infusion or were stored in Eppendorf vials at -15° C for
future analysis.
OTHER EMBODIMENTS
As described herein, the multiplexed u-TAS system of
the invention is particularly useful for electrospray
mass spectrometry analysis (ESI/MS). The system of the
invention may also be used for atmospheric pressure
CA 02357623 2001-07-06
WO 00/41214 PCT/US00/00470
-21-
chemical ionization mass spectrometry (APCI/MS), for
matrix assisted laser desorption ionization mass
spectrometry (particularly in a Time-Of-Flight
instrument), for nuclear magnetic resonance analysis
(NMR), for pneumatically or ultrasonically assisted spray
sample handling, for transfer to an off-chip detection
system, such as electrochemistry, conductivity or laser
induced fluorescence, or for collection of specific
fractions, e.g., in collection capillaries or on
collection membranes. Sample transfer may be by droplet,
spray or stream, as desired, or as suitable for the
instrument or device receiving the transferred sample.
The transferred fluid may be in the form of a liquid or a
gas.
While the present invention has been described in
conjunction with a preferred embodiment, one of ordinary
skill, after reading the foregoing specification, will be
able to effect various changes, substitutions of
equivalents, and other alterations to the compositions
and methods set forth herein. It is therefore intended
that the protection granted by Letters Patent hereon be
limited only by the definitions contained in the appended
claims and equivalents thereof.