Note: Descriptions are shown in the official language in which they were submitted.
CA 02359865 2004-04-20
65175-169
PROTON CONDUCTING MEMHRANE USING A SOLID ACID
Field
The present application describes a proton conducting
membrane formed using an solid acid in its solid phase. More
specifically, the present application teaches a proton conducting
membrane, formed using an solid acid mixed with a supporting
binder material, that is impermeable to fluids such as gas and
water, can operate without hydration, and has high proton
conductivity.
1
CA 02359865 2004-04-20
65175-169
Background
Proton conducting materials have a number of applications.
Proton conducting membranes are widely utilized in devices which
use a chemical reaction to produce or store electricity, or use
electricity to drive a chemical process. Materials which conduct
both protons and electrons ("mixed proton and electron
conductors") are utilized in related applications.
2
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Electrochemical devices depend on the flow of protons, or
the flow of both protons and electrons through a proton
conducting membrane. Exemplary electrochemical devices include a
fuel cell, an electrolysis cell, a hydrogen separation cell, a
battery, a supercapacitor, and a membrane reactor. There are
other electrochemical devices which also use a proton conducting
membrane.
An important use for proton conducting membranes is in fuel
cells. Fuel cells are attractive alternatives to combustion
engines for the generation of electricity because of their higher
efficiency and the lower level of pollutants they produce. A
fuel cell generates electricity from the electrochemical reaction
of a fuel e.g. methane, methanol, gasoline, or hydrogen, with
oxygen normally obtained from air.
There are three common types of fuel cells used at
temperatures close to ambient. A direct hydrogen/air fuel cell
system stores hydrogen and then delivers it to the fuel cell as
needed.
In an indirect hydrogen/air fuel cell, hydrogen is
generated on site from a hydrocarbon fuel, cleaned it of carbon
monoxide (CO), and subsequently fed to the fuel cell.
A direct methanol fuel cell ("DMFC"), feeds methanol/water
solution directly to the fuel cell, e.g., without any fuel
3
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
processing. One type of DMFC has been described, for example, in
U.S. Patent No. 5,559,638. There are various advantages and
disadvantages inherent within all three configurations. All are,
to a greater or lesser extent, limited by the performance of the
proton conducting membrane.
NafionT'", a perfluorinated sulphonic acid polymer, is often
used as a membrane material for fuel cells which operate at
temperatures close to ambient. Other hydrated polymers have also
been used as proton conductive materials. Membranes of modified
perfluorinated sulfonic acid polymers, polyhydrocarbon sulfonic
acid polymers, and composites thereof are also known. These and
related polymers are used in hydrated form. Proton transport
occurs by the motion of hydronium ions, H30+. Water is necessary
in order to facilitate proton conduction. Loss of water
immediately results in degradation of the conductivity.
Moreover, this degradation is irreversible - a simple
reintroduction of water to the system does not restore the
conductivity. Thus, the electrolyte membranes of these hydrated
polymer-based fuel cells must be kept humidified during
operation. This introduces a host of balance-of-plant needs for
water recirculation and temperature control.
4
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
A second limitation derives from the need to maintain water
in the membrane. In order to maintain hydration, the temperature
of operation cannot exceed 100°C without cell pressurization.
High temperature operation could be desirable, however, to
increase catalyst efficiency in generating protons at the anode
(in both HZ and direct methanol fuel cells) and to improve
catalyst tolerance to carbon monoxide ("CO"). CO is often
present in the fuel that is used in the fuel cells. The CO can
poison the precious metal catalysts. This is particularly
problematic in indirect hydrogen/air fuel cells for which
hydrogen is generated on site. High temperatures also benefit
the reduction reaction on the cathode.
Another limitation of hydrated polymer electrolytes occurs
in applications in methanol fuel cells. These polymers can be
permeable to methanol. Direct transport of the fuel (i.e.
methanol) across the membrane to the air cathode results in
losses in efficiency.
Alternate proton conducting materials, which do not require
humidification, which can be operated at slightly elevated
temperatures, and/or which are impermeable to methanol, are
desirable for.fuel cell applications.
5
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
In the field of hydrogen separation, a proton conducting
membrane is utilized to separate hydrogen from other gases such
as CO and/or C02. Palladium is often used for this application.
Palladium is permeable to molecular hydrogen, but not in general
to other gases. There are drawbacks to the use of this material.
It is expensive and the hydrogen diffusion rate is low. It
would be desirable to develop new materials which are less
expensive and exhibit higher proton/hydrogen transport rates.
In general, materials utilized in other electrochemical
devices such as electrolysis cells, batteries, supercapacitors,
etc., include liquid acid electrolytes, which are highly
corrosive, and solid polymer proton conductors, which require
humidification or exhibit insufficient proton conductivity. High
conductivity, high chemical and thermal stability solid membranes
with good mechanical properties are desirable for all of these
applications.
Summary
The present specification defines a new kind of material for
a proton conducting membrane. Specifically, a proton conducting
material is formed using an solid acid. The solid acid can be of
the general form MaHb(XOt)~ or MaHb(XOt)~~nH20,
where:
6
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
M is one or more of the species in the group consisting of
Li, Be, Na, Mg, K, Ca, Rb, Sr, Cs, Ba, T1 and NH4+ or Cu+;
X is one or more of the species in the group consisting of
Si, P, S, As, Se, Te, Cr and Mn; and
a, b, c, n and t are rational numbers.
Solid acids do not rely on the presence of hydronium ions
for proton transport, thus they do not require hydration for use
as proton conductors.
A preferred solid acid used according to this specification
is a solid phase solid acid that exhibits a superprotonic phase,
a phase in which the solid has disorder in its crystal structure
and a very high proton conductivity.
An embodiment uses a structural binder or matrix material to
enhance the mechanical integrity and/or chemical stability of the
membrane. That structural~binder can be many different kinds of
materials in the different embodiments. In particular, the
structural binder can be a polymer, a ceramic, or an oxide glass.
Another embodiment uses an electronically conducting
material as a matrix. This creates a membrane which conducts
both protons and electrons.
7
CA 02359865 2005-02-18
65175-169
The resulting material can be used for a proton
conducting material in a device that relies on the flow of
protons or the flow of both protons and electrons across a
membrane, herein an "electrochemical" device e.g. a fuel
cell, a hydrogen separation membrane, or a electrolysis
cell.
In a specific aspect the invention provides a
proton conducting membrane for use in the presence of H20 in
a device selected from the group consisting of fuel cells,
proton transport membranes, membrane reactors and sensors,
comprising: a solid acid material of the general formula:
MaHb(XOt)~ wherein M is a ration having a charge from +1 to
+2, X is selected from the group consisting of S, Se, P, As,
Si and Te, and a, b, c and t are non-zero integers, wherein
the solid acid material is capable of conducting protons in
a solid state through a superprotonic mechanism.
Brief Description of the Drawings
Figure 1 shows an exemplary hydrogen/air fuel cell
using a solid acid supported by a binder as its proton
conducting membrane;
Figure 2 shows an exemplary direct methanol fuel
cell using a solid acid supported by a binder as its proton
conducting membrane;
Figure 3 shows a hydrogen separation membrane for
the removal of CO and other gases from hydrogen;
Figure 4 shows another type of hydrogen separation
membrane made of a proton conducting composite; and
Figures 5 and 6 show a membrane reactor.
8
CA 02359865 2005-02-18
65175-169
Detailed Description
The present application teaches using a solid acid
as a proton conducting membrane.
A solid acid can be of the general formula
MaHb ( XOt ) c ' riH2O .
8a
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
where:
M is one or more of the species in the group consisting of
Li, Be, Na, Mg, K, Ca, Rb, Sr, Cs, Ba, Tl and NH4+;
X is one or more of the species in the group consisting of
Si, P, S, As, Se, Te, Cr and Mn; and
a, b, c, n and t are rational numbers; with t preferably
being 3 or 4, and where t~0..
The solid acids used herein are compounds, such as CsHS04,
whose properties are intermediate between those of a normal acid,
such as HZS04, and a normal salt, such as Cs2S04. In general, the
chemical formula of the solid acids of the type used according to
the present specification can be written as a combination of the
salt and the acid.
In general, solid acids are comprised of oxyanions; for
example 504, 503, Se04, Se03, Si04, P04 or As04, etc. , which are
linked together via O-H.... O hydrogen bonds. The structure may
contain more than one type of X04 or X03 group, and may also
contain more than one type of M species.
Certain solid acids are solid materials at room temperature.
9
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Many different solid acids are contemplated by this
specification. One example of a material that can be used as the
solid acid is CSHS04, which is intermediate between Cs2S04 (a
normal salt) and HzS04 (a normal acid). In this case, the solid
acid can be written as 0.5 Cs2S04 * 0.5 HZS04. Another example,
using the same salt and the same acid, is 1.5 Cs2S04 * 0.5 HzS04,
to give Cs3H ( S04 ) 2 .
Other examples are:
CsH2PO4, Cs5 (HSO4) 3 (HzP04) 2~ Cs2 (HSO4) (HaP04) ~ Cs3 (HSO4) 2 (HzP04) ,
CS3 (HSOg ) 2 (H1 .5 ( S0. 5P0.5 ) ~4 ) i CSSH3 ( S~4 ) 4 ' XH2O, T1HS04 ,
CsHSeO4 ,
Cs2 (HSeO4) (HzP04) , Cs3H (SeO4) a (NH4) sH (SO4) 2, (NH4) a (HSO4) (HzP04) ,
Rb3H (SO4) 2~ R.bsH (SeO4) 2, CSi.sLli.sH (SO4) 2, Cs2Na (HSO4) 3. T1H3 (SeO3)
2~
CsHzAs04 (NH4) 2 (HS04) (HZAs04) , CaNaHSi04
The preferred material for any specific electrochemical
device depends on the application. For example, Cs2 (HS04) (HZP04)
may be preferred for electrochemical devices where high
conductivity is critical. (NH4)3H(S04)Z may be preferred where low
cost is critical. CaNaHSi04 may be preferred where chemical
stability is critical.
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Solid acids have certain characteristics that can be
advantageous when used as a proton conducting membrane. The
proton transport process does not rely on the motion of hydronium
ions, thus solid acids need not be humidified and their
conductivity is substantially independent of humidity. Another
advantage is that solid acids are generally stable against
thermal decomposition at elevated temperatures. The thermal
decomposition temperature for some of the solid acids described
in this specification, e.g., CaNaHSi04, can be as high as 350°C.
Since solid acids need not be humidified, solid acid based
membranes can be operated at elevated temperatures, e.g.
temperatures above 100°C.
The conductivity of solid acids may be made purely
protonic, or both electronic and protonic depending on the choice
of the X element in the chemical formula MaHb (X04 ) ~ ~ nH20 or
MaHb (X03 ) ~ ~ nH20. That is, by using a given amount of a variable
valence element such as Cr or Mn for X, the solid acid can be
made to conduct electrons as well as protons.
11
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Another advantage is caused by the structure of the solid
acids themselves. Since solid acids are dense, inorganic
materials, they are impermeable to gases and other fluids that
may be present in the electrochemical environment, e.g., gases
and hydrocarbon liquids.
The materials are also relatively inexpensive.
This combination of properties: good conductivity in dry
environments, conductivity which can be controlled to be either
purely proton conducting or both electron and proton conducting,
impermeability to gases and hydrocarbon liquids, serviceability
at elevated temperatures, e.g. temperatures over 100°C and
relatively low cost, render solid acids as useful materials for
use as membranes in electrochemical devices.
Solid acids exhibit another advantageous property for
applications in proton conducting membranes. Under certain
conditions of temperature and pressure, the crystal structure of
a solid acid can become disordered. Concomitant with this
disorder is an high conductivity, as high as 10-3 to 10-2 S2-lcm-1.
Because of the high proton conductivity of the structurally
disordered state, it is known as a superprotonic phase. The
proton transport is believed to be facilitated by rapid X04 or
X03 group reorientations, which occur because of the disorder.
12
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Many solid acids enter a superprotonic state at a
temperature between 50 and 150°C at ambient pressures. The
transition into the superprotonic phase may be either sharp or
gradual. The superprotonic phase is marked by an increase in
conductivity, often by several orders of magnitude. At
temperatures above the transition temperature, the solid acid is
superprotonic and retains its high proton conductivity until the
decomposition or melting temperature is reached.
Solid acids that undergo a superprotonic transition include:
1~ CsHSO4, Csz (HSO4) (HzPO4) , Cs3 (HSO4) z (HzPO4) ,
CS3 (HSOq) 2 (H1.5 (SO.SP0.5) ~4) i CS5H3 (S04) 4'XHzO, CSHSeOg, CS3H (SeOq)
2,
(NH4)aH(S04)z. RbaH(Se04)z.
The superprotonic phases of solid acids have increased
conductivity. An interesting embodiment is a solid acid operated
at a temperature above the superprotonic transition temperature,
and below the decomposition or melt temperature.
Despite the many advantageous properties of solid acids,
problems can be encountered in trying to implement them in
electrochemical devices because many solid acids are water
soluble. They can also be difficult to process into large area
membranes, and they often have poor mechanical properties. Some
solid acids, such as CaNaHSi04 and other silicates, are not
soluble in water.
13
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Because of these difficulties, a disclosed embodiment
includes a composite comprised of an solid acid embedded in a
supporting matrix. The solid acid part of the composite provides
the desired electrochemical activity, whereas the matrix provides
mechanical support and also may increase chemical stability.
Different materials are contemplated herein for use as the
supporting matrix.
In light of the properties of solid acids outlined above,
the preferred embodiment is a composite material comprised of a
solid acid embedded in a supporting matrix and operated at a
slightly elevated temperature. In such a composite, the solid
acid is in its superprotonic phase, exhibits high conductivity,
and provides the desired electrochemical functions; the support
matrix may provide mechanical support, and it may also serve to
protect the solid acid from water in the environment. A high
temperature of operation can render the solid acid into its
superprotonic state. A high temperature of operation can also
ensure that any water present in the electrochemical device will
be present in the form of steam rather than liquid water, making
the H20 less likely to attack the solid acid.
Hydrogen/Air Fuel Cells
14
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
A hydrogen/air fuel cell is shown in Figure 1, in which the
proton conducting membrane is a solid acid/matrix composite of
the type described herein. Because the membrane need not be
humidified, the fuel cell system can be simpler than one which
uses a hydrated polymer membrane. The humidification system
normally required for fuel cell utilizing a Nafion or related
polymer membrane can be eliminated in Figure 1. Hence, less
rigid temperature monitoring and control may be used in the solid
acid based system as compared with Nafion based fuel cell
systems. These differences allow a less-costly fuel cell
system.
Because the membrane need not be humidified, the fuel cell
shown in Fig. 1 can be operated at temperatures above 100°C. The
tolerance of the Pt/Ru catalysts to carbon monoxide CO
poisoning increases with increasing temperature. Thus, a fuel
cell such as shown in Fig. 1, operated at a temperature above
100°C may withstand higher concentrations of CO in the hydrogen
fuel than a Nafion based fuel cell which is typically operated at
a temperature lower than 100°C.
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
The high temperature of operation also enhances the kinetics
of the electrochemical reactions, and can thereby result in a
fuel cell with higher overall efficiency than one based on Nafion
or other hydrated polymers.
Direct Methanol Fuel Cells
A direct methanol fuel cell is shown in Figure 2. The
proton conducting membrane is a solid acid/matrix composite of
the type described herein. Because the membrane need not be
humidified, the fuel cell system is much simpler and thus less
costly than state of the art direct methanol fuel cell systems.
The humidification system normally required for fuel cell
utilizing a Nafion or related polymer membrane is eliminated in
Figure 2. Furthermore, temperature monitoring and control in the
solid acid based system does not need to be as tight as in Nafion
based fuel cell systems. Because the solid acid based membrane
need not be humidified, the fuel cell may be operated at elevated
temperatures. High temperatures can enhance the kinetics of the
electrochemical reactions. This can result in a fuel cell with
very high efficiency.
16
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Another significant advantage of the fuel cell shown in
Figure 2 over state of the art direct methanol fuel cells results
from the decreased permeability of the membrane to methanol. In
state of the art direct methanol fuel cells, in which Nafion or
another hydrated polymer serves as the membrane, methanol cross-
over through the polymeric membrane lowers fuel cell
efficiencies. The impermeability of a solid acid membrane can
improve this efficiency.
Hydrogen Separation Membranes
The Ru/Pt catalyst in a hydrogen/air fuel cell is sensitive
to CO poisoning, particularly at temperatures close to ambient.
Therefore, in an indirect hydrogen/air fuel cell, the hydrogen
produced by the reformer is often cleaned, of e.g. CO to below
50ppm, before it enters the fuel cell for electrochemical
reaction.
In Figure 3, a hydrogen separation membrane is shown for the
removal of CO and other gases from hydrogen. The hydrogen
separation membrane is made of a mixed proton and electron
conducting membrane, as described herein. Hydrogen gas, mixed
with other undesirable gases, is introduced onto one side of the
membrane. Clean hydrogen gas is extracted from the other side of
the membrane.
17
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
On the inlet side of the membrane, hydrogen gas is
dissociated into H+ and e-. Because the membrane is both proton
conducting and electron conducting, both of these species can
migrate through the membrane. However, the membrane is
substantially impermeable to other gases and fluids. Hence, CO
and other undesirable gases or fluids cannot so migrate. On the
outlet side of the membrane, the H+ and e- recombine to form
hydrogen gas. The overall process is driven by the hydrogen
chemical potential gradient, which is high on the inlet side of
the membrane and low on the outlet side of the membrane.
18
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Another type of hydrogen separation membrane is shown in
Figure 4. The membrane is made of a proton conducting composite
of the type described herein, and is connected to a current
source. Hydrogen gas, mixed with other undesirable gases, is
introduced onto one side of the membrane and clean hydrogen gas
is extracted from the other side of the membrane. Application of
a current causes the hydrogen gas to dissociate into H+ and e-.
Because the membrane conducts only protons, these protons are the
only species which can migrate through the membrane. The
electrons migrate through the current source to the outlet side
of the membrane, where the H+ and e- recombine to form hydrogen
gas. The membrane is substantially impervious to other gases and
fluids. Hence, CO and other undesirable gases or fluids cannot
migrate through the proton conducting membrane. The overall
process is driven by electric current applied via the current
source.
Membrane Reactors
19
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
In Figure 5 a membrane reactor is shown, in which a mixed
proton and electron conducting membrane of the type described
herein is utilized. The general reaction is that reactants A + B
react to form products C + D, where D is hydrogen gas. Use of a
mixed proton and electron conducting membrane in this reactor can
enhance the reaction to give yields that exceed thermodynamic
equilibrium values. On the inlet side of the membrane reactor,
the reactants form products C + H2. Under equilibrium
conditions, the hydrogen concentration builds up and the forward
reaction is slowed. With the use of the mixed hydrogen and
electron conducting membrane, the hydrogen is immediately
extracted from the reaction region via transport through the
membrane, and the forward reaction is enhanced. Examples of
reactions in which yield could be enhanced by using such a
membrane reactor include (1) the steam reformation of methane
(natural gas) to produce syngas: CH4 + H20 -> CO + 3H2; (2) the
steam reformation of CO to produce C02 and H2: CO + H20 -> C02 +
H2; (3) the decomposition of H2S to H2 and S, (4) the
decomposition of NH3 to H2 and N2; (4) the dehydrogenation of
propane to polypropylene; and (5) the dehydrogenation of alkanes
and aromatic compounds to various products.
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
In Figure 6 a second type of membrane reaction is shown,
again, utilizing a mixed proton and electron conducting membrane
of the type described herein. In this case, the general reaction
is that the reactants A + B form the products C + D, where B is
hydrogen. The hydrogen enters the reaction region via transport
through the mixed conducting membrane, whereas the reactant A is
introduced at the inlet to the membrane reactor, and is mixed
with other species. The manner in which the hydrogen is
introduced into the reactant stream (through the membrane)
ensures that only the reactant A, and none of the other species
reacts with hydrogen. This effect is termed selective
hydrogenation.
The mixed proton and electron conducting membranes described
herein provide an advantage over state-of-the-art membranes in
that the conductivity is high at temperatures as low as 100°C,
and the membranes are relatively inexpensive. Selective
hydrogenation at temperatures close to ambient may have
particular application in synthesis of pharmaceutically important
compounds which cannot withstand high temperatures.
21
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
According to a first class of materials, the solid acid is
mixed with a supporting structure that is electrochemically
unreactive, to form a composite. A first embodiment uses a solid
acid mixed with a melt-processable polymer as the supporting
matrix structure.
The solid acid (CHS) was prepared from aqueous solutions
containing stoichiometric amounts of Cs2C03 and H2S04.
Crystalline CsHS04 and a small amount (~ 8 wto) of the related
compound Cs5H3(S04)4~xH20 (which also exhibits superprotonic
behavior) were obtained upon introduction of methanol into the
solution. Composite membranes of the solid acid and
poly(vinylidene fluoride) were prepared by simple melt-processing
methods. The two components were lightly ground together then
hot-pressed at 180 °C and 10 kpsi for 15 minutes. Volume ratios
of CHS:PVDF from 1000 CsHS04 to 1000 PVDF were prepared in 10
vol% increments.
Another example of a composite contains a solid acid and a
thermoset polymer, which can be mixed in with the solid acid in
monomer or prepolymer form, and then polymerized in situ.
22
CA 02359865 2004-04-20
65175-169
The solid acid (CHS) was prepared from aqueous solutions
containing stoichiometric amounts of Cs2C03 and HzS04.
Crystalline CsHS04 and a small amount (- 8 wt%) of the related
compound Cs5H3(S04)4~xH20 (which also exhibits superprotonic
behavior) were obtained upon introduction of methanol into the
solution. Composite membranes of the solid acid and the
polyester resin marketed under the Trade-mark Castoghas by Buehler,
Inc. were synthesized simply by lightly grinding the solid acid
and pre-polymer together and then adding the
polymerization/crosslinking catalyst. A material with a 50:50
volume ratio was prepared.
Another example of a thermoset polymer - solid acid
composite comprises the solid acid (NH3)3H(S04)2 and the polymer
poly(dicyclopentadiene) or poly DCPD.
The solid acid, TARS, was prepared from aqueous solutions of
(NH4)ZSO9 and HZS04. The solid acid was ground then mixed with
the monomer dicyclopentadiene. The polymerization catalyst was
introduced into the mixture, which was then poured onto a Teflon
plate and pressed into a thin film. The film was cured at 100°C
for approximately 2 hours. Materials with 25 and 17 vol % TARS
were prepared.
23
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Another method for preparing solid acid/polymer composites
is suspension coasting. For this, CsHS04 was dissolved in a
water/ethanol solution. The polymer PVDF was then dispersed into
this solution. A composite membrane was formed by casting the
suspension and allowing the solvents to evaporate. Composite
membranes comprised of a solid acid and a non-polymeric matrix
material, such as a ceramic or an oxide glass can be prepared in
the following manner. The solid acid is synthesized form aqueous
solution and the matrix material is synthesized separately. The
two components are mixed and ground together. The mixture is
then hot pressed, preferably at a temperature which causes the
solid acid to melt and flow, to yield a dense composite membrane.
The nature of the chemical bonding in solid acids of general
formula MaHb (X04 ) ~ ~ nH20 or MaHb (X03 ) ~ ~ nH20 where
M is one or more of the species in the group consisting of
Li, Be, Na, Mg, K, Ca, Rb, Sr, Cs, Ba, T1 and NH4+;
X is one or more of the species in the group consisting of
Si, P, S, As, Se, and Te; and
a, b, c, and n are rational numbers, and n can be zero.
leads to materials which are inherently poor conductors of
electrons. These compounds can be used in devices which require
both proton and electron transport directly through the membrane
if a mechanism for electron transport is introduced.
24
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
The first approach for introducing electronic conductivity
into solid acid based materials is to prepare a composite
comprised of the solid acid and a second substance which has a
high electronic conductivity. This second substance may be an
electronically conducting polymer, such as poly(aniline), or a
typical metal, such as aluminum or copper. Where the
electronically conducting component is a metal, it may be
advantageous to introduce a chemically and electrically inert
polymer into the composite simply to serve as a binder and
provide the membrane with good mechanical properties. The
processing methods described above may be used to prepare such
composite membranes.
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
The second approach for introducing electronic conductivity
into solid acid based materials is to perform direct chemical
substitutions with variable valence ions. For example, a portion
of the sulfur in CsHS04 may be replaced by chromium, which can be
present in an oxidation state of anywhere from 2+ to 6+.
Similarly, manganese may be introduced on the sulfur site, as
this ion exhibits valence states anywhere between 2+ and 7+.
Chemical substitution may also be performed with respect to the
cesium in a compound such as CsHS04. Large ions with variable
valence, such as thallium, indium, lead and tin can be used for
these substitutions. The solid acid so modified may be used in
an electrochemical device directly, or may be combined with a
supporting matrix material as described above.
26
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
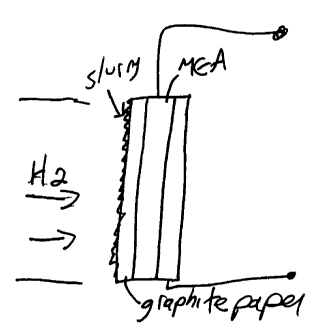
In the Figure 1 embodiment, a membrane-electrode assembly
(MEA) is prepared from the CHS-PVDF composite film in which the
solid acid to polymer volume ratio is 50:50. The electrodes are
formed of graphite paper which is impregnated with a complex
slurry of platinum powder, PVDF, the solid acid, and Nafion,
suspended/dissolved in a water and isopropanol solution. After
evaporation of the solvents, the electrodes so prepared are
hot-pressed onto the composite membrane. The MEA is placed in a
fuel cell test station at 140°C and hydrogen is introduced at the
anode and oxygen at the cathode. The open cell voltage (OCV)
obtained in this manner was 0.88 V. The same type of MEA may
also be used in the Fig 2 embodiment.
VII. Examples
Example 1
A Cs based solid acid such as CsHS04, CsHSe04 or
Cs5H3(S04)4~xH20 is ground and mixed with a melt-processable
polymer binder, such as poly(vinylidene fluoride), and hot-
pressed. The result forms a solid composite membrane which is
proton conducting even in dry atmospheres. The composite
membrane, being comprised of two components whicha re
substantially impermeable to fluids " may be less permeable than
Naf ionTM .
27
CA 02359865 2004-04-20
65175-169
Example 2
A Cs based solid acid such as Cs3 (HSO9) 2 (Hl,s (So.spo.s) 04) .
Cs3 (HS04) 2 (H2P04) , Css (HS04) 3 (HZP04) z or Cs2 (HS09) (HzP04) is ground
and mixed with a melt-processable polymer binder, such as
poly(vinylidene fluoride), and hot-pressed. The result forms a
solid composite membrane which is proton conducting even in dry
atmospheres. The membrane is also less permeable to fluids than
Naf ions"'
l0
Example 3.
A NH9 based solid acid such as (NH4 ) 3H (S04 ) 2 or (NH4 ) 3H ( Se04 ) 2
is ground and mixed with a melt-processable polymer binder, such
TM
as Crystar 101 thermoplastic, and hot-pressed. The result forms
IS a solid composite membrane which is proton conducting even in dry
atmospheres. The membrane is less permeable to fluids than
Nafion""' and is also less expensive.
' Example 4.
28
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
An solid acid silicate of general formula MaHbSi04, such as
CaNaHSi04, Cs3HSi04, (NH4)3HSi04, is used as a membrane. Some of
these materials are water insoluble and may have sufficient
structural integrity that a binder is not required in some
applications.
Example 5.
A Cs or NH4 based solid acid, such as CsHS04,
Cs2 ( HSO4 ) ( HZPO4 ) , CsSH3 ( SO4 ) 4 ~ X HzO Or (NH4 ) 3H ( SO4 ) z is
mixed Wlth
the prepolymer of a resin such as "castoglas", a commercial
product from Buehler, Inc. The polymerization/crosslinking
catalyst is added to the mixture, and a solid composite membrane
so formed. The in situ polymerization/crosslinking can lead to a
higher impermeability than composites formed by melt-processing.
Example 6.
29
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
A Cs or NHg based solid acid, such as CsHSOg,
Cs2 (HSOg) (H2POg) , Cs5H3 (SOg) g ~XHZO or (NHg) 3H (SOg) 2 is mixed Wlth a
monomer such as dicyclopentadiene. A polymerization catalyst is
then added to the mixture, and a solid composite membrane
comprised of the solid acid and poly(dicyclopentadiene) is
formed. The in situ polymerization of the polymer can lead to a
higher impermeability than composites formed by melt-processing.
Use of a NHg based solid acid can result in an inexpensive
membrane.
Example 7.
A Cs or NHg based solid acid, such as CSHSOg,
Csz (HSOg) (HZPOg) , Cs5H3 (SOg) g xH2O or (NHg) 3H (SOg) 2 1S dlSSOlVed In
water, and added to a suspension of an insoluble polymer such as
poly(vinylidene fluoride) suspended in a fluid such as ethanol.
The mixture is cast and the liquids (water and ethanol) allowed
to evaporate. This procedure yields a composite membrane which
is proton conducting even in dry atmospheres. The casting step
can produce very thin membranes, with thicknesses on the order of
one hundred microns.
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Example 8.
A Cs or NHg based solid acid, such as CsHSOg,
Cs2 (HSOg ) (H2POg ) , Cs5H3 ( SOg ) g ~XHZO Or (NHg ) 3H ( SOg ) 2 1S ground
and
mixed with a ceramic, such as A1z03, or an oxide glass, such as
amorphous Si02. The mixed powders are compressed by hot-
pressing. The resulting composite membrane may be stable to
higher temperatures than those in which the binder is a polymer.
Example 9.
A Cs or NHg based solid acid, such as CsHSOg,
CS2 (HSOg) (HzPOg) , Cs5H3 (SOg) g ~XH2O Or (NHg) 3H (SOg) 2 1S dlSSOlVed In
water. The solution is introduced into a porous membrane
comprised of an inert binder such as TeflonTM, Si02, or A1203.
The water is allowed to evaporate, leaving the solid acid to fill
the pores of the binder. The result is a composite membrane
which is proton conducting even in dry atmospheres.
Example 10.
31
CA 02359865 2001-07-10
WO 00/45447 PCT/iJS00/01783
A Cs or NH4 based solid acid, such as CsHS04,
Cs2 (HS04) (HZP04) , Cs5H3 (S04) 4 ~xHzO or (NH4) 3H (S04) 2, which is only
proton conducting, is ground and mixed with an electronically
conducting polymer such as poly(anylene). The composite membrane
formed can conduct both protons and electrons.
Example 11.
An solid acid silicate of general formula MaHbSi04, such as
CaNaHSi04, Cs3HSi04 or (NH4) 3HSi04, is ground and mixed with an
electronically conducting polymer such as poly(anilene). The
composite membrane formed can conduct both protons and electrons.
Example 12.
A proton conducting solid acid, such as CsHS04,
Csz (HS04) (HZP04) , (NH4) 3H (S04) 2 or CaNaHSi04, and a metal, such as
Ag, Au, or Cu, are ground and mixed. The mixed powders are
compressed by hot-pressing. The composite membrane formed can
conduct both protons and electrons, and may be stable to higher
temperatures than a composite in which the electron conducting
component is a polymer.
Example 13.
32
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
A proton conducting solid acid, such as CsHS04,
Cs2 (HS04) (H2P04) , (NH4) 3H (S04) z or CaNaHSi04, and a metal, such as
Ag, Au, or Cu, are ground and mixed. A polymeric material is
also added. A solid composite membrane is prepared either by
hot-pressing, if the polymer is melt-processable such as
poly(vinylidene fluoride), or by in situ polymerization, if the
polymer is in situ polymerizable such as poly(dicyclopentadiene).
The composite membrane is both proton and electron conducting,
and may have superior mechanical properties to a composite
containing only a solid acid and a metal.
Example 14.
A mixed electron and proton conducting solid acid, such as
CsHCrXSl_X04 or (NH4) 3H (CrXSl_X04) 2 in which one of the X elements
has a variable valence, is mixed with an inert polymeric binder.
If the polymer is melt-processable, such as poly(vinylidene
fluoride), a membrane is formed by hot-pressing. If the polymer
can be polymerized in situ, a membrane is formed by mixing the
solid acid, the monomer and the polymerization catalyst. The
resulting membrane conducts both protons and electrons, and may
be more stable in oxidizing atmospheres than a composite
containing metal particles.
33
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Example 15.
A Cs or NH4 based solid acid, such as CsHS04,
Cs2 ( HS04 ) ( HZ P04 ) , Cs5H3 ( S04 ) 4 ~xH20 or ( NH4 ) 3H ( S04 ) 2 i s
prepared f rom
aqueous solution, ground, and then pressed into a thin membrane.
The membrane is used in an electrochemical device at a
temperature above the superprotonic transition temperature and
above 100°C, so that the proton conductivity of the solid acid is
high and any Hz0 that may be present in the device exists in the
form of steam rather than liquid water.
Example 16.
A mixed electron and proton conducting solid acid, such as
CsHCrXSl_X04 or (NH4) 3H (CrXSl_X04) 2 in which one of the X elements
has a variable valence, is prepared from aqueous solution or by
solid state reaction. The powder is then ground and pressed into
a thin membrane. The membrane is used in an electrochemical
device at a temperature above the superprotonic transition
temperature and above 100°C, so that the conductivity of the
solid acid is high and any H20 that may be present in the device
exists in the form of steam rather than liquid water.
Example 17.
34
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
A composite comprised of one or more of the solid acids
listed in Table 1 and one or more of inert binders listed in
Table 2. If one or more of the components in the composite is
electronically conducting, the composite membrane will be capable
of conducting both protons and electrons. Electronically
conducting substances are indicated.
Table 1. Solid acid compounds.
Sulfates and selenates and silicates
sulfate-phosphates selenate phosphates
CsHS04 CsHSe04 CaNaHS i04
Cs3H ( S04 ) z Cs3H ( Se04 ) z CaH2Si04
CssH3 ( SO4 ) 4 ' xHzOCssH3 ( SeO4 ) 4 ' CsH3S1O4
xHzO
Cs3 (HS04) z (Hi.s Csa (HSe04) z (Hi.s CszH2Si04
(So.sPo. (Seo.sP
5) 04) 0.5) ~4)
Cs3 (HSO4) z (HzPO4) Cs3 (HSeO4) z (HzPO4) CS3HSlO4
Csz (HS04) (H2P04) Csz (HSe04) (H2P04) NH4H3Si04
CSS (HSO4) 3 (H2P~4) CS5 (HSeOg) 3 (H2P04) (NH4) 2H2SlOg
2 2
CsHzP04 (NH4) 3HSi04
NH4HS04 NH4HSe04 RbH3Si04
(NH4) sH (SO4) z (NH4) 3H (SeO4) 2 RbzHzSlOg
(NH4 ) sHa ( SO4 ) (NH4 ) sH3 ( SeO4 ) Rb3HSiO4
4 ' xHzO 4 ' xHzO
(NH4) z (HS04) (HzP04)(NH4) z (HSe04) (HZP04)KH3Si04
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
(NHg) HzPOg KZHzSiOg
RbHSOg RbHSeOg K3HS iOg
Rb3H ( SOg ) 2 Rb3H ( SeOg ) 2 NaH3Si0g
Rb5H3 ( SOg ) g ' Rb5H3 ( SeOg ) g ' Na2HzSi0g
xH20 xHzO
Rb2 ( HSOg ) ( HZ Rb2 ( HSeOg ) ( HzPOg Na3HSiOg
POg ) )
RbH2POg BaCsHSiOg
Table 2. Binder or matrix materials
Polymer ceramic/oxide metal or
glass semiconductor
poly(vinylidene fluoride) Si02 Ag*
poly(dicyclopentadiene) A1203 Au*
poly(tetraflouroethelyne) Mg0 Cu*
[Teflon]
poly(ether-ether ketone) cordierite Al*
poly(ether sulfone) Ni*
Silicones [dimethyl Fe*
siloxane polymers]
poly(pyrrole)* Zn*
poly(aniline)* graphite*
silicon*
electronically conducting
36
CA 02359865 2001-07-10
WO 00/45447 PCT/US00/01783
Other modifications are within the disclosed embodiment.
For example, the above has described the materials having a
superprotonic transition upon heating. Certain materials may
have their superprotonic transition temperature below room
temperature. Thus, there may be no apparent superprotonic
transition and the material would be disordered at room
temperature. These solid acids with structural disorder even
prior to heating are also contemplated.
37