Note: Descriptions are shown in the official language in which they were submitted.
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
1
VENOUS VALVE IMPLANT BIOPROSTHESIS AND
ENDOVASCULAR TREATMENT FOR VENOUS INSUFFICIENCY
This application claims the benefit of U.S. Provisional Application
Serial No. 60/119,995, which was filed February 12, 1999, the disclosure
of which is incorporated herein by this reference.
DESCRIPTION OF THE RELATED ART
s Two percent of the United States population suffers from severe
forms of venous insufficiency. It is a significant health problem since the
condition affects a wide range of ages, from pre-teenagers to the elderly.
Symptoms include dilated veins, leg pain, swelling, and stasis skin
changes such as discoloration, lipodermatosclerosis, ulcerations, and
to recurrent deep venous thrombosis (DVT). The disease carries a
significant morbidity that includes frequent hospitalizations and absence
from work, recurrent debilitating symptoms despite treatment and
changes in lifestyle.
The underlying pathophysiologic mechanism in chronic venous
is insufficiency is venous hypertension, particularly during the systolic
phase of the cardiac cycle. The venous hypertension may be due to
outflow obstruction, reflux or a mixed problem. Reflux, frequently the
sequelae of venous thrombosis, produces distal venous hypertension
equal to the hydraulic pressure resulting from a vertical column of blood
20 (extending from the heart to the ankle) in the upright position. Venous
reflux is the result of valvular dysfunction due to prior trauma (valves
become scarred or destroyed after thrombus), congenital absence, or
incompetence. After an episode of deep vein thrombosis (DVT), patients
may present years or even decades later with post-thrombotic syndrome.
zs The initiating event may have been prior surgery, trauma, fractures,
pregnancy, and/or prolonged standing or immobility.
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
2
The diagnostic evaluation of these patients may include
hypercoagulability testing, color duplex ultrasound, and ascending and
descending (i.e., contrast) venography.
Medical and surgical treatments are used to treat this condition
s with moderate success. Medical management aims to control symptoms
whereas surgical treatments attempt to restore normal physiologic
mechanism. The choice of surgical or non-surgical treatment is based
on the severity of symptoms and the anatomic systems) affected by the
disease process. Medical treatments include external compression
io (compression wraps, elastic compression stockings, and intermittent
pneumatic compression devices) and pharmacologic agents. External
compression blocks the transcapillary fluid flow during ambulatory
venous pressure cycle and causes an increase in the fibrinolytic activity
of the veins. Surgical treatments include ligation and stripping of the
is superficial system, subfascial ligation of incompetent perforating veins,
venous reconstructive surgery, crossover saphenofemoral venous
bypass, saphenous bypass in patients with isolated obstruction; venous
valvuloplasty, venous segment transfer, and vein valve transplantation.
Recently, some investigators described the use of endoscopic
zo venous valve transplantation and restoration of vein competence with a
xenograft monocusp valve. See, e.g., Garcia-Rinaldi R, et al., "Femoral
vein valve incompetence: treatment with a xenograft monocusp patch," J
Vasc Surg, 1986,3:932-935 and Ofenloch JC, et al., "Endoscopic venous
valve transplantation with a valve-stent device," Ann Vasc Surg,
2s 1997;11:62-67. Others have reported a technique of autogenous valve
reconstruction at the saphenofemoral junction by creating a proximal
saphenous stump, and invaginating it to create a bicuspid valve (Plagnol
P, et al., "Autogenous valve reconstruction technique for post-thrombotic
reflux," Ann Vasc Surg, 1999;13:339-342), or creating the valve by vein
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
3
wall intussusception (Wilson NM, et al., "In situ venous valve
construction," Br J Surg, 1991;78:595-600). Cardon JM, et al. have
described the use of ipsilateral saphenous vein as a valve transplant.
See "Use of ipsilateral greater saphenous vein as a valued transplant in
s management of post-thrombotic deep venous insufficiency: long term
results," Ann Vasc Surg, 1999;13:284-289. To date, the tatter produces
the most encouraging results, but it is limited by eventual degeneration of
the transplanted valves, or inadequate donor valves. In "Experimental
prosthetic vein valve," Int Angiol 1989;8:7-9, Taheri, et al. have described
to the use of an experimental prosthetic vein valve in a dog model as an
alternative to autogenous venous transplantation.
Despite these therapeutic options, the results have been mixed.
Medical treatment may be efficacious and cost effective, but demands
strict adherence to a program of ambulatory venous compression.
is Surgical treatment requires skillful, meticulous technique. Often patients
may require multiple interventions. Moreover, the use of compression
stockings is often required even after surgical intervention to ensure relief
of symptoms and durability of the operation. Some consider the need for
compression garments as a proof that surgery was unsuccessful. Thus,
2o it is evident that a need remains for the development of more effective
products and procedures for the treatment of chronic venous
insufficiency.
BACKGROUND AND SUMMARY OF THE INVENTION
Percutaneous techniques have emerged as less invasive options
2s in the treatment of vascular problems.
Martin, LW, et al. evaluated the feasibility of percutaneous
deployment of a venous stent valve in the bovine central venous system,
as reported at the SCVIR 22nd Annual Scientific Meeting, March 8-13,
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
4
1997, Washington, D.C. Martin et al. obtained two gluteraldehyde-fixed
bovine jugular veins with a single valvular apparatus from Baxter
Healthcare Corp. One vein valve segment measured 13.9 mm in
diameter. He trimmed this vein of excess tissue (no details given) and
s sutured it inside a self-expanding Nitinol stent (l5mm X 28mm). The
second vein valve segment measured 8.9 mm in diameter. He trimmed
it of excess tissue (no details given) and sutured it to a Gianturco-Rosch
Z stent (Cook, Inc., Bloomington, IN). In his single experiment, he
percutaneously placed the first bioprosthesis; reportedly confirmed newly
to established in vivo venous valve competence, inferior vena cava patency
and valve leaflet function; and then immediately sacrificed the animal.
The second prosthesis was saved for future use.
Martin et al. supplied to us and we used in our first experiment the
vein segment from his second prosthesis. We sutured that vein segment
Is to a self-expanding Nitinol stent using spot sutures as was done by
Martin et al. We prepared the remaining bioprostheses used in our
study, using different trimming techniques than those apparently used by
Martin (according to the bioprosthesis he supplied). Also, as detailed
hereinbelow, for our third and subsequent experiments we used a
2o different suturing technique and for all our experiments we used a
different delivery technique, than was utilized by Martin et al.
It was an object of the invention to develop a bioprosthesis for use
in providing or restoring valvular function in a biological duct of a patient.
More particularly, it was an object of the invention to provide a system for
2s potential use in treatment of chronic venous insufficiency by using
percutaneous techniques. The foregoing and other objects of the
invention have been realized by percutaneously placing an endovascular
device comprising a vein valve segment that has been substantially
trimmed to reduce a radial dimension thereof and sutured, preferably in a
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
running fashion, to a self-expanding stent, to treat chronic venous
insufficiency when it is due to incompetent venous leaflets.
More specifically, using the concept of endoluminal stent graft, a
bovine jugular vein with a valve was sutured to a Nitinol stent and
s deployed in the swine venous system. As noted above, for our first
experiment, we used the second bioprosthesis prepared by Martin et al.
To prepare the bioprostheses for our remaining experiments, segments
of glutaraldehyde-fixed bovine external jugular vein with valves were
substantially trimmed, as detailed hereinbelow, and sutured, as also
Io detailed herein, below to a self-expanding, Nitinol stent.
In our first series of experiments, each of eleven animals were
premedicated and anesthetized (n=11 ). Venography of the right external
jugular vein, inferior vena cava (IVC), and common iliac vein was
performed. Deployment was accomplished via a sheath (12F-24F) using
is fluoroscopic guidance. Eleven (11) bioprostheses were deployed in the
eleven (11 ) animals. Bioprostheses were deployed in the IVC (n=3) or
right external iliac vein (n=6). Animals were sacrificed immediately after
deployment (n=7), at one week (n=1 ), or at two (n=2) weeks. One
animal was found dead in the cage. At necropsy, each bioprosthesis
20 (n=4) was explanted, and histopathologic analysis performed. We used
the right external jugular vein as the entry site for percutaneous implant
delivery. It is potentially possible to place the device in the same manner
in human patients. However, since it is possible to construct small
bioprostheses with the techniques we have developed, as described
2s herein above, it would be possible to use alternative routes of delivery
such as the popliteal vein (posterior aspect of the knee) without the need
to predilate the vein, which can disadvantageously activate a myriad of
thrombogenic reactions in response to the balloon injury.
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
6
The deployments of the bioprostheses were successful in 9 of 11
swine. Two deployments were unsuccessful (one accidental deployment
in the right renal vein, one deployment in the IVC caused rupture of the
vein). Post-deployment venography (n=9) confirmed no reflux (in the
s recumbent position of the swine) of the valve leaflets and patency of the
vein inferior to the level of the bioprostheses. In the first group of
animals (n=5), valve leaflets were normal and competent. In the survival
animal group (n=4), the bioprostheses remained patent without evidence
of thrombus formation by ascending and descending venography. Gross
io inspection of the explanted bioprostheses (n=4) demonstrated grossly
normal valves that fully occluded the lumen. Complications included
hemarthrosis (n=1 ), death (n=1 ), and, in our first experiment,
bioprosthesis thrombosis immediately after deployment (n=1 ).
Histopathologic analysis showed endothelial cells covering the luminal
is surfaces. The wall of the bioprostheses had granulomatous response
and foreign body reaction. Bacterial contamination was noted in one
bioprosthesis.
Our studies show that deployment of a glutaraldehyde-fixed
bovine vein sutured to a self-expanding Nitinol stent in the swine iliac
2o vein or IVC is technically feasible and, in the cases where the vein
segment is substantially trimmed, will remain patent following
deployment. A venous bioprosthesis that can be placed percutaneously
may have important clinical applications as an endovascular treatment
for chronic venous insufficiency when it is due to valvular incompetence.
25 BRIEF DESCRIPTION OF THE DRAWINGS
These, as well as other objects and advantages of this invention,
will be more completely understood and appreciated by careful study of
the following more detailed description of the presently preferred
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
7
exemplary embodiments of the invention taken in conjunction with the
accompanying drawings, in which:
FIGURE 1 is a digital image of a segment of a glutaraldehyde-
fixed bovine jugular vein with leaflets;
s FIGURE 2 is a digital image of, in the order recited from the top of
the image, a bovine vein segment before trimming, a vein segment
axially and radially trimmed and sutured to a Nitinol stent thereby to
define a bioprosthesis embodying the invention, and a compressed and
loaded bioprosthesis within an introducer according to the present
io invention;
FIGURE 3 is a schematic illustration of an introducer sheath with
dilator;
FIGURE 4 is an schematic, exploded elevational view illustrating
the loading of the introducer sheath in an embodiment of the invention;
is FIGURE 5 is a schematic elevational view illustrating the
deployment of the bioprosthesis in an embodiment of the invention;
FIGURE 6 is a digital image showing a pre-deployment baseline
flow through the vein (venogram; injection rate l5cc/sec, total volume
30cc);
2o FIGURE 7 is a digital image showing the unsheathing of the
bioprosthesis at the level of the right iliac vein;
FIGURE 8 is a digital image after bioprosthesis deployment, with
the stent fully expanded;
FIGURE 9 is a digital image showing flow through the vein
2s (descending venography) two weeks after bioprosthesis deployment,
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
8
showing the column of contrast is interrupted at the level of the
competent leaflets;
FIGURE 10 is a digital image showing flow through the vein
(ascending venography) two weeks after bioprosthesis deployment,
s showing a continuous column of contrast and no thrombus formation
superior or inferior to the bioprosthesis;
FIGURE 11 is a digital image of bovine vein segment after fixation
and containing valve leaflets longitudinally bisected to show the leaflets
are normal in appearance, i.e., membranous, pleated and free of
to thrombus;
FIGURE 12 is a digital image of a microscopic view of a valve
segment (longitudinal view, 13X magnification, Masson's Trichome stain)
composed of densely collagenous connective tissue with thin bands of
smooth muscle, showing reactive endothelial cells are more prominent at
is the base (arrowheads) and commissure of the valve;
FIGURE 13 is a digital image showing foreign body reaction in the
outer two-thirds of the bovine graft (arrows); there is marked remodeling
of the normal stromal and cellular architecture. Dense nodular
aggregates of macrophages are seen in the ab-lumenal aspect of the
2o vein wall (small circle), as well as a large number of foreign body type
multinucleated giant cells (large circle).
DETAILED DESCRIPTION OF THE INVENTION
Segments of a glutaraldehyde-fixed bovine jugular veins (n=11 )
with leaflets were used. One glutaraldehyde-fixed bovine jugular vein
2s was supplied by Martin, as noted above, and without further trimming by
us was sutured with isolated sutures to a Nitinol mesh stent. The
remaining glutaraldehyde-fixed bovine jugular veins were obtained by us
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
9
from Venpro, Irvine, CA. (FIGURE 1). Bovine vein diameter ranged from
8.9 mm to 14 mm. Each segment obtained from Venpro was
substantially trimmed by us to remove at least about 50% of the excess
tissue around each vein. More specifically, we trimmed the vein segment
s to an axial length corresponding to or, more preferably, less than the
length of the stent, and we dissected the excess tissue so that the wall
thickness of the vein was reduced to at least about 50% of its original
thickness. By way of example, we removed approximately 1-3 mm of the
initial wall thickness of the veins. We recognized, and our experiments
io have confirmed, as detailed hereinbelow, that the trimming process is
important from a mechanical standpoint because a smaller, more
compressible design can be delivered via a smaller system, more
suitable for percutaneous techniques. Moreover, when histopathologic
analysis is performed, the advantage of having a thinner piece of foreign
Is tissue is that the "host" has to process this tissue and eventually convert
it into its own cellular elements. If the host is exposed to less tissue to
process, i.e. a substantially trimmed vein segment according to our
invention, this can be done in less time, increasing the chances of
patency and decreasing the possibility of thrombosis. Substantial
2o trimming according to our invention also helps to keep the functional
lumen of the bioprosthesis in close correspondence to the vein in which
the bioprosthesis is implanted. Moreover we have found that the
substantially trimmed vein segment can be more easily secured with
respect to the stent so as to closely appose the stent structure, so that
2s the secured vein segment and stent act as a one piece assembly. This
helps in the process of expansion of the bioprosthesis, obtaining a better
apposition of the bioprosthesis against the host vein and the
achievement and maintenance of a patent passage therethrough.
Self-expanding Nitinol stents (Symphony, Meditech, Boston
3o Scientific, Watertown, MA) were selected to match the diameter of each
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
of the vein segments. For our second and subsequent experiments, the
vein segment with leaflets, radially and axially trimmed as noted above,
was placed inside the Nitinol stent. As illustrated in FIGURE 2, the vein
segment is preferably trimmed to an axial length less than that of the
s stent. Providing a vein segment having a length less than that of the
stent defines a staged or stepped transition between the edge of the vein
segment, the stent, and the host vein. A staged transition is helpful to
anchor the device better and also to provide a smoother transition
between the device and the host vein, therefore minimizing turbulent flow
io in these areas thereby reducing the potential for thrombosis formation.
For our second experiment, the trimmed vein was sutured to the
stent using discrete sutures. Following implantation we observed that
while the prosthesis appeared patent, it appeared to have an irregular
diameter, suggesting that the vein segment was sagging between
is sutures. Accordingly, for our third and subsequent experiments, the
trimmed vein was sutured to the stent using 6-0 Prolene (Ethicon, Inc.,
Johnson & Johnson, Sommerville, NJ) in a running fashion. More
specifically, rather than placing isolated sutures in select locations as
was done by Martin et al. and for our first and second experiments, we
2o sutured the vein with at least one continuous suture along substantially
the entire stent, so that the vein is substantially completely apposed to
the stent. This assured that the vein segment would not collapse inside
the stent in the process of being delivered or following deployment.
Moreover, with the above described trimming and suturing technique, we
2s have found that neither stabilizing sutures nor mechanical dilation is
required since the device can substantially fully open and appose to the
host vein wall.
After constructing the bioprostheses, the competency of the valve
leaflets of each was tested by manually infusing normal saline with a
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
11
l0cc syringe in the direction opposite to the blood flow. Each
bioprosthesis was kept in a glutaraldehyde bath until the time of implant.
Eleven 25-35Kg female swine were used. The Animal Care and
Use Committee at our institution approved this research protocol. The
s day of experiment, each animal was premedicated with Acepromazine
Maleate 1.1 mg/Kg IM, Ketamine Hydrochloride 22 mg/Kg IM, and
Atropine Sulfate 0.8 mg/Kg IM. Thiopental 15 mg/Kg IV was used for
induction. Isoflurane 1.5%-2-5% was used for maintenance anesthesia.
The right external jugular vein was dissected and used as venous
io access. An 8.5F vascular sheath (C.R. Bard, Inc., Billerica, MA) was
advanced into the jugular vein. Venography of the right iliac vein and
IVC was performed using a 5F marker pigtail catheter (Cook Inc.,
Bloomington, IN) to correct for magnification. Contrast was injected at a
rate of 15 cc per sec for a total of 30 cc (FIGURE 6). Each animal was
is heparinized with 300-400 units/Kg administered intravenously. Prior to
implantation, each bioprosthesis was submerged in an ice bath to
facilitate crimping and placement inside the introducer/deployment
system. We loaded the cooled, reduced diameter bioprosthesis into an
introducer tube (FIGURE 2) to facilitate loading into the deployment
2o system, as described in greater detail herein below. The transverse
diameters of the right external iliac vein and inferior vena cava (IVC) at
specific locations were measured using an electronic caliper.
Measurements were obtained in the AP position. The selection of the
site for deployment was made to match the diameter of the swine's vein
2s (IVC or iliac vein) to the transverse diameter of the bioprosthesis. Prior
to bioprosthesis deployment, the vascular sheath and catheter were
removed over an Amplatz superstiff wire (Meditech, Boston Scientific,
Watertown, MA). With reference to FIGURE 3, a long deployment
system 10 (Cook, Bloomington, IN) ranging from 12F to 24F (12F (n=1),
30 16F (n=6), 18F (n=3), 24F (n=1 )) was advanced into the venous system,
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
12
using the right external jugular vein as the entry site, over the guidewire
12. The deployment sheath size was selected based on in vitro
experience. We developed the "n+4 French" rule. The rule states that
"n" are the diameter of the bioprosthesis, and the deployment sheath
s should be at least "n+4" French (F). We only used one 24F delivery
system (in the first experiment) since it was the only diameter that would
accommodate the bioprosthesis formed from the vein segment obtained
from Martin et al. For the remaining experiments we were able to use
smaller delivery systems due to our trimming and suturing techniques.
to With reference to the schematic illustrations of FIGURES 3-5, the
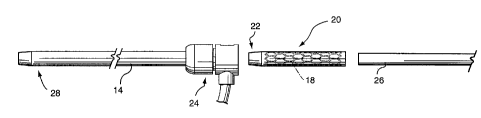
selected deployment sheath 14 with inner dilator 16 were advanced over
the wire 12. The inner dilator 16 and wire 12 were then removed. As
mentioned above, the bioprosthesis 18 (not shown in FIGURE 4) was
cooled to reduce its diameter and preloaded in an introduces tube 20.
is The introduces 20 has an inner diameter equal to or less than the inner
diameter of the deployment sheath 14 so that the bioprosthesis can be
readily loaded from the introduces to the sheath. We created an
introduces by cutting off the distal portion of the deployment sheath of
another deployment system of the same size as the selected deployment
2o system 10. However, the introduces could be created as an independent
component.
To load the bioprosthesis, the tapered tip 22 of the introduces 20
was pushed into the one-way valve 24 of the deployment system 10; and
the bioprosthesis was pushed into the deployment sheath 14 with the aid
2s of a pusher 26. The pusher has an outer diameter that can be
accommodated in the inner bore of the introduces and in the inner bore
of the sheath 14 and a length greater than that of the sheath so that the
pusher can displace the bioprosthesis from the introduces into the sheath
and along the sheath to the target portion of the vessel for deployment.
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
13
We created a pusher by cutting off the tapered end of the inner dilator 16
of the deployment system 10.
The deployment was accomplished by unsheathing the
bioprosthesis (FIGURES 5, 7,8). More specifically, once the
s bioprosthesis 18 was displaced by the pusher 26 to the distal end 28 of
the deployment sheath 14, the deployment sheath 14 was displaced
proximally, as shown by the arrow in FIGURE 5, relative to the
bioprosthesis 18 and pusher 26, so that the bioprosthesis 18 is disposed
in the vessel and is free to self-expand, due to the ambient temperature
to and its memory characteristics, to substantially fully open and appose
the host vein wall (FIGURE 8).
In our experiments, as detailed herein above, we used self-
expanding stents so that mechanically expansion such as with a balloon
catheter, which may damage the valve and/or vein segment, was not
is required. As also noted above, the self-expanding stents we selected
were Nitinol stents manufactured by a particular manufacturer. However,
as will be appreciated by those skilled in the art, self-expanding stents
formed from other material(s), having other structural configurations,
and/or produced by other manufactures could be used to advantage in
2o accordance with the invention. Thus, the invention is not to be limited to
the particular stent used in our experiments.
In our experiments, as described above, the delivery procedure
did not require and did not use an over the wire system to deploy the
bioprosthesis. The wire was used solely to place the deployment sheath.
2s This was advantageous in that it minimized the possibility of damage to
the delicate leaflets of the bioprosthesis or of potentially dislodging the
vein from the stent. Furthermore, as noted above, we did not have to
dilate any of the bioprostheses we prepared after delivery. The need to
dilate afterwards could be potentially damaging to the device. We found
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
14
that with our trimming and suturing techniques, neither stabilizing sutures
nor post deployment dilation were required since they could fully open
and appose the host vein wall.
Post-deployment ascending and descending venography were
s performed in the recumbent position. Venography was performed to
evaluate patency, thrombosis and valvular competency. Descending
venography was performed via right external jugular vein access.
Ascending venography was performed at the time of sacrifice by
exposing the right femoral vein by cutdown and placing a 6F vascular
Io sheath.
Seven animals were sacrificed immediately after implantation of
the bioprosthesis with an overdose of Thiopental IV and 30 cc of
supersaturated solution of Potassium Chloride (KCI). Gross examination
included evaluation of the valvular apparatus by infusing normal saline
is with a 10 cc syringe as it was done before implantation.
Four animals were selected for the survival group (four animals for
two weeks). Anticoagulation consisted of Warfarin Sodium, 2.5 mg orally
prior to the procedure and daily thereafter. Ten thousand units of
Heparin IV and 44,000 units/Kg of Penicillin G benzathine/Penicillin G
zo procaine were administered during the procedure. Each bioprosthesis
was deployed in the same fashion as previously described. Descending
venography was performed immediately after deployment. The right
external jugular vein was ligated and the incision was closed with 2-0
Vycril (Ethicon, Johnson & Johnson, Somerville, NJ). Each animal
2s received 60 mg SQ of Enoxaparin Sodium immediately afterwards.
Two of the four (4) animals that survived for two (2) weeks were
evaluated after bioprosthesis deployment with ascending and
descending venography (FIGURES 9,10). Explanted bioprostheses
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
(n=4) were submitted for light microscopic analysis. Each bioprosthesis
was longitudinally bisected between the leaflets. One segment was
infiltrated with, and imbedded in hard plastic. The surface was stained
with Hematoxylin and Eosin (HE) and saffron stains. In the other
s segment, the metallic components of the stent were carefully removed,
step-cut longitudinally, and the step sections imbedded in paraffin. Serial
sections were prepared and stained with HE, Masson's Trichome (MT)
and Verhoeff's Van Gieson (VVG) stains.
Nine of the eleven bioprostheses were percutaneously deployed.
to Six were deployed in the external iliac vein and three in the IVC. Two
inadvertent malpositions occurred, one in the right renal vein and one in
the peritoneal cavity. These malpositions occurred when attempting to
advance the delivery system into a better position for deployment after
the wire was removed.
is In the acute animal group (n=7), four descending venograms were
performed demonstrating competent leaflets, with interruption of the
column of contrast at the level of the leaflets. One bioprosthesis (the
one made using the vein segment supplied by Martin) was occluded by
thrombus. One was inadvertently placed in the right renal vein. One
2o bioprosthesis was found in the peritoneal cavity after inadvertent rupture
of the intrahepatic IVC during advancement of the deployment system.
The descending venogram showed contrast extravasation indicating IVC
rupture. Four ascending venograms were performed in the acute group.
These demonstrated fully retracted valve leaflets, without obstructing the
2s flow of contrast. The ascending venograms were omitted in the two
inadvertent malpositions and in the occluded bioprosthesis immediately
after deployment.
In the survival group (n=4), four descending venograms were
performed at the time of implantation of the bioprostheses. In all cases,
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
16
the leaflets appeared competent, the bioprostheses fully expanded and
free of thrombus. Of the four survival animals, all on warfarin therapy,
one animal had to be sacrificed prematurely at one week due to
spontaneous hemarthrosis as noted by limping, swelling and
s discoloration of the hind legs. One animal was found dead in the cage at
one (1 ) week, presumably from exsanguination due to the extensive
amount of blood found in the cage. Ascending and descending
venography was performed in three (3) of the four (4) animals. In all
descending venograms performed (n=3), the leaflets were competent,
to with interruption of the column of contrast at the level of the competent
leaflets. A continuous column of contrast was seen in all ascending
venograms performed (n=3), indicating that the valve leaflets were fully
retracted (FIGURES 9,10), and that there was no thrombus formation.
No migration of the bioprostheses was documented in the acute or the
is survival groups.
At necropsy, gross inspection at the time demonstrated competent
leaflets, fully expanded bioprostheses, and three (3) devices free of
thrombus. A single bioprosthesis had post-mortem thrombus entrapping
the valve leaflets, which appeared otherwise grossly normal. Light
Zo microscopic examinations of the leaflets showed normal tissue, i.e.,
membranous and variably pleated valves (FIGURE 11 ).
Endothelial cells were particularly prominent in the valve recesses
and commissures (FIGURE 12). However, microscopic examination
showed histologically normal valve leaflets (n=4). In the outer two thirds
2s of the bovine vein wall (n=4), inflammatory foreign body reaction was
most pronounced (FIGURE 13). Additionally, dystrophic mineralization
of the prosthetic collagen, infiltration by macrophages, granulocytes, and
a few lymphocytes were also seen. Microscopic examination of one of
the bioprosthesis showed marked hemorrhage dissecting the collagen
3o fibers of the bioprosthesis, purulent inflammation, and a few cocci.
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
17
Possibly, contamination at the time of bioprosthesis implantation could
explain the presence of bacteria. In all animals (n=4), lymph nodes
adjacent to the bioprostheses demonstrated marked histiocytosis.
Our histopathologic studies confirm the lack of thrombus formation
s within the vein leaflets, which appears intact, without fenestrations. A
foreign body reaction was noted,.particularly in the outer aspect of the
graft. Viable endothelial cells were present within the leaflets. The
implications of these observations are not clear, but may represent the
early attempts to transform the graft tissue into host tissue. It may also
to suggest that the leaflets are relatively protected from immunogenic
reaction, and therefore free of thrombus formation.
Our short-term animal experience suggests that, using a stent
skeleton, it is possible to implant a glutaraldehyde-fixed bovine vein with
leaflets into the swine central venous system, and to maintain valvular
is competence for at least two weeks. Thus, bovine vein is a reasonable
preliminary choice for bioprosthesis construction, and this belief is
supported by reports that glutaraldehyde fixation renders bovine vein
valve biocompatible and non-thrombogenic. See, e.g., DeLaria GA, et
al., "Hemodynamic evaluation of bioprosthetic venous prosthesis," J
2o Vasc Surg, 1993,18:577-586; and Wang SK, et al. "In vitro performance
of venous valve prostheses: an experimental model study, " ASA10
Journal, 1992:M213-M215.
Our study was preliminary in that it had several potential
shortcomings. First, the experiments were conducted in an animal model
2s with healthy veins. Second, the device was not tested in the upright
position. Finally, the need for a better anticoagulation regimen is
important based on our two anticoagulation-related complications. It is
possible that a better vein apparatus for construction of the bioprosthesis
may be an autologous glutaraldehyde-fixed or a cryopreserved vein
CA 02360175 2001-08-03
WO 00/47136 PCT/US00/03603
18
(Burkhart HM, et al., "Experimental repair of venous valvular insufficiency
using cryopreserved venous valve allograft aided by a distal
arteriovenous fistula," J Vasc Surg, 1997;26:817-822). In addition,
several issues such as bioprosthesis durability, immunogenicity and
s leaflet function should be evaluated with long term studies.
Nevertheless, our experience suggests that percutaneous placement of
a venous bioprosthesis is technically feasible and, on a short-term basis,
an effective means of restoring valve competence, particularly when the
implanted vein segment has been trimmed to substantially reduce its wall
io thickness.
Moreover we recognize that a percutaneously implantable
bioprosthesis has several potential advantages, including the minimally
invasive nature of the procedure; it does not preclude the possibility of
future re-intervention, either percutaneous, conservative treatments, or
is conventional surgical treatments; and it involves potentially lower costs.
While the invention has been described in connection with what is
presently considered to be the most practical and preferred embodiment,
it is to be understood that the invention is not to be limited to the
disclosed embodiment, but on the contrary, is intended to cover various
2o modifications and equivalent arrangements included within the spirit and
scope of the appended claims. Thus, for example, while we have
described the preparation of a venous valve prosthesis using a vein
segment obtained from a particular vessel in a particular biological
source other than the host or patient, the invention is not to be limited
2s thereto. Indeed, a different vessel could be the source of the vein
segment, and the donor could be the patient. Moreover, while the
invention has been described with reference to the implantation of a
bioprosthesis in a vein, it is to be understood that a bioprosthesis of the
type described herein could be implanted in an another biological duct to
3o provide/restore valvular function therein.