Note: Descriptions are shown in the official language in which they were submitted.
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
METHOD AND APPARATUS FOR CONTROLLING THE
STRATEGY OF COMPOUNDING PHARMACEUTICAL ADMIXTURES
The present invention generally relates to a method and apparatus
for preparing and accounting for pharmaceutical admixtures. More
particularly, it relates to strategies for preparing prescriptions for
parenteral
admixtures, for controlling the compounding apparatus, and for properly
accounting for the prepared admixture with the strategies being implemented in
computer software.
BACKGROUND OF THE INVENTION
Pharmaceutical parenteral admixtures are a combination of sterile
drugs that are mixed together under aseptic conditions and are intended for
intravenous infusion. These admixtures may be relatively simple or extremely
complex, with the complexity increasing with the inclusion of multiple active
ingredients. Nutrition admixtures are one example of complex parenteral
admixtures that are frequently prepared in a hospital pharmacy for treating
patients in the hospital. In general parenteral nutrition admixtures ("PN")
refer
to most types of nutritional solutions for intravenous feeding. Total
patenteral
nutrition admixtures ("TPN") generally refer to those PNs that do not contain
lipids as a component, and total nutritional admixtures ("TNA") refer to those
PN's that contain lipids.
The pharmacy of a hospital, a compounding center or a care
facility prepares or compounds a prescription which typically has been
detennined by a physician singularly or in conjunction with a dietician,
pharmacist or other care provider. The phannacy may be required to compound
large numbers of PN on a daily basis. The actual PN compounding is done
primarily by electomechanical mixing equipment called compounders which
are extremely sophisticated and at-e adapted to admix many different
-1-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
components in differing proportions as set forth in pharmaceutical
prescriptions.
Compounders include high volume compounders which are
adapted to prepare PN by transferring those components which are normally
found in relatively large volumes in a PN, for example amino acids, sterile
water, lipids and dextrose, at a relatively high speed. Such compounders
include the AUTOMIX 3 + 3 compounder manufactured by the Clintec
Nutrition Division of Baxter Healthcare Corporation.
Compounders also include low volume compounders such as
lo compounders from the same corporation marketed as a MICROMIX
compounder. The MICROMIX compounder is adapted to accurately transfer
those components that ar-e normally found in relatively small volumes in a
PNT.
It is comnlon for pharmacies to produce a prescribed PN by
utilizing both a MICROMIX and an AUTOMIX compounder, typically by
adding the high volume components to the final container or final bag with the
AUTOMIX compounder and then transferring the final bag to the MICROMIX
compounder for transfer-ring the smaller volume components. It should be
understood that a single compounder may have the capability of transferring
both high volume components and low volume components either sequentially
or concurrently. Alternatively, the single compounder could have both a high
volume module and a low volume module that could transfer fluid to a
common manifold, a common transfer tube connected to a final bag or
container, or into separate ports in a final bag.
To prepare such PN at an acceptable cost it is inlportant that the
PN are compounded as efficiently as possible. Efficiency is generally achieved
by seeking to maximize the nuniber of PN's prepared over a given period of
time or "throughput". Howevei-, the complexity of properly pr-eparing PN
-2-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
tends to slow down such throughput. Areas of complexity may be found in
determining the proper PN for a given prescription for a particular patient,
accurately preparing the PN and accounting or billing for the PN. However,
safety of the patient is paramount and efficient PN preparation must be
accomplished with little possibility of errors.
In preparing the proper prescription for a particular patient the
pharmacist must perform many tasks including evaluation and determination of
the proper components and their respective amounts. Patient specific factors
including the type of patient i.e. neonatal, and weight of patient, will be
io considered. Improving the ability of the pharmacist in making such
evaluations and determinations will increase the throughput and reduce the
possibility of errors. For record keeping purposes it is desirable and on
occasion required for the pharmacist to note in a permanent record why the
prescription differs from generally desired amounts of source solutions.
As is well known in pharmacy practice, much of the complexity
involved in preparing PN results from compatibility issues relating to the
components that are placed in the prescribed PN. Compatibility is defined as
the interaction between a drug and all other components with which that drua
comes into contact, including but not limited to the diluent, the container
and
other drugs in the same PN. Compatibility is divided into two subcategories
which are physical as well as chemical compatibility. Physical compatibility
is
defined as an incompatibility that will alter the physical appearance of the
drug, typically resulting in a visual change such as precipitation, gas
evolution
or a change in color. Chemical incompatibilities are not visually observed but
must be analytically tested. Chemical incompatibilities occur as a result of
changes in the active drug such as oxidation or photodegi-adation. Factors
that
can influence compatibility include, but are not limited to the total diluent
-3-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
volume, concentration levels, the order of admixing and the pH.
There are tivo steps in the evaluation of compatibility and
parenteral admixtures. First, compatibility of the entire PN over the period
between preparation of the admixture and completion of delivery to the patient
should be evaluated prior to compounding. Secondly, the compounding
preparation process must be planned in a way to allow for compatibility while
the compounding process is proceeding. For example, the compatibility
between a source solution being added to the final mixing container or any
intermediate mixing container and the solution present in that container
should
to be evaluated. In many instances source solutions which are packaged at
concentrations which are incompatible with other solutions must be diluted
before they come into contact with each other in such chambers.
As can be appreciated the higllest dilution will occur when the
greatest amount of diluting fluids are already present in the container into
which the solutions are being added. For example, amino acid or dextrose
source solutions will form a large portion of a PN and yet are typically
compatible with most additives. Thus it would seem to that these solutions
would be transferred first to the final containei- or to any intermediate
mixing
chambers to dilute added source solutions.
An additional complexity that must be considered is the
prevention of the contact between highly concentrated solutions which are
incompatible with each other along a common flowpath in the compounder. In
a representative instances, although source solutions may flow along separate
tubes for much of a transfer flowpatli, there may be a section along a
transfer
flowpath which is conlnlon to the two incompatible source solutions. This
common flow path nlay be found along any part of the flow path such as in an
intermediate mixing chamber or after the intermediate nlixing cllamber or
after
-4-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
a switching valve.
One method to reduce the possibility of a solution being
incompatible with a second solution along a common flow path is to flush the
common flow path after each solution has been transferred. Such flushing is
accomplished with a solution which is compatible with both the prior solution
as well as the solution to be added after the flush. As can be appreciated
frequent flushing dilute the incompatible solutions thereby making them
compatible but will also decrease throughput.
Also there must be a source of fluid for such a flushing scheme.
to The source of such flushing solutions may either be a compatible source
solution which forms a part of the prescription or the solution present in a
downstream chamber such as an admixture in the final mixing container.
However, in present pharmacy practices, the prescribed amount of solutions
which are used as flushing solutions are typically transferred first to the
final
container for expediency and dilution purposes and are not available as
flushing solutions. In this instance and by default the solution in the final
bag
must be compatible with the solution which is to be flushed, and the flushing
solution will be drawn from the final container. Drawing the flush from the
final bag and returning the flush to the final bag decreases throughput. On
the
other hand holding back an amount of the diluting source solutions for
flushing
may lead to instances where two incompatible solutions come into contact with
each other in the final mixing chamber without being properly diluted to a
compatible concentration.
In an effort to increase efficiency, a pharmacist will typically
group solution containers in dependence on the operating scheme of the
compounder so that inQredients which are compatible with each other (at
source solution concentration) are added together sequentially between rinses
-5-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
which are set by the pharmacist. After making a determination regarding the
compatibility of the various solutions the pharmacist may group a set of
compatible solutions on station 1-4, a second set of compatible of compatible
solutions on stations 5-8, etc with rinses set after station 4, station 8,
etc.
However, a particular admixture may require 5 solutions which are compatible.
Because setting rirjses requires greater time and effort, the pharmacist may
hang the fifth ingredient at a station which has rinses before and after
rather
than adjust the station an-angement and rinsing scheme. This however is not
optimal.
Another consideration for a pharnlacist when compounding
multiple prescriptions comprising a nlixture of TNA and TPN is lipid llazing
in
which a trace of lipid is present in a final solution which is not to contain
lipids. Hazing can be produced by lipids being present in amounts as low as
one to 3 parts per million. Such hazing will typically occur when a
is prescription containing lipids is compounded immediately prior to a
prescription which is not to contain lipids. Lipid hazing is not generally
believed to create a health hazard, however lipid hazing in a PN which is to
be
infused may be later mistaken for a PN with unacceptable precipitation arising
from an error in formulating a compatible PN solution, and the hazy solution
may be mistakenly discarded.
If lipid hazing is an issue the pharmacist may seek to avoid such
problems by flushing the compounder after each prescription containing lipids
is compounded. However, such flushing will decrease throughput and not be
totally effective. To increase throughput, the pharmacist may decrease such
flushing by grouping lipid prescriptions; however such groupings have a
negative impact on flexibility. If lipid hazing is not an issue, the
possibility of
lipid hazing should be communicated to persons who are compounding the
-6-
CA 02360788 2008-01-25
solution and who are administrating the PN to the patient to prevent the
administer from
mistakenly believing that the admixture has become unstable.
Other methods of seeking to prevent lipid hazing are to use a completely
separate flowpath for the lipids to the final mixing container. However, once
lipids are
present in a final container flushing or rinsing using solution from the final
container will
introduce lipids into the flowpath and may cause lipid hazing in a subsequent
PN.
Adding to the complexity of compounding the pharmacist must consider
is the accuracy limits of the compounders such that prescriptions which have
ingredients
in volume levels below the accuracy limit of the compounder will likely be
added by
hand utilizing a syringe. Such manual addition decreases throughput. Also
inefficiencies inherent in the administration of the PN to the patient such as
residual
volumes in administration sets must also be considered. Accounting for the
complexities
has proven to be time consuming and lead to inefficient activities and
practices.
For reimbursement and record keeping purposes, the prepared admixture
must be accounted for which is generally accomplished by reporting either
manually or
electronically transferring information into a facility's accounting system.
The steps the
pharmacist must perform to insure that an admixture is properly accounted for
should be
minimized to increase efficiency.
Thus, it is a primary object of an aspect of the present invention to
provide an improved method and apparatus for preparing and accounting for
parenteral
nutrition solutions. It is a further object of an aspect of the present
invention to provide a
method and apparatus for preparing a parenteral admixture to reduce instances
of
incompatibility, which preferably includes a software implementation of the
method that
will accommodate many known active ingredients and other components that are
set
forth in various prescription admixtures. More particularly, it is a related
object of an
-7-
CA 02360788 2008-01-25
aspect of the invention to provide strategies for preparing prescriptions for
parenteral
admixtures, for controlling the compounding apparatus, and for properly
accounting for
the prepared admixture with the strategies being implemented in computer
software.
It is another primary object of an aspect of the present invention to
provide such an improved method and apparatus for preparing a parenteral
admixture by
increasing the throughput of a compounder, principally by minimizing the
number of
rinses that have to be performed.
A related object of an aspect of the present invention lies in the provision
for selectively determining the order or reorder of a plurality of admixture
solutions or
compatibility groups that may reside in a prescription, to more efficiently
prepare the
prescriptions, such as by maximizing the number of prescriptions which may be
prepared
over a set time period.
A more detailed object of an aspect of the present invention is to provide
such an improved method and apparatus for controlling a compounder of the type
which
utilizes an intermediate mixing chamber such as a funnel for admixing
components prior
to transferring the contents of the chamber to the fmal mixing chamber such as
a final
bag
Another object of an aspect of the present invention lies in the provision
of patient specific data to enhance the ability of the pharmacist to
efficiently formulate
and safely compound admixtures from prescriptions.
Still another object of an aspect of the present invention is to provide an
improved method and apparatus for allowing the pharmacist to efficiently
compensate
for the accuracy limits of the compounder and inefficiencies in administering
the PN to
the patient such as the fluid which is lost in the administration sets.
Yet another object of an aspect of the present invention is to provide an
-8-
CA 02360788 2008-01-25
improved method and apparatus which includes a higher concentration
formulation of an
ingredient to be substituted for a prescribed formulation but determines
whether the
diluting fluid may be provided by compatible other ingredients so that the
volume of the
resultant admixture is minimized.
Another object of an aspect of the present invention is to provide such an
improved method and apparatus which controls the compounding to alert users
when
recommended limits are not being adhered to and such alerts are not
accidentally
ignored. A related object of an aspect of the invention is to provide for the
input and
recording of the rationale in overriding a warning.
Another object of an aspect of the present invention is to provide a
method and apparatus which reduces instances or alerts users to the
possibility of lipid
hazing and the possibility that such lipid hazing will be accidentally
mistaken for an
unacceptable precipitate.
Accordingly, in one aspect of the present invention there is provided
apparatus for use in controlling the operation of at least one pharmaceutical
compounder
adapted to selectively transfer prescribed amounts of pharmaceutical
components from
individual source containers through elongated hollow transfer means to a
final container
in order to prepare a prescription admixture, said apparatus comprising:
computing means having memory means for storing instructions for
operating the apparatus and for controlling the at least one compounder to
prepare a
prescribed admixture, said memory means including data relating to a plurality
of the
pharmaceutical components that may be transferred to prepare the prescription
admixture, and data concerning the operating characteristics of the at least
one
compounder;
-9-
CA 02360788 2008-01-25
said computing means including at least one communication port for
establishing a communication link with the at least one compounder;
said computing means being adapted to receive a prescription admixture,
identify the pharmaceutical components thereof, determine the compatibility of
the
pharmaceutical components relative to one another, determine the order in
which the
components are transferred in preparing the prescription admixture, and
communicate
the instructions for preparing the prescription admixture to the at least one
compounders
that is to be used in preparing the prescription admixture,
wherein the compatibility of the pharmaceutical components relative to
one another comprises a concentration dependant compatibility.
According to another aspect of the present invention there is provided a
method of controlling the operation of at least one pharmaceutical compounder
adapted
to selectively transfer prescribed amounts of pharmaceutical components from
individual
source containers through elongated hollow transfer means to a final container
in order to
prepare a prescription admixture, the method utilizing a computing means
having
memory means for storing instructions for operating the apparatus and for
controlling the
compounders to prepare a prescribed admixture, with the memory means including
data
relating to a plurality of the pharmaceutical components that may be
transferred to
prepare the prescription admixture, and data concerning the operating
characteristics of
at least one of the compounder that the apparatus is adapted to control, the
computing
means including at least one communication port for establishing a
communication link
with each compounder that is to be controlled, the method comprising the steps
of:
receiving a prescription admixture in the computing means;
identifying and determining the amounts of the pharmaceutical
components of the prescription admixture;
-9a-
CA 02360788 2008-01-25
determining the compatibility of the pharmaceutical components relative
to one another;
determining the order in which the components are transferred during the
preparation of the prescription admixture; and
communicating the instructions for preparing the prescription admixture
to the at least one compounder that is to be used in preparing the
prescription admixture;
wherein the compatibility of the pharmaceutical components relative to
one another comprises a concentration dependant compatibility.
These and other objects and aspects will become apparent to those of
ordinary skill in the art upon reading the following detailed description,
while referring
to the attached drawings, in which:
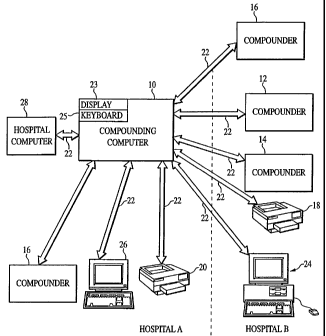
FIGURE 1 is a block diagram illustrating the apparatus of the present
invention shown in the context of a hospital having a central billing and
patient computer
which is networked to the apparatus of the present invention and with the
apparatus also
operatively connected to compounders and printers;
FIG. 2 is a perspective view of a representative compounder that may be
controlled by the method and apparatus of the present invention, and
particularly
showing the compounder having a funnel or interrnediate container in which
source
components are placed either sequentially or concurrently
-9b-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
before being transferred to a final bag; and,
FIG. 3 is a perspective view of a second representative
compounder that may be controlled by the method and apparatus of the
presentation.
FIGS. 4a-4h together comprise a flow chart for controlling the
compounding of a prescribed admixture in accordance with the present
invention.
DETAILED DESCRIPTION
Broadly stated, the present invention is directed to a method and
apparatus for controlling the compounding of pharmaceutical admixtures,
where the compounding is done by one or more compounders that may be
remotely located relative to the controller computer or processing means that
is
interconnected with the compounders. Referring to FIG. 1, the controller
computer or controller 10 has sufficient memory for storing pharmaceutical
data in the form of a database as well as operating software for use in
controlling compounders and other peripheral equipment. The computer 10 is
preferably a multi-user. multi-tasking computer that has a cominunication
interface for interconnecting to compounders 12, 14 and 16 and othel-
peripheral equipment such as printers 18 and 20 by comniunication links 22
that may be wired or wireless, may be part of a local area network, a wide
area
network or the Internet or a combination of the above. The computer 10 may
have a display 23 and keyboard 25 as well as other accessories and features
common to commercially available computers at this time.
Other peripheral equipment can include a dumb terminal 24
having a keyboard and display or other input device such as a laptop computer
26 or other handheld device that is adapted to enter prescriptions and input
-10-
CA 02360788 2008-01-25
instructions for operating the controller computer software. The compounders
may be
located in different areas of a healthcare facility such as a hospital, or on
different floors
of a hospital or even at different hospitals. The compounders 12 and 14 as
well as the
printer 18 and terminal 24 are located in hospital B in FIG. 1, whereas the
remainder of
the equipment is shown to be located in hospital A. There is preferably a
printer located
near each compounder or combination of compounders, as shown, for printing
labels that
are applied to the prescribed admixtures that are compounded. The controller
computer
is preferably interconnected with a general hospital computer 28 that may be
used to
prepare and record billing statements among other functions.
10 The present invention is adapted to control compounders such as
compounders 12, 14 and 16. The presence of two machines in hospital B is to
indicate
that two different types of compounders may be used in combination to prepare
prescription admixtures, such as by but one example, the aforementioned
AUTOMIXTM
and MICROMIXTM compounders. Thus, the compounders 12 may be a compounder
adapted for transfer of high volume additives and the compounder 14 may be a
compounder adapted for low volume additives. Moreover the compounder 16 may be
adapted to transfer both high volume and low volume amounts of ingredients.
Referring to FIG. 2, a perspective view of a low-flow module
compounder is illustrated and is adapted to transfer small volumes of
components such
as micro-nutrients and other drugs from individual source containers 30.
However,
prescription admixtures may be prepared by a single compounder 16 adapted to
transfer
high volume and low volume additives or multiple compounders attached to a
single
final bag 46.
In an embodiment when high accuracy is desired such as when low
-11-
CA 02360788 2008-01-25
volume additives are being added to a PICT the fluids from the containers 30
is
transferred through a separate individual fluid conduit 32 to a single
intermediate
container or funnel 34 that is suspended from a load cell assembly 36. The
load cell
assembly 36 weighs the total weight of the funnel 34 to develop an output
signal, which
is indicative of the amount of fluid in the funnel 36 at any given time. The
funnel 34 is
closed and is connected to a pressure conduit 38 that is connected to a
pressure means
and occlusion means such as a valve 40 by example. The pressure means is
preferably a
single peristaltic pump which can selectively create positive and negative
pressures in
the funnel 34 to control the direction and flow of fluid into and out of the
funnel 34. The
funnel 34 also is connected to an outlet conduit 42 that extends to a second
occlusion
means 44 that is interposed between the funnel 34 and the final bag or
container 46. By
selectively operating the occlusion means 40 and 44, and the pressure means,
fluid can
be drawn into the funnel and transferred out of it. These same portions of the
machine
can also control the direction of flow, so that fluid can be transferred into
the final bag 46
and can be removed from the final bag for the purpose of rinsing the funnel
34.
A detailed operation of an example of a compounder adapted to transfer
low volume components, at least as of approximately April 1990 is described in
US
Patent No. 5,228,485 which is assigned to the same assignee as the present
invention.
The current commercial MICROMIX compounder may embody certain improvements
compared to the '485 patent, but is believed to be similar to that described
in the patent.
The compounder 12 may also include an assembly that transfers the
additives utilizing other methods of operation such as one or more pumping
mechanisms
and switching mechanism alone or in combination with volumetric delivery
methods,
possibly including calibration such as the compounding devices supplied
commercially
by the BAXA Corporation of Englewood, Colorado.
-12-
CA 02360788 2008-01-25
Referring to Fig. 3 a further embodiment of a compounder 50 is
represented which is particularly suited for transferring large volume
additives. The
compounder 50 includes a number of individual pumping stations 52 which
cooperate
with a disposable transfer set 54 to pump fluids from individual source
containers 56 to a
final container 58. A detailed operation of an example of a compounder adapted
to
transfer low volume components, at least as of approximately 1999 is described
in US
Patent Nos. 4,712,590 and 5,927,349 which is assigned to the same assignee as
the
present invention. The current commercial AUTOMIX compounder may embody
certain improvements compared to the '485 and'349 patents, but is believed to
be similar
to that described in the patent.
Preparing the Prescription
In an example of a process for utilizing the preferred embodiment of the
present invention, a physician or other healthcare provider or group of
providers
determine what is the parenteral nutritional needs of a patient and arrive at
a prescription.
A pharmacist will then need to convert the prescription into particular
amounts or
concentration of additives in the PN which is to be administered to the
patient. These
amounts will vary in dependence on the particular patient. For example the
patient may
not be able to accept a large amount of fluid parenterally and, the
nutritional needs will
need to be accomplished within a minimal amount of fluid. One example of a
fluid
restricted patient is a neonatal patient. To administer the desired amount
-13-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
of an additive in a smaller total volume, the level of concentration of the
additive in the final bag 46 may be higher in the PN than if more of a diluent
volume could be used. This higher concentration may lead to a greater chance
of compatibility problems with other additives in the PN and may exceed
acceptable limits for that patient.
Referring to figure Figure 3a in the preferred embodiment, the
pharmacist enters patient identifying data such as a patient ID code into the
controller utilizing the keyboard 25 (block 70). The controller 10 then
requests
and accepts patient specific data (block 72) from a data storage location such
as the computer system 28 of the facility and displays such information on the
display 23. One type of patient specific data which is preferably utilized by
the
controller 10 in the preferred embodiment is the patient type such as
premature, neonatal, pediatric or adult, etc. In an alternate embodiment, the
provider enters the patieilt specific data directly into the controller 10 or
a
storage location therein.
The controller 10 also retains in a storage location preferred
ranges for the acceptable concentration levels of the different admixtures in
a
final bag 46. In an embodiment, the controller 10 may also retain in the data
storage location, concentration ranges for the ingredients for the various
patient
types and set the preferred concentration range with a type dependent range
foi-
that particular patient. In a further embodiment ranges corresponding to
patient attributes such as various patient ages and weights may be retained in
the data storage location and the controller 10 may set the preferred
concentration range with attribute specific ranges.
In a further embodiment, the controller may also contain an
algorithm for adjusting the concentration range in dependence on
predetermined patient specific factors such as the age of the patient.
-14-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
Thus, in dependence on the patient specific data, the controller
may evaluate whether the preferred ranges are appropriate for the patient
specific type and may then adjusts the range (block 74).
The health care provider will then enter the prescription (block
5 76) by, for example, utilizing the keyboard 25. In entering the
prescription, the
provider will set concentration levels of the ingredient solutions so that
upon
compounding the PN will correspond to the prescription. The controller 10
allows the prescription to be entered in several different formats. By way of
example the controller 10 may accept inputs of the ingredients in percent of
the
1o final solution, concentration per unit volume or an amount corresponding to
the
per unit weight of the patient.
As the provider is entering the prescription the controller 10
checks the entered concentrations against the determined ranges (block 78). If
a concentration is entered which is outside the range, an error message is
displayed on the display 23. In addition, if the provider enters an ingredient
with an inappropriate format an error or alarm message is displayed on the
display to alert the health care provider. An example of an inappropriate
forniat
is where the concentration is entered in units of n7easure per the patients
weight but the patients weight has not been input as part of the patent
specific
2o data.
In an alternate embodiment, after all the concentrations have
been entered the controller 10 may then revie ~ the various ingredients and
highlight with error messages those ingredients which fall outside the range
which has been set for that ingredient.
In a further enlbodiment, templates of various prescriptions
corresponding to various patient types may be retained in a storage location.
The pharmacist or controller 10 may then call up the template and either
accept
-15-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
or adjust the template. Ir a still furtl~er embodiment, a previous
prescription of
the patient may be retained in a storage location. The pharmaaist or
controller
may then call up the prior prescription and either adjust or accept the prior
prescription to be utilized as the present prescription.
5 When an alarm is displayed. Even though the concentration is
outside the range, the concentration may still, in the medical judgement of a
provider, be desired. The controller 10 may then in certain predetermined
instances allow the provider to override the alarm (block 82). The controller
10 will allow an override upon the occurrence of one or a combination of
1o certain factors. One factor is whether the provider entering the
prescription has
the clearance to override the particular alarm. An each alarm may require a
different level of clearance before the override is accepted. Some alarms may
not be overridden.
The identity and clearance level of the provider may be
established by a unique password that is requested by the controller 10 and
entered at an appropriate time such as at the beginning of the entry of the
prescription or at the occurrence of an alarm (block 84). Other methods of
establishing the identity of the provider are also contemplated such as
keycards, retina scans or the like.
In addition to establishing the clearance of the provider, to verify
that the provider is recognizing and appreciating the error message and for
record keeping purposes. The controller 10 may require that the rationale for
the override be entered into a note screen displayed on the display 23. For
certain alarm situations, the controller 10 does not allow any overrides even
with a rationale (block 86).
Compatibility Groupings
The preferred embodiment of the present invention evaluates the
-16-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
compatibilies of the ingredients and the solutions into which the ingredient
comes into contact during the compounding process and also the solution in the
final solution bag 46 after the compounding is complete.
In present practice, the evaluation of the final prepared PN is a
process that is routinely performed by pharmacists. The pharmacists compare
the components of the final prepared admixture to literature, which has
information concerning compatibility. Many times, the literature is not
sufficiently specific to the exact type of ingredients in the admixture being
prepared, which requires the pharmacist to use professional judgment in
to deciding whether the resulting admixture will be compatible.
In accordance with the present invention, the overall
compatibility evaluation for complex admixtures primarily focuses on the
compounding of parenteral nutrition, which broadly includes PN screening and
calcium phosphate solubility screening.
The process preferably involves a first screening step of
comparing all PN additives to limits set by the controller 10 which may
include
the steps of setting ranges preferred concentration limits as described above
A second step involves conlparing the final concentration of
amino acids, dextrose and lipid based components to the database of tested
2o admixtures. Amino acid comparisons are brand specific. Databases of
admixtures have been compiled through the testing of adnlixtures and also by
utilizing published literature. The admixture database preferably comprises
concentrations for both stable and unstable adnlixtures with a notation of the
study conditions such as time and temperature. Preferable the database
includes adniixtures having identified source components such as by example.
brand named amino acids.
In the second step, the prescribed admixture is compar-ed to the
-17-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
database of admixtures. Preferably the comparison is first carried out against
admixtures having identified source components. If the prescribed admixture
falls within some range of a stable admixture the present invention proceeds
to
the next step without generating a notice to pharmacists. The range may be set
by some variance amount, for example by a set percent of the amounts or
concentration levels of corresponding base components in a stable admixture.
However, admixtures with matches (or mixed stable and unstable
matches) to the unstable formulation contained in the database (to preferably
plus or minus a set variation of the amounts of base components) and yet have
io passed the first screening step may be designated as potentially unstable.
In an embodiment of the present invention, if the prescribed
admixture matches an unstable admixture a ftirther step may be performed
such as screening whether the study conditions of the matched admixture are
equivalent to the present conditions. The present invention provides a warning
to pharmacists that the admixture is equivalent to potentially unstable
admixture under the study conditions of that admixture.
In a further embodiment, admixtures that do not match any stable
or unstable admixtures contained in the database are re-evaluated. When this
is done, the amino acid brand is ignored and the admixture is then compared to
the entire database. The results of this compai-ison are handled following the
same steps that have been previously described. Preferably, the pharmacist
would be provided a warning about the ignoring of the amino acid brand of the
database admixture. If the admixture does not match the database after re-
evaluation of the entire database, the present invention will provide a
warning
notice to the pharmacist that no similar PN has been previously tested.
With regard to calcium phosphate solubility screening, the
solubility of calcium salts and phosphate salts in the same solution is
-18-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
dependent on many variables including, but unlimited to concentration,
temperature, salt form, order of mixing, pH, amino acids concentration, other
additives and time. It has been the practice in the prior art for the
pharmacist
to compare the final concentration of both the calcium salt and phosphate salt
to a solubility curve that is specific to a given amino acids brand and final
concentration.
In the present invention, the calcium phosphate solubility
screening in a complex compounding process is achieved by the controller 10
comparing the final concentration of both the calcium salt and phosphate salt
to
io a matrix of known compatibility. The matrix nlay be input into a storage
location by the Pharmacist or previously input into the database The present
invention uses the matrix to sort compatibility by the amino acids brand and
final concentration. For example, a calcium phosphate solubility matrix for a
specific amino acids brand may have compatible concentrations of calcium
salts and phosphate salts for a 1%, 2% and 4% final amino acid concentration.
The present invention determines the limits of solubility that have been
exceeded and will generate a warning to the pharmacist if it has.
In a further embodiment, the controller 10 may generate and
display on the display 2-5 a graph of a shape representing the calcium
phosphate solubility for that a particular amino acid and may also present a
designation of the prescribed admixture relatiN-e to the solubility shape to
assist
the pharmacist in achieving a prescription which is compatible.
However, in addition to the determining whether the prescription
present in the final bag is compatible, compatibility during the compounding
process must be evaluated. For example, the compatibilities of a solution
wit11
a second solution at the time of contact must be evaluated. The second fluid
may be found in a common conduit, intermediate mixing chamber or final bag.
-19-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
To overcome this potential problem ;he pharmacist may adopt gross rules for
the compounding process. For example it is common practice that all diluent
volumes are added to the final bag first so that all additives which are
present
in the final bag are diluted as much as possible at the time of the addition
of an
additional ingredient to the final bag. However, such a practice reduces the
ability to rinse from such a diluent during the compounding process.
In accordance with an important aspect of the present invention,
the controller computer 10 may utilize the known compatibilities of
components to enable concurrent compounding of such compatible
io components into the final bag or an intermediate mixing chamber. In
addition.
rinsing may be accomplished with a source solution which is compatible with
both the solutions flowing through the rinsed portion before and after the
rinsing. Thus, large volume additives may be transferred to the final
container
or bag or transferred to an intermediate mixing chamber at the same time as
small volume additives or used as rinsing fluids. Such compatibility screeninQ
and concurrent compounding enables the present invention to maximize the
speed in which admixtures are compounded which results in more efficient use
of the compounders, as -ell as the controller computer.
In accordance with an important aspect of the present invention,
testing of components for compatibility characteristics is used to build a
database that includes a plurality of groups, which represent concentration
dependent compatibility on the basis of testing of components. In an example,
there are seven groups of components identified in Table 1 set forth below,
based upon the current knowledge. It should be understood that many more
groups of components may be defined as greater knowledge about
compatibility characteristics of various ingredients are acquired, even to the
extent of having a group for each individual component, or even separate
-20-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
groups for the same component in different concentrations.
TABLE 1
Group Compatibility
Compatible Incompatible
Group
1 1,2,3,6 4,5,7
2 1,2,3,4,6,7 5
3 1, 2, 3, 4, 6, 5,7
4 2, 3, 4,6 1,5,7
5 6 1, 2,3,4,5,7
6 1,2,3,4,5,6,7 -
7 2,6,7 1,3,4,5
The compatibility groups may be known based on test results and
are contained in the database of the controller computer so that the
compounding process can be carried out with the information in the database.
It is preferred that the database be located only in the controller computer,
rather than be distributed to various locations so that it can be reliably
controlled, managed and modified as additional knowledge and information is
is gained through history, continued testing, and the addition of other drugs
and
components to the database.
In a preferred embodiment of the present invention, based upon
the database, the controller will logically group the fluids in the source
containers 30 (Fig. 2) into the compatibility groups regardless of their
physical
placement on one of the compounder 12, 14, 16.
In a further embodiment of the present invention, the controller
shall calculate for a particular prescription, the number of groups present
and
the sorting of the groups into sets of compatibility groups between which a
-21-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
rinse is required such that the total number of rinses is minimized.
In a still further embodiment of the present invention the
controller utilizes other inputs such as physical restraints of the system to
determine the proper compounding sequence to more efficiently utilize source
solutions as rinses as opposed to rinses from the final bag. Examples of a
physical restraint may include the volume of an intermediate chamber or
funnel 34 and the rinse volume for such a chamber.
In an example, the intermediate chamber has a funnel with a
volume of 60 ml and a rinse volume requirement of 30 ml. If the prescription
io calls for 5 ml of Group 1, 20 ml of Group 2; 20 ml of Group 3; 55 ml of
Group
4 and 40 rnl of Group 6, the controller 10 may adopt a compounding order of
Group 1, Group 2, Group 3, Group 6 and Group 4 instead of ascending
sequence.
By partially filling the funnel 34 with just Groups 1, Group 2,
is Group 3, and 10 ml of Group 6, then draining the partially filled funnel
before
the addition of the remainder of Group 6, at least 30 ml of Group 6 fluid is
remaining after the funnel 34 is first filled and this Group 6 fluid can serve
as a
rinse thereby removing the need to rinse from the final bag.
Other examples of sorting relationships or algorithms may be
2o defined and implemented by the controller to accomplish the desires of the
users, such as allocating the volume of a group to other compatible groups to
reduce the number of draining of the chamber or funnel 34 may be minimized.
In this regard and reiterating what was stated above, while seven
separate groups are contained in Table 1, it is expected that additional
groups
25 will be defined, which may be based on more sophisticated knowledge and
testing. The precise number of groups will eventually be a fiinction of the
sophistication of compatibility knowledge vis-a-vis the all other components
-22-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
that are used, and it is contemplated that a significantly larger number of
groups will be defined.
This will lead to the controller computer being able to more
accurately control the compounding steps that will result in yet increased
efficiency and speed of compounding. Additionally, the database may be
considered to be proprietary as its sophistication increases and control of
the
database at a single location is a significant protection that would not be
present if the database were to be distributed to a processor in each
compounder, for example.
Compounding Strategies
In a further embodiment of the present invention a mixing
strategy or method which recognizes the possibility of liquid hazing and
utilizing preferably minimizing rinses from the final bag is shown in FIGS. 4b-
4h, which illustrates the preferred embodiment of a method of defining the
operation of at least one compounder to provide a nutritional formula
admixture. The start (block 100) of the method or process is shown in FIG. 4b
and occurs after prescriptions have been entered into the controller computer.
In an alternate enlbodiment of the present inventions the
prescription are initially screened by the controller 10 in one or more of the
methods described above.
The next step is to decide the compounding strategy (block 102)
which is in part dependent upon the kind of compounding equipment that is
present.
In this regard, and as previously mentioned, a hospital, other
healthcare facility or phai-macy may have only a high-flow module
compounder 12 (Fig. 1) which is adapted to transfer high volume fluids at a
relatively higll flow rate. However, in the event that the facility also has a
lo -
-23-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
flow module compounder 12, then it can transfer solutions at a low flow rate,
which generally enables ;,ery small volumes or amounts of a component to be
added to a bag. Therefore, in instances where particularly adopted high
volume and low volume compounders are utilized, the controller decides
compounding strategy (block 2) determines which strategy to employ. The
program is adapted to control either a high flow rate (block 104) which would
control a high-flow module compounder for example, a low flow rate (block
106) which would control a Low-flow module compounder, for example, or a
high and low flow rate (block 108) which would result in both machines being
io used or for a single conipounder 16 suitable for both high volume and low
volume transfers, for example.
Referring initially to the high flow only, the controller performs
initial compounder calculations for high flow only compounder set up (block
104) which comprises several calculations that the program will execute for
each large volume component that will be part of the final bag. This includes
the calculation based on specific gravity to convert volume measure to weight
measure, if the transfer is carried out by utilizing the weight of a component
that is transfeiTed rather than volume that is transferred. In this regard, a
prescription nlay be written using measurenlents that are input by grams or
milliliters or a percentage of the final solution and the software may be
required to convert the measurements to weight, if the compounders transfers
in dependence on the sensed weight of the transferred component. For
example, the high-flow module 14 and low-flow module compounders 12
compound utilizing the -eight or change of weight of an intermediate or final
container.
After the calculations are made, line 110 extends to FIG. 4c
where a detei-inination is made whether a prescription containing lipids
should
-24-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
have the lipids transferred to the final bag first (block 112), which is a
user
setting. In this regard, users may wish the lipids to be first or last into
the final
bag, which is strictly an option that the user can specify. Such specification
is
preferably based on criteria that is set up initially before the compounder is
ever run in a facility.
This involves the sorting of all the additives into compatibility
groups such as compatibility groups and this is done by grouping common
compatibility components as shown in the Table 1 above. If lipids are
transferred first in the final bag, a determination of the number of
compatibility
meta-groups is made and the number of rinses N that will be required (block
114) and then the program specifies a sequence of large volume transfers with
lipids first. Once the sequence is determined, then line 118 extends to FIG.
4d
where the instructions for operating the compounder are transferred to the
compounder (block 120).
Alternately the controller 10 can transfer the fluids utilizing other
user settings including settings reflecting the general rules of mixing TPN's
(blocks 116, 124). With regard to the general rules of mixing total parenteral
nutrients, they include the following:
1. Phosphate salts are added before calciunl salts.
2. The determination of calcium phosphate solubility
should be made based on the volume of solution in the TPN bag at the time
calcium is added.
3. Unless lipids are required as the last additive,
calcium should always be the last additive to the TPN bag, holding out one
rinse, if possible.
4. Compatibility groups are numbered sequentially to
coincide with the order of mixing unless specific exceptions are identified.
-25-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
If the compounder 14 has separate conduits to the final bag for
each of the source solution the controller 10 set the order of pumping to
insure
that the fluid added to the final bag, the primary detennination of the order
of
pumping is the compatibility of the fluid enteriilg the bag with the fluid
present
in the bag.
Returning to FIG. 4c, if the lipids are not first in the final bag,
the number of compatibility groups is also determined, as is the number of
rinses required (block 122) and the sequence of transfers and rinses with
lipids
last is determined using one or more of the conlpounding methods is executed
io and the final step shown by line 126 that extends to FIG. 4d results in the
transfer instnictions being sent to the compounder (block 120).
Turning now to the low flow only path which begins with the
initiating compounding calculations for low flow only (block 106), this would
be used for compounding admixture prescriptions that would be done with a
Low-flow module compounder, for example. Even though it is likely that a
high flow compounding apparatus would exist in the same area, it is common
to choose the low flow compounder if the volume that is going to be added to
the final bag is relatively low, such as would occur foi- a neo-natal
prescription
or for a very small infusion.
An initial determination is made whether the final bag already
contains lipids (block 130). The reason that this deter-mination is made is
that
there may be a prescription that is conlpounded in two stages with large
volume flow components alreadv being transferred to a bag and the bag is then
placed on the Low-flo,~v module for transfer7-inQ micro-nutrients into it. If
lipids are already in the bag, that will make a difference as to how rinses
froni
the final bag are made into a funnel or intermediate mixing chamber which
may be present in a low-flow module compounder.
-26-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
The program determines from the prescription whether lipids are
contained in the bag and if so, the entire admixture prescription is checked
to
determine if lipids are in or will be in the final bag. If they are, then the
inquiry is made as to whether the user cares whether there is lipid haze in a
following admixture prescription (block 132). This is due to the fact that if
any
rinse is performed using fluid from the final bag some of the lipids will stay
behind in the funnel. These lipids may be transferred to a number of the
following bags and in an amount sufficient to produce a visible haze in the
solution. If the present bag is made with lipids and the next bag does not
have
1 o lipids and no rinse of surfaces which will come into contact with the
contents
of both bags occurs, then there is a possibility that the lipids would haze
the
next bag, particularly if the prior bag utilizes a rinse from the final bag.
In the
event that the facility does not desire lipid haze, then the compound will not
be
prepared at that time but will remain in the queue to be compounded at another
time (block 134). If the hospital accepts lipid haze, then a warning is
printed
by the printer (or by a visual display) indicating that there is a possibility
that
lipid hazing will exist in the funnel (block 136).
If the final bag does not contain lipids (block 130), or if they do
contain lipids but do not care about lipid haze (block 136), then using the
solubility and compatibility tables and proceed to calculate the compound
keeping in mind that rinses will be from the final bag (block 138). This step
is
intended to perform calculations that are designed to minimize the number of
rinses to maximize efficiency and may utilize one of the methods described
above. When this is done, line 140 extends to FIG. 4d and the transfer
instructions to the compounder are then sent to the conlpounder (block 142).
Tunling noNv to the high and low flow branch shown in FIG. 4b.
the initial step is to initiate the compounding set up (block 108) which
requires
-27-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
the converting calculations be carried out that have been described with
respect
to the high flow only routine (block 104) and line 144 extends to FIG. 4c,
where the number of compatibility groups and rinses is determined (block
146). Basically, it is a determination as to wllether there will be a problem
with the prescription if it is compounded in the way that it is written.
The determination is made as to whether lipids are included in
the final bag (block 152). If lipids are required, the determination is made
whether lipids are to be transferred first, last or otherwise optimized (block
154). Whether lipids are required to be first, last or optimized is a user
t o preference that is prograinmed in the sense that the user defines this
once and it
is thereafter not prescription dependent. Optinlize usually always means that
lipids would be placed first. Thus, the criteria for compounding that is
established by the user initially will determine the path of steps taken. If
they
are first or optimized, then line 156 extends to FIG. 4d and FIG. 4c to a step
that will be described later. If lipids are not included in the final bag,
then line
158 extends to FIGS. 4d and 4e for steps that wi11 also be described later. If
lipids are required to be last, then line 160 extends to FIG. 4d and the
determination is made v,-hether the prescription is stable without the lipid
volume (block 162).
If the prescription is not stable Without the lipid volume, the
program alerts the user that the prescription cannot be compounded if lipids
are
last and that a pharmacist check may be required (block 164). The program
then determines whether lipids can be transferred into the final bag first
(block
166), which if not, results in the compound not being prepared (block 168). If
the lipids can be transferred first, then line 170 extends to FIG. 3d wherein
the
number of rinses including the volume of lipids and lipids will be transferred
to
the final bag first (block 172).
-28-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
Returning to block 162, if the prescription is stable without
including the lipid volume, then the program calculates all solubilities
including the volume of lipids and the lipids will be transferred to the final
bag
last (block 174) (FIG. 4d). The calculation of solubilities not including the
volume of lipids (block 174) is done to calculate the calcium phosphate
solubility based on possibly less volume than what was included in the
original
screening. Therefore, for example, if there were 50 milliliters of lipids in a
200
milliliter total volume PN, then the phosphate calcium solubility evaluation
would be done on 150 milliliters.
After the compatibility groupings and rinses are calculated
(blocks 172 and 174), the program then determines whether the total volume
excluding lipids is more than the funnel volume (block 176). If yes, line 178
extends to FIG. 4f where the program determines whether lipids are first
(block
180) which if yes, results in the program determining whether the number of
rinses of a source rinse of base may be held for a rinse in an acceptable
sequence (block 182). If it can, the program breaks into compatibility groups
and proceeds with compounding with required rinses coming from the selected
source container (block 184) and the instructions are transferred to the
compounder (block 186). As described above the steps that are described in
2o blocks 182 and 184, while identified as separate steps in the flow chart,
are
really in actuality interrelated. This is because the number of rinses is a
function of the compatibility groups and the compatibility groups must be
determined in order to identify where rinses should occur as described
earlier.
If the total volume excluding lipids is not more than the funnel
volume, then the completion of the compounding is done using the low-flo-w
module and can be done in the funnel of the low-flow module compounder.
The program determines if a source rinse volume of a component solution can
-29-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
be held back for a final rinse (block 188) which if yes, results in holding
back
when source rinse volume of a base component and all other ingredients are
compounded in the funnel and transferred to the final bag and the funnel is
then to be rinsed with the reserve base (block 190) with the rinse transferred
to
the final bag and transfer instructions are sent to the compounder (block
192).
In order to determine whether a source rinse volume can be held
back, it is necessary to screen the fluid present during mixing in the funnel
without the diluting effect of the rinse to see if it is permissible to hold
anything out, i.e., whether the resulting admixture will be stable. Also, the
io capacity of the funnel is important with regard to the volume that can be
held
out for doing a complete rinse. For example, if the funnel capacity is 50
milliliters and only 30 milliliters can be held back, then there will not be a
full
funnel rinse and the decision as to whether this is adequate or not can be
made
from the user. It is also contemplated that the method of rinsing will see to
have the final rinse originate from a source component such as sterile water,
dextrose or amino acids and that intermediate rinses may be made using
solution from the final bag with the source rinse of a component volume being
held back for the final rinse so that the ftinnel would be cleaned as much as
possible.
If there is not sufficient source rinse volume, the program
determines whether any amount of the source rinse volume can be held back
for a final rinse (block 194) which if yes, it is done, and all other
ingredients
are compounded in the funnel and it is rinsed with the reserve base (block
196). The transfer instnictions are then sent to the compounder (block 198).
If there is ilo amount of source that can be held back for the final
rinse, all ingredients are then coinpounded into the funnel without any rinse
(block 200) and instructions are sent to the compoundei- (block 202).
-30-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
It should be appreciated that from block 152 if the answer is that
there are no lipids in the final bag, then the path through the flow chart
assuming that the total volume is in excess of the funnel volume results in
the
determination of whether lipids are first in block 180, which really is not
applicable because lipids are not present. In this case, the steps 182 and 184
may be carried out with the source rinse being from source container and/or
the
final bag. As described above the controller will preferably utilize rinsing
and
compounding sequence which eliminates the need to rinse from the final bag.
Returning to block 204, if the answer is no, or if the admixture
1 o contains lipids, the lipids are to be transferred first and a rinse from a
final bag
is required (block 182) the program determines if lipids may be transferred
last
(block 206) which if not, the program inquires whether lipid haze is
acceptable
(block 208), which if not, results in the compounding not be continued (block
210). If it is acceptable, the program produces a warning about lipid haze
(block 212).
If the lipids can be transferred last, then the program determines
whether the number of rinses of the source rinse can be held for a rinse and
an
acceptable sequence can be carried out with one rinse originating from the
final
bag (block 214). If it can, then the prescription is analyzed for
compatibility
groups and compounding will proceed with the rinses occurring at the
appropriate times with preferably the next to last rinse being done with the
final bag with all others from the source components (block 216). In this
manner upon rinsing with the contents of the final bag, the ingredients
present
in the final bag will be diluted as much as possible while allowing for a
final
source container rinse. The instructions are transferred to the compounder
(block 218).
If the answer from block 214 is no, line 220 which extends to -31-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
FIG. 4g results in the program determining if the number of rinses minus 2 (N-
2) times the source component rinse can be held for a rinse in an acceptable
sequence with two rinses frorn the final bag being determined (block 222). If
yes, then the compatibility group step is again executed (block 224) and
instructions to the compounder are issued (block 226). If no, then another
determination is made for the number of rinses minus 3(N-3) (block 228), with
compatibility analysis being done if yes (block 230) and transfer instructions
are sent to the compounder (block 232). If no, the determination is made
regarding N-4 (block 234). If the determination from block 234 is yes, the
io compatibility analysis is again conducted (block 236) and the instructions
are
transferred to the compounder (block 238). If the determination is no on line
240, the program then determines if a source rinse can be held for a final
rinse
(block 242) which if yes, results in the compatibility analysis being carried
out
once again (block 244) and the compounding instructions being issued (block
246).
If no, the program determines if any amount of source solution
can be held back to make the required volume excluding lipids below the
funnel volume (block 248). If the answer is yes, then that appropriate volume
is held back and the ingredients are compounded in the ftinnel and the held
2o base component is used to rinse the funnel (block 250) and the transfer
instructions are issued to the compounder (block 252). If not, the program
determines if lipids are in the prescription (block 254), which if not,
results in
the program compounding with all rinses originating from the final bag (block
256) and the instructions are issued to the compounder (block 258) but if
lipids
are present, the program determines whether lipid haze is cared about (block
258). If not, a warning is issued (block 260) and if it is, the compound will
not
be prepared (block 262). After issuing the warning, the compounding proceeds
-32-
CA 02360788 2001-07-27
WO 01/39874 PCT/US00/32651
with all rinses from the final bag (block 256) which results in the transfer
instructions being sent to the compounder (block 258).
In sending the instructions to the compounder (blocks 192, 186
etc) the compounder and controller may utilize several methods and
adaptations to perform the compounding. For example the controller 10 may
send instructions to a controller included as a part of the compounder 12, 14,
16 or the controller may directly operate the compounder or any combination
or similar method.
In addition to the compounding strategies that are carried out in
to the manner described in connection with the flow charts of FIGS. 4a through
4h, there are other functionalities that are carried out by the present
invention.
In this regard, the contr-oller computer 10 is adapted to examine the
composition of each prescription admixture that is present in a queue of such
prescription admixtures for which instructions are sent to the compounder that
is to prepare the admixture. By examining the components of each
prescription admixture in the queue to determine those admixtures which
contain lipids, for exanlple, those admixtures Ni-hich do contain lipids can
be
grotip together in order so that lipid hazing is not a concern until the last
of the
lipid containing admixtures is prepared.
As described in US patent no. 4.653,010 prescription admixtures
residing in queues may be sorted and grouped around common components. In
an embodiment of the invention other desired aroupings of admixtures such as
by patient type can be determined in a similar fashion. Such reordering of the
prescription admixtures in the queue can have the effect of increasing the
throughput due to the needs and requirements of a facility.
Another important aspect of the present invention involves the
ability of the computer 10 to adjust for a user defined overfill volunie by
-33-
CA 02360788 2001-07-27
WO 01/39874 PCT/USOO/32651
increasing the volume of each of the components that are to be added to the
prescription admixture by a predetermined anlount to achieve an admixture of
equal prescription but a slightly higher volume, and thereby compensate for
volume that is required to prime an administrator set or address accuracy
concerns when the prescription call for extremely small concentrations of an
admixture so that the correct amount of the component in the desired
concentration will in fact be delivered to the patient.
Yet another important aspect of the present invention involves
the capability of the computer 10 to receive a user switchable option, which
i o when activated, enables a diluted higher concentration ingredient to be
substituted for a prescribed lower concentration ingredient. In many instances
where the patient is not fluid constrained diluting a higher concentration
solution with a compatible rinse solution such as sterile water will produce
the
prescribed admixture with the minimum amount of potential instability. In a
is further embodiment of the present invention, particularly when the patient
is
fluid constrained, the stability of the admixture in eliminating or minimizing
the diluting solution by considering other ingredients as diluting fluids is
determined by the controller 10 which may employ one of the methods
described above to deterrnine stability during and after compounding.
20 In a further- embodiment of the method of the present invention, a
compounding strategy for overfilling of the final bag 46 may be performed. As
described previously, overfilling may be desired to compensate for the amount
of admixture, which may not be administered due to the system of
administration. For example some portion of the solution may be retained in a
25 final bag even after administration.
In keeping with an aspect of the present invention, the desired
method of setting the overfill may be particularly configured. By way of
-34-
CA 02360788 2001-07-27
WO 01/39874 PCTIUSOO/32651
example the overfill volume may be set in absolute amounts, by a percent or so
that the final bag will have a specific desired volume. In preparing such a
prescription, the method calculates the new amounts of ingredients required to
achieve an admixture substantially equivalent to the prescribed admixture but
at a slightly greater volume.
Upon determining the proper ingredient amounts of the
ingredients, the controller 10 may check the resulting admixture against
various criteria to detern-iine if the resulting admixture may be
administered.
For example for a fluid restricted patient, an overfill may generate an
lo admixture with an amount of fluid in excess of the allowable amount. An
alarm may be generated an error message may be displayed to the user.
In a further embodiment, the controller 10 may adjust or suggest
the adjustment of the volume of the admixture to avoid having one or more of
the ingredients in an amount less than a predetermined level such as that
which
corresponds to the minimum accuracy amount suggested for a compounder 12,
14, 16. By way of example, the amount of a component may be 90% of the
minimum suggested amount. The controller may then increase the total
volume of the admixture such that the amount of the component reaches the
minimum suggested amount and indicate to the user that only of a portion of
the resulting admixture is to be administered to the patient.
In should be understood that the arrangement of the steps in the
various preferred embodiments of the present invention may be altered. For
example the stability of the final admixtut-e may be determined before or
aftei-
the determination of the proper compounding strategy.
Reportin~
The compounder 12, 14, 16 may communicate to the controllei-
10 during and after the compounding process. For example, should a sensinQ
-35-
CA 02360788 2008-01-25
device as described in U. S. Patent no. 5,927,349, detect an incorrect source
solution
flowing through one of the conduits 32 an alarm may be communicated.
Similarly,
during and after compounding the exact quantities of the ingredients
transferred to the
final bag 46 may be transmitted to the controller 10.
Upon receiving the amounts of the ingredients transferred during
compounding, the controller 10 may present cost data to the pharmacist or
communicate
such data to the hospital computer system 28. The controller 10 may adjust the
cost data
to reflect the actual cost of providing the admixture. By way of example, some
ingredients may come in containers which can only be accessed once before
discarding.
Thus if such an ingredient is used in an amount less than that in a container,
the
controller 10 will indicate the cost of the entire container as opposed to
that portion of
the ingredient used in the admixture.
From the foregoing, it should be appreciated that an improved method and
apparatus for controlling the preparation of parenteral admixtures has been
described,
which results in faster, more efficient preparation of the same without
sacrificing safety
in any way. Moreover, several of the features provide added safeguards. The
present
invention employs an extensive analysis of admixture components and utilizes
known
characteristics of components in a novel fashion to control compounders so
that such
prescription admixtures can be reliably and safely prepared without violating
known
rules of preparation, but also in a manner consistent with certain user
defined
preferences.
While various embodiments of the present invention have been shown
and described, it should be understood that other modifications, substitutions
and
alternatives are apparent to one of ordinary skill in the art.
36