Note: Descriptions are shown in the official language in which they were submitted.
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
Method for Controlling Liposome Size
Field of the Invention
The present invention relates to a method for preparing a liposome
composition,
which provides control over the resulting liposome size. The invention further
relates to
compositions prepared according to the method.
Background of the Invention
Liposomes are spherical aqueous particles that are surrounded by at least one
fully
1 o closed lipid bilayer and typically have a diameter of from about 70 nm to
several 1000 nm.
Over the last decade, liposomes have become important as vehicles for
transporting
pharmaceutical and cosmetic agents. Such agents are enclosed within the
aqueous
compartment or are integrated within the liposome lipid bilayer, depending on
the
hydrophilicity of the agent.
15 Methods for preparing liposomes are already known in the art. Multilamellar
vesicles, that is liposomes having more than one lipid bilayer, are prepared
most simply by
depositing the vesicle-forming lipid or lipids from an organic solvent in a
thin film on the
wall of a flask by rotary evaporation under reduced pressure. An aqueous
buffer is added
and the lipids are hydrated at a temperature above the crystalline melting
temperature of the
2 0 lipid or above the higher crystalline melting temperature of the highest
melting component
in the lipid mixture. Addition of the aqueous buffer is accompanied with
agitation, e. g. ,
stirring, sonicating, vortexing, to obtain a suspension of liposomes.
After liposome formation, the liposomes are sized to obtain a smaller and/or
more
uniform distribution of particle size. One common sizing method is extrusion
of the
2 5 liposome suspension through a series of sized filters. Alternatively, the
liposomes are sized
by sonicating the suspension to break up the liposomes into smaller particles.
Small and large unilamellar vesicles (SUVs and LUVs) are liposomes with one
lipid
bilayer and can be prepared by a number of techniques. SUVs typically have
diameters in
the range of 20-50 nm and can be formed by rapid injection of a dilute
solution of lipid in
3o ethanol into an aqueous phase, the so-called ethanol injection procedure.
Another
technique for forming SUVs is by vigorous sonication of multilamellar
vesicles. Large
unilamellar vesicles can be from several hundred nanometers to several microns
in size and
are typically formed by a reverse phase evaporation procedure, where the
organic solvent
~4:58pm Fram-CARPMAELS anD RAnSFORD +uzurd~naui i-a~z r.u4m~
27-03-2001 CA 02361551 2001-08-07 US 000003039
4
la
#~om a water-in-oil emulsion of lipid, buffer and organic is removed under
reduced
pressure.
WO 91/16039 describes a process for the preparation of aqueous liposome
suspensions
containing active substances, the suspensions being prepared from a solution
of a
liposome-forming substance, or a mixture of such substances, in a lower
alcohol with a
ma.~cimum of thrse carbon atoms and an aqueous phase. The active ingredients
are
dissolved or suspended in the lipid solution and/or the aqueous phase. The
process is
characterized in that the aqueous phase is iacorporated, by mixing, in the
alcoholic
solution, and the lower alcohol removed by vacuum distillation. If necessary,
the
liposome suspension free2e-dried.
AMENDED SHEET
FMPFAhIGC7FtT 77 MaR 1A~(1~ ansllRIICKC7F1T 77 MdR lA~ffS
Lf-IAa~-W u4:poP~ rWm-bfW rmn~w rwv nn.wmr ......._... . .._ -. __ . ._.
. 27-03-2001 CA 02361551 2001-08-07 US 000003039
ee~~~
Stl~tii9rv Ai' t~1! ~OedtiOn
s wccordingly, is is au ob~cci of ti:e invention w provide a method for
preparing
liposoates wuh a desired diameter.
It a another object of the invcnuop to provide a method for obtaining
liposomes
having a selected siu.
It a a further object of the invention to provide a method for coauolling rhc
size of
io ligo~omes through xIeenon of the compo3~tion of the hydration composition.
in one aspic, the invention includes a method for obtaining a desired Ztposomc
particle ,~izc. The mrttwd includes hydrating a vesicle-foaming Lpid dissolved
is a lipid
solvent with a second solvent co form a hydration maxture containing as amouuc
of lipid
solvau between 10-50 weight percem. The a~rwuiu of lipid solvetu is selected
to obtain
zs the desired particle sue and the amount of lipid solvent is miscible with
tlve second
solvent is the hydration nuxturt.
Ia one embodiment, the lipid solvent is as alcohol, such as methanol, ethanol
or
butanol.
The vesicle-forming lipid is, is one embodiment, a charged vesicle-forming
hgid,
2o such as the cationic lipid DODAC or the aaianic lipid DSPE. Ia another
embodiment,
the vcsidc-forming lipid is a neuual vesicle-forming lipid.
In yet another embodiment, the hydrating fucthes includes hydrating a vcsicle-
forming lipid derivauzed with a hydroptuiu polymer chain, such as
polyerhylepegiycol.
The second solvent m the method of the invention, is one preferred embodiment,
is
25 Water.
Ln another embodiment, hydrating includes hydrating a therapeutic agent with
the
second solvent. Alternatively, hydrating can further include hydratixtg a
therapeutic
agent with the lipui solvent.
Ia a preferred embodiment, tde therapeutic agent is a radiosen,Wizxr
dcrivacized
~o mdi out ur mutt acyl chaia~. One preferred derivati2ed radiosensicizzr is
dipaluutoyI S-
iodo-2-dexoyuridine.
Ia another embodiment, the therapeutic agent is a nucleic acid.
In another aspect, the invention includes a liposotne composition prepared
according
2
EMPFANGSZEIT 27. MAR. fig; O~AMENDED SHEET~pRUCKSZEIT 27. MAR. 18:04
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
to the method described above which further includes a therapeutic agent.
In another aspect, the invention includes a method for obtaining liposomes
having a
minimum particle size, by mixing a vesicle-forming lipid in a lipid solvent;
and adding a
second solvent to achieve a lipid solvent amount greater than 10 weight
percent and less
than about 50 weight percent, where the amount of lipid solvent is selected to
obtain
liposomes smaller in size than liposomes prepared from a similar formulation
except
having a lipid solvent amount less than 10 weight percent or greater than 50
weight
percent.
In yet another aspect of the invention, a method for determining a liposome
1 o composition to form liposomes having a selected size is described and
claimed. The
method includes mixing a vesicle-forming lipid in a lipid solvent; and
hydrating the lipid
with an amount of a second solvent to obtain a suspension of liposomes. The
liposome
particle diameter of the suspension is measured and the steps of hydrating and
measuring
are repeated to obtain a profile of liposome particle diameter as a function
of amount of
lipid solvent. Based on the profile, the amount of lipid solvent required to
achieve a
selected liposome particle diameter is selected.
These and other objects and features of the invention will be more fully
appreciated
when the following detailed description of the invention is read in
conjunction with the
accompanying drawings.
Brief Description of the Drawings
Figs. lA-1B correspond to liposome suspensions prepared with egg
phosphatidylcholine, cholesterol, N,N-dioleoyl-N,N-dimethylammonium chloride
(DODAC) and ceramide derivatized with polyethyleneglycol of molecular weight
2000
daltons (CZOPEG-ceramide) with an entrapped oligonucleotide, where the
composition of
each suspension is indicated on the phase diagram of Fig. lA and the mean
particle
diameter, in nm, of the liposomes in each suspension are plotted in Fig. 1B as
a function of
both weight percent lipid and weight percent ethanol;
Figs. 2A-2B correspond to liposome suspensions prepared with partially
3 o hydrogenated soy phosphatidylcholine, cholesterol, DODAC and a neutral
lipid derivatized
with mPEG (mPEG-DS) and containing an entrapped oligionucleotide, where the
composition of each suspension is indicated on the phase diagram of Fig. 2A
and the mean
particle diameter, in nm, of the liposomes in each suspension are plotted in
Fig. 2B as a
3
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
function of both weight percent lipid and weight percent ethanol;
Figs. 3A-3B correspond to liposome suspensions prepared with partially
hydrogenated soy phosphatidylcholine, cholesterol, DODAC and an anionic lipid
(distearoyl phosphatidyl-ethanolamine, DPSE) derivatized with methoxy-
polyethylene
glycol (mPEG-DSPE) and having an entrapped oligionucleotide, where the
composition of
each suspension is indicated on the phase diagram of Fig. 3A and the mean
particle
diameter, in nm, of the liposomes in each suspension are plotted in Fig. 3B as
a function of
both weight percent lipid and weight percent ethanol;
Figs. 4A-4B correspond to liposome suspensions prepared with partially
1 o hydrogenated soy phosphatidylcholine, cholesterol and mPEG-DSPE, where the
composition of each suspension is indicated on the phase diagram of Fig. 4A
and the mean
particle diameter, in nm, of the liposomes in each suspension are plotted in
Fig. 4B as a
function of both weight percent lipid and weight percent ethanol;
Figs. SA-SB correspond to liposome suspensions prepared with partially
15 hydrogenated soy phosphatidylcholine and cholesterol, where the composition
of each
suspension is indicated on the phase diagram of Fig. SA and the mean particle
diameter, in
nm, of the liposomes in each suspension are plotted in Fig. SB as a function
of both weight
percent lipid and weight percent ethanol;
Figs. 6A-6B correspond to liposome suspensions prepared with partially
2 o hydrogenated soy phosphatidylcholine, cholesterol and mPEG-DSPE, where the
composition of each suspension is indicated on the phase diagram of Fig. 6A
and the mean
particle diameter, in nm, of the liposomes in each suspension are plotted in
Fig. 6B as a
function of both weight percent lipid and weight percent ethanol;
Figs. 7A-7B correspond to liposome suspensions prepared with partially
2 5 hydrogenated soy phosphatidylcholine and cholesterol, where the
composition of each
suspension is indicated on the phase diagram of Fig. 7A and the mean particle
diameter, in
nm, of the liposomes in each suspension are plotted in Fig. 7B as a function
of both weight
percent lipid and weight percent ethanol;
Fig. 8 is a synthetic reaction scheme for synthesis of 3',5'-dipalmitoyl-5-
iodo-2'-
3o deoxyuridine;
Fig. 9 is a plot of liposome size, in nm, as a function of weight percent
ethanol
during hydration of liposome lipids composed of HSPC, mPEG-DSPE and dpIUdR;
and
Fig. 10 is a phase diagram for formation of liposomes including dpIUdR in
ethanol,
4
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
water and shows a preferred operating region.
Detailed Description of the Invention
I. Definitions and Abbreviations
"Vesicle-forming lipid" refers to any lipid capable of forming part of a
stable
micelle or liposome composition and typically includes one or two hydrophobic
acyl
hydrocarbon chains or a steroid group and may contain a chemically reactive
group, such
as an amine, acid, ester, aldehyde or alcohol, at its polar head group.
"Lipid solvent" refers to any solvent capable of dissolving a vesicle-forming
lipid at
1o any temperature.
"Second solvent" refers to a solvent which in at least some proportion is
completely
or partially miscible with the lipid solvent.
"dpIUdR" refers to 3',5'-dipalmitoyl-5-iodo-2'-deoxyuridine.
"DODAC" refers to the cationic lipid N,N-dioleoyl-N,N-dimethylammonium
chloride.
"mPEG-DSPE" refers to distearoyl phosphatidyl-ethanolamine (DPSE) derivatized
with methoxy-polyethylene glycol (mPEG). The mPEG can have a molecular weight
of
between 500-20,000 daltons, preferably between 1,000-5,000 daltons.
"mPEG-DS" refers to a neutral vesicle forming lipid derivatized with mPEG.
II. Method of the Invention
In one aspect, the invention includes a method of preparing liposomes where
the
particle size of the liposomes is controlled. In another aspect, the method
provides a means
for obtaining liposomes having a desired particle size and a means for
obtaining a
liposomes suspension having a minimum particle size. These aspects will now be
described
by way of Examples 1-8.
Example 1 describes preparation of a suspension of liposomes, where the lipids
forming the liposomes were composed of egg phosphatidylcholine (EPC),
cholesterol,
DODAC and CZo-PEG-ceramide in a 50:25:15:10 molar ratio. The lipids were
dissolved in
3 o ethanol. Aliquots from the mixture of lipids were hydrated with an aqueous
solution
containing 20 mg/mL of an oligonucleotide 34 bases in length to form six
liposome
suspensions varying in ethanol concentration. The compositions of the
suspensions are
indicated on the phase diagram of Fig. lA, and as seen, are at either
approximately 0.6
5
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
weight percent lipid or at approximately 6 weight percent lipid. The weight
percentages of
ethanol at the 0.6% lipid were 9.5, 19.3 and 39. The weight percentages of
ethanol at the
6% lipid were 5.9, 15.8 and 35.5. Table 1 in Example 1 summarizes the
composition of
each liposome suspension.
s The mean particle diameter of each suspension was measured by quasi-elastic
light
scattering just after hydration. The results are reported in Table 1 (see
Example 1 below)
and are plotted in Fig. 1B against weight percent lipid and weight percent
ethanol. As seen
in the figure, for each of compositions at the two lipid levels (0.6 wt% and 6
wt%), there is
a range of lipid solvent where the liposome diameter is minimized. For the
liposomes
to having approximately 0.6wt% lipids, from about 12-35 wt%o ethanol the
liposome particle
size is less than 100 nm. For the liposomes having approximately 6 wt% lipids,
the effect
of a minimum particle size at a selected ethanol range is more evident, as the
liposome size
minimizes between 6-35 weight percent ethanol.
Example 2 provides a further example of the method of the invention, where the
15 particle size of the liposomes is controlled by the composition of the
hydration mixture,
e. g. , by the amount of lipid solvent and second solvent. In this example
liposomes
composed of partially hydrogenated soy phosphatidylcholine (PHSPC),
cholesterol,
DODAC and mPEG-DS in a 50:25:15:10 molar ratio were prepared. The lipids were
dissolved in ethanol and hydrated with an aqueous solution containing an
oligonucleotide 34
2 o bases in length. Nine liposome suspensions varying the composition of the
hydration
mixture were prepared, and each composition is shown on the phase diagram in
Fig. 2A.
The mean particle diameter of the liposomes in each suspension was measured
just
after hydration and again after storage overnight at 5 °C. The results
are shown in Table 2
below, and the diameters measured just after hydration are plotted in Fig. 2B
as a function
25 of weight percent ethanol and weight percent lipid. As seen in the figure,
there is a strong
relationship between amount of lipid solvent and liposome size. More
specifically, there is
an interval where the liposome diameter is minimized. This interval, for this
particular
lipid mixture, falls between 5-30 weight percent ethanol. The smallest
liposome size was at
12.9 weight percent ethanol, with the liposome size increasing as the amount
of ethanol
3 o increased or decreased from 12.9 weight percent.
Accordingly, in one aspect, the invention includes a method for obtaining
liposomes
having a minimum diameter by conducting a study as set forth in Example 2.
That is, a
vesicle-forming lipid, or mixture of lipids, are solvated with a lipid solvent
and hydrated
6
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
with a second solvent to form liposomes. The percentage of lipid solvent in
the resulting
hydration mixture is varied, and the liposome sizes are determined. A profile
of liposome
size as a function of lipid solvent content is established to determine the
composition of the
hydration mixture that results in the minimum liposome diameter. It will be
appreciated
that the profile can be used to determine the minimum liposome diameter at a
particular
lipid solvent concentration, and also to determine the lipid solvent content
in the hydration
composition to achieve any desired liposome size.
Further studies were done in support of the invention to examine the effect of
the
lipid composition on the method. In Example 3, liposomes were formed using a
charged,
1 o mono-anionic PEG lipid conjugate, mPEG-DSPE. Nine liposome suspensions
were
prepared and the composition of each is shown on the phase diagram of Fig. 3A.
The
particle size in each suspension was determined by light scattering, and the
values are
reported in Table 3, below, and plotted in Fig. 3B. The correlation between
liposome size
and lipid solvent content is readily apparent in viewing Fig. 3B, where at
ethanol
percentages between about 15-35 the liposome diameter is minimized.
In Example 1-3, the liposome compositions included a neutral vesicle-forming
lipid
or an anionic vesicle-forming lipid and a cationic vesicle-forming lipid. For
use in the
invention, any of the vesicle-forming lipids known to those of skill in the
art are suitable,
and include those lipids which can form spontaneously into bilayer vesicles in
water, as
2 o exemplified by the phospholipids, with the hydrophobic moiety in contact
with the interior,
hydrophobic region of the bilayer membrane, and the head group moiety oriented
toward
the exterior, polar surface of the membrane.
The vesicle-forming lipids of this type are preferably ones having two
hydrocarbon
chains, typically acyl chains, and a head group, either polar or nonpolar.
There are a
2 5 variety of synthetic vesicle-forming lipids and naturally-occurring
vesicle-forming lipids,
including the phospholipids, such as phosphatidylcholine,
phosphatidylethanolamine,
phosphatidic acid, phosphatidylinositol, and sphingomyelin, where the two
hydrocarbon
chains are typically between about 14-22 carbon atoms in length, and have
varying degrees
of unsaturation. The above-described lipids and phospholipids whose acyl
chains have
3 o varying degrees of saturation can be obtained commercially or prepared
according to
published methods. Other suitable lipids include glycolipids, cerebrosides and
sterols, such
as cholesterol.
7
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
Cationic lipids are also suitable for use in the liposomes of the invention,
where the
cationic lipid can be included as a minor component of the lipid composition
or as a major
or sole component. Such cationic lipids typically have a lipophilic moiety,
such as a sterol,
an acyl or diacyl chain, and where the lipid has an overall net positive
charge. Preferably,
the head group of the lipid carries the positive charge. Exemplary cationic
lipids include
N,N-dioleoyl-N,N-dimethylammonium chloride (DODAC); 1,2-dioleyloxy-3-
(trimethylamino) propane (DOTAP); N-[1-(2,3,-ditetradecyloxy)propyl]-N,N-
dimethyl-N-
hydroxyethylammonium bromide (DMRIE); N-[1-(2,3,-dioleyloxy)propyl]-N,N-
dimethyl-
N-hydroxy ethylammonium bromide (DORIE); N-[1-(2,3-dioleyloxy) propyl]-N,N,N-
to trimethylammonium chloride (DOTMA); 3 [N-(N',N'-dimethylaminoethane)
carbamoly]
cholesterol (DC-Chol); and dimethyldioctadecylammonium (DDAB).
The cationic vesicle-forming lipid may also be a neutral lipid, such as
dioleoylphosphatidyl ethanolamine (DOPE) or an amphipathic lipid, such as a
phospholipid, derivatized with a cationic lipid, such as polylysine or other
polyamine
lipids. For example, the neutral lipid (DOPE) can be derivatized with
polylysine to form a
cationic lipid.
Other lipids which are stably incorporated into lipid bilayers are also
contemplated,
and include steroids, such as cholesterol.
Also in the Examples 1-3, a vesicle-forming lipid derivatized with a
hydrophilic
2o polymer was included in the lipid mixture. As has been described, for
example in U.S.
Patent No. 5,013,556, including such a derivatized lipid in the liposome
composition
forms a surface coating of hydrophilic polymer chains around the liposome. The
surface
coating of hydrophilic polymer chains is effective to increase the in vivo
blood circulation
lifetime of the liposomes when compared to liposomes lacking such a coating.
Other
2 5 hydrophilic polymers conjugated to any of the lipids recited above are
suitable, and include
polyvinylpyrrolidone, polyvinylmethylether, polymethyloxazoline,
polyethyloxazoline,
polyhydroxypropyloxazoline, polyhydroxypropylmethacrylamide;
polymethacrylamide,
polydimethylacrylamide, polyhydroxypropylmethacrylate,
polyhydroxyethylacrylate,
hydroxymethylcellulose, hydroxyethylcellulose, polyethyleneglycol,
polyaspartamide and
3o hydrophilic peptide sequences. The polymers may be employed as homopolymers
or as
block or random copolymers, as described in U.S. Patent Nos. 5,395,619 and
5,631,018.
A preferred hydrophilic polymer chain is polyethyleneglycol (PEG), preferably
as a
8
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
PEG chain having a molecular weight between 500-10,000 daltons, more
preferably
between 1,000-5,000 daltons. Methoxy or ethoxy-capped analogues of PEG are
also
preferred hydrophilic polymers, commercially available in a variety of polymer
sizes,
e.g., 120-20,000 daltons.
Preparation of vesicle-forming lipids derivatized with hydrophilic polymers
has
been described, for example in U.S. Patent No. 5,395,619. Preparation of
liposomes
including such derivatized lipids has also been described, where typically,
between 1-20
mole percent of such a derivatized lipid is included in the liposome
formulation.
The selected vesicle-forming lipid or mixture of lipid is solvated in a "lipid
1 o solvent" . As used herein, "lipid solvent" refers to an organic solvent in
which the lipid
components of the liposome are soluble, at any temperature. Examplary lipid
solvents
include alcohols, such as methanol, ethanol, butanol, etc. and low molecular
weight
polyols, such as glycerol, propyleneglycol and ethyleneglycol. The lipids are
added to the
solvent in the desired molar ratio and mixed until dissolved, with heating as
necessary. An
amount of a second solvent is then added to the lipid solvent mixture to form
a hydration
mixture. The second solvent, as used herein, refers to a solvent that is
miscible with the
lipid solvent in some proportion, and preferably is miscible with the lipid
solvent in the
resulting hydration mixture. The second solvent is added to the lipid solvent
mixture in an
amount sufficient to bring the weight percentage of the lipid solvent to a
selected point
2 o between about 10-50 weight percent, more preferably in a lipid solvent
weight percent
range of 15-45, most preferably in a lipid solvent weight percent range of 20-
40, to obtain
liposomes having a desired size, as described above.
Examples 4-8 describe further studies using liposome lipid mixtures with no
cationic
lipid (DODAC) and with no polynucleotide. In Example 4, lipid mixtures of
HSPC,
cholesterol and mPEG-DSPE in a molar ratio of 50.6:44.3:5.1 were prepared. The
mixtures were hydrated with an aqueous solvent to form nine liposome
suspensions varying
in ethanol content at three lipid concentrations (0.9, 3.1 and 4.7 weight %).
The
compositions are shown in the phase diagram of Fig. 4A and reported in Table 4
below.
The liposome sizes were measured and are plotted in Fig. 4B as a function of
lipid and
3 o ethanol content. At the three lipid levels, the liposome size is minimized
at ethanol
concentrations between about 5-35 weight percent ethanol.
Example 5 provides a lipid mixture which does not include a lipid derivatized
with a
hydrophilic polymer. In Example 5, the lipid mixture of Example 4 except for
the mPEG
9
CA 02361551 2001-08-07
WO 00/45791 PCT/LTS00/03039
DSPE (HSPC:cholesterol 53.1:46.9 molar ratio) was used to prepare nine
liposome
suspensions at lipid weight percentages of 0.9, 2.9 and 4.3. The ethanol
content varied
between 4-39 weight percent, and the composition of each suspension prepared
is indicated
on the phase diagram of Fig. SA. The particle sizes as a function of lipid and
ethanol
content are shown in Fig. SB, and as seen, the method of the invention for
controlling
liposome size through composition of the hydration mixture, and specifically
by content of
the lipid solvent, is evident even in the absence of the derivatized lipid. As
before, between
5-35 weight percent of lipid solvent, the liposome size is correlated to the
lipid solvent
content.
1 o Example 6 describes preparation of liposome suspensions using a lipid
mixture of
HSPC, cholesterol and mPEG-DSPE in a molar ratio of 65.4:38.3:5.3. The
composition
of each suspension is shown in the phase diagram of Fig. 6A and the liposome
diameters
are plotted in Fig. 6B as a function of lipid and ethanol contents. The
liposome size is
minimized when the content of ethanol in the hydration mixture is between
about 5-35
weight percent. The minimum liposome size at each lipid concentration falls
between about
15-20 weight percent ethanol, with the size of the liposomes increasing as the
ethanol
content increases or decreases from this range.
Example 7 describes preparation of liposome suspensions like those of Example
6,
except that the mPEG-DSPE was omitted (HSPC:Chol; 59:41 molar ratio). The
phase
2o diagram of Fig. 7A shows the composition of each suspension, and the
liposome size is
plotted in Fig. 7B, with results similar to those obtained with mPEG-DSPE was
present in
the lipid mixture. These results and the results of Example 5 show that the
control over the
liposome size is provided regardless of the type of lipids present.
The method of the invention can be used to prepare liposomes entrapping a
variety of
therapeutic agents. The therapeutic agent can be mixed with the lipids and the
lipid solvent
or with the second solvent, depending on the nature of the agent and is
solubility in the
solvents employed. Agents contemplated are widely varied, and include both
therapeutic
applications and those for use in diagnostic applications. Therapeutic agents
include
natural and synthetic compounds having the following therapeutic activities:
3 o anti-angiogenesis, anti-aging, anti-arthritic, anti-arrhythmic, anti-
bacterial,
anticholinergic, anticoagulant, antidiuretic, antidote, antiepileptic,
antifungal, anti-
inflammatory, antimetabolic, antimigraine, antineoplastic, antiparasitic,
antipyretic,
antiseizure, antisera, antispasmodic, analgesic, anesthetic, beta-blocking,
biological
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
response modifying, bone metabolism regulating, cardiovascular, diuretic,
enzymatic,
fertility enhancing, growth-promoting, hemostatic, hormonal, hormonal
suppressing,
hypercalcemic alleviating, hypocalcemic alleviating, hypoglycemic alleviating,
hyperglycemic alleviating, immunosuppressive, immunoenhancing, muscle
relaxing,
neurotransmitting, parasympathomimetic, sympathominetric plasma extending,
plasma
expanding, psychotropic, thrombolytic and vasodilating.
In a preferred embodiment, the entrapped agent is a cytotoxic drug, that is, a
drug
having a deleterious or toxic effect on cells. Exemplary cytotoxic agents
include the
anthracycline antibiotics such as doxorubicin, daunorubicin, epirubicin and
idarubicin,
1 o and analogs of these, such as epirubidin and mitoxantrone; platinum
compounds, such as
cisplatin and carboplatin.
The entrapped agent can also be a nucleic acid, including ribonucleic acid or
synthetic analogues, analogues derivatized to peptides or containing one or
more
synthetically modified bases or base analogues, or containing a ligand
incorporated as
part of the sequence including plasmid DNA, synthetic oligonucleotides or
other oligo
analogues.
It will further be appreciated that the liposomes can include a targeting
ligand
covalently attached to the free distal end of the hydrophilic polymer chain,
which is
attached at its proximal end to a vesicle-forming lipid. Alternatively, for
composition
lacking a lipid derivatized with a hydrophilic polymer, targeting ligands can
be attached
directly to the liposome outer surface by attaching to a surface lipid
component. There
are a wide variety of techniques for attaching a selected hydrophilic polymer
to a selected
lipid and activating the free, unattached end of the polymer for reaction with
a selected
ligand, and in particular, the hydrophilic polymer polyethyleneglycol (PEG)
has been
2s widely studied (Allen, T.M., et al., Biochemicia et Biophysica Acta 1237:99-
108 (1995);
Zalipsky, _S., Bioconjugate Chem., 4(4):296-299 (1993); Zalipsky, S., etal.,
FEBSLett.
353:71-74 (1994); Zalipsky, S., et al., Bioconjugate Chemistry, 705-708
(1995); Zalipsky,
S., in STEALTH LIPOSOMES (D. Lasic and F. Martin, Eds.) Chapter 9, CRC Press,
Boca
Raton, FL (1995)).
3 o Generally, the PEG chains are functionalized to contain reactive groups
suitable for
coupling with, for example, sulfhydryls, amino groups, and aldehydes or
ketones
(typically derived from mild oxidation of carbohydrate portions of an
antibody) present in
a wide variety of ligands, such as those set forth in PCT Application No.
98/16202 and
11
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
which are incorporated by reference herein. Examples of such PEG-terminal
reactive
groups include maleimide (for reaction with sulfliydryl groups), N-
hydroxysuccinimide
(NHS) or NHS-carbonate ester (for reaction with primary amines), hydrazide or
hydrazine (for reaction with aldehydes or ketones), iodoacetyl (preferentially
reactive
with sulfhydryl groups) and dithiopyridine (thiol-reactive). Synthetic
reaction schemes
for activating PEG with such groups are set forth in U.S. Patent Nos.
5,631,018,
5,527,528, 5,395,619, and the relevant sections describing synthetic reaction
procedures
are expressly incorporated herein by reference.
Example 8 describes preparation of a liposome composition containing the
1 o radiosensitizer dpIUdR. 5-iodo-2'deoxyuridine, referred to herein as IUdR,
was
derivatized with a 16-carbon fatty acid, palmitic acid, at two positions on
the ribose sugar
of IUdR, as shown in Fig. 8. In the reaction scheme shown in the figure and
detailed in
Example 8A, IUdR was reacted with a small excess of palmitoyl chloride and 4-
dimethylpyridine catalyst in pyridine or in pyridine/chloroform as the solvent
to yield 3',5'-
dipalmitoyl-5-iodo-2'-deoxyuridine, referred to herein as dpIUdR.
As described in Example 8B, the lipids HSPC, mPEG-DSPE and dpIUdR were
dissolved in ethanol in an 89:5:6 molar ratio. This lipid stock solution was
used to prepare
liposome suspensions by hydrating an amount of the lipid solution with a
second solvent, an
aqueous buffer. Liposome suspensions were prepared in triplicate at final
ethanol weight
2o percentages of 8.1, 10.1, 12.2, 14.3, 16.5, 20.8, 25.3, and 44.1. The
average size of the
liposomes in each sample was measured by quasi-elastic light scattering, and
the results are
shown in Table 8.
Table 8
Sample No. Liposome t Ethanol
Size
in
nm
at
Weight
Percen
8.1 1Q:1 12:2 14.3 16.5 2Q:8 25:3 44.1
1 51200 1410 226 197 84 53700 100000012500
2 71 46 78 119 149 27300 11000 10500
3 405 78 157 1360 84 11400 21400 12500
AVERAGE 17225 511 154 559 106 30800 344133 11833
In viewing the last row of Table 8, it is apparent that there is a minimum in
the
liposome particle size at 16.5 weight percent ethanol. This data is shown
graphically in
12
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
Fig. 9 and the trough in liposome size beginning at about 10 weight percent
and ending at
about 25 weight percent is apparent. A profile such as the one in Fig. 9 of
lipid size as a
function of ethanol content can be employed to determine the ethanol amount
needed to
obtain a particular liposome size. For example, the minimum particle size of
106 nm is
obtained by hydrating the lipid-ethanol mixture to 16.5 weight percent
ethanol. A larger
particle size is obtained by hydrating to achieve more or less ethanol,
according to the
profile. Establishing such a profile, the target amount of lipid solvent to
achieve a
minimum particle size or a selected particle size is determined for any
mixture of lipids and
solvents, as will be further illustrated below.
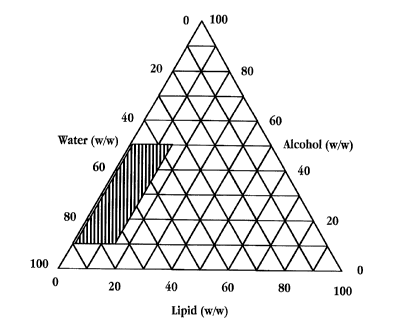
to Fig. 10 is a phase diagram showing the operating region for formation of
the
liposomes in accordance with the invention. In the diagram, the shaded region
corresponds
to formation of liposomes where the lipid solvent amount is between about 10-
50 weight
percent and the weight percent of the lipids is between 0.1-15. Depending on
the lipid
mixture and the lipid solvent, the point at which a minimum in liposome
particle size
15 occurs within the operating region can be determined. The liposome
suspensions in the
operating region are visually clear and contain submicron size liposomes. It
will be
appreciated that the operating region will vary slightly according to the
lipid, solvent and
drug components. One of skill in the art can readily conduct a study like that
set forth in
Example 8B and in Table 8 to determine the amount of lipid solvent that yields
a minimum
2o in the liposome size.
Liposomes formed by the above-described method can be, depending on the lipid
solvent amount in the hydration mixture, at a minimum particle size. Thus, in
some cases,
it may not be necessary to further size the liposomes via extrusion or other
technique. In
some cases, it may be desirable to further process the liposomes, for example
by extrusion.
2 5 The method of preparation is particularly useful for incorporation of
lipid-derivatized
drugs into liposomes at a high drug-to-lipid ratio, where obtaining a
pharmaceutically
useful particle size of between 90-150 nm is difficult due to an inability to
extrude the
mixture, as discussed above. Formation of liposomes according to the invention
overcomes
this limitation, since, the liposomes are at or near to the desired particle
size upon
3o hydration with the second solvent, and any further size processing is
minimized. Thus, a
higher lipid-derivatized drug load can be employed while still being able to
achieve the
desired liposome size.
13
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
This feature of the invention is illustrated by the study described in Example
8C.
Liposomes were prepared using the lipids HSPC, mPEG-DSPE and dpIUdR, where the
molar amounts of the formulations were 89/5/6, 85/5/10, 80/5/15, 70/5/25 and
55/5/40.
The lipids, including the dpIUdR, were dissolved in ethanol and then hydrated
with
sufficient water to bring the ethanol concentration in each mixture to 16.5
weight percent
(20 volume percent). Each liposome suspension was then extruded as described
in the
Example and after extrusion, the average particle size of the liposomes in
each suspension
was measured. The results are shown in Table 9.
1 o Table 9
Mole percent Conc. dpILldR Drug/Iipid rata~Liposome sate
dpi'(; dR (mglml) i ~ (nm)
6 1.25 1.67 99
2.32 3.17 95
4.09 4.65 103
6.90 8.91 142
40 - - -
*composition could not be extruded
Liposomes containing 6, 10 and 15 mole percent dpIUdR were readily sized to
15 about 100 nm when prepared according to the method of the invention by
hydrating the
lipid mixture to an amount of lipid solvent that gives a minimum particle
size. Importantly,
the liposomes are formed at the minimum particle size with a drug-to-lipid
ratio of between
about 1.5 and 5, more preferably between 2-4. In a preferred embodiment of the
invention, liposomes having the desired particle size are prepared using the
method to a
2 o drug-to-lipid ratio of greater than about 4, as achieved with the liposome
composition
having 15 mole percent dpIUdR.
From the foregoing it can be appreciated how various objects and features of
the
invention are met. Preparation of liposome according to the method provides a
means of
controlling the size of the particles in the suspension. More specifically,
there is a
2 5 relationship between liposome size and composition of the hydration
mixture, i. e. , amount
of lipid solvent in the hydration mixture, at greater than about 10 weight
percent and less
than 50 weight percent lipid solvent. In this region, there is a minimum in
the liposome
14
CA 02361551 2001-08-07
WO 00/45791 PCT/IJS00/03039
particle size.
III. Examples
The following examples illustrate the method of controlling liposome particle
size via
composition of the hydration mixture. The examples are in no way intended to
be limiting
to the scope and spirit of the invention.
Materials: The following materials were obtained from the indicated source:
egg
phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL); partially
hydrogenated soy
to phosphatidylcholine (Vernon Walden Inc., Green Village, NJ); cholesterol
(Solvay
Pharmaceuticals, The Netherlands); N,N-dioleoyl-N,N-dimethylammonium chloride
(DODAC) (Northern Lipids, Canada); PEG (MW 2000)-ceramide conjugate (CZOPEG
ceramide) (Northern Lipids, Canada); histidine (Seltzer Chemicals, Carlsbad,
CA); ethanol
(Quantum Chemical Co. Tuscola, IL). mPEG-DSPE was prepared as described in the
literature (for example, Zalipsky, S., et al, Bioconjugate Chem., 4:296-299
(1993)).
Methods
QUELS: quasi-elastic light scattering was performed using a Coulter model N4MD
(Coulter Corporation, Miami, FL) for Example 1; and a Brookhaven Instruments
Model
2 o B 1-200SM (Brookhaven Instruments Corporation, Holtsville, NY) for
Examples 2-7.
Example 1
1975 mg of egg phosphatidylcholine, 483.8 mg of cholesterol, 436.9 mg of DODAC
and 1337.5 mg of CZOPEG ceramide were mixed with 5 ml of ethanol and heated to
about
2 s 65-70 °C until dissolved. The composition of this lipid stock
solution was 51.7 weight
percent lipid and 48.3 weight percent ethanol. The total lipid concentration
was 496
mmoles/mL.
An oligonucletide having approximately 34 bases was dissolved in 10 mmolar
histidine, 0.9 % NaCI (pH 6.5) to a concentration of 20 mg/mL.
3 o Liposome suspensions were formed by mixing aliquots of the lipid solution
with
aliquots of the oligonucleotide stock solution. Both stock solutions were
diluted as needed
with their respective solvents just prior to mixing, in order to vary the
ethanol
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
concentration of the mixture while maintaining a fixed oligonucleotide and
lipid
concentration. Six liposome suspensions were formed from the stock solutions,
and the
composition of each mixture is indicated on the phase diagram of Fig. lA.
After mixing the mean particle diameters of the liposomes in the suspension
were
determined by Quasi-elastic light scattering. The results are shown in Table
1.
Table 1
Lipid after Mean Particle Diameter(nm)
s Ethanol 90/30 scattering
after
mixing s mixing
'W~% Wt%
4.6 35.5 628/681
5.0 15.8 322/357
5.9 5.9 932/767
0.46 39.0 183/381
0.50 19.3 87/161
0.59 9.5 136/243
to Fig. 1B is a plot showing the mean particle diameter in nm as a function of
both
weight percent lipid and weight percent ethanol.
Example 2
1183 mg of partially hydrogenated soy phosphatidylcholine, 290 mg of
cholesterol,
262 mg of DODAC and 795 mg of mPEG-DS were mixed with 0.47 mL of ethanol and
heated until dissolved. The composition of this lipid stock solution was 87.2
weight
percent lipid and 12.8 weight percent ethanol.
A stock solution containing 20 mg/mL of a 34 base oligonucleotide was prepared
in
10 mmolar histidine, 0.9 % NaCI, pH 6.5 aqueous solution.
2 o Aliquots of the lipid stock solution, diluted as needed with ethanol, were
hydrated
with aliquots of the aqueous oligonucleotide solution, diluted as needed with
the aqueous
phase, were taken to prepare suspensions of liposomes at the compositions
indicated in Fig.
2A.
Each liposome suspension was characterized by measuring the mean particle
diameter
using QELS just after hydration and after overnight incubation at 5 °C.
The results are
16
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
shown in Table 2.
Table 2
Sample Pnst ' Post ~ Mean ' Mean diameter
Pest
hydrationhydration by drationdiameter (nm) after
(nan)
weight weight weight after mixingovernight
~ i .
percent percent percent 90 degree/30~cubation at
; ~ 5
EtOH Lipid ' Oligo- degree ' degrees after
;
v nucleotidescattering axing 90
degree/30 degree
t scattering
1 32.6 7.2 1 425/534 1037/660
2 28.7 7.2 1 200/258 275/421
3 24.7 7.2 1 101/173 154/202
4 20.8 7.2 1 84/167 100/165
16.9 7.2 1 72/145 72/123
6 12.9 7.2 1 66/129 67/127
7 9.0 7.2 1 104/317 105/360
8 7.0 7.2 1 172/406 177/430
9 5.0 7.2 1 310/517 323/556
5
Fig. 2B is a plot showing the mean particle diameter in nm as a function of
both
weight percent lipid and weight percent ethanol.
Example 3
l 0 394 mg of partially hydrogenated soy phosphatidylcholine, 96.6 mg of
cholesterol,
87.4 mg of DODAC and 275 mg of mPEG-DSPE were mixed with 2.147 mL of ethanol
and heated until dissolved. The composition of this lipid stock solution was
33.5 weight
percent lipid and 66.5 weight percent ethanol.
A stock solution containing 6.7 mg/mL of a 34 base oligonucleotide was
prepared in
10 mmolar histidine, 0.9 % NaCI, pH 6.5 aqueous solution.
Aliquots of the lipid stock solution, diluted as needed with ethanol, were
hydrated
with aliquots of the aqueous oligonucleotide solution, diluted as needed with
the aqueous
phase, were taken to prepare suspensions of liposomes at the compositions
indicated in Fig.
3A.
17
CA 02361551 2001-08-07
WO 00/45791 PCT/LTS00/03039
Each liposome suspension was characterized by measuring the mean particle
diameter
using QELS just after hydration. The results are shown in Table 3.
Table 3
Sample Ethanol postTotal Lipid ~ ~ligo Mean Mean
hydration post hydrationpost diameter diameter
(wt%) (eat%) ~ hyd~cation90 30
(wt%) degree degree
scatter scatter
1 36.9 2.69 0.33 814 596
2 33.0 2.69 0.33 182.7 233
3 29.0 2.69 0.33 114 140.3
4 25.1 2.69 0.33 82.1 93.9
5 21.2 2.69 0.33 92.6 149
6 17.2 2.69 0.33 115.7 295
7 13.2 2.69 0.33 209.7 311.3
8 11.3 2.69 0.33 274.7 423.0
9 9.3 2.69 0.33 334.7 524.7
Fig. 3B is a plot showing the mean particle diameter in nm as a function of
both
weight percent lipid and weight percent ethanol.
1 o Example 4
599.6 mg of partially hydrogenated soy phosphatidylcholine, 257.2 mg of
cholesterol, and 214.2 mg of mPEG-DSPE were mixed with 1 mL of ethanol and
heated
until dissolved. The composition of this lipid stock solution was 57.6 weight
percent total
lipid in ethanol.
An aqueous phase for hydration was prepared and contained 10 mmolar histidine,
0.9 % NaCI, pH 6.5 aqueous solution.
Aliquots of the lipid stock solution, diluted as needed with ethanol, were
hydrated
with aliquots of the aqueous solution, to prepare suspensions of liposomes at
the
compositions indicated in Fig. 4A. Each of the compositions were prepared in
triplicate.
2 o Each liposome suspension was characterized by measuring the mean particle
diameter
using QELS just after hydration. The results are shown in Table 4.
18
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
Table 4
Sample 'Weight PercentWeight PercentMean particle Standard deviation
EtOH Total lipid :
diameter (nm)
1 7.0 0.94 413 91
2 18.8 0.94 161 21
3 38.5 0.94 537 227
4 4.9 3.12 1084 135
16.8 3.12 569 86
6 36.5 3.12 705 192
7 3.5 4 1435 76
.6
9
8 15.3 _ 737 146
_
4.69
9 35.0 4.69 735 108
Fig. 4B is a plot showing the mean particle diameter in nm as a function of
both
5 weight percent lipid and weight percent ethanol.
Example 5
Liposomes were prepared as described in Example 5 except the mPEG-DSPE was
omitted for the liposome lipid mixture. Thus, 629.8 mg of partially
hydrogenated soy
1 o phosphatidylcholine and 272 mg of cholesterol were mixed with 1 mL of
ethanol and
heated until dissolved. The composition of this lipid stock solution was 53.3
weight
percent total lipid in ethanol.
Aliquots of the lipid stock solution, diluted as needed with ethanol, were
hydrated
with aliquots of the aqueous solution (10 mmolar histidine, 0.9% NaCI, pH
6.5), to prepare
z5 suspensions of liposomes at the compositions indicated in Fig. SA. Each of
the
compositions were prepared in triplicate.
Each liposome suspension was characterized by measuring the mean particle
diameter
using QELS just after hydration. The results are shown in Table 5.
2 o Table 5
Sample Weight percentWeight percentli~Iean particle Standard
EtOH Total li id diameter (nm) deviation
1 7.1 0.87 365 150
2 18.9 0.87 495 504
3 38.7 0.87 1596 128
4 5.2 2.88 2891 986
5 17.0 2.88 305 68
6 36.7 2.88 2116 407
7 3.8 4.33 5710 516
8 15.6 4.33 719 244
9 35.4 4.33 2433 867
19
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
Fig. SB is a plot showing the mean particle diameter in nm as a function of
both
weight percent lipid and weight percent ethanol.
Example 6
668.3 mg of partially hydrogenated soy phosphatidylcholine, 222.3 mg of
cholesterol, and 222.6 mg of mPEG-DSPE were mixed with 1 mL of ethanol and
heated
until dissolved. The composition of this lipid stock solution was 55.5 weight
percent total
lipid in ethanol.
An aqueous phase for hydration was prepared and contained 10 mmolar histidine,
l 0 0.9 % NaCI, pH 6.5 aqueous solution.
Aliquots of the lipid stock solution, diluted as needed with ethanol, were
hydrated
with aliquots of the aqueous solution, to prepare suspensions of liposomes at
the
compositions indicated in Fig. 6A. Each of the compositions were prepared in
triplicate.
Each liposome suspension was characterized by measuring the mean particle
diameter
1 s using QELS just after hydration. The results are shown in Table 6.
Table 6
Sample Weight Mean particle Standard
1 Weight diameter (nm) deviation
percent
~ percent
otal
Et~H ~
lipid
1 7.0 0.95 411 57
2 18.8 0.95 127 9
3 38.6 0.95 1423 135
4 4.9 3.2 961 97
5 16.7 3.2 418 63
6 36.4 3.2 634 41
7 3.4 4.8 1725 127
8 15.2 4.8 634 35
9 35.0 4.8 837 74
2o Fig. 6B is a plot showing the mean particle diameter in nm as a function of
both
weight percent lipid and weight percent ethanol.
Example 7
Liposomes were prepared as described in Example 6 except the mPEG-DSPE was
CA 02361551 2001-08-07
WO 00/45791 PCT/LTS00/03039
omitted for the liposome lipid mixture. Thus, 629.8 mg of partially
hydrogenated soy
phosphatidylcholine and 272 mg of cholesterol were mixed with 1 mL of ethanol
and
heated until dissolved. The composition of this lipid stock solution was 54.3
weight
percent total lipid in ethanol.
Aliquots of the lipid stock solution, diluted as needed with ethanol, were
hydrated
with aliquots of the aqueous solution (10 mmolar histidine, 0.9% NaCI, pH 6.5)
to prepare
suspensions of liposomes at the compositions indicated in Fig. 7A. Each of the
compositions were prepared in triplicate.
Each liposome suspension was characterized by measuring the mean particle
diameter
1 o using QELS just after hydration. The results are shown in Table 7.
Table 7
Sample Weigbt Weight Mean particle Standard
percent percent totaldiaaneter (nm)deviation
EtO H li id i
1 7.1 0.88 222 22
2 18.9 0.88 219 24
3 38.6 0.88 3180 331
4 5.1 2.9 3993 1625
5 17.0 2.9 516 299
6 36.7 2.9 2619 1154
7 3.7 4.4 7014 27
8 15.5 4.4 604 106
9 35.3 4.4 1879 322
Fig. 7B is a plot showing the mean particle diameter in nm as a function of
both
weight percent lipid and weight percent ethanol.
Example 8
Liposomes Containing dpIUdR
2o A. Synthesis of 3',5'-dipalmitoyl-5-iodo-2'-deoxyuridine (dpIUdR)
Palmitoyl chloride was added slowly to a solution of deoxyuridine in pyridine
or in
pyridine/chloroform and 4-dimethylpyridine as a catalyst. The solution was
mixed until a
yellow in color and then left to stand overnight for precipitation. The
mixture was
dissolved in chloforma and washed with 10 % aqueous citric acid solution and
saturated
2 5 sodium bicarbonate solution. The chloroform was removed and methanol was
added to
yield a white precipitate, identified as 3',5'-dipalmitoyl-5-iodo-2'-
deoxyuridine (66%
21
CA 02361551 2001-08-07
WO 00/45791 PCT/US00/03039
yield). The synthetic reaction scheme is shown in Fig. 8.
B. Liposome Preparation Varying Ethanol Content
The lipids hydrogenated soy phosphatidyl choline (HSPC) and
distearoylphosphatidylcholine derivatized with methoxy-polyethyleneglycol
(DSPE-mPEG)
and dpIUdR in molar ratio of 89/5/6 were dissolved in ethanol at 70°C
until complete
dissolution was achieved (about 1 hour). This lipid stock solution, after
diluting as
necessary to maintain a fixed lipid concentration, was hydrated with a 10%
sucrose solution
at 70°C to vary the ethanol concentration from 8-44 weight percent.
Each of the liposome
to mixtures were stirred for one hour to form liposome suspensions. The
liposome particle
size of each mixture was determined using quasi-elastic light scattering and
the results are
shown in Table 8.
C. Liposome Preparation at 16.5 weight percent Ethanol
The lipids hydrogenated soy phosphatidyl choline (HSPC) and
distearoylphosphatidylcholine derivatized with methoxy-polyethyleneglycol
(DSPE-mPEG)
and dpIUdR in a molar ratios of 89/5/6, 85/5/10, 80/5/15, 70/5/25 and 55/5/40
were
dissolved in ethanol at 70°C until complete dissolution was achieved
(about 1 hour). The
lipid mixtures were hydrated with sufficient 10% sucrose solution at
70°C to achieve a
2 0 16.5 weight percentage ethanol content in the hydration mixture (20 %
(v/v) ethanol). The
mixtures were stirred for one hour prior to extruding the mixtures.
The liposome suspensions were extruded through polycarbonate membranes to
achieve a uniform size of 100 nm. The suspensions were then diafiltered
against 10 %
sucrose solution to reduce the ethanol concentration to below 400 ppm. The
suspensions
were then ultrafiltered to above the target drug concentration, assayed for
drug
concentration and diluted to target by adding 10 mM histidine buffer and
adjusting the pH
to 6.5.
The liposome particle sizes were measured by quasi-elastic light scattering
and the
results are shown in Table 9.
3 o Although the invention has been described with respect to particular
embodiments,
it will be apparent to those skilled in the art that various changes and
modifications can
be made without departing from the invention.
22