Note: Descriptions are shown in the official language in which they were submitted.
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
CARTRIDGE FOR DIALYSIS CONTAINING SODIUM
BICARBONATE
This invention relates to dialysis cartridges containing solid sodium
bicarbonate.
BACKGROUND
It has long been known to use cartridges containing drugs, or other
to substances, in solid form and to pass water or a solution through the
cartridge to dissolve the solid substance continuously, e.g. for continuous
administration to a patient.
Examples are WO-A-86/03417 and US-A-4432756.
It is also known, as disclosed in EP-A-0278100 to provide sodium
is bicarbonate in solid form for use as a buffer in haemodialysis. Sodium
bicarbonate is stored separately from the rest of a dialysis solution, which
contains calcium and magnesium ions, to prevent calcium and magnesium
carbonate precipitation. A cartridge of sodium bicarbonate powder is
inserted in a haemodialysis machine and water is passed through the
2o cartridge. The powder is gradually dissolved, so that a solution of sodium
bicarbonate is continuously produced. The solution is continuously
flowed through the machine, mixing with the rest of the dialysis solution
in-line upstream of the dialyzer. There is, therefore, only a short dwell
time in the machine after mixing, so that the problem of calcium and
25 magnesium carbonates being precipitated is avoided.
A problem does, however, arise with such cartridges. The pH of
the mixed dialysis solution is monitored upstream of the dialyzer. If the
pH falls outside a given range, then an alarm is triggered. It has been
found that this often happens during the first twenty minutes of flow,
3o when the machine is being set up for operation. After this period, no
~UBSTI~JTE SHEET (RULE 26)
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
2
problems are encountered. This causes substantial inconvenience to
personnel operating haemodialysis machines, since the problem has to be
investigated and the machine reset, each time the alarm is triggered.
s The inventors have discovered that the problem is probably caused
by contamination of the sodium bicarbonate powder with a small amount
of sodium carbonate. The bicarbonate is less soluble than the carbonate,
so that a high pH is caused by the dissolution of the carbonate in the early
stages. Once the carbonate has dissolved, the problem disappears. It is,
io however, difficult and expensive to produce a sodium bicarbonate powder,
which is not contaminated with sodium carbonate.
A possible solution to the problem once it was realised that sodium
carbonate precipitation was the cause, would be to introduce a further line
is upstream of the pH monitoring device to add dilute acid solution to the
dialysis solution during the first twenty minutes of use of the cartridge.
This could be done upstream or downstream of the cartridge. This
involves, however, modification of the dialysis solution, use of an
additional solution and additional operational control.
The inventors have found that the problem can be relatively simply
solved by modifying the contents of the cartridge.
SUMMARY OF THE INVENTION
2s The present invention relates to a cartridge having an operable
sealed inlet and an operable sealed outlet, for connection in-line in a
haemodialysis machine for passage of water, or a solution through the
cartridge, the cartridge containing sodium bicarbonate in solid form.
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
3
In accordance with the invention, the camidge additionally contains
an acid, or acid anhydride in solid form, or carbon dioxide gas.
When the cartridge is mounted in a haemodialysis machine and
s water is passed through the cartridge, the acid or acid anhydride
(including carbon dioxide) is gradually dissolved, decreasing the pH of the
resulting solution to counteract any temporary increase in pH caused by
sodium carbonate contamination.
to The amount of acid or acid anhydride provided is preferably
predetermined, so that it is leached from the cartridge during the initial 10
to 30 minutes, i.e. during the period that sodium carbonate is also likely to
be leached from the cartridge.
15 Carbon dioxide may be added to the cartridge, during manufacture,
in solid form, i.e. as dry ice, prior to sealing the cartridge.
Acids which may be used in solid form may be organic acids, e.g.
citric acid, or tartaric acid, citric acid being preferred for clinical
2o acceptability.
The cartridge may contain at least 0.2g of acid, or acid anhydride
per 1000g of sodium bicarbonate; preferably at least O.Sg per 1000g and
most preferably at least lg per 1000g. The preferred embodiment
2s contains 2.7g per 1000g.
DRAWINGS
The invention is described with reference to the accompanying
drawings, wherein:-
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
4
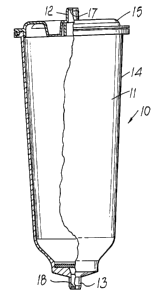
Fig 1. is a side elevation of a cartridge according to the invention,
shown partly in cross-section; and
Fig 2. is a diagrammatic illustration of the cartridge of fig 1
connected in a haemodialysis machine.
s
PREFERRED EMBODIMENTS
The currently preferred embodiments of the invention are now
described. The construction of haemodialysis machines is well known, as
is the construction of a sodium bicarbonate cartridge for use in a
haemodialysis machine. The machine and the cartridge are not, therefore,
described in detail. The cartridge may be of the type sold under the
trademark EASYCART by Bieffe Medital S.p.A. of Italy.
The cartridge 10 comprises a body 14, closed by a lid 15 and
is defining a chamber 11. The body and lid are injection moulded in
polypropylene. The chamber 11 contains sodium bicarbonate in granular,
crystalline form, although other solid forms are possible. The lid 15 is
sealed to the body 14 by ultrasonic welding. The lid 15 has an inlet 12
and the body had an outlet 13, both sealed closed in the as-moulded state,
2o by integral membranes 17, 18 respectively.
The cartridge 10 is connected in-line in a first line 20 for receiving
deionised water at 21 and supplying sodium bicarbonate solution to a main
line 22, at 23. The membranes 17, 18 are perforated during clamping of
2s the cartridge into the machine, by piercing means provided on the
machine. The main line 22 also receives deionised water at 24. A
container 30, containing a solution of the other ingredients of a dialysis
solution, is connected to the main line 22 by a second line 25 at 26. A
final dialysis solution is formed at point 26 and the main line 22 feeds this
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
to a dialyzer 40. A pH detector 50 is connected to the main line 22
downstream of point 26 and upstream of the dialyzer. The detector is
connected with a control system (not shown), which produces an alarm, if
a pH outside a predetermined range is exceeded. This range is usually 6.8
s to 7.9 pH may be monitored by other means, such as by conductivity
measurement.
The solution in the container 30 may contain any of the components
usually provided in a dialysis solution, such as calcium and magnesium
io chloride, sodium chloride and an osmotic agent, such as dextrose.
In accordance with the present invention, the cartridge contains, in
addition to the sodium bicarbonate, an acid or acid anhydride in solid
form, or carbon dioxide gas, so as to avoid any sodium carbonate
~5 contamination causing a temporary increase in the pH of the dialysis
solution to a degree sufficient to exceed the predetermined threshold and
trigger an alarm.
The preferred embodiment of a cartridge contains 750g sodium
2o bicarbonate and 2g citric acid, both in granular, crystalline form. A
similar weight ratio could be used with different amounts of sodium
bicarbonate.
Alternatives to citric acid are preferably provided in the same
2s weight ratio.
Tests were carried out using cartridges containing 750g sodium
bicarbonate and citric acid, tartaric acid, or carbon dioxide (added as dry
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
6
ice) respectively. These were compared with similar cartridges, to which
no acid or carbon dioxide had been added.
The tests were carried out by running an Integra (trademark)
s haemodialysis machine, using the various cartridges. Notes were made of
which cartridges produced an alarm signal, due to the pH of the mixed
dialysis solution falling outside the predetermined range. The actual
maximum pH of each solution was also recorded. "Acid" solutions, ie the
solutions carrying the other components of the dialysis solution, were
io standard solutions produced by Gambro. In each case, the pH of the
water supplied was 6.1. The results are shown in the tables below. There
were numerous false alarms with the reference cartridges, but no false
alarms with the cartridges according to the invention.
is Table 1
This shows the results using the reference cartridges, containing 750g
sodium bicarbonate and no added acid or carbon dioxide.
Sample No. Maximum pH of Alarm
dialysis solution Yes/No
1 7.9 No'
2 7.8 No
3 8 Yes
4 8.1 Yes
7.9 No
6 8 Yes
7 8.2 Yes
g 8 Yes
9 7.4 No
7.4 No
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
7
Table 2
This shows the results using cartridges according to the invention,
containing 2g citric acid and 750g sodium bicarbonate.
s
Sample No. Maximum pH of Alarm
dialysis solution Yes/No
11 7.5 No
12 7.5 No
13 7.5 No
14 7.5 No
15 7.4 No
16 7.4 No
17 7.4 No
18 7.4 No
19 7.4 No
20 7.4 No
21 7.4 No
Table 3
This shows the results using cartridges according to the invention,
to containing 0.5g or lg of carbon dioxide (dry ice) and 750g sodium
bicarbonate.
Sample No. Amount of COZ(g) Maximum pH Alarm
of
dialysis solutionYes/No
22 0.5 7.5 No
23 1.0 7.3 No
24 0.5 7.5 No
25 1.0 7.3 No
26 1.0 7.3 No
CA 02361641 2001-07-27
WO 00/44418 PCT/EP00/00130
g
Table 4
This shows the results using carnidges according to the invention,
containing lg tartaric acid and 750g sodium bicarbonate.
Sample No. Maxiumum pH of Alarm
dialysis solution Yes/No
27 7.5 No
2g 7.5 No
Other tests were carried out using different "acid" solutions, and water of
different pH. In each case, a cartridge according to the invention did not
cause any alarm due to either high or low pH.
to