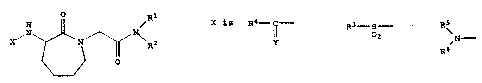Note: Descriptions are shown in the official language in which they were submitted.
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
LACTAM INHIBITORS OF FXa AND METHOD
Field of the Invention
The present invention relates to lactam inhibitors
of the enzyme Factor Xa which are useful as anticoagulants
in the treatment of cardiovascular diseases associated with
thromboses.
Brief Description of the Invention
In accordance with the present invention, novel
substituted lactam derivatives are provided which are
inhibitors of the enzyme Factor Xa and have the structure I
I.
O R1
H /
N ~ '
X~ N~ ~Ra
IOI
including pharmaceutically acceptable salts thereof and all
stereoisomers thereof, and prodrug esters thereof, wherein
R1 and R2 are the same or different and are
independently selected from alkyl, alkenyl, alkynyl, aryl,
heteroaryl, arylalkyl, heteroarylalkyl, cycloalkyl,
cycloalkylalkyl, polycycloalkyl, polycycloalkylalkyl,
cycloalkenyl, cycloheteroalkyl, cycloalkenylalkyl,
polycycloalkenyl, polycycloalkenylalkyl, or R1 and R2 can
be taken with the nitrogen to which they are attached to
form a cycloheteroalkyl ring; all optionally substituted
through available carbon atoms with 1, 2, 3 or 4 groups
selected from hydrogen, halo, alkyl, haloalkyl, alkoxy,
haloalkoxy, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl,
cycloheteroalkyl, cycloheteroalkylalkyl, aryl, heteroaryl,
arylalkyl, arylcycloalkyl, arylalkenyl, arylalkynyl,
aryloxy, aryloxyalkyl, arylalkoxy. arylazo, heteroaryloxo,
heteroarylalkyl, heteroarylalkenyl, heteroaryloxy, hydroxy,
nitro, cyano, amino, substituted amino, alkylamino,
dialkylamino, thiol, alkylthio, arylthio, heteroarylthio,
arylthioalkyl, alkylcarbonyl, arylcarbonyl,
- 1 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
arylaminocarbonyl, aminocarbonyl, alkynylaminocarbonyl,
alkylaminocarbonyl; alkenylaminocarbonyl, alkylcarbonyloxy,
arylcarbonyloxy, alkylcarbonylamino, arylcarbonylamino,
arylsulfinyl, arylsulfinylalkyl, arylsulfonyl,
alkylsulfonyl, arylsulfonylamino, heteroarylcarbonylamino,
heteroarylsulfinyl, heteroarylthio, heteroarylsulfonyl, or
alkylsulfinyl;
X is Rd-~- or R3-S-
oa
Y
RS
Y is O or S and R4 is ~N- ~ RIO- or R8
R6
R3 is selected from alkyl, alkenyl, alkynyl, aryl,
heteroaryl, arylalkyl, heteroarylalkyl, cycloalkyl,
cycloalkylalkyl, polycycloalkyl, polycycloalkylalkyl,
cycloalkenyl, cycloheteroalkyl, cycloalkenylalkyl,
polycycloalkenyl, or polycycloalkenylalkyl; all optionally
substituted through available carbon atoms with 1, 2, 3 or
4 groups selected from hydrogen, halo, alkyl, haloalkyl,
alkoxy, haloalkoxy, alkenyl, alkynyl, cycloalkyl,
cycloalkylalkyl, cycloheteroalkyl, cycloheteroalkylalkyl,
aryl, heteroaryl, arylalkyl, arylcycloalkyl, arylalkenyl,
arylalkynyl, aryloxy, aryloxyalkyl, arylalkoxy, arylazo,
heteroaryloxo, heteroarylalkyl, heteroarylalkenyl,
heteroaryloxy, hydroxy, nitro, cyano, amino, substituted
amino, alkylamino, dialkylamino, thiol, alkylthio,
arylthio, heteroarylthio, arylthioalkyl, alkylcarbonyl,
arylcarbonyl, arylaminocarbonyl, alkoxycarbonyl,
aminocarbonyl, alkynylaminocarbonyl, alkylaminocarbonyl,
alkenylaminocarbonyl, alkylcarbonyloxy, arylcarbonyloxy,
alkylcarbonylamino, azylcarbonylamino, arylsulfinyl,
arylsulfinylalkyl, arylsulfonyl, alkyl~ulfonyl,
azylsulfonylamino, heteroarylcarbonylamino,
heteroarylsulfinyl, heteroarylthio, heteroarylsulfonyl, or
alkylsulfinyl;
- 2 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
R5 and R6 are the same or different and are
independently selected from alkyl, alkenyl, alkynyl, aryl,
heteroaryl, arylalkyl, heteroarylalkyl, cycloalkyl,
cycloalkylalkyl, polycycloalkyl, polycycloalkylalkyl,
cycloalkenyl, cycloheteroalkyl, cycloalkenylalkyl,
polycycloalkenyl, polycycloalkenylalkyl, arylcarbonyl,
alkylcarbonyl, alkoxycarbonyl, aryloxycarbonyl,
arylsulfonyl, or alkylsulfonyl, or R5 and R6 can be taken
with the nitrogen to which they are attached to form a
cycloheteroalkyl ring; all optionally substituted through
available carbon atoms with 1, 2, 3 or 4 groups selected
from hydrogen, halo, alkyl, haloalkyl, alkoxy, haloalkoxy,
alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl,
cycloheteroalkyl, cycloheteroalkylalkyl, aryl, heteroaryl,
arylalkyl, arylcycloalkyl, arylalkenyl, arylalkynyl,
aryloxy, aryloxyalkyl, arylalkoxy, arylazo, heteroaryloxo,
heteroarylalkyl, heteroarylalkenyl, heteroaryloxy, hydroxy,
nitro, cyano, amino, substituted amino, alkylamino,
dialkylamino, thiol, alkylthio, arylthio, heteroarylthio,
arylthioalkyl, alkylcarbonyl, arylcarbonyl,
arylaminocarbonyl, alkoxycarbonyl, aminocarbonyl,
alkynylaminocarbonyl, alkylaminocarbonyl,
alkenylaminocarbonyl, alkylcarbonyloxy, arylcarbonyloxy,
alkylcarbonylamino, arylcarbonylamino, arylsulfinyl,
arylsulfinylalkyl, arylsulfonyl, alkylsulfonyl,
arylsulfonylamino, heteroarylcarbonylamino,
heteroarylsulfinyl, heteroarylthio, heteroarylsulfonyl, or
alkylsulfinyl;
R~ and R8 are independently selected from .alkyl,
alkenyl, alkynyl, aryl, heteroaryl, arylalkyl,
heteroarylalkyl, cycloalkyl, cycloalkylalkyl,
polycycloalkyl, polycycloalkylalkyl, cycloalkenyl,
cycloheteroalkyl, cycloalkenylalkyl, polycycloalkenyl,
polycycloalkenylalkyl, all optionally substituted through
available carbon atoms with l, 2, 3 or 4 groups selected
from hydrogen, halo, alkyl, haloalkyl, alkoxy, haloalkoxy,
alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl,
- 3 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
cycloheteroalkyl, cycloheteroalkylalkyl, aryl, heteroaryl,
arylalkyl, arylcycloalkyl, arylalkenyl, arylalkynyl,
aryloxy, aryloxyalkyl, arylalkoxy, arylazo, heteroaryloxo,
heteroarylalkyl, heteroarylalkenyl, heteroaryloxy, hydroxy,
nitro, cyano, amino, substituted amino, alkylamino,
dialkylamino, thiol, alkylthio, arylthio, heteroarylthio,
arylthioalkyl, alkylcarbonyl, arylcarbonyl,
arylaminocarbonyl, alkoxycarbonyl, aminocarbonyl,
alkynylaminocarbonyl, alkylaminocarbonyl,
alkenylaminocarbonyl, alkylcarbonyloxy, arylcarbonyloxy,
alkylcarbonylamino, arylcarbonylamino, arylsulfinyl,
arylsulfinylalkyl, arylsulfonyl, alkylsulfonyl,
arylsulfonylamino, heteroarylcarbonylamino,
heteroarylsulfinyl, heteroarylthio, heteroarylsulfonyl, or
alkylsulfinyl; with the proviso that
where in the formula I compounds
Rs
X is iN II or Re-~~
R Y IY
and (1) R1 and R2 are independently alkyl, cycloalkyl,
alkenyl, phenyl, benzyl, cyanoalkyl, alkoxycarbonylalkyl,
or phenyl mono- or disubstituted with lower alkyl, cyano,
hydroxy, dialkylamino, alkoxy, benzyloxy, alkylamino,
alkoxycarbonyl, pyrrolidino, morpholino, halogen, alkyl
substituted with one or more fluorines, then Y is S;
(2) where R1 and R2 are alkyl, then Y is S; and
(3) where one of R1 and R2 is alkyl and Y is O,
then the other is alkynyl, heteroazyl, heteroarylalkyl,
cycloalkenyl, cycloheteroalkyl, heteroaryloxy,
cycloalkenylalkyl, polycycloalkenyl, polycycloalkenylalkyl
or R1 and R2 can be taken with the nitrogen to which they
are attached to form a cycloheteroalkyl ring, all
optionally substituted through available carbon atoms with
1, 2, 3 or 4 substituents as defined for R1 and R2.
Thus, the compounds of formula I of the invention
can have the following structural formulae:
- 4 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
IA
IB
IC
ID
O R1
H
R3\S~N N~~Ra
Oa
O
O R1
FI /
Rs N II N N 11 \Ra
O
O R1
H /
R N N ~ Ra
O
O R1
H /
R N N ~ '1d Ra
II~II ~O
It is preferred that Y in the above formulae is S.
Preferred are compounds of formula IB wherein R1 and
R2 together with the nitrogen to which they are attached
form a cycloheteroalkyl ring, preferably a pyrrolidinyl
ring, Y is S, one of RS and R6 is hydrogen and the other of
RS and R6 is aryl, alkylaryl or alkoxyaryl such as phenyl,
3-methylphenyl or 3-methoxyphenyl, 4-cyanophenyl, 3-
fluorophenyl, 3-chlorophenyl, 4-chlorophenyl, 4-
methoxyphenyl, 3-chloro-4-methylphenyl, 3,5-dichlorophenyl,
3-iodophenyl, 3,5-dimethylphenyl or naphthyl.
In addition, in accordance with the present
invention, a method for preventing, inhibiting or treating
cardovascular diseases associated with thromboses is
provided, wherein a compound of formula I is administered
in a therapeutically effective amount which inhibits Factor
Xa.
- 5 _
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Detailed Description of the Invention
The following definitions apply to the terms as used
throughout this specification, unless otherwise limited in
specific instances.
Unless otherwise indicated, the term "lower alkyl",
"alkyl" or "alk" as employed herein alone or as part of
another group includes both straight and branched chain
hydrocarbons, containing 1 to 40 carbons (in the case of
alkyl or alk), preferably l to 20 carbons, more preferably
1 to 12 carbons (in the case of lower alkyl), in the normal
chain,such as methyl, ethyl, propyl, isopropyl, butyl, t-
butyl, isobutyl, pentyl, hexyl, isohexyl, heptyl, 4,4-
dimethylpentyl, octyl, 2,2,4-trimethylpentyl, rionyl, decyl,
undecyl, dodecyl, the various additional branched chain
isomers thereof, and the like as well as such groups
including 1 to 4 substituents which may be any of the R1 or
the R1 substituents set out herein.
Unless otherwise indicated, the term "cycloalkyl" as
employed herein alone or as part of another group includes
saturated or partially unsaturated (containing 1 or 2
double bonds) cyclic hydrocarbon groups containing 1 to 3
rings, including monocyclicalkyl, bicyclicalkyl and
tricyclicalkyl, containing a total of 3 to 20 carbons
forming the rings, preferably 4 to 12 carbons, forming the
ring and which may be fused to one aromatic ring as
described for aryl, which include cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl,
cyclodecyl and cyclododecyl, cyclohexenyl,
~ ~ '
any of which groups may be optionally substituted with 1 to
4 substituents which may be any of the R1 groups, or the R1
substituents set out herein.
The term "cycloalkenyl" as employed herein alone or
as part of another group ~~fers to cyclic hydrocarbons
- 6 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
containing 5 to 20 carbons, preferably 6 to 12 carbons and
1 or 2 double bonds. Exemplary cycloalkenyl groups include
cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl,
cyclohexadienyl, and cycloheptadienyl, which may be
optionally substituted as defined for cycloalkyl.
The term "aryl" as employed herein alone or as part
of another group refers to monocyclic and bicyclic aromatic
groups containing 6 to 10 carbons in the ring portion (such
as phenyl or naphthyl including 1-naphthyl and 2-naphthyl)
and may optionally include one to three additional rings
fused to a carbocyclic ring or a heterocyclic ring (such as
aryl, cycloalkyl, heteroaryl or cycloheteroalkyl rings) and
may be optionally substituted through available carbon
atoms with 1, 2, or 3 groups selected from hydrogen, halo,
haloalkyl, alkyl, haloalkyl, alkoxy, haloalkoxy, alkenyl,
trifluoromethyl, trifluoromethoxy, alkynyl, cycloalkyl-
alkyl, cycloheteroalkyl, cycloheteroalkylalkyl, aryl,
heteroaryl, arylalkyl, aryloxy, aryloxyalkyl, arylalkoxy,
arylthio, arylazo, heteroarylalkyl, heteroarylalkenyl,
heteroarylheteroaryl, heteroaryloxy, hydroxy, nitro, cyano,
amino, substituted amino wherein the amino includes 1 or 2
substituents (which are alkyl, aryl or any of the other
aryl compounds mentioned in the definitions), thiol,
alkylthio, arylthio, heteroarylthio, arylthioalkyl,
alkoxyarylthio, alkylcarbonyl, arylcarbonyl, alkyl-
aminocarbonyl, arylaminocarbonyl, alkoxycarbonyl,
aminocarbonyl, alkylcarbonyloxy, arylcarbonyloxy,
alkylcarbonylamino, arylcarbonylamino, arylsulfinyl,
arylsulfinylalkyl, arylsulfonylamino or arylsulfon-
aminocarbonyl or any of the R1 groups or the R1
substituents set out herein.
The term "aralkyl", "aryl-alkyl" or "aryllower
alkyl" as used herein alone or as part of another group
refers to alkyl groups as discussed above having an aryl
substituent, such as benzyl or phenethyl, or
naphthylpropyl, or an aryl as defined above.
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
The term "lower alkoxy", "alkoxy", "aryloxy" or
"aralkoxy" as employed herein alone or as part of another
group includes any of the above alkyl, aralkyl or aryl
groups linked to an oxygen atom.
The term "amino" as employed herein alone or as part
of another group may optionally be independently
substituted with one or two substituents, which may be the
same or different, such as alkyl, aryl, arylalkyl,
heteroazyl, heteroarylalkyl, cycloheteroalkyl,
cycloheteroalkylalkyl, cycloalkyl, cycloalkylalkyl,
haloalkyl, hydroxyalkyl, alkoxyalkyl o-r thioalkyl. These
substituents may be further substituted with a carboxylic
acid or any of the R1 groups or R1 substituents thereof as
set out above. In addition, the amino substituents may be
taken together with the nitrogen atom to which they are
attached to form 1-pyrrolidinyl, 1-piperidinyl, 1-azepinyl,
4-morpholinyl, 4-thiamorpholinyl, 1-piperazinyl,
4-alkyl-1-piperazinyl, 4-arylalkyl-1-piperazinyl,
4-diarylalkyl-1-piperazinyl, 1-pyrrolidinyl, 1-piperidinyl,
or 1-azepinyl, optionally substituted with alkyl, alkoxy,
alkylthio, halo, trifluoromethyl or hydroxy.
The term "lower alkylthio", alkylthio", "arylthio"
or "aralkylthio" as employed herein alone or as part of
another group includes any of the above alkyl, aralkyl or
aryl groups linked to a sulfur atom.
The term "lower alkylamino", "alkylamino",
"arylamino", or "arylalkylamino" as employed herein alone
or as part of another group includes any of the above
alkyl, aryl or arylalkyl groups linked to a nitrogen atom.
The term "acyl" as employed herein by itself or part
of another group, as defined herein, refers to an organic
0
radical linked to a carbonyl ~ ~ ~ group; examples of
acyl groups include any of ..__e R1 groups attached to a
carbonyl, such as alkanoyl, alkenoyl, aroyl, aralkanoyl,
heteroaroyl, cycloalkanoyl, cycloheteroalkanoyl and the
like.
- g -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
The term "alkanoyl" as used herein alone or as part
of another group refers to alkyl linked to a carbonyl
group.
Unless otherwise indicated, the term "lower alkenyl"
S or "alkenyl" as used herein by itself or as part of another
group refers to straight or branched chain radicals of 2 to
20 carbons, preferably 3 to 12 carbons, and more preferably
1 to 8 carbons in the normal chain, which include one to
six double bonds in the normal chain, such as vinyl, 2-
propenyl; 3-butenyl, 2-butenyl, 4-pentenyl, 3-pentenyl, 2-
hexenyl, ?-hexenyl, 2-heptenyl, 3-heptenyl, 4-heptenyl, 3-
octenyl, 3-nonenyl, 4-decenyl, 3-undecenyl, 4-dodecenyl,
4,8,12-tetradecatrienyl, and the like, and which may be
optionally substituted with 1 to 4 substituents, namely,
1S halogen, haloalkyl, alkyl, alkoxy, al;cenyl, alkynyl, aryl,
arylalkyl, cycloalkyl, amino, hydroxy, heteroaryl,
cycloheteroalkyl, alkanoylamino, alkylamido, arylcarbonyl-
amino, vitro, cyano, thiol, alkylthio or any of the R1
groups, or the R1 substituents set out herein.
Unless otherwise indicated, the term "lower alkynyl"
or "alkynyl" as used herein by itself or as part of another
group refers to straight or branched chain radicals of 2 to
20 carbons, preferably 2 to 12 carbons and more preferably
2 to 8 carbons in the normal chain, which include one
?S triple bond in the normal chain, suc'.: as 2-propynyl, 3-
butynyl, 2-butynyl, 4-pentynyl, 3-pentynyl, 2-hexynyl, 3-
hexynyl, 2-heptynyl, 3-heptynyl, 4-heptynyl, 3-octynyl, 3-
nonynyl, 4-decynyl,3-undecynyl, 4-dodecynyl and the like,
and which may be optionally substituted with 1 to 4
substituents, namely, halogen, haloalkyl, alkyl, alkoxy,
alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl, amino,
heteroaryl, cycloheteroalkyl, hydroxy, alkanoylamino,
alkylamido, arylcarbonylamino, ni~ro, cyano, thiol, and/or
alkylthio, or any of the R1 groups, or the R1 substituents
3S set out herein.
Where alkyl groups as defined above have single
bonds for attachment to other groups at two different
- g _
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
carbon atoms, they are termed "alkylene" groups and may
optionally be substituted as defined above for "alkyl".
Where alkenyl groups as defined above and alkynyl
groups as defined above, respectively, have single bonds
for attachment at two different carbon atoms, they are
termed "alkenylene groups" and "alkynylene groups",
respectively, and may optionally be substituted as defined
above for "alkenyl" and "alkynyl".
Suitable alkylene, alkenylene or alkynylene groups
(CH2)p (where, p is 1 to 8, preferably 1 to 5) (which may
include alkylene, alkenylene or alkynylene groups) as
defined herein, may optionally include 1, 2, or 3
substituents which include any of the R1 groups, or the R1
substituents set out herein.
Examples of alkylene, alkenylene and alkynylene
include
- CH- CH- CHZ- ~ - CHZCH- CH- 7 - C-C- CHZ-
CH2 II ~ CHZ CHZ- CHZ li
O O
CHg
- ~H2~ - CCHa - , - C- CH - CHZ-
- (CHZ)2- ~ -(CH2)3- ~ - (CH2)4-
CHg
- (CH2 ) 2- i - CH2CHz- ~ - CH2CH- ~ - CH2CHCH2-
CHg I$3 12H5
- iHCHz- ~ - iHCHZcH2- ~ -cHiHCHZ
CHg C2H5 I CH3
CHg
CHg F
-CHZ-C-CHZ- ~ -(CHZ)~ , -(CHZ)2-C-CHZ- ,
Cg3 F
- 10 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Cl CH3 CHg
-CHZ-CH-CHZ- ~ -(C$2)Z- i H- ~ -C$2-CH- ~- ~
CH3 CH3
- CHZ - CH - CH - C$Z ~ ~ CHZ - ~H- C$2 - C$- ,
CH3 C$3 CHg CH3
CH3 ~CH3
- CH- C82CH2- ~ - CH' CHZCHZ- ~ - CHZOCHZ-
- OC$ZCH2- ~ - CHZNHCHg- ~ - NHCHZCHZ-
i H3 - i - C$2CH2.-
- ( CHZ ) 3- CFZ- ~ - Cg2- N- CHZ- Or CH3
The term "halogen" or "halo" as used herein alone or
as part of another group refers to chlorine, bromine,
fluorine, and iodine as well as CF3, with chlorine or
fluorine being preferred.
The term "metal ion" refers to alkali metal ions
such as sodium, potassium or lithium and alkaline earth
metal ions such as magnesium and calcium, as well as zinc
and aluminum.
The term "cycloheteroalkyl" as used herein alone or
as part of another group refers to a S-, 6- or 7-membered
saturated or partially unsaturated ring which includes 1 to
2 hetero atoms such as nitrogen, oxygen and/or sulfur,
linked through a carbon atom or a heteroatom, where
possible, optionally via the linker (CH2)p (which is
defined above), such as
O' N ~O S,/
> > U
N~ O ~ N
' J '
0
- 11 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
N~ O~ Sue/
' , ,
and the like. The above groups may include 1 to 4
substituents such as alkyl, halo, oxo and/or any of of the
R1 groups, or the R1 substituents set out herein. In
addition, any of the above rings can be fused to a
cycloalkyl, aryl, heteroaryl or cycloheteroalkyl ring.
The term "heteroaryl" as used herein alone or as
part of another group refers to a 5- or 6- membered
aromatic ring which includes 1, 2, 3 or 4 hetero atoms such
as nitrogen, oxygen or sulfur,and such rings fused to an
aryl, cycloalkyl, heteroaryl or cycloheteroalkjrl ring (e. g.
benzothiophenyl, indolyl), and includes possible N-oxides.
The heteroaryl group may optionally include 1 to 4
substituents such as any of the R1 groups or the R1
substituents set out above. Examples of heteroaryl groups
include the following:
O
N~ S\ O
l
/ ~ ~ ~ ~ ~ '
( ~ NO /N ~ N~~ N ~ ~~ N 'l O ~ i
1
w'~U~~ I >> I J eo
S N
N N N ~ /
/ ~ N ~~O N ~~S o Nw
iN ~ N /N ~ ~ N ~ ~ ' ~ ' \N ~ / '
N H
N-N N-N N-N N N=N
/ S /O /N /O N
~I
N ~ ~ N , ~ N
I ~ ~ N I ,
- 12 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
CHg / CHgO
-CH3 I ~--CH3
\ ~ . \ ~ ,
and the like.
The term "cycloheteroalkylalkyl" as used herein
alone or as part of another gorup refers to
cycloheteroalkyl groups as defined above linked through a C
atom or heteroatom to a (CH2)p chain.
The term "heteroarylalkyl" or "heteroarylalkenyl" as
used herein alone or as part of another group refers to a
heteroaryl group as defined above linked through a C atom
or heteroatom to a -(CH2)p- chain, alkylene or alkenylene
as defined above.
The term "polyhaloalkyl" as used herein refers to an
"alkyl" group as defined above which includes from 2 to 9,
preferably from 2 to 5, halo substituents, such as F or C1,
preferably F, such as CF3CH2, CF3 or CF3CF2CH2.
The term "polyhaloalkyloxy" as used herein refers to
an "alkoxy" or "alkyloxy" group as defined above which
includes from 2 to 9, preferably from 2 to 5, halo
substituents, such as F or Cl, preferably F, such as
CF3CH20, CF30 or CF3CF2CH20.
The compounds of formula I can be present as salts, in
particular pharmaceutically acceptable salts. If the
compounds of formula I have, for example, at least one
basic center, they can form acid addition salts. These are
formed, for example, with strong inorganic acids, such as
mineral acids, for example sulfuric acid, phosphoric acid
or a hydrohalic acid, with strong organic carboxylic acids,
such as alkanecarboxylic acids of 1 to 4 carbon atoms which
are unsubstituted or substituted, for example, by halogen,
for example acetic acid, such as saturated or unsaturated
dicarboxylic acids, for example oxalic, malonic, succinic,
malefic, fumaric, phthalic or terephthalic acid, such as
hydroxycarboxylic acids, for example ascorbic, glycolic,
lactic, malic, ~-rtaric or citric acid, such as amino
- 13 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
acids, (for example aspartic or glutamic acid or lysine or
arginine), or benzoic acid, or with organic sulfonic acids,
such as (C1-C4)-alkyl- or aryl-sulfonic acids which are
unsubstituted or substituted, for example by halogen, for
example methane- or p-toluene-sulfonic acid. Corresponding
acid addition salts can also be formed having, if desired,
an additionally present basic center. The compounds of
formula I having at least one acid group (for example COOH)
can also form salts with bases. Suitable salts with bases
are, for example, metal salts, such as alkali metal or
alkaline earth metal salts, for example sodium, potassium
or magnesium salts, or salts with ammonia or an organic
amine, such as morpholine, thiomorpholine, piperidine,
pyrrolidine, a mono-, di- or tri-lower alkylamine, for
example ethyl-, tert-butyl-, diethyl-, diisopropyl-,
triethyl-, tributyl- or dimethyl-propylamine, or a mono-,
di- or trihydroxy lower alkylamine, for example mono-, di-
or triethanolamine. Corresponding internal salts may
furthermore be formed. Salts which are unsuitable for
pharmaceutical uses but which can be employed, for example,
for the isolation or purification of free compounds I or
their pharmaceutically acceptable salts, are also included.
Preferred salts of the compounds of formula I include
monohydrochloride, hydrogensulfate, methanesulfonate,
phosphate or nitrate.
All stereoisomers of the compounds of the instant
invention are contemplated, either in admixture or in pure
or substantially pure form. The compounds of the present
invention can have asymmetric centers at any of the carbon
atoms including any one of the R substituents.
Consequently, compounds of formula I can exist in
enantiomeric or diastereomeric forms or in mixtures
thereof. The processes for preparation can utilize
racemates, enantiomers or diastereomers as starting
materials. When enantiomeric or diastereomeric products
are prepared, they can be separated by conventional methods
for example, chromatographic or fractional crystallization.
- 14 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
It should be understood that the present invention
includes prodrug forms of the compounds of formula I such
as alkylesters of acids or any known prodrugs for lactam
derivatives.
The compounds of the instant invention may, for
example, be in the free or hydrate form, and may be
obtained by methods exemplified by the following
descriptions.
The compounds of formula I may be prepared by the
exemplary processes described in the following reaction
schemes. Exemplary reagents and procedures for these
reactions appear hereinafter and in the working Examples.
Compounds of formula I of the invention can be
prepared from the corresponding amine 1 by using the
sequence of steps outlined in Scheme I set out below.
Reaction Scheme I
O R1
Ha
N~~RZ + 2_ or 3_ or 4 or 5_
I IO
1
O O Ri
H ~ /
Amidation
--~ Re~N N~~R2
_1 + Re Cl II II ;
O O
2
°r
O R1
Sulfonylation Re~ ~N
1 + R3/ \ Cl ~ ~O N R2
O
3
or =A
- 15 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
O O Rl
H /
1 + R~ ~ Condensatioa / O N
\O~C1 R7 N ~R2
4 O O
or IC
R6 O 1
O ~ H .R
_1 + R5~ ~ Condensation RS/ N N -~!N
N Cl R
R6 O O
IB
Reaction of amine 1 in an inert organic solvent such
as dichloromethane, chloroform or tetrahydrofuran with
reactant acid chloride 2, sulfonyl chloride 3,
chloroformate 4 or carbamoylchloride 5_, employing a molar
ratio of reactant:amine 1 within the range from about S:1
to about 1:5, optionally in the presence of an acid
scavenger such as triethylamine, diisopropylethylamine,
pyridine, or polyvinylpyridine, forms compounds ID, IA, IC
or IB of the invention.
Starting compound 1 can be prepared by methods known
in the art as outlined in Reaction Scheme IA below.
Reaction Scheme IA
0
Boc
H oII
R1~N~Ra + Cl~Br
1~ 14 15
Acylation O
Et3N, CHZC12, O°C-r.t.,36h. R1~ N~Br
R~
- 16 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
O Rl
1) Alkylation HZ N
Li~S, THF N R
.1~ + 1~ O
r.t., ldh
2) Deprotection
TFA/CHZC12
Compound 1_ is a novel compound provided that Rl and RZ are
as defined herein, but excludes alkyl, alkenyl, aryl,
arylalkyl, cycloalkyl or polycycloalkyl.
ComFounds of formula I of the invention wherein
RS\
X is R4-li° ~ y is O and R4 is ,N°
R6
Y
R5
that is \ N- C
R6 OI
can be prepared from the corresponding acid 6 by using the
sequence of steps outlined in Scheme II (Procedures A and
B) set out below.
Reaction Scheme II
Procedure A
R O Amidation ~5 H O R1.
R5 ' ~~',, OH 1) H~SR6 (21) RG~N N~ ~R2
,N
N~ DIC/HOAt/CH2C12/DMF O
O O O
2) SCX Purification
Procedure B I$
Amidation
1) FINRIRS (21)
EDAC/DMAP/CHZCIz
2) SCX Purification
Procedure A: For amines where R1 and/or R2 contain
additional basic nitrogens.
Procedure H: For amines where R~ and/or RZ contain no
additional basic nitrogens.
In Procedure A (for amines where R1 and/or RZ contain
additional basic nitrogens), a mixture of a solution of
- 17 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
amine 21 in an inert organic solvent such as THF,
methylenechloride or chloroform, a carbodiimide such as
diisopropylcarbodiimide (DIC) and 7-aza-1-hydroxy-
benzotriazole (HOAt) is reacted with acid 20, employing a
molar ratio of amine 2l: acid 20 within the range from about
5:1 to about 1:5, preferably at about l:l.l, to form a
reaction mixture which is purified via an SCX column to
separate out compound IB of the invention.
The DIC will be employed in a molar ratio to acid 20
within the range from about 5:1 to about 1:5, preferably at
about 1.6:1, and the HOAt will be employed in a molar ratio
acid 20 within the range from about 5:1 to about 1:5,
preferably at about 1.6:1.
In Procedure B (for amines where R1 and/or R2 contain
no additional basic nitrogens) a mixture of a solution of
amine 21 in an inert organic solvent such as THF ,
methylenechloride or chloroform,
ethyldimethylaminopropylcarbodiimide (EDAC) and
dimethylaminopyridine (DMAP) with acid 20, employing a
molar ratio of amine 2l: acid 20 within the range from about
5:1 to about 1:5, preferably at about 1.5:1, to form a
reaction mixture which is purified via a SCX column to
separate out compound IB of the invention.
The EDAC will be employed in a molar ratio to acid
20 within the range from about 5:1 to about 1.5, preferably
at about 1.5:1, and the DMAP will be employed in a molar
ratio to acid 20 within the range from about 5:1 to about
1:5, preferably at about 1.5:1.
Starting compound 20 can be prepared by methods
known in the art as outlined in Reaction Scheme IIA.
- 18 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
React3.oa Scheme IIA
Alkylation
O O
O" N Br
OEt
O '--
LHMDS
THF
Deprotection O
H O HZ N~ 'OEt
'O"N N~ 'OEt 1 M HC ~1
O
O O Et20
RT
?
Coadensation O
H H
N N OEt
NCO ~ ~ ~ N
/ O O
ET3N
THF
RT
Saponification O
or Hydrolysis H H
N" N ~ 'OH
2 M NaOH ~ ~N
/ O O
THF
EtOH 2g
RT
1~
Compounds of formula I of the invention wherein
RS
X is R4-C- Y is O or S, and R4 is \N-
R6
Y
R~
that is _ _
R6 N ~~ or 5 N
O R
S
can be prepared from the corresponding amine 1 by using the
sequence of steps outlined in Scheme III set out below.
- 19 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Reaction Scheme III
Condensation
O R1
O R1 5 5 H H
1. R NCO or R NCS ~ N N/~
H2Zfi.. N~~RZ (~.~.) (~) RS N R
II O O
O CHZC1Z,RT,6h.
IB'
1 2. aminomethyl=
polystyreae (32) or
resin, 16h.,RT
O R1
H H
RS. N~ N/i, N~ ~ 2
II R
S O
IB"
Reaction of amine 1 (in an inert organic solvent
such as dichloromethane, chloroform or tetrahydrofuran)
with reactant 30 or 31 employing a molar ratio of 30 or
3l: amine _1 within the range of from about 5:1 to about 1:5,
followed by treatment with aminomethylpolystyrene (32),
affords the compound of the invention IB' or IB".
The compounds of the present invention are
inhibitors of the activated coagulation serine protease
known as Factor Xa and thus are useful for the treatment or
prophylaxis of those processes which involve the production
and/or action of Factor Xa. Thus, the compounds of the
invention are useful in the treatment or prevention of
thrombotic events associated with coronary artery and
cerebrovascular disease. This includes a number of
thrombotic and prothrombotic states in which the
coagulation cascade is activated which include, but are not
limited to, formation of atherosclerotic plaques, venous or
arterial thrombosis, coagulation syndromes, ischemia and
angina (stable and unstable), deep vein thrombosis (DVT),
disseminated intravascular coagulopathy, Kasabach-Merritt
syndrome, pulmonary embolism, myocardial infarction,
cerebral infarction, cerebral thrombos:_s, atrial
- 20 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
fibrillation, cerebral embolism, thromboembolic
complications of surgery (such as hip replacement,
introduction of artificial heart valves and endarterectomy)
and peripheral arterial occlusion. The compounds of the
invention are also useful as inhibitors of blood
coagulation such as during the preparation, storage and
fractionation of whole blood.
The present compounds may also be useful in
maintaining whole and fractionated blood in the fluid phase
such as required for analytical and biological testing.
Examples include, but are not limited to, ex vivo platelet
and other cell function studies, bioanalytical procedures
and quantitation of blood-containing components.
In addition, the compounds of the present invention
may be useful to prevent restenosis following arterial
injury induced by endogenous (rupture of an atherosclerotic
plaque) or exogenous (invasive cardiological procedure such
as vessel wall injury resulting from angioplasty) events.
The compounds of the present invention may also be
used as an anticoagulant in extracorpeal blood circuits,
such as those necessary in dialysis and surgery (such as
coronary artery bypass surgery).
In addition, the compounds of the present invention
may be useful for maintaining blood vessel patency in
conjunction with vascular surgery including bypass
grafting, arterial reconstruction, atherectomy, vascular
graft and stent patency, organ, tissue and cell
implantation and transplantation.
The compounds of the present invention may be useful
for the treatment of heparin-intolerant patients, including
those with congenital and acquired antithrcmbin III
deficiencies, heparin-induced thrombocytopenia, and those
with high levels of polymorphonuclear granulocyte elastase.
The compounds of the present invention may also be
useful for the treatment of inflammato?-y diseases and the
prevention of septic shock and vascular damage due to
bacterial and/or ~Tiral infections.
- 21 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
The compounds of the present invention may also be
useful in the treatment of malignancies, prevention of
metastases, prevention of prothrombotic complications of
cancer, and as an adjunct to chemotherapy.
The compounds of the present invention may also be
used in combination with prothrombolytic agents, such as
tissue plasminogen activator (natural or recombinant),
streptokinase, reteplase, activase, lanoteplase, urokinase,
prourokinase, anisolated streptokinase plasminogen
activator complex (ASPAC), animal salivary gland
plasminogen activators, and the like. The compounds of the
present invention may act in a synergistic fashion with one
or more of the above agents to prevent reocclusion
following a successful thrombolytic therapy and/or reduce
the time to reperfusion. The compounds of the present
invention may also allow for reduced doses of the
thrombolytic agent to be used and therefore minimize
potential hemorrhagic side-effects.
The compounds of the present invention may also
inhibit other serine proteases, for example, thrombin,
Factor VIIa, urokinase-type plasminogen activator
(urokinase), tryptase and/or tzypsin. As a result, these
compounds may additionally be useful as angiogenesis
inhibitors in the treatment of cancer, as antiinflammatory
agents particularly in the treatment of chronic asthma and
in the treatment or prevention of allergic rhinitis,
rheumatoid arthritis, inflammatory bowel disease,
psoriasis, and conjunctivitis and in the treatment or
prevention of pancreatitis.
The compounds of the present invention may also be
used in combination with other antithrombotic or
anticoagulant drugs such as thrombin inhibitors, platelet
aggregation inhibitors such as clopidogrel, ticlopidine,
PAI-1 inhibitors such as XR-330 and T-686, inhibitors of
a-2-antiplasmin such as anti-a-2-antiplasmin antibody and
thromboxane receptor antagonists (such as ifetroban),
prostacyclin mimetics, phosphodiesterase (PDE) inhibitors,
- 22 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
such as dipyridamole or cilostazol, PDE inhibitors in
combination with thromboxane receptor
antagonists/thromboxane A synthetase inhibitors (such as
picotamide), serotonin-2-receptor antagonists (such as
ketanserin), fibrinogen receptor antagonists, aspirin,
hypolipidemic agents, (such as HMG-CoA reductase inhibitors
for example pravastatin or simvastatin, or microsomal
triglyceride transport protein inhibitors such as disclosed
in U.S. Patent Nos. 5,739,135, 5,712,279 and 5,760,246),
antihypertensive agents, (such as angiotensin converting
enzyme inhibitors, for example, captopril, lisinopril or
fosinopril, angiotensin II receptor antagonists, for
example, irbesartan, losartan or valsartan, and ACE/NEP
inhibitors, for example omapatrilat), PDE inhibitors in
combination with aspirin, ifetroban, picotamide, ketanserin
or clopidogrel and the like.
The compounds of the invention can be administered
orally or parenterally such as subcutaneously or
intravenously, as well as by nasal application, rectally or
sublingually to various mammalian species known to be
subject to such maladies, e.g., humans, cats, dogs and the
like in an effective amount within the dosage range of
about 0.1 to about 100 mg/kg, preferably about 0.2 to about
50 mg/kg and more preferably about 0.5 to about 25 mg/kg
(or from about 1 to about 2500 mg, preferably from about 5
to about 2000 mg) on a regimen in single or 2 to 4 divided
daily doses.
The active substance can be utilized in a
composition such as tablet, capsule, solution or suspension
or in other type carrier materials such as transdermal
devices, iontophoretic devices, rectal. suppositories,
inhalant devices and the like. The composition or carrier
will contain about 5 to about 500 mg per unit of dosage of
a compound or mixture of compounds of formulas I, IA., IB,
IC and ID. They may be compounded in conventional matter
with a physiologically acceptable vehicle or carrier,
- 23 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
excipient, binder, preservative, stabilizer, flavor, etc.,
as called for by accepted pharmaceutical practice.
The following working Examples represent preferred
embodiments of the present invention.
Example 1
O N ~ /N
H
N~NIn N O
~\\(
O
A.
O
H
~O~N~~, N~ 'OEt
(\ ~O ~O
To a solution of 8.3 g (36 mmol,.l eq) of
0
a
~o~N~,,
II0
compound in 40 mL of dry THF was added
dropwise 72 mL (72 mmol, 2 eq) of a 1 M solution of lithium
hexamethyldisilazide (LHMDS) in THF over 1 h. After 10
min, a solution of 4.4 mL (40 mmol, 1.1 eq) of
bromoethylacetate in 10 mL of dry THF was added dropwise
over 10 min and the resulting reaction mixture was stirred
at RT for 17 h. The reaction mixture was diluted with
diethyl ether (100 mL) and washed twice with 5~ KHS04
(aq.), followed by saturated NaHC03 and brine. The organic
solution was dried (MgS04) and concentrated to afford 11.3
g (99~) of title compound as a viscous yellow brown oil.
1H and 13C NMR spectra were consistent with the desired
product and indicated the m~.-Trial was pure except for a
small amount of hexamethyldisilazane. The material was
used without further purification.
- 24 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
B.
0
H2Py,,, N~ 'OEt
I~IO
To a solution of 7.8 g (25 mmol, 1 eq) of Part A
compound in 10 mL of diethyl ether was added 50 mL (50
mmol, 2 eq) of a 1 M solution of hydrochloric acid in
diethyl ether. The reaction mixture was stirred at RT for
18 h. The resulting heterogeneous reaction mixture was
concentrated and the oily residue was triturated with
ether, dissolved in methanol and concentrated to afford 5.1
g (81~) of title compound as a yellow solid. 1H and 13C
NMR spectra were consistent with the desired product.
C.
O ,OEt
~/'~,~(~H
~N/~, N O
~,('0
IS
To a solution of 5.1 g (20 mmol, 1 eq) of Part B
compound in 120 mL of dry THF was added 5.7 mL (41 mmol, 3
eq) of triethylamine and 3.9 mL (30 mmol, 1.5 eq) of m-
tolylisocyanate. The reaction mixture was stirred at RT
for 18 h. The reaction mixture was concentrated and the
residue dissolved in methnol. An insoluble impurity was
removed by filtration and the crude product was again
concentrated. Flash chromatography (Si02) eluting with 9:1
CH2C12:ethyl acetate (EtOAc) afforded 3.3 g (48~) of title
compound as a light brown solid. 1H and 13C NMR spectra
were consistent with the desired product.
D.
0 off
H H _
N Nln N O
O
- 25 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
To a solution of 2.3 g (7 mmol, 1 eq) of Part C
compound in 30 mL of THF and 30 mL of EtOH was added 8.3 mL
(17 mmol, 2.5 eq) of 2 M sodium hydroxide in water. The
reaction mixture was stirred at RT for 18 h. The reaction
mixture was concentrated, the residue was dissolved in 20
mL of water and the pH was adjusted to 3 with 1 M HC1. The
resulting precipitate was collected by filtration, washed
with water (10 mL), washed with hexane (10 mL) and dried to
afford 1.7 g (82~) of title compound as a light yellow
solid. iH and 13C NMR spectra were consistent with the
desired product.
E.
O N ~ /N
H
N~N/~, N O
~\'(O
The title compound was prepared as part of an
automated solution phase run using a liquid handler
(Hamilton Microlab~ 2200) for reagent and starting material
addition using the following procedure.
To a 16 mm x 100 mm reaction tube was added via the
liquid handler 100 ~.L (3.9 mg, 0.036 mmol, 1 eq) of a stock
solution of 4-[2-(methylamino)ethyl]pyridine in THF, 300 ~.L
(7 mg, 0.057 mmol, 1.6 eq) of a stock solution of
diisopropylcarbodiimide in CH2C12, 300 ~.L (8 mg, 0.057
mmol, 1.6 eq) of a stock solution of 7-aza-1-hydroxy-
benzotriazole in DMF and 300 ~L (12 mg, 0.038 mmol, 1.05
eq) of a stock solution of Part D compound in CHZC12. The
tube was removed and mixed on an orbital shaker for 72 h.
The product was purified via solid phase extraction
using a Varian SCX cation exchange colu_~rz (1 g of sorbent
in 6 mL column, ~.3 meq/g) by the procedure outlined below:
- 26 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
1) Column conditioned with 2 x 7.5 mL of MeOH
(10 mL/min).
2) Reaction mixture (1 mL) loaded onto SCX column
(3 mL/min).
S 3) Column rinsed with 20 mL of MeOH (6 mL/min).
4) Column rinsed with 10 mL of 0.1 N ammonia in MeOH
(6 mL/min).
5) Product eluted with 8 mL of 2 N ammonia in MeOH into
a tared 16 x 100 tube (6 mL/min).
The product solution was concentrated using a speed
vac for 14 h to afford 17 mg of title compound (1090 as an
oil. Reverse phase analytical HPLC analysis indicated a
purity of 96~.
IS
MS (electrospray): m/z 438 (M+H).
Examples 2 to 4
Following the procedure of Example 1, the following
compounds of the invention were prepared.
Example Structure Mass Spec.
No. m/z (M+H)+
2 Q'' N Q Chiral 424
/ \ H N~N ,Nl
O
Chiral 4 3 8
O~- N H O ~ N
N
~./ H N~N
O
4 Chiral 47 9
Q, H H
/ \ N N N N
H ~J
0
- 27 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Example 5
Chiral
H N'~ N
O
Example 5 was prepared as part of an automated
solution phase run using a liquid handler (Hamilton
Microlab~ 2200) for reagent and starting material addition
using the following procedure.
To a 16 mm x 100 mm reaction tube was added via the
liquid handler 100 ~L (0.057 mmol, 1.5 eq) of a stock
solution of 1,2,3,6-tetrahydropyridine in THF, 300 ~.L of a
stock solution containing both ethyldimethylaminopropyl-
carbodiimide hydrochloride (0.057 mmol, 1.5 eq) and
dimethylaminopyridine (0.057 mmol, 1.5 eq) in CH2C12 and
600 ~.t,L ( 0 . 038 mmol, 1. 0 eq) of a stock solution of Example
1 Part D compound in CH2C12. The tube was removed and
mixed on an orbital shaker for 72 h.
The product was purified via solid phase extraction
using a Varian SCX cation exchange column (1 g of sorbent
in 6 mL column, 0.3 meq/g) by the procedure outlined below.
1) Column conditioned with 15 of MeOH (10 mL/min).
2) Reaction mixture (1 mL) was loaded onto SCX
column (3 mL/min) and effluent was collected into
a tared 16 mm x 100 mm tube.
3) Column rinsed with 6 mL of MeOH and collected
into tared tube (6 mL/min).
The product solution was concentrated using a speed
vac for 14 h to afford 14 mg of Example 5 compound (94~) as
an oil. Reverse phase analytical HPLC analysis indicated a
purity of 97~.
MS (electrospray): m/z 385 (M + H).
- 28 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Example 6 to 10
Following the procedure of Example 5, the following
compounds of the invention were prepared.
Example Structure Mass Spec.
No. m/z (M+H)+
O'' H Chiral 4 0 3
/ ~ ~-N O
H N'~ N
H"
O
HO
7 O Chiral 3 8 9
</ ~~-- ~'NHO
V H N~N~OH
O
8 O~ N H O Chiral 3 8 7
~N
H N'~ N
O
~.~ H H dal 427
~ ~-~- N O
~H N~N
O
O H Chiral 3 7 3
N NH O
N ~ N
I~O
5
Example 11
0
x x
N~ N/~, N~ N
ISI I IO
- 29 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
A.
0
Br
GN
0
To a solution of cl-c-cat-Br (55 g, 0.35 mol) in 400
mL of CH2C12 was added dropwise a solution of pyrrolidine
(25 g, 0.35 mol) and triethylamine (42.4 g, 0.42 mol) in
100 mL of CH2C12 at 0°C under argon over 5h. The reaction
mixture was allowed to slowly warm to room temperature with
stirring for an additional 14h. The reaction mixture was
washed with H20 (250 mLx3), 0.5 N HC1 (250 mL), saturated
NaCl (300 mLx3), and dried (Na2S04) and concentrated. The
resulting residue was purified by flash column
chromatography (elute with 1~ MeOH in CH2C12) to yield
title compound (46.1 g, 68.60 as off-brown solid.
Found: MH+: 191.7.
B.
0
HarY~,. N~'N
~O
O
BocBIJ~,,,
To a solution of (8.0 g, 35.1 mmol) in
600 mL of THF was added dropwise 70.2 mL of LHMDS (1.0 M in
THF) at room temperature under argon over 3h, followed by
adding dropwise a solution of Part B compound (7.4 g, 38.6
mmol) in 100 mL of THF over 2h. The reaction mixture was
stirred for an additional 14h at room temperature. The
reaction mixture was poured into 5~ KHS04 (300 mL), and
added ethylacetate (AcOEt) (300 mL). The organic layer was
washed with 5~ KHS04 (300 mL), saturated NaHC03 (300 mLx2),
H20 (300 mLx3), and dried (Na2S04) and concentrated to
yield title compound (11.1 g, 93.20 as yellow oil.
Found: MH+: 340.1.
- 30 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
C.
~HCl O
H2Z'Yi.. N~ N
IIO
To a solution of Part B compound (4.1 g, 12.1 mmol)
in 100 mL of CHzCl2 was added 100 mL of HC1 in Et20 (1.0 M)
at room temperature. The mixture was stirred for 14h. The
solvent was removed in vacuum and the resulting residue was
purified by ion-exchange resin column chromatography (elute
with 2~ ammonia in MeOH) to yield title compound (1.91 g,
66.00 as yellow oil. Found: MH+: 240.2.
D.
1$
0
H H
\ N~N/i, N N
ISI O
To a solution of Part C compound (90.8 mg, 0.38
mmol) in 3 mL of CHZC12 was added a solution of m-tolyl-
isothiocyanate (51.5 mg, 0.345 mmol) in 2 mL of CH2C12 at
room temperature. The reaction mixture was stirred for
0.5h and concentrated in vacuum. The resulting residue was
purified by flash column chromatography (eluted with 1~
MeOH in CH2C12) to yield title compound (130 mg, 97.00 as
white solid. Found: MH+: 389.1.
Examples 12 to 16
The following compounds of the invention were
prepared employing procedures described in Example 11.
- 31 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Example Structure Mass Spec.
No. m/z (M+H)+
12 . I ~ chiral 375
0
HN N ~N
~H N II
S ~ O
13 403
Chiral
O.
o N
H
N
H
N
S
O
14 chiral 420
o vN*~o_
O N
H
N
H ~N
S H
15 ~o I ~ 405
Chiral
o
N ~N
~H N II
S ~ O
16 chiral 400
0
O N
\\
HN
H
S
H
- 32 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Example 17
/ o
A
\ Nh, N N
O O
To 13.9 mg of polyvinylpyridine (9.0 mmol/g) was
added 0.400 mL of solution of Example 13, Part C compound
in dichloromethane (0.158 mmol/mL) and 0.400 mL of solution
of o-toluoyl chloride in dichloromethane 0.173 mmol/mL).
The mixture was shaken for 4h. at room temperature. The
reaction mixture was then added to 31.4 mg of
aminomethylpolystyrene (1.0 mmol/g) and 0.200 mL of
dichloromethane. The mixture was shaken for 14h at room
temperature. The reaction solution was collected and the
residue resins were washed with dichloromethane (0.400 mL).
The combined reaction solutions were dried by speed vacuum
to yield title compound (17.1 mg, 69~). Found: MH+: 358.1.
Examples 18, 19
The following compounds were prepared employing the
procedure as described in Example 17.
Example Structure Mass Spec.
No.
18 chiral 3 7 4
o
N
~~/~,
\ O' _ N N
O
19 chiral 430
0
g n
O N-S
II
~iIFiO
N~N
O
- 33 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
Examples 20 to 57
The following compounds were prepared employing
procedures as described in previous Examples.
Example Structure Mass Spec.
No. m/z (M+H)+
20 s chiral 409
N
N
O~ O
Cl
21 -chiral -. 405
CH3
O N N ~ ~ O
~~IH S
N 11 N
O
22 chiral 443
c1 / c1
0
N\/N N \\
H O
S
23 chiral 403
--i 3
N
O
S CH3
CNN
0
24 chiral 425
O
N
O N
~il H S
CNN
O
- 34 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
2 5 Chiral 3'7'7
0
O N
N
F
26 Chiral 437
o''
~ ~N
N
N
O' / O
~I / Br
27 chiral 409
S
0
N N~ N
O / C1
2 8 chiral 3 9 3
0
GN
s
o N
N
F
29 Chiral 409
0
GN
S
o N-
N
C1
- 35 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
3 0 chiral 443
0
GN
S
O N
N
F
F
F
3 1 chiral 393
s"
~.H
N
N
O~ O
~/ / F
32 chiral 405
H3C°O
N
o N
.n//H S
N 1\ N V
O
33 chiral 403
H3
N \ ~ CH3
N
~i~ H S
CNN
O
34 chiral 423
CHg
Cl
O
N N N
H O
S
- 36 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
35 chiral 443
s
0 ~
N H NI -N
O / C1
Cl
3 6 chiral 4 0 0
N
O~ o
N
N
H ~-- N
-N
37 chiral 439
0
~~N H
S
O N
N
CHg
cl ~ ~ o
38 Chiral 501
0
~N~
H g
O N
N
I
39 Chiral 481
H3C~ O
N
O N
N N ~IH
O
- 37 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
40 Chiral 433
0
,
CHg
N
O ~H~
S
CN 1\ N
O
41 chiral 417
0
CH3
N
O N--
~~H S
CN ~ N
O
42 Chiral 419
Hg C° O
N
O N''~
d H g CHg
CN 11 N
O
43 chiral 477
Cl g g
/ \
g
O
N"N I N
H o
s
44 Chiral 403
CH3
N \
N
S
N ~ N~.
c
O
- 38 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
45 Chirai 454
s
H ~--N Cl
N
N
O~ O
NOZ
4 6 chl=al 42 0
0
GN~G- x
s
O N J/
~N
N02
4~ Chiral 434
s
N
N
O / ~ NOZ
CS3
48 chiral 450
~~ N H3 O
~N
N
O' / O
~I/ NOZ
39
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
49 chiral 450
s
0
~N N~N
~/~ p / ~NOZ
H3G~ O
0 chiral 3 7 6
N
O
N N N
g O
S
51 chiral 393
/'o
N
N N
O ~ N
s o
52 chiral 415
p N~ \ /
~~ta s
CN 11 N
p
53 chiral 419
H3
CH3
O N \\ \ ~ O
~~IH S
CN ~ N
O
54 chiral 481
0 ,~/
N N N' \\
g O
S
- 40 -
CA 02361919 2001-08-O1
WO 00/47563 PCT/US00/01859
55 Chiral 437
0
0
O N --~
N
Br
56 chiral 387
o~ o
N
N
$ ~- N
O
57 chiral 427
ci / ci
0
N N N
H O
O
58 chiral 429
H H o
A _N
~ N ~N~~~ / ~''N
/ S O
O
59 chiral 413
H H O
w -N
~ N~N~~~ / ~1'N
O O
O
- 41 -