Note: Descriptions are shown in the official language in which they were submitted.
CA 02362390 2001-08-07
WO 00/47578
PCT/US99/18065
HETEROARYL AMIDINES, METHYLAMIDINES AND GUANIDINES AS PROTEASE INHIBITORS
Background of the Invention
Field of the Invention
The present invention relates to novel heteroaryl compounds that function
as enzyme inhibitors, and particularly to a new class of non-peptidic
inhibitors of
proteolytic enzymes such as urokinase (uPa).
Related Art
Proteases are enzymes that cleave proteins at single, specific peptide
bonds. Proteases can be classified into four generic classes: serine, thiol or
cysteinyl, acid or aspartyl, and metalloproteases (Cuypers et al., J. Biol.
Chem.
257:7086 (1982)). Proteases are essential to a variety of biological
activities,
such as digestion, formation and dissolution of blood clots, reproduction and
the
immune reaction to foreign cells and organisms. Aberrant proteolysis is
associated with a number of disease states in man and other mammals. The
human neutrophil proteases, elastase and cathepsin G, have been implicated as
contributing to disease states marked by tissue destruction. These disease
states
include emphysema, rheumatoid arthritis, corneal ulcers and glomerular
nephritis.
(Barret, in Enzyme Inhibitors as Drugs, Sandler, ed., University Park Press,
Baltimore, (1980)). Additional proteases such as plasmin, C-1 esterase, C-3
convertase, urokinase and tissue-type plasminogen activators, acrosin, and
kallikreins play key roles in normal biological functions of mammals. In many
CA 02362390 2001-08-07
WO 00/47578
-2-
PCT/US99/18065
instances, it is beneficial to disrupt the function of one or more proteolytic
enzymes in the course of therapeutically treating a mammal.
Serine proteases include such enzymes as elastase (human leukocyte),
cathepsin G, plasmin, C-1 esterase, C-3 convertase, urokinase and tissue-type
plasminogen activators, acrosin, chymotrypsin, trypsin, thrombin, factor Xa
and
kallikreins.
Human leukocyte elastase is released by polymorphonuclear leukocytes
at sites of inflammation and thus is a contributing cause for a number of
disease
states. Cathepsin G is another human neutrophil serine protease. Compounds
with the ability to inhibit the activity of these enzymes are expected to have
an
anti-inflammatory effect useful in the treatment of gout, rheumatoid arthritis
and
other inflammatory diseases, and in the treatment of emphysema. Chymotrypsin
and trypsin are digestive enzymes. Inhibitors of these enzymes are useful in
treating pancreatitis. Inhibitors of urokinase plasminogen activator are
useful in
treating excessive cell growth disease states, such as benign prostatic
hypertrophy,
prostatic carcinoma and psoriasis.
Urokinase (urinary-type plasminogen activator or uPA; International
Union of Biochemistry Classification Number: EC3.4.21.31) is a proteolytic
enzyme which is highly specific for a single peptide bond in plasminogen. It
is
a multidomain serine protease, having a catalytic "B" chain (amino acids (aa)
144-411), and an amino-terminal fragment ("ATF", as 1-143) consisting of a
growth factor-like domain (4-43) and a Kringle domain (aa 47-135). The uPA
Kringle domain appears to bind heparin, but not fibrin, lysine, or
aminohexanoic
acid. The growth factor-like domain bears some similarity to the structure of
epidermal growth factor (EGF) and is thus also referred to as "EGF-like"
domain.
The single chain pro-uPA is activated by plasmin, cleaving the chain into a
two-
chain active form that is stabilized by a disulfide bond.
Cleavage of the peptide bond in plasminogen by urokinase ("plasminogen
activation") results in the formation of a potent general protease, plasmin.
Many
cell types use urokinase as a key initiator of plasmin-mediated proteolytic
degradation or modification of extracellular support structures (e.g., the
CA 02362390 2001-08-07
WO 00/47578
-3-
PCT/US99/18065
extracellular matrix (ECM) and the basement membrane (BM)). Cells exist,
move, and interact with each other in tissues and organs within the physical
framework provided by the ECM and BM. Movement of cells within the ECM
or across the BM requires local proteolytic degradation or modification of
these
structures, allowing cells to "invade" into adjacent areas, that were
previously
unavailable.
Central to the ability of urokinase to mediate cellular migration and
invasiveness is the existence of specific high affinity urokinase receptors
(uPARs)
which concentrate urokinase on the cell surface, leading to the generation of
locally high plasmin concentrations between cells and ECM or BM (Blasi, F., et
al., Cell Biol. 104:801-804 (1987); Roldan, A.L., et al., EMBO J. 9:467-74
(1990)). The binding interaction is apparently mediated by the EGF-like domain
(Rabbani, S.A., et al., J. Biol. Chem. 267:14151-56 (1992)). Cleavage of
pro-uPA into active uPA is accelerated when pro-uPA and plasminogen are
receptor-bound. Thus, plasmin activates pro-uPA, which in turn activates more
plasmin by cleaving plasminogen. This positive feedback cycle is apparently
limited to the receptor-based proteolysis on the cell surface, since a large
excess
of protease inhibitors is found in plasma, including a2 antiplasmin, PAI-l and
PAI-2. High plasmin concentrations between invasive cells and ECM or BM are
necessary in order to overcome inhibitory effect of these ubiquitous plasmin
inhibitors. Thus, it is cell surface receptor-bound urokinase, and not simply
free
urokinase secreted by cells, which plays the predominant role in initiating
cellular
invasiveness.
Plasmin can activate or degrade extracellular proteins such as fibrinogen,
fibronectin, and zymogens, including matrix metalloproteinases. Plasminogen
activators thus can regulate extracellular proteolysis, fibrin clot lysis,
tissue
remodeling, developmental cell and smooth muscle cell migration, inflammation,
and metastasis. Cellular invasiveness initiated by urokinase is central to a
wide
variety of normal and disease-state physiological processes (reviewed in
Blasi, F.,
et al., J. Cell Biol.104:801-804 (1987); Dano, K., et al. , Adv. Cancer Res.
44:139-
266 (1985); Littlefield, B.A.,Ann. N. Y. Acad. Sci. 622:167-175 (1991);
Saksela,
CA 02362390 2001-08-07
WO 00/47578
_4_
PCT/US99/18065
O., Biochim. Biophys. Acta 823:35-65 (1985); Testa, J.E., and Quigley, J.P.,
Cancer Metast. Rev. 9:353-367 (1990)). Such processes include, but are not
limited to, angiogenesis (neovascularization), bone restructuring, embryo
implantation in the uterus, infiltration of immune cells into inflammatory
sites,
ovulation, spermatogenesis, tissue remodeling during wound repair, restenosis
and organ differentiation, fibrosis, local invasion of tumors into adjacent
areas,
metastatic spread of tumor cells from primary to secondary sites, and tissue
destruction in arthritis. Inhibitors of urokinase therefore have mechanism-
based
anti-angiogenic, anti-arthritic, anti-inflammatory, anti-restenotic, anti-
invasive,
anti-metastatic, anti-osteoporotic, anti-retinopathic (for angiogenesis-
dependent
retinopathies), contraceptive, and tumoristatic activities. Inhibitors of
urokinase
are useful agents in the treatment of a variety of disease states, including
but not
limited to, benign prostatic hypertrophy, prostatic carcinoma and psoriasis.
Beneficial effects of urokinase inhibitors have been reported using anti
urokinase monoclonal antibodies and certain other known urokinase inhibitors.
For instance, anti-urokinase monoclonal antibodies have been reported to block
tumor cell invasiveness in vitro (Hollas, W., et al., Cancer Res. 51:3690-
3695,
(1991); Meissauer, A., et al., Exp. Cell Res. 192:453-459 (1991)), tumor
metastasis and invasion in vivo (Ossowski, L., J. Cell Biol. 107:2437-2445
( 1988); Ossowski, L., et al., J. Cancer Res. 51:274-81 ( 1991 )), and
angiogenesis
in vivo (Jerdan, J. A., et al., J. Cell Biol. 1153 Pt 2J:402a (1991)). In
addition,
amiloride, a known urokinase inhibitor of only moderate potency, has been
reported to inhibit tumor metastasis in vivo (Kellen, J.A., et al., Anticancer
Res.
8:1373-1376 (1988)) and angiogenesis/capillary network information in vitro
(Alliegro, M.A., et al., J. Cell Biol. 1153 Pt 2J:402a (1991)).
Urokinase plays a significant role in vascular wound healing and arterial
neointima formation after injury, most likely affecting cellular migration.
Urokinase mediates plasmin proteolysis, which in turn promotes vascular wound-
healing and associated neointima formation (Carmeliet et al., Circ. Res.
81:829-
839 (Nov. 1997), Lupu et al., Fibrinolysis 10 Supp 2:33-35 (1996)). A viral
serine proteinase inhibitor, SERF-1, has been employed to reduce plaque
CA 02362390 2001-08-07
WO 00/47578
-5-
PCT/US99/18065
formation after primary balloon angioplasty in rabbits. This activity has been
attributed to the inhibition by SERF-1 of cellular proteinases, such as
plasmin or
urokinase (Lucas et al., Circulation 94:2890-2900 (1996)).
A need continues for non-peptidic compounds that are potent and
selective urokinase inhibitors, and which possess greater bioavailability and
fewer
side-effects than currently available urokinase inhibitors. Accordingly, new
classes of potent urokinase inhibitors, characterized by potent inhibitory
capacity
and low toxicity, are potentially valuable therapeutic agents for a variety of
conditions.
Summary of the Invention
1 S The present invention is broadly directed to the use of heteroaryl
amidines, methylamidines and guanidines having Formula 1 (below) as protease
inhibitors, preferably as urokinase inhibitors.
Compounds of the present invention exhibit anti-urokinase activity via
direct, selective inhibition of urokinase, or are intermediates useful for
forming
compounds having such activity. Compounds of the present invention inhibit
urokinase and are, therefore, useful anti-angiogenic, anti-arthritic, anti-
inflammatory, anti-restenotic, anti-invasive, anti-metastatic, anti-
osteoporotic,
anti-retinopathic (for angiogenesis-dependent retinopathies), contraceptive,
and
tumoristatic treatment agents. For example, such treatment agents are useful
in
the treatment of a variety of disease states, including but not limited to,
benign
prostatic hypertrophy, prostatic carcinoma, tumor metastasis and psoriasis.
Also provided are methods to inhibit extracellular proteolysis, methods
to treat benign prostatic hypertrophy, prostatic carcinoma, tumor metastasis,
psoriasis, and other conditions by administering the compound of Formula 1.
A number of the heteroaryl compounds described herein are novel
compounds. Therefore, the present invention is also directed to novel
compounds
of Formula I.
CA 02362390 2001-08-07
WO 00/47578
-6-
PCT/US99/18065
Further provided are pharmaceutical compositions comprising a
compound of Formula I and one or more pharmaceutically acceptable carriers or
diluents and said pharmaceutical compositions further comprising a
thrombolytic
agent such as tissue plasminogen activator and streptokinase.
Further provided are methods of synthesizing compounds of Formula I.
Detailed Description of the Preferred Embodiments
The present invention is broadly directed to a method of inhibiting
proteases, particularly serine proteases, by contacting a serine protease with
a
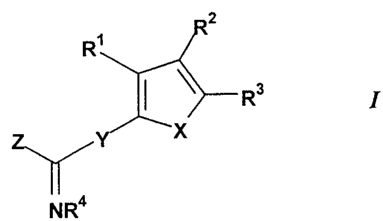
compound of the general Formula 1:
2
R~
~ y R3
1
X
NR4
or a solvate, hydrate or pharmaceutically acceptable salt thereof; wherein:
X is O, S orNR', where R' is hydrogen, alkyl, aralkyl, hydroxy(CZ_4)alkyl,
or alkoxy(CZ_4)alkyl;
Y is a direct covalent bond, CHz or NH;
Z is NRSR6, hydrogen or alkyl, provided that Y is NH whenever Z is
hydrogen or alkyl;
R' is hydrogen, amino, hydroxy, halogen, cyano, C,_4 alkyl or -CHzR,
where R is hydroxy, amino or C,_3 alkoxy;
Rz and R3 are independently:
i. hydrogen;
ii. halogen;
iii. hydroxy;
iv. nitro;
v. cyano;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
S vi. amino, monoalkylamino, dialkylamino, monoarylamino,
diarylamino, monoalkylmonoarylamino, monoaralkylamino, diaralkylamino,
monoalkylmonoaralkylamino, monoheterocycleamino, diheterocycleamino,
monoalkylmonoheterocycleamino, alkoxycarbonylamino,
aralkoxycarbonylamino, aryloxycarbonylamino, alkylsulfonylamino,
aralkylsulfonylamino, aralkenylsulfonylamino, arylsulfonylamino,
heteroarylsulfonylamino, di(aralkylsulfonyl)amino, di(aralkenylsulfonyl)amino,
di(arylsulfonyl)amino, or di-(heteroarylsulfonyl)amino, formylamino,
alkanoylamino, alkenoylamino, alkynoylamino, aroylamino, aralkanoylamino,
aralkenoylamino, heteroaroylamino, heteroaralkanoylamino, H(S)CNH-, or
thioacylamino, wherein any of the aryl or heteroaryl containing groups can be
optionally substituted on the aromatic ring and wherein any of the heterocycle
containing groups can be optionally ring substituted;
vii. aminocarbonyl, monoalkylaminocarbonyl, dialkylaminocarbonyl,
acyl, aminoacyl, monoarylaminocarbonyl, diarylaminocarbonyl or
monoalkylmonoarylaminocarbonyl;
viii. aminothiocarbonyl, monoalkylaminothiocarbonyl,
dialkylaminothiocarbonyl, thioacyl or aminothioacyl;
ix. aminocarbonylamino, mono- and dialkylaminocarbonylamino,
mono- and diarylaminocarbonylamino, or mono- and
diaralkylaminocarbonylamino;
x. aminocarbonyloxy, mono- and dialkylaminocarbonyloxy, mono-
and diarylaminocarbonyloxy, mono- and diaralkylaminocarbonyloxy;
xi. aminosulfonyl, mono- and dialkylaminosulfonyl, mono- and
diarylaminosulfonyl, or mono- and diaralkylaminosulfonyl;
xii. alkoxy, or alkylthio, wherein the alkyl portion of each group may
be optionally substituted,
xiii. aralkoxy, aryloxy, heteroaryloxy, aralkylthio, arylthio, or
heteroarylthio, wherein the aryl portion of each group can be optionally
substituted;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_g_
xiv. alkylsulfonyl, wherein the alkyl portion can be optionally
substituted;
xv. aralkylsulfonyl, aralkenylsulfonyl, arylsulfonyl or
heteroarylsulfonyl, wherein the aryl portion of each group can be optionally
substituted;
xvi. alkenyl, or alkynyl;
xvii. optionally substituted aryl;
xviii. optionally substituted alkyl;
xix. optionally substituted aralkyl;
xx. optionally substituted heterocycle; or
xxi. optionally substituted cycloalkyl; and
R4, RS and R6 are independently hydrogen, C,_4 alkyl, aryl, hydroxyalkyl,
aminoalkyl, monoalkylamino(Cz_,o)alkyl, dialkylamino(Cz_,o)alkyl,
carboxyalkyl,
cyano, amino, alkoxy, or hydroxy, or -COZR"", where
R"" is alkyl, cycloalkyl, phenyl, benzyl,
O O Rh
~O
or
O Rg O
Rd Re
where Rd and Re are independently hydrogen, C,_6 alkyl,
CZ_6 alkenyl or phenyl, Rf is hydrogen, C,_6 alkyl, CZ_6
alkenyl or phenyl, R~ is hydrogen, C,_6 alkyl, CZ_6 alkenyl
or phenyl, and R" is aralkyl or C,_6 alkyl.
The present invention is also directed to novel compounds of Formula 1,
where X, Y and R'-R6 are as defined above;
provided that at least one of RZ or R3 is selected from the group consisting
of:
(a) an optionally substituted alkyl group, preferably C,-C6 alkyl, more
preferably C,-C3;
(b) alkoxy, aryloxy, alkylthio or arylthio, any of which is optionally
substituted;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-9-
(c) optionally substituted C6-C,4 aryl, or optionally substituted aralkyl,
except that R3 is not nitrophenyl or aminophenyl, when R' and RZ are both
hydrogen or methyl;
(d) optionally substituted heterocycle; and
(e) optionally substituted cycloalkyl.
When an alkyl-containing group, heterocyclic-containing group or aryl-
containing group of RZ or R3 is optionally substituted, the optional
substituents
can be 1 to 4 non-hydrogen substituents, provided that the resulting compound
is
stable. Values of optional substituents on alkyl groups are halogen, hydroxy,
thiol, amino, monoalkylamino, dialkylamino, formylamino, aminoiminomethyl,
acylamino, aminoacyl, mono- or di- alkylaminocarbonyl, thiocarbonylamino,
thioacylamino, aminothiocarbonyl, alkoxy, aryloxy, aminocarbonyloxy, mono-
or di-alkylaminocarbonyloxy, mono- or diarylaminocarbonyloxy, mono- or
diaralkylaminocarbonyloxy, alkylsulfonyl, arylsulfonyl, aralkylsulfonyl,
alkylsulfonylamino, arylsulforiylamino, aralkylsulfonylamino,
alkoxycarbonylamino, aralkoxycarbonylamino, aryloxycarbonylamino, mono- or
di- alkylaminothiocarbonyl, aralkoxy, carboxy, carboxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, nitro, cyano, trifluoromethyl, alkylthio and arylthio.
Preferred values of optional substituents on an alkyl group are chloro,
hydroxy, amino, mono(C,_4)alkylamino, di(C,_4)alkylamino, formylamino, CZ_6
acylamino, aminocarbonyl, Cz_8 aminoacyl, C,_6 alkoxy, C6_,4 aryloxy, carboxy,
carboxy(C,_6)alkyl, CZ_g alkoxycarbonyl, nitro, cyano, trifluoromethyl, C,_6
alkylthio, C6_,4 arylthio, C,_6 alkylsulfonylamino, C~_,5
aralkylsulfonylamino, C6_,o
arylsulfonylamino, mono- or di(C,_6)alkylaminocarbonyloxy, mono- or di-
(C6_,o)arylaminocarbonyloxy, mono- or di(C.,_,5)aralkylcarbonyloxy, C,_6
alkoxycarbonylamino, C~-C,5 aralkoxycarbonylamino, and C6-C,o
aryloxycarbonylamino.
Preferred values of optional substituents on aryl-containing and
heterocyclic-containing groups include chloro, hydroxy, amino, mono(C,_4)
alkylamino, di(C,~)alkylamino, formylamino, CZ_6 acylamino, aminocarbonyl,
CZ_g
aminoacyl, C3_~ cycloalkyl, C,_6 alkyl, C,_6 alkoxy, C6_,4 aryloxy, carboxy,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-10-
carboxy(C,_6)alkyl, CZ_g alkoxycarbonyl, nitro, cyano, trifluoromethyl, C,_6
alkylthio, C6_,4 arylthio, C6_,4 aryl, substituted phenyl, tetrazolyl, thienyl
(further
optionally substituted by one, two or three of chloro, hydroxy, C,_4 alkyl,
C,_4
alkoxy, amino or carboxy), 3,4-methylenedioxy, 3,4-ethylenedioxy,
3,4-propylenedioxy, C,_6 alkylsulfonylamino, C~_,5 aralkylsulfonylamino, C,_6
arylsulfonylamino, C,_6 alkyl/sulfonyl, C6_,o arylsulfonyl, mono- or
di(C,_6)alkylaminocarbonyloxy, mono- or di- C6_,o arylaminocarbonyloxy, mono-
or di-(C~_,5)aralkylcarbonyloxy, C,_6 alkoxycarbonylamino, C~-C,5
aralkoxycarbonylamino, C6-Coo aryloxycarbonylamino, CZ_6 thioacylamino,
aminothiocarbonyl, and CZ_8 aminothioacyl.
Preferred values of R' include hydrogen, amino, hydroxy and fluoro.
A preferred value of RZ is Formula II:
R8
Ar 11
Rs
where Ar is phenyl, thiazolyl, thiazolinyl, oxazolyl, isothiazolyl,
isoxazolyl,
imidazolyl, pyridyl, pyrimidinyl, pyrazinyl, thienyl (thiophenyl), pyrrolyl,
oxazolinyl and benzothienyl.
Preferred values ofR3 include C,_4 alkyl (optionally substituted), halogen;
amino, acylamino, C,_6 alkylthio (such as methylthio or ethylthio), C,_6
alkoxy
(such as methoxy and ethoxy), trifluoromethyl, methylsulfonyl, and benzylthio.
A preferred value of X is divalent sulfur (S).
Preferred values of Y are a covalent bond or NH-, most preferably a
covalent bond.
Preferred values of R4, RS and R6 in Formula I are hydrogen, hydroxy,
cyano, C,_6 alkyl, or C,_6 alkoxy. Suitable values of R4, RS and R6 include
hydrogen, methyl, ethyl, propyl, n-butyl, hydroxy, methoxy, and ethoxy. In the
most preferred embodiments, R4, RS and R6 are each hydrogen.
Preferred values of R4, RS and R6 in Formula I also include prodrugs such
as -COzR''", where R'", in each instance, is preferably one of C,_4alkyl,
CQ_,cycloalkyl ~or benzyloxycarbonyl. Suitable values of R4, RS and R6 include
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-11-
hydrogen, methyl, ethyl, propyl, n-butyl, hydroxy, methoxy, ethoxy, cyano,
-COzCH3, -COzCHZCH3 and -COZCH~CH~CH3. In the most preferred
embodiments, R4, RS and R6 are each hydrogen.
Also preferred at R4, RS and R6 is the group -COZR"', where R'" is one of
° O Rh
or
~~O R9 O
Rdi~e
where Rd-R" are defined as above. When R4, RS and R6 are -COZRW, where RW is
one of one of these moieties, the resulting compounds are prodrugs that
possess
desirable formulation and bioavailability characteristics. A preferred value
for
each of Rd, Re and R~ is hydrogen, Rf is methyl, and preferred values for R"
include benzyl and tent-butyl.
Preferred values ofR' include hydrogen, C,_6 alkyl, C6_,o ar(C,_4)alkyl, and
Cz_6 hydroxyalkyl. Suitable values are hydrogen, methyl, ethyl, and benzyl.
The term "alkyl" as employed herein by itself or as part of another group
refers to both straight and branched chain radicals of up to 12 carbons, such
as
methyl, ethyl, propyl, isopropyl, butyl, t-butyl, isobutyl, pentyl, hexyl,
isohexyl,
heptyl, 4,4-dimethylpentyl, octyl, 2,2,4-trimethylpentyl, nonyl, decyl,
undecyl,
dodecyl.
The term "alkenyl" is used herein to mean a straight or branched chain
radical of 2-20 carbon atoms, unless the chain length is limited thereto,
including,
but not limited to, ethenyl, 1-propenyl, 2-propenyl, 2-methyl-1-propenyl,
1-butenyl, 2-butenyl, and the like. Preferably, the alkenyl chain is 2 to 10
carbon
atoms in length, more preferably, 2 to 8 carbon atoms in length most
preferably
from 2 to 4 carbon atoms in length.
The term "alkynyl" is used herein to mean a straight or branched chain
radical of 2-20 carbon atoms, unless the chain length is limited thereto,
wherein
there is at least one triple bond between two of the carbon atoms in the
chain,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-12-
including, but not limited to, acetylene, 1-propylene, 2-propylene, and the
like.
Preferably, the alkynyl chain is 2 to 10 carbon atoms in length, more
preferably,
2 to 8 carbon atoms in length, most preferably from 2 to 4 carbon atoms in
length.
In all instances herein where there is an alkenyl or alkynyl moiety as a
substituent group, the unsaturated linkage, i.e., the vinylene or acetylene
linkage
is preferably not directly attached to a nitrogen, oxygen or sulfur moiety.
The term "alkylthio" as employed herein by itself or as part of another
group refers to a straight or branched chain radical of 1 to 20 carbon atoms,
unless
the chain length is limited thereto, bonded to a sulfur atom, including, but
not
limited to, methylthio, ethylthio, n-propylthio, isopropylthio, and the like.
Preferably the alkylthio chain is 1 to 10 carbon atoms in length, more
preferably
1 to 8 carbon atoms in length.
The term "alkoxy" as employed herein by itself or as part of another group
refers to a straight or branched chain radical of 1 to 20 carbon atoms, unless
the
chain length is limited thereto, bonded to an oxygen atom, including, but not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy, and the like. Preferably
the
alkoxy chain is 1 to 10 carbon atoms in length, more preferably 1 to 8 carbon
atoms in length.
The term "cycloalkyl" as employed herein by itself or as part of another
group refers to cycloalkyl groups containing 3 to 9 carbon atoms. Typical
examples are cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
cyclooctyl and cyclononyl.
The term "halogen" or "halo"as employed herein by itself or as part of
another group refers to chlorine, bromine, fluorine or iodine with chlorine
being
preferred.
The term "acyl" as employed herein by itself or as part of another group
refers to the group -C(O)RD where R~ is alkyl, alkenyl, alkynyl, aryl.
aralkyl,
aralkenyl, heteroaryl, heteroarylalkyl or heteroarylalkenyl. Preferred acyl
groups
are alkanoyl, aralkanoyl and aroyl groups (-C(O)Rb where R6 is C,_8 alkyl,
C6_,o
aryl(C,~)alkyl or C6_,o aryl).
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-13-
The term "thioacyl" as employed herein by itself or as part of another
group refers to the group -C(S)R6 where Rs is alkyl, alkenyl, alkynyl, aryl,
aralkyl,
aralkenyl, heteroaryl, heteroarylalkyl or heteroarylalkenyl, preferably C,.B
alkyl.
The term "thiocarbonyl" as employed herein by itself or as part of another
group refers to the group -C(S)-.
The term "monoalkylamine" as employed herein by itself or as part of
another group refers to an amino group which is substituted with one alkyl
group
having from 1 to 6 carbon atoms.
The term "dialkylamine" as employed herein by itself or as part of another
group refers to an amino group which is substituted with two alkyl groups,
each
having from 1 to 6 carbon atoms
The term "aryl" as employed herein by itself or as part of another group
refers to monocyclic or bicyclic aromatic groups containing from 6 to 14
carbons
in the ring portion, preferably 6-10 carbons in the ring portion, such as
phenyl,
naphthyl or tetrahydronaphthyl.
The term "aralkyl" or "arylalkyl" as employed herein by itself or as part
of another group refers to C,_balkyl groups as discussed above having an aryl
substituent, such as benzyl, phenylethyl or 2-naphthylmethyl.
The terms "heterocyclic," "heterocyclo" or "heterocycle" as employed
herein by themselves or as part of larger groups refers to a saturated or
wholly or
partially unsaturated 3-7 membered monocyclic, or 7-10 membered bicyclic ring
system, which consists of carbon atoms and from one to four heteroatoms
independently selected from the group consisting of O, N, and S, wherein the
nitrogen and sulfur heteroatoms can be optionally oxidized, the nitrogen can
be
optionally quaternized, and including any bicyclic group in which any of the
above-defined heterocyclic rings is fused to a benzene ring, and wherein the
heterocyclic ring can be substituted on carbon or on a nitrogen atom if the
resulting compound is stable. Especially useful are rings containing one
oxygen
or sulfur, one to three nitrogen atoms, or one oxygen or sulfur combined with
one
or two nitrogen atoms. Examples of such heterocyclic groups include
piperidinyl,
piperazinyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolodinyl, 2-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-14-
S oxoazepinyl, azepinyl, pyrrolyl, 4-piperidonyl, pyrrolidinyl, pyrazolyl,
pyrazolidinyl, imidazolyl, imidazolinyl, imidazolidinyl, pyridyl, pyrazinyl,
pyrimidinyl, pyridazinyl, oxazolyl, oxazolidinyl, isoxazolyl, isoxazolidinyl,
morpholinyl, thiazolyl, thiazolidinyl, isothiazolyl, quinuclidinyl,
isothiazolidinyl,
indolyl; indanyl, quinolinyl, isoquinolinyl, benzimidazolyl, thiadiazoyl,
benzopyranyl, benzothiazolyl, benzoxazolyl, furyl, tetrahydrofuryl,
tetrahydropyranyl, thienyl, benzothienyl, thiamorpholinyl, thiamorpholinyl
sulfoxide, thiamorpholinyl sulfone, and oxadiazolyl. Morpholino is the same as
morpholinyl.
The term "heteroatom" is used herein to mean an oxygen atom ("O"), a
sulfur atom ("S") or a nitrogen atom ("N"). It will be recognized that when
the
heteroatom is nitrogen, it may form an NRyRZ moiety, wherein Ry and RZ are,
independently from one another, hydrogen or C, to Cg alkyl, or together with
the
nitrogen to which they are bound, form a saturated or unsaturated 5-, 6-, or 7-
membered ring.
The term "heteroaryl" as employed herein refers to groups having 5 to 14
ring atoms; 6, 10 or 14 ~ electrons shared in a cyclic array; and containing
carbon
atoms and 1, 2 or 3 oxygen, nitrogen or sulfur heteroatoms (where examples of
heteroaryl groups are: thienyl, benzo[b]thienyl, naphtho[2,3-b]thienyl,
thianthrenyl, furyl, pyranyl, isobenzofuranyl, benzoxazolyl, chromenyl,
xanthenyl,
phenoxathiinyl, 2H pyrrolyl, pyrrolyl, imidazolyl, pyrazolyl, pyridyl,
pyrazinyl,
pyrimidinyl, pyridazinyl, indolizinyl, isoindolyl, 3H indolyl, indolyl,
indazolyl,
purinyl, 4H quinolizinyl, isoquinolyl, quinolyl, phthalazinyl, naphthyridinyl,
quinazolinyl, cinnolinyl, pteridinyl, 4aH carbazolyl, carbazolyl, ~3-
carbolinyl,
phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl, phenazinyl,
isothiazolyl,
phenothiazinyl, isoxazolyl, furazanyl and phenoxazinyl groups).
The expression "prodrug" denotes a derivative of a known direct acting
drug, which derivative has enhanced delivery characteristics and therapeutic
value
as compared to the drug, and is transformed into the active drug by an
enzymatic
or chemical process. Useful prodrugs are those where R4, RS and/or R6 are
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-15-
-COZR'", where R"' is defined above. See, U.S. Patent No. 5,466,81 l and
Saulnier
et al., Bioorg. Med. Chem. Lett. 4:1985-1990 (1994).
The term "substituted", as used herein, means that one or more hydrogens
of the designated moiety are replaced with a selection from the indicated
group,
provided that no atom's normal valency is exceeded, and that the substitution
results in a stable compound. When a substituent is keto (i.e., =O), then 2
hydrogens attached to an atom of the moiety are replaced.
By "stable compound" or "stable formula" is meant herein a compound
that is sufficiently robust to survive isolation to a useful degree of purity
from a
reaction mixture and formulation into an efficacious therapeutic agent.
A first preferred group of compounds falling within the scope of the
present invention include compounds of Formulalwherein X is sulfur or oxygen;
Y is a covalent bond or -NH-; R' is hydrogen, amino, hydroxy or halogen; R4,
RS
and R6 are independently hydrogen, C,_4 alkyl, amino, cyano, C,_4 alkoxy or
hydroxy, and are preferably all hydrogen; one of RZ or R3 is hydrogen, C,_6
alkyl
(optionally substituted with hydroxy, amino, carboxy or aminocarbonyl), C,_6
alkylthio or C,_6 alkoxy; and the other of RZ or R3 is aminoacyl, acylamino,
aminosulfonyl, sulfonylamino, aminocarbonylamino, alkoxycarbonylamino,
optionally substituted oxazolyl, optionally substituted isoxazolyl, optionally
substituted benzothienyl, optionally substituted furanyl, optionally
substituted
pyrazolyl or optionally substituted pyridyl.
Specific compounds within the scope of the invention include the
compounds described in the Examples, such as the following:
4-[4-(4-chlorophenyl)thiazol-2-ylJ-5-methylthiothiophene-2-carboxamidine;
4-phenyl-5-methylthiothiophene-2-carboxamidine;
4-[4-(2,4-dichlorophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-(4-methylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
methyl 4-[4-(4-phenylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxylate;
4-[4-(3-methoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine,
4-[4-(3-hydroxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine,
4-(4-phenylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-16-
4-[4-(4-nitrophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine,
4-[4-(3,4-ethylenedioxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine,
4-[4-(3,4-propylenedioxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine,
4-[4-(4-(naphth-2-yl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine,
4-isopropylsulfonyl-5-methylthiothiophene-2-carboxamidine;
4-phenyl-5-methylthiothiophene-2-carboxamidine;
4-[4-(4-chlorophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(4-phenylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
1 S 4-[4-(4-methoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-(2-naphthylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[4-(4-chloro-3-methylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-(5-methyl-4-phenylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[4-(4-chloro-3-nitrophenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-(5-phenyloxazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[4-(3-fluoro-5-trifluoromethylphenyl)-S-methylthiazol-2-yl]-5-
methylthiothiophene-2-carboxamidine;
4-[4-(3,5-bis(trifluoromethyl)phenyl)-5-methyl-thiazol-2-yl]-5-
methylthiothiophene-2-carboxamidine;
4-[4-(3-fluoro-5-trifluoromethylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(3-bromophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(3,4-methylenedioxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(4-methylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(3,5-bis(trifluoromethyl)phenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(2-methoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_ 17_
4-(4-phenylimidazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[4-(2,4-dimethoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-(4-benzylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[4-(3,4-dichlorophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(3-methylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(3,5-dimethoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(2-methylphenyl)thiazol-2-yl]-S-methylthiothiophene-2-carboxamidine;
4-[4-(2,5-dimethoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-(4,5-diphenylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-(2-phenyl)thiazol-4-yl-5-methylthiothiophene-2-carboxamidine;
4-[4-(2-chloro-3-pyridyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(phenoxymethyl)thiazol-2-yl]-5-rriethylthiothiophene-2-carboxamidine;
4-(4-cyclohexylthiazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[4-(4-chlorophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(2-hydroxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-[4-(3-trifluoromethoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(2-chloro-4-pyridyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine;
4-(5-phenyl-2-pyridyl)-5-methylthiothiophene-2-carboxamidine;
4-[2-(2-chlorophenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(3-methoxyphenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(phenylamino)thiazol-4-yl]-5-methylthiothiophene-2-carboxamidine;
4-[2-(2,5-dimethoxyphenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-(2-aminothiazol-4-yl)-5-methylthiothiophene-2-carboxamidine;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-18-
4-[2-(4-chloro-2-methylphenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(4-dimethylaminophenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(4-methoxyphenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(4-hydroxy-3-methoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[4-(3-hydroxy-4-methoxyphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(2-fluorophenylamino)thiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(2,4,5-trimethylphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(3-chloro-2-methylphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(2-isopropylphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(4-benzyloxyphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(2-bromophenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(2,S-dichlorophenyl)aminothiazol-4-yl]-S-methylthiothiophene-2-
carboxamidine;
4-[2-(2-bromo-4-methylphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(2,3-dichlorophenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(3,4,5-trimethoxyphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-19-
4-[2-(2-piperidinylethyl)aminothiazol-4y1]-5-methylthiothiophene-2-
carboxamidine;
4-[2-(4-methylphenyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine;
4-(4-phenyloxazol-2-yl)-5-methylthiothiophene-2-carboxamidine;
4-[2-(diphenylmethyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine; and
4-[2-(3-phenylpropyl)aminothiazol-4-yl]-5-methylthiothiophene-2-
carboxamidine,
as well as pharmaceutically acceptable salts thereof, for example the
hydrochloride, hydrobromide and acetate salts thereof, or a prodrug thereof.
A second preferred group of compounds falling within the scope of the
present invention include compounds of Formula I wherein X is sulfur or
oxygen;
Y is a covalent bond or -NH-; Z is NRSR6; R' is hydrogen. amino, hydroxy or
halogen; R4, RS and R6 are independently hydrogen, C,_4 alkyl, amino, C,_4
alkoxy
or hydroxy, and are preferably all hydrogen; one of RZ or R3 is hydrogen, C,_6
alkylthio, C,_6 alkyl optionally substituted with OH, NH2, COOH or
aminocarbonyl, or C,_6 alkoxy; and the other of Rz or R3 is:
R$
Ar II
R9
where:
Ar is a group selected from the group consisting of phenyl, thiazolyl,
thiazolinyl, oxazolyl, isothiazolyl, isoxazolyl, furanyl, imidazolyl, pyridyl,
pyrimidinyl, pyrazinyl, thienyl (thiophenyl), tetrazolyl, pyrrolyl, pyrazolyl,
oxadiazolyl, oxazolinyl, isoxazolinyl, imidazolinyl, triazolyl, pyrrolinyl,
benzothiazolyl, benzothienyl, benzimidazolyl,1,3-oxazolidin-2-onyl, imidazolin-
2-onyl (preferably phenyl, thiazolyl, thiazolinyl, oxazolinyl, isothiazolyl,
isoxazolyl, imidazolyl, pyridyl, pyrimidinyl, thienyl, pyrrolyl, oxazolinyl
and
benzothienyl), any of which can optionally include an exocyclic = O (keto) or
=
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-20-
NR'' (imino) group, where R'' is alkyl, aryl, aralkyl, alkylamino, arylimino
or
aralkylimino; and
R8 and R9 are independently selected from the group consisting of
hydrogen, halogen, amino, mono(C,_4)alkylamino, di(C,_4)alkylamino, arylamino,
mono- and di- (C6_,4)arylamino, mono- and di-(C6_,4)ar(C,_6)alkylamino,
formylamino, Cz_6 acylamino, aminocarbonyl, CZ_g aminoacyl, CZ_6
thioacylamino,
aminothiocarbonyl, CZ_8 aminothioacyl, C,_6 alkyl, C3_8 cycloalkyl, C,_6
alkoxy,
carboxy, carboxy(C,_6)alkyl, CZ_8 alkoxycarbonyl, nitro, cyano,
trifluoromethyl,
thiazolyl, thiazolinyl, oxazolyl, isothiazolyl, isoxazolyl, furanyl,
imidazolyl,
pyridyl, pyrimidinyl, pyrazinyl, thienyl (thiophenyl), tetrazolyl, pyrrolyl,
pyrazolyl, oxadiazolyl, oxazolinyl, isoxazolinyl, imidazolinyl, triazolyl,
pyrrolinyl, benzothiazolyl, benzothienyl, benzimidazolyl, 1,3-oxazolidin-2-
onyl,
imidazolin-2-onyl, C6_,4 aryloxy, C,_6 alkylthio, C6_,4 arylthio, C6_,4 aryl,
or C6_,4
ar(C,_6)alkyl, wherein the aforementioned heteroaryl groups and the aryl
portions
of C6_,4 aryloxy, mono- and di C6_,4 aryl amino, mono- and di- C6_,a
ar(C,_6)alkylamino, C6_,4 arylthio, C6_,4 ar(C,_6)alkyl, and C6_,4 aryl can be
further
optionally substituted, preferably by one, two or three of halogen, hydroxy,
amino, mono(C,_4)alkylamino, di(C,_4)alkylamino, formylamino, C,_4acylamino,
C,_4aminoacyl, mono- or di-(C,_4)alkylaminocarbonyl, thiocarbonylamino,
C,_4thioacylamino, aminothiocarbonyl, C,_4alkoxy, C6_,oaryloxy,
aminocarbonyloxy, mono- or di(C,_4)alkylaminocarbonyloxy, mono- or
di(C6_,o)arylaminocarbonyloxy, mono- or di(C.,_,5)aralkylaminocarbonyloxy, C,_
~alkylsulfonyl, C6_,oarylsulfonyl, (C~_,5)aralkylsulfonyl,
C,_4alkylsulfonylamino,
C6_,oarylsulfonylamino, (C,_,5)aralkylsulfonylamino, aminosulfonyl, mono- and
di-alkylaminosulfonyl, mono- and di-arylaminosulfonyl, mono- and di-
aralkylaminosulfonyl, C,~ alkoxycarbonylamino, C~_,Saralkoxycarbonylamino,
C6_,oaryloxycarbonylamino, mono- or di-(C,_4)alkylaminothiocarbonyl, C~_
,Saralkoxy, carboxy, carboxy(C,_4)alkyl, C,_4alkoxycarbonyl, C,_
4alkoxycarbonylalkyl, carboxy(C,_4)alkoxy, alkoxycarbonylalkoxy, nitro, cyano,
trifluoromethyl, C,_4alkylthio and C6_,oarylthio, or by 3,4-methylenedioxy,
3,4-
ethylenedioxy, and 3,4-propylenedioxy.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-21-
Preferred values of R8 and R9 are halogen, C,_6 alkyl, C,_6 alkoxy, hydroxy,
nitro, trifluoromethyl, C6_,o aryl (further optionally substituted by one or
two of
chloro, halogen, C,_6 alkyl, C,_6 alkoxy, hydroxy, nitro, trifluoromethyl,
carboxy,
3,4-methylenedioxy, 3,4-ethylenedioxy, 3,4-propylenedioxy, or amino),
4-phenylphenyl (biphenyl), C,_6 aminoalkyl, carboxy, C,_6 alkyl,
3,4-methylenedioxy, 3,4-ethylenedioxy, 3,4-propylenedioxy, amino, C,_6
alkanoylamino, C6_,4 aroylamino, C,_6 hydroxyalkyl, thienyl (further
optionally
substituted by one or two of chloro, amino, methyl, methoxy, or hydroxy) and
tetrazolyl. More preferably, Rz is thienyl, oxazolyl, or thiazolyl, optionally
substituted by any of the aforementioned groups.
Examples of preferred R8 and R9 groups include 4-chlorophenyl,
2,4-dichlorophenyl, methyl, 4-nitrophenyl, 3-nitrophenyl, 4-methoxyphenyl,
3-methoxyphenyl, 2-methoxyphenyl, 3-(2,4-dimethylthien-5-yl)phenyl,
3-hydroxyphenyl, 5-(carboxymethyl)thien-2-yl, phenyl, 3,4-ethylenedioxyphenyl,
3,4-propylenedioxyphenyl, naphth-~-yl, 3-phenyl-4-(tetrazol-5-yl)phenyl,
2,4-dichlorophenyl), 4-phenylphenyl, 3-methoxyphenyl, 3-hydroxyphenyl,
3-phenylphenyl, phenylthiomethyl, 2-chloro-4,5-dimethoxyphenyl, 4-chloro-3-
methylphenyl, 5-methyl-4-phenyl, 4-chloro-3-nitrophenyl,
3-fluoro-5-trifluoromethylphenyl, 3,5-bis(trifluoromethyl), 3-fluoro-S-
trifluoromethylphenyl, 3-bromophenol, 3,4-methylenedioxyphenyl,
4-methylphenyl, 3-methylphenyl, 3,5-bis(trifluoromethyl)phenyl,
2-methoxyphenyl, 6-phenyl-2-pyridyl, 2,4-dimethoxyphenyl, 3,4-
dimethoxyphenyl, benzyl, 3,4-dichlorophenyl, 3-methylphenyl, 3,5-
dimethoxyphenyl, 2-methylphenyl, 2,5-dimethoxyphenyl, 2-chloro-3-pyridyl,
phenoxymethyl, cyclohexyl, 2-hydroxyphenyl, 3-trifluoromethoxyphenyl, 2-
chloro-4-pyridyl, 3-chloro-4-pyridyl, 2-chlorophenylamino, 3-
methoxyphenylamino, phenylamino, 2,5-dimethoxyphenylamino, amino, 4-
chloro-2-methylphenylamino, 4-dimethylaminophenylamino,
4-methoxyphenylamino, 4-hydroxy-3-methoxyphenyl, 3-hydroxy-4-
methoxyphenyl, 2-fluorophenylamino, 2,4,5-trimethylphenylamino, 3-chloro-2-
methylphenylamino, 2-isopropylphenylamino, 4-benzyloxyphenylamino, 2-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-22-
bromophenylamino, 2,5-dichlorophenylamino, 2-bromo-4-methylphenylamino,
2,3-dichlorophenylamino, 3,4,5-trimethoxyphenylamino,
2-piperidinylethylamino, 4-methylphenylamino, 2-thienyl, 2-5,6,7,8-
tetrahydronaphthyl, 3-(2-phenoxyacetic acid)phenyl, 2-(2-phenoxyacetic
acid)phenyl, diphenylmethylamino, 3-phenylpropylamino, 3-phenylphenyl,
phenylthiomethyl, 2-chloro-4,5-dimethoxyphenyl, and isopropyl.
A third preferred group of compounds are those of Formula I wherein:
X is sulfur;
Y is a covalent bond;
Z is NRSR6;
R' is hydrogen;
R3 is methylthio or methyl;
R4, RS and R6 are all hydrogen; and
RZ is Formula II, where Ar is phenyl, thiazolyl, oxazolyl,
benzothienyl, pyridyl, or imidazolyl; and R8 and R9 are independently
hydrogen,
or Cb_,o aryl or heterocycle, optionally substituted by one, two or three of
chloro,
hydroxy, C,_4 alkyl, C3_6 cycloalkyl, C,~ alkoxy, amino, carboxy, phenyl,
naphthyl,
biphenyl, hydroxyphenyl, methoxyphenyl, dimethoxyphenyl,
carboxyalkoxyphenyl, alkoxycarbonylalkoxy, carboxyethoxy;
alkylsulfonylaminophenyl, arylsulfonylaminophenyl, acylsulfonylaminophenyl,
aralkylsulfonylaminophenyl, heteroarylsulfonylaminophenyl where the heteroaryl
portion is optionally halo or C,_balkyl substituted, chlorophenyl,
dichlorophenyl,
aminophenyl, carboxyphenyl, nitrophenyl, or by 3,4-methylenedioxy, 3,4-
ethylenedioxy, and 3,4-propylenedioxy.
A fourth preferred group of compounds are those of Formula I wherein:
X is sulfur;
Y is a direct covalent bond;
Z is NRSR6;
R' is hydrogen;
RZ is alkyl, ar(alkyl), alkylsulfonyl, -SOZ-alkyl, amido, amidino,
or
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-23-
R$
Ar ll
Rs
where
Ar is an aromatic or heteroaromatic group selected from the group
consisting of phenyl, thiazolyl, oxazolyl, imidazolyl and pyridyl;
R8 and R9 are independently selected from the group consisting of
hydrogen, carboxy, phenyl, naphthyl, alkyl, pyridyl, oxazolyl, furanyl,
cycloalkyl
and amino, any of which may be optionally substituted with I to 3 substituents
independently selected from the group consisting of halogen, alkyl, haloalkyl,
alkaryl, heteroaryl, phenyl, naphthyl, alkoxy, aryloxy, hydroxy, amino nitro,
thiophenyl, benzothiophenyl, fluorenyl, 3,4-ethylenedioxy, 3,4-methylenedioxy,
1 S 3,4-propylenedioxy, arylsulfonamido, alkylsulfonamido and aryloxy, each of
said
1 to 3 substituents may be further optionally substituted with one or more
groups
selected from alkoxy, haloalkyl, halogen, alkyl, amino, acetyl, hydroxy,
dialkylamino, dialkylamino acyl, monoalkylaminoacyl, -SO~-heteroaryl,
-SOZ-aryl, or aryl;
R3 is -SOz-alkyl, trifluoromethyl, S(O)-alkyl, hydrogen, alkoxy,
alkylthio, alkyl, aralkylthio; and
R4, R5, R6 are hydrogen.
Preferred compounds of this embodiment are those where Ar is a
thiazolyl, preferably thiazol-2-yl or thiazol-4-yl, and at least one of R8 and
R9 is
substituted phenyl, most preferably on the 4-position of the thiazol-2-yl
group.
Also preferred are compounds where RZ is a 4-phenylthiazol-2-yl group wherein
said phenyl is further optionally substituted. and R3 is methylthio.
A fifth preferred group of compounds are those of Formula III:
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-24-
/ O R.
G'-~HZ)~-~C jrrr N~
R"
N
, III
S
NH
or a pharmaceutically acceptable salt or prodrug thereof, where
A is methylthio or methyl;
G' is -O-, -S-, NH-, or a covalent bond;
n is an integer from 1-10, preferably from 1-6;
m is an integer from 0-l; and
R' and R" are independently selected from hydrogen, alkyl, aryl or
aralkyl, or R' and R" are taken together with the N atom to which they are
attached form a 3-8 membered heterocyclic ring, optionally containing an
additional O, N, or S atom, and when said 3-8 membered heterocyclic ring
contains an additional N atom, said additional N atom is optionally
substituted by
hydrogen, C,_4alkyl, C6_,oaryl, C6_,oar(C,_4)alkyl, acyl, alkoxycarbonyl or
benzyloxycarbonyl.
Most preferred compounds of Formula III are those for which R' and R",
taken together with the N atom to which they are attached, form a ring
selected
from piperazinyl, pyrrolidinyl, piperidinyl or morpholinyl, which are further
optionally substituted with 1 to 4 non-hydrogen substituents selected from
halogen, hydroxy, amino, monoalkylamino, dialkylamino, formylamino,
acylamino, aminoacyl, mono- or di- alkylaminocarbonyl, thiocarbonylamino,
thioacylamino, aminothiocarbonyl, alkoxy, aryloxy, aminocarbonyloxy, mono-
or di-alkylaminocarbonyloxy, mono- or diarylaminocarbonyloxy, mono- or
diarakylaminocarbonyloxy, alkylsulfonyl, arylsulfonyl, aralkylsulfonyl,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-25-
alkylsulfonylamino, arylsulfonylamino, arakylsulfonylamino,
alkoxycarbonylamino, aralkoxycarbonylamino, aryloxycarbonylamino, mono- or
di- alkylaminothiocarbonyl, aralkoxy, carboxy, carboxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, nitro, cyano, trifluoromethyl, alkylthio and arylthio,
where
each of these substituents has the preferred values set forth for Formulae I
and 11
above.
Examples of preferred compounds of Formula Ill include:
5-methylthio-4-[4-(3-{ [N-(2-morpholin-4-
ylethyl)carbamoyl]methoxy } phenyl)( 1,3-thiazol-2-yl)]thiophene-2-
carboxamidine,
5-methylthio-4-{4-[3-(2-morpholin-4-yl-2-oxoethoxy)phenyl](1,3-thiazol-2-
yl)}thiophene-2-carboxamidine,
5-methylthio-4-{4-[3-(2-oxo-2-piperazinylethoxy)phenyl]( 1,3-thiazol-2-
yl)}thiophene-2-carboxamidine,
4-[4-(3- { [N-(2-aminoethyl)carbamoyl']methoxy } phenyl)( 1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxamidine,
4-(4-{ 3-[2-(4-acetylpiperazinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxamidine,
4-(4- { 3-(2-(4-methylpiperazinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxamidine,
the compound described in Example 151,
5-methylthio-4-[4-(3- { 2-oxo-2-[4-benzylpiperazinyl] ethoxy } phenyl)( 1,3-
thiazol-
2-yl)]thiophene-2-carboxamidine,
(D,L)-4-(4-{ 3-(2-(3-aminopyrrolidinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-
yl))-
S-methylthiothiophene-2-carboxamidine,
5-methylthio-4-{4-[3-(2-oxo-2-piperidylethoxy)phenyl](1,3-thiazol-2-
yl) } thiophene-2-carboxamidine,
(D,L)-ethyl 1-(2- { 3-[2-(5-amidino-2-methylthio-3 -thienyl)-1,3-thiazol-4-
yl]phenoxy}acetyl)piperidine-2-carboxylate,
5-methylthio-4-{4-[3-(2-oxo-2-pyrrolidinylethoxy)phenyl]( 1,3-thiazol-2-
yl)}thiophene-2-carboxamidine,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-26-
5-methylthio-4-[4-(3-{2-oxo-2-[4-benzylpiperidyl]ethoxy}phenyl)(1,3-thiazol-2-
yl)]thiophene-2-carboxamidine,
(D,L)-4-(4-{ 3-[2-(3-methylpiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxamidine,
4-(4-{ 3-[2-(4-methylpiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxamidine,
4-(4-{3-[2-(2-azabicyclo[4.4.0]dec-2-yl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-
yl))-
5-methylthiothiophene-2-carboxamidine,
(D,L)-ethyl 1-(2-{ 3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetyl)piperidine-3-carboxylate,
5-methylthio-4-{4-[3-(2-oxo-2-(1,2,3,4-tetrahydroquinolyl)ethoxy)phenyl](1,3-
thiazol-2-yl)}thiophene-2-carboxamidine,
ethyl 1-(2- { 3-[2-(5-amidino-2-methylthio-3-thienyl)-1, 3 -thiazol-4-
yl]phenoxy } acetyl)piperidine-4-carboxylate,
4-(4-{3-[2-((3R)-3-hydroxypiperidyl)-2~ oxoethoxy]phenyl}(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxamidine,
D,L-4-(4-{ 3-[2-(2-ethylpiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-S-
methylthiothiophene-2-carboxamidine,
4-(4-{ 3-[2-((3 S)-3-hydroxypyrrolidinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-
yl))-
5-methylthiothiophene-2-carboxamidine,
D,L-4-[4-(3-{2-[3-(hydroxymethyl)piperidyl]-2-oxoethoxy}phenyl)(1,3-thiazol-2-
yl)]-5-methylthiothiophene-2-carboxamidine,
4-{4-[3-(2-{(2R)-2-[(phenylamino)methyl]pyrrolidinyl}-2-
oxoethoxy)phenyl]( 1,3-thiazol-2-yl) }-5-methylthiothiophene-2-carboxamidine,
4-[4-(3-{2-[(3R)-3-(methoxymethyl)pyrrolidinyl]-2-oxoethoxy }phenyl)( 1,3-
thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine,
1-(2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetyl)piperidine-3-carboxamide, and
2-{ 3-[2-(5-{ [(tert-butoxy)carbonylamino]iminomethyl }-2-methyl-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy } acetic acid;
or pharmaceutically acceptable salts or prodrugs thereof.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-27-
A sixth preferred group of compounds are those of Formula IV:
R"'
HN
S N
IV
NH
NHZ
or a pharmaceutically acceptable salt or prodrug thereof, where
A is methylthio or methyl; and
R"' is hydrogen, C6_,4aryl, C,_6alkyl, C,_balkoxy (C6_,4)aryl,
amino(C6_,4)aryl,
monoalkylamino(C6_,4)aryl, dialkylamino(C6_,4)aryl, C6_,oar(C,_6)alkyl,
heterocycle(CZ_6)alkyl such as morpholinoalkyl, piperazinylalkyl and the like,
C,_balk(C6_,4)aryl, amino(C,_6)alkyl, mono(C,_6)alkylamino(C,_6)alkyl,
di(C,_6)alkylamino(C,_6)alkyl, hydroxy(C6_,4)aryl, or hydroxy(C,_6)alkyl,
where the
aryl and heterocyclic rings can be further optionally substituted by 1-4 non
hydrogen substituents selected from halogen, hydroxy, amino;
mono(C,_6)alkylamino, di(C,_6)alkylamino, formylamino, (C,_6)acylamino,
amino(C,_6)acyl, mono- or di-(C,_6)alkylaminocarbonyl, thiocarbonylamino,
(C,_6)thioacylamino, aminothiocarbonyl, (C,_6)alkoxy, (C6_,o)aryloxy,
aminocarbonyloxy, mono- or di-(C,_6)alkylaminocarbonyloxy, mono- or di-
(C6_,o)arylaminocarbonyloxy, mono- or di(C6_,o)ar(C,_6)alkylaminocarbonyloxy,
(C,_6)alkylsulfonyl, (C6_,o)arylsulfonyl, (C6_,o)ar(C,_6)alkylsulfonyl,
(C,_6)alkylsulfonylamino, C6_,o arylsulfonylamino,
(C6_,o)ar(C,_6)alkylsulfonylamino,(C,_6)alkoxycarbonylamino,
(C6_,o)ar(C,_6)alkoxycarbonylamino, C6_,oaryloxycarbonylamino, mono- or di-
(C,_
6)alkylaminothiocarbonyl, (C6-,o)ar(C,_6)alkoxy, carboxy, (C,_6)carboxyalkyl,
C,_balkoxycarbonyl, (C,_6)alkoxycarbonyl(C,_6)alkyl, nitro, cyano,
trifluoromethyl,
(C,_6)alkylthio and C6_,oarylthio.
Examples of preferred compounds of Formula IV include:
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-28-
4-{2-[(3-methoxyphenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-
carboxamidine,
4-{ 2-[(4-methoxyphenyl)amino]( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-
carboxamidine,
4-(2- { [4-(dimethylamino)phenyl] amino } ( 1, 3-thiazo 1-4-yl))-5-
methylthiothiophene-2-carboxamidine,
4- { 2-[(4-chloro-2-methylphenyl)amino] ( 1, 3-thiazol-4-yl) }-5-
methylthiothiophene-2-carboxamidine,
4-{2-[(diphenylmethyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-
carboxamidine,
5-methylthio-4-{2-[(3-phenylpropyl)amino](1,3-thiazol-4-yl)}thiophene-2-
carboxamidine,
5-methylthio-4-{2-[(2,4,5-trimethylphenyl)amino]( 1,3-thiazol-4-yl)}thiophene-
2-
carboxamidine,
4-{2-[(2-fluorophenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-
carboxamidine,
4-{2-[(3-chloro-2-methylphenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxamidine,
4-(2-{ [2-(methylethyl)phenyl]amino}(1,3-thiazol-4-yl))-5-methylthiothiophene-
2-
carboxamidine,
5-methylthio-4-(2-{[4-(phenylmethoxy)phenyl]amino}(1,3-thiazol-4-
yl))thiophene-2-carboxamidine,
4-{2-[(2-bromophenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-
carboxamidine,
4-{2-[(2,6-dichlorophenyl)amino](1,3-thiazol-4-yl)}-S-methylthiothiophene-2-
carboxamidine,
4-{2-[(2-bromo-4-methylphenyl)amino]( 1,3-thiazol-4-yl) }-5-
methylthiothiophene-2-carboxamidine,
5-methylthio-4- { 2-[(2-morpholin-4-ylethyl)amino] ( 1,3-thiazol-4-yl) }
thiophene-2-
carboxamidine,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-29-
4-{2-[(2,3-dichlorophenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-
carboxamidine,
5-methylthio-4-{2-[(3,4,5-trimethoxyphenyl)amino]( 1,3-thiazol-4-yl)}thiophene-
2-carboxamidine,
5-methylthio-4-{2-[(2-piperidylethyl)amino] ( 1,3-thiazol-4-yl) } thiophene-2-
carboxamidine,
4-(2- { [(4-methylphenyl)methyl] amino } ( 1, 3-thiazol-4-yl))-5-
methylthiothiophene-
2-carboxamidine,
4-(2-{ [4-(4-chlorophenoxy)phenyl]amino } ( 1,3-thiazol-4-yl))-5-
methylthiothiophene-2-carboxamidine,
4-(2-{[4-phenoxyphenyl]amino}(1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxamidine,
5-methylthio-4-(2-{ [4-(phenylamino)phenyl]amino } ( 1,3-thiazol-4-
yl))thiophene-
2-carboxamidine,
5-methylthio-4-(2-{ [4-benzylphenyl~'amino } ( 1,3-thiazol-4-yl))thiophene-2-
carboxamidine,
S-methylthio-4-(2-{ [4-(piperidylsulfonyl)phenyl]amino } ( 1,3-thiazol-4-
yl))thiophene-2-carboxamidine,
5-methylthio-4-[2-(3-quinolylamino)( 1,3-thiazol-4-yl)]thiophene-2-
carboxamidine,
5-methylthio-4-[2-(2-naphthylamino)(1,3-thiazol-4-yl)]thiophene-2-
carboxamidine,
4-[2-(2H-benzo [3,4-d] 1,3-dioxolan-5-ylamino)( 1,3-thiazol-4-yl)]-5-
methylthiothiophene-2-carboxamidine,
4-{2-[(7-bromofluoren-2-yl)amino] ( 1,3-thiazol-4-yl) }-5-methylthiothiophene-
2-
carboxamidine,
4- { 2-[(4-cyclohexylphenyl)amino]( 1,3-thiazol-4-yl) }-5-methylthiothiophene-
2-
carboxamidine,
5-methylthio-4-(2-{ [4-(phenyldiazenyl)phenyl]amino } ( 1,3-thiazol-4-
yl))thiophene-2-carboxamidine,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-30-
5-methylthio 4-(2-{[3-(hydroxymethyl)phenyl]amino}(1,3-thiazol-4-yl))-
thiophene-2-carboxamidine,
4-[2-( { 3-[(3-methylpiperidyl)methyl]phenyl } amino)( 1,3-thiazol-4-yl)]-5-
methylthiothiophene-2-carboxamidine,
4- { 2-[(3-hydroxyphenyl)amino] ( 1,3-thiazol-4-yl) } -S-methylthiothiophene-2-
carboxamidine,
4-(2-{ [4-(carbamoylmethoxy)phenyl] amino } ( 1, 3-thiazol-4-yl))-5-
methylthiothiophene-2-carboxamidine,
5-methyl-4-{2-[(3,4,5-trimethoxyphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-
carboxamidine,
5-methyl-4-{2-[(4-phenoxyphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-
carboxamidine,
5-methyl-4-[2-(phenylamino)(1,3-thiazol-4-yl)]thiophene-2-carboxamidine, and
4-(4-isoxazol-5-yl(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxamidine;
as well as pharmaceutically acceptable salts and prodrugs thereof.
A seventh preferred group of compounds are compounds of Formula I, or
a pharmaceutically acceptable salt or prodrug thereof, wherein:
X is sulfur or oxygen, preferably sulfur;
Y is a covalent bond or -NH-, preferably a covalent bond;
Z is NRSR6;
R' is hydrogen, amino, hydroxy or halogen, preferably hydrogen;
R4, RS and R6 are independently hydrogen, C,_4 alkyl, amino, C,_4 alkoxy
or hydroxy, and are preferably all hydrogen;
R3 is hydrogen, C,_6 alkylthio, C,_6 alkyl optionally substituted with OH,
NHZ, COOH or aminocarbonyl, or C,_6 alkoxy, preferably methylthio or methyl;
and
Rz is
alkylsulfonylamino, aralkylsulfonylamino,
aralkenylsulfonylamino, arylsulfonylamino,
heteroarylsulfonylamino, di(aralkylsulfonyl)amino,
di(aralkenylsulfonyl)amino, di(arylsulfonyl)amino, or di-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99118065
-31-
(heteroarylsulfonyl)amino, wherein any of the aryl or
heteroaryl containing groups can be optionally substituted
on the aromatic ring; or
amino, monoalkylamino, dialkylamino, monoarylamino,
diarylamino, monoalkylmonoarylamino,
monoaralkylamino, diaralkylamino,
monoalkylmonoaralkylamino, monoheterocycleamino,
diheterocycleamino, monoalkylmonoheterocycleamino,
wherein any of the aryl or heteroaryl containing groups
can be optionally substituted on the aromatic ring and
wherein any of the heterocycle containing groups can be
optionally ring substituted; or
alkanoylamino, alkenoylamino, alkynoylamino, aroylamino,
aralkanoylamino, aralkenoylamino, heteroaroylamino,
heteroarylalkanoylamino, any of which is optionally
substituted on the aromatic ring; or
alkoxy and alkylthio, either of which is optionally substituted, or
aryloxy, aralkoxy, arylthio, aralkylthio, arylsulfonyl,
aralkylsulfonyl, aralkenylsulfonyl, any of which is
optionally substituted on the aromatic ring; or
alkoxycarbonylamino, aralkoxycarbonylamino,
aryloxycarbonylamino, wherein any of the aryl containing
groups can be optionally substituted on the aromatic ring;
or
formylamino, H(S)CNH-, or thioacylamino.
Preferred optional substituents are halogen, C,_6 alkyl, C,_6 alkoxy,
hydroxy, nitro, trifluoromethyl, C6_,o aryl, C6_,o aryloxy, C6_,o arylmethoxy
(wherein the aryl groups on these aryl-containing substituents are further
optionally substituted by one or two of chloro, halogen, C,_6 alkyl, C,_6
alkoxy,
phenyl, hydroxy, nitro, trifluoromethyl, carboxy, 3,4-methylenedioxy,
3,4-ethyienedioxy, 3,4-propylenedioxy, or amino), C,_6 aminoalkyl, carboxy,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-32-
alkyl, 3,4-methylenedioxy, 3,4-ethylenedioxy, 3,4-propylenedioxy, amino, mono
or di- (C,_6)alkylamino, mono- or di- C6_,o arylamino, C, _~
alkylsulfonylamino, C6_
,o arylsulfonylamino, C,_8 acylamino, C,_8 alkoxycarbonyl, C,_6 alkanoylamino,
C6_
,4 aroylamino, C,_6 hydroxyalkyl, methylsulfonyl; phenylsulfonyl, thienyl
(further
optionally substituted by one or two of chloro, amino, methyl, methoxy, or
hydroxy) and tetrazolyl.
In one aspect of this embodiment, RZ is preferably C,_6
alkylsulfonylamino, C6_,o ar(C,_6)alkylsulfonylamino, C6_,o
ar(Cz_6)alkenylsulfonylamino, C6_,o arylsulfonylamino,
heteroarylsulfonylamino,
di(C6_,o ar(C,_6)alkylsulfonyl)amino, di(C6_,o ar(CZ_6)alkenylsulfonyl)amino,
di(C6_
,o arylsulfonyl)amino, or di-(heteroarylsulfonyl)amino, wherein any of the
aryl or
heteroaryl containing groups can be optionally substituted on the aromatic
ring.
Especially preferred RZ groups in this embodiment of the invention
include C6_,o arylsulfonylamino, di-(C6_,o arylsulfonyl)amino, C6_,o
ar(C,_3)alkylsulfonylamino, di-(C6_,o ar(C,_3)alkylsulfonyl)amino,
thienylsulfonylamino, any of which is optionally substituted on the aromatic
ring.
Useful values of RZ, when RZ is a substituted sulfonylamino group include
biphenylsulfonylamino, bis(biphenylsulfonyl)amino, naphth-2-ylsulfonylamino,
di(naphth-2-ylsulfonyl)amino, 6-bromonaphth-2-ylsulfonylamino, di(6-
bromonaphth-2-ylsulfonyl)amino, naphth-1-ylsulfonylamino, di(naphth-1-
ylsulfonyl)amino, 2-methylphenylsulfonylamino, di-(2-
methylphenylsulfonyl)amino, 3-methylphenylsulfonylamino, di-(3-
methylphenylsulfonyl)amino, 4-methylphenylsulfonylamino, di-(4-
methylphenylsulfonyl)amino, benzylsulfonylamino, 4-
methoxyphenylsulfonylamino, di-(4-methoxyphenylsulfonyl)amino, 4-
iodophenylsulfonylamino, di-(4-iodophenylsulfonyl)amino, 3,4-
dimethoxyphenylsulfonylamino, bis-(3,4-dimethoxyphenylsulfonyl)amino,
2-chlorophenylsulfonylamino, di-(2-chlorophenylsulfonyl)amino,
3-chlorophenylsulfonylamino, di-(3-chlorophenylsulfonyl)amino,
4-chlorophenylsulfonylamino, di-(4-chlorophenylsulfonyl)amino,
phenylsulfonylamino, di-(phenylsulfonyl)amino, 4-tert-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-33-
S butylphenylsulfonylamino, di-(4-tert-butylphenylsulfonyl)amino, 2-
phenylethenylsulfonylamino, and 4-(phenylsulfonyl)thien-2-ylsulfonylamino.
In another aspect of this embodiment, RZ is preferably amino,
mono(C,_6)alkylamino, di(C,_6)alkylamino, mono(C6_,o)arylamino,
di(C6_,o)arylamino, mono(C,_6)alkylmono(C6_,o)arylamino,
monoar(C,_6)alkylamino, di(C6_,o)ar(C,_6)alkylamino, mono(C,_6)alkylmono(C6_
,o)ar(C,_6)alkylamino, monoheteroarylamino, diheteroarylamino,
mono(C,_6)alkylmonoheteroarylamino, wherein any of the aryl or heteroaryl
containing groups can be optionally substituted on the aromatic ring.
Especially preferred RZ groups in this embodiment of the invention
IS include mono(C6_,o)arylamino, mono(C,_6)alkylmono(C6_~o)arylamino,
mono(C6_,o)ar(C,_3)alkylamino, mono(C,_6)alkylmono(C6_,o)ar(C,_3)alkylamino,
monoheteroarylamino, and mono(C,_6)alkylmonoheteroarylamino. Examples of
suitable heteroarylamino groups include 1,3-thiazol-2-ylamino, imidazol-4
ylamino, quinolin-2-ylamino and quinolin-6-ylamino.
Useful values of R2, when Rz is a substituted amino group include anilino,
naphth-2-ylamino, naphth-1-ylamino, 4-(biphenyl)thiazol-2-ylamino,
4-(phenyl)thiazol-2-ylamino, 4-phenyl-5-methylthiazol-2-ylamino, 4-hydroxy-4-
trifluoromethylthiazol-2-ylamino, 3-phenylphenylamino, pyrimidin-2-ylamino,
4-isopropylphenylamino, 3-isopropylphenylamino, 4-phenylphenylamino, 3-
fluoro-4-phenylphenylamino, 3,4-methylenedioxyphenylamino, n-
butylphenylamino, N-methyl-N-(2-methylphenyl)amino, 3-nitrophenylamino, 4-
methoxyphenylamino, 3-methoxyphenylamino, 2-methoxyphenylamino, 2-
methylphenylamino, 3-methylphenylamino, 3,4-dimethylphenylamino, 3-
chlorophenylamino, 4-chlorophenylamino, 4-(3-fluoro-4-methylphenyl)amino,
4-(indan-5-yl)amino, benzylamino, indanylmethylamino, 2,3-
dihydrobenzofuranylmethyl, 2-phenylimidazol-5-yl, 3-hydroxybenzyl, 3-
phenoxyphenylamino, 4-phenoxyphenylamino, 3-benzyloxyphenylamino, 4-
benzyloxyphenylamino, quinolin-6-ylamino, quinolin-3-ylamino, 4-
(phenylamino)phenylamino, 4-(4-ethylphenyl)phenylamino, 4-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-34-
S (dimethylamino)phenylamino, 4-cyclohexylphenylamino, 4-(9-ethylcarbazol-3
yl)amino, 4-(t-butyl)phenylamino, and 4-methylthiophenyl amino.
In another aspect of this embodiment, RZ is preferably an acylamino
group, such as alkanoylamino, alkenoylamino, aroylamino, aralkanoylamino,
aralkenoylamino, heteroaroylamino, heteroarylalkanoylamino, any of which is
optionally substituted on the aromatic ring.
Especially preferred RZ groups in this embodiment of the invention
include (C6_,o)arylcarbonylamino, C6_~o ar(C,_3)alkylcarbonylamino, C6_,o
ar(CZ_3)alkenylcarbonylamino, C6_,o aryloxy(C,_3)alkylcarbonylamino, C3_8
cycloalkylcarbonylamino, C,_6 alkylcarbonylamino, and heteroarylcarbonylamino,
such as furanylcarbonylamino, and quinolinylcarbonylamino.
Useful values of RZ, when R2 is an acylamino group include
3-hydroxyphenylcarbonylamino, 2-phenylethenylcarbonylamino,
phenylcarbonylamino, cyclohexylcarbonylamino, 4-methyl-3-
nitrophenylcarbonylamino, furan-2-ylcarbonylamino, tent-butylcarbonylamino,
5-(3,5-dichlorophenoxy)furan-2-ylcarbonylamino, naphth-1-ylcarbonylamino,
quinolin-2-ylcarbonylamino, 4-ethoxyphenylcarbonylamino,
phenoxymethylcarbonylamino, and 3-methylphenylcarbonylamino.
In another aspect of this embodiment, R'- is preferably C6_,o aryloxy, C6_,o
ar(C,_6)alkoxy, C6_,o arylsulfonyl, C6_,o ar(C,_6)alkylsulfonyl, or C6_,o
ar(CZ_6)alkenylsulfonyl, any of which is optionally substituted on the
aromatic
ring. Especially preferred RZ groups in this embodiment of the invention
include C6_,o aryloxy, and C6_,o arylsulfonyl.
Useful values of Rz, when RZ is an aryloxy or arylsulfonyl group include
phenoxy, naphthyloxy, phenylsulfonyl, and naphthylsulfonyl.
Representative compounds within the scope of this seventh embodiment
of the invention include:
5-methylthio-4-(6-quinolylamino)thiophene-2-carboxamidine
5-methylthio-4-[(3-phenylphenyl)amino]thiophene-2-carboxamidine
5-methylthio-4-(3-quinolylamino)thiophene-2-carboxamidine
5-methylthio-4-(pyrimidin-2-ylamino)thiophene-2-carboxamidine
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-35-
4-[(4-cyclohexylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
methyl 4-amino-5-methylthiothiophene-2-carboxylate
methyl 4-[(aminothioxomethyl)amino]-5-methylthiothiophene-2-carboxylate
5-methylthio-4-[(4-phenyl( 1,3-thiazol-2-yl))amino]thiophene-2-carboxamidine
5-methylthio-4- { [4-(4-phenylphenyl)( 1,3-thiazol-2-yl)]amino } thiophene-2-
carboxamidine
4-[(5-methyl-4-phenyl(1,3-thiazol-2-yl))amino]-5-methylthiothiophene-2-
carboxamidine
4-{[4-hydroxy-4-(trifluoromethyl)(1,3-thiazolin-2-yl)]amino}-5-
methylthiothiophene-2-carboxamidine
5-methylthio-4-(2-naphthylamino)thiophene-2-carboxamidine
4-[(4-chlorophenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-[(3-methylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-[(3-methoxyphenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-{ [3-(methylethyl)phenyl]amino}-5-rriethylthiothiophene-2-carboxamidine
5-methylthio-4-[(3-nitrophenyl)amino]thiophene-2-carboxamidine
4- { [4-(methylethyl)phenyl]amino }-5-methylthiothiophene-2-carboxamidine
4-[(3,4-dimethylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
5-methylthio-4-[(4-phenylphenyl)amino]thiophene-2-carboxamidine
4-[(3-fluoro-4-phenylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-(2H-benzo[d]1,3-dioxolen-5-ylamino)-S-methylthiothiophene-2-carboxamidine
4-[(4-butylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
5-methylthio-4-[benzylamino]thiophene-2-carboxamidine
4-(indan-S-ylamino)-5-methylthiothiophene-2-carboxamidine
4-(2,3-dihydrobenzo [b] furan-5 -ylamino)-S-methylthiothiophene-2-
carboxamidine
5-methylthio-4-[(2-phenylimidazol-4-yl)amino]thiophene-2-carboxamidine
5-methylthio-4-[(2-quinolylmethyl)amino]thiophene-2-carboxamidine
4-{ [(3-hydroxyphenyl)methyl]amino}-S-methylthiothiophene-2-carboxamidine
5-methylthio-4-(phenylcarbonylamino)thiophene-2-carboxamidine
4-((2E)-3-phenylprop-2-enoylamino)-5-methylthiothiophene-2-carboxamidine
4-[(4-chlorophenyl)carbonylamino]-5-methylthiothiophene-2-carboxamidine
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-36-
4-(cyclohexylcarbonylamino)-5-methylthiothiophene-2-carboxamidine
methyl 4-[(4-methyl-3-nitrophenyl)carbonylamino]-5-methylthiothiophene-2-
carboxylate
4-(2-furylcarbonylamino)-S-methylthiothiophene-2-carboxamidine
4-(2,2-dimethylpropanoylamino)-5-methylthiothiophene-2-carboxamidine
4-{[5-(3,5-dichlorophenoxy)(2-furyl)]carbonylamino}-5-methylthiothiophene-2-
carboxamidine
5-methylthio-4-(naphthylcarbonylamino)-thiophene-2-carboxamidine
5-methylthio-4-(2-quinolylcarbonyl-amino)thiophene-2-carboxamidine
4-[(3-methoxyphenyl)carbonylamino]-5-methylthiothiophene-2-carboxamidine
4-[2-(2-hydroxy-5-methoxyphenyl)acetylamino]-5-methylthiothiophene-2-
carboxamidine
4-[(4-ethoxyphenyl)carbonylamino]-5-methylthiothiophene-2-carboxamidine
S-methylthio-4-(2-phenoxyacetylamino)-thiophene-2-carboxamidine
4-[(3-methylphenyl)carbonylamino]-5-methylthiothiophene-2-carboxamidine
5-methylthio-4-{[3-(phenylmethoxy)phenyl]amino}thiophene-2-carboxamidine
5-methylthio-4-[(3-phenoxyphenyl)amino]thiophene-2-carboxamidine
5-methylthio-4-[(4-phenoxyphenyl)amino]thiophene-2-carboxamidine
4-[(2-methoxyphenyl)amino]-S-methylthiothiophene-2-carboxamidine
4-[(2-methylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-[(3-chlorophenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-(methylphenylamino)-5-methylthiothiophene-2-carboxamidine
S-methyl-4-(phenylamino)thiophene-2-carboxamidine
4-{ [4-(dimethylamino)phenyl]amino}-5-methylthiothiophene-2-carboxamidine
4-[(4-ethylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
5-methylthio-4-{[4-(phenylmethoxy)phenyl]amino}thiophene-2-carboxamidine
S-methylthio-4-{ [4-(phenylamino)phenyl]amino } thiophene-2-carboxamidine
4-[(4-methoxyphenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-[(3-fluoro-4-methylphenyl)amino]-5-methylthiothiophene-2-carboxamidine
4-(indan-5-ylamino)-5-methylthiothiophene-2-carboxamidine
4-[(9-ethylcarbazol-3-yl)amino]-5-methylthiothiophene-2-carboxamidine
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-3 7-
S 5-methylthio-4-{[(4-phenylphenyl)sulfonyl]amino}thiophene-2-carboxamidine
4-{bis[(4-phenylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxamidine
5-methylthio-4-[(2-naphthylsulfonyl)-amino]thiophene-2-carboxamidine
4-[bis(2-naphthylsulfonyl)amino]-S-methylthiothiophene-2-carboxamidine
4-{ [(6-bromo(2-naphthyl))sulfonyl]amino}-5-methylthiothiophene-2-
carboxamidine
4-{bis[(6-bromo(2-naphthyl))sulfonyl]amino }-5-methylthiothiophene-2-
carboxamidine
5-methylthio-4-[(naphthylsulfonyl)-amino]thiophene-2-carboxamidine
4-[bis(naphthylsulfonyl)amino]-5-methylthiothiophene-2-carboxamidine
4-{[(2-methylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxamidine
4- { bis [(2-methylphenyl)sulfonyl] amino } -5-methylthiothiophene-2-
carboxamidine
4-{ [(3-methylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxamidine
4-{bis[(3-methylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxamidine
4-{ [(4-methylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxamidine
4-{bis[(4-methylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxamidine
5-methylthio-4-{ [benzylsulfonyl]amino } -thiophene-2-carboxamidine
5-methylthio-4-phenoxythiophene-2-carboxamidine
5-methylthio-4-(phenylsulfonyl)thiophene-2-carboxamidine
as well as salts thereof, such as hydrochloride or trifluoracetate salts and
prodrugs
thereof.
Methods of Use and Pharmaceutical Compositions
For medicinal use, the pharmaceutically acceptable acid addition salts,
those salts in which the anion does not contribute significantly to toxicity
or
pharmacological activity of the organic cation, are preferred. The acid
addition salts are obtained either by reaction of an organic base of Formula I
with an organic or inorganic acid, preferably by contact in solution, or by
any
of the standard methods detailed in the literature available to any
practitioner
skilled in the art. Examples of useful organic acids are carboxylic acids such
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-3 8-
as malefic acid, acetic acid, tartaric acid, propionic acid, fumaric acid,
isethionic acid, succinic acid, cyclamic acid, pivalic acid and the like;
useful
inorganic acids are hydrohalide acids such as HCI, HBr, HI; sulfuric acid;
phosphoric acid and the like. Preferred acids for forming acid addition salts
include HCl and acetic acid.
The compounds of the present invention represent a novel class of
potent inhibitors of metallo, acid, thiol and serine proteases. Examples of
the
serine proteases inhibited by compounds within the scope of the invention
include leukocyte neutrophil elastase, a proteolytic enzyme implicated in the
pathogenesis of emphysema; chymotrypsin and trypsin, digestive enzymes;
1 S pancreatic elastase, and cathepsin G, a chymotrypsin-like protease also
associated with leukocytes; thrombin and factor Xa, proteolytic enzymes in the
blood coagulation pathway. Inhibition of thermolysin, a metalloprotease, and
pepsin, an acid protease, are also contemplated uses of compounds of the
present invention. The compounds of the present invention are preferably
employed to inhibit trypsin-like proteases.
Compounds of the present invention that inhibit urokinase
plasminogen activator are potentially useful in treating excessive cell growth
disease state. Compounds of the present that inhibit urokinase are, therefore,
useful as anti-angiogenic, anti-arthritic, anti-inflammatory, anti-invasive,
anti-
metastatic, anti-restenotic, anti-osteoporotic, anti-retinopathic (for
angiogenesis-dependent retinopathies), contraceptive, and tumoristatic
treatment agents. For example, such treatment agents are useful in the
treatment of a variety of disease states, including but not limited to, benign
prostatic hypertrophy, prostatic carcinoma, tumor metastasis, restenosis and
psoriasis. Also provided are methods to inhibit extracellular proteolysis,
methods to treat benign prostatic hypertrophy, prostatic carcinoma, tumor
metastasis, restenosis and psoriasis by administering the compound of Formula
I. For their end-use application, the potency and other biochemical parameters
of the enzyme inhibiting characteristics of compounds of the present invention
are readily ascertained by standard biochemical techniques well known in the
CA 02362390 2001-08-07
WO 00/47578 PCTIUS99/18065
-3 9-
art. Actual dose ranges for this application will depend upon the nature and
severity of the disease state of the patient or animal to be treated as
determined
by the attending diagnostician. It is to be expected that a general dose range
will be about 0.01 to 50 mg, preferably 0.1 to about 20 mg per kg per day for
an effective therapeutic effect.
An end use application of the compounds that inhibit chymotrypsin and
trypsin is in the treatment of pancreatitis. For their end-use application,
the
potency and other biochemical parameters of the enzyme-inhibiting
characteristics of the compounds of the present invention is readily
ascertained
by standard biochemical techniques well known in the art. Actual dose ranges
for their specific end-use application will, of course, depend upon the nature
and severity of the disease state of the patient or animal to be treated, as
determined by the attending diagnostician. It is expected that a useful dose
range will be about 0.01 to about 50 mg, preferably about 0.1 to about 20 mg
per kg per day for an effective therapeutic effect.
Compounds of the present invention that are distinguished by their
ability to inhibit either factor Xa or thrombin may be employed for a number
of therapeutic purposes. As factor Xa or thrombin inhibitors, compounds of
the present invention inhibit thrombin production. Therefore, these
compounds are useful for the treatment or prophylaxis of states characterized
by abnormal venous or arterial thrombosis involving either thrombin
production or action. These states include, but are not limited to, deep vein
thrombosis; disseminated intravascular coagulopathy which occurs during
septic shock, viral infections and cancer; myocardial infarction; stroke;
coronary artery bypass; fibrin formation in the eye; hip replacement; and
thrombus formation resulting from either thrombolytic therapy or percutaneous
transluminal coronary angioplasty (PCTA).
By virtue of the effects of both factor Xa and thrombin on a host of cell
types, such as smooth muscle cells, endothelial cells and neutrophils, the
compounds of the present invention find additional use in the treatment or
prophylaxis of adult respiratory distress syndrome; inflammatory responses;
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-40-
wound healing; reperfusion damage; atherosclerosis; and restenosis following
an injury such as balloon angioplasty, atherectomy, and arterial stmt
placement. The compounds of the present invention may be useful in treating
neoplasia and metastasis as well as neurodegenerative diseases, such as
Alzheimer's disease and Parkinson's disease.
When employed as thrombin or factor Xa inhibitors, the compounds of
the present invention may be administered in an effective amount within the
dosage range of about 0.1 to about 500 mg/kg, preferably between 0.1 to 30
mg/kg body weight, on a regimen in single or 2-4 divided daily doses.
Human leucocyte elastase is released by polymorphonuclear leukocytes
at sites of inflammation and thus is a contributing cause for a number of
disease states. Compounds of the present invention are expected to have an
anti-inflammatory effect useful in the treatment of gout, rheumatoid arthritis
and other inflammatory diseases, and in the treatment of emphysema. The
leucocyte elastase inhibitory properties of compounds of the present invention
are determined by the method described below. Cathepsin G has also been
implicated in the disease states of arthritis, gout and emphysema, and in
addition, glomerulonephritis and lung infestations caused by infections in the
lung. In their end-use application the enzyme inhibitory properties of the
compounds of Formula I is readily ascertained by standard biochemical
techniques that are well-known in the art.
The Cathepsin G inhibitory properties of compounds within the scope
of the present invention are determined by the following method. A
preparation of partially purified human Cathepsin G is obtained by the
procedure of Baugh et al., Biochemistry IS: 836 (1979). Leukocyte granules
are a major source for the preparation of leukocyte elastase and cathepsin G
(chymotrypsin-like activity). Leukocytes are lysed and granules are isolated.
The leukocyte granules are extracted with 0.20 M sodium acetate, pH 4.0, and
extracts are dialyzed against 0.05 M Tris buffer, pH 8.0 containing 0.05 M
NaCI overnight at 4°C. A protein fraction precipitates during dialysis
and is
isolated by centrifugation. This fraction contains most of the chymotrypsin-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-41-
like activity of leukocyte granules. Specific substrates are prepared for each
enzyme, namely N-Suc-Ala-Ala-Pro-Val p-nitroanilide and Suc-Ala-Ala-Pro-
Phe p-nitroanilide. The latter is not hydrolyzed by leukocyte elastase.
Enzyme preparations are assayed in 2.00 mL of 0.10 M Hepes buffer, pH 7.5,
containing 0.50 M NaCI, 10% dimethylsulfoxide and 0.0020 M Suc-Ala-Ala-
Pro-Phe p-nitroanilide as a substrate. Hydrolysis of the p-nitroanilide
substrate is monitored at 405 nm and at 25°C.
Useful dose range for the application of compounds of the present
invention as neutrophil elastase inhibitors and as Cathepsin G inhibitors
depend upon the nature and severity of the disease state, as determined by the
attending diagnostician, with a range of 0.01 to 10 mg/kg body weight, per
day, being useful for the aforementioned disease states.
Additional uses for compounds of the present invention include
analysis of commercial reagent enzymes for active site concentration. For
example, chymotrypsin is supplied as a standard reagent for use in clinical
quantitation of chymotrypsin activity in pancreatic juices and feces. Such
assays are diagnostic for gastrointestinal and pancreatic disorders.
Pancreatic
elastase is also supplied commercially as a reagent for quantitation of a,-
antitrypsin in plasma. Plasma a,-antitrypsin increases in concentration during
the course of several inflammatory diseases, and a,-antitrypsin deficiencies
are
associated with increased incidence of lung disease. Compounds of the
present invention can be used to enhance the accuracy and reproducibility of
these assays by titrametric standardization of the commercial elastase
supplied
as a reagent. See, U.S. Patent No. 4,499,082.
Protease activity in certain protein extracts during purification of
particular proteins is a recurring problem which can complicate and
compromise the results of protein isolation procedures. Certain proteases
present in such extracts can be inhibited during purification steps by
compounds of the present invention, which bind tightly to various proteolytic
enzymes.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-42-
S The pharmaceutical compositions of the invention can be administered
to any animal that can experience the beneficial effects of the compounds of
the invention. Foremost among such animals are humans, although the
invention is not intended to be so limited.
The pharmaceutical compositions of the present invention can be
administered by any means that achieve their intended purpose. For example,
administration can be by parenteral, subcutaneous, intravenous, intramuscular,
intraperitoneal, transdermal, buccal, or ocular routes. Alternatively, or
concurrently, administration can be by the oral route. The dosage administered
will be dependent upon the age, health, and weight of the recipient, kind of
concurrent treatment, if any, frequency of treatment, and the nature of the
effect desired.
In addition to the pharmacologically active compounds, the new
pharmaceutical preparations can contain suitable pharmaceutically acceptable
carriers comprising excipients and auxiliaries that facilitate processing of
the
active compounds into preparations that can be used pharmaceutically.
The pharmaceutical preparations of the present invention are
manufactured in a manner that is, itself, known, for example, by means of
conventional mixing, granulating, dragee-making, dissolving, or lyophilizing
processes. Thus, pharmaceutical preparations for oral use can be obtained by
combining the active compounds with solid excipients, optionally grinding the
resulting mixture and processing the mixture of granules, after adding
suitable
auxiliaries, if desired or necessary, to obtain tablets or dragee cores.
Suitable excipients are, in particular, fillers such as saccharides, for
example, lactose or sucrose, mannitol or sorbitol, cellulose preparations
and/or
calcium phosphates, for example, tricalcium phosphate or calcium hydrogen
phosphate, as well as binders, such as, starch paste, using, for example,
maize
starch, wheat starch, rice starch, potato starch, gelatin, tragacanth, methyl
cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose,
and/or polyvinyl pyrrolidone. If desired, disintegrating agents can be added,
such as, the above-mentioned starches and also carboxymethyl-starch, cross-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-43-
linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof, such
as,
sodium alginate. Auxiliaries are, above all, flow-regulating agents and
lubricants, for example, silica, talc, stearic acid or salts thereof, such as,
magnesium stearate or calcium stearate, and/or polyethylene glycol. Dragee
cores are provided with suitable coatings that, if desired, are resistant to
gastric
juices. For this purpose, concentrated saccharide solutions can be used, which
may optionally contain gum arabic, talc, polyvinyl pyrrolidone, polyethylene
glycol, and/or titanium dioxide, lacquer solutions and suitable organic
solvents
or solvent mixtures. In order to produce coatings resistant to gastric juices,
solutions of suitable cellulose preparations, such as, acetylcellulose
phthalate
or hydroxypropylmethyl-cellulose phthalate, are used. Dye stuffs or pigments
can be added to the tablets or dragee coatings, for example, for
identification
or in order to characterize combinations of active compound doses.
Other pharmaceutical preparations which can be used orally include
push-fit capsules made of gelatin, as well as soft, sealed capsules made of
gelatin and a plasticizer, such as, glycerol or sorbitol. The push-fit
capsules
can contain the active compounds in the form of granules that may be mixed
with fillers such as lactose, binders such as starches, and/or lubricants such
as
talc or magnesium stearate and, optionally, stabilizers. In soft capsules, the
active compounds are preferably dissolved or suspended in suitable liquids,
such as, fatty oils or liquid paraffin. In addition, stabilizers may be added.
Suitable formulations for parenteral administration include aqueous
solutions of the active compounds in water-soluble form, for example, water-
soluble salts, alkaline solutions and cyclodextrin inclusion complexes.
Especially preferred salts are hydrochloride and acetate salts. One or more
modified or unmodified cyclodextrins can be employed to stabilize and
increase the water solubility of compounds of the present invention. Useful
cyclodextrins for this purpose are disclosed in U.S. Patent Nos. 4,727,064,
4,764,604, and 5,024,998.
In addition, suspensions of the active compounds as appropriate oily
injection suspensions can be administered. Suitable lipophilic solvents or
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-44-
vehicles include fatty oils, for example, sesame oil, or synthetic fatty acid
esters, for example, ethyl oleate or triglycerides or polyethylene glycol-400
(the compounds are soluble in PEG-400). Aqueous injection suspensions can
contain substances that increase the viscosity of the suspension, for example,
sodium carboxymethyl cellulose, sorbitol, and/or dextran. Optionally, the
suspension may also contain stabilizers.
Methods of Making
Many synthetic methods used to form compounds of the present
invention generally involve the formation of an amidine from a carboxylic acid
derivative, such as an ester or a nitrite. In the process a Lewis acid, such
as
trimethylaluminum, is added to a source of ammonia, such as ammonium
chloride in an aprotic solvent, such as a toluene, under an inert atmosphere
(e.g., under an atmosphere of nitrogen or argon gas) at a temperature between
-15°C and 5°C, preferably at 0°C. An appropriate
carboxylic acid derivative
is added to the mixture and the mixture is heated at reflux for a
predetermined
period of time, preferably between 1 hr. and 24 hrs., and most preferably
between 1 hr. and 4 hrs. The resulting solution is allowed to cool to room
temperature and the amidine product isolated by known methods.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-45-
Description of Syntheses
The chemical schemes appear after the description of the schemes.
Scheme 1 a
Scheme la illustrates a general approach to compounds of Formula 1
where X = O or S, R3 = alkylthio, aralkylthio, arylthio, alkyloxy, aralkyloxy
or
aryloxy, Y = bond and Z = NRSR6. When R2z and RZ' of compounds 2 and 3
are retained in the final product, they correspond to RZ and R3 of Formula I,
respectively. Otherwise Rz2 and RZ' represent groups which, after further
transformations, will become RZ and R3 of Formula I.
Starting with the heterocycle where X = O or S appropriately
substituted by two leaving groups, the leaving groups can be sequentially
displaced by appropriate nucleophiles (preferably the anion of the group RZ'
or
Rzz to be substituted) to produce the mono- or disubstituted heterocycles.
Examples of leaving groups include halogens (chlorine, bromine or iodine),
sulfonates (methanesulfonate, toluenesulfonate or trifluoromethanesulfonate)
or sulfones (methylsulfonyl). Preferable nucleophiles include anions of thiols
or alcohols having as the counterion an alkali or alkali earth metal such as
sodium, lithium, potassium, magnesium or cesium, or in some cases, a
transition group metal such as zinc, copper or nickel. In certain cases where
the nucleophile used contains an anion on carbon, catalysis of the
displacement may be useful for this transformation. Examples of catalysts
would include compounds containing palladium, silver or Ni salts.
Scheme Ib
Scheme lb illustrates approaches to providing the functionality of
Y(CNR4)Z in compounds of Formula 1 where X = N, O or S, RZZ and Rz' are
defined as in Scheme la. Depending on the nature of the group W in 3,
several methods may be employed in the transformation of W to Y(CNR4)Z.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-46-
S When W in 3 is a cyano group (CN), primary amide (CONHZ) or ester
(CO~Rz3), direct conversion to an unsubstituted amidine 5 (i.e. Formula I
where Y = bond, Z = NR5R6 and R4, R5, R6 = H) can be effected by treatment
with a reagent consisting of a Lewis acid complexed to ammonia. This
complex is produced by treatment of ammonia or an ammonium salt,
preferably an ammonium halide and most preferably ammonium chloride or
bromide, with an appropriate Lewis acid, preferably a trialkylaluminum and
most preferably trimethyl- or triethylaluminum in a solvent inert to the Lewis
acid employed. For example, when a trialkylaluminum Lewis acid is
employed with an ammonium halide, reaction occurs with loss of one
equivalent of alkane to produce the dialkylhaloaluminum complex of ammonia
(see for example Sidler, D.R., et al, J. Org. Chem., 59:1231 (1994)). Examples
of suitable solvents include unsaturated hydrocarbons such as benzene,
toluene, xylenes, or mesitylene, preferably toluene, or halogenated
hydrocarbons such as dichloroethane, chlorobenzene or dichlorobenzene. The
amidination reaction is generally carried out at elevated temperatures,
preferably 40-200 °C, more preferably 80-140 °C, and most
preferably at the
reflux temperature of a solvent in the range of 80-120 °C.
When W is a cyano group (CN), direct conversion to a mono- or
disubstituted amidine 5 (R4, R5, R6 = H) is also possible by treatment with a
reagent consisting of a Lewis acid, preferably a trialkylaluminum, complexed
to a mono- or disubstituted amine HZNRS or HNRSR6 (Garigipati, R.,
Tetrahedron Lett. 31: 1969 (1990)). Alternatively the same addition of a
mono- or disubstituted amine may catalyzed by a copper salt such as Cu(I)
chloride (Rousselet, G., et al, Tetrahedron Lett. 34: 6395 (1993)).
When W in 3 is a carboxyl group (COzH), indirect conversion to an
unsubstituted amidine 5 can be carried out by initial esterification to 4 by
any
of a number of well-known dehydrating agents (for example,
dicyclohexylcarbodiimide) with an alcohol (RZ'OH). More preferably 4 can be
made by initial formation of an acid chloride by treatment of 3 with any of a
~ number of anhydrides of HCl and another acid, such as thionyl chloride,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-47-
POCl3, PCl3, PC15, or more preferably oxalyl chloride, with or without an
added catalyst such as N,N dimethylformamide (DMF), followed by the
alcohol R230H. Conversion to the unsubstituted amidine 5 (R4, R5, R6 = H)
can be carried out by treatment with a Lewis acid complexed to ammonia.
Amidines 5 also can be produced indirectly by conversion of 3 (W =
CN) to iminoethers 6 by exposure to a strong acid such as a hydrogen halide,
HBF4 or other non-nucleophilic acid, preferably gaseous HCl in the presence
of an alcohol R230H (R23 = alkyl, branched alkyl or cycloalkyl, preferably Me
or Et) and most preferably with the alcohol as solvent. Alternatively when W
= CONH2, conversion to an iminoether can be carried out by treatment with a
trialkyloxonium salt (Meerwein's salts). In either case, treatment of the
iminoether 6 with ammonia (R5, R6 = H) or a mono- or disubstituted amine
(HNRSR6) provides the corresponding unsubstituted or substituted amidines 5
(i.e. via classical Pinner synthesis: Pinner, A., Die Iminoaether and ihre
Derivate, Verlag R. Oppenheim, Berlin (1892)).
When W = NHZ in 3, treatment with a reagent Z(CNR4)L where Z =
alkyl and L is a leaving group such as O-alkyl and preferably OMe, provides
the subclass of amidines 135 (Z = alkyl ) which are isomeric to 5 (Formula 1,
where Y = NH, Z = H or alkyl). Examples of reagents for this reaction include
methyl or ethyl acetimidate hydrochloride. Alternatively treatment of 3 (W =
NHZ) with a trialkyl orthoformate ester, preferably trimethyl- or triethyl
orthoformate, followed by an amine R4NH2 affords the corresponding
formidines 135 (Z = H) (Formula I, where Y = NH, Z = H).
Also, when W = NH2, 3 can be treated with a reagent Z(CNR4)L where
R4 = H and Z = NRSR6 and L is a leaving group such as pyrazole,
methylpyrazole, S03H, S-alkyl, S-aryl, trifluoromethanesulfonate (OTf) or
trifluoromethanesulfonamide (NHTf), preferably pyrazole, S03H or
trifluoromethanesulfonamide (NHTf). Examples of these reagents include
aminoiminosulfonic acid (Miller, A.E. and Bischoff, J.J., Synthesis, 777
(1986) and 1H pyrazole-1-carboxamidine hydrochloride (Bernatowicz, M.S.,
et al., J. Org. Chem. 57:2497 (1992)). Such treatment provides guanidines
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_48_
136 directly (Formula I where Y = NH, Z = NRSR6). Alternatively a reagent
Z(CNP')L may be also used where Z = NHPZ and L again a leaving group such
as pyrazole, methylpyrazole, S03H, S-alkyl, S-aryl, trifluoromethanesulfonate
(OTf) or trifluoromethanesulfonamide (NHTf), to provide protected
guanidines (P', PZ = alkoxylcarbonyl, aralkoxycarbonyl or polymer-bound
alkoxylcarbonyl similar to those described below in Scheme 4a) where the
protecting groups P' and PZ can then be removed to give unsubstituted 136 (R4,
RS and R6 = H). Protected guanidines are advantageous when further
transformations are required after introduction of the guanidine functionality
where an unprotected guanidine would not be stable. Examples of these
protected reagents include reagents such as N,N'-bis(tert-butoxycarbonyl)-S-
methylthiourea (Bergeron, R.J. and McManis, J.S, J. Org. Chem. 52:1700
(1987)), N,N'-bis(benzyloxycarbonyl)-1 H pyrazole-1-carboxamidine or N,N'-
bis(tert-butoxycarbonyl)-1 H pyrazole-1-carboxamidine (Bernatowicz, M.S.,
et al., Tetrahedron Letters, 34: 3389 (1993)), N,N'-bis(benzyloxycarbonyl)-
N"-trifluoromethanesulfonylguanidine, and N,N'-bis(bis(tert-butoxycarbonyl)-
N"-trifluoromethanesulfonylguanidine (Feichtinger, K., et al, J. Org. Chem.
63:3804 (1998)). Detailed descriptions and examples of these protecting
groups and their use as protection for amidines are further outlined in
Schemes
4a, 4b and 5.
When W in 3 is an ester (COzR23) or carboxyl group (COZH), indirect
conversion to an N-substituted or unsubstituted methylamidine (Formula I
where Y = CHZ, Z = NRSR6) can be carried out by initial reduction of the ester
or carboxyl by any of a number of well-known reducing agents. When W in 3
is an ester (COzR23), examples of reducing agents include reducing agents such
lithium aluminum hydride (LAH) and lithium borohydride. When W in 3 is a
carboxyl group (C02H), examples of reducing agents include LAH and borane
complexed to THF, dimethyl sulfide, dimethylamine or pyridine. The
resulting hydroxymethyl derivative (W = CHZOH) is converted to a
cyanomethyl derivative (W = CHZCN) by initial formation of a leaving group
(W = CHzL) where the leaving group L is a halogen (chlorine, bromine or
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-49-
iodine) or sulfonate ester (for example methanesulfonate, toluenesulfonate or
trifluoromethanesulfonate). Displacement of L by cyanide can then be
performed by treatment with a metal cyanide such as LiCN, NaCN, KCN or
CuCN in a polar solvent such as DMF and with or without a catalyst such as a
crown ether, to afford the cyanomethyl derivative (see for example Mizuno,
Y., et al, Synthesis, 1008 (1980)). More preferably, the conversion of W =
CHZOH to W = CHZCN may be effected by a Mitsunobu reaction (Mitsunobu,
O., Synthesis, 1 ( 1981 )) using an azodicarboxylate ester such as diethyl
azodicarboxylate or diisopropyl azodicarboxylate, Ph3P and a source of
cyanide such as HCN or more preferably acetone cyanohydrin (Wilk, B.
Synthetic Commun. 23:2481 (1993)). Treatment of the resulting cyanomethyl
intermediate (W = CHZCN) under the conditions described for the conversion
of 3 (W = CN) to 5 (either directly or indirectly via 6) provides the
corresponding amidinomethyl products.
Scheme 1 c
When not commercially available, alkylthiothiophenes (3, X = S, R' _
OH or NHz, Rz' = SR54, W = CN, COZRz3, CONHz) can be synthesized by the
methods illustrated in Scheme lc. Condensation of carbon disulfide and a
malonic acid derivative (RSZCH2Rzz) in the presence of two alkylating agents
R54L and WCHZL and a base in a suitable medium provide 3 (Dolman, H.,
European Patent Application No. 0 234 622 Al (1987)). When Rzz = Rsz =
CN, the resulting R' will be NHz; when Rzz = Rsz = COZRz3, the resulting R'
will be OH; and when Rzz and Rsz = CN, COzRz3, the resulting R' can be
selected to be OH or NHz (and Rzz = CN or COZRz3) depending on the reaction
conditions and order of reagent addition. Examples of malonic acid
derivatives suitable for this transformation include but are not limited to
malonate diesters such as dimethyl malonate or diethyl malonate (Rsz, Rzz =
COZRz3, Rz3 = Me or Et), malononitrile (Rsz, Rzz = CN), or methyl or ethyl
cyanoacetate (RSZ = COZRz3, Rzz = CN, Rz3 = Me or Et). Leaving groups L
include halides such as chloride, bromide or iodide, preferably bromide or
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-5 0-
iodide, or sulfonates such as toluenesulfonate, benzenesulfonate,
methanesulfonate or trifluoromethanesulfonate. Examples of alkylating agent
R54L include primary or secondary alkyl, allyl or aralkyl halides or
sulfonates,
such as methyl iodide, isopropyl bromide, allyl bromide, benzyl chloride or
methyl trifluoromethanesulfonate, or a 2-haloacetate ester such as tert-butyl
2-
bromoacetate. Examples of alkylating agents WCHZL include 2-
chloroacetonitrile, methyl 2-bromoacetate or 2-bromoacetamide. Suitable
media are generally polar aprotic solvents, for example, N,N
dimethylformamide (DMF), N,N dimethylacetamide (DMA), N-
methylpyrrolidinone (NMP) or dimethylsulfoxide (DMSO), preferably DMF.
Alternatively compounds 3 (RZZ = CN) can be synthesized from
precursors 138 (derived from malononitrile, R54L and carbon disulfide), a
thioglycolate WCHSH and a base in a suitable polar solvent, preferably
methanol (Tominaga, Y., et al, J. Heterocyclic Chem. 31:771 (1994)).
When 3 contains an amino group at R', it can be diazotized with
subsequent loss of nitrogen to give 3, R' = H by treatment with a nitrosating
agent in suitable solvent. Nitrosating agents include nitrosonium
tetrafluoroborate, nitrous acid or, more preferably and alkyl nitrite ester
such
as tert-butyl nitrite. Suitable solvents are those which are stable to the
nitrosating agents, preferably DMF, benzene or toluene.
Scheme Id
When not commercially available, heterocyclic precursors 1 or 2 (X =
O, S; W = COZR23, COOH; L = halogen) used in Scheme la can be
synthesized by the methods illustrated in Scheme ld. Depending on the
conditions used, treatment of compounds such as 139 with elemental halogen
(Clz, Br2 or I2, preferably Br2) or an N-halosuccinimide reagent, preferably N-
bromosuccinimide (NBS), affords either 1 or 2 directly. Description of
suitable solvents and conditions to selectively produce 1 or 2 are found in
Karminski-Zamola, G. et al, Heterocycles 38:759 (1994); Divald, S., et al, J.
Org. Chem. 41:2835 (1976); and Bury, P., et al, Tetrahedron 50:8793 (1994).
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-51-
Scheme 2a
Scheme 2a illustrates the synthesis of compounds 12 representing the
subclass of compounds for which RZ is Formula II, where Ar = 2-thiazolyl, Y
= bond and Z = NRSR6. Starting with compound 1 (L = Br) and using the
sequential displacement methodology discussed for Scheme la, Rz' can be
first introduced to give 7. This is followed by a second displacement with a
metal cyanide such as copper (I) cyanide, sodium cyanide or lithium cyanide
and most preferably copper (I) cyanide at a temperature of 80-200 °C
and
preferably at 100-140 °C, in a polar aprotic solvent, preferably DMF or
DMSO, to give 8. After esterification by any of the means described for the
conversion of 3 to 4, conversion to the thioamide is carried out by treatment
of
the nitrite with any of the methods well known in the art (see for example
Ren,
W., et al., J. Heterocyclic Chem. 23:1757 (1986) and Paventi, M. and Edward,
J.T., Can. J. Chem. 65:282 (1987)). A preferable method is treatment of the
nitrite with hydrogen sulfide in the presence of a base such as a trialkyl or
heterocyclic amine, preferably triethylamine or pyridine, in a polar solvent
such as acetone, methanol or DMF and preferably methanol. Conversion to
the thiazole can be executed by classical Hantzsch thiazole synthesis followed
by amidine formation as discussed in Scheme lb.
Scheme 2b
Scheme 2b illustrates the synthesis of compounds representing the
subclass of compounds for which RZ is Formula 11 where, in addition to being
an alternate route to Ar = 2-thiazolyl (20) (see 12, Scheme 2a) also provide
compounds of Formula II where Ar = 2-oxazolyl (16) or 2-imidazolyl (18) (Y
= bond and Z = NRSR6). Starting with compound 9, a selective hydrolysis of
the nitrite with a tetrahalophthalic acid, preferably tetrafluoro- or
tetrachlorophthalic acid, can be used to give 7 according to the method of
Gribble, G.W. et al., Tetrahedron Lett. 29: 6557 (1988). Conversion to the
acid chloride can be accomplished using the procedures discussed for
conversion of 3 to 4, preferably with oxalyl chloride in dichloromethane in
the
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-52-
presence of a catalytic amount of DMF. Coupling of the acid chloride to an
aminoketone (RZ6COCH(RZ')NHz) can be performed in the presence of an acid
scavenger, preferably N,N diisopropylethylamine (DIEA) or pyridine in a
suitable solvent such as DMF, dichloromethane or tetrahydrofuran (THF) to
afford the common intermediate 14. Alternatively coupling of the acid
chloride to a less-substituted aminoketone (RZ6COCHZNH2) can be used
followed by optional alkylation with alkylating agent RZ'L in the presence of
a
base, preferably NaH or t-BuOK. Transformation of 14 to the corresponding
2-oxazolyl (15), 2-imidazolyl (17) or 2-thiazolyl (19) esters can carried out
by
the methodology of Suzuki, M., et al., Chem. Pharm. Bull. 34:3111 (1986)
followed by amidination according to Scheme lb. In addition, direct
conversion of ketoamide 14 to imidazolyl derivative 18 is possible under the
same conditions for conversion of 17 to 18 when conducted for extended
periods, preferably greater than 2 h.
Scheme Zc
Scheme 2c describes a general route to the synthesis of oxazoles,
imidazoles and thiazoles of structure 27, 29 and 31 respectively. Acid 2 (see
Scheme la) is converted to the ester by methods that are well known in the art
(Theodora W. Greene and Peter G. M. Wuts, John Wiley and Sons, Inc. 1991 ).
For example methyl ester 21 is formed by treating the acid in an appropriate
solvent such as methanol with trimethylsilyldiazomethane. Alternatively the
acid is treated with oxalyl chloride and catalytic amounts of
dimethylformamide (DMF) in an appropriate solvent such as dichloromethane
to form the acid chloride, which is then treated with methanol to give the
methyl ester. Ester 21 is treated with a palladium (0) catalyst such as
palladium
tetrakistriphenylphosphine, and an alkylstannane such as hexa-n-
butyldistannane or tri-n-butyltin chloride in an appropriate solvent such as
DMF at elevated temperatures (50 °C - 120 °C) to give the
arylstannane of
general structure 22 (Stifle, J.K., Angew. Chem. Int. Ed. Engl. 25:508-524
(1986)). The stannane 22 is then treated with acid chlorides in the presence
of
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-53-
S a palladium(0) catalyst to give ketone 23. The ketone is treated with
ammonia/ammonium chloride to give amine 24. Alternatively the ketone is
reacted with an azide such as sodium azide in a suitable solvent such as DMF,
and the resulting azidoketone is reduced to amine 23 with a suitable reducing
agent such as catalytic hydrogenation in the presence of palladium on carbon
and an acid such as HCl CChem. Pharm. Bull. 33:509-514 (1985)).
Ketoamides 25 are formed by coupling the ketoamine 24 with a variety of
suitably functionalized acid chlorides. Alternatively amide coupling may be
performed using any of a number of peptide coupling reagents such as 1,3-
dicyclohexylcarbodiimide (Sheehan, J. C. et al., J. Am. Chem. Soc., 77:1067
(1955)) or Castro's reagent (BOP, Castro, B., et al., Synthesis 413 (1976)):
In
another approach, amides 25 are formed directly from ketones 23 by reacting
with various amide salts in an appropriate solvent such as DMF. The amide
salts are generated by treating the amides with a suitable base such as sodium
hydride (NaH). For example acetamide is treated with NaH in DMF at 0 °C
to
give sodium acetamide. Keto amide 25 is cyclized to the oxazole 26, imidazole
28 and thiazole 30 using procedures similar to that shown in scheme 2b.
Oxazole 26, imidazole 28 and thiazole 30 are treated with trimethylaluminum
and ammonium chloride in refluxing toluene to give the amidines 27, 29 and
31 respectively.
Scheme 2d
Scheme 2d illustrates to the preparation of compounds of Examples
42-43, where RZ' and R43 correspond in Formula I to groups R3 and Rz,
respectively. The acids 2 can be converted to the stannane by treatment with
base, such as n-butyl lithium or sec-butyl lithium, followed by trimethyltin
chloride. The resulting acid can be then converted to the ester 22 by methods
that are well known in the art (Theodora W. Greene and Peter G. M. Wuts,
Protective Groups in Organic Chemistry, John Wiley and Sons, Inc. 1991).
For example the methyl ester can be made by treating the acid 2 in a suitable
solvent such as methanol with trimethylsilyldiazomethane. The stannane 22
CA 02362390 2001-08-07
WO 00/47578 PCT/LJS99/18065
-54-
can be reacted with suitable halides in the presence of catalytic amounts of a
palladium catalyst, such as palladium tetrakistriphenylphosphine, to give the
esters 32 (Stille, J.K., Angew. Chem. Int. Ed. Engl. 25:508-524 (1986)). These
esters are then treated with trimethylaluminum and ammonium chloride in
refluxing toluene to give the amidines 33. In the case where R43L~ (n = 2),
this
can be cross-coupled to an aryl, heteroaryl or vinyl boronic acid or ester to
give
compounds 34 (Miyaura, N. and Suzuki, A., Chem. Rev. 95:2457-2483
(1995)). This can usually be done in the presence of catalytic amounts of a
palladium (0) catalyst such as tetrakistriphenylphosphine palladium and a base
such as potassium carbonate in DMF at 90°C. Similar cross-coupling
reactions
can also be achieved by using aryl, heteroaryl and vinyl stannanes instead of
boronic acids or esters. These esters are converted to the amidines 35 in the
manner previously described.
Scheme 2e
Scheme 2e represents a modification to the methodology outlined in
Scheme 2b which allows synthesis of compounds of Formula 11 where Ar = 2-
thiazolyl, 2-oxazolyl or 2-imidazolyl (Y = bond and Z = NR'R6) but which are
regioisomeric to 16, 18 or 20 in the relative positions of substituents RZ6
and
RZ'. This is illustrated in Scheme 2b by the synthesis of 2-oxazolyl
derivative
39. Thus, acid 13 can be coupled to an hydroxy-containing amine
RZ'CH(NHZ)CH(R26)OH to give amide 36 by any of a number of amide
coupling reagents well known in the art (see Bodanszky, M. and Bodanszky,
A., The Practice of Peptide Synthesis, Springer-Verlag, New York ( 1984)).
More preferably 13 can be converted to the corresponding acid chloride using
any of the procedures mentioned for conversion of 3 to 4 followed by
treatment with the RZ'CH(NHZ)CH(R26)OH in the presence of an acid
scavenger, preferably N,N diisopropylethylamine (DIEA) or pyridine in a
suitable solvent such as DMF, dichloromethane or tetrahydrofuran (THF) to
give 36. Oxidation of the alcohol 36 to the aldehyde 37 (R'-6 = H) or ketone
37
(R26 = alkyl, aryl, aralkyl, heterocycle) can be effected by am~ of a number
of
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-5 5-
common methods known in the art (see for example F. Carey, F.A., Sundberg,
R.J. Advanced Organic Chemistry, Part B: Reactions and Synthesis, 3rd
Edition, Plenum Press, New York (1990)), preferably by a mild Moffatt-type
oxidation such as a Swern oxidation (Mancuso, A.J., Huang, S.L. and Swern,
D., J. Org. Chem. 3329 (1976)) or more preferably using Dess-Martin reagent
(Dess, D.B. and Martin, J.C., J. Org. Chem. 48:4155 (1983)). Conversion to
the heterocycle (in this case the oxazole) is effected with any of a number of
reagents including phosphorus oxychloride, Pz05 or thionyl chloride (see
Moriya, T., et al., J. Med. Chem. 31:1197 (1988) and references therein).
Alternatively closure of 37 with either Burgess reagent or under Mitsunobu
conditions affords the corresponding oxazolinyl derivatives (Wipf, P.and
Miller, C.P., Tetrahedron Lett. 3: 907 ( 1992)). Final amidination to 39 as in
Scheme lb completes the synthesis.
Scheme 2f
Scheme 2f illustrates a general approach to the synthesis of thiazoles of
structure 43 (Formula II, X = S, Ar = thiazolyl). Nitriles of structure 40 can
be
treated with hydrogen sulfide (HzS) in a suitable solvent such as methanol, or
pyridine in the presence of a base such as triethylamine to give thioamides 41
(Ren,W. et al., J. Heterocyclic Chem. 23:1757-1763 (1986)). Thioamides 41
can be then treated with various haloketones 42 preferably bromoketones
under suitable reaction conditions such as refluxing acetone or DMF heated to
50° C - 80° C to form the thiazoles 43 (Hantzsch, A. R. et al.,
Ber. 20:3118
( 1887)).
Scheme 2g
Scheme 2g illustrates one synthetic route to 2-haloketones of structure
42 which are employed in the synthesis of thiazolyl derivatives as in Schemes
2a and 2f. 2-Bromoketones 42 (L = Br) are prepared by treating the ketone 44
with a suitable brominating agent such as Br2 or N-bromosuccinimide in a
suitable solvent such as chloroform or acetic acid (EP 0393936 A1).
CA 02362390 2001-08-07
WO 00/47578
-56-
PCT/US99/18065
Alternatively, the ketone 44 is treated with a polymer-supported brominating
agent such as poly(4-vinyl)pyridinium bromide resin (Sket, B., et al.,
Synthetic
Communications 19:2481-2487 (1989)) to give bromoketones 42. In a similar
fashion 2-chloroketones are obtained by treating 44 with copper (II) chloride
in
a suitable solvent such as chloroform (Kosower, E. M., et,al., J Org. Chem.
28:630 (1963)).
CA 02362390 2001-08-07
WO 00/47578
_57_
Scheme 2h
PCT/US99/18065
Scheme 2h illustrates another synthetic route to 2-haloketones of
structure 42 which is particularly useful in that it employs acids 45 or
activated
carbonyl compounds such as 46 as precursors which are more readily available
than the ketones 44. The acid 45 is converted to the acid halide 46 (L = Cl,
Br
or OCOR39) by treating with a suitable halogenating reagent. For example, an
acid chloride is formed by treating 45 with oxalyl chloride and catalytic
amounts of DMF in dichloromethane. The acid chloride is converted to a
diazoketone by treatment with trimethysilyldiazomethane (Aoyama, T. et al.,
Tetrahedron Lett. 21:4461-4462 (1980)). The resulting diazoketone is
converted to a 2-haloketone of structure 42 by treatment with a suitable
mineral acid. For example a bromoketone is formed by treating the
diazoketone in a suitable solvent such as acetonitrile (CH3CN) with a solution
of 30% hydrogen bromide (HBr) in acetic acid (Organic Synthesis Collective
Vol III, 119, John Wiley and Sons, New York, Ed. Horning E. C.). In an
alternative approach the acid 45 is converted to the mixed-anhydride 46 by
treatment with a suitable chloroformate such as isobutyl chloroformate or tert-
butyl chloroformate in a suitable solvent, such as tetrahydrofuran or
dichloromethane, in the presence of a base such as N-methylmorpholine. The
mixed anhydride 46 is converted to a diazoketone by treatment with
trimethylsilyldiazomethane and the resulting diazoketone is converted to a
haloketone in the manner described above.
Scheme 2i
When amide coupling as described in Scheme 2e is followed directly
by amidination, compounds of Formula I where R' or R3 is aminoacyl or
aminoiminomethyl can be derived. Thus, coupling of acid 13 (or the
corresponding acid chloride as previously described) with an amine RS'RSZNH
can afford 130 which can be carried on to the amidine 131. Upon either longer
or more vigorous additional treatment (for example, higher temperatures) with
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-5 8-
a Lewis acid-ammonia reagent as described in Scheme lb, the amide group
can be converted to an aminoiminomethyl group to give a bisamidine
compound 132.
Scheme 3a
Acid 13 can also be converted to an amine 47 from which
sulfonamides, ureas and urethanes can be formed (Formula 1 where RZ or R3 =
NR3zSO2R3', NHCONRS'R52 or NHCOR3', respectively). Scheme 3a
illustrates this methodology for introduction of these three groups at RZ of
Formula 1. Conversion of the acid 13 to an intermediate acyl azide can be
followed by heating of such azide in the presence of an alcohol under Curtius
rearrangement conditions to form the carbamate ester of the alcohol.
Subsequent carbamate ester hydrolysis yields amine 47. The intermediate acyl
azide may be synthesized by coupling the acid 13 to hydrazine through the
acid chloride or by any of the amide coupling procedures discussed for
Scheme 2e followed by nitrosation of the resulting hydrazide by any of the
nitrosating agents discussed for conversion of 3 (R' = NHz) to 3 (R' = H) in
Scheme lc. More preferably conversion of 13 to 47 is carried out through
treatment of acid 13 with diphenylphosphoryl azide in the presence of an
alcohol, preferably tent-butanol, and a base, preferably triethylamine or
DIEA,
as shown in Scheme 3a, to give a tert-butylcarbamate that is readily
decomposed to the salt of amine 47 on exposure to an acid, preferably HCl or
trifluoroacetic acid in a suitable solvent such as CHZCIz. Further treatment
with a base such as NaOH or preferably KZC03 or NaHC03 provides the free
base 47. Treatment of amine 47 with a sulfonyl chloride R3' SO~CI in the
presence of an acid scavenger, such as pyridine or DIEA, followed by optional
alkylation on nitrogen with an alkylating agent R32L in the presence of a base
such as KZC03, DIEA or more preferably sodium hydride, in a solvent such as
THF, MeCN or CHZC12 affords the sulfonylamine functionaliy at RZ (48).
When necessary, this transformation can be catalyzed by the presence of 4-
dimethylaminopyridine for less reactive sulfonyl chlorides. Similar treatment
CA 02362390 2001-08-07
WO 00/47578
-59-
PCT/US99/18065
of amine 47 with an isocyanate RS'NCO or carbamyl chloride RS'RSZCOCI
affords the aminocarbonylamine functionality at RZ (50). Similar treatment of
amine 47 with an acid chloride R3'COCI affords the carbonylamine
functionality at RZ (52). Conversion of the esters in 48, 50 and 52 to
amidines
as previously mentioned gives the products 49, 51 and 53. Further conversion
of the acylamino group of 53 as discussed for synthesis of 132 also provides
access to the iminomethylamino group at RZ (54).
Scheme 36
Introduction of an aminosulfonyl group (including
monoalkylaminosulfonyl and dialkylaminosulfonyl groups) for Rz of Formula
1 can be carried out starting from amine such as 47 as well. Conversion to a
sulfonyl chloride by the method of Gengnagel, et al. (U.S. Patent No.
3,947,512 (1976)) and treatment with an amine R34NHz followed by optional
alkylation on nitrogen with R35L (under the sulfonylation and alkylation
conditions described in Scheme 3a) provides 56 which is further converted to
amidines 57 as previously described.
Scheme 3c
In addition to the synthesis outlined in Scheme 3a, amine 47 may also
be produced as illustrated in Scheme 3c. A nitrothienyl ester 122 (Dell'Erba,
C. and Spinelli, D., Tetrahedron, 21: 1061 (1965), Dell'Erba, C. et al., J.
Chem. Soc, Perkin Trans 2, 1779 (1989)) with a suitable leaving group L may
be substituted with an anion of RZ' to give intermediate 123. Amine 47 is then
derived from reduction of the nitro group. Appropriate reagents to effect
reduction of the nitro functionality include hydrogen gas in the presence of a
catalyst such as palladium or platinum metal deposited on carbon or barium
sulfate in any of a number of solvents such as methanol, ethanol, ethyl
acetate,
DMF or THF. More preferably, tin (II) chloride may be employed as a
reductant in solvents such as DMF or THF, or in the presence of HCl in a
solvent such as methanol or ethanol. Alternatively, metals such as zinc or
iron
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-60-
(Stanetty, P. and Kremslehner, M., Heterocycles 48: 259 (1998)) may also be
used.
Scheme 4a
Scheme 4a illustrates the preparation of the compounds of Formula 111
and Examples 48-59 and 61-77. The amidine moiety of compounds of
structure 60 can be protected with a protecting group P' that can be readily
removed from 62 and 64 using methods known to those skilled in the art
(Theodora W. Greene and Peter G. M. Wuts, John Wiley and Sons, Inc. 1991).
For example, a tert-butoxycarbonyl (BOC) protecting group can be removed
by exposure to strongly acidic medium such as hydrogen chloride in a suitable
solvent such as dioxane, or by trifluoroacetic acid in a suitable solvent such
as
methylene chloride. Benzyloxycarbonyl (Cbz) protecting groups can be
removed by catalytic hydrogenation using palladium on carbon as a catalyst in
solvents such as ethanol or tetrahydrofuran.
In some cases, P' can be a solid support such as polystyrene or
polyethyleneglycol-grafted polystyrene which can be attached to the amidine
moiety via a cleavable linker such as 4-(benzyloxy)benzyloxy-carbonyl (using
carbonate Wang resin). Attaching an amidine to a solid support can be
achieved by treating a solid support having a linker containing an
appropriately
activated functional group with the amidine under suitable conditions. For
example, an amidine can be attached to Wang resin by treating para-
nitrophenylcarbonate Wang resin with the amidine and a suitable base such as
DBU in a suitable solvent such as DMF. When D is OH or SH the protected
amidines 61 can be alkylated with carboxy-protected (protecting group is R36)
haloaliphatic acids, such as bromoacetic acid or bromopropionic acid in the
presence of a suitable base such as cesium carbonate~or DIEA, in a suitable
solvent such as DMF with heating when necessary to give compounds of
structure 62. When D is NO2, the nitro group can be reduced prior to
alkylation
using an appropriate reducing agent, such as tin (II) chloride, in a suitable
solvent such as DMF, or by catalytic hydrogenation using palladium on carbon
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-61-
as a catalyst in solvents such as ethanol or tetrahydrofuran. Other useful
carboxy protecting groups are well known in the art (Theodora W. Greene and
Peter G. M. Wuts, Protective Groups in Organic Chemistry, John Wiley and
Sons, Inc. 1991 ). For example, tent-butyl ester can be removed by exposure to
strongly acidic medium such as hydrogen chloride in a suitable solvent such as
dioxane, or such as trifluoroacetic acid in a suitable solvent such as
methylene
chloride. Benzyl ester can be removed by catalytic hydrogenation using
palladium on carbon as a catalyst in solvents such as ethanol or
tetrahydrofuran or by base hydrolysis.
When protecting groups P' and R36 in compounds 62 are orthogonal (as
1 S defined by the ability to remove one protecting group preferentially in
the
presence of the other), R36 can be preferentially removed to give acids 63.
For
example when P' is BOC and R36 is OMe, the methyl ester can be removed by
treating with a base such as sodium hydroxide in a suitable solvent such as
aqueous tetrahydrofuran leaving the BBC group intact. When protecting
groups PI and R36 in compounds 62 are not orthogonal, both protecting groups
are removed, and the amidine can be protected with a suitable protecting group
such as BOC or a suitably functionalized resin. The protected amidine 63 can
be treated with various amines under suitable amide coupling conditions, such
as in the presence 1-hydroxy-7-azabenzotriazole (HOAt), O-(7-
azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
(HATU) and DIEA to form amides of structure 64. The amidine protecting
group can be then removed, for example by treating with an acid, such as
trifluoroacetic acid in a suitable solvent such as methylene chloride, when a
BOC protecting group is employed, to give amidines 65.
Scheme 4b
Scheme 4b illustrates a specific example which utilizes the method
described in Scheme 4a. The amidine moiety of 66 can be monoprotected
with a tert-butyloxycarbonyl group. The monoprotected phenoxyamidine 67
can be alkylated on the phenolic hydroxy group with an ester of 2-bromoacetic
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-62-
acid to give 68. In the case where the ester can be removed by base, it can be
hydrolyzed with aqueous base, such as NaOH, to give the acid 69. This acid
can be treated with various amines in the presence of 1-hydroxy-7-
azabenzotriazole (HOAt), O-(7-azabenzotriazol-1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate (HATU) and DIEA to form amides
of structure 70. The amines are unsubstituted, di- or mono-substituted
aliphatic or aromatic amines. In some cases the amines are cyclic-amines such
as piperazine and piperidine. The amides 70 are then treated with
trifluoroacetic acid to give the amidines 71. In the case where the ester 68
is
acid-labile, it can be treated with trifluoroacetic acid to give the amidino-
acid
72. This amidine can be loaded on to an insoluble support, such as polystyrene
or polyethyleneglycol-grafted polystyrene via a cleavable linker, such as
Wang, which is functionalized as an activated carbonate such as p-
nitrophenylcarbonate or succinimidyl carbonate. Generally this can be done by
treating the activated carbonate resin with the amidine and a suitable base
such
as DBU in a suitable solvent such as DMF. The support-bound acid 73 can be
treated with various amines in the presence of 1-hydroxy-7-azabenzotriazole
(HOAt), O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium
hexafluorophosphate (HATU) and DIEA to form amides. These amides are
then cleaved from the solid support by treating with trifluoroacetic acid to
give
compounds of structure 71.
Scheme S
Scheme 5 illustrates a synthetic route to amidines containing di-
substituted thiazoles represented by compounds for which Rz is Formula II
and both R8 and R9 are non-hydrogen substituents . The ketoamide 74 can be
converted to the mono-bromoketoamide by treating with bromine in acetic
acid. Thiazoles 76 are formed by reacting the bromoketoamide with 10 under
suitable conditions, preferably by heating the mixture in DMF or acetone.
Amidines 77 are formed by heating 76 in toluene with trimethylaluminum and
ammonium chloride. The amidines 77 are treated with strong acid such as HCl
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-63-
S to give the acids 78. The amidines 78 are in one route protected with a
suitable protecting group such as BOC to give 79. The protected amidines 79
are treated with various amines under suitable coupling conditions, such as in
the presence of HOAt, HATU, and DIEA to form various amides. The amidine
protecting group can be then removed, for example by treating with
trifluoroacetic acid in a suitable solvent such as methylene chloride, when a
BOC protecting group is employed to give amidines 80. In a second route, the
amidines 78 can be loaded onto an insoluble support, such as polystyrene or
polyethyleneglycol-grafted polystyrene via a cleaveable linker, such as Wang
resin, which is functionalized as an activated carbonate ester, such as p-
nitrophenylcarbonate or succinimidyl carbonate, to give a resin-bound scaffold
81. The resin-bound acid 81 can be treated with various amines under suitable
coupling conditions such as in the presence of HOAT, HATU and DIEA to
form amides. These amides are then cleaved from the solid support by treating
with trifluoroacetic acid to give amidines 80.
Scheme 6a
Scheme 6a illustrates the preparation of compounds of Examples 144,
145, 146, 147, 148, 149, 150 and 151. Compounds of this invention
correspond to those of Formula I where Rz is Formula II and where Ar is
thiazole and R3' and R38 (Rg and R9 of Formula II) are phenyl, which can be
additionally substituted. Starting from 2,5-dibromothiophene 90, treatment
with lithium diisopropylamide followed by RZ'L, where L is a leaving group,
preferably a halogen, mesylate, tosylate, or methyl sulfate, and more
preferably
iodomethane or methyl sulfate, according to the procedure of Kano, et al.,
Heterocycles 20(10):2035 (1983-), gives 91. Compound 91 can be treated with
an appropriate base, preferably a lithium alkyl like n-butyllithium, sec-
butyllithium, or t-butyllithium, and more preferably n-butyllithium, followed
by carbon dioxide gas and conversion of the resulting carboxylate salt to the
free acid with a mineral acid, preferably hydrochloric acid. Conversion to
ester 21 can be carried out by preparation of the acid chloride using oxalyl
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_64_
chloride and treatment of this intermediate acid chloride with an alcohol R23
in
an appropriate solvent, preferably dichloromethane, with an appropriate base,
preferably pyridine. Compound 21 can be treated with copper (I) cyanide in
refluxing dimethylformamide to give compound 9. Compound 9 can be
treated with hydrogen sulfide gas in an appropriate solvent, preferably
methanol, containing an appropriate base, preferably triethylamine to give
compound 10. Compound 10 can be treated with an appropriate ketone where
L is a leaving group, preferably halogen, mesyl, or tosyl, and most preferably
bromo, refluxing in a suitable solvent, preferably, acetone,
dimethylformamide, dimethyl acetamide, methyl ethyl ketone, or other polar
aprotic solvents, and most preferably acetone to give compound 92.
Compound 92 is treated with an appropriate reagent, preferably the aluminum
amide reagent to give amidine 93.
Scheme 6b
Scheme 6b illustrates the preparation of the compound of Example
144, which corresponds to a compound for which RZ is Formula II, and where
Ar is thiazole and R8 and R9 (R3' and R38 in Scheme 6b) are phenyl, which can
be optionally substituted. Starting from 2,5-dibromothiophene 90, treatment
with n-butyllithium produces an anion which undergoes a rearrangement
(Kano, S., et al, Heterocycles 20:2035 (1983)). Quenching with carbon
dioxide gas and conversion of the resulting carboxylate salt to the free acid
with a mineral acid, preferably hydrochloric acid, gives acid 94. Conversion
to
ester 95 can be carried out by preparation of the acid chloride using oxalyl
chloride and treatment of this intermediate acid chloride with an alcohol Rz3-
OH in an appropriate solvent, preferably dichloromethane, with an appropriate
base, preferably pyridine. Compound 95 can be treated with copper (I)
cyanide in refluxing dimethylformamide to give compound 96. Compound 96
can be treated with hydrogen sulfide gas in an appropriate solvent, preferably
methanol, containing an appropriate base, preferably triethylamine to give
compound 97. Compound 97 can be treated with an appropriate ketone where
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-65-
L is a leaving group, preferably halogen, mesyl, or tosyl, and most preferably
bromo, refluxing in a suitable solvent, preferably, acetone,
dimethylformamide, dimethyl acetamide, methyl ethyl ketone, or other polar
aprotic solvents, and most preferably acetone to give compound 98.
Compound 98 is treated with an appropriate reagent, preferably the aluminum
amide reagent (Al(CH3)3/NH4Cl) to give amidine 99.
Scheme 7a
Scheme 7a illustrates the preparation of compounds for which RZ is
Formula 11 and Ar is thiazol-4-yl. As illustrated, the acids 13 can be
converted
to their acid chlorides by treatment with oxalyl chloride with
dimethylformamide catalysis in methylene chloride, or by using thionyl
chloride, either neat or in an organic solvent, at ambient or elevated
temperature. Compounds are then homologated to the desired a-haloketones
100 by sequential treatment with trimethylsilyldiazomethane and hydrogen
bromide. An alternative would be to substitute diazomethane (generated from
Diazald~, Aldrich Chemical Co., Milwaukee, WI) for the
trimethylsilyldiazomethane. Also, the conversion of 13 to 100 can be effected
using the procedure derived for the synthesis of compound 42 from compound
46.
The alpha-haloketones 100 are then allowed to react with the
appropriate thiourea (Scheme 7b) or thioamide derivative in an organic
solvent, preferably acetone or dimethylformamide at 70 ° C to give 2-
aminothiazoles or thiazoles 101.
The thiazoles 101 can be treated with the aluminum amine reagent
(Al(CH3)3/NH4Cl) formed at ambient temperature by the reaction of
trimethylaluminum with ammonium chloride in an organic solvent, preferably
toluene. The ester can then be converted to the amidines 102 at elevated
temperatures, preferably higher than 80 °C.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-66-
Scheme 7b
As shown in Scheme 7b, amines 110 (or their hydrochloride salts) can
be converted to their respective mono-substituted thioureas (methan-1-
thiones) 112 by treatment with thiophosgene to form the intermediate
isothiocyanates 111. Preferred conditions include treating the amine with
thiophosgene in a biphasic solvent system composed of a halogenated solvent
such as chloroform and an aqueous phase of saturated sodium bicarbonate.
Alternatively, the reaction may be effected by treatment of 110 with a
hindered
amine and thiophosgene such as triethylamine or diisopropylethylamine in an
organic solvent such as tetrahydrofuran or methylene chloride. Another
alternative to forming isothiocyanates 111 is the direct treatment of primary
amines and carbon disulfide in pyridine with dicyclohexylcarbodiimide
(Jochims, Chem. Ber. 101:1746 (1968)).
Isothiocyanates 111 can be converted to thioureas 112 by treatment
with an ammonia-alcohol solution, preferably a 2M ammonia in methanol or
ethanol solution, at room temperature or elevated temperatures (>70°C).
Alternatively, the thioureas 112 can be prepared directly form the appropriate
urea (or thioamide from the appropriate amide when R8 =alkyl or aryl)) by
treatment with Lawesson's reagent (Lawesson, S.-O., et. al. Bull. Soc. Chim.
Belg. 87:223, 293 (1978)).
Scheme 8
Scheme 8 illustrates the preparation of compounds of this invention
where Rz is Formula II and Ar is thiazole and R3' and R38 are phenyl which is
further substituted by a sulfonylamino or carbonylamino group. Starting from
thioamide 10, treatment with a nitro substituted 2-halo-acetophenone, where
the halogen is chloro, bromo, or iodo, preferably bromo, refluxing in a
suitable
solvent, preferably acetone, dimethylformamide, dimethyl acetamide, methyl
ethyl ketone, or other polar aprotic solvents, and most preferably acetone.
The
reduction of nitroaryl compound 113 can be carried out with a suitable
reducing agent, preferably tin (II) chloride, titanium (II) chloride, iron
(III)
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-67-
chloride, lithium metal, sodium metal, catalytic hydrogenation over platinum
or palladium catalyst, and most preferably 20% aqueous solution of titanium
(III) chloride. The acylation of aniline 114 can be carried out with an
appropriate acyl compound .R4zL where L is a halogen, preferably chloro, in an
appropriate solvent, preferably dichloromethane, containing a base, preferably
pyridine, N-methylmorpholine, or diisopropylethylamine. Alternatively, the
acylation of aniline 114 is carried out with an activated carboxylic acid
compound R42COL where L is hydroxy activated with
dicyclohexylcarbodiimide, ethyl-3-(diethylamino)propylcarbodiimide
(EDAC), O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate (HATU), or pentafluorophenyl. The sulfonylation of
aniline 114 can be carried out with and appropriate sulfonyl chloride
compound R4'SOZL in an appropriate solvent, preferably dichloromethane,
containing a base, preferably N-methyl morpholine, diisopropylethylamine, or
pyridine, most preferably N-methyl morpholine, with or without a
condensation catalyst, preferable dimethylaminopyridine (DMAP). The
amidinylation of compounds 115 and 117 can be carried out with an
appropriate reagent, preferably the aluminum amide reagent
(Al(CH3)3/NH4Cl).
Scheme 9
Scheme 9 illustrates the preparation of compounds of Formula I, for
which one of RS and R6 is a non-hydrogen substituent. The amidines 5 are
converted to the amidoximes 119 by heating with hydroxylamine in a suitable
solvent such as ethanol. The cyanoamidines 120 are prepared by heating the
amidines 5 with cyanamide in a suitable solvent such as ethanol. (Huffman,
K.R. and Schaeffer, F., J. Amer. Chem. Soc. 28:1812 (1963). Alternatively 5
can be heated with an amine such as methylamine to give the N-alkylated
amidines 121.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-68-
S Scheme 10
Scheme 10 illustrates an approach to compounds of Formula I where X
= S or O, RZ = arylamino, R3 = alkylthio, aralkylthio, arylthio, alkyloxy,
aralkyloxy, aryloxy, alkylamino, dialkylamino, aralkylamino, diaralkylamino,
arylamino or diarylamino, Y = bond and Z = NRSR6.
Aminothiophenes 47 (Formula I where X = S, or O; Rz =NHz) can be
reacted with an arylboronic acid (RS6B(ORS$)Z, R58 = H) or arylboronic ester
(RS6B(OR58)2, R58 = alkyl) in the presence of a copper catalyst, preferably
copper (II) acetate, and an amine base such as triethylamine or pyridine
(Chan,
D. M. T. et al., Tetrahedron Lett 39: 2933 (1998)) to give a thienylarylamine
124. Conversion of the ester to amidine 125 is carried out in the manner
previously described in Scheme lb.
Sclzeme ll
Another route to compounds of this invention, where RZ = arylamino or
alkylarylamino and alkylamino and where R3, Y and Z are as described in
Scheme 10, is shown in Scheme 11 where intermediate 2 (RZ' = R3, L =
leaving group) is aminated using conditions well known in the art. (See for
example: Ahman, J. and Buchwald, S. L., Tetrahedron Lett. 38: 6363 (1997)
and Wolfe, J. P. and Buchwald, S. L., Tetrahedron Lett. 38: 6359 (1997). For
reviews see: Frost, C. G and Mendonca, P., J. Chem. Soc, Perkin Trans l:
2615 (1998) and Wolfe, J. P. et al., Acc. Chem. Res. 31: 805 (1998).) Thus, 2
may be treated with an aniline R56R5'NH (R56 = aryl, RS' = H or alkyl) in the
presence of a palladium catalyst, a suitable palladium ligand and a base to
give
127. Suitable catalysts include any of a number of Pd(0) or Pd(II) salts, such
as tetrakis(triphenylphosphino) palladium (0),
dichlorobis(acetonitrile)palladium (II) or preferably palladium (II) acetate
or
tris(dibenzylideneacetone)dipalladium. The most appropriate ligands for any
given reaction are often compound-dependent and are discussed in detail in the
aforementioned references but may include l,l'-
bis(diphenylphosphino)ferrocene (DPPF), 1-[2-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-69-
(diphenylphosphino)ferrocenyl]ethyl methyl ether (PPF-OMe), or preferably
2,2'-bis(diphenylphosphino)-l,l'-binaphthyl (BINAP). Appropriate bases
include sodium t-butoxide or preferably cesium carbonate or potassium
phoshate. Useful solvents include DMF, dioxane, dimethoxyethane or
preferably toluene. Conversion of the ester to amidine 128 is carried out in
the
manner previously described in Scheme lb.
Scheme 12
The corresponding compounds of Formula I where RZ = alkylamino
and R3, Y and Z are as described in Scheme 10 are produced as shown in
Scheme 12 by initial reductive alkylation of amine 47 with an aldehyde
R59CH0 or ketone R59COR6° in the presence of any of a number of
suitable
reducing agents including sodium borohydride, sodium cyanoborohydride or,
more preferably, sodium or tetraalkylammonium salts of triacetoxyborohydride
to give 129. Depending on the reducing agent employed, suitable solvents
may include an alcohol, such as methanol, ethanol or isopropanol, or solvents
such as THF or dichloromethane. Conversion of the ester to the amidine 130
is again carried out in the manner previously described in Scheme lb.
Scheme 13
Scheme 13 illustrates an approach to compounds of Formula I where
Rz is a 2-thiazolylamino and R3, Y and Z are as described in Scheme 10. In
this
method, amine 47 can be first converted to a thiourea 131 using the various
procedures outlined in Scheme 7b. Further reaction of the thiourea with a
leaving group-substituted ketone R3'COCH(L)R3~, preferably a 2-haloketone
as described in Schemes 2f, 2g or 2h, can provide thiazolylaminothiophenes
132 which are then converted to the corresponding amidines 133 by the
previously described methodology of Scheme lb.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-70-
Scheme 1 a
(_ R21 R21
X ~ R21 X ~ R~ ~ X ~ 22
L ~ Br ~ R
w w w
W R1 1 W R1 2 W R1 3
X=O,S
W = CN, COOH, CONH2, CO2R23
L=Br
Scheme 1 b
Rz1 R~ R21
R°N X ~ ~ W = NH2 X ~ W = NHz RaN
R tR~ -~ R22
W ~
H R~ 135 R' 3 Z" NR° H R~ 136
Z = H, alkyl Z = NR5R6 Z = NR5R6
W = COOH W = CN, CONHz, or W = CN
(COCI)z then
Rzs OH C02Rz3 W = CN HCI I Rz30H
(or CH2Nz R5 = Rs = H R5 = alkyl, aryl, aralkyl
R23 = Me) AI(CH3)3 I NH4CI AI(CH3)3 I RSRsNH
Toluene I D Toluene I 4
Rz~ Rzi R2~
AI(CH3)3
Rza X ~ R~ NH4C~ i 5 X ~ R~ R~ i ~ X ~ Rzz
O
Toluene / 4 Rs/ ~ HCI
O R~ 4 R5 = Rs = H NH R1 5 NH R~ 6
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-71-
Scheme 1 c
R~ SR5°
base X ~ base ~ R~
CSz + RSZCHZRzz + R~L + WCHyL --a R~ ~-- WCHZSH + '~~--R~
W R~
Rzz = CN, COZRz3, CONHz R~ 3 zz - 138
R5z = CN, CO2Rz3 R - CN
W = CN, CO2Rz3, CONHz X = S
L = CI, Br, I, OTs, OMs, OTf Rz~ = SRS
R~ = OH, NHz
W = CN, CONHz, COZRza
Rzi
diazotization
R~
R~ = NHz W
X=S
R~ 3 R~ = H
Scheme 1 d
R21 L
X ~ L E X ~ X \ L
w w w
W W W
R1 2 139 R1 R1 1
R21 =H X=O,S L=Br
L = Br W = CN, COOH,
CONH2, CO2R23
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-72-
Sche~ze 2~c
L R~ 2~
X ~ Rz~M X ~ MCN X ~ CN
L ~ Br
M = Li, Na, K ~O ~ H,O
W
1 X=O,S O 7 8 O
W = COOH
(COCI)z
L= Br
then Rz30H
or CHZNz
(Rz3 = Me)
R~ Rz~ R2~
S R S
X \ \ ( RzaCOCH(Rz5)L X ~ HzS i ~ X ~ CN
~O ~ ~ D O ~ O w
f? R Rx~ NHz
O 11 O 10 O
L = CI, Br, 1, OMs, OTs
AI(CH3)3
NH4CI
Toluene I 4
R2~
S Rzs
X
HZN
N Rza
NH 12
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-73-
Scheme 2b
F
F COOH
Rzt
Rz1 1 ) ~ (COCI)z
X F ~ COOH 2) Rz6COCH(Rz7)NHz
CN F X ~ COOH
O
R2~ ~ RIO \ 1) (COCI)2
O 2) RzsCOCHZNHz
9 O 13
3) Optional alkylation
X = O, S with base / Rz7L
R2t
X ~ O ~ L = CI, Br, I, OMs, OTs
R
RIO w H
14 R2~
O
O
POCI3 NH40Ac PzSs
HOAc or Lawesson's
reagent
R2t Rzt Rzt
O R~ H R~ R2s
N X S
zs~0 ~\ \N' \ 27 230 X~\ N' \ 27 O
R R R R ~ 2'r
R R
O 15 O 17 19
O
AI(CH3)3/ NHyCI AI(CH3)3/ NH4C1 AI(CH3)3/ NH4C1
Toluene I D Toluene l o Toluene/ D
R2t R~ Rzt
O R2s X H Rzs S R~
X \ \ X
Hz w \ ~ Hz w \~ 27 H2
N Rz~ R N R~
NH
NH 16 1$ NH 20
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-74-
Scheme 2c
Ray Rzi
CHZNz
(R~= Me) X \ Pd(0) catalyst X ,
Br --~ ze
2 or (C~ RIO ~ (SnR~a)z Rz~O w SnR
then Ra30H O 21 O 22
X = O, S Rze=nBu
W = COOH
L = CI, Br, I, OTf
Rzi
Pd(0) catalyst X \ O NH3/ NH4CI
R~aCH(L)COCI R23/O ~ L 1) MN3
O R 2) H2 I Pd (cat)
L = CI, Br, I, OMs, OTs 23 HCI
R2~ Rzt
R3°CONHM
X ~ O R3°COCI X O
RIO ~ NHz RzaiO ~ ~NH
O R R3°
R ~HCI O O
24 25
POC13 NH40Ac
HOAc or Lawesson's
re a ge nt
1
Rzf R2s Rz~ R2s Ray Rzs
X ~ ~~N X ~ / N X
Ra~O ~ O' \R3o Rza~O ~ N' \R3o ~O ~ S 30
H R R
O 26 O 2g O 30
AI(CH3)3/ NH4C1 ~ AI(CH3)3 ! NH4C1 AI(CH3)3I NH4C1
Toluene I D Toluene I D Toluene I D
R2~ R2s Rz~ R2s Ra~ R2s
X ~ ~~N X ~ ~ N X ~ ~ IN
HZN w O ~ H2N w N ~ HaN w
R H R S Rao
NH 27 NH 29 NH 31
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-75-
Scheme 2d
Rz~ Br 1. base R2~ Sn(Rz8)s
(CH3)3SnCl R43L° (n = 1-2)
X / 2. (CH3)3SiCHNz S / Pd(PPh3)a
or DMF, 120°C
W Rz3Ubase ORzs
L = Br, I, OTf
W = COOH Raa = aryl, heteroaryl, allyl, vinyl
X = S, O 22 (Rz8 = Me)
2
R21 R4sL~_7
When n = 1
S / NH4CI
AI(CH3)s
32 Toluenel~
OMe
When n = 2 R°4B(OH)z Rz~ R4a
R4° = aryl, hetero-aryl Pd(PPh3)a
DMF, 90°C
K2CO3 S /
R2~ Raa R2~ Ra3 HN/ ~NHz
\ \R 33
R4° NH4CI
S / ~ AI(CH3)3 S /
Toluenel0
H NHz OMe
35 34
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-76-
Scheme 2e
Rz~
1) (COCI)z
x \ COOH 2) Rz~CH(NHz)CH(Rzs)OH
Rz~O
13
O Rzs
oxidation p0C13
Rzs
Rye R21
Rz~ AI(CH3)3/NH4C1 R2~
Toluene/e
x \ /~ --. x \ /
Rz~p w \ HZN w
Rzs Rzs
O 38 NH 39
Scheme 2f
42 R3s
NHz
CN R3~ S\ R3~
S ~se //~~N
S R triethylamine S R w S Rzt
VV'~~~ z~ HzSh / z~ -r
40 41 43
CA 02362390 2001-08-07
WO 00/47578 PCT/~JS99/18065
_77_
Scheme 2g
0 0
R37~R~ halogenation R3~ R3s
L
44 42 L = halogen
Scheme 2h
0
R3a
1) TMSCHN2 R3~
R3~COOH ~ R3~COL 2) HL L
3$ 42
45 46 L = CI, Br
R3s = H
L = CI, Br, OCOR39 L = CI, Br
(R39 = iBu, tBu)
Scheme 2i
AI(CH3)3/NH4CI
1) (COCI)z Toluene/e
2) RstR52NH RzrO
-Rs~
O R
130
X=N,O,S
Rz~ Rzt
O AI(CH INH CI NH
X 3)3 4
Tolueneh X
HzN ~ ~5t HzN
~s~
NH Rsz R i
131 NH 132
Rz~
O
X
w
N
sz
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_78_
Seheme 3a
Rz~
Rz~
1 ) (Ph0)zP(O)N~ X.
X ~ COOH ETzNIt-BuOH \ NHz
,O
2) CF~COOHICH2CIz R23
Rz~,O
13 3) aq NaHCO~
47
O
O
1) R31SOZC1
2) R32Ubase R51NC0 R31COCI
(optional) or R5lRszNCOCI
Rzi Rz~ Rzt
X ~ NRzzSOZR3~ X ~ NHCONR5~R5z X ~ NHCORz~
R O ~ RzrO w Rz3 ~ w
52
48 O 50
O
AI(CH3)zINHaCI AI(CHz)z/NHyCI AI(CH3)31NH4C1
Toluenel~ Toluenel~ Toluenel4
Rz~ Rz~ Rzt
X \ NRzzSO2Rz~ X \ NHCONRs~Rsz X \ NHCOR3~
Hz w Hz ~ Hz w
49 51 53
NH NH NH
R AI(CH~)zINHeCI
Toluene/4
X
NH
Hz ~ ~R3t
~,f ~//H
NH 54
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_79_
Scheme 3b
Rz~
HCIINaN031 X ~ 1) R3°NHz
CuSO~/NaHS03 SOZCI 35
47 z3.0 ~ 2) R Llbase
R (optional)
O
R21 R21
AI(CH3)aINH4Cl
X \ SOZNR~R35 Toluenelo X \ SOZNR34R3a
z3~0 w Hz w
56 ~ 57
O NH
Scheme 3c
L Rz~ R2~
X
NOz R~ X ~ NOz Reducti~ X ~ NH
2
O
Rz3 O 122 Rzs~ O 123 Rzs~ 47
O
X=O,S
L = CI, Br, I, OTs, OMs
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-80-
Scheme 4a
z, z,
Ar s--p Ar e--p 1. optional reduction
w ~ w ~ 2. Br(CHz)nCOR~s
X Rs protecting (P~) grou_p X Rs Cs,CO, _
introduction ~ DMF, 70°C
n = 1-4
H NHz H
D = OH, SH, NOz
X=S,O 61
When P~ and R3s
z~ are ortho onal
z,
Ar s--E~C~n R3s removal
Ar s--E~CH~
X
X Rs ~OH
When P~ and R3s ~~ff
P, 62 are not orthogonal ~ 63
H H E=O, S, NH H ~P
1. P~ and R3s removal H
2. protecting (P~) group
Introduction
HOAT
HATU HN ~'
DMF
I,
2,
Ar ~E-(CF~n
Ar e--ESC ,
X R ~~ ~ ~.. Pt removal X Rs
P~ 64
H N~z H N~
H
S1~BST(TUTE SHEET (RULE 26~
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-81-
Scheme 4b
H \ 'O"O"O\ / grCH2COR3s
IIDIEA II I \ CspC03
DMF DMF, 70°C
R3s = OBut, OMe
66 ",
When R3s = OMe
NaOH, THF
68
When R3s = OBu~
50% TFA/DCM HOAT
2% Hp0 DMF" "HN~
/R,
H
72 ~ , .-, ~..., NO
50% TFA/DCM
2°/ H20
Linker Insoluble support
DBU, DMF
1. HOAT
HATU HN~
DMF ~"
2. 50% TFA/DCM
2% Hp0
H
Linker Insoluble support TFA salt
73
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-82-
Scheme S
0 0 / o o /
BrzIAcOH ~ Acetone reflux
\ \ ~ \ \ S
H I I H Rz~
/ ~ Br ~ ~NHz
74 75 g /
~OR23
(Rz3 = Me)
AIMe3INH4Cl HCh
toluene, reflux A
H
76 77 78
O" O"O'
O O 1. HOAT ~R'
DIEA HATU HN
DMF DMF R"
2. 50% TFA/DCM
2% HZO
79
H
1. 50% TFA/DCM
2% HZO
NO 1. HOAT R'
/ ~ z HATU HN
DMF ~R~~
2. 50% TFA/DCM
Linker Insoluble support ~H 2% HZO
DBU, DMF
Rz~
Linker ~ Insoluble support
H 81
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-83-
Scheme 6a
Br
1 ) LDA, -78°C.
2) Rz~_L
Br S r L = CI, Br, l, OMs, OTs, OSOZOMe gr S Rz~
90 g1
1) base,-78°C
2) COz(g)
3) 6N HCI (aq)
1) base, -78°C
S L 2) Rz~-L S~R2~
L = CI, Br, I, OMs, OTs, OSOZOMe
1 HO
W = COOH W = COOH
L=Br L=Br
1) (COCI)z, CHZCIz, DMF
2) Rz3-OH, Pyridine
CN
O
S Rz~ ~ CuCN, DMF, reflux ~ S Rz~
Rz~ Rz3 O
21
Rz3 = Me, i-Pr Rz3 = Me, i-Pr
L = Br
HZS, TEA, MeOH R3i
S
R38
NHz L = CI, Br, I, OTs, OMs
O ~ Acetone, reflux
W S R21
R R;
Rz3 = Me, i-Pr AI(CH3)3, NH4C1
toluene, reflux
93
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-84-
Scheme 6b
1) Butyllithium, -78°C.
2) COZ(g)
3) 6N HCI (aq) ~ ~ ~ p
Br S r B ~S~
94 OH
1) (COCI)z, CHZCIz, DMF
2) Rz3-OH, Pyridine
O
CuCN, DMF, reflux ~ ~ O
NC g
Br S
96 O Rz3 95 O.-Rz3
Rz3 = Me, i-Pr
HZS, TEA, MeOH
R
R38
3
S ~ ~ O L = CI, Br, I, OTs, OMs R S ~ ~ O
S Acetone, reflux ~ ~ S
1N
HzN 9~ O-Rz3 R3~ 98 O-Rzs
AI(CH3)3, NHQCI
toluene, reflux
R3 NH
S
/ S
N
Ray NH2
99
SUBS'ITiUTE SHEET (RULE 26)
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-85-
Scheme 7a
1. oxalyl chloride
2. TMSCHNz, then
30% HBr-acetic acid
Rz~ R« Ry
R9=H
13 100
Rz3 = CH3
S
HZN R$
acetone or DMF
70°C
O R.",
AIMe3, NH4C1
3
toluene, 118°C
E
R$
R~, Rz~
102 101
Scheme 7b
s
thiophosgene
H2N R4° CHCI3, sat'd. NaHC03 ~~ NH3-solvent
HZ NHRa°
'Rao
110 111 112
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-86-
Scheme 8
i , S \
~N ~ NOz
Br I /
NHz OzN
_ S R2~
Rz~ Acetone, reflux Rz3-O
113
Rz3-~ 10
R23 = CH3
20% TiCl3(aq)
THF, rt
S \
NHz
\N I /
R2t
Rz3-O 114
CH2CIz
CHZCIz N-methyl morpholine (1.5 eq)
Pyridine DMAP (0.1 eq)
O
4 a (L.0 eq) Rai~--CI (1.0 eq)
O
O
S \ H ~~ S \
w N- C- Raz w N- SOz- Ray
I ~ \N I / ~ ~ 'N I /
wS/ ~Rzi WS/ ~Rz~
Rz3-O I 11 S Rz3-0 ~ 117
AI(CH3)3, NH4CI ( AI(CH3)3, NH4C1
toluene, reflux toluene, reflux
O
S \ H ~~ S \
~ N-G-R'tz ~ ~ N-Spz-R°~
HN ~ ~ N I / HN ~ ~ N I . /
ws/ ~R2t \S/ ~Rzi
116 118
HyN H2N
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_87_
Scheme 9
Rz~
Rzz
X /
Rso
HN/
(when R5° = H)
NHZOH NH2CN MeNHz
EtOH, o/ I EtOH, D \ MeOH, o
Rz~ Rzz Rz~ Rzz Rz~ Rzz
/ X
OH CN Me
HN/ H HN~ N/ HN/ H
H
119 120 121
Scheme 10
Rz~ Rz~ Rz~
AI(CHy)3 ~
X ~ NH RssB~ X \ ~R~ NHyCI ~ X ~R~
w 2 Cu(II) catalyst N~ Toluene / D \ N
R~ z ~ ~Rs~
H
R O 47 Rzs~ 0 124 NH 125
X = O~ S Rs~ = H Rsy H
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_88_
Sche~rze 11
AI(CH3)s /
RssRs~NH X \ ~R~ NH4CI X R~
Pd catalyst N~ Tolue~ ~ N~
ligand O ~ R'' H ~ ~ \Rs~
base R23
127 ", , 128
X=O,S
L = CI, Br, 1, OSOZCF3
Scheme 12
Rz~ Rz~ Rss Rso Rz~ Rss Rso
AI(CH3)3 I
X ~ RssCORs° X y NH4CI X
NHz reducing \ N Tolue~ ~ N
agent O H H ~ \H
R23~ ~~ 2
O 47 R O 129 130
NH
X=O,S
S Scheme 13
1) CSCIz ~~
CH2CI2 y--NHz
47 aq. NaHC03 ~H R3~COC
2) NH3 - MeOH
X=O,S
Rs~
N \ _R3s
AI(CH3)3 / ~\S~
NH4CI
Toluene / D N H
O -is~
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
89-
Example I
4-~4-(4-clzlorophenyl)(1,3-tlziazol 2 yl)J S-methylthiothiophene-2-
carboxamidine
Trimethylaluminum (2.0 M in toluene, 2 mL) was added dropwise over 10
min to a suspension of ammonium chloride (216 mg) in toluene (2 mL), stirred
under
NZ at 0 °C. When gas evolution moderated, the mixture was stirred at
25°C for 30
min, when most of the solid had dissolved, methyl 4-[4-(4-chlorophenyl)(1,3-
thiazol-
2-yl)]-5-methylthiothiophene-2-carboxylate (100 mg, Maybridge Chemical Co.,
Cornwall, U.K.) was added in one portion. This solution was heated to reflux
in stages
over 1 h. After 2.5 h of reflux, the reaction mixture was allowed to cool to
25°C, and
was poured on to a vigorously stirred slurry of silica gel (2 g) in CHC13 (20
mL). After
min the solids were collected by suction filtration, and washed with MeOH
(3x10
mL). The combined filtrates were evaporated to dryness, and the residual
yellow
solid was subjected to preparative thin-layer chromatography to obtain 77 mg
of 4-
[(4-chlorophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine as a
yellow
15 solid.'H-NMR (DMSO-db; 300 MHz) 8 2.80 (s, 3H), 7.55-7.59 (m, 1H), 8.04-
8.13
(m, 1 H), 8.31 (s, 1 H), 8.69 (s, 1 H), ), 9.2 (broad s, 4H). Mass spectrum
(MALDI-
TOF, m/z): Calcd. for C,SH,ZC1N3S3, 365.9 (M+H), found 366.9.
Example 2
20 S Methylthiotlzioplzene-2-carboxamidine
5-(Methylthio)thiophene-2-carbonitrile (100 mg, Maybridge Chemical
Company, Cornwall, UK) was taken in a dry 2 dram vial. To this a solution of
saturated HCl in anhydrous MeOH (4 mL) was added. The vial was tightly capped
and the mixture was stirred for 24 h. The vial was cooled in an ice bath,
uncapped
and Nz was bubbled through the solution to remove dissolved HCI. The solvent
was
removed under reduced pressure and the resulting residue was dried under high
vacuum for 24 h. A solution of methanolic ammonia (2M NH3 in MeOH) was added
to the vial, and the mixture was stirred for 3 days. Methanol was removed
under
vacuum and the resulting residue was subjected to preparative thin-layer
chromatography to obtain 5-(methylthio)thiophene-2-carboxamidine as a yellow
solid.
'H-NMR (DMSO-d6; 300 MHz) b 2.64 (s, 3H), 7.22 (d, J--3.8 Hz, 1H), 7.95 (broad
d,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-90-
J=3.33 Hz, 1H), 9.4 (broad s, 4H). Mass spectrum (MALDI-TOF, m/z): Calcd. for
C6H8NzS2, 172.3 (M+H), found 173Ø
Example 3
S-Methylthio-4 plzenylthiophene-2-carboxamidine
Methyl 5-methylthio-4-phenylthiophene-2-carboxylate (100 mg, Maybridge
Chemical Company, Cornwall, UK) was treated in a manner similar to that for
Example 1, to give 50 mg of 4-phenyl-5-rnethylthiothiophene-2-carboxamidine as
an
off white solid. 'H-NMR (DMSO-db; 300 MHz) b 2.65 (s, 3H), 7.39-7.60 (m, SH),
8.27 (s, 1H), 9.2 (broad s, 4H). Mass spectrum (MALDI-TOF, m/z): Calcd. for
C,ZH,ZNZS2, 248.4 (M+H), found 249Ø
Example 4
4-~4-(2,4-Dichlorophenyl)(1,3-thiazol 2 yl)J S-methyltlziothiophene-2-
carboxamidine
Methyl 4-[4-(2,4-dichlorophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxylate ( 100 mg, Maybridge Chemical Company, Cornwall, UK) was treated in
a
manner similar to that for Example 1, to give 60 mg of 4-[4-(2,4-
dichlorophenyl)thiazol-2-yl]-5-methylthiothiophene-2-carboxamidine as a yellow
solid. 'H-NMR (DMSO-d~; 300 MHz) 8 2.77 (s, 3H), 7.6 (dd, J--2.2 and 8.5 Hz,
1H),
7.79 (d, J--2.2 Hz, 1 H), 8.09 (d, J--8.5 Hz, 1 H), 8.3 (s, 1 H), 8.6 (s, 1
H). Mass
spectrum (MALDI-TOF, m/z): Calcd. for C,SH"N3S3C12, 400.0 (M+H), found 400.1.
Example S
4-(4-Methyl(1,3-thiazol 2 yl))-S-methylthiothiophene-2-carboxamidine
Methyl 4-(4-methyl( 1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxylate
(100 mg, Maybridge Chemical Company, Cornwall, UK) was treated in a manner
similar to that for Example 1, to give 40 mg of 4-(4-methylthiazol-2-yl)-5-
methylthiothiophene-2-carboxamidine as a yellow solid. 'H-NMR (DMSO-d6; 300
MHz) 8 2.43 (s, 3H), 2.7 (s, 3H), 7.38 (s, 1H), 8.28 (s, 1H). Mass spectrum
(MALDI-
TOF, m/z): Calcd. for C,oH"N3S3, 270.0 (M+H), found 270.1.
CA 02362390 2001-08-07
WO 00/47578 _9' _ PCT/US99/18065
Example 6
a) Methyl S-metlzylthio-4-(4-(2-naphthyl)(1,3-thiazol-2 yl))thiophene-Z-
carboxylate: Methyl4-(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate
(40 mg, Maybridge Chemical Company, Cornwall, UK) was reacted with 2-bromo-2'-
acetonaphthone (1.1 eq) in a manner similar to Example 13 step (a) to give 40
mg of
methyl 5-methylthio-4-(4-(2-naphthyl)(1,3-thiazol-2-yl))thiophene-2-
carboxylate. 'H-
NMR (CDCl3/CD30D; 300 MHz) 8 3.71 (s, 3H), 3.94 (s, 3H), 7.47-7.55 (m, 2H),
7.67
(s, 1H), 7.84-7.99 (m, 3H), 8.08 (dd, J--1.75 Hz and 8.6 Hz, 1H), 8.3 (s, 1H),
8.5 (s,
1 H).
b) 5-Methyltlzio-4-(4-(2-naphthyl)(1,3-thiazol 2 yl))thiophene-2-
carboxamidine: Methyl5-methylthio-4-(4-(2-naphthyl)(1,3-thiazol-2-
yl))thiophene-
2-carboxylate, (40 mg) as prepared in the previous step was treated in a
manner
similar to that for Example 1, to give 30 mg of 4-[4-(naphth-2-yl)thiazol-2-
yl]-5-
methylthiothiophene-2-carboxamidine. 'H-NMR (DMSO-db; 300 MHz) 8 2.83 (s,
3H), 7.52-7.69 (m, 2H), 7.95-8.01 (m, 2H), 8.05 (d, J--8.6 Hz, 1H), 8.24 (dd,
J--1.69
Hz and 8.6 Hz, 1 H), 8.4 (s, 1 H), 8.65 (s, 1 H), 8.74 (s, 1 H). Mass spectrum
(MALDI-
TOF, CHCA matrix, m/z): Calcd. for C,9H,SN3S3, 382.1 (M+H), found 382Ø
Example 7
S-Metlzyltlzio-4-~4-(4 plienylphenyl)(1,3-tlziazol 2 yl)Jthioplzene-2-
carboxamidine
hydrochloride
a) Met/:yl S-metlzyltlzio-4-~4-(4 phenylphenyl)(1,3-thiazol 2 yl)Jtlziophene-2-
carboxylate: 27 mg (0.109 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 2
mL of
reagent grade acetone. 4'-Phenyl-2-bromoacetophenone (33 mg; 0.120 mmol;
Aldrich
Chemical Co., Milwaukee, WI) was added and the solution was allowed to reflux
for 2.5 h.
The solution was allowed to cool and solid was filtered and washed with
methanol and dried
in vacuo to afford 30 mg (65% yield) of methyl 5-methylthio-4-[4-(4-
phenylphenyl)(1,3-
thiazol-2-yl)]thiophene-2-carboxylate. 'H-NMR (DMSO-db, 300 MHz) 8 8.28 (s, 1
H), 8.24
(s, 1H), 8.17 (d, J=8.5 Hz, 2H), 7.8 (d, J=B.SHz, 2H), 7.74-7.77 (m, 2H), 7.48-
7.53 (m, 2H),
7.40 (m, 1H), 2.78 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA matrix, m/z) Calcd.
for
CZZH~6NOZS3: 423.0 (M+H), found 424.4.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
.92_
b) 5-Methyltlzio-4-~4-(4 plzenylphenyl)(1,3-thiazol 2 yl)Jthiophene-2-
carboxamidine hydrochloride: To a stirred suspension of 0.473 mmol ( 25 mg) of
ammonium chloride (Fisher Scientific Pittsburgh, PA) in 2 mL of anhydrous
toluene (Aldrich
Chemical Co.) placed under nitrogen atmosphere at 0 °C, 237 ~L (0.473
mmol) of 2M
trimethylaluminum in toluene (Aldrich Chemical Co.) was added via syringe over
10 min and
then let stir at 0 °C for 30 min after which 20 mg (0.0473 mmol) of
methyl 5-methylthio-4-
[4-(4-phenylphenyl)(1,3-thiazol-2-yl)]thiophene-2-carboxylate was added to
solution and
allowed to reflux for 2.5 h. The reaction mixture was quenched by pouring over
a slurry of
500 mg of silica in 10 mL of chloroform. The silica was poured onto a sintered
glass funnel
and washed with a 10% methanol/CHZCl2 solution and concentrated. The crude
product was
purified on a 1 mm silica prep plate eluting with 10% methanol/CHZCl2 to
afford 10 mg (53%
yield) of 5-methylthio-4-[4-(4-phenylphenyl)(1,3-thiazol-2-yl)]thiophene-2-
carboxamidine
hydrochloride. Mass Spectrum (MALDI-TOF. CHCA matrix, m/z) Calcd. for
Cz,H"N3S3:
408.1 (M+H), found 408Ø
Examples 8 & 9
4 ~4-(3-Metlzoxyphenyl)(1,3-thiazol 2 yl)J S-metlzylthiothiophene-2-
carboxamidine
IZydrochloride and 4-~9-(3-Izydroxyphenyl)(1,3-thiazol-2 yl)J S
methyltlziothioplZene-2-carboxamidine IZydroclzloride
a) Metlzyl4-(4-(3-metlzoxyplzenyl)(1,3-thiazol 2 yl)J-S-metlzylthiothioplzene-
2-
carboxylate: 32 mg (0.133 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 2
mL of
reagent grade acetone. 3'-Methoxy-2-bromo acetophenone (0.155 mmol; 36 mg;
Aldrich
Chemical Co.) was added and the solution was allowed to reflux for 2.5 h The
solution was
allowed to cool and a solid was filtered and washed with methanol and dried in
vacuo. The
solid was purified on 1 mm silica plate eluting with 25% ethyl acetate/hexane
to afford 31 mg
(63% yield) of methyl 4-[4-(3-methoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxylate.
b) 4-~4-(3-Metlzoxyphenyl)(1,3-thiazol-2 yl)J S-methylthiothiophene-2-
carboxamidine hydrochloride and 4-~4-(3-hydroxyplzenyl)(1,3-thiazol-2 yl)J S-
methylthiothiophene-2-carboxamidine Jzydrochloride: To a stirred suspension of
0.821
mmol ( 44 mg) of ammonium chloride (Fisher Scientific) in 2 mL of anhydrous
toluene
CA 02362390 2001-08-07
WO 00/47578 _93_ PCT/US99/18065
(Aldrich Chemical Co.) placed under nitrogen atmosphere at 0 °C, was
added 411 pL (0.821
mmol) of 2M trimethylaluminum in toluene (Aldrich Chemical Co.) via syringe
over 10 min
and then let stir at 0 °C for 30 min after which 31 mg (0.0821 mmol) of
methyl 4-[4-(3-
methoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was
added to
solution and allowed to reflux for 2.5 h. The reaction mixture was quenched by
pouring over
a slurry of 500 mg of silica in 10 mL of chloroform. The silica was poured
onto a sintered
glass funnel and washed with a 10% methanol/CHZCIz solution and concentrated.
The crude
product was purified on a 1 mm silica prep plate eluting with 10%
methanol/CHZCI2~to afford
4.4 mg (15% yield) of 4-[4-(3-methoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine hydrochloride and 4.2 mg (15% yield) of 4-[4-(3-
hydroxyphenyl)(1,3-thiazol-
2-yl)]-5-methylthiothiophene-2-carboxamidine hydrochloride. 4-[4-(3-
methoxyphenyl)(1,3-
thiazol-2-yl)]-S-methylthiothiophene-2-carboxamidine hydrochloride: 'H-NMR
(CD30D;
300 MHz) 8 8.5 (s, 1H), 7.9 (s, 1H), 7. 59-7.65 (m, 2H), 7.33-7.38 (m, 1H),
6.91-6.95 (m,
1H), 3.87 (s, 1H), 2.8 (s, 3H) Mass Spectrum (MALDI-TOF, CHCA matrix, m/z)
Calcd. for
IS C,6H,SN3OS3: 361.5(M+H), found 362.2. 4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-
yl)]-5-
methylthiothiophene-2-carboxamidine hydrochloride: 'H-NMR (CD30D; 300 MHz) 8
8.50
(s, 1 H), 7. 81 (s, 1 H), 7.267.51 (m, 2H), 7.24 (m, 1 H), 6.79 (m, 1 H), 2. 8
(s, 3 H) Mass
Spectrum (MALDI-TOF, CHCA matrix, m/z) Calcd. for C,SH,3N30S;: 347.5(M+H),
found
348Ø
Example 10
5-Metlzylthio-4-(4 phenyl(1,3-thiazol-2 yl))thiophene-2-carboxamidine
hydrochloride
a) Methyl S-methylthio-4-(4 phenyl(1,3-thiazol 2 yl))tlziophene-2-carboxylate:
33
mg (0.133 mmol) methyl 4-(aminothioxomethyl)-5-methylthiothiophene-2-
carboxylate
(Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 2 mL of reagent
grade
acetone. 2-Bromoacetophenone (0.133 mmol; 27 mg; Aldrich Chemical Co.) was
added and
the solution was allowed to reflux for 2.5 h. The solution was allowed to cool
and the solid
was filtered and washed with methanol and dried in vacuo. The solid was
purified on 1 mm
silica plate eluting with 25% ethyl acetate/hexane mixture to afford 46 mg
(90% yield) of
methyl 5-methylthio-4-(4-phenyl( 1,3-thiazol-2-yl))thiophene-2-carboxylate.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-94-
b) S-Methylthio-4-(4 phenyl(1,3-thiazol 2 yl))tlziophene-2-carboxamidine
hydrochloride: To a stirred suspension of 1.32 mmol ( 71 mg) of ammonium
chloride
(Fisher Scientific) in 2 mL of anhydrous toluene (Aldrich Chemical Co.) placed
under
nitrogen atmosphere at 0 °C, 662 ~.L (1.32 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 10 min and then let stir at
0 °C for 30 min
after which 46 mg (0.133 mmol) of methyl 5-methylthio-4-(4-phenyl(1,3-thiazol-
2-
yl))thiophene-2-carboxylate was added to solution and allowed to reflux for
2.5 h. The
reaction mixture was quenched by pouring over a slurry of 500 mg of silica in
10 mL of
chloroform. The silica was poured onto a sintered glass funnel and washed with
a 10%
methanol/CHZCIz solution and concentrated. The crude product was purified on a
2 g silica
silica SPE column eluting with 10% methanol/CHZC12 to afford 32.5 mg (75%
yield) of 5-
methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamidine
hydrochloride. 'H-
NMR (DMSO-db; 300 MHz) 8 8.70 (s, 1H), 8.25 (s, 1H), 8.07-8.11 (m, 2H), 7.37-
7.53 (m,
3H), 2.8 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA matrix, m/z) Calcd. for
C,SH,3N3S3:
331.5(M+H), found 332.1.
Example ll
S-Methyltlzio-4 ~4-(4-nitrophenyl)(1,3-thiazol 2 yl)Jthiophene-2-carboxamidine
hydrochloride
a) Methyl S-methylthio-4-~4-(4-nitrophenyl)(1,3-thiazol-2 yl)Jthiophene-2-
carboxylate: 38 mg (0.141 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 2
mL of
reagent grade acetone. 2-Bromo-4'-nitroacetophenone (0.155 mmol; 38 mg;
Aldrich
Chemical Co.) was added and the solution was allowed to reflux for 2.5 h. The
solution was
allowed to cool and a solid was filtered and washed with methanol and dried in
vacuo. The
crude product was dissolved in CHzCl2 and 0.141 mmol of N-(2-
mercapto)aminoethyl
polystyrene resin (Calbiochem, San Diego, CA; 1.28mmo1/g; 110 mg) was added
and
allowed to stir overnight. The solution was filtered, concentrated and dried
to afford 60 mg
(90% yield) of crude methyl 5-methylthio-4-[4-(4-nitrophenyl)(1,3-thiazol-2-
yl)]thiophene-2-
carboxylate.
b) S Metlaylthio-4 ~4-(4-nitrophenyl)(1,3-thiazol-2 yl)Jtlzioplrene-2-
carboxamidine
hydrochloride: To a stirred suspension of 1.66 mmol (90 mg) of ammonium
chloride (Fisher
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-95
Scientific) in 2 mL of anhydrous toluene (Aldrich Chemical Co.) placed under
nitrogen
atmosphere at 0 °C, 830 pL (1.66 mmol) of 2M trimethylaluminum in
toluene (Aldrich
Chemical Co.) was added via syringe over 10 min and then let stir at 0
°C for 30 min after
which 60 mg (0.166 mmol) of 5-methylthio-4-[4-(4-nitrophenyl)(1,3-thiazol-2-
yl)]thiophene-
2-carboxylate was added to solution and allowed to reflux for 2.5 h. The
reaction mixture
was quenched by pouring over a slurry of 500 mg of silica in 10 mL of
chloroform. The
silica was poured onto a sintered-glass funnel and washed with a 10%
methanol/CHZCIz
solution and concentrated. The crude product was purified on a 1 mm silica
prep plate
eluting with 10% methanol/CHzCIz to afford 12 mg (19% yield) of S-methylthio-4-
[4-(4-
nitrophenyl)(1,3-thiazol-2-yl)]thiophene-2-carboxamidine hydrochloride. 'H-NMR
(CD30D,
300 MHz) 8 8.58 (s, 1H), 8.32-8.33 (m, 4H), 8.24 (s, 1H), 2.83 (s, 3H). Mass
Spectrum
(MALDI-TOF, CHCA matrix, m/z) Calcd. for C,SH,ZN4OZS3: 376.5(M+H), found
377.3.
Example 12
4 ~4-(3,4-Etlzylenedioxyphenyl)thiazol 2 ylJ S-methyltlziotlzioplzene-2-
carboxamidine hydrochloride
a) Methyl 4-(4-(2H,3H benzo~3,4-eJl,4-dioxin-6 yl)(1,3-thiazol 2 yl))-S-
metlrylthiothioplzene-2-carboxylate: 40 mg (0.162 mmol) of methyl 4-
(aminothioxomethyl)-
5-methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
dissolved in 2 mL of reagent grade acetone. 1-(2H,3H benzo[e] 1,4-dioxin-6-yl)-
2-
bromoethan-1-one (0.162 mmol; 42 mg; Maybridge Chemical Co. LTD., Cornwall,
U.K.)
was added and the solution was allowed to reflux for 3 h. The solution was
allowed to cool
and allowed to stir for 2 days after which the reaction solution was
concentrated in vacuo.
The crude product was dissolved in 50 mL of CHZCIz and partitioned between 50
mL of 1 N
NaOH (aq.). The organic layer was obtained and dried over sodium sulfate and
concentrated
to afford 60 mg (90% yield) of methyl 4-[4-(3,4-ethylenedioxyphenyl)thiazol-2-
yl]-5-
methylthiothiophene-2-carboxylate.
b) 4-~4-(3,4-Etlzylenedioxyphenyl)thiazol 2 ylJ 5-methyltlaiotlaiophene-2-
carboxamidine liydrocl:loride: To a stirred suspension of 1.62 mmol ( 86 mg)
of ammonium
chloride (Fisher Scientific) in 2 mL of anhydrous toluene (Aldrich Chemical
Co.) placed
under nitrogen atmosphere at 0 °C, 810 pL (1.62 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 10 min and then let stir at
0 °C for 30 min
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-96-
after which 60 mg (0.162 mmol) of methyl 4-[4-(3,4-ethylenedioxyphenyl)thiazol-
2-yl]-5-
methylthiothiophene-2-carboxylate was added to solution and allowed to reflux
for 2.5 h.
The reaction mixture was quenched by pouring over a slurry of 500 mg of silica
in 10 mL of
chloroform. The silica was poured onto a sintered glass funnel and washed with
a 10%
methanol/CHZC12 solution and concentrated. The crude product was purified on a
1 mm silica
prep plate eluting with 10% methanol/CHZCl2 to afford 47 mg (75% yield) of 4-
[4-(3,4-
ethylenedioxyphenyl)thiazol-2-yl)-S-methylthiothiophene-2-carboxamidine
hydrochloride.
'H-NMR (CD30D; 300 MHz) 8 8.53 (s, 1H), 7.73 (s, 1H), 7.56 (d, J= 2Hz, 1H),
7.50 (dd,
J--2.1 Hz and 8.4 Hz, 1 H), 6.89 (d, J=8.4 Hz, 1 H), 4.28 (s, 4H), 2.8 (s,
3H). Mass Spectrum
(MALDI-TOF, CHCA matrix, m/z) Calcd. for C"H,SN30zS3: 389.5(M+H), found 390.1.
Example 13
4-~4-(4-Methoxyplzenyl)(1,3-tJziazol 2 yl)J S-methylthiothiophene-2-
carboxamidine
hydrochloride
a) Methyl4 ~4-(4-methoxyphenyl)(1,3-thiazol 2 yl)J S-methylthiotlziophene-2-
carboxylate: 30 mg (0.122 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in
1.2 mL of
reagent grade acetone. 2-bromo-4'-methoxy acetophenone (0.146 mmol; 28 mg;
Aldrich
Chemical Co.) was added and the solution was allowed to reflux for 3 h. The
solution was
allowed to cool and a solid was filtered and washed with methanol and dried in
vacuo to
afford 46 mg (90% yield) of methyl 4-[4-(4-methoxyphenyl)(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxylate.
b) 4-~4-(4-Metlzoxyphenyl)(1,3-thiazol-2 yl)J 5-methylthiothiopJzene-2-
carboxamidine hydrochloride: To a stirred suspension of 1.22 mmol (66 mg) of
ammonium
chloride (Fisher Scientific) in 2 mL of anhydrous toluene (Aldrich Chemical
Co.) placed
under nitrogen atmosphere at 0 °C, 612 ~,L (1.22 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 10 min and then let stir at
0 °C for 30 min
after which 46 mg (0.122 mmol) of 4-[4-(4-methoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate was added to solution and allowed to reflux
for 2.5 h.
The reaction mixture was quenched by pouring over a slurry of 500 mg of silica
in 10 mL of
chloroform. The silica was poured onto a sintered glass funnel and washed with
a 10%
methanol/CHZC12 solution and concentrated. The crude product was purified on a
1 mm silica
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_97_
prep plate eluting with 10% methanol/CHZCIz to afford 32 mg (73% yield) of 4-
[4-(4-
methoxyphenyl)(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxamidine
hydrochloride.
'H-NMR (CD30D; 300 MHz) b 8.53 (s, 1H), 7.98 (d, J--7 Hz, 2H), 7.75 (s, 1H),
7.01 (d, J--5
Hz, 2H), 3.9 (s, 3H), 2.8 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA matrix, m/z)
Calcd.
for C,6H,SN3OS3: 362.0 (M+H), found 362.2.
Example 14
4-~4-(3,4-Propylenedioxyplzenyl)thiazol 2 ylJ S-methyltlziotlziophene-2-
carboxamidine
hydrochloride
a) Methyl 4-~4-(3,4 propylenedioxyphenyl)thiazol 2 ylJ S-methyltlziothiophene-
2-
carboxylate: 42 mg (0.170 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 5
mL of
reagent grade acetone. 3',4'-Propylenedioxy-2-bromoacetophenone (0.170 mmol;
28 mg;
Maybridge Chemical Co. LTD., Cornwall, U.K.) was added and the solution was
allowed to
reflux for 3 h. The solution was allowed to cool and a solid was filtered and
purified on a 1
mm silica prep plate eluting with 20% ethyl acetate/hexane and dried in vacuo
to afford 42
mg (59% yield) of methyl 4-[4-(3,4-propylenedioxyphenyl)thiazol-2-yl)-5-
methylthiothiophene-2-carboxylate.
b) 4-~4-(3,4-Propylenedioxyplzenyl)thiazol-2 ylJ-S-methylthiotlriophene-2-
carboxamidine hydrochloride: To a stirred suspension of 1.01 mmol (54 mg) of
ammonium
chloride (Fisher Scientific) in 2 mL of anhydrous toluene (Aldrich Chemical
Co.) placed
under nitrogen atmosphere at 0 °C, 510 pL (1.01 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 10 min and then let stir at
0 °C for 30 min
after which 42 mg (0.101 mmol) of methyl 4-[4-(3,4-
propylenedioxyphenyl)thiazol-2-yl)-5-
methylthiothiophene-2-carboxylate was added to solution and allowed to reflux
for 3 h. The
reaction mixture was quenched by pouring over a slurry of 500 mg of silica in
20 mL of
chloroform. The silica was poured onto a sintered glass funnel and washed with
a 10%
methanol/CHZCIz solution and concentrated to afford 20 mg (50% yield) of 4-[4-
(3,4-
propylenedioxyphenyl)thiazol-2-yl)-5-methylthiothiophene-2-carboxamidine
hydrochloride.
'H-NMR (CD30D; 300 MHz) 8 8.53 (s, 1 H), 7.78 (s, 1 H), 7.68 (d, J--2.2 Hz, 1
H), 7.60 (dd,
J--2.2 Hz and 8.4 Hz, 1 H), 7.00 (d, J--8.3 Hz; 1 H), 4.19-4.28 (m, 4H), 2.77
(s, 3H), 2.18-2.23
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-98-
(in, 2H). Mass Spectrum (MALDI-TOF, CHCA matrix, m/z) Calcd. for C,BH"N30zS3:
404.1 (M+H), found 404.1.
Example I S
S 5-Methylthio-4-(4-(2-tlzienyl)(1,3-tlziazol 2 yl))thioplzene-2-carboxamidine
acetate
a) 2-Bromo-1-(2-thienyl)ethan-1-one: To a solution of 500 mg (3.96 mmol) of 2-
acetyl thiophene (Aldrich Chemical Co.) dissolved in 20 mL of CHC13, was added
1 drop of
30% HBr/CH3COOH (Aldrich Chemical Co.) followed by 3.96 mmol (633 mg; 204 ~L)
of
bromine (Aldrich Chemical Co.) added dropwise over 30 min. The reaction was
allowed to
stir for 1 h. The solution was concentrated to an oil and dried in vacuo. The
crude product
was purified on 1 mm silica prep plates eluting with neat CHZCIz to obtain 300
mg (37%
yield) of 2-bromo-1-(2-thienyl)ethan-1-one. 'H-NMR (CDCl3; 300 MHz) 8 7.80 (m,
2H),
7.18 (m, 1H), 4.37 (s, 2H).
b) Metlryl S-methylthio-4-(4-(2-tlZienyl)(1,3-thiazol 2 yl))thiophene-2-
carboxylate:
44 mg ( 0.176 mmol) of methyl 4-(aminothioxomethyl)-5-methylthiothiophene-2-
carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 3
mL of
reagent grade acetone. 2-Bromo-1-(2-thienyl)ethan-1-one (0.176 mmol; 36 mg)
was added
and the solution was allowed to reflux for 3 h. The solution was allowed to
cool and was
concentrated. The crude product was dissolved in 20 mL of CHZC12 and washed
with 20 mL
of 1N HCl (aq.). The organic layer was obtained and dried over sodium sulfate
to afford 115
mg (80% yield) of crude methyl 5-methylthio-4-(4-(2-thienyl)(1,3-thiazol-2-
yl))thiophene-2-
carboxylate.
c) 5-Methylthio-4-(4-(2-thienyl)(1,3-thiazol 2 yl))tlztoplzene-2-carboxamidine
acetate: To a stirred suspension of 2.80 mmol (150 mg) of ammonium chloride
(Fisher
Scientific) in 5 mL of anhydrous toluene (Aldrich Chemical Co.) placed under
nitrogen
atmosphere at 0 °C, 1.5 mL (2.8 mmol) was added 2M trimethylaluminum in
toluene
(Aldrich Chemical Co.) via syringe over 15 min and then let stir at 0
°C for 25 min. after
which 115 mg (0.280 mmol) of methyl S-methylthio-4-(4-(2-thienyl)(1,3-thiazol-
2-
yl))thiophene-2-carboxylate in 5 mL of anhydrous toluene was added to solution
and allowed
to reflux for 1.5 h. The reaction mixture was quenched by pouring over a
slurry of silica in
CHZCl2. The silica was poured onto a sintered glass funnel and washed with a
10%
methanol/CHZCl2 solution and concentrated. The crude product was purified on a
1 mm silica
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_99_
prep plate eluting with 10% methanol/CHZCI2with 1% CH3COOH to afford 40 mg
(43%
yield) of 5-methylthio-4-(4-(2-thienyl)(1,3-thiazol-2-yl))thiophene-2-
carboxamidine acetate.
'H-NMR (CD30D; 300 MHz) 8 8.52 (s, 1 H), 7.74 (s, 1 H), 7.59 (dd, J 2 Hz and 5
Hz, 1 H),
7.42 (dd, J--2 Hz and 5 Hz, 1H), 7.11 (m, 1H), 2.79 (s, 3H). Mass Spectrum
(MALDI-TOF,
CHCA matrix, m/z) Calcd. for C,3H"N3S4: 338.0 (M+H), found 337.9.
Example 16
4-~4-(3-Bromophenyl)(1,3-thiazol 2 yl)J-S-methylthiothiophene-2-carboxamidine
hydrochloride
a) Metlzyl4-~4-(3-bromophenyl)(1,3-thiazol 2 yl)J S-methylthiothiophene-2-
carboxylate: 99 mg (0.400 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in
25 mL of
reagent grade acetone. 2-Bromo-3'-bromo acetophenone (0.4 mmol; 111 mg) was
added and
the solution was allowed to reflux for 3 h. The solution was allowed to cool
and a solid was
filtered and dissolved in 5 mL of hot tetrahydrofuran (THF), (Aldrich Chemical
Co.) and
purified on a 1 mm silica prep plate eluting with 20% ethyl acetate/hexane and
dried in vacuo
to afford 66 mg (40% yield) of methyl 4-[4-(3-bromophenyl)(1,3-thiazol-2-yl)]-
5-
methylthiothiophene-2-carboxylate.
b) 4 ~4-(3-Bromophenyl)(1,3-tlZiazol 2 yl)~ 5-methyltlZiothioplZene-2-
carboxamidine IZydrochloride: To a stirred suspension of 1.55 mmol ( 83 mg) of
ammonium
chloride (Fisher Scientific) in 10 mL of anhydrous toluene (Aldrich Chemical
Co.) placed
under nitrogen atmosphere at 0 °C, 774 pL (1.55 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 10 min and then let stir at
25°C for 20
min after which 66 mg (0.155 mmol) of 4-[4-(3-bromophenyl)(1,3-thiazol-2-yl)]-
5-
methylthiothiophene-2-carboxylate was added to solution and allowed to reflux
for 3 h. The
reaction mixture was quenched by pouring over a slurry of 5 g of silica in 25
mL of
chloroform. The silica was poured onto a sintered glass funnel and washed with
a 10%
methanol/CHZCl2 solution and concentrated. The crude product was purified on 1
mm silica
plates eluting with 10% methanol/CHZCIz to afford 63 mg (90% yield) of 4-[4-(3-
bromophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride. 'H-
NMR (CD30D; 300 MHz) 8 8.49 (s, 1H), 8.21 (m, 1H), 7.96 (m, 2H), 7.50 (m, 1H),
7.5 (m,
CA 02362390 2001-08-07
WO 00/47578 -100- PCT/US99/18065
1H), 7.34 (m, 1H), 2.8 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA matrix, m/z)
Calcd.
for C~SH~ZBrN3S3: 411.9 (M+H), found 411.9.
Example 17
4 ~4-(4-Chloro-3-nitrophenyl)(1,3-thiazol 2 yl)J S-methylthiothiopl:ene-2-
carboxamidine lZydroclzloride
a) Metltyl4 ~4-(4-chloro-3-nitrophenyl)(1,3-thiazol-2 yl)J 5-
methylthiothiophene-2-
carboxylate: 50 mg (0.202 mmol ) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in
10 mL of
reagent grade acetone. 2-Bromo-4'-chloro-3'-nitroacetophenone (0.212 mmol; 59
mg) was
added and the solution was allowed to reflux for 3 h. The solution was allowed
to cool and a
solid was filtered and dissolved in hot tetrahydrofuran (THF) and purified on
a 1 mm silica
prep plate eluting with 20% ethyl acetate/hexane and dried in vacuo to afford
60 mg (70%
yield) ofmethyl4-[4-(4-chloro-3-nitrophenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxylate.
b) 4-~4-(4-Chloro-3-nitrophenyl)(1,3-thiazol 2 yl)J-S-metlzylthiothioplzene-2-
carboxamidine hydrochloride: To a stirred suspension of 1.40 mmol ( 75 mg) of
ammonium
chloride (Fisher Scientific) in 10 mL of anhydrous toluene (Aldrich Chemical
Co.) placed
under nitrogen atmosphere at 0 °C, 700 p.L ( 1.40 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 10 min and then let stir for
20 min after
which 60 mg (0.140 mmol) of 4-[4-(4-chloro-3-nitrophenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate was added to solution and allowed to reflux
for 3 h. The
reaction mixture was quenched by pouring over a slurry of 5 g of silica in 50
mL of
chloroform. The silica was poured onto a sintered glass funnel and washed with
a 10%
methanol/CHzCl2 solution and concentrated. The crude product was purified on 1
mm silica
plates eluting with 10% methanol/CHzCl2 to afford 17 mg (32% yield) of 4-[4-(4-
chloro-3-
nitrophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride. 'H-
NMR (CD30D; 300 MHz) 8 8.53-8.58 (m, 2H), 8.26 (dd, J--2.2 Hz and 8.5 Hz, 1H),
8.16 (s,
1H), 7.72 (d, J= 8.5 Hz, 1H), 2.80 (s, 3H).
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-101
Example 18
4 ~4-(4-Chloro-3-methylphenyl)(1,3-thiazol 2 yl)J S-methylthiotlziophene-2
carboxamidine hydrochloride
a) Methyl4-~4-(4-chloro-3-methylphenyl)(1,3-thiazol-2 yl)J 5-
methylthiothiophene-
2-carboxylate: 155 mg (0.627 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
dissolved in 10 mL of reagent grade acetone. 2-Bromo-1-(4-chloro-3-
methylphenyl)ethan-1-
one (0.658 mmol; 163 mg) was added and the solution was allowed to reflux for
3 h. The
solution was allowed to cool and the reaction mixture was concentrated and
dissolved in 50
mL of CHzCl2. The organic layer was washed with SO mL of 1N HCl (aq.), dried
over
sodium sulfate and concentrated. The crude product was purified on a 1 mm
silica plate
eluting with 20% ethyl acetate/ hexane to afford 168 mg (68% yield) of methyl
4-[4-(4-
chloro-3-methylphenyl)( 1,3-thiazol-2-yl)J-5-methylthiothiophene-2-
carboxylate.
b) 4-~4-(4-Chloro-3-metlzylphenyl)(1,3-thiazol-2 yl)J S-methylthiotlziophene-2-
carboxamidine hydrochloride: To a stirred suspension of 4.24 mmol ( 227 mg) of
ammonium chloride (Fisher Scientific) in 1 S mL of anhydrous toluene (Aldrich
Chemical
Co.) placed under nitrogen atmosphere at 0 °C, 2.2 mL (4.24 mmol)
of 2M
trimethylaluminum in toluene (Aldrich Chemical Co.) was added via syringe over
10 min and
then let stir for 20 min at 25°C after which 168 mg (0.424 mmol) of
methyl 4-[4-(4-chloro-3-
methylphenyl)(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxylate was added
to solution
and allowed to reflux for 2.5 h. The reaction mixture was quenched by pouring
over a slurry
of Sg silica in chloroform. The silica was poured onto a sintered glass funnel
and washed
with a 10% methanol/CHZC12 solution and concentrated to afford 117 mg (73%
yield) of 4-
[4-(4-chloro-3-methylphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxamidine
hydrochloride. 'H-NMR (CD30D; 300 MHz) b 8.53 (s, 1H), 8.03 (dd, J= 1.2 Hz and
2.7 Hz,
1 H), 7.9 (s, 1 H), 7.85 (dd, J= 2 Hz and 8.5 Hz 1 H), 7.3 8 (dd, J= 8.3 Hz
and 17.4 Hz, 1 H), 2.8
(s, 3H) 2.45 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA matrix, m/z) Calcd. for
C,6H,4C1N3S3: 380.0 (M+H), found 380.3.
CA 02362390 2001-08-07
WO 00/47578 _~~2_ PCT/US99/18065
Example 19
4-(S-Methyl 4 pl:enyl(1,3-thiazol 2 yl))-5-methylthiothiophene-2-carboxamidine
hydrochloride
a) Methyl 4-(S-methyl 4 phenyl(1,3-thiazol 2 yl))-S-methylthiothiophene-2-
carboxylate: 48 mg (0.194 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U.K.) was dissolved in 5
mL of
reagent grade acetone. 2-Bromo-1-phenylpropan-1-one (0.223 mmol; 48 mg) was
added and
the solution was allowed to reflux for 5 h. The solution was allowed to cool
and the reaction
mixture was concentrated and dissolved in 50 mL of CHZCIz. The organic layer
was washed
with 50 mL of 1N HCl (aq.), dried over sodium sulfate and concentrated. The
crude product
was purified on a 1 mm silica plate eluting with 20% ethyl acetate/ hexane to
afford 53 mg
(76% yield) of methyl 4-(S-methyl-4-phenyl(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-
carboxylate.
b) 4-(5-Metlayl 4 phenyl(1,3-thiazol 2 yl))-5-methylthiothiophene-2-
carboxamidine
Izydrochloride: To a stirred suspension of 1.47 mmol (78 mg) of ammonium
chloride (Fisher
Scientific) in 5 mL of anhydrous toluene (Aldrich Chemical Co.) placed under
nitrogen
atmosphere at 0 °C, 735p.L (1.47 mmol) of 2M trimethylaluminum in
toluene (Aldrich
Chemical Co.) was added via syringe over 10 min and then let stir for 20 min
at 25°C then,
53 mg (0.147 mmol) of methyl 4-(S-methyl-4-phenyl(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxylate were added to solution and allowed to reflux
for 2.5 h.
The reaction mixture was quenched by pouring over a slurry of Sg silica in
chloroform. The
silica was poured onto a sintered glass funnel and washed with a 10%
methanol/CHzCl2
solution and concentrated to afford 26 mg (51% yield) of 4-(5-methyl-4-
phenyl(1,3-thiazol-2-
yl))-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H-NMR (CD30D; 300
MHz) 8
8.45 (s, 1H), 7.74-7.77 (m, 2H), 7.44-7.50 (m, 2H), 7.38-7.41 (m, 1H), 2.8 (s,
3H) 2.6 (s, 3H).
Mass Spectrum (MALDI-TOF, CHCA matrix, m/z) Calcd. for C,6H,SN3S3: 346.0
(M+H),
found 345.6.
CA 02362390 2001-08-07
WO 00/47578 _,~03_ PCT/US99/18065
Example 20
4-~4-(4-Methylphenyl)(1,3-thiazol 2 yl)J S-methyltlziothiophene-2-
carboxamidine
tr~uoroacetate
a) Metlayl4 (4-(4-metlaylplzenyl)(1,3-thiazol-2 yl)J 5-metlzylthiotlzioplzene-
2-
carboxylate: 103 mg (0.416 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
dissolved in 5 mL of reagent grade acetone. 2-Bromo-4'-methyl acetophenone
(0.416 mmol;
89 mg) was added and the solution was allowed to reflux for 3 h. The solution
was allowed
to cool and crude product was filtered and washed two times with acetone and
purified on a 1
mm silica plate eluting with 20% ethyl acetate/ hexane to afford 104 mg (69%
yield) of
methyl 4-[4-(4-methylphenyl)( 1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxylate.
b) 4-~4-(4-Methylphenyl)(1,3-tlziazol-2 yl)J S-metlzyltlziotlziophene-2
carboxamidine trifluoroacetate: To a stirred suspension of 2.87 mmol ( 154 mg)
of
ammonium chloride (Fisher Scientific) in 10 mL of anhydrous toluene (Aldrich
Chemical
Co.) placed under nitrogen atmosphere at 0 °C, 144~L (2.87 mmol)
of 2M
trimethylaluminum in toluene (Aldrich Chemical Co.) was added via syringe over
10 min and
then let stirred for 20 min at 25°C after which 104 mg (0.287 mmol) of
4-[4-(4-
methylphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was added
to solution
and allowed to reflux for 3 h. The reaction mixture was quenched by pouring
over a slurry of
5 g of silica in 50 mL of chloroform. The silica was poured onto a sintered
glass funnel and
washed with a 10% methanol/CHZCIz solution and concentrated. The crude product
was then
purified on a 1 mm silica prep plate eluting with 10% methanol/CHzCl2 with 1%
CH3COOH.
The product was then basified with aq. NaOH and extracted with CHCI~ and
concentrated.
TFA was added and the product was crystallized from methanol as 4-[4-(4-
methylphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
trifluoroacetate (20
mg; 30% yield). 'H-NMR (DMSO-db; 300 MHz) 8 8.62 (s, 1H), 8.12 (s, 1H), 7.98
(d, 1H, J--
8.1 Hz) 7.31 (d, 1H, J-- 8.1 Hz), 2.8 (s, 3H) 2.5 (s, 3H). Mass Spectrum
(MALDI-TOF,
CHCA Matrix, m/z) Calcd. for C,6H,SN3S3: 346.0 (M+H), found 346.1.
CA 02362390 2001-08-07
WO 00/47578 PCT/LTS99/18065
-104-
Example 21
4-~4-(2-methoxyphenyl)(1,3-thiazol-2 yl)J S-methylthiothiophene-2-
carboxamidine
hydrochloride
a) Metlzyl4-~4-(2-methoxyphenyl)(1,3-thiazol-2 yl)J-5-methyltlziotlziophene-2-
carboxylate: 105 mg (0.424 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
dissolved in 5 mL of reagent grade acetone. 2-Bromo-2'-methoxy acetophenone
(0.467
mmol; 110 mg) was added and the solution was allowed to reflux for 3 h. The
solution was
allowed to cool and the solution concentrated. The crude product was dissolved
in 100 mL of
CHzCl2 and washed one time with 50 mL of 1N NaOH. The organic layer was
obtained,
dried over sodium sulfate, concentrated and purified on a 1 mm silica plate
eluting with 20%
ethyl acetate/ hexane to afford 160 mg (95% yield) of methyl 4-[4-(2-
methoxyphenyl)(1,3-
thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate.
b) 4-~4-(2-Methoxyphenyl)(1,3-thiazol 2 yl)J S-methylthiothiophene-2-
carboxamidine hydrochloride: To a stirred suspension of 4.23 mmol ( 227 mg) of
ammonium chloride (Fisher Scientific) in 10 mL of anhydrous toluene placed
under nitrogen
atmosphere at 0 °C, 2.12 mL (4.23 mmol) of 2M trimethylaluminum in
toluene (Aldrich
Chemical Co.) was added via syringe over 10 min and then let stir for 20 min
at 25°C after
which 160 mg (0.287 mmol) of methyl 4-[4-(2-methoxyphenyl)(1,3-thiazol-2-yl)]-
5-
methylthiothiophene-2-carboxylate in a solution of 5 mL of anhydrous toluene
was added to
solution and allowed to reflux for 3 h. The reaction mixture was quenched by
pouring over a
slurry of 5 g of silica in 30 mL of chloroform. The silica was poured onto a
sintered glass
funnel and washed with a 10% methanol/CHZCIz solution and concentrated. The
crude
product was then purified on a 2 mm silica prep plate eluting with 10%
methanol/CHZC12
with 1 % NH40H. The product was then dissolved in 2 mL of 4N HCl/dioxane and
concentrated to afford 45 mg (29% yield) of 4-[4-(2-methoxyphenyl)(1,3-thiazol-
2-yl)]-5-
methylthiothiophene-2-carboxamidine hydrochloride. 'H-NMR (DMSO-db; 300 MHz) 8
8.68
( s, 1H), 8.36 (dd, J-- 1.6 Hz and 7.74 Hz, 1H), 8.21 (s, 1H), 7.36-7.42 (m,
1H), 7.05-7.22 (m,
3 H), 3.97 (s, 3H), 2.8 (s, 3H).
Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z) Calcd. for C,6H,SN3OS3: 362.0
(M+H),
found 361.7.
CA 02362390 2001-08-07
WO 00/47578
-'f 05-
PCT/US99/18065
Example 22
4-~4-(2,4-Dimethoxyphenyl)(1,3-thiazol-2 yl)J 5-methylthiothiophene-2
carboxamidine hydrochloride
a) Methyl 4-~4-(2,4-dimethoxyplzenyl)(1,3-thiazol-2 yl)J S-methylthiothiophene-
2-
carboxylate: 99 mg (0.424 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Co. LTD., Cornwall, U..K.) was dissolved in
5 mL of
reagent grade acetone. 2-Bromo-2',4'-dimethoxyacetophenone (0.440 mmol; 114
mg) was
added and the solution was allowed to reflex for 2.5 h. The solution was
allowed to cool and
the crude product was collected as a solid and washed with methanol and dried
yielding 91
mg (56% yield) of methyl 4-[4-(2,4-dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate.
b) 4-~4-(2,4-Dimethoxyphenyl)(1,3-thiazol-2 yl)~ 5-methylthiothiophene-2-
carboxamidine hydrochloride: To a stirred suspension of 2.23 mmol (119 mg) of
ammonium chloride (Fisher Scientific) in 10 mL of anhydrous toluene placed
under nitrogen
atmosphere at 0 °C, 1.1 mL (2.23 mmol) of 2M trimethylaluminum in
toluene was added via
syringe over 10 min and then let stirred for 20 min at 25°C after which
81 mg (0.223 mmol)
of methyl 4-[4-(2,4-dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-
2-
carboxylate was added to solution and allowed to reflex for 2.5 h. The
reaction mixture was
quenched by pouring over a slurry of silica in chloroform. The silica was
poured onto a
sintered glass funnel and washed with a 10% methanol/CHzCl2 solution and
concentrated.
The crude product was then purified on a 0.5 mm silica prep plate eluting with
10%
methanol/CHZCIz to afford 32 mg (37% yield) of 4-[4-(2,4-dimethoxyphenyl)(1,3-
thiazol-2-
yl)]-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H-NMR (CD30D; 300
MHz) 8
8.49 ( s, 1H), 8.31 (d, J-- 8.5 Hz, 1H), 7.93 (s, 1H), 6.64 (m, 2H), 3.97 (s,
3 H), 3.85 (s, 3H),
2.79 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z) Calcd. for
C"H,~N3OZS3~
392.1 (M+H), found 392.4.
CA 02362390 2001-08-07
WO 00/47578
-106-
Example 23
PCT/US99/18065
4-~4-(3,4-Dichlorophenyl)(1,3-thiazol-2 yl)J 5-metlzylthiothiophene-2
carboxamidine hydrochloride
a) Methyl4-~4-(3,4-dichlorophenyl)(1,3-thiazol-2 yl)J-5-methylthiotlziophene-2-
carboxylate: 176 mg (0.712 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co.,LTD., Cornwall,
U.K.) was
reacted with 2-bromo-3',4'-dichloroacetophenone (0.854 mmol; 330 mg) in a
manner similar
to Example 22, step (a) to afford 270 mg (91 % yield) of methyl 4-[4-(3,4-
dichlorophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate.
b) 4-~4-(3,4-Dichlorophenyl)(1,3-thiazol-2 yl)J-5-methylthiothiophene-2-
carboxamidine hydrochloride: 270 mg (0.648 mmol) of methyl 4-[4-(3,4-
dichlorophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was
treated in a
manner similar to Example 22, step (b) to afford 135 mg (52% yield) of 4-[4-
(3,4-
dichlorophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride.
'H-NMR (CD30D; 300 MHz) 8 8.54 (s, 1 H), 8.22 (d, J-- 2 Hz, 1 H), 8.02 (s, 1
H), 7.94 (dd,
J-- 2 Hz and 8.4 Hz, 1H), 7.58 (d, J-- 8.5 Hz, 1 H), 2.79 (s, 3H). Mass
Spectrum (MALDI-
TOF, CHCA Matrix, m/z) Calcd. for C,SH"ClzN3S3: 400.0 (M+H), found 400.6.
Example 24
4-~4-(3-Metlzylphenyl)(1,3-thiazol-2 yl)~ 5-metlzylthiothioplzene-2-
carboxamidine
hydrochloride
a) Methyl 4-~4-(3-methylphenyl)(1,3-thiazol 2 yl)J-5-methylthiotlziophene-2-
carboxylate: Methyl 4-(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate,
106 mg
(0.428 mmol) (Maybridge Chemical Co. LTD., Cornwall, U.K.) was reacted with 2-
bromo-3'
methylacetophenone (0.428 mmol, 91 mg) in a manner similar to Example 22, step
(a) to
afford 98 mg (63% yield) of methyl 4-[4-(3-methylphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate.
b) 4-~4-(3-Methylplzenyl)(1,3-thiazol-2 yl)J S-methylthiothiophene-2-
carboxamidine hydrochloride: 4-[4-(3-methylphenyl)( 1.3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate, (98 mg, 0.271 mmol) was treated in a
similar manner to
Example 22, step (b) to afford 75 mg (80% yield) of 4-[4-(3-methylphenyl)(1,3-
thiazol-2-
yl)]-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H-NMR (CD30D; 300
MHz) 8
CA 02362390 2001-08-07
W O 00/47578 _ ~ Q7-
PCT/US99/18065
8.56 (s, 1H), 7.88 (s, 1H), 7.86 (d, J-- 14 Hz, 2H), 7.33 (m, 1H), 7.19 (m, 1
H), 2.79 (s, 3H),
2.42 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z) Calcd. for
Ci6H,5N3S3v
346.0 (M+H), found 346.7
Example 25
5-Metlzylthio-4-(4-(2-5,6,7,8-tetrahydronaphthyl)(1,3-thiazol-2 yl))thiophene-
2
carboxamidine lZydroclZloride
a) Methyl 5-metlaylthio-4-(4-(2-5,6,7,8-tetrahydronaplathyl)(1,3-thiazol 2-
yl))thiophene-2-carboxylate: Methyl4-(aminothioxomethyl)-5-methylthiothiophene-
2-
carboxylate, (160 mg, 0.647 mmol) (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
reacted with 2-bromo-1-(2-5,6,7,8-tetrahydronaphthyl)ethan-1-one (0.712 mmol;
180 mg) in
a manner similar to Example 22, step (a) to afford 106 mg (41% yield) of
methyl 5-
methylthio-4-(4-(2-5,6,7,8-tetrahydronaphthyl)( 1,3-thiazol-2-yl))thiophene-2-
carboxylate.
b) 5-Methylthio-4-(4-(2-5,6,7,8-tetrahydronaphthyl)(1,3-thiazol 2
yl))thioplzene-2-
carboxamidine hydrochloride: 106 mg (0.264 mmol) of methyl 5-methylthio-4-(4-
(2-
5,6,7,8-tetrahydronaphthyl)(1,3-thiazol-2-yl))thiophene-2-carboxylate was
treated in a similar
manner to Example 22, step (b) to afford 88 mg (80% yield) of 5-methylthio-4-
(4-(2-5,6,7,8-
tetrahydronaphthyl)(1,3-thiazol-2-yl))thiophene-2-carboxamidine hydrochloride.
'H-NMR
(CD30D; 300 MHz) 8 8.49 (s, 1H), 7.78 (s, 1H), 7.73 (m, 2H), 7.11 (m, 1 H),
2.79 (m, 7H),
1.82-1.86 (m, 4H). Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z) Calcd. for
C,9H,9N3S3: 386.1 (M+H), found 386.2
Example 26
4-~4-(3,5-Dimethoxyphenyl)(1,3-thiazol-2 yl)J-5-metlzylthiotlaiophene-2-
carboxamidine hydroc)zloride
a) Methyl4-~4-(3,5-dimethoxyphenyl)(1,3-tlziazol-2 yl)J-S-metlzylthiothiophene-
2-
carboxylate: 100 mg (0.404 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
reacted with 2-bromo-3',5'-dimethoxy acetophenone (0.444 mmol) in a manner
similar to
Example 22, step (a) to afford 44 mg (27% yield) of methyl 4-[4-(3,5-
dimethoxyphenyl)(1,3-
thiazol-2-yl)J-5-methylthiothiophene-2-carboxylate.
CA 02362390 2001-08-07
PCT/US99/18065
WO 00/47578 _~~g_
b) 4-~4-(3,5-Dimethoxyphenyl)(1,3-thiazol 2 yl)J-S-methylthiothiophene-2-
carboxamidine hydrochloride: 44 mg (0.108 mmol) of methyl 4-[4-(3,5-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was
treated in a
manner similar to Example 22, step (b) to afford 25 mg (60% yield) of 4-[4-
(3,5-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride.
'H=NMR (CD30D; 300 MHz) 8 8.52 (s, 1H), 7.91 (s, 1H), 7.23 (d, J-- 2.2 Hz,
1H), 6.50 (t,
1H), 3.85 (s, 6 H), 2.89 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z)
Calcd.
for C"H"N30zS3: 392.11 (M+H), found 392.4.
Example 27
4-~4-(2-Metlzylphenyl)(1,3-tlaiazol-2 yl)J 5-methylthiothiophene-2-
carboxamidine
hydroclz loride
a) Methyl4-~4-(2-methylphenyl)(1,3-thiazol-2 yl)J S-methylthiothiophene-2-
carboxylate: 160 mg (0.647 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
reacted with 2-bromo-2'-methyl acetophenone (0.711 mmol, 152 mg) in a manner
similar to
Example 22, step (a) to afford 124 mg (53% yield) of methyl 4-[4-(2-
methylphenyl)(1,3-
thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate.
b) 4-~4-(2-Methylplzenyl)(1,3-thiazol-2 yl)J-5-methyltlziothiophene-2-
carboxamidine hydrochloride: 124 mg (0.343 mmol) of methyl 4-[4-(2-
methylphenyl)(1,3-
thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was treated in a manner
similar to
Example 22, step (b) to afford 60 mg (50% yield) of 4-[4-(2-methylphenyl)(1,3-
thiazol-2-
yl)]-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H-NMR (CD30D; 300
MHz) b
8.50 (s, 1H), 7.65 (m, 2H), 7.22-7.32 (m, 3H), 2.79 (s, 3H), 2.51 (s, 3H).
Mass Spectrum
(MALDI-TOF, CHCA Matrix, m/z) Calcd. for C,6H,SN3S3: 346.0 (M+H), found 346.2.
Example 28
4-~4-(2,5-Dimethoxyplzenyl)(1,3-tlziazol 2 yl)j-S-metlzylthiothiophene-2-
carboxamidine hydrochloride
a) Methyl 4-~4-(2,5-dimethoxyphenyl)(1,3-thiazol-2 yl)J-S-metlzylthiothiophene-
2-
carboxylate: 132 mg (0.534 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
CA 02362390 2001-08-07
wo ooi4~s~8 -~pg-
PCT/US99/18065
reacted with 2-bromo-2',5'-dimethoxy acetophenone (0.587 mmol; 152 mg) in a
manner
similar to Example 22, step (a) to afford 97 mg (45% yield) of methyl 4-[4-
(2,5-
dimethoxyphenyl)( 1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate.
b) 4-~4-(2,5-Dimethoxyplzenyl)(1,3-thiazol-2 yl)J-5-methylthiothiophene-2-
carboxamidine hydrochloride: 97 mg (0.238 mmol) of methyl 4-[4-(2,5-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2; carboxylate was
treated in a
manner similar to Example 22; step (b) to afford 30 mg (32% yield) of 4-[4-
(2,5-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride.
'H-NMR (CD30D; 300 MHz) 8 8.46 (s, 1H), 8.10 (s, 1H), 7.99 (d, J 3.2 Hz, 1H),
7.05 (d,
J-- 9 Hz, 1H), 6.93 (d, J 3.2 Hz, 1H), 6.90 (d, J-- 3.2 Hz, 1H), 3.94 (s, 3H),
3.83 (s, 3H), 2.51
(s, 3H). Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z) Calcd. for C"H"N3OZS3:
392.1
(M+I-I), found 392.1.
Example 29
4-~4-(4-Chloro(3 pyridyl))(1,3-thiazol 2 yl)J S-metlzyltlziothiophene-2-
carboxamidine lzydroclzloride
a) Metlzyl4-~4-(4-clzloro(3 pyridyl))(1,3-thiazol-2 yl)J-5-
methylthiothioplzene-2-
carboxylate: 240 mg (0.970 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene=2-carboxylate (Maybridge Chemical Co. LTD.. Cornwall,
U.K.) was
reacted with 2-bromo-1-(4-chloro(3-pyridyl))ethan-1-one (1.06 mmol; 250 mg) in
a manner
similar to Example 22, step (a) to afford 286 mg (77% yield) of methyl 4-[4-(4-
chloro(3-
pyridyl))(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate.
b) 4-(4-(4-Chloro(3 pyridyl))(1,3-thiazol 2 yl)J-5-methylthiothiophene-2-
carboxamidine hydrochloride: 286 mg (0.747 mmol) of methyl 4-[4-(4-chloro(3-
pyridyl))(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was treated
in a manner
similar to Example 22, step (b) to afford 134 mg (49% yield) of 4-[4-(4-
chloro(3-
pyridyl))(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride. Mass
Spectrum (MALDI- -TOF, CHCA Matrix, m/z) Calcd. for C,4H"N4C1S;: 366.9 (M+H),
found
366.6
CA 02362390 2001-08-07
PCT/US99/18065
WO 00/47578 -110-
Example 30
4-(4-(2H Benzo~dJl,3-dioxolen-S yl)(1,3-thiazol-2 yl))-S-metlaylthiothiophene-
2
carboxamidine hydrochloride
a) 1-(2H Benzo~3,4-dJl,3-dioxolen-S yl)-2-bromoethan-1-one: To a solution of
2.5
g (15.23 mmol) of 3,4-methylenedioxy acetophenone in 200 mL of anhydrous
methanol was
added 61 mmol (20 g) of poly (4-vinylpyridinium tribromide), Aldrich Chemical
Co., and
allowed to reflux for 2.5 h. The solution was filtered and concentrated. 1-(2H-
benzo[3,4-
d]1,3-dioxolen-5-yl)-2-bromoethan-1-one (1.4 g, 38% yield) was obtained
methylene
chloride/hexanes as off white crystals. 'H-NMR (DMSO-db; 300 MHz) 8 8.20 (s,
1H), 8.07
(s, 1H), 7.63 (m, 2H), 7.03 (dd, J-- 1.2 Hz and 7.1 Hz, 1H), 6.09 (s, 2H),
3.86 (s, 3H), 2.75 (s,
3H).
b) Methyl 4-(4-(2H benzo~dJl,3-dioxolen-S yl)(1,3-thiazol-2 yl))-5-
metlZyltlziothioplzene-2-carboxylate: 1.4 g (5.66 mmol) of methyl 4-
(aminothioxomethyl)-5-
1 S methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
reacted 1-(2H-benzo[3,4-d]1,3-dioxolen-5-yl)-2-bromoethan-1-one (5.66 mmol,
1.37 g) in a
manner similar to Example 22, step (a) to afford 1.55 g (70% yield) of methyl
4-(4-(2H-
benzo[d] 1,3-dioxolen-5-yl)(1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate.
c) 4-(4-(2H Benzo~dJl,3-dioxolen-5 yl) (1,3-thiazol-2 yl))-5-
metlzyltl:iothiophene-2-
carboxamidine IZydrochloride: 1.55 g (3.95 mmol) of methyl 4-(4-(2H-
benzo[d]1,3-
dioxolen-5-yl)(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxylate was
treated in a
manner similar to Example 22, step (b) to afford 130 mg (9% yield) of 4-(4-(2H-
benzo[d]1,3-
dioxolen-5-yl)(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxamidine
hydrochloride. 'H-
NMR (CD30D; 300 MHz) b 8.51 (s, 1H), 7.73 (s, 1H), 7.58 (m, 2H), 6.89 (d, J--
8 Hz, 1H),
6.00 (s, 2H), 2.79 (s, 3H). Mass Spectrum (MALDI-TOF, CHCA Matrix, m/z) Calcd.
for
C,6H,3N3OZS3: 376.0 (M+H), found 376.1.
Example 31
4-~4-(3,4-Dimetlzoxyplzenyl)(1,3-thiazol-2 yl)J-5-methylthiotlzioplzene-Z-
carboxamidine hydrochloride
a) 1-(3,4-Dimethoxyphenyl)-2-bromoetlzan-1-one: 2 g of 1-(3,4-
dimethoxyphenyl)ethan-1-one (11.1 mmol) was reacted in a manner similar to
Example 15 ,
step (a), to yield 1.2 g (42% yield) of 1-(3,4-dimethoxyphenyl)-2-bromoethan-1-
one.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-ttt-
b) Methyl4 ~4-(3,4-dimethoxyphenyl)(1,3-thiazol-2 yl)J 5-methylthiothiophene-2-
carboxylate: 1 OS mg (0.424 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Co. LTD., Cornwall,
U.K.) was
reacted with 1-(3,4-dimethoxyphenyl)-2-bromoethan-1-one (0.467 mmol; 120 mg)
in a
manner similar to Example 22, step (a) to afford 148 mg (85% yield) of methyl
4-[4-(3,4-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-S-methylthiothiophene-2-carboxylate.
c) 4-~4-(3,4-Dimethoxyphenyl)(1,3-thiazol 2 yl)J S-methylthiothiophene-2-
carboxamidine hydrochloride: 148 mg (0.363 mmol) of methyl 4-[4-(3,4-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate was
reacted in a
manner similar to Example 22, step (b) to afford 70 mg (50% yield) of 4-[4-
(3,4-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
hydrochloride.
'H-NMR (CD30D; 300 MHz) 8 8.50 (s, 1H), 7.76 (s, 1H), 7.61 (m, 2H), 7.31 (m,
1H), 7.01
(d, J-- 8 Hz, 1H), 3.9 (s, 3H) 3.86 (s, 3H), 2.78 (s, 3H). Mass Spectrum
(MALDI-TOF,
CHCA Matrix, m/z) Calcd. for C,~H"N30zS3: 392.1 (M+H), found 392.4.
Example 32
4-~4-(2-Clzloro(3 pyridyl))(1,3-thiazol 2 yl)~ 5-metlzyltlziothiophene-2
carboxamidine
a) Methyl4 ~4-(2-clZloro(3 pyridyl))(1,3-tlZiazol-2 yl)J-S-
methyltlZiothiophene-2-carboxylate: 2-Chloropyridine-3-carbonyl chloride (300
mg,
1.7 mmol) was dissolved in anhydrous CH3CN (4 mL). While stirring well with a
magnetic stirrer, trimethylsilyldiazomethane (4 mL, 2M solution in hexane, 8
mmol)
was dripped into the reaction mixture. The resulting yellow solution was
stirred for
2h at room temperature, at which time the mixture was cooled in an ice bath.
To the
cold solution, 30% HBr in acetic acid (2 mL) was added dropwise with vigorous
evolution of gas. This solution was stirred for lh during which time 2-bromo-1-
(2-
chloro(3-pyridyl))ethan-1-one precipitated. This solid was collected by
filtration and
dried under vacuum. The dry solid (142 mg, 0.6 mmol) was dissolved in acetone
(10
ml). To this solution S-(methoxycarbonyl)-2-(methylthio)-thiophene-3-
thiocarboxamide (100 mg, 0.4 mmol; Maybridge Chemical Company, Cornwall, UK)
was added and heated at reflux for 5 h. At this point the solid that
precipitated was
filtered off and washed with methanol and dried under vacuum to give 110 mg
(71%)
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-112-
of methyl 4-[4-(2-chloro(3-pyridyl))(1,3-thiazol-2-yl))-5-methylthiothiophene-
2-
carboxylate. 'H-NMR (CDCl3; 300 MHz) 8 2.70 (s, 3H), 3.92 (s, 3H), 7.39 (dd,
J--4.7 and 7.7 Hz, 1 H), 8.11 (s, 1 H), 8.22 (s, 1 H), 8.3 8 (dd, J--1.9 and
4.7 Hz, 1 H),
8.62 (dd, J--1.9 and 7.7 Hz, 1 H).
b) 4-~4-(2-Clzloro(3 pyridyl))(1,3-thiazol 2 yl)J S-methyltlziotlziopltene-2-
carboxamidine: Methyl4-[4-(2-chloro(3-pyridyl))(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate (100 mg, 0.26 mmol) as prepared in previous
step
was treated in a manner similar to that for Example 1, to give 50 mg (52%) of
4-[4-(2-
chloro(3-pyridyl))(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine as
a
solid. 'H-NMR (DMSO-db; 300 MHz) 8 2.79 (s, 3H), 7.62 (dd, J--4.9 and 7.4 Hz,
1 H), 8.41 (s, 1 H), 8.49 (m, 2H), 8.69 (s, 1 H), 9.1 (broad s, 2H), 9.4
(broad s, 2H).
Mass spectrum (ESI, m/z): Calcd. for C,4H"N4S3C1: 367.0 (M+H), found 369Ø
Example 33
4-(4-Cyclohexyl(1,3-thiazol 2 yl))-5-metlZyltlziotJZiopl:ene-2-carboxamidine
a) Methyl4-(4-cyclohexyl(1,3-thiazol-2 yl))-5-metlryltlZiothioplZene-2-
carboxylate: Cyclohexanecarbonyl chloride (300 mg, 2.0 mmol) was treated in a
manner similar to that for Example 32 to give 2-bromo-1-cyclohexylethan-1-one.
The
dry solid (125 mg) was dissolved in acetone (10 ml). To this solution 5-
(methoxycarbonyl)-2-(methylthio)-thiophene-3-thiocarboxamide ( 100 mg, 0.4
mmol,
Maybridge Chemical Company, Cornwall, UK) was added and heated at reflux for 5
h. At this point the solid that precipitated was filtered off and washed with
methanol
and dried under vacuum to give 100 mg (70%) of methyl 4-(4-cyclohexyl(1,3-
thiazol-
2-yl))-5-methylthiothiophene-2-carboxylate which was used without further
purification in the following step.
b) 4-(4-Cyclohexyl(1,3-thiazol-2 yl))-5-methylthiothioplZene-2-
carboxamidine: Methyl4-(4-cyclohexyl(1,3-thiazol-2-yl))-5-methylthiothiophene-
2-
carboxylate (100 mg, 0.28 mmol) as prepared in previous step was treated in a
manner
similar to that for Example 1, to give 60 mg (63%) of 4-(4-cyclohexyl(1,3-
thiazol-2-
yl))-5-methylthiothiophene-2-carboxamidine as a solid. 'H-NMR (DMSO-db; 300
MHz) 8 1.21-1.53 (m, SH), 1.61-1.78 (m, 3H), 2.05 (m, 2H), 2.7 (s, 3H), 2.74
(m,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
_113_
1 H), 7.33 (s, 1 H), 8.32 (s, 1 H). Mass spectrum (MALDI-TOF, m/z): Calcd. for
C,SH'gN3S3, 338.1 (M+H), found 338.1.
Example 34
$ 4-Phenyl-S-(trifluorometlzyl)thiophene-2-carboxamidine
Methyl 4-phenyl-5-(trifluoromethyl)thiophene-2-carboxylate (100 mg, 0.37
mmol, Maybridge Chemical Company, Cornwall, UK) was treated in a manner
similar to that for Example 1 to give 80 mg (85%) of 4-phenyl-5-
(trifluoromethyl)thiophene-2-carboxamidine as a solid. 'H-NMR (DMSO-db; 300
MHz) 8 7.45-7.52 (m, SH), 7.79 (d, J 1.4 Hz, 1H). Mass spectrum (MALDI-TOF,
m/z): Calcd. for C'ZH9F3NZS, 271.1 (M+H), found 271.2.
Example 35
5-Methylthio-4-(2 phenyl(1,3-thiazol 4 yl))thiophene-2-carboxamidine
a) Methyl4-(2-bromoacetyl)-S-methylthiotlziophene-2-carboxylate:
5-(Methoxycarbonyl)-2-methylthiothiophene-3-carboxylic acid (200 mg, 0.86
mmol)
as prepared in Example 95 was taken in a round bottomed flask and anhydrous
CHZC12 (10 mL) was introduced to the flask. This solution was cooled in an ice
bath
under an argon atmosphere. To this mixture oxalyl chloride (328 mg, 2.6 mmol)
was
added followed by anhydrous DMF (500 ~,L). The resulting solution was stirred
at
4°C for 30 min and then allowed to warm up to room temperature, while
monitoring
for the disappearance of the acid by TLC. After 2 h solvents were removed
under
vacuum and the residual oxalyl chloride was removed azeotropically with
toluene.
The resulting residue was dried under high-vacuum to give the acid chloride as
a gray
solid. This solid was dissolved in anhydrous CH3CN (8 mL). While stirring well
with a magnetic stirrer trimethylsilyldiazomethane (4 mL, 8 mmol, 2M solution
in
hexane) was dripped into the reaction mixture. The resulting yellow solution
was
stirred for 2h at room temperature, at which time the mixture was cooled in an
ice
bath. To the cold solution 30% HBr in acetic acid ( 2 mL) was added dropwise,
with
vigorous evolution of gas. This solution was stirred for lh, during which
methyl 4-(2
bromoacetyl)-5-methylthiothiophene-2-carboxylate precipitates. This solid was
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-114-
collected by filtration and dried under vacuum to give 120 mg (45%). 'H-NMR
(CDC13; 300 MHz) b 2.64 (s, 3H), 3.91 (s, 3H), 4.27 (s, 2H), 8.10 (s, 1H).
b) Methyl 5-methylthio-4-(2 phenyl(1,3-thiazol 4 yl))thiophene-2-
carboxylate: 5-(Methoxycaxbonyl)-2-(methylthio)-thiophene-3-thiocarboxamide
(100
mg, 0.4 mmol, Maybridge Chemical Company, Cornwall, UK) was dissolved in
acetone (20 ml). To this solution, methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-
2-carboxylate (112 mg) as prepared in previous step was added and heated at
reflux
for 3 h. At this point the solid that precipitated was filtered off and washed
with
acetone and dried under vacuum to give 82 mg (65%) of methyl 5-methylthio-4-(2-
phenyl(1,3-thiazol-4-yl))thiophene-2-carboxylate.'H-NMR (CDC13; 300 MHz) 8
2.67
(s, 3H), 3.91 (s, 3H), 7.44-7.49 (m, 3H), 7.61 (s, 1H), 8.03-8.06 (m, 2H),
8.28 (s, 1H).
c) S Metlzylthio-4-(2 plzenyl(1,3-thiazol 4 yl))thiophene-2-carboxamidine:
Methyl 5-methylthio-4-(2-phenyl(1,3-thiazol-4-yl))thiophene-2-carboxylate (80
mg)
as prepared in previous step was treated in a manner similar to that for
Example 1, to
give 50 mg of 5-methylthio-4-(2-phenyl(1,3-thiazol-4-yl))thiophene-2-
carboxamidine
as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 2.75 (s, 3H), 7.51-7.60 (m, 3H), 8.02
(s,
1 H), 8.06 (m, 2H), 8.70 (s, 1 H), 9.06 (broad s, 2H), 9.3 8 (broad s, 2H).
Mass
spectrum (MALDI-TOF, m/z): Calcd. for C,5H,3N3S3, 332.0 (M+H), found 332.1.
Example 36
4-~4-(2-Chloro(4 pyridyl))(1,3-thiazol 2 yl)J S-metlzylthiothioplzene-2
carboxamidine
a) Methyl4-~4-(2-clzloro(4 pyridyl))(1,3-thiazol 2 yl)J S-
methylthiothiophene-2-carboxylate: 2-Chloropyridine-4-carbonyl chloride (300
mg,
1.7 mmol) was dissolved in anhydrous CH3CN (4 mL). While stirring well with a
magnetic stirrer trimethylsilyldiazomethane (4 mL, 8 mmol, 2M solution in
hexane)
was dripped into the reaction mixture. The resulting yellow solution was
stirred for 2
h at room temperature, at which time the mixture was cooled in an ice bath. To
the
cold solution 30% HBr in acetic acid (2 mL) was added dropwise, with vigorous
evolution of gas. This solution was stirred for lh, during which time 2-bromo-
1-(2-
chloro(4-pyridyl))ethan-1-one precipitates. This solid was collected by
filtration and
dried under vacuum. The dry solid (142 mg, 0.6 mmol) was dissolved in acetone
(10
CA 02362390 2001-08-07
WO 00/47578 _~ ~ 5_ PCT/US99/18065
ml). To this solution 5-(methoxycarbonyl)-2-(methylthio)-thiophene-3-
thiocarboxamide (100 mg, 0.4 mmol, Maybridge Chemical Company, Cornwall, UK)
was added and heated at reflux for 5 h. At this point the solid that
precipitated was
filtered off and washed with methanol and dried under vacuum to give 100 mg of
methyl4-[4-(2-chloro(4-pyridyl))(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxylate. 'H-NMR (CD30D; 300 MHz) 8 2.73 (s, 3H), 3.94 (s, 3H, overlapping
H20 peak), 7.92-7.99 (m, 2H), 8.05 (s, 1 H), 8.24 (s, 2H), 8.48 (m, 1 H).
b) 4-~4-(2-Clzloro(4 pyridyl))(1,3-tlziazol 2 yl)J S-methylthiothiopJzene-2-
carboxamidine: Methyl4-[4-(2-chloro(4-pyridyl))(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate (100 mg, 0.26 mmol) as prepared in previous
step
was treated in a manner similar to that for Example 1, to give 50 mg of 4-[4-
(2-
chloro(4-pyridyl))(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine as
a
solid. 'H-NMR (CDCl3/CD30D; 300 MHz) 8 2.82 (s, 3H), 7.95 (dd, J--1.4 and 5.3
Hz, 1H), 8.08 (d, J--1.0 Hz, 1H), 8.23 (s, 1H), 8.42 (d, J--5.3 Hz, 1H), 8.56
(s, 1H).
Mass spectrum (MALDI-TOF, m/z): Calcd. for C,4H"NQS3Cl, 367.0 (M+H), found
367.1.
Example 37
4-~4-(4-Cl:lorophenyl)(1,3-thiazol-2 yl)J-5-(metlzylsulfonyl)thiophene-2-
carboxamidine
4-[4-(4-Chlorophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxamidine (35 mg, 0.1 mmol) prepared according to Example 1 was dissolved
in
a mixture of MeOH and CHzCl2 (1:1, 6 mL). While stirring well, m-
chloroperoxybenzoic acid (100 mg) was added in portions to this solution over
a 3h
period. The mixture was stirred for a further 2 h and the solvents were
removed under
vacuum. The resulting residue was dissolved in MeOH (8 mL). Strong anion
exchange resin (AG 1-X8, 5 ml, 1.4 meq/mL) was packed into a disposable
chromatography column and washed with H20 (5x5 mL) and MeOH (3x5 mL). The
methanolic solution from the reaction was slowly introduced into this column,
and the
column effluent was collected. The column was washed with MeOH (2x5 mL) and
these washings were also collected. The combined effluents were evaporated
under
vacuum and the residue was subjected to preparative thin layer chromatography
(silica
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-116-
gel, 10% MeOH in CHzCl2 with 2% acetic acid). The major band was isolated and
suspended in CHzCIz and filtered. The filtrate was collected and the residue
was
washed with 10% MeOH in CHZCI, saturated with NH3. The washings were
combined with the original filtrate and the solvents were removed under
vacuum. The
resulting solid was dissolved in 10% MeOH in CHC13 and filtered through a 0.45
micron filter. The filtrate was collected and evaporated under vacuum to give
20 mg
(53%) of an off white solid. 'H-NMR (CDC13/CD30D; 300 MHz) 8 3.78 (s, 3H),
7.47
(d, J--8.7 Hz, 2H), 7.96 (d, J--8.7 Hz, 1 H), 8.00 (s, 1 H), 8.3 5 (s, 1 H).
Mass spectrum
(MALDI-TOF, m/z): Calcd. for C,SH,ZOzN3S3C1, 398.0 (M+H), found 398Ø
Example 38
Hydrazino~5-methylthio-4-(4 phenyl(1,3-thiazol-2 yl))(2-thienyl)Jmethanimine
a) 5-Metlzylthio-4-(4 phenyl(1,3-tlziazol 2 yl))thiophene-2-carboxamide:
1 S Liquid ammonia (5 mL) was condensed into a cold (-78 °C) Teflon-
lined steel bomb.
Methyl 5-methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate (0.6
g,
1.7 mmol) as prepared in Example 10 step (a) was introduced in one portion and
the
bomb was sealed and heated in an oil bath at 80 °C for 48h. The bomb
was cooled to
-78 °C, opened and the ammonia was allowed to evaporate at room
temperature. The
residual solid was collected and dried under vacuum to give 0.5 g (88%) of
5-methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamide. 'H-NMR
(DMSO-db; 300 MHz) 8 2.75 (s, 3H), 7.38 (m, 1H), 7.40-7.51 (m, 2H), 8.04-8.18
(m,
2H), 8.19 (s, 1H), 8.20 (s, 1H).
b) 5-Methylthio-4-(4 plzenyl(1,3-thiazol-2 yl))thiophene-2-carbonitrile: A
slurry of P205 (2.7 g, 19 mmol) and hexamethyldisiloxane (6.7 mL) in
dichloroethane
(13 mL) was heated to 90 °C, while stirring under a NZ atmosphere.
After stirring for
2 h, the resulting clear solution was allowed to cool to 40 °C. 5-
methylthio-4-(4-
phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamide (0.9 g, 2.7 mmol) as prepared
in
previous step was added to this solution and the mixture was heated at
75°C for Sh.
This solution was cooled to room temperature and stirred with aqueous NaCI (6
M,
100 mL) for 10 min. As the aqueous solution is added a yellow solid
precipitated.
After 10 min this solid was separated by filtration, and dried under vacuum to
give
CA 02362390 2001-08-07
w0 00/47578 _~ ~ ~_ PCT/US99/18065
(0.5 g, 59%) of 5-methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-
carbonitrile
as a yellow solid.'H-NMR(DMSO-db; 300 MHz) b 2.76 (s, 3H), 7.38 (m, 1H), 7.48
(m, 2H), 8.07 (m, 2H), 8.22 (s, 1 H), 8.51 (s, 1 H).
c) Hydrazino(S-methylthio-4-(4 phenyl(1,3-thiazol 2 yl))(2-
thienyl)Jmethanimine: 5-Methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-
carbonitrile (100 mg, 0.32 mmol) as prepared in previous step was dissolved in
EtOH
( 10 mL). To this solution hydrazine monohydrate ( 10 eq) was added and the
mixture
was heated at reflux for 3h. The EtOH solution was concentrated down to 1 mL
and
water (2 mL) was added to this solution. This resulted in the formation of a
white
solid. The solid was collected by filtration washed with a small amount of
water and
dried under vacuum to give 50 mg (45%) of hydrazino[5-methylthio-4-(4-
phenyl(1,3-
thiazol-2-yl))(2-thienyl)]methanimine. 'H-NMR (CD30D/CDC13; 300 MHz) 8 2.69
(s, 3H), 7.39 (m, 1H), 7.47 (m, 2H), 7.52 (s, 1H), 7.98 (m, 2H), 8.10 (s, 1H).
Mass
spectrum (ESI, m/z): Calcd. for C,SH,4N4S3, 347.04 (M+H), found 347.1.
Example 39
(Imino~5-metlzylthio-4-(4 plzenyl(1,3-thiazol 2 yl))(2-
thienyl)Jmethyl~methylamine
5-Methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamidine (20
mg, 0.06 mmol) as prepared in Example 10 step (b) was dissolved in MeOH, and
to
this solution methylamine (0.6 mL, 2M solution in tetrahydrofuran) was added.
This
solution was refluxed for 6h, at which time the solvents were removed under
vacuum
to give a solid. This solid was dissolved in a small amount of MeOH. H20 was
added
dropwise to the methanolic solution until a precipitate was formed. This solid
was
isolated, washed with a small amount of water and dried under vacuum to give
15 mg
(72%) of {imino[5-methylthio-4-(4-phenyl(1,3-thiazol-2-yl))(2-
thienyl)]methyl}methylamine. 'H-NMR (DMSO-db; 300 MHz) 8 2.77 (s, 3H), 3.00
(s, 3H), 7.36-7.42 (m, 1H), 7.47-7.52 (m, 2H), 8.07-8.10 (m, 2H), 8.23 (s,
1H), 8.55
(s. 1H). Mass spectrum (ESI, m/z): Calcd. for C,~H,SN3S3, 346.5 (M+H), found
346.2.
CA 02362390 2001-08-07
WO 00/47578 _,~ ,) $_ PCT/US99/18065
Example 40
2-~3-~Z-(S Amidino-2-methylthio-3-thienyl)-1,3-tlziazol-4 ylJphenoxyJacetic
acid
a) 2-Bromo-I-(3-laydroxyphenyl)ethan-1-one: 2-Bromo-1-(3-
methoxyphenyl)ethan-1-one (2 g, 8.7 mmol) was taken in a round bottomed flask
equipped with magnetic stir bar. The flask was put under a NZ atmosphere and
CHZCIz was introduced into the flask. The resulting solution was cooled in a
dry ice-
acetone bath and BBr3 (27 mL, 1M in CHzCl2) was introduced dropwise. The
resulting solution was allowed to warm up to room temperature overnight. The
solvents were removed under vacuum and the residue was purified by passing
through
a short pad of silica gel (50 g) to give 1.3 g (69%) of 2-bromo-1-(3-
hydroxyphenyl)ethan-1-one as an oil. 'H-NMR (CDC13; 300 MHz) 8 4.47 (s, 2H),
6.21 (s, 1H), 7.14 (m, 1H), 7.35 (m, 1H), 7.52-7.82 (m, 2H).
b) Metlzyl4-~4-(3-hydroxyphenyl)(1,3-thiazol 2 yl)J S-methylthiothiophene-
2-carboxylate: 2-Bromo-1-(3-hydroxyphenyl)ethan-1-one (229 mg, 1.1 mmol) as
prepared in previous step was treated' in a manner similar to that of Example
13, step
(a) to give 225 mg (61%) ofmethyl 4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxylate as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 2.76
(s, 3H), 3.86 (s, 3H), 6.87 (m, 1H), 7.27 (t, J--7.8 Hz, 1H), 7.49 (m, 2H),
8.12 (s, 1H),
8.20 (s, 1 H).
c) (tent Butoxy)-N (~4 ~4-(3-hydroxyphenyl)(1,3-thiazol-2 yl)J S-
methyltlzio(2-thienyl)Jiminomethyl)carboxamide: 4-[4-(3-Hydroxyphenyl)(1,3-
thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine (2 g, 5.8 mmol), prepared
by
treating methyl 4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxylate in a manner similar to that for Example 1, was dissolved in
anhydrous
DMF (10 mL). To this solution di-tert-butyl dicarbonate (1.38 g, 6.3 mmol) and
DIEA
(2 mL, 11.5 mmol) was added, and the mixture was stirred at room temperature
for 18
h. DMF was removed under vacuum and the residue was purified by silica gel
column
chromatography to give 1.8 g (70%) of (tert-butoxy)-N-({4-[4-(3-
hydroxyphenyl)(1,3-
thiazol-2-yl)]-5-methylthio(2-thienyl)}iminomethyl)carboxamide as an oil. 'H-
NMR
(DMSO-db; 300 MHz) 8 1.58 (s, 9H), 2.81 (s, 3H), 6.81 (m, 1H), 7.28 (t, J--8.0
Hz,
1 H), 7.49-7.52 (m, 2H), 8.09 (s, 1 H), 8.71 (s, 1 H).
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-119
d) tent-Butyl2-~3 ~2-(S-~((tert-butoxy)carbonylaminoJiminomethylf-2-
methylthio-3-thienyl)-1,3-thiazol-4 ylJphenoxyJacetate: (tent-Butoxy)-N-({4-[4-
(3-
hydroxyphenyl)( 1,3-thiazol-2-yl)]-5-methylthio(2-thienyl) }
iminomethyl)carboxamide
(23 mg, 0.05 mmol) as prepared in previous step was dissolved in anhydrous DMF
(1
mL). To this solution tent-butyl 2-bromoacetate (20 mg, 0.1 mmol), CszC03
(33.5 mg,
0.1 mmol) and KI (5 mg) was added and the mixture was heated at 70 °C
for 18 h.
The solvents were removed under vacuum and the residue was~purified by
preparative
silica gel thin-layer chromatography to give 12 mg (42%) of tert-butyl 2-{3-[2-
(5-
{ [(tent-butoxy)carbonylaminoJiminomethyl }-2-methylthio-3-thienyl)-1,3-
thiazol-4-
yl]phenoxy}acetate which was used in the following step.
e) 2-~3-~2-(S Amidino-2-metJzylthio-3-thienyl)-1,3-tlziazol 4-
ylJphenoxy)acetic acid: tert-Butyl 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl }-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetate (12 mg, 0.02 mmol) as prepared in previous step was
dissolved in
1 ml 50% TFA in CHZC12 containing 2% H20 and stirred for 4h. The solvents were
removed under vacuum. The residual TFA was removed by azeotroping with toluene
to give 8.7 mg (100%) of 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-
thiazol-4-
yl]phenoxy}acetic acid as a buff colored solid. 'H-NMR (CD30D/CDC13; 300 MHz)
8 2.77 (s, 3H), 4.74 (S, 2H), 6.93 (m, 1H), 7.35 (t, J--7.9 Hz, 1H), 7.62 (m,
1H), 7.68
(M, 1H), 7.84 (s, 1H), 8.46 (s, 1H). Mass spectrum (ESI, m/z): Calcd. for
C"H,SN3O3S3, 406.5 (M+H), found 406.3.
Example 41
2-~2 ~2-(S Amidino-2-methylthio-3-thienyl)-1,3-tltiazol 4 ylJphenoxy~acetic
acid
a) tert-Butyl2-~2 ~2-(5-~~(tert-butoxy)carbonylaminoJiminomethylJ-2-
methylthio-3-thienyl)-1,3-thiazol 4 ylJphenoxyJacetate: 4-[4-(2-
Hydroxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine (100
mg,
0.29 mmol) as prepared in Example 196 step (b) was treated in a manner similar
to
that shown in Example 40 step (c) to give 100 mg (0.22 mmol, 77%) of (tert-
butoxy)-
N-({4-[4-(2-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-methylthio(2-
thienyl)}iminomethyl)carboxamide. This compound was treated in a manner
similar
to that shown in Example 40, step (d) to give 63 mg (50 %) of ten-butyl 2-{2-
[2-(5-
CA 02362390 2001-08-07
WO 00/47578 _' 20- PCT/US99/18065
{ [(tert-butoxy)carbonylamino]iminomethyl}-2-methylthio-3-thienyl)-1,3-thiazol-
4-
yl]phenoxy}acetate. 'H-NMR (CDC13; 300 MHz) 8 1.55 (s, 9H), 1.56 (s, 9H), 2.69
(s,
3H), 4.66 (s, 2H), 6.88 (dd, J 0.8 and 8.3 Hz, 1H), 7.14 (dt, J 1.0 and 7.6
Hz, 1H),
7.30 (m, 1 H), 8.08 (s, 1 H), 8.48 (dd, J--1.8 and 7.8 Hz, 1 H), 8.51 (s, 1
H).
b) 2-(2 ~2-(S Amidino-2-methylthio-3-thienyl)-1,3-thiazol 4-
ylJphenoxyJacetic acid: tert-Butyl 2-{2-[2-(5-{ [(tert-
butoxy)carbonylamino]iminomethyl}-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetate (60 mg, 0.12 mmol) as prepared in previous step was treated
in a
manner similar to that shown in Example 40, step (e) to give 22 mg (50 %) of 2-
{2-[2-
(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid. 'H-
NMR
(DMSO-db; 300 MHz) 8 2.80 (s, 3H), 4.90 (s, 2H), 7.17 (m, 2H), 7.36 (m, 1H),
8.41
(d, J--6.3 Hz, 1H), 8.60 (s, 1H), 8.62 (s, 1H), 9.00 (broad s, 2H), 9.37
(broad s, 2H).
Mass spectrum (ESI, m/z): Calcd. for C"H,SN3O3S3, 406.5 (M+H), Found 406.1.
Example 42
S-Methylthio-4-(6 phenyl(2 pyridyl))thiophene-2-carboxamidine
a) Metlzyl4-(l,l-dimethyll-stannaethyl)-5-methyltlaiothioplzene-2
carboxylate: 4-Bromo-5-methylthiothiophene-2-carboxylic acid (EP 0676395 A2)
(4.67 g, 18.4 mmol) was dissolved in anhydrous THF (30 mL), taken in a round
bottomed flask and cooled to -78 °C under a NZ atmosphere. To this
solution n-
butyllithium (20.3 mL, 40.6 mmol, 2M in cyclohexane) was introduced in a
dropwise
manner. The resulting solution was stirred at -78 °C for 45 min and
then allowed to
warm up to -60 °C. To this solution trimethyltin chloride (40.6 mL,
40.6 mmol, 1M
in THF) was added dropwise. This solution was stirred at -60 °C for 30
min and then
allowed to warm up to room temperature. The THF was removed under vacuum and
the residue was treated with HZO and extracted with hexane. The hexane layer
was
evaporated and the residue was dissolved in Et20. The EtzO solution was washed
with
10% HCl , saturated NaCI and dried over anhydrous MgS04. Et20 was removed
under
vacuum and the residue was taken in MeOH. The MeOH solution was treated with
trimethylsilyldiazomethane (18.5 mL, 2M in hexane) and stirred at room
temperature
for 1 h. The solvents were removed under vacuum to give 2 g (31 %) of methyl 4-
( 1,1-
CA 02362390 2001-08-07
WO 00/47578 _~ 21 _ PCT/US99/18065
dimethyl-1-stannaethyl)-5-methylthiothiophene-2-carboxylate as an oil. 'H-NMR
(CDC13; 300 MHz) 8 0.31 (s, 9H), 2.57 (s, 3H), 3.86 (s, 3H), 6.98 (s, 1H).
b) Methyl4-(6-bromo(2 pyridyl))-S-methylthiothiophene-2-carboxylate:
Methyl 4-(1,1-dimethyl-1-stannaethyl)-5-methylthiothiophene-2-carboxylate (195
mg,
0.56 mmol) as prepared in previous step, and 2,6-dibromopyridine (398 mg, 1.7
mmol) were taken in anhydrous DMF (2 mL). To this mixture
tetrakistriphenylphosphine-palladium (20 mg) was added and heated at 120
°C for 24
h. DMF was removed under vacuum and the residue was purified by preparative
silica
gel thin -layer chromatography to give 78 mg (41 %) of methyl 4-(6-bromo(2-
pyridyl))-5-methylthiothiophene-2-carboxylate as a solid. 'H-NMR (CDC13; 300
MHz) 8 2.60 (s, 3H), 3.78 (s, 3H), 7.19 (s, 1H), 7.47 (dd, J 1.1 and 7.7 Hz,
1H)), 7.58
(t, J--7.7, 1 H), 7.65 (dd, J 1.1 and 7.4 Hz, 1 H).
c) Methyl S-methyltltio-4-(6 phenyl(2 pyridyl))tltiopherte-2-carboxylate:
Methyl 4-(6-bromo(2-pyridyl))-5-methylthiothiophene-2-carboxylate (78 mg, 0.23
mmol) as prepared in previous step, phenylboronic acid (33 mg, 0.27 mmol) and
tetrakistriphenylphosphine-palladium (10 mg) were taken in DMF (1 mL). To this
solution KZC03 (75 mg, 0.54 mmol) and Hz0 (0.3 mL) were added and the mixture
was stirred and heated at 90 °C for 18h. Solvents were removed under
vacuum and the
residue was dissolved in EtOAc and extracted with HzO, washed with saturated
NaCI
and dried over anhydrous Na2S04. Thin-layer chromatography of the aqueous
layer
indicated the presence of some hydrolyzed product. Therefore the aqueous layer
was
separated acidified with 10% HCl and extracted with EtOAc. The EtOAc layer was
washed with saturated NaCI and dried over anhydrous Na2S04. This second EtOAc
fraction was evaporated and the residue was dissolved in MeOH and treated with
trimethylsilyldiazomethane (1.2 eq). This methanolic solution and the first
EtOAc
fraction were combined and evaporated. The residue was subjected to
preparative
thin-layer chromatography (10% EtOAc in Hexane) to give 40 mg (51%) of methyl
5-
methylthio-4-(6-phenyl(2-pyridyl))thiophene-2-carboxylate which was used
directly
in the next step.
d) S-Metltylthio-4-(6 phenyl(2 pyridyl))thiophene-2-carboxamidine: Methyl
5-methylthio-4-(6-phenyl(2-pyridyl))thiophene-2-carboxylate (40 mg, 0.12 mmol)
as
prepared in previous step was treated in a manner similar to that for Example
1, to
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-122-
give 10 mg of 5-methylthio-4-(6-phenyl(2-pyridyl))thiophene-2-carboxamidine as
a
solid. 'H-NMR (CD30D; 300 MHz) b 2.69 (s, 3H), 7.45-7.60 (m, 3H), 7.62 (s,
1H),
7.79 (dd, J--0.9 and 7.8 Hz, 1 H), 7.96 (dd, J 0.9 and 8.0 Hz, 1 H), 8.03-8.12
(m, 3H).
Mass spectrum (ESI, m/z): Calcd. for C,~H,SN3S2, 326.1 (M+H), found 326.1.
Example 43
S-Methylthio-4-(3 phenylphenyl)thiophene-2-carboxamidine
a) Methyl S-metl:ylthio-4-(3 phenylphenyl)thiophene-2-carboxylate:
Methyl 4-(1,1-dimethyl-1-stannaethyl)-S-methylthiothiophene-2-carboxylate (200
mg,
0.57 mmol, as prepared in Example 42, step a) and 1-bromo-3-phenylbenzene (266
mg, 1.14 mmol) were taken in anhydrous ]~MF (2 mL). To this mixture
tetrakistriphenylphosphine-palladium (20 mg) was added and heated at 120
°C for 24
h. DMF was removed under vacuum and the residue was purified by preparative
silica
gel thin -layer chromatography to give 39 mg (20 %) methyl 5-methylthio-4-(3-
phenylphenyl)thiophene-2-carboxylate as a solid. 'H-NMR (CD30D; 300 MHz)8
2.60 (s, 3H), 3.75 (s, 3H), 7.3-7.5 (m, 6H), 7.60-7.66 (m, 4H).
b) S-Methylthio-4-(3 phenylphenyl)thiophene-2-carboxamidine: Methyl 5-
methylthio-4-(3-phenylphenyl)thiophene-2-carboxylate (35 mg, 0.1 mmol) as
prepared in previous step was treated in a manner similar to that for Example
l, to
give 17 mg of 5-methylthio-4-(3-phenylphenyl)thiophene-2-carboxamidine as a
solid.
'H-NMR (CD30D; 300 MHz) 8 2.60 (s, 3H), 7.3-7.6 (m, lOH). Mass spectrum (ESI,
m/z): Calcd. for C,8H,6NZS2, 325.4 (M+H), found 325.2.
Example 44
S-Metlzylthio-4-~4-(phenylthiometlzyl)(1,3-thiazol-2 yl)Jtlziophene-2-
carboxamidine
a) Methyl S-methyltlZio-4 ~4-(phenylthiomethyl)(1,3-thiazol-2 yl) Jthiophene-
2-carboxylate: 2-Phenylthioacetyl chloride (1.0 g, 5.4 mmol) was treated in a
manner
similar to that for Example 32 step (a) to give 2-bromo-1-
phenylthiomethylethan-1-
one. The dry solid (1.3 g, 5.3 mmol) was dissolved in acetone (25 ml). To this
solution 5-(methoxycarbonyl)-2-(methylthio)-thiophene-3-thiocarboxamide (1.32
g,
5.3 mmol, Maybridge Chemical Co.) was added and heated at reflux for 5 h. At
this
point the solid that precipitated was filtered off and washed with acetone and
dried
CA 02362390 2001-08-07
WO 00/47578 _,~ 23_ PCT/US99/18065
under vacuum to give 1.5 g (71 %) of methyl 5-methylthio-4-[4-
(phenylthiomethyl)(1,3-thiazol-2-yl)]thiophene-2-carboxylate which was used
without
further purification in the following step.
b) S-MetIZylthio-4-~4-(plzenyltlziomethyl)(1,3-tlaiazol 2 yl)Jthiophene-2-
carboxamidir:e: Methyl5-methylthio-4-[4-(phenylthiomethyl)(1,3-thiazol-2-
yl)]thiophene-2-carboxylate (1.5 g, 3.8 mmol) as prepared in previous step was
treated
in a manner similar to that for Example l, however the product was purified by
crystallizing from methanol to give 0.86 g (60%) 5-methylthio-4-[4-
(phenylthiomethyl)(1,3-thiazol-2-yl)]thiophene-2-carboxamidine as a solid. 'H-
NMR
(DMSO-db; 300 MHz) 8 2.72 (s, 3H), 4.38 (s, 2H), 7.18-7.39 (m, 5H), 7.57 (s,
1H),
8.46 (s, 1H). Mass spectrum (MALDI-TOF, m/z): Calcd. for C~6H~SN3S4. 378.0
(M+H), found 378.1.
Example 45
4-~4-(2-Chloro-4,5-dimethoxyplzenyl)(1,3-thiazol-2 yl)J 5-metlzylthiothiophene-
2-
carboxamidine
a) Methyl4-~4-(2-chloro-4,5-dimethoxyphenyl)(1,3-tltiazol 2 yl)J 5
metlzyltlaiotlziophene-2-carboxylate: 2-Chloro-4,5-dimethoxybenzoic acid (0.5
g, 2.3
mmol) and PCIs (0.54 g, 2.6 mmol) were placed in a round bottomed flask fitted
with
a reflux condenser. The mixture was heated in an oil bath at 120 °C for
70 min. The
mixture was allowed to cool and the formed phosphorus oxychloride was removed
under vacuum to give 0.52 g (96%) of 2-chloro-4,5-dimethoxybenzoyl chloride as
a
solid. 2-Chloro-4,5-dimethoxybenzoyl chloride (0.52 g, 2.2 mmol) was treated
in a
manner similar to that for Example 32 step (a) to give 2-bromo-1-(2-chloro-4,5-
dimethoxyphenyl) ethan-1-one. The dry solid (0.65 g, 2.2 mmol) was dissolved
in
acetone (25 ml). To this solution 5-(methoxycarbonyl)-2-(methylthio)-thiophene-
3-
thiocarboxamide (0.55 g, 2.2 mmol) was added and heated at reflux for 5 h. At
this
point the solid that precipitated was filtered off and washed with acetone and
dried
under vacuum to give 0.53 g (54%) of methyl 4-[4-(2-chloro-4,5-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate. 'H-
NMR
(DMSO-db; 300 MHz) 8 2.73 (s, 3H), 3.83 (s, 3H), 3.84 (s, 3H), 3.85 (s, 3H),
7.13 (s,
1 H), 7.69 (s, 1 H), 8.13 (s, 1 H), 8.17 (s, 1 H).
CA 02362390 2001-08-07
WO 00/47578 _,~24' PCT/US99/18065
b) 4-~4-(2-Chloro-4,5-dimethoxyphenyl)(1,3-thiazol 2 yl)J S-
metlryltlZiotlziophene-2-carboxamidine: Methyl4-[4-(2-chloro-4,5-
dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxylate (0.53
g,
1.2 mmol) as prepared in previous step was treated in a manner similar to that
for
Example l, however the product was purified by crystallizing from methanol to
give
to give 0.3 g (60%) 4-[4-(2-chloro-4,5-dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxamidine as a solid. 'H-NMR (DMSO-db; 300 MHz)
8 2.77 (s, 3 H), 3.84 (s, 6H), 7.13 (s, 1 H), 7.71 (s, 1 H), 8.17 (s, 1 H),
8.69 (s, 1 H), 9.16
(broad s, 2H), 9.48 (broad s, 2H). Mass spectrum (MALDI-TOF, m/z): Calcd. for
lO C"H~6N3OZS3C1, 426.0 (M+H), found 426.6.
Example 46
4-~(Methylethyl)sulfonylJ S-methylthiothiophene-2-carboxamidine
Methyl 4-[(methylethyl)sulfonyl]-5-methylthiothiophene-2-carboxylate (100
mg, Maybridge Chemical Company, Cornwall, UK) was treated in a manner similar
to that for Example 1, to give 50 mg of 4-[(methylethyl)sulfonyl]-5-
methylthiothiophene-2-carboxamidine. 'H-NMR (DMSO-db; 300 MHz) b 1.21 (d,
J--6.77 Hz, 6H), 2.66 (s, 3H), 3.55 (m, 1H), 7.85 (s, 1H). Mass spectrum
(MALDI-
TOF, CHCA matrix, m/z): Calcd. for C9H'4NZO2S3, 279.0 (M+H), found 279.3.
Example 47
Methyl2-~3-~2-(S-amidino-2-methylthio-3-thienyl)-1,3-tlziazol-4
ylJphenoxyJacetate
tr~uoroacetate
To a solution of 42 mg (0.094 mmol) of (tert-butoxy)-N ({4-[4-(3-
hydroxyphenyl)( 1,3-thiazol-2-yl)]-5-methylthio(2-thienyl) }
iminomethyl)carboxamide,
prepared in a manner similar to Example 40, step (c), in 2 mL of anhydrous
N?V'-
dimethylformamide (DMF) was added potassium iodide (0.006 mmol, 1 mg, Aldrich
Chemical Co.), cesium carbonate (0.187 mmol, 61 mg, Aldrich Chemical Co.), and
methyl
bromoacetate (0.187 mmol, 18 ~L, Aldrich Chemical Co.) and heated to 60
°C overnight.
The reaction solution was concentrated and purified on a 1 mm silica prep
plate eluting with
3% methanol/CHZC12 to afford 11 mg (23% yield) of methyl 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methylthio-3-thienyl)-1,3-thiazol-4-
CA 02362390 2001-08-07
WO 00/47578 _,~25_ PCT/US99/18065
yl]phenoxy}acetate which was then subjected to a solution of SO%
trifluoracetic acid/CHZCIz
for 1 h then concentrated and triturated with diethyl ether and dried to
afford 7 mg (77%
yield) of methyl 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetate trifluoroacetate. 'H-NMR (CD30D; 300 MHz) 8 8.51 (s, 1H),
7.92 (s,
1H), 7.66 (m, 2H), 7.34-7.39 (t, 1H), 6.93 (m, 1H), 4.8 (s, 2H) 3.80 (s, 3H),
2.78 (s, 3H).
Mass Spectrum (LC-Q ESI, m/z) Calcd. for C,BH,~N3O3S3: 419.5 (M+H), found
420.3.
Example 48
5-Methylthio-4 j4-(3-~~N beuzylcarbamoylJmethoxyJphenyl)(1,3-thiazol 2
yl)Jthiophene-2-
carboxamidine tr~uoroacetate
100 mg (0.197 mmol) of 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl } -2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetic acid, as prepared in the previous step, were dissolved in 1
mL of
anhydrous DMF and PyBOP (0.396 mmol, 206 mg), benzylamine (0.396 mmol, 42
mg), and diisopropylethylamine (0.494 mmol; 86 ~,L) were added to the solution
and
stirred for 18 hrs after which the solution was concentrated and purified on a
2 g silica
SPE column and deprotected with 50% trifluoroacetic acid/ methylene chloride
to
afford 60 mg (67% yield) of 5-methylthio-4-[4-(3-{ [N-
benzylcarbamoyl]methoxy}phenyl)(1,3-thiazol-2-yl)]thiophene-2-carboxamidine
trifluoroacetate. 'H-NMR (CDCl3/TFA-d; 300 MHz) 8 8.97 (s, 1H), 7.86 (s, 1H),
7.53
(t, 1H), 7.33 (m, 7H), 7.17 (d, 1H), 4.79 (s, 2H) 4.59 (s, 2H), 2.95 (s, 3H).
Mass
Spectrum (ESI, m/z) Calcd. for CZ4HZZN4OZS3: 494.6 (M+H), found 495.2.
Example 49
4-~4-~3-(~1V ~(3,4-Dimethoxyphenyl)metlzylJcarbamoylJmethoxy)phenylJ(1,3-
tlziazol
2 yl)J-S-methyltlziotlzioplzene-2-carboxamidi~ze tr~uoroacetate
Dissolved 100 mg (0.197 mmol) of 2-{3-[2-(5-{ [(tert-
butoxy)carbonylamino]iminomethyl}-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetic acid, prepared in a manner similar to Example 48, step (c),
in 1 mL of
anhydrous DMF and added PyBOP (0.396 mmol, 206 mg), 3,4-dimethoxybenzylamine
(0.396 mmol,66 mg), and diisopropylethylamine (0.494 mmol; 86 ~,L) and let
stir for 18 hrs
after which solution was concentrated and purified on a 2 g silica SPE column
and
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-t26-
deprotected with 50% trifluoroacetic acid/ methylene chloride to afford 45 mg
(41 % yield) 4-
{4-[3-( {N-[(3,4-dimethoxyphenyl)methyl]carbamoyl } methoxy)phenyl]( 1,3-
thiazol-2-yl) }-5-
methylthiothiophene-2-carboxamidine trifluoroacetate. 'H-NMR (CDCl3/TFA-d: 300
MHz)
8 8.48 (s, 1H), 7.78 (s, 1 H), 7.72 (m, 1 H), 7.66 (d, 1 H), 7.39 (t, 1 H),
7.02 (d, 1 H) 4.68 (s,
2H), 4.43 (s, 2H), 3.75 (s, 3H). 3.56 (s, 3H). 2.78 (s, 3H). Mass Spectrum (LC-
Q ESI, m/z)
Calcd. for CZ6H26N4O4'~3~ 554.6 (M+H), found 555.2
Example SO
5-Metlayltlzio-4-~4-~3-(~1V ~2-
(pJzenylamino)ethylJcarbamoyl)methoxy)phenylJ(1,3-
thiazol-2 yl))thiophene-2-carboxamidine tr~uoroacetate
Dissolved 100 mg (0.197 mmol) of 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetic acid, prepared in a manner similar to Example 48, step (c),
in 1 mL of
anhydrous DMF and added PyBOP (0.396 mmol, 206 mg), N-phenylethylenediamine
(0.396
mmol, 54 mg), and diisopropylethylamine (0.494 mmol; 86 ~L) and let stir for
18 hrs after
which solution was concentrated and purified on a 2 g silica SPE column and
deprotected
with 50% trifluoroacetic acid/ methylene,chloride to afford 65 mg (63% yield)
5-methylthio-
4-{4-[3-( {N-[2-(phenylamino)ethyl]carbamoyl } methoxy)phenyl] ( 1,3-thiazol-2-
yl)}thiophene-2-carboxamidine trifluoroacetate 'H-NMR (CDC13/TFA-d; 300 MHz) 8
8.50
(s, 1 H), 7.82 (s, 1 H), 7.77 (s, 1 H), 7.66 (d, 1 H), 7.39 (t, l H), 7.02 (d,
1 H) 4.68 (s, 2H), 4.43 (s,
2H), 3.75 (s, 3H). 3.56 (s, 3H). 2.78 (s, 3H). Mass Spectrum (LC-Q ESI, m/z)
Calcd. for
C2sHzsNsOzSs: 523.6 (M+H), found 524.1
Example 51
5-Methylthio-4-~4-(3-~~N (2-morplzolin-4 ylethyl)carbamoylJmethoxy~phenyl)(1,3
tlZiazol 2 yl)Jthiophene-2-carboxamidine tr~uoroacetate
83 mg (0.164 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 40, step (c), was reacted with 2-morpholin-4-ylethylamine (0.328 mmol,
43 ~L) in
a manner similar to Example 48 to afford 46 mg (54% yield) of 5-methylthio-4-
[4-(3-{ [N-(2-
morpholin-4-ylethyl)carbamoyl]methoxy} phenyl)( 1,3-thiazol-2-yl)]thiophene-2-
carboxamidine trifluoroacetate. 'H-NMR (DMSO-db, 300 MHz) 8 9.38 (bs, 2H),
9.08 (bs,
CA 02362390 2001-08-07
WO 00/47578 _~ 27_ PCT/US99/18065
2H), 8.61 (s, 1 H), 8.45 (t, 1 H), 8.27 (s, 1 H), 7.72 (m, 2H) 7.45 (t, 1 H),
7.02 (d, J-- 8 Hz, 1 H),
4.62 (s, 2H), 3.53-3.64 (m, SH), 3.24-3.38 (m, SH), 2.80 (s, 3H), 1.1 (t, ZH).
Mass Spectrum
(ESI, m/z) Calcd. for C23Hz~N5O3S3: 517.6 (M+H), found 518.2.
Example 52
S-Metlzylthio-4-(4 ~3-(2-morpholin-4 yl-Z-oxoethoxy)phenylJ(1,3-thiazol-2
yl)Jthioplzene
2-carboxamidine tr~uoroacetate
73 mg (0.144 mmol) of 2-{3-[2-(5-{[(tent-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 48, step (c), was reacted with morpholine (0.288 mmol; 25 ~.L) in a
manner similar
to Example 48 step (b) to afford 50 mg (75% yield) 5-methylthio-4-{4-[3-(2-
morpholin-4-yl-
2-oxoethoxy)phenyl](1,3-thiazol-2-yl)}thiophene-2-carboxamidine
trifluoroacetate. 'H-NMR
(DMSO-db/TFA-d; 300 MHz) 8 9.38 (bs, 1H), 9.08 (bs, 2H), 8.66 (s, 1H), 8.22
(s, 1H), 7.72
(m, 2H) 7.42 (t, 1H), 6.98-7.00 (dd, J-- 2.3 Hz and 8.2 Hz, 1H), 4.95 (s, 2H),
3.53-3.67 (m,
8H), 2.82 (s, 3H). Mass Spectrum (ESI, m/z) Calcd. for CZ,HZZN4O3S3: 474.6
(M+H), found
475.2.
Example 53
S-Methylthio-4-(4-~3-(2-oxo-2 piperazinylethoxy)phenylJ(1,3-tlziazol 2-
yl)Jthiophene-2-carboxamidine tr~uoroacetate
100 mg (0.198 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 48, step (c), was reacted with tert-butyl piperazinecarboxylate (0.396
mmol; 74 mg)
in a manner similar to Example 48, step (b) to afford 40 mg (43% yield) of 5-
methylthio-4-
{4-[3-(2-oxo-2-piperazinylethoxy)phenyl](1,3-thiazol-2-yl) }thiophene-2-
carboxamidine
trifluoroacetate. 'H-NMR (DMSO-db/TFA-d; 300 MHz) 8 8.68 (s, 1H), 8.20(s, 1H),
7.75 (m,
2H) 7.43 (t, 1 H), 7.01 (dd, J-- 2.3 Hz and 8.1 Hz, 1 H), 5.02 (s, 2H), 3.76
(bs, 4H), 3.17-3.26
(m, 4H). 2.82 (s, 3H). Mass Spectrum (LC-Q ESI, m/z) Calcd. for CZ,HZ3NSOzS3:
473.6
(M+H), found 474.2.
CA 02362390 2001-08-07
WO 00/47578 _i28_ PCT/US99/18065
Example 54
4-~4-(3-~~N (2 Aminoetl:yl)carbamoylJmethoxyJphenyl)(1,3-thiazol 2 yl)J 5
metltylthiothiophene-2-carboxamidine hydrochloride
51 mg (0.101 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 48, step (c), was reacted with N-(2-aminoethyl)(tent-
butoxy)carboxamide (0.202
mmol; 32 mg) in a manner similar to Example 48, step (b) to afford 80 mg (80%
yield) of 4-
(4-{ 3-[(N-{2-[(tert-butoxy)carbonylamino]ethyl } carbamoyl)methoxy]phenyl } (
1,3-thiazol-2-
yl))-5-methylthiothiophene-2-carboxamidine which was then deprotected with 4N
HCl in
dioxane to afford 36 mg (68% yield) of 4-[4-(3-{[N-(2-
aminoethyl)carbamoyl)methoxy}phenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-
2-
carboxamidine hydrochloride. 'H-NMR (CD30D; 300 MHz) 8 8.55 (s, 1H), 7.95 (s,
1H),
7.73 (m, 2H) 7.41 (t, 1H), 7.05 (m, 1H), 4.80 (s, 2H), 3.51 (m, 2H), 3.13-3.31
(m, 2H), 2.83
(s, 3H). Mass Spectrum (ESI, m/z) Calcd. for C,9HZ,NSOzS3: 447.5 (M+H), found
448.2.
Example SS
4-(4-~3-~2-(4 Acetylpiperazinyl)-Z-oxoethoxyJphenylJ(1,3-thiazol-2 yl))-S
methyltlZiotlziophene-2-carboxamidine tr~uoroacetate
52 mg (0.103 mmol) of 2-{3-[2-(5-{[(tent-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 48, step (c), was reacted with 1-acetyl piperazine (0.154 mmol, 20
mg), 1-hydroxy-
7-azabenzotriazole (HOAt)) (0.154 mmol, 21 mg), O-(7-azabenzotriazol-1-yl)-
1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.154 mmol, 58 mg) and
diisopropylethylamine (0.258 mmol, 44 ~L) in DMF to afford crude product which
was then
purified on 1 mm silica prep plates eluting with 3% methanol/methylene
chloride to afford 28
mg (53% yield) ofN-{[4-(4-{3-[2-(4-acetylpiperazinyl)-2-oxoethoxy]phenyl}(1,3-
thiazol-2-
yl))-5-methylthio(2-thienyl)]iminomethyl}(tert-butoxy)carboxamide. This was
subsequently
reacted with a solution of trifluoroacetic acid: methylene chloride: water
(47.5%: 47.5%:
2.5%) for 1 hour, concentrated and purified on a silica SPE column eluting
with 15%
methanol/methylene chloride to afford 20 mg (80% yield) of 4-(4-{3-[2-(4-
acetylpiperazinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-2-
carboxamidine trifluoroacetate. 'H-NMR (CD30D; 300 MHz) 8 8.48 (s, 1H), 7.91
(s, 1H),
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-129-
7:69 (m, 2H) 7.38 (t, 1H), 6.99 (dd, J-- 2 Hz and 8.1 Hz, 1H), 4.93 (s, 2H),
3.52-3.67 (m, 8H),
2.78 (s, 3H), 2.12 (s, 3H). Mass Spectrum (ESI, m/z) Calcd. for C23H25N503S3~
515.6 (M+H),
found 516.2:
Example 56
4-(4-(3-~2-(4-Metlzylpiperazinyl)-2-oxoethoxyJphenyl~(1,3-thiazol 2 yl))-5
methylthiothiophene-2-carboxamidine tr~uoroacetate
54 mg (0.107 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 48, step (c), was reacted with N-methyl piperazine (0.128 mmol, 14
pL), 1-hydroxy-
7-azabenzotriazole (HOAt) (0.128 mmol, 17 mg), O-(7-azabenzotriazol-1-yl)-
1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.128 mmol, 49 mg) and
diisopropylethylamine (0.268 mmol, 56 ~L) in DMF to afford crude product which
was then
partitioned between methylene chloride and 1N NaOH and washed. The organic
layer was
obtained and similarly washed with 10 % citric acid and saturated aq. sodium
chloride, dried
over sodium sulfate and concentrated to a yellow oil. The oil was then
purified on 1 mm
silica prep plates eluting with 5% methanol/methylene chloride to afford (tert-
butoxy)-N-
{ imino [4-(4-{ 3-[2-(4-methylpiperazinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-
2-yl))-5-
methylthio(2-thienyl)]methyl}carboxamide. This was subsequently reacted with a
solution of
trifluoroacetic acid: methylene chloride: water (47.5%: 47.5%: 2.5%) for 1
hour, concentrated
and purified on a silica SPE column eluting with 10-15% methanol/methylene
chloride to
afford 17 mg (33% yield) of 4-(4-{3-[2-(4-methylpiperazinyl)-2-
oxoethoxy]phenyl}(1,3-
thiazol-2-yl))-5-methylthiothiophene-2-carboxamidine trifluoroacetate. 'H-NMR
(CD30D;
300 MHz) 8 8.52 (s, 1H), 7.91 (s, 1H), 7.69 (m, 2H), 7.38 (t, 1H), 6.98 (dd, J-
- 2.0 Hz and 8.1
Hz, 1H), 4.90 (s, 2H), 3.66 (t, 4H), 2.78 (s, 3H), 2.49-2.57 (m, 4H), 2.35 (s,
3H). Mass
Spectrum (ESI, m/z) Calcd. for CZZH2sNsOzS3: 487.6 (M+H), found 488.2
Example 57
S-Methylthio-4-~4-(3-~2-oxo-2-~4-benzylpiperazinylJethoxy)pheizyl)(1,3-
tlziazol2-
yl)JtlZiophene-2-carboxamidine tr~uoroacetate
54 mg (0.107 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
CA 02362390 2001-08-07
WO 00/47578 _~ 3~_ PCT/US99/18065
Example 48, step (c), was reacted with N-benzylpiperazine (0.128 mmol, 22 ~L),
1-hydroxy-
7-azabenzotriazole (HOAt) (0.128 mmol, 17 mg), O-(7-azabenzotriazol-1-yl)-
1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.128 mmol, 48 mg) and
diisopropylethylamine (0.267 mmol, 50 ~L) in DMF to afford crude product which
was then
partitioned between methylene chloride and 1N NaOH and washed. The organic
layer was
obtained and similarly washed with 10% citric acid and saturated aq. sodium
chloride, dried
over sodium sulfate and concentrated to a yellow oil. The oil was then
purified on 1 mm
silica prep plates eluting with 5% methanol/methylene chloride to afford (tert-
butoxy)-N-
(imino { 5-methylthio-4-[4-(3-{ 2-oxo-2-[4-benzylpiperazinyl]ethoxy}phenyl)(
1,3-thiazol-2-
yl)](2-thienyl)}methyl)carboxamide. This was subsequently reacted with a
solution of
trifluoroacetic acid: methylene chloride: water (47.5%: 47.5%: 2.5%) for 1
hour, concentrated
and purified on a 5 g silica SPE column eluting with 10-15% methanol/methylene
chloride to
afford 36 mg (60% yield) of 5-methylthio-4-[4-(3-{2-oxo-2-[4-
benzylpiperazinyl]ethoxy}phenyl)( 1,3-thiazol-2-yl)]thiophene-2-carboxamidine
trifluoroacetate. 'H-NMR (CD30D; 300 MHz) 8 8.54 (s, 1H), 7.93 (s, 1H), 7.71
(m, 2H),
7.50 (s, 5H) 7.39 (t, 1H), 6.99 (dd, J-- 2 Hz and 8.1 Hz, 1H), 4.94 (s, 2H),
4.37 (s, 2H), 3.3
(m, 4H), 2.81 (s, 3H), 2.49-2.57 (m, 4H), 2.35 (s, 3H). Mass (ESI, m/z) Calcd.
for
CZ8HZ9NSOzS3: 563.7 (M+H), found 564.3.
Example 58
(D,L)- 4-(4-~3-~2-(3 Aminopyrrolidinyl)-2-oxoetltoxyJphertyl~(1,3-thiazol-2
yl))-S
methylthiothiophene-2-carboxamiditze tr~uoroacetate
41 mg (0.081 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 48, step (c), was reacted with (D,L) (tert-butoxy)-N-pyrrolidin-3-
ylcarboxamide
(0.122 mmol, 23 mg), O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium
hexafluorophosphate) HATU (0.122 mmol, 46 mg), 1-hydroxy-7-azabenzotriazole
(HOAt)
(0.122 mmol, 17 mg) and diisopropylethylamine (0.203 mmol, 35pL) in a manner
similar to
Example 56 to afford 20 mg (53% yield) of (D,L)- 4-(4-{3-[2-(3-
aminopyrrolidinyl)-2-
oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxamidine
trifluoroacetate. 'H-NMR (CD30D; 300 MHz) 8 8.54 (s, 1H), 7.94 (s, 1H), 7.71
(m, 2H),
7.39 (t, 1H), 6.99 (dd, J-- 2.0 Hz and 8.1 Hz, 1H), 4.85 (s, 2H), 4.37 (s,
2H), 3.60-4.01 (m,
CA 02362390 2001-08-07
WO 00/47578 _,13~ _ PCT/US99/18065
SI-I), 2.81 (s, 3H), 2.15-2.71 (m, 2H). Mass Spectrum (ESI, m/z) Calcd. for
Cz,Hz3N5O,S3:
473.6 (M+H), found 474.3.
Example 59
S Methyltlzio-4-~4-~3-(2-oxo-2 piperidylethoxy)phenylJ(1,3-thiazol 2
yl)Jtlaiophene-
2-carboxamidine trifluoroacetate
33 mg (0.065 mmol) of 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-
methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid, prepared in a
manner similar to
Example 40, step (c), was reacted with piperidine (0.078 mmol, 8 p.L), O-(7-
azabenzotriazol-
1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.078 mmol, 30
mg), 1-
hydroxy-7-azabenzotriazole (HOAt) (0.078 mmol, 11 mg) and
diisopropylethylamine (0.163
mmol, 56~.L) in a manner similar to Example 57 to afford 15 mg (41 % yield) of
5-
methylthio-4-{4-[3-(2-oxo-2-piperidylethoxy)phenyl] ( 1,3-thiazol-2-yl) }
thiophene-2-
carboxamidine trifluoroacetate. 'H-NMR (CD30D; 300 MHz) 8 8.54 (s, 1H), 7.92
(s, 1H),
7.69 (m, 2H), 7.35-7.40 (t, 1H), 6.98 (dd, J-- 2 Hz and 8.1 Hz, 1H), 4.95 (s,
2H), 3.52-3.60
(m, 4H), 2.80 (s, 3H), 1.57-1.70 (m, 6H). Mass Spectrum (ESI, m/z) Calcd. for
CzzHzaNaOzS3: 472.6 (M+H), found 473.2.
Example 60
2-(3-(2-(S-(Imino(~(4 polystyryloxyphenyl)methoxyJcarbonylaminof methyl)-2
methyltlZio-3-thienylJ 1,3-thiazol 4 ylfplZenoxy)acetic acid
2 g (1.86 mmol) ofp-nitrophenyl carbonate Wang resin (0.93 nunol/g)
(Calbiochem-
Novabiochem, San Diego, CA) was suspended in 9 mL of a 2:1 mixture of
anhydrous
DMSO:DMF. 2 g (4.93 mmol) of 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-
thiazol-4-
yl]phenoxy}acetic acid was added to suspension followed by the addition of 1
mL of 1,8-
diazabicyclo[5.4.0]undec-7-ene, (DBU, 6.69 mmol) and let shake vigorously for
5 days after
which resin was washed thoroughly with DMF, MeOH, and diethyl ether and dried
in vacuo
to afford 2 g of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-
thiazol-4-
yl]phenoxy}acetic acid.
CA 02362390 2001-08-07
VVO 00/47578 PCT/US99/18065
-132-
Example 61
(D,L)-Etlayl 1-(2-~3-~2-(S-amidino-2-methylthio-3-thienyl)-1,3-thiazol 4
ylJpheuoxyJacetyl)piperidine-2-carboxylate trifluoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was suspended 1 mL of anhydrous DMF. O-(7-azabenzotriazol-1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg), ethyl piperidine-2-carboxylate (0.5 M;
78 pL) and
diisopropylethylamine (0.233 mmol, 40 pL) were added and allowed to shake
vigorously for
18 hrs, after which the resin was washed thoroughly with DMF, methanol,
methylene
chloride, and diethyl ether. After drying, crude product was removed from
resin by reaction
with a solution of trifluoroacetic acid: methylene chloride: water (47.5%:
47.5%: 2.5%) for 1
hour. The solution was filtered and concentrated to a yellow oil. After
purification on a 2 g
silica SPE column, eluting with a gradient of 3%-10% MeOH/methylene chloride,
15 mg
(30% yield) of (D,L)-ethyl 1-(2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-
thiazol-4-
yl]phenoxy}acetyl)piperidine-2-carboxylate trifluoroacetate was obtained. Mass
Spectrum
(ESI, m/z) Calcd. for CZSHZ8N4O4S3: 544.70 (M+H), found 545.2
Example 62
S-Methylthio-4-(4-~3-(2-oxo-2 pyrrolidinylethoxy)phenylJ(1,3-tlziazol 2-
yl)Jthioplzene-2-carboxamidine trifluoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was suspended 1 mL of anhydrous DMF. O-(7-azabenzotriazol-1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg), pyrrolidine (0.5 M; 42 p.L) and
diisopropylethylamine (0.233 mmol, 40 ~L) were added and allowed to shake
vigorously for
18 hours, after which the resin was washed thoroughly with DMF, methanol,
methylene
chloride, and diethyl ether. After drying, crude product was removed from
resin by reaction
with a solution of trifluoroacetic acid: methylene chloride: water (47.5%:
47.5%: 2.5%) for 1
hour. After trituration with diethyl ether and drying, 18 mg (42% yield) of 5-
methylthio-4-
{ 4-[3-(2-oxo-2-pyrrolidinylethoxy)phenyl] ( 1,3-thiazol-2-yl) }thiophene-2-
carboxamidine
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-133-
trifluoroacetate was obtained. Mass Spectrum (ESI, m/z) Calcd. for
CZ~HZZN4OzS3: 458.6
(M+H), found 459.2
Example 63
S-Methylthio-4-~4-(3-~2-oxo-2 (4-benzylpiperidylJethoxyfphenyl)(1,3-thiazol-2
yl)JtlZiophene-2-carboxamidine tr~uoroacetate
80 mg (0.074 mmol) of resin-bound 2-{3-[2-(S-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was suspended in 1 mL of anhydrous DMF. O-(7-azabenzotriazol-1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg), 4-benzyl piperidine (0.5 M; 88 pL) and
diisopropylethylamine (0.185 mmol, 32 ~,L) were added and allowed to shake
vigorously for
18 hrs, after which the resin was washed thoroughly with DMF, methanol,
methylene
chloride, and diethyl ether. After drying, crude product was removed from
resin by reaction
with a solution of trifluoroacetic acid: methylene chloride: water (47.5%:
47.5%: 2.5%) for 1
hour. After trituration with diethyl ether and drying, 17 mg (40% yield) of 5-
methylthio-4-[4-
(3- {2-oxo-2-[4-benzylpiperidyl]ethoxy}phenyl)( 1,3-thiazol-2-yl)]thiophene-2-
carboxamidine
trifluoroacetate was obtained. Mass Spectrum (ESI, m/z) Calcd. for
Cz9H3oNaOzS3~ 562.7
(M+H), found 563.3.
Example 64
(D,L)-4-(4-~3-~2-(3-Metlzylpiperidyl)-2-oxoethoxyJphenylf (1,3-thiazol 2 yl))-
S
methylthiothiophene-2-carboxamidine trifluoroacetate
80 mg (0.074 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with (+/-)-3-methyl piperidine (0.5 M, 59 p,L) and O-(7-
azabenzotriazol-1-
yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-
hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg) and diisopropylethylamine (0.185 mmol,
32 ~,L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 10 mg (28%
yield) of
4-(4-{ 3-[2-(3-methylpiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for
C23H26N402"S3~ 486.6 (M+H), found 487.3.
CA 02362390 2001-08-07
WO 00/47578 _,~ 34_ PCT/US99/18065
Example 65
4-(4-~3-~2-(4 Methylpiperidyl)-2-oxoethoxyJphenylJ(1,3-thiazol-2 yl))-S
methylthiothioplaene-2-carboxamidine tr~uoroacetate
80 mg (0.074 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with 4-methyl piperidine (0.5 M, 59 ~L) and O-(7-
azabenzotriazol-1-yl)-
1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-
hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg) and diisopropylethylamine (0.185 mmol,
32 ~L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 12 mg (33%
yield) of
4-(4-{ 3-[2-(4-methylpiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-S-
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for
C23H26N402s3 ~ 486.6 (M+H), found 487.3.
Example 66
4-(4-~3 ~2-(2 Azabicyclo~4.4.OJdec-2 yl)-2-oxoethoxyJphenylJ(1,3-tlziazol 2
yl))-S
methylthiothiopJzene-2-carboxamidine tr~uoroacetate
80 mg (0.074 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with decahydroquinoline (0.5 M, 75 ~,L) and O-(7-
azabenzotriazol-1-yl)-
1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-
hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg) and diisopropylethylamine (0.185 mmol,
32 ~L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 16 mg (41 %
yield) of
4-(4-{ 3-[2-(2-azabicyclo [4.4.0]dec-2-yl)-2-oxoethoxy]phenyl} ( 1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for
C26H30N4~2'~3~ 526.7 (M+H), found 527.2.
Example 67
(D,L)-Ethyl 1-(2-~3-~2-(5-amidiuo-2-methylthio-3-thienyl)-1,3-thiazol-4
ylJphenoxyJacetyl)piperidine-3-carboxylate tr~uoroacetate
80 mg (0.074 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
CA 02362390 2001-08-07
WO 00/47578 _' 35_ PCT/US99/18065 -
60, was reacted with ethyl nipecotate (0.5 M, 78 ~L) and O-(7-azabenzotriazol-
1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-hydroxy-7-
azabenzotriazole (HOAt) (0.5 M. 68 mg) and diisopropylethylamine (0.185 mmol,
32 ~L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 18 mg (45%
yield) of
ethyl 1-(2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetyl)piperidine-3-carboxylate trifluoroacetate. Mass Spectrum
(ESI, m/z)
Calcd. for CZSHZ8N4O4S3: 545.7 (M+H), found 545.2.
Example 68
S-Methylthio-4-~4 ~3-(2-oxo-2-(1,2,3,4-tetralzydroguinolyl)ethoxy)pheuylJ(1,3-
thiazol 2 yl)Jthiopheue-2-carboxamidiue tr~uoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with 1,2,3,4-tetrahydroisoquinoline (0.5M) and O-(7-
azabenzotriazol-1-yl)-
1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-
hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg) and diisopropylethylamine (0.233 mmol,
40 ~L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 20 mg (42%
yield) of
5-methylthio-4-{4-[3-(2-oxo-2-( 1,2,3,4-tetrahydroquinolyl)ethoxy)phenyl] (
1,3-thiazol-2-
yl)}thiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for
C26H24N4~2'S3~ 520.7 (M+H), found 521.2.
Example 69
EtIZyI 1-(2-(3-~2-(5-amidino-2-metlzyltlzio-3-thienyl)-1,3-thiazol 4-
ylJphenoxyJacetyl)piperidine-4-carboxylate trifluoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with ethyl isonipecotate (0.5 M, 77 mg) and O-(7-
azabenzotriazol-1-yl)-
1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-
hydroxy-7-
azabenzotriazole (HOAt) (0.5 M; 68 mg) and diisopropylethylamine (0.233 mmol,
40 ~.L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 21 mg (42%
yield) of
ethyl 1-(2- { 3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-
CA 02362390 2001-08-07
WO 00/47578 _~ 36_ PCT/US99/18065
yl]phenoxy}acetyl)piperidine-4-carboxylate trifluoroacetate. Mass Spectrum
(ESI, m/z)
Calcd. for Cz5H28N4O4S3: 545.7 (M+H), found 545.3.
Example 70
4-(4-~3-~2-((3R)-3-Hydroxypiperidyl)-2-oxoethoxyJphenylf (1,3-thiazol Z yl))-S
metlZylthiothiopheue-2-carboxamidiue trifluoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with R-(+)-3-hydroxy piperidine (0.5 M, 69 mg) and O-(7-
azabenzotriazol-1-
yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-
hydroxy-7-
azabenzotriazole (HOAt) (O.SM; 68 mg) and diisopropylethylamine (0.233 mmol,
40 p.L) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 16 mg (36%
yield) of
4-(4-{ 3-[2-((3R)-3-hydroxypiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for
C22H23N4~3S3~ 489.7 (M+H), found 489.2.
Example 71
D,L-4-(4-~3-~2-(2-Ethylpiperidyl)-2-oxoethoxyJphenylJ(1,3-thiazol 2 yl))-5-
methylthiothiophene-2-carboxamidine tr~uoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with 2-ethyl piperidine (O.SM) and O-(7-azabenzotriazol-1-yl)-
1,1,3,3-
tetramethyluronium hexafluorophosphate) HATU (0.5 M, 190 mg), 1-hydroxy-7-
azabenzotriazole (HOAt) (O.SM; 68 mg) and diisopropylethylamine (0.233 mmol,
40 pL) in
1 mL of anhydrous DMF in a manner similar to Example 63 to afford 11 mg (23%
yield) of
D,L-4-(4-{ 3-[2-(2-ethylpiperidyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for
C24HZ,N402S3: 501.4 (M+H), found 501.4.
CA 02362390 2001-08-07
WO 00/47578 _,~ 37_ PCT/US99/18065
Example 72
4-(4-(3 ~2-((3S)-3 Hydroxypyrrolidinyl)-2-oxoethoxyJphenyl~(1,3-thiazol 2 yl))-
S
methylthiothiophene-2-carboxamidine trifluoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-
thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a
manner
similar to Example 60, was reacted with R-(-)-3-pyrrolidinol (0.5 M, 62 mg)
and O-
(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate)
(HATU,
0.5 M, 190 mg), 1-hydroxy-7-azabenzotriazole (HOAt) (O.SM; 68 mg) and
diisopropylethylamine (0.233 mmol, 40 pL) in 1 mL of anhydrous DMF in a manner
similar to Example 63 to afford 10 mg (23% yield) of 4-(4-{3-[2-((3S)-3-
hydroxypyrrolidinyl)-2-oxoethoxy]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-
2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z) Calcd. for
CZ,HzZN4O3S3: 475.2 (M+H), found 475.2.
Example 73
S-Methylthio-4-(4-~3-~(N (5,6,7,8
tetrahydronaphtlayl)carbamoyl)methoxyJphenylJ(1,3-thiazol-2 yl))thiophene-2
carboxamidine trifluoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with 5,6,7,8-tetrahydro-1-naphthylamine (0.5 M, 73 mg) and O-
(7-
azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) (HATU,
0.5 M, 190
mg), 1-hydroxy-7-azabenzotriazole (HOAt) (0.5 M; 68 mg) and
diisopropylethylamine (0.233
mmol, 40 pL) in 1 mL of anhydrous DMF in a manner similar to Example 63 to
afford 15 mg
(30% yield) of 5-methylthio-4-(4-{3-[(N-(5,6,7,8-
tetrahydronaphthyl)carbamoyl)methoxy]phenyl } ( 1,3-thiazol-2-yl))thiophene-2-
carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z) Calcd. for
CZ,Hz6N4OzS3: 535.2
(M+I-I), found 535.3.
CA 02362390 2001-08-07
WO 00/47578 _,~ 38_ PCT/US99/18065
Example 74
D, L-4 ~4-(3-~2 ~3-(Hydroxymetlzyl)piperidylJ 2-oxoethoxy)phenyl)(1,3-thiazol
2 yl)J S
methylthiotlZiophene-2-carboxamidine tr~uoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-
thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a
manner
similar to Example 60, was reacted with 3-piperidine methanol (0.5 M, 58 mg)
and
O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate)
(HATU, 0.5 M, 190 mg), 1-hydroxy-7-azabenzotriazole (HOAt) (O.SM; 68 mg) and
diisopropylethylamine (0.233 mmol, 40 ~L in 1 mL of anhydrous DMF in a manner
similar to Example 40 to afford to 19 mg (40% yield) of D,L-4-[4-(3-{2-[3-
(hydroxymethyl)piperidyl]-2-oxoethoxy } phenyl)( 1,3-thiazol-2-yl)]-5-
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass Spectrum (ESI, m/z)
Calcd. for C23HZSN4O3S3: 503.2 (M+H), found 503.2.
Example 75
4-~4-~3-(2-((2R)-2-~(Phenylamino)methylJpyrrolidinyl~-Z-oxoethoxy)phenylJ(1,3
thiazol 2 yl)~-S-methylthiothiophene-2-carboxamidine tr~uoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with (S)-(+)-2-anilino methyl pyrrolidine (0.5 M, 88 mg) and O-
(7-
azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) (HATU,
0.5 M, 190
mg), 1-hydroxy-7-azabenzotriazole (HOAt) (0.5M; 68 mg) and
diisopropylethylamine (0.233
mmol, 40 pL) in 1 mL of anhydrous DMF in a manner similar to Example 63 to
afford 13 mg
(25% yield) of 4-{4-[3-(2-{(2R)-2-[(phenylamino)methyl]pyrrolidinyl}-2-
oxoethoxy)phenyl] ( 1,3-thiazol-2-yl) }-5-methylthiothiophene-2-carboxamidine
trifluoroacetate. Mass Spectrum (ESI, m/z) Calcd. for CZgH28N502S3: 563.8
(M+H), found
564.2.
CA 02362390 2001-08-07
WO 00/47578 _~ 39_ PCT/US99/18065
Example 76
4 ~4-(3-(2-J(3R)-3-(Methoxymethyl)pyrrolidinylJ Z-oxoethoxyJphenyl)(1,3-
thiazol
2 yl)J-S-methylthiotlziopheue-Z-carboxamidine tr~uoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with (S)-(+)-2-methoxymethyl pyrrolidine (0.5 M, 58 mg) and O-
(7-
azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) (HATU,
0.5 M, 190
mg), 1-hydroxy-7-azabenzotriazole (HOAt) (O.SM; 68 mg) and
diisopropylethylamine (0.233
mmol, 40 ~L) in 1 mL of anhydrous DMF in a manner similar to Example 63 to
afford 16
mg (35% yield) of 4-[4-(3-{2-[(3R)-3-(methoxymethyl)pyrrolidinyl]-2-
oxoethoxy} phenyl)( 1,3-thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine
trifluoroacetate. Mass Spectrum (ESI, m/z) Calcd. for C23HZ6N4O3S3: 503.2
(M+H), found
503.3.
Example 77
1-(2-~3-~2-(5 Amidino-2-methylthio-3-tlzienyl)-1,3-thiazol-4-
ylJphenoxyJacetyl)piperidine-3-carboxamide tr~uoroacetate
100 mg (0.093 mmol) of resin-bound 2-{3-[2-(5-amidino-2-methylthio-3-thienyl)-
1,3-
thiazol-4-yl]phenoxy}acetic acid (0.93 mmol/g), as prepared in a manner
similar to Example
60, was reacted with nipecotamide (0.5 M, 64 mg) and O-(7-azabenzotriazol-1-
yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate) (HATU, 0.5 M, 190 mg), 1-hydroxy-7-
azabenzotriazole (HOAt) (O.SM; 68 mg) and diisopropylethylamine (0.233 mmol,
40 ~,L) in 1
mL of anhydrous DMF in a manner similar to Example 63 to afford 11 mg (23%
yield) 1-(2-
{ 3-[2-(5-amidino-2-methylthio-3-thienyl)-1,3-thiazol-4-yl]phenoxy }
acetyl)piperidine-3-
carboxamide trifluoroacetate. Mass Spectrum (ESI, m/z) Calcd. for
C23H25N403S3~ 516.2
(M+H), found 516.3.
Example 78
S-Metlzylthio-4-(4-~3-(trifluorometl:oxy)phenylJ(1,3-tlaiazol-2 yl)ftlziophene-
2-
carboxamidine hydrochloride
a) Methyl S-methyltlaio-4-~4-~3-(tr~uoromethoxy)phenylJ(1,3-thiazol 2-
yl)~thioplzene-2-carboxylate: 435 mg (1.76 mmol) of methyl 4-
(aminothioxomethyl)-5-
CA 02362390 2001-08-07
w0 00/47578 _~4~_ PCT/US99/18065
methylthiothiophene-2-carboxylate was dissolved in 10 mL of reagent grade
acetone. 2-
bromo-3'-trifluoromethoxy acetophenone, prepared in a manner similar to
Example 95, step
(a), (1.76 mmol; 497 mg) was added and the solution was allowed to reflux for
3 h. The
solution was allowed to cool and concentrated to an oil which was then
dissolved in 150 mL
S of methylene chloride and washed with 50 mL of 10% HCl (aq.) and SO mL of 2N
NaOH
(aq.). The organic layer was obtained and dried over magnesium sulfate and
concentrated
affording 877 mg (90% yield) of a methyl- 5-methylthio-4-{4-[3-
(trifluoromethoxy)phenyl] ( 1, 3-thiazol-2-yl) } thiophene-2-carboxylate.
b) S-Metlzyltlzio-4-~4-~3-(triJluoromethoxy)phenylJ(1,3-thiazol 2
yl)Jthiophene-2-
carboxamidine hydrochloride: To a stirred suspension of 19.4 mmol ( 1.04 g) of
ammonium
chloride (Fisher Scientific) in 20 mL of anhydrous toluene (Aldrich Chemical
Co.) placed
under nitrogen atmosphere at 0 °C, 9.7 mL ( 19.4 mmol) of 2M
trimethylaluminum in toluene
(Aldrich Chemical Co.) was added via syringe over 15 min and then let stir at
0 °C for 30 min
after which 837 mg (1.94 mmol) of methyl- 5-methylthio-4-{4-[3-
(trifluoromethoxy)phenyl](1,3-thiazol-2-yl)}thiophene-2-carboxylate was added
to solution
and allowed to reflux for 3 h. The reaction mixture was quenched by pouring
over a slurry of
10 g of silica in 50 mL of chloroform. The silica was poured onto a sintered
glass funnel and
washed with ethyl acetate and eluting with a 15% methanol/CHzCh solution and
concentrated. The crude product was purified on 1 mm silica prep plates
eluting with 15%
methanol/CHZCl2 and treated with 4N HCl/dioxane to afford 37 mg (5% yield) of
5-
methylthio-4-{4-[3-(trifluoromethoxy)phenyl]( 1,3-thiazol-2-yl) } thiophene-2-
carboxamidine
hydrochloride. 'H-NMR (DMSO-db; 300 MHz) 8 9.43 ( bs, 1.9 H), 9.05 (bs, 1.9
H), 8.67 (s,
1 H), 8.43 (s, 1 H), 8.10 (m, 2H), 7.65 (t, 1 H), 7.40 (m, 1 H), 2.8 (s, 3H).
Mass Spectrum
(LCQ-ESI, m/z) Calcd. for C,6H,ZF3N3OS3: 415.5(M+H), found 416.2.
Example 79
S-Methyltlzio-4-(5 phenyl(1,3-oxazol 2 yl))thiophene-2-carboxamidine
hydrochloride
a) Methyl S-methylthio-4 ~N (2-oxo-2 pheuyletlryl)carbamoylJtl:iophene-2-
carboxylate: To a stirred suspension of 300 mg (1.29 mmol) of 5-
(methoxycarbonyl)-
2-methylthiothiophene-3-carboxylic acid (as prepared in Example 95) in 10 mL
of
anhyd CHZCIz (under a CaS04 drying tube) was added 135 mL (I .55 mmol) of
oxalyl
CA 02362390 2001-08-07
WO 00/47578 _t 4~ _ PCT/US99/18065
chloride followed by 30 mL of anhyd DMF. After stirring for 2 h at room
temperature, the mixture was concentrated in vacuo. The resulting yellow solid
was
dissolved in 10 mL of anhyd CHZCIz, cooled (0°C) and 266 mg (1.55 mmol)
of 2-
aminoacetophenone was added. N,N diisopropylethylamine (DIEA) (756 mL, 4.34
mmol) was added dropwise over 3 min and the mixture stirred for 1 h at room
temperature. The mixture was concentrated to an oil and partitioned between
125 mL
of EtOAc and 80 mL of 1 M HCI. The aqueous layer was extracted with ethyl
acetate
(2 x 30 mL) and the combined organic phases were washed with 1 M HCl (60 mL),
saturated NaHC03 (120 mL), and brine (120 mL) and dried over NazS04. After
removing the solvent in vacuo, the residue was recrystallized from MeOH to
afford
the title compound as a cream-colored powder (314 mg, 70%). 'H-NMR (300 MHz,
DMSO-db) 8 8.82 (t, 1 H, J--6 Hz), 8:43 (s, 1 H), 8.02 (d, 2H, J 7 Hz), 7.69
(t, 1 H, J--7
Hz), 7.57 (t, 2H, J--7 Hz), 4.72 (d, 2H, J--6 Hz), 3.84 (s, 3H) and 2.57 (s,
3H). Mass
spectrum (MALDI-TOF, a-cyano-4-hydroxycinnamic acid matrix) calcd. for
C,6H,SN04S2: 372.0 (M + Na). Found: 372.1.
b) Methyl S-metlzylthio-4-(5 phezzyl(1,3-oxazol 2 yl))thiophene-2-
carboxylate: To a cooled (0 °C) solution of 80.1 mg (0.229 mmol) of
methyl S-
methylthio-4-[N-(2-oxo-2-phenylethyl)carbamoyl]thiophene-2-carboxylate (as
prepared in the previous step) in 2 mL of anhyd DMF was added 26.7 mL (0.286
mmol) of phosphorus oxychloride. After stirring for 20 h at room temperature,
the
mixture was concentrated in vacuo. The resulting yellow solid was
recrystallized
twice from MeOH to afford the title compound as a beige powder (48.8 mg, 64
%).
'H-NMR (300 MHz, DMSO-db) ~ 8.26 (s, 1H), 7.88 (s, 1H), 7.86 (d, 2H, J--7 Hz),
7.51 (m, 2H), 7.40 (m, 1H), 3.86 (s, 3H), and 2.79 (s, 3H). Mass spectrum
(MALDI-
TOF, a-cyano-4-hydroxycinnamic acid matrix) calcd. for C,6H,3NO3S2: 332.0 (M +
H). Found: 331.9.
c) S-Metlzylthio-4-(S phenyl(1,3-oxazol 2 yl))tlziophene-2-carboxamidine
hydrochloride: Methyl5-methylthio-4-(5-phenyl(1,3-oxazol-2-yl))thiophene-2-
carboxylate (37.0 mg, 0.112 mmol, as prepared in the previous step) was
treated
according to the procedure in Example 10, step (b) using 59.9 mg (1.12 mmol)
of
ammonium chloride in 0.50 mL of toluene and 0.560 mL (1.12 mmol) of 2 M
trimethylaluminum in toluene. The resulting residue was chromatographed on a 5
g
CA 02362390 2001-08-07
WO 00/47578 _,~~2_ PCT/US99/18065
silica SPE column (Waters Sep-Pak) with 10% MeOH-CHZC12 to elute an impurity
followed by 20% MeOH-CHZCl2 to give 39 mg of a light yellow glass.
Crystallization from MeOH-MeCN afforded the title compound as a cream-colored
solid (33.4 mg, 85 %). 'H-NMR (300 MHz, DMSO-db) 8 9.45 (broad s, 2H), 9.13
(broad s, 2H), 8.72 (s, 1 H), 7.93 (s, 1 H), 7.84 (d, 2H, J--7 Hz), 7.53 (t,
2H, J 7 Hz),
7.42 (t, 1H, J--7 Hz), and 2.80 (s, 3H). Mass spectrum (MALDI-TOF, a-cyano-4-
hydroxycinnamic acid matrix) calcd. for C,SH,3N30Sz: 316.1 (M + H). Found:
316.5.
Examples 80 and 81
S-Methylthio-4-(4 phenylimidazol-2 yl)thiophene-2-carboxamidine
trifluoroacetate
and S-Metl:ylthio-4-~N (2-oxo-2 plzenylethyl)carbamoylJthiophene-2
carboxamidine trifluoroacetate
Methyl5-methylthio-4-[N-(2-oxo-2-phenylethyl)carbamoyl]thiophene-2-
carboxylate (39.4 mg, 0.100 mmol, as prepared in Example 79, step (a)) was
treated
according to the procedure in Example 10, step (b) using 64.2 mg (1.20 mmol)
of
ammonium chloride in 0.2 mL of toluene and 0.600 mL (1.20 mmol) of 2 M
trimethylaluminum in toluene. The resulting residue was chromatographed on a 5-
g
silica SPE column (Waters Sep-Pak) with a gradient of 5-20% MeOH-CHZCIz to
elute
an impurity followed by 20% MeOH-CHZC12 to give a yellow resin.
Crystallization
from MeOH-Et20-MeCN afforded 16 mg of a yellow solid consisting of two
products
by'H-NMR spectra. A portion of the mixture (11 mg) was purified by reverse-
phase
HPLC (Sm Cg column, 4.6 x 100 mm, gradient S-100% solvent B over 15 min,
solvent A = 0.1 % TFA/HZO, solvent B = 0.1 %TFA/MeCN, detection at 215 nm) to
afford 6 mg of 5-methylthio-4-(4-phenylimidazol-2-yl)thiophene-2-carboxamidine
trifluoroacetate as a colorless glass. 'H-NMR (300 MHz, CD30D) b 8.23 (s, 1H),
7.80
(s, 1H), 7.79 (d, 2H, J 7 Hz), 7.48 (m, 2H), 7.39 (m, 1H), and 2.78 (s, 3H).
Mass
spectrum (electrospray ionization) calcd. for C,SH,4N4S2: 315.1 (M + H).
Found:
315.3. Also isolated was 4 mg of 5-methylthio-4-[N-(2-oxo-2-
phenylethyl)carbamoyl]-thiophene-2-carboxamidine trifluoroacetate as a
colorless
glass. 'H-NMR (300 MHz, DMSO-db) 8 9.30 (broad s, 2H), 8.86 (broad s, 2H),
8.68
(t, 1 H, J--5.4 Hz), 8.43 (s, 1 H), 8.04 (d, 2H, .7 7 Hz), 7.70 (t, 1 H, J--7
Hz), 7.5 8 (t, 2H,
CA 02362390 2001-08-07
WO 00/47578 _' 43_ PCT/US99/18065
J 7 Hz), 4.78 (d, 2H, J--5.4 Hz), and 2.63 (s, 3H). Mass spectrum
(electrospray
ionization) calcd. for C,SH,SN3OZSz: 334.1 (M + H). Found: 334.3.
Example 82
4-(4-Phenyl-1,3-tl:iazol 2 yl)thiophene-2-carboxamidine hydrochloride
a) 4-Bromothiophene-2-carboxylic acid: To a cooled (0 °C) solution of
10.0
g (47.1 mmol based on 90 % purity) of 4-bromothiophene-2-carbaldehyde (Aldrich
Chemical Company, Milwaukee; WI) in 200 mL of t-butanol was added 100 mL of 20
% (w/v) NaH2P04 followed by 60 mL (0.566 mol) of 2-methyl-2-butene. Sodium
chlorite (70.8 mmol based on 80 % purity) in 60 mL of water was added with
stirring.
After stirring the two-phase mixture vigorously for 16 h at room temperature,
the pH
of the aqueous layer was adjusted to 1-2 with 20 % HCI. The layers were
separated
and the aqueous layer extracted with EtOAc (2 x 120 mL). The combined organic
layers were dried (Na2S04) and concentrated in vacuo to afford 9.8 g of an off
white
solid. Recrystallization from a minimum of MeCN (three crops) gave the title
compound as a white solid (9.02 g, 93%). 'H-NMR (300 MHz, CDC13) 8 7.79 (d,
1H,
J--1.5 Hz), and 7.55 (d, 1 H, J--1.5 Hz).
b) MetIZyl4-bromotlziophene-2-carboxylate: To a cooled (-20°C) solution
of
6.02 g (29.1 mmol) of 4-bromothiophene-2-carboxylic acid (as prepared in the
previous step) in 100 mL of anhyd MeOH under nitrogen was added 2.55 mL (34.9
mmol) of thionyl chloride dropwise at a rate to keep the temperature below -
5°C (ca.
8-10 min). After stirring for 1 h at room temperature, the mixture was
refluxed for 8
h, cooled, and concentrated in vacuo. The resulting 6.7 g of pale amber oil
was
passed through a 150 g pad of silica gel with ca. 600 mL of CHzCl2 (discarding
the
first 120 mL which contained a minor impurity) to afford, after concentration
in
vacuo, the title compound as a colorless oil (6.11 g, 95 %). 'H-NMR (300 MHz,
CDCl3) 8 7.69 (d, 1H, J--1.5 Hz), 7.45 (d, 1H, J--1.5 Hz), and 3.90 (s, 3H).
c) MetlZyl4-cyanothiophene-Z-carboxylate: To a solution of 3.82 g (17.3
mmol) methyl 4-bromothiophene-2-carboxylate (as prepared in the previous step)
in
10 mL of anhyd DMF was added 3.10 g (34.6 mmol) of copper (I) cyanide. The
mixture was heated to reflux with stirring for 18 h, cooled and poured into
100 mL of
10 % (w/v) KCN. The mixture was extracted with EtOAc (3 x 60 mL) and the
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-144
combined extracts were washed with 150 mL each of water and brine. The dark
solution was dried over Na2S04, treated with decolorizing carbon, filtered and
the
resulting colorless solution concentrated in vacuo. The resulting light yellow
solid
was recrystallized from MeOH to afford the title compound as a cream-colored
solid
(1.67 g, 58 %). 'H-NMR (300 MHz, CDCl3) 8 8.09 (d, 1H, J--1.4 Hz), 7.93 (d,
1H,
J--1.4 Hz), and 3.93 (s, 3H). IR (film): 2235 and 1712 cm'.
d) Methyl4-(aminothioxomethyl)thiophene-2-carboxylate: A solution of
1.32 g (7.89 mmol) of methyl 4-cyanothiophene-2-carboxylate (as prepared in
the
previous step) in 200 mL of reagent grade MeOH was degassed with nitrogen
through
a fritted gas dispersion tube for 10 min. Triethylamine (5.50 mL, 39.5 mmol)
was
added and hydrogen sulfide gas was bubbled into the solution at a vigorous
rate for 5
min and then at a minimal rate (as measured through an outlet oil bubbler) for
5 h
with stirring. The gas introduction was stopped and the mixture was capped and
stirred for 19 h at room temperature. The mixture was concentrated in vacuo to
a
yellow solid which was suspended in 10 mL of EtOH, cooled to -20 °C,
and filtered
washing with 5 mL of cold (-20 °C) EtOH. The resulting solid was dried
under
suction followed by high vacuum to afford the title compound as a beige solid
( 1.31 g,
82 %). 'H-NMR (300 MHz, DMSO-db) 8 9.85 (broad s, 1H), 9.51 (broad s, 1H),
8.50
(d, 1H, J--1.5 Hz), 8.28 (d, 1H, J--1.5 Hz), and 3.84 (s, 3H).
e) Methyl4 (4 phenyl 1,3-thiazol 2 yl)thiophene-2-carboxylate: To a
solution of 150 mg (0.745 mmol) of methyl 4-(aminothioxomethyl)-thiophene-2-
carboxylate (as prepared in the previous step) in 6 mL of acetone was added
148 mg
(0.745 mmol) of 2-bromoacetophenone. After refluxing for 2 h, the mixture was
concentrated by boiling to a volume of ca. 2 mL. The resulting mixture was
cooled (-
10 °C) and filtered washing with cold acetone (2 x 0.5 mL). A second
crop was
obtained from the mother liquors and the combined crops dried to afford the
title
compound as a beige solid (202 mg, 90 %). 'H-NMR (300 MHz, DMSO-db) 8 8.56
(d, 1 H, J--1.5 Hz), 8.25 (d, 1 H, J--1.5 Hz), 8.18 (s, 1 H), 8.04 (d, 2H, J--
7 Hz), 7.48 (t,
2H, J--7 Hz), 7.38 (t, 1H, J--7 Hz), and 3.89 (s, 3H). Mass spectrum (MALDI-
TOF, a-
cyano-4-hydroxycinnamic acid matrix) calcd. for C,SH"NOZS2: 302.0 (M + H).
Found: 301.8.
CA 02362390 2001-08-07
WO 00/47578 _,) ~5_ PCT/US99/18065
~ 4-(4-Phenyl 1,3-thiazol-2 yl)thiophene-2-carboxamidine hydrochloride:
Methyl 4-(4-phenyl-1,3-thiazol-2-yl)thiophene-2-carboxylate (160 mg, 0.531
mmol,
as prepared in the previous step) was treated according to the procedure in
Example
10, step (b) using 284 mg (5.31 mmol) of ammonium chloride in 2.6 mL of
toluene
and 2.65 mL (5.30 mmol) of 2 M trimethylaluminum in toluene. The resulting
light
yellow solid was chromatographed on a 10 g silica SPE column (Waters Sep-Pak)
with a gradient of 5-20% MeOH-CHZC12. The resulting pale amber glass was
triturated with CHZC12-MeCN and concentrated in vacuo to afford the title
compound
as a beige solid (68 mg, 45 %). 'H-NMR (300 MHz, DMSO-db) 8 9.51 (broad s,
2H),
9.09 (broad s, 2H), 8.71 (d, 1H, J--1.5 Hz), 8.61 (d, 1H, J--1.5 Hz), 8.21 (s,
1H), 8.05
(d, 2H, J--7 Hz), 7.50 (t, 2H, J--7 Hz), and 7.40 (t, 1H, J--7 Hz). Mass
spectrum
(MALDI-TOF, a-cyano-4-hydroxycinnamic acid matrix) calcd. for C,4H"N3Sz: 286.0
(M + H). Found: 286.3.
Example 83
5-Methylthio-4-~4-benzyl(1,3-thiazol-2 yl)Jtl:iophene-2-carboxamidine
Izydrocl:loride
a) Bromo-3 plzenylacetone: To a solution of 132 mL (1.00 mmol) of
phenylacetyl chloride in 1.0 mL of anhyd MeCN was added 1.05 mL (2.10 mmol) of
a
2 M solution of trimethylsilyldiazomethane in hexane. After stirring 1 h at
room
temperature, the mixture was cooled (0°C) and 300 mL (1.50 mmol) of 30
wt % HBr
in acetic acid was added dropwise (gas evolution). After stirring 15 min, the
mixture
was concentrated in vacuo and rapidly chromatographed on a 2 g silica SPE
column
(Waters Sep-Pak) with 50 % CHzCl2-hexane to afford the title compound as a
pale
yellow oil (201 mg, 94 %). 'H-NMR (300 MHz, CDC13) 8 7.2-7.4 (m, SH), 3.95 (s,
2H), 3.92 (s, 2H).
b) Methyl S-methylthio-4-~4-benzyl(1,3-thiazol-2 yl)Jtl:iophene-2-
carboxylate: Using a procedure similar to that of Example 10 with 171 mg
(0.690
mmol) of methyl 4-(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (as
prepared in Example 82, step (e)) in 4 mL of acetone and 147 mg (0.690 mmol)
of 1-
bromo-3-phenylacetone (as prepared in the previous step) afforded the title
compound
as a light tan powder (236 mg, 95 %). ~H-NMR (300 MHz, DMSO -d6) 8 8.11 (s,
CA 02362390 2001-08-07
WO 00/47578 -146- PCT/US99/18065
1H), 7.2-7.4 (m, SH), 4.11 (s, 2H), 3.84 (s, 3H), and 2.72 (s, 3H). Mass
spectrum
(MALDI-TOF, a-cyano-4-hydroxycinnamic acid matrix) calcd. for C,~H,SNOZS3:
362.0 (M + H). Found: 362.3.
c) S-Methyltlaio-4-~4-benzyl(1,3-thiazol 2 yl)Jthiophene-2-carboxamidine
hydroclzloride: Methyl5-methylthio-4-[4-benzyl(1,3-thiazol-2-yl)]thiophene-2-
carboxylate (60 mg, 0.166 mmol, as prepared in the previous step) was treated
according to the procedure in Example 10, step (b) using 88.8 mg (1.66 mmol)
of
ammonium chloride in 0.5 mL of toluene and 0.830 mL (5.30 mmol) of 2 M
trimethylaluminum in toluene to afford, after trituration from MeOH with Et20,
the
title compound as a yellow solid (38.2 mg, 60 %). 1H-NMR (300 MHz, CD30D)
8 8.43 (s, 1H), 7.16-7.33 (m, SH), 4.15 (s, 2H), and 2.75 (s, 3H). Mass
spectrum
(MALDI-TOF, a-cyano-4-hydroxycinnamic acid matrix) calcd. for C~6H15N3S3:
346.0
(M + H). Found: 346Ø
Example 84
S-Methyltlaio-4-(4 phenyl(1,3-oxazol-2 yl))thiophene-2-carboxamidine
I:ydroclZloride
a) Methyl 4 ~N (2-hydroxy-1 phenylethyl)carbamoylJ 5-
methylthiothiophene-2-carboxylate: To a stirred suspension of 1.23 g (5.29
mmol)
of 5-(methoxycarbonyl)-2-methylthiothiophene-3-carboxylic acid (as prepared in
Example 79, step (a)) in 20 mL of anhyd CHzCl2 (under a CaS04 drying tube) was
added 1.85 mL (21.2 mmol) of oxalyl chloride followed by 30 mL of anhyd DMF.
After stirring for 2 h at room temperature, the mixture was concentrated in
vacuo.
The resulting yellow solid was dissolved in 20 mL of anhyd CHzCl2, cooled
(0°C) and
1.85 mL of N,N diisopropylethylamine (10.6 mmol) and 1.02 g (7.41 mmol) of
phenylglycinol was added and the mixture stirred for 1 h at room temperature.
The
mixture was concentrated to an oil and partitioned between 200 mL of EtOAc and
200
mL of saturated NaHC03. The organic phase was washed with saturated NaHC03
. (200 mL), 10 % (w/v) citric acid, and brine (200 mL), and dried over Na2S04.
After
removing the solvent in vacuo, the residue was chromatographed on a 10 g
silica SPE
column (Waters Sep-Pak) with a gradient of 0-20 % EtOAc-CHZCIz to afford the
title
compound as a light yellow solid (1.26 g, 68 %). ~H-NMR (300 MHz,-CDCl3) S
8.00
CA 02362390 2001-08-07
WO 00/47578 _~47_ PCT/US99/18065
(s, 1 H), 7.30-7.42 (m, SH), 7.08 (d, 1 H, J--7.2 Hz), 5.26 (m, 1 H), 3.99 (t,
2H, J--5.4
Hz), 3.89 (s, 3H), 2.60 (s, 3H), and 2.33 (t, 1H, J 6.1 Hz). Mass spectrum
(electrospray ionization) calcd. for C~6H,~N04S2: 352.1 (M + H). Found: 352Ø
b) Methyl 5-methylthio-4-~N (2-oxo-1 phenylethyl)carbamoylJthiophene-2-
S carboxylate: To a solution of 505 mg (1.44 mmol) methyl 4-[N-(2-hydroxy-1-
phenylethyl)carbamoyl]-5-methylthiothiophene-2-carboxylate (as prepared in the
previous step) in 20 mL of anhydrous CHzCl2 was added 856 mg (2.02 mmol) of
Dess
Martin reagent (Omega Chemical Company, Inc., Levis (Qc) Canada). After
stirring
in an open flask for 1.5 h at room temperature, the mixture was concentrated
in vacuo.
to ca. 10 % volume and partitioned between 50 mL of EtOAc and 50 mL of
saturated
NaHC03-brine (1:1). The organic phase were washed with brine (200 mL), dried
over
NazS04 and concentrated in vacuo. Concentrated again from CHZC12 followed by
high vacuum afforded the title compound as a light yellow foam (495 mg, 98 %)
which was used in the next step without further purification. 'H-NMR (300 MHz,
CDC13) 8 9.64 (s, 1H), 8.04 (s, 1H), 7.59 (d, 1H, J--5 Hz), 7.36-7.46 (m, SH),
5.76 (d,
1H, J--5 Hz), 3.90 (s, 3H), and 2.62 (s, 3H).
c) Methyl S-methylthio-4-(4 phenyl(1,3-oxazol-2 yl))thiophene-2-
carboxylate: To a cooled (0°C) solution of 465 mg (1.33 mmol) methyl 5-
methylthio-4-[N-(2-oxo-1-phenylethyl)carbamoyl]thiophene-2-carboxylate (as
prepared in the previous step) in 6 mL of anhyd DMF was added 186 mL (2.00
mmol)
of phosphorus oxychloride. After stirring for 14 h at room temperature, the
mixture
was treated with 10 mL of saturated NaHC03 and concentrated to dryness under
high
vacuum. The resulting residue was partitioned between 80 mL of EtOAc and 60 mL
of water. The aqueous layer was extracted with EtOAc (2 x 10 mL) and the
combined
organic phases washed with brine (60 mL), and dried over Na2S04. The resulting
406
mg of amber-colored solid was recrystallized from CHzCl2-EtzO to remove the
majority of a polar impurity as a cream-colored solid. The remaining mother
liquors
were chromatographed on a 10 g silica SPE column (Waters Sep-Pak) with a
gradient
of 40-100 % CHZC12-hexane and the resulting residue triturated with EtzO-
hexane
(2:1)) to afford the title compound as a light beige solid (114 mg, 26 %). 'H-
NMR
(300 MHz, CDC13) 8 8.24 (s, 1H), 7.93 (s, 1H), 7.83 (m, 2H), 7.43 (m, 2H),
7.33 (m,
CA 02362390 2001-08-07
WO 00/47578 _~4$~ PCT/US99/18065
1H), 3.91 (s, 3H), and 2.72 (s, 3H). Mass spectrum (ESI) calcd. for
C,6H,3NO3Sz:
332.0 (M + H). Found: 332.2.
d) S-Metlzyltlzio-4-(4 phenyl(1,3-oxazol 2 yl))tltiophene-2-carboxamidine
hydrochloride: Methyl5-methylthio-4-(4-phenyl(1,3-oxazol-2-yl))thiophene-2-
S carboxylate (80.3 mg, 0.242 mmol, as prepared in the previous step) was
treated
according to the procedure in Example 10, step (b) using 155 mg (2.90 mmol) of
ammonium chloride in 1.45 mL of toluene and 1.45 mL (2.90 mmol) of 2 M
trimethylaluminum in toluene. The resulting light yellow solid was
chromatographed
on a 5 g silica SPE column (Waters Sep-Pak) with 10% MeOH-CHZCIz to give a
light
yellow resin. Crystallization from MeOH- EtzO (ca. 1:3) afforded the title
compound
as a yellow solid (62.2 mg, 82 %). 'H-NMR (300 MHz, DMSO-d6) 8 9.39 (broad s,
2H), 8.97 (broad s, 2H), 8.78 (s, 1H), 8.60 (s, 1H), 7.89 (d, 2H, J--7 Hz),
7.49 (t, 2H,
J--7 Hz), 7.38 (t, 1H, J--7 Hz), and 2.80 (s, 3H). Mass spectrum (ESI) calcd.
for
C,SH,3N30Sz: 316.1 (M + H). Found: 316.2.
Example 85
4 ~9-(4-lzydroxy-3-metlzoxyphenyl)(1,3-thiazol 2 yl)J S-metlrylthiotltiophene-
2
carboxamidine hydrochloride
a) 9-(Clzlorocarbonyl)-2-methoxyplzenyl acetate: To a stirred suspension of
1.00 g (4.76 mmol) of 4-acetoxy-3-methoxybenzoic acid (Pfaltz and Bauer, Inc.)
in 4
mL of anhyd CHZC12 (under a CaS04 drying tube) was added 4.15 mL (47.6 mmol)
of
oxalyl chloride followed by 25 mL of anhyd DMF. After stirring for 4 h at room
temperature, the mixture was concentrated in vacuo to afford the title
compound as
light yellow crystals (1.12 g, 103%). 'H-NMR (300 MHz, CDCl3) 8 7.81 (dd, 1H,
J--8.4, 2.1 Hz), 7.66 (d, 1H, 2.1 Hz), 7.19 (d, 1H, 8.4 Hz), 3.91 (s, 3H), and
2.35 (s,
3H).
b) 4-(2-Bromoacetyl)-2-metltoxyphenyl acetate: To a solution of 1.09 g (4.6
mmol) of 4-(chlorocarbonyl)-2-methoxyphenyl acetate (as prepared in the
precious
step) in 10 mL of anhyd CHzCl2 was added 10.0 mL (20.0 mmol) of a 2 M solution
of
trimethylsilyldiazomethane in hexane. After stirring 2 h at room temperature,
the
mixture was cooled (0 °C) and 3.20 mL (16.0 mmol) of 30 wt % HBr in
acetic acid
CA 02362390 2001-08-07
WO 00/47578 r~49_ PCT/US99/18065
was added dropwise (gas evolution). After stirring S min, the mixture was
concentrated in vacuo and rapidly chromatographed on a 10-g silica SPE column
(Waters Sep-Pak) with CHZCIz to afford the title compound as a light yellow
crystalline solid (1.28 g, 97 %). 1H-NMR (300 MHz, CDC13) 8 7.63 (d, 1H, 1.9
Hz);
7.59 (dd, 1H, J--8.2, 1.9 Hz), 7.16 (d, 1H, 8.2 Hz), 4.43 (s, 2H), 3.91 (s,
3H), and
2.35 (s, 3H).
c) 2-Methoxy-4-~2 ~S-(methoxycarbonyl)-2-methylthio(3-thienyl)J(1,3-
tlziazol-4 yl))plzenyl acetate: Using a procedure similar to that of Example
82, step
(e) with 1.00 g (4.04 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-
2-carboxylate (Maybridge Chemical Company, Cornwall, UK) in 1 S mL of reagent
acetone and 1.16 g (4.04 mmol) of 4-(2-bromoacetyl)-2-methoxyphenyl acetate
(as
prepared in the previous step) afforded the title compound as 1.42 g of a
yellow solid
which, according to the'H-NMR spectrum, consisted of a ca. l:l mixture of the
title
compound and the corresponding compound resulting from partial loss of the
acetate.
1H_NMR (300 MHz, DMSO -d6 ) 8 8.27 (s, 1H), 8.22 (s, 1H), 8.19 (s, 1H), 8.00
(s,
1 H), 7.78 (d, 1 H, 1.9 Hz), 7.67 (dd, 1 H, J 8.2, 1.9 Hz), 7.61 (d, 1 H, 1.9
Hz), 7.51 (dd,
1 H, J--8.2, 1.9 Hz), 7.19 (d, 1 H, 8.2 Hz), 6.86 (d, 1 H, 8.2 Hz), 8.87 (m,
12H), 2.76 (s,
3H), 2.75 (s, 3H), and 2.28 (s, 3H). Mass spectrum (ESI) calcd. for C,9H"NOSS3
and
C,~H,SN03S3 436.0 (M + H) and 394.1 (M + H). Found: 436.1 and 394.2. The
mixture was used without further purification in the following step where
formation of
the amidine involves concomitant removal of the acetate.
d) 4-~4-(4-hydroxy-3-methoxyphenyl)(1,3-thiazol 2 yl)J S-
methyltlziotlzioplzene-2-carboxamidine hydrochloride: A portion of the mixture
(500
mg, ca. 1.21 mmol as based on the'H-NMR integration) containing the 2-methoxy-
4-
{2-[5-(methoxycarbonyl)-2-methylthio(3-thienyl)](1,3-thiazol-4-yl)}phenyl
acetate
(as prepared in the previous step) was treated according to the procedure in
Example
10, step (b) using 610 mg (11.4 mmol) of ammonium chloride in 5.7 mL of
toluene
and 5.70 mL (11.4 mmol) of 2 M trimethylaluminum in toluene. After
chromatography of the resulting residue on a 10 g silica SPE column (Waters
Sep-
Pak) with a gradient of 5-20 % MeOH-CHzCl2 to obtain a yellow glass which was
recrystallized from MeOH-CHZCIz to afford the title compound as a pale yellow
solid
CA 02362390 2001-08-07
WO 00/47578 -150- PCT/US99/18065
(192 mg, 42 %). 1H-NMR (300 MHz, DMSO-ds) 8 9.35 (broad s, 2H), 9.27 (s, 1H),
8.97 (broad s, 2H), 8.62 (s, 1 H), 8.04 (s, 1 H), 7.62 (s, 1 H), 7.54 (d, 1 H
J--8.2 Hz),
6.88 (d, 1H, J--8.2 Hz), 3.87 (s, 3H), and 2.79 (s, 3H). Mass spectrum (ESI)
calcd. for
C,6H,SN3OzS3: 378.0 (M + H). Found: 378.1.
Example 86
4-~4-(3 Hydroxy-4-metl:oxyphertyl)(1,3-thiazol 2 yl)J S-metlzylthiothiophene-2
carboxamidine hydrochloride
a) 3 Acetyloxy-4-methoxybenzoic acid: To a suspension of 600 mg (3.57
mmol) of 3-hydroxy-4-methoxybenzoic acid (Aldrich Chemical Company,
Milwaukee, WI) in 5 mL of anhyd CHzCIz was added 1.31 mL (7.50 mmol) of N, N
diisopropylethylamine and the mixture stirred until homogeneous (ca. 5 min).
Acetyl
chloride (305 mL, 4.28 mmol) was added dropwise over 2 min followed by 2.0 mg
((0.016 mmol) of 4-dimethylaminopyridine. After stirring at room temperature
for 1
h, the mixture was poured into 50 mL of EtOAc and washed with 1 M HCl (3 x 25
mL). The organic phase was extracted with saturated NaHC03 (6 x 1 S mL) and
the
combined extracts saturated with solid NaCI and acidified to pH 2 with conc
HCI.
The resulting suspension was extracted with EtOAc (3 x 20mL) and the combined
extracts were dried over Na2S04 and concentrated in vacuo to afford the title
compound as a light beige powder (463 mg, 62 %). 'H-NMR (300 MHz, CDCl3)
b 8.00 (dd, 1 H, J--8.7, 2.0 Hz), 7.79 (d, 1 H, 2.0 Hz), 7.00 (d, 1 H, 8.7
Hz), 3 .91 (s,
3H), and 2.34 (s, 3H).
b) 3-(Chlorocarbonyl)-6-methoxyphenyl acetate: Using the procedure in
Example 85, step (a), 400 mg (1.90 mmol) of 3-acetyloxy-4-methoxybenzoic acid
(as
prepared in the previous step) was treated with 663 mL (7.60 mmol) of oxalyl
chloride and 25 mL of anhyd DMF for 2 h to afford, after workup, the title
compound
as a beige crystalline solid which was used in the following step without
further
purification.
c) 5-(2-Bromoacetyl)-2-metl:oxyphenyl acetate: Using the procedure in
Example 85, step (b), the entire sample of 3-(chlorocarbonyl)-6-methoxyphenyl
acetate (as prepared in the previous step) in 5 mL of anhyd CHZCI, was treated
with
2.09 mL (4.18 mmol) of a 2 M solution of trimethylsilyldiazomethane in hexane
and
CA 02362390 2001-08-07
WO 00/47578 _~ 5,) _ PCT/US99/18065
456 mL (2.28 mmol) of 30 wt % HBr in acetic acid. Chromatography as in Example
85, step (b) followed by recrystallization from CHZCIz-hexane afforded the
title
compound as a faintly yellow solid (366 mg. 67 %). 'H-NMR (300 MHz, CDC13)
8 7.79 (dd, 1 H, J--8.6, 2.2 Hz), 7.70 (d, 1 H, 2.2 Hz), 7.03 (d, 1 H, 8.6
Hz), 4.38 (s,
2H), 3.92 (s, 3H), and 2.34 (s, 3H).
d) 2-Methoxy-S-~2 ~5-(methoxycarbonyl)-2-methylthio(3-tlzienyl)J(1,3-
tlaiazol 4 yl)~phenyl acetate: Using a procedure similar to that of Example
82, step
(e) with 282 mg (1.14 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Company, Cornwall, UK)
in 4 mL of acetone and 3.27 mg (1.14 mmol) of 5-(2-bromoacetyl)-2-
methoxyphenyl
acetate (as prepared in the previous step) afforded a yellow solid (374 mg)
which,
according to the' H-NMR spectrum, consisted of a 3:7 mixture of the title
compound
and the corresponding compound resulting from partial loss of the acetate.
Mass
spectrum (ESI) calcd. for C,9H"NOSS3 and C,~H,SNO3S3 436.0 (M + H) and 394.1
(M
+ H). Found: 436.0 and 394Ø The mixture was used without further
purification in
the following step where formation of the amidine involves concomitant removal
of
the acetate.
e) 4-~4-(3-Hydroxy-4-methoxyplzenyl)(1,3-tliiazol 2 yl)J 5-
methyltltiothiophene-2-carboxamidine hydrochloride: A portion of the mixture
(320
mg, ca. 0.788 mmol as based on the 1H-NMR spectrum) containing the 2-methoxy-5-
{2-[5-(methoxycarbonyl)-2-methylthio(3-thienyl)](1,3-thiazol-4-yl)}phenyl
acetate
(as prepared in the previous step) was treated according to the procedure in
Example
10, step (b), using 415 mg (7.76 mmol) of ammonium chloride in 3.5 mL of
toluene
and 3.88 mL (7.66 mmol) of 2 M trimethylaluminum in toluene. After
chromatography of the resulting residue on a 10 g silica SPE column (Waters
Sep-
Pak) with 10-40 % MeOH-CHZCIz, a light yellow solid was obtained which was
dissolved in 45 mL of DMF and filtered to remove silica gel. Concentration
under
high vacuum and recrystallization from MeOH-Et20 afforded the title compound
as a
light tan solid (132 mg, 44 %). IH-NMR (300 MHz, DMSO-d6) S 9.49 (broad s,
2H),
9.16 (broad s, 2H), 8.67 (s, 1H), 7.98 (s, 1H), 7.5 (obscured m, 3H), 7.00
(obscured d,
1H, J--8.3 Hz), 3.82 (s, 3H), and 2.79 (s, 3H). Mass spectrum (ESI) calcd. for
C~6H~SN3OZS3: 378.0 (M + H). Found: 378.1.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-152-
Example 87
5-Methyltlzio-4-(N phenylcarbamoyl)thioplzene-2-carboxamidine hydrochloride
a) Methyl 5-metl:ylthio-4-(N phenylcarbamoyl)thiophene-2-carboxylate:
To 182 mg (0.785 mmol) of 5-(methoxycarbonyl)-2-methylthiothiophene-3-
carboxylic acid (as prepared in Example 95) in 4 mL of anhyd~ CHZCIZ was
treated
with 275 mL (3.15 mmol) of oxalyl chloride and 6 mL of anhyd DMF for 2 h
similar
to Example 79, step (a); followed by 206 mL (1.18 mmol) of N,N
diisopropylethylamine and 85.9 mL (0.942 mmol) of aniline in 3 mL of anhyd
CHzCl2
for 20 min. The mixture was poured into 25 mL of EtOAc and washed with 1 M HCl
(2 x 25 mL), saturated NaHC03 (2 x 25 mL), and brine (25 mL), and dried over
Na,S04. Removal of the solvent in vacuo, afforded the pure title compound as a
light
yellow solid (163 mg, 68 %). 'H-NMR (300 MHz, CDC13) b 8.23 (broad s, 1H),
8.10
1 S (s, 1 H), 7.63 (d, 2H, J--7 Hz), 7.3 6 (t, 2H, J--7 Hz), 7.1 S (t, 2H, J--
7 Hz), 3.90 (s, 3H),
and 2.64 (s, 3H).
b) S-Metlzylthio-4-(N phenylcarbamoyl)thiophene-2-carboxamidine
hydrochloride: Methyl5-methylthio-4-(N-phenylcarbamoyl)thiophene-2-carboxylate
(60.0 mg, 0.195 mmol , as prepared in the previous step) was treated similarly
to the
procedure in Example 10, step (b) using 310 mg (5.80 mmol) of ammonium
chloride
in 2 mL of toluene and 2.90 mL (5.80 mmol) of 2 M trimethylaluminum in toluene
for
6 h. Chromatography of the resulting residue on a 2 g silica SPE column
(Waters
Sep-Pak) with a gradient of 5-20 % MeOH-CHzCIZ, followed by crystallization
from
MeOH-Et20 afforded the title compound as a beige solid (40.3 mg, 71 %). 1H-NMR
(300 MHz, DMSO-db) 8 10.24 (s, 1H), 9.34 (broad s, 2H), 9.05 (broad s, 2H),
8.75 (s,
1H), 7.73 (d, 2H, J--8 Hz), 7.36 (t, 2H, J--8 Hz), 7.11 (m, 1H), and 2.67 (s,
3H). Mass
spectrum (ESI) calcd. for C,3H,3N3OSz: 292.1 (M + H). Found: 292.4.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-153-
Example 88 and 89
5-Metlzyltlzio-4-~N benzylcarbamoylJthioplzene-2-carboxamidinehydrochloride
and
4-~Imino~benzylaminoJmethylJ-5-methyltlziothiophene-2-carboxamidine
Izydrochloride
a) Methyl 5-metlzylthio-4 ~N benzylcarbamoylJthiophene-2-carboxylate:
The identical procedure of Example 87, step (a) was used with 103 mL (0.942
mmol)
of benzylamine and the same amounts of all other reagents to afford the title
compound as a light yellow solid (167 mg, 66 %). 1H-NMR (300 MHz, CDC13)
8 7.93 (s, 1H), 7.28-7.38 (m, SH), 6.58 (broad s, 1H), 4.62 (s, 2H, J 5.7 Hz),
3.87 (s,
3H), and 2.60 (s, 3H).
b) 5-Methyltlzio-4-~N benzylcarbamoylJthioplzene-2-
carboxamidinehydroclzloride and 4-~Imino~benzylaminoJmethylJ-5-
metlzyltlziotlziophene-2-carboxamidinelzydroclzloride: Methyl5-methylthio-4-[N-
benzylcarbamoyl)thiophene-2-carboxylate (62.7 mg, 0.195 mmol, as prepared in
the
previous step) was treated similarly to the procedure in Example 10, step (b)
using
310 mg (5.80 mmol) of ammonium chloride in 2 mL of toluene and 2.90 mL (5.80
mmol) of 2 M trimethylaluminum in toluene for 6 h.
Chromatography of the resulting residue on a 2 g silica SPE column (Waters
Sep-Pak) with a gradient of 5-20 % MeOH-CHZC12, followed by crystallization
from
MeOH-Et20 afforded 5-methylthio-4-[N-benzylcarbamoyl]thiophene-2-
carboxamidinehydrochloride as a beige solid (21.1 mg, 35 %). 'H-NMR (300 MHz,
DMSO-d6) 8 7.93 (s, 1H), 7.28-7.38 (m, SH), 6.58 (broad s, 1H), 4.62 (s, 2H, J-
-5.7
Hz), 3.87 (s, 3H), and 2.60 (s, 3H). Mass spectrum (ESI) calcd. for
C,4H,SN3OS2:
306.1 (M + H). Found: 306.6.
Also isolated and crystallized from MeOH-Et20 was the more polar 4-
{ imino [benzylamino]methyl }-5-methylthiothiophene-2-
carboxamidinehydrochloride
as a beige solid (32.0 mg, 54 %). 'H-NMR (300 MHz, DMSO-d6) consistent with
desired product as broad mixture of rotomers. Mass spectrum (ESI) calcd. for
3O C,4H,6N4S2: 305.1 (M + H). Found: 305.8.
CA 02362390 2001-08-07
WO 00/47578 _' S4_ PCT/US99/18065
Example 90 and 91
4-~N Methyl N benzylcarbamoylJ S-metlzyltlziothiophene-2-carboxamidine
hydrochloride and 4-~Imino~methylbenzylaminoJmethylJ-5-methylthiothiophene-2
carboxamidinehydroclzloride
a) Methyl 4-~N methyl N benzylcarbamoylJ S-metlzylthiothiophene-2-
carboxylate: The identical procedure of Example 87, step (a) was used with 122
mL
(0.942 mmol) of N benzylmethylamine and the same amounts of all other reagents
to
afford the title compound as a light yellow solid (169 mg, 64 %). IH-NMR (300
MHz, CDC13) 8 7.68 (s, 1H), 7.34 (m, SH), 4.6 (broad m, 2H), 3.86 (s, 3H),
2.91 (m,
3H), and 2.60 (s, 3H).
b) 4-(N Methyl N benzylcarbamoylJ 5-methylthiothiophene-2-
carboxamidine hydrochloride and 4-~Imino~methylbenzylaminoJmethylJ-S-
methyltlziotlzioplzene-2-carboxamidine Izydrochloride: Methyl 4-[N-methyl-N-
benzylcarbamoyl]-5-methylthiothiophene-2-carboxylate (65.4 mg, 0.195 mmol, as
prepared in the previous step) was treated similarly to the procedure in
Example 10,
step (a) using 310 mg (5.80 mmol) of ammonium chloride in 2 mL of toluene and
2.90 mL (5.80 mmol) of 2 M trimethylaluminum in toluene for 6 h.
Chromatography of the resulting residue on a 2 g silica SPE column (Waters
Sep-Pak) with a gradient of 5-20 % MeOH-CHZCIz afforded 4-[N-methyl-N-
benzylcarbamoyl]-5-methylthiothiophene-2-carboxamidine hydrochloride as a
amber-
colored glass (34.3 mg, 55 %). 1H-NMR (300 MHz, DMSO-d6) 8 9.32 (broad s, 2H),
9.06 (broad s, 2H), 8.11 (s, 1H), 7.36 (m, SH), 4.66 (m, 2H), 2.88 (s, 3H) and
2.66 (s,
3H). Mass spectrum (ESI) calcd. for C,SH,~N30Sz: 320.1 (M + H). Found: 320.4.
Also isolated and then crystallized from MeOH-Et20 was the more polar
4-{imino[methylbenzylamino]methyl}-5-methylthiothiophene-2-carboxamidine
hydrochloride as a beige solid (19.8 mg, 32 %). 1H-NMR (300 MHz, DMSO-d6)
consistent with desired product as broad mixture of rotomers. Mass spectrum
(ESI)
calcd. for C,SH,gN4S2: 319.1 (M + H). Found: 319.6.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-155-
Example 92 and 93
S Metliylthio-4 ~N (2 phenylethyl)carbamoylJthiophene-2-carboxamidine
lzydrochloride and 4-(Imino~(2 phenylethyl)aminoJmethylJ-S-
methyltlziotlziophene
2-carboxamidine hydrochloride
a) Methyl S-methyltl:io-4-~N (2 pltenylethyl)carbamoylJthioplzene-2-
carboxylate: The identical procedure of Example 87, step (a) was used with 118
mL
(0.942 mmol) of phenethylamine and the same amounts of all 'other reagents to
afford
the title compound as a light yellow solid (165 mg, 63 %). 'H-NMR (300 MHz,
CDC13) 8 7.86 (s, 1H), 7.30-7.35 (m, SH), 6.44 (m, 1H), 3.87 (s, 3H), 3.70 (q,
2H,
J--7 Hz), 2.93 (t, 2H, J--7 Hz), and 2.53 (s, 3H).
b) S-Methylthio-4 ~N (2 phenyletlzyl)carbamoylJthioplzene-2-carboxamidine
hydrochloride and 4-~Imino~(2 phenyletliyl)aminoJmethylJ-S-
methylthiotlziophene-
2-carboxamidine hydrochloride: Methyl S-methylthio-4-[N-(2-
phenylethyl)carbamoyl]thiophene-2-carboxylate (65.4 mg, 0.195 mmol , as
prepared
in the previous step) was treated similarly to the procedure in Example 10,
step (a)
using 310 mg (5.80 mmol) of ammonium chloride in 2 mL of toluene and 2.90 mL
(5.80 mmol) of 2 M trimethylaluminum in toluene for 6 h.
Chromatography of the resulting residue on a 2 g silica SPE column (Waters
Sep-Pak) with a gradient of S-20 % MeOH-CHZCl2, followed by crystallization
from
MeOH-Et20 afforded 5-methylthio-4-[N-(2-phenylethyl)carbamoyl]thiophene-2-
carboxamidine hydrochloride as a beige solid (17.4 mg, 28 %). 'H-NMR (300 MHz,
DMSO-d6) 8 8.8-9.3 (broad m, 4H), 8.48 (m, 1H), 8.35 (s, 1H), 7.26 (m, SH),
3.44 (m,
2H), 2.82 (t, 3H, J--7.5 Hz), and 2.61 (s, 3H). Mass spectrum (ESI) calcd. for
C,SH"N30S2: 320.1 (M + H). Found: 320.4.
Also isolated and crystallized from MeOH-EtzO was the more polar
4-{imino[(2-phenylethyl)amino]methyl}-5-methylthiothiophene-2-carboxamidine
hydrochloride as a beige solid (19.1 mg, 31 %). ~H-NMR (300 MHz, DMSO-db)
b 8.37 (s, 1H), 7.2-7.4 (m, SH), 3.70 (t, 2H, J--7.6 Hz), 2.96 (t, 2H, J--7.6
Hz), and
2.71 (s, 3H). Mass spectrum (ESI) calcd. for C,SH,8N4Sz: 319.1 (M + H). Found:
319.5.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-156
Example 94
3 Amino-2-aza-3-~S-methylthio-4-(4 phenyl(1,3-thiazol-2 yl))(2-
thienyl)Jprop-2-enenitrile
To 100 mg (0.302 mmol) of 5-methylthio-4-(4-phenyl(1,3-thiazol-2-
yl))thiophene-2-carboxamidine (as prepared in Example 10, step b) in 3 mL of
EtOH
was added 29.6 mg (0.604 mmol) of cyanamide as a solution in 0.3 mL of water.
The
mixture was heated to reflux and 0.302 mL (0.302 mmol) of 1 M aqueous KOH was
added. After 3 h, the mixture was cooled (0°C) and filtered washing
with ice-cold
EtOH. The resulting solid was dried in vacuo to afford the title compound as a
light
yellow powder (78.4 mg, 73 %). ~H-NMR (300 MHz. DMSO-db) 8 9.31 (broad s,
1 H), 8.70 (broad s, 1 H), 8.63 (s, 1 H), 8.19 (s, 1 H), 8.09 (d, 2H, J--7
Hz), 7.49 (t, 2H,
J 7 Hz), 7.39 (t, 1H, J--7 Hz), and 2.75 (s, 3H). Mass spectrum (MALDI-TOF, a-
cyano-4-hydroxycinnamic acid matrix) calcd. for C,6H,ZN4S3: 357.0 (M + H).
Found:
357.1.
Example 95
S-(Methoxycarbonyl)-2-methylthiothiophene-3-carboxylic acid
Methyl 4-cyano-5-methylthiothiophene-2-carboxylate (2.20 g, 10.3 mmol,
Maybridge Chemical Company, Cornwall, UK) and tetrafluorophthalic acid (2.45
g,
10.3 mmol) in an 8-mL sealable pressure tube (Ace Glass Company) with stir bar
was
heated to 160 °C. The molten mixture was stirred for 4 days, cooled and
the resulting
residue broken up and extracted by refluxing with 80 mL chloroform. The
mixture
was cooled, decolorizing carbon (ca. 0.5 g) was added and the mixture filtered
(Celite). The resulting solution was extracted with saturated NaHC03 (4 x 30
mL)
and the combined aqueous extracts acidified to pH 1-2 with conc HCl and
filtered to
provide a light tan solid. After dissolving the solid in a minimum of 1 M
KZC03 (35-
40 mL) and filtering (washing with 10-20mL of water) to clarify the solution,
it was
slowly acidified to pH 6.5-7.0 with stirring and filtered (Celite) to remove a
brown
precipitate. The pH adjustment and filtration was repeated and the resulting
solution
was saturated with solid NaCI and acidified to pH 1-2 with conc HCI. The
precipitate
was filtered, washed with water (3 x 10 mL) and dried over P,OS under high
vacuum
to afford the title compound as a cream-colored powder (1.24 g, 52 %). ~H-NMR
CA 02362390 2001-08-07
WO 00/47578 _,~ 5~_ PCT/US99/18065
(300 MHz, DMSO-db) 8 13.14 (broad s, 1H), 7.89 (s, 1H), 3.82 (s, 3H) and 2.64
(s,
3H). Mass spectrum (ESI, negative mode) calcd. for C8H804SZ: 232.0 (M-).
Found:
231.7.
Example 96
S-Etlzyltlzio-4-(4 plienyl(1,3-thiazol 2 yl))tlZioplaene-2-carboxamidine
laydrocl:loride S
a) Methyl4-(4 plaenyl(1,3-thiazol 2 yl))-5-(metlZylsulfonyl)thiophene-2-
carboxylate: Using the procedure of Example 141, step (a) with 600 mg (1.73
mmol)
of methyl 5-methylthio-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate
as
prepared in Example 10, step (a) afforded 642 mg (98 %) of the title compound
as a
light yellow powder. IH-NMR (300 MHz, CDCl3) 8 7.93 (s, 1H), 7.90 (m, 2H),
7.63
(s, 1H), 7.47 (m, 2H), 7.39 (m, 1H), 3.98 (s, 3H) and 3.73 (s, 3H). Mass
spectrum
(ESI, m/z): calcd. for C,6H,3NO4S3 380.0 (M+H), found 380.2.
b) 4-(4-Plzenyl)(1,3-thiazol 2 yl))-S-(metltylsulfonyl)thiophene-2-
carboxamidine IZydrochloride: Using the procedure of Example 141, step (b)
with
560 mg (1.48 mmol) methyl 4-[4-(4-chlorophenyl)(1,3-thiazol-2-yl)]-5-
(methylsulfonyl)thiophene-2-carboxylate as prepared in the previous step
afforded
392 mg (66 %) of the title compound as a off white solid. 'H-NMR (300 MHz,
DMSO-d6) 8 9.7 (broad s, 2H), 9.4 (broad s, 2H), 8.58 (s, 1H), 8.43 (s, 1H),
8.02 (d,
2H, J--7 Hz), 7.52 (t, 2H, J--7 Hz), 7.43 (t, 1H, J--7 Hz), and 3.90 (s, 3H).
Mass
spectrum (ESI, m/z): calcd. for C,SH,3N3OZS3 364.0 (M+H), found 364.1.
c) S-Etlaylthio-4-(4 phenyl(1,3-tlZiazol-2 yl))thioplzene-2-carboxamidine
hydrochloride: Using the procedure of Example 141, step (c) with 23.1 mg
(0.0578
mmol) of the 4-(4-phenyl)(1,3-thiazol-2-yl))-5-(methylsulfonyl)thiophene-2-
carboxamidine hydrochloride (as prepared in the previous step), 64.1 mL( 0.867
mmol) of ethanethiol (in 2 portions over 2 h) and 40.3 mL (0.231 mmol) of DIEA
in 3
mL of methanol gave a yellow resin which was chromatographed on a 2 g silica
SPE
column (Waters Sep-Pak) with a gradient of 0-15 % MeOH-CHZC12, followed by
trituration with CHZC12 to afford the title compound as an off white solid
(21.7 mg, 98
%). 'H-NMR (300 MHz, DMSO-db) 8 9.45 (broad s, 2H), 9.07 (broad s, 2H), 8.68
(s,
1 H), 8.28 (s, 1 H), 8.09 (d, 2H, J--7 Hz), 7.51 (t, 2H, J--7 Hz), 7.40 (t, 1
H, J--7 Hz),
CA 02362390 2001-08-07
w0 00/47578 _ ~ 58_ PCT/US99/18065
3:23 (q, 2H, J--7 Hz) and 1.42 (t, 3H, J--7 Hz). Mass spectrum (ESI) calcd.
for
C~6H,SN3S3: 346.1 (M+ H). Found: 346.2.
Example 97
5-Methyltlzio-4-~4-(phenoxymetl:yl)(1,3-thiazol Z yl)Jthiophene-2-
carboxamidine
hydrochloride
a) 3-Bromo-1 plzenoxyacetone: To a solution of 6.c (0.050 mmol) of
phenoxyacetyl chloride in 250 mL of anhyd MeCN in a 1-dram short vial (Wheaton
Glass) was added 50 mL (0.100 mmol) of a 2 M solution of
trimethylsilyldiazomethane in hexane and the vial capped with a PTFE-lined
cap.
After stirring 1 h at room temperature on a vortex shaker, the mixture was
cooled (0
°C) and 21 mL (0.105 mmol) of 30 wt % HBr in acetic acid was added
dropwise (gas
evolution). After vortexing for 10 min, the mixture was concentrated in vacuo
on a
vacuum centrifuge concentrator (Speed-Vac, Savant Instruments, Inc.) to
provide an
amber-colored oil which was used directly in the following step.
b) Methyl S-methylthio-4-~Q-(phenoxymethyl)(i,3-tlaiazol 2 yl)Jthiophehe-2-
carboxylate: To the 3-bromo-1-phenoxyacetone (as prepared in the previous step
in a
1-dram vial) was added 14.8 mg (0.060 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge Chemical Company, Cornwall, UK)
as 1.48 mL of a 10 mg /mL solution in acetone. The vial was tightly capped and
placed on a heated platform shaker (Innova model 4080, New Brunswick
Scientific
Co., Inc.) and vortexed at 55 °C and 250 rpm for 4 h. To the resulting
mixture was
added 50 mg (0.150 mmol) of diethylaminomethyl-polystyrene resin (Fluka
Chemika-
Biochemika, 3.0 mmol / g) as 0.50 mL of a 100 mg / mL suspension in acetone
and
the mixture vortexed briefly. Chloroacetylpolystyrene resin (30 mg, 0.150
mmol,
Advanced ChemTech Inc., 5.0 mmol / g) was then added followed by (0.750 mg,
0.005 mmol) NaI as 100 mL of a 7.5 mg / mL solution in acetone. The mixture
was
again capped tightly and placed on a heated platform shaker and vortexed at
55°C and
250 rpm for 22 h. The mixture was filtered through a 2 mL fritted column
(BioRad
Biospin minicolumn) washing with acetone (2 x 0.5 mL) and MeOH (2 x 0.5 mL)
into
a 2 dram vial and concentrated on a vacuum centrifuge concentrator to afford
21.0 mg
of the title compound as an off white solid. 1H-NMR (300 MHz, DMSO-d6) 8 8.17
CA 02362390 2001-08-07
WO 00/47578 _~ 59_ PCT/US99/18065
(s, 1 H), 7.82 (s, 1 H), 7.13 (m, 2H), 7.07 (m, 2H), 6.96 (m, 1 H), 5.22 (s,
2H), 3.85 (s,
3H), and 2.74 (s, 3H). Mass spectrum (MALDI-TOF, a-cyano-4-hydroxycinnamic
acid matrix) calcd. for C,~H,SN03S3: 378.0 (M + H). Found: 378.3.
c) S-Methyltlzio-4-~4-(phenoxymethyl)(1,3-tlaiazol-2 yl)Jtlziophene-2-
carboxamidine hydrochloride: The methyl 5-methylthio-4-[4-(phenoxymethyl)(1,3-
thiazol-2-yl)]thiophene-2-carboxylate (as prepared in the previous step)under
nitrogen
in a 2 dram vial with a micro magnetic stir bar) was capped with an open-top
phenolic
cap containing a PTFE-backed silicone septum. A 1 M solution of the reagent
freshly
prepared from trimethylaluminum and ammonium chloride in toluene according to
the
procedure in Example 10, step b (0.750 mL, 0.750 mmol) was added by syringe by
puncturing the septum once with the needle to allow venting of gas followed by
a
second puncture to inject the reagent. The vial was placed in an aluminum
heating
block under nitrogen (Fisher Scientific Dry Bath Incubator fitted with a
custom-made
nitrogen manifold cover). The manifold was flushed with nitrogen and the
reaction
stirred by means of a large magnetic stir motor placed inverted on top of the
manifold.
The reaction was heated to 100 °C for 4 h, and cooled to room
temperature over ca. 2
h. The contents of the vial were quenched carefully into 0.5 g of silica gel
in 2 mL of
CHzCIz, capped and shaken to homogeneity. The slurry was filtered through a 4-
mL
fritted column (Isolab microcolumn) into a 2-dram vial washing with CHZCIz (2
x 1
mL), CHzCIz-MeOH (1:1, 1 x 1 mL) and MeOH(2 x 1 mL) and the filtrate
concentrated on a vacuum centrifuge concentrator to a yellow solid. Filtration
through a 500 mg silica SPE column (Supelco LC-Si) with 10 % MeOH -CHZC12
afforded the title compound as a yellow solid (14.8 mg). 'H-NMR (300 MHz,
DMSO-d6) 8 9.45 (d, 2H, J--8.2 Hz), 9.11 (d, 2H, J--8.2 Hz), 8.97 (broad s,
2H), 8.65
(s, 1H), 7.90 (s, 1H), 7.0-7.5 (m, SH), 5.25 (s, 2H), and 2.79 (s, 3H). Mass
spectrum
(MALDI-TOF, gentisic acid matrix) calcd. for C,~H,SN03S3: 362.0 (M + H),
Found:
361.7.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-160-
Examples 98-126
Examples 98-104 were carried out using the procedure of Example 97, steps
(b) and (c) using 0.050 mmol of the reagent specified in the table. Examples
105-126
were carried out using the procedure of Example 97, steps (a), (b) and (c)
using 0.05
mmol of reagent.
Mass
Spectrum
(ESI)
ExampleReagent Compound Formula Calcd Found
(M+H)
98 1-bromo- 4-[4-(tent-butyl)(1,3-C13 H17 312.1 312.2
N3 S3
pinacolone thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
99 4-fluoro- 4-[4-(4- C15 H12 350.0 350.2
F N3
phenacyl fluorophenyl)(1,3-S3
bromide thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
100 4-cyano- 4-[4-(4- C16 H15 374.1 374.2
N5 S3
phenacyl amidinophenyl)(1,3-
bromide thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
101 3-fluoro- 4-[4-(3- C15 H12 350.0 350.2
F N3
phenacyl fluorophenyl)(1,3-S3
bromide thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
102 4-(diethylamino)-4-{4-[4-(diethylamino)-C19 H22 403.1 403.2
N4 S3
phenacyl phenyl](1,3-thiazol-2-
bromide yl)}-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
103 3-chloro- 4-[4-(3- C15 H12 366.0 366.1
Cl N3
phenacyl chlorophenyl)(1,3-S3
bromide thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-161-
104 3,4-difluoro-4-[4-(3,4- C15 H11 368.0 368.2
F2 N3
phenacyl difluorophenyl)(1,3-S3
bromide thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
105 2,6-difluoro-4-[4-(2,6- C15 HI 1 368.0 368.2
F2 N3
benzoyl difluorophenyl)(1,3-S3
chloride
thiazol-2-yl)]-5-
methylthiothiophene-2-'
carboxamidine
hydrochloride
106 4-ethoxy-benzoyl4-[4-(4- C17 H17 376.1 376.2
N3 O
chloride ethoxyphenyl)(1,3-S3
thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
107 4-chloro- 4-{4-[(4- C16 H14 396.0 396.1
Cl N3
phenoxyacetylchlorophenoxy)-O S3
chloride methyl](1,3-thiazol-2-
yl)}-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
108 cyclopentane-4-(4-cyclopentyl(1,3-C14 H17 324.1 324.2
N3 S3
carbonyl thiazol-2-yl))-5-
chloride
methylthiothiophene-2-
carboxamidine
hydrochloride
109 1-naphthoyl5-methylthio-4-(4-C19 H15 382.1 382.2
N3 S3
chloride naphthyl(1,3-thiazol-2-
yl))thiophene-2-
carboxamidine
hydrochloride
I 10 3,5-dichloro-4-[4-(3,5- C15 H11 400.0 400.1
C12 N3
benzoyl dichlorophenyl)(1,3-S3
chloride
thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
111 2,5- 4-[4-(2,5- C15 H1 I 368.0 368.2
F2 N3
difluorobenzoyldifluorophenyl)(1,3-S3
chloride thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
112 9-fluorenone-4-5-methylthio-4-[4-(9-C22 H15 434.1 434.2
N3 O
carbonyl oxofluoren-4-yl)(1,3-S3
chloride
thiazol-2-yl)]thiophene-
2-carboxamidine
hydrochloride
CA 02362390 2001-08-07
WO 00/47578 _~ 6z_ PCT/US99/18065
113 3- 4-{4-[(3- C17 H17 376.1 376.2
N3 O
methoxyphenyl-methoxyphenyl)methyl](S3
acetyl chloride1,3-thiazol-2-yl)}-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
114 4-methyl 4-[4-(3-methylbutyl)(1,3-C14 H19 326.1 326.2
valeroyl N3 S3
chloride thiazol-2-yl)]-S-
methylthiothiophene-2-
carboxamidine
hydrochloride
115 3-(2- 4-{4-[3-(2- C19 H15 447.0 447.1
CI N4
chlorophenyl)-5-chlorophenyl)-5-O S3
methylisoxazole-methylisoxazol-4-yl](1,3-
4-carbonyl thiazol-2-yl)}-S-
chloride methylthiothiophene-2-
carboxamidine
hydrochloride
116 4-n-amyloxy-5-methylthio-4-[4-(4-C20 H23 418.1 418.2
N3 O
benzoyl pentyloxyphenyl)(1,3-S3
chloride
thiazol-2-yl)]thiophene-
2-carboxamidine
hydrochloride
117 1-(4- 4-{4-[(4-chlorophenyl)-C20 H20 434.1 434.3
CI N3
chlorophenyl)-1-cyclopentyl](1,3-thiazol-S3
cyclopentanecarb2-yl)}-5-
onyl-chloridemethylthiothiophene-2-
carboxamidine
hydrochloride
118 4-(trifluoro-5-methylthio-4-{4-[4-C16 H12 416.0 416.1
F3 N3
methoxy)benzoyl(trifluoromethoxy)phenylO S3
chloride ](1,3-thiazol-2-
yl)}thiophene-2-
carboxamidine
hydrochloride
119 3-chloro- 4-[4-(3- C17 H12 422.0 422.1
CI N3
benzo[b] chlorobenzo[b]thiophen-S4
thiophene-2-2-yl)(1,3-thiazol-2-yl)]-
carbonyl 5-methylthiothiophene-2-
chloride
carboxamidine
hydrochloride
120 3-(2-chloro-6-4-{4-[3-(6-chloro-2-C19 H14 465.0 465.1
CI F N4
fluorophenyl)-5-fluorophenyl)-5-O S3
methylisoxazole-methylisoxazol-4-yl](1,3-
4-carbonyl thiazol-2-yl)}-5-
chloride methylthiothiophene-2-
carboxamidine
hydrochloride
121 3-cyanobenzoyl4-[4-(3- C I 6 H 374. 374.7
I S NS I
S3
chloride amidinophenyl)(1,3-
thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-163-
122 4- 4-{4-[(4- C17 H17 376.1 376.2
N3 O
methoxyphenyl-methoxyphenyl)-S3
acetyl methyl](1,3-thiazol-2-
chloride
yl)}-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
123 3-(t-butyl)-1-4-{4-[3-(tent- C16 H19 378.1 378.2
NS S3
benzylpyrazole-butyl)pyrazol-5-yl](1,3-
5- thiazol-2-yl)}-5-
carbonyl methylthiothiophene-2-
chloride_ carboxamidine
hydrochloride
124 3-(4- 5-methylthio-4-[4-(I-C12 H13 296.0 296.2
N3 S3
chlorophenyl)-methylvinyl)(1,3-thiazol-
2,2-dimethyl-2-yl)]thiophene-2-
propanoyl carboxamidine
chloride hydrochloride
125 n-(1- 5-methylthio-4-(4-{C27 H24 565.1 565.1
1- N4 02
naphthalene-[(naphthylsulfonyl)aminoS4
sulfonyl)-1-]-2-phenylethyl}(1,3-
phenylalanylthiazol-2-yl))thiophene-
chloride 2-carboxamidine
hydrochloride
126 2-bromo-5-, 4-[4-(2-bromo-5-C 16 H 14 440.0 440.2
Br N3
methoxybenzoylmethoxyphenyl)(1,3-O S3
chloride thiazol-2-yl)]-5-
methylthiothiophene-2-
carboxamidine
hydrochloride
Example 127
a) 1-~3,5-Bis(trifluoromethyl)phenyl~ 2-bromoetlzan-1-one: A stirred
suspension of 1 g (3.9 mmol) of 3,5-bis(trifluoromethyl)acetophenone
(Lancaster,
Windham, NH, USA) in dry methanol (20 mL) and 1 g (15 mmol, 2.6 eq) of poly(4-
vinyl pyridinium tribromide) (Aldrich, Milwaukee, WI, USA) was protected from
moisture with dry nitrogen, and heated at reflux for 70 min. The polymer was
filtered
from the cooled solution and washed with methanol and twice with
dichloromethane.
The solvents were removed in vacuo to give 1-[3,5-bis(trifluoromethyl)phenyl]-
2-
bromoethan-1-one (1.2 g, 92 %). 'H-NMR (DMSO-db; 300 MHz) 8 8.43 (m, 2H),
8.12 (m, 1H), 4.46 (s, 3H).
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-164
b) Methyl 4-~4-~3,5-bis(trifluoromethyl)phenylJ(1,3-thiazol 2 yl)J-5-
methyltlziotlzioplzene-2-carboxylate: A solution of 75 mg (0.3 mmol) of methyl
4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge, Cornwall,
UK) was reacted with 101 mg (0.3 mmol) of 1-[3,5-bis(trifluoromethyl)phenyl]-2-
bromoethane-1-one in a manner similar Example 8, step (a) to give methyl 4-{4-
[3,5-
bis(trifluoromethyl)phenyl](1,3-thiazol-2-yl)}-S-methylthiothiophene-2-
carboxylate
(7 mg, 5 %) as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.75 (s, 1H), 8.73 (m,
2H),
8.29 (s, 1H), 8.13 (m, 1H), 3.87 (s, 3H), 2.79 (s, 3H). Mass spectrum (MALDI-
TOF,
CHCA matrix, m/z): Calcd. for C,gH"NOZS3F6, 484.0 (M+H), found 484Ø
c) 4-~4-~3,5-Bis(tr~uoromethyl)phenylJ(1,3-thiazol 2 yl)J-S-
znethylthiothiophene-2-carboxamidizze: Methyl4-[4-3,5-
bis(trifluoromethyl)phenyl](1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (7
mg, 14.5 mmol) was treated in a manner similar to that for Example 10, step
(b), to
give 4-{4-[3,5-bis(trifluoromethyl)phenyl](1,3-thiazol-2-yl)}-5-
methylthiothiophene-
2-carboxamidine (6 mg, 89 %) as a yellow solid. 'H-NMR (DMSO-db; 300 MHz) 8.78
(s, 1 H), 8.74 (s, 2H), 8.62 (s, 1 H), 8.15 (s, 1 H), 2.82 (s, 3H). Mass
spectrum
(MALDI-TOF, CHCA matrix, m/z): Calcd. for C"H"N3S3F6, 468.0 (M+H), found
468Ø
Example 128
a) Z-Bromo-I-~3 Jluoro-S-(trifluoromethyl)phenylJethau-1-one: A stirred
suspension of 1 g (4.5 mmol) of 3-fluoro-5-(trifluoromethyl)acetophenone
(Lancaster,
Windham, NH, USA) was treated in a manner similar to that for Example 127,
step
(a) to give of a 1:1 mixture of 2-bromo-1-[3-fluoro-5-
(trifluoromethyl)phenyl]ethan-
1-one and dibrominated product (1.6 g, 100%). 'H-NMR (DMSO-db; 300 MHz)
8 8.25-7.52 (m, 6H), 6.54 (s, 1 H), 4.42 (s, 2H).
b) Methyl 4-~4-~3 fluoro-5-(tr~uoromethyl)phenylJ(1,3-thiazol 2 yl)~-S-
metlzyltlziothiopliezze-2-carboxylate: A solution of 75 mg (0.3 mmol) of
methyl 4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge, Cornwall,
UK) was reacted with of 86 mg (0.3 mmol) 2-bromo-1-[3-fluoro-5-
(trifluoromethyl)phenyl]ethan-1-one in a manner similar to Example 8, step (a)
to
give, methyl 4-{4-[3-fluoro-5-(trifluoromethyl)phenyl](1,3-thiazol-2-yl)}-5-
CA 02362390 2001-08-07
WO 00/47578 -165- PCT/US99/18065
methylthiothiophene-2-carboxylate (41 mg, 31 %) as a solid. 'H-NMR (DMSO-d~;
300 MHz) 8 8.59 (s, 1H), 8.29 (m, 1H), 8.27 (s, 1H), 8.25 and 8.21 (m, 1H, 1:1
ratio
conformers), 7.73 and 7.70 (m, 1H, l:l ratio conformers). Mass spectrum (MALDI-
TOF, CHCA matrix, m/z): Calcd. for C"H"NOZS3F4, 434.0 (M+H), found 434Ø
c) 4-~4-~3-Fluoro-S-(tr~uorometliyl)phenylJ(1,3-thiazol-2 yl))-5-
metlzylthiotlziophene-2-carboxamidine: Methyl4-{4-[3-fluoro-5-
(trifluoromethyl)phenyl](1,3-thiazol-2-yl)}-5-methylthiothiophene-2-
carboxylate (40
mg, 0.92 mmol) was treated in a manner similar to that for Example 10, step
(b), to
give 4-{4-[3-fluoro-5-(trifluoromethyl)phenyl](1,3-thiazol-2-yl)}-5-
methylthiothiophene-2-carboxamidine (31 mg, 81 %) as a yellow solid. 'H-NMR
(DMSO-db; 300 MHz) 8 9.36 (br s, 2H), 9.01 (br s, 2H), 8.68 (s, 1H), 8.63 (s,
1H),
8.30 (m, 1 H), 8.25 and 8.22 (m, 1 H, 1:1 ratio conformers), 7.75 and 7.73 (m,
1 H, 1:1
ratio conformers), 2.82 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z):
Calcd. for C,6H"N3S3F4, 418.5 (M+H), found 418Ø
Example 129
a) 2-Bromo-1-~3 fluoro-S-(tr~uoromethyl)phenylJpropan-1-one: A stirred
suspension of 1 g (4.5 mmol) of 1-[3-fluoro-5-(trifluoromethyl)phenyl]propan-1-
one
(Lancaster, Windham, NH, USA) was treated in a manner similar to that for
Example
127, step (a) to give 2-bromo-1-[3-fluoro-5-(trifluoromethyl)phenyl]propan-1-
one
(1.33 g, 99 %). 'H-NMR (DMSO-db; 300 MHz) 8 8.07 (m, 1H), 7.92 and 7.89 (m,
1 H, 1:1 ratio conformers), 7.57 and 7.55 (m, 1 H, 1:1 ratio conformers), 5.20
(q, 1 H,
J--6.6Hz), 1.93 (d, 3H, J--6.6 Hz).
b) MetlZyl4-(4-~3 fluoro-S-(trifluoromethyl)phenylJ S-methyl(1,3-thiazol 2-
yl)J-5-metlzyltlaiotl:iophene-2-carboxylate: A solution of 75 mg (0.3 mmol) of
methyl 4-(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge,
Cornwall, UK) was reacted with 90 mg (0.3 mmol) of 2-bromo-1-[3-fluoro-S-
(trifluoromethyl)phenyl]propan-1-one in a manner similar to Example 8, step
(a) to
give, methyl 4-{4-[3-fluoro-5-(trifluoromethyl)phenyl]-S-methyl(1,3-thiazol-2-
yl)}-5-
methylthiothiophene-2-carboxylate (31.9 mg, 24 %) as a solid. 'H-NMR (DMSO-d~;
300 MHz) 8 8.17 (s, 1H), 7.98 (m, 1H), 7.95 and 7.92 (m, 1H, 1:1 ratio
conformers),
7.77 and 7.74 (m, 1H, 1:1 ratio conformers), 3.87 (s, 3H), 2.75 (s, 3H), 2.70
(s, 3H).
CA 02362390 2001-08-07
WO 00/47578 _~ 66_ PCT/US99/18065
Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C,8H,3NOZS3F4, 448.0
(M+H), found 448Ø
c) 4-(4-(3-Fluoro-S-(trifluoromethyl)plzenylJ-S-methyl(1,3-thiazol 2 yl)J-5-
metlZylthiothiophene-2-carboxamidine: Methyl4-{4-[3-fluoro-5-
(trifluoromethyl)phenyl]-S-methyl(1,3-thiazol-2-yl)}-5-methylthiothiophene-2-
carboxylate (30 mg, 0.067 mmol) was treated in a manner similar to that for
Example
10, step (b), to give 4-{4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-methyl(1,3-
thiazol-2-
yl)}-5-methylthiothiophene-2-carboxamidine (32 mg, quantitive yield) as a
yellow
solid. 'H-NMR (DMSO-db; 300 MHz) b 9.42 (br s, 2H), 9.03 (br s, 2H), 8.60 (s,
1H),
7.98 (m, 1 H), 7.95 and 7.92 (m, 1 H, 1:1 ratio conformers), 7.79 and 7.76 (m,
1 H, 1:1
ratio conformers), 2.78 (s, 3H), 2.71 (s, 3H). Mass spectrum (MALDI-TOF, CHCA
matrix, m/z): Calcd. for C,~H,3N3S3F4, 432.0 (M+H), found 432.6.
Example 130
a) 1-~3,5-Bis(tr~uorometlZyl)plaenylJ-2-bromopropan-1-oue: A stirred
suspension of 1 g (3.7 mmol) of 1-[3,5-bis(trifluoromethyl)phenyl]-propan-1-
one
(Lancaster, Windham, NJ, USA) treated in a manner similar to that for Example
127,
step (a) to give 2-bromo-1-[3-fluoro-5-(trifluoromethyl)phenyl]propan-1-one
(1.1 g,
86 %). 'H-NMR (DMSO-db; 300 MHz) ~ 8.46 (m, 2H), 8.09 (m, 1), 5.26 (q, 1H,
J--6.6Hz), 1.96 (d, 3H, J--6.5 Hz). Mass spectrum (MALDI-TOF, CHCA matrix,
m/z):
Calcd. for C"H,OBrFb, 349.0 (M+H), found 348.9.
b) Methyl4-~4-~3,5-bis(triJluorometlzyl)pheuylJ S-methyl(1,3-tlziazol 2 yl)J-
5-methyltl:iothiophene-2-carboxylate: A solution of 75 mg (0.3 mmol) of methyl
4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge, Cornwall,
UK) was reacted with 105 mg 1-[3,5-Bis(trifluoromethyl)phenyl]-2-bromopropan-1-
one in a manner similar to Example 8, step (a) to give, after preparative thin-
layer
chromatrography purification, methyl 4-{4-[3,5-bis(trifluoromethyl)phenyl]-5-
methyl(1,3-thiazol-2-yl)}-5-methylthiothiophene-2-carboxylate (16.2 mg, 11 %)
as a
solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.41 (m, 2H), 8.18 (m, 2H), 3.86 (s, 3H),
2.75 (s, 3H), 2.71 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z):
Calcd. for C,9H,3NOZS3F6, 498.0 (M+H), found 497.6.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-167-
c) 4-~4-~3,5-Bis(trifluoromethyl)phenylJ S-methyl(1,3-thiazol-2 yl)~-5-
methylthiothiophene-2-carboxamidine: Methyl4-{4-[3;5-
bis(trifluoromethyl)phenyl]-5-methyl(1,3-thiazol-2-yl)}-5-methylthiothiophene-
2-
carboxylate (15 mg, 0.031 mmol) was treated in a manner similar to that for
Example
10, step (b), to give 4-{4-[3,5-bis(trifluoromethyl)phenyl]-5-methyl(1,3-
thiazol-2-
yl)}-5-methylthiothiophene-2-carboxamidine (13 mg, 88 %) as a yellow solid. 'H-
NMR (DMSO-db; 300 MHz) 8 9.39 (br s, 2H), 8.94 (br s, 2H), 8.58 (s, 1H), 8.40
(m,
2H), 8.19 (m, 1H), 2.79 (s, 3H), 2.73 (s, 3H). Mass spectrum (MALDI-TOF, CHCA
matrix, m/z): Calcd. for C,8H,3N3S3F6, 482.0 (M+H), found 482.5.
Example 131
a) 2-Bromo-1,2-diphenylethan-1-one: A stirred suspension of 0.2 g (1
mmol) of deoxybenzoin was treated in a manner similar to that for Example 127,
step
(a) to give 2-bromo-1,2-diphenylethan-1-one (270 mg, 98 %). 'H-NMR (DMSO-db;
300 MHz) 8 8.10-8.06 (m, 2H), 7.95-7.31 (m, 8H), 7.21 (s, 1H).
b) Methyl4-(4,5-diphenyl(1,3-thiazol 2 yl))-S-methylthiothiophene-2-
carboxylate: A solution of 75 mg (0.3 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge, Cornwall, UK) was reacted with
92
mg, 0.3 mmol) of 2-bromo-1,2-diphenylethan-1-one in a manner similar to
Example
8, step (a) to give, after preparative thin-layer chromatrography
purification, methyl 4-
(4,5-diphenyl(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxylate (9 mg, 7
%) as
a solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.94 (br s, 0.4H), 8.66 (s, 1H), 8.60 (br
s,
0.3 H), 8.08 (s, 1H), 7.93 and 7.20 (AB quartet, 2H, J--8.7 Hz), 7.68 and 7.35
(AB
quartet, 2H, J--8.2 Hz), 2.77 (s, 3H), ), 2.33 (s, 3H). Mass spectrum (MALDI-
TOF,
CHCA matrix, m/z): Calcd. for CzzH,~NO2S3, 424.0 (M+H), found 424.3.
c) 4-(4,5 DipIZenyl(1,3-tlZiazol 2 yl))-S-methylthiothiophene-2-
carboxamidine: Methyl4-(4,5-diphenyl(1,3-thiazol-2-yl))-5-methylthiothiophene-
2-
carboxylate (9 mg, 0.021 mmol) was treated in a manner similar to that for
Example
10, step (b), to give 4-(4,S-diphenyl(1,3-thiazol-2-yl))-5-methylthiothiophene-
2-
carboxamidine (3 mg, 35 %) as a brown oil. Mass spectrum (MALDI-TOF, CHCA
matrix, m/z): Calcd. for CZ,H"N3S3, 408.1 (M+H), found 408Ø
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-168-
Example 132
a) Methyl4-(4-benzo(bJthiophen-2 yl(1,3-thiazol 2 yl))-S-
methylthiothiophene-2-carboxylate: A solution of 75 mg (0.3 mmol) of methyl 4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate was reacted with 77 mg
(0.3 mmol) of 3-bromoacetylbenzo[b]thiophene (Maybridge, Cornwall, UK) in a
manner similar to Example 8, step (a) to give, after preparative thin-layer
chromatrography purification, methyl 4-(4-benzo[b]thiophen-2'-yl(1,3-thiazol-2-
yl))-
5-methylthiothiophene-2-carboxylate (28 mg, 23 %) as a solid. 'H-NMR (DMSO-db;
300 MHz) 8 8.63 (d, 1H, J--7.4 Hz), 8.30 (s, 1H), 8.25 (s, 1H), 8.22 (s, 1H),
7.53-7.46
(m, 2H), 3.87 (s, 3H), 2.78 (s, 3H).
b) 4-(4-Benzo~bJtltiophen-2 yl(1,3-thiazol 2 yl))-5-methyltltiothiophene-2-
carboxamidine: Methyl4-(4-benzo[b]thiophen-2-yl(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxylate (28 mg, 0.69 mmol) was treated in a manner
similar to that for Example 10, step (b), to give 4-(4-benzo[b]thiophen-2-
yl(1,3-
thiazol-2-yl))-5-methylthiothiophene-2-carboxamidine (17 mg, 64 %) as a brown
solid. 'H-NMR (DMSO-db; 300 MHz) 8 9.22 (br s, 4H), 8.68 (s, 1H), 8.66 (d, 1H,
J 7.6 Hz), 8.30 (s, 1 H), 8.25 (s, 1 H), 8.10 (d, 1 H, J--7.3 Hz), 7.55-7.45
(m,
2H), 2.81 (s, 3H). Mass spectrum (MALDI-TOF, GA matrix, m/z): Calcd. for
C"H,3N3S4, 388.0 (M+H), found 388.2.
Example 133
a) Methyl4-(4-benzo~dJbenzo~3,4-bJfuran-3 yl(1,3-thiazol 2 yl))-5-
methylthiothiophene-2-carboxylate: A solution of 75 mg (0.3 mmol) of methyl 4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge, Cornwall,
UK) was reacted with 86 mg (0.3 mmol) of 2-(bromoacetyl)-dibenzofuran
(Aldrich,
Milwaukee, WI, USA) in a manner similar to Example 8, step (a) to give, after
preparative thin-layer chromatrography purification, methyl 4-(4,5-
diphenyl(1,3-
thiazol-2-yl))-5-methylthiothiophene-2-carboxylate (45 mg, 36 %) as a solid.
'H-
NMR (DMSO-db; 300 MHz) 8 8.83-7.44 (m, 7H), 8.29 (s, 1H), 8.27 (s, 1 H), 3.88
(s,
3H), 2.80 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
CZZH~5NO3S3, 438.0 (M+H), found 438.5.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-169-
b) 4-4-Benzo~dJbenzo~3,4-bJfuran-3 yl(1,3-thiazol 2 yl))-5-
methylthiothiophene-2-carboxamidine: Methyl4-(4-benzo[d]benzo[3,4-b]furan-3-
yl(1,3-thiazol-2-yl))-5-methylthiothiophene-2-carboxylate (45 mg, 0.11 mmol)
was
treated in a manner similar to that for Example 10, step (b), to give 4-4-
benzo[d]benzo[3,4-b]furan-3-yl(1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxamidine (16.8 mg, 36 %) as a yellow solid. 'H-NMR (DMSO-db; 300 MHz)
8 9.72-9.10 (m, 3H), 8.84 - 7.31 (m, 9H), 2.84 (s, 3H). Mass spectrum (MALDI-
TOF,
CHCA matrix, m/z): Calcd. for CZ,H,SN3OS3, 422.0 (M+H), found 421.9.
Example 134
a) Metl:yl4-(4-(4-nitrophenyl)(l,~-tl:iazol 2 yl))-S-methylthiothiophene-2-
carboxylate: A solution of 1 g (4 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiothiophene-2-carboxylate (Maybridge, Cornwall, UK) was reacted with
987
mg (4 mmol) of 2-bromo-4'-nitroacetophenone in a manner similar to Example 8,
step
(a) to give methyl 4-(4-(4-nitrophenyl)(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-
carboxylate (1.7 g, quantitive yield) as a brown solid. 'H-NMR (DMSO-db; 300
MHz) ~ 8.57 (s, 1H), 8.34 (s, 4H), 8.25 (s, 1H), 3.94 (s, 3H), 3.81 (s, 3H).
Mass
spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C,6H,zN2O4S3 393.0 (M+H),
found 3 92.8.
b) Metliyl4-(4-(4-aminophenyl)(1,3-tlziazol Z yl))-5-metlzyltlziothioplzene-2-
carboxylate: Methyl4-(4-(4-nitrophenyl)(1,3-thiazol-2-yl))-5-
methylthiothiophene-
2-carboxylate (800 mg, 2 mmol) was dissolved in 150 mL tetrahydrofuran and
treated
with 20 % titanium chloride solution (Fisher Scientific, Pittsburgh, PA, USA)
for 1 h.
The mixture was poured into 2 M sodium hydroxide solution ( 100 mL), extracted
with dichloromethane (4 x 50 mL). The combined organic layers were washed with
saturated brine solution and dried over anhydrous sodium sulfate. The solid
was
filtered off, and the solvent removed in vacuo. This material was purified by
column
chromatography on silica gel (30 g) eluting with dichloromethane:methanol 98/2
(v:v)
to give methyl 4-(4-(4-aminophenyl)(1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (500 mg, 69 %) as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.17 (s,
1H), 7.77 (s, 1H), 7.74 and 6.62 (AB quartet, 2H, J--8.6 Hz), 5.35 (s, 2H),
3.86 (s,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-170-
3H), 2.74 (s, 3H). ). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
C,6H,4NZOZS3 363.0 (M+H), found 362.4.
c) Methyl 4-(4-~4 ~(methylsulfonyl)aminoJplaenylJ(1,3-thiazol-2 yl))-S-
methylthiothiophene-2-carboxylate: Methyl4-(4-(4-aminophenyl)(1,3-thiazol-2-
yl))-S-methylthiothiophene-2-carboxylate (200 mg, 0.55 mmol) was dissolved in
dry
dichloromethane (20 mL). To this, N methyl morpholine (150 ~L, 1.38 mmol) and
dimethylaminopyridine (6.1 mg, 0.055 mmol) were added, the mixture was cooled
on
an ice bath, and methanesulfonyl chloride (43 ~.L, 0.55 mmol) was added
dropwise.
The mixture was then stirred for 8 days at room temperature. The mixture was
partitioned between saturated sodium bicarbonate (SO mL) and dichloromethane
(20
mL). The aqueous layer was extracted with dichloromethane (3 x 20 mL), and the
combined organic layers were washed with saturated sodium bicarbonate (20 mL),
brine (2 x 20 mL), and dried over anhydrous sodium sulfate. The solvent was
removed in vacuo. Column chromatrography on silica gel ( 100 g) eluting with
dichloromethane:methanol 99/1 (v:v), gave methyl 4-(4-{4-
[(methylsulfonyl)amino]phenyl } ( 1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (155 mg, 64 %) as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 9.92 (s,
1H), 8.22 (s, 1H), 8.11 (s, 1H), 8.40 and 6.90 (AB quartet, 2H, J--8.7 Hz),
3.87 (s,
3H), 3.05 (s, 3H), 2.76 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix m/z):
Calcd. for C,~H,6Nz04S4441.0 (M+H), found 441.2.
d) Q (4-~4 ~(Methylsulfonyl)aminoJphenylJ(1,3-thiazol-2 yl))-5-
methyltl:iothiophene-2-carboxamidine: Methyl4-(4-{4-
[(methylsulfonyl)amino)phenyl} (1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (81 mg, 0.184 mmol) was treated in a manner similar to that for
Example
10, step (b), to give 4-(4-{4-[(methylsulfonyl)amino]phenyl}(1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxamidine (24.9 mg, 32 %) as a light brown solid. 'H-
NMR (DMSO-db; 300 MHz) 8 10.0 (br s, 1 H), 9.3 (br s, 2H), 8.98 (s, 1 H), 8.65
(s,
1H), 8.21 (s, 1H), 7.98 and 7.5 (AB quartet, 2H, J--8.6 Hz), 3.05 (s, 3H),
2.79 (s, 3H).
Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C~6H,6N4OZS4425.0
(M+H), found 425.1.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-111-
Example 135
a) Metlzyl 4-(4-~4 ~(phenylsulfonyl)aminoJphenylJ(1,3-thiazol 2 yl))-S-
methylthiotlaiophene-2-carboxylate: Methyl4-(4-(4-aminophenyl)(1,3-thiazol-2-
yl))-5-methylthiothiophene-2-carboxylate (100 mg, 0.28 mmol) was dissolved in
dry
dichloromethane (10 mL). To this, N-methyl morpholine (46 ~L, 0.42 mmol) and
dimethylaminopyridine (3.4 mg, 0.028 mmol) were added, the mixture was cooled
on
an ice bath, and benzenesulfonyl chloride 35 ~.L, 0.28 mmol) was added
dropwise.
The mixture was then stirred for 24 h at room temperature. Workup was carried
out
as in Example 134, step (c). Trituration with dichloromethane and methanol
gave
methyl4-(4-{4-[(phenylsulfonyl)amino]phenyl}(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxylate (44 mg, 31 %) as a crystalline solid. 'H-NMR
(DMSO-db; 300 MHz) 8 10.46 (s, 1H), 8.19 (s, 1H), 8.05 (s, 1H), 7.91 and 7.19
(AB
quartet, 2H, J--8.7 Hz), 7.81 (m, 2H), 7.64-7.54 (m, 3H) 3.85 (s, 3H), 2.74
(s, 3H).
Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for CZZH,gN2O4S4 504.2
(M+H), found 504.1
b) 4-(4-~4-~(Plzenylsulfonyl)aminoJplzenylJ(1,3-thiazol 2 yl))-5-
methylthiotlziophene-2-carboxamidine: Methyl4-(4-{4-
[(phenylsulfonyl)amino]phenyl } ( 1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (30 mg, 0.060 mmol) was treated in a manner similar to that for
Example
10, step (b), to give 4-(4-{4-[(phenylsulfonyl)amino]phenyl}(1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxamidine (12.6 mg, 43 %) as a yellow solid. 'H-NMR
(DMSO-db; 300 MHz) 8 9.13 (br s, 3H), 8.60 (s, 1H), 8.08 (s, 1H) 7.93 and 7.20
(AB
quartet, 2H, J--8.7 Hz), 7.82-7.79 (m, 2H), 7.65-7.53 (m, 3H) 3.85 (s, 3H),
2.74 (s,
3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for CZ,H,8N40zS4,
87.0 (M+H), found 487.7.
Example 136
a) Methyl 4-(4-~4 ~(tr~uorometlzylsulfonyl)aminoJphenyl~(1,3-thiazol 2-
yl))-S-methylthiothiophene-2-carboxylate: Methyl4-(4-(4-aminophenyl)(1,3-
thiazol-
2-yl))-5-methylthiothiophene-2-carboxylate (200 mg, 0.55 mmol) was dissolved
in
dry pyridine (20 mL). The mixture was cooled on an ice bath, and
trifluoromethanesulfonic anhydride (0.5 mL, 3 mmol) was added. The mixture was
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-172-
then stirred for 1.5 h at room temperature. Workup was carried out as in
Example
134, step (c). Column chromatrography on silica gel (30 g) eluting with
hexanes:ethyl
acetate 7/3 (v:v), followed by preparative thin layer chromatography eluting
with
dichloromethane:methanol 99/1 (v:v) gave methyl 4-(4-{4-
[(trifluoromethylsulfonyl)amino]phenyl}(1,3-thiazol-2-yl))-S-
methylthiothiophene-2-
carboxylate (160 mg, 59 %) as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.48 and
7.87(s, 3/2 ratio conformers, 1 H), 8. 23 (s, 1 H), 8.21 (s, 1 H), 8.29 and
7.84 (AB
quartet, 2H, 2/3 ratio conformers, J--8.7 Hz), 8.10 and 7.37 (AB quartet, 2H,
J--8.7
Hz), 3.87 and 3.86 (s, 2/3 ratio conformers, 3H), 2.77 and 2.76 (s, 2/3 ratio
conformers, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
C,~H,3NZO4S4F4 495.0 (M+H), found 495.6
b) 4-(4-~4-~(Tr~uoromethylsulfonyl)aminoJphenylJ(1,3-thiazol-2 yl))-S-
metlZylthiothiophene-2-carboxamidine: Methyl4-(4-{4-
[(trifluoromethylsulfonyl)amino]phenyl } ( 1,3-thiazol-2-yl))-5-
methylthiothiophene-2-
carboxylate (30 mg, 0.061 mmol) was treated in a manner similar to that for
Example
10, step (b), to give of 4-(4-{4-[(trifluoromethylsulfonyl)amino]phenyl}(1,3-
thiazol-2-
yl))-5-methylthiothiophene-2-carboxamidine (21.6 mg, 74 %) as a light brown
solid.
'H-NMR (DMSO-db; 300 MHz) 8 9.39 (br s, 2H), 8.97 (br s, 2H), 8.64 (s, 1H),
8.24
(s, 1H), 8.12 and 7.39 (AB quartet, 2H, J--8.7 Hz), 4.78 (br s, 1H), 2.79 (s,
3H). Mass
spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C~6H,3N4OZS4F3, 479.0
(M+H), found 479.5.
Example 137
a) Methyl 4-(4-~4 ~(toluenesulfonyl)aminoJplzenylJ(1,3-thiazol 2 yl))-5-
methylthiothiophene-2-carboxylate: Methyl4-(4-(4-aminophenyl)(1,3-thiazol-2-
yl))-5-methylthiothiophene-2-carboxylate (33 mg, 0.09 mmol) was dissolved in
dry
dichloromethane (5 mL). To this, N methyl morpholine (10 ~.L, 0.09 mmol) and p-
toluenesulfonyl chloride ( 17 mg, 0.09 mmol) was added and the mixture was
stirred at
room temperature for 5 days. Workup was carried out as in Example 134, step
(c).
Trituration with dichloromethane and methanol gave methyl 4-(4-{4-
[(toluenesulfonyl)amino]phenyl } ( 1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (20 mg, 43 %) as a brown solid. 'H-NMR (DMSO-db; 300 MHz) 8 10.39
CA 02362390 2001-08-07
WO 00/47578 _,~ ~3_ PCT/US99/18065
(s, 1 H), 8.19 (s, 1 H), 8.05 (s, 1 H), 7.91 and 7.18 (AB quartet, 2H, J--8.7
Hz), 7.68 and
7.35 (AB quartet, 2H, J--8.2 Hz), 3.85 (s, 3H), 2.74 (s, 3H), 2.27 (s, 3H).
Mass
spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C,3HZONZO4S4, 517.2
(M+H), found 517Ø
b) 4-(4-(4-~(Toluenesulfonyl)aminoJphenyl)(1,3-tlziazol 2 yl))-S-
methyltlziotlZiophene-2-carboxamidine: Methyl4-(4-{4-
[(toluenesulfonyl)amino]phenyl } ( 1,3-thiazol-2-yl))-5-methylthiothiophene-2-
carboxylate (15 mg, 0.029 mmol) was treated in a manner similar to that for
Example
10, step (b), to give 4-(4-{4-[(toluenesulfonyl)amino]phenyl}(1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxamidine (17.9 mg, 81 %) as a light brown solid. 'H-
NMR (DMSO-db; 300 MHz) 8 8.94 (br s, 0.4H), 8.66 (s, 1H), 8.60 (br s, 0.3 H),
8.08
(s, 1H), 7.93 and 7.20 (AB quartet, 2H, J--8.7 Hz), 7.68 and 7.35 (AB quartet,
2H,
J--8.2 Hz), 2.77 (s, 3H), 2.33 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix,
m/z): Calcd. for CZZH20N4~2'S4~ 501.1 (M+H), found 501.1.
Example 138
a) MetIZyl4 ~4-(4-clalorophenyl)(1,3-thiazol 2 yl)J S-
(methylsulfinyl)thiophene-2-carboxylate: To a stirred solution of 764 mg (2
mmol)
of methyl 4-[4-(4-chlorophenyl)(1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxylate (Maybridge, Cornwall, UK) dissolved in 1,1,1,3,3,3-
hexafluoroisopropanol (2.5 mL) was added 30% hydrogen peroxide (0.45 mL, 4
mmol). This solution was stirred for 45 h at room temperature. Dichloromethane
( 10
mL) was added after 2 hours. Additional hydrogen peroxide (2 x 0.45 mL
portions)
was added after 4 hours and 24 hours. The mixture was quenched with 10% sodium
sulfite in brine (4 mL). The organic layer was separated, dried over anhydrous
sodium sulfate, and the solvents removed in vacuum. Column chromatography on
silica gel (45 g), eluting with dichloromethane:methanol 99/1 (v:v) gave
methyl 4-[4-
(4-chlorophenyl)(1,3-thiazol-2-yl)]-5-(methylsulfinyl)thiophene-2-carboxylate
(720
mg, 90 %) as a solid. 'H-NMR (DMSO-db; 300 MHz) b 8.37 (s, 1H), 8.30 (s, 1H),
8.05 and 7.52 (AB quartet, 2H, J--8.6 Hz), 3.91 (s, 3H), 3.16 (s, 3H). Mass
spectrum
(MALDI-TOF, GA matrix, m/z): Calcd. for C,6H,ZN03S3C1:398.0 (M+H), found
397.8.
CA 02362390 2001-08-07
WO 00/47578 _ ~ 74_ PCT/US99/18065
b) 4-(4-(4-Chloropheuyl)(1,3-thiazol 2 yl)J 5-(methylsulfiuyl)thiophene-2
carboxamidine: Methyl4-[4-(4-chlorophenyl)(1,3-thiazol-2-yl)]-5-
(methylsulfinyl)thiophene-2-carboxylate (100 mg, 0.25 mmol) was treated in a
manner similar to that for Example 10, step (b), to give, after preparative
thin layer
chromatography purification eluting with dichloromethane:methanol:acetic acid
9/1/0.5 (v:v:v), 4-[4-(4-chlorophenyl)(1,3-thiazol-2-yl)]-5-
(methylsulfinyl)thiophene-
2-carboxamidine (18.2 mg, 19 %) as a solid. 'H-NMR (DMSO-d~; 300 MHz) 8 8.33
(s, 1H), 8.22 (s, 1H), 8.05 and 7.57 (AB quartet, 2H, J--8.6 Hz), 3.12 (s,
3H). Mass
spectrum (MALDI-TOF, CHCA matrix m/z): Calcd. for C,SH,ZN30S3C1382.0 (M+H),
found 3 82.1.
Example 139
a) Metlryl 4-cyano-S-(methylsulfonyl)thiophene-2-carboxylate: To a stirred
solution of (4.5 g, 21 mmol) of methyl 4-cyano-5-methylthiothiophene-2-
carboxylate
(Maybridge, Cornwall, UK) was dissolved in dichloromethane (250 mL) and
treated
with m-chloroperbenzoic acid (15.3 g, 90 mmol) at room temperature for 2.25 h.
The
mixture was filtered and the solid washed with dichloromethane (2 x 50 mL).
The
filtrate was washed with sodium bicarbonate (2 x 100 mL), sodium thiosulfate
(100
mL), sodium bicarbonate ( 100 mL), water ( 100 mL), brine ( 100 mL), and dried
over
anhydrous sodium sulfate. The solvent was removed in vacuo to give methyl 4-
cyano-S-(methylsulfonyl)thiophene-2-carboxylate (4.91 g, 95%) as a solid. 'H-
NMR
(DMSO-db; 300 MHz) 8 8.44 (s, 1H), 3.91 (s, 3H), 3.58 (s, 3H).
b) Metlzyl4-cyano-5-methoxythiophene-2-carboxylate: Methyl4-cyano-5-
(methylsulfonyl)thiophene-2-carboxylate (2 g, 8 mmol) was refluxed with 0.5 M
sodium methoxide in methanol (16 mL) for 15 minutes. The solution was cooled,
the
crystallized solid collected on a Buchner funnel and washed with methanol (50
mL) to
give methyl 4-cyano-5-methoxythiophene-2-carboxylate (1.145 g, 73%) as a
solid.
'H-NMR (DMSO-db; 300 MHz) 8 8.87 (s, 1H) 4.19 (s, 3H), 3.82 (s, 3H).
c) Methyl4-(aminothioxomethyl)-S-methoxythiophene-2-carboxylate:
Methyl 4-cyano-5-methoxythiophene-2-carboxylate (1 g, 5 mmol) was dissolved in
dry methanol (150 mL) and triethylamine (3.5 mL, 25.4 mmol) was added. After
degassing the solution with argon for 10 minutes, hydrogen sulfide gas was
bubbled
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-175-
through the solution for 5 h. After stirring 18 h at room temperature, the
solution was
degassed by bubbling argon (6 h), concentrated to 20 mL and acetone (20 mL)
was
added. The dark solid was collected on a Buchner funnel and washed with
acetone.
Recrystallize solid from hot ethanol ( 15 mL) to give methyl 4-
(aminothioxomethyl)-
5-methoxythiophene-2-carboxylate (683 mg, 59 %) as a brown oil. Mass spectrum
(MALDI-TOF, CHCA matrix, m/z): Calcd. for C8H9N03S2 232.0 (M+H), found
232.4
d) Methyl 5-methoxy-4-(4 phenyl(1,3-thiazol 2 yl))thiophene-2-carboxylate:
A solution of 400 mg (1.73 mmol) of methyl 4-(aminothioxomethyl)-5-
methoxythiophene-2-carboxylate was reacted with 345 mg (1.73 mmol) of 2-
bromoacetophenone (Aldrich, Milwaukee, WI, USA) in a manner similar to Example
8, step (a) to give methyl S-methoxy-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-
carboxylate (56 mg, 10 %) as a solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.22 (s,
1H),
8.14 (s, 1H), 8.05 (m, 2H), 7.47 (m, 2H), 7.36 (m, 1H), 4.26 (s, 3H), 3.85 (s,
3H).
e) S-Methoxy-4-(4 phenyl(1,3-thiazol 2 yl))thiophene-2-carboxamidine:
Methyl 5-methoxy-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate (55 mg,
0.16 mmol) was treated in a manner similar to that for Example 10, step (b),
to give 5-
methoxy-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamidine (36 mg, 69 %)
as a
yellow solid. 'H-NMR (DMSO-db; 300 MHz) 8 9.34 (br s, 2H), 8.94 (br s, 2H),
8.70
(s, 1H), 8.20 (s, 1H), 8.07 (m, 2H), 7.49 (m, 2H), 7.38 (m, 1H), 4.32 (s, 3H).
Mass
spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C,SH,3N3OSz 316.5 (M+H),
found 316.1
Example 140
a) Metltyl4-cyano-S-~(4-metltoxyphenyl)metlzylthioJthiophene-2-
carboxylate: To a stirred solution of 2.5 g ( 10 mmol) of methyl 4-cyano-5-
(methylsulfonyl)thiophene-2-carboxylate (Example 139, step (a)) in dry
methanol (15
mL) was addedp-methoxybenzylmercaptan (3.8 mL, 28 mmol) and triethylamine (1.4
mL, 10 mmol). This solution was refluxed for 15 min and cooled. The resulting
solid
was collected on a biichner funnel and washed with methanol (2 x 25 mL) to
methyl
4-cyano-5-[(4-methoxyphenyl)methylthio]thiophene-2-carboxylate (2.84 g, 89 %)
as a
solid.
CA 02362390 2001-08-07
WO 00/47578 _~ ~6_ PCT/US99/18065
b) Methyl 4-(aminothioxomethyl)-S ~(4-
methoxyphenyl)methylthioJthiophene-2-carboxylate: Methyl4-cyano-5-[(4-
methoxyphenyl)methylthio]thiophene-2-carboxylate (2.5 g; 7.8 mmol) was treated
as
in Example 139, step (c) to give methyl 4-(aminothioxomethyl)-S-[(4-
methoxyphenyl)methylthio]thiophene-2-carboxylate (1.32 g, 48 %) as a solid. 'H-
NMR (DMSO-db; 300 MHz) 8 9.64 (s, 1H), 9.28 (s, 1H), 8.08 (s, 1H), 7.35 and
6.92
(AB quartet, 2H, J--8.7 Hz), 4.27 (s, 2H), 3.82 (s, 3H), 3.75 (s, 3H).
c) Methyl S-(methoxyphenylthio)-4-(4 plzenyl(1,3-thiazol 2 yl))thiophene-2
carboxylate: A solution of 1.2 g (3.4 mmol) of methyl 4-(aminothioxomethyl)-5-
[(4
methoxyphenyl)methylthio]thiophene-2-carboxylate was reacted with 676 mg (3.4
mmol) of 2-bromoacetophenone (Aldrich, Milwaukee, WI, USA) in a manner similar
to'Example 8, step (a) to give methyl 5-(methoxyphenylthio)-4-(4-phenyl(1,3-
thiazol-
2-yl))thiophene-2-carboxylate (755 mg, 49 %) as a solid. 'H-NMR (DMSO-db; 300
MHz) S 8.26 (s, 1 H), 8.22 (s, 1 H), 8.04 (m, 2H), 7.48 (m, 2H), 7.3 8 (m, 1
H), 7.3 3 and
6.89 (AB quartet, 2H, J--8.7 Hz), 4.40 (s, 2H), 3.86 (s, 3H), 3.72 (s, 3H).
d) S-(Methoxyphenylthio)-4-(4 phenyl(1,3-tlziazol 2 yl))thiophene-2-
carboxamidine: Methyl5-(methoxyphenylthio)-4-(4-phenyl(1,3-thiazol-2-
yl))thiophene-2-carboxylate (100 mg, 0.22 mmol) was treated in a manner
similar to
that for Example 10, step (b), to give 5-methoxy-4-(4-phenyl(1,3-thiazol-2-
yl))thiophene-2-carboxamidine (94 mg, 91 %) as an orange solid. 'H-NMR (DMSO-
db; 300 MHz) 8 9.49 (br s, 2H), 9.15 (br s, 2H), 8.70 (s, 1 H), 8.26 (s, 1 H),
8.07 (m,
2H), 7.49 (m, 2H), 7.40 (m, 1H), 7.35 and 6.90 (AB quartet. 2H, J--8.7 Hz),
4.41 (s,
2H), 3.73 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
CZZH,9N3OS3 438.5 (M+H), found 438.1.
Example 141
a) Methyl4-~4-(4-chloroplzenyl)(1,3-thiazol-2 yl)J 5-
(methylsulfonyl)thioplzene-2-carboxylate: To a stirred solution of 1 g (2.6
mmol) of
methyl 4-[4-(4-chlorophenyl)( 1,3-thiazol-2-yl)]-5-methylthiothiophene-2-
carboxylate
(Maybridge, Cornwall, UK) was dissolved in dry dichloromethane (50 mL) and
treated with m-chloroperbenzoic acid (1.94 g, 11.3 mmol) at room temperature
for
1.5 h. The solution was filtered and the solid washed with dichloromethane.
The
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-177-
filtrate was washed with sodium bicarbonate solution (2 x 20 mL), sodium
thiosulfate
solution (20 mL), sodium bicarbonate solution (20 mL), brine (20 mL), and
dried over
anhydrous sodium sulfate. The solvent was removed in vacuo to give methyl 4-[4-
(4-
chlorophenyl)(1,3-thiazol-2-yl)]-5-(methylsulfonyl)thiophene-2-carboxylate
(826 mg,
77 %) as a tan solid. Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
C,6H,ZN04S3C1414.0 (M+H), found 414.8.
b) 4 ~4-(4-Chlorophenyl)(1,3-thiazol 2 yl)J S-(methylsulfonyl)thiophene-Z-
carboxamidine: Methyl4-[4-(4-chlorophenyl)(1,3-thiazol-2-yl)]-5-
(methylsulfonyl)thiophene-2-carboxylate (200 mg, 0.4 mmol) was treated in a
manner
similar to that for Example 10, step (b), to give 4-[4-(4-chlorophenyl)(1,3-
thiazol-2
yl)]-5-(methylsulfonyl)thiophene-2-carboxamidine (85 mg, 53 %) as a yellow
solid.
c) 4-~4-(4-Chlorophenyl)(1,3-thiazol-2 yl)J 5-(phenylmethylthio)thiophene
2-carboxamidine: A stirred solution of 80 mg (0.2 mmol) of 4-[4-(4-
chlorophenyl)( 1,3-thiazol-2-yl)]-5-(methylsulfonyl)thiophene-2-carboxamidine
benzyl mercaptan ( 11 S ~L, 0.980 ~mol) was treated in a manner similar to
that for
Example 140, step (a) to give, after silica gel column chromatography (20 g)
eluting
with dichloromethane:methanol:acetic acid 9/1/0.5 (v:v:v), 4-[4-(4-
chlorophenyl)(1,3-
thiazol-2-yl)]-5-(phenylmethylthio)thiophene-2-carboxamidine (75 mg, 85 %) as
a
pale orange solid. 'H-NMR (DMSO-db; 300 MHz) 8 9.44 (br s, 2H), 9.03 (br s,
2H),
8.67 (s, 1H), 8.33 (s, 1H), 8.08 and 7.56 (AB quartet, 2H, J 8.7 Hz), 7.54-
7.17 (m.
SH), 4.45 (s, 2H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
CZ,H,6N3S3C1442.0 (M+H), found 442.7.
Example 142
a) 1 ~S-(tert-Butyl)-2-methyl(3 furyl)J 2-bromoethan-1-one: A solution of 1
g (5 mmol) of 5-(tert-butyl)-2-methylfuran-3-carbonyl chloride (Maybridge,
Cornwall, UK) dissolved in dry acetonitrile (4 mL) and 6.25 mL (12.5 mmol) of
2 M
trimethylsilyldiazomethane in hexanes (Aldrich, Milwaukee, WI) was stirred
1.75 h at
room temperature and the mixture was cooled on an ice bath for 5 min. To this,
30%
hydrogen bromide in acetic acid (2 mL, 10 mmol) was added dropwise over 10
min.
This was stirred an additional 20 minutes on an ice bath. Evaporation of the
solvents
gave 1-[5-(tent-butyl)-2-methyl(3-furyl)]-2-bromoethan-1-one (1 g, 77 %) as a
brown
CA 02362390 2001-08-07
WO 00/47578 -' 78_ PCT/US99/18065
oil.'H-NMR (DMSO-d6; 300 MHz) 8 6.50 (s, 1H), 4.57(s, 2H), 2.52 (s, 1H), 1.24
(s,
9H). Mass spectrum (LCA, m/z): Calcd. for C"H,SOzBr, 259.1 and 261.1 (M+H),
found 259.1 and 261.1.
b) Methyl 4-~4-~5-(tert butyl)-2-methyl(3 furyl)J(1,3-thiazol 2 yl)J-S-
methylthiothiophene-2-carboxylate: A solution of 955 mg (3.86 mmol) of methyl
4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge, Cornwall,
UK) was reacted with 1 g (3.86 mmol) of 1-[5-(tert-butyl)-2-methyl(3-furyl)]-2-
bromoethan-1-one (1 g) in a manner similar to Example 8, step (a) to give
methyl 4-
{ 4-[5-(tert-butyl)-2-methyl(3-furyl)] ( 1,3-thiazol-2-yl) } -5-
methylthiothiophene-2-
carboxylate (999 mg, 64 %) as a red-brown solid. 'H-NMR (DMSO-db; 300 MHz)
8 8.14 (s, 1H), 7.74 (s, 1H), 6.46 (s, 1H), 3.86 (s, 3H), 2.74 (s, 3H), 2.66
(s, 3H), 1.27
(s, 9H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C,9HZ,NO3S3,
408.1 (M+H), found 408Ø
c) 4-~4-~S-(tert-Butyl)-2-methyl(3 furyl)J(1,3-thiazol 2 yl)f-S-
methyltlziotlziophene-2-carboxamidine: Methyl4-{4-[5-(tert-butyl)-2-methyl(3-
furyl)](1,3-thiazol-2-yl)}-5-methylthiothiophene-2-carboxylate (940 mg, 2.3
mmol)
was treated in a manner similar to that for Example 10, step (b) to give 4-{4-
[5-(tert-
butyl)-2-methyl(3-furyl)]( 1,3-thiazol-2-yl) }-5-methylthiothiophene-2-
carboxamidine
(930 mg, quantitive yield) as a yellow solid. 'H-NMR (DMSO-db; 300 MHz) 8 9.42
(br s, 2H), 9.03 (br s, 2H), 8.59 (s, 1H), 7.77 (s, 1H), 6.47 (s, 1H), 2.78
(s, 3H), 2.68
(s, 3H), 1.27 (s, 9H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
C~BHZ,N3OS3, 392.1 (M+H), found 392.1.
Example 143
a) 1 ~3-(tert Butyl)-1-benzylpyrazol S ylJ 2-bromoethan-1-one: A solution
of 1 g (3.6 mmol) of 3-(tert-butyl)-1-benzylpyrazole-5-carbonyl chloride
(Maybridge,
Cornwall, UK) was dissolved in dry acetonitrile (4 mL) and 4.5 mL (9 mmol) of
2 M
trimethylsilyldiazomethane in hexanes (Aldrich, Milwaukee, WI, USA) was added.
After stirring 1 h 20 min at room temperature, the mixture was cooled on an
ice bath
for 5 min. To this, 30% hydrogen bromide in acetic acid (2 mL, 10 mmol) was
added
dropwise over 15 min. This was stirred an additional 15 minutes on an ice
bath.
Filtration of the precipitated solid and evaporation of the solvents gave 1-[3-
(tert-
CA 02362390 2001-08-07
WO 00/47578 _,1 ~9_ PCT/US99/18065
butyl)-1-benzylpyrazol-5-yl]-2-bromoethan-1-one (1.47 g, quantitive yield) as
an
orange solid. 'H-NMR (DMSO-db; 300 MHz) 8 7.33-7.06 (m, SH), 7.08 (s, 1H),
5.64
(s, 2H), 4.57 (s, 2H), 1.28 (s, 9H). Mass spectrum (MALDI-TOF, CHCA matrix.
m/z): Calcd. for C,6H,9NZOBr, 335.1 and 337.1 (M+H), found 335.6 and 337.6.
b) Methyl 4-(4 ~3-(tert Butyl)-1-benzylpyrazol 5 y1J(1,3-thiazol Z yl)J-5-
methylthiothiophene-2-carboxylate: A solution of 823 mg (3.3 mmol of methyl 4-
(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate (Maybridge, Cornwall,
UK) was reacted with 1.36 g (3.3 mmol) of 1-[3-(tert-butyl)-1-benzylpyrazol-5-
yl]-2-
bromoethan-1-one in a manner similar to Example 8, step (a) to give methyl 4-
{4-[3-
(tert-butyl)-1-benzylpyrazol-5-yl](1,3-thiazol-2-yl)}-5-methylthiothiophene-2-
carboxylate (1.25 g, 79 %) as a crystalline solid. 'H-NMR (DMSO-db; 300 MHz)
8 8.11 (s, 1H), 8.05 (s, 1H), 7.28-6.99 (m, SH), 6.70 (s, 1H), 5.88 (s, 2H),
3.86 (s, 3H),
2.70 (s, 3H), 1.30 (s, 9H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z):
Calcd.
for C24H2SN302"~3~ 484.1 (M+H), found 483.9.
c) 4-~4-~3-(tert Butyl)-1-benzylpyrazol-5 ylJ(1,3-thiazol 2 yl)J-S-
methylthiotlziophene-2-carboxamidine: Methyl4-{4-[3-(tert-butyl)-1-
benzylpyrazol-5-yl](1,3-thiazol-2-yl)}-5-methylthiothiophene-2-carboxylate
(1.2 mg,
2.6 mmol) was treated in a manner similar to that for Example 10, step (b) to
give 4-
{4-[3-(tert-butyl)-1-benzylpyrazol-5-yl]( 1,3-thiazol-2-yl) }-5-
methylthiothiophene-2-
carboxamidine (1.21 g, quantitive yield) as a yellow solid. 'H-NMR (DMSO-db;
300
MHz) 8 9.43 (br s, 1 H), 9.07 (br s, 1 H), 8.60 (s, 1 H), 8.04 (s, 1 H), 7.37-
6.97 (m, SH),
6.70 (s, 1H), 5.92 (s, 2H), 2.73 (s, 3H), 1.30 (s, 9H). Mass spectrum (MALDI-
TOF,
CHCA matrix, m/z): Calcd. for C23H25NSS3, 468.1 (M+H), found 468.1.
Example 144
a) 4-Bromo-S-metlzylthiophene-2-carboxylic acid: A stirred solution of 1 g
(3.9 mmol) of 2-methyl-3,5-dibromothiophene (prepared by the method of Kano,
S.et
al., Heterocycles 20(10):2035, 1983) in dry tetrahydrofuran (10 mL) was cooled
to
78 °C and 2 M n-butyllithium in cyclohexane (1.93 mL, 3.87 mmol) was
added over 3
min. After stirring 3 min at -78 °C, the mixture was added to
tetrahydrofuran (100
mL) with dry ice suspended. This mixture was allowed to stir and warm to room
temperature. To this, 6 N hydrochloric acid (50 mL) was added carefully. Then,
CA 02362390 2001-08-07
WO 00/47578 _180_ PCT/US99/18065
water (50 mL) was added and the layers were separated. The aqueous layer was
extracted with diethyl ether (4 x 30 mL). The combined organic layers were
washed
with water, brine, and dried over anhydrous sodium sulfate. The solvents were
removed in vacuo to give an 85/15 mixture of 4-bromo-5-methylthiophene-2-
carboxylic acid and 5-bromothiophene-2-carboxylic acid (780 mg, 90 %) as a tan
solid. 'H-NMR (DMSO-db; 300 MHz) b 13.33 (br s, 1H), 7.62 (s, 1H), 7.56 and
7.34
(AB quartet, 0.35H, J--3.9 Hz), 2.41 (s, 3H). Gas Chromotography/Mass
spectroscopy (m/z): Calcd. for C6HSOzSBr, 220.9 and 222.9 (M+H), found 221.3
and
223.3. Calcd. for CSH30zSBr, 206.9 and 208.9 (M+H), found 207.3 and 209.3.
b) Methyl 9-bromo-5-methylthiophene-2-carboxylate: A solution of 780 mg
(3.5 mmol) of an 85/15 mixture of 4-bromo-5-methylthiophene-2-carboxylic acid
and
5-bromothiophene-2-carboxylic acid was dissolved in methanol (50 mL) and
treated
with 9 ml (18 mmol) 2 M trimethylsilyldiazomethane in hexanes (Aldrich,
Milwaukee, WI, USA). Evaporation of the solvents gave an 8/2 mixture of methyl
4-
bromo-5-methylthiophene-2-carboxylate and methyl 5-bromothiophene-2-
carboxylate
(858 mg, quantitive yield) as a brown oil. Gas Chromotography/Mass
spectroscopy
(m/z): Calcd. for C,H~OzSBr, 234.9 and 236.9 (M+H), found 235.3 and 237.3.
Calcd.
for C6H402SBr, 220.9 and 222.9 (M+H), found 221.3 and 223.3.
c) Methyl 4-cyano-S-methylthiophene-2-carboxylate: A solution of an 8/2
mixture of 823 mg (3.5 mmol) of methyl 4-bromo-5-methylthiophene-2-carboxylate
and methyl 5-bromothiophene-2-carboxylate was dissolved in dry
dimethylformamide
(5 mL) and refluxed with copper cyanide (345 mg, 3.9 mmol) for 7 hours. The
cooled
solution was poured into 0.1 M aqueous sodium cyanide solution (200 mL) and
extracted with diethyl ether (5 x 30 mL). The organic layers were washed with
brine
(2 x 30 mL), dried over anhydrous sodium sulfate, and the solvents removed in
vacuo.
The resulting brown solid was purified by column chromatography on silica geI
eluting with hexanes:ethyl acetate 9/1 (v:v) to give a 95/5 mixture of methyl
4-cyano-
5-methylthiophene-2-carboxylate and methyl 5-methylthiophene-2-carboxylate
(369
mg, 68 %) as a yellow solid. 'H-NMR (DMSO-db; 300 MHz) 8 8.06 (s, 1H), 8.05
and
7.90 (2H, 0.1 H, J--4.0 Hz, minor component), 3.87 (s, 3H, minor component),
3.84
(s, 3H) 2.68 (s, 3H).
CA 02362390 2001-08-07
WO 00/47578 _,l $' _ PCT/US99/18065
d) Methyl4-(aminothioxomethyl)-S-methylthiophene-2-carboxylate: A
stirred solution of 804 mg (4.4 mmol) of methyl 4-cyano-5-methylthiophene-2-
carboxylate was treated in a manner similar to Example 139, step (c) to give,
after
fractional crystallization ethanol of the unreacted starting nitrite, a 2:3
ratio of methyl
4-(aminothioxomethyl)-5-methylthiophene-2-carboxylate and methyl 4-cyano-5-
methylthiophene-2-carboxylate (457 mg, 48 %) as a light brown solid. 'H-NMR
(DMSO-db; 300 MHz) 8 9.93 (br s, 1H, minor), 9.34 (br s, 1H, minor), 8.06 (s,
1H,
major), 7.77 (s, 1H, minor component), 3.84 (s, 3H, minor), 3.81 (s, 3H,
major), 2.68
(s, 3H, major), 2.61 (s, 2H, minor). Mass spectrum (MALDI-TOF, CHCA matrix,
m/z): Calcd. for CgH9NOZS2 216.0 (M+H), found 216.4.
e) Methyl S-methyl 4-(4 phenyl(1,3-thiazol 2 yl))thiophene-2-carboxylate:
A solution of 200 mg (0.93 mmol) of methyl 4-(aminothioxomethyl)-5-
methylthiophene-2-carboxylate was reacted with 185 mg (0.93 mmol) of 2-
bromoacetophenone in a manner similar to Example 8, step (a) to give, after
purification by preparative thin layer chromatography eluting with
hexanes:ethyl
acetate 7/3 (v:v), a mixture of methyl 5-methyl-4-(4-phenyl(1,3-thiazol-2-
yl))thiophene-2-carboxylate and methyl 4-cyano-5-methylthiophene-2-carboxylate
(96 mg, 36 %) as a solid.
~ S-Methyl 4-(4 phenyl(1,3-thiazol-2 yl))thiophene-2-carboxamidine:
Methyl-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate (64 mg, 0.23
mmol)
was treated in a manner similar to Example 10, step (b) to give, after
preparative high
pressure liquid chromatography (Dynamax C18 column, 300 ~ pore size, 10 ~m
particle size, 40% to 100% acetonitrile over 30 minutes in 0.1% aqueous
trifluoroacetic acid) 5-methyl-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-
carboxamidine (0.6 mg, 0.9 %) as an an off white solid. 'H-NMR (CD30D, 300
MHz) b 8.44 (s, 1 H), 8.02 (m, 2H), 7.92 (s, 1 H), 7.45 (m, 2H), 7.36 (m, 1
H), 2.96 (s,
3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for C,SH,3N3Sz
300.1 (M+H), found 300.6.
g) 5-(4-Phenyl 1,3-thiazol 2 yl)tlziophene-2-carboxamide: From the HPLC
purified mixture in the previous step was isolated 5-(4-phenyl-1,3-thiazol-2-
yl)thiophene-2-carboxamide as an off white solid (2 mg). 'H-NMR (Methanol-d4;
300 MHz) ~ 7.99 (m, 2H), 7.97 (s, 1 H), 7.95 and 7.78 (AB quartet, 2H, J--4.2
Hz),
CA 02362390 2001-08-07
WO 00/47578 _1$2_ PCT/US99/18065
7.48-7.35 (m, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z): Calcd. for
C,4H"N3S2 286.0 (M+H), found 286.2.
Example 145
a) Methyl4 ~4-(3,4-dimethoxyphenyl)(1,3-thiazol 2 yl)J 5-metl:ylthiophene-
Z-carboxylate: A solution of 257 mg (0.48 mmol, based on a mixture containing
60%
nitrile) of methyl 4-(aminothioxomethyl)-5-methylthiophene-2-carboxylate was
reacted with 124 mg (0.48 mmol) of 2-bromo-(3',4'-dimethoxy)-acetophenone
(Example 31, step (a)) was reacted in a manner similar to Example 8, step (a)
to give
methyl4-[4-(3,4-dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiophene-2-
carboxylate (95 mg, 53 %) as a solid. Mass spectrum (MALDI-TOF, CHCA matrix,
m/z): Calcd. for C,$H"N04S2 376.1 (M+H), found 376.3.
b) 4-~4-(3,4 Dimethoxyplzenyl)(1,3-thiazol-2 yl)J S-methyltlziophene-2-
carboxamide: Methyl4-[4-(3,4-dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiophene-2-carboxylate (95 mg, 0.25 mmol) was treated in a manner
similar to
Example 10, step (b) to give 4-[4-(3,4-dimethoxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiophene-2-carboxamide (8 mg, 9 %) as a yellow solid. 'H-NMR (Methanol-
d4; 300 MHz) 8 8.42 (s, 1 H), 7.81 (s, 1 H), 7.61 (m, 2H), 7.03 (m, 1 H), 3.92
(s, 3H),
3.88 (s, 3H), 2.95 (s, 3H). Mass spectrum (MALDI-TOF, CHCA matrix, m/z):
Calcd. for C"H"N30zSz 360.1 (M+H), found 360.2.
Example 146
a) 4 Bromo-5-methylthiophene-2-carboxylic acid: A solution of 27.65 g
(108 mmol) of 2-methyl-3,5-dibromothiophene (prepared by the method of Kano,
S.et
al., Heterocycles 20(10):2035, 1983) was dissolved in dry tetrahydrofuran (280
mL),
cooled to -78 °C and 2 M n-butyl lithium in cyclohexane (54 mL, 108
mmol) was
added over 10 min. After stirring 20 min at -78 °C, dry carbon dioxide
gas was
bubbled through the solution for 1.5 h as the mixture was allowed to warm to
room
temperature. To this 6 N hydrochloric acid (100 mL) was added carefully. The
layers
were separated and the aqueous layer was extracted with diethyl ether (4 x 50
mL).
The combined organic layers were washed with brine, and dried over anhydrous
sodium sulfate. The solvents were removed in vacuo to give 4-bromo-5-
CA 02362390 2001-08-07
WO 00/47578 _~g3_ PCT/US99/18065
methylthiophene-2-carboxylic acid (22.4 g, 94 %) as an off white solid. 'H-NMR
(DMSO-db; 300 MHz) 8 13.34 (br s, 1H), 7.61 (s, 1H), 2.41 (s, 3H).
b) Isopropyl 4-bromo-S-methylthiophene-2-carboxylate: A solution of 5 g
(22.6 mmol) of 4-bromo-5-methylthiophene-2-carboxylic acid was dissolved in
dry
dichloromethane (200 mL) and reacted with oxalyl chloride (2 mL, 22.6 mmol)
and
dimethylformamide (100 ~.L) stirring on an ice bath for 30 min and then at
room
temperature for 2.5 h. The solvents were removed in vacuo and the residue was
passed through silica gel, eluting off with hexanes:ethyl acetate 7/3 (v:v),
ethyl
acetate, and dichloromethane. The solvents were removed in vacuo and the
resulting
oil dissolved in dry dichloromethane (100 mL). This solution was reacted with
dry
pyridine (9 mL, 113 mmol) and dry isopropanol (40 mL, 522 mmol) for 88 h. The
solvents were removed in vacuo and the residue partitioned between sodium
bicarbonate (150 mL) and dichloromethane (75 mL). The aqueous layers were
extracted with dichloromethane (2 x 20 mL), and the combined organic layers
were
washed with sodium bicarbonate (30 mL), brine (30. mL), and dried over
anhydrous
sodium sulfate. The solvents were removed in vacuo. The residue was purified
by
column chromatography eluting with hexanes:ethyl acetate 9/1 (v:v) to give
isopropyl
4-bromo-5-methylthiophene-2-carboxylate (1.91 g, 32 %) as a pale yellow oil.
'H-
NMR (DMSO-db; 300 MHz) 8 7.66 (s, 1H), 5.07 (septet, 1H, J--6.2 Hz), 2.42 (s,
3H),
1.29 (d, 6H, J--6.2 Hz). Mass spectrum (ESI, m/z): Calcd. for C9H"OZSBr 264.2
(M+H), found 264.8.
c) Isopropyl 4-cyaho-S-metlaylthiophene-2-carboxylate: A stirred solution
of 1.9 g (7.3 mmol) of isopropyl 4-bromo-5-methylthiophene-2-carboxylate was
dissolved in dry dimethylformamide (30 mL) and refluxed with copper cyanide
(785
mg, 8.8 mmol) for 16 hours. The cooled solution was poured into 0.1 M aqueous
sodium cyanide solution (300 mL) and extracted with diethyl ether (4 x 40 mL).
The
organic layers were washed with brine (2 x 40 mL), dried over anhydrous sodium
sulfate, and the solvents removed in vacuo. Column chromatography on silica
gel
eluting with hexanes:ethyl acetate 9/1 (v:v), gave isopropyl 4-cyano-5-
methylthiophene-2-carboxylate (960 mg, 63 %) as a yellow crystalline solid'H-
NMR
(DMSO-db; 300 MHz) 8 8.01 (s, 1H), 5.09 (septet, 1H, J--6.2 Hz), 2.67 (s, 3H),
1.29
(d, 6H, J--6.2 Hz).
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-t84-
d) Isopropyl4-(aminothioxomethyl)-S-methylthiophene-2-carboxylate: A
stirred solution of 960 mg (4.59 mmol) of isopropyl 4-cyano-5-methylthiophene-
2-
carboxylate was treated in a manner similar to Example 139, step (c) to give,
after
crystallization from diethyl ether, isopropyl 4-(aminothioxomethyl)-S-
methylthiophene-2-carboxylate (623 mg, 56 %) as a solid. 'H-NMR (DMSO-d6; 300
MHz) 8 9.93 (br s, 1 H), 9.34 (br s, 1 H), 7.54 (s, 1 H), 5.07 (septet, 1 H, J-
-6.2 Hz), 2.60
(s, 3H), 1.29 (d, 6H, J--6.2 Hz). Mass spectrum (MALDI-TOF, GA matrix, m/z):
Calcd. for C,°H,3NOZSz 244.0 (M+H), found 243.8.
e) Isopropyl S-methyl 4-(4 phenyl(1,3-thiazol 2 yl))thiophene-2-
carboxylate: A solution of 375 mg (1.54 mmol) of isopropyl 4-
(aminothioxomethyl)-
5-methylthiophene-2-carboxylate was reacted with 307 mg (1.54 mmol) of 2-
bromoacetophenone (Aldrich, Milwaukee, WI, USA) in a manner similar to Example
8, step (a) to give, after crystallization from methanol, isopropyl 5-methyl-4-
(4-
phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate (347 mg, 66%) as light brown
needles. 'H-NMR (DMSO-db; 300 MHz) 8 8.23 (s, 1H), 8.09 (s, 1H), 8.05 (m, 2H),
7.49 (m, 2H), 7.38 (m, 1H), 5.13 (septet, 1H, J--6.2 Hz), 2.86 (s, 3H), 1.33
(d, 6H,
J--6.2 Hz). Mass spectrum (ESI, m/z): Calcd. for C,BH"NOzSz 344.1 (M+H), found
344.1.
~ S-Methyl 4-(4 plZenyl(1,3-thiazol 2 yl))thiophene-2-carboxamidine:
Isopropyl 5-methyl-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate (340
mg,
0.99 mmol) was treated in a manner similar to Example 10, step (b) to give 5-
methyl-
4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamidine (360 mg, quantitive
yield)
as a yellow solid. This material was dissolved in dry methanol (20 mL) and
treated
with 1 M HCl (g) in diethyl ether. Evaporation of the solvents in vacuo and
recrystallization from methanol gave the hydrochloride salt of 5-methyl-4-(4-
phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamidine (252 mg, 76 %) as a light
brown
crystalline solid. 'H-NMR (DMSO-db; 300 MHz) 8 9.45 (br s, 2H), 9.10 (br s,
2H),
8.56 (s, 1H), 8.27 (s, 1H), 8.06 (m, 2H), 7.50 (m, 2H), 7.40 (m, 1H), 2.93 (s,
3H).
Mass spectrum (ESI, m/z): Calcd. for C,SH,3N3Sz 300.1 (M+H), found 300.2.
CA 02362390 2001-08-07
WO 00/47578 _~85_ PCT/US99/18065
Example 147
a) 2-Methyl S ~(methylethyl)oxycarbonylJthiophene-3-carboxylic acid: A
stirred mixture of 500 mg (2.39 mmol) of isopropyl 2-methyl-3-cyanothiophene-5-
carboxylate and tetrafluorophthalic acid (570 mg, 2.39 mmol) was heated in a
glass
bomb at 160 °C for 66 hours. The cooled residue was digested in hot
chloroform (30
mL), treated with norite, and filtered through celite. The celite was washed
with hot
chloroform (30 mL). The cooled chloroform extracts were filtered and extracted
with
saturated sodium bicarbonate (4 x 10 mL). The basic extracts were washed with
chloroform, filtered through celite, and acidified to pH 1 with concentrated
hydrochloric acid. The solid was collected by filtration and washed with water
(3 x
10 mL) to give 2-methyl-5-[(methylethyl)oxycarbonyl]thiophene-3-carboxylic
acid
(288 mg, 53 %) as a light brown solid. 'H-NMR (DMSO-db; 300 MHz) 8 13.03 (br
s,
1H), 7.85 (s, 1H), 5.08 (septet, 1H, J--6.2 Hz), 2.71 (s, 3H), 1.29 (d, 6H, J--
6.2 Hz).
Mass spectrum (ESI, m/z): Calcd. for C,oH~204S 229.1 (M+H), found 228.8
b) Isopropyl4-(2-bromoacetyl)-S-methyltlziophene-2-carboxylate: A stirred
solution of 300 mg (1.3 mmol) of 2-methyl-5-
[(methylethyl)oxycarbonyl]thiophene-
3-carboxylic acid was dissolved in dry dichloromethane (10 mL) and treated
with
oxalyl chloride (174 p.L, 2 mmol) and dimethylformamide (50 pL). The mixture
was
stirred at room temperature for 1.25 h, the solvents removed in vacuo, and the
residue
passed through silica gel (1 inch in a 60 mL sintered-glass Buchner funnel)
and eluted
off with dichloromethane (150 mL). This material was treated in a manner
similar to
Example 142, step (a) to give isopropyl 4-(2-bromoacetyl)-5-methylthiophene-2-
carboxylate (266 mg, 67 %) as a solid.
c) Isopropyl 4-(2-amino(1,3-thiazol-4 yl))-S-metl:ylthiophene-2-carboxylate:
A solution of 260 mg (0.85 mmol) of isopropyl 4-(2-bromoacetyl)-5-
methylthiophene-2-carboxylate was reacted with 65 mg (0.85 mmol) of thiourea
in a
manner similar to Example 8, step (a) to give isopropyl 4-(2-amino(1,3-thiazol-
4-yl))-
5-methylthiophene-2-carboxylate (257 mg, quantitive yield) as a white solid.
'H-NMR
(DMSO-db; 300 MHz) 8 7.90 (s, 1H), 6.93 (s, 1H), 5.09 (septet, 1H, J--6.2 Hz),
2.61
(s, 3H), 1.29 (d, 6H, J--6.2 Hz). Mass spectrum (ESI, m/z): Calcd. for
C,,H,4NzOzS2
283.1 (M+H), found 283.1
CA 02362390 2001-08-07
WO 00/47578 _~86_ PCT/US99/18065
d) 4-(2 Amino(1,3-thiazol-4 yl))-S-metlzylthiophene-2-carboxamidine:
Isopropyl 4-(2-amino(1,3-thiazol-4-yl))-5-methylthiophene-2-carboxylate (240
mg,
0.85 mmol) was treated in a manner similar to Example 10, step (b) to give 4-
(2-
amino(1,3-thiazol-4-yl))-5-methylthiophene-2-carboxamidine (20 mg, 10 %) as a
solid. 'H NMR (DMSO-db, 300 MHz): 8 9.30 (br s, 2H), 8.99 (bs, 2H), 8.28 (s,
1H),
6.78 (s, 1H), 2.71 (s, 3H); Mass Spectrum (ESI, m/z) calcd. for C9H,oN4S2,
238.8
(M+H), found 239.2.
Example 148
a) 4-Bromo-5-ethylthiophene-2-carboxylic acid: A stirred solution of 10 g
(35 mmol) of 4,S-dibromothiophene-2-carboxylic acid (Lancaster, Windham, NH,
USA) in dry THF (100 mL) was cooled to -78 °C. To this, 35 mL (70 mmol)
of 2.0
M n-butyllithium in cyclohexane (Aldrich, Milwaukee, WI, USA) was added
dropwise over 15 min, and the reaction was allowed to stir for 15 min at -78
°C. The
mixture was quenched with ethyl iodide (2.8 mL, 35 mmol) and allowed to warm
to
room temperature. The mixture was carefully poured into 6N hydrochloric acid
(100
mL) and extracted with diethyl ether (4 x 50 mL). The organic layers were
washed
with water (2 x 50 mL), brine (50 mL), and dried over anhydrous sodium
sulfate. The
solvents were removed in vacuo to give 2-ethyl-3-bromo-thiophene-5-carboxylate
(7
g, 85 %) as a dark solid. 'H-NMR (DMSO-db; 300 MHz) 8 13.25 (br s, 1H), 7.62
(s,
1H), 2.80 (q, 2H, J--7.5 Hz), 1.23 (t, 3H, J--7.5 Hz).
b) Isopropyl4-bromo-S-etlzylthiophene-2-carboxylate: A solution of 7 g (30
mmol) of 4-bromo-5-ethylthiophene-2-carboxylic acid was dissolved in dry
dichloromethane (200 mL) and treated with oxalyl chloride (3.2 mL, 36 mmol)
and
dimethylformamide (0.5 mL) for 18.5 h. The solvents were removed in vacuo and
the
residual brown oil was passed through silica gel (2 inches in a 350 mL
scintered-glass
Buchner funnel) and eluted with 700 mL of hexanes:ethyl acetate 9/1 (v:v). The
elutate was concentrated in vacuo and the oil dissolved in dry dichloromethane
(200
mL). This solution was treated with pyridine (12 mL, 150 mmol) and dry
isopropanol
(60 mL, 750 mmol) for 4 h at room temperature. The solvents were removed in
vacuo
and the residue partioned between dichloromethane ( 100 mL) and water (200
mL).
The aqueous layers were extracted with dichloromethane (2 x 30 mL). The
combined
CA 02362390 2001-08-07
WO 00/47578 _18~_ PCT/US99/18065
organic layers were extracted with sodium bicarbonate (2 x 30 mL), brine (30
mL),
and dried over anhydrous sodium sulfate. The solvent was removed in vacuo.
Purification by column chromatography on silica gel (250 g) eluting with
hexanes:ethyl acetate 95/5 (v:v) gave isopropyl 2-ethyl-3-bromo-thiophene-5-
carboxylate (4 g, 48 %) as a yellow oil. 'H-NMR (DMSO-db; 300 MHz) 8 7.66 (s,
1 H), 5.89 (septet, 1 H, J--6.2 Hz), 2.80 (q, 2H, J--7.5 Hz), 1.29 (d, 6H, J
6.0 Hz), 1.24
(t, 3H, J--7.5 Hz).
c) Isopropyl 4-cyano-S-ethylthiophene-2-carboxylate: A stirred solution of 4
g ( 14.4 mmol) of isopropyl 4-bromo-5-ethylthiophene-2-carboxylate was
refluxed in
dry dimethylformamide (50 mL) with copper cyanide (1.94 g, 22 mmol) for 8
hours.
The cooled mixture was poured into 0.1 M sodium cyanide (500 mL) and extracted
with diethyl ether (4 x 50 mL). The organic layers were washed twice with
brine (50
mL) and dried over anhydrous sodium sulfate. The solvents were removed in
vacuo.
Column chromatography on silica gel (400 g), eluting with hexanes:ethyl
acetate 9/1
(v:v) gave isopropyl 2-ethyl-3-cyano-thiophene-5-carboxylate (1.7 g, 53 %) as
a pale
yellow oil. 'H-NMR (DMSO-db; 300 MHz) 8 8.03 (s, 1H), 5.10 (septet, 1H, J--6.2
Hz), 3.04 (q, 2H, J--7.5 Hz), 1.31 (t, 3H, J--7.5 Hz), 1.30 (d, 6H, J--6.2
Hz). Mass
spectrum (ESI m/z): Calcd. for C"H,3NOZS 224.1 (M+H), found 224Ø
d) Isopropyl4-(aminothioxomethyl)-S-etlzylthiophene-2-carboxylate: A
stirred solution of 1.7 g (7.6 mmol) of isopropyl 4-cyano-5-ethylthiophene-2-
carboxylate was treated as in Example 139, step (c) to give isopropyl 5-ethyl-
4-
(aminothioxomethyl)-5-ethylthiophene-2-carboxylate ( 1.45 g, 74 %) as a yellow
solid.
'H-NMR (DMSO-db; 300 MHz) 8 9.93 (br s, 1H), 9.39 (br s, 1H), 8.04 (s, 1H),
5.08
(septet, 1H, J--6.2 Hz), 3.08 (q, 2H, J--7.5 Hz), 1.29 (d, 6H, J--6.2 Hz),
1.24 (t, 3H,
J--7.5 Hz).
e) Isopropyl S-ethyl-4-(4 phenyl(1,3-thiazol 2 yl))thiophene-2-carboxylate:
A solution of 450 mg (1.75 mmol) of isopropyl S-ethyl-4-(aminothioxomethyl)-5-
ethylthiophene-2-carboxylate was reacted with 348 mg (1.75 mmol) of 2-
bromoacetophenone (Aldrich, Milaukee, WI, USA) in a manner similar to Example
8,
step (a) to give isopropyl 5-ethyl-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-
carboxylate (303 mg, 49%) as an off white solid. 'H-NMR (DMSO-db; 300 MHz)
b 8.22 (s, 1 H), 8.07 (s, 1 H), 8.03 (m, 2H), 7.49 (m, 2H), 7.3 8 (m, 1 H),
5.13 (septet,
CA 02362390 2001-08-07
WO 00/47578 _~ $8~ PCT/US99/18065
1H, J 6.2 Hz), 3.34 (q, 2H, J 7.4 Hz), 1.39 (t, 3H, J--7.4 Hz), 1.33 (d, 6H, J
6.2 Hz).
Mass spectrum (ESI, m/z): Calcd. for C,9H,9NOZS2 358.1 (M+H), found 358.1.
,~ S-Etlzyl 4-(4 phenyl(1,3-thiazol 2 yl))thiophene-2-carboxamidine:
Isopropyl 5-ethyl-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxylate (250
mg,
0.70 mmol) was treated in a manner similar to that for Example 10, step (b),
to give 5-
ethyl-4-(4-phenyl(1,3-thiazol-2-yl))thiophene-2-carboxamidine (148 mg, 67 %)
as a
yellow solid. 'H-NMR (DMSO-d6; 300 MHz) 8 9.44 (br s, 2H), 9.07 (br s, 2H),
8.54
(s, 1 H), 8.26 (s, 1 H), 8.05 (m, 2H), 7.50 (m, 2H), 8.70 (s, 1 H), 7.40 (m, 1
H), 3.44 (q,
2H, J--7.4 Hz), 1.42 (t, 3H, J--7.4 Hz). Mass spectrum (ESI, m/z): Calcd. for
C,6H,SN3Sz 314.1 (M+H), found 314.2.
Example 149
a) Isopropyl 4 ~4-(3-hydroxyphenyl)(1,3-thiazol 2 yl)J S-methylthiophene-
2-carboxylate: A solution of 1.97 g (8.1 mmol) of isopropyl 4-
(aminothioxomethyl)-
S-methylthiophene-2-carboxylate was reacted with 1.74 g (8.1 mmol) of 3'-
hydroxy-
2-bromoacetophenone (Example 40, step (a)) were reacted in a manner similar to
Example 8, step (a) to give, after column chromatography on silica gel eluting
with
hexane:ethyl acetate 7/3 (v:v), crystallization from acetonitrile, and
recrystallization
from hexanes, isopropyl 4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiophene-2-carboxylate (1.4 g, 48%) as brown solid. 'H-NMR (DMSO-db;
300 MHz) 8 9.57 (br s, 1H), 8.14 (s, 1H), 8.08 (s, 1H), 7.46 (m, 2H), 7.26 (m,
1H), ),
6.78 (m, 1H), 5.12 (septet, 1H, J--6.2 Hz), 2.85 (s, 3H), 1.33 (d, 6H, J 6.2
Hz). Mass
spectrum (ESI, m/z): Calcd. for C,BH,~N03S2 360.1 (M+H), found 360.1.
b) 4 ~4-(3 Hydroxyphenyl)(1,3-thiazol 2 yl)J 5-methylthiophene-2-
carboxamide: Isopropyl 4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiophene-2-carboxylate (1.4 g, 3.89 mmol) was treated in a manner
similar to
Example 10, step (b) to give 4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-
methylthiophene-2-carboxamide (360 mg, 31 %) as a brown solid. 'H-NMR (DMSO-
db; 300 MHz) 8 9.62 (br s, 1H), 9.45 (br s, 2H), 9.09 (br s, 2H), 8.53 (s,
1H), 8.16 (s,
1H), 7.47 (m, 2H), 7.27 (m, 1H), 6.80 (m, 1H), 2.93 (s, 3H). Mass spectrum
(ESI,
m/z): Calcd. for C,SH,3N3 OSz 316.1 (M+H), found 316.2.
CA 02362390 2001-08-07
WO 00/47578 _189_ PCT/US99/18065
Example 1 SO
a) (tert Butoxy) N ((4-~4-(3-hydroxyphenyl)(1,3-thiazo! 2 yl)J S-methyl(2-
thienyl))iminomethyl)carboxamide: A stirred solution of 320 mg (1 mmol) of 4-
[4-
(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-5-methylthiophene-2-carboxamide was
dissolved in dry dimethylformamide (50 mL) and treated with 262 mg (1.2 mmol)
of
di-tert-butyl-dicarbonate (Acros, Pittsburgh, PA, USA) and
diisopropylethylamine
(261 ~L, 1.5 mmol) for 64 hours at room temperature. The mixture was poured
into
sodium bicarbonate solution (200 mL) and extracted with dichloromethane (6 x
30
mL). The organic extracts were washed twice with brine (50 mL) and dried over
anhydrous sodium sulfate. The solvents were in vacuo and column chromatography
on silica gel (100 g) eluting with dichloromethane:methanol 95/5 (v:v) gave
(tert-
butoxy)-N-( { 4-[4-(3-hydroxyphenyl)( 1,3-thiazol-2-yl)]-5-methyl(2-
thienyl)}iminomethyl)carboxamide (247 mg, 59 %) as a yellow oil. 'H-NMR
(DMSO-db; 300 MHz) ~ 9.56 (s, 1H), 9.12 (br s, 2H), 8.47 (s, 1H), 8.09 (s,
1H), 7.46
(m, 2H), 7.26 (m, 1H), 6.78 (m, 1H), 2.83 (s, 3H), 1.45 (s, 9H). Mass spectrum
(ESI,
m/z): Calcd. for CZ°HZ~N3O3Sz 416.1 (M+H), found 415.7
b) Metlryl2-~3-~2-(S-~~(tert butoxy)carbonylaminoJiminometlrylJ-2-methyl
3-thienyl)-1,3-thiazol 4 ylJphenoxyJacetate: A stirred solution of 247 mg
(0.595
mmol) of (tert-butoxy)-N-({4-[4-(3-hydroxyphenyl)(1,3-thiazol-2-yl)]-~-
methyl(2-
thienyl)}iminomethyl)carboxamide was dissolved in dry dimethylformamide (4 mL)
and treated with cesium carbonate (291 mg, 0.89 mmol) and methyl bromoacetate
(136 mg, 0.89 mmol) for 3 h at 60 °C. The mixture was poured into water
(50 mL)
and extracted with dichloromethane (9 x 10 mL). The organic extracts were
washed
with brine (10 mL) and dried over anhydrous sodium sulfate. The solvents were
removed in vacuo and column chromatography on silica gel (50 g) eluting with
dichloromethane:methanol 98/2 (v:v) gave methyl 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methyl-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetate (178 mg, 61 %) as an oil. Mass spectrum (ESI, m/z): Calcd.
for
C23HZSN30sS2 488.1 (M+H), 388.1 ((M-BOC)+H), found 487.8, 388.2.
. c) Methyl 2-~3-~2-(S-amidino-2-methyl 3-tlzienyl)-1,3-thiazol-4-
ylJphenoxyfacetate: Methyl2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-
2-methyl-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetate (15 mg, 0.031 mmol)
treated
CA 02362390 2001-08-07
WO 00/47578 _,) 90_ PCT/US99/18065
with dichloromethanearifluoroacetic acid 1/1 (v:v) with 2.5% water added at
room
temperature for 1.5 h. Removal of the solvents in vacuo gave methyl 2-{3-[2-(5-
amidino-2-methyl-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetate (8.1 mg, 52 %) as
a
brown solid. 'H-NMR (DMSO-d6; 300 MHz) 8 9.38 (br s, 2H), 8.94 (br s, 2H),
8.51
S (s, 1 H), 8.31 (s, 1 H), 7.62 (m, 2H), 7.41 (m, 1 H), 6.96 (m, 1 H), 4.89
(s, 2H), 3.72 (s,
3H), 2.92 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for C,gH"N303S2 388.1
(M+H),
found 388.3.
Example 1 SI
a) 2-~3 ~2-(S-~~(tert-Butoxy)carbonylaminoJiminomethylJ-2-methyl-3-
thienyl)-1,3-thiazol 4 ylJphenoxyJacetic acid: A stirred solution of 50 mg
(0.11
mmol) of methyl 2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-methyl-
3-
thienyl)-1,3-thiazol-4-yl]phenoxy}acetate was dissolved in tetrahydrofuran (10
mL)
and treated 2M aqueous sodium hydroxide solution (2 mL) at room temperature
for 1
h 10 min. The solvents were removed in vacuo. Purification by passing the
solid
through silica gel (1 inch in a 60 mL sintered-glass Buchner funnel) eluting
with
dichloromethane:methanol 8/2 (v:v) gave 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methyl-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetic acid (44 mg, 88 %) as a yellow solid. 'H-NMR (DMSO-db; 300
MHz) 8 9.3 8 (br s, 2H), 8.94 (br s, 2H), 8.51 (s, 1 H), 8.31 (s, 1 H), 7.62
(m, 2H), 7.41
(m, 1H), 6.96 (m, 1H), 4.89 (s, 2H), 3.72 (s, 3H), 2.92 (s, 3H). Mass spectrum
(ESI,
m/z): Calcd. for CZZH23N3~5'~2 474.1 (M+H), 374.1 ((M-BOC)+H) found 374.2,
473.7.
b) 2-~3-~2-(5 Amidino-2-methyl-3-thienyl)-1,3-thiazol-4 ylJplzenoxyJacetic
acid: Methyl2-{3-[2-(5-{[(tert-butoxy)carbonylamino]iminomethyl}-2-methyl-3-
thienyl)-1,3-thiazol-4-yl]phenoxy}acetate (4 mg, 0.0084 mmol) was treated with
dichloromethanearifluoroacetic acid 1/1 (v:v) with 2.5% water added at room
temperature for 2 h 35 min. Removal of the solvents in vacuo gave 2-{3-[2-(5-
amidino-2-methyl-3-thienyl)-1,3-thiazol-4-yl]phenoxy}acetic acid (2.9 mg, 71
%) as a
solid. Mass spectrum (ESI, m/z): Calcd. for C"H,SN3O3S2 373.1 (M+H), found
374.2.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-191-
c) tent Butyl4-(2-(3 ~2-(S-(~(tert butoxy)carbonylaminoJiminomethylf-2-
methyl 3-thienyl)-1,3-thiazol 4 ylJphenoxyJacetyl)piperazinecarboxylate: A
stirred
solution of 40 mg (0.084 mmol) of 2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methyl-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetic acid dissolved in dry dimethylformamide (5 mL) was treated
with
hydroxybenzotriazole (23 mg, 0.17 mmol), 32 mg (0.17 mmol) of N tert-
butoxycarbonyl-piperazine (Lancaster, Wiridham, NH, USA), ~ 65 mg (0.17 mmol)
of
O-(7-azabenzotriazol-1-yl)-N,N,N;N-tetramethyluronium hexafluorophosphate
(HATU) at room temperature for 20 h. The mixture was partitioned between
dichloromethane (50 mL) and brine (50 mL). The aqueous layers were extracted
twice with dichloromethane (50 mL) and the combined organic layers were washed
with brine (50 mL) and dried over anhydrous sodium sulfate. The solvents were
removed in vacuo. Purification preparative thin layer chromatography eluting
with
dichloromethane:methanol 95/5 (v:v) gave tert-butyl 4-(2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methyl-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetyl)piperazinecarboxylate (25 mg, 46%) as a white solid. 'H-NMR
(DMSO-db; 300 MHz) 8 9.13 (br s, 2H), 8.50 (s, 1H), 8.20 (s, 1H), 7.63 (m,
2H), 7.39
(m, 1H), 6.95 (m, 1H), 4.93 (s, 2H), 3.47-3.34 (m, 8H), 2.82 (s, 3H), 1.45 (s,
9H), 1.42
(s, 9H). Mass spectrum (ESI, m/z): Calcd. for C3,H39NSO6S2 642.3 (M+H), 542.3
((M-BOC)+H), 442.3 ((M-2 BOC)+H), found 642.0, 542.2, 442.3.
d) S Metlzyl 4-~4 ~3-(2-oxo-2 piperazinylethoxy)phenylJ(1,3-thiazol 2-
yl)Jthiophene-2-carboxamidine: tert-Butyl4-(2-{3-[2-(5-{[(tert-
butoxy)carbonylamino]iminomethyl}-2-methyl-3-thienyl)-1,3-thiazol-4-
yl]phenoxy}acetyl)piperazinecarboxylate (25 mg, 0.039 mmol) treated with
dichloromethanearifluoroacetic acid 1/1 (v:v) with 2.5% water added at room
temperature for 2 h. Removal of the solvents in vacuo gave 5-methyl-4-{4-[3-(2-
oxo-
2-piperazinylethoxy)phenyl](1,3-thiazol-2-yl)}thiophene-2-carboxamidine (27.4
mg,
quantitive yield) as an off white solid. 'H-NMR (Methanol-d4; 300 MHz) 8 8.41
(s,
1 H), 7.94 (s, 1 H), 7.67 (m, 2H), 7.39 (m, 1 H), 7.00 (m, 1 H), 4.96 (s, 2H),
3.88 (m,
4H), 3.25 (m, 4H), 2.95 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
Cz~H23N5O2S2
442.1 (M+H), found 442.4.
CA 02362390 2001-08-07
WO 00/47578 _~92_ PCT/US99/18065
Example 152
Methyl 4-(2-bromoacetyl)-S-methylthiothiophene-2-carboxylate
To a stirring slurry of 2-methylthio-(5-carbomethoxy)-thiophene-3-carboxylic
acid (2.0 g, 8.61 mmol) in 28 mL of CHZCIz under NZ containing 0.8 mL DMF at 0
°C
was added oxalyl chloride (1.9 equiv, 16.3 mmol) slowly via syringe. The
reaction
was allowed to warm to ambient temperature after 1 h, and then stirred an
additional 1
h. The reaction mixture was filtered through a 20 cm pad of silica gel in a 30
mL
sintered glass funnel wetted with 50% ethyl acetate-hexanes and further eluted
with
the same solvent system until the eluent showed no product by UV
visualization. The
solvent was concentrated in vacuo, azeotroped with toluene (lx), and dried
under
vacuum to afford the acid chloride (1.52 g) as a light yellow solid. The acid
chloride
was dissolved in 20 mL of CH3CN , cooled to 0 °C, and treated with
TMSCHNz (2.1
equiv, 6.3 mL, 2 M in hexanes) dropwise via syringe. The reaction was allowed
to
warm to ambient temperature (0.5 h), cooled back to 5°C and immediately
treated
with 30% HBr-acetic acid (0.66 mL) dropwise via an addition funnel. After 15
min. at
0 °C, the reaction diluted with 20 mL of ether, filtered and thoroughly
washed with
ether (3x20 mL). The yellow solids were dried under vacuum to afford methyl 4-
(2-
bromoacetyl)-5-methylthiothiophene-2-carboxylate (1.0 g, 37% yield) as a
yellow
powder.'H NMR (DMSO-db, 300 MHz) b 2.66 (s, 3H), 3.84 (s, 3H), 5.03 (s, 2H),
8.29 (s, 1 H).
Example 153
Isopropyl 4-(2-bromoacetyl)-S-methylthioplzene-2-carboxylate
To a stirring slurry of 2-methyl-(5-carboisopropoxy)-thiophene-3-carboxylic
acid (0.40 g, 1.75 mmol) in 15 mL of CHZCl2 under NZ containing 0.8 mL DMF at
0
°C was added oxalyl chloride (1.9 equiv, 3.32 mmol,) slowly via
syringe. The reaction
was allowed to warm to ambient temperature after 1 h, and then stirred an
additional 1
h. The solvent was concentrated in vacuo, azeotroped with toluene (lx), and
dried
under vacuum to afford the acid chloride (0.397 g, 1.60 mmol) as a light
yellow solid.
The acid chloride was dissolved in 7 mL of CH3CN , cooled to 0 °C, and
treated with
TMSCHNZ {2.1 equiv, 1.68 mL, 2 M in hexanes) dropwise via syringe. The
reaction
CA 02362390 2001-08-07
WO 00/47578 _~ 93_ PCT/US99/18065
was allowed to warm to ambient temperature (0.5 h), cooled back to S°C
and
immediately treated with 30% HBr-acetic acid (0.5 mL) dropwise via an addition
funnel. After 15 min. at 0 °C, the reaction mixture was filtered
through a 10 cm pad of
silica gel in a 15 mL sintered glass funnel wetted with 50% ethyl acetate-
hexanes and
further eluted with the same solvent system until the eluent showed no product
by UV
visualization. The solvent was concentrated in vacuo dried under vacuum to
afford
isopropyl- 4-(2-bromoacetyl)-5-methylthiophene-2-carboxylate (0.329 g, 61%
yield)
as an oil which solidified upon standing to a tan solid. 'H NMR (DMSO-d6, 300
MHz)
8 1.31 (d, 6H, J--6.3 Hz), 2.71 (s, 3H), 4.60 (s, 2H), 5.09 (m, 1H), 8.08 (s,
1H).
Example 154
a) Methyl 5-methylthio-4-~2-(phenylamino)-(1,3-thiazol-4 yl)J thiophene-2-
carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-methylthiothiophene-2-
carboxylate (60.5 mg, 0.19 mmol) was slurried in 4 mL of acetone with phenyl
thiourea (1 equiv, 30 mg) and heated to 70 °C . After 3 h the reaction
was allowed to
cool to room temperature, filtered, and dried in vacuo to give 62.5 mg (69%
yield) of
methyl 5-methylthio-4-[2-(phenylamino)-( 1,3-thiazol-4-yl)]-thiophene-2-
carboxylate
hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 2.65 (s, 3H), 3.83 (s, 3H), 6.95-
6.99
(m, 1 H), 7.28-7.35 (m, 4H), 7.67 (d, 1 H, J-- 1.4, 7.7 Hz), 8.06 (s, 1 H),
10.54 (s, 1 H);
Mass Spectrum (ESI) m/z calcd. for C,6H,4NZOzS3, 362.49 (M+H), found 363.7.
b) S-Metlzylthio-4 ~2-(phenylamino)(1,3-thiazol-4 yl)Jthiophene-2-
carboxamidine hydrochloride: To a flame-dried flask containing 57.8 mg (8
equiv,
1.08 mmol) of NHqCI under NZ was charged 1.3 mL of toluene. AlMe3 (8 equiv,
2M/hexanes, 0.54 mL) was added dropwise to the stirred slurry over a 3 min.
period,
and allowed to stir another 5 min. At this time methyl 5-methylthio-4-[2-
(phenylamino)-(1,3-thiazol-4-yl)]-thiophene-2-carboxylate hydrobromide (1
equiv, 60
mg, 0.135 mmol) was quickly added in one portion and the resultant mixture was
immersed in a 120 °C oil bath. After 2 h 10 min. at this temperature,
TLC (silica gel
60 FZS4, Merck KGaA, Darmstadt, Germany, 9:1:0.5 CHZC12-MeOH-AcOH eluent)
indicated the reaction to be complete by disappearance of the starting
material. The
reaction was allowed to cool to ambient temperature, then added via pipette to
a
stirred slurry of 1.3 g of Si02 in 20 mL of CHC13. The residual residue in the
flask was
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-194-
rinsed with 4 mL of MeOH, briefly sonicated and added to the Si02 slurry. The
slurry
was stirred for 10 min. and then filtered through a 15-mL sintered glass
funnel
containing 20 cm of SiOz with SO% CHCl3-MeOH. The yellow fraction was
collected,
discarding the forerun. TLC indicated the product was essentially pure. The
solvent
S was removed in vacuo, and the residue triturated with 10% MeOH-CHZCIz. The
solids
were removed by filtration. The solvent was concentrated in vacuo to give 30.1
mg
(66% yield) of 5-methylthio-4-[2-(phenylamino)-(1,3-thiazol-4-yl)]thiophene-2-
carboxamidine hydrochloride as a red-brown powder. 'H NMR (DMSO-d~, 300 MHz)
8 2.73 (s, 3H), 6.94- 7.00 (m, 1 H), 7.15 (s, 1 H), 7.30-7.3 S (m, 1 H), 7.78
(d, 1 H, J--8.7
Hz), 8.49 (s, 1H), 8.87 (bs, 2H), 9.31 (bs, 2H), 10.38 (s, 1H); Mass Spectrum
(ESI)
m/z calcd. for C,SH,4N4S3, 346.50 (M+H), found 347.2.
Example 1 SS
a) Methyl4-(2-~(2-chlorophenyl)aminoJ(1,3-thiazol 4 yl)~-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (50 mg) was allowed to react with 2-
chlorophenyl
thiourea (26.7 mg) as described in Example 154, step (a), to give 58 mg (75%)
of
methyl 4-{2-[(2-chlorophenyl)amino]-( 1,3-thiazol-4-yl) } -5-
methylthiothiophene-2-
carboxylate hydrobromide.'H NMR (DMSO-db, 300 MHz) 8 2.66 (s, 3H), 3.82 (s,
3H), 7.04 (m, 1H), 7.32-7.38 (m, 2H), 7.47 (dd, 1H, J-- 1.4, 8.7 Hz), 8.12 (s,
1H), 8.56
(dd, 1H, J--1.4, 8.3 Hz), 9.75 (s, 1H) ; Mass Spectrum (ESI) m/z calcd. for
C,6H,3C1NZOZS3, 396.94 (M+H), found 397.1.
b) 4-(2 ~(2-Chloroplzenyl)aminoJ(1,3-thiazol 4 yl))-5-metl:ylthiothioplzene
2-carboxamidine hydrochloride: Methyl 4-{2-[(2-chlorophenyl)amino]-(1,3-
thiazol
4-yl)}-5-methylthiothiophene-2-carboxylate hydrobromide (40 mg, 0.08 mmol) was
treated as described in Example 154, step (b) to give 24 mg (71.8%)of 4-{2-[(2-
chlorophenyl)amino]-( 1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxamidine
hydrochloride.'H NMR (DMSO-db, 300 MHz) 8 2.71 (s, 3H), 7.04 (td, 1H, J--1.4 ,
7.8 Hz), 7.21 (s, 1H), 7.35 (t, 1H, J--8.5 Hz), 8.42 (s, 1H), 8.57 (dd, 1H, J--
1.3, 8.3
Hz), 8.80 (bs, 2H), 9.26 (bs, 2H), 9.79 (s, 1H); Mass Spectrum (ESI) m/z
calcd. for
C,SH,4N4S3C1, 380.94 (M+H), found 381.1.
CA 02362390 2001-08-07
WO 00/47578 _' 95_ PCT/US99/18065
Example 156
a) Methyl 4-(2-amino(1,3-thiazol 4 yl))-5-methylthiotlziophene-2-
carboxylate Izydrobromide: Methyl 4-(2-bromoacetyl)-5-methylthiothiophene-2-
carboxylate (50 mg, 0.16 mmol) was allowed to react with thiourea (12 mg) as
described in Example 154, step (a), to give 54 mg (70% yield) of methyl 4-(2-
amino
(1,3-thiazol-4-yl))-5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR
(DMSO-db, 300 MHz) 8 2.69 (s, 3H), 3.83 (s, 3H), 7.00 (s, 1H), 8.05 (s, 1H);
Mass
Spectrum (ESI) m/z calcd. for C,oH,oO2S3N~, 286.41 (M+H), found 287.1;
b) 4-(2 Amino-(1,3-thiazol-4 yl))-S-methylthiothioplzene-2-carboxamidine
hydroclzloride: Methyl4-(2-amino-(1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate hydrobromide (110 mg, 0.29 mmol) was treated as described in
Example
154, step (b). The resultant amidine (74 mg) was stirred in 3 mL of dry
methanol
under NZ and treated with ca. 1mL of ether saturated with dry HCl gas. Dry
ether (1.5
mL) was then added and the result was allowed to sit for 2 h at ambient
temperature
and then filtered to give 40 mg (45% yield) of 4-(2-amino-(1,3-thiazol-4-yl))-
5-
methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz)
b 2.69 (s, 3H), 6.90 (s, 1H), 8.44 (s, 1H), 9.20, 9.42 (s, 4H, NH); Mass
Spectrum
(ESI) m/z calcd.C9H,oN4S3, 270.4 (M+H), found 271.2.
Example 157
a) Metlzyl9-~2-~(2,5-dimetlzoxyphenyl)aminoJ(1,3-thiazol-4 yl)f-5-
methylthiothiophene-2-carboxylate )zydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (49.4 mg, 0.15 mmol) was allowed to react
with
2,5-dimethoxy phenyl thiourea (37.2 mg) as described in Example 154, step (a),
to
give 65.5 mg (87% yield) of methyl 4-{2-[(2,5-dimethoxyphenyl)amino](1,3-
thiazol-
4-yl)}-5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300
MHz) 8 2.66 (s, 3H), 3.76 (s, 3H), 3.81 (s, 3H), 3.83 (s, 3H), 6.49 (dd, 1H, J-
-3.0, 8.8
Hz), 6.92 (d, 1 H, J--8.9 Hz), 7.26 (s, 1 H), 8.17 (s, 1 H), 8.37 (d, 1 H. J--
3.1 Hz), 9.70 (s,
1H); Mass Spectrum (ESI) m/z calcd. for C,8H,8N204S3, 422.54 (M+H), found
423.1.
b) 4-~2-~(2,5-Dimethoxyphenyl)aminoJ(1,3-thiazol-4 yl)J-S-
methyltlziothiophene-2-carboxamidine: Methyl4-{2-[(2,5-
dimethoxyphenyl)amino]-( 1,3-thiazol-4-yl) } -5-methylthiothiophene-2-
carboxylate
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-196-
hydrobromide (45.5 mg, 0.09 mmol) was treated as described in Example 154,
step
(b), followed by preparative thin layer chromatography (500 mm silica gel
plate, J.T.
Baker, Phillipsburg, NJ, 10%-methanol-CH~C12-sat'd. NH3 eluent) to give 9.9 mg
(27% yield of 4-{2-[(2,5-dimethoxyphenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxamidine. 'H NMR (DMSO-db, 300 MHz) 8 2.60 (s,
3H), 3.73 (s, 3H), 3.81 (s, 3H), 6.48 (dd, 1H, J--3.1, 8.8 Hz), 6.92 (d, 1H, J-
-7.9 Hz),
7.05 (s, 1 H), 7.5 (bs, 2H), 8.04 (s, 1 H), 8.34 (d, 1 H, J--1.0 Hz), 9.6 (bs,
1 H); Mass
Spectrum (ESI) m/z calcd. for C"H,8N4OZS3, 406.55 (M+H), found 407.1.
Example 158
a) Methyl4-~2-~(3-methoxyphenyl)aminoJ(1,3-thiazol 4 yl)J-S-
metlzylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (53.3 mg, 0.17 mmol) was allowed to react
with
2-methoxy phenyl thiourea (34.5 mg) as described in Example 154, step (a), to
give
61 mg (76% yield) of methyl 4-{2-[(3-methoxyphenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz)
8 2.67 (s, 3H), 3.78 (s, 3H), 3.83 (s, 3H), 6.53 (d, 1H, J--6.8 Hz), 7.13-7.24
(m, 2H),
7.29 (s, 3H), 7.59 (m, 1H), 8.16 (s, 3H), 10.32 (s, 1H); Mass Spectrum (ESI)
m/z
calcd. for C"H,6NZO3S3, 392.52 (M+H), found 393.2.
b) 4-~2-~(3 Metlzoxyplzenyl)aminoJ(1,3-thiazol-4 yl)J-S-
methylthiothiophene-2-carboxamidine Izydrochloride: Methyl 4-{2-[(3-
methoxyphenyl)amino]-( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-carboxylate
hydrobromide (54.6 mg, 0.11 mmol) was treated as described in Example 154,
step
(b) to give 25.2 mg (56%) of 4-{2-[(3-methoxyphenyl)amino](1,3-thiazol-4-yl)}-
5-
methylthiothiophene-2-carboxamidine hydrochloride.'H NMR (DMSO-db, 300 MHz)
8 2.71 (s, 3H), 3.77 (s, 3H), 6.54 (m, 1H), 7.15 (s, 3H), 7.19-7.28 (m, 2H),
7.47 (m,
1H), 8.46 (s, 1H), 8.86 (bs, 2H), 9.28 (bs, 2H), 10.36 (s, 1H); Mass Spectrum
(ESI)
m/z calcd. for C~6H,6N4OS3, 376.52 (M+H), found 377.2.
Example 159
a) MetJzyl4-(2 ~(4-methoxyphenyl)aminoJ(1,3-thiazol 4 yl)~-5-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
CA 02362390 2001-08-07
WO 00/47578 _19~_ PCT/US99/18065
inethylthiothiophene-2-carboxylate (41.3 mg, 0.13 mmol) was allowed to react
with
5-methoxy phenyl thiourea (26.8 mg) as described in Example 154, step (a) to
give 25
mg (41% yield) of methyl 4-{2-[(4-methoxyphenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) b
2.64, 2.68 (s, 3H rotomer) ), 3.72, 3.73 (s, 3H rotomer), 3.83 (s, 3H), 6.91
(dd, 2H,
J--6.7, 8.8 Hz), 7.21 (s, 1 H), 7.59 (d, 1 H, J--9.0 Hz), 7.67 (d, 1 H, J--9.0
Hz), 8.05, 8.13
(s, 1 H rotomer), 10.16, 10.34 (bs, 1 H, rotomer); Mass Spectrum (ESI) m/z
calcd. for
C,~H,6NzOzS3, 392.52 (M+H), found 393.1.
b) 4-(2 ~(4-Methoxyphenyl)aminoJ(1,3-thiazol 4 yl))-S-
methyltlaiotJziophene-2-carboxamidine hydrochloride: Methyl 4-{2-[(4-
methoxyphenyl)amino]-(1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxylate
hydrobromide (22 mg, 0.046 mmol) was treated as described in Example 154, step
(b)
to give 11.5 mg (61% yield) of 4-{2-[(4-methoxyphenyl)amino](1,3-thiazol-4-
yl)}-5-
methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz)
8 2.72 (s, 3H), 3.73 (s, 3H), 6.91 (d, 2H, J 9.0 Hz), 7.08 (s, 1H), 7.69 (d,
2H, J--9.1
Hz), 8.44 (s, 1 H), 8.83 (bs, 2H), 9.28 (bs, 2H), 10.15 (s, 1 H);Mass Spectrum
(ESI)
m/z calcd. for C,6H,6N4OS3, 376.52 (M+H), found 377.1.
Example 160
a) Methyl4-(2-~~4-(dimethylamino)phenylJamino)(1,3-thiazol 4 yl))-S-
metlZylthiotlZiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (50 mg, 0.16 mmol) was allowed to react with
4-
N,N-dimethylaminophenyl thiourea (31.5 mg) as described in Example 154, step
(a),
to give 53.2 mg (75% yield) of methyl 4-(2-{[4-
(dimethylamino)phenyl]amino}(1,3-
thiazol-4-yl))-5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-
db, 300 MHz) 8 2.69 (s, 3H), 3.15 (s, 6H), 3.83 (s, 3H), 7.36 (s, 1H), 7.55
(bs, 2H),
7.88 (d, 2H, J--8.3 Hz), 8.16 (s, 1H), 10.56 (bs, 1H); Mass Spectrum (ESI) m/z
calcd.
for C,8H~9N3OzS3, 405.56 (M+H), found 406.1.
b) 4-(2-(~4-(Dimethylamino)phenylJaminof (1,3-thiazol 4 yl))-S-
methyltl:iotlziophene-2-carboxamidine hydrochloride: Methyl 4-(2-{[4-
(dimethylamino)phenyl]amino } ( 1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate hydrobromide (50 mg, 0.10 mmol) was treated as described in
Example
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-198-
154, step (b) to give 9.4 mg (22% yield) of 4-{2-[(4-methoxyphenyl)amino](1,3-
thiazol-4-yl)}-S-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR
(DMSO-db, 300 MHz) 2.70 (s, 3H), 2.84 (s, 6H), 6.75 (d, 2H, J--9.2 Hz), 7.00
(s, 1H),
7.56 (d, 2H, J--9.1 Hz), 8.31 (s, 1 H), 8.68 (bs, 3H), 9.92 (bs, 1 H).
Example 161
a) Methyl4-(2-~(4-chloro-2-metlzylphenyl)aminoJ(1,3-thiazol 4 yl)J-S-
metlZylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (50 mg, 0.16 mmol) was allowed to react with
2-
methyl-4-chlorophenyl thiourea (32.1 mg) as described in Example 154, step
(a), to
give 62.2 mg (79% yield) of methyl 4-{2-[(4-chloro-2-methylphenyl)amino](1,3-
thiazol-4-yl)}-5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-
db, 300 MHz) ~ 2.28, 2.29 (s, 3H rotomer), 2.62, 2.66 (s, 3H rotomer), 3.82
(s, 3H),
7.21-7.29 (m, 3H), 8.04, 8.11 (s, 1H rotomer), 8.17 (d, 1H, J--8.8 Hz), 8.30
(d, 1H,
J--8.4 Hz), 9.44 (s, 1H), 9.59 (s, 1H); Mass Spectrum (ESI) m/z calcd. for
C"H,SCINzO2S3, 410.96 (M+H), found 411.1.
b) 4-~2-~(4-Chloro-Z-methylplzenyl)aminoJ(1,3-thiazol 4 yl)J-S-
metl:ylthiothiophene-2-carboxamidine hydrocl:loride: Methyl 4-{2-[(4-chloro-2-
methylphenyl)amino]-( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-carboxylate
hydrobromide (55 mg, 0.17 mmol) was treated as described in Example 154, step
(b)
to give 16 mg (22% yield) of 4-{2-[(4-chloro-2-methylphenyl)amino](1,3-thiazol-
4-
yl)}-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300
MHz) b 2.30 (s, 3H), 2.70 (s, 3H), 7.15 (s, 1H), 7.23 -7.29 (m, 2H), 8.34 (d,
1H, J--8.6
Hz), 8.44 (s, 1H), 8.86 (bs, 2H), 9.29 (bs, 2H), 9.47 (s, 1H); Mass Spectrum
(ESI) m/z
calcd. for C,6H~SC1N4S3, 394.97 (M+H), found 395.1.
Example 162
a) Methyl4-(2-~(diphenylmethyl)aminoJ(1,3-thiazol 4 yl)J-S-
methylthiotlziophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (50 mg, 0.16 mmol) was allowed to react with
diphenylmethane thiourea (38 mg) as described in Example 154, step (a), to
give 145
mg (100% yield) of methyl 4-{2-[(diphenylmethyl)amino](1,3-thiazol-4-yl)}-5-
CA 02362390 2001-08-07
WO 00/47578 _,~ 9~_ PCT/US99/18065
methylthiothiophene-2-carboxylate hydrobromide after removal of solvent in
vacuo.
'H NMR (DMSO-db, 300 MHz) 8 2.50 (s, 3H), 2.80 (s, 3H), 6.13, 6.18 (d, 1H
rotomer, J--7.9 Hz), 7.23-7.41 (m, 11H), 8.00, 8.02 (s, 1H rotomer), 8.73,
8.86 (d, 1H,
rotomer, J--8.0 Hz); Mass Spectrum (ESI) m/z calcd. for C23H20N202S3, 452.62
(M+H), found 453Ø
b) 4-~2-~(Diphenylmethyl)aminoJ(1,3-thiazol 4 yl)~-S-methylthiothiophene-
2-carboxamidine: Methyl4-{2-[(diphenylmethyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxylate hydrobromide. (96.3 mg. 0.18 mmol) was
treated
as described in Example 154, step (b) to give 16 mg (20% yield) of 4-{2-
[(diphenylmethyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-
carboxamidine
hydrochloride. 'H NMR (DMSO-d6, 300 MHz) 2.59 (s, 3H), 6.23 (d, 1H, J--7.9
Hz),
6.84 (s, 1H), 7.22-7.40 (m, 10 H), 8.09 (bs, 3H), 8.12 (s, 1H), 8.68 (d, 1H, J-
-8.4 Hz);
Mass Spectrum (ESI) m/z calcd. for CZZH20N4S3, 436.62 (M+H), found 437.1.
Example l63
a) Methyl S-metlZylthio-4-~2 ~(3 phenylpropyl)aminoJ(1,3-thiazol 4-
yl))tlZiopIZene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate ( 131 mg, 0.42 mmol) was allowed to react
with
propylphenyl thiourea (82.3 mg) in DMF as described in Example 154, step (a),
then
filtered through a 5 cm pad of silica gel in a 15 mL glass fritted funnel with
10%
methanol-CHC13. Concentration of the solvent in vacuo gave 203 mg (100% yield)
of
methyl 5-methylthio-4-{ 2-[(3-phenylpropyl)amino]( 1,3-thiazol-4-yl)
}thiophene-2-
carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 1.89 (m, 2H), 2.62 (s,
3H), 2.63-2.71 (m, 2H), 3.27-3.39 (m, 2H), 3.82 (s, 3H), 6.97 (s, 1H), 7.15-
7.31 (m,
SH), 8.06 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,9Hz°NzO2S3,
404.57 (M+H),
found 405.1.
b) 5-Metlzylthio-4-~2-~(3 phenylpropyl)aminoJ(1,3-thiazol-4 yl)f thiophene-
2-carboxamidine hydrochloride: Methyl -5-methylthio-4-{2-[(3-
phenylpropyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate hydrobromide
(112
mg, 0.23 mmol) was treated as described in Example 154, step (b) to give 16 mg
(16% yield) of 4-{2-[(diphenylmethyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxamidine hydrochloride, which was further purified
by
CA 02362390 2001-08-07
WO 00/47578 -200- PCT/US99/18065
preparative thin layer chromatography using 20%-methanol-CHZC12-sat'd. NH3 as
eluent. 'H NMR (DMSO-db, 300 MHz) 8 1.89 (m, 2H), 2.54 (s, 1H), 2.66 (at, 2H,
J--7.3 Hz), 3.31 (m, 2H), 6.69 (bs, 3H), 6.76 (s, 1H), 7.15-7.31 (m, SH), 7.69
(m, 1H),
7.84 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,8H2°N4S3, 388.58
(M+H), found
389.2.
Example 164
a) Methyl S-methylthio-4-~2 ~(2,4,5-trimethylphenyl)aminoJ(1,3-thiazol 4-
yl))tlziophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.21 mmol) was allowed to react with
2,4,5-trimethylphenyl thiourea as described in Example 154, step (a) to give
42.3 mg
(41% yield) of methyl 5-methylthio-4-{2-[(2,4,5-trimethylphenyl)amino](1,3-
thiazol-
4-yl)}thiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 2.16
(s, 3H), 2.18 (s, 3H), 2.19 (s, 3H), 2.64 (s, 3H), 3.82 (s, 3H), 6.97 (s, 1H),
7.18 (s,
1 H), 7.86 (s, 1 H), 8.12 (s, 1 H), 9.29 (s, 1 H); Mass Spectrum (ESI) m/z
calcd. for
C~9HZpN2OzS3, 404.57 (M+H), found 405.1.
b) S-Methylthio-4-(Z ~(2,4,5-trimethylphenyl)aminoJ(1,3-thiazol 4-
yl)ftlziophene-2-carboxamidine hydrochloride: Methyl -5-methylthio-4-{2-
[(2,4,5-
trimethylphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate hydrobromide
(37.3 mg, 0.07 mmol) was treated as described in Example 154, step (b) to give
28.3
mg (95% yield) of 5-methylthio-4-{2-[(2,4,5-trimethylphenyl)amino](1,3-thiazol-
4-
yl)}thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 2.16
(s, 3H), 2.19 (s, 3H), 2.20 (s, 3H), 2.68 (s, 3H), 6.97 (s, 1H), 7.03 (s, 1H),
7.84 (s,
1H), 8.41 (s, 1H), 8.84 (bs, 2H), 9.26 (bs, 3H); Mass Spectrum (ESI) m/z
calcd. for
C,BHz°N4S3, 388.58 (M+H), found 389.2.
Example 165
a) Methyl 4-~2 ~(2 Jluorophenyl)aminoJ(1,3-thiazol-4 yl))-S-
metlaylthiothioplZene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2-
fluorophenyl thiourea as described in Example 154, step (a) to give 55.6 mg
(70%
yield) of methyl 4-{2-[(2-fluorophenyl)amino](1,3-thiazol-4-yl)}-5-
CA 02362390 2001-08-07
w0 00/47578 -201- PCT/US99/18065
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8
2.68 (s, 3H), 3.83 (s, 3H), 6.96-7.04 (m, 1H), 7.14-7.29 (m, 3H), 7.35 (s,
1H), 8.06,
8.14 (s, 1 H rotomer), 8.61 (td, 1 H rotomer, J 1.5, 8.5 Hz), 10.14. 10.3 0
(s, 1 H
rotomer); Mass Spectrum (ESI) m/z calcd. for C,6H,3FNZOZS3, 380.48 (M+H),
found
381.1.
b) 4-~2-~(2-Fluorophenyl)aminoJ(1,3-thiazol 4 yl)J-5-methylthiothiophene-
2-carboxamidine IZydrocl:loride: Methyl 4-{2-[(2-fluorophenyl)amino](1,3-
thiazol-
4-yl)}-5-methylthiothiophene-2-carboxylate hydrobromide (55.6 mg, 0.13 mmol))
was treated as described in Example 154, step (b) to give 12.4 mg (24 %) of 4-
{2-[(2-
fluorophenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxamidine
hydrochloride. 'H NMR (DMSO-db, 300 MHz); 8 2.72 (s, 3H), 3.16 (s, 3H), 6.97-
7.08 (m, 1 H), 7.18-7.36 (m, 4H), 8.49 (s, 1 H), 8.70 (td, 1 H, 1.4, 8.4 Hz),
8.92 (bs,
2H), 9.32 (bs, 2H), 10.18 (d, 1H, J--1.6 Hz); Mass Spectrum (ESI) m/z calcd.
for
C,SH,3FN4S3, 364.49 (M+H), found 365.1.
Example 166
a) Methyl4-~2 ~(3-chloro-2-methylphenyl)aminoJ(1,3-thiazol-4 yl)J-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2-
methyl-3-chlorophenyl thiourea (39 mg) as described in Example 154, step (a)
to give
61.8 mg (66% yield) of methyl 4-{2-[(3-chloro-2-methylphenyl)amino](1,3-
thiazol-4-
yl)}-5-methylthiothiophene-2-carboxylate hydrobromide. Mass Spectrum (ESI) m/z
calcd. for C,~H,SCINZOzS3, 410.96 (M+H), found 411.1.
b) 4-~2-~(3-Chloro-2-methylphenyl)aminoJ(1,3-thiazol-4 yl)J-5-
methyltlaiothiophene-2-carboxamidine hydrochloride: Methyl 4-{2-[(3-chloro-2-
methylphenyl)amino]( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-carboxylate
hydrobromide (61.8 mg, 0.12 mmol) was treated as described in Example 154,
step
(b) to give 46.7 mg (90% yield) of 4-{2-[(3-chloro-2-methylphenyl)amino](1,3-
thiazol-4-yl)}-5-methylthiothiophene-2-carboxamidine hydrochloride.'H NMR
(DMSO-db, 300 MHz) 8 2.34 (s, 3H), 2.69 (s, 3H), 7.15 (s, 1 H), 7.18-7.26 (m,
2H),
8.12 (d, 1 H, J--7.9 Hz), 8.41 (s, 1 H), 8.84 (bs, 2H), 9.27 (bs, 2H), 9.61
(s, 1 H); Mass
Spectrum (ESI) m/z calcd. for C,6H,SC1N4S3, 394.97 (M+H), found 395.1.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-202
Example 167
a) Methyl 4-(2-~~2-(methylethyl)phenylJamino)(1,3-thiazo! 4 yl))-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2-
isopropyl phenyl thiourea (40 mg) as described in Example 154, step (a) to
give 33.1
mg (36% yield) of methyl 4-(2-{[2-(methylethyl)phenyl]amino}(1,3-thiazol-4-
yl))-5-
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz)
8 1.17 (d, 6H, J--6.7 Hz), 2.60, 2.65 (s, 3H rotomer), 3.27 (s, 1H), 3.82 (s,
3H), 7.13
(s, 1 H), 7.14-7.25 (m, 2H), 7.34-7.37 (m, 1 H), 7.78 (m, 1 H), 7.99, 8.08 (s,
1 H
rotomer), 9.52, 9.61 (bs, 1 H rotomer); Mass Spectrum (ESI) m/z calcd. for
C,9H2°NZOZS3, 404.57 (M+H), found 405.1.
b) 4-(2-~(2-(Metlzylethyl)phenylJamino)(1,3-thiazol 4 yl))-S-
methylthiothiophene-2-carboxamidine hydrochloride: Methyl 4-(2-{[2-
(methylethyl)phenyl]amino}(1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate
hydrobromide (33.1 mg, 0.06 mmol) was treated as described in Example 154,
step
(b) to give 22.4 mg (88%) of 4-(2-{[2-(methylethyl)phenyl]amino}(1,3-thiazol-4-
yl))-
5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-d6, 300
MHz) 8 1.19 (d, 6H, J--6.8 Hz), 2.70 (s, 3H), 3.32 (m, 1 H), 7.04 (s, 1 H),
7.14-7.25 (m,
2H), 7.35 (dd, 1H, J--1.4, 7.5 Hz), 7.86 (dd, 1H, J--1.4, 7.9 Hz), 8.37 (s,
1H); Mass
Spectrum (ESI) m/z calcd. for C,gH2°N4S3, 388.58 (M+H), found
389.2.
Example 168
a) Methyl S-methylthio-4-(2-~~Q-(phenylmethoxy)phenylJamino)(1,3-thiazol
4 yl))tltiophene-2-carboxylate: Methyl 4-(2-bromoacetyl)-5-methylthiothiophene-
2-
carboxylate (336.3 mg, 1.08 mmol) was allowed to react with 4-benzyloxyphenyl
thiourea (279 mg) as described in Example 154, step (a) to give 450 mg (76%
yield)
of methyl 4-(2-{[4-phenylmethoxyphenyl]amino}(1,3-thiazol-4-yl))-5-
methylthiothiophene-2-carboxylate hydrobromide. Mass Spectrum (ESI) m/z calcd.
for Cz3H20N2O3'S3~ 468.61 (M+H), found 469.2.
b) S-Methyltlzio-4-(2-(~4-(phenylmethoxy)phenylJamino)(1,3-tlziazol4-
yl))thiophene-2-carboxamidine hydrochloride: Methyl 4-(2-{ [4-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-203-
phenylmethoxyphenyl]amino } ( 1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate hydrobromide ( 100 mg, 0.18 mmol) was treated as described in
Example
154, step (b) to give 23.9 mg (27% yield) 5-methylthio-4-(2-{ [4-
(phenylmethoxy)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxamidine
S hydrochloride. 'H NMR (DMSO-d6, 300 MHz) 8 2.73 (s, 3H), 5.08 (s, 2H), 7.00
(d,
2H, J--8.2 Hz), 7.09 (s, 1H), 7.31-7.47 (m, SH), 7.70 (d, 2H, J--8.0 Hz), 8.47
(s, 1H),
8.88 (bs, 2H), 9.30 (bs, 2H), 10.20 (s, 1H); Mass Spectrum (ESI) m/z calcd.
for
CZZHZ°N4OS3, 452.62 (M+H), found 453.1.
Example 169
a) Methyl4-(2 ~(Z-bromophenyl)aminoJ(1,3-thiazol 4 yl))-S-
methyltlZiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2-
bromophenyl thiourea (44 mg) as described in Example 154, step (a) to give
63.1 mg
(64% yield) of methyl 4-{2-[(2-bromophenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8
2.65 (s, 3H), 3.82 (s, 3H), 7.00 (m, 1 H), 7.33 (s, 1 H), 7.40 (m, 1 H), 7.64
(dd, 1 H,
J--1.4, 7.9 Hz), 8.04, 8.11 (s, 1H rotomer), 8.27, 8.37 (dd, 1H 9.60, 9.80
(bs, 1H
rotomer, J--1.5, 8.2 Hz), Mass Spectrum (ESI) m/z calcd. for C,6H,3BrN,OzS3,
441.39
(M+H), found 441.1.
b) 4-(2-~(2-Bromophenyl)aminoJ(1,3-thiazol-4 yl)~-5-metlrylthiothiophene-
2-carboxamidine hydrochloride: Methyl 4-{2-[(2-bromophenyl)amino](1,3-thiazol-
4-yl)}-5-methylthiothiophene-2-carboxylate hydrobromide (63.1mg, 0.12 mmol)
was
treated as described in Example 154, step (b) to give 47.9 mg (86% yield) of 4-
{2-[(2-
bromophenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxamidine
hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 2.70 (s, 3H), 7.01 (m 1H), 7.20 (s,
1 H), 7.40 (m, 1 H), 7.65 (dd, 1 H, J--1.5, 8.0), 8.3 8 (dd, 1 H, J--1. S, 8.3
Hz), 8.44 (s,
1H), 8.89 (bs, 2H), 9.30 (bs, 2H), 9.62 (s, 1H); Mass Spectrum (ESI) m/z
calcd. for
C,SH,3BrN4S3, 425.39 (M+H), found 425.1.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-2D4
Example 170
a) Methyl4-~2 ~(2,6-dichlorophenyl)aminoJ(1,3-thiazol-4 yl)J-5-
metltylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2,6-dichlorophenyl thiourea (42 mg) as described in Example 154, step (a) to
give
63.1 mg (65% yield) of methyl 4-{2-[(2,6-dichlorophenyl)amino](1,3-thiazol-4-
yl)}-
5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz)
b 2.59 (s, 3H), 3.8 (s, 3H), 7.15 (s, 1H), 7.36 (m, 1H), 7.61 (m, 2H), 7.97
(s, 1H);
Mass Spectrum (ESI) m/z calcd. for C,6H,ZC12NZOZS3, 431.38 (M+H), found 431Ø
b) 4-~2-~(2,6-Dichlorophenyl)aminoJ(1,3-thiazol 4 yl)f-S-
methylthiothiophene-2-carboxamidine hydrochloride: Methyl 4-{2-((2,6-
dichlorophenyl)amino]( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-carboxylate
hydrobromide (43 mg, 0.08 mmol) was treated as described in Example 154, step
(b)
to give 14.5 mg (40% yield) of 4-{2-[(2,6-dichlorophenyl)amino](1,3-thiazol-4-
yl)}-
5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300
MHz) b 2.69 (s, 3H), 7.15 (s, 1H), 7.18-7.26 (m, 2H), 8.13 (d, 1H, J--7.~ Hz),
8.41 (s,
1H), 8.84 (bs, 2H), 9.27 (bs, 2H), 9.61 (bs, 1H); Mass Spectrum (ESI) m/z
calcd. for
C,SH,ZC12N4S3, 415.39 (M+H), found 415.1.
Example 171
a) Metlzyl4-(2-~(2-bromo-4-methylplzenyl)aminoJ(1,3-tlziazol-4 yl)J-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2-
bromo-4-methylphenyl thiourea (47 mg) as described in Example 154, step (a) to
give
62 mg (61% yield) of methyl 4-{2-[(2-bromo-4-methylphenyl)amino](1,3-thiazol-4-
yl)}-5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300
MHz) 8 2.28 (s, 3H), 3.82 (s, 3H), 7.21 (m, 1 H), 7.27 (s, 1 H), 7.48 (m, 1
H), 8.14, 8.17
(s, 1H rotomer), 9.52, 9.72 (bs, 1H rotomer); Mass Spectrum (ESI) m/z calcd.
for
C,~H,SBrNzO2S3, 455.42 (M+H), found 455Ø
b) 4-(2 I(2-Bromo-4-methylphenyl)aminoJ(1,3-thiazol 4 yl)J-5-
methyltltiothiophene-2-carboxamidine hydrochloride: Methyl 4-{2-[(2-bromo-4-
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-205-
rriethylpheny)amino]( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-carboxylate
hydrobromide (62 mg, 0.11 mmol) was treated as described in Example 154, step
(b)
to give 26 mg (50% yield) of 4-{2-[(2-bromo-4-methylphenyl)amino](1,3-thiazol-
4-
yl)}-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db,
300 MHz) b 2.28 (s, 3H), 2.70 (s, 3H), 7.14 (s, 1H), 7.21 (dd, 1H, J--1.6, 8.5
Hz), 7.49
(d, 1H, J 1.5 Hz), 8.16 (d, 1H, 8.3 Hz), 8.41 (s, 1H), 8.85 (bs, 2H), 9.28
(bs, 2H),
9.53 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,6H,SBrN4S3, 439.42 (M+H),
found
439.1.
Example 172
a) Methyl 5-methylthio-4-~2-~(2-morpholin-4 yletltyl)aminoJ(1,3-thiazol 4-
yl))thiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (100 mg, 0.32 mmol), was allowed to react
with
1-ethylmorpholinothiourea (61.2 mg) as described in Example 154, step (a) to
give
120.8 mg (79% yield) methyl 5-methylthio-4-{2-[(2-morpholin-4-
ylethyl)amino](1,3-
thiazol-4-yl)}thiophene-2-carboxylate hydrobromide.'H NMR (CD30D, 300 MHz) 8
2.64 (s, 3H), 3.43-3.52 (m, SH), 3.83-3.86 (m, lOH), 6.95 (s, 1H), 8.04 (s,
1H); Mass
Spectrum (ESI) m/z calcd. for C,6HZ,N3O3S3, 399.55 (M+H), found 400.1.
b) S-Metlzyltlzio-4-~2-~(2-morpholin-4 ylethyl)aminoJ(1,3-thiazol 4-
yl))thiophene-2-carboxylate hydrochloride: Methyl- 5-methylthio-4-{2-[(2-
morpholin-4-ylethyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate
hydrobromide
(62 mg, 0.12 mmol) was treated as described in Example 154, step (b) to give
26 mg
(52% yield) of 5-methylthio-4-{2-[(2-morpholin-4-ylethyl)amino](1,3-thiazol-4-
yl)}thiophene-2-carboxylate hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 2.69
(s, 3H), 3.16-3.95 (m, 1 SH), 6.96 (s, 1 H), 8.01 (bs, 1 H), 8.49 (s, 1 H),
8.84 (bs, 2H),
9.28 (bs, 2H), 10.49 (bs, 1H); Mass Spectrum (ESI) m/z calcd. for C~SHZ,NSOS3,
383.56 (M+H), found 384.2.
Example 173
a) Methyl 4-(2-~(2,3-dichlorophenyl)aminoJ(1,3-thiazol-4 yl)~-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
CA 02362390 2001-08-07
WO 00/47578 -206- PCT/US99/18065
2,3-dichlorophenylthiourea (42 mg) as described in Example 154, step (a) to
give 60.5
mg (62% yield) methyl 4-{2-[(2,3-dichlorophenyl)amino](1,3-thiazol-4-yl)}-S-
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8
2.66 (s, 3H), 3.82 (s, 3H), 7.27 (dd, 1H, J--1.5, 6.5 Hz), 7.36 (d, 1H, J--8.2
Hz), 7.43
(s, 1H), 8.14 (s, 1H), 8.62 (dd, 1H, J--1.5, 8.4 Hz), 9.95 (bs, 1H); Mass
Spectrum
(ESI) m/z calcd. for C,6H,zC12NzO2S3, 431.38 (M+H), found 431.1.
b) 4-~2-~(2,3-Dichlorophenyl)aminoJ(1,3-thiazol 4 yl))-S-
metlZyltlZiothiophene-2-carboxamidine hydrochloride: Methyl 4-{2-[(2,3-
dichlorophenyl)amino)(1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxylate
hydrobromide (60.5 mg, 0.11 mmol) was treated as described in Example 154,
step
(b) to give 15 mg (30% yield) of 4-{2-[(2,3-dichlorophenyl)amino](1,3-thiazol-
4-yl)}-
5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-d6, 300
MHz) b 2.71 (s, 3H), 7.27-7.28-7.41 (m, 2H), 8.45 (s, 1H), 8.63 (dd, 1H, J--
1.5, 8.4
Hz), 8.84 (bs, 2H), 9.29 (bs, 2H), 9.99 (s, 1H); Mass Spectrum (ESI) m/z
calcd. for
IS C~SH,ZCIzN4S3, 415.34 (M+H), found 415.1.
Example 174
a) Methyl S-methylthio-4-~2-~(3,4,5-trimethoxyphenyl)aminoJ(1,3-thiazol 4-
yl)f thiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
2,3,4-trimethoxyphenylthiourea (46 mg) as described in Example 154, step (a)
to give
61.8 mg (63% yield) of methyl 5-methylthio-4-{2-[(3,4,5-
trimethoxyphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate
hydrobromide.
'H NMR (DMSO-db, 300 MHz) 8 2.67 (s, 3H), 3.81 (s, 6H), 3.82 (s, 3H), 7.11 (s,
2H),
7.25 (s, 1H), 8.19 (s, 1H), 10.25 (s, 1H); Mass Spectrum (ESI) m/z calcd. for
C~8H2°N4O3S3, 436.56 (M+H), found 437.1.
b) S Methylthio-4-~2-~(3,4,5-trimethoxyphenyl)aminoJ(1,3-thiazol 4-
yl)Jthiophene-2-carboxamidine hydrochloride: Methyl 5-methylthio-4-{2-[(3,4,5-
trimethoxyphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate hydrobromide
(61.8 mg, 0.11 mmol) was treated as described in Example 154, step (b) to give
14 mg
(27% yield) of 5-methylthio-4-{2-[(3,4,5-trimethoxyphenyl)amino](1,3-thiazol-4-
yl)}thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8
CA 02362390 2001-08-07
WO 00/47578 _20~_ PCT/US99/18065
2:70 (s, 3H), 3.61 (s, 3H), 3.80 (s, 6H), 7.08 (s, 2H), 7.14 (s, 1H), 8.44 (s,
1H), 8.84
(bs, 2H), 9.26 (bs, 2H), 10.29 (s, 1H); Mass Spectrum (ESI) m/z calcd. for
C,BHz°N~03S3, 436.56 (M+H), found 437.1.
S Example 175
a) Methyl S-methylthio-4-(2-~(2 piperidylethyl)aminoJ(1,3-thiazol 4-
yl)ftlzioplzene-2-carboxylate IZydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (100 mg, 0.32 mmol) was allowed to react
with N-
ethylpiperidylthiourea (60.6 mg) as described in Example 154, step (a) to give
90 mg
(59% yield) of methyl S-methylthio-4-{2-[(2-piperidylethyl)amino](1,3-thiazol-
4-
yl)}thiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 1.41
(m, 2H), 1.70-1.79 (m, 6H), 2.65 (s, 3H), 2.95 (m, 2H), 3.52 (m, 2H), 3.73 (m,
2H),
3.82 (s, 3H), 7.08 (s, 1H), 7.96 (at, 1H, J--5.3 Hz), 8.09 (s, 1H), 9.40 (bs,
1H); Mass
Spectrum (ESI) m/z calcd. for C"H23N3OZS3, 397.6 (M+H), found 398.1.
b) 5 MethyltlZio-4-~2 ~(2 piperidylethyl)aminoJ(1,3-thiazol 4 yl)Jthiophene-
2-carboxamidine hydrochloride: Methyl 5-methylthio-4-{2-[(2-
piperidylethyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate hydrobromide
(72
mg, 0.15 mmol) was treated as described in Example 154, step (b) to give 26.8
mg
(43% yield) of 5-methylthio-4-{2-[(2-piperidylethyl)amino](1,3-thiazol-4-
yl)}thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 1.40
(m, 2H), 1.72-1.79 (m, 6H), 2.69 (s, 3H), 2.96 (m, 2H), 3.51 (m, 2H), 3.76 (m,
2H),
6.97 (s, 1 H), 8.08 (t, 1 H, J--5.5 Hz), 8.60 (s, 1 H), 8.95 (bs, 1 H), 9.3 5
(bs, 2H), 10.25
(s, 1H); Mass Spectrum (ESI) m/z calcd. for C,6Hz3N5S3, 381.1 (M+H), found
382.2.
Example 176
a) Methyl4-(2-(((4-methylphenyl)methylJaminoJ(1,3-thiazol 4 yl))-5-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (111 mg, 0.35 mmol) was allowed to react
with 4-
methylphenylmethylthiourea as described in Example 154, step (a) to give 125
mg
(81% yield) of methyl 4-(2-{[(4-methylphenyl)methyl)amino}(1,3-thiazol-4-yl))-
5-
methylthiothiophene-2-carboxylate hydrobromide. Mass Spectrum (ESI) m/z calcd.
for C,gH,gN202Sz, 358.5 (M+H), found 359.1.
CA 02362390 2001-08-07
WO 00/47578 _208- PCT/US99/18065
b) 4-(2-~~(4 Methylphenyl)methylJamirrof (1,3-thiazol 4 yl))-S-
methylthiothiophene-2-carboxamidirre hydrochloride: Methyl 4-(2-{[(4-
methylphenyl)methyl]amino } ( 1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate
hydrobromide (118 mg, 0.26 mmol) was treated as described in Example 154, step
(b)
to give 58.2 mg (54% yield) of 4-(2-{[(4-methylphenyl)methyl]amino}(1,3-
thiazol-4-
yl))-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db,
300 MHz) 8 2.27 (s, 3H), 2.66 (s, 3H), 4.49 (d, 2H, J--5.7 Hz), 6.88 (s, 1H),
7.13 (d,
2H, J--7.8 Hz), 7.27 (d, 2H, J--8.0 Hz), 8.20 (t, 1 H, J--5.8 Hz), 8.42 (s, 1
H), 8.90 (bs,
2H), 9.27 (bs, 2H); Mass Spectrum (ESI) m/z calcd. for C"H,gN4S3, 374.55
(M+H),
found 375.2.
Example 177
a) Amirro~~4-(4-chlorophenoxy)phenylJaminoJmethane-1-thione: Unless
otherwise indicated, all thioureas, isothiocyanates, thioamides and amines
were
purchased from Maybridge Chemical Co. Ltd.(Cornwall, U.K.), Transworld
Chemical
Co. (Rockville, MD), or Aldrich Chemical Co., (Milwaukee, WI). (i) 4-Amino-
4'-chlorodiphenylether (TCI America, Portland OR, 520 mg, 2.03 mmol) was
slurried
in 10 mL of ether and treated with ca. 1 mL of ether saturated with HCl gas.
After 5
min. the solvent was removed in vacuo. To a stirring biphasic solution amine-
HCl
salt in 20 mL CHCl3-sat'd NaHC03 (1:1, v/v) at ambient temperature was added
thiophosgene (1.2 equiv, 2.4 mmol) in 5 mL of CHCl3 dropwise via an addition
funnel. The reaction was vigorously stirred for 1 h (TLC, 50% ethyl acetate-
hexanes
indicates clean conversion to a higher Rf spot), at which time the layers were
separated, the aqueous layer extracted with CHC13 (1x20 mL), and the combined
organic layers washed with brine (1x20 mL) and dried (NazS04). Concentration
of the
solvent in vacuo yielded the crude 4-(4-chlorophenoxy)-phenylisothiocyanate
(414
mg). (ii) The 4-(4-chlorophenoxy)-phenylisothiocyanate was transferred to an
Ace
Glass pressure tube equipped with a Teflon coated stir bar and treated with a
2.0 M
solution of NH3 in 5 ml methanol (Aldrich Chemical Co., Milwaukee, WI)). The
tube
was sealed and immersed in a 80 °C oil bath. After 2 h, the reaction
was cooled to 0
°C in an ice bath. The precipitates were filtered and dried under
vacuum to yield
amino{[4-(4-chlorophenoxy)phenyl]amino}methane-1-thione (328 mg, 79%). 'H
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-209-
NMR (DMSO-db, 300 MHz) b 7.02 (m, 4H), 7.41 (m, 4H), 9.65 (s, 1 H); Mass
Spectrum (ESI) m/z calcd. for C,3H"C1NZOS, 278.8 (M+H), found 279.4.
b) Methyl4-(2-~~4-(4-chlorophenoxy)phenylJamirzoJ(1,3-thiazol 4 yl))-S
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5
methylthiothiophene-2-carboxylate (309 mg, 1.0 mmol) was allowed to react with
amino{[4-(4-chlorophenoxy)phenyl]amino}methane-1-thione (297 mg) as described
in Example 154, step (a) to give 410 mg (72% yield) of methyl-4-(2-{[4-(4-
chlorophenoxy)phenyl]amino} (1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate hydrobromide. Mass Spectrum (ESI) m/z calcd. for CZZH"C1N203S3,
489.1 (M+H), found 489.1.
c) 4-(2-~J4-(4-Chlorophenoxy)phenylJaminoJ(1,3-thiazol 4 yl))-S-
methylthiotlriophene-2-carboxamidine hydrochloride: Methyl 4-(2-{[4-(4-
chlorophenoxy)phenyl]amino } ( 1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate hydrobromide (300 mg, 0.52 mmol) was treated as described in
Example
154, step (b) to give 129.9 mg (49% yield) of 4-(2-{ [4-(4-
chlorophenoxy)phenyl]amino } ( 1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxamidine hydrochloride. 'H NMR (DMSO-d6, 300 MHz) 8 2.72 (s, 3H), 6.97
(m, 2H), 7.07 (m, 2H), 7.15 (s, 1H), 7.40 (m, 2H), 7.85 (m, 2H), 8.46 (s, 1H),
8.82
(bs, 2H), 9.27 (bs, 2H), 10.43 (bss, 1 H); Mass Spectrum (ESI) m/z calcd. for
Cz,H"C1N40S3, 473.1 (M+H), found 473.2, 475.1.
Example 178
a) Metltyl S-metlZylthio-4 (2-(~4 ~5-(tr~uorometlzyl)(2-
pyridyloxy)JphenylJamino)(1,3-thiazol 4 yl)JtIZiophene-2-carboxylate: Methyl 4-
(2-
bromoacetyl)-5-methylthiothiophene-2-carboxylate (70 mg, 0.23 mmol) was
allowed
to react with 4-[5-(trifluoromethyl)pyrid-2-yloxy]thiobenzamide (50 mg) as
described
in Example 154, step (a) to give 115 mg (98% yield) of methyl 5-methylthio-4-
[2-({4-
[5-(trifluoromethyl)(2-pyridyloxy)]phenyl } amino)( 1,3-thiazol-4-
yl)]thiophene-2-
carboxylate. 'H NMR (DMSO-db, 300 MHz) 8 2.70 (s, 3H), 3.85 (s, 3H), 7.38 (m,
3H), 8.10 (m, 1H), 8.18 (s, 1H), 8.28 (dd, 1H, J--2.7, 8.8 Hz), 8.32 (s, 1H),
8.60 (m,
1H); Mass Spectrum (ESI) m/z calcd. for CZZH,SF3NZO3S3, 508.56 (M+H), found
509.2.
CA 02362390 2001-08-07
WO 00/47578 _2~~~ PCT/US99/18065
b) S MetlzyltJzio-4-(2-(~4 ~5-(tr~uoromethyl)(2-
pyridyloxy)JphenylJamino)(1,3-thiazol 4 yl)Jtlziophene-2-carboxamidine
Izydroclzloride: Methyl5-methylthio-4-[2-({4-[5-(trifluoromethyl)(2-
pyridyloxy)]phenyl}amino)(1,3-thiazol-4-yl)]thiophene-2-carboxylate (95 mg,
0.18
mmol) was treated as described in Example 154, step (b) to give 30.3 mg (32%
yield)
of 5-methylthio-4-[2-({4-[5-(trifluoromethyl)(2-pyridyloxy)]phenyl}amino)(1,3-
thiazol-4-yl)]thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-d6, 300
MHz) 8 2.75 (s, 3H), 7.34 (d, 1H, J 8.7 Hz), 7.41 (m, 2H), 8.01 (s, 1H), 8.10-
8.14 (m,
2H), 8.29 (dd, 1 H, J--2.5, 8.4 Hz), 8.60 (m, 1 H), 8.63 (s, 1 H); 8.91 (bs,
2H), 9.31 (bs,
2H); Mass Spectrum (ESI) m/z calcd. for CZ,H~SF3N4OS3, 492.6 (M+H), found
493.1.
Example 179
a) Methyl 4-(2-~~4 phenoxyphenylJaminoJ(1,3-tltiazol 4 yl))-5-
methylthiotlziophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (200 mg, 0.64 mmol) was allowed to react
with 4-
phenoxyphenylthiourea (158 mg) as described in Example 154, step (a) to give
300
mg (88% yield) of methyl 4-(2-{[4-(phenoxy)phenyl]amino}(1,3-thiazol-4-yl))-5-
methylthiothiophene-2-carboxylate hydrobromide. Mass Spectrum (ESI) m/z calcd.
for CZZH,8NZO3S3, 454.6 (M+H), found 455.2.
b) 4-(2-~~4 PhenoxyphenylJaminoJ(1,3-tliiazol 4 yl))-S-
methylthiotlzioplzene-2-carboxamidine Izydrochloride: Methyl 4-(2-{[4-
(phenoxy)phenyl]amino } ( 1,3-thiazol-4-yl))-5-methylthiothiophene-2-
carboxylate
hydrobromide (230 mg, 0.42 mmol) was treated as described in Example 154, step
(b)
and purified by preparative thin layer chromatography (20% methanol-CHZCIz-
sat'd.
NH3, 500 mm silica gel plate, J.T. Baker, Phillipsburg, NJ) to give 86 mg (47%
yield)
of the product. A 46 mg aliquot was dissolved in 1 mL of methanol, treated
with 3
drops of ether saturated with HCl gas, and concentrated in vacuo with toluene
(2x5mL) to give 42.3 mg (21% yield) of 4-(2-{[4-phenoxyphenyl]amino}(1,3-
thiazol-
4-yl))-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-d6,
300 MHz) 8 2.71 (s, 3H), 6.97-7.11 (m, 4H), 7.15 (s, 1H), 7.36 (m, 2H), 7.72,
7.85 (d,
2H rotomer, J--8.7 Hz), 8.36, 8.55 (s, 1H rotomer), 9.00 (bs, 2H), 9.35 (bs,
2H), 10.49
(s, 1H); Mass Spectrum (ESI) m/z calcd. for CZ,H,8N4OS3, 438.6 (M+H),, found
439.2.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-211-
Example 180
a) Amino((4-(phenylamino)phenylJaminoJmethane-1-thione: 4-
Aminodiphenylamine (500 mg, 2.71 mmol) was treated as described in Example
177,
step (a) and recrystallized from toluene to give 350 mg (53% yield) of
amino{[4-
(phenylamino)phenyl]amino}methane-1-thione. 'H NMR (DMSO-d6, 300 MHz) 8
6.80 (m, 1H), 7.01-7.24 (m, 8H), 8.15 (s, 1H), 9.45 (s, 1H); Mass Spectrum
(ESI) m/z
calcd. for C'3H,3N3S, 243.33 (M+H), found 244.2.
b) Methyl S-metlzylthio-4-(2-((4-(phenylamino)phenylJaminoJ(1,3-thiazol 4-
yl))tlziophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (90 mg, 0.28 mmol) was allowed to react with
amino{[4-(phenylamino)phenyl]amino}methane-1-thione (70.8 mg) as described in
Example 154, step (a) to give 71 mg (47% yield) of methyl 5-methylthio-4-(2-{
[4-
(phenylamino)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide.'H NMR (DMSO-db, 300 MHz) b 2.66 (s, 3H), 3.82 (s, 3H), 6.73 (m,
1 H), 6.96-7.24 (m, 9H), 7.63 (d, 1 H, J--8.6 Hz), 8.12 (s, 1 H), 10.13 (bs, 1
H); Mass
Spectrum (ESI) m/z calcd. for CZZH,9N3OZS3, 453.60 (M+H), found 454.2.
c) S-Methylthio-4-(2-((4-(phenylamino)phenylJamino~(1,3-thiazol-4-
yl))tlZiopIZene-2-carboxamidine hydrochloride: Methyl 5-methylthio-4-(2-{ [4-
(phenylamino)phenyl]amino}(1,3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide (71 mg, 0.13 mmol) was treated as described in Example 154, step
(b)
to give 23.3 mg (38% yield) of 5-methylthio-4-(2-{[4-
(phenylamino)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxamidine
hydrochloride. 'H NMR (DMSO-d6, 300 MHz) b 2.72 (s, 3H), 6.74 (t, 1H, J--7.3
Hz),
6.98 (d, 1 H, J--7.6 Hz), 7.08 (m, 2H), 7,18 (m, 2H), 7.66 (d, 2H, J--8.9 Hz),
7.99 (s,
1H), 8.45 (s, 1H), 9.03 (bs, 4H), 10.17 (s, 1H); Mass Spectrum (ESI) m/z
calcd. for
CZ,H,~N5S3, 437.59 (M+H), found 438.2.
Example 181
a) Amino((4-benzylphenylJaminoJmethane-1-thione: 4-Benzylphenylamine
(500 mg, 2.73 mmol) was treated as described in Example 177, step (a) to give
410
mg (62% yield) of amino{[4-benzylphenyl]amino}methane-1-thione.'H NMR
CA 02362390 2001-08-07
WO 00/47578 _2~ 2_ PCT/US99/18065
(DMSO-db, 300 MHz) 8 3.89 (s, 2H), 7.14-7.28 (m, 9H), 9.59 (s, 1H); Mass
Spectrum
(ESI) m/z calcd. for C,4H,4NZS3, 242.1 (M+H), found 243.2.
b) Methyl S-methylthio-4-(2-~~4-benzylphenylJaminoJ(1,3-thiazol 4-
yl))thiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (90 mg, 0.28 mmol) was allowed to react with
amino{[4-benzylphenyl]amino}methane-1-thione (70.5 mg) as described in Example
154, step (a) to give 70.1 (47% yield) of methyl 5-methylthio-4-(2-{ [4-
benzylphenyl] amino } ( 1, 3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide. ' H
NMR (DMSO-d6, 300 MHz) 8 2.66 (s, 3H), 3.82 (s, 3H), 3.87 (s, 2H), 7.14 -7.30
(m,
8H), 7.66 (d, 2H, J--8.5 Hz), 8.12 (s, 1H), 10.23 (s, 1H); (Mass Spectrum
(ESI) m/z
calcd. for CZZH,9N3O2S3, 453.6 (M+H), found 454.2.
c) S-Methylthio-4-(2-~~4-benzylphenylJaminoJ(1,3-thiazol 4 yl))thiophene-
2-carboxamidine Izydrochloride: Methyl 5-methylthio-4-(2-{ [4-
benzylphenyl]amino}(1,3-thiazol-4-yl))thiophene-2-carboxylate hydrobromide
(82.2
mg, 0.15 mmol) was treated as described in Example 154, step (b) to give 33.4
mg
(47% yield) of 5-methylthio-4-(2-{[4-benzylphenyl]amino}(1,3-thiazol-4-
yl))thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 2.72
(s, 3H), 3.89 (s, 2H), 7.12 (s, 1H), 7.16-7.29 (m, 7H), 7.69 (d, 2H, J--8.6
Hz), 8.43 (s,
1H), 9.02 (bs, 4H), 10.28 (s, 1H); Mass Spectrum (ESI) m/z calcd. for
C,2H2°N4S3,
436.6 (M+H), found 437.2.
Example 182
a) ((4-((Aminotlzioxomethyl)aminoJphenyl)sulfonyl)piperidine: 4-
Aminophenylsulphonylpiperidine (500 mg, 2.08 mol) was treated as described in
Example 177, step (a) to give 382 mg (61% yield) of ({4-
[(aminothioxomethyl)amino]phenyl}sulfonyl)piperidine.'H NMR (DMSO-db, 300
MHz) 8 1.34 (m, 2H), 1.53 (m, 4H), 2.85 (m, 4H), 7.62 (m, 2H), 7.78 (m, 2H),
10.10
(bs, 1H); Mass Spectrum (ESI) m/z calcd. for C,ZH,~N302Sz, 299.4 (M+H), found
3 00.2.
b) Metlzyl 5-methyltliio-4-(2-~~4-(piperidylsulfonyl)plzenylJaminof (1,3-
thiazol-4 yl))tlziophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-
5-
methylthiothiophene-2-carboxylate (90 mg, 0.28 mmol) was allowed to react with
CA 02362390 2001-08-07
w0 00/47578 _2~ 3_ PCT/US99/18065
({4-[(aminothioxomethyl)amino]phenyl}sulfonyl)piperidine (87.1 mg) as
described in
Example 154, step (a) to give 105 mg (63% yield) of methyl 5-methylthio-4-(2-
{[4-
(piperidylsulfonyl)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 1.33 (m, 2H), 1.52 (m, 4H), 2.69 (s,
3H), 2.84 (m, 4H), 3.82 (s, 3H), 7.43 (s, 1H), 7.66 (m, 2H), 7.98 (m, 2H),
8.16 (s, 1H),
10.85 (s, 1H); (Mass Spectrum (ESI) m/z calcd. for Cz,Hz3N3O4S4, 509.69 (M+H),
found 510.2.
c) S-Methylthio-4-(2-~~4-(piperidylsulfonyl)phenylJaminoJ(1,3-thiazol4-
yl))thioplZene-2-carboxamidine hydrochloride: Methyl 5-methylthio-4-(2-{ [4-
(piperidylsulfonyl)phenyl]amino}(1,3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide (105 mg, 0.17 mmol) was treated as described in Example 154, step
(b)
to give 30.3 mg (34% yield) of 5-methylthio-4-(2-{[4-
(piperidylsulfonyl)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-
carboxamidine
hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 1.36 (m, 2H), 1.54 (m, 4H), 2.76
(s,
3H), 2.86 (m, 4H), 7.30 (s, 1H), 7.68 (d, 2H, J--8.8 Hz), 8.03 (d, 2H, J--8.8
Hz), 8.51
(s, 1 H), 8.84 (bs, 2H), 9.28 (bs, 2H), 10.94 (s, 1 H); Mass Spectrum (ESI)
m/z calcd.
for Cz°H23NSO2'~5~ 493.69 (M+H), found 494.2.
Example 183
a) Amino(3-quinolylamino)methane-1-thione: 3-Aminoquinoline (500 mg,
3.46 mmol) was treated as described in Example 177, step (a) to give 285 mg
(41
yield) of amino(3-quinolylamino)methane-1-thione. 'H NMR (DMSO-db, 300 MHz)
8 7.57 (m, 1 H), 7.67 (m, 1 H), 7.94 (m, 2H), 8.41 (d, 1 H, J--2.4 Hz), 8.85
(d, 1 H, J--2.5
Hz), 10.03 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,°H9N3S, 203.3
(M+H),
found 204.1.
b) Methyl S-metlzyltlaio-4 ~2-(3-quinolylamino)(1,3-thiazol-4 yl)Jtlaiophene-
2-carboxylate: Methyl 4-(2-bromoacetyl)-5-methylthiothiophene-2-carboxylate
(90
mg, 0.28 mmol) was allowed to react with amino(3-quinolylamino)methane-1-
thione
(59.1 mg) as described in Example 154, step (a) to give 107.5 mg (78% yield)
of
methyl5-methylthio-4-[2-(3-quinolylamino)(1,3-thiazol-4-yl)]thiophene-2-
carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 2.75 (s, 3H), 3.84 (s,
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-214-
3H), 7.52 (s, 1H), 7.92-8.05 (m, 2H), 8.22 (s, 1H), 9.22 (m, 2H); Mass
Spectrum (ESI)
m/z calcd. for C,9H,SN3OZS3, 413.54 (M+H), found 414.1.
c) S-Metltylthio-4-(2-(3-quinolylamino)(1,3-thiazol 4 yl)Jthiophene-2-
carboxamidine hydrochloride: Methyl 5-methylthio-4-[2-(3-quinolylamino)(1,3-
thiazol-4-yl))thiophene-2-carboxylate hydrobromide (107.5 mg, 0.21 mmol) was
treated as described in Example 154, step (b) to give 4.5 mg (4.9% yield) of 5-
methylthio-4-[2-(3-quinolylamino)(1,3-thiazol-4-yl))thiophene-2-carboxamidine
hydrochloride.'H NMR (DMSO-d6, 300 MHz) 8 2.80 (s, 3H), 7.29 (s, 1H), 7.59 (m,
2H), 7.93 (m, 2H), 8.54 (s, 1 H), 8.89 (bs, 2H), 8.91 (m, 1 H), 9.16 (m, 1 H),
9.29 (bs,
2H), 10.97 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,BH,SNSS3, 397.5
(M+H),
found 398.1.
Example 184
a) Metlzyl S-metlzylthio-4 ~2-(2-naplzthylamino)(1,3-thiazol 4 yl)Jthiophene-
2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-methylthiothiophene-2-
carboxylate (65 mg, 0.21 mmol) was allowed react with 2-napthylthiourea (42.4
mg)
as described in Example 154, step (a) to give 82.5 mg (80% yield)of methyl 5-
methylthio-4-[2-(2-naphthylamino)(1,3-thiazol-4-yl)]thiophene-2-carboxylate
hydrobromide. 'H NMR (DMSO-d6, 300 MHz) 8 2.67 (s, 3H), 3.83 (s, 3H), 7.31 (s,
1 H), 7.5 0-7. 67 (m, 4H), 7.93 (m, 1 H), 8 .15 (s, 1 H), 8 .31-8.3 5 (m, 1
H), 8 .46 (d, 1 H,
J--7.6), 10.22 (s, 1H)); Mass Spectrum (ESI) m/z calcd: for
CZ°H,6NZOZS3, 412.6
(M+H), found 413.1.
c) S-Methylthio-4 ~2-(2-naphthylamino)(1,3-thiazol 4 yl)Jthiophene-2-
carboxamidine hydrochloride: Methyl 5-methylthio-4-[2-(2-naphthylamino)(1,3-
thiazol-4-yl)]thiophene-2-carboxylate hydrobromide (42.7 mg, 0.086 mmol) was
treated as described in Example 154, step (b) to give 5.8 mg ( 16% yield) of 5-
methylthio-4-[2-(2-naphthylamino)( 1,3-thiazol-4-yl)]thiophene-2-carboxamidine
hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 2.72 (s, 3H), 7.12-7.27 (m, 3H),
7.50-7.68 (m, 3H), 7.94 (m, 1H), 8.32-8.35 (m, m, 1H), 8.51 (s, 1H), 8.97 (bs,
2H),
9.34 (bs, 2H), 10.26 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,9H,6N4S3,
396.6
(M+H), found 397.2.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-z~ 5-
Example 185
a) Methyl4-~Z-(2H benzo~3,4-dJl,3-dioxolan-S ylamino)(1,3-thiazol 4 yl)J
S-methyltl:iotlziophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (65 mg, 0.21 mmol) was allowed to react with
2,3-methylenedioxyphenylthiourea (41.2 mg) as described in Example 154, step
(a) to
give S1 mg (50% yield) ofmethyl 4-[2-(2H-benzo[3,4-d]1,3-dioxolan-5-
ylamino)(1,3-
thiazol-4-yl)]-5-methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-
db, 300 MHz) 8 2.66 (s, 3H), 3.83 (s, 3H), 5.98 (s, 2H), 6.84-6.89 (m, 1H),
6.96, 7.04
(dd, 1 H rotomer, J--2.2, 8.5 Hz), 7.25 (s, 1 H), 7.46, 7.60 (d, 1 H rotomer,
J--2.1 Hz),
8.05, 8.13 (s, 1H rotomer), 10.19, 10.34 (s, 1H, rotomer); Mass Spectrum (ESI)
m/z
calcd. for C"H,4NZO4S3, 406.5 (M+H), found 407.1.
b) 4-~2-(2H Benzo~3,4-dJl,3-dioxolan-5 ylamino)(1,3-tlziazol 4 yl)J S-
metlzylthiothiophene-Z-carboxamidine hydrochloride: Methyl 4-[2-(2H-benzo[3,4-
d] 1,3-dioxolan-5-ylamino)( 1,3-thiazol-4-yl)]-5-methylthiothiophene-2-
carboxylate
1 S hydrobromide (S 1 mg, 0.10 mmol) was treated as described in Example 154,
step (b)
to give 16.6 mg (39% yield) of 4-[2-(2H-benzo[3,4-d] 1,3-dioxolan-5-
ylamino)(1,3-
thiazol-4-yl)]-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR
(DMSO-db, 300 MHz) 8 2.71(s, 3H), 5.98 (s, 2H), 6.87 (d, 1H, J--8.2 Hz), 7.09-
7.13
(m, 2H), 7.67 (d, 1H, J--2.4 Hz), 8.50 (s, 1H), 8.95 (bs, 2H), 9.33 (bs, 2H),
10.30 (s,
1H); Mass Spectrum (ESI) m/z calcd. for C,6H,4N402S3, 390.51 (M+H), found
391.2;
Example 186
a) Amino~(7 bromofluoren-2 yl)aminoJmetlaane-1-thione: 2-Amino-7-
bromofluorene (500 mg, 1.90 mmol) was treated as described in Example 177,
step
(a) to give 128 mg (21% yield) of amino[(7-bromofluoren-2-yl)amino]methane-1-
thione.'H NMR (DMSO-db, 300 MHz) 8 3.35 (s, 2H), 7.35 (d, 1H, J--8.3 Hz), 7.54
(d, 1H, J--8.0 Hz), 7.66 (s, 1H), 7.77-7.87 (m, 3H), 9.80 (s, 1H); Mass
Spectrum (ESI)
m/z calcd. for C,4H"BrN2S, 319.2 (M+H), found 320.1, 321.1.
b) Methyl4-~2 ~(7 bromofluoren-2 yl)aminoJ(1,3-thiazol 4 yl)~-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (90 mg, 0.28 mmol) was allowed to react with
amino[(7-bromofluoren-2-yl)amino]methane-1-thione (92.8 mg) as described in
CA 02362390 2001-08-07
w0 00/47578 _2,16_ PCT/US99/18065
Example 154, step (a) to give 141 mg (82% yield) of methyl 4-{2-[(7-
bromofluoren-
2-yl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxylate
hydrobromide.
'H NMR (DMSO-db, 300 MHz) 8 2.70 (s, 3H), 3.83 (s, 3H), 3.93 (s, 2H), 7.33 (s,
1H),
7. S 1 (dd, 1 H, J--1.9, 8.0 Hz), 7.65 (dd, 1 H, J--2.0, 8.4 Hz), 7.74 (ad,
2H, J 8.3 Hz),
S 7.83 (ad, 1 H, J--8.4 Hz), 8.18 (s, 1 H), 8.23 (d, 1 H, J--1.4 Hz), 10.47
(s, 1 H).
c) 4-~2 ~(7 Bromofluoren-2 yl)aminoJ(1,3-thiazol 4 yl)J-S-
methylthiothiophene-2-carboxamidine hydrochloride: Methyl 4-{2-[(7-
bromofluoren-2-yl)amino] ( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-
carboxylate
hydrobromide ( 100 mg, 0.15 mmol) was treated as described in Example 154,
step (b)
to give 3.3 mg (4% yield) of 4-{2-[(7-bromofluoren-2-yl)amino](1,3-thiazol-4-
yl)}-5-
methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz)
8 2.76 (s, 3H), 3.95 (s, 2H), 7.18 (s, 1H), 7.54 (dd, 1H, J--1.8, 10.0 Hz),
7.67-7.76 (m,
3H), 7.85 (d, 1H, J--8.2 Hz), 8.23 (s, 1H), 8.50 (s, 1H), 10.53 (s, 1H); Mass
Spectrum
(ESI) m/z calcd. for CZZH"BrN4S3, 513.5 (M+H), found 513.1, 515.1.
Example 187
a) Methyl 4-(2-~(4-cyclolzexylplzenyl)aminoJ(1,3-tlziazol 4 yl)J-S-
methylthiothiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (65 mg, 0.21 mmol) was allowed to react with
4-
cyclohexylphenylthiourea (49.2 mg) as described in Example 154, step (a) to
give 45
mg (41% yield) of methyl 4-{2-[(4-cyclohexylphenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8
1.23-1.39 (m, SH), 1.71-1.79 (m, SH), 2.68 (s, 3H), 3.83 (s, 3H), 7.16 (d, 2H,
J--8.6
Hz), 7.26 (s, 1 H), 7.65 (d, 2H, J--8.7 Hz), 8.14 (s, 1 H), 10.19 (s, 1 H);
Mass Spectrum
(ESI) m/z calcd. for CZZH24N2~2s3~ 444.64 (M+H), found 445.2.
b) 4-~2-((9-Cyclolzexylphenyl)aminoJ(1,3-tlziazol 4 yl)~-S-
methylthiothiophene-2-carboxamidine hydrochloride: Methyl 4-{2-[(4-
cyclohexylphenyl)amino]( 1,3-thiazol-4-yl) }-5-methylthiothiophene-2-
carboxylate
hydrobromide (31.1 mg, 0.059 mmol) was treated as described in Example 154,
step
(b) to give 12.8 mg (47% yield) of 4-{2-[(4-cyclohexylphenyl)amino](1,3-
thiazol-4-
yl)}-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300
MHz) 8 1.33-1.40 (m, SH), 1.68-1.79 (m, SH), 2.44 (m, 1H), 2.73 (s, 3H), 7.12
(s,
CA 02362390 2001-08-07
WO 00/47578 _2~ ~_ PCT/US99/18065
1H), 7.18 (d, 2H, J--8.7 Hz), 7.68 (d, 2H, J--8.7 Hz), 8.47 (s, 1H), 8.85 (bs,
2H), 9.32
(bs, 2H), 10.28 (s, 1H); Mass Spectrum (ESI) m/z calcd. for CZ,H24N4S3, 428.64
(M+H), found 429.2.
S Example 188
a) Amino~~4-(phenyldiazenyl)phenylJaminoJmethane-1-thione: 4-
Phenylazophenylisothiocyanate (314 mg, 1.30 mmol) was treated as described in
Example 177, step (a), part (ii), to give 295 mg (88% yield) of amino{[4-
(phenyldiazenyl)phenyl]amino}methane-1-thione. 'H NMR (DMSO-db, 300 MHz)
8 6.84 (m, 1H), 7.57 (m, 2H), 7.73 (m, 2H), 7.85-7.89 (m, 4H), 10.04 (s, 1H);
Mass
Spectrum (ESI) m/z calcd. for C,3H,ZN4S, 256.3 (M+H), found 257.2.
b) Methyl S-methylthio-4-(2-~~4-(phenyldiazenyl)phenylJamino)(1,3-thiazol
4 yl))thiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (65 mg, 0.21 mmol) was allowed to react with
amino{[4-(phenyldiazenyl)phenyl]amino}methane-1-thione (53.8 mg) as described
in
Example 154, step (a) to give 80.6 mg (70% yield) of methyl 5-methylthio-4-(2-
{ [4-
(phenyldiazenyl)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide. 'H NMR (DMSO-d6, 300 MHz) 8 2.72 (s, 3H), 3.84 (s, 3H), 7.46 (s,
1H), 7.49-7.61 (m, 3H), 7.84 (m, 2H), 7.91-8.02 (m, 4H), 8.20 (s, 1H), 10.83
(s, 1H);
Mass Spectrum (ESI) m/z calcd. for CZZH,8N402S3, 466.6 (M+H), found 467.1.
c) S-Metlzylthio-4-(2-~~4-(plzenyldiazenyl)plzenylJaminoJ(1,3-tl:iazol4-
yl))tlZiophene-2-carboxamidine hydrochloride: Methyl 5-methylthio-4-(2-{[4-
(phenyldiazenyl)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxylate
hydrobromide (47.7 mg, 0.087 mmol) was treated as described in Example 154,
step
(b) to give 32.8 mg (77% yield) of 5-methylthio-4-(2-{[4-
(phenyldiazenyl)phenyl]amino } ( 1,3-thiazol-4-yl))thiophene-2-carboxamidine
hydrochloride. ' H NMR (DMSO-db, 300 MHz) b 2.78 (s, 3H), 7.26 (s, 1H), 7.49-
7.63 (m, 3H), 7.66-7.74 (m, 3H), 7.84-8.08 (m, 3H), 8.60 (s, 1H), 11.02 (bs,
1H);
Mass Spectrum (ESI) m/z calcd. for Cz,H,gN6S3, 450.6 (M+H), found 451.1.
CA 02362390 2001-08-07
WO 00/47578 _2,1$_ PCT/US99/18065
Example 189
a) ~3-~(Aminothioxomethyl)aminoJphenylf methan-1-ol: 3-Aminobenzyl
alcohol (550 mg, 4.46 mmol) was treated as described in Example 177, step (a)
to
give 618 mg (76% yield) of {3-[(aminothioxomethyl)amino]phenyl}methan-I-ol. 'H
NMR (DMSO-db, 300 MHz) 8 4.47 (d, 2H, J--5.6 Hz), 5.19 (t, 1H, J--5.7 Hz),
7.06 (d,
1H, J--6.2 Hz), 7.18-7.30 (m, 3H), 9.73 (s, 1H).
b) Methyl S-metlzylthio4-(2-~~3-(hydroxymethyl)phenylJamino)(1,3-thiazol
4 yl))-thiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (1.01 g, 3.26 mmol) was allowed to react
with of
{3-[(aminothioxomethyl)amino]phenyl}methan-1-of as described in Example 154,
step (a) to give 1.42 g (92% yield) of methyl-5-methylthio4-(2-{[3-
(hydroxymethyl)phenyl]amino } ( 1,3-thiazol-4-yl))-thiophene-2-carboxylate
hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 2.67 (s, 3H), 3.83 (s, 3H), 4.49 (s,
2H), 6.92 (m, 1 H), 7.23-7.31 (m, 2H), 7.60 (m, 1 H), 7.81 (bs, 1 H), 8.17 (s,
1 H), I 0.29
(bs, 1 H).
c) S-Methylthio 4-(2-~~3-(hydroxymethyl)phenylJamino)(1,3-thiazol 4 yl))-
thiophene-2-carboxamidine hydrochloride: Methyl-5-methylthio4-(2-{ [3-
(hydroxymethyl)phenyl] amino } ( 1,3-thiazol-4-yl))-thiophene-2-carboxylate
hydrobromide (700 mg, 1.47 mmol) was treated as described in Example I54, step
(b)
using 1:9:1 methanol-CHzCl2-DMF as eluent to give 195 mg (32% yield) of 5-
methylthio 4-(2-{[3-(hydroxymethyl)phenyl]amino}(1,3-thiazol-4-yl))-thiophene-
2-
carboxamidine hydrochloride. ' H NMR (DMSO-db, 300 MHz) 8 2.71 (s, 3H), 4.50
(s, 2H), 6.93 (d, 1 H, J--7.6 Hz), 7.15 (s, 1 H), 7.21-7.27 (m, 1 H), 7.38
(bs, l H), 7.65
(d, 1H, J--8.1 Hz), 7.80 (s, 1H), 8.53 (s, 1H), 8.94 (bs, 2H), 9.32 (bs, 2H),
10.37 (s,
1H); Mass Spectrum (ESI) m/z calcd. for C,6H,6N4OS3, 376.5 (M+H), found 377.2.
Example 190
a) (tert-Butoxy)-N ~(4-~2 ~(3-hydroxymethylphenyl)aminoJ(1,3-thiazol 4-
yl)f-S-methylthio(2-thieuyl))iminomethylJ carboxamide: 5-Methylthio 4-(2-{[3-
(hydroxymethyl)phenyl]amino}(1,3-thiazol-4-yl))-thiophene-2-carboxamidine (103
mg, 0.27 mmol) was slurried in THF (4 mL) and treated with 0.5 mL of 0.5 N
NaOH.
tert-Butyldicarbonate (0.40 mmol) was added in one portion and the resulting
mixture
CA 02362390 2001-08-07
WO 00/47578 _2~9_ PCT/US99/18065
v~ias stirred overnight. The reaction was partitioned in CHZC12 and water. The
organic
layer was separated and washed with brine ( 1 x20 mL) and dried (Na2S04).
Removal
of the solvent in vacuo, followed by purification on preparative thin layer
chromatography (500 mm silica gel plate, J.T.Baker, Phillipsburg, NJ, 1%
methanol-
CHZCIz), gave 45 mg (35% yield) of ((tert-Butoxy)-N-[(4-{2-[(3-
hydroxymethylphenyl)amino] ( 1,3-thiazol-4-yl) }-5-methylthio(2-
thienyl))iminomethyl]-carboxamide. ' H NMR (DMSO-db, 300 MHz) 8 1.44 (s, 9H),
2.66 (s, 3H), 4.49 (d, 2H, J--5.7 Hz), 5.15 (t, 1H, J--S.5 Hz), 6.92 (d, 1H, J-
-7.5 Hz),
6.96 (s, 1 H), 7.26 (m, 1 H), 7.66 -7.75 (m, 2H), 8.38 (s, 1 H), 8.98 (bs,
2H), 10.24 (s,
1H).
b) (tert-Butoxy) N (imino(4-~2-(~3-~(3-
methylpiperidyl)methylJphenylJamino)(1,3-thiazol 4 yl)J S-methylthio(2-
tl:ienyl)~methyl)carboxamide: To a stirring solution of ((tert-butoxy)-N-[(4-
{2-[(3-
hydroxymethylphenyl)amino] ( 1,3-thiazol-4-yl) }-5-methylthio(2-
thienyl))iminomethyl]-carboxamide (45 mg, 0.094 mmol) under NZ was added
triethylamine (2 equiv, 26.3 p,l), followed by methansulfonyl chloride
(Aldrich
Chemical Co., Milwaukee, WI, 0.13 mmol, 10.2 ~l ). The reaction was stirred
for 1 h,
at which time the reaction was partitioned in CHzCl2-water. The organic layer
was
washed with brine (20 mL), filtered through a 5-cm pad of silica gel in a 1 S-
mL fritted
glass funnel and dried (NazS04). Removal of the solvent in vacuo afforded the
crude
mesylate (44 mg) which was used immediately without further purification. To
25.3
mg (0.045 mmol) of the mesylate in 0.5 mL of DMF was added 3-methyl piperidine
(0.18 mmol, 21.4 p.L ) and the result was heated to 65°C in an oil bath
for 4 h. The
reaction was concentrated in vacuo and purified by preparative thin layer
chromatography (250 mm silica gel plate, 10% methanol-CHZCIZ, J.T.Baker,
Phillipsburg, NJ) to give 8.2 mg (32% yield) of (tert-butoxy)-N-(imino{4-[2-
({3-[(3-
methylpiperidyl)methyl]phenyl } amino)( 1,3-thiazol-4-yl)]-5-methylthio(2-
thienyl)}methyl)carboxamide. Mass Spectrum (ESI) m/z calcd. for CZ,H35NSOZS3,
557.8 (M+H), found 557.9, 458.2 (-C(O)OC(CH3)3.
c) 4-~2-((3-((3-Metltylpiperidyl)methylJphenylJamino)(1,3-thiazol 4 yl)J 5-
methyltl:iothiophene-2-carboxamidiue hydrochloride: (tert-Butoxy)-N-(imino{4-
[2-
( { 3-[(3-methylpiperidyl)methyl]phenyl } amino)( 1,3-thiazol-4-yl)]-5-
methylthio(2-
CA 02362390 2001-08-07
WO 00/47578 -220- PCT/US99/18065
thienyl)}methyl)carboxamide (8.2 mg, 0.014 mmol) was stirred 2 mL of a 10% 3N
HCl-ethyl acetate solution at 0 °C for 30 min., at which time the
solvent was removed
in vacuo to give 8 mg (100% yield) of the 4-[2-({3-[(3-
methylpiperidyl)methyl]phenyl } amino)( 1,3-thiazol-4-yl)]-5-
methylthiothiophene-2-
carboxamidine hydrochloride. ' H NMR (DMSO-d6, 300 MHz) 80.83 (d, 3H, J--5.6
Hz), 1.54-2.48 (m, SH), 2.52-2.63 (m, 4H), 2.66 (s, 3H), 4.23 (d, 2H, J--4.8
Hz), 7.15-
7.23 (m, 2H), 7.41 (t, 1 H, J--7.8 Hz), 7.86-7.92 (m, 2H), 8.63 (s, 1 H), 9.01
(bs, 2H),
9.42 (bs, 2H), 10.63 (s, 1H); (Mass Spectrum (ESI) m/z calcd. for CZZHz,N5S3,
457.7
(M+H), found 458.2.
Example 191
a) Methyl S-methylthio-4-~2-~(3-Izydroxyphenyl)aminoJ(1,3-thiazol 4 yl)J-
thiophene-2-carboxylate hydrobromide: Methyl 4-(2-bromoacetyl)-5-
methylthiothiophene-2-carboxylate (60 mg, 0.19 mmol) was allowed to react with
3-
hydroxyphenylthiourea (32.6 mg) as described in Example 154, step (a) to give
80.2
mg (92% yield) of methyl-5-methylthio-4-{2-[(3-hydroxyphenyl)amino](1,3-
thiazol-
4-yl)}-thiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 2.67
(s, 3H), 3.83 (s, 3H), 6.38 (d, 1H, J--7.6 Hz), 7.06-7.12 (m, 2H), 7.20-7.29
(m, 2H),
8.14 (s, 1 H), 10.17 (s, 1 H).
b) 4-~2 ~(3 Hydroxyphenyl)aminoJ(1,3-thiazol 4 yl)J-5-
methylthiothiophene-2-carboxamidine hydrochloride: Methyl-5-methylthio-4-{2-
[(3-hydroxyphenyl)amino](1,3-thiazol-4-yl)}-thiophene-2-carboxylate
hydrobromide
(460 mg, 1.0 mmol) was treated as described in Example 154, step (b) to give
215 mg
(54% yield) of 4-{2-[(3-hydroxyphenyl)amino](1,3-thiazol-4-yl)}-5-
methylthiothiophene-2-carboxamidine hydrochloride. (Mass Spectrum (ESI) m/z
calcd. for C,SH,4N4OS3, 362.5 (M+H), found 363.2.
c) (tert-Butoxy) N ~(4-~2 ~(.4-hydroxyphenyl)aminoJ(1,3-thiazo!-4 yl)f-5-
methyltlzio(2-thienyl))iminometlzylJcarboxamide: To a stirring solution of 4-
{2-[(3-
hydroxyphenyl)amino](1,3-thiazol-4-yl)}-5-methylthiothiophene-2-carboxamidine
hydrochloride (215 mg, 0.48 mmol) in 4 mL of CHZCIz-DMF (3:1, v/v) was added
di-
isopropylethylamine (1.2 equiv). Di-tert-butyl dicarbonate (1.2 equiv, 127 mg,
Aldrich Chemicals, Milwaukee, WI) was then added dropwise in 1 mL CHZC12 via
an
CA 02362390 2001-08-07
WO 00/47578 _2a~ _ PCT/US99/18065
addition funnel. The reaction was allowed to stir overnight, partitioned in
CHZCIz-
H20, and the layers separated. The organic layer was dried (Na2S04) and
concentrated
in vacuo. The residue was purified by flash chromatography (1% methanol-
CHzCIz)
to give 60 mg (27% yield) of (tert-butoxy)-N-[(4-{2-[(4-
hydroxyphenyl)amino](1,3-
thiazol-4-yl)}-S-methylthio(2-thienyl))iminomethyl]carboxamide. 'H NMR (DMSO-
db, 300 MHz) 8 1.44 (s, 9H), 2.72 (s, 3H), 6.38 (m, 1H), 6.96 (s, 1H), 7.06-
7.12 (m,
2H), 7.28 (m, 1 H), 8.35 (s, 1 H), 9.00 (bs, 2H), 9.28 (s, 1 H), 10.11 (s, 1
H); Mass
Spectrum (ESI) m/z calcd. for CZ°HZZN4O3S3, 462.6 (M+H), found 462.7,
363.2 [-
C(O)OC(CH3)3]~
d) (tert-Butoxy) N ~~4-(2-~~3-(carbamoylmethoxy)phenylJaminoJ(1,3-
thiazol 4 yl))-S-methylthio(2-tlzienyl)JiminomethylJcarboxamide: To stirring
solution of (tert-butoxy)-N-[(4-{2-[(4-hydroxyphenyl)amino](1,3-thiazol-4-yl)}-
5-
methylthio(2-thienyl))iminomethyl]carboxamide (65 mg, 0.14 mmol) in 1.5 mL of
DMF was added sequentially CsZC03 (l.s equiv, 60.1 mg, Aldrich Chemicals,
Milwaukee, WI), bromoacetamide (1.2 equiv, 20.4 mg, Aldrich Chemicals,
Milwaukee, WI), and a catalytic amount of KI. The reaction was warmed to 58
°C in
an oil bath, stirred for 48 h, at which time another 0.6 equiv of
bromoacetamide was
added. Stirring was continued for another 24 h, at which time the reaction was
filtered
and concentrated in vacuo. The residue was purified by preparative thin layer
chromatography (50% ethyl acetate-hexanes) to give 9 mg (12% yield) of (tert-
butoxy)-N-{ [4-(2- { [3-(carbamoylmethoxy)phenyl]amino } ( 1,3-thiazol-4-yl))-
5-
methylthio(2-thienyl)]iminomethyl}carboxamide. Mass Spectrum (ESI) m/z calcd.
for
C22H25N504'S3~ 519.7 (M+H), found 519.7, 420.7 [-C(O)OC(CH3)3].
e) 4-(2-~~4-(Carbamoylmethoxy)phenylJaminoJ(1,3-thiazol 4 yl))-5-
methylthiothiophene-2-carboxamidine tr j~uoroacetate: To a stirring suspension
of(tert-butoxy)-N-{ [4-(2-{ [3-(carbamoylmethoxy)phenyl]amino } ( 1,3-thiazol-
4-yl))-5-
methylthio(2-thienyl)]iminomethyl}carboxamide (ca. 4 mg, 0.007 mmol) in CHZCIz-
DMF (4 mL, 3:1 v/v) at 0 °C was added 1 mL of trifluoroacetic
acid. The
homogeneous solution was stirred an additional 40 min. at this temperature,
warmed
to ambient temperature over a 30 min. period and concentrated in vacuo to give
4 mg
(100% yield) of 4-(2-{[4-(carbamoylmethoxy)phenyl]amino}(1,3-thiazol-4-yl))-5-
methylthiothiophene-2-carboxamidine trifluoroacetate. 'H NMR (DMSO-db, 300
CA 02362390 2001-08-07
WO 00/47578 -222- PCT/US99/18065
MHz) 8 2.75 (s, 3H), 4.21 (d, 2H, J--5.7 Hz), 6.64 (dd, 1 H, J--2.4, 8.2 Hz),
6.97 (dd,
1 H, J--1.1, 8.2 Hz), 7.16 (s, 1 H), 7.22 (m, 1 H), 7.60-7.63 (m, 1 H), 7.69-
7.72 (m, 1 H),
7.88 (t, 1H, J--2.1 Hz), 8.42 (s, 1H); Mass Spectrum (ESI) m/z calcd. for
C"H"NSOZS3, 419.6 (M+H), found 420.1.
Example 192
a) Isopropyl 5-methyl-4-(2 ~(3,4,5-trimethoxyphenyl)aminoJ(1,3-thiazol-4-
yl))thiophene-2-carboxylate hydrobromide: Isopropyl-4-(2-bromoacetyl)-5-
methylthiophene-2-carboxylate (84 mg, 0.27 mmol) was allowed to react with
3,4,5-
trimethoxyphenylthiourea (66.5 mg) as described in Example 154, step (a) to
give 68
mg (48% yield) of isopropyl 5-methyl-4-{2-[(3,4,5-trimethoxyphenyl)amino](1,3-
thiazol-4-yl)}thiophene-2-carboxylate hydrobromide. Mass Spectrum (ESI) m/z
calcd.
For CZ,H24NZOSS2, 448.56 (M+H), found 449Ø
b) S-Metlzyl4-~2-~(3,4,5-trimethoxyphenyl)aminoJ(1,3-thiazol-4-
yl))tlziophene-2-carboxamidine hydrochloride: Isopropyl 5-methyl-4-{2-[(3,4,5-
trimethoxyphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate hydrobromide
(59 mg, 0.11 mmol) was treated as described in Example 154, step (b) to give
24.4 mg
(50% yield) of S-methyl-4-{2-[(3,4,5-trimethoxyphenyl)amino](1,3-thiazol-4-
yl)}thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-db, 300 MHz) 8 2.81
(s, 3H), 3.61 (s, 3H), 3.77 (s, 6H), 7.04 (s, 2H), 7.09 (s, 1H), 8.40 (s, 1H);
Mass
Spectrum (ESI) m/z calcd. for C,gH2°N403S2, 404.5 (M+H), found
405.2.
Example 193
a) Isopropyl S-methyl-4-~2-~(4 phenoxyphenyl)aminoJ(1,3-thiazol 4-
yl)fthioplzene-2-carboxylate IZydrobromide: Isopropyl- 4-(2-bromoacetyl)-5-
methylthiophene-2-carboxylate (91 mg, 0.29 mmol) was allowed to react with 4-
phenoxyphenylthiourea (72.6 mg) as described in Example 154, step (a) to give
115
mg (75% yield) of isopropyl 5-methyl-4-{2-[(4-phenoxyphenyl)amino](1,3-thiazol-
4-
yl)}thiophene-2-carboxylate hydrobromide. 'H NMR (DMSO-db, 300 MHz) 8 1.28
(d, 6H, J--6.2 Hz), 2.70 (s, 3H), 6.06 (quintet, 1H, J--6.2 Hz), 6.92-7.09 (m,
SH), 7.15
(s, 1H), 7.30-7.37 (m, 2H), 7.56-7.70 (m, 2H), 7.98 (s, 1H); Mass Spectrum
(ESI) m/z
calcd. for C24HZZNzO3S2, 450.6 (M+H), found 451.2, 409.2 [-CH(CH3)2].
CA 02362390 2001-08-07
WO 00/47578 -223- PCT/US99/18065
b) S-Methyl 4-~2-~(4 phenoxyphenyl)aminoJ(1,3-thiazol-4 yl))thiophene-2-
carboxamidine hydrochloride: Isopropyl 5-methyl-4-{2-[(4-
phenoxyphenyl)amino](1,3-thiazol-4-yl)}thiophene-2-carboxylate hydrobromide
(95.5 mg, 0.17 mmol) was treated as described in Example 154, step (b) to give
23.8
mg (32% yield) of 5-methyl-4-{2-[(4-phenoxyphenyl)amino](1,3-thiazol-4-
yl)}thiophene-2-carboxamidine hydrochloride. 'H NMR (DMSO-d6, 300 MHz) 8
2.76 (s, 3H), 6.95-7.12 (m, 6H), 7.34-7.39 (m, 2H), 7.72-7.78 (m, 2H), 8.33
(s, 1H),
8.98 (bs, 3H), 10.29 (bs, 1H); Mass Spectrum (ESI) m/z calcd. for
CZ,H,8N4OzS3,
406.5 (M+H), found 40?.2.
Example 194
a) Isopropyl 5-metlzyl 4-~2-(plzenylamino)(1,3-thiazol 4 yl)J thiophene-2-
carboxylate Izydrobromide: Isopropyl 4-(2-bromoacetyl)-5-methylthiophene-2-
carboxylate (64 mg, 0.21 mmol) was allowed to react with phenylthiourea (32.1
mg)
as described in Example 154, step (a) to give 80 mg (87% yield) of isopropyl 5-
methyl-4-[2-(phenylamino)(1,3-thiazol-4-yl)]-thiophene-2-carboxylate
hydrobromide.
Mass Spectrum (ESI) m/z calcd. for C,BH,gN202Sz, 358.5 (M+H), found 359.2.
b) 5-Metlzyl 4-~2-(plzenylamino)(1,3-thiazol 4 yl)Jthiophene-2-
carboxamidine hydrochloride: Isopropyl 5-methyl-4-[2-(phenylamino)(1,3-thiazol-
4-yl)]-thiophene-2-carboxylate hydrobromide (74 mg, 0.16 mmol) was treated
with
phenylthiourea (24.3 mg) as described in Example 154, step (b) to give 15 mg
(28%
yield) (of 5-methyl-4-[2-(phenylamino)(1,3-thiazol-4-yl)]thiophene-2-
carboxamidine
hydrochloride, which was further purified by recrystallization from methanol-
water.
'H NMR (DMSO-db, 300 MHz) 8 2.79 (s, 3H), 6.96 (t, 1H, J--7.2 Hz), 7.09 (s,
1H),
7.33 (t, 2H, J--7.5 Hz), 7.71 (d, 2H, J--7.7 Hz), 8.39 (s, 1H), 8.95 (bs, 2H),
9.33 (bs,
2H), 10.37 (s, 1H); Mass Spectrum (ESI) m/z calcd. for C,SH,4N4S3, 314.4
(M+H),
found 315.2.
Example 195
a) Methyl 4-(4-isoxazol 5 yl(1,3-thiazol 2 yl))-S-metlrylthiotl:iopl:ene-2-
carboxylate: Methyl4-(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate
(872 mg, 2.51 mmol) was allowed to react with 2-bromo-1-isoxazol-S-ylethan-1-
one
(737 mg, prepared from from isoxazole-S-carbonyl chloride [Maybridge
Chemicals,
CA 02362390 2001-08-07
WO 00/47578 -224- PCT/US99/18065
Cornwall, UK] as described in Example 177, step (a) as described in Example
154,
step (a) to give 704 mg (83% yield) of methyl 4-(4-isoxazol-5-yl(1,3-thiazol-2-
yl))-5-
methylthiothiophene-2-carboxylate. 'H NMR (DMSO-db, 300 MHz) b 2.75 (s, 3H),
3.85 (s, 3H), 6.93 (d, 1H, J--1.8 Hz), 8.22 (s, 1H), 8.38 (s, 1H), 8.70 (d,
1H, J--1.8
Hz).
b) 4-(4 Isoxazol-5 yl(1,3-thiazol-2 yl))-5-methylthiothiophene-2-
carboxamidine hydrochloride: Methyl 4-(4-isoxazol-5-yl(1,3-thiazol-2-yl))-5-
methylthiothiophene-2-carboxylate (350 mg, 1.03 mmol) was treated as described
in
Example 154, step (b) to give 290 mg (78% yield) of 4-(4-isoxazol-5-yl(1,3-
thiazol-2-
yl))-5-methylthiothiophene-2-carboxamidine hydrochloride, of which an aliquot
was
further purified by recrystallization from methanol-isopropanol-water
(3:1:0.2, v/v/v).
'H NMR (DMSO-db, 300 MHz) 8 2.79 (s, 3H), 6.93 (d, 1H, J 1.9 Hz), 8.45 (s,
1H),
8.74 (m, 2H), 9.23 (bs, 2H), 9.53 (bs, 2H); Mass Spectrum (MALDI-TOF, CHCA
matrix) m/z calcd. for C,ZH,oN40S3, 322.4 (M+H), found 323.3.
Example 196
a) Methyl4-~4-(2-Izydroxyphenyl)(1,3-thiazol 2 yl)J S-methylthiothiophene-
2-carboxylate: Methyl4-(aminothioxomethyl)-5-methylthiothiophene-2-carboxylate
(808 mg, 3.26 mmol) was allowed to react with 2-(2-bromoacetyl)hydroxybenzene
(925 mg, prepared from 2-(chlorocarbonyl)phenyl acetate [Aldrich Chemicals,
Milwaukee, WI] as described in Example 177, step (a)) as described in Example
154,
step (a) to give 433 mg (37% yield) of methyl 4-[4-(2-hydroxyphenyl)(1,3-
thiazol-2-
yl)]-5-methylthiothiophene-2-carboxylate. 'H NMR(DMSO-db, 300 MHz) 8 2.77 (s,
3H), 3.86 (s, 3H), 6.91-7.00 (m, 2H), 7.23 (m, 1H), 8.14-8.19 (m, 2H), 8.24
(s, 1H);
Mass Spectrum (ESI) m/z calcd. for C,6H,3NO3S3, 363.48 (M+H), found 364.2.
b) 4 ~4-(2-Hydroxyplzenyl)(1,3-thiazol-2 yl)J S-methylthiothiophene-2-
carboxamidine hydrochloride: Methyl 4-[4-(2-hydroxyphenyl)(1,3-thiazol-2-yl)]-
5-
methylthiothiophene-2-carboxylate (400 mg, 1.1 mmol) was treated as described
in
Example 154, step (b) to give 173 mg (41% yield) of4-[4-(2-hydroxyphenyl)(1,3-
thiazol-2-yl)]-5-methylthiothiophene-2-carboxamidine hydrochloride. 'H NMR
(DMSO-db, 300 MHz) 8 2.81 (s, 3H), 6.92-7.02 (m, 2H), 7.22 (m, 1H), 8.20 (dd,
1H,
J--1.7, 7.8 Hz), 8.27 (s, 1 H), 8.65 (s, 1 H), 9.00 (bs, 2H), 9.41 (bs, 2H),
10.58 (s, 1 H);
Mass Spectrum (ESI) m/z calcd. for C,SH,3N3OS3, 347.48 (M+H), found 348.2.
CA 02362390 2001-08-07
WO 00/47578 -225- PCT/US99/18065
Example 197
S-Methyltl:io-4-(6-quinolylamino)thiophene-2-carboxamidine hydrochloride
a) Methyl5-methylthio-4-(6-quinolylamino)thioplZene-2-
S carboxylate: To an oven-dried glass vial with stir bar was added a mixture
of
65.2 mg (0.244 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a)), 5.2 mg'(9.5 mol %) of
palladium (II) acetate, 22.2 mg (14.6 mol %) of racemic-2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl (BINAP), 125 mg (0.384 mmol) of
cesium carbonate and 50.3 mg (0.349 mmol) of 6-aminoquinoline. The vial
was transferred to a glove bag, flushed with dry argon and anhydrous toluene
(488 ~L) was added. The vial was capped with a Teflon-lined screw cap and
heated at 100°C for 48 h. To the cooled suspension was added ethyl
acetate (4
mL), the mixture filtered (Celite), washing with ethyl acetate (2 x 2 mL), and
the solvents removed in vacuo. The resulting residue was purified by
chromatography on a 10-g silica SPE column with a gradient of 5-12 % ethyl
acetate-CH2Cl2 to afford 53.3 mg (66%) of the title compound as a pale yellow
resin. ~ H-NMR (CDCl3, 400 MHz) 8 8.77 (dd, 1 H, J = 4.2, 1.6 Hz), 8.04 (d,
1 H, J = 9.4 Hz), 8.02 (d, 1 H, J = 8.4 Hz), 7.90 (s, 1 H), 7.41 (dd; 1 H, J =
9.0,
2.6 Hz), 7.36 (dd, 1H, J = 8.3, 4.2 Hz), 7.27 (d, 1H, J = 2.6 Hz), 3.92 (s,
3H)
and 2.4~ (s, 3H). Mass spectrum (ESI, m/z): Calcd. For C,6H15NZOZS2, 331.1
(M+H), found 331.2.
b) 5-Methyltlzio-4-(6-quinolylamino)thioplzene-Z-carboxamidine
IZydrochloride: Trimethylaluminum (2.0 M in toluene, 0.76 mL, 1.52 mmol)
was added dropwise to a suspension of ammonium chloride (85.6 mg, 1.60
mmol) in anhydrous toluene (0.76 mL) under Ar at 0°C. The mixture was
stirred at 25°C for 30 min and then 50.2 mg (0.152 mmol) of methyl 5-
mehtylthio-4-(6-quinolylamino)thiophene-2-carboxylate (as prepared in
previous step) was added. The reaction mixture was heated slowly to
100°C
and stirred for 4 h. The cooled mixture was added to a vigorously stirred
slurry of silica gel (3 g) in chloroform (15 mL). The suspension was filtered
(Celite) washing with 25% MeOH-CHZCIz (2 x 5 mL), 50% MeOH-CHZC12 (2
x 5 mL) and 75% MeOH-CH2Clz (2 x 5 mL). The combined washings were
concentrated and the resulting residue was purified on a 5-g silica SPE column
CA 02362390 2001-08-07
w0 00/47578 -226- PCT/US99/18065
with a gradient of 10-15% MeOH-CH2Clz to afford 42.2 mg (79%) of the title
compound as a yellow solid. 'H-NMR (DMSO-db, 400 MHz) b 9.39 (br s,
2H), 9.12 (br s, 2H), 8.63 (dd, 1 H, J = 4.2, 1.6 Hz), 8.44 (s, 1 H), 8.16 (m,
2H),
7.89 (d, 1H, J = 8.5 Hz), 7.54 (dd, 1H, J = 9.1, 2.6 Hz), 7.39 (dd, 1H, J =
8.3,
4.2 Hz), 7.20 (d, 1H, J = 2.5 Hz) and 2.55 (s, 3H). Mass spectrum (ESI, m/z):
Calcd. For C15H,4N4S2, 315.1 (M+H), found 315.2.
Example 198
S-Methylthio-4-~(3 phenylphenyl)aminoJtlziphene-2-carboxamidine
hydrochloride
a) Methyl S-methylthio-4 ~(3 phenylplZenyl)aminoJtlZiophene-2-
carboxylate: The same procedure as in Example 197, step (a), was followed
using 62.2 mg (0.233 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a)). 4.7 mg (9.0 mol %) of
palladium (II) acetate, 20.0 mg (13.8 mol %) of racemic-BINAP, 140 mg
(0.430 mmol) of cesium carbonate, 48.2 mg (0.285 mmol) of 3-aminobiphenyl
and 466 p.L of toluene, and chromotographed as before using 20-40%
CHZC12-hexane to afford 52.3 mg (63%) of the title compound as a yellow
resin. 1H-NMR (CDCl3, 400 MHz) 8 7.81 (s, 1H), 7.61 (m, 2H), 7.46 (m, 2H),
7.38 (m, 2H), 7.21 (m, 2H), 7.03 (m, 1H), 6.22 (s, 1H), 3.90 (s, 3H), 2.43 (s,
3H). Mass spectrum (ESI, m/z): Calcd. For C19H,~NOZS2, 356.1 (M+H),
found 356.2.
b) 5-MetIZylthio-4 ~(3 plzenylplzenyl)aminoJthiophene-2-
carboxamidine hydrochloride: The same procedure as in Example 197,
step (b) was followed using 46.4 mg (0.131 mmol) of methyl 5-methylthio-4-
[(3-phenylphenyl)amino]thiophene-2-carboxylate (as prepared in previous
step), 0.76 mL of trimethylaluminum (2.0 M in toluene, 1.57 mmol), 87.7 mg
of ammonium chloride (1.64 mmol) and 0.79 mL of toluene, and purified on a
5-g silica SPE column with 5-10% MeOH-CHZC12 to afford 46.8 mg (95%) of
the title compound as a yellow foam. 'H-NMR (DMSO-db, 400 MHz) 8 9.04
(br s, 4H), 8.10 (s, 1H), 8.06 (s, 1H), 7.62 (m, 2H), 7.46 (m, 2H), 7.35 (m,
2H), 7.19 (t, 1 H, J = 1.9 Hz), 7.12 (d, 1 H, J = 8.2 Hz), 6.95 (dd, 1 H, J =
7.8,
1.9 Hz), 2.53 (s, 3H). Mass spectrum (ESI, m/z): Calcd. For C,gH,~N3S2,
340.1 (M+H), found 340.2.
CA 02362390 2001-08-07
WO 00/47578 -22~_ PCT/US99/18065
Example 199
5-Metlzylthio-4-(3-quinolylamino)thiophene-2-carboxamidine hydrochloride
a) Methyl5-methylthio-4-(3-quinolylamino)thiophene-2-
carboxylate: The same procedure as in Example 197, step (a) was followed
using 104 mg (0.389 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a)), 7.1 mg (8.1 mol %) of
palladium (II) acetate, 29.3 mg (12.1 mol%) of racemic-BINAP, 192 mg
(0.589 mmol) of cesium carbonate, 70.5 mg (0.489 mmol) of 3-
aminoquinoline and 778 ~.L of toluene, and chromatographed as before using
3-8% ethyl acetate-CHZC12 to afford 34.4 mg (27%) of the title compound as a
yellow resin. iH-NMR (CDC13, 400 MHz) 8 8.73 (d, 1H, J = 2.5 Hz), 8.04 (d,
1 H, J = 8.2 Hz), 7.8 5 (d, 1 H, J = 4.0 Hz), 7.71 (d, 1 H, J = 7.9 Hz), 7.62
(m,
1H), 7.56 (m, 2H), 6.34 (s, 1H), 3.93 (s, 3H) and 2.46 (s, 3H). Mass spectrum
(ESI, m/z): Calcd. For C16H14N202S2, 331.1 (M+H), found 331.3.
b) 5-Metlayltlzio-4-(3-quinolylamino)tlzioplzene-2-carboxamidine
l:ydrochloride: The same procedures as in Example 197, step (b) was
followed using 32.3 mg (0.0977 mmol) of methyl 5-methylthio-4-(3-
quinolylamino)thiophene-2-carboxylate (as prepared in previous step), 0.586
mL of trimethylaluminum (2.0 M in toluene, 1.17 mmol) and 65.8 mg of
ammonium chloride (1.26 mmol) and 0.59 mL of toluene and purified on a 5-g
silica SPE column with 5-12% MeOH-CHZC12 to afford, after concentration
once from MeOH-MeCN ( 1:1 ), 17.3 mg (51 %) of the title compound as a light
tan crystalline solid. ~H-NMR (DMSO-db, 400 MHz) ~ 9.09 (br s, 4H), 8.79
(s, 1 H), 8.56 (s, 1 H), 8.12 (s, 1 H), 7.89 (m, 1 H), 7.79 (m, 1 H), 7.56 (s,
1 H),
7.50 (m, 2H) and 2.55 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C~SH~4NqSz, 315.1 (M+H), found 315.4.
Example 200
S-Metlzyltlzio-4-(pyrimidin-2 ylamino)thioplzene-2-carboxamidine
hydrochloride
a) Methyl5-methyltlZio-4-(pyrimidin-2 ylamino)thiophene-2-
carboxylate: The same procedure as in Example 197, step (a) was followed
using 50.9 mg (0.191 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
CA 02362390 2001-08-07
WO 00/47578 _228_ PCT/US99/18065
carboxylate (as prepared in Example 241, step (a)), 2.7 mg (6.3 mol %) of
palladium (II) acetate, 11.3 mg (9.5 mol %) of racemic-BINAP, 101 mg
(0.310 mmol) of cesium carbonate, 25.9 mg (0.270 mmol) of 2-
aminopyrimidine and 381 p,L of dioxane, and chromatographed as before
using 5-10% ethyl acetate-hexane to afford 16.7 mg (31%) of the title
compound as a yellow crystalline solid: 1H-NMR (CDCl3, 400 MHz) b 8.72
(s, 1 H), 8.49 (d, 1 H, J = 4.8 Hz), 6.80 (t, 1 H, J = 4.8 Hz), 3.92 (s, 3H),
2.42 (s,
3H) and 1.28 (br s, 2H). Mass spectrum (ESI, m/z): Calcd. for C~IHlIN3OZS2,
282.0 (M+H), found 282.3.
b) 5-MetlZyltlaio-4-(pyrimidin-2 ylamino)tlziophene-2-
carboxamidine hydroclzloride: The same procedure as in Example 197, step
(b) was followed using 15.2 mg (0.0540 mmol) of methyl 5-methylthio-4-
(pyrimidin-2-ylamino)thiophene-2-carboxylate (as prepared in previous step),
0.324 mL or trimethylaluminum (2.0 M in toluene, 0.648 mmol) and 36.4 mg
of ammonium chloride (0.680 mmol) and 0.32 mL of toluene, and purified on
a 2-g silica SPE column with 5-15% MeOH-CH2C12 to afford, after
concentration once from MeOH-MeCN (1:10), 11.4 mg (70%) of the title
compound as a light yellow crystalline solid. 1H-NMR (DMSO-d6, 300 MHz)
8 9.24 (br s, 2H), 8.85 (br s, 2H), 8.45 (d, 1H, J = 4.8 Hz), 8.25 (s, 1H),
6.87 (t,
1H, J = 4.8 Hz) and 2.53 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
CIOH»NSS2, 266.1 (M+H), found 266.2.
Example 201
4-~(4-Cyclohexylphenyl)aminoJ-S-methylthiotlzioplzene-2-carboxamidine
hydrochloride
a) Methyl 4-~(4-cyclolzexylphenyl)amino~ 5-metl:yltlziotl:iophene
-2-carboxylate: The same procedure as in Example 197, step (a) was followed
using 122 mg (0.457 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a)), 9.9 mg (9.7 mol %) of
palladium (II) acetate, 42.3 mg (14.9 mol %) of racemic-BINAP, 206 mg
(0.632 mmol) of cesium carbonate, 102 mg (0.582 mmol) of 4-
cyclohexylaniline and 913 ~L of toluene, and chromatographed as before
using 20-40% CH2C12-hexane to afford 73.8 mg (45%) of the title compound
as a light green resin: 'H-NMR (CDCl3, 400 MHz) 8 7.74 (s, 1H), 7.15 (d,
CA 02362390 2001-08-07
WO 00/47578 -229- PCT/US99/18065
2H, J = 8.4 Hz), 6.98 (d, 2H, J = 8.4 Hz), 6.12 (s, 1H), 3.88 (s, 3H), 2.48
(m,
1H), 2.39 (s, 3H), 1.87 (m, 4H), 1.76 (br d, 1H, J = 12:5 Hz), 1.41 (m, 4H)
and
1.28 (m, 1H). Mass spectrum (ESI, m/z): Calcd. for C,9H23NOZSz, 362.1
(M+H), found 362.4.
b) 4-~(4-Cyclohexylphenyl)aminoJ S-metlzyltlziotlzioplzene-2-
carboxamidine hydrochloride: The same procedure as in Example 197, step
(b) was followed using 70.2 mg (0.194 mmol) of methyl 4-[(4-
cyclohexylphenyl)amino]-5-methylthiothiophene-2-carboxylate (as prepared
in previous step), 0.970 mL or trimethylaluminum (2.0 M in toluene, 1.94
mmol), 109 mg of ammonium chloride (2.04 mmol) and 0.97 mL of toluene,
and purified on a 10-g silica SPE column with 4-8% MeOH-CH2C12 to afford
57.7 mg (78%) of the title compound as a yellow foam. 'H-NMR (DMSO-db,
400 MHz) 8 8.45 (br s, 4H), 7.97 (s, 1H), 7.86 (s, 1H), 7.08 (d, 2H, J = 8.5
Hz), 6.92 (d, 2H, J = 8.5 Hz), 2.48 (s, 3H), 1.65-1.85 (m, SH) and 1.35 (m,
SH). Mass spectrum (ESI, m/z): Calcd. for C~gH23N3S2, 346.1 (M+H), found
346.4.
Example 202
Metlzyl 4-amino-S-metlzylthiotlziophene-2-carboxylate
To a pressure tube (Ace Glass, Vineland, NJ) containing 1.0 g (4.30
mmol) of 5-(methoxycarbonyl)-2-methylthiothiophene-3-carboxylic acid (as
prepared in Example 95), 1.01 mL (1.1 equiv, 4.73 mmol) of
diphenylphosphoryl azide, and I .57 mL (2.1 equiv, 9.03 mmol) of N,N-
diisopropylethylamine was charged 7 mL of t-butanol. The resultant mixture
was sealed and heated to 80°C in an oil bath for 6 h. The dark reaction
mixture was cooled to ambient temperature and concentrated in vacuo. The
crude oil was dissolved in 3 mL of CH2Cl2 and then treated with 2 mL of 1:1
CH2C12-trifluoroacetic acid followed by 0.5 mL H20. After 6 h, the mixture
was concentrated in vacuo, dissolved in 50 mL of CH2C12, washed with sat'd.
NaHC03, dried (Na2S04), and eluted through a pad of silica gel with 50%
ethyl acetate-hexanes. The solvent was concentrated in vacuo and the crude
amine was purified by preparative thin layer chromatography (20% ethyl
acetate-hexanes, 2000 qm Si02 gel) to yield 210 mg (24%) of methyl 4-
CA 02362390 2001-08-07
WO 00/47578 -230- PCT/US99/18065
amino-S-methylthiothiophene-2-carboxylate as a honey-colored oil. 1H NMR
(DMSO-db, 300 MHz) b 2.28 (s, 3H), 3.77 (s, 3H), 5.36 (bs, 2H), 7.24 (s, 1H).
Mass spectrum (ESI, mlz): Calcd. for C~H9NOZS2, 204.02 (M+H), found
204Ø
Example 203
Metlzyl4-~(aminotlzioxomethyl)aminoJ S-metlayltlziothioplzene-2-carboxylate
To a stirring 5 mL biphasic CHZC12-NaHC03 (1:1, vlv) mixture of 98
mg (0.48 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
added 43 ~L (1.2 equiv, 0.57 mmol) of thiophosgene (Aldrich Chemical,
Milwaukee, WI). The reaction was stirred vigorously overnight, diluted with
CHZC12 (50 mL), and the layers separated. The organic layer was washed with
NaHC03 (1x15 mL), brine (1x15mL), and dried (NaZS04). Concentration of
the solvent in vacuo yielded the crude isothiocyanate, which was dissolved in
5 mL of 2M NH3 in MeOH and stirred overnight. The.reaction was
concentrated to '/2 volume and filtered. The filtered solids were washed with
acetone and dried, yielding 79.8 mg (63.4%) of methyl 4-
[(aminothioxomethyl)amino]-5-methylthiothiophene-2-carboxlyate as a light
tan solid. IH NMR (DMSO-db, 300 MHz) b 2.51 (s, 3H), 3.81 (s, 3H), 7.41
(bs, 2H), 8.03 (s, 1 H) and 9.27 (bs, 1 H). Mass spectrum (ESI, m/z): Calcd.
for C8H1oN2O2S3, 263.00 (M+H), found 263.2.
Example 204
S-Metlayltlaio-4-~(4 pl:enyl(1,3-thiazol-2 yl))aminoJthiophene-2-
carboxamidine
a) Methyl S-methyltlzio-4-~(4 pl:enyl(1,3-tlziazol-2-
yl)aminoJthioplzene-2-carboxylate: To a 25-mL round bottom flask
containing 40 mg (0.15 mmol) of methyl 4-[(aminothioxomethyl)amino]-5-
methylthiothiophene-2-carboxylate and 30.3 mg (1 equiv, 0.15 mmol) of
bromoacetophenone was added 2 mL of acetone, and the resultant mixture was
heated to reflux for 18 h. The reaction was cooled to room temperature and
filtered to give 50 mg (92%) of methyl 5-methylthio-4-[(4-phenyl(1,3-thiazol-
2-yl))amino]thiophene-2-carboxylate, which was used without further
CA 02362390 2001-08-07
WO 00/47578 _23~ _ PCT/US99/18065
purification. 1H NMR (DMSO-d6, 300 MHz) 8 2.49 (s, 3H), 3.84 (s, 3H), 7.09
(s, 1H), 7.26-7.48 (m, 3H), 7.85 (m, 2H), 8.63 (s, 1H), 10.06 (bs, 1H). Mass
spectrum (ESI, m/z): Calcd. for C,6H,4N2O2S3, 363.03 (M+H), found 363.4.
b) 5 Methyltlzio-4 ~(4 phenyl(1,3-thiazol 2 yl)aminoJthiophene
2-carboxamidine: Using a procedure similar to that of Example 154, step (b),
47 mg (0.13 mmol) ofmethyl S-methylthio-4-[(4-phenyl(1,3-thiazol-2-
yl))amino]thiophene-2-carboxylate was allowed to react with 0.5 mL (8 equiv,
1.04 mmol) of the AlMe3/NH4C1 reagent and purified by preparative thin layer
chromatography (20% MeOH-CHC13-sat'd. NH3, 500 p.m Si02 gel plate) to
give 19 mg (42%) of 5-mehtylthio-4-[(4-phenyl(1,3-thiazol-2-
yl))amino]thiophene-2-carboxamidine as a yellow solid. 1H NMR (DMSO-d6,
300 MHz) b 2.43 (s, 3H), 7.27-7.42 (m, 4H), 7.90 (d, 2H, J = 7.1 Hz), 8.41 (s,
1H). Mass spectrum (ESI, m/z): Calcd. for C~SH~4N4S3, 347.05 (M+H), found
347.1.
Example 205
S-Methyltlzio-4-(~4-(4 plzenylplzenyl)(1,3-thiazol 2 yl)Jaminoftlziophene-2
carboxamidine
a) Methyl S-metlzyltlzio-4-~~4-(4 plzenylphenyl)(1,3-tlziazol-2-
yl)JaminoJtlzioplzene-2-carboxylate: Using a procedure similar to Example
204, step (a) 53 mg (0.2 mmol) of methyl 4-[(aminothioxomethyl)amino]-5-
methylthiothiophene-2-carboxylate was allowed to react with 55.6 mg (0.2
mmol) of 4-phenyl-bromoacetophenone for 3 h to afford 57 mg (65%) of
methyl5-methylthio-4-{[4-(4-phenylphenyl)(1,3-thiazol-2-
yl)]amino}thiophene-2-carboxylate. 'H NMR (DMSO-d6, 300 MHz) 8 2.51
(s, 3H), 3.86 (s, 3H), 6.93 (s, 1H rotomer), 7.10 (s, 1H rotomer), 7.27 (s, 1H
rotomer), 7.37-7.50 (m, 3H rotomer), 7.72-7.76 (m, 4H rotomer), 8.4 (d, 2H,
8.4 Hz), 8.66 (s, 1H rotomer), 10.10 (bs, 1H). Mass spectrum (ESI, m/z):
Calcd. for CZZH~gN202S3, 439.06 (M+H), found 439.2.
b) 5-Methyltlzio-4-~J4-(4 plzenylphenyl(1,3-thiazol-2-
yl)JaminoJthiophene-2-carboxamidine: Using a procedure similar to that of
Example 154, step (b), 57 mg (0.12 mmol) of methyl 5-methylthio-4-{ [4-(4-
phenylphenyl)(1,3-thiazol-2-yl)]amino}thiophene-2-carboxylate was allowed
to react with 6.7 equiv (0.87 mmol) of the AlMe3/NH4C1 reagent and purified
CA 02362390 2001-08-07
WO 00/47578 -232- PCT/US99/18065
by preparative thin layer chromatography (20% MeOH-CHCI3-sat'd. NH3, 500
pm Si02 plate) to give 20.7 mg (40.7%) of 5-methylthio-4-{ [4-(4-
phenylphenyl)(1,3-thiazol-2-yl))amino}thiophene-2-carboxamidine. 'H NMR
(DMSO-db, 400 MHz) 8 2.51 (s, 3H), 6.93 (s, 1H), 7.10 (s, 1H), 7.27 (s, 1H),
7.35-7.50 (m, 4H), 7.72-7.76 (m, 4H), 7.94-7.96 (m, 2H), 8.66 (s, 1H), 10.11
(bs, 1H). Mass spectrum (ESI, m/z): Calcd. for C2~H,gNqS3, 423.08 (M+H),
found 423.2.
Example 206
4-((5-Methyl 4 phenyl(1,3-thiazol 2 yl))aminoJ S-methylthiothiophene-2-
carboxamidine
a) Metlzyl 4-~(S-methyl-4 plaenyl(1,3-tl:iazol-2 yl)aminoJ S-
metlzyltltiothiopltene-2-carboxylate: Using a procedure similar to Example
204, step (a), 51 mg (0.19 mmol) of methyl 4-[(aminothioxomethyl)amino]-5-
methylthiothiophene-2-carboxylate was allowed to react with 41.4 mg (0.38
mmol) of 2-bromopropiophenone (Aldrich Chemical Co., Milwaukee. WI) in
2 mL of DMF for 4 h. Concentration in vacuo of the reaction mixture
afforded 73 mg (100%) of methyl-4-[(5-methyl-4-phenyl(1,3-thiazpl-2-
yl))amino]-5-methylthiothiophene-2-carboxylate. Mass spectrum (ESI, m/z):
Calcd. for C,~H,6NZO2S3, 377.05 (M+H), found 377.2.
b) 4-~(S-Methyl-4 phenyl(1,3-thiazol 2 yl))aminoJ-S-
methylthiothiophene-2-carboxamidine: Using a procedure similar to
Example 154, step (b), 73 mg (0.19 mmol) of methyl-4-[(5-methyl-4-
phenyl(1,3-thiazol-2-yl))amino]-5-methylthiothiophene-2-carboxylate was
allowed to react with 8 equiv ( 1.5 mmol) of the AIMe3/NH4C1 reagent and
purified by preparative thin layer chromatography (20 %-MeOH-CHCI;-sat'd.
NH3, 500 qm Si02 plate) to afford 17.9 mg (26 %) of 4-[(5-methyl-4-
phenyl(1,3-thiazol-2-yl))amino]-5-methylthiothiophene-2-carboxamidine. ~H
NMR (DMSO-d6, 300 MHz): 8 2.40 (s, 3H), 2.51 (s, 3H rotomer), 2.73 (s, 3H
rotomer), 7.29-7.44 (m, 2H rotomer), 7.64-7.73 (m, 3H rotomer), 7.95 (s, 1H
rotomer), 8.06 (s, 1 H rotomer). Mass spectrum (ESI, m/z): Calcd. for
C16Hi6N4S3, 361.06 (M+H), found 361.2.
CA 02362390 2001-08-07
WO 00/47578 -233- PCT/US99/18065
Example 207
9-~~4-Hydroxy-4-(tr~uorometlzyl)(1,3-tlziazolin-2 yl)JaminoJ-S
methylthiotlziophene-2-carboxamidine
a) Methyl4-(~4-Izydroxy-4-(trifluorometlzyl)(1,3-thiazolin-2-
yl)JaminoJ-S-metlzylthiothiophene-2-carboxylate: Using a procedure similar
to Example 204, step (a), 56 mg (0.21 mmol) of methyl 4-
[(aminothioxomethyl)amino]-5-methylthiothiophene-2-carboxylate was
allowed to react with 40 mg (0.21 mmol) of bromotrifluoroacetone (Aldrich
Chemical Co., Milwaukee, WI) to afford 40.3 mg (54 %) of methyl 4-{[4-
hydroxy-4-(trifluoromethyl)( 1,3-thiazolin-2-yl)] amino }-5-
methylthiothiophene-2-carboxylate. Mass spectrum (ESI, m/z): Calcd. for
C"H~1F3N2O3S3, 373.00 (M+H), found 373Ø
b) 4-~~4-Hydroxy-4-(tr~uorometlzyl)(1,3-thiazolin-2 yl)Jamino~-
S-metlzyltlziotlzioplzene-2-carboxamidine: Using a procedure similar to
Example 154, step (b), 40 mg (0.11 mmol) of methyl 4-{[4-hydroxy-4-
(trifluoromethyl)( 1,3-thiazolin-2-yl)]amino }-S-methylthiothiophene-2-
carboxylate was allowed to react with 8 equiv (0.89 mmol) of the
AlMe3/NH4Cl reagent and purified by preparative thin layer chromatography
(20 %-MeOH-CHC13-sat'd. NH3, 500 ~m Si02 plate) to afford 11 mg (28 %)
of 4-{[4-hydroxy-4-(trifluoromethyl)(1,3-thiazolin-2-yl)]amino}-5-
methylthiothiophene-2-carboxamidine as a ca. l : l mixture of cyclized aminal
and open imine tautomers. IH NMR (DMSO-db, 300 MHz) 8 2.73 (s, 3H
tautomer), 2.89 (s, 3H tautomer), 3.36 (d, 2H, J--6.5 Hz), 3.62 (d, 2H, J--7.2
Hz), 7.95 (s, 1H), 8.36 (bs, 2H), 9.79 (bs, 1H). Mass spectrum (ESI, m/z):
Calcd. for CIOHliF3N4OS3, 357.01 (M+H), found 357.2.
Example 208
S-Metlzyltlzio-4-(2-naphthylamino)thiopliene-2-carboxamidine
a) Metlzyl5-nzetltyltlzio-4-(Z-naplzthylamino)thioplzene-2-
carboxylate: To an oven-dried round bottom flask equipped with Teflon-
coated stir bar and rubber septum was added 190 mg (0.93 mmol) of methyl 4-
amino-5-methylthiothiophene-2-carboxylate, 320 mg (2 equiv, 1.86 mmol) of
2-napthalene boronic acid (Lancaster Synthesis, Windham, NH), and 168 mg
CA 02362390 2001-08-07
WO 00/47578 _23~_ PCT/US99/18065
(1 equiv, 0.93 mmol) of Cu(OAc)2 (Aldrich Chemical Co., Milwaukee, WI).
The flask was flushed with Ar, then charged with 4 mL CHZC12 followed by
259 ~.L (2 equiv, 1.86 mmol) of NEt3. The mixture was stirred vigorously for
48 h and then filtered through a small pad of Si02, eluting with 50 % ethyl
acetate-hexanes. Concentration of the solvent in vacuo, and purification of
the
residue by preparative thin layer chromatography (2S % ethyl acetate-hexanes,
1000 gM Si02 plate) afforded 170 mg (SS %) of methyl S-methylthio-4-(2-
naphthylamino)thiophene-2-carboxylate and 54 mg (28 %) of recovered
methyl 4-amino-5-methylthiothiophene-2-carboxylate. IH NMR (CDC13, 400
MHz) 8 2.43 (s, 3H), 3.92 (s, 3H), 6.29 (s, 1H), 7.21 (dd, 1H, J-- 2.35, 8.7
Hz),
7.33-7.37 (m, 2H), 7.45 (m, 1H), 7.71 (d, 1H, J--8.2 Hz), 7.78 (m, 2H), 7.88
(s,
1H). Mass spectrum (ESI, m/z): Calcd. for C~7H15NO2S2, 330.06 (M+H),
found 330.1.
b) 5-Methyltltio-4-(2-naphthylamino)thiophene-2-
1 S carboxamidine hydrochloride: Using a procedure similar to Example 154,
step(b),730 mg (2.21 mmol) of methyl 5-methylthio-4-(2-
naphthylamino)thiophene-2-carboxylate was allowed to react with 8 equiv
(17.7 mmol) of the AlMe3/NH4C1 reagent and purified by preparative thin
layer chromatography (20 %-MeOH-CHCl3-sat'd. NH3, 1000 ~m Si02 plate)
to afford 5-methylthio-4-(2-naphthylamino)thiophene-2-carboxamidine, which
was dissolved in 4 mL of dry MeOH , cooled to 0°C and carefully treated
with
1.6 mL( 1.5 equiv, 3.31 mmol) of 2M HCl in ether. The reaction was stored at
5°C overnight, then concentrated in vacuo with toluene (3x10mL) and
then
hexanes (2x10 mL). The yellow solid was dried under vacuum to afford 415
2S mg (53.6 %) of S-methylthio-4-(2-naphthylamino)thiophene-2-carboxamidine
hydrochloride. 1H NMR (DMSO-d6, 400 MHz) 8 2.53 (s, 3H), 7.20 (d, 1H,
J=2.2 Hz), 7.24-7.31 (m, 2H), 7.3 8 (m, 1 H), 7.69 (d, 1 H, 8.1 Hz), 7.75-7.79
(m, 2H), 8.13 (s, 1 H), 8.24 (s, 1 H), 9.06 (bs, 2H), 9.33 (bs, 2H). Mass
spectrum (ESI, m/z): Calcd. for C,6H~5N3S2, 314.08 (M+H), found 314.5.
CA 02362390 2001-08-07
WO 00/47578 _23~_ PCT/US99/18065
Example 209
4-~(4-Chloroplzenyl)aminoJ S-methylthiotlziophene-2-carboxamidine
a) Metlryl4-~(4-clzloroplzenyl)aminoJ-5-metltylthiothiophene-2-
carboxylate: Using a procedure similar to Example 208, step (a), 66.6 mg
(0.32 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
allowed to react with 100 mg (2 equiv, 0.64 mmol) of 4-chlorophenyl boronic
acid to give 11.8 mg (11.7 %) of methyl 4-[(4-chlorophenyl)amino]-5-
methylthiothiophene-2-carboxylate and 21 mg (31.5 %) of unreacted starting
material. 'H NMR (CDC13, 400 MHz) 8 2.41 (s, 3H), 3.89 (s, 3H), 6.09 (bs,
1 H), 6.94 (d, 2H, J--8.6 Hz), 7.25 (d, 2H, J--8.6 Hz), 7.70 (s, 1 H).
b) 4-~(4-chlorophenyl)aminoJ-5-methyltlziothiophene-2-
carboxamidine hydrochloride: Using a procedure similar to Example 154,
step (b), 11.8 mg (0.037 mmol) of methyl 4-[(4-chlorophenyl)amino]-5-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (2.96
mmol) of the AlMe3/NH4C1 reagent to afford 13 mg ( 100 %) of 4-[(4-
chlorophenyl)amino]-5-methylthiothiophene-2-carboxamidine. 'H NMR
(DMSO-d6, 400 MHz) 8 2.41 (s, 3H), 6.91-6.95 (m, 2H), 7.10-7.13 (m, 2H),
7.64 (s, 1H), 7.93 (s, 1H), 8.67 (bs, 2H), 9.11 (bs, 2H). Mass spectrum (ESI,
m/z): Calcd. for C,2H12C1N3S2, 298.02 (M+H), found 298.1.
Example 210
4-((3-Metlzylphenyl)aminoJ-5-metltylthiothiophene-2-carboxamidine
a) Metlzyl4-~(3-metlzylphenyl)aminoJ-S-methylthiothioplzene-2-
carboxylate: Using a procedure similar to Example 208, step (a), 55.7 mg
(0.27 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
allowed to react with 73.4 mg (2 equiv, 0.54 mmol) of 3-methylphenyl
boronic acid to give 29.2 mg (36.8 %) of methyl 4-[(3-methyl)amino]-5-
methylthiothiophene-2-carboxylate. IH NMR (CDC13, 400 MHz) 8 2.35 (s,
3H), 2.40 (s, 3H), 3.89 (s, 3H), 6.11 (bs, 1H), 6.80-6.86 (m, 3H), 7.20 (m,
1H),
7.77 (s, 1H). Mass spectrum (ESI, m/z): Caled. for C,4H,SNOZS2, 294.06
(M+H), found 294.1.
CA 02362390 2001-08-07
WO 00/47578 -236- PCT/US99/18065
b) 4-~(3-Methylphenyl)aminoJ S-methylthiothiophene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 29.2 mg
(0.098 mmol) of methyl 4-[(3-methyl)amino]-5-methylthiothiophene-2-
carboxylate was allowed to react with 8 equiv (0.78 mmol) of the
AlMe3/NH4C1 reagent and purified by preparative thin layer chromatography
(20 %-MeOH-CHCl3-sat'd. NH3, 500 ~m Si02 plate) to afford 27 mg (100 %)
of 4-[(3-methylphenyl)amino]-5-methylthiothiophene-2-carboxamidine. 1H
NMR (CDC13, 400 MHz) 8 2.24 (s, 3H), 2.50 (s, 3H), 6.65 (d, 1H, J--7.3 Hz),
6.74-6.76 (m, 2H), 7.10 (m, 1 H), 7.88 (s, 1 H), 7.97 (s, 1 H), 9.07 (bs, 3
H).
Mass spectrum (ESI, m/z): Calcd. for C13H~SN3S2, 278.08 (M+H), found
278.2.
Example 211
4-~(3-Methoxyphenyl)aminoJ-5-methylthiotlziophene-2-carboxamidine
a) Methyl 4 ~(3-metlzoxyplaenyl)aminoJ 5-methyltlaiothioplaene-
2-carboxylate: Using a procedure similar to Example 208, step (a), 73.2 mg
(0.35 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
allowed to react with 109 mg (2 equiv, 0.70 mmol) of 3-methoxyphenyl
boronic acid to give 25.2 mg (23 %) of methyl 4-[(3-methoxyphenyl)amino]-
5-methylthiothiophene-2-carboxylate. 'H NMR (CDC13, 400 MHz) 8 2.40 (s,
3H), 3.81 (s, 3H), 3.89 (s, 3H), 6.12 (s, 1H), 6.43-6.63 (m, 2H), 7.20 (m,
1H),
7.78 (s, 1H). Mass spectrum (ESI, m/z): Calcd. for C,4H~SN03S2, 310.06
(M+H), found 310.1.
b) 4-~(3-Methylphenyl)aminoJ S-methylthiothiophene-2-
carboxamidine hydrochloride: Using a procedure similar to Example 154,
step (b), 25.2 mg (0.081 mmol) of methyl 4-[(3-methyl)amino]-S-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (0.64
mmol) of the AlMe3/NH4C1 reagent to afford 27 mg (100 %) of 4-[(3-
methoxyphenyl)amino]-5-methylthiothiophene-2-carboxamidine. lH NMR
(DMSO, 400 MHz) 8 2.49 (s, 3H), 3.71 (s, 3H), 6.41 (dd, 1H, J--2.1, 8.0 Hz),
6.49 (m, 1 H), 6.50-6.54 (m, 1 H), 7.12 (m , 1 H), 7.97 (s, 1 H), 8.01 (s, 1
H),
8.88 (bs, 2H), 9.23 (bs, 2H). Mass spectrum (ESI, m/z): Calcd. for
C13H,5N3OS2, 294.07 (M+H), found 294.1.
CA 02362390 2001-08-07
WO 00/47578 _23~_ PCT/US99/18065
Example 212
4-~(3-(Methyletltyl)phenylJaminoJ-5-methyltltiothiophene-2-carboxamidine
a) MetJtyl4-~~3-(methylethyl)phenylJamino)-5-
methylthiothiophene-2-carboxylate: Using a procedure similar to Example
208, step (a), 74.4 mg (0.36 mmol) of methyl 4-amino-5-methylthiothiophene-
2-carboxylate was allowed to react with 118 mg (2 equiv, 0.72 mmol) of 3-
isopropylphenyl boronic acid to give 22.6 mg (19.5 %) of methyl 4-[(3-
methylethylphenyl)amino]-5-methylthiothiophene-2-carboxylate. ~ H NMR
(CDCl3, 400 MHz) 8 1.27 (d, 6H, J--6.9 Hz), 2.40 (s, 3H), 2.89 (m, 1H), 3.88
(s, 3H), 6.15 (s, 1H), 6.86-6.89 (m, 3H), 7.24 (m, 1H), 7.77 (s, 1H).
b) 4-~~3-(Methyletlzyl)phenylJaminoJ-5-methylthiotlziophene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 22.6 mg
(0.07 mmol) of methyl 4-{[3-(methylethyl)phenyl]amino}-5-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (0.56
mmol) of the AlMe3/NH4Cl reagent to afford 18.9 mg (78.8 %) of ) 4-{ [3-
(methylethyl)phenyl]amino]-5-methylthiothiophene-2-carboxamidine. 'H
NMR (DMSO-d6, 400 MHz) 8 1.18 (d, 6H, J--9.2 Hz), 2.51 (s, 3H), 2.81 (m,
1 H), 6.71-6.77 (m, 2H), 6.85 (s, 1 H), 7.14 (m, 1 H), 7.98 (s, 1 H), 8.32 (s,
1 H),
8.88 (bs, 2H), 9.23 (bs, 2H). Mass spectrum (ESI, m/z): Calcd. for
C~SH19N3S2, 306.11 (M+H), found 306.2.
Example 213
S Metlzyltlzio-4-~(3-nitroplzenyl)aminoJtlzioplzene-2-carboxamidine
a) Methyl5-methylthio-4-~(3-nitrophenyl)antinoJtlziophene-2-
carboxylate: Using a procedure similar to Example 208, step (a), 74.4 mg
(0.36 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
allowed to react with 120 mg (2 equiv, 0.72 mmol) of 3-nitrophenyl boronic
acid to give 14.5 mg (12.4 %) of methyl 5-methylthio-4-[(3-nitrophenyl)
amino]thiophene-2-carboxylate. 1H NMR (CDC13, 400 MHz) 8 2.45 (s, 3H),
3.93 (s, 3H), 6.21 (s, 1H), 7.41-7.47 (m, 2H), 7.73-7.78 (m, 3H).
b) S-Methylthio-4-~(3-nitrophenyl)aminoJthiophene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 14.5 mg
CA 02362390 2001-08-07
WO 00/47578 _23$_ PCT/US99/18065
(0.04 mmol) of methyl 5-methylthio-4-[(3-nitrophenyl)amino]thiophene-2-
carboxylate was allowed to react with 8 equiv (0.35 minol) of the
AlMe3/NH4Cl reagent to afford 4.3 mg (34.8 %) of 5-methylthio-4-[(3-
nitrophenyl)amino]thiophene-2-carboxamidine. Mass spectrum (ESI, m/z):
Calcd. for C12H12N4~2s2~ 309.05 (M+H), found 309.2.
Example 214
4-(~4-(Methylethyl)phenylJamino)-5-methyltlziothiophene-2-carboxamidine
a) _ Metlzyl4-~~4-(methylethyl)phenylJamino~-5-
metlzylthiothiophene-2-carboxylate: Using a procedure similar to Example
208, step (a), 74.4 mg (0.36 mmol) of methyl 4-amino-5-methylthiothiophene-
2-carboxylate was allowed to react with 118 mg (2 equiv, 0.72 mmol) of 4-
isopropylphenyl boronic acid to give 14.5 mg ( 12.5 %) of methyl 4-[(4-
methylethylphenyl)amino]-5-methylthiothiophene-2-carboxylate. 1H NMR
(CDCl3, 400 MHz) 8 1.26 (d, 6H, J--6.2 Hz), 2.39 (s, 3H), 2.89 (m, 1H), 3.89
(s, 3H), 6.98-7.01 (m, 2H), 7.17-7.19 (m, 2H), 7.73 (s, 1H).
b) 4-~~4-(Methylethyl)phenylJaminoJ-5-metltylthiotlziophene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 14.5 mg
(0.045 mmol) ofmethyl4-{[4-(methylethyl)phenyl]amino}-5-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (0.36
mmol) of the AlMe3/NH4Cl reagent to afford 11.4 mg (74 %) of 4-{ [4-
(methylethyl)phenyl]amino}-5-methylthiothiophene-2-carboxamidine. 1H
NMR (DMSO-db, 400 MHz) 8 1.17 (d, 6H, J--9.2 Hz), 2.51 (s, 3H), 2.81 (m,
1H), 6.92 (d, 2H, J 11.4 Hz), 7.10 (d, 2H, J--11.2 Hz), 7.88 (s, 1H), 7.96 (s,
1H), 8.89 (bs, 2H), 9.22 (bs, 2H). Mass spectrum (ESI, m/z): Calcd. for
C,SH,9N3S2, 306.11 (M+H), found 306.2.
Example 215
4-x(3,4-Dimethylplzenyl)anzinoJ S-methylthiothiophene-2-carboxamidine
a) Methyl4-((3,4-dimetlzylplzenyl)aminoJ S-
metlzylthiothiophene-2-carboxylate: Using a procedure similar to Example
208, step (a), 74.4 mg (0.36 mmol) of methyl 4-amino-5-methylthiothiophene-
CA 02362390 2001-08-07
WO 00/47578 _239_ PCT/US99/18065
2-carboxylate was allowed to react with 108 mg (2 equiv, 0.72 mmol) of 3, 4-
dimethylphenyl boronic acid to give 135.9 mg (32.4 %) of methyl 4-[(3,4-
dimethylphenyl)amino]-5-methylthiothiophene-2-carboxylate. 'H NMR
(CDC13, 400 MHz) b 2.24 (s, 3H), 2.26 (s, 3H), 2.38 (s, 3H), 3.88 (s, 3H),
6.11
(bs, 1 H), 6.80 -6.84 (m, 2H), 7.07 (d, 1 H, J--7.9 Hz), 7.71 (s, 1 H).
b) 4-x(3,4 DimethylpJ:enyl)aminoJ 5-metlzyltl:iothioplzene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 35.6 mg
(0.116 mmol) of methyl 4-[(3,4-dimethylphenyl)amino]-5-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (0.93
mmol) of the AlMe3/NH4Cl reagent to afford 26.1 mg (68.5 %) of 4-[(3,4-
dimethylphenyl)amino]-5-methylthiothiophene-2-carboxamidine.'H NMR
(DMSO-d6, 400 MHz) 8 2.13 (s, 3H), 2.16 (s, 3H), 2.51 (s, 3H), 6.69-6.78 (m,
2H), 6.99 (d, 1 H, J--10.8 Hz), 7.76 (s, 1 H), 7.91 (s, 1 H), 8.82 (bs, 2H),
9.17
(bs, 2H). Mass spectrum (ESI, m/z): Calcd. for C,qH»N3S2, 292.09 (M+H),
found 292.2.
Example 216
S-Methylthio-4-~(4 plienylplzenyl)aminoJthioplzene-2-carboxamidine
a) Methyl S-methyltlZio-4-~(4 plaenylplzenyl)aminoJthiophene-2-
carboxylate: Using a procedure similar to Example 208, step (a), 74.4 mg
(0.36 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
allowed to react with 142.5 mg (2 equiv, 0.72 mmol) of 4-phenylphenyl
boronic acid to give 24.5 mg (19.1 %) of methyl 4-[(4-phenylphenyl)amino]-
5-methylthiothiophene-2-carboxylate. 'H NMR (CDCl3, 400 MHz) 8 2.45 (s,
3H), 3.92 (s, 3H), 6.38 (bs, 1H), 7.08-7.14 (m, 2H), 7.33 (m, 1H), 7.43-7.46
(m, 2H), 7.54-7.60 (m, 4H), 7.82 (s, 1 H).
b) S-Methyltlzio-4-~(4 plzenylplzenyl)aminoJtlrioplzene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 24.5 mg
(0.07 mmol) of methyl 4-[(4-phenylphenyl)amino]-5-methylthiothiophene-2-
carboxylate was allowed to react with 8 equiv (0.56 mmol) of the
AlMe3/NH4C1 reagent to afford 16.9 mg (64.1 %) of 5-methylthio-4-[(4-
phenylphenyl)amino]thiophene-2-carboxamidine. 'H NMR (DMSO-db, 400
MHz) 8 2.51 (s, 3H), 7.03 (d, 2H, J--8.6 Hz), 7.26-7.61 (m, 7H), 8.04 (s, 1H),
CA 02362390 2001-08-07
w0 00/47578 _2~o PCT/US99/18065
8.15 (s, 1H), 8.88 (bs, 2H), 9.25 (bs, 2H). Mass spectrum (ESI, m/z): Calcd.
for C18H17N3S2, 340.09 (M+H), found 340.2.
Example 217
4-~(3-Fluoro-4 phenylphenyl)aminoJ-S-methylthiothiophene-2-
carboxamidine
a) Methyl4-~(3 fluoro-4 plzenylphenyl)aminoJ 5-
methylthiothiophene-2-carboxylate: Using a procedure similar to Example
208, step (a), 74.4 mg (0.36 mmol) of methyl 4-amino-5-methylthiothiophene-
2-carboxylate was allowed to react with 155.5 mg (2 equiv, 0.72 mmol) of 3-
fluoro-4-phenylphenyl boronic acid to give 50.6 mg (41.6 %) of methyl 4-[(3-
fluoro-4-phenylphenyl)amino]-5-methylthiothiophene-2-carboxylate. 1H NMR
(CDC13, 400 MHz) 8 2.44 (s, 3H), 3.91 (s, 3H), 6.19 (s, 1H), 6.78-6.86 (m,
2H), 7.32-7.39 (m, 2H), 7.73-7.47 (m; 2H), 7.55 (d, 1H, J--6.9 Hz), 7.82
(s, 1 H).
b) 4 ~(3-Fluoro-9 phenylplzenyl)aminoJ S-metltyltl:iothiophene-
2-carboxamidine: Using a procedure similar to Example 154, step (b), 50.6
mg (0.13 mmol) of methyl 4-[(3-fluoro-4-phenylphenyl)amino]-5-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (1.08
mmol) of the AlMe3/NH4Cl reagent to afford 39 mg (76.1 %) of 4-[(3-fluoro-
4-phenylphenyl)amino]-5-methylthiothiophene-2-carboxamidine. 1H NMR
(DMSO-db, 400 MHz) 8 2.51 (s, 3H), 6.75-6.87 (m, 2H), 7.30-7.50 (m, 6H),
8.06 (s, 1 H), 8.37 (s, 1 H), 8.90 (bs, 2H), 9.27 (bs, 2H). Mass spectrum
(ESI,
m/z): Calcd. for C~gH,6FN3SZ, 358.08 (M+H), found 358.2.
Example 218
4-(2H Benzo~dJl,3-dioxolen-S ylamino)-S-methyltlziothiophene-2
carboxamidine
a) Methyl 4-(2H benzo~dJl,3-dioxolen-5 ylamino)-S-
methylthiotltiophene-2-carboxylate: Using a procedure similar to Example
208, step (a), 74.4 mg (0.36 mmol) of methyl 4-amino-5-methylthiothiophene-
2-carboxylate was allowed to react with 119.4 mg (2 equiv, 0.72 mmol) of
3,4-methylenedioxyphenyl boronic acid to give 24.4 mg (20.9 %) of methyl 4-
(2H-benzo[d] 1,3-dioxolen-5-ylamino)-5-methylthiothiophene-2-carboxylate .
CA 02362390 2001-08-07
w0 00/47578 -241- PCT/US99/18065
IH NMR (CDC13, 400 MHz) 8 2.39 (s, 3H), 3.87 (s, 3H), 5.96 (s, 2H), 6.00
(bs, 1 H), 6.52 (dd, 1 H, J--2.3, 8.3 Hz), 6.63 (d, 1 H, J--2.2 Hz), 6.76 (d,
1 H,
J 8.3 Hz), 7.59 (s, 1H).
b) 4-(2H Benzo(dJl,3-dioxolen-S ylamino)-S-
S methyltlziothiophene-2-carboxamidine: Using a procedure similar to Example
154, step (b), 24.4 mg (0.075 mmol) of methyl 4-(2H-benzo[d]1,3-dioxolen-5-
ylamino)-5-methylthiothiophene-2-carboxylate was allowed to react with 8
equiv (0.6 mmol) of the AlMe3/NH4C1 reagent to afford 7.7 mg (29.7 %) 4-
(2H-benzo [d] 1,3-dioxolen-5-ylamino)-5-methylthiothiophene-2-
carboxamidine. 1H NMR (DMSO-db, 400 MHz) 8 2.51 (s, 3H), 5.95 (s, 2H),
6.46 (dd, 1 H, J--3.0, 11.2 Hz), 6.65 (d, 1 H, J--2.8 Hz), 6.79 (d, 1 H, J
11.0
Hz), 7.80 (s, 1 H), 7.87 (s, 1 H), 8.91 (bs, 2H), 9.24 (bs, 2H). Mass spectrum
(ESI, m/z): Calcd. for C13Hi3N3O2S2, 308.05 (M+H), found 308.2.
1 S Example 219
4-~(4-Butylpl:enyl)aminoJ S-metlzylthiotlziophene-2-carboxamidine
a) Methyl4-~(4-butylphenyl)aminoJ S-metlayltl:iotlzioplaene-2-
carboxylate: Using a procedure similar to Example 208, step (a), 74.4 mg
(0.36 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate was
allowed to react with 128 mg (2 equiv, 0.72 mmol) of 4-butylphenyl boronic
acid to give 22.2 mg ( 18.3 %) of methyl 4-[(4-butylphenyl)amino]-5-
methylthiothiophene-2-carboxylate. 'H NMR (CDC13, 400 MHz) 8 0.97 (t,
2H, J--7.4 Hz), 1.38 (m, 2H), 1.59 (m, 2H obscured by water), 2.39 (s, 3H),
2.58 (t, 2H, J--7.6 Hz), 3.90 (s, 3H), 6.12 (bs, 1H), 6.97 (d, 2H, J--8.2 Hz),
7.12 (d, 2H, J--8.4 Hz), 7.73 (s, 1 H).
b) 4-~(4-Butylplzenyl)aminoJ-5-metlzyltlaiothiophene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 22.2 mg
(0.06 mmol) of methyl 4-[(4-butylphenyl)amino]-5-methylthiothiophene-2-
carboxylate was allowed to react with 8 equiv (0.52 mmol) of the
AlMe3/NH4Cl reagent to afford 18.9 mg (88 %) of 4-[(4-butylphenyl)amino]-
S-methylthiothiophene-2-carboxamidine. 1H NMR (DMSO-db, 400 MHz) 8
0.89 (t, 2H, J--9.7 Hz), 1.23-1.33 (m, 2H), 1.51 (m, 2H), 2.47-2.50 (m, 2H
obscured by DMSO-d6), 2.51 (s, 3H), 6.90 (d, 2H, J--11.3 Hz), 7.05 (d, 2H,
CA 02362390 2001-08-07
WO 00/47578 -242- PCT/US99/18065
J--11.2 Hz), 7.86 (s, 1 H), 7.94 (s, 1 H), 8.78 (bs, 2H), 9.21 (bs, 2H). Mass
spectrum (ESI, m/z): Calcd. for C16H2~N3S2, 320.13 (M+H), found 320.2.
Example 220
S-Methylthio-4-~benzylaminoJthioplzene-2-carboxamidine
a) Methyl5-methylthio-4-~benzylaminoJthiophene-2-
carboxylate: To a 2-dram vial equipped with a stir bar and septum cap was
weighed 60 mg (0.29 mmol) of methyl 4-amino-5-methylthiothiophene-2-
carboxylate and 30.7 mg (0.29 mmol) of benzaldehyde. The vial was charged
with 1 mL CH2C12-DMF (2:1, v/v) and 135 mg (2.2 equiv, 0.63 mmol) of
NaHB(OAc)3 was added. The reaction was flushed with Ar and allowed to stir
for 48 h. At this time 2 mL of CH30H was added, the reaction stirred an
additional 15 min then diluted with 20 ml of CH2Cl2. The organic layer was
washed with water (2x20 mL), dried (Na2S04), and concentrated in vacuo into
an oven-dried 2 dram vial to give the crude methyl-5-methylthio-4-
[benzylamino]thiophene-2-carboxylate together with unreduced imine. The
crude reaction mixture was converted to the amidine without further
purification. Mass spectrum (ESI, m/z): Calcd. for C14H,SNOZS2, 294.06
(M+H), found 292.2 (imine), 294.2.
b) S-Methylthio-4-~benzylaminoJthiophene-2-carboxamidine:
To a 2-dram vial containing a stir bar and methyl-5-methylthio-4-
[benzylamino]thiophene-2-carboxylate (assume 0.29 mmol) was added 2 mL
of toluene, followed by 8 equiv (2.32 mmol) of the AIMe3/NH4CI reagent. The
resultant yellow mixture was heated to 110°C for 3 h, cooled to ambient
temperature, and then added to a slurry of 1 g of Si02 gel in 10 mL of CHC13.
After stirring for 15 min, the slurry was eluted through a 15-mL sintered
glass
funnel containing a pad of silica gel with 50 % CHCl3- CH30H. The solvent
was removed in vacuo and the residue was triturated with 10 % CH30H -
CHCl3 and filtered. Removal of the solvent in vacuo gave the crude product
which was purified by preparative thin layer chromatography (S00 ~m Si02,
20 % CH30H-CHCl3-sat'd. NH3) 14.8 mg (18.3 % from methyl 4-amino-5-
methylthiothiophene-2-carboxylate) of 5-methylthio-4-[benzylamino]
thiophene-2-carboxamidine. 'H NMR (DMSO-db, 400 MHz) 8 2.49 (s, 3H),
CA 02362390 2001-08-07
WO 00/47578 -2q,g_ PCT/US99/18065
4.35 (d, 2H, J--6.7 Hz), 5.91 (t, 1H, J--6.8 Hz), 7.20-7.38 (m, 6H). Mass
spectrum (ESI, m/z): Calcd. for C,3H,SN3S2, 278.08 (M+H), found 278.3.
Example 221
4-(Indan-S ylamino)-S-methylthiothiophene-2-carboxamidine
a) Methyl4-(indan-S ylamino)-S-methyltJziothiophene-2-
carboxylate: Using the procedure described in Example 220, step (a), 60 mg
(0.29 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate, 42.3
mg (0.29 mmol) of 5-indancarboxaldehyde, and 135 mg (2.2 equiv, 0.63
mmol) of NaHB(OAc)3 were allowed to react to give methyl 4-(indan-5-
ylamino)-5-methylthiothiophene-2-carboxylate. Mass spectrum (ESI, m/z):
Calcd. for C1~H19N02S2, 334.09 (M+H), found 332.3 (imine), 333.4.
b) 4-(Indan-S ylamino)-S-methyltJ:iothiopJzene-2-
carboxamidine: Using the procedure described in Example 220, step (b), 22.0
mg (27.3 % from methyl 4-amino-S-methylthiothiophene-2-carboxylate) of 4-
(indan-5-ylamino)-5-methylthiothiophene-2-carboxamidine was obtained. ~H
NMR (DMSO-d6, 400 MHz) 8 1.94-2.01 (m, 2H), 2.49 (s, 3H), 2.77-2.82 (m,
4H), 4.29 (d, 2H, J--5.6 Hz), 5.78 (t, 1H, .I 8.1 Hz), 7.08 (d, 1H, J--7.8
Hz),
7.14 (d, 1H, J--7.5 Hz), 7.20 (s, 1H), 7.23 (s, 1H). Mass spectrum (ESI, m/z):
Calcd. for C~6H~9N3S2, 318.11 (M+H), found 318.3.
Example 222
4-(2,3-Dihydrobenzo~bJfuran-S ylamino)-S-methylthiotJiiophene-2-
carboxamidine
a) Methyl 4-(2,3-dihydrobenzo~bjfuran-S ylamino)-S-
methylthiothiophene-2-carboxylate: Using the procedure described in
Example 220, step (a), 60 mg (0.29 mmol) of methyl 4-amino-5-
methylthiothiophene-2-carboxylate, 42.9 mg (0.29 mmol) of 2,3-
dihydrobenzo[b]furan-5-carboxaldehyde, and 135 mg (2.2 equiv, 0.63 mmol)
of NaHB(OAc)3 were allowed to react to give methyl 4-(2,3-
dihydrobenzo[b]furan-5-ylamino)-5-methylthiothiophene-2-carboxylate. Mass
spectrum (ESI, m/z): Calcd. for CI6H»NO3S2, 336.07 (M+H), found 334.3
(imine), 335.3.
CA 02362390 2001-08-07
WO 00/47578 -244- PCT/US99/18065
b) 4-(2,3-Dilzydrobenzo~bJfuran-S ylamino)-S-
methylthiothiophene-2-carboxamidine: Using the procedure described in
Example 220, step (b), 21.8 mg (23.5 % from methyl 4-amino-5-
methylthiothiophene-2-carboxylate) of 4-(2,3-dihydrobenzo[b]furan-5-
ylamino)-5-methylthiothiophene-2-carboxamidine was obtained. ~H NMR
(DMSO-d6, 400 MHz) 8 2.49 (s, 3H), 3.13 (t, 2H, J--8.7 Hz), 4.24 (d, 2H,
J--6.6 Hz), 4.48 (t, 2H, J 8.7Hz), 5.69 (t, 1 H, J--6.7 Hz), 6.68 (d, 1 H, J--
12.4
Hz), 7.06 (d, 1 H, J--7.4 Hz), 7.21 (s, 1 H), 7.26 (s, 1 H). Mass spectrum
(ESI,
m/z): Calcd. for C~SH»N30S2, 320.09 (M+H), found 320.3.
Example 223
S-Metltylthio-4 ~(2 pltenylimidazol-4 yl)aminoJtlzioplzene-2-carboxamidine
a) Methyl S-methyltltio-4-~(2 plzenylimidazol 4 yl)
1 S aminoJthiophene-2-carboxylate: Using the procedure described in Example
220, step (a), 60 mg (0.29 mmol) of methyl 4-amino-5-methylthiothiophene-2-
carboxylate, 49.9 mg (0.29 mmol) of 4-formyl-2-phenylimidazole, and 135
mg (2.2 equiv, 0.63 mmol) of NaHB(OAc)3 were allowed to react to give )
methyl 5-methylthio-4-[(2-phenylimidazol-4-yl)amino]thiophene-2-
carboxylate. Mass spectrum (ESI, m/z): Calcd. for C,~H,~N302S2, 360.08
(M+H), found 360Ø
b) S-Methyltlzio-4 ~(2 plzenylintidazol 4 yl)aminoJthiophene-2-
carboxamidine: Using the procedure described in Example 220, step (b), 30.9
mg (30 % from methyl 4-amino-5-methylthiothiophene-2-carboxylate) of 5-
methylthio-4-[(2-phenylimidazol-4-yl)amino]thiophene-2-carboxamidine was
obtained. 'H NMR (DMSO-db, 400 MHz) 8 2.49 (s, 3H), 4.30-4.38 (m, 3H),
7.09 (bs, 1H), 7.32 (m, 1H), 7.40-7.44 (m, 3H), 7.90-7.95 (m, 3H), 8.43 (bs,
3H). Mass spectrum (ESI, m/z): Calcd. for C~6H»N;S2, 344.10 (M+H), found
344.2.
CA 02362390 2001-08-07
WO 00/47578 -245- PCT/US99/18065
Example 224
5-Metlzylth io-4-~(2-quinolylmethyl) aminoJth ioph ene-2-carboxamidin a
a) Metlzyl S-metlzyltlzio-4-~(2-quinolylmetlzyl)aminoJthioplzene-
2-carboxylate: Using the procedure described in Example 220, step (a), 60
mg (0.29 mmol) of methyl 4-amino-5-methylthiothiophene-2-carboxylate,
45.5 mg (0.29 mmol) of 2-quinolinecarboxaldehyde, and 135 mg (2.2 equiv,
0.63 mmol) of NaHB(OAc)3 were allowed to react to give methyl 5-
methylthio-4-[(2-quinolylmethyl)amino]thiophene-2-carboxylate. Mass
spectrum (ESI, m/z): Calcd. for C,~HI6N2O2S2, 345.07 (M+H), found 343.3
(imine), 345.2.
b) 5-Metlzylthio-4-~(2-quinolylmethyl)aminoJtlziophene-2-
carboxamidine: Using the procedure described in Example 220, step (b), 2.5
mg (2.6 % from methyl 4-amino-5-methylthiothiophene-2-carboxylate) of S-
methylthio-4-[(2-quinolylmethyl)amino]thiophene-2-carboxamidine was
obtained. Mass spectrum (ESI, m/z): Calcd. for C~6H~6N4S2, 329.09 (M+H),
found 329.3.
Example 225
4-(~(3-Hydroxyplzenyl)metlzylJaminof-5-metlzyltlziotlziophene-2-
carboxamidine
a) Metlzyl4-(~(3-Izydroxyphenyl)methylJamino~-5
methyltlziothiophene-2-carboxylate: Using the procedure described in
Example 220, step (a), 61.6 mg (0.30 mmol) of methyl 4-amino-5-
methylthiothiophene-2-carboxylate, 49.5 mg (0.30 mmol) of 3-
acetoxybenzaldehyde, and 135 mg (2.2 equiv, 0.63 mmol) of NaHB(OAc)3
were allowed to react to give methyl 4-{[(3-hydroxyphenyl)methyl]amino}-5-
methylthiothiophene-2-carboxylate. Mass spectrum (ESI, m/z): Calcd. for
3O Ci4H~6NO3S2, 352.07 (M+H), found, 350.2 (imine), 352.1.
b) Methyl4-~~(3-hydroxyphenyl)methylJaminof-5-
metlzylthiotlziopltene-2-carboxylate: Using the procedure described in
Example 220, step (b), 7.9 mg (8.9 % from methyl 4-amino-5-
methylthiothiophene-2-carboxylate) of methyl 4-{ [(3-hydroxyphenyl)
CA 02362390 2001-08-07
WO 00/47578 -246- PCT/US99/18065
methyl]amino}-5-methylthiothiophene-2-carboxylate; Mass spectrum (ESI,
m/z): Calcd. for C13HISN3OS2, 294.07 (M+H), found 294.3.
Example 226
S-Methylthio-4-(phenylcarbonylamino)thiophene-2-carboxamidine
a) Metlzyl S-metlzylthio-4-(phenylcarbonylamino)thiophene-2-
carboxylate: To a stirring solution of 114 mg (0.55 mmol) of methyl 4-amino-
5-methylthiothiophene-2-carboxylate in 4 mL of CHZC12 at 0 °C was added
142 ~.L (1.5 equiv, 0.82 mmol) of N,N diisopropylethylamine via syringe,
followed by 71.3 ~L (1.1 equiv, 0.61 mmol) of benzoyl chloride. The reaction
was allowed to warm to room temperature, and stirred an additional 4h. At this
time the reaction was partitioned in 40 mL of 1:1 CH2Cl2-sat'd. NaHC03 (v/v)
and the organic layer was separated, washed with 20 mL of brine, dried
(Na2S04), and concentrated in vacuo to afford 113 mg (66.8 %) of methyl S-
methylthio-4-(phenylcarbonylamino)thiophene-2-carboxylate which was used
without further purification. 1H NMR (DMSO-db, 400 MHz) 8 2.55 (s, 3H),
3.83 (s, 3H), 7.47-7.56 (m, 2H), 7.64 (m, 1H), 7.88 (s, 1H), 7.93-7.99 (m,
2H),
10.12 (s, 1H). Mass spectrum (ESI, m/z): Calcd. for C~4H~3NO3S2, 308.04
(M+H), found 308.2.
b) 5-Metlzylthio-4-(plzenylcarbonylamino)thiophene-2-
carboxamidine: Using a procedure similar to Example 154, step (b), 100 mg
(0.32 mmol) of methyl 4-{[4-(methylethyl)phenyl]amino}-5-
methylthiothiophene-2-carboxylate was allowed to react with 8 equiv (2.58
mmol) of the AlMe3/NH4C1 reagent to afford 95.4 mg (100 %) of 5-
methylthio-4-(phenylcarbonylamino)thiophene-2-carboxamidine. 'H NMR
(DMSO-d6, 400 MHz) 8 2.59 (s, 3H), 7.30-7.64 (m, 3H), 7.98-8.00 (m, 2H),
8.23 (s, 1H), 9.19 (bs, 2H), 9.41 (bs, 2H), 10.35 (s, 1H). Mass spectrum (ESI,
m/z): Calcd. for C13H,4N3OS2, 292.06 (M+H), found 292.2.
Example 227-290: To each of a series of 2-dram vials equipped a
with a stir bar and Teflon septum was added between 0.3 and 0.6 mmol an
acid chloride ( 1 equiv), followed by 1 equiv of methyl 4-amino-5-
methylthiothiophene-2-carboxylate as a 1 M CHZC12 solution. An additional 2
mL of CH2Cl2was charged into each vial, followed by 1.5 equiv of IV,N
CA 02362390 2001-08-07
WO 00/47578 _24~, PCT/US99/18065
diisopropylethylamine. Each vial was swept with Ar and allowed to stir for 3
h. At this time 4 mL of sat'd. NaHC03 was added to each vial and stirring was
continued for 5 min. The aqueous layers were removed by pipette and Na2S04
was added to each vial. The vials were allowed to stand overnight, and the
contents then eluted through 5-g silica gel (SPE column) cartridges with 0.5
MeOH-CHZC12. The amides were concentrated in vacuo into pre-weighed 2-
dram vials equipped with a stir bar and Teflon septum for the subsequent
amidination reactions. The vials were purged with Ar and charged with 2 mL
of toluene, followed by 8 equiv of the AlMe3/NH4Cl reagent as a 1M solution
in toluene. The reactions were heated to 110°C in a heating block for 3
h.
They were then cooled to ambient temperature and each was added by pipette
to a slurry of 1.5 g silica gel in 15 mL of CH2Cl2. Each slurry was vigorously
stirred for 15 min, at which time they were filtered through a 15 mL sintered
glass funnel containing 20 cm of silica gel with 50 % CHCI3-MeOH. The
yellow fractions were collected and concentrated in vacuo. The solids were
triturated with 10 % MeOH-CHCl3 and filtered. Concentration in vacuo
yielded the crude amidines, which were purified by preparative thin layer
chromatography (20 % MeOH-CHCI3-sat'd. NH3, 500 ~m Si02) to afford the
amidines as their respective free bases.
CA 02362390 2001-08-07
WO 00/47578 _24$_ PCT/US99/18065 -
ExampleAcid Chloride Amidine Product % Yield
227 cinnamoyl chloride4-((2E)-3-phenylprop-2-enoylamino)-15.3
5-methylthiothiophene-2-
carboxamidine
228 4-chlorobenzoyl 4-[(4-chlorophenyl)carbonylamino]-5-44.6
chloride
methylthiothiophene-2-carboxamidine
229 cyclohexoyl chloride4-(cyclohexylcarbonylamino)-5-17.8
methylthiothiophene-2-carboxamidine
230 3-nitro-4-methylbenzoylmethyl 4-[(4-methyl-3- 8.8
chloride nitrophenyl)carbonylamino]-5-
methylthiothiophene-2-carboxylate
231 2-furoyl chloride4-(2-furylcarbonylamino)-5-13.3
methylthiothiophene-2-carboxamidine
232 2,2-dimethyl-propanoyl4-(2,2-dimethylpropanoylamino)-5-8.5
chloride methylthiothiophene-2-carboxamidine
233 5-(3,5-dichloro- 4-{[5-(3,5-dichlorophenoxy)(2-22.9
phenoxy)furan-2- furyl)]carbonylamino}-5-
carbonyl chloridemethylthiothiophene-2-carboxamidine
234 1-napthoyl chloride5-methylthio-4- 3.1
(naphthylcarbonylamino)-thiophene-2-
carboxamidine
235 2-quinolinyl chlorideS-methylthio-4-(2-quinolylcarbonyl-6.8
amino)thiophene-2-carboxamidine
236 3-methoxybenzoyl 4-[(3-methoxyphenyl)carbonylamino]-6.8
chloride 5-methylthiothiophene-2-
carboxamidine
237 2-(2,5- 4-[2-(2-hydroxy-5- 18.3
dimethoxyphenyl)acetylmethoxyphenyl)acetylamino]-5-
chloride methylthiothiophene-2-carboxamidine
238 4-ethoxybenzoyl 4-[(4-ethoxyphenyl)carbonylamino]-5-34
chloride methylthiothiophene-2-carboxamidine
239 2-phenoxyacetyl 5-methylthio-4-(2- 10
chloride phenoxyacetylamino)-thiophene-2-
carboxamidine
240 3-methylbenzoyl 4-[(3-methylphenyl)carbonylamino]-5-21.1
chloride methytthiothiophene-2-carboxamidine
" Yield calculated from starting methyl 4-amino-5-methylthiotl:iophene-2-
carboxylate.
CA 02362390 2001-08-07
WO 00/47578 _249 PCT/IJS99/18065 -
Example 227
4-((2E)-3-Plzenylprop-2-enoylamino)-S-methylthiotlziophene-2
carboxamidine
a) Methyl4-((2E)-3 phenylprop-2-enoylamino)-5-
methylthiothiophene-2-carboxylate: yield: 100%. IH NMR (DMSO-db, 400
MHz) 8 2.49 (s, 3H), 3.83 (s, 3H), 7.12 (d, 1H, J--15.7 Hz), 7.41-7.66 (m,
6H),
8.24 (s, 1H), 9.92 (s, 1H). Mass spectrum (ESI, m/z): Calcd. for
C,6H~SNO3S2, 334.06 (M+H), 334.1.
b) 4-((2E)-3-Phenylprop-2-enoylamino)-S-methylthiothiophene-
2-carboxamidine: 1H NMR (DMSO-d6, 400 MHz) 8 2.54 (s, 3H), 7.13 (d, 1H,
J--15.7 Hz), 7.41-7.51 (m, 3H), 7.59-7.66 (m, 2H), 8.40 (s, 1H), 8.81 (bs,
3H),
10.02 (bs, 1H). Mass spectrum (ESI, m/z): Calcd. for CISH~SN30SZ, 318.07
(M+H), 318.2.
Example 228
4-~(4-Chlorophenyl)carbonylaminoJ S-methylthiothiophene-2-carboxamidine
a) Metlzyl4-~(4-chloroplzenyl)carbonylaminoJ 5-
metlzylthiothiophene-2-carboxylate: yield: 53%. 1H NMR (DMSO-db, 400
MHz) 8 2.55 (s, 3H), 3.83 (s, 3H), 7.62 (d, 2H, J--8.5 Hz), 7.87 (s, 1H), 7.97
(d, 2H, J--8.5 Hz), 10.21 (s, 1 H).
b) 4-~(4-Chlorophenyl)carbonylaminoJ 5-methylthiothiophene-
2-carboxamidine: 1H NMR (DMSO-db , 400 MHz) 8 2.59 (s, 3H). 7.63-7.66
(m, 2H), 7.98-8.01 (m, 2H), 8.99 (bs, 2H), 9.33 (bs, 2H), 10.39 (s, 1 H). Mass
spectrum (ESI, m/z): Calcd. for C,3H~2C1N30S2, 326.02 (M+H), found 326.2.
Example 229
4-(Cyclohexylcarbonylamino)-S-methylthiotlzioplzene-2-carboxamidine
a) Methyl4-(cyclohexylcarbonylamino)-S-ntethyltlziothiophene-
2-carboxylate: yield: 69.9%. ~H NMR (DMSO-db, 400 MHz) 8 1.22-1.81 (m,
11H), 2.51 (s, 3H), 3.82 (s, 3H), 7.97 (s, 1H), 9.55 (s, 1H). Mass spectrum
(ESI, m/z): Calcd. for C~qH~9NO3S2, 314.09 (M+H), found 314.2.
CA 02362390 2001-08-07
WO 00/47578 -250- PCT/US99/18065
b) 4-(Cyclohexylcarbonylamino)-S-methylthiotlziophene-2-
carboxamidine: ~H NMR (DMSO-db, 400 MHz) b 2.59 (s, 3H), 7.63-7.66 (m,
2H), 7.98-8.01 (m, 2H), 8.99 (bs, 2H), 9.33 (bs, 2H), 10.39 (s, 1H). Mass
spectrum (ESI, m/z): Calcd. for C~3HZON30S2, 298.10 (M+H), found 298.2.
Example 230
Metlzyl 4-~(4-methyl 3-nitrophenyl)carbonylaminoJ-5-methylthiotlziophene-2
carboxylate
a) Metlzyl4-~(4-metlzyl3-nitroplzenyl)carbonylaminoJ-S-
methylthiothiophene-2-carboxylate: yield: 80%. 'H NMR (DMSO-d6, 400
MHz) ~ 2.56 (s, 3H), 2.61 (s, 3H), 3.82 (s, 3H), 7.70 (d, 1H, J--8.1 Hz), 7.86
(s, 1H), 8.19 (dd, 1H, J--1.7, 8.0 Hz), 8.56 (d, 1H, J--1.7 Hz), 10.41 (s,
1H).
Mass spectrum (ESI, m/z): Calcd. for C,SH14NZO5S2, 367.42 (M+H), found
367.2.
b) Methyl4-~(4-metlzyl3-nitrophenyl)carbonylaminoJ-5
methyltJziothiophene-Z-carboxylate: 1H NMR (DMSO-db, 400 MHz) 8 2.47
(s, 3H), 2.61 (s, 3H), 7.12 (bs, 3H), 7.69-7.73 (m, 2H), 8.20 (dd, 1H, J--1.6,
7.9 Hz), 8.57 (d, 1H, J--1.6 Hz). Mass spectrum (ESI, m/z): Calcd. for
2O C,4H~4N4O3S2, 351.06 (M+H), found 351.2.
Example 231
4-(2-Furylcarbonylamino)-5-methyltlziothioplzene-2-carboxamidine
a) Metlzyl4-(2 furylcarbonylamino)-5-methylthiothiophene-2-
carboxylate: yield: 100%; IH NMR (DMSO-db, 400 MHz) 8 2.54 (s, 3H),
3.83 (s, 3H), 6.71 (dd, 1H, J--1.8, 3.4 Hz), 7.33 (d, 1H, J 3.5 Hz), 7.87 (s,
1 H), 7.95 (m, 1 H), 9.93 (s, 1 H). Mass spectrum (ESI, m/z): Calcd. for
C~2H~,N04S2, 298.02 (M+H), found 298.3.
b) 4-(2-Furylcarbonylamino)-S-methyltlziothioplzene-2-
carboxamidine: IH NMR (DMSO-d6, 400 MHz) b 2.51 (s, 3H), 6.71 (dd, 1H,
J--1.8, 3.5 Hz), 7.18 (bs, 3H), 7.32 (d, 1 H, J--3.4 Hz), 7.79 (s, 1 H), 7.96
(m,
1H). Mass spectrum (ESI, m/z): Calcd. for C"H1,N302S2, 282.04 (M+H),
found 282.2.
CA 02362390 2001-08-07
WO 00/47578 -251- PCT/US99/18065 -
Example 232
4-(2,2-Dimethylpropanoylamino)-S-methylthiothioplzene-Z-carboxamidine
a) Methyl4-(2,2-dimethylpropanoylamino)-5-
methylthiothiophene-2-carboxylate: yield: 93.4%. 'H NMR (DMSO-d6, 400
MHz) 8 1.23 (s, 9H), 2.51 (s, 3H), 3.81 (s, 3H), 7.74 (s, 1H), 9.04 (s, 1H).
Mass spectrum (ESI, m/z): Calcd. for C12H»NO3S2, 288.07 (M+H), found
288.1.
b) 4-(2,2-Dimethylpropanoylamino)-5-metlzylthiothiophene-2-
carboxamidine: 1H NMR (DMSO-db, 400 MHz) 8 1.24 (s, 9H), 2.55 (s, 3H),
8.05 (s, 1 H), 9.0 (bs, 3H), 9.1 (s, 1 H). Mass spectrum (ESI, m/z): Calcd.
for
C,~H1~N30S2, 272.09 (M+H), found 272.2.
Example 233
4-~~5-(3,5-Dichloropltenoxy)(2 furyl)JcarbonylaminoJ-5-
methylthiothiophene-2-carboxamidine
a) Methyl4-~~5-(3,5-dicltlorophenoxy)(2 furyl)JcarbonylaminoJ-
5-metlryltl:iothioplzene-2-carboxylate: yield: 96.9%. Mass spectrum (ESI,
m/z): Calcd. for C,gH,3C,ZNO5S2, 457.97 (M+H), found 457.9.
b) 4-~~5-(3,5-Dicltlorophenoxy)(2 frrryl)Jcarbonylamino~-5-
methylthiothiophene-2-carboxamidine: ~H NMR (DMSO-db, 400 MHz) 8
2.53 (s, 3H), 6.12-6.17 (m, 1H), 6.79 (d, 1H, J--1.8 Hz), 7.40-7.43 (m, 2H),
7.70 (m, 1 H), 8.13 (s, 1 H), 8.92 (bs, 2H), 9.21 (bs, 1 H), 10.06 (s, 1 H).
Mass
spectrum (ESI, m/z): Calcd. for C~7H,4C12N3O3S2, 441.99 (M+H), found
442.2.
Example 234
5-Methylthio-4-(naphtliylcarbonylamino)thiophene-2-carboxamidine
a) Metlzyl5-metlzylthio-4-(naplitltylcarbonylamino)thioplzene-2-
carboxylate: yield: 80.8 %. 'H NMR (DMSO-db, 400 MHz) b 7.59-7.67 (m,
3H), 7.80 (d, 1H, J--6.8 Hz), 8.02-8.34 (m, 4H), 10.38 (s, 1H).
b) 5-Methylthio-4-(naphtlzylcarbonylamino)thiopherte-2-
carboxamidine: ~H NMR (DMSO-d6, 400 MHz) ~ 2.50 (s, 3H), 7.60 (m, 3H),
CA 02362390 2001-08-07
WO 00/47578 -252- PCT/US99/18065
7.76 (d, 1 H, J--6.7 Hz), 7.94 (s, 1 H), 8.03 (d, 1 H, J--6.8 Hz), 8.09 (d, 1
H, 8.3
Hz), 8.30 (d, 1H, J--8.8 Hz). Mass spectrum (ESI, m/z): Calcd. for
C»H1sN30S2, 342.07 (M+H), found 342.2.
Example 235
5-Methylthio-4-(2-quinolylcarbonylamino)tlziophene-2-carboxamidine
a) Methyl5-methylthio-4-(2-quinolylcarbonylamino)thiophene-
2-carboxylate: yield: 80.9 %. 1H NMR (DMSO-db, 400 MHz) 8 2.59 (s, 3H),
3.86 (s, 3H), 8.03-8.06 (m, 3H), 8.24-8.29 (m, 3H), 9.58 (s, 1H), 10.63 (s,
1H).
b) 5-Metlzyltlzio-4-(2-quinolylcarbonylamino)thiophene-2-
carboxamidine: 1H NMR (DMSO-d6, 400 MHz) 8 2.53 (s, 3H), 7.21 (bs; 3H),
7.74 (s, 1H), 7.96-7.98 (m, 2H), 8.19-8.22 (m, 4H), 9.77 (s, 1H). Mass
spectrum (ESI, m/z): Calcd. for C~6H,4N4OS2, 343.45 (M+H), found 343.1.
Example 236
4-~(3 Metlzoxyphenyl)carbonylaminoJ 5-methylthiothiophene-2
carboxamidine
a) Metlzyl4-~(3-methoxyplzenyl)carbonylaminoJ-5-
metlzylthiothiophene-2-carboxylate: yield: 90.3 %. 1H NMR (DMSO-d6, 400
MHz) 8 2.55 (s, 3H), 3.83 (s, 3H), 3.85 (s, 3H), 7.19 (m, 1H), 7.39-7.59 (m,
3H), 7.85 (s, 1H), 10.09 (s, 1H). Mass spectrum (ESI, m/z): Calcd. for
CisHisNOaS2, 338.05 (M+H), found 338.3.
b) 4-((3-Metlzoxyphenyl)carbonylaminoJ S-methylthiothiophene-
2-carboxamidine: 1H NMR (DMSO-db, 400 MHz) 8 2.58 (s, 3H), 3.84 (s,
3H), 7.19 (dd, 1H, J--2.1, 8.1 Hz), 7.45-7.57 (m, 3H), 8.15 (s, 1H), 9.11 (bs,
4H), 10.32 (bs, 1H). Mass spectrum (ESI, m/z): Calcd. for C,4H1sN3O2S2,
322.07 (M+H), found 322.2.
Example 237
4-~2-(2, S-Dimethoxyphenyl)acetylaminoJ 5-methylthiothioplrene-2
carboxamidine
a) Methyl4-~2-(2,5-dimethoxyphenyl)acetylanzinoJ S=
methyltlziothiophene-2-carboxylate: 1H NMR (DMSO-db, 400 MHz) 8 2.47
CA 02362390 2001-08-07
WO 00/47578 _253_ PCT/US99/18065 -
(s, 3H), 3.67 (s, 2H), 3.70 (s, 3H), 3.75 (s, 3H), 3.80 (s, 3H), 6.81 (dd, 1H,
J 3.0, 8.8 Hz), 6.87 (d, 1 H, J--3.0 Hz), 6.93 (d, 1 H, J--8.9 Hz), 8.04 (s, 1
H),
9.62 (s, 1 H);
b) 4-~2-(2,5-Dimetlzoxyphenyl)acetylaminoJ-5-
methyltltiotlziophene-2-carboxamidine: ~H NMR (DMSO-db, 400 MHz) 8
2.38 (s, 3H), 3.66 (s, 2H), 3.70 (s, 3H), 3.76 (s, 3H), 6.81 (dd, 1H, J=3.3,
8.0
Hz), 6.88-6.94 (m, 2H), 7.91 (s, 1 H), 9.42 (bs, 1 H).
Example 238
4-~(4-Ethoxyphenyl)carbonylaminoJ 5-methylthiotl:iophene-2-carboxamidine
a) Metlryl4-~(4-ethoxyphenyl)carbonylaminoJ S-
methylthiotlziophene-2-carboxylate: 'H NMR (DMSO-db, 400 MHz) ~ 1.36
(t, 3H, J--7.0 Hz), 2.54 (s, 3H), 3.83 (s, 3H), 4.13 (q, 2H, J--7.0 Hz), 7.05
(d,
2H, J--8.8 Hz), 7.87 (s, 1 H), 7.93 (d, 2H, J--8.8 Hz), 9.93 (s, 1 H). Mass
spectrum (ESI, m/z): Calcd. for C~6H1~NO4S2, 352.07 (M+H), found 352.2.
b) 4-~(4-Etlzoxyplzenyl)carbonylaminoJ-S-methyltlziothiophene-
2-carboxamidine: 'H NMR (DMSO-db, 400 MHz) 8 1.36 (t, 3H, J--7.0 Hz),
2.55 (s, 3H), 4.13 (q, 2H, J--7.0 Hz), 7.04-7.08 (m, 2H), 7.94-7.97 (m, 2H),
8.09 (s, 1H);8.73 (bs, 3H), 10.01 (bs, 1H). Mass spectrum (ESI, m/z): Calcd.
for C~SH~7N302S2, 336.08 (M+H), found 336.2.
Example 239
5-Methylthio-4-(2 phenoxyacetylamino)tlziophene-2-carboxamidine
a) Methyl5-methylthio-4-(2 pl:enoxyacetylamino)thioplzene-2-
carboxylate: yield: 79 %. 'H NMR (DMSO-d6, 400 MHz) ~ 2.48 (s, 3H), 3.82
(s, 3H), 4.78 (s, 2H), 6.97-7.02 (m, 2H), 7.31-7.35 (m, 2H), 8.05 (s, 1H),
9.80
(s, 1 H).
b) S-Methylthio-4-(2 plzenoxyacetylamino)thiophene-2-
carboxamidine: 'H NMR (DMSO-d~, 400 MHz): 8 2.52 (s, 3H), 4.81 (s, 2H),
6.97-7.04 (m, 3H), 7.31-7.35 (m, 2H), 8.26 (s, 1H), 8.84 (bs, 4H). Mass
spectrum (ESI, m/z): Calcd. for C~qH,SN3O2S2, 322.43 (M+H), found 322.2.
CA 02362390 2001-08-07
WO 00/47578 -254- PCT/US99/18065
Example 240
4-~(3-Metlzylph enyl) carbonylamin oJ-5-metlzylth ioth ioph en e-2-
carboxamidin a
a) Methyl4-((3-methylphenyl)carbonylaminoJ-5-
metlayltlaiothioplzene-2-carboxylate: yield: 79 %. 'H NMR (DMSO-d6, 400
MHz) 8 2.40 (s, 3H), 2.55 (s, 3H), 3.83 (s, 3H), 4.78 (s, 2H), 7.42-7.43 (m,
2H), 7.47-7.77 (m, 2H), 7.86 (s, 1H), 10.06 (s, 1H). Mass 'spectrum (ESI,
m/z): Calcd. for C~SH,SN03S2, 322.06 (M+H), found 322.2.
b) 4 ~(3-Metlzylphenyl)carbonylaminoJ-S-metlzyltlziothioplzene-
2-carboxamidine: 'H NMR (DMSO-d6, 400 MHz) 2 2.40 (s, 3H), 2.55 (s,
3H), 7.43-7.44 (m, 2H), 7.75-7.78 (m, 2H), 8.05 (s, 1H), 8.52 (bs, 3H), 10.12
(bs, 1H). Mass spectrum (ESI, m/z): Calcd. for C,4H~SN3OS2, 306.07 (M+H),
found 306.2.
Example 241
a) MetlZyl4-bromo-S-methyltlZiotIZiophene-2-carboxylate: To a
stirred solution of 4-bromo-5-methylthiothiophene-2-carboxylic acid (87
mmol), prepared according to the procedure of Kleemann, et al., EP .
0676395A2, in dry methanol (750 mL) was added thionyl chloride (7 mL, 96
mmol) dropwise. After stirring for 10 min at room temperature, the solution
was heated to reflux and stirred 7.5 h. The solution was cooled and the
solvents were removed in vacuo. The resulting solid was dissolved in
dichloromethane (1500 mL) and washed with saturated sodium bicarbonate (2
x 300 mL), water (300 mL), saturated brine (300 mL), and dried over
anhydrous sodium sulfate. The solvents were removed in vacuo. The
resulting solid was recrystallized twice from hexane/ethyl acetate to give
methyl 4-bromo-5-methylthiothiophene-2-carboxylate (4.4 g, 19 %). 'H-
NMR (CDCl3, 400 MHz) ~ 7.66 (s, 1H), 3.90 (s, 3H), 2.60 (s, 3H).
b) Methyl S-metlzyltlzio-4-~~3-(phenylmetlroxy)plzenylJaminoJ
thiopl:ene-2-carboxylate: A dry mixture of 60 mg (0.225 mmol) of methyl 4-
bromo-5-methylthiothiophene-2-carboxylic acid, as prepared in the previous
step, 3.0 mg (6 mole %) of palladium (II) acetate (Aldrich Chemical Co.,
Milwaukee, WI), 12.6 mg (9 mole %) of racemic-2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl (Strem, Newburyport, MA), 110 mg
CA 02362390 2001-08-07
WO 00/47578 -255- PCT/US99/18065 -
(0.34 mmol, 1.5 eq) of cesium carbonate (Aldrich Chemical Co., Milwaukee,
WI), and 54 mg (0.29 mmol, 1.3 eq) of 3-benzyloxyaniline (Aldrich Chemical
Co., Milwaukee, WI) was added to an oven-dried 1-dram glass vial. This vial
was flushed with dry argon in a glove bag, dry toluene (450 ~L, 0.5 M) was
added, and the assembly was heated at 100°C for 36 h. To the cooled
suspension ethyl acetate (4 mL) was added, the mixture passed through 1 inch
of Celite, washed with ethyl acetate (2 x 4 mL) and the solvents removed in
vacuo. Purification by preparative thin-layer chromatography ( 1:1
dichloromethane/hexanes) gave 13 mg of the title compound (15 °%) as a
pale
yellow solid. 1H-NMR (CDC13, 400 MHz) 8 7.77 (s, 1H), 7.47-6.59 (m, 9H),
6.11 (s, 1H), 5.07 (s, 2H), 3.89 (s, 3H), 2.47 (s, 3H). Mass spectrum (ESI,
m/z): Calcd. for C2pHI9NO3S2, 386.1 (M+H), found 386.3.
c) S-MetlZylthio-4-~~3-(plzenylmethoxy)plzenylJamino)thioplzene-
2-carboxamidine: Trimethylaluminum (2.0 M in toluene, 2 mL) was added
dropwise over 10 min to a suspension of ammonium chloride (216 mg) in
toluene (2 mL), stirred under dry nitrogen at 0°C. After the mixture
was
stirred at 25°C for 30 min, when most of the solid had dissolved, this
mixture
was taken up in a syringe and added to 13 mg (0.03 mmol) of methyl 5-
methylthio-4-{ [3-(phenylmethoxy)phenyl]amino}thiophene-2-carboxylate.
The reaction mixture was heated to reflux in stages and stirred for 2h 10 min.
The cooled mixture was poured in to a vigorously stirred slurry of silica gel
(2
g) in chloroform (20 mL). To this suspension methanol (50 mL) was added,
the mixture was passed through 1 inch of silica gel in a sintered glass
Buchner
funnel, washed with methanol (50 mL), and the solvents removed in vacuo.
The crude product was purified on a 5 g silica gel SPE column washing first
with dichloromethane and then eluting the product off with 10 % methanol in
dichloromethane. The product was further purified by preparative High
Pressure Liquid Chromatography (HPLC) on a Dynamax C 18 column, 60 ~
pore size, 10 ~.M particle size, 40 to 100 % methanol over 30 min in 0.1
trifluoroacetic acid to give 5.4 mg of the title compound (45 %) as a yellow
solid. ~ H-NMR (CD30D, 400 MHz) ~ 7.84 (s, 1 H), 7.44-6.60 (m, 9H), 5.08
(s, 2H), 2.48 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for C19H,9N3OS2,
370.1 (M+H), found 370.2.
CA 02362390 2001-08-07
WO 00/47578 -256- PCT/US99/18065 -
Example 242
a) Methyl S-methylthio-4-((3 pltenoxyphenyl)aminoJthiophene-
2-carboxylate: A stirred suspension of 80 mg (0.299 mmol) of methyl 4-
bromo-5-methylthiothiophene-2-carboxylate and 72 mg (0.389 mmol, 1.3 eq)
of 3-phenoxyaniline (Aldrich, Milwaukee, WI) was treated as in Example 241,
step (b). Further purification of the product by preparative thin layer
chromatography eluting with 10 % ethyl acetate in hexane gave 36 mg of the
title compound (32 %) as a yellow oil. 'H-NMR (CDCl3, 400 MHz) S 7.76 (s,
1H), 7.40-6.65 (m, 9H), 6.26 (s, 1H), 3.89 (s, 3H), 2.40 (s, 3H). Mass
spectrum (ESI, m/z): Calcd. for C19H,7NO3S2, 372.1 (M+H), found 372.2.
b) 5-Methyltltio-4 I(3 pltenoxyplzenyl)aminoJthiopliene-2-
carboxamidine: Methyl5-methylthio-4-[(3-phenoxyphenyl)amino]thiophene-
2- .carboxylate (36 mg, 0.097 mmol) was treated as in Example 241. step (c),
but without HPLC purification to give 30 mg of the title compound (86 %) as
an orange glass. 1H-NMR (CDCl3, 400 MHz) 8 9.28 (s, 2H), 8.11 (s, 2H),
7.99 (s, 1H), 7.34-6.50 (m, 9H), 6.29 (s, 1H), 2.35 (s, 3H). Mass spectrum
(ESI, m/z): Calcd. for C~8H~7N30S2, 356.1 (M+H), found 356.2.
Example 243
a) 5 Metltylthio-4 ~(4 phenoxyplzenyl)aminoJthiophene-2-
carboxamidine: A stirred suspension of 80 mg (0.299 mmol) of methyl 4-
bromo-5-methylthiothiophene-2-carboxylate and 72 mg (0.389 mmol, 1.3 eq)
of 4-phenoxyaniline (Aldrich, Milwaukee, WI) was treated as in Example 241,
step (b). Further purification of the product by preparative thin layer
chromatography eluting with 10 % ethyl acetate in hexane gave 53 mg of the
title compound (48 %) as a yellow oil. iH-NMR (CDCl3, 400 MHz) b 7.70 (s,
1H), 7.34-7.00 (m, 9H), 6.11 (s, 1H), 3.89 (s, 3H), 2.42 (s, 3H). Mass
spectrum (ESI, m/z): Calcd. for C~9H1~NO3S2, 372.1 (M+H), found 372.1.
b) 5-Methylthio-4 ~(4 phenoxyphenyl)aminoJthiophene-2-
carboxamidine: Methyl5-methylthio-4-[(4-phenoxyphenyl)amino]thiophene-
2-carboxylate (53 mg, 0.14 mmol) was treated as in Example 241, step (c), but
without HPLC purification to give 58 mg of the title compound (quantitative
yield) as an orange glass. 1H-NMR (CDC13, 400 MHz) 8 8.89 (s, 2H), 8.59 (s,
CA 02362390 2001-08-07
WO 00/47578 _25~- PCT/US99/18065
2H), 8.00 (s, 1H), 7.25-6.87 (m, 9H), 6.20 (s, 1H), 2.27 (s, 3H). Mass
spectrum (ESI, m/z): Calcd. for C18H17N30S2, 356.1 (M+H), found 356.2.
Example 244
a) Methyl 4 ~(2-methoxypheityl)aminoJ S-methylthiothiophene-
2-carboxylate: A stirred suspension of 103 mg (0.386 mmol) of methyl 4-
bromo-5-methylthiothiophene-2-carboxylate and 57 mg (0:46 mmol, 1.2 eq)
of 2-methoxyaniline (Aldrich, Milwaukee, WI) was treated in a manner
similar to Example 241, step (b) to give 78 mg the title compound (65 %) as a
yellow oil. IH-NMR (CDCl3, 400 MHz) 8 7.82 (s, 1H), 7.12-6.52 (m, 4H),
6.52 (s, 1H), 3.92 (s, 3H), 3.87 (s, 3H), 2.40 (s, 3H). Mass spectrum (ESI,
m/z): Calcd. for C14H15N03S2, 310.1 (M+H), found 310.2.
b) 4-~(2-Methoxyphenyl)aminoJ 5-methylthiotlziophene-2-
carboxamidine: Methyl4-[(2-methoxyphenyl)amino]-5-
methylthiothiophene-2-carboxylate (78 mg, 0.25 mmol) was treated as in
Example 241, step (c), but without HPLC purification to give 75 mg of the
title compound (quantitative yield) as an orange glass. 1H-NMR (CD30D, 400
MHz) 8 7.91 (s, 1H), 7.15-6.93 (m, 4H), 3.93 (s, 3H), 2.48 (s, 3H). Mass
spectrum (ESI, m/z): Calcd. for C~3H~SN3OS2, 294.1 (M+H), found 294.2.
Example 245
a) Metltyl4-~(2-metlzylplzenyl)aminoJ-5-methylthiothiophene-2-
carboxylate: A dry mixture of 100 mg (0.374 mmol) of methyl 4-bromo-5-
methylthiothiophene-2-carboxylate, 51 mg (14.9 mole %) of
tris(dibenzylideneacetone)dipalladium (Lancaster, Pelham, NH), 52 mg (22.3
mole %) of racemic-2,2'-bis(diphenylphosphino)-l,l'-binaphthyl (Strem,
Newburyport, MA), 183 mg of (0.56 mmol, 1.5 eq) cesium carbonate (Aldrich
Chemical Co., Milwaukee, WI), and 71 ~L (0.49 mmol, 1.3 eq) of 2-
methylaniline (Aldrich Chemical Co., Milwaukee, WI) was added to an oven-
dried 1-dram glass vial. This vial was flushed with dry argon in a glove bag,
dry toluene (750 ~L, 0.5 M) was added, and the assembly was heated at
100°C
for 40 h. To the cooled suspension ethyl acetate (4 mL) was added, the
mixture passed through 1 inch of Celite, washed with ethyl acetate (2 x 4 mL)
and the solvents removed in vacuo. Purification by preparative thih-layer
CA 02362390 2001-08-07
WO 00/47578 _25$_ PCT/US99/18065
chromatography (1:1 dichloromethane/hexanes) gave 67 mg of the title
compound (61 %) as a yellow oil. IH-NMR (CDCl3, 400 MHz) 8 7.64 (s, 1H),
7.23-6.94 (m, 4H), 5.91 (br s, 1H), 3.88 (s, 3H), 2.41 (s, 3H), 2.31 (s, 3H).
Mass spectrum (ESI, m/z): Calcd. for C,4H~SN02S2, 294.1 (M+H), found
294.2.
b) 4 ~(2-Methylphenyl)aminoJ S-metlzylthiotlziophene-2-
carboxamidine: Methyl4-[(2-methylphenyl)amino]-S-methylthiothiophene-
2-carboxylate (67 mg, 0.23 mmol) was treated as in Example 241, step (c), but
without HPLC purification to give 20 mg of the title compound (30 %) as a
yellow glass. 1H-NMR (CD30D; 400 MHz) 8 7.56 (s, 1H), 7.24-6.99 (m, 4H),
2.49 (s, 3H), 2.29 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for C,3H,SN3Sz,
278.1 (M+H), found 278.2.
Example 246
a) Methyl 4-~(3-chlorophenyl)aminoJ S-methylthiotlziophene-2-
carboxylate: A stirred suspension of 80 mg (0.299 mmol) of methyl 4-bromo-
5-methylthiothiophene-2-carboxylate and 41 ~L (0.389 mol, 1.3 eq) of 3-
chloroaniline (Aldrich, Milwaukee, WI) was treated in a manner similar to
Example 241, step (b) to give 47 mg of the title compound (50 %) as a yellow
oil. 1H-NMR (CDCI3, 400 MHz) b 7.75 (s, 1H), 7.23-6.89 (m, 4H), 6.10 (s,
1H), 3.89 (s, 3H), 2.42 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C,3H12N02S2C1, 314.0 (M+H), found 314.1.
b) 4 ~(3-Chlorophenyl)aminoJ S-methylthiothiophene-2-
carboxamidine: Methyl4-[(3-chlorophenyl)amino]-5-methylthiothiophene-2-
carboxylate (47 mg, 0.15 mmol) was treated as in Example 241, step (c) to
give 33 mg of the title compound (75 %) as a light yellow solid. 1H-NMR
(DMSO-d6, 400 MHz) 8 9.22 (s, 2H), 8.81 (s, 2H), 8.22 (s, 1 H), 7.99 (s, 1 H),
7.24-6.82 (m, 4H), 2.53 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C,ZH12N3S2C1, 298.0 (M+H), found 298.3.
Example 247
a) Methyl4-(metlzylphenylamino)-S-methylthiothioplrene-2-
carboxylate: A stirred suspension of 100 mg (0.374 mmol) of methyl 4-
bromo-5-methylthiothiophene-2-carboxylate and 72 gL (0.487 mmol, 1.3 eq)
CA 02362390 2001-08-07
WO 00/47578 _259_ PCT/US99/18065
of N-methylaniline (Aldrich Chemical Co., Milwaukee, WI) was treated in a
manner similar to Example 245, step (a) to give 23 mg of the title compound
(21 %) as a yellow oil. 'H-NMR (CDC13, 400 MHz) 8 7.61 (s, 1 H), 7.26-6.68
(m, SH), 3.89 (s, 3H), 3.25 (s, 3H), 2.50 (s, 3H). Mass spectrum (ESI, m/z):
Calcd. for C,4H15NOZS2, 294.1 (M+H), found 294.3.
b) 4-(Methylphenylamino)-S-methyltlziotlziophene-2-
carboxamidine: Methyl4-[(2-methylphenyl)amino]-5-methylthiothiophene-
2-carboxylate (23 mg, 0.078 mmol) was treated as in Example 241, step (c),
but without HPLC purification to give 5.6 mg of the title compound (26 %) as
a yellow glass. IH-NMR (CD30D, 400 MHz) b 7.83 (s, 1H), 7.24-6.71 (m,
4H), 3.27 (s, 3H), 2.57 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C~3H,SN3S2, 278.1 (M+H), found 278.3.
Example 248
a) Methyl5-metlzyl4-(phenylamino)tlziophene-2-carboxylate: A
stirred suspension of 400 mg (1.7 mmol) methyl 5-methyl-4-bromo-thiophene-
2-carboxylate and 192 pL (2.1 mmol, 1.25 eq) of aniline (Aldrich, Milwaukee,
WI) was treated in a manner similar to Example 241, step (b) to give 66 mg of
the title compound (16 %) as a brown glass. 'H-NMR (DMSO-db, 400 MHz)
8 7.70 (s, 1H), 7.56 (s, 1H), 7.17 (m, 2H), 6.72 (m, 3H), 3.79 (s, 3H), 2.31
(s,
3H). Mass spectrum (MALDI, gentisic acid matrix, m/z): Calcd. for
C13H,3N02S, 248.1 (M+H), found 247.5.
b) S-Metlzyl-4-(phenylamino)tlzioplzene-2-carboxamidine:
Methyl 4-(methylphenylamino)-5-methylthiothiophene-2-carboxylate (66 mg,
0.27 mmol) was treated as in Example 241, step (c), but without HPLC
purification to give 57 mg of the title compound (91 %) as a brown glass. ~H-
NMR (DMSO-db, 400 MHz) 8 9.17 (s, 2H), 8.85 (s, 2H), 7.98 (s, 1 H), 7.85 (s,
1H), 7.21-6.73 (m, SH), 2.39 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C~ZH~3N3S, 232.1 (M+H), found 232.2.
Example 249
a) Methyl4-~~4-(dimetlzylamino)phenylJaminoJ-S-
methylthiothiophene-2-carboxylate: A stirred suspension of 100 mg (0.267
mmol) methyl 5-methyl-4-bromo-thiophene-2-carboxylate and 66 mg (0.35
CA 02362390 2001-08-07
w0 00/47578 -260- PCT/US99/18065
mmol, 1.3 eq) of 4-amino-N,N-dimethylaniline (Fluka, Milwaukee, WI) was
treated in a manner similar to Example 241, step (b), but eluting with 1:1
ethyl
acetate/hexane for preparative thin-layer chromatrography purification, to
give
86 mg of the title compound (quantitative yield) as an orange glass. 1H-NMR
(CDC13, 400 MHz) 8 7.53 (s, 1H), 7.16 and 6.62 (AB quartet, 4H, J=8.9 Hz),
5.99 (s, 1H), 3.86 (s, 3H), 2.94 (s, 6H), 2.39 (s, 3H). Mass spectrum (ESI,
m/z): Calcd. for CISH~gN202S2, 323.1 (M+H), found 323.3.
b) 4-~~4-(Dimethylamino)plzenylJaminoJ-5-metlzyltlaiotlziophehe-
2-carboxamidine: Methyl4-(methylphenylamino)-S-methylthiothiophene-2-
carboxylate (86 mg, 0.267 mmol) was treated as in Example 241, step (c), but
without HPLC purification. This material was further purified by passing
through 1 inch of basic alumina and eluting with 10 % methanol in
dichloromethane ( 1 S mL) to give 62 mg of the title compound (76 %) as a
brown glass. 'H-NMR (DMSO-db, 400 MHz) 8 8.95 (s, 4H), 7.75 (s, 1H),
7.56 (s, 1 H), 6.97 and 6.72 (AB quartet, 4H, J=8.9 Hz), 2.83 (s, 6H), 2.44
(s,
3H). Mass spectrum (ESI, m/z): Calcd. for C14H~8N4S2, 307.1 (M+H), found
307.3.
Example 250
4-~(4-Etlzylplzenyl)aminoJ 5-methylthiotlzioplzene-2-carboxamidine
hydrochloride
a) Methyl4-~(4-ethylplzenyl)aminoJ S-methylthiotlziophene-2-
carboxylate: To an oven-dried glass vial with stir bar was added a mixture of
100 mg (0.374 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a)), 5.8 mg (6.9 mol %) of
palladium (II) acetate, 21.7 mg (9.3 mol %) of racemic-2,2'-
bis(diphenylphosphino)-l,l'-binaphthyl , 171.5 mg (0.526 mmol) of cesium
carbonate and 59 mg (0.487 mmol) of 4-ethylaniline. The vial was transferred
to a glove bag, flushed with dry argon and anhydrous toluene (749 ~L) was
added. The vial was capped with a Teflon-lined screw cap and heated at 100
°C for 48 h. The cooled suspension was filtered (Celite) washing with
ethyl
acetate (2 x 2 mL), and the solvents removed in vacuo. The resulting residue
was purified on 1 mm silica prep plates eluting with 40% methylene chloride-
hexanes to afford 14 mg (12 %) of methyl 4-[(4-ethylphenyl)amino]-5-
CA 02362390 2001-08-07
w0 00/47578 PCT/US99/18065
-261
methylthiothiophene-2-carboxylate as a pale yellow resin which was used
directly in the following step.
b) 4-~(4-Etlzylphenyl)aminoJ 5-methylthiotlziophene-2-
carboxamidine hydrochloride: Trimethylaluminum (2.0 M in toluene, 0.182
mL, 0.363 mmol) was added dropwise to a suspension of ammonium chloride
(19 mg, 0.363 mmol) in anhydrous toluene (1 mL) under Ar at 0°C. The
mixture was stirred at 25 °C for 30 min and then 14 mg (0.036 mmol) of
methyl 4-[(4-ethylphenyl)amino]-5-methylthiothiophene-2-carboxylate (as
prepared in previous step) was added. The reaction mixture was heated slowly
to 100 C and stirred for 4 h. The cooled mixture was added to a vigorously
stirred slurry of silica gel (1.3 g) in chloroform (20 mL). The suspension was
filtered (silica) washing with 50 % MeOH-CH2C12 (2 x 50 mL). The washings
were concentrated and the resulting residue was purified on a 0.5 mm silica
prep plate eluting with a 10 % MeOH-CH2C12 to afford 8 mg (67 %) of 4-[(4-
ethylphenyl)amino]-5-methylthiothiophene-2-carboxamidine hydrochloride as
a yellow oil. 'H-NMR (CD30D, 400 MHz) 8 7.84 (s, 1H), 7.14 (d, 2H, 8 Hz),
7.01 (d, 2H, 8 Hz), 2.55 (q, 2H, 65.5 Hz), 2.48 (s, 3H), 1.23 (t, 3H, 15.2
Hz).
Mass spectrum (ESI, m/z): Calcd. for C~4H~7N3S2, 292.1 (M+H), found 292.5.
Example 251
S-Methylthio-4-(~4-(phenylmethoxy)phenylJaminoJthioplzene-2-
carboxamidine hydrochloride
a) Methyl S-methylthio-4-~~4-(phenylmethoxy)plZenylJ
aminoJtlaioplzene-2-carboxylate: The same procedure as in Example 250,
step a was followed using 100 mg (0.374 mmol) of methyl 4-bromo-5-
methylthiothiophene-2-carboxylate (as prepared in Example 241, step (a)), 5.5
mg (6.5 mol %) of palladium (II) acetate, 23.6 mg (10.1 mol %) of racemic-
2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, 194 mg (0.59 mmol) of cesium
carbonate, 97.3 mg (0.488 mmol) of 4-benzyloxyaniline and 749 ~L of
toluene, and chromatographed as before using 40 % CH2Cl~-hexane to afford
7 mg (5 %) of methyl 5-methylthio-4-{[4-(phenylmethoxy)phenyl]
amino}thiophene-2-carboxylate as a yellow resin which was used directly in
the following step.
b) 5-Methyltlaio-4-~~4-(phenylmetlzoxy)plzenylJamino~thiophene
2-carboxamidine hydrochloride: The same procedure as in Example 250,
CA 02362390 2001-08-07
WO 00/47578 -262- PCT/US99/18065
step (b) was followed using 7 mg (0.018 mmol) of methyl S-methylthio-4-{[4-
(phenylmethoxy)phenyl]amino}thiophene-2-carboxylate (as prepared in
previous step), 0.091 mL of trimethylaluminum (2.0 M in toluene, 0.182
mmol), 10 mg of ammonium chloride (0.182 mmol) and 1 mL of toluene, and
purified on a 0.5 mm silica prep plate eluting with 10 % MeOH-CHZCIz to
afford 3 mg (41 %) of 5-methylthio-4-{[4-
(phenylmethoxy)phenyl]amino}thiophene-2-carboxamidirie hydrochloride as a
yellow oil. 1H-NMR (CD30D, 400 MHz) 8 7.72 (s, 1H), 7.45 (d, 2H, 7 Hz),
7.39 (t, 2H, 9 Hz), 7.37 (d, 1H, 12 Hz), 7.06 (d, 2H, l2Hz), 6.97 (d, 2H,
l2Hz), 5.08 (s, 2H), 2.46 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C,9H,9N3OS2, 370.1 (M+H), found 370.3.
Example 252
S-Metltyltlzio-4-~~4-(phenylamino)phenylJaminoJthiophene-2-carboxamidine
Izydrochloride
a) Methyl S-methyltltio-4-~~4-(phenylanzino)phenylJaminoJ
thiophene-2-carboxylate: The same procedure as in Example 250, step (a)
was followed using 100 mg (0.374 mmol) of methyl 4-bromo-5-
methylthiothiophene-2-carboxylate (as prepared in Example 241, step a), 5.5
mg (6.5 mol %) of palladium (II) acetate, 21.6 mg (9.3 mol %) of racemic-
2,2'-bis(diphenylphosphino)-1,1'-binaphthyl , 173.7 mg (0.533 mmol) of
cesium carbonate, 92.3 mg (0.500 mmol) of N-phenyl-1,4-phenylenediamine,
and 749 uL of toluene, and chromatographed as before using 40 % CH2C12-
hexane to afford 58 mg (42 %) of methyl 5-methylthio-4-{[4-
(phenylamino)phenyl]amino}thiophene-2-carboxylate as a brown solid. ~H-
NMR (DMSO-db, 400 MHz) 8 7.85 (s, 1H), 7.61 (s, 1H), 7.48 (s, 1H), 7.14 (t,
2H, 16 Hz), 6.99 (d, 2H, 16 Hz), 6.90 (q, 4H, 44 Hz), 6.70 (t, 2H, 4 Hz), 3.77
(s, 3H), 2.43 (s, 3H).
b) S-Methylthio-9-(~4-(plzenylamino)pltenylJamino~thioplzene-2-
carboxamidine l:ydroclzloride: The same procedure as in Example 250, step
(b) was followed using 58 mg (0.156 mmol) of methyl 5-methylthio-4-{[4-
(phenylamino)phenyl]amino}thiophene-2-carboxylate (as prepared in previous
step), 0.783 mL of trimethylaluminum (2.0 M in toluene, 1.56 mmol), 84 mg
of ammonium chloride (1.56 mmol) and 10 mL of toluene, and purified by
CA 02362390 2001-08-07
WO 00/47578 -263- PCT/US99/18065
passing through a pad of silica eluting with 50 % MeOH-CH2C12 to afford 50
mg (75 %) of the 5-methylthio-4-{[4-(phenylamino)phenyl]amino}thiophene-
2-carboxamidine hydrochloride as a brown solid. 1H-NMR (DMSO-d6, 400
MHz) 8 7.91 (d, 2H, 12 Hz), 7.78 (s, 1H), 7.20 (t, 3H, 12 Hz), 7.04-6.94 (m,
SH), 6.71 (m, 1H), 2.47 (s, 3H). Mass spectrum (ESI, m/z): Calcd. for
C,gH18N4S2, 355.1 (M+H), found 355.4.
Example 253
4-~(4-Metlzoxyplzenyl)aminoJ S-methylthiotlziophene-2-carboxamidine
Hydrochloride
a) Metlzyl4-~(4-metlioxyphenyl)aminoJ S-methyltlziothiophene-
2-carboxylate: To an oven-dried glass vial with stir bar was added a mixture
of 120 mg (0.449 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a)), 7.1 mg (7 mol %) of
palladium (II) acetate, 29.4 mg (10.5 mol %) of racemic-2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl, 205 mg (0.629 mmol) of cesium
carbonate and 69.1 mg (0.561 mmol) of p-anisidine. The vial was transferred
to a glove bag, flushed with dry argon and anhydrous toluene (0.9 mL) was
added. The vial was capped with a Teflon-lined screw cap and heated at 100
°C for 48 h. To the cooled suspension was added ethyl acetate (4 mL),
the
mixture filtered (Celite) washing with ethyl acetate (2 x 2 mL), and the
solvents removed in vacuo. The resulting residue was purified by silica gel
preparative thin layer chromatography (40% CH2C12 in hexane) to afford 83
mg (60 %) of the title compound as a yellow oil. 'H-NMR (CDC13, 400 MHz)
8 2.39 (s, 3H), 3.82 (s, 3H), 3.87 (s, 3H), 6.03 (s, 1H), 6.89 (m, 2H), 7.03
(m,
2H), 7.58 (s, 1H).
b) 4-~(4-Metltoxyplzenyl)aminoJ 5-metltylthiotlziophene-2-
carboxamidine hydrochloride: Trimethylaluminum (2.0 M in toluene, 2 mL,
4 mmol) was added dropwise to a suspension of ammonium chloride (216 mg,
4 mmol) in anhydrous toluene ( 1 mL) under Ar at room temperature. The
mixture was stirred at 25 °C for 30 min and then 80 mg (0.259 mmol) of
methyl 4-[(4-methoxyphenyl)amino]-5-methylthiothiophene-2-carboxylate (as
3~ prepared in previous step) in anhydrous toluene (1 mL) was added. The
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-264-
reaction mixture was heated slowly to 100 °C and stirred for 2.5 h. The
cooled mixture was added to a vigorously stirred slurry of silica gel (3 g) in
chloroform (20 mL). The suspension was filtered washing with MeOH (4 x 5
mL) and 50 % MeOH-CH2Cl2 (4 x S mL). The combined washings were
concentrated and the resulting residue was purified on a 2-g silica SPE column
with S % MeOH-CHZCl2 to afford 50 mg (59 %) of the title compound as an
orange solid. 1H-NMR (DMSO-db; 400 MHz) 8 2.44 (s, 3H), 3.69 (s, 3H),
6.84 (m, 2H), 6.98 (m, 2H), 7.73 (s, 1 H), 7.84 (s, 1 H), 9.01 (br s, 2H),
9.24 (br
s, 2H). Mass spectrum (ESI, m/z): Calcd. for C13H15N3OS2, 294.1 (M+H),
found 294.2.
Example 254
4 ~(3-Fluoro-4-methylplzenyl)aminoJ-S-metlzyltlziotlziophene-2-
carboxamidine
a) Methyl4-~(3;fluoro-4-methylphenyl)aminoJ 5-
methylthiothiophene-2-carboxylate: To an oven-dried glass vial with stir bar
was added a mixture of 120 mg (0.449 mmol) of methyl 4-bromo-5-
methylthiothiophene-2-carboxylate (as prepared in Example 241, step (a), 41
mg (10 mol %) of tris-(dibenzylidineacetone)dipalladium, 42 mg (15 mol %)
of racemic-2,2'-bis(diphenylphosphino)-l,l'-binaphthyl , 205 mg (0.629
mmol) of cesium carbonate and 70 mg (0.56 mmol) of 3-fluoro-4-
methylaniline. The vial was transferred to a glove bag, flushed with dry argon
and anhydrous toluene (0.9 mL) was added. The vial was capped with a
Teflon-lined screw cap and heated at 100 °C for 48 h. To the
cooled
suspension was added ethyl acetate (4 mL), the mixture filtered (Celite)
washing with ethyl acetate (2 x 2 mL), and the solvents removed in vacuo.
The resulting residue was purified by silica gel preparative thin layer
chromatography (10% Et20 in hexane) to afford 103 mg (78 %) of the title
compound as a yellow oil 1H-NMR (CDCl3, 400 MHz) 8 2.22 (d, 3H, J = 1.6
Hz), 2.40 (s, 3H), 3.89 (s, 3H), 6.09 (s, 1H), 6.68 (m, 1H), 6.71 (s, 1H),
7.08
(m, 1 H), 7.72 (s, 1 H).
b) 4 ~(3-Fluoro-4-methylphenyl)aminoJ 5-methylthiothiophene-
2-carboxamidine: The same procedure as in Example 253, step (b) was
CA 02362390 2001-08-07
WO 00/47578 -265- PCT/US99/18065
followed using 103 mg (0.349 mmol) of methyl 4-[(3-fluoro-4-
methylphenyl)amino]-5-methylthiothiophene-2-carboxylate (as prepared in
previous step), 2 mL of trimethylaluminum (2.0 M in toluene, 4 mmol), 216
mg of ammonium chloride (4 mmol) and 2 mL of toluene, and purified on a
2-g silica SPE column with 5 % MeOH-CH2Cl2 to afford 45 mg (44 %) of the
title compound as a yellow foam'H-NMR (DMSO-d6; 400 MHz) 8 2.13 (s,
3H), 2.50 (s, 3H ), 6.70 (m, 2H), 7.10 (m, 1 H), 7.98 (s, 1 H), 8.09 (s, 1 H),
9.16
(br s, 4H). Mass spectrum (ESI, m/z): Calcd. for C,3H~4FN3SZ, 296.1 (M+H),
found 296.2.
Example 255
4-(Indan-5 ylamino)-S-metlzylthiothioplZene-2-carboxamidine
a) Methyl4-(indan-5 ylamino)-S-methyltlaiothiophene-2-
carboxylate: The same procedure as in Example 254, step (a) was followed
using 120 mg (0.449 mmol) of methyl 4-bromo-5-methylthiothiophene-2-
carboxylate (as prepared in Example 241, step (a), 41 mg (10 mol %) of tris-
(dibenzylidineacetone)dipalladium, 42 mg (15 mol %) of racemic-2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl , 205 mg (0.629 mmol) of cesium
carbonate and 74.8 mg (0.56 mmol) of 5-aminoindan in 900 ~L of toluene,
and chromatographed as before using 40 % CH2Clz-hexane to afford 100 mg
(73 %) of the title compound as a yellow resin. 'H-NMR (CDC13, 400 MHz) 8
2.05-2.12 (m, ZH), 2.85-2.90 (m, 4H), 3.86 (s, 3H), 6,09 (s, 1H), 6.82 (d, 1H,
J
= 8.0 Hz), 6.93 (s, 1 H), 7.14 (d, 1 H, J = 8.0 Hz), 7.70 (s, 1 H).
b) 4-(Indan-5 ylamino)-5-metJ:yltlaiotlaiophene-2-
carboxamidine: The same procedure as in Example 253, step (b) was
followed using 100 mg (0.33 mmol) of methyl 4-(indan-5-ylamino)-5-
methylthiothiophene-2-carboxylate (as prepared in previous step), 2 mL of
trimethylaluminum (2.0 M in toluene, 4 mmol), 216 mg of ammonium
chloride (4 mmol) and 2 mL of toluene, and purified on a 2-g silica SPE
column with 5 % MeOH-CH2C12 to afford 65 mg (65 %) of the title compound
as a yellow foam. 'H-NMR (DMSO-db, 400 MHz) 8 1.99 (m, 2H), 2.48 (s,
3H), 2.78 (m, 4H), 6.77 (dd, 1 H, J = 8.0, 1.78 Hz), 6.86 (s, 1 H), 7.08 (d, 1
H, J
CA 02362390 2001-08-07
WO 00/47578 -266- PCT/US99/18065 -
= 8.1 Hz), 7.80 (s, 1 H), 7.94 (s, 1 H), 9.13 (br s, 4H). Mass spectrum (ESI,
m/z): Calcd. for CISH1~N3S2, 304.1 (M+H), found 304.3.
Example 256
4-~(9-Ethylcarbazol 3 yl)aminoJ S-methyltlziothioplzene-2-carboxamidine
a) Methyl 4-~(9-ethylcarbazol 3 yl)aminoJ S-.
methyltlziotlZiophene-2-carboxylate: The same procedure as in Example 254,
step (a) was followed using 120 mg (0.449 mmol) of methyl 4-bromo-5-
methylthiothiophene-2-carboxylate (as prepared in Example 241, step (a), 41
mg (10 mol %) of tris-(dibenzylidineacetone)dipalladium, 42 mg (15 mol %)
of racemic-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl , 205 mg (0.629
mmol) of cesium carbonate and 118 mg (0.56 mmol) of 3-amino-9-
ethylcarbazole in 900 pL of toluene, and chromatographed as before using 40
CHZC12-hexane to afford 80 mg (47 %) of the title compound as a yellow
resin. 1H-NMR (CDCl3, 400 MHz) 8 1.46 (t, 3H, J = 7.2 Hz), 2.44 (s, 3H),
3.85 (s, 3H), 4.39 (q, 2H, J = 7.2 Hz), 6.25 (s, 1H), 7.24 (m, 1H), 7.28 (s,
1H),
7.40 (m, 2H), 7.49 (m, 1 H), 7.61 (s, 1 H), 7.83 (d, 1 H, J = 2.1 Hz), 8.06
(d, 1 H,
J = 7.8 Hz).
b) 4-~(9-Ethylcarbazol 3 yl)aminoJ 5-methylthiotlziophene-2-
carboxamidine: The same procedure as in Example 253, step (b) was
followed using 80 mg (0.21 mmol) of methyl 4-[(9-ethylcarbazol-3-yl)amino]-
5-methylthiothiophene-2-carboxylate (as prepared in previous step), 2 mL of
trimethylaluminum (2.0 M in toluene, 4 mmol), 216 mg of ammonium
chloride (4 mmol) and 2 mL of toluene, and purified on a 2-g silica SPE
column with 5 % MeOH-CH2Cl2 to afford 56 mg (70 %) of the title compound
as a yellow foam. 'H-NMR (DMSO-db, 400 MHz) 8 1.31 (t, 3H, J = 7.0 Hz),
2.50 (s, 3 H), 4.42 (q, 2H, J = 7.0 Hz), 7.14 (m, 1 H), 7.27 (dd, 1 H, J =
8.7, 2.1
Hz ), 7.43 (m, 1 H), 7.56 (m, 2H), 7.82 (d, 1 H, J = 2.0 Hz), 7.87 (s, 1 H),
7.92
(s, 1 H), 8.10 (d, 1 H, J = 7.7 Hz), 9.11 (br s, 4H). Mass spectrum (ESI,
m/z):
Calcd. for C2oH2oNaS2, 381.1 (M+H), found 381.3.
CA 02362390 2001-08-07
WO 00/47578 r26~_ PCT/US99/18065
Examples 257 and 258
S-Methylthio-4-(~(4 phenylplzenyl)sulfonylJaminoJthioplzene-2
carboxamidine tr~uoroacetate
4-(Bis~(4 phenylphenyl)sulfonylJaminoJ-S-methylthiothiophene-2
carboxamidine tr~uoroacetate
a) Methyl S-methylthio-4-~(phenylsulfonyl)aminoJthiophene-2-
carboxylate and methyl 4-(bis~(4 phenylphenyl)sulfonylJaminoJ-S-
metlzyltlaio-thioplzene-2-carboxylate: To an oven-dried round bottom flask
with stir bar was added a mixture of 50 mg (0.24 mmol) of methyl 4-amino-5-
methylthiothiophene-2-carboxylate (as prepared in Example 202), 68 mg (0.27
mmol) of 4-biphenylsulfonyl chloride and SO mg (0.49 mmol) of 4-
dimethylaminopyridine. The flask was flushed with dry argon and anhydrous
acetonitrile (3 mL) was added. The reaction was refluxed for 3 hours and then
the solvent was removed in vacuo. The crude of the reaction was extracted
with ethyl acetate (2 x 25 mL) and 1N HCl (SO mL), The organic layer was
collected, dried (NaZS04), filtered and concentrated under vacuum to yield a
foam that was chromatographed on silica with 30 % ethyl ether-hexane to
obtain 143 mg of a mixture of methyl 5-methylthio-4-
[(phenylsulfonyl)amino]thiophene-2-carboxylate and methyl 4-{bis[(4-
phenylphenyl)-sulfonyl]amino}-5-methylthiothiophene-2-carboxylate. This
mixture was used in the next reaction without further purification. Mass
spectrum (ESI, m/z): Calcd. for C19H,7NO4S3, 420.0 (M+H), found 419.7.
b) S-MethyltJzio-4-(~(4 phenylphenyl)sulfonylJaminofthioplzene-
2-carboxamidine tr~uoroacetate and 4-(bis~(4 phenylphenyl)sulfonylJ
amino)-S-metlzyltlziothiophene-2-carboxamidine tr~uoroacetate:
Trimethylaluminum (2.0 M in toluene, 1.36 mL, 2.72 mmol) was added
dropwise to a suspension of ammonium chloride (155 mg, 2.89 mmol) in
anhydrous toluene (2.0 mL) under Ar at 0 °C. The mixture was stirred at
25
°C for 30 min and then 143 mg of a mixture of methyl S-methylthio-4-
[(phenylsulfonyl)amino]thiophene-2-carboxylate and methyl 4-{bis[(4-
phenylphenyl)sulfonyl]amino}-5-methylthiothiophene-2-carboxylate (as
prepared in previous step) in anhydrous toluene (2.0 mL) was added. The
reaction mixture was heated slowly to 100 °C and stirred for 4 h. The
cooled
mixture was added to a vigorously stirred slurry of silica gel (3 g) iri
CA 02362390 2001-08-07
WO 00/47578 _268_ PCT/US99/18065 -
chloroform ( 15 mL). The suspension was filtered (Celite) washing with 25
MeOH-CH2Cl2 (2 x 5 mL), 50 % MeOH-CH2C12 (2 x 5 mL) and 75
MeOH-CH2Cl2 (2 x 5 mL). The combined washings were concentrated and
the resulting residue was purified on a 10-g silica SPE column with a gradient
of 10-15 % MeOH-CHZC12 saturated with ammonia to afford 66 mg of a
mixture of the title compounds as a yellow solid. This mixture was
chromatographed by preparative reverse phase HPLC performed with a Rainin
SD-1 Dynamax system and a 2-in. C18 reverse phase Dynamax 60A column
using a gradient of 30% MeOH /0.1% TFA in water to 100% MeOH and a
flow rate of 50 mL/ min. to yield 15 mg 5-methylthio-4-{[(4-
phenylphenyl)sulfonyl]amino}thiophene-2-carboxamidine trifluoroacetate;
mass spectrum (ESI, m/z): Calcd. for C~gH~7N3O2S3, 404.0 (M+H), found
404.1; and 11 mg of 4-{bis[(4-phenylphenyl)sulfonyl]amino}-S-
methylthiothiophene-2-carboxamidine trifluoroacetate. Mass spectrum (ESI,
m/z): Calcd. for C3pH25N3~4s4, 619.8 (M+H), found 620.2.
Examples 259 to 282
The same methods as for Examples 257 and 258 were used to synthesize the
following compounds:
CA 02362390 2001-08-07
WO 00/47578 -269- PCT/US99/18065
Mass
spec.
ESI.
m/z
Examp Reagent Compound Formula Calc. Found
M+H
259 1- 5-Methylthio-4-[(2-naphthylsulfonyl)-C16H15N302S3378.0
378.1
Naphthalenesulfonylamino]thiophene-2-carboxamidine
chloride
260 1- 4-[Bis(2-naphthylsulfonyl)amino]-5-C26H21N304S4568.0 568.1
Naphthalenesulfonylmethylthiothiophene-2-carboxamidine
chloride
261 7-Bromonaphthalene4-{[(6-Bromo(2- C16H14BrN302S3455.9
sulfonyl chloridenaphthyl))sulfonyl]amino}-5-
methylthiothiophene-2-carboxamidine
262 7-Bromonaphthalene4-{Bis[(6-bromo(2- C26H19Br2N304S723.9
sulfonyl chloridenaphthyl))sulfonyl]amino}-5-4
methylthiothiophene-2-carboxamidine
263 2- 5-Methylthio-4-[(naphthylsulfonyl)-C16H15N302S3378.0 378.1
Naphthalenesulfonylamino]thiophene-2-carboxamidine
chloride
264 2- 4-[Bis(naphthylsulfonyl)amino]-5-C26H21N304S4568.7 568.3
Naphthalenesulfonylmethylthiothiophene-2-carboxamidine
chloride
265 o-Toluenesulfonyl4-{ [(2-Methylphenyl)sulfonyl)amino}-C 13H
15N302S3342.4 342.1
chloride 5-methylthiothiophene-2-
carboxamidine
266 o-Toluenesulfonyl4-{Bis[(2- C20H21N304S4496.6 496.1
chloride methylphenyl)sulfonyl)amino}-5-
methylthiothiophene-2-carboxamidine
267 m-Toluenesulfonyl4-{[(3-Methylphenyl)sulfonyl]amino}-C13H15N302S3342.0
342.1
chloride 5-methylthiothiophene-2-
carboxamidine
268 m-Toluenesulfonyl4-{Bis[(3- C20I-I21N304S4496.6 496.0
chloride methylphenyl)sulfonyl)amino}-5-
methylthiothiophene-2-carboxamidine
269 p-Toluenesulfonyl4-{[(4-Methylphenyl)sulfonyl]amino}-C13I-115N302S3342.0
342.1
chloride 5-methylthiothiophene-2-
carboxamidine
270 p-Toluenesulfonyl4-{Bis[(4- C20H21N304S4496.6 496.1
chloride methylphenyl)sulfonyl]amino}-5-
methylthiothiophene-2-carboxamidine
271 a-Toluenesulfonyl5-Methylthio-4- C13HISN302S3342.0 342.1
chloride {[benzylsulfonyl]amino}-thiophene-2-
carboxamidine
SUB;5T1TUTE SHEET (RULE 26)
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065 -
-270-
Mass
spec,
ESI,
m/z
Examp Reagent Compound Formula Calc,Found
M+H
272 4-Methoxybenzene-4-{[(4-Methoxyphenyl)sulfonyl]amino}-
C13H15N303S3358.0358.1
sulfonyl 5-methylthiothiophene-2-carboxamidine
chloride
273 4-Methoxybenzene-4-{Bis((4- C20H21N306S4528.0528.0
sulfonyl methoxyphenyl)sulfonyl]amino}-5-
chloride
methylthiothiophene-2-carboxamidine
274 4- 4-{[(4-Iodophenyl)sulfonyl]amino}-5-C12H12IN302S3453.9454.0
Iodobenzenesulfonymethylthiothiophene-2-carboxamidine
1 chloride
275 3,4- 4-{((3,4- C14H17N304S3388.0388.1
DimethoxybenzeneDimethoxyphenyl)sulfonyl]amino}-5-
sulfonyl methylthiothiophene-2-carboxamidine
chloride
276 3,4- 4-{bis[(3,4- C22H25N308S4588.0588.1
DimethoxybenzeneDimethoxyphenyl)sulfonyl]amino}-5-
sulfonyl methylthiothiophene-2-carboxamidine
chloride
277 2- 4-{[(2-Chlorophenyl)sulfonyl]amino}-5-
C12H12CIN302S3361.9362.1
Chlorobenzenesulfomethylthiothiophene-2-carboxamidine
nvl chloride
278 3-Chlorobenzene-4-{[(3-Chlorophenyl)sulfonyl]amino}-5-
C12H12C1N302S3361.9362.1
sulfonyl methylthiothiophene-2-carboxamidine
chloride
279 3-Chlorobenzene-4-{Bis[(3-chlorophenyl)sulfonyl]amino}-
C18H15C12N304S535.9537.9
sulfonyl 5-methylthiothiophene-2-carboxamidine4
chloride
280 4-Chlorobenzene-4-{[(4-Chlorophenyl)sulfonyl]amino}-5-
C12FI12CIN302S3361.9362.1
sulfonyl methylthiothiophene-2-carboxamidine
chloride
281 4-Chlorobenzene-4-{Bis[(4-chlorophenyl)sulfonyl]amino}-
C18H15C12N304S535.9
sulfonyl 5-methylthiothiophene-2-carboxamidine4
chloride
282 Benzenesulfonyl5-Methylthio-4-((phenylsulfonyl)amino]-
C12H13N302S3328.0328.1
chloride thiophene-2-carboxamidine
Mass
spec,
ESI,
m/z
Examp Reagent Compound Formula Calc,Found
M+H
283 Benzenesulfonyl4-[Bis(phenylsulfonyl)amino]-5-C18H17N304S4468.0467.9
chloride methylthiophene-2-carboxamidine
284 4-tert- 4-({[4-(Tert- C16H21N302S3384.0384.2
Butylbenzene-butyl)phenyl]sulfonyl}amino)-5-
sulfonyl methylthiothiophene-2-carboxamidine
chloride
285 4-tert- 4-(Bis{[4-(tert- C26H33N304S4580.1580.2
Butylbenzene-butyl)phenyl]sulfonyl}amino)-5-
sulfonyl methy(thiothiophene-2-carboxamidine
chloride
286 Trans-~3-styrene4-{[((lE)-2- C14H15N302S3354.0
sulfonyl Phenylvinyl)sulfonyl]amino}-5-
chloride
methylthiothiophene-2-carboxamidine
287 4-benzensulfonyl-5-Methylthio-4-({ [4-(phenylsulfonyl)(2-C 16H
15N304S5473.9474.1
thiophene-2-thienyl)]sulfonyl}amino)thiophene-2-
sulfonyl carboxamidine
chloride
* Mass spectral data inconclusive.
SUB.STnUTE SHEET (RULE 26)
CA 02362390 2001-08-07
WO 00/47578 _27~ _ PCT/US99/18065
Example z88
S Methylthio-4 phenoxythiophene-2-carboxamidine trifluoroacetate.
a) Methyl 5-methylthio-4 phenoxythiophene-2-carboxylate: To
an oven-dried round bottom flask with stir bar was added a mixture of 100 mg
(0.37 mmol) of methyl 4-bromo-5-methylthiothiophene-2-carboxylate (as
prepared in Example 241), 20 mg of Cu (0) (Brewster, R.Q. and Groening T.,
Organic Syntheses, Vol. II, Note l, pp 445-446) and 42 mg (0.46 mmol) of
phenol. The flask was flushed with dry argon and anhydrous tetrahydrofuran
(5 mL) was added. The reaction was refluxed for 48 hours and then the solvent
was removed in vacuo. The resulting residue was purified on a 10-g silica SPE
column with a gradient of 50-100 % CH2Cl2-hexane to yield 48 mg of methyl
5-methylthio-4-phenoxythiophene-2-carboxylate (37%). ~H-NMR (CDC13,
400 MHz) 8 7.39 (s, 1 H), 7.32 (m, 2H), 7.09 (m, 2H), 6.97 (d, 1 H, J = 8.4
Hz),
3.86 (s, 3H) and 2.49 (s, 3H).
b) S-Metlzyltlzio-4 plzenoxythiophene-2-carboxamidine
trifluoroacetate: The same procedure as in Example 257, step (b) was
followed using 48.0 mg (0.17 mmol) of methyl 5-methylthio-4-
phenoxythiophene-2-carboxylate (as prepared in the step before), 78 mg of
ammonium chloride (1.5 mmol), 0.68 ml of trimethylaluminum (2.0 M in
toluene, 1.3 mmol) and 3 ml of anhydrous toluene and chromatographed as
before using preparative reverse phase HPLC performed with a Rainin SD-1
Dynamax system and a 2-in. C 18 reverse phase Dynamax 60A column using a
gradient of 30% MeOH /0.1% TFA in water to 100% MeOH and a flow rate
of 50 mL/ min. 1H-NMR (CD30D, 400 MHz) 8 7.66 (s, 1H), 7.39 (t, 2H, J =
7.5 Hz), 7.17 (t, 2H, J = 7.4 Hz), 7.02 (d, 1 H, J = 7.7 Hz) and 2.5 8 (s, 3
H).
Mass spectrum (ESI, m/z): Calcd. C,2H,ZN2OS2, 265.0 (M+H), found 262.2.
Example 289
S-Methyltlzio-4-(phenylsulfonyl)thiopJzene-2-carboxamidine tr~uoroacetate.
a) 4-Bromo-5-metlzylthiothioplzene-2-carboxylic acid: To 1.0 g
(3.7 mmol) of methyl 4-bromo-5-methylthiothiophene-2-carboxylate (as
prepared in Example 241, step (a) dissolved in 25 ml of MeOH was added 450
CA 02362390 2001-08-07
WO 00/47578 _2~2_ PCT/US99/18065
mg of NaOH dissolved in 10 ml of H20. The reaction was stirred for 5 hours
at room temperature, and then the solvents were removed under vacuum. The
residue of the reaction was extracted with ethyl acetate (2 x 50 mL) and 1N
HCI. The organic layer was collected, dried (NaZS04), filtered and
concentrated under vacuum to yield 833 mg (89%) of 4-bromo-5-
methylthiothiophene-2-carboxylic acid as a white solid.
b) S-Methylthio-4-(phenylsudfonyl)thiophene-Z-carboxylic acid:
To an oven-dried round bottom flask with stir bar was added 100 mg (0.39
mmol) 4-bromo-5-methylthiothiophene-2-carboxylic acid (as prepared in
Example before). The flask was flushed with dry argon and anhydrous
tetrahydrofuran (3 mL) was added. Then the solution was cooled at -78
°C
before adding 511 ~L of tert-butyl lithium (0.87 mmol, 1.7 M in
tetrahydrofuran). The mixture was stirred for a period of 45 minutes and 77
mg of benzenesulfonyl flouride (0.39 mmol) was added and the reaction was
allowed to rise to room temperature. The reaction was stirred for 12 hours and
then quenched carefully with HZO. The solvents were removed under vacuum
and the residue of the reaction was extracted with ethyl acetate (2 x 50 ml)
and
1N HCI. The organic layer was collected, dried (Na2S04), filtered and
concentrated under vacuum to yield 130 mg of a solid. This solid was used in
the next step without further purification.
c) Methyl5-methylthio-4-(phenylsulfonyl)thiopltene-2-
carboxylate: To a solution of 25 mg of the mixture from the previous step
dissolved in 3 mL of MeOH wa added dropwise 397 ~L of
trimethylsilyldiazomethane (0.79 mmol, 2 M solution in hexanes) and the
reaction was stirred for a period of 1 hour. The solvents were removed under
vacuum. The resulting residue was purified on a 10-g silica SPE column with
a gradient of 50-100 % ethyl acetate-hexane to yield 13.8 mg of methyl 5-
methylthio-4-(phenylsulfonyl)thiophene-2-carboxylate. Mass spectrum (ESI,
m/z): Calcd. C13H,2O4S3, 329.0 (M+H), found 329Ø
d) S-Metltylthio-4-(plzenylsulfonyl)tlziophene-2-carboxamidine
tr~uoroacetate: The same procedure as in Example 257, step (b) was
followed using 13.8 mg (0.044 mmol) of methyl 5-methylthio-4-
(phenylsulfonyl)thiophene-2-carboxylate (as prepared in the step before), 20
mg of ammonium chloride (0.376 mmol), 0.176 ml of trimethylaluminum (2.0
CA 02362390 2001-08-07
WO 00/47578 _2~3_ PCT/US99/18065
M in toluene, 0.353 mmol) and 3 ml of anhydrous toluene and
chromatographed as before by preparative reverse phase HPLC performed
with a Rainin SD-1 Dynamax system and a 2-in. C18 reverse phase Dynamax
60A column using a gradient of 30% MeOH /0.1% TFA in water to 100%
MeOH and a flow rate of SO mL/ min to yield 2.3 mg of 5-methylthio-4-
(phenylsulfonyl)thiophene-2-carboxamidine . 'H-NMR (CD30D, 400 MHz) 8
8.42 (s, 1 H), 8.04 (m, 2H), 7.70 (m, 2H), 7.62 (m, 1 H) and 2.70 (s, 3 H).
Mass
spectrum (ESI, m/z): Calcd. CIZHiaN202S3, 313.0 (M+H), found 313.2.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-274-
Example 290
Tablet Preparation
Tablets containing 25.0, 50.0, and 100.0 mg, respectively, of the
following active compounds are prepared as illustrated below:
a. 4-(4-methylthiazol-2-yl)-S-methylthiothiophene-2-
carboxamidine;
b. 4-[4-(4-phenylphenyl)thiazol-2-yl]-5-methylthiothiophene-2-
carboxamidine.
TABLET FOR DOSES CONTAINING FROM
25-100 MG OF THE ACTIVE COMPOUND
Amount-m~
Active Compound 25.0 50.0 100.00
Microcrystalline cellulose 37.25 100.0 200.0
Modified food corn starch 37.25 4.25 8.5
Magnesium stearate 0.50 0.75 1.5
All of the active compound, cellulose, and a portion of the cornstarch
are mixed and granulated to 10% corn starch paste. The resulting granulation
is sieved, dried and blended with the remainder of the corn starch and the
magnesium stearate. The resulting granulation is then compressed into tablets
containing 25.0, 50.0, and 100.0 mg, respectively, of active ingredient per
tablet.
Example 291
Intravenous Solution Preparation
An intravenous dosage form of the above-indicated active compounds
is prepared as follows:
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-275-
Active Compound 0.5-10.0 mg
Sodium Citrate 5-50 mg
Citric Acid I-IS mg
Sodium Chloride I-8 mg
Water for Injection (USP) ~ q.s. to 1 ml
Utilizing the above quantities, the active compound is dissolved at
room temperature in a previously prepared solution of sodium chloride, citric
acid, and sodium citrate in Water for Injection (USP, see page 1636 of United
States Pharmacopeia/National Formulary for 1995, published by United States
Pharmacopeial Convention, Inc., Rockville, Maryland (1994).
Example 292
lu vitro Inhibition of Purified Enzymes
Reagents: All buffer salts were obtained from Sigma Chemical Company (St.
Louis, MO), and were of the highest purity available. The enzyme substrates,
N-benzoyl-Phe-Val-Arg p-nitroanilide (Sigma B7632),
N-benzoyl-Ile-Glu-Gly- Arg p-nitroanilide hydrochloride (Sigma B2291 ),
N p-tosyl-Gly-Pro-Lys-p-nitroanilide (Sigma T6140), N-succinyl-Ala-Ala-
Pro-Phe p-nitroanilide (Sigma 57388) and N-CBZ-Val-Gly-Arg p-nitroanilide
(Sigma C7271 ) were obtained from Sigma. N-Succinyl-Ala-Ala-Pro-Arg-
p-nitroanilide (BACHEM L-1720) and N-succinyl-Ala-Ala- Pro-Val-
p-nitroanilide (BACHEM L-1770) were obtained from BACHEM (King of
Prussia, PA).
Human a-thrombin, human factor Xa and human plasmin were
obtained from Enzyme Research Laboratories (South Bend, Indiana). Bovine
a-chymotrypsin (Sigma C4129), bovine trypsin (Sigma T8642) and human
kidney cell urokinase (Sigma U5004) were obtained from Sigma. Human
leukocyte elastase was obtained from Elastin Products (Pacific, MO).
K; Determinations: All assays are based on the ability of the test compound
to inhibit the enzyme catalyzed hydrolysis of a peptide p-nitroanilide
substrate.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065 -
-276-
S In a typical K; determination, substrate is prepared in DMSO, and diluted
into
an assay buffer consisting of 50 mM HEPES, 200 mM NaCI, pH 7.5. The
final concentrations for each of the substrates is listed below. In general,
substrate concentrations are lower than the experimentally determined value
for Km. Test compounds are prepared as a 1.0 mg/mL solution in DMSO.
Dilutions are prepared in DMSO yielding 8 final concentrations encompassing
a 200 fold concentration range. Enzyme solutions are prepared at the
concentrations listed below in assay buffer.
In a typical K; determination, into each well of a 96 well plate is
pipetted 280 pL of substrate solution, 10 ~.L of test compound solution, and
1 S the plate allowed to thermally equilibrate at 37 °C in a Molecular
Devices plate
reader for > 15 minutes. Reactions were initiated by the addition of a 10 ~L
aliquot of enzyme and the absorbance increase at 405 nm is recorded for 15
minutes. Data corresponding to less than 10% of the total substrate hydrolysis
were used in the calculations. The ratio of the velocity (rate of change in
absorbance as a function of time) for a sample containing no test compound is
divided by the velocity of a sample containing test compound, and is plotted
as
a function of test compound concentration. The data are fit to a linear
regression, and the value of the slope of the line calculated. The inverse of
the
slope is the experimentally determined K; value.
Thrombin: Thrombin activity was assessed as the ability fo hydrolyze the
substrate N-succinyl-Ala-Ala-Pro-Arg p-nitroanilide. Substrate solutions were
prepared at a concentration of 32 ~M (32 ~M«Krn = 180 ~M) in assay
buffer. Final DMSO concentration was 4.3%. Purified human a-thrombin
was diluted into assay buffer to a concentration of 15 nM. Final reagent
concentrations were: [thrombin] = 0.5 nM, [substrate
N-succinyl-Ala-Ala-Pro-Arg-p-nitroanilide] = 32 ~.M.
Factor X [FXa]: FXa activity was assessed as the ability to hydrolyze the
substrate N-benzoyl-Ile-Glu-Gly-Arg p-nitroanilide hydrochloride. Substrate
solutions were prepared at a concentration of 51 ~M (51 « Km = 1.3 mM) in
assay buffer. Final DMSO concentration was 4.3%. Purified activated human
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065 -
-277-
S Factor X was diluted into assay buffer to a concentration of 300 nM. Final
reagent concentrations were: [FXa] = 10 nM, [N-benzoyl-Ile-Glu-Gly-Arg-
p-nitroanilide hydrochloride] = 51 ~M.
Plasmin: Plasmin activity was assessed as the ability to hydrolyze the
N p-Tosyl-Gly-Pro-Lys p-nitroanilide. Substrate solutions were prepared at a
concentration of 37 ~.M (37 ~M« Km= 243 ~M) in assay buffer. Final
DMSO concentration was 4.3%. Purified human plasmin was diluted into
assay buffer to a concentration of 240 nM. Final reagent concentrations were:
[Plasmin] = 8 nM, [N p-Tosyl-Gly-Pro-Lys p-nitroanilide] = 37 ~M.
Chymotrypsin: Chymotrypsin activity was assessed as the ability to
hydrolyze N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide. Substrate solutions
were prepared at a concentration of 14 ~M (14 ~.M« Km= 62 ~.M) in assay
buffer. Final DMSO concentration was 4.3%. Purified bovine chymotrypsin
was diluted into assay buffer to a concentration of 81 nM. Final reagent
concentrations were: [Chymotrypsin] = 2.7 nM,
[N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide] = 14 ~M.
Trypsin: Trypsin activity was assessed as the ability to hydrolyze
N-benzoyl-Phe-Val-Arg p-nitroanilide. Substrate solutions were prepared at a
concentration of 13 ~.M (13 g.M« Km = 291 ~M) in assay buffer. Final
DMSO concentration was 4.3%. Purified bovine trypsin was diluted into
assay buffer to a concentration of 120 nM. Final reagent concentrations were:
[Trypsin] = 4 nM, [N-benzoyl-Phe-Val-Arg p-nitroanilide] = 13 ~M.
Elastase: Elastase activity was assessed as the ability to hydrolyze
N-succinyl-Ala-Ala-Pro-Val p-nitroanilide. Substrate solutions were prepared
at a concentration of 19 ~M (19 ~M« Km = 89 ~M) in assay buffer. Final
DMSO concentration was 4.3%. Purified human leukocyte elastase was
diluted into assay buffer to a concentration of 750 nM. Final reagent
concentrations were: [Elastase] = 25 nM,
[N-succinyl-Ala-Ala-Pro-Val p-nitroanilide] = 19 ~M.
Uro'nase: Urokinase activity was assessed as the ability to hydrolyze
N-CBZ-Val-Gly-Arg p-nitroanilide. Substrate solutions were prepared at a
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-278-
concentration of 100 ~M (100 ~M < Km = l.2mM) in assay buffer. Final
DMSO concentration was 4.3%. Purified human kidney urokinase was diluted
into assay buffer to a concentration of 1.2 ~M. Final reagent concentrations
were: [Urokinase] = 40 nM, and N-CBZ-Val-Gly-Arg p-nitroanilide] = 100
mM.
The results of exemplary assays are shown in the following table.
CA 02362390 2001-08-07
WO 00/47578 PCT/US99/18065
-279
Protease Inhibition Data
Protease Ki Exam
le#
micromolar
Trypsin 0.858 8
Trypsin 0.474 52
Factor Xa 2.73 94
Factor Xa 3.00 119
Chymo- 4.90 11
trypsin
tPA 9.49 1
Plasmin 7.31 12
C 1 S 0.940 283
Additionally, the following compounds have Ki values in the range of
0.016 to 3.5 micromolar for uPA:
Ex. # 28, 40, 53, 79, 84, 89, 131, 138, 139, 140, 143, 145, 172, 187,
200, 204, 206, 208, 213, 220, 222, 223, 227, 233, 235, 239, 260, 281 and 288.
The results indicate that the compounds of the present invention are
inhibitors of proteases, including urokinase.
Having now fully described this invention, it will be understood to
those of ordinary skill in the art that the same can be performed within a
wide
and equivalent range of conditions, formulations, and other parameters without
affecting the scope of the invention or any embodiment thereof. All patents
and publications cited herein are fully incorporated by reference herein in
their
entirety.