Note: Descriptions are shown in the official language in which they were submitted.
CA 02362632 2004-12-14
28888-47D
- 1 -
R-SUBSTITUTED-2- OR 3-AMINOMETHYL-4-AMINO-
3,4-DIHYDRO-2H-1,4-BENZOXAZINES AS
INTERMEDIATES FOR PRODUCING ANALGESIC AGENTS
BACKGROUND OF THE INVENTION
This is a divisional application of Canadian
Patent Application Serial No. 2,006,769 filed on December
28, 1989.
(a) Field of the Invention
This invention relates to 2- and 3-aminomethyl-6-
arylcarbonyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazines
which are useful as analgesic agents.
The subject matter of this application relates to
novel R-substituted-2- or 3-aminomethyl-4-amino-3,4-dihydro-
2H-1,4-benzoxazine compounds of formula (IV) described
hereinunder. Acid-addition salts thereof and racemic
mixtures and d- and 1-enantiomers thereof are also the
subject matter of this divisional application. The claimed
subject of the parent application is restricted to 2- and 3-
aminomethyl-6-arylcarbonyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-
benzoxazine compounds of formula (I) described hereinunder.
However, it should be understood that the expression "the
invention", or the like, encompasses the subject matter of
both the parent and the divisional application.
(b) Information Disclosure Statement
Brennan and Saxton, Tetrahedron Lett. 26 (14),
1769-72 (1985) and Tetrahedron 43 (1), 191-205 (1987)
disclose compounds having the structures:
CA 02362632 2004-12-14
28888-47D
- 1a -
\NH2
N'~COOR ~ N'~CH3
OCH3 OCH
3
as intermediates in the total synthesis of the alkaloid
obscurinervidine.
French Application 2,567,126, published January
20, 1986, discloses the compound having the formula:
CA 02362632 2001-11-19
22749-359
-2- D.N. 74368
N~ COOC2H5
O
useful as an intermediate for the preparation of compounds
having antidepressant activity.
Thus, so far as the inventors are aware, compounds
of the 2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine class
are not known to have utility for any purpose except as
synthetic intermediates, and furthermore, so far as is known,
such compounds having an arylcarbonyl function in any posi-
tion or an aminoalkyl function in any position other than
the 6-position are unknown.
SUMMARY
In a compound aspect, this invention relates
to 5-R5-R-substituted-2- and 3-aminomethyl-6-arylcarbonyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazines and their
acid addition salts, which are useful as analgesic agents.
In a further compound aspect, the invention
relates to R-substituted-2- and 3-aminomethyl-4-amino-3,4-
dihydro-2H-1,4-benzoxazines, which are useful as interme-
diates for the preparation of the final products of the
invention.
In a further compound aspect, the invention
relates to 5-R5-R-substituted-2- and 3-aminomethyl-6-phenyl-
thio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazines, which
are useful as intermediates for the preparation of the final
products of the invention.
CA 02362632 2001-11-19
22749-359
-3- D.N. 7436H
In a still further compound aspect, the inven-
tion relates to 5-R5-R-substituted-2- and 3-aminomethyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazines, which are
useful as intermediates for the preparation of the final
products of the invention.
In a method aspect, the invention relates to a
method for the relief of pain in a patient requiring such
treatment comprising administering to such patient a composi-
tion containing an effective analgesic amount of a 5-85-
R-substituted-2- or 3-aminomethyl-6-arylcarbonyl-2,3-dihydro-
pyrrolo-[1,2,3-de]-1,4-benzoxazine.
In a composition aspect, the invention relates
to analgesic compositions for the relief of pain comprising
a pharmaceutically acceptable carrier and an effective analgesic
15~ amount of a said 5-R5-R-substituted-2- or 3-aminomethyl-
6-arylcarbonyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine.
In a process aspect, the invention relates to
a process for preparing a 5-R5-R-substituted-2- or 3-amino-
methyl-6-arylcarbonyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine
of the invention comprising reacting a 5-R5-R-substituted-
2- or 3-aminomethyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzox-
azine with an aryl carboxylic acid halide in the presence
of a Lewis acid.
In a further process aspect, the invention relates
to a process for preparing a 5-R5-R-substituted-2- or 3-
aminomethyl-6-arylcarbonyl-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine which comprises reacting a R-substituted-2-
CA 02362632 2001-11-19
-4- D.N. 7436A
or 3-aminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine
with a 1-aryl-1,3-diketone or a S-aryl-S-ketopropionaldehyde,
R5COCH2COR6, under dehydrating conditions and heating the
resulting hydrazone in an acid medium.
DETAILED DESCRIPTION INCLUSIVE OF THE PREFERRED EMBODIMENTS
More specifically, the invention relates to 5-
R5-R-substituted-2- and 3-aminomethyl-6-arylcarbonyl-2,3-
dihydropyrrolo-[1,2,3-de]-1,4-benzoxazines, useful as analgesic
agents, having the formula:
6 CO-R
R~ ~ ~ 6
RS
2-N=B
I
where:
R is hydrogen or from one to two substituents
selected from the group consisting of lower-alkyl,
lower-alkoxy, hydroxy or halogen in the 7-, 8- or 9-
positions;
R5 is hydrogen or lower-alkyl;
R6 is phenyl, methylenedioxyphenyl (or phenyl
substituted by from one to two substituents selected
from the group consisting of chlorine, bromine, fluorine,
.lower-alkoxy, hydroxy, lower-alkyl, lower-alkylmercapto,
lower-alkylsulfinyl or lower-alkylsulfonyl), 1- or
CA 02362632 2001-11-19
22749-359
-5- D.N. 7436B
2-naphthyl (or 1- or 2-naphthyl substituted by from
one to two substituents selected from the group con-
sisting of lower-alkyl, lower-alkoxy, hydroxy, bromine,
chlorine, fluorine, dower-alkylmercapto, lower-alkyl-
sulfinyl or lower-alkylsulfonyl) or 2-, 3-, 4-, 5-,
6-, 7- or 8- quinolyl; and
N=B is amino, N-lower-alkylamino, N,N-di-lower-
alkylamino, 4-morpholinyl, 4-thiomorpholinyl, 1-piperidinyl,
1-pyrrolidinyl, 1-azetidinyl, 4-lower-alkyl-1-pipera-
zinyl or 1-(hexahydro-4H-1,4-diazepinyl).
Preferred compounds of Formula I above are those
where:
R is hydrogen;
R5 is hydrogen or lower-alkyl;
R6 is phenyl, phenyl substituted by lower-alkoxy
or halogen, 1-naphthyl, 1-naphthyl substituted by halogen
or 6-quinolyl; and
N=B is 4-morpholinyl.
As used herein the terms lower-alkyl and lower-
alkoxy mean monovalent aliphatic radicals, including branched
chain radicals, of from one to four carbon atoms, for example
methyl, ethyl, propyl, isopropyl, butyl, sec.-butyl, methoxy,
ethoxy, propoxy, isopropoxy, butoxy or sec.-butoxy.
As used herein, the term, halo or halogen includes
fluorine, chlorine or bromine.
The compounds of Formula I are prepared by the
following sequence of reactions:
CA 02362632 2001-11-19
-6- D.N. 7436A
R / ( R / I
NH . Hh02 , \ NNO
O O
CH2-N=B CH2-N=B
II III
V
/ S C6H5
R I I R / I
~ N RS ~ ~ N-NH2
O 0
CH2-N=B CH2-N=B
V IV
[H] R6COCH2COR5
v V
/ / CO-R6
R ~ I ' R ~ I
~ N ~ R5 ~ N RS
0 O
CH2-N=B CH2-N=B
VI I
CA 02362632 2001-11-19
22749-359
-7- D.N. 74368
where R, R5, R6 and N=B have the meanings given above.
In a first step, a R-substituted-2- or 3-amino-
methyl-3,4-dihydro-2H-1,4-benzoxazine of Formula II is reacted
with an alkali .metal nitrite in an acidic aqueous medium
to produce a respective R-substituted-2- or 3-aminomethyl-
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine of Formula III.
The reaction is carried out at a temperature from 0°C to
about 10°C with stirring. The compounds of Formula II where
R is hydrogen and N=B is 3-(4-morpholinyl), 3-(1-piperidinyl)
and 3-(diethylamino) and the preparation thereof are described
by Kurihara et al., Yakugaku Zasshi, 88 (9), 1118-1122 (1968);
Chem. Abs., 70, 37748w (1968). Compounds where R and N=B
have the other meanings given above can be similarly
prepared.
The Kurihara et al, method of preparing the R-
substituted-3-aminomethyl-3,4-dihydro-2H-1,4-benzoxazines
of Formula II where N=H is, for example, 1-piperidinyl,
diethylamino or 4-morpholinyl, involves reaction of an appro-
priate amine _(HN=H) with o-n~itrophenyl glycidyl ether, reduction
of the resulting 1-(o-nitrophenoxy)-3-(N=B)-2-propanol with
tin in the presence of a mineral acid and cyclization of
the resulting 1-(o-aminophenoxy)-3-(N=H)-2-propanol with
concentrated sulfuric acid. The method thus provides an
unambiguous route to the 3-aminomethyl compounds uncontam-
inated by the isomeric 2-aminomethyl species.
CA 02362632 2001-11-19
22749-359
-g- D.N. 7436B
The 3-aminomethyl compounds of Formula II
can also be prepared by the method described by Potter and
Munro, J. Het. ,Chem. 9, 299-301 (1972). In the latter
method, 2-acetamidophenol is reacted with epichlorohydrin
in the presence of a strong base (sodium ethoxide). The
proton on the,acetamido nitrogen atom is insufficiently
acidic to react with the epichlorohydrin, and the only pro-
duct apparently isolated by Potter and Munro was that obtained
by reaction of the epichlorohydrin at the phenolic group,
i.e. 2-(2,3-epoxypropoxy)acetanilide. The latter, on treat-
ment with sodium hydride in DMF followed by hydrolysis with
dilute hydrochloric acid afforded 3-hydroxymethyl-2,3-di-
hydro-2H-1,4-benzoxazine uncontaminated by the isomeric
2-hydroxymethyl compound. The hydroxymethyl compound was
thereafter reacted with p-toluenesulfonyl chloride in the
presence of pyridine. The resulting p-toluenesulfonate
can then be reacted with any desired amine, HN=B, to provide
the 3-aminomethyl compounds of Formula II.
We have found that, by using 2-methanesulfonamido-
phenol or 2-(p-toluenesulfonamido)phenol in the Potter and
Munro synthesis (the starting materials being prepared by
reaction of 2-aminophenol with methanesulfonyl chloride
or p-toluenesulfonyl chloride in the presence of pyridine),
the proton on the sulfonamido nitrogen atom is sufficiently
acidic to afford significant yields of 2-hydroxymethyl-4-
CA 02362632 2004-12-14
28888-47D
' -9- D.N, 7436A
methanesulfonyl- [or 4-(p-toluenesulfonyl)-] 2,3-dihydro-
2H-1,4-benzoxazine, resulting from initial condensation
of the epichlorohydrin at the sulfonamido function followed
by ring closure between the phenolic and epoxide functions,
along with the isomeric 3-hydroxymethyl-4-methanesulfonyl
[or 4-(p-toluenesulfonyl)-] 2,3-dihydro-2H-1,4-benzoxazine,
resulting from initial condensation of the epichlorohydrin
at the phenolic function followed by ring closure between
the sulfonamide and epoxide functions. Conversion of the
products to the p-toluenesulfonate ester as before, followed
by reductive cleavage of the sulfonamide group affords the
desired 2- or 3-hydroxymethyl-2,3-dihydro-2H-1,4-benzoxazine
p-toluenesulfonates which, on reaction with an appropriate
amine, HN=B, after separation from one another by conven-
tional means such as fractional crystallization or chromato-
graphy, affords the 2- or 3-aminomethyl-2,3-dihydro-2H-1,4-
benzoxazines of Formula II.
The compounds of Formula iII are thereafter reduced
to the corresponding R-substituted-2- or 3-aminomethyl-4-
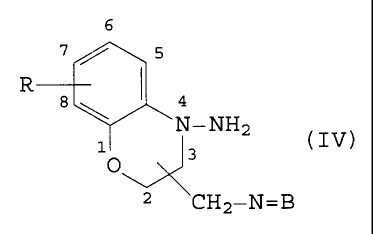
amino-3,4-dihydro-2H-1,4-benzoxazines of Formula IV. The
reduction can be.carried out either catalytically, using
for example a Raney nickel or palladium-on-charcoal catalyst
and hydrogen at pressures from about 30 to 50 p.s.i.g, at
ambient temperature and in an inert organic solvent, for
example a lower-alkanol or, alternatively, the reduction
can be effected chemically with an alkali metal aluminum
hydride, for example lithium aluminum hydride, or an alkali
metal bis-(2-methoxyethoxy) aluminum hydride, for example
*Trade-mark
CA 02362632 2001-11-19
-10 -
sodium bis-(2-methoxyethoxy) aluminum hydride. In the latter
case, the reaction is carried out in an inert organic solvent,
for example tetrahydrofuran (THF), dioxane, diethyl ether,
di-butyl ether or toluene at temperatures in the range from
0°C to the boiling point of the solvent used.
The compounds of Formula IV are converted to the
5-R5-R-substituted-2- or 3-aminomethyl-6-phenylthio-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazines of Formula V using
the Fischer indole synthesis by reaction of the compounds
of Formula IV with a phenylthiomethyl-R5-ketone or aldehyde,
C6H5SCH2COR5, where R5 has the meanings given above. The
reaction is carried out at a temperature in the range from
about 20°C to about 150°C in an organic solvent inert under
the conditions of the reaction, for example methanol, ethanol,
isopropanol and the like and in the presence of an acid
catalyst, for example sulfuric acid, hydrochloric acid or
glacial acetic acid. It is preferred to carry out the reaction
in glacial acetic acid, which not only provides the necessary
acidic medium, but also serves as solvent.
Removal of the a-phenylthio group from the com-
pounds of Formula V to produce the 5-R5-R-substituted-2-
or 3-aminomethyl-2,3-dihydropyrrolo[1,2,3-de]-I,4-benzox-
azines of Formula VI is effected by heating the compounds
of Formula V in an organic solvent, for example a lower-
alkanol, in the presence of Raney nickel at the reflux temper-
ature of the solvent.
*Trade-mark
CA 02362632 2001-11-19
-11- D.N. 7436A
The final products of Formula I are prepared from
the 5-R5-R-substituted-2- or 3-aminomethyl-2,3-dihydropyrrolo-
[1,2,3-de]-1,4-benzoxazin:~s of Formula VI by reaction of
the latter with an appropriate arylcarboxylic acid halide
(R6C0-X, where X represents halogen) in the presence of
a Lewis acid, such as aluminum chloride or ethyl aluminum
chloride, and in an organic solvent inert under the condi-
tions of the reaction. Suitable solvents are chlorinated
hydrocarbons, such as methylene dichloride (hereinafter
MDC) or ethylene dichloride (hereinafter EDC). The reaction
is carried out at a temperature from 0°C up to the boiling
point of the solvent used.
Alternatively, as indicated by the reaction sequence
above, the intermediates of Formula IV can be converted
directly to the final products of Formula I by reaction
of the compounds of Formula IV with a ~-aryl-S-ketopropion-
aldehyde or a 1-aryl-1,3-diketone, R6COCH2COR5, where R5
is, respectively, hydrogen or lower-alkyl and R6 has the
meanings given above, using the same Fischer indole synthesis
conditions described above.
It will be appreciated that the compounds of Formula I
as well as the intermediates of Formulas II, III, IV, V
and VI have a chiral center at the 2- or 3-positions of
the respective R-substituted-2- and 3-aminomethyl-3,4-di-
hydro-2H-1,4-benoxazines (of Formulas II, III or IV) or
the 2- or 3-positions of the 2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazines (of Formulas I, V and VI) and thus can
CA 02362632 2001-11-19 ,
-12- D.N. 7436
exist as optically active stereoisomers. Such stereoisomers
of the various compounds of Formulas I, IV, V and VI are
considered to be within the purview of the present invention.
The isolation of either the intermediates or the final pro-
s ducts in their optically active forms can be accomplished
by application of general principles well-known in the art.
The compounds of Formulas I, IV, V and VI in free
base form are converted to the acid-addition salt form by
interaction of the base with an acid. In like manner, the
free base can be regenerated from the acid-addition salt
form in conventional manner, that is by treating the salts
with cold, weak aqueous bases, for example alkali metal
carbonates and alkali metal bicarbonates. The bases thus
regenerated can be interacted with the same or a different
acid to give back the same or a different acid-addition
salt. Thus the novel bases and all of their acid-addition
salts are readily interconvertible.
It will thus be appreciated that Formulas I, IV,
V and VI not only represent the structural configuration
of the bases of Formulas I, IV, V and VI but are also repre-
sentative of the structural entities which are common to
all of the compounds of Formulas I, IV, V and VI, whether
in the form of the free base or in the form of the acid-
addition salts of the base. It has been found that, by
virtue of these common structural entities the bases of
formula I and their acid-addition salts have inherent pharma-
cological activity of a type to be more fully described
CA 02362632 2001-11-19
-13- D.N. 7436A
hereinbelow. This inherent pharmacological activity can
be enjoyed in useful form for pharmaceutical purposes by
employing the free bases themselves or the acid-addition
salts formed from~pharmaceutically acceptable acids, that
is acids whose anions are innocuous to the animal organism
in effective doses of the salts so that beneficial properties
inherent in the common structural entity represented by
the free bases are not vitiated by side effects ascribable
to the anions.
In utilizing this pharmacological activity of
the salts of the invention, it is preferred, of course,
to use pharmaceutically acceptable salts. Although water
insolubility, high toxicity or lack of crystalline character
may make some particular salt species unsuitable or less
desirable for use as such in a given pharmaceutical applica-
tion, the water-insoluble or toxic salts can be converted
to the corresponding pharmaceutically acceptable bases by
decomposition of the salts with aqueous base as explained
above, or alternatively they can be converted to any desired
pharmaceutically acceptable acid-addition salt by double
decomposition reactions involving the anion, for example
by ion-exchange procedures.
Moreover, apart from their usefulness in pharma-
ceutical applications, the salts are useful as character-
izing or identifying derivatives of the free bases or in
isolation or purification procedures. Like all of the acid-
CA 02362632 2001-11-19
-14- D.N. 7436A
addition salts, such characterizing or purification salt
derivatives can, if desired, be used to regenerate the pharma-
ceutically acceptable free bases by reaction of the salts
with aqueous base; or alternatively they can be converted
to a pharmaceutically acceptable acid-addition salt by,
for example, ion-exchange procedures.
The novel feature of the compounds of the inven-
tion, then, resides in the concept of the bases and cationic
forms of the new 5-R5-R-substituted-2- and 3-aminomethyl-
6-arylcarbonyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazines
of Formula I, the R-substituted-2- and 3-aminomethyl-4-amino-
3,4-dihydro-2H-1,4-benzoxazines of Formula IV and the 5-
R5-R-substituted-2- and 3-aminomethyl-2,3-dihydropyrrolo-
[1,2,3-de]-1,4-benzoxazines of Formulas V and VI and not
in any particular acid moiety or acid anion associated with
the salt forms of the compounds; rather, the acid moieties
or anions which can be associated with the salt forms are
in themselves neither novel nor critical and therefore can
be any acid anion or acid-like substance capable of salt
formation with the bases.
Thus appropriate acid-addition salts are those
derived from such diverse acids as formic acid, acetic acid,
isobutyric acid, alpha-mercaptopropionic acid, malic acid,
fumaric acid, succinic acid, succinamic acid, tartaric acid,
citric acid, lactic acid, benzoic acid, 4-methoxybenzoic
acid, phthalic acid, anthranilic acid, 1-naphthalenecarboxylic
acid, cinnamic acid, cyclohexanecarboxylic acid, mandelic
CA 02362632 2001-11-19
-15- D.N. 7436A
acid, tropic acid, crotonic acid, acetylenedicarboxylic
acid, sorbic acid, 2-furancarboxylic acid, cholic acid,
pyrenecarboxylic acid, 2-pyridinecarboxylic acid, 3-indole-
acetic acid, quinic acid, sulfamic acid, methanesulfonic
acid, isethionic acid, benzenesulfonic acid, p-toluenesulfonic
acid, benzenesulfinic acid, butylarsonic acid, diethylphos-
phonic acid, p-aminophenylarsinic acid, phenylstibnic acid,
phenylphosphinous acid, methylphosphinic acid, phenylphosphinic
acid, hydrofluoric acid, hydrochloric acid, hydrobromic
acid, hydriodic acid, perchloric acid, nitric acid, sulfuric
acid, phosphoric acid, hydrocyanic acid, phosphotungstic
acid, molybdic acid, phosphomolybdic acid, pyrophosphoric
acid, arsenic acid, picric acid, picrolonic acid, barbituric
acid, boron trifluoride and the like.
The acid-addition salts are prepared by reacting
the free base and the acid in an organic solvent and isolating
the salt directly or by concentration of the solution.
In standard pharmacological test procedures, the
compounds of Formula I have been found to possess analgesic
activity and are thus useful as analgesic agents.
The test procedures used to determine the analgesic
activities of the compounds have been described in detail
in the prior art and are as follows: The acetylcholine-
induced abdominal constriction test, which is a primary
analgesic screening test designed to measure the ability
of a test agent to suppress acetylcholine-induced abdominal
CA 02362632 2001-11-19
-16- D.N. 7436A
constriction in mice, described by Collier et al., Brit.
J. Pharmacol. Chemotherap. 32, 295 (1968): a modification
of the anti-bradykinin test, which is also a primary analgesic
screening procedure, described by Berkowitz et al., J. Pharmacol.
Exptl. Therap. 177, 500-508 (1971), Hlane et al., J. Pharm.
Pharmacol 19, 367-373 (1967), Botha et al., Eur. J. Pharmacol.
6, 312-321 (1969) and Deffenu et al., J. Pharm. Pharmacol.
18, 135 (1966); the rat paw flexion test, described by Kuzuna
et al., Chem. Pharm. Hull., 23, 1184-1191 (1975), Winter
et al., J. Pharm. Exptl. Therap., 211, 678-685 (1979) and
Capetola et al., J. Pharm. Exptl. Therap. 214, 16-23 (1980);
and the mouse vas deferens adrenergic transmission test,
described by Henderson et al., Brit. J. Pharmacol., 46,
764-766 (1972).
In order to further detail the analgesic profiles
of the compounds of the invention, their activities in the
inhibition of acetic acid-induced writhing assay in the
rat, the inhibition of cyclooxygenase assay and a modifica-
tion of the Randall-Selitto analgesic test were also deter-
mined. The test procedure used to determine the inhibition
of acetic acid-induced writhing in the rat is described
as follows.
Male Sprague Dawley rats (Taconic Farms) weighing
between 160 and 2009 were treated in a random and blind
fashion with 1% gum tragacanth vehicle or test agent suspended
or dissolved in 1% gum tragacanth. Rats were individually
housed in plastic cages (28 x 18 x 12 cm) throughout the
experiment. Forty-five minutes after drug treatment, 0.5 ml
CA 02362632 2001-11-19
-17- D.N. 7436A
of 9% (w/v) acetic acid was administered intraperitoneally,
and animals were returned to their cages for 10 minutes.
Writhing is not normally seen during this 10 minute period.
Each animal was then observed for a writhing response during
a subsequent 10 minute interval (55-65 minutes post-drug
treatment). A writhing response was defined as arching
of the back with extension of the hindlimbs and contraction
of abdominal muscles. The number of writhing responses
exhibited by each animal was recorded and the mean ~ SE
for each experimental group was calculated. Data were expressed
as percent inhibition of the mean number of writhes in the
vehicle treated control group. Data were evaluated for
statistical significance by Dunnett's test, and ED50~s for
inhibition of acetic acid-induced writhing were determined
by the graded dose-response analysis of Tallarida and Murray,
Manual of Pharmacologic Calculations with Computer Programs,
Springer Verlag, New York (1981).
The test procedure used to determine cyclooxygenase
inhibitory activity is described as follows.
Male mice were decapitated and stomach and brain
(cerebral cortex, hypothalamus and medulla) were rapidly
dissected over ice. The brains were homogenized in 3 volumes
of ice cold 0.1 M potassium phosphate buffer, pH 7.4 containing
10 mM EDTA and 1% HSA (w/v). The homogenate was centri-
fuged at 10,000 xg for 15 minutes. The resulting supernatant
was decanted and centrifu5ed at 100,000 x9 for 60 minutes
yielding a crude microsomal pellet. The pellet was washed
CA 02362632 2001-11-19
-18- D.N. 7436A
superficially three times in 3 volumes of 0.1 M potassium
phosphate buffer before final resuspension in 1.5 volumes
of 0.1 M potassium phosphate buffer containing 1 mM norepine-
phrine and 1 mM reduced glutathione. The microsomes (50 ~1)
were preincubated with 50 ~1 of indomethacin (0.3 ~M), test
compound or norepinephrine/glutathione-containing buffer
for 5 minutes at room temperature. The enzyme reaction
was initiated by the addition of 50 ~1 of 14C arachidonic
acid to yield a final concentration of 10~M. After a further
incubation of 15 minutes at 37°C, the reaction was stopped
by the addition of 501 of 4M formic acid. Blank values
were determined by using microsomes boiled for 5 minutes
but otherwise were treated similarly. The 14C-labelled
material was extracted 3 times with 0.5 ml of ethyl acetate
with intermediate centrifugations at 500 xg for 5 minutes
to clarify the aqueous and organic phases. This procedure
extracted >95% of the 14C-labelled materials. The ethyl
acetate washes were added to tubes containing 5 ~g each
of PGE2, PGF2, PGD2, and 10 ~g PGI2 in 25 ~1 of ethanol.
The mixture was evaporated to dryness under nitrogen and
the residue dissolved in 150 ~1 of chloroform: methanol (2:1).
This solution was spotted on 5 x 20 cm silica gel plates
and chromatographed with an ethyl acetate, trimethylpentane,
acetic acid and water mixture (110:50:20:100 by volume)
prepared by shaking the solvents and separating and discarding
the organic phase. The prostaglandins were visualized in
CA 02362632 2001-11-19
-19- D.N. 7436A
iodine vapor, the various spots were scraped, and the 14C-
labelled prostaglandins quantitated by liquid scintillation
spectroscopy in 10 ml of BIOFLUOR~ or FLUORODYNE~.
The modification of the Randall-Selitto analgesic
test procedure used is described as follows:
Female Sprague-Dawley rats, weighing 60 to 95
grams, were routinely used. In three experiments, male
Sprague-Dawley rats of comparable weights were used. The
rats were housed 10 per cage with food and water available
ad libitum.
The Randall-Selitto Test device used was a UGO
Hasile Analgesy Meter (catalog no. 7200), with the flat-
topped plinth on the bottom, acting as a platform upon which
the hind paw of the rat was placed and the round-pointed
pusher oriented downward. The gap between the pusher and
the plinth was set at the thickness of approximately 0.1 cm
for normal paws and approximately 0.2 cm for inflamed paws.
After placing the hind paw on the plinth, gradually increasing
pressure was applied to the paw by a motor-driven weight
sliding along a calibrated scale.
Testing was accomplished by holding the rat upright
in one hand by the scruff of the neck, then guiding the
test paw into position on the plinth, under the pusher.
The paw was positioned so that the pusher made contact with
it at a point where the metatarsals and phalanges meet,
between the third and fourth digits. Application of gradually
increasing force to the paw was accomplished by operating
the device with a Eoot pedal.
CA 02362632 2001-11-19
-20- D.N. 7436A
A rat was considered to have responded to the
pressure (pain stimulus) if it reacted in any one of the
following ways:
1. if the rat pulled the paw free of the test
dev ice
2. if the rat began to struggle in such a way
that the operator would have difficulty holding
onto it; or
3. if the rat vocalized.
The test involved a total of sixty rats, which
were prescreened in the following manner. Both paws of
each naive rat were tested twice as described above. The
first test acclimatized the animal to the apparatus; the
second established a baseline response for comparison with
later inflamed-paw responses. Any rat responding to less
than 60 grams of pressure during this second trial of the
left paw was discarded and replaced.
Hyperalgesia was induced by administration of
brewers' yeast (Fisher Scientific Company, Lot 785660) 20%
suspension in 0.85$ saline. Each of the rats selected as
described above was given a 0.1 cc sub-plantar injection
of this suspension in the left paw, using a 23 gauge, 1
inch needle.
Animals were given the test drug, p.o., two hours
after the sub-plantar injection, four doses customarily
being given, along with vehicle control and a positive control
CA 02362632 2001-11-19
-21- D,N, 7436A
(Zomepirac). Ten animals were used in each experimental
group. Treatments were assigned in such a way that both
the range and the means of the baseline readings were as
nearly equal as possible for all treatments.
Final testing was performed two hours after the
administration of the test drug. The observer was unaware
of the treatment any particular rat had received. In this
portion of the study, all the inflamed (left) paws were
tested first, as a group, and then the non-inflamed (right)
paw was tested.
Results were analyzed with the Student's t-test.
The minimally effective dose was defined as the lowest dose
tested which met 2 criteria: i) did not produce a response
significantly (>0.05) different from the second (baseline)
preliminary reading, and ii) permitted application of signi-
ficantly (p<0.05) greater pressure than that observed for
the hyperalgesic response in vehicle-treated rats. Thus,
the dose had to completely antagonize the hyperalgesia.
The compounds of formula I of the invention can
be prepared for pharmaceutical use by incorporating them
in unit dosage form as tablets or capsules for oral or parenteral
administration either alone or in combination with suitable
adjuvants such as calcium carbonate, starch, lactose, talc,
magnesium stearate, gum acacia and the like. Still further,
the compounds can be formulated for oral or parenteral admin
istration either in aqueous solutions of the water soluble
CA 02362632 2001-11-19 ,
-22- D.N, 7436A
salts or in aqueous alcohol, glycol or oil solutions or
oil-water emulsions in tr:e same manner as conventional medicinal
substances are prepared.
The percentages of active component in such compo-
sitions may be varied so that a suitable dosage is obtained.
The dosage administered to a particular patient is variable,
depending upon the clinician's judgment using as criteria:
the route of administration, the duration of treatment,
the size and the physical condition of the patient, the
potency of the active component and the patient's response
thereto. An effective dosage amount of the active component
can thus only be determined by the clinician after a consider-
altion of all criteria and using his best judgment on the
patient's behalf.
The molecular structures of the compounds of the
invention were assigned on the basis of study of their infra-
red, ultraviolet and NMR spectra. The structures were con-
firmed by the correspondence between calculated and found
values for elementary analyses for the elements.
The following examples will further illustrate
the invention without, however, limiting it thereto. All
melting points are uncorrected.
CA 02362632 2001-11-19
-23- D.N. 7436A
EXEMPLARY DISCLOSURE
PREPARATION OF INTERMEDIATES
Preparation 1 - The Compounds of Formula II
A. The compounds of Formula II can be prepared using
the method described by Kurihara et al, ibid. The procedure
used for the preparation of 3-(4-morpholinylmethyl)-3,4-
dihydro-2H-1,4-benzoxazine was as follows.
A five liter round bottom flask was charged with
163 g (1.0 mole) of o-nitrophenol (15% moisture content)
and 193 g (1.4 moles) of potassium carbonate in 1.5 liters
of DMF. The reaction mixture was heated on a steam bath
for 30 minutes, treated with 176 ml (2.25 moles) of epichloro-
hydrin added rapidly in three portions and then heated with
stirring for an additional 2 hours. The reaction mixture
was then cooled to room temperature, filtered to remove
a solid, and the filtrate concentrated in vacuo. The residual
dark oil was taken into ethyl acetate, the solution washed
with brine, and the organic phase was separated, dried over
magnesium sulfate and concentrated in vacuo. The resulting
solid was suspended in 750 ml of diethyl ether and stirred
overnight. The insoluble material was removed by filtra-
tion, and the filtrate was diluted with hexane to give three
crops of product (139.9 g) consisting of 2-(1,2-epoxy-3-
propyloxynitrobenzene.
The resulting product (133 g, 0.68 mole) was added
to a two liter round bottom flask containing 133 ml (1.53
moles) of morpholine and 750 ml of toluene. The reaction
CA 02362632 2001-11-19
-24- D.N. 7436A
mixture was stirred for 2 hours at 95%, then overnight at
room temperature and then concentrated in vacuo to afford
a yellow oil which was dissolved in ethyl acetate. The
resulting solution was washed with brine, dried over magne-
sium sulfate and concentrated in vacuo, the residual morpholine
being removed under high vacuum (0.2 mm) at 70°C. The residual
oil was crystallized from 400 ml of diethyl ether to give
two crops, totalling 160.3 g, of 2-[3-(4-morpholinyl)-2-
hydroxy-1- ropyloxy]nitrobenzene.
A five liter round bottom flask was charged with
63.8 ml (0.45 mole) of trifluoroacetic anhydride and 400
ml of MDC under nitrogen. The resulting solution was cooled
to -65°C with a dry ice bath, treated with 42.6 g (0.54
mole) of dimethylsulfoxide over a period of 15 minutes,
stirred for 30 minutes, and then treated with a solution
of 90 g (0.32 mole) of 2-[3-(4-morpholinyl) -2-hydroxy-1-
propyloxy]nitrobenzene in 200 ml of MDC over a 35 minute
period. The reaction mixture was stirred for 30 minutes,
quenched with 261 ml of diisopropylethylamine, the reaction
temperature allowed to rise to -10°C and the mixture treated
with one liter of diethyl ether. The pale yellow solid
which separated was collected by filtration, slurried in
1.5 liters of diethyl ether and the product collected and
dried to give 73.7 g of 2-[3-(4-morpholinyl)-2-keto-1- ro-
pyloxy]nitrobenzene.
The product (20.4 g, 0.73 mole) and 5 g of 5%
platinum on carbon sulfided was placed in a two liter stir-
rable Parr pressure reactor under nitrogen containing 1 liter
CA 02362632 2001-11-19
-25- ~ D.N. 7436A
of ethyl acetate. The reactor was pressurized to 100 psi
of hydrogen and stirred for 20 hours. The catalyst was
then removed by filtration, the filtrate treated with 14 g
of oxalic acid d'ihydrate, and the salt which separated was
collected by filtration and then neutralized with a solution
of sodium bicarbonate. The mixture was extracted with ethyl
acetate, and the organic solution was dried over magnesium
sulfate and concentrated in vacuo to give 9.3 g of 3-(4_
mor holinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine, m,p,
117-121 °C.
The 3-(4-morpholinylmethyl)-3,4-dihydro-2H-1,4-
benzoxazine described above was resolved into its d- and
1-enantiomers as follows:
The racemic material (45.5 g, 0.19 mole) was dis-
solved in 600 ml of hot acetone, and 70 g (0.19 mole) of
d-dibenzoyltartaric acid monohydrate was added and the mixture
stirred until all material had dissolved. The solution
was then concentrated to about 200 ml, diluted with 500 ml~
of ethyl acetate and cooled. The solid which separated
was collected and dried to give 36 g of the crude 1-base.d-
acid salt, the filtrate being set aside for further processing.
Several recrystallizations of the crude salt from methanol
afforded 6.3 g of (-)-3-(4-morpholinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine.(+)-dibenzoyltartrate, [alDS = +45.8°
(1$ in DMF), m.p. 176-178°C.
The latter (26.9 g) was partitioned between ethyl
acetate and saturated aqueous sodium carbonate, and the
CA 02362632 2001-11-19
-26- D.N. 7436A
organic layer was dried over magnesium sulfate, filtered
and taken to dryness to yield 15.1 g of a white solid which
was recrystallized from ethyl acetate/hexane to give 6.7 g
of (-)-3-(4-morpholinylmethyl)-3,4-dihydro-2H-1,4-benzoxa-
zine, [alDS = -28.0° (1% in CHC13), m.p. 93-95°C.
The filtrate set aside from the preparation of
the 1-base d-acid salt as described above was combined with
all the other liquors from the recrystallizations thereof
and partitioned between ethyl acetate and aqueous sodium
carbonate, and the organic layer was dried over magnesium
sulfate, filtered and taken to dryness. The residue (13.7 g,
0.059 mole) was dissolved in methanol, 22 g (0.059 mole)
of (-)-dibenzoyltartaric acid monohydrate was added and
the solution diluted with hexane. The solid which separated
was collected and recrystallized from methanol to give the
2:1 salt of ~+)-3-(4-mor holinylmethyl)-3,4-dihydro-2H-1,4-
benzoxazine (-)-dibenzoyltartrate, [alDS = -39.8° (1% in
DMF), m.p. 177.5-179.5°C. Another batch, prepared similarly,
showed (a]D5 = -42.0° (1% in DMF).
The free base was liberated from 31.6 g of the
latter by partitioning the salt between ethyl acetate and
saturated sodium carbonate and isolation of the base from
the organic layer. Recrystallization of the product thus
obtained from ethyl acetate/hexane afforded 9.4 g of (~)-
3-(morpholinylmethyl)-3.4-dihydro-2H-1,4-benzoxazine, [a~25 =
D
+28.1° (1% in CHC13), m.p. 94-96°C.
CA 02362632 2001-11-19
-27- D,N. 7436A
Proceeding in a manner similar to that described
above in Preparation 1A and by Kurihara et al., substituting
for the 2-nitrophenyl glycidyl ether used by Kurihara et
al., a molar equivalent amount of 6-methoxy-2-nitrophenyl
glycidyl ether or 6-chloro-2-nitrophenyl glycidyl ether
and substituting for the piperidine, diethylamine or morpholine
used by Kurihara et al. a molar equivalent amount of ammonia,
methylamine, thiomorpholine, pyrrolidine, azetidine, 1-methyl-
piperazine or hexahydro-4H-1,4-diazepine, there can be obtained
8-methoxy-3-aminomethyl-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-methylaminomethyl-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-(4-thiomorpholinylmethyl)-3,4-dihydro-2H-1,4-
benzoxazine, 8-methoxy-3-(1-pyrrolidinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine, 8-methoxy-3-(1-azetidinylmethyl)-3,4-
dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(4-methyl-1-pipera-
zinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-
[1-(hexahydro-4H-1,4-diazepinyl)methyl]-3,4-dihydro-2H-1,4-
benzoxazine, 8-chloro-3-aminomethyl-3,4-dihydro-2H-1,4-benzox-
azine, 8-chloro-3-methylaminomethyl-3,4-dihydro-2H-1,4-benzox-
azine, 8-chloro-3-(4-thiomorpholinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinylmethyl)-3,4-
dihydro-2H-1,4-benzoxazine, 8-chloro-3-(1-azetidinylmethyl)-
3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-(4-methyl-1-piper-
azinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine and 8-chloro-
3-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-3,4-dihydro-2H-
1,4-benzoxazine.
CA 02362632 2001-11-19
-28- D.N, 7436A
B. To a stirred suspension of 109 g (1.0 mole) of
2-aminophenol in 1 liter of MDC containing 79 g of pyridine
was added, over a 20 minute period with stirring and while
maintaining the temperature below 38°C, 115 g (1.0 mole)
of methanesulfonyl chloride. When addition was complete
the reaction mixture was washed with water, then with saturated
sodium bicarbonate and the organic solution taken to dryness
and the residue diluted with xylene and taken to dryness
again to remove residual pyridine. There was thus obtained
106 g of 2-methanesulfonamidophenol.
The latter (10 g, 0.053 mole) was dissalved in
40 ml of N-methylpyrrolidone containing 4.0 g (0.06 mole)
of solid potassium hydroxide, and the mixture was treated
over a five minute period while warming on a steam bath
with 10 ml (0.127 mole) of epichlorohydrin. The mixture
was extracted with isopropyl acetate, and the organic extracts
were washed two times with water, dried and the organic
layer taken to dryness to give 15 g of a mixture of 2- and
3-hydroxymethyl-4-methanesulfonyl-3,4-dihydro=2H-1,4-benzoxazine.
The crude product (about 120 g, 0.5 mole) was
dissolved in 1 liter of MDC, cooled to 10°C in an ice bath
and treated first with 125 ml of triethylamine followed
by a solution of 97 g (0.5 mole) of p-toluenesulfonyl chloride
in 200 ml of MDC. The reaction mixture was stirred at ambient
temperature for about twelve hours and then washed first
with water, then with saturated sodium carbonate and taken
to dryness. The residue was dissolved in toluene, the solution
CA 02362632 2001-11-19
_2g_ D.N. 7436A
washed again with water, dried and taken to near dryness,
and the residue was diluted with diethyl ether and filtered.
The solid material was rinsed with diethyl ether, then triturated
with methanol and collected and dried to give 113 g of crude
product consisting of a mixture of 2- and 3-hydroxymethyl-
4-methanesulfonyl-3,4-dihydro-2H-1,4-benzoxazine p-toluene-
sulfonate. The latter was recrystallized from 150 ml of
MDC to give 47 g of 3-hydroxymethyl-4-methanesulfonyl-3.4-
dihydro-2H-1.4-benzoxazine p-toluenesulfonate. The mother
liquors from the latter crystallization were set aside,
allowed to cool for about twelve hours, and the solid which
separated was collected by filtration and dried to give
19 g of 2 hydroxymethyl-4-methanesulfonyl-3.4-dihydro-2H-
1,4-benzoxazine -p-toluenesulfonate.
A solution of 18.5 g (0.047 mole) of 2-hydroxy-
methyl-4-methanesulfonyl-3,4-dihydro-2H-1,4-benzoxazine
p-toluenesulfonate in 40 ml of N-methylpyrrolidone and 50
ml of morpholine was heated under reflux for about one and
a half hours and then taken to dryness. zne res~aue was
extracted with ethyl acetate, and the solution was washed
first with water, then with brine and taken to dryness to
give 15 g of an oil which crystallized on standing. The
solid was triturated with diethyl ether and collected by
filtration to give 11.1 g of 2-(4-morpholinylmethyl)-4-methane-
sulfonyl-3.4-dihydro-2H-1.4-benzoxazine.
CA 02362632 2001-11-19
-30- D.N. 7436A
To a stirred solution of 10.8 g (0.035 mole) of
the latter in 100 ml of toluene at ambient temperature was
added dropwise and with stirring over a twenty minute period
a solution of 8 g~(0.040 mole) of a 70% solution of sodium
bis-(2-methoxyethoxy)aluminum hydride in toluene. The mixture
was stirred for thirty minutes at ambient temperature, warmed
on a steam bath for five minutes, cooled, quenched with
water and 35% sodium hydroxide, and extracted with toluene.
The combined organic extracts were washed with water, dried
and taken to dryness to give the crude product as a syrup
which was chromatographed on silica gel, the product being
eluted with 40:60 ethyl acetate: hexane. There was thus
obtained 8 g of 2-(4-morpholinvlmethvl)-3.4-dihvdro-2H-1,4-
benzoxazine.
In the same manner, the isomeric 3-hydroxymethyl-
4-methanesulfonyl-3,4-dihydro-2H-1,4-benzoxazine p-toluene-
sulfonate obtained above (40.8 g, 0.10 mole) was reacted
with 60 ml of morpholine in 100 ml of N-methylpyrrolidone
to give 22.5 g of 3-(4-morpholinylmethvl)-4-methanesulfonyl-
3,4-dihydro-2H-1,4-benzoxazine.
The latter (22 g, 0.07 mole), on reduction with
56.5 g (0.28 mole) of a 70% solution of sodium bis-(2-methoxy-
ethoxy) aluminum hydride in toluene, gave 11.8 g of 3-(4-
morpholinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine.
CA 02362632 2001-11-19
-31- D.N. 7436A
Proceeding in a manner similar to that described
above in Preparation 1B for the preparation of 2-(4-morpho-
linylmethyl)-3,4-dihydro-2H-1,4-benzoxazine by reaction
of 2-hydroxymethyl-4-methanesulfonyl-3,4-dihydro-2H-1,4-
benzoxazine p-toluenesulfonate with morpholine and reduction
of the resulting 2-(4-morpholinylmethyl)-4-methanesulfonyl-
3,4-dihydro-2H-1,4-benzoxazine with sodium bis-(2-methoxy-
ethoxy) aluminum hydride, substituting for the morpholine
used therein a molar equivalent amount of piperidine, diethyl-
amine, ammonia, methylamine, thiomorpholine, pyrrolidine,
azetidine, 1-methylpiperazine or hexahydro-4H-1,4-diazepine,
there can be obtained, respectively, 2-(1-piperidinylmethyl)-
3,4-dihydro-2H-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-
3,4-dihydro-2H-1,4-benzoxazine, 2-aminomethyl-3,4-dihydro-
2H-1,4-benzoxazine, 2-methylaminomethyl-3,4-dihydro-2H-1,4-
benzoxazine, 2-(4-thiomorpholinylmethyl)-3,4-dihydro-2H-
1,4-benzoxazine, 2-(1-pyrrolidinylmethyl)-3,4-dihydro-2H-
1,4-benzoxazine, 2-(1-azetidinylmethyl)-3,4-dihydro-2H-1,4-
benzoxazine, 2-(4-methyl-1-piperazinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)-
methyl]-3,4-dihydro-2H-1,4-benzoxazine.
Preparation 2 - The Compounds of Formula III
A. A solution of (-)-3-(4-morpholinylmethyl)-3,4-
dihydro-2H-1,4-benzoxazine in 250 ml of 2N aqueous hydro-
chloric acid was cooled to 0°C and treated with a solution
of 4.8 g (0.070 mole) of sodium nitrite in 50 ml of water.
CA 02362632 2001-11-19
-32- D.N. 7436A
The solution was stirred for one hour at 0°C, poured into
a mixture of 1500 ml of water and 1 liter of ethyl acetate
and neutralized carefully by the addition of solid sodium
bicarbonate. The~organic layer was separated, and the aqueous
layer was extracted with an additional 400 ml of ethyl acetate.
The combined organic extracts were dried over magnesium
sulfate, filtered and taken to dryness to give (-)-3-(4-
morpholinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
which was used as such in the next synthetic step without
further purification or characterization.
Similarly 17.4 g (0.074 mole) of (+)-3-(4-morpho-
linylmethyl)-3,4-dihydro-2H-1,4-benzoxazine in 300 ml of
2N aqueous hydrochloric acid was treated with 5.6 g (0.081
mole) of sodium nitrite in 60 ml of water at 0°C to produce
~~)-3-(4-mor holinylmethyl)-4-nitroso-3,4-dihydro-2H-1.4-
benzoxazine, which was used as such in the next synthetic
step without further purification or characterization.
Similarly 10.9 g (0.047 mole) of racemic 3-(4-
morpholinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine in 250
ml of 2N aqueous hydrochloric acid was treated with 3.9 g
(0.057 mole) of sodium nitrite in 50 ml of water at 0°C
to produce racemic 3-(4-morpholinylmethyl)-4-nitroso-3~4-
dihydro-2H-1.4-benzoxazine, which was used as such in the
next synthetic step without further purification or character-
ization.
CA 02362632 2001-11-19
-33- D.N. 7436A
B. Similarly 8 g (0.034 mole) of racemic 2-(4-morpho-
linylmethyl)-3,4-dihydro-2H-1,4-benzoxazine in about 130
ml, of 2N aqueous hydrochloric acid was treated with 1.3 g
(0.019 mole) of sodium nitrite in about 100 ml of water
at 0°C to produce racemic 2-14-morpholinylmethyl)-4-nitroso-
3.4-dihydro-2H-1,4-benzoxazine, which was used as such in
the next synthetic step without further purification or
characterization.
Proceeding in a manner similar to that described
above in Preparation 2A, substituting for the (+) or (-)-
3-(4-morpholinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine used
therein a molar equivalent amount of 3-(1-piperidinylmethyl)-
3,4-dihydro-2H-1,4-benzoxazine (Kurihara et al.), 3-(N,N-
diethylaminomethyl)-3,4-dihydro-2H-1,4-benzoxazine (Kurihara
et al.), 8-methoxy-3-aminomethyl-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-methylaminomethyl-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-(4-thiomorpholinylmethyl)-3,4-dihydro-2H-1,4-
benzoxazine, 8-methoxy-3-(1-pyrrolidinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine, 8-methoxy-3-(1-azetidinylmethyl)-3,4-
dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(4-methyl-1-pipera-
zinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-
(1-(hexahydro-4H-1,4-diazepinyl)methyl]-3,4-dihydro-2H-1,4-
benzoxazine, 8-chloro-3-aminomethyl-3,4-dihydro-2H-1,4-benzox-
azine, 8-chloro-3-methylaminomethyl-3,4-dihydro-2H-1,4-benzox-
azine, 8-chloro-3-(4-thiomorpholinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinylmethyl)-3,4-
CA 02362632 2001-11-19
-34- D.N. 7436A
dihydro-2H-1,4-benzoxazine, 8-chloro-3-(1-azetidinylmethyl)-
3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-(4-methyl-1-pipera-
zinylmethyl)-3,4-dihydro-2H-1,4-benzoxazine and 8-chloro-
3-[1-(hexahydro-4~-1,4-diazepinyl)methyl]-3,4-dihydro-2H-
1,4-benzoxazine, there can be obtained, respectively, 3-
(1-piperidinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
3-(N,N-diethylaminomethyl)-4-nitroso-3,4-dihydro-2H-1,4-
benzoxazine, 8-methoxy-3-aminomethyl-4-nitroso-3,4-dihydro-
2H-1,4-benzoxazine, 8-methoxy-3-methylaminomethyl-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(4-thiomorpho-
linylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-
methoxy-3-(1-pyrrolidinylmethyl)-4-nitroso-3,4-dihydro-2H-
1,4-benzoxazine, 8-methoxy-3-(1-azetidinylmethyl)-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(4-methyl-1-
piperazinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-[1-(hexahydro-4H-1,4-diazepinyl)methylj-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-aminomethyl-4-
nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-methyl-
aminomethyl-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-
chloro-3-(4-thiomorpholinylmethyl)-4-nitroso-3,4-dihydro-
2H-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinylmethyl)-4-
nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-(1-azeti-
dinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-
chloro-3-(4-methyl-1-piperazinylmethyl)-4-nitroso-3,4-dihydro-
2H-1,4-benzoxazine and 8-chloro-3-[1-(hexahydro-4H-1,4-diaze-
pinyl)methylj-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine.
CA 02362632 2001-11-19
-35- D.N. 7436A
Proceeding in a manner similar to that described
in Preparation 2B above, substituting for the 2-(4-morpho-
linylmethyl)-3,4-dihydro-2H-1,4-benzoxazine used therein
a molar equivalent amount of 2-(1-piperidinylmethyl)-3,4-
dihydro-2H-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-3,4-
dihydro-2H-1,4-benzoxazine, 2-aminomethyl-3,4-dihydro-2H-
1,4-benzoxazine, 2-methylaminomethyl-3,4-dihydro-2H-1,4-
benzoxazine, 2-(4-thiomorpholinylmethyl)-3,4-dihydro-2H-
1,4-benzoxazine, 2-(1-pyrrolidinylmethyl)-3,4-dihydro-2H-
1,4-benzoxazine, 2-(1-azetidinylmethyl)-3,4-dihydro-2H-1,4-
benzoxazine, 2-(4-methyl-1-piperazinylmethyl)-3,4-dihydro-
2H-1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)-
methylj-3,4-dihydro-2H-1,4-benzoxazine, there can be obtained,
respectively, 2-(1-piperidinylmethyl)-4-nitroso-3,4-dihydro-
2H-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 2-aminomethyl-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 2-methylaminomethyl-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-pyrrolidinyl-
methyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-azeti-
dinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 2-
(4-methyl-1-piperazinylmethyl)-4-nitroso-3,4-dihydro-2H-
1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)methylj-
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine.
Preparation 3 - The Compounds of Formula IV
A. To a suspension of 56 g of lithium aluminum hydride
in 500 ml of THF was added the (-)-3-(4-morpholinylmethyl)-
CA 02362632 2001-11-19
-36- D.N. 7436A
4-nitroso-3,4-dihydro-2H-1,4-benzosazine described above
while cooling the reaction mixture to 0°C with an external
cooling bath. When addition was complete the cooling bath
was removed, the reaction mixture was stirred for one hour
at ambient temperature, cooled again to 0°C and the excess
hydride carefully quenched by the dropwise addition of 5
ml of water, followed by 8 ml of 10% aqueous sodium hydroxide,
followed by an additional 5 ml of water. The mixture was
stirred, filtered through filter aid, and the filtrate taken
to dryness to afford 14.3 g of ~-)-3-(4-mor holinylmethvl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, which was used as
such in the next synthetic step without further characteri-
zation or purification.
Similarly the crude (+) (0.074 mole) and racemic
(0.047 mole) 3-(4-morpholinylmethyl)-4-nitroso-3,4-dihydro-
2H-1,4-benzoxazines described above were reduced with lithium
aluminum hydride in THF to give the corresponding + and
racemic 3-(4-morpholinylmethyl)-4-amino-3,4-dihydro-2H-1,4-
benzoxazines, (15.5 g, 0.062 mole and 8.8 g, 0.035 mole,
respectively) which were used as such in the next step with-
out further characterization or purification.
B. To a solution of 16 g (0.055 mole) of a 70% solution
of sodium bis-(2-methoxyethoxy) aluminum hydride diluted
with 75 ml of toluene was added with stirring at 0°C over
a thirty minute period a solution of 2-(4-morpholinylmethyl)-
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine in 80 ml of toluene.
CA 02362632 2001-11-19
-37- D.N, 7436A
The mixture was stirred at ambient temperature for two hours,
then quenched by the dropwise addition of 1N sodium hydroxide.
The organic layer was washed with additional 1N sodium hydroxide,
then with water, dried and taken to dryness to give 7.8
g of a syrup which was chromatographed on silica gel to
give 4.6 g of 2-(4-morpholinylmethyl)-4-amino-3~4-dihydro-
2H-1,4-benzoxazine.
Following a procedure similar to that described
above in Preparation 3A, substituting for the (-)-3-(4-morpho-
linylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine used
therein a molar equivalent amount of 3-(1-piperidinylmethyl)-
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 3-(N,N-diethyl-
aminomethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-
methoxy-3-aminomethyl-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-methylaminomethyl-4-nitroso-3,4-dihydro-2H-1,4-
benzoxazine, 8-methoxy-3-(4-thiomorpholinylmethyl)-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(1-pyrrolidinyl-
methyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-
3-(1-azetidinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-(4-methyl-1-piperazinylmethyl)-4-nitroso-3,4-
dihydro-2H-1,4-benzoxazine, 8-methoxy-3-[1-(hexahydro-4H-
1,4-diazepinyl)methylj-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
8-chloro-3-aminomethyl-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
8-chloro-3-methylaminomethyl-4-nitroso-3,4-dihydro-2H-1,4-
benzoxazine, 8-chloro-3-(4-thiomorpholinylmethyl)-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinyl-
CA 02362632 2001-11-19
-3g- D.N. 7436A
methyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-
3-(1-azetidinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
8-chloro-3-(4-methyl-1-piperazinylmethyl)-4-nitroso-3,4-
dihydro-2H-1,4-benzoxazine and 8-chloro-3-[1-(hexahydro-
4H-1,4-diazepinyl)methyl]-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine,
there can be obtained, respectively, 3-(1-piperidinylmethyl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 3-(N,N-diethylamino-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-
3-aminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-
methoxy-3-methylaminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-(4-thiomorpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine, 8-methoxy-3-(1-pyrrolidinylmethyl)-4-
amino-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(1-azeti-
dinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-
3-(4-methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-2H-
1,4-benzoxazine, 8-methoxy-3-[1-(hexahydro-4H-1,4-diaze-
pinyl)methyl]-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-
chloro-3-aminomethyZ-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
8-chloro-3-methylaminomethyl-4-amino-3,4-dihydro-2H-1,4-
benzoxazine, 8-chloro-3-(4-thiomorpholinylmethyl)-4-amino-
3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinyl-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-
3-(1-azetidinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
8-chloro-3-(4-methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine and 8-chloro-3-[1-(hexahydro-4H-1,4-diaze-
pinyl)methyl]-4-amino-3,4-dihydro-2H-1,4-benzoxazine.
CA 02362632 2001-11-19
-39- D,N. 7436A
Following a procedure similar to that described
above in Preparation 3B for the preparation of 2-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, substi-
tuting for the 2-(4-morpholinylmethyl)-4-nitroso-3,4-dihydro-
2H-1,4-benzoxazine used therein a molar equivalent amount
of 2-(1-piperidinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-
benzoxazine, 2-(N,N-diethylaminomethyl)-4-nitroso-3,4-di-
hydro-2H-1,4-benzoxazine, 2-aminomethyl-4-nitroso-3,4-di-
hydro-2H-1,4-benzoxazine, 2-methylaminomethyl-4-nitroso-
3,4-dihydro-2H-1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-pyrrolidinyl-
methyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-azeti-
dinylmethyl)-4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, 2-
(4-methyl-1-piperazinylmethyl)-4-nitroso-3,4-dihydro-2H
1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)methyl]
4-nitroso-3,4-dihydro-2H-1,4-benzoxazine, there can be obtained,
respectively, 2-(1-piperidinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-4-amino-3,4-
dihydro-2H-1,4-benzoxazine, 2-aminomethyl-4- amino-3,4-di-
hydro-2H-1,4-benzoxazine, 2-methylaminomethyl-4-amino-3,4-
dihydro-2H-1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-pyrrolidinyl-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-azeti-
dinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(4-
methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine
or 2-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-4-amino-3,4-
dihydro-2H-1,4-benzoxazine.
CA 02362632 2001-11-19
-40- D.N. 7436A
Preparation 4 - The Compo~~nds of Formula V
A. A solution of 37.8 g (0.15 mole) of 3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine and
25 g (0.15 mole) of phenylthioacetone in 500 ml of glacial
acetic acid was heated under reflux for one hour, then cooled,
diluted with water and ethyl acetate and then carefully
neutralized by the addition of solid sodium carbonate.
The two phases were separated, and the aqueous phase was
extracted with ethyl acetate. The combined organic extracts
were washed with sodium carbonate, then saturated brine,
filtered and taken to dryness to give 14.1 g of crude product,
about 5 g of which was recrystallized from ethyl acetate/
hexane to give 3.2 g of 3-(4-morpholinylmethyl)-5-methyl-
6-phenylthio-2.3-dihydropyrrolo[1,2.3-de]-1,4-benzoxazine,
m.p. 161-164°C.
B. Similarly 15.1 g (0.06 mole) of (+)-3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine was
reacted with 12 g (0.07 mole) of phenylthioacetone in I25
ml of glacial acetic acid and the product recrystallized
from ethanol to give 8.3 g of (+)-3-(4-morpholinylmethyl)-
5-methyl-6-phenylthio-2,3-dihydro yrrolo[1,2.3-de]-1,4-benzoxazine,
m.p. 126.5-127.5°C, [a]D5 = +53.0°.
Similarly 2.5 g (0.01 mole) of (-)3-(4-morpholinyl-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine was reacted
with 1.7 g (0.01 mole) of phenylthioacetone in 25 ml of
glacial acetic acid and the product recrystallized from
CA 02362632 2001-11-19
-41- D,N. 7436A
ethanol to give 1.3 g of (-)-3-(4-mor holinylmethyl)-5-methyl-
6-phenylthio-2,3-dihvdropyrrolo[1,2,3-deJ-1,4-benzoxazine,
m.p. 127.5-128.5°C, [aJDS = -49.4°.
Proceeding in a manner similar to that described
above in Preparation 4A, substituting for the 3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine used
therein a molar equivalent amount of 3-(1-piperidinylmethyl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 3-(N,N-diethylamino-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-
3-aminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-
methoxy-3-methylaminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
8-methoxy-3-(4-thiomorpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine, 8-methoxy-3-(1-pyrrolidinylmethyl)-4-
amino-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-3-(1-azeti-
dinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-methoxy-
3-(4-methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-2H-
1,4-benzoxazine, 8-methoxy-3-[1-(hexahydro-4H-1,4-diazepinyl)-
methylJ-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-
3-aminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-
chloro-3-methylaminomethyl-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
8-chloro-3-(4-thiomorpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinylmethyl)-4-
amino-3,4-dihydro-2H-1,4-benzoxazine, 8rchloro-3-(1-azeti-
dinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 8-chloro-
3-(4-methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-2H-
1,4-benzoxazine and 8-chloro-3-[1-(hexahydro-4H-1,4-diaze-
CA 02362632 2001-11-19
-42- D.N, 7436A
pinyl)methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, there
can be obtained, respectively, 3-(1-piperidinylmethyl)-5-
methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
3-(N,N-diethylaminomethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-3-aminomethyl-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-methoxy-3-methylaminomethyl-5-methyl-6-phenylthio-2,3-
dihydropyrrolo[1,2,3-de)-1,4-benzoxazine, 8-methoxy-3-(4-
thiomorpholinylmethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-3-(1-pyrroli-
dinylmethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-(1-azetidinylmethyl)-5-
methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-methoxy-3-(4-methyl-1-piperazinylmethyl)-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-(1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-
aminomethyl-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-methylaminomethyl-5-methyl-
6-phenylthio-2,3-dihydropyrrolo(I,2,3-de]-1,4-benzoxazine,
8-chloro-3-(4-thiomorpholinylmethyl)-5-methyl-6-phenylthio-
2,3-dihydro[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-(1-pyrroli-
dinylmethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-(1-azetidinylmethyl)-5-methyl-
6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-chloro-3-(4-methyl-1-piperazinylmethyl)-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de)-1,4-benzoxazine and 8-chloro-
3-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-6-phenylthio-
2,3-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine.
CA 02362632 2001-11-19
-43- D.N. 7436A
Proceeding in a manner similar to that described
above in Preparation 4A, substituting for the 3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine used
therein a molar equivalent amount of 2-(1-piperidinylmethyl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(N,N-diethylamino-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-aminomethyl-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-methylaminomethyl-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(4-thiomorpholinyl-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-pyrroli-
dinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-
azetidinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
2-(4-methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-2H-
1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, there can be obtained,
respectively, 2-(1-piperidinylmethyl)-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(N,N-diethyl-
aminomethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-aminomethyl-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-methylamino-
methyl-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-5-methyl-6-
phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(1-pyrrolidinylmethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(1-azetidinylmethyl)-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(4-methyl-1-piperazinylmethyl)-5-methyl-6-phenylthio-2,3-
CA 02362632 2001-11-19
-44- D. N. 7436A
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine or 2-[1-(hexahydro-
4H-1,4-diazepinyl)methyl]-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine.
Preparation 5 - The Co ounds of Formula VI
A. To a solution of 10.6 g (0.028 mole) of 3-(4-morpho-
linylmethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo(1,2,3-
de]-1,4-benzoxazine in 600 ml of ethanol was added seven
teaspoons of Raney nickel. The mixture was heated under
reflux for two hours and then filtered through filter aid.
Evaporation of the filtrate to dryness and recrystallization
of the residue from ethyl acetate afforded 4.7 g of 3-(4-
morpholinylmethyl)-5-methyl-2,3-dihydro yrrolo[1,2,3-de]
1.4-benzoxazine, m.p. 178-180°C.
B. Similarly 7.9 g of (+)-3-(4-morpholinylmethyl)-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine
in 300 ml of ethanol was reduced with three teaspoons of
Raney nickel to give 5.1 g of ~+)-3-(4-mor holinylmethyl)-
5-methyl-2,3-dihydro yrrolo 1,2,3-de]-1,4-benzoxazine, (a]25
D
- +64.8°.
C. Similarly 1.0 g of (-)-3-(4-morpholinylmethyl)-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine
in 100 ml of ethanol was reduced with one teaspoon of Raney
nickel to give 0.9 g of ~-)-3-(4-morpholinylmethyl)-5-methyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, [a]25 = -63°.
D
CA 02362632 2001-11-19
-45- D.N. 7436A
Proceeding in a manner similar to that described
above in Preparation 5A, substituting for the 3-(4-morpho-
linylmethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine used therein a molar equivalent amount
of 3-(1-piperidinylmethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(N,N-diethylaminomethyl)-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de)-1,4-benzoxazine,
8-methoxy-3-aminomethyl-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-3-methylamino-
methyl-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 8-methoxy-3-(4-thiomorpholinylmethyl)-5-
methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-methoxy-3-(1-pyrrolidinylmethyl)-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-(1-azetidinylmethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-3-(4-methyl-
1-piperazinylmethyl)-5-methyl-6-pheny.lthio-2,3-dihydropyrrolo[1,2,3
de]-1,4-benzoxazine, 8-methoxy-3-[1-(hexahydro-4A-1,4-diaze-
pinyl)methyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-aminomethyl-5-methyl-6-phenyl-
thio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-
3-methylaminomethyl-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-(4-thiomorpholinylmethyl)-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-chloro-3-(1-pyrrolidinylmethyl)-5-methyl-6-phenylthio-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-
CA 02362632 2001-11-19
-46- D.N, 7436A
3-(1-azetidinylmethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-(4-methyl-
1-piperazinylmethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine and 8-chloro-3-[1-(hexahydro-4H-1,4-
diazepinyl)methyl]-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, there can be obtained, respectively,
3-(1-piperidinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 3-(N,N-diethylaminomethyl)-5-methyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-aminomethyl-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-
benzoxazine, 8-methoxy-3-methylaminomethyl-5-methyl-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-3-(4-
thiomorpholinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-(1-pyrrolidinylmethyl)-
5-methyl-2,3-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, 8-
methoxy-3-(1-azetidinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-(4-methyl-1-piperazinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-methoxy-3-I1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-
aminomethyl-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-chloro-3-methylaminomethyl-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-(4-thiomorpholinylmethyl)-
5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-
chloro-3-(1-pyrrolidinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-(1-azetidinylmethyl)-5-methyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-
CA 02362632 2001-11-19
-47- D.N. 7436A
(4-methyl-1-piperazinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine and 8-chloro-3-[1-(hexahydro-4H-1,4-
diazepinyl)methyl]-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine.
Proceeding in a manner similar to that described
above in Preparation 5A, substituting for the 3-(4-morpho-
linylmethyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine used therein a molar equivalent amount
of 2-(1-piperidinylmethyl)-5-methyl-6-phenylthio-2,3-di-
hydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(N,N-diethylamino-
methyl)-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 2-aminomethyl-5-methyl-6-phenylthio-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-methylamino-
methyl-5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-5-methyl-6-
phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(1-pyrrolidinylmethyl)-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(1-azetidinylmethyl)-
5-methyl-6-phenylthio-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(4-methyl-1-piperazinylmethyl)-5-methyl-6-phenylthio-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine or 2-[1-(hexahydro-
4H-1,4-diazepinyl)methyl]-5-methyl-6-phenylthio-2,3-dihydro-
pyrrolo(1,2,3-de]-1,4-benzoxazine, there can be obtained,
respectively, 2-(1-piperidinylmethyl)-5-methyl-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)
5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2
CA 02362632 2001-11-19
-48- D.N. 7436A
aminomethyl-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-methylaminomethyl-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-5-methyl-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(1-pyrrolidinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(1-azetidinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-(4-methyl-1-piperazinylmethyl)-5-
methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine or 2-
[1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine.
PREPARATION OF THE FINAL PRODUCTS
Via the Com ounds of Formula VI
Example 1
A. To a suspension of 7.5 g (0.056 mole) of aluminum
chloride in 100 ml of methylene dichloride (MDC) was added,
rapidly with stirring, a solution of 6.2 ml (0.038 mole)
of 4-methoxybenzoyl chloride. When addition was complete
the mixture was stirred for one hour at room temperature
and the resulting solution then added dropwise over a ten
minute period with stirring to a solution of 8.5 g (0.031
mole) of 3-(4-morpholinylmethyl)-5-methyl-2,3-dihydropyrrolo-
[1,2,3-de]-1,4-benzoxazine in 100 ml of MDC. The resulting
mixture was then heated under reflux for thirty minutes,
cooled and poured into 300 ml of ice water with stirring.
The organic solvent was removed in vacuo, and the residual
CA 02362632 2001-11-19
-49- D.N. 7436A
aqueous medium was diluted with ethyl acetate and neutralized
by the addition of saturated aqueous sodium bicarbonate.
The organic layer was separated, dried over magnesium sulfate,
filtered through silica, taken to dryness in vacuo and chroma
tographed on silica gel, eluting with 1:1 hexane: ethyl acetate.
There was thus obtained about 7 g of crude product which
was recrystallized from ethyl acetate/hexane to give 6.0 g
of 3-(4-morpholinylmethyl)-5-methyl-6-(4-methoxybenzoyl)-
2,3-dihydro yrrolo[1,2,3-de]-1,4-benzoxazine, m.p. 182-189°C.
B. Following a procedure similar to that described
in Example 1A, 3-t4-morpholinylmethyl)-5-methyl-6-(1-naphthyl-
carbonyl)-2,3-dihydro yrrolo[1,2,3-de]-1,4-benzoxazine
methanesulfonate (1.4 g), m,p. 257-260°C (from methanol)
was prepared by reaction of 2.5 g (0.009 mole) of 3-(4-morpho-
linylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine
in 50 ml of MDC with 1.9 ml (0.013 mole) of 1-naphthoyl
chloride in 50 ml of MDC in the presence of 2.2 g (0.017
mole) of aluminum chloride and recrystallization of the
product from methanol/diethyl ether. The free base, recrys-
tallized from ethanol showed m.p. 158.0-159.0°C,
C, Following a procedure similar to that described
in Example 1A, 3-(4-morpholinylmethyl)-5-methyl-6-(6-cruinolyl-
carbonyl)-2,3-dihvdropyrrolo(1,2,3-de]-1,4-benzoxazine (3.9 g),
m.p. 192-198°C, was prepared by reaction of 6.9 g (0.025
mole) of 3-(4-morpholinylmethyl)-5-methyl-2,3-dihydropyrrolo-
CA 02362632 2001-11-19
-50- D,N. 7436A
(1,2,3-de]-1,4-benzoxazine in 200 ml of MDC with 11.4 g
(0.05 mole) of 6-quinoline carboxylic acid chloride in 100
ml of MDC in the presence of 10 g (0.075 mole) of aluminum
chloride and recrystallization of the product from ethyl
acetate/hexane.
D, Following a procedure similar to that described
in Example 1A, ~+)-3-(4-morpholinylmethyl)-5-methyl-6-(5.7-
dibromonaphthylcarbonyl)-2.3-dihydropyrrolo(1.2,3-de~-1.4-
benzoxazine methanesulfonate (1.98 g), m.p. 256.0-257.0,
[a]D5 = +38,9°, was prepared by reaction of 4.09 g (0.015
mole) of (+) -3-(4-morpholinylmethyl) -5-methyl-2,3-dihydro-
pyrrolo[1,2,3-de)-1,4-benzoxazine with 5.19 g (0.16 mole)
of 5,7-dibromonaphthylcarboxylic acid chloride in 100 ml
of MDC in the presence of 15 ml of ethyl aluminum chloride
and recrystallization of the product from ethanol.
E, Following a procedure similar to that described
above in Example 1A, (-)-3-(4-morpholinylmethyl)-5-methyl-
6-(5,7-dibromonanhthylcarbonyl)-2.3-dihydro yrrolo[1,2,3-
de)-1.4-benzoxazine methanesulfonate (0.3 g), m.p, 252.0-
253.0, [a)D5 = -35.7.°, was prepared by reaction of 0.63
g (0.002 mole) of (-) -3-(4-morpholinylmethyl) -5-methyl-2,3-
dihydropyrrolo[1,2,3-de)-1,4-benzoxazine with 0.88 g (0.0025
mole) of 5,7-dibromonaphthylcarboxylic acid chloride in
15 ml of MDC in the presence of 2.6 ml of ethyl aluminum
chloride and crystallization of the product from diethyl
ether/ethyl acetate.
CA 02362632 2001-11-19
-51- D,N. 7436A
Following a procedure similar to that described
in Example 1A above, substituting for the 3-(4-morpholinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine
used therein a molar equivalent amount of 3-(1-piperidinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
3-(N,N-diethylaminomethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-(aminomethyl)-5-methyl-
2,3-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-methylaminomethyl-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 8-methoxy-3-(4-thiomorpholinylmethyl)-5-
methyl-2,3-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-(1-pyrrolidinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-(1-azetidinylmethyl)-5-
methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-(4-methyl-1-piperazinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-[1-(hexahydro-4H-1,4-diaza-
pinyl)methyl]-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-
benzoxazine, 8-chloro-3-aminomethyl-5-methyl-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-methylamino-
methyl-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-chloro-3-(4-thiomorpholinylmethyl)-5-methyl-2,3-dihydropyrrolo-
[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinylmethyl)-
5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-
chloro-3-(1-azetidinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-chloro-3-(4-methyl-1-piperazinylmethyl)-
5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine and
CA 02362632 2001-11-19
-52- D,N, 7436A
8-chloro-3-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, there can
be obtained, respectively, 3-(1-piperidinylmethyl)-5-methyl-
6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
3-(N,N-diethylaminomethyl) -5-methyl-6-(4-methoxybenzoyl) -
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-(aminomethyl)-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-3-(methylamino-
methyl)-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 8-methoxy-3-(4-thiomorpholinylmethyl)-
5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 8-methoxy-3-(1-pyrrolidinylmethyl)-5-methyl-
6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-methoxy-3-(1-azetidinylmethyl)-5-methyl-6-(4-methoxybenzoyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-(4-methyl-1-piperazinylmethyl)-5-methyl-6-(4-methoxybenzoyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-methoxy-
3-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-6-(4-
methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
8-chloro-3-aminomethyl-5-methyl-6-(4-methoxybenzoyl)-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-methyl-
aminomethyl-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-
de)-1,4-benzoxazine, 8-chloro-3-(4-thiomorpholinylmethyl)-
5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 8-chloro-3-(1-pyrrolidinylmethyl)-5-methyl-
6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
CA 02362632 2001-11-19
-53- D.N. 7436A
8-chloro-3-(1-azetidinylmethyl)-5-methyl-6-(4-methoxybenzoyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 8-chloro-3-
(4-methyl-1-piperazinylmethyl)-5-methyl-6-(4-methoxybenzoyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine and 8-chloro-
3-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-5-methyl-6-(4-
methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine.
Ether cleavage of each of the foregoing 8-methoxy-
3-CH2N=H-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazines by heating the latter with pyridine
hydrochloride or with aqueous hydrogen bromide affords the
corresponding 8-hydroxy-3-CH2N=B-5-methyl-6-(4-hydroxybenzoyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazines.
Following a procedure similar to that described
in Example 1A above, substituting for the 3-(4-morpholinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine
used therein a molar equivalent amount of 2-(1-piperidinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(N,N-diethylaminomethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-(aminomethyl)-5-methyl-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 2-methylaminomethyl-5-
methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(4-
thiomorpholinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-(1-pyrrolidinylmethyl)-5-methyl-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(1-azetidinyl-
methyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(4-methyl-1-piperazinylmethyl)-5-methyl-2,3-dihydropyrrolo[1,2,3-
CA 02362632 2001-11-19
-54- D.N. 7436A
de]-1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)-
methyl]-5-methyl-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
there can be obtained, respectively, 2-(1-piperidinylmethyl)-
5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-5-methyl-6-(4-
methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(aminomethyl)-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo-
[1,2,3-de]-1,4-benzoxazine, 2-methylaminomethyl-5-methyl-
6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benz-
oxazine, 2-(4-thiomorpholinylmethyl)-5-methyl-6-(4-methoxy-
benzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-
(1-pyrrolidinylmethyl)-5-methyl-6-(4-methoxybenzoyl)-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(1-azetidinyl-
methyl)-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-(4-methyl-1-piperazinylmethyl)-5-
methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-
5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine.
Via the Compounds of Formula IV
Example 2
A. To a suspension of 8.8 g (0.18 mole) of a 50%
dispersion of sodium hydride in hexane was added 1 ml of
ethanol followed by the addition, over a period of five
minutes, of a solution of 30 g (0.2 mole) of 4-methoxyaceto-
phenone in 50 ml of diethyl ether. 50 ml of ethyl propionate
CA 02362632 2001-11-19
-55- D.N. 7436A
was then added rapidly, and the solution was heated under
reflux for three hours and then cooled. An additional 7 g
of sodium hydride dispersion was added, the reaction was
allowed to subside, and the mixture was then cooled and
diluted with 500 ml of water with stirring. The solid which
separated was collected by filtration, washed with water,
then with diethyl ether and dried in a vacuum oven at 60°C
to give 18.3 g of 5-(4-methoxyphenyl)-3.5-pentanedione as
the sodium salt. -
A solution of 22.2 g (0.089 mole) of 3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine and
18.3 g (0.08 mole) of the sodium salt of 5-(4-methoxyphenyl) -
3,5-pentanedione in 250 ml of glacial acetic acid was heated
under reflux for four hours, then cooled and poured into
1500 ml of water and 600 ml of ethyl acetate. The mixture
was neutralized by the addition of solid sodium carbonate
and solid sodium bicarbonate with stirring, the organic
layer was separated from the aqueous layer and dried over
magnesium sulfate, filtered and taken to dryness. The crude
product was purified by chromatography on silica gel, eluting
with 4:1 hexane/ethyl acetate, the first 700 ml of eluate
being discarded, and the next 800 ml, containing the product,
being retained. Evaporation of the latter to dryness and
recrystallization of the residue from ethyl acetate afforded
3.9 g of 3-(4-morpholinylmethyl)-5-ethyl-6-(4-methoxybenzoyl)-
2.3-dihydropyrrolo[1,2,3-de]-1.4-benzoxazine, m.p. 185.5-
187.0°C.
CA 02362632 2001-11-19 ,
-56- D.N. 7436A
H. Following a procedure similar to that described
in Example 2A above, ~+)-3-(4-morpholinylmethyl)-5-methvl-
6-(1-naphthvlcarbonyl)-2,3-dihvdropvrrolo~1,2,3-de]-1,4-
benzoxazine methariesulfonate (11.6 g from methanol/diethyl
ether), m.p. 256-259°C, [a]D5 = +40.2° (1% in DMF) was pre-
pared by reaction of 15.5 g (0.062 mole) of (+)-3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine with
15.8 g (0.075 mole) of 4-(1-naphthyl)-2,4-butanedione in
500 ml of toluene in the presence of a catalytic amount
of pyridine 3-nitrobenzenesulfonate (the 2,4-diketone being
prepared by reaction of methyl 1-naphthyl ketone with ethyl
acetate in the presence of sodium hxdride and ethanol in
diethyl ether), followed by cyclization of the resulting
hydrazone by refluxing the latter in 300 ml of glacial acetic
acid and recrystallization of the product from methanol/
diethyl ether.
Similarly (-) -3-(4-mor holinylmethyl) -5-methyl-
6-(1-naphthylcarbonyl)-2,3-dihydro vrrolo[1,2,3-de]-1,4-
benzoxazine methanesulfonate (7.2 g), m.p. 256-260°C, [a]p5 -
-39.4° (1% in DMF) was prepared by reaction of 9.3 g (0.037
mole) of (-)3-(4-morpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine with 9.5 g (0.045 mole) of 4-(1-naphthyl)-
2,4-butanedione in toluene in the presence of a catalytic
amount of pyridine 3-nitrobenzenesulfonate followed by cycliz-
ation of the resulting hydrazone by refluxing in glacial
acetic acid and recrystallization of the product from methanol/
diethyl ether.
CA 02362632 2001-11-19
-57- D.N. 7436A
C. 3-(4-mor holinylmethyl)-6-(4-methoxybenzoyl)-2~3-
dihydro yrrolo[1.2.3-de]-1.4-benzoxazine, m.p. 209-214°C
(1.5 g from ethyl acetate) was prepared by reaction of 13 g
(0.052 mole) of 3-(4-morpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine with 10.2 g (0.057 mole) g-(4-methoxy-
phenyl)-S-ketopropionaldehyde in glacial acetic acid (the
S-ketopropionaldehyde being prepared by reaction of 4-methoxy-
acetophenone with methyl formate in diethyl ether in the
presence of ethanol and sodium hydride).
D. 3-(4-morpholinylmethyl)-5-methyl-6-(2-fluorobenzoyl)-
213-dihydropyrrolo[1,2.3-de]-1.4-benzoxazine methanesulfonate,
m.p. 241-245°C (3.2 g from methanol/diethyl ether) prepared
by reaction of 11 g (0.044 mole) of 3-(4-morpholinylmethyl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine with 8.7 g (0.048
mole) of 4-(2-fluorophenyl)-2,4-butanedione in toluene in
the presence of a catalytic amount of acid (the 2,4-diketone
being prepared by reaction of 2-fluoroacetophenone with
ethyl acetate in diethyl ether in the presence of ethanol
and sodium hydride).
E. 3-(4-morpholinylmethyl)-5-methyl-6-(4-bromo-1-
naphthylcarbonyl)-2.3-dihydropyrrolo[1.2,3-de]-1,4-benzoxazine
methanesulfonate, m.p. 281-286°C (2.0 g from methanol/diethyl
ether) prepared by reaction of 9.5 g (0.038 mole) of 3-(4-
morpholinylmethyl)-4-amino-3.4-dihydro-2H-1,4-benzoxazine
with 12.2 g (0.042 mole) of 4-(4-bromo-1-naphthyl)-2,4-butanedione
in toluene in the presence of a catalytic amount of pyridine
CA 02362632 2001-11-19
-58- D.N. 7436A
toluenesulfonate (the 2,4-diketone being prepared by reaction
of 1-acetyl-4-bromonaphthalene with ethyl acetate in diethyl
ether in the presence of ethanol and sodium hydride) followed
by cyclization of'the resulting hydrazone by refluxing in
glacial acetic acid.
F. 3-(4-morpholinylmethyl) -6-~1-naphthylcarbonyl) -
2,3-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, m.p. 190-193°C
(2.6 g from ethyl acetate) prepared by reaction of 10.7 g
(0.04 mole) of 3-(4-morpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine with 9.4 g (0.048 mole) of s-(1-naphthyl)-
S-ketopropionaldehyde in toluene in the presence of a catalytic
amount of pyridine toluenesulfonate (the S-ketopropional-
dehyde being prepared by reaction of 1-acetylnaphthalene
with methyl formate in diethyl ether in the presence of
ethanol and sodium hydride) followed by cyclization of the
resulting hydrazone by refluxing in glacial acetic acid.
G. (+)-3-(4-morpholinylmethyl)-5-methyl-6-(5,7-dibromo-
1-naphthylcarbonyl)-2,3-dihvdropvrrolo~1,2,3-del-1,4-benzoxazine
m.p. 120°C (239 mg), [a]D5 = +44.4° (CHC13), prepared by
reaction of 1.4 g (0.0056 mole) of (+) -3-(4-morpholinylmethyl) -
4-amino-3,4-dihydro-2H-1,4-benzoxazine with 1.9 g (0.0051
mole) of 4-(5,7-dibromo-1-naphthyl)-2,4-butanedione in toluene
in the presence of a catalytic amount of pyridine 3-nitro-
benzenesulfonate (the diketone being prepared by reaction
of 1-acetyl-5,7-dibromonaphthalene with ethyl acetate in
diethyl ether in the presence of ethanol and sodium hydride)
followed by cyclization of the resulting hydrazone in refluxing
glacial acetic acid.
CA 02362632 2001-11-19
-59- D.N. 7436A
The 1-acetyl-5,7-dibromonaphthalene used in the
foregoing procedure was prepared by dibromination of benz[cd]-
indol-2(1H)-one with bromine in glacial acetic acid to give
41 g of 6~8 -dibromobenz[cd)indol-2(1H)-one, m. p. 259-260°C.
The latter (12.8 g) was heated under reflux for one hour
in two liters of 5% potassium hydroxide, the reaction mixture
was cooled, filtered and then treated with 2.8 g of a solution
of sodium nitrite in 50 ml of water. The resulting solution,
cooled to 0°C, was added dropwise to 800 ml of a 40% solution
of sulfuric acid while maintaining the temperature at -5°C.
The reaction mixture was stirred for one hour, then poured
slowly into a solution of 140 g of sodium hypophosphite
in 800 ml of water at 0°C with stirring. The solid which
separated was collected by filtration and dissolved in 3
liters of 2% sodium carbonate solution, and the resulting
solution was filtered, acidified with concentrated hydro-
chloric acid and the mixture extracted with ethyl acetate.
The combined organic extracts were dried and taken to dry-
ness to give 8 g of 5,7-dibromo-1-naphthalenecarboxylic
acid which was heated with thionyl chloride in the presence
of a small amount of DMF. The resulting crude 5,7-dibromo-
1-naphthalenecarboxylic acid chloride, together with 6.0
g of methoxymethylamine hydrochloride in 750 ml of MDC,
was treated dropwise at 0°C with 60 ml of triethylamine
in 250 ml of MDC. The reaction mixture was filtered, the
filtrate was taken to dryness, and the resulting residue
CA 02362632 2001-11-19
-60- D.N. 7436A
(12.0 g) was dissolved in THF and treated at 0°C with stirring
with a solution of 29.5 ml of 3M methyl magnesium bromide.
The reaction mixture was stirred for ten minutes, quenched
with water, taken-to dryness and the residue partitioned
between ethyl acetate and water. The mixture was filtered,
the organic extracts were separated, dried and taken to
dryness to give 9.9 g of 1-acetyl-5.7-dibromona hthalene.
H~ (-? -3-(4-morpholinylmethyl) -5-methyl-6-(4-methoxybenzoyl) -
2,3-dihydropyrrolo[1,23-de]-1.4-benzoxazine, m.p. 149-150°C
(3.7 g from diethyl ether), [a]25 = -53.8°
D ( 1% in CHC13 ) ,
prepared by reaction of 5.8 g (0.023 mole) of (-) -3-(4-morpho-
linylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine with
5.0 g (0.026 mole) of 4-(4-methoxyphenyl)-2,4-butanedione
in toluene in the presence of a catalytic amount of pyridine
3-nitrobenzenesulfonate (the diketone being prepared by
reaction of 4-methoxyacetophenone with ethyl acetate in
diethyl ether in the presence of ethanol and sodium hydride)
followed by cyclization of the resulting hydrazone in refluxing
glacial acetic acid.
Similarly (~) -3-(4-mor holinylmethvl) -5-methvl-
6-(4-methoxvbenzoyl)-2,3-dihvdropvrrolo(1,2.3-de]-1.4-benzoxazine,
m.p. 152-153°C (3.88 g from diethyl ether), [a]D5 = +55.1°
(1% in CHC13), was prepared by reaction of 5.84 g (0.023
mole) of (+)-3-(4-morpholinylmethyl)-4-amino-3,4-dihydro-
2H-1,4-benzoxazine with 5.0 g (0.026 mole) of 4-(4-methoxy-
phenyl)-2,4-butanedione in toluene in the presence of a
catalytic amount of pyridine 3-nitrobenzenesulfonate followed
by cyclization of the resulting hydrazone in refluxing glacial
acetic acid.
CA 02362632 2001-11-19
-61- D.N. 7436A
I. 2-(4-morpholinylmethyl)-5-methyl-6-(1-na hthylcarbonvl)-
213-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, m.p. 158-159°C
(2.1 g from ethanol), prepared by reaction of 4.6 g (0.019
mole) of 2-(4-morpholinylmethyl)-4-amino-3,4-dihydro-2H-
1,4-benzoxazine with 4.0 g (0.019 mole) of 4-(1-naphthyl)-
2,4-butanedione in 50 ml of glacial acetic acid.
Proceeding in a manner similar to that described
in Example 2A above, substituting for the 5-(4-methoxyphenyl)-
3,5-pentanedione used therein a molar equivalent amount
of 4-(4-methylphenyl)-2,4-butanedione, 4-(4-methylmercapto-
phenyl)-2,4-butanedione, 4-(4-methylsulfinylphenyl)-2,4-
butanedione, 4-(4-methylsulfonylphenyl)-2,4-butanedione,
4-(3,4-methylenedioxyphenyl)-2,4-butanedione, 4-(2-naphthyl)-
2,4-butanedione, 4-(7-methyl-2-naphthyl)-2,4-butanedione,
4-(7-methoxy-2-naphthyl)-2,4-butanedione, 4-(7-methylmercapto-
2-naphthyl)-2,4-butanediane, 4-(7-methylsulfinyl-2-naphthyl)-
2,4-butanedione, 4-(7-methylsulfonyl-2-naphthyl)-2,4-butane-
dione, 4-(2-quinolyl)-2,4-butanedione, 4-(3-quinolyl)-2,4-
butanedione, 4-(4-quinolyl)-2,4-butanedione, 4-(5-quinolyl)-
2,4-butanedione, 4-(7-quinolyl)-2,4-butanedione or 4-(8-
quinolyl)-2,4-butanedione, there can be obtained, respectively,
3-(4-morpholinylmethyl)-5-methyl-6-(4-methylbenzoyl)-2,3-
dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, 3-(4-morpholinyl-
methyl)-5-methyl-6-(4-methylmercaptobenzoyl)-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpholinylmethyl)-
5-methyl-6-(4-methylsulfinylbenzoyl)-2,3-dihydropyrrolo[1,2,3-
CA 02362632 2001-11-19
-62- D.N. 7436A
de]-1,4-benzoxazine, 3-(4-morpholinylmethyl)-5-methyl-6-
(4-methylsulfonylbenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-
1,4-benzoxazine, 3-(4-morpholinylmethyl)-5-methyl-6-(3,4-
methylenedioxybenzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-
benzoxazine, 3-(4-morpholinylmethyl)-5-methyl-6-(2-naphthyl-
carbonyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
3-(4-morpholinylmethyl)-5-methyl-6-(7-methyl-2-naphthylcarbonyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpho-
linylmethyl)-5-methyl-6-(7-methoxy-2-naphthylcarbonyl)-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpholinyl-
methyl)-5-methyl-6-(7-methylmercapto-2-naphthylcarbonyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpho-
linylmethyl)-5-methyl-6-(7-methylsulfinyl-2-naphthylcarbonyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpho-
linylmethyl)-5-methyl-6-(7-methylsulfonyl-2-naphthylcarbonyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpho-
linylmethyl)-5-methyl-6-(2-quinolylcarbonyl)-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpholinylmethyl)-
5-methyl-6-(3-quinolylcarbonyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 3-(4-morpholinylmethyl)-5-methyl-6-
(4-quinolylcarbonyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
3-(4-morpholinylmethyl)-5-methyl-6-(5-quinolylcarbonyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 3-(4-morpho-
linylmethyl)-5-methyl-6-(7-quinolylcarbonyl)-2,3-dihydro- .
pyrrolo[1,2,3-de]-1,4-benzoxazine and 3-(4-morpholinylmethyl)-
5-methyl-6-(8-quinolylcarbonyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine.
CA 02362632 2001-11-19
-63- D.N. 7436A
Ether cleavage of the foregoing 3-(4-morpholinyl-
methyl)-5-methyl-6-(4-methoxybenzoyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine and 3-(4-morpholinylmethyl)-5-methyl-
6-(7-methoxy-2-naphthylcarbonyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine by heating the latter with pyridine
hydrochloride or with aqueous hydrogen bromide affords,
respectively, 3-(4-morpholinylmethyl)-5-methyl-6-(4-hydroxy-
benzoyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine and
3-(4-morpholinylmethyl)-5-methyl-6-(7-hydroxy-2-naphthylcarbonyl)-
2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine.
Proceeding in a manner similar to that described
in Example 2A above, substituting for the 3-(4-morpholinyl-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine used therein
a molar equivalent amount of 2-(1-piperidinylmethyl)-4-amino-
3,4-dihydro-2H-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-aminomethyl-4-
amino-3,4-dihydro-2H-1,4-benzoxazine, 2-methylaminomethyl-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(4-thiomorpholinyl-
methyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-pyrroli-
dinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine, 2-(1-
azetidinylmethyl)-4-amino-3,4-dihydro-2H-1,4-benzoxazine,
2-(4-methyl-1-piperazinylmethyl)-4-amino-3,4-dihydro-2H-
1,4-benzoxazine or 2-[1-(hexahydro-4H-1,4-diazepinyl)methyl]-
4-amino-3,4-dihydro-2H-1,4-benzoxazine, and substituting
for the 5-(4-methoxyphenyl)-3,5-pentanedione used therein
CA 02362632 2001-11-19
-64- D.N. 7436A
a molar equivalent amount of 4-(1-naphthyl)-2,4-butanedione,
there can be obtained, respectively, 2-(1-piperidinylmethyl)-
5-methyl-6-(1-naphthylcarbonyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-(N,N-diethylaminomethyl)-5-methyl-
6-(1-naphthylcarbonyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-
benzoxazine, 2-(aminomethyl)-5-methyl-6-(1-naphthylcarbonyl)-
2,3-dihydropyrrolo(1,2,3-de]-1,4-benzoxazine, 2-(methylamino-
methyl)-5-methyl-6-(1-naphthylcarbonyl)-2,3-dihydropyrrolo[1,2,3-
de]-1,4-benzoxazine, 2-(4-thiomorpholinylmethyl)-5-methyl-
6-(1-naphthylcarbonyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-
benzoxazine, 2-(1-pyrrolidinylmethyl)-5-methyl-6-(1-naphthyl-
carbonyl)-2,3-dihydropyrrolo[1,2,3-de]-1,4-benzoxazine,
2-(1-azetidinylmethyl)-5-methyl-6-(1-naphthylcarbonyl)-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine, 2-(4-methyl-1-
piperazinylmethyl)-5-methyl-6-(1-naphthylcarbonyl)-2,3-dihydro-
pyrrolo[1,2,3-de]-1,4-benzoxazine or 2-[1-(hexahydro-4H-
1,4-diazepinyl)methyl]-5-methyl-6-(1-naphthylcarbonyl)-2,3-
dihydropyrrolo[1,2,3-de]-1,4-benzoxazine.
BIOLOGICAL TEST RESULTS
Data obtained with the compounds of the invention
in the acetylcholine-induced abdominal constriction test
(ACH) (expressed as the ED50 in mg/kg or as the percent
inhibition at a given dose level), the anti-bradykinin test
(BDK) (expressed as the ED50 or as the percent inhibition
at a given dose level), the acetic acid-induced writhing
CA 02362632 2001-11-19
-65- D.N. 7436A
assay in the rat (RW) (expressed as the ED50 or the percent
inhibition at a given dose level), the inhibition of cyclo-
oxygenase assay (CO) (expressed as the percent inhibition
at a g iven dose level, e. g. 19 % I at 30 ~M) and the Randall-
Selitto (RS) paw pressure test (expressed as the minimum
effective dose, MED, in mg/kg ED50) are given in the table
below. Negative values in the cyclooxygenase inhibition
assay indicate increases in cyclooxygenase activity over
controls that have not been demonstrated to differ signifi-
cantly from controls. In the acetylcholine-induced abdominal
constriction test, the test compounds were administered
either as suspensions in gum tragacanth (GT) or as aqueous
solutions prepared by addition of just sufficient dilute
aqueous methanesulfonic acid (MS) or dilute aqueous lactic
acid (LA) to dissolve the compound. The compounds are identified
by the example number above where their preparations are
described. All data in the anti-bradykinin, rat writhing
and Randall-Selitto tests were obtained on oral administra-
tion.
CA 02362632 2001-11-19
-66- D.N. 7436A
M
cn o
i o
x .
0
N !~
H
dP dP
dP V~ N dP
dP dp 01
.
r1 M \ O 10
O
\ ~ \ N \
~' F
o 3 o 3
' .
o o
.-i 0
M O
O M
r-1 M
O
O O O
O r-I O
r3M \ ~O tl1 r-1 1!1
\ da. . \
dP 1pO !~ dP M
M N
N O r1
O
CaO O
~.M
Qi\ ~1
trldP M
O
..
E~
O (,9
O ...
U
\ o
dP
0
M r
-11
n \
dP
O
..
... .. .-. ~ .-.
.1,' ~ ~
~i
'~ p v v v v a
O cn~ v v
O O n M o o
~ ~ o o
o
oo o \ o~, c \ \ p
\ \ ~ M ~ \ \
\ ~
do 0P dP ~ dP dP dP dP \
dP O O \ O dP
O dp O O O
O r,.~ tn O
O
N M M1 O N M M M
M r1
tJ1 M
, M r-1.
~I' N N a!1\ \ O CO
C1 O
O \ dP dP\ .
,~ p dP O O dP e-1 M N
M
O O e-ierO
M N
ri
I~ ~ ~ N 'C ~ U ~ 'C U L1 W W
W ri N N N N N
-r
~1 O vf1 O
r-i r1 N
CA 02362632 2001-11-19
-67- D.N, 7436A
Data obtained-on the compounds of the invention
in the mouse vas deferens adrenergic transmission test (MVD)
expressed as the IC50 in ~M (the inhibitory concentration
which produces 50% of the maximum response) or as the percent
inhibition at a given dose level are given in the table
below. As before, the compounds are identified by the example
number above where their preparations are described.
Ex. MVD
1A 0.123 0.013
1B (base) 0.004 0.002
1B (CH3S03H) 0.006 0.0006
1C 0.021 0.00065
1E 2.62 t 1.7
2A >10 (27%/lO~tM)
2B(d) 0.00043 t 0.0001
2B(1) >10 (37%/10~M)
2C 0.044 t 0.0098
2D 0.076 + 0.004
2E 0.0022 t 0.0002
2F 0.002 0.00003
2G 0.04 0.0054
2H(1) >10 (44%/104tM)
2H(d) 0.044 0.013
2I 0.004 t 0.002