Note: Descriptions are shown in the official language in which they were submitted.
CA 02363031 2005-06-21
IMPROVED METHODS FOR LEACHING ORES
Background of the Invention
Oxides of cobalt (Co), nickel (Ni), titanium (Ti), copper (Cu), molybdenum
(Mo), lead
(Pb), zinc (Zn), gold (Au), and silver (Ag) are important minerals. Various
methods exist for
recovering these compounds from the ores where they are found. For example,
autoclave
methods are often used to recover Co, Ni and Ti oxides. These methods are
capital and labor
intensive. Mo oxide has been leached by hydrochloric acid methods. Cyanide,
thiosulfate,
thiourea and halides are used in leaching Au and Ag metals and oxides. Cu, Zn
and Pb can
be leached with sulfuric acid.
Rutile (Ti02) is a mineral used for many purposes. Amongst other uses, it is a
source
of titanium metal and a paint pigment. Synthetic rutile is generally
considered as any rutile
created from another mineral, usually ilmenite, that has at least 90% Ti02.
High purity ruble
is 99.9%+Ti02. High purity rutile generally carries a commercial value
premium.
Ilmenite (FeTi03) is most often converted to synthetic rutile by high
temperature
leaching with hydrochloric acid in an autoclave. Leaching temperatures are
generally between
800 to 900°C. Ferric chloride is sometimes used in these autoclave
leaches to increase the
reaction rates at the lower temperatures.
Zoumei Jin et al. (B. Mishra and G. J. Kiporous eds, In: Titanium Extraction
and
Processing, The Mineral, Metals & Materials Society (1997) pg 122-128)
reported that 4 to 6
Normal hydrochloric acid at 110 to 140° C., will dissolve the iron (Fe)
from Ilmenite from the
Sichuan province of China in 6 hours. They found that the reaction rate is 0.4
order with
respect to initial Fe+Z concentration. They postulate a surface reaction
control model with an
apparent activation energy of 56.97 kilojoules per mol.
Conventional autoclave technology is capital, maintenance and energy
intensive. The
process disclosed in Zoumei Jin et al. process involves the use of large
amounts of
hydrochloric acid, which is expensive, difficult to handle and requires
special stainless steel
equipment. There is a clear need for more efficient processes for leaching of
ores to obtain
valuable minerals.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
2
Cyanide is the most commonly used leachant for gold. Two molecules of cyanide
complex with every molecule of gold. Copper also complexes with cyanide, but
it takes 4
molecules of cyanide for every copper molecule. Copper is often present in
copper gold ores
in the one tenth to one percent range. Gold in these ores is in the one to 10
parts per million
range. The copper consumes so much cyanide it needs to be recovered by
hydrogen cyanide
distillation, an expensive and dangerous operation. Systems have been proposed
where sulfuric
acid is used to leach the copper first. Then, the heap or batch is
neutralized. Cyanide can then
be used to leach the gold. The problem, of course, is the expense of
neutralization. In heap
operations, the additional worry of incomplete neutralization is present.
In other ores, the gangue, or unwanted material, can be an acid consumer.
Copper oxide
in limestone rich rock is an example.
Brief Summarv of the Invention
The subject invention pertains to novel and highly efficient methods for
leaching
valuable minerals, such as cobalt (Co), nickel (Ni), titanium (Ti), copper
(Cu), molybdenum
(Mo), lead (Pb), zinc (Zn), gold (Au), and silver (Ag) from ores.
One aspect of the present invention concerns methods for recovering titanium
from ores.
One embodiment of the subject method uses an acidic solution, such as sulfuric
acid, to leach
titanium oxides from a mineral feed. Additional modifications and/or steps,
including, for
example, grinding of the ore, addition of an alkali metal halide, addition of
a carbon source, and
adjustment of pressure and/or temperature, can be incorporated in the process.
In a preferred
embodiment, a mineral feed is contacted with an acid and an alkali metal
halide to leach titanium
oxides from the feed. High purity titanium dioxide having a commercial premium
over synthetic
ruble can be produced using the methods of the subject invention.
Another aspect of the present invention concerns methods for recovering
transition
metals other than titanium from ores. In one embodiment, the present invention
provides a
method for recovery of nickel and cobalt from a mineral feed by leaching the
feed with an acidic
solution. In an exemplified embodiment, a mixture of sulfuric acid and an
alkali metal halide
are used to leach out cobalt and nickel from a laterite ore. The subject
methods can also be used
to recover cobalt, nickel, copper, etc. by leaching these elements from scrap
metal.
The subject invention also concerns methods for recovering multiple metals or
metal
oxides in separate solutions. In one embodiment, ore is contacted with an acid
solution, such
as sulfuric acid. Solid residue is then collected and contacted with an alkali
metal halide and
acid solution. In an exemplified embodiment, the subject method is used to
recover copper
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
separately from gold and silver. The copper is recovered primarily in the
first acid solution,
while the gold and silver are recovered in the alkali metal halide and acid
solution.
Brief Description of the Drawings
Figure 1 shows the kinetics of leaching titanium and iron from ilmenite.
Figure 2 shows the results of four consecutive one-hour leaches of titanium
and iron
from ilmenite.
Figure 3 shows pulp density relationships in the leaching of titanium and iron
from
ilmenite.
Figure 4 shows the results of experiments evaluating the effect of an alkali
metal halide
(NaCI) on the sulfuric acid leaching process.
Figure 5 shows the results of experiments evaluating the effects of grinding
the ore on
recovery rates.
Figure 6 shows the results of experiments evaluating the effect of adding a
carbon
source during the sulfuric acid leaching process.
Figure 7 shows the results of experiments evaluating the effect of an alkali
metal halide
on the sulfuric acid leaching process of leaching nickel from an initial
laterite feed (Laterite-1).
Figure 8 shows the results of experiments evaluating the effect of an alkali
metal halide
on the sulfuric acid leaching process of leaching cobalt from an initial
laterite feed (Laterite-1).
Figure 9 shows the results of experiments evaluating the effect of an alkali
metal halide
on the sulfuric acid leaching process of leaching nickel from a second
laterite feed (Laterite-2).
Figure 10 shows the results of experiments evaluating the effects of an alkali
metal
halide on the sulfuric acid leaching process of leaching cobalt from a second
laterite feed
(Laterite-2).
Detailed Disclosure of the Invention
The subject invention provides novel materials and methods useful for the
recovery of
minerals from ores. An important component of the leaching processes of the
subject invention
is the use of an acidic solution. In one embodiment, the acid is sulfuric
acid. Sulfuric acid used
in the leaching procedures can be at a concentration ranging from about 20
grams per liter to
about 500 grams per liter. In a preferred embodiment, the concentration of
sulfuric acid ranges
from about 150 grams per liter to about 250 grams per liter. Preferably, the
concentration of
sulfuric acid is approximately 200 grams per liter.
In addition to using sulfuric acid solutions in the leaching processes of the
subject
invention, particularly preferred embodiments of the subject invention utilize
additional factors
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
4
including, for example, the use of an alkali metal halide, grinding the ore,
addition of a carbon
source, and/or adjustment of the temperature at which the process is carried
out.
In accordance with the subject invention, the efficiency of the leaching
process can be
improved by grinding the ore prior to treatment. In a preferred embodiment,
the ore is ground
so that it can pass through a 200 mesh sieve.
In a further embodiment, an alkali metal salt can be added to the leach
solution to
improve recovery. The alkali metal salt can be for example, an alkali metal
halide, alkali metal
nitrite, alkali metal nitrate, alkali metal sulfite or alkali metal thionite.
The metal halide can be,
for example, NaCI, KCI, NaBr or KBr, or mixtures of one or more of these. The
metal sulfites
can be, for example, sodium sulfite, sodium metabisulfite, sodium bisulfate,
sodium dithionite,
or other alkali metal or ammonium sulfite, metabisulfite, bisulfite or
dithionite. The ordinarily
skilled artisan, having the benefit of the teachings disclosed herein, can
readily determine those
alkali metal salts that can be used in conjunction with the particular acid
solution used in the
solubilization step of the process.
A further embodiment of the subject invention involves the use of a carbon
source to
improve recovery. The carbon source can be, for example, graphite or activated
carbon. The
source of this material can be, for example, from coconut shell or wood.
The present invention accordingly provides in one embodiment a method for
recovering
titanium oxides) from a titanium and iron-containing mineral feed, the method
including the
steps of:
(a) solubilizing titanium and iron by leaching the feed with an acidic
solution in the
presence of an alkali metal halide;
(b) selectively precipitating titanium oxide(s), and
(c) recovering titanium oxide(s).
Typically, the titanium oxides) may be titanium dioxide.
The titanium-containing mineral feed is typically post heavy mineral
concentration
products. The feed will include titanium mineralization. Typical examples of
this titanium
mineralization are ilmenite (FeTi03), leucoxene, perovskite (CaTi03 ) and
titano magnetite.
The feed may in an alternative embodiment comprise a bulk ilmenite
concentrate. Other
titanium-containing mineral feed materials are contemplated within the scope
of the invention.
The present invention provides in another separate embodiment a method for
recovering
synthetic ruble (TiOz), from a mineral feed comprising ilmenite (FeTi03), the
method including
the steps of:
(a) solubilizing titanium and iron by leaching the ilmenite with an acidic
solution
in the presence of an alkali metal halide;
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
S
(b) selectively precipitating titanium oxide(s), and
(c) recovering titanium oxide as Ti02.
In step (a) of the method, the acidic solution preferably includes sulfuric
acid. The sulfuric acid
used in the leaching step is typically at a concentration in the range of from
about 20 grams per
liter to about 500 grams per liter. In a preferred embodiment, the
concentration of sulfuric acid
is in the range of from about 150 grams per liter to about 250 grams per
liter. Most preferably
the concentration of sulfuric acid is about 200 grams per liter. Other acids
contemplated for use
in step (a) of the present invention include, but are not limited to, a halide
acid such as
hydrochloric acid or hydrobromic acid. The typical concentration of halide
acid used is in the
range of from about 150 to about 350 grams per liter.
Step (a) is typically carried out in the presence of an alkali metal halide at
a ratio of
alkali metal halide to ilmenite in the feed in the range of from about 1:1 to
2:1. Preferably, the
ratio of alkali metal halide is from about 1:1 to 1.5:1. More preferably, the
ratio is about 1.2:1.
Suitable alkali metal halides include, but are not limited to, NaCI, KCl or
KBr or mixtures of one
or more of these.
In the methods of the present invention, the alkali metal halide can be added
directly to
the leach solution. Alternatively, the alkali metal halide can be combined
with the feed prior to
introduction of the leaching solution. In this case, the feed may be subjected
to a boildown
pretreatment (i.e., by boiling down to approximate dryness) in the presence of
the alkali metal
halide whereby the feed (e.g., ilmenite surfaces) are coated with alkali metal
halide prior to
leaching. Optionally, a combination of the foregoing, i.e., direct addition of
alkali metal halide
to the feed and combination of alkali metal halide with the feed prior to
leaching, can be used
in the subject methods. Thus, for example, a proportion of the alkali metal
halide is combined
with the feed prior to step (a) and a proportion of the alkali metal halide is
added directly to the
leach solution. Typically, steps (a) and (b) may be conducted simultaneously
or separately once
solubilization commences. It is particularly preferred to concurrently remove
some of the
pregnant solution from the leach residue to permit precipitation to take place
away from the
leach residue. In this way, the precipitate may be restricted from coating the
leach residue which
could potentially decrease the efficiency of the process.
In one embodiment, the precipitation step (b) can be regulated by adjustment
of
temperature and/or pH of the solution. Typically, step (a) is carried out at a
temperature in the
range of from about 80°C to about 120°C and, preferably, is in
the range of from about 90°C
to about 110°C. In a preferred embodiment, the operating temperature
for step (a) is about
100°C.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
6
In one embodiment, the leach solution in step (a) has a solids content of up
to about 60%
by weight. Preferably, the leach solution has a solids content of from about
10% to about 40%.
To facilitate more rapid leaching, the feed may be ground into finer
particles. In a
preferred embodiment, the feed may be subjected to fine grinding. Preferably,
the majority of
particles in the feed are capable of passing through a 75 micron sieve after
grinding.
Optionally, a source of carbon may be provided in the subject method. The
carbon may
be in the form of any commercially available carbon source including, for
example, activated
carbon, coal, coke, charcoal or graphite. A preferred source of carbon is
activated carbon
derived from coconut shell. The ratio of carbon to feed (e.g., ilmenite) is
typically between
about 0.01:1 to 1:1.
Methods according to the present invention may be carried out at or above
atmospheric
pressure. When elevated pressures are used, the typical elevated pressures and
temperatures at
which the present methods may be performed are in the range of from about 1
bar to about 30
bar. Preferably, pressures are in the range of from about 1 bar to about 5
bar. Temperatures
used in the subject methods range from about 100°C to about
235°C. Preferably temperatures
range from about 100°C to about 150°C.
The leach residue produced from step (a) can be subjected to further leaching
to
solubilize undissolved iron and/or titanium in the residue. The further
leaching can be
performed using fresh acidic solution. In an alternative embodiment, spent
leach liquor or a
combination of fresh acidic solution and spent leach liquor, can be used.
In another embodiment, step (a) of the subject method can be perfornied in the
presence
of ferrous and/or fernc ions to promote dissolution of the iron
mineralization. Ferrous ions will
generally be present in recirculated process plant solutions.
If desired, iron may be removed from the leachant solution using standard
techniques,
such as precipitation. The purpose is to remove soluble iron from any process
solutions. Solvent
extraction, ion exchange, reverse osmosis or other techniques can also be used
to remove soluble
iron.
The leach time for this embodiment is generally relatively long, and typically
is in the
range of from about SO to about 120 hours. Preferably, leach time is from
about 60 to about 100
hours. However, the operating conditions are much milder than conventional
autoclave
techniques, leading to large capital and operating cost advantages. Sulfuric
acid and alkali metal
halides are easier to handle than the hydrochloric acid used in the Zoumei Jin
et al. process
referred to above.
The present invention provides in another separate embodiment, a method for
recovering
titanium from a titanium and iron-containing mineral feed, the method
including the steps of
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
7
(a) solubilizing titanium and iron by leaching the feed with an acidic
solution in the
presence of an alkali metal halide and a source of activated carbon;
(b) selectively precipitating titanium oxide(s), and
(c) recovering titanium oxides) from the leach residue.
The present invention provides in another separate embodiment a method for
recovering
titanium from a mineral feed comprising ilmenite (FeTi03), the method
including the steps of
(a) solubilizing titanium and iron in the ilmenite by leaching the ilmenite
with an
acidic solution in the presence of an alkali metal halide and a source of
activated carbon;
(b) selectively precipitating titanium oxide(s), and
(c) recovering titanium oxide from the leach residue as TiO~.
The present invention provides in another separate embodiment a method for
recovering
titanium oxides) from a mineral feed comprising ilmenite (FeTi03), the method
including the steps of
(a) leaching the ilmenite with an acidic solution at a temperature in the
range of
from about 80 to 120°C in the presence of an alkali metal halide for a
predetermined time, the leach solution containing up to about 60% by weight
solids to produce a leachant solution containing iron and titanium ions;
(b) separating the iron from the titanium in the leachant solution; and
(c) recovering the separated titanium as TiO,.
As mentioned above, maintaining the titanium in solution rather than allowing
it to
report to the residue as a precipitate has been observed to further enhance
the likelihood of the
titanium being recovered as a pure product. Where most of the titanium reports
to the residue,
other materials that may be found in proximity with the ilmenite mineral
including chromite,
lime, magnesia, silica or silicates, manganese, alumina, vanadium, phosphate
and zirconium will
also tend to remain in the residue along with undissolved iron. The presence
of such materials
is likely to dilute the purity of titanium recoverable from the residue.
Depending on the metals content of the leach solution, a typical reaction time
for step
(a) of this embodiment is up to about an hour. Preferably, the reaction time
of step (a) is up to
about half an hour. More preferably, the reaction time is in the range of from
about 5 to about
15 minutes. It has been observed that titanium solubility reaches a peak
during reaction times
of approximately that length.
Optionally, step (a) above may be repeated to solubilize unleached titanium in
the
residue obtained following step (a) in order to obtain cumulative maximum
solubility of
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
8
titanium. Fresh acidic solution and alkali metal halide can be used when step
(a) is repeated.
Step (a) may in one embodiment comprise a type of countercurrent leach
circuit.
The acidic solution in this embodiment can be supplemented with hydrochloric
acid in
one or more steps of a repeated leach sequence to assist in enhancing the
titanium solubility
profile.
In another separate embodiment the present invention provides a method for
recovering
titanium from a titanium and iron-containing mineral feed, the method
including the steps o~
(a) contacting the feed material with an halide acid solution or an acid -
alkali
halide solution for a period of time sufficient to solubilize the titanium but
insufficient to allow the titanium to appreciably precipitate;
(b) selectively precipitating titanium oxide(s); and
(c) recovering titanium oxide(s).
The halide acid used in step (a) can be, for example, hydrochloric acid or
hydrobromic
acid. The concentration of halide acid used can be in the range of from about
150 to about 350
grams per liter acid.
Any precipitated titanium reporting to the leach residue of this embodiment
may be
recovered in subsequent leaching operations.
The present invention provides in another separate embodiment a method for
recovering
titanium from a feed comprising finely ground ilmenite (FeTi03), the method
including the steps
of:
(a) leaching the ilmenite with an acidic solution containing sulfuric acid at
a
temperature of about 100°C in the presence of an alkali metal halide
selected
from the group consisting of NaCI, KCl and KBr and in the presence of a source
of activated carbon for up to about half an hour to produce a leachant
solution
containing iron and titanium ions, the ratio of alkali metal halide to
ilmenite in
the feed being about 1.2:1; and the ratio of activated carbon to ilmenite in
the
feed being about 0.01:1, the solids content of the leach solution being up to
about 60% by weight;
(b) repeating step (a);
(c) separating at least some of the pregnant solution from the leach residue;
(d) selectively precipitating the titanium oxides) from the pregnant solution;
and
(e) recovering the titanium oxide as TiOz.
In a particularly preferred embodiment, the present invention provides
multistage
leaching of iron and titanium from an iron and titanium-bearing mineral feed,
the method
comprising the following steps:
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
9
(a) contacting the feed material with an acid - alkali halide solution for a
period
of time sufficient to solubilize the titanium but not so long as to allow the
titanium to appreciably precipitate;
(b) separating the pulp from the leach liquor;
(c) contacting the pulp with fresh leach liquor and repeating steps (a) and
(b) until
all economically feasible titanium is leached; and
(d) selectively recovering the titanium and iron in separate stages from the
leach
solutions by precipitation, solvent extraction or other means.
The conditions of step (a) can involve percent solids on a weight/weight basis
of
between about 1 percent and about 60 percent. The typical percent solids are
in the range of
from about 10% to 40%. The solids may be ground to fme size to facilitate
leaching, typically
so that the feed passes a 73 micron sieve. The acid used is most typically
sulfuric acid. The acid
concentration can range from about 20 to about 300 grams per liter acid. Most
typically the acid
concentration ranges from about 150 to 230 gram per liter.
The alkali halide can be any alkali halide. Preferably, the alkali halide is
NaCI, KCI,
NaBr, or KBr. The concentration of alkali halide can range from about 50 grams
per liter to
about 400 grams per liter. Preferably, the alkali halide concentration is
about 100 to about 200
grams per liter.
The leaching is most typically carried out at about room pressure. The
temperature can
be between about 40°C and about 110°C at room pressure.
Preferably, leaching temperature is
between about 90°C and about 105°C. Leaching at room pressure
will typically be performed
in a leach vessel with a condenser to limit the loss of halide acid generated
in the leach solution.
The titanium is allowed to reach a concentration as high as possible before it
begins to re-
precipitate onto the leach feed material. This is typically slightly over four
(4) grams of titanium
per liter of solution. The leach time to accomplish this solubilization will
depend on the various
aforementioned parameters but will usually range from about 10 minutes to 1
hour.
The method of solid - liquid separation in step (b) can be any method that
makes a
good separation of the solids from the leach liquor in a relatively short
time. These include
methods such as cyclones, filters, centrifuges, magnetic separators, and
settlers. The list is not
meant to exclude any unnamed method.
The fresh leach liquor in step (c) can be leach liquor from which the titanium
content
has been reduced or eliminated. 'The iron content of liquor should be
controlled so that no
precipitation of an iron compound occurs during the leaching.
The titanium can be totally or partially removed from the leach liquor in step
(d) by the
method that makes the most economic sense for any given plant. The methodology
available
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
includes, but is not limited to, precipitation by seeding or pH adjustment,
crystallization, solvent
extraction, and ion exchange.
The iron can be removed in a similar fashion in a step before or after the
titanium
recovery. Titanium and iron are recovered as separate products, in separate
stages. The titanium
5 would be recovered as a titanium salt, most typically TiOZ. The iron would
most typically be
recovered as an iron salt such as ferrous chloride or sulphate.
In addition to titanium and iron leaching, the present invention also concerns
methods
for the recovery of other minerals, such as nickel, cobalt, copper,
molybdenum, lead, zinc, gold
or silver from ore, soil, concentrate, slag or residue. In one embodiment, a
method is provided
10 for the dissolution of nickel and cobalt from a nickel, cobalt and iron-
containing mineral feed,
the method comprising solubilizing the nickel, cobalt and iron in the feed by
leaching the feed
with an acidic solution. In a further embodiment, an alkali metal salt can be
added to the leach
solution to improve recovery. The alkali metal salt can be for example, an
alkali metal halide,
alkali metal nitrite, alkali metal nitrate, alkali metal sulfite or alkali
metal thionite. The metal
halide can be, for example, NaCl, KC1, NaBr or KBr, or mixtures of one or more
of these. The
metal sulfites can be, for example, sodium sulfite, sodium metabisulfite,
sodium bisulfate,
sodium dithionite, or other alkali metal or ammonium sulfite, metabisulfite,
bisulfite or
dithionite. The ordinarily skilled artisan, having the benefit of the
teachings disclosed herein,
can readily determine those alkali metal salts that can be used in conjunction
with the paa-ticular
acid solution used in the solubilization step of the process. In another
embodiment, the method
of the invention can be conducted at above ambient temperatures and at or
above atmospheric
pressures prior to metal extraction by precipitation, solvent extraction or
other means.
Where the metals of interest are nickel and cobalt, the nickel and cobalt-
containing
mineral feed is typically post beneficiation by comminution and thickening
products. A typical
example of nickel and cobalt mineralization is a laterite ore. Alternatively,
the feed may
comprise a bulk laterite concentrate.
One embodiment of the present method provides for recovering nickel and cobalt
from
a mineral feed comprising laterite, the method including the step of
solubilizing nickel and
cobalt and iron in the laterite by leaching the laterite with an acidic
solution in the presence of
an alkali metal halide at a temperature not exceeding about 150°C at
normal pressures prior to
nickel and cobalt extraction by established precipitation, solvent extraction
or other means.
Preferably, the acidic solution contains sulfuric acid. The sulfuric acid used
in the
leaching step is typically at a concentration in the range of from about 20
grams per liter to about
500 grams per liter. In a preferred embodiment, the concentration of sulfuric
acid is in the range
of from about 150 grams per liter to about 250 grams per liter. Preferably,
the concentration of
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
11
sulfuric acid is about 200 grams per liter. Other acids contemplated for use
in the present
invention include halide acids, for example, hydrochloric acid or hydrobromic
acid. The typical
concentration of halide acid used is in the range from about 50 to about 350
grams per liter acid.
The process is typically carned out in the presence of an alkali metal halide
at a ratio
of alkali metal halide to laterite in the feed in the range of from about
0.05:1 to about 4:1.
Preferably, the ratio is about 0.1:1, and most preferably about 0.2:1.
In any of the described embodiments of the invention, including those methods
directed
towards leaching of titanium and non-titanium transition elements from a
mineral feed, the alkali
metal salt may be added directly to the leach solution. Alternatively, the
alkali metal salt is
combined with the feed prior to introduction of the leaching solution. In this
case, the feed may
be subjected to a boildown pre-treatment (i.e., by boiling down to approximate
dryness) in the
presence of the alkali metal salt whereby the feed (e.g., laterite) surfaces
are coated with alkali
metal salt prior to leaching. In another alternative embodiment the solution
of alkali salt may
be sprayed on a heap of lateritic ore and allowed to evaporate. Further a
combination of the
foregoing may be adopted. Namely, a proportion of the alkali metal salt is
combined with the
feed prior to solubilization and a proportion of the alkali metal salt is
added directly to the leach
solution. It is particularly preferred to concurrently remove some of the
pregnant solution from
the leach residue to permit separation of the nickel and cobalt to take place
away from the leach
residue.
Typically, the process is carried out at a temperature in the range of from
about 80°C
to about 120°C. Preferably, the temperature is in the range of from
about 90°C to about 110°C.
A typical operating temperature for the process is about 100°C.
The leach solution in the subject process preferably has a solids content of
up to about
60% by weight. Preferably, the leach solution has a solids content of from
about 10 to 40%.
To facilitate rapid leaching, the feed can be ground into smaller particles.
It is preferred
that the feed be subjected to fme grinding. Preferably, the majority of
particles in the feed are
capable of passing through 75 micron sieve. Typically, at least 75% of the
particles in the feed
are of a size that can pass through 75 micron sieve openings.
Methods according to the present invention may be carried out at or above
atmospheric
pressure. When elevated pressures are used, the typical elevated pressures and
temperatures at
which methods according to the invention may be performed are in the range of
from about 1
bar to about 30 bar. Preferably, pressures are in the range of from about 1
bar to about 5 bar and
temperatures range from about 100 ° C to about 235 ° C.
Preferably in the range of from about
100°C to about 150°C. The methods described in the embodiments
of the present invention do
not conflict with known autoclave technology as the present invention involves
the use of alkali
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
12
metal halides in combination with sulfuric acid whereas known autoclave
technology utilizes
pure acid or ammoniacal solutions to leach the nickel and cobalt from
lateritic feed ores.
The leach residue produced by the present process may be subjected to further
leaching
to solubilize undissolved iron and/or nickel and cobalt in the residue. The
further leaching can
be performed using fresh acidic solution. In an alternative embodiment, spent
leach liquor, or
a combination of fresh acidic solution and spent leach liquor, may be used in
the process.
Additionally, the process may be performed in the presence of ferrous and/or
ferric ions
to promote dissolution of the iron mineralization. Ferrous ions will generally
be present in
recirculated process plant solutions.
Depending on the metals content of the leach solution, a typical reaction time
for the
process of this embodiment is up to about six hours. Preferably, the reaction
time is up to about
two hours. More preferably, the reaction time is in the range of from about 15
minutes to about
3 hours. It has been observed that nickel and cobalt solubility reaches a peak
during reaction
times of approximately that length. A person of ordinary skill in the art can
vary leach time so
as to leach less of an unwanted species such as manganese or iron at the
expense of some cobalt
and nickel recovery.
The process above may be repeated to solubilize unleached nickel or cobalt in
the
residue in order to obtain cumulative maximum solubility of nickel and cobalt.
Fresh acidic
solution and alkali metal halide may be used when the process is repeated. The
process may in
one embodiment comprise a type of countercurrent leach circuit.
The acidic solution may in this embodiment be supplemented with hydrochloric
acid in
one or more steps of a repeated leach sequence to assist in enhancing the
nickel or cobalt
solubility profile.
In another embodiment of the present invention, a metal halide salt may be
used either
to precondition an aqueous slurry or it may be sprayed onto the feed material
and allowed to
evaporate prior to contacting with sulfuric acid.
Upon contact with sulfuric acid the resultant slurry is permitted to leach for
a short time
(typically less than about fifteen minutes) but most preferably about five
minutes or less. The
liquid is then separated and sent for cobalt recovery. This flash leaching
process utilizes the
selective nature of the leach to achieve a cobalt rich solution containing
only minor amounts of
nickel, manganese, iron, etc.
The residue from the flash leach is subsequently leached with the metal halide
sulfuric
acid mixture for longer periods of time to solubilize the nickel and any
remaining cobalt.
In another embodiment super alloy scrap and other recycled metal alloys may be
leached
by treating with a halide salt of the metal and sulfuric acid. The
concentrations of the metal
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
13
halide salt and the sulfuric acid will be dependent upon the specific scrap
mixture. This
embodiment can be utilized to selectively leach specific metals or to place
all the metals into
solution. This embodiment may also be used to solubilize radio-nucleosides of
such metal as
nickel from a radiated scrap. Oxygen or other oxidizing gasses such as
chlorine can be added
to the system to oxidize the metal.
For some oxide ores containing minerals that contain multivalent transition
metals such
as Co and Mn in an high oxidation state species, the alkali metal halide may
be substituted with
a sulfur-based reducing chemical. For example, sodium sulfite, sodium
metabisulfite, sodium
bisulfite, sodium dithionite, or other alkali metal or ammonium sulfite,
metabisulfite. bisulfite
or dithionite can be used in place of the alkali metal halide. These sulfur
based reducing
chemicals will facilitate the reduction of the transition metal, opening the
ore up to attack by the
sulfuric acid. The metal of economic interest need not always be the one
reduced. Alkali metal
nitrates or nitrites may be used with sulfuric acid to leach most metals.
These techniques may
be used to leach metals from sulfide minerals or from scrap, residue, slags,
concentrates, or soils.
In another embodiment the process utilizing a metal halide salt and sulfuric
acid may
be used, with minor modifications, in currently existing counter current
decantation (CCD)
circuits. Such an embodiment would utilize fresh feed material to achieve
neutralization to a
pH adequate to retain iron in solution. After a liquid-solid separation has
been effected, the
resultant leach liquor may be further neutralized to precipitate iron as a
hydroxide in the
presence of a binding material. The iron precipitate may then be partially
dried and pelletised
to produce pig iron feed stocks.
The method of solid-liquid separation can be any method that produces a good
separation of the solids from the leach liquor in a relatively short time.
These include, but are
not limited to, methods such as cyclones, filters, centrifuges, magnetic
separators, and settlers.
The nickel or cobalt can be totally or partially removed from the leach liquor
by the
method that makes the most economic sense for any given plant. The methodology
available
includes, but is not limited to, precipitation by seeding or pH adjustment,
crystallization, solvent
extraction, and ion exchange.
The subject invention also concerns methods for recovering multiple metal or
metal
oxides in separate solutions. Mineral species of economic value are often
associated with species
that consume the chemical reagents that are used to leach them. Sometimes even
though the
consuming species is of economic value, the overall leach becomes uneconomic.
The most
common example of this is copper-gold ores.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
14
Following are examples which illustrate procedures for practicing the
invention. These
examples should not be construed as limiting. All percentages are by weight
and all solvent
mixture proportions are by volume unless otherwise noted.
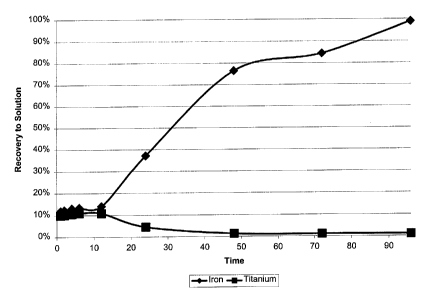
Example 1- Leaching of Titanium and Iron from Ilmenite
Kinetics experiments on the leaching of titanium and iron from ilmenite shows
that both
are leached early on and that the titanium then precipitates and slows the
iron leaching. An
experiment with 100 grams of ilmenite, ground to -200 mesh was conducted. The
tests were
conducted with 1000 grams 200 gram per liter (g/I) sulfuric acid with 120 g/1
NaCI solution. 100
grams activated carbon were added and the solution heated to 100°C. The
Fe and Ti
concentrations were monitored during the course of the 96 hour leach. The
results are presented
in Figure 1. The results present a mechanism of initial Ti leaching into the
liquor with
subsequent hydroxylation and subsequent precipitation. While this occurs it
slows leaching of
the iron. The Ti appears to be leached within one hour.
In a separate but analogous experiment a 100 gram quantity of ilmenite with a
head
assay of 34.0% Fe and 27.0% Ti, and particle size such that 100% of the
particles pass through
a 75 micron screen, was leached for 72 hours at 100° C in 1 liter of
200 gram per liter HZSO4-
120 gram per liter alkali metal halide solution. A 100 gram quantity of
activated carbon was
also present in the leach solution. The leach liquor was monitored
periodically for Ti and Fe
content. The results of the experiment are shown in Table 1. Titanium is
dissolved then
observed to subsequently precipitate. The final assay of the 57.4 gram residue
showed that it
contained only 0.67% Fe and 46.6% Ti. Thus, 98.9% of the iron had been
extracted into the
solution while 99.7% of the titanium remained in the residue. The experiment
indicates that due
to the initial solubilization of Ti, both Ti and Fe can ideally be extracted
from ilmenite by
repeated short duration leaches.
35
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
Table y Dissolution Ilmenite
1. of
Concentration Iron
of from
Titanium
in
Residue
b
Fe Ti
Time m g/1 Volume gm Cumulativegm Cumulative
liters
Hours Fe Ti Extraction Extraction
5 1 5600 3720 0.720 4.03 11.9 268 9.9
2 5650 3700 0.720 4.07 12.3 266 10.0
4 5950 3810 0.720 4.28 13.1 274 10.6
6 6010 3880 0.720 4.33 13.4 279 10.9
12 6220 3830 0.720 4.48 14.0 276 10.9
10 24 16900 1410 0.720 12.17 37.1 1.02 4.5
48 35000 212 0.720 25.20 76.5 0.15 1.3
72 38200 121 0.720 27.50 84.4 0.09 1.1
96 37600 90 0.720 27.07 98.9 0.06 1.0
Wash 5000 20 1.000 5.00 14.7 0.02 0.1
1
15 ~ Wash 375 1 ~ 0.990 0.37 1.1 0.00 0.0
2 ~ ~ ~
Example 2 - Consecutive One-Hour Leaching of Titanium and Iron from Ilmenite
Using the data in Example 1 allowed the development of a new leach procedure
for
ilmenite. The procedure comprises leaching ilmenite for one hour or less and
then contacting
it with fresh leach solution. In this manner both the iron and titanium is
leached together. This
was tested using the same conditions as in the 96-hour test. The results of
four (4) consecutive
one-hour leaches on the same ore sample are shown in Figure 2. As can be seen,
approximately
the same amount of iron and ilmenite was leached in each step. The ordinarily
skilled artisan,
having the benefit of the teachings described herein, can determine the proper
reagent
concentration, temperature, particle size of the ore, whether to include
carbon and its form (e.g.,
activated carbon or graphite), or atmospheric pressure (typically < 3
atmospheres) that is
optimum for a particular ore. The technique of separating the Ti as TiO, with
short leach times
followed by precipitation of TiO~ is also applicable to other leach systems
such as the
hydrochloric acid leach system.
The following two experiments further demonstrate methodology for leaching
both the
titanium and iron from ilmenite in a multistage fashion:
Experiment A comprises a leach solution of 60 grams alkali metal halide, 100
grams
HZ,S04, and 350 grams of HZO heated to 100°C in Erlenmeyer flasks on a
stirring hotplate to
which is added 50 grams of minus 75 microns particle size ilmenite resulting
in a 9% pulp
density.
Experiment B comprises a leach solution of 60 grams alkali metal halide, 100
grams
H,S04, and 350 grams of H20 heated to 100°C in Erlenmeyer flasks on a
stirring hotplate to
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
16
which is added 100 grams of minus 75 microns particle size ilmenite resulting
in a 16% pulp
density.
The ilmenite had an assay head of 30% titanium and 34% iron.
The following procedure steps are applied separately to Experiment A and
Experiment
B:
Step 1. A condenser is placed on the Erlenmeyer containing the slurry
comprising
the prescribed solution and ilmenite feed;
Step 2. The slurry is stirred vigorously with a magnetic stirrer for 30
minutes with
the temperature maintained at 100°C;
Step 3. 'The Erlenmeyer and contents are cooled for a couple of minutes in a
room
temperature water bath;
Step 4. The Erlenmeyer solution is decanted into a centrifuge rube and
centrifuged
at 4,000 rpm for 5 minutes;
Step 5. The liquor in the ccntrifugc tube is decanted and separated from the
solids
into a sample bottle, volume and weight determined and retained for further
test work including analysis;
Step 6. The remaining solids in the centrifuge tube are weighed and then
washed,
with 510 grams of fresh leach solution, back into the residue remaining in
the Erlenmeyer after Step 4;
Step 7. The reconstituted slurry is stirred and the slurry temperature
increased to
100°C;
Step 8. The procedure is continued by repeating Steps 1 through 7 inclusive, a
total
of seven times and thus equating to a total leach duration of 4 hours;
Step 9. The post centrifuging liquors collected at each repetition of Step 5
are
individually subsampled and analysed for titanium and iron;
Step 10. Calculations are conducted to determine titanium and iron contents of
both
solids and liquors and comparisons made with the respective elemental
assay values of the ilmenite ore feed;
Step 11. The individual liquors remaining after the subsampling conducted in
Step
9 are combined in a flask and subsampled and analysed for titanium and
iron;
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
17
Step 12. The titanium can be totally or partially removed from the leach
liquor by
the method that makes the most economic sense for any given plant. The
methodologies available include, but are not limited to, precipitation by
seeding or pH adjustment, crystallization, solvent extraction, and ion
exchange.
The results of the experiments are shown in Table 2 and Figure 3. For both
levels of
percent solids the trend is for roughly a constant amount of titanium to be
extracted at each step.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
18
Table
2.
Pulp
Density
Relationships
on
the
Leaching
of
Titanium
and
Iron
from
Ilmenite
9%
Solids
on
a
weight/weight
basis
g/1 Fe Ti
LeachTime Volume gm Cumulative Cumulative
Hours Fe Ti Liters Extractiongm Extraction
1 0.5 6.32 3.96 0.400 2.53 14.8 1.58 10.6
%
2 0.5 3.97 3.32 0.423 1.68 24.7 1.40 19.9
3 0.5 3.14 2.88 0.412 1.29 32.3 1.19 27.8
4 0.5 2.15 2.18 0.410 0.88 37.4 0.89 33.8
0.5 1.77 1.85 0.415 0.73 41.7 0.77 38.9
l~ 6 0.5 1.71 1.81 0.412 0.70 45.9 0.75 43.9
7 0.5 1.50 1.75 0.412 0.62 49.5 0.72 48.7
8 0.5 1.34 1.61 0.417 0.56 52.8 0.67 53.2
9 0.5 1.44 1.69 0.410 0.59 56.2 0.69 57.8
0.5 1.06 1.28 0.415 0.44 58.8 0.53 61.3
IS 11 0.5 0.90 1.18 0.409 0.37 61.0 0.48 64.5
Wash 0.00 0.00 0.390 0.00 61.0 0.00 64.6
I
12 0.5 1.03 1.040 0.403 0.42 63.4 0.42 67.3
13 0.5 0.91 0.960 0.415 0.38 65.6 0.40 70.0
14 0.5 0.86 0.93 0.410 0.35 67.7 0.38 72.5
0.5 0.85 0.89 0.412 0.35 69.8 0.37 75.0
16 0.5 0.77 0.75 0.420 0.32 71.7 0.32 77.1
17 0.5 0.63 0.64 0.415 0.26 73.2 0.27 78.9
18 0.5 0.65 71.0% 0.402 0.26 74.7 0.29 80.8
19 0.5 0.58 0.65 0.415 0.24 76.1 0.27 82.6
2$ 20 0.5 0.52 0.6 0.412 0.21 77.4 0.25 84.2
21 0.5 0.53 0.58 0.415 0.22 78.7 0.24 85.8
22 0.5 0.48 0.53 0.417 0.20 79.9 0.22 87.3
23 0.5 0.41 0.48 0.413 0.17 80.8 0.20 88.6
Wash 0.002 0.0024 0.540 0.00 80.9 0.00 88.6
2
24 0.5 0.37 0.43 0.410 0.15 81.7 0.18 89.8
Wash 0.0023 0.0019 0.590 0.00 81.7 0.00 89.8
3
16%
Solids
on
a
weight/weight
Basis
g1 Fe Ti
Time Fe Ti Volume gm Cumulativegm Cumulative
35 LeachHours Liters Extraction Extraction
0.25 7.07 4.00 0.010 0.07 0.2 0.04 0.1
1 0.50 8.16 4.52 0.380 3.10 9.3 1.72 5.9
0.25 4.77 4.63 0.010 0.05 9.4 0.05 6.0
2 0.50 5.78 5.44 0.402 2.32 16.3 2.19 13.3
3 0.50 4.74 4.54 0.418 1.98 22.1 1.90 19.6
4 0.50 4.16 4.13 0.419 1.74 27.2 1.73 25.4
5 0.50 3.62 3.63 0.418 1.51 31.6 1.52 30.5
6 0.50 3.30 3.27 0.425 1.40 35.7 1.39 35.1
Wash 0.05 0.04 0.511 0.03 35.8 0.02 35.2
1
45 7 0.50 2.54 2.65 0.408 1.04 38.8 1.08 38.8
8 0.50 2.08 2.41 0.417 0.87 41.4 1.00 42.1
Wash 0.50 0.08 0.09 0.450 0.04 41.5 0.04 42.3
2
Example 3 - Effect of Alkali Metal Halide
50 Experiments were conducted to evaluate the effect of an alkali metal halide
on the
recovery of iron from ore using the sulfuric acid process of the subject
invention. The results
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
19
are shown in Figure 4. In this case, the salt which was used was NaCl at 0%,
5%, 15% and 25%
(w/w). These tests were performed using 200 gram per liter sulfuric acid
solution and no
activated carbon at 100°C on unground ore. The addition of salt speeds
the reaction rate.
However, at around 15 to 20% salt (150 to 200 grams per liter), NaCl appears
to become
counterproductive. The total percentage of iron leached actually falls after
15% NaCI is reached.
In a separate but analogous experiment to further demonstrate the effect of an
alkali
metal halide on the leaching of iron out of ilmenite, the samples of 100 grams
of ilmenite feed
were leached with 200 grams of sulfuric acid, 700 grams of water at
100° C and varying
amounts of alkali metal halide for 72 hours. The amounts of alkali metal
halide were 0, 50, 150
and 250 grams representing 0, 5, 15, and 25% (w/w) alkali metal halide
solutions. The leach rate
of the iron was observed. The results are shown in Table 3.
CA 02363031 2001-08-17
WO 00/48944 PCTIUS00/04333
Table 3. The
effect of alkali
metal halide
on the sul huric
acid leachin
rocess
No Alkali Metal
Halide
Time Hours Fe mg/L Volume Fe Extraction
liters
m Cumulative
0 86 0.700 0.06 0.2%
3 990 0.700 0.69 2.0%
6 1460 0.700 1.02 3.0%
12 3000 0.700 2.10 6.2%
24 4000 0.700 2.80 8.2%
10 36 6100 0.700 4.27 12.6%
48 10200 0.700 7.14 21.0%
60 14600 0.700 10.22 30.1
72 20700 0.720 14.90 46.5%
Wash I 860 1.020 0.88
1$ Wash 2 28 0.990 0.03
5% Alkali Metal
Halide
Time Hours Fe mg/I Volume Fe Extraction
liters
m Cumulative
0 100 0.700 0.07 0.2%
20 3 1110 0.700 0.78 2.4%
6 1860 0.700 1.30 4.0%
12 3500 0.700 2.45 7.6%
24 4700 0.700 3.29 10.2%
36 6500 . 0.700 4.55 14.1%
48 10100 0.700 7.07 21.8%
60 18600 0.700 13.02 40.2%
72 21700 0.790 17.14 56.5%
Wash 1 1100 1.000 1.10
Wash 2 34 1.000 0.03
15% Al kali Metal
Halide
Time Hours Fe mg/1 Volume Fe Extraction
liters
m Cumulative
0 189 0.700 0.13 0.4%
3 1910 0.700 1.34 4.3%
6 2500 0.700 1.75 5.7%
12 4400 0.700 3.08 10.0%
24 5600 0.700 3.92 12.8%
36 7900 0.700 5.53 18.0%
48 12300 0.700 8.61 28.0%
60 18200 0.700 12.74 41.4%
72 25300 0.720 18.22 63.9%
Wash I 1350 1.020 1.38
Wash 2 61 0.990 0.06
25% Al kali Metal
Halide
Time Fe Volume Fe Extraction
Hours mg/1 liters m Cumulative
0 250 0.700 0.18 0.5%
3 2600 0.700 1.82 5.2%
6 4200 0.700 2.94 8.4%
J~0 12 9800 0.700 6.86 19.7%
24 11700 0.700 8.19 23.5%
36 14500 0.700 10. I 5 29.1
48 17300 0.700 12.11 34.7%
60 18900 0.700 13.23 37.9%
Jr5 72 18700 0.720 13.46 47.9%
Wash 1 2960 1.020 3.02
Wash 2 230 0.990 0.23
60 Example 4 - Effect of Grinding of Ore
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
21
In accordance with the subject invention, grinding of the ore can be used to
increase the
reaction rate of leaching iron from ilmenite. This is shown in Figure 5 and
Table 4. Both tests
were performed using a 100 gram quantity of ilmenite placed in one liter of
200 grams per liter
sulfuric acid and 150 grams per liter alkali metal halide solution heated to
100°C. The
experiments were conducted on two samples of the same ilmenite feed. One
experiment used
course ilmenite ( 100% retained on a 75 micron screen) and the other
experiment used fine
ilmenite (100% passing through a 75 micron screen). The slurry was vigorously
stirred for 72
hours and the iron concentration periodically monitored. The ground ore (finer
particle sized
samples) had faster early and late leach kinetics than the unground ore
(coarser particle sized
sample). The kinetics of the ore during the 5 to 25 hour time period was
similar in both cases.
Table 4.
The Results
of Feed
Particle
Size on
the Sulphuric
Acid Leaching
Process
Coarse Ilmenite
Time Fe Volume Fe Extraction
IS Hours mg/1 liters gm Cumulative
0 189 0.700 0.13 0.4%
3 1910 0.700 1.34 4.3
6 2500 0.700 1.75 5.7
I 2 4400 0.700 3.08 10.0%
2,0 24 5600 0.700 3.92 12.8
36 7900 0.700 5.53 18.0
48 12300 0.700 8.61 28.0
60 18200 0.700 12.74 41.4
72 25300 0.720 18.22 59.3
25 Wash 1 1350 1.020 1.38
Wash 2 61 0.990 0.06
Fine Ilmenite
Time Fe Volume Fe Extraction
Hours mg/1 liters gm Cumulative
0 0 2300 0.700 1.61 4.8
3 5000 0.700 3.50 10.5
6 -- 5100 - p.700 3.57 10.7
12 5400 0.700 3.78 11.3
24 6600 0.700 4.55 13.6
48 20000 0.700 14.00 42.0
60 24000 0.700 16.80 50.4
72 29300 0.815 23.88 76.3
Wash 1 1550 0.995 1.54
Wash 2 ~ 44 ~ I .000 0.04
Example S - Addition of Carbon Source
The addition of a carbon source in the form of activated carbon or graphite
speeds the
kinetics of the leaching reaction. The ratios of carbon to ore tried were 1:2
and 1:1. The results
of tests carried out at 100°C with 120 grams per liter salt and 200
grams per liter sulfuric acid
are shown in Figure 6. The most cost effective carbon to ore ratio will depend
on final leach
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
22
conditions. A person skilled in the art, having the benefit of the current
disclosure, can identify
the optimal carbon to ore ratio for a particular process.
In a separate but analogous experiment to demonstrate the effect of the
addition of a
carbon source to the leaching of iron from ilmenite to leave a TiOz
concentrate residue, a 150
gram per liter alkali metal halide solution was used, as opposed to the 120
grams per liter salt
used in the aforementioned experiment. The experiment used ilmenite having a
particle size
wherein 100% of particles passed through a 75 micron screen. A varying amount
of coconut
shell activated carbon was placed in each container. The same carbon to sample
ratios were
evaluated as in the aforementioned experiment. The amounts were 0, 50 and 100
grams of
carbon for carbon to sample ratios of 0, 1:2 and 1:1, respectively. The slurry
was vigorously
stirred for 72 hours and the iron concentration periodically monitored. The
results are shown
in Table 5. The leach with the 1:1 ratio of carbon to feed material had
slightly better kinetics
than the other two conditions.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
23
Table 5. The f adding
Results o Carbon during
the Sulphuric
Acid Leaching
Process
No Carbon
Source
Time (Hours) Fe mg/1 Volume (liters)Fee Extraction
gm Cumulative
0 3000 0.700 2.10 6.0
3 5700 0.700 3.00 11.5
6 5900 0.700 4.13 11.9%
12 6200 0.700 4.34 12.5
24 14500 0.700 10.15 29.1
48 25000 0.700 17.50 50.3%
60 27000 0.700 18.90 54.3
72 36500 0.740 27.01 83.6
Wash 1 2060 0.995 2.05
Wash 2 60 1.010 0.06
1:2 Carbon:Ilmenite
Time Fe Volume Fe Extraction
Hours mg/1 liters gm Cumulative
0 4100 0.700 2.87 8.4
3 8200 0.700 5.74 16.7
6 11100 0.700 7.77 22.6
12 12300 0.700 8.61 25.1
24 20200 0.700 14.14 41.2
48 24000 0.700 16.80 49.9
60 27000 0.700 18.90 55.I
72 39300 0.695 27.31 89.3
Wash 1 3240 0.980 3.18
Wash 2 150 1.000 0.15
1:1 Carbon:Ilmenite
Time Fe Volume Fee Extraction
Hours mg/1 liters gm Cumulative
0 4800 0.700 3.36 10.0
3 8000 0.700 5.60 16.7
6 10100 0.700 7.07 21.1
12 14500 0.700 10.15 30.3
24 24000 0.700 16.80 50.2
48 27000 0.700 16.90 56.4
60 31000 0.700 21.70 64.8
72 45400 0.550 24.97 93.5
Wash 1 6040 0.990 5.98
Wash 2 ~ 355 ~ 0.990 0.35
Example 6 - Leachin ogY f Copper and Nickel from Laterite Ore with a Sulfuric
Acid-Halide-
Carbon S, s
This ore has an assay head of 2.36% Co, 1.26% Ni, 11.00% Fe, 10.80% Mn. A
sample
of 100 grams of ground, -200 mesh ore was first treated with 200 grams of NaCI
dissolved in
650 grams of water. The water was evaporated on a hot plate. This procedure is
a speeded up
simulation of spaying a heap of ore with a salt solution and letting it
evaporate naturally. The
ore-salt solids were then slurried in 200 grams of sulfuric acid in 700 grams
of water solution.
The stirred slurry was brought to 100°C on a stirring hot plate, and
then 100 grams of+65 mesh,
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
24
coconut shell, activated carbon was added. The test was run for 48 hours with
aliquots of
solution taken at l, 4, 6, and 24 hours. The results are shown in Table 6. The
extraction of cobalt
was probably complete within the first hour. The cobalt was probably
precipitated by the ionic
strength of the solution and not recovered until the wash solution dissolved
it. After 120 hours
of leaching the ore under the same conditions except for omitting the NaCI the
Co recovery was
63.9% and the Ni recovery was 58.2%.
Table 6. Percenttracted fromte Ore
Ex Lateri Leach
Time hrs Co Ni Fe Mn
1 81.5% 67.5% 18.8% 63.4%
2 81.5% 78.2% 25.5% 71.0%
4 81.5% 85.5% 31.5% 71.0%
6 81.5% 84.8% 36.0% 72.6%
24 85.0 % 87.5 % 54.8 % 73.3
48 81.5 % 86.2 % f 7.5 % 71.0
Washl 13.1% 12.9% 18.5% 11.5%
Wash2 5.4% 0.6% 2.2% 0.4%
Final Li 100.0 99.6 % 88.2% 82.9%
uors: %
Example 7 - Effect of Alkali Metal Halide on the Leaching of Nickel and Cobalt
from Laterite 1
Experiments were conducted on two samples of 100 grams of laterite-1 feed,
comprising
1.0 percent nickel and 0.1 percent cobalt of a particle size of approximately
80% passing 75
microns.
In the first experiment the 100 g sample was leached with 200 grams of
sulfuric acid,
800 grams of water and no alkali halide at 100°C.
In the second experiment the 100 g sample was leached with 200 grams of
sulfuric acid,
800 grams of water at 100°C, and 200 grams of alkali metal halide
(sodium chloride).
Each experiment was run for a total of 6 hours with solution sampling being
carried out
at 0.25, 0.5, 1.0, 2.0, 4.0, and finally 6.0 hours. The results are shown in
Table 7 and Figures 7
and 8. The second experiment that utilized the halide showed significantly
better results for both
nickel and cobalt, and particularly cobalt.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
Table 7.
Leaching
of Nickel
and Cobalt
from Laterite-1
No Alkali
Metal
Halide
Ni Co
Time Hoursmg/1 Volume Cumulative Cumulative
Ni liters mg Extraction mg Extraction
Co
0.25 240.0 20.0 0.010 2.40 21.3% 0.20
20.3%
0.5 380.0 30.0 0.010 3.80 33.7% 0.30
30.5%
1 550.0 39.0 0.010 5.50 48.8% 0.39
39.6%
2 740.0 53.0 0.010 7.40 65.8% 0.53
53.8%
4 820.0 64.0 0.010 8.20 72.7% 0.64
65.0%
l~ 6 hr PLS 1060.075.0 0.780 826.80 94.0% 58.50
78.2%
Wash 1 116.0 40.0 0.670 77.72 26.80
Wash 2 12.0 1.3 0.700 8.40 0.91
Included
Alkali
Metal
Halide
Ni Co
IS Time Hoursmg/1 Volume Cumulative Cumulative
Ni liters mg Extraction mg Extraction
Co
0.25 741.7 100.0 0.010 7.42 69.4% 1.00
97.7%
0.5 867.2 100.0 0.010 8.67 81.2% 1.00
97.7%
1 958.4 100.0 0.010 9.58 89.7% 1.00
97.7%
2 1026.9I 00.00.010 10.27 96.1 1.00
% 97.7%
2~ 4 1049.7100.0 0.010 10.50 98.3% 1.00
97.7%
6 hr PLS 1050.0100.0 0.765 803.25 98.3% 76.50
97.7%
Wash I 103.8 10.0 0.670 69.57 6.70
Wash 2 60.5 5.7 0.590 35.68 3.36
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
26
Example 8 - Effect of Alkali Metal Halide on the Leaching of nickel and cobalt
from Laterite-2
Experiments were conducted on two samples of 100 grams of laterite-2
comprising 1.1
percent nickel and 0.1 percent cobalt feed of a particle size of approximately
80% passing 75
microns.
In the first experiment the 100 g sample was leached with 200 grams of
sulfuric acid,
800 grams of water and no alkali halide at 100°C.
In the second experiment the 100 g sample was leached with 200 grams of
sulfuric acid,
800 grams of water at 100°C, and 200 grams of alkali metal halide
(sodium chloride).
Each experiment was run for a total of 6 hours with solution sampling being
carned out
at 0.25, 0.5, 1.0, 2.0, 4.0, and finally 6.0 hours. The results are shown in
Table 8 and Figures 9
and 10. The alkali metal halide (sodium chloride) test showed significantly
better results for
both nickel and cobalt, particularly with regard to the speed with which full
( 100%) dissolution
is achieved.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
27
Table 8.
Leaching
of Nickel
and Cobalt
from Laterite-2
No Alkali
Metal
Halide
Ni Co
Time Hoursmg/1 Volume Cumulative Cumulative
Ni liters mg Extraction mg Extraction
Co
$ 0.25 738.9 45.0 0.010 7.39 0.45
60.0% 51.6%
0.5 915.3 54.0 0.010 9.15 0.54
74.3% 61.9%
1 1036.665.0 0.010 10.37 0.65
84.2% 74.5%
2 I I 80.0 0.010 11.14 0.80
13.8 90.4% 91.7%
4 1146.980.0 0.010 11.47 0.80
93.1 91.7%
%
1~ 6 hr PLS I 180.085.0 0.725 855.50 61.63
95.8% 97.4%
Wash 1 264.7 21.0 0.530 140.28 11.13
Wash 2 51.8 4.1 0.695 36.02 2.85
Included
Alkali
Metal
Halide
Ni Co
IS Time Hoursmg/I Volume Cumulative Cumulative
Ni liters mg Extraction mg Extraction
Co
0.25 906.5 80.0 0.010 9.07 0.80 88.9%
76.4%
0.5 1054.190.0 0.010 10.54 0.90 100.0%
88.8%
1 1149.090.0 0.010 11.49 0.90 100.0%
96.8%
2 1159.590.0 0.010 11.60 0.90 100.0%
97.7%
2~ 4 1170.090.0 0.010 11.70 0.90 100.0%
98.6%
6 hr PLS 1170.090.0 0.750 877.50 67.50 100.0%
98.6%
Wash 1 177.1 13.0 0.800 141.67 10.40
Wash 2 25.3 2.1 0.795 9.07 1.67
The above two experiments demonstrate the results for leaching both the nickel
and
cobalt from two different nickel-cobalt laterite samples.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
28
The following procedure steps have been applied separately to each of the
laterite
samples:
Step 1. A condenser is placed on the Erlenmeyer containing the slurry
comprising the
prescribed solution and laterite feed;
Step 2. The slurry is stirred vigorously with a magnetic stirrer for the
duration of the test
with the temperature maintained at 100 ° C;
Step 3. The test is sampled at predetermined times, eg., 15 minutes, 30
minutes, etc., by
pipetting 10 ml of the hot slurry from the Erlenmeyer into a centrifuge tube
and
centrifuge at 4,000 rpm for 5 minutes;
Step 4. The centrifuged timed leach solution is transferred into a sample tube
for later
analysis;
Step 5. 10 ml of make-up leach solution is used to wash the centrifuged
residue back into the
Erlenmeyer, while the Erlenmeyer continues to be agitated at 100 °C on
the hot plate;
Step 6. At the end of the test (e.g., 6 hours) the contents of the Erlenmeyer
is poured into two
centrifuge tubes, using an additional very small amount of distilled water to
wash out
any residue remaining on the inside lip of the Erlenmeyer, and then
centrifuged;
Step 7. The centrifuged liquid contents (pregnant leach solution - PLS) from
both centrifuge
tubes is decanted into a graduated cylinder and allow to cool;
Step 8. Then having read the volume of PLS solution, approximately 20 ml is
transferred
into a sample tube and analysed for nickel and cobalt;
Step 9. Calculations are conducted to determine nickel and cobalt contents of
the liquors and
comparisons made with the respective elemental assay values of the laterite
ore feed;
Step 10. Nickel and cobalt can be totally or partially removed from the leach
liquor by the
method that makes the most economic sense for any given plant. The
methodologies
available variously include, but are not limited to, precipitation of metallic
salts by
seeding, pH adjustment, or crystallisation; solvent extraction and
electrowinning of
elemental metal; and ion exchange.
Example 9 - Leaching of Silver
This example shows the leaching of silver from a copper refinery pilot plant's
slimes.
The test was conducted at 100°C with 200 gram per liter sulfuric acid
and 200 gram per liter
NaCI. Samples of 50 grams of slimes were leached in 500 milliliters of
solution. The leaching
was conducted for 48 hours. The results are shown in the Table 9.
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
29
Table 9.
Results
of 48
hour Leaching
of Silver
from Refinery
Slimes
Sample Head Ag, ppm Liquor Ag, % Recovery
ppm
Slime 1 14.75 18 80
Slime 2 58.9 59 65
ppm = parts per million
Example 10- Leachin og f Molybdenum
A sample of molybdenum oxide ore with a head grade of 0.070% Mo was ground to
minus 200 mesh and leached with agitation for 48 hours at room temperature
with a solution of
100 grams per liter sulfuric acid and 100 grams per liter sodium chloride.
This leach recovered
89% of the molybdenum in the sample.
Another sample of unground ore from the same mine ore was screened to select
the
minus 18,850 plus 833 micron (minus 3/4 inch plus 20 mesh) fraction. This
fraction was placed
in a column and the same 100 g/1 sulfuric acid, 100 g/1 sodium chloride was
applied to the ore
for 56 days at 0.05 gallons per minute per square foot The leach solution was
recirculated
continuously. This leach scheme obtained 82% recovery of the molybdenum.
Example 11- Two Stage Leaching of Different Metals into Two Separate Leach
Liquors
An oxide copper ore sample, ground to minus 200 mesh, with a head grade of
0.91%
Cu, 2.0 grams/ton Au, and 2.4 grams/ton Ag was leached with 100 gram per liter
sulfuric acid
for 72 hours in a stirred vessel at room temperature. The solid residue was
then filtered and put
into another vessel and leached for 30 hours at room temperature with a
solution of 50 gram per
liter of potassium bromide and enough sulfuric acid (6 ml) to adjust the pH to
1.0 with agitation.
The results are shown in Table 10.
Table 10.
Percent Recovery
in Stage
Leach Stage Cu Au Ag
100 g/1 H~S0471 0 0
KBr- H,S04 28 100 100
CA 02363031 2001-08-17
WO 00/48944 PCT/US00/04333
A person skilled in the art, having the benefit of the teachings of this
disclosure, can
adjust the acid concentration and temperature to achieve complete recovery of
the copper in the
first stage while maintaining excellent recovery of the Au and Ag in the
second stage. The
subject method can also be readily adapted to heap leaching.
5
It should be understood that the examples and embodiments described herein are
for
illustrative purposes only and that various modifications or changes in light
thereof will be
suggested to persons skilled in the art and are to be included within the
spirit and purview of this
application and the scope of the appended claims.