Note: Descriptions are shown in the official language in which they were submitted.
CA 02363487 2001-12-04
METHOD FOR REMOVAL OF NITROGEN OXIDES FROM EXHAUST GAS
This application is a division of copending Canadian
patent Application Serial No. 2,138,133 filed April 28, 1994.
This invention relates to purification of an
industrial exhaust gas by removal of nitrogen oxides
therefrom. More. particularly, it relates to the
purification of the industrial exhaust gas emanating as from
a boiler, a power generation plant, an industrial plant, or
an internal combustion engine like a gasoline or diesel
engine by the removal of nitrogen oxides therefrom.
In recent years, the 'exhaust gases such as are
emanating from internal combustion engines of automobiles,
boilers, or industrial plants contain noxious substances
such as nitrogen oxides (hereinafter occasionally referred
to collectively as "NOx") which form the cause for air
pollution. Generally, the NOx are not easily reduced or
decomposed in an oxidizing atmosphere (the atmosphere
embracing the exhaust gas and having an oxygen supply more
than necessary for complete combustion of the unburnt
portion of the fuel entrained in the exhaust gas). As a
result, the removal of the NOx from the exhaust gas is
attained only with difficulty. Thus, the removal of NOx
from a varying exhaust gas has been the subject of a
scientific study in various fields.
For the purification of the exhaust gas from
automobiles, it has been heretofore customary to adopt the
method of treating the exhaust gas with a three-way catalyst
thereby simultaneously removing NOx, hydrocarbons (HC), and
carbon monoxide (CO). By this method, the atmosphere of the
exhaust gas to be treated is in the neighborhood of
stoichiometry (which is the theoretical air-fuel ratio,
namely the ratio of air required for complete combustion of
the fuel). When the internal combustion engine is operated
with the air introduced in an amount in excess of this
-1-
CA 02363487 2001-12-04
theoretical air-fuel ratio, the oxygen is present at the
site of treatment more than necessary for complete
combustion of the unburnt portions such as of hydrocarbons
and carbon monoxide in the exhaust gas. In the exhaust gas
present in the oxidizing atmosphere of this kind, therefore,
it is difficult to reduce, decompose, and remove the NOx.
In respect to the diesel engines, boilers, etc., it
is general to use a reducing agent such as ammonia,
hydrogen, or carbon monoxide. This method, however, entails
the problem of necessitating a special device for recovering
and disposing of the unaltered portion of the reducing
agent. It, therefore, cannot be effectively applied easily
to a small NOx generating sources such as engines in
automobiles or cogeneration systems in buildings.
Particularly in the removal of NOx of a relatively low
concentration,- this method operates with notably low
efficiency.
Recently as a means for the removal of NOx, the
method resorting to use of a NOx decomposing catalyst made
of a copper ion-containing crystalline aluminosilicate has
been proposed (as in JP-A-60-125,250, US-A-4,297,328, etc.).
This method is depicted therein simply as being capable of
decomposing nitrogen monoxide (NO) into nitrogen (N2) and
oxygen (02). It does not easily obtain effective removal of
NOx from the exhaust gas which emanates under actual
conditions. Further, it is known that aluminosilicates are
generally so deficient in resistance to heat as to offer no
infallible use at elevated temperatures.
JP-A-63-100,919 has a mention to the effect that
when the exhaust gas is treated with a copper-containing
catalyst under an oxidizing atmosphere in the presence of a
hydrocarbon, the reaction of the exhaust gas proceeds
preferentially :with the hydrocarbon and, therefore, the
removal of NOx. is obtained with high efficiency. It is
remarked that the hydrocarbon to be used in this method may
b.e either the hydrocarbon which is contained per se in the
-2-
CA 02363487 2001-12-04
exhaust gas or the hydrocarbon which is added as occasion
demands from an external source. Specifically, this method
is carried out by first bringing the exhaust gas into
contact with the copper-containing catalyst thereby removing
NOx therefrom and then causing the residual exhaust gas to
contact an oxidizing catalyst thereby removing hydrocarbons,
carbon monoxide, etc. If the exhaust gas to be treated by
this method happens to have an unduly low hydrocarbon
content, the method will necessitate continuous introduction
of the hydrocarbon into the exhaust gas. Further, in the
oxidizing atmosphere, since the combustion of HC proceeds
preferentially over the reaction of the hydrocarbon with
NOx, thorough removal of the NOx from the exhaust gas
requires the hydrocarbon to be introduced in a large amount.
JP-A-04-250,822 discloses a method for purifying
such an NOx-containing air as generated in a tunnel by
passing the air through an absorption tower thereby
effecting absorption and concentration of the NOx and then
introducing the separated NOx into a reaction tower packed
with an NOx reducing catalyst. This method requires two
devices, the one for absorption and the other for reduction
of the NOx.
A method which is capable of efficiently decomposing
and removing the NOx from the exhaust gas and enabling a
catalyst to manifest excellent durability to tolerate high
temperatures and ideal packability in a reactor remains yet
to be developed. Such is the existing state of the art.
This invention, therefore, is directed towards the provision
of a method which is capable of efficiently removing NOX from
exhaust gas in an oxidizing atmosphere while precluding the
drawbacks mentioned above.
In accordance with one aspect of the present invention, there
is provided a method for the removal of nitrogen oxides from an
exhaust gas, which comprises causing the exhaust gas in an
oxidizing atmosphere to contact a catalyst comprising a refractory
-3-
CA 02363487 2001-12-04
inorganic oxide and catalytically active components, the.
components- comprising 0.1 to 30 g as metal per liter of the
catalyst of at least one noble metal selected from the group
consisting of platinum, palladium, rhodium and ruthenium or
a compound of the noble metal, and 1 to 80 g as metal per
liter of the catalyst of at least one metal selected from
the group consisting of lithium, potassium, sodium,
rubidium, cesium, beryllium, magnesium, calcium, strontium,
and barium or a compound of the metal, thereby inducing the
catalyst to adsorb thereon the nitrogen oxides in the
exhaust gas and, subsequently introducing a reducing
substance intermittently into the exhaust gas thereby
purifying the exhaust gas by reducing the nitrogen oxides
adsorbed on the catalyst. This aspect of the invention forms the
subject of and is claimed in the parent Serial No. 2,138,133.
This invention further contemplates the method
mentioned above, wherein as the component at least one heavy
metal selected from the group consisting of manganese,
copper, cobalt, molybdenum, tungsten, and vanadium or a
compound of the heavy metal is further contained in an
amount in the range of 0.1 to 50 g per liter of the
catalyst. This invention also contemplates the method
mentioned above, wherein the catalyst has a capacity for
adsorbing the riitrogen oxides to saturation in the range of
6 to 30 m.mols per liter of the catalyst. This invention
contemplates the method mentioned above, wherein the
introduction of the reducing substance to the exhaust gas is
effected by introducing a gas containing the reducing
substance in an amount of 1 to 10 mols per mol of the
nitrogen oxides (as NO) adsorbed on the catalyst for a
period in the range of 0.1 to 20 seconds at intervals of 7
seconds to 60 minutes, preferably 7 seconds to 20 minutes.
This invention further contemplates the method mentioned
above, wherein the introduction of the reducing substance is
effected before the amount of nitrogen oxides adsorbed on
the catalyst reaches 50% of the capacity of the catalyst for
adsorbing nitrogen oxides to saturation. This invention
-4-
CA 02363487 2001-12-04
also contemplates the method mentioned above, wherein the
exhaust gas is that from an internal combustion engine.
This invention further contemplates the method mentioned
above, wherein the introduction of the reducing substance
into the exhaust gas is accomplished by lowering the air to
fuel ratio in the suction system of the internal combustion
engine. This invention also contemplates the method
mentioned above, wherein the introduction of the reducing
substance into the exhaust gas is accomplished by setting
the air to fuel ratio in the suction system of the internal
combustion engine at a theoretical level or an air-rich
level. This invention further contemplates the method
mentioned above, wherein the reducing substance is
introduced into the exhaust gas from an external source.
In accordance with a further aspect of the present invention, there is
provided a method for the removal of nitrogen oxides from an exhaust gas,
which
comprises installing on the upstream side of the flow of the exhaust gas a
catalyst
comprising 50 to 400 g per liter of the catalyst of a refractory inorganic
oxide and
catalytically active components, the components comprising 0.1 to 30 g as
metal per
liter of the catalyst of at least one noble metal selected from the group
consisting of
platinum, palladium, rhodium and ruthenium or a compound of the noble metal,
and 1
to 80 g as metal per liter of the catalyst of at least one metal selected from
the group
consisting of lithium, potassium, sodium, rubidium, cesium, beryllium,
magnesium,
calcium, strontium, and barium or a compound of the metal and, at the same
time,
installing an oxidizing catalyst or a three-way catalyst on the downstream
side of the
flow of the exhaust gas.
In accordance with an additional aspect of the present invention, there is
provided a method for the removal of nitrogen oxides from an exhaust gas,
which
comprises installing on the upstream side of the flow of the exhaust gas a
catalyst
comprising 50 to 400 g per liter of the catalyst of a refractory inorganic
oxide and
catalytically active components, the components
-5-
CA 02363487 2001-12-04
comprising 0.1 to 30 g as metal per liter of the catalyst of
at least one noble metal selected from the group consisting
of platinum, palladium, rhodium and ruthenium or a compound
of the noble metal, 1 to 80 g as metal per liter of the
catalyst of at least one metal selected from the group
consisting of lithium, potassium, sodium, rubidium, cesium,
beryllium, magnesium, calcium, strontium, and barium or a
compound of the metal, and 0.1 to 50 g per liter of the
catalyst of at least one heavy metal selected from the group
consisting of manganese, copper, cobalt, molybdenum,
tungsten, and vanadium or a compound of the heavy metal and,
at the same time, installing an oxidizing catalyst or a
three-way catalyst on the downstream side of the flow of the
exhaust gas.
This invention further contemplates the method
mentioned above, wherein the oxidizing catalyst comprises
0.1 to 10 g per liter of the catalyst of at least one noble
metal selected: from the group consisting of platinum and
palladium and 10 to 300 g per liter of the catalyst of a
refractory oxide.
This invention also contemplates the method
mentioned above, wherein the oxidizing catalyst further
comprises 0.1 to 150 g per liter of the catalyst of at least
one oxide of an element selected from the group consisting
of rare earth elements, nickel, cobalt, and iron. This
invention contemplates the method mentioned above, wherein
the three-way catalyst comprises 10 to 300 g per liter of
the catalyst of a refractory inorganic oxide and catalytic
components, the components comprising 0.1 to 10 g as metal
per liter of the catalyst of noble metal(s) selected from
the group consisting of (a) palladium, (b) platinum and
rhodium, (c) palladium and rhodium, and (d) platinum,
palladium, and rhodium and 10 to 150 9 as Ce02 per liter of
the catalyst of ceria. This invention further contemplates
the method mentioned above, wherein the three-way catalyst
further comprises 0.1 to 50 g as oxide per liter of the
-6-
CA 02363487 2001-12-04
catalyst of at least one member selected from the group
consisting of zirconia and rare earth elements except for
cerium. This invention also relates to a method for the
removal of nitrogen oxides from an exhaust gas characterized
by installing a three-way catalyst or an oxidizing catalyst on
the upstream side of the exhaust gas, a catalyst for the
removal of the nitrogen oxides next thereto, and a three-way
catalyst or an oxidizing catalyst on the downstream side.
In accordance with a further aspect of the present
invention, there is provided a method for the removal of
nitrogen oxides from an exhaust gas characterized in that a
three-way catalyst or oxidizing catalyst is disposed on the
upstream side of the flow of said exhaust gas, a catalyst
comprising 50 to 400 g per liter of said catalyst of a
refractory inorganic oxide and catalytically active
components, said components comprising 0.1 to 30 g as metal
per liter of said catalyst of at least one noble metal
selected from the group consisting of platinum, palladium,
rhodium and ruthenium or a compound of said noble metal, and 1
to 80 g as metal per liter of said catalyst of at least one
metal selected from the group consisting of lithium,
potassium, sodium, rubidium, cesium, beryllium, magnesium,
calcium, strontium, and barium or a compound of said metal is
disposed next thereto, and said three-way catalyst or
oxidizing catalyst is disposed farther on the downstream side.
In accordance with an additional aspect of the present
invention, there is provided a method for the removal of
nitrogen oxides from an exhaust gas characterized in that a
three-way catalyst or oxidizing catalyst is disposed on the
upstream side of the flow of said exhaust gas, a catalyst
comprising 50 to 400 g per liter of said catalyst of a
7
CA 02363487 2001-12-04
refractory inorganic oxide and catalytically active
components, said components comprising 0.1 to 30 g as metal
per liter of said catalyst of at least one noble metal
selected from the group consisting of platinum, palladium,
rhodium and ruthenium or a compound of said noble metal, 1 to
80 g as metal per liter of said catalyst of at least one metal
selected from the group consisting of lithium, potassium,
sodium, rubidium, cesium, beryllium, magnesium, calcium,
strontium, and barium or a compound of said metal, and 0.1 to
50 g per liter of said catalyst of at least one heavy metal
selected from the group consisting of manganese, copper,
cobalt, molybdenum, tungsten, and vanadium or a compound of
said heavy metal is disposed next thereto, and said three-way
catalyst or oxidizing catalyst is disposed farther on the
downstream side.
Reference is made in the following description to the
accompanying drawings, wherein:
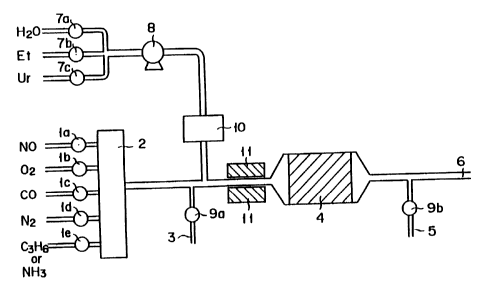
Fig. 1 is a schematic diagram illustrating an
apparatus for experimenting a method for the removal of
nitrogen oxides according to this invention.
7A
CA 02363487 2001-12-04
First, the principle of this invention will be
described. In this invention, an exhaust gas containing NOx
is brought into contact with a component manifesting an
oxidizing activity in an oxidizing atmosphere so that N0,
N20, etc. which are generally present at high proportions in
the NOx components of the exhaust gas are oxidized or
activated into. N02. The N02 thus resulting from the
oxidation or activation is then adsorbed on a component
possessing an NO2 adsorbing ability. By introducing a
reducing substance instantaneously into the exhaust gas
enveloping the NOx accumulated on the adsorbent component,
the adsorbed NOx is reduced or decomposed to complete the
removal of NOx. It is the catalyst contemplated by this
invention that discharges the function of reducing or
decomposing the:NOx.
The reducing substances which are effectively usable
in this invention include hydrocarbons, aleohols, urea and
other similar organic substances, and ammonia and other
similar inorganic substances, for example. As examples of
particularly preferable reducing substances, the
hydrocarbons may be saturated, unsaturated, linear, or
branched hydrocarbons. They may be in a gaseous or liquid
state at normal room temperature. The hydrocarbons which
7B
CA 02363487 2001-12-04
assume a gaseous state at normal room temperature are
hydrocarbons having a carbon chain of C1 to C4 and those
which assume a liquid state at normal room temperature are
hydrocarbons having a carbon chain of C5 to C20. As
occasion demands, such mixtures of hydrocarbons as gasoline,
kerosene, and gas oil may be used. Likewise, the
aforementioned alcohols may be saturated, unsaturated,
linear, or branched alcohols. They have a carbon chain of
C1 to C6. They may be dihydric or trihydric alcohols
besides monohydric alcohols. The inorganic substances may
be hydrogen and carbon monoxides besides ammonia.
The amount of the reducing substance to be
introduced into the exhaust gas is preferable to be in the
range of 1 to 10 mols, preferably 1 to 5 mols, as reducing
substance per mol (as NO) of the nitrogen oxides adsorbed on
the catalyst. This amount is computed based on one molecule
of the reducing substance when the reducing substance is an
inorganic substance or on one carbon atom thereof when the
reducing substance is an organic substance.
This reducing substance generally is desired to be
introduced in a gaseous state onto the catalyst. If the
reducing substance is in a liquid state, however, it may be
directly introduced in a sprayed state onto the catalyst
with the aid of a nozzle, for example.
If the amount of the reducing substance to be
introduced is less than 1 mol on the molar ratio defined
above, the effect of the invention is not fully manifested.
Conversely, if this amount exceeds 10 mols, the excess
supply produces an unaltered portion of the reducing
substance and poses a problem of disposal of the unaltered
reducing substance, although the effect of the invention
itself is infallibly manifested. The upper limit imposed on
the introduction of the reducing agent, however, may shift
within some extent, since the reducing agent treating
activity of the catalyst is also concerned with the
disposal.
-8-
CA 02363487 2001-12-04
While the aforementioned magnitude is expressed on
the basis of the molar ratio of the reducing substance when
the reducing substance is an inorganic substance, it is
expressed on the basis of the number of carbon atoms of the
reducing substance when the reducing substance is an organic
substance. When the reducing substance happens to be urea,
the magnitude expressed as 2 mols of ammonia is adopted.
The determination of the amount of the adsorbed
nitrogen oxides may be attained, for example, by carrying
out a preparatory experiment as described below. To be
specific, this amount can be determined either directly in a
given internal combustion engine operated under the
conditions for working the method of this invention or
indirectly in a desk-top apparatus adapted to simulate the
temperature, composition, flow volume, etc. of the exhaust
gas emanating from the internal combustion engine. Now, the
procedure for the determination is shown below. First, in
an exhaust gas:pipe having nitrogen oxide analyzing meters
disposed one each in front of and behind the prospective
site for a catalyst bed, a catalyst conforming to this
invention is packed in a prescribed amount to form the
catalyst bed. Then, a mixed gas of oxygen and nitrogen set
at the same temperature and flow volume as those of a given
exhaust gas under the working conditions of the catalyst is
passed through the apparatus until this apparatus is fully
stabilized. Thereafter, a gas containing nitrogen oxides at
a concentration under the working conditions of the catalyst
is introduced instead of the mixed gas into the catalyst
bed. The nitrogen oxide concentrations are measured
continuously with the analyzing meters until the scale
reading of the analyzing meter disposed behind the catalyst
bed ceases to vary. Each momentary differences between the
nitrogen oxide concentrations before and after the catalyst
bed are integrated and the integrated amount is recorded as
the amount of nitrogen oxides adsorbed on the catalyst.
-9-
CA 02363487 2001-12-04
The catalyst possibly has an ability to decompose
nitrogen oxides during the measurement of the amount of
nitrogen oxides to be adsorbed thereon. If the catalyst in
use happens to possess this ability, the determination of
the amount of adsorption cannot be based on the
concentration of nitrogen oxides found in front of the
catalyst bed. In this case, therefore, the amount of
adsorption is computed, though in the same manner as
described above, on the basis of the constant concentration
of nitrogen oxides found behind the catalyst bed during the
measurement of the amountof adsorption in the place of the
concentration found in front of the catalyst bed.
When the reducing agent is intermittently
introduced, the amount of the reducing agent to be
introduced may be suitably selected indue consideration of
the flow volume and flow rate of the exhaust gas, the
concentration of the nitrogen oxides in the exhaust gas, and
the capacity of the catalyst for adsorbing nitrogen oxides.
It is preferable to introduce the reducing agent to the
catalyst bed before the catalyst has adsorbed nitrogen
oxides to saturation. This introduction before the
saturation of adsorption is attained by preparatorily
computing the amount of nitrogen oxides in the exhaust gas
per unit time based on the flow volume and flow rate of the
exhaust gas and the concentration of nitrogen oxides
therein, estimating the maximum length of time within which
the amount of nitrogen oxides adsorbed on the catalyst does
not reach the level of saturation, and continuing the
introduction of=the reducing agent until the time expires.
The adsorption prior to reaching this state of
saturation is preferable to be in the range of 5% to 90%,
preferably 15% to 80%, of the saturated amount of
adsorption. More preferably, it is in the range of 15% to
48%. If the amount of adsorption is less than 5%, the
frequency of introducing the reducing agent must be
increased possibly to the extent of jeopardizing the
-10-
CA 02363487 2001-12-04
operating conditions of the apparatus. Conversely, if this
amount exceeds 90%, on account of the approximation thereof
to the saturated amount of adsorption, the adsorption of
nitrogen oxides does not easily proceed and the amount of
nitrogen oxides suffered to pass through the catalyst is
consequently increased so much as to diminish the effect of
this invention.
The reducing agent, depending on the quality
thereof, has the possibility of being oxidized with the
oxygen in the air and consequently prevented from
manifesting an effect commensurate with the amount of its
introduction. In this case, the deficiency is preferable to
be compensated by preparatorily measuring the susceptibility
of the reducing agent to the oxidation under the working
conditions thereof as by use of an inert carrier and
increasing the amount of the reducing agent being introduced
proportionately to the degree of the susceptibility found as
above.
The reducing substance may be introduced at any time
when the result of actual measurement as mentioned above
reaches an appropriate level, with an amount correlative to
the level. Alternatively, as a convenient procedure, the
reducing substance can be introduced with a prescribed time
and amount schedule determined by repeating the former
procedure suitable times, averaging the results of the
mesurements, and deciding the amount of the reducing
substance correlative to the average.
Preferably, the reducing agent for use in this
invention is introduced at intervals in the range of 7
seconds to 60 minutes, preferably in the range of 10 seconds
to 20 minutes. If the intervals are less than 10 seconds,
the frequency of introduction will be so high as to force a
sacrifice of efficiency or economy. Conversely, if they
exceed 60 minutes, the capacity of the catalyst of this
-11-
CA 02363487 2001-12-04
invention for adsorption of NOx will be surpassed possibly
to the extent of bringing about an adverse effect on the
purification of the exhaust gas by the removal of NOx.
The duration of this introduction is in the range of
0.1 to 20 seconds, preferably 1 to 10 seconds. If. this
duration is less than 0.1 second, thorough removal of the
adsorbed NOx will not be possibly obtained. If it exceeds
20 seconds, the disadvantage arises that the reducing
substance will possibly fail to operate effectively.
Incidentally, the intervals of the introduction and
the duration of the introduction of the reducing substance
which are specified above as preferable for this invention
are variable to a certain extent due to the kind of the
catalyst to be used. They may be suitably varied,
therefore, within the ranges mentioned above.
In the method of this invention, the N0x adsorbed on
the catalyst in consequence of oxidation or activation is
concentrated in an activated state on the catalyst as
compared with the conventional method and, therefore, can be
reduced by the catalyst with high selectivity unlike the
method which comprises continuous introduction of the
reducing agent. Thus, the method of this invention affords
a saving in the total amount of the reducing agent to be
introduced at all. The continued introduction of the
reducing agent has the possibility of barring the oxidation
or adsorption of the N0x and rather degrading the efficiency
of purification of the exhaust gas by the removal of NOx.
In the suction system of the internal combustion engine, the
N0x accumulated therein can be removed by instantaneously
decreasing the amount of air being aspirated or supplying
the fuel in an excess amount thereby enabling the exhaust
gas to form a reducing atmosphere. By repeating the cycle
described above, the removal of the NOx from the exhaust gas
can be carried out continuously.
More preferably, the reducing substance is
introduced under conditions such that the catalyst will
-12-
CA 02363487 2001-12-04
adsorb NOx thereon while the exhaust gas is in the state of
containing excess air and, during the conversion of the
adsorbed NOx into less harmful substances, the oxygen
concentration during the introduction of the reducing agent
will be lowered to the extent of allowing the reaction of
the accumulated nitrogen oxides with the reducing agent to
proceed more readily than the oxidation of the reducing
agent. Thus, the removal of the accumulated nitrogen oxides
can be carried out more efficiently.
The exhaust gas to which the method of this
invention is applied is not particularly limited. The
method is effective when the exhaust gas to be treated forms
an atmosphere of excess oxygen containing NOx in the range
of 1 to 5,000 ppm. It is more effective when the exhaust
gas forms an atmosphere of excess oxygen containing N0x in
the range of 100 to 3,000 ppm. The conventional method
suffices when the exhaust gas to be treated does not form an
oxidizing atmosphere. If the N0x content is less than- 1
ppm, the adsorption side will be at a disadvantage from the
stoichiometric standpoint of adsorption. If it exceeds
5,000 ppm, the disadvantage will arise that the introduction
of the reducing substance must be frequently carried out.
This invention does not require the presence of an oxidizing
atmosphere throughout the entire period of the purification
of the exhaust. gas. It can be effectively utilized even
where an oxidizing atmosphere and a reducing atmosphere are
alternated repeatedly.
This invention in principle is capable of
accomplishing the removal of N0x from the exhaust gas under
treatment without reference to the NOx concentration in the
exhaust gas. When the exhaust gas under treatment contains
nitrogen oxides at a high concentration, the duration of
introduction of the reducing agent or the duration of
impartation of a reducing atmosphere to the exhaust gas must
be shortened. For this invention, the space velocity (S.V.)
of the exhaust gas under treatment relative to the catalyst
-13-
CA 02363487 2001-12-04
bed is preferable to be in the range of 1,000 to 300,000/hr,
preferably 10,000 to 200,000/hr. If the space velocity
exceeds 300,000/hr, the catalyst will manifest ample
reactivity with difficulty. Conversely, if it falls short
of 1,000/hr, the catalyst will have to be increased.in
volume and, moreover, the diffusion in the flow path of gas
will bring about the influence of nullifying the effect of
intermittently introducing the reducing substance or
imparting a reducing atmosphere to the exhaust gas.
The catalyst to be used in this invention comprises
(A) catalytically active components composed of (a) 0.1 to
30 g as metal per liter of the catalyst of at least one
noble metal selected from the group consisting of platinum,
palladium, rhodium, and ruthenium or a compound of the noble
metal and (b) 1 to 80 g as metal per liter of the catalyst
of at least one alkali or alkaline earth metal selected from
the group consisting of lithium, potassium, sodium,
rubidium, cesium, beryllium, magnesium, calcium, strontium,
and barium or a compound of the metal and (B) a refractory
inorganic oxide and optionally further comprises as another
catalytically active component 0.1 to 50 g per liter of the
catalyst of at least one heavy metal selected from the group
consisting of manganese, copper, cobalt, molybdenum,
tungsten, and vanadium or a compound of the heavy metal.
In the components mentioned above, such noble metals
as platinum, palladium, rhodium, and ruthenium, particularly
platinum and/or palladium, are effective in oxidizing NOx in
an oxidizing atmosphere. These noble metals function to
reduce and decompose N0x in the presence of a reducing
substance or in a reducing atmosphere besides functioning to
oxidize Nq{ in an oxidizing atmosphere. By using these noble
metals, therefore, the oxidation or activation of NOx in an
oxidizing atmosphere and the removal of the adsorbed NOx,
particularly N02, due to the intermittent introduction of a
reducing substance or in a reducing atmosphere can be
carried out with high efficiency. The amount of such a
-14-
CA 02363487 2001-12-04
noble metal to be used is in the range of 0.1 to 30 g,
preferably 0.5 to 5 g, as metal per liter of the catalyst.
If this amount is less than 0.1 g, the oxidation of NOx will
not easily proceed and the amount of NOx to be adsorbed will
be unduly small and the reduction and removal of the
adsorbed NOx will not be amply effected. Conversely, if it
exceeds 30 g, the excess noble metal will produce no
proportionate addition to the effect of noble metal and will
increase the cost of material possibly to the extent of
impairing the economy of the operation.
As the component for adsorbing the oxidized and
activated NOx, particularly NO2, alkali metals such as
lithium, sodium, potassium, rubidium, and cesium or
compounds thereof and/or alkaline earth metals such as
magnesium, calcium, strontium, and barium or compounds
thereof, particularly the compounds of alkali metals, are
effectively used. The amount of this component to be used
is in the range of 1 to 80 g, preferably 5 to 50 g, as metal
per liter of the catalyst. If this amount is less than 1 g,
the component acquires no sufficient capacity for adsorption
of NOx and, therefore, manifests an unduly low capacity for
treatment of NOx. Conversely, if the amount exceeds 80 g,
the capacity for treatment of NOx will be degraded because
the basicity grows fairly strong possibly to the extent of
enhancing the fast adsorption of NOx, the extent of curbing
the oxidation of NOx and the reduction of N0x by the noble
metal. Among other alkali metals mentioned above, potassium
and sodium prove particularly preferable. In this
specification, the amount of the alkali metal mentioned
above will be indicated as reduced to metal unless otherwise
specified.
The purification of the exhaust gas by the removal
of NOx can be carried out with greater efficiency when at
least one metal selected from the group consisting of
manganese, copper, cobalt, molybdenum, tungsten, and
vanadium or a compound of the metal is used as another
-15-
-_,
CA 02363487 2001-12-04
catalytically active component in addition to the components
mentioned above. This catalytically active component is
thought to play the part of promoting the oxidation and
adsorption of NOx in an oxidizing atmosphere and/or
promoting the reduction and decomposition of the adsorbed
NOx in the presence of a reducing agent or in an oxidizing
atmosphere.
The amount of this component to be used is in the
range of 0.1 to 50 g, preferably 1 to 20 g, per liter of the
catalyst. If this amount is less than 0.1 g, neither the
adsorption of nitrogen oxides nor the reduction of the
adsorbed NOx will be amply promoted. Conversely, if the
amount exceeds 50 g, the excess component will not bring
about any proportionate addition to the capacity for N0x
adsorption or the capacity for N0x reduction.
As the refractory inorganic oxide, any of the
inorganic oxides which are generally used as carriers for
catalysts can be adopted. As typical examples of these
inorganic oxides, a-alumina, or B-activated
alumina, titania, zirconia, ceria, lanthana, or silica,
mixtures thereof, and complex oxides may be cited. The
amount of the refractory inorganic oxide to be used is in
the range of 50 to 400 g, preferably 100 to 300 g, per liter
of the catalyst. The weight of the oxide will be computed
based on stable oxide unless otherwise specified. The
refractory inorganic oxide is generally in a powdery form.
The Brunauer-Emmett-Teller (hereinafter referred to as
"BET") surface area of the oxide is in the range of 10 to
400 m2/g, preferably 50 to 300 m2/g.
For the purpose of enabling the adsorption and
oxidation of ,NOx to proceed continuously with high
efficiency, the oxidizing component, the adsorbing
component, and the auxiliary component of the catalyst are
preferable to be carried in a homogeneously mixed state on
the refractory inorganic oxide instead of being locally
distributed.
-16-
CA 02363487 2001-12-04
In this invention, the mixture comprising the
catalytically active components and the refractory inorganic
oxide, in the actual treatment of the exhaust gas, is used
in the unmodified powdery form, in the molded form thereof
such as pellets or honeycombs, or in the coating form to a
three-dimensional structure base. Among other forms
mentioned above, the form using the three-dimensional
structure for coating proves particularly preferable. As
typical examples of the three-dimensional structure, pellets
and honeycomb carriers may be cited. Among other three-
dimensional structures, monolithically molded honeycomb
structures prove particularly preferable. As typical
examples of the monolithically molded honeycomb structures,
monolithic honeycomb carriers, metal honeycomb carriers, and
plug honeycomb carriers may be cited.
The monolithic carriers may be any of those which
are generally~ called the ceramic honeycomb carriers.
Particularly, the honeycomb carriers which are made of such
materials as cordierite, mullite, a-alumina, zirconia,
titania, titanium phosphate, aluminum titanate, bellite,
spodumene, aluminosilicate, and magnesium silicate prove
preferable. Among other examples cited above, the honeycomb
carriers made of cordierite prove especially preferable.
The monolithically molded structures which are made of such
metals resistant to oxidation and proof against heat as
stainless steel and Fe-Cr-Al alloy are also usable.
These monolithic carriers are produced by the
extrusion molding technique or the process of tightly
rolling a sheetlike material. The openings (cells) formed
therein for passage of a gas under treatment may be in a
hexagonal, tetragonal, or triangular cross section or in a
corrugated cross section. A cell density (number of
cells/unit cross section) which falls in the range of 100 to
600 cells/square inch, preferably 200 to 500 cells/square
inch, suffices for effective use of the monolithic carriers.
-17-
CA 02363487 2001-12-04
The amount of the mixture of the catalytically
active components and the refractory inorganic oxide to be
deposited on the honeycomb structure is in the range of 50
to 500 g, preferably 100 to 300 g, per liter of the
catalyst. If this amount is less than 50 g, the mixture
fails to manifest ample activity because of an unduly small
amount thereof. If the amount exceeds 500 g, the
disadvantage arises that the exhaust gas under treatment
will suffer as from loss of pressure.
The amount of the catalytically active components to
be used per liter of the catalyst is computed based on the
volume of the molded structure itself when those components
are molded by themselves or based on a three-dimensional
structure when the components are deposited on the three-
dimensional structure base.
Any of - the examples of method which will be adduced
hereinbelow can be adopted for the preparation of the
catalyst. Some other method than the methods shown below
may be adopted providing it causes no deviation from the
spirit of this invention. The examples are (a) a method for
obtaining a finished catalyst by impregnating a refractory
inorganic oxide with a mixed solution of catalytically
active components, drying the resultant impregnated
composite, optionally ealcining the dry composite thereby
obtaining a powder, wet pulverizing the powder together with
water added thereto and consequently forming a slurry,
applying the slurry to a honeycomb structure and allowing
the applied coat of slurry to dry, and optionally calcining
the coated honeycomb structure, (b) a method for obtaining a
finished catalyst by wet pulverizing a refractory inorganic
oxide together with water added thereto thereby forming a
slurry, applying the slurry to a honeycomb structure and
drying the applied coat of slurry, optionally calcining the
resultant dry coated honeycomb structure, then impregnating
the coated honeycomb structure with a mixed solution of
catalytically active components, drying the impregnated
-18-
CA 02363487 2001-12-04
honeycomb structure, and optionally calcining the dry
honeycomb structure, and (c) a method for obtaining a
finished catalyst by impregnating a refractory inorganic
oxide with a mixed solution of part(s) of catalytically
active components (such as, for example, an NOx oxidizing
component), drying and optionally calcining the resultant
impregnated composite thereby forming a powder, wet
pulverizing the powder together with water added thereto and
consequently forming a slurry, applying the slurry to a
honeycomb structure and drying the applied coat of slurry,
optionally calcining the dry coated honeycomb structure,
then impregnating the structure with a mixed solution of
remaining part(s) of catalytically active components (such
as, for example, an NOx adsorbing component), and drying and
optionally calcining the impregnated structure. The raw
materials which are usable for the formation of
catalytically active components include nitrides, chlorides,
sulfates, carbonates, and acetates, for example.
When the exhaust gas under treatment contains
hydrocarbons, carbon monoxide, etc. at high concentrations,
the catalyst mentioned above may be used in combination with
an oxidizing catalyst or a three-way catalyst. In this
case, the catalyst described above may be disposed in the
leading stage and the oxidizing catalyst or three-way
catalyst in the trailing stage relative to the inlet for the
exhaust gas. Particularly when the exhaust gas contains
hydrocarbons, carbon monoxide, etc. at high concentrations,
the three-way catalyst is used for the purpose of enhancing
the stoichiometric conversion of NOx with the oxidizing
catalyst.
When the exhaust gas under treatment has a low
temperature, the ratio of purification of the exhaust gas is
low as when an automobile is being started. The low
temperature is undesirable because it causes particularly
the ratios of removal of CO and HC to be conspicuously
increased. In this case, the ratio of conversion of HC can
-19-
CA 02363487 2001-12-04
be enhanced by preparatorily elevating the temperature of
the exhaust gas. The purification of the exhaust gas may be
carried out with a three-way catalyst or an oxidizing
catalyst installed as a warm-up catalyst.
In this case, the removal of nitrogen oxides from
the exhaust gas may be effected by a method of disposing the
three-way catalyst or oxidizing catalyst on the upstream
side of the flow of the exhaust gas, then the catalyst set
forth in claim 1 next thereto, and the three-way catalyst or
oxidizing catalyst farther on the downstream side.
The oxidizing catalyst has no particular
restriction. It is only required to be capable of oxidizing
hydrocarbons and carbon monoxide. The catalytic components
which are effectively usable in the oxidizing catalyst
include noble metals such as platinum and/or palladium and
refractory inorganic oxides such as alumina, titania, and
silica, for example. One or more members selected from
among rare earth oxides such as lanthanum oxide (La203) and
from among metals such as iron, cobalt and nickel may be
additionally used. The amount of the noble metal to be
preferable is- desired to be in the range of 0.1 to 5 g per
liter of the catalyst. The amount of the refractory
inorganic oxide to be carried is preferable to be in the
range of 10 to 300 g per liter of the catalyst. When the
oxide of a rare earth element is additionally incorporated
in the catalyst, the amount thereof is preferable to be
within the range of >0 to 150 g per liter of the catalyst.
If the amount of the noble metal is less than 0.1 g per
liter, the catalyst will acquire only an unduly low capacity
for the purification. If it exceeds 5 g per liter, the
excess supply ~of the noble metal will bring about no
proportionate addition to the effect. If the amount of the
refractory inorganic oxide to be added is less than 10 g per
liter, the ability to disperse the noble metal will be
degraded intolerably. If it exceeds 300 g per liter, the
excess supply of the inorganic oxide will bring about the
-20-
CA 02363487 2001-12-04
adverse effect of clogging a honeycomb being used as a
carrier for the refractory inorganic oxide. The oxide of a
rare earth element is added for the purpose of improving the
thermal stability of the refractory inorganic oxide. If the
amount of this oxide to be added exceeds 150 g per liter of
the catalyst, the excess supply of the oxide will bring
about the adverse effect of intolerably degrading the
strength with which the catalytic component is carried. In
the present invention, when the catalyst comprising at least
one metal selected from the group consisting of platinum and
palladium, at least one metal selected from the group
consisting of potassium, sodium, rubidium, and cesium, and a
refractory inorganic oxide is disposed on the upstream side
of the flow of the exhaust gas and then an oxidizing
catalyst is disposed-next thereto, the contents. of CO, HC,
etc. in the exhaust gas can be further lowered than when no
oxidizing catalyst is used.
Generally, (a) palladium, (b) platinum and rhodium,
(c) palladium and rhodium, or (d) platinum, palladium, and
rhodium as noble metal component(s), a refractory inorganic
oxide such as alumina, titania, or silica, and ceria are
essential catalytic components for the three-way catalyst.
This three-way catalyst may additionally incorporate therein
zirconia and/or the oxide of a rare earth element other than
cerium such as, for example, lanthanum oxide (La203). The
three-way catalyst is generally prepared by having the
catalytic components deposited on a honeycomb which is
generally used as a carrier for a catalyst. The amount of
the noble metal to be deposited is preferable to be in the
range of 0.1 to 5 g, the amount of such a refractory
inorganic oxide as alumina, titania, or silica.to be in the
range of 10 to:300 g, the amount of ceria (Ce203) to be in
the range of 10 to 150 g, and the amount of the oxide of a
rare earth element other than cerium to be in the range of 0
to 50 g respectively per liter of the catalyst. If the
amount of the noble metal is less than 0.1 g per liter, the
-21-
CA 02363487 2001-12-04
catalyst will acquire an unduly low capacity for the
purification. If the amount exceeds 5 g per liter, the
excess supply of the noble metal will bring about no
discernible addition to the effect of noble metal. If the
amount of the refractory inorganic oxide is less than 10 g
per liter, the insufficient supply of this oxide will
produce the adverse effect of impairing the ability thereof
to disperse the noble metal, for example. If this amount
exceeds 300 g per liter, the excess supply of this oxide
will bring about the adverse effect of clogging a honeycomb
being used as a carrier for the refractory inorganic oxide.
If the amount of ceria is less than 10 g per liter, the
effect of ceria manifested in storing and discharging oxygen
will not be fully manifested throughout the entire volume of
the catalyst. If this amount exceeds 150 g per liter, the
excess supply of ceria will produce the adverse effect of
degrading the strength with which the catalytic components
are carried. The addition of the oxide of a rare earth
element other than cerium is intended to improve the thermal
stability of the refractory inorganic oxide. If the amount
of this oxide to be added exceeds 50 g per liter, the excess
supply of the. oxide will produce the adverse effect of
degrading the strength with which the catalytic components
are carried because ceria is deposited to some extent on the
three-way catalyst. The three-way catalyst removes NOx in a
stoichiometric condition. It fails to effect the removal of
N0x in a lean condition. This invention, however, allows
the removal of NOx to proceed in a stoichiometric condition
to a greater extent when a catalyst comprising at least one
metal selected from the group consisting of platinum and
palladium, at least one metal selected from the group
consisting of potassium, sodium, rubidium, and cesium, and a
refractory inorganic oxide is disposed on the upstream side
of the flow of the exhaust gas and a three-way catalyst is
disposed next thereto than when no three-way catalyst is
disposed at all.
-22-
CA 02363487 2001-12-04
Typical examples of the three-way catalyst of this
kind are cited below.
(a) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 10 to 300
g of activated alumina and catalytically active components
composed of 0.5 to 30 g of palladium, 0.1 to 50 t of an
alkaline earth metal oxide, 10 to 150 g of cerium oxide, and
0.1 to 50 g of zirconium oxide per liter of the catalyst
(Japanese Patent Application No. 04-82,311).
(b) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 10 to 300
g of activated alumina and catalytically active components
composed of 0.5 to 30 g of palladium, 0.1 to 50 g of an
alkaline earth metal oxide, 0.1 to 50 g of lanthanum oxide,
to 150 g of eerium oxide, and 0.1 to 50 g of zirconium
oxide per liter of the catalyst (Japanese Patent Application
No. 04-149,400).
(c) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 10 to 300
g of activated alumina and catalytically active components
composed of 0.5 to 30 g of palladium, 0.1 to 50 g of an
alkaline earth metal oxide, 10 to 150 g of cerium oxide, 0.1
to 50 g of zirconium oxide, and 1 to 150 g of titanium oxide
per liter of the catalyst (Japanese Patent Application No.
04-166,383).
(d) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 10 to 300
g of activated alumina and catalytically active components
composed of 0.5 to 30 g of palladium, 0.1 to 50 g of an
alkaline earth metal oxide, 10 to 150 g of cerium oxide, 0.1
to 50 g of zirconium oxide, and 0.1 to 50 g of silicon oxide
per liter of the catalyst (Japanese Patent Application No.
04-166,460) .
(e) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 10 to 300
g of activated alumina and catalytically active components
-23-
___.
CA 02363487 2001-12-04
composed of 0.5 to 30 g of palladium, 0.1 to 50 g of an
alkaline earth metal oxide, 10 to 150 g of cerium oxide, 0.1
to 50 g of zirconium oxide, 1 to 150 g of titanium oxide,
and 0.05 to 50 g of silicon oxide per liter of the catalyst
(Japanese Patent Application No. 04-167,136).
(f) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 10 to 300
g of activated alumina and catalytically active components
composed of 0.1 to 20 g of the oxide of at least one metal
selected from the group consisting of iron, cobalt, and
nickel, 0.5 to 30 g of palladium, 0.1 to 50 g of an alkaline
earth metal oxide, 10 to 150 g of cerium oxide, and 0.1 to
50 g of zirconium oxide per liter of the catalyst (Japanese
Patent Application No. 04-167,363).
(g) A three-way catalyst having carried on a
monolithic structure carrier a mixture comprising 20 to 200
g of activated alumina and catalytically active components
composed of 0.1, to 5 g as a total of platinum and palladium,
0.01 to 1 g of rhodium, and 10 to 150 g of cerium oxide per
liter of the catalyst (JP-A-62-91,244).
(h) A three-way catalyst having carried on a
monolithic structure carrier 0.1 to 10 g of noble metal, 1
to 150 g of cerium oxide, and 50 to 200 g of a refractory
inorganic oxide (JP-A-01-27,643).
Now, the present invention will be described more
specifically below with reference to working examples. It
is to be distinctly understood that the invention is not
limited thereto, but may be otherwise variously embodied and
practiced without departure from the spirit of this
invention.
Referential Example 1
A powder was obtained by mixing 200 g of activated
alumina having a BET surface area of 100 m2/g with an
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum, drying the resultant mixture at 120 C for 2 hours,
and calcining the dry mixture at 500 C for 2 hours. This
-24-
CA 02363487 2001-12-04
powder was wet pulverized in a ball mill to obtain an
aqueous slurry:
A cordierite honeycomb carrier (the product of
Nippon Glass Co., Ltd.: containing 400 gas flow cells per
square inch of cross section and measuring 33 mm in
diameter, 76 mm in length, and 65 ml in volume) was dipped
in the aqueous slurry, then removed therefrom, and blown
with compressed air to expel excess slurry. Then, the
carrier coated with the slurry was dried at 120 C for 2
hours and calcined at 500 C for 2 hours to obtain a
honeycomb carrier coated with a platinum-carrying alumina
powder. The honeycomb carrier was dipped in an aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter, removed therefrom, blown with compressed air to
expel excess aqueous solution therefrom, dried at 120 C, and
calcined at 500 C to obtain a finished catalyst (1).. This
catalyst was found to have deposited on the carrier 3 g of
platinum, 10 g of sodium (as metal), and 200 g of activated
alumina per liter of the carrier.
Referential Example 2
A finished catalyst (2) was obtained by following
the procedure of Referential Example 1 while using an
aqueous palladium nitrate solution containing 3 g of
palladium in the place of the aqueous
dinitrodiammineplatinum solution containing 3 g of platinum.
This catalyst was found to have deposited on the carrier 3 g
of palladium, 10 g of sodium, and 200 g of activated alumina
per liter of the carrier.
Referential Example 3
A finished catalyst (3) was obtained by following
the procedure :of Referential Example 1 while using an
aqueous rhodium nitrate solution containing 3 g of rhodium
in the place of the aqueous dinitrodiammineplatinum solution
containing 3 g of platinum. This catalyst was found to have
deposited on the carrier 3 g of rhodium, 10 g of sodium, and
200 g of activated alumina per liter of the carrier.
-25-
CA 02363487 2001-12-04 -
Referential Example 4
A finished catalyst (4) was obtained by following
the procedure of Referential Example 1 while using an
aqueous ruthenium chloride solution containing 3 g of
ruthenium in the place of the aqueous
dinitrodiammineplatinum solution containing 3 g of platinum.
This catalyst was found to have deposited on the carrier 3 g
of ruthenium, 10 g of sodium, and 200 g of activated alumina
per liter of the carrier.
Referential Example 5
A finished catalyst (5) was obtained by following
the procedure of Referential- Example 1 while using an
aqueous solution containing lithium nitrate at a
concentration of 14.4 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 10 g of lithium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 6
A finished catalyst (6) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution containing potassium nitrate at a
concentration of 2.6 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 10 g of potassium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 7
A finished catalyst (7) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution containing rubidium nitrate at a
concentration of 1.2 mols/liter in the place of the aqueous
solution containing so-dium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 10 g of rubidium, and 200 g of
activated alumina per liter of the carrier.
-26-
CA 02363487 2001-12-04
Referential Example 8
A finished catalyst (8) was obtained by following
the procedure; of Referential Example 1 while using an
aqueous solution containing cesium nitrate at a
concentration of 0.8 mol/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 10 g of cesium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 9
A finished catalyst (9) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution containing beryllium nitrate at a
concentration of 11.1 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 10 g of beryllium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 10
A finished catalyst (10) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution containing magnesium nitrate at a
concentration of 8.2 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 20 g of magnesium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 11
A finished catalyst (11) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution containing calcium nitrate at a
concentration of 5.0 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 20 g of calcium, and 200 g of
activated alumina per liter of the carrier.
-27-
CA 02363487 2001-12-04
Referential Example 12
A finished catalyst (12) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution containing strontium nitrate at a
concentration of 2.3 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst was found to have deposited on
the carrier 3 g of platinum, 20 g of strontium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 13
A finished catalyst (13) was obtained by following
the procedure of Referential Example 1 while using an
aqueous solution. containing barium acetate at a
concentration of 1.5 mols/liter in the place of the aqueous
solution containing sodium nitrate at a concentration of 4.3
mols/liter. This catalyst, was found to have deposited on
the carrier 3 g of platinum, 20 g of barium, and 200 g of
activated alumina per liter of the carrier.
Referential Example 14
A finished catalyst (14) was obtained by following
the procedure of Referential Example 1 while using a mixed
aqueous solution containing dinitrodiammineplatinum
incorporating 3 g of platinum therein and copper nitrate
incorporating 2 g of copper therein in the place of the
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum. This catalyst was found to have deposited on the
carrier 3 g of platinum, 2 g of copper, 10 g of sodium, and
200 g of activated alumina per liter of the carrier.
Referential Example 15
A finished catalyst (15) was obtained by following
the procedure of Referential Example 1 while using a mixed
aqueous solution containing dinitrodiammineplatinum
incorporating 3 g of platinum therein and cobalt nitrate
incorporating 2 g of cobalt therein in the place of the
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum. This catalyst was found to have deposited on the
-28-
CA 02363487 2001-12-04
carrier 3 g of platinum, 2 g of cobalt, 10 g of sodium, and
200 g of activated alumina per liter of the carrier.
Referential Example 16
A finished catalyst (16) was obtained by following
the procedure of Referential Example 1 while using a mixed
aqueous solution containing dinitrodiammineplatinum
incorporating 3 g of platinum therein and manganese nitrate
incorporating 2 g of manganese therein in the place of the
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum. This catalyst was found to have deposited on the
carrier 3 g of platinum, 2 g of manganese, 10 g of sodium,
and 200 g of activated alumina per liter of the carrier.
Referential Example 17
A finished catalyst (17) was obtained by preparing a
catalyst (A) in the same manner as in Referential Example 1,
dipping this catalyst in an aqueous solution containing
ammonium molybdate at a concentration of 0.5 mol/liter,
removing the catalyst therefrom, blowing the wet catalyst
with compressed air to expel excess aqueous solution
therefrom, drying the catalyst at 120 C, and calcining the
dry catalyst at 500 C. This finished catalyst was found to
have deposited. on the carrier 3 g of platinum, 10 g of
sodium, 5 g of molybdenum, and 200 g of activated alumina
per liter of the carrier.
Referential Example 18
A finished catalyst (18) was obtained by preparing a
catalyst (A) in the same manner as in Referential Example 1,
dipping this catalyst in an aqueous solution containing
ammonium tungstate at a concentration of 0.3 mol/liter,
removing the catalyst therefrom, blowing the wet catalyst
with compressed air to expel excess aqueous solution
therefrom, drying the catalyst at 120 C, and calcining the
dry catalyst at 500 C. This finished catalyst was found to
have deposited on the carrier 3 g of platinum, 10 g of
sodium, 5 g of tungsten, and 200 g of activated alumina per
liter of the carrier.
-29-
CA 02363487 2001-12-04
Referential Example 19
A finished catalyst (19) was obtained by preparing a
catalyst (A) in the same manner as in Referential Example 1,
dipping this catalyst in an aqueous solution containing
vanadyl oxalate at a concentration of 1.0 mol/liter,
removing the catalyst therefrom, blowing the wet catalyst
with compressed air to expel excess aqueous solution
therefrom, drying the catalyst at 120 C, and calcining the
dry catalyst at 500 C. This finished catalyst was found to
have deposited on the carrier 3 g of platinum, 10 g of
sodium, 5 g of vanadium, and 200 g of activated alumina per
liter of the carrier.
Referential Example 20
A powder was obtained by mixing 100 g of the same
activated alumir-ia as used in Referential Example 1 with a
mixed solution of an aqueous dinitrodiammineplatinum
solution containing 2 g of platinum and an aqueous rhodium
nitrate solution containing 0.4 g of rhodium, drying the
resultant mixture at 120 C for two hours, and calcining the
dry mixture at 500 C for two hours. This powder and 50 g of
cerium oxide were wet pulverized together in a ball mill to
obtain an aqueous slurry. The same honeycomb carrier as
used in Referential Example 1 was dipped in the aqueous
slurry, removed therefrom, and blown with compressed air to
expel excess aqueous slurry. Then the resultant composite
was dried at 120 C for 2 hours to obtain a finished catalyst
(20). This catalyst was found to have deposited on the
carrier 2 g of platinum, 0.4 g of rhodium, 50 g of cerium
oxide, and 100 g of activated alumina per liter of the
carrier.
Referential Example 21
Zeolite, grade ZSM-5, was prepared in accordance
with the information reported in literature (Rapid
Crystallization Method, Proceedings 8th International
Congress on Catalysis, Berin, 1984, Vol. 3, P 569). By the
.X-ray analysis, the zeolite thus obtained was identified as
-30-
CA 02363487 2001-12-04
the product of grade ZSM-5. To the mixture obtained by
stirring 1.5 kg of this zeolite, grade ZSM-5, and 6 liters
of purified water added thereto at 98 C for two hours, an
aqueous solution containing copper ammine complex at a
concentration of 0.2 mol/liter was slowly added dropwise at
80 C. After the addition was completed, the resultant
mixture was stirred continuously at 80 C for 12 hours. It
was cooled to normal room temperature and then filtered to
separate the zeolite. The zeolite was thoroughly washed and
dried at 120 C for 24 hours. The powder consequently
obtained was wet pulverized in a ball mill to obtain an
aqueous slurry. Thereafter, a finished catalyst (21) was
obtained by following the procedure of Referential Example 1
while using the aqueous slurry instead. This catalyst was
found to have deposited on the carrier 120 g of the zeolite,
grade ZSM-5, and 6.9 g of copper per liter of the carrier.
Referential Example 22
A finished catalyst (22) was obtained by following
the procedure of Referential Example 6 while using 0.05 g of
platinum in the place of the aqueous dinitrodiammineplatinum
solution containing 3 g of platinum. This catalyst was
found to have deposited on the carrier 0.05 g of platinum,
g of potassium, and 200 g of activated alumina per liter
of the carrier.
Referential Example 23
A finished catalyst (23) was obtained by following
the procedure of Referential Example 6 while using 0.2 g of
platinum in the place of the aqueous dinitrodiammineplatinum
solution containing 3 g of platinum. This catalyst was
found to have deposited on the carrier 0.2 g of platinum, 10
g of potassium, and 200 g of activated alumina per liter of
the carrier.
Referential Example 24
A finished catalyst (24) was obtained by following
the procedure of Referential Example 6 while using 25 g of
platinum in the place of the aqueous dinitrodiammineplatinum
-31-
CA 02363487 2001-12-04
solution containing 3 g of platinum. This catalyst was
found to have deposited on the carrier 25 g of platinum, 10
g of potassium, and 200 g of activated alumina per liter of
the carrier.
Referential Example 25
A finished catalyst (25) was obtained by following
the procedure of Referential Example 6 while using 40 g of
platinum in the place of the aqueous dinitrodiammineplatinum
solution containing 3 g of platinum. This catalyst was
found to have deposited on the carrier 40 g of platinum, 10
g of potassium, and 200 g of activated alumina per liter of
the carrier.
Referential Example 26
A finished catalyst (26) was obtained by following
the procedure of Referential Example 6 while using a mixed
solution of an aqueous dinitrodiammineplatinum solution
containing 3 g of platinum and an aqueous palladium nitrate
solution containing 2 g of palladium in the place of the
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum. This catalyst was found to have deposited on the
carrier 3 g of platinum, 2 g of palladium, 10 g of
potassium, and 200 g of activated alumina per liter of the
carrier.
Referential Example 27
A finished catalyst (27) was obtained by following
the procedure of Referential Example 6 while using a mixed
solution of an aqueous dinitrodiammineplatinum solution
containing 3 g of platinum and an aqueous rhodium nitrate
solution containing 0.3 g of rhodium in the place of the
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum. This catalyst was found to have deposited on the
carrier 3 g of platinum, 0.3 g of rhodium, 10 g of
potassium, and :200 g of activated alumina per liter of the
carrier.
Referential Example 28
-32-
CA 02363487 2001-12-04
A finished catalyst (28) was obtained by following
the procedure of Referential Example 6 while using a mixed
solution of an aqueous dinitrodiammineplatinum solution
containing 3 g of platinum and an aqueous ruthenium nitrate
solution containing 0.3 g of ruthenium in the place of the
aqueous dinitrodiammineplatinum solution containing 3 g of
platinum. This catalyst was found to have deposited on the
carrier 3 g of platinum, 0.3 g of ruthenium, 10 g of
potassium, and 200 g of activated alumina per liter of the
carrier.
Referential Example 29
A finished catalyst (29) was obtained by following
the procedure of Referential Example 6 while using a mixed
solution of an aqueous palladium nitrate solution containing
g of palladium and an aqueous rhodium nitrate solution
containing 0.3: g of rhodium in the place of the aqueous
dinitrodiammineplatinum solution containing 3 g of platinum.
This catalyst was found to have deposited on the carrier 5 g
of palladium, 0.3 g of rhodium, 10 g of potassium, and 200
g of activated alumina per liter of the carrier.
Referential Example 30
A finished catalyst (30) was obtained by following
the procedure of Referential Example 6 while using an
aqueous solution containing potassium nitrate at a
concentration of 0.13 mol/liter in the place of the aqueous
solution containing potassium nitrate at a concentration of
2.6 mols/liter. This catalyst was found to have deposited
on the carrier .3 g of platinum, 0.5 g of potassium, and 200
g of activated alumina per liter of the carrier.
Referential Example 31
A finished catalyst (31) was obtained by following
the procedure of Referential Example 6 while using an
aqueous solution containing potassium nitrate at a
concentration of 0.52 mol/liter in the place of the aqueous
solution containing potassium nitrate at a concentration of
2.6 mols/liter. This catalyst was found to have deposited
-33-
CA 02363487 2001-12-04
on the carrier 3 g of platinum, 2 g of potassium, and 200 g
of activated alumina per liter of the carrier.
Referential Example 32
A finished catalyst (32) was obtained by following
the procedure'of Referential Example 6 while using an
aqueous solution containing potassium nitrate at a
concentration of 18.2 mols/liter in the place of the aqueous
solutioncontaining potassium nitrate at a concentration of
2.6 mols/liter. This catalyst was found to have deposited
on the carrier 3 g of platinum, 70 g of potassium, and 200 g
of activated alumina per liter of the carrier.
Referential Example 33
A finished catalyst (33) was obtained by following
the procedure of Referential Example 6 while using an
aqueous solution containing potassium nitrate at a
concentration of 23.4 mols/liter in the place of the aqueous
solution containing potassium nitrate at a concentration of
2.6 mols/liter. This catalyst was found to have deposited
on the carrier;3 g of platinum, 90 g of potassium, and 200 g
of activated alumina per liter of the carrier.
Referential Example 34
A finished catalyst (34) was obtained by following
the procedure of Referential Example 15 while using a mixed
aqueous solution of cobalt nitrate containing 0.05 g of
cobalt in the place of the aqueous solution of cobalt
nitrate containing 2 g of cobalt. This catalyst was found
to have deposited on the carrier 3 g of platinum, 0.05 g of
cobalt, 10 g of sodium, and 200 g of activated alumina per
liter of the carrier.
Referential Example 35
A finished catalyst (35) was obtained by following
the procedure of Referential Example 15 while using a mixed
aqueous solution of cobalt nitrate containing 0.2 g of
cobalt in the place of the aqueous solution of cobalt
nitrate containing 2 g of cobalt. This catalyst was found
to have deposited on the carrier 3 g of platinum, 0.2 g of
-34-
CA 02363487 2001-12-04
cobalt, 10 g of sodium, and 200 g of activated alumina per
liter of the carrier.
Referential Example 36
A finished catalyst (36) was obtained by following
the procedure of Referential Example 15 while using a mixed
aqueous solution of cobalt nitrate containing 25 g of cobalt
in the place of the aqueous solution of cobalt nitrate
containing 2 g of cobalt. This catalyst was found to have
deposited on the carrier 3 g of platinum, 25 g of cobalt, 10
g of sodium, and 200 g of activated alumina per liter of the
carrier.
Referential Example 37
A finished catalyst (37) was obtained by following
the procedure of Referential Example 15 while using a mixed
aqueous solution of cobalt nitrate containing 40 g of cobalt
in the place of the aqueous solution of cobalt nitrate
containing 2 g.of cobalt. This catalyst was found to have
deposited on the carrier 3 g of platinum, 40 g of cobalt, 10
g of sodium, and 200 g of activated alumina per liter of the
carrier.
Referential Example 38
A powder was obtained by mixing 100 g of the same
activated alumina as used in Referential Example 1 with an
aqueous dinitrodiammineplatinum solution containing 1 g of
platinum, drying the resultant mixture at 120 C for 2 hours,
and calcining the dry mixture at 500 C for 2 hours. An
aqueous slurry was obtained by wet pulverizing the powder, 2
g of lanthanum:oxide, and 2 g of iron oxide together in a
ball mill. Thereafter, a finished catalyst (38) was
obtained by following the procedure of Referential Example
20 while using the aqueous slurry instead. This finished
catalyst was found to have deposited on the carrier 1 g of
platinum, 2 g of lanthanum oxide, 2 g of iron oxide, and 100
g of activated alumina per liter of the carrier.
Example 1
-35-
CA 02363487 2001-12-04
The catalysts obtained in Referential Examples 1 to
37 were subjected to a test conforming to this invention
(hereinafter referred to as "Test") as shown hereinbelow and
to a test for comparison (hereinafter referred to as
"Control").
A sample catalyst was packed in a stainless steel
pipe 34.5 mm in diameter and 300 mm in length. A reaction
gas of the following composition was introduced into the
stainless steel pipe at a spatial velocity of 20,000/hr.
Preparatory Test
In preparation for the Test indicated below, the
sample catalyst was analyzed to determine the capacity
thereof for adsorption of nitrogen oxides. For the
determination, an apparatus having chemical emission type
nitrogen oxide analyzers (capable of determining the NO and
NO2 contents :as the total NOx content) (omitted from
illustration) connected one each via conduits 3 and 5 to the
pipe at the points in front of and behind an enclosure for a
catalyst bed 4 as illustrated in Fig. 1 was used. The
enclosure for a catalyst bed 4 in the apparatus kept heated
with an electric furnace 11 was filled with the sample
catalyst. Then, a gas composed of 2.0% by volume of oxygen
fed via a regulating valve 1b, 10% by volume of water fed
via a regulating valve 7a, and the balance to make up 100%
by volume of nitrogen fed via a regulating valve 1d was
introduced at 400 C into the apparatus. After the flow
volume of this gas had stabilized, nitrogen monoxide (NO)
was introduced into the apparatus so as to give the gas a
nitrogen monoxide content of 500 ppm. In the course of this
operation, the gas was analyzed continuously to determine
the NO contents-of the gas before and after the catalyst bed
4, with the determined NO contents accumulated to find the
capacity of the catalyst for adsorption of nitrogen oxides.
The results are shown in Table 1.
Then, with the inlet temperature of the catalyst bed
kept at 400 C, the operation was continued under a varying
-36-
CA 02363487 2001-12-04
set of conditions prescribed for a relevant test to find the
average ratio of removal of NOx per hour.
Test 1
A reaction gas was formed by feeding 500 ppm of
nitrogen monoxide (NO), 2.0% by volume of oxygen, 2,000 ppm
of carbon monoxide, and the balance to make up 100% by
volume of nitrogen respectively via regulating valves la,
1b, 1c, and ld to a gas mixer 2. By the action of a liquid
supply pump 8 and an evaporator 10, water was advanced via a
liquid supply valve 7a at a ratio calculated to give the
reaction gas a water content of 10% by volume and introduced
into the catalyst bed 4. Propylene (C3H6) was fed for a
duration of 10 seconds once per minute via a regulating
valve le at a ratio calculated to give the reaction gas a
propylene content of 3,000 ppm (as methane) and introduced
into the reaction gas in the gas mixer 2. The reaction gas
finally formed:was supplied at a spatial velocity of 20,000
hr-1 to the cat-alyst bed 4, with the inlet temperature of
the catalyst kept at 400 C with the electric furnace 11 to
carry out Test 1. The exhaust gas emanating from the
catalyst bed was discharged through a gas outlet 6. In the
course of this:operation, samples of the gas were extracted
through the conduit 5 via a sampling valve 9b and introduced
into the analyzer to determine the ratios of removal of NOx.
The results are shown in Table 1.
Test 2
This test was performed by following the procedure
of Test 1 while adjusting the oxygen concentration at 0.4%
by volume only during the introduction of the reducing agent
and at 2.0% :by volume during the absence of this
introduction. The results are shown in Table 1.
Control 1
This control was performed by following the
procedure of Test 1 while constantly adding propylene at a
ratio of 1,000 ppm (as methane) instead of intermittently
introducing propylene. The results are shown in Table 1.
-37-
CA 02363487 2001-12-04
Control 2
This control was performed by following the
procedure of Test 1 while omitting the use of propylene.
The results are shown in Table 1.
Table 1
Amount of NOx Ratio of removal of N0g (~)
Catalyst adsorbed to
saturation
(m.mol/lit)*1 Test 1 Test 2 Control 1 Control 2
1 12 75 90 40 10
2 10 70 88 36 12
3 11 78 90 40 11
4 12 77 91 35 13
10 72 88 34 8
6 13 73 92 35 11
7 10 70 88 38 12
8 11 75 90 30 12
9 10 71 88 30 10
10 69 89 28 11
11 12 75 90 30 11
12 12 72 91 35 12
13 13 72 90 34 11
14 15 80 93 44 11
14 79 93 43 11
16 18 71 95 42 12
17 16 76 96 45 11
18 15 75 96 46 10
19 15 82 97 45 12
1 20 25 20 11
*1: Amount of nitrogen oxides adsorbed (m.mol) per
liter of the catalyst.
-38-
CA 02363487 2001-12-04
Table 1 (continued)
Amount of NOx Ratio of removal of N0X ( G)
Catalyst adsorbed to
saturation
(m.mol/lit)*l Test 1 Test 2 Control 1 Control 2
21 5 41 45 40 31
22 6 40 63 18 8
23 11 70 86 24 8
24 11 68 88 40 18
25 7 54 72 44 20
26 18 85 98 42 12
27 16 82 94 40 13
28 12 72 88 36 14
29 11 76 90 36 12
30 5 42 64 30 10
31 10 72 86 30 12
32 28 70 84 20 12
33 10 40 60 16 11
34 = 12 72 88 38 8
35 14 78 92 42 11
36 15 78 92 42 11
37 14 64 80 30 6
*1: Amount of nitrogen oxides adsorbed (m.mol) per
liter of the catalyst.
Test 3
This test was performed by following the procedure
of Test 2 while using the catalyst obtained in Referential
Example 1 and varying the interval of introduction and the
duration of introduction of propylene, the molar ratio of
the amount of the reducing substance to the amount of NOx
adsorbed, and the kind of reducing substance. The results
are shown in Tables 2 to 5.
-39-
CA 02363487 2001-12-04
Table 2
Interval of introduction of reducing 20 60 60 60
substance (second)
Amount of NOx adsorbed (m.mol/lit) 2.2 6.1 6.1 6.1
per liter of the catalyst*l
Degree of saturation 17 48 48 48
of adsorption(%)*2
Reducing substance*3 Pr Pr Pr Pr
Concentration of reducing substance 3000 3000 1500 4500
(ppmC1) in the reaction gas
Duration of introduction of reducing 3 10 20 10
substance (second)
Amount of reducing substance 3.0 8.3 8.2 12.3
introduced (m.mol/liter) per liter
of the catalyst*4
Amount of reducing substance 1.4 1.3 1.3 2.0
introduced / Amount of NOx adsorbed
(molar ratio)
Ratio of removal of NOx M 94 90 88 91
*1: Amount of NOx adsorbed in one process of
introduction of reducing substance per liter of the
catalyst.
*2: (Amount of nitrogen oxides adsorbed/amount of
nitrogen oxides adsorbed by the catalyst to saturation) x
100(%)
*3: Reducing substance - Pr: propylene (gas)
*4: Amount of reducing substance introduced in one
process of introduction of reducing substance per liter of
the catalyst.
-40-
CA 02363487 2001-12-04
Table 3
Interval of introduction of 5 15 3000 4800 10 10 45 120
reducing substance (second)
Amount of NOx adsorbed
(m.mol/lit) 0.7 1.7 12 12 1.2 1.2 4.4 10.1
per liter of the catalyst*1
Degree of saturation of 6 13 100 100 10 10 37 81
adsorption (%)*2
Reducing substance*3 Pr Pr Pr Pr Pr Pr Pr Pr
Concentration of reducing
substance (ppmCl) 3000 3000 3000 3000 9000 9000 1500 1500
in the reaction gas
Duration of introduction of 1 3 20 20 0.05 0.5 15 30
reducing substance (second)
Amount of reducing substance
introduced (m.mol/liter) 0.8 2.4 16.5 16.5 0.12 1.2 6.2 12.3
per liter of the catalyst*4
Amount of reducing substance
introduced / Amount of NOx 1.1 1.3 1.3 1.3 0.1 1.0 1.4 1.2
adsorbed (molar ratio)
Ratio of removal of NOA (~) 40 88 50 32 18 88 71 42
*1: Amount of NOx adsorbed in one process of
introduction of reducing substance per liter of the
catalyst.-
*2: (Amount of nitrogen oxides adsorbed/amount of
nitrogen oxides adsorbed by the catalyst to saturation) x
100(%)
*3: Reducing substance - Pr: propylene (gas)
*4: Amount of reducing substance introduced in one
process of introduction of reducing substance per liter of
the catalyst.
-41-
CA 02363487 2001-12-04
Table 4
Interval of introduction of reducing 60 60 240 360
substance (second)
Amount of NOx adsorbed (m.mol/lit) 6.1 6.1 11.8 12
per liter-of the catalyst*1
Degree of saturation of adsorption 48 48 95 100
(%)*2
Reducing substance*3 Pr Pr Pr Pr
Concentration of reducing substance 3000 3000 9000 9000
(ppmC1) in the reaction gas
Duration of introduction of reducing 4 15 29 73
substance (second)
Amount of reducing substance
introduced (m.mol/liter) per liter 3.2 12.2 70.9 180
of the catalyst*4
Amount of reducing substance
introduced / Amount of NOx adsorbed 0.5 2 6 15
(molar ratio)
Ratio of removal of NOx (~) 44 91 60 44
*1: Amount of NOx adsorbed in one process of
introduction of reducing substance per liter of the
catalyst.
*2: (Amount of nitrogen oxides adsorbed/amount of
nitrogen oxides adsorbed by the catalyst to saturation) x
100M
*3: Reducing substance - Pr: propylene (gas)
*4: Amount of reducing substance introduced in one
process of introduction of reducing substance per liter of
the catalyst.
-42-
CA 02363487 2001-12-04
Table 5
Interval of introduction of reducing 120 60 60 60
substance (second)
Amount of NOx adsorbed (m.mol/lit) 10.1 6.1 6.1 6.1
per liter -of the catalyst*1
Degree of saturation of adsorption 81 48 48 48
(%)*2
Reducing substance*3 Pr An Et Ur
Concentration of reducing substance 3000 3000 - -
(ppmC1) in the reaction gas
Duration of introduction of reducing 20 10 - -
substance (second)
Amount of reducing substance
introduced (m.mol/liter) per liter 15.6 6.1 12.2 6.1
of the catalyst*4
Amount of reducing substance
introduced / Amount of NOx adsorbed 1.5 1.0 2.0 1.0
(molar ratio)
Ratio of removal of NOx (~) 70 90 89 89
*1: Amount of NOx adsorbed in one process of
introduction of reducing substance per liter* of the
catalyst.
*2: (Amount of nitrogen oxides adsorbed/amount of
nitrogen oxides adsorbed by the catalyst to saturation) x
100W
*3: Reducing substance - Pr: propylene (gas), An:
Ammonia (ga), Et: Ethanol (introduced by spraying 0.56 g of
liquid per liter of catalyst), Ur: Urea (introduced by
spraying 0.46 'g of aqueous 40 wt% solution per liter of
catalyst)', providing 1 mol of urea computed as 2 mols of
ammonia.
*4: Amount of reducing substance introduced in one
process of introduction of reducing substance per liter of
the catalyst.
-43-
CA 02363487 2001-12-04
Example 2
The samples of the catalysts obtained in Referential
Examples 6, 20, and 38 were disposed in varying orders
indicated in Table 6 in the direction from the upstream to
the downstream side of the flow of the exhaust gas and
tested by following the procedure of Example 1. The results
are shown in Table 6.
Table 6
Ratio of removal of NOX
Cat. Cat. Cat. Amount of W
up- mid- down- NOx adsorbed
stream stream stream (m.mol/lit)*1 Test 1 Test 2 Con- Con-
trol 1 trol 2
6 - 20 14 86 99 44 13
20 - 6 13 85 98 42 12
6 - 6 26 75 94 37 11
20 - 20 1 22 26 22 12
6 - 38 19 86 99 44 13
38 - 6 18 85 98 42 13
20 6 20 16 88 99 44 14
38 6 38 22 88 99 44 14
*1: Amount of nitrogen monoxide adsorbed (m.mol)
per liter of the catalyst.
-44-
CA 02363487 2001-12-04
INDUSTRIAL APPLICABILITY
This invention pertains to a method for the removal
of nitrogen oxides from an exhaust gas. characterized by
causing the exhaust gas in an oxidizing atmosphere to
contact a catalyst comprising a refractory inorganic oxide
and catalytically active components, the components
comprising 0.1 to 30 g as metal per liter of the catalyst of
at least one noble metal selected from the group consisting
of platinum, palladium, rhodium, and ruthenium or a compound
of the noble metal and 1 to 80 g as metal per liter of the
catalyst of at least one metal selected from the group
consisting of lithium, potassium, sodium, rubidium, cesium,
beryllium, magnesium, calcium, strontium, and barium or a
compound of the metal, thereby inducing the catalyst to
adsorb thereon the nitrogen oxides in the exhaust gas and,
subsequently introducing a reducing substance intermittently
into the exhaust gas thereby purifying the exhaust gas by
reducing the nitrogen oxides adsorbed on the catalyst. It,
therefore, permits efficient removal of NOx from an exhaust
gas under treatment in an oxidizing atmosphere by means of
oxidation and adsorption and then enables the N0x adsorbed
on the catalyst to be efficiently removed therefrom by
either intermittent introduction of a reducing agent to the
catalyst or imparting a reducing atmosphere to the exhaust
gas. Further, the method of this invention permits
efficient disposal of NOx by use of a reducing agent only in
a small amount without requiring any special device.
Besides., the catalytic system contemplated by this
invention, when used in combination with an oxidizing
catalyst and/or a three-way catalyst, produces the advantage
of further exalting the efficiency of the removal of
hydrocarbons and carbon monoxide.
-45-