Note: Descriptions are shown in the official language in which they were submitted.
CA 02363648 2001-11-22
A METHOD FOR THE CONTINUOUS ELECTROWINNING OF PURE
TITANIUM METAL FROM MOLTEN TITANIUM SLAG, ILMENITE AND
OTHER SEMICONDUCTIVE TITANIUM OXIDE COMPOUNDS
TECHNICAL FIELD
This invention relates to a method for the continuous electrowinning of pure
titanium metal from titanium slag and other electrically semiconductive
titanium
mixed oxide compounds particularly ilmenite.
BACKGROUND ART
Titanium metal has been produced and manufactured on a commercial scale
since the early 1950s for its unique set of properties : (i) high strength-to-
weight
ratio, (ii) elevated melting point, and (iii) excellent corrosion resistance
in various
harsh chemical environmentst. Actually, about 55% of titanium metal produced
worldwide is used as structural metal in civilian and military aircraft and
spacecraft
such as jet engines, airframes components, and space and missile
applicationsz.
Titanium metal is also employed in the chemical process industries (30%),
sporting
and consumer goods ( 14%), and in a lesser extend power generation, marine,
ordnance, architecture, and medical3. Titanium sponge which is the primary
metal
form of titanium is produced industrially by the Kroll's process which is a
metallothermic reduction of gaseous titanium tetrachloride with magnesium.
Potential huge markets such as automotive parts are still looking forward to
seeing
the cost of the primary metal to decrease by 50-70%. Nevertheless, this cost
is only
maintained high due to the expensive metallothermic process used to win the
metal.
Even if the Kroll's process was greatly improved and modernized since its
first
industrial introduction it still exhibits several drawbacks : ( 1 ) it is
performed under
~ CARDARELLI, F (2001 ) - Materials Handbook : A Concise Desktop Reference. -
Springer-Verlag, London,
New York, pages 115-135.
2 GAMROGI, J. - Titanium and Titanium Dioxide - from - Mineral Commodity
Summaries.- U.S. Bureau of
Mines (1995) p.180.
3 GAMBOGI, J. - Annual Report : Titanium-1992 - U.S. Bureau of Mines ( 1993) p
CA 02363648 2001-11-22
2
batch conditions leading to expensive downtimes, (2) the inefficient contact
between reactants leads to slow reaction kinetics, (3) it requires the
preparation,
purification, and use the volatile and corrosive titanium tetrachloride
(TiCl4) as the
dominant feed with its associated health and safety issues, (4) the process
can only
accept expensive rutile or ruble substitutes (e.g., upgraded titanium slag,
synthetic
ruble) as raw materials, (5) the magnesium and chlorine must be recovered from
reaction products by electrolysis in molten salts accounting for 6% of the
final cost
of the sponge, (6) the specification of low residual oxygen and iron content
of the
final ingot requires expensive and complex refining steps (e.g., vacuum
distillation,
and/or acid leaching) of the titanium sponge in order to remove entrapped
inclusions accounting for about 30% of the final cost of the ingot, finally
(7) it only
produces dendritic crystals or powder requiring extensive reprocessing before
usable mill products can be obtained (i.e., remelting, casting, forging) and
wastage
of 50% is common in fabricating titanium parts. For all these reasons, since
the
early 1970s there is a strong commitment of the titanium industry in synergy
with
several academic institutes for actively researching new routes to produce
titanium
metal with a focus on developing a continuous process to produce high-purity
and
low-cost titanium powder for metallurgical applications. Although a plethora
of
alternative methods have been examined beyond a laboratory stage or have been
considered for preparing titanium crystals, sponge, powder, and alloys, none
have
reached the industrial production. Included in those processes were : (i)
gaseous
and plasma reduction, (ii) tetraiodide decomposition, (iii) calcio- and
aluminothermic reduction, (iv) disproportionation of TiCl3 and TiCl2, (v)
carbothermic reduction, and (vi) electrowinning in molten salts. Most were
considered by the authoring National Materials Advisory Board committee
(NMAB)4 panel to be unlikely to progress to production in the near future
except
electrowinning which seemed to be the most promising alternative route.
Actually,
4 NATIONAL MATERIALS ADVISORY BOARD Committee on Direct Reduction Processes
for the Production of
Titanium Metal. 1974. Report # NMAB-304, National Academy of Sciences,
Washington, DC.
CA 02363648 2001-11-22
3
the extraction and preparation of pure metals from ores using an electrolytic
process is known as electrowinning. This process is based on the
electrochemical
reduction of metal cations present in a suitable electrolyte by electrons
supplied by
a negative electrode (i.e., cathode, -) according to the Faraday's law of
electrolysis,
while at the positive electrode (i.e., anode, +) an oxidation reaction occurs
(e.g.,
anode dissolution, gas evolution, etc.). Today among the current industrial
electrolytic processes several utilize an aqueous electrolyte to
electrodeposit the
metal (e.g., Cu, Zn, Ni, Pb, Au). Unfortunately, aqueous electrolytes which
exhibit
a narrow electrochemical span are unsuitable for preparing highly
electropositive
metals such as titanium. Actually, when cathodic (i.e., negative) potentials
are
applied to the electrode, the competitive process of the electrochemical
reduction of
protons occurs accompagnied with the evolution of hydrogen gas. This main
parasitic reaction consumes the major part of the reduction current decreasing
drastically the overall current efficiency. Despite the availability of
cathode
materials exhibiting a large hydrogen evolution overpotential (e.g., Cd, Hg,
Pb), it
is quite impossible to electrodeposit such metals despite numerous attempts
reported in the literature about the electrodeposition of titanium metal from
aqueous solutionss 6 7 g 9. prganic electrolytes were also testedlo II I2 but
despite
their wide decomposition potential limits, organic solvents in which an
appropriate
supporting electrolyte has been dissolved have not yet been used industrially
owing
to their poor electrical conductivity which increases ohmic drop between
electrode
5 KOLTHOFF, M., and THOMAS, J. - J. Electrochem. Soc. 9( 1964) 1065.
6 SINHA, N.H., and SWARUP, D. -Indian Mining J. Spec. 1(1975)134.
KUDRYATSEV, V.N., LYAKHOV, B.F., ANUFRIEV, W.G., and PEDAN, K.S. - Hydrog.
Met. Proc. Int. Cong.
2nd. Pergamon Press, Oxford 1977, page 5.
g SOFRONKOV, A.N., PRVII, E.N., PRESNOV, V.N., and SEMIZOROV, N.F. -Zh. Prikl.
Khim. 51(1978)607
9 BRIBIESCA, S.L., CONTRERAS, E.S., and TAVERA, F.J. -Electrowinning of
Titanium from Sulfuric Acid
Titanium Solutions. -Proc. Titanium'92, The Minerals, Metals, and Materials
Society 1993, pages 2443-
2444.
~° BIALLOZOR, S., and LISOWSKA, A. -Electrochim. Acta 25(1980)1209.
~ ~ LISOWSKA, A., and BIALLOZOR, S. - Electrochim. Acta 27( 1982) 105.
~Z ABBOTT, A.P., BETTLEY, A., and SCHIFFRIN, D.J. -J. Electroanal. Chem.
347(1993)153-164.
CA 02363648 2001-11-22
4
gap, the low solubility of inorganic salts, their elevated cost and toxicity.
By
contrast, molten salt based electrolytes were already used industrially since
the
beginning of the 1900s in the electrolytic preparation of important structural
metals
(e.g., Al, Mg), and in a lesser extend for the preparation of alkali and
alkali-earth
metals (e.g., Na, Li, and Be). Actually, fused inorganic salts exhibit
numerous
attractive features 13 14 over aqueous electrolytes, these advantages are as
follows
( 1 ) they produce ionic liquids having a wide electrochemical span between
decomposition limits (i.e., high decomposition potential) allowing the
electrodeposition of highly electropositive metals such as titanium. (2) Based
on the
Arrhenius law, the high temperature required to melt the inorganic salt
promotes
fast electrochemical reaction kinetics suitable to increase hourly yields. (3)
The
faradaic efficiencies are usually close to 100%. (4) Due to their ionic state
molten
salts possess a high electrical ionic conductivity which minimizes the ohmic-
drop
and induces lower energy consumption. (5) The elevated solubility of
electroactive
species in the bath allows to utilize high solute concentrations allowing to
operate
at high cathodic current densities. Therefore, the best route for
electrowinning
titanium is to develop an electrolytic process conducted in molten salt
electrolytes.
However, despite the numerous attempts performed until today there are still
no
current electrolytic processes in molten salts for producing titanium metal
industrially. In order to reach industrial success the new electrochemical
route must
solve the major issues of the expensive and labor intensive Kroll's process
and also
overcome the pitfalls that have lead to unsuccess until today and abandon of
all
industrial electrolytic pilot plants.
Actually, several attempts were made in the industry to produce primary
titanium metal by an electrolytic process. First of all, in 1956 at the former
U.S.
Bureau of Mines (USBM) in Boulder City, Nevada a small pilot was built to
~3 DELIMARSKII, Iu.K., and MARKOV, B.F. (1961) - Electrochemistry ofFused
Salts. - Sigma Press
Publishing, New York.
" LANTELME, F., INMAN, D., and LOVERII~1G, D.G. (1984) - Electrochemistry-I,
in Molten Salt Techniques,
LovERING, D.G., GALE, R.J. (Eds.) Vol. 2., Plenum Press, New York, pp. 138-
220.
CA 02363648 2001-11-22
investigate the electrowinning of titanium's. It consisted of a 12-inch
cylinder
vessel lined with pure iron containing the molten electrolyte made of a
mixture of
LiCI-KC1 approximately at the eutectic composition with TiCl2 added. Three
equally spaced openings in the cell top accomodated : (i) the replaceable
anode
5 assembly, (ii) the titanium tetrachloride feed unit, and (iii) the cathode.
Three slide
valves combined with air-locks allowed the quick and easy introduction or
removal
of assemblies without contaminating the cell. The desired solute (i.e., TiCl2)
was
produced in-situ either by the chemical reduction of stoichiometric amount of
TiCl4
with titanium metal scrap or by direct electrochemical reduction of TiCl4 at
the
cathode. Actually, TiCl4 which is a covalent compound does not ionize and must
be
converted to a ionic compound such as TiCl2. The concentration was then
increased
by operating only the feed cathode and anode and feeding one mole of TiCl4 per
two faradays of charge. In all cases gaseous TiCl4 was introduced into the
bath
close to the cathode with a feed nickel tube plated with molybdenum and dipped
below the surface level of the melt. In order to avoid the oxidation of the
newly
formed Ti2+ and dragout of the dissolved TiCl4 with the chlorine evolved at
the
anode, a porous ceramic diaphragm made of alundum~ (i.e., 86 wt.% A1203-12
wt.%Si02)~6 surrounded the immersed graphite anode forming distinct anolyte
and
catholyte compartments. The optimum operating conditions identified were : ( 1
) an
operating temperature above 500°C to prevent the precipitation of
K2TiF6, and
below 550°C to avoid severe corrosion of the alundum diaphragm, usually
520°C,
(2) a solute content comprises between 2 and 4 wt.% TiCl2, (3) a cathodic
current
density of 1 to 5 kA.lri Z, while the anodic current density was comprised
between 5
and 10 kA.rri 2, (4) a diaphragm current density of 1.5 kA.rri 2. By
conducting
experiments with the above conditions USBM claimed that high-purity titanium
was electrowon with a Brinell hardness as low as 68 HB and a current
efficiency of
~5 LEONE, O.Q., KNUDSfiN, H., and COUCH, D.E. - High-purity titanium
electrowon from titanium
tetrachloride. -J. O. M. 19(1967)18-23
CA 02363648 2001-11-22
6
60%. However frequent failures of the diaphragm that became periodically
plugged
or loaded with titanium crystals proved troublesome. As the titanium content
increased, the ceramic diaphragm became conductive and then acted as a bipolar
electrode and had to be removed rapidly from the bath. In 1972, the same
authors"
build a larger rectangular cell containing 226.8 kg (i.e., 500 1b.) of bath in
order to
use assess the actual performances of two kind of diaphragm materials : (i)
solid
materials composite diaphragms, and (ii) loose fill materials composite
diaphragms.
For solid diaphragms it was observed that alundum coated nickel screen showing
no deterioration but however it was subject to the same current density
limitation as
the porous alundum diaphragm. On the other hand, cemented coated nickel screen
with loose fill material such as alumina was the best material in terms of
strength,
flexibility, resistance to corrosion, and low replacement of titanium (0.2 to
1.0
wt.%).
In 1968, Priscut8 of the Titanium Metal Corporation (TIMET) has designed
and operated a new electrowinning cellt9 in Henderson, Nevada. This
electrolytic
cell was a unique pilot based on a non diaphragm basket cathode type. The cell
used a suspended central metal basket cathode with sixteen anodes peripheral
to the
basket. The central basket cathode was a cubic box with the four sides made of
perforated steel plates, while the bottom and top were blind plates. Four
steel rods
were used in the basket to act as cathode collectors while TiCl4 was fed using
a
tube positionned at the center of the basket. TiCl4 intially was fed at a low
rate into
the center of the basket walls. This porous sidewall deposit served as a
diaphragm
to keep the reduced TiCl2 inside the basket while a mechanical system for
'6 COUCH, D.E., LEONE, O.Q., LANG, R.S., and BLUE, D.D. - Evaluation of
diaphragm materials for
electrowinning high-purity titanium. Proc. Extractive Met. Div. Symp., Met.
Soc. RIME Chicago IL
December 11-13 1967, pp. 309-323.
" LEONE, O.Q, and COUCH, D.E. (1972) - Use of Composite Diaphragms in the
Electrowinning of Titanium.
- Report Investigation #7648, U.S. Dept. of Interior, Bureau of Mines,
Washington D.C.
'8 PRISCU, J.C. - Symp. on Electrometallur~, Proc. AIMS Extractive Metallurgy
Div, Cleveland Ohio,
December 1968, page 83.
'9 TIMET - An electrolytic cell for electrolysis of titanium tetrachloride. -
French Pat. 1,496,806 Aug. 24,
1966.
CA 02363648 2001-11-22
7
withdrawing the large cathode deposits into an inert-gas-filled chamber,
installing a
new cathode, and reclaiming the inert gas for reuse. The average valence of
dissolved titanium canons was maintained very low generally no greater than
2.1 to
obtain the electrodeposition of premium-grade titanium metal. TIMET claimed
that
later models of pilot-plants have produced until 363 to 408 kg (i.e., 800 to
900 1b.)
of titanium metal in one cathode deposit.
Later in 1971, Hashimoto et al. have published a series of three
comprehensive articles2° ZI z2 regarding the electrowinning of titanium
from its
oxides or mixed oxides. Titanium solute was introduced in a molten fluoride
bath,
as a solid compound such as Ti02, CaTi03, FeTi03, or MgTi03. The melts tested
were CaF2, MgF2, BaF2, NaF and their mixtures. The first electrolysis study
was
conducted at temperatures above 1600°C with a graphite anode and
cathode. Only
in the cases of the CaF2-Ti02 (1-10% wt.) and CaF2-CaTi03 (10% wt.) systems
molten titanium was obtained but largely contaminated by carbon and oxygen (2-
4
wt.%). In other cases, fine titanium powder was obtained. After the
preliminary
results, they focussed on the electrowinning of titanium from pure Ti02
carried out
in molten salt baths made of CaF2, BaF2, MgF2, CaF2-MgF2, CaF2-NaF, CaF2-
MgF2-NaF, CaFz-MgF2-NaF2, and CaF2-MgF2-SrF2 at 1300-1420°. The
titanium
electrodeposited in CaF2 and BaF2 baths was considerably contaminated by
carbon
owing to graphite electrodes. In NaF-containing fused salts such as CaFz-NaF
and
CaF2-MgF2-NaF, only fine powdery deposits were obtained due to simultaneous
sodium reduction. In the baths of MgF2, CaF2-MgF2, CaF2-MgF2-BaF2, and CaF2-
MgF2- SrF2, dendritic deposits were obtained. They pointed out that best
result was
obtained in the CaF2-MgF2 bath, but the purity of the deposit was not as high
as
z° HASHIMOTO, Y.; URII'A, K.; and KOIVO, R. - Electrowinning of
titanium from its oxides. Part I. Fused salt
electrolysis at temperatures above the melting point of the metal. - Denki
Kagaku 39(6) (1971)516-522.
z' HASI-IImoTO, Y. - Electrowinning of titanium from its oxides. Part II.
Influences of fluoride salt baths on
fused-salt electrodeposition of titanium metal from titanium dioxide. - Denki
Kagaku 39(12) (1971)938-943.
z2 HASHIMOTO, Y. - Electrowinning of titanium from its oxides. Part III.
Electrowinning of titanium from
titanium dioxide or calcium titanate in calcium fluoride-magnesium fluoride
molten salt baths. - Denki
Kagaku 40(1) (1972) 39-44.
CA 02363648 2001-11-22
8
that of the common grade titanium sponge required in the industry. In the
third
article, electrowinning of titanium was carried out in CaF2-MgF2 (50-50 wt.%)
molten salt baths at 1020-1030°C in an argon atmosphere by using a
completely
enclosed cell. In electrowinning from Ti02, the form of the electrodeposited
metal
changed from crystaline to spongelike with an increase in current density, or
cell
voltage, but when CaTi03 was used, deposits were spongelike. The hardness of
the
deposits was influenced by the bath temperature of the last stage of
electrolysis and
by the duration of electrolysis at the given temperature. The material yield
of
titanium was superior to 95 wt.% but do not meet the requirements of
commercial
sponge. Moreover, Hashimoto used a graphite basket to contain the titanium
compounds at the anode because it was unable to make a conductive soluble
anode.
Later in 1980, the Dow Chemical Company in a close working relationship
with the HOWMET group (i.e., subsidiary of the French Pechiney Ugine
Kuhlmann Group) founded the D-H Titanium Company for producing continuously
high-purity electrolytic titanium at Freeport, Texas. Cell design, operating
procedure, metal quality, proposed production, and economic projections have
been
described by Cobel et a1.23. The technology utilized was based on the cell
designed
by the U.S. Bureau of Mines, and on the previous work done at Dow by Juckniess
et a124. Actually, an alleged major cell improvement in the D-H Titanium
design
was the fabrication of a metal screen diaphragm that was electroless-plated
with
cobalt or nickel to give the required electrical and flow characteristics. The
cell
operated with argon blanketed LiCI-KC1-TiCl2 (ca. 2 wt.% TiCl2) at 520-
600°C
molten salt electrolyte. TiCl4 was fed continuously into a pre-reduction
cathode
compartment where reduction to dichloride TiCl2 takes place at a separate feed
cathode within the cell. Final reduction to metal is continuously done on
separate
deposition cathodes which are periodically removed hot into an inert
atmosphere
z3 CosEl., G.; FISHER, J.; and SNYDBR, L.E - Electrowinning of titanium from
titanium tetrachloride: pilot
plant experience and production plant projections 1969-1976. - Conference:
Titanium '80, Science and
Technology, Vol. 3, Kyoto, Japan, 19-22 May 1980 TMS/AIME, Warrendale, Pa.
CA 02363648 2001-11-22
9
stripping machine and then replaced within the cell. Metal-working cathodes
were
individually pulled, stripped, and replaced in the cell, in an argon
atmosphere, by a
self positioning and automatically operated mechanical device. A sealed, argon-
sheilded hopper containing the titanium crystals and entrained electrode was
cooled
before being opened to discharge its contents. Crystalline metal and dragout
salts
were crushed to 3/8-inch size and leached in dilute 0.5 wt.% HC1 solution.
Then the
spent solution was neutralized with a mixture of Li2C03 and KOH in a ratio
equivalent to that used in the electrolyte. Dragout of electrolyte varied with
the
titanium crystal sizes to about 1 kg per kg of fine titanium for coarse washed
metal.
Dragout was dried and passed over a magnetic separator, and metal fines were
removed by screening to about 80 mesh (177 gm). They claimed that the sponge
produced exhibited both a low residual oxygen, nitrogen, iron and chlorine
content,
had a Brinell hardness of 60 to 90 HB and excellent melting characteristics.
According to Cobel et a1.25, the direct current required for electrowinning (
17.4
kWh/kg) appears to be only about half that required for the Kroll process.
Although
titanium sponge of excellent purity was claimed to be produced in relatively
small
pilot-plant cells with a daily titanium capacity of up to 86 kilograms per day
the
electrowinning of titanium was far from the industrial scale. Unfortunately,
in
December 30th, 1982, according to the American Metal Market (AMM), the
expenses for completing the joint program and the economic climate at that
time
have forced the dissolution of the D-H Titanium Company. With the breakup each
company (i.e., Dow and Howmet) Dow has continued some research and
development work on the electrolytic process but without success while Howmet
apart some work done in France and applied for patent coverage2627 have later
focused in the metal fabrication area.
za JucKNIESS, P.R., and JotINSON, D.R. - Method for Electrowinning Titanium. -
U.S. Pat. 4,118,291;
October 3 (1978).
zs MAY COBEL G., FISHER J., and SNYDER L. (1980) - Electrowinning of Titanium
from Titanium
Tetrachloride. - 4th International Conference on Titanium, May 1980, Kyoto,
Japan.
zs A~ANO, M. - Process for the Preparation of Titanium by Electrolysis. - U.
S. Pat. 4,381,976, May 3
( 1983).
CA 02363648 2001-11-22
In 1988, the Italian company Elettrochimica Marco Ginatta S.p.A. (EMG)
owned by the Italian scientist and business man Marco Vincenzo Ginatta2g
applied
for patent on a new electrowinning process largely inspired from the three
previous
pilots of USBM, TIMET, and DH29. This new upgraded process for the
electrolytic
5 preparation of titanium uses always the dissolution and cathodic reduction
of
titanium tetrachloride in an electrolyte made of alkali or alkaline-earth
metal
halides and the electrodeposition of the dissolved titanium cations. The
process was
supported by RMI Titanium, and the company built a pilot plant. Ginatta
claimed
that the current production capacity of this plant reached 70 tonnes per year
in
10 19853°. Unfortunately, in 1990 RMI closed the plant owing to
inability to solve
"engineering issues". More recenty, the same company, now renamed Ginatta
Torino Technology (GTT) applied for patent on a new process for electrowinning
titanium based on the recovery of the molten metal using a pool cathode like
for
aluminium. The main idea of Ginatta is to avoid common dendritic
electrodeposits
by producing the electrodeposited titanium metal in the liquid state such as
for
aluminium. Marco Ginatta continues to pursue chloride process and has built a
pilot
in Torino, Italy.
Simultaneously, in the period 1997-2000 Kawakami et a1.31 have proposed
an electroslag remelting process. The main idea was to avoid common dendritic
electrodeposits by producing the electrodeposited titanium metal in its liquid
state.
Direct electrowinning of liquid titanium metal was the investigated techniques
by
using a direct current Electro-Slag Remelting (i.e., DC-ESR) apparatus. A
small
2' ARMAND, M. - Novel Apparatus and process for the TiCl4 feed to electrolysis
cells for the preparation of
titanium. - U.S Pat. 4,396,472, August 2 (1983).
z8 GINATTA, M. V.; ORSELLO, G.; and BERRUTI, R. - A method for the
electrolytic production of a polyvalent
metal and equipment for carrying out the method. - PCT Int. Appl., 33 pp. WO
8910437 (1990).
Z9 GINATTA, M.V.; and ORSELLO, G. - Plant for the electrolytic production of
reactive metals in molten salt
baths. - Eur. Pat. Appl. EP 210961 Apr. 02, 1987.
3o pIMARIA, E. - RMI Gets License to Make New Type of Titanium. - Metalworking
News, February I S'.,
1988.
3~ KAwAIC.4MI, M.; OOISHI, M.; TAKE1JAKA, T.; and SUZUKI, T. - The possibility
of electrowinning of liquid
titanium using ESR apparatus. - Proc. Int. Conf. Molten Slags, Fluxes Salts
'97, Sth (1997) 477-482 Iron and
Steel Society, Warrendale, PA.
CA 02363648 2001-11-22
11
scale DC-ESR unit of 110 mm inner diameter was operated in d.c. reverse
polarity
mode, where a graphite rod was used as anode and a steel or a copper base-
plate
was used as cathode. The used slag was Ca0-CaF2-Ti02 mixture. The current was
approximately 1.5 kA. Under certain experimental conditions, some amount of
titanium was electrodeposited in the metal pool. From the view point of heat
balance, the sufficient heat was supplied by the Joule heating in molten slag
phase.
It can be seen from these results that the present process is possible in
principle but
unfortunately most of the deposit was obtained as TiC and the current
efficiency for
the reduction was only 1.5%. In 1999, the process was improved32, the current
efficiency for the reduction was up to 18% with the proper distance between
the
electrodes. Some amount of titanium was electrodeposited on the base-plate
though
its state changed with the electrolytic condition. Pure titanium metal pieces
were
obtained in the solidified salt after the run with the bigger distance. It was
concluded that the electrowinning of liquid titanium metal by the present
process
was possible if sufficient heat to form a metal pool can be supplied at the
bigger
distance between the electrodes. The DC-ESR process was patented in 1988 and
reconducted in 2000, and then recently presented at ECS33.
Recently in 2000, based on early results obtained by Fray, Farthing, and
Chen34 3sat the Dept. of Materials Science of the Cambridge university, and
later on
early trials that were conducted and patented36 3~at the Defence Evaluation
and
32 TAKENAKA, T.; SUZUKI, T.; ISHIKAWA, M.; FUKASAWA, E.; and KAWAKAMI, M. -
The new concept for
electrowinning process of liquid titanium metal in molten salt. -
Electrochemistry 67(6) (1999) 661-668.
33 TAKENAKA, T.; ISHIKAWA, M.; and KAWAKAMI, M. - Direct electrowinning of
liquid titanium metal by
using direct current electro slag remelting apparatus. - Proc. Electrochem.
Soc. (Molten Salts XIL) (2000) 99-
41 578-584.
3a CHEN, G.Z, FRAY, D.J., and FARTHING, T.W - Direct electrochemical reduction
of titanium dioxide to
titanium in molten calcium chloride - Nature 407 (2000) 361-364.
ss FRAY, D.J., FARTHING, T. W., and CHEN, G.Z. - Removal of Oxygen from Metal
Oxides and Solids
Solutions by Electrolysis in a Fused Salt. - British Pat. Appl. WO 99/64638 16
December 1999.
36 WARD-CLOSE, C. M., and GODFREY, A.B. - Electrolytic reduction of metal
oxides such as titanium
dioxide and process applications. - U.S. Pat. Appl. W001/62996, 20 Feb. 2001.
3' WARD-Cl.osE, C. M., and GODFREY, A.B. - Method of Manufacture for Ferro-
Titanium and Other Metal
Alloys Electrolytic Production. - U.S. Pat. Appl. W001/62994, 19 Feb. 2001
CA 02363648 2001-11-22
12
Research Agency (DERA) at Farnborough (Hampshire, U.K.) a new company
British Titanium (BTi) has been formed to commercialize the newly discovered
process of refining metallic titanium3g. The inventors have proposed an
entirely
novel electrolytic route, that the scientific litterature has already called
the Fray's or
FFC process, the process claims to avoids conversion of the titanium dioxide
to the
tetrachloride or the dissolution of the feedstock into a molten electrolyte.
The
inventors have demonstrated at the laboratory scale that the reduction
reaction
proceeds at 950°C from a cathode made originally of solid Ti02 while
oxidation of
oxygen anions occurs at the graphite anode with evolution of carbon dioxide.
The
molten salt electrolyte selected is made of pure calcium chloride (CaCl2) bath
which exhibits both a high solubility for oxygen and excellent migration
transport
properties for oxygen anions. According to inventors, the process for the
production of pure titanium metal consists of the following sequences of
operations. The titanium dioxide powder is mixed with an appropriate binder to
form a past or slip, and cast into a rectangular shape cathodes using one of
the
techniques common in the ceramic industry, such as rolling or slip casting.
The
green cathode will be then fired in an air kiln to initiate sintering in order
to
produce a solid ceramic material. After sintering the shapes give solid
cathodes.
Reduction of titanium occurs in an enclosed electrolytic cell with inert gas
filling.
The cell is designed for continuous operation with cathodes at different
stages in
their cycles being inserted and removed through an automated air lock. By
controlling the cathode potential, oxygen can be removed from titanium dioxide
allowing to leave behind a high purity metal which is morphologically similar
to
the Kroll's sponge. The cell voltage is roughly 3 V, which is just below the
decomposition voltage of CaCl2 (3.25 V at 950°C), avoiding chlorine
evolution at
the anode but well above the decomposition voltage of Ti02 (1.85 V at
950°C).
Sufficient overpotential is necessary to reduce the oxygen content of the
titanium
38 Financial Times December 21 st, 2000, page 12.
CA 02363648 2001-11-22
13
metal. The inventors claim39 that stoichiometric mixture of other metal oxides
with
Ti02 into the original cathode are also concurrently reduced to metal leading
to the
possibility to produce valuable titanium alloys although the microstructure
may not
be the same. The process has been demonstrated in a bench-scale reactor (i.e.,
1
kilogram per day). Whilst there are obvious difficulties to be overcome in
scaling-
up the vessel, primarily in reducing the diffusion path for oxygen through the
metal
leading to several hours to completely reduce a porous pellet made of sintered
Ti02, and increase the extremely low energy efficiency. It is a single-stage
process,
since the waste CaCl2 can be removed of the titanium by water leaching after
the
completion of the reaction. They claim that perating costs will be somewhat
reduced by lower labour requirements, and by the elimination of the vacuum
distillation stage. Unfortunately, the specific energy consumption of the
process is
not provided and the process requires pigment-grade TiOz or synthetic Ti02,
such
as upgraded titanium slag, though the impurities will report to the finished
metal; or
on mixtures of oxides.
The Fray's process4° claimed that it overcomes several of the
issues
encountered by its predecessors but its poor energy efficiency, its extremely
low
hourly yield related to the slow diffusion kinetics of oxygen into titanium
metal,
and the mandatory use of a graphite anode are important pifalls for a future
commercial development. Actually, the electrodeposited of titanium metal at
the
surface of the cathode impedes the diffusion of oxygen anions and represent
the
rate determining step in the overall process. Moreover, the use of a graphite
anode
leads to an elevated anode overpotential, leading to high cell voltage and
thus
elevated specific energy consumption. Finally, the anodic oxidation mechanisms
leads to severe corrosion of the graphite with contamination of the melt by
floating
39 FRAY, D.J., FARTHING, T. W., and CHEN, G.Z. - Removal of Oxygen from Metal
Oxides and Solids
Solutions by Electrolysis in a Fused Salt. - British Pat. Appl. WO 99/64638 16
December 1999.
ao CHHN, G.Z, FRAY, D.J., arid FARTHING, T.W - Direct electrochemical
reduction of titanium dioxide to
titanium in molten calcium chloride - Nature 407 (2000) 361-364
CA 02363648 2001-11-22
14
flakes of graphite leading to short circuits between electrodes. In addition
the
evolution of gases such as carbon dioxide and chlorine gives the so-called
"anode
effect" at elevated anodic current densities.
Therefore, the present invention would seek to resolve most if not all of the
previous issues related to the electrolytic production of the titanium metal
by using
an anode and a cathode made of titanium slag such as the Sorelslag~ and
Richard's
Bay Slag~ which are produced at QIT and Richard's Bay by the reduction of
ilmenite with anthracite by smelting into an electric arc furnace (EAF).
Actually,
titanium slag is mainly composed of sub-stoichiometric titanium oxides with
the
typical Andersson-Magnelli crystal structure4~ having the global chemical
formula
Ti02_X, (e.g., Ti305, Ti40~, and Ti509) and its melting range is comprised
between
1640-1660°C. This composition was already confirmed by the work of
Desjardins42. Hence, these oxides exhibit both a good corrosion resistance and
a
low electrical resistivity similar to that of pure graphite (i.e., as low as
630 ~S2.cm)
and their mechanical and electroconductive properties can be compared to those
of
the electrode material named Ebonex~43 and produced commercially by the
British
company Atraverda Ltd. since the 1980s.
The major benefits to use a soluble anode made of titanium slag are : ( 1 )
the
anodic dissolution of the titanium slag feed continuously providing the
electrochemical reactor with titanium electroactive species avoiding the
tedious
introduction of a solute (i.e., the electrode acts as an electroactive species
reservoir)
and hence preventing the anodic evolution of chlorine or carbon dioxide. (2)
The
cell operating voltage being maintained extremely low resulting in a low
specific
energy consumption. (3) Finally, the more active impurities (e.g., Si, Ca, Mg)
4~ ANDERSSON, S., COLLEN, B., KUYLENSTIERNA, U., and MAGNELLI, A. Acta Chem.
Scand. 11(1957)1641
az pESdARDINS, J. F. ( 1986) - Study of Insoluble Formation in Richards Bay
Slag. - QIT Internal Report No
R-I1-86.
as CARDARELLI, F (2001 ) - Materials Handbook : A Concise Desktop Reference. -
Springer-Verlag Ltd.,
London, pages 329-330
CA 02363648 2001-11-22
contained in the titanium slag remaining dissolved in the bath while the more
noble
(e.g., Fe, V, Mn, and Cr) not being dissolved and being recovered in the
sludge at
the bottom of the cell (i.e., electrorefinning). While the major benefits of a
molten
titanium slag cathode is its low viscosity allowing the droplets of liquid
titanium
5 metal to fall by gravity to the bottom of the cell and forming a pool of
liquid
titanium acting as a current collector and that does not impedes the oxygen
diffusion allowing rapid reduction of the titanium slag. In addition
conducting the
electrolysis in inorganic fluoride melts which are strong complexing ligands
solves
the issue of disproportionation of titanium species and stabilizes the high
valence
10 states of titanium in the form of the T1F62~. Other benefits provided by
fluoride
melts that are utilized in this invention are44 : ( 1 ) wide decomposition
potentials, (2)
low dynamic viscosities, (3) low melting range when used in mixtures with the
eutectic composition, (4) elevated ionic conductivities, and (5) capability to
dissolve large amount of solute leading to operate at elevated cathodic
current
15 densities. Therefore, the soluble titanium slag anode and molten titanium
slag
cathode overcomes all the issues of the prior art FFC process such as the poor
hourly yield and the low energy efficiency and utilization of crude feedstock
materials.
DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic flow sheet of the entire process.
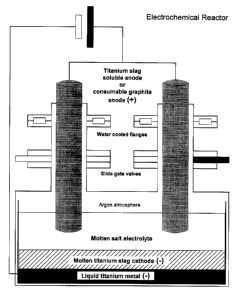
Figure 2 is a schematic illustration of the electrochemical reactor.
MODES OF CARRYING OUT THE INVENTION
Generally speaking, This invention relates to a method for the continuous
electrowinning of pure titanium metal from titanium slag and other
electrically
semiconductive titanium mixed oxide compounds particularly ilmenite. The
'4 CARDARELLI, F., T.axiL, P., and SAVALL, A. - Tantalum Protective Thin
Coating Techniques for the
Chemical Process Industry : Molten Salts Electrocoating as New Alternative -
Int. J. Refractory Metals &
Hard Materials 14(1996)365-381.
CA 02363648 2001-11-22
16
method involves crushing and grinding a mass of crude titanium slag,
separating
magnetically the major impurities in the ground slag, melting the purified and
conductive titanium slag at elevated temperature, pouring the molten titanium
slag
at a bottom of an electrolytic cell to form a liquid cathode pool covered with
a layer
of molten salt electrolyte. Reducing cathodically by direct current
electrolysis at
high temperatures the molten titanium slag to produce droplets of pure liquid
titanium metal. Owing to the slightly higher density of the liquid titanium
compared to that of the molten titanium slag or molten ilmenite, the droplets
fall by
gravity at the bottom of the electrolytic cell forming after coalescence a
pool of
liquid titanium metal. The liquid titanium metal is continuously siphoned or
tapped
under an inert atomosphere and cast into a dense, coherent, and pure ingot.
The first step consists in comminuting (i.e., crushing and grinding) the crude
titanium slag (i.e., 78-82 wt.% Ti02) or other semiconductive titanium
compounds
such as ilmenite to a final particle size comprised preferably between 0.075
mm
and 0.42 mm (i.e., 40 and 200 mesh Tyler), but most preferably between 0.105
mm
and 0.150 mm (100 and 150 mesh Tyler). The comminution step is necessarily
required in order to render easier the removal of inert minerals present in
the crude
titanium slag (e.g., silicates, sulfides) and facilitate the removal of
associated
chemical impurities (e.g., Fe, Si, Ca, Mg). The removal of these impurities is
suitable for both improving the electrical conductivity of the titanium slag
or
ilmenite and for enhancing its titanium concentration.
Secondly, the finely ground titanium slag or ilmenite produced undergoes a
common magnetic separation step. The strong ferromagnetic phases such as for
instance free metallic iron entrapped in the titanium slag during the smelting
process and the intimately binded silicate minerals are efficiently removed
using a
low magnetic induction of 0.3 tesla and separated with the magnetic fraction
which
is discarded or rerouted. Then the remaining materials undergoes a second
magnetic separation conducted with a stronger magnetic induction of 1 tesla.
The
CA 02363648 2001-11-22
17
non magnetic fraction that contains all the diamagnetic mineral phases (e.g.,
silica,
silicates, etc.) is also discarded. The remaining material consists of a
finely purified
ground titanium slag (i.e., 85-88 wt.% Ti02) or finely ground ilmenite mainly
composed of semiconductive titanium oxide with the Andersson-Magnelli crystal
structure (i.e., titanium oxides having the general stoichiometry Ti02_X, such
as
Ti305, Ti40~, and Ti509).
Thirdly, the purified ground titanium slag or ilmenite is melted above
1700°C during at least 2 hours. Once totally liquid the molten purified
titanium slag
or molten ilmenite is introduced at the bottom of an electrolytic cell and
covered by
a layer of molten salt electrolyte.
The electrolytic cell which is designed for continuous operation consists of a
closed reactor vessel set into a high temperature furnace. with soluble
titanium slag
anodes or consumable graphite anodes that can be inserted and removed from the
electrochemical reactor at different stages in their cycles being without any
entries
of air and moisture through airtight locks which are closed by means of large
gate
valves. The electrochemical reactor is heated by Joule effect during
electrolysis.
The inert atmosphere within the cell is performed by an argon stream purified
by
passing it through a water and oxygen traps (i.e., getter).
The electrolytic bath is made of inorganic salts or their mixtures preferably
selected from the group consisting of M"Xm wherein M = Li, Na, K, Rb, Cs, Be,
Mg, Ca, Sr, Ba and X = F-, Cl-, Br , I', SO42-, N03-, CO32-, B032-, P043-or
mixtures
thereof, preferably alkali-metals and alkali-earth metals halides, but more
preferably alkali-metals and alkali-earth metals chlorides or fluorides with a
final
preference for CaCl2, CaF2, or the following mixtures of salts with the
eutectic
composition (e.g., CaF2-CaCl2, CaF2-CaCl2-CaO, CaF2-LiCI-Ca0).
CA 02363648 2001-11-22
18
The electrolysis is performed under galvanostatic conditions (i.e., at
constant
current) by imposing a direct current between the soluble titanium slag anode
or
consumable graphite anode (+) and the molten titanium slag or molten ilmenite
cathode (-) by mean of an electric power supply or a rectifier . Usually
cathodic and
anodic current densities of 20 kA.rri Z are imposed with a cell voltage of
less than 5
volts. The electric current is maintained until the desired electric charge
has
circulated. During the cathodic reduction of the titanium slag or of the
molten
ilmenite, owing to the high operating temperature which is slightly above the
melting point of titanium metal (1660°C) and the slightly higher
density of the
liquid titanium (4110 kg.m-3) compared to that of the molten titanium slag
(4000
kg.m-3), the electrodeposited titanium forms small liquid metal droplets that
fall by
gravity at the bottom of the electrolytic cell forming a pool of pure liquid
titanium
metal that also acts as an efficient current collector and hence it does not
impedes
the diffusion of oxygen from the titanium slag to the electrolyte. While at
the anode
dissolution of the titanium slag anode or combustion of the graphite anode
occurs.
The level of molten titanium slag in the electrolytic cell is permanently
adjusted in
order to insure continuous electrolysis. The liquid titanium metal is
continuously
siphoned or tapped under an inert argon atmosphere and cast into pure, dense,
and
coherent ingot. The titanium ingot produced exhibited a high purity (i.e., 99
wt.%
Ti) and other characteristics that satisfies the grade EL-110 in accordance
with the
standard B299-99 from the AmeYican Society for Testing Materials (ASTM)45 such
as a low residual oxygen, nitrogen, iron and chlorine content, a Brinell
hardness of
60 HB. The electrowinning process exhibits a low specific energy consumption
of
15 kWh/kg of titanium metal produced.
as ASTM B299-99 - Standard Specifrcation for Titanium Sponge. - American
Society for Testing and
Materials (ASTM)
CA 02363648 2001-11-22
19
EXAMPLE
The electrolytic cell consists to a molten titanium slag or molten ilmenite
acting as
a liquid cathode (-) covered by a thick layer of a molten salt electrolyte
such as
CaF2-CaCl2-CaO. The anode (+) is either a consumable graphite anode or a
soluble
titanium slag anode. The electrolysis is performed at temperature of
1680°C under
galvanostatic conditions (i.e., at constant current) by imposing a direct
current
between the electrodes by mean of an electric power supply or a rectifier .
Usually
cathodic and anodic current densities of 20 kA.rri 2 are imposed with a cell
voltage
of less than 5 volts. The electric current is maintained until the desired
electric
charge has circulated. During the cathodic reduction of the titanium slag or
of the
molten ilmenite, owing to the high operating temperature which is slightly
above
the melting point of titanium metal (1660°C) and the slightly higher
density of the
liquid titanium (4110 kg.rri 3) compared to that of the molten titanium slag
(4000
kg.rri 3), the electrodeposited titanium forms small liquid metal droplets
that fall by
gravity at the bottom of the electrolytic cell forming a pool of pure liquid
titanium
metal that acts as an efficient current collector and hence it does not
impedes the
diffusion of oxygen required by the reduction from the titanium slag to the
electrolyte. While at the anode, the dissolution of the titanium slag or the
combustion of the graphite anode forming carbon dioxide occurs. The level of
molten titanium slag in the electrolytic cell is permanently adjusted in order
to
insure continuous electrolysis. The liquid titanium metal is continuously
siphoned
or tapped under an inert argon atmosphere and cast into pure, dense, and
coherent
ingot. The titanium ingot produced exhibited a high purity (i.e., 99 wt.% Ti)
and
other characteristics that satisfies the grade EL-110 in accordance with the
standard
B299-99 from the American Society for Testing Materials (ASTM)46 such as a low
residual oxygen, nitrogen, iron and chlorine content, a Brinell hardness of 60
HB.
a6 ASTM B299-99 - Standard Specification for Titanium Sponge. - American
Society for Testing and
Materials (ASTM)
CA 02363648 2001-11-22
The electrowinning process exhibits a low specific energy consumption of 15
kWh/kg of titanium metal produced.