Note: Descriptions are shown in the official language in which they were submitted.
CA 02363658 2001-11-21
1
TITLE OF INVENTION
Improved process for the preparation of Ramipril.
FIELD OF INVENTION
The present invention relates to a process for the production of inhibitors of
ACE
(Angiotensin Converting Enzyme) and, in particular, to a process for
separation of
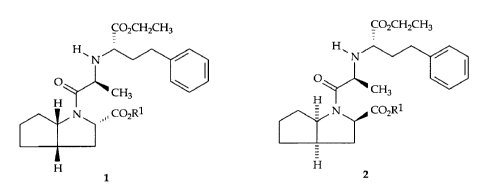
diastereomeric mixtures of compounds of formula 1 and 2.
COZCHZCH3 COZCH2CH3
H H.
~N / N /
O \ ~ O
CH3 \
CH3
H H
N ,,, C02R1 N COZR1
H H
1 2
wherein Rl = H or Rl denotes a carboxyl-esterifying group, such as Ci-C6
alkyl, or
C~-Cs aralkyl.
BACKGROUND OF THE INVENTION
The previously reported syntheses of Ramipril (1, wherein Rl = H) use two
approaches. The first approach utilizes the reaction of racemic amino esters
4a and 4b
(R2 denotes a carboxyl-esterifying group, such as Ci-C6 alkyl, C~-Cs aralkyl,
preferably
benzyl or tert-butyl)
CA 02363658 2001-11-21
2
H H
H I H I
N ,~~,, C02R2 N C02R2
H H
4a 4b
with a compound of formula 3, wherein the atoms indicated with an asterisk
have the S
configuration,
CO2CH2CH3
H~ N
HO *
CH3
O
3
using amide formation methods known in peptide chemistry (such as those
described
in CA 1,338,162, EP 79022, US 5977380, ES 549789 and ES 2004804, for example)
to
prepare the mixture of compounds 5 and 6,
C02CHZCH3 COZCHzCH3
H ~ ~ H ~ I
O~ ~ O
_CH3 ~ _CH3
~COz~ ~ N~COzRz
H H
5 6
wherein R2 denotes a carboxyl-esterifying group, such as CZ-C6 alkyl, or C~-Ca
aralkyl.
This route gives a 1:1 mixture of diastereomers 5 and 6 from which the desired
diastereomer 5 is separated using silica gel chromatography. Subsequent
removal of the
CA 02363658 2001-11-21
3
protecting group by hydrogenolysis or treatment with an acid or base yields
Ramipril
(compound of formula 1, R1 = H). This approach is disclosed in EP 79022, for
example.
This procedure suffers from the disadvantage of requiring two additional
synthetic
steps to install the carboxyl protecting group of 4a and 4b and to remove the
ester
group on 5, and the disadvantage of requiring costly and hard-to-implement
silica gel
chromatographic purification to separate 5 and 6.
The compound of the formula 3 is well known (for example in European patent
037, 231) and is accessible in various ways. Several routes for the synthesis
of the
racemic mixture 4a and 4b have been disclosed in the patent literature (Patent
EP 79022,
Patent EP 50800, Patent ES 549251, Patent CN 1106386 for example) and in the
literature
(V. Teetz et al Tetrahedron Letters,1984, 25(40), 4479-4482, for example).
The second approach utilizes enantiopure amino ester 4a (RZ denotes a carboxyl-
esterifying group, such as Ci-C6 alkyl, C~-Cs aralkyl, preferably benzyl or
tert-butyl) as
one partner in a coupling reaction with compound 3, using the methods commonly
known in peptide chemistry (such as those described in CA 1,338,162, EP 79022,
US
5977380, ES 549789 and ES 2004804, for example) to prepare 5 wherein R2
denotes a
carboxyl-esterifying group, such as Ci-C6 alkyl or C~-Cs aralkyl.
C02CH2CH3
H
H H C_02CHzCH3
,,COzRz H~ ' ~ w
N ~ ~ ~~CH3
HO ,C02R2
H CHs
O
H
4a 3 5
When the compound of formula 5 (wherein R2 denotes a carboxyl-esterifying
group, such as CZ-C6 alkyl or C~-Cs aralkyl) is prepared using a coupling of
4a with 3,
the ester protecting group (R2) is removed by hydrogenolysis or treatment with
an acid
CA 02363658 2001-11-21
4
or base, then the resulting product (1, Ramipril where R1 = H) is crystallized
from a
substantially pure solution. However, this general route also has its
difficulties. Firstly,
the efficient large-scale enantioselective synthesis of chirally pure 4a has
not been
reported. However, there are two reported enantioselective syntheses of
compound 4a
or derivatives. An enantioselective synthesis of 7a was reported by L. M.
Harwood and
L. C. Kitchen (Tetrahedron Letters, 1993, 34(41), 6603-6606) but the chemistry
does not
appear suitable to scale-up and the overall yield is low (13 %).
H
H I
,, C02H
H
7a
An enantioselective (but not diastereoselective) route, reported by H. Urbach
and
R. Henning, (Heterocycles, 1989, 28(2), 957 - 965) gave 4a (RZ = Benzyl) in an
overall
yield of 5.5 %, which appears to be too low for commercial implementation.
There are three reported methods for the resolution of bicyclic amino acids of
this type. An enantioenriched sample of amino acid 7a was obtained by
resolution of 7a
and 7b is reported in patent ES 549251. Amino acid 7a can be converted to
amino ester
4a by methods known to those skilled in the art.
H H
H I H I
"C02H ~~=~COzH
H H
7a 7b
This resolution removes 7b from a mixture of 7a and 7b, giving, after removal
of
the chiral base by acidification of the residue, a 52% yield of material (7a)
in unspecified
optical purity. However, this resolution uses an expensive chiral amine (S)-1-
(1-
naphthyl)ethylamine.
CA 02363658 2001-11-21
The other reported resolution methods resolve the racemic mixture of 4a and
4b.
A resolution separating 4a (R2 = carboxyl esterifying group) from a racemic
mixture of
4a and 4b using N-acyl derivatives of optically active R or S amino acids
containing a
phenyl nucleus has been disclosed in patent EP 115345. This procedure gives 4a
5 (wherein R1 = Benzyl) in a yield corresponding to 102.1 % of theoretical,
with an optical
purity of 87%. This resolution, as disclosed, uses the toxic solvent
dichloromethane and
an expensive anti-solvent cyclohexane.
A resolution protocol for the obtention of 4a from a racemic mixture of 4a and
4b
has been reported using S-mandelic acid (J. Martens and S. Lubben, Journal fur
Prakt.
Chemie 1990, 332(6), 1111-1117). This protocol returns 4a in high purity
(>98%) but in
lower yield (43% for the mandelic acid salt). Unfortunately, these two
approaches
require a sequence of steps when used in the production of Ramipril, as it is
reported:
protection of the amino acid as a carboxylic ester, free-basing the HCl salt,
formation of
the salt with the resolving agent, isolation of the diastereomeric salt, free-
basing the
diastereomeric salt, and formation of the HCl salt. Using this many steps to
produce the
material consumes expensive plant time and reduces efficiency.
SUMMARY OF THE INVENTION
According to a first aspect of the present invention there is provided a
process
for separating diastereomeric mixtures of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-phenylpropyl]amino]propanoyl]octahydro-cyclopenta[b]pyrrole-
2-
carboxylic acid derivative of compound formula 1 and (2R, 3aR,6aR)-1-[(S)-2-
[[(S)-1-
(ethoxycarbonyl)-3-phenylpropyl]amino]propanoyl] octahydrocyclopenta[b]
pyrrole-2-
carboxylic acid derivative of compound of formula 2, the process comprising:
(a) treating the mixture of 1 and 2 with a solvent or a mixture of solvents
selected from a group consisting of CZ-C~ nitrite solvents, Ci-C6 alcohol
solvents, C6-Cs aromatic hydrocarbon solvents, C3-CZO ether solvents, C3-
CA 02363658 2001-11-21
6
C6 ketone solvents, CZ-C~ ester solvents, Ci to Cs chlorinated solvents, and
C5-Cio hydrocarbon solvents,
(b) adding an organic or inorganic acid , if desired , selected from a group
consisting of benzoic acid, mandelic acid, malefic acid, fumaric acid,
methane sulfonic acid, toluene sulfonic acid, hydrochloric acid,
hydrobromic acid, sulfuric acid, and phosphoric acid,
(c) allowing the compound of formula 1 to precipitate and filtering the slurry
to obtain a solid compound of formula 1, where R1 = H or R1 denotes a
carboxyl-esterifying group, such as Ci-C6 alkyl, preferably tert-butyl, and
C~-Cs aralkyl, preferably benzyl.
C02CH2CH3 COZCH2CH3
~N / ( ~N
H H /
O \ O \
~ CHs H CH3
H
N C02R1
N , ~,~ C02R1
H H
1 2
The process involves the treatment of an equal or unequal amount of
diastereomeric compounds 1 and 2 with a solvent or a mixture of solvents,
treating the
mixture of 1 and 2 in a solvent with an inorganic or organic acid, if
necessary, stirring
the mixture of 1 and 2 in a solvent or a mixture of solvents, allowing the
desired isomer
to precipitate with or without seeding, adding a solvent (or a mixture of
solvents) if
desired, and isolating the desired substantially pure compound 1 at a
temperature of -
50 to 50°C as a solid. The isolated product 1 may be treated with an
acid or base if
necessary, or subjected to hydrogenolysis if necessary to give Ramipril.
CA 02363658 2001-11-21
When Rl is benzyl , for example, the preferred acid salt is malefic acid and
the
salt produced is (2S,3aS,6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-
phenylpropyl]-
amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid benzyl ester
malefic
acid salt. This salt is new and is a useful intermediate for manufacturing the
compound
Ramipril.
According to a second aspect of the present invention there is provided a
process
for separation of a mixture of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
derivatives of the formula 1 and (2R, 3aR, 6aR)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
derivative of formula 2, wherein Rl = H, the process comprising:
(a) treating the mixture of 1 and 2 with a solvent or mixture of solvents
selected from a group consisting of CZ-C4 nitrite solvents, Ci-C6 alcohol
solvents, C6-Cs aromatic hydrocarbon solvents, C3-Cio ether solvents, Cs-
C6 ketone solvents, CZ-C~ ester solvents, Ci-C3 halogenated solvents, and
C5-Cio hydrocarbon solvents,
(b) adding an organic or inorganic base selected from a group consisting of
sodium hydroxide, lithium hydroxide, potassium hydroxide, sodium
bicarbonate, sodium carbonate, potassium carbonate, tent-butylamine,
triethylamine, piperidine, aniline, n-butylamine or dicyclohexylamine,
then filtering the slurry to obtain a solid salt of compound of formula 1.
Suitable solvents or solvent mixtures of the present invention include but are
not
limited to C2-C4 nitrite solvents such as acetonitrile, propionitrile and the
like, Cl-C6
alcohols such as ethanol, methanol and the like, C6-C9 aromatic hydrocarbons
such as
benzene, toluene, xylenes and the like, Cs-CZO ethers such as dimethoxyethane,
diethyl
ether, tetrahydrofuran, diisopropyl ether and the like, Cs-C6 ketone solvents
such as
CA 02363658 2001-11-21
8
methyl isobutyl ketone, methyl isopropyl ketone and the like, CZ-C~ ester
solvents such
as ethyl acetate, ethyl propionate, isopropyl acetate and the like, Cs-CZO
hydrocarbons
such as hexanes, heptanes, octanes, and the like; and Ci to C3 chlorinated
solvents such
as dichloromethane, chloroform and the like. Suitable organic acids for this
process
include but are not limited to Cl-Cs acids and diacids such as benzoic acid,
mandelic
acid, malefic acid, fumaric acid, and the like. Suitable inorganic acids for
this process
include but are not limited to hydrochloric acid, hydrobromic acid, sulfuric
acid,
phosphoric acid, and the like. Suitable inorganic and organic bases for this
process
include but are not limited to sodium hydroxide, lithium hydroxide, potassium
hydroxide, sodium bicarbonate, sodium carbonate, potassium carbonate, tert-
butylamine, triethylamine, piperidine, aniline, n-butylamine or
dicyclohexylamine, and
the like.
The preferred organic acid, when required, is malefic acid, especially when Rl
is a
carboxyl esterifying group and preferably when Rl is benzyl. The preferred
solvents
are, for instance, ethyl acetate or a mixture containing ethyl acetate,
acetonitrile, butyl
acetate, and isopropyl acetate, which may be present in a mixture with, for
instance,
diisopropyl ether and/ or ethanol and/ or acetonitrile. The preferred
precipitation
temperature range is -15 to 30°C and the ratio of the diastereomeric
mixture is
preferably between 8:1 to 1:5 for compound of formula 1 and 2 respectively.
DETAILS OF THE INVENTION
In the present invention, a (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoylJoctahydrocyclopenta[b]pyrrole-2-carboxylic acid
derivative of formula 1 is separated from an equal or unequal amount of (2R,
3aR, 6aR)-
1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]propanoyl]-
octahydrocyclopenta[b]pyrrole-2-carboxylic acid derivative of formula 2,
wherein Rl =
H or R1 denotes a carboxyl-esterifying group, such as (Ci-C6) alkyl, (C~-Cs)
aralkyl by
CA 02363658 2001-11-21
9
treatment with a solvent (or a mixture of solvents) at a temperature between -
50 and
50°C, treating the mixture with an organic or inorganic acid if
necessary or an inorganic
or organic base if necessary, stirring the mixture in a solvent or a mixture
of solvents at
-50 to 50°C, allowing compound of formula 1 to precipitate at -50 to
50°C with or
without seeding, adding a solvent or a mixture of solvents, if desired, to
give
substantially pure compound of formula 1, at a temperature between -50 and
50°C, as a
solid. Scheme 1 depicts this process.
COZCH2CH3
H
O
~CH3
H
N ,CO2R~
COZCH2CH3
H
H 1 ) Solvent
2) Acid or base, if dewed O
CH3
3) solvent, if desred H
C02CH2CH3 4)filtration N ,COZR~
H
N
O' ~ H
~CH3
H N COZR~
H
2
The isolated compound of formula 1, where Rl is defined as above, may be
treated with an acid or base if necessary, or subjected to hydrogenolysis if
necessary, to
give Ramipril (1, where R1 = H).
Suitable solvents or solvent mixtures for this separation include but are not
limited to CZ-C4 nitrite solvents such as acetonitrile, propionitrile and the
like; Ci-C6
alcohols such as ethanol, methanol and the like; C6-C9 aromatic hydrocarbons
such as
benzene, toluene, xylenes and the like; C3-Cio ethers such as dimethoxyethane,
diethyl
ether, tetrahydrofuran, diisopropyl ether and the like; C3-C6 ketone solvents
such as
CA 02363658 2001-11-21
methyl isobutyl ketone, methyl isopropyl ketone and the like; Cz-C~ ester
solvents such
as ethyl acetate, ethyl propionate, isopropyl acetate and the like; Cs-Cio
hydrocarbons
such as hexanes, heptanes, octanes, and the like; Ci to C3 chlorinated
solvents such as
dichloromethane, and the like. Suitable organic acids for this process include
but are
5 not limited to Ci-Cs acids and diacids such as benzoic acid, mandelic acid,
malefic acid,
fumaric acid, and the like. Suitable inorganic acids for this process include
but are not
limited to hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric
acid, and the
like. Suitable inorganic and organic bases for this process include but are
not limited to
sodium hydroxide, lithium hydroxide, potassium hydroxide, sodium bicarbonate,
10 sodium carbonate, potassium carbonate, tert-butylamine, triethylamine,
piperidine,
aniline, n-butylamine or dicyclohexylamine, and the like.
The preferred carboxyl esterifying group for this process is R1 = benzyl. The
preferred acid salt, when required, is malefic acid. The preferred solvents
are, for
instance, toluene, dimethoxyethane, ethyl acetate, isopropyl acetate, which
may or be
present in a mixture with, for instance, diisopropyl ether and/ or ethanol
and/ or
acetonitrile. The precipitation temperature can range between -50 and 50
°C and the
preferred temperature range is -15 to 30°C.
The following non-limiting examples show the process for separating 1 from 2
(Rl defined as above), via the processes of the present invention.
Example 1
Separation of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-
amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid from an
approximately equimolar amount of (2R, 3aR, 6aR)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
using
ethyl acetate/ diisopropyl ether solvent.
CA 02363658 2001-11-21
11
A reaction solution of an approximately equimolar mixture of 1 (R1= H) and 2
(R1
= H) (theoretical yield of 1 + 2 = 3.68 g) was prepared in ethanol solution
using methods
described in the prior art. This mixture was evaporated on the rotary
evaporator at a
bath temperature of 40°C to give a light yellow syrup. This syrup was
dissolved in 10
mL of ethyl acetate. The resulting solution was seeded with 10 mg of ramipril
seed
crystals, and the resulting light suspension was stirred at a bath temperature
of 19°C.
Over the next two hours,11 mL of diisopropyl ether was added to the reaction,
in small
portions. The reaction mixture was then filtered and washed with three 5-mL
portions
of 1:1 ethyl acetate/ diisopropyl ether. The solids were dried under vacuum
overnight
at 40 - 45°C, to give 1.13 g (30.6 % yield, 61.2 % of theoretical
recovery) of Ramipril (1,
R1 = H). The proton NMR spectrum indicated that no 2 (Rl = H) was present.
Example 2
Separation of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-
amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid from an
approximately equimolar amount of (2R, 3aR, 6aR)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
using
isopropyl acetate solvent.
A reaction solution of an approximately equimolar mixture of 1 (Rl = H) and 2
(Rl = H) (theoretical yield of 1 + 2 =2.29 g) was prepared in
ethanol/acetonitrile
solution using methods described in the prior art. This mixture was evaporated
on the
rotary evaporator to a mass of 4.45 g. A 25-mL portion of isopropyl acetate
was added
to the mixture, and the mixture was evaporated to a mass of 5.1 g, using the
rotary
evaporator. A 25-mL portion of isopropyl acetate was added to the mixture,
which was
then evaporated to a mass of 5.65 g on the rotary evaporator. A 3.41 g portion
of
isopropyl acetate was added to the mixture. The resulting solution was stirred
at 20-25
°C, and the reaction was seeded with approximately 5 mg of Ramipril.
After stirring
CA 02363658 2001-11-21
12
and allowing solids to precipitate for 6 hours, the mixture was filtered and
washed with
three 0.5-mL portions of isopropyl acetate. After drying overnight at 40 -
45°C, 0.53 g
(45.8 % of theoretical, 23 % yield) of Ramipril (1, Rl = H) was obtained. The
proton
NMR spectrum showed none of isomer 2 (Rl - H) was present, and the
chromatographic purity of the sample exceeded 99.0 %.
Example 3
Separation of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-
amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid from an
approximately equimolar amount of (2R, 3aR, 6aR)-1-[(S)-2-[((S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
using
butyl acetate solvent.
Substituting the isopropyl acetate in EXAMPLE 2 with butyl acetate resulted in
a
0.49 g (42 % of theoretical, 21 % yield) of Ramipril (1, Rl = H). The proton
NMR
spectrum showed none of the undesired isomer (2, Rl = H) was present, and the
chromatographic purity of the sample exceeded 99.0 %.
Example 4
Separation of (2S, 3aS, 6aS)-1-((S)-2-([(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-
amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid from an
approximately equimolar amount of (2R, 3aR, 6aR)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
using
ethyl propionate solvent.
Substituting the isopropyl acetate in EXAMPLE 2 with butyl acetate resulted in
a
0.51 g (44 % of theoretical, 22 % yield) of Ramipril (1, R1 = H). The proton
NMR
spectrum showed that none of the undesired isomer (2, Rl = H) was present, and
the
chromatographic purity of the sample exceeded 99.0 %.
CA 02363658 2001-11-21
13
Example 5
Separation of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-
amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid benzyl ester
malefic
acid salt 1 (Rl = benzyl) from an approximately equimolar amount of (2R, 3aR,
6aR)-1-
[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]propanoyl]-
octahydrocyclopenta[b]pyrrole-2-carboxylic acid benzyl ester malefic acid salt
2 (R1 =
benzyl) using butyl acetate/ diethyl ether solvent.
A solution of an equimolar mixture of 1 (R1 = Benzyl) and 2 (Rl = Benzyl)
(theoretical yield of 1 + 2 = 4.50 g, 9.14 mmol) in ethyl acetate solution
(Vtoc = 35 mL),
was prepared using methods described in the prior art, particularly EP 79022
and ES
2004804. To this solution was added 1.16 g (9.76 mmol) of malefic acid. This
slightly
cloudy organic solution was evaporated to approximately 5 mL, and was filtered
to
remove solid particles. The filtrate was evaporated to give a light yellow
syrup. A 10-
mL portion of butyl acetate was added to the syrup, giving a clear yellow
solution. The
solution was cooled to -10 °C, and 10 mL of diethyl ether was added
slowly. The
mixture was seeded with 10 mg of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
benzyl ester malefic acid salt 1 (R1 = Benzyl). The mixture was stirred at -10
to -5 °C for
1 hour. The mixture was placed in the refrigerator at -15 °C overnight.
After removal
from the refrigerator at -15 °C and stirring at -15 °C for 0.5
hours, the mixture was
filtered, and the solids washed with ethyl ether/ethyl acetate (1:1). After
drying at 40 -
45 °C in a vacuum oven, 1.52 g (27.6 % yield, 55.2 % of theoretical) of
ramipril benzyl
ester malefic acid salt (1, R1 = Benzyl) was obtained as a white solid. The
proton NMR
spectrum indicated that no undesired isomer (2, R1 = Benzyl) was present.
CA 02363658 2001-11-21
14
Example 6
Separation of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-
aminoJpropanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid benzyl ester
malefic
acid salt 1 (Rl = Benzyl) from an approximately equimolar amount of (2R, 3aR,
6aR)-1-
[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]propanoyl]-
octahydrocyclopenta[bJpyrrole-2-carboxylic acid benzyl ester malefic acid salt
2 (R1 =
Benzyl).
A reaction solution of an equimolar mixture of 1 (R = Benzyl) and 2 (R =
Benzyl)
(theoretical yield of 1 + 2 = 4.50 g) in ethyl acetate solution (Vtot = 35
mL), was prepared
using methods described in the prior art, particularly EP 79022 and ES
2004804. This
solution was evaporated to an oil at a bath temperature of 40 - 45 °C.
To this oil was
added 1.2 g of malefic acid dissolved in 15 mL of hot ethyl acetate. The
mixture was
cooled to -10 °C and was seeded with 0.1 g of (2S, 3aS, 6aS)-1-[(S)-2-
[[(S)-1-
(ethoxycarbonyl)-3-phenylpropylJamino]propanoyl]octahydro-cyclopenta[b]pyrrole-
2-
carboxylic acid benzyl ester malefic acid salt 1. After 2.5 hours at -5 to -10
°C, the solid
was filtered and washed with ether/ethyl acetate and dried to give 1.4 g (23.6
% yield,
47.2 % recovery) of ramipril benzyl ester malefic acid salt (1, R = Bn), as a
white solid.
The proton NMR spectrum indicated that none of the undesired isomer (2) was
present.
Example 7
Preparation of Ramipril from (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-
3-
phenylpropyl]amino]propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
benzyl ester malefic acid salt 1 (Rl = Benzyl).
A 1.0 g portion of (2S, 3aS, 6aS)-1-[(S)-2-[[(S)-1-(ethoxycarbonyl)-3-
phenylpropyl]aminoJpropanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid
benzyl ester malefic acid salt 1 was placed into 100 mL of dichloromethane,
and was
CA 02363658 2001-11-21
cooled to 0 - 5°C. Triethylamine (0.19 g) was added to the mixture. The
resulting
mixture was washed with water, and the organic phase was evaporated to give an
oil.
This oil was deprotected using hydrogenolysis, as per examples stated in EP
079022 to
give Ramipril as a white solid.
5 While the foregoing provides a detailed description of a preferred
embodiment
of the invention, it is to be understood that this description is illustrative
only of the
principles of the invention and not (imitative. Furthermore, as many changes
can be
made to the invention without departing from the scope of the invention, it is
intended
that all material contained herein be interpreted as illustrative of the
invention and not
10 in a limiting sense.