Note: Descriptions are shown in the official language in which they were submitted.
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
DESCRIPTION
ENZYME-LINKED IMMUNO-MAGNETIC ELECTROCHEMICAL BIOSENSOR
FIELD OF THE INVENTION
The present invention relates to devices and methods
for detecting and quantitating specific analytes in a
sample.
BACKGROUND OF THE INVENTION
The detection and quantitation of specific analytes in
a sample is in important activity in environmental, health,
biotechnology, industrial chemistry and other fields. The
assays have also found use in high throughput screening,
screening of oligo libraries in the field of functional
genomics analysis, combinatorial chemistry screening, and
other such fields. The analytes detected or quantitated may
be any compound of interest for which there is a specific
recognition molecule. Well known recognition molecules
include proteins, such as receptors, immuno-globulins, and
the like, nucleic acids, their analogs, and the like,
haptens, hormones, polypeptides, certain drugs and other
such molecules.
Devices and techniques for detecting analytes are well
known in the art. These including ELISAs, RIAs, PCR, and
the like. Although these techniques have proven very
powerful, effective and valuable, they suffer from
drawbacks.
Most devices and techniques presently used for the
detection of analytes require relatively long reaction
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
times, complex processes and laboratory conditions. For
example, temperatures above room temperature, reaction times
in excess of 30 minutes, and strict time limitations. Other
drawbacks have included auto-fluorescence of reagents or
analytes, in particular in the field of combinatorial
chemistry, and when screening small peptide libraries using
optical methods.
Decreasing the time necessary to perform an assay while
maintaining the precision, sensitivity, reliability and
dose-dependent results that can obtained using conventional
methods presents great economic advantages, and the
patient's well-being in the case of laboratory medicine.
The use of an electrochemical sensor rather than an optical
sensor further presents other advantages, including avoiding
auto-fluorescence and turbidity problems.
SUMMARY OF THE INVENTION
In a first, independent aspect of the present
invention, an electrochemical sensor includes an
interdigitated array of electrodes on a substantially
dielectric substrate and a means for concentrating reagents
on the surface of the interdigitated array of electrodes.
In a second, independent aspect of the present
invention, an electrochemical reporter system includes a
first recognition molecule linked to a magnetic bead,
wherein the first recognition molecule can specifically bind
an analyte; a second recognition molecule linked to an
enzyme, for coupling with specificity the enzyme to the
analyte or the first recognition molecule/analyte complex; a
substrate, which in the presence of the enzyme is processed
into an electrochemical reporter molecule capable of redox
recycling; a sensor for detecting the electrochemical
2
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
reporter molecule, wherein the sensor has a configuration
such that electrochemical reporter molecules, if present,
exhibit redox recycling; and a magnetic field generating
device positioned such that the magnetic field it generates
can attract to the surface of the sensor magnetic beads in
solution over the sensor.
In a third, independent aspect of the present
invention, an electrochemical reporter device includes a
chamber for receiving an analytical reaction having magnetic
beads; a sensor on a surface of the chamber, the sensor for
detecting electrochemical reporter molecules within the
chamber, the sensor having a configuration such that it
causes redox recycling of reporter molecules capable of
exhibit redox recycling; and a magnetic field generating
device capable of generating a magnetic field that attracts
magnetic beads present within the chamber onto the sensor.
In a fourth, independent aspect of the present
invention, an electrochemical reporter system includes a
magnetic bead; a first recognition molecule capable of
specifically binding an analyte, the first recognition
molecule being linked to the magnetic bead; an enzyme; a
coupling element, or second recognition molecule, for
coupling with specificity the enzyme to the analyte or the
first recognition molecule/analyte complex; a substrate,
which in the presence of the enzyme is cleavable into an
electrochemical reporter molecule capable of exhibiting
redox recycling; a sensor for detecting the electrochemical
reporter molecule and having a configuration such that the
reporter molecule will exhibit redox recycling; and a
magnetic field generating device positioned such that the
magnetic beads may be attracted to the vicinity of the
sensor.
3
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
In a fifth, independent aspect of the present
invention, an assay for detecting or quantitating a specific
analyte in a sample comprises the following steps: a primary
incubation, wherein magnetic beads coated with a recognition
molecule that specifically binds an analyte are incubated
with a sample; a secondary incubation, wherein the magnetic
beads are then incubated with a conjugate comprising an
enzyme and a molecule that specifically binds the analyte,
or the analyte/recognition molecule complex; capturing the
magnetic beads with a magnetic field generating device over
a sensor capable of producing redox recycling of an
electrochemical capable of undergoing redox recycling;
adding a substrate, wherein the substrate in the presence of
the enzyme is cleaved into an electrochemical reporter
molecule capable of undergoing redox recycling; detecting
the presence or measuring the amount of electrochemical
present in the solution with the sensor.
In a sixth, independent aspect of the present
invention, an electrochemical immunoassay for detecting an
analyte in a sample includes the steps of providing an
antigen linked to a magnetic bead and an antibody specific
for an analyte bound to the antigen, wherein the antibody is
coupled to an enzyme or has a coupling element such that it
can be specifically coupled to an enzyme; contacting the
magnetic bead/antigen/antibody/enzyme complex with a sample
to be analyzed; attracting the magnetic
bead/antigen/antibody/enzyme complex to the vicinity of a
sensor; adding a substrate to the collected magnetic
bead/antigen/antibody/enzyme complex, wherein the substrate
in the presence of the enzyme is cleaved into an
electrochemical reporter molecule capable of exhibiting
redox recycling; detecting the presence or measuring the
4
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
amount of reporter molecule with the sensor, wherein the
sensor is an interdigitated array of electrodes capable of
producing redox recycling of the reporter molecule.
In a seventh, independent aspect of the present
invention, an electrochemical immunoassay for detecting a
specific analyte in a sample includes the steps of providing
a recognition molecule linked to a magnetic bead, wherein
the recognition molecule is capable of specifically binding
the analyte; contacting the magnetic bead with a sample to
be analyzed; coupling with specificity an enzyme to the
analyte or the recognition molecule/analyte complex;
attracting the magnetic bead/recognition
molecule/analyte/coupling element-enzyme complex to the
vicinity of a sensor with a device capable of generating a
magnetic field; adding a substrate, which in the presence of
the enzyme is cleaved into an electrochemical reporter
molecule capable of exhibiting redox recycling; detecting
the presence or measuring the amount of electrochemical with
the sensor, wherein the sensor is an interdigitated array of
electrodes capable of producing redox recycling of the
electrochemical reporter molecule.
In an eighth, independent aspect of the present
invention, an electrochemical reporter system includes a
magnetic bead; a recognition molecule capable of
specifically binding an analyte, the recognition molecule
being linked to the magnetic bead; an enzyme; a coupling
element, for coupling with specificity the enzyme to the
analyte or recognition molecule/analyte complex; a
substrate, which in the presence of the enzyme is cleavable
into a reporter molecule capable of exhibiting redox
recycling; a sensor, for detecting the electrochemical
reporter molecule and having a configuration such that the
5
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
reporter molecule will exhibit redox recycling; a magnetic
field generating device positioned such that the magnetic
beads will be attracted to the vicinity of the sensor.
BRIEF DESCRIPTION OF THE FIGURES
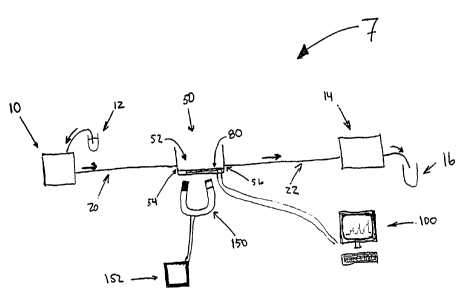
FIG. 1 is a schematic representation of a device in
accordance with the present invention for a detecting and/or
quantitating a specific analyte in a sample.
FIG. 2 is a graphic representation of the change in current
measured when assaying in accordance with the present
invention serum samples having low, medium and high anti-p24
levels.
FIG. 3 is a graph plotting the slope of the kinetic
measurement (nA/s) against the original concentration
(mIU/ml) of HBsAg in the sample.
FIG. 4 is a dose response curve using electrochemical
measurement according to the present invention.
FIG. 5 are measurements of different concentrations using
electrochemical measurement with a device without the
magnetic beads and magnet of the present invention.
FIG. 6 is a comparison of assays using different number of
magnetic beads per sample.
FIG. 7 is a flow chart of methods and devices in accordance
with the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Turning in detail to the drawings, FIG. 1 is a
schematic representation of a device 7 in accordance with
the present invention for detecting and/or quantitating a
specific analyte in a sample.
A first pump 10 pumps a processed sample 12 through an
inflow tubing, or first tubing segment 20, into the
6
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
electrochemical sensing module 50. The first pumping device
may be any device that can move fluids containing small
particles, or a slurry, through a tubing segment.
Preferably, the first pumping device 10 is an adjustable
5 speed peristaltic pump.
The first tubing segment 20 may be any tubing that can
carry a fluid having small particles without clogging, or
transport a fluid/particulate slurry. It is preferably made
from an inert material, i.e., a material that will not
10 interact detrimentally with the fluids and reagents flowing
within it. Most preferably, the first tubing segment 20 is
TYGON tubing.
The diameter of the first tubing segment 20 will depend
on the rate of flow desired. When the first pumping device
10 is a peristaltic pump, the rate of flow of fluids through
the tube 20 can be increased by using tubing having a wider
inner diameter, or by increasing the speed of the
peristaltic pump. Conversely, a slower rate of flow can be
achieved by using tubing having a smaller diameter or by
decreasing the speed of the peristaltic pump. Preferably
the first tubing segment has an inner diameter such that an
appropriate rate of flow may be achieved for the specific
pump being used. The diameter of the tubing used will also
be a function of the size of the beads and the fluid being
delivered.
The electrochemical sensing module 50, includes an
inflow orifice 54, a chamber 52 for holding the sample, an
outflow orifice 56 and a sensor 80. The outflow orifice 56
is connected to the outflow tubing, or second tubing segment
22. The second tubing segment 22 may be connected at its
other end to a second pumping (drawing) device 14 (shown in
the figure), or alternatively the first pumping device 10
7
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
may also be used for this purpose, in which case the system
preferably is hermetically sealed.
The second tubing segment 22 advantageously terminates
at a waste receptacle 16, collecting chamber, or the like.
The properties of the outflow tubing 22 are preferably the
same as the inflow tubing 20, described supra. If two pumps
are used, less flexible, more inert material can be used for
the tubing, including TEFLON, or the like.
The electrochemical sensing module 50 may be a
disposable, single use unit, in which case the module 50
preferably is adapted to slide and/or snap into and out of
the device 7 for easy replacement. Upon sliding and/or
snapping into place, the electrochemical sensing module 50
is adapted such that a tight seal is formed between the
inflow tubing 20 and the inflow orifice 54, and between the
outflow orifice 56 and the outflow tubing 22.
Alternatively, the inflow and/or outflow tubing 20, 22 are
part of the sensing module 50, and are also discarded and
replaced after each use. The waste receptacle 16,
collecting chamber, or the like, may also be part of the
disposable sensing module 50. The electrical contacts for
the IDA are also preferably adapted to plug-in to the
controller 100 and/or a power supply once the module slides
or snaps into place.
The sensor 80 may be any device that can detect and/or
measure an electrochemical reporter that can undergo redox
recycling, while providing for redox recycling of the
electrochemical reporter. See, for example, WO 99/07879 and
United States Patent No. 5,670,031. Preferred is an
interdigitated array of electrodes (IDA) with a spacing
between the electrodes of about 800~m, or smaller. Most
preferred is an array of electrodes with a spacing between
8
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
the electrodes between about 200~m and about 400~,m, for
example, an array of electrodes with a spacing between the
electrodes of about 300~m.
The sensor 80 may have one or more IDAs. More IDAs
provide for greater sensitivity, but are not necessarily
indispensable, depending on the assay. When more than one
array is present, (i.e., a "ganged" IDA sensor) the arrays
may be linked in series or in parallel. Independent
combinations thereof may also be used.
The sensor 80 is linked to a system controller 100
including a multipotentiostat that provides a specified
potential across the IDA or IDAs and measures the dose
dependent current resulting from redox recycling of
electrochemical reporter molecules proximal to the IDA.
Alternatively, the information may be derived by scanning
voltammetry, or the like. The system controller is thus
capable of measuring and preferably also recording the
change in voltage, and/or current, and the like, in the IDA.
If more than one IDA is present in series, the system
controller 100 preferably can measure and also record the
change occurring in each independent IDA or the sum of such
changes. The system controller may advantageously be part
of a computer network, such that processes and results can
be order, monitored, controlled, retrieved and/or analyzed
remotely.
A magnetic field generating device 150, or the like, is
positioned relative to the electrochemical sensing module 50
and is capable of generating a magnetic field of such
strength that when a fluid having magnetic beads is
circulated within the chamber 52, a quantity of magnetic
beads adequate for the detection or quantitation of the
9
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
analyte of interest will be attracted onto the sensor's 80
surface .
The magnetic field generating device 150 may be
activated/deactivated by an on/off switch 152, or the like.
The switch may be under the control of a system controller
100, or the like.
Alternatively, the magnetic field generating device 150
may be a permanent magnet. In that case it is preferable
that the magnet be moveable such that in at least a first
position the magnetic field it generates affects magnetic
particles within chamber 52, such that it may cause magnetic
beads to be attracted onto the sensor surface 80. When the
magnet is moved into a second position the magnetic field
does not significantly affect magnetic particles within the
chamber 52, such that the magnetic beads are no longer
attracted onto the sensor surface 80, to facilitate clearing
the magnetic beads from the sensor after the detection
and/or quantitation of analyte has been achieved. An
activatable/deactivatable magnetic field generating device
may be used; but is not necessarily required, when a single
use/disposable electrochemical sensing module 50 is used.
In use, a buffer is pumped through the system and over
the sensor 80 to establish a baseline. The buffer is flowed
over the sensor 80 at any effective rate, however, a slow
rate (about 0.2mL/min) is preferred. Any effective buffer
may be used, but enzyme substrate buffer (ESB), described
below, is preferred.
The magnet 150 under the sensor 80 may be activated
152, or, alternatively, a magnet may be placed under the
sensor 80, at any time prior to the flow of the sample
including the magnetic beads over the sensor 80. The magnet
150 should be able to generate an applied field such that an
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
adequate amount of magnetic beads may be drawn and captured
on the surface of the sensor 80.
Next, the processed sample to be tested 12 is
circulated over the sensor 80. The processed sample may be
prepared by any effective method. One method for preparing
a processed sample is described in more detail infra. In
general, the processed sample includes magnetic beads, or
the like, and an enzyme indirectly linked to the magnetic
beads by the analyte, the recognition molecule or the
recognition molecule/analyte complex.
The processed sample may be circulated over the sensor
80 surface for any effective amount of time, preferably
until an adequate quantity of beads is captured by the
magnet 150 over the sensor 80 surface. Preferably, the bead
solution is circulated for approximately 2 minutes at medium
to fast flow rates (approximately 0.38mL/min). The effect
of the magnet 150 and the flow rate should be such that an
adequate concentration of beads is captured over the sensor
surface 80.
A substrate is then circulated over the sensor 80. The
substrate may be circulated at any effective rate, however,
a slow flow rate (approximately 0.2mL/min) is preferred.
The flow is then preferably stopped while substrate solution
is over the sensor 80 and the signal is measured and/or
recorded by the system controller 100, with no flow, for the
desired period of time.
The signal may be measured for any adequate amount of
time. In general, however, the signal may be measured for
about 90 to about 100 seconds or about 60 seconds of useable
data. Longer or shorter measurements may be used if
necessary. It is within the skill in the art to determine
11
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
the optimal length of time the measurement should take place
for a given set of conditions and samples.
The substrate will depend on the enzyme and the
conditions used. Any effective substrate may be used. A
non-exclusive list of enzyme/substrate pairs that may be
used in accordance with the present invention is disclosed
in WO 99/07879. Any effective concentration of substrate
may be used. The preparation of the preferred substrate
solution is described in greater detail infra.
Once the sample has been assayed, the beads may be
cleared from the sensor 80 surface by deactivating 152, or
removing, the magnet 150 from the proximity of the sensor
80, and circulating fresh buffer at a sufficiently rapid
rate of flow over the sensor 80.
Alternatively, if a disposable sensor is being used,
once the sample has been assayed the sensor module 50 may be
removed and discarded.
In case the magnetic beads are to be cleared from the
sensor, any effective buffer may be used, but ESB is
preferred. The flow rate is preferably about 0.43mL/min.
The addition of bubbles to the buffer flow has been found to
assist the clearance of the beads. The washing buffer may
be applied for any effective amount of time, however,
generally between about 45 and 60 seconds has been found to
be sufficient. Once the beads are washed out, fresh buffer
may be recycled over the sensor 80 until the baseline
equilibrates again. This step generally takes about 30
seconds. The sensor 80 is then ready for a new sample.
Another aspect of the present invention is a fast and
reliable assay for measuring and quantitating analytes in a
sample. The method is particularly effective when used with
the device of the present invention.
12
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
Analytes that may be detected or quantitated include
any compound of interest for which there is a specific
recognition molecule. Well known recognition molecules
include proteins, such as receptors, immuno-globulins, and
the like; nucleic acids, their analogs, and the like;
haptens; hormones; polypeptides; certain drugs; and other
such molecules.
In general, the assay uses magnetic beads, or the like,
which are commercially available. Any effective magnetic
beads may be used, however Tosyl-activated DYNABEADSM-450
(DYNAL Inc, 5 Delaware Drive, Lake Success, NY 11042 Prod
No. 140.03, 140.04,) or the like, are preferred. The
magnetic beads may be of any size that can be held to the
chip surface with a magnetic field.
The magnetic beads are generally coated with a
recognition molecule that binds with specificity and high
affinity to the analyte to be detected or quantitated.
Methods for coating magnetic beads with specific recognition
molecules are well known in the art. The magnetic beads are
generally coated by dissolving the coating material in
carbonate buffer (pH 9.6, 0.2 M) or the like, or any other
well known in the art method.
For the DYNABEADS, the instructions provided by the
manufacturer may be used. Briefly, the magnetic beads are
first resuspended and homogenized by vortexing, or the like,
and a volume corresponding to the number of beads desired is
pipetted into a test tube. The magnetic beads are
concentrated using a magnet, and the supernatant is pipetted
off, leaving the magnetic beads undisturbed.
The beads are then resuspended in an ample volume
(preferably greater than original volume) of any effective
buffer. It is within the skill in the art to determine the
13
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
most effective buffer for the recognition molecule to be
used. Buffers that may be used include, for example,
phosphate buffer pH 7.4, borate buffer pH 9.5 or acetate
buffer pH 4.0 with molarities between 0.1M and 0.5M.
The beads are mixed gently with the final coating
solution for any effective period of time. Generally, the
beads are mixed with the final coating solution for about 2
minutes.
The magnetic beads are once more concentrated with a
magnet, and the supernatant pipetted off leaving the beads
undisturbed. The beads are then resuspended in an
appropriate volume of any effective buffer. Effective
buffers include, among other buffers, phosphate buffer pH
7.4, borate buffer pH 9.5 or acetate buffer pH 4Ø The
beads are now ready for coating.
For coating, the magnetic beads are thoroughly
resuspended in any effective buffer. Effective buffers
include, among other buffers, phosphate buffer pH 7.4,
borate buffer pH 9.5 or acetate buffer pH 4Ø From between
about leg to about 10~g of the pure recognition molecule, if
it is a protein, polypeptide or the like, per 10~ magnetic
beads may be added to the magnetic bead/buffer solution.
Preferably, about 5~g of the pure recognition molecule, if
it is a protein, polypeptide or the like, per 10~ magnetic
beads is added to the magnetic bead/buffer solution. The
solution is then vortexed for 1-2 minutes. The manufacturer
of DYNABEADS recommends a concentration of 4-10 x 10g
DYNABEADS per ml final coating solution (including the
antibody or other recognition molecule).
Preferably the salt concentration in the final coating
solution is greater than about 0.05M. Higher pH and/or
higher temperature will give a quicker formation of chemical
14
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
bonds. The upper pH and temperature limit is determined
based on the recognition molecule used to coat the magnetic
beads.
The magnetic beads/recognition molecule solution may
then be incubated for 16-24 hours at 37°C with slow tilt
rotation, or the like. Lower temperatures may be used for
temperature sensitive recognition molecules. Higher
temperatures and shorter incubation times may be used for
stable recognition molecules. Preferably the magnetic beads
are not permitted to settle during the incubation period.
Phosphate buffer pH 7.4 (0.1M) may be prepared by
dissolving 2.62 g NaH2P04xH20 (MW 137.99) and 14.42 g
Na~HP09x2H20 (MW 177.99) in distilled water and adjusting the
volume to 1000 ml.
Borate buffer pH 9.5 (0.1M) may be prepared by
dissolving 6.183 g H3B03 (MW 61.83) in 800 ml distilled
water, adjusting the pH to 9.5 using 5M NaOH and then
adjusting the volume to 1000 ml with distilled water.
Acetate buffer pH 4.0 (0.1M) may be prepared by
dissolving 2.86 ml acetic acid (CH3COOH), in 900 ml distilled
water, adjusting the pH to 4.0 using 5M NaOH and adjusting
the volume to 1000 ml with distilled water.
These buffers may be used for prewashing and coating of
DYNABEADSM-450 Tosylactivated. It is preferred that no
proteins, sugars, or the like be added to these buffers.
Recognition molecules other than proteins or
polypeptides may also be directly or indirectly used to coat
the magnetic beads. For example, nucleic acids and their
analogs can be attached to the magnetic beads by an avidin
biotin link, or the like; by binding the nucleic acid or
analog to a protein like albumin or the like, which is then
used to coat the magnetic beads; or by other methods well
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
known in the art. Other recognition molecules, including
hormones, haptens, sugars, polypeptides and the like may
similarly be bound to the magnetic beads using strategies
well known by those of skill in the art.
After the incubation with the coating solution, the
magnetic beads are concentrated using a magnet, and the
supernatant is pipetted off. The coated beads are then
washed, preferably a total of four times. Twice in buffer D
for 5 minutes at 4°C, once in buffer E for 24 h at 20°C or
for 4 hours at 37°C, and once in buffer D for 5 minutes at
4°C. The beads should be coated and ready for use after
this procedure. The amount of specific recognition
molecules bound to the beads may be established by
radioactive labeling, immunofluorescent methods,
spectrophotometry, or any other method known in the art.
The beads may be stored in buffer D at 4°C, usually for
months, depending on the stability of the immobilized
material. If the beads are stored for more than two weeks,
it is preferred that they be washed twice in PBS/BSA for
five minutes before use.
Buffer D consists generally of PBS pH 7.4 with O.lo w/v
bovine serum albumin (BSA) or human serum albumin (HSA). It
may be made by dissolving 0.888 NaCl (MW 58.4) and 0.1o
(w/v) BSA or HSA to 80m1 0.01M Na-phosphate pH 7.4 (see
above). The solution is then mixed thoroughly and the
volume adjusted to 100 ml with O.OlM Na-phosphate pH 7.4.
Buffer D is generally used for washing precoated
DYNABEADS. According to the manufacturer, this buffer or
any buffer containing protein or amino-groups (glycine, Tris
etc.) should preferably not be used for pre-washing or
coating of DYNABEADSM-450 Tosylactivated.
16
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
If a preservative is needed in the coated product, an
effective amount of sodium azide (NaN3) may be added to
buffer D. Preferred is a final concentration of 0.020
(w/v). This preservative is cytotoxic and should be
carefully removed before use by washing. Required safety
precautions should be followed when handling this material.
Buffer E: 0.2M Tris pH 8.5 with 0.10 (w/v) BSA (HSA),
may be made by dissolving 2.92g Tris in 80 ml distilled
water, and adjusting the pH to 8.5 using 1 M HCl, then
dissolving O.lo BSA/HSA and adjusting the volume to 100m1.
All reagents should preferably be analytical grade.
To test a sample for the presence or quantity of an
analyte, the sample in which the analyte is to be detected
or quantitated is combined with the coated beads in a
primary incubation. The primary incubation in general
consists of adding the sample to be analyzed to the magnetic
beads pre-coated with a first recognition molecule. In
general, the volume in which the primary incubation is
carried will depend on the number of beads to be used and
the final volume at which the reaction will take place.
Any effective number of beads per volume may be used in
the primary incubation. The desired number of beads coated
with the appropriate recognition molecule are pipetted and
then washed in modified buffer E (MBE), which consists of
0.2 m Tris buffer, pH 8.5, with l.Oo (w/v) BSA, and are then
resuspended in the desired volume of MBE. In general
between about 4-5x104 and about 4-5x101 beads in 20.1 may be
used for an assay having a final volume of 401.
Preferably, between about 4-5x10' and about 4-5x10' beads in
20~~1 may be used for an assay having a final volume of 401.
Most preferred is the use of between about 4-5x106 and about
17
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
1x10 beads in 20,1 for an assay having a final volume of
40y1 .
Any sample generally tested using conventional
techniques may also be tested using the methods and devices
of the present invention. The sample may be diluted in MBE
if necessary. In general, for example, it has been found
that serum samples may be diluted 1:2 or 1:4, or even
greater, if the analyte is present in sufficient
concentrations. Diluting the sample has been found to
decrease the background.
The sample and the beads are mixed, generally in a 1:1
(v/v) ratio. Preferably, 201 of beads and 201 of sample
are mixed, for a total reaction volume of 401.
Several experiments have been performed in which the
primary incubation time period was examined, with time
incubation time intervals ranging from about 0.5 of a minute
to about 30 minutes. Although longer incubations were found
to yield more sensitive results, in general primary
incubations of about 10 minutes or less were found to yield
highly sensitive results. Primary incubations of about 5
minutes or less were also found to yield highly sensitive
results. Most preferred are primary incubation of between
about 1 and about 2 minutes.
After the primary incubation the beads are preferably
washed twice with MBE (1001 per 401 in the primary
incubation may be used).
The secondary incubation with a conjugate, generally a
second recognition molecule that specifically binds the
analyte (or the first recognition molecule/analyte complex)
and is conjugated or may be conjugated to an enzyme, is then
effectuated. Other methods, for example, complementation of
polypeptide fragments of beta-galactosidase, or the like,
18
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
may also be used. Any effective amount and concentration of
the conjugate or second recognition molecule may be used.
Preferably, however, the secondary incubation takes place in
the same volume a-s the primary incubation. The conjugate
may be diluted in MBE, as necessary.
Secondary incubations ranging in time from about 0.5 of
a minute to about 30 minutes were tried. Although longer
incubations were found to yield more sensitive results, in
general secondary incubations of about 10 minutes or less
were found to yield highly sensitive results. Secondary
incubations of about 5 minutes or less were also found to
yield highly sensitive results. Most preferred are
secondary incubations of between about 1 and about 2
minutes. The solution is preferably gently rocked during
the procedure to ensure mixing of the reaction components.
It was found that when the systems and methods of the
present invention are used, the primary and secondary
incubations may be performed at room temperature (17°C -
25°C), with excellent results. Higher or lower temperatures
may be used if appropriate.
After the secondary incubation the liquid phase may be
discarded and the reaction washed. In general, the reaction
is washed three times in PBS with 0.050 TWEEN 20. Prior to
injection into the device, the reaction is washed with
Enzyme substrate buffer (ESB), (0.1 M Phosphate, 0.1 M NaCl,
pH 6.8), and the reaction resuspended in ESB. In general,
with the device described above, the reaction is resuspended
in 200~~1.
The substrate to be used will depend on the enzyme in
the conjugate. In general, if the enzyme is beta-
galactosidase, an effective substrate is P-aminophenyl-beta-
D-galactopyranoside (PAPG). A concentration of 2mM is
19
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
preferred. A non-exclusive list of enzymes and substrates
is disclosed in WO 99/07879.
Other assay formats known in the art may also be
adapted for use in accordance with the present invention.
See, e.g., WO 99/07879.
Yet another embodiment of the present invention is a
kit including reagents to perform the assays of the present
invention. The kit may include any combination of reagents
used in performing the assays. It may include, for example,
a first vial or the like having magnetic beads pre-coated
with a recognition molecule for the analyte of interest; a
second vial or the like having a second recognition
molecule, specific for the analyte or the first recognition
molecule/analyte complex, the second recognition molecule
being conjugated or conjugatable to an enzyme; a substrate,
which in the presence of the enzyme generates an
electrochemical capable of redox recycling. The kit may
also contain a single use electrochemical sensor module.
Preferably the kit also includes buffers, positive controls,
negative controls, and other reagents for use in the assay.
EXAMPLES
Experiments were conducted to evaluate faster, more
sensitive devices and methods for detecting and quantitating
analytes based on the proportional production of an
electrochemical capable of undergoing redox recycling and
the measurement of the electrochemical with an IDA having a
conformation such that the electrochemical will undergo
redox recycling.
Unless otherwise specified, the following materials
were used. M450 Tosyl-activated magnetic beads (Dynal,
Product No. 140.04). Coating buffer 0.1 M Phosphate Buffer
Saline (PBS), pH 7.9. Post-coating washing buffer PBS, pH
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
7.4 with 0.10 (w/v) bovine serum albumin (BSA)(lx
crystallized, Sigma, cat# A-4378). Storage buffer, PBS, pH
7.4 with O.lo (w/v) BSA and 0.020 (w/v) sodium azide. Tosyl
blocking buffer, 0.2 M Tris Buffer, pH 8.5, with O.lo (w/v)
BSA. Recombinant HIV-1 p24 antigen (Devaron, Inc., cat#
301-8-2, clone # AR-DEV). Human serum derived hepatitis B
surface antigen AD subtype (adHBsAg)(Genzyme Diagnostics,
Cat# ABH0707, Lot#M-22975). Recombinant HBsAg (ayw subtype)
(Genzyme Diagnostics, Cat#ABH0705, Lot# M-22756). Goat
anti-human (IgG H+L-specific) conjugated to beta-
galactosidase (American Qualex, cat# A110GN, lot# GG017).
P-aminophenyl-beta-D-galactopyranoside (Sigma, cat# A-9545)
at 2mM, in enzyme substrate buffer (0.1 M Phosphate, 0.1 M
NaCl, pH 6.8).
Modified buffer E (MBE), 0.2 m Tris buffer, pH 8.5,
with 1.00 (w/v) BSA, is used in the coating to block the
unbound, active tosyl groups. It has been found that by
using Tris buffer with BSA, the assay is less likely to
produce non-specific binding.
. The experiments were performed with the following
positive and negative controls. Human serum with antibody
to p24: negative a-p24 (98-058-08445), approximate titer of
0; low + a-p24 (98-053-01456), approximate titer of 261;
medium + a-p24 (98-062-07940), approximate titer of 1,515;
and high + a-p24 (98-058-07537), approximate titer of
104,186. Human serum with antibody to HBsAg: high + a-HBsAg
(98-306-04981), approximate concentration of 4742 mIU/ml;
negative a-HBsAg (98-306-05415). Dilutions of high + a-
HBsAg with the negative serum were used to produce samples
with lower a-HBsAg titers.
21
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
A colorimetric assay was performed on the samples for
comparative purposes. This colorimetric assay is a widely
utilized non-electrochemical detection technique. For the
colorimetric bead optical endpoint assays, peroxidase-
Affinipure Flab) fragment mouse anti-human IgG Fc (gamma)
fragment specific (Jackson Immunoresearch, code 209-036-098,
lot 25206) was used and OPD was obtained from Abbott kit
products (OPD tablets no. 7181E, OPD diluent no. 5695).
Example 1
The desired number of beads were washed in MBE and
resuspended in the desired volume of MBE. In particular, 4-
5x106 magnetic beads were used for an electrochemical
reaction, while 1x106 beads were used in the optical
reaction.
The primary incubation in general consists of adding
the sample to be analyzed to beads (201) pre-coated with
the recognition molecule. For the test indicated below, the
serum sample was diluted 1:4 in MBE (201 per well) for a
total reaction volume of 401.
Several experiments have been performed in which the
primary incubation time period was examined, with time
incubation time intervals ranging from about 0.5 of a minute
to about 30 minutes. Although longer incubations were found
to yield more sensitive results, in general primary
incubations of 1-2 minutes were found to yield a high
sensitivity.
The reaction was then washed twice with MBE (1001) and
the beads incubated (secondary incubation) in goat-anti-
human beta-galactosidase conjugate (40~.~1 per well, 1:1000
dilution in MBE). Incubations ranging in time from about
0.5 of a minute to about 30 minutes were tried. Although
longer secondary incubations were found to yield more
22
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
sensitive results. In general, however, incubations of 1-2
minutes were found to yield a high sensitivity. The
solution is preferably shaken during the procedure to ensure
mixing of the reaction components.
It was found that when the systems and methods of the
present invention are used, the primary and secondary
incubations may be performed at room temperature (17°C -
25°C), with excellent results.
After the secondary incubation, the liquid phase was
discarded and the reaction washed three times (100.1) in PBS
with 0.050 TWEEN 20 (PBST). The reaction was then washed
once (1001) with ESB, and the reaction resuspended in ESB
(200~~1) .
The sensor (a single array of an interdigitated array
of electrodes, as generally described in United States
Patent No. 5,670,031) was activated and ESB flowed over the
sensor at a slow rate (about 0.2mL/min) until a stable
baseline was achieved. The magnet was then placed under the
sensor. The magnet was placed such that it generated a
field of force sufficient to capture magnetic beads on the
surface of the sensor.
The processed sample was then circulated over the
sensor. The bead solution was circulated for approximately
2 minutes at medium to fast flow rates (approximately
0.38mL/min). Due to the magnet, a high concentration of
beads was captured over the sensor surface.
The substrate (2mM PAPG, 1001) was then circulated
over the sensor at a slow flow rate (approximately
0.2mL/min). The flow was then stopped while substrate
solution was over the sensor and the signal was measured
with no flow for the desired period of time. The signal was
23
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
measured for about 90 to about 100 seconds for 60 seconds of
useable data.
The beads were cleared from the sensor by removing the
magnet from the proximity of the sensor and circulating
fresh ESB at a high flow rate (approximately 0.43mL/min)
over the sensor. The addition of bubbles to the ESB flow
was found to assist the clearance of the beads. The washing
step generally took between about 45 and 60 seconds. Once
the beads were washed out, fresh ESB was recycled over the
sensor until the baseline equilibrated. This step generally
took about 30 seconds. The sensor was then ready for a new
sample.
After coating the magnetic beads with p24, the beads
were incubated for one minute with the serum to be tested
(primary incubation), washed, incubated for one minute with
goat anti human beta-galactosidase IgG (secondary
incubation) and washed. The procedure described above was
then used to concentrate the beads over the sensor and the
substrate was added.
Figure 2 is a graphical representation of the measured
change in voltage over time. The first peak, starting at
about t 11900 and ending at about t 12200 corresponds to the
measurement of anti p24 in the low titer serum. The second
peak, starting at about t 12500 and ending at about t 12800
corresponds to the measurement of anti p24 in the medium
titer serum. The third peak, starting at about t 13200 and
ending at about t 13500 corresponds to the measurement of
anti p24 in the high titer serum.
The average slope was calculated from the data graph
(nAmp = y-axis; time (seconds) - x-axis) for data points
acquired from the 20th second through the 100th second of
measurement. Data was acquired at the rate of 2
24
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
observations per second and recorded as spreadsheet entries
by the acquisition program (Origin Software). For the low
titer serum the average slope was estimated to be 0.061, the
average slope for the medium titer serum was estimated to be
0.112, and the average slope for the high titer serum was
estimated to be 0.344. These values can be compared to the
optical measurements obtained using a commercially available
kit. The optical measurement provided values of 0.147,
0.291 and 0.495 for the low, medium and high titer serums
respectively. Advantageously, a tight correlation therefore
was observed between the results obtained using the present
invention and those obtained using commercially available
systems. The results obtained using the present invention,
however, required only a fraction of the time required for
the commercially available system and method.
Example 2
An experiment was conducted to find out the sensitivity
of the systems and methods of the present invention under
the conditions described below. Serial dilutions of human
serum having concentrations equivalent to 0, 15, 50, 100,
200, 400 and 800 mIU/ml anti-HBsAg were prepared. Magnetic
beads (DYNABEADS M450) which had previously been coated with
HBsAg were washed and resuspended in MBE.
For the primary incubation, the serum samples were
diluted 1:1 with MBE, and 25y1 of each diluted sample was
dispensed in a microtiter plate well. 5x10 coated beads in
25~.~1 MBE were then added to each well. The samples were
incubated for 2 minutes with gentle rocking at room
temperature. The samples were then washed twice with MBE.
For the secondary incubation, 501 of a 1:1000 dilution
of goat anti human beta galactosidase conjugate in MBE was
added to each well. The samples were incubated for 2
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
minutes with gentle rocking at room temperature. The
samples were then washed twice with MBE, twice with PBST,
once with ESB, and then resuspended in 2501. The samples
were then individually loaded onto the chip. PAPG 2mM in
ESB was then added to the system and the voltage in the
sensor recorded.
Figure 3 is a graph plotting the slope of the kinetic
measurment (nA/s) against the original concentration
(mIU/ml) of HBsAg in the sample. The results indicate a
correlation having an RZ equal to 0.8626. Qualitative
results are obtainable for concentrations at least as low as
mIU/ml, with semi-quantitative results obtainable from 50
mIU/ml or greater under these conditions. As shown in
Example 4 infra, more sensitive results may be obtained by
15 slightly varying the conditions.
Example 3
In this set of experiments, hepatitis B surface antigen
(HBsAg) levels in human serum were measured. The
measurement of dilutions corresponding to 0, 15, 50, 100,
200, 400 and 800mIU/ml were obtained using side by side
matched conditions for all reagents. A direct comparison
was made between the sensitivity of the methods and devices
in accordance with the present invention and the devices and
methods disclosed in WO 99/07879, which are at least as
sensitive and reliable as the colorimetric assay
commercially available, to obtain a direct comparison
between the system and method with and without the novel
aspects of the present invention.
Preliminarily, DYNABEADS (M450) (4x108) were coated with
200yg of HBsAg (100~~g of ad subtype obtained from human
plasma and 100~.g of ayw recombinant HBsAg) in a 850.1
reaction volume following the same protocol used for p24 in
26
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
Example 1. In parallel, a substantially identical surface
area of a microtiter plate was also similarly coated.
Sets of microbeads and microtiter plate wells were then
incubated (primary incubation) with the different dilutions
of HBsAg serum samples for two minutes at room temperature.
After two minutes the samples were removed and the different
sets of microbeads and the wells of the microtiter plates
were washed. The microbeads and microtiter plate wells were
then subjected to a two minutes secondary incubation with
goat anti-human (3-galactosidase. The conjugate was then
removed and excess conjugate was washed off.
The wicrobead samples corresponding to the different
dilutions of the sample were then individually measured by
capturing the microbeads over the sensor, adding the
substrate solution and measuring the change in voltage over
a period of 60 seconds. Figure 4 is graphic representation
of the results obtained.
Similarly, the matched pairs measured in the microtiter
plates were assessed by stopping the reaction after two
minutes, and measuring in a traditional manner the
electrochemical generated. Figure 5 is a graphic
representation of the results obtained.
As can be seen by comparing figure 4 to figure 5, the
method and device of the present invention provides under
these conditions a linear dose response that can
qualitatively detect as low as 200mIU/ml anti-HBsAg at 2
standard deviations uncertainty, with a linear dose response
up to 800 mIU/ml. In contrast, the results obtained using
the traditional method showed no statistically significant
difference between the samples, i.e., the traditional method
under these conditions does not demonstrate a measurable
dose dependent increase in electrochemical, and in fact the
27
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
traditional method under these conditions cannot
qualitatively detect the analyte at a concentration below
800 mIU/ml.
The sensitivity and reliability of the method and
device, in particular as demonstrated by the results
obtained using the short primary and secondary incubations
at room temperature was much greater than expected. These
properties of the methods and devices of the present
invention are valuable because they permit faster, cheaper,
less cumbersome analysis of a sample.
Example 4
Having determined the unexpected and valuable
properties of the system and method of the present
invention, experiments were performed in order to optimize
the procedure. In this experiment, the effect of the
concentration of magnetic beads per sample to be analyzed
was evaluated.
The experiment was generally set up as in Example 2.
Serum samples having three concentrations (100 mIU/ml, 500
mIU/ml, 2000 mIU/ml) of HBsAg were tested using either
800,000 beads per sample, as in Example 2, or 5,000,000
beads per serum sample. The data was derived as in Example
2, and is shown graphically in Figure 6.
The data from the experiment indicates that the
sensitivity of the device and method can be further greatly
enhanced by increasing the number of magnetic beads per
volume of sample. This presents a further advantage over
the traditional method since it permits an increase in the
surface area over which reactions can take place. As may be
seen from Figure 6, a concentration of 100 mIU/ml can easily
be detected using the larger amount of beads. Lower
concentrations were not tested, but the linearity of the
28
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
response indicates that concentrations as low as 15 mIU/ml
should be easily obtained with the increased number of
beads.
As sho~~in in Fig. 7, another embodiment of the
invention comprises forming linearly disposed discrete
solution compartments within a conduit. Each solution
compartment may be defined by interposing a separator, such
as a gas bubble, within a carrier fluid at predetermined
points. In this manner, the carrier fluid may be divided up
into solution compartments, each of which is formed or
sandwiched between two opposing gas bubbles within the
conduit. The conduit, such as an inert tube, may be
parallel to a ground surface, vertical to a ground surface,
or even at an angle thereto. Preferably, the conduit is
vertically positioned relative to a ground surface.
Each solution compartment may contain a different
composition of materials, such as a sample or a conjugate,
to respectively define a sample solution compartment or a
conjugate solution compartment. At least one of the
solutions compartments contains an attractable bead coated
with a recognition molecule to define a coated bead solution
compartment.
In operation, each of the solution compartments are
transported over time, from left to right as seen in Fig. 7,
within the conduit via a peristaltic pump or the like. An
attraction device, such as a magnet, electromagnet, or the
like, is disposed about the conduit. The attraction device,
when actuated, is capable of attracting one or more of the
attractable beads for processing/testing. The attraction
device preferably contains a sensor, or IDA chip as
described in detail supra. The sensor is capable of
measuring the manipulated and processed beads after they
29
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
have been transported through the conduit and/or subjected
to the "conveyor belt" of discrete solution compartments.
Advantageously, as each of the solution compartments
are transported within the conduit, due to the placement of
the attraction device, the attraction device is capable of
selectively retaining at least some of the attractable beads
within the conduit. In this manner, the attracted
attractable beads are effectively separated from the carrier
fluid. As the carrier fluid continues to flow through the
conduit, the next linearly disposed solution compartment can
manipulate the temporarily restrained beads. For example,
if the next linear solution compartment comprises a wash
solution, the temporarily restrained beads will be washed.
Similarly, if the solution compartment preceding the wash
solution compartment contains a substrate/carrier fluid, the
bathed and temporarily restrained beads can be subjected to
the substrate/carrier fluid within the conduit.
As is apparent to one of ordinary skill in the art,
such a conduit arrangement allows for the implementation of
separate processing steps in an endless sequence that can be
manipulated depending on the assay. The preferred linear
order of the solution compartments, as illustrated in Fig. 7
from left to right, is as follows: a substrate/carrier
fluid compartment, a conjugate/carrier fluid compartment, a
sample/carrier fluid compartment, and a bead compartment.
Most preferably, a wash solution compartment separates each
of the four identified material-containing solution
compartments. Multiple attraction devices are also
preferably used to facilitate improved processing
techniques.
In sum, the preferred operational steps of this
embodiment of the invention, as illustrated in Fig. 7, are
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
as follows: (1) transporting a coated bead solution
compartment to a first separation station having an
actuatable attraction device; (2) actuating the attraction
device to attract some of the coated beads in the coated
bead solution compartment such that some of the coated beads
are temporarily restrained within the first separation
station and separated from the carrier fluid; (3) flowing a
wash solution compartment into the first separation station;
(9) flowing a sample/carrier fluid solution compartment over
the attracted beads; (5) actuating the attraction device to
release the temporarily restrained beads into the sample
solution compartment; (6) flowing the sample/bead mixture
from the first separation station preferably to a second
separation station having an actuatable attraction device;
(7) actuating the second attraction device to attract some
of the coated beads such that some of the coated beads are
temporarily restrained within the second separation station;
(8) flowing a wash solution compartment into the second
separation station; (9) flowing a conjugate/carrier fluid
solution compartment over the attracted beads; (10)
actuating the second attraction device to release the
temporarily restrained beads into the conjugate/carrier
fluid solution compartment; (11) flowing the conjugate/bead
mixture from the second separation station to a third
separation station that preferably has a third actuatable
attraction device having a sensor; (12) actuating the third
actuatable attraction device to attract some of the beads,
or more specifically, some of the
bead/antigen/antibody/enzyme complex, to the vicinity of the
sensor; (13) flowing a wash solution compartment into the
third separation station; (14) flowing a substrate/carrier
fluid solution compartment over the attracted beads, which
31
CA 02364288 2001-09-10
WO 00/47983 PCT/US00/03485
in the presence of the enzyme is cleaved into a reporter
molecule capable of exhibiting redox recycling, and (15)
measuring the presence or amount of electrochemical with the
sensor, wherein the sensor produces redox recycling of the
electrochemical.
Thus, devices and methods for detecting and
quantitating analytes in a sample are disclosed. While
embodiments and applications of this invention have been
shown and described, it will be apparent to those skilled in
the art that many modifications are possible without
departing from the inventive concepts herein. The
invention, therefore is not to be restricted except in the
spirit of the appended claims.
32