Note: Descriptions are shown in the official language in which they were submitted.
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
PURIFICATION OF COBALT SOLUTIONS CONTAINING IRON AND MANGANESE WITH OXIDATION
MIXTURE
OF So2 AND OXYGEN
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present invention relates to techniques for the production of high quality
cobalt-
bearing materials, such as cobalt metals, salts and the like.
2. DESCRIPTION OF THE RELATED ART
The production of high quality cobalt metal or cobalt salts, such as the
carbonate, chloride
and sulphate forms thereof, requires the cobalt solution or electrolyte to be
purified for metals
such as iron, copper, aluminum, nickel, manganese and zinc. For example, in
the Republic of
Congo, Gecamines plants, where a large portion of the world cobalt has been
produced, the feed
solution for cobalt recovery goes to a series of hydrolysis steps, to remove
in succession copper,
then iron, aluminum, silica followed by sulphide precipitation to remove zinc
and nickel. In
Zambia, similar feed solutions go through a copper hydrolysis, followed by
iron and
aluminum/silica hydrolysis, zinc solvent extraction with DEHPA (a trademark)
and nickel removal
by ion exchange with DOWEX 4185 (a trademark). None of these processes remove
manganese
from solution. The purified solutions containing cobalt and manganese are
thereafter
''electrowon", a term well known in the art involving an electric potential
driven cathodic and
anodic reactions. Manganese is oxidized at the anode and forms Mn02, while
cobalt is deposited
on the cathode in the form of cobalt metal. Some of the manganese dioxide
formed at the anode
peels off thereby requiring frequent clean up of the cell to minimize
manganese inclusion in the
cathode.
Recent progresses in solvent extraction have led to the development of
extractants for
cobalt. One such reagent commercially used for cobalt solvent extraction
(hereinafter referred to
as "Co/SX") is CYTEC's CYANEX 272 (a trademark). One of the drawbacks of
CYANEX 272
is that it is not selective against manganese. Rather, any manganese present
in the solution fed to
SUBSTITUTE SHEET (RULE 2~)
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
SX will be loaded together with the cobalt, decreasing the loading capacity of
the solvent for
cobalt. Manganese will be stripped together with cobalt and report to the
electrolyte, leading to
similar problems as mentioned earlier.
Iron hydrolysis is also a problem. Ferrous (Fe2+) precipitation does not occur
at the low
pH levels (that is below 3) used in typical processing plants. Instead, iron
must be oxidized to its
ferric (Fe3~) form to eliminate it completely prior to Co/SX or cobalt
electrowinning (hereinafter
referred to as "Co/EW"). Oxidation is performed by sparging air or oxygen
through the solution.
This process is inefficient and takes up to 10 hours to achieve satisfactory
results.
In the case of Manganese, a proposed solution involves oxidizing, and then
precipitating,
manganese prior to Co/SX or Co/EW. Oxidants suggested to conduct this
operation are
expensive and usually difficult to handle, such as ozone, hydrogen peroxide
and hydrogen
peroxysulphate (known as Caro's acid.
Among the literature are two processes which relate to the use of SOZ with air
as an
oxidant in processes to precipitate certain ionic species from solution. For
example, US
2.816,819 to Wallis et al. discloses a system which uses SOZ/Air to
precipitate iron from a cobalt-
bearing solution. Canadian Patent 935,650 discloses a technique by which a
mixture of SOZ/Air is
used to precipitate a number of impurities from a cobalt solution. However,
neither reference
makes any suggestion toward the selective precipitation of iron or manganese
from a cobalt
solution in a manner that minimizes the precipitation of cobalt, along with
the subject iron or
manganese.
It is therefore an object of the present invention to obviate or mitigate
these
disadvantages.
SUMMARY OF THE INVENTION
2
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
Briefly stated, the present invention involves a process for selectively
removing an iron
constituent and a manganese constituent from a cobalt-bearing composition,
comprising the steps
of:
(a) subjecting the composition to a first oxidation mixture of SOZ and oxygen,
at
conditions sufficient to oxidize the iron constituent;
(b) hydrolyzing the iron constituent;
(c) subjecting the composition to a second oxidation mixture of SO, and oxygen
at
conditions sufficient to oxidize the manganese constituent; and
(d) hydrolyzing the manganese constituent,
1 S (e) wherein, in steps (a) and (b), the composition is maintained at a pH
sufficient to
precipitate iron and not manganese nor cobalt, and
(f) wherein, in steps (c) and (d), the composition is maintained at a pH
sufficient to
precipitate manganese and not cobalt.
In another aspect of the present invention, there is provided a process for
selectively
removing an iron constituent and a manganese constituent from a cobalt-bearing
composition,
comprising the steps of:
(a) subjecting said composition to a first oxidation mixture of S02 and
oxygen, at
conditions sufficient to oxidize said iron constituent;
(b) hydrolyzing said iron constituent;
3
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
(c) subjecting said composition to a second oxidation mixture of SOz and
oxygen at
conditions sufficient to oxidize said manganese constituent; and
(d) hydrolyzing said manganese constituent,
S
(e) wherein, in steps (a) and (b), the composition is maintained at a pH
sufficient to
precipitate iron while minimizing precipitation of manganese or cobalt, and
(f) wherein, in steps (c) and (d), the composition is maintained at a pH
sufficient to
precipitate manganese while minimizing precipitation of cobalt.
In another aspect of the present invention, there is provided a process for
removing a
manganese constituent form a cobalt-bearing composition comprising the steps
of
- subjecting the composition to an oxidation mixture of SOz and oxygen, at
conditions
sufficient to oxidize the manganese constituent and at a pH sufficient to
precipitate
manganese and not cobalt; and
- hydrolyzing the manganese constituent.
In still another aspect of the present invention, there is provided a process
of removing
iron and manganese constituents from a cobalt-bearing solution comprising the
steps of:
(a) converting substantially all of the iron to an Fe'+ valence state;
(b) precipitating the iron from solution, while leaving substantially all of
the manganese
and cobalt in solution; and thereafter
(c) converting substantially all of the manganese to an Mn4+ state;
4
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
(d) precipitating the manganese from solution, while leaving substantially all
of the cobalt
in solution.
In yet another aspect of the present invention, there is provided a process of
removing iron
and manganese impurities from a cobalt solution, comprising the steps of:
(a) reacting the solution with an oxidation mixture of SOZ and oxygen at a pH
sufficient to
oxidize the iron impurity, while leaving the manganese impurity and the cobalt
in a
substantially unreacted state;
(b) precipitating the iron impurity from solution, and thereafter
(c) reacting the solution with an oxidation mixture of SO, and oxygen at a pH
sufficient to
oxidize the manganese impurity, while leaving the cobalt in a substantially
unreacted state;
(d) precipitating the manganese impurity from solution, wherein substantially
all of the
cobalt remains in solution.
BRIEF DESCRIPTION OF THE DRAWINGS
Several preferred embodiments of the present invention will now be described,
by way of
example only, with reference to the appended drawings in which:
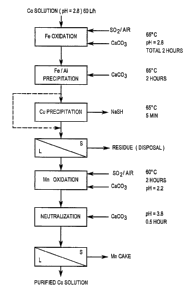
Figure 1 is a schematic view of a process to purify cobalt;
Figure 2 is a schematic view of another process to purify cobalt;
Figure 3 is a plot of iron removal versus retention time;
5
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
Figure 4 is another plot of iron removal versus retention time;
Figure 5 is a schematic view of still another process to purify cobalt;
Figure 6 is another plot of iron removal versus retention time for the process
of figure 5;
and
Figure 7 is a plot of manganese removal versus retention time for the process
of figure 5.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
As will be described, the present invention, in one of its aspects, involves a
process for
selectively removing an iron constituent and a manganese constituent from a
cobalt-bearing
composition, comprising the steps of:
(a) subjecting the composition to a first oxidation mixture of SOz and oxygen,
at
conditions sufficient to oxidize the iron constituent;
(b) hydrolyzing the iron constituent;
(c) subjecting the composition to a second oxidation mixture of S02 and oxygen
at
conditions sufficient to oxidize the manganese constituent; and
(d) hydrolyzing the manganese constituent,
(e) wherein, in steps (a) and (b), the composition is maintained at a pH
sufficient to
precipitate iron and not manganese nor cobalt, and
(f) wherein, in steps (c) and (d), the composition is maintained at a pH
sufficient to
6
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
precipitate manganese and not cobalt.
Preferably, the pH is maintained between 2.5 and 3.5, more preferably 2.8 and
3.3 and still
more preferably 3.
In one embodiment, the oxygen is in the form of O2. Preferably, the oxidation
mixture
includes Air, with O, being a constituent thereof. In this embodiment, the SOz
is at a
concentration from 0.1 percent to 2 percent, with the balance being Air. More
preferably, the
SO, is at a concentration from 0.2 to 1.4 percent, still more preferably from
0.4 to 0.6 percent.
Preferably, steps (a) and (c) occur at a temperature ranging from 40 to
90°C, more
preferably, at a temperature ranging from SO to 75°C and still more
preferably at a temperature
ranging from 58 to 64°C. Most preferably, steps (a) and (c) occur at
60°C.
In another embodiment, the oxygen is in the form of substantially pure OZ. In
this
embodiment, the SOz is at a concentration from 0.5 percent to 10 percent, with
the balance being
0, . More preferably, the SO, is at a concentration from 1 to 8 percent, still
more preferably from
2 to 3 percent.
In another aspect of the present invention, there is provided a process for
removing a
manganese constituent from a cobalt-bearing composition comprising the steps
of
- subjecting the composition to an oxidation mixture of SO, and oxygen, at
conditions
sufficient to oxidize the manganese constituent and at a pH sufficient to
precipitate
manganese and not cobalt; and
- hydrolyzing the manganese constituent.
In still another aspect of the present invention, there is provided a process
of removing
7
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
iron and manganese constituents from a cobalt-bearing solution comprising the
steps of:
(a) converting substantially all of the iron to an Fe3+ valence state;
(b) precipitating the iron from solution, while leaving substantially all of
the manganese
and cobalt in solution; and thereafter
(c) converting substantially all of the manganese to an Mn4+ state;
(d) precipitating the manganese from solution, while leaving substantially all
of the cobalt
in solution.
In yet another aspect of the present invention, there is provided a process of
removing iron
and manganese impurities from a cobalt solution, comprising the steps of:
(a) reacting the solution with an oxidation mixture of SOZ and oxygen at a pH
sufficient to
oxidize the iron impurity, while leaving the manganese impurity and the cobalt
in a
substantially unreacted state;
(b) precipitating the iron impurity from solution, and thereafter
(c) reacting the solution with an oxidation mixture of SO, and oxygen at a pH
sufficient to
oxidize the manganese impurity, while leaving the cobalt in a substantially
unreacted state;
(d) precipitating the manganese impurity from solution, wherein substantially
all of the
cobalt remains in solution.
As will be described herein below, the present invention provides an improved
process to
purify cobalt, particularly from solutions containing such impurities as iron
and manganese. This
8
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
is achieved, for example, by improving the efficiency by which iron as well as
manganese are
isolated from the solution, along with other impurities therein, leaving the
cobalt constituent for a
final isolation step thereof.
Furthermore, the present process isolates, in one embodiment manganese
selectively from
cobalt compositions, and in another embodiment both iron and manganese
selectively, that is
substantially one at a time, for example with only trace amounts of manganese
or cobalt, if any,
precipitated with the iron, and trace amounts cobalt, if any, precipitated
with the manganese.
Trace amounts in this case would vary from 0 to 4 percent of the total cobalt
present in the initial
solution.
In one example, a gas mixture of SOZ and oxygen are applied to the solution
first to
oxidize the iron into its ferric form. Thereafter, the iron is hydrolyzed with
an hydroxide bearing
agent such as lime, to yield an easily removed iron-bearing precipitate.
Thereafter, manganese is
removed in a similar manner. In this case, both steps involve a relatively
inexpensive and plentiful
oxidant, a gas mixture of O,/SO,, or alternatively Air/SOZ, or still
alternatively 100% pure Air can
be used together with equivalent amounts of SOz, preferably added as SO, in a
gaseous or liquid
form, or added as a constituent in a solution containing, for example, sodium
metabisulphite,
ammonium metabisulphite, potassium metabisulphite or other suitable forms of
metabisulphite.
The oxidant can be a 0.1-5% SO2, 99.9-95% OZ mixture, a 0.02-1% SOZ, 99-99.98%
Air
mixture. Alternatively, 100% pure Air can be used together with equivalent
amounts of SO,,
preferably added as SO, in a gaseous or liquid form, or added as a constituent
in a solution
containing, for example, sodium metabisulphite, ammonium metabisulphite,
potassium
metabisulphite or other suitable forms of metabisulphite.
Iron Oxidation/Hydrolysis
The oxidation reaction of ferrous can be conducted at temperatures ranging
from 30 to
9
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
95°C, but better results are obtained between 50 and 60°C. The
oxidation of ferrous occurs via
the reaction:
2FeS0~+ SOZ + O, -~ Fe2(S04)3 (1)
The oxidation occurs even at high acid content, but is more efficient at pH's
above pH 2.0
to minimize the effects of an unwanted side reaction as shown in (2) which
consumes SO2.
SO,+ H,O +'/20, --~H,S04 (2)
Once oxidized, the iron can then be eliminated from solution by hydrolysis as
per reaction
(3):
Fez (SO~)3+ 4H,0 --~ 2Fe00H + 3HZS04 (3)
In reaction (3), iron is shown to be hydrolyzed as goethite. To maintain the
efficiency of
the process, the acid generated in (2) and (3) can be neutralized, for example
with lime, limestone,
or any other material consuming acid.
The oxidation and the hydrolysis operations can be practiced one step after
the other, or
together. In the latter case, the overall reaction of the oxidation/hydrolysis
of ferrous when using
this oxidation process can be written as reaction (4):
2FeS0~+SO,+Oz+ 4HZ0--~2Fe00H + 3HzS04 (4)
Another way to enhance the oxidation reaction is to add small quantities of
ferric ion to
the solution being purified. Either fresh ferric sulphite solution can be
added or better, some bleed
of the oxidized solution as shown in Figure 2. This occurs because the ferric
ion tends to act as a
catalyst for further oxidation.
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
This process is particularly interesting if the oxidation has to be operated
in batch mode or
at the start up of a continuous operation. Under the conditions described
above and at a
temperature greater than 60°C, the iron precipitate formed is mostly
goethite and is relatively
easy to settle and filter.
Manganese Oxidation/Hydrolysis
Another feature of the present invention is the removal of manganese prior to
the cobalt
recovery system (precipitation, cobalt SX, cobalt EW) using
oxidation/hydrolysis. The oxidant
used is advantageously the same as the one used for iron oxidation, namely
SOz/Air or SO2/Oz or
metabisulphite/Air.
Similar to the oxidation of iron, the proportion of SOZ in the gas mixture is
0.1 to 5%
SO,, 95-99.9% O, (preferably 2% SO,, 98% OZ) or equivalent proportions when
using SOZ
O,/Air or metabisulphite/Air. Temperature ranges between 30-90°C
preferably between 50 and
60°C. The oxidation occur, even at high acidities but efficiency
increases with increasing pH.
Optimum pH is around pH = 2.5. Here too, it is preferable to neutralize acid
generated (during
oxidation).
The oxidation reaction for manganese can be written as shown in reaction (5).
MnSO~+SO, + Oz -a Mn~'+ 2S04- (5)
The oxidized manganic ion is hydrolyzed as MnO~ (reaction 6). The resulting
MnOz is
easy to settle and to filter.
MnSO~ +SO, + O,+ 2H,0 --~ MnO, + 2H,S0~ (6)
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
It is critical that the mixture of gas be well mixed to maintain efficiency.
The gas mixture
may be introduced under the impeller, or using a porous gas sparger, or any
other device
providing good gas-mixing.
Embodiments of the present invention will be described with reference to the
following
Examples which are presented for illustrative purposes only and are not
intended to limit the
scope of the invention.
EXAMPLES
Example 1- PRIOR ART
A sample of cobalt solution produced during the acid leaching of a copper-
cobalt ore from
Africa contained 7 g/L Co, 0.7 g/L Al, 2.5 g/L Fe, 0.6 g/L Si and 0.7 g/L Mn.
The iron was batch
oxidized by blowing pure oxygen through the liquid. The oxidized iron was
hydrolyzed with lime.
The graph in Figure 3 shows the kinetics of iron oxidation/hydrolysis using
oxygen. After 10
hours oxidation with pure oxygen, there was still 1.2 g/L Fe left in solution.
This amount of iron is
not compatible with downstream processing to recover pure cobalt.
Example 2
The same solution as described in Example 1 was batch oxidized using the
present
process. A mixture of 99.6% Air, 0.4% SOZ (vol) was sparged through the liquid
at 60°C. All
other conditions were similar to those of Example 1. The kinetics of iron
removal are shown in
Figure 4. In 3 hours, all the iron was removed.
Example 3
A sample of cobalt solution produced during the acid leach of a copper-cobalt
ore sample
12
CA 02366294 2001-09-24
WO 00/56943 PCT/CA00/00284
from Africa was treated to remove iron, aluminum and silica. After treatment,
the c b It solution -J
assayed: 3.1 g/L Co, 0.226 g/L Mn, 1.4 mg/L Fe, 1 1 mg/L Al. The solution
sample, still
containing manganese, was batch oxidized/hydrolyzed using SOz/Air. The
solution temperature
was held at 60°C. The proportion in the gas mixture was 0.4% SOz ,
99.6% Air. The kinetics of
S manganese removal are illustrated in Table 1. Further removal of manganese
occurs with longer
retention times. Results indicate a very selective process and minor cobalt
losses, that is in the
order of 0.5 to 1 % of the total cobalt in the initial solution.
Example 4
A large sample of the same cobalt solution as described in Examples 1 and 2
was
continuously treated during a pilot plant at a feed rate of 60 L/h. The
flowsheet to treat the
solution included the new process of this invention, namely iron and manganese
were
oxidized/hydrolyzed using SO,/Air mixtures. The overall process flowsheet is
illustrated in Figure
1~
From a solution containing an average 6321 mg/L Co, 1767 mg/L Fe, 639 mg/L Al,
103
mg/L Cu and 568 mg/L Mn, the present process was used incorporating SO,/Air
oxidation/hydrolysis for both the iron and the manganese, and produced a
purified cobalt solution
?0 assaying 6442 mg/L Co, 1.2 mg/L Fe, 5.4 mg/L Al, 8.4 mg/L Cu and I 1.5 mg/L
Mn. Overall
cobalt losses throughout the purification circuit were limited to between 2
and 4 % of the total
cobalt.
TABLE 1:
TIME (MIN) SOLUTION _ % REMOVAL
ANALYSIS -
m IL
Co Mn Co Mn
0 30 3240 226 0
30 3222 11.1 -0 95.1
13
SUBSTITUTE SHEET (RULE 26)