Note: Descriptions are shown in the official language in which they were submitted.
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
DUAL FUNCTION ASSAY DEVICE
S This application claims the benefit of U.S. Provisional Application No.
60/127,442, filed April 1, 1999, and entitled "Glucose Assay Method and
Device," the
entirety of which is incorporated herein by reference.
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to an apparatus and method for detecting substances
including glucose in a fluid to be collected from tissue.
Discussion of the Art
Glucose is an important substance for biological activities. For example, in
individuals who may be affected by diabetes, there is a need to detect or
measure the
presence and amount of glucose as part of a daily routine. However, currently
available
measurement techniques often involve invasive testing. One method of glucose
testing
includes blood based assay testing. The "finger stick" blood assay testing
technique
currently is one widely accepted methodology for glucose testing results in
the United
States. Of course, this invasive approach requires that the drawing of blood
to perform
the test. This is quite uncomfortable to patients, especially to young
patients.
Moreover, this approach is time consuming.
Therefore, it is desirable to provide non-invasive or minimally-invasive
techniques for measuring substances, such as glucose concentration, from
fluids. such
as blood and interstitial fluid.
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
2
SUMMARY OF THE INVENTION
Briefly, the present invention is directed to a system and method for
detecting
substances, such as glucose, in a fluid to be collected from a tissue. In one
aspect, the
system according to the present invention has an assay device and an optical
apparatus.
The assay device is suitable for attachment to the tissue, wherein the assay
device is a
dual function device that includes a reactive region that is responsive to at
least one
substance in fluid to be collected from the tissue when the fluid is in
contact with the
reactive region, and which reactive region is also responsive to a.first type
of optical
energy suitable to heat up and transfer heat by conduction to the tissue to
ablate the
tissue and form at least one opening in the tissue from which fluid is
collected. The
optical apparatus has an activation head to which the assay device is
attached, and a
first optical energy source that outputs the first type of optical energy. An
optical
detecting device is included in the optical apparatus to measure a
characteristic of the at
least one substance from the response of the reactive region when the reactive
region is
in contact with the at least one substance in fluid.
In another aspect, the present invention provides a method for detecting a
substance, such as glucose, in a fluid from a tissue. The method includes the
steps of
placing an assay on an activation head of an optical instrument, wherein the
assay is
responsive to at least one substance, positioning the activation head to the
surface of the
tissue so that the assay is in contact with the surface of the issue, forming
at least one
opening underneath the assay through the surface of the tissue, thereby to
allow the
fluid from the tissue to flow through the at least one opening and make
contact with the
assay, and detecting the response of the assay to the fluid to measure the
presence of the
at least one substance in the fluid. The method can be practiced by using the
system in
accordance with a preferred embodiment of the present invention.
According to yet another aspect of the present invention, an assay device is
provided that includes a base having a first side and a second side, and a
reactive region
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
3
disposed or deposited on the first side of the base. The reactive region
comprises a
photosensitizing material that is placed in contact with the surface of the
tissue and is
responsive to a suitable electromagnetic energy emitted thereon so as to heat
up and
conductively transfer heat to the surface of the tissue to form at least one
opening,
thereby to allow fluid from the tissue through the at least one opening to
contact with
the assay. Moreover, the photosensitizing material is further responsive to at
least one
substance in the fluid, from which a characteristic of the at least one
substance is
detected based upon electromagnetic energy scattered and/or reflected
therefrom.
Additional advantages and features of the invention will be set forth in part
in
the description which follows, and in part will be obvious from the
description, or may
be learned by practice of the invention. The advantages of the invention will
be
realized and attained by means of the elements and combinations particularly
pointed
out in the appended claims. It is to be understood that both the foregoing
general
description and the following detailed description are exemplary and
explanatory only
and are not restrictive of the invention, as claimed. These and other features
and
advantages of preferred forms of the present invention are described herein
with
reference to the drawing figures.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic diagram of a system for detecting at least one substance
in
a fluid to be collected from a tissue according to the present invention.
2~ FIG. 2 shows a first side of an assay device in connection with the system
shown in Fig. 1 according to the present invention.
FIG. 3 shows a second side of an assay device in connection with the system
shown in Fig. 1 according to the present invention.
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
4
FIG. 4 shows a cross-sectional side view of an assay device in connection with
the system shown in Fig. 1 according to the present invention.
FIG. 5 is a flow chart generally depicting the overall process employing the
method according to the present invention.
FIG. 6 shows a cross-sectional, partial view of the assay device and
activation
head of the optical apparatus shown in Fig. 1 in operation.
FIG. 7 shows a cross-sectional, bottom view of a first embodiment of an
activation head of the optical apparatus shown in Fig. 1 according to the
present
invention.
FIG. 8 shows a cross-sectional, bottom view of a second embodiment of an
1 S activation head of the optical apparatus shown in Fig. 1 according to the
present
invention.
DETAILED DESCRIPTION OF THE DRAWINGS
Definitions
As used herein, the term "biological fluid" or "fluid" at least includes
"interstitial fluid" (ISF), which is the clear fluid that occupies the space
between the
cells in the body, or blood.
As used herein, the term "tissue" means an aggregate of cells of a particular
kind, together with their intercellular substance, that form a structural
material of an
animal or plant. At least one surface of the tissue must be accessible to
electromagnetic
radiation so that the invention can be carried out. The preferred tissue is
the skin.
Other tissues suitable for use with this invention include mucosal tissue and
soft organs.
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
As used herein, the term "glucose" means a monosaccharide also known as D-
glucose, D-glucopyranose, grape sugar, corn sugar, dextrose, and cerelose.
Glucose
occurs in the animal body fluids, for example, in blood, lymphy, or
interstitial fluid.
Glucose enters the bloodstream by absorption from the small intestine. It is
carried via
the portal vein to the liver, where part is stored as glycogen, the remainder
reentering
the circulatory system. Another site of glycogen storage is muscle tissue.
As used herein, "analyte," "substance," or any such similar term means a
component that is being detected or measured in an analysis. In particular,
the analyte
may be any chemical or biological material or compound suitable for passage
through a
biological membrane technology known in the art, of which an individual might
want
to know the concentration or activity inside the body. Glucose is a specific
example of
an analyte because it is a sugar suitable for passage through the skin.
Individuals, for
example those having diabetes, might want to know their blood glucose levels.
Other
examples of analytes include, but are not limited to, such compounds as
sodium,
potassium, billirubin, urea, ammonia, calcium, lead, iron, lithium,
salicylates,
pharmaceutical compounds, and the like.
As used herein, "poration," "microporation," or any such similar term means
the
formation of a small hole or pore or opening to a desired depth in or through
the
biological membrane, such as skin or mucous membrane, or the outer layer of an
organism to lessen the barner properties of this biological membrane to the
passage of
biological fluids, such as analytes from below the surface for analysis.
Preferably the
hole or micropore will be no larger than about 1 mm (1000 um) in diameter, and
will
extend to a selected depth, as described hereinafter.
As used herein, "micropore" or "pore" means an opening formed by the
microporation method.
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
6
As used herein, the term "reagent," "active component," or any other similar
term means any chemical material or compound suitable for use by the methods
previously known in the art and/or by the methods taught in the present
invention, that
induces a desired effect, such as a biological, or optical effect, or other
observable
effect, which may include but is not limited to (1) producing a detectable
shift in this
compound or formulation's measurable response to the application of energy to
this
area which may be electromagnetic, mechanical, thermal, optical or acoustic
when in
contact with at least one substance in a fluid to be collected from a tissue,
(2) producing
a response when in contact with at least one substance in a fluid to be
collected from a
tissue so as to allow a characteristic of the at least one substance can be
measured or
detected from the response; and/or (3) being responsive to a type of
electromagnetic
energy emitted thereon when in contact with a tissue to heat up and transfer
heat by
conduction to the issue to ablate the tissue and form at least one opening in
the tissue
from which a fluid can be collected. As used herein, the term
"photosensitizing
material" means a material that contains at least one reagent or active
component,
which at least is responsive to at least one substance in a fluid and to a
type of
electromagnetic energy emitted thereon when in contact with a tissue to heat
up and
transfer heat by conduction to the issue to ablate the tissue.
The present invention is directed to a system and method for detecting at
least
one substance in a fluid to be collected from a tissue. For example, the
system and
method are described in connection with an application for detecting glucose
in
interstitial fluid or blood collected from a human being. Obviously, the
system and
method according to the present invention can be used to detect other
substances) in
any biological fluids.
Specifically, Fig. 1 shows a system 100 which utilizes a disposable assay
device
20 in combination with an optical apparatus 50 for detecting a substance such
as
glucose in a fluid to be collected from a tissue 40. The optical apparatus 50
includes a
housing 52 that is approximately the size of a human hand. A first energy
source 54, a
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
7
second energy source 56, and a detecting instrument 58 are located inside
housing 52.
First energy source 54, second energy source 56, and detecting instrument 58
are
coupled to an activation head 70 via optical fiber (s) 60. Activation head 70
is received
in an open end 74 of a holder 72 of housing 52. Holder 72 can have any shape
depending, among other things, on the shape of activation head 70 and hence
may
alternatively be referred to as an activation head receiving element. In a
preferred
embodiment as shown in Fig. 1, holder 72 is cone-shaped. Holder 72 can be a
separate
piece or part of housing 52. It is preferable that holder 72 be capable of
receiving
activation head 70, to allow a glucose measurement to be made by using a
disposable
assay device 20, but then allowing disposable assay device 20 to be readily
removed
after a measurement performed, and then allowing a new assay device 20 to be
attached
to the activation head 70 again so that system 100 is ready to perform a new
measurement. The optical apparatus SO shown in Fig. 1 is derived from an
apparatus
disclosed in commonly assigned U.S. Patent No. 5,792,049, which is
incorporated
herein by reference.
In a preferred embodiment, first energy source 54 transmits a first type of
energy in the form of electromagnetic radiation 39 with sufficient intensity.
Preferably,
the first energy source 54 is an optical energy source, such as a laser, that
provides
stimulated emission of radiation and operates in the infrared, visible, or
ultraviolet
region and is suitable for practicing the present invention. Alternatively,
the first
energy source 54 can be a laser diode, a radio signal generator, a microwave
signal
generator, an acoustic signal generator, a visible signal generator, an
ultraviolet signal
generator, an x-rays generator, a y-rays generator, an a-rays generator, a ~-
rays
generator, or any other type of electromagnetic signal generator.
The second energy source 56 provides a second type of energy as output to a
subject, i.e., the assay device 20. Preferably, the second energy source 56 is
an optical
energy source such as a light bulb, a tungsten halogen bulb, a noble gas
filled tungsten
bulb, one or several LEDs, or other similar optical devices covering the
desired regions
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
8
of a target optical spectrum. The second energy source S6 transmits the second
type of
energy to the activation head 70 through optical fibers) 60. The activation
head 70
projects the second type of energy onto the assay device 20. Alternatively,
the second
energy source S6 can be placed at a location within housing S2 and near the
holder
portion 72 to output the second type of energy to the assay device 20
directly. For the
embodiments where the second energy source S6 provides optical energy, the
optical
energy is output to the assay device 20 through the activation head 70 to
illuminate the
assay device 20, which is in contact with fluid from the tissue 40. Optical
energy
scattered and/or reflected from the assay device 20 can be collected and
transmitted to
the detecting instrument S8 through activation head 70 to detect and/or
measure the
presence of at least one substance in the fluid from the tissue 40, such as
glucose. Note
that although in the embodiment shown in Fig. l, first and second energy
sources S4,
S6 are separate elements, it is also envisioned that a single energy source
may provide
both first and second types of energy. An example of such an energy source is
a laser
with an adjustable intensity and bandwidth. The optical apparatus SO can
include a
control unit (not shown) to control application of the first type of energy
from the first
energy source S4, the second type of energy from the second energy source S6
and
processing of energy received by the detecting instrument S8.
Still refernng to Fig. l, detecting instrument S8 is an optical detecting
device,
such as a spectrometer. The spectrometer can, for example, include a
microspectrometer offered by American Laubscher Corporation of Farmingdale,
New
York, called the VIS/NIP microspectrometer. The detecting instrument 58 can be
other
kinds of electromagnetic signal detectors such as specified band detector(s).
The
2S detecting instrument 58 is coupled to the activation head 70 through one of
the optical
fibers 60 to detect and/or measure a characteristic of at least one substance
such as
glucose in a fluid collected from the tissue 40 based on energy spectrum
corresponding
to an interaction between the assay device 20 and the glucose in a fluid
collected from
the tissue 40. The energy spectrum includes electromagnetic energy scattered
and/or
reflected from the assay device 20 which is irradiated by at least one of the
first energy
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
9
source 54 and the second optical energy source 56. For the embodiment where
the
second energy source 56 is used to illuminate the assay device 20, the energy
spectrum
includes light within a waveband indicative of the substance, such as glucose,
in the
fluid scattered and/or reflected from the assay device 20, and the desirable
optical
interaction can include the appearance and/or change of color (visible or
invisible) in a
region of the assay device 20. Alternatively, the presence of a substance can
be
measured if the energy spectrum detected by the detecting instrument 58 does
not have
a component with a specific waveband otherwise indicative of the substance.
Furthermore, depending on the types of first and second energy sources 54, 56
and/or
the type of photosensitizing material used in the assay device 20 as discussed
in more
detail below, the presence of a substance in a fluid, such as glucose, can be
measured
using Fluorescence intensity, Fluorescence lifetime, surface plasmon
resonance,
Fluorescence polarization, circular dichroism, Raman scattering and other
known
technologies, or a combination of at least two of these technologies in
conjunction with
the embodiments of the present invention. The assay device 20 has a reactive
region
that is responsive to glucose and in contact with the fluid as discussed in
more detail
below. The detecting instrument 58 preferably has a sensor (not shown)
responsive to
energy reflected from and/or scattered by the assay device 20, and a processor
(not
shown) coupled to the sensor for receiving and processing an output of the
sensor to
determine the presence of the at least one of the substances. Further a
display (LCD or
other type) disposed on the exterior of the optical apparatus 50 may be
coupled to the
detecting instrument to display a measurement.
Optical fibers) 60 can be a single flexible transparent fiber device
containing a
2~ bundle of optical fibers or a bundle of flexible transparent fiber devices.
Preferably,
optical fibers) 60 are light guides having fiber properties and requirements
for image
transfer, in which information is continuously transmitted over relatively
short
distances. Optical fibers) 60 can be any one, or a combination of multimode,
stepped
refractive index profile fibers, graded index multimode fiber, and a single-
mode,
stepped index fiber. Preferably, however, optical fibers) 60 is a single or
combination
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
of multimode, stepped refractive index profile fibers. For example, optical
fibers made
by 3M Corporation, having a diameter range of 1-1000 microns, can be used to
practice
the present invention.
S In one embodiment of the present invention as shown in Fig. 7, optical
fibers)
60 includes optical fibers 60a, 60b, and 60c for electromagnetic energy
transmission for
the first energy source 54, the second energy source 56 and the detecting
instrument 58,
respectively. In another embodiment of the present invention as shown in Fig.
8,
optical fibers) 60 includes a bundle of several flexible transparent fiber
devices 60a,
10 60b, and 60c. For example, optical fiber 60a couples the first energy
source 54 to the
activation head 70, optical fibers 60b couple the second energy source 56 to
the
activation head 70, and optical fibers 60c couple the detecting instrument 58
to the
activation head 70. Note that as shown in Fig. 8, there are several optical
fibers 60b
and 60c to enhance the ability of the activation head 70 to output the second
type of
energy to, and collect energy scattered and/or reflected from, the assay
device 20.
Referring back to Fig. 1, curved portion 66 of housing 52 allows a user's hand
to comfortably hold and position system 100 which includes the optical
apparatus 50
with attached assay device 20 so as to press the assay device 20 firmly
against the tissue
40 to conduct a measurement. A person can initiate a measurement as the case
may be,
by pressing a push button 61 with his or her thumb.
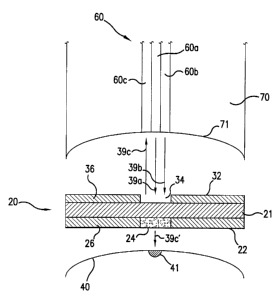
The activation head 70 has a concavely-curved portion 71, as shown in Fig. 6.
Note that Fig. 6 shows for explanatory purposes the activation head 70 being
spaced
from the tissue 40; in actual operation, the assay device 20 is attached to
the activation
head 70 and is in contact with the tissue 40. A concavely-shaped activation
head 70
allows the assay device 20 closely in contact with the tissue 40 when the
assay device
20 is pressed against the tissue 40 by the activation head 70. Moreover, the
activation
head 70 is preferably made of material suitable for absorbing heat from the
tissue
generated by the reactive region 24 during operation. The activation head 70
thus
CA 02366746 2001-09-27
WO OOI59371 PCT/C1S00/08530
11
serves as a heat sink to reduce the sensation to the subject, such as a
patient, by
removing the heat from the tissue incidentally created during the operation
process.
The material of the activation head 70 is aluminum or other suitable metals or
alloys
that have good heat sinking characteristics.
Referring now to Figs. 2-4 in conjunction with Fig. 6, according to a
preferred
embodiment of the present invention, the assay device 20 includes a base or
support
member 21 having a first side 22 and a second side 32. The base 21 can be a
small
disk-shaped member made from fiber or other suitable materials) transparent to
the
first and second types of energy output by the first and second energy sources
54, 56.
Alternatively, base 21 can be oval, square, triangular, or any other geometric
shape.
Likewise, base 21 can be made from plastics, polymers, thin film of metal,
paperboards,
or other types of materials. As shown in Figs. 2, 4, and 6, the first side 22
of the assay
device 20 has a reactive region 24 or a microdot disposed or deposited on the
first side
22. Preferably, the reactive region 24 is substantially located at the center
area of the
first side 22. In a preferred embodiment of the present invention, the
reactive region 24
includes a layer of photosensitizing material, which is responsive to the
electromagnetic
energy output by the first energy source 54 so as to heat up and conductively
transfer
heat to the surface of the tissue 40 to form at least one opening or micropore
41 as
shown in Fig. 6, thereby to allow fluid from the tissue 40 through the at
least one
opening or micropore 41 to contact with the first side 22 of the assay device
20. This
microporation technique is described in commonly assigned U.S. Patent No.
5,885,211,
which is incorporated herein by reference. Moreover, the reactive region 24 or
the layer
of photosensitizing material is responsive to a substance of interest in the
fluid, to alter
in a detectable manner electromagnetic energy scattered by and/or reflected
from the
reactive region 24 in response to application of the second type of optical
energy
thereby indicating a characteristic of the at least one of the substances in
the tissue 40.
The first side 22 of the assay device 20 optionally has adhesive material 26
disposed or deposited thereon as to leave the reactive region 24 substantially
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
12
uncovered, as shown in Fig. 2. The adhesive material 26 can be used to attach
the assay
device 20 to the tissue 40 when the activation head 70 presses the assay
device 20 to the
tissue 40. The assay device 20 optionally has adhesive material 36 deposited
on the
second side 32. The adhesive material 36 can be used to attach the assay
device 20 to
the activation head 70 of the optical apparatus 50. Optionally, the adhesive
material 36
is disposed on the base 21 to form a mask around a window 34 opposite the
reactive
region 24 of the first side 22. The window 34 allows the output
electromagnetic energy
39a from the first energy source 54, such as a laser, to reach and heat up the
reactive
region 24 of the first side 22, which then transfers heat 39c' to the surface
of the tissue
40 to form at least one opening or a micropore 41 as shown in Fig. 6, thereby
to allow
fluid from the tissue through the at least one opening 41 to contact with the
reactive
region 24 of the first side 22. The window 34 also allows the output
electromagnetic
energy 39b from the second energy source 56 to reach the reactive region 24
and cause
a desirable optical interaction with the reactive region 24 that can then be
detected from
1 S scattered by and/or reflected energy 39c, as explained above.
Referring back to Figs. 2 and 3, optionally, the assay device 20 has a tear
tab 28.
Tear tab 28 can be an integral part of the base 21, or a separate component
attached to
the base 21 by glue or other kind of adhesive material or heat sealing, etc.
Tear tab 28
can be used to handle or transport the assay device 20, prior, during or after
a
measurement. For example, prior to a measurement to be performed, tear tab 28
can be
used to attach the assay device 20 to the activation head 70 of the optical
apparatus 50.
Likewise, once a measurement has been performed, tear tab 28 can be used to
peel the
assay device 20 away from the activation head 70. A new assay device 20 can
then be
attached to the activation head 70 and system 100 is now ready to make another
measurement on tissue 40.
The photosensitizing material used in the reactive region 24 preferably
includes
a formulation of active components and/or inactive components. As explained
above,
the formulation of the photosensitizing material provides at least two
ftinctions: one
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
13
function to react with one or more substances of interest to allow for
detection thereof
by electromagnetic means; and a second function to absorb a certain type of
electromagnetic energy focused thereon to heat up and conductively transfer
heat to
adjacent tissue and form at least one opening therein. In one embodiment of
the present
invention, the inactive components include a number of well-known polymeric
binders
that can both stabilize and hold the active components in place. These
polymeric
binders include, but are not limited to, polyvinylpyrrolidone, polyvinyl
alcohol,
polyethylene glycol, bovine serum albumin, and collagen. Optionally, a
surfactant that
will allow for more even spreading and quicker re-solubilization of the active
components can be added as an inactive component. There are many choices for
the
surfactant suitable for the present invention, such as sodium dodecyl sulfate,
Triton X-
100, cholate, dioctylsulfosuccinate, polyoxyethylenesorbitans such as Tween 20
and
Span 20; and polyoxyethylene ethers such as Brij 3~, etc.
In another preferred embodiment of the present invention, a buffer can be
included in the formulation as an inactive component. Commonly used buffers
are
citrate, phosphate and a variety of "biological buffers" such as HEPES, MES,
Bis-Tris,
BES, ADA, ACES, MOPSO, MOPS, Bis-Tris propane, TES, etc. The addition of a
buffer to the formulation can improve the stability and performance of the
photosensitizing material. However, the choice of the buffer system will
greatly
depend on the choice of an indicator system as discussed below.
The active components of the layer of photosensitizing material include an
enzyme system and an indicator of the at least one of the substances in the
tissue 40 to
be measured. In a preferred embodiment of the present invention, the active
components include specific enzymes or compounds with a high binding affinity
for
glucose and can include an auxiliary enzyme or mediator. These components are
used
m conjunction with one or more indicators such as chromogens or fluorescent
probes to
produce a change in the absorption or absorption and emission spectra,
respectively.
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
14
One enzyme system that is useful in a preferred embodiment of the present
invention is the glucose oxidase\peroxidase system. This enzyme system can be
used
in conjunction with a variety of indicators such as either 4-aminoantipyrine
(4-AAP) or
3-methyl-2-benzothiazolone hydrazone (MBTH) with a variety of derivatives of
phenol
or aniline. These derivatives include phenol, p-hydroxybenzoic acid, p-
hydroxybenzene sulfonate, aniline, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-
dimethyl
aniline, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methyl aniline, N-ethyl-N-(2-
hydroxy-
3-sulfopropyl) aniline, N-(2-hydroxy-3-sulfopropyl) aniline, etc. Some
indicator
systems that can be used with the glucose oxidase\peroxidase system require
just one
chromogen and can be used without 4-AAP or MBTH. Examples of such indicators
are
ortho-dianisidine, ortho-toluidine, 3,3',5,5'-tetramethylbenzidine, ABTS and
others.
Another enzyme system that is useful in a preferred embodiment of the present
invention is glucose dehydrogenase and NAD. This enzyme system can either be
used
as is with ultraviolet detection of NADH or coupled with either an electron
mediator (or
diaphorase) with a chromogen. The electron mediator can come form the class of
compounds such as ferrocyanide, phenazine methosulfate or phenazine
ethosulfate.
The indicator can be one of the common tetrazolium dyes, such as iodo-
nitrotetrazolium, neo-tetrazolium blue, nitro-tetrazolium blue or some of the
newer
water-soluble tetrazoliums (WSTs).
There exists a large class of stains (dyes and pigments) used for cytological
staining that can be used with either the glucose oxidase/peroxidase system or
the
glucose dehydrogenase system to serve the function of absorbing
electromagnetic
(optical) energy of the first type to form openings in the tissue, as
described above.
Moreover, to detect the presence of glucose, instead of using enzymes, glucose
binding proteins can be used in a preferred embodiment of the present
invention. Such
glucose binding proteins are nondestructive and are based on a signal change
upon
glucose binding. The glucose detecting system that utilizes glucose binding
proteins as
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
active components is commonly fluorescence based. At least two types of
glucose
binding proteins can be used in the present invention. One is a single
molecule system,
and the other is a bimolecular or multimolecular system.
5 In a single molecule system, according to one embodiment of the present
invention, the binding molecule has conjugated to it two fluorophores with the
property
that the emission spectrum of one of the fluorescent dyes (donor) overlaps
with the
absorption spectrum of the other dye (acceptor). Upon binding there is a
usually a
conformational change in the protein molecule that changes the relative
distance
10 between the two dyes. Typically, the dyes move closer to each other.
Glucose binding
proteins that are candidates for this type of work are Glucose-Galactose
Binding Protein
(GGBP), hexokinase (in the absence of ATP) and apo-glucose oxidase. Any of a
large
number of molecules that undergo conformational change upon glucose binding
that
can be used to practice the present invention. Upon irradiation with a
wavelength that
15 excites the donor dye, the proximity of the two dyes determines what
percentage of the
excited donor dyes will be nonradiatively transferred to the acceptor dye; the
closer the
two dyes, the more of this quantum energy transfer occurs. This process is
called
Fluorescence Resonance Energy Transfer (FRET). The amount of FRET measured is
directly related to the glucose concentration. This nonradiative transfer can
be
measured in a number of ways: by measuring the intensities of the light
emitted from
the donor and acceptor dyes, by measuring the fluorescence lifetime of the
donor dye,
and/or by measuring the decrease in fluorescence polarization relative to the
incident
light.
According to another preferred embodiment of the present invention, in a
bimolecular system, a macromolecule that includes a single or multiple glucose
molecules) is conjugated with a donor or acceptor fluorescent dye. While a
glucose
binding protein is conjugated with the other dye, i.e., if the glucose bearing
molecule is
conjugated with a donor dye, then the glucose binding protein is conjugated
with the
acceptor dye. A common glucose binding protein used in this application is
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
16
Concanavalin A. Other lectins and GGBP, hexokinase and apo-glucose oxidase can
also be used to bind glucose in this system. Again, the amount of FRET that
occurs in
this bimolecular system is proportional to the glucose concentration and is
measured in
the same ways as in the monomolecular system described above.
The photosensitizing material is disposed or deposited onto the base 21 as a
thin
film, or as a microdot, as known to those skilled in the art, or as an
aggregation of
powders containing a formulation of inactive components and active components
as
described above. The reactive region 24 is formed and defined by the
photosensitizing
material.
FIG. 5 depicts the steps involved for using system 100 to perform a
measurement on tissue 40 according to the present invention. In particular,
step 502
involves placing an assay device 20 on activation head 70 of optical apparatus
50. As
discussed above, the assay device 20 is responsive to at least one substance
in a fluid
from tissue 40. One application of the present invention is where system 100
is used to
measure the presence of glucose in a fluid to be collected from tissue 40; in
this case
the assay device 20 is responsive to glucose. The adhesive material 36
attaches the
assay device 20 to the activation head 70 to maintain a proper position during
the
measurement.
Step 504 involves positioning the activation head 70 to the surface of the
tissue
40 so that the assay device 20 is in contact with the surface of the tissue
40. It is
preferable to press the activation head 70 firmly but gently against the
tissue 40 so that
the reactive region 24 is in direct contact with the surface of the tissue 40.
Step 506 involves forming at least one opening or micropore 41 underneath the
assay 20 through the surface of the tissue 40, thereby to allow the fluid from
the tissue
40 to flow through the at least one opening 41 and make contact with the assay
20 so as
to wet the reactive region 24. In particular, refernng to Fig. 6, step 506
involves
CA 02366746 2001-09-27
WO 00/59371 PCT/US00/08530
17
irradiating the reactive region 24 of the base 21 with energy 39a, whereby the
photosensitizing material in the reactive region 24 is responsive to the
energy 39a so as
to heat up and conductively transfer heat 39c' to the surface of the tissue 40
to form the
at least one opening 41. Alternatively, multiple openings or micropores spaced
apart
from each other in the tissue may be formed. The micropore is formed through a
surface of the tissue, such as skin, to a predetermined depth range into the
tissue. One
type of depth control of the micropore is described in more detail in commonly
assigned U.S. Patent No. 6,022,316, which is incorporated herein by reference.
After
the openings) is/are formed, the activation head 70 may be pressed against the
tissue
40 to assist in drawing the fluid from the tissue 40 into the assay device 20.
Step 508 involves detecting the response of the assay device 20 to the fluid
to
measure the presence of the at least one of the substances in the tissue 40.
Referring to
Fig. 6, step 508 involves irradiating the assay device 20 with energy, such as
optical
energy 39b or light from the second energy source 56, detecting energy 39c
reflected
and/or scattered from the reactive region 24 of the assay device 20, and
evaluating the
reflected and/or scattered energy 39c to determine the presence (and/or
measurement)
of the at least one substance in the tissue 40. The detection can be performed
by an
optical instrument or detecting unit 58.
Optionally, after a measurement on tissue 40 is performed, the assay device 20
can be removed from the optical apparatus 50 and disposed. Steps 502-508 as
discussed above can then be repeated to perform a new measurement.
Various modifications and alterations of this invention will become apparent
to
those skilled in the art without departing from the scope and spirit of this
invention, and
it should be understood that this invention is not to be unduly limited to the
illustrative
embodiments set forth herein.