Note: Descriptions are shown in the official language in which they were submitted.
CA 02367232 2002-O1-09
~,i
FIELI3 OF INVL~1~TTIOIvI~
This in~rention relates to a wound heating antiscaning topical
composition of ccntclla and ginscng.
This invention also r~e~atcs to a pxoctss for the preparation of
the topical composition, a wound heating ~sc~ing, medicated pad of
ccnsclla and ginseng and a nu~thod of malting the medicated pad.
The topical compositior~lmeclic~tec~, pad of the invcntivn.:s for
therrapeutic use on wound, kcal cut, ulcer or the like.
PRIER ART
The herb ccntella a~dica is w~ known to hsve mcdic,-inal
propel. Its viz centella e~ct is repoxtecl to be need in prieve~
~d healing of verio~aes types of wounds or ulcers, diction thereof. uz
by mgoneration or s~tim'ul~ion of skin, a»d also as a scar
minimising agent C"~ical study of a new snt~eluid ~gepL",' Annals of
Plastic Surgery, ~''ol 3, No l, pp 13 t~c~ 21,, by $osse J P etal, French
PateQt Na ,
'.594690A. Burope~t Patent No 22046,, Bc~puxn Patent No 69941aA). For
therapeutic uge, u~elly the centella e~r~t isolated, from the herb is
p'tuified
further by nanofilt~on, liquid-liquid ext~caon or colurmA, c~tron~tography
to obtain, an ex~at enriched with ~ts~ ~ es, k~p~ ~,ves via
s~~icoside, s~imic acid. end medecassic acid.. Such puri,fed ce~elts extract
comb 1°rb of the trues is Icntown to be fosmmul~ed to ~ ct~t or gel
rwith Purmulnti~ ~~ such as la~toline or propylene g'~aol o~tesrete.
2
CA 02367232 2002-O1-09
,. r
Alteniati~ely ~ the time of use, such cream or gel of purified centells
fact may be n~us~,~y applied oi~tu a permeable mat~iui strip end abed
as b~da~ge to tl~e ai~f'ec~ed area of skin. Purification of centella e~r~et,
is
laborious, t~iime corg ~d ~penaive thor rendes~sg puri~tcd centclla
every expensive. Although pfd 11e~ ext~ac;t is reporrred to show
efficacy ~t ._ I% of dze penea, Ehe cost of the purred extract and that
too et. 1°,~o is high and fiu~her increases kho cost of the
formnls~kions.
The extinct obtsitied ;from the raiots of the ~ p~ is
reported. to be main3y effective in scar elirrun~ore~'reduction, preen of
roughening of sly s:rd is also rcportcd, to hmre use ~tn cog ulcer
(CPatents 'Nod I 1052G~~4., 1 I04536A ar~,d 1080181, Enrapcen. P-ai,crtt
IrTo 6135?_2~! J '
a p'a~nts loos 6239959A, 5262635A. add 58044o58A, and
US F'nt No SfiS85'J8A). ~ The active of the ~ e~ gated to
the. saponins contained therein. t,'~,aig m O.OaS _ to % is,repcx~ted to
o~crs ar uY~NTiarr
An objccx of flue invention is to provide a wound ,~ ' ~~,g
~sc~ing topical composition of c~ ~d which shows
~d ~vouad lee~g y~ rednc,~ed scar foam and let too at low
c~caions of the actives.
a
CA 02367232 2002-O1-09
~t
Another object of the invention is tv provide a ground hea~irr~
~tisc~ring Gopicat compo~tion of cand which is
comparatively less expensive.
Another object of the mvct~tion is to provide a proc;a~ for the
prGp~rativn of a wound healing antiscazring top~al cornpo~~on of centella
~d ~ ,ginseng which. shows enhanced wound hcaliilg vviih reduced scar
forn~cion and that tnp at iow concenrmtions of the sc~ves.
Another object of the invention is to ,provide a process for the
P~Pon of a wound healing a~g topical compo~on of centella
~.d ginseng which i~ compa~tively ,less expe~tsive,
Another object of the inver~ioi~, i~ to provide a wound healing
antisca:riarg rnedicatcd pad of centclla ~rnd ,gittsea~g, which shows
er~hartced
wound healing with reduced scar forn~tion. and that too a~t low
concenbrat;,ons of the activyes.
Another object of the invention is to provide a wound heali~
anbiscarring medicated pad of ~ ,artd ginae~, which, is compmnti~reiy
less cxp '
eostve.
Another object of the invention Is to provide .a method of
m~ing a womid h,e~g a~pad of ceWells ~mcl ~~en~
4
CA 02367232 2002-O1-09
which shows enhanced wound hec~Iing with reduced scar formation and that
too at low conccn~tions of the acaives.
Another object of the inve~naon i~ to provide a method of
m~r~g a wound heolvig ~tiscarning medicated pad of ccnkclla and ,g~cn~,
'which ~ c;umparatively Ie~,c expen~ye.
A,~ccording to the invention, Chere is provided a around healing
~tsc~rrin~ topical compvsitzon of ccntclla and ginsang cvng centella
whale extrsct in 0_0! - Palo by weight and Sinseng extract in 0.01 - 0.5~.~ by
weight as actives optionally in conjunction with formul~ing agents.
,~ccaxding to the invention. there is alto provided a process for
the pre~pof a twonnd heaii~ ~picsl. eompoai~ion of
ccntclla and ginseng conrp~risu~g, mixing the actives centella whole e~taa in
0.01 - 1°fa by weight and ginseng e.~, 0.01 - O.S~o by weight apt~n~ly
W1t11 f0'Il~'lJtll,~lllg, 'I~i ~ '~-1~0°C_
according to the ion there i~ aLso provided s wound
B ~~~ng medicsted pad of cen~la ~, ,gu~~ compii~rg a
p~ ~p 'mpm~h ee~ella whole ext~ct in a.01 -
lalo weaghtlweif~,t and ~n~g c~; 0.01 ~. 0.5'x'0 ,tas
acxiv~.
s
CA 02367232 2002-O1-09
V
A,cco~to the invthere is sl'so provided a methrod of
a~g a wound heg antisc~g medic~ed pad of cend gia~eng
com~risi~ a permeable material strip imprceged with centeZlawwhole
e~sct an 0.01 - 1% weight~lweight ~d ginseng extract in 0.01 - Q.99jo
~e~ghtlweight as activess, the method compxi~g:
a) solving the actives ceitt~lls whole e~acx and gutse~n8
eat. irt a solvent. such that the solrxt~,on contains O.OOS - 0.8'!0 and 0.008
Q.4°/'0 ~,ghthove~ight of the actives respeckive~ly,
itnprognthe pcunesble material drip with the
solnticm of the acxives such that, the percentage weight g~ick-up or ed~d-oa
weight the is 130 ~ 40 gb; aid
c~ dryi~ the merdica~d drip to c~porate the s~Ivcnt.
wThs term. whole pct of ccnt~s means exude isoif~ted
from, plant in kao~vn mmner or com~rnecdaIly avaslable sad ~purised.
The whol~ eC,~ of Vie, and g malr be water or glycolic
r~a~ spray dpi yr ot'herArise.
The cosnposli3on of the inwent~lan may be cn~nverueci to forms
such as gel or cre~n, in. grown manner wing f~rmul -a4in~ sv~ch as
s~di~ua mid plwsphate, propylene g~rcol, methyl or pmpy9, p~b~
gi~'eeni~te, nilrosol; poiyethYlen~e glycol andlor polysorbate ~0.
s
CA 02367232 2002-O1-09
r~ ,
The cenrElla whole exact m the composition may be in,
preferabxy 0.1 - 1'Yo by weight.
The ginseng extract in the coirlposition ms~.y be in, preferably 0.1
- 0.5°r~o by weight.
The pezmeable mated. strip in the medicated pad camgnses
p~efcrably 0.1- 1°rru weight!'weiglit of the oentGlla whole eat ~d 0.~ -
1°~"'0
hE of the Wing e.
The permeable mot~ial strip may be $a~sletts oioth or nori
proven fabric.
Salvent~ need fnr di~scrh~ing the rcntella. whole e~roct or trie
girrsang ext. may be ptopm~ol, ethyl scet~ andlvr watear. ~refesably
centelta and ginseng whole ease di~Q~,~red in water: propanol: ethyl
aaeta~e :: 2:1:1.
p~ '~ ~o~, of the a ~r~es comb 4.5 - 0,8% by
weight of the aent~a arho~le ex~ra~x aid. 0:3 - 0.49ro by t of the
Profer~bly the p~centagc weigut l~lcup crr mld-~n weight vn
the permeable m~e~l. strip is 1300 .
z
CA 02367232 2002-O1-09
The medicated strip may be used ss such es s b$ndaga.
Alta~s~vely the medicated snip may be used im, a typical adhesive bandage
which co~nprixs a bang layer provided with breathing perforations and
coated Sikh sn adhesive at the inner surface thereof. The medicated
permeable strip of the inmen~ion can. b~ stuc'h to the iltner a~a~ of the
bacl~g layer ertd coveted wsth a non-stick coves and peel-off strips. The
m~~c~c~ of making the typical adhesive L~endag~ except the medicated
permeable stzip, mgy be in known manner: The medicated. pad msty ~so be
of the non-cx:Glu~ive type es c~sc~ribed in our Indian Patent Applzceriou No
851lM'IJ14JJ2000 . The nori-occlusive medicated pod rnay also used in a
typical
adhesive bandage as dascnibed above. These combina~itons are to be
constr<ted to be ~ the scopo of the invention..
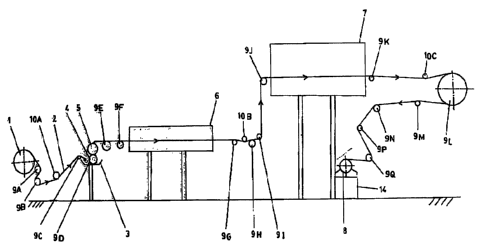
Fig 1 of the accontpmzyi~g dravvartgs is a soh~m~.o flow
dia~n of an impreg~aon system used for ~ryjr~,g nut the method of tha
invention. The gnadoa system ie known ~nd worlds is knoarn meauLer.
In F'~g 1, 1 is the roll far unwinding a permeable materiel roll
2. 3 is a trough containing a solution (not shown) of awes v~ centeaaa
whole w~kract errd giasertg ear s mof w~cr, prapanol and
~hyle_ d end 5 m~e ccmtra-rotnang squee~g rollnts disposed in the
trough o~s~e above the ot6ar de~ng s Trip or clew knot shown)
there'bet~veea. s ~d ? ~ drying ovens. s is a roller for winding the
dried perrneeble mgt roTt impregnated with the actives. 9A~9N> 9P mzd
9C~ tine ,guider rollers. 14A, 10B and lOC ace te~on, rollers.
s
CA 02367232 2002-O1-09
c
l ~1C ~ rt7u ~ ,~a9Se~ thto~i$~1 l~i~e 5i011'I~LOri
compris~r~ tho octives cor~teatted in trough. 3 it gets impre$~ted with the
activeq. The nig betr'rvcxn the squeezing rollcr~ is ~tdju~d to control the
pcrcentsge weight pick-up or add-on weight on the permeable mri roll.
The sqllToners also ensure a 11I11fUI111 ltlLj3ICgJl~l011 Of t~lG ecti.'vcs on
the parmcable material roll. The impregnated and dried permeable materiel
xoll on roller R ix cut tin strips (riot shown) of the required .
Olhtr krtc~wst devices tn~y bo us~cd for imp~gt~.ot~. of the
persruasble strip. Sach ion is the scope of the uiventzon.
The ~n~la whale exlrad ~d ~s~g d oombitof
the invention in Ire weir pctcenae elated hue. exhibits feet wound
he~g ~nr~h. ~duCed gar fob The me~pad of the ion
uniformly impregnated with such cambin~n. also exhi"i~its 'Bast wound
hea'~aqg with radu~d e~ faanatian. The cfficacy of the coaipo 'scion of the
i~verrtion is con~ne~d from the folltowing invitxo ~ntd irtvivo sh~di~. Tit
in comb~on have been found to chow a#~ivily at as low as 0.01°ro
aonoa itself. The cost of the ae~ells, arhole extxuct beit~, qtly one
9
CA 02367232 2002-O1-09
hundredth that of the puaiiied extract, the co~positiona'medie~ed pad of the
urvenkion is rendered comparatively leis exp~.ve. ~c~idee, he use of the
whol~ extra of rentellr; elimnuc~es the pur~f"xcakion step and se~res dime
thorcsby lu~iher redu~g the cost of the compositic~n~'medicatrd ~d of the
invention. The pxesence of other in centella whole e'be~des
the iril~pe~, also may be responsible fcrr the enhanced a~nund bes~lin~
cam reduced sco~ng properties of the eompositiox~lmedics~cd pad of the
invention and the too at lout enncentr.~ic~xts.
The follot~rin~ experimental examples are illustt~adve of the
invention but not lQnita~ive of the soaps f.
Example 1
Cen~ls ~Cj whole e~ot [of Amsar I'sivatc United, India,
0.0?4 gm~ and ginseng (G) jof Indens Puwet$ Lsmitedo.oz~
gs~t] ware mixed at 30°C to obtaht 2~4~ ml of topieai con~po~tion ~cyg
0.1%'by aof each of C whole exmZd tlr cad.
~o
CA 02367232 2002-O1-09
fixamgle 2
The following ing~reds~r~ts were mixed at 65°C to obt~n. ~ topical
gel:
Sodium acid phosplte . 0.8 !o
nri:ed scadinm phosphate - 0.04 ~o
Glyce~n~e - 11.25 Y
Propylene glycol - 3.75 !o
'Prc~yl ~ - O.U4 J6
Methyl p~eb~, - 0.1 "y6
I~~trosol - 1.98 !o
.
Ed~te dz godiam - ' O.OIS %
Prod - 81.84 %
Cwl~e e~d~d - 0.1 ~'lo
_ 0.1 !o
Wig, ext. -
E~3 ,
The procure of Exe~le 1 was followed with 0.0012 gxn of
east of C whole ~t ~d C~ e~ract to abt~n 2.4 m1 of topica'i
compoeidon oompris~g 0.0056 by Weights of each of C who'I~ d end ~"r
Ex~a~p~le 4
The vir~g ir~odi~ants ~ mvaed s~. 65°"C to o~mm topical
~1.
i~
CA 02367232 2002-O1-09
Sodiiun acid phosphate - 0.8 ~
Dricd sodium phosphate - ' 0.0~ ~'o
Glyc:Grine - 11.25
Propylene gXycol - 3.7s ~'fv
I'zopyl p~be31 - 0.04 a,~'n
Methyl ,p-aceben - 0.1
Nidrosol 1.98
Sodium alginate - 0.001 vfo
~d~r~ di ~o~~ ~ - a.01 s ~ro
r~~~a. ~r~r - s0.s~~ ~o
Ccnte~,La whole ex<aract - u. l vfo
G~e~ c - o.os ~fa
Faa~npie s
The proccdttrrc of Examplc 1 wag followsd wish Q'.ala 8,m of
each of C whole extrarct and G ~ to obt~. 24 ml ~f topical compv~licut
comprising O.p9°y6 by weirs of cash of C whole extra,Gt and G ex~ck
ple s
C whole ~ (4:OZA. kg) and ~C e~ct (0.012 kg~ were
di.~oived. in a mixauG of water ( 1:5 lg~, prop$noI (9.5 kg,) anal
ethylacetote
(?.5 kg) to contra, 0.0'f°h~ by weights of each of C wha~l~e extract
snd G
east. in the ~Intion_ The perce~c~ weight pioh-up or add-ott weight on
the permcablc drip comprising #l~elette cloth wyas 130". and the medicated
fl~nelette cloth slap was dried in o» progressively at 50 - 8t1°C. The
iltied mcdicatcd. staip piccc was fotmd to coin 0.1°fo vcteightJ~re'~t
of C
whole extract arid 0.0,9°~o weightlweight of G
~Z
CA 02367232 2002-O1-09
.
fixample 7
The procedure of Example 6 was followed by dissolving 0.012
kg of cash of C whole cxt~ ~d G extract in solveis~t. (water =15 kg,
propanol = 9.5 kg, ethylacetate = 7.5 kg). The percentage weight pick op or
add-an weight on the .ilannclette cloth strip was 130°x'0. The
medicated ship
piece was found to co~uain 0.1% weightlweight of each. of C whole extract
ltd Qr C7ItI'aCt.
ire-~ra~o sTV~~~s:
The following par~et~ were studied to test the efficacy of the
composstions of the invcniion.
. gtudics on eonversfon of Lotcnt TGF - beta to active form:
' Active TGF..beta (Transforming C',rrowZh Fir-bets is an
important factor 3n the process of scarring and its inhi ' 'oa reduces
scarnirtg_ Lipopolysa~~hatLde (LPS) atimulaGed mouse macrophweze
fi'brobl~ast conditioned. Th~csc were acatcd with 4.401°r6, 4.4
f°~6 ~d Q. l°~6 by
weights of each of whoje extract..c of f: (Mfid by .Am.aar Private Limited,
India), G exacts (Mfd by Inaena Private L>mited, India) and purified C
ext:~ts (Mfd by Indena Private Limited, India) anal O.a01°i6 of a
GOt111?p'~J~LOII of C whole et ~ e; composition of E~mpl~e 3
eompri~ag O.Olofo by wit of C whole exact + G end ,o~,on
of Exaurnple S con~ris~ing 4.1°fo by vvei~,hl, or C whole e~dr$c~. -~-
G exact,
13
CA 02367232 2002-O1-09
The reductions in. TGF-beta lEVels were as follows in Table 1:
Table 1
cone- 10 Ng 10U Ng 1000 erg
to.aomy ~o.oi~ey ~0.1~~
tio~n
C
whole .4'756 al% sib9~
exaace
___ .._ .__
axtract No No 2,696
effec! e~aet
C I~PdWC
extrsd t 19.5x"0 49.4g!5 61.'T~'o
G e~i
C
pm~ed No No . No
~'~Ct effe~Ct e$eC! ffe~
From the above results it is clear that C ~hol~e eat O.I°ro
and 0.01°rn showed markmd rednction in the conve~sinn of latent TGk'-
beta
to tbc active fonts while G e~auot ahowcd slig~rt =cducttaon and apt Q.19~0.
The
composidan$ of Examples 3 and 5 showed ~. 50~./o and. ~ 6~./0 suppr~ion
of TGF-beta activation respectively and also showed better e~ivity when.
compared to the purified C extract which had no effect at all in $11 the
canccntrations stated abo!vc. It is unp~t~t to note that cv~ though 0.1%
and U.Olnfo of G whole extrarctg caused nearly total. eupprre~ssion, of TGF-
beta actin du 62'r6 irrlub~,ion o'bt~n,ed using the cumpositipn of
fixantple S is sttffident to inhibit seamng while allow:ix~.g the low levels
of
s.~
CA 02367232 2002-O1-09
TGF-beta to funcLic~n in the nvr~rtal ptuces-s of ~tcaZir~,g. Thsrrefore txse
cotrcerittatxvns of the actives in the compositi~rra as per the inver~io~n are
optsmst to be highly effective in hea'!i~ of waund anal ~ the same time
reduce scat formatson.
Studs showin,~ the end--inflammatory (a~ntiscerrina)
..~..~ s..
llrIs~cxophages co»ditiorred in 1~1V11 + Z U°~o FBS medium were
utilised for inhibition of IL-~6 (Inixrlrukin - 6) lrr~Gh by ~rGa~ing with
0.001%,
4.(31°!o ~d O.l~Jo by weights of each of whole extracts of C ( Mfd by
Amsa~r Private Lnnited): G eats ~.Vlfd by Indan$ Private Limixed, W diaj
and purified C extrocts (Mfd by Indens~, private Limited; Iradia~ m~.d
0.001°i6 of a composition of C whv~le cxt~Cl + G' cxLraci; cvsnpo~-
ition of
Exatnp3e 3 comp~ri~irrg 0.0 i~ by weight of C whole extract + ~G eared
composition of Ex~nple 5 compris~r~g 4: ~% by of C vrhvle ca4~ct t
C cadract:
The results of rcduetion in xI. - 8 levels were as follows in Table
2.
~s
CA 02367232 2002-O1-09
v
v
TaublG 2
xo ~ y ~ _. ~~ ~ _
trasionr (0.00~~6) (o,oyy to.i~ .
whose S_6% 22% 27.8%
a
G
~aet Nn Nu ' X7.796
effect effect
r
e~t~t + No 22% 359
t3 wc~'et:lc1t'cct
.~
jmtiliCd No No No
e7~'aCt effect et~aet effect
From the about x~csults it i~ y!cry c,~ee~ tlx~ the cxxapc~ili~s of
Bxampies 3 and 5 of thre invert~ioo, speclelly that of ~ 5 was found
to be more c3~cclivc in inhibi#~g IL..b lavels. This impiias more eff~cscy in
seducing inf~an~mstory ~d inr~sad rata of wound heal~,g rhea
compered to rho xxidividuai C and CI whole eadracts, why p'~d C
e,~cts showed ao erect ~ e11.
In-vayo atn~e~ .
Slx~c'i~s 't~ conc~ucttd vn surg~icat founds of sizes 2" x 1"
10" x 1" of 30 ~ Volm. 15 YOh~teess ice trca~cd with 8 pla~ccbo
base (piss, gcl,) while 15 others weo~ with the composudon of the
' 16
CA 02367232 2002-O1-09
mvcntion of Example 5. The data was teed wing the "t" t~cst ett 9596
co~tfidence level.
The mean hea~i~,g times of the placebo group ~d groups
treated !rxith the Composition of E~arnpl~e 5 we.~e 13.64 days end lQ.fr4 days
respectively: These xesult~s t~aiag the cosnpositsor~s of the invention were
found to be ~cally sigxtificent. 30 vulunire~ visuall~r n~.ed the degree of
scox~ a scale from I - 5 ( 1 rated low scaa~g and 5 rdt~. very high
scarring), bawd on photographs taken befom and after the stitches were
removed attar healing tames. The avemg~e rating by the volutes for the
tscated sets was 2:664 whereas for the placebo sets aras 4.541. This
showed that: the degree of sce~ring is the treated sett~ was mv~ch lei wh~an.
competed tn placebo.
The centelta wile e~ui e~ct coni4n as
per the invention has been found to be effective is both Ever wound pooling
std, reduced sag even st low ~ncettu~~tions of the eaiv!es x.e. from
a.ai~~.