Note: Descriptions are shown in the official language in which they were submitted.
CA 02367376 2001-09-17
WO 00/54821 , PCT/US00/06773
TITLE OF THE INVENTION
MOLDED IMPLANTS FOR ORTHOPEDIC APPLICATIONS
BACKGROUND OF THE INVENTION
Field of the Invention:
This invention relates to an implant and methods for making and using the
implant
to fill void defects in bone and to accomplish orthopedic fusions.
Background Information:
In the field of orthopedics, it is desirous to be able to fill bony defects
and to be
able to fuse joints together using grafting procedures. One procedure that is
frequently required is the repair of skeletal void defects. In particular, it
is
frequently required that bony defects be filled or repaired after trauma or
disease
has destroyed the native bone. This need may arise from trauma, as in a
compound or complex fracture, through removal of diseased tissue, as in, for
example, removal of a cancerous growth, or any of a number of other
degenerative
or damaging conditions. It is common practice in spinal surgery to effect the
fusion of adjacent vertebrae by placing bone graft between the vertebrae. This
need may arise from a condition such as severe scoliosis, from trauma in which
the back is severely damaged, or in the common instance of degenerative disk
disease.
Prior to the present invention, the filling of bone defects was usually
accomplished
through the use of metallic fixation and reinforcement devices or the
combination
of metallic devices with autograft or allograft.
Recurrent problems in the methods known in the art are the lack of
incorporation
of the metallic graft materials, the pain associated with autograft harvest,
the lack
of sufficient amounts of autograft for harvesting, the labor-intensive nature
of
CA 02367376 2001-09-17
WO 00/54821 2 PCT/LJS00/06773
autograft and allograft preparation, and the relatively poor performance of
commonly acquired allografts.
A recurring problem in the methods known in the art for repairing, for
example,
the acetabular surface is that frequently, upon insertion into the acetabulum
of
metallic or polymeric implant materials, voids remain between the back surface
of
the implant and the pelvic bone remaining in the original femoral socket.
In one method known in the art, generally referred to as "impaction grafting"
(see,
for example, Elting, et al., Clinical Orthopaedics and Related Research,
319:159-
167, 1995), compressed morselized cancellous allograft bone is used to fashion
implants for insertion, for example, into the intramedullary canal of
recipients.
However, problems associated with that technique include subsidence and the
need to use synthetic "glues" such as polymethylmethacrylate. While cortical
cancellous chips combined with metallic mesh and circlage wires have been used
successfully to fill voids in the acetabulum and proximal femur, and while
incorporation of bone chips and de novo bone formation at the impaction
grafting
site has been observed, cortical-cancellous chips handle poorly. The chips
tend to
behave like gravel and do not stay in the location into which they are placed
unless enclosed by wire mesh or another retaining device. Furthermore, when
methyl methacrylate or like cement is pressurized in impaction grafting, large
amounts of bone chips become sequestered and therefore are biologically
inactive.
In one recent patent, (see US Patent No. 5,824,078 and references cited
therein),
an apparatus was described for fashioning composite allograft by impaction of
cancellous bone and added cement to form acetabular cups. These methods are
limited in applicability in that the impacted implant, once formed, is no
longer
moldable and has limited pliability. The result of such inflexibility is that
voids
remain, even after the impacted graft is positioned in an appropriate location
in a
recipient. In addition, the impaction procedure itself requires specialized
equipment (such as the rack-and-pinion device to which the 5,824,078 patent is
directed) or time consuming in-surgery impaction of bone particles (see the
Elting
CA 02367376 2001-09-17
WO 00/54821 2 PCT/US00/06773
et al., article, which describes a six-step, in-situ, procedure which requires
iterative packing and tamping of bone particles).
In US Patent No. 5,439,684, methods of making variously shaped pieces of
demineralized swollen bone are disclosed. The shaped bone pieces are composed
of large machined pieces of bone of specific shape and are thus not moldable
and
are not composed of cortical-cancellous bone chips.
This invention provides a solution to 'the above-noted, long-standing problems
by
providing specific shapes and compositions of biomaterials for filling of
tissue
voids, in particular in bony tissue, in an easy to use and effective format.
BRIEF DESCRIPTION OF THE DRAWINGS:
Figure 1 is a representation of a first embodiment of the invention, wherein a
disk-shaped bioimplant is provided for insertion into the acetabular socket or
other
location to fill voids that remain upon insertion of a metallic or other
implant.
Figure 2A is a representation of a second embodiment of the invention, wherein
a
substantially disk-shaped bioimplant is provided, but wherein a sector of the
disk-
shaped implant has either been removed or has not been included when initially
created, so that upon insertion into the acetabluar socket, a substantially
cone-
shaped or hemisphere-shaped implant, figure 2B, is formed.
Figure 3 provides representations of a number of further embodiments of the
invention: Fig. 3A depicts a thin "U"-shaped implant useful in knee revision
surgeries; Fig. 3B depicts a thicker "U"-shaped implant useful in spinal
fusion
procedures; Fig. 3C depicts a thin oval implant useful in knee revision and
other
surgical procedures; Fig. 3D depicts an implant shape useful in posterior
lumbar
interbody fusion ("PLIF") procedures; Fig. 3E depicts a dowel shaped implant,
useful in spinal and joint fusions; Fig. 3F depicts a tapered dowel shaped
implant,
useful in spinal and joint fusions.
Figure 4 provides representations of a number of further embodiments of the
invention: Fig. 4A depicts a femoral or tibial ring shaped implant useful in
interbody fusion procedures; Fig. 4B depicts a round, plug-shaped implant
useful
CA 02367376 2001-09-17
WO 00/54821 4 PCT/US00/06773
in cranial burr-hole repairs; Fig. 4C depicts a thin "U"-shaped implant which
may
be folded to provide a cone-shaped or hemisphere-shaped implant depicted in
Fig.
4D, useful in knee replacement procedures; Fig. 4E depicts a thin embodiment
of
the implant depicted according to figure 2, and Fig. 4F depicts the implant
when it
is folded onto itself to form a cone or hemisphere, useful in acetabular cup
reconstruction and other procedures.
Figure 5 provides representations of a number of further embodiments of the
invention: Fig. SA depicts an implant similar to that shown in figures 2 and
4A,
except that an asymmetric sector has been removed or excluded from the
otherwise circular implant shape; Fig. SB depicts the implant of Fig. SA when
folded upon itself to form a cone, or hemisphere, useful in acetabular cup and
like
reconstructions; Fig. SC depicts a "donut"-shaped implant comprising a flat
circular implant having a co-axial void, useful in acetabular cup
reconstruction
and like procedures where the implant is molded or press-fit to the void
space;
Fig. SD depicts a hemi-shell shaped implant which may be press-fit into a bone
void, such as in the acetabular cup; Fig. SE depicts a cone-shaped or
hemisphere-
shaped implant which may be press-fit into a bone void, such as in the
acetabular
cup; Fig. 5F depicts a tube which, depending on diameter, may be press-fit or
used
in an impaction grafting procedure in a bone intramedullary canal; Fig. SG
depicts a nested pair of tubes or cones which may be used for repair of large
femoral defects, optionally in association with impaction grafting procedures.
Figure 6 provides representations of a number of further embodiments of the
invention: Fig. 6A depicts a sheet while Fig. 6B depicts a strip for repair of
traumatic fractures, for cranial and flat-bone repair applications, and for
inter-
transverse process fusions; Fig. 6C depicts a cord-shaped implant for wrapping
or
grouting of severe trauma defects, for spinal fusions, inter-transverse
process
fusions and the like; Fig. 6D depicts a wedge-shaped implant for tibial
plateau
repairs, joint fusions, and intervertebral body fusions; Figs. 6E, 6F and 7
depict
different embodiments of restrictive devices, useful in restricting cement or
other
flowable materials in plugged intramedullary canals and the like, as in
femoral
canals during impaction procedures; Fig. 6G depicts an ovoid or football
shaped
implant useful in repairing cystoid or like bone defects; Fig. 6H depicts a
hemi-
ovoid or hemi-football shaped implant useful in repairing cystoid or like bone
CA 02367376 2001-09-17
WO 00/54821 ~ PCT/US00/06773
defects; Fig. 6I depicts a spherical implant useful in repairing cystoid or
like bone
defects; Fig. 6J depicts a hemi-spherical implant useful in repairing cystoid
or like
bone defects.
Figure 7 depicts an implant useful as a restrictive device for insertion into
a canal,
such as the intramedullary canal of a long bone, for example during a
cementous
impaction procedure.
Figures 8A-C provide X-ray evidence of the efficacy of an acetabular implant
according to this invention.
Figures 9A-10 provide photomicrographs of the composition of this invention,
before and after implantation.
Figures l0A-D provide further photomicrographs of the composition of this
invention, before and after implantation.
Figures 11A-H provides a series of photographs and X-rays showing repair of a
severe tibial complex compound fracture after removal of antibiotic loaded
methacrylate beads and implantation of the composition according to this
invention.
Figure 12A and 12B provide photographs of one embodiment of the implant
according to this invention, and its moldability.
SUMMARY OF THE INVENTION:
This invention provides shaped implants and methods for making and using the
implants to repair a wide variety of orthopedic defects or lesions, including,
for
example, acetabular cup damage or repair procedures. The implant may be made
from any of a number of known materials, by employing the specific shapes and
methods provided herein. Alternatively, specific novel compositions disclosed
herein may be used for this purpose. In one embodiment of this invention, the
implant is placed in the acetabular socket or other defect requiring repair,
and is
molded to create a perfect fit between an overlay implant to be inserted into
the
acetabulum and the bone surface of the pelvis or other overlay implant and
basal
bony structure.
CA 02367376 2001-09-17
WO 00/54821 6 PCT/LTS00/06773
Accordingly, it is one object of this invention to provide a wide variety of
desirably shaped implants for a wide variety of orthopedic applications.
It is another object of this invention to provide implant devices optimized in
shape
for repair of acetabular cup defects.
It is a further object of this invention to provide a preferred method for
making a
wide variety of desirably shaped implants useful in a wide variety of
orthopedic
applications.
It is a further object of this invention to provide a preferred method for
repair of
acetabular and other orthopedic defects.
It is yet a further object of this invention to provide desirably shaped
implants
which may be molded to create a perfect fit at the site of implantation.
Other objects and advantages of this invention will become apparent from a
review of the complete disclosure and the claims appended to this disclosure.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS:
Any material having the following required characteristics may be employed to
produce a device having the shapes and utilities disclosed herein. However, it
will
be appreciated by those skilled in the art that acceptable implant materials
having
the shapes and utilities disclosed herein may be prepared even though one or
more
of the desired characteristics is absent. Essentially, the following list of
desirable
characteristics would be displayed by an ideal composition, of which certain
compositions are disclosed herein:
a. The composition should be bioabsorbable.
b. The composition should be osteogenic.
c. The composition should be osteoinductive.
d. The composition should be osteoconductive.
CA 02367376 2001-09-17
WO 00/54821 ~ PCT/iJS00/06773
e. The composition should be malleable or flexible prior to and shortly after
implantation so that any desired shape may be produced.
~ The composition should be able to withstand freezing, freeze-drying or other
methods of preservation and be able to withstand sterilization.
g. Upon implantation, the materials should fill voids and, if malleable prior
to
implantation, should then set-up as a hard material in the shape of the voids
that have been filled.
Those skilled in the art will appreciate that any autograft, allograft or
xenograft
material that is molded, machined, cast or otherwise formed into the shapes
for
use according to this disclosure come within the scope of this invention.
However, disclosed herein are specific compositions of preferred
characteristics.
Refernng now to figure l, there is provided a representation of a first
embodiment
100 of a device that may be prepared and used for acetabular implantation. The
device 100 is substantially disk-shaped, having an upper surface 101, a lower
surface 102, each of which is substantially circular, with a diameter 110. The
diameter 110 is preferably in the range between about 35 and 55 mm, and most
preferably is about 45 mm. The disk 100 has a height 120, which is preferably
in
the range between about 1 mm and about 10 mm, and is most preferably about 5
mm in height. Furthermore, the disk 100 may be composed of particulate matter
130 embedded or suspended in a base or carrier material 140. The particulate
matter may be collagen sponge, cortical bone chips, cancellous bone chips,
cortico-cancellous bone chips, hydroxyapatite or like ceramics, bioactive
glass,
growth factors, including but not limited to bone morphogenetic protein, PDGF,
TGF(3, cartilage-derived morphogenetic proteins (CDMPs), vascular growth
factors, and the like, demineralized bone, or any other material considered to
be
beneficial in the filling of bone or cartilaginous voids and the remodeling
thereof
into solid, healthy bone or cartilage through the processes of
osseointegration
(including osteogenesis, osteoinduction, or osteoconduction, as these terms
are
recognized in the art). The base or carrier material 140 may be any material,
which retains a given form upon implantation into the void being filled behind
an
acetabular implant or in any other orthopedic application. Thus, for example,
CA 02367376 2001-09-17
WO 00/54821 o PCT/C1S00/06773
fibrin-containing compositions, which coagulate, may be included in the
carrier
material 140, as may be various collagen formulations, hydroxylapatite,
pleuronic
polymers, natural or synthetic polymers, or carboxymethylcellulose, and
combinations thereof. Preferably, the carrier material 140 comprises a
sufficiently
high concentration of gelatin, derived from human or animal tissue, or
transgenic
sources, such that prior to or upon implantation, the gelatin sets up to form
a solid
or semi-solid material of the desired shape. Use of gelatin as the base Garner
material is considered desirable because, by simply heating a pre-formed
device
according to any of the embodiments of this invention, the implant device
becomes flexible or malleable, and may be caused to precisely fit into the
shape of
any existing void or defect.
Where gelatin is employed as the base or carrier material, and cortical,
cancellous
or cortico-cancellous bone chips or demineralized bone is included in the
Garner,
the following percentages, on a weight basis, are considered desirable for
formation of the variously shaped implants disclosed herein: the gelatin is
preferably present at between about 12 to 27 weight percent. Demineralized
bone
is preferably present at between about 15 to 33 weight percent. Finally,
cancellous bone chips, cortical bone chips or cortico-cancellous bone chips
are
preferably present at between about 70 to 100 volume percent. The bone chips
soak up the gelatin/demineralized bone material so that approximately equal
volumes of the gelatin/demineralized bone and bone chips are preferably
combined to produce the final preferred composition. Devices formed from this
composition meet all of the requirements of a desirable implant material set
forth
above. Naturally, those skilled in the art will appreciate that a wide variety
of
supplemental constituents may be included in the composition. Thus, for
example, growth factors, antibiotics, anti-inflammatory or other biologically
active agents may be included at percentages that may be defined through
routine
experimentation, so long as the basic properties of the implant material is
not
adversely affected.
Using the appropriate concentration of gelatin, demineralized bone (to provide
osteogenic factors) and cortical-cancellous bone chips (to provide structural
CA 02367376 2001-09-17
WO 00/54821 9 PCT/LJS00/06773
strength and bone void filling capacity), a composition that is malleable
above
body temperature may be produced. Upon implantation or upon cooling, a solid
device forms which may be machined or warmed for molding into any desired
shape.
Referring now to figure 2A, there is shown a further embodiment 200 of the
device according to this invention. This device is similar to that shown in
figure
l, in that it has an upper surface 201, a lower surface 202, both of which are
substantially circular. However, from this embodiment of the invention, a
sector
203 has been removed or has not been included in the formation of the device,
resulting in what will be referred to herein as a "filled-C-shape". The
purpose of
this design modification is discussed in connection with the description of
figure
2B below. The composition of the device shown in figure 2A and that of figure
1
may be similar, as are its desirable characteristics. The diameter 210 of the
device 200 is preferably between about 50 mm and about 150 mm, and is most
preferably between about 75 mm and 90 mm. The height 220 of the device is
between about 1 mm and about 10 mm, and is most preferably about 5 mm. In
addition, the particulate materials 230, when included, are similar to the
particulate materials 130. The base or carrier material 240 is likewise
similar to
the carrier or base material 140. The angle formed between the adjacent sides
204
and 205 of the device 200 that exist by virtue of the absent sector 203 may be
any
angle greater than zero degrees and less than three-hundred and sixty degrees,
and
is preferably between about 90 and 150 degrees, and is most preferably about
120
degrees.
In figure 2B, there is shown the device 200, wherein the adjacent sides 204
and
205 have been brought into contact, to form a substantially cone-shaped or
hemisphere-shaped implant 260. Desirably, the device retains thermoplastic
behavior for a limited amount of time after formation, so that the desired
shape
may be formed from the cone-shaped implant 260.
Based on the foregoing disclosure, it will be apparent to one skilled in the
art that
a wide variety of shapes and orthopedic applications may be addressed
according
CA 02367376 2001-09-17
WO 00/54821 10 PCT/CTS00/06773
to this invention. As examples of the wide-variety of applications and shapes
that
may be addressed by this invention, reference is made to figures 3 through 7
included with this disclosure. Thus, Figure 3 provides representations of a
number of further embodiments of the invention: Fig. 3A depicts a thin "U"-
shaped implant 300 useful in knee revision surgeries. Fig. 3B depicts a
thicker
"U"-shaped implant 310 useful in spinal fusion procedures. Fig. 3C depicts a
thin
oval implant 320 useful in knee revision and other surgical procedures. Fig.
3D
depicts an implant shape 330 useful in posterior lumbar interbody fusion
("PLIF")
procedures. Fig. 3E depicts a dowel shaped implant 340, useful in spinal and
joint
fusions. Fig. 3F depicts a tapered dowel shaped implant 350, useful in spinal
and
joint fusions. According to the methods disclosed above, various percentages
of
particulate materials may be included in each of these disclosed shapes, as
defined
by routine experimentation, for particular applications. In addition, methods
for
conducting posterior lumbar interbody fusions, spinal fusions induced by
dowels
1 S and the like may be earned out according to methods known in the art, but
using
the novel devices disclosed herein.
Further examples of implant shapes that may be produced and used according to
the present disclosure are depicted in Figure 4. Thus, Fig. 4A depicts a
femoral or
tibial ring shaped implant 400 useful in interbody fusion procedures. Fig. 4B
depicts a round, plug-shaped implant 410 useful in cranial burr-hole repairs.
Fig.
4C depicts a thin "U"-shaped implant 420 which may be folded to provide a cone-
shaped or hemisphere-shaped implant 430 depicted in Fig. 4D, useful in knee
replacement procedures. Fig. 4E depicts a thin embodiment 440 of the implant
depicted according to figure 2, and Fig. 4F depicts the implant 450 when it is
folded onto itself to form a cone, or hemisphere, useful in acetabular cup
reconstruction and other procedures.
Additional examples of implant shapes that may be produced and used according
to the present disclosure are depicted in Figure 5. Thus, Fig. SA depicts an
implant 510 similar to that shown in figures 2 and 4A, except that an
asymmetric
sector 511 has been removed or excluded from the otherwise circular implant
shape. Fig. SB depicts the implant of Fig. SA when folded upon itself to form
a
CA 02367376 2001-09-17
WO 00/54821 11 PCT/US00/06773
cone or hemisphere 520, useful in acetabular cup and like reconstructions.
Fig.
SC depicts a "donut"-shaped implant 530 comprising a flat circular implant
having
a co-axial void, useful in acetabular cup reconstruction and like procedures
where
the implant is molded or press-fit to the void space. Fig. SD depicts a hemi-
shell
shaped implant 540 which may be press-fit into a bone void, such as in the
acetabular cup. Fig. SE depicts a cone-shaped or hemisphere-shaped implant
550,
which may be press-fit into a bone void, such as in the acetabular cup. Fig.
SF
depicts a tube 560 which, depending on diameter, may be press-fit or used in
an
impaction grafting procedure in a bone intramedullary canal. Fig. SG depicts a
nested pair of tubes or cones 570, which may be used for repair of large
femoral
defects, optionally in association with impaction grafting procedures. Each of
these shapes may be fashioned by hand, molded, extruded or formed by other
means known in the art. In addition, solid materials may be machined to
produce
the desired shapes, or because of the thermoplastic properties of gelatin, the
desired shapes may be produced by known stereolithographic processes.
Yet further examples of the shapes that may be produced and used according to
this invention are depicted in Figure 6. Thus, Fig. 6A depicts a sheet 600
while
Fig. 6B depicts a strip 610 for repair of traumatic fractures, for cranial and
flat-
bone repair applications, and for inter-transverse process fusions. Fig. 6C
depicts
a cord-shaped implant 620 for wrapping or grouting of severe trauma defects,
for
spinal fusions, inter-transverse process fusions and the like. Fig. 6D depicts
a
wedge-shaped implant 630 for tibial plateau repairs, joint fusions, and
intervertebral body fusions; Figs. 6E, 6F and 7 depict different embodiments
of
restrictive devices, 640, 650, 700, useful in restricting cement or other
flowable
materials in plugged intramedullary canals and the like, as in femoral canals
during impaction procedures. The flow restrictor 640 has a classic "cork"
stopper
shape. The implant 650 has a tapered shape like that of the "cork" 640, but
the
device 650 is formed by a plurality of stacked "ribs" 651-655 of decreasing
diameter. Naturally, the ribs may be formed by molding, such that separate
elements 651-655 need to be separately produced. The implant 700 comprises an
upper, solid portion 710 having a substantially "cork" shaped configuration.
Affixed at seam 720 to the upper solid portion 710 is a thin, hollow, lower
portion
CA 02367376 2001-09-17
WO 00/54821 12 PCT/US00/06773
730. The thin lower portion 730 folds upward about seam 720 upon insertion of
the implant 700 into a lumen 780 of a bone 790 to form a tight seal 740
surrounding the upper plug portion 710. Fig. 6G depicts an ovoid or football
shaped implant 660 useful in repairing cystoid or like bone defects. Fig. 6H
depicts a hemi-ovoid or hemi-football shaped implant 670 useful in repairing
cystoid or like bone defects. Fig. 6I depicts a spherical implant 680 useful
in
repairing cystoid or like bone defects. Fig. 6J depicts a hemi-spherical
implant
690 useful in repairing cystoid or like bone defects.
Having generally described the invention, including the best mode and
preferred
embodiments thereof, the following section provides specific exemplary support
for the invention as disclosed and claimed. However, the specifics of these
examples are not to be considered as limiting on the general aspects of this
invention as disclosed and claimed.
Example 1: REPAIR OF AN ACETABULAR CUP DEFECT:
A patient presents with a severe osteolytic lesion behind a primary acetabular
implant, due to wear-debris induced osteolysis. In this case, a revision
surgery
was indicated to replace the worn acetabular component and to remove the
lesion.
After removing the original acetabular component, the bone lesion was curetted
out leaving a healthy bleeding bone mass. A cone- or hemisphere-shaped device
was made from 100% v/v cortical-cancellous chips mixed with 68% v/v
demineralized bone matrix in a gelatin Garner (24% w/w demineralized bone
matrix, 26% w/w gelatin, 50% w/w water) was heated to soften the implant,
which
was then folded to form a cone or hemisphere. This softened cone or hemisphere
of allograft was then forced into the curetted lesion and compressed with the
forgers or a trial acetabular cup. A trial cup or a reamer was used to shape
the
allograft into the form of the back of the new acetabular component. Once the
material hardened, the new acetabular component was placed on top of the
allograft cup and screwed into place. The resulting efficacy is plainly
evident in a
series of X-rays of a patient that underwent this procedure. See figure 8.
CA 02367376 2001-09-17
WO 00/54821 13 PCT/LTS00/06773
Figure 8A shows the pre-operative condition of an implant in which the
osteolytic
defect surrounding the implant articulating surface is clearly evident as the
absence of bone mass in the X-ray. Figure 8B shows an immediate post-operative
X-ray, showing the implant with the above-described composition located where
the osteolytic defect existed. Figure 8C shows the same patient six months
after
completion of the osteolytic defect repair operation. Growth of new bone and
repair of the defect is clearly evident.
Example 2: PLACEMENT OF A PRIMARY HIP ACETABULAR CUP:
Press-fit implants are used in younger patients because the long-term success
of
these implants is improved over those that are cemented into place using
methacrylate bone cement. The reason for this improved long-term success is
that
the bone directly bonds to the surface of the implant. Because bone-to-implant
bonding is improved by the incorporation of a porous coat in the implant, most
press-fit orthopedic implants now have a porous coating. However, even with a
porous coating, after explantation, most implants are found to only have
bonded to
the bone over approximately 20% of the surface area. Research has also shown
that the long-term success of the implant is roughly correlated with degree of
host-
implant bonding. The degree of host-implant bonding is severely affected by
the
quality of the fit between the bone and the implant. If there is too much play
in
the bone-implant fit, then little or no bonding occurs and it will be
necessary to
cement the implant into place. By contrast, the osteoinductive,
osteoconductive or
osteogenic matrix according to this invention, which closely and concurrently
interdigitates with both the porous surface of the implant and the bone into
which
the implant is inserted, facilitates repair of even poorly cut cavities in
bone for
press-fit insertion of implants. Interdigitation between the porous implant
surface
and bone causes bone to be induced or conducted from the bleeding bone into
the
porous coating and thereby induce much better bone-implant bonding. Bearing
these considerations in mind, a young, otherwise healthy, patient presenting
with
osteoarthritis of the hip is treated as follows: It is noted that the degree
of
advancement of osteoarthritic bone destruction is such that drug-therapy is
insufficient to relieve pain and the patient has limited mobility. In this
case, a
primary press-fit hip replacement is indicated. Through standard surgical
CA 02367376 2001-09-17
WO 00/54821 14 PCT/US00/06773
techniques, the natural hip is removed and prepared for replacement with a
metallic hip. The acetabulum is prepared by carefully reaming out a space that
fits
to the back of the acetabulum. A doughnut-shaped acetabular implant (Fig. 4A
or
SC) is prepared by warming in a water bath. The warm doughnut-shaped implant
is placed into the patient's prepared acetabulum. While the doughnut-shaped
implant is still warm, the porous acetabular cup is placed on top of the
doughnut-
shaped implant and is hammered into place. The particle size and viscosity of
the
doughnut-shaped implant material allows the material to easily flow into the
porous coating of the implant and into the host's cancellous bone.
Figure 9A shows a photomicrograph (40-X) of stained (H&E) composition
according to this invention. Based on the staining, the different components
of
this composition are identified. Note the preferred relative uniformity,
preferably
between about 125 ~.m to about Smm, and preferably, between about 500 pm to
about 1 mm or between about lmm to about 3.35 mm. We have found that bone
chips uniformly formed within these preferred size ranges result in
surprisingly
improved induction and conduction of new bone formation and improved
handling of the composition. In figure 9B, the same material is viewed under
higher magnification (100X), showing the interpenetration of gelatin into and
onto
the cortical-cancellous chips and demineralized bone matrix of the
composition.
Figure 9C shows a biopsy after implantation of this composition in a human
female, 6 months after implantation, showing new bone formed onto the surface
of a piece of allograft (H&E, 100X). Noticeable are the numerous cutting cones
within the mineralized allograft, indicating that the allograft bone will
continue to
be fully remodeled over time. Figure 9D shows a biopsy of new woven bone
between mineralized allograft chips (H&E, 100X). It should be noted that the
area
between the spicules would normally be filled with healthy marrow. However, in
this case, it can be seen that these areas are filled with fibrous
inflammatory tissue
cause by wear debris from a failed prosthesis. Figure l0A shows additional
photomicrographs of a biopsy from a human female six months after implantation
of the composition of this invention. This photograph shows details of a
cutting
cone in a piece of mineralized allograft (H&E, 400X), revealing the presence
of
osteoclasts, osteoblasts and a cement line, whereby implant material is
remodeled
CA 02367376 2001-09-17
WO 00/54821 15 PCT/US00/06773
into normal healthy recipient bone. Figure l OB shows a detailed
photomicrograph
of a cement line between mineralized allograft and new bone (H&E, 400X),
revealing osteoblasts at the periphery of the allograft. Figure l OC is a
photomicrograph of normal marrow found in areas adjacent newly formed bone,
unaffected by wear debris (H&E, 400X). Figure l OD provides a detail of the
filamentous wear debris found in the fibrous inflammatory tissue (H&E, 400X).
These photomicrographs clearly demonstrate that the composition of this
invention, whether provided in a pre-formed shape, or molded to fit precisely
into
a recipient implant site, results in rapid remodeling and osteoinductive and
osteoconductive effects. Accordingly, gaps that might otherwise prevent new
bone formation and ingrowth may be filled with the composition of this
invention
to induce union between bone and implant materials. Thus, in one specific
embodiment of this invention, a porous implant or an implant having a porous
coating is contacted with the composition according to this invention. For
example, in a total knee arthroplasty, typically an implant having 500-700 pm
metal beads contacted with the sawn-off end of the femur. By application of
the
composition of this invention at the union surface, rapid ingrowth of bone
into the
metal bead interstices is induced by driving the implant surface into a pre-
formed
or molded shape formed from the composition according to this invention.
Example 3: REPAIR OF A COMPLEX COMPRESSION FRACTURE:
Complex compression fractures are frequently associated with significant bone
loss because the nature of the fracture is such that the bone is shattered and
many
of the bone fragments are irretrievable. Current practice dictates the
collection of
as many bone pieces as possible and the placement of those pieces back into
the
fracture site. Missing pieces are normally replaced with morselized autograft
taken from the hip, from the rib, or from the fibula. Occasionally, artificial
grafting materials are used with limited success. Allografts have also been
used,
with varying success, largely dependent upon the nature of the allograft and
its
source. The application of malleable or moldable pre-formed and appropriately-
shaped implants to this type of repair allows the surgeon to effectively
replace the
CA 02367376 2001-09-17
WO 00/54821 16 PCT/US00/06773
lost bone, without inducing additional trauma by harvesting autograft from
another surgical site.
Accordingly, a complex fracture, such as one in the radius, is repaired by
following standard surgical techniques to clean the fracture site followed by
placement within the fracture of malleable allograft implant material of this
invention in the form of a football, sphere, hemi-football, hemisphere, or
sheet/strip. Shattered bone particles are packed around the malleable
material.
Alternatively, the shattered particles of bone are placed into the fracture
site and
then strips or cords of malleable implant material according to this invention
are
laid over the fracture site. Malleable cord-shaped implant material of this
invention is optionally used as an adjunct or in place of circlage wires to
fix the
fracture fragments into place.
Figure 11 shows a surgical procedure in a tibia of a patient who experienced a
complex compound fracture into which, for a period of four weeks, had been
implanted gentamycin impregnated polymethylmethacrylate "beads on a string".
Figure 11A shows circular structures in the center of the photograph which are
the
beads, implanted in an effort to treat a local infection at a fracture site.
Figure
11B shows a pre-operative X-ray of the surgical set-up, again with the
implanted
beads visible in the bone void. Figure 11C shows the intra-operative procedure
whereby the implanted beads were removed. Figure 11D shows the large cavity
remaining after removal of the beads. Figure 11E shows a photograph of the
composition according to this invention, formed in the shape of two dry eight
cubic centimeter disks, prior to implantation. Figure 11F is an intra-
operative
photograph, after implantation of sixteen cubic centimeters of the composition
of
this invention. The implant material is clearly visible, and as can be seen
from
this photograph, is moistened by body fluids, but is not soluble and is not
washed
away. Figure 11 G shows the implant site immediately post-implantation. The
site
of the implant within the void can be discerned as a faint cloud within the
void.
Figure 11H is an X-ray photograph of the implant site six-weeks post
implantation. It can clearly be seen that the implant material has remodeled
to
CA 02367376 2001-09-17
WO 00/54821 1~ PCT/US00/06773
form solid bone mass, while a portion of the void into which implant material
was
not or could not be implanted remains a void.
Example 4: REPAIR OF OSTEOLYTIC CYSTS:
Osteolytic cysts and other growths on bone that must be removed are typically
difficult to replace. Traditional practice dictates that large cystic defects
be filled
with weight- bearing allograft or autograft. Alternative techniques have
employed
synthetic materials with limited success.
In this application of the malleable implant material of this invention,
cystic
defects are repaired after removal of the cyst by placing warm, malleable
implant
material according to this invention onto the defect and forming it to
completely
fill the void. The material according to a preferred embodiment of this
invention
remodels into natural bone in a period ranging from between about 6 weeks to
about 9 months.
Example 5: 1NTERTRANSVERSE PROCESS SPINAL FUSION:
Intertransverse process spinal fusion is generally accomplished by the joint
application of both metallic fixation devices and the use of auiagraft, which
is
generally harvested from the patient's hip. The autograft harvest is
associated
with a high rate of morbidity (21%). The use of a grafting material that is
effective without the necessity of harvesting autograft would greatly benefit
patients in need of such procedures.
Accordingly, after standard surgical preparation including rigorous
decortication
of the transverse processes and the facets of two adjoining vertebrae, a
malleable
pre-molded form (strips or cords) of the malleable implant material of this
invention are lain gutter alongside the vertebral bodies. Local bone reamings
are
optionally mixed or intermingled with the still warm and malleable implant
material and then the implant material is pressed into the bleeding bone bed.
CA 02367376 2001-09-17
WO 00/54821 1 g PCT/CTS00/06773
Example 6: FILLING OF CRANIAL BURR HOLES:
Cranial burr-holes are created whenever it is necessary to cut into the skull
in
order to gain access to the brain. Current technique dictates the use of
plaster of
Paris-like substances, metallic meshes, and bone waxes to fill these holes, or
to not
fill them at all. None of the commonly employed products and procedures induce
bone to grow across the defect, and some of these products and procedures
actually inhibit the growth of the bone.
Accordingly, in this application, a disk-shaped piece of pre-molded implant
material according to this invention is placed, warm, into the burr-hole
defect,
with a small lip of the implant material remaining above the surface to serve
as a
temporary support for the material. It is anticipated that the temporary
support is
unnecessary after a period of several days, after which the plug is expected
to
remain in place on its own. It is anticipated that new bone grows into the
remaining gap to completely bridge the gap within about 6 weeks to about 9
months.
Example 7: MOLDING OF THE COMPOSITION OF THIS INVENTION:
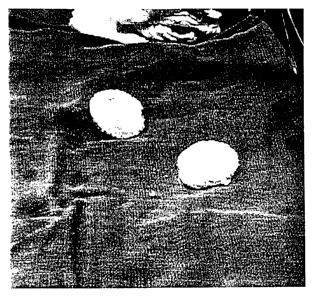
Figure 12 shows the formability and moldability of the composition of this
invention. Figure 12A shows a dry cone or hemisphere of the composition. Upon
hydration and heating to about 43 to about 49 degrees centigrade, the material
becomes moldable, and re-sets at body temperature, as shown in figure 12B,
where the moldable material is being press-fit by finger pressure into a
cavity.
Once set-up, the material is easily reamed or drilled for placement of any
desired
prosthesis.
CA 02367376 2001-09-17
WO 00/54821 19 PCT/US00/06773
Example 8: PRODUCTION OF CORTICAL, CANCELLOUS OR CORTICAL-
CANCELLOUS BONE CHIPS FOR INCLUSION IN THE COMPOSITION OF
THIS INVENTION:
Corticocancellous chips were processed from allograft obtained from the iliac
crest, iliac crest segments and from metaphyseal cancellous bone. When
metaphyseal ends and iliac crests are used, an approximate mixture of 20%:80%
to about 50%:50% cortical:cancellous bone chips is obtained. The bone chips
are
produced after debridement and antiniicrobial treatment in a class 10 or class
100
cleanroom. Appropriately cleaned and sectioned bone was ground in a bone mill
fitted with a sieve, to ensure that all collected bone chips are of a fairly
uniform
size between about 125 ~m and about 5 mm. Preferably, the collected bone chips
are in the size range of about 125 pm to about 1 mm or between about 1 mm and
3.35 mm. The ground bone chips were soaked in peroxide, with sonic treatment.
The peroxide treatment was repeated until no more fat or blood was visible,
the
peroxide was decanted and the chips were soaked in povidone iodine solution.
The chips were then rinsed with water, and then soaked in an ascorbic acid
solution, followed by treatment with isopropanol, with sonic treatment.
Finally,
the chips were treated with a further peroxide soak, followed by a water
rinse, and
then lyophilization. The dried chips were then sieved to select the desired
size
range of bone chips desired. Samples were cultured to ensure sterility.
Example 9' PREPARATION OF THE COMPOSITION OF THIS INVENTION
FOR MOLDING INTO DESIRED SHAPES:
A known weight of ground lyophilized gelatin of up to 850~m particle size was
mixed with a known weight of demineralized bone particles of between about
250~m and 850pm. A known weight of water was added to the combined gelatin
and demineralized bone, and thoroughly mixed. The gelatin, water,
demineralized
bone composition was then warmed to form a paste of known volume, and a fifty-
percent to 100 percent volume of corticocancellous bone chips of between about
125~m and 5 mm particle size was then added and the entire composition was
thoroughly mixed, with repeated warming steps as needed to ensure thorough
CA 02367376 2001-09-17
WO 00/54821 2~ PCT/US00/06773
mixing. The mixed composition was then molded into desired shapes, which are
stored in sealed sterile pouches or like containers. Upon use, a surgeon uses
the
shaped material in its pre-formed shape, or warms the material until it
becomes
moldable, before implanting the material into a desired implant site.