Note: Descriptions are shown in the official language in which they were submitted.
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
Method of Treating Eating Disorders
This invention relates to a method of treating eating disorders.
According to the present invention there is provided a method of treating
eating disorders, in which a therapeutically effective amount of a compound of
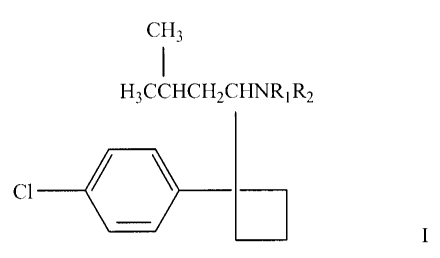
formula I
CI H3
H3CCHCH2CHNR1R2
CI
including enantiomers and pharmaceutically acceptable salts thereof, in which
R,
and R2 are independently H or methyl, is administered in conjunction with a
pharmaceutically acceptable diluent or carrier to a human in need thereof.
Eating disorders which may advantageously be treated with a compound
of formula I include anorexia nervosa, bulimia nervosa, weight-gain after
smoking
cessation, snacking, binge eating and other related disorders known to those
skilled in the art.
A preferred compound of formula I is N,N-dimethyl-1-[1-(4-
chlorophenyl)cyclobutyl]-3-methylbutylamine or a salt thereof, for example the
hydrochloride salt. A preferred form of this hydrochloride is its monohydrate.
The preparation and use of compounds of formula I, such as N,N-
dimethyl-1 -[1 -(4-chlorophenyl)cyclobutyl]-3-methylbutylamine and salts
thereof, in
the treatment of depression is described in British Patent Specification
2098602.
The use of compounds of formula I such as N,N-dimethyl-1-[1-(4-
1
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
chlorophenyl)cyclobutyl]-3-methylbutylamine and salts thereof in the treatment
of
Parkinson's disease is described in published PCT application WO 88/06444.
The use of N,N-dimethyl-l-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine
and
salts thereof in the treatment of cerebral function disorders is described in
US
Patent 4939175. The use of N,N-dimethyl-l-[1-(4-chlorophenyl)cyclobutyl]-3-
methylbutylamine hydrochloride in the treatment of obesity is described in
published PCT application W090/061 10. A particularly preferred form of this
compound is N, N-dimethyl-l-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine
hydrochloride monohydrate (sibutramine hydrochloride) which is described in
European Patent Number 230742. The use of N,N-dimethyl-1-[1-(4-
chlorophenyl)cyclobutyl]-3-methylbutylamine and salts thereof for improving
the
glucose tolerance of humans having Impaired Glucose Tolerance or Non-Insulin
Dependent Diabetes Mellitus is described in published PCT application
W095/20949.
It will be appreciated by those skilled in the art that compounds of formula
I contain a chiral centre. When a compound of formula I contains a single
chiral
centre it may exist in two enantiomeric forms. The present invention includes
the
use of the individual enantiomers and mixtures of the enantiomers. The
enantiomers may be resolved by methods known to those skilled in the art, for
example by formation of diastereoisomeric salts or complexes which may be
separated, for example, by crystallisation; via formation of diastereoisomeric
derivatives which may be separated, for example, by crystallisation, gas-
liquid or
liquid chromatography; selective reaction of one enantiomer with an enantiomer-
specific reagent, for example enzymatic oxidation or reduction, followed by
separation of the modified and unmodified enantiomers; or gas-liquid or liquid
chromatography in a chiral environment, for example on a chiral support, for
example silica with a bound chiral ligand or in the presence of a chiral
solvent. It
will be appreciated that where the desired enantiomer is converted into
another
chemical entity by one of the separation procedures described above, a further
step is required to liberate the desired enantiomeric form. Alternatively,
specific
2
CA 02367666 2001-09-14
WO 00/54765 PCTIUSOO/07115
enantiomers may be synthesised by asymmetric synthesis using optically active
reagents, substrates, catalysts or solvents, or by converting one enantiomer
to
the other by asymmetric transformation.
Preferred compounds of formula I are N,N-dimethyl-l-[1-(4-chlorophenyl)-
cyclobutyl]-3-methylbutylamine, N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-
methylbutyl}-N- methylamine, and 1-[1-(4-chlorophenyl)cyclobutyl]-3-
methylbutylamine including racemates, individual enantiomers and mixtures
thereof, and pharmaceutically acceptable salts thereof.
The individual enantiomers can be prepared by enantioselective synthesis
from optically active precursors, or by resolving the racemic compound which
can
be prepared as described above. Enantiomers of secondary amines of the
formula I can also be prepared by preparing the racemate of the corresponding
primary amine, resolving the latter into the individual enantiomers, and then
converting the optically pure primary amine enantiomer into the required
secondary amine by methods described in British Patent Specification 2098602.
Specific examples of compounds of formula I are:
(+)-N-[1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine;
(-)-N-{1-[1-(4-chlorophenyl)cyclobutyl-3-methylbutyl}-N-methylamine;
(+)-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine;
(-)-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine;
(+)-N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-N-dimethylamine;
(-)-N-{ 1 -[ 1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-N-dimethylamine.
The hydrochloride salts are preferred in each case, but the free bases
and other pharmaceutically acceptable salts are also suitable.
3
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
The compound of formula I may be administered in any of the known
pharmaceutical dosage forms. The amount of the compound to be administered
will depend on a number of factors including the age of the patient, the
severity of
the condition and the past medical history of the patient and always lies
within the
sound discretion of the administering physician but it is generally envisaged
that
the dosage of the compound to be administered will be in the range 0.1 to 50
mg
preferably 1 to 30 mg per day given in one or more doses.
Oral dosage forms are the preferred compositions for use in the present
invention and these are the known pharmaceutical forms for such
administration,
for example tablets, capsules, granules, syrups and aqueous or oil
suspensions.
The excipients used in the preparation of these compositions are the
excipients
known in the pharmacist's art. Tablets may be prepared from a mixture of the
active compound with fillers, for example calcium phosphate; disintegrating
agents, for example maize starch; lubricating agents, for example magnesium
stearate; binders, for example microcrystalline cellulose or
polyvinylpyrrolidone
and other optional ingredients known in the art to permit tableting the
mixture by
known methods. The tablets may, if desired, be coated using known methods
and excipients which may include enteric coating using for example
hydroxypropylmethylcellulose phthalate. The tablets may be formulated in a
manner known to those skilled in the art so as to give a sustained release of
the
compounds of the present invention. Such tablets may, if desired, be provided
with enteric coatings by known methods, for example by the use of cellulose
acetate phthalate. Similarly, capsules, for example hard or soft gelatin
capsules,
containing the active compound with or without added excipients, may be
prepared by known methods and, if desired, provided with enteric coatings in a
known manner. The contents of the capsule may be formulated using known
methods so as to give sustained release of the active compound. The tablets
and capsules may conveniently each contain 1 to 50 mg of the active compound,
preferably 10 mg or 15 mg.
4
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
Other dosage forms for oral administration include, for example, aqueous
suspensions containing the active compound in an aqueous medium in the
presence of a non-toxic suspending agent such as sodium carboxy-
methylcellulose, and oily suspensions containing a compound of the present
invention in a suitable vegetable oil, for example arachis oil. The active
compound may be formulated into granules with or without additional
excipients.
The granules may be ingested directly by the patient or they may be added to a
suitable liquid carrier (for example, water) before ingestion. The granules
may
contain disintegrants, eg an effervescent couple formed from an acid and a
carbonate or bicarbonate salt to facilitate dispersion in the liquid medium.
The therapeutically active compounds of formula I may be formulated into
a composition which the patient retains in his mouth so that the active
compound
is administered through the mucosa of the mouth.
Dosage forms suitable for rectal administration are the known
pharmaceutical forms for such administration, for example, suppositories with
cocoa butter or poiyethylene glycol bases.
Dosage forms suitable for parenteral administration are the known
pharmaceutical forms for such administration, for example sterile suspensions
or
sterile solutions in a suitable solvent.
Dosage forms for topical administration may comprise a matrix in which
the pharmacologically active compounds of the present invention are dispersed
so that the compounds are held in contact with the skin in order to administer
the
compounds transdermally. A suitable transdermal composition may be prepared
by mixing the pharmaceutically active compound with a topical vehicle, such as
a
mineral oil, petrolatum and/or a wax, e.g. paraffin wax or beeswax, together
with
a potential transdermal accelerant such as dimethyl sulphoxide or propylene
glycol. Alternatively the active compounds may be dispersed in a
5
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
pharmaceutically acceptable cream, gel or ointment base. The amount of active
compound contained in a topical formulation should be such that a
therapeutically
effective amount of the compound is delivered during the period of time for
which
the topical formulation is intended to be on the skin.
The therapeutically active compound of formula I may be formulated into
a composition which is dispersed as an aerosol into the patients oral or nasal
cavity. Such aerosols may be administered from a pump pack or from a
pressurised pack containing a volatile propellant.
The therapeutically active compounds of formula I used in the method of
the present invention may also be administered by continuous infusion either
from an external source, for example by intravenous infusion or from a source
of
the compound placed within the body. Internal sources include implanted
reservoirs containing the compound to be infused which is continuously
released
for example by osmosis and implants which may be (a) liquid such as an oily
suspension of the compound to be infused for example in the form of a very
sparingly water-soluble derivative such as a dodecanoate salt or a lipophilic
ester
or (b) solid in the form of an implanted support, for example of a synthetic
resin or
waxy material, for the compound to be infused. The support may be a single
body containing all the compound or a series of several bodies each containing
part of the compound to be delivered. The amount of active compound present in
an internal source should be such that a therapeuticaliy effective amount of
the
compound is delivered over a long period of time.
In some formulations it may be beneficial to use the compounds of the
present invention in the form of particles of very small size, for example as
obtained by fluid energy milling.
6
CA 02367666 2007-12-06
In the compositions of the present invention the active compound may, if
desired,
be associated with other compatible pharmacologically active ingredients.
In one aspect of the invention, there is provided the use of a compound of
formula
I
CH3
(
H3CCHCH2CHNRiR2
Cl
7
I
an enantiomer or a phannaceutically acceptable salt thereof in which R, and R2
are
independently H or methyl for treating eating disorders selected from anorexia
nervosa,
bulimia nervosa, weight-gain after smoking cessation and binge eating.
The invention further provides the use of compounds of formula I, an
enantiomer
or a pharmaceutically acceptable salt thereof, in the manufacture of a
medicament for
treating eating disorders, for example anorexia nervosa, bulimia nervosa,
weight-gain
after smoking cessation, snacking, binge eating and other related disorders
known to
those skilled in the art.
In another aspect, the invention further provides a phanmaceutical composition
for
treating anorexia nervosa, bulimia nervosa, weight-gain after smoking
cessation,
snacking, binge eating and other related disorders known to those skilled in
the art
comprising a compound of formula I, an enantiomer or a pharmaceutically
acceptable salt
thereof, in conjunction witli a pharmaceutically acceptable diluent or
carrier.
The efficacy of compounds of formula I in treating eating disorders is
demonstrable through clinical trials in a relevant population set and the data
presented
below.
7
CA 02367666 2007-12-06
Monoaminc rcuptake inhibitors have been used to treat certain of the disorders
described in the present invention. Itowever, these compounds are known to
suffer from a
number of disadvantages. Firstly, such compounds are not effective in all
patients.
Secondly, where the compounds are effective they may not provide a complete
cure of the
disorder. Thirdly, there are many undesirable side-effects known with this
type of
compound. Such side-effects include nausea, sexual dysfunction, light
headedness,
somnolence, sweating, tremor, dry mouth, asthenia, insomnia, diarrhea,
headache,
vomiting, anxiety, drowsiness, dizziness, fever, rash or allergic reactions,
arthralgia,
myalgia, convulsions, hypomania and mania.
7a
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
Sibutramine (Formula I, R, = CH3, R2 = CH3) has a pharmacological
profile which is unique amongst monoamine reuptake inhibitors. Through its
pharmacologically active metabolites, (metabolite 1, R, = H, R2 = CH3 in
Formula
I and metabolite 2, R, = H, R2 = H in Formula I) sibutramine inhibits the
reuptake
of all three monoamines differentiating it from serotonin (5-HT)-selective
reuptake
inhibitors, e.g. fluoxetine, noradenaline-selective reuptake inhibitors, e.g.
desipramine, dopamine-selective reuptake inhibitors, e.g. bupropion, and
serotonin-noradenaline reuptake inhibitors, e.g. venlafaxine (Table 1). It is
this
unique combination of pharmacological actions which renders sibutramine, and
the other compounds of formula I, efficacious in the treatment of anorexia
nervosa, bulimia nervosa, weight-gain after smoking cessation, snacking and
binge eating.
The assays below are performed in a similar manner to those described
in WO98/41528.
8
CA 02367666 2001-09-14
WO 00/54765 PCT/US00/07115
TABLE 1
Comparison of the in vitro monoamine reuptake inhibition profiles of Examples
1
and 2, and various reference monoamine reuptake inhibitors in rat brain tissue
Ki (nM)
[3H]Noradenaline [3H]5-HT [3H]Dopamine
Example 1 3 18 24
Example 2 5 26 31
Bupropion 2590 18312 409
Desipramine 2 200 4853
Fluoxetine 320 11 2025
Venlafaxine 196 26 2594
The results are the means of >_3 separate determinations
Example 1 R, = H, R2 = CH3 in Formula I
Example 2 R, = H, R2 = H in Formula I
9