Note: Descriptions are shown in the official language in which they were submitted.
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-1-
AZAINDOLE DERIVATIVES FOR THE TREATMENT OF DEPRESSION
FIELD OF INVENTION
This invention relates to compounds useful for the treatment of diseases
affected by disorders of the serotonin-affected neurological systems, such as
depression and anxiety. More specifically, the present invention is directed
to aryl
piperazinyl cyclohexyl derivatives useful for the treatment of such disorders.
BACKGROUND OF INVENTION
Pharmaceuticals which enhance the neurotransmission of serotonin (5-HT) are
useful for the treatment of many psychiatric disorders, including depression
and
anxiety. The first generation of non-selective serotonin-affecting drugs
operated
through a variety of physiological means which caused them to possess numerous
undesired side-effects. The more recently prescribed drugs, the selective
serotonin
reuptake inhibitors (SSRIs), act predominately by inhibiting 5-HT, which is
released
at the synapses, from being actively removed from the synaptic cleft via a
presynaptic
serotonin transport carrier. Since SSRIs require several weeks before they
exert their
full therapeutic effect, this 5-HT blockade mechanism cannot fully account for
their
therapeutic activity. It is speculated that this two week induction which
occurs before
a full antidepressant effect is observed, is due to the involvement of the 5-
HTlA
autoreceptors which suppress the firing activity of 5-HT neurons, causing a
dampening of the therapeutic effect. Studies suggest that after several weeks
of SSRI
administration, a desensitization of the 5-HT autoreceptors occurs allowing a
full
antidepressant effect in most patients. (See, e.g., Le Poul et al., Arch.
Pharmacol.,
352:141 (1995)). Hence, it is believed that overriding this negative feedback
by using
5HT1A antagonists would potentially increase and accelerate the clinical
antidepressant response. Recent studies by Artigas et al., Trends Neurosci.,
19:378
383 (1996), suggest a combination of 5-HT1A activity and inhibition of 5-HT
uptake
within a single molecular entity can achieve a more robust and fast-acting
antidepressant effect.
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-2-
European Patent Application No. 0714894A1 discloses the preparation of the
following compounds as SHTlA agonists for the treatment of migraine headaches.
X ~-~ (CH2)n Y- Ar
$A
\ /
N
H
wherein:
A-B is -CH-CH2 or -C=CH-;
X is H, halo, C,-CQ alkoxy, C,-CQ alkylthio, C,-C4 alkyl, benzyloxy, hydroxy
or
carbaxamido;
Y is O, S or a bond;
n is 1-4; and
Ar is 1-naphthyl, 2-naphthyl, phenyl or phenyl, mono-substituted with a
substituent
selected from the group consisting of halo, C,~ alkoxy, C,~ alkylthio, C,~
benzyloxy
hydroxy or trifluoromethyl.
U.S. Patent No. 5,627,196 discloses compounds of the following formula as
having effects on serotonin-related systems.
X
I
O-(CH2)r- CH-CH2(CHZ)s R
wherein r is 0-4;
s is 0-1; and
D is a residue which combines with the carbon atoms to which it is attached to
complete a pyrrolyl, imidazolyl, pyridinyl, pyrazinyl, pyridazinyl or
pyrimidinyl
group;
WO 00/64898 CA 02367681 2001-10-22 pCT~S00/10628
-3-
X is hydrogen, phenyl, hydroxy or methoxy provided that X is hydrogen or
phenyl
where r is 0; and
R is -NH-R,,
Rg
,/ 2 ~ n
-N~R - ~N-R5 or
~J ~
R3
Malleron et al., J. Med. Chem. 36:1194-1202 (1983)) discloses indole
derivatives as serotonin uptake inhibitors having the basic formula:
F
\ ~ I N
N ~A
H
wherein A may be:
O
N- O~S O N-
\ .
\ i I/
O~N- \ % H3
\ . O~S-N
~ ;
\ /
O
O~~S~O O~S~N-
\ '
N_ ; or ~ ~ ~ ~
CH3
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-4-
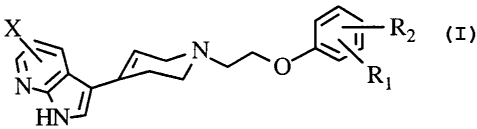
Thus, according to the present invention there are provided compounds of
Formula I:
i
X ~ R2
\\Ri
~J
I
wherein:
R, and Rz form a carbocyclic or heterocyclic ring of 5 to 7 atoms, wherein
said ring
may be saturated or unsaturated ; and
X is independently hydrogen, cyano, carbamoyl, halogen or alkoxy;
or pharmaceutically acceptable salts thereof.
Preferred compounds of the present invention are preferably those of Formula
I, wherein:
R, and RZ form a carbocyclic or heterocyclic ring of 5-6 atoms; and X is
hydrogen;
or pharmaceutically acceptable salts thereof.
Most preferably, the compounds of the present invention are selected from the
following:
3-{ 1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-ethyl]-1,2,3,6-tetrahydro-
pyridin-4-
yl }-1H-pyrrolo[2,3-b]pyridine;
3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahydro-pyridin-4-yl}-1H-
pyrrolo[2,3-
b]pyridine; and
5-{ 2-[4-( 1 H-Pyrrolo[2,3-b]-pyridine-3-yl)-3,6-dihydro-2H-pyridin-1-yl]-
ethoxy }-
quinoline.
As used herein, the term "alkoxy" is meant to include both straight and
branched carbon chains containing 1-6 carbon atoms. The term "halogen" is
meant to
include fluorine, chlorine, bromine and iodine. The term "heterocyclic ring"
mean
saturated or unsaturated rings containing one or more heteroatoms, preferably
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-5-
selected from oxygen, nitrogen and sulfur. Preferred examples contain, 5 or 6
atoms,
particular examples, are 1,4-dioxane, pyrrole and pyridine. The term
"carbocyclic
ring" means saturated or unsaturated carbon rings such as aryl or cycloalkyl,
preferably containing 5 or 6 carbon atoms..
The compounds of Formula I also may be used in the form of a
pharmaceutically acceptable acid addition salt having the utility of the free
base.
Such salts, prepared by methods well known to the art are formed with both
inorganic
or organic acids, for example: fumaric, malefic, benzoic, ascorbic, pamoic,
succinic,
bismethylenesalicylic, methanesulfonic, ethanedisulfonic, acetic, oxalic,
propionic,
tartaric, salicyclic, citric, gluconic, lactic, malic, mandelic, cinnamic,
citraconic,
aspartic, stearic, palmitic, itaconic, glycolic, p-aminobenzoic, glutamic,
benzene-
sulfonic, hydrochloric hydrobromic, sulfuric, cyclohexylsulfamic, phosphoric
and
nitric acids.
The compounds of the present invention may be prepared by any suitable
method which will be recognized by those skilled in the art. However, the
present
compounds may be advantageously prepared according to Scheme 1 set forth
below.
WO 00/64898 CA 02367681 2001-10-22 pCT~S00/10628
-6-
Scheme 1
\ i\
+ H~O
H~ H~ H
1
~I ~ ~I ~I
HO ~ OH ~ OH ~ O~CI
OH
2 3
H
~ o~
Ex. 1
H
I ~ ~ I ~ I ~ ~ ~ /
/ ~Cl '
H OH H O H O
4 Ex. 2
H
I ~ ~ / v
~ I off ~ I o'~CI ~ ~ o
Ex. 3
Processes for the preparation of compounds of formula I form a further aspect
5 of the present invention. Specific exemplification of the production of
representative
compounds of this invention is provided in the following procedures.
WO 00/64898 CA 02367681 2001-10-22 pCT~S00/10628
_7_
INTERMEDIATE 1
3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine
7-azindole (10 g, 85 mmol), 4-piperidone (34 g,0.22 mol) and potassium
hydroxide (16.83 g, 0.3 mol) were heated to reflux in 150 ml methanol
overnight.
The reaction was cooled, filtered and concentrated to give an orange slurry.
The
slurry was then extracted with methylene chloride and washed with water. The
organic layer was dried over anhydrous magnesium, filtered and concentrated to
afford 14.2 g (84%) of product as a solid: mp 195-199°C.
INTERMEDIATE 2
5-Hydroxy-(2,3)-dihydrobenzo[1,4]dioxine
Pyrogallol (5 g, 0.04 mol) was dissolved in 2-butanone (600 ml) to which
potassium carbonate (1.82 g, 0.013 mol) was added. The mixture was stirred at
reflux
while 1,2-dibromoethane (2.48 g, 1. 14 ml, 0.013 mol) was slowly added
dropwise.
The reaction was allowed to stir overnight and then cooled to room
temperature. The
mixture was poured into water ( 100 ml) and extracted with methylene chloride
(200
ml). The organic layer was dried over anhydrous sodium sulfate, filtered, and
the
solvent was removed under vacuum. Chromatography (5% methanol-methylene
chloride) afforded 2.74 g (45%) of product as a clear oil. MS EI mle 152 (M+)
INTERMEDIATE 3
5-(2-Chloroethoxy)-(2,3)-dihydrobenzo-[1,4]dioxane
To solution of 5-hydroxybenzodioxane (1.0 g, 6.5 mmol) and 2-chloroethanol
(0.79 g, 9.9 mmol), triphenylphosphine (2.6 g, 9.9 mmol) in tetrahydrofuran
(50 ml)
was slowly added diisopropylazidodicarbimide (DIAD) (2.0 g, 9.8 mmol). After 2
hours, another 1.5 eq of triphenylphosphine, DIAD, and 2-chloroethanol was
added
and the reaction stirred for another 2 hours. The reaction mixture was poured
into
water (100 ml), and extracted with methylene chloride (100 ml). The organic
layer
was separated and dried over anhydrous magnesium sulfate, filtered, and the
solvent
removed under vacuum. Chromatography (20% ethyl acetate-hexanes) afforded 1.7
g
(76%) of product as a white solid: mp 70.5-72.5°C.
CA 02367681 2001-10-22
WO 00!64898 PCT/US00/10628
_g_
Elemental analysis for C,oH"C103
Cal'd C, 55.96; H, 5.17
Found: C, 55.57; H, 5.20
INTERMEDIATE 4
2-(1H-Indol-4-yloxy)ethylchloride
To a solution of 4-hydroxyindole (4 g, 30 mmol), 2-chloroethanol (4.83 g, 60
mmol), triphenylphosphine (15.7 g, 60 mmol) in anhydrous tetrahydroufuran (40
ml)
was slowly added diisopropyl azodicarboxylate (l2.lg, 60 mmol). The reaction
was
allowed to stir for 2.5 hours at room temperature, then poured into methylene
chloride
(250 ml), washed with water (3 x 100 ml) and dried over anhydrous sodium
sulfate,
filtered and the solvent was removed under vacuum. Chromatography (20% hexanes-
ethyl acetate) to remove triphenylphosphine (20% methylene chloride-hexanes)
afforded 2.94 g (50%) of product as a white solid: mp 69.5-72°C.
INTERMEDIATE 5
5-(2-Chloroethoxy)-quinoline
A 100 ml three-neck oven dry flask was cooled under nitrogen. 5-Hydroxy
quinoline (2 g, 14 mmol) was added as well as triphenylphosphine (5.42 g, 21
mmol)
suspended in 50 ml anhydrous tetrahydrofuran. 2-Chloroethanol ( 1.3 ml, 21
mmol)
was slowly added to above reaction mixture via syringe, followed by adding
DEAD
(2.98 ml, 21 mmol) via syringe. A second 1.5 eq of 2-chloroethanol, triphenyl-
phosphine and DEAD was added. The reaction mixture was poured into 100 ml
water and extracted by methylene chloride. The organic layer was dried over
anhydrous sodium sulfate and concentrated. Chromatographed (20% ethyl acetate-
hexanes) afforded 2.31 g (82%) of product as a solid: mp 75-78°C.
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-9-
EXAMPLE 1
3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-ethyl]-1,2,3,6-tetrahydro-
pyridin
4-yl}-1H-pyrrolo[2,3-b]pyridine
A solution of 5-(2-chloroethoxy)-(2,3)-dihydrobenzo [1,4]dioxane (0.5 g, 23
mmol), 3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine (0.56 g,
28
mmol) and triethylamine (0.65 ml, 46 mmol) in anhydrous dimethylsulfoxide (20
ml)
was allowed to stir for 4 hours at 105°C. The mixture was poured into
water (100 ml)
and extracted with methylene chloride (3 x 100 ml). The organic layer was
washed
with water (3 x 150 ml), sodium bicarbonate and dried over anhydrous sodium
sulfate, filtered, and the solvent was removed under vacuum. Chromatography (
10%
methanol-methylene chloride) afforded 0.80 g (92%) of product as a yellow oil.
The oxalate salt was prepared in ethanol: mp 164-167°C
Elemental analysis for CZZHz3N303~2CzHz04~0.7Hz0
Calc'd: C, 54.77; H, 5.02; N, 7.37
Found: C, 54.77; H, 4.97; N, 7.23
EXAMPLE 2
3-{ 1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahydro-pyridin-4-yl}
-1H-pyrrolo[2,3-b]pyridine
A solution of 2-(1H-indol-4-yloxy)ethylchloride (0.5 g, 26 mmol), 3-(1,2,3,6-
tetrahydro-pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine (0.61 g, 31 mmol) and
triethyl-
amine (0.71 ml, 52 mmol) in anhydrous dimethylsulfoxide (20 ml) was allowed to
stir
for 4 hours at 80°C. The mixture was poured into water and extracted
with ethyl
acetate (3 x 100 ml). The organic layer was washed with water (3 x 100 ml),
sodium
bicarbonate and dried over anhydrous sodium sulfate, filtered, and the solvent
was
removed under vacuum. Chromatography ( 10% methanol-methylene chloride)
afforded 0.95 g (97%) of product as a green oil.
The oxalate salt was prepared in ethanol: mp 106-109°C
Elemental analysis for C2zHz2N40~2CzH204
Calc'd: C, 57.99; H, 4.87; N, 10.40
Found: C, 57.62; H, 5.03; N, 10.36
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
- 10-
EXAMPLE 3
5-{2-[4-(1H-Pyrrolo[2,3-b]pyridin-3-yl)-3,6-dihydro-2H
pyridin-1-yl]-ethoxy}-quinoline
A solution of 5-(2-chloroethoxy)quinoline (0.5 g, 24 mmol), 3-(1,2,3,6-tetra-
hydro-pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine (0.58 g, 29 mmol) and
triethylamine
(0.67 ml, 48 mmol) in anhydrous dimethylsulfoxide (20 ml) was allowed to stir
for 4
hours at 80°C. The mixture was poured into water dilute with sodium
hydroxide
solution and extracted with ethyl acetate (3 x 100 ml). The organic layer was
washed
with water (3 x 100 ml), sodium bicarbonate and dried over anhydrous sodium
sulfate, filtered, and the solvent was removed under vacuum to give a pale
yellow
solid, which was triturated with ethanol-ethyl ether to afford a pale yellow
solid: mp
202-205°C.
The oxalate salt was prepared in ethanol: mp 202-205°C
Elemental analysis for C23HZZN40'C2Hz04~O.SH20
Calc' d: C, 72.80; H, 6.11; N, 14.77
Found: C, 72.71; H, 5.97; N, 15.37
The activity of the present compounds is demonstrated by the
following standard pharmacological test procedures.
The PCR cloning of the human 5-HT,A receptor subtype from a human
genomic library has been described previously by Chanda et al., Mol.
Pharmacol.,
43:516 (1993). A stable Chinese hamster ovary cell line expressing the human 5-
HT,A
receptor subtype (5-HT,A.CHO cells) was employed throughout this study. Cells
were
maintained in DMEM supplemented with 10% fetal calf serum, non-essential amino
acids and penicillin/ streptomycin.
Cells were grown to 95-100% confluency as a monolayer before membranes
were harvested for binding studies. Cells were gently scraped from the culture
plates,
transferred to centrifuge tubes, and washed twice by centrifugation (2000 rpm
for 10
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-11-
min., 4°C) in buffer (50 mM Tris; pH 7.5). The resulting pellets were
aliquoted and
maintained at -80°C. On the day of assay, the cells were thawed on ice,
and
resuspended in buffer. Studies were conducted using [3H]8-OH-DPAT as the
radioligand. The binding assay was performed in 96 well microtiter plates in a
final
total volume of 250 ~,L of buffer. Comparison experiments were performed by
using
7 concentrations of unlabelled drug and a final ligand concentration of 1.5 nM
. Non-
specific binding was determined in the presence of 10 ~.M SHT. Saturation
analysis
was conducted by using [3H]8-OH-DPAT at concentrations ranging from 0.3-30 nM.
Following a 30 minute incubation at room temperature, the reaction was
terminated
by the addition of ice cold buffer and rapid filtration using a M-96 Brandel
Cell
Harvester (Gaithersburg, MD) through a GF/B filter presoaked for 30 minutes in
0.5% polyethyleneimine.
A protocol similar to that used by Cheetham et al., Neuropharmacol. , 32:737
(1993) was used to determine the affinity of compounds for the serotonin
transporter.
Briefly, frontal cortical membranes prepared from male Sprague-Dawley rats
were
incubated with 3H-paroxetine (0.1 nM) for 60 min at 25°C. All tubes
also contained
either vehicle, test compound (one to eight concentrations), or a saturating
concentration of fluoxetine ( 10 ~M) to define specific binding. All reactions
were
terminated by the addition of ice cold Tris buffer followed by rapid
filtration using a
Tom Tech filtration device to separate bound from free 3H-paroxetine. Bound
radioactivity was quantitated using a Wallac 1205 Beta Plate~ counter.
Nonlinear
regression analysis was used to determine ICso values which were converted to
Ki
values using the method set forth in Cheng and Prusoff, Biochem. Pharmacol.,
22:3099 (1973) (Ki = IC50/((Radioligand conc.)/(1 + KD)).
The [35S]-GTFyS binding assay was similar to that used by Lazareno and
Birdsall, Br. J. Pharmacol. 109:1120 (1993). Briefly, 5-HT,A cloned receptor
membrane fragments (as used for 5-HT,A receptor binding assays) were stored at
-70°C. When needed, membranes were rapidly thawed, centrifuged at
40,000 x g for
10 minutes and resuspended at 4 °C for 10 minutes in assay buffer (25
mM HEPES, 3
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-12-
mM MgClz, 100 mM NaCI, 1 mM EDTA, 10 uM GDP, 500 mM DTT, pH 8.0).
These membranes were then incubated for 30 min at 30 °C with [35S]GTPgS
(1 nM) in
the presence of vehicle, test compound (one to eight concentrations), or
excess 8-OH-
DPAT to define maximum agonist response. All reactions were terminated by the
addition of ice cold Tris buffer followed by rapid filtration using a Tom
Tech~
filtration device to separate bound from free [35S]GTPgS. Agonists produced an
increase in the amount of ['SS]GTPgS bound whereas antagonists produced no
increase in binding. Bound radioactivity was counted and analyzed as above.
The following assays were performed by incubating the cells with DMEM
containing 25 mM HEPES, 5 mM theophylline and 10 ~.M pargyline for a period of
minutes at 37°C. Functional activity was assessed by treating the cells
with
forskolin (1 uM final concentration) followed immediately by test compound (6
concentrations) for an additional 10 min at 37°C. In separate
experiments, 6
15 concentrations of antagonist were preincubated for 20 min prior to the
addition of 10
nM 8-OH-DPAT and forskolin. The reaction was terminated by removal of the
media and addition of 0.5 ml ice cold assay buffer. Plates were stored at -
20°C prior
to assessment of cAMP formation by a cAMP SPA assay (Amersham).
Example 5-HT ST GTFyS ED50 o~P ED50
No. (Ki~lnM) (Ki, ~M,) (oEmax) (Emax)
1 43.9 18.0 (30~)
2 10.9 1.46 38.9(9.00 12.4 (0~)
3 71.6 6.89 181 (0~) 90.1 (0~)
As demonstrated by the results set forth above, the compounds of the present
invention are active towards SHT,A receptors and generally elevate serotonin
levels by
inhibiting 5-HT transport. Accordingly, the present compounds should be useful
in
treating disorders related to defects in serotonin concentration.
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
-13-
The compounds of formula I for use in methods of treatment or therapy form
further aspects of the present invention.
The compounds of this invention may be administered orally or parenterally,
neat or in combination with conventional pharmaceutical carriers. Applicable
solid
carriers can include one or more substances which may also act as flavoring
agents,
lubricants, solubilizers, suspending agents, fillers, glidants, compression
aids, binders
or tablet-disintegrating agents or an encapsulating material. In powders, the
carrier is
a finely divided solid which is in admixture with the finely divided active
ingredient.
In tablets, the active ingredient is mixed with a carrier having the necessary
compression properties in suitable proportions and compacted in the shape and
size
desired. The powders and tablets preferably contain up to 99% of the active
ingredient. Any of the solid carriers known to those skilled in the art may be
used
with the compounds of this invention. Particularly suitable solid carriers
include, for
example, calcium phosphate, magnesium stearate, talc, sugars, lactose,
dextrin,
starch, gelatin, cellulose, methyl cellulose, sodium carboxymethyl cellulose,
polyvinylpyrrolidone, low melting waxes and ion exchange resins.
Liquid carriers may be used in preparing solutions, suspensions, emulsions,
syrups and elixirs of the compounds of this invention. The compounds of this
invention can be dissolved or suspended in a pharmaceutically acceptable
liquid
Garner such as water, an organic solvent, a mixture of both or
pharmaceutically
acceptable oils or fat. The liquid carrier can contain other suitable
pharmaceutical
additives such as solubilizers, emulsifiers, buffers, preservatives,
sweeteners,
flavoring agents, suspending agents, thickening agents, colors, viscosity
regulators,
stabilizers or osmo-regulators. Suitable examples of liquid carriers for oral
and
parenteral administration include water (particularly containing additives as
above,
e.g., cellulose derivatives, preferably sodium carboxymethyl cellulose
solution),
alcohols (including monohydric alcohols and polyhydric alcohols, e.g.,
glycols) and
their derivatives and oils (e.g., fractionated coconut oil and arachis oil).
For
parenteral administration, the carrier can also be an oily ester such as ethyl
oleate and
isopropyl myristate. Sterile liquid Garners are used in compositions for
parenteral
administration.
Liquid pharmaceutical compositions which are sterile solutions or
suspensions can be utilized by, for example, intramuscular, intraperitoneal or
CA 02367681 2001-10-22
WO 00/64898 PCT/US00/10628
- 14-
subcutaneous injection. Sterile solutions can also be administered
intravenously.
Compositions for oral administration may be either liquid or solid composition
form.
Preferably, the pharmaceutical compositions containing the compounds of this
invention are in unit dosage form, e.g., tablets or capsules. In such form,
the
compositions may be sub-divided in unit doses containing appropriate
quantities of
the present compounds. The unit dosage forms can be packaged compositions, for
example, packeted powders, vials, ampoules, prefilled syringes or sachets
containing
liquids. Alternatively, the unit dosage form can be, for example, a capsule or
tablet
itself, or it can be the appropriate number of any such compositions in
package form.
The therapeutically effective amount of the compounds of this invention that
is administered and the dosage regimen depends on a variety of factors,
including the
weight, age, sex, and medical condition of the subject, the severity of the
disease, the
route and frequency of administration, and the specific compound employed, and
thus
may vary widely. However, it is believed that the pharmaceutical compositions
may
contain the compounds of this invention in the range of about 0.1 to about
2000 mg,
preferably in the range of about 0.5 to about 500 mg and more preferably
between
about 1 and about 100 mg. Projected daily dosages of active compound are about
0.01 to about 100 mg/kg body weight. The daily dose can be conveniently
administered two to four times per day.
Pharmaceutical compositions comprising the compounds of formula I and a
pharmaceutically acceptable carrier form a further aspect of the present
invention.
The present invention may be embodied in other specific forms without
departing from the spirit and essential attributes thereof and accordingly,
reference
should be made to the appended claims, rather than to the foregoing
specification, as
indicating the scope of the invention.