Note: Descriptions are shown in the official language in which they were submitted.
BASF Aktiengesellschaft 20000104 0.2. 0050/51174 DE
,.
~i
1
Prodrugs of thrombin inhibitors
The present invention relates to prodrugs of pharmacologically
active heterocyclic amidines, from which in vivo compounds result _
which are competitive inhibitors of trypsin-like serine
proteases, particularly thrombin, their preparation and their use
as medicaments. The invention also relates to pharmaceutical
compositions which contain the prodrugs of the active compounds
as constituents, and to the use of the compounds as thrombin
inhibitors, anticoagulants and as antiinflammatory agents.
Thrombin belongs to the serine proteases group and plays a
central role as a terminal enzyme in the blood-clotting cascade.
Both the intrinsic and the extrinsic clotting cascades lead, via
a number of amplification stages, to the formation of thrombin
from prothrombin. The thrombin-catalyzed cleavage of fibrinogin
to fibrin then initiates blood clotting ,and the aggregation of
the platelets, which for their parts increase the formation of
thrombin by means of the binding of platelet factor 3 and
clotting factor XIII and also a whole series of highly active
mediators.
Thrombin formation and action are central events in the genesis
both of white, arterial thrombi and of red, venous thrombi and
are therefore potentially effective points of attack for
pharmaceuticals. In contrast to heparin, thrombin inhibitors are
able, independently of cofactors, simultaneously to completely
inhibit the actions of free thrombin and thrombin bound to
platelets. In the acute phase, they can prevent thromboembolic
events after percutaneous transluminal coronary angioplasty
(PTCA) and lysis and can be used as anticoagulants in the
extracorporeal circulation (heart-lung machine, hemodialysis).
They can also be generally used for thrombosis prophylaxis, for
example after surgical interventions.
It is known that synthetic arginine derivatives influence the
enzyme activity of the thrombin by interacting with the active
serine residue of the protease thrombin. Peptides based on
Phe-Pro-Arg, in which the N-terminal amino acid is present in the
D form, have proven particularly favorable. D-Phe-Pro-Arg
isopropyl ester is described as a competitively acting thrombin
inhibitor (C. Mattson et. al. Folia Haematol, 1~, 43 to 51,
1983).
CA 02368830 2001-09-26
,. _2_
TWO 94/29336, EP 0601459, WO 95/23609, EP 0672658, WO 97/23499,
WO 98/06740 and WO 95/35309 represent a further development in which the
agmatine residue is replaced by an arylamidine residue.
Although these compounds have significant antithrombotic action, it is
ladvantageous to improve their pharmacokinetic properties after oral or
Iparenteral administration.
Inter alia, the influencing of the following pharmacokinetic properties
:is desirable:
I. The improvement of the absorption from the gastrointestinal tract
with the aim of a high bioavailability.
;II. The minimization of the inter- and intraindividual variability of
the bioavailability by means of constant absorption
i
i
;III. The achievement of therapeutically relevant activity levels, which
are as constant as possible, over the time course. With respect to
the therapeutic breadth, plasma concentrations which are as
constant as possible over the time course are indispensable, as
variations which are too great can lead to undesired side effects.
If the plasma concentration of the active compound is too high,
bleeding can be expected; if the concentration is too low the risk
of thrombus formation increases.
I IV. The prolongation of the duration of action of the active compound:
Active compound is understood as meaning the pharmacologically
active substance (drug) in comparison to the substance (prodrug),
which first has to be converted into the active compound
metabolically.
V. Reduction of trypsin inhibition: since the prodrugs affect the
digestive enzyme trypsin markedly less, fewer side effects are to
be. expected with the prodrugs.
A further advantage of the prodrugs compared with the drugs lies in the
fact that high local concentrations of the drugs do not occur outside
the target site. Moreover, with less selective drugs side effects are
minimized, as, for example, in the gastrointestinal tract no further
serine proteases are inhibited if the drug is essentially formed by
metabolism of the prodrug only after or during gastrointestinal passage.
CA 02368830 2001-09-26
,, SASF' Aktiengesellschaft- 20000104 O.Z. 0050/51174 DE
3
The aim of this invention is the improvement of the
pharmacokinetic properties of the thrombin inhibitors mentioned
in particular in WO 95/35309 and WO 96/25426 by means of suitable
prodrugs.
35
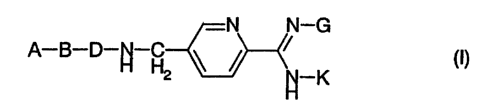
The invention relates to compounds of the formula.I
N N-G
io A B ~ p HZ ~ ~ "-K U)
in which A, B, D, G and K have the following meanings:
A is: R100C-~H2-, R100C-CH2-CHZ-, R100C-CH(CH3)-, HO-CH2-CH2-,
R2R3N(O)C-CH2-, R2R3N-O-CO-CHZ-, R2N(OH)-CO-CH2-, where R2 and
R3 independently of one another.are.H, Cl-C6-alkyl,
C3-C8-cycloalkyl, C3-C8-cycloalkyl-C~-C3-alkyl, or benzyl, or
RZ and R3 together form a C4-C6-alkylene chain,
in which
R1 ia: H-, C1-C16-alkyl-, H3C-(0-CH2--CH2)q (q = 1-4),
Clo-tricycloalkyl-, Clo-tricycloalkyl-CH2-, C3-CB-cycloalkyl-,
C3-Cg-cycloalkyl-C1-C3-alkyl-, where a phenyl ring can be
fused to the cycloalkyl ring, pyranyl-, piperidinyl-, aryl-
or phenyl-C1-C3-alkyl-, where except for H all radicals
mentioned can optionally carry up to 4 identical or different
radicals selected from C1-C4-alkyl, CF3, F, Cl, N02, HO or
Cl-C4-alkoxy radicals, or
R1 is (2-oxo-1,3-dioxolen-4 yl)methyl-, which can be
substituted in the 5-position by C1-Cls-alkyl or aryl,
or
R1 is: R4-C(O)O.-C(R5)Z-, R4-C(O)NR2-C(R5)Z-, where R4 can be
C1-C4-alkyl-, C3-CB-cycloalkyl-C1-C3-alkyl-, C3-C8-cycloalkyl-,
C1-C4-alkyloxy-, C3-C8--cycloalkyl-C1-C3-alkyloxy-,
C3-C8-cycloalkyloxy-, aryl- or phenyl-C1-C6-alkyl-, the two
radicals R5 independently of one another are H, CH3 or C2H5,
and R2 has the meaning indicated above,
R600C~1-C6-alkyl, R6R~N(O)C-C1-C6-alkyl-, R6R~N-CZ-C6-alkyl-.
and in which R6 and R~ independently of one another are H or
Cl-C6-alkyl, or
CA 02368830 2001-09-26
BASF Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
,
r
4
if Rl is R6R~N(O)C-C1-Cs-alkyl-, Rs and R~ together form a
C4-Cs-alkylene chain,
or A is:
C1-C4-alkyl-S02-(CHZ)2-s- ~ H03S-(CH2)4-6m 5-tetrazolyl-(cH2)i-s-.
C1-Cq-alkyl-0-(CHZ)2-fi-r R2R3N-(CH2)2-6-i R2S-(CH2)2-6-i
R2R3NS02-(CH2)2-6-i HO-(CHy)2-6-r
B is
(CHAP
-N_H_GO_
Ra
p is0,1,2
R8 is H-, R1o00C- where R1~= C1-ls-alkyl-, phenyl-,
C3-C8-cycloalkyl-, phenyl-C1-C4-alkyl-, R11C(O)-0-CHZ-,
R11C(0)-O-CH(CH3)-, where R11 can be C1-C4-alkyl-, phenyl-,
benzyl-, C3-Ce-cycloalkyl- or ayclohexyl-CHZ-,
R9 is C3_$-cycloalkyl-, which can carry up to four identical or
different C1_4-alkyl radicals,
D is
30 ~ ~ ~
\NJ~ (II) ~N/~/ (III)
O ~ O
G is: -H, -OH, -OR12,
in which
R1z is: -C1_8-alkyl, -C3-C8-cycloalkyl,
-C1-C3-alkyl-C3-C8-cycloalkyl, -aryl or -C1-Cs-alkylphenyl,
which can optionally carry up to three C1-C4-alkyl, CF3, F,
C1, or C1-C4-alkoxy radicals,
K is: H,
or G and K together form a -C(O)O-group,
CA 02368830 2001-09-26
BASt~ Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
their configurational isomers, tautomers and their salts with
physiologically tolerable acids,
where the following applies:
5 (i)
if D = (II) or (III) and G = -H, -OH, -OR12,
in which
R1z is: -C1-Ce-alkyl, -C1-C3-alkyl-C3-C8-cycloalkyl, -aryl or
-C1-C6-alkylphenyl, which can optionally carry up to three
C1-C4-alkyl, CF3, F, C1, or C1-C4-alkoxy radicals
R is : H,
or G and R together form a -C(O)O-group, then A and 8 have the
following meanings: '
A: R100C--CH2-, R100C-CH2-CHZ-, R100C-CH(CHg)-, HO-CH2-CHZ-,
RZaR3aN(O)C-CH2-, R2R3N-O-CO-CHZ-, RZN(OH)-CO-CHZ-, where RZ
and R3 independently of one another.are H, C1-C6-alkyl,
C3-C8-cycloalkyl or benzyl or R2 and R3 together form a
C4-C6-alkylene chain, R2a is equal to H and R3a is C5-C8-alkyl,
C3-C8-cycloalkyl or benzyl;
in which
R1 is: C5-C16-alkyl-, H3C-[O-CHZ-CH2]q (q = 1-4),
Clp-tricycloalkyl-, Clo-tricycloalkyl-CHZ-, C3-C8-cycloalkyl-,
C3-Ce-cycloalkyl-C1-C3-alkyl-, where the phenyl ring can be
fused to the cycloalkyl ring, pyranyl-, piperidinyl-, or
aryl-, where except for H all radicals mentioned can
optionally carry up to four identical or different radicals
selected from C1-C4-alkyl, CF3, F, C1, N02, HO or Ci-C4-alkoxy
radicals, or
Rl is 2~xo-l,3~iioxolen-4-yl)methyl- which can be
substituted in the 5-position by C1-Cls-alkyl or aryl,
or
R1 is: R4-C(O)0-C(RS)2-, R'~--C(O)NR2-C(R5)2-, where R4 can be
C1-C4-alkyl-, C3~8-cycloalkyl-C1-C3-alkyl-, C3-CB-cycloalkyl-,
4.0 C1-C4-alkyloxy-, C3-CB-cycloalkyl-C1-C3-alkyloxy-,
C3-C8-cycloalkyloxy-, aryl- or phenyl-C1-C6-alkyl-, the two
radicals R5 independently of one another are H, CH3 or CZHS.
and R2 has the meaning indicated above,
R600C-C1-C6-alkyl-, R6R~N(O).C-C1-C6-alkyl-, R6R~N-C2--C6-alkyl-,
and in which R6 and R~ independently of one another are H or
C1-C6-alkyl, or
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
,
6
if Rl is R6R~N(0)C-C1-C6-alkyl-, R6 and R~ together form a
C4-C6-alkylene chain,
or A is:
C1-C4-alkyl-S02-(CH2)2-s- . H03S-(CH2)4-6-, 5-tetrazolyl-(CH2)1_6-.
C1-C4-alkyl-O-(CH2)2-6w R2R3N-(CH2)2-6w R2S-(CH2)2-6-~
R2R3NS02-(CH2)2-6-r HO-(CH2)2-6w
B is
Rs
(CHAP
is
p is 0,1, 2
R$ is H-, R1~OOC- where R1~= C1_ls-alkyl-, phenyl-,
C3-Cg-cycloalkyl-, phenyl-C1-C4-alkyl-, R11C(O)-O-CH2-,
R11C(O)-O-CH(CH3)-, where R11 can be C1-C4-alkyl-, phenyl-,
benzyl-, C3-CB-cycloalkyl- or cyclohexyl-CH2-,
R9 is C3_8-cycloalkyl-, which can carry up to four identical or
different C1_4-alkyl radicals,
ia)
Preferred compounds of the formula I in i) are those in which A,
B, D, G and K have the following meanings:
A is: R100C-CH2-, R100C-CH2-CH2-, R100C-CH(CH3)-,
in which
R1 iS: C5-C16-alkyl-, H3C-(O-CH2-CH2)q (q = 1-4),
Clo-tricycloalkyl-, Cio-tricycloalkyl-CH2-, C3-Ce-cycloalkyl-,
C3-Ce-cycloalkyl-C1-C3-alkyl-, where a phenyl ring can be
fused to the cycloalkyl ring, pyranyl- piperidinyl-, where
except for H all radicals mentioned can optionally carry up
to four identical or different radicals selected from CH3,
CF3, F, Cl, HO or methoxy radicals, or
R1 is (2~xo-1,3-dioxolen-4-yl)methyl-, which can be
substituted in the 5-position by Ci-C3-alkyl or aryl,
or
CA 02368830 2001-09-26
,, BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
R1 is: R4-C(O)O-C(R5)2-, where R4 Cl-C4-alkyl-, C3-CB-cycloalkyl-,
C1-~4-alkyloxy, _ , .C3-C8-cycloalkyl-C1-C3-alkyloxy-,
C3-Cg-cycloalkyloxy-, or aryl- the two radicals R5
independently of one another are H, CH3 or C2H5,
R600C-C1-C6-alkyl-, R6R~N(0)C-C1-C6-alkyl-, R6R~N-C2-C6-alkyl-,
and in which R6 and R~ independently of one another are H or
C1-C6-alkyl or,
if R1 is R6R~N(O)C-C1-C6-alkyl-, R6 and R~ together form a
C4-C6-alkylene chain,
B is
R9
(CH~p
i5 _
R8
p is o,l
Ra is H-, R1o00C- and R1~= Ci_e-alkyl-, phenyl-, C3-CB~ycloalkyl-,
phenyl-C1-C4-alkyl-,
R9 is C3_8-cycloalkyl-, which can carry up to four identical or
different C1_4-alkyl radicals,
D = (II)
and G = -H, -OH, -O-C1--Cg-alkyl,
K is: H
or G and K together form a -C(O)O-group.
(ii)
if D = (II) or (III) and G = -OR12,
in which
R12 is: -C5-C8-alkyl, -C3-CB-cycloalkyl,
-C1-C3-alkyl-C3-C8-cycloalkyl, -aryl or -C1-C6-alkylphenyl,
which can optionally carry up to three C1-C4-alkyl, CF3, F,
C1, or C1-C4-alkoxy radicals,
K is: H,
or G and K together form a -C(O)O-group, then A and H have the
following meanings:
7
CA 02368830 2001-09-26
,. BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
_ . 8
A: R100C-CHZ-, R100C-CHy-CH2-, R100C--CH(CH3)-, R2aR3aN(0)C-CH2-,
where R2a and R3a independently of one another are.H,..Cl-C6-alkyl,
C3-CB-cycloalkyl or benzyl or RZa and R3a together form a
C4-C6-alkylene chain,
in which
R1 is: H-, C1-C4-alkyl- or phenyl-C1-C4-alkyl-, where except for H
all radicals mentioned can optionally carry up to four
identical or different radicals selected from C1-C4-alkyl,
CF3, F, C1, N02, HO or C1-C4-alkoxy radicals,
B, p and R8, R9, Rl~ and R11 have the meaning indicated in i).
iia)
Preferred compounds of the formula I in ii) are those in Which A,
B, D, G and K have the following meanings: .
A is: R100C-CH2-, RlOOC-CHZ-CH2-, R100C-CH(CH3)-, R2aR3aN(0)C-CH2-,
where R2a and R3a independently of one another are H,
C1-C6-alkyl, C3-C8-cycloalkyl or benzyl, or R2a and R3a
together form a C4-C6-alkylene chain,
in which
R1 is: H-, C1-C4-alkyl- or phenyl-C1-C4-alkyl-, where except for H
all radicals mentioned can optionally carry up to four
identical or different radicals selected from CH3, CF3, F, Cl,
HO or methoxy radicals,
B is
Rs
~CH~p
-N-H-CO-
R8
p is 0,1
Rs is H-, R1o00C- and Rlo= C1_ls-alkyl-, phenyl-, C3-C~ycloalkyl-,
benzyl-,
and R9 has the meaning indicated in i)
D = (II)
G = -OR12 ,
CA 02368830 2001-09-26
,, BASF Aktieagesellschaft ~ 20000104 O.Z. 0050/51174 DE
9
in which
R12 is: -CS-C8-alkyl, -C3-CB-cycloalkyl,
-C1-C3-alkyl-C3-Ce-cycloalkyl, -aryl or -C1-C6-alkylphenyl,
which can optionally carry up to three CH3-, CF3-, F-, C1-, or
methoxy radicals,
K is: H,
or G and K together form a -C(O)0-group.
Particularly preferred prodrugs of the formula I are those where
A, B, D, G and K have the following meanings:
A is: R100C-CH2-, R100C-CHy--CHy-, R100C-CH(CH3)-,
in which
R1 is: CS-Clo-alkyl-, C4-C~-cycloalkyl-, C4-C~_cycloalkyl-CH2-,
where all radicals mentioned can optionally carry up to four
identical or different radicals selected from CH3- and
methoxy-,
B is
R9
(CH~p
Ra
p is 0,1,
RB is H-,
R9 is C4_~-cycloalkyl-, which can carry up to four identical or
different methyl or ethyl radicals
D is:
-_
(II)
I (,~ .
G is: -OH,
K is: H.
CA 02368830 2001-09-26
CA 02368830 2001-09-26
ZO
The aforementioned compounds belong to three groups of substances:
- The first group comprises prodrugs of thrombin inhibitors (e.g. G
equals -OH, -OR12) which is a substance to only a negligible
antithromotic effect, which, however, are converted in the organism
into the active substance (G equals H). These compounds are included
in all claims. The advantage of the prodrugs lies in their improved
pharmacokinetic and pharmacodynamic behaviour in the organism.
Compounds wherein G equals -OH, -OR12, and simultaneously A equals
R100C-CHZ-, R100C-CHZ-CHZ-, R100C-CH (CH3 ) - etc . are double prodrugs
which are converted in the organism into the respective drug (G
equals -H, A equals HOOC-CHz- etc.) by converting both prodrug
groups.
- The second group comprises prodrugs of thrombin inhibitors which
show already as a prodrug a thrombin-inhibiting effect (e.g. A
equals R100C-CHZ-, R100C-CHZ-CHZ-, R100C-CH (CH3) - etc. in combination
with G equals -H). The effective substance formed in the organism
(drug; A equals HOOC-CHZ-, HOOC-CHZ-CHZ-~, HOOC-CH (CH3) - etc., G
equals -H) shows also a thrombin-inhibiting effect. These are in
part compounds of claims 1 (i), 2 and 5. The advantage of these
prodrugs lies also in their improved pharmacokinetic and
pharmacodynamic behaviour in the organism.
- The third group comprises thromin inhibitors which per se show the
antithrombotic effect (e. g. A equals Cl_9-alkyl-SOz- (CHz) 2_s-, H03-S-
(CHZ) 2_s-, 5-tetrazolyl- (CHZ) 1_s-, C1_9-alkyl-O- (CHZ) 2-s-. RZR3N- (CHz)
2_s-r
RzS- (CHZ) z-s-r R2R3NS0z- (CHZ) z_s-, in combination with G equals -H) .
Such compounds are included in claim 1 (i).
BASS Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
11
The following compounds, their configurational isomers,
tautomers, and salts with physidlogically tolerable acids are
furthermore the subject of this invention:
HOOC-EHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
H3C0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
Et0-OC-CH2-(D)-Cha-Pyr-NH-3-(6-am-(OH)]-pico
nPrO-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
iPrO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
nBuO-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
iBuO-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
tHuO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
Bn0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
HOOC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
H3C0-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
EtO-OC-CH2-(D)-Chg-Pyr-NH-3-['6-am-(OH)]-pico
nPrO-OC-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
iPrO-OC-CHZ-(D)-Chg-Pyr-NH-3-[6~am-(OH)]-pico
nBuO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
iBuO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
tBuO-OC-CHy-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
H3C0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
Et0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
nPrO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
iPrO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
nBuO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
iHuO-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
t8u0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(H)]-pico
H3C0-OC-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(H)]-pico
Et0-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(H)]-pico
nPrO-OC-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(H)]-pico
nBuO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(H)]-pico
iBuO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(H)]-pico
tBuO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(H)]-pico
HOOC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(O-Allyl)]-pico
H3C0-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OCH3)]-pico
iPrO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OCHg)]-pico
45
CA 02368830 2001-09-26
,. BASF' Aktiengesellschaft 20000104 O.Z. 0050/51174 D~
' 12
The following substances are particularly preferred:
1. CH3-(CHZ)150-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
2. CH3-(CHZ)loo-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
3. Piperidin-1-yl-O-OC-CH2-(D)-Gha-Pyr-NH-3-[6-am-(OH)]-pico
4. Piperidin-4-yl-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
5. Decalinyl-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
tBu-cHexyl-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
7. Ada-CHZ-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
8. 4-tBu-cHexy7.-CHy-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH))-pico
9. ~Hept-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
10. 3,3,5,5-TetraMe-cHex-O-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
11. 4-Pyranyl-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
12. 2,4-DiMe-3-Pentyl-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
13. 1-Me-cPentyl-O-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
14. a,a-Di-cHex-CHz-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
15.~ t8u N-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
16. nHex-N-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
17. HO-NH-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
18. cPent-CHy-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
19. cHex-CHZ-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
20. cHex-N(OH)-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
21. iPr-N(OH)-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
22. CH3-N(OH)-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
23. H2N-O-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
24. c(CHZ)5N-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
25. N-Me-4-Pip-O-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
26. (CH3)3C-COz-CH2-O-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
27. (CH3)3C-C02-CHZ-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
28. (CH3)3C-C02-CH2-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
29. (CH3)3C-COy-CH2-O-OC-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
30.~ CYclopropylmethyl-O-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-
pico
31. 13-Dioxol-2-on-4-enyl-0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-
(OH)]-pico
32. CYclopropylmethyl-O-OC-CH2-(D)-Chg-Pyr-
NH-3-[6-am-(OH)]-pico
33, 1.3-Dioxol-2-on-4-enyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-
(OH)]-pico
34. CYclopropylmethyl-0-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-
(OH)]-pico
35.
. 1,3-Dioxol-2-on-4-enyl-0-OC-CHZ-(D)-Cha-Dep-NH-3-[6-am-
(OH)]-pico -
36. Cyclopropylmethyl-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-
(OH)]-pico
37. 1,3-Dioxol-2-on-4-enyl-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-
(OH)]-pico
38. CH3-(CH2)150-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
39.' GHg-(CH2)loo-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
40. Piperidin-1-yl-O-OC-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
41. Piperidin-4-yl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
42. Decalinyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
CA 02368830 2001-09-26
BAS1~ Aktieagesellschaft 20000104 O.Z. 0050/51174 D8
13
43. tBu-cHexyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)j-pico
. 44. Ada-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)j-pico
45. 4-tBu-cHexyl-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-('OH)]-pico
46. cHept-O-OC-CH2-(D)-Ghg-Pyr-NH-3-[6-am-(OH)j-pico
47, 3,3,5,5-TetraMe-cHex-0-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-
( off ) .] -pico
48.. 4-Pyranyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
49, 2,4-DiMe-3-Pentyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-
pico
50. 1-Me-cPentyl-O-OC-CHy-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
.51. a,a-Di-cHex-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)j-pico
52. ~tBu N-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
53. nHex-N-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
54. HO-NH-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
55. cPent-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
56. cHex-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
57. cHex-N(OH)-OC-CH2-(D)-Ghg-Pyr-NH-3-[6-am-(OH)j-pico
58. iPr-N(OH)-OC-CH2-(D)-Ghg-Pyr-NH-3-[6-am-(OH)]-pico
59. CH3-N(OH)-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
60. H2N-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
61. c(CH2)5N-O-OC-CHy-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
62. N-Me-4-Pip-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
63. (CH3)3C-C02-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
64. (CH3)gC-C02-CH2-O-OC-CHy-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
65. (CH3)gC-C02-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
66. (CHg)gC-COy-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
67. nOctO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
68. cfiex-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
69. neoPentO~-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
70. CH3-O(CH2)20-OC-CH2-(D)-Ghg-Pyr-NH-3-[6-am-(OH)]-pico
71. CH3-[O(CH2)2120-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
72. cHex-CH2-O-OC-GH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
73. cOctO~OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
74. 4-Me-cHexyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
75. nHexO-OC-CH2-(D)-Ghg-Pyr-NH-3-[6-am-(OH)]-pico
76. cPentO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
77. 4-Me0-cHexyl-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
78. 2,3-DiMe-2-Bu-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
79.
2-Me-1,3-DioXane-5-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-
pico
80. 2,4-DiMe-3-Pent-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)j-pico
.
81. 2-Indan-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
82. 2,6-DiMe-4-Hept-O-OC-CHy-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
83. Pyrr-N-CO-(CHZ)3-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
84, cHex-N=CO-CH2-O-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(OH)j-pico
85. CH3-(CH2)150-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)j-pico
86. CH3-(CH2)loo-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
87. Piperidin-1-yl-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
88'~ Pa.peridin-4-yl-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
89. Decalinyl-0-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
90. tBu-cHexyl-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
91. Ada-CH2-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
CA 02368830 2001-09-26
BASS Aktiengesellschaft 20000104 O.Z. 0050/51174 D8
14
92. 4-tBu-cHexyl-CHZ-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
93. cHept-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
94. 33,5,5-TetraMe-cHex-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-
pico
95. 4-Pyranyl-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
2,4-DiMe-3-Pentyl-O-OC-C~2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-
pico
97. 1-Me-cPentyl-O-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
98. a,a-Di-cHex-CHZ-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
99. tBu-N-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
100. nHex-N-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
101. HO-NH-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
102. cPent-CH2-O-OC-CHy-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
103. cHex-CHZ-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
104. cHex-N(OH)-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
105. iPr-N(OH)-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
106...CH3-N(OH)-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
107. H2N-O-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
108. c(CHy)sN-O-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
109. N-Me-4-Pip-O=OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
110. (CH3)3C-COy-CHy-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
111. (CH;)gC-COq-CH2-O-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
112. (CH3)3C-C02-CH2-O-OC-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
113. (CH3)3C-C02-CHy-O-OC-CH2-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
114. CH3-(CH2)is0-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
115. CH3-(CHZ)1o0-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
116. Piperidin-1-yl-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
117. Piperidin-4-yl-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
118. Decalinyl-O-OC-CHy-(D)-Cha-Dep-NH-3-[6-am=(OH)]-pico
119. tBu-cHexyl-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
120. Ada-CHZ-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
121. 4- Bu-cHexyl-CHZ-O-OC-CHZ-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
122. cHept-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
123. 33,5,5-TetraMe-cHex-0-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-
pico
124. 4-Pyranyl-O-OC-CHZ-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
125. 24-DiMe-3-Pentyl-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-
pico
126. 1-Me-cPentyl-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
127. a,a-Di-cHex-CH2-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
128. tBu N-OC-CHy-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
129. nHex-N-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
130. HO-NH-OC-CHZ-(D)-Cha-Dep=NH-3-[6-am-(OH)]-pico
131. cPent-CH2-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
132. cHex-CHZ-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-{OH)]-pico
133. cHex-N(OH)-OC-CHy-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
134. iPr-N(OH)-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
135. CH3-N(OH)-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH))-pico
136: H2N-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
137. c(CH2)sN-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
138. N-Me-4-Pip-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
139. (CHg)gC-COZ-CHZ-O-OC-CHZ-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pica
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
140. (CH3)3C-C02-CHy-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
14 (CH3)3C-COZ-CHZ-O-OC-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
1.
142. (CH3)3C-C02-CH2-O-OC-CHZ-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
143. cHN4C-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
5 144. cHN4C-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
.
145. NHy-CHy-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
146. NH2-CHy-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
147. NH2-CH2-CHZ-(D)-Chg-Dep-NH-3-[6-am-(OH)]-pico
148. (CH3)yN-CH2-CHy-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
149. (CH3)2N-CH2-CHy-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
10 150. (CH3)2N-CH2-CHZ-(D)-Cha-pep-NH-3-[6-am-(OH)]-pico
151. CH3-NH-SOy-CHZ-CH2-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
152. CH3-NH-S02-CH2-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
153. CH3-NH-SOy-CH2-CHZ-(D)-Cha-Dep-NH-3-[6-am-(OH.)]-pico
154. H2N-S02-CH2-CHZ-(D)-Cha-Pyr-NH-3-[6-am-(OH)]-pico
15 155. H2N-SOy-CHy-CHZ-(D)-Chg-Pyr-NH-3-[6-am-(OH)]-pico
156: H2N-SOZ-CH2-CH2-(D)-Cha-Dep-NH-3-[6-am-(OH)]-pico
157. HO-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(-COO-)]-pico
158. Me0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(-COO-)]-pico
159. HO-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(-COO-)]-pico
160. Me0-OC-CH2-(D)-Chg-Pyr-NH-3-[6-am-(-COO-)]-pico
161. Et0-OC-CH2-(D)-Cha-Pyr-NH-3-[6-am-(-COO-)]-pico
162. cHexO-OC-CHy-(D)-Cha-Pyr-NH-3-[6-am-(-COO-)]-pico
List of abbreviations:
Adaala: Adamantylalanine
Adagly: Adamantylglycine
AIBN: Azobisisobutyronitrile
Ac: Acetyl
Ala: Alanine
am: Amidino
Asp: Aspartic acid
Aze: Azetidinecarboxylic acid .
Bn: Benzyl
Boc: tert-Butyloxycarbonyl
Bu: Butyl
Cbz: Benzyloxycarbonyl
Cha: Cyclohexylalanine
Chea: Cycloheptylalanine
Cheg: Cycloheptylglycine
Chg: ~ Cyclohexylglycine
Cog: Cyclooctylglycine
Cpa: Cyclopentylalanine
Cpg: Cyclopentylglycine
DC: Thin-layer chromatography
DCC: Dicyclohexylcarbodiimide
Dch: Dicyclohexylalanine
CA 02368830 2001-09-26
w BASg Aktiengesellschaft 20000104 O.Z. 0050/511?4 D8
16
Dcha: Dicyclohexylamine
DCM: Dichlormethane
Dep: 4,5-Dehydropipecolic acid
DMF: Dimethylformamide
~
DIPEAc Diisopropylethylamine
Et: Ethyl
Eq: Equivalent
Gly: Glycine
ham: Hydroxyamidino
10HOSucc: Hydroxysuccinimide
HPLC: High-performance liquid chromatography
iPr: Isopropyl
Lsg: Solution
Me: Methyl
15bb~ie2Cha: 2-Amino-3-cyclohexyl-3~nethylbutyric acid
or
bb-Dimethylcyclohexylalanine
4-MeCha: (4-Methylcyclohex-1-yl)alanine
g-MeCha: (1-Methylcyclohex-1-yl)alanine
203,3-Me2Cha: (3,3 Dimethylcyclohex-1-yl)alanine
4-MeChg: (4-Methylcyclohex-1-yl)glycine
3,3-Me2Chg: (3,3-Dimethylcyclohex-1-yl)glycine
MPLC: Medium-pressure liquid chromatography
MTBE: Methyl-tert butyl ether
25NBS: N-Hromsuccinimide
Nog: Norbornylglycine
Oxaz: Oxazole
Ph: Phenyl
phe: Phenylalanine
30Pic: Pipecolic acid
pico: Picolyl
PPA: ~ Propylphosphonic anhydride
pro: Proline
Py: Pyridine
35Pyr: 3,4 Dehydroproline
pyraz: Pyrazole
pyrr: Pyrrole
RT: Room temperature
RP-18 Reversed phase C-18
40t: Tertiary
tBu: tertiary-Butyl
tert: Tertiary
TBAB: Tetrabutylammonium bromide
TEA:. ~ Triethylamine
45TFA: Trifluoroacetic acid .
TFAA: Trifluoroacetic anhydride
thiaz: Thiazole
CA 02368830 2001-09-26
~~} BASF Akt3.engesellschaft 20000104 O.Z. 0050/51174 DE
17
' thioph: Thiophene
TOTU: 0-(Cyanoethoxycarbonylmethylene)amino-]-
N,N,N',N' tetramethyluronium
tetrafluoroborate
Z: - Benzyloxycarbonyl
nPent: ~ n-Pentyl
neoPent: neo-Pentyl (2,2-dimethyl-1-propyl)
nHex: n-Hexyl
cHex: Cyclohexyl
c-Pent Cyclopentyl
cHN4C- Tetrazolyl- (3-tetrazolyl- or
5-tetrazolyl)
c(CHZ)5N- N-Piperidinyl
nOct: n-Octyl
O-p-Me-Bn: p-Methylbenzyloxy-
N-Me~4-Pip-OH N-Methyl-4-piperidinyl alcohol
Me0-tetraethoxy: Tetraethylene glycolyl monomethyl ether
CH3-(CH2)150:~ Hexadecyloxy
CH3-(~CH2)1o0 Undecyloxy
4-Pip-O: 4-Piperidinyloxy
1-Pip-O: 1-Piperidinyloxy
~ tBu-cHexyl-O: 4-tert-Butyl-cyclohexyloxy
Ada-CH2-O: 1-Adamanthylmethyloxy
4-tBu-cHexyl-CH2-O: 4-tert-Butylcyclohexylmethyloxy
cHept-O: Cycloheptyloxy
3,3,5,5-tetraMe-cHex-0:3,3,5,5-Tetramethylcyclohexanyloxy~
4-Pyranyl-O: 4-Pyranyloxy
nPrO: n-Propyloxy
nBu-O: n-Butyloxy
iBu-O: t-Butyloxy
2,4-diMe-3-Pentyl-O: 1-Isopropyl-2-methylpropyloxy
1-Me-cPentyl-O: 1-Methyl-cyclopentyloxy
a,a-di- c8ex-CH2-O: Dicyclohexylmethoxy ',
tBu~T : tert-Butylamino
nHex-N: n-Hexylamino
HZN-3-[6-am-(-COO-)]-pico:3-[5-(aminomethyl)-2-pyridinyl]-1,2,4-oxadiazol-5-
one
HZN-3-[6-am-(OH)]-pico: 5-(aminomethyl)-N'-hydroxy-2-pyridin-
carboximidamide
CA 02368830 2001-09-26
''~ '' BASF Aktiengesellschaft 20000104 O.Z. 0050/51174
;'
1$
In the description and the claims, the following definitions
apply to the individual substituents:
The term "cycloalkyl" per se or as part of another substituent
includes saturated, cyclic hydrocarbon groups which contain the
number of carbon atoms indicated and in which up to, two CHZ groups
can be replaced by oxygen, sulfur or nitrogen atoms.
C3_8-cycloalkyl relates to saturated alicyclic rings having 3 to 8
C atoms such as, for example, cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, 4-methylcyclohexyl, cyclohexylmethylene,
cycloheptyl or cyclooctyl, pyrrolidine, piperidine, morpholine.
Pure carbocycles are preferred.
The term "alkyl" per se or as part of another substituent denotes
a linear or branched alkyl chain radical of the length indicated
in each case, which can be saturated or unsaturated and in which
up to 5 CH2 groups can be replaced by oxygen, sulfur or nitrogen
atoms. In this case, the heteroatoms are separated from one
another by at least two carbon atoms. Thus C1_4-alkyl is, for
example, methyl, ethyl, 1-propyl, 2-propyl, 2-methyl-2-propyl,
2-methyl-1-propyl, 1-butyl, 1-but 2-enyl, 2-butyl, C1_6-alkyl, for
example, Cl_4-alkyl, pentyl, 1-pentyl, 2-pentyl, 3-pentyl,
1-hexyl, 2-hexyl, 3-hexyl, 4-methyl-1-pentyl or
3,3-dimethylbutyl. C1_8-alkyl is additionally the radicals
indicated for C1~-alkyl, e.g. C1_6-alkyl, heptyl,
2-(2-methoxyethoxy)ethyl or octyl. The saturated alkyl chains
without heteroatoms are preferred.
The term "alkoxy" per se or as part of another substituent
denotes a linear or branched alkyl chain radical of the length
indicated in each case, which can be saturated or unsaturated and
is bonded to the respective parent compound via an oxygen atom.
Thus C1_4-alkoxy is, for example, methoxy, ethoxy, 1-propoxy,.
2-propoxy, 2-methyl-2-propoxy, 2-methyl-1-propoxy, 1-butoxy,
2-butoxy.
The term "aryl" per se or as part of another substituent includes
mono-, bi- or tricyclic aromatic hydrocarbons, such as phenyl.
naphthyl, tetralinyl, indenyl; fluorenyl, indanyl, anthracenyl,
phenanthrenyl.
The compounds of the formula I can be present as such or in the
form of their salts with physiological tolerable acids. Examples
of acids of this type are: hydrochloric acid, citric acid,
tartaric acid, lactic acid, phosphoric acid, methanesulfonic
acid, acetic acid, formic acid, malefic acid, fumaric aid,
succinic acid, hydroxysuccinic acid, sulfuric acid, glutaric
CA 02368830 2001-09-26
,.. BASF Aktieagesellschaft 20000104 O.Z. 0050/511?4 DE
19
acid, aspartic acid, pyruvic acid, benzoic, acid, glucuronic acid,
oxalic acid, ascorbic acid and acetylglycine.
The novel compounds of the formula I can be employed in the
following indications:
- disorders whose pathological mechanism is based directly or
indirectly on the proteolytic action of thrombin,
- disorders whose pathological mechanism is based on the
thrombin-dependent activation of receptors and signal
transduction,
- disorders which are accompanied by stimulation [e.g. by
PAI-l, PDGF (platelet-derived growth factor, P-Selectin,
ICAM-1, Tissue factor] or inhibition (e.g. NO synthesis in
smooth muscle cells) of gene expression in body cells,
- disorders which are based on the mitogenic action of
thrombin,
- disorders which are based on a thrombin-dependent
contractility and permeability change in epithelial cells
(e. g. vascular endothelial cells),
- thrombin-dependent, thromboembolic events such as deep vein
thrombosis, pulmonary embolism, myocardial or cerebral
infarct, atrial fibrillation, bypass occlusion,
- disseminated intravasal coagulation (DIC),
reocclusion and for the reduction of the reperfusion time in
the case of comedication with thrombolytics such as ,
streptokinase, urokinase, prourokinase, T-PA, APSAC,
plasminogen activators from the salivary glands of animals,
and the recombinant and mutated forms of all these
substances,
- the occurrence of earlier~reocclusion and later restenosis
after PTCA,
- the thrombin-dependent proliferation of smooth muscle cells,
- the accumulation of active.thrombin in the CNS (e.g. in
Alzheimer's disease),
CA 02368830 2001-09-26
$ASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
20
- tumor growth and against the adhesion and metastasis'of tumor
cells.
In particular, the novel compounds can be employed for the
5 therapy and prophylaxis of thrombin-dependent thromboembolic
events such as deep vein thromboses, pulmonary embolisms,
myocardial or cerebral infarcts and unstable angina, and
furthermore for the therapy of disseminated intravasal
coagulation (DIC). They are furthermore suitable for combination
10 therapy with thrombolytics such as atreptokinase, urokinase,
prourokinase, t PA, APSAC and other plasminogen activators for
reduction of the reperfusion time and prolongation of the
reocclusion time.
15 Further preferred application areas are the prevention of
thrombin-dependent early reocclusion and late restenosis after
percutaneous transluminal coronary angioplasty, the prevention of
thrombin-induced proliferation of smooth muscle cells, the
prevention of the accumulation of active thrombin in the CNS
20 (e. g. in Alzheimer~s disease), tumor control and the prevention
of mechanisms which lead to adhesion and metastasis of tumor
cells.
The novel compounds can further be employed in disorders whose
25 pathological mechanism is based directly or indirectly on the
proteolytic action of kininogenases, in particular kallikrein,
e.g. in inflammatory conditions such as asthma, pancreatitis,
rhinitis, arthritis, urticaria and other internal inflammatory
conditions.
The compounds according to the invention can be orally
administered in the customary manner. Administration can also be
carried out through the nasopharyngeal space using vapors or,
sprays.
The dose depends on the age, condition and weight of the patient
and on the manner of administration. As a rule, the daily dose of
active compound per person is between approximately 10 and
2000 mg in the case of oral administration. This dose can be
given in 2 to 4 individual doses or once daily as a slow-release
form.
The novel compounds can be administered in solid or liquid form
in the customary pharmaceutical.administration forms, e.g. as
tablets, film-coated tablets, capsules, powders, granules, coated
tablets, solutions or sprays. These are prepared in the customary
manner. The active compounds can in this case be processed with
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 D8
21
the customary pharmaceutical excipients such as tablet binders,
fillers, preservatives, tablet disinteqrants, flow regulators,
plasticizers, wetting agents, dispersants, emulsifiers, solvents,
release-delaying agents, antioxidants and/or propellants (cf. H.
Sucker et al.: Pharmazeutische Technologie (Pharmaceutical
Technology], Thieme-Verlag, Stuttgart, 1978). The administration
forms thus obtained normally contain the active compound in an
amount from 0.1 to 99% by weight.
Experimental section
Pharmacological tests
The absorption rate of orally administered medicaments from the
gastrointestinal tract (GIT) is an important factor with respect
to the bioavailability of the medicament. A prerequisite for a
high bioavailability is a good absorption rate.
A number of in vitro models are available for the study of
intestinal absorption. Thus, the human colon adenocarcinoma cell
lines THE-29, Caco-2 and.T84 are routinely employed in order to
investigate various intestinal transport processes (Madara
et al., Am. J. Physiol. 1988 ,254: 6416-6423; R. L. Audus et al,
Pharm. Res. 1990, 7, 435-451). The IEC-18 cell line also proved
to be a suitable model for the investigation of the permeability
of hydrophibic substances through the intestinal membrane (Ma et
al., J. Lab. Clin. Med. 1992; Duizer et al., J. Coatr. Rel.
1997). ~ .
For the transport experiments (Materials, Methods: see R.T.
Borchardt, P.L. Smith, G. Wilson, Models for Assessing Drug
Absorption and Metabolism, 1st Edition, Plenum Press New York and
London, 1996, Chapter 2), the cells are cultured for 17-24 days
on Transwell polycarbonate membranes. The experimental chamber is
arranged such that the membrane separates the apical compartment
from the basolateral compartment. The transport of the test
substances from the apical side through the cell layer to the
basolateral side can be measured as a function of the pH
gradient, e.g. apical (pH 6.0] basolateral (pH 8.0).
After incubation of the cells with the test substance, samples
are removed from the apical and basolateral sides after a defined
time interval (e. g. 24 h). The~content of test substance employed
and possible metabolites in each of the two compartments is
determined by HPLC (comparison of retention times) and HPLC-MS
CA 02368830 2001-09-26
BASF Aktieagesellsehaft 20000104 O.Z. 0050/51174 DE
22
(elucidation of metabolites) analysis. The transport rate is
calculated.
With the aid of the values which these tests produce, it is
possible to divide the test substances into the following
categories:
+++ : very good transport
++ . good transport
+ : moderate transport
o . poor transport
In the table below; the division into the categories mentioned
has been carried out for selected examples:
Ex. No. Transport
O1 ++
03 ++
05 +++
07 +
14 +++
15 +++
31 0
32 +
Pharmacokinetics and clotting parameters in rats
The test substances are dissolved in isotonic saline solution
immediately before administration to conscious Sprague Dawley
rats. The administration volumes are 1 ml/kg for intravenous
bolus injection into the tail vein and 10 ml/kg for oral
administration, which is carried out by stomach tube. If not
mentioned otherwise, taking of blood is carried out 1 h after
oral administration of 21.5 mg~kg-1 or intravenous administration
of 1.0 mg~kg-1 of the test substance or of the corresponding
vehicle (control). Five minutes before taking blood, the animals
are anesthetized by i.p. administration of 25% strength urethane
solution (dose 1 g~kg-1 i.p.) in physiological saline solution.
The carotid artery is dissected and catheterized and blood
samples (2 ml) are taken in citrate tubes (1.5 parts of citrate
plus 8.5 parts of blood). Directly after taking samples, the
ecarin clotting time (ECT) in whole blood is determined. After
the preparation of the plasma by centrifugation, the plasma
CA 02368830 2001-09-26
BASF Aktieages~llschaft 20000104 O.Z. 0050/51174 DE
23
thrombin time and the activated partial thromboplastin time
(APTT) are determined with the aid of a coagulometer.
Clotting parameters:
Ecarin clotting time (ECT): 100 ~,1 of citrated blood are
incubated for 2 min at 37°C in a coagulometer (CL 8, ball type,
Bender & Hobein, Munich, FRG). After the addition of 100 ~,l of
prewarmed (37°C) ecarin reagent (Pentapharm), the time until
formation of a fibrin clot is determined.
Activated thromboplastin time (APTT): 50 ~l of citrate plasma and
50 ~l of the PTT reagent (Pathrombin, Behring) are mixed and
incubated for 2 min at 37°C in a coagulometer (CI. 8, ball type,
Bender & Hobein, Munich, FRG). After the addition of 50 ~l of
prewarmed (37°C) calcium chloride, the time until formation of a
fibrin clot is determined.
Thrombin time (TT): 100 ~.l of citrate-treated plasma are
incubated for 2 min at 37°C in a coagulometer (CL-8, ball type,
Bender & Hobein, Munich, FRG). After the addition of 100 ~1 of
prewarmed (37°C) thrombin reagent (Boehringer Mannheim), the time
until the formation of a fibrin clot is determined.
pharmacokinetics and clotting parameters in dogs
The test substances are dissolved in isotonic saline solution
immediately before administration to conscious mongrel dogs. The
administration volumes are 0.1 ml/kg for intravenous bolus
injection and 1 ml/kg for oral administration, which is carried
out by stomach tube. Before and 5, 10, 20, 30, 45, 60, 90, 120,
180, 240, 300 and 360 min (if required after 420, 480 min and
24 h) after intravenous administration of 1.0 mg/kg or before and
10, 20, 30, 60, 120, 180, 240, 300, 360, 480 min and 24 h after
oral administration of 4.64 mg/kg, samples of venous blood (2 ml)
are taken in citrate tubes. Directly after taking the samples,
the ecarin clotting time (ECT) in the whole blood is determined.
After the preparation of the plasma by centrifugation, the plasma
thrombin time and the activated partial thromboplastin time
(APTT) are determined with the aid of a coagulometer.
The anti-F IIa activity (ATU/ml) and the concentration of the
substance are additionally determined by its anti-F-IIa activity
in the plasma by means of chromogenic (S-2238) thrombin assay,
calibration curves with r-hirudin and the test substance being
employed.
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
2~
The plasma concentration of the test substance is the basis for
the calculation of the pharmacokinetic parameters: time of
maximum plasma concentration (T max), maximum plasma
concentration; plasma half-life, to.5; area under the curve (AUC);
absorbed part of the test substance (F).
Clotting parameter:
Ecarin clotting time (ECT): 100 ~1 of citrate-treated blood are
incubated for 2 min at 37°C in a coagulometer (CL 8, ball-type,
Bender & Hobein, Munich, FRG). After the addition of 100 ~1 of
prewarmed (37°C) ecarin reagent '(Pentapharm), the time until the
formation of a fibrin clot is determined.
Activated thromboplastin time (APTT): 50 ~l of citrate-treated
plasma and 0 ~1 of the PTT reagent (Pathrombin, Behring) are
mixed and incubated for 2 min at 37°C in a.coagulometer (CL 8,
ball type, Bender & Hobein, Munich, FRG). After the addition of
50 ~1 of prewarmed (37°C) calcium chloride, the time until the
formation of a fibrin clot is determined.
Thrombin time (TT): 100 ~1 of citrate-treated plasma are
incubated for 2 min at 37°C in a coagulometer ((CL 8, ball type,
Bender & Hobein, Munich, FRG). After the addition of 100 ~1 of
prewarmed (37°C) thrombin reagent (Boehringer Mannheim), the time
until the formation of a fibrin clot is determined.
As the prodrugs in some cases are very poor thrombin inhibitors,
the proportion of active compound (drug) formed is determined
directly by means of the determination of the clotting
parameters. The kinetics therefore include the absorption of the
prodrug, its metabolization and excretion, and the conversion
into the active compound and its metabolization and excretion.
The compounds of the formula I can be prepared according to
Scheme's I-III.
The.units A, B and D are preferably synthesized separately and
employed in suitably protected form (see schemes I-III, use in
each case of orthogonal protective groups (P or P*) compatible
with the synthesis method used.
-
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
Scheme I
A B E
5 L
L
L
10 L
L
(P) L
(P) L
H ~G
fK
(P = protective group, (P) = protective group or H)
Hcheme I~describes the linear synthesis of the molecule I by
protective group removal from P-D-L (L is equal to CONH2, CSNH2,
CN)~, coupling of the amine H-D-L with the N-protected amino acid
P-E-0H to give P-E-D-L, removal of the N-terminal protective
groups to give H-E-D-L, coupling with the N-protected amino acid
P-B-OH to give P-B-E-D-L, removal of the protective group P to
give H-B-E-D-L, subsequent coupling or alkylation with the
optionally protected (P)~r-U unit (U = leaving group) or
reductive alkylation with (P) A'-U (U = aldehyde, ketone) or
Michael addition with a suitable P A"~=C- derivative to give
(P)-A-B-E-D-L. If L is an amide function, this can be converted
into the corresponding nitrile function at the in each case
protected stages by dehydration using trifluoroacetic anhydride.
Amidine syntheses of compounds of the structural type I starting
from the corresponding carboximides, nitriles, thiocarboxamides
and hydroxyamidines are described in a number of patent
applications (see, for example WO 95/35309, WO 96/17860,
WO 96/24609, WO 96/25426, WO 98/09950). Protective groups which
may still be present are then removed.
CA 02368830 2001-09-26
BASF Aktiengesellschaft . 20000104 O.Z. 0050/51174 DE
Scheme II
26
A B E D
(P*) L*
15 (p*) L*
(P*)
(P*)
(P*)
(P*)
(P*)
(p**)
Scheme II describes the linear synthesis of the molecule I by
coupling, alkylation, reductive amination or Michael addition of
H-B-P to correspondingly suitable optionally protected (P*) A
units ((P*) A-LJ (U = leaving group) or (P*) A'-U (U = aldehyde,
ketone), or (P*) A"-C=C-derivative] to give (P*)-A-B-P. After
removal of the C-terminal protective group to give (P*)-A-B-OH,
coupling with H-E-P to give (P*)-A-B-E-P, removal of the
C-terminal protective group again to give (P*)-A-H-E-OH and
coupling with H-D-L* (L* is equal to CONH2, CSNHZ, CN,
C(=NH)NH-R*; R* is equal to a hydrogen atom or protective group)
to give (P*)-A-B-E-D-L*, the reaction of this intermediate to
give the final product is carried out analogously to Scheme I.
The synthesis of the hydroxyl-, alkoxy- or aryloxyamidines (G =
OH, OR) is carried out by reaction of the corresponding nitriles
or iminothioester salts with hydroxylamine hydrochloride or
O-substituted hydroxylamine derivatives. (P**) is then introduced
by transesterification or stating from the free acid. For the
synthesis of the oxadiazolones (G and K together form a
COO-group), in particular of the three-substituted
1,2,4-oxadiazol-5-ones, the corresponding amidoximes are reacted,
with addition of bases (e. g. NaOH, pyridine, tertiary amines),
with carbonic acid derivatives such as, for example, phosgene,
di- and triphosgene, carbonyldiimidazole or chloroformic acid
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DL
27
esters (R.E. Bolton et al., Tetrahedron Lett. 1995, 36, 4471; R.
Rehse, F. Brehme, Arch. Pharm. Med. Chem. 1998, 331, 375).
Scheme III
(P*I L*
(P*) _'*
H
(P*) H2
H-G
(P*)
H-R
Scheme III describes a very efficient route for the preparation
of the compounds I by a convergent synthesis. The appropriately
protected units (P*) 1~B-OH and H-E-D-L* are coupled to one
another and the resulting intermediates (P*) 1~8-E=D-L* are
reacted to give the final product analogously to scheme I and
scheme II.
N-terminal protective groups employed are Boc, Cbz or Fmoc;
C-terminal protective groups are methyl, tert-butyl and benzyl
esters. Amidine protective groups are preferably Boc and Cbz. If
the intermediates contain olefinic double bonds, protective
groups which are removed hydrogenolytically are unsuitable.
The required coupling reactions and the customary reactions of
protective group introduction and removal are carried out
according to.standard conditions of peptide chemistry (see
M. Bodanszky, A. Bodanszky nThe Practice of Peptide Synthesis",
2nd Edition, Springer Verlag Heidelberg, 1994).
Boc protective groups are removed by means of dioxane/HC1,
diethyl ether/HCl, dichloromethane/HC1 or TFA/DCM, Cbz protective
groups hydrogenolytically or using HF, and Fmoc protective groups
using piperidine. Ester functions are hydrolyzed using LiOH in an
alcoholic solvent or in dioxane/water. t-Butyl esters are cleaved
using TFA or dioxane/HC1.
CA 02368830 2001-09-26
A B E D
BASS Aktieagesellschaft 20000104 O.Z. 0050/51174
28
The reactions were checked by means of TLC, the following eluents
customarily being used:
A. DCM/MeOH 95:5
8. DCM/MeOH 9;1
C. DCM/MeOH 8:2
D. DCM/MeOH/50% strength HOAc 40:10:5
E. DCM/MeOH/50% strength HOAc 35:15:5
F. cyclohexane/EA 1:1
w
Lf column-chromatographic separations~are mentioned, these
separations were carried out on silica gel, for which the
abovementioned eluents were used.
Reversed phase HPLC separations were carried out using
acetonitrile/water and HOAc buffer.
The starting compounds can be prepared according to the following
methods:
Units A prepared for alkylation are, for example, tert-butyl
a-bromoacetate, adamantyl a-bromoacetate, tert-butyl
~-bromopropionate, tert-butyl a-bromopropionate, tert-butyl
a-bromobutyrate, 2,3-dimethyl-2-butyl a-bromoacetate,
THP-protected bromoethanol, N-tert-butyl-a-bromoacetamide and
N,N,-diethyl-a-bromoacetamide. The tert-butyl esters mentioned,
if they cannot be purchased commercially, ar.e prepared
analogously to G. Uray, W. Lindner, Tetrahedron 1988, $l,
4357-4362. The bromoacetic acid esters, if they are not
obtainable commercially, were prepared by reaction of bromoacetyl
bromide with the appropriate alcohols with addition of pyridine
as a base.
8 units:
A variety of possibilities are available in the literature for
the general and specific synthesis of amino acids. Volume
El6d/Part 1 - H Houben-Weyl, pp. 406 et seq., inter alia, gives a
general overview of this. -
Frequently employed starting materials were ethyl
benzophenoneiminoacetate, diethyl acetamidomalonate and ethyl
isonitriloacetate.
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
29
The preparation of various glycine and alanine derivatives was
carried out, for example, starting from ethyl isonitroloacetate
and an appropriate ketone or aldehyde (see (H. J. Pratorius,
J. Flossdorf, M. R. Kula Chem. Ber. 1975, ~Q$, 3079).
The syntheses of cyclooctylglycine, 4-isopropylcyclohex-1-yl-
alanine, 4~nethylcyclohex-1-ylalanine and
4-methylcyclohex-1-ylglycine were carried out via the
corresponding ethyl 2-formylaminoacrylate (U. Schollkopf and R.
~10 Meyer, Liebigs Ann. Chem. 1977, 1174) starting from ethyl
isocyanoacetate using the respective carbonyl compounds
cyclooctanone, 2-norbornanone, 1-formyladamantane,
1-formyl-1-methylcyclohexane, 1-formyl-4-isopropylcyclohexane,
1-formyl-4-methylcyclohexane and 4-methylcyclohexanone according
to the following general procedures:
General working procedure for the synthesis of ethyl
2-formylaminoacrylates.
The solution of 100 mmol of ethyl isocyanoacetate in 50 ml of TEF
was added dropwise at 0 to -10~C to 100 mmol of potassium
tert-butoxide in 150 ml~of THF. After 15 min, 100 mmol of the
appropriate carbonyl compound in 50 ml of THF were added, the
reaction mixture was slowly allowed to rise to RT and the solvent
was stripped off on a rotary evaporator. The residue was mixed
with 50 ml of water, 100 ml of acetic acid and 100 ml of DCM and
the product was extracted with DCM. The DCM phase was dried over
Na2S04 and the solvent was stripped off on a rotary evaporator. If
necessary, the products obtained in almost pure form were further
purified by column chromatography on silica gel (eluent: mixtures
of ether/petroleum ether).
General procedure for the synthesis of the amino acid
hydrochlorides starting from the ethyl 2-formylaminoacrylates
100 mmol of the ethyl 2-formylaminoacrylate were hydrogenated
until reaction was complete using Pd/C (10%)/hydrogen in 200 ml
of glacial acetic acid. The catalyst was then filtered off, the
acetic acid was removed as extensively as possible on the rotary
evaporator and the residue was heated tv reflux for 5 h in 200 ml
of semiconcentrated hydrochloric acid. The hydrochloric acid was
stripped off on the rotary evaporator, and the product was dried
at 50~C in vacuo and washed several times with ether. The
hydrochlorides were obtained as slightly colored crystals.
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
Starting from 18.9 g (150 mmol) of cyclooctanone, 25.0 g of
cyclooctylglycine hydrochloride were obtained. Starting from
16.5 g (150 mmol) of 2-norbornanone, 26.6 g of 2-norbornylglycine
hydrochloride were obtained. Starting from 19.7 g (120 mmol) of
5 1-formyladamantane, 26.0 g of adamantylalanine hydrochloride were
obtained. Starting from 12.61g (100 mmol) of 1-formyl-1-methyl-
cyclohexane, 16.6 g of y~nethylcyclohexylalanine hydrochloride
were obtained. Starting from 16.8 g (150 mmol) of
4~nethylcyclohexanone, 25.9 g of 4-methylcyclohexylglycine
l0 hydrochloride were obtained. Starting from 15 g of
trans-1-formyl-4~nethylcyclohexane, 18 g of trans-4~nethylcyclo-
hex-1-yl-alanine hydrochloride were obtained. Starting from 9 g
of 3,3-dimethyl-1-formylcyclohexane, 10 g of 3,3~limethylcyclo-
hex-1-ylalanine hydrochloride were obtained.
The aldehyde needed for the synthesis, 1-formyl- 3,3-dimethyl-
cyclohexane, was prepared following Moskal and Lensen (Rec. Trav.
Chim. Pays Bas 1987, ~, 137-141):
A solution of n-butyllithium in n-hexane was added dropwise in
the course of 10 min at -60~C to a stirred solution of diethyl
isocyanomethyl phosphonate (17 ml, 105 mmol) in 280 ml of
anhydrous diethyl ether. The resulting suspension was stirred at
-60~C for 15 min. and treated in the course of 10 min with a
solution of 3,3-dimethylcyclohexanone (13 g, 105 mmol) in 100 ml
of anhydrous diethyl ether, the temperature being kept below
-45~C. The reaction mixture was allowed to come to O~C and was
stirred at this teperature for 90 min., and 150-200 ml of 38%
strength aqueous hydrochloric acid were cautiously added. To
complete the hydrolysis, the mixure was vigorously stirred at
room temperature for 15 h. The organic phase was separated off
and washed with 200 ml each of water, saturated sodium
hydrogencarbonate solution and saturated sodium chloride
solution. It was dried over magnesium sulfate, filtered and
concentrated on a rotary evaporator to remove the solvents. The
resulting residue was employed without further purification as a
starting material for the synthesis of the amino acid.
Preparation of cycloheptylglycine, cyclopentylglycine,
4-isopropylcyclohexylglycine and 3,3-dimethylcyclohexylglycine
These amino acids were prepared by reaction of cycloheptanone,
cyclopentanone, 4-isopropylcyclohexanone or 3,3-dimethylcyclo-
hexanone with ethyl isonitriloacetate according to a procedure of
H.J. Pratorius (H. J,. Pratorius, J. Flossdorf, M. Kula, Chem. Ber.
1985, 1~8, 3079 ) .
CA 02368830 2001-09-26
' BASF Aktieagesellsahaft 20000104 O.Z. 0050/51174 DE
31
Preparation of H-D,Ir-Chea-OH
4.0 g of cycloheptylmethyl methanesulfonate (19.39 mmol),
prepared from cycloheptylmethanol and methanesulfonyl chloride,
were heated to reflux in an inert gas atmosphere for 10 h with
4.9 of benzophenone imine glycine ethyl ester (18.47 mmol), 8.9 g
of dry finely powdered potassium carbonate (64.65 mmol) and 1 g
of tetrabutylammonium bromide (3 mmol) in 50 ml of dry
acetonitrile. The potassium carbonate was then filtered off, the
filtrate was evaporated to dryness and the crude product was
hydrolyzed directly at RT with stirring for 1.5 h using 20 ml of
2N hydrochloric acid in 40 ml of ethanol. After dilution of the
reaction solution, benzophenone was extracted with ethyl acetate
in the acidic range, then H-D,L-Chea-OEt was extracted with DCM
in the alkaline range (pH = 9), and the solution was dried over
magnesium sulfate and concentrated in a rotary evaporator. Yield
3.7 g = 95% of theory.
The amino acids mentioned were converted into the in each case
Boc-protected form according to generally known processes using
di-tert-butyl dicarbonate in water/dioxane and then
recrystallized from ethyl acetate/hexane mixtures or purified by
column chromatography on silica gel (eluent: ethyl
acetate/petroleum ether mixtures).
The Boc-protected amino acids were employed as B units according
to Scheme I.
As B units,. the amino acids mentioned were in some cases also
converted into the corresponding benzyl esters and linked to the
appropriately protected A units. In the case of compounds having
a still free NH function, this was then protected by a Boc group,
the benzyl ester group was removed by hydrogenation and the unit
A-B-OH was purified by crystallization, salt precipitation or
column chromatography. This route is described by way of example
for tBu00C--CH2-(Boc)(D)Cha-OH below.
Synthesis of D-cyclohexylalanine benzyl ester
A suspension of 100 g (481 mmol) of D-cyclohexylalanine
hydrochloride, 104 g (962 mmol) of benzyl alcohol and 109.7 g
(577 mmol) of p-toluenesulfonic acid monohydrate in 2200 ml of
toluene was slowly heated to reflux in a water separator. In a
temperature range from 80-90aC,.evolution of hydrogen chloride and
the dissolution of the suspension to give a clear solution was
observed. When water no longer separated (about 4 h), 500 ml of
toluene were distilled off, the reaction mixture was allowed to
CA 02368830 2001-09-26
BASS Aktieagesellschaft 20000104 O.Z. 0050/51174 D~
32
' cool overnight, and the resulting residue was filtered off and
washed twice with 1000 ml each of hexane. The resulting residue
(195 g) was then suspended in 2000 ml of dichloromethane, treated
with 1000 ml of water and adjusted to pH 9-9.5 with stirring by
successive addition of 50% strength sodium hydroxide solution.
The organic phase was separated off, washed twice with 500 ml
each of water, dried over sodium sulfate, the drying agent was
filtered off and the filtrate was concentrated, whereby 115 g
(94%) of the title compound were obtained as a pale oil.
N-(.tert Butyloxycarbonylmethylene)-D-cyclohexylalanine benzyl
ester
115 g (440 mmol) of D-cyclohexylalanine benzyl ester were
dissolved in 2000 ml of acetonitrile, treated at room temperature
with 607.5 g (4.40 mmol) of potassium carbonate and 94.3 g
(484 mmol) of tert-butyl bromoacetate and stirred at this
temperature for 3 days. The carbonate was filtered off and washed
with acetonitrile, the mother liquor was concentrated (30~C,
20 mbar), the residue was taken up in 1000 ml of methyl
tert butyl ether and the organic phase was extracted with 5%
strength citric acid and saturated sodium hydrogencarbonate
solution. The organic phase was dried over sodium sulfate, the
drying agent was filtered off, the filtrate was concentrated and
the oil obtained (168 g) was employed directly in the following
reaction.
N-Boc N-(tert Butyloxycarbonylmethylene)-D-cyclohexylalanine
benzyl ester
The oil obtained in the previous synthesis (168 g, 447 mmol) was
dissolved in 1400 ml of acetonitrile, treated with 618 g
(4.47 mmol) of potassium carbonate powder and 107.3 g (492 mmol)
of di-tert-butyl dicarbonate and the mixture was stirred at room
temperature for 6 days. The potassium carbonate was filtered off
with suction, washed with about 1000 ml of acetonitrile and the
filtrate was concentrated. 230 g of the desired product were
obtained.
N-Hoc-N-(tert butyloxycarbonylmethylene)-D-cyclohexylalanine
cyclohexylammonium salt
115 g of N-Boc-N-(tert-butyloxycarbonylmethylene)-D-cyclo-
hexylalanine benzyl ester were dissolved in 1000 ml of pure
ethanol and hydrogenated at normal pressure with hydrogen for 2 h
at 25-30~C in the presence of 9 g of 10% strength Pd on active
carbon. After filtration and removal of the solvent in a rotary
CA 02368830 2001-09-26
BASS Akti~ngesellschaft 20000104 O.Z. 0050/51174 DE
33
evaporator, 100 g (260 mmol) of a yellow oil were obtained, which
was taken up in 1600 ml of acetone and heated to reflux. The
heating bath was removed and a solution of 27 g (273 mmol) of
cyclohexylamine in acetone was added rapidly through a dropping
funnel. On cooling the reaction mixture to room temperature, the
desired salt crystallized out.. The solid was filtered off, washed
with 200 ml of acetone and recrystallized once more from acetone
for final purification. After drying the residue in a vacuum
drying oven at 30~C, 70.2 g of the desired salt were obtained as a
white powder.
N-Boc-N-(tert-butyloxycarbonylmethylene-D-cyclohexylglycine
cyclohexylammonium salt was prepared in an analogous manner from
cyclohexylglycine as starting material.
N-Boc-N-(tert butyloxycarbonylethylene) D-cyclohexylalanine
cyclohexylammonium salt
a) tert-Butyl 3-bromopropionate
16.64 g (109 mmol) of bromopropionic acid, 150 ml of
condensed 2-methylpropene and 2 ml of concentrated sulfuric
acid were added at ~30°C in a nitrogen countercurrent to a
glass vessel suitable for an autoclave, the vessel was firmly
sealed and the mixture was stirred at room temperature for
72 h. For working-up, the reaction vessel was again cooled to
-30°C and the reaction solution was cautiously poured into
200 ml of an ice-cold, saturated sodium hydrogencarbonate
solution. Excess 2-methylpropene was allowed to evaporate
with stirring, the residue was extracted three times with
50 ml each of dichloromethane, the combined organic phases
were dried over sodium sulfate, the drying agent was filtered
off and the filtrate was concentrated in a water-jet vaouum.
The oil residue was purified by column chromatography (eluent
N-hexane, later N-hexane/diethyl ether 9:1)'. 18.86 g of the
title compound were obtained.
b) N-(tert Butyloxycarbonylethylene)-D-cyclohexylalanine benzyl
ester
49.4 g (189 mmol) of D-cyclohexylalanine benzyl ester were
dissolved in 250 ml of acetonitrile, treated at room
temperature with 31.6 g (151 mmol) of tert-butyl
bromopropionate and the mixture was refluxed for 5 days. The
resulting precipitate was filtered off, washed repeatedly
with acetonitrile, the filtrate was concentrated in a
water-jet vacuum, the residue was taken up in 350 ml of
CA 02368830 2001-09-26
~
T
r, ~' BASF' Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
. ,. . .
34
dichloromethane and the organic phase was extracted with 5%
strength citric acid and saturated sodium hydrogencarbonate
solution. The organic phase was dried over sodium sulfate,
the drying agent was filtered off and the filtrate was
5 concentrated. The oily residue was purified by column
chromatography (eluent: dichloromethane, later
dichloromethanelmethanol 95:5). A slightly impure oil was
obtained, which Was employed directly in the following
reaction.
c) N-Boc-N-(tert butyloxycarbonylethylene)-D-cyclohexylalanine
benzyl ester
The oil obtained in the preceding synthesis (30 g, max.
70 mmol) was dissolved in 150 ml of acetonitrile, treated
with 28 ml (160 mmol) of diisopropylethylamine and 19.2
(88 mmol) of di-tert-butyl Bicarbonate and stirred at room
temperature for 3 days. The reaction mixture was concentrated
on the rotary evaporator in a water-jet vacuum, the residue
was taken up in n-hexane and washed five times with 3 ml each
of a 5% strength citric acid solution, the combined organic
phases were dried over sodium sulfate, the drying agent was
filtered off, the filtrate was concentrated and the residue
was subjected to purification by column chromatography
(eluent: hexane/ethyl acetate 95:5). 32.66 g (64 mmol) of the
desired product were obtained.
d) N-Boc-N-(tert butyloxycarbonylethylene-D-cyclohexylalanine
cyclohexylammonium salt
32.66 g (64 mmol) of N-Boc-N-(tert butyloxycarbonylethylene)-
D-cyclohexylalanine benzyl ester were dissolved in 325 ml of
pure ethanol and hydrogenated at 25 to 30oC with hydrogen at
normal pressure for 14 h in the presence of 3 g of 10%
strength Pd on active carbon. After filtration of the
solution through Celite~, Washing with ethanol and removal of
the solvent in a rotary evaporator, 26.7 g of a yellow oil
were obtained, which was taken up in acetone and heated to
reflux. The heating bath was removed and the solution of 7 g
(70 mmol) of cyclohexylamine in~acetone was added rapidly
through a dropping funnel. On cooling the reaction mixture to
room temperature, the desired salt crystallized out. The
solid was filtered off, washed with 25 ml of acetone and
.recrystallized once more from acetone for final purification.
After drying the residue in a vacuum drying oven at 30~C,
CA 02368830 2001-09-26
BASF Aktieagesellschaft 20000104 O.Z. 0050/51174 D~
26.6 g (54 mmol) of the desired salt were obtained as a white
powder. '
N-Boc-N-(tert-butyloxycarbonylmethylene)-(D)-cyclohexylalanyl-
5 3,4-dehydroproline:
a) N-Boc-Pyr-OH (5 g, 23.45 mmol) was dissolved in MeOH (50 ml)
and treated with HCl in dioxane (4N, 30 ml). The mixture was
then heated under reflux for 12 h. The solvent was removed in
10 a rotary evaporator and H-Pyr-OMe hydrochloride was obtained
as the product. Yield: 3.84 g (100%).
b) N-(t Bu02C-CH2) N-Boc-(D)-Cha-OH (8 g, 20.75 mmol) was
dissolved in dichloromethane (75 ml) and treated at -10°C
15 with ethyldiisopropylamine (15.5 ml, 89.24 mmol). After
storing at this temperature for~5 min., a solution of
H-Pyr-OMe hydrochloride (3.4 g, 20.75 mmol) in
dichloromethane (25 ml) was added dropwise. A solution of
propanephosphonic anhydride in ethyl acetate (50% strength,
20 20 ml, 26.96 mmol) was then added dropwise and the mixture
was stirred at -10 to 0°C for 2 h. The batch was diluted with
dichloromethane and washed with saturated sodium
hydrogencarbonate solution (2 x 80 ml, 5% strength citric
acid solution (2 x 15 ml) and saturated sodium chloride
25 solution (1 x 20 ml). The organic phase was dried over sodium
sulfate and the solvent was removed in a rotary evaporator.
The crude product was purified by means of flash
chromatography (silica gel, dichloromethane/methanol 95/5).
.Yield: 6.2 g (60%).
c) N-(t-Bu02C-CH2) N-Boc-(D)-Cha-Pyr-OMe (5.5 g, 11.12 mmol) was
dissolved in dioxane (40 ml), treated with sodium hydroxide
solution (1N, 22.2 ml, 22.24 mmol) and stirred at room .
temperature for 2 h. The dioxane was removed in a rotary
evaporator, and the aqueous phase was washed with ethyl
acetate and acidified to pH 1 to 2 with potassium
hydrogensulfate solution (20% strength). The aqueous phase
was extracted with dichloromethane and the combined organic
phases were dried over sodium sulfate. Yield: 5 g (94%),
colorless foam. Recrystallization from n-hexane saturated
with water afforded colorless crystals (m. p. - 158 to 160°C).
N-Boc-N-(tert-butyloxycarbonylmethylene)-(D)-cyclohexylglycyl-
3,4-dehydroproline
CA 02368830 2001-09-26
BASF Rktiengesellschaft .20000104 O.Z. 0050/51174 D8
36
15
This compound was prepared from N-Boc~(tert butyloxycarbonyl-
methylene)-(D)-cyclohexylglycine and 3,4-dehydroproline methyl
ester in an analogous manner.
5 The (x)3,4-dehydroproline employed as the D unit can be obtained
commercially; the (D, L)-4,5-dehydropipecolic acid can be prepared
according to A. Burgstahler, C.E. Aiman J. Org. Chem. ~ (1960),
489 or C. Herdeis, W. Engel Arch. Pharm ~, (1993), 297 and then
converted into Hoc-(D, L)-Dep-OH using (Boc)20.
The synthesis of 3-(6-cyano)picolylamine has been described in
WO 96/25426 and WO 96/24609.
3-(6-Cyano)picolylamine
The preparation of this component was carried out as described in
WO 96/25426 and WO 96/24609.
Example 1:
N-(tert-butoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
A suspension of 1.22 g (17.6 mmol) of hydroxylamine hydrochloride
in 50 ml of ethanol was treated with 1.3 g of conc. ammonia,
stirred for 30 min. and the deposited precipitate (ammonium
chloride) was filtered off with suction. 4.3 g (8.9 mmol) of
N-(tert-butoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-cyano-3-picolyl)amide (WO 96/25426, Example 93,
stage a) were then added to the alcoholic hydroxylamine solution
and it was allowed to stand at room temperature for one hour.
According to TLC (eluent: dichloromethane/ethanol = 9:1 or
dichloromethane/methanol/conc. ammonia = 45:5:0.3), starting
material was no longer detectable. After distilling off the .
solvent in vacuo, the residue was dissolved in 100 ml of
dichloromethane, and the solution was washed with water and
aqueous sodium hydrogencarbonate solution and dried over sodium
sulfate. After concentration, 4.1 g (87%) of an amorphous residue
remained.
FAB-MS (M+H+): 529
Example 2:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride
3.5 g (6.6 mmol) of N-(tert-butoxycarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)-
amide (see Ex. 1) were dissolved in 15 ml of dichloromethane,
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z: 0050/51174 DE
37
treated with 25 ml of a 4N solution of hydrogen chloride in
dioxane and allowed to stand at room temperature overnight. After
distilling off the solvent in vacuo (toward the end with addition
of toluene), the amorphous residue was digested repeatedly with
diethyl ether. After drying, 3.1 g (90% of theory) of a white
amorphous powder remained.
FAB-MS (M+H+): 473
Example 3:
N-(Ethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide
2.5 g (5.3 mmol) of N-(hydroxycarbonylmethylene)-(D)-cyclohexyl-
alanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
hydrochloride (see Ex. 2) were dissolved in 50 ml of dry ethanol,
treated with 3 ml of a 4N solution of hydrogen chloride in
dioxane, refluxed for 4 hours and allowed to stand at room
temperature for 2 days.
After distilling off the solvent in vacuo at a bath temperature
of 35°C, the residue was digested repeatedly with diethyl ether,
then taken up in ethyl acetate and extracted with saturated
sodium hydrogencarbonate solution. The organic phase was dried
over sodium sulfate and concentrated, and the residue was
purified by column chromatography (eluent dichloromethane/ethanol
= 9:1, toward the end 4:1). After distilling off the solvent,
1.85 g (70% of theory) of a white amorphous powder remained,
FAB-MS (M+H+); 501
Example 4:
N-(Methoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 3, the residue
digested with ether first being dissolved in methanol and
converted into the acetic acid salt by means of ion exchanger
before it was purified by column chromatography (eluent
dichloromethane/methanol = 9:1).
FAB-MS (M+H+): 487
Example 5:
N-(Isopropyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This. compound was prepared analogously to Example 3, the starting
material N-(hydroxycarbonylmethylene)- (D)-cyclohexyl-.
alanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
hydrochloride (see Ex. 2) being dissolved in isopropanol and
CA 02368830 2001-09-26
. BASF' Aktiengesellschaft 20000104 O.Z. 0050/51174
38
hydrogen chloride being introduced. The work-up and purification
was carried out analogously to Example 4.
FAB-MS (M+H+): 515
Example 6:
N-(Benzyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride (see Ex.
2) and benzyl alcohol.
FAH-MS (M+H+): 563
Example 7:
N-(Ethyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-[(6-amidino)-3-picolylamide] hydrochloride
HOZC-CH2-(D)-Cha-Pyr-NH-3-(6-am)-pico (4.93 g, 10 mmol;
preparation: WO 96/25426, Example 93) was dissolved in 60 ml of
ethanol, treated with HCl in ether (4.5N, 15 ml) and stirred at
60°C for 6 h. As according to TLC (methylene chloride/methanol/
acetic acid (50% strength in water): 35/15/7) the conversion was
still not complete, a,further 25 ml of 4.5N hydrogen chloride in
ether and 50 ml of ethanol were added and the mixture was stirred
at 60°C again for 5 h. After concentrating the reaction mixture in
vacuo in a rotary evaporator, it was codistilled a number of
times with ethanol and ether in order to remove adhering
hydrochloric acid. The product was then washed by stirring in a
little acetone/methylene chloride, and the residue was filtered
off with suction and dried in vacuo. 5.4 g of the title compound
were obtained as a white, hygroscopic solid substance.
FAB-MS (M+H+): 485
Example 8:
N-(Methyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-[(6-amidino)-3-picolylamide] hydrochloride
This compound was prepared analogously to Example 7 by
esterification of H02C-CH2-(D)-Cha Pyr NH-3-(6-am)-pico with
methanol.
FAB-MS (M+H+): 471
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
39
Example 9:
N-(n-Propyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-[(6-amidino)-3-picolylamide] hydrochloride
This compound was prepared analogously to Example 7 by
esterification of H02C-CH2-(D)-Cha-Pyr~lH-3-(6-am) pico with
n-propanol.
FAB~IS ( M+H+ ) ; 4 9 9
Example 10:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-methoxy-amidino-3-picolyl)amide
a) N-(tert-Butoxycarbonylmethylene)-(Boc)-(D)~yclohexylalanyl-
3,4-dehydroprolyl-(6-aminothiocarbonyl-3-picolyl)amide
t-Hu02C-CHZ-(Boc)-(D)-Cha-Pyr-NH-3-(6-CN)-pico (WO 96/25426,
Ex. 93, stage b) was reacted with hydrogen sulfide in
pyridine/triethylamine to~give the corresponding thioamide
t-Bu02C~H2-(Boc)-(D)-Cha-Pyr-NH-3-(6-CSNH2) pico according to
WO 96/25426, Ex. 93, stage c).
b) N-(tert-eutoxycarbonylmethylene)-(Hoc)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-S-~nethyliminothiocarbonyl-3-picolyl)-
amide hydroiodide
The product t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr-NH-3-(6-CSNHy) pico
obtained from a) was reacted with methyl iodide to give
t-BuOZC-CHZ-(Boc)-(D)-Cha-Pyr-NH-3-(6-C=NH(SCH3)) pico x HI
analogously to WO 96/25426, Ex. 93, Stage d.
c) N-(tert-Butoxycarbonylmethylene)-(Boc)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-~nethoxyamidino-3-picolyl)amide
O-Methylhydroxylamine hydrochloride (0.9 g, 8.1 mmol) was
dissolved in 30 ml of methanol and converted into the
corresponding acetic acid salt by means of an ion exchanger
(Fluka: acetate on polymeric support, 3.0 mmol of acetate per
g). t-Bu02C-CH2-(Boc)-(D)=Cha-Pyr-NH-3-(6~=NH(SCH3))-pico x
HI (4.8 g, 6.2 mmol; see b) was added to this methanolic
solution and the reaction mixture was stirred at room
temperature overnight. After concentrating in vacuo, the
residue was taken up in 200 ml of ethyl acetate, washed three
.times with 30 ml each of water, twice with 20 ml each of 20%
strength sodium hydrogensulfate solution and once.with 30 ml
of saturated sodium chloride solution and then purified by
CA 02368830 2001-09-26
BASF Aktieagesellschaft 20000104 O.Z. 0050/51174 DS
40 _
column chromatography on silica gel, 0.9 g of the desired
product being isolated.
d) N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-methoxyamidino-3-picolyl)amide
The product t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr NH-
3-(6-C=NH(NHOCH3))-pico (0.9 g, 0.7 mmol) obtained according
to c) was dissolved in 10 ml of absolute dioxane, cooled to
0°C and treated with 5 ml of a 4N solution.of hydrogen
chloride in dioxane. The mixture was stirred at room
temperature for 6 h with exclusion of moisture, then
dissolved in water and subjected.to salt exchange by means of
an acetate exchanger and the aqueous phase was freeze-dried.
0.38 g of the title compound was obtained as a white powder.
FAB-MS (M+H+): 487
Example 11:
N-(Methoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
ZO prolyl-(6-methoxy-amidino-3-picolyl)amide
A solution of 1.5 g (2.9 mmol) of N-(hydroxycarbonylmethylene)-
(D)-cyclohexylalanyl-3,4-dehydroprolyl-(6-methoxyamidino-3-
picolyl.)amide (see Ex. 10 d) in methanol was treated with a 4N
solution of hydrogen chloride in dioxane and stirred at room
temperature for 2 days. It was concentrated,' the residue was
codistilled twice with diethyl ether in order to remove excess
acid and the crude product was purified by column chromatography.
FAB-MS (M+H+): 501
Example 12:
N-(Isopropyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydr
oprolyl-(6~nethoxyamidino-3-picolyl)amide .
This compound was prepared analogously to Example 11 by
esterification of N-(hydroxycarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-(6-methoxyamidino-3-picolyl)-
amide (see Ex. lOd) with isopropanol.
FAB-MS (M+H+): 529 -
45
CA 02368830 2001-09-26
BASF Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
41
Example 13:
N-(n-Octyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydropro7.y1-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
grolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride (see
Ex. 2) and n-octanol.
FAB-MS (M+H+): 585
Example 14:
N-(c-Hexyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 3 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride
(see Ex. 2) and c-hexanol.
FAB-MS (M+H+): 555
Example 15:
N-(Neopentyloxycarbonylmethylene)-(D)~yclohexylalanyl-
3:,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 3 from
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride (see
Ex. 2) and neopentyl alcohol.
FAB-MS (M+H+): 543
Example 16:
N-(Methoxyethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide-hydrochloride
(see Ex. 2) and ethylene glycol monomethyl ether.
FAB-MS (M+H+): 531
Example 17:
N-(O-Methyldiethoxyoxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
CA 02368830 2001-09-26
,, BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 D8
42
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl- .
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride
(see Ex. 2) and diethylene glycol monomethyl ether.
FAB-MS (M+H+): 575 ,
Example 18:
N-(Cyclohexylmethyloxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehyd=oprolyl-(6-hydroxyamidino-3-picolyl)amide-hydro-
chloride (see Ex. 2) and cyclohexylmethanol, the residue digested
with ether being filtered off and purified by means of reversed
phase HPLC.
FAB-MS (M+H+): 569
Example 19:
N-(Cyclooctyloxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride (see
Ex. 2) and cyclooctanol. As the purification by column
chromatography using the eluent dichloromethane/methanol = 9:1
failed, a second purification was carried out (eluent ethyl
acetate) by means of MPLC (silica gel). The title compound was
obtained as a white powder.
FAB-MS (M+H+): 583
Example 20: ,
N-(traps-4-Methylcyclohexyloxycarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)-
amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(-D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride
(see Ex. 2) and traps-4-methylcyclohexanol. As the purification
by column chromatography using the eluent dichloromethane/
methanol = 9:1 and 95:5 failed, a third purification was carried
out (eluent ethyl acetate) by means of MPLC (silica gel). The
title compound was obtained as a white powder.
FAB-MS (M+H+): 569
CA 02368830 2001-09-26
BASt~ Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
43
Example 21:
N-(n-Hexyloxycarbonylmethylene)-(D)-cyclohexylalanyl-.3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cycloheXylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide hydrochloride
(see Ex. 2) and n-hexanol, the purification by column
chromatography being carried out on silica gel (MPLC; eluent
ethyl acetate/n-hexane a 7:3).
FAB-MS (M+H+): 557
Example 22:
N-(c-Pentyloxycarbonylmethylene)-(D)--cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide hydro-
chloride (see Ex. 2) and c-pentanol, the purification by column
chromatography being carried out on silica gel (MPLC; eluent
ethyl acetate/n-hexane = 1:1).
FAB-MS (M+H+): 541
Example 23:
N-(4-Methoxycyclohexyloxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 4 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide hydro-
chloride (see Ex. 2) and 4-methoxycyclohexanol, the purification
by column chromatography being carried out silica gel (MPLC:,
eluent ethyl acetate/methanol = 99:1, with an increase in the
proportion of methanol of 0.1% per minute). The title compound
was obtained as a cis/trans mixture (according to HPLC the ratio
of the two isomers was 29:71).
FAB-MS (M+H+): 585
Example 24:
N-(1,1,2-Trimethylpropyloxycarbonylmethylene)-(D)-cyclo-
hexylalanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
a) 1,1,2-Trimethylpropyl bromoacetate
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
44
3.5 ml (l.l equivalents) of pyridine and, at -5°C, 7.9 g
(39 mmol) of brornoacetyl bromide were. added at room
temperature to a solution of 4.0 g (39 mmol) of
2,3-dimethyl-2-butanol in 20 ml of dichloromethane. During
the addition of the bromide, which proceeded in a strongly
exothermic manner, a pale precipitate was formed. During the
course of this, the temperature rose to 20°G. The mixture was
stirred at room temperature for 1 hour, diluted with ethyl
acetate and extracted three times with 5 ml each of saturated
sodium chloride solution. The ethyl acetate phase was dried
.over magnesium sulfate and concentrated, and the residue
obtained was employed in the next reaction without further
purification.
b) N-(1,1,2-Trimethylpropyloxycarbonylmethylene)-(D)-cyclo-
hexylalanyl-3,4-dehydroprolyl-(6-cyano-3-picolyl)amide
30 ml of a 4N solution of hydrogen chloride in dioxane was
added at -5°C to a solution of 14.1 g (29.3 mmol) of
Boc-(D)-Cha-Pyr-NH-3-(6-CN)-pico (WO 96/25426, Ex. 32, stage
d) in 30 ml of dioxane. The mixture was stirred at room
temperature for 3 hours and concentrated. The residue was
taken up a total of three times in dichloromethane and
concentrated again in order to remove the excess hydrogen
chloride. After the residue had been taken up in 50 ml of
ethyl acetate and had been treated with 200 ml of diethyl
ether, the product precipitated. It was filtered off and
washed with diethyl ether. After drying, 12.0 g (98%) of the
compound H-(D)-Cha-Pyr-NH-3-(6-CN)-pico were obtained as the
hydrochloride.
2.3 g (5.5 mmol) of H-(D)-Cha-Pyr-NH-3-(6-CN)-pico x HC1 were
dissolved in 20 ml of dichloromethane, and the solution.was
treated at room temperature with 7.6 g (54.7 mmol) of
potassium carbonate and dropwise at -5°C with 1.22 g
(5.5 mmol) of 1,1,2-trimethylpropyl bromoacetate. It was
allowed to come to room temperature and stirred for 3 days.
The reaction mixture was concentrated in vacuo in a rotary
evaporator, taken up in ethyl acetate, and the solution was
washed three times with a little Water and once with
saturated sodium chloride solution. The organic phase was
dried and concentrated. The residue was purified by column
chromatography (eluent dichloromethane/methanol = 9:1) by
.means of MPLC (silica gel). 1.82 g (64%) of the title
compound were obtained as a white powder.
FAB-MS (M+H+): 524
CA 02368830 2001-09-26
BAS1~ Aktiengesellscbaft 20000104 O.Z. 0050/51174 DE
c) N-(1,1,2-Trimethylpropyloxycarbonylethylene)-
(D)-cyclohexylalanyl-3,4-~iehydroprolyl-(.6-hydroxy-
.amidino-3-picolyl)amide
5 0.17 g (1.32 mmol) of diisopropylethylamine and 73 mg
(1.05 mmol) of hydroxylamine hydrochloride were added at room
temperature to a solution of 460 mg (0.88 mmol) of the
compound obtained in b) in 10 ml of dichloromethane. The
mixture was stirred at room temperature for 4 hours, diluted
10 with dichloromethane and extracted twice with 5 ml each of 5%
strength citric acid solution. The organic phase was dried
and concentrated. The residue was purified by column
chromatography by means of reverse phase HPLC. The title
compound was obtained as a white powder.
15 FAB-MS (M+H+): 557
Example 25:
N-(2-Methyl-1,3-dioxan-5-yloxycarbonylmethylene)-(D)-cyclohexyl-
alanyl-3,4-dehydroprolyl-(6 hydroxyamidino-3-picolyl)amide~
This compound was prepared analogously to Example 24 starting
from H-(D)-Cha-Pyr-Nfi-3-(6-CN)-pico x HCl and
2-methyl-1,3-dioxan-5-ol, the hydroxylamine addition being
carried out in acetonitrile and the purification by column
chromatography on silica gel (MPLC; eluent ethyl acetate/methanol
- 99:1, with an increase in the proportion of methanol of 0.1%
per minute). The title compound was obtained as a white powder.
FAB-MS (M+H+): 573
Example 26:
N-(1-Isopropyl-2-methylpropyloxycarbonylmethylene)-(D)-cyclohexyl
alanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 24 starting
from H-(D)-Cha-Pyr-NIi-3-(6-CN)-pico x HC1 and 2,4-dimethyl-
3-pentanol, the hydroxylamine addition being carried out in
acetonitrile and the purification by column chromatography on
silica gel (MPLC; eluent ethyl acetate/methanol = 99:1, with an
increase in the proportion of~methanol of 0.1% per minute). The
title compound was obtained as a white powder.
FAB-MS (M+H+): 571
CA 02368830 2001-09-26
SASE Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
46
Example 27:
t~(2-Indanyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 25 starting
from H-(D)-Cha-Pyr-NH-3-(6-CN)-pico x HC1 and 2-indanol.
FAB-MS (M+H+): 589
. Example 28:
N-(1-Isobutyl-3-methyloxycarbonylmethylene)-(D)--cyclohexyl-
alanyl-3,4-dehydroprolyl-(6 hydroxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 25 starting
from H-(D)-Cha-Pyr-NH-3-(6-CN)-pico x HCl and 2,6.-dimethyl
heptan-4-ol.
FAB-MS (M+H+): 599
Example 29:
N-[4-Oxo-4-(1-pyrrolidinyl)butyloxycarbonylmethylene)-(D)-cyclo-
hexylalanyl-3,4-dehydroprolyl-(6 hydroxyamidino-3-picolyl)amide
a) 4-Oxo-4-(1-pyrrolidinyl)-1-butanol
A mixture of 7.1 g (82 mmol) of y-butyrolactone and 11.7 g
(164.5 mmol) of pyrrolidine was stirred at room temperature
for 3 hours. The pyrrolidine was distilled off in vacuo in a
rotary evaporator to the greatest possible extent, and the
residue was dissolved in toluene a number of times and
concentrated again in order to remove traces of the base. The
product obtained was employed in the following reaction
without purification.
b) 4-Oxo-4-(1-pyrrolidinyl)butyl bromoacetate .
The product obtained a) was reacted with bromoacetyl bromide
analogously to Example 24a), 4-dimethylaminopyridine being
employed as a base instead of pyridine.
The title compound was obtained analogously to Example 24
starting from H-(D)-Cha-Pyr-NH-3-(6-CN)-pico x HCl and the
4-oxo-4-(1-pyrrolidinyl)butyl bromoacetate prepared in b).
FAB-MS (M+H+): 612
CA 02368830 2001-09-26
BASF Aktieagesellschaft 20000104 O.Z. 0050/51174
47
Example 30:
N-[2-(Cyclohexylamino)-2-oxoethyloxyoarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)-
amide
a) N-Cyclohexyl-2-hydroxyacetamide
A mixture of 1.2 g (10 mmol) of 1,4-dioxane-2,5-dione and
4.0 g (40 mmol) of cyclohexylamine was stirred at a
temperature of 100°C for 3 hours.. The cyclohexylamine was
distilled off in vacuo in a rotary evaporator to the greatest
possible extent, and the residue was dissolved a number of
times in toluene and concentrated again in order to remove
traces of the base. The product obtained was dissolved in
diethyl ether and added dropwise to petroleum ether, a
precipitate being deposited. The precipitate was filtered off
and employed in the following reaction without further
purification.
b) 2-(Cyclohexylamino)-2-oxoethyl bromoacetate
The product obtained in a) was reacted with bromoacetyl
bromide analogously to Example 24a), 4-dimethylaminopyridine
being employed as base instead of pyridine.
Analogously to Example 24, the title compound was obtained
starting from $-(D)-Cha-Pyr-N$-3-(6-CN)-pico x $Cl and the
2-(cyclohexylamino)-2-oxoethyl.bromoacetate prepared in b).
FAB-MS (M+$+): 612
Example 31:
N-($ydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-{5-[2-(1,2,4-oxadiazol-3-yl-5-on)]-pyridyl}methylamide
hydrochloride
a) N-(tert-butoxycarbonylmethylene)-(Boc)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-hydroxyamidino-3-picolyl)amide
11.9 g (20 mmol) of N-(tert-butoxycarbonylmethylene)-(Boc)-
(D)-cyclohexylalanyl-3,4-dehydroprolyl-(6-cyano-3-picolyl)-
amide (WO 96/25426, Example 93, Stage b), 2.78 g (40 mmol) of
hydroxylamine hydrochloride and 4.65 g (36 mmol) of
diisopropylethylamine were dissolved in 100 ml of ethanol and
.heated at 55-60°C for 5 hours. The solution was concentrated
in vacuo, the residue was taken up in 100 ml of ethyl acetate
and the mixture was washed twice with saturated sodium
chloride solution. After drying and distilling off the
CA 02368830 2001-09-26
BASg Aktieagesellschaft 20000104 O.Z. 0050/51174 D8
48
solvent, 11.3 g (90% of theory) of slightly yellowish,
amorphous residue remained..
b) N-(tert-Butoxycarbonylmethylene)-(Boc)-(D)-cyclohexyl-
alanyl-3,4-dehydroprolyl-{5-[2-(1,2,4-oxadiazol-3-yl-5-one)]-
pyridyl}methylamide
10.2 g (16.2 mmol) of the above amidoxime were dissolved in
60 ml of pyridine and, after addition of 2.9 g (17.9 mmol) of
carbonyldiimidazole, heated under reflux for 3 hours. The
pyridine was distilled off in vacuo, the residue was taken up
in methyl tert-butyl ether, and the solution was washed with
5% strength citric acid solution and finally with saturated
sodium chloride solution. After drying and distilling off the
solvent, 10 g (94% of theory) of amorphous residue remained)..
c) N-(Hydroxycarbonylmethylene)=(D)-cyclohexylalanyl-3,4-
dehydroprolyl-{5-[2-(1,2,4-oxadiazol-3-yl-5-one)]-pyridyl}-
methylamide hydrochloride
10 g (15.3 mmol) of the compound obtained in b) were
dissolved in 80 ml of glacial acetic acid, treated with 80 ml
of a 4N solution of hydrogen chloride in dioxane and allowed
to stand at room temperature overnight.
After distilling off the solvent in vacuo (toward the end
with addition of toluene), the amorphous residue was purified
by column chromatography (eluent: ethanol/25% strength
ammonia = 50:2.5). The residue was dissolved in a mixture of
water and dioxane (ratio 3:7), treated with one equivalent of
32% strength hydrochloric acid and concentrated to dryness.
The residue was digested with acetonitrile and then filtered
off with suction. 3.9 g (48% of theory) of a white powder
were isolated;
FAB-MS (M+H+): 499
Example 32:
N-(Methoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-{5-[2-(1,2,4-oxadiazol-3-yl-5-one)]-pyridyl}methylamide
hydrochloride
1.9 g (3.6 mmol)of N-(hydroxycarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-{5-[2-(1,2,4-oxadiazol-3-yl-5-
one)]-pyridyl}methylamide hydrochloride (see Example 31) were
dissolved in 100 ml of methanol and, with addition of 10 ml of a
CA 02368830 2001-09-26
BAS1~ Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
49
4N solution of hydrogen chloride in dioxane, heated under reflux
for 8 hours.
The residue was digested with acetonitrile and then filtered off
with suction. 1.65 g (85% of theory) of a white powder were.
isolated;
FAB-MS (M+H+): 513
Example 33:
N-(Neopentyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-amidino-3-picolyl)amide
This compound was prepared analogously to Example 7 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-amidino-3-picolyl)amide hydrochloride (Preparation:
w0 9625426, Example 93) and neopentyl alcohol.
FAB-MS (M+H+): 527
Example 34:
N-(n-Hexyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-amidino-3-picolyl)amide
This compound was prepared analogously to Example 7 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-amidina-~3-picolyl)amide hydrochloride (Preparation:
WO 9625426, Example 93) and n-hexanol.
FAB-MS (M+H+): 541
Example 35:
N-(c-Hexyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-amidino-3-picolyl)amide
This compound was prepared analogously to Example 7 from -
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-amidino-3-picolyl)amide hydrochloride (Preparation:
WO 9625426, Example 93) and c-hexanol.
FAB-MS (M+H+): 539
Example 36:
N-(methoxyethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-amidino-3-picolyl)amide
This compound was prepared analogously to Example 7 from
N-(hydroxycarbonylmethylene)-(D)--cyclohexylalanyl-3,4-
dehydroprolyl-(6-amidino-3-picolyl)amide hydrochloride
(Preparation: WO 9625426, Example'93) and ethylene glycol
monomethyl ether.
CA 02368830 2001-09-26
BASF Akt3engesellschaft 20000104 O.Z. 0050/51174 DE
FAH-MS (M+H+): 515
Example 37:
N-(O-Methyldiethoxyoxycarbonylmethylene)-(D)-cyclohexylalanyl-
5 3,4-dehydroprolyl-(6-amidino-3-picolyl)amide .
This compound was prepared analogously to Example 7 from
N-(hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydroprol
yl-(6-amidino-3-picolyl)amide hydrochloride (Preparation:
10 WO 9625426, Example 93) and diethylene glycol monomethyl ether.
FAB-MS (M+H+): 559
Example 38:
N-(Methoxyethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
15 dehydroprolyl-(6-methoxyamidino-3-picolyl)amide
This compound was prepared starting from N-(tert-butoxycarbonyl=
methylene)-(Boc)-(D)-cyclohexylalanyl-3,4-dehydroprolyl-(6-
methoxyamidino-3-picolyl)amide (see Ex. lOc).
The removal of the protective groups and the transesterification/
esterification of the carboxyl function in
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr-NH-3-(6-C=NH(NHOCHg)) pico was
achieved by treating with a 4N solution of hydrogen chloride in
dioxane and a large excess of ethylene glycol monomethyl ether.
The work-up and purification of the compound obtained was carried
out analogously to Ex. 11.
FAB-MS (M+H+): 545
Example 39:
N-(n-Octyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-methoxyamidino-3-picolyl)amide
This compound was prepared starting from N-(tert-butoxycarbonyl-
methylene)-(Boc)-(D)-cyclohexylalanyl-3,4-dehydroprolyl-
(6~nethoxyamidino-3-picolyl)amide (see Ex. lOc).
The removal of the protective groups and the transesterification/
esterification of the carboxyl-function in
t-BuOyC-CH2-(Hoc)-(D)-Cha-Pyr-NH-3-(6-C=NH(NHOCH;))-pico was
achieved by treating with a 4N solution of hydrogen chloride in
dioxane and a large excess of n-octanol. The work-up and
purification of the compound obtained was carried out analogously
to Ex. 11.
FAB-MS (M+H+): 599
CA 02368830 2001-09-26
a
BASF' Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
51
Example 40:
N-(c-Hexyloxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-methoxyamidino-3-picolyl)amide
This compound was prepared starting from N-(tert-butoxycarbonyl-
methylene)-(Boc)-(D)-cyclohexylalanyl-3,4-dehydroprolyl-(6-
methoxyamidino-3-picolyl)amide (see Ex. lOc).
The removal of the protective groups and the
transesterification/esterification of the carboxyl function in
t Bu02C-CHZ-(Boc)-(D)-Cha-Pyr-NH-3-(6-C=NH(NHOCH3))-pico was
achieved by treating with a 4N solution of hydrogen chloride in
. dioxane and a large excess of cyclohexanol. The work-up and
purification of the compound obtained was carried out analogously
to Ex. 11.
FAB-MS (M+H+): 569
Example 41:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-allyloxyamidino-3-picolyl)amide
a) N-(tert-butoxycarbonylmethylene)-(Hoc)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-aminothiocarbonyl--3-picolyl)amide
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr-NH-3-(6-CN)-pico (WO 96/25426,
Ex. 93, stage b) was reacted with hydrogen sulfide in
pyridine /triethylamine to give the corresponding thioamide
t Bu02C-CH2-(Boc)-(D)-Cha-Pyr NH-3-(6-CSNHy) pico according to
WO 96/25426, Ex. 93, Stage c).
b) N-(tert-Butoxycarbonylmethylene)-(Boc)-(D)--cyclohexylalanyl-
3,4-dehydroprolyl-(6-S-methyliminothiocarbonyl-3-picolyl)-
amide hydroiodide
The product t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr-NH-3-(6-CSNH2)-pico
obtained from a) was reacted with methyl iodide to give
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr NH-3-(6-C=NH(SCH3))-pico x HI
analogously to WO 96/25426, Ex. 93, Stage d).
c) N-(tert Hutoxycarbonylmethylene)-(Boc)-(D)-cyclohexylalanyl-
3,4-dehydroprolyl-(6-allyloxyamidino-3-picolyl)amide
O-Allylhydroxylamine hydrochloride (0.93 g, 7.0 mmol) were
dissolved in 20 ml of methanol and converted into the
corresponding acetic acid salt by means of an ion exchanger
.(Fluka: acetate on polymeric support, 3.0 mmol of acetate per
g). t-Bu02C-CHZ-(Boc)-(D)-Cha-Pyr NH-3-(6-C=NH(SCH3))-pico x
HI (4.5 g, 5.8 mmol; see b) was added to this methanolic
solution and the reaction mixture was stirred at room
CA 02368830 2001-09-26
BAS1~ Aktieagesellschaft 20000104. O.Z. 0050/51174 DE
52
,temperature overnight. After concentrating in vacuo, the
residue was taken up in ethyl acetate, washed three times
with 30 ml each of water, twice with 20 ml each of 20%
strength sodium hydrogensulfate solution and once with 30 ml
of saturated sodium chloride solution and then purified. by
column chromatography on silica gel, 2.1 g of the desired
product being isolated.
d) N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-(6-allyloxyamidino-3-picolyl)amide
The product t-Bu02C-CHZ-(Boc)-(D)-Cha-Pyr-NH-
3-(6-C=NH(NHO-allyl))~ico (2.1 g, 3.1 mmol) obtained as in
c) was dissolved in 5 ml of absolute dioxane, cooled to 0°C
and treated with 5 ml of a 4N solution of hydrogen chloride
in dioxane. The mixture was stirred at room temperature for
6 h with exclusion of moisture and concentrated, then the
residue was dissolved in water, the solution was subjected to
salt exchange by means of an acetate exchanger and the
aqueous phase was freeze dried. 0.69 g of the title compound
was obtained as a white powder.
FAB-MS (M+H+): 513
Example 42:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-(6-benzyloxyamidino-3-picolyl)amide
This compound was prepared analogously to Example 41, the product
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr NH-3-(6-C=NH(SCHg))-pico x HI
obtained in 41c) being reacted with O-benzylhydroxylamine
(corresponding to Ex. 40 subjected to salt exchange from the
hydrochloride to the acetate) at 35°C in the course of 30 min.
Work-up was carried out analogously to Ex. 41. As the
purification by column chromatography by means of MPLC (silica
gel) using the eluent ethyl acetate/cyclohexnae = 1:1 failed, a
second purification was carried out by means of MPLC (eluent
ethyl acetate/cyclohexane = 3:7). 1.5 g of the title compound
were obtained as a white powder. The removal of the Boc
protective group and the hydrolysis of the tert-butyl ester were
carried out using a solution of hydrogen chloride in diethyl
ether.
FAB-MS (M+H+): 563
CA 02368830 2001-09-26
BASF Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
53
Example 43:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-[6-(m-methoxy-benzyloxy)amidino-3-picolyl]amide
This compound was prepared analogously to Example 41, the product
t Bu02C-CHZ-(Boc)-(D)-Ch~Pyr~iH-3-(6-C=NH(SCHg))~ico x HI
obtained in 41c) being reacted with 0-(m-methoxybenzyl)-
hydroxylamine (corresponding to Ex. 40 subjected to salt exchange
from the hydrochloride to the acetate) at 35°C in the course of 30
min. Work-up was carried out analogously to Ex. 41. As the
purification by column chromatography by means of MPLC (silica
gel) using the eluent ethyl acetate/cyclohexnae = 1:1 failed, a
second purification was carried out by means of MPLC (eluent
ethylacetate/cyclohexane = 3:7). 1.5 g of the title compound were
obtained as a white powder. The removal of the Boc protective
group and the hydrolysis of the tert-butyl ester were carried out
using a solution of hydrogen chloride in diethyl ether.
FAB-MS (M+H+); 563
Example 44:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-[6-(4-chlorophenyl)hexyloxy)amidino-3-picolyl]-
amide
This compound was prepared analogously to Example 41, the product
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr NH-3-(6-C=NH(SCH3)) pico x HI
obtained in 41 c) being reacted with O-[6-(4-chlorophenyl)hexyl]-
hydroxylamine at 20°C in the course of 10 hours. The removal of
the~Boc protective group and the hydrolysis of the tert-butyl
ester were carried out using a 4N solution of hydrogen chloride
in dioxane.
FAB-MS (M+H+); 667
Example 45:
N-(Ethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro
prolyl-[6-(4-chlorophenyl)hexyloxy)amidino-3-picolyl]amide
This compound was prepared analogously to Example 11 by
esterification of N-(hydroxycarbonylmethylene)-(D)-cyclohexyl-
alanyl-3,4-dehydroprolyl-[6-(4-chlorophenyl)hexyloxyj-amidino-3-
picolyl]amide (Ex. 44) with ethanol in a 4N solution of hydrogen
chloride in dioxane.
FAB-MS (M+H+): 695
CA 02368830 2001-09-26
BASF Aktiengesellschaft 20000104 O.Z. 0050/51174 DE
54
Example 46:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-[6-(p-methyl-benzyloxy)amidino-3-picolyl]amide
This compound was prepared analogously to Example 43, the product
t-HuOzC-CH2-(Boc)-(D)-Cha-Pyr NH-3-(6-C=NH(SCH3)) pico x HI
obtained in 41 c) being reacted with O-(p-methylbenzyl)-
hydroxylamine. The removal of the Boc protective group and the
hydrolysis of the tert-butyl ester were carried out using a 4N
solution of hydrogen chloride in dioxane.
FAB-MS (M+H+): 577
Example 47:
N-(Ethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-[6-(p-methylbenzyloxy)amidino-3-picolyl]arnide
This compound was prepared analogously to Example 11 by
esterification of 1~(hydroxycarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-[6-(p-methylbenzyloxy)amidino-
3-picolyl]amide (Ex. 46) with ethanol in a 4N solution of
hydrogen chloride in dioxane.
FAB-MS (M+H+): 605
Example 48:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-dehydro-
prolyl-[6-phenyloxyamidino-3-picolyl]amide
This compound was prepared analogously to Example 43, the product
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr-NH-3-(6-C=NH(SCH3))-pico x HI
obtained in 41c) being reacted with O-phenylhydroxylamine
hydrochloride in the presence of two equivalents of
diisopropylethylamine. The removal of the Boc protective group
and hydrolysis of the tert-butyl ester was carried out using~a 4N
solution of hydrogen chloride in dioxane.
FAB-MS (M+H+): 549
Example 49:
N-(Ethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-[6-phenyloxyamidino-3-picolyl]amide
This compound was prepared analogously to Example 11 by
esterification of N-(hydroxycarbonylmethylene)-(D)-
cyclohexylalanyl-3,4-dehydroprolyl-[6-phenyloxyamidino-3-
pico].yl]amide (Ex. 48) with ethanol in a 4N solution of hydrogen
chloride in dioxane.
FAB-MS (M+H+): 577
CA 02368830 2001-09-26
~~, BASF' Aktieagesellschaft 20000104 O.Z. 0050/51174 DE
Example 50:
N-(Hydroxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-[6-isopentyloxyainidino-3-picolyl)amide
5 This Eompound was prepared analogously to Example 43, the product
t-Bu02C-CH2-(Boc)-(D)-Cha-Pyr-NH-3-(6-C=NH(SCH3))-pico x HI
obtained in 41c) being reacted with 0-isopentylhydroxylamine
hydrochloride in the presence of six equivalents of
diisopropylethylamine. The removal of the Boc protective group
10 and the hydrolysis of the tert-butyl ester was carried out using
a solution of hydrogen chloride in diethyl ether.
FAB-MS (M+H+): 543
Example 51:
15 N-(Ethoxycarbonylmethylene)-(D)-cyclohexylalanyl-3,4-
dehydroprolyl-[6-isopentylvxyamidino-3-picolyl]amide
This compound was prepared analogously to Example 11 by
esterification of N-(hydroxycarbonylmethylene)-(D)-
20 cyclohexylalanyl-3,4-dehydroprolyl-[6-isopentyloxyamidino-3-
picolyl)-amide (Ex. 50) with ethanol in a 4N solution of hydrogen
chloride in dioxane.
FAB-MS (M+H+): 571
30
40
CA 02368830 2001-09-26