Note: Descriptions are shown in the official language in which they were submitted.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
FAECAL COLLECTOR WITH A RELIABLE, COMFORTABLE FLANGE
10
Field of the invention
The present invention relates to human waste management devices for babies,
children or adults. Said devices are provided with a flange to allow for safe
attachment, comfortable wearing and low skin exposure.
Back4round of the invention
Human waste management devices are known articles of manufacture that are
designed to be worn principally by incontinence sufferers and in particular by
bedridden patients. Such human waste management devices are attached to the
perianal or uro-genital region of the wearer and are intended to entrap and
immediately contain faecal material, urine and other bodily discharges. Such
devices, as they are mostly known today are constituted of a bag at one
extremity of which is positioned the aperture and the attachment device, which
typically is adhesive.
Faecal management devices are disclosed in for example the following
documents: US 3,577,989, which details a disposable plastic bag for
incontinence sufferers. US 4,784,656, which describes a receptacle for
collecting
faecal matter. The receptacle is formed from two sheets of thermoplastic film
that
are heat sealed along their side edges. GB 2 152 387, which teaches a faecal
collector for incontinence sufferers comprising a collection bag consisting of
a
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
2
pair of panels of thermoplastic sheet material joined at their margins. EP 245
064
discloses bags substantially consisting of a front and a rear wall, which are
made
of a synthetic plastic material, such as PVC. GB 2 215 605 discloses an
ostonomy bag comprising panels of synthetic plastic material, the rear bag
wall
further comprising a needled film.
Urine management devices are, for instance, disclosed in the following
documents: GB 1 092 274 discloses a pediatric urine collector for female use
comprising a collector bag of plastic material opening. The collector is
secured to
the body of the wear by adhesive material. GB 2 268 882 discloses a urostomy
pouch/bag of plastic material provided with a circular stomal orifice which is
surrounded by a first coupling member, by which the pouch can be affixed to a
counterpart coupling member, which can be attached to a wearer. US 4,804,377
discloses a collector for urine specimens from children. The collector
comprises
a rectangular flange for adhesive attachment. EP 140 478 discloses a
disposable diaper having a water proof barrier preferably polypropylene or
polyethylene formed as a flattened bag having a single opening. US 1,092,274
and US 3,292,626 disclose a urine collector for female infants. Chinese patent
application CN 1079381 discloses urine bags for infants with circular and
elliptical apertures.
A problem naturally associated with these devices is their secure attachment
to
the human body whilst allowing for the faecal matter or urine to be safely
contained within the bag. The approach which is mostly used in the field and
described in most of the above cited documents is to provide the device with a
flange which surrounds an aperture and provide adhesive on the flange, which
will stick to the perianal or uro-genital area. Further considerations
regarding the
selection of the flange and the adhesive are disclosed in the following
documents:
US 3,734,096 teaches to provide the flange from a compressable and flexible
material, such as a plastic sheet of about 0.4 mm thickness. The flexibility
is
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
3
portrayed as to contribute to allow the flange to follow the movement of the
sphincter muscle during defaecation. The thickness of the adhesive means is
said to be of little consequence.
EP 0 753 290 A2 teaches to provide the flange from a heat-sealable,
stretchable
material, such as a film or a non-woven fabric. For attachment of the flange
to
the human body a hydrocolloid adhesive is disclosed. As known in the art, such
hydrocolloid layers are typically thick, having a caliper of about 1 mm or
above.
Specified in one example is a non-woven material with a caliper of 0.33 mm and
a hydrocolloid layer with a caliper of 1.02 mm.
WO 99/00089 discloses numerous materials for the flange, the preferable choice
being an open celled polyurethane foam. The thickness of the adhesive layer to
be used for attachement is not disclosed.
Despite the above considerations in selecting the flange and adhesive, the
problem of unintentional detachment of faecal management devices is a problem
known in the art, as disclosed for example in GB 2 116 849.
Thus, considering the possible unintended detachment and the handling after
use, a small aperture is desirable. Moreover, a small aperture prevents faecal
matter from coming into contact with large areas of the wearer's skin. This
reduces skin irritation problems, which may occur due to the age of typical
wearers of such devices, whether very old or very young. On the other hand,
the
aperture should be large enough as to allow complete collection of faecal
material, even in case of non ideal placement. This is, for example, addressed
in
WO 99/00089.
Hence, there still exists a need for a human waste management device which
ensures reliable adhesive attachment, which can be easily be placed and
ensures low skin exposure.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
4
In attempting to overcome all the aforementioned problems relating to the
prior
art, it has now been found that adhesive human waste management devices can
be provided with particularly selected flange characteristics.
Summary of the invention
The present invention relates to human waste management devices for babies,
children or adults. The invention resides principally in providing such
devices with
a flange (12) which is comfortable to wear and ensures safe attachment to the
body. The overall caliper of the flange (12) and the adhesive layer (20)
according
to the present invention is less than 1.3 mm while the transversal diameter of
the
aperture (21 ) is less than 80 mm.
Brief descrietion of the drawinas
It is believed that the invention will be better understood from the foregoing
description in conjunction with the accompanying drawings in which:
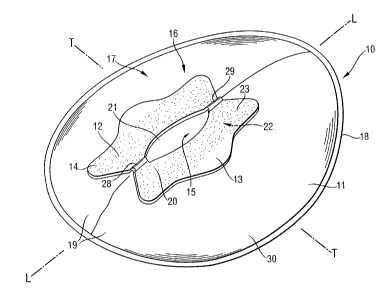
Figure 1 is a perspective view of a preferred embodiment of a faecal
management device according to the present invention.
Figure 2 is a cross sectional view along line T-T of the preferred embodiment
depicted in Figure 1. c denotes the overall caliper of the flange (12) and the
adhesive layer (20).
Figure 3 is an on top view onto the flange of a faecal management device
according to the present invention. d denotes the transversal diameter of the
aperture (21 ).
Figure 4 is a schematic representation of a cast of the anal region.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
Detailed description of the invention
The invention relates to human waste management devices. Such a human
waste management device may be a faecal management device (10), which is
designed for attachment to the anal area and mainly used for collecting
faeces,
5 or it may be a urine management device, which is attached to the urinary
duct
and mainly used for collecting urine. A human waste management device may
also be a device to collect both urine and faeces and thus be attached to both
of
the above areas. All of the above human waste management devices are
preferably designed for single use and disposal thereafter.
A faecal management device (10) is shown in Figure 1.
Description of the human waste management device as a whole
Typically human waste management devices comprise a bag (11 ) having an
aperture (21 ) and a flange (12) surrounding the aperture for preferably
adhesive
attachment to the perianal area of a wearer as visible from Figure 1. Any
human
waste management device known in the art can be provided according to the
present invention.
The bag (11 ) as used herein is a flexible receptacle for the containment of
excreted faecal matter or urine. The bag (11 ) is designed to safely contain
any
entrapped material, typically it will be liquid impermeable, yet it may be
breathable. The bag (11 ) is designed of sufficient strength to withstand
rupture in
use, also when pressure on the bag (11 ) is exerted in typical wearing
conditions,
such as sitting.
According to the present invention the bag material can comprise one or
multiple
layers, preferably two or three layers. The layer on the inside of the bag (11
),
which will typically at least partially come in contact with faecal material
or urine
is called the inner layer. The outermost layer of the bag, which will
typically at
least partially come in contact with the skin to the wearer and the garments
of
the wearer, is called the outer layer.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
6
The layers of the bag material may comprise any material, preferably so that
the
bag is liquid impervious. The layers may in particular comprise any material
such
as non-wovens or films. In a preferred embodiment of the present invention a
laminate may be formed from a non-woven layer and a film. The laminate can be
formed by means known to the man skilled in the art.
Any non-woven layer can comprise felt fabrics, spunlaced fabrics, fluid jet
entangled fabrics, air-laid fabrics, wet-laid fabrics, dry-laid fabrics, melt-
blown
fabrics, staple fibre carding fabrics, spunbonded fabrics, stitch-bonded
fabrics,
apertured fabrics, combinations of the above or the like.
Suitable film materials for any of said layers preferably comprise a
thermoplastic
material. The thermoplastic material can be selected from among all types of
hot-melt adhesives, polyolefins especially polyethylene, polypropylene,
amorphous polyolefins, and the like; material containing meltable components
comprising fibres or polymeric binders including natural fibres such as
cellulose -
wood pulp, cotton, jute, hemp; synthetic fibres such as fibreglass, rayon,
polyester, polyolefin, acrylic, polyamid, aramid, polytetrafluroethylene
metal,
polyimide; binders such as bicomponent high melt/low melt polymer, copolymer
polyester, polyvinyl chloride, polyvinyl acetate/chloride copolymer, copolymer
polyamide, materials comprising blends wherein some of the constituent
materials are not meltable; air and vapour permeable materials including
microporous films such as those supplied by EXXON Chemical Co., III, US
under the designation EXXAIRE or those supplied by Mitsui Toatsu Co., Japan
under the designation ESPOIR NO; and monolithic breathable materials such as
HytreIT"" available from DuPont and PebaxT"' available from ELF Atochem,
France.
In a preferred embodiment a film, which is comprised in any layer, is
preferably
permeable to gases such as air and to vapour such as water vapour in order to
avoid the problem of entrapment and condensation of moisture vapour given off
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
7
by the body of the wearer and thus, the hot, clammy and uncomfortable
conditions after a short period of use.
The outer layer of the bag material may comprise a non-woven layer. Such
material layers present an uneven surface to the skin of the wearer and thus
reduce significantly the problem of occlusion and greatly improve skin
healthiness.
In one preferred embodiment of the present invention the bag material
comprises two layers. Preferably the outer layer comprises a non-woven layer
and the inner layer comprises a film.
In yet another preferred embodiment of the present invention, the bag material
comprises three layers, preferably one film and two non-woven layers. In an
even more preferable embodiment the film is interposed between the two non-
woven layers. This sequence of layers results in a closed fibrous structure,
which has a particularly pleasing sensation on contact with the skin of the
wearer. In yet another preferred embodiment the inner layer comprises a film
and the other two layers comprise non-wovens.
Any non-woven layer or the non-woven layers comprised by the bag material
may be hydrophobic or hydrophilic. If the bag material does not comprise a
film
layer, preferably at least one non-woven layer is hydrophobic. As a
consequence, fluid penetration is resisted through the bag (11 ) of the human
waste management device (10). If the bag material comprises a film or a
hydrophobic non-woven layer, further non-woven layers may be hydrophilic.
Typically, the non-woven layer is treated with a surface active material, such
as
a fluorchemical or other hydrophobic finishings, to provide the requisite
hydrophobicity. The non-woven layer, however, may equally be treated with
coatings of liquid impervious materials such as hot-melt adhesives or coatings
of
silicone or other hydrophobic compounds such as rubbers and vegetable and
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
8
mineral waxes or it may be physically treated using nano-particulates or
plasma
coating techniques, for example.
Any non-woven layer can also be treated with agents to improve the tactile
perceivable softness of the bag (11 ). The agents include but are not limited
to
vegetable, animal or synthetic oils, silicone oils and the like. The presence
of
these agents are known to impart a silky or flannel-like feel to the non-woven
layer without rendering it greasy or oily to the tactile sense of the wearer.
Additionally, surfactant material, including anionic, nonionic, cationic and
amphoteric surfactants, may be added to further enhance softness and surface
smoothness.
Furthermore, the non-woven layer may be impregnated with a lotion to provide
desirable therapeutic or protective coating lotion benefits. The lotion
coating on
the bag (11 ) is transferable to the skin of the wearer by normal contact and
wearer motion and/or body heat. Generally, mineral oil in the form of a lotion
is
recognised as being effective in imparting a soothing, protective coating to
the
skin of the wearer. It is also possible to impregnate the non-woven layer with
a
solid oil phase of cream formulation or to incorporate into the non-woven
layer
an array of pressure- or thermal- or hydrorupturable capsules containing for
example, baby oil.
According to the present invention, depending on the shape of the bag (11 )
required, the bag (11 ) may be provided from a unitary piece of material or a
number of separate pieces of material, which may be identical or different and
which are sealed at their respective peripheries. The preferred shape of the
bag
depends in particular on the intended use thereof, i.e. whether the device is
intended for bedridden patients or active patients suffering from incontinence
or
requiring an artificial bowel or for infants.
The bags described herein preferably have a wearer facing portion (16) and a
garment facing portion (17), which both comprise separate pieces of material.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
9
The wearer facing portion (16) and the garment facing portion (17) are sealed
at
the periphery of the bag (11 ), thus creating a bag peripheral rim (18). The
wearer
facing portion (16) and the garment facing portion (17) may each independently
comprise more than one section of material. Preferably the garment facing
portion (17) comprises only one section of material; most preferably also the
wearer facing portion (16) comprises only one section of material.
The wearer facing portion (16), the garment facing portion (17) and the pieces
of
material comprised by either of these portions are secured to each other by
means known to the man skilled in the art, such as adhesive, thermobonding or
pressure bonding in order to provide the desired bag configuration. The rim
(18),
at which the wearer facing portion (16) and the garment facing portion (17)
are
sealed together, may be provided inside the bag (11 ) rather than outside the
bag
(11 ), thus being coextensive with the inner surface (15) of the bag (11 )
rather
than with the outer surface (30) of the bag (11 ).
Hence a variety of shapes of the bag is within the scope of the present
invention.
Particularly, preferred shapes are flat circular type bags, cone shaped bags,
truncated cone shaped bags and pyramidal or truncated pyramidal shaped bags
and flat T shaped bags.
In one embodiment of the present invention the bag (11 ) may contain absorbent
material. The absorbent material may comprise any absorbent material which is
capable of absorbing and retaining liquids. The absorbent material may
comprise a wide variety of liquid-absorbent materials commonly used in
disposable diapers and other absorbent articles such as comminuted wood pulp,
which is generally referred to as airfelt. Examples of other suitable
absorbent
materials include creped cellulose wadding; meltblown polymers, including
coform; chemically stiffened, modified or cross-linked cellulosic fibers;
tissue,
including tissue wraps and tissue laminates; absorbent foams; absorbent
sponges; superabsorbent polymers; absorbent gelling materials; or any other
known absorbent material or combinations of materials.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
The absorbent material may be positioned in the bag (11 ) in any suitable
manner. For example, the absorbent material may be loosely arranged within the
bag or may be secured to the inner surface (15) of the bag (11 ). Any known
5 techniques for securing absorbent material to nonwoven and film substrates
may
be used to secure the absorbent material to the inner surface (15) of the bag.
The absorbent material may also be arranged to have any desired shape or
configuration (e.g., rectangular, oval, circular, etc.).
10 The flange (12) is attached to the bag (11 ) according to any means known
to the
man skilled in the art which may provide permanent or releasable attachment.
Preferably however, the flange is attached to the bag by adhesive. Typically,
the
bag will be attached to the flange, towards the outer periphery of flange so
as
not to cause any obstruction for the entering faecal matter or urine.
The flange may be provided in any size depending on the wearer group for
which the device is intended. Similarly the flange may be provided in any
shape
and preferably has a symmetrical shape preferably comprising a plurality of
lobes (13).
The flange comprises a garment facing portion (22) and a wearer facing portion
(23). In an preferred embodiment these are two large, substantially flat
surfaces,
however, the flange (12) may also comprise projections, a front projection
(28)
and/or a rear projection (29), in case of a faecal management device (10)
designed to fit the perineal and/or coccygeal area of the wearer and in case
of a
urine management device (10) designed to fit the genital and/or perineal area.
If
a human waste management device is to be used by a female wearer the
presence of a projection to fit the perineum has been found very beneficial.
According to the present invention the human waste management device (10)
further comprises an attachment means to secure the device to the wearer.
Such means comprise a body-compatible pressure sensitive adhesive provided
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
11
as an adhesive layer (20) applied to the wearer facing portion (23) of the
flange
(12) and may also comprise straps.
According to the present invention any medically approved water resistant
pressure sensitive adhesive may be used to attach the device to the perianal
or
uro-genital area of the wearer, such as hydrocolloid adhesives and hydrogel
adhesives. Particularly effective adhesives in providing the desired adhesive
properties to secure the flange to the skin of the wearer at the sensitive
perianal
area, whilst allowing for relatively painless application and removal, are
formed
from crosslinking polymers with a plastisicer to form a 3-dimensional matrix.
The adhesive layer (20) can be applied to the wearer facing portion (23) of
the
flange (12) by any means known in the art such as slot coating, spiral, or
bead
application or printing. Typically the adhesive layer (20) is applied at a
basis
weight of from 20g/m2 to 2500g/mz, more preferably from 100 g/m2 to 1000g/m2
depending on the end use envisioned and the adhesive caliper desired. For
example, for human waste management devices (10) to be used for babies the
amount of adhesive may be less than for human waste management devices
(10) designed for active adult incontinence sufferers.
The adhesive layer (20) is preferably covered with a release means (not shown)
in order to protect the adhesive layer (20), such as siliconized paper. The
adhesive (20) can cover the entire wearer facing portion (23) of the flange
(12),
more preferably the flange (12) has at least one, preferably two to six non-
adhesive portions. These portions may be adhesive free or may contain
inactivated or covered adhesives. As is evident from Figure 1, the adhesive is
in
one preferred embodiment not applied to the entire wearer facing portion (23)
of
the flange (12), so as to provide lobes (13) on either side of the flange (12)
which are non-adhesive and can thereby serve to facilitate placement and
removal of the device whilst avoiding contact with the adhesive. These lobes
(13) are however preferably also covered by the release means. Before
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
12
application of the human waste management device (10) to the skin of the
wearer, the release means if present is removed.
The human waste management device (10) of the present invention has been
found to be particularly useful and beneficial when used in conjunction with a
garment, or diaper, preferably a disposable diaper.
To allow a more detailed and clear description of the device (10), in the
following
paragraphs firstly a few terms will be defined, as used herein.
Regarding in particular the flange (12) the longitudinal axis for a faecal
management device (10) is to be understood as follows: The direction which is
substantially defined by the anal groove in the intended wearing position
shall
define the longitudinal direction. The longitudinal axis is an axis in the
longitudinal direction, which crosses the centre of the aperture (21 ). The
most
preferred indication of the intended wearing position is the presence of one
or
two projections (28) and/or (29) designed to fit the perineal and/or coccygeal
area of the wearer, a less preferred indication of the intended wearing
position is
a fold in said flange (12) prior to use intended to be placed in parallel to
the anal
groove when placing the product. The longitudinal axis is typically also an
axis of
symmetry. Regarding in particular the flange (12) the longitudinal axis for a
urine
management device is to be understood as follows: The direction which is
substantially defined by a line connecting the coccyx, the perineum and the
genital area for a wearer with the urine management device (10) in the
intended
wearing position defines the longitudinal direction. The longitudinal axis is
an
axis in the longitudinal direction, which crosses the centre of the aperture
(21 ).
The longitudinal axis is typically also an axis of symmetry.
The transversal axis is an axis perpendicular to said longitudinal axis, which
crosses the centre of the aperture (21 ). The transversal direction, which is
defined by the transversal axis, is generally aligned with the wearer's hips.
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
13
Unbent is used with regard to the flange (12). The flange (12) is typically
bent
along a longitudinal axis to place it onto the perianal area of the wearer. In
an
unbent state the flange (12) is typically flattest.
Centre is used to describe a point of an object or a part of an object, which
coincides with the centre of mass, if said object or part were of uniform
density.
Thus for the aperture (21 ), the centre is to be determined when the area
within
the contour of the aperture (21 ) is considered to be filled with a material
of
uniform thickness and density, when the flange (12) is unbent.
A diameter of the aperture (21 ) is the length of a line through the centre of
the
aperture (21 ), whose ends lie on the inner periphery (25) of the aperture (21
),
when the flange is unbent. The diameter of a flexible aperture (21 ) has to be
measured when no outer forces are present which could affect the shape of the
aperture (21 ) (apart from normally unavoidable forces such as gravity). The
longitudinal diameter of the aperture (21 ) is measured along the longitudinal
axis. The transversal diameter of the aperture (21 ) is measured along the
transversal axis.
While the present invention comprises urine management devices as well as
faecal management devices the following description focuses on faecal
management devices. However, the person skilled in the art has no difficulties
to
adapt a urine management device to the teachings of the present invention.
It has been found that certain flanges greatly improve wearing comfort and
safe
attachment while minimising skin exposure.
Flanges (12) according to the present invention can be provided from numerous
materials which are soft, flexible, pliable and malleable. Moreover, the
material is
preferably elastic (inter alia to follow movements of the sphincter muscle),
stretchable, contractible and breathable. Even more preferred materials allow
to
CA 02369171 2001-10-12
WO 00/61039 PCT/iJS99/07908
14
securely embed a hydrogel adhesive, for example by having a three-dimensional
microstructure in the surface region. A suitable material is a plastic film.
Such a
plastic film may be provided with a layer of pressure sensitive adhesive to
which
a hydrogel adhesive will more firmly bond. Another preferred material is a
laminate of a plastic film and a non-woven. Other preferred materials include
wovens, open celled thermoplastic foams, closed-cell thermoplastic foams,
composites of open celled foams and stretch nonwoven, and films. Preferably,
such foams have a basis weight of 5 to 250 g/m2, more preferably 50 g/m2.
Other
thermoplastic foam materials, or other suitable plastics sheet materials
having
the described properties might also be used. Highly preferred materials are
non-
woven materials, e.g. spun-bonded or melt-blown non-wovens. The materials
preferably have a basis weight from 15g/m2 to 30g/mz.
The caliper of such material providing the flange (12) is preferably from 0.01
mm
to 1.25 mm, more preferably from 0.05 mm to 0.5 mm, yet more preferably from
0.1 mm to 0.3 mm.
Flanges according to the present invention comprises an adhesive for
attachment to the human body. The adhesive is deposited on the wearer facing
portion (23) of the flange (12) as to form an adhesive layer (20). Preferably
the
adhesive layer (20) is a continuous layer. In an alternative embodiment the
adhesive layer (20) is deposited in a discontinuous pattern, e.g. in a dotted
pattern. Preferably the caliper of the adhesive layer (20) is uniform. If the
caliper
of the adhesive layer (20) is non uniform, the average caliper of the adhesive
layer (20) is herein referred to as the caliper of the adhesive layer (20).
The
adhesive layer (20) should also be thin, preferably having a caliper from 0.01
mm to 1.25 mm, more preferably from 0.5 mm to 1.1 mm, most preferably from
0.8 mm to 1.0 mm.
According to the present invention the overall caliper of the flange (12) is
not
more than 1.3 mm, preferably from 0.1 mm to 1.3 mm , more preferably from 0.9
mm to 1.25 mm, yet more preferably from 1.0 mm to 1.2 mm. The term overall
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
caliper, as used herein, denotes the caliper of the flange (12) and the
adhesive
layer. If the caliper is not uniform, the term overall caliper, as used
herein,
denotes the average overall caliper. For the particular embodiment of a faecal
management device (10) of Figure 2 the overall caliper is marked as c.
5
The bag (11 ) is provided with an aperture (21 ) whereby faecal matter or
urine is
received from the body prior to storage within the bag cavity. The aperture
(21 )
is surrounded by a flange (12) and may be provided in any shape or size, such
as circular, oblong, heart shaped and may be symmetrical or asymmetrical,
10 preferably the aperture has an oblong configuration either in the
longitudinal or
in the transversal direction. However, the transversal diameter of the
aperture
(21 ) must be less than 80 mm. Preferably, a flange (12) according to the
present
invention should have a transversal diameter from 5 to 60 mm, more preferably
from 30 mm to 55 mm, yet more preferably from 40 mm to 50 mm. For the
15 particular embodiment of a faecal management device (10) of Figure 3 the
transversal diameter is marked as d.
The thin overall caliper of the flange (12) in combination with a small
transversal
diameter of the aperture (21 ) ensures that the faecal material is transferred
into
the human waste management device close to the anal opening. The anal
groove tightly surrounds the anal opening towards the buttocks essentially in
a
V-shape. In order to collect faecal material close to the anal opening, only a
thin
flange can be positioned close to the anal opening, since the buttocks would
otherwise need to be spread apart in a way, which is both inconvenient for the
wearer and difficult to achieve for a caretaker placing such a device.
This interdependence of flange (12) caliper, aperture (21 ) transversal
diameter
and close to source human waste collection is further illustrated by the
following
experimental data relating to a faecal management device (10) for a male or
female baby. The data can be best understood from the schematic
representation of the experiments given in Figure 4: Represented is a cast
(220)
of the anal region taken from a sitting baby boy, adjacent to the anal opening
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
16
(210). Faecal matter excreted by a wearer will have a typical diameter,
represented as d, of about 2 cm for example for a baby.
Attachment of the flange of a faecal management device (10) can only be so
close to the anal opening (210) so as not to obstruct faecal matter which is
to be
entrapped in the bag (11 ) of the faecal management device (10). The flange
caliper contributes to the obstruction of faecal matter. Hence, an ideal
theoretical
flange having an overall caliper of 0 mm could be closest to the anal opening
(210). The ideal distance is represented in Figure 4 as x, corresponding to a
transversal flange diameter of about 2x. A thin flange (230) in accordance
with
the present invention, due to its non-vanishing caliper cannot be worn so
close
to the anal opening (210), the distance to the anal opening (210) being y > x,
corresponding to a transversal flange diameter of about 2y. A conventional
thick
flange (240) can be worn only at a distance to the anal opening of z > y,
corresponding to an also greater transversal flange diameter of about 2z.
Typically for a baby x has a value of approximately 25 mm, y of 28 mm and z of
33 mm.
Conventional flanges have an overall caliper which does not allow for
positioning
close to the anal opening and hence require a larger aperture. This leads to
an
unnecessary and undesirable area of exposes skin, i.e. all the skin within the
area of the aperture. Such skin exposure favours skin irritations. Moreover,
it has
been found that less skin exposure reduces the need for frequent changes of
human waste management devices, thus giving a considerable convenience,
economical and ecological benefit. It has further been found that less skin
cleaning is required after the detachment of the device, which largely
improves
the convenience of such devices for the caretaker, such as a baby's mother.
In another aspect, the present invention allows the safer attachment by
providing
a larger flange (12) surface area. The flange (12) surface area available for
adhesive attachment of the device (10) is determined by the area within the
aperture (21 ) and the area within the outer periphery of the flange (12).
With
CA 02369171 2001-10-12
WO 00/61039 PCT/US99/07908
17
regard to the outer periphery, the flange (12) can have only a limited size as
determined by the anatomy of the intended wearers and comfort considerations.
Namely for babies only small flanges (12) are suitable. For a given outer
periphery of the flange (12) the most surface area is available for an
adhesive
(20), when the area within the aperture (21 ) is small. A small transversal
diameter of the aperture (21 ) contributes to such a small area within the
aperture
(21 ).
In yet another aspect, a small transversal diameter of the aperture (21 )
lessens
the risk of leakage after the detachment of the device and allows for easier
closing and sealing of the aperture (21 ).
Moreover, in the defaecation process pressure onto builds up at the inner
periphery (25) of the flange (12), namely at the edges of the flange. The edge
area of the flange (12) which is subjected to such pressure depends on the
overall caliper of the flange (12) as visible from Figure 4. Hence, a thin
flange
(12) reduces the risk of detachment in comparison with a thick flange (12).