Note: Descriptions are shown in the official language in which they were submitted.
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
PULMONARY ADMINISTRATION OF DRY POWDER FORMULATIONS
FOR TREATING INFERTILITY
This application claims the benefit of priority of U.S. Provisional Patent
Application Serial No. 60/129,121 filed April 13, 1999 and Serial No.
60/130,099,
filed April 20, 1999, both of which are incorporated in their entirety by
reference.
Field of the Invention
The present invention is directed to pulmonary administration of stable, dry
powder compositions comprising a follicle stimulating protein (FSP) and
suitable for
delivery to the deep lung, to methods for preparing and administering such
compositions, and to methods for treating infertility.
Background of the Invention
Follicle stimulating hormone (FSH) is a heterodimeric gonadotropic hormone
secreted by the pituitary gland, and is one of three anterior pituitary
glycoprotein
hormones including, in addition to FSH, thyroid-stimulating hormone (TSH) and
luteinizing hormone (LH). FSH consists of two non-covalently bound subunits
referred
to as the alpha (a.) and beta ((3) subunits. The alpha subunit is the same
amongst the
three hormones, while the beta subunit is unique to each hormone and confers
specificity. In humans, the mature alpha subunit consists of 92 amino acid
residues and
possesses two carbohydrate chains. The corresponding FSH mature beta subunit
is
composed of 111 amino acids and also possesses two carbohydrate chains.
Together,
the two subunits have a molecular weight of about 31 kD measured by mass
spectroscopy and about 35-45 kD measured by PAGE or gel chromatography
depending upon the state of glycosylation. FSH directly regulates the
metabolic activity
of granulosa cells of the ovary and Sertoli cells of the testis.
Pharmaceutical preparations of follicle stimulating hormone (FSH) play an
important role in the treatment of human infertility, and administration of
FSH, either
alone or in combination with other biologically active compounds and proteins,
has
been employed for treating infertility problems since the early 1960s. In
females, FSH
promotes ovarian follicular development and pharmaceutical preparations of FSH
are
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
used primarily for ovulation induction and in in vitro fertilization
procedures. [Speroff,
L. Glass R.H.,et al., Clnical Gynecologic Endocrinology and Ir fer-tibilitv,
583-609
(1989); Jones, H.W., et al., Fertil. Ster-il, 38:14-21 (1982)]. In males, such
compositions are used to initiate and maintain spermatogenesis in
hypogonadotropic
hypogonadism [Witcomb, R.W., et al., J. Clin Endocrinol Metab, 70:3-7 (1990)].
In early infertility treatment methods, FSH-containing formulations were
administered via injection into deep muscle. Such injections were typically
given with
the aid of the patient's partner or healthcare provider and required the use
of a needle up
to five times the size of a typical subcutaneous needle and were very painful.
More
recently, formulations have been developed in which purified or recombinant
FSH is
administered subcutaneously, often by patient self administration. Although
subcutaneously administered FSH offers an advantage over intrasmuscularly
delivered
drug by allowing the patient greater independence through self administered
treatment,
many patients are reluctant or unwilling to undergo infertility treatments
requiring the
subcutaneous administration of FSH, due to the inconvenience, discomfort, or
even
inherent dislike associated with needle-based delivery methods.
Pulmonary delivery has received much attention as an attractive alternative to
subcutaneous injection, because this approach eliminates the necessity for
needles,
limits irritation to the skin and body mucosa (common side effects of
transdermally,
iontophoretically, and intranasally delivered drugs), and eliminates the need
for nasal
and skin penetration enhancers (typical components of intranasal and
transdermal
systems that often cause skin irritations/dermatitis). Pulmonary
administration is also
economically attractive, amenable to patient self administration, and is often
preferred
by patients over other alternative modes of administration. However, due to
their
high molecular weight and low lipophilicity, peptide or protein based drugs
have not
traditionally been among those drugs that are administered by inhalation for
deposition in and absorption from the lung, although various aerosol
formulations
have been suggested. Moreover, a previous attempt to administer FSH in dry
powder
form via intratracheal delivery resulted in apparently low bioavailability -
0.6 percent
relative to intravenous administration - suggesting the undesirability of the
pulmonary
route for delivering gonadotropin hormones such as FSH [Komada, F., et al.,
JPharnr
Sciences, 83, (6):863-867 (1994)].
Another often-encountered problem in formulating proteins for administration
is
their tendency towards inactivation. With the recent advent of more effective
2
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
purification and recombinant techniques, highly purified forms of both urinary-
derived
[Arpaia, G., et. al.~ U.S. Patent No. 5,128,453, July 7, 1992) and recombinant
[Loumaye, E., et al., Fertil. Steril., 63:77-86 (1995)] FSH have become
available,
making this problem even more pronounced. Although these highly purified forms
of
FSH offer many potential advantages over less purified forms, including
improved
batch-to-batch consistency, high specific activity due to the absence of
luteinizing
hormone and other competing proteins, improved efficacy, good local tolerance
to
injections, and low immunogenicity, preparations containing very pure FSH are
often
highly unstable. These compositions often degrade in a relatively short time,
with a
partial or even complete loss of bioactivity. Moreover, although excipients
have been
described which are capable of stabilizing FSH-containing solid formulations
[e.g.,
Samaritani, et al., U.S. Patent No. 5,650,390, July 22, 1997] for
reconstitution to
injectable forms, such solid formulations typically lack the features
necessary for
pulmonary delivery.
Thus, even with the amount of work that has been done to optimize pulmonary
delivery of proteins, there still does not exist an effective system and
method of
pulmonary delivery of FSH that (i) provides a sufficiently stabilized,
respirable, dry
powder form of FSH, (ii) eliminates the need for cold storage, (iii) provides
powders
having superior aerosol properties, (iv) requires neither propellants to aid
in
dispersion nor enhancer compounds to enhance absorption in the lower
respiratory
tract, and (v) exhibits good pulmonary bioavailability.
Summary of the Invention
The present invention provides a stabilized FSH dry powder composition for
delivery to the systemic circulation via the deep lung. The dry powder of the
invention, when aerosolized and administered via inhalation, is useful in
therapies
and/or treatments for infertility.
The respirable, dry powder composition of the invention includes a
pharmacologically effective amount of a follicle stimulating protein (FSP) and
a
pharmaceutically acceptable excipient. More specifically, the dry powder of
the
invention includes a mammalian urinary-derived or recombinant FSP.
The powders of the invention exhibit good bioavailabilities when aerosolized
and administered by inhalation to the deep lung. A dry powder of the invention
is
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
characterized by a relative pulmonary bioavailability of at least about 1 %,
with
relative pulmonary bioavailability values typically ranging from about 1% to
60%.
In another aspect, the invention is directed to a method of preparing a
stabilized dry powder FSP composition as described above. The method includes
the
steps of mixing FSP and an excipient with a solvent to form a solution or
suspension,
and drying the solution or suspension to form a bioactive powder comprising
dry
particles containing FSP and the excipient material. Particular solvents for
use in the
method include water and alcohols. In a preferred embodiment of the method,
the
FSP composition is produced by spray drying.
In yet another aspect, the invention provides a method for delivering FSP to a
mammalian subject in need thereof, where the method includes administering by
inhalation a FSP dry powder composition as previously described in aerosolized
form.
The invention also encompasses, in yet another aspect, a method for treating
infertility in a mammalian subject, where a therapeutically effective amount
of a FSP
dry powder composition in accordance with the invention is administered to the
subject by inhalation for deposition in and absorption from the lung. In a
specific
embodiment, the subject is an infertile female, and the FSP dry powder
composition is
administered periodically by inhalation into the subject's lungs for one or
more cycles
of treatment extending over a time course of at least 3-20 days, where, as a
result of
said administering, the subject exhibits a level of follicular growth that is
increased
relative to such level measured prior to said administering. In one particular
embodiment, the therapeutically effective amount of FSP is from about 10 to
1000 IL1
of FSP per day. . In another embodiment, the therapeutically effective amount
of FSP
is from about 50 to about 3000 IU of FSP per day. In yet another embodiment,
in
particular relating to super ovulation therapies (i.e., in vitro
ferilization), the
therapeutically effective amount of FSP is from about 200 to 12,000 ILT of FSP
per
day.
These and other objects and features of the invention will become more fully
apparent when the following detailed description is read in conjunction with
the
accompanying figures and examples.
4
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Brief Description of the Figures
Fig. 1 is a graph showing the relationship between fine particle fraction
(FPF,
less than 3.3 microns) and aerosol particle size (MMAD) for three exemplary
FSP
powder formulations (L2017, L2010, L2018) utilized in a one-month stability
study,
as described in detail in Example 7C.
Figs. 2A and 2B are graphs demonstrating the results of an in vitro bioassay
to determine the bioactivity of representative uFSH dry powder formulations
compared to an FSH standard (Example 8). Fig. 2A demonstrates bioactivities of
powders L2013 and L2010; Fig. 2B demonstrates bioactivities of powders L2006
and
L2008.
Fig. 3 provides a graphical comparison of the in vivo bioavailability in rats
of
uFSH administered intratracheally (IT) and subcutaneously (SC) (Example 9).
Fig. 4 provides a graphical representation of mean serum concentrations
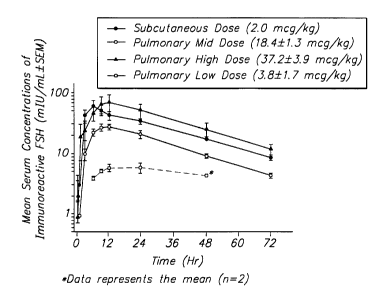
(~SEM) of immunoreactive FSH in cynomolgus monkeys following pulmonary and
subcutaneous administration of human uFSH (Example 10).
Fig. 5 provides a graphical representation of mean serum concentrations
(~SEM) of an immunoreactive a hFSH variant in cynomolgus monkeys following
pulmonary and subcutaneous administration (Example 17).
Fig. 6 provides a graphical comparison of the mean serum concentrations
(+SEM) of an immunoreactive a hFSH variant or uFSH following subcutaneous
administration (Example 17).
Detailed Description of the Invention
I. Definitions
"Bioactive powder" refers to a powder having either in vitro or in vivo
activity, and typically refers to a powder containing one or more bioactive or
pharmaceutical agents, such as FSP. "Bioactivity" of the powders of the
invention
can be generally measured using FSH assays known in the art, such as signal
transduction assays, FSH receptor binding assay, or a version of the
Steelman/Pohley
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
in vivo rat ovarian weight assay, FSH half life measurement and/or FSH
bioactivity
assay [Zlokarnik et al., Science, ?79, 84-88 (1998); Dahl, K. et al.. Journal
of
Audrology, 13:11-22 (1992); Roth and Dias, Biochern. 35:7928 (1996); Valvore,
et
al., Endocrinol 135:2657 (1994); Sprengel, et al., Mol. Endocrinol. 4:525
(1990):
Tilly, et al., Endocrinol. 131:799-806 (1992); Steelman and Pohley,
Endocrinol.
53:604 (1953); Bishop et al., Endocrine. 136:2635 (1995); Minders, et al.,
Biologicals, 25:269 (1997); Fares, PNAS, 89:4304 (1992); LaPolt, et al.,
Erzdocrinol.
131:2514 (1992)]. Further examples of such assays for in vitro biological
activities,
include, but are not limited to, measurement of estradiol production by
granulosa cells
using a rat granulosa cell aromatase assay [Dahl, et al., Meth. Enrymol.
169:414
(1989)]; and a FSH receptor activation assay, such as that described in
Kelton, et al.,
Molec. Cell. Endocrirtol. 89:141 (1992).
"Relative pulmonary bioavailability" is the percentage of the FSP dry powder
dose deposited in the lungs that is absorbed and enters the blood of a mammal
relative
to the percent that is absorbed into the blood from an intramuscular or
subcutaneous
injection site. Representative model systems for determining relative
pulmonary
bioavailabilities include rat, rabbit, and monkey. The FSP dry powder
composition of
the invention is characterized by a relative pulmonary bioavailability of at
least about
1 % in plasma or blood, with relative pulmonary bioavainabilities generally
ranging
from about 1 to 20% , and preferably from about 1 % to about 60%. Relative
pulmonary bioavailability may be estimated by measuring absorption from direct
intratracheal administration or by inhalation of an FSP-dry powder
composition.
"Distribution phase", in reference to the half life of FSP, refers to the
initial
rapid phase during which the hormone disappears from the plasma. The terminal
slow or elimination phase half life refers to the terminal slow phase during
which
hormone is eliminated from the body.
"Pharmaceutically acceptable salt" includes, but is not limited to, salts
prepared with inorganic acids, such as chloride, sulfate, phosphate,
diphosphate,
hydrobromide, and nitrate salts, or salts prepared with an organic acid, such
as malate,
maleate, fumarate, tartrate, succinate, ethylsuccinate, citrate, acetate,
lactate,
methanesulfonate, benzoate, ascorbate, para-toluenesulfonate, panmoate,
salicylate
and stearate, as well as estolate, gluceptate and lactobionate salts.
Similarly salts
containing pharmaceutically acceptable cations include, but are not limited
to,
6
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
sodium, potassium, calcium, aluminum, lithium, and ammonium (including
substituted ammonium).
"Citrate salt" refers to any pharmaceutically acceptable salt of citric acid
with
canons such as sodium, potassium, ammonium (including alkyl ammonium salts),
calcium, and the like.
"Amino acid" refers to any compound containing both an amino group and a
carboxylic acid group. Although the amino group most commonly occurs at the
position
adjacent to the carboxy function, the amino group may be positioned at any
location
within the molecule. The amino acid may also contain additional functional
groups,
such as amino, thio, carboxyl, carboxamide, imidazole, etc. The amino acids
may be
synthetic or naturally occurring, and may be used in either their racemic or
optically
active (D-, or L-) forms.
"Enhancer" refers to a compound that enhances the absorption of FSP through
the epithelium of the lower respiratory tract and into the systemic
circulation. In the
presence of an enhancer, the amount of FSP absorbed into the systemic
circulation is
greater than the amount absorbed for a dry powder lacking such enhancer, and
preferably
is increased by at least about 150% of the amount absorbed in the absence of
enhancer.
"Delivered dose efficiency" or "DDE" provides an indication of the delivery
of a dry powder from the mouthpiece of a suitable inhaler device after a
firing or
dispersion event. DDE is synonymous with the term "emitted dose" or "ED." More
specifically, the DDE (ED) is a measure of the percentage of powder which is
drawn
out of a unit dose package and which exits the mouthpiece of an inhaler
device. DDE
(ED) is defined as the ratio of the dose delivered by an inhaler device to the
nominal
dose (i. e., the mass of powder per unit dose placed into a suitable inhaler
device prior
to firing). DDE (ED) is an experimentally determined parameter, and is
typically
determined using a device that mimics patient dosing. To determine a DDE
value, a
nominal dose of dry powder, typically in unit dose form, is placed into a
suitable dry
powder inhaler (such as that described in U.S. Patent No. 5,785,049, assigned
to
Inhale Therapeutic Systems) which is then actuated, dispersing the powder. The
resulting aerosol cloud is then drawn by vacuum from the device, where it is
captured
on a fared filter attached to the device mouthpiece. The amount of powder that
reaches the filter constitutes the delivered dose. For example, for a 5 mg,
dry powder-
containing dosage form placed into an inhalation device, if dispersion of the
powder
results in the recovery of 4 mg of powder on a fared filter as described
above, then the
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
DDE for the dry powder composition is: 4 mg (delivered dose)/5 mg (nominal
dose) x
100 = 80%. For non-homogenous powders, DDE values provide an indication of the
delivery of drug from an inhaler device after firing rather than of dry
powder, and are
based on amount of drug rather than on total powder weight.
"Fine particle fraction" or "FPF" is defined as the mass percent of
aerosolized
powder particles having an aerodynamic diameter less than 3.3 Vim, typically
determined by measurement in an Andersen cascade impactor. This parameter
provides an indication of the percent of particles having the greatest
potential to reach
the deep lung of a patient for systemic uptake of a drug substance.
"Aerosol performance coefficient" or "APC" is an estimate of the total powder
delivery efficiency from a unit dosage form (e.g., a blister pack) to the deep
lung, and
is determined experimentally using a short stack Anderson cascade impactor
operated
at a vacuum of 28.3 liters per minute. APC is synonymous with the term, "fine
particle dose" or "FPD". The APC is defined as the total mass, in milligrams,
of
aerosolized powder having a particle size less than 3.3 micrometers, relative
to the
mass of powder contained in a unit dosage form (e.g., blister or capsule), in
milligrams, and expressed as a percentage: APC (%) = total aerosolized powder
mass
less than 3.3 ~m (mg) = unit dosage form fill mass X 100.
"Dry powder" refers to a composition that contains finely dispersed solid
particles that are free flowing and capable of (i) being readily dispersed in
an
inhalation device and (ii) inhaled by a subject so that a portion of the
particles reach
the lungs to permit penetration into the alveoli. Such a powder is considered
to be
"respirable" or suitable for pulmonary delivery. A dry powder contains from
about
0.1 % to 10% moisture, and typically contains less than about 10 percent
moisture,
preferably less than 5% moisture, and more preferably contains less than about
3
percent moisture. Unless otherwise stated, a "dry powder composition for
delivery to
the deep lung" is one that, when aerosolized, is administered as dry powder
particles.
"Follicle stimulating protein" or "FSP" means a protein or a mixture of
proteins having structure and function similar to that of human follicle
stimulating
hormone ("hFSH"). FSP includes hFSH, amino acid analogs of hFSH ("hFSH
analogs"), glycoforms of hFSH, glycoforms of hFSH analogs, and mixtures of
proteins selected from hFSH, hFSH analogs, glycoforms of hFSH, and glycoforms
of
hFSH analogs.
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
FSH purified to various extents from human urine ("uFSH") is a mixture of
proteins having structure and function similar to that of hFSH, and therefore
is within
the definition of FSP. UFSH is a mixture of hFSH and hFSH analogs, each also
having heterogeneity of glycosylation.
So-called recombinant FSP ("rFSP") is a mixture of proteins having structure
and function similar to that of hFSH and is biosynthesized in a host cell
modified
using recombinant or related techniques to express the hFSH amino acid
sequence or
the amino acid sequence of a hFSH analog [Keene, et al., J. Biol. Chent.,
264:4769-
4775 ( 1989); Chappel, S., et al., Proceedings of the 3rd World Congress on
Gynocological Endocrinology, 179-184 (1992); or Reddy, V. B., et al., U.S.
Patent
No. 5,156,957, October 20, 1992]. The heterogeneity of rFSH is caused by
infidelity
in transcription and heterogeneity of post-translational modifications
characteristic of
the host cell or resulting from culture conditions, purification, formulation,
and
storage, among other causes.
Structurally, hFSH is a heterodimeric glycoprotein consisting of two non-
covalently bound subunits referred to as the alpha (a) and beta ([3) subunits.
The
human FSH alpha subunit consists of a chain of 92 amino acids, five
intrasubunit
disulfide bonds, and two N-linked glycosyl side chains. The amino acid
sequence of
human FSH alpha subunit is given as SEQ ID NO:S herein. The human FSH beta
subunit consists of a chain of 111 amino acids, six intrasubunit disulfide
bonds, and two
N-linked glycosyl side chains. Its amino acid sequence is given as SEQ ID N0:6
herein. Disulfide bonds link residues 7 and 31, 10 and 60, 28 and 82, 32 and
84, and 59
and 87 in the hFSH alpha subunit, and they link residues 3 and 51, 17 and 66,
20 and
104, 28 and 82, 32 and 84, and 87 and 94 in the hFSH beta subunit. The sites
of N-
glycosylation are at positions 52 and 78 in the a subunit (SEQ ID NO:S) and at
residues 7 and 24 in the (3 subunit (SEQ ID N0:6). Together, the two subunits
have a
molecular weight of about 31 kD measured by mass spectroscopy and about 35-45
kD
measured by PAGE or gel chromatography depending upon the state of
glycosylation.
Pertinent sequence information (along with references) can be found in the
Entrez
Protein Database, which is provided and maintained by the National Center for
Biotechnology Information (NCBI). An illustrative protein sequence for the
precursor a-subunit corresponds to, e.g., Entrez Accession No. 69160 (116
amino
acids, of which the C-terminal 92 amino acids constitute the mature a-subunit
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
sequence); representative sequences for the precursor (3-subunit are
identified by the
following Entrez Accession Nos.: 120552, 476441, 182767, 182762, and 511854
(129
amino acids, of which the C-terminal 111 amino acids constitute the mature ~i-
subunit
sequence).
Functionally, the activity of FSP is determined as described herein for
bioactive
powders. "FSP activity," "FSP bioactivity," "FSH activity," "FSH bioactivity,"
and
like concepts of functionality can be measured by heterodimer stability;
activity in an
FSH-related signal transduction assay; FSH receptor binding; a version of the
Steelman/Pohley in vivo rat ovarian weight assay; in vivo pharmacokinetic
measures,
such as in vivo half life; in vivo pharmacodynamic testing, as known in the
art and as
described herein, such as increased follicular growth. The specific activity
of FSP
contained in the dry powders of the invention may range from about 100-175 ILJ
/mg
protein (typical activity ranges for uFSH, which generally contains LH or
other co-
purified proteins) to about 1000 to 13,500 IU /mg protein for ultra-pure
urinary FSH
(Arpaia, et al., 1992) or recombinant material, and will depend upon the
source and
degree of purification of the hormone.
FSP is meant to include deglycosylated, unglycosylated, modified
glycosylated, and other glycoforms. Glycosylated forms are preferred.
Glycosylated
FSP usually is heterogeneous to some extent in its glycosylation, having
within any
sample a multiplicity of glycosylated species. Glycosyl heterogeneity can be
extensive depending on the type of cell in which the FSP was biosynthesized
and on the
conditions to which FSP was exposed post-translation, post-secretion, during
purification and storage, as described by Baenziger and Green, Biochem.
Biophys.
Acta, 947, 287-306 (1998); Bishop, et al., Mol. Endocrinol., 8, 722-731
(1994);
Thotakura and Blithe, Glvcobiology, 5, 3-10 (1995); Dahl, et al., Scierace,
239, 72-74
(1988); Ulloa-Aguirre, et al., Eradocr. Rev., 1 G, 765-787 (1995); Stanton, et
al.,
Endocrinology, 130, 2820-2832 (1992); Beitens and Padmanabhan, Trends
Endocrinol. Metab., 2, 145-151 (1991); Hard, et al. Eur. J. Biochem., 193,
1064-1069
( 1990); Harris, et al., Mol. Hum. Reprod., 2, 807-811 ( 1996); Hakola, et
al., Mol.
Cell. Endocrinol., 1?7, 59-69 (1997). A glycoform is a form of glycoprotein
having
variation in the type or pattern of glycosylation compared with the type or
pattern of
glycosylation of hFSH. A glycoform can be thought of as a carbohydrate analog
(as
opposed to an amino acid analog).
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
FSP includes amino acid analogs of hFSH (i.e., hFSH analogs) such as those
described in Monahan, M., et al., Biocheniistrl~, 12:4616-4620 (1973): Nestor,
J.J., et
al., U.S. Patent No. 4,234,571, Nov. 18, 1980; Fujino, M., et al, J. Med.
Chent.,
16:1144-1147 (1973); Coy, D.H., et al., Bioclzem. Biophys. Res. Comn Tun.,
67:576-
582 (1975); Combarnous, Y., Endocrine Reviews, 13(4):670-691 (1992), and
Antoni,
F., et al., U.S. Patent No. 4,552,864, Nov. 12, 1985. A hFSH analog is a
compound
structurally and functionally similar to hFSH, but differing structurally from
hFSH in
that one or more amino acids have been substituted, deleted, added, or
otherwise
modified compared with hFSH. A hFSH analog herein must exhibit at least 10%
bioactivity by some measure compared with hFSH. Examples of hFSH analog
subunits
include, but are not limited to, those in SEQ ID NOS: 1-4 and 7-31. Amino
acids in a
FSH analog that are essential for function can be identified by methods known
in the
art, such as site-directed mutagenesis or alanine-scanning mutagenesis
[Cunningham
and Wells, Science, 244:1081-1085 (1989)]. The latter procedure introduces
single
alanine mutations at every residue in the molecule. The resulting mutant
molecules
are then tested for biological activity. Sites that are critical for ligand-
protein binding
can also be identified by structural analysis such as crystallography, nuclear
magnetic
resonance spectroscopy, or photoaffinity labeling [Smith, et al., J. Mol.
Biol.,
224:899-904 (1992); de Vos, et al, Science, 255:306-312 (1992)].
Particularly preferred hFSH analogs for use in the present invention are
"hFSH variants." A hFSH variant is a FSP differing structurally from hFSH in
that
one or more amino acids are deleted from one or more of the four termini of
hFSH
(the alpha and beta subunits each have two termini, an N-terminus and a C-
terminus).
In hFSH variants, one or more amino acid additions, substitutions, or
deletions may
also be present internally, i.e., not at the termini, in the alpha subunit,
the beta subunit,
or both, compared with hFSH. Examples of amino acid sequences of hFSH variant
a
subunits include, but are not limited to, those in SEQ ID NOS:29-31,
optionally
comprising at least one further substitution, addition, or deletion. Examples
of amino
acid sequences of hFSH variant (3 subunits include, but are not limited to,
those in
SEQ ID NOS: 11-28, optionally comprising at least one further substitution,
addition,
or deletion. Certain variants are well known in the art as described in
Hoffmann, J.
A., et al., International Patent Publication WO 00/04913, Feb. 3, 2000.
11
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Preferred hFSH variants are: 1) those wherein one or both subunits have
terminal deletions, but wherein any deletions are limited to one, two, three,
four, five,
or six amino acids from the N-terminus of the alpha subunit, one, two, three,
four, or
five amino acids from the C-terminus of the alpha subunit, one or two amino
acids
from the N-terminus of the beta subunit, and one, two, three, four, five, six,
or seven
amino acids from the C-terminus of the beta subunit; 2) those wherein one or
both
subunits have one or more deletions described in 1 ) above except that the C-
terminus
of the alpha subunit does not have deletions; 3) those having deletions at the
C-
terminus of the beta subunit, and in that case more preferably those having at
least
three amino acids deleted from the C-terminus of the beta subunit; 4) those
whose
subunits have no deletions at the N-termini, or at most one or two amino acids
deleted
at the N-termini, and in that case more preferably at the N-terminus of the
beta
subunit; 5) those having an alpha subunit of full length (92 amino acids)
combined
with a variant beta subunit, preferably having one to seven amino acids
deleted from
the C-terminus and either no amino acids deleted from the N-terminus or one or
two
amino acids deleted from the N-terminus; all optionally having one or more
deletions,
substitutions, or additions of amino acids internally (i.e., not at the
termini) as
compared with hFSH. The most preferred hFSH variants are those comprised of an
alpha subunit having the amino acid sequence of any one of SEQ ID NOS:S, 29-31
and a beta subunit having an amino acid sequence of any one of SEQ ID NOS:11-
28,
and those comprised of an alpha subunit having the amino acid sequence of any
one
of SEQ ID NOS:29-31 and a beta subunit having an amino acid sequence of SEQ ID
N0:6.
For hFSH variant nomenclature herein, reference will be made to SEQ ID NO.
or to the amino acids deleted, using either "des" followed by the position
numbers)
or by the three letter codes) and positions) of the deleted amino acids)
(e.g.,
desAsnl) or by simply indicating the removed amino acids) in one or three
letter
codes) and the affected positions) (e.g., 1N or Asn~). The SEQ ID NOS are
identified in below.
12
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
FSP DNA FSP DNA
SEQ SEQ ID SEQ ID SEQ
ID ID
NO: NO: Subunit NO: NO: Subunit
1 - Bovine a 17 42 Human ~i variant
Des 1, 111
2 - Bovine (3 18 43 Human ~3 variant
Des 1, 2, 11
I
3 - Equine a 19 44 Human (3 variant
Des l, 110,
111
4 - Equine (3 20 45 Human (3 variant
Des 1, 2, 110,
111
32, 37 Human a 21 46 Human (3 variant
Des 1, 109-111
6 36 Human (3 22 47 Human (3 variant
Des 1, 2, 109-111
7 - Porcine a 23 48 Human (3 variant
Des 1, 108-111
8 - Porcine (3 24 49 Human (3 variant
Des 1, 2, 108-111
9 - Ovine a 25 50 Human (3 variant
Des I, 107-111
- Ovine (3 26 51 Human ~3 variant
Des 1, 2, 107-1
I 1
11 33, 38 Human (3 variant27 52 Human (3 variant
Des 109, 110, Des l, 106-111
111
12 34 Human (3 variant28 53 Human ~3 variant
Des 110, 111 Des 1, 2, 106-1
I 1
13 35 Human (3 variant29 54 Human a variant
Des 1 I 1 Des Ala'
14 39 Human ~3 variant30 55 Human a variant
Des 108-111 DesAla'Pro'
13
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
FSP DNA FSP DNA
SEQ ID SEQ ID SEQ SEQ ID I
ID
NO: NO: Subunit NO: NO: Subunit
15 40 Human (3 variant31 56 Human a variant
Des 107-111 DesAla~Pro''Asp3
16 41 Human (3 variant
Des 106-111
"Mass median diameter" or "MMD" is a measure of mean particle size, since
the powders of the invention are generally polydisperse (i.e., consist of a
range of
particle sizes). MMD values as reported herein are determined by centrifugal
sedimentation, although any number of commonly employed techniques can be used
for measuring mean particle size (e.g., electron microscopy, light scattering,
and laser
diffraction).
"Mass median aerodynamic diameter" or "MMAD" is a measure of the
aerodynamic size of a dispersed particle. The aerodynamic diameter is used to
describe an aerosolized powder in terms of its settling behavior, and is the
diameter of
a unit density sphere having the same settling velocity, generally in air, as
the particle.
The aerodynamic diameter encompasses particle shape, density and physical size
of a
particle. As used herein, MMAD refers to the midpoint or median of the
aerodynamic
particle size distribution of an aerosolized powder determined by cascade
impaction.
"Pharmaceutically acceptable excipient or carrier" refers to an excipient that
can be taken into the lungs in association with FSH with no significant
adverse
toxicological effects to the subject, and particularly to the lungs of the
subject.
"Pharmacologically effective amount" or "physiologically effective amount of
FSP" is the amount of FSP present in a dry powder composition as described
herein that
is needed to provide a desired level of drug in the bloodstream of a subject
to be treated
to give an anticipated physiological response when such composition is
administered by
inhalation for deposition in and absorption from the lung. The precise amount
will
depend upon numerous factors, e.g., the source of FSP (urinary or
recombinant), the
specific activity of the composition, the delivery device employed, physical
characteristics of the powder, its intended use, and patient considerations,
and can
14
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
readily be determined by one skilled in the art, based upon the information
provided
herein.
Before therapy, certain infertile female subjects will generally possess small
follicles, i.e., follicles in each ovary's largest section which are less than
10 mm in
diameter, typically determined by ultrasound performed using a vaginal probe.
"Increased follicular growth," in the context of treatment for female
infertility, is a level
of follicular growth in a subject that is increased relative to its baseline
level prior to
administering an FSP dry powder composition of the invention. An increase in
follicular
growth is measured by an increase in the number of follicles having a mean
diameter of
mm or greater. Preferably, increased follicular growth is indicated by 1 or
more
follicles demonstrating growth to a mean diameter of 10 mm or greater. In
ovulation
induction-type therapies, increased follicular growth is typically indicated
by 1 - 2
follicles or more demonstrating growth to a mean diameter of 10 mm or greater.
In
super ovulation-type therapies (e.g., in-vitro fertilization), increased
follicular growth is
preferably indicated by 2 or more follicles demonstrating growth to a mean
diameter of
10 mm or greater, more preferably 3 to 5 follicles having increased in size to
a diameter
of 10 mm or greater, and even more preferably 6 to 8 follicles or more
exhibiting such
size characteristics. Over the course of treatment (particularly super
ovulation
treatment), one indication of full follicular development in a subject is an
average of 9
follicles or more having a diameter greater than 10 mm, where the largest
growing
follicle has matured to a mean diameter of greater than or equal to 16 mm. The
efficacy
of FSH therapy can also be evaluated by other methods, e.g., ultrasound and
subject
estradiol levels.
"Stabilized dry powder composition", particularly in reference to a dry powder
for aerosolized delivery to the deep lung, is a powder which (i) contains FSH
and an
excipient material or materials, where the excipient is not melezitose, (ii)
possesses a
specific activity of at least 50 ILJ/mg FSH and more preferably a specific
activity of at
least 100 IU/mg FSH, and (iii) maintains at least about 70% of its initial
bioactivity
when stored for one month at room temperature under ambient conditions. That
is to
say, a stabilized dry powder in accordance with the invention is one that
substantially
maintains in vivo and/or in vitro FSH activity upon formulation and long term
storage
over a period of at least one month. Stabilized dry powder compositions
containing a
salt of a carboxylic acid having two or more ionizable protons (e.g., citrate,
glutamate,
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
tartrate, and the like) as a buffer are prepared by methods known in the art,
but not by
lyophilization.
"Polymeric macromolecule" is a high molecular weight polymeric compound
that can be naturally occurnng (e.g., proteins, carbohydrates, nucleic acids)
or
synthetically-produced (e.g., polyethylene glycols, polyvinylpyrrolidones,
Ficolls,
and the like, as known in the art.)
"Therapeutically effective amount" is the amount of FSP, which when delivered
by inhalation for deposition in and absorption from the lung in the form of a
dry
powder composition as described herein, provides the desired biological
effect.
"Cascade impactor efficiency" or "CI Eff ' is the fraction of aerosolized
powder recovered in the cascade impactor. CI Eff values are typically less
than DDE
values due to losses to the throat or walls of the cascade impactor.
"Percent left" means the percent of powder originally in a blister pack (BP)
that remains in the BP after dispersion of the powder dose.
"Percent collected" means the percent of powder dispersed from a BP that
deposits on a collection filter. "Percent collected" accounts for powder that
may be
lost to deposition in the device and chamber.
"Bulk density" refers to the density of a powder prior to compaction (i.e.,
density of an uncompressed powder), and is typically measured by a well-known
USP
method.
II. FSP Dry Powders
The present invention provides stable, dispersible dry powder compositions
for pulmonary delivery of FSP. The compositions developed by the applicants
overcome many of the problems often encountered heretofore in formulating
proteins,
particularly gonadotropin hormones, for delivery to the deep lung. The FSP dry
powder compositions described herein are readily dispersed (i.e., demonstrate
good
aerosol performance), are stable against both physical and chemical
degradation (i) in
solution, before powder manufacture, (ii) during powder manufacture and
processing,
and (iii) upon storage, and exhibit good bioavailabilities when delivered by
inhalation
for deposition in and absorption from the lung. The dry powder compositions
according to the present invention generally include FSP and a
pharmaceutically
acceptable excipient, although dry powders composed of neat FSP (i.e.,
respirable
powders composed of FSP and essentially lacking any additional excipients or
16
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
additives) are also envisioned. Components of FSP dry powders suitable for
delivery
to the deep lung will now be described.
A. Follicle Stimulating Proteins
1. Naturally occurring FSP. The FSP contained in the solid preparations may
be at least partially purified from natural sources (i.e., may be highly
purified and may
optionally include LH or other co-purified proteins), such as human urinary
FSH
(uFSH). Urinary-derived FSH may be obtained from a commercial source (e.g.,
Vitro
Diagnostics, Boulder, CO) or purified by immunopurification as described in
Arpaia,
G., et al., 1992. A number of naturally occurring FSP heterodimers are known
and
are suitable for use in the dry powder of the invention. Such exemplary FSP
heterodimers, i.e., comprising one alpha subunit and one beta subunit, include
but are
not limited to SEQ ID NOS: 1 and 2 (bovine FSH); 3 and 4 (equine FSH); 5 and 6
(hFSH); 7 and 8 (porcine FSH); 9 and 10 (ovine FSH).
2. Synthetic or Recombinant FSP. Alternatively, FSP may be produced by
chemical synthesis or may be biosynthesized in host cells appropriately
engineered
using conventional techniques of molecular biology (i.e., recombinant
technology).
Recombinant FSP, a preferred form of FSP for use in the dry powder
compositions of
the invention, may be prepared using conventional techniques such as those
described
in Keene, et al., 1989; Boime, et al., Seminars in Reproductive Endocrinology,
10:45-
50 (1992); Chappel, et al., 1992; or Reddy, et al., 1992; Shome, B., et al.,
J. Prot.
Chern., 7:325-339 (1988); Saxena, B.B. and Rathnam, P., J.Biol. Chenr.,
251:993-
1005 (1976); Watkins, et al., DNA, 6:205-212 (1987); Shome, B. and Parlow,
A.F., J.
Clin. Endocrinol. Metab., 39(1):203-205 (1974); Beck, et al., DNA, 4:76
(1985);
Boime, et al., U.S. Patent No. 5,405,945 (1995); and Reddy, V.B., et al., U.S.
Patent
No. 5,639,640 (1997); Sambrook, et al. Molecular Cloning: A Laboratory manual,
2"d Edition, ( 1989); Ausubel, et al., Eds., Current Protocols in Molecular
Biology,
(1987-1998), Chapters 10, 12, 13, 16, 18 and 20, the contents of which are
incorporated herein by reference. Exemplary preparations of FSH variants are
provided
in Examples 13, 14, 18 and 19.
More specifically, FSP products may be prepared by recombinant techniques
in a prokaryotic or eucaryotic host, including, for example, bacterial, yeast,
[Sherma,
F., et al., Methods in Yeast Genetics, (1992)] higher plant, insect
[Schneider, J.
17
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Enibwol. E.rp. Morphol., ?7:353-365 (1987)] and mammalian cells such as CHO,
COS and Bowes melanoma cells. Depending upon the host employed in a
recombinant production procedure, the rFSP molecules thus produced may be
glycosylated or can be non-glycosylated. In addition, a rFSP subunit may also
include an initiating methionine residue. Such methods are described in many
standard laboratory manuals.
FSP may be expressed in modified forms, such as a fusion protein, and can
include not only secretion signals, but also additional heterologous
functional regions.
For instance, a region of additional amino acids, particularly charged amino
acids, can
be added to the N-terminus to improve stability and persistence in the host
cell, during
purification, or during subsequent handling and storage. Also, peptide
moieties can
be added to an FSP molecule to facilitate purification. Such regions can be
removed
prior to final preparation of a polypeptide. Such methods are described in
many
standard laboratory manuals, such as Sambrook, supra, Chapters 17.29-17.42 and
18.1-18.74; Ausubel, supra, Chapters 16, 17 and 18.
Briefly, the expression of isolated nucleic acids encoding a FSP used in the
present invention will typically be achieved by operably linking, for example,
the DNA
or cDNA to a promoter (which is either constitutive or inducible), followed by
incorporation into an expression vector. The vectors can be suitable for
replication and
integration in either prokaryotes or eucaryotes. Typical expression vectors
contain
transcription and translation terminators, initiation sequences and promoters
useful for
regulation of the expression of the DNA encoding a protein of the present
invention. To
obtain high level expression of a cloned gene, it is desirable to construct
expression
vectors which contain, at the minimum, a strong promoter to direct
transcription, a
ribosome binding site for translational initiation, and a
transcription/translation
terminator.
Expression of a FSP in yeast cells is carried out by well-known methods
[Shernia, et al., (1982)]. Two widely utilized yeast for production of
eucaryotic proteins
are Saccharomyces cerevisiae and Pichia. Vectors, strains, and protocols for
expression
in Saccharonryces and Pichia are known in the art and available from
commercial
suppliers (e.g., Invitrogen, Inc.). Suitable vectors usually have expression
control
sequences, such as promoters, including 3-phosphoglycerate kinase or alcohol
oxidase,
and an origin of replication, termination sequences and the like.
18
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Polynucleotide sequences encoding FSP can also be ligated to various
expression
vectors for use in transfecting mammalian cells. Mammalian cell systems often
will be
in the form of monolayers of cells although mammalian cell suspensions may
also be
used. A number of suitable host cell lines capable of expressing FSP include
the
HEK293, BHK21, AV12 and CHO cell lines. Expression vectors for these cells can
include expression control sequences, such as an origin of replication, a
promoter (e.g.,
CMV promoter, EF 1 alpha promoter, or a HSV tk promoter or phosphoglycerate
kinase
promoter), an enhancer, and processing information sites, such as ribosome
binding
sites, RNA splice sites, polyadenylation sites (e.g., an SV40 large T Ag poly
A addition
site), and transcriptional terminator sequences. Other animal cells useful for
production
of FSP are available, for instance, from the American Type Culture Collection
Catalogue
of Cell Lines and Hybridomas (7th edition, 1992).
Appropriate vectors for expressing FSP in insect cells are usually derived
from
the SF9 baculovirus. Suitable insect cell lines include mosquito larvae.
silkworm,
armyworm, moth and Drosophila cell lines such as a Schneider cell line
[Schneider,
supra, 1987].
Introduction of a vector construct into a host cell can be effected by calcium
phosphate transfeetion, DEAE-dextran mediated transfection, cationic lipid-
mediated
transfection, electroporation, transduction, infection or other methods. Such
methods
are described in many standard laboratory manuals, such as Sambrook, supra,
Chapters 1-4 and 16-18; Ausubel, supra, Chapters 1, 9, 13, 15, 16, entirely
incorporated herein by reference.
Alternatively, polynucleic acids encoding FSP can be expressed in host cells
by
introducing, by homologous recombination into the cellular genome at a
preselected
site, DNA which includes at least a regulatory sequence, an exon and a splice
donor
site. These components are introduced into the chromosomal (genomic) DNA in
such
a manner that this, in effect, results in production of a new transcription
unit (in which
the regulatory sequence, the exon and the splice donor site present in the DNA
construct are operatively linked to the endogenous gene). As a result of
introduction
of these components into the chromosomal DNA, the expression of the desired
endogenous gene is altered. Such methods are well known in the art, e.g., as
described
in U.S. Patent Nos. 5,580,734, 5,641,670, 5,733,746, and 5,733,761, entirely
incorporated herein by reference.
19
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Recombinant FSP can be recovered and purified from recombinant cell
cultures by well-known methods including ammonium sulfate or ethanol
precipitation, acid extraction, anion or cation exchange chromatography,
phosphocellulose chromatography, hydrophobic interaction chromatography,
affinity
chromatography, hydroxylapatite chromatography, reverse phase chromatography,
dye chromatography and lectin chromatography. Monitoring of the purification
process can be accomplished by using Western blot techniques or
radioimmunoassay or
other standard immunoassay techniques.
3. hFSH Variants. The dry powder composition of the invention may contain
a hFSH variant. hFSH variants can be produced by a number of techniques well
known to those skilled in the art such as by chemical synthesis, recombinant
biosynthesis, and in vivo or in vitro processing of naturally occurnng or
recombinant
FSH by amino- and/or carboxy-peptidases to expose one or more internal amino
acids.
B. Nucleic Acid Molecules Encoding FSP
Polynucleic acid molecules encoding FSP described herein are illustrative, not
limiting, of sequences useful for preparing FSP by recombinant expression and
they
may be incorporated into the dry powder compositions of the invention, for
expression of FSP in vivo, as described in greater detail below. Other
polynucleic
acids may be used to produce FSP without affecting the present invention. The
polynucleic acid molecules can be in the form of RNA, such as mRNA, hnRNA, or
any other form, or in the form of DNA, including, but not limited to, cDNA and
genomic DNA obtained by cloning or produced synthetically, or any combination
thereof. The DNA can be triple-stranded, double-stranded or single-stranded,
or any
combination thereof. Any portion of at least one strand of the DNA or RNA can
be
the coding strand, also known as the sense strand, or it can be the non-coding
strand,
also referred to as the anti-sense strand.
Isolated polynucleic acid molecules for use in expressing a FSP comprise an
open reading frame (ORF) shown in at least one of SEQ ID NOS: 32, 37, 54, 55,
or
56, or a nucleic acid molecule having a sequence complementary thereto, for
expressing an alpha subunit, or comprise an ORF in at least one of SEQ ID
N0:33,
34, 35, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, or 53, or
a nucleic
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
acid molecule having a sequence complementary thereto, for expressing a beta
subunit.
The polynucleic acid sequences can be made using (a) standard recombinant
methods, (b) synthetic techniques, (c) purification techniques, or
combinations thereof,
as well known in the art. The polynucleic acids may conveniently comprise
sequences
in addition to those exemplary sequences provided herein. For example, a multi-
cloning
site comprising one or more endonuclease restriction sites may be inserted
into the
nucleic acid to aid in isolation of the polynucleotide. Also, translatable
sequences may
be inserted to aid in the isolation of a translated FSH polynucleotide.
Additional
sequences may be added to such cloning and/or expression sequences to optimize
their
function in cloning and/or expression, to aid in isolation of an FSP
polynucleotide, or to
improve the introduction of the polynucleotide into a cell.
Polynucleic acids encoding FSP can be prepared by direct chemical synthesis
methods such as the phosphotriester method of Narang, et al., Meth. Enzymol.,
68:90-99
(1979); the phosphodiester method of Brown et al., Meth. Enzvmol., 68:109-151
(1979);
the diethylphosphoramidite method of Beaucage et al., Tetra. Letts., 22:1859-
1862
(1981); the solid phase phosphoramidite triester method described by Beaucage
and
Caruthers, Tetra. Letts., 22(20):1859-1862 (1981), e.g., using an automated
synthesizer,
e.g., as described in Needham-VanDevanter, et al., Nucleic Acids Res., 12:6159-
6168
(1984); and the solid support method of U.S. Patent No. 4,458,066. Chemical
synthesis
generally produces a single-stranded oligonucleotide, which may be converted
into
double-stranded DNA by hybridization with a complementary sequence, or by
polymerization with a DNA polyrnerase using the single strand as a template.
Although
chemical synthesis of DNA is limited to sequences of about 100 bases, longer
sequences
may be obtained by the ligation of shorter sequences.
Representative polynucleotide sequences described in sequences 32-56 herein
encode either an alpha or a beta subunit. The DNA of SEQ ID NO: 37 and 38 is
designed and constructed from ligated oligonucleotides. The differences
between
SEQ ID NO: 32 and SEQ ID NO: 37 do not change the encoded amino acid sequence
of the alpha subunit protein. Likewise, the differences between SEQ ID NO: 38
and
SEQ ID NO: 33 do not change the encoded amino acid sequence of the beta
variant
subunit protein. The skilled person will know a multitude of sequences
equivalent to
the sequences described in SEQ ID NO: 32-56 exist because of the degeneracy of
the
genetic code.
21
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
The dry powder of the invention may contain a polynucleotide encoding FSP
(e.g., genes encoding both the a- and (3-subunits of FSP), a fragment thereof
(e.g., the
(3-subunit, which confers biological specificity), and/or a variant as
described herein,
where the polynucleotide or FSP-coding region is operably linked to suitable
transcriptional regulatory or control sequences (e.g., promoters, enhancers,
and the
like) to permit FSP transgene expression in target host cells of a subject,
e.g.,
mammalian cells from the pulmonary regions of the lung (e.g., lung epithelial
cells,
alveolar type cells), or other suitable subject host cells. In addition to the
sequences
provided herein, representative gene sequences encoding FSP, its subunits, or
precursor proteins, are found, for example, in the Entrez Nucleotide Database
(as
described above). Such exemplary coding sequences have the following Accession
Nos.: J00152 V00487 (a subunit, bases 1-397), M54912 M38644 M21219 M18536
((3-subunit gene, exon 1), M54913 M21220 M18536 ((3-subunit gene, exon 2),
M54914 M38646 M21221 M18536 ((3-subunit gene, exon 3).
In a preferred embodiment of this aspect of the invention, regulatory
sequences operably linked to a polynucleotide coding sequence will function to
express FSP in a pulsatile fashion. Polynucleotides encoding FSP may
optionally be
contained in a viral or other conventional gene therapy vector, or may
comprise a
lipid-FSP transgene complex, as described in Eljamal, M., et al.,
International Patent
Publication WO 96/32116, October 17, 1996; in McDonald, R.J., et al., Pharrn.
Res.,
15(5):671-9 (1998), and in Eastman, et. al., Hum. Gene Ther., 8(6):765-73
(1997), the
contents of which are expressly incorporated herein by reference. Generally,
FSP
expression levels in the target tissue will roughly correspond to the
quantities of FSP
described herein for direct incorporation into the dry powders of the
invention; such
expression levels will characteristically correspond to a therapeutically
effective
amount of FSP. Representative quantities of nucleic acid constructs for
incorporation
in the dry powders of the invention for achieving the desired expression
levels are
described in Eljamal, M., et al., ibid..
C. Excipients and Other Additives
In the powders of the invention, FSP is generally combined with one or more
pharmaceutical excipients that are suitable for respiratory and pulmonary
administration. Such excipients may serve simply as bulking agents when it is
desired
22
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
to reduce the active agent concentration in the powder that is being delivered
to a
patient. An excipient may also serve to improve the dispersibility of the
powder
within a powder dispersion device in order to provide more efficient and
reproducible
delivery of the active agent and to improve the handling characteristics of
the active
agent (e.g., flowability and consistency) to facilitate manufacturing and
powder filling
into unit dosage forms. In particular, the excipient materials can often
function to
improve the physical and chemical stability of FSP, to minimize the residual
moisture
content, hinder moisture uptake, and to regulate particle size, degree of
aggregation,
particle surface properties (i.e., rugosity), ease of inhalation, and
targeting of the
resultant particles to the deep lung.
Alternatively, FSP may be formulated in an essentially neat form, wherein the
composition contains FSP particles within the requisite size range and
substantially
free from other biologically active components, pharmaceutical excipients, and
the
like.
Pharmaceutical excipients and additives useful in the present composition
include but are not limited to proteins, peptides, amino acids, lipids, and
carbohydrates (e.g., sugars, including monosaccharides, di-, tri-, tetra-, and
oligosaccharides; derivatized sugars such as alditols, aldonic acids,
esterified sugars
and the like; and polysaccharides or sugar polymers such as Ficolls), which
may be
present singly or in combination. Preferred are excipients that are soluble in
either
water (e.g., sugars, peptides, amino acids, and salts), alcohol (such as
pectin, lecithin,
povidone) or acetone (e.g., citric acid, PLGA). Also preferred are excipients
having a
glass transition temperature (Tg), above about 35° C, preferably above
about 45° C,
more preferably above about 55 °C. Illustrative excipients suitable for
use in the FSP
dry powders of the invention include those disclosed in Inhale Therapeutic
Systems'
International Patent Application No. W098/16207.
Exemplary protein excipients include but are not limited to serum albumin
such as human serum albumin (HSA), recombinant human albumin (rHA), gelatin,
casein, hemoglobin, and the like. Polypeptides and proteins suitable for use
in the dry
powder composition of the invention are provided in Inhale Therapeutic
Systems'
International Patent Publication No. W096/32096. HSA is a preferred
proteinaceous
excipient, and has been shown to increase the dispensability of dry powders
for
aerosolized delivery to the lungs (WO 96/32096, ibic~.
23
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Representative amino acid/polypeptide components, which may optionally
function in a buffering capacity, include alanine, glycine, arginine, betaine,
histidine,
glutamic acid, aspartic acid, cysteine, lysine, leucine, isoleucine, valine,
methionine,
phenylalanine, aspartame, threonine, tyrosine, tryptophan and the like.
Preferred are
amino acids and peptides that function as dispersing agents. Amino acids
falling into
this category include hydrophobic amino acids such as leucine (leu), valine
(val),
isoleucine (isoleu), norleucine (nle), tryptophan (try), alanine (ala),
methionine (met),
phenylalanine (phe), tyrosine (tyr), histidine (his), and proline (pro).
Examplary FSP
formulations containing a variety of amino acid excipients are provided in
Example
23. Leucine is one particularly preferred amino acid excipient for the FSP
compositions described herein. Leucine, when used in the formulations
described
herein includes D-leucine, L-leucine, and racemic leucine. Exemplary FSP dry
powders containing leucine are described in Examples I 1, 21, 22 and 23.
Dispersibility enhancing peptides for use in the invention include dimers,
trimers,
tetramers, and pentamers composed of hydrophobic amino acid components such as
those described above, e.g., dileucine, trileucine, and the like.
Carbohydrate excipients suitable for use in the invention exclude melezitose
and include, for example, monosaccharides such as fructose, maltose,
galactose,
glucose, D-mannose, sorbose, and the like; disaccharides, such as lactose,
sucrose,
trehalose, cellobiose, and the like; polysaccharides, such as raffinose,
maltodextrins,
dextrans, starches, and the like; and alditols, such as mannitol, xylitol,
maltitol,
lactitol, xylitol, sorbitol (glucitol), myoinositol and the like. Preferred
carbohydrate
excipients for use in the present invention are mannitol, trehalose, and
raffinose.
Preferred powder compositions in accordance with the invention are those that
are
stable in the absence of sucrose, and particularly those that are stable in
the absence of
combinations of sucrose and glycine.
The dry powder compositions may also include a buffer or a pH-adjusting
agent. Typically, the buffer is a salt prepared from an organic acid or base.
Representative buffers include salts of citric acid, ascorbic acid, gluconic
acid,
carbonic acid, tartaric acid, succinic acid, acetic acid, or phthalic acid and
Tris,
tromethamine hydrochloride, or phosphate buffers. Preferred buffers for use in
the
present compositions are organic acid salts such as citrate.
Additionally, the FSP dry powders of the invention may include polymeric
excipients such as polyvinylpyrrolidones, Ficolls (a polymeric sugar),
hydroxyethyl
24
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
starch, dextrates, polyamino acids (e.g., polyleucine, polyglutamic acid),
and/or
polyethylene glycols, where such polymers are present as additives rather than
as
encapsulating agents. The dry powder may also optionally contain flavoring
agents,
antimicrobial agents, sweeteners, antioxidants, antistatic agents, steroids
(e.g.,
cholesterol), and chelating agents (e.g., EDTA). Other pharmaceutical
excipients
and/or additives suitable for use in the FSP compositions according to the
invention
are listed in Remington, The Science & Practice ofPharmacy, 19'h ed., (1995),
and in
the Physician's Desk Reference, 52"d ed., (1998), the disclosures of which are
herein
incorporated by reference.
In contrast to previously reported dry powders that are poorly absorbed into
the pulmonary vasculature and into the systemic circulation when lacking one
or more
enhancer compounds [Backstrom, et al., U.S. Patent No. 5,128,453, July 7,
1992], the
dry powder of the invention is surprisingly well-absorbed through the lung,
and does
not require the addition of absorption enhancers. This is evidenced by the
bioavailability data provided herein, e.g., in Examples 10 and 17. Thus, the
dry
powders of the invention preferably lack typical enhancer compounds, such as
surfactants (e.g., salts of a fatty acids), bile salts, alkyl glycosides,
cyclodextrins, and
phospholipids, or, if such compounds are present, they are typically present
in very
low concentrations--less than about 20% by weight relative to FSP, and more
preferably less than about 1 S% to 10% by weight relative to FSP--such that
they do
not function to noticeably enhance absorption.
Preferred carrier or excipient materials are carbohydrates (e.g., saccharides
and alditols) and buffers (e.g., citrate) or polymeric agents, optionally in
combination
with an amino acid or di- or tripeptide as described above.
In accordance with the invention, the solid state matrix formed by the
excipient and the protein imparts a stabilizing environment to the FSP, and
may
optionally aid in dispersivity of the composition. The stabilizing matrix may
be
crystalline, an amorphous glass, or a mixture of both forms. Preferred are
compositions that, irrespective of their percent crystallinity, are stable
with respect to
this percentage over time. Most suitable are dry powder formulations which are
substantially amorphous (glasses) or substantially crystalline (i.e., are
crystalline to
the greatest extent possible, after adjusting for the amount of FSP contained
in the
powder, since FSH does not crystallize). More generally, preferred powders are
substantially crystalline, such as FSP powder L2017 (Example 6.C.), i.e., 51
to 99%
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
crystalline, 60% to 95% crystalline, 65% to 90% crystalline, or any range or
value
therein, or are substantially amorphous glasses, such as powders L2010 and
L2018
(Example 6.C.), i. e., at least about 70% of the solid is an amorphous glass,
preferably
at least about 75% is an amorphous glass, and more preferably at least about
85% is
an amorphous glass.
For FSP dry powder formulations which are substantially amorphous,
preferred are those formulations exhibiting glass transition temperatures (T~)
above
about 30°C, preferably above about 40°C, and more preferably
above about 60°C.
Glass transition temperatures for representative FSP dry powder compositions
are
presented in Table 6 (Example 6.B.) As can be seen from the values presented
therein, for those compositions for which Tg values were determined, 22
formulations/lot numbers exhibited Tg values above 30 °C, while 19
formulations/lot
numbers possessed Tg values about 60°C. Formulations having Tg values
at or above
60°C included the following: L2001, L2004, L2006, L2008, L2009, L2010,
L2012,
L2013, L2014, L2016, L2020 and L2018. Preferred storage temperatures for
substantially amorphous powders are at least about I 0°C lower than the
Tg of the
composition, as set forth in Inhale Therapeutic Systems' International Patent
Publication WO 98/16205.
FSP contained in the dry powder formulations is present in a quantity
sufficient to form a pharmacologically effective amount when administered by
inhalation to the lung. The dry powders of the invention will generally
contain from
about 0.1 % by weight to about 99.9% by weight FSP, more typically from about
0.5
to 80% by weight FSP, and preferably from about 1 to 75% by weight FSP.
Preferred
compositions contain from about I .5 to 70% FSP, and more preferably from
about 12
to 60% by weight FSP, depending upon the specific activity of FSP contained in
the
formulation and the type of treatment (i.e., ovulation induction versus super
ovulation
therapy). In one preferred embodiment of the invention, the dry powder
contains
about 5% by weight FSP. In an alternate embodiment, the dry powder contains
about
15% by weight FSP. Correspondingly, the amount of excipient materials) will
range
from about 99.9 to 0.1 % by weight, more typically from about 99.50% to 20% by
weight, and preferably from about 99% to 25% by weight. Preferred compositions
contain from about 98.5 to 30% by weight excipient material, and more
preferably
contain from about 88 to 40% by weight excipient material. Leucine-containing
FSP
26
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
powders will typically contain from about 15-85% by weight leucine, preferably
from
about 20-80% by weight leucine, and more preferably from about 40-60% by
weight
leucine.
The composition of representative FSP dry powders for pulmonary delivery is
provided at least in Examples 2 and 11. As can be seen from the data provided
in
Example 2, Table 1, compositions containing various combinations and relative
amounts of FSP and excipient(s) resulted in powders having good particle size
characteristics (all powders possessed MMDs less than 3.5 microns, while 80
percent
of powders possessed MMDs less than 1.5 microns) and which exhibited a minimal
change in higher order aggregate formation of FSP upon powder manufacture
(Example 3). The findings detailed in Example 3 provide evidence of another
surprising feature of the FSP compositions of the invention, i. e., their
ability to
stabilize monomeric FSP against higher order aggregate formation during powder
manufacture and processing. This is important, since the formation of higher
order
aggregates of FSP can adversely affect its bioactivity, due to impaired
receptor
binding, which will in turn reduce the potency of the resultant powders.
III. Preparing FSP Dry Powders
Dry powder FSP formulations are preferably prepared by spray drying under
conditions that result in a substantially amorphous glassy or a substantially
crystalline
bioactive powder as described above. Spray drying of the FSP-solution
formulations
is carried out, for example, as described generally in the Spray Drying
Handbook, S'~'
ed., (1991), and in Platz, R., et al., International Patent Publication No. WO
97/41833,
Nov. 13, 1997.
To prepare an FSP solution for spray drying, FSP is generally dissolved in a
physiologically acceptable aqueous buffer, e.g., a citrate buffer or a citrate
glyeine
buffer, typically having a pH range from about 2 to 9. The pH range of pre-
dried
solutions is generally maintained between about 4 and 10, with near neutral pH
being
preferred, since such pHs may aid in maintaining the bioactivity or
physiological
compatibility of the powder after dissolution of powder within the lung. The
aqueous
formulation may optionally contain additional water-miscible solvents, such as
alcohols and the like. Representative alcohols include methanol, ethanol,
propanol,
isopropanol, and the like. FSP solutions will generally contain FSP dissolved
at a
27
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
concentration from 0.01 % (weight/volume)to about 10% (weight/volume), usually
from 0.1 % to 1 % (weightlvolume).
In turning now to the results provided in Example 1, it can be seen that
representative solution formulations of FSP prior to spray drying showed no
appreciable decline in bioactivity upon storage for several days, and after
repeated
freeze-thaw cycles, indicating the relatively high stability of such solution
formulations.
The FSP-containing solutions are then spray dried in a conventional spray
drier, such as those available from commercial suppliers such as Niro A/S
(Denmark),
Buchi (Switzerland) and the like, resulting in a stable, FSP dry powder.
Optimal
conditions for spray drying the FSP solutions will vary depending upon the
formulation components, and are generally determined experimentally. The gas
used
to spray dry the material is typically air, although inert gases such as
nitrogen or argon
are also suitable. Moreover, the temperature of both the inlet and outlet of
the gas
used to dry the sprayed material is such that it does not cause deactivation
of FSP in
the sprayed material. Such temperatures are typically determined
experimentally,
although generally, the inlet temperature will range from about 50° C
to about 200° C
while the outlet temperature will range from about 30° C to about
150° C.
Alternatively, FSP dry powders may be prepared by first drying a solution
containing FSP and exciptient(s) by lyophilization, vacuum drying, spray
freeze
drying, super critical fluid processing, or other forms of evaporative drying,
and then
further processing the dry material to obtain a FSP dry powder having aerosol
properties suitable for administration into the deep lung and an acceptable
ED, by
blending, grinding or jet milling the dried formulation. In some instances, it
is
desirable to make FSP dry powder formulations possessing improved
handling/processing characteristics, e.g., reduced static, better flowability,
low caking,
and the like, by preparing compositions composed of fine particle aggregates,
that is,
aggregates or agglomerates of the above-described FSP dry powder particles,
where
the aggregates are readily broken back down to the fine powder components for
pulmonary delivery, as described, e.g., Johnson, et al., U.S. Patent No.
5,654,007,
Aug. 5, 1997, incorporated herein by reference. Alternatively, the FSP powders
may
be prepared by agglomerating the powder components, sieving the materials to
obtain
the agglomerates, spheronizing to provide a more spherical agglomerate, and
sizing to
28
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
obtain a uniformly-sized product, as described, e.g., and in Ahlneck, C.; et
al.,
International PCT Publication No. W095/09616, April 13, 1995, incorporated
herein
by reference. The FSP dry powders of the invention may also be prepared by
blending, grinding or jet milling formulation components directly in dry
powder form.
The FSP dry powders are preferably maintained under dry (i.e., relatively low
humidity) conditions during manufacture, processing, and storage.
Irrespective of the drying process employed, the process will preferably
result
in particles composed of FSP in non-aggregated form, or having an extent of
higher -
order aggregate formation that is substantially unchanged from that observed
in the
pre-dried material, as illustrated in Example 3. Moreover, processes suitable
for
preparing the FSP formulations of the invention are those in which the
bioactivity of
the FSP is not adversely affected, i.e., the bioactivity of the resulting
powder is
reduced by no more than about 40-50%, preferably by no more than about 30-40%,
and more preferably by no more than about 15%-30% in comparison to the
bioactivity
of the FSP formulation prior to drying/sizing. Illustrative FSP dry powders
which
exhibited no significant loss in bioactivity upon drying are described in
Example 8.
Provided in Example 2, and more specifically in Table 1, are exemplary FSP
compositions in accordance with the invention. As can be seen, FSP dry powders
were obtained in high yields, typically between about 70 and 80%, illustrating
the
ability to reproducibly prepare large quantities of FSP dry powders suitable
for
pulmonary delivery. Moreover, the extent of FSP sialylation was shown to be
unaffected by spray drying (Example 5), illustrating the chemical stability of
FSP
under conventional spray drying conditions. This is important since the
prolonged
bioactive half life of FSP is a result of its oligosaccharide content; FSP
asialoglycoproteins (i.e., proteins stripped of sialic acid) are cleared
rapidly by the
liver.
IV. Features of FSP Dry Powders
The FSP powders of the invention are further characterized by several
features, most notably, the ability of the powder to penetrate to the tissues
of the
lower respiratory tract (i.e., the alveoli) for subsequent entry into the
bloodstream
(see, e.g., Examples 10 and 18). It has been found that certain physical
characteristics
of the FSP dry powders, to be described more fully below, are important in
maximizing the efficiency of aerosolized delivery of such powders to the deep
lung.
29
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
The FSP dry powders are composed of particles effective to penetrate into the
alveoli
of the lungs, that is, having a mass median diameter (MMD) from about 0.1 to
20 pm.
Typically, the MMD of the particles is less than about 10 pm (e.g., ranging
from
about 0.1 to 10 pm), preferably less than 7.5 pm (e.g., ranging from about 0.5
to 7
microns), and most preferably less than 5 pm, and usually being in the range
of 0.1
pm to 5 pm in diameter. Preferred powders are composed of particles having an
MMD from about 1 to 3.5 Vim. Numerous examples of respirable FSP powders of
varying concentrations of active agent and excipients and composed of
particles
within this preferred size range have been prepared (e.g., Table 1, column 10;
Example 2). In some cases, the FSP powder will also contain non-respirable
carrier
particles such as lactose, where the non-respirable particles are typically
greater than
about 40 microns in size.
The FSP powders of the invention are further characterized by an aerosol
particle size distribution less than about 10 pm mass median aerodynamic
diameter
(MMAD), and preferably less than 5 pm, and more preferably less than about 3.5
pm.
The mass median aerodynamic diameters of the powders will characteristically
range
from about 0.5 - 5.0 pm, preferably from about 1.0 - 4.0 um MMAD, more
preferably
from about 1.5 - 3.5 pm MMAD, and even more preferably from about 1.5 to 3.0
pm.
To further illustrate the ability to prepare FSP powders having an aerosol
particle size
distribution within a range suitable for pulmonary administration, exemplary
FSP dry
powders composed of particles having an aerosol particle size distribution
less than
about 5 pm MMAD, and more specifically, characterized by MMAD values less than
3.5 pm, are illustrated in Table 8 (Example 7), in Table 16 (Example 11), in
Table 30
(Example 21 ) and in Table 31 (Example 22).
FSP dry powders of the invention are characterized, in another respect, by a
relative pulmonary bioavailability between about 1 % to 60%. The relative
pulmonary
bioavailability of a powder of the invention will typically fall between
about: 1-60%,
1-30%, 5-30%, 10-30%, 15-30%, 15-25%, 20-25%, 1-20%, 2-20%, 3-20%, 4-20%, 1-
15%, 1-10%, 2-10%, 3-10% and 4-10%. Preferably, an FSP powder will exhibit a
relative pulmonary bioavailability between about I-30% and more preferably
between
about 1-20%, as supported by the data provided herein.
The FSP dry powders generally have moisture content below about 10% by
weight, usually below about 5% by weight, and preferably below about 3% by
weight.
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Such low moisture-containing solids tend to exhibit a reduced tendency towards
degradation of protein. The moisture content of representative FSP dry powders
prepared as described herein is provided in Example 6.B., Table 6.
The delivered dose efficiency (DDE) of these powders is greater than 30% and
usually greater than 40%. More preferably, the DDE of the FSP powders of the
invention is greater than 50%, and is often greater than 55%. Even more
preferably,
the DDE of an FSP powder is greater than 60%. Highly preferred are powders
having
DDE values greater than 70%, and even more preferred are powders having DDE
values greater than about 75%. In looking at Example 7, Table 7,and Example
11,
Tables 14 and 15, it can be seen that the applicants have successfully
prepared a large
number of representative FSP dry powders with DDE values greater than or equal
to
50%. Formulations which exhibited and maintained particularly good powder
dispersibilities, as indicated by their DDE values, included L2001, L2005,
L2006,
L2008, L2010, L2012, L2014, L2016, L2017, L2018, and L2020, the compositions
of
which are provided in Table 6, and 99348, 99349, 99423, 99425, 99426, 99455,
99454, and 99457 (Tables 14, 15).
The FSP powders of the invention will typically possess a bulk density value
ranging from about 0.10 to 10 gram/cubic centimeter, preferably from about
0.15 to 5
gram/cubic centimeter, more preferably from about 0.15 to 4.0 grams/cubic
centimeter, even more preferably from about 0.17 to 1 gram/cubic centimeter,
even
more preferably from about 0.17-0.75 gram/cubic centimeter, and most
preferably
from about 0.2 to 0.75 gram/cubic centimeter.
Powders of the invention will possess a wide range of specific bioactivity
values, depending upon the type of FSP (e.g., impure FSP mixtures containing
FHP
and other proteins versus highly purified forms of FSP) contained in the
powder.
Powders of the invention will generally possess a specific bioactivity greater
than 100
IU per gram of powder. In most instances, the specific bioactivity of the FSP
powder
is: greater than 250 IL1 per gram of powder, greater than 500 ILJ per gram of
powder,
greater than 1,000 IU per gram of powder, greater than 2,000 IU per gram of
powder,
greater than 5000 IU per gram of powder, greater than 10,000 IU per gram of
powder,
greater than 25,000 IU per gram of powder, greater than 50,000 ILJ per gram of
powder, greater than 100,000 IU per gram of powder, greater than 250,000 IL1
per
gram of powder, greater than 500,000 IL1 per gram of powder, greater than
1,000,000
31
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
per gram of powder, greater than 2,500,000 IU per gram of powder, greater than
5,000,000 IL1 per gram of powder, or greater than 10,000,000 IU per gram of
powder.
An additional measure for characterizing the overall aerosol performance of a
dry powder is the aerosol performance coefficient (APC). The APC value for FSP
powders as described herein is greater than 0.10, typically greater than 0.15,
and more
preferably greater than 0.25. As can be seen from the APC values provided in
Tables
8 and 14, FSP dry powders have been prepared which are particularly well
suited for
pulmonary delivery, as evidenced by APC values greater than 0.25. Such powders
contain a large proportion of small aerosol particle sizes and are thus
extremely
effective when delivered aerosolized form, in (i) reaching the alveolar region
of the
lung, followed by (ii) diffusion to the interstitium and (iii) subsequent
passage into the
bloodstream through the endothelium.
V. Pulmonary Administration of FSP Compositions
The FSP dry powder formulations described herein may be delivered using
any suitable dry powder inhaler (DPI), i.e., an inhaler device that utilizes
the patient's
inhaled breath as a vehicle to transport the dry powder drug to the lungs.
Preferred
are Inhale Therapeutic Systems' dry powder inhalation devices as described in
Patton,
J.S., et al., U.S. Patent No. 5,458,135, Oct. 17, 1995; Smith, A. E., et al.,
U.S. Patent
No. 5,740,794, Apr. 21, 1998; and in Smith, A. E., et. al., U.S. Patent No.
5,785,049,
July 28, 1998, herein incorporated by reference. When administered using a
device of
this type, the powdered medicament is contained in a receptacle having a
puncturable
lid or other access surface, preferably a blister package or cartridge, where
the
receptacle may contain a single dosage unit or multiple dosage units.
Convenient
methods for filling large numbers of cavities (i.e., unit dose packages) with
metered
doses of dry powder medicament are described, e.g., in Parks, D. J., et al.,
International Patent Publication WO 97/41031, Nov. 6, 1997, incorporated
herein by
reference.
Also suitable for delivering the FSP powders described herein are dry powder
inhalers of the type described, for example, in Cocozza, S., et al., U.S.
Patent No.
3,906,950, Sept. 23, 1974, and in Cocozza, S., et al., U.S. Patent No.
4,013,075,
March 22, 1977, incorporated herein by reference, wherein a pre-measured dose
of
FSP dry powder for delivery to a subject is contained within a hard gelatin
capsule.
32
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Other dry powder dispersion devices for pulmonary administration of FSP dry
powders include those described, for example, in Newell, R. E., et al.
European Patent
No. EP 129985, Sept. 7, 1988); in Hodson, P. D., et al., European Patent No.
EP472598, July 3, 1996; in Cocozza, S., et al., European Patent No. EP 467172,
April
6, 1994, and in Lloyd, L.J. et al., U.S. Patent No. 5,522,385, June 4, 1996,
incorporated herein by reference. Also suitable for delivering the FSP dry
powders of
the invention are inhalation devices such as the Astra-Draco "TURBUHALER".
This
type of device is described in detail in Virtanen, R., U.S. Patent No.
4,668,218, May
26, 1987; in Wetterlin, K., et al., U.S. Patent No. 4,667,668, May 26, 1987;
and in
Wetterlin, K., et al., U.S. Patent No. 4,805,811, Feb. 21, 1989, all of which
are
incorporated herein by reference. Other suitable devices include dry powder
inhalers
such as Rotahaler~ (Glaxo), Discus~ (Glaxo), SpirosT"' inhaler (Dura
Pharmaceuticals), and the Spinhaler~ (Fisons). Also suitable are devices which
employ the use of a piston to provide air for either entraining powdered
medicament,
lifting medicament from a carrier screen by passing air through the screen, or
mixing
air with powder medicament in a mixing chamber with subsequent introduction of
the
powder to the patient through the mouthpiece of the device, such as described
in
Mulhauser, P., et al, U.S. Patent No. 5,388,572, Sept. 30, 1997, incorporated
herein
by reference.
The FSP dry powders may also be delivered using a pressurized, metered dose
inhaler (MDI), e.g., the Ventolin~ metered dose inhaler, containing a solution
or
suspension of drug in a pharmaceutically inert liquid propellant, e.g., a
chlorofluorocarbon or fluorocarbon, as described in Laube, et al., U.S. Patent
No.
5,320,094, June 14, 1994, and in Rubsamen, R.M., et al, U.S. Patent No.
5,672,581
(1994), both incorporated herein by reference. When administered by a metered
dose
inhaler, the FSP composition is preferably absent a surfactant (e.g., fatty
acids, bile
salts, phospholipids, or alkyl saccharides) for enhancing the systemic
absorption of
FSP, since, when administering the compositions of the invention, such
compounds
are unnecessary for achieving therapeutic levels of FSP in the bloodstream.
Alternatively, the powders described herein may be dissolved or suspended in
a solvent, e.g., water or saline, and administered by nebulization. Nebulizers
for
delivering an aerosolized solution include the AERxTM (Aradigm), the
Ultravent~
(Mallinkrodt), and the Acorn II~ (Marquest Medical Products).
33
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Prior to use, the FSP dry powders are generally stored under ambient
conditions, and preferably are stored at a temperature at or below about
25°C, and
relative humidity (RH) ranging from about 30 to 60%. More preferred relative
humidity conditions, e.g., less than about 30%, may be achieved by the
incorporation
of a desiccating agent in the secondary packaging of the dosage form. The FSP
powders of the invention demonstrate no significant loss of bioactivity upon
storage;
moreover, accelerated stability studies carried out on three representative
dry powder
formulations (Example 7.C., Table 9, and Fig. 1 ) illustrate the ability to
prepare
respirable FSP dry powders characterized not only by good aerosol performance,
but
having good stability, as well. Moreover, both preliminary in vitro results
for
intratracheally administered FSP solutions in rats (Example 9) and pulmonary
administration of FSP powders to monkeys (Examples 10 and 17) revealed
reasonably
high bioavailability values, i.e., from about 5% to 20% and from 18% to 26%,
relative
to administered subcutaneously FSP, further indicating the operability of
treating
infertility by pulmonary administration of dry powders of the present
invention.
VI. Therapeutic Applications
The FSP dry powder of the invention is useful, when administered by
inhalation for deposition in and absorption from the lung in a therapeutically
effective
amount, for treating infertility in both male and female subjects. More
specifically,
the methods of the present invention are particularly useful in therapeutic
applications
for the treatment of patients who are deficient in, or could otherwise benefit
from,
levels of FSP that are augmented over those produced endogenously.
When inhaled into the deep lung in dry powder form, FSP is effective to
stimulate ovarian follicular growth in women who are not suffering from
primary
ovarian failure. Following pulmonary delivery of FSP, measurement of plasma
inhibin levels can be used to provide a pharmacodynamic marker of FSP
activity.
Levels of follicular growth (an increase in the number of follicles greater
than about
lOmm in diameter, (e.g., determined by ultrasound) and estradiol secretion
(i.e.,
serum estradiol levels) can also be used to assess the effects of FSP; in
males, Serotoli
cell production levels can also provide an additional indicator of the
efficacy of
treatment. Preferably, pulmonary delivery of an FSP dry powder formulation as
described herein is effective to result in a level of one or more of the above
markers
34
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
(follicular growth, serum estradiol, inhibin, Serotoli cell production) that
is increased
relative to its baseline level measured prior to FSP treatment.
The FSP powders of the invention are also useful, when administered to the
deep lung, for Assisted Reproductive Technologies (ART). In such instances,
FSP is
administered to ovulatory infertile females undergoing stimulation of multiple
follicular development for In Vitro Fertilization (IVF), Embryo Transfer (ET),
and
other assisted reproductive technologies. Generally, for use in female
infertility-
related conditions, FSP is administered to the deep lung for one or more
treatment
cycles of a period of from about 7 to 21 days or more to stimulate follicular
growth.
In the case of infertile females, in order to effect final maturation of the
follicle and
ovulation in the absence of a LH (luteinizing hormone) surge, human chorionic
gonadotropin (hCG) is administered after sufficient follicle development has
occurred
(as described above). When used for treating male infertility, the FSP dry
powder is
typically administered for at least about six months, and typically for at
least about a
year.
Typically, treatment of the above-described conditions is effected by
administering therapeutically effective doses of FSP dry powder that, on
average,
range from at least about 0.5 to 35 micrograms FSP/kilogram of patient daily,
and
preferably at least about 1 to 20 micrograms FSP/kilogram of patient daily,
depending
upon the specific activity of FSP contained in the composition. Suitable
dosages are
known to medical practitioners and will, of course, depend upon the particular
disease
state, specific activity of the composition being administered, and the
particular
patient undergoing treatment. For example, the amount of FSP administered per
unit
dosage form will generally range from about 5 ILJ to about 12,000 IU FSP,
preferably
from 5 IU to about 1000 IU FSP more preferably from about 37 IU to 500 IU, and
even more preferably from about 50 IU to 300 IU FSP. Preferably, a
therapeutically
effective amount will range, on average, from about: 10 to 1000 IU FSP per
day, 50
to 3,000 IU FSP per day, 75 ILJ to about 600 IU of FSP per day, or from about
200 IU
to 12,000 IU FSP per day, depending upon the dosing regimen followed by the
subject (i.e., the number of aerosolized doses delivered over a period of 24-
hours or
greater) and the type of treatment. In some instances, to achieve the desired
therapeutic amount, it may be necessary to provide for daily repeated
administration,
i.e., repeated individual inhalations of a particular metered dose per day,
where the
individual administrations are repeated until the desired daily dose is
achieved.
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
The following examples illustrate, but in no way are intended to limit the
scope of the present invention.
All articles, books, patents and other publications referenced herein are
hereby
incorporated by reference in their entirety.
Examples
Materials and Methods
Materials
uFSH. FSH derived from human urine (uFSH) was used as a source of FSP in
certain experiments. Lyophilized uFSH powder (Vitro Diagnostics, Inc.,
Boulder,
CO) was typically reconstituted to 5 mg/ml prior to preparation of formulation
solutions. Initial formulations (powder lots no. 898025 through 898054) were
prepared directly from the vendor-supplied uFSH material. Formulations 898067
through 898112 were prepared from uFSH that was further purified by
ultrafiltration
(Centricon 10, 10 kD molecular weight cutoff) to remove residual ammonium
bicarbonate present in the vendor-supplied material. Glycoprotein integrity
after
desalting was confirmed by SE-HPLC and the percent glycoprotein recovery was
determined by UV. The remaining solution was tested for solution stability at
4° C
overnight and for 3- freeze-thaw cycles.
Excipients. Excipients were obtained from commercial sources as follows:
mannitol (Mallinckrodt Specialty Chemical Co., Paris, Kentucky; U.S.P; FW
180),
sodium citrate~2 HZO (J.T.. Baker Inc., Phillipsburg, NJ; U.S.P; FW 294.1),
citric
acid~Hz0 (J.T. Baker, Phillipsburg, NJ; U.S.P; FW 210.14), sucrose (EM
Science,
Gibbstown, NJ; FW 342.2), raffinose~5H20 (Pfanstiehl Lab., Inc., Waukegan, IL;
FW
594.5), glycine (J.T. Baker, Inc., Phillipsburg, NJ; FW 75.07), trehalose~2Hz0
(Pfanstiehl Lab., Inc., Waukegan, IL; FW 350.3), leucine (Sigma, St. Loius,
MO)
Sterile water for Irrigation (USP) was used for preparation of formulation
solutions and reconstituted uFSH drug substance.
Methods
Spectroscopy. The concentrations of stock uFSH solutions, pre-spray dried
solutions, and reconstituted powders were determined by ultraviolet/visible
(UV/Vis)
spectrophotometry. Sample solutions were diluted to give absorbance values
between
36
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
0.1-1.5 unit at 276 nm. Solution turbidity at 400 nm was used as a measure of
insoluble particle/aggregate formation.
Particle size measurement. Mass median diameters (MMD) of the powders
were measured using a Horiba CAPA-700 particle size analyzer (Horiba
Instruments
inc., Irvine, CA). Measurements were based upon centrifugal sedimentation of
dispersed particles in suspending medium. Mass median diameter, which is based
on
the particle's Stokes' diameter, was calculated using the particle densiy and
the
density and viscosity of the suspending medium. The density of the powder was
set
as 1.5 g/cm3 for all powders. (This nominal value was used for all powders
analyzed
and is within a range that is typical for spray dried powders). Particle size
measurements were conducted with about 5 - 10 mg powder suspended in 5 ml
Sedisperse A-11 (Micromeritics, Norcross, GA) and dispersed by sonication for
10
minutes. The range over which particle size data was gathered was set to 0.4
to 10.0
Vim.
Differential Scanning Calorimetr~(DSC). The thermal behavior of spray
dried powders (2 -10 mg in hermetically sealed aluminum pans) was analyzed by
a
TA modulated differential scanning calorimeter 2920. Typically, samples were
equilibrated at -60°C for 10 min and heated at 2°C/min to
200°C under a NZ stream.
The modulation amplitude was ~I°C or + 0.3°C and the modulation
period was 60 sec
where at least four cycles during a transition were achieved. The instrument
was
calibrated by Indium for temperature and heat capacity. A baseline scanning
was
performed before sample running.
Andersen Cascade Impactor. An Andersen cascade impactor (a sieve-like
apparatus with a series of stages that capture particles on plates by inertial
impaction
according to their size) was used to determine the MMAD and particle size
distribution of aerosolized powder formulations in an air stream. The plates
were
weighed before and after testing and the mass of powder deposited on the plate
of
each stage was determined. Unless otherwise indicated, studies were undertaken
using a traditional Andersen cascade impactor having eight stages (from top to
bottom
stages 0 to 7) with cut-off sizes ranging from 9.0 to 0.4 pm, and a final
filter stage that
37
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
traps particles < 0.4 ~m when operated at a flow rate of 28.3 L/min. The
device test
set-up was similar to the DDE test, except that the cascade impactor and a USP
(United States Pharmacopoeia, USP 23, chapter <601 >) were attached to the
device
mouthpiece rather than to a filter. Multiple dispersions were typically
conducted for
each cascade impaction run to achieve gravimetrically accurate data, e.g., 20
FSP-
filled blister packs (2 mg fill weight per blister pack).
Andersen Short Stack (SS~Method. In the SS method (used to determine
APCs), the order in which the stages were placed was altered from the
conventional
Andersen cascade impactor set-up as described above. From the top, stage 0 was
utilized for inlet cone attachment to connect the throat. Stage 3 was
positioned next,
beneath stage 0, followed by the filter stage (stage F). The powder-containing
stream
of air passes only through stages 0 and 3; air (but not powder) flows through
the other
stages, which are placed under stage F to hold the remainder of the assembly
in place.
A pre-weighed filter was placed on stage F and captured particles < 3.3 Vim. A
second filter was placed on an inverted plate under stage 3, and captured
particles >
3.3 pm. For the studies described herein, one BP (blister pack) containing 2
mg of
FSP powder composition was dispersed in an aerosol delivery device and a
vacuum
was pulled at 28.3 L/min as per USP methodology. This process was then
repeated
two times for a target mass of 6 mg per run. The filters were then removed and
weighed to determine the amount of powder deposited.
EXAMPLE 1
Pre-formulation evaluation of uFSH: Characterization of Drug Properties
Pre-formulation activities were undertaken to evaluate drug properties and to
evaluate the stability of solution samples stored prior to analysis. Pre-
formulation
activities included evaluating glycoprotein stability to freeze-thaw cycling,
adsorption
to surfaces, solubility, and solution stability of (i) reconstituted bulk uFSH
material,
(ii) formulated uFSH in solution (pre-spray drying), and (iii) reconstituted
solutions of
uFSH powders. The pH range of pre-spray dried solutions (as described in Table
1 )
was maintained between 5 and 8. This pH range was investigated in order to
monitor
the effects of pH on glycoprotein stability and solid state properties. Near
neutral pH
was selected to help maintain physiological compatibility after dissolution of
powder
38
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
within the lung. Preliminary results indicated that all formulated uFSH
solutions were
stable when stored at 4°C for 4 to 7 days, or when stored at ambient
temperature for 1
day, or after repeated freeze-thawing cycles. Preliminary data indicated that
the
adsorptive loss of glycoprotein to silicon tubing was minimal. The first 2 mL
of 175
~g/mL uFSH formulation solution resulted in 10 pg of uFSH adsorption to the
tubing
(2 m x 4.6 mm i.d.) used with the Buchi spray dryer. No further loss of uFSH
was
observed in subsequent aliquots.
EXAMPLE 2
Preparation of Representative uFSH Solution Formulations and Preparation of
Powders for Pulmonary Delivery
Formulation solutions were prepared at a total solids content of 0.5% (w/v).
The pH of each solution was determined, and solutions were then inspected for
clarity
prior to spray drying. Table 1 lists the compositions of all pre-spray dried
solutions.
Mannitol was the sugar unless otherwise noted. Powders were produced by spray
drying an aqueous solution of uFSH and excipient(s) using a Buchi 190 mini
spray
dryer (Buchi Labortechnik AG, Meierseggstrasse, Switzerland) equipped with a
customized nozzle (as described in Platz, et al., 1997) and cyclone. Typical
formulations were prepared from solutions containing less than 300 mg of total
solids.
High collection efficiencies, usually between 70 to 80%, were attained,
resulting in a
satisfactory powder supply for subsequent powder characterizations.
Table 1. Description of spray dried solutions and powder processing results.
Form. Lot uFSH :Sugar:CitratepH pH YieldYield MMD"
ID # (wt:wt:wt) bsdz asd3 (g) (%) (pm)
- 898023 0:15:85 6.7 6.7 0.15 15 nd
- 898024 0:15:85 6.7 6.7 0.17 17 nd
L2001 898025 3.5:15:81 7.7 7.7 0.17 81 0.98
L2002 89802653.5:81:15 7.8 7.4 0.03 13 nd
L2003 898027 3.5:48:48 7.9 7.4 0.16 77 1.20
L2004 898028 3.5:32:64 7.8 7.4 0.16 76 1.00
L2005 898029 3.5:95:0 7.8 7.3 0.11 50 1.44
39
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Form. Lot uFSH':Sugar:CitratepH pH Yield Yield MMD"
ID # (wt:wt:wt) bsd' asd3 (g) (%) (gym)
L2006 898030 3.5:15:81 7.0 6.9 0.16 74 1.04
L2006 898037 3.5:15:81 6.9 6.8 0.17 80 1.38
L2007 898038 3.5:95:0 7.9 7.7 0.18 79 1.10
(sucrose)
L2008 898039 3.5:95:0 7.8 7.6 0.17 74 1.00
(raffmose)
L2006 898040 3.5:15:81 6.8 6.9 0.18 77 1.31
L2010 898046 5:15:80 7.0 7.0 0.27 77 1.21
A
L2010 898046 5:15:80 7.0 7.0 0.29 79 0.96
B
L2011 898047 5:80:15 7.7 7.4 0.16 45 3.16
L2012 898048 5:15:80 5.3 5.3 0.27 78 1.20
L2013 898049 10:14:76 7.4 na 0.27 82 1.08
L2014 898050 5:31:64 7.1 7.0 0.27 78 0.94
~,
L2015 898051 5:80:15 6.3 5.8 0.10 29 3.38
'
L2006 89805253.5:15:81 6.9 6.9 0.26 75 1.27
'
L2006 898053 3.5:15:81 6.9 6.9 0.35 81 1.06
',
L2016 898054 5:15:80 7.7 7.4 0.25 81 1.06
L2020 898067 10:14:76 7.1 7.1 0.19 77 1.10
'
L2012 898068 5:15:80 5.1 5.1 0.18 71 1.20
L2016 898069 5:15:80 7.6 7.5 0.17 69 1.07
L2006 898070 3.5:15:81 7.0 7.0 0.17 68 1.18
L2010 898084 5:15:80 7.1 7.1 0.33 77 1.07
L2017 898085 5:95:0 7.5 7.1 0.25 59 1.59
L2018 898086 5:95:0 7.4 7.2 0.33 77 0.99
(raffmose)
L2019 89808755:64:31 7.4 7.1 0.07 16 2.66
(raffinose)
L2010 898110 5:15:80 6.9 6.9 0.98 81 1.03
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Form. Lot uFSH :Sugar:CitratepH pH YieldYield MMD''
#
ID (wt:wt:wt) bsdz asd3 (g) (%) (gm)
L2017 898111 5:95:0 7.0 6.7 0.80 65 1.44
L2018 898112 5:95:0 7.2 7.0 0.97 80 0.83
(raffinose)
target uFSH content, error was approximately ~ 0.2 %; z solution pH before
spray
drying; 3 pH of reconstituted powder; 4 determined by centrifugal
sedimentation
(Horiba); 5 lot excluded from testing.
The pH of the reconstituted powders (after spray drying, Table 1 ) from early
lots demonstrated an unexpected drop in pH after spray drying, especially in
unbuffered (i.e., no citrate) formulations. This was attributed to the
presence of
ammonium bicarbonate in the drug substance material. The pH drop was minimized
upon use of purified drug substance and was insignificant in formulations
containing
buffer.
EXAMPLE 3
Chemical Stability Evaluation of Spray Dried Powders: Loss Of Monomeric
uFSH Via Formation Of Higher Order Aggregates.
The chromatographic purity and content of formulated uFSH solutions were
assessed using a SE-HPLC (size exclusion high performance liquid
chromatography)
methodology, as described below.
Table 2. SE-HPLC Parameters
Column: Tosohaas GW3000XL or GW2000XL , 5 um, 7.8x30mm
Mobile Phase: ~ O.1M sodium phosphate / 5% IPA, pH 7.5
Column Temperature: ~ Ambient
Flow Rate: ~ 0.5 mL/min
Typical Sample: ~ 3.5-10.0 p.g
Detection: I 214 nm
The intact monomer unit, its dissociated subunits and higher order aggregates
eluted at retention times of 18.6 minutes, 20.2 minutes and 16.2 minutes
(GW3000XL
41
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
column), respectively, under the SE-HPLC conditions specified above. Retention
times were decreased on the GW2000XL column.
The precision of the SE-HPLC method with the GW3000XL column was
evaluated by six replicate injections of a single concentration of uFSH drug
substance
(173 ~g/mL). The relative standard deviation was 0.0% and 0.2% for purity and
response factor, respectively. The linearity of the SE-HPLC method was
evaluated in
the range of 0.87 to 17.4 mg of uFSH. A single concentration of uFSH solution
(173
pg/mL) was injected at various volumes onto the column; each injected volume
was
applied in duplicate. The response indicated linearity with Rz=0.99999.
Chromatographically, the a, and (3 subunits co-eluted and were not well
resolved from the monomeric form of uFSH. Resolution profiles were maintained
between 1 ~,g and 10 ~g loading. Thus, quantitation of uFSH was determined by
integration of the combined peak areas of the monomer and subunit peaks. The
higher order aggregates were better resolved from monomer and were integrated
separately. In summary, this method was utilized only to indicate the loss of
monomeric uFSH through the formation of higher order aggregates.
Table 3. SE-HPLC Analysis of Bulk Powder and After Spray Drying
Higher
order
uFSH
Aggregates
(by area)
ID Lot % bsd' % asd' change
in
L2001 98025 0.34 1.98 1.64
L2002 98026 0.35 0.65 0.30
L2003 98027 0.32 1.07 0.75
L2004 98028 0.30 1.11 0.81
L2005 98029 0.30 1.50 1.20
L2006 98030 0.28 0.83 0.55
L2006 98037 0.35 0.49 0.14
L2007 98038 0.31 0.36 0.05
L2008 98039 0.18 0.23 0.05
L2009 98040 0.20 0.37 0.17
L2010 98046a0.61 2.20 1.59
42
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Higher
order
uFSH
Aggregates
(by
area)
ID Lot % bsd % asd changein
L2010 98046b 0.61 6.72 6.11
L2011 98047 0.56 0.98 0.42
L2012 98048 0.64 1.88 1.24
L2013 98049 0.69 1.03 0.34
L2014 98050 0.54 1.07 0.53
L2015 98051 0.60 1.77 1.17
L2006 98052 0.48 1.22 0.74
L2006 98053 0.55 1.13 0.58
L2016 98054 0.55 1.88 1.33
L2020 98067 0.70 1.23 0.53
L2012 98068 0.58 1.08 0.50
L2016 98069 0.70 1.25 0.55
L2006 98070 0.68 1.50 0.82
L2010 98084 0.43 1.02 0.59
L2017 98085 0.49 0.51 0.02
L2018 98086 0.37 0.52 0.15
L2019 98087 0.44 0.54 0.10
L2010 98110 0.82 1.68 0.86
L2017 98111 0.78 1.01 0.23
L2018 98112 0.80 0.78 -0.02
~bsd=before spray drying; Zasd=after spray drying
Table 3 above summarizes the change in percent higher order aggregate that
occurred due to spray drying. Higher order aggregates were present as
impurities in
bulk drug substance (<0.8% purity by SE-HPLC) and increased slightly after
spray
drying. Less than 2% increase in higher order aggregate was observed for all
uFSH
formulations after spray drying, except lot R98046b which showed >6% higher
order
aggregate (and was considered an outlier). No clear trend in higher order
aggregate
formation with pH, or citrate, or uFSH content was observed.
43
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
EXAMPLE 4
One Month and Long Term Stability of Packaged, Spray Dried Powders
The real time and accelerated stability of packaged powders were determined
on the basis of % change in higher order aggregates between initial and 1-
month time
points (Table 4). Powders were hand-filled to a total mass of 2 mg in blister
packs
(BPs). The blister packs were placed in Petri dishes (20-60 BPs/dish). The
Petri
dishes were then secondarily packaged in foil pouches with desiccant.
Table 4. uFSH Packaged Powder Stability Analyzed by SE-HPLC
Form/Lot (Size) Time point Higher
order
aggregate
%Total (+/-) %Change(+/-)
i
Pre-spray 0.68 0.02 na na
L2010/R98084 Initial 1.03 0.01 na na
(0.5 g)
1 month (40C)1.76 0.01 0.74 0.01
Pre-spray 0.60 0.02 na na
L2017/R98085 Initial 0.55 na na na
(0.5 g)
1 month (40C)1.25 0.05 0.70 0.04
Pre-spray 0.5 0.00 na na
8
L2018/R98086 Initial 0.65 0.02 na na
(0.5 g)
1 month (40C)0.69 0.03 0.05 0.03
Pre-spray 0.82 0.01 na na
L2010/R98110 Initial 1.70 0.01 na na
(1.25 g)
1 month (30C)1.83 0.02 0.16 0.02
1 month (40C)2.27 0.01 0.59 0.02
Pre-spray 0.78 0.01 na na
L2017/R98111 Initial 1.02 0.05 na na
( 1.25 g)
1 month (30C)1.18 0.07 0.17 0.07
1 month (40C)1.76 0.12 0.74 0.10
Pre-spray 0.80 0.01 na na
L2018/R98112 Initial 0.78 0.02 na na
(1.25 g)
1 month (30C)0.71 0.08 -0.08 0.10
1 month (40C)0.85 0.01 0.06 0.02
44
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Several of the representative packaged formulations demonstrated good
storage stability. Urinary FSH formulated with raffinose exhibited good
stability
under both sets of conditions. Mannitol/citrate and mannitol-based
formulations were
stable at 30°C for 1-month as supported by the insignificant increase
in higher order
aggregate (<0.2%). The higher order aggregate further increased as temperature
increased to 40°C for both formulations with a total of percentage
change <0.8%
after 1-month storage, indicating that both the mannitol/citrate and the
mannitol
formulations have comparable stability.
Long term stability (48-52 weeks) was similarly measured by SE-HPLC for
certain mannitol-citrate blister packaged uFSH powders (5% uFSH/15%
mannitol/80% citrate: L2012/powder lot 98048; L2010/powder lot 98110) stored
at
ambient temperatures and < 5% relative humidity. Powder L2012, which
demonstrated an initial higher order aggregate level after spray drying of
1.8%,
remained virtually unchanged after 52 weeks, with total higher order
aggregates at
1.5%. Similarly, the higher order aggregate level for powder L2010, initially
at 1.7%
after spray-drying, increased to only 2.6 percent when measured after storage
for 48
weeks in a sealed blister package.
EXAMPLE 5
Extent of uFSH Sialylation After Spray Drying
Due to the presence of potentially labile terminal sialic acid residues in
uFSH,
a colorometric method was utilized to investigate the integrity of uFSH
sialylation
after spray drying. In carrying out the method, terminal sialic acid groups
were
cleaved at low pH, followed by a copper catalyzed reaction with resorcinol.
The
absorbance of the product was measured at 560 nm and subsequently converted to
mnol sialic acid based on a calibration curve generated with N-
acetylneuraminic acid
(NANA). Triplicate measurements were carned out for both standards and
glycoprotein samples. Prior to the color forming reaction, the intact
sialylated
glycoproteins were separated from any free sialic acid by passing the sample
through
a NICK column. An aliquot of each eluted fraction was used for the reaction.
The linear range for this method extended to 80 nmol. The lower limit of
detection was found to be 2 nmol of sialic acid, which corresponds to the
ability to
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
detect a 1-2% loss for a 3.5% uFSH powder. The accuracy of the method was
tested
with fetuin as a control. The resulting sialic acid concentration was 380
nmol/mg
protein, which compared well with the average value of 370~35 nmol/mg reported
in
a comprehensive inter-laboratory ABRF study on Fetuin (ACS National Meeting,
April 1997).
The colorometric method was used to evaluate tvvo uFSH powders (898026,
898029). A 2 mM citrate solution was used to reconstitute the powders and to
elute
the sialylated glycoproteins from the NICK column. The resulting elution
profiles for
the two lots indicated the absence of free sialic acid in both of the
formulations. A
Bradford assay was conducted to confirm the presence of protein in fractions 1-
3.
Since cleavage of sialic acid is more likely under acidic conditions, the same
assay was employed to ascertain whether sialic acid cleavage would occur in
formulations prepared at lower pH. For this assay, powders 898046 and 898048
were utilized. The profiles obtained were similar to those observed at higher
pH.
The concentrations of the intact sialic acid for all four lots (Table 5) were
similar to the sialic acid concentration for the initial stock solution of
urinary FSH,
indicating that the extent of uFSH sialylation was unaffected by spray drying.
Thus,
the results in Table 5 illustrate the chemical stability of uFSH towards
desialylation
under conventional spray drying conditions.
Table 5. Quantitative Results from Sialic Acid Assay
Composition Sialic Acid
(%)
Lot uFSH MannitolCitrate (nmollmg protein)pH
898026 3.5 81 15 266 24 7.7
898029 3.5 96 0 282 33 7.8
898046 5 15 80 285 26 7.0
898048 5 15 80 288 12 5.3
stock 0.5 - - 297 1 S -
The results in Table 5 demonstrate that at near neutral or slightly acidic pH
values there is no loss of sialylation of uFSH in representative formulations
as a result
of spray drying. The average sialic acid content measured by this assay (8.8%
by
weight) is comparable to that for uFSH (8.96% by weight).
46
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
EXAMPLE 6
Solid State Characterization of uFSH Powders for Pulmonary Delivery
Representative uFSH powder formulations were further characterized to
explore the effects of various excipients and residual moisture on the solid
state
properties of the resulting powders, and their combined effect on protein
stability.
Modulated differential scanning calorimetry~DSC). DSC was used to
determine the heat flux profile of a given spray dried powder sample, to
measure its
glass transition temperatures (Tg), and to estimate the degree of
crystallinity. Glass
transition temperatures were also determined to provide an indication of the
potential
solid state stability of particular formulations, since high Tg values may
indicate a
greater potential for physical stability than low Tgs. DSC measurements were
conducted as described under the Materials and Methods section above. Table 6
summarizes the DSC data and the glass transition temperatures obtained for
representative uFSH powder formulations.
Looking at the data presented in Table 6, the amorphous raffmose
formulations showed a distinct glass transition near 100°C. In
addition, high Tgs (70
to 90°C) were observed for the high-citrate content formulations (>80%
citrate); the
Tg was followed by an exotherm attributed to crystallization of an anhydrous
polymorph of citrate. The mannitol/citrate formulations with less citrate
(<60%
citrate) exhibited lower Tgs (30 to 60°C), an exotherm at ~ 90°C
due to crystallization
of mannitol, and the subsequent melting of mannitol. Highly crystalline 95%
mannitol formulations exhibited no observable Tg; only an endotherm at
160°C
characteristic of the melting of crystalline mannitol. Looking more generally
at the
data, the effect of residual ammonium bicarbonate from impure uFSH appeared to
depress Tg values. Although citric acid (low pH) is reported to depress the Tg
of
citrate glasses [Lu, Q., et al., Pharm. Res., 15(8):1202-1206 (1998)], this
was not
readily apparent in the pH range investigated. These data demonstrate the
ability to
prepare stable uFSH powders of varied Tgs and degrees of crystallinity.
Thermo~ravimetric Analysis. Thermogravimetric analysis (TGA), a technique
for monitoring the weight loss from a sample upon heating from room
temperature to
200°C, was employed to determine the residual water content of the
above-described
uFSH dry powders. Weight loss observed upon heating from 2~ to 150°C
was
ascribed to the loss of residual moisture. The moisture content of spray dried
powder
47
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
(2-10 mg in hermetically sealed aluminum pans packed in glove box) was
analyzed
with a TA 2950 thermogravimetric analyzer. The tops of the sealed pans were
pierced immediately prior to loading into the TGA furnace to minimize water
uptake
from room air. Results are provided in Table 6. In looking at the results in
Table 6,
formulations prepared with impure uFSH exhibited a fairly high weight loss
upon
heating, which was later attributed to the presence of residual ammonium
bicarbonate
in the powder (ammonium bicarbonate begins to decompose at 60°C).
Moisture
content was less than 2% for sodium citrate-free powders prepared with
purified
uFSH, while high-citrate content powders generally contained between 2 and 6%
water.
Table 6. Solid state characteristics of uFSH Powders
Composition pH TGA Karl DSC
Form Form (wt/wt/wt) Fisher
ID Lot uFSH/mannitol/citrate bsd' ~ asd' rate H20 ~ H20 ~ Tg
min % % C
98023 0:15:85 na na
98024 0:85:15 na na
L200198025 3.5:15:81 7.7 7.7 6.7 6 69
L200298026 3.5:81:15 7.8 7.4 1.0 na 28
L200398027 3.5:48.5:48.57.9 7.4 3.8 4.1 SI
L200498028 3.5:32:64 7.8 7.4 5.0 na 60
L200598029 3.5:96 7.8 7.3 6.5 na none
L200698030 3.5:15:81 7.0 6.9 5.1 na 79
L200698037 3.5:15:81 6.9 6.8 4.5 na
L200798038 3.5:96:0 7.9 7.7 nd na
sucrose
L200898039 3.5:96:0 7.8 7.6 1.8 na 100
raffinose
L200998040 3.5:15:81 6.8 6.9 4.5 na 83
L201098046a 5:15:80 7.0 7.0 4.6 na
L201098046b 5:15:80 7.0 7.0 3.9 na 84
L201198047 5:80:15 7.7 7.4 na 33
48
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
Composition pH TGA Karl DSC
Form Form (wt/wt/wt) Fisher
ID Lot uFSH/mannitol/citratebsd'asd' rateH20 H20 Tg
min% % C
L2012 98048 5:15:80 5.3 5.3 0.5 na 73
L2013 98049 10:14:76 7.4 7.3 5.1 na 88
L2014 98050 5:31:64 7.1 7.0 2.6 na 75
L2015 98051 5:80:15 6.3 5.8 1.4 na
L2006 98052 3.5:15:81 6.9 6.9 4.1 rza
L2006 98053 3.5:15:81 6.9 6.9 4.0 na 82
L2016 98054 5.0:15:80 7.7 7.4 7.4 na 83
L2020 98067 10:14:76 7.1 7.1 3.5 na 82
L2012 98068 5:15:80 5.1 5.1 2.4 na 65
L2016 98069 5:15:80 7.6 7.5 3.6 rza 84
L2006 98070 3.5:15:81 7.0 7.0 5.6 na 73
L2010 98084 5:15:80 7.1 7.1 10 3.3 na 90
L2017 98085 5:95:0 7.5 7.1 10 0.6 na nd
L2018 98086 5:95:0 7.4 7.2 10 1.4 na 99
raffinose
L2019 98087 5:64:31 na na 10 1.2 na na
cycloneraffinose
L2019 98087 5:64:31 7.4 7.1 10 0.3 na 43
collectorraffinose
L2010 98110 5:15:80 6.9 6.9 10 2.8 na 83
L2017 98111 5:95:0 7.0 6.7 10 0.5 na nd
L2018 98112 5:95:0 7.2 7.0 10 0.7 na 98
raffinose
~ pH of formulation solution before spray drying, Z pH of reconstituted powder
X-ray Diffraction. Powder X-ray diffraction studies were performed on a
Shimadzu XRD6000 diffractometer to characterize the extent of crystallinity of
representative powder samples (L2010, L2017, L2018). The uFSH/mannitol/citrate
49
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
formulation (L2010) was determined to be largely amorphous as indicated by the
absence of distinct diffraction peaks. The uFSH/mannitol formulation (L2017)
appears highly crystalline in light of a diffraction pattern characteristic of
the a-
polymorph of mannitol. The uFSH/raffinose formulation (L2018) was completely
amorphous, as determined by the absence of distinct diffraction peaks. These
data are
consistent with the DSC results described above.
Moisture Sorption. Moisture sorption analyses were conducted to provide an
indication of the hygroscopicity of certain uFSH powder samples. Specifically,
the
samples were held at constant temperature and exposed to an excursion of
relative
humidity, from 0% to 80%, then back to 0% RH. Each sample was positioned on a
microbalance to monitor the equilibrium weight gain as a function of RH. The
moisture sorption results of two illustrative uFSH powders were as follows. A
high-
citrate content powder (L2006, uFSH/mannitol/citrate) showed a sharp change in
the
moisture uptake profile between 30 and 40% RH, which was attributed to the
formation of citrate dihydrate. A 95% mannitol formulation showed a small
transition
around 60% RH which was attributed to the crystallization of a small amount of
remaining amorphous mannitol. (Small amounts of amorphous mannitol (<5%)
associated with the protein are difficult to detect using DSC or X-ray
diffraction).
Isothermal microcalorimetry (TAM) data also depicted the presence of mannitol
crystallization at 60% RH. Crystallization of mannitol was also detected in a
mannitol/citrate formulation by isothermal microcalorimetry. The results of
these
moisture sorption studies reveal a preferred mode of handling the resultant
powders,
i.e., keeping the powders dry during manufacture, packaging, and on storage.
EXAMPLE 7
Aerosol Performance of uFSH Dry Powders
The aerosol performance of representative uFSH powders was evaluated,
using various measurable parameters, to determine their suitability for
delivery to the
lung.
Delivered Dose Efficienc~Measurements (DDE). A single blister pack (BP)
filled with 2 mg of uFSH powder was loaded into a dry powder inhaler as
described in
Smith, A. E., et al., U.S. Patent No. 5,740,794, Apr. 21, 1998. The device was
then
fired, dispersing the powder into the device chamber. Particles forming the
resultant
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
aerosol cloud were then drawn from the device chamber by vacuum at a rate of
30
L/min for 2.5 seconds and were captured on a pre-weighed filter attached to
the
device mouthpiece. The particle-containing filter was weighed again to provide
the
mass of powder from the blister pack that reached the filter. The fired BP was
also
weighed after dispersion to determine the amount of powder that remained in
the
blister package following the event. For the data reported below, one test set
was
composed of 5 to 10 BP dispersions.
Aerodynamic Particle Size Measurement. A modified short stack (SS) method
with the Andersen cascade impactor was utilized during the early screening
phase of
uFSH powder formulations. The SS method was utilized to provide the FPF, the
APC
and CI Eff values. The full-sized Andersen cascade impactor was utilized for
analysis
of the accelerated stability batches. Aerosol performance results carried out
as
described above are provided for representative uFSH-containing powder
compositions in the Tables below.
Table 7: Percent emitted dose (mean, SD, and range), percent powder left in
the
BP, and percent powder collected that left the BP for each powder lot
Emitted % %
Dose Left Collected
(%)
(DDE)
Form Lot n MeanSD min max meanSD mean SD
No No
. .
L2001 98025 5 68 10 57 82 8 4 73 9
L2003 98027 5 9 5 2.5 16 44 17 15 6
L2004 98028 5 25 17 7 45 18 24 29 15
L2005 98029 5 66 5 58 71 11 4 74 4
L2006 98030 5 71 10 60 83 19 5 88 11
L2006 98037 5 58 3 55 64 16 3 69 4
L2007 98038 5 15 8 7 24 23 14 19 9
L2008 98039 5 50 4 45 54 26 6 68 7
L2006 98040 5 64 6 58 74 19 7 79 5
L2010 98046A 5 54 2 53 57 12 3 62 2
L2010 98046B 6 50 3 46 56 22 5 65 5
L2011 98047 5 29 4 24 33 4 4 30 5
L2012 98048 5 69 7 63 79 8 3 75 7
51
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
L2013 98049 5 41 5 36 48 19 3 51 4
L2014 98050 5 52 5 44 58 9 2 57 6
L2015 98051 5 46 6 41 55 9 5 51 5
L2006 98053 4 53 6 48 61 18 3 64 5
L2016 98054 5 42 3 39 45 26 7 57 3
L2020 98067 5 71 2 68 74 12 2 81 2
L2012 98068 5 68 2 65 70 9 3 75 1
L2016 98069 5 69 2 67 72 17 4 84 3
L2006 98070 5 63 10 46 70 12 6 71 8
L2010 98084 6 62 3 55 66 28 3 84 5
L2017 98085 6 64 11 47 75 18 5 77 10
L2018 98086 6 60 5 55 69 27 7 83 6
In looking at the above data, formulations exhibiting superior aerosol
performance were L2006, L2010, and L2020, each composed ofmannitol:citrate
(1:5,
pH 7) containing 3.5, 5, and 10% uFSH, respectively. The first four screen
sets all
included L2006 (3.5% uFSH), and its DDE averaged 62 t 7% for the five lots
prepared; the averages for individual lots varied from 53 to 71 %. The DDE of
the
L2010 (5% uFSH) lots averaged 55 ~ 5% (n=5), and the DDE of individual lots
ranged from 47 to 66%. The 10% uFSH-content powder had an average DDE of
71%, but upon re-testing showed an unexplained drop to 55%. Other powder
formulations exhibiting particularly good powder dispersabilities, i.e.,
having DDE
values of 60% or greater included L2001, L2005, L2006, L2012, L2016, L2006,
L2010, L2017, and L2018. The DDE of the raffinose powders L2018 and L2008
averaged 50 t 10% (n=3 lots). Although the DDE of the raffinose formulations
was
lower than some of the other uFSH formulations, these powders were
distinguished
by the fine nature of their aerosol clouds. As an additional indicator of
aerosol
performance, Andersen short stack test results are provided in Table 8 below
showing
mean Fine Particle Fraction (FPF or %<3.3 Vim), mean Aerosol Performance
Coefficient (APC), mean cascade impactor (CI) efficiency and estimated MMAD
for
three measurements (three blister packs per measurement).
52
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Table 8: Short Stack Andersen Particle Size Measurements
Andersen Short Stack CI
Form No. Lot No. n ~' ~ FPF (%<3.3um) APC CI % MMAD
mean mean Efficiency (gym)
mean
L2001 98025 3 81 0.44 54 2.2
L2005 98029 3 58 0.33 57 3.0
L2006 98030 3 83 0.43 52 2.1
L2006 98037 3 81 0.42 52 2.2
L2008 98039 3 91 0.49 53 1.8
L2006 98040 3 79 0.42 53 2.2
L2012 98048 3 59 0.34 57 2.9
L2020 98067 3 61 0.37 60 2.9
L2012 98068 3 51 0.33 64 3.2
L2016 98069 3 64 0.39 61 2.8
L2006 98070 4 74 0.37 51 2.4
L2010 98084 2 80 0.38 47 2.2
L2017 98085 2 50 0.30 59 3.3
L2018 98086 2 89 0.44 50 1.9
3 blister packs were used per measurement (n)
The Andersen short stack data indicated good powder performance
characteristics for the powders tested. All formulations exhibited FPF values
equal to
or greater than 50%, indicating that 50% or greater of the particles have
sizes below
3.3 microns. Such powders are desirable for delivery to the deep lung for
subsequent
systemic uptake. Half of the formulations tested exhibited FPF values of 75%
or
more, indicating the high percentage of small sized particles present in the
formulations. These formulations included L2001, L2006, L2008, L2010, and
L2018.
Nearly all powders also exhibited particularly good APC values, preferably
0.36 or
greater. These formulations included L2001, L2006, L2008, L2020, L2016, L2010,
and L2018. The APC of L2006 consistently exceeded 0.36 and ranged from 0.37 to
0.42, with corresponding values for FPF (%<3.3 Vim) between 74 to 83%. The APC
53
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
values for L2010 were very similar and ranged from 0.34 to 0.38, with 70 to
80%
FPF.
The raffinose formulations gave very high values for APC, from 0.37 to 0.49,
with corresponding FPF values of 90%. Mannitol formulations demonstrated
slightly
lower APCs (0.26 to 0.33). Thus, mannitol formulations appeared to produce the
largest aerosol particle sizes, while the mannitol/citrate formulations were
mid-sized,
and the raffinose formulations were of the smallest aerosol particle size.
Accelerated Stability Study. Three powder formulations, L2010, L2017,
L2018 were set up for one month at 30°C and 40°C and a stress
cycling condition of
2-40°C. Previously generated short stack data was confirmed when the
stability
batches were tested for particle size distribution with the full stack set-up.
Table 9
contains the data from the accelerated stability study.
Table 9: Accelerated Stability Summary of Data
Emitted % % Collected
Dose Left
(%)
Form/LotConditionn mean SD min max mean SD mean SD
2-40 10 52.2 4.8 41 57 29 2 74 6
L2010 30 10 55.7 3.7 49 60 27 3 76 3
98110 40 11 51.4 5.0 43 84 28 10 75 6
t=0 10 50.5 5.1 40 59 28 8 71 7
2-40 11 48.2 3.9 39 52 20 5 60 3
L2017 30 11 50.9 3.2 46 57 14 3 59 4
98111 40 10 49.9 3.3 44 54 14 4 58 4
t=0 11 47.2 2.8 41 52 15 4 56 3
2-40 10 45.1 4.0 37 54 23 9 60 5
L2018 30 11 47.0 6.0 35 54 24 6 62 4
98112 40 9 49.8 5.3 41 59 23 5 64 3
t=0 10 41.1 4.8 34 50 30 5 59 3
54
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Andersen
Short Stack
CI
Form/LotConditionn FPF (%<3.3um)APC CI
mean mean Efficiency
mean
2-40 3 68 0.32 47
L2010 30 3 72 0.32 45
98110 40 3 75 0.35 47
r-0 3 70 0.34 49
2-40 3 53 0.25 47
L2017 30 3 53 0.24 46
98111 40 3 50 0.25 50
t=0 3 53 0.26 50
2-40 3 87 0.35 41
L2018 30 3 86 0.34 40
98112 40 3 87 0.35 40
t=0 3 89 0.37 41
In the accelerated stability study, initial DDE values were lower than for
previous batches. On testing at one month, however, there was no decline in
DDE for
any of the powder formulations held at any of the stability conditions. Fig. 1
shows
the relationship between FPF and MMAD for three uFSH powder formulations
employed in this one-month stability study (L2010, L2017, L2018). Looking at
the
clusters of data points presented in Fig. 1 and proceeding from left to right
along the
x-axis, the data clusters correspond to samples L2017, L2010, and L2018,
respectively. The APC values for both the initial and one month measurements
were
quite consistent within each powder formulation and were consistent with the
APC
values of earlier batches. Thus, the exemplary powder formulations described
herein
exhibit good aerosol performance and stability.
EXAMPLE 8
Bioactivity of uFSH Dry Powders
The bioactivity of four representative dry powder uFSH formulations, L2013
(10% uFSH, 14% raffmose, 76% citrate), L2010 (5% uFSH, 15% raffinose, 80%
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
citrate), L2006 (3.5% uFSH, 15% mannitol, 81% citrate), and L2008 (3.~% uFSH,
95% raffinose) was determined by a (3-lactamase sensitive reporter cell assay.
The
four uFSH powder formulations retained significant bioactivity comparable to a
uFSH
standard. Other suitable in vitro assays are mentioned herein.
These results are depicted graphically in Figs. 2A and 2B.
EXAMPLE 9
Bioavailability of Subcutaneous (SC) and Intratracheally (IT) Administered FSP
in Rats
The following studies were undertaken to provide an indication of the
bioavailability of intratracheally administered FSP in rats relative to SC-
administered
FSP.
Protocol 1 (FSP-SC). Human FSH from pituitary was obtained from Sigma
(St. Louis, MO, USA, lot #34H0069 product # F 4021 ). The diluent was PBS
(phosphate-buffered saline) containing 1 % albumin. Male Sprague-Dawley rats
weighing between 280-300 grams were used for the single exposure study. Two
animals were used per data collection time point for a total of 6 time points.
Animals
were administered either 0.9 units/200 ~L or 9.0 units/200~L, killed at the
appropriate
times and a total blood sample was obtained. Individual rats were used for
each time
point. Blood samples were collected at 0, 4, 8, 24, 48, and 96 hours. Samples
were
centrifuged and the supernatants were frozen at -80 °C until analyses
using RIA kits
from ICN Pharmaceuticals (Costa Mesa, CA USA).
Protocol 2 (uFSH- SC and IT). Active agent administered to the animals was
"FERTINORM HP" 75IU (Urofollitropin iFSH) im/sc, obtained from Serono
Laboratories (Norwell, MA, USA). Active agent (ten vials of uFSH each
containing
mg of lactose) was reconstituted by addition of 1 ml of NaCI solution supplied
by
the vendor, to which was added sufficient PBS (phosphate-buffered saline) to
prepare
solutions having a concentration of 9.0 units/200 pL FSH. Male Sprague-Dawley
rats
weighing 280-300 grams were used for the single exposure study. Each group was
composed of thirty six animals, with a control group of 4 animals for each
day. Drug
solutions containing 9.0 units/200 pL uFSH were administered to the animals at
given
time points for both the IT and SC studies. Two animals were used per data
collection time point for a total of 6 time points.
56
CA 02369262 2001-10-02
VVO 00/61178 PCT/US00/09869
On the day of the experiment, rats were anaesthetized for IT dosing and were
then allowed to recover. Animals were then sacrificed at the given times and a
total
blood sample was obtained. Individual rats were used for each time point.
Blood
samples were collected at 0, l, 2, 4, 6, 9, 24 hours (day 1), and at 48 (day
2), 72 (day
3), and 96 (day 4) hours. Samples were centrifuged and the supernatants were
frozen
at -80 °C until analyses using RIA kits from ICN Biomedicals (Costa
Mesa, CA).
The results are summarized in Table 10 below and graphically in Fig. 3.
Table 10. Bioavailability of SC and IT Administered FSP in Rats
Dose/Source Route Tmax, hourCmax AUC f
mIU/ml mIU/ml.hr(%)
0.9 U/* SC 8 6.6 94.05
9.0 U/* SC 4 13.1 420.37
9.0 U/** SC 9 10.25 251.1
9.0 U/** IT 6 4.14 53.63
21.4
Source: * = human pituitary-derived;** = human urinary-derived
FSP Dose = Units/200~,L/rat
Tmax: time at which maximum drug concentration in plasma is reached
Cmax: maximum drug concentration reached (at Tmax)
AUC: total area under the drug level-time curve for elimination of drug
f: bioavailability in % = AUC,T/AUCsc.
The above results provide an indication of the bioavailability of
intratracheally-delivered FSP, and reveal a relative IT bioavailability of
about 20
percent in comparison to SC-delivered FSP. The results of this preliminary
study
indicate that FSP has a reasonable bioavailability when instilled in liquid
form into
the rat trachea. This data further suggests that FSP can be administered to
the lung,
and absorbed through the lower respiratory tract and into systemic
circulation.
57
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
EXAMPLE 10
Pharmacokinetics of uFSH following Pulmonary and Subcutaneous
Administration in Cynomolgus Monkeys
The following in vivo pharmacokinetic (PK) study was conducted to examine
the bioavailability of follicle stimulating protein (uFSH) in cynomolgus
monkeys
when administered by the pulmonary route as an alternative to subcutaneous or
intramuscular injection.
Each of five female cynomolgus monkeys was administered uFSH by a
subcutaneous injection followed by three separate pulmonary doses of
aerosolized
uFSH powder composition 2010 (2 mg dose; 5% FSH/ 15% mannitol/ 80% citrate)).
For pulmonary administration of the powder, the monkeys were placed within a
helmet-type exposure apparatus and the FSH dry powder was dispersed directly
into
the helmet using a dry powder inhaler device of the type described in Smith,
A. E., et
al., U.S. Patent No. 5,740,794, Apr. 21, 1998. Inhaled doses of increasing
concentrations of FSH were administered over 5 or 15 minute exposure periods.
The
actual averages of inhaled doses were 3.8 ~ 1.7 pg/kg, 18.4 ~ 1.3 ~g/kg and
37.2 ~
3.9 ~g/kg, which were calculated post-treatment based on the aerosol
concentration,
the mean minute volume, and the exposure duration for each monkey during each
inhalation. Dose administrations were separated by at least one week.
Whole blood samples were collected at 0 hours (predose), 0.08, 0.25, 0.5, 1,
3,
6, 9, 12, 24, 48 and 72 hours post-dose. Serum concentrations of
immunoreactive
uFSH were determined by a validated, modified immunoradiometric assay ((IRMA),
906DM-052-O1), employing a commercial FSH kit, (Coat-A-Counto FSH IRMA,
DPC~ Ltd., Los Angeles, CA). Percent relative bioavailability was calculated
by
Microsoft Excel using the following relationship [Wagner, J., Pharmacokinetics
for
the Pharmaceutical Scientist, 188-189 (1993)]:
(AUCpulmonary X DOSEs~)
X 100
(AUCs~ x DOSEp~~"o~ary)
AUCO_~ was used in this calculation. Individual concentrations of
immunoreactive uFSH in monkey serum are reported in Table 11. The results of
the
pharmacokinetic analysis are reported in Table 12. Mean serum concentrations
(~
SEM) are depicted graphically in Figure 4.
58
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Table 11. Serum Concentrations of Immunoreactive FSH in Cynomolgus
Monkeys Following Pulmonary and Subcutaneous Administration of uFSH and
Pharmacokinetic Statistics
SC - 2 ~g/kg Animal Number/Concentration (mIU/mL)
Time (h) 82841 82861 83871 8392184761 Mean SIJM
0 BQL BQL BQL BQL BQL 0
1 5.3 4.6 BQL 5.3 BQL 3.0 1.3
3 20.9 63.7 28.2 65.5 36.3 42.9 9.2
6 25.4 75.7 28.9 97.1 68.5 59.1 13.9
9 20.2 58.3 31.5 71.3 76.0 51.5 11.0
12 18.7~2~46.8~2~33.4~2~55.0~2~54.3~z~41.6 6.9
24 20.7~''~36.8~''~29.1~2~38.0~2~40.5~2~33.0 3.6
48 15.7~'~20.8~2~17.4~2~17.4~z~14.5~2~17.2 1.1
72 9.2~'~9.8~z~8.4~2~8.6~2~5.8~2~8.4 0.7
AUCO_ t (mIU*h/mL)1197.12198.41544.42294.22078.11862.42lU.y
AUCO_~ (mIU*h/mL)2013.42594.51932.52566.82231.32267.7136.9
Half Life (h) 55.5 26.6 29.8 22.3 18.1 26.4 4.3
CMS (mIU/mL) 25.4 75.7 33.4 97.1 76.0 61.5 13.7
TM~ (h) 6.0 6.0 12.0 6.0 9.0 7.8 1.2
Dose (~g/kg) 2 2 2 2 2 2
59
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
PUL - 18.4 ~ 1.3 ~g/kg Animal Number/Concentration (mIU/mL)
Time (h) 82841 82861 83871 83921 84761 Mean SEM
0 BQL BQL BQL BQL BQL 0
0.08 BQL BQL BQL BQL BQL 0
0.25 BQL BQL BQL BQL BQL 0
0.5 BQL BQL BQL BQL BQL 0
1 BQL BQL BQL BQL 4.4 0.9 0.9
3 6.9 13.1 6.4 5.5 16.6 9.7 2.2
6 20.0 21.0 16.6 18.8 33.0 21.9 2.9
9 29.1 24.3 19.4 17.2 40.1 26.0 4.1
12 26.6~z~25.2~z~24.4~''~20.4~z~40.O~z~27.3 3.3
24 19.6~z~20.4~z~13.7~z~16.3~z~31.3~z~20.3 3.0
48 7.4~z~9.3~z>6.9~z~9.O~z~10.7~z~8.7 0.7
72 3.9~''~4.5~z~3.O~z~5.3~z~4.2~z~4.2 0.4
AUCO_ t (mIU*h/mL)941.3 1001.8755.2847.8 1436:9996.6 117.8
AUCp_~ (mIU*h/mL)1053.51157.9843.41079.31546.81136.0115.1
Half Life (h) 20.9 23.5 20.5 30.4 17.83 21.9 1.8
CMax (m~/mL) 29.1 25.2 24.4 20.4 40.1 27.8 3.4
TM~ (h) 9.0 12.0 12.0 12.0 9.0 10.8 0.7
Dose (~g/kg) 19 19 22 14 18 18.4 1.3
Percent Bioavailability5.5 4.7 4.0 6.0 7.7 5.6 0.6
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
PUL - 37.2 3.9 Animal
~.g/kg Number/Concentration
(mIU/mL)
Time (h) 8284182861 83871 8392184761 Mean SEM
0 BQL BQL BQL BQL BQL 0
0.33 BQL BQL BQL BQL BQL 0
0.5 BQL BQL 4.3 BQL BQL 0.86 0.86
0.75 3.8 BQL 6.6 BQL BQL 2.1 1.4
1.25 57.6 BQL 31.0 4.7 BQL 18.7 11.3
3.25 32.7 10.4 50.1 12.2 13.4 23.8 7.7
6.25 69.3 22.7 85.2 23.7 29.0 46.0 13.1
9.25 82.4 30.6 132.8 33.8 36.8 63.3 19.8
12.25 72.5~Z~36.0~2~155.0~2~36.7~z~39.8~z~68.0 22.8
24.25 55.4~''~32.7~''~94.6~'~30.4~2~36.6~2~49.9 12.0
48.25 29.3~2~17.5~'~47.8~2~15.5~2~14.2~z~24.9 6.4
72.25 14.1 9.2~Z~19.4~2~8.6~Z~6.0~2~11.5 2.4
~''~
AUCO_ t (mIU*h/mL)3023.61574.95069.11506.51601 2555.0689.6
AUCO_~ (mILJ*h/mL)3554.71981.55659.11845.21790.42968.0747.9
Half Life (h) 25.3 29.4 20.52 27.9 20.9 24.3 1.8
CMS (mILJ/mL) 82.4 36.0 155.0 36.7 39.8 70.0 23.0
TM~ (h) 9.3 12.3 12.3 12.3 12.3 11.7 0.6
Dose (~g/kg) 38 47 43 33 25 37.2 3.9
Percent Bioavailability9.3 3.2 13.6 4.4 6.4 7.4 1.9
61
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
PUL - 3.8 1.7 Animal
pg/kg Number/Concentration
(mIU/mL)
Time (h) 82841 8286183871 83921 84761 Mean SEM
0 BQL BQL BQL BQL BQL 0
0.08 BQL BQL BQL BQL BQL 0
0.25 BQL BQL BQL BQL BQL 0
0.5 BQL BQL BQL BQL BQL 0
1 BQL BQL BQL BQL BQL 0
3 BQL BQL BQL BQL BQL 0
6 BQL 3.1 4.5 3.7 3.7 3.8 0.3
9 BQL 4.5 6.1 4.9 4.3 5.0 0.4
12 BQL 4.6 8.1 5.2 4.7 5.7 0.8
24 BQL 4.2 8.5 5.6 3.9 5.6 1.1
48 BQL BQL 4.9 3.0 BQL 4.0 NC~3~
72 BQL BQL BQL BQL BQL 0
AUCp_ t (mIU*h/mL)0 132.9363.2 237.6 129.5 172.6 60.7
AUCp_~ (mIU*h/mL)NC NC NC NC NC NC NC
Half Life (h) NC NC NC NC NC NC
CM~ (mIUImL) 0 4.6 8.5 5.6 4.7 4.7 1.4
TM~ (h) 0 12 24 24 12 14.4 4.5
Dose (~g/kg) 6 2 3 S 3 3.8 1.7
Percent BioavailabilityNC NC NC NC NC NC
h=hour; mItJ=mini-International Units; mL=milliliter; SEM=standard error of
mean;
wg=microgram; kg=killogram; Cmax=maximum concentration; AUCp_ t=area under
the curve extrapolated from time 0 to tau; AUCp_~,=area under the curve
extrapolated
from time 0 to infinity; Tmax=time to maximum plasma concentration.
~I~BQL means below the limit of quantitation, which is, defined as 3.0 ng/mL
and for
calculations, BQL values were assigned a value of zero.
~Z~Time points used for calculating the half life.
~3~NC means not calculated.
62
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Table 12 Pharmacokinetic Parameters for Cynomolgus Monkeys after
Pulmonary and Subcutaneous Administration of uFSH
SC Pulmonary Pulmonary Pulmonary
Mid High Dose Low
Dose Dose
Mean SEM Mean SEM Mean SEM Mean SEM
AUCO_ t (mIU*h/mL)1862 211 997 118 2555 690 173 61
AUCp_~ (mIU*h/mL)2268 137 1136 115 2968 748 NC NC
Half Life (h) 26.4 4.3 21.9 1.8 24.3 1.8 NC
C;,,~aX (mIU/mL)61.5 13.727.8 3.4 70.0 23.0 4.7 1.4
Tn,,~ (h) 7.8 1.2 10.8 0.7 11.7 0.6 14.4 4.5
Dose (~g/kg) 2 - 18.4 1.3 37.2 3.9 3.8 1.7
Bioavailability100 5.6 0.6 7.4 1.9 NC
(%)
The serum pharmacokinetic results demonstrated that the serum concentration
time profiles following pulmonary delivery were similar to subcutaneous
administration. The terminal half life following pulmonary delivery was
similar to
that obtained with subcutaneous administration (approximately 24 hours).
Within the
pulmonary dose groups (mid and high doses) the serum AUCp_~ and Cm~ increased
in relation to increasing dose administered to the lung. Finally, the average
percent
bioavailability of aerosol formulated uFSH delivered by the pulmonary route
compared to subcutaneous administration averaged approximately 6.5% based on
the
inhaled dose. The corresponding in-lung bioavailability, or relative pulmonary
bioavailability, is estimated to be 26% based on the measurements of pulmonary
deposition of 2 ~m MMAD particles in monkeys using gamma scintography. The
low dose pulmonary PK was not used in these calculations because there were
too few
samples taken when the FSH blood levels were measurable. These findings
indicate
that dry powders of FSP can be successfully administered by the pulmonary
route to
cynomolgus monkeys, to other mammals, and particularly to other primates such
as
humans, resulting in serum concentrations and profiles similar to those
obtained by
the widely practiced subcutaneous route.
63
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
EXAMPLE 11
Respirable FSH Dry Powders Containing Leucine
Powder lots 99348 and 99349 were spray dried under conditions as described
in Example 2 for the L2010 powders. These formulations each incorporated
leucine
at 40% (w/w); the remainder of the formulation matrix was mannitol and sodium
citrate in a similar relative ratio as is present in L2010. The uFSH content
in the
powders was 5% and 2%, for lots 99348 and 99349, respectively. For the
composition containing 2% FSH, the fill mass in the blister pack was 5 mg,
while for
the 5% FSH dry powder composition, the fill mass in the blister pack was 2 mg.
Each
of the two spray-dried leucine formulations possessed good uFSH stability,
comparable to those of non-leucine containing formulations as determined by
SEC-
HPLC (data for non-leucine-containing formulations can be found in Table 3).
Table 13. Effect of Spray Drying on Higher Order Aggregate (HOA) content in
uFSH Powders Formulated with L-Leucine
total HOA
uFSH:citrate:mannitol:leucine Before After spray
spray
(wt:wt:wt:wt) Lot Form. drying drying
# #
:48: 7 :40 99348 L2029 0.6 1.8
2 :50: 8 :40 99349 L2030 0.6 1.8
2 :83:15: 0 99347 L2028 0.4 2.3
The two leucine formulations showed superior aerosol perrormance compares
to all other formulations tested. A 14 to 30 % absolute increase in DDE was
observed
and was accompanied by a decrease in the "% Left" in the blister pack. The
aerosol
performance coefficient (APC) increased significantly, to more than 0.60.
64
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
Table Aerosol taining
14. performance powders
of
L-Leucine-con
Lot BP DescriptionDDE (%) SD %Left SD APC
fill
mass
99348 2 mg 5% uFSH 71.1 5.7 15.2 3.7 0.64
w/ leucine
99349 5 mg 2% uFSH 74.2 5.0 13.3 4.2 0.60
w/ leucine
99295 2 mg 5% uFSH 56.9 10.7 24.2 10.8 0.37
99347 5 mg 2% rFSH 44.5 2.7 17.1 3.3 0.32
Additionally, the morphology of the particles, as viewed by SEM, was altered
by the addition of the amino acid. The particles seem more "collapsed" and
thus the
surface folding was more pronounced. In the limited fields of view available
with
these micrographs, the particle size distribution seemed more homogenous with
few
very fine particles evident. Additional FSP-leucine formulations were prepared
as
above, to further examine their aerosol and stability properties.
Table 15. Comparison of ED values for leucine and non-leucine powders.
Composition ED (%)'
(wt %) mean (n = 10)
Lot # FSP citrate mannitol raffinose leucine initial 4 months
99420 5 80 15 0 0 60.8 n.d.
99421 5 42.5 10 42.5 0 62.9 n.d.
99422 5 20 0 75 0 53.2 n.d.
99423 5 0 0 55 40 70.9 65.3
99425 5 0 0 35 60 74.4 75.8
99426 5 55 0 0 40 65.3 67.1
99455 5 47.7 7.3 0 40 71.6 66.3
994542 5 47.7 7.3 0 40 72.7 n.d.
99457 5 25 0 10 60 70.4 71.5
" , , ~ ~.,_ " ___ _ r_m __ :_L~
am ~r~wucm cvaiuawu mum. iu~ i,u vw,ym.
Z lot 99454 made with uFSH.
n.d. indicates ED testing not performed at 4 months.
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Each of the six leucine-containing formulations demonstrated a higher DDE
from the mouthpiece of the device than the three formulations that did not
contain
leucine. No significant changes in DDE were observed after 4 months of storage
at
40°C, indicating that these leucine containing formulations retain
their highly
dispersible nature, even under accelerated stability testing conditions. The
properties
of additional similarly prepared FSP leucine-containing dry powders are
provided in
the tables below.
Table 16. Initial and one month aerosol performance of four exemplary FSP Dry
Powder Formulations containing 15% FSH and placed on accelerated stability
(40°C/75% RH in foil pouch)
Composition ED (%) MMAD
(wt %) initial, (gym)
1 month'
Lot FSH citratemannitolraffinoseleucine
# ~ ~
99513 15 42.5 0 42.5 0 55.7, 2.8,
56.3 3.0
99514 15 45 0 0 40 63.9, 2.2,
65.1 2.3
99515 15 2 0 43 40 68.7, 2.3,
66.1 2.3
99516 15 72 13 0 0 58.6, 2.8,
59.9 2.9
~ all powders evaluated with 3 mg fill weights.
Table 17. Physical Characterization of Leucine-Containing Dry Powders
ID Lot FSP LeucineCitrateYield MMD HZO T~ L.C.
(%) (Etm) (%) (C) (%)
L2041 99598 2 40 58 79 1.2,1.62.7 106 35
L2042 99599 2 80 18 72 1.3 1.3 100 62
L2043 99600 2 95 3 71 1.1 0.5 n.o. 80
L2044 99601 8 60 32 78 1.2 1.7 112 45
L2032 99602 15 40 45 83 1.4 2.4 t.b.d.322
L2045 99603 15 80 5 79 1.4 1.0 n.o. 54'
L.C. = leucine crystallinity
66
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Table 18. ED and Percent Fine Particle as a Function of BP Fill Weight and
Formulation for Leucine Containing Dry Powders of FSP
Lot No. Formulation BP fill % ED %Fine
wt.
FSP:citrate:leucine(mg) mean (RSD)Particle
(n = 16) (n =
3)
99598 2:58:40 2 76 (6) 53
3 74 (4)
4 76 (5) 53
5 76 (5)
99599 2:18:80 2 80 (7) 51
3 81 (4)
4 81 (4) 41
5 81 (3)
99600 2:3:95 2 83 (7) 50
99601 8:32:60 2 75 (6) 55
4 78 (4) 52
99602 15:45:40 2 77 (8) 53
3 73 (8)
4 74 (5) 51
99603 ( 15:5:80 2 78 (5) 57
4 80 (4) 54
EXAMPLE 12
Expression of an FSH Variant in AV12 Cells
An expression cassette vector pGTH was used for expression of the FSH alpha
subunit in AV 12. Briefly, pGTH contains several elements in sequence: the
SV40
early promoter/ori, E. coli hygromicin resistance, SV40 small "t" antigen
splice
site/poly-A site, pBR322 cloning remnant, BK virus (strain P2) cloning
remnant,
Poly-CAzo/GTZO element (synthetic oligonucleotide), BK virus (strain P2)
enhancer,
AD2 major late promoter/spliced tripartite leader, Bcll insertion site for the
FSH
alpha subunit coding sequence (including stop codon), SV40 small "t" antigen
splice
site/poly-A site; and pBR322 ampicillin resistance/ori.
67
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
The plasmid construct pGTH-alpha was generated to express the encoded the
human alpha subunit sequence (SEQ ID NO: S) by cloning a 362-by BcII FSH
variant
cDNA fragment into the unique BclI site of the vector. The FSH variant alpha
cDNA
fragment DNA was generated by PCR amplification using the shuttle plasmid
pLGD637 as template (pLGD637 contains a synthetic/oligonuclotide-assembled FSH
variant alpha cDNA sequence (SEQ ID NO: 37). The integrity of BcII insert was
confirmed by sequencing followed by comparison to the GenPept database
(Accession Number 31869).
An expression cassette vector pGTD was used to express a human beta subunit
FSH variant sequence encoding SEQ ID NO: 11 (i.e., nucleotide SEQ ID NO: 38).
pGTD contains several elements for expression in AV 12 cells: sequentially,
the BK
virus (strain P2) cloning remnant, Poly-CAZO/GTZO element (synthetic
oligonucleotide), BK virus (strain P2) enhancer, AD2 major late
promoter/spliced
tripartite leader, Bcl l insertion site for the FSH variant beta subunit
coding sequence
(including stop codon), SV40 small "t" antigen splice site/poly-A site; SV40
early
promoter ori, Murine dihydrofolate reductase cDNA, SV40 small "t" antigen
splice
site/poly-A site, and pBR322 ampicillin resistance/ori.
The plasmid construct pGTD-bCD3 was generated by cloning a 393-by BcII
FSH variant beta-bCD3 cDNA fragment into the unique BclI site of the pGTD
vector
(see SEQ ID NO: 38). The FSH variant beta-CD3 cDNA fragment DNA was
generated by PCR amplification, using the shuttle plasmid pLGD638 as template
(pLGD638 contains a synthetic/oligonucleotide-assembled FSH variant beta cDNA
sequence). The integrity of the construct was confirmed by sequencing and
compared
with the human beta subunit sequence deposited in the GenPept database
(Accession
Number 476441 ).
In brief, the pGTH-alpha and pGTD-bCD3 plasmids were linearized,
repurified, and then co-transfected into adherent AV23-RGT18 cells. Following
selection with medium containing 0.25uM methotrexate and 100 ~g/ml hygromicin-
B, along with 200 ~g/ml 6418 to maintain the glutamate transporter genotype of
the
AV 12-RGT18 cells, individual stable clones were isolated either manually or
via
flow-assisted cell sorting. Highest producing clones were identified by
analysis of
conditioned medium with a commercial FSH ELISA kit to measure FSH variant
production. Several clones were adapted to serum-free suspension and further
amplified to obtain isolatable quantities of the FSH variant heterodimer.
68
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
EXAMPLE 13
1~T-terminal Truncated FSH Variant Forms
Analysis of Protein Sequence b~N-terminal Sequencing. Multiple lots of
recombinant hFSH variant were produced in fermentation in the laboratory and
quantified using a variety of assays. The host (CHO cells) was designed to
express an
alpha subunit encoded by SEQ ID N0:37 and a beta subunit encoded by SEQ ID
N0:38. All the N-terminal sequencing was done using either solutions or
blotted
samples. Only relative ratios of intact and N-2 alpha and beta subunits can be
obtained from N-terminal sequence data. For example, the presence of Cys is
not
quantifiable by N-terminal sequencing and Ser exhibits a lower than average
recovery, which makes it more difficult to accurately estimate relative ratios
of intact
and N-2 beta-subunits by this method (beta-subunits have Ser and Cys at
positions 2
and 3 respectively). Relative amounts of amino termini are estimate using the
second
cycle recoveries, e.g., Ser and Glu. Some representative results are
summarized in
Table 19 below.
Table 19. Truncation at N-terminus of CHO-derived FSH
Protein a-subunita (%N-2)(3-subunit (%N-2)b
WUJ-3 8 7.9 5 8
11 60
WUQ-115 10 59
WWG-15 9.5 59
WWG-30 9.2 59
WWG-SO 8.7
FSH 8.6 66
a Estimates based on recoveries in the first cycle. Examination of tryptic
peptides for
WUJ-38 and WL1Q-115 by LC/MS indicates 94 and 92.7% respectively of intact N-2
alpha subunit.
b Estimates. Examination of tryptic peptides for WUJ-38 and WUQ-115 by LC/MS
indicates a 1:1 ratio of intact and N-2 variant beta subunits.
69
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
EXAMPLE 14
C-terminal Truncated FSH Variant Forms Quantitated by Tryptic Mapping and
LC/MS
Six lots of Chinese Hamster Ovary cells modified to biosynthesize hFSH
variant having an alpha subunit encoded by DNA of SEQ ID N0:37 and having a
beta
subunit encoded by DNA of SEQ ID N0:38 (coding for 108 amino acids) were grown
at laboratory scale. The expressed FSH variant proteins were purified and were
analyzed for heterogeneity of the beta subunit C-terminus. C-terminus sequence
data
from three independent LC/MS runs on FSH variant preparations are shown below
in
Table 20.
Table 20. Truncation of the C-terminus of FSH variant beta subunit
Lot No. 108 amino acids107 amino acids106 amino acids
WUJ-38a' 84.2 1.5 14.2
WUQ-90 96 2.2 1.7
WUQ-115' 3.8 7.8 88.4
WWG-15 62 15 22
WWG-30 94 2 4
WWG-50 63.3 12.1 24.6
°In a separate run for WUJ-38, 73.5, 7, and ZU"/° was obtamea
for the tnree rorms,
respectively.
b In a separate run for WUJ-38, 83% and 17% for 108 and 106 amino acid were
obtained, respectively.
'In a separate run for WUQ-115, 25 and 75% were obtained for CD3 and CDS,
respectively.
EXAMPLE 15
Biological Activity of FSH Variants
In vivo activity data obtained on the different lots of the FSH variant showed
similar potency of the hormone in a rat ovarian weight gain assay and are
shown in
Table 21. ht vivo activity of FSH variants compared to urinary FHS (uFSH) was
measured by the Steelman/Pohley rat ovarian weight gain assay (Steelman and
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Pohley, 1953, Endocrinology, 53, 604-616). Hypophysectomized immature female
rats (23-25 days) were housed with controlled lighting. The rats were given
daily
subcutaneous treatment with hFSH variant or vehicle (PBS with 0.1% BSA, ~ 0.25
mL). A dose of 0.1, 0.3, 1.0 or 3.0 pg/day was used. The treatment was
repeated at
24 hour intervals for 4 days. The rats were weighed and sacrificed 24 hr after
4th
dose, the ovaries and uterus were trimmed of fat and weighed. Six rats were
used for
each dose. FSH variant samples were prepared as follows: samples were dialyzed
in
PBS buffer (GIBCOBRL) for 3 hrs with 10,000 MWCO dialysis cassettes from
Pierce. The concentration of the sample was determined with an AVIV UV
spectrometer using an extinction coefficient of 1.06 at 278 nm for 1 mg/mL
solution
in 1-cm path length cell.
Table 21. In vivo activity of FSP (FSH variants) as measured by rat ovarian
weight (Ov wt) and uterine weight (Ut wt) gain as a function of FSH dose
uFSH WUJ-38 WUQ-090 WUQ-115
Dose Ov wt Ut wt Ov wt Ut Ov Ut Ov wt Ut
wt wt wt wt
(w~daY)(mg) (mg) (mg) (mg) (mg) (mg) (mg) (mg)
0 11.2 15.0 11.2 15.0 11.7 13.2 11.7 13.2
0.1 31.5 33.0 26.8 33.7 22.0 26.7 22.4 17.0
0.3 35.0 62.0 40.2 66.2 33.0 49.3 44.5 63.3
1 49.2 78.8 38.2 77.2 36.5 72.5 39.7 70.2
3 41.2 82.7 42.8 77.5 40.7 87.0 42.0 75.7
EXAMPLE 16
Pharmacokinetics of uFSH Compared to an FSH Variant In Female Fisher Rats
The following studies measure the pharmacokinetics (PK) of a hFSH variant
compared with uFSH in Fisher Rats following intravenous (IV) and subcutaneous
(SC) administration. Female Fisher Rats were administered uFSH or a hFSH
variant
( 15 pg/kg) by SC and IV routes. The hFSH variant was recombinantly produced
in
cells expressing an alpha subunit having the amino acid sequence of SEQ ID
N0:5
and a beta subunit having the amino acid sequence of SEQ ID NO:11. Whole blood
samples were collected at 0.08, 0.25, 0.5, 1, 3, 6, 9, 12, and 24 hrs post-
dose. Serum
71
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
concentrations of immunoreactive uFSH or hFSH variant were determined by
immunoradiometric assay employing a modified commercial FSH kit, FSH IRMA,
DPC~ Ltd. (Los Angeles, CA). RIASYS, a software program that employs a
weighted 4-parameter logistic model algorithm, was used to analyze IRMA
binding
data. Serum concentrations of immunoreactive uFSH or hFSH variant were
estimated
from a standard curve. Standards in rat serum ranged between 3.5 and 3500
mIU/mL.
The lower limit of quantitation was ~ mIU/mL. The upper limit of quantitation
was
750 mIU/mL. A pharmacokinetic analysis of the serum concentration data was
conducted using a statistical package. Individual concentrations of
immunoreaetive
uFSH and hFSH variant in rat serum following SC and IV administration are
reported
in Table 23 and Table 24, respectively. Pharmacokinetic (PK) values are
reported in
Table 25.
Table 23. Serum Concentrations (mIU/mL) of Immunoreactive uFSH in
Female Fisher Rats Following Subcutaneous or Intravenous Administration of
15.0 pg/kg of uFSH
Time
(h)
uFSH 0.08 0.25 0.5 1 3 6 9 12 24
IV
Rat
1 1894 645 109
2 2435 856 78
3 2447 920 85
4 2992 477 84
103 308 145
6 1223 351 131
7 1604 224 38
8 2031 198 24
9 1888 200 23
Mean 2258 1439 1841 807 379 207 91 120 28
SEM 182 841 125 83 51 8.4 9.5 18.5 4.7
uFSH 0.08 0.25 0.5 1 3 6 9 12 24
SC
72
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Rat
5.5 37.9 143
11 BQL 23.8 165
12 BQL 34.8 117
13 BQL 95 126
14 15.8 139 144
9.3 170 153
16 15.4 138 75.4
17 37.2 168.1 70.1
18 15.2 150.4 74.5
Mean 1.8 8.4 22.6 32.2 135 152 142 141 73.3
SEM 1.8 4.6 7.3 4.3 21.7 8.7 13.9 7.9 1.6
Table 24. Serum Concentrations (mIU/mL) of Immunoreactive FSH variant
in Female Fisher Rats Following Subcutaneous or Intravenous Administration of
15.0 ~g/kg of FSH variant
Time
(h)
FSH 0.08 0.25 0.5 1 3 6 9 12 24
variant
IV
Rat
1 3689 1150 79
2 3994 1348 90
3 3816 1248 85
4 2671 407 54
5 2743 432 56
6 2399 316 36
7 2047 150 19
8 1937 145 20
9 1947 166 17
Mean 3833 2604 1977 1249 385 154 84 49 18.7
SEM 88 105 35 57 35 6.1 3.0 6.3 1.0
73
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Time
(h)
FSH 0.08 0.25 0.5 1 3 6 9 12 24
variant
SC
Rat
BQL 36.5 152
11 BQL 36.7 172
12 BQL 28.1 139
13 10.2 112 111
14 12.2 215 123
5.8 116 100
16 17.9 162 53.8
17 23.0 175 62.4
18 28.1 183 62.9
Mean 0 9.4 23.0 33.8 148 173 154 111 59.7
SEM 0 1.9 2.9 2.8 34 6.1 9.5 6.8 3.0
Table 25. Pharmacolanetic Parameters for Female Fisher Rats after
Intravenous and Subcutaneous Administration of uFSH or FSH variant
Treatment Dose Cmax AUCp_ t AUCp_~ Tmax T1/2
(~g/kg)(mIU/mL)(mIUh/mL) mILJh/mL)(h) (h)
(
UFSH IV 15 2258 5093 5373 0.1 6.8
UFSH SC 15 152 2767 4566 6.0 16.4
FSH variant15 3833 5318 5472 0.1 6.3
IV
FSH variant15 173 2598 3575 6.0 11.5
SC
Abbreviations: ng, nanograms; h, hour; mL, milliliter; gig, microgram; kg,
kilogram;
Cmax~ maximum concentration; AUCp_ t, Area under the curve extrapolated from
time 0 to 24h; AUCp_~, Area under the curve extrapolated from time 0 to
infinity;
Tmax~ time to maximum serum concentration; T 1 /2, terminal elimination half
life.
74
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Serum concentration time profiles demonstrate similar profiles for SC and IV
administration for the FSH variant and uFSH. AUC, terminal half life, and Tmax
were also similar.
EXAMPLE 17
Pharmacokinetics of Subcutaneous (SC) Delivery of uFSH compared to
Pulmonary (P) and Subcutaneous (SC) FSH variant in Cynomolgus Monkeys
The following studies were undertaken to measure the bioavailability and
pharmacokinetics of subcutaneous and pulmonary administration of a hFSH
variant
relative to subcutaneous administration of uFSH in Cynomolgus monkeys.Protocol
1
(Subcutaneous uFSH). Cynomolgus monkeys were administered uFSH (2 ~g/kg) by
the SC route. The solution contained uFSH, PBS, and 0.1% human serum albumin .
Whole blood samples were collected at 0.08, 0.25, 0.5, 1, 3, 6, 9, 12, 24, 48,
and 72
hrs post-dose. Samples were collected from the femoral vein or artery and
placed in
collection tubes without anticoagulants. The serum from the whole blood
samples
was obtained by centrifugation. Serum concentrations of immunoreactive uFSH
were
determined by a modified immunoradiometric assay employing a modified
commercial FSH kit, FSH IRMA, DPC~ Ltd. (Los Angeles, CA). IRMA binding
data were analyzed by using a validated data reduction software that employs a
4 or S
parameter logistic algorithm (StatLIA~, Brendan Scientific Corporation). Serum
concentrations of immunoreactive uFSH were estimated from a standard curve
with
uFSH for monkeys administered uFSH. The standards were prepared in cynomolgus
monkey serum with concentrations ranging from 0.5 ng/mL - 75 ng/mL. The lower
limit of quantitation was 0.5 ng/mL. The upper limit of quantitation was 75
ng/mL.
A pharmacokinetic analysis of the serum concentration data was conducted using
conventional techniques. Means and SEM were sometimes calculated using
Microsoft Excel.
Protocol 2 (Subcutaneous and Pulmonary rFSH variant). Cynomolgus
Monkeys were administered a recombinantly produced hFSH variant (2 pg/kg) by
SC
and by pulmonary routes. The recombinant host was designed to express the
alpha
subunit of SEQ ID NO:S and the beta subunit of SEQ ID NO:11 and active variant
was purified from the fermentation broth. The powder formulation was comprised
of
FSP, leucine, and sodium citrate in weight ratio of 12.5:80:7.5. Pulmonary
delivery
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
was carried out using an Inhale dry powder inhalation device (as described in
Example 10 above) to disperse powder from blister packs into the monkey head
dome
for a 2-minute exposure, as described by Allen, D. L., et al., J. Appl.
Toxicol.,
15(1):13-17 (1995). Serum levels of rFSH variant were then measured after the
2-
minute exposure. The inhaled doses each monkey received were calculated post-
treatment based on the aerosol concentration and the mean minute volume of
each
monkey during the 2 minutes of inhalation. These calculated pulmonary doses
vary
between monkeys (see Table 26). A subcutaneous administration of rFSH variant
was also administered to obtain relative pulmonary bioavailability following
calculations of pharmacokinetic parameters. The formulation for subcutaneous
administration contained FSP, PBS, and 0.1% human serum albumin.
Whole blood samples were collected at 0.08, 0.25, 0.5, 1, 3, 6, 9, 12, 24, 48,
and 72 hrs post-dose. Samples were collected from the femoral vein or artery
and
placed in collection tubes without anticoagulants. The serum from the whole
blood
samples was obtained by centrifugation. Serum concentrations of immunoreactive
rFSH variant were determined by a modified immunoradiometric assay employing a
modified commercial FSH kit, FSH IRMA, DPC~ Ltd. (Los Angeles, CA). IRMA
binding data were analyzed by using a valid data reduction software that
employs a 4
or 5 parameter logistic algorithm (StatLIA~, Brendan Scientific Corporation).
Serum
concentrations of immunoreactive rFSH variant were estimated from a standard
curve
with rFSH variant for monkeys administered rFSH variant. The standards were
prepared in cynomolgus monkey serum with concentrations ranging from 0.5 ng/mL
-
75 ng/mL. The lower limit of quantitation was 0.5 ng/mL. The upper limit of
quantitation was 75 ng/mL.
The purpose of this study was to measure the percent bioavailability of an
exemplary dry powder formulation (leucine/citrate) of a hFSH variant in
cynomolgus
monkeys following pulmonary administration, relative to the subcutaneous
administration of FSH. In this study, each monkey received one pulmonary
administration of rFSH variant and two separate subcutaneous injections [2
~g/kg of
hFSH variant and uFSH]. The subcutaneous injection of uFSH was administered to
compare to the rFSH variant. Blood samples were obtained at 0, 0.08, 0.25,
0.5, 1, 3,
6, 9, 12, 24, 48, and 72 hours following pulmonary administration. Blood
samples for
subcutaneous administration were taken at 0, 1, 3, 6, 9, 12, 24, 48, and 72
hours. A
76
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
modified commercial IRMA kit was used to determine the serum concentrations of
immunoreactive hFSH variant or uFSH. These concentrations are found in Tables
26
through 29.
The average percent bioavailability of hFSH variant in the exemplary
pulmonary formulation (leucine/citrate) was approximately 4.6% relative to
subcutaneous administration based on inhaled doses. The corresponding in-lung
bioavailability, or relative pulmonary bioavailability, in this study with
hFSH variant
is estimated to be about 18.4% based on the measurements of pulmonary
deposition
of 2 ~m MMAD particles in monkeys using gamma scintography. In a monkey study
with uFSH in a mannitol/citrate formulation, the average relative
bioavailability was
6.5% based on inhaled dose (26% relative pulmonary bioavailability). Pulmonary
administration of rFSH variant produced peak concentrations of immunoreactive
rFSH variant at an average of 10.8 hours compared to 5.4 hours following
subcutaneous administration. Pulmonary delivery of uFSH in a mannitol/citrate
formulation also reached peak serum concentrations at approximately 10 hours.
The pharmacokinetic parameters of hFSH variant following pulmonary and
subcutaneous administration were similar (Tables 26, 27). The pharmacokinetic
parameters for the two molecules (uFSH and hFSH variant) following
subcutaneous
administration were similar (Table 29). These parameters were also comparable
to
those found in the Example 10 herein following subcutaneous administration of
2 p
g/kg of uFSH.
Table 26. Serum Concentrations (ng/mL) of Immunoreactive rFSH variant
in Cynomolgus Monkeys Following Pulmonary Administration of rFSH variant.
Pulmonary rFSH variant
Animal Number (sex)
Time 8717 8720 8791 8794 8795 Mean SEM N
(hr) (M) (M) (F) (F) (F)
0 BQL BQL BQL BQL BQL 0 0 5
0.08 BQL BQL BQL BQL BQL 0 0 5
0.25 BQL BQL BQL BQL 0.89 0.18 0.18 5
0.5 BQL BQL BQL 0.55 1.29 0.37 0.25 5
77
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
1 0.80 1.04 0.96 1.28 2.20 1.26 0.25 5
3 4.94 4.58 3.07 4.14 5.68 4.48 0.43 5
6 7.78 7.09 5.73 6.30 6.30 6.64 0.36 5
9 10.08 6.77 6.34 4.37 7.92 7.10 0.94 5
12 10.88~'~7.74~'~7.25~'~5.73~'~14.44~'~9.21 1.55 5
24 5.90~'~ 4.00~'~5.41~'~3.80~'~5.81~'~ 4.98 0.45 5
48 1.92~'~ 2.01~'~1.94~'~2.32~'~1.28~'~ 1.89 0.17 5
72 0.72~'~ 0.86~'~0.86~'~1.11~'~2.28~'~ 1.17 0.29 5
Table 27. Serum Concentrations (ng/mL) of Immunoreactive rFSH variant
in Cynomolgus Monkeys Following Subcutaneous Administration of rFSH
variant.
Subcutaneous
rFSH
variant
(2~g/kg)
Animal
Number
(sex)
Time 8717 8720 8791 8794 8795 Mean SEM N
(hr) (M) (M) (F) (F) (F)
0 BQL BQL BQL BQL BQL 0 0 5
1 0.53 BQL BQL BQL NR 0.13 0.13 4
3 2.76 1.32 2.66 2.66 7.03 3.29 0.97 5
6 5.53 3.65 3.80 4.32 6.14 4.69 0.49 5
9 4.39 2.74 3.46 3.29 4.42 3.66 0.33 5
12 2.74~'~2.04~'~2.64~'~2.68~'~3.81~'~ 2.78 0.29 S
24 2.59~'~1.86~'~1.57~'~2.57~'~2.64~'~ 2.25 0.22 5
48 0.80~'~1.29~'~1.19~'~1.06~'~1.26~'~ 1.12 0.09 5
72 BQL 0.57 0.61 BQL BQL 0.24 0.14 5
Abbreviations: M = male; F = female; BQL = below the quantitation limit; NR =
no
result due to insufficient amount of sample; hr = hour; ng = nanogram; mL =
milliliter; ~g = microgram; kg = kilogram.
(1) Time point used for calculating the half life .
78
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Table 28. Serum Concentrations (ng/mL) of Immunoreactive FSH in
Cynomolgus Monkeys Following Subcutaneous Administration of uFSH
Subcutaneous
uFSH
(2~g/kg)
Animal
Number
(sex)
Time 8717 8720 8791 8794 8795 Mean SEM N
(hr) (M) (M) (F) (F) (F)
0 BQL BQL BQL BQL BQL 0 0 5
1 0.77 0.67 0.73 2.93 1.23 1.27 0.43 5
3 5.05 4.38 3.57 6.71 3.55 4.65 0.59 5
6 6.31 4.52 4.76 6.53 3.60 5.14 0.56 5
9 6.30 4.28 4.14 4.85 3.55 4.62 0.47 5
12 4.46~'~4.04~'~4.11~'~3.39~'~2.81~'~3.76 0.29 5
24 2.61~'~2.87~'~2.83~'~3.06~'~2.85~'~2.84 0.07 5
48 1.15~'~1.10~'~1.65~'~1.04~'~1.65~'~1.32 0.14 5
72 BQL BQL 0.86 0.61 0.70 0.43 0.18 5
Abbreviations: M = male; F = female; BQL = below the quantitation limit; hr =
hour;
ng = nanogram; mL = milliliter; ~g = microgram; kg = kilogram.
( 1 ) Time point used for calculating the half life
Table 29. Pharmacokinetic Parameters for Cynomolgus Monkeys after
Pulmonary and Subcutaneous Administration of rFSH variant and
Subcutaneous Administration of uFSH
rFSH variant Subcutaneous Dose
Statistics\Subject8720 8791 8794 8795 Mean SEM N
ID 8717
AUCp_ 72hr 123.8 109.1 112.4 121.3 159.1 125.1
8.92 5
(ng*hr/mL)
AUCO_~ 130.9 144.5 132.1 139.3 174.3 144.2
7.92 5
(ng*hr/mL)
Half Life 52.9533.6125.3422.5427.05 4.26
(hr) 19.09 5
CMS (ng/mL) 3.653.80 4.32 7.03 4.87 0.63
5.53 5
TM~ (hr) 6 6 6 6 3 5.40 0.60
5
Dose (~g/kg) 2 2 2 2 2
2
79
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
rFSH variant Pulmonary Dose
Statistics\Subject8720 8791 8794 8795 Mean SEM
ID 8717 N
AUCp_ 72hr 309.5 242.9 253.7 224.5 331.2 272.4
20.42 5
(ng*hr/mL)
AUCO_~ 324.3 268.5 282. 6 297.5 337.9 302.1
12.84 5
(ng*hr/mL)
Half Life (hr) 19.7018.9226.2221.3219.691.71 5
15.33
CMax (ng/mL) 10.887.74 7.25 6.3014.449.32 1.49 5
Two (hr) 12 12 12 6 12 10.8 1.20 5
Ave. Minute Vol. 2.07 3.57 3.082.36
2.45
(L)
Dose (~g/kg) 69 85 124 114 87 95.8 10.1 5
Percent 7.18 4.37 3.45 3.754.50 4.64 0.66 5
Bioavailability
uFSH
Subcutaneous
Dose
Statistics\Subject87208791 8794 8795 Mean SEM N
ID 8717
AUCO_ 72hr 159.6 146.7168.4168.1152.6159.14.27 5
(ng*hr/mL)
AUCO_~ 169.3 161.6204.3178.4215.1185.710.3 5
(ng*hr/mL)
Half Life (hr) 18.9027.7920.0643.5123.223.21 5
18.66
CM~ (ng/mL) 6.31 4.524.76 6.71 3.60 5.18 0.58 5
TM~ (hr) 6 6 6 3 6 5.40 0.60 5
Dose (~g/kg) 2 2 2 2 2 2 5
Abbreviations: hr = hour; mL = milliliter; SEM = standard error of the mean;
ng =
nanogram; ~g = microgram; kg = kilogram; N = number of observations; Cm~ _
maximum concentration; AUCO_ t = Area under the curve extrapolated from time 0
to
tau (72hr); AUCO_~ = Area under the curve extrapolated from time 0 to
infinity;
Tmax = time to maximum serum concentration.
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Examule 18
Expression of hFSH variant in CHO-Kl Cells
One skilled in the art will be aware of many ways to express FSP using
suitable expression vectors and host cell lines. One way is to use a Chinese
Hamster
Ovary cell line, such as the CHO-K1 cell line (LONZA Biologics). These cells
may
be designed to express hFSH variant comprised of the alpha subunit having the
sequence of SEQ ID NO: 5 and the beta subunit having the sequence of SEQ ID
NO:
11.
An expression vector is constructed by standard techniques. Vector DNA to
support the transfection may be prepared in GibcoBRL ElectroMax DH10B cells
(Cat. # 18290-015), using the Qiagen QiaFilter Maxi Prep kit (Cat. # 12263)
and the
vector confirmed by an appropriate diagnostic digest (e.g., Hind III/Xho I).
Sequence
analysis of both genes would confirm that the DNA sequences designed to
express the
alpha subunit of SEQ ID NO:S and the variant beta subunit of SEQ ID NO:11 are
those given in SEQ ID NOS:37 and 38 herein, respectively. The expression of
each
subunit can be controlled by a different promoter or by the same promoter. The
sequences may also use a polyA tail. The vector may contain a selectable
marker.
The vector is used to transfect CHO-K1 cells using known techniques.
The cell line is grown in suitable medium, such as GibcoBRL's CD CHO
media, under selective pressure. ELISA assays may be used to identify master
wells
expressing hFSH variant, which are cloned and amplified using standard
procedures.
These procedures will lead to clones that have suitable expression levels. For
example, in shake flasks (20-60 mL), active hFSH variant at 30 mg/L is
produced
after 7 to 8 days.
Example 19
Purification of FSP
Purification of recombinant FSP, such as for example, the hFSH variant
comprised of an alpha subunit of SEQ ID NO: 5 and a beta subunit of SEQ ID:l
1, can
be accomplished by a number of methods described and known in the art from
monolayer or suspension cultures of transformed host cells, such as CHO-K1 or
AV 12 cell lines or other production lines suitably available. One method for
isolating
FSP from the culture medium comprises subjecting the culture medium to cation
or
anion exchange chromatography, dye affinity chromatography, reverse phase
81
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
chromatography, gel filtration chromatography, or to some combination of these
methods. In the case of suspension cultures, which may contain detergents,
additional
purification steps may be needed, such as a ion exchange step. The
chromatographic
steps can be optimized for pH, conductivity, buffer composition and running
conditions (column dimensions, flow rates, etc). Purity and yield can be
analyzed by
SDS-PAGE gels (both Coomassie staining and Western blotting), ELISA assays,
exclusion chromatography and protein concentration by UV absorbance at 277nm
or
other known techniques. Using standard purification steps, FSP having purity
greater
than 95% by Coomassie and silver-stained gels can be obtained.
Examule 20
Subchronic Inhalation Study of Leucine as an Excipient for Pulmonary
Administration
The pharmaceutical acceptability of leucine in a powder for pulmonary
delivery of FSP was studied in domestic-reared cynomolgus monkeys (Macaca
fascicularis). The target inhaled dose of leucineaodium citrate (90:10) was
1.66
mg/kg/day. The animals were exposed for 15 minutes per day, for 15 days. The
monkeys were obtained from Charles River BRF, Inc - Mauritius. Monkeys were
housed upon arrival and isolated for at least 6 weeks prior to being assigned
to the
study. Four monkeys (two of each sex) were arbitrarily assigned to treatment
groups
and were assigned to individual cages. Monkeys were housed in a room in which
the
thermostats were set to maintain a temperature of 72 ~ 8°F. The
environmental
control system was designed to maintain a minimum relative humidity of 20% and
a
maximum of 80%. The photo period was 12 hours light and 12 hours dark,
changing
at 0600 and 1800 hours. Monkeys were individually housed in aluminum cages
with
suspended floors. Feces and urine were manually washed away daily.
Approximately
to 20 biscuits of Certified Primate Chow 5048 (PMI Nutrition International,
Inc)
were offered to the monkeys twice daily during the week, and a double ration
was
offered once daily on weekends. Each monkey received fresh fruit at least 3
times
each week. City water was available ad libitum from an automatic watering
system
except during exposures. Each monkey was conditioned to sit in a restraint
chair at
least 9 times prior to beginning the exposures. The conditioning was
accomplished
with the use of food treats as positive reinforcement.
82
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
The monkeys were placed into restraint chairs prior to each exposure. Two
sheets of latex (0.30 in. thick) were placed around the animals' necks to form
a seal.
An approximately 7.7-liter head-dome was placed over the animals' heads.
Airflow of
approximately 12 L/min was maintained through the head-dome via a calibrated
transvector on the exhaust port. The leucine-citrate powder (90% leucine/10%
sodium citrate) was generated using a Wright Dust Feed generator. Eight to ten
liters/minute of air were run through the generator. The aerosol was passed
through a
cyclone then entered the head-dome for the monkey to breathe.
The concentration of leucine was determined with gravimetric samples taken
from the head dome during exposure. These samples were collected by drawing 1
LPM of air through a 25-mm Gelman Type A/E glass fiber filter that was
connected
to the head dome. One particle size determination was completed. The particle
size
was determined from samples collected using a Sierra Model 218K Cascade
Impactor
fitted with Gelman Type A/E glass fiber filters. Airflow though the Cascade
Impactor
was 3 LPM for a duration of 5 minutes. Inhaled doses were calculated as
follows:
(average minute volume x exposure time) x (average gravimetric concentration)
body weight.
Flow-derived parameters (tidal volume, minute volume, and breathing
frequency) were monitored using a size '0' pneumotachograph connected to a
port on
the head-dome. The signals were collected on a personal computer using the
XA software from Buxco Electronics, Inc. At least 15 minutes of pre-exposure
data
were collected before the exposures began, followed by data collection
throughout the
exposure period. All pulmonary function data were collected as 5-minute
averages.
These data were downloaded into a Microsoft Excel spreadsheet and graphed to
look
for trends in breathing patterns.
Each monkey was observed prior to, during, and after exposures. Any unusual
behavior was noted. Body weights were recorded (initial body mass - males: 2.9
to
4.1 kg; females: 2.7 to 2.8 kg). These data were averaged and used to
calculate the
dose of leucine delivered to the monkeys' lungs. Blood samples were obtained
from
each monkey prior to initiation of dosing and on Day 15. These blood samples
were
obtained prior to feeding. In addition, bronchoalveolar lavage (BAL) was
performed
prior to initiation of dosing and on Day 15. The animals were dosed with
glycopyrrolate 0.01 S mg/kg IM, followed a few minutes later with ketamine HCl
83
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
mg/kg IM. A pediatric bronchoscope was then inserted into the right or left
lower
lobe and wedged into an airway. Two 10-mL aliquots of normal saline were put
into
the lung then gently hand-suctioned out with a 20-mL syringe. The recovered
fluid
was put on ice and sent to the clinical pathology laboratory for analysis.
Blood analyses included: erythrocyte count, hemoglobin, hematocrit (packed
cell volume), mean corpuscular volume, mean corpuscular hemoglobin, mean
corpuscular hemoglobin concentration, reticulocyte count, blood cell
morphology,
total leukocyte count, leukocyte differential, platelet (thrombocyte) count,
total
leukocyte count, leukocyte differential, prothrombin time, activated partial
thromboplastin time, glucose, blood urea nitrogen, creatinine, total
bilirubin, alkaline
phosphatase, alanine transaminase, aspartate transaminase, gamma
glutamyltransferase, creatine phosphokinase, calcium, inorganic phosphorus,
sodium,
potassium, chloride, cholesterol, triglycerides, total protein, albumin,
globulin,
microprotein. Urine was analyzed for specific gravity and protein.
The average gravimetric concentration across the 14 days of exposure was
425.83 ~ 232.90 p.g/L (Mean ~ SD). The mass median equivalent aerodynamic
diameter was 1.00 pm with a geometric standard deviation of 2.46. The average
inhaled dose relative to body weight was 2.13 ~ 0.56 mg/kg/day.
There were no unusual behaviors noted in any animal during the study. There
were no treatment-related increases in breathing frequency, tidal volume, or
minute
volume. An apparent increase in breathing frequency on the first day of
exposure is
most likely due to a non-specific response of the monkeys to the appearance of
the
dust cloud in the dome. By the next measurement day, the animals had
acclimated to
the dust in the dome and the difference in breathing frequency disappeared.
There were no compound-related effects on hematology parameters, clinical
chemistry parameters, urinalysis parameters, or on bronchoalveolar lavage
(BAL)
parameters. One monkey had minimal elevation in protein level, total white
blood
cell count, neutrophil, lymphocyte and macrophage counts, and red blood cell
count
in the BAL fluid compared to other monkeys in the study and to historical
reference
values. These findings were considered unrelated to the compound and likely
reflect
a mild, undetermined, preexisting pulmonary condition in this monkey as
evidenced
by an elevated BAL fluid protein level in the pretreatment BAL.
84
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Example 21
FSP Powders made using a Niro Spray Dryer
Powders containing leucine, sodium citrate, and recombinant FSP
biosynthesized and purified essentially as described herein were prepared
using a Niro
mobile minor spray dryer fitted with a t'~~o-fluid nozzle. Powder
characteristics were
measured as described elsewhere herein. Free subunit composition was measured
essentially as described in Example 2. The composition of each of the FSP
powders
is provided in Table 30 below. Batch sizes were ten grams for the first six
lots, and 5
grams for the last three lots in Table 30. Yields for the powders ranged from
62%-
78%.
Table 30. Niro FSP Powder Characteristics
Lot Powder MMD MMAD Sub. HZO ED
#
Composition
FSP L C (pm) (pm) (%) (%)
2011 1.9 38 2.3 3.5 8.2 1.4
6
0
2012 1.9 38 2.2 3.6 6.6 0.8
6
0
2013 1.9 38 2.1 3.5 7.9 1.3
6
0
2014 1.9 38 2.6 3.8 7.7 1.2
6
0
2015 1.9 38 2.0 3.4 6.1 1.2
6
0
2016 1.9 38 1.7 3.0 4.5 1.7
6
0
2017 1.9 58 1.7 2.3 3.2 82
4
0
2018 1.9 38 1.4 2.2 3.8 77
6
0
2021 11 29 1.8 2.2 2.8 78
6
0
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
2022 11 4 49 1.6 2.3 2.9 82
0
2095 1.9 6 38 1.5 2.4 5.5
0
' 2096 1.9 4 58 1.5 2.1 4.0
0
FSP=follicle stimulating protein; L=leucine; C=sodium citrate; Sub.=subunit
content
Example 22
Effects of Scale and Formulation Components on Characteristics of FSP
Powders
Lots 00023-00030 were produced using a Buchi spray dryer (Model 190)
fitted with a modified two fluid nozzle. Analytical measurements were made
after
filling blister packs by hand. A hFSH variant (cell designed to express the
alpha
subunit having the sequence of SEQ ID N0:5 and the beta subunit having the
sequence of SEQ ID NO:11) was used for all lots. Lots 1952-1954 were produced
using a Niro Mobile Minor spray dryer fitted with a modified two fluid nozzle.
Table 31.
Lot FSP L C Lot [FSP] MMD MMAD APC
size
(g) l~~mL (pm) (wm) (%)
1952 1.75 40 58 5 170 1.9 2.2 56
1953 1.75 40 58 5 340 2.2 2.8 46
1954 8 80 12 3 800 1.8 3.6 39
86
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Table
32.
Powder ED (%)
Composition
Lot FSP L C mean SubunitHOA
(%) (%) (%) (SD) (%) (%)
00023 1.0 40 59.0 76.2 (8.5) 6.5 0.16
00024 1.5 60 38.5 83.1 (4.0) 5.6 0.18
00025 1.5 50 48.5 4.8 0.24
00026 4.0 80 16.0 85.7 (2.5) 5.4 0.12
00027 4.0 40 56.0 82.3 (5.0) 3.6 0.07
00028 12.0 60 28.0 3.3 0.03
00030 1.5 60 38.5 81.5 (3.2) 5.6 0.08
1952 1.7 40 58.3 78.1 (6.6) 4
1953 1.7 40 58.3 75.6 (6.5) 5
1954 8.0 80 12.0 76.9 (3.5) 9
L=leucine; C=sodium citrate
Example 23
Effect of Various Amino Acid Excipients on FSP Powder Characteristics
FSP powders containing various amino acid excipients, optionally in
combination with citrate, prepared essentially as described herein, with
yields ranging
from about 54% to 78%. Selected features of the resultant powders are provided
in
Table 33 below.
Table 33.
Lot Powder Composition HZO Subunit HOA MMD
(%) (%) (%) (%) (~.lm)
000047 1.3 36.4 0.18 1.32
FSP:alanine:citrate
2:80:18
000048 0.6 33.1 0.05 1.89
FSP:leucine:citrate:methionine
2:80:13:5
87
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
000049FSP:leucine:citrate 0.6 15.1 0.12 1.72
2:80:18
000050FSP:leucine:citrate:mannitol0.3 15.4 0.08 1.76
2:80:13:5
000051FSPari-leucine:citrate2.3 4.5 0.03 1.43
2:40:58
000052FSP:leucine:citrate:isoleucine0.5 11.1 0.05 1.78
2:80:13:5
000053FSP:leucine:citrate:arginine0.7 6.9 0.05 1.40
2:80:13:5
00029 FSP:valine:citrate - 13.6 0.30 -
1.5:60:38:5
88
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<110> ELI LILLY AND COMPANY AND IT: HALE T!-IFJRAPEUTIC SYS'='EMS, IivC.
<120> PULMONARY ADMINISTRATION OF DRY POPdDER FORMLiLATIONS FOR TREATING
INFERTILITY
<130> 032055-019
<140>
<141>
<150> 60/130,099
<151> 1999-04-20
<160> 56
<170> PatentIn Ver. 2.1
<210> 1
<211> 96
<212> PRT
<213> mammalian
<400> 1
Phe Pro Asp Gly Glu Phe Thr Met Gln Gly Cys Pro Glu Cys Lys Leu
1 5 10 15
Lys Glu Asn Lys Tyr Phe Ser Lys Pro Asp Ala Pro Ile Tyr Gln Cys
20 25 30
Met Gly Cys Cys Phe Ser Arg Ala Tyr Pro Thr Pro Ala Arg Ser Lys
35 40 45
Lys Thr Met Leu Val Pro Lys Asn Ile Thr Ser Glu Ala Thr Cys Cys
50 55 60
Val Ala Lys Ala Phe Thr Lys Ala Thr Val Met Gly Asn Val Arg Val
65 70 75 80
Glu Asn His Thr Glu Cys His Cys Ser Thr Cys Tyr Tyr His Lys Ser
85 90 95
<210> 2
<211> 111
<212> PRT
<213> mammalian
<400> 2
Arg Ser Cys Glu Leu Thr Asn Ile Thr Ile Thr Val Glu Lys Glu Glu
1 5 10 15
Cys Gly Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys
20 25 30
Tyr Thr Arg Asp Leu Val Tyr Arg Asp Pro Ala Arg Pro Asn Ile Gln
35 40 45
89
CA 2001-10-02
02369262
WO 0/61178 PCT/US00/09869
0
LysThrCys ThrPhe LysGluLeu ValTyrGlu ThrVal LysValPro
50 55 60
GlyCysAla HisHis AlaAspSer LeuTyrThr TyrPro ValAlaThr
65 70 75 80
GluCysHis CysSer LysCysAsp SerAspSer ThrAsp CysThrVal
g5 g0 95
ArgGlyLeu GlyPro SerTyrCys SerPheArg GluIle LysGlu
100 105 110
<210> 3
<211> 96
<212> PRT
<213> mammalian
<400> 3
Phe Pro Asp Gly Glu Phe Thr Thr Gln Asp Cys Pro Glu Cys Lys Leu
1 5 10 15
Arg Glu Asn Lys Tyr Phe Phe Lys Leu Gly Val Pro Ile Tyr Gln Cys
20 25 30
Lys Gly Cys Cys Phe Ser Arg Ala Tyr Pro Thr Pro Ala Arg Ser Arg
35 40 45
Lys Thr Met Leu Val Pro Lys Asn Ile Thr Ser Glu Ser Thr Cys Cys
50 55 60
Val Ala Lys Ala Phe Ile Arg Val Thr Val Met Gly Asn Ile Lys Leu
65 70 75 80
Glu Asn His Thr Gln Cys Tyr Cys Ser Thr Cys Tyr His His Lys Ile
85 90 95
<210> 4
<211> 111
<212> PRT
<213> mammalian
<400> 4
Asn Ser Cys Glu Leu Thr Asn Ile Thr Ile Ala Val Glu Lys Glu Gly
1 5 10 15
Cys Gly Phe Cys Ile Thr Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys
20 25 30
Tyr Thr Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Asn Ile Gln
35 40 45
Lys Thr Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Lys Val Pro
50 55 60
Gly Cys Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Ala Cys His Cys Gly Lys Cys Asn Ser Asp Ser Thr Asp Cys Thr Val
85 90 95
Arg Gly Leu Gly Pro Ser Tyr Cys Ser Phe Gly Asp Met Lys Glu
100 105 110
<210> 5
<211> 92
<212> PRT
<213> Homo Sapiens
<400> 5
Ala Pro Asp Val Gln Asp Cys Pro Glu Cys Thr Leu Gln Glu Asn Pro
1 5 10 15
Phe Phe Ser Gln Pro Gly Ala Pro Ile Leu Gln Cys Met Gly Cys Cys
20 25 30
Phe Ser Arg Ala Tyr Pro Thr Pro Leu Arg Ser Lys Lys Thr Met Leu
35 40 45
Val Gln Lys Asn Val Thr Ser Glu Ser Thr Cys Cys Val Ala Lys Ser
50 55 60
Tyr Asn Arg Val Thr Val Met Gly Gly Phe Lys Val Glu Asn His Thr
65 70 75 80
Ala Cys His Cys Ser Thr Cys Tyr Tyr His Lys Ser
85 90
<210>
6
<211>
111
<212>
PRT
<213>
Homo
Sapiens
<400>
6
Asn CysGlu LeuThrAsn IleThrIle AlaIle GluLysGlu Glu
Ser
1 5 10 15
Cys PheCys IleSerIle AsnThrThr TrpCys A1aGlyTyr Cys
Arg
20 25 30
Tyr ArgAsp LeuValTyr LysAspPro AlaArg ProLysIle Gln
Thr
35 40 45
Lys CysThr PheLysGlu LeuValTyr GluThr ValArgVal Pro
Thr
50 55 60
Gly AlaHis HisAlaAsp SerLeuTyr ThrTyr ProValAla Thr
Cys
65 70 75 80
Gln HisCys GlyLysCys AspSerAsp SerThr AspCysThr Val
Cys
85 90 95
Arg LeuGly ProSerTyr CysSerPhe GlyGlu MetLysGlu
Gly
100 105 110
91
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<210> 7
<211> 96
<212> PRT
<213> mammalian
<400> 7
Phe Pro Asp Gly Glu Phe Thr Met Gln Gly Cys Pro Glu Cys Lys Leu
1 5 10 15
Lys Glu Asn Lys Tyr Phe Ser Lys Leu Gly Ala Pro Ile Tyr Gln Cys
20 25 30
Met Gly Cys Cys Phe Ser Arg Ala Tyr Pro Thr Pro Ala Arg Ser Lys
35 40 45
Lys Thr Met Leu Val Pro Lys Asn Ile Thr Ser Glu Ala Thr Cys Cys
50 55 60
Val Ala Lys Ala Phe Thr Lys Ala Thr Val Met Gly Asn Ala Arg Val
65 70 75 80
Glu Asn His Thr Glu Cys His Cys Ser Thr Cys Tyr Tyr His Lys Ser
85 90 95
<210>
8
<211> 1
11
<212>
PRT
<213> mmalian
ma
<400>
8
Asn CysGlu LeuThrAsn IleThrIle ThrValGlu LysGluGlu
Ser
1 5 10 15
Cys PheCys IleSerIle AsnThrThr TrpCysAla GlyTyrCys
Asn
20 25 30
Tyr ArgAsp LeuValTyr LysAspPro AlaArgPro AsnIleGln
Thr
35 40 45
Lys CysThr PheLysGlu LeuValTyr GluThrVal LysValPro
Thr
50 55 60
Gly AlaHis HisAlaAsp SerLeuTyr ThrTyrPro ValAlaThr
Cys
65 70 75 80
Glu HisCys GlyLysCys AspSerAsp SerThrAsp CysThrVal
Cys
85 90 95
Arg LeuGly ProSerTyr CysSerPhe SerGluMet LysGlu
Gly
100 105 110
<210> 9
<211> 96
<212> PRT
<213> mammalian
92
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<400> 9
Phe Pro Asp Gly Glu Phe Thr Met Gln Gly Cys Pro Glu Cys Lys Leu
1 5 10 15
Lys Glu Asn Lys Tyr Phe Ser Lys Pro Asp Ala Pro Ile Tyr Gln Cys
20 25 30
Met Gly Cys Cys Phe Ser Arg Ala Tyr Pro Thr Pro Ala Arg Ser Lys
35 40 45
Lys Thr Met Leu Val Pro Lys Asn Ile Thr Ser Glu Ala Thr Cys Cys
50 55 60
Val Ala Lys Ala Phe Thr Lys Ala Thr Val Met Gly Asn Val Arg Val
65 70 75 80
Glu Asn His Thr Glu Cys His Cys Ser Thr Cys Tyr Tyr His Lys Ser
85 90 95
<210> 10
<211> 111
<212> PRT
<213> mammalian
<400> 10
Arg Ser Cys Glu Leu Thr Asn Ile Thr Ile Thr Val Glu Lys Glu Glu
1 5 10 15
Cys Ser Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys
20 25 30
Tyr Thr Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Asn Ile Gln
35 40 45
Lys Ala Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Lys Val Pro
50 55 60
Gly Cys Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr
65 70 75 80
Glu Cys His Cys Gly Lys Cys Asp Arg Asp Ser Thr Asp Cys Thr Val
85 90 95
Arg Gly Leu Gly Pro Ser Tyr Cys Ser Phe Ser Asp Ile Arg Glu
100 105 110
<210> 11
<211> 108
<212> PRT
<213> Homo Sapiens
<900> 11
Asn Ser Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu
1 5 10 15
93
CA
02369262
2001-10-02
WO 0/61178 PCT/US00/09869
0
CysArg PheCysIle SerIleAsn ThrThr TrpCysAla GlyTyr Cys
20 25 30
TyrThr ArgAspLeu ValTyrLys AspPro AlaArgPro LysIle Gln
35 40 45
LysThr CysThrPhe LysGluLeu ValTyr GluThrVal ArgVal Pro
50 55 60
GlyCys AlaHisHis AlaAspSer LeuTyr ThrTyrPro ValAla Thr
65 70 75 80
GlnCys HisCysGly LysCysAsp SerAsp SerThrAsp CysThr Val
85 90 95
ArgGly LeuGlyPro SerTyrCys SerPhe GlyGlu
100 105
<210> 12
<211> 109
<212> PRT
<213> Homo sapiens
<400> 12
Asn Ser Cys Glu Leu Thr Asn Ile Thr_Ile Ala Ile Glu Lys Glu Glu
1 5 10 15
Cys Arg Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys
20 25 30
Tyr Thr Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile Gln
35 40 45
Lys Thr Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Arg Val Pro
50 55 60
Gly Cys Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr
65 70 75 80
Gln Cys His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val
85 90 95
Arg Gly Leu Gly Pro Ser Tyr Cys Ser Phe Gly Glu Met
100 105
<210>
13
<211>
110
<212>
PRT
<213>
Homo
sapiens
<400>
13
Asn Ser Glu Thr Asn Thr IleAla Ile LysGlu
Cys Leu Ile Glu Glu
1 5 10 15
Cys Arg Cys Ser Ile Thr ThrTrp Cys GlyTyr
Phe Ile Asn Ala Cys
20 25 30
Tyr Thr Asp Val Tyr Asp ProAla Arg LysIle
Arg Leu Lys Pro Gln
35 90 45
94
CA
02369262
2001-10-02
WO PCT/US00/09869
00/61178
Lys Thr ThrPhe LysGluLeu ValTyr GluThrVal ArgVal Pro
Cys
50 55 60
Gly Cys HisHis AlaAspSer LeuTyr ThrTyrPro ValAla Thr
Ala
65 70 75 80
Gln Cys CysGly LysCysAsp SerAsp SerThrAsp CysThr Val
His
85 90 95
Arg Gly GlyPro SerTyrCys SerPhe GlyGluMet Lys
Leu
100 105 110
<210>
14
<211> 7
<212>
PRT
<213> mo ns
Ho sapie
<400>
14
Asn CysGlu LeuThrAsn IleThr IleAlaIle GluLysGlu Glu
Ser
1 5 10 15
Cys PheCys IleSerIle AsnThr ThrTrpCys AlaGlyTyr Cys
Arg
20 25 30
Tyr ArgAsp LeuValTyr LysAsp ProAlaArg ProLysIle Gln
Thr
35 40 45
Lys CysThr PheLysGlu LeuVal TyrGluThr ValArgVal Pro
Thr
50 55 60
Gly AlaHis HisAlaAsp SerLeu TyrThrTyr ProValAla Thr
Cys
65 70 75 80
Gln HisCys GlyLysCys AspSer AspSerThr AspCysThr Val
Cys
85 90 95
Arg LeuGly ProSerTyr CysSer PheGly
Gly
100 105
<210> 15
<211> 106
<212> PRT
<213> Homo
Sapiens
<900> 15
Asn Ser Glu LeuThrAsn IleThrIle AlaIleGlu LysGluGlu
Cys
1 5 10 15
Cys Arg Cys IleSerIle AsnThrThr TrpCysAla GlyTyrCys
Phe
20 25 30
Tyr Thr Asp LeuValTyr LysAspPro AlaArgPro LysIleGln
Arg
35 40 45
Lys Thr Thr PheLysGlu LeuValTyr GluThrVal ArgValPro
Cys
50 55 60
Gly Cys His HisAlaAsp SerLeuTyr ThrTyrPro ValAlaThr
Ala
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Gln Cys His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val
85 90 95
Arg Gly Leu Gly Pro Ser Tyr Cys Ser Phe
100 105
<210> 16
<211> 105
<212> PRT
<213> Homo Sapiens
<400> 16
Asn Ser Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu
1 5 ~ 10 15
Cys Arg Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys
20 ~ 25 30
Tyr Thr Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile Gln
35 40 45
Lys Thr Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Arg Val Pro
50 55 60
Gly Cys Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr
65 70 75 80
Gln Cys His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val
85 90 95
Arg Gly Leu Gly Pro Ser Tyr Cys Ser
100 105
<210> 17
<211> 109
<212> PRT
<213> Homo apiens
S
<400> 17
Ser Cys LeuThrAsn IleThrIle AlaIle GluLysGlu GluCys
Glu
1 5 10 15
Arg Phe IleSerIle AsnThrThr TrpCys AlaGlyTyr CysTyr
Cys
20 25 30
Thr Arg LeuValTyr LysAspPro AlaArg ProLysIle G1nLys
Asp
35 40 45
Thr Cys PheLysGlu LeuValTyr GluThr ValArgVal ProGly
Thr
50 55 60
Cys Ala HisAlaAsp SerLeuTyr ThrTyr ProValAla ThrGln
His
65 70 75 80
Cys His GlyLysCys AspSerAsp SerThr AspCysThr ValArg
Cys
85 90 95
96
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<210> 18
<211> 108
<212> PRT
<213> Homo Sapiens
<400> 18
Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu Cys Arg
1 5 10 15
Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys Tyr Thr
20 25 30
Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile Gln Lys Thr
35 40 45
Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Arg Val Pro Gly Cys
50 55 60
Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr Gln Cys
65 70 75 80
His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val Arg Gly
85 90 95
Leu Gly Pro Ser Tyr Cys Ser Phe Gly Glu Met Lys
100 105
<210> 19
<211> 108
<212> PRT
<213> Homo
Sapiens
<400> 19
Ser Cys LeuThrAsn IleThr IleAlaIle GluLysGlu GluCys
Glu
1 5 10 15
Arg Phe IleSerIle AsnThr ThrTrpCys AlaGlyTyr CysTyr
Cys
20 25 30
Thr Arg LeuValTyr LysAsp ProAlaArg ProLysIle GlnLys
Asp
35 40 45
Thr Cys PheLysGlu LeuVal TyrGluThr ValArgVal ProGly
Thr
50 55 60
Cys Ala HisAlaAsp SerLeu TyrThrTyr ProValAla ThrGln
His
65 70 75 80
Cys His GlyLysCys AspSer AspSerThr AspCysThr ValArg
Cys
85 90 95
Gly Leu ProSerTyr CysSer PheGlyGlu Met
Gly
100 105
<210> 20
97
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<212>
PRT
<213>
Homo
Sapiens
<400>
20
Cys LeuThr AsnIle ThrIleAla IleGluLys GluGlu CysArg
Glu
1 5 10 15
Phe IleSer IleAsn ThrThrTrp CysAlaGly TyrCys TyrThr
Cys
20 25 30
Arg LeuVal TyrLys AspProAla ArgProLys IleGln LysThr
Asp
35 40 45
Cys PheLys GluLeu ValTyrGlu ThrValArg ValPro GlyCys
Thr
50 55 60
Ala HisAla AspSer LeuTyrThr TyrProVal AlaThr GlnCys
His
65 70 75 80
His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val Arg Gly
85 90 95
Leu Gly Pro Ser Tyr Cys Ser Phe Gly Glu Met
100 105
<210>
21
<211> 7
<212> T
PR
<213> mo apiens
Ho S
<400>
21
Ser GluLeu ThrAsn IleThrIle AlaIleGlu LysGlu GluCys
Cys
1 5 10 15
Arg CysIle SerIle AsnThrThr TrpCysAla GlyTyr CysTyr
Phe
20 25 30
Thr AspLeu ValTyr LysAspPro AlaArgPro LysIle GlnLys
Arg
35 40 45
Thr ThrPhe LysGlu LeuValTyr GluThrVal ArgVal ProGly
Cys
50 55 60
Cys HisHis AlaAsp SerLeuTyr ThrTyrPro ValAla ThrGln
Ala
65 70 75 80
Cys CysGly LysCys AspSerAsp SerThrAsp CysThr ValArg
His
85 90 95
Gly GlyPro SerTyr CysSerPhe GlyGlu
Leu
100 105
<210> 22
<211> 106
<212> PRT
<213> Homo sapiens
<400> 22
98
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
1 5 lU 15
PheCys IleSer IleAsnThr ThrTrpCys AlaG~-yTyrCysTyr Thr
20 25 30
ArgAsp LeuVal TyrLysAsp ProAlaArg ProLys IleGlnLys Thr
35 40 45
CysThr PheLys GluLeuVal TyrGluThr ValArg ValProGly Cys
50 55 60
AlaHis HisAla AspSerLeu TyrThrTyr ProVai AlaThrGln Cys
65 70 75 80
HisCys GlyLys CysAspSer AspSerThr AspCys ThrValArg Gly
g5 90 95
LeuGly ProSer TyrCysSer PheGlyGlu
100 105
<210> 23
<211> 106
<212> PRT
<213> Homo Sapiens
<400> 23
Ser Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu Cys
1 5 10 15
Arg Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys Tyr
20 25 30
Thr Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile Gln Lys
35 40 45
Thr Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Arg Val Pro Gly
50 55 60
Cys Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr Gln
65 70 75 80
Cys His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val Arg
85 90 95
Gly Leu Gly Pro Ser Tyr Cys Ser Phe Gly
100 105
<210> 24
<211> 105
<212> PRT
<213> Homo Sapiens
<900> 24
Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu Cys Arg
1 5 10 15
Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys Tyr Thr
20 25 30
99
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
ArgAsp LeuVal TyrLysAsp ProAiaArg ProLys IleGlnLys Thr
35 40 45
CysThr PheLys GluLeuVal TyrGluT:!rValArg ValProGly Cys
50 55 60
AlaHis HisAla AspSerLeu TyrThrTyr ProVal AlaThrGln Cys
65 70 75 80
HisCys GlyLys CysAspSer AspSerThr AspCys ThrValArg Gly
85 90 95
LeuGly ProSer TyrCysSer PheGly
100 105
<210>
25
<211>
105
<212>
PRT
<213> Sapiens
Homo
<400>
25
Ser Cys Leu ThrAsn IleThrIle AlaIle GluLysGlu GluCys
Glu
1 5 10 15
Arg Phe Ile SerIle AsnThrThr TrpCys AlaGlyTyr CysTyr
Cys
20 25 30
Thr Arg Leu ValTyr LysAspPro AlaArg ProLysIle GlnLys
Asp
35 40 45
Thr Cys Phe LysGlu LeuValTyr GluThr ValArgVal ProGly
Thr
50 55 60
Cys Ala His AlaAsp SerLeuTyr ThrTyr ProValAla ThrGln
His
65 70 75 80
Cys His Gly LysCys AspSerAsp SerThr AspCysThr ValArg
Cys
85 90 95
Gly Leu Pro SerTyr CysSerPhe
Gly
100 105
<210> 26
<211> 104
<212> PRT
<213> Homo Sapiens
<400> 26
Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu Cys Arg
1 5 10 15
Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys Tyr Thr
20 25 30
Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile Gln Lys Thr
35 40 45
Cys Thr Phe Lys Glu Leu Va1 Tyr Glu Thr Val Arg Val Pro Gly Cys
100
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr ~_.. Cys
65 70 75 80
His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val _rg Gly
85 90 95
Leu Gly Pro Ser Tyr Cys Ser Phe
100
<210> 27
<211> 104
<212> PRT
<213> Homo sapiens
<400> 27
Ser Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys G1u Glu Cys
1 5 10 15
Arg Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys Tyr
20 25 30
Thr Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile ~ln Lys
35 40 45
Thr Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Arg Val Pro Gly
50 55 60
Cys Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala T hr Gln
65 70 75 80
Cys His Cys Gly Lys Cys Asp Ser Asp Ser Thr Asp Cys Thr Val Arg
85 90 95
Gly Leu Gly Pro Ser Tyr Cys Ser
100
<210> 28
<211> 103
<212> PRT
<213> Homo sapiens
<400> 28
Cys Glu Leu Thr Asn Ile Thr Ile Ala Ile Glu Lys Glu Glu Cys Arg
1 5 10 15
Phe Cys Ile Ser Ile Asn Thr Thr Trp Cys Ala Gly Tyr Cys Tyr Thr
20 25 30
Arg Asp Leu Val Tyr Lys Asp Pro Ala Arg Pro Lys Ile Gln Lys Thr
35 40 45
Cys Thr Phe Lys Glu Leu Val Tyr Glu Thr Val Arg Val Pro Gly Cys
50 55 60
Ala His His Ala Asp Ser Leu Tyr Thr Tyr Pro Val Ala Thr Gln Cys
65 70 75 80
101
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
Leu Gly Ser TyrC,esSer
Pro
100
<210> 29
<211> 91
<212> PRT
<213> Homo
Sapiens
<400> 29
Pro Asp Gln AspCys ProGluCys ThrLeuGln GluAsn ProPhe
Val
1 5 10 15
Phe Ser Pro GlyAla ProIleLeu GlnCysMet GlyCys CysPhe
Gln
20 25 30
Ser Arg Tyr ProThr ProLeuArg SerLysLys ThrMet LeuVal
Ala
35 40 45
Gln Lys Val ThrSer GluSerThr CysCysVal AlaLys SerTlrr
Asn
50 55 60
Asn Arg Thr ValMet GlyGlyPhe LysValGlu AsnHis ThrAla
Val
65 70 75 80
Cys His Ser ThrCys TyrTyrHis LysSer
Cys
85 90
<210> 30
<211> 90
<212> PRT
<213> Homo Sapiens
<400> 30
Asp Val Gln Asp Cys Pro Glu Cys Thr Leu Gln G1u Asn Pro Phe Phe
1 5 10 15
Ser Gln Pro Gly Ala Pro Ile Leu Gln Cys Met Gly Cys Cys Phe Ser
20 25 30
Arg Ala Tyr Pro Thr Pro Leu Arg Ser Lys Lys Thr Met Leu Val Gln
35 40 45
Lys Asn Val Thr Ser Glu Ser Thr Cys Cys Val Ala Lys Ser Tyr Asn
50 55 60
Arg Val Thr Val Met Gly Gly Phe Lys Val Glu Asn His Thr Ala Cys
65 70 75 80
His Cys Ser Thr Cys Tyr Tyr His Lys Ser
85 90
<210> 31
<211> 89
<212> PRT
<213> Homo Sapiens
102
CA 02369262 2001-10-02
WO PCT/US00/09869
00/61178
<400>
Va1 31 AspCys ProGlu CysThrLeu GlnGluAsn ProPhe PheSer
1 Gln 5 10 15
Gln ProGlyAla ProIle LeuGlnCys MetGlyCys CysPhe SerArg
20 25 30
Ala TyrProThr ProLeu ArgSerLys LysThrMet LeuVal GlnLys
35 40 45
Asn ValThrSer GluSer ThrCysCys ValAlaLys SerTyr AsnArg
50 55 60
Val ThrValMet GlyGly PheLysVal GluAsnHis ThrAla CysHis
65 70 75 80
Cys SerThrCys TyrTyr HisLysSer
85
<210> 32
<211> 276
<212> DNA
<213> Homo Sapiens
<400> 32
gctcctgatg tgcaggattg cccagaatgc acgctacagg aaaacccatt cttctcccag 60
ccgggtgccc caatacttca gtgcatgggc tgctgcttct ctagagcata tcccactcca 120
ctaaggtcca agaagacgat gttggtccaa aagaacgtca cctcagagtc cacttgctgt 180
gtagctaaat catataacag ggtcacagta atggggggtt tcaaagtgga gaaccacacg 240
gcgtgccact gcagtacttg ttattatcac aaatct 276
<210> 33
<211> 324
<212> DNA
<213> Homo Sapiens
<400> 33
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtgagagtgc ccggctgtgc tcaccatgca gattccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
324
cccagctact gctcctttgg tgaa
<210> 34
<211> 327
<212> DNA
<213> Homo Sapiens
<400> 39
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtgagagtgc ccggctgtgc tcaccatgca gattccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctcctttgg tgaaatg 327
103
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<210> 35
<211> 330
<212> DNA
<213> Homo Sapiens
<400> 35
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtgagagtgc ccggctgtgc tcaccatgca gattccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctcctttgg tgaaatgaaa 330
<210> 36
<211> 333
<212> DNA
<213> Homo sapiens
<400> 36
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtgagagtgc ccggctgtgc tcaccatgca gat~ccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctcctttgg tgaaatgaaa gaa 333
<210> 37
<211> 276
<212> DNA
<213> Homo Sapiens
<400> 37
gctcctgatg tgcaggattg cccagaatgc acgctacagg aaaacccatt cttctcccag 60
ccgggtgccc caatacttca gtgcatgggc tgctgcttct caagagcata tcccactcca 120
ctaaggtcca agaagacgat gttggtccaa aagaacgtca cctcagagtc cacttgctgt 180
gtagctaaat catataacag ggtcacagta atggggggtt tcaaagtgga gaaccacacg 240
gcgtgccact gcagtacttg ttattatcac aaatct 276
<210> 38
<211> 324
<212> DNA
<213> Homo Sapiens
<400> 38
aacagctgtg agctcaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
atatcgatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccggccc gtcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtacgcgtgc ccggctgtgc tcaccatgca gattccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctcctttgg tgaa 324
<210> 39
<211> 321
<212> DNA
<213> Homo Sapiens
104
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 18C
gtgagagtgc ccggctgtgc tcaccatgca gattccttgt atacataccc a~~ggccacc 24C
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctcctttgg t 321
<210> 40
<211> 318
<212> DNA
<213> Homo Sapiens
<400> 40
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtgagagtgc ccggctgtgc tcac~~atgca gattccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctccttt 318
<210> 41
<211> 315
<212> DNA
<213> Homo Sapiens
<400> 41
aatagctgtg agctgaccaa catcaccatt gcaatagaga aagaagaatg tcgtttctgc 60
ataagcatca acaccacttg gtgtgctggc tactgctaca ccagggatct ggtgtataag 120
gacccagcca ggcccaaaat ccagaaaaca tgtaccttca aggaactggt atatgaaaca 180
gtgagagtgc ccggctgtgc tcaccatgca gattccttgt atacataccc agtggccacc 240
cagtgtcact gtggcaagtg tgacagcgac agcactgatt gtactgtgcg aggcctgggg 300
cccagctact gctcc 315
<210> 42
<211> 327
<212> DNA
<213> Homo Sapiens
<400> 42
agctgtgagc tgaccaacat caccattgca atagagaaag aagaatgtcg tttctgcata 60
agcatcaaca ccacttggtg tgctggctac tgctacacca gggatctggt gtataaggac 120
ccagccaggc ccaaaatcca gaaaacatgt accttcaagg aactggtata tgaaacagtg 180
agagtgcccg gctgtgctca ccatgcagat tccttgtata catacccagt ggccacccag 240
tgtcactgtg gcaagtgtga cagcgacagc actgattgta ctgtgcgagg cctggggccc 300
agctactgct cctttggtga aatgaaa 327
<210> 43
<211> 324
<212> DNA
<213> Homo Sapiens
<400> 43
tgtgagctga ccaacatcac cattgcaata gagaaagaag aatgtcgttt ctgcataagc 60
atcaacacca cttggtgtgc tggctactgc tacaccaggg atctggtgta taaggaccca 120
gccaggccca aaatccagaa aacatgtacc ttcaaggaac tggtatatga aacagtgaga 180
gtgcccggct gtgctcacca tgcagattcc ttgtatacat acccagtggc cacccagtgt 240
10$
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
tactgctcct ttggtgaaat gaga 324
<210> 44
<211> 324
<212> DNA
<213> Homo Sapiens
<400> 44
agctgtgagc tgaccaacat caccattgca atagagaaag aagaatgtcg tttctgcata 60
agcatcaaca ccacttggtg tgctggctac tgctacacca gggatctggt gtataaggac 120
ccagccaggc ccaaaatcca gaaaacatgt accttcaagg aactggtata tgaaacagtg 180
agagtgcccg gctgtgctca ccatgcagat tccttgtata catacccagt ggccacccag 240
tgtcactgtg gcaagtgtga cagcgacagc actgattgta ctgtgcgagg cctggggccc 300
agctactgct cctttggtga aatg 324
<210> 45
<211> 321
<212> DNA
<213> Homo Sapiens
<400> 45
tgtgagctga ccaacatcac cattgcaata gagaaagaag aatgtcgttt crgca~aagc 60
atcaacacca cttggtgtgc tggctactgc tacaccaggg atctggtgta taaggaccca 120
gccaggccca aaatccagaa aacatgtacc ttcaaggaac tggtatatga aacag~gaga 180
gtgcccggct gtgctcacca tgcagattcc ttgtatacat acccagtggc cacccagtgt 240
cactgtggca agtgtgacag cgacagcact gattgtactg tgcgaggcct ggggcccagc 300
tactgctcct ttggtgaaat g 321
<210> 46
<211> 321
<212> DNA
<213> Homo Sapiens
<400> 46
agctgtgagc tgaccaacat caccattgca atagagaaag aagaatgtcg tttctgcata 60
agcatcaaca ccacttggtg tgctggctac tgctacacca gggatctggt gtataaggac 120
ccagccaggc ccaaaatcca gaaaacatgt accttcaagg aactggtata tgaaacagtg 180
agagtgcccg gctgtgctca ccatgcagat tccttgtata catacccagt ggccacccag 240
tgtcactgtg gcaagtgtga cagcgacagc actgattgta ctgtgcgagg cctggggccc 300
agctactgct cctttggtga a 321
<210> 47
<211> 318
<212> DNA
<213> Homo sapiens
<400> 47
tgtgagctga ccaacatcac cattgcaata gagaaagaag aatgtcgttt ctgcataagc 60
atcaacacca cttggtgtgc tggctactgc tacaccaggg atctggtgta taaggaccca 120
gccaggccca aaatccagaa aacatgtacc ttcaaggaac tggtatatga aacagtgaga 180
gtgcccggct gtgctcacca tgcagattcc ttgtatacat acccagtggc cacccagtgt 240
cactgtggca agtgtgacag cgacagcact gattgtactg tgcgaggcct ggggcccagc 300
tactgctcct ttggtgaa 318
<210> 48
106
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
<212> DNA
<213> Homo Sapiens
<400> 98
agctgtgagc tgaccaacat caccattgca atagagaaag aagaatgtcg tttctgcata 60
agcatcaaca ccacttggtg tgctggctac tgctacacca gggatctggt gtataaggac 120
ccagccaggc ccaaaatcca gaaaacatgt accttcaagg aactggtata tgaaacagtg 180
agagtgcccg gctgtgctca ccatgcagat tccttgtata catacccagt ggccacccag 240
tgtcactgtg gcaagtgtga cagcgacagc actgattgta ctgtgcgagg cctggggccc 300
agctactgct cctttggt 318
<210> 49
<211> 315
<212> DNA
<213> Homo Sapiens
<400> 49
tgtgagctga ccaacatcac cattgcaata gagaaagaag aatgtcgttt ctgcataagc 60
atcaacacca cttggtgtgc tggctactgc tacaccaggg atctggtgta taaggaccca 120
gccaggccca aaatccagaa aacatgtacc ttcaaggaac tggtatatga aacagtgaga 180
gtgcccggct gtgctcacca tgcagattcc ttgtatacat acccagtggc cacccagtgt 240
cactgtggca agtgtgacag cgacagcact gattgtactg tgcgaggcct ggggcccagc 300
tactgctcct ttggt 315
<210> 50
<211> 315
<212> DNA
<213> Homo Sapiens
<400> 50
agctgtgagc tgaccaacat caccattgca atagagaaag aagaatgtcg tttctgcata 60
agcatcaaca ccacttggtg tgctggctac tgctacacca gggatctggt gtataaggac 120
ccagccaggc ccaaaatcca gaaaacatgt accttcaagg aactggtata tgaaacagtg 180
agagtgcccg gctgtgctca ccatgcagat tccttgtata catacccagt ggccacccag 240
tgtcactgtg gcaagtgtga cagcgacagc actgattgta ctgtgcgagg cctggggccc 300
agctactgct ccttt 315
<210> 51
<211> 312
<212> DNA
<213> Homo sapiens
<400> 51
tgtgagctga ccaacatcac cattgcaata gagaaagaag aatgtcgttt ctgcataagc 60
atcaacacca cttggtgtgc tggctactgc tacaccaggg atctggtgta taaggaccca 120
gccaggccca aaatccagaa aacatgtacc ttcaaggaac tggtatatga aacagtgaga 180
gtgcccggct gtgctcacca tgcagattcc ttgtatacat acccagtggc cacccagtgt 240
cactgtggca agtgtgacag cgacagcact gattgtactg tgcgaggcct ggggcccagc 300
tactgctcct tt 312
<210> 52
<211> 312
<212> DNA
<213> Homo sapiens
<400> 52
107
CA 02369262 2001-10-02
WO 00/61178 PCT/US00/09869
agctgtgagc tgaccaacat caccattgca atagagaaag aagaatgtcg tttctgcata 60
agcatcaaca ccacttggtg tgctggctac tgctacacca gggatctggt gtataaggac 120
ccagccaggc ccaaaatcca gaaaacatgt accttcaagg aactggtata tgaaacagtg 180
agagtgcccg gctgtgctca ccatgcagat tccttgtata catacccagt ggccacccag 240
tgtcactgtg gcaagtgtga cagcgacagc actgattgta ctgtgcgagg cctggggccc 300
312
agctactgct cc
<210> 53
<211> 309
<212> DNA
<213> Homo sapiens
<400> 53
tgtgagctga ccaacatcac cattgcaata gagaaagaag aatgtcgttt ctgcataagc 60
atcaacacca cttggtgtgc tggctactgc tacaccaggg atctggtgta taaggaccca 120
gccaggccca aaatccagaa aacatgtacc ttcaaggaac tggtatatga aacagtgaga 180
gtgcccggct gtgctcacca tgcagattcc ttgtatacat acccagtggc cacccagtgt 240
cactgtggca agtgtgacag cgacagcact gattgtactg tgcgaggcct ggggcccagc 300
309
tactgctcc
<210> 59
<211> 273
<212> DNA
<213> Homo Sapiens
<400> 54
cctgatgtgc aggattgccc agaatgcacg ctacaggaaa acccattctt ctcccagccg 60
ggtgccccaa tacttcagtg catgggctgc tgcttctcta gagcatatcc cactccacta 120
aggtccaaga agacgatgtt ggtccaaaag aacgtcacct cagagtccac ttgctgtgta 180
gctaaatcat ataacagggt cacagtaatg gggggtttca aagtggagaa ccacacggcg 240
tgccactgca gtacttgtta ttatcacaaa tct 273
<210> 55
<211> 270
<212> DNA
<213> Homo Sapiens
<400> 55
gatgtgcagg attgcccaga atgcacgcta caggaaaacc cattcttctc ccagccgggt 60
gccccaatac ttcagtgcat gggctgctgc ttctctagag catatcccac tccactaagg 120
tccaagaaga cgatgttggt ccaaaagaac gtcacctcag agtccacttg ctgtgtagct 180
aaatcatata acagggtcac agtaatgggg ggtttcaaag tggagaacca cacggcgtgc 240
cactgcagta cttgttatta tcacaaatct 270
<210> 56
<211> 267
<212> DNA
<213> Homo Sapiens
<400> 56
gtgcaggatt gcccagaatg cacgctacag gaaaacccat tcttctccca gccgggtgcc 60
ccaatacttc agtgcatggg ctgctgcttc tctagagcat atcccactcc actaaggtcc 120
aagaagacga tgttggtcca aaagaacgtc acctcagagt ccacttgctg tgtagctaaa 180
tcatataaca gggtcacagt aatggggggt ttcaaagtgg agaaccacac ggcgtgccac 240
tgcagtactt gttattatca caaatct 267
108