Note: Descriptions are shown in the official language in which they were submitted.
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
MEDICINAL COMPOSITIONS AND THEIR METHOD OF PREPARATION
FIELD OF THE INVENTION
The present invention relates to medicinal
compositions and in particular therapeutic compositions
comprising glycoalkaloids. Such compositions may be used
in the treatment, control and diagnosis of cancers and
tumors in mammals, contraception and termination of
pregnancy. The present invention is particularly
directed towards a composition comprising a mixture of
solasodine glycosides.
The present invention is also directed towards
a method of preparing a medicinal composition and a
method of treatment, control or diagnosis of cancers and
tumors in mammals.
BACKGROUND ART
Glycoalkaloids are steroidal alkaloids which
have a sugar moiety bound to the alkaloid moiety. The
sugar moiety can be a monosaccharide, disaccharide,
oligosaccharide or polysaccharide. Certain
glycoalkaloids derived from plants have been observed to
have anti-cancer properties.
Of particular interest are glycoalkaloids
extracted from the Solanum genus. Glycoalkaloids from
the species Solanum Sodomaeum L. have been shown to be
active against cancer in animals and skin tumors in
humans. The glycoalkaloids extracted from the fruit of
Solanum Sodomaeum L. include the triglycosides
solasonine,[(22R, 25R) - spiro-5-en-3(3-yl-a-L-
rhamnopyranosyl-(1->2ga1)-0-(3-D-glucopyranosyl-(1->3ga1)-
(3-D-galactopyranose] (33%) , solamargine' (22R, 25R) -spiro-5-
en-3(3-yl-a-L-rhamnopyranosyl-(1->2glu)-O-a-L-
rhamnopyranosyl-(1->4glu)-(3-D-gluco-pyranose](33%), and
their corresponding di- and monoglycosides (34%). All
the glycosides contain the same aglycone, solasodine.
This mixture of glycosides which includes solasonine and
solamargine is commonly referred to as BEC. The
structures of solasonine and solamargine are shown below:
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
2
H7C N H
H ~ C ~~~CH3
I
N1C
H0 Ol
OH < I0
0 ~~0
HO
HO . 0
OH OH
~; ~~oH
CH3 So(asonine
OH
H
HOC H
H3 C ..CH3
HOC ~
OH
~ ~0
0.%''~0 .
H,C 0 HO~ ~0
HO OH
OH OH OOH
cH~ Solama~gine
HO
The anti-cancer properties of BEC has been
studied in vivo with mice inoculated with murine sarcoma
180 and in cell culture studies. BEC was observed to
selectively destroy tumor cells relative to normal cells.
The efficacy and specificity of BEC was also observed to
be dependent upon the type of tumor. These observations
were attributed to the presence of endogenous endocytic
lectins or EEL' s present on the membranes of those cells
observed to be susceptible to BEC. EEL's are endogenous
receptors which have been reported to be expressed during
human embryogenesis and carcinogenesis. Interaction of
the EEL with a molecule or ligand for which it is a
receptor results in internalization of the EEL and bound
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
3
ligand.
It is believed that the tumor cells susceptible
to BEC have EEL receptors specific for the glycoside
portion of the glycoalkaloids in BEC. These EEL's
selectively recognize and bind the sugar moiety of the
glycoalkaloid. The glycoalkaloid is subsequently
internalized and the result is destruction of the cell.
The mechanism of cell destruction is believed to be by
cell lysis.
That there is an EEL specific for the glycoside
moiety of the glycoalkaloid is supported by a number of
observations. First, the aglycone, solasodine, when
administered at levels at which BEC is effective is
ineffective against tumor cells. The sugar portion of
the glycoalkaloid on its own is also ineffective.
Second, competition studies have also shown that at least
a three fold molar excess of the sugar rhamnose is
required to inhibit the cytotoxicity of BEC.
Solamargine contains two molecules of rhamnose and
solasonine, one molecule. It should be noted that
rhamnose is a plant sugar and is rarely found in
mammalian cells. Thus, it is unlikely that normal
mammalian cells have a receptor for rhamnose.
The aforementioned competition studies were
conducted on mice with murine sarcoma 180. Untreated
mice died in 2-3 weeks. Four doses of 8mg/kg BEC given
on consecutive days resulted in survival of virtually all
animals. Five mg rhamnose/kg decreased the survival to
75%, lOmg rhamnose/kg decreased the survival to 50o and
l5mg rhamnose/kg decreased the survival to 42%. Similar
concentrations of rhamnose were observed to have no
effect on 5180 activity in the absence of BEC.
Acute toxicity studies for BEC were also
carried out in mice. These studies showed that for
single intraperitoneal (ip) doses of BEC, the LDso was
30mg/kg. For administration of 14 daily single ip doses,
the LDSO for mice was lOmg/kg. In contrast it was shown
that the EDso (quantal effective level for 500 of the
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
4
population after given a single dose) for a single dose
of BEC was 9mg/kg. With 3 and 4 administrations at
9mg/kg BEC to mice with Sarcoma 180, greater than 95 0 of
the mice were rendered cancer free for the remainder of
their life span. The quantal effective levels (EDso) of
BEC for single administrations were similar to the lethal
levels (LDSO) for multiple administrations of lOmg/kg at
14 daily ip doses. BEC has also been observed to be
effective for melanoma and ovarian tumor cells grown in
cell culture. The therapeutic index (LDso/EDSO) for these
cell culture trials was about 3.
It can be seen that a disadvantage of BEC is
the toxicity of the preparation when administered at the
very high levels required to successfully treat internal
cancers. It would therefore be desirable to obtain a
glycoalkaloid composition which is more effective than
BEC for the treatment of cancers.
The above toxicity studies also provide further
support for the EEL mediated activity of glycoalkaloids
against cancer cells. Mice with advanced cancer activity
could tolerate up to three times the LDloo of BEC. This
could be explained by selective absorption of BEC by the
cancer cells which were present in abundance. Thus the
bioavailablity of BEC to normal cells could be reduced.
These toxicity studies also showed that ingestion of BEC
into normal cells can occur by routes other than
selective recognition by EEL's at high concentrations.
For example, BEC at high concentrations may diffuse
through the plasma membrane of the cell.
Treatment of premalignant and malignant skin
lesions with BEC in humans has also been studied.
Topical application of BEC has been observed to be
effective for the treatment of lesions consisting of
keratosis, basal cell carcinoma and squamous cell
carcinoma. Creams containing 10% and 0.005°s BEC when
applied topically showed complete clinical and
histological regression when applied twice daily over
treatment periods of up to about three months. Although
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
the final result of the 10% and 0.005% treatments were
comparable in relation to regression of the disease, the
duration of the treatment with the 0.005% BEC required
for regression of the lesions was considerably longer
5 than for the 10% BEC. Typically the treatment period
required for the low concentration of BEC was about 13 to
14 weeks.
The extended duration of the treatment for the
low concentration BEC formulations has a number of
disadvantages. First there is a difficulty with patient
compliance. For optimum effectiveness, the BEC
formulation must be applied at regular intervals,
typically twice a day, until clinical regression is
observed. Many patients find it difficult to comply with
such a regime for up to 14 weeks. During this period,
patients may experience an unacceptable amount of pain
due to high salicyclic acid concentrations. Further,
during treatment, as the affected cells undergo lysis,
the lesions ulcerate and should be covered by a dressing.
From a cosmetic and patient comfort perspective it would
be desirable to be able to reduce the duration of
treatment. Although such a reduction can be achieved by
increasing the dose of BEC, this is undesirable in view
of the toxicity of BEC. Still further, as large amounts
of plant product are required to produce small amounts of
BEC, the lOoBEC preparation is quite expensive to
produce . It is therefore desirable to be able to obtain
a low dose glycoalkaloid composition for the treatment of
skin conditions, which results in clinical regression in
a relatively short period of time and is also cost
effective.
OBJECT OF THE INVENTION
It is therefore an object of the present
invention to provide an improved glycoalkaloid
composition for interaction with target cells and which
may be used for the treatment of cancer and tumors in
mammals and which may at least partially overcome the
above disadvantages or provide the public with a useful
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
6
choice.
SUMMARY OF THE INVENTION
Glycoalkaloids can undergo degradation in which
the glycoside moiety or a saccharide unit thereof is
cleaved from the alkaloid. Where the glycoside moiety of
the glycoalkaloid includes two or more saccharide units,
there are a number of possible degradation products
including free sugars such as monosaccharides,
disaccharides and trisaccharides; the aglycone and mono
and diglycosides.
It has been surprisingly and unexpectedly
discovered that the efficacy of a glycoalkaloid
formulation against cancer, other abnormal cells or other
target cells having EEL's can be inhibited by very low
amounts of free sugars which may be produced as a result
of degradation of the glycoalkaloid.
In the present specification and claims, the
term "free sugars" refers to any sugar such as a mono, di,
trisaccharide, oligosaccharide or polysaccharide or
derivative thereof which is not bound to an alkaloid.
According to a first broad form of the
invention, there is provided a medicinal composition
comprising at least one compound which can interact with
a target cell, the at least one compound being a
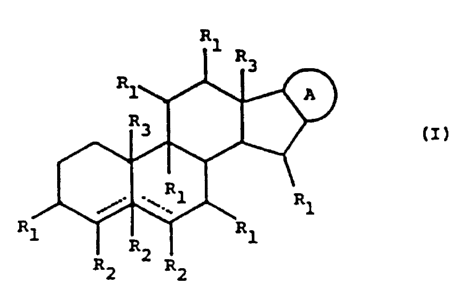
glycoalkaloid of the general formula I:
R
I1 R3
A
3 o R3 i i r
~~ Rl Rl
Rl ~R~ Rl
R2 R2
wherein:
either one of the dotted lines represents a double bond,
and the other a single bond, or both represent single
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
7
bonds;
A: represents a radical selected from the following
radicals of general formulae ( II ) to (V)
R3 N R3 R3 X ~R3
R_'~O O
R1
(II) (III)'
H
R R1 R3 N
R3
or
N ~O ORQ
R3
(IV) (V)
each of R1 is a radical separately selected from the group
consisting of hydrogen, amino, oxo and OR4; each of Rz is
a radical separately selected from the group consisting
of hydrogen, amino and OR4~ each of R3 is a radical
separately selected from the group consisting of
hydrogen, alkyl and R4-alkylene; each of R4 is a radical
separately selected from the group consisting of
hydrogen, carbohydrate and a carbohydrate derivative;" X"
is a radical selected from the group comprising -CHZ-, -O-
and -NHZ - ;
wherein the compound includes at least one R4 group in
which R' is a carbohydrate or a derivative thereof;
together with a pharmaceutically acceptable carrier,
adjuvant, excipient and/or diluent, wherein the
composition is essentially without free sugars of the
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
8
type which inhibit the interaction between the at least
one glycoalkaloid and a target cell.
Preferred carbohydrate radicals R' are glyceric
aldehyde; glycerose; erythrose; threose; ribose;
arabinose; xylose; lyxose; altrose; allose; gulose;
mannose; glucose; idose; galactose; talose; rhamnose;
dihydroxyactone; erythrulose; ribulose; xylulose;
psicose; fructose; sorbose; tagatose; and other hexoses
( C6H12O6 ) ; heptoses ( C,H140, ) ; octoses ( C8H1608 ) ; nanoses
(C9H18O9) ; decoses (CloHzoOlo) : deoxysugars with branched
chains (eg. apiose, hamamelose, streptose, cordycepose,
mycarose and cladinose); compounds wherein the aldehyde,
ketone or hydroxyl groups have been substituted (eg. N-
acetyl, acetyl, methyl, replacement of CHZOH); sugar
alcohols; sugar acids; benzimidazoles; the enol salts of
the carbohydrates; saccharinic acids; sugar phosphates.
The more preferred compounds are solasonine,
solamargine, solanine and tomatine.
Other preferred compounds of the general
formula (1) are solanocapsine and 26-aminofurostane.
It will be appreciated that the various
compounds referred to throughout this specification may
be chiral and the present invention relates both to the
individual stereoisomers and to any mixtures thereof
including mixtures of enantiomers and/or
diastereoisomers.
A preferred composition of the present
invention is a solasodine glycoside composition which
includes solasonine, solamargine and their di and
monoglycosides in the same or similar proportion as the
aforementioned BEC.
The composition of the present invention
typically comprises naturally occurring glycoalkaloids
extracted from a plant source. Generally, the plant
extract is treated to remove essentially all of any free
sugars which can inhibit the efficacy of the
glycoalkaloids prior to formulation of the composition of
the present invention. Although it may be possible that
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
9
one or more free sugars do not inhibit the efficacy of
the glycoalkaloids and do not need to be removed,
typically all of the free sugars will be removed from the
plant extract.
According to a further broad form of the
invention there is provided a method of preparing a
glycoalkaloid preparation comprising at least one
glycoalkaloid according to formula I, as hereinbefore
defined, the method including extracting the at least one
glycoalkaloid from a suitable plant material to form a
crude extract, and removing essentially all free sugars
from the crude extract.
The crude extract may be obtained by any
suitable method. When the plant material is Solanum
Sodomaeum a preferred method is to extract coarsely
ground plant material with acetic acid. The extract is
filtered and the pH adjusted to about 9 to 10 to obtain a
precipitate. The precipitate may be dissolved in acetic
acid and re-precipitated at high pH. The precipitate is
typically further extracted with ethanol to provide the
solasodine glycoside mixture or BEC as a semicrystalline
powder.
The free sugars may be removed from the plant
extract by any suitable method. A preferred method is to
wash the crude extract in water or other suitable
solvent. Generally, the free sugars are removed to below
detectable limits or are at least removed to a level
below which an inhibitory effect can be detected.
Generally, the composition of the present invention is
essentially without all free sugars. However, it will be
appreciated that free sugars which do not inhibit the
cytotoxicity of the glycoalkaloids may be present.
The composition of the present invention may
also be formulated from a synthetic glycoalkaloid or a
mixture of glycoalkaloids. In this case, the synthetic
glycoalkaloids would typically be treated prior to
formulation of the composition to remove any sugars
present as a result of glycoalkaloid degradation.
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
The glycoalkaloids in the composition of the
present invention may also be obtained from chemical
modification of naturally occurring glycoalkaloids. In
this case, the naturally occurring sugar moiety of the
5 glycoalkaloid can be modified by removing or adding a
saccharide unit or units. Suitable methods of
carbohydrate modification are known and include chemical
or enzymatic hydrolysis. Alternatively, the sugar moiety
may be completely removed and replaced with a different
10 sugar moiety. An advantage of such modification of the
sugar group of a glycoalkaloid is to be able to modify
the efficacy or selectivity of that glycoalkaloid towards
a desired target cell.
It is believed that the mode of action of
glycoalkaloids against target cells is by EEL mediated
endocytosis in which an EEL recognizes the sugar moiety
of the glycoalkaloid and subsequent internalization of
the EEL and glycoalkaloid. Thus, by identifying those
sugars which can be recognized by receptors on a desired
target cell, a modified glycoalkaloid may be derived
which is specific to that receptor. In this way a
glycoalkaloid can be designed to target a desired cell
type.
The products of glycoalkaloid degradation may
also include the aglycone. Preferably, any aglycone is
also removed prior to formulation of the therapeutic
compositions of the present invention. Removal of the
aglycone may be conducted by any suitable means and is
typically removed by solvent extraction. Suitable
solvents include the chlorinated hydrocarbon solvents and
chloroform is particularly preferred.
Under normal storage conditions, some
degradation of glycoalkaloids in a pure or semi-pure
crystalline or semicrystalline form can occur. Thus, it
is preferred, that where storage has occurred, the
aforementioned sugar removal and if desired aglycone
removal of stored glycoalkaloid be conducted immediately
prior to formulation of the therapeutic compositions of
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
11
the invention. Typically the composition is stabilized
against glycoalkaloid degradation. Typically, the
composition is acidic and preferably includes acetic or
lactic acid. The acidic conditions minimize degradation
to produce free sugars.
Alternatively, sugar free glycoalkaloid
preparations including the crystalline form may be
prepared and then stored under stable conditions prior to
formulation of the therapeutic composition of the present
invention. The sugar free preparation may be stored in
an acidic solution and/or at low temperature.
According to a further broad. form of the
present invention, there is provided a method of
preparing a therapeutic composition which comprises a
therapeutically effective amount of at least one
glycoalkaloid according to formula I, as hereinbefore
defined, the method including obtaining at least one
glycoalkaloid, removing any free sugars from the
glycoalkaloid and mixing the glycoalkaloid with a
pharmaceutically acceptable stabilizer.
The amount of the glycoalkaloid present in the
therapeutic composition of the present invention may
depend on the dose rate, patient, the type of condition
being treated and in the case of a tumor the type, size
and position of the tumor to be treated. In the
preferred composition which includes solasodine
glycosides, a typical composition for the treatment of
skin tumors would typically include between about 5 to
about 0.001%, preferably about 0.005% solasodine
glycosides.
The therapeutic composition of the present
invention may be used in the treatment and control of
conditions which may be treated or controlled by
selective cellular destruction or modification. Such
uses include the treatment or control of cancer,
contraception, termination of pregnancy, removal of
pathogenic organisms and removal of abnormal cellular
growth.
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
12
According to a further broad form of the
present invention there is provided a method for the
treatment or control of cancer, contraception,
termination of pregnancy, removal of pathogenic organisms
and removal of abnormal cellular growth in a mammal
requiring such treatment, the method comprising
administering to the mammal an effective amount of a
medicinal composition or preparation of the present
invention.
The medicinal composition of the present
invention may be formulated in any suitable manner
including injectable compositions, tablets,
suppositories, capsules and topical formulations.. In a
preferred formulation for the treatment of skin tumors or
lesions, the formulation is a cream for topical
administration or an injectable formulation. In the case
of an internal cancer or sarcoid, the composition may be
an injectable formulation for intraperitoneal or
intralesional injection.
Typically, the injectable composition is
administered in an amount of between about 50 to about
200 mg of sugar free glycoalkaloid composition per kg of
tumour. Animal and human studies (as illustrated in the
following examples) show that successful treatment of
some tumors and cancers may be accomplished with as few
as two to four injections. The injection may be given at
one, two or three daily intervals, preferably the
treatment is given twice, at day 1 and day 3. Treatment
by injection may also be given in association with
topical administration if desired or considered
necessary.
It has also been surprisingly discovered that
the therapeutic composition of the present invention may
also be used to diagnose skin conditions before such
conditions can be detected by visual inspection.
Such diagnosis may be carried out by broadly
applying a composition of the present invention to an
area of skin to be tested. The composition is left on
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
13
the skin for a pre-determined period of time. During
this time, any abnormal cells are selectively destroyed.
This produces a detectable inflammation of the affected
areas which may then be identified and treated.
According to a further broad form of the
present invention there is provided a method of
diagnosing a skin condition in a mammal, the skin
condition being caused by the presence of abnormal cells,
wherein the method includes applying an effective amount
of a composition or preparation of the present invention
to an area of skin to be diagnosed, leaving the
composition on the skin for a pre-determined period of
time, removing the composition and detecting any change
to any areas of skin.
The diagnostic method of the present invention
is particularly suitable for diagnosing skin conditions
of humans. Typical conditions which may be diagnosed
include Keratoses, basal cell carcinomas, squamous cell
carcinomas, melanomas or other skin cancers.
A particularly preferred diagnostic composition
is a solasodine glycoside mixture having about the same
glycoside composition as BEC but without free sugars or
the aglycone, solasodine. In trials conducted by the
present inventor it has been observed that the normal
healthy skin tissue is unaffected by the composition.
This demonstrates the selectivity of glycoalkaloids for
abnormal cells.
This method of diagnosis allows skin conditions
to be detected and treated at an early stage, typically
before the condition produces visible skin lesions.
It should be appreciated that such a method of
diagnosis would not be possible with conventional skin
treatment compositions which adversely affect all cells.
A further advantage of such specificity is that during
application, should the composition be inadvertently
applied to a patients' healthy skin, the healthy skin
will not be damaged. This does not occur with
conventional skin treatment where care must be exercised
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
14
to avoid contact with healthy skin.
Further, in view of the suprisingly improved
efficacy of the present invention in treatment of skin
conditions, the diagnosis can be conducted using very low
concentrations of solasodine glycosides.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1 and 3 and Figures 2 and 4 illustrate
HPLC spectra for unwashed and washed BEC respectively.
Figures 5 to 8 illustrate a sarcoid tumor in a
horse, before (Figure 5), during (Figures 6 and 7) and
after (Figure 8) treatment with a preferred composition
of the present invention.
Figures 9 to 12 illustrate the stages in the
treatment of a horse with a penile sarcoma before (Figure
9) during (Figures 10 and 11) and after (Figure 12)
treatment with a preferred composition of the present
invention.
Figures 13 to 15 illustrate stages in the
treatment of a human with squamous cell carcinoma before
(Figure 13) and during (Figures 14 and 15) treatment.
BEST MODE
The present invention will now be
described with reference to the following non-limiting
examples.
Example 1
A sugar free solasodine glycoside preparation
was prepared according to the following:
50kg Solanum Sodomaeum berries are put through
commercial meat mincer (fitted with 1.HP electric motor
1425 rpm) with a sieve size of 3mm.
The slurry is diluted with 3o acetic acid (pH
2.5) (food grade) to a volume of 200L. This semi-solid
solution is treated with a Silverson homogenizer for 15
minutes. Mixing is continued for another 4 hours using a
SS rod with arms mixer at room temperature at 30 rpm
(Flamingo CMG 0.75kw variable speed control meter).
The solution is allowed to stand overnight
without mixing. The solution is subsequently filtered
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
through a muslin cloth. The filtrate is then subjected
to a flow through centrifuge (3.5HP) at 1455 rpm. The
clear filtrate is heated to 50°C in a stainless steel
double jacketed bowl. Concentrated ammonia (L R Grade)
5 is added until pH ~ 10. A precipitate is observed. The
precipitate is allowed to settle and cool (approx. 24
hrs). The supernatant is carefully decanted. The
precipitate is dissolved in 25L 3% aqueous acetic acid.
The solution is centrifuged through flow through
10 centrifuge as above. The supernatant is collected in an
SS double jacketed bowl and heated to 50°C with
continuous stirring (30 rpm, 30min).
The glycoalkaloids are re-precipitated by the
addition of concentrated ammonia solution until pH ~ 10.
15 The solution is allowed to cool and the precipitate is
allowed to settle (approx. 24 hrs). The supernatant is
carefully decanted and the precipitate is washed with 50L
water and allowed to settle for 24 hrs as before. The
supernatant is decanted and this procedure is repeated
four times.
The precipitate is finally dissolved in lOL
alcohol at 75°C and filtered whilst hot through Whatman
No. 1 filter paper. The supernatant is dried at 50°C.
This yields a fine, semicrystalline powder. The yield is
5058 which is l.Olo.
Any aglycone, solasodine, is removed by washing
the extract in chloroform. The solasodine is soluble in
the chloroform phase and the sugars are soluble in the
aqueous phase. The glycoalkaloids remain insoluble under
all these conditions.
Example 2
Cream formulations were prepared from the sugar
free solasodine glycoside preparation from Example 1 as
follows
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
16
Percentage Function
Composition
Active
ingredient
Solasodine 0.005 % w/w Antineoplast
Glycosides (BEC)
Other
ingredients
Cetomacrogol 15.0% w/w Emulsifying agent
emulsifying wax
White soft paraffin 10.0% w/w Cream base
Liguid paraffin 10.0% w/w Cream base
Salicylic acid 10.0% w/w Keratolytic
Urea 5.0% w/w Keratolytic
Propylene glycol 5.0% w/w Emollient
Chlorocresol 0.1% w/w Preservative
Acetic or lactic qs Solvent
acid
Purified water qs Solvent/
Cream base
Emulsifying wax, white soft paraffin, liquid
paraffin, propylene glycol and water were used to provide
a cream base of a suitable consistency and viscosity.
Chlorocresol was included in the formulation as a
preservative. Salicylic acid and urea were included as
keratolytic agents and were considered to be excipients
in the cream formulation because their primary function
was to enhance the bioavailability of the active
ingredient by clearing tissue from around the tumor, thus
allowing a higher concentration of the active ingredients
to reach the tumor. (The International Pharmaceutical
Excipients Council definition of an excipient includes
substances which are included in a drug delivery system
to protect, support or enhance stability, bioavailability
or patient acceptability and to enhance any other
attribute of the overall safety and effectiveness of the
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
17
drug during storage or use).
Acetic or lactic acid was present in the final
formulation because a 3°s solution of acetic acid is used
as the solvent for the active ingredients during the
manufacturing process.
Example 3
White soft paraffin, liquid paraffin and
cetomacrogol emulsifying wax were weighed into a
sanitized stainless steel container ("Phase A"). This
mixture was gently heated on a low burner until the
temperature reached 70°C.
Purified water at 70°C, urea, chlorocresol and
propylene glycol were added to a suitable stainless steel
container and mixed for 2 minutes using a Silverson mixer
("Phase B") .
The melted Phase A was slowed added to Phase B
and thoroughly mixed using a Silverson mixer or follow-
through homogenizer. The mixture was allowed to cool to
about 50°C and then the salicylic acid was added.
The freshly washed solasodine glycosides were
dissolved in acetic acid or lactic acid solution and
added to the cream, with mixing to ensure even
dispersion. The cream was allowed to cool to room
temperature with occasional mixing to ensure an even,
smooth texture.
The formulated cream had the following
specifications:
Description Smooth, white or
slightly off-white
cream
BEC assay 0.0046 - 0.0054%
Salicylic acid assay 9.5 - 10.50
pH Less than 3
Example 4
The solasodine glycoside preparation from
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
18
Example 1 and creams from examples 2 and 3 were analyzed
for hydrolysis products by MS and HPLC according to the
following procedure.
Sample Preparation:
Standard was prepared in 50o CH3CN/Hz0 at lmg/ml
and 100ug/ml. Cream was prepared by dissolving 100mg
cream in 2m1 methylene chloride, after centrifugation
100u1 of the aqueous phase was removed and made up to lml
with methanol.
HPLC Conditions:
A Waters Alliance system was used consisting of
a 2690 separations module, and 996 diode .array detector.
A Micromass Waters Platform LCZ mass spectrometer was
interfaced and the whole system was controlled by
MassLynx chromatography software.
Solvent:
Isocractic analysis was performed with 750
CH3CN/Hz0
Gradients were run from 80% CH3CN to 50 o CH3CN
over 7 minutes
Flow Rate was 1.0 or 0.5 ml/min
W Detection was from 205nm to 320nm (205nm was
extracted for alkaloids and 254 for salicylic acid and
chlorocresol)
Mass Spec full scans from 400-900 m/z were used
for TIC chromatograms and single channels of 869 and 885
were used for quantitation.
Full detailed conditions including cone
voltages are attached.
Column
High Performance Carbohydrate column (4um) 0.46
*25cm was used.
Results
The standard gave three peaks corresponding to
Solasonine, Solamargine and an unidentified peak at mass
722. Some smaller peaks were also observed but no
indication of mass 414 consistent with the aglycone,
hence no obvious hydrolysis of the samples. The cream
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
19
showed both actives as well as propylene glycol,
salicylic acid and chlorocresol. MS results were
approximately 1000 times more sensitive than UV.
Example 5
HPLC studies were also conducted on BEC
preparations obtained by conventional extraction
procedures (i.e. without any washing steps) and stored
for a period of up to about 6 - 8 months. HPLC analysis
was conducted on the stored BEC both before and after
washing to remove free sugars and solasonine.
Figures 1 to 4 illustrate HPLC spectra of
unwashed (Figures 1 and 3) and washed (Figures 2 and 4)
BEC respectively.
Compounds marked as I and II had the same
elution times as solasonine and solamargine standards.
Figures 1 and 2 show that the unwashed BEC
includes a number of further peaks. By comparison with
Figures 3 and 4, it can be seen that these peaks have
been removed or significantly decreased upon washing with
water and chloroform. These further peaks have been
assigned to the various sugar degradation products of
BEC.
The compounds represented by peaks I and II
have increased in height relative to the remaining peaks.
It can be seen that BEC undergoes degradation
under normal storage conditions. These degradation
products may be removed by washing the stored BEC with
water and chloroform.
Example 6
A BEC extract was obtained from Solanum
Sodomaeum according to conventional extraction
procedures. The degradation of the BEC extract was
estimated by measuring the change in solamargine and
solasonine levels over time. Although degradation of BEC
could also be measured by an increase in sugar levels, in
practice HPLC analysis for solamargine and solasonine
allowed a more quantative analysis to be conducted and
was therefore chosen for this study.
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
The results are illustrated in the following
Table.
Degradation (~) mean
+.S.D.
Time/Years Solamargine Solasonine
0 0 0
0.5 4+4 5+5
8+5 10+5
2 12+7 15+8
3 15+8 19+9
1910 2110
5 22+10 23+11
It can be seen that degradation of the
solasodine glycosides of the BEC occurs over time. The
effectiveness of cream formulations prepared from this
BEC was observed to decrease with the time the BEC was
stored prior to formulating the cream. This decrease in
efficacy resulted in an increase in the duration of
treatment required for regression of skin conditions
treated by the cream.
It will be appreciated that even after 5 years
the relative amounts of free sugars produced by
degradation of solamargine and solasonine are present in
relatively low amounts. Any inhibition at these low
levels could not have been predicted from the observation
that a large molar excess of rhamnose in the
aforementioned studies. It should also be noted that the
decrease in efficacy observed with stored BEC is
inconsistent with what would be predicted from the small
decrease in concentration of the active agents,
solamargine and solasonine.
Example 7
The survival of mice with sarcoma 180 when
treated with varying doses of 7mg and 8mg unwashed and
washed BEC/kg.
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
21
Washed BEC was prepared according to Example 1
and administered immediately after preparation.
Unwashed BEC was prepared in a similar manner
but was not washed prior to use. The unwashed
crystalline BEC was stored under ambient conditions for
about four weeks prior to administration to the mice. By
reference to the degradation studies provided in the
previous example, the % degradation over the four weeks
can be estimated to be between less than 4 to 5% for
solamargine and solasonine respectively.
Although, this degree of degradation may be
considered to be negligible, it can be seen that there is
a significant decrease in efficiency. Thus, BEC should be
washed prior to formulation even after storage for even
short periods of time (such as about four days).
12 mice with sarcoma 180 were treated with
7mg/kg doses given on consecutive days. The results are
shown in the following table.
COMPOUND DOSE NUMBER SURVIVAL ANIMALS
OF DOSES TIME SURVIVED
TREATED
- - - 20.9~5.6 0/12
BEC
Unwashed 7 1 20.96.0 0/12
7 2 29.1+6.6 2/12
7 3 37.516.2 4/12
7 4 42.0+17.1 6/12
BEC
Washed 7 1 25.36.1 0/12
7 2 44 14.2 7/12
7 3 53.010.0 11/12
7 4 56.0 12/12
BEC
Unwashed 8 1 20.9 5.5 0/12
8 2 30.1 15.8 4/12
8 3 48.0 16.2 11/12
8 4 53.0 12.6 11/12
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
22
BEC
Washed 8 1 38.3 10.2 7/12
8 2 55.1 6.8 11/12
8 3 56.0 12/12
8 4 56.0 12/12
Each value is the mean ~ S D obtained in groups
of twelve tumour - bearing mice treated intraperitoneally
0.5h after tumour implantation (5 x 105 cells/mouse).
a The criterion of survival was taken as 56 days
because it was shown that if the treatment was
effective against sarcoma 180 for this period, the
mice then had a normal life span (approximately 3
years ) .
b Doses given on consecutive days.
Animals surviving after eight weeks.
It can be seen that the % survival for animals
treated with the washed BEC was superior when compared
with animals treated with an equivalent dose of unwashed
BEC. For example, the % survival rate for four doses of
unwashed BEC is 50% as compared with 100% for washed BEC.
Example 8
The effect of washed and unwashed BEC or human
ovarian cancer cells was compared. The washed and
unwashed BEC were prepared as described for Example 8.
Cells (5 x 104) were transferred (200 ~,1/chamber
of a microscope slide (Lab Tek Miles Scientific).
Controls received 50 ~1 HIFCS/TCM and experimental
chambers 50 ~l of solasodine glycosides (BEC) 1.5 - 3.8
~M/L, washed and unwashed after 7-h preincubation and
incubated for a further 17h and 3-15.3 ~M/12L h
preincubation and incubated for a further 3h. Similarly,
the cells were treated with the aglycone solasodine 19.4-
96.8 ~M/L. The cells were fixed and examined by the
Papanicolaou method.
The results are shown in the following table.
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
23
BEC unwashed %survival BEC washed %survival
~g/mL ~g/ml
0 100 0 100
1 92 1 80
2 95 2 30
3 92 3 10
4 77 4 3
15 5 1
6 3 6 1
8 1 8 1
1 10 1
Solasodine % survival
~g/ml
0 100
3 100
6 100
9 100
12 100
100
18 100
24 100
These results again illustrate the surprisingly
superior efficiency of the washed BEC.
Example 9
A patient with no visible lesions on the face had a cream
as prepared in Example 2 applied to the skin of the face.
The cream was left for 30 minutes before being washed
away. The patient's face was then examined. Areas of
redness were noted which were identified as pre-malignant
or malignant skin lesions in the very early stages of
development. The affected areas of skin were
subsequently treated with the same cream.
Example 10
A human patient was diagnosed with an intra
epithelial penile tumour. The prognosis was that no
treatment was available and that amputation was the only
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
24
option. The patient commenced treatment with the cream
as prepared in Exampl e 2 and was applied to the tumour
twice daily. Necrosis of
the tumour was observed
to
occur shortly after treatment commenced. Within six
weeks, the patient was observed to be free of the tumour.
Example 11
The use of a preferred composition of the
present invention was trialed on solid tumors in animals
and humans as follows:
Formulation: A sugar and aglycone free
solasodine glycoside
preparation which was prepared
according to Example 1 in
DMSO. DMSO is used for its
aprotic characteristics and
because when pure is sterile.
Models: Horses, Dogs, Humans.
Lesions: Solid Sarcoids and squamous
cell carcinoma (SCC).
Procedure: The approximate weight of the
sarcoid or SCC is assessed
then 100mg of the sugar and
aglycone free solasodine
glycosides preparation of
Example 1 (100mg/ml DMSO of
stock solution) is injected
intralesionally to lkg tumor
weight. Two days later this
procedure is repeated.
Results: At day 2 after the first
injection, massive necrosis is
observed. Two weeks later
ablation of tumor is achieved.
Figures 5, 6, 7 and 8 show an example of a
sarcoid tumor in a horse, before, during and after
treatment. Figure 5 illustrates the sarcoma before
treatment. Figures 6 and 7 show the sarcoma after
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
injection with the above composition. Necrosis of the
sarcoma can be seen. Figure 4 shows that the sarcoma has
fully regressed after treatment.
Figures 9 to 12 show a further example of the
5 treatment of a horse with a penile tumor before, during
and after treatment. Figure 9 shows the horse prior to
treatment. The horse was anesthetized and the tumor
injected with the above formulation. Figure 10 shows the
response of the tumor to the composition. The tumor then
10 separated entirely and fell off as shown in Figure 11.
Figure 12 illustrates the penis after the treatment.
Figures 13, 14 and 15 show an example of the
treatment of a human SCC. Figure 13 shows the SCC
located on the patient's scalp. The patient was treated
15 with a single injection and recovery of the SCC shortly
after treatment occurred as illustrated in Figures 14 and
15.
In the above examples, it can be seen that a
composition of the present invention was successful in
20 the treatment of solid sarcoids in animals and SCC in
humans. During treatment, necrosis of the lesion was
observed to begin almost immediately after injection.
It was also observed that similar treatment
with BEC which contains free sugars was less effective
25 than the inventive composition. Treatment with BEC
required much higher dosages before any effect was
observed.
The dosages of the compositions in the above
examples is 100mg of solasodine glycosides per lkg tumor.
A typical tumor is 1008 such that a typical injection
contains lOmg solasodine glycosides. This dose for a
500kg horse corresponds to 0.02mg/kg body weight.
It can be seen that a therapeutic composition
of the present invention provides a suprising and
unexpected improvement in efficacy of glycoalkaloids in
the treatment of cancers and tumors. This increase in
efficacy allows disease conditions to be treated with
dosages which are well below the threshold level of
CA 02369272 2001-10-05
WO 00/61153 PCT/AU00/00300
26
toxicity for normal cells. This is advantageous for the
patient and also allows the inventive compositions to be
used as diagnostic tools. Still further, the improved
efficacy enables the duration of treatments to be reduced
and total dosages to be decreased. This is advantageous
for the overall safety and comfort of the patient and
also provides a superior treatment regime in terms of
cost effectiveness.
Throughout the specification (including claims
if present) unless the context requires otherwise, the
word " comprise" or variations such as " comprising"
will be understood to imply the inclusion of a stated
integer or group of integers but not the exclusion of any
other integer or group of integers.
It will be appreciated that modifications and
changes may be made to the embodiments described therein
without departing from the spirit and scope of the
invention as herein described.