Note: Descriptions are shown in the official language in which they were submitted.
CA 02369340 2001-10-03
WO 00/64527 PCTIUSOO/10905
VALVED INTRODUCER SHEATH AND RELATED METHODS
Technical Field
This relates to a medical device used to aid the introduction of another
medical device
into the body, and to methods of making and using such an introduction-aiding
device. More
particularly, this relates to an introducer system useful in the placement of
a catheter into the
body, and to methods of making and using such a system.
Background Information
In general, sheaths used to facilitate the insertion of devices, such as
catheters, into the
body are known. A catheter (or other medical device) can be inserted, with the
use of a known
sheath, percutaneously into the body, and the sheath can be torn off after
insertion of the catheter.
Tearable sheaths generally are referred to as peelable sheaths. There are also
non-peelable
lo sheaths. Peelable sheaths generally are used to introduce devices which are
left in (or at least
partially within) the patient after the procedure is completed, and a dialysis
catheter is one
example of a medical device that is left within the patient after a placement
procedure. Non-
peelable sheaths generally are used when the introduced device is removed at
the end of the
procedure.
Known peelable sheaths generally include a hub located at the proximal end of
the sheath,
and the hub can be manually grasped and pulled apart to permit the
longitudinal severance of the
hub and the sheath thereby to allow the removal of the sheath and hub from
about the catheter
extending therethrough and from the body. Such known peelable sheath/hub
combinations
typically are provided with a dilator, and the whole package generally is
referred to as a peelable
introducer system or simply an introducer system. Introducer systems with non-
peelable sheaths
also are known.
Some introducer systems, with the dilator inserted into and through the
peelable
sheath/hub, are designed to be advanced over a guide wire that is indwelling
in a vein, artery, or
other body cavity of a medical patient. In general, whether or not used with a
guide wire, an
introducer system is a medical device for insertion into the body (e.g., into
a vein, such as the
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-2-
jugular vein, of a patient). In one possible use, the introducer system is
placed into a patient's
vein in order to allow a flexible catheter to be placed later within the same
vein through the
sheath/hub of the introducer system after the dilator component of the
introducer system is
removed from the sheath/hub component.
Various types of peelable and non-peelable sheaths and introducer systems are
available
from Boston Scientific Corporation (Natick, MA) and other companies. Boston
Scientific
Corporation provides certain peelable sheaths and introducer systems in its
commercially-
available Vaxcel PICC kits.
Some known introducer systems include a valve to provide a seal around the
catheter
introduced through the sheath.
In general, known valved peelable introducer systems include two basic
components: (1)
a peel-away sheath/hub, where the hub is fixed to the proximal end of the
sheath; and (2) a more
rigid dilator that is sized to slide and fit snugly into a lumen extending
through the sheath/hub.
The hub generally is provided either as two identical separate halves (split
along the length of the
hub) or as a single unit with scoring or perforations on both sides along its
length to allow the
sheath hub to be broken into two identical separate halves. The dilator has a
proximal hub
portion and a longer tubular portion, and a passage typically extends through
the dilator such that
the dilator can be passed over a guide wire, for example. The tubular portion
of the dilator is
designed to extend through the sheath and the hub, and the proximal hub of the
dilator can lock
(e.g., via a luer lock) onto the hub of the sheath. A valve is incorporated
into the hub of the
sheath, and the dilator passes tllrough this valve when the dilator is
inserted into and through the
sheath/hub. The valve typically is a thin, disk-like membrane.
In a typical use, a known valved introducer system (with the dilator inserted
into the
sheath/hub and through the valve) is inserted into a patient's vein (usually
over a guide wire that
is already in the vein) up to the sheath's hub such that most or all of the
sheath is within the
patient's vein and most of the dilator also is within the patient's vein,
while the hubs of the
sheath and the dilator are outside of the patient's body. The dilator is then
removed from the
sheath and from the patient's body. With the dilator removed, the valve is
supposed to close and
prevent air from entering the vein, which generally would happen if the
passage through the
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-3-
sheath was open, because of negative pressure in the vein. The catheter (or
other medical device)
is now supposed to be inserted through the hub, valve, and sheath and thus
into the patient's
vein.
Known valved introducer systems generally have at least two issues. First, the
valve may
be too rigid to allow flexible devices, such as flexible catheters, to be
inserted therethrough.
Many catheters do not have enough column strength or rigidity to be passed
through a tight
valve. Second, if the valve is sufficiently flexible to allow a flexible
catheter to be inserted
therethrough, the valve may become malpositioned after the dilator is removed.
That is, the
valve may not close properly after removal of the dilator, and thus the danger
of air entering the
body or blood leaving the body is not avoided by use of the valve.
Summary of the Invention
In general, the invention relates to a valved introducer sheath and associated
methods of
making and using such a sheath. The sheath can include a body portion and a
proximal hub
portion, with a passageway extending through the entire length of the sheath.
The passageway is
sized to receive a dilator that generally is more rigid than the body portion
of the sheath. The
elongated body portion of the sheath can be generally circular in cross-
section and can have a
first section that has a larger diameter than a second section. The body
portion of the sheath
could have one or more non-circular cross-sectional shapes. Along and within
at least a portion
of the passageway of the sheath, an elongated valve extends longitudinally.
This elongated valve
can be formed of foam or other compliant material that will allow the dilator
to pass therethrough
and that will form a seal therearound, and that will also close and form a
reliable seal after the
dilator is removed. The elongated valve of the invention also will allow a
device more flexible
than the dilator (e.g., a catheter) to be passed therethrough, and it will
form a seal around such a
device and then close and seal after the device is removed.
The valved introducer sheath of the invention can be "peelable" in that the
body portion,
hub, and valve can be manually broken apart, split, and peeled from around the
device that
extends therethrough. Once peeled from around the inserted device and removed
from the body
of the patient, the two halves of the now-split valved introducer sheath
typically are discarded. In
an alternative embodiment, the valved introducer sheath of the invention is
not designed to be
peelable.
CA 02369340 2008-03-14
-4-
The elongated valve provides better performance than existing valves in terms
of at least the valved introducer sheath's ability to prevent reliably the
influx of air
(and/or the outflow of blood) as the dilator is being removed and after it is
removed.
The elongated valve of the invention can be opened and closed (by, for
example,
inserting and removing a dilator or other more flexible device such as a
catheter)
repeatedly without losing its ability to provide a reliable seal. Also, when
the dilator is
removed from the valve, and no medical device is present in the valve, the
valve
material is in an unstressed state.
In one aspect, the invention involves a method of producing a valved sheath.
The sheath comprises a proximal hub portion, an elongated body portion
extending
distally from the proximal hub portion, and a passageway extending through the
proximal hub and elongated body portions. A foam material is injected into at
least
some of the passageway. In the foam material, one or more self-sealing slits
are
formed. The foam material and the one or more slits serve as a valve in the
passageway
of the sheath.
Embodiments according to this aspect of the invention can include the
following features. The foam material can be injected such that the length of
the foam
material within the passageway is greater than the width of the foam material
at any
point within the passageway. The foam material can be a closed cell foam. The
foam
material can be affixed to at least some of an inner surface of the sheath.
The self-
sealing slits can be formed such that none of them extends in width to an
inner surface
of the sheath. The self-sealing slits substantially prevent the flow of gas
into the
passageway of the sheath. Scorings or lines can extend at least some of the
length, and
on opposite sides, of the sheath, such that the sheath is separable along the
scorings or
lines. The proximal hub portion can comprise a pair of wings that extend
substantially
perpendicular to the elongated body portion of the sheath.
Thus, in another aspect, there is provided a method of producing a valved
sheath, comprising: (a) providing a sheath comprising a proximal hub portion,
an
elongated body portion extending distally from the proximal hub portion, and a
CA 02369340 2008-03-14
-5-
passageway extending through the proximal hub and elongated body portions; (b)
injecting a closed-cell foam material into at least some of the passageway,
the length of
the foam material within the passageway being greater than the width of the
foam
material at any point within the passageway, the foam material being affixed
to a
portion of an inner surface of the sheath that defines the passageway; (c)
forming in the
foam material one or more self-sealing slits, the foam material and the one or
more slits
serving as a valve in the passageway of the sheath which substantially
prevents the flow
of gas into the passageway of the sheath; and (d) scoring a pair of lines
extending at
least some of the length, and on opposite sides, of the sheath such that the
sheath is
separable along the lines.
In another aspect, the invention relates to apparatus for facilitating the
insertion
of a flexible medical device into a body, comprising: (a) a sheath comprising
a
proximal hub portion, an elongated body portion extending distally from the
proximal
hub portion, at least some of the elongated body portion capable of being
placed into
the body, a passageway extending through the proximal hub and elongated body
portions, the passageway being defined by an inner surface of the sheath, and
a pair of
lines extending at least some of the length, and on opposite sides, of the
sheath, at least
the sheath being separable along the lines; and (b) a valve comprising a foam
material
filling at least some of the length of the passageway in the elongated body
portion, the
length of the foam material within the passageway being greater than the width
of the
foam material at any point within the passageway, and one or more self-sealing
slits in
the foam material, none of the slits extending in width to the inner surface
of the sheath,
the one or more slits capable of allowing the flexible medical device to pass
therethrough and sealing around the device.
Embodiments according to this other aspect of the invention can include the
following features. The foam material can include a proximal section and a
distal
section, where the one or more slits in the distal section remain sealed as
the flexible
medical device is introduced first into the one or more slits in the proximal
section. The
foam material can define a depression in the proximal section, and the
depression can
have a conical shape for receiving the flexible medical device. The foam
material can
CA 02369340 2008-03-14
-6-
be a closed cell foam. The foam material can be affixed to at least some of an
inner
surface of the sheath. A pair of scorings or lines can extend at least some of
the length,
and on opposite sides, of the sheath, such that at least the sheath is
separable along the
lines. The valve, too, can be separable with the sheath and can be split into
two halves
along one of the self-sealing slits. The elongated body portion can comprise
at least a
first section and a second section, where a first cross-sectional area of the
first section is
greater than a second cross-sectional area of the second section. The
elongated body
portion also can comprise a shoulder disposed within the passageway and
between the
first and second sections. The proximal hub portion can comprise a pair of
wings which
extend substantially perpendicular to the elongated body portion of the
sheath.
Thus, in another aspect, there is provided apparatus for facilitating the
insertion
of a flexible medical device into a body, comprising: (a) a sheath comprising
a
proximal hub portion, an elongated body portion extending distally from the
proximal
hub portion, a passageway extending through the proximal hub and elongated
body
portions; and a pair of lines extending at least some of the length, and on
opposite sides,
of the sheath such that the sheath is separable along the lines; and (b) a
valve
comprising a closed-cell injected foam material filling at least some of the
length of the
passageway, the length of the foam material within the passageway being
greater than
the width of the foam material at any point within the passageway, the foam
material
being affixed to a portion of an inner surface of the sheath that defines the
passageway,
and one or more self-sealing slits in the foam material, the foam material and
the one or
more slits serving as a valve in the passageway of the sheath which
substantially
prevents the flow of gas into the passageway of the sheath.
In yet another aspect, the invention involves apparatus for facilitating the
insertion of a flexible medical device into a body, comprising: (a) a sheath
comprising a
proximal hub portion, an elongated body portion extending distally from the
proximal
hub portion, at least some of the elongated body portion capable of being
placed into
the body, a passageway extending through the proximal hub and elongated body
portions, the passageway being defined by an inner surface of the sheath, and
a pair of
lines extending at least some of the length, and on opposite sides, of the
sheath, at least
CA 02369340 2008-03-14
- 6a-
the sheath being separable along the lines; and (b) a valve comprising a foam
material
filling at least some of the length of the passageway in the elongated body
portion, the
length of the foam material within the passageway being greater than the width
of the
foam material at any point within the passageway, the foam material being
affixed to a
portion of the inner surface of the sheath, and one or more self-sealing slits
in the foam
material, none of the one or more self-sealing slits in the foam material
extend in width
to the inner surface of the sheath, the one or more slits capable of allowing
the flexible
medical device to pass therethrough and sealing around the device.
Embodiments according to this aspect of the invention can include the
following features. The foam material can include a proximal section and a
distal
section, where the one or more slits in the distal section remain sealed as
the flexible
medical device is introduced first into the one or more slits in the proximal
section. The
foam material can define a depression in the proximal section, and the
depression can
have a conical shape for receiving the flexible medical device. The foam
material can
be a closed cell foam. A pair of scorings or lines can extend at least some of
the length,
and on opposite sides, of the sheath, such that at least the sheath is
separable along the
lines. The valve, too, can be separable with the sheath and can be split into
two halves
along one of the self-sealing slits. Each of the halves of the valve remain
affixed to the
respective halves of the sheath when the sheath and valve are separated. None
of the
one or more self-sealing slits in the foam material extend in width to the
inner surface
of the sheath. The proximal hub portion can comprise a pair of wings which
extend
substantially perpendicular to the elongated body portion of the sheath.
In still another aspect, the invention relates to apparatus for facilitating
the
insertion of a flexible medical device into a body, comprising: (a) a sheath
comprising a
proximal hub portion, an elongated body portion extending distally from the
proximal
hub portion, a passageway extending through the proximal hub and elongated
body
portions, and a pair of lines extending at least some of the length, and on
opposite sides,
of the sheath, at least the sheath being separable along the lines; and (b) a
valve
comprising: an injected foam material filling at least some of the length of
the
passageway in the elongated body portion, the length of the foam material
within the
CA 02369340 2008-03-14
- 6b -
passageway being greater than the width of the foam material at any point
within the
passageway, and one or more self-sealing slits in the foam material, the foam
material
and the one or more slits serving as a valve in the passageway of the sheath,
and
wherein none of the one or more self-sealing slits extends in the width to an
inner
surface of the sheath that defines the passageway.
Embodiments according to this aspect of the invention include the following
features. The foam material can include a proximal section and a distal
section, where
the one or more slits in the distal section remain sealed as the flexible
medical device is
introduced first into the one or more slits in the proximal section. The foam
material
can define a depression in the proximal section, and the depression can have a
conical
shape for receiving the flexible medical device. The foam material can be a
closed cell
foam. In one embodiment, none of the one or more self-sealing slits extends in
width to
an inner surface of the sheath that defines the passageway. The one or more
self-sealing
slits substantially prevent the flow of gas into the passageway of the sheath.
A pair of
scorings or lines can extend at least some of the length, and on opposite
sides, of the
sheath, such that at least the sheath is separable along the lines. The valve,
too, can be
separable with the sheath. The proximal hub portion can comprise a pair of
wings
which extend substantially perpendicular to the elongated body portion of the
sheath.
The foregoing and other objects, aspects, features, and advantages of the
invention will become more apparent from the following description and from
the
claims.
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-7-
Brief Description of the Draw~
In the drawings, like reference characters generally refer to the same parts
throughout the
different views. Also, the drawings are not necessarily to scale, emphasis
instead generally being
placed upon illustrating the principles of the invention.
FIG. 1 is a partial sectional view of a valved introducer sheath according to
the invention,
with a dilator inserted into the sheath and thus with a valve of the sheath
open.
FIG. 2 is a partial sectional view of the sheath of FIG. 1, with the dilator
of FIG. 1 partially
removed from the sheath and thus with the valve of the sheath closed along a
distal part of its
length.
FIG. 3 is a partial sectional view of the sheath of FIG. 1, without the
dilator of FIG. 1
inserted into the sheath and thus with the valve of the sheath completely
closed along its entire
length.
FIG. 4 shows the dilator of FIG. 1 when removed from the sheath of FIG. 1.
FIG. 5 is a partial sectional view of a conventional valved sheath, with a
slit valve.
FIG. 6 is a top view of the conventional valved sheath of FIG. 5, showing two
cross slits
that form the slit valve of the sheath of FIG. 5.
FIG. 7 is a partial sectional view of the conventional valved sheath of FIG.
5, with a dilator
passing through the slit valve of the sheath and extending partially into the
sheath.
FIG. 8 is a partial sectional view of the conventional valved sheath of FIG.
5, with the
2o dilator of FIG. 7 completely removed from the sheath and the slit valve
left in a malpositioned and
open state that will allow air influx and/or blood outflow.
FIG. 9 shows a possible non-circular cross-sectional shape of a portion of the
sheath of FIG.
l.
FIG. 10 is a partial sectional view of the valved introducer sheath of FIG. 1,
with a flexible
catheter inserted into the sheath and thus with the valve of the sheath open.
FIG. 11 is a partial sectional view of the sheath of FIG. 10, with the
catheter of FIG. 10
partially removed from the sheath and thus with the valve of the sheath closed
along a distal part of
its length.
FIG. 12 is a partial sectional view of the sheath of FIG. 10, without the
catheter of FIG. 10
inserted into the sheath and thus with the valve of the sheath completely
closed along its entire
length.
FIG. 13 shows a peelable sheath with scoring or tear lines for separating the
sheath.
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-8-
FIG. 14 is a flow diagram illustrating a method of making a valved introducer
sheath
according to one embodiment of the invention.
FIG. 15 is a top view, along the longitudinal axis of the sheath, of an
elongated valve
comprising one slit according to one embodiment of the invention.
FIG. 16 is a top view, along the longitudinal axis of the sheath, of an
elongated valve
comprising two slits according to one embodiment of the invention.
FIG. 17 is a top view, along the longitudinal axis of the sheath, of an
elongated valve
comprising three slits according to one embodiment of the invention.
FIG. 18 is a three-dimensional perspective view of an elongated valve
comprising two slits
according to one embodiment of the invention.
Description
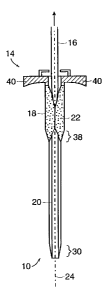
Referring to FIGS. 1-4 and 10-12, a valved introducer sheath 10 according to
the invention
includes a body portion 12 and a proximal hub portion 14, with a passageway
extending through
the entire length of the sheath 10. The hub portion 14 comprises a pair of
wings 40 that extend
substantially perpendicular to the body portion 12. The passageway is sized to
receive a dilator
16 that generally is more rigid than the body portion 12 of the sheath 10. The
body portion 12 of
the sheath 10 includes at least a first section 18 and a second section 20,
where the first section
18 has a larger cross-sectional area than the second section 20, such that the
transition 38 from
the first section 18 to the second section 20 defines a shoulder or other
discontinuity. For
example, if the elongated body portion 12 of the sheath 10 is generally
circular in cross-section,
the first section 18 has a larger diameter than the second section 20. The
body portion 12 of the
sheath 10 could have non-circular cross-sectional shapes, such as the oblong
shape shown in
FIG. 9. In an alternative embodiment, the body portion 12 has a single
constant cross-sectional
area along its entire length. In other embodiments, the body portion 12 can
taper (proximal-to-
distal, or distal-to-proximal, for example) or otherwise have a varying cross-
sectional area along
its length. Along and within at least a portion of the passageway (such as
some or all of the
length of the first section 18), an elongated valve 22 extends longitudinally
along the lengthwise
axis 24 of the first section 18 and the sheath 10 generally. In the disclosed
embodiment, the
elongated valve 22 extends along and within the entire length of the first
section 18. In this
embodiment, the first section is about 2 cm in length and about 0.230 inches
in diameter, and the
second section is about 14 cm in length and about 0.200 inches in diameter,
and thus the
CA 02369340 2001-10-03
WO 00/64527 PCTIUSOO/10905
-9-
elongated valve 22 is approximately 2 cm in length. Also, in this embodiment,
the thickness of
the wall of the body portion 12 is about 0.010 inches, and can range
generally, for example, from
about 0.008 inches to about 0.0 15 inches in thickness.
In the disclosed embodiment, the ratio of the length of the valve 22 to the
diameter of the
valve 22 is about 3.5, because 2 cm divided by 0.230 inches equals about
3.423. In general, and
in accordance with the invention, this ratio for any elongated valve 22 of the
invention will be
greater than about 2, and preferably greater than about 3. This is in contrast
to the
length/diameter ratio of known disc-like valves which generally is less than
or equal to about 2,
and usually less than or equal to about 1. One of ordinary skill will
recognize the design trade-
offs between the additional sealing capability due to the length of the valve
and the additional
friction resulting from extending the length of the valve. Depending on the
valve material(s), a
variety of valve ratios are within the scope of this invention.
This elongated valve 22 of the invention can be formed of a foam material or
other
compliant material that will allow the dilator 16 to pass therethrough and
that will form a seal
therearound, but that will also close and form a reliable seal after the
dilator 16 is removed. The
elongated valve 22 of the invention also will allow a device more flexible
than the dilator 16,
such as a flexible catheter 36, to be passed therethrough, and it will form a
seal around such a
device and then close and seal after the device is removed. The larger cross-
sectional area of the
first section 18 of the body portion 12 of the sheath 10, as compared to the
second section 20,
provides space for the material (e.g., foam) of the elongated valve 22 to
compress and compact
when a dilator or other medical device (e.g., the flexible catheter 36) is
inserted through the
elongated valve 22 and into and through the second section 20 of the body
portion 12 of the
sheath 10. This extra space in the first section 18 thus allows the elongated
valve 22 to open
fully and seal around the medical device inserted through the sheath 10, even
when the inserted
medical device has a diameter (or cross-sectional area) that is equal to,
about the same as, or
more likely slightly smaller than the inner diameter (or cross-sectional area)
of the second section
20 of the body portion 12 of the sheath 10.
Referring to FIG. 13, in one embodiment, the valved introducer sheath 10 of
the
invention is "peelable" in that the body portion 12, hub 14, and valve 22 can
be manually broken
3o apart, split, and peeled or separated along scorings or lines 44 that
extend at least some of the
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-10-
length and, on opposite sides, of the sheath. The lines or scorings 44 are
shown extending the
entire length of the sheath 10, but they may instead extend less than the
entire length. The sheath
can be separated from around the device that extends therethrough (such as the
dilator 16 or,
more typically, a catheter such as the flexible catheter 36). In another
embodiment, the sheath 10
is not peelable. In the peelable embodiment, once peeled from around the
inserted device and
removed from the body of the patient, the two halves of the now-tom valved
introducer sheath 10
are discarded.
The dilator 16 includes a substantially rigid (at least generally more rigid
than the body
portion 12 of the sheath 10) and elongated portion 26 and a hub portion 28.
The hub portion 28
of the dilator 16 is designed to be received by the proximal hub portion 14 of
the sheath 10. In
the disclosed embodiment, the hub 28 of the dilator 16 can be locked into the
hub 14 of the
sheath 10 by inserting the elongated portion 26 of the dilator 16 into the
sheath 10 and fitting the
hub 28 of the dilator 16 and then turning the dilator 16 (or perhaps turning
just the hub 28 of the
dilator 16) to lock it into the receiving structure on the hub 14 of the
sheath 10. In other
embodiments, the hub 26 of the dilator 16 locks to the hub 14 of the sheath 10
in a different
fashion, or they do not lock or twist together at all and instead the hub 26
of the dilator 16 just
contacts the hub 14 of the sheath 10 when the dilator 16 is fully inserted
into the sheath 10.
The valved introducer sheath 10 of the invention, along with the dilator 16,
can be used to
place the catheter 36 (or other medical device) into the body of a patient.
For example, a large
Central Venous Catheter (CVC) can be inserted into a patient's vein with the
help of the valved
introducer sheath 10. Also, the sheath 10 can be used to help place a dialysis
catheter into a
patient. During the time in which the dilator 16 is removed from the sheath
10, but prior to
insertion of the catheter into the sheath 10, there is a risk that air can be
aspirated into the
patient's venous system through the sheath, causing major medical
complications. Also, when
placing a catheter 36 or other device into the body of a patient, there may be
a risk that blood or
other bodily material or fluid can escape from the patient. In any event, and
in general, the
valved introducer sheath 10 of the invention can be used to place such medical
devices into the
body without the risk of substantially anything entering into or escaping from
the body. The
elongated valve 22 of the invention provides a reliable and effective seal
that is easy to
manufacture and that will seal properly even after repeatedly being opened and
closed by
CA 02369340 2001-10-03
WO 00/64527 PCTIUSOO/10905
-11-
inserting and removing a dilator 16 and/or catheter 36 and/or other medical
device through the
elongated valve 22.
The elongated valve 22 of the invention will seal before substantially any air
or other
material can either enter or exit the body of the patient, thereby
substantially eliminating and
preventing any risk to the patient, such as the risk of air embolism. The
elongated valve 22
provides a seal that is more robust than known valves, such as slit valves,
because the elongated
valve 22 has a large surface area that is oriented along the length of the
sheath 10. The elongated
valve 22 generally is easier to peel apart than known valves, such as slit
valves, because the
elongated valve 22 tears in half more easily along its length. Also, the
elongated valve 22 is not
part of the hub 14 of the sheath 10, and thus the hub 14 of the sheath 10
generally is easy to
manufacture and easy to break in half, as compared to conventional valved
sheaths that use disc-
like slit valves incorporated into the hub of the sheath. It is easier and
less expensive to
manufacture the hub 14 of the invention (whether it can be peeled apart or
not) than it is to make
the more-complex hub of a known sheath (whether it can be peeled apart or not)
which includes
the slit valve as part of the hub assembly.
The elongated valve 22 of the invention establishes a seal even before the
dilator 16,
catheter 36, or other medical device is removed from the sheath 10, as
illustrated in FIG. 2.
Also, the lengthwise structure of the elongated valve 22 provides a high
security seal after the
dilator 16 or other medical device is removed even if the body portion 12 of
the sheath 10 is bent
or twisted during the rest of the clinical procedure.
Known slit valves 505 formed into the hub of known valved sheaths, as shown in
FIGS.
5-8, can become malpositioned when the dilator 16, catheter 36, or other
medical device is
removed, even after the first time the dilator 16, catheter 36, or other
medical device is inserted
and removed. This malpositioning results in a slit valve 505 that is not
completely closed upon
removal of the dilator 16, catheter 36, or other medical device (FIG. 8), and
thus air and/or blood
and/or other material can enter and/or exit the body of the patient through
the deformed slit valve
805. The conventional slit valve 505 is disc-like and is not extended along
the longitudinal
length of the sheath 10 or the hub 14, and thus it cannot provide the
multitude of benefits
provided by the elongated valve 22 of the invention.
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-12-
The elongated valve 22 of the invention can be formed of a foain material or
other
material or combination of materials. The elongated valve 22 must allow the
dilator 16 or other
medical device, such as the catheter 36. to pass therethrough, and it must
form a seal
therearound. The elongated valve 22 also must close and form a reliable seal
after the dilator 16
or other medical device is removed, and it must form a reliable seal even
after repeated insertions
and removals of dilators 16 and/or catheters 36 and/or other devices. If a
foam material is
selected as the material to be used to form the elongated valve 22, the foam
material should be
very light (i.e., its volume should be largely occupied by gas) to minimize
resistance to passage
during the insertion and removal of dilators, catheters, and other devices.
Also, the foam
lo material selected should be very elastic in that it consistently springs
closed when the devices
(e.g., dilators, catheters, etc.) are removed. The foam material should be
closed cell to minimize
absorption of fluids (i.e., liquids or gasses). The foam material also should
be tough in that it
does not wear, degrade, or generate debris (for example, pieces should not
break off) as a result
of friction associated with devices being inserted into and removed from the
valve 22. The foam
material used to form the valve 22 could be lubricated to minimize friction.
The foam material
could be carried in a solvent which enables it to adhere to the inner walls of
the first section 18 of
the body portion 12 of the sheath 10, and the foam material should be bio-
compatible and
capable of being sterilized. Some foam materials that possibly could be used,
as long as they
meet all or most of the above-identified requirements, include silicones,
polyurethanes,
polyethylenes, and the like.
The body portion 12 of the sheath 10, which can be extruded to form its shape,
can be
made from any of a variety of materials or combination of materials. The body
portion 12 can be
made, for example, of polyethylene or other thermoformable plastic, and such a
material would
allow the body portion 12 of the sheath 10 to be set into a curved or bent
configuration rather
than the depicted straight geometry. In some applications, such as accessing
the left internal
jugular vein, a pre-curved or pre-bent body portion 12 of the sheath 10 would
have clinical
advantages including ease of placing and lack of kinking which might happen if
one were to
bend a straight sheath. If the body portion 12 of the sheath 10 were made from
a thermoset
Teflon type material, which it could be, it generally would not be able to be
pre-curved or pre-
3o bent and likely would have to be provided straight.
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
- 13 -
Referring to FIG. 14, one possible method of manufacturing the valved
introducer sheath
of the invention would include the following steps.
First, the body portion 12 of the sheath 10 is extruded (Step 1010). The hub
14 of the
sheath 10 also is formed (Step 1020), and then the hub 14 is molded onto the
proximal end of the
5 sheath 10 (i.e., the proximal end of the first section 18 of the body
portion 12 of the sheath 10)
(Step 1025), as shown in FIGS. 1-3 and 10-12.
The body portion 12 of the sheath 10 then can be scored to allow the sheath 10
to be
peeled into two halves (Step 1030). Alternatively, if the material(s) from
which the body portion
12 is formed is/are "highly oriented," there possibly would be no need to
score the length of the
10 body portion 12 as such material(s) would tend to peel apart naturally into
two halves. If the
sheath 10 is not to be peelable, it would not be necessary to perform the
scoring step or to select a
highly oriented material.
A mandrel can then be inserted into the distal end 30 of the body portion 12
of the sheath
10 until it enters the first section 18 (which is larger than the second
section 20 of the body
portion 12 of the sheath 10) or until it reaches the transition point between
the first and second
sections 18, 20 (Step 1040). The mandrel is then adjusted so that it provides
a seal between itself
and the inner wall of the second section 20 of the body portion 12 -- the
mandrel can be equipped
with a balloon which can be inflated to contact the inner wall of the second
section 20 near or at
the transition point between the first and second sections 18, 20, for
example. Regardless of
whether the mandrel or some other mechanism is used, the point is to seal off
a section of the
body portion 12 (Step 1050) so that the foam can be placed in the first
section 18 to form the
elongated valve 22.
A nozzle can now be inserted into the proximal end of the sheath 10 through
the hub 14
and into the first section 18 of the body portion 12, and liquid foam material
(e.g., a polyurethane
material) can be injected into the first section 18 of the body portion 12 of
the sheath 10 (Step
1060). The foam material should be allowed to set and to adhere to the inner
wall of the first
section 18 (Step 1070). If necessary to set (or better set) the foam material
and/or to cause it to
adhere (or better adhere) to the inner wall of the first section 18 of the
body portion 12 of the
sheath 10, heat can be applied for an appropriate amount of time and at an
appropriate
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
-14-
temperature or temperatures. During the time the foam material is allowed to
set and adhere to
the inner wall of the first section 18, the nozzle used to inject the foam
material into first section
18 of the body portion 12 of the sheath 10 may remain inserted into the first
section 18 of the
body portion 12 in order create a conical or other shaped depression in the
proximal end of foam
material. Referring to FIG. 3, the mandrel that is used to seal off the second
section 20 from the
first section 18 can also be used to create a conical or other shaped
depression in the distal end of
the foam material. This depression facilitates easy advancement of the sheath
10 over a flexible
medical device, such as a hubless catheter, that is inserted into the sheath
10 at the distal end.
Alternatively, at least the distal end of the foam material can be formed
without any type of
shaped depression, and can instead be flat, as indicated by the short
horizontal dotted line 46.
The shaped (e.g., conical) depression in the proximal end of the foam material
can be used to
guide easily various medical devices into the one or more slits. This
depression facilitates easy
entry of a flexible medical device, such as the catheter 36, into the
elongated valve 22.
Once properly set and adhered, a scalpel type device is used to create at
least one slice or
slit 50 (FIGS. 15-18) longitudinally through the center of the foam material
while leaving the
block of foam in place, thereby creating the elongated valve 22 (Step 1080).
If the sheath 10 is
peelable, at least one slit 50 should be oriented to coincide with the
scoring, or the natural tear
line 44, of the body portion 12 of the sheath 10 to allow the elongated valve
22, the body portion
12, and the hub 14 to separate into two halves easily, neatly, and evenly
(which can be formed as
described below).
The very distal end of the body portion 12 of the sheath 10 could then be
tapered
appropriately (Step 1090). As seen in FIGS. 1-3 and 10-12, the distal end 30
of the body portion
12 of the sheath 10 is tapered, and this generally helps reduce any abrupt
transitions between the
end of the body portion 12 and the dilator 16, catheter 36, or other medical
device that extends
therefrom, to allow the entire assembly to slide more easily into the body of
the patient (over a
guide wire, typically). Step 1090 is performed last to prevent potential
damage to the distal end
from the insertion of the mandrel (Step 1040). However, step 1090 could be
executed earlier.
Referring to FIG. 16, whether the sheath 10 is peelable or not, one embodiment
of the
elongated valve 22 is formed by two slits 50 that cross each other and are
oriented ninety degrees
30 with respect to each other to form an "X" or "+" configuration when viewed
straight down from
CA 02369340 2001-10-03
WO 00/64527 PCT/US00/10905
- 15-
the top of the sheath 10 along the longitudinal axis of the sheath 10 and the
elongated valve 22.
Elongated valves of the invention can be formed with one or more longitudinal
slits 50 oriented
in a variety of configurations. For example, one (FIG. 15), two (FIG. 16, 18),
three (FIG. 17), or
more slits 50 can be used to form the elongated valve 22.
Referring to FIG. 18, none of the one or more slits 50 in the foam material
extend in
width to the inner wall of the sheath. That is, there is an unbroken "band" of
foam material on
the edge of valve 22 along its entire length, as indicated by the distance "b"
in FIG. 18.
Still referring to FIG. 18, the valve 22, if it could be removed in one piece
from the body
portion 12 of the sheath 10 (which it cannot, because it is affixed or adhered
to at least a portion
of the inner surface to the sheath 10 that defines the passageway through the
sheath 10), would
have the same shape it has when in place within the sheath 10. The removed
valve 22 would not
expand, as it is not held in a compressed state within the sheath 10, as with
some inserts that are
used in known sheaths. The removed valve 22 would retain the shape and
configuration it has
when it is in the sheath 10, with the slit(s) 50 of the valve 22 closed when
nothing is inserted into
the slit(s) 50.
Elongated valves (22) in accordance with the invention could be used in other
types of
medical devices instead of the introducer sheath 10 described herein. In
general, any type of
medical device requiring valving could benefit from the use of an elongated
valve 22 of the type
described herein. Elongated valves 22 of the invention eliminate or at least
minimize the
undesired transfer of fluids and/or gasses, and such elongated valves 22 could
be useful in a
variety of medical procedures when incorporated into any of a variety of
different medical
devices.
Variations, modifications, and other implementations of what is described
herein will
occur to those of ordinary skill in the art without departing from the spirit
and the scope of the
invention. Accordingly, the invention is not to be defined solely by the
preceding illustrative
description.
What is claimed is