Note: Claims are shown in the official language in which they were submitted.
We claim:
1. A composition for controlling the corrosion of metals in contact with an
aqueous system having a pH of about 6 or greater which comprises a combination
of:
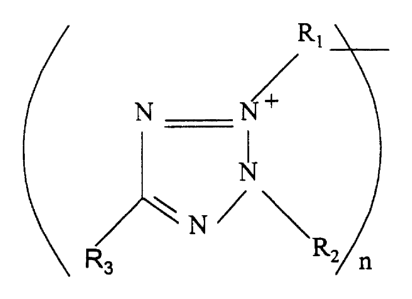
(a) a tetrazolium compound of the formula:
Image
wherein R1, R2 and R3 are selected from the group consisting of lower alkyl,
branched lower
alkyl, aryl, substituted aryl, alkylaryl, substituted alkylaryl and
heterocyclic substituted aryl,
with the proviso that neither R1, R2, or R3 contain more than 14 carbon atoms;
and n is 1 or 2,
such tetrazolium compound optionally having associated water soluble ionic
species if needed
to obtain a neutral charge, and
(b) at least one other aqueous system treatment material chosen so that the
material
does not substantially reduce the tetrazolium compound.
2. A composition as recited in claim 1 wherein said other aqueous system
treatment material is selected from the group consisting of inorganic
phosphates, borates,
nitrites, compounds that release a metal anion in water, 2,3-dihydroxybenzoic
acid, 1,10-
phenanthroline, polycarboxylates, akyl hydroxycarboxylic acids,
aminohydroxysuccinic acids,
carboxyamines, polyepoxysuccinic acids, modified polyepoxysuccinic acids,
monophosphonic
acids, diphosphonic acids, phosphonocarboxylic acids,
hydroxyphosphonocarboxylic acids,
aminophosphonic acids, phosphonomethylamine oxides, polymeric amine oxides,
polyetherpolyaminomethylene phosphonates, polyetherpolyamino-methylene
phosphonate N-
oxides, iminoakylenephosphonic acids, long chain fatty acid derivatives of
sarcosine;
telomeric, co-telomeric, polymeric, or copolymeric phosphorus-containing
carboxylates, alkali
metal silicates, monofluorophosphate, amines, diamines, alkanolamines, ether
amines, fatty
amines and diamines, quaternized amines, oxyalkylated amines, akyl pyridines,
tetrazoles,
imidazoline and substituted imidazolines, amidoamines, polyamines,
polyakylenepolyamines,
-43-
alkyl derivatives of benzene sulfonic acid, benzoates and substituted
benzoates,
aminobenzoates, salicylates, dimer-trimer acids, petroleum oxidates,
borogluconates; lignins,
lignosulfonates, tannins; straight chain C5-C11 monocarboxylates and C4-C15
.alpha.,.omega.-
dicarboxylates; amine salts of carboxylic acids and mercaptocarboxylic acids,
amino acids,
polyamino acids, hydroxyether acids and related lactone compounds, N-
acyliminodiacetic
acids; triazine di- and tri-carboxylic acis, phospho- and phosphate esters;
and
monofluorophosphates; water soluble salts thereof, and mixtures thereof.
3. A composition as recited in claim 1 wherein said tetrazolium
compound is selected from the group consisting of the water soluble salts of
Nitro Blue
Tetrazolium (2,2'-Di-p-nitrophenyl-5,5'-distyryl-3,3'-[3,3'-dimethoxy-4,4'-
biphenylene]
ditetrazolium), Distyryl Nitroblue Tetrazolium (2,2'-Di-p-nitrophenyl-5,5'-
distyryl-3,3'-[3,3'-
dimethoxy-4,4'-biphenylene] ditetrazolium), Tetranitro Blue Tetrazolium (3,3'-
(3,3'-
Dimethoxy-4,4'-biphenylene)-bis-[2,5-p-nitrophenyl-2H-tetrazolium) and
Iodonitro
Tetrazolium (2-(4-lodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium).
4. A composition as recited in claim 2 wherein said tetrazolium compound is
selected from the group consisting of the water soluble salts of Nitro Blue
Tetrazolium (2,2'-
Di-p-nitrophenyl-5,5'-distyryl-3-,3'-[3,3'-dimethoxy-4,4'-biphenylene]
ditetrazolium),
Distyryl Nitroblue Tetrazolium (2,2'-Di-p-nitrophenyl-5,5'-distyryl-3,3'-[3,3'-
dimethoxy-
4,4'-biphenylene] ditetrazolium), Tetranitro Blue Tetrazolium (3,3'-(3,3'-
Dimethoxy-4,4'-
biphenylene)-bis-[2,5-p-nitrophenyl-2H-tetrazolium) and Iodonitro Tetrazolium
(2-(4-lodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium).
5. A composition as recited in claim 1 wherein said water soluble ionic
species
are anions selected from the group consisting of halogens, nitrates, nitrites,
carbonates,
bicarbonates, sulfates, phosphates, and transition metal oxygenates.
6. A composition as recited in claim 5 wherein said halogens are selected from
the
group consisting of chlorides, fluorides, bromides and iodides.
7. A composition as recited in claim 6 wherein said halogen is chloride.
8. A composition as recited in claim 5 wherein said transition metal oxygenate
is
selected from the group consisting of molybdate, chromate, and tungstate.
9. A composition as recited in claim 8 wherein said transition metal oxygenate
is
molybdate.
-44-
10. A composition as recited in claim 2 wherein said inorganic phosphates are
orthophosphates, polyphosphates, water soluble salts thereof and mixtures
thereof.
11. A composition as recited in claim 2 wherein said inorganic phosphates are
a
mixture of orthophosphoric acid and pyrophosphoric acid or the water-soluble
salts thereof.
12. A composition as recited in claim 2 wherein said borate is a water-soluble
borate selected from the group consisting of tetraborates, metaborates, and
orthoborates.
13. A composition as recited in claim 12 wherein said water-soluble borate is
sodium tetraborate or a hydrate of sodium tetraborate.
14. A composition as recited in claim 2 wherein said nitrite is sodium
nitrite.
15. A composition as recited in claim 2 wherein the metal anion releasing
compounds are selected from the group consisting of the water soluble salts of
molybdate,
tungstate, vanadate, metavanadate, and chromate.
16. A composition as recited in claim 15 wherein the water soluble salt of a
molybdate is sodium molybdate or a hydrate of sodium molybdate.
17. A composition as recited in claim 2 wherein said polycarboxylates comprise
aliphatic compounds containing between about 4 and about 20 carbon atoms which
are
multiply substituted with carboxylate groups or water soluble salts thereof.
18. A composition as recited in claim 17 wherein said polycarboxylate is
1,2,3,4-
butanetetracarboxylic acid.
19. A composition as recited in claim 2 wherein said polycarboxylate is a
homopolymer obtained from the polymerization of an ethylenically unsaturated
monomer
containing one or more carboxyl groups.
20. A composition as recited in claim 19 wherein said homopolymer is
polyacrylic
acid or its water soluble salts.
21. A composition as recited in claim 19 wherein said homopolymer is
polymaleic
acid or its water soluble salts.
22. A composition as recited in claim 19 wherein said homopolymer is
polymaleic
anhydride or its water soluble salts.
23. A composition as recited in claim 2 wherein said polycarboxylate is a
copolymer obtained from the polymerization of two or more different
ethylenically
unsaturated monomers, each of said monomers containing one or more carboxyl
groups.
24. A composition as recited in claim 2 wherein said alkyl hydroxycarboxylic
acid
has the generalized formula
HOOC -(R B1)a -( R B2)b -(R B3)c - R B4
where a, b, and c are integers from 0 to 6 and (a+b+c)>0 where R B1, R B2, R
B3 comprise C=O or
CYZ, where Y and Z are separately selected from the group of H, OH, CHO, COON,
CH3,
CH2(OH), CH(OH)2, CH2(COOH), CH(OH)COOH, CH2(CHO) and CH(OH)CHO, so selected
that the molecule has a minimum of one OH group when written in its fully
hydrated form and
R B4 is either H or COOH, including the various stereoisomers and chemically
equivalent
cyclic, dehydrated, and hydrated forms of these acids and hydrolyzable esters
and acetals that
form the above compounds in water or the water soluble salts of such alkyl
hydroxycarboxylic
acids.
25. ~A composition as recited in claim 24 wherein said akyl
hydroxycarboxylic acid is chosen from the group consisting of tartaric acid,
mesotartaric acid,
citric acid, gluconic acid, glucoheptonic acid, ketomalonic acid, saccharic
acid and the water
soluble salts thereof.
26. ~A composition as recited in claim 2 wherein the said other aqueous
system treatment materials is a mixture of orthophosphoric acid or its water-
soluble salts and
at least one alkyl hydroxycarboxylic acid having the generalized formula
HOOC -(R B1)a -(R B2)b -(R B3)c -R B4
where a, b, and c are integers from 0 to 6 and (a+b+c)>p where R B1, R B2, R
B3 comprise C=O or
CYZ, where Y and Z are separately selected from the group of H, OH, CHO, COOH,
CH3,
CH2(OH), CH(OH)2, CH2(COOH), CH(OH)COOH, CH2(CHO) and CH(OH)CHO, so selected
that the molecule has a minimum of one OH group when written in its fully
hydrated form and
R B4 is either H or COOH, including the various stereoisomers and chemically
equivalent
cyclic, dehydrated, and hydrated forms of these acids and hydrolyzable esters
and acetals that
form the above compounds in water or the water soluble salts of such alkyl
hydroxycarboxylic
acids, and the water soluble salts thereof.
27. A composition as recited in claim 26 wherein the hydroxycarboxylic acid is
selected from the group consisting of tartaric acid, mesotartaric acid, citric
acid, gluonic acid,
glucoheptonic acid, ketomalonic acid, saccharic acid and the water soluble
salts thereof.
28. A composition as recited in claim 2 wherein said aminohydroxysuccinic acid
has the generalized formula
-46-
Image
wherein R C1 is H or C1 to C4 alkyl, optionally substituted with optionally
substituted with -OH,
-CO2H, -SO3H, or phenyl, C4 to C7 cycloalkyl, or phenyl which is optionally
substituted with
-OH or -CO2H, and R C2 is H, C1 to C6 alkyl, optionally substituted with -OH
or -CO2H
(specifically including the moiety -CH(CO2H)CH(OH)(CO2H)); and
Image
wherein R C2 is as above, and Z C is selected from the group consisting of
i)- (CH2)k wherein k is an integer from 2 to 10,
ii) -(CH2) 2 -X C -(CH2)2 wherein X C is -O-, -S-, -NR C3 , wherein R C3 is
selected from the
group consisting of H, C1 to C6 alkyl, hydroxyalkyl, carboxyalkyl, acyl, and -
C(O)OR C4
wherein R C4 is selected from the group consisting of C1 to C6 alkyl or benzyl
and a residue
having the general formula:
Image
wherein R C2 is as above,
iii) a residue having the generalized formula
-47-
Image
wherein Y is H, C1 to C6 alkyl, alkoxy, halogen, - CO2H, or - SO3H, m is
independently 0 or 1,
and p is 1 or 2, and
iv) a residue having the generalized formula:
Image
wherein R C5 and R C6 are independently H or C1 to C6 alkyl, Q is H or C1 to
C6 alkyl, s is 0, 1 or
2, t is independently 0, 1, 2, or 3, q is 0, 1, 2, or 3, and r is 1 or 2 or
water soluble salts thereof.
29. The composition as recited in claim 28 wherein the aminohydroxysuccinic
acid
is selected from the group consisting of iminodi(2-hydroxysuccinic acid), N,
N'-Bis(2-
hydroxysuccinyl)-1,6-hexanediamine, N,N'-Bis(2-hydroxysuccinyl)-m-
xylylenediamine, and
the water-soluble salts thereof.
30. A composition as recited in claim 2 wherein said other aqueous system
treatment material is a mixture of orthophosphoric acid or its water-soluble
salts and at least
one aminohydroxysuccinic acid wherein said aminohydroxysuccinic acid has the
generalized
formula
Image
wherein R C1 is H or C1 to C4 alkyl, optionally substituted with optionally
substituted with -OH,
-CO2H, -SO3H, or phenyl, C4 to C7 cycloalkyl, or phenyl which is optionally
substituted with
-OH or -CO2H, and R C2 is H, C1 to C6 alkyl, optionally substituted with -OH
or -CO2H
(specifically including the moiety -CH(CO2H)CH(OH)(CO2H)); and
-48-
Image
wherein R C2 is as above, and Z C is selected from the group consisting of
i)- (CH2) k - wherein k is an integer from 2 to 10,
ii) -(CH2)2 -X C-(CH2)2 wherein X C is -O-, -S-, -NR C3, wherein R C3 is
selected from the
group consisting of H, C1 to C6 alkyl, hydroxyalkyl, carboxyalkyl, acyl, and -
C(O)OR C4
wherein R C4 is selected from the group consisting of C1 to C6 alkyl or benzyl
and a residue
having the general formula:
Image
wherein R C2 is as above,
iii) a residue having the generalized formula
Image
wherein Y is H, C1 to C6 alkyl, alkoxy, halogen, - CO2H, or - SO3H, m is
independently 0 or 1,
and p is 1 or 2, and
iv) a residue having the generalized formula:
-49-
Image
wherein R C5 and R C6 are independently H or C1 to C6 alkyl, Q is H or C1 to
C6 alkyl, s is 0, 1 or
2, t is independently 0, 1, 2, or 3, q is 0, 1, 2, or 3, and r is 1 or 2 or
water soluble salts thereof.
31. A composition as recited in claim 30 wherein the aminohydroxysuccinic acid
is
selected from the group consisting of iminodi(2-hydroxysuccinic acid), N, N'-
Bis(2-
hydroxysuccinyl)-1,6-hexanediamine, N,N'-Bis(2-hydroxysuccinyl)-m-
xylylenediamine, and
the water-soluble salts thereof.
32. A composition as recited in claim 2 wherein the polyepoxysuccinic acid has
the
generalized formula
Image
where 1 ranges from about 2 to about 50, M T is hydrogen or a water soluble
cation and R T is
hydrogen, C1-4 alkyl or C1-4 substituted alkyl, or water soluble salts
thereof.
33. A composition as recited in claim 32 wherein R T is hydrogen and 1 ranges
from
about 2 to about 10.
34. A composition as recited in claim 32 wherein R T is hydrogen and 1 is from
about 4 to about 7.
35. A composition as recited in claim 2 wherein the said other aqueous system
treatment material is a mixture of orthophosphoric acid or its water-soluble
salts and a
polyepoxysuccinic acid having the generalized formula
Image
-50-
where 1 ranges from about 2 to about 50, M T is hydrogen or a water soluble
cation and R T is
hydrogen, C1-4 alkyl or C1-4 substituted alkyl, or water soluble salts
thereof.
36. A composition as recited in claim 35 wherein said polyepoxysuccinic acid
has
R T as hydrogen and 1 is from about 2 to about 10.
37. A composition as recited in claim 35 wherein said polyepoxysuccinic acid
has
R T as hydrogen and 1 is from about 4 to about 7.
38. A composition as recited in claim 2 wherein the modified polyepoxysuccinic
acid has the generalized formula
Image
wherein R D1, when present, is H, a substituted or non-substituted alkyl or
aryl moiety having a
carbon chain up to the length where solubility in aqueous solution is lost, or
a repeat unit
obtained after polymerization of an ethylenically unsaturated compound; R D2
and R D3 each
independently are H, C1 to C4 alkyl or C1 to C4 substituted alkyl; Z D is O,
S, NH, or NR D1,
where R D1 is as described above, n is a positive integer greater than 1; f is
a positive integer;
and M D is H, a water soluble cation, or a non-substituted lower alkyl group
having from 1 to 3
carbon atoms (when R D1 is not present, Z D may be M D O3S, where M D is as
described above).
39. The composition as recited in claim 38 wherein R D1 is the meta-xylylene
moiety (meta-CH2-C6H4-CH2-), R D2 and R D3 are both H, Z D is -NH, M D is Na
or H, and f=2,
and u is a positive integer greater than 1.
40. A composition as recited in claim 2 wherein said monophosphonic acid has
the
generalized formula
-51-
Image
wherein R F is a C1 to C12 straight or branched chain alkyl residue, a C2 to
C12 straight or
branched chain alkenyl residue, a C5 to C12 cycloalkyl residue, a C6 to C10
aryl residue, or a C7
to C12 aralkyl residue, and where R F may additionally be singly or multiply
substituted with
groups independently selected from the group consisting of hydroxyl, amino,
and halogen, or
the water soluble salts thereof.
41. A composition as recited in claim 2 wherein said diphosphonic acid has the
generalized formula
Image
wherein R K is a C1 to C12 straight or branched chain alkylene residue, a C2
to C12 straight or
branched chain alkenylene residue, a C5 to C12 cycloalkylene residue, a C6 to
C10 arylene
residue, or a C7 to C12 aralkylene residue where R K may additionally be
singly or multiply
substituted with groups independently selected from the group consisting of
hydroxyl, amino,
and halogen, or the water soluble salts thereof.
42. A composition as recited in claim 41 wherein said diphosphonic acid is 1-
hydroxyethane-1,1-diphosphonic acid or the water soluble salts thereof.
43. A composition as recited in claim 2 wherein said phosphonocarboxylic acid
has the
generalized formulas
Image
-52-
Image
where R H1 is H, alkyl, alkenyl, or alkinyl radical having 1 to 4 carbon
atoms, an aryl,
cycloalkyl, or aralkyl radical, or the radical selected from the following:
Image
where R H2 is H, alkyl radical of 1 to 4 carbon atoms, or a carboxyl radical;
and X H is
Image
selected from the following:
and where the -PO3H2 group is the phosphono group
Image
or the water-soluble salts thereof.
44. A composition as recited in claim 43 wherein said phosphonocarboxylic acid
is 2-phosphonobutane-1,2,4-tricarboxylic acid or the water soluble salts
thereof.
-53-
45. A composition as recited in claim 2 wherein said
hydroxyphosphonocarboxylic
acid has the generalized formula
Image
wherein R E is H, a C1 to C12 straight or branched chain alkyl residue, a C2
to C12 straight or
branched chain alkenyl residue, a C5 to C12 cycloalkyl residue, a C6 to C10
aryl residue, or a C7
to C12 aralkyl residue, X E is an optional group, which when present is a C1
to C10 straight or
branched chain alkylene residue, a C2 to C10 straight or branched chain
alkenylene residue, or a
C6 to C10 arylene residue or water soluble salts thereof.
46. A composition as recited in claim 45 wherein said
hydroxyphosphonocarboxylic acid is 2-hydroxy-phosphonoacetic acid or the water
soluble
salts thereof.
47. A composition as recited in claim 2 wherein said aminophosphonic acid has
the
generalized formula
Image
where R G2 is a lower alkylene having from about one to about four carbon
atoms, or an amine,
hydroxy, or halogen substituted lower alkylene; R G3 is R G2 - PO3H2, H, OH,
amino, substituted
amino, or R F, where R F is a C1 to C12 straight or branched chain alkyl
residue, a C2 to C12
straight or branched chain alkenyl residue, a C5 to C12 cycloalkyl residue, a
C6 to C10 aryl
residue, or a C7 to C12 aralkyl residue, and where R F may additionally be
singly or multiply
substituted with groups independently selected from the group consisting of
hydroxyl, amino,
and halogen, R G4 is R G3 or the group represented by the generalized formula:
-54-
Image
where R G5 and R G6 are each independently selected from the group consisting
of H, OH,
amino, substituted amino, and R F as previously defined; R G7 is R G5, R G6,
or the group R G2 -
PO3H2 with R G2 as previously defined; v is an integer from 1 to about 15; and
w is an integer
from 1 through about 14 or water soluble salts thereof.
48. A composition as recited in claim 47 wherein said aminophosphonic acid is
diethylenetriamine penta(methylenephosphonic acid) or the water soluble salts
thereof.
49. A composition as recited in claim 2 wherein said phosphonomethyl amine
oxide has
the generalized formula
Image
wherein either R A1 is selected from the group consisting of hydrocarbyl, and
hydroxy-
substituted, alkoxy-substituted, carboxyl-substituted and sulfonyl-substituted
hydrocarbyl; and
R A2 is selected from the group consisting of hydrocarbyl, and hydroxy-
substituted, alkoxy-
substituted, carboxyl-substituted and sulfonyl-substituted hydrocarbyl, -
CH2PO3H2, and
Image
-55-
or R A1 and R A2 together form an alicyclic ring having 3 to 5 carbon atoms in
the ring or a
water-soluble salt of said phosphonomethyl amine oxide, wherein said
hydrocarbyl includes
alkyl, aryl, and alkaryl groups which do not render the amine oxide insoluble
in water.
50. A composition as recited in claim 49 wherein said phosphonomethyl amine
oxide is
N,N-bis-phosphonomethylethanolamine N-oxide or the water soluble salts
thereof.
51. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
an oligomer, polymer, co-oligomer, or copolymer obtained from the
polymerization of one or
more unsaturated monomers in the presence of a phosphorus containing compound,
said
monomers containing one or more carboxyl groups or containing one or more
groups that
have been transformed after polymerization into carboxyl groups, and in which
the resulting
phosphorus containing carboxylate contains phosphorus incorporations that are
predominantly
or exclusively present as end-type phosphino species or the water soluble
salts thereof.
52. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
an oligomer, polymer, co-oligomer, or copolymer obtained from the
polymerization of one or
more unsaturated monomers in the presence of a phosphorus containing compound,
said
monomers containing one or more carboxyl groups or containing one or more
groups that
have been transformed after polymerization into carboxyl groups, and in which
the resulting
phosphorus containing carboxylate contains phosphorus incorporations that are
predominantly
or exclusively present as phosphono species or the water soluble salts
thereof.
53. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
an oligomer, polymer, co-oligomer, or copolymer obtained from the
polymerization of one or
more unsaturated monomers in the presence of a phosphorus containing compound,
said
monomers containing one or more carboxyl groups or containing one or more
groups that
have been transformed after polymerization into carboxyl groups, and in which
the resulting
phosphorus-containing carboxylate contains phosphorus incorporations that are
predominantly
or exclusively present as dialkylphosphino species or the water soluble salts
thereof.
54. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
an oligomer, polymer, co-oligomer, or copolymer obtained from the
polymerization of one or
more unsaturated monomers in the presence of a phosphorus containing compound,
said
monomers containing one or more carboxyl groups or containing one or more
groups that
have been transformed after polymerization into carboxyl groups, and in which
the resulting
phosphorus-containing carboxylate contains phosphorus incorporations which are
present as a
-56-
mix of phosphono, end-type phosphino, and dialkylphosphino species or the
water soluble
salts thereof.
55. A composition as recited in claim 51 wherein said unsaturated monomers are
chosen
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
56. A composition as recited in claim 51 wherein acrylic acid is the sole
unsaturated
monomer.
57. A composition as recited in claim 51 wherein the sole unsaturated monomer
is selected
from the group consisting of maleic acid, itaconic acid, and maleic anhydride.
58. A composition as recited in claim 51 wherein one unsaturated monomer is
acrylic acid
and the other unsaturated monomer is selected from the group consisting of
maleic acid,
itaconic acid, and maleic anhydride.
59. A composition as recited in claim 52 wherein said unsaturated monomers are
selected
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, S-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
60. A composition as recited in claim 52 wherein acrylic acid is the sole
unsaturated
monomer.
61. A composition as recited in claim 52 wherein the sole unsaturated monomer
is selected
from the group consisting of maleic acid, itaconic acid, and maleic anhydride.
62. A composition as recited in claim 52 wherein one unsaturated monomer is
acrylic acid
and the other unsaturated monomer is selected from the group consisting of
maleic acid,
itaconic acid, and maleic anhydride.
-57-
63. A composition as recited in claim 53 wherein said unsaturated monomers are
seleted
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydxophthalic
anhydride.
64. A composition as recited in claim 53 wherein acrylic acid is the sole
unsaturated
monomer.
65. A composition as recited in claim 53 wherein the sole unsaturated monomer
is selected
from the group consisting of maleic acid, itaconic acid, and maleic anhydride.
66. A composition as recited in claim 53 wherein one unsaturated monomer is
acrylic acid
and the other unsaturated monomer is selected from the group consisting of
maleic acid,
itaconic acid, and maleic anhydride.
67. A composition as recited in claim 54 wherein said unsaturated monomers are
selected
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-.tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
68. A composition as recited in claim 54 wherein acrylic acid is the sole
unsaturated
monomer.
69. A composition as recited in claim 54 wherein the sole unsaturated monomer
is selected
from the group consisting of maleic acid, itaconic acid, and maleic anhydride.
70. A composition as recited in claim 54 wherein one unsaturated monomer is
acrylic acid
and the other unsaturated monomer is selected from the group consisting of
maleic acid,
itaconic acid, and maleic anhydride.
71. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
a co-oligomer or copolymer obtained from the polymerization of two or more
unsaturated
monomers in the presence of a phosphorus containing compound, a major
proportion of
-58-
residues (more than 50% by weight) of the phosphorus-containing carboxylate
being derived
from carboxyl monomers which contain one or more carboxyl groups or which
contain one or
more groups that have been transformed after polymerization into carboxyl
groups, the
remaining residues being obtained from non-carboxyl monomers, and in which the
resulting
phosphorus-containing carboxylate contains phosphorus incorporations that are
predominantly
or exclusively present as end-type phosphino species or the water soluble
salts thereof.
72. A composition as recited in claim 71 wherein the non-carboxyl monomers are
selected
from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-hydroxy-3-
(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
and C1-C4 alkyl esters of acrylic or methacrylic acid, acrylamides, alkyl
substituted
acrylamides, allyl alcohol, 2-vinyl pyridine, 4-vinyl pyridine, N-
vinylpyrrolidone, N-
vinylformamide, N-vinylimidazole, vinyl acetate, hydrolyzed vinyl acetate, and
styrene.
73. A composition as recited in claim 71 wherein said carboxyl monomers are
selected
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic-acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
74. A composition as recited in claim 73 wherein the carboxyl monomer is
selected from
the group consisting of acrylic acid, maleic acid, itaconic acid, and maleic
anhydride.
75. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
a co-oligomer or copolymer obtained from the polymerization of two or more
unsaturated
monomers in the presence of a phosphorus containing compound, a major
proportion of
residues (more than 50% by weight) of the phosphorus-containing carboxylate
being derived
from carboxyl monomers which contain one or more carboxyl groups or which
contain one or
more groups that have been transformed after polymerization into carboxyl
groups, the
remaining residues being obtained from non-carboxyl monomers, and in which the
resulting
phosphorus-containing carboxylate contains phosphorus incorporations that are
predominantly
or exclusively present as phosphono species or the water soluble salts
thereof.
-59-
76. A composition as recited in claim 75 wherein the non-carboxyl monomers are
chosen
from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-hydroxy-3-
(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
and C1-C4 alkyl esters of acrylic or methacrylic acid, acrylamides, alkyl
substituted
acrylamides, allyl alcohol, 2-vinyl pyridine, 4-vinyl pyridine, N-
vinylpyrrolidone, N-
vinylformamide, N-vinylimidazole, vinyl acetate, hydrolyzed vinyl acetate, and
styrene.
77. A composition as recited in claim 75 wherein said carboxyl monomers are
chosen from
the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid, itaconic
acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic acid,
mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
78. A composition as recited in claim 77 wherein the carboxyl monomer is
chosen from
the group consisting of acrylic acid, maleic acid, itaconic acid, and maleic
anhydride.
79. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
a co-oligomer, or copolymer obtained from the polymerization two or more
unsaturated
monomers in the presence of a phosphorus containing compound, a proportion of
residues of
more than 50% by weight of the entire compound, in the phosphorus-containing
carboxylate
being derived from monomers containing one or more carboxyl groups or
containing one or
more groups that have been transformed after polymerization into carboxyl
groups, the
remaining residues being obtained from monomers which do not contain either
carboxyl
groups or groups that have been transformed after polymerization into carboxyl
groups or non-
carboxyl monomers, and in which the resulting phosphorus-containing
carboxylate contains
phosphorus incorporations that are predominantly or exclusively present as
dialkylphosphino
species or the water soluble salts thereof.
80. A composition as recited in claim 79 wherein the non-carboxyl monomers are
selected
from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-hydroxy-3-
(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
-60-
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
and C1-C4 alkyl esters of acrylic or methacrylic acid, acrylamides, alkyl
substituted
acrylamides, allyl alcohol, 2-vinyl pyridine, 4-vinyl pyridine, N-
vinylpyrrolidone, N-
vinylformamide, N-vinylimidazole, vinyl acetate, hydrolyzed vinyl acetate, and
styrene.
81. A composition as recited in claim 79 wherein said carboxyl monomers are
selected
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
82. A composition as recited in claim 81 wherein the carboxyl monomer is
selected from
the group consisting of acrylic acid, maleic acid, itaconic acid, and maleic
anhydride. 83.
A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is a
co-oligomer or copolymer obtained from the polymerization of two or more
unsaturated
monomers in the presence of a phosphorus containing compound, a major
proportion of
residues (more than 50% by weight) of the phosphorus-containing carboxylate
being derived
from carboxyl monomers which contain one or more carboxyl groups or which
contain one or
more groups that have been transformed after polymerization into carboxyl
groups, the
remaining residues being obtained from non-carboxyl monomers, and in which the
resulting
phosphorus-containing carboxylate contains phosphorus incorporations that are
present as a
mixture of phosphono, end-type phosphino, and dialkylphosphino species or the
water soluble
salts thereof.
84. A composition as recited in claim 83 wherein the non-carboxyl monomers are
selected
from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-hydroxy-3-
(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
esters of acrylic or methacrylic acid, C1-C4 alkyl esters of acrylic or
methacrylic acid,
acrylamides, alkyl substituted acrylamides, allyl alcohol, 2-vinyl pyridine, 4-
vinyl pyridine,
N-vinylpyrrolidone, N-vinylformamide, N-vinylimidazole, vinyl acetate,
hydrolyzed vinyl
acetate, and styrene.
-61-
85. A composition as recited in claim 83 wherein said carboxyl monomers are
chosen from
the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid, itaconic
acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic acid,
mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
86. A composition as recited in claim 85 wherein the carboxyl monomer is
selected from
the group consisting of acrylic acid, maleic acid, itaconic acid, and maleic
anhydride.
87. A composition as recited in claim 2 wherein said phosphorus-containing
carboxylate is
a phosphonic polymer having the generalized formula
Image
wherein X j is H, an alkali metal atom, an alkaline earth metal atom, or an
ammonium or amine
residue; and R j1 is a copolymer residue comprising two different residues
Image
wherein z is an integer ranging from 2 to 100, and wherein, in the first
residue, R j2 is -COOH,
and in the second residue, R j2 is -CONHC(CH3)2CH2SO3X j wherein X j is as
hereinbefore
defined.
88. A composition as recited in claim 2 wherein the aqueous system treatment
material is a
composition of up to 50% by weight of a phosphonosuccinic acid, based on the
weight of the
composition, a phosphonated dimer of alkali metal maleate, not more than a
minor proportion
by weight, based on the weight of the dimer, of higher phosphonated oligomers
of maleate;
and from 0.5 to 5% by weight of the composition of an alkali metal phosphate.
89. A composition as recited in claim 2 wherein the long chain fatty acid
derivative
of a sarcosine is chosen to be N-Lauroylsarcosine or the water soluble salts
thereof.
90. A composition as recited in claim 1 wherein the composition includes
water.
-62-
91. A composition as recited in claim 2 wherein the composition additionally
includes
water.
92. A composition as recited in claim 2 wherein said composition additionally
contains at
least one additive chosen from the group consisting of:
i) one or more dispersants
ii) one or more copper corrosion inhibitors
iii) one or more aluminum corrosion inhibitors
iv) one or more water-soluble metal salts of metals chosen from the group
zinc,
manganese, aluminum, tin, nickel, yttrium, and the rare earth metals
v) one or more water-soluble organic metal chelates of metals ions chosen from
the group
zinc, manganese, aluminum, tin, nickel, yttrium, and the rare earth metals,
where the organic
chelant is chosen to impart a desired level of water solubility of the metal
ion
vi) one or more scale control agents
vii) one or more sequestering agents
viii) one or more anti-foaming agents
ix) one or more oxidizing biocides
x) one or more non-oxidizing biocides
xi) one or more water-soluble alcohols capable of lowering the freezing point
of an
aqueous system
xii) one or more ionic freezing point depressants
xiii) one or more p H adjusting agents
xiv) one or more inert tracers
xv) one or more active tracers
xvi) one or more water insoluble organic lubricants
xvii) one or more water soluble lubricants
xviii) one or more surfactants
xix) one or more calcium hardness adjusting agents, and
xx) one or more coloring agents
93. A composition as recited in claim 92 wherein the composition additionally
includes
water.
94. A composition as recited in claim 92 where the dispersant is a water-
soluble sulfonated
polymer or copolymer obtained from the polymerization of one or more
ethylenically
unsaturated monomers.
-63-
95. A composition as recited in claim 94 where the water-soluble sulfonated
copolymer is
about a 3:1 weight ratio copolymer of acrylic acid and allyl hydroxy propyl
sulfonate ether or
the water soluble salts thereof.
96. A composition as recited in claim 92 where the dispersant is a copolymer
of
diiosbutylene and malefic anhydride with molecular weight < 10,000 or its
water soluble salts.
97. A composition as recited in claim 92 where the copper corrosion inhibitor
is
tolyltriazole.
98. A composition as recited in claim 92 where the copper corrosion inhibitor
is a mixed
tolyltriazole composition including at least 65% of the 5-methylbenzotriazole
isomer by
weight.
99. A composition as recited in claim 92 where the copper corrosion inhibitor
is
benzotriazole.
100. A composition as recited in claim 92 where the copper corrosion inhibitor
is
mercaptobenzothiazole.
101. A composition as recited in claim 92 where the copper corrosion inhibitor
is an
akyl or alkoxy substituted benzotriazole wherein the substitution occurs on
the 4 or 5 position
of the benzene ring.
102. A composition as recited in claim 101 wherein the substituent is chosen
from
the group consisting of a n-butyl and hexyloxy.
103. A composition as recited in claim 92 where the copper corrosion inhibitor
is 1-
phenyl-5-mercaptotetrazole.
104. A composition as recited in claim 92 where the copper corrosion inhibitor
is a
halogen-tolerant azole.
105. A composition as recited in claim 104 where the halogen-tolerant azole is
chloro-tolyltriazole.
106. A composition as recited in claim 92 where the aluminum corrosion
inhibitor is
a water-soluble nitrate salt.
107. A composition as recited in claim 106 where the water-soluble nitrate
salt is
sodium nitrate.
108. A composition as recited in claim 92 where the water-soluble metal salt
is
obtained from zinc.
109. A composition as recited in claim 108 where the zinc salt is the sulfate,
chloride, acetate, or nitrate salt.
-64-
110. A composition as recited in claim 92 where the metal salt is obtained
from
manganese in the +2 oxidation state.
111. A composition as recited in claim 110 where the manganese salt state is
the
sulfate, chloride, acetate, or nitrate salt.
112. A composition as recited in claim 92 where the metal salt is obtained
from
lanthanum or a mixture of rare earth metals containing lanthanum.
113. A composition as recited in claim 112 where the lanthanum salt or mixture
of
rare earth metal salts containing lanthanum are independently selected from
the group
consisting of sulfate, chloride, acetate, and nitrate salts.
114. A composition as recited in claim 92 where the sequestering agent is
selected
from the group consisting of ethylenediaminetetra(acetic acid)
nitrolotriacetic acid, N,N-di(2-
hydroxyethyl)glycine and the water soluble salts thereof.
115. The composition as recited in claim 2 wherein the alkali metal silicate
is
sodium metasilicate.
116. A composition as recited in claim 92 where the anti-foaming agent is
selected
from the group consisting of silicones, polydimethylsiloxanes,
distearylsebacamide,
distearyladipamide, fatty alcehols; and ethylene oxide condensates of fatty
alcohols.
117. A composition as recited in claim 92 where the oxidizing biocide is
selected
from the group consisting of chorine, hypochlorite, bromine, hypobromite,
chlorine donor
compounds, bromine donor compounds, peracetic acid, inorganic peroxides and
peroxide
generators, chlorine dioxide, ozone and mixtures thereof.
118. A composition as recited in claim 92 where the non-oxidizing biocide is
selected from the group consisting of amines, quaternary ammonium compounds, 2-
bromo-2-
nitropropane-1,3-diol, .beta.-bromonitrostyrene, dodecylguanidine
hydrochloride, 2,2-dibromo-3-
nitrilopropionamide, gluteraldhyde, chlorophenols, sulphones, methylene bis
thiocyanates,
methylene bis carbamates, isothiazolones, brominated propionamides, triazines,
phosphonium
compounds, organometallic compounds and mixtures thereof.
119. A composition as recited in claim 92 where the non-oxidizing biocide is a
mixture of (a) 2-bromo-2-nitropropane-1,3-diol (BNPD) and (b) a mixture of
about 75% 5-
chloro-2-methyl-4-isothiazolin-3-one and about 25% 2-methyl-4-isothiazolin-3-
one, the
weight ratio said BNPD (a) to said mixture (b) being about 16:1 to about 1:1.
-65-
120. A composition as recited in claim 92 where the water-soluble alcohol
freezing
point depressant is selected from the group consisting of ethylene glycol,
propylene glycol,
ethanol, glycerol, isopropanol, methanol and mixtures thereof.
121. A composition as recited in claim 92 where the ionic freezing point
depressant
is selected from the group consisting of calcium chloride, sodium chloride,
lithium bromide,
and lithium chloride.
122. A composition as recited in claim 92 where the pH adjusting agent is
selected
from the group consisting of sodium hydroxide, potassium hydroxide, lithium
hydroxide,
hydrochloric acid, sulfuric acid, nitric acid, carbon dioxide, ammonia,
organic acids such as
oxalic acid, alkali metal carbonates, and akali metal bicarbonates.
123. A composition as recited in claim 92 where the inert tracer is selected
from the
group consisting of soluble lithium salts, transition metals, and fluorescent
materials.
124. A composition as recited in claim 92 where the active tracer is selected
from
the group consisting of fluorescently tagged polymers, polymers containing a
photo-inert,
latently detectable moiety, water soluble molybdate salts, and azole-based
copper corrosion
inhibitors.
125. A composition as recited in claim 92 where the water insoluble organic
lubricant is selected from the group consisting of naturally occurring oils
and synthetic oils.
126. A composition as recited in claim 92 where the surfactant is selected
from the
group consisting of anionic, cationic, amphoteric, and nonionic surfactants.
127. A composition as recited in claim 92 where the calcium hardness adjusting
agent is selected from the group consisting of the bicarbonate, carbonate,
chloride, sulfate, and
acetate salts of calcium, calcium hydroxide and calcium oxide.
128. A composition as recited in claim 92 where the coloring agent is a water
soluble dye.
129. A composition as recited in claim 2 wherein said monofluorophosphate is
sodium monofluorophosphate.
130. A method for controlling the corrosion of metals in contact with an
aqueous
system having a pH of about 6 or greater which comprises introducing into said
system a
combination of:
(a) a tetrazolium compound of the formula:
-66-
Image
wherein R1, R2 and R3 are selected from the group consisting of lower alkyl,
branched lower
alkyl, aryl, substituted aryl, alkylaryl, substituted alkylaryl and
heterocyclic substituted aryl,
with the proviso that neither R1, R2, or R3 contain more than 14 carbon atoms;
and n is 1 or 2,
such tetrazolium compound optionally having associated water soluble ionic
species if needed
to obtain a neutral charge, and
(b) at least one other aqueous system treatment material chosen so that the
material does
not substantially reduce the tetrazolium compound.
131. The method as recited in claim 130 wherein said other aqueous system
treatment material is selected from the group consisting of inorganic
phosphates, borates,
nitrites, compounds that release a metal anion in water, 2,3-dihydroxybenzoic
acid, 1,10-
phenanthroline, polycarboxylates, hydrocarbyl polycarboxylates, akyl
hydroxycarboxylic
acids, aminohydroxysuccinic acids, carboxyamines, polyepoxysuccinic acids,
modified
polyepoxysuccinic acids, monophosphonic acids, diphosphonic acids,
phosphonocarboxylic
acids, hydroxyphosphonocarboxylic acids, aminophosphonic acids,
phosphonomethylamine
oxides, polymeric amine oxides, polyetherpolyaminomethylene phosphonates,
polyetherpolyamino-methylene phosphonate N-oxides, iminoakylenephosphonic
acids, long
chain fatty acid derivatives of sarcosine; telomeric, co-telomeric, polymeric,
or copolymeric
phosphorus-containing carboxylates, alkali metal silicates,
monofluorophosphate, amines,
diamines, alkanolamines, ether amines, fatty amines and diamines, quaternized
amines,
oxyalkylated amines, akyl pyridines, tetrazoles, imidazoline and substituted
imidazolines,
amidoamines, polyamines, polyakylenepolyamines, alkyl derivatives of benzene
sulfonic acid,
benzoates and substituted benzoates, aminobenzoates, salicylates, dimer-trimer
acids,
petroleum oxidates, borogluconates; lignins, lignosulfonates, tannins;
straight chain C5-C11
-67-
monocarboxylates and C4-C15 .alpha.,.omega.-dicarboxylates; amine salts of
carboxylic acids and
mercaptocarboxylic acids, amino acids, polyamino acids, hydroxyether acids and
related
lactone compounds, N-acyliminodiacetic acids; triazine di- and tri-carboxylic
acis, phospho-
and phosphate esters; and monofluorophosphates; water soluble salts thereof,
and mixtures
thereof.
132. A method as recited in claim 130 wherein said tetrazolium compound is
selected from the group consisting of the water soluble salts of Nitro Blue
Tetrazolium (2,2'-
Di-p-nitrophenyl-5,5'-distyryl-3,3'-[3,3'-dimethoxy-4,4'-biphenylene]
ditetrazolium),
Distyryl Nitroblue Tetrazolium (2,2'-Di-p-nitrophenyl-5,5'-distyryl-3,3'-[3,3'-
dimethoxy-
4,4'-biphenylene] ditetrazolium), Tetranitro Blue Tetrazolium (3,3'-(3,3'-
Dimethoxy-4,4'-
biphenylene)-bis-[2,5-p-nitrophenyl-2H-tetrazolium) and Iodonitro Tetrazolium
(2-(4-
lodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium).
133. A method as recited in claim 131 wherein said tetrazolium compound is
selected from the group consisting of the water soluble salts of Nitro Blue
Tetrazolium (2,2'-
Di-p-nitrophenyl-5,5'-distyryl-3,3'-[3,3'-dimethoxy-4,4'-biphenylene]
ditetrazolium),
Distyryl Nitroblue Tetrazolium (2,2'-Di-p-nitrophenyl-5,5'-distyryl-3,3'-[3,3'-
dimethoxy-
4,4'-biphenylene] ditetrazolium);-Tetranitro Blue Tetrazolium (3,3'-(3,3'-
Dimethoxy-4,4'-
biphenylene)-bis-[2,5-p-nitrophenyl-2H-tetrazolium) and Iodonitro Tetrazolium
(2-(4-
lodophenyl)-3-(4-nitrophenyl)-S-phenyltetrazolium).
134. A method as recited in claim 130 wherein said water soluble ionic species
are
anions selected from the group consisting of halogens, nitrates, nitrites,
carbonates,
bicarbonates, sulfates, phosphates, and transition metal oxygenates.
135. A method as recited in claim 134 wherein said halogens are selected from
the
group consisting of chlorides, fluorides, bromides and iodides.
136. A method as recited in claim 135 wherein said halogen is chloride.
137. A method as recited in claim 134 wherein said transition metal oxygenate
is
selected from the group consisting of molybdate, chromate, and tungstate.
138. A method as recited in claim 137 wherein said transition metal oxygenate
is
molybdate.
139. A method as recited in claim 131 wherein said inorganic phosphates are
orthophosphates, polyphosphates, water soluble salts thereof and mixtures
thereof.
-68-
140. A method as recited in claim 131 wherein said inorganic phosphates are a
mixture of orthophosphoric acid and pyrophosphoric acid or the water-soluble
salts thereof.
141. A method as recited in claim 131 wherein said borate is a water-soluble
borate
selected from the group consisting of tetraborates, metaborates, and
orthoborates. 142. A
method as recited in claim 141 wherein said water-soluble borate is sodium
tetraborate or a
hydrate of sodium tetraborate.
143. A method as recited in claim 131 wherein said nitrite is sodium nitrite.
144. A method as recited in claim 131 wherein the metal anion releasing
compounds
are selected from the group consisting of the water soluble salts of
molybdate, tungstate,
vanadate, metavanadate, and chromate.
145. A method as recited in claim 144 wherein the water soluble salt of a
molybdate
is sodium molybdate or a hydrate of sodium molybdate.
146. A method as recited in claim 131 wherein said polycarboxylates comprise
aliphatic compounds containing between about 4 and about 20 carbon atoms which
are
multiply substituted with carboxylate groups or water soluble salts thereof.
147. A method as recited in claim 146 wherein said polycarboxylate is 1,2,3,4-
butanetctracarboxylic acid.
148. A method as recited in claim 131 wherein said polycarboxylate is a
homopolymer obtained from the polymerization of an ethylenically unsaturated
monomer
containing one or more carboxyl groups.
149. A method as recited in claim 148 wherein said homopolymer is polyacrylic
acid or its water soluble salts.
150. A method as recited in claim 148 wherein said homopolymer is polymaleic
acid or its water soluble salts.
151. A method as recited in claim 148 wherein said homopolymer is polymaleic
anhydride or its water soluble salts.
152. A method as recited in claim 131 wherein said polycarboxylate is a
copolymer
obtained from the polymerization of two or more different ethylenically
unsaturated
monomers, each of said monomers containing one or more carboxyl groups.
153. A method as recited in claim 131 wherein said alkyl hydroxycarboxylic
acid
has the generalized formula
HOOC -(R B1)a -(R B2)b -(R B2)c - R B4
-69-
where a, b, and c are integers from 0 to 6 and (a+b+c)>0 where R B1, R B2, RB3
comprise C=O or
CYZ, where Y and Z are separately selected from the group consisting of H, OH,
CHO,
COOH, CH3, CH2(OH), CH(OH)2, CH2(COOH), CH(OH)COOH, CH2(CHO) and
CH(OH)CHO, so selected that the molecule has a minimum of one OH group when
written in
its fully hydrated form and R B4 is either H or COOH, including the various
stereoisomers and
chemically equivalent cyclic, dehydrated, and hydrated forms of these acids
and hydrolyzable
esters and acetals that form the above compounds in water or the water soluble
salts of such
alkyl hydroxycarboxylic acids.
154. A method as recited in claim 153 wherein said akyl hydroxycarboxylic acid
is
chosen from the group consisting of tartaric acid, mesotartaric acid, citric
acid, gluconic acid,
glucoheptonic acid, ketomalonic acid, saccharic acid and the water soluble
salts thereof.
155. A method as recited in claim 131 wherein the said other aqueous system
treatment materials is a mixture of orthophosphoric acid or its water-soluble
salts and at least
one alkyl hydroxycarboxylic acid having the generalized formula:
HOOC -(R B1)a -(R B2)b -(R B3)c - R B4
where a, b, and c are integers from 0 to 6 and (a+b+c)>0 where R B1, R B2, R
B3 comprise C=O or
CYZ, where Y and Z are separately selected from the group of H, OH, CHO, COOH,
CH3,
CH2(OH), CH(OH)2, CH2(COOH), CH(OH)COOH, CH2(CHO) and CH(OH)CHO, so selected
that the molecule has a minimum of one OH group when written in its fully
hydrated form and
RB4 is either H or COOH, including the various stereoisomers and chemically
equivalent
cyclic, dehydrated, and hydrated forms of these acids and hydrolyzable esters
and acetals that
form the above compounds in water or the water soluble salts of such alkyl
hydroxycarboxylic
acids, and the water soluble salts thereof.
156. A method as recited in claim 155 wherein the hydroxycarboxylic acid is
selected from the group consisting of tartaric acid, mesotartaric acid, citric
acid, gluonic acid,
glucoheptonic acid, ketomalonic acid, saccharic acid and the water soluble
salts thereof.
157. A method as recited in claim 131 wherein said aminohydroxysuccinic acid
has
the generalized formula
-70-
Image
wherein R C1, is H or C1 to C4 alkyl, optionally substituted with -OH, -CO2H, -
SO3H, or phenyl,
C4 to C7 cycloalkyl, or phenyl which is optionally substituted with -OH or -
CO2H, and R C2 is
H, C1 to C6 alkyl, optionally substituted with -OH or -CO2H (specifically
including the moiety
-CH(CO2H)CH(OH)(CO2H)); and
Image
-71-
wherein R c2 is as above, and Z c is selected from the group consisting of
i)- (CH2)k wherein k is an integer from 2 to 10,
ii) -(CH2)2-X c-(CH2)2 wherein X c is -O-, -S-, -NR C3-, wherein RC3 is
selected from the
group consisting of H, C1 to C6 alkyl, hydroxyalkyl, carboxyalkyl, acyl, and -
C(O)ORC4
wherein R C4 is selected from the group consisting of C to C6 alkyl or benzyl
and a residue
having the general formula:
Image
wherein RC2 is as above,
iii) a residue having the generalized formula
Image
wherein Y is H, C1 to C6 alkyl, alkoxy, halogen, - CO2H, or - SO3H, m is
independently 0 or 1,
and p is 1 or 2, and
iv) a residue having the generalized formula:
Image
wherein R C5 and R C6 are independently H or C1 to C6 alkyl, Q is H or C1 to
C6 alkyl, s is 0, 1 or
2, t is independently 0, 1, 2, or 3, q is 0, 1, 2, or 3, and r is 1 or 2 or
water soluble salts thereof.
158. A method as recited in claim 157 wherein the aminohydroxysuccinic acid is
selected from the group consisting of iminodi(2-hydroxysuccinic acid), N, N'-
Bis(2-
-72-
hydroxysuccinyl)-1,6-hexanediamine, N,N'-Bis(2-hydroxysuccinyl)-m-
xylylenediamine, or
the water-soluble salts thereof.
159. A method as recited in claim 131 wherein said other aqueous system
treatment material is a mixture of orthophosphoric acid or its water-soluble
salts and at least
one aminohydroxysuccinic acid wherein said aminohydroxysuccinic acid has the
generalized
formula
Image
wherein RC1, is H or C1 to C4 alkyl, optionally substituted with optionally
substituted with -OH,
-CO2H, -SO3H, or phenyl, C4 to C7 cycloalkyl, or phenyl which is optionally
substituted with
-OH or -CO2H, and RC2 is H, C1 to C6 alkyl, optionally substituted with -OH or
CO2H
(specifically including the moiety -CH(CO2H)CH(OH)(CO2H)); and
Image
wherein RC2 is as above, and Z c is selected from the group consisting of
i)- (CH2)k wherein k is an integer from 2 to 10,
ii) -(CH2)2 X c-(CH2)2 wherein X c is -O-, -S-, or -NR C3, wherein RC3 is
selected from
the group consisting of H, C1 to C6 alkyl, hydroxyalkyl, carboxyalkyl, acyl, -
C(O)ORC4
wherein RC4 is selected from the group consisting of C1 to C6 alkyl or benzyl
and a residue
having the general formula:
-73-
Image
wherein R C2 is as above,
iii) a residue having the generalized formula
Image
wherein Y is H, C1 to C6 alkyl, alkoxy, halogen, -CO2H, or - SO3H, m is
independently 0 or 1,
and p is 1 or 2, and
iv) a residue having the generalized formula:
Image
wherein RC5 and RC6 are independently H or C1 to C6 alkyl, Q is H or C1 to C6
alkyl, s is 0, 1 or
2, t is independently 0, 1, 2, or 3, q is 0, 1, 2, or 3, and r is 1 or 2 or
water soluble salts thereof.
160. A method as recited in claim 159 wherein the aminohydroxysuccinic acid is
selected from the group consisting of iminodi(2-hydroxysuccinic acid), N, N'-
Bis(2-
hydroxysuccinyl)-1,6-hexanediamine, N,N'-Bis(2-hydroxysuccinyl)-m-
xylylenediamine, and
the water-soluble salts thereof.
161. A method as recited in claim 131 wherein the polyepoxysuccinic acid has
the generalized formula:
-74-
Image
where 1 ranges from about 2 to about 50, M T is hydrogen or a water soluble
cation and R T is
hydrogen, C1-4 alkyl or C1-4 substituted alkyl.
162. A method as recited in claim 161 wherein R T is hydrogen and 1 ranges
from
about 2 to about 10.
163. A method as recited in claim 161 wherein R T is hydrogen and 1 is from
about 4
to about 7.
164. A method as recited in claim 131 wherein the said other aqueous system
treatment material is a mixture of orthophosphoric acid or its water-soluble
salts and a
polyepoxysuccinic acid having the generalized formula:
Image
where 1 ranges from about 2 to about 50, M T is hydrogen or a water soluble
cation and R T is
hydrogen, C1-4 alkyl or C1-4 substituted alkyl, or the water soluble salts
thereof.
165. A method as recited in claim 164 wherein said polyepoxysuccinic acid has
R T
as hydrogen and 1 is from about 2 to about 10.
166. A method as recited in claim 164 wherein said polyepoxysuccinic acid has
R T
as hydrogen and 1 is from about 4 to about 7.
167. A method as recited in claim 131 wherein the modified polyepoxysuccinic
acid
has the generalized formula
-75-
Image
wherein R D1, when present, is H, a substituted or non-substituted alkyl or
aryl moiety having a
carbon chain up to the length where solubility in aqueous solution is lost, or
a repeat unit
obtained after polymerization of an ethylenically unsaturated compound; R D2
and R D3 each
independently are H, C1 to C4 alkyl or C1 to C4 substituted alkyl; Z D is O,
S, NH, or NR D1,
where R D1, is as described above, n is a positive integer greater than 1; f
is a positive integer;
and Mp is H, a water soluble cation, or a non-substituted lower alkyl group
having from 1 to 3
carbon atoms (when R D1, is not present, Z D may be M D O3S, where M D is as
described above).
168. A method as recited in claim 167 wherein R DI, is the meta-xylylene
moiety
(meta-CH2-C6H4 CH2-), R D2 and R D3 are both H, Z D is -NH, M D is Na or H,
and f=2, and a is a
positive integer greater thank.
169. The method as recited in claim 131 wherein said monophosphonic acid has
the
generalized formula:
Image
wherein R F is a C1 to C12 straight or branched chain alkyl residue, a C2 to
C12 straight or
branched chain alkenyl residue, a C5 to C12 cycloalkyl residue, a C6 to C10
aryl residue, or a C7
to C12 aralkyl residue, and where R F may additionally be singly or multiply
substituted with
groups independently chosen from hydroxyl, amino, or halogen, or the water
soluble salts
thereof.
170. A method as recited in claim 131 wherein said diphosphonic acid has the
generalized formula:
-76-
wherein R K is a C1 to C12 straight or branched chain alkylene residue, a C2
to C12 straight or
branched chain alkenylene residue, a C5 to C12 cycloalkylene residue, a C6 to
C10 arylene
residue, or a C7 to C12 aralkylene residue where R K may additionally be
singly or multiply
substituted with groups independently chosen from hydroxyl, amino, or halogen,
or the water
soluble salts thereof.
171. A method as recited in claim 170 wherein said diphosphonic acid is is 1-
hydroxyethane-1,1-diphosphonic acid or the water soluble salts thereof.
172. A method as recited in claim 131 wherein said phosphonocarboxylic acid
has
the generalized formulas
Image
where R H1, is H, an alkyl, alkenyl, or alkinyl radical having 1 to 4 carbon
atoms, an aryl,
cycloalkyl, or aralkyl radical, or the radical selected from the following:
Image
where R H2 is H, alkyl radical of 1 to 4 carbon atoms, or a carboxyl radical;
and X H is selected
from the following:
-77-
Image
and where the -PO3H2 group is the phosphono group
Image
or the water-soluble salts thereof.
173. A method as recited in claim 172 wherein said phosphonocarboxylic acid is
2-
phosphonobutane-1,2,4-tricarboxylic acid or the water soluble salts thereof.
174. A method as recited in claim 131 wherein said hydroxyphosphonocarboxylic
acid has the generalized formula
Image
wherein RE is H, a C1 to C12 straight or branched chain alkyl residue, a C2 to
C12 straight or
branched-chain alkenyl residue; a C5 to C12 cycloalkyl residue, a C6 to C10
aryl residue, or a C7
to C12 aralkyl residue, X E is an optional group, which when present is a C1
to C10 straight or
branched chain alkylene residue, a C2 to C10 straight or branched chain
alkenylene residue, or a
C6 to C10 arylene residue or water soluble salts thereof.
175. A method as recited in claim 174 wherein said hydroxyphosphonocarboxylic
acid is 2-hydroxy-phosphonoacetic acid or the water soluble salts thereof.
176. A method as recited in claim 131 wherein said aminophosphonic acid has
the
generalized formula:
-78-
Image
where R G2 is a lower alkylene having from about one to about four carbon
atoms, or an amine,
hydroxy, or halogen substituted lower alkylene; R G3 is R G2 ~ PO3H2, H, OH,
amino, substituted
amino, or R F, where R F is a C1 to C12 straight or branched chain alkyl
residue, a C2 to C12
straight or branched chain alkenyl residue, a C5 to C12 cycloalkyl residue, a
C6 to C10 aryl
residue, or a C7 to C12 aralkyl residue, and where R F may additionally be
singly or multiply
substituted with groups independently selected from the group consisting of
hydroxyl, amino,
and halogen, R G4 is R G3 or the group represented by the generalized formula:
Image
where R G5 and R G6 are each independently selected from the group consisting
of H, OH,
amino, substituted amino, and R F as previously defined; R G7 is R G5, R G6,
or the group R G2-
PO3H2 with R G2 as previously defined; v is an integer from 1 to about 15; and
w is an integer
from 1 through about 14 or water soluble salts thereof.
177. A method as recited in claim 176 wherein said aminophosphonic acid is
diethylenetriamine penta(methylenephosphonic acid) or the water soluble salts
thereof.
178. A method as recited in claim 131 wherein said phosphonomethyl amine oxide
has the generalized formula
Image
-79-
wherein either R A1 is selected from the group consisting of hydrocarbyl, and
hydroxy-
substituted, alkoxy-substituted, carboxyl-substituted and sulfonyl-substituted
hydrocarbyl; and
R A2 is selected from the group consisting of hydrocarbyl, and hydroxy-
substituted, alkoxy-
substituted, carboxyl-substituted and sulfonyl-substituted hydrocarbyl, -
CH2PO3H2, and
Image
or R A1 and R A2 together form an alicyclic ring having 3 to 5 carbon atoms in
the ring or a
water-soluble salt of said phosphonomethyl amine oxide. Hydrocarbyl includes
alkyl, aryl,
and alkaryl groups which do not render the amine oxide insoluble in water.
179. A method as recited in claim 178 wherein said phosphonomethyl amine oxide
is N,N-bis-phosphonomethylethanolamine N-oxide or the water soluble salts
thereof.
180. A method as recited in claim 131 wherein said phosphorus-containing
carboxylate is an oligomer, polymer, co-oligomer, or copolymer obtained from
the
polymerization of one or more unsaturated monomers in the presence of a
phosphorus
containing compound, said monomers containing one or more carboxyl groups or
containing
one or more groups that have been transformed after polymerization into
carboxyl groups, and
in which the resulting phosphorus containing carboxylate contains phosphorus
incorporations
that are predominantly or exclusively present as end-type phosphino species or
the water
soluble salts thereof.
181. A method as recited in claim 131 wherein said phosphorus-containing
carboxylate is an oligomer, polymer, co-oligomer, or copolymer obtained from
the
polymerization of one or more unsaturated monomers in the presence of a
phosphorus
containing compound, said monomers containing one or more carboxyl groups or
containing
one or more groups that have been transformed after polymerization into
carboxyl groups, and
in which the resulting phosphorus containing carboxylate contains phosphorus
incorporations
that are predominantly or exclusively present as phosphono species or the
water soluble salts
thereof.
182. A method as recited in claim 131 wherein said phosphorus-containing
carboxylate is an oligomer, polymer, co-oligomer, or copolymer obtained from
the
-80-
polymerization of one or more unsaturated monomers in the presence of a
phosphorus
containing compound, said monomers containing one or more carboxyl groups or
containing
one or more groups that have been transformed after polymerization into
carboxyl groups, and
in which the resulting phosphorus-containing carboxylate contains phosphorus
incorporations
that are predominantly or exclusively present as dialkylphosphino species or
the water soluble
salts thereof.
183. A method as recited in claim 131 wherein said phosphorus-containing
carboxylate is an oligomer, polymer, co-oligomer, or copolymer obtained from
the
polymerization of one or more unsaturated monomers in the presence of a
phosphorus
containing compound, said monomers containing one or more carboxyl groups or
containing
one or more groups that have been transformed after polymerization into
carboxyl groups and
in which the resulting phosphorus-containing carboxylate contains phosphorus
incorporations
which are present as a mix of phosphono, end-type phosphino, and
dialkylphosphino species
or the water soluble salts thereof.
184. A method as recited in claim 180 wherein said unsaturated monomers are
chosen from the group consisting of acrylic acid, maleic acid, maleic
anhydride, methacrylic
acid, itaconic acid, crotonicacid; vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic
acid, acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic
acid, cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-
tetrahydrophthalic
anhydride, 5-norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-
2,3-dicarboxylic
anhydride, 3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic anhydride.
185. A method as recited in claim 180 wherein acrylic acid is the sole
unsaturated
monomer.
186. The method as recited in claim 180 wherein the sole unsaturated monomer
is
selected from the group consisting of maleic acid, itaconic acid, and maleic
anhydride.
187. A method as recited in claim 180 wherein one unsaturated monomer is
acrylic
acid and the other unsaturated monomer is selected from the group consisting
of maleic acid,
itaconic acid, and maleic anhydride.
188. A method as recited in claim 181 wherein said unsaturated monomers are
selected from the group consisting of acrylic acid, maleic acid, maleic
anhydride, methacrylic
acid, itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid,
citraconic acid, mesaconic
acid, acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic
-81-
acid, cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-
tetrahydrophthalic
anhydride, 5-norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-
2,3-dicarboxylic
anhydride, 3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic anhydride.
189. A method as recited in claim 181 wherein acrylic acid is the sole
unsaturated
monomer.
190. A method as recited in claim 181 wherein the sole unsaturated monomer is
selected from the group consisting of maleic acid, itaconic acid, and maleic
anhydride.
191. A method as recited in claim 181 wherein one unsaturated monomer is
acrylic
acid and the other unsaturated monomer is selected from the group consisting
of maleic acid,
itaconic acid, and maleic anhydride.
192. A method as recited in claim 182 wherein said unsaturated monomers are
selected from the group consisting of acrylic acid, maleic acid, maleic
anhydride, methacrylic
acid, itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid,
citraconic acid, mesaconic
acid, acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic
acid, cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-
tetrahydrophthalic
anhydride, 5-norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-
2,3-dicarboxylic
anhydride, 3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic anhydride.
193. A method as recited in claim 182 wherein acrylic acid is the sole
unsaturated
monomer.
194. A method as recited in claim 182 wherein the sole unsaturated monomer is
selected from the group consisting of maleic acid, itaconic acid, and maleic
anhydride.
195. A method as recited in claim 182 wherein one unsaturated monomer is
acrylic acid and
the other unsaturated monomer is selected from the group consisting of maleic
acid, itaconic
acid, and maleic anhydride.
196. A method as recited in claim 183 wherein said unsaturated monomers are
selected from the group consisting of acrylic acid, maleic acid, maleic
anhydride, methacrylic
acid, itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid,
citraconic acid, mesaconic
acid, acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic
acid, cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-
tetrahydrophthalic
anhydride, 5-norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-
2,3-dicarboxylic
-82-
anhydride, 3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic anhydride.
197. A method as recited in claim 183 wherein acrylic acid is the sole
unsaturated
monomer.
198. A method as recited in claim 183 wherein the sole unsaturated monomer is
selected from the group consisting of maleic acid, itaconic acid, and maleic
anhydride.
199. A method as recited in claim 183 wherein one unsaturated monomer is
acrylic
acid and the other unsaturated monomer is selected from the group consisting
of maleic acid,
itaconic acid, and maleic anhydride.
200. A composition as recited in claim 131 wherein said phosphorus-containing
carboxylate is a co-oligomer or copolymer obtained from the polymerization of
two or more
unsaturated monomers in the presence of a phosphorus containing compound, a
major
proportion of residues (more than 50% by weight) of the phosphorus-containing
carboxylate
being derived from carboxyl monomers which contain one or more carboxyl groups
or which
contain one or more groups that have been transformed after polymerization
into carboxyl
groups, the remaining residues being obtained from non-carboxyl monomers, and
in which the
resulting phosphorus-contain-ing carboxylate contains phosphorus
incorporations that are
predominantly or exclusively present as end-type phosphino species or the
water soluble salts
thereof.
201. A method as recited in claim 200 wherein the non-carboxyl monomers are
selected from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-
hydroxy-3-(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
and C1-C4 alkyl esters of acrylic or methacrylic acid, acrylamides, alkyl
substituted
acrylamides, allyl alcohol, 2-vinyl pyridine, 4-vinyl pyridine, N-
vinylpyrrolidone, N-
vinylformamide, N-vinylimidazole, vinyl acetate, hydrolyzed vinyl acetate, and
styrene.
202. A method as recited in claim 200 wherein said carboxyl monomers are
selected
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
-83-
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
203. A method as recited in claim 202 wherein the carboxyl monomer is selected
from the group consisting of acrylic acid, maleic acid, itaconic acid, and
maleic anhydride.
204. A composition as recited in claim 131 wherein said phosphorus-containing
carboxylate is a co-oligomer or copolymer obtained from the polymerization of
two or more
unsaturated monomers in the presence of a phosphorus containing compound, a
major
proportion of residues (more than 50% by weight) of the phosphorus-containing
carboxylate
being derived from carboxyl monomers which contain one or more carboxyl groups
or which
contain one or more groups that have been transformed after polymerization
into carboxyl
groups, the remaining residues being obtained from non-carboxyl monomers, and
in which the
resulting phosphorus-containing carboxylate contains phosphorus incorporations
that are
predominantly or exclusively present as phosphono species or the water soluble
salts thereof.
205. A method as recited in claim 204 wherein the non-carboxyl monomers are
chosen from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-
hydroxy-3-(2-
propenyloxyl propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
and C1-C4 alkyl esters of acrylic or methacrylic acid, acrylamides, alkyl
substituted
acrylamides, allyl alcohol, 2-vinyl pyridine, 4-vinyl pyridine, N-
vinylpyrrolidone, N-
vinylformamide, N-vinylimidazole, vinyl acetate, hydrolyzed vinyl acetate, and
styrene.
206. A method as recited in claim 204 wherein said carboxyl monomers are
chosen
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
207. A method as recited in claim 206 wherein the carboxyl monomer is chosen
from the group consisting of acrylic acid, maleic acid, itaconic acid, and
maleic anhydride.
208. A composition as recited in claim 131 wherein said phosphorus-containing
carboxylate is a co-oligomer or copolymer obtained from the polymerization of
two or more
-84-
unsaturated monomers in the presence of a phosphorus containing compound, a
major
proportion of residues (more than 50% by weight) of the phosphorus-containing
carboxylate
being derived from carboxyl monomers which contain one or more carboxyl groups
or which
contain one or more groups that have been transformed after polymerization
into carboxyl
groups, the remaining residues being obtained from non-carboxyl monomers, and
in which the
resulting phosphorus-containing carboxylate contains phosphorus incorporations
that are
predominantly or exclusively present as dialkylphosphino species or the water
soluble salts
thereof.
209. A method as recited in claim 208 wherein the non-carboxyl monomers are
selected from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-
hydroxy-3-(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
and C1-C4 alkyl esters of acrylic or methacrylic acid, acrylamides, alkyl
substituted
acrylamides, allyl alcohol, 2-vinyl pyridine, 4-vinyl pyridine, N-
vinylpyrrolidone, N-
vinylformamide, N-vinylimidazole, vinyl acetate, hydrolyzed vinyl acetate, and
styrene.
210. A method as recited in claim 208 wherein said carboxyl monomers are
selected
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile, alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
211. A method as recited in claim 210 wherein the carboxyl monomer is selected
from the group consisting of acrylic acid, maleic acid, itaconic acid, and
maleic anhydride.
212. A composition as recited in claim 131 wherein said phosphorus-containing
carboxylate is a co-oligomer or copolymer obtained from the polymerization of
two or more
unsaturated monomers in the presence of a phosphorus containing compound, a
major
proportion of residues (more than 50% by weight) of the phosphorus-containing
carboxylate
being derived from carboxyl monomers which contain one or more carboxyl groups
or which
contain one or more groups that have been transformed after polymerization
into carboxyl
groups, the remaining residues being obtained from non-carboxyl monomers, and
in which the
-85-
resulting phosphorus-containing carboxylate contains phosphorus incorporations
that are
present as a mixture of phosphono, end-type phosphino, and dialkylphosphino
species or the
water soluble salts thereof.
-86-
213. A method as recited in claim 212 wherein the non-carboxyl monomers are
selected from the group consisting of 2-acrylamido-2-methylpropanesulfonic, 2-
hydroxy-3-(2-
propenyloxy)propanesulfonic acid, 2-methyl-2-propene-1-sulfonic acid,
allylsulfonic acid,
allyloxybenzenesulfonic acid, styrenesulfonic acid, vinylsulfonic acid,
allylphosphonic acid,
vinylphosphonic acid, isopropenylphosphonic acid, phosphoethyl methacrylate,
hydroxyalkyl
esters of acrylic or methacrylic acid, C1-C4 alkyl esters of acrylic or
methacrylic acid,
acrylamides, alkyl substituted acrylamides, allyl alcohol, 2-vinyl pyridine, 4-
vinyl pyridine,
N-vinylpyrrolidone, N-vinylformamide, N-vinylimidazole, vinyl acetate,
hydrolyzed vinyl
acetate, and styrene.
214. A method as recited in claim 212 wherein said carboxyl monomers are
chosen
from the group consisting of acrylic acid, maleic acid, maleic anhydride,
methacrylic acid,
itaconic acid, crotonic acid, vinyl acetic acid, fumaric acid, citraconic
acid, mesaconic acid,
acrylonitrile, methacrylonitrile,'alpha-methylene glutaric acid,
cyclohexenedicarboxylic acid,
cis-1,2,3,6-tetrahydrophthalic anhydride, 3,6-epoxy-1,2,3,6-tetrahydrophthalic
anhydride, 5-
norbornene-2,3-dicarboxylic anhydride, bicyclo[2.2.2]-5-octene-2,3-
dicarboxylic anhydride,
3-methyl-1,2,6-tetrahydrophthalic anhydride, and 2-methyl-1,3,6-
tetrahydrophthalic
anhydride.
215. A method as recited in claim 214 wherein the carboxyl monomer is selected
from the group consisting of acrylic acid, maleic acid, itaconic acid, and
maleic anhydride.
216. A method as recited in claim 131 wherein said phosphorus-containing
carboxylate is a phosphonic polymer having the generalized formula
Image
wherein X J is H, an alkali metal atom, an alkaline earth metal atom, or an
ammonium or amine
residue; and R J1 is a copolymer residue comprising two different residues
Image
wherein z is an integer ranging from 2 to 100, and wherein, in the first
residue, R J2 is -COOH,
and in the second residue, R J2 is -CONHC(CH3)2CH2SO3X J, wherein X J is as
hereinbefore
defined.
-87-
217. A method as recited in claim 131 wherein the aqueous system treatment
material is a composition of up to 50% by weight of a phosphonosuccinic acid,
based on the
weight of the composition, a phosphonated dimer of alkali metal maleate, not
more than a
minor proportion by weight, based on the weight of the dimer, of higher
phosphonated
oligomers of maleate; and from 0.5 to 5% by weight of the composition of an
alkali metal
phosphate.
218. A method as recited in claim 131 wherein the long chain fatty acid
derivative
of a sarcosine is chosen to be N-Lauroylsarcosine or the water soluble salts
thereof.
219. A method as recited in claim 130 wherein the composition includes water.
220. A method as recited in claim 131 wherein the composition additionally
includes water.
221. A method as recited in claim 131 wherein said composition additionally
contains at least one additive chosen from the group consisting of:
i. one or more dispersants
ii. one or more copper corrosion inhibitors
iii. one or more aluminum corrosion inhibitors
iv. one or more water-soluble metal salts of metals chosen from the group
zinc,
manganese, aluminum, tin, nickel, yttrium, and the rare earth metals
v. one or more water-soluble organic metal chelates of metals ions chosen from
the group zinc, manganese, aluminum, tin, nickel, yttrium, and the rare earth
metals, where the organic chelant is chosen to impart a desired level of water
solubility of the metal ion
vi. one or more scale control agents
vii. one or more sequestering agents
viii. one or more anti-foaming agents
ix. one or more oxidizing biocides
x. one or more non-oxidizing biocides
xi. one or more water-soluble alcohols capable of lowering the freezing point
of an
aqueous system
xii. one or more ionic freezing point depressants
xiii. one or more pH adjusting agents
xiv. one or more inert tracers
xv. one or more active tracers
-88-
xvi. one or more water insoluble organic lubricants
xvii. one or more water soluble lubricants
xviii. one or more surfactants
xix. one or more calcium hardness adjusting agents, and
xx. one or more coloring agents.
222. A method as recited in claim 221 wherein the composition additionally
includes water.
223. A method as recited in claim 221 where the dispersant is a water-soluble
sulfonated polymer or copolymer obtained from the polymerization of one or
more
ethylenically unsaturated monomers.
224. A method as recited in claim 223 where the water-soluble sulfonated
copolymer is about a 3:1 weight ratio copolymer of acrylic acid and allyl
hydroxy propyl
sulfonate ether or the water soluble salts thereof.
225. A method as recited in claim 221 where the dispersant is a copolymer of
diiosbutylene and maleic anhydride with molecular weight<10,000 or its water
soluble salts.
226. A method as recited in claim 221 where the copper corrosion inhibitor is
tolyltriazole.
227. A method as recited in claim 221 where the copper corrosion inhibitor is
a
mixed maleic composition including at least 65% of the 5-methylbenzotriazole
isomer
by weight.
228. A method as recited in claim 221 where the copper corrosion inhibitor is
benzotriazole.
229. A method as recited in claim 221 where the copper corrosion inhibitor is
mercaptobenzothiazole.
230. A method as recited in claim 221 where the copper corrosion inhibitor is
an
akyl or alkoxy substituted benzotriazole wherein the substitution occurs on
the 4 or 5
position of the benzene ring.
231. A method as recited in claim 230 wherein the substituent is chosen from
the
group consisting of n-butyl and hexyloxy.
232. A method as recited in claim 221 where the copper corrosion inhibitor is
1-
phenyl-5-mercaptotetrazole.
233. A method as recited in claim 221 where the copper corrosion inhibitor is
a
halogen-tolerant azole.
-89-
234. A method as recited in claim 233 where the halogen-tolerant azole is
chloro-
tolyltriazole.
235. A method as recited in claim 221 where the aluminum corrosion inhibitor
is a
water-soluble nitrate salt.
236. A method as recited in claim 235 where the water-soluble nitrate salt is
sodium
nitrate.
237. A method as recited in claim 221 where the water-soluble metal salt is
obtained
from zinc.
238. A method as recited in claim 237 where the zinc salt is the sulfate,
chloride,
acetate, or nitrate salt.
239. A method as recited in claim 221 where the metal salt is obtained from
manganese in the +2 oxidation state.
240. A method as recited in claim 239 where the manganese salt state is the
sulfate,
chloride, acetate, or nitrate salt.
241. A method as recited in claim 221 where the metal salt is obtained from
lanthanum or a mixture of rare earth metals containing lanthanum.
242. A method as recited in claim 241 where the lanthanum salt or mixture of
rare
earth metal salts containing lanthanum are independently selected from the
group consisting of
the sulfate, chloride, acetate, and nitrate salts.
243. A method as recited in claim 221 where the sequestering agent is selected
from
the group consisting of ethylenediaminetetra(acetic acid), nitrolotriacetic
acid, N,N-di(2-
hydroxyethyl)glycine and the water soluble salts thereof.
244. A method as recited in claim 131 wherein the alkali metal silicate is
sodium
metasilicate.
245. A method as recited in claim 221 where the anti-foaming agent is selected
from
the group consisting of silicones, polydimethylsiloxanes,
distearylsebacamides,
distearyladipamide, fatty alcohols, and ethylene oxide condensates of fatty
alcohols.
246. A method as recited in claim 221 where the oxidizing biocide is selected
from
the group consisting of chorine, hypochlorite, bromine, hypobromite, chlorine
donor
compounds, bromine donor compounds, peracetic acid, inorganic peroxides and
peroxide
generators, chlorine dioxide, ozone and mixtures thereof.
247. A method as recited in claim 221 where the non-oxidizing biocide is
selected
from the group consisting of amines, quaternary ammonium compounds, 2-bromo-2-
-90-
nitropropane-1,3-diol, .beta.-bromonitrostyrene, dodecylguamctme hydrochlonde,
2,2-dibromo-3-
nitrilopropionamide, gluteraldhyde, chlorophenols, sulphones, methylene bis
thiocyanates,
methylene bis carbamates, isothiazolones, brominated propionamides, triazines,
phosphonium
compounds, organometallic compounds and mixtures thereof.
248. A method as recited in claim 221 where the non-oxidizing biocide is a
mixture
of (a) 2-bromo-2-nitropropane-1,3-diol (BNPD) and (b) a mixture of about 75% 5-
chloro-2-
methyl-4-isothiazolin-3-one and about 25% 2-methyl-4-isothiazolin-3-one, the
weight ratio
said BNPD (a) to said mixture (b) being about 16:1 to about 1:1.
249. A method as recited in claim 221 where the water-soluble alcohol freezing
point depressant is selected from the group consisting of ethylene glycol,
propylene glycol,
ethanol, glycerol, isopropanol, and methanol, or mixtures thereof.
250. A method as recited in claim 221 where the ionic freezing point
depressant is
selected from the group consisting of calcium chloride, sodium chloride,
lithium bromide, and
lithium chloride.
251. A method as recited in claim 221 where the pH adjusting agent is selected
from
the group consisting of sodium hydroxide, potassium hydroxide, lithium
hydroxide,
hydrochloric acid, sulfuric acid, nitric-acid, carbon dioxide, ammonia,
organic acids such as
oxalic acid, alkali metal carbonates, and alkali metal bicarbonates.
252. A method as recited in claim 221 where the inert tracer is selected from
the
group consisting of soluble lithium salts, transition metals, and fluorescent
materials.
253. A method as recited in claim 221 where the active tracer is selected from
the
group consisting of fluorescently tagged polymers, polymers containing a photo-
inert, latently
detectable moiety, water soluble molybdate salts, and azole-based copper
corrosion inhibitors.
254. A method as recited in claim 221 where the water insoluble organic
lubricant is
selected from the group consisting of naturally occuring oils and synthetic
oils.
255. A method as recited in claim 221 where the surfactant is selected from
the
group consisting of anionic, cationic, amphoteric, and nonionic surfactants.
256. A method as recited in claim 221 where the calcium hardness adjusting
agent is
selected from the group consisting of the bicarbonate, carbonate, chloride,
sulfate, and acetate
salts of calcium, calcium hydroxide and calcium oxide.
257. A method as recited in claim 221 where the coloring agent is a water
soluble
dye.
-91-
258. A method as recited in claim 131 wherein said monofluorophosphate is
sodium
monofluorophosphate.
259. A method as recited in claim 155 wherein the weight ratio of ortho-
phosphate
species to pyrophosphate species is in the range of about 20:1 to about 1:20,
when both
species are expressed as PO4 -3.
260. A method according to claim 130 where the aqueous system is a cooling
water
system.
261. A method according to claim 260 where the cooling system is an open,
evaporative cooling water system.
262. A method according to claim 260 where the cooling system is a once-
through
system.
263. A method according to claim 260 where the cooling system is closed loop
cooling system.
264. A method according to claim 263 where the closed loop cooling system is
the
cooling system of an internal combustion engine.
265. A method according to claim 263 where the closed loop cooling system is a
brine-based system which captains at least one additive selected from the
group consisting of
calcium chloride, lithium chloride, lithium bromide, and sodium chloride.
266. A method according to claim 263 where the closed loop cooling system is a
system which contains at least one additive chosen from the group consisting
of ethylene
glycol, propylene glycol, ethanol, glycerol, isopropanol, and methanol.
267. A method according to claim 130 where the aqueous system is a hot water
heating system.
268. A method according to claim 130 where the aqueous system is selected from
the group consisting of pulping and papermaking systems, food and beverage
systems, boiler
systems, refinery systems, petrochemical processing systems, mining systems,
and metal
machining systems which utilize aqueous metal working fluids.
270. A method according to claim 130 where the aqueous system contains a fluid
that is at least 5 percent by weight water.
271. A method according to claim 130 where the aqueous system contains a fluid
that is at least 50 percent by weight water.
272. A method according to claim 130 where the aqueous system contains a fluid
that is at least 90 percent by weight water.
-92-
273. A method according to claim 130 where the aqueous system contains
dissolved
oxygen.
274. A method according to claim 130 where the aqueous system is substantially
or
completely free of dissolved oxygen.
275. A method according to claim 130 where the aqueous system contains at
least
one dissolved gas chosen from group consisting of oxygen, carbon dioxide,
hydrogen sulfide,
and ammonia.
276. A method according to claim 130 where the aqueous system contains ferrous
metal.
277. A method according to claim 276 where the ferrous metal is at least one
metal
selected from the group of cast iron, mild steel, low alloy steel, and
stainless steel.
278. A method according to claim 130 where the aqueous system contains non-
ferrous metal.
279. A method according to claim 278 where the non-ferrous metal is at least
one
metal selected from the group consisting of aluminum, copper, and the copper-
based alloys.
280. A method according to claim 130 where the aqueous system contains both
ferrous and non-ferrous metals.
281. A method according to claim 130 where the components are introduced into
the
system at an effective concentration by a slug feed.
282. A method according to claim 130 where the components are fed into the
system
using a combination of intermittent and continuous methods.
283. A method according to claim 130 where some of the components are fed into
the system on a continuous basis and the remaining components are fed on an
intermittent
basis.
284. A method according to claim 130 where components are introduced into the
aqueous system at an effective concentration by a controlled release delivery
system.
285. A method according to claim 130 where the combination of components is
introduced into said aqueous system at a total concentration of about 0.5 to
about 10,000 parts
per million by weight.
286. A method according to claim 130 where the combination of components is
introduced into said aqueous system at a total concentration of about 10 to
about 1,000 parts
per million by weight.
-93-
287. A method according to claim 130 where the weight ratio of component b) to
component a) is from about 100:1 to about 1:20.
288. A method according to claim 130 where the weight ratio of component b) to
component a) is from about 20:1 to about 1:1.
289. A method according to claim 130 where the pH of said aqueous system is
from
about 6 to about 10.
290. A method of controlling corrosion, deposition, and scale in an aqueous
system
having a pH of about 6 or greater which comprises introducing into said system
a combination
of:
(a) a tetrazolium compound of the formula:
Image
wherein R1, R2 and R3 are selected from the group consisting of lower alkyl,
branched lower
alkyl, aryl, substituted aryl, alkylaryl, substituted alkylaryl and
heterocyclic substituted aryl,
with the proviso that neither R1, R2, or R3 contain more than 14 carbon atoms;
and n is 1 or 2,
such tetrazolium compound optionally having associated water soluble ionic
species if needed
to obtain a neutral charge, and
(b) at least one other aqueous system treatment material chosen so that the
material
does not substantially reduce the tetrazolium compound, and additionally
selected so that at
least one of these treatments is effective in inhibiting scale and/or
deposition.
291. A method for controlling corrosion of stainless steel in contact with an
aqueous
system which comprises introducing into said system at least one tetrazolium
compound of the
formula:
-94-
Image
wherein R1, R2 and R3 are selected from the group consisting of lower alkyl,
branched lower
alkyl, aryl, substituted aryl, alkylaryl, substituted alkylaryl and
heterocyclic substituted aryl,
with the proviso that neither R1, R2, or R3 contain more than 14 carbon atoms;
and n is 1 or 2,
such tetrazolium compound optionally having associated water soluble ionic
species if needed
to obtain a neutral charge.
292. The method as recited in claim 300, wherein the aqueous system includes
at least
one other aqueous system treatment material chosen so that the material does
not substantially
reduce the tetrazolium compound.
293. The method as recited in claim 130 or 291 wherein the aqueous system
contains
dissolved oxygen.
294. The method as recited in claim 130 or 291 wherein the at least one
tetrazolium is
added to the aqueous system at active treatment levels ranging from about 0.1
to about 50
parts per million.
295. The method as recited in claim 294 wherein the at least one tetrazolium
compound is added to the aqueous system at active treatment levels ranging
from about 1 to
about 25 parts per million.
296. The method as recited in claim 130 or 291 wherein the at least one
tetrazolium
compound is added to the aqueous system at active treatment levels ranging
from about 0.1 to
about 50 parts per million and the aqueous system contains oxygen.
297. The method as recited in claim 296 wherein the at least one tetrazolium
compound is added to the aqueous system at active treatment levels ranging
from about 1 to
about 25 parts per million.
-95-