Note: Descriptions are shown in the official language in which they were submitted.
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
METHOD OF IMPROVING THE AESTHETIC APPEARANCE
OF EPITHELIA
BACKGROUND OF THE INVENTION
This application claims priority in U.S. Patent Application Ser. No.
09/301,570, filed April 29, 1999.
1. Field Of The Invention
The present invention relates to a method of improving or re-
moisturizing the epithelia. More particularly, the present invention relates
to a method of improving lip color and reducing the number and depth of lip
lines on the surface of the lips, as well as re-moisturizing the vagina and
lips. This is achieved by using a composition comprising a retinoid, such
as retinol, and a penetration enhancing agent in a cosmetically acceptable
carrier. The present invention also relates to a composition, which
comprises a retinoid, and to a process for preparing the composition.
2. Description Of The Prior Art
Retinol is known for its beneficial effects in the treatment of acne. In
the field of repair of damage caused either by age or overexposure to the
sun, retinol has been proven beneficial. Repeated application of cosmetic
compositions containing retinol has been used to smooth the skin surface,
repair small cracks in the epidermis and to remove wrinkles or minimize the
formation thereof.
Because the anatomy and physiology of vagina and the lips differ
greatly from the anatomy and physiology of skin proper, the use of retinol
for re-moisturizing, treating a color deficiency and/or treating vertical
lines,
does not have an obvious correlation to skin aging pathologies. As known
in the art, vertical lip lines are visually distinguishable from general
wrinkling of the lips. As the term "vertical" implies, vertical lip lines
appear
as substantially vertical creases, whereas "wrinkling" has no such
discernible form.
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
However, it is well known that orally administered retinoids, such as,
for example, retinol and retinoic acid, dry out the epithelia and cause
cornification of mucosal epithelia.
Research on beneficial effects of retinol has been directed to
cosmetic "skin" to produce a reduction in wrinkles and other skin effects
related to or resulting from aging. However, there are no reports relating to
a method of re-moisturizing the epithelia, improving lip color or reducing
the number and depth of lip lines, particularly vertical lip lines, using
retinol.
Moreover, there are no reports of reversing age-associated cornification of
the vaginal or lip epithelia.
Surprisingly, it has been discovered that topically applying a
composition including retinoid, such as retinol, and a penetration
enhancing agent to mucosal or semi-mucosal epithelium that is dried out
and/or cornified, re-moisturizes the epithelium. Furthermore, with repeated
application, the cornified epithelia returns to its original mucosal state.
Related U.S. Patent Nos. 5,656,672 and 5,800,596 provide a water-
in-oil emulsion cream, containing retinol, for use as a nourishing and
repairing care product for damaged and wrinkled lips.
U.S. Patent No. 4,826,828 is directed to a water-in-oil emulsion
containing retinol, a volatile silicone and a solvent for both the retinol and
the volatile silicones. This patent also provides preparation of a retinol
emulsion by adding a solution containing retinol to a water-in-oil emulsion.
However, to avoid degradation, the retinol is added to the emulsion just
prior to or at the time of use. It is apparent that the stability of retinol
in a
composition of this type is insufficient for prolonged storage prior to use.
2
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
U.S. Patent No. 5,124,313 provides topical compositions having a
retinyl ester-polypeptide complex, specifically a retinyl palmitate-
polypeptide complex.
WO 93/00085 provides stabilization of retinol in cosmetic
compositions by addition to the latter a stabilizing complex comprising, in
combination, an antioxidant and a chelating agent for chelating metal ions.
The stability of the retinol appears enhanced due to considerable amounts
of stabilizing antioxidants and chelating agents in the composition.
WO 97/02814 provides the preparation of an antibacterial
medicament containing a retinoid for rapid bactericidal action, particularly
on Gram positive bacteria, which accelerates the repair of small lesions.
WO 97/02030 provides cosmetic, antimycotic compositions
containing a glycol or glyceryl ester of retinoic acid. These compositions
are used to produce visible reduction in wrinkles and visible improvement
in tone, firmness and luminosity of skin.
Thus, there as been a need for a topical retinol composition that
provides a method for restoring the aged, cornified vaginal epithelia and
cornified human lips to their original mucosal state.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a method of re-
moisturizing epithelia, comprising topically applying to vaginal or lip
epithelia an effective amount of a composition comprising a retinoid. The
composition can further comprise a cosmetically acceptable carrier.
It is another object of the present invention to provide such a
method of re-moisturizing vaginal and lip epithelia in older women,
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
improving lip color, lip clarity and lip dryness, and reducing the number and
depth of lip lines.
It is yet another object of the present invention to provide a
composition that can be used to re-moisturize vaginal and lip epithelia in
older women and improve lip color, lip clarity and lip dryness, and reducing
the number and depth lip lines.
These and other objects of the present invention will become
apparent in the course of the following description of the preferred
embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a plot of the average scores for dryness of lips;
Figure 2 is a plot of the average scores for dry appearance of lips;
Figure 3 is a plot of the average scores for clarity of lips;
Figure 4 is a plot of the average scores for color of lips;
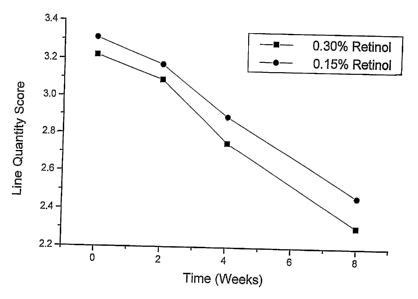
Figure 5 is a plot of the average scores for line quantity of lips; and
Figure 6 is a plot of the average scores for line depth of lips.
DETAILED DESCRIPTION OF THE INVENTION
The anatomy and physiology of vagina and the lips of one's mouth
differ in many ways from "skin" proper. Because of such differences, the
use of a composition having a retinoid, such as retinol, for treating vaginal
and lip dryness or treating color deficiency and/or lines of the lip is not an
obvious correlation to the treatment of aging pathologies associated with
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
other types of skin. Thus, the present invention is the first to demonstrate
a clinical benefit when applied topically to a vaginal epithelium and to a
semi-mucosal epithelium, which is within and up to the vermilion border of
the lips. In addition, the present invention is the first to demonstrate that
the aesthetic appearance of epithelia is improved by applying a topical
composition having both a retinoid, particularly retinol, in an amount
effective to improve the aesthetic appearance of the epithelial and a
penetration enhancing agent in an amount effective to enhance
penetration of the retinoid into the epithelia. The applicants have
unexpectedly discovered an effective method for improving human lip color
(i.e., increasing redness) and reducing the number and depth of lip lines
(i.e., line quantity), as well as re-moisturizing vaginal and lip epithelia in
older women. As used in the context of the present invention, the term
"older" refers to post-menopausal women and/or women who suffer from
age-related vaginal and lip dryness or cornification.
When used as a vaginal treatment for re-moisturizing vaginal
epithelia, the method of the present invention must be applied almost
exclusively to older women who are experiencing routine vaginal dryness.
The composition is preferably applied daily to inner surfaces of the vagina,
before bedtime.
Without being bound by any theory, it is believed that, as they age,
epithelial cells may lose responsiveness to circulating retinoids, such as
retinol, causing a fundamental change in the structure of the cells, i.e.,
squamous metaplasia. With a supply of excess retinoid available to the
cells, the cells respond and return to their mucosal state. The penetration
enhancing agent provides improved delivery of the retinoid to the "active"
site located at the epithelial cell.
A beneficial re-moisturizing effect is obtained on repeated
application of the composition to the vaginal epithelia. To produce the
5
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
beneficial re-moisturizing effect of the method of the present invention, the
composition is preferably applied to the vaginal epithelia in the form of a
cream.
On repeated application of the composition to the lips, particularly
aging lips, in accordance with the method of the present invention, a visible
improvement in lip condition, moisturization, color, clarity and a measurable
reduction in the number and depth of lip lines is observed within a short
period of time, which can be as little as two (2) weeks, when applied once
or twice a day. The improvement in the color of the lips is manifested by
an increase in the redness of the lips, whereas the reduction in the number
of lines is directly measured and reduction in the dryness and depth of the
lines estimated by direct observation as described below.
In the context of the present invention, the term retinoid includes the
following classes of compounds: retinol; esters of retinol with carboxylic
acids of 1 to 24 carbon atoms, such as retinyl acetate, retinyl propionate,
retinyl butyrate, retinyl octanoate, retinyl laurate, retinyl palmitate,
retinyl
oleate, retinyl linoleate; esters of retinol with alpha-hydroxy carboxylic
acids; ether derivatives of retinol, including alkyl ethers, ethers derived
from glycolic acid and glycolate esters and amides, such as retinyl glycolyl
ether (retinyl glycolic acid ether); retinaldehyde; retinoic acid; esters of
retinoic acid with alcohols of 1 to 24 carbon atoms; isotretinoin as well as
synthetic retinoid mimics, and derivatives of the foregoing, as well as
others that bind to RAR receptors; cis- and trans-isomers thereof; salts
thereof; and mixtures thereof. Preferably, the retinoid is retinol. More
preferably, the retinoid is the trans-isomer of retinol. Retinol may be
prepared by well-known methods such as those described in U.S. Patent
No. 3,060,229, the content of which is incorporated herein by reference.
Retinyl esters, which generally are less potent than other retinoids,
are less preferred retinoids for the purposes of the present invention.
6
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
Specifically, retinyl ester-polypeptide complexes, such as those described
in U.S. Patent No. 5,124,313, are not contemplated as retinoids within the
context of the present invention.
The amount of retinoid in the composition of the present invention is
preferably in the range from about 0.001 to about 1.5 weight percent (wt%)
of the total composition, more preferably from about 0.06 wt% to about 0.3
wt%, and most preferably about 0.1 wt% to about 0.2 wt% of the
composition. However, as stated above, the amount of retinoid may be
adjusted, based upon the potency of the retinoid, without departing from
the present invention.
The penetration enhancing agent is present in an amount effective
to either enhance the penetration of the selected retinoid into the epithelia
or increase the rate (i.e., speed) of penetration of the selected retinoid
into
the epithelia. Preferably, the penetration enhancing agent does both. The
selection of the penetration enhancing agent will depend on formulation
factors, such as the chemical properties of the selected retinoid and the
vehicle (e.g., solution, emulsion, stick). Non-limiting examples of such
penetration enchancing agents include: organic solvents, such as ethanol,
glycols (e.g., propylene, butylene, pentylene), pyrrolidones (e.g., 2-
pyrrolidone, 1-methyl-2-pyrrolidone, 5-methyl-2-pyrrolidone, 1,5-dimethyl-2-
pyrrolidone, 1-ethyl-2-pyrrolidone, 2-pyrrolidone carboxylic acid), dimethyl
sulfoxide, dimethylacetamide, and dimethylformadide); alkyl sulfoxide;
phosphine oxide; sugar esters (e.g., sucrose acetate, sucrose octanoate);
anionic surfactants; nonionic surfactants; AzoneT~" (e.g., 1-dodecylazaclo-
heptan-2-one); N-substituted di-isopropanolamines; fatty acids (e.g., oleic
acid, linoleic acid); and mixtures thereof. Additional resources are
available to those in the art to assist with the selection of the penetration
enhancing agent. One such resource is available at pages 160 through
172 of Dermatological Formulations, B.W. Barry (Marcel Decker, 1983),
which is incorporated herein by reference.
7
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
In addition to the retinoid and the penetration enhancing agent, the
composition of the invention can contain a cosmetically acceptable carrier.
The carrier can be water, a humectant, a thickener, a gelling agent, an oil,
an emulsifier, or mixtures thereof. The resulting composition can be in the
form of a cream, dispersion, emulsion, foam, gel, solution, stick
suspension, spray, patch, powder or in a towelette. The emulsion can be
either an oil-in-water emulsion or a water-in-oil emulsion.
Preferably, the retinoid is formulated into an appropriate vehicle
consistent with consumer requirements. For example, the composition can
preferably be formulated as a stick or cream for treatment of the lips. For
the treatment of the vaginal epithelia, the composition would preferably be
formulated as a cream.
The oil phase of the emulsion preferably has one or more organic
compounds, including emollients. The aqueous phase has humectants,
such as propylene glycol and glycerin; other water-dispersible or water-
soluble components including thickeners such as veegum or hydroxyalkyl
cellulose; gelling agents, such as high MW polyacrylic acid, i.e. carbopol
934; and mixtures thereof. The emulsion has one or more emulsifiers
capable of emulsifying the various components present in the composition,
including the retinoids. Because of the light, heat and air sensitivity of
retinoids, retinoids are generally added last in the preformed emulsion so
as to minimize exposure to light, heat and oxygen.
Non-limiting examples of organic compounds suitable for use in the
oil phase include emollients, skin conditioning agents, solvents that are
capable of dissolving retinol or retinoids without reducing the stability
thereof, and mixtures thereof. The compounds suitable for use in the oil
phase include one or more of the following: an alcohol including cetyl
alcohol; ester including cetyl recinoleate, sterol esters; carboxylic acid;
mineral oil; wax; hydrocarbon; paraffin; isoparaffin; petrolatum; low taste
8
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
petrolatum for application on lips; hydrogenated polydecene; silicone-
containing compound such as dimethyl polysiloxane; and mixtures thereof.
The emulsifier can be an emulsifying wax, an emulsifying polyhydric
alcohol, a polyether polyol, a polyether, a mono- or di-ester of a polyol, an
ethylene glycol mono-stearate, a glycerin mono-stearate, glycerin di-
stearate, a silicone-containing emulsifier, a soya sterol, a fatty alcohol
such
as cetyl alcohol, a fatty acid such as stearic acid, a fatty acid salt, and
mixtures thereof. The preferred emulsifiers include soya sterol, cetyl
alcohol, stearic acid, emulsifying wax, and mixtures thereof.
The emulsifying waxes that are suitable for use in the present
invention are well known to persons skilled in the art. The emulsifying wax
includes compositions such as those containing about 80 wt% cetearyl
alcohol, about 10 wt% polysorbate 60, about 5 wt% stearate, and about 5
wt% steareth-20.
In the emulsion, the aqueous phase is preferably from about 60 wt%
to about 90 wt%, the oil phase is preferably from about 5 wt% to about 30
wt% of the emulsion, and the emulsifier is preferably from about 5% to
about 10 wt% of the emulsion.
The emulsion according to the present invention has pH preferably
in the range from about 6.5 to about 7.5.
The composition according to the present invention can be prepared
by dissolving a retinoid, such as retinol, in a medium comprising an organic
solvent, and optionally water, and adding the resulting homogeneous
solution to the emulsion. The composition produced thereby preferably
has from about 0.001 wt% to about 1.0 wt% retinol, on an active basis, and
about 0.5 wt% to about 1.0 wt% organic solvent. More preferably, the
composition has from about 0.06 wt% to about 0.3 wt% retinol.
9
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
The present invention includes a process for preparing a
composition comprising a retinoid, such as retinol, in the form of a cream,
dispersion, emulsion, foam, gel, solution, stick or suspension.
The process for preparing a composition for re-moisturizing
epithelia, comprises:
preparing a first mixture comprising water, a humectant, a thickener,
and a gelling agent;
preparing a second mixture comprising an oil and an emulsifier;
adding the second mixture and the first mixture together, preferably
mixing the second mixture in the first mixture, at a temperature and period
of time sufficient to produce a stable emulsion;
cooling the stable emulsion; and
adding a retinoid to the stable emulsion to produce the composition.
In addition, the present invention may include a secondary
component. The secondary component is preferably selected from one or
more of the following thirteen groups.
1. Rexinoids: Rexinoids include compounds, such as all-trans
retinoic acid, 9-cis retinoic acid, phytanic acid and others, that bind to RXR
receptors.
2. An estrogen synthetase (aromatase) stimulating compound:
Examples of such a compound include caffeine and/or derivatives thereof,
and any mixture thereof. Caffeine is the more preferred of such
compounds.
3. A compound capable of inhibiting 5 alpha-reductase activity:
Examples of such a compound include linolenic acid, linoleic acid,
finasteride, and mixtures thereof.
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
4. An exfoliation promoting compound: Suitable examples include
alpha hydroxy acids; beta hydroxy acids; oxa acids as disclosed in U.S.
Patent No. 5,847,003 (the disclosure of which is incorporated herein by
reference); oxa diacids as disclosed in U.S. Patent No. 5,834,513 (the
disclosure of which is incorporated herein by reference); mechanical
exfoliation compounds, such as bamboo exfoliant extract; salicylic acid;
benzoyl peroxide; keto acids, such as pyruvic acid, 2-oxopropanoic acid, 2-
oxobutanoic acid, and 2-oxopentanoic acid; and mixtures thereof.
The preferred exfoliation promoting compounds are lactic acid,
glycolic acid, 3,6,9-trioxaundecanedioic acid, and any mixture thereof.
When the present invention includes an exfoliation promoting compound,
the composition comprises about 1 wt% to 20 wt%, preferably about 1 wt%
to about 15 wt%, more preferably about 4 wt% to about 10 wt% acid, and
most preferably about 4 wt%, of the exfoliation promoting compound.
5. An ultraviolet (UV) light protectina/sunscreen agent: Examples
include organic and inorganic sunscreens, such as titanium dioxide, zinc
oxide, methyl benzylidene camphor and/or its derivatives, octocrylene,
anthranilates, benzophenones, butylmethoxydibenzoylmethane
(avobenzone), naphtholsulphonates, benzoic acid derivatives, salicylates,
cinnamic acid derivatives, terephthalylidene dicamphor sulfonic acids, and
mixtures thereof. Of these, butylmethoxydibenzoylmethane, octocrylene,
octylsalicylate, octylmethoxycinnamate, oxybenzone, titanium dioxide, and
mixtures thereof are preferred. Butylmethoxydibenzoylmethane,
oxybenzone, octylmethoxycinnamate, terephthalylidene dicamphor sulfonic
acids, and mixtures thereof are most preferred. Salts, esters and other
derivatives of the aforementioned sunscreen agents, which are compatible
with the composition, are also contemplated in practicing the present
invention. A preferred UV absorber includes a hydroxybenzophenone
11
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
derivative, a benzotriazole derivative, a dibenzoyl methane derivative, an
oxanilide derivative, a hydroxy cinnamate derivative, and mixtures thereof.
Co-formulation with an ultraviolet light protecting/sunscreen agent is
particularly desirable when the present invention is used for treating lip
epithelia, particularly for users who engage in activities, particularly
outdoor
activities, which expose the user's lip epithelia to UV radiation. Non-
limiting
examples of such activities include indoor tanning, sunbathing, and skiing.
It is preferred that the sunscreen comprises from about 2 wt% to about 20
wt%, more preferably about 2 wt% to about 15 wt%, of the total weight of
the composition.
6. Barrier function enhancing agents: Examples include ceramides;
essential fatty acids and their esters, especially glycerides, a-hydroxy fatty
acids and their esters, ~-hydroxy fatty acids and their esters;
phospholipids; cholesterol and its esters, such as cholesteryl
hemisuccinate, cholesteryl phosphate; and cholestanol and its derivatives.
The barrier function enhancing agent can be added to a topical
composition either as singular molecular entities or as a complex mixture of
lipids derived from either synthetic, animal or plant sources.
7. Collagen enhancingi agents: These agents prevent epithelia
"sagging" by promoting a net increase in collagen, either by reducing
collagen breakdown or by promoting collagen formation. Examples of such
agents include Clara extract (Sophora augustifolia), ascorbyl-phoshoryl-
cholesterol, ascorbic acid, ascorbic acid derivatives, and mixtures thereof.
8. Elastase inhibitors: Examples of these inhibitors include fatty
acids, such as oleic acid, perinaric acid, and Honeysuckle extract (Lonicera
caprifolium). These inhibitors act to prevent sagging of the epithelia.
12
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
9. Skin lightenina agents: Examples include kojic acid,
hydroquinone, licorice derivatives, ascorbic acid/ascorbic acid derivatives
(e.g. magnesium ascorbyl phosphate), arbutin, bearberry (Arctostaphylos
uva ursi), Glycyrrhiza glabra and its derivatives, Chlorella vulgaris extract,
and mixtures thereof.
10. Antioxidants: Examples include compounds having phenolic
hydroxy functions, such as ascorbic acid, ascorbic acid derivatives; gallic
acid derivatives (e.g. propyl gallate); ferulic acid derivatives (e.g. ethyl
ferulate, sodium ferulate); nitrones; N-tertbutyl-nitrone; I-(4-pyridyl-1-
oxide)-
N-tertbutyl-nitrone; curcumin, tetrahydrocurcumin; 6-hydroxy-
2,5,7,tetramethylchroman-2-carboxylic acid; uric acid; reductic acid; tannic
acid; rosmarinic acid; tocopherol and its derivatives; catechins; and
mixtures thereof. Other suitable antioxidants are those that have one or
more thiol functions (-SH), in either reduced or non-reduced form, such as
glutathione, lipoic acid, thioglycolic acid, and other sulfhydryl compounds.
The antioxidant may be inorganic, such as sulfites, bisulfites, metabisulfite,
or other inorganic salts and acids containing sulfur. Preferably, the
antioxidant is selected from the group consisting of: a phenolic antioxidant
such as butylated hydroxytoluene; butylated hydroxyanisole; an alkyl
paraben such as methyl, ethyl or propyl paraben; and any mixtures thereof.
11. Skin warming agents: Examples include vanillyl butylamid,
capsaicin, and mixtures thereof.
12. Skin cooling compounds: Examples include menthol, menthyl
glycerol, asymmetrical carbonates, thiocarbonates and urethanes, N-
substituted carboxamides, ureas or phosphine oxides, menthyl lactate,
menthone glycerine acetal, and any mixtures thereof.
13
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
13. Anti-pruretic/Anti-itch compounds: Non-limiting examples of
such compounds include capsaicin, nonivamde, and corticosteroids. Co-
formulation with an anti-pruretic/anti-itch compound may be desirable when
the present invention is applied to itchy vaginal epithelia. A non-limiting
example of when such co-formulation may be desirable includes when the
user has a concurrent condition commonly referred to as a yeast (Candida
albicans) infection.
The addition of the secondary component enhances the
dermatological benefits achieved and the utilization for compositions of the
present invention. The compositions of the present invention may include
at least two secondary components, with each secondary component being
selected from a different group.
The compositions of the present invention can include other
cosmetic and pharmaceutical actives and excipients. Such suitable
cosmetic and pharmaceutical agents include, but are not limited to, one or
more of erythromycins, tetracyclines, salicylic acids, antifungals, vitamins,
anti-inflammatory agents, antimicrobials, analgesics, nitric oxide synthase
inhibitors, self-tanning agents, surfactants, moisturizers, stabilizers,
preservatives, antiseptics, chelating agents, thickeners, emulsifiers,
lubricants, humectants, chelating agents, skin penetration enhancers, skin
cooling agents, emollients, fragrances, colorants, flavoring agents,
pigments, and mixtures thereof.
Other conventional constituents including cosmetic and
pharmaceutical additives may be added to the composition. These
additives include: vitamins, such as tocopherol and ascorbic acid; vitamin
derivatives such as ascorbyl monopalmitate; thickeners such as
hydroxyalkyl cellulose; gelling agents; structuring agents such as bentonite,
smectite, magnesium aluminum silicate and lithium magnesium silicate;
14
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
metal chelating agents such as EDTA; pigments such as zinc oxide and
titanium dioxide; colorants; emollients; and humectants.
When the present invention is used to improve the aesthetic
appearance of lip epithelia, the compositions of the present invention may
be non-pigmented or pigmented. Lip compositions, such as lipsticks, often
have pigments incorporated therein. An example of a pigment is iron
oxides. However, when the composition is pigmented, it is preferred that
the composition includes an ascorbyl-phosphoryl-cholesterol (APC)
compound. Examples of suitable APC compounds are disclosed in WO
97/42960, which is commonly assigned and is incorporated herein by
reference.
The addition of an APC compound in pigmented compositions of the
present invention is particularly desirable when the pigment is an iron
oxide. An example of such a pigmented composition may have from about
0.2 wt% to about 20 wt% of iron oxides in addition to the APC compound.
One preferred example of such a topical composition has from about 5
wt% to about 7 wt% of iron oxides and about 1 wt% of the APC compound
in a suitable vehicle. In the preferred example, the iron oxides are selected
from the group consisting of iron oxide red 2259-preserved, iron oxides
(yellow), iron oxides (black), and mixtures thereof. Additional advantages
of including an APC compound are set forth in PCT WO 00/06091, which
is commonly assigned and is incorporated herein by reference.
When the present invention is applied to lip epithelia, particularly in
the form of a pigmented composition, it is preferred that the weight
percentage of retinoid in the pigmented composition is adjusted to
accommodate numerous re-application as may occur with topical lip
compositions, such as lipsticks and lip balms.
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
Compositions
of the present
invention
can be applied
to epithelia
for
a period time to improve the aesthetic appearance of the
of epithelia. The
improveme nt in aesthetics can include at least one of
the following:
a. reducing intrinsic aging;
b. reducing photoaging;
c. decreasing epithelial fragility;
d. preventing and reversing loss of collagen;
e. preventing epithelial atrophy;
f. promoting/accelerating cell turnover;
g. improving epithelial firmness/plumpness;
h. improving epithelial texture;
i. decreasing fine lines;
j. decreasing wrinkles;
k. improving epithelial tone;
I. enhancing epithelial thickness;
m increasing moisture retention;
n. minimizing epithelial discoloration; and
o. reversing age-associated cornification of epithelia.
Other improvements in the aesthetic appearance of epithelia are provided
by the present invention. The above improvements are only examples of
the improvements made possible by the present invention and are set forth
for illustration only.
The Examples that follow are intended for illustrating the present
invention and not for limiting the scope thereof.
Clinical Study Results
Efficacy of retinol-containing creams in reducing visible signs of
aging lips was demonstrated as follows.
16
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
Two lip creams, Lip Cream A containing 0.15% active retinol and Lip
Cream B containing 0.30% active retinol were prepared as shown below in
TABLE 1.
TABLE 1
Preparation Of Lip Cream A And Lip Cream B
INGREDIENTS (Wt%) LIP CREAM A LIP CREAM
B
Retinol Cream
Retinol 0.30 0.15
Acrylates Copololymer 1.10 0.55
Carbopol/thickeners 0.90 0.90
Disodium EDTA 0.20 0.20
Glycerin 5.00 5.00
Propylene Glycol 0.56 0.56
Emollients 13.50 13.50
Emulsifiers 8.50 8.50
Preservatives 1.40 1.40
Anti-oxidants 0.10 0.10
Triethanolamine 1.00 1.00
Demineralized Water qs qs
The study was a double blind monadic design. A total of 36 female
subjects who met the inclusion criteria ranging in age from 33 to 64 years
were selected for this study. The subjects were in good general health,
with no known allergies or sensitivities to lip products. The subjects had
skin types I-III (predominantly I-II), were not pregnant or nursing, had full
lips, exhibiting acceptable appearance of aging lips, including paleness,
mild dryness and flaking of the surface, and some vertical lines, but not
premature aging.
17
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
Each subject's lips were visually examined (baseline examination)
and signs of dryness, flaking, paleness, fullness and lip lines were
recorded.
The subjects were randomly divided into two groups and each group
was asked to use one Lip Cream once a day, at night, shortly before
bedtime, after cleansing their face prior to using the Lip Cream. They were
asked to apply a small amount of product onto a finger and spread the
product on both the upper and lower lip, avoiding the outer edges of the
lips, with the lips closed, to avoid getting the product into the mouth. The
use of usual lip products such as lipstick and/or lip balm was permitted
except on examination days.
At baseline the following visual parameters were graded (5-point
subjective scoring):
(1 ) Dryness (flaking): 0=no visible flakes, 5=severe flaking;
(2) Dry appearance (visible tightness): 0=none, 5=severe;
(3) Color: 0=very pale, 5=dark red;
(4) Clarity (transparency): 0=clear, 5=highly opaque;
(5) Number of lines: 0=none, 5=numerous; and
(6) Depth of lines: 0=shallow, 5=deep.
Lip Cream A and Lip Cream B were then dispensed and use
instructions administered. Briefly, subjects were instructed to apply the test
product to their lips once a day, before bedtime.
Subjects returned for follow-up visual grading after 2, 4 and 8
weeks. In addition, 35mm frontal lip photos were taken at baseline and
after 8 weeks. Finally, a self-perception questionnaire was administered at
8-weeks.
18
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
TABLE 2 compares the average visual scores of the two treatment
groups.
TABLE 2
Efficacy of Retinol-Containing Creams in
Reducing Visible Signs of Aging Lips
Average Visual Scores
FORMULA A FORMULA B
Feature EvaluatedWeek 0.3% Retinol 0.15% Retinol
Mean N=18 Mean N=18
D ness 0 1.00 1.06
2 1.09 1.08
4 0.44 0.56
8 0.34 0.58
D A earance 0 2.41 2.58
2 2.44 2.53
4 1.63 1.83
8 1.09 1.36
Clarit 0 3.84 3.86
2 3.53 3.58
4 2.69 2.89
8 1.91 2.00
Color 0 1.84 1.86
2 2.06 2.06
4 2.69 2.50
8 3.09 2.83
Number of Lines 0 3.22 3.31
2 3.09 3.17
4 2.75 2.89
8 2.31 2.47
Line De th 0 1.84 2.03
2 1.69 1.75
4 1.50 1.67
- -
8 1.28 I 1.50
From the results obtained, it can be seen that:
(1) there was no statistical difference between the two treatment
groups;
(2) both Lip Creams were clinically well tolerated without any
adverse clinical response attributable to the use of Lip Cream A or Lip
Cream B;
(3) all parameters improved after 8 weeks;
(4) most parameters improved at 4 weeks, except number of
lines, dryness and the 0.15% retinol (Formula B) line depth score; and
19
CA 02370633 2001-10-16
WO 00/66077 PCT/US00/11529
(5) the percent improvement, calculated from the average scores
within an interval, was always numerically superior for Lip Cream A
containing 0.3% retinol than for Lip Cream B containing 0.15% retinol.
Calculation of percent improvement for Lip Cream A containing
0.3% retinol revealed a 68% improvement in lip color and 28% reduction in
the number of vertical lip lines and 30% reduction in the depth of vertical
lip
lines within 8 weeks of treatment.
While the difference in efficacy between Lip Cream A and Lip
Cream B was not large, the magnitude of improvements in dryness, dry
appearance, color, clarity and lines of the lips obtained from Lip Cream A
containing 0.3% retinol was greater for all measured parameters. In
addition, both Lip Cream A and Lip Cream B were clinically well tolerated.
Figures 1 through 6 chart the average scores for each parameter.
These graphs demonstrate that, for all parameters, the most dramatic
improvement was observed between 2 and 4 weeks of Lip Cream use.
Features associated with aging lips, including dryness (flaking/taut),
dry appearance, color, clarity and number and depth of lines were all
significantly improved by the use of the retinol-containing Lip Cream A and
Lip Cream B according to the present invention.
Obvious modifications and variations of the present invention are
possible in light of the above teachings. It is therefore to be understood
that such modifications not specifically described herein are within the
scope of the appended claims. Also, singular used in the application can
also mean plural of the same ingredient.
20