Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE I)E CETTE DEMANDE OU CE BREVETS
COMPRI~:ND PLUS D'UN TOME.
CECI EST ~.E TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter 1e Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional vohxmes please contact the Canadian Patent Oi~ice.
CA 02372197 2001-07-12
WO 00142045 PCTIUS99130434
-1-
FUNCTIONALIZED HETEROCYCLES AS CHEMOKINE
RECEPTOR MODULATORS
BACKGROUND OF THE INVENTION
The present invention relates to functionalized heterocycles useful as
modulators of the chemokine receptors, including CCR-1, CCR-2, CCR-2A,
CCR-2B, CCR-3, CCR-4, CCR-5, CXCRI, CXCR2, CXCR-3, and/or CXCR4
and to pharmaceutical compositions which include these compounds and a
pharmaceutically acceptable carrier. More particularly, the present invention
is
directed to methods for inhibiting HIV infectivity.
Chemokines mediate a range of proinflammatory effects on leukocytes,
such as chemotaxis, degranulation, and intigran activation (Baggiolini et al.,
Aclv.
Inzzzzunol., 1994;55:97-179; Oppenheim et al., Annu. Rev. Inzmunol., 1991;
9:617-48; Miller et al., Crit. Rev. Inzmunol., 1992;12:17-46). These effects
are
mediated by binding to the seven-transmembrane-spanning G-protein coupled
I S receptors (Baggiolini et al., Adv. Immzznol., 1994;55:97-179; Murphy,
Annu. Rev.
Izrtmzznol., 1994;12:593-633; Schall et al., Cttrr. OPin. I7711n1(I101.,
1994;6:865-73;
Gerard et al., Curr. Opizt. Immunol., 1994;6;140-5; Mackay, Czzrr. Bio., In
press).
Chemokine receptors also serve as coreceptors for HIV-1 entry into cells. This
came from observations that RANTES, MIP-la, and MIP-1(3 suppressed infection
of susceptible cells in vitro by macrophage-tropic primary HIV-1 isolates
(Cocchi et al., Science (Wash. DC), 1995;270:1811-5). The chemokine receptor
CXCR-4 was found to support infection and cell fusion of CD4+ cells by
laboratory-adapted, T-tropic HIV-1 strains (Feng et al., Science (Wash. DC),
1996;272:872-7). CCR-5, a RANTES, MIP-la, and MIP-1(3 receptor, was
subsequently identified as the principle coreceptor for primary macrophage-
tropic
strains (Choe et al., Cell, 1996;85:1135-48; Alkhatib et al., Science (Wash.
DC),
1996;272:1955-8; Doranz et al., Cell, 1996;85:1149-58; Deng et al., Nattn-e
(Load.) 1996;381:661-6; Dragic et al., Natzzre (Loud.), 1996;381:667-3). The
importance of CCR-5 for HIV-l transmission was underscored by the observation
that certain individuals who had been repeatedly exposed to HIV-I but remained
CA 02372197 2001-07-12
WO 00/42045 PCT/US99t30434
-2-
uninfected had a defect in CCR-5 expression (Liu et al., Cell, 1996; 86:367-
77;
Samson et al., Nature (Lond.), 1996;382:722-5; Dean et al., Science (Wash.
DC),
1996;273:1856-62; Huang et al., Nature Med., 1996;2:1240-3). These
noninfectable individuals were found to be homozygous for a defective CCR-5
allele that contains an internal 32-base pair deletion (CCR-5 X32). The
truncated
protein encoded by this gene is apparently not expressed at the cell surface.
CCR-5 X32 homozygous individuals comprise ~1% ofthe Caucasian population
and heterozygous individuals comprise ---20%. In studies of about 2700 HIV-1
infected individuals, no X32 homozygotes were found. Individuals who are
heterozygous for X32 CCR-5 allele have been shown to progress more slowly to
AIDS than wild-type homozygous individuals (Samson et al., Nature (Loud.),
1996;382:722-5; Dean et al., Science (Wash. DC), 1996;273:1856-62;
Huang et al., Nature Med., 1996;2:1240-3). Thus, the identity of CCR-5 as the
principle coreceptor for primary HIV isolates provides an opportunity to
1 S understand disease pathogenesis, and more importantly to identify a new
avenue
for the treatment of HIV-1 infection.
The instant invention is a series of functionalized heterocycles that block
the CD-4/GP-120 interaction with CCR-5 receptor, and thus can be useful in the
treatment of HIV infection manifested in AIDS.
SUMMARY OF THE INVENTION
The compounds of the invention are useful in a method of modulating
chemokine receptor activity in a patient in need of such modulation comprising
the administration of an effective amount of the compound.
The present invention is directed to the use of the foregoing substituted
heterocycles as modulators of chemokine receptor activity. In particular,
these
compounds are useful as modulators of the chemokine receptors, including
CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4, CCR-5, CXCR-3, CXCR1,
CXCR2, and/or CXCR-4. In particular, the compounds of the present invention
are preferred as modulators of the chemokine receptor CCR-5.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-3-
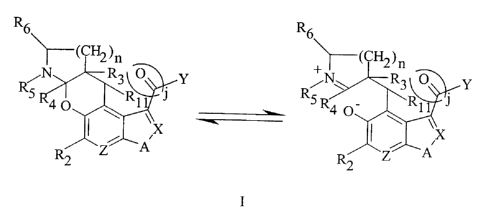
The compounds of the instant invention are those of Formula I which may
exist in both closed and open form.
R6 R6\
~(Cl~)n ~(CH2)n
/N R3 O Y /N R3 O Y
RS 40 Rl\ J RSR40 ~R11
\ \
~X ~ ~ ~X
R Z A R Z~A
2 2
ring close I ring open
or a pharmaceutically acceptable salt thereof wherein:
A is O, S, and additionally A is NR1 when X is C-R~;
X is N when A is NRl or
X is C-R9 wherein R~ is halogen, hydrogen, alkyl, -CF3, CH2F, CHF2,
-(CH2)m-OR1, aryl, arylalkyl, -(CH~)m-NR~Rg, or
CH2) r
(CH2)q wherein m is an integer of from 0 to 2 and
each occurrence of m is independently an integer of from 0 to 2,
q is an integer of from 0 to l, and r is an integer of from 0 to 3;
Y is hydrogen, alkyl, arylalkyl, aryl, (CH2)n~-NR~Rg, -N(Rl)-(CH2)v-
C(R~Rg)-aryl, or ORlO wherein Rl0 is hydrogen, alkyl,
cycloalkyl, cycloalkyl fused to an aryl ring, aryl, (CH2)saryl,
-CH2CF3, (CH2)tC(R7R8)-(Cl-I2)uarYl~
O
R (CH2)m NR~R8 R (CH2)mC- ORS
~'I ~, Aryl
O
R
~~(CH2)m C-NR~Rg
~z, Aryl
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99/30434
-4-
I I
R (CH2)m NR~RB R O
l~~(CH2)m C-ORS
~'z, alkyl ~ ~/'alkyl
O
R I I
1~~(CH2)m C-NR~RB
~z, alkyl
O
RI (CH2)m NR~RB RM (CH2)m C-ORS
'~cycloalkyl ~'~cycloalkyl
O
R I I
(CH2)m C-NR~RB
' ~~~CCI
cycloalkyl 3
CH3
~z,~C=N ~ ~~~C---CH , , or
~N~
S
R2 ~ ~ (CH2)w wherein s is an integer of from 1 to 3, t is
an integer of from 0 to 3, a is an integer of from 0 to 3, v is an
integer of from 1 to 3, and w is an integer of from 0 to 2;
ZisCRorN;
RI is hydrogen or alkyl and each occurrence of RI is independently
hydrogen or alkyl;
R and R2 are each independently selected from:
hydrogen,
alkyl,
halogen,
-CN,
-N02
-(CH2)m-~7R8>
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-5-
-(CH2)m-COORS,
-(CH2)m-CONR~Rg,
O
(CH2)m N~R7 '
O
-(CH2)mN-S-R7
O
-(CH2)m-OR~,
-(CH2)m-S02NR~Rg, and
-(CH2)m-S(O)pR~ wherein each occurrence of R~ and Rg are each
independently hydrogen, alkyl, aryl, arylalkyl, -CF3, or R~
and Rg may be taken together to form a cyclic ring of from
3 to 7 atoms which ring may have O, S, or NR1 and p is an
1 S integer of from 0 to 2;
R3 is hydrogen or alkyl;
R4 is hydrogen, alkyl, aryl, or aralkyl;
RS is alkyl, aryl, arylalkyl, acyl; or
R4 and RS are taken together with the atoms to which they are attached to
form a cyclic ring of from 5 to 7 atoms;
Rg is hydrogen or alkyl;
RS when not taken together with R4 can be taken together with R6 with
the atoms to which they are attached to form a ring of from 5 to
7 atoms;
N-RS is also the corresponding N-oxide;
R11 is hydrogen or alkyl;
n is an integer of from 1 to 3;
j is an integer of from I to 2, and j is the integer 0 when Y is hydrogen,
alkyl, arylalkyl, or aryl;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-6-
with the proviso that pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-
carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
ethyl ester is not included.
Preferred compounds are those of Formula 1 above wherein
R1 is hydrogen.
Other preferred compounds are those of Formula 1 above wherein
R1 is hydrogen and
X is C-R9.
Still other preferred compounds are those wherein
R1 is hydrogen and
X is C-R9, wherein R9 is alkyl.
Still other preferred compounds are those wherein
Rl is hydrogen,
X is C-R~, wherein R9 is alkyl;
R4 and R5 are taken together with the atoms to which they are
attached to form a ring of from 5-7 atoms; and
Y is ORlO.
Still other preferred compounds are those wherein
R1 is hydrogen,
X is C-Rc~, wherein R~ is alkyl;
R4 and R5 are taken together to form a 6-membered ring; and
Y is OR10 wherein R10 is alkyl, aryl or -(CH2)saryl,
-(CH2)t-C(R7R8)-(CH2)u-aryl.
Still other preferred compounds are those wherein
R1 is hydrogen,
X is C-R9, wherein R9 is Me;
R4 and R5 are taken together to form a 6-membered ring;
R6 is hydrogen;
n is 2; and
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_7_
Y is OR10 wherein R10 is alkyl, aryl or Rlp is -(CH2)t-C(R7Rg)-
(CH2)u-aryl wherein t is 0, R7 and Rg can each
independently be
H,
alkyl,
-(CH2)vOH or (CH2)uCOOR7, and -(CH2)vNRlR2
where a and v are as defined above.
More preferred compounds are those of Formula I and selected from:
Pyrrolo[3',2':S,G][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, methyl ester;
Pyrrolo[3',2':5,6][l]benzopyrxno[3,2-i]quinolizine-1-carboxylic acid,
5-bromo-3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-; ethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13, I 4,14x,15-decahydro-2-methyl-, propyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-methylpropyl ester;
Pyrrolo[3',2':S,G][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-methyl-, 2,2-dimethylpropyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, l 5-decahydro-2-methyl-, phenylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-methylcthyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9, I 0,12,13,14,14x,15-decahydro-2-methyl-, cyclopropylmethyl ester;
Pyrrolo[3',2':S,G][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-(1-piperidinyl)ethyl ester;
Pyrrolo[3',2':S,G][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-(phenylmethyl)-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid, 2-
ethyl-3,7,8,9,10,12,13,14,14x,15-decahydro-, ethyl ester;
Pyrrolo[3',2':S,G][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid, 2-
cyclopropyl-3,7,8,9,10,12,13,14,14x,15-decahydro-, ethyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_g_
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-propyl-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-(2-methylpropyl)-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x, l 5-decahydro-2-methyl-, I, I -dimethylethyl ester;
2,6x,7-Trimethyl-7,8,9,10,1 Oa, I 1-hexahydro-3H,6aH-6-oxa-3,7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester;
7-Ethyl-2,6x-dimethyl-7,8,9,10,1 Oa, l l -hexahydro-3H,GaH-6-oxa-
3,7-diaza-cyclopenta[a]anthracene-1-carboxylic acid ethyl ester;
6a-Ethyl-2,7-dimethyl-7,8,9,10,1 Oa, l l-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyelopenta[a]anthracene-1-carboxylic acid ethyl ester;
6x,7-Diethyl-2-methyl-7, 8,9,10,1 Oa, l l -hexahydro-3H,GaH-6-oxa-
3,7-diaza-cyclopenta[a]antbracene-1-carboxylic acid ethyl ester;
7-Benzyl-2,6x-dimethyl-7,8,9,10,1 Oa, l l -hexahydro-3H,6aH-6-oxa
3,7-diaza-cyclopenta[a]anthraccne-I-carboxylic acid ethyl ester;
2,7-Dimethyl-Ga-phenyl-7,8,9,10,1 Oa, l l-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyclopenta[a]anthracene-I-carboxylic acid ethyl ester;
Pyrrolo[3',2':S,G][1]benzopyrano[3,2-a]indole-I-carboxylic acid,
8,9,1 I , I 2, I 3,13a,14,14a-octahydro-2-methyl-, ethyl ester;
3H,7H-Pyrrolizino[1',8':S,G]pyrano[3,2-a]indole-I-acetic acid,
8,9,11,12,12x,13-hexahydro-2-methyl-, ethyl ester;
2-Methyl-8,9,10,1 Oa,11,12,12x,13-octahydro-3H,6aH,7H-6-oxa-3,6b-
diaza-benzo[a]cyclopcnta[h]anthracene-1-carboxylic acid ethyl ester;
3H-Pyrido[1",2":1'2']azepino[3'2':5,6]pyrano[3,2-a]indole-1-acetic acid,
7,8,9,10,12,13,14,15,15x,16-decahydro-2-methyl-, ethyl ester or
7H-Azepino[1",2":1'2']pyrido[3',2':5,6]pyrano[3,2-a]indole-1-acetic acid,
3,8,9,10,11,13,14,15,15x,16-decahydro-2-methyl-, ethyl ester;
Pyrrolo[3',2':S,G] [ 1 ]benzopyrano[3,2-i]quinolizine-1-carboxamide,
8,9,11,12,13,13x,14,14x-octahydro-2-methyl-N-(phenylmethyl)-;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxamide, N-
ethyl-8,9,11,12,13,13 a,14,14a-octahydro-2-methyl-;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-9-
Pyrrolo[3',2': S, 6][ 1 ]benzopyrano[3,2-i]quinolizine-1-carboxaldehyde,
8,9,11,12,13,13a,14,14a-octahydro-2-methyl-;
Pyrrolo[3',2' :5,6] [ 1 ]benzopyrano[3,2-i]quinolizine-1-
carboxamide,8,9,1 l,12,13,13a,14,14a-octahydro-N,2-dimethyl-;
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-1-carboxylic acid,
8,9, I 1,12,13,13a, l4, l4a-octahydro-2-methyl-, (4-fluorophenyl)-methyl
ester;
Indazolo[4',S':5,6]pyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I S-decahydro-2-methyl-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,12,12-trimethyl-, phenylmethyl ester,
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7, 8, 9,10,12,13,14,14a,15-decahydro-2,10, I 0-trimethyl-, phenyl methyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7, 8, 9,10,12, I 3,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester;
12H-Furo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-7,8,9,10,13,14,14a,15-octahydro-2-methyl-, ethyl ester;
Pyrrolo(3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
4,5-difluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
4,5-dichloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano(3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-4,5-dimethoxy-2-methyl-, phenylmethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, phenylmethyl ester;
Pyrrolo[3',2':5,6](1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, phenylmethyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/I7S99130434
-10-
Pyrrolo(3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, I-(4-fluorophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-
(methoxycarbonyl)phenyl]ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(3-carboxyphenyl)ethyl
ester;
1-Propanaminium, N,N,N-trimethyl-, salt with 1-(3-carboxyphenyl)ethyl
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-pyrrolo[3',2':5,6] [ I ]-
benzopyrano[3,2-i]quinolizine-1-carboxylate (I:1);
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-nitrophenyl)methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(3-cyanophenyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14, I 4a,15-decahydro-2-methyl-,
1-[3-[(dimethylamino)carbonyl]phenyl]ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-[(dimethylamino)methyl)-
phenyl]ethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,1,13,14,14a,15-decahydro-2-methyl-,2-phenylethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [4-(methoxycarbonyl)phenyl]-
methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,-methyl-, (4-carboxyphenyl)methyl
ester;
I -[3-(4-Carboxy-benzyloxycarbonyl)-5-hydroxy-2-methyl-1 H-indol-
4-ylmethyl]-1,2,3,4,6,7,8,9-octahydro-quinolizinylium; chloride;
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [4-(hydroxymethyl)phenyl]-
methyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-1 I-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-2-carboxylic acid,
3,78,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-naphthalenylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1S-decahydro-2-methyl-, [3-(methoxycarbonyl)phenyl]-
methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,IS-decahydro-2-methyl-, [3-(hydroxymethyl)phenyl]-
methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-carboxyphenyl)methyl
ester);
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (3-carboxypheny)-
methyl 3,7,8,9,10, I 2,13,14,14a, I S-decahydro-2-methylpyrrolo[3',2':5,6][ I
]-
benzopyrano[3,2-i]quinolizine-I-carboxylate (1:1);
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9, I 0,12,13,14,14a,15-decahydro-2-methyl-, [3-[(dimethylamino)methyl]-
phenyl]methyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [3-[(dimethylamino)carbonyl]-
phenyl]methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [2-(4-morpholinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[1,1'-biphenylJ-4-ylethyl
ester;
Pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (2,6-difluorophenyl)methyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1-phenyl-2,2,2-
trifluoro)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-(trifluoromethyl)phenyl]-
ethyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-12-
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-(dimethylamino)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-(1-pyrrolidinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(1-naphthalenyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-phenylcyclobutyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-phenylcylopropyl ester;
Pyn-olo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-pyrazinylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(4-quinolinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-pyrimidinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-chloro-3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, phenylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-chloro-3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl, 1-(4-
fluorophenyl)ethyl
ester;
Quinolizinium, 1-[[(4-fluorophenyl)methoxy]carbonyl]-5-hydroxy-
2-methyl-1H-indol-4-yl]methyl]-1,2,3,4,6,7,8,9-octahydro-, chloride;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-1,2-dimethyl-, (4-fluorophenyl)methyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-phenylpropyl ester;
Quinolizinium, 1,2,3,4,6,7,8,9-octahydro-1-[[5-hydroxy-2-methyl-
3-[(phenylmethoxy)carbonyl]-1H-indol-4-yl]methyl]-, chloride;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-methyl-, (4-nitrophenyl)methyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-13-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, l 5-decahydro-2-methyl-, ( 1 R)- I -phenyl ethyl
ester;
Pyrrolo(3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-(4-fluorophenyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13, I 4,14x,15-decahydro-2-methyl-, phenyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2,2,2-trifluoro-I-phenyl-
I-(trifluoromethyl)ethyl ester;
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, bicyclo[2.2.1]hept-2-yl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9, I 0,12,13,14,14x,15-decahydro-2-methyl-, 1-(4-fluorophenyl)-
1-methylethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-phenylcyclopentyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-phenylcyclohexyl ester;
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9, I 0,12,13,14,14x,15-dccahydro-2-methyl-, 3-(hydroxymethyl)phenyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decalaydro-2-methyl-, (3-hydroxyphenyl)methyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (IS)-I-(4-pyridinyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, [6-(methoxycarbonyl)-
2-pyridinyl]methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-pyridinylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (6-carboxy-2-pyridinyl)methyl
ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-14-
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (6-carboxy-
2-pyridinyl)methyl 3,7, 8,9,10, I 2,13,14,14x,15-decahydro-2-
methylpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylate (1:1);
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, [5-(methoxycxrbonyl)-
3-pyridinyl]methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (5-carboxy-3-pyridinyl)methyl
ester;
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (5-carboxy-
3-pyridinyl)methyl 3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-
methylpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylate (I:1);
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(4'-methyl[ I ,1'-biphenyl]-
3-yl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2,6-dimethylphenyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S,2R)-2-(dimethylamino)-
1-phenylpropyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (IR,2S)-2-(dimethylamino)-
1-phenylpropyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-naphthalenyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-methyl-, diphenylmethyl ester;
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1R)-2,3-dihydro-1H-inden-1-yl
ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-1 S-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,2-dihydro-1-acenaphthylenyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, cyclohexyl(phenyl)methyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 9H-fluoren-9-yl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,2,3,4-tetrahydro-
1-naphthalenyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [(2R,3R)-
3-phenyloxiranyl]methyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-oxo-1,2-diphenylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 10,11-dihydro-5H-
dibenzo[a,d]cyclohepten-5-yl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (2-methylphenyl)phcnylmethyl
ester;
Pymolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-m ethyl-,
cyclopropyl(4-fluorophenyl)methyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3,4-dihydro-2H
1-benzothiopyran-4-yl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S)-1-(2-bromophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2,2,2-trifluoroethyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-16-
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, [(2S,3S)-
3-phenyloxiranyl]methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
S 3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2,2,2-trifluoro-1-methyl-
1-(trifluoromethyl)ethyl ester;
Pyrrolo[3°,2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic
acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2,2,2-trifluoro-
1-(4-fluorophenyl)-1-(trifluoromethyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-cyclopentyl-1-phenylcthyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-[1,1'-biphenyl]-4-yl-
1-methylethyl ester;
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-methyl-1-phenyl-2-propynyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-methyl-, 1,1-diphenylethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-methyl-1,2-diphenylethyl
ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(4-fluorophenyl)cyclohexyl
ester;
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,2-diphenylethyl ester;
Pyn-olo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-methyl-, 1-phenyl-2-propynyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, [1,1'-biphenyl]-4-ylmethyl
ester;
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99/30434
_17_
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 4-pyridinylmethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,2,3,4-tetrahydro-
7,8-dimethoxy-2-methyl-4-isoquinolinyl ester;
Pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-[3-(dimethylamino)phenyl]-
ethyl ester;
Pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,1-dimethyl-2-pyrazinylethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 4-(dipropylamino)-
1,1-dimethyl-2-butynyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (IS)-2,3-dihydro-1H-inden-1-yl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S,2S)-2-(dimethylamino)-
I-phenylpropyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, [4-(trifluoromethyl)phenyl]-
methyl ester;
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (4-chlorophenyl)methyl ester;
Pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-, phenylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-hydroxyethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)qninolizine-1-carboxylic acid,
3,7,8,9, I 0,12, I 3,14,14x,15-decahydro-2-methyl-, (3-methylphenyl)methyl
ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/L1S99/30434
-18-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, ( I S)-1-phenylethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-phenylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carbothioic acid,
3,7,8.,9,10,12,13,14,14x,15-decahydro-2-methyl-, S-(phenylmethyl) ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-pyridinylmethyl ester;
Pyrrolo[3',2':5,6](1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-[4-(trifluoromethyl)phenyl]-
ethyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-(pentafluorophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2,6-difluorophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S)-I-(2-furanyl)cthyl ester
Pyrrolo[3',2':5,6][1]benzopvrano[3,2-i]quinolizine-1-carboxylic acid,
3, 7,8,9,10,12,13,14, I 4x,15-decahydro-2-methyl-, 2-(4-morpholinyl)-
1-phenylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1R)-I-(2-furanyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-methoxy-2-oxo-I-phenylethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(3-pyridinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (S)-carboxy(phenyl)methyl
ester;
CA 02372197 2001-07-12
WO 00!42045 PCT/US99130434
-19-
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (S)-
carboxy(phenyl)methyl, 3,7,8,9,10,12,13,14,14x,15-decahydro-2-
methylpyrrolo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylate (1:1);
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1R)-2-methoxy-2-oxo-
1-phenylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (R)-carboxy(phenyl)methyl
ester;
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (R)-
carboxy(phenyl)methyl, 3,7,8,9,10,12,13,14,14x,15-decahydro-
2-methylpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxyl ate
(I:I);
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 4-pyridinylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-(4-pyridinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-pyridinyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-thienylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S)-1-(4-fluorophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1R)-1-(4-fluorophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-pyridinylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-ethoxy-2-oxoethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-furanylmethyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCTNS99/30434
-20-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (2-nitrophenyl)methyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I S-decahydro-2-methyl-, 2-furanylmethyl ester;
S Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-(2-chlorophenyl)ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I 5-decahydro-2-methyl-, carboxymethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2,6-dichlorophenyl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-methoxyphenyl)ethyl
ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-(5-carboxy-3-pyridinyl)ethyl
ester;
1,3-Benzenedicarboxylic acid, 5-[([(3,7,8,9,10,12,13,14,14a,15-
decahydro-2-methylpyrrolo[3',2':5,6][ 1 ]benzopyrano(3,2-i]quinolizin-
1-yl)carbonyl]oxy]methyl]-, diethyl ester;
1,3-Benzenedicarboxylic acid, 5-[[[(3,7,8,9,10,12,13,14,14a,15-
decahydro-2-methylpyrrolo[3',2':5,6] [ 1 ]benzopyrano[3,2-i]quinolizin-
1-yl)carbonyl]oxy]methyl]-;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (2-chloro-5-nitrophenyl)methyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (2-chloro-5-nitrophenyl)methyl
ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (5-xmino-
2-chlorophenyl)methyl ester;
Pyrrolo(3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-I-acetic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-.alpha.-oxo-, ethyl ester;
CA 02372197 2001-07-12
WO 00/42045 PCTNS99130434
-21-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4,5-trimethyl-, phenylmethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,1-dimethyl-2-propynyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2,2,2-trichloro-
1,1-dimethylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, tricyclo[3.3.1.137]dec-1-yl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-methyl-1-phenylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-methylcyclohexyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,1,2-trimethylpropyl ester;
Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-methylcyclopentyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-cyclohexyl-1-methylethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,4-dimethyl-4-piperidinyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 4-fluorophenyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-dccahydro-2-methyl-, 3-methylphenyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, phenyl ester;
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-(methoxycarbonyl)phenyl
ester;
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-22-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-pyridinyl ester;
Pyrrolo(3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12, I 3,14,14a,15-decahydro-2-(trifluoromethyl)-, ethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [5-[(dimethylamino)methyl]-
2-furanyl]methyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-carboxy-2-methylpropyl
ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-(dimethylamino)-
2,2-dimethylpropyl ester;
Propanedioic acid, monoanhydridc with 3,7,8,9,10,12,13,14,14a,15-
decahydro-2-methylpyrrolo[3',2':5,6] [ 1 ]-benzopyrano[3,2-i)quinolizine-
1-carboxylic acid, I,1-dimethylethyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(dimethylamino)-
2-methylpropyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(1H-imidazol-1-yl)ethyl
ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-benzofuranylmcthyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (IR,2S)-2-(dimethylamino)-I-
phenylpropyl ester;
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S,2R)-2-(dimethylamino)-1-
phenylpropyl ester;
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-fluorophenyl)cyclopropyl
ester;
CA 02372197 2001-07-12
WO 00142045 PCT/tJS99/30434
-23-
Pyridinium, 3-[[[(3,7,8,9,10,12,13,14,14a,15-decahydro-2-
methylpyrrolo[3',2':5,6] [ 1 ]benzopyrano [3,2-i]quinolizin-1-
yl)carbonyl]oxy]methyl]-1-methyl-, methanesulfonate;
Pyrrolo[3',2':5,6] [ 1 ]benzopyrano[3,2-i]quinol izinium,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,11-dimethyl-1-[(S)-( 1-
phenylethoxy)carbonyl]-, methanesulfonate; and
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S)-1-phenylethyl ester,
11-oxide.
The instant invention includes pharmaceutical compositions of compounds
of Formula 1 and methods of using the compounds for modulating chemokine
receptor activity, for preventing or treating infection by HIV, delaying the
onset of
AIDS, treating AIDS, and treating inflammatory disease.
DETAILED DESCRIPTION OF THE INVENTION
In this present invention, compounds of Formula I can exist in two forms
(close and open form) at the bicyclic aminal moiety. The equilibrium between
these two forms is pH dependent. At a neutral or basic pH (pH ?7.0), these
compounds predominantly exist in the closed form. However, at an acidic pH
range (pH <7.0), these molecules may exist as a mixture of both close and open
form. The ratio of closed and open form may depend on pH and solvent, as well
as
the nature of substituents R, R2-R6, and n.
In the compounds of Formula I, the term alkyl means a straight or
branched hydrocarbon radical having from 1 to 8 carbon atoms and includes, for
example, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl,
tert-butyl, n-pentyl, n-hexyl, ti-heptyl, n-octyl, and the like. The alkyl can
be
substituted with fluorine, for example, additionally the alkyls can be
substituted
with from 1 to 3 substituents selected from alkoxy, carboxy, hydroxy, nitro,
halogen, amino, and substituted amino to provide other active compounds. Alkyl
includes cycloalkyl of from 3 to 7 carbons which can be substituted with, for
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-24-
example, I to 3 substituents selected from alkyl, alkoxy, carboxy, hydroxy,
nitro,
halogen, and amino and substituted amino. Cycloalkyl can be fused to an aryl
ring
such as phenyl, pyridyl, and the like.
Alkoxy is O-alkyl of from 1 to 6 carbon atoms as defined above for alkyl.
O
Acyl is -C-alkyl, wherein alkyl is as defined above.
The term aryl means an aromatic radical which is a phenyl group, a phenyl
group substituted by 1 to 4 substituents selected from alkyl as defined above,
alkoxy as defined above, hydroxy, halogen, trifluoromethyl, amino, alkylamino
as
def ned above for alkyl, dialkylamino as defined for alkyl, nitro, cyano,
carboxy,
O O O
S03H, CHO, -C-alkyl as defined above for alkyl, -C-NH2, -C-NH-alkyl as
O
defined above for alkyl, -C-N(alkyl)2 as deCned above for alkyl, -(CH2)112-NH2
wherein n2 is an integer of 1 to 5, -(CH2)1~2-NH-alkyl as defined above for
alkyl
and n2, -(CH2)112-N(alkyl)2 as defined above for alkyl and n2. The terns
further
includes heteroaryl which is a mono or bicyclic heteroaromatic radical having
5 to
10 atoms which may contain one or more of heteroatom such as N, O, S,
including, for example, 2- or 3-thienyl, 2- or 3-furanyl, 2- or 3-pyrrolyl, 2-
, 3-, or
4-pyridinyl, 2-pyrazinyl, 2-, 4-, or 5-pyrimidinyl, 3- or 4-pyridazinyl, or 2-
, 3-, 4-,
5-, 6-, or 7-indolyl. The heteroaryls can be unsubstituted or substituted as
above
for aryl.
fhe term aralkyl or arylalkyl means an aryl radical attached to an alkyl
radical wherein aryl and alkyl are as defined above, for example, benzyl,
fluorenylmethyl, and the like.
Halogen is fluorine, chlorine, bromine, or iodine.
Some of the compounds of ring close Formula I are capable of further
forming N-oxides and N-quaternary alkyl salts with the ring nitrogen atom at
N-11. Further, some of the compounds of ring close Formula I are capable of
further forming N-oxides and N-quaternary alkyl salts with nitrogen atoms
CA 02372197 2001-07-12
WO 00/42045 PCT/U599/30434
-25-
optionally present in R10. These structural forms are within the scope of the
present invention.
Some of the compounds of Formula I are capable of further forming both
pharmaceutically acceptable acid addition and/or base salts. All of these
forms are
within the scope of the present invention.
Pharmaceutically acceptable acid addition salts of the compounds of
Formula I include salts derived from nontoxic inorganic acids such as
hydrochloric, nitric, phosphoric, sulfuric, hydrobromic, hydroiodic,
hydrofluoric,
phosphorous, and the like, as well as the salts derived from nontoxic organic
acids, such as aliphatic mono- and dicarboxylic acids, phenyl-substituted
alkanoic
acids, hydroxy alkanoic acids, alkanedioic acids, aromatic acids, aliphatic
and
aromatic sulfonic acids, etc. Such salts thus include sulfate, pyrosulfate,
bisulfate,
sulfite, bisulfate, nitrate, phosphate, monohydrogenphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide,
acetate, trifluoroacetate, propionate, caprylate, isobutyrate, oxalate,
malonate,
succinate, suberate, sebacate, fumarate, maleate, mandelate, benzoate,
chlorobenzoate, methylbenzoate, dinitrobenzoate, phthalate, benzenesulfonate,
toluenesulfonate, phenylacetate, citrate, lactate, maleate, tartrate,
methanesulfonate, and the like. Also contemplated are salts of amino acids
such as
arginate and the like and gluconate, galacturonate (see, for example, Berge
S.M.
et al., "Pharmaceutical Salts,"J. ofPhczrj~ta. Sci., 1977;66:1).
The acid addition salts of said basic compounds are prepared by contacting
the free base form with a sufficient amount of the desired acid to produce the
salt
in the conventional manner. The free base form may be regenerated by
contacting
the salt form with a base and isolating the free base in the conventional
manner.
The free base forms differ from their respective salt forms somewhat in
certain
physical properties such as solubility in polar solvents, but otherwise the
salts are
equivalent to their respective free base for purposes of the present
invention.
Pharmaceutically acceptable base addition salts are formed with metals or
amines, such as alkali and alkaline earth metals or organic amines. Examples
of
metals used as canons are sodium, potassium, magnesium, calcium, and the like.
Examples of suitable amines are N,N'-dibenzylethylenediamine, chloroprocaine,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-26-
choline, diethanolamine, dicyclohexylamine, ethylenediamine,
N-methylglucamine, and procaine (see, for example, Berge supra., 1977}.
The base addition salts of said acidic compounds are prepared by
contacting the free acid form with a sufficient amount of the desired base to
produce the salt in the conventional manner. The free acid form may be
regenerated by contacting the salt form with an acid and isolating the free
acid in
the conventional manner. The free acid forms differ from their respective salt
forms somewhat in certain physical properties such as solubility in polar
solvents,
but otherwise the salts are equivalent to their respective free acid for
purposes of
the present invention.
Certain of the compounds of the present invention can exist in unsolvated
forms as well as solvated forms, including hydrated forms. In general, the
solvated
forms, including hydrated forms, are equivalent to unsolvated forms and are
intended to be encompassed within the scope of the present invention.
Certain of the compounds of the present invention possess one or more
chiral centers and each center may exist in the R or S configuration. The
present
invention includes all diastereomeric, enantiomeric, and epimeric forms as
well as
the appropriate mixtures thereof. Additionally, the compounds of the present
invention may exist as geometric isomers. The present invention includes all
cis,
trans, syn, anti, entgegen (E), and zusammen (Z) isomers as well as the
appropriate mixtures thereof.
The compounds of the present invention can be prepared and administered
in a wide variety of oral and parenteral dosage forms. Thus, the compounds of
the
present invention can be administered by injection, that is, intravenously,
intramuscularly, intracutaneously, subcutaneously, intraduodenally, or
intraperitoneally. Also, the compounds of the present invention can be
administered by inhalation, for example, intranasally. Additionally, the
compounds of the present invention can be administered transdermally. It will
be
obvious to those skilled in the art that the following dosage forms may
comprise
as the active component, either a compound of Formula I or a corresponding
pharmaceutically acceptable salt of a compound of Formula I.
For preparing pharmaceutical compositions from the compounds of the
present invention, pharmaceutically acceptable carriers can be either solid or
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99130434
-27-
liquid. Solid form preparations include powders, tablets, pills, capsules,
cachets,
suppositories, and dispersible granules. A solid carrier can be one or more
substances which may also act as diluents, flavoring agents, solubilizers,
lubricants, suspending agents, binders, preservatives, tablet disintegrating
agents,
or an encapsulating material.
In powders, the earner is a finely divided solid which is in a mixture with
the finely divided active component.
In tablets, the active component is mixed with the carrier having the
necessary binding properties in suitable proportions and compacted in the
shape
and size desired.
The powders and tablets preferably contain from five or ten to about
seventy percent of the active compound. Suitable carriers are magnesium
carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch,
gelatin,
tragacanth, methylcellulose, sodium carboxymethyleellulose, a low melting wax,
cocoa butter, and the like. The term "preparation" is intended to include the
formulation of the active compound with encapsulating material as a carrier
providing a capsule in which the active component, with or without other
carriers,
is surrounded by a carrier, which is thus in association with it. Similarly,
cachets
and lozenges are included. Tablets, powders, capsules, pills, cachets, and
lozenges
can be used as solid dosage forms suitable for oral administration.
For preparing suppositories, a low melting wax, such as a mixture of fatty
acid glyceridcs or cocoa butter, is first melted and the active component is
dispersed homogeneously therein, as by stirring. The molten homogenous mixture
is then poured into convenient sized molds, allowed to cool, and thereby to
solidify.
Liquid form preparations include solutions, suspensions, and emulsions,
for example, water or water propylene glycol solutions. For parenteral
injection,
liquid preparations can be formulated in solution in aqueous polyethylene
glycol
solution.
Aqueous solutions suitable for oral use can be prepared by dissolving the
active component in water and adding suitable colorants, flavors, stabilizing,
and
thickening agents as desired.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-28-
Aqueous suspensions suitable for oral use can be made by dispersing the
finely divided active component in water with viscous material, such as
natural or
synthetic gums, resins, methylcellulose, sodium carboxymethylcellulose, and
other well-known suspending agents.
Also included are solid form preparations which are intended to be
converted, shortly before use, to liquid form preparations for oral
administration.
Such liquid forms include solutions, suspensions, and emulsions. These
preparations may contain, in addition to the active component, colorants,
flavors,
stabilizers, buffers, artificial and natural sweeteners, dispersants,
thickeners,
solubilizing agents, and the like.
The pharmaceutical preparation is preferably in unit dosage form. In such
form, the preparation is subdivided into unit doses containing appropriate
quantities of the active component. The unit dosage form can be a packaged
preparation, the package containing discrete quantities of preparation, such
as
packeted tablets, capsules, and powders in vials or ampoules. Also, the unit
dosage form can be a capsule, tablet, cachet, or lozenge itself, or it can be
the
appropriate number of any of these in packaged form.
The quantity of active component in a unit dose preparation may be varied
or adjusted from 1 mg to 1000 mg, preferably 10 mg to 10U mg according to the
particular application and the potency of the active component. The
composition
can, if desired, also contain other compatible therapeutic agents.
In therapeutic use as agents for the treatment of HIV infection, the
compounds utilized in the pharmaceutical method of this invention can be
administered at the initial dosage of about 1 mg to about 100 mg per kilogram
daily. A daily dose range of about 25 mg to about 75 mg per kilogram is
preferred.
The dosages, however, may be varied depending upon the requirements of the
patient, the severity of the condition being treated, and the compound being
employed. Determination of the proper dosage for a particular situation is
within
the skill of the art. Generally, treatment is initiated with smaller dosages
which are
less than the optimum dose of the compound. Thereafter, the dosage is
increased
by small increments until the optimum effect under the circumstance is
reached.
For convenience, the total daily dosage may be divided and administered in
portions during the day if desired.
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-29-
The compounds of Formula I are valuable antagonists of the CCR-5
chemokine receptor. Compounds which are antagonists of the CCR-5 chemokine
receptor are expected to have efficacy in inhibiting HIV infection and are
thus
useful in the treatment of AIDS. The compounds of the present invention were
evaluated in a CCR-5 receptor binding assay.
CCR-5 Receptor Binding Assay
The 1251-gp 120/sCD4/CCR-5 binding assay was carried out similarly as
described in Wu et al., Nature, 1996;384:179-183. Briefly, the envelope gp120
protein derived from HIV-1 JR-FL (Trkola et al., Nature, 1996;384:184-186), a
M-tropic strain, was iodinated using solid phase lactoperoxidase to a specific
activity of 20 pCi/pg. For each binding reaction (in a final volume of 100 p.L
binding buffer [50 mM HEPES, pH 7.5, 1 mM CaCl2, 5 mM MgCl2, and 0.5%
BSA]), 25 pL (2.5 pg) of membranes prepared from CCR-5/L 1.2 cells were
mixed with 25 pL (3 nM) sCD4, followed by 25 ~tL (0.1 nM) radio-labeled gp120
in the presence or absence of 25 pL compound dissolved in DMSO (final
concentration of DMSO 0.5%). The reactions were incubated at room temperature
for 45 to 60 minutes and stopped by transferring the mixture to GFB filter
plates,
which were then washed 3 to 4 times with binding buffer containing 0.5 M NaCI.
The plates were dried and MicroScint scintillation fluid was added before
, counting.
The compounds of present invention, represented by Formula I, block the
sCD-4/GP-120 binding to CCR-5 receptor with affinity less than or equal to
200 ~M.
Synthesis of CCR-5 Analogs
Synthesis of the final target compounds is shown in Scheme 1.
Compound II in a protic solvent, preferably ethanol, was treated with aqueous
forn~aldehyde and dimethylamine at temperatures which ranged from 0-
90°C,
preferably at 25-60°C, to give the Mannich base III. Condensation of
III with a
enamine at temperatures which ranged from 50-110°C, preferably at 80-
100°C,
under nitrogen atmosphere in an aprotic solvent, preferably dioxane, gives
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-3 0-
compound IV. Alkylation of IV with NaH and alkylhalides in an aprotic solvent,
preferably DMF, under nitrogen atmosphere at temperatures which ranged from
-10 to 25°C, preferably at 0-25°C, provides compound I (where R1
~ H).
The preparation of indole intermediates is shown in Scheme II. Reaction of
bromoacetate with nitriles in an aprotic solvent, preferably THF, in the
presence
of activated Zn at reflux under nitrogen atmosphere gives amino crotonates V.
Alternatively, amino crotonates V can be obtained by reacting the
corresponding
(3-ketoester with ammonia in EtOH. The (3-ketoestcrs can be derived from 2,2,6-
trimethyl-4H-1,3-dioxin-4-one and the con-esponding alcohols. Condensation of
amino crotonates V with substituted benzoquinone in a solvent, preferably
acetic
acid, ethanol, or nitromethane at temperatures which ranged from 25°C
to reflux
affords substituted 5-hydroxyindoles VI. The indole ester VI is hydrolyzed to
the
corresponding acid VII using aqueous NaOH at temperatures which ranged from
50-100°C, preferably at reflux, under nitrogen. To suppress the
decarboxylation
reaction, it is important that after the reaction is done the reaction mixture
was
cooled to 0°C in an ice-water bath and acidified with a concentrated
acid,
preferably HCI, at 0°C to generate the acid. Esters or amides IX can be
made from
acid VIII following several standard esterification procedures or a standard
procedure for amide synthesis using HBTU as the coupling reagent. For the
ester
synthesis, Mitsunobu procedure is preferred where appropriate alcohols, DEAD,
and Ph3P are used, and the reaction is carried out at ambient temperature.
Another
preferred procedure to make esters is treating the acid with a base,
preferably
DBU, and alkylhalides or arylalkylhalides in a polar solvent, preferably DMF
or
acetonitrile at ambient temperature.
The following schemes are illustrative of the procedures useful in the
preparation of final compounds. Variations known to skilled chemists are
considered part of the invention.
CA 02372197 2001-07-12
WO 00/42045 PCT/~JS99/30434
-31-
Scheme 1
Preparation of Final Target Compounds
R6
-(CH2 )n
O Y /N 8110 RS N / R3
HO / I ~ R11CH0 Y R4
X HO
N
R2 R H Me2NH ~ I N X dioxane
R2 H
R
II III
c6 R6~
~(CH2 )n ~(CH2 )n
N R ~O
/ 3 NaH /N R3 O ~,
R5 4 O / 1 \ Y ----~ RS 4 O / R11
X R1X I \X
N ~ '
N
R2 R H R2 R
R1
IV I
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-32-
Scheme 2
Preparation of Substituted Indole Derivatives
O
O xylenes O O
+ R'OH ~ ~
O 150°C / v \0R'
NH3
EtOH
O O
C02R'
O activated Zn
1. Br~ + R~-CN ~ R2 R
OR' THF, reflux H N R
2 7
V
O OR~ O OH
HO / HO
N~R,/ Nap \ I N~R
7
R2 R H R2 R H
VI VII
O O
OH y
2. R'0 R'O
\X ester or amide ~ ~ \X
R2 \ N formation R2
R H R H
R' = H or CI-I3C0
VIII IX
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-33-
Scheme 3
O R3 O 02R~2
R %'~ , HO
2 H2N OR 2 /
\ I O>-- R3
O R3=Me, Ph R2 C
A
C(O)XR'2 ~N C(O)XR'2
HO / HO
/
\ ~R3 ~ \ ~ ~ R3
R2 R2 O
D E
X=O,N
~/ Z,
F 2
N
~2
G
Substituted S-hydroxybenzofurans (C).
S The substituted 5-hydroxybenzofurans (C) were prepared by condensing
the appropriate 1,4-benzoquinone (A) with the appropriate 3-aminocrotonate (B)
in acetic acid. The solvent was removed in vacuo, and the product was purified
by
recrystallization or flash chromatography on silica gel.
Mannich Bases (E).
The S-hydroxybenzofuran (C, D) was treated with aqueous dimethylamine
and aqueous formaldehyde in ethanol at 50°C or, alternatively, with
N,N,N',N'-
tetramethyldiaminomethane in refluxing dioxane until the reaction was
complete.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-34-
The solution was concentrated under reduced pressure, and the product was
purified by recrystallization.
Benzofurans (G).
The mannich base (E) was added to a dioxane solution of enamine (F),
which was freshly prepared by treating its perchlorate salt with aqueous
sodium
hydroxide, extracting the enamine into ether, drying and concentrating the
extracts
in vacuo. The resulting solution was heated between 80-100°C until the
reaction
was complete. The mixture was concentrated in vacuo and the product purified
by
recrystallization or flash chromatography on silica gel.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-35-
Scheme 4
Synthesis of 7-Azaindoles Analogs
OH H ~ 'pH 1 OH H3C
II \J~' 1Z I ~ OH
P~N=N N pd/C, AcOH HZN N AcOH, rt HZN N Pd(dppf)C12
LiCI, NaZC03
DMF, 100°C
Pd(dppf)C12 3
LiCI, NaZC03 O
DMF, 100°C H3C-
OH
O O
HO \ I I OH IC MnOq HO \ I I O R---~ O \ I I
_ OR
N H KZ O N~ HBTU N
H H
6
N~
O N
N O
CHZO HO ~ I I O O ~ OR
w~ ~ w
HN(CH ) N N
32 H N H
7 8
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-36-
Procedures:
Starting with commercially available 3-hydroxy-2-phenylazopyridine (1) the
corresponding aminopyridine is synthesized by reducing 1 in the presence of
H2 (57 bar) and Pd/C in acetic acid at 65°C (Synthesis, 1990: 681) to
afford
amine 2. Amine 2 is then transformed into 2-amino-5-hydroxy-3-iodopyridine
through reaction with iodine and acetic acid (Synthesis, 1990:681) at room
temperature. The iodopyridine 3 is then converted to the azaindole 4 via
palladium
catalyzed cyclization with the appropriately substituted alkyne (Tetrahedron
Lett.,
1998;39:5355; Tetrahedron Lett., 1993;34:2823). Conversion of 3 to 4 is
followed
by oxidation of the hydroxymethylene to the corresponding acid 5 using
KMn04 in the presence of K2C03 (Gazz. Chim. Itnl., 1932;62:844)
Alternatively, direct conversion of 3 to 5 is accomplished by using the
carboxy
substituted alkyne. Esterification of 5 to the desired ester is effected using
diimide
coupling reagents and the desired alcohol (J. Of g. CJrem., 1995;60:5214).
Substitution of the pyridine ring using formaldehyde and dimethyl amine
(Tetrahedron Lett., 1966:4459) afforded 7. Intermediate 7 is then converted to
the
final azaindole analog 8 by reacting 7 and the quinolizidine imine shown in
refluxing ethanol (J. Net. Chenz., 1970;7:131).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-37
Scheme 5
Preparation of Final Target Compounds From Novel Intermediate
R6
1. oxalyl chloride Rg
2. NHR~R8
Kl ~ RI
I II
R6
---(CH2 ) n
1. oxalyl chloride /N R3 O O-R~
RS
2. HORS
R2
R1 IV Rl
I O III
~ 'O~
Cl~ R
'0I
O \
Halogen
7
base
Z6
--(CH2)n
R,N R3
CORD
\X
N
R2
R1
CA 02372197 2001-07-12
WO 00/42045 PCTJUS99/30434
-38-
Compound I in an aprotic solvent, preferably Et20, CH2C12, or THF, was treated
with a solution of oxalyl chloride in the same aprotic solvent at temperatures
ranged from -10°C to 30°C, preferably at 0°C to
25°C, followed by treatment of
an amine of choice in an aprotic solvent to give the desired product II. The
desired
product III can be obtained by reacting compound I with oxalyl chloride in an
aprotic solvent, preferably Et20, CH2Cl2, or THF, at temperatures ranged from
-10°C to 30°C, preferably at 0°C to 25°C, followed
by treatment of an alcohol of
choice in an aprotic solvent. Alternatively, the desired product III can be
made by
reacting compound I with compound IV in an aprotic solvent such as Et20,
CH2Cl2, or THF.
CA 02372197 2001-07-12
WO 00!42045 PCT/US99/30434
-39
Scheme 6
Preparation of the Mixed Anhydride
R6 R6.
CH2)n ~ ~ ~ CH2)n
N R O
RS 3 O Pd/C (20%) R/N R3 O
R4 O -~' S R4
%X THF, Hz O / ~ X
N
R2 ' N
R R2
I RI
I II
R6
O ~-- CH2)n
O
R/N R3 O O
Cl R4 O /
\\X
Et3N, THF i
".
K2
Rl
III
General Description
5 The benzylester I is subjected to hydrogenolysis reaction conditions in
aprotic polar solvents, preferably THF, at ambient temperature to give acid
II. The
acid II is treated subsequently with benzoyl chloride in presence of an
organic
base, such as Et3N, to afford the mixed anhydride III.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-40-
Scheme 7
Synthesis of Esters From the Mixed Anhydride
R6
% 10
RS O
RS R4 O\ ~ ~O + RIO -OH 100-180°C
R2
1Z1 ~ R1
I II
General Description
The mixed anhydride I is mixed with the desired alcohol, the resultant
reaction mixture was heated to 100°C to 180°C until the mixed
anhydride is
consumed affording the corresponding ester.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-41-
The present invention is further directed to combinations of the present
compounds with one or more agents useful in the prevention or treatment of
AIDS. For example, the compounds of this invention may be effectively
administered, whether at periods of pre-exposure and/or post-exposure, in
combination with effective amounts of the anti-HIV compounds,
immunomodulators, anti-infectives, or prophactic or therapeutic vaccines known
to those of ordinary skill in the art.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-42
ANTIVIRALS
Drug Name Manufacturer Indication
097 Hoechst/Bayer HIV infection, AIDS,
ARC (non-
nucleoside reverse transcriptase
(RT) inhibitor)
GW141 W94/ Glaxo Wellcome HIV infection, AIDS,
ARC
VX478 (protease inhibitor)
Amprenavir
GW 1592U89 Glaxo Wellcome HIV infection, AIDS,
ARC
Abacavir (RT inhibitor)
Acemannan Carrington LabsARC
(Irving, TX)
Acyclovir Burroughs WellcomeHIV infection, AIDS,
ARC, in
Combination with AZT
AD-439 Tanox BiosystemsHIV infection, AIDS,
ARC
AD-519 Tanox BiosystemsHIV infection, AIDS,
ARC
Adefovir dipivoxilGilead SciencesHIV infection
AL-721 Ethigen ARC, PGL HIV positive,
AIDS
(Los Angeles,
CA)
Alpha InterferonGlaxo Wellcome Kaposi's sarcoma, HIV
in
combination
Alferon InterferonInterferon SciencesKaposi's sarcoma, HIV
in
combination
Ansamycin Adria LaboratoriesARC
LM 427 (Dublin,'OH)
Erbamont
(Stamford, CT)
Antibody whichAdvanced BiotherapyAIDS, ARC
neutralizes Concepts
pH
labile alpha (Rockville,
MD)
aberrant
Interferon
AR177 Aronex Pharm HIV infections, AIDS,
ARC
beta-Iluoro-ddA Nat'I Cancer Institute AIDS-associated diseases
CA 02372197 2001-07-12
WO 00!42045 PCT/US99/30434
-43-
ANTIVIRALS (cont'd)
Dnig Name Manufacturer Indication
BMS-232623 Bristol-Myers HIV infection, AIDS,
ARC
(CGP-73547) SquibblNovartis(protease inhibitor)
BMS-234475 Bristol-Myers HIV infection, AIDS,
ARC
(CGP-61755) Squibb/Novartis(protease inhibitor)
(-)6-Chloro-4(S)-Merck HIV infection, AIDS,
ARC
cyclopropylethynyl- (non-nucleoside reverse
4(S)-trifluoro- transcriptase inhibitor)
methyl-1,4-dihydro-
2H-3,1-benzoxazin-
2-one
CI-1012 Warner-Lambent HIV-1 infection
Cidofovir Gilead Science CMV retinitis, herpes,
papillomavirus
Combivir AZT+3TCGlaxo Wellcome HIV infection, AIDS,
ARC
Curdlan sulfateAJI Phanna USA HIV infection
CytomegalovirusMedImmune CMV retinitis
immune globin
Cytovene GanciclovirSyntex/Roche Sight threatening CMV,
peripheral CMV, retinitis
Delaviridine Pharmacia-UpjohnHIV infection, AIDS,
ARC
(RT inhibitor)
Dextran SulfateUeno Fine Chem.AIDS, ARC, HIV positive
Ind.
Ltd. (Osaka, asymptomatic
Japan)
HIVID (ddc) Hoffman-La RocheHIV infection, AIDS,
ARC
Dideoxycytidine
ddI DideoxyinosineBristol-Myers HIV infection, AIDS,
Squibb ARC;
combination with AZT/d4T
DMP-450 Triangle HIV infection, AIDS,
ARC
Pharmaceutical (protease inhibitor)
Efavirenz (DMPDuPont Merck HIV infection, AIDS,
266) ARC
(non-nucleoside RT
inhibitor)
EL10 Elan Corp, PLC HIV infection
(Gainesville,
GA)
Famciclovir Smith Kline Herpes zoster, herpes
simplex
Foscavir/FoscarnetAstra CMV, HSV 1-2
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-44-
ANTIVIRALS (cont'd)
Drug Name Manufacturer Indication
FTC Triangle HIV infection, AIDS,
ARC
Pharmaceutical (reverse transcriptase
inhibitor)
GS 840 Gilead HIV infection, AIDS,
ARC
(reverse transcriptase
inhibitor)
HBY097 Hoechst Marion HIV infection, AIDS,
ARC
Roussel (non-nucleoside reverse
transcriptase inhibitor)
Hypericin VIMRx Pharm. HIV infection, AIDS,
ARC
Recombinant Triton BiosciencesAIDS, Kaposi's sarcoma,
Human ARC
Interferon (Almeda, CA)
Beta
Interferon Interferon SciencesARC, AIDS
alpha-n3
Indinavir Merck HIV infection, AIDS,
ARC,
asymptomatic HIV
positive,
also in combination
with
AZT/ddI/ddC
ISIS 2922 ISIS PharmaceuticalsCMV retinitis
JE 2147 (KNI-764)Japan Energy/ HIV infection, AIDS,
ARC
Protease inhibitorAgouron PI (reverse transcriptase
inhibitor);
also with AZT
KNI-272 Nat'1 Cancer HIV-associated diseases
Institute
Lamivudine, Glaxo Wellcome HIV infection, AIDS,
3TC ARC
(reverse transcriptase
inhibitor);
also with AZT
Lobucavir Bristol-Myers CMV infection - HBV
Squibb infection
Nelfinavir Agouron HIV infection, AIDS,
ARC
Pharmaceuticals (protease inhibitor)
Nevirapine Boeheringer HIV infection, AIDS,
ARC
Ingleheim (RT inhibitor)
Novapren Novaferon Labs, HIV inhibitor
Inc.
(Akron, OH)
Peptide T Peninsula Labs AIDS
Octapeptide (Belmont, CA)
Sequence
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-45
ANTIVIRALS (cont'd)
Drug Name Manufacturer Indication
PNU-140690 Pharmacia UpjohnHIV infection, AIDS,
ARC
(protease inhibitor)
Probucol Vyrex HIV infection, AIDS
RBD-CD4 Sheffield Med. HIV infection, AIDS,
Tech ARC
(Houston, TX)
Ritonavir Abbott HIV infection, AIDS,
ARC
(protease inhibitor)
S-1153 Agouron/ShionogiNnRTI
Saquinavir Hoffmann-La RocheHIV infection, AIDS,
ARC
(protease inhibitor)
Stavudine; Bristol-Myers HIV infection, AIDS,
d4T Squibb ARC
Didehydrodeoxy-
thymidine
Valaciclovir Glaxo Wellcome Genital HSV & CMV infections
Virazole RibavirinViratek/ICN Asymptomatic HN positive,
(Costa Mesa, LAS, ARC
CA)
Zidovudine; Glaxo Wellcome HIV infection, AIDS,
AZT ARC,
Kaposi's sarcoma, in
combination with other
therapies
IMMUNO-MODULATORS
Drug Name Manufacturer Indication
AS-101 Wyeth-Ayerst AIDS
Bropirimine Pharmacia UpjohnAdvanced AIDS
Acemannan Carrington Labs,AIDS, ARC
Inc.
(Irving, TX)
CL246,738 American CyanamidAIDS, Kaposi's sarcoma
Lederle Labs
EL10 Elan Corp, PLC HIV infection
(Gainesville,
GA)
FP-21399 Fuki ImmunoPharmBlocks HIV fusion with
CD4+ cells
Gamma InterferonGenentech ARC, in combination
w/TNF
(tumor necrosis factor)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-46-
IMMUNO-MODULATORS (cont'd)
Drug Name Manufacturer Indication
Granulocyte Genetics InstituteAIDS
Macrophage 5andoz
Colony Stimulating
Factor
Granulocyte Hoeschst-RousselAIDS
Macrophage Immunex
Colony Stimulating
Factor
Granulocyte Schering-PloughAIDS, combination w/AZT
Macrophage
Colony Stimulating
Factor
HIV core ParticleRorer Seropositive HIV
Immunostimulant
IL-2 Interleukin-2Cetus AIDS, in combination
wIAZT
IL-2 Interleukin-2Hoffman-La AIDS, ARC, HIV, in
roche
Immunex combination w/AZT
IL-2 Interleukin-2Chiron AIDS, increase in CD4
cell
(aldeslukin) counts
Immune GlobulinCutter BiologicalPediatric AIDS, in combination
Intravenous (Berkeley, w/AZT
CA)
(human)
IMREG-1 Imreg AIDS, Kaposi's sarcoma,
ARC,
(New Orleans, PGL
LA)
IMREG-2 Imreg AIDS, Kaposi's sarcoma,
ARC,
(New Orleans, PGL
LA)
Imuthiol DiethylMerieux InstituteAIDS, ARC
Dithio Carbamate
Alpha-2 InterferonSchcring PloughKaposi's sarcoma w/AZT,
AIDS
Methionine- TNI PharmaceuticalAIDS, ARC
Enkephalin (Chicago, IL)
MTP-PE Muramyl-Ciba-Geigy Kaposi's sarcoma
Corp.
Tripeptide
Granulocyte Amgen AIDS, in combination
Colony wIAZT
Stimulating
Factor
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_47_
IMMLJNO-MODULATORS (cont'd)
Drug Name Manufacturer Indication
Remune Immune ResponseImmunotherapeutic
Corp.
rCD4 RecombinantGenentech AIDS, ARC
Soluble Human
CD4
rCD4-IgG hybrids AIDS, ARC
Recombinant Biogen AIDS, ARC
Soluble
Human CD4
Interferon AlfaI-Ioffman-La Kaposi's sarcoma AIDS,
2a Roche ARC,
in combination w/AZT
SK&FI06528 Smith Kline HIV infection
Soluble T4
Thymopentin Immunobiology HIV infection
Research Institute
(Annandale,
NJ)
Tumor Necrosis Genentech ARC, in combination
w/gamma
Factor; TNF Interferon
ANTI-INFECTIVES
Drug Name Manufacturer Indication
Clindamycen Pharmacia UpjohnPCP
with
Primaquine
Fluconazole Pfizer Cryptococcal meningitis,
candidiasis
Pastille NystatinSquibb Corp. Prevention of oral
candidiasis
Pastille
Ornidyl EflornithineMerrell Dow PCP
Pentamidine LyphoMed PCP treatment
Isethionate (Rosemont, IL)
(IM &
IV)
Trimethoprim Antibacterial
Trimethoprim/sulfa Antibacterial
Piritrexim Burroughs WellcomePCP treatment
Pentamidine Fisons CorporationPCP prophylaxis
isethionate
for
inhalation
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-4g
ANTI-INFECTIVES (cont'd)
Drug Name Manufacturer Indication
Spiramycin Rhone-Poulenc Cryptosporidial diarrhea
Intraconazole- 7anssen Pharm. Histoplasmosis; cryptococcal
RS 1211 meningitis
Trimetrexate Warner-Lambert PCP
OTHER
Drug Name Manufacturer Indication
Daunorubicin NeXstar, SequusKarposi's sarcoma
Recombinant Ortho Pharm. Severe anemia associated
Human Corp. with
Erythropoietin AZT therapy
Recombinant Serono AIDS-related wasting,
Human cachexia
Growth Hormone
Megestrol AcetateBristol-Myers Treatment of anorexia
Squibb
associated w/AIDS
Testosterone Alza, Smith AIDS-related wasting
Kline
Total Enteral Norwich Eaton Diarrhea and malabsorption
Nutrition Pharmaceuticalsrelated to AIDS
It will be understood that the scope of combinations of the compounds of
this invention with AIDS antivirals, immunomodulators, anti-infectives or
vaccines is not limited to the list in the above table, but includes in
principle any
combination with any pharmaceutical composition useful for the treatment of
AIDS.
The following examples are illustrative of the intermediate and final
compounds and methods for their preparation. They are not intended to limit
the
scope of the invention.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-49-
EXPERIMENTALS
Synthesis of Intermediate Indole derivatives
5-Acetoxy-2-methyl-1H-indole-3-carboxylic acid (A)
5-Hydroxy-2-methyl-1-H-indole-carboxylic acid (8.54 g, 44.7 mmol) was
dissolved in aqueous sodium hydroxide (2N, 45 mL). 1-acetyl-1H-2,3-
triazolo(4,5-b-)-pyridine (7.24 g, 44.7 mmol) was dissolved in THF (30 mL),
and
the solution was added to the solution of 5-hydroxy-2-methyl-1-H-indole-
carboxylic acid. The mixture was stirred until little or no starting material
remained, ~30 minutes; a white precipitate formed. The mixture was cooled to
0°C and concentrated HCl was added dropwise until the pH was ~1. The
resulting
white solid was filtered, washed with water (2 x 50 mL), recrystallized from
ethanol, and dried under vacuum, yield 6.95 g (67%); mp 233-235°C
(dec); IR:
3331, 1740, 1642, 1234, 1207 cm-l. 1H NMR (DMSO-d6) 8: 2.21 (s, 3H
CH3C02), 2.59 (s, 1H, ArCH3), 6.79 (d, J= 6.84 Hz, 1H, ArH), 7.27 (d,
J= 8.55 Hz, 1H, ArH), 7.53 (s, 1H, ArH), 11.77 (s, 1H, NH) 11.93 (s, lI-I,
COOH). MS(APCI+): m/z 234.1 (MH+). Analysis calculated for C12H11N104~
C, 61.80; H, 4.75; N, 6.01. Found: C, 61.48; H, 4.66; N, 5.86.
Procedure A. General procedure for the preparation of esters
5-Hydroxy-2-methyl-1-H-indole-carboxylic acid or 5-acetoxy-2-methyl-
1H-indole-3-carboxylic acid (4-28 g) was combined with enough THF to effect
dissolution. Triphenylphosphine (1 eq) and the alcohol of interest (2.5-4.0
eq,
depending on solubility) were added to the THF solution.
Diethylazodicarboxylate
(DEAD, 1 eq) was added dropwise to the mixture over the course of 1-1.5 hour.
The mixture was stirred overnight at ambient temperature. The solution was
concentrated in vacuo to give an oily mixture; a solution of 1:1 hexanelcthyl
acetate was used to redissolve the oil. The desired product was purified by
flash
chromatography. Residual diethylhydrazinedicarboxylate remaining in the
product
was removed by trituration with hot water; the resulting solid was dried under
vacuum at 40°C. For compounds made with 5-acetoxy-2-methyl-1H-indole-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-50-
3-carboxylic acid, the 5-acetyl group was removed in the following manner: the
protected ester (1 eq) was dissolved in a small amount of MeOH. NaOMe (4 eq)
was added and the mixture stirred until no starting material remained
(~45 minutes). The pH of the solution was adjusted to 1 with the addition of
S aqueous HCI, and a copious white precipitate occurred. The solid was
filtered,
washed with water (2 x 20 mL), and dried under vacuum at 40°C.
Alternatively,
the pH of the solution was adjusted to 1 with the addition of aqueous HCI, and
the
solution was extracted with ethyl acetate (2 x 25 mL). The organic layer was
dried
over Na2S04 and evaporated to give a solid. The solid may be further purified
by
recrystallization from appropriate solvents. According to the Procedure A,
Intermediates B-G were synthesized.
5-Acetoxy-2-methylindole-3-carboxylic acid benzyl ester (B) Yield: 8.32 g
(21.6%); mp 152-154°C; IR: 3310, 1752, 1662, 1226, 1094 cm-1; 1H NMR
(DMSO-d6) 8 2.21 (s, 3H, CH3C02), 2.60 (s, 3H, ArCH3), 5.28 (s, 2H, CH2Ph),
6.82 (dd, J= 8.55, 2.44 Hz, IH, ArH), 7.26-7.41 (m, 6H, ArH), 7.53 (d,
J= 2.44 Hz, 1H, ArH), 11.9 (s, 1H, NH). MS(APCI+): 324.1 (MH+). Analysis
calculated for CI~H17N104: C, 70.58; H, 5.30; N, 4.33. Found: C, 70.47; H,
5.43; N, 4.24.
5-Hydroxy-2-methylindole-3-carboxylic acid benzyl ester (C) Yield: 5.03 g
(76%); mp 191-193°C; IR: 3227, 1654, 1472, 1429, 1094 cm-I. IH NMR
(DMSO-d6) 8 2.54 (s, 3H, alkyl CH3 ), 5.26 (s, 2H, PhCH2), 6.55 (d, J= 6.10
Hz,
1 H, ArH), 7.08 (d, J= 8.8 Hz, 1 H, ArH), 7.24-7.42 (m, 6H, ArH), 8.82 (s, 1
H,
aromatic OH), 11.55 (s, 1H, NH). MS(APCI+): ntlz 282.0 (MI-I+). Analysis
calculated for C17H15N103: C, 71.71; H, 5.44; N, 4.92. Found: C, 71.72; H,
5.49; N, 4.85.
Alternatively, Intermediate C can be synthesized from Intermediate B according
to the procedure described in Example 9, Step A.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-51-
5-Acetoxy-2-methylindole-3-carboxylic acid propyl ester (D) Yield: 2.16 g
(37%); mp 134-136°C; IR: 3263, 2966, 1758, 1677, 1657, 1215 cm-1. 1H
NMR
(DMSO-d6) 8 0.99 (t, J= 7.51 Hz, 3H, CH2CH2CH3), 1.71 (sextet, J= 7.33 Hz,
3H, CH2CH2CH3), 2.26 (s, 3H, CH3C0), 2.63 (s, 3H, ArCH3), 4.17 (t,
S J= 6.41 Hz, 2H, CII2CH2CH3), 6.86 (dd, J= 8.61, 2.20 Hz, 1H, ArH), 7.34 (d,
J= 8.61 Hz, IH, ArH), 7.55 (d, J= 2.20 Hz, 1H, ArH), I 1.9 (s, 1H, NH).
MS(APCI+): m/z 276.0 (MH+). Analysis calculated for CISH17N104: C, 65.44;
H, 6.22; N, 5.09. Found: C, 65.13; H, 6.28; N, 5.10.
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 2-isopropyl ester (E) Yield:
0.720 g (12%); mp 188-189°C; IR: 3409, 3391, 1663, 1467, 1181, 1095 (cm-
1).
1H NMR (DMSO-d6) 8 1.27 (d, J= 6.35 Hz, 6H, CH(CH3)2) , 2.52 (s, 3H, CH3),
5.04 (septet, J= 6.35 Hz, 1H, CH(CH3)2), 6.53 (dd, J= 8.55, 2.44 Hz, 1H, ArH),
7.06 (d, J= 8.55 Hz, 1H, ArH), 7.25 (d, J= 2.44 Hz, 1H, ArH), 8.77 (s, 1H, OH)
I 1.4 (s, 1H, NH). MS(APCI+): m/z 234.1 (MH+). Analysis calculated for
C 13H 15N103 ~ C~ 66.94; H, 6.48; N, 6.00. Found: C, 66.79; H, 6.53; N, 5.88.
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid cyclopropylmethyl ester (F)
Yield: 0.532 g (8.3%); mp 187-188°C; IR: 3388, 3297, 1663, 1466,
1179,
1094 cm-l. 1H NMR (DMSO-d6) 8 0.00-0.04 (m, 2H, cyclopropyl CH2CH2),
0.22-0.26 (m, 2H, cyclopropyl CH2CH2), 0.86-0.93 (m, 1H, CH2CH), 2.27 (s,
3H, ArCH3), 3.72 (d, J= 7.32 Hz, 2H, CH2CH), 6.27 (dd, J= 8.55, 2.44 Hz, 1H,
ArH), 6.79 (d, J---- 8.55, 1H, ArH), 6.99 (d, J= 2.20, IH, ArH), 8.51 (s, 1H,
OH),
11.2 (s, 1H, NH). MS(APCI+): rnlz 246.1 (MH+). Analysis calculated for
C14H15N103~ C~ 68.56; H, 6.16; N, 5.71. Found: C, 68.50; H, 6.19; N, 5.67.
S-Acetoxy-2-methylindole-3-carboxylic acid I-phenyl-propyl ester (G) Yield:
1.36 g (23%); mp 144-145.5°C; IR: 3289, 1755, 1661, 1459, 1216, 1204,
1089 cm-1. 1H NMR (DMSO-d6) 8 0.863 (t, J= 7.32 Hz, 3H, CH2CH3),
1.82-1.98 (m, 2H, CHCH2CH3), 2.22 (s, 3H, CH3C0), 2.63 (s, 3H, ArCH3),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-52-
5.80 (t, J= 6.84 Hz, 1H, benzylic CH), 6.83 (dd, J= 8.79, 2.20 Hz, 1H, ArH),
7.21-7.3G (m, 6H, ArH), 7.60 (d, J= 2.20 Hz, 1H, ArH), 11.9 (s, 1H, NH).
MS(APCI-): m/z 350.1 (M-1). Analysis calculated for C21H21N104~ C> 71.78;
H, G.02; N, 3.99. Found: C, 71.53; H, 6.02; N, 3.81.
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid isobutyl ester (H) 5-Hydroxy-
2-methyl-1H-indole-3-carboxylic acid isobutyl ester was synthesized according
to
the general procedure A and was recrystallized from hexanelCH2Cl2 to give
1.39 g (26.2%) of white solid: mp 188-190°C; IR (KBr) 3385, 3272, 2963,
1655,
1630, 1464, 1174, 1094 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 0.95 (d,
J= G.84 Hz, 6H, CH(CH3)2), 1.94 (n, J= 6.84 Hz, 1H, CH(CH3)2), 2.54 (s, 3H,
CCH3), 3.95 (d, J= 1.95 Hz, 2H, OCH2), 6.54 (dd, J= 8.55, 2.44 Hz, 1H, ArH),
7.07 (d, J = 8, 1 H, ArH), 7.25 (s, 1 H, ArH), 8.81 (s, 1 H, OH), 11.49 (s, 1
H, NH);
MS(APCI+): m/z 248.1 (MH+). Analysis calculated for C 14H 17N03: C, 68.00; H,
6.93; N, 5.66. Found: C, 67.92; H, 6.87; N, 5.54.
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 2,2-dimethyl-propyl ester (I)
5-I-Iydroxy-2-methyl-1H-indole-3-carboxylic acid 2,2-dimethyl-propyl ester was
synthesized according to the general procedure A and was recrystallized from
CH2CI2 to give 2.31 g (33.8%) of white solid: mp 195-196°C; IR
(KBr) 3262,
2960, 1652, 1464, 1170, 1094 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 0.97 (s,
9H, C(CH3)3), 2.55 (s, 3H, ArCH3), 3.87 (s, 2H, OCH2), 6.55 (dd, J= 8.55,
2.44 Hz, 1H, ArCH), 7.07 (d, J= 8.55 Hz, 1H, ArCH), 7.29 (s, 1H, ArH), 8.82
(s,
1H, OH), 11.50 (s, 1H, NH); MS(APCI+); m/z 2G2.1(MH+). Analysis calculated
for C15H19N03~ C, 68.94; H, 7.33; N', 5.36. Found: C, 68.55; H, 7.23; N, 5.41.
Procedure B: An Alternative General Procedure for the Preparation of Esters
A solution of 5-acetoxy-2-methyl-1H-indole-3-carboxylic acid (Aldrich,
5.00 g, 21.4 mmol) and diethyl azodicarboxylate (Aldrich, 3.73 g, 21.4 mmol)
in
7 mL of THF was added dropwise to a mixture of triphenyl phosphine (Aldrich,
5.62 g, 21.4 mmol) and an alcohol of choice (Aldrich, 2.00-4.00 g, 32.2 mmol)
in
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-53-
32 mL of THF over an hour. After stirring at room temperature for 24 hours,
the
mixture was concentrated. The product was purified by flash column
chromatography on silica gel (10% MeOH,CHCl3) to give the corresponding
ester.
S 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 2-piperdin-1-yl-ethyl ester
(J)
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 2-piperdin-lyl-ethyl ester was
synthesized according to the procedure B and was recrystallized from
hexane/ethyl acetate to give 0.350 g (26.9%) of white solid: mp 210-
212°C; IR
(KBr) 3203, 2934, 1690, 1455, 1175, 1067 cm-1; 1H NMR (400 MHz,
DMSO-d6) 8 1.31 (m, 2H, NCH2CH2CH2), 1.42 (m, 4H, NCH2CH2), 2,38 (m,
4H, NCH2CH2CH2), 2.53 (s, 3H, ArCH3), 2.59 (t, J= 6.10 Hz, 2H, OCH2CH2),
4.22 (t, J= 6.10 Hz, 2H, OCH2CH2N), 6.54 (dd, J= 8.66, 2.32 Hz, 1H, ArH),
7.06 (d, J= 8.55 Hz, 1H, ArH), 7.27 (s, 1H, ArH), 8.77 (s, 1H, OH), 11.48 (s,
1H,
NH); MS(APCI+); m/z 303.1(MH+). Analysis calculated for C17H22N203: C,
67.05; H, 7.28; N, 9.20. Found: C, 66.93; H, 7.28; N, 9.00.
Procedure C: A General Procedure for the Synthesis of ethyl 3-alkyl-
3-aminocrotonates (K-O)
Activation of Zn: To a stirred 3N HCl solution (50 mL) was added Zn
(20 g) and stirred at room temperature for 15 minutes. The HCl solution was
decanted, and this was repeated two times. The activated Zn was washed with
distilled H20 (2x, 100 mL), ethanol (2x, 50 mL), and ether (2x, 50 mL). The
activated Zn was then placed under reduced pressure for 12 hours at
40°C. To a
stirred solution of dry THF (30 mL) and activated Zn (3.27 g, 50 mmol) in a
flame
dried 100 mL round bottom flask under an inert atmosphere was added 0.2 mL of
ethylbromoacetate (1) at room temperature. The reaction was then heated to
reflux. After the solution turned green (15-30 min), the alkyl cyanide (10
mmol)
was added at once, and ethylbromoacetate (4.44 mL, 40 mmol) was added
dropwise over 30 minutes and refluxed for an additional 30 minutes and then
allowed to cool to room temperature. To the stirred solution was added THF
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-54-
(30 mL) and K2C03 (13 mL, 50% w/w) and stirred vigorously for 30 minutes.
The solution was then placed in a centrifuge tube and centrifuged. The
supernatant
was decanted, and the pellet was resuspended in THF (30 mL), shaken vigorously
and centrifuged (procedure repeated twice). The combined supernatant were
dried
over MgS04, filtered, and concentrated under reduced pressure to yield of
ethyl
3-alkyl-3-aminocrotonate as a crude product which was used directly in the
next
step.
Ethyl 3-amino-3-benzylcrotonate (K) 1H NMR (250 MHz, CDC13) b 1.26 (t,
J= 7.1 Hz, 3H), 3.46 (s, 2H), 4.12 (q, J= 7.15 Hz, 2H), 4.64 (s, 1H), 7.27 (m,
5H).
Ethyl 3-amino-3-ethylcrotonatc (L) 1H NMR (250 MHz, CDC13) 8 1.47 (t, J=
7.5 Hz, 3H), 1.26 (t, J= 7.1 Hz, 3H), 2.16 (q, 5.7 Hz, 2H), 4.11 (q, J= 5.4
Hz,
2H), 4.55 (s, 1H). 13C NMR (62.5 MHz, CDCI3) c5 12.0, 14.5, 29.3, 30.3, 58.5,
82.6, 164.9, 170.5.
Ethyl 3-amino-3-cyclopropylcrotonate (M) IH NMR (250 MHz, CDCI3) 8
0.74 (m, 2H), 0.86 (m, 2H), 1.25 (t, J= 7 Hz, 3H), 2.27 (s, 1H), 4.10 (q, J= 7
Hz,
2H), 4.45 (s, 1H). 13C NMR (62.5 MHz, CDC13) 8 7.1, 14.6, 15.8, 58.5, 80.7,
165.1, 170.4. LC/MS (150 mm x 4.6 mm, C-18, S micron, 10 mM NH40Ac/
CH3CN, APCI+) t = 7.24 min, mlz = 156 (M+1).
Ethyl 3-amino-3-propylcrotonate (N) 1H NMR (250 MHz, CDCl3) 8 0.95 (t,
J= 7.3 Hz, 3H), 1.26 (t, J= 7.1 Hz, 3H), 1.56 (s, J= 7.3 Hz, 2H), 2.10 (d,
J= 7.3 Hz, 2H), 4.1 (q, J= 7.2 Hz, 2H), 4.53 (s, 1H). 13C NMR (62.5 MHz,
CDC13) 6 13.5, 14.5, 21.1, 38.4, 58.30, 84 LC/MS (150 mm x 4.6 mm, C-18,
5 micron, 10 mM NH40Ac/CH3CN, APCI+) t = 8.02 min, rnlz = 158.4 (M+1).
CA 02372197 2001-07-12
WO 00142045 PCT/ITS99130434
-55-
Ethyl 3-amino-3-isobutylcrotonate (O) 1H NMR (250 MHz, CDC13) 8 0.95 (d,
J= 6.4 Hz, 6H), 1.26 (t, J= 7.1 Hz, 3H), 1.9 (m, IH), 1.96 (d, J= 7.0 Hz, 2H),
4.1 I (q, J= 7.1 Hz, 2H), 4.51 (s, 1H). LC/MS (150 mm x 4.6 mm, C-18,
micron, 10 mM NH40Ac/CH3CN, APCI+) t = 8.69 min, m/z = 172.4 (M+1 ).
5 Procedure D: General procedure for the synthesis of ethyl 2-alkyl-5-hydroxy-
3-indolecarboxylates (P-T)
To a stirred solution of ethyl 3-alkyl-3-aminocrotonate (15.3 mmol) in
acetic acid (50 mL) was added 1,4-benzoquinone (3.3 g, 30.5 mmol). The
solution
was stirred overnight at room temperature and then filtered through a frit.
The
solid was washed with cold distilled water and dried in an Abderhalden over
P205 to afford the ethyl 2-alkyl-5-hydroxy-3-indolecarboxylate.
Ethyl 2-benzyl-5-hydroxy-3-indolecarboxylate (P) 70% yield (from starting
nitrile) of a white powder. 1 H NMR (250 MHz, DMSO) ~ 1.32 (t, J= 7.08 Hz,
3H), 4.26 (q, J= 7.05 Hz, 2H), 4.41 (s, 2H), 6.63 (dd, J= 8.5, 2.2 Hz, 1H),
7.25 (m, 7H), 8.88 (s, 1H), 11.64 (s, 1H). 13C NMR (62.5 MHz, DMSO) 8 14.5,
17.3, 32.8, 58.7, 102.1, 105.4, 111.8, 126.2, 127.8, 128.4, 129.3, 139.0,
146.1,
152.3, 165.1. LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM
NH40AclCH3CN, APCI+) t = 7.83, ntlz = 296.3 (M+1 ).
Ethyl 2-ethyl-5-hydroxy-3-indolecarboxylate (Q) 54% yield (from starting
nitrile)
as a white powder. 1H NMR (250 MHz, DMSO) b 1.23 (t, J= 7.6 Hz, 3H),
1.33 (t, J= 7.1 Hz, 3H), 3.04 (q, J= 7.5 Hz, 2H), 4.24 (q, J= 7.1 Hz, 2H), 6.5
(dd,
J= 8.7, 2.4 Hz, 1H), 7.14 (d, J= 8.5 Hz, 1H), 7.33 (d, .7= 2.4 Hz, 1H), 8.82,
(s,
IH), 11.48 (s, IH). 13C NMR (62.5 MHz, DMSO) 8 13.6, 14.4, 20.7, 58.4, 105.3,
111.3, I I 1.6, 127.9, 128.9, 150.0, 152.2, 165Ø LC/MS (150 mm x 4.6 mm, C-
18,
5 micron, 10 mM NH40Ac/CH3CN, APCI+) t = 7.60 min, m/z = 234.3 (M+1 ).
Ethyl 2-cyclopropyl-5-hydroxy-3-indolecarboxylate (R) 83% yield (from staring
nitrile) as a white powder. 1H NMR (250 MHz, DMSO) 8 0.97 (m, 2H), 1.10 (m,
2H), 1.35 (t, .I= 7.1 Hz, 3H), 3.00 (m 1H), 4.26 (q, J= 7.0 Hz, 2H), 6.59 (dd,
8.7,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-56-
2.4 Hz, 1H), 7.8 (d, J= 8.7 Hz, 1H), 7.30 (d, J= 2.4 Hz, IH), 8.81 (s, 1H),
10.93 (s, 1H). 13C NMR (62.5 MHz, DMSO) 8 8.8, 9.2, 14.5, 17.2, 21.0, 58.5,
102.9, 105.1, 111.1, 111.4, 127.9, 128.8, 150.1, 152.1, 165.4, 171.9. LC/MS
(150 mm x 4.6mm, C-18, 5 micron, 10 mM NH40Ac/CH3CN, APCI+)
t = 7.03 min, m/z = 246.3 (M+1).
Ethyl 5-hydroxy-2-propyl-3-indolecarboxylate (S) 62% yield (from starting
nitrile) as a white powder. /H NMR (250 MHz, DMSO) 8 0.93 (t, J= 7.3 Hz,
3H), 1.35 (t, J= 7.1 Hz, 3H), 1.67 (m, 2H) 3.01 (t, J= 9.0 Hz, 2H), 4.26 (q,
J= 7.1 Hz, 2H), 6.77 (dd, J= 8.7, 2.2 Hz, 1H), 7.16 (d, J= 8.6 Hz, 1H), 7.35
(d,
J= 2.2 Hz, 1H), 8.86 (s, 1H), I 1.51 (s, 1H). 13C NMR (62.5 MHz, DMSO) 8
13.7, 14.4, 17.2, 22.3, 29.2, 58.4, 105.2, I I I .3, 115.5, 127.9, 128.9,
148.4, 149.6,
152.1, LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM NH40Ac/CH3CN,
APCI+) t = 7.21 min, mlz = 248.4 (M+1 ).
Ethyl 2-isobutyl-5-hydroxy-3-indolecarboxylate (T) 68% yield (from starting
nitrile) the as a white powder. 1H NMR (250 MHz, DMSO) 8 0.91 (d, J= 6.6 Hz,
6H), 1.35 (t, J= 7.1 Hz, 3H), 2.06 (m, 1H), 2.91 (d, J= 7.2 Hz, 2I I), 4.25
(q,
J= 7.1 Hz, 2H), 6.62 (dd, J= 8.6, 2.4 Hz, 1H), 7.16 (d, J= 8.5 Hz, 1H), 7.35
(d,
J= 2.4 Hz, 1H), 8.85 (s, 1H), 11.5 (s, 1H). 13C NMR (62.5 MHz, DMSO) a 14.4,
17.2, 22.3, 28.6, 36.2, 58.4, 102.0, 105.2, I 11.2, I 11.4, 127.9, 128.8,
147.6, 152.1,
165Ø LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM NH40Ac/CH3CN,
APCI+) t = 7.69 min, »i/z = 262.4 (M+1 ).
Procedure E: General procedure for the preparation of indolc amides
5-Acetoxy-2-methyl-1H-indole-3-carboxylic acid (I.0 g, 4.3 mmol) was
dissolved in 10 mL of DMF, and Et3N (0.6 mL, 1 eq) was added. The solution
was stirred for 5 minutes. The solution was cooled to 0°C and HBTU
(1.63 g,
4.3 mmol) was added, then stirred for 15 minutes. The amine (2 N in THF, 4 eq)
was added, and the solution stirred until the starting material was consumed,
~1 hour water was added. The pH of the resulting mixture was adjusted to 5
with
HCl (1N), and extracted with ethyl acetate. The organic layer was dried and
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-57-
evaporated to give the crude product which can be further purified by flash
chromatography or recrystallization.
Intermediates U-V were synthesized according to Procedure E. Acetic acid
2-methyl-3-methylcarbamoyl-1H-indol-5-yl ester (U) Yield: 0.093 g (18%);
mp 201-203°C; IR: 3402, 1748, 1609, 1218, 1170 cm-1. 1H NMR (DMSO-d6) 8
2.21 (s, 3H, CH3C0), 2.45 (s, 3H, ArCH3), 2.72 (d, J= 4.39 Hz, 3H, NHCH3),
6.76 (dd, J = 8.79, 1.46 Hz, 1 H, ArH), 7.24 (d, J = 8.79 Hz, 1 H, ArH), 7.25
(bs,
1 H, CONHCH3), 7.41 (s, 1 H, ArH), 11.5 (s, 1 H, indole NH). MS(APCI+); m/z
247.1 (MH+); Analysis calculated for C13H14N203'0.9 H20; C, 59.49; H, 6.07;
N, 10.67. Found: C, 59.51; H, 6.12; N, 10.55.
Acetic acid 3-benzylcarbamoyl-2-methyl-1H-indol-S-yl ester (V) Yield: 0.454 g
(33%); mp 182-184°C; IR: 3413, 3319, 3222, 3191, 1750, 1609, 1228,
1216,
1170 cm-1. 1H NMR (DMSO-d6) c5 2.20 (s, 3H, CH3C0), 2.54 (s, 3H, ArCH3),
4.42 (d, J= 6.1 Hz, 2H, NHCH2Ph), 6.77 (dd, J= 8.55, 1.95 Hz, 1H, ArH),
7.1 S-7.19 (m, 1 H, ArH), 7.25-7.34 (m, SH, ArH), 7.45 (d, J= 1.71 Hz, 1 H,
ArH),
7.89 (t, J= 6.10 Hz, 1H, CONIICH2Ph), 11.5 (s, 1H, indole NH). MS(APCI+):
m/z 323.2 (MH+); Analysis calculated for C 19H 1 gN203: C, 70.79, H, 5.63, N,
8.69. Found: C, 70.62, H, 5.78, N, 8.60.
Procedure F: General procedure for deacylation of the amides
The amide of interest (1 eq) was dissolved in a small amount of MeOH.
MeONa (4 eq) was added and the mixture stirred until no starting material
remained, ~45 minutes. The pH of the solution was adjusted to 1 with the
addition
of aqueous HCI, and the solution extracted with 2 x 25 mL of ethyl acetate.
The
organic layer was dried and evaporated to give a solid. Recrystallization from
ethyl acetate yields a white solid.
Intermediates W-X were synthesized according to Procedure F.
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid methyl amide (W) Yield:
0.201 g (70%); mp 226-227°C;1R: 3366, 1602, 1558, 1552, 1215, 1198 cm-
l. 1H
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-5 8-
NMR (DMSO-d6) 8 2.45 (s, 3H, ArCH3~ obscured by DMSO peak), 2.70 (d,
J= 4.40 Hz, 3H, CONHCH3), 6.51 (d, J= 8.55 Hz, I H, ArH), 7.02 (d,
J= 8.55 Hz, 1H, ArH), 7.07 (s, 1H, ArH), 7.13-7.14 (m, 1H, CONHCH3), 8.65 (s,
1 H, OH), I 1.0 (s, I H, indole NH). MS(APCI+): m/z 205. I (MH+); Analysis
S calculated for CI IH12N202~ C~ 64.69; H, 5.92; N, 13.72. Found: C, 64.53;
H, 5. 91; N, 13.44.
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl amide (X) Yield:
0.228 g (65.7%); mp: 194-196°C; IR: 3392, 3246, 1610, 1528, 1465, 1214,
I 188 cm-1. 1H NMR (DMSO-d6) S: 2.48 (s, 3H, ArCH3), 4.41 (d, J= 5.86 Hz,
1U 2H, NHCH2Ph), 6.52 (d, J= 8.06, 1H, ArH), 7.04 (d, J= 8.55, 1H, ArH), 7.12
(s,
1H, ArH) 7.17-7.31 (m, SH, ArH), 7.71-7.78 (m, 1H, CONHCH2Ph), 8.68 (s, 1H,
OH), 11.1 (s, 1H, indole NH). MS(APCI+): r~t/z 281.1 (MH+); Analysis
calculated
for C17H16N202: C, 72.84; H, 5.75; N, 9.99. Found: C, 72.78; H, 5.70; N, 9.87.
Example 1
15 Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, ethyl ester
H
_ y
H3C \ ~ / ~ O
O, ~N
O
C
CH3
Synthesized according to procedures published in J. Het. Chem.,
1970;7:131 I-1319.
20 Example 2
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
8,9, I 1,12,13, I 3a,14,14a-octahydro-2-methyl-, (4-fluorophenyl)methyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-59-
/ -F
:,H3
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-fluoro-benzyl ester
To a solution of 5-hydroxy-2-methyl-1-H-indole-carboxylic acid (4.6 g,
24.1 mmol) in DMF (100 mL) was added DBU (3.67 g, 24.1 mmol) followed by
4-fluorobenzyl bromide (5.0 g, 26.5 mmol). The resulting mixture was stirred
at
room temperature under nitrogen for 3 days and then partitioned between ethyl
acetate (200 mL) and water (200 mL). The organic phase was separated, washed
with water (2 x 100 mL) and then dried over Na2S04 and concentrated in vcacuo
to give a white solid. Recrystallization from ethyl acetate gave 3.4 g (47%)
of pure
titled compound as a white solid: mp 209-210°C; IR 3412, 3377, 3305,
1667,
1512, 1466, 1221, 1176, 1094 cm-1; IH NMR (DMSO-d6) 8 2.53 (s, 3H, CH3),
5.23 (s, 2H, CH2), 6.55 (dd, J = 8.79, 2.20 Hz, 1 H, ArH), 7.08 (d, J= 8.79
Hz,
1H, ArH), 7.14-7.17 (m, 2H, ArH), 7.19 (d, J= 2.20 Hz, l II, ArH), 7.44-7.48
(m,
2H, ArH), 8.81 (s, 1H, OH), I 1.55 (s, lI-I, NII); MS(APCI+): m/z 300.1 (MH+).
Analysis calculated for C17H14F N 03: C, 68.22; H, 4.71; N, 4.68. Found:
C, 67.91; H, 4.65; N, 4.59.
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid 4-fluoro-benzyl ester
5-Hydroxy-2-methyl-IH-indole-3-carboxylic acid 4-fluoro-benzyl ester
(2.90 g, 9.69 mmol) and aqueous Me2NH (40%, 2.67 mL, 21.3 mmol) were
mixed with 22 mL of EtOH, aqueous HCHO (37%, 0.940 g, 11.6 mmol) was then
added. The resulting reaction mixture was heated with a heatgun until a clear
solution was obtained. The reaction mixture was stirred at 50°C for 16
hours. The
reaction mixture was allowed to stand at 4°C for 15 hours, white
precipitate
formed. Filtration and drying under vacuum gave 1.56 g (45%) of pure titled
compound as a white solid: mp 131-133°C (dec.); IR 3376, 3214, 1693,
1686,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-60-
1513, 1424, 1259, 1227, 1085, 806 cm-1; IH NMR (DMSO-dG) 8 2.12 (s, 6H,
N(CH3)2), 2.45 (s, 3H, ArCH3), 3.97 (s, 2H, ArCH2NMe2), 5.23 (s, 2H,
C02CH2Ar), 6.56 (d, J= 8.G1 Hz, 1H, ArH), 7.06 (d, J= 8.61 Hz, 1H, ArH),
7.18-7.24 (m, 2H, ArH), 7.49-7.54 (m, 2H, ArH), 11.5 (bs, 1H, exchangeable
S proton); MS(APCI+): m/z 357.2 (MH+). Analysis Calculated for
C20H21N203F1 ~O.1H20: C, 67.06; H, 5.97; N, 7.82; F, 5.30; H20, 0.50. Found:
C, 66.90; H, 5.81; N, 7.53; F, 5.33; H20, 0.20.
Step C: Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
8,9,11,12,13,13a,14,14a-octahydro-2-methyl-, (4-fluorophenyl)methyl
ester
To a mixture ofperchlorate salt (1.27 g, 5.36 mmol, Example 3, Step B)
and 50 mL of ether was added 50 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 50 mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 15 mL of dioxane, then indole mannich base (1.47
g,
4.12 mmol) was added; the resulting reaction mixture was rcfluxed under
nitrogen
for 5.5 hours. The reaction mixture was cooled to ambient temperature and
concentrated in vacuo affording a brown solid. Recrystallization from CH3CN
gave 1.G3 g (88%) of pure titled compound as a brown solid: mp 209-
214°C
(decomposed); IR 2934, 1704, 1152, 1431, 1235, 1148, 1078, 827 cm-1; 1H NMR
(DMSO-d6) 8 1.06-1.67 (m, 10H, aliphatic CH2 and CH), 1.81-1.86 (m, 1H,
aliphatic CH), 2.30-2.46 (m, 2H, obscured by DMSO peak, aliphatic CH), 2.46
(s,
3H, ArCH3), 2.G0-2.70 (m, 2H, aliphatic CH), 2.84-2.91 (m, 1H, aliphatic CH),
3.17 (dd, J= 18.3, G.78 Hz, 1H, aliphatic CH), 5.21 (ABq, Jab = 11.9 Hz,
vab = 19.0 Hz, 2H, C02CH2Ar), 6.59 (d, J-- 8.61 Hz, 1H, ArH), 7.03 (d,
.I= 8.G1 Hz, IH, ArH), 7.17-7.25 (m, 2H, ArH), 7.48-7.52 (m, 2H, ArH), 11.5
(bs,
1H, NH); MS(APCI+): mlz 449.3 (MH+). Analysis calculated for
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-61-
C27H29N203F1~0.08CH3CN: C, 72.20; H, 6.52; N, 6.45; F, 4.20. Found:
C, 71.88; H, 6.35; N, 6.42; F, 4.15.
Example 3 (Intermediate)
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine, 3,7,8,9,10,12,13,14,14a,15-
decahydro-
Step A: 4-Dimethylaminomethyl-IH-indol-5-0l
5-Hydroxyindole (Aldrich, Milwaukee, WI, 5.09 g, 38.2 mmol) was
dissolved in 25 mL of EtOH, aqueous Me2NH (40%, 5.28 mL, 42.1 mmol) was
added followed by aqueous HCHO (37%, 3.65 g, 45.9 mmol). The resulting
reaction mixture was stirred at ambient temperature for 1.5 hours during which
time a precipitate formed. Filtration and drying under vacuum gave 4.13 g
(57%)
ofpure titled compound as a beige solid: mp 137-139°C; IR 3316, 1625,
1592,
1523, 1450, 1239, l 198, 724 cm-l; 1H NMR (DMSO-d6) 8 2.25 (s, 6H,
CH2N(CH3)2, 3.76 (s, 2H, CH2N(CH3)2), 6.29-6.30 (m, 1H, ArH), 6.54 (d,
J= 8.61 Hz, 1 H, ArH), 7.10 (d, J = 8.60 Hz, 1 H, ArH), 7.18-7.20 (m, 1 H,
ArH),
10.8 (bs, 1H, exchangeable proton); MS(APCI+): m/z 191.1 (MH+). Analysis
calculated for C 11 H 14N20- C, 69.45; H, 7.42; N, 14.72. Found: C, 69.36; H,
7.38; N, 14.71.
Step B: 1,2,3,4,6,7,8,9-Octahydro-quinolizinylium perchlorate
N
C104 ~
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-62-
The synthesis is found in David A. Evans, A new endocyclic enamine
synthesis. JAC,S, 1970;92:7593-7595 and Leonard N.J., Hay A.S., Fulmer R.W.,
Gash V.W., Unsaturated amines. III. Introduction of a,(3-unsaturation by means
of
mercuric acetate: X1(10)-dehydroquinolizidine, J. Am. Che»t. Suc., 1955;77:439-
444.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine,
3,7,8,9,10, I 2,13,14,14x,15-decahydro-
To a mixture of perchlorate salt (406 mg, 1.71 mmol, Example 3, Step B)
and 20 mL of ether was added 30 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 30 mL).
The
combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 5 mL ofdioxane, then 4-dimethylaminomethyl-1H-
indol-S-of (250 mg, 1.31 mmol) was added, the resulting reaction mixture was
refluxed under nitrogen for 4 hours. The reaction mixture was cooled to
ambient
temperature and a precipitate formed. Filtration and recrystallization from
EtOAc
gave 0.17 g (46%) of pure titled compound as a beige solid: mp >250°C;
IR 3414,
3148, 1454, 1242, 1148, 888 cm-1; 1H NMR (DMSO-d6) S 1.13-1.65 (m, 9H,
aliphatic CH2 and CH), 1.76-1.91 (m, 2H, aliphatic CH), 2.36-2.52 (m, obscured
by DMSO peak, 3H, aliphatic CH), 2.68-2.77 (m, 1H, aliphatic CH),
2.88-2.96 (m, 1H, aliphatic CH), 3.06 (dd, J= 17.6, 6.78 Hz, 1H, aliphatic
CH),
6.22-6.23 (m, 1H, ArH), 6.56 (d, J= 8.61 Hz, lI-I, ArH), 7.07 (d, J= 8.61 Hz,
1H,
ArH), 7.19-7.21 (m, 1H, ArH), 10.8 (bs, 1H, NH); MS(APCI+): m/z 383.1 (MH+)
Analysis calculated for CI gH22N20~0.1Ii20: C, 76.08; H, 7.87; N, 9.86; H20,
0.63. Found: C, 76.09; H, 7.81; N, 9.83; H20, 0.74
Example 4 (Intermediate)
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine, 3,7,8,9,10,12,13,14,14a,15-
decahydro-2-methyl-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-63-
11
Step A: 2-Methyl-1H-indol-5-0l
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl ester (Aldrich,
Milwaukee, WI, 20.0 g, 91.2 mmol) was mixed with aqueous NaOH (2N, 365 mL,
730 mmol), the resulting reaction mixture was refluxed under nitrogen for 1
hour.
After cooling to 70°C, the reaction solution was treated with
concentrated aqueous
HCl until pH = 1. The resulting dark brown solution was extracted with ether
(3 x
300 mL). Combined ether solution was dried over Na2S04 and concentrated
iu vucuo affording a brown solid. Recrystallization from EtOAc/CH2C12 gave
11.7 g (87%) ofpure titled compound as a light brown solid: mp 129-
130°C; IR
3387, 3333, 1588, 1453, 1368, 1173, 783 cm-1; 1 H NMR (DMSO-d6) 8 2.29 (s,
3H, ArCH3), 5.88-5.89 (m, 1H, ArH), 6.45 (dd, J= 8.42, 2.38 Hz, 1H, ArH),
6.68 (d, J = 2.38 Hz, 1 H, ArH), 7.00 (d, J= 8.42 Hz, 1 H, ArH), 8.44 (s, 1 H,
NH),
10.5 (bs, 1H, OH); MS(APCI+): mlz 148.1 (MH+). Analysis calculated for
C9H9N0: C, 73.45; H, 6.16; N, 9.52. Found: C, 7.13; H, 6.18; N, 9.41.
Step B: 4-Dimethylaminomethyl-2-methyl-1H-indol-5-0l
2-Methyl-1H-indol-5-0l (5.00 g, 34.0 mmol) was dissolved in 20 mL of
EtOH, aqueous Me2NH (40%, 9.40 mL, 74.7 mmol) was added followed by
aqueous HCHO (37%, 3.30 g, 40.8 mmol). The resulting reaction mixture was
stirred at ambient temperature for 2 hours, then mixed with 50 mL of water,
precipitate formed. Filtration and recrystallization from ethanol
(<SO°C) gave
3.0 g (43%) ofpure titled compound as a white solid: mp 133-135°C; IR
3404,
3385, 1598, 1515, 1428, 1271, 1204, 798, 778 cm-1; 1H NMR (DMSO-d6) 8
2.23 (s, 6H, N(CH3)2), 2.30 (s, 3H, ArCH3), 3.68 (s, 2H, CH2N), 5.98 (s, 1H,
ArH), 6.42 (d, J= 8.42 Hz, 1H, ArH), 6.95 (d, J= 8.79 Hz, IH, ArH), 10.6 (bs,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-64-
IH, exchangeable proton); MS(APCI+): f~zlz 205.2 (MH+). Analysis calculated
for
C12H16N20- C, 70.56; H, 7.90; N, 13.71. Found: C, 70.39; H, 7.87; N, 13.75.
Step C: Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine,
3,7,8,9,10,12, I 3,14,14a,15-decahydro-2-methyl-
To a mixture ofperchlorate salt (973 mg, 4.10 mmol, Example 3, Step B)
and 30 mL of ether was added 40 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 40 mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 7 mL of dioxane, then 4-dimethylaminomethyl-
2-methyl-1H-indol-5-0l (697 mg, 3.41 mmol) was added, the resulting reaction
mixture was refluxed under nitrogen for 16 hours. The reaction mixture was
cooled to ambient temperature and concentrated in vaca~o affording a brown
solid.
Trituration with EtOAc gave 1.01 g (59%) of pure titled compound as a beige
solid: mp 267-270°C (dec.); IR 3407, 3189, 2926, 1435, 1212, 1197, 774
cm-1;
1H NMR (DMSO-d6) 8 1.13-1.64 (m, 9H, aliphatic CH2 and CH), 1.74-1.89 (m,
2H, aliphatic CH), 2.31 (s, 3H, CH3), 2.35-2.50 (m, obscured by DMSO peak, 3H,
aliphatic CH), 2.67-2.75 (m, IH, aliphatic CH), 2.87-3.31 (m, 2H, aliphatic
CH),
5.92 (m, 1 H, ArH), 6.45 (d, J = 8.61 I Iz, 1 H, ArH), 6.94 (d, J = 8.79 Hz, 1
H,
ArH), 10.6 (bs, 1H, exchangeable proton); MS(APCI+): ntlz 297.1 (MH+)
Analysis calculated for C19H24N2O: C, 76.99; H, 8.16; N, 9.45. Found: C,
76.79;
H,8.19;N,9.35.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-65-
Example 5
Pyrrolo[3',2':5,6)[1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 5-bromo-
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, ethyl ester
O
O~CH3
Br N CHa
H
Step A: 6-Bromo-4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-
carboxylic acid ethyl ester
6-Bromo-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl ester,
prepared according to the literature procedure [Bell M.R.; Oesterlin R.;
Beyler
A.L.; Handing H.R.; Potts G.O., J. Merl. Chent., 1967;10:264-266], (3.01 g,
10.1 mmol) and aqueous Me2NH (40%, 2.79 mL, 22.2 mmol) were mixed with
30 mL of EtOH, the mixture was heated with a heatgun until a clear solution
was
obtained. After cooled to ambient temperature, aqueous HCHO (37%, 0.982 g,
12.1 mmol) was added. The resulting reaction mixture was stirred at ambient
temperature for 48 hours during which time white precipitate formed.
Filtration
and drying under vacuum Gave 1.91 g (53%) of pure titled compound as a white
solid: mp 179-180°C (dec.); IR 3339, 1700, 1688, 1426, 1092, 833 cm-1;
1H
NMR (DMSO-d6) 8 1.30 (t, J= 7.14 Hz, 3H, CH2CH3), 2.26 (s, 6H, N(CH3)2),
2.49 (s, 3H, obscured by DMSO peak, ArCH3), 4.16 (s, 2H, ArCH2NMe2),
4.23 (q, J= 6.96 Hz, 2H, CH2CH3), 7.38 (s, 1H, ArH), 11.6 (bs, 1H,
exchangeable proton); MS(APCI+): rnlz 355.0 (MH+). Analysis calculated for
C15H19N203Br: C, 50.72; H, 5.39; N, 7.89; Br, 22.49. Found: C, 50.71; H, 5.31;
N, 7.75; Br, 22.67.
Step B:
To a mixture ofperchlorate salt (1.40 g, 5.90 mmol, Example 3, Step B)
and 50 mL of ether was added 50 mL of aqueous NaOH (2N). The resulting
N
O ~
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-66-
were separated, and the aqueous layer was extracted with ether (2 x SO mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 20 mL of dioxane, then bromoindole mannich base
(1.61 g, 4.54 mmol) was added, the resulting reaction mixture was refluxed
under
nitrogen for 4 hours. The reaction mixture was cooled to ambient temperature
and
concentrated in vacuo affording a thick oil. The crude product was further
purified
by chromatography (50% EtOAc in hexanes) to give a white foam, trituration
with
EtOAc/hexanes gave 1.42 g (54%) of pure titled compound as a white solid: mp
184-185°C; IR 3295, 2930, 1662, 1426, 1185, I I 10, 1081, 869 cm-1; 1H
NMR
(CDCl3) 8 1.15-2.08 (m, 11H, aliphatic CH2 and CH), 1.39 (t, J= 7.14 Hz, 3H,
CH2CH3), 2.45-2.65 (m, 2H, aliphatic CH), 2.60 (s, 3H, ArCH3), 2.85-3.00 (m,
2H, aliphatic CH), 3.17-3.30 (m, 1H, aliphatic CH), 3.50 (dd, J= 18.0, 6.96
Hz,
1H, aliphatic CH), 4.34 (q, J= 7.14 Hz, CH2CH3), 7.35 (s, 1H, ArH), 8.10 (bs,
IH, NH); MS(APCI+): nTlz 447.1 (MH+). Analysis calculated for
C22H27N303Br: C, 59.06; H, 6.08; N, 6.26; Br, 17.86. Found: C, 59.11; H, 6.07;
N, 6.07; Br, 17.97.
Example 6
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12, I 3,14,14a, I S-decahydro-2-methyl-, propyl ester
N O
I~l'O~CH3
N CHs
H
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid propyl ester
5-Acetoxy-2-methyl-IH-indole-3-carboxylic acid propyl ester
(intermediate D, 2.04 g, 7.42 mmol) was mixed with 20 mL of methanol,
NaOCH3 (1.60 g, 29.6 mmol) was then added. The resulting reaction mixture was
stirred at ambient temperature for 1.5 hour. The reaction mixture was then
mixed
with 20 mL of water, the resulting reaction mixture was treated with 5% HCl
until
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-67-
pH = 1 affording a white precipitate. The solid was isolated by filtration and
recrystallized from EtOAc/hexanes to give 1.39 g (80%) pure titled compound as
a beige solid, mp 188-189°C (dec.); IR 3381, 3297, 1661, 1457, 1178,
1090, 794,
783 cm-1; 1H NMR (DMSO-d6) 8 0.989 (t, J= 7.51 Hz, 3H, CH2CH2CH3),
1.72 (sextet, J= 7.14 Hz, 2H, CH2CH2CH3), 2.57 (s, 3H, ArCH3), 4.14 (t,
J= 6.41 Hz, 2H, CH2CH2CH3), 6.58 (dd, J= 8.42, 2.20 Hz, 1H, ArH), 7.11 (d,
J = 8.61 Hz, 1 H, ArH), 7.29 (d, J = 2.20 Hz, 1 H, ArH), 8.83 (s, 1 H, OH), 1
I .5 (bs,
1 H, NH); MS(APCI+): rnlz 234.1 (MH+). Analysis calculated for
C13HISN03~0.06H20: C, 66.63; H, 6.50; N, 5.98. Found: C, 66.27; H, 6.38; N,
5.84.
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1 H-indole-3-carboxylic
acid propyl ester
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid propyl ester (1.27 g,
5.43 mmol) and aqueous Me2NH (40%, 1.50 mL, 12.0 mmol) were mixed with
10 mL of EtOH, the mixture was heated with a heat gun until a clear solution
was
obtained. After cooled to ambient temperature, aqueous HCHO (37%, 0.528 g,
6.52 mmol) was added. The resulting reaction mixture was stirred at ambient
temperature for 3 days. The reaction mixture was then concentrated in vaccro
to
reduce the volume by half. Precipitate formed. Filtration and drying under
vacuum
gave 0.86 g (54%) of pure titled compound as a white solid: mp 135-
137°C (dec.);
IR 3217, 2969, 1684, 1420, 1141, 1075 cm-1; IH NMR (DMSO-d6) 8 0.953 (t,
J= 7.32 Hz, 3H, CH2CH2CH3), 1.70 (sextet, J= 7.33 Hz, 2H, CH2CH2CH3),
2.19 (s, 6H, N(CH3)2), 2.49 (s, 3H, obscured by DMSO peak, ArCI-I3), 4.06 (s,
2H, ArCH2NMe2), 4.13 (t, J= 6.78 Hz, 2H, CH2CH2CH3), 6.56 (d, J= 8.61 Hz,
1H, ArH), 7.07 (d, J= 8.42 Hz, 1H, ArH), 1 I.5 (bs, 1H, exchangeable proton);
MS(APCI+): ntlz 291.1 (MH+). Analysis calculated for C16H22N203~ C~ 66.19;
H, 7.64; N, 9.65. Found: C, 65.94; H, 7.67; N, 9.31.
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-68-
Step C:
To a mixture of perchlorate salt (0.763 g, 3.21 mmol, Example 3, Step B)
and 30 mL of ether was added 30 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 30 mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in S.0 mL of dioxane, then indole Mannich base
(0.717 g, 2.47 mmol) was added; the resulting reaction mixture was refluxed
under nitrogen for 3 hours. The reaction mixture was cooled to ambient
temperature and concentrated in vacuo affording a thick oil. The crude product
was further purified by chromatography (50% EtOAc in hcxanes) to give a white
solid. Recrystallization from CH3CN gave 0.67 g (70%) of pure titled compound
as a white solid: mp 162-164°C; IR 3329, 2931, 1702, 1665, 1434, 1235,
1200,
1149, 1079, 948, 781 cm-1; 1H NMR (CDC13) d 0.992 (t, J= 7.32 Hz, 3H,
CH2CH2CH3), 1.76 (sextet, J= 7.08 Hz, 2H, CH2CH2CH3), 1.29-1.86 (m, l OH,
aliphatic CH2 and CH), 2.11 (d, J-- 13.43 Hz, 1H, aliphatic CH), 2.27-2.58 (m,
2H, aliphatic CH), 2.58 (s, 3H, ArCH3), 2.82-2.86 (m, 2H, aliphatic CH),
3.00-3.10 (m, 1H, aliphatic CH), 3.46 (dd, J= 18.1, 6.84 Hz, 1H, aliphatic
CH),
4.21 (t, J= 6.84 Hz, CH2CH2CH3), 6.73 (d, J- 8.79 Hz, 1H, ArFI), 7.01 (d,
J= 8.79 Hz, lI-I, ArH), 8.06 (bs, 1H, NH); MS(APCI+): mlz 383.1 (MH+)
Analysis calculated for C23H30N203~ C~ 72.22; H, 7.91; N, 7.32. Found:
C, 72.19; H, 7.88; N, 7.36.
Example 7
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-methylpropyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-69-
CH3
N O
v O CHa
O
\~CHa
N
H
Step A:
S-Hydroxy-2-methyl-1H-indole-3-carboxylic acid isobutyl ester
(intermediate H, 1.03 g, 4.17 mmol) and aqueous Me2NH (40%, 1.15 mL,
9.17 mmol) were mixed with 4 mL of EtOH, aqueous HCHO (37%, 0.406 g,
5.01 mmol) was then added. The resulting reaction mixture was heated with a
heat
gun until a clear solution was obtained. The reaction mixture was stirred at
50°C
for 4.5 hours. The reaction mixture was then allowed to stand for 16 hours at
4°C.
Cotton-like white crystals formed. Filtration and drying under vacuum gave
0.62 g
(49%) ofpure titled compound as a white solid: mp 122-124°C (dec.); IR
3229,
2957, 1686, 1424, 1242, 1085, 1000 cm-l; IH NMR (DMSO-d6) b 0.951 (d,
J= 6.59 Hz, 6H, CH2CH(CH3)2), 1.98 (m, J= 6.59 Hz, 1H, CH2CH(CH3)2),
2.18 (s, 6H, N(CH3)2), 2.50 (s, 3H, obscured by DMSO peak, ArCH3), 3.97 (d,
J= 6.59 Hz, 2H, CH2CH(CH3)2), 4.07 (s, 2H, ArCH2NMe2), 6.56 (d,
J= 8.61 Hz, 1H, ArH), 7.06 (d, J= 8.42 Hz, 1H, ArH), 11.5 (bs, 1H,
exchangeable proton); MS(APCI+): m/z 305.2 (MH+). Analysis calculated for
C17H24N203M.03H20: C, 63.23; H, 8.13; N, 8.67. Found: C, 62.84; H, 7.30; N,
8.44.
Step B:
To a mixture of perchlorate salt (0.458 g, I .93 mmol, Example 3, Step B)
and 30 mL of ether was added 30 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 30 mL).
Combined ether layer was dried over Na2S04 and concentrated irr vacuo. The
residual oil was dissolved in 5.0 mL of dioxane, then indole mannich base
(0.451 g, 1.48 mmol) was added, the resulting reaction mixture was refluxcd
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-70-
under nitrogen for 3 hours. The reaction mixture was cooled to ambient
temperature and concentrated in vaczso affording a thick oil. The crude
product
was further purified by chromatography (50% EtOAc in hexanes) to give 0.40 g
(52%) of pure titled compound as a white solid: mp 203-204.5°C; IR
3341, 2933,
1700, 1673, 1434, 1236, 1082, 886, 781 cm-l; 1H NMR (CDC13) 8 0.984 (d,
J= 6.84 Hz, 6H, CH2CH(CH3)2), 1.32-1.90 (m, l OH, aliphatic CH2 and CH),
2.04 (m, J= 6.59 Hz, 1H, CH2CH(CH3)2), 2.08-2.18 (m, 1H, aliphatic CH),
2.40-2.60 (m, 2H, aliphatic CH), 2.59 (s, 3H, ArCH3), 2.84-2.88 (m, 2H,
aliphatic
CH), 2.97-3.10 (m, 1H, aliphatic CH), 3.46 (dd, J= 18.1, 6.84 Hz, 1H,
aliphatic
CH), 4.00-4.09 (m, 2H, CH2CH(CH3)2), 6.73 (d, J= 8.79 Hz, 1H, ArH), 7.02 (d,
J= 8.79 Hz, IH, ArH), 8.05 (bs, 1H, NH); MS(APCI+): mlz 397.2 (MH+)
Analysis calculated for C24H32N203 ~ C> 72.70; H, 8.13; N, 7.06. Found:
C, 72.85; H, 8.19; N, 7.00.
Example 8
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2,2-dimethylpropyl ester
C H3
Ov ~ ~CH3
v O CHa
~/ ~~CH3
~ ~N
H
Step A:
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 2,2-dimethyl propyl
ester (intern~ediate I, 1.55 g, 5.93 mmol) and aqueous Me2NH (40%, 1.64 mL,
13.1 mmol) were mixed with 4 mL of EtOH, aqueous HCHO (37%, 0.406 g,
5.01 mmol) was then added. The resulting reaction mixture was heated with a
heat
gun until a clear solution was obtained. The reaction mixture was stirred at
50°C
for 4.5 hours. The reaction mixture was mixed with 50 mL of EtOAc, the mixture
was washed with water (2 x 50 mL), the organic phase was dried over
Na2S04 and concentrated in vacato affording a thick oil. The cnide product was
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-71-
further purified by flash chromatography (10%-20% methanol in CHC13 to give
0.90 g (48%) of pure titled compound as a white solid: mp I 50-151 °C
(dec.); IR
3251, 2953, 1690, 1424, 1238, 1081, 801 cm-1; 1H NMR (DMSO-d6) 8 0.970 (s,
9H, CH2C(CH3)3), 2.18 (s, 6H, N(CH3)2), 2.52 (s, 3H, ArCH3), 3.91 (s, 2H,
CH2C(CH3)3), 4.08 (s, 2H, ArCH2NMe2), 6.57 (d, J= 8.42 Hz, IH, ArH),
7.07 (d, J= 8.61 Hz, 1H, ArH), 11.5 (bs, 1H, exchangeable proton); MS(APCI+):
m/z 319.2 (MH+). Analysis calculated for CI8H26N203: C, 67.90; H, 8.23;
N, 8.80. Found: C, 67.53; H, 8.04; N, 8.57.
Step B:
To a mixture of perchlorate salt (0.710 g, 2.23 mmol, Example 3, Step B)
and 30 mL of ether was added 30 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 30 mL).
Combined ether layer was dried over Na2S04 and concentrated in v~icuo. The
residual oil was dissolved in 5.0 mL of dioxane, then indole Mannich base
(0.690 g, 2.90 mmol) was added, the resulting reaction mixture was refluxed
under nitrogen for 4 hours. The reaction mixture was cooled to ambient
temperature and concentrated iy7 vacuo affording a thick oil. The crude
product
was further purified by chromatography (50% EtOAc in hexanes) to afford a
white solid. Recrystallization from CH3CN gave 0.92 g (44%) ofpure titled
compound as a white solid: mp 240-243°C; IR 3187, 2934, 1700, 1433,
1235,
1077, 883, 780 cm-1; 1H NMR (DMSO-d6) 8 0.96 (d, 9H, CH2C(CH3)3),
1.19-1.57 (m, 9H, aliphatic CH2 and CH), 1.70-1.80 (m, 1H, aliphatic CH),
1.76-1.85 (m, 1H, aliphatic CH), 2.34-2.45 (m, ZH, obscured by DMSO peak
aliphatic CH), 2.53 (s, 3H, ArCH3), 2.63-2.79 (m, 2H, aliphatic CH),
2.85-2.95 (m, 1H, aliphatic CH), 3.25-3.35 (m, 1H, obscured by water peak
aliphatic CH), 3.94 (ABq, Jab = 10.62 Hz, vab = 24.1 Hz, 2H, CH2C(CH3)3),
6.61 (d, J= 8.42 Hz, 1H, ArH), 7.04 (d, J= 8.61 Hz, 1H, ArH), 11.51 (bs, 1H,
CA 02372197 2001-07-12
WO 00/42045 PCT/IIS99130434
-72-
NH); MS(APCI+): nilz 411.3 (MH+). Analysis calculated for C24H32N203v
C, 73.14; H, 8.35; N, 6.82. Found: C, 73.16; H, 8.52; N, 6.77.
Procedure G: General procedure for the Mannich reaction
The 5-hydroxy indole ester of choice, (2.2-17.9 mmol, 1 eq) was dissolved
in EtOH by stirnng while warming the solution; the solution was cooled.
Aqueous
HCHO (37%, 1.2 eq) and Me2NH (40%, 2.2 eq) were added, and the reaction was
stirred at 50°C until the ratio of starting material to product was
constant. The
ethanol was removed in vacuo, the brown oil was purified by flash
chromatography (using MeOH/CHC13 as the eluent) to afford the desired product.
Example 9
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,?,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl ester
N O
~~ O
O~.
~ CHs
;y N
H
Step A:
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
benzyl ester was synthesized from intermediate C according to Procedure G
Yield: 3.36 g (55%); 1H NMR (DMSO-d6) 8 2.10 (s, 6H, CH2N(CH3)2), 2.45 (s,
3H, ArCH3), 3.97 (s, 2H, CH2NMe2), 5.21 (s, 2H, C02CH2Ph), 6.53 (d,
J= 8.30 Hz, 1H, ArH), 7.04 (d, J= 8.55 Hz, 1H, ArH), 7.29-7.43 (m, 5H, ArH),
11.5 (s, 1 H, NH). MS(APCI+): m/z 339.1 (MH+)
Step B:
By a procedure similar to that described in Example 7, Step C
Yield: 3.30 g (77%); mp 162-164°C; IR: 2930, 2855, 1700, 1432,
1077 cm-1.
1H NMR (DMSO-d6) 8 1.11-1.67 (m, 10H, aliphatic CH2 and CH), 1.82-1.86 (m,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-73-
IH, aliphatic CH), 2.30-2.48 (m, 2H, obscured by DMSO peak, aliphatic CH),
2.48 (s, 3H, ArCH3), 2.62-2.70 (m, 2H, aliphatic CH), 2.86-2.92 (m, IH,
aliphatic
CH), 3.19 (dd, J= 18.3, 6.78 Hz, IH, aliphatic CH), 5.23 (ABq, Jab = 12.1 Hz,
vab = 16.4 Hz, 2H, C02CH2Ph), 6.59 (d, J= 8.61 Hz, 1H, ArH), 7.03 (d,
J= 8.61 Hz, 1H, ArH), 7.30-7.46 (m, 5H, ArH), 11.5 (bs, 1H, NH); MS(APCI+):
431.2 (MH+). Analysis calculated for C27H3pN203: C, 75.35; H, 7.02; N, 6.51.
Found: C, 75.16; H, 6.97; N, 6.47.
Example 10
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-methylethyl ester
HsC CHa
O
v O
O
~~--CHs
~~ N
H
Step A:
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
isopropyl ester was synthesized from intermediate E according to Procedure G.
Yield: 0.490 g (61 %); I H NMR 8 1.26 (d, J= 6.35 Hz, 6> I, CH(CH3)2), 2.17
(s,
6H, CH2N(CH3)2), 2.45 (s, 31-i, ArCH3), 4.03 (s, 2H, CH2NMe2), 5.04 (sextet,
J= 6.35, 1H, C02CH(CH3)2), 6.52 (d, J= 8.55 Hz. 1FI, ArH), 7.02 (d,
J= 8.55 Hz, 1H, ArH), 11.4 (s, 1H, NH). MS(APCI+): m/z 291.1 (MH+)
Step B:
By a procedure similar to that described in Example 7, Step C.
Yield: 0.390 g (60.4%); mp 186-188°C; IR: 2976, 2930, 2856, 1703,
1433,
1079 cm-I. 1H NMR (DMSO-d6) 8 1.10-1.57 (m, 9H, aliphatic CH2 and CH),
1.23 (d, J= 5.62 Hz, 3H, CH3), 1.25 (d, J= 5.86 Hz, 3H, CH3), 1.71-1.74 (m,
1H,
aliphatic CH), 1.85-1.88 (m, 1H, aliphatic CH), 2.32-2.44 (m, 2H, obscured by
CA 02372197 2001-07-12
WO 00/42045 PCT/IIS99/30434
_74_
DMSO peak, aliphatic CH), 2.44 (s, 3H, ArCH3), 2.63-2.74 (m, 2H, aliphatic
CH), 2.83-2.89 (m, 1H, aliphatic CH), 3.21-3.28 (m, 1H, aliphatic CH, obscured
by water peak), 5.01 (septet, 1H, C02CH(CH3)2), 6.56 (d, J= 8.79 Hz, 1 H,
ArH),
6.99 (d, J= 8.80 Hz, 1H, ArH), 11.4 (bs, 1H, NH); MS(APCI+): 383.1 (MH+).
Analysis calculated for C23H30N2~3~ C~ 72.22; H, 7.91; N, 7.32. Found:
C, 71.98; H, 7.85; N, 7.29.
Example 11
Pymolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, cyclopropylmethyl ester
N O
v-O
O
C H3
N
H
Step A:
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
cyclopropyl methyl ester was synthesized from intermediate F according to
Procedure G. Yield: 0.406 g (62.1%); 1H NMR (DMSO-d6) ~ 0.309-0.346 (m,
2H, cyclopropyl CI-I2CH2), 0.538-0.584 (m, 2H, cyclopropyl CH2CH2),
1.16-1.24 (m, 1H, cyclopropyl CH), 2.23 (s, 6H, CH2N(CH3)2), 2.52 (s, 3H,
ArCH3), 4.02 (d, J= 7.32, 2H, C02CH2CH), 4.10 (s, 2H, CH2NMe2), 6.58 (d,
J= 8.55 Hz, 1H, ArH), 7.09 (d, J= 8.55 Hz, 1H, ArH), 11.5 (s, 1H, NH).
MS(APCI+): m/z 303.1 (MH+).
Step B:
By a procedure similar to that described in Example 7, Step C. Yield:
0.269 g (50.7%); mp 199-200°C; IR: 3376, 3337, 2932, 2857, 1698, 1669,
1433,
1081 cm-1. lII NMR (CDC13) 8 0.337-0.373 (m, 2H, cyclopropyl CH2CH2),
0.596-0.641 (m, 2H, cyclopropyl CH2CH2), 1.21-1.89 (m, 10H, aliphatic
CI-I2 and CH), 2.15-2.18 (m, 1H, aliphatic CH), 2.48-2.64 (m, 2H, obscured by
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-75-
ArCH3 peak, aliphatic CH), 2.64 (s, 3H, ArCH3), 2.86-2.96 (m, 2H, aliphatic
CH), 3.00-3.10 (m, 1H, aliphatic CH), 3.52 (dd, J= 18.1, 6.84 Hz, 1H,
aliphatic
CH), 4.07-4.17 (m, 1H, C02CH2), 6.77 (d, J= 8.55 Hz, 1H, ArH), 7.06 (d,
J= 8.79 Hz, IH, ArH), 8.10 (bs, 1H, NH); MS(APCI+): 395.1 (MH+). Analysis
calculated for C24H30N203 ~ C~ 73.07; H, 7.66; N, 7.10. Found: C, 72.96; H,
7.70; N, 6.97.
Example 12
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(1-piperidinyl)ethyl ester
Ov O
i
~ CH3
H
Step A:
5-Hydroxy-2-methyl-IH-indolc-3-carboxylic acid 2-piperdin-I-yl-ethyl
ester (intermediate J, 0.770 g, 2.55 mmol) and aqueous Me2NH (40%, 0.704 mL,
5.60 mmol) were mixed with 2 mL of EtOH, aqueous HCIIO (37%, 0.248 g,
3.06 mmol) was then added. The resulting reaction mixture was heated with a
heatgun until a clear solution was obtained. The reaction mixture was stirred
at
50°C for 2 days. The reaction mixture was then diluted with EtOAc, then
washed
with water and dried over Na2S04. The solution was concentrated in vacuo
affording an oil. The crude product was further purified by flash
chromatography
(10%-20% MeOH in CHCl3) to give an oil (402 mg, 44% crude yield) which was
the desired product with minor impurities: IH NMR (DMSO-d6) 8 1.31-1.33 (m,
2H, piperidine CH2), 1.40-1.45 (m, 4H, 2 x piperidine CH2), 2.16 (s, 6H,
CH2N(CH2N(CH3)2), 2.30-2.40 (m, 4H, 2 x piperidine CH2), 2.47 (s, 3H,
ArCH3), 2.52-2.55 (m, 2H, OCH2CH2N), 4.01 (s, 2H, CH2N(CH3)2), 4.22 (t, .7=
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-76-
5.86 Hz, 2H, OCH2CH2N), 6.52 (d, J= 8.55 Hz, 1H, ArH), 7.02 (d, J= 8.55 Hz,
IH, ArH), 11.4 (bs, 1H, exchangeable proton); MS (APCI+): m/z 360.2 (MH+)
Step B:
By a procedure similar to that described in Example 7, Step C. Yield:
0.137 g (37%); mp 169-171°C; IR: 2928, 1696, 1434, 1094, 1081 cm-1. 1H
NMR
(DMSO-d6) b 1.11-1.54 (m, 15H, aliphatic CH2 and CH), 1.71-1.74 (m, 1H,
aliphatic CH), 1.86-1.90 (m, 1H, aliphatic CH), 2.34-2.47 (m, 6H, obscured by
DMSO peak, aliphatic CH), 2.47 (s, 3H, ArCH3), 2.52 (t, J= 5.62 Hz, 2H,
OCII2Cf12N), 2.62-2.71 (m, 2H, aliphatic CH), 2.84-2.89 (m, 1H, aliphatic CH),
3.24-3.33 (m, IH, aliphatic CH, obscured by waterpcak), 4.14-4.26 (m, 2H,
OCH2CH2N), 6.56 (d, J= 8.79 I-Iz, 1H, ArH), 7.00 (d, J= 8.55 Hz, 1H, ArH),
11.5 (bs, 1H, NH); MS(APCI+): m/z 452.3 (MH+). Analysis calculated for
C27H37N303~0.15H20: C, 71.38; H, 8.28; N, 9.25; H20, 0.59. Found: C, 71.08;
H, 8.25; N, 9.02; H20, 0.21.
Procedure H: General Procedure for the Synthesis of Ethyl 2-alkyl-4-
(dimethylamino)methylene-5-hydroxy-3-indolecarboxylate. To a stirred solution
of ethyl 2-alkyl-5-hydroxy-3-indolecarboxylate (2.63 mmol) in ethanol (8 mL)
was added formaldehyde (0.24 mL, 3.16 mmol) and dimethylamine (0.73 mL,
5.80 mmol). The solution was stirred at 45°C for 3 hours, cooled and
concentrated
under reduced pressure. The residue was subjected to flash column
chromatography (Si02, 1:1 ethyl acetate/hexane then 10:1 ethyl
acetate/ethanol)
to afford the desired product.
Procedure I: General Procedure for the Synthesis of Ethyl 2-alkyl-
[(pyrano[2,3-b]quinolizidine)[5,6-a]]indole-3-carboxylate. To a stin-ed
solution of
NaOH (50% w/w, 100 mL) and ether (20 mL) was added iminium perchlorate salt
(0.42 g, 1.78 mmol, Example 3, Step B). The solution was extracted with ether
( 10 x 100 mL), dried over MgS04 and concentrated under reduced pressure to
afford the enamine as a white solid. To a stirred solution of dioxane
CA 02372197 2001-07-12
VVO 00/42045 PCT/US99/30434
_77_
(2.5 mL/mmol) was added the enamine (0.197 g, 1.43 mmol) and indole
(1.43 mmol). The solution was refluxed overnight, cooled to room temperature,
concentrated under reduced pressure and subjected to flash column
chromatography (Si02, 99:1 dichloromethane/methanol) to afford the desired
product.
Example 13
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I S-decahydro-2-(phenylmethyl)-, ethyl ester
~~CH3
Step A:
Ethyl 2-benzyl-4-(dimethylamino)methylene-5-hydroxy-
3-indolecarboxylate 84% yield as a white solid was synthesized from
intermediate P according to Procedure H. 1H NMR (250 MHz, CD30D) cS 1.38 (t,
J= 7.2 Hz, 3H), 4.32 (q, J= 8.5 Hz, 2H), 4.46 (s, 2H), 6.68 (dd, J= 6.8, 2.5
Hz,
1H), 7.17 (m, SH) 7.47 (d, J =2.25 Hz, 1H). LC/MS (150 mm x 4.6 mm, C-18,
S micron, 10 mM NH40Ac/CH3CN, APCI+) t = 7.86 min, mlz = 353.2 (M+1 ).
Step B:
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-(phenylmethyl)-, ethyl ester was
synthesized according to Procedure I. 68% yield as a white solid. IH NMR
(250 MHz, CDCl3) b 1.36 (t, J= 7.2 Hz, 3H), 1.42 (m, 3H), 1.68 (m, SH),
1.92 (m, 2H), 2.15 (d,J= 13.3 Hz, 1H), 2.53 (m, 2H), 2.85 (m, 2H), 3.09 (m,
IH),
3.48 (dd, J= 20.0, 7.5 Hz, 1H), 4.35 (q, J= 7.1 Hz, 2H), 4.40 (s, 2H), 6.75
(d,
J= 8.6 Hz, 1H), 6.96 (d, J= 8.7 Hz, 1H), 7.29 (m, SH), 7.90 (s, 1H). 13C NMR
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
_78_
(62.5 MHz, CDCl3) S 14.5, 19.7, 25.0, 25.5, 27.0, 30.2, 31.2, 34.5, 36.7,
49.6,
59.9, 67, 87.2, 107, 109.6, 111.4, 114.1, 126, 127.0, 128.9, 129.1, 138, 144,
149,
167. LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM NH40Ac/CH3CN,
APCI+) t = 10.73, mlz = 445.6 (M+1 ) Elemental Analysis Calculated: C, 75.64;
H,
7.25; N, 6.30. Found: C, 75.71; H, 7.34; N, 6.23.
Example 14
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid, 2-ethyl-
3,7,8,9,10,12,13,14,14a,15-decahydro-, ethyl ester
,N
' o O
I ~~ O~CH3
i N y.~CHa
H
Step A:
Ethyl 4-(dimethylamino)methylene-2-ethyl-5-hydroxy-
3-indolecarboxylate was synthesized from intermediate Q according to
Procedure H 55% yield as a white solid. I H NMR (250 MHz, DMSO) 8 I .23 (t,
J= 7.4 Hz, 3H), 1.32 (t, J= 7.0 Hz, 3H), 2.46 (s, 6H), 2.91 (q, J= 7.6 Hz,
2H),
1 S 4.30 (q, J= 7.1 Hz, 2H), 4.44 (s, 2H), 6.61 (d, J= 8.6 Hz, 1 H), 7.11 (d,
J
= 8.4 Hz, 1H), 9.70 (bs, 1H). 13C NMR (62.5 MHz, DMSO) d 14.1, 14.3, 17.3,
21.1, 58.2, 59.1, 103.2, 110.8, 111.6, 112.1, 125.3, 129.4, 148.1, 153.0,
165.6.
LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM NH40Ac/CH3CN, APCI+)
t = 6.25 min, rnlz = 291.3 (M+1 ).
Step B:
Pyrrolo[3',2':5,6J[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
2-ethyl-3,7,8,9,10,12,13,14,14a,15-decahydro-, ethyl ester was synthesized
according to Procedure I 43% yield as a white solid. 1H NMR (250 MHz, CDC13)
~ 1.34 (t, J= 7.6 Hz, 3H), 1.36 (t, J= 7.2 Hz, 3H), 1.63 (m, 5H), 1.88 (m,
3H),
2.15 (d, J= 13.3 Hz, 1H), 2.47 (m, 2I-I), 2.82 (m, 2H), 3.02 (q, J= 7.5 Hz,
2H),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
_79-
3.48 (dd, J= 17.9, 6.8 Hz, 1H), 4.35 (q, J= 7.2 Hz, 2H), 6.77 (d, J= 8.7 Hz,
1H),
7.06 (d, J= 8.7 Hz, 1H), 7.26 (s, 1H), 8.19 (s, 1H). 13C NMR (62.5 MHz, CDC13)
8 13.7, 14.4, 19.7, 21.8, 25.0, 25.5, 27.0, 30.1, 31.1, 36.7, 49.6, 59.8,
67.0, 87.1,
106, 109.4, 113.8, 126, 130, 148, 149, 167. LC/MS (150 mm x 4.6 mm, C-18,
5 micron, 10 mM NH40Ac/CH3CN, APCI+) t = 10.75, mlz = 383.5 (M+1 )
Elemental Analysis: Calculated C, 72.22; H, 7.90; N 7.32. Found C, 72.03; H,
7.96; N, 7.19.
Example 15
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
2-cyclopropyl-3,7,8,9,10,12,13,14,14a,15-decahydro-, ethyl ester
>~CH3
Step A:
Ethyl 2-cyclopropyl-4-(dimethylamino)methylene-5-hydroxy-
3-indolecarboxylate was synthesized from intermediate R according to
Procedure H. 64% yield as a white solid. 1H NMR (250 MHz, DMSO) ~ 0.92 (m,
2H), 1.04 (m, 2H), 1.33 (t, J= 7.1 Hz, 3H), 2.22 (s, 6H), 3.98 (s, 2H), 4.27
(q, J=
7.1 Hz, 2H), 6.58 (d, J- 8.4 Hz, 1H), 7.14 (d, J= 8.8 Hz, 1H), 10.88 (s, 1H).
13C
NMR (62.5 MHz, DMSO) 8 8.4, 8.9, 14.3, 1703, 43.9, 58.3, 59.2, 110.6, 111.3,
112.0, 129. l , 147.3, 152.8, 165.9.
Step B:
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
2-cyclopropyl-3,7,8,9,10,12,13,14,14a,15-decahydro-, ethyl ester was
synthesized
according to Procedure I. 60% yield as a white solid. 1H NMR (250 MHz,
CDC13) 8 0.79 (m, 2H), 1.07 (d, J= 8.54, 2H), 1.37 (m, 3H), 1.40 (t, J= 7.2
Hz,
3H), 1.61 (m, 9H), 1.92 (m, 1H), 2.17 (d, J---- 15.0 Hz, 2H), 2.60 (m, 3H),
2.83 (m,
CA 02372197 2001-07-12
WO 00/42045 PCT/IJS99/30434
-80-
2H), 3.48 (dd, J= 17.9, 6.8 Hz, 1H), 4.37 (q, J= 7.1 Hz, 2H), 6.75 (d, J= 8.6
Hz,
1H), 7.03 (d, J= 8.7 Hz, 1H), 7.84 (s, 1H). 13C NMR (62.5 MHz, CDC13) 8 4.8,
6.0, 7.5, 7.7, 9.5, 14.5, 19.7, 25.0, 25.5, 27.0, 30.0 ,36.7, 49.6, 59.8,
71.1, 74.8,
75.3, 75.8, 76.0, 77.5, 87.1, 109.4, 111.1, 113.8. LC/MS (150 mm x 4.6 mm, C-
18, 5 micron, 10 mM NH40Ac/CH3CN, APCI+) t = 10.70 min,
m/z = 395.5 (M+1). Elemental Analysis: Calculated (as hydrate) C, 69.88; H,
7.82; N, 6.79. Found C, 69.92; H, 7.87; N, 6.67.
Example 16
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-propyl-, ethyl ester
~~CH3
H CHs
Step A:
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-propyl-, ethyl ester was synthesized
from
intermediate S according to Procedure H. 24% yield as a white solid. LC/MS
(150 mm x 4.6mm, C-18, 5 micron, 10 mM NH40Ac/CI-I3CN, APCl+)
t = 7.45 min, mlz = 305.3 (M+1).
Step B:
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-propyl-, ethyl ester was synthesized
according to Procedure I. 28% yield as a white solid. 1H NMR (250 MHz,
CDC13) 8 0.99 (t, J= 7.3 Hz, 3I-I), 1.40 (m, 4H), 1.44 (t, J= 7.1 Hz, 3H),
1.68 (m,
7H), 1.92 (m, 1H), 2.12 (d, J= 12.0 Hz, 1H), 2.55 (m, 2H), 2.92 (m, 5H),
3.47 (dd, J= 16.7, 6.6 Hz, 1H), 4.35 (q, J= 7.1 Hz, 2H), 6.77 (d, J= 8.7 I-Iz,
1H),
7.05 (d, J= 8.7 Hz, 1H), 8.12 (s, 1H). 13C NMR (62.5 MHz, CDC13) 8 13.9, 14.4,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-81-
19.8, 23.0, 25, 26, 27.0, 30.1, 30.5, 36.7, 49.6, 59.7, 87.1, I 10, 111,
113.8, 127,
129, 146, 149, 167. LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM
NH40Ac/CH3CN, APCI+) t = 11.56 min, mlz = 397.5 (M+1 ). Elemental
Analysis: Calculated C, 72.69; H, 8.13; N, 7.06. Found C, 72.30; H, 8.18; N,
6.79.
Example 17
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14, I 4a, I S-decahydro-2-(2-methylpropyl)-, ethyl ester
~N O
O
W ~ wO~CHs
N
H
H3C ~ CH3
Step A:
Ethyl 2-isobutyl-4-(dimethylamino)methylene-5-hydroxy-
3-indolecarboxylate was synthesized from intermediate T according to
Procedure H. 39% yield as a white solid. LC/MS (150 mm x 4.6 mm, C-18,
5 micron, 10 mM NH40Ac/CH3CN, APCI+) t -- 7.86 min, mlz = 3 I 9.3 (M+1 ).
Step B:
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-(2-methylpropyl)-, ethyl ester was
synthesized according to Procedure I. 27% yield as a white solid. l H NMR
(250 MHz, CDCl3) cS 0.95 (d, J= 4.3 Hz, 3H), 0.98 (d, J= 4.3 Hz, 3H) 1.23 (m,
IH), 1.39 (t, J= 7.1 Hz, 3H), 1.42 (m, 6H), 1.63 (m, 6H), 1.88 (m, 1H), 2.00
(m,
1H), 2.16 (d, J= 13.3 Hz, 1H), 2.53 (m, 2H), 2.86 (m, 4H), 3.01 (m, 1H),
3.47 (dd, J= 17.4, 7.2 Hz, 1H), 4.34 (q, J= 7.1, 2H), 6.77 (d, J= 8.6 Hz, 1H),
7.06 (d, J= 8.7 Hz, IH), 8.04 (s, 1H). 13C NMR (62.5 MHz, CDC13) 8 14.5, 19.8,
22.5, 22.6, 25.0, 25.5, 27.0, 29.4, 30.1, 31.2, 36.7, 37.5, 49.5, 59.7, 87.1,
109.3,
1 I 1.4, 113.8, 166.1. LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-82-
NH40Ac/CH3CN, APCI+) t = 6.43 min, mlz = 411.4 (M+1 ). Elemental Analysis:
Calculated C, 73.14; H, 8.35; N, 6.82. Found C, 73.04; H, 8.55; N, 6.60.
Example 18
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,1-dimethylethyl ester
H3C
H
Step A:
tent-Butyl 3-amino-3-methylcrotonate Activation of Zn: To a stirred 3N
HCI solution (50 mL) was added Zn (20 g) and stirred at room temperature for
15 minutes. The HCl solution was decanted, and this was repeated two times.
The
activated Zn was washed with distilled H20 (2x, 100 mL), ethanol (2x, 50 mL),
and ether (2x, 50 mL). The activated Zn was then placed under reduced pressure
for 12 hours at room temperature. To a stirred suspension of anhydrous THF
(10 mL) and activated Zn (0.83 g, 13 mmol) in a flame dried 100 mL round
bottom flask was added 5 drops of tert-butyl bromoacetate at room temperature.
1'he mixture was then heated to reflux for 15 minutes. 0.60 mL (6 mmol)
Acetonitrile was added at once and tert-butyl bromoacetate (1.50 mL, 10 mmol)
was added dropwise over 30 minutes. The reaction mixture turned to green when
about 2/3 of tert-butyl bromoacetate was added. The mixture was refluxed for
an
additional 30 minutes and then allowed to cool to room temperature. To the
stirred
solution was added THF (30 mL) and K2C03 (2 g dissolved in 3 mL water) and
stirred vigorously for 30 minutes. The solution was then placed in a
centrifuge
tube and centrifuged. The supernatant was decanted and the pellet was
resuspended in THF (30 mL), shaken vigorously and centrifuged (2x). The
combined supernatant was dried over MgS04, filtered, and concentrated under
reduced pressure to yield 0.78 g (83%) of tert-butyl 3-amino-3-methylcrotonate
as
light yellow liquid which solidifies at 0°C to a light yellow solid. 1H
NMR
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-83-
(250 MHz, CDC13) 8 1.45 (s, 9H), 1.84 (s, 3H), 4.43 (s, 2H). 13C NMR
(62.5 MHz, CDCI3) b 22.3, 28.6, 78.1, 86.0, 158.9, 171.1.
Step B:
tent-Butyl 5-hydroxy-2-methyl-3-indolecarboxylate. 1,4-Benzoquinone
S (3.30 g, 30 mmol) in ethanol (15 mL) was heated up until all solid was
dissolved.
tent-Butyl 3-amino-3-methylcrotonate (5.50 g, 35 mmol) in ethanol (15 mL) was
added to the hot solution, and the reaction mixture was refluxed for 6 hours,
cooled, and concentrated under reduced pressure. The residue was subjected to
flash column chromatography (A1203, ethyl acetate) to afford 3.57 g of title
compound (14.4 mmol, 48%) as a brown crystal. mp 114.0-116.0°C. 1H NMR
(250 MHz, DMSO) ~ 1.57 (s, 9H ), 2.55 (s, 3H), 6.57 (dd, J= 8.6, 2.3 Hz, 1H),
7.09 (d, .I= 8.6 Hz, 1H,), 7.28 (d, J= 2.3 Hz, 1H), 8.78 (s, 1H), I 1.41 (s,
1H). 13C
NMR (62.5 MHz, d6-MeOH) 8 14.4, 29.1, 80.6, 105.3, 106.8, 112.2, 129.9, 131.1,
145.9, 153.1, 161.7, 167.9.
Step C:
tert-Butyl 4-(dimethylamino)methylene-5-hydroxy-2-methyl-
3-indolecarboxylate. To a stirred solution of tent-butyl 5-hydroxy-2-methyl-
3-indolecarboxylate (1.48 g, 6.0 mmol) in ethanol (4.5 mL) was added
formaldehyde (0.55 mL, 7.2 mmol) and dimethylamine (1.66 mL, 13.2 mmol).
The solution was stirred at 60°C for 10 hours, cooled and
concentrated under
reduced pressure. The residue was extracted with ether (30 mL), filter,
evaporated
solvent under reduced pressure again to afford 1.54 g of title compound
(5.05 mmol, 84%) as a brown crystal. mp 151.0°C (decompose). 1H NMR
(250 MHz, d6-MeOH) c5 1.63 (s, 9H), 1.88 (s, 3H), 2.61 (s, 3H), 2.90 (s, 6H),
4.76 (s, 2H), 6.81 (d, J= 8.7 FIz, 1H), 7.27 (d, J= 8.6 Hz, 1H). 13C NMR
(62.5 MHz, d6-MeOH) 8 16.1, 24.2, 29.0, 43.1, 55.4, 82.0, 107.7, 112.4, 115.4,
131.6, 146.5, 154.1, 161.7. LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM
NH40Ac/CH3CN, APCI+) t = 7.75 min, mlz = 305.4 (M+1 ).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-84-
Step D:
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,1-dimethylethyl ester To a
stirred solution of NaOH (50% w/w, 100 mL) and ether (20 mL) was added
0.082 g (0.20 mmol) of iminium salt (Example 3, Step B). The solution was
extracted with ether (3 x 30 mL), dried over MgS04 and concentrated under
reduced pressure to afford the enamine as a white solid. 1.0 mL dioxane was
added to the enamine and then tort-butyl 4-(dimethylamino)methylene-5-hydroxy-
2-methyl-3-indole carboxylate (0.061 g, 0.20 mmol) followed by 0.5 mL dioxane.
The solution was refluxed overnight, cooled to room temperature, concentrated
under reduced pressure and subjected to flash column chromatography (Si02,
1:1 hexane/ethyl acetate) to afford 0.031 g desired product (0.08 mmol, 40%)
as a
white solid; mp 214.0°C (decompose). /H NMR (250 MHz, CDC13) b 1.45 (m,
4H), 1.61 (s, 9H), 1.69 (m, 5H), 1.89 (m, 1H), 2.14 (d, J= 15 Hz, 1H), 2.49
(m,
2H), 2.56 (s, 3H), 2.84 (d, J= 6 Hz, 2H), 3.06 (m, 1H), 3.48 (dd, J= 18, 7 Hz,
1 H), 6.74 (d, J = 8.6 Hz, 1 H), 7.01 (d, J = 8.6 Hz, 1 H), 8.07 (s, 1 H). 13
C NMR
(62.5 MHz, CDCl3) b 14.8, 19.8, 25.0, 25.5, 27.1, 28.6, 30.2, 31.2, 36.7,
49.6,
66.4, 80.0, 87.2, 108.0, 109.2, 111.3, 113.6, 126.0, 129.2, 140.6, 148.7,
165.6.
LC/MS (150 mm x 4.6 mm, C-18, 5 micron, 10 mM NH40Ac/CH3CN, APCI+) t
=7.98 min, rnlz = 397.4 (M+1 ). Elemental Analysis: Calculated C, 72.70; H,
8.13;
N, 7.06. Found C, 72.28; H, 8.23; N, 6.72.
Example 19
2,6a,7-Trimethyl-7,8,9,10, I Oa, l l -hexahydro-3 H,6aH-6-oxa-3,7-diaza-
cyclopenta[aJanthracene-1-carboxylic acid ethyl ester
3
HaCH C~' O~~p'CH
3 O.
~~ CH3
N
H
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_85_
Step A: 1,6-Dimethyl-1,2,3,4-tetrahydro-pyridine
1,6-Dimethyl-1,2,3,4-tetrahydro-pyridine was synthesized according to the
procedure published in Lipp A., Liebigs AnrT. Chem., 1898;289:216.
Step B: 2,6a,7-Trimethyl-7,8,9,10,10a,11-hexahydro-3H,6aH-G-oxa-3,7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
A solution of 1,6-dimethyl-1,2,3,4-tetrahydropyridine (0.100 g,
0.899 mmol, Example 19, Step A) and 4-dimethylaminomethyl-5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid ethyl ester (0.200 g, 0.724 mmol) in
dioxane (0.800 mL) under N2 was heated at 100°C for 4 hours. An
additional
1.0 mL of dioxane was added, and heating was continued for 24 hours. The
solution was cooled to room temperature, concentrated, and the residue was
purified by flash column chromatography on silica gel using 50%-75% ethyl
acetate:hexane and recrystallized with ethyl acetate to give 12 mg (4.8%) of
2,6a,7-trimethyl-7,8,9,10,10a,11-hexahydro-3H,6aH-6-oxa-3,7-diaza-
cyclopenta[aJanthracene-1-carboxylic acid ethyl ester as a white powder: mp
169-173°C; 1H NMR (400 MHz, CDC13) 8 1.34 (m, 2H), 1.36 (t, J= 7.08 Hz,
3H, CH2CH3), 1.45 (s, 3H, CH3C(O)N), 1.56 (m, 2H), 1.66 (m, 1 H), I .93 (m,
1H), 2.56 (s, 3H, CH3), 2.58 (s, 3H, CH3), 2.87 (bd, J= 17.58 Hz, 2H), 3.50
(dd,
J= 18.19, 6.72 Hz, 1H,), 4.31 (q, J= 7.08 Hz, 2H, CH2CH3), 6.68 (d,
J= 8.79 Hz, 1H, ArH), 7.01 (d, J= 8.30 Hz, 1 H, ArH), 8.03 (bs, 1H, NFL; MS
(APCI+) m/z 343.2 (MH+). Analysis calculated for C2pH26N203'0.17 H20:
C, 69.53; H, 7.68; N, 8.11. Found: C, 69.52; H, 7.33; N, 7.84.
Example 20
7-Ethyl-2,6a-dimethyl-7,8,9,10,1 Oa, l l -hexahydro-3H,6aH-6-oxa-3, 7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
HsCuN~~. O ~-CHs
HsC DLO
O~~
~~~ ~ ~CH3
N
H
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-86-
Step A: I-Ethyl-6-methyl-2,3,4,5-tetrahydropyridinium perchlorate
I-Ethyl-6-methyl-2,3,4,5-tetrahydropyridinium perchlorate was
synthesized according to the procedure published in Ladenburg A., Liebigs Ann.
Chem., 1899;304:54.
S Step B: 7-Ethyl-2,6a-dimethyl-7,8,9,10,10a,11-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyclopenta[a)anthracene-1-carboxylic acid ethyl ester
1-Ethyl-6-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate (0.245 g,
1.08 mmol, Example 20, Step A) was dissolved in a-minimum amount of water
and treated with 50% aqueous NaOH until strongly basic. The aqueous solution
was extracted with 4 x 10 mL of Et20, and the combined extracts were washed
with 1 x 10 mL of saturated aqueous NaCI, dried with MgS04, filtered, and
concentrated into the reaction flask to give 90 mg (0.724 mmol) of I-ethyl-
6-methyl-1,2,3,4-tetrahydro-pyridine. The residue was dissolved in dioxane
(0.750 mL) and 4-dimethylaminomethyl-5-hydroxy-2-methyl-IH-indole-
3-carboxylic acid ethyl ester (0.200 g, 0.724 mmol) was added. The resulting
solution was heated at reflux under N2 for 4 hours, cooled to room
temperature,
and concentrated. The crude residue was purified by flash column
chromatography on silica gel using 100% ethyl acetate and recrystallized with
ethyl acetate to give 98 mg (38%) of 7-ethyl-2,6a-dimethyl-7,8,9,10,10a,11-
hexahydro-3H,6aH-6-oxa-3,7-diaza-cyclopenta[a)anthracene-1-carboxylic acid
ethyl ester as a white powder: mp 173-174°C; IR (KBr) 3298, 2930, 2856,
1669,
1433, 1238, 1094 cm-l; 1H NMR (300 MHz, CDC13) 8 1.16 (m, 3H, CH3CH2N),
1.37 (m, IH), 1.40 (m, 1H), 1.48 (s, 2H), 1.56 (s, 2H), 1.64 (m, 2H), 1.97 (m,
1H),
2.61 (s, 3H, CH3), 2.72 (m, 1H), 2.84 (m, IH), 2.90 (bd, J= 19.04 Hz, 2H),
3.16 (m, 1H), 3.54 (dd, J= 18.31, 6.78 Hz, 1H), 4.35 (qd, .I= 7.14, 1.65 Hz,
2H,
OCH2CH3), 6.70 (d, J= 8.61 Hz, 1H, ArH), 7.03 (d, J= 8.61 Hz, ArH), 8.06 (bs,
1H, NI~; MS (APCI+) nrlz 357.1 (MH+). Analysis calculated for C21H28N2p3:
C, 70.76; H, 7.92; N, 7.86. Found: C, 70.49; H, 7.80; N, 7.66.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_87_
Example 21
I CHa
H3C. N
t
CH3 I ~ CH
t 3
N
H
Isomer A:
6a-Ethyl-2,7-dimethyl-7,8,9,10,1 Oa, I l -hexahydro-3H,6aH-6-oxa-3,7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
Step A: 6-Ethyl-I-methyl-2,3,4,5-tetrahydropyridinium perchlorate
6-Ethyl-I-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate was
synthesized according to the procedure published in Leonard N.J.; Hauck, Jr.,
F.P., J. Ana. Cheat. Soc., 1957;79:5279.
Step B: 6a-Ethyl-2,7-dimethyl-7,8,9,10,IOa,ll-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyelopenta[a]anthracene-I-carboxylic acid ethyl ester
6-Ethyl-1-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate (0.712 g,
3.16 mmol, Example 21, Step A) was dissolved in a minimum amount of water
and treated with 50% aqueous NaOH until strongly basic. The aqueous solution
was extracted with 4 x 15 mL of Et2O, and the combined extracts were washed
with 1 x 15 mL of saturated aqueous NaCI, dried with MgS04, filtered, and
concentrated into the reaction flask to 6-ethyl-1-methyl-1,2,3,4-tetrahydro-
pyridine. The residue was dissolved in dioxane (2.1 mL) and
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl
ester (0.581 g, 2.10 mmol) was added. The resulting solution was monitored by
tlc
and MS as it was stirred at room temperature under N2 for 2 hours, heated at
50°C
for 24 hours, at 60°C for 3 hours, at 70°C for 17 hours, and
between 89-90°C for
4.5 hours. The darkening solution was cooled to room temperature, and
concentrated. The crude residue was purified by flash column chromatography on
silica gel using 20-60% ethyl acctate:hexanes to give 56 mg (7.5 %) of a
single
isomer A of 6a-ethyl-2,7-dimethyl-7,8,9,10, I Oa, l l-hexahydro-3H,6aH-6-oxa-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_88_
3,7-diaza-cyclopenta[a]anthracene-I-carboxylic acid ethyl ester with a larger
Rf
value as a white powder: mp 144-147°C; IR (KBr) 3375, 2975, 2937, 2858,
1671,
1470, 1432, 1245, 1202 cm-I; IH NMR (400 MHz, CDCl3) b 0.91 (m, 3H,
CH3CH2C(O)N), 1.34 (m, 2H), 1.36 (m, 3H, OCH2CH3), 1.54 (bs, 1H), 1.63 (m,
S 1 H), 1.76 (m, 1 H), 1.84 (m, 1 H), 2.06 (m, I H), 2.46 (s, 3H, CH3), 2.52
(m, 1 H),
2.57 (2, 3H, CH3), 2.79 (d, J= 18.07 Hz, 1H), 2.88 (m, IH), 3.37 (dd, J=
18.07,
6.59 Hz, 1H), 4.31 (m, 2H, OCH2CH3), 6.67 (d, J= 8.79 Hz, 1H, ArH), 6.99 (d,
J= 8.55 Hz, 1H, ArH), 8.03 (bs, 1H, NH); MS (APCI+) mlz 357.2 (MH+).
Analysis calculated for C21H2gN203~0.04 H20: C, 70.61; H, 7.92; N, 7.84;
water, 0.22. Found: C, 70.27; H, 7.92; I~T, 7.58; water, 0.22.
Example 22
C H3
H3C.N ~y_p
C H3 \
N
H
Isomer B:
6a-Ethyl-2,7-dimethyl-7,8,9, 10,10a, I 1-hexahydro-3H,6aH-6-oxa-3,7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
6-Ethyl-1-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate (0.712 g,
3.16 mmol, Example 21, Step A) was dissolved in a minimum amount of water
and treated with SO% aqueous NaOH until strongly basic. The aqueous solution
was extracted with 4 x 1 S mL of Et20, and the combined extracts were washed
with 1 x 15 mL of saturated aqueous NaCI, dried with MgS04, filtered, and
concentrated into the reaction flask to give 6-ethyl-1-methyl-1,2,3,4-
tetrahydro-
pyridine. The residue was dissolved in dioxane (2.1 mL) and
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl
ester (0.581 g, 2.10 mmol) was added. The resulting solution was monitored by
tlc
and MS as it was stirred at room temperature under N2 for 2 hours, heated at
50°C
for 24 hours, at 60°C for 3 hours, at 70°C for 17 hours, and
between 89-90°C for
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-89-
4.5 hours. The darkening solution was cooled to room temperature, and
concentrated. The crude residue was purified by flash column chromatography on
silica gel using 20-60% ethyl acetate:hexanes to give 37 mg (4.9 %) of a
single
isomerB of6a-ethyl-2,7-dimethyl-7,8,9,10,10a,11-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyclopenta[a]anthracene-1-carboxylic acid ethyl ester with a smaller
Rf
value as a fine off white powder: mp 158-161 °C; IR (KBr) 3310, 2957,
2928,
2862, 1654, 1436, 1419, 1204 cm-1; 1H NMR (400 MHz) 8 1:08 (m, 3H,
CH3CH2C(O)N), 1.20 (m, 1H), 1.37 (m, 3H, OCH2CH3), 1.41 (m, 1H), 1.52 (s,
3H, CH3), 1.59 (m, 2H), 1.85 (bd, J-- 13.18 Hz, 1H), 2.30 (m, 1H), 2.35 (m,
2H),
2.58 (s, 3H, CH3), 2.91 (m, 1H), 3.11 (m, 2H), 4.32 (m, 2H, OCH2CH3), 6.72 (d,
J= 8.55 Hz, 1H, ArH), 7.00 (d, J= 8.79 Hz, IH, ArH), 8.03 (bs, 1H, NH); MS
(APCI+) ntlz 357.2 (MH+). Analysis calculated for C21H28N203: C, 70.76; H,
7.92; N, 7.86. Found: C, 71.45; H, 8.44; N, 6.65. HPLC (ALLTECH/ALLTIMA
C-18 I :I H20/CH3CN + 0.05% TFA): retention time = 4.940 min, 99.40% purity.
1 S Example 23
6a, 7-Diethyl-2-methyl-7,8,9,10,1 Oa, l l -hexahydro-3H,6aH-6-oxa-3, 7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
I
N\ O /~-CH3
O
CH O
3CH~ ~ ~ \ CHa
N
H
Step A: 1,6-Diethyl-2,3,4,5-tetrahydro-pyridinium perchlorate
1,6-Diethyl-2,3,4,5-tetrahydro-pyridinium perchlorate was synthesized
according to the procedure published in Leonard N.J., Hauck, Jr., F.P., J.
Arn.
Cheat. Soc., 1957;79:5279.
Step B: 6a,7-Diethyl-2-methyl-7,8,9,10,10a,11-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
1,6-Diethyl-2,3,4,5-tetrahydro-pyridinium perchlorate (0.485 g,
2.02 mmol, Example 23, Step A) was dissolved in a minimum amount of water
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-90-
and treated with 50% aqueous NaOH until strongly basic. The aqueous solution
was extracted with 4 x 15 mL of Et20, and the combined extracts were washed
with 1 x I S mL of saturated aqueous NaCI, dried with MgS04, filtered, and
concentrated into the reaction flask to give 207 mg (1.49 mmol) of 1,6-diethyl-
1,2,3,4-tetrahydro-pyridine. The residue was dissolved in dioxane (1.3 mL) and
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl
ester (0.373 g, 1.35 mmol) was added. The resulting solution was heated at
100°C
under N2 for 7 hours, cooled to room temperature, and concentrated. The crude
residue was purified by flash column chromatography on silica gel using 20-
100%
ethyl acetate:hexanes and recrystallized with ethyl acetate to give 123 mg
(25%)
of 6a,7-diethyl-2-methyl-7,8,9, I 0,1 Oa,11-hexahydro-3H,6aH-6-oxa-3,7-diaza-
cyclopcnta[a]anthracene-1-carboxylic acid ethyl ester as a white crystalline
solid:
mp 162-163°C; IR (KBr) 3390, 2972, 2929, 2859, 1654, 1432, 1201, 1097
cm-/;
1H NMR (400 MHz, CDC13) cS 0.900 (m, 2H, CH3CH2C(O)N), 1.11 (m, 2H,
CH3CH2N), 1.34 (m, 2H), 1.36 (m, 3H, CH3CH20), 1.57 (m, 2H), 1.85 (m, 2H),
2.06 (m, 1H), 2.57 (s, 3H, CH3), 2.70 (m, 2H), 2.79 (bd, J= 18.31 Hz, 1H),
2.84 (m, 1H), 3.00 (m, 1H), 3.37 (m, 1H), 4.31 (m, 2H, CH3CH20), 6.64 (d,
J= 8.55 Hz, 1H, ArH), 6.98 (d, J---- 8.55 Hz, 1 H, Ar~~, 8.00 (bs, 1 H, NH);
MS
(APCI+) m/z 371.1 (MH+). Analysis calculated for C22H30N203v C> 71.32;
H, 8.16; N, 7.56. Found: C, 71.28; H, 7.77; N, 7.32.
Example 24
7-Benzyl-2,6a-dimethyl-7,8,9,10,1 Oa,11-hexahydro-3H,6aI-I-6-oxa-3,7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
/I \
,f
Ha( 7
CH3
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-91-
Step A: 1-Benzyl-6-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate
1-Benzyl-6-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate was
synthesized according to the procedure published in Mohrle H.; Dwuletzki H.Z.,
Nc~tatrforsch., B: Anorg. Chem., Org. Chenz, 1986;41b:1323.
Step B: 7-Benzyl-2,6a-dimethyl-7,8,9,10,10a,11-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyclopenta[a]anthracene-I-carboxylic acid ethyl ester
An excess of 1-benzyl-6-methyl-2,3,4,5-tetrahydro-pyridinium perchlorate
(Example 24, Step A) was dissolved in a minimum amount of water and treated
with 50% aqueous NaOH until strongly basic. The aqueous solution was extracted
with 4 x 20 mL of Et20, and the combined extracts were washed with 1 x 20 mL
of saturated aqueous NaCI, dried with MgS04, filtered, and concentrated to
give
the enamine. 1-Benzyl-2-methyl-1,2,3,4-tetrahydro-pyridine (0.447 g, 2.39
mmol)
was dissolved in dioxane (2.4 mL) and 4-dimcthylaminomethyl-5-hydroxy-2-
methyl-1H-indole-3-carboxylic acid ethyl ester (0.330 g, 1.20 mmol) was added.
The resulting solution was heated at 80-90°C under N2 for 20 hours,
cooled to
room temperature, and concentrated. The crude residue was purified by flash
column chromatography on silica gel using 10-20% ethyl acetate:hexanes and
recrystallized with ethyl acetate to give 208 mg (42%) of 7-benzyl-2,6a-
dimethyl-
7,8,9,10,1 Oa,11-hexahydro-3H,6aH-6-oxa-3,7-diaza-cyclopenta[a]anthracene-
1-carboxylic acid ethyl ester of an off white powder: mp 196-198°C; IK
(KBr)
3397, 2983, 2923, 2897, 2854, 1668, 1432, 1200, 1097 cm-l; 1H NMR
(400 MHz, CDC13) ~ 1.36 (m, 1H), 1.37 (m, 3H, OCH2CH3), 1.50 (s, 3H,
CH3C(O)N), 1.53 (m, 3H), 2.03 (m, 1H), 2.56 (m, 1H), 2.58 (s, 3H, CH3),
2.71 (m, 1H), 2.94 (d, J- 18.07 Hz, 1H), 3.53 (dd, J= 18.07, 6.84 Hz, 1H),
3.58 (d, J = 15.14 Hz, 1H, NCH(H)Ph), 4.32 (m, 2H, OCH2CH3), 4.47 (d,
J= 14.89 I-Iz, 1H, NCH(H)Ph), 6.71 (d, J= 8.55 Hz, 1H, ArH), 7.01 (d,
J= 8.55 Hz, 1H, ArH), 7.18 (t, J= 7.08 Hz, IH, PhH), 7.28 (m, 2H, PhI~,
7.35 (d, J= 7.33 Hz, 2I-I, PhH), 8.06 (bs, 1H, NH); MS (APCI+) m/z
419.2 (MH+). Analysis calculated for C26H3pN203~H20: C, 74.39; H, 7.24; N,
6.67; water, 0.30. Found: C, 74.27; H, 7.25; N, 6.54; water, 0.31.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99l30434
-92-
Example 25
H3C
H3C-N O\
CH3
Isomer A:
2,7-Dimethyl-6a-phenyl-7,8,9,10,10a,11-hexahydro-3H,6aI-I-6-oxa-3,7-diaza-
cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
Step A: 1-Methyl-6-phenyl-2,3,4,5-tetrahydro-pyridinium perchlorate
A procedure for the synthesis of 1-methyl-6-phenyl-2,3,4,5-tetrahydro-
pyridinium perchlorate is published in Leonard N..T.; Hauck Jr. F.P., J. Am.
Chern.
Soc., 1957;79:5279.
Step B: 2,7-Dimethyl-6a-phenyl-7,8,9,10,10a,11-hexahydro-3H,6aH-6-oxa-
3,7-diaza-cyclopenta[a]anthracene-1-carboxylic acid ethyl ester
An excess of 1-methyl-6-phenyl-2,3,4,5-tetrahydro-pyridinium perchlorate
(Example 25, Step A) u-as dissolved in a minimum amount of water and treated
with 50% aqueous NaOH until strongly basic. The aqueous solution was extracted
with 4 x 20 mL of Et20, and the combined extracts were washed with 1 x 20 mL
of saturated aqueous NaCI, dried with MgS04, filtered, and concentrated to
give
the enamine. 1-Methyl-6-phenyl-1,2,3,4,-tetrahydro-pyridine(0.428 g, 2.47
mmol)
was dissolved in dioxane (1.8 mL) and 4-dimethylaminomethyl-5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid ethyl ester (0.342 g, 1.24 mmol) was
added. The reaction mixture was heated at 75-80°C under N2 for 16
hours, 1.0 mL
of dioxane was added and heating was continued at 90°C for 5 hours.
Dried
toluene (1.0 mL) was added to the mixture and it was heated at 100°C
for
26 hours, cooled to room temperature, and concentrated. The crude residue was
purified by flash column chromatography on silica gel using 10-50% ethyl
acetate:hexanes to give a single isomer A with a smaller R f value, which was
recrystallized with cther:hexanes to give 73 mg (15%) of 2,7-dimethyl-6a-
phenyl-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-93-
7, 8,9,10,1 Oa, I 1-hexahydro-3H,6aH-6-oxa-3,7-diaza-cyclopcnta[a] anthracene-
1-carboxylic acid ethyl ester as an off white powder; mp 176-177°C; IR
(KBr)
3400, 2942, 2930, 2859, 1647, 1434, 1206, 1099, 1079 cm-l; 1H NMR
(400 MHz, CDCI3) 8 1.20 (m, 3H, OCH2CH3), 1.48 (m, 2H), 1.67 (d,
J= 13.18 Hz, 1H), 1.84 (m, 1H), 2.13 (s, 3H, CH3), 2.35 (m, 1H), 2.52 (s, 3H,
CH3), 2.58 (m, IH), 2.68 (m, 1H), 2.81 (d, J= 17.33 Hz, 1H), 2.90 (m, 1H),
4.17 (q, J= 7.08 Hz, 2H, OCH2CH3), 6.89 (d, J= 8.79 Hz, 1H, ArH), 7.06 (d,
J= 8.79 Hz, 1H, ArH), 7.15 (m, 3H, PhH), 7.35 (m, 2H, Pl>H), 8.07 (bs, lI-I,
NH);
MS (APCI+) ntlz 405.2 (MH+). Analysis calculated for C25H28N203: C, 74.23;
H, 6.98; N, 6.93. Found: C, 73.93; H, 7.11; N, 6.66.
Example 26
H3C
H3C-N O , O
O
CH3
N
H
Isomer B:
2,7-Dimethyl-6a-phenyl-7,8,9,10, I Oa, l I -hcxahydro-3H,6aH-6-oxa-3,7-diaza-
cyclopenta[a]anthracene-I-carboxylic acid ethyl ester
An excess of 1-methyl-6-phenyl-2,3,4,5-tetrahydro-pyridinium perchlorate
(Example 25, Step A) was dissolved in a minimum amount of water and treated
with 50% aqueous NaOH until strongly basic. The aqueous solution was extracted
with 4 x 20 mL of Et20, and the combined extracts were washed with I x 20 mL
of saturated aqueous NaCI, dried with MgS04, filtered, and concentrated to
give
the enamine. I-Methyl-6-phenyl-1,2,3,4,-tetrahydro-pyridine(0.428 g, 2.47
mmol)
was dissolved in dioxane (1.8 mL) and 4-dimethylaminomethyl-5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid ethyl ester (0.342 g, 1.24 mmol) was
added. The reaction mixture was heated at 75-80°C under N2 for 16
hours, 1.0 mL
of dioxane was added and heating was continued at 90°C for 5 hours.
Dried
toluene (1.0 mL) was added to the mixture and it was heated at 100°C
for
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-94-
26 hours, cooled to room temperature, and concentrated. The crude residue was
purified by flash column chromatography on silica gel using 10-50% ethyl
acetate:hexanes, recrystallized with ether:hexanes and the filtrate further
purified
by silica gel chromatography using 1-10% ethyl acetate:hexanes to give 70 mg
(14%) of a single isomer B oft,7-dimethyl-6a-phenyl-7,8,9,10,10a,11-hexahydro-
3H,6aH-6-oxa-3,7-diaza-cyclopenta[a)anthracene-1-carboxylic acid ethyl ester
with a larger Rfvalue as a course peach powder: mp 184-186°C; IR (KBr)
3380,
2926, 1698, 1684, 1436, 1073 cm-1; 1H NMR (400 MHz, CDCl3) 8 1.37 (t,
J= 7.08 Hz, 3H, OCH2CH3), 1.49 (m, 2H), 1.64 (m, 1H), 1.76 (m, 1H), 2.29 (m,
1H), 2.31 (s, 3H, CH3), 2.59 (s, 3H, CH3), 2.97 (d, J= 17.58 Hz, 1H), 3.52 (m,
1H), 3.77 (m, 1H), 4.32 (m, 2H, OCH2CH3), 4.89 (bs, 1H), 6.74 (d, J= 8.55 Hz,
1H, ArH), 7.02 (d, J= 8.55 Hz, 1H, ArH), 7.33 (m, SII, PhH), 8.06 (bs, 1H,
NH);
MS (APCI+) m/z 405.2 (MH+). Analysis calculated for C25H28N203: C, 74.23;
H, 6.98; N, 6.93. Found: C, 74.18; H, 6.90; N, 6.72
Example 27
1H,7H-Indolizino[8',8a':5,6]pyrano[3,2-a]indole-1-carboxylic acid,
8,9,11,12,13,13 a,14,14a-octahydro-2-methyl-, ethyl ester
N o rC"3
0
C H3
N
H
Step A: 2,3,5,6,7,8-Hexahydro-1H-indolizinylium perchlorate
2,3,5,6,7,8-Hexahydro-1H-indolizinylium perchlorate was synthesized
according to the procedure published in Reinecke M.G.; Kray L.R., J. Org.
Chem., 1964;29:1736.
Step B: 1H,7H-Indolizino[8',8a':5,6]pyrano[3,2-a]indole-1-carboxylic acid,
8,9,11,12,13,13 a, l 4,14a-octahydro-2-methyl-, ethyl ester
2,3,5,6,7,8-Hexahydro-1 H-indolizinylium perchlorate (0.330 g,
1.48 mmol, Example 27, Step A) was dissolved in a minimum amount of water
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-95-
and treated with 50% aqueous NaOH until strongly basic. The aqueous solution
was extracted with 4 x I S mL of Et20, and the combined extracts were washed
with I x 15 mL of saturated aqueous NaCI, dried with MgS04, filtered, and
concentrated into the reaction flask to give 143 mg (1.16 mmol) of enamine.
S 1,2,3,5,6,7-Hexahydro-indolizine was dissolved in dioxane (1.5 mL) and
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl
ester (0.321 g, 1.16 mmol) was added. The resulting solution was heated at
reflux
under N2 for S hours, cooled to room temperature, and concentrated. The crude
residue was purified by flash column chromatography on silica gel using 100%
acetone and recrystallized with ethyl acetate to give 129 mg (31%) of 1H,7H-
Indolizino[8',8a':5,6]pyrano[3,2-a]indole-I-carboxylic acid,
8,9,11,12,13,13a,14,14a-octahydro-2-methyl-, ethyl ester as an off white
powder:
mp 170-172°C; IR (KBr) 3396, 3353, 2929, 2853, 1668, 1434, 1156, 1096,
1075 cm-1; IH NMR (300 MHz, CDC13) c~ 1.38 (m, 2H), 1.40 (t, J= 7.14 Hz, 3H,
OCH2CH3), 1.68 (m, 3H), 1.90 (m, 3H), 2.05 (m, IH), 2.61 (s, 3H, CH3),
2.74 (m, 1H), 2.84 (m, IH), 2.97 (d, J= 17.40 Hz, 1H), 3.09 (m, 2H), 3.47 (dd,
J= 17.76, 6.78 Hz, IH), 4.36 (m, 2H, OCH2CH3), 6.67 (d, J= 8.61 Hz, 1H,
ArH), 7.03 (d, J= 8.61 Hz, 1 H, Atl~, 8.06 (bs, 1 H, NH); MS (APCI+) m/z
355.2 (MH+). Analysis calculated for C21H26N203'0.23H20: C, 70.34; H, 7.44;
N, 7.81. Found: C, 70.35; H, 7.48; N, 7.61.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-96-
Example 28
3H,7H-Pyrrolizino[1',8':5,6]pyrano[3,2-a]indole-1-acetic acid,
8,9,11,12,12a,13-
hexahydro-2-methyl-, ethyl ester
N O ~CH3
O
O
\~ CHa
N
H
S Step A: 1,2,3,5,6,7-Hexahydro-pyrrolizinylium perchlorate
1,2,3,5,6,7-Hexahydro-pyrrolizinylium perchlorate was synthesized
according to the procedure published in Miyano S. et al., Syjithesis,
1978;9:701.
Step B: 3H,7H-Pyrrolizino[I',8':5,6]pyrano[3,2-a]indole-1-acetic acid,
8,9,11,12,12a,13-hexahydro-2-methyl-, ethyl ester
1,2,3,5,6,7-Hexahydro-pyrrolizinylium perchlorate (0.904 g, 4.31 mmol,
Example 28, Step A) was dissolved in a minimum amount of water and treated
with 50% aqueous NaOH until strongly basic. The aqueous solution was extracted
with 4 x 15 mL of Et20, and the combined extracts were washed with 1 x 15 mL
of saturated aqueous NaCl, dried with MgS04, filtered, and concentrated into
the
reaction flask to give 325 mg (2.98 mmol) of enamine, 2,3,5,6-tetrahydro-1H-
pyrrolizine. The residue was dissolved in dioxane (2.8 mL) and
4-dimethylaminomethyl-5-hydroxy-2-methyl-lI-I-indole-3-carboxylic acid ethyl
ester (0.794 g, 2.88 mmol) was added. The resulting solution was heated at
80°C
under N2 for 17 hours, cooled to room temperature, and concentrated. The cmde
residue was purified by flash column chromatography on silica gel using I S%
MeOH:CH2C12 and recrystallized with ether to give 105 mg (11%) of 3H,7H-
pyrrolizino[1',8':5,6]pyrano[3,2-a]indole-I-acetic acid, 8,9,11,12,12a,13-
hexahydro-2-methyl-, ethyl ester as a white powder; mp 206-207°C; IR
(KBr)
2972, 2901, 2864, 2828, 1694, 1429, 1196, 1087 cm-I v IH NMR (400 MHz,
CDCl3) S 1.37 (t, J= 7.08 Hz, 3H, OCH2CH3), 1.60 (m, 2H), 1.91 (m, 1H),
1.99 (m, 2H), 2.10 (m, 1H), 2.27 (m, 2H), 2.46 (m, 1H), 2.55 (m, 1H), 2.60 (s,
3H,
CH3), 3.22 (m, 1H), 3.35 (m, 1H), 3.36 (d, J= 4.88 Hz, 1H), 4.34 (m, 2H,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
_97_
OCH2CH3), 6.76 (d, J= 8.55 Hz, 1H, ArH), 7.00 (d, J= 8.55 Hz, 1H, ArH),
8.26 (m, 1H, NH); MS (APCI+) m/z 341.1 (MH+). Analysis calculated for
C20H24N203~ C~ 70.57; H, 7.1 l; N, 8.23. Found: C, 70.40; H, 7.27; N, 7.94.
Example 29
2-Methyl-8,9,10,10a,11,12,12a,13-octahydro-3H,6aH,7H-6-oxa-3,6b-diaza-
benzo[a]cyclopenta[h]anthracene-I-carboxylic acid ethyl ester
~ CH3
7
CH3
Step A: 1,3,4,8,9,9a-Hexahydro-2H-quinolizine
1,3,4,8,9,9a-Hexahydro-2H-quinolizine was synthesized according to the
procedure published in Bohlmann F. et al., Cheat. Ber., 1973;106:3026.
Step B: 2-Methyl-8,9,10,10a,11,12,12a,13-octahydro-3H,6aH,7H-6-oxa-3,6b-
diaza-benzo[a]cyclopenta[h]anthracene-1-carboxylic acid ethyl ester
1,3,4,8,9,9a-Hexahydro-2H-quinolizine (0.372 g, 2.71 mmol, Example 29,
Step A) was dissolved in dioxane (2.7 mL) and 4-dimethylaminomethyl-5-
hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl ester (0.374 g, 1.36 mmol)
was added. The resulting solution was heated at 80°C under N2 for 17
hours, at
reflux for 24 hours, cooled to room temperature, and concentrated. The crude
residue was purified by flash column chromatography on silica gel using 10-20%
ethyl acetate:CH2Cl2 and recrystallized with cyclohexane to give 71 mg (l4%)
of
2-methyl-8,9,10,IOa,11,12,12a,13-octahydro-3H,6aH,7H-6-oxa-3,6b-diaza-
benzo[a]cyclopenta[h]anthracene-1-carboxylic acid ethyl ester as an off white
powder: mp 178-180°C; IR (KBr) 3372, 2929, 2859, 1669, 1654, 1435,
1198,
1095, 1079 cm-1; 1H NMR (400 MHz, CDCl3) 8 1.15 (m, 1H), 1.35 (t,
J= 7.08 Hz, 3H, OCH2CH3), 1.36 (m, 4H), 1.49 (m, 2H), 1.65 (m, 3H), 2.20 (m,
1H), 2.58 (s, 3H, CH3), 2.68 (m, IH), 2.82 (m, 1H), 2.89 (d, J= 17.58 Hz, 1H),
CA 02372197 2001-07-12
WO 00/42045 PCT/U599/30434
-98-
3.05 (m, 1H), 3.45 (m, 1H), 4.31 (m, 2H, OCH2CH3), 4.68 (s, 1H, CH(O)N),
6.68 (d, J= 8.79 Hz, 1H, ArH), 6.99 (d, J= 8.55 Hz, 1H, ArH), 8.03 (bs, I H,
NH); minor diastereomer diagnostic peaks I H NMR (400 MHz, CDCl3) 8
6.75 (d, J= 8.55 Hz, 1H, ArH); MS (APCI+) nilz 369.1 (MH+). Analysis
calculated for C22H28N203: C, 71.71; H, 7.66; N, 7.60. Found: C, 71.96; H,
7.92; N, 7.09. HPLC (ALLTECH/ALLTIMA C-18 150 mm x 4.6 mm column,
1:1 H20/CH3CN + 0.5% TFA): retention time = 3.526 min (11.26%), 3.882 min
(85.82 %) (diastereomers), 97.08 % purity. HPLC (Alltima Silica 5 micron,
150 mm x 4.5 mm column, 95:5 hexane + 0.05 % Et2NH,ethanol + 0.05%
Et2NH): retention time = 5.19 min (84.08 %), 5.87 min (8.63 %)
(diastereomers),
92.71 % purity.
Example 30
3H-pyrido(1",2":1'2']azepino[3'2':5,6]pyrano[3,2-a]indole-1-acetic acid,
7,8,9,10,12,13,14,15,15a,16-decahydro-2-methyl-, ethyl ester, or
7H-Azepino[I",2":1"2']pyrido[3',2':5,6]pyrano[3,2-eJindole-1-acetic acid,
3,8,9,10,11,13,14,15,15a,16-decahydro-2-methyl-, ethyl ester
O ~ CH: ~ CH3
i v/ O 7
O
\>_ _CH3 CH3
N
H H
Step A: 2,3,4,6,7,8,9,10-Octahydro-pyrido[1,2-a]azepine perchlorate
A synthesis of 2,3,4,6,7,8,9,10-octahydro-pyrido[1,2-a]azepine perchlorate
is published in McIntosh J.M. et al., CafT. J Chent., 1983;61:2016.
Step B:
An excess oft,3,4,6,7,8,9,10-octahydro-pyrido[1,2-a]azepine perchlorate
(Example 30, Step A) was dissolved in a minimum amount of water and treated
with 50% aqueous NaOH until strongly basic. The aqueous solution was extracted
with 4 x 20 mL of Et20, and the combined extracts were washed with 1 x 20 mL
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-99-
of saturated aqueous NaCI, dried with MgS04, filtered, and concentrated to
give
582 mg (3.85 mmol) of the crude enamine. The residue was dissolved in dioxane
(3.8 mL) and 4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-
3-carboxylic acid ethyl ester (0.797 g, 2.88 mmol) was added. The reaction
mixture was heated at 80°C under N2 for 6 hours, cooled to room
temperature,
and concentrated. The crude residue was purified by flash column
chromatography on silica gel using 10% ethyl acetate:CH2Cl2-100% ethyl acetate
and recrystallization with iso-octane to give 18 mg (2%) of a single compound,
either 3H-pyrido[1",2":1'2']azepino[3'2':5,6]pyrano[3,2-a]indole-1-acetic
acid,
7, 8,9,10,12,13,14,15, I 5a,1 C-decahydro-2-methyl-, ethyl ester or 7H-
azepino[I",2":1'2']pyrido[3',2':5,6]pyrano[3,2-a]indole-I-acetic acid,
3,8,9,10, I 1,13,14, I 5,15a, l 6-decahydro-2-methyl-, ethyl ester as a fine
white
powder: mp 118-121°C; IR (KBr) 3379, 3306, 2926, 2853, 1671, 1435,
1151,
1094, 10?3 cm-I; IH NMR (400 MHz, CDC13) b 0.86 (m, 1H), 1.35 (m, 3H),
1.36 (m, 3H, OCH2CH3), 1.45 (m, 3H), 1.60 (m, 3H), 1.69 (dd, J= 14.65,
10.01 Hz, 1 H), 1.95 (m, 1 H), 2.19 (m, 1 H), 2.41 (m, 1 H), 2.54 (m, 1 H),
2.57 (s,
3H, CH3), 2.80 (d, J= 18.56 Hz, 1H), 3.11 (m, 1H), 3.34 (m, 1H), 3.48 (m, 1H),
4.31 (m, 2H, OCH2CH3), 6.70 (d, J-- 8.55 Hz, 1 H, ArH), 6.98 (d, J= 8.55 Hz,
IH, Ar~l), 8.01 (bs, IH, NH); MS (APCI+) ntla 383.1 (MH+). Analysis calculated
for C23H30N203v C, 72.22; H, 7.91; N, 7.32. Found: C, 72.14; H, 7.95; N, 6.97.
Procedure J: General procedure for the Mannich reaction: The amide (1.I mmol),
1 eq) was dissolved in EtOH by stirring while warming the solution: the
solution
was cooled. Aqueous HCHO (37%, 1.32 mmol, 1.2 eq) and Me2NH (40%,
2.42 mmol, 2.2 cq) were added, and the reaction was allowed to stir. After
several
hours, a white precipitate begins to form in the solution; the reaction may be
heated to 5°C to speed the reaction. Upon completion, there is little
or no starting
material present. Water was added to the solution, and the mixture was then
cooled in an ice bath. The resulting white solid was collected by filtration
and
dried under vacuum.
CA 02372197 2001-07-12
WO 00142045 PCT1US99/30434
-100-
Procedure K: General procedure for the condensation reaction: 1,2,3,4,6,7,8,9-
octahydro-quinolizinylium perchlorate (17.8 mmol, 1.2 eq) was converted to the
enamine in the following manner: the imine was dissolved in 1N NaOH (10 mL)
and the solution extracted with 2 x 20 mL of diethyl ether. The extracts were
combined dried, and evaporated under vacuum to yield a white solid. The solid
was dissolved in dioxane (10 mL). A solution of the 4-dimethylaminomethyl-5-
hydroxy-2-methyl-1H-indole-3-carboxylic acid methyl or benzyl amide
(14.8 mmol, 1 eq) was dissolved in dioxane (10 mL) and added to the enamine.
The solution was refluxed for 19 hours, resulting in the formation of a white
precipitate. The mixture was cooled in an ice bath, and the resulting solid
was
collected by filtration. The solid was washed sparingly with CH3CN and dried
under vacuum at 50 degrees for 24 hours.
Example 31
8,9,11,12,13,13a, I 4, I 4a-Octahydro-N,2-dimethyl-pyrrolo[3',2' :5,6] [ 1 ]-
benzopyrano[3,2-i]quinolizine-1-carboxamide
N O CH3
H
O
~~-CH3
N
H
Step A:
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
methyl amide was synthesized from Intermediate W according to Procedure J.
Yield: 0.186 g (63.2%); mp: decomposition at >210°C; IR: 3319,
1615, 1515,
1433, 1218, 801 cm-1. 1H NMR (DMSO-d6) 8: 2.14 (s, 6H, CH2N(CH3)2),
2.28 (s, 3H, ArCH3), 2.70 (d, J= 3.91 Hz, 3H, CONHCH3), 3.69 (s, 2H,
CH2N(CH3)2), 6.48 (d, J= 8.55 Hz, 1H, ArH), 6.97 (d, J= 8.55 Hz, 1H, ArH),
8.06 (s, 1H, CONHCH3), 10.88 (s, 1H, indole NH). MS(APCI+): m/z
262.1 (MH+); Analysis calculated for C14H1c~N302: C, 64.35, H, 7.33, N, 16.08.
Found: C, 64.26, H, 7.44, N, 15.87.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-101-
Step B:
8,9,11,12,13,13a,14,14a-Octahydro-N,2-dimethyl-
pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxamide was
synthesized according to Procedure K. Yield: 0.057 g (9.8%); mp: decomposition
at >210°C; IR: 3392, 3057, 2935, 2857, 1625, 1429, 1215 cm-1; 1H NMR
(DMSO-d6) 8 1.08-1.54 (m, 9H, aliphatic CH2 and CH), 1.63-1.69 (m, 1H,
aliphatic CH), 1.81-1.93 (m, 1H, aliphatic CH), 2.27 (s, 3H, ArCH3),
2.31-2.45 (m, 3H, obscured by DMSO peak, aliphatic CH), 2.63-2.69 (m, 1H,
aliphatic CH), 2.68 (d, J= 3.91 Hz, 3H, CONHCH3), 2.87-2.95 (m, 1H, aliphatic
CH), 3.05 (dd, J= 18.3, 5.37 Hz, IH, aliphatic CH), 6.49 (d, J= 8.79 Hz, 1H,
ArH), 6.94 (d, J= 8.79 Hz, 1 H, ArH), 7.70-7.75 (m, 1 H, CONHCI I3), 10.9 (s,
1 H,
NH); MS(APCI+): m/z 354.2 (MH+); Analysis calculated for
C21H27N302~O.SC4H802(CH3C02Et): C, 69.49, H, 7.86, N, 10.57. Found:
C, 69.64, H, 7.75, N, 10.54.
Example 32
8,9,11,12,13,13 a,14, I 4a-octahydro-2-methyl-N-(phenylmethyl)-
pyrrolo[3',2':5,6][ I ]benzopyrano[3,2-i]quinolizine-I-carboxamide
N O
v-N
O H
i
~ CH3
N
H
Step A:
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
benzyl amide was synthesized from Intermediate X according to Procedure J.
Yield: 0.582 g (69.2%); mp: 208-210 °C; IR: 3312, 1610, 1510, 1437,
1207, 747,
697 (cm-1). 1H NMR (DMSO-d6) 8: 2.05 (s, 6H, CH2N(CH3)2), 2.31 (s, 3H,
ArCH3), 3.67 (s, 2H, CH2N(CH3)2), 4.40 (d, J= 5.86 Hz, 2H, CONHCH2),
6.46 (d, J= 8.55, IH, ArH), 6.98 (d, J= 8.55, 1H, ArH), 7.18-7.34 (m, 5H,
ArH),
8.63 (t, J= 5.86, 1H, CONHCH2), 10.76 (s, 1H, aromatic OH), 10.91 (s, 1H,
CA 02372197 2001-07-12
WO 00/42045 PCT/IJS99/30434
-102-
indole NH). MS(APCI+): m/z 338.2 (MH+); Analysis calculated for
C20H23N302~ C~ 71.19, H, 6.87, N, 12.45. Found: C, 70.82, H, 6.86, N, 12.24.
Step B:
Example 32 was synthesized according to Procedure K. Yield: 0.228 g
(35.9%); mp: 235-237°C; IR: 3177, 2929, 1627, 1429, 1089 cm-/. 1H NMR
(DMSO-d6) 8 1.08-1.17 (m, 3H, aliphatic CH2 and CH), 1.35-1.61 (m, 7H,
aliphatic CH2 and CH), 1.85-1.88 (m, 1H, aliphatic CH), 2.26-2.45 (m, 3H,
obscured by DMSO peak, aliphatic CH), 2.29 (s, 3H, ArCH3), 2.61-2.67 (m, 1H,
aliphatic CH), 2.82-2.88 (m, 1H, aliphatic CH), 2.97 (dd, J= 17.6, 6.59 Hz,
IH,
aliphatic CH), 4.33-4.43 (m, 2H, CONHCH2Ph), 6.49 (d, J= 8.55 Hz, 1H, ArH),
6.94 (d, J= 8.55 Hz, 1H, ArH), 7.17-7.31 (m, 5H, ArH), 8.36 (t, J= 6.10 Hz,
1H,
CONHCH2Ph), 10.9 (s, 1H, indole NH); MS(APCI+): m/z 430.2 (MH+); Analysis
calculated for C27H31N302~O.1C4Hg02: C, 75.07, H, 7.31, N, 9.59. Found:
C, 74.99, H, 7.33, N, 9.54.
Example 33
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxamide, N-ethyl-
8,9,1 I ,12, I 3,13 a,14,14a-octahydro-2-methyl-
_N~ O /-CHs
H
O
~~CH3
N
H
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid ethylamide
5-Hydroxy-2-methyl-1-H-indole carboxylic acid (3.28 g, 17.2 mmol) was
dissolved in dry DMF (20 mL) under nitrogen atmosphere and cooled to
0°C in an
ice-water bath. To this solution were added in succession triethylamine (2.39
mL,
17.2 mmol) and solid O-benzotriazol-I-yl-N,I~T,I~T',N'-tetramethyluronium
hexafluorophosphate (6.51 g, 17.2 mmol). The resulting reaction mixture was
stirred at that temperature for 15 minutes, gaseous ethylamine was bubbled in
for
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-103-
minutes. After sequentially 15 minutes stirnng at 0°C and 15 minutes at
ambient temperature, reaction mixture was mixed with 60 mL of EtOAc, the
resulting mixture was successively washed with 1N HCl aqueous solution
(2 x 60 mL), brine (2 x 60 mL), and was dried over Na2S04. The solution was
5 concentrated in vacuo affording a solid. The crude product was further
purified by
flash chromatography (100% EtOAc) followed by recrystallization from EtOAc to
provide 0.81 g (18%) of pure titled compound as a white solid: mp 199-
201°C
(dec.); IR 3372, 1609, 1523, 1464, 1246, 1216, 1193 cm-1; 1H NMR (DMSO-d6)
~ 1.1 l (t, J= 7.14 Hz, 3I-I, CH2CH3), 2.48 (s, 3H, ArCH3), 3.25 (quintet,
10 J= 6.96 Hz, 2H, NHCH2CH3), 6.54 (dd, J= 8.61, 2.20 Hz, 1H, ArH), 7.06 (d,
J= 8.42 Hz, 1H, ArH), 7.09 (d, J= 2.01 Hz, 1 H, ArH), 7.23 (t, J= 5.68 Hz, 1H,
NHEt), 8.70 (s, 1H, NH), 11.1 (bs, 1H, OH); MS(APCI+): m/z 219.1 (MH+).
Analysis calculated for C12H14N202'0.13H20: C, 65.34; H, 6.52; N, 12.70.
Found: C, 65.00; H, 6.38; N, 12.64.
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid ethylamide
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid ethylamide (0.708 g,
3.24 mmol) was mixed with 7 mL of EtOH, aqueous Me2NH (40%, 0.895 mL,
7.13 mmol) was added followed by aqueous HCHO (37%, 0.315 g, 3.89 mmol). A
clear reaction solution was obtained. The resulting reaction mixture was
stirred at
ambient temperature for 2 hours during which time precipitate formed.
Filtration
and drying under vacuum gave 0.298 g (33%) of pure titled compound as a white
solid: mp 198-200°C (dec.); IR 3346, 3189, 2986, 1615, 1436, 1215, 801
cm-1;
1H NMR (DMSO-d6) 8 1.11 (t, J= 7.14 Hz, 3I-I, CH2CH3), 2.18 (s, 6H,
N(CH3)2), 2.32 (s, 3H, ArCH3), 3.24 (quintet, J= 6.78 Hz, 2H, CH2CH3), 3.78
(s, 2H, ArCH2NMe2), 6.50 (d, J= 8.42 Hz, 1H, ArH), 7.01 (d, J= 8.61 Hz, 1H,
ArH), 10.6 (bs, 1H, exchangeable proton), 10.9 (bs, 1H, exchangeable proton);
MS(APCI+): m/z 276.1 (MH+). Analysis calculated for C15H21N302~ C~ 65.43;
H, 7.69; N, 15.26. Found: C, 65.24; H, 7.73; N, 14.92.
CA 02372197 2001-07-12
WO 00/42045 PCTliJS99/30434
-104-
Step C: Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxamide, N-
ethyl-8,9,11,12,13,13a,14,14a-octahydro-2-methyl-
To a mixture of perchlorate salt (263 mg, 1.11 mmol, Example 3, Step B)
and 30 mL of ether was added 40 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 40 mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 20 mL of dioxane, then 4-dimethylaminomethyl-
5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethylamide (234 mg,
0.851 mmol) was added, the resulting reaction mixture was refluxed under
nitrogen for 16 hours. The reaction mixture was cooled to ambient temperature
and concentrated in vacuo affording a brown solid. The crude product was
recrystallized from CH3CN, and then further purified by chromatography (10%
MeOH in HCCl3) to give 0.070 g (17%) of pure titled compound as a yellow
solid: mp 264-266°C (dec.); IR 3313, 2930, 1623, 1604, 1435, 1216, 872
cm-I;
1H NMR (DMSO-d6) 8 1.08 (t, J= 7.14 Hz, 3H, CH2CH3), 1.18-1.58 (m, 9H,
aliphatic CH2 and CI-I), 1.71-1.75 (m, 1H, aliphatic CH), 1.92-1.96 (m, 1H,
aliphatic CH) 2.32 (s, 3H, ArCH3), 2.38-2.49 (m, obscured by DMSO peak, 3H,
aliphatic CH), 2.66-2.73 (m, IH, aliphatic CH and CH2), 2.90-2.94 (m, 1H,
aliphatic CH), 3.10 (dd, J= 18.3, 6.78 Hz, 1H, aliphatic CH), 3.23 (quintet,
J= 6.78 Hz, NHCH2CH3), 6.53 (d, J= 8.79 Hz, 1H, ArH), 6.98 (d, J= 8.61 Hz,
1H, ArH), 7.87 (t, J= 5.68 Hz, 1H, NHEt), 10.9 (bs, 1H, exchangeable proton);
MS(APCI+): m/z 368.2 (MH+). Analysis calculated for C22H29N302~ C> 71.90;
H, 7.95; N, 11.43. Found: C, 71.52; H, 7.97; N, 11.25.
Example 34
Pyrrolo[3',2':5,6][ I ]benzopyrano[3,2-i]quinolizine-1-carboxaldehyde,
8,9,11,12,13,13a,14, I 4a-octahydro-2-methyl-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-105-
N
_O
O
\~ ~~--CH3
N
H
To a solution of DMF (642 pL, 8.29 mmol) in CH2C12 was added POC13
(736 pL, 7.89 mmol) dropwise under nitrogen atmosphere. After stirring at
ambient temperature for 10 minutes, pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]-
quinolizine, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl- (Example 4, 1.17
g,
3.95 mmol) was added. When reaction was done as shown by TLC, the reaction
mixture was poured into 300 mL of saturated aqueous NaHC03 solution and
stirred vigorously for 10 minutes. The resulting mixture was extracted with
CHC13 (4 x 100 mL), the combined organic phase was washed with water (1 x
200 mL) and brine (1 x 200 mL), dried over Na2S04, and concentrated in vacuo
affording a golden solid. The crude product was further purified by flash
chromatography (25% acetone in EtOAc). Recrystallization from EtOH/Et20
gave 0.63 g (49%) of pure titled compound as a white solid: mp 262°C
(dce.); IR
3178, 2931, 1633, 1617, 1484, 1474, 1436, 1391, 1130, 1085, 868, 772 cm-1; 1H
NMR (DMSO-d6) 8 1.14-1.59 (m, 9H, aliphatic CH2 and CH), 1.75-1.87 (m, 2H,
aliphatic CH), 2.34-2.54 (m, obscured by DMSO peak, 2H, aliphatic CH), 2.56
(s,
3H, ArCH3), 2.63-2.70 (m, 1H, aliphatic CH), 2.83-2.90 (m, 2H, aliphatic CH),
3.22-3.29 (m, 1H, obscured by water peak, aliphatic CH), 6.60 (d, J= 8.55 Hz,
1H, ArH), 7.04 (d, J= 8.55 Hz, 1H, ArH), 10.0 (s, 1H, ArCHO), 11.9 (bs, 1H,
exchangeable proton); MS(APCI+): m/z 325.2 (MH+). Analysis calculated for
C20H24N202v C~ 74.05; H, 7.46; N, 8.63. Found: C, 73.97; II, 7.48; N, 8.58.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-106-
Example 35
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7, 8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-, methyl ester
N O CH3
O
O
~~-CHs
N
H
Step A: 5-Hydroxy-2-methyl-1H-indolc-3-carboxylic acid methyl ester
Synthetic procedure is available in: Studies on the Nenitzescu synthesis of
5-hydroxyindoles. Patrick, James B.; Saunders, Elizabeth K., Tetrahedron
Lett.,
1979;42:4009-4012.
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1 H-indole-3-carboxylic
acid methyl ester
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid methyl ester (10.0 g,
49.0 mmol) and aqueous Me2NH (40%, 12.0 mL, 107 mmol) were mixed with
32 mL of EtOH, aqueous HCHO (37%, 4.75 mL, 58 mmol) was then added. After
stirring at ambient temperature for 16 hours, the reaction mixture was mixed
with
I 5 I 00 mL of water. The resulting mixture was extracted with EtOAc (3 x
100), the
combined organic phase was washed with water (l x 100 mL) and brine (1 x
100 mL), dried over Na2S04 and filtered. The filtrate was treated with HCl
gas,
precipitate formed and was isolated by filtration. Trituration in hot acetone
(150 mL) gave 3.88 g of white solid. The white solid was suspended in 150 mL
of
EtOAc and mixed with 100 mL of 10% aqueous K2C03 solution, the mixture was
stirred until a clear solution is obtained, two layers were separated, the
aqueous
layer was extracted with EtOAc (50 mL). The combined organic phase was over
Na2S04 and concentrated ijz vacuo to give 3.22 g (25%) of light tan crystals.
Recrystallization of small portion of the crude product from acetone/water
gave
pure titled compound as white crystals: mp 145-146°C; IH NMR (CDC13) 8
2.33 (s, 6H, N(CH3)2), 2.55 (s, 3H, ArCH3), 3.84 (s, 3H, C02CH3), 4.19 (s, 2H,
ArCH2NMe2), 6.72 (d, J= 8.55 Hz, 1H, ArH), 7.04 (d, J= 8.55 Hz, 1H, ArH);
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-107-
MS(APCI+): nilz 263,1 (MH+). Analysis calculated for C14HI8N2O3: C, 64.11;
H, 6.92; N, 10.68. Found: C, 63.77; H, 6.85; N, 10.54.
Step C:
To a mixture of perchlorate salt (2.17 g, 9.10 mmol, Example 3, Step B)
and 50 mL of ether was added 50 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solid had dissolved. Two
layers
were separated, and the aqueous layer was extracted with ether (2 x 50 mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 8 mL of dioxane, then indole mannich base (2.00
g,
7.60 mmol) was added, the resulting reaction mixture was refluxed under
nitrogen
for 2.5 hours followed by stirring at ambient temperature for 16 hours. The
reaction mixture was concentrated in vacuo affording a thick oil.
Crystallization
from CH3CN gave 1.75 g (63%) ofpurc titled compound as a white solid: mp
205-205.5°C; IR 3242, 2938, 1696, 1441, 1236, 1079, 884 cm-I~ 1H NMR
(CDC13) 8 1.21-1.80 (m, 9H, aliphatic CH2 and CH), 1.82-1.95 (m, 1H, aliphatic
CH), 2.09-2.13 (m, 1H, aliphatic CH), 2.41-2.77 (m, 2H, obscured by ArCH3
peak, aliphatic CH), 2.77 (s, 3H, ArCH3), 2.82-2.86 (m, 2II, aliphatic CH),
3.00-3.07 (m, IH, aliphatic CH), 3.44 (dd, J= 18.1, 6.59 Hz, IH, aliphatic
CH),
3.83 (s, 3H, C02CH3), 6.73 (d, J= 8.55 Hz, 1H, ArH), 7.01 (d, J= 8.79 Hz, 1H,
ArH), 8.12 (bs, 1H, NH); MS(APCI+): m/z 355.2 (MH+). Analysis calculated for
C21H26N203v C~ 71.16; H, 7.39; N, 7.90. Found: C, 71.17; H, 7.32; N, 8.00.
Example 36
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,12,12-trimethyl-, phenylmethyl ester
and/or
Pyrrolo(3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14, I 4a,15-decahydro-2,10,10-trimethyl-, phenylmethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-108-
or
I-I
Step A: 4,4-Dimethyl-1,2,3,4,6,7,8,9-octahydro-quinolizinylium perchlorate
'NJ
0
ClO4O
The synthesis of 4,4-dimethyl-1,2,3,4,6,7,8,9-octahydro-quinolizinylium
perchlorate from 1-chloro-3-iodopropane and 2,3,4,5-tetrahydro-2,2,6-
trimethylpyridine was adapted from the procedure described in Evans, D.A.;
Domeier, L.A. Org Synth Coll Vol v1, p 819. 1H NMR (400 MHz, CDC13)
8 1.52 (s, 6H, C(CH3)2), 1.75-1.86 (m, 4H, aliphatic CII), 1.88-2.00 (m, 4H,
aliphatic CH), 2.80-2.87 (m, 4H, aliphatic Cll), 2.65-2.75 (m, 2H, NCH2); MS
(APCI+) m/z 166.0 (parent MH+)
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,12,12-trimethyl-, phenylmethyl
ester
and/or
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,10,10-trimethyl-, phenylmethyl
ester
One equivalent of 4,4-dimethyl-1,2,3,4,6,7,8,9-octahydro-quinolizinylium
perchlorate (1.45 mmol, 0.385 g) was dissolved in a minimum amount of water
and treated with 50% aqueous NaOH until strongly basic. The aqueous solution
was extracted with 4 x 20 mL of Et20 and the combined extracts were washed
with 1 x 20 mL of saturated aqueous NaCl, dried with MgS04, filtered, and
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-109-
concentrated to give the enamine. The residue was dissolved in dioxane (14 mL)
and 4-dimethylaminomethyl-5-hydroxy-2-methyl-1H indole-3-carboxylic acid
benzyl ester (1.45 mmol, 0.490 g) was added. The reaction mixture was heated
at
90°C under N2 for I 8 hours, cooled to room temperature, and
concentrated. The
crude residue was purified by flash column chromatography on silica gel using
100% ethyl acetate and trituration with ether to give 90 mg (14%) of
pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2,12,12-trimethyl-, phenylmethyl ester
and/or pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2,10,10-trimethyl-, phenylmethyl ester as
a
yellow foam: IR (KBr) 3383, 2932, 2857, 1675, 1432, 1090, 1072 cm-1; 1H NMR
(400 MHz, DMSO-d6) 8 1.07 (s, 3H, CH3), 1.23 (s, 3II, CH3), 1.09-1.26 (m, SH,
aliphatic CH), 1.42-1.61 (m, 6H, aliphatic CH), 1.90 (d, J--12.70 Hz, 1H,
aliphatic
CH), 2.58 (d, J--18.56 Hz, 1H, aliphatic CH), 2.64-2.75 (m, 3H, aliphatic CI~,
3.11-3.19 (m, 2H, aliphatic CH), 3.24-3.30 (m, IH, aliphatic CH), 5.15-5.25
(m,
2H, OCH2Ar), 6.52 (d, J--8.79 Hz, 1H, ArH), 6.99 (d, J--8.55 Hz, IH, ArH),
7.30-7.42 (m, SH, ArH), 11.50 (s, 1H, NH); MS (APCI+) ntlz 459.3 (MH+). Anal.
Calcd for C2c~H34N203~0.19 H20: C, 75.39; H, 7.50; N, 6.06; H20, 0.74. Found:
C, 75.00; H, 7.73; N, 5.79; H20, 0.36.
Example 37
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, ethyl ester
1e
CA 02372197 2001-07-12
WO 00/42045 PCT/IJS99I30434
-110-
Step A: 6-Fluoro-5-hydroxy-2-methyl-IH-indole-3-carboxylic acid ethyl ester
O
O
HO
i
Me
F N
H
6-Fluoro-5-hydroxy-2-methyl-IH indole-3-carboxylic acid ethyl ester was
synthesized according to the procedure published in Littell, R.; Allen, G.R.,
Jr.
J. Org. Chem. 1968;33:2064.
Step B: 4-Dimethylaminomethyl-6-fluoro-5-hydroxy-2-methyl-1H-indole-
3-carboxylic acid ethyl ester
Me2N O
O
HO
i
Me
F ~ N
H
4-Dimethylaminomethyl-6-fluoro-5-hydroxy-2-methyl-IH indole-
3-carboxylic acid ethyl ester was prepared according to Procedure G from
6-fluoro-S-hydroxy-2-methyl-1H indole-3-carboxylic acid ethyl ester (4.51
mmol,
1.07 g). The product precipitated out of the reaction solution upon reducing
the
volume by one-third and was recrystallized from acetonitrile to give a yellow-
orange solid (0.510 g, 38%): mp 174-176°C (dec); IR (KBr) 3278, 2975,
1692,
1443, 1124, 1078 cm-1; 1H NMR (400 MHz, DMSO-d6) b 1.32 (t, J--7.08 Hz,
3H, OCH2CH3), 2.26 (s, 6H, N(CH3)2), 2.50 (s, 3H, ArCH3), 4.17 (s, 2H,
NCH2Ar), 4.24 (q, J--7.08 Hz, 2H, OCH2CH3), 7.05 (d, J--10.50 Hz, 1H, ArH),
11.56 (bs, 1H, NH); 19F NMR (DMSO-d6) 8 -141.69 (d, J--10.68 Hz); MS
(APCI+) fnlz 295.1 (MH+). Anal. Calcd for C15H1yF1N203: C, 61.21; H, 6.51;
N, 9.52; F, 6.45. Found: C, 61.32; H, 6.55; N, 9.51; F, 6.61.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-111-
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, ethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, ethyl ester was
synthesized according to Procedure I from 4-dimethylaminomethyl-6-fluoro-
5-hydroxy-2-methyl-1H indole-3-carboxylic acid ethyl ester (1.66 mmol,
0.488 g). The compound was purified by silica gel flash column chromatography
(50:50 ethyl acetate:hexanes) and recrystallized from ethyl acetate to give a
white
solid (32%): mp 184-186°C; IR (KBr) 3367, 2932, 2858, 1670, 1456, 1437,
1135 cm-1; 1H NMR (400 MHz, CDC13) 8 1.37 (t, J--7.08 Hz, 3H, OCH2CH3),
1.25-1.47 (m, 4H, aliphatic CH), 1.60-1.78 (m, 5H, aliphatic CH), 1.85-1.95
(m,
1H, aliphatic CH), 2.09 (bd, J--13.43 Hz, 1H, aliphatic CH), 2.43-2.49 (m, 2H,
aliphatic CH), 2.57 (s, 3H, ArCH3), 2.87-2.92 (m, 2H, aliphatic CH),
3.05-3.18 (m, 1H, aliphatic CH), 3.43-3.50 (m, 1H, aliphatic CH), 4.32 (q,
J--7.08 Hz, 2H, OCH2CH3), 6.86 (d, J--10.01 Hz, 1H, ArH), 8.09 (bs, 1H, NH);
19F NMR (CDCI3) 8 -140.62 (d, J--10.68 Hz); MS (APCI+) m/z 387.1 (MH+).
Anal. Calcd for C22H27F1N2O3: C, 68.37; H, 7.04; N, 7.25; F, 4.92. Found: C,
68.30; H, 7.11; N, 7.09; F, 4.97.
Example 38
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 5-fluoro-
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl ester
H
CA 02372197 2001-07-12
W0 00142045 PCT/US99/30434
-112-
Step A: 6-Fluoro-5-hydroxy-2-methyl-IH indole-3-carboxylic acid
O
OH
HO
i
Me
F \ H
6-Fluoro-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid ethyl ester
(Example 37, Step A, 9.02 mmol, 2.14g) was dissolved in 40 mL of 2N sodium
hydroxide and heated at reflux for 1 hour. The solution was cooled to
0°C and
carefully acidified to pH 9 with concentrated HCI. The solution was extracted
with CH2CI2, the extracts were discarded and the aqueous layer was further
acidified at 0°C to pII 4 with concentrated HCI. The precipitate was
filtered off
and dried in vacuo for 18 hours to afford a tannish pink solid (1.16 g, 62%):
mp
L0 202-204°C (dec); IR (KBr) 3584, 3358, 1649, 1471, 1109 cm-l; 1H
NMR
(400 MHz, DMSO-d6) cS 2.53 (s, 3H, ArCH3), 7.04 (d, J--11.23 Hz, 1H, ArH),
7.46 (d, J--9.03 Hz, I H, ArH), 9.17 (s, 1 H, ArOH), 11.46 (s, 1 H, NH or
COOK,
11.76(s, 1H, COOH or NH); 19F NMR (DMSO-d6) 8 -141.60 (t, J--10.68 Hz);
Ms (APCI-) ~~iZ 2os.o (M-1).
Step B: 6-Fluoro-5-hydroxy-2-methyl-1H indole-3-carboxylic acid benzyl ester
To a suspension of 6-fluoro-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid (4.92 mmol, 1.03 g) in 10.0 mL of DMF at room temperature under N2 was
added dropwise via syringe 1,8-diazabicyclo[5.4.0]undec-7-ene (4.92 mmol,
0.736 mL) followed by benzyl bromide (5.42 mmol, 0.644 mL). After 48 hours,
water (10 mL) was added, and the precipitate was filtered off, dried, and
recrystallized from chloroform to give a white, cottony solid (0.677 g, 46%):
mp
191-193°C; IR (KBr) 3384, 3254, 1662, 1475, 1327, 1129, 1098 cm-1; 1H
NMR
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-113-
(400 MHz, DMSO-d6) 8 2.59 (s, 3H, ArCH3), 5.32 (s, 2H, OCH2C6H5), 7.12 (d,
J--10.99 Hz, 1H, ArH), 7.31-7.49 (m, 6H, ArH), 9.29 (s, 1H, ArOH), 11.67 (s,
1H,
NH); 19F NMR (DMSO-d6) b -140.96-141.01 (m); MS (APCI-) m/z 298.1 (M-1 ).
Anal. Calcd for C17H14F1N103'0.04 H20: C, 68.06; H, 4.73; N, 4.67; F, 6.33;
H20, 0.24. Found: C, 67.69; H, 4.63; N, 4.57; F, 6.61; H20, 0.10.
Step C: 4-Dimethylaminomethyl-6-fluoro-5-hydroxy-2-methyl-1H indole-
3-carboxylic acid benzyl ester
~l
MeZN O
O
HO
~>-Me
F ~ N
H
A solution of 6-fluoro-5-hydroxy-2-methyl-1H indole-3-carboxylic acid
benzyl ester (2.26 mmol, 0.677 g) and N,N,N',N'-tetramethyldiaminomethane
(2.49 mmol, 0.34 mL) in 5 mL of dioxane under N2 was heated at reflux for
21 hours. An additional aliquot ofN,N,N',N'-tetramethyldiaminomethane
(2.49 mmol, 0.34 mL) was added, and the reaction was continued at reflux for
24 hours, cooled to room temperature, and concentrated. The residue was
recrystallized from ethyl acetate to afford a light yellow solid (0.260 g,
32%): mp
167-169°C; IR (KBr) 3280, 2951, 1692, 1443, 1123, 1081 cm-1; 1H NMR
(400 MHz, DMSO-d6) 8 2.12 (s, 6H, N(CH3)2), 2.44 (s, 3H, ArCH3), 4.04 (s,
2H, NCH2Ar), 5.23 (s, 2H, OCH2C6H5), 7.01 (d, J--10.50 Hz, 1H, ArH),
7.31-7.44 (m, 5I-I, ArH), 11.57 (s, 1H, NH); 1~F NMR (DMSO-d6) 8 -141.55 (d,
J 10.68 Hz); MS (APCI+) m/z 357.1 (MH+). Anal. Calcd for
C20H21F1N203-0.07 C4H802: C, 67.18; H, 5.99; N, 7.73; F, 5.24. Found: C,
66.81; H, 6.20; N, 7.74; F, 5.49.
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-114-
Step D: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl ester
was
synthesized according to Procedure I from 4-dimethylaminomethyl-6-fluoro-
5-hydroxy-2-methyl-1H indole-3-carboxylic acid benzyl ester (0.601 mmol,
0.214 g), heating at 80°C for 40 hours. The product was purified by
silica gel flash
column chromatography (30-50% ethyl acetate/hexanes) and recrystallized from
ether to give a white solid (0.169 g, 63%): mp 179-181 °C; IR (KBr)
2932, 2857,
1699, 1453, 1131, 1075 cm-1; 1H NMR (400 MHz, CDCl3) 8 1.17-1.38 (m, 3H,
aliphatic CH), 1.41-1.48 (m, 1H, aliphatic CH), 1.55-1.85 (m, 6H, aliphatic
CH),
2.03 (bd, J--12.94 Hz, 1H, aliphatic CH), 2.42-2.48 (m, 2H, aliphatic CH),
2.55 (s,
3H, ArCH3), 2.79 (d, J--17.58 Hz, 1H, aliphatic CH), 2.85-2.92 (m, 1H,
aliphatic
CH), 3.04-3.17 (m, 1H, aliphatic CH), 3.35 (dd, J--18.31, 6.51 Hz, 1H,
aliphatic
CH), 5.25-5.37 (m, 2H, OCH2C6H5), 6.86 (d, J--10.25 Hz, 1H, ArH),
7.28-7.38 (m, 3H, ArH), 7.40-7.44 (m,2H, ArH), 8.07 (bs, 1H, NH); 19F NMR
(CDCl3) 8 -142.0 (m); MS (APCI+) nTlz 449.1 (MH+). Anal. Calcd for
C27H29F1N203: C, 72.30; H, 6.52; N, 6.25; F, 4.24. Found: C, 72.28; H, 6.45;
N, 6.09; F, 4.50.
Example 39
12H-Furo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 5-
fluoro-
7,8,9,10,13,14,14a,15-octahydro-2-methyl-, ethyl ester
1e
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-115-
Step A: 6-Fluoro-5-hydroxy-2-methyl-benzofuran-3-carboxylic acid ethyl ester
O
O
HO
i
\ Me
F ~ O
To a solution oft-fluoro-[1,4]benzoquinone (38.3 mmol, 4.82 g) in
300 mL of glacial acetic acid was added 3-amino-but-2-enoic acid ethyl ester
(31.9 mmol, 4.12 g). The solution was heated at reflux for 1.5 hours, cooled
to
room temperature, and concentrated. The product was isolated by silica gel
flash
column chromatography (30-50% ethyl acetate/hexanes) to afford a yellow solid
(0.456 g, 6%): mp 138-139°C; IR (KBr) 3284, 2991, 1680, 1469, 1422,
1326,
I 110 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.36 (t, J--7.08 Hz, 3H,
OCH2CH3), 2.68 (s, 3H, ArCH3), 4.32 (q, J--7.08 Hz, 2H, OCH2CH3), 7.43 (d,
J--8.79 Hz, 1 H, ArH), 7.55 (d, J--10.74 Hz, 1 H, ArH), 9.82 (s, 1 H, ArOH); I
~F
NMR (DMSO-d6) 8 -137.42 (t, J--9.16 Hz); MS (APCI-) tnlz 237.1 (M-1). Anal.
Calcd for C12H11F104~ C~ 60.50; H, 4.65; F, 7.98. Found: C, 60.50; H, 4.46; F,
8.20.
Step B: 4-Dimethylaminomethyl-6-fluoro-5-hydroxy-2-methyl-benzofuran-
3-carboxylic acid ethyl ester
Me2N O
O
HO
Me
p ~ O
A solution of 6-fluoro-5-hydroxy-2-methyl-benzofuran-3-carboxylic acid
ethyl ester (1.90 mmol, 0.452 g) and N,N,N;N'-tetramethyldiaminomethane
(2.09 mmol, 0.285 mL) in 4 mL of dioxane under N2 was heated at reflux for
4.5 hours, cooled to room temperature, and concentrated. Water (10 mL) was
added to the residue, and the resultant precipitate was filtered off, dried,
and
recrystallized from t-butyl methyl ether to give a light yellow solid (0.225
g,
40%): mp 120-122°C; IR (KBr) 2989, 1706, 1446, 1384, 1322, 1120 cm-1;
1H
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-I 16-
NMR (400 MHz, DMSO-d6) 8 1.34 (t, J 7.08 Hz, 3H, OCH2CH3), 2.21 (s, 6H,
N(CH3)2), 2.57 (s, 3H, ArCH3), 4.03 (s, 2H, NCH2Ar), 4.32 (q, J--7.08 Hz, 2H,
OCH2CH3), 7.48 (d, J--10.25 Hz, 1H, Arl~; 19F NMR (DMSO-d6) 8 -137.69 (d,
J--10.68 Hz); MS (APCI+) rnlz 296. I (MH+). Anal. Calcd for C 15H 18F I N I
04:
C, 61.01; H, 6.14; N, 4.74; F, 6.43. Found: C, 61.06; H, 6.07; N, 4.61; F,
6.47.
Step C: 12H-Furo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
5-fluoro-7, 8,9,10,13,14,14a,15-octahydro-2-methyl-, ethyl ester
12H-Furo[3',2':5,6][IJbenzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-fluoro-7,8,9,10,13,14,14a,15-octahydro-2-methyl-, ethyl ester was
synthesized
according to Procedure I (90°C, 21 hours) from 4-dimethylaminomethyl-6-
fluoro-
5-hydroxy-2-methyl-benzofuran-3-carboxylic acid ethyl ester (0.603 mmol,
0.178 g). The product was purified by silica gel flash column chromatography
(30% ethyl acetate/hexanes) and recrystallized from ether to afford a white
solid
(0.210 g, 90%): mp 147-149°C; IR (KBr) 2936, 2848, 1717, 1453, 1242,
1125 cm-1; IH NMR (400 MHz, CDC13) 8 1.25-1.37 (m, 2H, aliphatic CH),
1.38 (t, J--7.08 Hz, 3H, OCH2CH3), 1.40-I .52 (m, ZH, aliphatic CH),
1.57-1.80 (m, SH, aliphatic CH), 1.84-1.96 (m, 1H, aliphatic CH), 2.02 (d,
J--13.43 Hz, IH, aliphatic CH), 2.42-2.58 (m, 2H, aliphatic CH), 2.61 (s, 3H,
ArCH3), 2.83-2.91 (m, 1H, aliphatic CH), 2.90 (d, J--17.82 Hz, IH, aliphatic
CH),
3.06-3.16 (m, 1H, aliphatic CH); 3.34-3.40 (m, 1H, aliphatic CH), 4.34 (q,
J--7.08 Hz, 2H, OCH2CH3), 7.03 (d, J--9.77 Hz, 1H, ArH); 19F NMR (CDC13) 8
-137.90 (d, J--9.16 Hz); MS (APCI+) rn/z 388.2 (MH+). Anal. Calcd for
C22H26FIN104~ C~ 68.20; H, 6.76; N, 3.62; F, 4.90. Found: C, 68.12; H, 6.84;
N, 3.56; F, 4.96.
Example 40
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 4,5-
difluoro-
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-117-
O
O
O
~~Me
F ~ N
H
F
Step A: 2,3-Difluoro-benzene-1,4-diol
OH
F
F
OH
2,3-Difluorophenol (Aldrich, 183.2 mmol, 24.32 g) was oxidized using
potassium persulfate nitrate following the procedure of Feiring, A.E.;
Sheppard,
W.A. J. Org. Chem. 1975;40:2543. The crude material was purified by silica gel
flash column chromatography (10% acetonitrile/chloroform) and recrystallized
from chloroform to give a light yellow solid (7.32 g, 27%): mp 156-
158°C; IR
(KBr) 3343 (br), 1514, 1505, 1259, 1199, 1041 cm-1; 1H NMR (400 MHz,
DMSO-d6) ~ 6.52 (d, J--5.37 Hz, 2H, Arl~, 9.45 (s, 2H, ArOl~; 1 ~F NMR
(DMSO-d6) 8 -159.78 (d, J--4.58 Hz); MS (APCI-) m/z 145.0 (M-1). Anal. Calcd
for C6H4F202: C, 49.33; H, 2.76; F, 26.01. Found: C, 49.09; H, 2.73; F, 26.37.
Step B: 2,3-Difluoro-[1,4)benzoquinone
O
F
~F
O
2,3-Difluoro-benzene-1,4-diol (49.1 mmol, 7.17 g) was oxidized using
ammonium cerium(IV) nitrate following the procedure of Feiring, A.E.;
Sheppard,
W.A. J. Org. Client. 1975;40:2543 to afford a bright yellow solid (6.63 g,
94%):
mp 97.0-98.5°C; IR (KBr) 3352, 1684, 1333 cm-1; 1H NMR (400 MHz, DMSO-
d6) 8 6.98 (s, 2H, ArI~; 1'~F NMR (DMSO-d6) 8 -144.85 (s); MS (APCI-) m/z
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-118-
144.0 (M-). Anal. Calcd for C6H2F202: C, 50.02; H, 1.40; F, 26.37. Found: C,
49.89; H, 1.31; F, 26.19.
Step C: 6,7-Difluoro-5-hydroxy-2-methyl-111-indole-3-carboxylic acid benzyl
ester
O
O
HO
i
~>--Me
F \ N
F H
To a solution of2,3-difluoro-[1,4]benzoquinone (1.26 mmol, 0.180 g) in
3.4 mL of glacial acetic acid was added 3-amino-but-2-enoic acid benzyl ester
(1.05 mmol, 0.200 g). The mixture was heated at 50°C for 18 hours,
cooled to
room temperature, and the precipitate was filtered off. The beige solid was
washed
with glacial acetic acid and dried in vaczco to give clean product. The
filtrate was
neutralized, water (20 mL) was added, and the solution was extracted with
ethyl
acetate (4 x 20 mL). The extracts were dried over MgS04, filtered,
concentrated
and the residue purified by silica gel flash column chromatography (20% ethyl
acetate/hexancs). The pure portions were combined to give 0.174 mg (52%) of
off white solid, which was recrystallized from acetonitrile: mp 215-
217°C; IR
(KBr) 3457, 3243, 1658, 1480, 1338, 1150 cm-1; 1H NMR (400 MHz, DMSO-
d6) 8 2.58 (s, 3H, ArCH3), 5.31 (s, 2H, OCH2C6H5), 7.29-7.45 (m, 6H, ArH),
9.75 (s, 1H, ArOH), 12.13 (s, 1H, NH); MS (APCI+) m/z 318.0 (MH+). Anal.
Calcd for C17H13F2N103~0.04 C2H402: C, 64.17; H, 4.15; N, 4.38; F, 11.89.
Found: C, 63.80; H, 4.15; N, 4.05; F, 12.18.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-119-
Step D: 4-Dimethylaminomethyl-6,7-difluoro-5-hydroxy-2-methyl-1H indole-
3-carboxylic acid benzyl ester
Me2N O
O
HO
>--Me
F \ H
F
4-Dimcthylaminomethyl-6,7-difluoro-5-hydroxy-2-methyl-I H-indole-
3-carboxylic acid benzyl ester was prepared according to Procedure G from
6,7-difluoro-5-hydroxy-2-methyl-1H indole-3-carboxylic acid benzyl ester
(8.08 mmol, 2.56 g). The reaction was heated at 50°C for 21 hours, the
precipitate
was filtered off, washed with ethanol, and dried to give a light yellow solid
(1.49 g, 49%): mp 159-160°C (dec); IR (KBr) 3235, 1688, 1438, 1365,
1301,
1123, 1083 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 2.13 (s, 6H, N(CH3)2),
2.46 (s, 3H, ArCH3), 4.00 (s, 2H, ArCH2N), 5.24 (s, 2H, OCH2C6H5),
7.27-7.38 (m, 3H, ArH), 7.41-7.44 (m, 2H, ArH), 12.08 (s, IH, NH); 1~F NMR
(DMSO-d6) 8 -168.26 (d, J--21.4 Hz), -159.68 (d, J--21.4 Hz); MS (APCI+) m/z
375.0 (MH+). Anal. Calcd for C2pH2pF2N2O3: C, 64.16; H, 5.38; N, 7.48; F,
10.15. Found: C, 64.00; H, 5.44; N, 7.36; F, 10.15.
Step E: Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-l-carboxylic acid,
4,5-difluoro-3,7,8,9, I 0,12, I 3,14,14a,15-decahydro-2-methyl-,
phenylmethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
4,5-difluoro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester
was synthesized according to Procedure I (90°C, 18 hours) from
4-dimethylaminomethyl-6,7-difluoro-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid benzyl ester (3.82 mmol, 1.43 g). The product was purified by silica gel
flash
column chromatography (5-10% ethyl acetate/dichloromethane) to give 1.37 g
(77%) of cream colored solid. A portion was recrystallized from t-butyl methyl
ether/hexanes to afford a white solid: mp 159-160°C; IR (KBr) 3297,
2933, 2857,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-120-
1704, 1455, 1141, 1124 cm-1; 1H NMR (400 MHz, CDCl3) ~ 1.17-1.36 (m, 3H,
aliphatic CH), 1.43-1.49 (m, 1H, aliphatic CI~, 1.57-1.78 (m, 6H, aliphatic
CH),
2.00 (d, J--13.7 Hz, 1H, aliphatic CH), 2.46 (d, J--9.77 Hz, 1H, aliphatic
CH),
2.53-2.57 (m, IH, aliphatic CH), 2.57 (s, 3H, ArCH3), 2.71 (d, J--18.31 Hz,
1H,
aliphatic CH), 2.83-2.88 (m, 1H, aliphatic CH), 3.06-3.16 (m, 1H, aliphatic
CH),
3.24-3.30 (m, 1H, aliphatic CH), 5.24-5.36 (m, 2H, OCH2C6H5), 7.32-7.38 (m,
3H, ArH), 7.42 (d, J--6.84 Hz, 2H, ArH), 8.21 (s, 1H, NH); 19F NMR (CDC13) ~
-166.80 (d, J--19.84 Hz), -162.52 (d, J--21.36 Hz); MS (APCI+) m/z 467.1
(MH+).
Anal. Calcd for C27H28F2N203: C, 69.51; H, 6.05; N, 6.00; F, 8.14. Found: C,
69.36; H, 5.99; N, 5.88; F, 8.37.
Example 41
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
4,5-dichloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester
1e
Step A: 6,7-Dichloro-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl
ester
O
O
HO
~--Me
Cl \ H
Cl
6,7-Dichloro-5-hydroxy-2-methyl-IH indole-3-carboxylic acid benzyl
ester was prepared from 2,3-dichloro-[1,4]benzoquinone (16.8 mmol, 2.98 g) and
3-amino-but-2-enoic acid benzyl ester (25.3 mmol, 4.83 g) following the
procedure for the corresponding ethyl ester reported by Grinev, A.N.; Zaitsev,
CA 02372197 2001-07-12
W0 00/42045 PCT/US99/30434
-121-
LA.; Shvedov, V.L; Terent'ev, A.P. J. Org. Chem. USSR (English) ;28:439. Yield
0.649 g (11%): mp 235-236°C; IR (KBr) 3421, 3281, 1651, 1098 cm-1; IH
NMR
(400 MHz, DMSO-d6) 8 2.59 (s, 3H, ArCH3), 5.29 (s, 2H, OCH2C6H5), 7.30 (t,
J--7.08 Hz, 1H, Arl~, 7.34-7.38 (m, 2H, ArH), 7.42 (d, J--7.32 Hz, 2FI, ArH),
7.51 (s, 1H, ArH); MS (APCI-) m/z 348.0 (M-1). HPLC (ALLTECH/ALLTIMA
C-18, 1:1-2:98 H20/CH3CN + 0.05% TFA): retention time=6.573 min, 98.41
purity.
Step B: 4-Dimethylaminomethyl-6,7-dichloro-5-hydroxy-2-methyl-IH indole-
3-carboxylic acid ben~yl ester
MezN O
O
HO
i
~~--Me
CI ~ N
H
I 0 C1
4-Dimethylaminomethyl-6,7-dichloro-5-hydroxy-2-methyl-1H-indole-
3-carboxylic acid benzyl ester was prepared according to Procedure G from
6,7-dichloro-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
(3.06 mmol, I .07 g). The reaction was heated at 50°C for 22.5 hours,
concentrated
and purified by silica gel flash column chromatography (30-50%
acetone/hexanes)
to afford a golden yellow solid (0.830 g, 67%): mp 167-170°C; IR (KBr)
3328,
1695, 1438, 1409, 1330, 1281, I 107 cm-1; 1H NMR (400 MHz, DMSO-d6) 8
2.18 (s, 6H, N(CH3)2), 2.52 (s, 3H, ArCH3), 4.06 (s, 2H, ArCH2N), 5.27 (s, 2H,
OCH2C6H5), 7.30-7.39 (m, 3H, ArH), 7.41-7.47 (m, 2H, ArH), 11.84 (s, 1H,
NH); MS (APCI+) m/z 407.1 (M+). Anal. Calcd for C2pH2pC12N203: C, 58.98;
H, 4.95; N, 6.88; Cl, 17.41. Found: C, 58.87; H, 4.96; N, 6.68; Cl, 17.23.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-122-
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
4,S-dichloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
phenylmethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
S 4,S-dichloro-3,7,8,9,10,12,13,14,14a,1S-decahydro-2-methyl-, phenylmethyl
ester
was synthesized according to Procedure I (80°C, 18 hours) from
4-dimethylaminomethyl-6,7-dichloro-S-hydroxy-2-methyl-1H indole-
3-carboxylic acid benzyl ester (1.82 mmol, 0.742 g). The product was purified
by
silica gel flash column chromatography (10% acetone/hexanes) to give 0.684 g
(7S%) peach colored foam: mp 100-lOS°C; IR (KBr) 3424, 2932, 2853,
1685,
1430, 1125, 1076 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.00-1.30 (m, 3H,
aliphatic CH), 1.37-1.76 (m, 8H, aliphatic CH), 2.37-2.40 (m, 1H, aliphatic
CH),
2.46-2.49 (m, 1H, aliphatic CH), 2.50 (s, 3H, ArCH3), 2.63-2.69 (m, 2H,
aliphatic
CH), 2.95-2.99 (m, 1H, aliphatic CH), 3.17 (dd, J--18.31, 6.74 Hz, 1I-/,
aliphatic
1S CH), 5.23 (dd, J--28.08, 12.21 Hz, 2H, OCH2C6HS), 7.31-7.39 (m, 3H, ArH),
7.42-7.44 (m, 2H, ArH), 11.85 (s, 1 H, NH); MS (APCI+) ntlz 499.1 (M/-/+).
Anal.
Calcd for C27H28C12N203~0.07 H20: C, 64.77; H, 5.66; N, S.S9; Cl, 14.16;
H20, 0.25. Found: C, 64.37; H, 5.64; N, S.S1; C1, 13.96; H20, 0.32.
Example 42
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1S-decahydro-4,S-dimethoxy-2-methyl-, phenylmethyl
ester
CA 02372197 2001-07-12
W0 00/42045 PCT/US99/30434
-123-
Step A: 6,7-Dimethoxy-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl
ester
O
O
HO
~>--Me
Me0 ~ N
H
OMe
2,3-Dimethoxy-[1,4]benzoquinone (10.4 mmol, 1.74 g) was added to a
solution of 3-amino-but-2-enoic acid benzyl ester (8.62 mmol, 1.65 g) in
ethanol
(25 mL) at 0°C. The reaction was warmed to room temperature and then
heated at
reflux for 18 hours. The solvent was removed and the product was purified by
silica gel flash column chromatography (30-50% ethyl acetate/hexanes) and
recrystallized from toluene to give a peach colored solid (0.621 g, 21%): mp
154-156°C; IR (KBr) 3336, 3267, 1660, 1468, 1327, 1146, 1081 cm-I; IH
NMR
(400 MHz, DMSO-d6) 8 2.56 (s, 3H, ArCH3), 3.73 (s, 3H, OCH3), 3.89 (s, 3H,
OCH3), 5.29 (s, 2H, OCH2C6H5), 7.1 I (s, 1 H, ArH), 7.29-7.43 (m, 5H, ArH),
8.79 (s, 1H, ArOH), 11.59 (s, 1H, NH); MS (APCI-) m/z 340.0 (M-1). Anal. Calcd
for C19H19N105: C, 66.85; H, 5.61; N, 4.10. Found: C, 66.69; H, 5.55; N, 3.79.
Step B: 4-Dimethylaminomethyl-6,7-dimethoxy-5-hydroxy-2-methyl-1H-indole-
3-carboxylic acid benzyl ester
MezN O
O
HO
t
~>--Me
Me0 ~ N
H
OMe
4-Dimethylaminomethyl-6,7-methoxy-5-hydroxy-2-methyl-1H indole-
3-carboxylic acid benzyl ester was prepared according to procedure G from
6,7-dimethoxy-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
( 1.69 mmol, 0.577 g). The reaction was heated at 50°C for 18 hours,
concentrated
and purified by silica gel flash column chromatography (0-3%
triethylamine/ethyl
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-124-
acetate) and recrystallized from cyclohexane to afford a lemon yellow solid
(0.274 g, 41%): mp 132-134°C; IR (KBr) 3312, 1698, 1440, 1415, 1290,
I 129 cm-1; 1H NMR (400 MHz, DMSO-d6) ~ 2.10 (s, 6H, N(CH3)2), 2.44 (s,
3H, ArCH3), 3.73 (s, 3H, ArOCH3), 3.86 (s, 3H, ArOCH3), 3.94 (s, 2H,
ArCH2N), 5.21 (s, 2H, OCH2C6H5), 7.29-7.38 (m, 3H, ArH), 7.41-7.43 (m, 2H,
ArH), 11.48 (s, 1H, NH); MS (APCI+) m/z 399.0 (MH+). Anal. Calcd for
C22H26N205~ C~ 66.32; H, 6.58; N, 7.03. Found: C, 66.47; H, 6.58; N, 6.73.
Step C: Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-4, 5-dimethoxy-2-methyl-,
phenylmethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10, I 2,13,14,14x,15-decahydro-4,5-dimethoxy-2-methyl-, phenylmethyl
ester was synthesized according to Procedure I (90°C, 21 hours) from
4-dimethylaminomethyl-6,7-dimethoxy-5-hydroxy-2-methyl-1 H-indole-
3-carboxylic acid bcnzyl ester (0.595 mmol, 0.237 g). The product was purified
by
silica gel flash column chromatography (50% ethyl acetate/hexanes) and
recrystallized from 2,2,4-trimethylpentane to give 0.089 g (30%) white solid:
mp
130-136°C; IR (KBr) 3307, 2931, 2856, 1833, 1700, 1684, 1448, 1418,
1282,
I 137, 1123 cm-I; IH NMR (400 MHz, CDCl3) 8 1.24-1.38 (m, 4H, aliphatic
CH), 1.44-1.55 (m, IH, aliphatic CSI), 1.59-1.80 (m, 6H, aliphatic CH), 2.09
(d,
J--13.98 Hz, IH, aliphatic CH), 2.51 (d, J--10.37 Hz, 1H, aliphatic CH), 2.58
(s,
3H, ArCH3), 2.78 (d, J--18.08 Hz, IH, aliphatic CH), 2.85-2.91 (m, IH,
aliphatic
CH), 3.08-3.14 (m, 1H, aliphatic CH), 3.29-3.36 (m, 1H, aliphatic CH), 3.96
(s,
3H, OCH3), 4.05 (s, 3H, OCH3), 5.26-5.37 (m, 2H, OCH2C6H5), 7.31-7.40 (m,
3H, ArH), 7.44 (d, J--6.99 Hz, 2H, ArH), 8.29 (s, 1H, NH); MS (APCI+) m/z
491.1 (MH+). HPLC (ALLTECH/ALLTIMA C-18, 1:1-2:98 H20/CH3CN +
0.05 % TFA): retention time=4.527 min, 100.00% purity.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-125-
Example 43
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, phenylmethyl ester
O
O
O
t
Me
Me ~ N
H
Step A: 6-Methyl-5-hydroxy-2-methyl-IH indole-3-carboxylic acid benzyl ester
o ~l
0
HO
t
~ Me
Me
6-Methyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
(2.42 g, 10%) was obtained from methyl-[1,4]benzoquinone (Aldrich, 81.9 mmol,
10.0 g) and 3-amino-but-2-enoic acid benzyl ester (79.5 mmol, 15.2 g)
following
the procedure for the corresponding ethyl ester reported by Allen, G.R., Jr.;
Pidacks, C.; Weiss, M.J. J. Am. Chem. Soc. 1966;88:2536. The crude reaction
product consisted of a mixture of the desired 6-methyl-5-hydroxy-2-methyl-1H-
indole-3-carboxylic acid benzyl ester and the regioisomer, 7-methyl-5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid benzyl ester. The regioisomers were
separated following the procedure for the corresponding ethyl esters reported
by
Poletto, J.F.; Allen, G.R., Jr.; Sloboda, A.E.; Weiss, M.J. J. Med. Chem.
1973;16:757. Each isomer was separately recrystallized from acetone to give
X-ray quality crystals. Single crystal X-ray analysis indicated that the
higher Rf
isomer (silica gel, 50% ethyl acetatc/hexanes) was 6-methyl-5-hydroxy-2-methyl-
1H-indole-3-carboxylic acid benzyl ester: mp 196-197°C; 1R (KBr) 3399,
3314,
1655, 1469, 1438, 1086 cm-I; IH NMR (400 MHz, DMSO-d6) b 2.14 (s, 3H,
ArCH3), 2.53 (s, 3H, ArCH3), 5.28 (s, 2H, OCH2C6H5), 6.99 (s, IH, ArH),
7.28-7.42 (m, 6H, ArH), 8.81 (s, 1 H, ArOH), 11.42 (s, 1 H, NII); MS (APCI+)
ntlz
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-126-
296.0 (MH+). Anal. Calcd for C18H17N103~0.25 H20: C, 72.10; H, 5.88; N,
4.67. Found: C, 71.72; H, 5.54; N, 4.74.
Step B: 4-Dimethylaminomethyl-6-methyl-S-hydroxy-2-methyl-1H indole-
3-carboxylic acid benzyl ester
MeZN O
O
HO
i
~ Me
Me \ H
4-Dimethylaminomethyl-6-methyl-S-hydroxy-2-methyl-1H indole-
3-carboxylic acid benzyl ester was prepared according to Procedure G from
6-methyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
(7.48 mmol, 2.21 g) with the addition of 49.4 mmol of dimethylamine and
26.9 mmol of formaldehyde. The reaction was heated at 50°C for 70
hours,
concentrated and purified by silica gel flash column chromatography (0-5%
triethylamine/ethyl acetate) to afford a tan solid (1.67 g, 63%). A portion
was
recrystallized from ethyl acetate to give a light yellow solid: mp 162-
164°C; IR
(KBr) 3313, I 688, 1432, 1227, 1119, 1075 cm l ; 1 H NMR (400 MHz, DMSO-
d6) 8 2.13 (s, 9H, N(CH3)2 and ArCH3), 2.44 (s, 3H, ArCH3), 4.01 (s, 2H,
ArCH2N), 5.22 (s, 2H, OCH2C6H5), 6.95 (s, 1H, Arll), 7.29-7.44 (m, 5H, ArH),
11.40 (s, 1H, NI~; MS (APCI+) m/z 353.1 (MH+). Anal. Calcd for
C21H24N203: C, 71.57; H, 6.86; N, 7.95. Found: C, 71.30; H, 6.92; N, 7.87.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, phenylmethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, phenylmethyl ester was
synthesized according to Procedure I (90°C, 24 hours) from
4-dimethylaminomethyl-6-methyl-5-hydroxy-2-methyl-IH indole-3-carboxylic
acid benzyl ester (4.45 mmol, 1.57 g). The product was purified by silica gel
flash
column chromatography (20-50% acetone/hexanes then 20-40% ethyl
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-127-
acetate/dichloromethane) to give 0.953 g (48%) yellow solid: mp 110-
115°C; IR
(KBr) 3379, 2931, 2857, 1673, 1425, 1123, 1071 cm-1; 1H NMR (400 MHz,
DMSO-d6) 8 1.05-1.16 (m, 2H, aliphatic CH), 1.21-1.25 (m, 1H, aliphatic CH),
1.33-1.42 (m, 2H, aliphatic CH), 1.45-1.66 (m, 5H, aliphatic CH), 1.78 (d,
J--13.67 Hz, 1H, aliphatic CH), 2.18 (s, 3H, ArCH3), 2.34 (d, J 9.28 Hz, 1H,
aliphatic CH), 2.44 (s, 3H, ArCH3), 2.41-2.46 (m, 1H, aliphatic CH),
2.62-2.70 (m, 2H, aliphatic CH), 2.87-2.95 (m, 1H, aliphatic CH), 3.16 (dd,
J--18.07, 6.59 Hz, 1H, aliphatic CI~, 5.19 (dd, J--25.15, 12.21 Hz, 2H,
OCH2C6H5), 6.90 (s, 1H, ArH), 7.28-7.37 (m, 3H, ArH), 7.41 (d, J--6.84 Hz, 2H,
ArH), 11.37 (s, 1H, NH); MS (APCI+) mlz 445.3 (MH+). Anal. Calcd for
C28H32N203~0.16 H20: C, 75.16; H, 7.28; N, 6.26; H20, 0.64. Found: C, 74.76;
H, 7.35; N, 6.07; H20, 0.34.
Example 44
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, phenylmethyl ester
N O
O
O
i
~Me
'N
N
Me
Step A: 7-Methyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
O \
O
HO
---Me
N
H
Me
7-Methyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
(2.04 g, 8.7% yield) was obtained as an off white powder from methyl-
[1,4]benzoquinone (81.9 mmol, 10.0 g) and 3-amino-but-2-enoic acid benzyl
ester
CA 02372197 2001-07-12
WO 00142045 PCT/US99130434
-128-
(79.5 mmol, 15.2 g) as in Example 43, Step A. Single crystal X-ray analysis
indicated that the lower R f isomer (silica gel, 50% ethyl acetate/hexanes)
was
7-methyl-5-hydroxy-2-methyl-1H indole-3-carboxylic acid benzyl ester: mp
188-189°C; IR (KBr) 3430, 3286, 1641, 1446, 1294, 1153, 1098 cm-1; 1H
NMR
(400 MHz, DMSO-d6) b 2.32 (s, 3H, ArCH3), 2.58 (s, 3H, ArCH3), 5.27 (s, 2H,
OCH2C6H5), 6.39 (d, J--1.22 Hz, 1H, ArH), 7.10 (d, J--1.71 Hz, I H, ArH),
7.26-7.31 (m, 1H, ArH), 7.34-7.38 (m, 2H, ArH), 7.42 (d, J--7.81 Hz, 1H, ArH),
8.71 (s, 1H, ArOH), 11.42 (s, 1H, NH); MS (APCI-~) rnlz 296.0 (MH+). Anal.
Calcd for C18H17N103: C, 73.20; H, 5.80; N, 4.74. Found: C, 73.02; H, 5.70; N,
4.40.
Step B: 4-Dimethylaminomethyl-7-methyl-5-hydroxy-2-methyl-1H-indole-
3-carboxylic acid benzyl ester
MezN O
O
HO
>--Me
N
H
Me
4-Dimethylaminomethyl-7-methyl-S-hydroxy-2-methyl-1 H-indole-
3-carboxylic acid benzyl ester was prepared according to procedure G from
7-methyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester
(6.62 mmol, 1.85 g), 41.3 mmol of dimethylamine, and 22.5 mmol of
formaldehyde. The reaction was heated at SO°C for 46 hours,
concentrated,
purified by silica gel flash column chromatography (0-5% triethylamine/ethyl
acetate) and recrystallized from ethyl acetate to give a light tan solid
(0.900 g,
41%): mp 161-164°C; IR (KBr) 3307, 1684, 1292, 1220, 1070 cm-1; IH NMR
(400 MHz, DMSO-d6) 8 2.08 (s, 6H, N(CH3)2), 2.30 (s, 3H, ArCH3), 2.47 (s,
3H, ArCH3), 3.89 (s, 2H, ArCH2N), 5.22 (s, 2H, OCH2C6H5), 6.37 (s, 1H, ArH),
7.28-7.38 (m, 3H, ArH), 7.43 (d, J--7.08 Hz, 2H, ArH), 11.28 (s, 1H, NH); MS
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-129-
(APCI+) m/z 353.2 (MH+). Anal. Calcd for C21H24N203~ C~ 71.57; H, 6.86; N,
7.95. Found: C, 71.20; H, 6.82; N, 7.79.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, phenylmethyl ester
Pyrrolo[3',2':5,6](1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I 5-decahydro-2,4-dimethyl-, phenylmethyl ester was
synthesized according to Procedure I (80°C, 39 hours) from
4-dimethylaminomethyl-7-methyl-S-hydroxy-2-methyl-IH-indole-3-carboxylic
acid benzyl ester (2.55 mmol, 0.900 g). The product was purified by silica gel
flash column chromatography (30% ethyl acetatelhexanes then 20-50% ethyl
acetate/dichloromcthane) to give 0.569 g (50%) off white solid: mp 183-
187°C;
IR (KBr) 3325, 2929, 1664, 1431, 1279, 1221, I 107, 1075 cm-/; 1H NMR
(400 MHz, DMSO-d6) 8 1.06-1.15 (m, 2H, aliphatic CH), 1.19-1.22 (m, 1H,
aliphatic CH), 1.31-1.62 (m, 8H, aliphatic CII), 1.81 (d, J--12.94 Hz, IH,
aliphatic
CH), 2.30 (s, 3H, ArCH3), 2.33-2.42 (m, 1H, aliphatic CH), 2.45 (s, 3H,
ArCH3),
2.55 (d, J--17.82 Hz, 1H, aliphatic CH), 2.61-2.66 (m, 1H, aliphatic CH),
2.82-2.88 (m, 1H, aliphatic CII), 3.09-3.15 (m, 1H, aliphatic CII), 5.20 (dd,
.l--24.90, 12.21 Hz, 2H, OCH2C6H5), 6.41 (s, IH, ArH), 7.28-7.43 (m, SH, ArH),
11.31 (s, 1 H, NH); MS (APCI+) na/z 445.3 (MH+). Anal. Calcd for
C28H32N203: C, 75.65; H, 7.26; N, 6.30. Found: C, 75.62; H, 7.34; N, 6.31.
Example 45
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, 1-(4-fluorophenyl)ethyl
ester
Me
N O \
O
O
i
~ Me
Me \ N
H
CA 02372197 2001-07-12
WO 00142045 PCT/US99130434
-130-
Step A: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, anhydride with
benzoic acid
H
$ To a solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-,
phenylmethyl ester (1.88 mmol, 0.837 g) and triethylamine (1.88 mmol,
0.262 mL) in 18 mL of tetrahydrofuran was added 20% Pd(OH)2lC (0.200 g,
24 wt%). The mixture was stirred under a hydrogen atmosphere (balloon) at room
temperature for 20 minutes and filtered through celite, rinsing with
tetrahydrofuran, to give the carboxylic acid: MS (APCI+) m/z 355.2 (MH+). To
the filtrate under N2 at room temperature was added benzoyl chloride (1.88
mmol,
0.218 mL) dropwise. After 60 hours at room temperature, the precipitate was
filtered off, the filtrate was concentrated, and the residue was triturated
with ether
to afford a light peach solid (0.517 g, 60%): IH NMR (400 MHz, DMSO-d6)
selected diagnostic peaks 8 2.23 (s, 3H, ArCH3), 2.48 (s, 3H, ArCH3), 7.00 (s,
1H, ArH), 7.57 (t, J 7.81 Hz, 2H, ArH), 7.73 (t, J--7.57 Hz, 1H, ArH), 8.06
(d,
J--7.32 Hz, 2I-I, ArH), 11.94 (s, 1H, NH); MS (APCI+) mlz 459.2 (MH+).
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, 1-(4-
fluorophenyl)ethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,5-dimethyl-, anhydride with benzoic
acid
(1.13 mmol, 0.517 g) was added to 4-fluoro-a-methylbenzyl alcohol (3.38 mmol,
0.427 mL) and heated with stirnng at 150°C for 2 minutes. A homogeneous
solution formed, was cooled to room temperature, dissolved with ethyl acetate
CA 02372197 2001-07-12
WO 00/42045 PCT/U599/30434
-131-
(10 mL), and stirred with a saturated aqueous solution of NaHC03. The layers
were separated, and the aqueous phase was extracted with 3 portions (10 mL) of
ethyl acetate. The combined extracts were concentrated, and the product was
purified by silica gel flash column chromatography (10-1S% acetone/hexanes) to
S give a shiny beige powder (0.256 g, 48%): mp 124-128°C; IR (KBr)
3378, 2933,
1675, 1425, 1228, 1127, 1059 cm-1; 1H NMR (400 MHz, DMSO-d6) 8
1.07-1.28 (m, 3H, aliphatic CH), 1.42-1.65 (m, 6H, aliphatic CH), 1.57 (d,
J--6.75 Hz, 3H, OCH(CH3)Ar), 1.71-1.74 (m, 1H, aliphatic CH), 1.79-1.87 (m,
1H, aliphatic CH), 2.21 & 2.22 (s, 3H, ArCH3, diastereomers), 2.38 (d,
J--11.09 Hz, 1H, aliphatic CH), 2.46-2.49 (m, 1H, aliphatic CH), 2.50 & 2.52
(s,
3H, ArCH3, diastereomers), 2.64-2.72 (m, 2H, aliphatic CH), 2.92-2.98 (m, 1H,
aliphatic CH), 3.10-3.29 (m, 1H, aliphatic CH), 5.94-6.00 (m, 1H,
OCII(CH3)Ar),
6.92 & 6.93 (s, 1H, ArH, diastereomers), 7.16-7.22 (m, 2H, ArH), 7.45-7.51 (m,
2H, ArH), 11.40 (s, 1H, NH); 19F NMR (DMSO-d6) -115.13 & -114.94 (s,
1S diastereomers); MS (APCI+) nTlz 477.3 (MH+). Anal. Calcd for C2~H33F1N2O3:
C, 73.09; H, 6.98; N, 5.88; F, 3.99. Found: C, 72.84; H, 7.02; N, 5.78; F,
3.89.
Example 46
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1S-decahydro-2,4-dimethyl-, 1-(4-fluorophenyl)ethyl
ester
F
O
Me
Me
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-132-
Step A: Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, anhydride with
benzoic acid
1
1e
Following the procedure in Example 45, Step A,
pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, phenylmethyl ester
(1.10 mmol, 0.491 g) was converted to the mixed anhydride intermediate (cream
colored solid, 0.512 g, 100%): MS (APCI+) m/z 459.3 (MH+).
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, 1-(4-
fluorophenyl)ethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9, I 0,12,13, I 4,14a, I 5-decahydro-2,4-dimethyl-, 1-(4-
fluorophenyl)ethyl
ester was synthesized following the procedure in Example 45, Step B from
pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,4-dimethyl-, anhydride with benzoic
acid
(1.12 mmol, 0.512 g). The product was purified by silica gel flash column
chromatography (10-20% acetone/hexanes) to give a fluffy off white powder
(0.227 g, 43%): mp 105-108°C; IR (KBr) 3325, 2931, 1674, 1512, 1433,
1222,
1109, 1059 cm-1; IH NMR (400 MHz, DMSO-d6) b 1.12-1.26 (m, 3H, aliphatic
CH), 1.39-1.72 (m, 7H, aliphatic CH), 1.57 (d, .l--6.51 Hz, 3H, OCH(CH3)Ar),
1.82-1.91 (m, IH, aliphatic CH), 2.31-2.36 (m, 1H, aliphatic CH), 2.33 & 2.34
(s,
3H, ArCH3, diastereomers), 2.43-2.47 (m, 1H, aliphatic CH), 2.54 & 2.56 (s,
3H,
ArCH3, diastereomers), 2.58-2.70 (m, 2H, aliphatic CH), 2.86-2.91 (m, 1H,
aliphatic CH), 3.06-3.25 (m, 1 H, aliphatic CH), 5.94-6.01 (m, 1 H,
OCH(CH3)Ar),
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-133-
6.43 & 6.45 (s, 1H, ArH, diastereomers), 7.16-7.22 (m, 2H, ArH), 7.45-7.51 (m,
2H, ArH), 11.31 & 11.32 (s, 1H, NH, diastereomers); 19F NMR (DMSO-d6)
-(I 15.13-115.08) & -(114.92-I 14.91) (m, diastereomers); MS (APCI+) m/z
477.3 (MH+). Anal. Calcd for C29H33F1N203~0~12 H20: C, 72.75; H, 7.00; N,
5.85; F, 3.97; H20, 0.45. Found: C, 72.38; H, 7.13; N, 5.73; F, 3.61; H20,
0.31.
Example 47
Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-
(methoxycarbonyl)phenyl]ethyl ester
)ZMe
I-i
Step A: 3-(1-Hydroxyethyl)benzoic acid methyl ester
HO ~ C02Me
A 500 mL stainless steel reactor was purged with N2 and charged
successively with I-(3-bromophenyl)ethanol (0.126 mol, 25.4 g), DMSO
(150 mL), methanol (3.70 mol, 150 mL), triethylamine (0.143 mol, 20.0 mL),
Pd(OAc)2 (2.78 mmol, 0.625 g), and 1,3-bis(diphenylphosphino)propane
(2.62 mmol, 1.08 g). The reactor was sealed, purged with N2 and then with CO,
pressurized to 663 psi with CO, rocked and heated to 80°C for 12 hours.
The
reactor was re-pressurized to 724 psi with CO, rocked and heated to I
00°C for
70 hours. The reaction was cooled to room temperature, filtered through
celite,
concentrated, and partitioned between H20 and CH2C12. The aqueous phase was
extracted with CH2C12 (3 x 100 mL), and the extracts were washed with water
(3 x 100 mL), dried over MgS04, and concentrated to an oil. The product was
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-134-
purified by silica gel flash column chromatography (10-25% ethyl
acetate/hexanes) to give 3-(1-hydroxyethyl)benzoic acid methyl ester (15.1 g,
66%): IH NMR (400 MHz, CDCl3) 8 1.50 (d, J--6.59 Hz, 3H, CH(CH3)OH),
1.86 (d, J--3.66 Hz, 1H, CH(CH3)OH), 3.90 (s, 3H, C02CH3), 4.92-4.97 (m, IH,
CH(CH3)OH), 7.39-7.43 (m, IH, ArF~, 7.57 (d, J 7.57 Hz, 1H, ArH), 7.92 (d,
J--7.57 Hz, 1 H, ArH), 8.02 (s, 1 H, ArH).
Step B: Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I 5-decahydro-2-methyl-,
1-[3-(methoxycarbonyl)phenyl]ethyl ester
A 100 mL flask was charged with 3-(I-hydroxyethyl)bcnzoic acid methyl
ester (18.0 mmol, 3.24 g) followed by pyrrolo[3',2':5,6][1 ]benzopyrano[3,2-
i]quinolizine-1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-
,
anhydride with benzoic acid (Example 86, 4.50 mmol, 2.00 g) and heated with
stirring at 150°C until a homogeneous solution was obtained (4.5 min).
The
solution was cooled to room temperature, dissolved in ethyl acetate and
stirred
with a saturated aqueous solution of NaHC03. A white solid precipitated out
and
was filtered away. The layers of the filtrate were separated, the aqueous
phase was
extracted with ethyl acetate (3 x 25 mL), and the combined extracts were dried
and concentrated to give a thick oil. The product was purified by silica gel
flash
column chromatography (20% acetone/hexanes) to give a white solid (0.776 g,
34%): mp 100-103, 155-156°C (diastereomers); IR (KBr) 3375, 2931, 2855,
1725,
1704, 1432, 1200, 1078, 1065 cm-1; 1H NMR (400 MHz, CDCI3) 8 1.20-1.29 (m,
1H, aliphatic CH), 1.32-1.34 (m, 2H, aliphatic CH), 1.38-1.47 (m, 1H,
aliphatic
CH), 1.53-1.58 (m, 2H, aliphatic CH), 1.62-1.82 (m, 4H, aliphatic CH), 1.68
(d,
J--6.59 Hz, 3H, OCI-I(CH3)Ar), 2.01-2.12 (m, 1H, aliphatic CH), 2.41-2.45 (m,
IH, aliphatic CH), 2.50-2.53 (m, 1H, aliphatic CH), 2.60 & 2.62 (s, 3H, ArCH3,
diastereomers), 2.69-2.85 (m, 2H, aliphatic CH), 2.98-3.04 (m, 1H, aliphatic
CH),
3.29-3.43 (m, 1H, aliphatic CH), 3.89 (s, 3H, C02CH3), 6.09-6.17 (m, 1H,
OCH(CH3)Ar), 6.73 & 6.74 (d, J--8.55 Hz, 1H, ArH, diastereomers), 7.01 &
7.02 (d, J--8.79 & 8.55 Hz, 1 H, ArH, diastereomers), 7.41 & 7.42 (t, J--7.81
&
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-135-
7.57 Hz, 1H, ArH, diastereomers), 7.61 & 7.63 (d, J--7.57 Hz, 1H, ArH,
diastereomers), 7.93-7.95 & 7.95-7.97 (m, IH, ArH, diastereomers), 8.06 (bs,
IH,
NH), 8.10 & 8.13 (s, 1H, ArH, diastereomers); MS (APCI+) nalz 503.1 (MH+).
Anal. Calcd for C3pH34N2O5: C, 71.69; H, 6.82; N, 5.57. Found: C, 71.58; H,
6.95; N, 5.42.
Example 48
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(3-carboxyphenyl)ethyl ester
zH
A solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-
(methoxycarbonyl)phenyl]ethyl ester (1.43 mmol, 0.717 g) in methanol (18 mL)
and 1N NaOH (5.71 mmol, 5.71 mL) was heated at 55-60°C for 1 hour. The
solution was cooled to room temperature, the methanol was removed by rotary
evaporation, and the aqueous phase was neutralized with 1N HCI. A precipitate
formed and was filtered off and purified by silica gel flash column
chromatography (10-15% MeOH/CHC13) to afford a cream colored powder
(0.522 g, 75%): mp 244-250°C (dce); IR (KBr) 3413-3229 (b), 2931, 1685,
1432,
1195, 1150, 1078, 1063 cm-1; lI-I NMR (400 MHz, DMSO-d6) ~ 1.03-1.22 (m,
3H, aliphatic CH), 1.38-1.72 (m, 7H, aliphatic CH), 1.57 (d, J--6.35 Hz, 3H,
OCH(CH3)Ar), 1.78-1.88 (m, 1H, aliphatic CH), 2.28-2.38 (m, 1H, aliphatic CH),
2.40-2.47 (m, 1H, aliphatic CH), 2.51 & 2.55 (s, 3H, ArCH3, diastereomers),
2.56-2.69 (m, 2H, aliphatic CH), 2.81-2.92 (m, 1H, aliphatic CH), 3.06-3.23
(m,
IH, aliphatic CH), 5.96-6.04 (m, 1H, OCH(CH3)Ar), 6.57 & 6.58 (d, J--8.55 &
8.30 Hz, 1H, ArH, diastereomers), 7.01 & 7.02 (d, J 8.30 Hz, 1H, ArH,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-136-
diastereomers), 7.45-7.50 (m, IH, ArH, diastereomers), 7.64 & 7.67 (d, J--
10.25 &
8.55 Hz, 1H, ArH, diastereomers), 7.83 & 7.85 (d, J--6.10 & 7.32 Hz, 1H, ArH,
diastereomers), 7.96 & 7.98 (s, 1H, ArH, diastereomers), 11.54 (s, 1H, NH),
13.00 (s, 1H, C02H); MS (APCI+) rnlz 489.1 (MH+). Anal. Calcd for
C29H32N205'0.50H20~0.20 Si02: C, 68.35; H, 6.53; N, 5.50; H20, 1.77. Found:
C, 67.96; H, 6.81; N, 5.22; H20, 1.81. HPLC (ALLTECH/ALLTIMA C-18,
60:40-20:80 H20/CH3CN + 0.05% TFA): retention time=4.843 & 4.970 min
(diastereomers), 95.53% purity.
Example 49
I-Propanaminium, N,N,N-trimethyl-, salt with I-(3-carboxyphenyl)ethyl
3,7,8,9,10,12,13,14,14a, I S-decahydro-2-methylpyrrolo[3',2':5,6)[ 1 ]-
benzopyrano[3,2-i]quinolizine-1-carboxylate (1:1)
NMe3
Ol-1
H
An aqueous solution of choline bicarbonate (0.935 mmol, 0.165 mL,
5.66 M) was added to a suspension ofpyrrolo[3',2':5,6][I]benzopyrano[3,2-
i]quinolizine-1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-
,
I-(3-carboxyphenyl)ethyl ester (0.935 mmol, 0.457 g) in ethanol (23 mL), and
the
mixture was heated at 75-80°C for 30 minutes, cooled to room
temperature, and
filtered. The filtrate was concentrated. The residue was stirred vigorously
with
ether and filtered to give a light yellow solid. The solid was dissolved in
hot
CHC13, filtered, concentrated, and dried at 60°C in vacuo to give a
yellow powder
(0.233 g, 42%): mp 210-216°C; IR (KBr) 3428-3211 (b), 2930, 1684, 1566,
1432,
1878, 1870, 1079, 1063 cm-/; IH NMR (400 MHz, DMSO-d6) 8 1.03-1.26 (m,
3H, aliphatic CH), 1.36-1.72 (m, 6H, aliphatic CH), I .55 (d, J--6.35 Hz, 3H,
OCH(CH3)Ar), 1.80-1.88 (m, 1H, aliphatic CH), 2.30-2.42 (m, 2H, aliphatic CH),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-137-
2.51 & 2.52 (s, 3H, ArCH3, diastereomers), 2.61-2.70 (m, 2H, aliphatic CH),
2.81-2.92 (m, 1H, aliphatic CH), 3.07 (s, 9H, N(CH3)3), 3.11-3.23 (m, 1H,
aliphatic CH), 3.26-3.35 (m, 2H, CH20H & aliphatic CH), 3.36 (t, J--4.88 Hz,
2H,
OCH2CH2N(CH3)3), 3.77-3.83 (m, 2H, OCH2CH2N(CH3)3), 5.91-5.99 (m, 1H,
OCH(CH3)Ar), 6.54-6.58 (m, 1H, ArH), 7.01-7.04 (m, IH, ArH), 7.21-7.28 (m,
1H, ArH), 7.32-7.38 (m, 1H, ArH), 7.71-7.79 (m, 1H, ArH), 7.91 & 7.93 (s, 1H,
ArH, diastereomers), 11.70 (s, IH, NH); MS (APCI-) m/z 487.1 (parent M-I).
Anal. Calcd for C29H31N205'0~88 C5H14N0~0.50 H20~0.50 CHCI3: C, 62.84;
H, 6.97; N, 6.23; H20, 1.39. Found: C, 62.92; H, 6.17; N, 6.93; H20, 1.72.
Example 50
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-nitrophenyl)methyl ester
NOZ
O
Me
H
Following the procedure from Example 47, Step B, 3-nitrobenzyl alcohol
(18.0 mmol, 2.81 g) and pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, anhydride
with benzoic acid (Example 86, 4.50 mmol, 2.00 g) were converted to
pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-nitrophenyl)methyl ester.
The
product was purified by silica gel flash column chromatography (25-50% ethyl
acetate/hexanes) and recrystallized from ethyl acetate to afford a light
yellow
powder (1.10 g, 51%): mp 203-205°C; IR (KBr) 3383, 3181, 2929, 2855,
1709,
1532, 1430, 1351, 1236, 1075, 886 cm-I; 1H NMR (400 MHz, DMSO-d6) 8
1.02-1.16 (m, 2H, aliphatic CH), 1.19-1.24 (m, IH, aliphatic CH), 1.32-1.62
(m,
7H, aliphatic CH), 1.82 (d, .I 13.18 Hz, 1H, aliphatic CH), 2.33 (d, J--9.76
Hz,
CA 02372197 2001-07-12
WO 00/42045 PCT/OS99/30434
-138-
1H, aliphatic CH), 2.41 (d, J--11.23 Hz, 1H, aliphatic CH), 2.48 (s, 3H,
ArCH3),
2.60-2.67 (m, 2H, aliphatic CH), 2.84-2.89 (m, 1H, aliphatic CH), 3.15-3.21
(m,
IH, aliphatic CH), 5.32-5.40 (m, 2H, OCH2Ar), 6.59 (d, J--8.79 Hz, 1H, ArH),
7.03 (d, J 8.55 Hz, 1H, ArH), 7.65-7.69 (m, 1H, ArH), 7.89 (d,.l--7.57 Hz, IH,
ArH), 8.18 (d, J--8.06 Hz, 1H, ArH), 8.29 (s, 1H, ArH), 11.58 (s, 1H, NH); MS
(APCI+) m/z 476.1 (MH+). Anal. Calcd for C27H29N305: C, 68.20; H, 6.15; N,
8.84. Found: C, 67.89; H, 6.20; N, 8.74.
Example 51
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, l 5-decahydro-2-methyl-, 1-(3-cyanophcnyl)ethyl ester
I-1
Step A: 3-(1-Hydroxyethyl)benzonitrile
HO I ~ CN
To a solution of 3-acetylbenzonitrile (68.9 mmol, 10.0 g) in MeOH
(230 mL) at 0°C under N2 was added NaBH4 (68.9 mmol, 2.61 g) in
portions.
After 2 hours of gradual warming to room temperature, 1 N HCI was added and
the solvent removed under reduced pressure. Dichloromethane (50 mL) was
added, the layers were separated, and the aqueous phase was extracted with
3 portions of CH2C12 (50 mL). The combined extracts were washed with
NaHC03 (1 x 50 mL), saturated NaCl (1 x 50 mL), dried over MgS04, and
concentrated under vacuum to give pure product as a yellow oil (10.0 g, 98%):
1H NMR (400 MHz, CDCl3) 8 1.47 (d, J--6.35 Hz, 3H, ArCH(CH3)OH),
1.99 (bs, 1H, OH), 4.92 (q, J--6.35 Hz, 1H, ArCH(CH3)OH), 7.43 (t, J 7.81 Hz,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-139-
1H, ArH), 7.53 (d, J--7.57 Hz, 1H, ArH), 7.59 (d, J--7.81 Hz, 1H, ArH), 7.66
(s,
1 H, ArI~.
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-,
1-(3-cyanophenyl)ethyl ester
Following the procedure from Example 47, Step B, 3-(1-hydroxyethyl)-
benzonitrile (13.5 mmol, 1.98 g) and pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)-
quinolizine-1-carboxylic acid, 3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-,
anhydride with benzoic acid (Example 86, 3.37 mmol, 1.50 g) were converted
pyrrolo[3',2':5,6][1]-benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(3-cyanophenyl)ethyl ester.
The product was purified by silica gel flash column chromatography (20-30%
acetone/hexanes) to afford a cream colored powder (0.614 g, 39%): mp
131-134 and 171-173°C (diastereomers); IR (KBr) 3377, 2931, 2856, 2230,
1702,
1432, 1148, 1077, 1066 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.02-1.25 (m,
3H, aliphatic CH), 1.31-1.72 (m, 7H, aliphatic CI~, 1.57 (d, J--6.35 Hz, 3H,
OCH(CH3)Ar), 1.79-1.88 (m, 1H, aliphatic CH), 2.32-2.44 (m, 2H, aliphatic CI~,
2.46 (s, 3H, ArCH3), 2.56-2.69 (m, 2H, aliphatic CH), 2.80-2.91 (m, 1H,
aliphatic
CH), 3.02-3.28 (m, 1H, aliphatic CH), 5.93-6.01 (m, 1H, OCH(CH3)Ar),
6.56-6.60 (m, 1 H, ArH), 7.00-7.04 (m, 1 H, ArH), 7.51-7.59 (m, 1 H, ArH),
7.71-7.80 (m, 2H, ArH), 7.86 & 7.90 (s, 1H, ArH, diastereomers), 11.56 (s, 1H,
NH); MS (APCI+) m/z 470.1 (MH+). Anal. Calcd for C2yH31N303~0.25 H2O:
C, 73.47; H, 6.70; N, 8.86; H20, 0.95. Found: C, 73.18; H, 6.73; N, 8.56, H20,
0.58.
Example 52
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-,
1-[3-[(dimethylamino)carbonyl]phenyl]ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-140-
O NMe2
O
Me
Step A: 3-(1-Hydroxyethyl)benzoic acid
OH O
~OH
A mixture of 3-(1-hydroxyethyl)benzonitrile (Example 51, Step A,
6.79 mmol, 1.00 g) in 10% aqueous NaOH (68 mL) was heated at reflux for
4.5 hours, cooled to room temperature, and extracted with ether (2 x 50 mL).
The
extracts were discarded. The aqueous phase was acidified with concentrated
HCI,
extracted with ether (3 x 50 mL), and the combined extracts were washed with
brine, dried over MgS04, and concentrated to give a white solid (0.98 g, 87%):
mp 107-110°C; 1H NMR (400 MHz, DMSO-d6) S 1.29 (d, J=6.35 Hz, 3H,
ArCH(CH3)OH), 4.71-4.78 (m, 1H, ArCH(CH3)OH), 5.25 (d, J--4.40 Hz, 1H,
OH), 7.39 (t, J=7.57 Hz, 1H, ArH), 7.53 (d, J=7.57 Hz, 1H, ArH), 7.76 (d,
J 7.57 Hz, 1H, Arty, 7.91 (s, 1H, Arln, 12.87 (s, lII, COOH); MS (APCI-) mlz
165.1 (M-1 ).
Step B: 3-(1-Hydroxyethyl)-N,N dimethylbenzamide
OH O
~NMe2
To a solution of 3-(1-hydroxyethyl)benzoic acid (54.8 mmol, 9.10 g),
dimethylamine (54.8 mmol, 27.4 mL of 2.0 M THF solution), and iPr2NEt
(109 mmol, 19.1 mL) in 55 mL of DMF at 0°C under N2 was added HBTU
(54.8 mmol, 20.8 g) in two portions. After 45 minutes, 1 N HC1 (50 mL) and
ether
(50 mL) were added, and the layers were separated. The aqueous phase was
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-141-
further acidified with 1N HCI and extracted with ether (4 x 50 mL). The
extracts
were combined and concentrated. The aqueous phase was concentrated also. The
concentrates were combined, dissolved in CH2C12, washed with 10% HCl (1 x
20 mL), saturated NaHC03 (1 x 20 mL), brine (1 x 20 mL), dried over
MgS04 and concentrated. The residue was purified by silica gel flash column
chromatography (50-100% ethyl acetate/hexanes) to give a mixture of
3-(1-hydroxyethyl)-N,N dimethylbenzamide, iPr2NEt, and N,N,N',N'-
tetramethylurea. The yield based on 1H NMR was 6.56 g (62%). A small portion
was rechromatographed to give pure product as a clear, colorless oil: 1H NMR
(400 MHz, DMSO-d6) 8 1.28 (d, J--6.59 Hz, 3H, ArCH(CH3)OH), 2.8G (s, 3H,
NCH3), 2.93 (s, 3H, NCH3), 4.67-4.73 (m, 1H, ArCH(CH3)OH), 5.20 (d,
J--4.15 Hz, 1H, OH), 7.18-7.20 (m, 1H, ArH), 7.31-7.37 (m, 3H, Arl~; MS
(APCI+) m/z 194.0 (MH+).
Step C: Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
1-[3-[(dimethylamino)carbonyl]phenyl]ethyl ester
Following the procedure from Example 47, Step B, 3-(1-hydroxyethyl)-
N,N dimethylbenzamide (14.9 mmol, 2.88 g) and pyrrolo[3',2':5,6][1]-
benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-
decahydro-2-methyl-, anhydride with benzoic acid (Example 86, 3.73 mmol,
1.66 g) were combined with xylenes (10 mL) and heated at 150°C for 5
minutes to
form pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-[(dimethylamino)-
carbonyl]phenyl]ethyl ester. The product was purified by silica gel flash
column
chromatography (0-5% Et3N/ethyl acetate), dissolved in ether/ethyl acetate and
washed with water (15 x 20 mL) to remove unreacted 3-(1-hydroxyethyl)-N,N
dimethylbenzamide. The organic phase was concentrated and the residue was
recrystallized from acetonitrile to give a white powder (1.65 g, 86%): mp
199-202°C; IR (KBr) 3416 (br), 3219 (br), 2930, 2855, 1684, 1622, 1432,
1188,
1146, 1091, 1062 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.02-1.28 (m, 3H,
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
- I 42-
aliphatic CH), 1.32-1.70 (m, 7H, aliphatic CH), 1.57 (d, J--6.84 Hz, 3H,
OCH(CH3)Ar), 1.76-1.88 (m, 1H, aliphatic CH), 2.32-2.43 (m, 2H, aliphatic CH),
2.50 & 2.51 (s, 3H, ArCH3, diastereomers), 2.52-2.689 (m, 2H, aliphatic CH),
2.84 (s, 3H, NCH3), 2.84-2.89 (m, 1H, aliphatic CH), 2.92 (s, 3H, NCH3),
3.06-3.30 (m, 1H, aliphatic CH), 5.94-6.01 (m, 1H, OCH(CH3)Ar), 6.57 &
6.58 (d, J--8.55 Hz, 1H, ArH, diastereomers), 7.01 & 7.02 (d, J--8.55 Hz, IH,
ArH, diastereomers), 7.29 (t, J--7.33 Hz, 1H, ArH), 7.37-7.50 (m, 3H, ArH),
11.53 (s, 1 H, NH); MS (APCI+) m/z S 16.3 (MH+). Anal. Calcd for
C31H37N304: C, 72.21; H, 7.23; N, 8.15. Found: C, 71.96; H, 7.25; N, 8.22.
Example 53
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13, I 4,14a,15-decahydro-2-methyl-,
I-[3-[(dimethylamino)methyl]phenyl]ethyl ester
NMe2
O
Me
H
Step A: 1-(3-Dimethylaminomethylphenyl)ethanol
OH
~NMe2
i
To a suspension of LiAlH4 (23.3 mmol, 0.931 g/95 %) in THF (40 mL) at
0°C under N2 was added a solution of 3-(I-hydroxyethyl)-N,N-
dimethylbenzamide (Example 52, Step B, 15.5 mmol, 3.00 g) in 10 mL of THF.
After the evolution of H2 had ceased, the reaction was warmed to room
temperature. After 4 hours at room temperature, the mixture was cooled to
0°C
and ethyl acetate (0.74 mL) and 10% aqueous NaOH were sequentially added.
The mixture was warmed to room temperature for 30 minutes, MgS04 and celite
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99/30434
-143-
were added with stirring, and the mixture was filtered through celite, washing
with
20 mL of THF. The filtrate was concentrated to afford 2.73 g (99%) of pure
1-(3-dimethylaminomethylphenyl)ethanol: 1H NMR (400 MHz, DMSO-d6) 8
1.26 (d, J--6.59 Hz, 3H, ArCI-I(CII3)OH), 2.09 (s, 6H, N(CH3)2), 3.31 (s, 2H,
ArCH2N), 4.64-4.67 (m, 1 H, ArCH(CH3)OH), 5.08 (d, J--4.1 S Hz, 1 H, OH),
7.07 (d, J--7.08 Hz, 1 H, ArH), 7.1 S-7.25 (m, 3H, ArH); MS (APCI+) m/z
180.0 (MH+)
Step B: Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,1 S-decahydro-2-methyl-,
1-[3-[(dimcthylamino)methyl]phenyl)ethyl ester
Following the procedure from Example 47, Step B 1-(3-
dimethylaminomethylphenyl)ethanol (15.2 mmol, 2.73 g) and
pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, anhydride with benzoic acid
(Example 86, 3.81 mmol, 1.69 g) were combined with xylenes (10 mL) and
heated at 150°C for 5 minutes to form
pyrrolo(3',2':5,6)[1]benzopyrano[3,2-
i)quinolizine-I -carboxylic acid, 3,7,8,9,10,12,13,14,14x,1 S-decahydro-2-
methyl-,
1-[3-[(dimethylamino)methyl]phenyl)ethyl ester. The usual extractive work-up
(ethyl acetate) was followed. The aqueous phase was diluted with 100 mL of
acetone and the inorganic salts filtered off. The filtrate was concentrated.
The
organic and aqueous residues were combined, mixed with 15 mL of CH2CI2 at
room temperature under N2 and treated r~~ith pyridine (68.5 mmol, 5.54 mL) and
acetic anhydride (34.3 mmol, 3.23 mL) sequentially. After 48 hours, the white
precipitate was filtered off, and the filtrate was washed with water (3 x 20
mL).
2S The aqueous washes were concentrated and the product was purified by silica
gel
flash column chromatography (0-5% Et3N/ethyl acetate) to give a yellow solid
(0.160 g, 8.4%): mp 95-100°C; IR (KBr) 2931, 2857, 1678, 1430, 1237,
1195,
1147, 1062 cm-1. 1H NMR (400 MHz, DMSO-d6) 8 1.09-1.27 (m, 3H, aliphatic
CH), 1.32-1.68 (m, 7H, aliphatic CH), 1.54 (d, J--6.35 Hz, 3H, OCH(CH3)Ar),
1.79-1.84 (m, 1H, aliphatic CH), 2.07 & 2.08 (s, 6H, N(CH3)2, diastcreomers),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-144-
2.29-2.43 (m, 2H, aliphatic CH), 2.49 & 2.50 (s, 3H, ArCH3, diastereomers),
2.59-2.69 (m, 2H, aliphatic CH), 2.81-2.92 (m, 1H, aliphatic CH), 3.02-3.17
(m,
1H, aliphatic CH), 3.33 & 3.34 (s, 2H, ArCH2N, diastereomers), 5.90-5.98 (m,
1H, OCH(CH3)Ar), 6.56-6.59 (m, 1H, ArH), 7.00-7.03 (m, 1H, ArH),
7.12-7.21 (m, 1H, ArH), 7.26-7.33 (m, 3H, ArH), 11.50 & 11.51 (s, 1H, NH,
diastcreomers); MS (APCI+) m/z 502.2 (MH+). Anal. Calcd for
C31H39N303'0.27 C4H802: C, 73.33; H, 7.90; N, 8.00. Found: C, 72.94; H,
7.90; N, 8.10.
General Procedure J.
Diethyl azodicarboxylate (Aldrich, 1 eq.) was added dropwise to a mixture of
5-hydroxy-2-methyl-1H-indole-3-carboxylic acid (1 eq.), triphenylphosphine
(1 eq.), and an alcohol of choice (1.5 eq.) in THF over an hour period. After
stirring at room temperature for 24 hours, the mixture was concentrated. The
product was purified by recrystallization or flash column chromatography on
silica gel (45% ethyl acetate:hexanes) to give the corresponding ester.
General Procedure K.
Under a nitrogen atmosphere, DBU (Aldrich, 1 eq.) was added to a solution of
5-acetoxy-2-methyl-1H-indole-3-carboxylic acid (1 eq.) in DMF by the syringe
technique. To this reaction mixture, a solution of an alkyl halide of choice
(Aldrich, 1.1 eq.) in DMF was added. After stirring at room temperature for
24 hours, the reaction mixture was diluted with CH2CI2 which induced
precipitation of the desired product. The solid product was filtered off and
washed
with H20.
General Procedure L.
Dimethylamine (2.2 eq.) and formaldehyde (1.2 eq.) were added to a solution of
the ester of choice (1.0 eq.) in 10 mL of denatured ethanol. The mixture was
stirred under a nitrogen atmosphere at reflux for 24 hours. After the reaction
was
complete according to MS and TLC, the mixture was concentrated. The product
CA 02372197 2001-07-12
WO 00!42045 PCT/US99/30434
-145-
was purified by flash column chromatography on silica gel (30%
MeOH:CH2Cl2).
General Procedure M.
1,2,3,4,6,7,8,9-Octahydroquinolizinylium perchlorate (1.7 eq.) was dissolved
in
2N NaOH (excess). The solution was extracted with diethyl ether. The combined
organic layers were concentrated to give a clear oil. This oil was dissolved
in
dioxane and added to a solution of the desired Mannich base (1 eq.) dioxane.
After
heating at 1 lOoC for 24 hours, the reaction was cooled to room temperature
and
dioxane was removed to afford a brown oil. The product was purified by flash
column chromatography on silica gel (40% hexanes:ethyl acetate).
General Procedure N.
A mixture of the mixed anhydride (4 eq.) and an alcohol of choice (4 eq.) was
heated at 1 SO°C for 5 minutes or until the reaction was homogeneous.
After being
cooled to room temperature, the oil was diluted with ethyl acetate and washed
with saturated NaHC03. The organic layer was separated and concentrated to
afford a brown oil. The product was purified by flash column chromatography on
silica gel.
General Procedure O.
To a solution of a ester (1 eq.) in methanol was added 2N NaOH (4 eq.). After
heating at 55°C for 1 hour, the reaction became clear. The reaction
mixture was
cooled to 0°C and neutralized with concentrated HCI. The product
precipitated out
of solution as a white solid and was filtered off. The mother liquor was
extracted
with ethyl acetate and the solvent removed to afford additional product.
General Procedure P.
To a suspension of Mannich base of choice (1 eq.) in anhydrous THF was added
slowly solid LiBH4 (5 eq.). MeOH (5 eq.) was immediately added dropwise via
syringe. The resulting mixture was heated at reflux for 1 hour. After cooling
to
0°C, 1N HCl was added to neutralize the reaction. The product
precipitated out of
solution as a white solid. The mother liquor was extracted with CH2C12. The
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-146-
combined organic layers were dried with MgS04, and solvent was removed to
yield more product.
Example 54
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,1,13,14,14a,15-decahydro-2-methyl-,2-phenylethyl ester
N O
O
O
w I y--
N
H
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid phenylethyl ester
O
O
HO
N
H
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid phenylethyl ester was
synthesized according to Procedure J from 2-phenyl-ethanol (6.39 g, 52.3 mmol)
and recrystallized from hexane/ethyl acetate to give 2.40 g (31.1 %) of fine
white
powder: mp 186-188°C; IR (KBr) 3231, 2978, 1644, 1463, 1173, 1101 cm-1;
1H NMR (400 MHz, DMSO-d6) 8 2.49 (s, 3H, CCH3), 3.03 (t, J--6.99 Hz, 2H,
OCH2CII2), 4.40 (t, J--6.75 Hz, 2H, OCH2CH2), 6.57 (dd, J--8.60, 2.29 Hz, 1H,
ArH), 7.09 (d, J--8.68 Hz, 1H, ArH), 7.20 (d, J--6.99 Hz, 1H, ArH), 7.26-7.33
(m,
5H, ArH), 8.81 (s, IH, OH), 11.49 (s, 1H, NH); MS(APCI+): nalz 326.1 (MH+)
Anal. Calcd for C18H17N103: C, 73.20; H, 5.80; N, 4.74. Found: C, 73.23; H,
5.74; N, 4.67.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-14?-
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid phenylethyl ester
l~
/N O
O
HO /
N
H
4-Dimethylaminomethyl-5-hydroxy-2-methyl-IH-indole-3-carboxylic acid
phenethyl ester was synthesized according to Procedure L from 5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid phenylethyl ester (2.89 g, 9.78 mmol) and
recrystallized from ethyl acetate to give 0.250 g ( 15.0%) of a white solid:
mp
200-201°C; iR (KBr) 3138, 2971, 1673, 1585, 1437, 1279, 1099 cm 1; 1H
NMR
(400 MHz, DMSO-d6) 8 2.34 (s, 3H, CCI13) 2.68 (s, 6H, N(CH3)2), 3.00 (t,
J--6.59 Hz, 2H, OCH2CH2), 4.43 (t, J--6.59 Hz, 2H, OCH2CH2), 4.67 (s, 2H,
NCH2Ar), 6.81 (d, J 8.79 Hz, 1H, ArH), 7.17-7.20 (m, 1H, ArH), 7.23-7.28 (m,
SH, ArH), 11.93 (s, 1H, NH); MS(APC1+): ntlz 353.2 (MH+). Anal. Calcd for
C21H24N203~ C> 61.35; H, 7.48; N, 6.81. Found: C, 60.96; H, 6.66; N. 6.42.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,1,13,14,14a,15-decahydro-2-methyl-,2-phenylethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,1,13,14,14a,15-decahydro-2-methyl-,2-phenylethyl ester was
synthesized according to Procedure M from 4-dimethylaminomethyl-5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid phenethyl ester (0.250 g, 0.709 mmol) and
recrystallized from ethyl acetatelhexanes to give 0.170 g (54.0%) of a white
solid:
mp 185-186°C; IR (KBr) 3297, 2931, 2856, 1673, 1432, 1199, 1080 cm-l;
1H
NMR (400 MHz, CDC13) 8 1.20-1.81 (m, 10H, aliphatic CH), 2.07 (d,
J--13.92 Hz, 1H, aliphatic CH), 2.41 (s, 3H, CCH3), 2.44-2.53 (m, 2H,
aliphatic
CH), 2.73-2.84 (m, 2H, aliphatic CH), 2.98-3.04 (m, 1H, aliphatic CH),
CA 02372197 2001-07-12
WO 00/42045 PCT1CTS99/30434
-148-
3.05-3.08 (m, 2H, OCH2CH2), 3.38 (dd, J--17.95, 6.71 Hz, 1H, aliphatic CH),
4.50-4.53 (m, 2H, OCH2CH2), 6.72 (d, J--8.79 Hz, 1 H, ArH), 6.99 (d, J--8.79
Hz,
1H, ArF~, 7.17-7.29 (m, 5H, ArH), 7.99 (s, IH, NH); MS(APCI+): m/z
445.3 (MH+). Anal. Calcd for C28H32N203: C, 75.56; H, 7.26; N, 6.30. Found:
C, 75.36; H, 7.28; N, 6.13.
Example 55
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-l-carboxylic acid,
3,7,8,9, I 0,12,13,14,14a,15-decahydro-2-methyl-,
[4-(methoxycarbonyl)phenyl]methyl ester
O
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-methoxycarbonyl-
bcnzyl ester
O
O \ ~ O
O
HO
N
H
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-methoxycarbonyl-
1 S benzyl ester was synthesized according to Procedure K from 4-bromomethyl-
benzoic acid methyl ester (6.64 g, 29.0 mmol) and precipitated out of diethyl
ether
to give 4.88 g (55.0%) of light lavender powder: mp 232-234°C; IR (KBr)
3322,
2950, 1696, 1654, 1465, 1288, 1093 em-1; 1H NMR (400 MHz, DMSO-d6) 8
2.57 (s, 3H, CCH3), 3.80 (s, 3I-I, OCH3), 5.34 (s, 2H, OCH2Ar), 6.55 (dd, J--
8.67,
2.32 Hz, 1H, ArH), 7.09 (d, J--8.55 Hz, 1H, ArH), 7.24 (d, J--2.20 Hz, 1 H,
ArH),
7.54 (d, J--8.06 Hz, 2H, ArH), 7.94 (d, J--8.30 Hz, 2H, ArH), 8.83 (s, 1 H,
OH),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-149-
11.59 (s, IH, NH); MS(APCI+): m/z 340.1 (MH+). Anal. Calcd for
C19H17N105: C, 67.25; H, 5.05; N, 4.13. Found: C, 66.88; H, 5.06; N, 4.05.
Step B: 4-Dimethylaminomcthyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid 4-methoxycarbonyl-benzyl ester
O
N O ~ I \O
O
HO
N
H
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
4-methoxycarbonyl-benzyl ester was synthesized according to Procedure L from
5-hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-methoxycarbonyl-benzyl ester
(17.2 g, 50.7 mmol) and triturated with ethyl acetate to give 8.50 g (43.0%)
of
white solids: mp 125-126°C; IR (KBr) 3142, 2959, 1672, 1585, 1434,
1285,
1095 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 2.12 (s, 6H, N(CH3)2), 2.49 (s,
3H, CCH3), 3.84 (s, 3H, OCH3), 4.02 (s, 2H, NCH2Ar), 5.34 (s, 2H, OCH2Ar),
6.58 (d, J--8.44 Hz, 1H, ArH), 7.09 (d, J--8.44 Hz, 1H, ArH), 7.60 (d, J--8.20
Hz,
2H, ArH), 7.98 (d, J 8.20 Hz, 2H, ArH), 11.56 (s, 1H, NH); MS(APCI+): na/z
397.2 (MH+). Anal. Calcd for C22H24N205~ C> 66.41; H, 6.12; N, 7.04. Found:
C,66.41;H,6.OI;N,6.97.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-dccahydro-2-methyl-,
[4-(methoxycarbonyl)phenyl]methyl ester
Pyrrolo(3',2':5,6][I)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I S-decahydro-2-methyl-,[4-(methoxycarbonyl)
phenyl]methyl ester was synthesized according to Procedure M from
4-dimethylaminomcthyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
4-methoxycarbonyl-benzyl ester (0.200 g, 0.505 mmol) and recrystallizcd from
t-butyl methyl ether to give 0.090 g (23.0%) of granular off white solids: mp
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-150-
179-180°C; IR (KBr) 3173, 2930, 2856, 1725, 1615, 1433, 1275, 1079 cm-
1;
1 H NMR (400 MHz, CDCl3) 8 1.21-1.81 (m, 10H, aliphatic CH), 2.06 (d,
J--8.79 Hz, 1H, aliphatic CH), 2.43 (d, J--12.70 Hz, IH, aliphatic CH), 2.50-
2.53
(m, 1H, aliphatic CH), 2.55 (s, 3H, CCH3), 2.73-2.84 (m, 2H, aliphatic CH),
2.96-3.05 (m, 1H, aliphatic CH), 3.38 (dd, J--17.70, 7.20 Hz, 1H, aliphatic
CH),
3.89 (s, 3H, OCH3), 5.34 (dd, J--16.36, 12.70 Hz, 2H, OCH2Ar), 6.73 (d,
J 8.79 Hz, I H, ArH), 7.01 (d, J-- 8.79 Hz, 1 H, ArH), 7.47 (d, J--8.06 Hz,
2H,
ArH), 8.01 (d, J--8.30 Hz, 2H, ArH), 8.06 (s, 1H, NH); MS(APCI+); rnlz
489.3 (MH+). Anal. Calcd for C2gH32N205: C, 71.29; H, 6.60; N, 5.73. Found:
C, 70.94; H, 6.26; N, 5.57.
Example 56
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2,-methyl-, (4-carboxyphenyl)methyl ester
O
OH
N O
O
O
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (4-carboxyphenyl)methyl ester
was synthesized according to Procedure O and triturated with methanol to give
0.030 g (11.0%) of fine off white powder : mp 243-244°C; IR (KBr) 2932,
2863,
1698, 1592, 1432, 1077 cm-1; IH NMR (400 MHz, DMSO-d6) 8 1.05-1.26 (m,
3H, aliphatic CH) 1.39-1.66 (m, 6H, aliphatic CH), 1.85 (d, J--13.67 Hz, 1H,
aliphatic CH), 2.44 (d, J--11.23 Hz, 1H, aliphatic CH), 2.47-2.48 (m, lI-I,
aliphatic
CH), 2.48 (s, 3H, CCH3), 2.63-2.69 (m, 2H, aliphatic CH), 2.85-2.91 (m, 1H,
aliphatic CH), 3.19 (dd, J--18.07, 6.84 Hz, 1H, aliphatic CH), 3.41-3.43 (m,
1H,
aliphatic CH), 5.31 (dd, J--20.02, 12.94 Hz, 2H, OCH2Ar), 6.60 (d, .l--8.55
Hz,
1H, ArH), 7.04 (d, J--8.55 Hz, IH, ArH), 7.54 (d, J--8.30 Hz, 2H, ArH), 7.94
(d,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-151-
J-- 8.30 Hz, 2H, ArH), 11.57 (s, 1H, NIA, 12.98 (s, 1H, C02H); MS(APCI+): mlz
475.3 (MH+). Anal. Calcd for C28H3pN205: C, 70.17; H, 6.42; N, 5.85. Found:
C, 69.78; H, 6.50; N, 5.62.
Example 57
1-[3-(4-Carboxy-benzyloxycarbonyl)-5-hydroxy-2-methyl-l II-indol-4-ylmethyl]-
1,2,3,4,6,7,8,9-octahydro-quinolizinylium; chloride
O
OH
To a solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-
carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
[4-(methoxycarbonyl) phenyl]methyl ester (Example 56, Step C, 1.00 g,
2.05 mmol) in 25.6 mL of methanol was added 2N NaOH (4.09 mL, 8.19 mmol).
After heating at 55°C for 1 hour, the mixture turned to a clear
solution. The
reaction mixture was cooled to 0°C and concentrated HCl was added
slowly until
pH 3. The aqueous layer Was extracted with ethyl acetate (5 x 50 mL), and the
combined organic layers were dried with MgS04, and solvent was removed. The
residue was triturated with dichloromethane to give 0.967 g (92.0%) of fine
white
powder: mp 230-231°C; IR (KBr) 3361, 2941, 2360, 1709, 1589, 1428,
1227,
1083 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.20-1.30 (m, lI-I, aliphatic CH)
1.43-1.51 (m, 4H, aliphatic CH), 1.69-2.30 (m, 5H, aliphatic CH), 2.48 & 2.49
(s,
3H, CCH3, opened and closed forms) , 2.48-2.55 (m, 2H, aliphatic CI-I), 2.85-
3.30
(m, 2H, aliphatic CH), 3.40-3.80 (m, 3H, aliphatic CH), 5.33-5.36 (m, 2H,
OCH2Ar), 6.79 & 6.82 (d, J--8.55 & 8.06 Hz, 1H, ArH, opened & closed forms),
7.11 & 7.20 (d, J--8.55 & 8.55 Hz, IH, ArH, opened & closed forms), 7.54-7.56
(m, 2H, ArH), 7.94-7.96 (m, 2H, ArH), 9.19 (s, 1H, OH, opened), 11.79 &
11.85 (s, 1H, NH, opened & closed forms), 13.00 (s, 1H, C02H); MS(APCI+):
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-152-
m/z 475.3 (MH+). Anal. Calcd for C28H31N205C11: C, 65.35; H, 6.12; N, 5.44.
Found: C, 65.06; H, 6.17; N, 5.15.
Example 58
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
[4-(hydroxymethyl)phenyl]methyl ester
N O \ . ~ ~O H
O
O
N
H
Step A: 4-Dimcthylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid 4-hydroxymethyl-bcnzyl ester
I OH
/N O
O
HO /
.
N
H
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
4-hydroxymethyl-benzyl ester was synthesized according to Procedure P from
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
4-methoxycarbonyl-benzyl ester (Example 56, Step B, 2.32 g, 5.84 mmol) and
triturated with acetone to give 0.993 g (47.0%) of a white solid: mp 140-
143°C;
IR (KBr) 3140, 2777, 1686, 1583, 1435, 1092 cm-1; IH NMR (400 MHz, DMSO-
d6) 8 2.51 (s, 3H, CH3), 2.71 (s, 6H, N(CH3)2), 4.47 (d, J--5.13 Hz, 2H,
ArCH20H), 4.73 (s, 2H, NCH2Ar), 5.18 (t, J 5.62 Hz, 1H, ArCH20H), 5.25 (s,
2H, OCH2Ar), 6.84 (d, J--8.55 Hz, 1 H, Arll), 7.28 (d, J--8.55 Hz, 1 H, ArH),
7.31 (d, J--7.81 Hz, 2H, ArH ), 7.40 (d, J--7.81 Hz, 2H, ArH), 8.60 (s, 1 H,
OH),
11.97 (s, IH, NH); MS(APCI+): m/z 369.2 (MH+). HPLC (ALLTCH/ALLTIMA
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-153-
C-18 1:1-2:98 H20/CH3CN + 0.05% TFA): rentention time=4.57 min, 95.23%
purity.
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-,
[4-(hydroxymethyl)phenyl]methyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [4-(hydroxymethyl)-
phenyl]methyl ester was synthesized according to Procedure M from
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
4-hydroxymethyl-benzyl ester (0.969 g, 2.63 mmol) and triturated with acetone
to
give 0.890 g (49.0%) of a white solid: mp 198-200°C; IR (KBr) 3405,
3237, 2931,
1660, 1424, 1237, 1081 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.1 l-1.27 (m,
3H, aliphatic CH),1.39-1.70 (m, 7I-I, aliphatic CH), 1.85 (d, J--13.50 Hz, 1H,
aliphatic CH), 2.36 (d, J--10.37 Hz, 1H, aliphatic CH), 2.41-2.43 (m, 1H,
aliphatic
CH), 2.47 (s, 3H, CCH3), 2.65-2.69 (m, 2H, aliphatic CH), 2.86-2.92 (m, 1H,
aliphatic CH), 3.23 (dd, J--16.64, 9.64 Hz, 1H, aliphatic CH), 4.48 (d, J--
5.78 Hz,
2H, ArCl120H), 5.17 (t, J--5.55 Hz, 1 H, ArCH20H), 5.21 (dd, J--22.42, 12.06,
Hz, 2H, OCH2Ar), 6.59 (d, J 8.44 Hz, 1H, ArH), 7.03 (d, J--8.68 Hz, 1H, ArH),
7.31 (d, J-- 7.72 Hz, 2H, ArFl), 7.39 (d, J=7.96 Hz, 2H, ArH), 11.52 (s, 1 H,
NFL;
MS(APCI+): mlz 461.2 (MH+). Anal. Calcd for C28H32N204: C, 73.02; H, 7.00;
N, 6.08. Found: C, 72.62; H, 6.84; N, 5.83.
Example 59
Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-2-carboxylic acid,
3,78,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-naphthalenylmcthyl ester
2S
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-154-
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid naphthalen-
2-ylmethyl ester
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid naphthalen-2-ylmethyl
S ester was synthesized according to Procedure K from 2-bromomethyl-
naphthalene
(7.63 g, 34.5 mmol) and precipitated out of CH2C12 to give 5.01 g (48.1 %) of
pale
lavender powder: mp 243-245°C; IR (KBr) 3408, 3306, 3058, 1665, 1596,
1465,
1172, 1093 cm-/; IH NMR (400 MHz, DMSO-d6) 8 2.59 (s, 3H, CCH3), 5.46 (s,
2H, OCH2Ar), 6.59 (dd, J--8.44, 2.41 Hz, IH, ArH), 7.12 (d, J 8.44 Hz, 1H,
ArH), 7.30 (d, J--2.41 Hz, IH, ArH), 7.51 (dd, J--6.03, 3.38 Hz, IH, ArH),
7.59 (d, J 1.69 Hz, IH, ArH), 7.61 (d, J--1.69 Hz, 1H, ArH), 7.90-7.96 (m, 4H,
ArH), 8.83 (s, IH, OH), 11.58 (s, 1H, NH); MS(APCl+): rr~lz 332.2 (MH+). HPLC
(ALLTCH/ALLTIMA C-18 35:65-2:98 H20/CH3CN + 0.05% TFA): rentention
time=3.70 min, 95.81 % purity.
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid naphthalen-2-ylmethyl ester
/N O
O
HO
N
H
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
naphthalen-2-ylmethyl ester synthesized according to the Procedure L from
5-hydroxy-2-methyl-1H-indole-3-carboxylic acid naphthalen-2-ylmethyl ester
(Example 57, Step A, 3.38 g, 10.2 mmol) and recrystallized from CH2Cl2 to give
CA 02372197 2001-07-12
W0 00/42045 PCT/US99/30434
-155-
0.750 g (19.0%) of a white solid: mp 170-171°C; IR (KBr) 3120, 2964,
2853,
1675, 1592, 1431, 1257, 1088 cm-1; 1H NMR (400 MHz, DMSO-d6) S 2.11 (s,
6H, N(CH3)2), 2.49 (s, 3H, CCH3), 4.03 (s, 2H, NCH2Ar), 5.43 (s, 2H,
OCH2Ar), 6.57 (d, J--8.68 Hz, 1H, ArH), 7.08 (d, J--8.44 Hz, 1H, ArH),
7.51-7:53 (m, 2H, ArH), 7.60 (d, J--8.44 Hz, 1H, ArH), 7.91-7.99 (m, 4H, ArH),
I 1.55 (s, 1H, NH); MS(APCI+): m/z 389.2 (MH+). HPLC (ALLTCH/ALLTIMA
C-18 55:45-15:85 H20/CH3CN + 0.05% TFA): rentention time=4.80 min,
94.36% purity.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-2-carboxylic acid,
3,78,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-naphthalenylmethyl
ester
Pyrrolo[3',2':5,6][I]bcnzopyrano[3,2-i]quinolizine-2-carboxylic acid,
3,78,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-naphthalenylmethyl ester was
synthesized according to Procedure M from 4-dimcthylaminomethyl-5-hydroxy-
2-methyl-1H-indole-3-carboxylic acid naphthalen-2-ylmethyl ester (0.620 g,
1.62 mmol) and recrystallized from ethyl acetate/hexanes to give 0.690 g
(59.0%)
of granular a white solid: mp 141-143°C; IR (KBr) 3177, 3056, 2929,
1702, 1430,
1146, 1079 cm-1; 1H NMR (400 MI-Iz, CDC13) 8 1.19-1.45 (m, 4H, aliphatic CH)
1.55-1.78 (m, 6H, aliphatic CH), 2.07 (d, J--13.99 Hz, IH, aliphatic CH),
2.43-2.46 (m, 1H, aliphatic CH), 2.52-2.55 (m, 1H, aliphatic CH), 2.58 (s, 3H,
CCH3), 2.77-2.86 (m, 2H, aliphatic CH), 3.01-3.07 (m, 1H, aliphatic CH),
3.41 (dd, J--18.08, 6.75 Hz, IH, aliphatic CH), 5.48 (dd, J=19.53, 10.61 Hz,
2H,
OCH2Ar), 6.75 (d, J--8.68 Hz, 1 H, ArH), 7.03 (d, J--8.68 Hz, 1 H, ArH),
7.48-7.50 (m, 2H, ArH), 7.55-7.57 (m, 1H, ArH), 7.82-7.86 (m, 3H, ArFl), 8.11
(s,
1 H, NH); MS(APCI+): m/z 481.3 (MH+). Anal. Calcd for C3 I H32N203 ~ C
77.47; H, 6.71; N, 5.83. Found: C, 77.13; H, 6.84; N, 5.51.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-156-
Example 60
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9, I 0,12, I 3,14,14a,15-decahydro-2-methyl-,
[3-(methoxycarbonyl)phenyl]methyl ester
O
H
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 3-methoxycarbonyl-
benzyl ester
O
O
O
O
HO
N
H
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 3-methoxycarbonyl-
benzyl ester was synthesized according to Procedure K from 3-bromomethyl-
benzoic acid methyl ester (5.49 g, 24.0 mmol) and triturated with ethyl
acetate to
give 2.01 g (27.0%) of light gray powder: mp 160-162°C; IR (KBr) 3383,
3310,
1721, 1666, 1465, 1289, 1095 cm-1; 1H NMR (400 MHz, DMSO-d6) b 2.56 (s,
3H, CCH3), 3.81 (s, 3H, OCH3), 5.35 (s, 2H, OCH2Ar), 6.57 (dd, J--8.55,
2.20 Hz, IH, ArH), 7.10 (d, J--8.55 Hz, 1H, ArH), 7.25 (d, J 2.20 Hz, 1 H,
ArH),
7.53 (t, J--7.81 Hz, 1 H, ArH), 7.70 (d, J--7.81 Hz , I H, ArH), 7.89 (d, J
7.5 7 Hz,
1H, ArH), 8.01 (s, 1H, ArH), 8.82 (s, 1H, OH), 11.58 (s, 1H, NH); MS(APCI+):
rnlz 340.1 (MH+). Anal. Calcd for C19H17N105: C, 67.25; H, 5.05; N, 4.13.
Found: C, 67.22; H, 4.75; N, 4.05.
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-1S7-
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1 H-indole-3-carboxylic
acid 3-methoxycarbonyl-benzyl ester
o /
0
0
0
HO /
N
H
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
3-methoxycarbonyl-benzyl ester was synthesized according to Procedure L from
S-hydroxy-2-methyl-1H-indole-3-carboxylic acid 3-methoxycarbonyl-benzyl ester
(7.26 g, 21.4 mmol) and triturated with ethyl acetate to give 4.80 g (56.6%)
of fine
white powder: mp 164-167°C; IR (KBr) 3031, 2951, 1725, 1683, 1432,
1285,
1077 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 2.10 (s, 6H, N(CH3)2), 2.49 (s,
3H, CCH3), 3.81 (s, 3H, OCH3), 3.96 (s, 2H, NCH2Ar), 5.31 (s, 2H, OCH2Ar),
6.55 (d, J--8.55 Hz, 1H, ArH ), 7.06 (d, J--8.55 Hz, 1H, ArH), 7.52 (t, J--
7.81 Hz,
1H, ArH), 7.72 (d, J--7.57 Hz, 1H, ArH), 7.90 (d, J--7.81 Hz, 1H, Arf~, 8.03
(s,
1H, ArH), 11.54 (s, 1H, NH); MS(APCI+): ntlz 397.0 (MH+). HPLC
(ALLTCH/ALLTIMA C-18 65:3$-15:85 H20/CH3CN + 0.05% TFA): rentention
time=4.91 min, 92.97% purity.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12, I 3,14,14a,15-decahydro-2-methyl-,
[3-(methoxycarbonyl)phenyl]methyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1S-decahydro-2-methyl-, [3-(methoxycarbonyl)-
phenyl]methyl ester was synthesized according to Procedure M from
4-dimethylaminomethyl-S-hydroxy-2-methyl-1H-indole-3-carboxylic acid
3-methoxycarbonyl-benzyl ester (2.09 g, 5.27 mmol) and recrystallized from
t-butyl methyl ether to give 1.21 g (47.1 %) of fine white powder: mp 169-
170°C;
IR (ICBr) 3380, 2931, 2855, 1721, 1433, 1289, 1076 cm-1; 1H NMR (400 MHz,
DMSO-d6) 8 1.03-1.21 (m, 3H, aliphatic CH) 1.36-1.63 (m, 7H, aliphatic CH),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-158-
1.81 (d, J--13.43 Hz, 1H, aliphatic CH), 2.33 (d, J--10.50 Hz, 1H, aliphatic
CH),
2.40-2.42 (m, 1H, aliphatic CH), 2.46 (s, 3H, CCH3), 2.56-2.67 (m, 2H,
aliphatic
CH), 2.82-2.88 (m, 1H, aliphatic CH), 3.14 (dd, J--18.07, 6.84 Hz, IH,
aliphatic
CH), 3.81 (s, 3H, OCH3), 5.29 (dd, J--25.15, 12.45 Hz, 2H, OCH2Ar), 6.57 (d,
S J--8.79 Hz, 1 H, ArH), 7.02 (d, J-- 8.79 Hz, 1 H, ArH), 7.52 (t, J--7.57 Hz,
1 H,
ArH), 7.71 (d, J--7.57 Hz, 1H, ArH), 7.90 (d, J--7.81 Hz, 1H, ArH), 8.02 (s,
1H,
ArH), 11.55 (s, 1H, NH); MS(APCI+): m/z 489.2 (MH+). Anal. Calcd for
C29H32N205~ C~ 71.29; H, 6.60; N, 5.73. Found: C, 71.22; H, 6.91; N, 5.43.
Example 61
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7, 8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-,
[3-(hydroxymethyl)phenyl]methyl ester
OH
Step A: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid 3-hydroxymethyl-benzyl ester
OH
o ~I
O
HO
N
H
4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
3-hydroxymethyl-benzyl ester was synthesized according to procedure P from
4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid
3-methoxycarbonyl-benzyl ester (Example 60, Step B, 2.21 g, 5.58 mmol) and
triturated with CH2Cl2 to give 1.11 g (54.0%) of white powder: mp 199-201
°C;
IR (KBr) 3065, 2884, 1681, 1586, 1432, 1090 cm-1 ~ I H NMR (400 MHz, DMSO-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-159-
d6) b 2.25 (s, 3H, CCH3) 2.72 (s, 6H, N(CH3)2), 4.48 (d, J 5.37 Hz, 2H,
ArCH20H), 4.75 (s, 2H, NCH2Ar), 5.22 (t, J--5.62 Hz, IH, ArCH20H), 5.28 (s,
2H, OCH2Ar), 6.87 (d, J--8.55 Hz, 1H, ArH), 7.25-7.34 (m, 4H, ArH), 7.40 (s,
1H, ArH ), 8.59 (s, 1H, ArOH), 12.06 (s, 1H, NH); MS(APCI+): m/z
369.2 (MH+). HPLC (ALLTCH/ALLTIMA C-18 98:2-75:25 H20/CH3CN +
0.05% TFA): retention time=2.11 min, 98.74% purity.
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10, I 2,13,14,14a,15-decahydro-2-methyl-,
[3-(hydroxymethyl)phenyl]methyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [3-(hydroxymethyl)-
phenyl]methyl ester was synthesized according to Procedure M from
4-dimethylaminomethyl-5-hydroxy-2-methyl-IH-indole-3-carboxylic acid
3-hydroxymethyl-benzyl ester (0.980 g, 2.66 mmol) and triturated with t-butyl
I S methyl ether to give 0.420 g (44.2%) of a light yellow green crystal: mp
183-185°C; IR (KBr) 3381, 3197, 2931, 1682, 1430, 1249, 1076 cm-1; 1H
NMR
(400 MHz, DMSO-d6) 8 1.06-1.37 (m, 3H, aliphatic CH) 1.40-1.65 (m, 6H,
aliphatic CH), 1.82 (d, J--13.43 Hz, 1H, aliphatic CH), 2.33 (d, J--10.01 Hz,
1H,
aliphatic CH), 2.40-2.43 (m, 1H, aliphatic CH), 2.46 (s, 3H, CCI13), 2.61-2.68
(m,
2H, aliphatic CH), 2.83-2.89 (m, 1H, aliphatic CH), 3.12-3.16 (m, 1H,
aliphatic
CH), 3.18 (dd, J--18.31, 6.84 Hz, 1H, aliphatic CH), 4.46 (d, J--5.62 Hz, 2H,
ArCH20H), 5.18 (t, J--6.10 Hz, 1H, ArCH20H), 5.21 (dd, J--20.27, 12.21 Hz,
2H, OCH2Ar), 6.58 (d, J--8.79 Hz, 1H, ArH), 7.01 (d, J--8.55 Hz, IH, ArH),
7.23-7.32 (m, 3H, ArH), 7.36 (s, 1H, ArH), 11.51 (s, 1H, NH); MS(APCI+): ntlz
461.1 (MH+). Anal. Calcd for C28H32N204: C, 73.02; H, 7.00; N, 6.08. Found:
C, 72.84; H, 7.07; N, 5.91.
CA 02372197 2001-07-12
WO 00/42045 PCTNS99/30434
-160-
Example 62
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-carboxyphenyl)methyl ester)
0
OH
N O
O
O /
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-carboxyphenyl)methyl
ester was synthesized according to Procedure O from pyrrolo[3',2':5,6][I]-
benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-
decahydro-2-methyl-, [3-(methoxycarbonyl)phenyl]methyl ester (Example 60,
Step C, 0.923 g, 1.89 mmol) and triturated with ethyl acetate to give 0.456 g
(50.7%) of white powder: mp 205-208°C; IR (KBr) 2932, 2859, 1693, 1563,
1432, 1076 cm-1; 1H NMR (400 MHz, DMSO-d6) ~ 1.08-1.36 (m, 3H, aliphatic
CH) 1.39-1.63 (m, 7H, aliphatic CH), 1.81 (d, J--13.18 Hz, 1H, aliphatic CH),
2.33 (d, J--11.23 Hz, 1H, aliphatic CH), 2.40-2.43 (m, IH, aliphatic CH), 2.47
(s,
IS 3H, CCH3), 2.56-2.67 (m, 2H, aliphatic CH), 2.84-2.88 (m, IH, aliphatic
CH),
3.15 (dd, J=17.82, 6.35 Hz, 1 H, aliphatic CH), 5.28 (dd, J--24.17, 12.45 Hz,
2H,
OCH2Ar), 6.57 (d, J--8.79 Hz, 1H, ArH), 7.02 (d, J 8.55 Hz, 1H, ArH), 7.48 (t,
J--7.57 Hz, 1H, ArH), 7.65 (d, J 7.57 Hz, 1H, ArH), 7.87 (d, J--7.81 Hz, IH,
Arl~, 8.00 (s, 1H, ArH), 11.55 (s, 1H, NH); MS(APCI+): m/z 475.1 (MH+). Anal.
Calcd for C28H3pN2O5: C, 69.18; H, 6.50; N, 5.75. Found: C, 68.83; H, 6.40; N,
5.63.
Example 63
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (3-carboxypheny)methyl
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methylpyrrolo[3',2' :5,6] [ 1 ]-
benzopyrano[3,2-i]quinolizine-I-carboxylate (1:1)
CA 02372197 2001-07-12
WO 00/42045 PCT/1JS99130434
-161-
~+
o ~ ~~oH
N o
0
o ,
N
H
To a suspension ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-
carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
(3-carboxyphenyl)methyl ester (Example 62, 0.100 g, 0.211 mmol) in 5 mL of
EtOH was added choline carbonate (0.037 mL, 0.211 mmol, 5.66 M). The
reaction mixture was heated at reflux for 1 hour resulting in a clear
solution. After
cooling to room temperature, the mixture was concentrated to give a yellow
oil.
Triturating this oil with diethyl ether yielded 0.097 g (88.0%) of beige
solid: mp
165-170°C; IR (KBr) 2930, 2855, 1685, 1566, 1436, 1077 cm-1; 1H NMR
(400 MHz, DMSO-d6) c5 1.09-1.28 (m, 3H, aliphatic CH) 1.39-1.70 (m, 7H,
aliphatic CH), 1.86 (d, J--13.50 Hz, IH, aliphatic CH), 2.35 (d, J--10.37 Hz,
1H,
aliphatic CH), 2.42-2.45 (m, 1H, aliphatic CH), 2.47 (s, 3H, CCH3), 2.65-2.72
(m,
2H, aliphatic CH), 2.87-2.91 (m, IH, aliphatic CH), 3.09 (s, 9H, N(CH3)3),
3.23 (dd, J--15.19, 8.44 Hz, 1 H, aliphatic CH), 3.35-3.40 (m, 2H, NCH2CH20H),
3.81-3.85 (m, 2H, NCH2CH20H), 5.20 (dd, J--23.63, 12.30 Hz, 2H, OCHZAr),
6.55 (d, J--8.44 Hz, 1 H, ArH), 7.03 (d, J-- 8.44 Hz, 1 H, ArH), 7.22 (t, J--
7.47 Hz,
IH, ArH), 7.29 (d, J--7.23 Hz, 1H, ArH), 7.75 (d, J--7.48 Hz, 1H, ArH), 7.89
(s,
1H, ArH), 11.55 (s, 1H, NH); MS(APCI+): ntlz 475.1 (MH+). Anal. Calcd for
C33H43N306~ C~ 65.23; H, 7.68; N, 6.92. Found: C, 64.95; H, 7.68; N, 6.92.
Example 64
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12, I 3,14, I 4a,15-decahydro-2-methyl-,
[3-[(dimethylamino)methyl]phenyl]methyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-162-
N
N O
O
O
N
H
To a stirred solution of Ph3P (0.161 g, 0.616 mmol) and
pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [3-(hydroxymethyl)phenyl]-
methyl ester (Example 61, Step B, 0.284 g, 0.616 mmol) in 2.93 mL of anhydrous
THF at -18°C was added dropwise a solution of N-bromosuccinimide
(0.110 g,
0.616 mmol) in 2 mL of THF. After 10 minutes, the cold bath was removed, and
dimethylamine was introduced in one portion. The reaction was heated at
80°C for
1 hour in a stainless steel vessel. The THF was removed, and the product was
purified by flash column chromatography on silica gel (20% MeOH:CH2Cl2) and
recrystallized from CH2Cl2 to give 1.21 g (40.3%) of coarse off white powder:
mp 87-90°C; IR (KBr) 2930, 2854, 1700, 1589, 1432, 1077 cm-/; IH NMR
(400 MHz, DMSO-d6) ~ 1.06-1.36 (m, 3H, aliphatic CH) 1.39-1.63 (m, 7H,
aliphatic CH), 1.82 (d, J--13.18 Hz, IH, aliphatic CH), 2.11 (s, 6H, N(CH3)2)
2.34 (d, J 10.74 Hz, 1H, aliphatic CH), 2.40-2.46 (m, IH, aliphatic CH), 2.45
(s,
3H, CC113), 2.59-2.67 (m, 2H, aliphatic CH), 2.83-2.89 (rri, IH, aliphatic
CH),
3.15 (dd, J--18.31, 6.84 Hz, IH, aliphatic CH), 3.37 (s, 2H, ArCH2N(CH3)2),
5.20 (dd, J 23.93, 12.21 Hz, 2H, OCH2Ar), 6.57 (d, J 8.79 Hz, 1H, ArH),
7.02 (d, J--8.79 Hz, 1H, ArH), 7.22-7.31 (m, 3H, ArH), 7.34 (s, 1H, ArH),
11.53 (s, IH, NH); MS(APCI+): m/z 488.1 (MH+). Anal. Calcd for
C3pH37N303: C, 71.89; H, 7.65; N, 8.62. Found: C, 71.89; H, 7.65; N, 8.20.
Example 65
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10, I 2, I 3,14,14a,15-decahydro-2-methyl-,
[3-[(dimethylamino)carbonyl]phenyl]methyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-163-
N
Step A: 3-Hydroxymethyl-N,N-dimethyl-benzamide
0
HO ~ ~ N~
To a mixture of 3-(hydroxymethyl)benzoic acid (9.87 g, 64.9 mmol),
dimethylamine (2 M in THF, 32.4 mL, 64.9 mmol), N,N-diisopropyl ethylamine
(22.6 mL, 130 mmol) in 20 mL of DMF was added a solution of HBTU (24.6 g,
64.9 mmol) in 55 mL of DMF at 0°C. After 5 minutes, the yellow solution
turned
orange. After stirring at 0°C for 1 hour, the reaction mixture was
diluted with
diethyl ether, washed with 10% HCI, saturated NaHC03, brine, dried with
MgS04, and concentrated to give a brown oil. The product was purified by flash
column chromatography on silica gel (3% MeOH:CH2C12) to afford 7.5 g
(64.5%) of a yellow oil: 1H NMR (400 MHz, DMSO-d6) 8 2.88 (s, 3H, NCH3),
2.96 (s, 3H, NCH3), 4.51 (d, J--5.55 Hz, 2H, OCH2Ar), 5.25 (t, J 5.79 Hz, 1H,
OH), 7.21-7.25 (m, 1H, ArH), 7.31-7.47 (m, 3H, ArH); MS(APCI+): m/z
180.1 (MH+).
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
[3-[(dimethylamino)carbonyl]phenyl]methyl ester
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [3-[(dimethylamino)carbonyl]-
phenyl]methyl ester was synthesized according to Procedure N from
3-hydroxymethyl-N,N-dimethyl-benzamide (1.04 g, 5.81 mmol) and triturated
with acetone to give 0.250 g (25.7%) of fluffy white powder: mp 112-
116°C; IR
(KBr) 2929, 2854, 1698, 1624, 1432, 1077 cm-1; 1H NMR (400 MHz, DMSO-
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-164-
d6) 8 1.09-1.62 (m, 10H, aliphatic CH), 1.82 (d, J--14.16 Hz, IH, aliphatic
CH),
2.33 (d, J--10.25 Hz,lH, aliphatic CH), 2.40-2.43 (m, 1H, aliphatic CH), 2.45
(s,
3H, CCH3), 2.46 (s, 6H, N(CH3)2), 2.59-2.67 (m, 2H, aliphatic CH),
2.86-2.93 (m, 1H, aliphatic CH), 3.16 (dd, J--18.80, 7.81 Hz, IH, aliphatic
CH),
5.24 (dd, J 20.75, 12.45 Hz, 2H, OCH2Ar), 6.57 (d, J--8.55 Hz, 1H, ArH),
7.02 (d, J--8.55 Hz, 1H, ArH), 7.33 (d, J--7.57 Hz, 1H, ArH), 7.40-7.44 (m,
2H,
Arl~, 7.49 (d, J--7.57 Hz, 1H, ArH), 11.54 (s, 1H, NH); MS(APCI+): m/z
502.2 (MH+). Anal. Calcd for C3pH35N3O4: C, 71:83; H, 7.03; N, 8.38. Found:
C, 71.44; H, 7.05; N, 8.21.
Example 66
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [2-(4-morpholinyl)ethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [2-(4-morpholinyl)ethyl ester
was synthesized according to Procedure N from 2-morpholin-4-yl-ethanol
(0.300 mL, 2.64 mmol) and triturated with CH2C12 to give 0.122 g (41.0%) of a
white solid: mp 188-190°C; IR (KBr) 2931, 2854, 1696, 1588, 1432, 1148
cm 1;
1H NMR (400 MHz, DMSO-d6) 8 1.14-1.75 (m, 10H, aliphatic CH), 1.90 (d,
J--13.18 Hz, IH, aliphatic CH), 2.34-2.46 (m, 2H, aliphatic CH), 2.46 (s, 4H,
N(CH2CH2)20), 2.49 (s, 3H, CCH3), 2.59 (t, J--5.62 Hz, 2H, C02CH2CH2),
2.64-2.73 (m, 2H, aliphatic CH), 2.86-2.91 (m, 1H, aliphatic CH), 3.29 (dd,
J--19.29, 10.25 Hz, 1H, aliphatic CH), 3.52 (s, 4H, N(CH2CH2)20), 4.18-4.30
(m, 2H, C02CH2CI-I2), 6.58 (d, J-- 8.55 Hz, 1H, ArH), 7.02 (d, J 8.55 Hz, IH,
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-165-
ArH), 11.48 (s, 1H, NH); MS(APCI+): rnlz 454.1 (MH+). Anal. Calcd for
C26H35N304~ C~ 68.60; H, 7.79; N, 9.23. Found: C, 68.23; H, 7.80; N, 9.02.
Example 67
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[1,1'-biphenyl]-4-ylethyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[1,1'-biphenyl]-4-ylethyl
ester
was synthesized according to Procedure N from 1-biphenyl-4-yl-ethanol (2.68 g,
13.5 mmol) and triturated with diethyl ether to give 0.619 g (35.4%) of white
powder: mp 132-135°C; IR (KBr) 2929, 2855, 1677, 1432, 1078 cm-1; 1H
NMR
(400 MHz, DMSO-d6) 8 1.11-1.67 (m, 10H, aliphatic CH), 1.59 (d, J--6.59 Hz,
2H, OCHCH3), 1.79-1.89 (m, 2H, aliphatic CH), 2.33-2.47 (m, 1H, aliphatic CH),
2.53 & 2.54 (s, 3H, CCH3, diastereomers), 2.60-2.67 (m, 2I-I, aliphatic CH),
2.79-2.95 (m, 1H, aliphatic CH), 3.14 (dd, J--28.08, 10.12 I-Iz, 1H, aliphatic
CH),
5.96-6.03 (m, 1H, OCHCH3), 6.55-6.59 (m, 1H, ArH), 7.00-7.03 (m, 1H, ArH),
7.31-7.34 (m, 1/-I, Arl~, 7.40-7.51 (m, 4H, ArH), 7.60-7.64 (m, 4H, ArH),
11.53 (s, 1H, NH); MS(APCI+): nZ/z 521.1 (MH+). Anal. Calcd for
C34H36N203: C, 78.25; H, 7.36; N, 5.14. Found: C, 78.20; H, 7.76; N, 4.85.
Example 68
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (2,6-difluorophenyl)methyl
ester
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-166-
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,(2,6-difluorophenyl)methyl
ester
was synthesized according to Procedure N from (2,6-difluoro-phenyl)-methanol
(1.50 mL, 13.5 mmol) and triturated with diethyl ether to give 1.07 g (68.0%)
of
fluffy white powder: mp 219-220°C; IR (KBr) 3330, 2928, 1664, 1473,
1059 cm-I; 1H NMR (400 MHz, DMSO-d6) 8 1.03-1.35 (m, 3H, aliphatic CH),
1.38-1.56 (m, 7H, cylic CH), 1.78 (d, J--13.18 Hz, 2H, aliphatic CH), 2.32-
2.34
(m, 2H, aliphatic CH), 2.41 (s, 3H, CCH3), 2.55-2.66 (m, 2H, aliphatic CH),
2.83-2.88 (m, 1H, aliphatic CH), 3.08 (dd, J--18.31, 7.08 Hz, 1H, aliphatic
CH),
5.20 (d, J 11.96 Hz, 1 H, OCHZAr), 6.57 (d, J--8.55 Hz, 1 H, ArH), 7.01 (d,
J--8.55 Hz, 1H, ArH), 7.16 (t, J 7.81 Hz, 2H, ArH), 7.48-7.53 (m, 1H, ArH),
11.57 (s, 1H, NH); MS(APCI+): m/z 467.1 (MH+). Anal. Calcd for
C27H28N203F2: C, 69.32; H, 6.19; N, 5.80. Found: C, 69.51; H, 6.05; N, 6.00.
Example 69
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12, I 3,14,14a,15-decahydro-2-methyl-, ( I -phenyl-2,2,2-tri
fluoro)ethyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1-phenyl-2,2,2-
trifluoro)ethyl
ester was synthesized according to Procedure N from 2,2,2-trifluoro-1-phenyl-
ethanol (1.58 g, 9.00 mmol) and triturated with t-butyl methyl ether to give
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-167-
0.356 g (31.8%) of fluffy white powder: mp 115-117°C; IR (KBr) 3382,
2931,
1718, 1432, 1067 cm-1; 1H NMR (400 MHz, DMSO-d6) 8 1.07-1.68 (m, l OH,
aliphatic CH), 1.87-1.90 (m, 2H, aliphatic CH), 2.35-2.46 (m, 2H, aliphatic
CH),
2.58-2.62 (m, 1H, aliphatic CH), 2.61 & 2.62 (s, 3H, CCH3, diastereomers),
2.62-2.90 (m, 1H, aliphatic CH), 3.18 (dd, J--17.82, 6.84 Hz, 1H, aliphatic
CH),
6.52-6.57 (m, 1H, OCHCF3), 6.61-6.63 (m, 1H, ArH), 7.05-7.07 (m, 1H, ArH),
7.43-7.44 (m, 3H, ArH), 7.55-7.57 (m, 2H, ArH), 11.83 (s, 1H, NH); MS(APCI+):
m/z 499.1 (MH+). Anal. Calcd for C28H29N203F3: C, 67.46; H, 5.86; N, 5.62.
Found: C, 67.40; H, 6.03; N, 5.44.
Example 70
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
1-[3-(trifluoromethyl)phenyl]ethyl ester
F F
F
N O
O
O /
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[3-(trifluoromethyl)phenyl]-
ethyl ester was synthesized according to Procedure N from 1-(3-trifluoromethyl-
phenyl)-ethanol (2.06 mL, 13.5 mmol) and triturated with diethyl ether to give
0.793 g (45.8%) ofwhite solid: mp 102-105°C; IR (KBr) 3380, 2931, 1680,
1432,
1075 cm-l; 1H NMR (400 MHz, DMSO-d6) d 1.05-1.59 (m, 10H, aliphatic CH)
1.59 (d, J--6.59 Hz, 3H, OCHCll3), 1.77-1.88 (m, 1H, aliphatic CH), 2.33 (d,
J--9.03 Hz, 1H, aliphatic CH), 2.41 (d, J--8.55 Hz, 1H, aliphatic CH), 2.51 &
2.52 (s, 3H, CCH3, diastereomers), 2.51-2.67 (m, 2H, aliphatic CH), 2.83-2.86
(m, 1H, aliphatic CH), 3.06 (dd, J--18.07, 6.84 Hz, 1H, aliphatic CH), 6.01-
6.05
(m, 1H, OCHCH3), 6.58-6.60 (m, 1H, ArH), 7.01-7.04 (m, 1H, ArH), 7.57-7.67
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99130434
-168-
(m, 2H, ArH), 7.71-7.75 (m, 2H, ArH), 11.56 (s, 1H, NH); MS(APCI+): m/z
513.1 (MH+). Anal. Caled for C29H31N203F3: C, 67.69; H, 6.08; N, 5.44.
Found: C, 67.34; H, 6.35; N, 5.24.
Example 71
S Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-dccahydro-2-methyl-, 2-(dimethylamino)ethyl ester
N
N O
O
O
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(dimethylamino)ethyl ester
was synthesized according to Procedure N from 2-dimethylamino-ethanol
(1.36 mL, 13.5 mmol) and triturated with diethyl ether to give 0.489 g (34.9%)
of
granular off white solid: mp 190-191 °C; IR (KBr) 3274, 2950, 1653,
1518,
1248 cm-I; 1H NMR (400 MHz, DMSO-d6) c5 1.13-1.59 (m, 10H, aliphatic CH),
1.71-1.76 (m, 1H, aliphatic CH), 1.90 (d, J--13.43 Hz, IH, aliphatic CH), 2.05
(s,
6H, N(CH3)2), 2.35 (d, J--10.25 Hz, lI-I, aliphatic CH), 2.48 (s, 3H, CCH3),
2.52
(t, J--5.86 Hz, 2H, OCH2CH2), 2.64-2.74 (m, 2H, aliphatic CH), 2.85-2.89 (m,
1H, aliphatic CH), 3.31 (dd, J--18.56, 7.08 Hz, 1H, aliphatic CH), 4.14-4.27
(m,
2H, OCHZCH2), 6.58 (d, J--8.55 Hz, 1H, ArH), 7.02 (d, J--8.79 Hz, 1H, ArH),
11.56 (s, 1H, NH); MS(APCI+): m/z 412.2 (MH+). Anal. Calcd for
C24H33N303~ C~ 70.04; H, 8.08; N, 10.21. Found: C, 70.01; H, 8.20; N, 9.98.
Example 72
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(1-pyrrolidinyl)ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-169-
Pyrrolo[3',2':5,6][IJbenzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(1-pyrrolidinyl)ethyl ester
was
synthesized according to Procedure N from 2-pyrrolidin-I-yl-ethanol (1.02 g,
8.85 mmol) and triturated with t-butyl methyl ether to give 0.253 g (26.0%) of
white solid: mp 195-196°C; IR (KBr) 3377, 2930, 1700, 1432, 1081 cm-/;
IH NMR (400 MHz, DMSO-d6) 8 1.12-1.78 (m, 10H, aliphatic CH), 1.61-1.69
(m, 8H, cyclic CHZ), 1.92 (d, J--13.26 Hz, IH, aliphatic CH), 2.37 (d, J--
10.61 Hz,
1H, aliphatic CH), 2.47-2.49 (m, 1H, aliphatic CH), 2.50 (s, 3H, CH3), 2.66-
2.76
(m, 2H, aliphatic CH), 2.72 (t, J 6.51 Hz, 2H, OCH2CH2), 2.87-2.93 (m, 1H,
aliphatic CH), 3.32 (dd, ,l--19.05, 13.02 Hz, 1H, aliphatic CH), 4.19-4.30 (m,
2H,
OCH2CH2), 6.60 (d, J--8.68 Hz, 1 H, ArH), 7.04 (d, J--8.68 Hz, 1 H, ArH),
11.49
(s, 1H, NH); MS(APCI+): m/z 438.2 (MH+). Anal. Calcd for C26H35N303v C
71.37; H, 8.06; N, 9.60. Found: C, 70.99; H, 8.13; N, 9.49.
Example 73
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(1-naphthalenyl)ethyl ester
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, I-(1-naphthalenyl)ethyl ester
was synthesized according to Procedure N from I-naphthalen-1-yl-ethanol
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-170-
(2.32 g, 13.5 mmol) and triturated with diethyl ether to give 0.707 g (40.9%)
of
fine white powder: mp 140-145°C; IR (KBr) 3387, 2929, 1682, 1432, 1078
cm-l;
1H NMR (400 MHz, DMSO-d6) 8 0.94-1.72 (m, 10H, aliphatic CH), 1.72 (d,
J--2.69 Hz, 3H, OCHCH3), 1.83 (d, J--12.94 Hz, IH, aliphatic CH), 2.30-2.63
(m,
3H, aliphatic CH), 2.50 & 2.52 (s, 3H, CCH3, diastereomers), 2.83-2.88 (m, 2H,
aliphatic CH), 3.21 (dd, J 32.72, 26.12 Hz, 1H, aliphatic CH), 6.52-6.58 (m,
1H,
ArH), 6.74-6.75 (m, IH, OCHCH3), 6.98-7.03 (m, 1H, ArH), 7.46-7.65 (m, 4H,
ArH), 7.84-7.89 (m, 1 H, ArH), 7.93-7.96 (m, 1 H, ArH), 8.12-8.14 (m, 1 H,
ArH),
11.54 (s, 1H, NH); MS(APCI+): m/z 513.1 (MH+). Anal. Calcd for
C32H34N2O3: C, 76.66; H, 6.92; N, 5.58. Found: C, 76.29; H, 6.96; N, 5.40.
Example 74
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcyclobutyl ester
N O
O
O
N
H
Step A: I-Phenyl-cyclobutanol
OOH
To a solution ofphenylmagnesium bromide (1 M in THF, 148 mL,
148 mmol) in 87 mL of anhydrous diethyl ether was added cyclobutanone ( 10.0
g,
143 mmol) in 15 ml, of ether at 0°C. The reaction mixture was stirred
in an ice
bath for I hour. Saturated ammonium chloride was added and stirred for
10 minutes. The reaction mixture was washed with H20 (2 x 250 mL), dried with
MgS04, and concentrated to give a yellow oil. The product was purified by
flash
column chromatography on silica gel (10% acetone:hexanes) to give 10.1 g
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-171-
(47.7%) of a yellow oil: IH NMR (400 MHz, CDCl3) 8 1.62-1.73 (m, 1H,
CCH2CH2), 1.94-2.09 (m, 1H, CCH2CH2), 2.29-2.38 (m, 2H, CCH2), 2.50-2.57
(m, 2H, CCH2), 2.68 (s, 1H, OH), 7.21-7.29 (m, IH, ArH), 7.31-7.38 (m, 2H,
ArH), 7.38-7.50 (m, 2H, ArH); MS(APCI+): m/z 171.5 (MH+).
Step B: Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcyclobutyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-i-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcyclobutyl ester was
synthesized according to Procedure N from i-phenyl-cyclobutanol (1.33 g,
9.00 mmol) and triturated with diethyl ether to give 0.201 g ( 19.0%) of white
powder: mp 218-220°C; IR (KBr) 3328, 2930, 1674, 1434, 1080 cm-1; 1H
NMR
(400 MHz, DMSO-d6) 8 1.09-1.84 (m, 10H, aliphatic CH), 1.94-2.00 (m, 1H,
aliphatic CH), 2.33 (d, J--9.03 Hz, 1H, aliphatic CH), 2.40-2.46 (m, IH,
aliphatic
CH), 2.46 (s, 3H, CCH3), 2.56 (s, 6H, cyclic C(CH2)3Ar), 2.52-2.64 (m, 2H,
aliphatic CH), 2.83-2.88 (m, 1H, aliphatic CH), 3.15 (dd, J--18.80, 7.08 Hz,
IH,
aliphatic CH), 6.56 (d, J--8.55 Hz, 1 H, ArFl), 7.00 (d, .1--8.55 Hz, I H,
ArH),
7.22 (t, J--7.08 Hz, 1H, ArH), 7.33 (t, J--7.57 Hz, 2H, ArF~, 7.47 (d, .I--
7.32 Hz,
2H, ArH), 11.48 (s, 1H, NH); MS(APCI+): nrlz 671.1 (MH+). Anal. Calcd for
C3pH35N2O3: C, 74.72; H, 7.56; N, 5.81. Found: C, 74.43; H, 7.26; N, 5.41.
Example 75
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcylopropyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-172-
Step A: 1-Phenyl-cyclopropanol
~oH
i
I-Phenyl-cyclopropanol was synthesized according to the procedure
published in Kulinkovich, O.G.; Sviridov, S.V.; Vasilevskii, D.A.; Savchenko,
A.L; Pritytskaya, T.S. J. Org. Chem. USSR (Engl.) 1991;27:250-253.
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcylopropyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, I-phenylcylopropylesterwas
synthesized according to Procedure N from 1-phenyl-cyclopropanol (1.22 g,
9.00 mmol) and triturated with diethyl ether to give 0.078 g (7.57%) of shiny
yellow powder: mp 130-135°C; 1R (KBr) 3384, 2929, 1690, 1431, 1069 cm-
l; 1H
NMR (400 MHz, DMSO-d6) 8 1.06-1.69 (m, I OH, aliphatic CH), 1.86 (d,
.13.43 Hz, 1H, aliphatic CH), 2.34 (d, J--10.01 Hz, 1H, aliphatic CH),
2.41-2.44 (m, 1H, aliphatic CH), 2.46 (s, 3H, CCH3), 2.52 (s, 4H, cyclic
C(CII2)2Ar), 2.63-2.70 (m, 2H, aliphatic CH), 2.81-2.89 (m, 1H, aliphatic CH),
3.20 (dd, J--18.56, 7.33 Hz, I H, aliphatic CH), 6.59 (d, J--8.55 Hz, 1 H,
Arf~,
7.03 (d, J--8.55 Hz, 1H, ArH), 7.15-7.19 (m, 3H, Arl~, 7.26-7.30 (m, 2H, ArH),
11.57 (s, 1H, NH); MS(APCI+): mlz 457.1 (MH+). Anal. Calcd for
C29H32N203v C~ 76.29; H, 7.06; N, 6.14. Found: C, 76.12; H, 7.39; N, 5.83.
Example 76
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-pyrazinylethyl ester
N
N O ~ /N
O
O
CA 02372197 2001-07-12
WO 00142045 PCTlUS99/30434
-173-
Step A: 1-Pyrazin-2-yl-ethanol
OH
CN
i
N
To a solution of I-pyrazin-2-yl-ethanone (5.00 g, 40.9 mmol) in 100 mL of
MeOH at 0°C was added NaBH4 (0.774 g, 20.5 mmol) in portions. After
stirnng
at room temperature for 24 hours, the reaction mixture was quenched with 1 N
HCl
and extracted with CH2C12 (3 x 100 mL). The organic layers were dried with
Na2S04 and concentrated to give 2.60 g (51.2%) of a yellow oil: 1H NMR
(400 MHz, CDC13) 8 1.54 (d, J--6.59 Hz, 3H, CHCH3), 3.54 (s, l I-I, OH), 4.97
(q,
J=6.59 Hz, I H, CH3CII), 8.49 (s, 2H, NCHCHN), 8.65 (s, 1 H, CCNN);
MS(APCI+): ~?z/z 125.1 (MH+)
Step B: Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-pyrazinylethyl ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, I-pyrazinylethyl ester was
synthesized according to Procedure N from 1-pyrazin-2-yl-ethanol (1.12 g,
9.00 mmol) and triturated with cold acetone to give 0.303 g (30.3%) of coarse
white powder: mp 224-225°C; IR (KBr) 3172, 2930, 1704, 1431, 1073 cm-1;
1H
NMR (400 MHz, DMSO-d6) b 1.06-1.63 (m, l OH, aliphatic CH), 1.61 (d,
J--3.17 Hz, 3H, OCHCH3), 1.77-1.98 (m, 1H, aliphatic CH), 2.34 (d, J--10.25
Hz,
1H, aliphatic CH), 2.40-2.43 (m, 1H, aliphatic CH), 2.52 & 2.53 (s, 3H, CCH3,
diastereomers), 2.62-2.68 (m, III, aliphatic CH), 2.84-2.87 (m, 1H, aliphatic
CH),
3.12 (dd, J--19.04, C.84 Hz, IH, aliphatic CI-I), 5.99 (q, J--7.08 Hz, 1H,
OCHCH3), 6.56-6.60 (m, 1H, ArH), 7.01-7.04 (m, IH, ArH), 8.57 (s, 1H, ArH),
8.62 (s, 1 H, Arll), 8.71 & 8.73 (s, 1 H, ArH, diastereomers), I 1.56 (s, I H,
NH);
MS(APCI+): n1/z 447.1 (MH+). Anal. Calcd for C26H30N403 ~ C~ 69.90; H, 6.79;
N, 12.50. Found: C, 69.51; H, 6.78; N, 12.35.
CA 02372197 2001-07-12
WO 00142045 PCT1US99/30434
-174-
Example 77
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-quinolinyl)ethyl ester
w
N
N O \
O
O
N
H
Step A: 1-Quinolin-4-yl-ethanol
W
~oH
N
To a solution of quinoline-4-carbaldehyde (5.00 g, 31.8 mmol) in 127 mL
of anhydrous THF at -40°C was added methylmagnesium bromide (13.8 mL,
41.4 mmol). After stirnng for 5 hours, the reaction mixture was quenched with
saturated NH4C1 and extracted with ethyl acetate (5 x 100 mL). The organic
layers were washed with brine, dried with Na2S04, and concentrated to give a
purple solid. The solid was triturated with acetone to yield 4.52 g (82.2%) of
light
purple solid: 1H NMR (400 MHz, CDC13) 8 1.60 (d, J--6.35 Hz, 3H, CHCH3),
3.70 (s, 1H, OH), 5.62 (q, J=6.35 Hz, 1H, CHCH3), 7.50 (t, J--7.57 Hz, 1H,
CCCHCH), 7.56 (d, J--4.40 Hz, 1H, NCHCH), 7.63 (t, J--7.32 Hz, 1H,
NCCHCH), 7.97 (d, J--8.55 Hz, 1H, CCCHCH), 8.05 (d, J--8.30 Hz, 1H,
NCCHCH), 8.73 (d, J--4.39 Hz, 1H, NCHCH), MS(APCI+): m/z 174.1 (MH+)
Step B: Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1~-decahydro-2-methyl-, 1-(4-quinolinyl)ethyl
ester
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-quinolinyl)ethyl ester
was
synthesized according to Procedure N from 1-quinolin-4-yl-ethanol (1.56 g,
9.00 mmol) and triturated with acetone to give 0.192 g (17.1 %) of pale yellow
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-175-
powder: mp 165-168°C; IR (KBr) 2930, 2853, 1690, 1429, 1074 cm-/; 1H
NMR
(400 MHz, DMSO-d6) 8 1.00-1.46 (m, l OH, aliphatic CH), 1.70 (d, J--6.35 Hz,
3H, OCHCH3), 1.85 (d,J--13.18 Hz, IH, aliphatic CH), 2.32-2.63 (m, 3H,
aliphatic CH), 2.57 & 2.58 (s, 3H, CCH3, diastercomers), 2.83-2.86 (m, 2H,
aliphatic CH), 3.20 (dd, J--18.31, 6.84 Hz, 1 H, aliphatic CH), 6.55-6.58 (m,
1H,
ArH), 6.65 (q, J--6.59 Hz, IH, OCHCH3), 7.01-7.05 (m, 1H, ArH), 7.50-7.54 (m,
I H, ArH), 7.63-7.67 (m, .I H, ArH), 7.75-7.79 (m, I H, ArH), 8.04-8.06 (m, 1
H,
ArH), 8.21=8.23 (m, 1 H, ArH), 8.84-8.88 (m, I H, ArH), 11.61 (s, I H, NH);
MS(APCI+): rnlz 496.2 (MH+). Anal. Calcd for C31H33N303v C> 74.12; II, 7.00;
N, 7.83. Found: C, 73.73; H, 7.09; N, 7.44.
Example 78
Pyrrolo[3',2':5,6][1 ]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(2-pyrimidinyl)ethyl ester
N
N O
O N
O
N
H
IS Step A: 1-Pyrirnidin-2-yl-ethanone
0II
N
\ N
1-Pyrimidin-2-yl-ethanone was synthesized according to the procedure
published in Naumenko, I. L; Mikhaleva, M. A.; Mamaev, V. P. Chem.
Het. Crnpcls. 1981;17:710-714.
Step B: I-Pyrimidin-2-yl-ethanol
N\ ~
~OH
SIN
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-176-
To a solution of 1-pyrimidin-2-yl-ethanone (2.34 g, 19.2 mmol) in 65 mL
of MeOH at 0°C was added NaBH4 (0.726 g, 19.2 minol) in portions. After
stirring at room temperature for 4 hours, the reaction mixture was quenched
with
1N HCI and extracted with CH2C12 (3 x 100 mL). The organic layers were dried
with Na2S04 and concentrated to afford 0.984 g (41.3%) of a yellow oil: IH
NMR (400 MHz, DMSO-d6) 8 1.36 (d, J--6.59 Hz, 3H, CHCH3), 4.73 (q,
J 6.59 Hz, 1H, CH3CH), 5.21 (s, 1H, OI~, 7.33-7.36 (m, 1H, NCHCH),
8.73-8.77 (m, 2H, NCHCH); MS(APCI+): m/z 125.1 (MH+)
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-pyrimidinyl)ethyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, l-(2-pyrimidinyl)ethyl ester
was
synthesized according to Procedure N from 1-pyrimidein-2-yl-ethanol (1.01 g,
8.10 mmol) and triturated with acetone to give 0.360 g (29.9%) of fine off
white
powder: mp 220-222°C; IR (KBr) 3176, 2932, 1686, 1426, 1078 cm-1; IH
NMR
(400 MHz, DMSO-d6) cS 1.06-1.54 (m, l OH, aliphatic CH), 1.59 (d, J--6.59 Hz,
3H, OCHCH3), 1.84 (d, J--13.92 Hz, IH, aliphatic CH), 2.34-2.55 (m, 2H,
aliphatic CII), 2.58 (s, 3H, CCH3), 2.64-2.69 (m, 2H, aliphatic C.H), 2.81-
2.86 (m,
1H, aliphatic CI I), 3.17 (dd, J--18.31, 6.59 Hz, 1H, aliphatic CH), 5.84 (q,
J--6.84 Hz, 1H, OCHCH3), 6.57 (d, J--8.79 Hz, 1H, ArH), 7.02 (d, J=9.03 Hz,
1H,
ArH), 7.37-7.38 (m, 1H, ArFl), 8.76-8.77 (m, 2H, ArH), 11.53 (s, 1H, NH);
MS(APCI+): m/z 447.1 (MH+}. Anal. Calcd for C26H30N403~ C~ 69.86; H, 7.04;
N, 12.07. Found: C, 69.50; H, 6.94; N, 1 I .71.
Example 79
Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-I-carboxylic acid, 5-chloro-
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, phenylmethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-177-
N O
O
O
CI \ ~ N
H
Step A: 6-Chloro-5-hydroxy-2-methyl-lII-indole-3-carboxylic acid benzyl ester
0
0
HO
CI \ ~ N
H
To a solution of 3-amino-but-2-enoic acid benzyl ester (10.1 g, 52.9 mmol)
in 211 mL of EtOH was added 2-chloro-1,4-benzoquinone (9.04 g, 63.4 mmol).
After heating at 50°C for 24 hours, the mixture was cooled to room
temperature
and concentrated to afford a brown oil. The product was purified by flash
column
chromatography on silica gel (20% ethyl acetate:hexanes) and recrystallized
from
ethyl acetate to give 1.02 g (5.12%) of light yellow powder: nip 221-
224°C; IR
(KBr) 3409, 3226, 1642, 1461, 1181 cm-1; 1H NMR (400 MHz, DMSO-d6) 8
2.55 (s, 3H, CCH3), 5.29 (s, 2H, OCH2Ar), 7.27 (s, 1H, ArH), 7.30 (d, J--6.84
Hz,
1H, ArH), 7.36 (t, J--8.30 I-Iz, 2H, ArH), 7.42 (d, J--7.57 Hz, 2H, ArH), 7.50
(s,
1H, ArH), 9.56 (s, 1H, OH), 11.67 (s, 1H, NH); MS(APCI+): m/~ 316.1 (MH+)
HPLC (ALLTCH/ALLTIMA C-18 50:50-2:98 H20/CH3CN + 0.05% TFA):
rentention time=5.47 min, 95.86% purity.
Step B: 6-Chloro-4-dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-
3-carboxylic acid benzyl ester
6-Chloro-4-dimethylaminomethyl-5-hydroxy-2-methyl-1 H-indole-
3-carboxylic acid benzyl ester was synthesized according to Procedure L from
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-178-
6-chloro-5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester (7.77 g,
24.6 mmol) and triturated with cold EtOH to give 6.778 (73.8%) of a white
solid:
mp 178-180°C; IR (KBr) 3298, 2951, 1687, 1425, 1437, 1264, 1078 cm-1;
1H
NMR (400 MHz, DMSO-d6) 8 2.14 (s, 6H, N(CH3)2) 4.06 (s, 2H, NCH2Ar),
5.23 (s, 2H, OCH2Ar), 7.22 (s, 1H, ArH), 7.31-7.39 (m, 3H, ArH), 7.44 (d,
J--6.84 Hz, 2H, ArH), 11.85 (s, 1 H, NH); MS(APCI+): mlz 373.1 (MH+). HPLC
(ALLTCH/ALLTIMA C-18 50:50-2:98 H20/CH3CN + 0.05% TFA): retention
time=3.10 min, 99.09% purity.
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-chloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
5-chloro-3,7,8,9,10,12,13, l 4,14a,15-decahydro-2methyl-, phenylmethyl ester
was
synthesized according to Procedure M from 6-chloro-4-dimethylaminomethyl-
5-hydroxy-2-methyl-1H-indole-3-carboxylic acid benzyl ester (6.77 g, 18.2
mmol)
and triturated with acetone to give 6.05 g (71.7%) of shiny white powder: mp
90-93°C; IR (KBr) 3291, 2933, 2858, 1673, 1427, 1076 cm-1; 1H NMR
(400 MHz, DMSO-d6) 8 1.01-1.71 (m, l OH, aliphatic CH), 1.75 (d, J--13.43 Hz,
1H, aliphatic CH), 2.37 (d,.1--10.50 Hz, 1H, aliphatic CH), 2.45-2.46 (m, 1H,
aliphatic CH), 2.46 (s, 3H, CC~13), 2.64-2.74 (m, 2H, aliphatic CH), 2.94-2.99
(m,
1H, aliphatic CH), 3.38 (dd, J--18.56, 7.08 Hz, 1H, aliphatic CH), 5.21 (dd,
J--26.12, 12.21 Hz, 2H, OCH2Ar), 7.18 (s, 1H, ArH), 7.29-7.43 (m, 5H, ArH),
11.62 (s, 1H, NH); MS(APCI+); m/z 465.2 (MH+). Anal. Calcd for
C27H29N203C11: C, 69.74; H, 6.29; N, 6.02. Found: C, 69.45; H, 6.68; N, 5.82.
Example 80
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 5-chloro-
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl, 1-(4-fluorophenyl)ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-179-
F
N O
O
O
CI \ ~ N
H
Step A: Pyrrolo[3',2':5,6][M]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-chloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl, anhydride
with benzoic acid
S CI H
In a 250 mL, three-necked, round bottom flask was added
pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid, 5-chloro-
3,7,8,9,10,12,13,14,14a,15-decahydro-2methyl-, phenylmethyl ester (Example 79,
Step C, 5.44 g, 11.7 mmol), THF (58.6 mL, 0.2 M), Et3N (1.63 mL, 11.7 mmol),
and 10% Pd(OH)2/C (1.26 g) sequentially. The mixture was stirred under a
H2 atmosphere (balloon) for 1 hour. The reaction mixture was filtered through
a
pad of celite, and the yellow filtrate was carried on the next step. To this
yellow
filtrate was added benzoyl chloride in a dropwise fashion (1.36 mL, 11.7
mmol).
The mixture was stirred at room temperature for 48 hours and the solvent
removed
to give a brown oil. Trituration with acetone afforded 3.50 g (62.4%) of
yellow
powder: mp 160-165°C; 1H NMR (400 MHz, DMSO-d6) ~ 1.10-1.58 (m, 10H,
aliphatic CH), 1.83 (d, J--12.94 Hz, 1H, aliphatic CH), 2.41-2.46 (m, 2H,
aliphatic
CH), 2.51 (s, 3H, CCH3), 2.66-2.71 (m, 1H, aliphatic CH), 2.98-3.01 (m, 2H,
aliphatic CH), 3.38 (dd, J--17.82, 6.59 Hz, 1H, aliphatic CH), 7.29 (s, IH,
Ark,
7.58 (t, J--7.57 Hz, 2H, Arl~, 7.74 (t, J--7.57 Hz, 1H, ArI~, 8.07 (d, J--7.08
Hz,
2H, Arl~, 12.14 (s, 1H, NI~; MS(APCI+): na/z 479.1 (MH+).
Step B: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
0
N O
O
O
N
5-chloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl,
1-(4-fluorophenyl)ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCTNS99/30434
-180-
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-1-carboxylic acid,
5-chloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2methyl-, 1-(4-
fluorophenyl)ethyl
ester was synthesized according to Procedure N from 1-(4-fluoro-phenyl)-
ethanol
(0.900 g, 7.16 mmol) and pyrrolo[3',2':5,6][I]-benzopyrano[3,2-i]quinolizine-1-
carboxylic acid, 5-chloro-3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl,
anhydride with benzoic acid (0.857 g, 1.79 mmol). The product was
recrystallized
from t-butyl methyl ether to give 0.210 g (23.6%) of white powder: mp
102-107°C; IR (KBr) 2934, 2859, 1674, 1428, 1055 cm-I; 1H NMR (400 MHz,
DMSO-d6) S 1.06-1.81 (m, 11H, aliphatic CH), 1.55 (d, J--6.59 Hz, 3H,
OCHCH3), 2.37 (d, J--10.25 Hz, IH, aliphatic CH), 2.44-2.46 (m, 1H, aliphatic
CH), 2.46 (s, 3H, CCH3), 2.63-2.75 (m, 2H, aliphatic CH), 2.97-3.14 (m, IH,
aliphatic CH), 3.20 (dd, J--13.18, 6.59 Hz, 1H, aliphatic CH), 5.95 (q, J--
6.59 Hz,
1H, OCHCH3), 7.15-7.19 (m, 3H, ArH), 7.43-7.49 (m, 2H, ArH), 11.62 (s, IH,
NH); MS(APCI+): ratlz 497.2 (MH+). Anal. Calcd for C28H3pN203FIC11: C,
66.82; H, 6.15; N, 5.57. Found: C, 66.96; H, 6.39; N, 5.46.
Example 81
Quinolizinium, I-[[(4-fluorophenyl)methoxy]carbonyl]-5-hydroxy-2-methyl-1H-
indol-4-yl]mcthylJ-1,2,3,4,6,7,8,9-octahydro-, chloride
ct~
~F
I-I
To a solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1
carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
(4-fluorophenyl)methyl ester (0.500 g, 1.11 mmol) in 125 mL of CH2C12 was
added ethereal HCl in portions until the solution turned cloudy. After the
solvent
was removed, the yellow residue was triturated with acetone to give 0.307 g
(61.0%) ofwhite powder: mp 179-185°C; IR (KBr) 3408, 3193, 2934, 1697,
1431, 1152 cm-1; 1H NMR (400 MHz, CDC13) ~ 1.37-1.52 (m, IH, aliphatic
CH), 1.52-I.77 (m, 8H, aliphatic CH), 2.07-2.15 (m, IH, aliphatic CH), 2.26
(d,
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-181-
J 14.65 Hz, 1H, aliphatic CH), 2.40-2.54 (m, 2H, aliphatic CH), 2.58 (s, 3H,
CCH3), 3.10-3.18 (m, 2H, aliphatic CH), 3.34-3.48 (m, 2H, aliphatic CH),
5.28 (dd, J--14.65, 12.45 Hz, 2H, OCHZAr), 6.78 (d, J--14.65 Hz, 1H, ArH),
7.05 (t, J--8.55 Hz, 1H, ArH), 7.14 (d, J--8.79 Hz, IH, ArH), 7.41 (t, J--5.37
Hz,
1H, ArH), 8.58 (s, IH, OH), 12.52 (s, 1H, NH); MS(APCI+): m/z 449.3 (MH+).
Anal. Calcd for C27H3pN203F1C11: C, 66.87; H, 6.23; N, 5.78; Cl, 7.31; F,
3.92.
Found: C, 66.37; H, 6.27; N, 5.69; Cl, 7.64; F, 4.02.
Example 82
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-1,2-dimethyl-, (4-fluorophenyl)methyl
ester
~F
N O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinoli?ine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (4-fluorophenyl)methyl ester
(0.500 g, 1.11 mol) in 20 mL of DMF was added to NaH (60% dispersion in
mineral oil, 0.049 g, 1.23 mmol, washed with hexanes) and was stirred at room
temperature for I hOlll'. Methyl iodide (0.076 mL, 1.23 mol) was added to the
reaction mixture. The reaction was stirred for 2 hours, quenched with 15 mL of
H20 and extracted with diethyl ether (5 x50 mL). The organic layers were
concentrated to afford a yellow solid which was triturated with acetone to
give
0.274 g (52.7%) of white solid: mp 179-180°C; IR (KBr) 3466, 2932,
2854, 1673,
1482, 1155 cm-1; 1 H NMR (400 MHz, CDCl3) S 1.06-1.21 (m, 3H, aliphatic
CH), 1.36-1.58 (m, 7H, aliphatic CH), 1.79 (d, J--14.20 Hz, IH, aliphatic CH),
2.34 (d, J--10.74 Hz, 1H, aliphatic CH), 2.42-2.49 (m, 2H, aliphatic CH), 2.49
(s,
3H, CCH3), 2.64 (t, J--10.74 Hz, IH, aliphatic CH), 2.86 (t, J--11.48 Hz, 1H,
aliphatic CH), 3.10 (dd, .l--18.31, 6.84 Hz, 1H, aliphatic CH), 3.59 (s, 3H,
NCH3),
5.21 (dd, J--29.05, 11.96 Hz, 2H, OCH2Ar), 6.64 (d, J 8.79 Hz, 1H, ArH),
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-182-
7.16-7.22 (m, 3H, Arl~, 7.48 (t, J--7.81 Hz, 1H, Arl~; MS(APCI+): mlz 463.1
(MH+). Anal. Calcd for C28H31N203F1: C, 72.71; H, 6.76; N, 6.06. Found: C,
72.89; H, 6.72; N, 5.92.
Example 83
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylpropyl ester
N O
O
O
N
H
Step A: 5-Acetoxy-2-methyl-1H-indole-3-carboxylic acid 1-phenyl-propyl ester
W
O O
O
O
N
H
This compound was made according to Procedure A. White solid, mp
144-145.5°C; MS(APCI-): mlz 350.1 (M-H).
Step B: S-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 1-phenyl-propyl ester
O
O
O
N
5-Acetoxy-2-methyl-1H-indole-3-carboxylic acid, 1-phenyl-propyl ester
(1.36 g, 3.86 mmol) was mixed with 10 mL of methanol, NaOCH3 (0.834 g,
CA 02372197 2001-07-12
WO 00/42045 PCT/ITS99/30434
-183-
15.4 mmol) was then added. The resulting reaction mixture was stirred at
reflux
for 1 minute, then allowed to cool to ambient temperature. The reaction
mixture
was then mixed with 10 mL of water, the resulting reaction mixture was treated
with 5% HCl until pH = 1 affording a white precipitate. The mixture was
6 extracted with EtOAc (2 x 60 mL). The combined organic mixture was dried
over
Na2S04 and concentrated in vacuo to give a black thick oil which was further
purified by chromatography using 10% MeOH in HCCl3 as the eluant to give
1.13 g (98%) of the desired product as a brown solid:..l H NMR (DMSO-d6) ~
0.917 (t, J= 7.33 Hz, 3H, CHCH2CH3), 1.84-2.03 (m, 2H, CHCH2CH3), 2.59 (s,
3H, ArCH3), 5.84 (t, J= 5.68 Hz, 2H, CHCH2CH3), 6.59 (dd, J= 8.61, 2.38 Hz,
1 H, ArH), 7.12 (d, J= 8.61 Hz, 1 H, ArH), 7.23-7.41 (m, 6H, ArH), 8.87 (s, 1
H,
exchangeable proton), 11.6 (bs, 1H, exchangeable proton).
Step C: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid, 1-phenyl-propyl ester
O O
HO
N
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid, 1-phenyl-propyl ester
(1.07 g, 3.60 mmol) and aqueous Me2NII (40%, 0.99 mL, 7.92 mmol) were
mixed with 2.4 mL of EtOH, the mixture was heated with a heatgun until a clear
solution was obtained. After cooled to ambient temperature, aqueous HCHO
(37%, 0.35 g, 4.3 mmol) was added. The resulting reaction mixture was stirred
at
50°C for 4 hours, then at ambient teperature for 12 hours. The racoon
mixture was
diluted with EtOAc (30 mL), washed with water (2 x 30 mL), and dried over
Na2S04. Concentration in vacuo followed by chromatography using 100%
EtOAc, then 10% MeOH in HCC13 as the eluants gave 0.50 g (38%) ofpure titled
compound as a yellow foam: mp 50-62°C (dec.); MS(APCI+): ntlz 367.2 (MI-
I+).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-184-
Step D: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-, 1-phenylpropyl ester
H
To a mixture of perchlorate salt (0.38 g, 1.6 mmol, Example 3, Step B) and
30 mL of ether was added 30 mL of aqueous NaOH (2N). The resulting mixture
was shaken in a separatory fimnel until all solid had dissolved. Two layers
were
separated, and the aqueous layer was extracted with ether (2 x 30 mL).
Combined
ether layer was dried over Na2S04 and concentrated in vacuo. The residual oil
was dissolved in 10 mL of dioxane, then indole mannich base (0.45 g, 1.2 mmol)
was added, the resulting reaction mixture was refluxed under nitrogen for
18 hours. The reaction mixture was cooled to ambient temperature and
concentrated in vacuo affording a thick oil. The crude product was further
purified
by chromatography (50% EtOAc in hexanes) to 0.40 g (71 %) of titled compound
as a white foam: mp 90-115°C; MS(APCI+): m/z 459.3 (MH+).
Example 84
Quinolizinium, 1,2,3,4,6,7,8,9-octahydro-1-[[5-hydroxy-2-methyl-
3-[(phenylmethoxy)carbonyl]-1H-indol-4-yl]methyl]-, chloride
CI
O
~--O
HO~
To a solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-
carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl
ester (0.209 g, 0.485 mmol) in CH2Cl2 was added etheral HC1. After stirring at
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-185-
ambient temperature for 1 minute, the reaction mixture was concentrated in
vacuo.
The residue was triturated with 2-butanone. Filtration followed by drying
under
vacuum gave 0.18 g (79%) of the desired product as a white solid: MS(APCI+):
m/z 431.3 (MH+). Anal. Calcd for C27H3pN203M.0 HC1~0.3H20: C, 68.65; H,
6.74; N, 5.93; Cl, 7.50; H20, 1.14. Found: C, 68.62; H, 6.80; N, 6.00; Cl,
7.54;
H20, 0.93.
Example 85
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10, I 2,13,14,14x,15-decahydro-2-methyl-, (4-nitrophenyl)methyl ester
N
O O
0
N O
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, phenylmethyl ester (0.149 g,
0.341 mmol) was dissolved in 15 mL of THF, to the solution was added N,N-
dimethylacetamide dimethyl acetal (0.5 mL), and Pd(OH)2/C (20%, 0.125 g). The
resulting reaction solution was stirred at ambient temperature under hydrogen
atmosphere until the benzyl ester was completely consumed. The catalyst was
removed by filtration, and the filtrate was concentrated in vc~cuo at ambient
temperature and used in the next step without further purification.
To a solution of crude product of debenzylation reaction in DMF were
added para-nitrobenzylbromide and DBU. The resulting reaction solution was
stirred at ambient temperature for 16 hours. The reaction mixture was diluted
with
50 mL of EtOAc and washed successively with saturated aqueous NaHC03 (3 x
50 mL) and water (3 x 50 mL). After drying over Na2S04, the solution was
concentrated in vacuo and purified by chromatography twice using 10% MeOH in
CA 02372197 2001-07-12
WO 00/42045 PCTNS99/30434
-186-
HCCL3 and 50% EtOAc in hexanes to give 28 mg (17%) of desired product as a
yellow solid: mp 240-242°C; MS(APCI+): m/z 476.3 (MH+).
Example 86
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, anhydride with benzoic acid
O
N O
O
O
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, phenylmethyl ester (10.15 g,
23.58 mmol) was dissolved in 110 mL of THF, and the solution was transferred
to
a round bottom flask equipped with a stir bar and a three-way stopcock
connected
to a hydrogen balloon. To the solution was added triethylamine (3.287 mL,
23.58 mmol), followed by Pd(OH)2/C (20%, 2.7 g). The flask was purged with
hydrogen gas several times. The resulting reaction solution was stirred at
ambient
temperature under hydrogen atmosphere until the benzyl ester was completely
consumed (2 hours in this case). The catalyst was removed by filtration, and
the
filtrate was used in the next step.
To the filtrate was added benzoyl chloride (2.737 mL, 23.58 mmol). The
resulting
reaction solution was stirred at ambient temperature for 16 hours under
nitrogen.
White precipitate formed was removed by filtration. The filtrate was
concentrated
in vacuo affording thick oil; trituration with Et20 gave 9.188 (88% over two
steps) the desired product as a white solid: mp 159-160°C; MS(APCI+):
ntlz
443.3 (MH+).
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-187-
General procedure Q: ester synthesis from the mixed anhydride
The mixed anhydride (1 eq.) was mixed with the corresponding alcohol
(>2 eq.), the resulting slurry was heated at 120-150°C until a clear
solution was
obtained. After cooling down to ambient temperature, the solution was diluted
with EtOAC, then mixed with aqueous NaHC03 (saturated). The mixture was for
5 minutes. Two layers were separated, and the organic layer was washed with
brine and water, then dried over MgS04. Purification with flash chromatography
or recrystallization gave the desired product.
Example 87
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, ( 1 R)-l -phenylethyl ester
N O
~-O
O
~N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R)-1-phenylethyl ester was
synthesized according to procedure Q from (R)-(+)-1-phenylethanol. The crude
product was chromatographed on a preparative silica gel plate using 100%
acetonitrile as eluant to give 25 mg of the desired product as a white solid:
mp
100-112°C; MS(APCI+): nr/z 445.3 (MH+).
Example 88
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-fluarophenyl)ethyl ester
CA 02372197 2001-07-12
W0 00/42045 PCT/US99/30434
-188-
F
N O
O
O
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-fluorophenyl)ethyl ester
was synthesized according to procedure Q from I-(para-fluorophenyl)ethanol.
The
crude product was chromatographed first on a silica gel column (30% of EtOAc
in
hexanes as eluant) then on a preparative silica gel plate (100% acetonitrile
as
eluant) to give 54.5 mg (37%) of the desired product as a yellow foam: mp
98-110°C; MS(APCI+): m/z 463.1 (MH+).
General procedure R: parallel synthesis of 6 esters from the mixed anhydride
The mixed anhydride (1 eq.) and the corresponding alcohol (2 eq.) were
mixed in a VWR 60826-202 tube. The tube was loosely capped and submerged in
oil-bath at 120°C for 7 minutes. After cooling to ambient temperature,
10 mL of
ether and 10 mL of saturated aqueous Na2S04 were added to the tube. The
mixture was stirred for 1 minute, then the ether layer was transferred into a
new
tube with MgS04. After 10 minutes, the MgSO4 was removed by filtration. The
filtrate was blown down with a nitrogen stream and the residue was re-
dissolved
in 0.2 mL of ether and transferred onto a SPE cartridge containing 1 g of
silica
gel. The short column was eluded with 20 mL of a 10% of EtOAc in hexanes
solution. The fractions collected contained mainly the corresponding alcohol
and
were dicarded. The column was then eluded with 5 mL of a 10% of EtOAc in
hexanes solution. The fraction collected was concentrated down (blown down
with a nitrogen stream) to give the crude product.
Example 89
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,I3,14,14a,15-decahydro-2-methyl-, phenyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-189-
i
N O
O
O
N
H
Pyrrolo[3',2':5,6J[I]benzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, phenyl ester was synthesized
according to procedure R from phenol. The crude product was not further
purified: white solid; MS(APCI+): m/z 417.1 (MH+).
Example 90
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,IS-decahydro-2-methyl-, 2,2,2-trifluoro-1-phenyl-
I-(trifluoromethyl)ethyl ester
F FF F
F
F
N O
O
~N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-deeahydro-2-methyl-, 2,2,2-trifluoro-1-phenyl-
1-(trifluoromethyl)ethyl ester was synthesized according to procedure R from
1,1,1,3,3,3-hexfluoro-2-phenyl-2-propanol. The crude product was not further
purified: white solid; MS(APCI-): m/z 565.1 (M-H).
Example 91
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x, I S-decahydro-2-methyl-, bicyelo[2.2.1 ]kept-2-yl
ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-190=
O
O
O
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, bicyclo[2.2.1 )hept-2-yl ester
was synthesized according to procedure R from exo-norborneol. The crude
product was not further purified: white solid; MS(APCI-): ntlz 433.2 (M-H).
Example 92
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-fluorophenyl)-
1-methylethyl ester
N
O
O ~ O ~ / F
~l
N
H
Pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-fluorophenyl)-
I-methylethyl ester was synthesized according to procedure Q from
2-(4-fluoropbenyl)-2-propanol. The crude product was chromatographed on a
silica gel column (SO-70% of ether in hexanes as eluant) to give 0.15 g (9%)
of the
desired product as a white solid mp 110-112°C; MS(APCI+): m/z 477.1
(MH+).
Example 93
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-, 1-phenylcyclopentyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-191-
N
O
O O
N
H
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcyclopentyl ester was
synthesized according to procedure Q from 1-phenyl-1-cyclopentanol. The crude
S product was chromatographed on a silica gel column (8O% of ether in hexanes
as
cluant) to give 0.15 g (6%) of the desired product as a white solid: mp 20~-
206°C;
MS(APCI+): m/z 483.1 (MH+)
Example 94
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcyclohexyl ester
N
O
O
O
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylcyclohexyl ester was
synthesized according to procedure Q from I-phenylcyclohexanol. The cmde
product was chromatographed on a silica gel column (50-70% of ether in hexanes
as eluant) to give 0.13 g (5%) of the desired product as a yellow solid: mp
217-219°C; MS(APCI-): m/z 497 (M-H).
Example 95
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-(hydroxymethyl)phenyl ester
CA 02372197 2001-07-12
WO 00142045 PCTNS99/30434
-192-
W
N O
O OH
O /
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-(hydroxymethyl)phenyl ester
was synthesized according to procedure Q from 3-hydroxybenzyl alcohol. The
crude product was chromatographed on a silica gel column (50-100% of EtOAc in
hexanes as eluant) to give 0.196 g (16%) of the desired product as a white
foam:
mp 138-140°C; MS(APCI+): m/z 447.2 (MH+)
Example 96
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (3-hydroxyphenyl)methyl ester
OH
N O
O
O
N~-
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (3-hydroxyphenyl)methyl ester
was synthesized according to procedure Q from 3-hydroxybenzyl alcohol. The
crude product was chromatographed on a silica gel column (50-100% of EtOAc in
hexanes as eluant) to give 0.4818 g (39%) of the desired product as a white
solid:
mp 231-233°C; MS(APCI+): nilz 447.1 (MH+).
Example 97
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S)-1-(4-pyridinyl)ethyl
ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-193-
N O
I
O / O 1 N
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S)-1-(4-pyridinyl)ethyl
ester
was synthesized according to procedure Q from (S)-(-)-1-(4-pyridyl)ethanol.
The
crude product was chromatographed on a silica gel column (100% EtOAc as
eluant) to give 0.09 g of the desired product as a yellow foam: mp 105-1 I
5°C;
MS(APCI+): ~at/z 447.2 (MH+).
Example 98
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [6-(methoxycarbonyl)-
2-pyridinyl]methyl ester
O
N O N~ O
1
O O /
N
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [6-(methoxycarbonyl)-
2-pyridinyl]methyl ester was synthesized according to procedure Q from
6-(hydroxymethyl)-picolinic acid ethyl ester. The EtOAc solution (40 mL) of
the
crude product was mixed with 40 mL of 0.5 N HCI solution in a reparative
funnel
and shaken well, then the aqueous phase was basified with 2N NaOH solution to
pH = 1, shaken well. The two layers were then separated. The organic layer was
dried over Na2S04. Chromatography on a silica gel column (50-100% of EtOAc
in hexanes as eluant) to give 0.87 g (53%) of the desired product as a white
foam:
mp 90-100°C; MS(APCI+): ntlz 490.1 (MH+).
CA 02372197 2001-07-12
WO 00142045 PCT1US99/30434
-194-
Example 99
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-pyridinylmethyl ester
N O ~ l
O N
O
N
H
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-pyridinylmethyl ester was
synthesized according to procedure Q from 2-pyridylmethanol. The EtOAc
solution (40 mL) of the enzde product was mixed with 40 mL of 0.5N HCl
solution in a reparative funnel and shaken well, then the aqueous phase was
basified with 2N NaOH solution to pH = 1, shaken well. The two layers were
then
separated. After this process was repeated for three times, the organic layer
was
separated and dried over Na2S04. Chromatography on a silica gel column
(60-100% of EtOAc in hexanes as eluant) to give 1.39 g (72%) of the desired
product as a white foam: mp 85-95°C; MS(APCI+): m/z 432.2 (MH+)
Example 100
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (6-carboxy-2-pyridinyl)methyl
ester
O
N O N OH
I
O ~ ~ ~ O
~N~
H
To a solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
[6-(methoxycarbonyl)-2-pyridinyl]methyl ester (797.4 mg, 1.629 mmol) in MeOH
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-195-
(20 mL) was added 1N NaOH (6.5 mL, 6.5 mmol). The resulting reaction mixture
was refluxed for 15 minutes, then concentrated in vacato. The residue was
purified
by chromatography (10-30% MeOH in HCCI3 as eluant) to give 0.62 g of a
mixture of free acid and the sodium salt. 0.5 g of the mixture was dissolved
in
MeOH/HCCl3, then mixed with 0.64 mL of 1N HCI. The mixture was
concentrated iiz vacuo, the residue was purified by chromatography (10-30%
MeOH in HCC13 as eluant) followed by recrystallization from MeOH to give
0.25 g of the desired product as a white solid: MS(APCI-): m/z 474.1 (M-H).
Example 101
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (6-carboxy-2
pyridinyl)methyl 3,7,8,9, I 0,12,13,14,14a,1 S-decahydro-2
methylpyn-olo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylate (1:1)
o
N
O N O_
O ~ ~ O
N
H
~~+
N
OOH
To a suspension ofPyrrolo[3',2':5,6](1]benzopyrano[3,2-i]quinolizine-
I-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (6-carboxy-
2-pyridinyl)mcthyl ester (167.5 mg, 0.3522 mmol) in ethanol was added aqueous
choline bicarbonate (5.66 M, 0.056 mL, 0.32 mmol). The resulting mixture was
refluxed until a clear solution was obtained. The reaction mixture was
concentrated ijz vacaro, and the residue was dissolved in 2 mL of ethanol and
then
diluted with 70 mL of ether. After cooling in an ice-bath, the precipitate was
collected by filtration to give 0.15 g (74%) of the desired product as a
yellow
solid: mp 120-130°C; MS(APCI-): m/z 474.1 (M-H). Anal. Calcd for
C27H28N305'1.OCSH14N101~0.2CSH15N102M.8H20: C, 62.38; H, 7.71; N,
9.26. Found: C, 62.40; H, 7.58; N, 9.03.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-196-
Example 102
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [5-(methoxycarbonyl)-
3-pyridinyl]methyl ester
O
N O O
I
O / ~ ~ O
N~
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [5-(methoxycarbonyl)-
3-pyridinyl]methyl ester was synthesized according to procedure Q from
5-hydroxymethyl-nicotinic acid methyl ester. The EtOAc solution (40 mL) of the
crude product was mixed with 40 mL of 0.5 N HCl solution in a reparative
funnel
and shaken well, then the aqueous phase was basified with 2N NaOH solution to
pH = 1, shaken well. The two layers were then separated. After this process
was
repeated twice, the organic layer was separated and dried over MgS04.
Chromatography on a silica gel column (50-100% of EtOAc in hexanes as eluant)
followed by crystallization from ether gave 0.4254 g (25%) of the desired
product
as a yellow solid: mp 195-197°C; MS(APCI+): nz/z 490.1 (MH+).
Example 103
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (5-carboxy-3-pyridinyl)methyl
ester
O
N O OH
I
O / O
N II N
~H
To a solution ofPyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99/30434
-197-
[5-(methoxycarbonyl)-3-pyridinyl]methyl ester (391.6 mg, 0.7999 mmol) in
MeOH (30 mL) was added IN NaOH (3.2 mL, 3.2 mmol). The resulting reaction
mixture was stirred at 50°C for 60 minutes. After cooling down to
ambient
temperature, 3.2 mL of IN aqueous HCl was added, then concentrated iji vacuo.
The residue was purified by chromatography (10-30% MeOH in HCCl3 as eluant)
followed by trituration with ether to give 0.25 g (67%) of the desired product
as a
white solid: mp 224-227°C; MS(APCI-): rnlz 474.1 (M-H).
Example 104
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (5-carboxy-
3-pyridinyl)methyl 3,7,8,9,10,12,13,14,14a,15-decahydro-
2-methylpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylate (1:l)
0
N O O
I
O / O ~ i
N
N
H
~ ~.
,N
~ OH
To a suspension ofPyr-rolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (5-carboxy-
3-pyridinyl)methyl ester (170.3 mg, 0.3580 mmol) in ethanol was added aqueous
choline bicarbonate (5.66 M, O.OS69 mL, 0.322 mmol). The resulting mixture was
refluxed until a clear solution was obtained. The reaction mixture was
concentrated in vacuo, and the residue was dissolved in 2 mL of ethanol and
then
diluted with 70 mL of ether. After cooling in an ice-bath, the precipitate was
collected by filtration to give 0.163 g (79%) of the desired product as a
beige
solid: mp 147-152°C; MS(APCI-): m/z 474 (M-H). Anal. Calcd for
C27H28N305'1.OCSH14N101-0.28C4H100~1.2Si102M.4H20: C, 57.09; H,
6.89; N, 8.04. Found: C, 57.09; H, 6.70; N, 7.77.
CA 02372197 2001-07-12
WO 00/42045 PCTNS99/30434
-198-
Example 105
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4'-methyl[1,1'-biphenyl]-
3-yl)ethyl ester
H
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4'-methyl[I,l'-biphenyl]-
3-yl)ethyl ester was synthesized according to procedure Q from I-(4'-methyl-
biphenyl-3-yl)-ethanol. The crude product was chromatographed on a silica gel
column (40% of EtOAc in hexanes as eluant) to give 0.67 g (36%) of the desired
product as a white foam: mp 105-115°C; MS(APCI+): m/z 535 (MH+).
Example 106
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(2,6-dimethylphenyl)ethyl
ester
N o \
0
0
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(2,6-dimethylphenyl)ethyI
ester was synthesized according to procedure Q from 1-(2,6-dimethyl-phenyl)-
ethanol. The crude product was chromatographed on a silica gel column (30-50%
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-199-
of EtOAc in hexanes as eluant) to give 1.02 g of the desired product which was
impure as a yellow foam: mp 100-105°C; MS(APCI+): rnlz 473.3 (MH+).
Procedure S: procedure for the array synthesis:
The mixed anhydride (1 eq.) and the corresponding alcohol (2-4 eq.) were
mixed in a VWR 60826-202 tube. The tube was loosely capped and submerged in
oil-bath at 120°C until a clear solution was obtained (generally 5-7
minutes). After
cooling to ambient temperature, 6 mL of EtOAc and 5 mL of saturated aqueous
Na2S04 were added to the tube. The mixture was shaken and stin-ed for 1
minute,
then the organic layer was pipeted out and filtered through a pack of MgS04
(packed in a syringe filter) followed by washing with 1 mL of EtOAc. The
filtrate
was collected in a 2-dram vial and sample was blown dry with a stream of
nitrogen. The residue was chromatagraphed on a silica gel column using ISCO
system to give the desired product.
The Example 107 through Example 142 were made following Procedure S
in a parallel fashion:
Example 107
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S,2R)-2-(dimethylamino)-
1-phenylpropyl ester
\N'
N O
O
O
N
H
MS(APCI+): rn/z 502 (MH+)
CA 02372197 2001-07-12
WO 00142045 PCT/US99I30434
-200-
Example 108
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R,2S)-2-(dimethylamino)-
1-phenylpropyl ester
i
N
O~--O
i
w /~
MS(APCI+): m/z 502 (MH+)
Example 109
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-naphthalcnyl ester
i
i
N O
O
O
N
H
Ms(APCr+): nz/z 467 (MH+)
Example 110
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, diphenylmethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-201-
N
O
O
O
N
H
MS(APCI+): ntlz 507 (MH+).
Example 111
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R)-2,3-dihydro-1H-inden-1-yl
ester
w
N O
O
O
N
H
MS(APCI+): ntlz 457 (MH+)
Example 112
Pyrrolo[3',2':5,6][1]bcnzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,2-dihydro-1-acenaphthylenyl
ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-202-
N O
O
O
N
H
MS(APCI+): m/z 493 (MH+).
Example 113
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, cyclohexyl(phenyl)methyl ester
N
O
O
O
\
N
H
MS(APCI+): m/z 513 (MH+).
Example 114
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 9H-fluoren-9-yl ester
N O
O
O
N
H
MS(APCI+): nz/z 505 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-203-
Example 11 S
Pyrrolo(3',2':5,6][1]benzopyrano(3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,2,3,4-tetrahydro-
1-naphthalenyl ester
MS(APCI+): rnlz 471 (MH+)
Example 116
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [(2R,3R)-
3-phenyloxiranyl]methyl ester
/O1 _
N O ~,~~~n
O
O
N
H
MS(APCI+): m/z 473 (MH+).
Example 117
Pyrrolo[3',2':5,6)(1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-oxo-1,2-diphenylethyl ester
CA 02372197 2001-07-12
WO 00142045 PCT1US99130434
-204-
MS(APCI+): rrtlz 535 (MH+)
Example 118
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 10,11-dihydro-SII-
dibenzo[a,d]cyclohepten-5-yl ester
O
O
O
N
H
MS(APCI+): ntlz 533 (MH+)
Example 119
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (2-methylphenyl)phenylmcthyl
ester
CA 02372197 2001-07-12
WO 00/42045 PCTIUS99/30434
-205
N O i
O
O
N
H
MS(APCI+): rralz 521 (MH+)
Example 120
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14, I 4a,15-decahydro-2-methyl-,
cyclopropyl(4-fluorophenyl)mcthyl ester
N
O
O
O
/~
H
MS(APCI+): m/z 489 (MH+).
Example 121
Pyrrolo[3',2':S,6J[1]benzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, l 5-decahydro-2-methyl-, 3,4-dihydro-2H-
1-benzothiopyran-4-yl ester
i S
N
O~-O
O
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-206-
MS(APCI+): m/z 489 (MH+).
Example 122
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S)-1-(2-bromophenyl)ethyl
ester
Br
N
O
O
O
N
H
MS(APCI+): n~/z 524 (MH+)
Example 123
Pyrrolo[3',2':5,6](1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2,2,2-trifluoroethyl ester
F
~F
N ' IO
O F
O
N
H
MS(APCI+): nalz 423 (MH+).
Example 124
Pyrrolo[3',2':5,6][1]benzopyrano(3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [(2S,3S)-
3-phenyloxiranyl]methyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-207-
O
i"",
O
N
H
MS(APCI+): m/z 475 (MH+)
Example 125
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2,2,2-trifluoro-1-methyl-
1-(trifluoromethyl)ethyl ester
F
F FF
N O ~F
O F
O
N
H
Ms(aPCI+): ~~Z/~ sos (MH+).
Example 126
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2,2,2-trifluoro-
I-(4-fluorophenyl)-1-(trifluoromethyl)ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCTJUS99/30434
-208-
F
F
F F
F F
w 0,
F
MS(APCI+): m/z 585 (MH+).
Example 127
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-cyclopentyl-1-phenylethyl
ester
L /
N
H
MS(APCI+): m/z 513 (MH+).
Example 128
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-[1,1'-biphenyl]-4-yl-
1-methylethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99l30434
-209-
N O ~ / /
O '--i
O
N
H
MS(APCI+): mlz 535 (MH+).
Example 129
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-methyl-1-phenyl-2-propynyl
ester
..
/
N O
O
O
N
H
MS(APCI+): m/z 469 (MH+)
Example 130
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,1-diphenylethyl ester
N O
O
O
N
H
MS(APCI+): m/z 521 (MH+).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-210-
Example 131
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-methyl-1,2-diphcnylethyl
ester
w
N O
O
O
N
H
MS(APCI+): m/z 535 (MH+)
Example 132
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-fluorophenyl)cyclohexyl
ester
N O I \
O
O / F
I
N
H
MS(APCI+): nilz 517 (MH+).
Example 133
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,2-diphenylethyl ester
CA 02372197 2001-07-12
WO 00/42045
-211-
N O
O
O ~
N
I-I
MS(APCI+): »i/z 521 (MH+)
Example 134
PCT/US99/30434
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenyl-2-propynyl ester
N O
O
O
N
H
MS(APCI+): m/z 455 (MH+).
Example 135
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, [1,1'-biphenyl]-4-ylmethyl
ester
N O
O
O
N
H
MS(APCI+): nalz 507 (MH+).
CA 02372197 2001-07-12
WO 00/42045 PCTlUS99130434
-212-
Example 136
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 4-pyridinyhnethyl ester
y
N
N O
O
O
N
H
MS(APCI+): nt/z 432 (MH+).
Example 137
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1,2,3,4-tetrahydro-
7,8-dimethoxy-2-methyl-4-isoquinolinyl ester
H
MS(APCI+): ~aT/z 546 (MH+).
Example 138
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
1-[3-(dimethylamino)phenyl]ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-213-
H
MS(APCI+): m/z 488 (MH+).
Example 139
Pywolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1,1-dimethyl-2-pyrazinylethyl
ester
MS(APCI+): m/z 475 (MH+).
Example 140
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,1 S-decahydro-2-methyl-, 4-(dipropylamino)-
1,1-dimethyl-2-butynyl ester
N
~N
CA 02372197 2001-07-12
WO 00!42045 PCT/US99/30434
-214-
N O --
O N
O
/ N
H
MS(APCI+): m/z 520 (MH+)
Example 141
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decabydro-2-methyl-, (1S)-2,3-dihydro-IH-inden-I-yl
ester
N O
O
O
N
H
MS(APCI+): m/z 457 (MH+)
Example 142
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S,2S)-2-(dimethylamino)-
I-phenylpropyl ester
\N'
N O
O
O
/~T~~
MS(APCI+): m/z 502 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-215-
Example 143
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-,
[4-(trifluoromethyl)phenyl]methyl ester
CF3
N O
O
O /
N
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid
4-trifluoromethylbenzyl ester
CF3
O
O
HO
N
To a mixture of 5-hydroxy-2-methyl-1H-indole-3-carboxylic acid (4.S g,
23.54 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (3.58 g, 23.54 mmol) in
DMF (50 mL) was added 2'-bromo-2,2,2-trifluoro-p-xylene (6.2 g, 25.89 mmol).
The mixture was stirred at room temperature for 2 days and then partitioned
between ethyl acetate and water. The organic phase was washed with water and
brine, dried over Na2S04 and concentrated i~z vaci.~o to give a residue, which
was
recrystallized from ethyl acetate to give 4.26 g (S2%) of the desired product
as a
white solid: mp 224-225°C; MS(APCI+): m/z 350.1 (MII+); Anal. Calcd for
C18H14F3N103: C, 61.89; H, 4.04; N, 4.01. Found: C, 61.87; H, 4.00; N, 3.98.
Step B: 4-Dimethylaminomethyl-S-hydroxy-2-methyl-I H-indole-3-carboxylic
acid 4-trifluoro-methylbenzyl ester
CA 02372197 2001-07-12
WO 00142045 PCT/US99130434
-216-
CF3
O
/N O
O
N
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-trifluoromethylbenzyl
ester (3.0 g, 8.59 mmol) and aqueous dimethylamine (40%, 2.37 mL, 18.9 mmol)
were mixed with 6.7 mL of ethanol. The mixture was heated with a heatgun until
a clear solution was obtained. After cooled to ambient temperature, aqueous
HCHO (37%, 0.83 g, 10.31 mmol) was added. The resulting reaction mixture was
stirred at 50°C overnight. The reaction mixture was concentrated irt
vacavo to half
volume to give a solid, which was filtered. The solid was washed with ethanol-
water and dried ift vacuo to give 1.8 g (52%) of pure titled compound as an
off
white foam: MS(APCI+): m/z 407.2 (MH+)
Step C: Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
[4-(trifluoromethyl)phenyl]methyl ester
CF3
1 S To a mixture of perchlorate salt (1.37 g, 5.75 mmol, Example 3, step B)
and 100 mL of ether was added 150 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solids had dissolved. Two
layers were separated, and the aqueous layer was extracted with ether (2 x
50 mL). Combined ether layer was dried over Na2S04 and concentrated in vacuo.
The residual oil was dissolved in 25 mL of dioxane, then indole mannich base
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-217-
(1.8 g, 4.42 mmol) was added, the resulting reaction mixture was refluxed
under
nitrogen for 6 hours. The reaction mixture was cooled to ambient temperature
and
concentrated in vacuo affording a thick oil. The crude product was further
purified
by chromatography (10%-30% ethyl acetate in hexanes) to give 1.21 g (SS%) of
titled compound as a white foam: mp 97-99°C; MS(APCI+): m/z 499.2
(MH+);
Anal. Calcd for C28H29F3N203: C, 67.46; H, 5.86; N, 5.62; F, 11.43. Found: C,
67.13; H, 5.86; N, 5.45; F, 11.32.
Example 144
Pyrrolo[3',2':5,6)[1)benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (4-chlorophenyl)methyl ester
\ CI
N O
O
O
N
Step A: S-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-chlorobenzyl ester
Ci
0
0
HO
N
To a mixture of 5-hydroxy-2-methyl-1H-indole-3-carboxylic acid (5.0 g,
26.15 mmol) and 1,8-diazabicyclo[5.4.0)undec-7-ene (3.98 g, 26.15 mmol) in
DMF (50 mL) was added 4-chlorobenzyl bromide (5.9 g, 28.77 mmol). The
mixture was stirred at room temperature for 2 days and then partitioned
between
ethyl acetate and water. The organic phase was washed with water and brine,
dried over Na2S04 and concentrated in vacuo to give a residue, which was
recrystallized from ethyl acetate to give 5.0 g (61 %) of the desired product
as an
off white solid: mp 236-237°C; MS(APCI-): m/z 314.1 (M-H).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-218-
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid 4-chlorobenzyl ester
CI
O
/N O
HO
N
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 4-chlorobenzyl ester
(4.0 g, 12.7 mmol) and aqueous dimethylamine (40%, 3.5 mL, 27.8 mmol) were
mixed with 10.4 mL of ethanol. The mixture was heated with a heatgun until a
clear solution was obtained. After cooled to ambient temperature, aqueous HCHO
(37%, 1.24 g, 15.2 mmol) was added. The resulting reaction mixture was stirred
at
50°C overnight. The reaction mixture was concentrated in vact~o to give
a residue,
which was chromatographed using 100% ethyl acetate as eluant to give 2.3 g
(49%) of pure titled compound as an off white foam: MS(APCI+): m/z
373.2 (MH+).
Step C: Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
(4-chlorophenyl)methyl ester
Cl
N O
O
O
N
To a mixture of perchlorate salt (1.9 g, 8.02 mmol, Example 3, Step B) and
150 mL of ether was added 200 mL of aqueous NaOH (2N). The resulting mixture
was shaken in a separatory funnel until all solids had dissolved. Two layers
were
separated and the aqueous layer was extracted with ether (2 x 100 mL).
Combined
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-219-
ether layer was dried over Na2S04 and concentrated in vacuo. The residual oil
was dissolved in 30 mL of dioxane, then indole mannich base (2.3 g, 6.17 mmol)
was added, the resulting reaction mixture was refluxed under nitrogen for 6
hours.
The reaction mixture was cooled to ambient temperature and concentrated
in vacuo to give a thick oil. The crude product was further purified by
chromatography (20%-25% ethyl acetate in hexanes) to give 1.7 g (59%) of
titled
compound as a white solid: mp 220-221 °C; MS(APCI+): m/z 465.3 (MH+).
Example 145
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-, phenylmethyl ester
N O ~J
O
O
N
Step A: 5-Hydroxy-1H-indole-3-carboxylic acid benzyl ester
O a
O
HO
N
To a mixture of 5-hydroxy-1H-indole-3-carboxylic acid (4.5 g, 25.4 mmol)
and 1,8-diazabicyclo[5.4.0]undec-7-ene (3.87 g, 25.4 mmol) in DMF (50 mL) was
added benzyl bromide (4.78 g, 27.94 mmol). The mixture was stirred at room
temperature for 2 days and then partitioned between ethyl acetate and water.
The
organic phase was washed with water and brine, dried over Na2S04 and
concentrated in vacuo to give a residue, which was recrystallized from ethyl
acetate-hexanes to give 2.4 g (36%) of the desired product as an off white
solid:
mp 184-186°C; MS(APCI-): m/z 266.1 (M-H).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-220-
Step B: 4-Dimethylaminomethyl-5-hydroxy-1 H-indole-3-carboxylic acid benzyl
ester
O
/N O
HO
y
N
5-Hydroxy-1H-indole-3-carboxylic acid benzyl ester (2.3 g, 8.6 mmol) and
aqueous dimethylaminc (40%, 2.37 mL, 18.9 mmol) were mixed with 6.67 mL of
ethanol. The mixture was heated with a heatgun until a clear solution was
obtained. After cooled to ambient temperature, aqueous HCHO (37%, 0.84 g,
10.32 mmol) was added. The resulting reaction mixture was stirred at
50°C
overnight. The reaction mixture was concentrated in vacuo to give a residue,
which was chromatographed using 50%-100% ethyl acetate in hexanes as eluant
to give 2.16 g (77%) of pure titled compound as an off white foam: MS(APCI+):
m/z 325.3 (MH'~).
Step C: Pyrrolo[3',2':5,6)[1)benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9, I 0,12,13,14,14a,15-decahydro-, phenylmethyl ester
-
To a mixture of pcrchlorate salt (1.91 g, 8.02 mmol, Example 3, Step B)
and 150 mL of ether was added 200 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solids had dissolved. Two
layers were separated and the aqueous layer was extracted with ether (2 x
100 mL). Combined ether layer was dried over Na2S04 and concentrated
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-221-
in vacuo. The residual oil was dissolved in 30 mL of dioxane, then indole
mannich
base (2.0 g, 6.17 mmol) was added, the resulting reaction mixture was refluxed
under nitrogen for 6 hours. The reaction mixture was cooled to ambient
temperature and concentrated in vacuo to give a thick oil, which was
recrystallized from acetonitrile to give 1.2 g (47%) of titled compound as an
off
white solid: mp 255-257°C; MS(APCI+): m/z 417.3 (MH+).
Example 146
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12, I 3,14,14a,15-decahydro-, ethyl ester
Step A: 5-Hydroxy-IH-indole-3-carboxylic acid ethyl ester
O
O
HO
N
To a mixture of 5-hydroxy-1 H-indole-3-carboxylic acid (4.5 g, 25.4 mmol)
and 1,8-diazabicyclo[5.4.0]undec-7-ene (3.87 g, 25.4 mmol) in DMF (50 mL) was
added iodoethane (4.36 g, 27.94 mmol). The mixture was stirred at room
temperature overnight and then partitioned between ethyl acetate and water.
The
organic phase was washed with water and brine, dried over Na2S04 and
concentrated in vacuo to give a residue, which was recrystallized from ethyl
acetate-hexanes to give 2.2 g (42%) of the desired product as a light brown
solid:
MS(APCI+): m/z 206.2 (MH+).
Step B: 4-Dimethylaminomethyl-5-hydroxy-1H-indole-3-carboxylic acid ethyl
ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-222-
O
/N O
HO
N
5-Hydroxy-1H-indole-3-carboxylic acid ehtyl ester (2.1 g, 10.23 mmol)
and aqueous dimethylamine (40%, 2.83 mL, 22.51 mmol) were mixed with
7.7 mL of ethanol. The mixture was heated with a heatgun until a clear
solution
was obtained. After cooled to ambient temperature, aqueous HCHO (37%, 0.99 g,
12.28 mmol) was added. The resulting reaction mixture was stirred at
50°C
overnight. The reaction mixture was concentrated in vacuo to give a residue,
which was chromatographed using SO%-100% ethyl acetate in hexanes as eluant
to give 2.1 g (78%) of pure titled compound as a gum: MS(APCI+): zn/z
263.1 (MH+)
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-, ethyl ester
N O
O
N
To a mixture of perchlorate salt (2.36 g, 9.9 mmol, Example 3, Step B) and
1 SO mL of ether was added 250 mL of aqueous NaOH (2N). The resulting mixture
was shaken in a separatory funnel until all solids had dissolved. Two layers
were
separated and the aqueous layer was extracted with ether (2 x 100 mL).
Combined
ether layer was dried over Na2S04 and concentrated in vacuo. The residual oil
was dissolved in 30 mL of dioxane, then indole marmich base (2.0 g, 7.6 mmol)
was added, the resulting reaction mixture was refluxed under nitrogen for 6
hours.
The reaction mixture was cooled to ambient temperature and concentrated
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-223-
in vczcuo to give a thick oil, which was recrystallized from acetonitrile to
give
1.7 g (63%) of titled compound as an off white solid: mp 242-244°C;
MS(APCI+): m/z 355.3 (MH+).
Example 147
Pyrrolo[3',2':5,6J[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-hydroxyethyl ester
OH
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid hydroxyethyl ester
OH
HO
To a mixture of 5-hydroxy-2-methyl-1 H-indole-3-carboxylic acid (5.0 g,
26.15 mmol) and 1,8-diazabicyclo[5.4.0]undee-7-ene (3.91 g, 26.15 mmol) in
DMF (100 mL) was added 2-bromoethanol (3.6 g, 28.77 mmol). The mixture was
stirred at room temperature for 7 days and then partitioned between ethyl
acetate
and water. The organic phase was washed with water and brine, dried over
Na2S04 and concentrated in vacuo to give a residue, which was
choromatographed using 30%-100% ethyl acetate in hexanes as eluant to give
2.4 g (39%) of the desired product as a gum: MS(APCI+): m/z 236.1 (MH+).
Step B: 4-Dimethylaminomethyl-5-hydroxy-2-methyl-1H-indole-3-carboxylic
acid hydroxyethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-224-
OH
O
/N O
HO
N
5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid hydroxyethyl ester
(2.3 g, 9.8 mmol) and aqueous dimethylamine (40%, 2.7 mL, 21.5 mmol) were
mixed with 7.4 mL of ethanol. The mixture was heated with a heatgun until a
clear solution was obtained. After cooled to ambient temperature, aqueous HCHO
(37%, 0.95 g, 11.7 mmol) was added. The resulting reaction mixture was stirred
at
50°C overnight. The reaction mixture was concentrated in vacuo to give
a residue,
which was chromatographed using 100% ethyl acetate followed by 20% methanol
in methylene chloride as eluant to give 2.0 g (70%) of pure titled compound as
a
gum: MS(APCI+): ntlz 293.2 (MH+).
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-hydroxyethyl ester
OH
O
O
O
N
To a mixture of perchlorate salt (2.1 g, 8.9 mmol, Example 3, step B) and
150 mL of ether was added 250 mL of aqueous NaOH (2N). The resulting mixture
was shaken in a separatory funnel until all solids had dissolved. Two layers
were
separated and the aqueous layer was extracted with ether (2 x 100 mL).
Combined
ether layer was dried over Na2S04 and concentrated in vacuo. The residual oil
was dissolved in 25 mL of dioxane, then indole mannich base (2.0 g, 6.84 mmol)
was added, the resulting reaction mixture was refluxed under nitrogen for 6
hours.
The reaction mixture was cooled to ambient temperature and concentrated
CA 02372197 2001-07-12
WO 00142045 PCT/US99/30434
-225-
in vacuo to give a residue as thick oil, which was recrystallized from
acetonitrile
to give 1.6 g (61 %) of titled compound as a light brown solid: mp 221-
223°C;
MS(APCI+): m/z 385.2 (MH+).
Example 148
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (3-methylphenyl)methyl ester
N O
O
O
N
Step A: 5-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 3-methylbenzyl ester
O
O
HO
N
To a mixture of 5-hydroxy-2-methyl-1H-indole-3-carboxylic acid (4.0 g,
20.92 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (3.18 g, 20.92 mmol) in
DMF (100 mL) was added a-bromo-m-xylene (4.28 g, 23.1 mmol). The mixture
was stirred at room temperature for 7 days and then partitioned between ethyl
acetate and water. The organic phase was washed with water and brine, dried
over
Na2S04 and concentrated in vacuo to give a residue, which was chromatographed
using 20%-50% ethyl acetate in hexane as eluant to give 3.0 g (48%) of the
desired product as a tan solid: mp 165-167°C; MS(APCI+): m/z 296.2
(MH+).
Step B: 4-Dimethylaminomethyl-S-hydroxy-2-methyl-1H-indole-3-carboxylic
acid 3-methylbenzyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-226-
O
/N o
HO
N
S-Hydroxy-2-methyl-1H-indole-3-carboxylic acid 3-methylbenzyl ester
(2.7 g, 9.14 mmol) and aqueous dimethylamine (40%, 2.52 mL, 20.1 mmol) were
mixed with 7.4 mL of ethanol. The mixture was heated with a heatgun until a
clear solution was obtained. After cooled to ambient temperature, aqueous HCHO
(37%, 0.89 g, 10.97 mmol) was added. The resulting reaction mixture was
stirred
at 50°C overnight. The reaction mixture was concentrated in vacuo to
give a
residue, which was chromatographed using 50%-100% ethyl acetate in hexanes as
eluant to give 2.0 g (62%) of pure titled compound as a gum: MS(APCI+): m/z
353.3 (MH+).
Step C: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7, 8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
(3-mcthylphenyl)methyl ester
N O
O
O
N
To a mixture of perchlorate salt (1.75 g, 7.38 mmol, Example 3, step B)
and 100 mL of ether was added 150 mL of aqueous NaOH (2N). The resulting
mixture was shaken in a separatory funnel until all solids had dissolved. Two
layers were separated and the aqueous layer was extracted with ether (2 x 50
mL).
Combined ether layer was dried over Na2S04 and concentrated in vacuo. The
residual oil was dissolved in 25 mL of dioxane, then indole mannich base (2.0
g,
CA 02372197 2001-07-12
W0 00142045 PCT/US99/30434
-227-
5.67 mmol) was added, the resulting reaction mixture was refluxed under
nitrogen
for 6 hours. The reaction mixture was cooled to ambient temperature and
concentrated in vacuo affording a thick oil. The crude product was further
purified
by chromatography (10%-25% ethyl acetate in hexanes) to give 1.8 g (55%) of
titled compound as a white solid: mp 80-82°C; MS(APCI+): m/z 445.4
(MH+).
Example 149
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (IS)-1-phenylethyl ester
N O
O
O
N
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (IS)-1-phenylethyl ester was
synthesized according to procedure Q from (S)-(-)-1-phenylethanol. The crude
product was chromatographed using 30% ethyl acetate in hexanes as eluant to
give 75 mg of the desired product as a white solid: mp 98-100°C;
MS(APCI+):
mlz 445.2 (MH+).
Example 150
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-phenylethyl ester
N O
O
O
N
CA 02372197 2001-07-12
WO 00/42045 PCT/tJS99/30434
-228-
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, I-phenylethyl ester was
synthesized according to procedure Q froml-phenylethanol. The crude product
was chromatographed using 40% ether in hexanes followed by 50% ethyl acetate
in hexanes as eluant to give 480 mg of the desired product as a white solid:
mp
89-90°C; MS(APCI+): m/z 445.2 (MH+).
Example 151
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carbothioic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, S-(phenylmethyl) ester
-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carbothioic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, S-(phenylmethyl) ester was
synthesized according to procedure Q from benzyl mercaptan. The crude product
was chromatographed using 50% ether in hexanes as eluant to give 150 mg of the
desired product as a white solid: MS(APCI+): m/z 447.1 (MH+)
Example 152
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-pyridinylmethyl ester
N O ~ N
O
O
N
CA 02372197 2001-07-12
WO 00142045 PCT1US99/30434
-229-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1 S-decahydro-2-methyl-, 3-pyridinylmethyl ester was
synthesized according to procedure Q from 3-pyridylcarbinol. The crude product
was chromatographed using 50% ethyl acetate in hexanes followed by 10%
methanol in ethyl acetate as eluant to give 400 mg of the desired product as a
white solid: MS(APCI-): m/z 430.1 (M-H).
Example 153
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7, 8,9,10,12,13, I 4,14a,15-decahydro-2-methyl-,
1-[4-(trifluoromethyl)phenyl]ethyl ester
''. CFs
N O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-[4-(trifluoromethyl)phenyl]-
ethyl ester was synthesized according to procedure Q from 4-trifluoro-a-
methylbenzyl alcohol. The crude product was chromatographed using 40%-50%
ether in hexanes as eluant to give 180 mg of the desired product as a white
solid:
mp 104-106°C; MS(APCI-): m/z 511.1 (M-H).
Example 154
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13, I 4,14a,15-decahydro-2-methyl-, 1-(pentafluorophenyl)ethyl
ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-230-
F
F
F
N O
~F
O
N
Pyrrolo[3',2'a,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1 s-decahydro-2-methyl-, 1-(pentafluorophenyl)ethyl
ester
was synthesized according to procedure Q from pentafluoro-a-methylbenzyl
alcohol. The crude product was chromatographed using 40% ether in hexanes as
eluant to give 160 mg of the desired product as a white solid: mp 93-
9s°C;
Ms(APCI+): ~n/Z s3s.1 (MH+)
Example 1s5
Pyrrolo[3',2'a,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1s-decahydro-2-methyl-, 1-(2,6-difluorophcnyl)ethyl
ester
F
N O
O F/
O
N
Pyrrolo[3',2'a,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1s-decahydro-2-methyl-, 1-(2,6-difluorophcnyl)ethyl
ester was synthesized according to procedure Q from 2,6-difluoro-a-
methylbenzyl
alcohol. The crude product was chromatographed using 40% ether in hcxanes as
eluant to give 180 mg of the desired product as a white solid: mp 8s-
87°C;
MS(APCI+): m/z 481.1 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-231-
Example 156
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I 5-decahydro-2-methyl-, ( 1 S)-1-(2-furanyl)ethyl
ester
O
~N O~O
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, ( 1 S)-1-(2-furanyl)ethyl
ester
was synthesized according to procedure Q from S(-)-1-(2-furyl)ethanol. The
crude
product was chromatographed using 40%-60% ether in hexanes as cluant to give
300 mg of the desired product as a white solid: mp 84-86°C; MS(APCI+):
m/z
435.1 (MH+)
Example 157
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(4-morpholinyl)-
1-phenylethyl ester
O
N
~O
~ ~N
Pyrrolo[3',2':5,6][lJbcnzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-(4-morpholinyl)-
1-phenylethyl ester was synthesized according to procedure Q from a-phenyl-
CA 02372197 2001-07-12
WO 00!42045 PCT/US99/30434
-232-
4-morpholineethanol. The crude product was chromatographed using 40%-60%
ether in hexanes as eluant to give 130 mg of the desired product as a white
solid:
mp 251-252°C; MS(APCI-): m/z 528.2 (M-H).
Example 158
Pyrrolo[3',2':5,6](1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R)-1-(2-furanyl)ethyl ester
O
~N \\,O
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R)-1-(2-furanyl)ethyl ester
was synthesized according to procedure Q from R(+)-1-(2-furyl)ethanol. The
cnide product was chromatographed using 40%-60% ether in hexanes as eluant to
give 300 mg of the desired product as a white solid: mp 79-81 °C;
MS(APCI+):
ntlz 435.1 (MH+).
Example 159
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-methoxy-2-oxo-1-phenylethyl
ester
O'
O
N~ ~ O
O / \
i
N
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-233-
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-methoxy-2-oxo-1-phenylethyl
ester was synthesized according to procedure Q from (S)-(+)-methyl mandelate.
The crude product was chromatographed using 40%-60% ether in hexanes as
eluant to give 309 mg of the desired product as a white solid: mp 103-
105°C;
MS(APCI+): m/z 489.2 (MH+).
Example 160
Pyn-olo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(3-pyridinyl)ethyl ester
p ~N
\\r O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(3-pyridinyl)ethyl ester was
synthesized according to procedure Q from 1-(3-pyridyl)ethanol. The crude
product was chromatographcd using 50% ether in hexanes followed by 10%
methanol in methylene chloride as eluant to give 300 mg of the desired product
as
a white solid: mp 78-80°C; MS(APCl+): fnlz 446.2 (MH+).
Example 161
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (S)-carboxy(phenyl)methyl
ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-234-
OH
O
N O
O
O
N
To a solution ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-methoxy-
2-oxo-1-phenylethyl ester (650 mg, 1.33 mmol) in methanol (60 mL) was added
S 1N NaOH solution (5.32 mL, 5.32 mmol). The resulting reaction mixture was
stirred at 50°C for 1 hour, then concentrated iu vaczro. The residue
was purified by
chromatography (10-30% methanol in chloroform as eluant) to give 0.58 g of a
mixture of free acid and the sodium salt. All of the mixture (0.58g) was
dissolved
in methanol-chloroform, then mixed with 0.53 mL of 1N HCI. The mixture was
concentrated in vacuo, the residue was purified by chromatography (10-30%
methanol in chloroform as eluant) followed by recrystallization from methanol
to
give 0.35 g of the desired product as a white solid: mp 250-251 °C;
MS(APCI-):
nZ/z 473.1 (M-H), Anal. Calcd for C2gH3pN205~0.36H20: C, 69.91; H, 6.44; N,
5.82. Found: C, 69.96; H, 6.33; N, 5.59.
Example 162
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (S)-
carboxy(phenyl)methyl, 3,7,8,9,10,12,13,14,14a,15-decahydro-
2-methylpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylate (1:1)
O
O-:
N O
\\r O
p / H3C\N Ha
H3C~ ~OH
N
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-235-
To a suspension of pyrrolo[3',2':5,6] [ 1 ]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-,
(S)-carboxy(phenyl)methyl ester (181 mg, 0.38 mmol) in ethanol (5 mL) was
added aqueous choline bicarbonate (5.66 M, 0.06 mL, 0.34 mmol). The resulting
mixture was refluxed until a clear solution was obtained. The reaction mixture
was concentrated in vacuo, and the residue was dissolved in 2 mL of ethanol
and
then diluted with 70 mL of ether. After cooling in an ice-bath, the
precipitate was
collected by filtration to give 0.17 g (77%) of the desired product as an off
white
solid: mp 223-225°C; MS(APCI+): m/z 475.2 (MH+). Anal. Calcd for
C2gH29N205~C5H14NOM.2H20: C, 66.13; H, 7.64; N, 7.01. Found: C, 65.96;
H, 7.56; N, 6.78.
Example 163
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1R)-2-methoxy-2-oxo-
1-phenylethyl ester
O'
O
N O
O
O /
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1R)-2-methoxy-2-oxo-
1-phenylethyl ester was synthesized according to procedure Q from (R)-(-)-
methyl
mandelate. The cnide product was chromatographed using 40%-60% ether in
hexanes as eluant to give 750 mg of the desired product as a white solid: mp
106-108°C; MS(APCI+): m/z 489.2 (MH+).
CA 02372197 2001-07-12
WO 00/42045 PCT/IJS99/30434
-236-
Example 164
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (R)-carboxy(phenyl)methyl
ester
OH
O
N O
\\~ O
N
To a solution of pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R)-
2-methoxy-2-oxo-1-phenylethyl ester (670 mg, 1.37 mmol) in methanol (60 mL)
was added 1N NaOH (5.5 mL, 5.5 mmol). The resulting reaction mixture was
stirred at 50°C for 1 hour and 1N HCl (5.5 mL) was added. The mixture
was
concentrated in vacuo to give a residue, which was purified by chromatography
(10-30% methanol in chloroform as eluant) followed by recrystallization from
methanol to give 0.35 g of the desired product as a white solid: mp 248-
250°C;
MS(APCI-): n~lz 473.1 (M-H).
Example 165
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with (R)-
carboxy(phenyl)methyl, 3, 7,8,9,10,12,13,14,14x,15-decahydro-
2-methylpyn-olo[3',2':5,6][1]benzopyrano[3,2-i)quinolizine-1-carboxylate (l:1)
O
O-
O
\\-O
O
~OH
N
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-237-
To a suspension ofpyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-
1-carboxylic acid, 3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (R)-
carboxy(phenyl)methyl ester (210 mg, 0.44 mmol) in ethanol (5 mL) was added
aqueous choline bicarbonate (5.66 M, 0.07 mL, 0.40 mmol). The resulting
mixture
was refluxed until a clear solution was obtained. The reaction mixture was
concentrated in vacuo, and the residue was dissolved in 2 mL of ethanol and
then
diluted with 70 mL of ether. After cooling in an ice-bath, the precipitate was
collected by filtration to give 0.2 g (78%) of the desired product as an off
white
solid: mp 215-217°C; MS(APCI+): m/z 475.2 (MH+). Anal. Calcd for
C28H2c~N205~C5H14N0~1.8H20: C, 64.96; H, 7.7.70; N, 6.89. Found: C, 65.04;
H, 7.59; N, 6.55.
Example 166
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 4-pyridinylmethyl ester
w
/N
N O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 4-pyridinylmethyl ester was
synthesized according to procedure Q from 4-pyridylcarbinol. The crude product
was chromatographed using 50% ethyl acetate in hexanes followed by 100% ethyl
acetate as eluant to give 260 mg of the desired product as a white solid: mp
199-201 °C; MS(APCI+): m/z 432.5 (MH+).
Example 167
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-pyridinyl)ethyl ester
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-238-
w
/N
N O
O
O
N
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(4-pyridinyl)ethyl ester was
synthesized according to procedure Q from 1-(4-pyridyl)ethanol. The crude
product was chromatographed using 50% ethyl acetate in hexanes followed by
100% ethyl acetate as eluant to give 210 mg of the desired product as a white
solid: mp 219-221 °C; MS(APCI+): m/z 446.2 (MH+).
Example 168
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I 5-decahydro-2-methyl-, I -(2-pyridinyl)ethyl ester
N O N
O
O /
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(2-pyridinyl)ethyl ester was
synthesized according to procedure Q from I-(2-pyridyl)ethanol. The crude
1 S product was chromatographed using 50%-75% ethyl acetate in hexanes as
eluant
to give 200 mg of the desired product as a white solid: mp 87-89°C;
MS(APCI+):
ntlz 446.2 (MH+).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-239-
Example 169
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-thienylmethyl ester
N O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 3-thienylmethyl ester was
synthesized according to procedure Q from 3-thiophenemethanol. The crude
product was chromatographed using 50% ether in hexanes as eluant to give
330 mg of the desired product as a white solid: mp 115-116°C;
MS(APCI+): m/z
437.5 (MH+).
Example 170
Pyrrolo[3',2':5,6][lJbenzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (IS)-I-(4-fluorophenyl)ethyl
ester
F
N \~ O
O
I
~N
Pyrrolo[3',2':5,6][1]benzopyrxno[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S)-1-(4-fluorophenyl)ethyl
ester was synthesized according to procedure Q from S(-)-1-(4-
florophenyl)ethanol. The crude product was chromatographed using 30%-50%
ether in hexanes as eluant to give 300 mg of the desired product as a white
solid:
mp 108-I 10°C; MS(APCI+): ntlz 463.3 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-240-
Example 171
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1R)-1-(4-fluorophenyl)ethyl
ester
' F
,.
-O
~/ ~ N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (IR)-1-(4-fluorophenyl)ethyl
ester was synthesized according to procedure Q from R(+)-1-(4-
florophcnyl)ethanol. The crude product was chromatographed using 30%-50%
ether in hexanes as eluant to give 300 mg of the desired product as a white
solid:
MS(APCI+): m/z 463.3 (MH+).
Example 172
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-pyridinylmethyl ester
N O ~N
O
O
~I
'N
Pyrrolo[3',2':5,6)[I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-pyridinylmethyl ester was
synthesized according to procedure Q from 3-pyridylcarbinol. The crude product
was chromatographed (1:1 hexane/EtOAc) to give 1.43g (49.4%) of desired
product as a solid: mp 73-80°C; MS(APCI +): m/z 432.6 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT1US99/30434
-241-
Example 173
Pyrrolo[3',2':5,6)[1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-ethoxy-2-oxoethyl ester
N
O
O ~ O~O~
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-ethoxy-2-oxoethyl ester was
synthesized according to procedure Q from ethyl glycolate. The crude product
was
chromatographed (I :1 hexane/EtOAc) to give 0.52878 (36.8%) of desired product
as a solid: mp 60-70°C; MS(APCI +): m/z 427.2 (MH+).
Example 174
Pyridinium, 3-[[[(3,7,8,9,10,12,13,14,14a, I 5-decahydro-2-
methylpywolo[3',2':5,6] [ 1 ]benzopyrano[3,2-i]quinolizin-1-
yl)carbonyl]oxy]methyl]-1-methyl-, methanesulfonate
N O ~ N~
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13, I 4,14a,15-decahydro-2-methyl-, 3-pyridinylmethyl ester ( 1
eq)
and methyl methanesulfonate (4 eq) was mixed together in 1,2 dichloroethane
and
refluxed at 88°C for 30 minutes. After cooling down to ambient
temperature the
precipitate was isolated by suction filtration and washed with ether to give
86m8
(41.2%) of pure desired product as crystals: mp 228-232°C; MS(APCI +):
m/z
446.2 (M+).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-242-
Some of the compounds of ring close Formula I are capable of further
forming N-oxides and N-quaternary alkyl salts with the ring nitrogen atom at
N-I 1. Further, some of the compounds of ring close Formula I are capable of
further forming N-oxides and N-quaternary alkyl salts with nitrogen atoms
optionally present in R10. These structural forms are within the scope of the
present invention.
Example 175
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizinium,
3,7,8,9,10,12,13,14,14a,15-
dccahydro-2,I 1-dimethyl-1-[(S)-(1-phenylethoxy)carbonyl]-, methanesulfonate
~N + O
O
O
N
Pyrrolo[3',2':S,G][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (1S)-1-phenylethyl ester (0.6
g,
1.349 mmol) and methyl methanesulfonate (1.089 g, 10.7964 mmol) were mixed
together in 1,2 dichlorocthane (8m1) and refluxed at 88°C for 18 hours.
The
IS reaction mixture was concentrated I71 VC1C110. The crude product was
chromatographed (2:10 MeOH/CH2C12) to give 0.41 g (G 1.5%) of desired product
as a solid: mp 160-170°C;
MS(APCI +): m/z 459.3 (M+).
Some of the compounds of ring close Formula I are capable of further
forming N-oxides and N-quaternary alkyl salts with the ring nitrogen atom at
N-1 I . Further, some of the compounds of ring close Fornmla I are capable of
further forming N-oxides and N-quaternary alkyl salts with nitrogen atoms
optionally present in RI O. These structural forms are within the scope of the
present invention.
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-243-
Example 176
Pyrrolo[3',2':5,6][1)benzopyrano[3,2-i)quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-furanylmethyl ester
-1
N o ~ °
0
0
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 3-furanylmethyl ester was
synthesized according to procedure Q from furan-3-yl-methanol. The crude
product was chromatographed (I:1 hexane/EtOAc) to give 0.40 g (42.4%) of
desired product as a solid: mp 174-177°C; MS(APCI +): m/z 421.4 (MH+).
Example 177
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, (2-nitrophenyl)methyl ester
O
I+
O /N
i
N O
O
O
~I
'N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a, I 5-decahydro-2-methyl-, (2-nitrophenyl)methyl ester
was
synthesized according to procedure Q from 2-nitrobenzylalcohol. The crude
product was chromatographed (1:1 hexane/EtOAc) to give 0.39 g (35.9%) of
desired product: mp 200-203°C; MS(APCl +): m/z 476.5 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99130434
-244-
Example 178
Pyrrolo(3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,1 S-decahydro-2-methyl-, 2-furanylmethyl ester
N O O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 2-furanylmethyl ester was
synthesized according to procedure Q from furan-2-yl-methanol. The crude
product was chromatographed (I :1 hexane/EtOAc) to give 0.24 g (24.8%) of
desired product as a solid: mp 65-77°C; MS(APCI +): m/z 421.4 (MH+).
Example 179
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-chlorophenyl)ethyl ester
CI
N O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,1S-decahydro-2-methyl-, 1-(2-chlorophenyl)ethyl ester
was synthesized according to procedure Q from 1-(2-chlorophenyl) ethanol. The
crude product was chromatographed (1:1 hexane/cthcr) to give 80 mg (S.0%) of
desired product as a solid: mp 95-105°C; MS(APCI +): m/z 479.2 (MH+)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-245-
Example 180
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, carboxymethyl ester
O
N O / -O
/ 1 ~O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizinc-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 2-ethoxy-2-oxoethyl ester
(0.47 g, 0.96 mmol) was mixed with 1N NaOH (5.73 mmol, 5.73 mL) in MeOH
and kept it in a oil bath at 50°C for 3 hours. Then the reaction
mixture was cooled
to ambient temperature followed by addition of 1N HC1 to neutralize. The
reaction
mixture was concentrated in vacuo, and the erode product was chromatographed
(initially 1:1 hexane/ether, then 10% MeOH in CHCl3) to give 0.3748 of cmde
solid. The crude solid was redissolved in CHCl3 and filtered to get 78 mg
(20.6%)
of desired product as a solid: MS(APCI +): mp 260°C; m/z 399.1 (MH+)
Example 181
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(2,6-dichlorophenyl)ethyl
ester
CI
N o
o
CI
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(2,6-dichlorophenyl)ethyl
ester was synthesized according to procedure Q from 1-(2,2-dichloro phenyl)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-246-
ethanol. The crude product was chromatographed ( 1:1 hexane/ether) to give
93 mg (5.30%) of desired product as a solid: mp 130-135°C; MS(APCI +):
m/z
513.1 (M+)
Example 182
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-I-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-methoxyphenyl)ethyl ester
O
N O
O
O
N
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, 1-(2-methoxyphenyl)ethyl ester
was synthesized according to procedure Q from 1-(2-methoxyphenyl) ethanol.
The crude product was chromatographed (1:1 hexane/ether) to give 0.90 g (42%)
of desired product as a solid; mp I 15-125°C; MS(APCI +): m/~ 475.2
(MH+).
Example 183
Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S)-1-phenylethyl ester,
11-oxide
o~N+ o
0
o
Pyrrolo[3',2':5,6][I]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14x,15-decahydro-2-methyl-, (1S)-1-phcnylethyl ester (0.3
g)
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-247-
was mixed with 50% H202 in MeOH and the mixture was stirred at room
temperature for 3 hours. Then excess H2O2 was destroyed using Pd/C. The
reaction solution was filtered and concentrated in vacuo. The crude product
was
chromatographed (10% MeOH in CH2CI2) to give 52 mg (48.2%) ofpure desired
product as a solid: mp 180-190°C; MS(APCI +): m/z 461.2 (MH+).
Some of the compounds of ring close Formula I are capable of further
forming N-oxides and N-quaternary alkyl salts with the ring nitrogen atom at
N-11. Further, some of the compounds of ring close Formula I are capable of
further forming N-oxides and N-quaternary alkyl salts with nitrogen atoms
optionally present in R10. These structural forms are within the scope of the
presentinvention.
Example 184
Pyrrolo[3',2':5,6J[lJbenzopyrano[3,2-iJquinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-, 1-(5-carboxy-3-pyridinyl)ethyl
ester
O
O
N p ~ N
O
O
N
Step A: 1-(5-Bromo-pyridin-3-yl)-ethanone
3-(5-Bromo-pyridin-3-yl)-3-oxo-propionic acid methyl ester (8.75 g,
30 mmol) was mixed with 2N H2S04 and the mixture was refluxed for 24 hours.
The reaction solution was then neutralized with solid NaHC03 and extracted
with
ether. The organic phase was dried and ether was evaporated to give 6.06 g
(89.5%) of the pure desired product as crystals: MS(APCI +): m/z 201 (MH+).
CA 02372197 2001-07-12
WO 00/42045 PCT/US99/30434
-248-
Step B: 1-(5-Bromo-pyridin-3-yl)-ethanol
Compound 1-(5-bromo-pyridin-3-yl)-ethanone (5.85 g, 29.2 mmol) was
dissolved in MeOH (100 mL) and NaBH4 (1.104 g, 29.19 mmol) was added
slowly. The reaction was monitored by TLC. When the starting material was
completely consumed mixture was neutralized by NaHC03 solution and extracted
with ether to give the crude product. Crude product was mixed with 1:1 hexane/
ethyl acetate and filtered. The filtrate was concentrated to give the pure
desired
product. MS(APCI +): m/z 203 (MH+).
Step C: 5-(1-Hydroxy-ethyl)-nicotinic acid methyl ester
1-(5-Bromo-pyridin-3-yl)-ethanol (6.0 g), Palladium acetate (0.14 g),
DPPP (0.28 g), Triethylamine(6 mL), DMSO (60 mL), and MeOH (60 mL) were
mixed together and the reaction mixture was stirred at 100-110°C for 18
hours.
End of the reaction mixture was filtered to remove the solids and the filtrate
was
concentrated under vacuum. The residue was triturated with EtOAc and filtered,
the filtrate and the solid were collected. The solid was dissolved in NaI-IC03
and
extracted with EtOAc. The extracts and the filtrate were combined together and
dried over Na2S04. Concentration in vacuo gave the crude product which was
chromatographed with 1:1 hexane/EtOAc to give 3.32 g (61.8%) of the pure
desired product: MS(APCI+): m/z 182 (MH+).
Step D: Pyrrolo[3',2':5,6][1]benzopyrano[3,2-i]quinolizine-1-carboxylic acid,
3,7,8,9,10,12,13,14,14a,15-decahydro-2-methyl-,
1-[5-(methoxycarbonyl)-3-pyridinyl]ethyl ester
O
O
N O ~ N
O
O
N
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPRI~:ND PLUS D'UN TOME.
CECI EST L,E TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter 1e Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional valumes please contact the Canadian Patent Office.