Note: Descriptions are shown in the official language in which they were submitted.
CA 02372639 2002-03-07
DEUTERATED CYCLOSFORINE ANALOGS AND THEIR USE AS IMMUIYOMODULATING
AGENTS
This application is divided from Canadian Patent Application 2, 298,572, tiled
October 8,1998.
Cyclosporin derivatives of the present invention are disclosed which possess
enhanced efficacy and
reduced toxicity over naun~ally occurring and other presently known
cyclosporins and cyclosporine derivatives.
The cyclosporin derivatives of the present invention are produced by chemical
and isotopic substiwtion of the
cycIosporine A (CsA) molecule by:
I. Chemical substitution and optionally deuterium substitution of amino acid
I; and
2. Deuterium substitution at key sites of metabolism of the cyclosporine A
molecule such
as amino acids 1, 4, 9.
The most acri~ie derivatives of the invention were those possessing both
chemical and deuterium
substitution.
The cyclosporins are a family o~ neutral, hydrophobic cyclic undecapepddes,
containing a novel
nine-carbon amino acid (MeBmt) at position 1 of the ring that exhinit potent
immunosuppressive, antiparasitic,
fungicidat, and chronic anti-iafIammatory;properties. The naturally occurring
members of this icy of
structurally related compounds are produced by various fungi impcrfecti.
CycIosporines A and C; are the major
coniponeats. Cyclosporine A, which is discussed further below, is a
particularly important member of the
cycIosporin family of compounds. Twenty four minor metabolites, also
oligopeptides, have been identified:
Lawen et aI,_J. Antcbiotics 42,1283 (I989); Traber et sI, Helv. Chim. Acta 70,
13 (1987); Von VJartburg and
20. Traber Prop. Med: Chem., 25,1 (I988).
Isolation of cyclosporines A and C, as well as the structure of A were
reported by A. Raegger d sL,
Heiv. Chim. Acts 59, I075(19,76); M. Dreyfuss et al., J. Appl. MierobioL 3,125
(197f~ Crystal and molecular
structures of the iodo derivative of A have been reported by T. J. Petcher ei
al., Helv. Chim. Acts 59, 1480
ZS (1976). The strucuue of C was reported by R Traber et al., tbid. 60;1247
(I977j. Production of A and C has
been reported by E. Harri ct aL, U.S. Pat. No. 4, I 17, I I8 (1978 to Saadoz~
Isolation, characta~ization and
aatifungal activity of H, D, E, as well as the structures of A through D nave
been reported by R Traber of al.,
Helv. Chief. Acts 60,1568(1977). Isolation and siructurts ofE, F, G, H, I:
cidem, ibid. 65, 1655 (1982).
Preparation of (2-Deutcro-3-fluoro-D-Alaj=-CsA is disclosed by Paxchett d a1
in GB 2,206, I99A which was
30 published on Dec. 29, 1988.
Cyclosporin was discovered to be immunosuppressive when it was observed to
suppress ant:'body
production in mice during the screening of fungal extracu. Specifically, its
suppressive effects appear to be
retazed to the inhibition of T-cell receptor-tnodiated activation events. It
accomplishes this by iniarupting
calcium dependent signal transduction during T-cell activation by inactivating
eahaodulin and eyelophdin, a
CA 02372639 2002-03-07
peptidly propyi isomerase. It also inhibits IymphoIcine production by T:
helper cells in vitro and arrests the
development of mature CD8 and CD4 cells in the thymus. Other in vitro
properties include inhibition of IL~2
producing T-lymphocytes and cytotoxic T lymphocytes, inhibition of IL 2
released by activated T-cells,
inhibition of resting T-Iyrnphocytes in response to alloantigen and exogenous
lymphokine, inhibition of IL-I
production, and inIn'bition of m'rtogen activation of IL-2 producing T-
lymphoCytes..Further evidence indicates
that the above effects involve the T-lymphocytes at the activation and
mauuatio~ stages.
Stimulation of TCR (T cell receptor) by foreign antigen on a major
histocampat~t~ity (MHC}
molecule on the surface of the T cell results in the aemration of a TCR signal
transmission pathway (exact
method of transmission unlatown) through the cytoplasm causing the signal
results in the activation of nuclear
ID transcription factors, i.e. nuclear factors of activated T-cells (I~IF-AT}
which regulate transcription of T-cell
activation genes. These genes include that of lyraphokine imerleulin 2 (IL-2~
Translation of the message is
followed by secretion of.IL-2. T-cell activation also involves the appearance
of the Iyrapholcine receptor IL 2R
on the cell srface. After iL. 2 binds to IL-2It, a lymphokine receptor (LKR}
signal transmission pathway is
activated. Tlie immunosuppressive drug, rapamycim, inht'bits this pathway.
15 CsA is a potent inhibitor of TCR-mediated signal transduction pathway. It
inhibits binding of NF AT
to the IL 2 enhances, and thus inhibits transcriptions! activation. CsA binds
to cyclophilin, which binds to
calcineurin, which is a key enzyme in the T-cell signal transductioa cascade:
Cycloplu'lin is found in prokaryotic and eukarotic organisms and is ubiduitous
and abundant.
. Cyclophilin is a single poiypeptide chain vrith 165 amino acid residues. It
has a molecular mass of I7.8 kD. A
20 roughly spherical molecule with a radius of I 7 angstroms, cyclophilin has
a eight-stranded antiparallel beta
barrel. Inside the barrel, the tightly pgcked core contains mostly hydrophobic
side chains: CsA has numerous
hydrophobic side chains which allow it to fit into the cyclophiIin beta
barrel. Cyclophillin catalyzes the
interconversion of the cis and traps-rotamets of the peGIFdyI-prolyl amide
bond of peptide and protein
substrates. Cyclophilin is identical in structure with peptidyl prolyl cis-
traps isomerase and bears structural
25 resemblance to the superfamily of proteins that transports Iigands such as
retinoi-binding protein (RBP}. These
proteins carry the ligand in the barrel core. But cyclophiIin actually carries
the ligand binding site on the
outside of the barnl. The tetrapepdde ligand binds in a long deep groove on
the proteia surface between one
face of the beta barrel and the Thrl ld-GIy130 loop.
Further properties have also been reported in studies of the biological
activity of CsA: 3. F. Borel et
30 al., Agents tlctions 6, 468 (1976). Pharmacology: Eidem. immunology 32,
l01? (i977}; R Y. CaIne, Clin.
F.xp. Immunol. 35,1 (I979). human studio: R. Y. Calve et al., Lancet
2,1323(1978); R L: Powles et aL, bid.
1327; R. L. Powles ei aL, ibid 1, 327 (I980). In vitro activity (porcine T-
ceiLs): D. J. White et at.,
Transplantation 2?, 55 (1979}. Effects on human lymphoid and myeloid cells: M.
Y. Gordon, J. W. Singer,
Afature 279, 433(1979}. Clinical study of CsA in graft-versus-host disease: P.
J. Tutschka et aL,'Hlood 61,
35 3i8(i983).
Mechanism of Cvclosoorine A Action
Cyclosporine A-CycIophilin A coneplex
CsA, as discussed above, binds to the cyciophdin beta barrel. Thirteen CyP A
residues define the
CA 02372639 2002-03-07
CsA binding site. These residues are Arg 55, Phe b4, Met 6I, Gln 63, Gly 72,
AIa I01, Asn 102, AIa 143, GIa
i i i, Phe 1 I3, Trp 121, Leu 122, Isis 128. The largest side-chaixt movements
are I .3 A for Arg 55 and np to
0.7 A for Phe 60, Gln 63, and Trp I2I. There are four direct hydrogen bonds
between the CyP A aad CsA.
Residues 4, 5, '6, ?, 8 of CsA protrude out into the solvem and are thought to
be involved in binding the
effecior protein, salcineurin (Pflugl, G., KalIen, J., Schirmer,.T.,
Jansonius, J.H., Zurini, M.G:M., F~ .
Walkinshaw, M.Dc (1993)Namre 36I, 91-94.)
Function of CsA-CyP A complex..
The C$A-CyP A complex inhibits the phosphatase activity of the heterodimeric
protein serine!
threonine phosplzatase or cakineurin (I:iu, J., Farmer, J.D., Lane, W.S.,
Friedmaatt, J:, Weissrnan, L, &
Schreiber, S.L. (1991) Celt 66, 807-IS:; Swatuon, S.K., Born, T., Zydowsky,
C.D., Cho, H., Chang, Fi.Y., &
Walsh, C.T. (I992) Proc. Nail. Acad. Sci:USA 89, 368&90): CyP A binds CsA with
an amity of ca.10 nM.
The complex is then presented to calcineurin (Liu, J., Farmer, J.D., Lane,
W.S., Friedmate, J., Weissman, L, &
Scltreiber, S.L. (199.I) Cell 66, 807-15.).
13 Calcineurin dephosphoryhues the transcription factor NEAT found in the
cytoplasm of T-cells.
Dephosphorylation allows NFAT to translocate to the mucleus, combine with jun/
fos genes and activate the
transcription of the IL-2 gene responsible for cell cycle progression, leading
to immune response. CsA-CyP A
complex inhibits the phosphatase activity of calcinettrist and ulrimately
immunosuppression (Rtzkorn,.F. A.,
Chang, Z, Stalz, L.A., 8cWaL~I~ C.T. (1994) Sioeheznistry 33, 2380-2388.).
Neither CsA or CyI? A alone are
20. ~ . important immunologically. Only their complex is important (Liu, J.,
Farmer, J.D., Lane, W.S., Friedrnan, J.,
Weissman, L, & Schceiber, S.L. (I99I) Cell 6&, 807-15).
Metabolism Qf Cy elospurine:
Cyclasporine is tnetaboliied in liver, small imestine and kidney to more than
30 metatioIites. The
25_ structure of I3 metabolites and 2 phase II metabolites have been
identified amdat least.23 further metabolises
have beet isolated by FiPLC arid their structures characterized by mass
spearotnetry. The, reactions involved in
phase I metabolism of cyclosporine are &ydmxyIation, demethylation a$ well as
oxidation and cyclisation at
. amino acid 1. Several clinical studies and reports showed an association
between blood concentrations of
cyclosporine metabolites and neuro- or. nephrotoxicity. In vitro experiments
indicate that metabolites are
30 considerably less immsmosupi'eg5ive and more toxic than CsA.
As exemplified by the ever expanding list of indications for which CsA has
bees found useful, the
cyctosporin fam4y of cvzepounds stud ut~y in the prevention of rejection or
organ and bone marrow
transpIants;~and in the treatment of psoriasis, and a number of autoiaunune
disorders such as type 1 diabetes
mellitus, multiple sclerosis, autoimmune,meitis, and~rheumatoid aathritis.
Additional indications are discussed
35 infra,
As is generally accepted by-those of slaZl in the art, inhibition of secretion
of interleukin-2 (IL-2) and
other Iympholtines from Lymphocytes, is a useful Indicator of intrinsic
immudosuppressive actirilry of a
cyelosporin analoc For a recent review of cyclosporia uses and meebartisms of
action see Wenger et a1
Cyclosporine: Chemistry, Structure-Activity Relationships and Mode of Acdoa,
Progress in Clinical
CA 02372639 2002-03-07 '
Biochemistry and Medicine; wol. 2,'176 (198.
4
Cyclosporin A is a cyclic peptide which contains several N-methyl amino acids
and, at position-8,
contains a D-alanine. The structtn~e of CycIosporin A' is given below:
IH3C~ yH
C .-
~eLea 10 MsVat-'t1 ~ MeBMt 1
C~ lC~ C~ /C~ H~~~'t2 C..,,
CH CH C CH ~
CHz CH2 C~, ~ H~ ~' j H ~~; H2
CH=-CO -N --CH -CO-N-CH-CO-N-CFt-CO-N--~tt pbu 2
CH3 i ~~ CHI Cti9 H CD
H C-~H CH3-N
3
I ~ I H3 j~ ~eay 3
l~ c.ata tt .
CO--CH-! -CO-CH-N-CO--CH-N--CO- ;H-N-CO
C~ H ~~ / \ H
. j H tt3C CH3 CN
ti3C Cti~. H3C ~~
Ata T Met.eu 6 Yal 5 NteLeu 4
24
'Unless otherv~ise specified, each of the amino acids of the disclosed
ayclosporin is. of the L-canfignration.
As is the practice in the field; a particular cyclasporin analog may be named
using a shorthand
notation identifying bow the analog differs from cyclosporin A. Thus,
cyclosporia C which differs frotn
cyciosporin A by the dtreonine at position-2 may be identified as [1'hr]~-
cycIosporin or [Thr]a-CsA. ~imiia~iy,
25 cyclosporin H is [Ala~-CsA; cyclosporin D i's [Yal]=-CsA; cycIosporin E is
[Yan"-CsA; cyclosporin F is [3-
DesoxyMeHmtj'-CsA; cyclosporirt G is [NVaj~-CsA; and cycIosporin8 is [D-
MeVal]'~-CsA.
D-Serine and D-Threoniae have been inirodaced into the 8-position of
cyclosporin A by biosynthesis
t~esu3ting in active compounds. See R Traber et al. J. Antibtotics 42, 59I
(f989~ D-Chloroalanitu has a~ .
been introduced irno position-8 of Cyclos~rin A by biosynthesis. See A. Lawen
et'aI J. :antibiotics 52, 1283
30 (1989).
Fadications for Cyclosnorine Z'heranv
Immunoregulatory abnormalities have been sown to exist in a wide variety of
autoiatmune and
chronic inflamr<ratory diseases, including systemic lupus erythematosis,
chronic rheumatoid arthritis, type 1
35 diabetes mellitus, 'm#lammatory bowel disease, bt'liary cirrhosis, uveitis,
multiple sclerosis and other disorders
such as Crohn's disease, ulcerative colitis; bulious pemphigoid, sarcoidosis,
psoriasis, ichthyosis, and Graves
aphtbaImopathy. Although the. underlying pathogenesis of each of these
condiiioas may be cite different; they
have iu common the appearance, of a variety of autoantibodies and self
reactive lymphocytes ;5neh self
reacdviry shay be due, lit part, ~to a loss of the homeostatic controls under
which the normal izmnune system
CA 02372639 2002-03-07
OpeLateS.
Similarly, following a bone marrow or aworgan transplantation, the host
lymphocytes recognize the
foreign tissue antigens and begin to produce antibodies which lead-to graft
rejection.
One end result of as autoimmune or a rejection process is tissue destruction
caused by inflaznmatory
S cells and the mediators they release. Asiti i~Iammatory agents, such as
NSATCYs (Non-Steroidal Ami-
inflammatory Dnigs), and corticosteroids ac; principally by blocking the
effect of'~ or secretion oi; these
mediators, but do nothing to modify the itmnunologic basis of the disease. On
the other hand, cytotoxic agents,
such as cyclophosphamide, act in such a nonspecific fashion that both the
normal and antoimmune responses
are shut ofd Indeed, patients treated with such nonspecific itnmunosuppressive
agents are as fkeiy to succumb
to infection as they are to their autoimmune disease. .
Generally, a cyclosporin, such as cyclosporine A, is not cytotnxic nor
myelomxic. It does not inhibit
migration of monocytes nor doe's it inhibit granulacyres and macrophage
action. Its action is specific and
leaves most established immune responses intact, However, it is nephrotaxic
and is known to cause the
following undesirable side effects:
(I ) abnormal liver function;
(2) hirsutism;
(3) gum hypertrophy;
(4) tremor;
(5) neurotoxicity;.
(~ hyperaesthesia; and
(7} gastrointestinal discomfort.
A number of cyclosporines and analogs have been described in the patent
literature:
U.S: Pat. No. 4,108,985 issued to Ruegger, ei al, on Aug. 22,1978 entitled,
"Dihydracyclosporin C",
discloses d~ydrocyclosporin C, which can be produced by hydrogenation of
cycIosporia C.
U.S. Pat. No. 4,117,118 issued to Harri, et al. on Sep. 26, T978 entitled,
"Organic Compounds",
discloses cyciosporins A and B, and the production thereof by fermentation.
U.S. PaL..No. 4,210,581 issued to Rue~er, et al. on TuL 1,1980 entitled,
"Organic Compounds",
discloses cyclasporin C and d~3ydracyclosporin C which can be produced by
hydrogenation of cyclosporin C:
U.S. Pat. No. 4,220,641, issued to Tiab~; et al. on Sep. 2,1980 etttiiled,
"Organic Compounds",
discloses cycFosporin D, dihydrocyclosporin D, and isocyclosporin~D.
U.S. Pat. No. 4,288,431 ~ issued to Traber, et aL on Sep. 8,1981 entitled,
"Cyclosporin Derivatives,
Their Production and Pharmaceutical Cotupositiarrs Containing Them", discloses
cyciosporirt G,
d~ydrocylaspoiin G, and isocyciasporin G.
U.S, Pat. No. 4,289,851, issued to T'raber, et al. oa Seg. 15, I981 entitled,
"Process for Producing
Cyclosporin Derivatives", discloses c. yc~osporin D, d~ydrocyclospor'ra D, and
isoGyclosporia D, and a process
for producing same.
U.S, Pat. No. 4,384,996, issued to Bolliager, et al. on May 24,1983 entitled
"Alovel Cyclosporims",
discloses cyclasporins baying a ~i-vinylene-a-amino acid residue at the 2-
position andlor a -~-hydroxy
CA 02372639 2002-03-07
x-amino acid residue at the 8~position. The cycIosporins disclosed included
either MeBmt or dihydro-MeBmt
at the I position.
U.S. Pat. No. 4,396,542, 'issued to Wenger on Auo. 2,1983 entitled, "Method
for the Total Symhesis
of Cyclosporins, Novel CycIosporins and Novel Intermediates and Methods for
their Production", discloses the
synthesis of cyclosporins, wherein the residue at the 1 position is either
MeBmt; d'hydro-MeBtnt, and
protected intermediates.
IJ.S. Pat. No. 4,639,434, issued to Wenger, et al on Ian. 27, I98~,
entitled'~Vovel Cyciospotins~,
discloses cyclosporins with substitumd residues at positions I,, 2, 5 and 8.
U.S. Pat. No. 4,681,754, issued to Siegel on Jul. 21,1987 entitled,
"Counteracting CycIosporin Grgan
Toxicity", discloses methods of use of cyclosporin comprising ca-dergotxnae.
U.S. Pat: No. .4,703,033 issued to Seebach on OcK. 2'~, 198'7 entitled,
"l~Iovet Cyclosporins", discloses
cyclosporins witfi substituted residues at positions 1, 2 and ~3. The
substitutions at position-3 include halogen.
H. Kobel and R Traber, Directed Biosynthesis of Cyclosporiru, European J.
Apple: MicrobioI
Biotechnol., l4, 237B240 (1982), discloses the biosynthesis of cycIosporins A,
_B, C, D & G by fermentation.
IS . Additional cyclosparin analogs are disclosed in U.S. Pat: No.
4,798,823,.issued to Witzel, entitled,
New Cyclosporin Analogs with Modified "C-9 amino acids", ~ubich discloses
cyclosporin analogs with sulfur-
containing amino acids at position-1.
SUMMARY OP THE INVENTION
The present invention concerns chemically substituted and deuterated analogs
of cyclosporine A and
related cyclosporines.
An object of the present invention is to provide new eyclosporine analogs
vsrhieh have enhanced
e~cacy and aliened pharmaeolcinetic and pharmacodynamic parameters. Another
object, of the present
invention is to provide a cyclosporine analog for the care of immunoregulatory
disordersand diseases,
ZS including the prevention, control and trea:merit thereof. An additional
atiject of the present invention is to
provide phatmaceuticai compositions far administering to a pollen in_ need of
the treatment one or more of the
active immuno~ive agents afthe present invemion. StdI a further object of this
invention Is to provide
a method of controlling graft rejection, autoimmune and clsranic
immflaxnmatory diseases by administering a
stz~cient amourn of one or more of the novel immunosuppressive agents in a
mammalian species in need of
such treatment. Finally, it is the object of this invention to provide
processes for the preparation of the active
compounds of the presegt invention.
Substitution and deuteration of the cyclosporine molecule results in altered
physicochemical and
pharmacokinetic properties which enhance its usefulness in the treatment of
transplantation xejectioa, host vs.
graft disease, graft vs. host disease, apIastic anemia, focal and segmental
glomerulosclerosis, myasthenia
3 S grouts, psoriatic arthritis, relapsing poiychandritis and ulcerative
colitis.
Embodiments of the invention include CsA derivatives wherein one or more
hydrogen atoms in the I ,
3 and 9 amino acid positions are substituted with a deuterium atom and wherein
the cyclosporine A derivatives
arc optionally chemically substituted at the amino acid 9 position. A further
specific embodiment of the
invention is the CsA derivative repxeserned by formula 1:
CA 02372639 2002-03-07
7
CHR
v
CH
H3C ~CH3 CH2
CH H3C ~CH3 R,\ CH-CH3 CH3
CHZ CH CH CH2
-N-CH-CO-N-CH-CO-N-CH-CO-N-Cti
CH3 CH3 CH3 H CO tn
H3C IV Y
C,
CH3 ~ Z
-N-CO-CH-N--CO-CH-N-GO- i H-N'-CO
3H CH2 NCH H CH2 CH3
~CH H3C ~3 ,CH
H3C CH3 H3C CH3
where R is (i) a deuterium or (ii) a saturated or unsaturated straight or
branched aliphatic chain of
from I to 16 carbon atoms and containing one or more deuterium atoms or an
ester, ketone or
alcohol of the carbon chain and optionally containing one or more substituents
selected from
halogen, nitro, amino, amindo, aromatic, and heterocyclic, or (iii) R is an
aromatic or
heterocyclic group optionally containing a deuterium atom or (iv) R is a
methyl group; and X, Y,
and Z are hydrogen or deuterium provided that at least one of X, Y, or Z is
deuterium; and R' is
an OH or an ester or is an O and together with a carbon adjacent to a double
bond on amino acid
1 form a heterocyclic ring such as 5-membered rings where the heteroatom is
oxygen.
Preferably, R is an unsaturated straight or branched aliphatic carbon chain of
from 2 to 3
carbons, X, Y and Z are hydrogen or deuterium, and R' is an -0H or acetoxy.
Other specific
embodiments of the present invention include the CsA derivative of formula I
where R is a
saturated or unsaturated carbon chain of from 2 to 3 carbons containing one or
more deuterium
atoms.
CA 02372639 2002-03-07
The invention also specifically contemplates the compounds, and mixtures
thereof,
represented by the structures A and B:
R
w
HO,~ f
MeLeu-MeVah ~N Abu-Sar
O
MeLeu-D-Ata-Ala-MeLeu-Val-MeLeu
(A)
R
HO.,r
MeLeu-MeVal->~ ~--Abu-Sar
O
MeLeu-O AIa-Ala-MeLeu-Val-MeLeu
CB)
wherein R is selected from the group consisting of : (i)-CH=CH-CH3, (ii)-
CH=CH2, and
(iii)-CD=CD2.
Further specific embodiments include those of formulas Sg and Se below:
(5e)
MeLeu -MeVat '-~r MeLeu-MeVat
MeLeu -D-Ala -Ala -MeLeu -Vat-MeLeu MeLeu-D-,41a-
CA 02372639 2002-03-07
DESCRIPTION OF THE FIGURES
Figure I is the structure of cyclosparine A showing a site of deuteration at
the amino acid 3 position.
l figure 2 is the structure of cyelospotzne A showing a site of deuteration at
the amino acid 9 position.
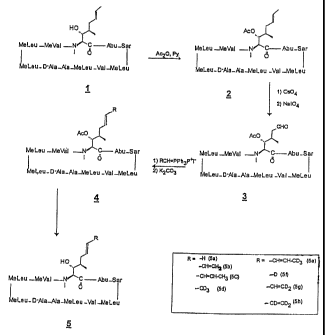
Figure 3 is scheme I of the syathesis~ of the cyclosporine derivatives.
S Figure 4 is scheane II of the synthesis ofthe cyclosporine derivatives.
Figure 5~ is a graph of the results of the calcineurin assay of Example 9:
Figctre d is a graph of the results of a mixed lysnpliocyte reaction assay
ofF.xampIe 10.
DETAILED DESCRIPTION OF THE INVENTION
I4 Substitution of deuterium for ordinary hydrogen and deuterated substrates
for protio metabolites can
produce profound changes in biosystems. Isatopicaliy altered drugs have shown
widely divergent
pharmacological effects. Pettersen et aL, found increased anti-cancer effect
with deuterated 5,6-benzylidene-d1-
L-ascorbic acid (Zilascorb} [Anticancer Res. I2, 33 (1992?].
Substitution of deuterium in methyl groups of ayclosporine wi'Il result in a
slower rate~of a~idaaon of
I S the C-D bond relative to the rate of oxidation of a non-deuterium
substituted C-H bond. Tlie isotopic effect
acts to reduce formation of demethylated metabefrtes and thereby alters the
pharmacokinetic parameters.of the
drub. Lower rates of oxidation, metabolism and clearance result in greater and
mare sustained biological
activity. Deuteration is targeted at various sites of the cycIosporin molecule
to increase the potency of drug,
reduce toxicity of the drug, reduce the clearance of the pharmacologically
active moiety and improve the
20 stability of the molecule.
Isotonic Substitution: .
Stable isotopes (eg., deuteriura; t'C, tsN, is ~) are nonradioactive isotopes
which cantaln one
25 additions! neutron than the normally abundant isotope of the respective
atom. Deuterated corupounds have
been used in pharmaceutical research to investigate the in vivo metabolic fate
of the compounds by evaluation
of the mechanism of action and W etabolic pathway of the non deuterated parent
compound (Blake et al. J.
Pharm. Sci. 64, 3, 36T-391,I97S7: Such axetabalic studies are ~ in the design
of safe, eil~ive
therapeutic drugs, either because the in vipo active cornpaund administered~to
the p~atiert or because the
30 metabolites produced from the parent compound prove to be toxic or
carcinogenic (Foster et al., Advances in
Drug Research Vol. I4, pp. 2 36, Academic press, London,1985~
Incorporation of a heavy atom p~rticulariy substitution of deuterium for
hydrogen, can give rise to an
isotope effect that could alter the pharmacokinetics of the drug. This effect
is nsualty insignificant if the IabeI is
placed at a metabolically inert position of the molecule.
35 Stable isotope labeling of a drug can alter its physico-chemical pzoperties
such as. pKa and lipid
solubility. These changes may influence the fate of the drug at different
steps along its passage through the
body. Absorption, distr~benion, metabolism or excretion can be changed:
Absorption and distn'bettion are
processes that depend primary on the moleGaIar size and the IipophiIicity of
the substance. These effects and
alterations can affect the pbarmacodynamic response of the drug molecule if
the isotopic substitution affects a
CA 02372639 2002-03-07
region involved in a ligand-receptflr interaction.
I~
Drug metabolism can give rise to large isotopic effect if the breaking of a
chemical bond to a
deuterium atom is the rate limiting step in the process. Wlu'le some of the
physical properties of a stable
isotope-labeled molecnIe are different from those of the unlabeled one, the
chenuca~ and hiaIogicai properties
are the same, with one important exception: because of the increased mass of
the heavy isotope, any ~bopd
involving the heavy~isotope and another atom wi'II be stronger than the same
bond between the light isotope
and that atom. In any reaction is which he breaking of this bond is the rate
limiting step, the reacti~ will
proceed slower for the molecule v~rith the heavy isotope due to "Iinetic
isotope effect" A reaction involving
breaking a C D bond can he up to 700 per cent slower than a similar reaction
involving breaking a C H bond.
If the C D bond is. not involved in arty of the steps leading to the
metabolite , there may not be any effect to
alter the behavior of the drug. If a deuterium is placed .at a site involved
in the metabolism of a drug , an
isotope effect will tie observed only if breaking of the G-D band is the rate
limiting step There is evidence to
suggest that whenever cleavage of an aliphatic C-H bond occurs, usually by
oxidation catalyzed by a raixed
function oxidase, replacement of the hydrogen by deuterium will lead to
observable isotope effect. It is also
I S raigortant to understand that the incorporation of deuterium at the site
of metabolism slows its raft to the point
where another metabolite produced by attack at a carbon atom not substituted
by deuterium becomes the nor
pathway a process called "metabolic switching". It is also observed that one
of the most important metabolic
pathways of compounds containing aromatic systems is hydroxylation leading to
a phenolic group in the 3 or 4
position to carbon substituents. Ahhough this pathway involves cleavage of the
C-H bond, it is often not
accompanied by an isotope effect, because the cleavage of this bond mostly not
involved in the rate limiting
step. The substitution of hydrogen by deuterium at the stereo center wilt
induce a greater effect oa the activity
of the drug.
Synthesls of Cyclos~SOrIne Derivatives.~
ZS The staring material for the preparation of the compounds of this invention
is cyclosporine A: The
process for preparing the compounds of the present invention are illustrated
as shown in scheme I in figure 3:
It~wilI be readily apparent to one of ordinary skill in the art reviewing the
syrnhetic;oute depicted helow that
other compounds with formula h can be synthesized by suvstiwtion of
appropriate reactants and agents in the
synthesis shown below.
The first step in the process for making deuterated cyclosporin analogs is the
pre~ration of the key
inte;mecilate 3 and 6 . This can be achieved by the oaddation of the double
bond in the amino acid 1.
Treatixnent of cyclosporh~ with acetic anhydride and excess of
dimethylaminopyridine provided the hydroxyl
~ ~~YI cyclosporia. 2 .Although cleavage of the double bond could then be
accomplished by
treatment of 2 with ozone; or KMnO,J NaIO,~, it was found out that Os04INaI0,
was the reagent of choice
3f for the transformation to the aIdehyde product 3. The reaction was
generally found to be cleaner, producing
the required material gad to proceed in higher yield. The drawback to this
reaction is that OsOa is expensive
gad highly toatic, so that its use is limited : But the results can be
accomplished morn economically by the use
of HzO, with Os04 present in catalytic amounts. t butyl hydroxide in alkaline
solution and N-
methylmorgholine-N-oxide can be substituted for HZOI in this pmcess. The
aIdehyde compound 3_ was fiu~h~
CA 02372639 2002-03-07
lI
trued with various deuterated alkyl or aryl triphenyl phosphonium
derivattves(wittig reagents) and hydrolysis
by alkaline solution provided the final derivatives ( 5_ a-~: We also
developed a general procedure to obtain
various compounds as shown in Scheme ll in figure 4. .
In ibis approach , the aldehyde derivative 3_ was treated with the Witdg
reagent prepared by using .
standard procedure. The restthant product on mt'Id acid hydrolysis provided
the key irdermediate sldehyde
product G_ This was further treated with second deu~rated aikyl or aryl
tripheirylpbosphflnium halide reagents
and on mild acid hydrolysis yielded the. required products. This method
provides control over the extension of
the diene system. By using this approch; olefinic double bonds can be
introduced step by step.
A third approach to prepare the deuterated compounds Sa-b- is by heating non
deuterated cyclosporiQ
analogs descn'bed earlier, in a deueraied Solvent such as deuterated water,
deuterated acetic acid in ibe
presence of acid or Base catalyst.
Preferred cyclosporins of the prGSent invention are those which both contain s
deuterium and a chemical
substitution on amino acid 1 such as those of formula II: .
X-Abu-Sar-MeLeu-Vat =MeLeu-Ata-(D)Ata-MeLeu-Meteu -Me
1 2 a 4 b B 7 8 9 40 11 .
Where X is
R~'CHZ
HC~ ~CH1CH,
CH a
-H-CH-Ca-
And R~ -CHO, -CDO, -CH=CD-CDa : C~CD-CD3 ,-CH~H-CHAD-CD3 ; CD--CH-CD~D.CD3 ,
-CH~H-CH=CD=,-CD=CH-CD=CDr,-CHI,-CH=CHiand-CII~CCI3i.
EXAMPLES:
Example 1.
To a stirred solution of cycIasporme 1(1.018, 4.84mmoI) in acetic anhydride
(20mL) at room temperature was
added DMAP (150mg, I23mrnol, LSeq) After stirring overnight, the reaction
mixture was partitioned
between EtOAc (~Om1) and water (25m1). The separated EtOAc layer was then
washed with water (SOmL} and
brine (SOmh), dried (MgS04) and the solvent removed irr vacuo to give the
crude product as a glassy solid.
Purification by flash chromatography through a short column of silica (2%
MeOFIIDCM} and Iyophitlisation
-from benzene yielded 2_ (1.0~4g, 0.84mmol, quart.} as a fluffy, colourless
solid; ta~ -305.? (c. 03; CI3CI3),
CA 02372639 2002-03-07
12
vo,~, (CFIC13 cast)iciri i 3328m, 2963m, I746m,1627s, I528m; I472m, 1233m; SH
(600MHz, C6D~ 8.73 (1H,
d, J= 9SHz, NHS, 8.30 (IH, d, J=7.OHz, NI-~1, ?.92 (IH, d,J= 75Hz; NCI,), 7 49
(IH, d, J=7.SHz, NI-~,
. 6.05 (IH, d, J~ 11 SHz), 5.$8 (1H, dd, J= 3S,1l.SHz), 5.82 (1H, d, J=
II.SHz), 5:65 (1H; dd, J=4:0,
t2.OHz), 5.60 (1H, dd, J= 3.5, l2SHz), 5.63-5.57 (1H, m], 5SI-5.45 (IH, m);
5.37 (1>3, dd, J= 5.5, 8SHz),
S.OS-5.01 (2H, complex 499 (1H, d, J= I l.OHz}, 4.7fi (IH, p; J~ 7.OHz), 4.58
(1H; pJ= 7.OHz), 4.02 (IF3,
~d, J=13.5p1z), 3.47 (3H, s), 3.30 (3H, s), 3.I? (3H, s), 3.11 (3H, s), 2.98
(3H, s), 2.68-2.62 (1H, m),2.63 (3H,
s); 2.51-239 (2H, complex), 234-2.25 (8H; coatpIex), 2.03 (3H; s},19?-L85
(2H,'coanple~c),1.$3 (3H, dd, J
=1.0, 6.SHz},1.82 1.77 (ZH, complex),1.68-L6I (3H, complex), L55 (3H,. d, J=
7,OHz),.1.55-1.51.(1H, m),
1.44-L3$ (1H, m), 132-1.20 {SH, complex),1.29 {3H, d; J= 7.OHz), 121 (3H, d,
J= 6SHz),1.I7 (3H, d, J=
I O 6SHz),1:14 (3H, d, J= 6SHz), 1:08 (3H, d,-J= 6.5Hz),1.04 {3Hf, d, J=
6.OHz),1.03 (3H, d, J=:7.Oliz),
I .00 (3H, d, J=?.OHz), 0.93 (3H, d; J= 6:OHz), 0.92 (3H, d; J= 6:SHz), 0.88-
0.84 (9H; complex), 0.76 (3H,
d, J= 6SHz); 0.5? (3H, d,J= 6SHz); ~ {'75MHz, C6D~
I73.b,173.2,172.8,172.6,1713, ~I7l.l,.T70.71,
170.67,170.4, I70.2,.I69.8, Ifi7:9 LCD), I29.0,126:2 (C-C,~, 73.1 (C_OAc],
58.1, 57.i, 56.0, 55.0, 54.6,
34:2, 50.3, 499, 48.6, 48.1, 47.8, 44.5, 40.8, 3.9.1, 35.7, 33.6, 32.9, 32.1;
3 IS, 31.2, 30.0, 29.7, 29.5, 293,
I5 24:9, 24.6, 24.4, 24.0, 23.6, 23.4, 233, 21,7, 21.1, 21.0,
20.6,20.3,19.5,18.5,18.0,17.7,17.5, 1?.4, I4.9; .
9.7; rrr~ (Eleczmspray)
ExamnTe 2
To a solution of compound 2 (289tag, 0?3~no>) in a 1:1 mixture of dloxar~e
arid water (5mL) was added
20 firstly sodium metaperiodate (IOOmg, 0.47mmo1, 2eq) and secondly a solution
of osmium tetraoxide (3mL;
0.5g OsO., iu 250tnL of solvent). 'Iwo-phase work up, purif canon by flash
cohimri ciuomatography (40%
acetone in petroleum ether) and lyophilisation from benzene gave compound 3_.
(22fimg; O.I8mmol, 80%) as a
fluffy, colourless solid; ~a~ 260.0 (c. 0.1, CHC13); v",~ (CHC13 cast)Iciri '
3325m, 2962m; I748w,1724w;
1677rn,1626s, IZ'8ra, 755; 8x (300MHz, C6I?~ $.63 (iH, d, J= 9SHz, N~, 8.16
(IH, d, J=?.OHz, N~,I),
25 795 (1H; d, J= 7SHz, NHS, 7.48~(IH, d; J= 9.pliz, NHS, 5.93 (I H, d, J=
7.SHz), 5.84 {I H, dd, J= 4:0,
I LSHz), 5.70 (lI#, d, J=1 I:SHz), 5.56-5.54 (IH, m), 532 (1H, dd, J= 5.5,
S.OHz), 5.07-4.88 (3H, complex),
4.T2 (IH, p, J=?.OHz), 4.49 (1H, p, J=7:UHz), 3.98 (IH, d; J=14.0Hz), 3.42
(3H, s, Cue, 3.27 {3H, s,
CH I~, 3.12 {3H; s, CSI I~, 3.07 (3H; s, CH~1~, Z.91 (3H, s; CI"~NN), 2.79
(3H;~ s, CSI 1V)2.59 (3H, s, CHIN},
2.42 208 (IOH, complex),1.94 (3H, s, CF~,,~I CO~,1:47 (3H, d,J=?:OHz), L24
{3H, 7.OHz),1.14-L09 (9H,
30 . Iex),1.04 (3H, d, J= 6.SHz}, LOI (3H, d, J= 7.Oliz), 0.96 (3H, d, J=
6.SHz), 0.92 (3H, d, J= 6.SHz),
4.91 (3H, d, J= fi.SHz), 0.89 (3H, d, J= fi.OHz); 0.83 (6H, d, J= 6.5Hz), 0.74
(3H, d, J= 6SHz}, 0.59 (3H, d,
J= 6.SHz); 8c (TSMFIz, C6D~ 202.5 (C_HO),1?4.4,174.0; I73.7,1?2.8,17I.6,
I?I.S, I?I2, I7L1,170.6;
1702, 170.2,168.1, 73.0, 58.7, 57.6; 56.7, 55.5, 55:0, 545, 49.4, 4$:9, 48.5,
48.1, 45.0, 44.6, 413, 39.8, 38.8,
3?.7; 362, 32.5, 32.0, 31.6, 30.9, 30.3, 30.0; 29.8, 29:6, 25.6, 25.3, 25.0,
24.8, 245, 24.0, 23.8, 23.4, 22.0,
35- 21.7, 2I Z, 205, 20.0,19.8,18.8,18.5,182,17.4,15:2,10.0; m/3
{Electrospray) 1232.8 (MH''', 100°i6).
CA 02372639 2002-03-07
13
Exaenule 3
Method A: To a solution of compound 3_( 3 l5mg, 026nunoI) in THF (5mL) at
0°C iwas added a solution of the
deutero-phosphorus ylid (2,67mmol, ~lOec~, Prepared from
drethyhriphenylghosphonium~iodide. After w~k-
up, purification by Sash column chromatography (30% to 60% acetone in FE) and
HPLC (60% to 65% MeCN
in water) , then Iyoplu'lisation from benzene yielded compound 4 (153mg,
0.12mmo1, 47%) as a fluffy,
colourless solid,
Method B: To a stirred salution of compound 3 (287mg, 0.23thmoI) in THF (5mL)
under Ar at 78°C was
carefully added a soIt>tion of phosphanu yIid (formed by the addition of
sodium hexamethyldis'ly3arrtide
(1.OM; 2.25tnL, 2.2Smtnol,--I Oeq) to a.suspension of d~-
ethyltripheaylphosphoaiurn iodide (480mg,
~ 1.l3mmol; ~5eq) in THF (lOmL) under Ar at room temperature). After stirring
for 2hr with gradual warming
to room temperatt>re; the reaction mixture was cooled to 0°C and was
quenched by the addition of I O°!o
AeOHfTHF (IOtnL).,The reaction mixture was concentrated in varuo and
partitioned between water (20tnL)
and EtOAc (20mL). The aqueous layer was further extracted with EtOAc (20nL)
and the combined organic
extracts were then washed with IN HCl (20mL) and water (24m1,), dried (MgSOd)
and the solvent removed in
I S vacuo to give the crude product. Purification by flash column
chromatogaphy (40% acetone in petroleum
ether) and lyophilisation from benzene yielded compound 4d (84mg, 67~rmol,
29%) as a fluffy, colourless
solid; It'X~ -283.0 (c. 0.1, CHCl3x v;"~ (CHC13 cast)lari f 3320th, 301 Om,
2959x, 2924s, 287Im, 2853an,
I743nt,1626s, 756x; &H.(b00MHz, CsD6) 8.78 (1H, d, J= 9.SHz), 8.33 (II~i, d,
J= 7.UHz), 7.99 (IH, d, J=
7.SHz), 7.59 (IH, d, J= 9.OHz), 6.09 (IH, d, J=1 LSHz~ 5.92 (IH, dd, J= 4.0, I
I .OHz), 5.86 (1H; d; J=
1 LSHz), 5.72-5.64 (ZH, complex), 5.b2 (IH, dd, J= 3.5, I2.5Hz~ 5.40 (IH, dd,
J= 5.5; 8.SHz), 5.10-5.02
(3H, complex), 4.80 (tli, q, J= 7.0Hz), 4.60 (IH, q, J= 7.OHz), 4.05 (1H, d,
J=14.OHx), 3 S I (3H, s~~ 331
(3H, s), 3.20 (3H, s~ 3.13 (3H, s~ 3.01 (3H, s), 2.87 (3H, s), 2.64 (3H, s),
2.45 (IH, dt, J= 4.0, L2.3Hz~ 236-
2.20 (I OH, cornpIex), 2.06 (3H, s), L93-1.79 (3H, complex); c~ (84MHz, C6H~ ,
Sc (125MHz,'CsD~ 17.4.5,173.7, 173.6,. i73.I,17,1:7,171.4, 170.9;170.7,170.6,
170.3;170.0,168.4,1302
(CSC), 123.8 (C'~, ?3:8 (Mel3rat C 3); 58.7, 58.1, 57.6, 57.1, 55.5, 55.0,
54.5, 49.4, 49.0, 48.6, 48.2, 45.0,
41.4, 39.9, 39.0, 37.8, 342, 33.9, 32.6, 32.3, 32.0, 31.4, 309, 30.8, 30:2,
30.1, 30.0, 299, 29.8, 29.6, 28.5,
25.6, 25.3, 25.0, 249, 24.8, 24.1, 23.9, 23.8, ?3.6, 23.1, 22.1, 21.7, 2I:4,
20.7, 20.0,19.9, I9.&, 18.9,1$.7, .
18.6,18.3,17.4,153,143,102; mlz (Elec~cospray) 1270 ([M+Naj'',100%j,1286
([M+Kj ; 20~
ExamEle 4
To a stirred sohttion of 4d (84ing, 67pmol) in MeOH (5m1.) and water (2.5mL)
at room te~nper~re was
added potassium carbonate (99mg; 0.72mmol, ~IOeq?: After stirring overnight,
the MeOH was removed fn
vacuo and the aqueous residue was partitioned between EiOAc (I OmL) and Solo
citric acid solution (IOmL).
The EtOAc layer was thetl washed with water (I OmL) and brine (IOmL), dried
(MgSO,j and the solvent
removed in varuo to give the crude product. I3PLC purification (60% to 65%
MeCN in water) and
Iyophilisation from benzene yielded compound 5d (59mg, 49pmo1, ?0%) as a
fluffy, colourless solid; ~Gx~
262.0 (c. O.OS, CHC13); v",~ (CHCI3 cast~ari' 3318m, 3008m; 29GOs, 2872ia,
I627s,1519m, I470m, I4I I m,
CA 02372639 2002-03-07
14
I295m, I095m, 754m; ~ (b00MHz, Cue) 8~.2? (1H, d, J= 9.Slllz~ 796 (IH, d, J=
7.SHx), 7.63 (1H, d, J=
B.OHz~ 7.45 (IH, d, J= 9.0Hz),.5.87 (IH, dd, J= 3.5, ILOHz}, 5.74 (IH, d, J=
7.SHz~ 5.73-5.69 (ll~, m),
5.66-5.64 (1H, br.d,.J= ll.OHz), 5.79 {IH, dd, J=4:0; Il.SHz), 3.39 (IH, dd,
J= S.S,10.5Hz), 533 (IH, dd,
J= 3.5, 8.5Hz), 524.(1H, d, J=1 i.OHz~ 5:12 (1H, dt, J= 7.5, I0.0Hz), 4.88-
4.79 (3H, complex), 4.22 (I H,
dd, J= 5.5, 7.SHz~ 4.00 (1H, d, I3.SHz~ 3.72 (3H, s), 322 (3H, s~ 3.06 (3H;
s), 2.97 {3H, s), 2:92 (3H, s~
2.85 (3H; s), 2.67-2.60 (IH, m), 2S8 (3H, s~ 2.56-2S0 (IH, br m~ 2.33-2.23
{4H, complcac~ 220-2.07 (4H,
camplex),1.80-1.74 (3H, complex); L67 (3H, d, J= 7.OHz~ 1.56-L50 (2H,
complex),1.46-i 23 (9H,
cornplex~ 1.i7~L13 (16H, complex), i.06 {3H, d, J= 6.SHz),1.02 (3H, d,
J=7.0I~Tz~, 0:98 (3H, d; J=
6.SHz), 0.96 (3H, d, Ja ?.OHz~ 092-0.89 (9H eomplex~ 0.86 (3H, t, J= 7.SHz),
0.83 (3Ii, d, J= 6.OHz~
0.64 (3H, d, J= 6.SHz~ ~ (84MHz, C6H~ 1.64 (CDR); &c (75MHz, e6Fi~ 174.2,174.
i; 174.0, I73.7,.17i.8,
171.4,171.2,170:5,170.4, I?0.3,169.>1,130.2, I24.I, (99.2,) 74.3, (67.1,) 663,
66.1, 6I .0, 59.5, 58.3, 57.8,
55:7,.55.5, 55,4, 49.4, 49.0, 48.4, 453; 414, 39.6, 39.0, 37.8; 365, 36.I,
35.8, 33.?, 31.6, 30.8, 30.4, 30.i,
29:9, 29.3, 29.4, 23.5, 25.2, 25.0, 24.9,.24.5, 24.2, 23.8, 23.?, 23.6, 22.0,
2L4; 20:0,18.8,18.5, 17.8, I6.0,
I O.I; m!a (EIectrospray) 1206 ((M-i-H]', 30%),.1228 ([M+Naj'',100), 1244
(HIV!+KI+, 25).
13
Example 5
To a vigorously stirred tnixmre of compound 3 (49mg; 39.8Iemol) ands
deuterated d~-
alIyiiripheuylphosphonium ltromide (3I lmg, 8l2prnot, ~20e~ in benzene (3mL.)
at room tempera'dzre was
added IN NaOH {3mL~ Stirring was continued at room temperature for 5days,
ailer which time the 2 layers
were separated, the benzene Layer was washed with water (SmL), dried (MgS~~ a~
the soIveut removed in
vacuo to give the crude pradud. Purifu~ion by HP1.C (20% to 60% MeCN is water)
and Iyophdisation from
benzene yielded compound 4g (23mg,183pno1, 47%) as a flufi~, colourless solid;
~a~ Z64:2.(c. 0.24,
CHCl3); v""~ (CHC13 cast}lari' 3322m, 2959m, ,1?44m, I626s, 1231 m, 754m; 8H
(300MI<3z, C6D~} complex
due to I :I ratio of geometrical isomers 8.73 (d, J= 9.5Hz, NHS, 8.T2 (d, J=
9.SHz, N~1, 829 {d, J= 6SHz,
N~), 8.26 {d, J= b.SHz, N_H}, ?.92 (d, J=?.Sllz, NCI , ?.86 (d, J= 7:SHz, NH ;
T:53 (d, J= 9.OHz, NI-~I , ?.49
(d, J= 9.0Hz, NH,,, 7.10-6.70 (complex); 6:33 (br t, J= Z I.OHz), 6.18 (d, J
=10.5Hz~ 6.12 (d, J = i 0,5Idz),
6.05 (d, J= I I.OHz~ 6.03 (d,.J=1 LOHz~-5.90-5.53 (complex 537 (dd, J= 6.0,-
8.OHz~ 5.20 (d, J=
12.OHz), 5.14 (d, J=12.OHz~ 5.07-497 (complex), 4.80-4.70 (complex), 4.57 (p;
J= 7.OHz), 4.02 (d, J=
I4.OHz), 4.01 (d, J= I4.OHz~ 3.47 (s), 3:46 (s~ 328 {s), 3.26 (s), 3.16 (s),
3.15 (s~ 3.09 (s), 2.97 (s), 2.96 (s),
2.84 (s), 2.62 (s~ 2.48 2.23 (complex), 2:05 (s), 2:03 (x),1.95-I .59
(comglex),.1;54 (d, J= 7_.OHz), I .53-0.80
(coanFlex), 0.?7 (d, J= 6.5Hz~ 0.58 (d, J= 6.SHz}, ,0.5? (d, J= 6.3Hz); &c
(?SMHz, CsDs) 174.5,174.0,
I73.9,173.6,173.5,173.I,17L7, IZL6, I7L4,170.9,170.8, 170.6, 170.6,170,3,
I69.8,:I69.?, 168.4, 13?9,
133.9,133.5,132:8,1323, 131.6; 130.1, I I6.9; I 15.0, 73.6,.58.6, 57.6, 57.0,
56.8, 55.7; 55.5, 55,0, 549,
54.7, 54.5, 49.4, 48.9, 48.5, 48.2, 48..I, 44.9, 41.5, 39:9, 39.0, 38.9; 37.8,
37.6, 36.6, 363, 34.1, 33.7, 32.?,
32;1, 32.0, 31.5, 30.9, 30.7, 30.0, 29.8, 29.6, 25.6, 25.5, 253, 25.2, 25.0,
24.9, 24.1, 239, 23.7, 23.6, 22.1,
21,7, 2L6, 21.4, 21.3, 20.7, 20.0,19.9,189,18.6,18.3,17.6, 15.3,102; rrrla
(Electrospray) 1258.8 (MHO,
100%).
CA 02372639 2002-03-07
Example 6
To a vigorously stirred mixhu~e of compound 3 (56mg, .45.Slunot) and
deuterated d~-
cmtyitriphenylghosphonium bromide (360mg, 907iunol, ZOeq) in benzene (3mL) at
room temperature was
added IN NaOH (3mL). Stirring was continued at roam temperature for 5days;
after which time the 2 layers
S were sepmated, the benzene layer was washed with water (3mi.), dried (MgS04)
and the solvent removed in
vacuo to give tile. exude product. Purification by IdPLC (20'/o to 60°~
MeCN in water) and lyophilisatioa from
benzene yielded compouad 4e (33mg, IB.Ipmol, 40%) as a fluffy, colourless
solid; ~Cx~s -236.0 (c. 0~,
CHCI3); v,",x (CHCI3 cast~cni ~ 3324m, 2959m, 287Im, 1T45w, 1626s,123Im; &H
(300MHz, CsD~ complex
due to presence of 4 isomers 8.76 (d, J= 6.OHz), 8.?3 (d, J= 6.OHz); 8.29 (d,
J= 7.OHz), ?:93 (d, J= 7.SI3z~
10 7.88 (d, J= 7.SHz}, 7.53 (d, J= 9.SHz~ 7:62-731 {I H, complex), 7.16-6.88
(2H, complex 6.59-.6:39
(complex), 6.28 (t, J=1 I.OHz~ 6.15 {d; J=-IO.SHz), 6.09 (d, J=10:5IIz), 6,05
(d, J=~ 1 LSHz), 6.03 (d, J=
I I.SHz), 5.90-5.82~(complex), S.b8:5.3S (complex), 5.08-4.97 {complex), 4.81-
4.?2 (ca~rrpiex), 4.63-4.53.
(complex), 4.03 (d, J= l4.Olviz), 3.47 (s), 3.46 (s), 3.28 (s), 3.26 (s}, 3.17
(s), 3:I5 (s), 3.09 (s~ 2.98 (s~ 2.97
(s), 2.83 (s), 2.63 {s), 2.62 (s), ? 7I 2.Sb (complex), 2.47-2.23 (campiex),
2.05 (s), 2.04 (s); 2.03 (s); 2.02 (s},
15 L98-0.82 (complex), 0.77 {d, J= 6.SHz), fl.58 (d, J= 6.SIiz), 0.58 (d, J=
b.SHz); ml: (Electrospray) I273,8
(' 1~)~
Examnte'I
To a stirred solution of compound 4g (20mg, I5.9pmot) in methanol {5mL) and
water (1mL) at room
temperature was added potassium carbonate {30mg, 217pmol). After stirring
overnight, the reaction mixture
was partitioned lieZween EtOAe (I OmL) and S% aqueous citric acid {1 OmL). The
aqueous layer was further
- extracted with EtOAc (SmL}, the combined or~nic layers were tl~ washed with
5% citric acid (1 OmL) and
brine (IOmL), dried {MgSO,} and the solvem iemoved in vacuo to give the crude
producK. Purification by
HPLC (85~o MeCN) and tyophilisation from benzene yielded compound Sg (I Omg,
8.2~tmol, 52%) as a fluffy,
colourless solid; ~lz~ -2852 {c. 0.Z9, CHCI~; v~ {CHCI; cast~ari ~ .3500-
3200bc, 33 I9m, 2958m,, 2927m,
I b26s,1520m, I4b8m, 754m; &n (300MHz, C6T),~ complex due to the presence of 2
isomers 8:25 (d, J=
I O.OHz, ~i~, 8.I3 (d, J=10.0Hz, I~B~, ?93 (d, J= 7.0Hz, NHS, 7.84 {d, J=
7.OHz, NI~, 7.67 (d, J= 8.0Hz,
Ice, 7.61 (d, J= 8.OHz, N~i , 7.55 (d, J= 8.SHz, N~, x.54 (d, J= B.SHz, NCI ,
6.84 (t,.J= IO.SHz), 6.79 (t,
..J=1Q.SHz~ 6.58 (t, J=10.SHz), 6.52 (t, J= IO.SHz~ 6.30-6.14 (complex), 5.88-
5.78 (complex 5.75-5.66
{complex 5.44-4.74 (complex), 4.22-4.15 (complex), 3.95.(d, J= I4.UHz), 3.93
{d, J=14.OHa), 3.72 (s),
3.68 {s~ 3.I9 (s), 3.I7 (s~ 3.05 ~(s~ 3.03 (s}; 2.94 (s}, 2.93 (s}, 2.89 (s~
2.8b (s), 2.82 {s), 2.81 (s), 2.72 2.53
(complex 2.55 {s), 2_54 {s); 2.49-236 (compl~~ 2.32 2.03 (eomplelc), I.BI-0:81
{complex), 0.65 (d, J=
6.5Hz)), m!z (Electmspray) 1216.8 (AdH*, 1009~0~ 607.9 ([M+2HJ~', _15'""~
CA 02372639 2002-03-07
Examvle 8
16
To a stirred solution of compound 4e {l8mg14.2~no1) iti methanol (SmL) and
water (1mL) at room
te~xtpeiature was added potassium carbo~te (35mg, 254ptnoi). After stirring
ovetuight, the reaction mixture
was partitioned between F..tOAc (lOmL) and S°1o aqueous citric acid (l
OmL). The aqueous layer was f~nthar
extracted with EtUAc (SmL~ the combined organic layers were then washed with
5°Yo citric acid {lOmL) and .
brine (1 OmL~ dried (Mg50~ and the solvent removed i» vacrre to give the cmde
product Purification by
HP1.C (65°/. MeG'1~ and lsrophilisatioa from be»zene yielded end Se
(IOmg, 8.I pnnol, 57%) as a fh~'y,
coiouriess solid; ~Cr~ 2853.(c. 0.1 I, CHCIs); 8x (3t)OMIiz, C°D~
complex due to presence of4 isomem.
831 (d, J = 9.SHz~ 8:28 (d, J= 9.,SIia), 8.16 (d, J ~ 9S1~Iz~ 8.14 (d, J =
9:SIix~ 7:96 {d, J= 7SHz), 795 (d,
IO .T= 7.SHz~ 7.86 (d, J= 9.SHz), 7.85 (d, J= l.SHz), 7.63 (d, J=7.SHx), 7.59
(d, J=7.SHx}x.50-7.44
(complex), 6.60-6.49 (complex 632-6.I 1 (complex}, 5.88-5:83 (complex), 5.76-
5:?I (complex), 5.64-5,22
(complex), S.I7-5.08 (complex 49I-4.77 (complex), 4.26-4.18 (complex), 3.99
(d, J=14.OHz), 397 (d, J=
I4.OHx), 3.74 (s~ 3.T3 (s), 3.71 (s~ 3.69 (s~ 3.22 (s), 3.21 (s~ 320 (s~ 3.i9
(s~ 3.07 (s), 3.06 (s), 3.05 (s~
2.97 (s}, 2.96 (s~ 2.95 (s), 2.92 (s), 2:9I (s}; 2.89 (s), 2.84 (s), 2.83 (s),
2.69-zo~ (complex), 3.58 {s); 2.57 (s),
I.84-0:81 (complex), 0.64 (d, J= 6.SHz); mla (Electrospray) 1269.8 ([M+Kj~',
5%);1253.8 ([M+Na]+, 30),
1231.8 (MFi')
Examyle 9
The immunosupressive activity was tested for deuterated cycIosporin analogs as
descn'bed below.
24 ~ Compound Se and compound Sg are more potent than the parent cyclosporin.
Calcineurin nativity was
assayed using a modification of the method;previously described by Fruman et
al. (Proc Natl Acad Sci
USA, 89,3686-3690, 1992). Whole blood lysates were evaluated for their ability
to dephosphorylate a
szp_IabeIled 19 amino acid peptide substrate in the presence of okadaic acid,
a phosphatase type I and 2
inhibitor. Background phosphatase 2C activity (CsA and okadaic acid resistant
activity) was determined
ZS and subtracted from each sample, with the assay performed in the presence
and absence of excess added
CsA. The remaining phosphatase activity was taken as calcineurin activity. The
results of the calcineurin
assay are presented in figure 5. The results are expressed as means t the
standard error of the mean. The
results are plotted as CsA derivative concentration in ug/L versus percentage
of caIcineurin inhabitation.
The structrues of the compounds assayed include:
MeLeu--WIeVat Abw-Sar
AIieLeu-D-Ala-AIa--Meleu-=VaI-Mefeu (t~~~d 2)
CA 02372639 2002-03-07
17
Ha~~
MeLeu--MeVat i ~-- Abu-,Sar
O.
MeLeu--D-Ala--A!a-MeLeu-Vat-MeLeu (~pc"md 3j
Hb
MeLeu-.fiAeVat---=~ Abu-far
.I ° i
MeLeu--D Ala~- Ala-~-MeLeer-VaHNeLeu (Compound 6)
S ExamQie 10
A mixed Lymphocyte ceacrion (MLR) assay was performed with cyclosporine and
compounds Se and
Sg. The rest are presented in figure 6 and are plotted as the means of four
experhnents showing
concentration of cyclospmine or derivative versus percent inhbition.
The MLR assay is useful for ide~ify~g CsA derivxrsves with biolo~Cai
('mmmosuppressive) activity
1 ~ and to qtietuifyr this activity relative to the immunosuppressive
acrivit3r of the. parent CsA molecule.
An example of a Lymphocyte proliferation assay procedure useful for this
p~pose is as .follows:
Ttt .
1. Collect blood from two individuals (20m~s each) and isolate lymghocytes
using ~coll-Paque (Pharma~ia
Biotech).
2. ~ Count lymphocytes at 1:I0 di7uv'on is 2 ~b acetic acid (vlv):
IS 3. Prepare lOmIs of each lymphocyte pogulati~s (A +B) at 1xI06 nl is DMEM
l20 % FCS (vlv~
4. Set up a 96 well ster0e tissue true plate, $~ bott~t (Sarstedt, cwt #
83.1835. To each well add:
5. Aliquot I OOp.I per well lymphocyte population A
6. Aliquot I QOltt per well lymphocyte population B
7. Aliquot 20pi per well of drag (CSA and CSA derivatives) at 0, 2.5, ~, I 0,
25, SO and 100ug/L in triplicate in
2t1 DMEM with no supplements.
8. To measure the effect ofdrug on proliferation,,iacubate the plate for 5
days at 3T° C in 5 % COi atmoe.
9. On day 5, prepare 3.2nals of 1:50 cf lotion of Methyl-'Ii-Ihymi~ (Aarersbam
Life Science, c~i # TRK 1Z0)
in DNIEM with no supplements. Add 30111 per well and incubate for I8 hours at
37° C in 5 % COi
aunosphere,
ZS 10: On day-7 celis~arc.harvested onto glass microfiber filters GFIA
(Whattnaa, cat # 1820024) using a CeII-
TM
Hxcvestor (Ska~a, cat # 11019). RTash cells 3x with 1.0-ral sterile dist;'lled
water.
Note All pros are done using ttrile fadmiques-in a biolbgical flow hood.
TM
i I_ Pla<x fdtas in Scint~ion vials and add 1.5m1s of SaraSafe Plus 50 %
scinaIatiOn fluid (F'>sher, cat # SX 25- -
CA 02372639 2002-03-07
Ig
I2. Measure the amount of radioactivity incorporated in the lymphocytes using
a beta counter (Micromedic
System Inc., TAURUS Automatic Liquid Scintiiation Counter) for L0 minute.
13. Calculate averages and standard deviations for each dmg and express
results as:
~Yo lrdubition ~ [1~ Ave CRM of test drug-] x I00
Ave CPM ofzero drug
°lo Proliferation ~ 100 ~ % Inhr'bition
The MLR assay caa be trtiIized to select anh'bodies of the invention which
bind biologically active
CSA metabolites and the parent CSA molecule. Arnt'bodies could also be
selected for reacrivitY to biologically
inactive metabolites.
From the results of the caieineurin assay and the mixed lyraphocyte reaction
assay, it was found that
cyciosporines that have been chemically substittuect and deuterated at the
amino acid 1 position can possess
significant imrnunosc~piessant activity. la the case of the derivatives Se and
Sg, isnmunost~pressant activity that
is significantly greater than GSA was obtained.
ExamQte 11
Other cyciosporine derivatives of tire invention which have been prepared
include the following:
STRUCTURE
MeLe~i-MeVal Abu--Sar
MeLeu-~D-Ala--Afa-MeLeu-Va!-Mel.eti
~'r/
MeLeu-lllleVal ! Abu-Sar
MeLetH--D-Aia-Vila-u--Va(~-~AAeiets
CA 02372639 2002-03-07
19
MeLeu-AAeVat---- i Abu-Sar
O
MeLeu-t)-A~~--Aia--AAeLeu-Vat-MeLeu
0
a~~
MeLect-MeVal i Abet-Sar
MeL~-~-Ai~~,ta-Meteu-Vat--MeLeu
HZ~i
HO,j
MeLeu-fiAsVat --- ~ Abu--Sat
MeLsu--D~la-A1a-MeLeu-Vai-MeLeu
MeLeu-MeVai Abu-Sar
MeLeu-D~tla-Afa-MeLeu-Vat-MeLeu
CA 02372639 2002-03-07
20
AcQ~/
MeLeu-MeVaf I ~ Abu-Sar
MeLeu-D=Ata-~41a-MeLeu-Vat-MeLeu
>u--Sar
MeLeu-D Ata~4la-MeLeu-Vat-MeLeu
MeLeu-MeVaE ~r--Sar
MeLeu-D-Ala-Ala-Meixu--Va!-MeLeu
MeLeu-MeVai -lit Abu-Sar
Q
MeLeu-t)-Ata-Ala-MeLeu-Vai-Mel.eu
CA 02372639 2002-03-07
21
MeLeu-MeVai ~u-Sar
MeLeu-~-~-Ata Ata-MeLeu=Val-.-MeLeu
HC~r~
MeLeu-MeVat j ~ ~---- Abu--Sar
Melee-D-Ala--Vila-MeLeu-Vat-MeLeu
bu=Sar
MeLeu-fl-Ala- Ala-Melee-Vaf-MeLeu
CA 02372639 2002-03-07
22
ar
MeLeu-D Ata-Ata-MeLeu-Val-MeLeu
ACCl~/
Melee-MeVa! i ~ ~-- Ab~i-~Sar
MeLeu-D Ala--Ala~-MeLeu--Val-NIeLeu
~ar
MeLeu-D-Ala Ata-MeLeu-Val-MeLeu
MeLeu~-~D-Ata-Ala-Mele~,~-Vat -MeLeu
CA 02372639 2002-03-07
23
HOi~
MeLeu--MeVal ~' ~"" Abu--Sar
MeLeu--~.-Aia- AtaE--MeLeu-Vat-MeLeu
MeLeu--MeVai Abu-Sar
Met.eu--D-Ata-Vila--AAeLeu--Vat--IUleLeu
HOy~
MeLeu-MeVa! ~ ~--- Abu--Sar
MeLeu-D-Ala-Ala-MeLeu--Vat--MeLeu
MeLeu-MeVa1 Atsu--Sar
MeLeu--D-Ata--~41a-MeLeu-Va!-MeLeu
CA 02372639 2002-03-07
24
MeLeu-MeVat i Abu--Sar
Mete::---D~lta-AIa--MeLeu-V~--MeLeu
DraE Composition Farmulation and Elicitationlof Immunosunressfion
- Detezmination of the physicochemical, pharmacodynamic, toxicological and
phar~cokinetic
properties Of the cyclosporine derivatives disclosed can be made using
standard chemical and biological assays
and through the use of mathematical modeling Lechniqnes which are W own in the
chemical and
pharmacologicalhoxicologieal arts. The therapeutic utility and dosing regimen
can be extrapolated front the
results of such techniques and through the use of appropriate pharmacokinetic
andlor pharmacodynamic
I0 models.
The compounds of this invention may be administe;ed neat or with a
pharmaceutical carrier to a warm
blooded anir~l in need thereof. The pharmaceutical carrier may be solid or
liquid.
This invention also relates to a method oftreatment for patients suffering
from immunoregiilaiory
abnoiwalities involving the administration of the disclosed cyclosporines as
the active constituent
I S . For the treatm~t of these conditions atxl diseases by
imraunoitregularity, a deuterated
ryclosporin map ~e ad~aistered orally, topically, parenteralty, by inhalation
spray or reetaIly in dosage unit
formulations containing ~nventional non-toxic pharmaceutically acceptably
carriers, adjttvants and vehicles.
The term parentezal, as used herein, includes sinus injections, intravenous,
iatramuscnlar, intrasternal .
injection ar infusion techniques.
20 The pharmaceutical compositions cog the active ingredient may be in a form
suitable for oral
use, for example, as tablets, tt, Ioyges, aqueous or oily suspensions,
disln~le powders or ~annles,
emulsions, hard or soft capsules, or syrups or elixirs. Compositions intended
for oral use may be ~.
according to auy method known to the art for the mant~acaue of pharmaceutical
compositions and such
compositions may contain. one or more_agems selected from the group,
consisting of sweetening agents,
23 ~ flavoring agerns, coloring agems and preserving agents 'm order to
provide pharmaceutically elegant and
palatable preparation.. Tablets containing the active ingredient in admixture
wish non-toxic pharmaceutically
acceptable excipients may also be manufactured by known methods. The
excipiei~ts used may be for example,
(1 ) inert diluents such as calcitmt carbonate, lcctose, calcium phosphate or
sodium phosphate; (2) granulating
and disintegrating agents such as com starch, cr alginic acid; {3) binding
agents such as star, gelatin or
30 ~ acacia, and (4) lubricating agents such as anagnesium stearate, stearic
acid or talc. The tablets rnay be uncoated
CA 02372639 2002-03-07
or they maybe coated by known techniques to delay disintegration arid
absorption in the, gastrointestinal tract
and thereby provide a sustained action over a longerperiod. For example, a
time delay material such as
glyceryl monostearate or glyceryl distearate may be employed. Tliey may also
be coated by the techniques
described in the U.S. Pat. Nos. 4,256,108; 4;160,452; and 4,26S,874 to form
osmotic therapeutic tablets for
5 controlled release.
In some cases, formulations for oral use may ix in the form of hard gelatin
capsules ~ the
active ingredient is'mixed with an inert solid dilnenx, for example, calcium
carbonate, calcium phosphate or
kaolin. They may also be in the form of soft gelatin capsules wherein the
active ingredient is mixed with water
or an oil medium, for-example pearnti orl, liquid paraffin, or olive off.
10 ~ Aqueous suspensions normally contain the active materials, in admixture
with excipients suitable foi
the manufacture of aqueous suspensions. Such excipients may he
(1 ) suspending agents such as sodium carbaxymetnylcellulose, methylcellulose,
hY~~YPreFY~e~yIceilulose, sodium alginate, polyviaylpytrrolidoue, gum
tragacanth and gum acacia;
(2) dispersing or wetting agerts which may be
~15 (a) a naturally occurring phosphatide such as lecithin;
(b) a condensation product of an alkylene oxide with a fatty acid, for
example,
polyoxyethyIene steatate,
(c) ~a condensation product of ethylene oxide with a long chain aliphatic
alcohol, for
example, heptadecaethyleneoxycetanol,
20 (d) a condensation product of ethylene oxide with a partial ester derived
from a fatty acid
and a hexitol such as polyoxyethyIene sorbitoi monooleate, or
(e) a condensation product of ethylene oxide with a partial ester derived from
a fatty acid and
a hexitoI anhydride, for example poIyoxyethylene sorbitan monooleate.
The aqueous suspensions may also contain one or more preservatives, for
exaasple, ethyl or a-propyl
25 p-hydroxybenzoate; one or more coloring agents; one or more flavoring
agents; and one or more sweetening
agents such as sucrose, aspartame or saccharin.
Oily suspension tnay be formulated by suspending the active ingredient in a
vegetable oil, for exaanple
arachis oil, olive oil; sesame oil or coconut oil, or in a mineral oil such as
liquid paraffin. :The o~7y suspensions
may contain a thickensng agent, for example beavvax, hard paraffin or cetyl
alcohol..Sweetening agents and
30' :flavoring agents may be added to provide a palatable oral preparation.
These ~compositiorta may he preserved
by the addition of an antioxidant such as ascorbic acid.
Dispezsible powders and granules are suitable for the preparation of an
aqueous suspension. They
provide the active ingredient in admixteue with a dispersing or wetting agent,
a suspending agent and one or
more preservatives. Suitable dispersing or wetting alts and suspending agents
are exemplified by those
3S already mentioned above. Additional excipients, for example, those
sweetening, flavoring and coloring agents
descn'bed above may also be present.
The pharri~aceuiical compositions of the invention may also be in the form of
oil-in-water emulsions.
The oily phase may be a vegetable oil sack as olive oil or arachis oils, ar a
mineral oil such as liquid gara~n
or a mixture thereof Suitable emulsifying agents may be (I) naturally
occurring Gums such as gum acacia and
CA 02372639 2002-03-07
26
gum tragacanth, (2} naturally-occurring phosphatides such as soy bean and
lecithin, (3)
esters or partial esters derived from fatty acids and hexitol anhydrides, for
example,
sorbitan monooleate, (4) condensation products of said partial esters with
ethylene oxide,
for example, polyoxyethylene sorbitan monooleate. The emulsions may also
contain
sweetening and flavoring agents.
Syrups and elixirs may be formulated with sweetening agents, for example,
glycerol, propylene glycol, sorbitol, aspartame or sucrose. Such formulations
may also
contain a demulcent, a preservative and flavoring and coloring agents.
The pharmaceutical compositions may be iw the form of a sterile injectable
aqueous
or oleagenous suspension. This suspension may be formulated according to known
methods using those suitable dispersing or wetting agents and suspending
agents which
have been mentioned above. The sterile injectable preparation may also be a
sterile
injectable solution or suspension in a non-toxic parenterally-acceptable
diluent or solvent,
for example as a solution in I,3-butanediol. Among the acceptable vehicles and
solvents
I5 that may be employed are water, Ringer's solution and isotonic sodium
chloride solution.
In addition; sterile, fixed oils are conventionally employed as a solvent or
suspending
medium. For this purpose any bland fixed oil may be employed including
synthetic mono=
or diglycerides. In addition, fatty acids such as oleic acid find use in the
preparation of
injectables.
The disclosed cyclosporins may also be administered in the form of
suppositories
for rectal administration of the drug. These compositions can be prepared by
mixing the
drug with a suitable non-irritating excipient which is solid at ordinary
temperatures but
liquid at the rectal temperature and will therefore melt in the rectum to
release the drug.
Such materials are cocoa butter and polyethylene glycols.
For topical use, creams, ointments, jellies, solutions or suspensions, etc.,
containing
the disclosed cyclosporins are employed.
Dosage levels of the order from about 0.05 mg to about 50 mg per kilogram of
body
weight per day are useful in the treatment of the above-indicated conditions
(from about 2.5
mg to about 2.5 gms. per patient per day}.
CA 02372639 2002-03-07
27
The amount of active ingredient that maybe combined with the carrier materials
to
produce a single dosage form will vary depending upon the host treated and the
particular
mode of administration. For example, a formulation intended for the oral
administration of
humans may contain from 2.5 mg to 2.5 gm of active agent compounded with an
appropriate and convenient amount of carrier material which may vary from
about 5 to
about 95 percent of the total composition. Dosage unit forms will generally
contain
between from about 5 mg to about 500 mg of active ingredient.
It will be understood, however, that the specific.dose level for any
particular patient
will depend upon a variety of factors including the activity of the specific
compound
employed,: the age, body weight, general health, sex, diet, time of
administration, route of
administration, rate of excretion, drug combination and the severity of the
particular disease
undergoing therapy.
In the case of conflict between the references listed herein and this
application, the
text of the application is controlling. Modifications and changes of the
disclosed
compounds and methods will be apparent to one skilled in the art. Such
modifications and
changes are intended to be encompassed by this disclosure and the claims
appended hereto.