Note: Descriptions are shown in the official language in which they were submitted.
CA 02373045 2001-11-02
WO 00/66177 1
PCT/IB00/00653
GENE THERAPY USING TGF¨P
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a method of introduc-
ing at least one gene encoding a member of the transforming
growth factor p superfamily into at least one mammalian
connective tissue for use in regenerating connective tissue
in the mammalian host. The present invention also relates to
a connective tissue cell line that harbors a DNA vector
molecule containing a gene encoding a member of the
transforming growth factor p superfamily.
2. Brief Description of the Related Art
In the orthopedic field, degenerative arthritis or
osteoarthritis is the most frequently encountered disease
associated with cartilage damage. Almost every joint in the
body, such as the knee, the hip, the shoulder, and even the
wrist, is affected. The pathogenesis of this disease is the
degeneration of hyaline articular cartilage (Mankin et al., J
Bone Joint Surg, 52A: 460-466, 1982). The hyaline cartilage
of the joint becomes deformed, fibrillated, and eventually
excavated. If the degenerated cartilage could somehow be
regenerated, most patients would be able to enjoy their lives
without debilitating pain. There has been no method reported
to date to regenerate damaged hyaline cartilage.
Traditional routes of drug delivery, such as oral,
intravenous or intramuscular administration, to carry the
drug to the joint are inefficient. The half-life of drugs
injected intra-articularly is generally short. Another
disadvantage of intra-articular injection of drugs is that
frequent repeated injections are necessary to obtain
acceptable drug levels at the joint spaces for treating a
CA 02373045 2001-11-02
WO 00/66177 2
PCT/IB00/00653
chronic condition such as arthritis. Because therapeutic
agents heretofore could not be selectively targeted to
joints, it was necessary to expose the mammalian host to
systemically high concentrations of drugs in order to
achieve a sustained, intra-articular therapeutic dose.
Exposure of non-target organs in this manner exacerbated the
tendency of anti-arthritis drugs to produce serious side
effects, such as gastrointestinal upset and changes in the
hematological, cardiovascular, hepatic and renal systems of
the mammalian host.
In the orthopedic field, some cytokines have been
considered as candidates for the treatment of orthopedic
diseases. Bone morphogenic protein has been considered to be
an effective stimulator of bone formation (Ozkaynak et al.,
EMBO J, 9:2085-2093, 1990; Sampath and Rueger, Complications
in Ortho, 101-107, 1994), and TGF-P has been reported as a
stimulator of osteogenesis and chondrogenesis (Joyce et al.,
J Cell Biology, 110:2195-2207, 1990).
Transforming growth factor-P (TGF-P) is considered to be
a multifunctional cytokine (Sporn and Roberts, Nature
(London), 332: 217-219, 1988), and plays a regulatory role in
cellular growth, differentiation and extracellular matrix
protein synthesis (Madri et al., J Cell Biology, 106: 1375-
1384, 1988). TGF-P inhibits the growth of epithelial cells and
osteoclast-like cells in vitro (Chenu et al., Proc Natl Acad
Sci, 85: 5683-5687, 1988), but it stimulates enchondral
ossification and eventually bone formation in vivo (Critchlow
et al., Bone, 521-527, 1995; Lind et al., A Orthop Scand,
64(5): 553-556, 1993; and Matsumoto et al., In vivo, 8: 215-
220, 1994). TGF-P-induced bone formation is mediated by its
stimulation of the subperiosteal pluripotential cells, which
eventually differentiate into cartilage-forming cells (Joyce
CA 02373045 2001-11-02
W000/66177
PCT/IB00/00653
3
et al., J Cell Biology, 110: 2195-2207, 1990; and Miettinen
et al., J Cell Biology, 127-6: 2021-2036, 1994).
The biological effect of TGF-P in orthopedics has been
reported (Andrew et al., Calcif Tissue In. 52: 74-78, 1993;
Borque et al., Int J Dev Biol., 37:573-579, 1993; Carrington
et al., J Cell Biology, 107:1969-1975, 1988; Lind et al., A
Orthop Scand. 64(5):553-556, 1993; Matsumoto et al., In vivo,
8:215-220, 1994). In mouse embryos, staining shows that TGF-P
is closely associated with tissues derived from the
mesenchyme, such as connective tissue, cartilage and bone. In
addition to embryologic findings, TGF-P is present at the site
of bone formation and cartilage formation. It can also
enhance fracture healing in rabbit tibiae. Recently, the
therapeutic value of TGF-P has been reported (Critchlow et
al., Bone, 521-527, 1995; and Lind et al., A Orthop Scand,
64(5): 553-556, 1993), but its short- term effects and high
cost have limited wide clinical application.
Intraarticular injection of TGF-P for the treatment of
arthritis is not desirable, because the injected TGF-P has a
short duration of action, as TGF-P is degraded into inactive
form in vivo. Therefore, a new method for long-term release
of TGF-P is necessary for the regeneration of hyaline
cartilage.
There have been reports of regeneration of articular
cartilage with autotransplantation of cartilage cells
(Brittberg et al., New Engl J Med 331: 889-895, 1994), but
this procedure entails two operations with wide excision of
soft tissues. If intraarticular injection is enough for the
treatment of degenerative arthritis, it will be of great
economic and physical benefit to the patients.
CA 02373045 2001-11-02
WO 00/66177
PCT/I1300/00653
4
Gene therapy, which is a method of transferring a
specific protein to a specific site, may be the answer to
this problem (Wolff and Lederberg, Gene Therapeutics ed. Jon
A. Wolff, 3-25, 1994; and Jenks, J Natl Cancer Inst, 89(16):
1182-1184, 1997).
United States Patents 5,858,355 and 5,766,585 disclose
making a viral or plasmid construct of the IRAP
(interleukin-1 receptor antagonist protein)
gene;
transfecting synovial cells (5,858,355) and bone marrow
cells (5,766,585) with the construct; and injecting the
transfected cells into a rabbit joint, but there is no
disclosure of using a gene belonging to the TGF-P superfamily
to regenerate connective tissue.
United States Patents 5,846,931 and 5,700,774 disclose
injecting a composition that includes a bone morphogenesis
protein (BMP), which belongs to the TGF p "superfamily",
together with a truncated parathyroid hormone related
peptide to effect the maintenance of cartilaginous tissue
formation, and induction of cartilaginous tissue. However,
there is no disclosure of a gene therapy method using the
BMP gene.
In spite of these prior art disclosures, there remains
a very real and substantial need for a method of introducing
at least one gene encoding a product into at least one cell
of a connective tissue of a mammalian host in vitro, or
alternatively in vivo, for use in treating the mammalian
host. Further, there is a need for a process wherein a gene
encoding a member of the transforming growth factor p
superfamily is used to regenerate connective tissue in the
mammalian host. More specifically, there is a need for a
process where a gene coding for a TGF-P superfamily of
CA 02373045 2001-11-02
WO 00/66177
PCT/I1300/00653
proteins is expressed in host connective tissue cells in
vivo.
SUMMARY OF THE INVENTION
The present invention has met the hereinbefore
5 described need. A method of introducing at least one gene
encoding a product into at least one cell of a mammalian
connective tissue for use in treating a mammalian host is
provided in the present invention. This method includes
employing recombinant techniques to produce a DNA vector
molecule containing the gene coding for the product and
introducing the DNA vector molecule containing the gene
coding for the product into the connective tissue cell. The
DNA vector molecule can be any DNA molecule capable of being
delivered and maintained within the target cell or tissue
such that the gene encoding the product of interest can be
stably expressed. The DNA vector molecule preferably
utilized in the present invention is either a viral or
plasmid DNA vector molecule. This method preferably includes
introducing the gene encoding the product into the cell of
the mammalian connective tissue for a therapeutic use.
The present invention is directed to a method of
treating arthritis comprising:
a) generating a recombinant viral or plasmid vector
comprising a DNA sequence encoding a member of a
transforming growth factor superfamily of proteins
operatively linked to a promoter;
b) transfecting in vitro a population of cultured
connective tissue cells with said recombinant vector,
resulting in a population of transfected connective tissue
cells; and
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
6
c) transplanting the transfected connective tissue
cells by intraarticular injection to an arthritic joint
space of a mammalian host, such that expression of the DNA
sequence within the joint space results in regenerating
connective tissue.
The recombinant vector may be, but not limited to, a
retroviral vector, preferably a retroviral vector. The
vector may also be a plasmid vector.
The method of the invention includes storing a
population of transfected connective tissue cells prior to
transplantation. The cells may be stored in 10% DMSO under
liquid nitrogen prior to transplantation.
The connective tissue cells include, but are not
limited to, fibroblast cells, mesenchymal
cells,
osteoblasts, or chondrocytes. The fibroblast cells may be
NIH 3T3 cells or human foreskin fibroblast cells.
The connective tissue includes, but is not limited to,
cartilage, ligament, or tendon. The cartilage may be hyaline
cartilage.
The method of the present invention uses a member of
the transformation growth factor superfamily, which includes
transforming growth factor p (TGF-P). The member of the
transformation growth factor superfamily may be TGF-131, TGF-
132, TGF-03, BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, or BMP-7.
Preferably, TGF-P is human or porcine TGF-01, TGF-02 or TGF-
133.
The present invention is also directed to a method of
regenerating hyaline cartilage, comprising:
a)
generating a recombinant viral or plasmid vector
comprising a DNA sequence encoding a member of a
transforming growth factor superfamily of proteins
operatively linked to a promoter;
CA 02373045 2001-11-02
W000/66177
PCT/IB00/00653
7
b) transfecting in vitro a population of cultured
connective tissue cells with the recombinant vector,
resulting in a population of transfected connective tissue
cells; and
c) transplanting the transfected connective tissue
cells by intraarticular injection to joint space of a
mammalian host, such that expression of the DNA sequence
within the joint space results in regenerating hyaline
cartilage.
The transfection method may be accomplished by methods
such as liposome encapsulation, calcium phosphate
coprecipitation, electroporation and DEAE-dextran mediation.
The method of the invention includes using preferably
the plasmid pmTP1.
The present invention is also directed to a connective
tissue cell line comprising a recombinant viral or plasmid
vector comprising a DNA sequence encoding a member of the
transforming growth factor superfamily. The connective
tissue cell line may include, but is not limited to, a
fibroblast cell line, a mesenchymal cell line, a chondrocyte
cell line, an osteoblast cell line, or an osteocyte cell
line. The fibroblast cell line may be a human foreskin
fibroblast cell line or NIH 3T3 cell line.
The connective tissue cell line according to the
invention comprises a member of the transforming growth
factor superfamily. Preferably, a member of the transforming
growth factor superfamily is TGF-31, TGF-132, TGF-33, BMP-2,
BMP-3, BMP-4, BMP-5, BMP-6, or BMP-7. More preferably, the
member is human or porcine TGF-P1, TGF-P2 or TGF-P3.
The connective tissue cell line of the invention also
may comprise cells harboring the recombinant vector pmTP1.
CA 02373045 2001-11-02
WO 00/66177 8
PCT/M00/00653
These and other objects of the invention will be more
fully understood from the following description of the
invention, the referenced drawings attached hereto and the
claims appended hereto.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 - Expression of TGF-131 mRNA. Total RNA was
isolated from NIH 3T3 cells or NIH 3T3 cells stably
transfected with pmT131, a TGF-131 expression vector, which were
grown in the absence or presence of zinc. Total RNA (15 mg)
was probed with either the TGF-131 cDNA or p actin cDNA as a
control.
Figs. 2A-2B - Gross findings of regenerated cartilage.
2A. A rectangular partial cartilage defect was made
on the femoral condyle and the knee joint was injected with
NIH 3T3 cells without TGF-131 transfection. The defect was not
covered.
2B. At 6 weeks after injection of NIH 3T3-TGF-131
cells, the defect was covered by newly formed tissue. The
color of the regenerated tissue was almost identical to that
of the surrounding cartilage.
Fig. 3A-3D - Microscopic findings of regenerated
cartilage (X 200).
3A and 3B. Hematoxilin-eosine (H&E) analysis of
defect area 4 and 6 weeks after injection with control cells.
No tissue covered the initial defect area.
3C and 3D. Hematoxilin-eosine (H&E) analysis of
defect area 4 and 6 weeks after injection of TGF-Pl-
transfected cells. At 4 weeks, partial defect area was
covered by hyaline cartilage after injection of TGF-01-
transfected cells. At 4 weeks and 6 weeks after injection,
CA 02373045 2001-11-02
WO 00/66177
PCT/I1100/00653
9
the regenerated tissue became thicker and its height was
almost identical to normal cartilage at 6 weeks.
Histologically, the regenerated cartilage (arrow) was
identical to the surrounding hyaline cartilage.
Figs. 4A-4B - Immunohistochemical analysis for TGF-$31
expression in rabbit joint x 200.
Brown immunoperoxidase reaction product indicates
high levels of recombinant TGF-Pl expression in the NIH 3T3-
TGF-Pl cells (4B).
4A show hyaline cartilage in a rabbit joint
injected with control cells.
Figs. 5A-5B - Microscopic findings (X 200) of
regenerated tissues with H&E staining (A) and Safranin-O
staining (B).
5A. In the partially damaged area, the regenerated
hyaline cartilage is shown by H&E staining (black arrow).
5B. In the completely denuded cartilage area, the
regenerated tissue (white arrow) was fibrous collagen.
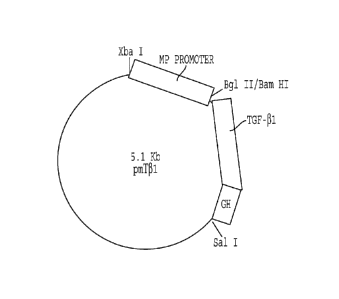
Fig. 6 - Plasmid map of pmT31.
Figs. 7A-7D - Gross morphology of rabbit achilles tendon
injected with TGF-31 transfected cells.
7A. Tendon injected with control cells.
7B. Tendon injected with TGF-131 transfected cells,
six weeks after injection.
7C. Cross-sectional view of the tendon pictured in
7A.
7D. Cross-sectional view of the tendon pictured in
7B.
CA 02373045 2001-11-02
W000/66177
PCT/IB00/00653
Figs. 8A-8F - Microscopic findings of regenerated tissue
in rabbit achilles tendon with H&E staining.
8A, 8B and 8C show tendon injected with control
5 cells 6 weeks after injection. 8A. x50 magnification. 8B.
x200 magnification. 80. x600 magnification.
8D, 8E and 8F show tendon injected with TGF-P1
transfected cells 6 weeks after injection. 8D. x50
magnification. 8E. x200 magnification. 8F.
x600
10 magnification. The TGF-P1 transfected cells injected into the
tendon appear to be more round than the endogenous tendon
cells. Fibrous collagen was produced by autocrine and
paracrine modes of action, and the tendon was enlarged. The
tendon was enlarged after the injection of TGF-Pl transfected
cells.
Figs. 9A-9B - Microscopic findings of regenerated tissue
in rabbit achilles tendon with H&E staining (A) and
immunohistochemical staining (B) with TGF-Pl antibody. Brown
immunoperoxidase reaction product indicates high levels of
recombinant TGF-Pl expression in the NIH 3T3-TGF-P1 cells.
DETAILED DESCRIPTION OF THE INVENTION
As used herein, the term "patient" includes members of
the animal kingdom including but not limited to human
beings.
As used herein, the term "mammalian host" includes
members of the animal kingdom including but not limited to
human beings.
As used herein, the term "connective tissue" is any
tissue that connects and supports other tissues or organs,
CA 02373045 2001-11-02
W000/66177
PCT/IB00/00653
11
and includes but is not limited to a ligament, a cartilage,
a tendon, a bone, and a synovium of a mammalian host.
As used herein, the term "connective tissue cell" or
"cell of a connective tissue" include cells that are found
in the connective tissue, such as fibroblasts, cartilage
cells (chondrocytes), and bone cells (osteoblasts/
osteocytes), which secrete collagenous extracellular matrix,
as well as fat cells (adipocytes) and smooth muscle cells.
Preferably, the connective tissue cells are fibroblasts,
cartilage cells, and bone cells. More preferably, the
connective tissue cells are fibroblast cells. Connective
tissue cells also include mesenchymal cells which are also
known as immature fibroblasts. It will be recognized that
the invention can be practiced with a mixed culture of
connective tissue cells, as well as cells of a single type.
As used herein, "connective tissue cell line" includes
a plurality of connective tissue cells originating from a
common parent cell.
As used herein, "hyaline cartilage" refers to the
connective tissue covering the joint surface. By way of
example only, hyaline cartilage includes, but is not limited
to, articular cartilage, costal cartilage, and nose
cartilage.
In particular, hyaline cartilage is known to be self-
renewing, responds to alterations, and provides stable
movement with less friction. Hyaline cartilage found even
within the same joint or among joints varies in thickness,
cell density, matrix composition and mechanical properties,
yet retains the same general structure and function. Some of
the functions of hyaline cartilage include surprising
stiffness to compression, resilience, and exceptional
CA 02373045 2001-11-02
WO 00/66177 12
PCT/M00/00653
ability to distribute weight loads, ability to minimize peak
stress on subchondral bone, and great durability.
Grossly and histologically, hyaline cartilage appears
as a slick, firm surface that resists deformation. The
extracellular matrix of the cartilage comprises
chondrocytes, but lacks blood vessels, lymphatic vessels or
nerves. An elaborate, highly ordered structure that
maintains interaction between chondrocytes and the matrix
serves to maintain the structure and function of the hyaline
cartilage, while maintaining a low level of metabolic
activity. The reference O'Driscoll, J. Bone Joint Surg.,
80A: 1795-1812, 1998 describes the structure and function of
hyaline cartilage in detail, which is incorporated herein by
reference in its entirety.
As used herein, the "transforming growth factor-P (TGF-
p) superfamily" encompasses a group of structurally-related
proteins which affect a wide range of differentiation
processes during embryonic development. The family includes,
Miillerian inhibiting substance (MIS), which is required for
normal male sex development (Behringer, et al., Nature,
345:167, 1990), Drosophila decapentaplegic (DPP) gene
product, which is required for dorsal-ventral axis formation
and morphogenesis of the imaginal disks (Padgett, et al.,
Nature, 325:81-84, 1987), the Xenopus Vg-1 gene product,
which localizes to the vegetal pole of eggs (Weeks, et al.,
Cell, 51:861-867, 1987), the activins (Mason, et al.,
Biochem, Biophys. Res. Commun., 135:957-964, 1986), which
can induce the formation of mesoderm and anterior structures
in Xenopus embryos (Thomsen, et al., Cell, 63:485, 1990),
and the bone morphogenetic proteins (BMP's, such as BMP-2,
3, 4, 5, 6 and 7, osteogenin, OP-1) which can induce de novo
cartilage and bone formation (Sampath, et al., J. Biol.
CA 02373045 2001-11-02
W000/66177 13
PCT/IB00/00653
Chem., 265:13198, 1990). The TGF-P gene products can
influence a variety of differentiation processes, including
adipogenesis, myogenesis, chondrogenesis, hematopoiesis, and
epithelial cell differentiation (for a review, see Massague,
Cell 49:437, 1987), which is incorporated herein by
reference in its entirety.
The proteins of the TGF-P family are initially
synthesized as a large precursor protein which subsequently
undergoes proteolytic cleavage at a cluster of basic
residues approximately 110-140 amino acids from the C-
terminus. The C-terminal regions of the proteins are all
structurally related and the different family members can be
classified into distinct subgroups based on the extent of
their homology. Although the homologies within particular
subgroups range from 70% to 90% amino acid sequence
identity, the homologies between subgroups are significantly
lower, generally ranging from only 20% to 50%. In each case,
the active species appears to be a disulfide-linked dimer of
C-terminal fragments. For most of the family members that
have been studied, the homodimeric species has been found to
be biologically active, but for other family members, like
the inhibins (Ung, et al., Nature, 321:779, 1986) and the
TGF-P's (Cheifetz, et al., Cell, 48:409, 1987), heterodimers
have also been detected, and these appear to have different
biological properties than the respective homodimers.
Members of the superfamily of TGF-P genes include TGF-
P3, TGF-P2, TGF-P4 (chicken), TGF-P1, TGF-P5 (Xenopus), BMP-
2, BMP-4, Drosophila DPP, BMP-5, BMP-6, Vgrl, OP-1/BMP-7,
Drosophila 60A, GDF-1, Xenopus Vgf, BMP-3, Inhibin-PA,
Inhibin-PB, Inhibin-a, and MIS. These genes are discussed in
CA 02373045 2001-11-02
ViC000/66177
PCT/IB00/00653
14
Massague, Ann. Rev. Biochem. 67:753-791, 1998, which is
incorporated herein by reference in its entirety.
Preferably, the member of the superfamily of TGF-P
genes is TGF-P. More preferably, the member is TGF-P1, TGF-
[32, TGF-P3, BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, or BMP-7.
Even more preferably, the member is human or porcine TGF-P.
Still more preferably, the member is human or porcine TGF-
131,
or TGF-03. Most preferably, the member is human
or porcine TGF-31.
As used herein, "selectable marker" includes a gene
product that is expressed by a cell that stably maintains
the introduced DNA, and causes the cell to express an
altered phenotype such as morphological transformation, or
an enzymatic activity. Isolation of cells that express a
transfected gene is achieved by introduction into the same
cells a second gene that encodes a selectable marker, such
as one having an enzymatic activity that confers resistance
to an antibiotic or other drug. Examples of selectable
markers include, but are not limited to, thymidine kinase,
dihydrofolate reductase, aminoglycoside phosphotransferase,
which confers resistance to aminoglycoside antibiotics such
as kanamycin, neomycin and geneticin, hygromycin B
phosphotransferase, xanthine-guanine
phosphoribosyl
transferase, CAD (a single protein that possesses the first
three enzymatic activities of de novo uridine biosynthesis -
carbamyl phosphate synthetase, aspartate transcarbamylase
and dihydroorotase), adenosine deaminase, and asparagine
synthetase (Sambrook et al. Molecular Cloning, Chapter 16.
1989), incorporated herein by reference in its entirety.
As used herein, a "promoter" can be any sequence of DNA
that is active, and controls transcription in an eucaryotic
cell. The promoter may be active in either or both
CA 02373045 2001-11-02
W000/66177
PCT/IB00/00653
eucaryotic and procaryotic cells. Preferably, the promoter
is active in mammalian cells. The promoter may be
constitutively expressed or inducible. Preferably, the
promoter is inducible. Preferably, the promoter is inducible
5 by an external stimulus. More preferably, the promoter is
inducible by hormones or metals. Still more preferably, the
promoter is inducible by heavy metals. Most preferably, the
promoter is a metallothionein gene promoter. Likewise,
"enhancer elements", which also control transcription, can
10 be inserted into the DNA vector construct, and used with the
construct of the present invention to enhance the expression
of the gene of interest.
As used herein, the term "DC-chol" means a cationic
liposome containing cationic cholesterol derivatives. The
15 "DC-chol" molecule includes a tertiary amino group, a medium
length spacer arm (two atoms) and a carbamoyl linker bond
(Gao et al., Biochem. Biophys. Res, Commun., 179:280-285,
1991).
As used herein, "SF-chol" is defined as a type of
cationic liposome.
As used herein, the term "biologically active" used in
relation to liposomes denotes the ability to introduce
functional DNA and/or proteins into the target cell.
As used herein, the term "biologically active" in
reference to a nucleic acid, protein, protein fragment or
derivative thereof is defined as an ability of the nucleic
acid or amino acid sequence to mimic a known biological
function elicited by the wild type form of the nucleic acid
or protein.
As used herein, the term "maintenance", when used in
the context of liposome delivery, denotes the ability of the
introduced DNA to remain present in the cell. When used in
CA 02373045 2001-11-02
WO 00/66177
PCT/I1300/00653
16
other contexts, it means the ability of targeted DNA to
remain present in the targeted cell or tissue so as to
impart a therapeutic effect.
The present invention discloses ex vivo and in vivo
techniques for delivery of a DNA sequence of interest to the
connective tissue cells of the mammalian host. The ex vivo
technique involves culture of target connective tissue
cells, in vitro transfection of the DNA sequence, DNA vector
or other delivery vehicle of interest into the connective
tissue cells, followed by transplantation of the modified
connective tissue cells to the target joint of the mammalian
host, so as to effect in vivo expression of the gene product
of interest.
As an alternative to the in vitro manipulation of
fibroblast cells, the gene encoding the product of interest
is introduced into liposomes and injected directly into the
area of the joint, where the liposomes fuse with the
connective tissue cells, resulting in an in vivo gene
expression of the gene product belonging to the TGF-p
superfamily.
As an additional alternative to the in vitro
manipulation of connective tissue cells, the gene encoding
the product of interest is introduced into the area of the
joint as naked DNA. The naked DNA enters the connective
tissue cell, resulting in an in vivo gene expression of the
gene product belonging to the TGF-P superfamily.
One ex vivo method of treating a connective tissue
disorder disclosed throughout this specification comprises
initially generating a recombinant viral or plasmid vector
which contains a DNA sequence encoding a protein or
biologically active fragment thereof. This recombinant
vector is then used to infect or transfect a population of
CA 02373045 2001-11-02
WO 00/66177 17
PCT/11300/00653
in vitro cultured connective tissue cells, resulting in a
population of connective cells containing the vector. These
connective tissue cells are then transplanted to a target
joint space of a mammalian host, effecting subsequent
expression of the protein or protein fragment within the
joint space. Expression of this DNA sequence of interest is
useful in substantially reducing at least one deleterious
joint pathology associated with a connective tissue
disorder.
It will be understood by the artisan of ordinary skill
that the preferred source of cells for treating a human
patient is the patient's own connective tissue cells, such
as autologous fibroblast cells.
More specifically, this method includes employing as
the gene a gene capable of encoding a member of the
transforming growth factor p superfamily, or a biologically
active derivative or fragment thereof and a selectable
marker, or a biologically active derivative or fragment
thereof.
A further embodiment of the present invention includes
employing as the gene a gene capable of encoding at least
one of a member of transforming growth factor p superfamily
or a biologically active derivative or fragment thereof, and
employing as the DNA plasmid vector any DNA plasmid vector
known to one of ordinary skill in the art capable of stable
maintenance within the targeted cell or tissue upon
delivery, regardless of the method of delivery utilized.
One such method is the direct delivery of the DNA
vector molecule, whether it be a viral or plasmid DNA vector
molecule, to the target cell or tissue. This method also
includes employing as the gene a gene capable of encoding a
CA 02373045 2001-11-02
W000/66177 18
PCTAB00/00653
member of transforming growth factor p superfamily or
biologically active derivative or fragment thereof.
Another embodiment of this invention provides a method
for introducing at least one gene encoding a product into at
least one cell of a connective tissue for use in treating
the mammalian host. This method includes employing non-viral
means for introducing the gene coding for the product into
the connective tissue cell. More specifically, this method
includes a liposome encapsulation, calcium phosphate
coprecipitation, electroporation, or DEAE-dextran mediation,
and includes employing as the gene a gene capable of
encoding a member of transforming growth factor superfamily
or biologically active derivative or fragment thereof, and a
selectable marker, or biologically active derivative or
fragment thereof.
Another embodiment of this invention provides an
additional method for introducing at least one gene encoding
a product into at least one cell of a connective tissue for
use in treating the mammalian host. This additional method
includes employing the biologic means of utilizing a virus
to deliver the DNA vector molecule to the target cell or
tissue. Preferably, the virus is a pseudovirus, the genome
having been altered such that the pseudovirus is capable
only of delivery and stable maintenance within the target
cell, but not retaining an ability to replicate within the
target cell or tissue. The altered viral genome is further
manipulated by recombinant DNA techniques such that the
viral genome acts as a DNA vector molecule which contains
the heterologous gene of interest to be expressed within the
target cell or tissue.
A preferred embodiment of the invention is a method of
delivering TGF-P to a target joint space by delivering the
CA 02373045 2001-11-02
W000/66177 19
PCT/IB00/00653
TGF-P gene to the connective tissue of a mammalian host
through use of a retroviral vector with the ex vivo
technique disclosed within this specification. In other
words, a DNA sequence of interest encoding a functional TGF-
p protein or protein fragment is subcloned into a retroviral
vector of choice, the recombinant viral vector is then grown
to adequate titer and used to infect in vitro cultured
connective tissue cells, and the transduced connective
tissue cells, preferably autografted cells, are transplanted
into the joint of interest, preferably by intra-articular
injection.
Another preferred method of the present invention
involves direct in vivo delivery of a TGF-P superfamily gene
to the connective tissue of a mammalian host through use of
either an adenovirus vector, adeno-associated virus (AAV)
vector or herpes-simplex virus (HSV) vector. In other words,
a DNA sequence of interest encoding a functional TGF-P
protein or protein fragment is subcloned into the respective
viral vector. The TGF-P containing viral vector is then
grown to adequate titer and directed into the joint space,
preferably by intra-articular injection.
Direct intra-articular injection of a DNA molecule
containing the gene of interest into the joint results in
transfection of the recipient connective tissue cells and
hence bypasses the requirement of removal, in vitro
culturing, transfection, selection, as well as transplanting
the DNA vector containing-fibroblast to promote stable
expression of the heterologous gene of interest.
Methods of presenting the DNA molecule to the target
connective tissue of the joint includes, but is not limited
to, encapsulation of the DNA molecule into cationic
liposomes, subcloning the DNA sequence of interest in a
CA 02373045 2001-11-02
vamwiwn 20
PCT/IB00/00653
retroviral or plasmid vector, or the direct injection of the
DNA molecule itself into the joint. The DNA molecule,
regardless of the form of presentation to the knee joint, is
preferably presented as a DNA vector molecule, either as
recombinant viral DNA vector molecule or a recombinant DNA
plasmid vector molecule. Expression of the heterologous gene
of interest is ensured by inserting a promoter fragment
active in eukaryotic cells directly upstream of the coding
region of the heterologous gene. One of ordinary skill in
the art may utilize known strategies and techniques of
vector construction to ensure appropriate levels of
expression subsequent to entry of the DNA molecule into the
connective tissue.
In a preferred embodiment, fibroblasts recovered from
the knee joint are cultured in vitro for subsequent
utilization as a delivery system for gene therapy. It will
be apparent that Applicants are not limited to the use of
the specific connective tissue disclosed. It would be
possible to utilize other tissue sources for in vitro
culture techniques. The method of using the gene of this
invention may be employed both prophylactically and in the
therapeutic treatment of arthritis. It will also be apparent
that Applicants are not limited to prophylactic or
therapeutic applications in treating only the knee joint. It
would be possible to utilize the present invention either
prophylactically or therapeutically to treat arthritis in
any susceptible joint.
In another embodiment of this invention, a compound for
parenteral administration to a patient in a therapeutically
effective amount is provided that contains a gene encoding a
TGF-13 superfamily protein and a suitable pharmaceutical
carrier.
CA 02373045 2001-11-02
WO 00/66177 1
PCT/11300/00653
2
Another embodiment of this invention provides for a
compound for parenteral administration to a patient in a
prophylactically effective amount that includes a gene
encoding a TGF-0 superfamily protein and a suitable
pharmaceutical carrier.
A further embodiment of this invention includes the
method as hereinbefore described including introducing the
gene into the cell in vitro. This method also includes
subsequently transplanting the infected cell into the
mammalian host. This method includes after effecting the
transfecting of the connective tissue cell but before the
transplanting of the infected cell into the mammalian host,
storing the transfected connective tissue cell. It will be
appreciated by those skilled in the art that the infected
connective tissue cell may be stored frozen in 10 percent
DMSO in liquid nitrogen. This method includes employing a
method to substantially prevent the development of arthritis
in a mammalian host having a high susceptibility of
developing arthritis.
Another embodiment of this invention includes a method
of introducing at least one gene encoding a product into at
least one cell of a connective tissue of a mammalian host
for use in treating the mammalian host as hereinbefore
described including effecting in vivo the infection of the
cell by introducing the viral vector containing the gene
coding for the product directly into the mammalian host.
Preferably, this method includes effecting the direct
introduction into the mammalian host by intra-articular
injection. This method includes employing the method to
substantially prevent a development of arthritis in a
mammalian host having a high susceptibility of developing
arthritis. This method also includes employing the method on
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
22
an arthritic mammalian host for therapeutic use. Further
this method also includes employing the method to repair and
regenerate the connective tissue as hereinbefore defined.
It will be appreciated by those skilled in the art,
that the viral vectors employing a liposome are not limited
by cell division as is required for the retroviruses to
effect infection and integration of connective tissue cells.
This method employing non-viral means as hereinbefore
described includes employing as the gene a gene capable of
encoding a member belonging to the TGF-P superfamily and a
selectable marker gene, such as an antibiotic resistance
gene.
Another embodiment of the present invention is delivery
of a DNA sequence encoding a member of the TGF-P superfamily
to the connective tissue of a mammalian host by any of the
methods disclosed within this specification so as to effect
in vivo expression of collagen to regenerate connective
tissue, such as cartilage.
In a specific method disclosed as an example, and not
as a limitation to the present invention, a DNA plasmid
vector containing the TGF-P coding sequence was ligated
downstream of the metallothionein promoter.
Connective tissues are difficult organs to target
therapeutically. Intravenous and oral routes of drug
delivery that are known in the art provide poor access to
these connective tissues and have the disadvantage of
exposing the mammalian host body systemically to the
therapeutic agent. More specifically, known intra-articular
injection of proteins to joints provides direct access to a
joint. However, most of the injected drugs in the form of
encapsulated proteins have a short intra-articular half-
life. The present invention solves these problems by
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
23
introducing into the connective tissue of a mammalian host
genes coding for proteins that may be used to treat the
mammalian host. More specifically, this invention provides a
method for introducing into the connective tissue of a
mammalian host genes coding for proteins with anti-arthritic
properties.
In the invention, gene therapy was applied to solve the
problem of short duration of action and high cost associated
with administering TGF-P. The transfected cells could survive
for more than 6 weeks in tissue cultures without
morphological change. To determine the viability and duration
of action, the cells were injected into rabbit achilles
tendon. If the nutritional supply is adequate for the cells
in vivo, the cells could survive and produce TGF-P for a long
enough period of time to stimulate the surrounding cells. The
cells were functional in both the intratendinous and
intraarticular environment.
The concentration of transfected cells is an important
factor for local action. In a previous experiment (Joyce et
al., supra, 1990), the dose of TGF-P determined the type of
tissue formed. In particular, the ratio of cartilage
formation to intramembranous bone formation decreased as the
dose was lowered. TGF-P is also biphasic in stimulation of
primary osteoblasts and MC3T3 cells (Centrella et al.,
Endocrinology, 119:2306-2312, 1986). That is, it can be both
stimulatory and inhibitory according to the concentration
(Chenu et al., Proc Natl Acad Sci, 85:5683-5687, 1988). In
the Examples provided herein, the NIH 3T3-TGF-P1 cells
stimulated collagen synthesis in different concentrations of
104, 105, and 106 cells/ml. The tendon was enlarged mostly with
the concentration of 106 cells/ml.
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
24
In the Examples, the joint was injected with 0.3 ml of
106 cells/ml concentration. The specimens were harvested from
2 weeks to 6 weeks after injection. The environment in the
joint is different from that of the tendon. The cells can
move freely within the joint. They will move to the area with
specific affinity for the cells. The synovium, meniscus and
cartilage defect areas are the possible sites for cellular
adhesion. At six weeks after injection, the regenerated
tissues were observed at the partially and completely damaged
cartilage defect areas, but not at the synovium or the
meniscus. This specific affinity for the damaged area is
another advantage for clinical application. If degenerative
arthritis can be cured with just injection of cells into the
joint, the patients can be treated conveniently without major
surgery.
The TGF-P secreted by injected cells can stimulate
hyaline cartilage regeneration by two possible ways. One is
that the cartilage cells remaining in the damaged area
produce the TGF-P receptors at their cell surface (Brand et
al., J Biol Chem, 270:8274-8284, 1995; Cheifetz et al., Cell,
48:409-415, 1987; Dumont et al., M Cell Endo, 111:57-66,
1995; Lopez-Casillas et al., Cell, 67:785-795, 1991;
Miettinen et al., J Cell Biology, 127:6, 2021-2036, 1994; and
Wrana et al., Nature, 370:341-347, 1994). These receptors may
have been stimulated by TGF-P secreted by injected cells,
which adhere to the damaged area. Because TGF-P is secreted in
a latent form in vivo (Wakefield et al., J Biol Chem, 263,
7646-7654, 1988), the latent TGF-P needs an activation
process. The other way is that the latent TGF-P or the TGF-P
secreted from the transfected cells may have bound to the
TGF-P binding protein (LTBT) at the extracellular matrix of
CA 02373045 2001-11-02
WO 00/66177 25
PCT/IB00/00653
partially damaged cartilage layers (Dallas et al., J Cell
Biol, 131:539-549, 1995).
Whatever the mechanism of action is, the finding of
hyaline cartilage synthesis indicates that a long duration of
high TGF-P concentration can stimulate hyaline cartilage
regeneration. The vehicle for local high concentration may
not be the critical factor for local stimulation, but
theoretically, the cartilage cell may be the most suitable
vehicle for delivering TGF-P to damaged areas of the cartilage
(Brittberg et al., New Engl J Ned 331:889-895, 1994). The
collagen bilayer matrix is another possible vehicle for local
distribution of transfected cells (Frenkel et al., J Bone J
Surg (Br) 79-B:831-836, 1997).
The properties of newly formed tissue were determined by
a histological method. In H&E staining, the newly formed
tissue was identical to surrounding hyaline cartilage (Fig.
4). To evaluate the properties of newly formed tissue, the
tissues were stained with Safranin-O (Rosenburg, J Bone Joint
Surg, 53A:69-82, 1971). In contrast to the white color of
fibrous collagen, the newly formed tissue stained red,
suggesting that it is hyaline cartilage (Fig. 5).
The cells in the completely damaged area produced
fibrous collagen. The surrounding osteoblastic cells may not
have been stimulated because of the osteoid matrix barrier to
TGF-P stimulation. Instead of stimulating surrounding cells,
the NIH 3T3-TGF-P1 cells produced the fibrous collagen by
autocrine stimulation. The fact that the cells were
stimulated by both autocrine and paracrine activation
increases the likelihood of treatment of degenerative
arthritis with chondrocytes that have been stably transfected
with TGF-P1 expression constructs.
CA 02373045 2001-11-02
WO 00/66177 26
PCT/IB00/00653
The cell lines stably transfected with TGF-31 expression
constructs can survive in tendons and knee joints. The cell
lines produce fibrous collagen in the tendon and the
completely damaged cartilage area. However, the cell lines
produce hyaline cartilage in the partially damaged articular
cartilage. The mechanism of stimulation by autocrine and
paracrine modes of action indicates that gene therapy with a
member of the TGF-P superfamily of genes is a new treatment
method for hyaline cartilage injury.
The inventors made stable fibroblast (NIH 3T3-TGF-131,
and human foreskin fibroblast TGF-01) cell line by
transfecting TGF-131 expression constructs. These TGF-p-
producing cells maintained high concentration of active TGF-P
concentration in vivo for a long duration.
The first question to be answered regarding the
possibility of gene therapy and, in particular, cell-mediated
gene therapy is the viability of the cells in vivo. Even
though TGF-P can suppress the immune cells in vitro, the cells
may not be able to survive in the tissue of other species
with highly effective immune surveillance systems. Secondly,
the optimum concentration for gene expression in vivo should
be evaluated. We injected the cells into rabbit achilles
tendon in three different concentrations to answer this
question. The concentration of intraarticular injection to be
used was determined from the optimal concentration for
intratendinous injection. The third question is how the cells
stimulate the regeneration of cartilage within the joint.
There are two modes of action for the injected cells.
One is the activation of surrounding cells by secreted TGF-p
(paracrine activation) (Snyder, Sci Am, 253(4): 132-140,
1985), and the other is self-activation (autocrine
CA 02373045 2001-11-02
WO 00/66177 27
PCT/I1300/00653
activation). The concentration of cells may affect the
pathways, but the surrounding environment may be the most
important factor for the determination of action mode.
Intraarticular joint fluid and the interior of a ligament are
two different environments in terms of blood supply,
nutritional supply and surrounding cells. The transfected
cells were injected into two different environments to find
out the mode of action of the cells. The overall purpose of
this study was to evaluate TGF-P-mediated gene therapy for
orthopedic diseases and to ascertain the mode of action in
vivo.
The following examples are offered by way of
illustration of the present invention, and not by way of
limitation.
EXAMPLES
EXAMPLE I - MATERIALS AND METHODS
Plasmid Construction
To generate the metallothionein expression construct
(PM) the metallothionein I promoter (-660/+63) was
generated by polymerase chain amplification using genomic
DNA using Xba I and Bam HI restriction sites built into the
oligonucleotides used for amplification. The amplified
fragment was subcloned into Xba I-Barn HI sites of
pBluescript (Stratagene, La Jolla, CA). The plasmid pmT31
was generated by subcloning a 1.2-kb Bgl II fragment
containing the TGF-31 coding sequence and a growth hormone
poly A site at the 3' end into the Barn HI-Sal I sites of pM.
Cell Culture and Transfections - The TGF-P cDNA was
transfected into fibroblasts (NIH 3T3-TGF-P1) or human
foreskin fibroblast/TGF-131. They were cultured in Dulbecco's
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
28
Modified Eagle's Medium (GIBCO-BRL, Rockville, MD) with 10%
concentration of fetal bovine serum. The TGF-131 cDNA sequence
was added into the pmTP1 vector with a metallothionein gene
promoter. A neomycin resistance gene sequence was also
inserted into the vector.
The calcium phosphate method to insert this vector into
the cells was used. To select the cells with the transfected
gene sequence, neomycin (300 g/ml) was added into the medium.
Then, the surviving colonies were selected and the expression
-- of TGF-131 mRNA was confirmed by Northern analysis and TGF-3l
ELISA assay (R & D Systems). The cells with TGF-P1 expression
were stored in liquid nitrogen and cultured just before the
injection.
Northern Blot analysis - Total RNA was isolated from
-- cells with guanidium isothiocyanate/phenol/chloroform. 10 g
of RNA was electrophoresed on a 1.0 % agarose gel containing
0.66M formaldehyde, transferred to a DURALON-UV membrane, and
cross linked with a UV STRATALINKER (STRATAGENE). Blots were
prehybridized and hybridized in a solution of 1% bovine serum
-- albumin, 7% (w/v) SDS, 0.5 M sodium phosphate, and 1 mM EDTA
at 65 C. Hybridized blots were washed in 0.1 % SDS, 1 X SSC
for 20 minute periods at 50 C before film exposure. RNA blots
were hybridized with 32P-labelled cDNA probes for human TGF-
pl. A probe for 13-actin was used to control for sample
loading.
Injection of cells into rabbit - New Zealand white
rabbits weighing 2.0 - 2.5 kg were selected as the animal
model. After anesthetization with ketamine and roumpun, each
rabbit was draped in a sterile manner. The achilles tendon
CA 02373045 2001-11-02
WO 00/66177 29
PCT/IB00/00653
was exposed, and 0.2 - 0.3 ml of cells with 104, 105 and 106
cells/ml concentrations were injected into the mid-portion of
the tendon. Zinc sulfate was added to the drinking water of
the rabbits for the expression of transfected DNA. After
determining the optimal concentration with achilles tendon
experiments, the intraarticular injection was performed. The
knee joint was exposed, and partial and complete cartilage
defects were made with a knife. The partial defects were made
on the hyaline cartilage layer with caution not to expose the
subchondral bone. The complete defects were made to expose
the subchondral bone after removing all of the hyaline
cartilage. After closing the surgical wound, the cells with
106 cells/ml concentration were injected intraarticularly, and
zinc sulfate was added to the drinking water.
Histological examination - After harvesting the tendons
and knee joints, the specimens were fixed in formalin and
decalcified with nitric acid. They were embedded in a
paraffin block and cut into 0.8 m thickness slices.
Hematoxilin-eosine, and Safranin-O staining were utilized to
observe the regenerated tissue microscopically.
EXAMPLE II - RESULTS
Stable cell line - Transfection was carried out by using
the calcium phosphate coprecipitation method (Fig. 1). About
80% of the surviving colonies expressed the transgene mRNA.
These selected TGF-Pl-producing cells were incubated in a zinc
sulfate solution. When the cells were cultured in 100 M zinc
sulfate solution, they produced mRNA. The TGF-P secretion rate
was about 32 ng/106cells/24 hr.
CA 02373045 2001-11-02
W000/66177
PCT/IB00/00653
Regeneration of Rabbit Articular Cartilage Defect - The
rabbit achilles tendons were observed to check the viability
of NIH 3T3-TGF-131 cells. At 106 cells/ml concentration, the
tendon was grossly thicker than at the other two
5 concentrations of 104 and 105. After making partial and
complete cartilage defects, 0.3 ml of 106 cells/ml of the NIH
3T3-TGF-131 cells were injected into knee joints. The joint was
examined 2 to 6 weeks after injection. In partially damaged
cartilage, we found newly formed hyaline cartilage; two weeks
10 after injection, hyaline cartilage appeared and six weeks
after injection, the cartilage defects were covered by
hyaline cartilage (Fig. 2). The thickness of the regenerated
cartilage became thicker as time passed (Fig. 3). The
injected cells secreted TGF-P1, that could be observed by
15
immunohistochemical staining with TGF-Pl antibody (Fig. 3).
The contralateral side injected with normal fibroblasts
without TGF-Pl transfection was not covered by hyaline
cartilage. In the partially damaged area, the regenerated
hyaline cartilage was colored red in Safranin-O staining
20 (Fig. 4). (The depth of newly formed cartilage was almost the
same as that of the defect.) This finding suggests that the
injected cells activate the surrounding normal cartilage
cells through a paracrine mode of action.
The regenerated tissues in completely damaged cartilage
25 were not hyaline cartilage but fibrous collagen. Their color
in Safranin-O staining was white instead of the red color
obtained with hyaline cartilage (Fig. 5). The cartilage was
covered by fibrous tissue, which means that these cells were
activated only by the autocrine mode. The surrounding
30 osteocytes, which can be stimulated by TGF-P, appeared to have
been blocked from being stimulated by TGF-p by the presence of
CA 02373045 2014-11-25
= '
31
a thick calcified bone matrix. The injected cells may have
been unable to stimulate the osteocytes because of this
barrier.
TGF-Pl transfected cells were injected into rabbit
achilles tendon. The tendon so manipulated exhibited a
grossly thicker morphology (Fig. 7) than the control tendon.
H&E staining of a section of the tendon showed, under
microscopic examination, the injected NIH 3T3-TGF-11 cells
survived and produced fibrous collagen in rabbit achilles
tendon (Fig. 8). Microscopic examination of the regenerated
tendon tissue stained immunohistochemically with TGF-Pl
antibody showed the expression of TGF-01 in the tendon (rig.
9).
Whereas particular embodiments of this invention have
been described above for purposes of illustration, it will
be evident to those persons skilled in the art that numerous
variations of the details of the present invention may be
made without departing from the invention as defined in the
appended claims.
CA 02373045 2001-11-02
WO 00/66177 32
PCT/IB00/00653
REFERENCES
Andrew JG, Hoyland J, Andrew SM, Freemont AJ and Marsh D:
Demonstration of TGF-131 m-RNA by in situ hybridization in
normal fracture healing. Calcif Tissue Int, 52: 74-78,
1993.
Bourque WT, Gross M and Hall BK: Expression of four growth
factors during fracture repair. Int J Dev Biol, 37: 573-
579, 1993.
Brand T and Schneider MD: Inactive type II and type I
receptors: TGF-P are dominant inhibitors of TGF-P dependent
transcription. J Biol Chem, 270: 8274-8284, 1995.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson 0 and
Peterson L: Treatment of deep cartilage defects in the knee
with autologous chondrocyte transplantation. New Engl J Med
331: 889-895, 1994.
Carrington JL, Roberts AB, Flanders KC, Roche NS and Reddi
AH: Accumulation, localization and compartmentation of TGF-
p during enchondral bone development. J Cell Biology, 107:
1969-1975, 1988.
Centrella M, Massague J and Canalis E: Human platelet-derived
transforming growth factor-3 stimulates parameters of bone
growth in fetal rat calvariae. Endocrinology, 119: 2306-
2312, 1986.
Cheifetz S, Weatherbee JA, Tsang MLS, Anderson JK, Lucas R,
Massague J: Transforming growth factor beta system, a
complex pattern of cross-reactive ligands and receptors.
Cell, 48: 409-415, 1987.
Chenu C, Pfeilschifter J, Mundy GR and Roodman GD: TGF-P
inhibits formation of osteoclast-like cells in long-term
human marrow cultures. Proc Natl Acad Sci, 85: 5683-5687,
1988
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
33
Critchlow MA, Bland YS and Ashhurst DE: The effect of
exogenous transforming growth factor-132 on healing
fractures in the rabbit. Bone, 521-527, 1995.
Dallas SL, Miyazono K, Skerry TM, Mundy GR and Bonewald LF:
Dual role for the latent transforming growth factor beta
binding protein (LTBP) in storage of latent TGF-P in the
extracellular matrix and as a structural matrix protein. J
Cell Biol, 131: 539-549, 1995.
Dumont N, O'Connor M and Philip A: Transforming growth factor
receptors on human endometrial cells: identification of the
type I and II receptors and glycosyl-phosphatidylinositol
anchored TGF-P binding proteins. M Cell Endo, 111: 57-66,
1995.
Frenkel SR, Toolan B, Menche D, Pitman MI and Pachence JM:
Chondrocyte transplantation using a collagen bilayer matrix
for cartilage repair. J Bone J Surg [Br] 79-B: 831-836,
1997.
Heine UI, Munoz EF, Flanders KC, Ellingsworth LR, Peter Lam
H-Y, Thompson NL,Roberts AB and Sporn MB: Role of
Transforming Growth Factor-13 in the development of the
mouse embryo. J Cell Biology, 105: 2861-2876, 1987.
Jenks S: Gene therapy: mastering the basics, defining details
[news]. J Natl Cancer Inst, 89(16): 1182-1184, 1997.
Joyce ME, Roberts AB, Sporn MB and Bolander ME: Transforming
Growth Factor-13 and the initiation of chondrogenesis and
osteogenesis in the rat femur. J Cell Biology, 110: 2195-
2207, 1990.
Lind M, Schumacker B, Soballe K, Keller J, Nelsen F, and
Bunger: Transforming growth factor-j3 enhances fracture
healing in rabbit tibiae. A Orthop Scand, 64(5): 553-556,
1993.
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
34
Lopez-Casillas F, Chifetz S, Doody J, Andres JL, Lane WS
Massague J: Structure and expression of the membrane
proteoglycan component of the TGF-P receptor system. Cell,
67: 785-795, 1991.
Madri JA, Pratt BM and Tucker AM: Phenotypic modulation of
endothelial cells by Transforming Growth Factor-0 depends
upon the composition and organization of the extracellular
matrix. J Cell Biology, 106: 1375-1384, 1988.
Mankin HJ: The response of articular cartilage to mechanical
injury. J Bone Joint Surg, 52A: 460-466, 1982.
Massague, Ann. Rev. Biochem. 67:753-791, 1998.
Matsumoto K, Matsunaga S, Imamura T, Ishidou Y, Yosida H
Sakou T: Expression and distribution of transforming growth
factor-P during fracture healing. In vivo, 8: 215-220,
1994.
Miettinen PJ, Ebner R, Lopez AR and Derynck R: TGF-P induced
transdifferentiation of mammary epithelial cells to
mesenchymal cells: involvement of type I receptors. J Cell
Biology, 127-6: 2021-2036, 1994.
O'Driscoll, J. Bone Joint Surg., 80A: 1795-1812, 1998.
Ozkaynak E, Rueger DC, Drier EA, Corbett C and Ridge RJ: OP-1
cDNA encodes an osteogenic protein in the TGF-P family.
EMBO J, 9: 2085-2093, 1990.
Rosenburg L: Chemical basis for the histological use of
Safranin-O in the study of articular cartilage. J Bone
Joint Surg, 53A: 69-82, 1971.
Sampath TK, Rueger DC: Structure, function and orthopedic
applications of osteogenic protein-1 (0P-1). Complications
in Ortho, 101-107, 1994.
Snyder SH: The molecular basis of communication between
cells. Sci Am, 253(4): 132-140, 1985.
CA 02373045 2001-11-02
WO 00/66177
PCT/IB00/00653
Sporn MB and Roberts AB: Peptide growth factors are
multifunctional. Nature (London), 332: 217-219, 1988.
Wakefield LM, Smith DM, Flanders KC and Sporn MB: Latent
transforming growth factor-0 from human platelets. J Biol
5 Chem, 263, 7646-7654, 1988.
Wolff JA and Lederberg J: A history of gene transfer and
therapy. John A. Wolff, Editor. Gene Therapeutics, 3-25,
1994. Birkhauser, Boston.
Wrana JL, Attisano L, Wieser R, Ventura F and Massague J:
10 Mechanism of activation of the TGF-P receptor. Nature, 370:
341-347, 1994.