Note: Descriptions are shown in the official language in which they were submitted.
CA 02373562 2002-02-27
Eon dt Ep PROCESS FOR BLEACHING OF CHEMICAL PULP
RELATED APPLICATION
This application claims priority from Provisional A.pplieation Serial Numbar
60/2? 1,987 filed on February 27, 2001.
BACKC3~ROUND OF THE INVENTION
Historically the treatment of wood chips to form a chemical pulp has been
divided into two prOGO$5e5. The first process is pulping and the ascend
process is
1.0 bleaching.
Pulping is the changigg of wood chips or othar wood particulate math to
fibrous form. Chemical pulping includes partial removal of lignin and other
materials associated with the wood.
Bleaching is the treatment of the partially delignifiod cellulosic fibers with
chemicals to remove or slter the coloring matter associated therewith.
Bleaching
brightens the fibers in order to reflect white light more truly.
Throughout the evolution of pulp bleaching, caustic soda (NaOH) has boon
used as the primary alkali source in bleaching of chemical pulp. Caustic soda
is
a highly soluble alkali that readily provides an. optimum reaction pH and
facilitates
the dissolution of lignin from pulp in pulp bleaching. Since caustic soda is a
strong base, carbohydrate degradation can occur resulting in a decrease in
pulp
viscosity and as increase in chemical oxygen demand (COD).
In conventional pulp bleaching, a D- E~-D-E,P-D bleaching saquence is
often employed far brightening chemical pulp. E~ bleaching typically utilizes
hydrogen pQroxide, oxygen, caustic soda cad magnesium sulfate as the bleaching
chemicals. F,~ bleaching typically utilizes hydrogen peroxide ~d caustic soda
s~s
the bleaching chemicals. Conventional E~, and EP bleaching suffer tom the
drawback of increased COD which is described above.
Accordingly, there is s need for a 8~ and Ep bleaching sequences which
overcome the above-described drawbadG,
CA 02373562 2002-02-27
SUMhfARY OF THE INVENTION
This ixrve~ion is directed to an E~, and an Ep process for bleaching
chemical pulp which overcome drawbacks associated with conventional bleaching.
The subject process comprises providing a bleached chemical pulp
produced by bleaching sequences in which E°p and/or the EP aqueous
bleaching
solutions employed therein include magnesium hydroxide in place of a
substantial
portion of the NaOH, and as a total replacement for any magnesium sulfate. The
E°p aqueous bleaching solution can comprise a peroxide compound, an
oxygen-
containing materiel, sodium hydroxide and magnesium hydroxide, in the absence
of magnesium sulfate. Attematiwely, the E~ aqueous chemical solution for
bleaching chemical pulp can eomprisc an E~ aqueous bleaching solution
consisting essentially of a peroxide compound, an oxygen-containing material,
sodium hydroxide and magnesium hydroxide. In any case, chemical pulp is
bleached with sa E~ aqueous bleaching solution to form an E°y bleached
chemical
pulp. The E~ aqueous bleaching solution of this invention can be employed in a
D-E°P D-Ep-D bleaching sequence. The subject invention is also
di~re,~ted to a
bleaching sequence which includes the EP bleaching of chemical pulp. The F.~,
aqueous bleaching solution can comprise a peroxide compound, sodium hydroxide
and magnesium hydroxide, in the absence of magnesium sulfate. Stated another
way, an Ep aqueous bleaching solution can be provided consisting essentially
of a
peroxide compound, sodium hydroxide and magnesium hydroxide. In certain
bleaching sequences, for example, the ED aqueous bleaching solution of the
subject
invention can be provided for bleaching E~ bleached pulp to form an Ep
bleached
chemical pulp. The EP aqueous bleaching solution of this invention can also be
employed in a D-E~ -D- Ep -D bleaching sequence.
In the E°P and/or Ep processes of the present invention, the
amount of
sodium hydroxide in the E°p and/or aqueous bleaching solution is
preferably not
more than about 8 % by weight, more preferably not more than about 5 % by
weight, and most preferably not mass than about 3 % by weight, based on the
O.D.
weight of said chemical pulp,
CA 02373562 2002-02-27
Furthermore, the ratio of sodium hydroxide to magnesium hydroxide in
said E~ aqueous bleaching solution ie not ttrore than about S:1 (based on an
OH'
molar ratio), prefierably not more than about 3:1 (based on an OFi' molar
ratio), and
most preferably not more than about 1:2 (based on an OH' molar ratio). As for
the
ratio of sodium hydroxide to magnesium hydroxide in said F,~, aqueous
bleaching
solution" it is not mono than about 1:1 (based oa an OH' molar ratio),
preferably
not more than about 1:3 (based on an OH' molar ratio). Most preferably about
100% magnesium hydroxide is employed without substantially any sodium
hydroxide.
Both the E~ and P~, bleaching pmeassos of this invartioa preferably
coatemplatos that the pulp viscosity of a final bleached chemical pulp is at
least
substantially the same as the pulp viscosity of a final bleached chemical pulp
which is bleached with the same total amount of an E~ aqueous blaa~chiag
solution
comprising a peroxide compound, oxygen-eontainirtg material, sodium hydroxide,
with or without magnesium sulfat~, is the absoacc of magnesium hydroxide, on
the
one hand, and/or an F,~ aqueous blaaehing solution comprising a peroxide
compound and sodium hydroxide, with or without magnesium sulfate, in the
absence of magnesium hydroxide, on the other hand. Pulp viscosity is measured
in
centipoises using the 0.5°~ CED viscosity test method described is
TAPPI T-X30.
2o Also, the preferred E~, process generates a bleach oi~luent which has a
COD which is less Than the COD of a bleach e~luont from an E~ process which
esaploys an Eq, aqueous bleaching solution comprising the peroxide compound,
oxygen-containing material, sodium hydroxide, with or without magnesium
sulfate, in the absence of magnesium hydroxide_ More specifically, the COD of
the bleach effluent produead wring the E.q, aqueous bleaching solution of the
present invention is preferably at least about 5%, morn preferably at least
about
8%, and most preferably at least about 10%, less than the COD geacratad by as
E~ stage which uses an E~ aqueous bleaching solution comprising said peroxide
compound, oxygen-containing material, sodium hydroxide, with or without
n~gneaium sulfate, in the absence of:nagnosium hydroxide. COD is measured in
mglL using a HACH test ldt.
3
CA 02373562 2002-02-27
Moreover, the bleached chemical pulp of x bleach aequenco containing the
subject process preferably has a final ISO brightness (% ISO) of which is at
least
substantially the same as the dal ISO brightness (% ISO) of bleached chemical
pulp which is bleached with the same bleach sequence but with as E~ aqueous
bleaching solution comprising a peroxide compound, oxygen-containing
nzsterial,
sodium hydroxide, with or without magnesium sulfate, in the absence of
magnesium hydroxide, on the ono hand, and/or an EP aqueous bleaching solution
comprising a peroxide compound and sodium hydroxide, with or without
magnesium sulfate, in the absence of magnesium hydroxide, on the other h~tad.
1 o ISO brightness is measured using the teat method deacribod in TAPPI ?-45Z.
The process of this invention also produces a bleached charnical pulp
having a preferred wet zero span tensile strength which is at least
substatltixlly the
same as the wet zero span tensile strength of bleached chemical pulp which is
bleached with the same total amount of an E~ aqueous bleaching solution
comprising a peroxide compound, oxygen-containing material, sodium hydroxide,
with or without magnesium sulfate, in the absence of magnesium hydroxide, on
the
ono hand, arul/or as Ep aqueous bleaching solutian comprising a pe~roxidc
carnpound and sodium hydroxide, with or without magnesium sulfate, in the
absence of magnesium hydroxide, on the other hand Wet zero spats tensile
strength is measured in bn using a Pulmac wet zero-spars tensile apparxtus_
E~ and P~, bleaching are typically part of a broader overall ehecr~ical pulp
bleaching sequmco_ Thus, there can be one or mono additional chemical pulp
bleaching stages that occur prior to Fey, and F,~, bleaching, as well as one
or more
additional chemical pulp bleaching stages that occur aubsequcnt to F.ro,, and
Ep
bleaching. Usually, these additional chemical pulp bleaching stages are
conducted
employing oonvontional bleaching technology utilizing bleaching ch~aicaia such
as CIOz, Oz, Cl= and peroxide.
The foregoing and other objects, features and advantages of the invention
will become morn apparent from the detailed description of a preferred
embodiment of the invention below which proceeds with reference to the
accompanying drawings.
d
CA 02373562 2002-02-27
DESCRIPTION OF THE Dn,A,W'>T1GS
FIG. 1 is a schematic diagrarrt comparing pulp brightness for various levels
of bleaching solution in an E~, ble~ehing process.
FIC3, 2 is a schacnatic diagram comparing pulp viscosity for various levels
of bleaching solution in an P,,~ bloacbing process.
FIG. 3 is a schematic diagratri comparing pulp wet tern-span tensile
strength for various levels of bleaching solution in an E°,, bleaching
process.
FIG. 4 is a sehomatic diagram tampering pulp COD for various levels of
bleaching solution irr an E,~ blaaahing psocess.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
As set forth in Tables I-III, an aqueous slurry containing magnesium
hydroxide was employed as a partial substitution fnr caustic ands and a
complete
replacement for magnesium sulfate in the e~ctraction-oxygen-peroxide stage
(E~,,)
of the bleaching of northeast softwood kraft pulp. More specifically,
laboratory
experiments examined the bleachability of the pulp through a complete DBopDEpD
bleach aequance with complete replacement ofmagaesium Sulfate and partial
substitution of NaOH on an OH' molar basis using CellGuard""OP (sec
description
of CellC~uard""OP below) in the F.q, stage. Three magnesium hydroxide
substitution levels were evaluated in the E~, stage: 0°/a, 259°,
and 30°/p of the OH'
derived from CollGuard"~OP and the balance Srom NaOH.
In Table III, an aQucous slurry containing magnesium hydroxide was also
employed as a partial substitution for caustic soda and a complete replaaoment
for
magnesium sulfate in the oactraction-peroxide stage (F.~) of the bleaching of
northeast soRwood kraft pulp. More speciEcally, laboratory experiments
examined the bleachsbility of the pulp through a complete D E"~ D F.,, I7
bleach
sequence with complata raplacearent of magnesium sulfate and partial
substitution
of NaOH on an OH- molar basis using CellCiuard'~OP (see description of
CcllC~uard~"OP below) is both the F.°,, and Bp stages. Four magnesium
hydroxide
c
CA 02373562 2002-02-27
substitution levels were evaluated is the EP stage: 0%, 25%, SOor6, 7S% and
100'/0
of the OH' derived frtrm CallGuard"~OP and the balsaee, if nay, from NaOH.
Bleaching experiments for the ~ stage were performed in a 2-liter,
medium consistency oxygen reactor equipped with a mixer. The oxygen reactor
was treated with nitric acid to passivate the surface to avail contamination
from
metals. The Do, D,, F,p, and Dx stages were performed by traditional bag
bleaching
methods. The bmwnstock ktaR pulp to be bleached bad a kappa number of 29.2, a
brightness of 26.9° ISO, and viscosity of 48.0 cps. This ktaR pulp was
bleach~l
under the conditions outlined in Tables I-III. Chemical doasges were based on
the
weight of oven-dried pulp. The oxporinaeatal procedure for oath stage is
summarized as follows:
egg. Standard conditions as sot forth in Tables I-III wore employed
for the Do stage. The kappa factor was adjusted with 2.44% C10= to reach the
desired kappa number out of the Do stage. The pulp was then washed to pH 7.5.
E. After the Do stage, the pulp was exttact~d with a conventional
F.~ stage which employed 1.7% NaOH sad 0.1 % MgSO,s. For comparison
purposes in Tables I and II, bleaching experiments using CcllGuard"'OP as a
replacement fo* MgS04 and as a partial substitute for NaOH were conducted,
Caustic sale replacement was based on an OH' molar basis. For example, at 25%
substitution where 25% of the OH' comes 8rom Mg(OH)z, the respective alkali
dosages used were 1.275% NaOH sad 0.31~o CellGuard'~"OP. Cax~atio soda
contains 42.51 % by weight OH' ions while maguosium hydroxide contains
58.32°Yo
by weight OH' ions, For the E°,, stage in Tables I-III, bleaching
occurred at 138
kPa of 02 pressure for 20 minutes followed by bleaching at atmospheric
pressure
for the remaining 40 minutes.
Martin Marietta Magnesia Specialties, LLC is the maaufaaturer of
CcllGuard~OP Magaesiium Hydroxide Slung for use in pulp bleaching. The
CeIlGuard""OP Mag~nosium Hydroxide Slurry, which is produced $om a dolomitie
limo and magnesium brine process, contains 62% by weight Mg(OIT)z solids
suspended in water. The purity of the magnesium hydroxide is over 98% with low
levels of transition motels. This product has a fine particle size (3 microns
as
6
CA 02373562 2002-02-27
toeasured by Micromeritics Sedigtaph 5100) which promotes high reactivity and
excellent suspension stability.
I~1~3
Pulp Time Temp Pressure Chemical
Stage Consistency(mm) (C) (kPa) % on O.D.
pulp
a
Do 12 45 60 -__ 2,44% Cl
0.5% HsOa
138 kPa of Oz 1.7/ NaOH
for or
EoP 12 60 70 20 mur, then Mg(OH~/NaOH
arm 0.1 % MgS04
(for Contro:
onI
D 12 180 80 -- _ QZ
1.2% C1
EP 12 40 70 --~-- (1. I5% HaOs
0.5% NaOH
Dz I2 100 80 --- 0.6% CIOz
DmF.~,~,~'I~T es I and I11-Standard conditions ware employed for
the Dt-F.~-D= stages shown in Tables I and II. The pulp and filtrate following
each
bleaching stage were tested for I,SO brightness sad end pH, respectively. The
pH
of the residual liquor was dettrrnined using a pH meter and appropriate buffer
solutions to calibrate the pH meter. Pulp samples from the E~" D~, and D2
stages
ZO were tested for viscosity and wet zero-span tensile strength. The
respective
filtrates were analyzed for chemical oxygen demand (COD).
After the Do stage and washing stop, the lcsppa number decre~aeed from
29.2 to 11.9. Pulp brightness iaCreased fi~ 26,9% ISO to 35.9% ISO.
The effects of CcllGuard"~"OP substitution in the P~ stage on each of the pulp
15 parameters were measured. Table II contains a summary of results for each
bleaching test performed.
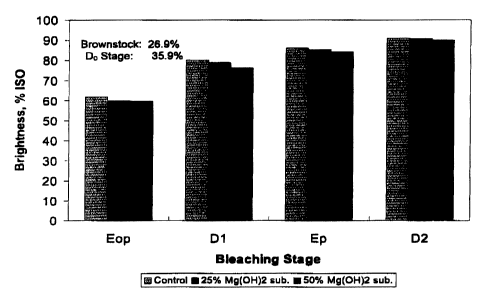
FIG. 1 compares pulp brightness for the various levels of CellGusrd~"OP
substitution in tire E~ stage. The data shows the increasing trend is
brightness
with each subsequent bleaching stage for all substitution cases. The control
20 sample, which employed 100°i6 4H' from NaOH (or 4°Yo OH' from
C~llGuard"'~OP)
7
CA 02373562 2002-02-27
and MgSO,, yielded as increase in brightness from 62.1 % ISO after the
E°P stage
to a final pulp brightness of 90.8% ISO after the D2 stage.
At 25% substitution with CellGuard""OP in the E~ stage, brightness slier
this bleaching step (60.1% ISO) was lowar than the control. However,
equivalent
final pulp brightness after the Da stage resulted in 90.5% ISO at the same
C10= and
HZOZ charge in the Dl and EP stages respectively ats the control sample.
At 50% substitution with CollC~ruatd~"OP, Figure 1 shows that the
brightness after the Pte,,, stage was 59.7% ISO. Even with the use of a mild
alkali in
the extraction stage which lowers the bleaching pH to 10.1 for
25°!° substitution
and 9.3 for 50% substitution, comparable final brightness was achieved as
eridence by the final 89.9% ISO brightness result after the Da stage.
At the end of the E~,, stage, the control sartnpIe yielded a pulp viscosity of
27.8 cps. As shown in FIG. 2, both the 25% and SO% substitution runs with
CellGuard"'OP produced higher viscosity results of 28.6 cps and 29,3 cps
respectively after the E~ stage. These results indicate that CellGusrd'7"OP
servos a
dual role as a peroxide activator and cellulose pmtoctor. In a conventional E~
system where NaOH and MgS04 are utilized, CellGuard"~OP Mg(OH)2 can reduce
the NaOH consumption and eliminate MgS04. As soon in FIG. 2, the control
sample's final viscosity was 16.5 cps, the 25% and 50% substitution samples
with
CellGuard~OP produced pulps with respcctivo viscosities of 17.5 cps and 18_6
cps. By utilizing CellGuard"'OP Magnesium Hydroxide in the E%, stage, a one to
two point increaso in pulp viscosity over the conventional E°,, stage
was achieved.
Fiber strength was maintained in both CollGuard~OP substitution cases as
shown in Figure 3. Wet zero-spas tensile strength for all cases ranged from
10.9 -
11.0 km for the E~ stage, 10.2 - 10.6 for the D~ stage, and 10.3 - 10.4 inn
for the
DZ stage.
High chomical oxygen demand (COD) is gonorated in the E~ stage where
the most of the extraction occurs (ace FIG. 4). Partially substituting the
NaOH
charge with a weak alkali such as Mg(OH)2 reduces the organic loading in the
effluent from the E~, stage. 'The highcFr substitution rate with CellGuard~OP
CA 02373562 2002-02-27
yielded a noticeable ~da~otion itt COD whoa cared to tho control aa:aple. Then
control sample generated 4635 mgt COD vatsus 4105 mg/L COD for the 50%
substitution cast, which roprosenta an 11% roduction. Lower COD loading can
contribute to reduced e~'tuent treatment costs dowaatream.
By employing partial substitution of CellGuard'~OP Magnesium Hydsoxide
Slurry for eausdc soda and eliminating magnesium sulfate in the Eop stage, a
strong
alkali is present to facilitate lignin removal while a mild allca~lii is added
to promote
peroxide bleaching arid eelluloac protecdon. CallGuard~'"OP can replace up to
50% of the caustic soda requirement in the Eq, stage producing final pulp from
the
Da stage with similar brightness, permanganate number, and wet aem-span
tensile
strength a: pulp bleached with a 100% caustic soda charge.
At 50% substitution of caustic soda, the use of Cdlt~luard~OP improves the
pulp viscosity by 12% and reduces the COD after the E~, stage; by 1 I %. Since
only 0.73 kg of magneaiurn hydroxide provides the equivalent amount of
hydroxyl
ions as 1 kg of caustic soda, Ce11C3uard~"OP reduces bleaching costs in many
cases.
At no added cost beyond the amount required for caustic soda replacement,
Cell(~uard'~"OP also elitniaates the steed for magnesium sulfate for i~ther
reduction in chmaical costs_
9
CA 02373562 2002-02-27
RESULTS OF PARTIAL SUBS'ITTUZTON OF
CAUSTIC SODw vViT~i MAaIvtESItJM HYDROXIDE SLURRY
IN THE EOP STA(i8 Og A DEopDEpD BLEACH SEQUENCE
..
Star Chemical Timo Tamp PressureEnd ~ $ COD
,...
a (r6 on (~) (~ (I~'a)pfY ~ ~ ~ ~ ~, (~)
pulp)
'
3
Brown- , ; 26 48 (~~ 5
9 0 ~ 12
' . . .
stock , ; ~ .
. ~; .
.. '
7.4
0.22 kf 45 ~0 2.3Z 35.9 ' (11.9
as
D Stage D (2.44% , ~.
C10~ kappa)
0.5~6 FIz02 138
kPa
Oa
1.7% NaOH for
~ 70 ,20 11.3 62.1 27.8 3.1 10.9 4635
Eop 0.1 % MBS04 ~jn,~
thaa
0~~ Mg(OH)i
Coatrol
N
OH +
a
M~SO~ 1.2% CIOs 180 80 2.3 80.2 21.4 1.1 10.2 1352
D
0. I S~b 40 70 ~ 11.4 86.0
Hi0=
E 0.5 % NaOH
0.6% CIO= 100 80 ~ 3.3 90.8 16.5 O.B 10.3 176
D
I 38
0.5% H=Os Id?a
pr
1.275/b fpr
NaOH
60 70 20 10.1 60.1 28.6 3.4 I0.8 4555
Eop 0.31~o min,
then
25% MB(OH)z
a~
Substirution
_
CellGuard'~ 1.2% CIOa 180 80 . ' 2.3 78.9 22.1 1.3 10.3 1326
.
in Eop D,
0.15% Ii=O=~ 70 ~' 11 85 ' . .
4 3 '
E 0.5 9~o . . . ' ' . ' . ..
NaOH '
D 0.6% C10~ 100 80 '' 3.3 90.5 17.5 0.6 10.2
'
0.5% I~ZOz
138
kPa
0.85% NaOH
~ 70 20 9.3 59.7 29.3 3.5 11.0 4105
Eop 0.62% min,
~a
MB(O~t
50% a~
S
b
rit
ti
u ,
s
on
u
CellGuard'"D 1.2% CIOz 180 80 : 2.3 76.3 22.0 1.4 10.6 1420
i
E
op
n
0.15% Hl0=
40 70 ' 3 83
11 9
B 0.5% NaOH . . .
D 0.6% CIOi 100 80 . 3.2 89.9 18.6 0.7 10.4 184
CA 02373562 2002-02-27
' % on oven-dried pulp
= After waahiag, pH was 7.5
All experimaata wore conducted at 12% pulp consistency.
B°P -DZ-E~DZS~ag, ~ ('Tabl~IIn-Standard conditions were employed
for
the D~ and DZ stages of bleaching processes shown in Table III. In the
experiments involving substitution of magnesium hydroxide for NaOlI in the Ep
stage, magatsium hydroxide was substituted for all of the magaeaium sulfate
sad a
portion of the NaOH (25%, 50%, 75% or 100%). The E~ atagc employed a 50%
substitution of magnesium hydroxide for NaOH, and 100% substitution for all of
the magnesium sulfate, in all the bleaching experiments. The pulp and filtrate
following each bleaching stage were tested for IS4 brightness and end pH,
respectively. The pH of the residual liquor was detenainod using a pH motor
and
appropriate buffer solutions to calibrate the pH meter. Pulp samplas from the
Eq,,
D~, and DZ stages were also tested for viscosity and K#. As seen in Table IIi,
the
~rLal ISO brightness, viscosity and K# of the bleached chemical pulp of the
process
of the present ~invontion is comparable or greater than the the final ISO
brightness,
viscosity and K# of bleached chemical pulp which is bleached with the same
total
amount of an F.~, aqueous bleaching solution comprising said paroxide compound
and sodium hydroxide, but in the absence of magneaium hydroxide.
11
CA 02373562 2002-02-27
Table III. RESULTS OF SUBS~TT~J'TION OF CAUSTIC SODA
AND MAC~NESIIJM SULFATE WITH MAC3NESIUM
HYDROXIDE SLURRY
In E~ and Ep Stages of a DES DEpD Bleach Sequence
StaOe C~tmital Tlms T >cure Kappa KB !SO Vit-
e,~ (rp(p)$ (pd~ 25 Brl=bt eaa-
m!
aea~ ity
Ye ep
BS $rpWI~t~Ck 30.4 Z6.1 51.9
D
After W,flblp/~
O 13.7 8.6 34.3 48.Z
Z2kf
~, ~,5 pD D .
.54% CIO
g O.s'l,H=O= 60 70 138Kpa 3.5 61.6 41.9
I00% OH- 1.7% NaO~i
CONTROL trem lr,o7~to.t~ Mgso,
NaOH + o/. wtg (OHh thoa
avn
MgS04
Sequence Di 1.2%QO~ t80 80 1.3 79.6 Z9.s
E & E sta
es ~ 70 86.4
g
s
OH only Eo0rl, OH 0,4896 aOH
N
from NaOH 0% Mg (OH)s
DZ 0.6/ CI 100 80 0.6 90.7 22.0
B 0.5/.H=Oz 60 70 1381Cpa 4.0 34.9 42.6
50~ OH 0.85% NaOH pf
Z
from Me(Oli~0.6T% Mp min.
(OHj=
Substitution den arm
Scquence
1.2% C10= 180 80 1.6 74.9 32.9
op
50%
0.15%H=0= 40 '~0
StrbStltatl0nBv
Coptrol 0.48% NaOH
o% M~ (off:
Ep
v~ysn~
Snbstitvtian DZ 0.6% C10= 100 s0 0.7 90.3 24.6
l2
CA 02373562 2002-02-27
Ro 0.15'yG~i~Oa40 70 83.d
=3% O~ 0.36iG NWH
prom M8(OI~Lh0.0915 M8
(0F1),
DZ 0.6lfi CID, 100 80 0.'7 90.2 ?4.d
E
O.15YHiOi 40 70
3.1
s0% OH- 0.?4% NaOH
from M8(OH~0.18% Mg
(OHM
ps 0.6% CIO= 100 80 0.8 89.9 25.2
Coot-
ME(UH)= ~ o.is~x~ 40 ~o e~.s
~~~ oe-
Subatitutionfrom Maoab o
~ ~ (o
~
sequence Hh
~' P D= 0.6% CIO, 100 80 89.5 25.3
50%
Substitution~ o.isx~to, 40 ~o ~8.s
1006 OH- 09~, Na013
E prom o 0.3s% Ma
p (OH)=
Varying
Sll~lat~ttlt~4n 0.6IG CIO, 100 SO 0.8 88.6 7R.0
D2
13