Note: Descriptions are shown in the official language in which they were submitted.
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
1
Multi-Stage Reforming Process Using
Rhenium-Containing Catalyst in the Final Stage
Field of the Invention
1o This invention relates to a process for conversion of hydrocarbons.
More specifically, the invention relates to a process for upgrading a
hydrocarbon feedstock by reforming followed by hydrodealkylation. A
multi-stage reforming process using a rhenium-containing catalyst in the
final stage is disclosed. The final stage produces additional benzene,
toluene and xylenes by dealkylating alkylated aromatics.
Background of the Invention
The reformate upgrading process of this invention, and its
2o background, is more completely described in U.S. Pat. No. 5,865,986,
which is incorporated by reference in this application. In the instant
application, however, the catalyst of the final stage is loaded with rhenium
rather than palladium or platinum, providing significant cost advantages.
Catalytic reforming of naphtha feedstocks is well known in the
petroleum refining industry. Most naphtha feeds contain large quantities
of naphthenes and paraffins and consequently they have low octane
numbers. In catalytic reforming these components go through a variety
of hydrocarbon conversions resulting in a gasoline product of improved
octane number. Some of the more important conversion reactions
3o include dehydrogenation of naphthenes to aromatics and
dehydrocyclization of normal paraffins to aromatics. Less desirable
reactions which commonly occur include hydrocracking of paraffins and
naphthenes to produce gaseous hydrocarbons such as methane and
ethane. Because of these less desirable reactions, an important
objective of catalytic reforming is to rearrange the structure of the
hydrocarbon molecules to form higher octane products without any
significant change in the carbon number distribution of the stock.
12-06-2001 ~ - - ~ US 00001368E
. CA 02374233 2001-11-16
2
The reforming reactions are, typically, catalyzed by catalysts
comprising porous supports, such as alumina, that have dehydrogenation
promoting metal components impregnated or admixed therewith. Platinurn
on alumina and more recently bimetallics such as platinum and rhenium on
alumina are examples of these catalysts. Such catalysts are described in
U.S. Pat. Nos. 3,415,737 and 3,953,3$8.
U.S. Pat. No. 5,744,674 discloses the preparation of benzene, toluene
and xylene from C9* heavy aromatics using ZSM-5 loaded with rhenium, tin
and platinum or palladium. This reference is not concerned with naphtha
upgrading, however. There is no teaching of a mufti-stage reforming
p rocess.
U.S. Pat. No. 4,877,514 discloses the preparation of catalyst suitable
for use in fluid catalytic cracking or reduced crude conversion hydrocarbon
conversion operations. These catalysts may comprise zeolites and may
further incorporate rhenium oxide.
U.S. Pat, No. 4,855,03fi discloses a process for fluid catalytic cracking
which employs a catalyst comprising a large pore zeolite. The zeolite is
prepared by contact with a fluoroanion. U.S. Pat. Nos. 4,642,408;
4,654,x57; and 4,499,321 disclose dealkyiation of 1,4 dialkylbenzene with
use of a zeolite catalyst such as ZSM-5. This catalyst may be nlodifred with
a Group Vllb element such as rhenium.
U.S. Pat. No. 4,467,129 discloses catalytic dealkylation of
ethylbenzene, where the ethylbenzene is mixed with xyiene. The catalyst
comprises mordenite and a zeolite such as ZSM-5. Rhenium may be added
for hydrogenation purposes. .
None of these patents discloses the concept of increasing ttte yield of
benzene,~toluene and xylene products from a mufti-stage naphtha reforming
process by use of a molecular sieve loaded with a Group ViIB metal in the
last bed, as in the instant invention.
'AMENDED SHEET ~~°'~~~~~~°~"~o'°'ph).aoc
Emufiangsteit l2.Juni 21:0
12-06-2001 l~-'"' "- ~ .. .. US 00001368E
CA 02374233 2001-11-16
3
It is known that pollution can be reduced by lowering gasoline
endpoint, resulting in a product endpoint where, in a standard ASTM
distillation, 90 volume percent of the gasoline distills below about
27a°F
(132°G) to 350°F (177°C) (T90). Based on this, there have
been regulatory
proposals, particularly in the State of California, to require gasoline to
meet
a maximum T90 specification of 300°F (149°G). Meeting this T90
permits
only 10% of the hydrocarbons in gasoline to boil above 300°F
(149°C). A
signifcant boiling range conversion of heavy naphthas will be required to
meet this goal.
Brief Description of the Invention
A process has been discovered for producing benzene; toluene and
xylenes while enhancing the octane value of the gasoline boiling range
materials of a naphtha fraction of low octane value and high gasoline end
boiling range.
The process of this invention can increase the benzene production of
a reformer by more than 10% while producing fewer G9* hydrocarbons,
through hydrodealkylation reactions.
The invention is directed to a multistage integrated process for
upgrading a petroleum naphtha comprising the steps of:
(a) introducing the naphtha to a catalytic reforming stage
comprising a plurality of operatively connected catalyst zones
including a first catalyst zone and a last catalyst zone, the last catalyst
zone being maintained under reforming conditions of temperature
ranging from at least 800°F (427°C) to 1050°F
(565°C) and pressure of
50 psig (44fi kPa) to 500 psig (3,549 kPa) to provide an intermediate
product comprising aromatics and paraffins;
(b) transferring at least a portion of the intermediate product of the
last catalyst zone to a benzene and toluene synthesis zone comprising
at least one benzene and toluene synthesis catalyst operatively
connected to the last catalyst zone of the reforming stage of step (a),
the benzene and toluene synthesis zone being maintained under
Empfa~gsteit l2,Ju~i 21:07 AMENDED SHEET
12-06-2001 ~ -°' - US 00001368E
CA 02374233 2001-11-16
4
conditions of hydrogen-to-hydrocarbon mole ratio and pressure
compatible with the fast catalyst zone of the reforming stage and
temperature of greater than 8Q0°F (427°C), the benzene and
toluene
synthesis catalyst zone containing a catalyst, which comprises a
hydrogenation component from Group VIIB and further comprises a
molecular sieve of Ivw acid activity, as determined by an alpha value
of less than 60, to provide a hydrocarbon product comprising more
benzene, toluene, xylenes content than the intermediate product of the
last catalyst zone of the reforming stage; wherein the intermediate
product of step (a) that is fed to the benzene, toluene, and xylenes
synthesis zone of step (by has not been subjected to intermediate
separation.
The hydrogenation component of Group VIIB in step (b) is preferably
rhenium.
The catalytic reforming zone and the benzene and toluene synthesis
zone are in series flaw arrangement, preferably without intermediate
separation of the reformer effluent so that the two zones are operated undeF
compatible conditions including hydrogen circulation rate and pressure.
In one embodiment of the invention, a low acidity molecular sieve can
be provided by using a deactivated catalyst from another refinery process.
in this respect, the other refinery process provides the catalyst treatment
conditions needed to reduce catalyst acidity.
Prior to the contacting with the reformate, the deactivated catalyst cart
be regenerated by conventional techniques such as by burning in an
oxygen-containing gas to remove at feast a major part of the accumulated
coke from the catalyst or by hydrogen regeneration.
Brief Description of the Drawings
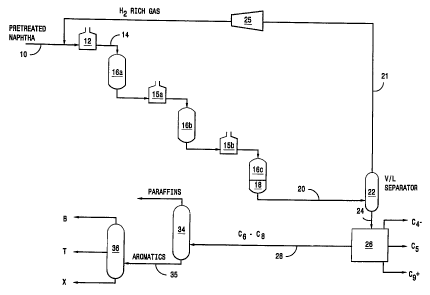
FIG. 1 is a simpl~ed scherrvatic flow diagram of the process of the
invention_
EmDfaoBSteit l2.Juei 21:O;AMENDEDSHEET
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
5 FIG. 2 is a simplified schematic flow diagram of an alternative
embodiment of the invention.
Detailed Description of the Invention
o A more detailed discussion of reforming, reformate upgrading, and
catalyst composition is provided in U.S. Pat. No. 5,865,986.
In the present invention a petroleum naphtha characterized by a
boiling range of C5 to about 450°F (232°C), typically boiling up
to about
400°F (204°C), is contacted with a reforming catalyst under
reforming
~5 conditions selected to produce a reaction product comprising aromatics
and paraffins. Typically, the hydrocarbon feed contains a percentage of
components which boil above 300°F (149°C). The components
boiling
above 300°F (149°C) usually comprise at least 10% of the entire
feed. In
general, the feed can be further characterized by the presence of C9+
2o hydrocarbons which are usually present in an amount of less than about
40 wt. %, typically 25 wt. % to 40 wt. %, based on the entire weight of
the feed. Yield advantages can be achieved by increasing the cut-point
of the reformer feed to boost C9+ aromatics. Alternatively, a C9+ aromatic
cofeed can be employed in which case the feed can contain over 40 wt.
25 % C9+ hydrocarbons, typically, up to 50 wt. % C9' hydrocarbons. Since
Cs components are olefin precursors, yield loss is minimized by
removing them from the feed. Thus, the feed can be substantially devoid
of Cs hydrocarbons.
The reforming process can be continuous, cyclic or
3o serniregenerative. The process can be in a fixed bed, moving bed,
tubular, radial flow or fluid bed. Typically, a hydrogen to hydrocarbon
mole ratio of up to 8:1 is employed to maintain a reasonable catalyst
cycle length.
The conditions of reforming typically include temperatures of at
35 least about 800°F (427°C) to about 1050°F
(565°C) and pressures from
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
6
about 50 psig (446 kPa) to about 500 psig (3,549 kPa), more specifically
from about 50 psig (446 kPa) up to and including 450 psig (3,204 kPa).
It may often be preferred to employ pressures in the lower ranges e.g.
50 psig (446 kPa) to about 125 psig (963 kPa) to encourage formation of
aromatics which supply precursors for the preferred reactions of the
o benzene and toluene synthesis zone and enhance yield of the preferred
products. The hydrogen-to-hydrocarbon ratio ranges from about 0.5 to
about 20 and the liquid hourly space velocity can be in the range of
about 0.1 to 10, usually about 0.5 to 5.
It is contemplated that any molecular sieve having a pore size
s appropriate to admit the bulky C9+ hydrocarbons and catalytically
dealkylate the aromatics can be employed in this reformate upgrading
process. More detailed information concerning appropriate molecular
sieves for this invention is found in U.S. Pat. No. 5,865,986. The
hydrogenation component which is preferred in this invention is rhenium,
2o which produces results comparable to those produced using platinum or
palladium but at a lower cost.
The molecular sieve which catalyzes these reactions is usually an
intermediate or large pore size zeolite having a silica-to-alumina mole
ratio of at least about 12, specifically from about 12 to 2000. The zeolite
25 is usually characterized by a Constraint Index of about 0.5 to 12
specifically about 1 to 12 as described in U.S. Pat. No. 4,088,605.
Typically, the molecular sieve of choice is a zeolite. Zeolites
contemplated include ZSM-5, ZSM-11, ZSM-12, ZSM-35, ZSM-38,
zeolite beta and other similar materials. U.S. Pat. No. 3,702,886
3o describing and claiming ZSM-5 is incorporated herein by reference.
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
7
Process Configuration
In the multi-step integrated process the petroleum naphtha is
catalytically reformed and the reformate is cascaded to the
hydrodealkylation reaction zone.
o FIG. 1 is a simplified schematic flow diagram of one useful process
configuration. Referring to FIG. 1, a petroleum naphtha supplied by line
is charged to reformer heater 12 which elevates the temperature of
the feed to a temperature suitable for reforming. The heated feed is
charged to a plurality of reformer reaction zones 16a, 16b and 16c with
~5 interstage heaters 15a and 15b.
Although three reformer reaction zones are shown, there can be
less than three or more than three reaction zones. The bottom portion of
the last reformer reaction zone 18 is loaded with the hydrodealkylation
catalyst. The feed passes over the hydrodealkylation catalyst just before
2o it exits the reformer to produce a product of increased benzene content
as compared to the effluent of the last reforming catalyst zone 16c.
The hydrodealkylation catalyst of reaction zone 18 is typically
isolated from the reforming catalyst to maximize its opportunity to work
on the products of reforming as opposed to the reformer feed. This can
25 be accomplished by providing a separate reactor or by segregating the
catalysts within the same reactor.
However, intermingling of the hydrodealkylation catalyst and the
reforming catalyst will be difficult to avoid and will not be detrimental in
the last part of the final reactor.
3o Usually when the hydrodealkylation catalyst is located within the
reformer, regardless of where the hydrodealkylation catalyst is located, a
radial flow reactor is particularly suitable to maintain a low pressure
drop. The radial flow reactor, particularly in combination with smaller
particle size hydrodealkylation catalyst, contributes to improved flow
35 distribution in the last bed of the reformer.
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
8
In some operations it will be useful to employ a small particle size
catalyst, typically when reactor volume is small or to alleviate pressure
drop. A self bound zeolite such as self-bound ZSM-5 is specifically
contemplated.
FIG. 2 shows an embodiment of the invention in which the
o hydrodealkylation catalyst is located in a separate reactor 19 associated
with switching valves 17a and 17b which, optionally, enable the catalyst
zone to be removed from on-line contact during at least a portion of
regeneration of the reformer catalyst. Optionally, heater 15c is located
between the last reactor of the reformer and the hydrodealkylation
~ 5 catalyst reactor 19.
Referring to both FIGS. 1 and 2, after cooling, the aromatics rich
product is passed to vapor/liquid separator 22 which separates a
hydrogen-rich gas via hydrogen compressor 25 for recycling to the
reformer via line 21. Via line 24, the liquid product is conveyed from
2o separator 22 to fractionator 26 typically a series of fractionators that
separate the product into C4 , C5, C6 Ce and C9+ hydrocarbon streams.
The C9+ aromatics can be separated and recycled to the reformer or the
hydrodealkylation reactor to increase yield.
The Cs to CB stream of fractionator 26 is transferred by line 28 to a
25 paraffin separator 34 which separates the paraffins from the aromatics,
typically, by solvent extraction. The aromatics extract can then be
conveyed via line 35 to separation zone 36 which separates the extract
into benzene, toluene and xylenes streams. An important advantage of
the invention is a low consumption of hydrogen. Typically, hydrogen
3o consumption is less than about 200 SCFB (35.6n.1.1.< - 1 > ), more
typically, ranging from about 0 SCFB (0 n.1.1< - 1 > ) to about 100
S.C.F.B. (17.8 n.1.1.< - 1 > ), more typically less than about 50 SCFB (8.9
n.1.1.< - 1 > ). This low hydrogen consumption can be particularly
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
9
advantageous when there is a need to balance a high hydrogen
consumption in the reformer.
The hydrodealkylation catalyst can be exposed to the conditions of
the reformer during rejuvenative treatment of the reformer catalyst.
Typically, the reformer catalyst is rejuvenated by oxychlorination but any
o rejuvenating method is contemplated.
The hydrodealkylation catalyst may be reactivated by the
rejuvenative treatment of the reformer catalyst. However, other methods
known for reactivating the catalyst may be employed such as burning
with oxygen, regeneration with hydrogen or an inert gas such as
nitrogen.
Examples
Example 1
A reformate was obtained which had the following composition.
Example 2
The catalyst used in this study was prepared by steaming an
alumina bound ZSM-5 base (65/35) at 1200°F for 15 hours. The
alpha activity of this catalyst after steaming is 2.6. This steamed
catalyst was then impregnated (incipient wetness impregnation)
3o with an aqueous solution of ammonium perrhenate to yield a
CA 02374233 2001-11-16
WO 00/71642 PCT/L1S00/13686
5 catalyst which contains 0.3% rhenium by weight (measured as the
metal). This catalyst was then dried and calcined for one hour at
975°F in a rotary calciner. This dried catalyst is herein referred to
as 0.3% Re/ZSM-5.
1o Example 3
The hydrocarbon mixture of Example 1 was used as feed in
a fixed-bed, laboratory reactor filled with the catalyst of Example 2.
The catalyst was first oxychlorided with a mixture of 1300 ppmv
chlorine and 7% oxygen in nitrogen at 990°F, followed by reduction
with hydrogen at 700°F in a glass-lined, fixed bed reactor to
simulate commercial reformer catalyst reactivation conditions.
Five grams of the oxychlorided and reduced catalyst were
then transferred to a 0.68" ID, stainless steel tube, fixed-bed reactor
operated in an adiabatic fashion. The catalyst was sulfided with
400 ppmv hydrogen sulfide in Hydrogen at 750°F prior to feeding
the hydrocarbon mixture to the reactor to simulate commercial
reformer catalyst preparation.
The conditions for the experiment were 24 WHSV, ca. 6:1
2s H2:HC, 940°F WABT, and ca. 300 psig. The hydrocarbon feed
mixture was combined with makeup hydrogen and recycle gas to
simulate the conditions present in the last reactor of a commercial
catalytic reformer. The reactor product is cooled and flashed in a
separator. A portion of the flash separator overhead gas is
3o recycled to the inlet of the reactor by a compressor. On-line, gas
chromatography is used to analyze the gaseous and liquid products
from the flash separator and calculate yields of the various
hydrocarbon molecules.
CA 02374233 2001-11-16
WO 00/71642 PCT/US00/13686
11
After nine days on stream, the following yields were
obtained:
Note that there are increases in benzene, toluene, and xylenes
1 o versus the feed composition.