Note: Descriptions are shown in the official language in which they were submitted.
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
TITLE: ENHANCED STRESS TOLERANCE IN MAIZE VIA
MANIPULATION OF CELL CYCLE REGULATORY GENES
FIELD OF THE INVENTION
This invention relates generally to the field of plant molecular biology.
More specifically, this invention relates to methods and reagents for the
temporal
and spatial expression of genes that enhance cell division in plants,
especially
transgenic plants, to increase yield and health of crop plants in general as
well as
in periods of stress.
BACKGROUND OF THE INVENTION
Cell division plays a crucial role during all phases of plant development.
The continuation of organogenesis and growth responses to a changing
environment require precise spatial, temporal and developmental regulation of
cell division activity in meristems (and in cells with the capability to form
new
meristems, such as in lateral root formation). Control of cell division is
also
important in organs themselves (i.e., separate from meristems per se), for
example, in leaf expansion, secondary growth, and endoreduplication.
A complex network controls cell division in eukaryotes. Various regulatory
2 0 pathways communicate environmental constraints such as nutrient
availability,
mitogenic signals such as growth factors or hormones, or developmental cues
such
as the transition from vegetative to reproductive growth. Ultimately, these
regulatory pathways control the timing, rate, plane, and position of cell
division.
Cell division in higher eukaryotes is controlled by two main checkpoints in
2 5 the cell cycle which prevent the cell from entering either M- or S-phase
prematurely. Evidence from yeast and mammalian systems has repeatedly shown
that over-expression of key cell cycle genes can either trigger cell division
in non-
dividing cells, or stimulate division in previously dividing cells (i.e., the
duration
of the cell cycle is decreased and cell size is reduced). Examples of genes
whose
3 0 over-expression has been shown to stimulate cell division include cyclins
(see, e.g.,
Doerner, P. et al., Nature (1996) 380:520-523; Wang, T.C. et al., Nature
(1994)
369:669-671; Quelle, D.E. et al., Genes Dev. (1993) 7:1559-1571); E2F
1
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
transcription factors (see, e.g., Johnson, D.G. et al., Nature (1993) 365:349-
352;
Lukas, J. et al., Mol. Cell. Biol. (1996) 16:1047-1057) , cdc25 (see, e.g.,
Bell, M.H. et
al., Plant Mol. Bio. (1993) 23:445-451; Draetta, D. et al., BBA (1996) 1332:53-
63),
and mdm2 (see, e.g., Teoh, G. et al., Blood (1997) 90:1982-1992). Conversely,
other gene products have been found to participate in checkpoint control,
effectively blocking or retarding progression through the cell cycle (Cebolla
et al.,
EMBO 18(16):4476-84(1999)).
The basic mechanism of cell cycle control is conserved among eukaryotes.
A catalytic protein kinase and an activating cyclin subunit control progress
through the cell cycle. The protein kinase is generally referred to as a
cyclin-
dependent-kinase (CDK), whose activity is modulated by phosphorylation and
dephosphorylation events and by association with regulatory subunits called
cyclins. CDKs require association with cyclins for activation, and the timing
of
activation is largely dependent upon cyclin expression.
Eukaryote genomes typically encode multiple cyclin and CDK genes. In
higher eukaryotes, different members of the CDK family act in different stages
of
the cell cycle. Cyclin genes are classified according to the timing of their
appearance during the cell cycle. In addition to cyclin and CDK subunits, CDKs
are often physically associated with other proteins which alter localization,
2 0 substrate specificity, or activity. A few examples of such CDK interacting
proteins
are the CDK inhibitors, members of the Retinoblastoma-associated protein (Rb)
family, and the Constitutive Kinase Subunit (CKS).
The protein kinase activity of the complex is regulated by feedback control
at certain checkpoints. At such checkpoints the CDK activity becomes limiting
for
further progress. When the feedback control network senses the completion of a
checkpoint, CDK is activated and the cell passes through to the next
checkpoint.
Changes in CDK activity are regulated at multiple levels, including reversible
phosphorylation of the cell cycle factors, changes in subcellular localization
of the
complex, and the rates of synthesis and destruction of limiting components.
3 0 Regulation of the cell cycle by the cyclin/CDK complex is noted
particularly at the
G1/S phase transition and at the G2lM phase transition. P.W. Doerner, Cell
Cycle Regulation in Plants, Plant Ph,~. (1994) 106:823-827.
2
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
Plants have unique developmental features that distinguish them from
other eukaryotes. Plant cells do not migrate, and thus only cell division,
expansion, and programmed cell death determine morphogenesis. Organs are
formed throughout the entire life span of the plant from specialized regions
called
meristems. In addition, many differentiated cells have the potential both to
dedifferentiate and to reenter the cell cycle. There are also numerous
examples of
plant cell types that undergo endoreduplication, a process involving nuclear
multiplication without cytokinesis. The study of plant cell cycle control
genes is
expected to contribute to the understanding of these unique phenomena. O.
Shaul
et al., Regulation of Cell DiUision in Arabidopsis, Critical Reviews in Plant
Sciences, 15(2):97-112 (1996).
Current methods for genetic engineering in maize require a specific cell
type as the recipient of new DNA. These cells are found in relatively
undifferentiated, rapidly growing callus cells or on the scutellar surface of
the
immature embryo (which gives rise to callus). There is evidence to suggest
that
cells must be dividing for transformation to occur. Therefore, to optimize
transformation it would be desirable to provide a method for increasing the
number of cells undergoing division.
It has also been observed that dividing cells represent only a fraction of
2 0 cells that transiently express a transgene. Regardless of the delivery
method
currently used, DNA is introduced into literally thousands of cells, yet
transformants are recovered at frequencies of 10-~ relative to transiently-
expressing cells. The presence of damaged DNA in non-plant systems (similar
to DNA introduced by particle gun or other physical means) has been well
2 5 documented to rapidly induce cell cycle arrest. Siede, W., Cell cycle
arrest in
response to DNA damage: lessons from yeast. Mutation Res. 337(2):73-84 (1995).
An increase in understanding and control of the cell cycle could also help to
further increase the rate of recovery of transformants.
Anthesis is generally recognized as the critical period of ear and kernel
3 0 development in maize. Varied experimental approaches demonstrate that
treatments, which decrease the cell division around anthesis, decrease grain
yield. For example, large yield losses occur when maize plants are subjected
to
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
abscisic acid (ABA) (Myers, P.N. et al., 1990; Mambelli and Setter, 1998),
thermal stress (Jones, R.J. et al., 1985; Cheikh and Jones, 1994), water-
deficits
(Artlip, T.S. et al., 1995) or exposed to high plant density around anthesis.
(See
Zinselmeier, C. and J.E. Habben, Use of mRNA-Profiling Technology to
Determine Gene Expression Patterns in Developing Maize Ears that Differ in
Yield, Plant Physiology Abstracts (1998); Prine, G.M. A Critical Period for
Ear
Development in Maize, Crop Science 11:782-786 (1971).) Conversely, treatments
that increase plant cell division around anthesis increase grain yield. For
example, application of cytokinins (Lejeune, P. et al., 1998). In most cases,
the
variation in yield was related to the number of kernels that developed.
Collectively, these results suggest that kernel number and size may be limited
by cell division, particularly during drought or high density stress at
anthesis.
According to the invention, enhancing cell division of the immature ear and
grain would maintain ear and seed growth, and as a consequence, buffer this
important vulnerable period of yield formation.
The tissues targeted for transgenes are in the maize female inflorescence,
since relative to other organs, it is frequently the most sensitive to abiotic
stress.
For example, transient water stress prior to pollination has been shown to
arrest
the growth of ears, embryo sacs, and silks. After pollination, drought stress
can
2 0 inhibit endosperm cell division, which peaks at 8 to 10 days after
pollination. As a
result, both kernel set and endosperm development are inhibited. This effect
is
most pronounced in the apical region of the ear. Retarded endosperm
development can result in aborted apical kernels, because of reduced cell
division
and decreased endoreduplication. Not surprisingly, both of these events have
2 5 been shown to be controlled by cyclin dependent protein kinases.
Barrennesss (the lack of ear development) is one of the most common
manifestations of maize plants grown at high densities. Another prevalent
trait
in density stressed plants is an increase in the anthesis/silking interval,
which
has been shown to be the result of retarded ear growth. Based on this and
other
3 0 information, one key to producing a viable ear under plant-population
stress is to
maintain its growth rate. Since cell division is a key component of organ
growth,
4
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
the cell cycle regulatory mechanism in the female inflorescence is the target
for
expression of transgenes.
Traditional methods of improving yield formation have centered around
breeding techniques. As with any valuable plant species, breeders have long
used
conventional breeding techniques to improve yield. While improvements have
been achieved, breeding techniques are laborious and slow because of the time
required to breed and grow successive plant generations. Furthermore, certain
phenotypes may be impossible to obtain by conventional techniques. Thus, it
would be desirable to utilize recombinant DNA technology to produce new plant
varieties and cultivars in a controlled and predictable manner. It would be
especially desirable to produce crop and ornamental plants with improved seed
set
over a range of environmental conditions to increase yield potential.
It can be seen from the foregoing that a need exists in the art for a
transgenic method of increasing yield potential in plants.
It is an object of the present invention to provide expression constructs
which when expressed in a temporal and spatial manner in a transgenic plant
increase yield potential, as well as resistance to stress through regulation
of cell
division.
It is another object of this invention to provide transgenic plant lines with
2 0 heritable phenotypes which are useful in breeding programs designed to
increase
yield potential in crop plants over a range of environmental conditions.
It is yet another object of this invention to produce seed which will produce
plants with increased yield potential.
It is a further object of this invention to provide plants, plant cells, and
2 5 plant tissues containing the expression constructs of the invention.
Other objects of the invention will become apparent from the description of
the invention which follow.
SUMMARY OF THE INVENTION
3 0 The present invention comprises the spatial and temporal expression of a
nucleotide sequence which will enhance stress tolerance (buffer female
inflorescence), particularly high density and drought stresses, in plants at
critical
5
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
times in plant development such as the vulnerable time of anthesis. In
particular,
this invention relates to polynucleotides which encode proteins involved in
the
regulation of the cell cycle. More particularly, the polynucleotides encode
proteins
which enhance cell division in maize ears and kernels by directly increasing
the
activities of cyclin dependent protein kinases or indirectly by augmenting the
activity of enzymes which control CDK activity.
Cell division in higher eukaryotes is controlled by a well-conserved
mechanism. The principal control factor of this mechanism is a protein
threonine/serine kinase complex that is composed of cyclin (the regulatory
subunit) and CDK (the catalytic subunit). This complex controls cell division
by
phosphorylating target proteins. Eukaryotes have evolved an elaborate
regulatory network to safeguard the fluctuation of CDK activities in the cell
cycle.
Cyclins oscillate in abundance as a result of both transcriptional and post-
transcriptional regulation. This provides an on/off control for CDK, since the
association of cyclin is absolutely required for kinase activity.
Phosphorylation
and dephosphorylation of CDK occurs. Three important phosphorylation sites are
involved in modulating CDK activities. Phosphorylation of Tyrl61 by the CDK
activating kinase (CAK) activates CDK, while phosphorylation of Thrl4 and
Tyrl5 by Myt1 and Weel, respectively, inactivates CDK (Mueller, P.R. et al.,
Mol.
2 0 Biol. Cell 6, 119(1995); Mueller, P.R. et al., Science 270, 86 (1995)).
CDC25, a
protein tyrosine phosphatase dephosphorylates Tyrl5 and activates CDK
(Kumagai, A. and Dunphy, W.G., Cell 70, 139 (1992). Both Weel and CDC25 are
in turn regulated by phosphorylation. Niml, a protein kinase identified in S.
pombe is able to phosphorylate Weel (this inhibits Weel activity), while Plxl
is
able to use CDC25 as a substrate and enhance CDC25 activity, a positive
feedback
loop for CDK regulation. The CDK complex interacts with CDK inhibitors (CKIs).
A number of proteins can physically bind to CDK and inhibit CDK activity. Well-
characterized inhibitors in human systems include p21, p27, p57, p16, and p19.
Identification of rate-limiting pathways influenced by abiotic stresses are
3 0 important in determining which ones to target. Carbohydrate and nitrogen
metabolic pathways, as well as hormonal pathways, have been found to be
modulated by stress. A recent study of wheat (Schuppler, U. et al., "Effect of
6
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
Water Stress on Cell Division and Cell-Division-Cycle 2-Like Cell-Cycle Kinase
Activity in Wheat Leaves," Plant Physiol. 117: 667-678 (1998)) showed
convincing
evidence that proteins encoded by cell cycle genes can be targets of water
stress.
When a transient drought was imposed on the wheat seedlings, the mesophyll
cells of leaves were arrested at the G1 phase. Enzyme assays revealed that
there
was a 50% decrease in CDK activity in the cells, which was caused by an
increased level of Tyrl5 phosphorylation.
Apical kernel abortion is a common characteristic of maize subjected to
drought stress. Research has shown that the plant hormone cytokinin, is able
to
reduce apical kernel abortion. Concurrently, it was shown that cytokinin can
enhance CDK activities by reducing the extent of CDK phosphorylation at TyrlS.
Other research has shown that the cell cycle regulatory mechanism is highly
conserved among all eukaryotes. Cell cycle genes from maize, Arabidopsis, and
alfalfa are able to rescue yeast mutants that are defective in cell cycle
genes.
Likewise, yeast cell cycle genes, such as CDC25, are able to promote cell
division
in higher plants. Thus, heterologous genes will work in transgenic maize
events.
In one embodiment, the invention comprises a genetic construct which
upon expression in plant cells provides a DNA sequence encoding a gene product
useful for directing the phosphorylation or activation state of CDK of a plant
or
plant tissue. Particularly, B-type and D-type cyclins, CDC25, Niml, and Plxl
will be over expressed in order to promote cell division under stress. In
another
embodiment, the invention comprises a genetic construct which provides a DNA
sequence encoding a gene product useful for co-suppressing Weel in order to
promote cell division of a plant or plant tissue.
2 5 Kernel abortion increases when unfavorable environments occur around
flowering, thereby decreasing genetic yield potential in plants. Typically,
developing female florets are more prone to abiotic stress compared to male
florets. CDKs are critical enzymes that determine maize floral cell division.
Modification of female cell division by altering the activation of CDKs in a
tissue
3 0 and temporal specific manner should increase the likelihood of vigorous
female
floral development and also improve the consistency of seed set under
unfavorable
conditions.
7
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
Thus the invention contemplates expression of cell division enhancing
nucleotide sequences during vulnerable periods, primarily those involved with
anthesis development, where yield is most significantly affected by stress.
Definitions
As used herein the term "anthesis development" shall include any period in
plant development where yield may be more significantly impacted by stress.
This can include the exponential growth phase of the ear during which biomass
is
accumulated and the lag phase of kernel development as more fully described
herein and in the following references. ("Set and Flower Synchrony within the
Ear of Maize II. Plant Population Effects", Crop Science, 37: 448-455 (March-
April
1997); and Shaw, Robert "Climate Requirement", Corn Improvement, 3rd ed.,
Chapter 10, pp. 609-638). As shown in Figure 1, reprinted from Corn and Corn
Improvement, plant yields are most vulnerable to moisture stress at a time
period
centered around flowering (0-10 DAP). Typically, this period will be
approximately 14 days prior to flowering through approximately 14 days after
flowering.
The examples and discussion herein may specifically reference maize,
however the teachings herein are equally applicable to any other grain or
2 0 flowering crop.
As used herein the term "ear" shall not be limited to maize and shall
include any developing female inflorescence from a plant.
As used herein the term "kernel" shall also not be limited to maize but shall
include grain, or seed within a fruit.
2 5 As used herein the term "cell division enhancing nucleotide sequence"
shall
mean any nucleotide sequence, (DNA, RNA, coding and/or antisense) the
expression of which increases the rate of a particular plant tissue's cell
division as
compared to the rate without the expression of said sequence.
According to the invention, a genetic construct is disclosed which causes
3 0 expression of the cell division enhancing nucleotide sequence at a time
and
location to maximize cell division typically during very vulnerable periods
primarily, around anthesis. The spatial and temporal expression of genes
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
affecting cell division of tissues can be achieved using different types of
promoters.
Promoters useful for the invention are promoters which would cause the
temporal
and spatial expression of a gene product during anthesis as defined herein and
can be constitutive, inducible, or tissue specific.
For example, seed specific promoters can be used to enhance cell division
during seed development, pre-pollination promoters can also be used or stress
inducible promoters can be used to enhance cell division during periods of
stress.
The optimization of promoters to achieve the objectives of the invention is
considered routine and easily ascertainable by those of skill in the art and
is
intended to be within the scope of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic diagram (reproduced from Shaw, Robert "Climate
Requirement", Corn Improvement, 3rd ed., Chapter 10, pp. 609-638). As shown in
Figure 1, reprinted from Corn and Corn Improvement from p. 614) of the
relationship between age of crop and percentage yield decrement due to 1 day
of
moisture stress. The top and bottom lines represent the highest and lowest
yield
reductions obtained in stress experiments, the middle line the average
reduction.
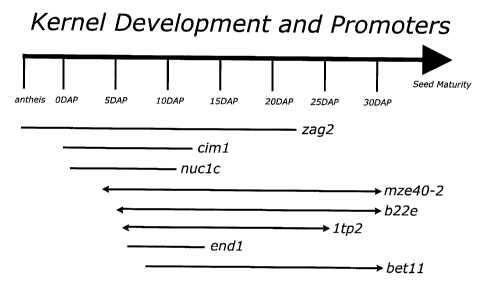
Figure 2 is a chart depicting expression timing of various promoters useful
2 0 for the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The present invention is based on isolation and characterization of genes
affecting CDKs or enzymes which control CDKs which control cell division in
2 5 plants. Any nucleotide sequence encoding an enzyme in the CDK
activation/modulation (phosphorylation/dephosphorylation) pathways may be
used in accordance with the present invention. Nucleotide sequences encoding
these enzymes are easily ascertainable to those of skill in the art through
Genbank or the references disclosed herein. Other reactions and pathways may
3 0 be utilized by different organs in a plant or by different plant species.
By
changing the levels or activity of a component in the
9
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
activation/deactivation/modulation pathway, it is possible to affect the
levels of
cell division in the plant, plant organ, or plant tissue.
Many different types of CDKs have been identified in plants. Several
cDNAs encoding functional homologs of cdc2 kinase have been isolated by
reduced
stringency hybridization or reverse transcription coupled polymerase chain
reaction from a number of plant species, including pea (Feiler and Jacobs,
1990),
alfalfa (flirt et al., 1991, 1993), Arabidopsis (Ferreira et al., 1991;
Hirayama et al.,
1991), soybean (Miao et al., 1993), Antirrhinum (Fobert et al., 1994), and
maize
(Colasanti et al., 1991). ). Soni, R. et al., "A Family of Cyclin D Homologs
from
Plants Differentially Controlled by Growth Regulators and Containing the
Conserved Retinoblastoma Protein Interaction Motif', The Plant Cell, 7:86
(1995).
Several other CDKs have been cloned and are easily accessible to those of
skill in
the art.
At least three different types of cyclins have been identified in plants: A-
type homologs, B-type homologs, and D-type homologs (Renaudin, J-P et al.,
"Plant cyclins: a unified nomenclature for plant A-, B- and D-type cyclins
based on
sequence organization", Plant Mol. Biol., 32:1003-1018 (1996)). A-type cyclins
are
broken down into three structural groups (A1, A2, and A3). Cyclin Al has been
isolated from maize. (Renaudin et al., Table 1). B-type cyclins are broken
down
2 0 into two structural groups (B1, and B2). Cyclins B1 and B2 have been
isolated
from maize. (Renaudin et al., Table 1). D-type cyclins contain three
structural
groups (Dl, D2, and D3). A number of cDNA sequences encoding plant mitotic
cyclins with A- or B-type characteristics of having mixed A- and B-type
features
have been isolated from various species, including carrot (Hata et al., 1991),
2 5 soybean (Hata et al., 1991), Arabidopsis (Hermerly et al., 1992; Day and
Reddy,
1994), alfalfa (flirt et al., 1992), Antirrhinum (Fobert et al., 1994) and
maize
(Redaudin et al., 1994; Sun, Y. et al., 1997, CycZmIn from maize endosperm
(GenBank #U66607), CycZmel, GenBank #U66608). Soni, R. et al., "A Family of
Cyclin D Homologs from Plants Differentially Controlled by Growth Regulators
3 0 and Containing the Conserved Retinoblastoma Protein Interaction Motif",
The
Plant Cell, 7:86 (1995). Several other cyclins have been cloned and are easily
accessible to those of skill in the art.
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
At its simplest, the invention comprises a nucleotide construct comprising a
cell division enhancing nucleotide sequence, a regulatory promoter to regulate
temporal tissue and spatial expression during anthesis development and
termination sequences operably linked to said cell division enhancing
sequence.
A non-exclusive list of enzymes that might be candidates for such
intervention include Mytl, Weel, Niml, CDC25, Plxl, CKIs, CAK, and cyclins.
Identification of other polynucleotides which may be useful in the invention
will typically be based on screening for procaryotic or eucaryotic organisms
with
altered levels of cell division using assays standard in the art and described
herein. For example, and not limited to, plant hormones such as cyktokinins,
ABA (Myers, P.N. et al. 1990), and auxin (Trehin et al., planta (1998)
206(2):215-
224)
The polynucleotides useful in the invention can be formed from a variety of
different polynucleotides (e.g., genomic or cDNA, RNA, synthetic
oligonucleotides,
and polynucleotides), as well as by a variety of different techniques. As used
herein, a polynucleotide is a sequence of either eukaryotic or prokaryotic
synthetic
invention.
In a preferred embodiment, the invention comprises use of one or more
nucleotide sequences which, when expressed together enhance reproductive cell
2 0 division. This can allow for hybrid plant or seed production, once
transgenic
inbred parental lines have been established. For this embodiment, the
invention
comprises a DNA sequence encoding B- or D-type cyclins, CDC25, Niml, and/or
Plx1 capable of promoting cell division by activating or modulating the
activity of
CDKs in critical, stress sensitive periods of plant development. In a second
embodiment, DNA sequence encoding for suppression of Wee1 capable of
promoting cell division by modulating the activity of CDKS, is provided for
increasing yield, seed development, flowering or resistance to stress.
The invention is not limited to any plant type and can be used for any crop
or ornamental plant species for which it is desirable to increase yield. The
3 0 methods of the invention may be applicable to any species of seed-bearing
plant to
enhance yield potential by affecting the cell division in seed tissue.
11
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
The nucleotide constructs of the present invention will share similar
elements, which are well known in the art of plant molecular biology. For
example, in each construct the DNA sequences of interest will preferably be
operably linked (i.e., positioned to ensure the functioning of) to a promoter
which
allows the DNA to be transcribed (into an RNA transcript) and will comprise a
vector which includes a replication system. In preferred embodiments, the DNA
sequence of interest will be of exogenous origin in an effort to prevent co-
suppression of the endogenous genes.
Promoters (and other regulatory elements) may be heterologous (i.e., not
naturally operably linked to a DNA sequence from the same organism).
Promoters useful for expression in plants are known in the art and can be
inducible, constitutive, tissue-specific, derived from eukaryotes,
prokaryotes, or
viruses, or have various combinations of these characteristics.
In choosing a promoter to use in the methods of the invention, it may be
desirable to use a tissue-specific or developmentally regulated promoter. A
tissue-
specific or developmentally regulated promoter is a DNA sequence which
regulates the expression of a DNA sequence selectively in the cells/tissues of
a
plant critical to seed set and/or function and/or limits the expression of
such a
DNA sequence to the period of seed maturation in the plant. Any identifiable
2 0 promoter may be used in the methods of the present invention which causes
expression during anthesis development as defined herein. It may also be
advantageous to use a stress inducible promoter to provide expression of the
construct during periods of stress.
Differential screening techniques can be used to isolate promoters
2 5 expressed in developing female reproductive organs (kernels and/or
immature
ears) from around 14 days before pollination to approximately 12 days after
pollination. Promoters predicted to operate in this manner include LTP2, gamma-
zero, and ZAG2.
Promoters preferred for the invention would be acceptably timed to 14 days
3 0 before and 12 days after anthesis when both immature ear and mitotically
active
kernel are most susceptible to the stress. Promoters predicted to operate
during
these developmental stages include LTP2, MZE40, nucl and ZAG2. For example,
12
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
LTP2 promoter from Barley (Kalla et al., 1994, Plant J.6(6):849-860) confers
the
specificity of aleurone expression. Pioneer Researchers have shown that this
promoter is also functional in maize. When fused with a GUS reporter gene,
LTP2
promoter directed aleurone specific expression of GUS activity in maize
kernels
(Niu and Tome, unpublished). Aleurone is a single celled, out-most layer of
endosperm that retains mitotic activity when the central region of endosperm
ceased division and committed to endoreduplication. Therefore, LTP2 promoter
will allow us to manipulate endosperm cell division when fused with cell
division
regulatory genes. B22E: 69 NAL Call No. 442.8 Z34 "Primary Structure of a
Novel Barley Gene Differentially Expressed in Immature Alleurone Layers,"
Klemsdae, S.S. et al., Springer Int'1 1991 Aug., Molecular and General
Genetics,
Vol. 228(1/2) p. 9-16, 1991. Expression of B22E is specific to the pedicel in
developing maize kernels, Zag2: 134 NAL Call. No.: QK725.P532 Identification
and molecular characterization of ZAG1, the maize homolog of the Arabidopsis
floral homeotic gene AGAMOUS. Schmidt, R.J.; Veit, B.; Mandel, M.A.; Mena, M.;
Hake, S.; Yanofsky, M.F. Rockville, MD: American Society of Plant
Physiologists,
c1989-; 1993 Jul. The Plant Cell v: 5(7): p 729-737; 1993 Jul. includes
references.
Zag2 transcripts can be detected 5 days prior to pollination to 7 to 8 DAP,
and
directs expression in the carpel of developing female inflorescences and Ciml
which is specific to the nucellus of developing maize kernels. Ciml transcript
is
detected 4 to 5 days before pollination to 6 to 8 DAP. Other useful promoters
include any promoter which can be derived from a gene whose expression is
maternally associated with developing female florets.
Table 1 shows a list of preferred promoters including their timing of
2 5 expression (DAP = days after pollination).
Promoter Expression Summary
Promoter Source Primary Tissue Temporal
ltp2 barley aleurone <6 - 24+ DAP
cDNA
ciml maize pericarp (under silk scar) 0 - 12+ DAP
EST
13
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
mze40-2 maize gloom, pericarp, pedicel <4 - 28+ DAP
forming
EST region, low in scutellum
b22e barley aleurone, embryo scutellum, <5 - 30+ DAP
genomic pedicel forming region
zag2 maize, floret, ovule <0 - 22 DAP
EST
endl maize, endosperm transfer cells 6 - 14 DAP
cDNA
betll maize, endosperm transfer cells 8 - 30+ DAP
cDNA
Figure 2 also depicts the timing of various preferred promoters and kernel
development.
For example a construct useful for the present invention might include the
maize B-cyclin gene operably linked to the ZAG2 promoter for expression of B-
cyclin <0 to 22 days after pollination.
Other promoters which are seed or embryo specific and may be useful in
the invention include patatin (potato tubers) (Rocha-Sosa, M. et al. (1989)
EMBO
J. 8:23-29), convicilin, vicilin, and legumin (pea cotyledons) (Rerie, W.G.,
et al.
(1991) Mol. Gen. Genet. 259:149-157; Newbigin, E.J., et al. (1990) Planta
180:461-
470; Higgins, T.J.V., et al. (1988) Plant. Mol. Biol. 11:683-695), zero (maize
endosperm) (Schemthaner, J.P., et al. (1988) EMBO J. 7:1249-1255), phaseolin
(bean cotyledon) (Segupta-Gopalan, C. et al. (1985) Proc. Natl. Acad. Sci.
U.S.A.
82:3320-3324), phytohemagglutinin (bean cotyledon) (Voelker, T. et al. (1987)
EMBO J. 6:3571-3577), B-conglycinin and glycinin (soybean cotyledon) (Chen, Z-
L
et al. (1988) EMBO J. 7:297-302), glutelin (rice endosperm), hordein (barley
endosperm) (Marris, C. et al. (1988) Plant Mol. Biol. 10:359-366), glutenin
and
gliadin (wheat endosperm) (Colot, V. et al. (1987) EMBO J. 6:3559-3564), and
sporamin (sweet potato tuberous root) (Hattori, T. et al. (1990) Plant Mol.
Biol.
2 0 14:595-604). Promoters of seed-specific genes operably linked to
heterologous
coding regions in chimeric gene constructions maintain their temporal and
spatial
expression pattern in transgenic plants. Such examples include Arabidopsis
thaliana 2S seed storage protein gene promoter to express enkephalin peptides
in
14
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
Arabidopsis and Brassica napus seeds (Vanderkerckhove et al., Bio/Technolo~y
7:L929-932 (1989)), been lectin and bean (3-phaseolin promoters to express
luciferase (Riggs et al., Plant Sci. 63:47-57 (1989)), and wheat glutenin
promoters
to express chloramphenicol acetyl transferase (Colot et al., EMBO J 6:3559-
3564
(1987)).
Any inducible promoter can be used in the instant invention to temporarily
express a particular construct during reproductive development. See Ward et
al.
Plant Mol. Biol.22: 361-366 (1993). Exemplary inducible promoters include, but
are not limited to, that from the ACEl system which responds to copper (Mett
et
al. PNAS 90: 4567-4571 (1993)); In2 gene from maize which responds to
benzenesulfonamide herbicide safeners (Hershey et al., Mol. Gen. Genetics 227:
229-237 (1991) and Gatz et al., Mol. Gen. Genetics 243: 32-38 (1994)) or Tet
repressor from TnlO (Gatz et al., Mol. Gen. Genet. 227: 229-237 (1991). A
particularly preferred inducible promoter is a promoter that responds to an
inducing agent to which plants do not normally respond. An exemplary inducible
promoter is the inducible promoter from a steroid hormone gene, the
transcriptional activity of which is induced by a glucocorticosteroid hormone.
Schena et al., Proc. Natl. Acad. Sci. U.S.A. 88: 0421 (1991).
Many different constitutive promoters can also potentially be utilized in
2 0 the instant invention. Exemplary constitutive promoters include, but are
not
limited to, the promoters from plant viruses such as the 35S promoter from
CaMV
(Odell et al., Nature 313: 810-812 (1985) and the promoters from such genes as
rice actin (McElroy et al., Plant Cell 2: 163-171 (1990)); ubiquitin
(Christensen et
al., Plant Mol. Biol 12: 619-632 (1989) and Christensen et al., Plant Mol.
Biol. 18:
2 5 675-689 (1992)): pEMU (Last et al., Theor. Appl. Genet. 81: 581-588
(1991)); MAS
(Velten et al., EMBO J. 3: 2723-2730 (1984)) and maize H3 histone (Lepetit et
al.,
Mol. Gen. Genet. 231: 276-285 (1992) and Atanassova et al., Plant Journal 2 3
291-300 (1992)).
The ALS promoter, a Xbal/Ncol fragment 5' to the Brassica napus ALS3
3 0 structural gene (or a nucleotide sequence that has substantial sequence
similarity
to said XballNcol fragment), represents a particularly useful constitutive
promoter. See PCT application W096/30530.
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
Transport of protein produced by transgenes to a subcellular compartment
such as the nucleus, chloroplast, vacuole, peroxisome, glyoxysome, cell wall
or
mitochondrion, or for secretion into the apoplast, is accomplished by means of
operably linking the nucleotide sequence encoding a signal sequence to the 5'
and/or 3' region of a gene encoding the protein of interest. Targeting
sequences at
the 5' and/or 3' end of the structural gene may determine, during protein
synthesis
and processing, where the encoded protein is ultimately compartmentalized. The
presence of a signal sequence directs a polypeptide to either an intracellular
organelle or subcellular compartment or for secretion to the apoplast. Many
signal sequences are known in the art. See, for example, Sullivan, T.,
"Analysis of
Maize Brittle-1 Alleles and a Defective Suppressor-Mutator-Induced Mutable
Allele", The Plant Cell, 3:1337-1348 (1991), Becker et al., Plant Mol.
Biol.20: 49
(1992), Close, P.S., Master's Thesis, Iowa State University (1993), Knox, C.,
et
al., "Structure and Organization of Two Divergent Alpha-Amylase Genes From
Barley", Plant Mol.Biol. 9: 3-17 (1987), Lerner et al., Plant Physiol.9l: 124-
129
(1989), Fontes et al.,Plant Cell 3: 483-496 (1991), Matsuoka et al., Proc.
Natl.
Acad. Sci. 88: 834 (1991), Gould et al., J. Cell Biol 108: 1657 (1989),
Creissen et
al., Plant J. 2: 129 (1991), Kalderon, D., Robers, B., Richardson, W., and
Smith A.,
"A short amino acid sequence able to specify nuclear location", Cell 39: 499-
509
2 0 (1984), Stiefel, V., Ruiz-Avila, L., Raz R., Valles M., Gomez J., Pages
M., Martinez-
Izquierdo J., Ludevid M., Landale J., Nelson T., and Puigdomenech P.,
"Expression of a maize cell wall hydroxyproline-rich glycoprotein gene in
early leaf
and root vascular differentiation", Plant Cell 2: 785-793 (1990).
Selection of an appropriate vector is relatively simple, as the constraints
2 5 are minimal. The minimal traits of the vector are that the desired nucleic
acid
sequence be introduced in a relatively intact state. Thus, any vector which
will
produce a plant carrying the introduced DNA sequence should be sufficient.
Typically, an expression vector contains (1) prokaryotic DNA elements encoding
for a bacterial replication origin and an antibiotic resistance marker to
provide for
3 0 the growth and selection of the expression vector in a bacterial host; (2)
DNA
elements that control initiation of transcription, such as a promoter; (3) DNA
elements that control the processing of transcripts such as transcription
16
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
termination/polyadenylation sequences; and (4) a reporter gene. Useful
reporter
genes include ~-glucuronidase, (3-galactosidase, chloramphynical
acetyltransferase, luciferase, kanamycin or the herbicide resistance genes PAT
and BAR. Preferably, the selectable marker gene is kanamyacin or the herbicide
resistance genes PAT and BAR. The BAR or PAT gene is used with the selecting
agent Bialaphos, and is used as a preferred selection marker gene for plant
transformation (Spencer, et al. (1990) J. Thero. Appl'd Genetics 79:625-631).
(5)
The target or structural gene of interest.
One commonly used selectable marker gene for plant transformation is the
neomycin phosphotransferase II (nptll) gene, isolated from transposon TnS,
which
when placed under the control of plant regulatory signals confers resistance
to
kanamycin. Fraley et al., Proc. Natl. Acad. Sci. U.S.A., 80: 4803 (1983).
Another
commonly used selectable marker gene is the hygromycin phosphotransferase
gene which confers resistance to the antibiotic hygromycin. Vanden Elzen et
al.,
Plant Mol. Biol., 5: 299 (1985).
Additional selectable marker genes of bacterial origin that confer
resistance to antibiotics include gentamycin acetyl transferase, streptomycin
phosphotransferase, aminoglycoside- 3' -adenyl transferase, the bleomycin
resistance determinant. Hayford et al., Plant Physiol. 86: 1216 (1988), Jones
et
2 0 al., Mol. Gen. Genet., 210: 86 (1987), Svab et al., Plant Mol.. Biol.. 14:
197 (1990),
Hille et al., Plant Mol. Biol. _7.~ 171 (1986). Other selectable marker genes
confer
resistance to herbicides such as glyphosate, glufosinate or broxynil. Comai et
al.,
Nature 317: 741-744 (1985), Cordon-Kamm et al., Plant Cell 2: 603-618 (1990)
and
Stalker et al., Science 242: 419-423 (1988).
2 5 Other selectable marker genes for plant transformation are not of
bacterial
origin. These genes include, for example, mouse dihydrofolate reductase, plant
5 -
enolpyruvylshikimate-3 -phosphate synthase and plant acetolactate synthase.
Eichholtz et al., Somatic Cell Mol. Genet. 13: 67 (1987), Shah et al., Science
233:
478 (1986), Charest et al., Plant Cell Rep. 8: 643 (1990).
3 0 Another class of marker genes for plant transformation require screening
of
presumptively transformed plant cells rather than direct genetic selection of
transformed cells for resistance to a toxic substance such as an antibiotic.
These
17
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
genes are particularly useful to quantify or visualize the spatial pattern of
expression of a gene in specific tissues and are frequently referred to as
reporter
genes because they can be fused to a gene or gene regulatory sequence for the
investigation of gene expression. Commonly used genes for screening
presumptively transformed cells include (3-glucuronidase (GUS), (3-
galactosidase,
luciferase and chloramphenicol acetyltransferase. Jefferson, R.A., Plant Mol.
Biol.
Rep. 5: 387 (1987)., Teeri et al., EMBO J. 8: 343 (1989), Koncz et al., Proc.
Natl.
Acad. Sci. U.S.A. 84:131 (1987), De Block et al., EMBO J. 3: 1681 (1984).
Another approach to the identification of relatively rare transformation
events has
been use of a gene that encodes a dominant constitutive regulator of the Zea
mays
anthocyanin pigmentation pathway. Ludwig et al., Science 247: 449 (1990).
Recently, in uiuo methods for visualizing GUS activity that do not require
destruction of plant tissue have been made available. Molecular Probes
Publication 2908, Imagene Green, p. 1-4 (1993) and Naleway et al., J. Cell
Bio1.115: 151a (1991). However, these in uivo methods for visualizing GUS
activity have not proven useful for recovery of transformed cells because of
low
sensitivity, high fluorescent backgrounds, and limitations associated with the
use
of luciferase genes as selectable markers.
More recently, a gene encoding Green Fluorescent Protein (GFP) has been
2 0 utilized as a marker for gene expression in prokaryotic and eukaryotic
cells.
Chalfie et al., Science 263: 802 (1994). GFP and mutants of GFP may be used as
screenable markers.
Genes included in expression vectors must be driven by a nucleotide
sequence comprising a regulatory element, for example, a promoter. Several
types
2 5 of promoters are now well-known in the transformation arts, as are other
regulatory elements that can be used alone or in combination with promoters.
A general description of plant expression vectors and reporter genes can be
found in Gruber, et al. (Gruber et al. (1993) Vectors for Plant
Transformation. In:
Methods in Plant Molecular Biolo~y and Biotechnolo~y. Glich et al., eds. (CRC
3 0 Press), pp. 89-119.)
Expression vectors containing genomic or synthetic fragments can be
introduced into protoplast or into intact tissues or isolated cells.
Preferably
18
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
expression vectors are introduced into intact tissue. General methods of
culturing
plant tissues are provided, for example, by Maki, et al. (Maki, et al. (1993)
Procedures for Introducing Foreign DNA into Plants: In: Methods in Plant
Molecular Biolo~y & Biotechnolo~y; Glich et al. eds. (CRC Press), pp. 67-88;
Philips, et al. (1988) Cell-Tissue Culture and In Vitro Manipulation. In Corn
&
Corn Improvement, 3rd ed. Sprague, et al. eds. (American Society of Agronomy
Inc.), pp. 345-387).
Methods of introducing expression vectors into plant tissue include the
direct transfection or co-cultivation of plant cell with Agrobacterium
tumefaciens
(Horsch et al. (1985) Science, 227:1229). Descriptions of Agrobacterium vector
systems and methods for Agrobacterium-mediated gene transfer are provided by
Gruber et al. su ra).
Numerous methods for plant transformation have been developed,
including biological and physical, plant transformation protocols. See, for
example, Miki et al., "Procedures for Introducing Foreign DNA into Plants" in
Methods in Plant Molecular Biology and Biotechnology, Glick, B.R. and
Thompson, J.E. Eds. (CRC Press, Inc., Boca Raton, 1993) pages 67-88. In
addition,
expression vectors and in vitro culture methods for plant cell or tissue
transformation and regeneration of plants are available. See, for example,
Gruber
2 0 et al., "Vectors for Plant Transformation" in Methods in Plant Molecular
Biology
and Biotechnology, Glick, B.R. and Thompson, J.E. Eds. (CRC Press, Inc., Boca
Raton, 1993) pages 89-119.
A. A~robacterium-mediated Transformation
One method for introducing an expression vector into plants is based on
2 5 the natural transformation system of Agrobacterium. See, for example,
Horsch et
al., Science 227: 1229 (1985). A. tumefaciens and A. rhizogenes are plant
pathogenic soil bacteria which genetically transform plant cells. The Ti and
Ri
plasmids of A. tumefaciens and A. rhizogenes, respectively, carry genes
responsible
for genetic transformation of the plant. See, for example, Kado, C.L, Crit.
Reu.
3 0 Plant. Sci.10: 1 (1991). Descriptions of Agrobacterium vector systems and
methods for Agrobacterium-mediated gene transfer are provided by Gruber et
al.,
19
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
supra, Miki et al., supra, and Moloney et al., Plant Cell Reports 8: 238
(1989). See
also, U.S. Patent No. 5,591,616, issued Jan. 7, 1997.
B. Direct Gene Transfer
Despite the fact the host range for Agrobacterium-mediated
transformation is broad, some major cereal crop species and gymnosperms have
generally been recalcitrant to this mode of gene transfer, even though some
success has recently been achieved in rice and maize. Hiei et al., The Plant
Journal 6: 271-282 (1994); U.S. Patent No. 5,591,616, issued Jan. 7, 1997.
Several
methods of plant transformation, collectively referred to as direct gene
transfer,
have been developed as an alternative to Agrobacterium-mediated
transformation.
A generally applicable method of plant transformation is microprojectile-
mediated transformation wherein DNA is carried on the surface of
microprojectiles measuring 1 to 4 mm. The expression vector is introduced into
plant tissues with a biolistic device that accelerates the microprojectiles to
speeds
of 300 to 600 m/s which is sufficient to penetrate plant cell walls and
membranes.
Sanford et al., Part. Sci. Technol. 5: 27 (1987), Sanford, J.C., Trends
Biotech. 6:
299 (1988), Klein et al., BiolTechnology 6: 559-563 (1988), Sanford, J.C.,
Physiol
Plant 79: 206 (1990), Klein et al., Biotechnology 10: 268 (1992). In maize,
several
target tissues can be bombarded with DNA-coated microprojectiles in order to
2 0 produce transgenic plants, including, for example, callus (Type I or Type
II),
immature embryos, and meristematic tissue.
Another method for physical delivery of DNA to plants is sonication of
target cells. Zhang et al., BiolTechnology 9: 996 (1991). Alternatively,
liposome
or spheroplast fusion have been used to introduce expression vectors into
plants.
Deshayes et al., EMBO J., 4: 2731 (1985), Christou et al., Proc Natl. Acad.
Sci.
U.S.A. 84: 3962 (1987). Direct uptake of DNA into protoplasts using CaCl2
precipitation, polyvinyl alcohol, or poly-L-ornithine have also been reported.
Hain
et al., Mol. Gen. Genet.199: 161 (1985) and Draper et al., Plant Cell
Physiol.23: 451
(1982). Electroporation of protoplasts and whole cells and tissues have also
been
3 0 described. Donn et al., In Abstracts of VIIth International Congress on
Plant Cell
and Tissue Culture IAPTC, A2-38, p 53 (1990); D'Halluin et al., Plant Cell 4:
1495-
1505 (1992) and Spencer et al., Plant Mol. Biol. 24: 51-61 (1994).
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
Following transformation of maize target tissues, expression of the above-
described selectable marker genes allows for preferential selection of
transformed
cells, tissues and/or plants, using regeneration and selection methods now
well
known in the art.
After transformation of a plant cell or plant, plant cells or plants
transformed with the desired DNA sequences integrated into the genome can be
selected by appropriate phenotypic markers. Phenotypic markers are known in
the art and may be used in this invention.
Confirmation of transgenic plants will typically be based on an assay or
assays or by simply measuring growth rate. Transformed plants can be screened
by biochemical, molecular biological, and other assays. Various assays may be
used to determine whether a particular plant, plant part, or a transformed
cell
shows an increase in enzyme activity. Typically, the change in expression or
activity of a transformed plant will be compared to levels found in wild type
(e.g.,
untransformed) plants of the same type. Preferably, the effect of the
introduced
construct on the level of expression or activity of the endogenous gene will
be
established from a comparison of sibling plants with and without the
construct.
Cyclin, CDC25, Niml, and Plxl transcript levels can be measured, for example,
by Northern blotting, primer extension, quantitative or semi-quantitative PCR
2 0 (polymerase chain reaction), and other methods well known in the art (See,
e.g.,
Sambrook, et al. (1989). Molecular Cloning, A Laboratory Manual, second
edition
(Cold Spring Harbor Laboratory Press), Vols. 1-3). Protein can be measured in
a
number of ways including immunological methods (e.g., by Elisa or Western
blotting). CDK activity can be measured in various assays as described in Sun
et
al., Proc. Nat'1. Acad. Sci. U S A. 96(7):4180-85 (1999). Cell division of a
plant cell
or tissue can be measured in a variety of ways including those described in
Myers
et al., Plant Physiol. 94:1330-36 (1990) and Artlip, et al., Plant Cell and
Environ
18:1034-40 (1995).
Normally, regeneration will be involved in obtaining a whole plant from a
3 0 transformation process. The term "regeneration" as used herein, means
growing a
whole plant from a plant cell, a group of plant cells, a plant part, or a
plant piece
(e.g., from a protoplast, callus, or a tissue part).
21
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
The foregoing methods for transformation would typically be used for
producing transgenic inbred lines. Transgenic inbred lines could then be
crossed,
with another (non-transformed or transformed) inbred line, in order to produce
a
transgenic hybrid maize plant. Alternatively, a genetic trait which has been
engineered into a particular maize line using the foregoing transformation
techniques could be moved into another line using traditional backcrossing
techniques that are well known in the plant breeding arts. For example, a
backcrossing approach could be used to move an engineered trait from a public,
non-elite line into an elite line, or from a hybrid maize plant containing a
foreign
gene in its genome into a line or lines which do not contain that gene. As
used
herein, "crossing" can refer to a simple X by Y cross, or the process of
backcrossing, depending on the context.
Various plants will be suitable targets for enhancing cell division in female
reproductive organs with the identified genes. In particular, the methods of
the
invention described herein may be applicable to any crop species including but
not
limited to barley, sorghum, wheat, maize, soybean, and rice.
In a most preferred embodiment, transformation is carried out in maize
plants according to the method of Agrobacterium.
Parts obtained from the regenerated plant, such as flowers, pods, seeds,
2 0 leaves, branches, fruit, and the like, are covered by the invention,
provided that
these parts comprise cells which have been so transformed. Progeny and
variants,
and mutants of the regenerated plants are also included within the scope of
this
invention, provided that these parts comprise the introduced DNA sequences.
Cyclin, CDC25, Niml,and Plxl levels and the activity of CDK are
2 5 preferably determined as set forth in the examples.
Once a transgenic plant is produced having a desired characteristic, it will
be useful to propagate the plant and, in some cases, to cross to inbred lines
to
produce useful hybrids.
In seed propagated crops, mature transgenic plants may be self crossed to
3 0 produce a homozygous inbred plant. The inbred plant produces seed
containing
the genes for the newly introduced trait. These seeds can be grown to produce
22
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
plants that will produce the selected phenotype. All articles cited herein and
in
the following list are hereby expressly incorporated in their entirety by
reference.
23
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
CITATIONS
Artlip, T.S. et al., "Water deficit in developing endosperm of maize: cell
division and
nuclear DNA endoreduplication", Plant, Cell, and Environ. 18:1034-1040
(1995).
Cheikh and Jones, "Disruption of Maize Kernel Growth and Development by Heat
Stress", Plant Physiol. 106:45-51 (1994).
Doerner, P. et al., "Control of root growth and development by cyclin
expression",
Nature 380:520-523 (1996).
Doonan et al., "Conserved and novel regulators of the plant cell cycle", Curr.
Opin. in
Cell Biol. 9:824-830 (1997).
Hoffmann, I. et al., "Phosphorylation and activation of human cdc25-Cby cdc2-
cyclin
B and its involvement in the self-amplification of MPF at mitosis", EMBO J. 12
(1):53-63 (1993).
Jinno, S. et al., "Cdc25A is a novel phosphatase functioning early in the cell
cycle",
EMBO J. 13(7):1549-1556 (1994).
Jones, R.J. et al., "Thermal Environment During Endosperm Cell Division in
Maize:
Effects on Number of endosperm Cells and Starch Granules", Crop Science
25:830-834 (1985).
Kalla et al., 1994, Plant J.6(6):849-860
Kumagai, A. and Dunphy, W.G., "Purification and Molecular Cloning of Plxl, a
Cdc25-Regulatory Kinase from Xenopus Egg Extracts", Science 273:1377-1380
(1996).
Lammer, C. et al., "The cdc25B phosphatase is essential for the G2/M phase
transition in human cells", J. Cell Sci. 111:2445-2453 (1998).
Lee, K.S. et al., "Plk is a Functional Homolog of Saccharomyces cerevasiae
CdcS, and
Elevated Plk Activity Induces Multiple Septation Structures", Mol. Cell. Biol.
17(6):3408-3417 (1997).
Lejeune, P. et al., "Hormonal Control of ear abortion in a stress-sensitive
maize (Zea
mays) inbred", Aust. J. Plant Physiol., 25:481-488 (1998).
Mambelli and Setter, "Inhibition of maize endosperm cell division and
endoreduplication by exogenously applied abiscisic acid", Physiologic Plant.
24
CA 02374431 2002-03-22
WO 01/23594 PCT/US00/26405
104:266-272 (1998).
McKibbin, R.S. et al., "Expression of fission yeast cdc25 alters the frequency
of lateral
root formation in transgenic tobacco", Plant Mol. Biol. 36:601-612 (1998).
Morgan, D., "Cyclin-Dependent Kinases: Engines, Clocks, and Microprocessors",
Annu. Rev. Cell Dev. Biol. 13:261-91 (1997).
Myers, P.N. et al., "Abscisic Acid Inhibition of Endosperm Cell Division in
Cultured
Maize Kernels", Plant Physiol. 94, 1330-1336 (1990).
Prine, G.M. 1971. A Critical Period for Ear Development in Maize. Crop
Science.
11:782-786.
Renaudin, J-P et al., "Plant cyclins: a unified nomenclature for plant A-, B-,
and D-
type cyclins based on sequence organization", Plant Mol. Biol., 32:1003-1018
(1996).
Schuppler, U. et al., "Effect of Water Stress on Cell Division and Cell-
Division-Cycle
2-Like Cell-Cycle Kinase Activity in Wheat Leaves," Plant Physiol. 117: 667-
678 (1998).
Soni, R. et al., "A Family of Cyclin D Homologs from Plants Differentially
Controlled
by Growth Regulators and Containing the Conserved Retinoblastoma Protein
Interaction Motif', The Plant Cell, 7:86 (1995).
Sun, Y. et al., "Alternative splicing of cyclin transcripts in maize
endosperm", Gene
195:167-175 (1997).
Sun, Y. et al., "Identification of Wee1 in Maize (Zea mays L.) and Its
Involvement in
Endoreduplication", Proc. Nat'1 Acad. Sci. U.S.A., 1999 96(7):4180-4185.
Zhang et al., "Cytokinin controls the cell cycle at mitosis by stimulating the
tyrosine
dephosphorylation and activation of p34~a~2_like H1 histone kinase", Planta
200:2-12 (1996).
Trehin et al., Planta (1998) 206(2):215-224)
Zinselmeier, C. and J.E. Habben. 1998. Use of mRNA-Profiling Technology to
Determine Gene Expression Patterns in Developing Maize Ears that Differ in
Yield. Plant Physiology Abstracts.
All references cited herein are hereby expressly incorporated in their
entirety by
reference.