Note: Descriptions are shown in the official language in which they were submitted.
65920-120
CA 02375225 2001-11-26
1
MUTUAL SALT OF AMLODIPINE AND ATORVASTATIN
This invention relates to a mutual salt of
amlodipine and atorvastatin, pharmaceutical compositions
thereof and methods of using said salt and/or said
compositions to treat subjects suffering from angina
pectoris, atherosclerosis, combined hypertension and
hyperlipidemia and to treat subjects presenting with
symptoms of cardiac risk, including humans.
BACKGROUND OF THE INVENTION
The conversion of 3-hydroxy-3-methylglutaryl-
coenzyme A (HMG-CoA) to mevalonate is an early and rate-
limiting step in the cholesterol biosynthetic pathway. This
step is catalyzed by the enzyme HMG-CoA reductase. Statins
inhibit HMG-CoA reductase from catalyzing this conversion.
As such, statins are collectively potent lipid lowering
agents.
Atorvastatin calcium, disclosed in U.S. Patent No.
5,273,995, is currently sold as Lipitor° and has the formula
O
O_
/~
Caz+
z
Atorvastatin calcium is a selective, competitive inhibitor
of HMG-CoA. As such, atorvastatin calcium is a potent lipid
65920-120
CA 02375225 2001-11-26
2
lowering compound. The free carboxylic acid form of
atorvastatin exists predominantly as the lactone of the
formula
O
~~'OH
and is disclosed in U.S.Patent No. 4,681,893.
Amlodipine and related dihydropyridine compounds
are disclosed in U.S. Patent No. 4,572,909, as potent
antiischemic and antihypertensive agents. U.S. Patent No.
4,879,303, discloses amlodipine benzenesulfonate salt (also
termed amlodipine besylate). Amlodipine and amlodipine
besylate are potent and long lasting calcium channel
blockers. As such, amlodipine, amlodipine besylate and
other pharmaceutically acceptable acid addition salts of
amlodipine have utility as antihypertensive agents and as
antiischemic agents. Amlodipine and its pharmaceutically
acceptable acid addition salts are also disclosed in U.S.
Patent No. 5,155,120 as having utility in the treatment of
congestive heart failure. Amlodipine besylate is currently
sold as Norvasc~. Amlodipine has the formula
65920-120
CA 02375225 2001-11-26
2a
H
CH20CHZCH2NH2
CH30
COzCH2CH3
'1
Atherosclerosis is a condition characterized by
irregularly distributed lipid deposits in the intima of
arteries, including coronary, carotid and peripheral
arteries.
CA 02375225 2001-11-26
WO 00/73271 -3- PCT/IB00/00590
Atherosclerotic coronary heart disease (hereinafter termed "CND") accounts for
about
53% of all deaths attributable to a cardiovascular event. CHD accounts for
nearly
one-half (about $50-60 billion) of the total U.S. cardiovascular healthcare
expenditures and about 6% of the overall national medical bill each year.
Despite
attempts to modify secondary risk factors such as, inter alia, smoking,
obesity and
lack of exercise, and treatment of dyslipidemia with dietary modification and
drug
therapy, CHD remains the most common cause of death in the United States.
High levels of blood cholesterol and blood lipids are conditions involved in
the
onset of atherosclerosis. It is well known that inhibitors of 3-hydroxy-3-
methylglutaryl-
coenzyme A reductase (HMG-CoA reductase) are effective in lowering the level
of
blood plasma cholesterol, especially low density lipoprotein cholesterol (LDL-
C), in
man (Brown and Goldstein, New England Journal of Medicine, 1981, 305, No. 9,
515-
517). It has now been established that lowering LDL-C levels affords
protection from
coronary heart disease (see, e.g., The Scandinavian Simvastatin Survival Study
Group: Randomised trial of cholesterol lowering in 4444 patients with coronary
heart
disease: the Scandinavian Simvastatin Survival Study (4S), Lancet, 1994, 344,
1383-
89; and Shepherd, J. et al., Prevention of coronary heart disease with
pravastatin in
men with hypercholesterolemia, New England Journal of Medicine, 1995, 333,
1301-
07).
Angina pectoris is a severe constricting pain in the chest, often radiating
from
the precordium to the left shoulder and down the left arm. Often angina
pectoris is
due to ischemia of the heart and is usually caused by coronary disease.
Currently the treatment of symptomatic angina pectoris varies significantly
from country to country. In the U.S., patients who present with symptomatic,
stable
angina pectoris are frequently treated with surgical procedures or PTCA.
Patients
who undergo PTCA or other surgical procedures designed to treat angina
pectoris
frequently experience complications such as restenosis. This restenosis may be
manifested either as a short term proliferative response to angioplasty-
induced
trauma or as long term progression of the atherosclerotic process in both
graft
vessels and angioplastied segments.
The symptomatic management of angina pectoris involves the use of a
number of drugs, frequently as a combination of two or more of the following
classes:
beta blockers, nitrates and calcium channel blockers. Most, if not all, of
these patients
require therapy with a lipid lowering agent as well. The National Cholesterol
CA 02375225 2001-11-26
WO 00/73271 -4- PCT/IB00/00590
Education Program (NCEP) recognizes patients with existing coronary artery
disease
as a special class requiring aggressive management of raised LDL-C.
Amlodipine helps to prevent myocardial ischemia in patients with exertional
angina pectoris by reducing Total Peripheral Resistance, or afterload, which
reduces
the rate pressure product and thus myocardial oxygen demand at any particular
level
of exercise. In patients with vasospastic angina pectoris, amlodipine has been
demonstrated to block constriction and thus restore myocardial oxygen supply.
Further, amlodipine has been shown to increase myocardial oxygen supply by
dilating
the coronary arteries.
Hypertension frequently coexists with hyperlipidemia and both are considered
to be major risk factors for developing cardiac disease ultimately resulting
in adverse
cardiac events. This clustering of risk factors is potentially due to a common
mechanism. Further, patient compliance with the management of hypertension is
generally better than patient compliance with hyperlipidemia. It would
therefore be
advantageous for patients to have a single therapy which treats both of these
conditions.
Coronary heart disease is a multifactorial disease in which the incidence and
severity are affected by the lipid profile, the presence of diabetes and the
sex of the
subject. Incidence is also affected by smoking and left ventricular
hypertrophy which
is secondary to hypertension. To meaningfully reduce the risk of coronary
heart
disease, it is important to manage the entire risk spectrum. For example,
hypertension intervention trials have failed to demonstrate full normalization
in
cardiovascular mortality due to coronary heart disease. Treatment with
cholesterol
synthesis inhibitors in patients with and without coronary artery disease
reduces the
risk of cardiovascular morbidity and mortality.
The Framingham Heart Study, an ongoing prospective study of adult men and
women, has shown that certain risk factors can be used to predict the
development of
coronary heart disease. (see Wilson et al., Am. J. Cardiol. 1987, 59(14):91 G-
94G).
These factors include age, gender, total cholesterol level, high density
lipoprotein
(HDL) level, systolic blood pressure, cigarette smoking, glucose intolerance
and
cardiac enlargement (left ventricular hypertrophy on electrocardiogram,
echocardiogram or enlarged heart on chest X-ray). Calculators and computers
can
easily be programmed using a multivariate logistic function that allows
calculation of
the conditional probability of cardiovascular events. These determinations,
based on
CA 02375225 2001-11-26
WO 00/73271 -5- PCT/IB00/00590
experience with 5,209 men and women participating in the Framingham study,
estimate coronary artery disease risk over variable periods of follow-up.
Modeled
incidence rates range from less than 1 % to greater than 80% over an
arbitrarily
selected six year interval. However, these rates are typically less than 10%
and
rarely exceed 45% in men and 25% in women.
Kramsch et al., Journal of Human Hypertension (1995) (Suppl. 1 ), 53-59
disclose the use of calcium channel blockers, including amlodipine, to treat
atherosclerosis. That reference further suggests that atherosclerosis can be
treated
with a combination of amlodipine and a lipid lowering agent. Human trials have
shown that calcium channel blockers have beneficial effects in the treatment
of early
atherosclerotic lesions. (see, e.g., Lichtlen, P.R. et al., Retardation of
angiographic
progression of coronary artery disease by nifedipine, Lancet, 1990, 335, 1109-
13;
and Waters, D. et al., A controlled clinical trial to assess the effect of a
calcium
channel blocker on the progression of coronary atherosclerosis, Circulation,
1990, 82,
1940-53.) U.S. 4,681,893 discloses that certain statins, including
atorvastatin, are
hypolipidemic agents and as such are useful in treating atherosclerosis.
Jukema et
al., Circulation, 1995 (Suppl. 1 ), 1-197, disclose that there is evidence
that calcium
channel blockers act synergistically in combination with lipid lowering agents
(e.g.,
HMG-CoA reductase inhibitors), specifically pravastatin. Orekhov et al.,
Cardiovascular Drugs and Therapy, 1997, 11, 350 disclose the use of amlodipine
in
combination with lovastatin for the treatment of atherosclerosis.
Commonly assigned International Patent Application Publication Number
W099/11259 discloses pharmaceutical compositions comprising a combination of
amlodipine and atorvastatin.
CA 02375225 2001-11-26
WO 00/73271 -6- PCT/IB00/00590
SUMMARY OF THE INVENTION
This invention is directed to a compound which is an atorvastatin salt of
amlodipine. It is to be understood that the phrase "amlodipine salt of
atorvastatin" is
synonymous with the phrase "atorvastatin salt of amlodipine." Those phrases
are
used interchangeably throughout this specification and appendant claims.
This invention is particularly directed to an atorvastatin salt of amlodipine
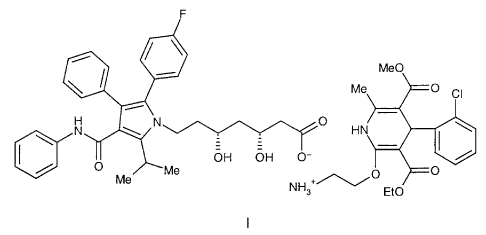
which is a compound of formula I,
~~~ Me
N ~ \N O H N \
O OH OH O-
~Me O O
Me +/~/
NH3 Et0
This invention is still more particularly directed to a compound of formula I
wherein the carbon atom at the 4-position of the dihydropyridine ring has the
(R)-
configuration, e.g., 7-[2-(4-fluoro-phenyl)-5-isopropyl-3-phenyl-4-
phenylcarbamoyl-
pyrrol-1-yl]-3,5-dihydroxy-heptanoate salt with 2-(2-amino-ethoxymethyl)-4(R)-
(2-
chloro-phenyl)-6-methyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid 3-ethyl
ester 5-
methyl ester.
This invention is also more particularly directed to a compound of formula I
wherein the carbon atom at the 4-position of the dihydropyridine ring has the
(S)-
configuration, 7-(2-(4-fluoro-phenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoyl-
pyrrol-1-
yl]-3,5-dihydroxy-heptanoate salt with 2-(2-amino-ethoxymethyl)-4(S)-(2-chloro-
phenyl)-6-methyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid 3-ethyl ester 5-
methyl
ester.
This invention is also directed to pharmaceutical compositions comprising a
compound of formula I and a pharmaceutically acceptable carrier, vehicle or
diluent.
This invention is also directed to methods of treating angina pectoris in a
mammal suffering from angina pectoris comprising administering to said mammal
an
angina pectoris treating effective amount of a compound of formula I.
CA 02375225 2001-11-26
WO 00/73271 _~_ PCT/IB00/00590
This invention is also directed to methods of treating angina pectoris in a
mammal suffering from angina pectoris comprising administering to said mammal
an
angina pectoris treating effective amount of a pharmaceutical composition
comprising
a mutual salt of amlodipine and atorvastatin and a pharmaceutically acceptable
carrier, vehicle or diluent.
This invention is also directed to methods of treating hypertension and
hyperlipidemia in a mammal suffering from hypertension and hyperlipidemia
comprising administering to said mammal a hypertension and hyperlipidemia
treating
effective amount of a compound of formula I.
This invention is also directed to methods of treating hypertension and
hyperlipidemia in a mammal suffering from hypertension and hyperlipidemia
comprising administering to said mammal a hypertension and hyperlipidemia
treating
effective amount of a pharmaceutical composition comprising a mutual salt of
amlodipine and atorvastatin and a pharmaceutically acceptable carrier, vehicle
or
diluent.
This invention is also directed to methods of treating atherosclerosis in a
mammal suffering from atherosclerosis comprising administering to said mammal
an
antiatherosclerosis effective amount of a compound of formula I. This
invention is
particularly directed to those cases where said antiatherosclerotic effect is
manifested
by a slowing of the progression of atherosclerotic plaques, including wherein
said
atherosclerotic plaque formation is slowed in coronary arteries, carotid
arteries or in
the peripheral arterial system. This invention is also particularly directed
to those
cases where said antiatherosclerotic effect is manifested by a regression of
atherosclerotic plaques, including wherein said regression occurs in the
coronary
arteries, in the carotid arteries and/or in the peripheral arterial system.
This invention is also directed to methods of treating atherosclerosis in a
mammal suffering from atherosclerosis comprising administering to said mammal
an
antiatherosclerosis effective amount of a pharmaceutical composition
comprising a
mutual salt of amlodipine and atorvastatin and a pharmaceutically acceptable
carrier,
vehicle or diluent.
This invention is also directed to methods of managing cardiac risk in a
mammal at risk of suffering an adverse cardiac event, comprising administering
to
said mammal a cardiac risk treating effective amount of a compound of formula
I.
CA 02375225 2001-11-26
WO 00/73271 _8_ PCT/IB00/00590
This invention is also directed to methods of managing cardiac risk in a
mammal at risk of suffering an adverse cardiac event, comprising administering
to
said mammal a cardiac risk treating effective amount of a pharmaceutical
composition comprising a mutual salt of amlodipine and atorvastatin and a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions comprising an
amount of a compound of formula I and amlodipine or a pharmaceutically
acceptable
salt thereof, e.g., amlodipine besylate. This invention is also directed to
methods of
treating diseases and conditions in mammals comprising administering to said
mammals, e.g., humans, such a pharmaceutical composition. Diseases and
conditions which may be treated with such pharmaceutical compositions include
angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and
cardiac risk.
This invention is also directed to pharmaceutical compositions comprising an
amount of a compound of formula I and atonrastatin or a pharmaceutically
acceptable
salt thereof, e.g., the hemicalcium salt of atorvastatin. This invention is
also directed
to methods of treating diseases and conditions in mammals comprising
administering
to said mammals, e.g., humans, such pharmaceutical compositions. Diseases and
conditions which may be treated with such pharmaceutical compositions include
angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and
cardiac risk.
This invention is also directed to methods of delivering amlodipine in vivo by
administering to a mammal, e.g., a human, a compound of formula I.
This invention is also directed to methods of delivering atorvastatin in vivo
by
administering to a mammal, e.g., a human, a compound of formula I.
This invention is also directed to methods of treating a mammal comprising
administering to said mammal an amount of a compound of formula I and an
amount
of amlodipine or a pharmaceutically acceptable salt thereof.
This invention is also directed to methods of treating a mammal comprising
administering to said mammal an amount of a compound of formula I and an
amount
of atorvastatin or a pharmaceutically acceptable salt thereof.
This invention is also directed to methods of treating a mammal with
amlodipine comprising administering to said mammal a compound of formula I.
CA 02375225 2004-07-30
65920-120
-9-
This invention is also directed to methods of treating a mammal with
atorvastatin comprising administering to said mammal a compound of formula I.
Amlodipine is a racemic compound due to the symmetry at position 4 of the
dihydropyridine ring. The R and S enantiomers may be prepared as described by
Arrowsmith et al., J. Med. Chem., 1986, 29, 1696. The calcium channel blocking
activity of amlodipine is substantially confined to the S(-) isomer and to the
racemic
mixture containing the R(+) and S(-) forms. The R(+) isomer has little or no
calcium channel blocking activity. However, the R(+)
isomer is a potent inhibitor of smooth muscle cell
migration. Thus, tire R(+) isomer is useful in the treatment or prevention of
atherosclerosis. Based on the above, a skilled person could choose the R(+)
isomESr,
the S(-) isomer or the racemic mixture of the R(+) isomer
and the S(-) isomer for use in the combination of this
invention.
Where used herein and in the appendant claims, the term "cardiac risk"
means the likelihood that a subject will suffer a future adverse cardiac event
such as,
e.g., myocardial infarction, cardiac arrest, cardiac failure and/or cardiac
ischaemia,
Cardiac risk is calculated using the Framingham Risk Equation as set forth
above.
The term "cardiac risk management" means that the risk of future adverse
cardiac
events is substantially reduced.
65920-120
CA 02375225 2001-11-26
DETAILED DESCRIPTION OF THE INVENTION
The mutual salt of amlodipine and atorvastatin of
this invention and pharmaceutical compositions thereof may
5 be readily prepared as set forth in the following
description and in the Examples below.
Specifically, the mutual salt of atorvastatin and
amlodipine is prepared by dissolving the free base of
amlodipine in an appropriate reaction inert solvent and
10 adding the resulting solution to a solution of the free acid
of atorvastatin in an appropriate reaction inert solvent.
As used herein, the expressions "reaction inert solvent" and
"inert solvent" refer to a solvent or mixture of solvents
which does not interact with starting materials, reagents,
intermediates or products in a manner which adversely
affects the yield of the desired product. Preferred
solvents for amlodipine include but are not limited to
acetone, water/acetone mixtures, alcoholic solvents such as
methanol and ethanol. An especially preferred solvent for
amlodipine is a one to one mixture of water and acetone.
Preferred solvents for atorvastatin include but are not
limited to ethyl acetate, toluene, acetone and alcoholic
solvents such as methanol and ethanol. An especially
preferred solvent for atorvastatin is ethyl acetate. The
reaction mixture is stirred vigorously at ambient
temperature. Crystallization is accomplished by the
addition of less polar solvents such as toluene (20m1) to
afford the mutual salt of amlodipine and atorvastatin. It
will be understood by a person skilled in the art that
amlodipine is a racemic mixture of two enantiomers and that
the salt formed using the racemic mixture of amlodipine will
result in a diastereomeric mixture of the salt of amlodipine
CA 02375225 2004-07-30
65920-120
11
amlodipine and atorvastatin. An optically pure form of the
salt of amlodipine and atorvastatin can be prepared using
the same procedure set forth above but substituting the
(R)-enanitiomer of amlodipine for the racemic mixture or by
substituting the (S)-enantiomer of amlodipine for the
racemic mixture.
Amlodipine may readily be prepared as described in
U.S. Patent No. 4,572,909. Amlodipine besylate, which is
currently sold as Norvasc~, may be prepared as described in
U.S. Patent No. 4,879,303. Amlodipine and amlodipine
besylate are potent and long lasting calcium channel
blockers.
The R and S enantiomers of amlodipine may be
prepared as described by Arrowsmith et al., J. Med. Chem.,
1986, 29, 1696.
Atorvastatin may readily be prepared as described
in U.S. Patent No. 4,681,893. The hemicalcium salt of
atorvastatin, which is currently sold as Lipitor~, may
readily be prepared as described in U.S. Patent No.
5,273,995.
In addition, the mutual salt of amlodipine and
atorvastatin of this invention may occur as a hydrate or as
a solvate. Said hydrates and solvates are also within the
scope of this invention.
The mutual salt, pharmaceutical compositions and
methods of this invention are all adapted to therapeutic use
as agents in the treatment of atherosclerosis, angina
pectoris, and a condition characterized by the presence of
both hypertension and hyperlipidemia in mammals,
particularly humans. Further, since these diseases and
conditions are closely related to the development of cardiac
CA 02375225 2005-07-26
72222-606
lla
disease and adverse cardiac conditions, the mutual salt,
compositions and methods, by virtue of their action as
antiatherosclerotics, antianginals, antihypertensives and
antihyperlipidemics, are useful in the management of cardiac
risk.
According to another aspect of the present
invention there is provided a commercial package comprising
a compound or pharmaceutical composition of the invention
and instructions for its use in treating the previously
described conditions.
The utility of the salt and compositions of the
present invention as medical agents in the treatment of
atherosclerosis in mammals (e.g. humans) is demonstrated by
the activity of the compounds of this invention in
conventional assays and the clinical protocol described
below.
Effect of the Mutual Salt of Amlodipine and Atorvastatinon
the Treatment of Atherosclerosis
This study is a prospective randomized evaluation
of the effect of a combination of a mutual salt of
amlodipine and atorvastatin on the progression/regression of
coronary and carotid artery disease. The study is used to
show that a mutual salt of amlodipine and atorvastatin is
effective in slowing or arresting the progression or causing
regression of existing coronary artery disease (CAD) as
evidenced by changes in coronary angiography or carotid
ultrasound, in subjects with established disease.
This study is an angiographic documentation of
coronary artery disease carried out as a double-blind,
placebo-controlled trial of a minimum of about 500 subjects
and preferably of about 780 to about 1200 subjects. It is
65920-120
CA 02375225 2001-11-26
llb
especially preferred to study about 1200 subjects in this
study. Subjects are admitted into the study after
satisfying certain entry criteria set forth below.
CA 02375225 2001-11-26
WO 00/73271 -12_ PCT/IB00/00590
Entry criteria: Subjects accepted for entry into this trial must satisfy
certain
criteria. Thus, the subject must be an adult, either male or female, aged 18-
80 years
of age in whom coronary angiography is clinically indicated. Subjects will
have
angiographic presence of a significant focal lesion such as 30% to 50% on
subsequent evaluation by quantitative coronary angiography (OCA) in a minimum
of
one segment (non-PTCA, non-bypassed or non-MI vessel) that is judged not
likely to
require intervention over the next 3 years. It is required that the segments
undergoing analysis have not been interfered with. Since percutaneous
transluminal
cardiac angioplasty (PTCA) interferes with segments by the insertion of a
balloon
catheter, non-PTCA segments are required for analysis. It is also required
that the
segments to be analyzed have not suffered a thrombotic event, such as a
myocardial
infarct (MI). Thus the requirement for non-MI vessels. Segments that will be
analyzed include: left main, proximal, mid and distal left anterior
descending, first and
second diagonal branch, proximal and distal left circumflex, first or largest
space
obtuse marginal, proximal, mid and distal right coronary artery. Subjects will
have an
ejection fraction of greater than 30% determined by catheterization or
radionuclide
ventriculography or ECHO cardiogram at the time of the qualifying angiogram or
within the previous three months of the acceptance of the qualifying angiogram
provided no intervening event such as a thrombotic event or procedure such as
PTCA has occurred.
Generally, due to the number of patients and the physical limitations of any
one facility, the study is carried out at multiple sites. At entry into the
study, subjects
undergo quantitative coronary angiography as well as B-mode carotid artery
ultrasonography and assessment of carotid arterial compliance at designated
testing
centers. This establishes baselines for each subject. Once admitted into the
test,
subjects are randomized to receive amlodipine besylate (10 mgs) and placebo or
atorvastatin calcium (10 mgs) and placebo or a mutual salt of amlodipine and
atorvastatin (about 5 to 160 mgs). All doses set forth in this protocol are
per day
doses. The amount of amlodipine besylate, atonrastatin calcium and mutual salt
of
amlodipine and atorvastatin may be varied as required. Generally, a subject
will
begin taking 10 mg and the amount will be titrated down to as little as 5 mg
as
determined by the clinical physician.
The subjects are monitored for a one to three year period, generally three
years being preferred. B-mode carotid ultrasound assessment of carotid artery
CA 02375225 2001-11-26
WO 00/73271 -13- PCT/IB00/00590
atherosclerosis and compliance are performed at regular intervals throughout
the
study. Generally, six month intervals are suitable. Typically this assessment
is
performed using B-mode ultrasound equipment. However, a person skilled in the
art
may use other methods of performing this assessment.
Coronary angiography is performed at the conclusion of the one to three year
treatment period. The baseline and post-treatment angiograms and the
intervening
carotid artery B-mode ultrasonograms are evaluated for new lesions or
progression of
existing atherosclerotic lesions. Arterial compliance measurements are
assessed for
changes from baseline and over the 6-month evaluation periods.
The primary objective of this study is to show that the mutual salt of
amlodipine and atonrastatin reduces the progression of atherosclerotic lesions
as
measured by quantitative coronary angiography (QCA) in subjects with clinical
coronary artery disease. QCA measures the opening in the lumen of the arteries
measured.
The primary endpoint of the study is the change in the average mean
segment diameter of the coronary artery tree. Thus, the diameter of an
arterial
segment is measured at various portions along the length of that segment. The
average diameter of that segment is then determined. After the average segment
diameter of many segments has been determined, the average of all segment
averages is determined to arrive at the average mean segment diameter. The
mean
segment diameter of subjects taking the mutual salt of atorvastatin and
amlodipine
will decline more slowly, will be halted completely, or there will be an
increase in the
mean segment diameter. These results represent slowed progression of
atherosclerosis, halted progression of atherosclerosis and regression of
atherosclerosis, respectively.
The secondary objective of this study is to show that the mutual salt of
amlodipine and atorvastatin reduces the rate of progression of atherosclerosis
in the
carotid arteries as measured by the slope of the maximum intimal-medial
thickness
measurements averaged over 12 separate wall segments (Mean Max) as a function
of time. The intimal-medial thickness of subjects taking the mutual salt of
atorvastatin
and amlodipine will increase more slowly, will cease to increase or will
decrease.
These results represent slowed progression of atherosclerosis, halted
progression of
atherosclerosis and regression of atherosclerosis, respectively.
CA 02375225 2001-11-26
WO 00/73271 -14- PCT/IB00/00590
The utility of the mutual salts and compositions of the present invention as
medical agents in the treatment of angina pectoris in mammals (e.g., humans)
is
demonstrated by the activity of the compounds of this invention in
conventional
assays and the clinical protocol described below.
Effect of the Mutual Salt of Amlodipine and Atorvastatin
on the Treatment of Angina
This study is a double blind, parallel arm, randomized study to show the
effectiveness of a mutual salt of amlodipine and atorvastatin in the treatment
of
symptomatic angina.
Entry criteria: Subjects are males or females between 18 and 80 years of age
with a history of typical chest pain associated with one of the following
objective
evidences of cardiac ischemia: (1 ) stress test segment elevation of about one
millimeter or more from the ECG; (2) positive treadmill stress test; (3) new
wall
motion abnormality on ultrasound; or (4) coronary angiogram with a significant
qualifying stenosis. Generally a stenosis of about 30-50% is considered to be
significant.
Each subject is evaluated for about ten to thirty-two weeks. At least ten
weeks are generally required to complete the study. Sufficient subjects are
used in
this screen to ensure that about 200 to 800 subjects and preferably about 400
subjects are evaluated to complete the study. Subjects are screened for
compliance
with the entry criteria, set forth above, during a four week run in phase.
After the
screening criteria are met, subjects are washed out from their current anti-
anginal
medication and stabilized on a long acting nitrate such as, for example,
nitroglycerin,
isosorbide-5-mononitrate or isosorbide dinitrate. The term "washed out", when
used
in connection with this screen, means the withdrawal of current anti-anginal
medication so that substantially all of said medication is eliminated from the
body of
the subject. A period of eight weeks is preferably allowed for both the wash
out
period and for the establishment of the subject on stable doses of said
nitrate.
Subjects having one or two attacks of angina per week while on stable doses of
long
acting nitrate are generally permitted to skip the wash out phase. After
subjects are
stabilized on nitrates, the subjects enter the randomization phase provided
the
subjects continue to have either one or two angina attacks per week. In the
randomization phase, the subjects are randomly placed into one of the four
arms of
the study set forth below. After completing the wash out phase, subjects in
CA 02375225 2001-11-26
WO 00/73271 _~ 5_ PCT/IB00/00590
compliance with the entry criteria undergo twenty four hour ambulatory
electrocardiogram (ECG) such as Holter monitoring, exercise stress testing
such as a
treadmill and evaluation of myocardial perfusion using PET (photon emission
tomography) scanning to establish a baseline for each subject. When conducting
a
stress test, the speed of the treadmill and the gradient of the treadmill can
be
controlled by a technician. The speed of the treadmill and the angle of the
gradient
are generally increased during the test. The time intervals between each speed
and
gradient increase is generally determined using a modified Bruce Protocol.
After the baseline investigations have been completed, subjects are initiated
on one of the following four arms of the study: (1 ) placebo; (2) atorvastatin
calcium
(about 2.5 mg to about 160 mg); (3) amlodipine besylate(about 2.5 mg to about
20
mg); or (4) a mutual salt of amlodipine and atorvastatin (about 5 to 160 mg).
The
subjects are then monitored for two to twenty four weeks.
After the monitoring period has ended, subjects will undergo the following
investigations: (1 ) twenty four hour ambulatory ECG, such as Holter
monitoring; (2)
exercise stress testing (e.g. treadmill using said modified Bruce Protocol);
and (3)
evaluation of myocardial perfusion using PET scanning. Patients keep a diary
of
painful ischemic events and nitroglycerine consumption. It is generally
desirable to
have an accurate record of the number of anginal attacks suffered by the
patient
during the duration of the test. Since a patient generally takes nitroglycerin
to ease
the pain of an anginal attack, the number of times that the patient
administers
nitroglycerine provides a reasonably accurate record of the number of anginal
attacks.
To demonstrate the effectiveness of the compounds and compositions of this
invention, and to determine the dosage amounts of the compounds and
compositions
of this invention, the person conducting the test will evaluate the subject
using the
tests described. Successful treatment will yield fewer instances of ischemic
events
as detected by ECG, will allow the subject to exercise longer or at a higher
intensity
level on the treadmill, or to exercise without pain on the treadmill, or will
yield better
perfusion or fewer perfusion defects on photoemission tomography (PET).
The utility of the mutual salts and compositions of the present invention as
medical agents in the treatment of hypertension and hyperlipidemia in mammals
(e.g., humans) suffering from a combination of hypertension and hyperlipidemia
is
CA 02375225 2001-11-26
WO 00/73271 _16_ PCT/IB00/00590
demonstrated by the activity of the compounds of this invention in
conventional
assays and the clinical protocol described below.
Effect of the Mutual Salt of Amlodipine and Atorvastatin
on the Treatment of Subjects Having Both
Hypertension and Hyperlipidemia
This study is a double blind, parallel arm, randomized study to show the
effectiveness of a mutual salt of amlodipine and atorvastatin in controlling
both
hypertension and hyperlipidemia in subjects who have mild, moderate, or severe
hypertension and hyperlipidemia.
Each subject is evaluated for 10 to 20 weeks and preferably for 14 weeks.
Sufficient subjects are used in this screen to ensure that about 400 to 800
subjects
are evaluated to complete the study.
Entry criteria: Subjects are male or female adults between 18 and 80 years of
age having both hyperlipidemia and hypertension. The presence of
hyperlipidemia is
evidenced by evaluation of the low density lipoprotein (LDL) level of the
subject
relative to certain positive risk factors. If the subject has no coronary
heart disease
(CHD) and has less than two positive risk factors, then the subject is
considered to
have hyperlipidemia if the LDL of the subject is greater than or equal to 190.
If the
subject has no CHD and has two or more positive risk factors, then the subject
is
considered to have hyperlipidemia if the LDL of the subject is greater than or
equal to
160. If the subject has CHD, then the subject is considered to have
hyperlipidemia if
the LDL of the subject is greater than or equal to 130.
Positive risk factors include (1 ) male over 45, (2) female over 55 wherein
said
female is not undergoing hormone replacement therapy (HRT), (3) family history
of
premature cardiovascular disease, (4) the subject is a current smoker, (5) the
subject
has diabetes, (6) an HDL of less than 45, and (7) the subject has
hypertension. An
HDL of greater than 60 is considered a negative risk factor and will offset
one of the
above mentioned positive risk factors.
The presence of hypertension is evidenced by a sitting diastolic blood
pressure (BP) of greater than 90 or sitting systolic BP of greater than 140.
All blood
pressures are generally determined as the average of three measurements taken
five
minutes apart.
Subjects are screened for compliance with the entry criteria set forth above.
After all screening criteria are met, subjects are washed out from their
current
CA 02375225 2001-11-26
WO 00/73271 _1 ~_ PCT/IB00/00590
antihypertensive and lipid lowering medication and are placed on the NCEP ATP
II
Step 1 diet. The NCEP ATP II (adult treatment panel, 2nd revision) Step 1 diet
sets
forth the amount of saturated and unsaturated fat which can be consumed as a
proportion of the total caloric intake. The term "washed out" where used in
connection
with this protocol, means the withdrawal of current antihypertensive and lipid
lowering
medication so that substantially all of said medication is eliminated from the
body of
the subject. Newly diagnosed subjects generally remain untreated until the
test
begins. These subjects are also placed on the NCEP Step 1 diet. After the four
week wash out and diet stabilization period, subjects undergo the following
baseline
investigations: (1 ) blood pressure and (2) fasting lipid screen. The fasting
lipid
screen determines baseline lipid levels in the fasting state of a subject.
Generally,
the subject abstains from food for twelve hours, at which time lipid levels
are
measured.
After the baseline investigations are performed subjects are started on one of
the following: (1 ) a fixed dose of amlodipine besylate, generally about 2.5
to 10 mg;
(2) a fixed dose of atorvastatin calcium, generally about 10 to 80mg; or (3) a
mutual
salt of amlodipine and atorvastatin (about 5 to 160 mgs). Subjects remain on
these
doses for a minimum of six weeks, and generally for no more than eight weeks.
The
subjects return to the testing center at the conclusion of the six to eight
weeks so that
the baseline evaluations can be repeated. The blood pressure of the subject at
the
conclusion of the study is compared with the blood pressure of the subject
upon
entry. The lipid screen measures the total cholesterol, LDL-cholesterol, HDL-
cholesterol, triglycerides, apoB, VLDL (very low density lipoprotein) and
other
components of the lipid profile of the subject. Improvements in the values
obtained
after treatment relative to pretreatment values indicate the utility of the
drug
combination.
The utility of the mutual salts and compositions of the present invention as
medical agents in the management of cardiac risk in mammals (e.g., humans) at
risk
for an adverse cardiac event is demonstrated by the activity of the compounds
of this
invention in conventional assays and the clinical protocol described below.
Effects of the Mutual Salt of Amlodipine and Atorvastatin
on SubLects at Risk of Future
Cardiovascular Events
CA 02375225 2001-11-26
WO 00/73271 _1 g_ PCT/IB00/00590
This study is a double blind, parallel arm, randomized study to show the
effectiveness of the mutual salt of amlodipine and atonrastatin in reducing
the overall
calculated risk of future events in subjects who are at risk for having future
cardiovascular events. This risk is calculated by using the Framingham Risk
Equation. A subject is considered to be at risk of having a future
cardiovascular
event if that subject is more than one standard deviation above the mean as
calculated by the Framingham Risk Equation. The study is used to evaluate the
efficacy of a mutual salt of amlodipine and atorvastatin in controlling
cardiovascular
risk by controlling both hypertension and hyperlipidemia in patients who have
both
mild to moderate hypertension and hyperlipidemia.
Each subject is evaluated for 10 to 20 weeks and preferably for 14 weeks.
Sufficient subjects are recruited to ensure that about 400 to 800 subjects are
evaluated to complete the study.
Entry criteria: Subjects included in the study are male or female adult
subjects
between 18 and 80 years of age with a baseline five year risk which risk is
above the
median for said subject's age and sex, as defined by the Framingham Heart
Study,
which is an ongoing prospective study of adult men and women showing that
certain
risk factors can be used to predict the development of coronary heart disease.
The
age, sex, systolic and diastolic blood pressure, smoking habit, presence or
absence
of carbohydrate intolerance, presence or absence of left ventricular
hypertrophy,
serum cholesterol and high density lipoprotein (HDL) of more than one standard
deviation above the norm for the Framingham Population are all evaluated in
determining whether a patient is at risk for adverse cardiac event. The values
for the
risk factors are inserted into the Framingham Risk equation and calculated to
determine whether a subject is at risk for a future cardiovascular event.
Subjects are screened for compliance with the entry criteria set forth above.
After all screening criteria are met, patients are washed out from their
current
antihypertensive and lipid lowering medication and any other medication which
will
impact the results of the screen. The patients are then placed on the NCEP ATP
II
Step 1 diet, as described in the hypertension and hyperlipidemia screen above.
Newly diagnosed subjects generally remain untreated until the test begins.
These
subjects are also placed on the NCEP ATP II Step 1 diet. After the four week
wash
out and diet stabilization period, subjects undergo the following baseline
investigations: (1 ) blood pressure; (2) fasting; (3) lipid screen; (4)
glucose tolerance
CA 02375225 2001-11-26
WO 00/73271 .19_ PCT/IB00/00590
test; (5) ECG; and (6) cardiac ultrasound. These tests are carried out using
standard
procedures well known to persons skilled in the art. The ECG and the cardiac
ultrasound are generally used to measure the presence or absence of left
ventricular
hypertrophy.
After the baseline investigations are performed patients will be started on
one
of the following: (1 ) a fixed dose of amlodipine (about 2.5 to 10 mg); (2) a
fixed dose
of atorvastatin (about 10 to 80mg); or (3) a mutual salt of amlodipine and
atorvastatin
(about 5 to 160 mgs). Patients are kept on these doses and are asked to return
in six
to eight weeks so that the baseline evaluations can be repeated. At this time
the new
values are entered into the Framingham Risk equation to determine whether the
subject has a lower, greater or no change in the risk of future cardiovascular
event.
The above assays demonstrating the effectiveness of the mutual salt of
amlodipine and atorvastatin in the treatment of angina pectoris,
atherosclerosis,
hypertension and hyperlipidemia together, and the management of cardiac risk,
also
provide a means whereby the activities of the compounds of this invention can
be
compared between themselves and with the activities of other known compounds.
The results of these comparisons are useful for determining dosage levels in
mammals, including humans, for the treatment of such diseases.
The following dosage amounts and other dosage amounts set forth elsewhere
in the specification and in the appendant claims are for an average human
subject
having a weight of about 65 kg to about 70 kg. The skilled practitioner will
readily be
able to determine the dosage amount required for a subject whose weight falls
outside the 65 kg to 70 kg range, based upon the medical history of the
subject and
the presence of diseases, e.g., diabetes, in the subject. All doses set forth
herein,
and in the appendant claims, are daily doses.
In general, in accordance with this invention, the mutual salt of amlodipine
and atorvastatin is generally administered in a dosage of about 2.5 mg to
about 20
mg.
The mutual salt of the present invention is generally administered in the form
of a pharmaceutical composition comprising the mutual salt of this invention
together
with a pharmaceutically acceptable carrier, vehicle or diluent. Thus, the
mutual salt of
this invention can be administered in any conventional oral, parenteral or
transdermal
dosage form.
CA 02375225 2001-11-26
WO 00/73271 -2~- PCT/IB00/00590
For oral administration a pharmaceutical composition can take the form of
solutions, suspensions, tablets, pills, capsules, powders, and the like.
Tablets
containing various excipients such as sodium citrate, calcium carbonate and
calcium
phosphate are employed along with various disintegrants such as starch and
preferably potato or tapioca starch and certain complex silicates, together
with
binding agents such as polyvinylpyrrolidone, sucrose, gelatin and acacia.
Additionally, lubricating agents such as magnesium stearate, sodium lauryl
sulfate
and talc are often very useful for tabletting purposes. Solid compositions of
a similar
type are also employed as fillers in soft and hard-filled gelatin capsules;
preferred
materials in this connection also include lactose or milk sugar as well as
high
molecular weight polyethylene glycols. When aqueous suspensions and/or elixirs
are
desired for oral administration, the compounds of this invention can be
combined with
various sweetening agents, flavoring agents, coloring agents, emulsifying
agents
and/or suspending agents, as well as such diluents as water, ethanol,
propylene
glycol, glycerin and various like combinations thereof.
The mutual salt of this invention may also be administered in a controlled
release composition such as a slow release or a fast release formulation. Such
controlled release dosage formulations of the mutual salts of this invention
may be
prepared using methods well known to those skilled in the art. The method of
preferred administration will be determined by the attendant physician or
other person
skilled in the art after an evaluation of the subject's condition and
requirements.
For purposes of parenteral administration, solutions in sesame or peanut oil
or in aqueous propylene glycol can be employed, as well as sterile aqueous
solutions
of the corresponding water-soluble salts. Such aqueous solutions may be
suitably
buffered, if necessary, and the liquid diluent first rendered isotonic with
sufficient
saline or glucose. These aqueous solutions are especially suitable for
intravenous,
intramuscular, subcutaneous and intraperitoneal injection purposes. In this
connection, the sterile aqueous media employed are all readily obtainable by
standard techniques well-known to those skilled in the art.
Methods of preparing various pharmaceutical compositions with a certain
amount of active ingredient are known, or will be apparent in light of this
disclosure, to
those skilled in this art. For examples, see Reminaton's Pharmaceutical
Sciences,
Mack Publishing Company, Easton, Pa., 19t" Edition (1995).
CA 02375225 2001-11-26
WO 00/73271 _21 _ PCT/IB00/00590
Pharmaceutical compositions according to the invention may contain 0.1 %-
95% of the compounds) of this invention, preferably 1 %-70%. In any event, the
composition to be administered will contain a quantity of a compounds)
according to
the invention in an amount effective to treat the condition or disease of the
subject
being treated.
EXAMPLE ONE
Mutual Salt of Amlodipine and Atorvastatin. To a solution of the free acid of
atorvastatin (5.0g, 8.9mM) in ethyl acetate (300m1) is added the free base of
racemic
amlodipine (3.7g, 8.9mM) in (1:1 ) water-acetone (25m1,25m1) or methanol (25
ml)
with vigorous stirring at room temperature. Crystallization is accomplished by
the
addition of a less polar solvent such as toluene (20m1) to afford the
diastereomeric
mutual salt of amlodipine and atorvastatin.
It should be understood that the invention is not limited to the particular
embodiments described herein, but that various changes and modifications may
be
made without departing from the spirit and scope of this novel concept as
defined by
the following claims.