Note: Descriptions are shown in the official language in which they were submitted.
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
METHODS FOR MANIPULATING THE AVIAN GENOME
BACKGROUND OF THE INVENTION
The invention relates to transgenic avian animals.
The ability to create transgenic animals form different species has had great
impact on
both biomedical research and the biotechnology industry. Transgenic technology
has been
applied to both laboratory and domestic species for the study of human disease
(see, for
example. Svnder, B. W., et al., Mol. Reprod. and Develop. 40:419-428 ( 1995)),
develop
improved agricultural livestock (see, for example, Ebert, K.M. et al., Animal
Biotechnology
1:145-1 ~9 ( 1990)), production of pharmaceuticals in milk (see, for review
article, Ebert, K.M.
~o and J.P. Selgrath, "Changes in Domestic Livestock through Genetic
Engineering" in
Applications in Mammalian Development. Cold Spring Harbor Laboratory Press,
1991 )), and
xenotransplantation (see, for example, Osman, N., et al., Proc. Natl. Acad.
Sci USA 94:14677-
14682 (1997)). However, the basic technique of microinjection used to create
many of these
transgenic animals is inefficient, costly, and not easily applicable to all
species such as the
~5 chicken (see, for example, Love, J., et al.. BioTechnology 12:60-63
(1994)).
SUMMARY OF THE INVENTION
The invention features methods of manipulating genomic DNA in avian species
and to
generate transgenic avian animals. A method for introducing a nucleic acid
molecule into the
zo genome of an avian species is carried out by contacting in vivo a
blastodermal cell of a
fertilized hard shelled egg with the nucleic acid molecule. Preferably, the
nucleic acid
molecule is not associated with a viral coat protein, e.g., the nucleic acid
is not delivered in a
viral particle. The nucleic acid molecule is introduced directly into the
germinal disc of the
egg. To avoid disrupting the germinal disc (or blastoderm) or dispersing the
blastodermal
25 cells, the nucleic acid is delivered in a volume that is less than the
volume of the germinal disc
(approximately 100 microliters or less). For example, the volume is greater
than 1 microliter
and less than 0.5 milliliters and introduction of the nucleic acid does not
rupture the area opaca
(membrane or sheath surrounding the germinal disc or blastoderm). In preferred
embodiments, the nucleic acid is delivered in a volume of 5-100 microliters,
more preferably
30 40-60 microliters, and most preferably, 10-20 microliters. To target
blastodermal cells, the
nucleic acid is delivered directly into the blastoderm or germinal disc and
not to an area
adjacent to or below the blastoderm. The thick albumen around the germinal
disc is not
-1-
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
removed before, during, or after the delivery process. The nucleic acid is
delivered by passing
a needle or microinjection pipet directly through the shell and underlying
membrane. Little or
no air is introduced into the egg, and the hole in the membrane left by the
pipet or needle is
small and self sealing. Accordingly, the method does not require deposition of
an aqueous
liquid over the opening of the egg to minimize the inadvertent introduction of
air into the egg
and does not require sealing the opening of the egg after nucleic acid
delivery. Optionally, the
blastodermal cell is exposed to an electrical current in vivo, e.g., by
applying an electrical
current across the egg.
The method is useful to introduce a nucleic acid such as DNA into the genome
of any
~o avian species such as a chicken, an ostrich, an emu, a turkeys, a duck, a
goose, a quail a parrot,
a parakeet, a cockatoo, or a cockatiel to produce therapeutic proteins or to
make an avian
model for a non-avian, e.g., human, disease state. For example, the method is
used to deliver
DNA to blastodermal cells in a fertilized egg of any breed of chicken or any
hybrid breed of
chicken. Chicken breeds include White Leghorn, White Plymouth Rock, Barred
Plymouth
~ 5 Rock, Rhode Island Red, New Hampshire and Dark Cornish.
Nucleic acid delivery is timed to optimize uptake of the nucleic acid by
blastodermal
(totipotent) cells and minimize (or eliminate) uptake by cells which have
begun to differentiate
into various tissue types. Thus, a blastodermal cell in a fertilized egg is
contacted with nucleic
acid during developmental stage X of the egg. Delivery takes place at a time
after oviposition
2o but before incubation of the egg (at which time cell division and cell
differentiation takes
place).
The nucleic acid encodes a polypeptide or antisense molecule. For example, the
nucleic acid contains a sequence encoding an antibody or fragment thereof. The
term antibody
encompasses an intact tetrameric antibody (e.g., a monoclonal antibody) as
well as an
z5 immunologically active antibody fragment, e.g., a Fab or (Fab), fragment,
an engineered single
chain F~. molecule, a chimeric molecule (e.g., an antibody which contains the
binding
specificity of one antibody, e.g., of murine origin, and the remaining
portions of another
antibody, e.g., of human origin. Preferably, the antibody or fragment thereof
is of human
origin. Other polypeptides include an insulin polypeptide (such as a human or
porcine insulin
so polypeptide), a growth hormone polypeptide, a calcitonin polypeptide, or a
serum albumin
polypeptide. For example, the transgenic nucleic acid encodes a porcine single
chain insulin
polypeptide.
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
To direct transcription of the transgenic nucleic acid in blastodermal cells
of an egg,
the nucleic acid construct contains an egg-specific transcriptional regulatory
element.
Transcriptional regulatory sequence is a generic term used throughout the
specification to refer
to DNA sequences, such as initiation signals, enhancers, and promoters, which
induce or
control transcription of protein coding sequences to which they are operably
linked.
Transcription of the recombinant gene or transgene is under the control of a
promoter sequence
as well as other transcriptional regulatory sequences. For example, an
ovalbumin promoter
sequence is used to direct expression of a transgene and a regulatory
sequences derived from a
chicken lysozvme gene is used to direct equal or equivalent expression of both
chains of a
io transgenic tetrameric antibody molecule. By "promoter" is meant a minimal
DNA sequence
sufficient to direct transcription. Promoters may be constitutive or
inducible, and may be
coupled to other regulatory sequences or "elements" which render promoter-
dependent gene
expression cell-type specific, tissue-specific or inducible by external
signals or agents; such
elements may be located in the 5' or 3' region of the native gene, or within
an intron.
i 5 A transgene expression construct contains one or more of the following
elements: a
chicken lysozyme or ovalbumin promoter, a chicken lysozyme enhancer, and a
matrix
attachment region of a chicken lysozyme gene. The matrix attachment region
(U.S. Patent No.
5, 731,178, hereby incorporated by reference) preferably includes a region of
the chicken
lysozyme gene spanning from position -11.7 to -8.8 or from position +5.3 to
+9Ø One or
zo both sequences are connected to the heavy and/or light chain of a
tetrameric antibody to
provide equal or equivalent expression of the two antibody subunits.
Preferably, the region
from -11.7 to -8.8 and/or +5.3 to +9.0 of the chicken lysozyme gene is
attached to the 5'
and/or 3' end of a construct with a heavy and light chain antibody coding
region each under
the control of its own promoter. The invention includes a transgene expression
cassette in
25 which the heavy and light chain coding regions of an antibody are ligated
together, each under
the direction of its own promoter operably linked to a matrix attachment
region. By "operably
linked" is meant that a coding sequence and a regulatory sequences) are
connected in such a
way as to permit gene expression when the appropriate molecules (e.g.,
transcriptional
activator proteins) are bound to the regulatory sequence(s).
so The invention also encompasses transgenic avian animals. The term
"transgenic avian
animal" refers to a member of an avian species (e.g., a chicken) that contains
a transferred
nucleic acid sequence, including a transferred protein-encoding and/or
regulatory sequence,
-3-
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
such that the transferred sequence is integrated into a host chromosome. As a
result of such
transfer and integration, the transferred sequence may be transmitted through
germ cells to the
offspring of a transgenic chicken. Thus, transgenic chickens are created by
introducing by a
method of transfer, new nucleic acid sequences into germ cells.
A transgenic avian animal is any bird in which one or more, and preferably all
of the
cells of the animal, includes a transgene. The transgene is introduced into
the cell, directly or
indirectly by introduction into a precursor of the cell, e.g., a totipotent
blastodermal cell of an
avian egg, by way of deliberate genetic manipulation. The term genetic
manipulation does not
include classical cross-breeding, or irr vitro fertilization, but rather is
directed to the
io introduction of a recombinant DNA molecule into a blastodermal cell of an
avian egg. This
molecule may be integrated within a chromosome, or it may be
extrachromosomally
replicating DNA. Transgenic avians which include one or more transgenes
encoding one or
more polypeptides are within the scope of this invention. For example, a
double or triple
transgenic bird, which includes t<vo or three transgenes can be produced.
15 A transgenic avian animal contains a nucleic acid sequence encoding a non-
avian
antibody polypeptide, e.g., a human antibody, in its genome. Other transgenic
avian animals
include those in which the genome has been engineered to contain a nucleic
acid sequence
encoding a non-avian insulin polypeptide such as a human or porcine insulin
polypeptide or
one which contains a nucleic acid encoding a calcitonin polypeptide, a growth
hormone
2o polypeptide, or a serum albumin polypeptide. Preferably, the polypeptides
are of human
origin. The transgenic avian animals express the transgenic nucleic acid, and
the cells of the
transgenic animal produce the transgenic polypeptides. Accordingly, the
invention
encompasses an isolated avian cell, e.g., an avian blastodermal cell, which
contains a transgene
encoding an antibody, an insulin, a growth hormone, a serum albumin, or a
calcitonin
25 polypeptide. The term "isolated" used in reference to a cell means that the
cell is substantially
free of other cell types or compositions with which it naturally occurs in a
tissue of an animal.
The nucleic acids described herein are isolated. An isolated nucleic acid,
e.g., an
isolated gene, or a fragment thereof, to be transferred into a blastodermal
cell of an egg is
isolated by any of several methods well known to the art. For example, the
nucleic acid
so molecules are recombinant and/or have been purified from the sequences
which flank it in a
naturally occurring state, i.e., a DNA has been removed from the sequences
which are
normally adjacent to the fragment, e.g., the sequences adjacent to the
fragment in the genome
-4-
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
in which it naturally occurs. Thus, an isolated nucleic acid molecule is
produced synthetically,
or by treating mRNA derived from the transcription of the gene with a reverse
transcriptase so
as to produce a cDNA, or by the direct isolation of the nucleic acid from
cells, bacterial clones,
or from other sources.
The invention includes sequences which hybridize under stringent conditions,
with all
or pan of the sequence reported in a reference sequence and retains a
biological function of the
reference nucleic acid. For example, the nucleic acid may contain one or more
sequence
modifications in relation to a reference sequence. Such modifications may be
obtained by
mutation, deletion and/or addition of one or more nucleotides compared to the
reference
io sequence. Modifications are introduced to alter the activity of the
regulatory sequence, e.g., to
improve promoter activity, to suppress a transcription inhibiting region, to
make a constitutive
promoter regulatable or vice versa. Modification are also made to introduce a
restriction site
facilitating subsequent cloning steps, or to eliminate the sequences which are
not essential to
the transcriptional activity. Preferably, a modified sequence is at least 70%
(more preferably
~5 at least 80%, more preferably at least 90%, more preferably at least 95%,
more preferably at
least 99%) identical to a reference sequence. In the case of a transcriptional
regulatory
element, the modifications do not substantially alter the transcription
promoter function
associated with the reference sequence (or a naturally-occurring lactofernn
promoter
sequence). For example, promoter modifications are engineered to avoid the
site of initiation
20 of transcription and the TATA box. Modified transcriptional regulatory
sequences such as
enhancers retain at least 50%, preferably at least 75%, preferably at least
95%, and most
preferably 100% of the enhancer activity of the reference sequence.
Alternatively, the
modified sequence directs a level of transcription that is greater than that
of the reference
sequence. For example, a modified enhancer directs at least 110% of the level
of transcription
25 associated with the reference enhancer sequence.
Nucleotide and amino acid comparisons are carried out using the Lasergene
software
package (DNASTAR, Inc., Madison, WI). The MegAlign module used was the Clustal
V
method (Higgins et al., 1989, CABIOS 5(2):151-153). The parameter used were
gap penalty
10, gap length penalty 10.
3o Alternatively, the nucleic acids described herein hybridize at high
stringency to a
strand of DNA having the reference sequence, or the complement thereof and
have
transcription regulatory activity. Hybridization is carned out using standard
techniques, such
-5-
WO 00/75300 CA 02375441 2001-11-22 pCT/US00/40059
as those described in Ausubel et al. (Current Protocols in Molecular Biology,
John Wiley &
Sons, 1989). "High stringency" refers to nucleic acid hybridization and wash
conditions
characterized by high temperature and low salt concentration, i.e,
hybridization at 42 degrees
C, and in 50% formamide; a first wash at 65 degrees C, 2X SSC, and 1% SDS;
followed by a
second wash at 65 degrees C and 0.1 % x SSC. Lower stringency conditions
suitable for
detecting DNA sequences having about 50% sequence identity to a reference gene
or sequence
are detected by, for example, hybridization at 42 degrees C in the absence of
formamide; a first
wash at 42 degrees C, in 6X SSC, and 1% SDS; and a second wash at 50 degrees
C, in 6X
SSC, and 1 % SDS.
~o Other features and advantages of the invention will be apparent form the
description
and the drawings and from the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
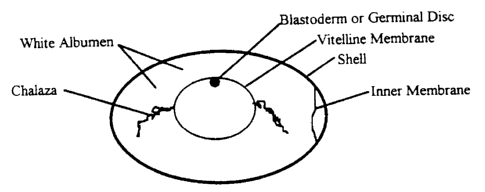
Fig. 1 is a diagram of a fertilized hen's egg.
Fig. 2 is a diagram of a transgene expression cassette.
~5 DETAILED DESCRIPTION OF THE INVENTION
The avian reproductive system is distinct from mammalian reproductive systems
in that
the female can store sperm and fertilize a single ovum at a time. The new
fertilized ovum is
large, fragile, and filled with yolk as it enters the reproductive track.
Early embryonic
development occurs in the oviduct as the egg is formed around the ovum. As the
ovum travels
2o down the reproductive tract, it is surrounded by a protective layer of
white albumen followed
by an inner membrane and hardened shell before being laid (Fig. 1 ). At the
time of
oviposition, the ovum has matured from a single cell into a blastoderm (also
known as the
germinal disc) composed of 40-60,000 cells and development arrests until the
hen has laid
enough eggs and beings to roost. In the blastoderm state, all cells are
totipotent and equally
z5 capable of contributing to the germ line of the developing chick.
Oviposition is the time at which the egg is laid. In the chicken, oviposition
occurs at
stage X (a freshly laid egg; about 20 hours of uterine age). The time at which
a nucleic acid is
introduced into the blastoderm or germinal disc is after oviposition but
before incubation of
the egg, i.e., before the first cell division after the egg is incubated. To
effectively target
so blastodermal cells, DNA is therefore introduced into the blastoderm of an
egg which has been
incubated for 6 hours or less. Early stages of development of the egg are
indicated by roman
numerals, whereas stage of development of an embryonic chick are indicated by
arabic
-6-
CA 02375441 2001-11-22
WO 00/75300 PCT1US00/40059
numerals. For example, stage X refers to a stage of development that occurs at
about 20 hours
uterine age and is characterized by oviposition, whereas stage 10 refers to a
stage of embryonic
chick development (characterized by tissue differentiation). No tissue
differentiation has
occurred in stage X of development. Accordingly, genetic manipulation occurs
before
s differentiation of blastodermal cells into embryonic tissue.
The germinal disc is distinguished from the germinal crescent region in that
the
germinal disc contains undifferentiated blastodermal cells (at stage X or
before), whereas the
germinal crescent region appears in the early stages of chick embryo
development (i.e., stages
3-~ or 9-11 of chick embryo development).
io The cells of the blastoderm are genetically manipulated both in vitro or in
vivo using
gene delivery techniques and then used to produce transgenic or chimeric
chickens by
allowing development in the egg, transferring to a recipient unfertilized egg,
or transferring to
the testes of a sterile rooster for development into spermatogonia. The
optimum time for
transfection is beriveen oviposition and several hours of activation of the
egg in order to reduce
i5 the number of target cells for transfection.
Nucleic acid delivery and production of trans~enic animals
The blastoderm is accessed by cutting or drilling a small hole in the egg
shell (sitting
upright) with a scapel or drill and gently peeling back the inner membrane to
expose the white
albumen. The blastoderm automatically orients to the top of the yolk and is
visualized under
20 light. For the production of chimeric or transgenic chickens, the cells of
the blastoderm are
transfected ifi vivo by infusing DNA directly into the blastoderm using a
syringe and small
gauge needle. The DNA is naked or complexed with lipids or other suitable
compounds to
facility DNA uptake (e.g., DEAE-dextran). If the DNA is naked, the
transfection efficiency is
increased by passing an electrical current across the blastoderm or whole egg
with a device
2s such as a human heart defibrillator. If a current is passed across the
whole egg, two additional
holes are made in the egg shell to expose the inner membrane to the current
since the shell will
not conduct electricity.
Alternatively, the blastoderm is removed from the egg and pooled with the
cells from
several eggs using a small pipet. In vitro, DNA uptake by blastodermal cells
is facillitated by
so such techniques as electroporation, DEAE-dextran treatment, calcium
phosphate treatment, or
lipofection. Following transfection, the cells are transferred into the
germinal disc of an
unfertilized egg for development of a transgenic chick or into the testes of a
sterile rooster to
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
induce development in spermatogonia and sperm for breeding. Chicks produced
are tested for
the presence of the transgene according to known methods, e.g, by the
polymerase chain
reaction or southern blot analysis.
The overall efficiency of the nucleic acid delivery procedure depends on the
method
and timing of gene delivery. Transfection efficiency is optionally increase by
methods such as
1 ) subjecting the blastoderm or cells derived from the blastoderm to several
rounds of
transfection. 2) adding a selectable marker such as but not limited to an
antibiotic gene to the
DNA vector and infusing the antibiotic into the yolk or testes following
transfection or cell
transfer.
io The method is applicable to all birds including, but not limited to,
chickens, turkeys
ostriches, and geese. The critical factor for the efficiency of gene delivery
is the timing of egg
activation in relation to transfection. The optimal time to transfect the
cells is after oviposition
and within six hours of activation (post-incubation) so that the cells have
started to grow but
have not undergone a cell division.
The following are examples of the preferred embodiments of the inventions and
specifically related to the production of transgenic chickens for the
production of
pharmaceuticals in eggs. The structure or composition of the transgene has
little or no effect
on the transfection efficiency of the methods described. Preferred transgene
constructs include
those carrying the ovalbumin promoter operatively linked to the human serum
albumin gene,
2o human insulin gene, native or modified porcine insulin gene, calcitonin
gene, or and gene
encoding and antibody variable region. The invention includes transgenic
chickens carrying
any of the above mentioned genes directed to expression in the egg.
Example 1: IfZ vivo nucleic acid delivery
Fertilized eggs are laid and collected (day 1). The eggs are shipped at room
z5 temperature (unincubated) (day 2). Optionally, the eggs are allowed to site
upright for at least
24 hours (day 3) at room termperature (unincubated) prior to manipulation for
nucleic acid
delivery.
A fertilized hen's egg was collected and placed in a humidified incubator for
between 1
and 6 hours to activate the egg. A hole was cut in the egg shell using a
dremel and the inner
3o membrane was folded back. The egg was placed on a light to visualize the
germinal disc
which was infused with a lipid/DNA complex. The inner membrane was folded into
place and
the egg sealed with parafilm. The egg was placed back into the incubator and
allowed to
_g_
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
develop to term. A blood sample or inner membrane sample was taken from the
resulting
chick and tested for the presence of the transgene by the polymerase chain
reaction with
transgene specific primers. .A mosaic or chimeric chick is bred to a rooster
to produce fully
transgenic offspring.
Example 2: In vitro DNA delivery of blastodermal cells and transfer of
transfected eras to an
unfertilized era
Ten to twenty fertilized hen's eggs are placed in a humidified incubator for
between 1
and 6 hours to activate cell division. A hole is cut in the egg shell using a
dremel and the inner
membrane folded back in order to visualize the blastoderm. The blastoderms are
removed
~o from the eggs with a large bore pipet tip and combine in a conical tube.
The yolk is removed
by washing with phosphate buffered saline. and the blastoderms collected by
centrifugation.
The cells are dispersed by treatment with collagenase or gentle pipetting and
collect by
centrifugation. The cells are resuspended in serum free media containing 1-5 g
of transgene
and allowed to sit at room temperature in an electroporation cuvette. The
cells are gently
i5 resuspended and electroporated at 300-500V and 250-960uF. Following
transfection, the cells
are transferred into the germinal disc of an unfertilized egg by cutting a
hole in the egg shell,
folding back the inner membrane, and infusing the cells into the disc with a
syringe and large
gauge needle. The egg is sealed and incubated in a humidified incubator to
allow
development. A blood sample is taken from the resulting chick and tested for
the presence of
2o the transgene by the polymerase chain reaction with transgene specific
primers using standard
methods.
Example 3 ~ In vitro DNA delivery of blastodermal cells and transfer of
transfected e~Qs to
rooster testes
Ten to twenty fertilized hen's eggs are placed in a humidified incubator for
between 1
25 and 6 hours to activate cell division. A hole is cut in the egg shell using
a dremel and the inner
membrane folded back in order to visualize the blastoderm. The blastoderms are
removed
from the eggs with a large bore pipet tip and combined in a conical tube. The
yolk is removed
by washing with phosphate buffered saline, and the blastoderms collected by
centrifugation.
The cells are suspended in serum free media containing 1-5 g of transgene and
allowed to sit
so at room temperature in an electroporation cuvette. The cells are gently
resuspended and
electroporated at 300-500V and 250-96uF. Following transfection, the cells are
transferred
-9-
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
into the testes of a sterilized rooster to induce development into
spermatogonia. The rooster is
monitored for breeding and sperm collected to test for the presence of the
transgene.
Example 4: Vector Construction
To target expression of insulin to the magnum cells of an egg ~Iaying hen, a
genomic
s sequence encoding human insulin was operatively linked to a promoter which
directs
transcription of a nucleic acid to which it is operably linked in avian eggs.
For example, the
promoter is a human lactoferrin promoter. The construction of a human
lactoferrin expression
cassette is described in patent application USSN 09/490,801, the contents of
which are hereby
incorporated by reference.
~o For example, transcription regulatory elements are derived from a milk
specific
promoter, e.g., a mammalian lactoferrin gene promoter. The expression cassette
contains a
promoter region derived from the human lactoferrin gene operably linked to a
heterologous
sequence. A heterologous sequence is one that does not encode a lactoferrin
polypeptide. The
promoter region includes at least 20 nucleotides of the nucleotide sequence of
SEQ ID NO:1.
~s For example, the promoter region contains nucleotides 1-154 of SEQ ID NO:1
or 2.
Table 1: Human Lactoferrin promoter region
1 CTGGATCCTCAAGGAACAAGTAGACCTGGCCGCGGGGAGT
41 GGGGAGGGAAGGGGTGTCTATTGGGCAACAGGGCGGCAAA
zo 81 GCCCTGAATAAAGGGGCGCAGGGCAGGCGCAAGTGCAGAG
121 CCTTCGTTTGCCAAGTCGCCTCGAGACCGCAGACATGAAA
161 GCATGTCTCCGCGGAAAA (SEQ ID NO:l)
BamHl restriction site GGATCC (nucleotides 5-8) and XhoI site (nucleotides 140-
25 145) are italicized. These restriction sites may be altered, e.g., replaced
with other restriction
sites or with nucleotides that do not represent restriction enzyme recognition
sites.
Table 2: Human Lactoferrin promoter re, 'on
1 CTr~IVNNNNTCAAGGAACAAGTAGACCTGGCCGCGGGGAGT
30 41 GGGGAGGGAAGGGGTGTCTATTGGGCAACAGGGCGGCAAA
81 GCCCTGAATAAAGGGGCGCAGGGCAGGCGCAAGTGCAGAG
121 CCTTCGTTTGCCAAGTCGCrI2~TNNNNACCGCAGACATGAAA
GCATGTCTCCGCGGAA.A.A (SEQ ID N0:2)
ss Optionally, the lactoferrin-derived promoter regions described above are
linked to the
nucleotide sequence of SEQ ID N0:3 (GENBANKT'~ accession no. 552659).
-10
RECTIFIED SHEET (RULE 91)
ISA/EP
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
Table 3: ~' Region of human lactoferrin gene
1 cgaggatcat ggctcactgc caccttcatc tcccaggctc aaatggtcct
cccactttag
61 cctcccaagt agctgggacc ataggcatac accaccatgc tgggctaatt
tttgtatttt
121 ttgtagagat gggggtttcc ctatgaagcc caggctagtc ttgaactcct
gggctcaagc
181 gatcctccca tcttggcctc ccaaagtgct gggattacag gcatgagcca
ctgtgccctg
241 cctagttact cttgggctaa gttcacatcc atacacacag gatattcttt
ctgaggcccc
301 caatgtgtcc cacaggcacc atgctgtatg tgacactccc ctagagatgg
atgtttagtt
361 tgcttccaac tgattaatgg catgcagtgg tgcctggaaa catttgtacc
tggggtgctg
~0 421 tgtgtcatgg gaatgtattt acgagatgta ttcttagaag cagtattcta
gcttttgaat
481 tttaaaatct gacatttatg gcgattgtta aaatgaggtt accatttcct
attgaatact
541 atcaacacca aaaaagaaga aggaggagat ggagaaaaaa aagacaaaaa
aaaaaaaagt
601 ggtagggcat cttagccata gggcatcttt ctcattggca aataagaaca
tggaaccagc
661 cttgggtggt ggccattccc ctctgaggtc cctgtctgtt ttctgggagc
tgtattgtgg
i s 721 gtctcagcag ggcagggaga taccccatgg gcagcttgcc tgagactctg
ggcagcctct
781 cttttctctg tcagctgtcc ctaggctgct gctgggggtg gtcgggtcat
cttttcaact
841 ctcagctcac tgctgagcca aggtgaaagc aaacccacct gccctaactg
gctcctaggc
901 accttcaagg tcatctgctg aagaagatag cagtctcaca ggtcaaggcg
atcttcaagt
961 aaagaccctc tgctctgtgt cctgccctct agaaggcact gagaccagag
ctgggacagg
zo 1021 gctcaggggg ctgcgactcc taggggcttg cagacctagt gggagagaaa
gaacatcgca
1081 gcagccaggc agaaccagga caggtgaggt gcaggctggc tttcctctcg
cagcgcggtg
1141 tggagtcctg tcctgcctca gggcttttcg gagcctggat cctcaaggaa
caagtagacc
1201 tggccgcggg gagtggggag ggaaggggtg tctattgggc aacagggcgg
ggcaaagccc
1261 tgaataaagg ggcgcagggc aggcgcaagt ggcagagcct tcgtttgcca
agtcgcctcc
z5 1321 agaccgcaga catgaaactt gtcttcctcg tcctgctgtt cctcggggcc
ctcggtgagt
1381 gcaggtgcct gggggcgcga gccgcctgat gggcgtctcc tgcgccctgt
ctgctaggcg
1441 ctttggtccc tgtgtccggt tggctgggcg cggggtctct gcgccccgcg
gtcccagcgc
1501 ctacagccgg gaggcggccc ggacgcgggg ccagtctctt tcccacatgg
ggaggaacag
1561 gagctgggct cctcaagccg gatcggggca cgcctagctc tgctcagagc
ttctcaaaag
30 1621 gcctcccagg cccctgtccc tttgtgtccc gcctaaggat ttggtcccca
ttgtattgtg
1681 acatgcgttt tacctgggag gaaagtgagg ctcagagagg gtgagcgact
agctcaagga
1741 ccctagtcca gatcctagct cctgcgagga ctgtgagacc ccagcaagac
cgagccttta
1801 tgagacttag tttcttcact taaagaaacg gcctaaccat gggtccacag
ggttgtgagg
1861 aggagatggg gcattcgcac accttccgtg gcagagggtt gtggaggggt
gcggtgctcc
35 1921 tgatggaacc ctgtgtcaga gggtttgaga gggaaatgtc agccaaacag
aaggaaggag
1981 cagaaggaag gaaacaattg tcagttccat aaccaaagta atttctcggg
tgctcagagg
2041 gcactcccca gcgctgcaca ttagtgacct aaatgcgtga gtgcgg
(SEQ ID NO: 3)
The nucleic acid molecules and constructs described herein are isolated. By
"isolated"
ao is meant a nucleic acid molecule that is free of the genes which, in the
naturally-occurnng
genome of the organism, flank the sequence of interest. The term therefore
includes, for
example, a recombinant DNA which is incorporated into a vector; into an
autonomously
replicating plasmid or virus; or into the genomic DNA of a procaryote or
eucaryote; or which
exists as a separate molecule (e.g., a cDNA or a genomic or cDNA fragment
produced by PCR
as or restriction endonuclease digestion) independent of other sequences. It
also includes a
-11-
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
recombinant DNA which is part of a hybrid gene encoding additional polypeptide
sequence.
The term excludes large segments of genomic DNA, e.g., such as those present
in cosmid
clones, which contain a given DNA sequence flanked by one or more other genes
which
naturally flank it in a naturally-occurnng genome.
Lactoferrin-derived transcription regulatory sequences, are attached to a
nominal
promoter (e.g., the nominal lactoferrrin promoter or a heterologous promoter)
which in tum is
operably linked to a sequence to be transcribed. The heterologous sequence to
be transcribed
is a polypeptide-encoding sequence or antisense sequence. When incorporated
into a
transgenic mammal such as a member of an avian species, the regulatory
sequences of the
~o invention operably linked to a polypeptide-encoding sequence direct
production of the
encoded polypeptide in avian tissues. The lactofernn-derived regulatory
sequence, e.g.,
promoter sequence is positioned 5' to a heterologous nucleic acid sequence,
e.g., a transgene,
in a transcription unit. Portions of the lactoferrin-derived promoter region
are tested for their
ability to allow tissue-specific and elevated expression of a transgene using
assays known in
15 the art, e.g., standard reporter gene assays using luciferase, beta-
galactosidase, or expression of
an antibiotic resistance gene as a detectable marker for transcription.
All or part of one of the nucleotide sequences specified in a reference
sequence, e.g.,
SEQ ID NO:1 or 2, its complementary strand or a variant thereof may be used in
to direct
transcription of a heterologous nucleic acid sequence such as a transgene in a
transgenic
zo mammal. A nucleic acid fragment is a portion of at least 20 continuous
nucleotides identical
to a portion of length equivalent to one of the reference nucleotide sequences
or to its
complement.
Other promoters which direct transcription in eggs of an avian animal are
known in the
art, e.g., the chicken ovalbumin promoter (GENBANKT''~ J00895 or M24999) and
the chicken
Zs lysozyme promoter (GENBANKT"' J00886 or V00429). An expression vector is
constructed
using a chicken ovalbumin promoter for expression of cloned sequences (Gannon
et al.,
Organisation and sequences at the 5'end of a cloned complete ovalbumin gene.
Nature
278:428434; Lai et al., The ovalbumin gene: Structural sequence in native
chicken DNA are
not contiguous. Proc. Natl. Acad. Sci. USA 75(5): 2205-2209; and Kaye et al.,
EMBO J.
so 3:1137-I 144). Other regulatory elements which direct transcription of
transgenes include a
nuclear DNA attachment element which mediates elevated and position-
independent gene
activity (Stief, A., et al., Nature 341:343-345) and an attachment element for
stimulation of
- I 2-
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
eucaryotic expression systems (Sippel et al., U.S. Patent No. 5,731,178).
Other promoters
useful to direct transcription of transgenes in eggs include the conalbumin,
ovomucoid, and
ovotransferrin promoters known in the art. For expression in blood or liver
tissues, a promoter
derived from the chicken beta globin gene is used (Foley et al., Proc. Natl
Acad Sci USA
91:7252-7256).
Protein or polypeptide products to be expressed by the transgene include human
insulin
(GENBANKTMV00565), human calcitonin (GENBANKTM X15943; Broad et al., Nucl.
Acids
Res. 17:6999-7011), human serum albumin (GENBANKTM M12523, J04457), and a
porcine
single chain insulin. The nucleic acid sequence of the porcine single chain
insulin sequence
~o are shown below in Tables 4 and 5.
Table 4: Porcine Single Chain Insulin sequence 1
CTCGAGATGAAAAGATTCGTTAACCAACACTTGTGCGGTTCCCACTTGTTGAAGCT
TTGTACTTGGTTTGCGGTGAAAGAGGTTTCTTCTACACTCCTAAGGCTGCTAAGGG
TATTGTCGAACAATGCTGTACCTCCATCTGCTCCTTGTACCAATTGGAAAACTACT
GCAACTAGACTCGAG (SEQ ID N0:4)
Table 5: Porcine Single Chain Insulin sequence with lysozyme sienal seguence
2o CTCGAGATGAGGTCTTTGCTAATCTTGGTGCTTTGCTTCCTGCCCCTGGCTGC
TCTGGGGAAAAGATTCGTTAACCAACACTTGTGCGGTTCCCACTTGTTGAAGCTT
TGTACTTGGTTTGCGGTGAAAGAGGTTTCTTCTACACTCCTA.AGGCTGCTAAGGGT
ATTGTCGAACAATGCTGTACCTCCATCTGCTCCTTGTACCAATTGGAAAACTACTG
CAACTAGACTCGAG (SEQ ID N0:5; bold type indicates signal sequence)
The invention includes sequences which hybridize under stringent conditions,
with all
or pan of the sequence reported in a reference sequence and retains
transcription regulatory
function. For example, the nucleic acid may contain one or more sequence
modifications in
relation to a reference sequence. Such modifications may be obtained by
mutation, deletion
so and/or addition of one or more nucleotides compared to the reference
sequence. Modifications
are introduced to alter the activity of the regulatory sequence, e.g., to
improve promoter
activity, to suppress a transcription inhibiting region, to make a
constitutive promoter
regulatable or vice versa. Modification are also made to introduce a
restriction site facilitating
subsequent cloning steps, or to eliminate the sequences which are not
essential to the
transcriptional activity. Preferably, a modified sequence is at least 70%
(more preferably at
least 80%, more preferably at least 90%, more preferably at least 95%, more
preferably at least
99%) identical to a reference sequence. The modifications do not substantially
alter the
-13-
WO 00/75300 CA 02375441 2001-11-22 PCT/US00/40059
transcription promoter function associated with the reference sequence (or a
naturally-
occurring lactoferrin promoter sequence). For example, modifications are
engineered to avoid
the site of initiation of transcription and the TATA box.
Example 5: Construction of a lactoferrin expression cassette
A lactoferrin expression cassette was constructed using lactoferrin
transcription
regulatory elements. The cassette contained 3Kb of promoter and 7Kb of 3'
flanking sequence
with unique SaII and NotI restriction sites at the 5' and 3'ends,
respectively. In addition, the
vector contained a unique XhoI site for the addition of heterologous coding
sequences.
io The human insulin gene was PCR amplified from human genomic DNA with the
primers HINF3 (~'GCCCTCGAGGACAGGCTGCATCAGAA3'; (SEQ ID N0:6))/HINR3
(5'CTCGGTGCTCGAGGCGGCGGGTGT3'; SEQ ID N07) cloned into the vector pCR2.1
(Invitrogen, Carlsbad,CA) according to the manufacturer's instructions. The
gene sequenced
using the Amplicycle Sequencing kit (PE Applied Biosystems, Foster City, CA)
to confirm
i5 that no base mutations had occurred during amplification. The gene was
excised from the
vector pCR2.1 as and XhoI fragment and cloned into the XhoI site of the human
lactofernn
expression cassette. The orientation of the insulin gene was confirmed by
restriction analysis
and DNA sequencing. The completed vector was designated HL31 and could be
excised from
the bacterial backbone as a SaII to NotI fragment.
2o Example 6: Preparation of HL31 for Transfection
To remove bacterial sequences from the vector HL31, it was digested with SaII
and
NotI to completion, extracted with phenol/chloroform, chloroform, and
fractionated on a 1%
Tris Acetate agarose gel. The transgene was excised from the gel and electro-
eluted in dialysis
tubing containing 1 x Tris-Acetate buffer. The eluted DNA was transferred into
an eppendorf
zs tube and precipitated by adding 1/lOth volume 3M sodium acetate pH5.2, 2.2
volumes ethanol,
and incubating at -20 degrees C for 24 hrs. The DNA was collected by
centrifugation at
13,000 x g for 10 minutes and resuspended in distilled sterile water. The
concentration of
DNA was estimated by Tris-borate gel electrophoresis against a known quantity
of lambda
HindIII digested standard. The DNA was stored at 4 degrees C.
so Example 7: Production of Chimeric Transeenic Chickens
Developmental stage X chicken eggs from white leghorn chickens were obtained
from
a commercial vendor (Charles River/SPAFAS, North Franklin, CT). The eggs were
allowed
-14-
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
to acclimate for 24 hrs at room temperature prior to transfection to allow the
germinal
disc/blastoderm to orient to the top of the egg. Using a dremel, a 1-2cm
circular cut was made
in eggshell over the air sac region. Before proceeding, the transgene was
prepared for
injection at the following concentrations; DNA 5-15 ng/ 1, 10-15% phospholipid
(Life
Technologies, Grand Island, NY), 50% Dulbecco's Modified Eagles media, and the
remaining
volume with phosphate buffered saline. The DNA/lipid is allowed to stand at
room
temperature for a minimum of l5minutes prior to injection into the egg.
Just prior to injection, the eggshell was removed to expose the inner shell
membrane
and allow the visualization of the germinal disc. If the germinal disc could
not be visualized a
~o small opening was made in the membrane. Following location of the germinal
disc, the
DNA/lipid solution was injected directly into the center of the disc. Nucleic
acid solutions
(construct encoding human insulin) were injected in a volume of SO-100 1 with
a lcc syringe
and 27 gauge needle or in a volume of 10-20 1 with a microinjection needle
attached to a
Hamilton syringe. Table 6 shows data from representative experiments. 228 eggs
were
injected and 26 chicks were hatched (Table 6)
Table 6: Summary of Egr~ Injection Experiments
Experiment Conc. Of Percent # of Eggs # of Chicks
i DNA
I
hospolipid njected atched
I ng/ 1)
i
32 I ~ 15 16 3
33 i 10 15 22 2
l
34 5 20 22 9
i
i
34a i 7.5 10 22 2
I
35 i 15 10 21 1
36 i 15 10 21 3
I
37 ~I 10 5 30 4
38 ! 5 10 30 2
39 j 10 10 30 0
'
40 10 10 30 0
-15-
WO 00/7$300 CA 02375441 2001-11-22 PCT/US00/40059
Chimerism of the hatched chicks was tested by PCR analysis of genomic DNA
isolated
from the inner shell membrane (post-hatching), or the liver, kidney, and
reproductive organs
upon necropsy (Table 7). Out of the 3 chicks tested in experiment 32, 2 showed
a strong PCR
signal in the inner shell membrane sample. Upon maturation, these chicks are
bred to confirm
germline transmission of the transgene. PCR analysis of necropsy samples
(membrane and
reproductive organs) taken from 4 chicks derived from experiments 32 & 33
revealed that 1
out of 4 chicks had detectable levels of the transgene its reproductive
organs.
Table 7 shows results from a representative analysis of tissue expression of
human
insulin transgene in chimeric chickens produced as described above.
io Table 7: PCR analysis of Genomic DNA Isolated from Chimeric Chicks
Experiment Chick Tissue Sample Status
Inner Reproductive
Membrane Organ
32 C NEG. Not tested Alive
E POS. Not tested Alive
M POS. Not tested Alive
N NEG. NEG. Dead
O POS. NEG. Dead
I 33 B Not tested Not tested Alive
BB POS. POS. Dead
FF POS. NEG. Dead
34 AA NEG. Not tested Alive
CC POS. Not tested Alive
EE NEG. Not tested Alive
I, II NEG. ~ Not tested Alive
KK NEG. Not tested Alive
QQ NEG. Not tested Alive
! VV POS. Not tested Alive
I ZZ POS. Not tested Alive
AAA POS. I Not tested Alive
QQQ POS. ' Not tested Alive
Marker transgenes such as MT-beta-Gal, and CMV-GFP were also transfected into
blastodermal cells in vivo. Chicks were produced and tested for tissue
expression of the
i5 transgene as described above. The transgenic DNA was detected in the
following tissues
which were tested: heart, liver, and kidney.
These data indicate that the methods and constructs described herein are
useful to
transfect avian blastodermal cells and to make transgenic avian animals which
produce a
desired transgenic polypeptide.
-16-
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
Example 8: Method for the production of tetrameric antibodies
Unlike many prior art methods for production of transgenic chickens, the
methods of
the invention do not utilize retroviral particles. Many of those methods use
non-replicating
retroviruses, which contain a gene of interest, to infect a developing egg.
This process has
s several limitations which include a restriction on the size of the gene
packaged in the viral
coat, and instability of expression from retroviral vectors. In addition,
retroviral gene delivery
is severely limited in its ability to produce high levels of multimeric
proteins since each coding
region would optimally require its own promoter. To circumvent this problem,
some
researchers have proposed the use of IRES sequences which allow for
bicistronic mRNAs;
~o however, the expression from these types of constructs is generally low
unless a selection
marker is used.
To overcome these drawbacks, a technology was developed which employs
in vitro gene delivery methods to the creation of transgenic chimeric
chickens. This method
has the advantage of being able to deliver a transgene of up to 1 OOKb and
allows for high level
i5 production of multimeric proteins such as antibodies. The flexibility in
vector design allows
the construction of transgene similar to those used in the mammary gland
expression system
which employ full length promoters, genomic coding regions, and 3'flanking
regions. The
method described herein utilizes the full 8Kb ovalbumin promoter, genomic or
cDNA coding
regions, and 3'flanking sequence. In addition, high level production of
multimeric proteins is
2o made possible because expression of each subunit is directed by its own
promoter. The
transgenes are transfected individually or ligated together to ensure co-
integration which
greatly facilitates germline transmission of all subunits (Step 1 and 2 of
Fig. 2).
To ensure the equal expression of both subunits of the antibody, the transgene
is
insulated from the effects of the surrounding chromatin structure by including
elements such
25 as matrix attachment regions (Step 3 of Fig. 2). Unlike prior art methods
which have utilized
matrix attachment region, the constructs described herein result in equal
expression of
multimeric proteins. The elements are called A-elements, MAR for matrix
attachment regions,
SAR, scaffolding attachment regions, DCR for dominant control region, and
insulators.
Other features, objects, and advantages of the invention will be apparent from
the
so description and from the claims. In the specification and the appended
claims, the singular
forms include plural references unless the context clearly dictates otherwise.
Unless defined
otherwise, all technical and scientific terms used herein have the same
meaning as commonly
-17-
CA 02375441 2001-11-22
WO 00/75300 PCT/US00/40059
understood by one of ordinary skill in the art to which this invention
belongs. All patents and
publications cited in this specification are incorporated by reference.
Other embodiments are within the following claims.
-18-