Note: Descriptions are shown in the official language in which they were submitted.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-1-
METHOD FOR ENHANCING HEMATOPOIESIS
PRIORITY CLAIM
This application claims priority from U.S. Provisional Application No.
60/140,628, filed on
June 23, 1999, herein incorporated by reference.
FIELD OF THE INVENTION
This invention relates to the field of hematopoietic stem cells, specifically
to the use of
vascular tissue to reconstitute hematopoiesis in a subject.
BACKGROUND OF THE INVENTION
All the blood cells that circulate in the peripheral blood are derived from
primitive
mesenchymal cells referred to as hematopoietic stem cells. In the adult, most
of these cells are
located in the bone marrow. In the marrow of a healthy person, most stem cells
are neither dividing
nor differentiating. These cells are considered to be in a prolonged
intermitotic interval and comprise
the reserve stem cell pool that can be induced to divide upon hematopoietic
stress.
Hematopoietic stem cells are self regenerating, and also pluripotent in that
they differentiate
into several lineages, including lymphoid, myeloid and erythroid lineages. The
lymphoid lineage,
comprising B-cells and T-cells, provides for the production of antibodies,
regulation of the cellular
immune system, detection of foreign agents in the blood, detection of cells
foreign to the host, and the
like. The myeloid lineage, which includes monocytes, granulocytes,
megakaryocytes as well as other
cells, monitors for the presence of foreign bodies in the blood stream,
provides protection against
neoplastic cells, scavenges foreign materials in the blood stream, produces
platelets, and the like.
The erythroid lineage provides the red blood cells, which act as oxygen
carriers. Exposure to growth
factors is believed to induce a stem cell to be dedicated to differentiate
into a specific lineage.
The stem cell population is known to constitute only a small percentage of the
total number
of leukocytes in bone marrow. Recently, the mouse stem cell has been obtained
in at least highly
concentrated, if not a purified form, where fewer than about 30 cells obtained
from bone marrow
were able to reconstitute all of the lineages of the hematopoietic system of a
lethally irradiated
mouse. Indeed, one injected cell should be able to reconstitute all of the
hematopoietic lineages.
SUMMARY OF THE INVENTION
There is a strong interest in identifying sources of hematopoietic stem cells,
as possession of
these sources will allow for identification of growth factors associated with
its self regeneration. In
addition, there may be as yet undiscovered growth factors associated (1) with
the early steps of
dedication of the stem cell to a particular lineage; (2) the prevention of
such dedication; and (3) the
negative control of stem cell proliferation. The availability of new sources
of stem cells can be
extremely useful as a substitute for bone marrow transplantation, as well as
in transplantation of other
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-2-
organs currently performed in association with transplantation of bone marrow.
In addition, stem
cells are important targets for gene therapy, where the inserted genes promote
the health of the
individual into whom the stem cells are transplanted. Identification of a new
source of stem cells thus
provides additional means of isolating cells useful in gene therapy. Isolation
of a novel
hematopoietic stem cell, or a novel intermediate in hematopoiesis, also
provides new avenues for
treatment of lymphomas and leukemias, as well as other neoplastic conditions,
e.g., breast cancer.
Model systems to isolate and test stem cells and hematopoiesis also provide a
means for testing
agents that affect stem cells.
A method is provided for enhancing hematopoiesis. The method includes
transplanting at
least a therapeutically effective portion of an isolated vascular tissue into
a subject, wherein the
vascular tissue enhances hematopoiesis.
In one embodiment, a method is provided for detecting agents that affect
hematopoiesis.
The method includes transplanting a portion of an isolated vascular tissue
into a subject, wherein the
portion of the isolated vascular tissue is sufficient to enhance
hematopoiesis. The vascular tissue is
treated with a candidate agent, and hematopoiesis is monitored in the subject.
Hematopoiesis in the
subject may be compared with hematopoiesis in a control subject, or compared
to expected reference
values, with reference to hematopoiesis in general or certain cell types (such
as leukocytes, for
example lymphocytes). A change in hematopoiesis in the subject as compared to
the control
indicatives that the agent affects hematopoiesis.
In another embodiment, a method is provided for isolating a hematopoietic
growth factor.
The method includes transplanting a portion of a vascular tissue into a
subject, wherein the portion of
the vascular tissue promotes hematopoiesis in the subject; and isolating a
hematopoietic growth factor
from the subject. Another embodiment is the hematopoietic growth factor
itself, whether isolated by
this approach or directly from the blood vessel itself.
In a further embodiment, a method is provided for isolating a hematopoietic
stem cell. The
method includes transplanting a portion of a vascular tissue into a subject,
and isolating a
hematopoietic stem cell from the subject.
A pharmaceutical composition is provided for promoting hematopoiesis that
includes a
therapeutically effective amount of an isolated vascular tissue in a
pharmaceutically acceptable
carrier. A method of making a pharmaceutical is also disclosed. The method
includes obtaining a
portion of a vascular tissue for use in enhancing hematopoiesis.
A kit is disclosed for promoting hematopoiesis. The kit includes a carrier
means comprising a
container which includes a portion of an isolated vascular tissue in a medium,
such as a preservative
medium that does not interfere with the hematopoietic activity of the vascular
tissue. The use of an
isolated blood vessel for hematopoiesis, is also disclosed, and this use can,
for example, be disclosed
in instructions included with the kit.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-3-
A non-human animal model is also disclosed for testing agents that affect
hematopoiesis. The
model system is a non-human animal deficient for hematopoiesis transplanted
with an isolated
portion of a vascular tissue, wherein the portion of the vascular tissue is
sufficient to promote
hematopoiesis. A method for generating the non-human animal model is also
disclosed.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a bar graph showing the spleen colony forming activity of
transplanted vascular
tissue. Irradiated recipients were transplanted with either 100uL untreated
PB, 1 x 105 BM cells, a 4
mm thoracic aorta (TA) or vena cava (VC) graft, or a 4mm segment of VC with no
irradiation
treatment. Spleens were harvested on day 14 post-transplant and the number of
CFU-S per spleen
were determined. Mean and SEM for each treatment group are shown. No colonies
were seen in
non-irradiated transplant recipients. PB= peripheral blood. BM= bone marrow.
TA= thoracic aorta.
VC= vena cava. No XRT= TA or VC grafts in nonirradiated recipients.
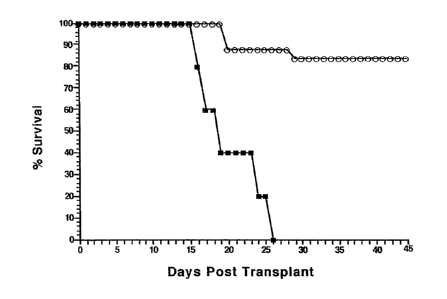
Fig. 2 is a graph of percent survival of transplanted mice tested using a
Radioprotection
assay. Irradiated recipients were transplanted with 4 mm segments of aorta or
vena cava (O).
Control animals recieved I OOpL untreated PB (~). The percent surviving over
time is shown.
Fig. 3 is a graph showing the results of a peripheral blood analysis.
Irradiated recipients
were transplanted with 4 mm segments of thoracic aorta or vena cava. Control
animals received
radiation alone. The peripheral blood of recipient mice was examined on day
7,14, 21, and 56. Each
group of mice was examined at one time point only to eliminate the effects of
serial phlebotomy.
Panel A documents the detection of red blood cells (hemoglobin). Panel B
documents the detection
of white blood cells (WBC). Panel C documents detection of platelets. (Mean ~
SEM for each group
are shown).
Fig. 4 shows plots of data obtained by fluorescence activated cell sorting
(FACS). These
plots demonstrate the detection of donor derived cells in transplant
recipients. Panel A shows the
percent donor cells (Ly5.2) detected in the PB of a vena cava recipient three
weeks after
transplantation. Panels B-D show multilineage reconstitution in the spleen of
an aorta graft recipient
four months after transplantation. The percentage of donor cells expressing
B220 (Pannel B), CD3 (a
T cell marker, Panel C), or Mac-1/Gr-I (a macrophage/granulocyte marker, Panel
D) are shown.
Fig. 5 is a graph showing the percentage of donor derived cells in the
peripheral blood.
Irradiated cogenic recipients were transplanted with 4 mm thoracic aorta (TA),
vena cava (VC) or
abdominal aorta (AA) grafts under the kidney capsule and the presence of donor
cells was analyzed at
the time points indicated. The percentage of Ly5.2 (donor) cells is shown.
Data is shown as the
mean + SEM. Five to 11 vascular recipients were analyzed per group.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-4-
DETAILED DESCRIPTION OF THE SPECIFIC EMBODIMENTS
The following definitions and methods are provided to better define the
present invention,
and to guide those of ordinary skill in the art in the practice of the present
invention. As used herein
and in the appended claims, the singular forms "a", "an", and "the" include
plural referents unless the
context clearly dictates otherwise. Thus, for example, reference to "a cell"
includes a plurality of
such cells and reference to "the growth factor" includes reference to one or
more growth factors, and
so forth.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood to one of ordinary skill in the art to which
this invention belongs.
Definitions
Agent that affects hematopoiesis: A compound, antibody, nucleic acid molecule
or protein
that affects hematopoiesis. In one embodiment, the agent affects the growth,
proliferation,
maturation, or differentiation of hematopoietic cells. An agent can be a
naturally occurring molecule
or a synthetic molecule. In one specific, non-limiting example, the agent is a
pharmaceutical
compound. In another specific non-limiting example, the agent is a protein,
such as a growth factor.
In yet another specific non-limiting example, the agent is a nucleic acid
molecule, such as an
antisense or ribozyme molecule.
Animal: Living multicellular vertebrate organisms, a category which includes,
for example,
mammals and birds.
B Cell: A B cell is a lymphocyte, a type of white blood cell (leukocyte), that
develops into a
plasma cell, which produces antibodies.
Blood Vessel: The vessels through which blood circulates. In general, blood
vessels are
elastic tubular channels that are lined with endothelium. Blood vessels
include the arteries, veins, and
capillaries. Specific, non-limiting examples of a blood vessel include a vena
cava, a thoracic aorta, a
saphanous vein, a mammary artery, the brachial artery, and a capillary. In
another embodiment, a
blood vessel includes the smaller arteries and veins. In yet another
embodiment, a blood vessel is a
capillary of the microvascular circulation
Differentiation: The process by which cells become more specialized to perform
biological
functions. Differentiation is a property that is often totally or partially
lost by cells that have
undergone malignant transformation.
Enhancement (enhancing): An increase in a particular parameter of a cell or
organism. In
one embodiment, enhancement refers to a 25%, 50%, 100% or greater than 100%
increase in a
parameter. In one specific, non-limiting example, enhancement of hematopoeisis
refers to an
increase in a population of the cells of a hematopoietic lineage (e.g. B
cells, T cells, macrophages,
monocytes, or a hematopoietic intermediate cell), such as a 25%, 50%, or 100%
increase in the
population of cells or the response of the population of cells.
Growth Factor: A "growth factor" is a substance that affects the growth of a
cell or
organism. In general, growth factors stimulate cell proliferation or
maturation when they bind to
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-5-
their receptor ("growth factor receptor"). In one embodiment, growth factors
are a complex family of
polypeptide hormones or biological factors that are control growth, division
and maturation of
hematopoietic cells. In another embodiment, growth factors regulate the
division and proliferation of
cells and influence the growth rate of neoplastic tissue (e.g. cancers). A
growth factor can be a
naturally occurring factor or a factor synthesized using molecular biology
techniques. In one
specific, non-limiting example, a growth factor can be used stimulate
lymphocyte production or
differentiation, and thus can be used following chemotherapy or bone marrow
transplantation.
Examples include growth factors, epidermal growth factor, platelet-derived
growth factor,
fibroblast growth factor. Insulin and somatomedin are also growth factors, the
status of nerve growth
factor is more uncertain. Perturbation of growth factor production or of the
response to growth factor
is important in neoplastic transformation.
A growth factor that affects the development (maturation), differentiation,
division, or
proliferation of hematopoietic cells is a "hematopoietic growth factor."
A "stem cell growth factor" is a growth factor that affects hematopoietic stem
cells. Specific
nonlimitiing examples of a stem cell growth factor are c-kit ligand (e.g.
steel factor, stem cell factor)
also FLT-3 ligand and LIF.
Hematopoiesis: The formation and development of blood cells. Hematopoiesis
involves
the proliferation and differentiation from stem cells. In adult mammals
hematopoeisis is known to
occur in bone marrow. Hematopoiesis is the production of hematopoietic cells
including B Cells, T
cells, cells of the monocyte macrophage lineage, and red blood cells.
Immunologically Normal: "Immunologically normal" denotes an individual that
displays
immune system characteristics typical for the species to which the individual
belongs. These
characteristics would typically include, among others, functioning B-cells and
T-cells as well as
structural cell components, called cell surface antigens, which act as the
immunologic signature for a
particular organism.
The use of such immunologically normal recipients means that an
immunologically normal
recipient's immune system, via its B- (humoral response) and T- (cellular
response) cells, will
identify the cell surface antigens of a foreign cell or an engrafted tissue as
foreign. This recognition
leads ultimately to an immune response against the cell or tissue, resulting
in destruction of the cell or
rejection of the graft. An immune response against an allogeneic tissue is
known as host-versus-graft
rejection.
Immunologically Compromised: An "immunologically compromised recipient" is a
subject with a genotypic or a phenotypic immunodeficiency. A genotypically-
immunodeficient
subject has a genetic defect which result in the inability to generate either
humoral or cell-mediated
response. A specific, non-limiting example of a genotypically immunodeficient
subject is a
genotypically immunodeficient mouse, such as a SCID mouse or a bg/nu/xid mice
(Andriole et al., J.
Immunol. 135:2911, 1985; McCune et al., Science 241:1632, 1988). In one
embodiment, a
genotypically immunodeficient subject is unable to react against a foreign
cell or engrafted allogeneic
tissue. A "phenotypically-immunodeficient subject" is a subject which is
genetically capable of
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-6-
generating an immune response, yet has been phenotypically altered such that
no response is seen. In
one specific, non-limiting example, a phenotypically-immunodeficient recipient
is irradiated. In
another specific, non-limiting example, a phenotypically -immunodeficient
subject has been treated
with chemotherapy.
Inhibition (inhibiting): An decrease in a particular parameter of a cell or
organism. In one
embodiment, inhibition refers to a 25%, 50%, 100% or greater than 100%
decrease in a parameter. In
one specific, non-limiting example, inhibition of hematopoeisis refers to a
decrease in a population of
the cells of a hematopoietic lineage (e.g. B cells, T cells, macrophages,
monocytes, or a
hematopoietic intermediate cell), such as a 25%, 50%, or 100% decrease in the
population of cells or
the response of the population of cells.
Isolated: An "isolated" biological component (such as a nucleic acid molecule,
protein
or portion of a vascular tissue) has been substantially separated or purified
away from other
biological components in the cell of the organism in which the component
naturally occurs. Vascular
tissue that has been isolated includes separation by surgical and/or enzymatic
methods. Nucleic acids
and proteins that have been "isolated" include nucleic acids and proteins
purified by standard
purification methods. The term also embraces nucleic acids and proteins
prepared by recombinant
expression in a host cell as well as chemically synthesized nucleic acids.
Lymphocytes: A type of white blood cell that is involved in the immune
defenses of the
body. There are two main types of lymphocytes: B-cell and T-cells.
Lymphoproliferation: An increase in the production of lymphocytes.
Mammal: This term includes both human and non-human mammals. Similarly, the
term
"subject" includes both human and veterinary subjects.
Monocyte: A large white blood cell in the blood that ingests microbes or other
cells and
foreign particles. When a monocyte passes out of the bloodstream and enters
tissues, it develops into
a macrophage.
Pharmaceutically acceptable carriers: The pharmaceutically acceptable carriers
useful in this invention are conventional. Remington's Pharmaceutical
Sciences, by E. W. Martin,
Mack Publishing Co., Easton, PA, 15th Edition (1975), describes compositions
and formulations
suitable for pharmaceutical delivery of the fusion proteins herein disclosed.
In general, the nature of the carrier will depend on the particular mode of
administration
being employed. For instance, parenteral formulations usually comprise
injectable fluids that include
pharmaceutically and physiologically acceptable fluids such as water,
physiological saline, balanced
salt solutions, aqueous dextrose, glycerol or the like as a vehicle. For solid
compositions (e.g.,
powder, pill, tablet, or capsule forms), conventional non-toxic solid carriers
can include, for example,
pharmaceutical grades of mannitol, lactose, starch, or magnesium stearate. In
addition to
biologically-neutral carriers, pharmaceutical compositions to be administered
can contain minor
amounts of non-toxic auxiliary substances, such as wetting or emulsifying
agents, preservatives, and
pH buffering agents and the like, for example sodium acetate or sorbitan
monolaurate.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
Progenitor Cell: A "progenitor cell" is a cell that gives rise to progeny in a
defined cell
lineage. A "hematopoietic progenitor cell" is a cell that gives rise to cells
of the hematopoietic
lineage. One specific non-limiting example of a hematopoietic progenitor cell
is a "common
lymphoid progenitor cell," which is a progenitor cell that gives rise to
immature and mature lymphoid
cells. Another specific, non-limiting example of a hematopoietic progenitor
cells is a "T cell
progenitor cell," which gives rise to immature and mature T cells. Yet another
specific, non-limiting
example of a progenitor cell is a "stromal progenitor cell," which is a
progenitor cell that gives rise to
stromal elements.
Stem Cell: A "stem cell" is a pluripotent cell that gives rise to progeny in
all defined
hematolymphoid lineages. In addition, limiting numbers of cells are capable of
fully reconstituting a
seriously immunocompromised subject in all blood cell types and their
progenitors, including the
pluripotent hematopoietic stem cell by cell renewal.
Subject: Any subject that has a vascular system and has hematopoietic cells in
the wild-
type organism. In one embodiment, the subject is a non-human mammalian
subject, such as a
monkey, mouse, rat, rabbit, pig, goat, sheep or cow. In another embodiment,
the subject is a human
subject.
Supernatant: The culture medium in which a cell is grown. The culture medium
includes
material from the cell, including secreted growth factors.
T Cell: A white blood cell critical to the immune response. T cells include,
but are not
limited to, CD4+ T cells and CD8+ T cells. A CD4+ T lymphocyte is an immune
cell that carries a
marker on its surface known as "cluster of differentiation 4" (CD4). These
cells, also known as
helper T cells, help orchestrate the immune response, including antibody
responses as well as killer T
cell responses. CD8+ T cells carry the "cluster of differentiation 8" (CD8)
marker. In one
embodiment, a CD8 T cells is a cytotoxic T lymphocytes. In another embodiment,
a CD8 cell is a
suppressor T cell.
Therapeutically effective portion or sufficient portion of a vascular tissue:
An
amount of a vascular tissue, that can be determined by various methods,
including generating an
empirical dose-response curve, predicting potency and efficacy of using
modeling, and other methods
used in the biological sciences. In general, a therapeutically effective
portion of a blood vessel is an
amount sufficient to reconstitute hematopoeisis in an immunocompromised
subject. In one
embodiment, a thereapeutically effective amount of vascular tissue is a blood
vessel that includes at
least one of the intima, media, or adventitia.
In one specific, non-limiting example, the graft is a vessel that is 1 mm to
about 10 cm
in length, or is less than I cm in length, or is about 2 mm to about 4 mm in
length. In another
specific, non-limiting example, the vessel has a circumference of less than
about 0.5 mm, or a
circumference of about 0.5 mm to about 2.5 mm. In one embodiment, the graft
has a circumference
of about 1.0 mm to about 2.0 mm.
In yet another specific, non-limiting, example the area of the vascular tissue
utilized is about
1 mm'' to about 1 cmr, or is about 1 mm' to about 100 mm', or is about 1 to
about 10 mm', or is about
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
_g_
6 to about 8 mm'', or is about 7 mm''. In a further specific, non-limiting,
example the vascular tissue
weighs about 1 to about 50 mg, or weighs about 5 to about 20 mg, or weights
about 10 mg. In
another specific, non limiting, example the vascular tissue contains about 1 x
105 cells to about 1 x
109 cells, or contains about 1 x 106 to about 1 x 10$ cells, or contains about
1 x 10' cells. In one
embodiment, the therapeutically effective amount of a vascular tissue is a 4
mm long portion of vena
cava or a thoracic aorta.
The therapeutically effective portion of a vascular tissue will be dependent
on the type of
vascular tissue utilized (e.g. artery, vein or capillary), the subject being
treated (e.g. the species or
size of the subject), the degree that the subject is immunocompromised, and
the location of the
transplant (e.g. intraperitoneal, kidney capsule, etc).
Specific assays for determining the therapeutically effective portion of a
vascular tissue
are provided herein. In one specific, non-limiting example, different lengths,
volumes or weights of
vascular tissue are transplanted under the kidney capsule of an
immunocompromised subject, and the
presence and/or quantity of hematopoietic cells (which can include subtypes of
hematopoietic cells)
is detected and/or analyzed. The methods disclosed in the present invention
have equal application in
medical and veterinary settings. Therefore, the general term "subject being
treated" is understood to
include all animals (e.g. humans, apes, dogs, cats, mice, rats, rabbits,
sheep, pigs, and cows) and
reconstitution of hematopoiesis is monitored using the assays described
herein. For example, the
presence of B cells, T cells, or macrophages following transplantation can be
determined.
Transplantation: The transfer of a tissue or an organ, or a portion thereof,
from one body
or part of the body to another body or part of the body. An "allogeneic
transplantation" or a
"heterologous transplantation" is transplantation from one individual to
another, wherein the
individuals have genes at one or more loci that are not identical in sequence
in the two individuals.
An allogeneic transplantation can occur between two individuals of the same
species, who differ
genetically, or between individuals of two different species. An "autologous
transplantation" is a
transplantation of a tissue or a portion thereof from one location to another
in the same individual, or
transplantation of a tissue or a portion thereof from one individual to
another, wherein the two
individuals are genetically identical.
Vascular Tissue: Tissue consisting of, or containing, vessels as an essential
part of a
structure. Vascular tissue operates by means of, or is made up of an
arrangement of, vessels.
Vascular tissue includes the arteries, veins, capillaries, lacteals, etc. In
one embodiment, vascular
tissue includes a highly vascularized organ (e.g. the lung). In another
embodiment, vascular tissue is
a blood vessel, or a portion thereof. A "portion" of a blood vessel is any
length less than the full
length of the vessel in its native location, and includes portions of the
blood vessel that do not form
an enclosed lumen. Cells isolated from a vascular tissue are a population of
cells isolated from the
remaining components of the tissue. One specific, non-liminting example of
cells from a vascular
tissue are endothelial cells isolated from vascular tissue, such as a blood
vessel.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-9-
Method for Enhancing Hematopoiesis
A method is provided herein for enhancing hematopoiesis, comprising
transplanting at least a therapeutically effective portion of an isolated
vascular tissue into a subject,
wherein the vascular tissue enhances hematopoiesis. The minimum
therapeutically effective amount
of a vascular tissue can be determined using the model system described
herein. The minimum
therapeutically effective amount of a vascular tissue can be utilized to
enhance hematopoiesis, or a
larger portion of a vascular tissue can be utilized.
In one embodiment, the subject can be an immunologically normal subject. In
another
embodiment the subject is immunocompromised (e.g. a phenotypcially or
genetically
immunocompromised subject). The subject may be generally immunocompromised, so
that the
responses of all of the cells of the immune system are impaired, or the
subject may be
immunocompromised in one particular aspect of the immune response, such as the
responsiveness of
a specific cell type. In one specific, non-limiting example, T cell responses
are impaired. In another
specific, non-limiting example, B cell response is impaired. In yet another
specific, non-limiting
example, a monocyte or macrophage response is impaired. It should be noted
that there are many
assays to test responsiveness of specific cells of the immune system, and that
one of skill in the art
can readily determine a test to assess responsiveness of a population of cells
of the immune system in
a subject. Specific non-limiting examples of tests to assess the immune system
are a test for
cytotoxic T lymphocyte activity (CTL assays) such as a chromium release assay,
an assay for mixed
lymphocyte response (MLR assay), an assay for helper cells, an assay for
cytokine secretion, an assay
for immunoglobulin production, or an assay to detect antigen presenting cells
(APCs). One specific
non-limiting example of an immunocompromised subject is a subject deficient in
hematopoiesis as a
result of irradiation or the use of chemotherapeutic agents.
A vascular tissue is transplanted into the subject, or from one location in
the subject to
another ectopic (away from its normal) location in the subject. In one
embodiment, the vascular
tissue is an isolated vessel, or an hematopoietic effective portion thereof.
Specific non-limiting
examples of vessels are the vena cava, the thoracic aota, the mammary artery,
the saphenous vein, or
a capillary. In another embodiment, the vascular tissue is a portion of a
highly vascularized organ.
One of skill in the art can readily determine a therapeutically effective
portion of vascular tissue using
the model system derived herein.
The site of transplantation is selected so that hematopoeisis is enhanced by
the grafted
vascular tissue. In one embodiment, the vascular tissue is transplanted under
the kidney capsule. In
another embodiment, the vascular tissue is transplanted into the peritoneum.
In a further
embodiment, the vascular tissue is transplanted subcutaneously.
Enhancement of hematopoiesis can be measured by any assay known to one of
skill in the
art. Specific, non-limiting examples of assays are the examination of the
spleen for macroscopic
colonies (CFU-S). Alternatively, fluorescence activated cell sorting can be
performed to assess the
presence of a population of lymphocytes. Assays for B and T cell activity can
also be utilized. As
described above, specific non-limiting examples of assays for hematopoietic
cells are an assay for
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-10-
cytotoxic T lymphocyte (CTL assay, such as a chromium release assay), an assay
for mixed
lymphocyte response (MLR assay), an assay for helper cells, an assay for
cytokine secretion, an assay
for immunoglobulin production, or an assay to detect antigen presenting cells
(APCs).
Animal Models
In one embodiment, the methods described herein are used to produce a non-
human animal model for
testing agents that affect hematopoiesis. To make the model, a non-human
animal deficient for
hematopoiesis is transplanted with an isolated portion of a vascular tissue,
wherein the portion of the
vascular tissue is sufficient to promote hematopoiesis.
In one embodiment, non-human animal is a mammal. Specific non-limiting
examples of a
mammal is a mouse, rat, rabbit, cat, dog, pig, goat, sheep, or a monkey. The
non-human animal is
deficient in hematopoiesis, and can be genetically or phenotypically
immunocompromised. Several
species of animals are known to have a genotypic immunodeficiency. One
specific, non-limiting
animal with a genotypic immunodeficiency is a SCID mouse. Similarly, methods
are known in the
art to create a phenotypic immunodeficiency. Specific, non-limiting examples
of treatments to create
a phenotypic immunodeficiency are the irradiation of a non-human mammal or the
treatment with a
chemotherapeutic agent of a non-human animal.
The methods and model systems described herein can be used to assess the
effect of an agent on
hematopoiesis. In order to assess the effect of an agent on hematopoiesis, a
therapeutically effective
portion of an isolated vascular tissue is transplanted into a subject, such as
a genetically or
phenotypically immunocompromised subject. In one embodiment, the portion of
the isolated
vascular tissue is known to be an amount sufficient to enhance hematopoiesis.
The vascular tissue is
treated with the agent. Treatment can occur prior to, or subsequent to,
engraftment of the vascular
tissue. If the vascular tissue is treated after transplantation, treatment
with the agent can be local or
systemic. Systemic treatment can occur by any route known to one of skill in
the art, including
intravenous, subcutaneous, intramuscular, or intraperitoneal injection.
Following treatment with the agent, hematopoiesis is detected in the animal.
Hematopoiesis
can be detected by any means known to one of skill in the art. One specific
non-limiting example of
an assay of use is fluorescence activated cell sorting. FACS analysis can be
performed to detect for
any population of hematopoietic cells (e.g., T cells, B cells, granulocytes,
macrophages, an immature
population of T or B cells). Another specific, non-limiting example of an
assay of use is a CFU-S
assay (see the Examples below). Yet another specific, non-limiting assay of
use is an assay the
measures red blood cells or a parameter thereof, such as a test to measure
hemoglobin concentration,
red blood cell count and red blood cell half life (such as conventional
detection of a peripheral red
blood cell count).
Hematopoiesis in the treated subject is then compared with a control value of
hematopoiesis.
In one embodiment, the control value is obtained from a normal subject, that
is not transplanted nor
treated with the agent. In another embodiment, the control value is obtained
from an
immunologically compromised subject transplanted with a therapeutically
effective portion of an
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-11-
isolated vascular tissue, but not treated with the agent. In yet another
embodiment, the control value
is obtained from non-transplanted subject that is treated with the agent. In a
further embodiment, the
control value is a compilation of data points, the value of which is used for
comparison purposes (e.g.
a standard value). Any change in any parameter of hematopoiesis in the treated
subject as compared
to the control is indicative that the agent affects hematopoiesis.
Kits
The methods of the invention are ideally suited for the preparation of a kit.
Such a kit may
comprise a carrier means, such as a box, bag, or plastic carton, containing
one or more container
means such as vials, tubes, and the like. Each of the container means can
include a separate element
to be used in a method. For example, one of the container means can include a
portion of a vascular
tissue. The kit may also contain a container means with a buffer or vehicle
for the introduction of the
vascular tissue. In addition, instructions can be provided to detail the use
of the components of the
kit, such as written instructions, video presentations, or instructions in a
format that can be opened on
a computer (e.g. a diskette or CD-ROM disk).
Without further elaboration, it is believed that one skilled in the art can,
using this
description, utilize the present invention to its fullest extent. The
following examples are illustrative
only, and not limiting of the remainder of the disclosure in any way
whatsoever.
SPECIFIC EXAMPLES
EXAMPLE 1
Production of an Animal Model
The yolk sac is the first site of recognizable hematopoiesis in the developing
mammalian
embryo. In the mouse, mesodermal cells aggregate and, by day 7 of gestation,
form the
extraembryonic blood islands. Differentiation of these mesodermal elements
gives rise to the
endothelium and the first primitive globinized erythrocytes. As the yolk sac
vascular network grows
it directly communicates with the dorsal aorta of the developing embryo,
thereby linking the
extraembryonic and intraembryonic vascular systems. The yollc sac is the
primary site of
erythropoiesis during embryonic development, then hematopoietic precursors are
thought to migrate
and subsequently colonize the liver beginning as early as day 9. The liver
then becomes the primary
site of hematopoiesis during fetal development until late in gestation, when
the medullary cavity of
the bones is formed and seeded with hematopoietic precursors from the fetal
liver. By the end of
gestation, the bone marrow has become the principal site of hematopoiesis and
continues to produce
blood cells throughout the lifetime of higher vertebrates. This model is based
on the orderly
migration of hematopoietic precursors as determined primarily by morphologic
studies of
erythropoiesis during embryonic and fetal development. Modern transplantation
studies however
have revealed that hematopoietic stem cell development is more complex.
Although the bone marrow is thought to be the principal site of hematopoiesis
in adult
mammals, in a number of disease states significant blood cell production
occurs outside the
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-12-
medullary cavity of the bone. This extramedullary hematopoiesis (EMH) is
encountered most
frequently in myelofibrosis, a neoplastic disorder characterized by the
replacement of the marrow
cavity with fibrous tissue. In addition, the abnormal hemoglobin synthesis
found in severe (3-
thalassemia results in a marked increase of ineffective red blood cell
production and the subsequent
expansion of hematopoiesis to extramedullary sites (Cardia et al., Pediatr.
Neurosurg. 20:186-189,
1994). It has been postulated that quiescent hematopoietic stem cells reside
in the adult liver or,
alternatively, that bone marrow derived HSC can migrate to the liver as a
consequence of
hematopoietic stress.
During embryogenesis, the close proximity of developing blood vessels and
early sites of
hematopoiesis have led to the hypothesis of a common precursor cell or
hemangioblast that is present
in the embryo. In order to determine if a population of hematopoietic
progenitor/stem cells persists in
association with the vascular system of the adult, an assay was performed to
determine if functional
hematopoietic cells were present in the blood vessels of normal adult mice.
It is known that when mice are phenotypically immunocompromised, such as by
treatment with
high doses of radiation, a marked depletion of hematopoietic progenitor cells
and stem cells within the
bone marrow is observed. This depletion of cells is accompanied by the
production of a number of
cytokines and growth factors that facilitate engraftment of transplanted donor
hematopoietic cells (such as
stem cells isolated from the bone marrow) into the irradiated hematopoietic
microenvironment. It has
previously been demonstrated that irradiated recipient mice will die within 3
weeks from bone marrow
failure if they are not transplanted with a su~cient number of primitive donor
hematopoietic cells.
Conversely, almost three logs more donor cells must be transplanted into
intact non-irradiated mice before
donor cells will engraft in active sites in the bone marrow and contribute to
steady state hematopoiesis
(Ramshaw et al., Blood 86:924-929, 1995).
A model system was thus developed, using irradiated mice, to demonstrate for
the first time that
functional hematopoietic cells are present in the vascular tissue. In order to
demonstrate that the
hematopoietic microenvironment in irradiated mice could stimulate and recruit
hematopoietic progenitor
cells from vascular tissue, 4 mm segments of proximal inferior vena cava (VC)
or thoracic aorta (TA) were
harvested from normal adult donor mice. The isolated vascular tissue was
transplanted intact under the
kidney capsule of lethally irradiated adult recipient mice.
The transplanted mice were sacrificed at 14 days post-transplant, and
histogical sections
were prepared of the graft and the surrounding tissue. The examination of the
vascular implant
revealed significant disruption of the normal architecture of the vessel wall.
A large accumulation of
a diverse population of cells was observed within the wall of the graft. In
addition to the presence of
a diffuse infiltrate of morphologically indistinct cells, the clear
development of thin walled vascular
channels, similar to hemangiomas, was observed. In contrast, when VC or TA
tissue was
transplanted under the kidney capsule of non-irradiated mice, the normal
architecture was essentially
maintained, indicating that the accumulation of cells within the wall of the
transplanted vascular
tissue was specifically induced by radiation treatment of the recipient. The
accumulation of cells and
the development of vascular channels in the wall of the vascular graft were
consistent with the
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-13-
proliferation and differentiation of a population of cells within the vascular
graft. However, is also
possible that a population of host cells in the irradiated recipient may have
migrated to and
proliferated within the transplanted vascular tissue.
EXAMPLE 2
Cells Migrate from Transplanted Vascular Grafts
To determine if cells from the vascular grafts migrated and differentiated, a
portion of the
vena cava or thoracic aorta was surgically isolated from ROSA26 mice, which
constituitively
expressed a (3-galactosidase transgene with a CMV promoter (Zambrowicz et al.,
Proc. Natl. Acad
Sci. 94:3789-3794, 1997). The isolated vascular tissue was transplanted into
the kidney of C57BL/6
mice. The recipient kidney containing the vascular graft was removed 14 days
after transplantation,
fixed, sectioned, and incubated with the enzyme substrate X-gal, which is
converted to a blue
precipitate in the presence of (3-galactosidase.
The majority of the cells in the ROSA-26 TA graft were blue and therefore
donor derived.
In addition, a significant population of donor cells was observed migrating
under the kidney capsule
away from the initial transplantation site, consistent with a response to
signals from the irradiated
host microenvironment. To demonstrate that large numbers of host cells had not
infiltrated into the
donor vascular tissue, a portion of the vena cava from normal adult donors was
transplanted under the
kidney capsule of irradiated ROSA26 recipients. These vascular grafts did not
significantly react
with X-gal confirming that this accumulation of cells was primarily of donor
origin. Histologic
evaluation of the ROSA26 grafts confirmed the presence of primarily donor-
derived cells in these
vascular grafts. Taken together, the results demonstrate an expansion of donor-
derived cells in both
donor vena cava and thoracic aorta following transplantation into irradiated
recipients.
EXAMPLE 3
Vascular tissue grafts give rise to colony forming cells in the spleen
Diverse sources of hematopoietic stem cells including bone marrow (BM), fetal
liver, and
peripheral blood (PB), produce macroscopic spleen colonies (CFU-S) 12-14 days
after injection into
irradiated mice. Each colony is clonal and an average of S colonies per spleen
arise from the
injection of 1x105 BM cells (see Till and McCulloch, Radiat. Res. 14:213,
1961; Siminovitch et al., J.
Cell. Comp. Physiol. 62:327, 1963). The potential of both vena cava (VC) and
thoracic aorta (TA)
grafts to give rise to CFU-S was determined and directly compared to 1x105 BM
cells or 100 p1 of
unfractionated normal PB. Cohorts of irradiated recipient mice received either
a 4 mm vascular graft
(TA or VC) under the kidney capsule, Ix105 BM cells by i.v. injection, or
100pL PB by i.v. injection.
Spleen colonies were enumerated on day 14; the mean number of colonies derived
was similar for
transplanted VC, TA, and BM ranging from 6.1-7.8 colonies per spleen (Figure
1). In this model
system, radiation (e.g. an immunocompromised subject) was required for the
development of day 14
CFU-S in recipients of VC or TA grafts.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-14-
To ensure that endogenous spleen colonies did not arise in animals treated
with this radiation
dose (1100cGy), the spleens from non-transplanted, irradiated control animals
were examined and no
colonies were detected. In contrast with the VC, TA and BM transplants, a mean
of only 1.2 colonies
per spleen were detected following the injection of 100 p1 of PB.
Normal adult peripheral blood does contain small numbers of circulating
hematopoietic stem
cells (Goodman and Hodgson, Blood 19, 702-714. 1962). Although the vascular
tissue grafts were
extensively rinsed to remove residual donor blood, we directly demonstrated
that contaminating
donor PB was not a significant source of day 14 spleen colonies in these
experiments. The maximum
volume of blood in the lumen of a VC or TA graft (4 mm X 0.7 mm) is 1.5 p1.
The injection of
almost 66-fold excess ( 100 ~1) of the maximal amount of blood present in
these vascular grafts gave
rise to only 1.2 colonies per spleen. This experiment indicates that the
frequency of cells that give
rise to day 14 CFU-S is > 300-fold higher in the transplanted vascular grafts
than in normal
peripheral blood.
EXAMPLE 4
Vascular grafts confer protection against lethal irradiation: A portion of a
vascular tissue is
sufficient
The injection of a su~cient number of hematopoietic stem cells into lethally
in-adiated recipient
mice protects them from death secondary to bone marrow failure. To evaluate
the potential of transplanted
vascular tissue to protect mice from radiation induced death, sections of 4mm
in length (an area of 1.88
mm'' ) of either VC or TA were transplanted under the kidney capsule of
lethally irradiated recipients
( 1100cGy in 2 fraction given 3 hours apart). Control mice were injected with
100 p1 of unfractionated PB.
Transplant recipients were maintained on antibiotic water under sterile
conditions and their survival
monitored daily for 45 days (Figure 2). All mice transplanted with 100 p1 of
PB died between day 15 and
day 26, consistent with the time course of radiation induced marrow failure. A
similar result was seen in
mice that were irradiated but not transplanted. In contrast, 85% of the
recipients of vascular grafts
survived. These results demonstrate a dramatic increase in survival with the
transplantation of relatively
small explants of normal large blood vessels (VC and TA) have the capacity to
protect mice from radiation
induced bone marrow failure.
EXAMPLE 5
Vascular grafts attenuate radiation induced bone marrow failure
Lethally irradiated mice primarily die due to severe anemia and bleeding from
low platelet
counts. To evaluate the effects of the transplantation of vascular tissue on
the leukocytes, red cells
and platelets in the peripheral circulation, cohorts of mice were treated with
radiation and then
transplanted with VC or TA grafts. A group of control mice was irradiated but
not transplanted. At
the time points indicated, cohorts of mice were bled and analyzed. Each group
of mice was analyzed
at one time point only to avoid the effects of repeated phlebotomy on the
hemoglobin (Hb)
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-15-
concentration. Irradiated control mice were evaluated only until day 18 as an
insufficient number of
mice survived longer than this time point. The results are shown in Figure 3.
When the Hb
concentration was assayed at day 7, a similar decrease was observed in both
transplant recipients and
control mice. By day 14 however, the Hb was 6.2 g/dL in the vascular graft
recipients but only 3.4
g/dL in the control group. Only a modest further decline was noted in
irradiated non-transplanted
recipients that survived until day I 8 (a Hb of ~3.Og/dL represents the
severest anemia compatible
with survival in this mouse strain). Vascular graft recipients maintained a Hb
concentration of about
2-fold higher than the threshold of 3.Og/dL, consistent with the production of
donor derived red blood
cells. When the number of circulating platelets was determined, a similar
decrease in platelet count
was observed in both the transplanted and non-transplanted groups up to day
14. By day 21 however,
the number of platelets had increased 7-fold above the day 14 value in the
vascular graft recipients.
Evaluation of the number of white blood cells revealed that a higher number of
circulating cells was
observed in the vascular graft recipients at all time points examined. As
shown in Figure 2, irradiated
non-transplanted mice typically died from complications of pancytopenia within
21 days after
radiation treatment. In contrast, the majority of VC and TA recipients
survived and demonstrated a
normal Hb, platelet and white blood cell counts 2 months after
transplantation.
EXAMPLE 6
Multilineage hematopoietic reconstitution after vascular transplantation
The role of donor derived cells in reconstituting the hematopoietic system of
irradiated mice was
further investigated using donor and recipient mice that were congenic at the
Ly5 locus (CD45). Ly5.2
donor VC or TA grafts were implanted under the kidney capsule of irradiated
Ly5.1 recipients. Peripheral
blood and spleen tissue was harvested at different time intervals and the
frequency of donor derived cells
was evaluated. Using flow cytometry, the frequency of Ly5.1 host cells and
Ly5.2 donor cells expressing
T-cell (CD3), B-cell (B220) and myeloid (Mac-1/Gr-I) markers was determined
(see Fig. 4).
As early as 21 days after the transplant of a VC graft, up to 67% of the cells
in the circulation of
recipient mice were donor derived (Figure 4A). When the spleens of vascular
graft recipients were
evaluated 4 months after transplant, multilineage donor derived hematopoiesis
was routinely observed
(Figure 4B-D). These results demonstrate that vascular derived donor cells
contribute significantly to early
hematopoietic reconstitution and persist long term, for at least 4 months, in
the recipient animals.
However, although the vascular derived donor cells can contribute
significantly to early
hematopoietic reconstitution, recipient cells could also play a role in
hematopoiesis. Thus, additional
transplantations were performed using donor and recipient mice that were
congenic at the Ly5 locus
(CD45). Ly5.2 donor VC or TA grafts were implanted under the kidney capsule of
irradiated LyS. I
recipients. The percentage of Ly5.2 donor cells present in the peripheral
blood was then evaluated (see
Fig. 5). The results demonstrated that recipient cells could also participate
in hematopoiesis. The results
also demonstrate that the levels of donor cells decline over time. This
indicates a very high degree of host
recovery, and demonstrates a highly protective effect of components of the
vascular graft on the host stem
cells. This protective effect may be cytokine mediated. It should be noted
that the use of higher doses of
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-16-
radiation can further compromise the host stem cells, and thus can ensure that
donor cells predominate
over t~rr~e.
EXAMPLE 7
Blood vessel derived hematopoiesis in other tissues
The experiments described above were performed using the largest blood vessels
found in
the murine circulatory system. To determine whether cells with hematopoietic
potential were found
in differentiated tissues within the adult mouse, explants of normal ROSA26
adult heart and lung
were transplanted into irradiated mice. Any means of sampling the vascularized
tissue can be utilized
to sample differentiated tissues, including surgical removal or punch biopsy.
In the instant example,
the tissue was dissected from donor animals, and a portion of the heart or
lung (approximately 10 mg)
was utilized. Fourteen days after transplantation, the recipients were
sacrificed, and cross sections
were prepared of the cardiac tissue or pulmonary tissue transplants.
Transplantation of cardiac or lung tissue into either the peritoneal space or
under the kidney
capsule stimulated the development of hematopoiesis. Thus, several
transplantation sites can be used
for the introduction of vascular tissue.
In histological sections prepared from the transplanted explants, thin walled
vascular
structures that appear to have coalesced together to form large vascular
channels were routinely
observed in explants of cardiac tissue. As with the thoracic aorta transplants
(see Example 2), a
significant population of migrating donor cells was observed following
transplantation of ROSA26
lung tissue under the kidney capsule. Histologic evaluation of the
transplanted lung tissue
demonstrates the ROSA26 staining of donor cells is clearly distinguishable
from host cells.
Remarkably, as little as 10 mg of donor lung tissue or the equivalent of 50%
of the total cardiac tissue
from a single donor mouse conferred protection from lethal irradiation. The
results demonstrate that
hematopoietic cells arise from a number of highly vascular organs when these
tissues are transplanted
into an irradiated microenvironment that stimulates and supports hematopoietic
stem cell
differentiation.
EXAMPLE 8
Localization of early proliferating cells within the donor vascular tissue
explants
To identify and purify the progenitor cell population of vascular tissue, the
cellular
proliferation within the vascular graft after transplantation was examined.
Segments of proximal,
inferior vena cava, (VC) or thoracic aorta (TA) were transplanted under the
kidney capsule of
irradiated (1100 cGy) or non-irradiated mice. Recipients were treated with
bromodeoxyuridine
(BrdU) and the kidney containing the vascular graft was removed, fixed and
evaluated for the uptake
of BrdU by immunochemistry. As early as 48 hours after transplantation, BrdU
positive,
proliferating cells were identified in the endothelial layer of both the TA
and VA grafts transplanted
into irradiated recipients. In contrast, BrdU labeled cells were not
identified following
transplantation into non-irradiated mice. To ensure that non-specific binding
of the BrdU antibody
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-17-
was not induced by transplantation into irradiated animals, tissue sections
were incubated with DAB
substrate or VIP substrate. No positive cells were observed in the absence of
the primary antibody to
BrdU.
These results demonstrate that the transplantation of donor vascular tissue
into irradiated
animals leads to the proliferation of a population of cells within the intima
of the transplanted vessel
wall by 48 hours. In addition, the absence of BrdU positive cells in the
intima of vascular grafts in
non-irradiated mice indicates that the proliferation of this cell populations)
is dependent on specific
signals in the immunocompromised recipient.
Thus, BrdU can be used to readily detect an anatomically distinct population
of proliferating cells
in transplanted vascular grafts. In addition the bone marrow, spleen, liver
and thymus are examined for
proliferating donor derived cells beginning as early as 24 hours after
transplantation of the vascular tissue.
To confirm that the BrdU identified cells are the progenitor cells, the cells
are purified (using H033342).
H033342 is a viable dye that stains DNA (Fleming et al., J Cell. Biol.
122:897, 1993). This dye is used in
conjunction with FACS to sort cells that are actively cycling (as compared to
cell in GONG 1 phase of the
cell cycle). The sorted cells can then be transplanted into recipient animals
and their developmental
potential.
Taken together, these studies described above demonstrate that hematopoietic
progenitor/stem cells are present in the vascular tissue of normal adult
animals.
EXAMPLE 9
Evaluation of the expression of hematopoietic cell and vascular markers on
donor cells in the
transplanted vascular tissue
Over the last few years, several cell surface markers have been found to be
useful for the
enrichment of HSC. Using combinations of these markers in conjunction with
intracellular markers
indicative of a quiescent non-proliferating cells (rhodamine low, Hoeschst
low) it has been shown
that the injection of as few as 5 cells can continuously give rise to more
than 50% of the mature cells
in the peripheral blood (e.g. see Morrison and Weissman, Immunity 1:661-
673,1994). 1n order to
identify a candidate stem cell population from the vascular grafts, the
phenotype of the cells that arise
within the wall of the transplanted blood vessels is analyzed. The cell cycle
status of donor cells is
used as a guide to determine to localize cell populations that have the
earliest proliferative response to
the microenvironment of an irradiated host. Using immunohistochemical
techniques, cells are
localized within the transplanted vascular tissue that express several well
described HSC markers
including Sca-1, c-kit and AA.41. Cell surface markers of endothelial
progenitors including Flk-I,
TIE, TEK and TAL will similarly be assayed in the grafted vascular tissue. In
the event that a
particular marker is not detected in at least some cells by immunochemistry,
in situ hybridization
studies are performed to confirm this finding. In addition, ROSA26 donor mice
are employed to
demonstrate that these phenotypically defined cell populations are derived
from the vascular graft
donor and not a population of host cells that have subsequently migrated into
the transplanted
vascular tissue.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-18-
Immunochemistry provides tissue specific localization of phenotypically
defined cells
within the transplanted vascular graft. The primary limitation of this
technique is that it is difficult to
study the co-expression of more than 2 markers on an individual cell. To
overcome this limitation,
flow cytometry is used to examine the simultaneous expression of up to 4 cell
surface markers on
individual donor cells. Suspensions of cells are prepared from the vascular
grafts and incubated with
the antibodies listed above and analyzed by flow cytometry, in order to
accurately quantitate the
frequency of combinations of hematopoietic and vascular cell surface marker
expression (see below,
Example 11 ). To confirm that phenotypically defined cells are donor derived,
the cell population of
interest is sorted using a FACS Vantage cell sorter. The purity of the sorted
product is evaluated by
re-analysis of the sorted cells. Cytospin preparations are made and
phenotypically defined cells are
incubated with X-gal to demonstrate the presence of the ROSA26 donor cell
marker. These
combined approaches identify cell populations that express HSC markers,
vascular progenitor
markers or a combination of both in vascular tissue grafts. Normal, non-
transplanted VC and TA are
also assessed for the presence of these phenotypically defined progenitors.
EXAMPLE 10
Functional analysis of blood vessel derived, candidate hematopoietic
progenitorlstem cells
The phenotypic analysis outlined above (Example 9) identifies cell populations
that express
cell surface markers associated with both vascular progenitors and
hematopoietic cell progenitors.
The expression of these cell markers is then utilized to identify and isolate
candidate hematopoietic
progenitor cells that arise in the transplanted vascular tissue. When
determining whether a specific
combination of cell surface markers are informative for the identification of
hematopoietic progenitor
cells, it is important to ensure that a subpopulation of functional donor
cells is not excluded by this
approach. Therefore, donor derived cells are isolated from the wall of the
vascular tissue implants
and evaluated for the functional activity of each cell surface marker
independently.
The minimum number of total donor derived vascular cells required to protect a
recipient
mouse from lethal irradiation is determined by limiting dilution analysis.
Using cell sorting for a
particular hematopoietic stem cell marker (e.g. Sca-1), populations of Sca-1+
cells and Sca-1- cells
are purified. Proportionate numbers of each population are then injected into
irradiated recipient
mice. For instance, if it is determined that 1x106 total vascular cells are
required to provide
radioprotection, and the frequency of Sca-1+ cells in the vascular cell
population to be tested is 10%,
then following cell sorting, one group of mice is transplanted with 1x105 Sca-
1+ cells and the second
cohort of mice eceives 9x105 Sca-1- cells. If Sca-1 marks the majority of
cells with hematopoietic
progenitor cell activity, then all mice transplanted with 1x10' Sca-1+ cells
would be expected to
survive.
However, if Sca-1 expression is not found on these progenitor cells, then the
radioprotective
capacity would be confined to the Sca-1- cell populations. Survival of
recipients of both 1x105 Sca-1+
cells and 1x105 Sca-1- cells indicates that Sca-1 is not an informative marker
of HSC activity in this
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-19-
system. Using this approach the utility of c-kit, AA.41, TIE1, TEK, Flk-I and
TAL will be tested for
HSC activity. Combinations of these markers will be evaluated to further
define this population.
This approach utilizes radioprotection as an assay for HSC activity. To ensure
that
multipotent cells that do not have the capacity for radioprotection but will
read out in a multilineage
hematopoietic assay are not excluded from analysis, irradiated recipient mice
are injected with a
limiting dose of marked (host) bone marrow to ensure radioprotection (1x105
cells/mouse) and an
appropriate number of vascular donor cells. The blood, bone marrow and spleen
of these recipient
mice are evaluated for the presence of donor cells at 1 and at 6 months post
transplant. This
competitive repopulation assay allows for the identification of blood vessel
derived cells that have a
functional capacity to repopulate irradiated hosts independent of the
requirement of providing
radioprotection.
EXAMPLE 11
Further Characterization and Isolation of a Stem Cell from Transplanted
Vascular Tissue and
the Use of these Stem Cells
Vascular tissue is transplanted into a subject as described above. In one
embodiment,
human vascular tissue (e.g. a portion of a saphenous vein, a portion of a
mammary artery, or a piece
of lung tissue obtained by biopsy) is transplanted into an immunocompromised
mouse (e.g. a SCID
mouse).
Various techniques are then employed to separate the cells by initially
removing cells of
dedicated lineage (see U.S. Patent 5,914,108). Monoclonal antibodies are
particularly useful for
identifying markers (surface membrane proteins) associated with particular
cell lineages (termed "lin"
antibodies, and a cell which does not express these marker is term a "lin-"
cell) and/or stages of
differentiation. The antibodies may be attached to a solid support to allow
for crude separation. The
separation techniques employed should maximize the retention of viability of
the fraction to be
collected. For "relatively crude" separations, that is, separations where up
to 10%, usually not more
than about 5%, preferably not more than about 1 %, of the total cells present
having the marker, may
remain with the cell population to be retained, various techniques of
different efficacy may be
employed. The particular technique employed will depend upon efficiency of
separation, cytotoxicity
of the methodology, ease and speed of performance, and necessity for
sophisticated equipment and/or
technical skill.
Procedures for separation may include magnetic separation, using antibody-
coated magnetic
beads, affinity chromatography, cytotoxic agents joined to a monoclonal
antibody or used in
conjunction with a monoclonal antibody, e.g., complement and cytotoxins, and
"panning" with
antibody attached to a solid matrix, e.g., plate, or other convenient
technique. Techniques providing
accurate separation include fluorescence activated cell sorters, which can
have varying degrees of
sophistication, e.g., a plurality of color channels, low angle and obtuse
light scattering detecting
channels, impedance channels, etc.
CA 02375527 2001-12-18
WO 00/78930 PCT/C1S00/17427
-20-
One procedure utilized with human vascular tissue transplanted into a
immunocompromised
host is incubating the cells recovered from spleen or bone marrow at reduced
temperatures, generally
about 4° C., with saturating levels of antibodies specific for a
particular cell type (e.g., CD3 and 8 for
T-cell determinants). The cells are then washed with a fetal calf serum (FCS)
cushion. The cells are
suspended in a buffer medium and separated by means of the antibodies for the
particular
determinants, using various proteins specific for the antibodies or antibody-
antigen complex.
Conveniently, the antibodies may be conjugated with markers, such as magnetic
beads,
which allow for direct separation, biotin, which can be removed with avidin or
streptavidin bound to
a support, fluorochromes, which can be used with a fluorescence activated cell
sorter, or the like, to
allow for ease of separation of the particular cell type. Any technique may be
employed which is not
unduly detrimental to the viability of the remaining cells.
After substantial enrichment of the cells lacking the mature cell markers,
generally by at
least about 50%, or at least about 70%, the cells may now be separated by a
fluorescence activated
cell sorter (FACS) or other methodology having high specificity. Multi-color
analyses may be
employed with the FACS. The cells are separated on the basis of the level of
staining for the
particular antigens.
In one embodiment, in a first separation, starting with at least about 1X 10
~° to 3X 10'°
cells, antibodies are used for the various dedicated lineages (B cell, T cell,
macrophage, etc.). These
antibodies are each conjugated to different fluorochromes. Fluorochromes which
may find use in a
mufti-color analysis include phycobiliproteins, e.g., phycoerythrin and
allophycocyanins, fluorescein,
Texas red, etc. While each of the lineages may be separated in a separate
step, it is also possible to
separate the lineages are separated at the same time. Generally, the number of
cells obtained will be
fewer than about 1 % of the original cells, generally fewer than about 0.5%
and may be as low as
0.2% or less.
The cells can also be further separated by positively selecting for the
expression of Thy
(Thy+), where the cells will generally be fewer than 0.5% of the original
cells, generally in the range
of 0.01-0.5%. The cells may be selected against dead cells, by employing dyes
associated with dead
cells (propidium iodide, LDS). In one embodiment, the cells are collected in a
medium comprising
2% fetal calf serum. Other techniques for positive selection may be employed,
which permit accurate
separation, such as affinity columns, and the like. The method should permit
the removal to a
residual amount of less than about 20%, preferably less than about 5%, of the
non-stem cell
populations.
In one embodiment, the cells are also selected for the expression of a known
stem cell
antigen. In a specific, non-limiting example, cells derived from human
vascular tissue are selected
for the expression of CD34.
In another embodiment cells are selected based on light-scatter properties as
well as their
expression of various cell surface antigens. In one specific non-limiting
example CD34+Lin- and the
CD34+Lin- Thy-1+ have low side scatter and low forward scatter profiles by
FACS analysis.
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-21-
Cytospin preparations show the stem cell to have a size between mature
lymphoid cells and mature
granulocytes. Cells of interest are thus isolated base on the side and forward
scatter profile.
In general, the particular order of separation is not critical. However, in
one embodiment,
the cells are initially separated by a coarse separation, followed by a fine
separation, negative
selection for markers associated with lineage committed cells. If positive
selection of a marker
associated with stem cells is utilized, it is performed at the same time as
the negative selection, or
following negative selection. This separation is followed by an assay to
determine if the cellular
composition has multi-lineage potential and enhanced self regeneration
capability.
An isolated cell is then tested to determine if it provides for production of
myeloid cells and
lymphoid cells in appropriate cultures. In one embodiment cultures providing
hydrocortisone for
production of myeloid cells (Dexter-type cultures) are used. In another
embodiment, cultures for B
lymphocytes, lacking hydrocortisone (Whitlock-Witte type cultures), are used.
In each of the
cultures, stromal cells are provided (e.g. mouse or human stromal cells). In
one embodiment, the
stromal cells are mouse or human and come from various strains (e.g. AC3 or
AC6), or the stromal
cells are derived from mouse or human fetal bone marrow by selection for the
ability to maintain
human stem cells, and the like. The medium employed for the culturing of the
cells is conveniently a
defined enriched medium, such as IMDM (Iscove's Modified Dulbecco's Medium), a
50:50 mixture
of IMDM and RPMI, and will generally be composed of salts, amino acids,
vitamins, 5X 10-5 M 2-
ME (beta mercaptoethanol), streptomycin/penicillin, and 10% fetal calf serum.
The media is changed
from time to time, generally at least about once to twice per week. In one
procedure, by transferring
cells from one culture with hydrocortisone, to the other culture without
hydrocortisone, and
demonstrating the production of members of the different lineages in the
different cultures, the
presence of the stem cell and its maintenance is supported. In this manner,
the production of both
myeloid cells and B-cells is demonstrated.
In identifying myeloid and B-cell capability, the population to be tested is
introduced first
into a hydrocortisone containing culture and allowed to grow for six weeks in
such culture. In one
embodiment, the medium employed includes a 50:50 mixture of RPMI 1640 and IMDM
containing
10% FCS, 10% horse serum, streptomycin/penicillin, glutamine, and 5X 10:1 M
hydrocortisone.
During a six week period, in the absence of progenitor cells, all of the
mature cells would die.
However, if at the end of six weeks, myeloid cells are still observed, a
progenitor cell is present
which provides for the continuous differentiation to myeloid cells. At this
time, the medium is
changed, so that the medium now lacks hydrocortisone, an the growth of B-cells
is thus selected.
After 3-4 weeks the presence of B-cells is demonstrated by FACS analysis. If
the presence of B cells
is demonstrated, then the progenitor cells which are capable of producing
myeloid cells are also
capable of producing B-cells.
To demonstrate differentiation to T-cells, fetal thymus is isolated (e.g.
mouse fetal thymus,
human fetal thymus from abortion tissue, pig, monkey, sheep, cow, rat, or
rabbit fetal thymus) and
the thymus is cultured for 4-7 days at about 25°C in order to deplete
the lymphoid population of the
fetal thymus. The cells to be tested are then microinjected into the thymus
tissue, where the HLA of
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-22-
the population which is injected is mismatched with the HLA of the thymus
cells. The thymus tissue
may then be transplanted into a scid/scid mouse as described in EPA 0 322 240,
particularly
transplanting in the kidney capsule.
In order to determine if a cell can differentiate into red blood cells,
conventional techniques
can be used to identify BFU-E units, for example methylcellulose culture
(Metcalf (1977) In: Recent
Results in Cancer Research 61. Springer-Verlag, Berlin, pp 1-227). The
procedures demonstrate that
the cells are capable of developing the erythroid lineage.
Once stem cells have been isolated, they are propagated by growing in
conditioned medium
from stromal cells, such as stromal cells that can be obtained from bone
marrow, fetal thymus or fetal
liver, and are shown to provide for the secretion of growth factors associated
with stem cell
maintenance, coculturing with such stromal cells, or in medium comprising
maintenance factors
supporting the proliferation of stem cells. The stromal cells may be
allogeneic or xenogeneic. Before
using in the coculture, the mixed stromal cell preparations may be freed of
hematopoietic cells
employing appropriate monoclonal antibodies for removal of the undesired
cells, e.g, with antibody-
toxin conjugates, antibody and complement, etc. Alternatively, cloned stromal
cell lines may be used
where the stromal lines may be allogeneic or xenogeneic.
Stem cells obtained using these methods can be used in a variety of
therapeutic treatments.
In one embodiment, the cells are used to reconstitute fully an irradiated host
and/or a host subject to
chemotherapy. Stem cells can also be used as a source of cells for specific
lineages, by providing for
their maturation, proliferation and differentiation into one or more selected
lineages by employing a
variety of factors, such as erythropoietin, colony stimulating factors, e.g.,
GM-CSF, G-CSF, or M-
CSF, interleukins, e.g., IL-1, -2, -3, -4, -5, -6, -7, -8, etc., Leukemia
Inhibitory Factory (LIF), Steel
Factor (SLF), or the like. Alternatively stromal cells associated with the
stem cells becoming
committed to a particular lineage, or with their proliferation, maturation and
differentiation can be
employed. The stem cells may also be used in the isolation and evaluation of
factors associated with
the differentiation and maturation of hematopoietic cells. Thus, the stem
cells may be used in assays
to determine the activity of media, such as conditioned media, evaluate fluids
for cell growth activity,
involvement with dedication of particular lineages, or the like.
The stem cells can be used for the treatment of genetic diseases. Genetic
diseases associated
with hematopoietic cells may be treated by genetic modification of autologous
or allogeneic stem
cells to correct the genetic defect. For example, diseases such as B-
thalassemia, sickle cell anemia,
adenosine deaminase deficiency, recombinase deficiency, recombinase regulatory
gene deficiency,
etc. may be corrected by introduction of a wild-type gene into the stem cells,
either by homologous or
random recombination. With allogeneic stem cells, normal cells lacking the
genetic defect can be
used as a therapy. Other indications of gene therapy are introduction of drug
resistance genes to
enable normal stem cells to have an advantage and be subject to selective
pressure, e.g. the multiple
drug resistance gene (MDR). Diseases other than those associated with
hematopoietic cells may also
be treated, where the disease is related to the lack of a particular secreted
product such as a hormone,
enzyme, interferon, factor, or the like. By employing an appropriate
regulatory initiation region,
CA 02375527 2001-12-18
WO 00/78930 PCT/US00/17427
-23-
inducible production of the deficient protein may be achieved, so that
production of the protein will
parallel natural production, even though production will be in a different
cell type from the cell type
that normally produces such protein. It is also possible to insert a ribozyme,
antisense or other
message to inhibit particular gene products or susceptibility to diseases,
particularly
hematolymphotropic diseases.
Alternatively, in order to remove a particular variable region of a T-cell
receptor from the T-
cell repertoire, homologous recombination, or antisense or ribozyme sequence
which prevents
expression is used to inhibit expression of the particular T-cell receptor may
be inhibited. For
treatment with hematotropic pathogens, such as HIV, HTLV-I and II, etc. the
stem cells are
genetically modified to introduce an antisense sequence or ribozyme which
would prevent the
proliferation of the pathogen in the stem cell or cells differentiated from
the stem cells.
Methods for recombination in mammalian cells may be found in Molecular
Cloning, A
Laboratory Manual (1989) Sambrook, Fritsch and Maniatis, Cold Spring Harbor,
N.Y.
Stem cells obtained using a model system or a method of the invention may be
frozen at
liquid nitrogen temperatures and stored for long periods of time, being thawed
and capable of being
reused. The cells will usually be stored in 10% DMSO, 70% autologous plasma
(irradiated with
2500 rad), 20% Tc199 (Tissue culture medium). Cells are frozen in a
programmable cell freezer to -
180° C. in liquid nitrogen. Once thawed, the cells may be expanded by
use of growth factors
or stromal cells associated with stem cell proliferation and differentiation.
The hematopoietic stem cells, either autologous or allogeneic, may be used for
treatment of
various diseases where toxic therapies may be involved. For example, in the
treatment of neoplasia,
bone marrow may be removed from the patient (autologous) or from a "matched"
donor
("allogeneic") and the stem cells isolated and optimally frozen. The patient's
bone marrow may be
partially or wholly ablated using irradiation and/or chemotherapy. Once the
treatment is completed,
the stem cells may be thawed, if appropriate, administered to the patient by
any convenient means,
e.g., intravascularly, in a physiologically acceptable medium. The patient may
then be monitored for
signs of engraftment.
The stem cells may be grown in culture, whereby the stem cells may be
expanded. In this
way, one can repetitively administer stem cells during a course of a toxic
therapy. In addition, the
expanded stem cells can be used to study growth factors in culture, or can be
used to test a method of
introducing exogenous nucleic acid into stem cells.
EXAMPLE 12
Using Transplants of Vascular Tissue to Isolate Growth Factors
The transplantation of vascular tissue into immunocompromised subjects may
also be
used to isolation and evaluate factors associated with the growth,
differentiation and maturation of
hematopoietic cells. Thus, the grafts are harvested from the recipients, and
are then placed in culture
and used in assays to determine the activity of media, such as conditioned
media, evaluate fluids for
growth factor activity, involvement with dedication of lineages, or the like.
The subject grafts may
CA 02375527 2001-12-18
WO 00/78930 PCTNS00/17427
-24-
also be used in the identification of supportive cells for the isolation and
evaluation of factors
associated with the self renewal of hematopoietic cells. Thus, the model
system of the invention may
be used in assays to determine either autocrine or paracrine regulatory
signals and evaluate responses
to growth factor either from external or intrinsic protein sources; and to
determine the activity of
media, such as conditioned media, evaluate fluids for cell growth activity,
involvement with
dedication of particular lineages, or the like.
The grafted vascular tissue produces and/or is responsive to a factor that
permits migration
and replication of hematopoietic stem cells. This factor may be isolated from
biological samples
isolated from recipient subjects, by separating or fractionating (e.g.
chromatographically) a biological
fluid (e.g. blood), or by sampling, and extracting a biopsy sample.
Alternatively, a tissue sample (e.g.
a graft 14 days after transplantation) is harvested from a recipient and
cultured in vitro, and
conditioned media is collected. In one embodiment, the conditioned media or
biological sample is
fractionated. The active fraction containing the desired factor is identified
by measuring the growth
and differentiation of stem cells in the presence and absence of such
fractions, or alternatively, using
comparative analysis of fluid.
Thus, in a yet further embodiment, the invention provides for a stem cell
growth factor,
characterized as facilitating and/or promoting growth of a stem cell
population and/or protecting the
stem cell from the effects of lethal irradiation. This factor is further
characterized in that it is capable
of being isolated from a vascular graft or an extract thereof, such as a
supernatant form an in vitro
culture derived from a vascular graft. The factor thus isolated is preferably
in pure or substantially
pure form, e.g. at least 90%, preferably at least 95%, most preferably at
least 99% pure form
A factor that protects normal stem cells from lethal irradiation may have a
variety of
clinical applications (e.g. to rescue the marrow of humans exposed to lethal
irradiation, or to treat
pateints with hematologic malignancies, such as by protecting normal stem
cells while abnormal cells
are removed).
Having illustrated and described the principles of using vascular tissue to
enhance
hematopoeisis in a subject, the art of the invention can be modified in
arrangement and detail without
departing from such principles. In view of the many possible embodiments to
which the principles of
my invention can be applied, it should be recognized that the illustrated
embodiments are only
examples of the invention and should not be taken as a limitation on the scope
of the invention.
Rather, the scope of the invention is in accord with the following claims. I
therefore claim as my
invention all that comes within the scope and spirit of these claims.