Note: Descriptions are shown in the official language in which they were submitted.
CA 02375719 2002-05-10
GLASS FIBER COMPOSITION
Background of the invention
1. Field of the Invention
The present invention relates to glass compositions for making glass
fibers; and more particularly to glass compositions having lowered liquidus
and forming temperatures.
2. Technical Considerations
The most common glass composition for making continuous glass fiber
strands for textiles and glass fiber reinforcements is "E" glass. The
requirements as to what type of composition constitutes an E-glass
composition are included in ASTM D578-98. An advantage of using E-glass
is that its liquidus temperature is well below its forming temperature, i.e.
typically greater than 100°F (56°C) and generally between
1'50°F (83°C} to
200°F (711 °C}. As used herein, the terms "forming temperature"
and "TFORM"
mean the temperature of the,glass at which the viscosity of the glass is log
3,
or 1000 poise, and the terms "liquidus temperature" and "T~,Q" mean the
temperature at which solid phase (crystals) and liquid phase (melt) are in
equilibrium. The difference between TFORnn and TL,Q, referred to herein as
"delta T" or "OT", is a common measure of the crystallization potential of a
given melt composition. In the glass fiber forming industry, DT is typically
maintained at a temperature of at least 90°F (50°C) in order to
prevent
devitrification of the molten glass in the bushing area of a glass fiber
forming
operation.
Boron and fluorine containing glass were developed to meet these
operating conditions. More specifically, the boron and fluorine were included
CA 02375719 2002-05-10
-2-
in the glass batch materials to act as fluxes during the glass melting
operation: However; these materials are volatilized during melting and boron
and fluorine emissions are released to the atmosphere. Since boron and
fluorine are considered pollutants, these emissions are closely controlled by
environmental regulations, which; in turn, requires careful control of the
furnace operations and the use of expensive pollution control equipment. tn
response to this, low boron and/or low fluorine E-glasses were developed. As
used herein, "low boron" means that the glass composition is no greater than
5 weight percent boron, and preferably boron-free and "low fluorine"means
that the glass composition is no greater than 1 weight percent fluorine, and
preferably is fluorine-free.
For example, U.S. Patent No. 3,929,497 disctoses a boron-tree and
fluorine-free glass composition containing titanium dioxide in the range of
0.5
to 5 percent by weight and Fe2G3 in the range of 5 to 15 percent by weight.
U.S: Patent No. 4,199,364 discloses a boron-free and fluorine-free
glass composition that contains Li20 in the range of 0.1 to 1.5 percent by
weight and may also include barium oxide. The liquidus temperature of the
compositions is over 2200°F.
U.S. Patent No. 4,542,106 discloses a boron-free and fluorine-free
glass composition that contains 1 to 5 percent by weight Ti02. The fibers also
have a seed count of 5 seeds or less per cubic centimeter of glass and an
electrical leakage value of 2.8 nanoamperes or less.
U.S. Patent No. 5,789,329 discloses a boron-free and fluorine-free
glass composition that contains up to 0.9 percent by weight Ti02 and has a
DT of at least 100°F (56°C).
For additional information concerning glass compositions and methods
for fiberizing the glass composition, see K: Loewenstein, The Manufacturina
Technolo4y of Continuous Glass Fibres, (3d Ed. 1993) at pages 30-44, 47-60,
115-122 and 126-135, and F. T. Walienberger (editor), Advanced inorganic
CA 02375719 2002-05-10
-3-
Fibers: Processes. Structures, Properties. Replications, (2000) at pages 81-
102 and 129-168.
Because the actual fiber forming operation is conducted at high
temperatures, there are high energy costs associated with its production. in
addition, the high temperatures accelerate the degradation of the refractory
used in the glass melting furnace as well as the bushings used to form the
fbers. The bushings include precious metals that cannot be recovered from
the glass as the bushings corrode. It would be advantageous to produce the
glass fibers at the lowest possible forming and liquidus temperatures so as to
reduce the energy costs and thermal load on the furnace refractory and
bushings, while at the same time provide the DT required to ensure an
uninterrupted glass fiber forming, operation. Reducing the forming and
liquidus temperatures of the glass compositions can also result in
environmental benefits, such as but not limited to, a reduction in the amount
of fuel required to generate the energy necessary for the fiber forming
operation, as well as a reduction in the flue gas temperature. In addition,'
it
would be advantageous if the glass compositions are !ow fluoride andlor low
boron compositions, and preferably are fluorine-free arydlor boron-free, so as
to reduce or eliminate the environmental pollutants associated with these
materials.
Summary of the invention
The present innovation provides a glass fiber composition comprising:
52 to 62 percent by weight Si02, '0 to 2 percent by weight Na20, 16 to 25
percent by weight CaO, 8 to 16 percent by weight A1203, 0.05 to 0.80 percent
by weight Fe203, 0 to 2 percent by weight K20, 1.7 to 2.9 percent by weight
MgO; O to 10 percent by weight B203, 0 to 2 percent by weight Ti02, 0 to 2
percent by weight BaO, 0 to 2 percent by weight Zr02, and'0 to 2 percent by
weight SrO, wherein the glass composition has a forming.temperature of no
CA 02375719 2002-05-10
-4-
greater than 2280°F based on an NIST 714 reference standard and a
liquidus
temperature of no greater than 2155°F. In one noniirniting embodiment
of the
invention, the glass fiber composition . further includes at least one
material
selected from the group consisting of: 0.05 to 1.5 percent by weight Li20,
'0.05
to 1.5 percent by weight ZnO, 0:05 to 3 percent by weight MnO, and 0.05 to 3
percent by weight Mn02.
The present invention also provides a glass fiber composition -
consisting essentially of: 52 to 62 percent by weight Si02, 0 to 2 percent by
weight Na20, 16 to 25 percent by weight CaO, 8 to 16 percent by weight
9 0 A1203, 0.05 to 0.80 percent by weight Fe203, O to 2 percent by weight KZO;
2.2
to 2.9 percent by weight MgO, 0 to 10 percent by weight 823, 0 to 2 percent
by weight Ti02, 0 to 2 percent by weight BaO, 0 to 2 percent by weight Zr02,
and O to 2 percent by weight SrO, wherein the glass composition has a
forming temperature of no greater than 2280°F based on an N1ST 714
reference standard and a liquidus temperature of no greater than
2155°F.
The present innovation provides a glass fiber composition comprising:
52 to 62 percent by weight Si02, 0 to 2 percent by weight Na20, 1'6 to 25
percent by weight CaO, 8 to 16 percent by weight AI203, 0.05 to 0.80 percent
by weight Fe203; 0 to 2 percent by weight K20, 1.7 to 2.6 percent by weight
MgO, 0 to 10 percent by weight B203, 0 to 2 percent by weight Ti02, 0 to..2
percent by weight BaO, 0 to 2 percent by weight Zr02; and 0 to 2 percent ;by
weight SrO, and further including at least one material selected from the
group consisting of: 0.05 to 1.5 percent by weight Li20, 0.05 to 1.5 percent
by
weight ZnO, 0.05 to 3 percent by weight MnO, and 0.05 to 3 percent by
weight Mn02, wherein the glass composition has a forming temperature of no
greater than 2280°F based on an N)ST 714 reference standard and a
liquidus
temperature of no greater than 2155°F. In one nonlimiting embodiment of
the
invention, the Si02 content is 57 to 59 percent by weight, the Na2~ content is
up to 1 percent by weight, the Ca0 content is 22 to 24 percent by weight, the
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
_5_
A1203 content is 12 to 14 percent by weight, the Fe203 content is up to 0.4
percent by weight, and the K20 content is up to 0.1 percent by weight, and
the composition includes at least one material selected from the group
consisting of: 0.2 to 1 percent by weight LizO, 0.2 to 1 percent by weight
ZnO,
up to 1 percent by weight MnO, and up to 1 percent by weight Mn02.
Brief Description of the Figure
Figures 1 and 2 are graphs showing the relation between the amount
of Mg0 and the liquidus temperature for glass fiber compositions of the
present invention.
Detailed Description of the Invention
The base composition for the glass fibers of the present invention
suitable for textiles and glass fiber reinforcements includes the following
main
constituents in weight percent (wt%) based on the total weight of the final
glass composition.
broad range preferred range most preferred range
Si02 (wt%)52 to 62 55 to 61 57 to 59
Na20 (wt%)0 to 2 up to 1.5 up to 1
Ca0 (wt%) 16 to 25 20 to 25 22 to 24
AI203 (wt%)8 to 16 11 to 14 12 to 14
Fe203 (wt%)0.05 to 0.80up to 0.5 up to 0.4
K20 (wt%) 0 to 2 up to 1 up to 0.1
It should be appreciated that numerical values discussed herein, such
as but not limited to weight percent of materials, length of time or
temperatures, are approximate and are subject to variations due to various
factors well known to those skilled in the art such as, but not limited to,
measurement standards, equipment and techniques. For example, where it
states in the present application that the range for Si02 is 52 to 62 weight
percent, this range is about 52 to about 62 weight percent, and where it
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-6-
states that the forming temperature of a glass composition should be no
greater than 2280°F ( 1249°C), the temperature is about
2280°F.
Mg0 is also a main constituent of the glass compositions of the
present invention. It has been found that the heating and melting profile of a
glass fiber composition, and in particular the liquidus temperature, can be
controlled and in particular optimized by controlling the amount of MgO, which
an object of the present invention and will be discussed later in more detail.
Oftentimes additional materials are added to the glass composition to
modify the melt properties of the glass. For example, and without limiting the
present invention, Li20, Zn02, Mn0 and Mn02 can be added to the glass fiber
composition to reduce T~,Q. In one nonlimiting embodiment of the glass fibers
of the present invention, the glass composition can include one or more of
these materials in the following amounts.
broad range preferred range
Li20 (wt%) 0.05 to 1.5 0.2 to 1
Zn0 (wt%) 0.05 to 1.5 0.2 to 1
Mn0 (wt%) 0.05 to 3 up to 1
Mn02 (wt%) 0.05 to 3 up to 1
It is believed that levels of these materials less than 0.05 wt% would be
considered either tramp amounts or so low that they will not materially impact
the glass melt properties.
Boron is another material that can be added to glass fiber
compositions to reduce T~,Q. However, as discussed earlier, the inclusion of
boron results in the production of particulates that, depending on the
particulate level, may have to be removed from a melting furnace exhaust
stream before being released into the environment. Although the amount of
B203 in a glass fiber composition can be as high as 10 wt%, one nonlimiting
embodiment of a glass composition of the present invention includes no
greater than 3 wt% Bz03, preferably no greater than 2 wt% BZ03, and more
CA 02375719 2002-05-10
preferably no greater than 1 wt% B203. fn another nonlimiting embodiment of
the invention, the glass composition is essentially boron-free, i.e. it
includes
no more than a trace amount of B203, which is considered herein to be up to
0.05 wt% B203, and preferably does not include any B203.
It should be appreciated that glass fiber compositions can include other
constituents and the present invention contemplates the inclusion of other
materials in the glass fiber compositions, such as but not limited to, 0 to
2 wt% each of Ti02, BaO, Zr02 and SrO.
1n addition, because of the environmental concerns discussed earlier,
although not limiting in the present invention, the glass compositions are
preferably low fluorine compositions, i.e. no greater than 0.30 wt% fluorine,
and more preferably are fluorine-free; i.e. it includes no more than a trace
amount of fluorine, which is considered herein to be up to 0.05 wt% fluorine,
and preferably does not include any fluorine.
It should be appreciated that the glass compositions disclosed herein
can also include small amounts of other materials, for example melting and
refining aids, tramp materials or impurities. For example and without limiting
the present invention, melting and fining aids, such as S03, are useful
during production of the glass, but their residual amounts in the glass can
20,. vary and have no material effect on the properties of the glass product.
In
addition, small amounts of the additives discussed above can enter the glass
composition as tramp materials or impurities included in the raw materials of
the main constituents.
Commercial glass fibers of the present invention can be prepared in
the conventional manner well known in the art, by blending the raw materials
used to supply the specific oxides that form the composition ofithe fibers.
For
example, typically sand is used for Si02, clay for AI203, lime or limestone
for
CaO, and dolomite for MgO and some of the CaO. As discussed earlier, the
glass can include other additives that are added to modify the glass
CA 02375719 2002-05-10
properties as well as small amounts of melting and refining aids, tramp
materials or impurities.
After the ingredients are mixed in the proper proportions to provide the
desired weight of each constituent for the: desired glass, the batch is melfed
in
a conventional glass fiber melting furnace and the resulting molten glass is
passed along a conventional forehearth and into a glass fiber forming bushing
located along the bottom of the forefiearth; as is weft known to those skilled
in
the art. During the glass melting phase, the glass is typically heated to a
temperature of at least 2550°F (1400°C). The molten glass is
then drawn or
pulled through a plurality of holes in the bottom of the bushing. The streams
of molten glass are attenuated to filaments by winding a strand of filaments
on a. forming tube mounted on a rotatable collet of a winding machine.
Alternatively, the fiber forming apparatus can be, for example, a forming
device for synthetic textile fibers or strands in which fibers are drawn from
nozzles, such as but not limited to a spinneret, as is known to those skilled
in
the art. Typical forehearths and glass fiber forming arrangements are shown:
in K. Loewenstein, The Manufacturin4 Technology of Continuous Glass
Fibres, (Third Edition 1993) at pages 85-107 and pages 115-135.
Tables 1-7 show laboratory examples of glass fiber compositions that
illustrate the effect of Mg0 on the liquidus temperature of the glass
compositions. Boron and fluorine were not included in these compositions.
The gtass fiber compositions shown in Tables 1-7 were prepared from
reagent grade oxides (e.g.; pure silica or calcia). The batch size for each
example was 1000 grams. The individual batch ingredients were weighed
out, combined and placed in a tightly sealed jar. The sealed jar was then
placed in a paint shaker for 15 minutes to effectively mix the ingredients. A
portion of the batch was then place into a platinum crucible, filling no more
than 314 of its volume. The crucible was then placed in a furnace and heated
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
_g_
to 2600°F (1425°C) for 15 minutes. The remaining batch was then
added to
the hot crucible and heated to 2600°F (1425°C) for 15 to 30
minutes. The
furnace temperature was then raised to 2700°F (1482°C) and held
there for 4
hours. The molten glass was then fritted in water and dried. The forming
temperature, i.e. the glass temperature at a viscosity of 1000 poise, was
determined by ASTM method C965-81, and the liquidus temperature by
ASTM method C829-81.
The weight percent of the constituents of the compositions shown in
Tables 1-7 are based on the weight percent of each constituent in the batch.
It is believed that the batch weight percent is generally about the same as
the
weight percent of the melted sample, except for glass batch materials that
volatilize during melting, e.g. boron and fluorine. For boron, it is believed
that
the weight percent of B203 in a laboratory samples will be 5 to 10 percent
less
than the weight percent of B203 in the batch composition. For fluorine, it is
believed that the weight percent of fluorine in a lab melt will be about 50
percent less than the weight percent of fluorine in the batch composition. It
is
further believed that glass fiber compositions made from commercial grade
materials and melted under conventional operating conditions will have
similar batch and melt weight percents as discussed above, except that the
batch and melt weight percents for the volatile components of the composition
will actually be closer to each other than the batch and melt wt% of the
laboratory melts because in a conventional melting operation, the materials
are exposed to the high melting temperatures for less time than the 4 hours of
exposure for the laboratory melts.
Determination of the high temperature viscosity, TFORM, was based on
the glass samples being compared against physical standards supplied by
the National Institute of Standards and Testing (NIST). In Tables 1-7, TFoRnn
is
reported based on comparison to either NIST 717A, which is a borosilicate
glass standard, or NIST 714, which is a soda lime glass standard. Although
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-10~
either standard can be used, it is believed that the NIST 714 reference
standard is more reliable because it has been observed that the NIST
borosilicate standard 717A deteriorates at temperatures greater than
2150°F
(1177°C). In comparing TFORM based on the two different standards,
generally
TFORM based on NIST 714 is 20°F to 25°F (11°C to
16°C) higher than TFORM
based on NIST 717A. The T~,o is unaffected by the NIST standard.
Examples 1-7 in Table 1 show the change in the liquidus temperature
of a typical glass fiber forming composition that further includes 1 wt% Ti02,
as the amount of Mg0 is varied from 1.82 to 3 wt%. Examples 8-14 in
Table 2 and Examples 15-30 in Table 3 show the change in the liquidus
temperature of a typical glass fiber forming composition that further includes
1.5 or 1.1 wt% Ti02, respectively, and 0.90 wt% Li20, as the amount of Mg0
is varied from 1.7 to 3.5 wt%. Examples 31-38 in Table 4 show the change in
the liquidus temperature of a typical glass fiber forming composition that
further includes 0.5 wt% Ti02 and 0.90 wt% LizO, as the amount of Mg0 is
varied from 1.7 to 3.12 wt%. Examples 39-49 in Table 5 show the change in
the liquidus temperature of a typical glass fiber forming composition that
further includes 1.1 wt% TiOz, 0.45 wt% Li20 and 0.45 wt% ZnO, as the
amount of Mg0 is varied from 1.7 to 3.1 wt%. Examples 47-54 in Table 6
show the change in the liquidus temperature of a typical glass fiber forming
composition that further includes 1.1 wt% Ti02 and 0.90 wt% ZnO, as the
amount of Mg0 is varied from 1.7 to 3.1 wt%. Examples 59-66 in Table 7
show the change in the liquidus temperature of a typical glass fiber forming
composition that further includes 1.1 wt% Ti02, 0.30 wt% Na20, 0.60 wt%
Li20 and 0.25 wt% Fe203 as the amount of Mg0 is varied from 1.7 to 3.1 wt%.
In addition, the forming temperatures for selected glass compositions are also
included in Tables 1-7.
CA 02375719 2001-11-28
WO 00/73231 P~T/US00/14155
-11-
Tables 1-7 also include the ratio SiO2/RO, which is the ratio of the silica
content of the batch, expressed as SiOZ, to the sum of the calcia and
magnesia content, expressed as Ca0 and MgO, respectively.
TABLE 1
Ex.1 Ex.2* Ex.3 Ex.4 Ex.S Ex.6 Ex.7
SiOz (wt%)59.83 59.61 60.13 60.43 60.43 59.61 59.61
AIz03 12.21 12.16 12.27 12.39 12.33 12.16 12.16
(wt%)
Ca0 (wt%)22.80 23.51 22.92 23.14 23.03 24.31 24.31
Mg0 (wt%)3.00 2.62 2.50 2.00 2.00 1.82 1.82
Ti02 (wt%)1.00 1.00 1.00 1.00 1.00 1.00 1.00
Na20 (wt%)0.96 0.90 0.98 1.01 1.01 0.90 0.90
Fe203 0.20 0.20 0.20 0.20 0.20 0.20 0.20
(wt%)
SiOz/RO 2.32 2.28 2.37 2.40 2.41 2.28 2.28
TFORM 2265 2309 2278
(F)
NIST 714
TuQ(F) 2160 2138 2127 2149 2154 2161 2182
4T (F) 127 182 94
* average of five samples
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
- 12-
TABLE 2
Ex.8 Ex.9 Ex.10 Ex.11 Ex.12 Ex.13 Ex.14
Si02 (wt%)59.62 59.30 59.97 60.09 60.21 60.33 60.75
AI203 (wt%)12.12 12.10 12.19 12.22 12.24 12.24 12.35
Ca0 (wt%) 22.12 22.60 22.25 22.30 22.34 22.39 22.55
Mg0 (wt%) 3.50 3.40 2.90 2.70 2.50 2.30 1.70
Ti02 (wt%)1.50 1.50 1.50 1.50 1.50 1.50 1.50
Li20 (wt%)0.90 0.90 0.90 0.90 0.90 0.90 0.90
Fe203 (wt%)0.25 0.20 0.25 0.25 0.25 0.25 0.25
Si02/RO 2.33 2.28 2.38 2.40 2.42 2.44 2.51
NIST 717A
TFORM(F) 2196 2197 2192 2196 2223 2239
T~,Q (F) 2158 2136 2122 2152 2124 2120 2131
4T (F) 38 75 40 72 103 108
NIST 714
TFORM(F) 2219 2223 2215 2219 2248 2264
T~,Q (F) 2158 2136 2122 2152 2124 2120 2131
0T (F) 61 101 63 95 128 133
TABLE 3
Ex.15 Ex.16 Ex.17 Ex.18 Ex.19 Ex.20 Ex.21 Ex.23
Si02 (wt%)59.61 59.61 59.73 59.85 59.97 60.09 60.10 60.10
AI203 (wt%)12.92 12.92 12.92 12.95 12.97 13.00 13.00 13.00
Ca0 (wt%) 21.91 21.96 22.00 22.04 22.09 22.13 22.15 22.15
Mg0 (wt%) 3.50 3.30 3.10 2.90 2.70 2.50 2.50 2.50
Ti02 (wt%)1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10
Li20 (wt%)0.90 0.90 0.90 0.90 0.90 0.90 0.9 0.9
Fe203 (wt%)0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25
SiOz/RO 2.35 2.36 2.38 2.40 2.42 2.44 2.44 2.44
NIST 717A
TFORM (
F)
T~,q (F) 2154 2138 2127 2122 2120 2111
4T (F)
NIST 714
TFORM(F) 2215 2215 2217 2217 2226 2233 2255 2259
T~,o(F) 2154 2138 2127 2122 2120 2111 2070 2077
~
4T (F) 61 77 90 95 106 122 185 182
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
- 13-
TABLE 3 (cont.)
Ex.23 Ex.24 Ex.25 Ex.26 Ex.27 Ex.28 Ex.29 Ex.30
Si02 (wt%)60.21 60.00 60.33 59.95 60.00 59.90 60.57 59.85
AI203 13.02 12.40 13.05 12.40 12.50 12.40 13.10 12.0
(wt%)
Ca0 (wt%)22.18 22.05 22.22 23.30 23.70 23.55 22.31 23.80
Mg0 (wt%)2.30 2.30 2.10 2.10 1.90 1.90 1.70 1.70
TiOz (wt%)1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10
LizO (wt%)0.90 0.90 0.90 0.90 0.90 0.90 0.90 0.90
Fez03 0.25 0.25 0.25 0.25 0.25 0.25 0.26 0.25
(wt%)
Si02/RO 2.46 2.46 2.48 2.36 2.34 2.35 2.52 2.35
NIST 717A
TFORM(F) 2226 2221 2273 2223 2237 2235 2210
T~,Q(F) 2088 2095 2077 2106 2082 2120 2086 2113
DT (F) 138 126 196 117 155 149 97
NIST 714
TFORM(F) 2251 2246 2296 2248 2262 2235 2262 2235
T~,Q(F) 2088 2095 2077 2106 2082 2113 2086 2113
4T(F) 163 151 219 142 180 122 176 122
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
TABLE 4
Ex.31Ex.32 Ex.33Ex.34 Ex.35 Ex.36Ex.37 Ex.38
Si02 (wt%)60.0559.97 60.0960.21 60.23 60.2360.33 60.76
AI203 12.9812.19 12.2212.24 12.25 12.2512.27 12.35
(wt%)
Ca0 (wt%)22.1423.56 23.3123.35 23.36 23.3623.40 23.56
Mg0 (wt%)3.12 2.90 2.70 2.50 2.50 2.50 2.30 1.70
Ti02 (wt%)0.55 0.50 0.50 0.50 0.51 0.51 0.50 0.50
Li20 (wt%)0.91 0.90 0.90 0.90 0.9 0.9 0.90 0.90
Fe203 0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25
(wt%)
SiOz/RO 2.38 2.27 2.31 2.33 2.33 2.33 2.35 2.41
NIST 717A
TFORM(F) 2264 2181 2197 2192 2196 2201
T~,Q(F) 2136 2138 2125 2124 2120 2116 2131 2149
~T (F) 128 43 72 68 65 52
NIST 714
TFORM(F) 2205 2223 2215 2228 2235 2221 2226
T~,o(F) 2136 2138 2125 2124 2120 2116 2131 2149
OT (F) 68 98 91 108 119 90 77
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-15-
TABLE 5
Ex.39 Ex.40Ex.41Ex.42 Ex.43Ex.44 Ex.45Ex.46Ex.47 Ex.48Ex.49
SiO~ (wt%)59.73 59.8559.9760.09 60.2160.33 59.5460.4559.47 60.5759.40
AIz03 12.92 12.9512.9713.00 13.0213.05 12.1613.0812.16 13.1012.16
(wt%)
Ca0 (wt%)22.00 22.0422.0922.13 22.18~ 22.2223.9522.2724.22 22.3124.49
Mg0 (wt%)3.10 2.90 2.70 2.50 2.30 2.10 2.10 1.90 1.90 1.70 1.70
Ti02 (wt%)1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10
Li20 (wt%)0.45 0.45 0.45 0.45 0.45 0.45 0.45 0.45 0.45 0.45 0.45
Zn0 (wt%)0.45 0.45 0.45 0.45 0.45 0.45 0.45 0.45 I 0.450.45 ~
0.45
Fe~03 0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25
(wt%) ~ I ~ I I
i
SiOz/RO 2.38 2.40 2.42 2.44 2.46 2.48 2.29 2.50 2.28 2.52 2.27
I I ~ ~ I
NIST 717A
TFORM 2242 2248 2250 2259 2257 2273 2241 2273 2278 2248
(F)
T~,Q(F) 2142 2134 2109 2097 2091 2084 2111 2082 2118 2075 2134
OT(F) 100 114 141 162 166 189 130 191 203 114
I
NIST 714
, TFORM(F)2268 2275 2275 2284 2284 2300 2266 2300 2224 2305 2273
I
T~,o(F) 2142 2134 2109 2097 2091 2084 2111 2082 21 2075 2134
8
0T(F) 124 141 166 187 193 216 155 218 106 230 139
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-16-
TABLE 6
Ex.50 Ex.51 Ex.52 Ex.53 Ex.54 Ex.55 Ex.56 Ex.57 Ex.58
Si02 (wt%)59.73 59.85 59.97 58.80 60.09 60.21 60.33 60.45 60.57
AIz03 (wt%)12.92 12.95 12.97 13.33 13.00 13.02 13.05 13.08 13.10
Ca0 (wt%) 22.00 22.04 22.09 23.45 22.13 22.18 22.22 22.27 22.31
Mg0 (wt%) 3.10 2.90 2.70 2.50 2.50 2.30 2.10 1.90 1.70
Ti02 (wt%)1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10
Zn0 (wt%) 0.90 0.90 0.90 0.9 0.90 0.90 0.90 0.90 0.90
Fe203 (wt%)0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25
Si02/RO 2.38 2.40 2.42 2.27. 2.44 2.46 2.48 2.50 2.52
NIST 717A
TFORM(F) 2282 2286 2296 2287 2304 2296 2307 2316 2298
TuQ(F) 2138 2131 2118 2129 2115 2131 2136 2128 2140
4T(F) 144 155 178 158 189 165 171 178 158
NIST 714
TFORM(F) 2309 2313 2323 2314 2332 2323 2336 2345 2327
TuQ(F) 2138 2131 2118 2129 2115 2131 2136 2138 2140
~T(F) 171 182 205 185 217 192 200 207 187
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
_17_
TABLE 7
Ex.59 Ex.60 I Ex.61Ex.62 Ex.63 Ex.64 Ex.65 Ex.66
Si02 (wt%)59.80 59.75 59.70 59.65 59.60 59.55 59.50 59.45
AI203 (wt%)12.25 12.25 12.25 12.25 12.25 12.25 12.25 12.25
Ca0 (wt%) 22.69 22.85 22.85 23.35 23.60 23.85 24.10 24.35
Mg0 (wt%) 3.10 2.90 2.70 2.50 2.30 2.10 1.90 1.70
Ti02 (wt%)1.10 1.10 1.10 1.10 1.10 1.10 1.10 1.10
NazO (wt%)0.30 0.30 0.30 0.30 0.30 0.30 0.30 0.30
Li20 (wt%)0.60 0.60 0.60 0.60 0.60 0.60 0.60 0.60
Fe203 (wt%)0.25 0.25 0.25 0.25 0.25 0.25 0.25 0.25
Si02/RO 2.33 2.32 2.34 2.31 2.30 2.29 2.29 2.28
NIST 717A
TFORM(F) 2239 2242 2232 2235 2228 2228 2228
T~,(F) 2113 2113 2111 2118 2133 2143 2158 2178
4T (F) 126 131 113 103 85 70 50
NIST 714
TFORM(F) 2264 2257 2268 2257 2260 2253 2253 2253
T~,(F) 2113 2113 2111 2118 2133 2143 2158 2178
4T (F) 151 144 157 139 127 110 95 75
Figures 1 and 2 present curves illustrating the relationship between
the forming temperature of the compositions shown in Tables 1-7 versus
the amount of MgO, as discussed below in more detail. These curves are
2nd order polynomial curves generated using Microsoft~ Excel 97 SR-2(f).
Each of the curves shows that the amount of Mg0 impacts the liquidus
temperature and in particular, there is a eutectic, i.e. minimum, in the
liquidus
temperature vs. the amount of MgO, indicating that the amount of Mg0 can
be controlled to generate a minimum liquidus temperature for a glass fiber
forming composition.
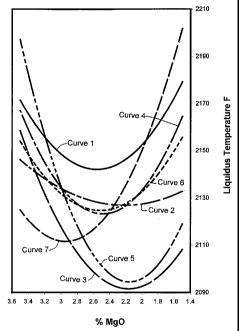
More specifically, Curve 1 in Figure 1 illustrates the relationship
between the liquidus temperature and the amount of Mg0 in the glass
compositions shown in Examples 1-7 in Table 1. In order to expand the Mg0
range to 3.5 weight percent, a control composition was also included in the
curve. The control composition included 59.37 wt% Si02, 12.94 wt% AI203,
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-18-
21.00 wt% CaO, 3.5 wt% MgO, 1.42 wt% Ti02, 1.01 wt% Na0 and 0.22 wt%
Fe203, and had a TL,Q of 2158°F. As can be seen, based on this
curve, the
liquidus temperature approaches a minimum in the range of 2.2 to 2.9 wt%
MgO, and reaches a minimum temperature at 2.45 to 2.65 wt% MgO.
Curve 2 in Figure 1 illustrates the relationship between the liquidus
temperature and the amount of Mg0 in the glass compositions shown in
Examples 8-14 in Table 2. As can be seen, based on this curve, the liquidus
temperature approaches a minimum in the range of 1.85 to 2.6 wt% MgO,
and reaches a minimum temperature at 2.0 to 2.45 wt% MgO.
Curve 3 in Figure 1 illustrates the relationship between the liquidus
temperature and the amount of Mg0 in the glass compositions shown in
Examples 15-30 in Table 3. As can be seen, based on this curve, the liquidus
temperature approaches a minimum in the range of 1.8 to 2.5 wt% MgO, and
reaches a minimum temperature at 2.0 to 2.3 wt% MgO.
Curve 4 in Figure 1 illustrates the relationship between the liquidus
temperature and the amount of Mg0 in the glass compositions shown in
Examples 31-38 in Table 4. As can be seen, based on this curve, the liquidus
temperature approaches a minimum in the range of 2.3 to 2.7 wt% MgO, and
reaches a minimum temperature at 2.35 to 2.6 wt% MgO.
Curve 5 in Figure 1 illustrates the relationship between the liquidus
temperature and the amount of Mg0 in the glass compositions shown in
Examples 39-49 in Table 5. As can be seen, based on this curve, the liquidus
temperature approaches a minimum in the range of 1.8 to 2.5 wt% MgO, and
reaches a minimum temperature at 2.0 to 2.3 wt% MgO.
Curve 6 in Figure 1 illustrates the relationship between the liquidus
temperature and the amount of Mg0 in the glass compositions shown in
Examples 50-58 in Table 6. As can be seen, based on this curve, the liquidus
temperature approaches a minimum in the range of 2.3 to 2.7 wt% MgO, and
reaches a minimum temperature at 2.4 to 2.6 wt% MgO.
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
_19_
Curve 7 in Figure 1 illustrates the relationship between the liquidus
temperature and the amount of Mg0 in the glass compositions shown in
Examples 59-66 in Table 7. The control sample discussed above was
incorporated into Curve 7. As can be seen, based on this curve, the liquidus
temperature approaches a minimum in the range of 2.7 to 3.2 wt% MgO, and
reaches a minimum temperature at 2.8 to 3.1 wt% MgO.
As can be seen in Curves 1-7, the amount of Mg0 impacts the liquidus
temperature and in particular, the amount of Mg0 can be controlled to
generate a minimum liquidus temperature for a glass fiber forming
composition.
Figure 2 Illustrates the relationship between the liquidus temperature
and the amount of Mg0 for various combinations of Examples 8-58 in Tables
2-6. More specifically, Curve A plots the liquidus temperature versus the
amount of Mg0 for the glass compositions shown in Tables 2, 3, and 4,
Curve B plots the liquidus temperature versus the amount of Mg0 for the
glass compositions shown in Tables 3, 5 and 6, and Curve C plots the
liquidus temperature versus the amount of Mg0 for the Glass compositions
shown in Tables 2-6. It is appreciated that Curves A, ~i and C combine the
liquidus temperature for different glass compositions. More specifically, the
glass compositions represented by Curve A have the same Li20 level but
differ in the amount of Ti02, the glass compositions represented by Curve B
have the same amount of Ti02 but differ in the amount of LizO and Zn0
(although the total amount of Li20+Zn0 is the same), and the glass
compositions represented by Curve C differ in the amounts of Ti02, Li20
and/or ZnO. However, these combinations are offered to illustrate the trend
in the liquidus temperature as the amount of Mg0 varies.
Referring to Figure 2, it can be seen that the liquidus temperature for
Curves A, B and C approaches a minimum in the range of 1.7 to 2.65 wt%
MgO, and reaches a minimum temperature at 1.90 to 2.55 wt% MgO.
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-20-
The fact that the glass compositions of Tables 2-6 exhibit a minimum
liquidus temperature (as shown in Curves 2-6, A, B and C) than the glass
compositions of Table 1 (as shown in Curve 1 ) is to be expected since
Examples 8-58 in Tables 2-6 all included additives, and in particular, LiZO
and/or ZnO, which reduce liquidus temperature. However, of particular
significance is the fact that the minimum liquidus temperatures for the glass
compositions of Tables 2-6 are generally within an Mg0 range lower than that
of the glass compositions of Table 1.
In viewing Curves 1-7 and A-C in Figures 1 and 2, it is clear that the
amount of Mg0 impacts the heating and melting profile of a glass fiber
forming compositions, and in particular, the Mg0 content can be used to
minimize the liquidus temperature of a glass fiber forming composition and
allow for a lower forming temperature while maintaining the OT required to
facilitate a continuous and uninterrupted glass fiber forming operation.
Examples 67-98 in Table 8 are additional examples of glass
compositions of the present invention having 2.3 to 2.55 wt% Mg0 and a OT
of greater than 90°F based on a NIST 714 reference standard. These
laboratory samples include up to 3 wt% B203, up to 0.9 wt% Na20, up to 1.1
wt% Ti02, up to 0.9 wt% Li20, up to 1 wt% ZnO, up to 3 wt% MnO, and up to
3 wt% Mn02 based on their batch composition, as discussed earlier. The
samples were made in the same manner as those in Tables 1-7.
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-21 -
TABLE 8
Ex.67 Ex.68**Ex.69 Ex.70 Ex.71 Ex.72 Ex.73 Ex.74
Si02 (wt%)60.12 59.61 59.11 58.61 60.12 59.01 59.31 59.61
AIz03 13.00 12.16 12.16 12.16 13.00 12.04 12.10 12.16
(wt%)
Ca0 (wt%)21.13 23.50 23.00 23.50 21.13 23.27 23.38 23.50
Mg0 (wt%)2.50 2.50 2.50 2.50 2.50 2.48 2.49 2.50
Ti02 (wt%)1.10 1.10 1.10 1.10 1.10 1.09 1.09 1.10
8203 (wt%)1.00 0.30 1.00 1.00 1.00 1.00 0.50 0.50
Na20 (wt%) 0.89 0.89
Li20 (wt%) 0.20 0.90 0.90 0.90 0.50
Zn0 (wt%)0.90 0.20
Mn0 (wt%) 0.20
Mn02 (wt%)
Fe203 0.25 0.23 0.23 0.23 0.25 0.23 0.23 0.23
(wt%)
Si02/RO 2.54 2.29 2.32 2.25 2.54 2.29 2.29 2.29
NIST 717A
TFORM(F) 2235 2247 2178 2172 2318 2260 2255 2221
T~,Q(F) 2082 2099 2088 2088 2172 2152 2154 2111
0T (F) 153 148 90 79 146 108 101 110
NIST 714
TFORM(F) 2260 2272 2201 2194 2345 2286 2280 2246
T~,Q(F) 2082 2099 2088 2088 2172 2152 2154 2111
4T (F) 178 173 113 106 173 134 126 135
** average of two samples
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-22-
TABLE 8 (cont.)
Ex. Ex. Ex. Ex. Ex. Ex. Ex. Ex.
75 76 77 78 79** 80** 81 82
**
Si02 (wt%)59.40 59.20 59.61 59.61 59.61 57.72 59.61 58.61
AI203 12.16 12.16 12.16 12.16 12.16 11.80 12.16 12.16
(wt%)
Ca0 (wt%)23.49 23.69 23.50 23.50 20.50 22.80 20.50 23.50
Mg0 (wt%)2.30 2.30 2.50 2.50 2.50 2.52 2.50 2.50
Ti02 (wt%)1.10 1.10 1.10 1.10 1.10 1.07 1.10 1.10
BZO3 (wt%) 0.30 0.45 1.00
NazO (wt%)0.40 0.40 0.90 0.87 0.90
Li20 (wt%)0.45 0.45 0.30 0.30
Zn0 (wt%)0.45 0.45 0.30 0.45 0.30
Mn0 (wt%) 3.00 0.30
Mn02 (wt%) 3.00 3.00
Fe203 0.25 0.25 0.23 0.23 0.23 0.22 0.23 0.23
(wt%)
Si02/RO 2.30 2.28 2.29 2.29 2.59 2.28 2.59 2.25
NIST 717A
TFORM(F) 2246 2250 2235 2271 2221 2196 2224 2226
T~,Q(F) 2118 2105 2113 2113 2117 2125 2118 2082
4T (F) 128 145 122 158 104 71 106 144
NIST 714
TFORM(F) 2273 2277 2260 2298 2245 2219 2249 2251
T~,a(F) 2118 2105 2113 2113 2117 2125 2118 2082
4T (F) 155 172 147 185 128 94 131 169
** average of two samples
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-23-
TABLE 8 (cont.)
Ex.83 Ex.84 Ex.85 Ex.86 Ex.87 Ex.88 Ex.89 Ex.90
Si02 (wt%)58.96 58.70 58.35 57.65 58.15 57.95 58.20 58.10
AIz03 (wt%)13.24 13.35 13.20 13.40 13.20 13.20 13.03 13.03
Ca0 (wt%) 23.65 23.50 23.65 24.15 22.85 24.05 23.64 23.74
Mg0 (wt%) 2.5 2.50 2.55 2.55 2.55 2.55 2.50 2.50
Ti02 (wt%).50 0.50 1.10 1.10 1.10 1.10 0.50 0.50
B20g(Wt%)
Na20 (wt%) 0.30
Li20 (wt%)0.90 0.90 0.90 0.90 0.90 0.90 0.90 0.90
Zn0 (wt%) 1.00 1.00 1.00 1.00
Mn0 (wt%)
Mn02 (wt%)
Fez03 (wt%)0.25 0.25 0.25 0.25 0.25 0.25 0.23 0.23
Si02/RO 2.25 2.26 2.23 2.16 2.29 2.18 2.23 2.21
NIST 717A
TFORM (
F)
Tua (F)
4T (F)
NIST 714
TFORM(F) 2214 2212 2212 2203 2205 2183 2201 2203
T~,Q (F) 2116 2107 2095 2109 2077 .' 'a~E.~842098 2091
4T (F) 98 105 117 94 128 99 103 112
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
TABLE 8 (cont.)
Ex.91 Ex.92 Ex.93 Ex.94 Ex.95 Ex.96 Ex.97 Ex.98
Si02 (wt%)58.00 58.10 58.30 57.60 56.25 55.75 56.00 57.75
AIz03 13.03 13.03 13.03 13.23 13.20 13.20 13.60 13.20
(wt%)
Ca0 (wt%)23.84 23.74 23.54 23.84 23.75 23.25 24.25 24.25
Mg0 (wt%)2.50 2.50 2.50 2.50 2.50 2.55 2.50 2.55
Ti02 (wt%)0.50 0.50 0.50 0.50 1.10 1.10 0.50 1.10
8203 (wt%)1.00 1.00 1.00 1.20 2.00 3.00 2.00
NazO (wt%) 0.10 0.90 0.90 0.90 0.90
Li20 (wt%)0.90 0.90 0.90 0.80
Zn0 (wt%)
Mn0 (wt%)
Mn02 (wt%)
Fe203 0.23 0.23 0.23 0.23 0.25 0.25 0.25 0.25
(wt%)
SiOz/RO 2.20 2.21 2.24 2.19 2.14 2.16 2.09 2.15
NIST 717A
TFORM
(F)
TuQ (F)
4T (F)
NIST 714
TFORM(F) 2178 2181 2183 2185 2235 2199 2215 2250
T~~Q(F) 2079 2075 2084 2071 2100 2060 2077 2131
DT (F) 99 106 99 114 135 139 138 119
Table 9 includes several glass fiber melt compositions made in a
commercial glass melting operation. The amount of each constituent in
the table is the weight percent in the actual melt. The weight percent for
the LizO was determined using wet chemical analysis techniques, the
weight percent for the B203 was determined using Neutron Transmission
analysis techniques, and the weight percent for the remaining constituents
was determined using X-ray fluorescence analysis (also referred to as
"XRF analysis"), all of which are well know to those skilled in the art.
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-25-
TABLE 9
Ex.99 Ex.100 Ex.101Ex.102
Si02 (wt%)57.97 56.83 58.57 58.46
AI203 12.28 13.28 12.44 12.32
(wt%)
Ca0 (wt%)24.15 23.71 23.85 23.70
Mg0 (wt%)2.6 2.45 2.42 2.48
Ti02 (wt%)1.16 0.55 0.51 0.50
8203 (wt%) 2.10 1.37 1.2
Na20 (wt%)0.89 0.87
Li20 (wt%) 0.84 0.88
K20 (wt%)0.06 0.06 0.07 0.07
~
Fez03 0.28 0.28 0.27 0.275
(wt%)
Si02/RO 2.17 2.17 2.23 2.23
NIST 714
TFORM 2253 2224 2183 2185
(F)
Tuo (F) 2140 2061 2082 2080
4T (F) 113 164 101 105
Based on the above, in one nonlimiting embodiment of the present
invention, the glass fiber compositions have a base composition of Si02,
CaO, A1203 and Fe203, and optionally NazO, as discussed above, and a
Mg0 content ranging from 1.7 to 2.9 wt%, preferably from 1.8 to
2.9 wt%, and more preferably 1.8 to 2.7. In another nonlimiting
embodiment, the glass fiber compositions have a Mg0 content ranging
from 1.7 to 2.7 wt%, and preferably from 1.9 to 2.65 wt%.
In one nonlimiting embodiment of the invention, for glass
compositions that do not include any liquidus temperature reducing
additives other than Mg0 or only trace amounts of these additive, i.e. less
than 0.05 wt, the Mg0 range is 2.2 to 2.9, preferably from 2.4 to 2.8
wt%, and more 2.45 to 2.65 wt%. In another embodiment of glass
compositions with little or no liquidus temperature reducing additives
other than MgO, the Mg0 range is from 2.2 to 2.7, and preferably from
2.3to2.6wt%.
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
_26_
In still another nonlimiting embodiment of the invention, in glass
compositions that include a total amount of at least 0.05 wt% of liquidus
temperature lowering additives, the Mg0 content ranges from 1.7 to
2.65 wt%, and preferably between 1.8 and 2.6 wt%, and more
preferably 1.9 to 2.55 wt%. In another nonlimiting embodiment of glass
compositions that include liquidus temperature lowering additives, the
Mg0 content ranges from 1 .7 to 2.5 wt%, and preferably between 1.8
and 2.3 wt%. In one nonlimiting embodiment of the present invention,
the liquidus temperature lowering additives in the glass fiber compositions
include, but are not limited to, Li20, ZnO, MnO, Mn02 and/or B203 in the
amounts discussed earlier.
It should be appreciated that although other commercially available
glasses reduce environmental hazards due to boron and fluorine emissions
by reducing or eliminating these materials from the batch, these glasses
are processed at higher forming temperatures that conventional E-glass.
As a result, they require additional energy for production. The present
invention provides glass compositions that include little or no boron and/or
fluorine and have a forming temperature generally lower than other low
boron and/or fluorine glass compositions, and boron-free and fluorine-free
glass compositions, and more specifically, have forming temperatures
approaching that of E-glass. In one nonlimiting embodiment of the
invention, the forming temperature of the glass compositions of the
present invention should be no greater than 2280°F ( 1249°C),
and
preferably no greater than 2260°F ( 1238°C), and more preferably
no
greater than 2230°F (1221°C), based on the NIST 714 reference
standard. In one particular nonlimiting embodiment of the invention, the
forming temperature is no greater than 2200°F ( 1204°C), based
on the
NIST 714 reference standard.
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
-27-
In addition, in one nonlimiting embodiment of the present invention,
the liquidus temperature of the glass compositions of the present
invention should be no greater than 2155°F ( 1 179°C), and
preferably no
greater than 2145°F ( 1 174°C), and more preferably no greater
than
2130°F (1166°C).
As discussed earlierP in the glass fiber forming industry, 0T should
be maintained in a range sufficient to prevent devitrification of the molten
glass in the bushing area of a glass fiber forming operation and stagnant
areas of the glass melting furnace. In the present invention, it 0T should
be at least 65°F (36°C), preferably at least 90°F
(50°C), and more
preferably at least 100°F (56°C). In addition, although not
required, in
order to maintain the overall heating and melting requirements of the glass
fiber composition low, it is preferred that 4T be no greater than 1
50°F
(83°C) and more preferably no greater than 125°F (69°C).
This will
maintain a lower forming temperature for a given liquidus temperature and
result in good energy efficiency. If required, the amounts of Si02 and Ca0
can be modified to change the forming temperature ~ar~rl provide a desired
4T. More specifically, reducing the silica content while simultaneously
maintaining or increasing the calcia content (thus reducing the Si02/RO
ratio) will reduce the forming temperature and thus reduce DT. This type
of modification would be of value if, for example, ~T was much greater
than 100°F and could be reduced without adversely affecting the glass
melting and forming operation. Conversely, increasing the silica content
and while simultaneously maintaining or reducing the calcia content (thus
increasing the Si02/RO ratio) will raise the forming temperature and thus
increase 4T. This type of modification would be of value if, for example,
0T was too low and had to be increased to at least 100°F. Compositional
adjustments of the silica and/or calcia (and the resulting Si02/RO ratio) in
CA 02375719 2001-11-28
WO 00/73231 PCT/US00/14155
_2g_
either direction are possible until the ~T is obtained that is deemed to
facilitate the pursuit of a safe industrial melt forming process.
As discussed above, the Si02/RO ratio can be manipulated to
further assist in achieving the goal of lowering the overall processing
temperature, and in particular lowering the forming temperature while
providing a OT required to facilitate continuous fiber forming operation.
Although not limiting in the present invention, the glass fiber compositions
of the present invention have an Si02/RO ratio of no greater than 2.3,
preferably no greater than 2.25, and more preferably no greater than 2.2.
The invention has been described with reference to specific
embodiments, but it should be understood that variations and
modifications that are known to those of skill in the art may be resorted to
within the scope of the invention as defined by the claims.