Note: Descriptions are shown in the official language in which they were submitted.
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
1
DERIVATIVES OF 4'-DEMYCAROSYL-8a-AZA-8a-HOMOTYLOSIN
Technical Field
IPC: A 61 K 31/70
C 07 H 17/08
Technical Problem
The present invention relates to novel compounds from the class of 17-membered
azalides having an antibacterial action. More particularly, the invention
relates to
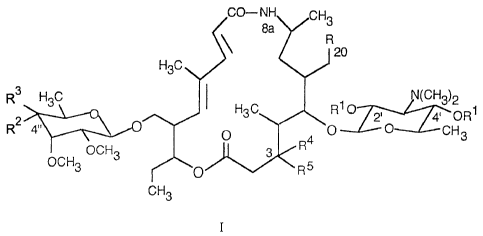
derivatives of 4'-demycarosyl-8a-aza-8a-homotylosin of the formula I
~ O-NH CH3
8a
R
120
H3C
R2 H3C N(CH3)2
H3C R1 ~ 2' 4 OR1
R3 4" ~ O \O O CH3
v ~ R4
OCH3 3
OCH3 n R5
CH3
I
wherein R represents CHO, CH(OCH3)2 or CH2N[CH2(C6H5)]~,
Rl represents H or C1-C3 acyl,
R2 represents OR6 and R6 represents H or C1-C3 acyl,
R3 represents H or RZ and R3 together represent =O,
R4 represents OH,
RS represents H or R4 and RS together represent =O,
and to a process for the preparation thereof.
Pr~io~ Apt
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
7
4'-Demycarosyl-8a-aza-8a-homotylosin, a novel semisynthetic macrolide from the
class of 17-membered azalides, was prepared by a double transformation of C-9
ketone of the 16-membered antibiotic 4'-demycarosyl-tylosin (R. L. Hamill,
Antibiotics and Chemotherapy 11, 328 (1961); A. Narandja et al, EP 0 287 082
B1; N.
Lopotar et al, EP 0 410 433 B 1). By reductive amination of C-20 aldehyde
group in
the presence of formic acid (Wallach reaction, J. March: "Advanced Organic
Chemistry", third ed. 6-15 p. 799 Wiley, New York, 1985) there was prepared 4'-
demycarosyl-20-deoxo-20-dibenzylamino-8a-aza-8a-homotylosin (N. Lopotar, HR
Patent Application P940962A, 30.11.1994).
C1-C3 acyl esters of 4'-demycarosyl-8a-aza-8a-homotylosin and of 4'-
demycarosyl-
20-deoxo-20-dibenzylamino-8a-aza-8a-homotylosin as well as 4"-deoxy-4"-oxo-
and
3-deoxy-3-oxo derivatives of 4'-demycarosyl-8a-aza-8a-homotylosin and of 4'-
demycarosyl-20-deoxo-20-dibenzylamino-8a-aza-8a-homotylosin, C1-C3 acyl esters
thereof and a process for the preparation thereof have hitherto not been
disclosed in
Prior Art.
Detailed Description of the Invention
According to the present invention derivatives of 4'-demycarosyl-8a-aza-8a-
homotylosin of the formula I
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
3
CO-
8a
\ R
I 20
R2 H3C N(CH3)2
H3C Rj p 2~ 4~ OR1
R3 4~~ ~ ) 4 O O CH3
OCH3
OCH3 R5
I
wherein R represents CHO, CH(OCH3)2 or CH2N[CHZ(C6H5)]2,
Rl represents H or C1-C3 acyl,
R2 represents OR6 and R6 represents H or CI-C3 acyl,
R3 represents H or R2 and R3 together represent =O,
R4 represents OH,
RS represents H or R4 and RS together represent =O,
may be prepared in such a way that
4'-demycarosyl-8a-aza-8a-homotylosin 20-dimethylacetal of the formula IIa and
4'-
demycarosyl-20-deoxo-20-dibenzylamino-8a-aza-8a-homotylosin of the formula IIb
O-NH CH8
8a
R
I 20
H3C N(CH3)2
H3C HO 2, 4~ OW
HO 4~ O ~Q O CH3
OCH3 3
OCH3 ~ OH
CH3
IIa R = CH(OCH3)2
IIb R = CH2N[CHZ(C6H5)]2
CH3
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
4
are subjected to
A) an O-acylation with anhydrides of Cl-C3 carboxylic acids, preferably with
acetic
acid anhydride in methylene chloride during 15 minutes to 1 hour at room
temperature, and the obtained compounds of the formula I, wherein R represents
CH(OCH3)~ or CH2N[CH2(C6H5)]2, R1 represents COCH3, R2 represents OR6,
wherein R6 represents H, R3 and RS are the same and represent H and R4
represents
OH,
are optionally subjected to
Al) an O-acylation with anhydrides of C1-C3 carboxylic acids, preferably with
acetic
acid anhydride in methylene chloride in the presence of an organic base,
preferably
triethyl amine and 4-dimethylaminopyridine as a catalyst during 30 hours at
room
temperature, and the obtained compounds of the formula I, wherein R represents
CH(OCH3)2 or CH2N[CHZ(C6H5)]2, R1 represents COCH3, RZ represents OR6,
wherein R6 represents COCH3, R3 and RS are the same and represent H and R4
represents OH,
are optionally subjected to
B) an oxidation reaction with N(3-dimethylamino-propyl)-N'ethyl carbodiimide
hydrochloride in the presence of dimethylsulfoxide and pyridine
trifluoroacetate as a
catalyst in an inert solvent, preferably methylene chloride, during 2 to 6
hours at a
temperature from 10°C to room temperature, and the obtained compounds
of the
formula I, wherein R represents CH(OCH3)2 or CH2N[CH2(C6H5)]2, R' represents
COCH3, R2 represents OR6, wherein R6 represents COCH3, R3 represents H and R4
and RS together represent =O,
are optionally subjected to
C) methanolysis at room temperature for 2 days and the obtained compounds of
the
formula I, wherein R represents CH(OCH3)Z or CH2N[CH~(C6H5)]Z, R1 and R3 are
the
same and represent H, R2 represents OR6, wherein R6 represents COCH3, and R4
and
RS together represent =O,
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
are optionally subjected to
C1) an alkaline methanolysis in a mixture of methanol and 25% ammonia (4:1) at
a
temperature from 5°C to room temperature during 20 to 60 hours to
obtain compounds
of the formula I, wherein R represents CH(OCH3)~ or CH2N[CH2(C6H5)]2, Rl and
R3
are the same and represent H, R2 represents OR6, wherein R6 represents H, and
R4 and
RS together represent =O;
or the compound obtained according to process C 1
of the formula I, wherein R represents CH(OCH3)~, R1 and R3 are the same and
represent H, Rz represents OR6, wherein R6 represents H, and R4 and R'
together
represent =O,
is optionally subjected to
D) a hydrolysis of the acetal in a mixture of acetonitrile and 0.1 N
hydrochloric acid
(1:l) for 2 hours at room temperature to obtain the compound of the formula I,
wherein R represents a CHO group, Rl and R3 are the same and represent H, R2
represents OR6, wherein R6 represents H, and Ra and RS together represent =O;
or compounds obtained according to process A
of the formula I, wherein R represents CH(OCH3)~ or CH2N[CHZ(C6H5)]2, R1
represents COCH3, R2 represents OR6, wherein R6 represents H, R3 and R5 are
the
same and represent H and R4 represents OH,
are optionally subjected to oxidation in the manner disclosed in B,
and the obtained compounds of the formula I, wherein R represents CH(OCH3)2 or
CH2N[CHZ(C6H5)]2, Rl represents COCH3, R2 and R3 together represent =O, R4
represents OH and RS represents H,
are optionally subjected to methanolysis in the manner disclosed in C,
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
6
to obtain compounds of the formula I, wherein R represents CH(OCH3)2 or
CH2N(CH2(C6H5)]2, Rl and R5 are the same and represent H, R'' and R3 together
represent =O and R4 represents OH;
or the compound obtained according to process B
of the formula I, wherein R represents a CH(OCH3)2 group, Rl represents COCH3,
R2
and R3 together represent =O, R'~ represents OH and RS represents H,
is optionally subjected to a hydrolysis of acetal in the manner disclosed in
D,
and the obtained compound of the formula I, wherein R represents a CHO group,
Rl
represents COCH3, R2 and R3 together represent =O, R4 represents OH and R5
represents H,
is optionally subjected to methanolysis in the manner disclosed in C,
to obtain the compound of the formula I, wherein R represents a CHO group, Rl
and
R5 are the same and represent H, R2 and R3 together represent =O and R4
represents
OH;
or the compound obtained according to process A
of the formula I, wherein R represents CH(OCH3)~, R1 represents COCH3, R''
represents OR6, wherein R6 represents H, R3 and RS are the same and represent
H and
R4 represents OH,
is optionally subjected to a hydrolysis of acetal in the manner disclosed in
D,
to obtain a compound of the formula I wherein R represents CHO, Rl represents
COCH3, R'' represents OR6, wherein R6 represents H, R3 and RS are the same and
represent H and R4 represents OH;
or compounds obtained according to process Al
of the formula I, wherein R represents CH(OCH3)2 or CH2N(CH2(C6H;)]2, Rl
represents COCH3, R2 represents OR6, wherein R6 represents COCH3, R3 and RS
are
the same and represent H and R4 represents OH,
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
7
are optionally subjected to methanolysis in the manner disclosed in C,
to obtain compounds of the formula I, wherein R represents CH(OCH3)2 or
CH2N[CH2(C6H5)]2, Rl, R3 and R' are the same and represent H, R2 represents
OR6,
wherein R6 represents COCH3, and R4 represents OH;
or the compound obtained according to process Al
of the formula I, wherein R represents CH(OCH3)2, Rl represents COCH3, Rz
represents OR6, wherein R6 represents COCH3, R3 and RS are the same and
represent
H and R4 represents OH,
is optionally subjected to a hydrolysis of acetal in the manner disclosed in
D,
and the obtained compound of the formula I, wherein R represents CHO, R'
represents COCH3, R2 represents OR6, wherein R6 represents COCH3, R3 and RS
are
the same and represent H and R4 represents OH,
is optionally subjected to methanolysis in the manner disclosed in C,
to obtain the compound of the formula I. wherein R represents CHO, Rl, R3 and
RS
are the same and represent H, R2 represents OR6, wherein R~ represents COCH3,
and
R4 represents OH.
According to the present invention novel compouds are isolated by conventional
processes of extraction from aqueous solutions of halogenated hydrocarbons
such as
methylene chloride or chloroform and by evaporating the organic solvent to a
dry
residue. Optionally, the separation of the reaction products or the
purification of the
products for spectral analyses is carried out by flash chromatography on a
silica gel
column (Merck & Co., Silicagel 60, 230-400 mesh/ASTM) in a solvent sistem:
CH2C12-CH30H-conc. NH40H (90:9:1.5, system A), CH2C12-CH30H (90:9, system
B) or CHC13-CH3COCH3 (7:3, system C).
The structure of the novel compounds was confirmed by spectrometric methods
and
mass analysis.
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
8
The novel compounds show antibacterial action and may be also used as
intermediates
for preparing novel 17-membered azalide antibiotics.
The invention is illustrated and in no way limited by the following Examples.
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
9
Example 1
4'-Demycarosyl-2',4'-di-O-acetyl-8a-aza-8a-homotylosin 20-dimethylacetal (1)
4'-Demycarosyl-8a-aza-8a-homotylosin 20-dimethylacetal (5.0 g, 6.02 mmol) was
dissolved in dry methylene chloride (50 ml), acetic anhydride (2.0 ml) was
added and
it was stirred for 15 minutes at room temperature. The reaction mixture was
poured
into a water/ice mixture (500 ml) and extracted twice with methylene chloride
at pH
8.5. The combined organic extracts were washed with a saturated NaHC03
solution
and water, dried (K2C03) and evaporated at reduced pressure to give a TLC
homogeneous product (1) (5.38 g; 97.8 %).
TLC: Rf (B) 0.44; Rf (C) 0.22.
IR (KBr) cm 1 1749, 1657, 1620, 1544, 1455, 1375, 1229, 1170, 1063.
1H NMR (CDC13) 8 ppm 7.16 (H-11), 5.69 (H-10), 5.66 (H-13), 4.96 (8a-NH)
exchangeable with D20, 4.88 (H-2'), 4.76 (H-4'), 4.63 (H-20), 4.58 (H-1"),
4.33 (H-1'), 4.17 (H-8), 3.61 (3"-OCH3), 3.47 (2"-OCH3), 3.56 (2x20-OCH3),
2.33 /3'-N(CH3)2/, 2.05 (COCH3), 2.03 (COCH3), 1.74 (H-22), 1.17 (H-21).
isC NMR (CDC13) b ppm 179.1 (C-1), 169.8, 169.4 (2xCOCH3), 166.2 (9-CONH),
144.7 (C-11), 138.2 (C-13), 134.9 (C-12), 119.2 (C-10), 103.5 (C-20), 102.0
(C-1'), 100.9 (C-1"), 72.5 (C-4"), 71.4 (C-4'), 70.3 (C-2'), 65.6 (C-3), 61.5
(3"-OCH3), 59.4 (2"-OCH3), 50.4 (2x20-OCH3), 42.7 (C-8), 42.5 (C-4), 41.0
/3'-N(CH3)Z/, 40.5 (C-2), 34.3 (C-19), 21.8, 20.9 (2xCOCH3), 21.9 (C-21),
12.6 (C-22), 8.3 (C-18).
FAB (MH+) 917.
Example 2
4'-Demycarosyl-2',4'-di-O-acetyl-20-deoxo-20-dibenzylamino-8a-aza-8a-
homotylosin (2)
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
4'-Demycarosyl-20-deoxo-20-dibenzylamino-8a-aza-8a-homotylosin (2.8 g, 2.90
mmol) was dissolved in dry methylene chloride (30 ml), acetic anhydride (1.3
ml,
13.76 mmol) was added and it was stirred for 15 minutes at room temperature.
The
reaction mixture was poured into a water/ice mixture (300 ml) and extracted
twice
with methylene chloride at pH 6.5. The combined organic extracts were washed
with a
saturated NaHC03 solution and water, dried (KZC03) and evaporated at reduced
pressure to give a TLC homogeneous product (2) (3.02 g; 98.9 %).
TLC: Rf (B) 0.38; Rf (C) 0.23.
IR (KBr) cm 1 1749, 1651, 1633, 1548, 1454, 1374, 123 l, 1169; 1059.
1H NMR (CDC13) 8 ppm 7.25 ~ 7.41 (phenyl), 7.10 (H-11), 5.70 (H-13), 5.65 (H-
10),
4.89 (8a-NH) exchangeable with DSO, 4.84 (H-2'), 4.74 (H-4'), 4.60 (H-1"),
4.15 (H-1'), 3.62 (3"-OCH3), 3.61 (20-N-CH2-phenyl), 3.58 (20-CH2-phenyl),
3.51 (2"-OCH3), 2.32 /3'-N(CH3)2/, 2.06 (COCH3), 2.00 (COCH3), 1.72 (H-
22), 1.12 (H-21).
i3C NMR (CDCl3) 8 ppm 173.4 (C-1), 169.9, 169.5 (2xCOCH3), 166.1 (9-CONH),
144.8 (C-11), 137.9 (C-13), 135.2 (C-12), 119.3 (C-10), 102.3 (C-1'), 101.0
(C-1"), 72.5 (C-4"), 71.4 (C-4'), 70.4 (C-2'), 66.0 (C-3), 61.5 (3"-OCH3),
59.5
(2"-OCH3), 52.2 (C-20), 42.9 (C-8), 42.4 (C-4), 41.0 /3'-N(CH3)2/, 38.7 (C-2),
29.4 (C-19), 21.8 (C-21), 21.1, 21.0 (2xCOCH3), 12.7 (C-22), 8.4 (C-18),
20-N(CH2C6H5)2, 139.8, 129.1, 128.0, 126.6, 57.9.
FAB (MH+) 1052.
Example 3
4'-Demycarosyl-2',4',4"-tri-O-acetyl-8a-aza-8a-homotylosin 20-dimethylacetal
(3)
Compound 1 (4.0 g, 4.37 mmol) was dissolved in dry methylene chloride (100
ml),
triethyl amine (7.0 ml), 4-dimethylaminopyridine (0.12 g) and acetic anhydride
(0.42
ml, 4.45 mmol) were added and then the reaction solution was left to stand for
26
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
11
hours at room temperature. The isolation of the product was carried out in the
manner
disclosed in Example 1 to give a TLC homogeneous product (3) (4.08 g; 97.7 %).
TLC: Rf (A) 0.65; Rf (C) 0.54.
IR (KBr) cm 1 1749, 1655, 1618, 1546, 1454, 1374, 1233, 1171, 1052.
1H NMR (CDC13) 8 ppm 7.16 (H-11), 5.69 (H-10), 5.65 (H-13), 4.89 (8a-NH)
exchangeable with D20, 4.88 (H-2'), 4.76 (H-4'), 4.64 (H-1"), 4.59 (H-20),
4.33 (H-1'), 4.18 (H-8), 3.52 (3"-OCH3), 3.46 (2"OCH3), 3.36 (20-OCH3),
3.35 (20-OCH3), 2.33 /3'-N(CH3)2/, 2.12 (COCH3), 2.05 (COCH3), 2.03
(COCH3), 1.74 (H-22), 1.16 (H-21).
i3C NMR (CDC13) 8 ppm 173.1 (C-1), 170.1, 169.8, 169.4 (3xCOCH3), 166.1
(9-CONH), 144.7 (C-11 ), 13 8.0 (C-13 ), 134.9 (C-12), 119.2 (C-10), 103 .7
(C-20), 102.1 (C-1'), 100.9 (C-1"), 74.5 (C-4"), 71.4 (C-4'), 70.3 (C-2'),.
65.6
(C-3), 61.3 (3"-OCH3), 59.3 (2"-OCH3), 53.7 (20-OCH3), 50.6 (20-OCH3),
42.7 (C-8), 42.6 (C-4), 41.0 /3'-N(CH3)2/, 40.5 (C-2), 34.5 (C-19), 21.9, (C-
21), 21.1, 21.0, 20.7 (3xCOCH3), 12.7 (C-22), 8.3 (C-18).
FAB (MH+) 959.
Example 4
4'-Demycarosyl-2',4',4"-tri-O-acetyl-20-deoxo-20-dibenzylamino-8a-aza-8a-
homotylosin (4)
Compound 2 (2.8 g, 2.66 mmol) was dissolved in dry methylene chloride (60 ml),
triethyl amine (3.7 ml), 4-dimethylaminopyridine (0.07 g) and acetic anhydride
(0.25
ml, 1.64 mmol) were added and then the reaction solution was left to stand for
26
hours at room temperature. The isolation of the product was carried out in the
manner
disclosed in Example 1 to give a TLC homogeneous product (4) (2.7 g; 92.9 %).
TLC: Rf (B) 0.55; Rf (C) 0.47.
IR (KBr) cm' 1747, 1651, 1632, 1538, 1453, 1372, 1233, 1170, 1051.
1H NMR (CDCl3) 8 ppm 7.22 ~ 7.41 (phenyl), 7.10 (H-11), 5.70 (H-13), 5.65 (H-
10),
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
12
4.91 (8a-NH) exchangeable with D20, 4.86 (H-2'), 4.74 (H-4'), 4.66 (H-1"),
4.46 (H-4"), 4.15 (H-1'), 3.61 (2x20-N-CH2-phenyl), 3.53 (3"-OCH3), 3.50
(2"-OCH3), 2.32 /3'-N(CH3)2/, 2.12 (COCH3), 2.06 (COCH3), 2.00 (COCH3),
1.72 (H-22), 1.12 (H-21), 0.78 (H-18).
mC NMR (CDC13) b ppm 173.3 (C-1), 170.1, 169.9, 169.5 (3xCOCH3), 166.1
(9-CONH), 144.8 (C-11), 137.9 (C-13), 135.2 (C-12), 119.3 (C-10), 102.3
(C-1'), 101.0 (C-1"), 74.6 (C-4"), 71.4 (C-4'), 70.4 (C-2'), 66.0 (C-3), 61.5
(3"-OCH3), 59.3 (2"-OCH3), 52.2 (C-20), 42.9 (C-8), 42.4 (C-4), 41.0
/3'-N(CH3)2/, 38.7 (C-2), 29.4 (C-19), 21.8 (C-21), 21.1, 21.0, 20.7
(3xCOCH3), 12.7 (C-22), 8.4 (C-18),
20-N(CH~C6H5)2, 139.8, 129.1, 128.0, 126.6, 57.9.
FAB (MH+) 1094.
Example 5
4'-Demycarosyl-2',4'-di-O-acetyl-4"-deoxy-4"-oxo-8a-aza-8a-homotylosin 20-
dimethylacetal (5)
A solution of pyridine trifluoroacetate ( 1.0 g, 5.24 mmol) in methylene
chloride ( 10
ml) was added drop by drop at 15°C to a solution of the compound 1 (1.0
g, 1.09
mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.0 g,
5.22
mmol) and dimethyl sulfoxide ( 1.0 ml, 14.10 mmol) in methylene chloride (20
ml).
The reaction mixture was stirred for 3 hours at room temperature, then poured
into
water (150 ml) and after separating the organic layer, it was extracted two
more times
with methylene chloride. The combined organic extracts were washed with a
saturated
NaHC03 solution and water, dried (K2C03) and evaporated at reduced pressure to
a
dry residue. The obtained crude product (0.95 g) was purified by flash
chromatography on a silica gel column using the solvent system B to give a TLC
homogeneous product (5) (0.45 g).
TLC: Rf (B) 0.52.
IR (KBr) cm 1 1749, 1657, 1620, 1542, 1455, 1375, 1230, 1172, 1060.
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
13
1H NMR (CDC13) 8 ppm 7.16 (H-11), 5.71 (H-10), 5.64 (H-13), 4.97 (8a-NH)
exchangeable with D20, 4.88 (H-2'), 4.76 (H-4'), 4.60 (H-20), 4.63 (H-1"),
4.33 (H-1'), 4.17 (H-8), 3.98 (H-5"), 3.78 (H-3"), 3.58 (3"-OCH3), 3.52
(2"-OCH3), 3.36 (20-OCH3), 3.35 (20-OCH3), 3.30 (H-2"), 2.33 /3'-N(CH3)2/,
2.05 (COCH3), 2.03 (COCH3), 1.76 (H-22), 1.34 (H-6"), 1.17 (H-21).
i3C NMR (CDC13) ~ ppm 202.4 (C-4"), 173.1 (C-1), 169.9, 169.5 (2xCOCH3), 166.1
(9-CONH), 144.6 (C-11), 137.6 (C-13), 135.3 (C-12), 119.5 (C-10), 103.6
(C-20), 103.0 (C-1"), 102.1 (C-1'), 85.3 (C-3"), 84.2 (C-2"), 73.3 (C-5"),
71.3
(C-4'), 70.3 (C-2'), 65.6 (C-3), 60.2 (3"-OCH3), 59.1 (2"-OCH3), 53.7
(20-OCH3), 50.5 (20-OCH3), 42.7 (C-8), 42.6 (C-4), 41.0 /3'-N(CH3)2/, 40.7
(C-2) 34.4 (C-19), 21.9 (C-21), 21.1, 21.0 (2xCOCH3), 14.0 (C-6"), C-12.7
(C-22), 8.3 (C-18).
FAB (MH+) 915.
Example 6
4'-Demycarosyl-2',4'-di-O-acetyl-4"-deoxy-4"-oxo-20-deoxo-20-dibenzylamino-
8a-aza-8a-homotylosin (6)
A solution of pyridine trifluoroacetate (0.6 g, 3.11 mmol) in methylene
chloride (6 ml)
was added drop by drop at 15°C to a solution of the compound 2 (0.6 g,
0.57 mmol),
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.6 g, 3.14 mmol)
and
dimethyl sulfoxide (0.45 ml, 6.35 mmol) in methylene chloride (20 ml). The
reaction
mixture was stirred for 5 hours at room temperature. The isolation of the
product was
carried out in the manner disclosed in Example 5. The obtained crude product
(0.54 g)
was purified by flash chromatography on a silica gel column using the solvent
system
B to give a TLC homogeneous product (6) (0.28 g).
TLC: Rf (B) 0.48; Rf (C) 0.33.
IR (KBr) cm 1 1747, 1651, 1633, 1548, 1454, 1372, 1231, 1058.
1H NMR (CDC13) 8 ppm 7.25 ~ 7.41 (phenyl), 7.12 (H-11), 5.70 (H-13), 5.65 (H-
10),
4.94 (8a-NH) exchangeable with D20, 4.82 (H-2'), 4.74 (H-4'), 4.65 (H-1"),
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
14
4.15 (H-1'), 3.98 (H-5"), 3.78 (H-3"), 3.62 (20-N-CH2-phenyl), 3.58
(20-CH2-phenyl), 3.55 (3"-OCH3), 3.49 (2"-OCH3), 2.32 /3'-N(CH3)2/, 2.06
(COCH3), 2.00 (COCH3), 1.74 (H-22), 1.36 (H-6"), 1.12 (H-21).
13C NMR (CDC13) 8 ppm 202.4 (C-4"), 173.4 (C-1), 169.8, 169.3 (2xCOCH3), 166.1
(9-CONH), 144.6 (C-11), 137.0 (C-13), 135.6 (C-12), 119.6 (C-10), 103.0
(C-1"), 102.2 (C-1'), 85.3 (C-3"), 84.8 (C-2"), 73.3 (C-5"), 71.4 (C-4'), 70.4
(C-2'), 65.9 (C-3), 60.3 (3"-OCH3), 59.1 (2"-OCH3), 52.2 (C-20), 42.9 (C-8),
42.4 (C-4), 40.9 /3'-N(CH3)2/, 38.7 (C-2), 29.4 (C-19), 21.8 (C-21), 21.1,
21.0
(2xCOCH3), 14.0 (C-6"), 12.8 (C-22), 8.4 (C-18),
20-N(CH2C6H5)2 139.6, 129.9, 128.0, 126.6, 57.8.
FAB (MH+) 1050.
Example 7
4'-Demycarosyl-2',4',4"-tri-O-acetyl-3-deoxy-3-oxo-8a-aza-8a-homotylosin 20-
dimethylacetal (7)
A solution of pyridine trifluoroacetate (3.0 g, 15.72 mmol) in methylene
chloride (30
ml) was added drop by drop at 15°C to a solution of the compound 3 (2.0
g, 2.09
mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (3.0 g,
15.66
mmol) and dimethyl sulfoxide (2.9 ml, 40.89 mmol) in methylene chloride (50
ml).
The reaction mixture was stirred for 3 hours at room temperature. The
isolation of the
product was carried out in the manner disclosed in Example 5. The obtained
crude
product ( 1.95 g) was purified by flash chromatography on a silica gel column
using
the solvent system C to give a TLC homogeneous product (7) (1.3 g).
TLC: Rf (C) 0.58.
IR (KBr) cm 1 1749, 1655, 1618, 1546, 1454, 1374, 1233, 1171, 1052.
'H NMR (CDC13) 8 ppm 6.90 (H-11), 5.76 (H-10), 5.43 (H-13), 4.96 (8a-NH)
exchangeable with D20, 4.89 (H-2'), 4.79 (H-4'), 4.66 (H-1"), 4.40 (H-1'),
4.18 (H-8), 3.55, 3.32 (H-2), 3.52 (3"-OCH3), 3.49 (2"-OCH3), 3.30
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
(20-OCH3), 3.29 (20-OCH3), 2.34 /3'-N(CH3)2/, 2.12 (COCH3), 2.06 (COCH3),
2.03 (COCH3), 1.75 (H-22), 1.10 (H-21), 1.07 (H-18).
i3C NMR (CDCl3) ~ ppm 205.6 (C-3), 172.9 (C-1), 170.1, 169.8, 169.4 (3xCOCH3),
166.1 (9-CONH), 144.1 (C-11), 138.0 (C-13), 134.9 (C-12), 119.6 (C-10),
103 . 7 (C-20), 102.1 (C-1' ), 100. 9 (C-1 "), 74. 5 (C-4"), 71.4 (C-4' ), 70.
3 (C-2' ),
61.3 (3"-OCH3), 59.3 (2"-OCH3), 53.7 (20-OCH3), 50.6 (20-OCH3), 46.5
(C-2), 44.2 (C-4), 42.0 (C-8), 41.0 /3'-N(CH3)2/, 34.5 (C-19), 21.9, (C-21),
21.1, 21.0, 20.7 (3xCOCH3), 17.6 (C-18), 12.7 (C-22).
FAB (MH+) 957.
Example 8
4'-Demycarosyl-2',4',4"-tri-O-acetyl-3-deoxy-3-oxo-20-deoxo-20-dibenzylamino-
8a-aza-8a-homotylosin (8)
A solution of pyridine trifluoroacetate (2.0 g, 10.36 mmol) in methylene
chloride (10
ml) was added drop by drop at 15°C to a solution of the compound 4 (1.0
g, 1.09
mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (2.04 g,
10.44
mmol) and dimethyl sulfoxide (1.6 ml. 22.56 mmol) in methylene chloride (20
ml).
The reaction mixture was stirred for 6 hours at room temperature. The
isolation of the
product was carried out in the manner disclosed in Example 5. The obtained
crude
product (0.96 g) was purified by flash chromatography on a silica gel column
using
the solvent system B to give a TLC homogeneous product (8) (0.62 g).
TLC: Rf (B) 0.60.
IR (KBr) cm 1 1748, 1633, 1538, 1454, 1373, 1231, 1052.
1H NMR (CDC13) 8 ppm 7.22 ~ 7.40 (phenyl), 6.89 (H-11), 5.66 (H-10), 5.49 (H-
13),
4.96 (8a-NH) exchangeable with D20, 4.81 (H-2'), 4.74 (H-4'), 4.66 (H-1"),
4.42 (H-4"), 4.15 (H-1'), 4.12 (H-8), 3.78, 3.38 (H-2), 3.51 (2x20-N-CHZ-
phenyl, 3"-OCH3), 3.48 (2"-OCH3), 2.32 /3'-N(CH3)2/, 2.22 (H-4), 2.09
(COCH3), 2.06 (COCH3), 2.00 (COCH3), 1.72 (H-22), 1.10 (H-21), 1.08
(H-18).
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
16
i3C NMR (CDC13) 8 ppm 206.7 (C-3), 172.7 (C-1), 170.1, 169.9, 169.5 (3xCOCH3),
166.1 (9-CONH), 144.0 (C-11),. 136.5 (C-12), 135.0 (C-13), 119.9 (C-10),
102.7 (C-1'), 100.9 (C-1"), 74.6 (C-4"), 71.3 (C-4'), 70.3 (C-2'), 61.3
(3"-OCH3), 59.3 (2"-OCH3), 51.7 (C-20), 47.7 (C-2), 44.5 (C-4)), 42.0 (C-8),
41.0 /3'-N(CH3)2/, 28.6 (C-19), 22.0 (C-21), 21.0, 20.7 (3xCOCH3), 17.8
(C-18), 13.1 (C-22),
20-N(CHZC6H2), 140.1, 128.9, 128.0, 126.4, 57.9.
FAB (MH+) 1092.
Example 9
4'-Demycarosyl-4"-deoxy-4"-oxo-8a-aza-8a-homotylosin 20-dimethylacetal (9)
The compound 5 (0.65 g, 0.71 mmol) was dissolved in methanol (20 ml) and left
to
stand at room temperature for 48 hours. To the reaction solution a saturated
NaHC03
solution was added and it was extracted twice with chloroform. The combined
organic
extracts were dried (K2C03) and evaporated at reduced pressure to a dry
residue. The
obtained crude product (0.45 g) was purified by flash chromatography on a
silica gel
column using the solvent system A to give a TLC homogeneous product (9) (0.20
g).
TLC: Rf (A) 0.27.
IR (KBr) cm 1 1749, 1657, 1620, 1542, 1455, 1375, 1230, 1172, 1060.
1H NMR (CDC13) ~ ppm 7.16 (H-11), 5.72 (H-10), 5.67 (H-13), 4.99 (8a-NH)
exchangeable with D20, 4.60 (H-20), 4.63 (H-1"), 4.33 (H-1'), 4.17 (H-8),
3.98 (H-5"), 3.78 (H-3"), 3.58 (3"-OCH3), 3.52 (2"-OCH3), 3.46 (H-2'), 3.36,
3.35 (2x20-OCH3), 3.30 (H-2"), 3.06 (H-4'), 2.33 /3'-N(CH3)2/, 1.76 (H-22),
1.34 (H-6"), 1.17 (H-21).
i3C NMR (CDCl3) 8 ppm 202.4 (C-4"), 173.1 (C-1), 166.1 (9-CONH), 144.6 (C-11),
137.6 (C-13), 135.3 (C-12), 119.5 (C-10), 103.6 (C-20), 103.0 (C-1"), 102.1
(C-1'), 85.3 (C-3"), 84.2 (C-2"), 73.3 (C-5"), 65.6 (C-3), 60.2 (3"-OCH3),
59.1
(2"-OCH3), 53.7 (20-OCH3), 50.5 (20-OCH3), 42.7 (C-8), 42.6 (C-4), 41.0
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
17
/3'-N(CH3)2/, 4f.7 (C-2), 34.4 (C-19), 21.9 (C-21), 14.0 (C-6"), 12.7 (C-22),
8.3 (C-18).
FAB (MH+) 831.
Example 10
4'-Demycarosyl-4"-deoxy-4"-oxo-20-deoxo-20-dibenzylamino-8a-aza-8a-
homotylosin (10)
The compound 6 (0.30 g, 0.73 mmol) was dissolved in methanol (20 ml) and left
to
stand at room temperature for 30 hours. After addition of water (50 ml) the
product
was isolated by a gradient extraction with chloroform at pH 4.5 and 7.5. The
combined chloroform extracts at pH 7.5 were dried (K2C03) and evaporated at
reduced pressure and the obtained product (0.17 g) was purified by flash
chromatography on a silica gel column using the solvent system A to give a TLC
homogeneous product (10) (0.08 g).
TLC: Rf (A) 0.49.
IR (KBr) cm I 1715, 1655, 1619, 1542, 1454, 1377, 1168, 1082.
'H NMR (CDC13) ~ ppm 7.25 ~ 7.41 (phenyl), 7.12 (H-11), 5.70 (H-13), 5.65 (H-
10),
4.94 (8a-NH) exchangeable with D20, 4.84 (H-2'), 4.74 (H-4'), 4.60 (H-1"),
4.15 (H-1'), 3.98 (H-5"), 3.78 (H-3"), 3.62 (3"-OCH3), 3.61 (20-N-CH2-
phenyl), 3.58 (20-CHZ-phenyl), 3.51 (2"-OCH3), 3.46 (H-2'), 3.01 (H-4'), 2.32
/3'-N(CH3)2/, 1.72 (H-22), 1.12 (H-21).
i3C NMR (CDC13) ~ ppm 202.4 (C-4"), 173.4 (C-1), 166.1 (9-CONH), 144.7 (C-11),
137.1 (C-13), 135.6 (C-12), 119.7 (C-10), 104.2 (C-1'), 103.0 (C-1"), 85.4
(C-3"), 84.9 (C-2"), 73.3 (C-5"), 66.4 (C-3), 59.8 (3"-OCH3), 58.6 (2"-OCH3),
52.2 (C-20), 43.3 (C-8), 42.3 (C-4), 41.5 /3'-N(CH3)2/, 38.7 (C-2), 29.4 (C-
19),
22.0 (C-21), 14.1 (C-6"), 12.8 (C-22), 9.1 (C-18),
20-N(CH2C6H5)2 139.8, 129.1, 128.0, 126.6, 58Ø
FAB (MH+) 967.
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
18
Example 1l
4'-Demycarosyl-4"-O-acetyl-3-deoxy-3-oxo-8a-aza-8a-homotylosin 20-
dimethylacetal (11)
The compound 7 (0.70 g, 0.73 mmol) was dissolved in methanol (50 ml) and left
to
stand at room temperature for 24 hours. The isolation of the product was
carried out in
the manner disclosed in Example 9 and the obtained crude product (0.62 g) was
purified by flash chromatography on a silica gel column using the solvent
system A to
give a TLC homogeneous product (11) (0.40 g).
TLC: Rf (A) 0.44.
IR (KBr) cm 1 1749, 1657, 1620, 1544, 1455, 1375, 1229, 1170, 1063.
1H NMR (CDC13) 8 ppm 6.87 (H-11), 5.77 (H-10), 5.44 (H-13), 5.18 (8a-NH)
exchangeable with D20, 4.88 (H-2'), 4.64 (H-1"), 4.44 (H-4"), 4.30 (H-1'),
4.17 (H-8), 3.93 (H-5"), 3.89 (H-3"), 3.53 (3"-OCH3), 3.50, 3.26 (H-2), 3.48
(2"-OCH3), 3.30 (20-OCH3), 3.29 (20-OCH3), 2.53 /3'-N(CH3)2/, 2.12
(COCH3), 1.75 (H-22), 1.25 (H-18).
isC NMR (CDC13) ~ ppm 205.4 (C-3), 172.9 (C-1), 170.1 (COCH3), 167.4 (9-
CONH), 143.4 (C-11), 136.2 (C-12), 134.6 (C-13), 120.7 (C-10), 104.2 ,(C-1'),
103.9 (C-20), 100.8 (C-1"), 74.5 (C-4"), 70.9 (C-2'), 70.5 (C-2'), 61.3 (3"-
OCH3), 59.0 (2"-OCH3), 52.6 (20-OCH3), 52.1 (20-OCH3), 45.9 (C-2), 44.4
(C-4), 42.5 (C-8), 41.4 /3'-N(CH3)Z/, 33.8 (C-19), 22.0 (C-21), 20.7 (COCH3),
17.5 (C-18), 12.9 (C-22).
FAB (MH+) 873.
Example 12
4'-Demycarosyl-4"-O-acetyl-3-deoxy-3-oxo-20-deoxo-20-dibenzylamino-8a-aza-
8a-homotylosin (12)
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
19
The compound 8 ( 1.20 g, 10.99 mmol) was dissolved in methanol ( 100 ml) and
left to
stand at room temperature for 24 hours. To the reaction solution water ( 100
ml) was
added and it was extracted with methylene chloride at pH 6.5. The combined
organic
extracts were dried (K2C03) and evaporated at reduced pressure and the
obtained
crude product ( 1.0 g) was purified by flash chromatography on a silica gel
column
using the solvent system A to give a TLC homogeneous product (12) (0.52 g).
TLC: Rf (A) 0.65.
IR (KBr) cm 1 1745, 1650, 1622, 1537, 1454, 1373, 1233, 1166, 1058.
1H NMR (CDC13) 8 ppm 7.25 ~ 7.41 (phenyl), 6.90 (H-11), 5.67 (H-10), 5.52 (H-
13),
4.98 (8a-NH) exchangeable with DSO, 4.67 (H-1"), 4.45 (H-4"), 4.17 (H-1'),
4.02 (H-8), 3.61 (20-CH2-phenyl), 3.53 (3"-OCH3), 3.52 (20-CH,-phenyl),
3.50 (2"-OCH3), 3.76, 3.32 (H-2), 2.52 /3'-N(CH3)2/, 2.12 (COCH3), 1.73 (H-
22), 1.21 (H-18), 1.08 (H-21).
isC NMR (CDC13) ~ ppm 205.3 (C-3), 172.5 (C-1), 170.1 (COCH3), 167.2 (9-
CONH), 143.9 (C-11), 135.9 (C-12), 135.4 (C-13), 120.0 (C-10), 103.9 (C-1'),
100.9 (C-1"), 74.6 (C-4"), 70.7 (C-4'), 70.4 (C-2'), 61.3 (3"-OCH3), 59.3 (2"-
OCH3), 51.6 (C-20), 46.1 (C-2), 44.5 (C-4), 43.3 (C-8), 41.5 /3'-N(CH3),/,
2s.s (C-19), 22.0 (C-21), 20.7 (COCH3), 17.g (C-18), 12.9 (C-22), 20-
N(CH2C6H)2 139.9, 128.8, 128.0, 126.5, 58Ø
FAB (MH+) 1008.
Example 13
4'-Demycarosyl-4"-O-acetyl-8a-aza-8a-homotylosin 20-dimethylacetal (13)
The compound 3 (0.5 g, 0.52 mmol) was dissolved in methanol (20 ml) and left
to
stand at room temperature for 24 hours. The isolation of the product was
carried out in
the manner disclosed in Example 9 and the obtained crude product (0.43 g) was
purified by flash chromatography on a silica gel column using the solvent
system A to
give a TLC homogeneous product (13) (0.32 g).
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
TLC: Rf (A) 0.32.
IR (KBr) cm 1 1739, 1656, 1616, 1541, 1455, 1376, 1237, 1170, 1062.
1H NMR (CDC13) 8 ppm 7.15 (H-11), 5.71 (H-10), 5.66 (H-13), 4.97 (8a-NH)
exchangeable with D20, 4.64 (H-1"), 4.62 (H-20), 4.44 (H-4"), 4.24 (H-1'),
4.18 (H-8), 3.53 (3"-OCH3), 3.47 (2"-OCH3), 3.37 (20-OCH3), 3.36 (20-
OCH3), 2.50 /3'-N(CH3)2/, 2.12 (COCH3), 1.75 (H-22), 1.17 (H-21).
FAB (MH+) 875.
Example 14
4'-Demycarosyl-4"-O-acetyl-20-deoxo-20-dibenzylamino-8a-aza-8a-homotylosin
(14)
The compound 4 (0.75 g, 0.69 mmol) was dissolved in methanol (20 ml) and left
to
stand at room temperature for 24 hours. The isolation of the product was
carried out in
the manner disclosed in Example 12 and the obtained crude product (0.66 g) was
purified by flash chromatography on a silica gel column using the solvent
system A to
give a TLC homogeneous product (14) (0.45 g).
TLC: Rf (A) 0.50.
IR (KBr) cm 1 1740, 1657, 1621, 1538, 1454, 1373, 1236, 1169, 1054.
1H NMR (CDCl3) ~ ppm 7.25 ~ 7.41 (phenyl), 7.10 (H-11), 5.69 (H-13), 5.65 (H-
10),
4.96 (8a-NH) exchangeable with DzO, 4.66 (H-1"), 4.45 (H-4"), 4.14 (H-8),
4.07 (H-1'), 3.59 (20-N-CH2-phenyl), 3.56 (20-CH2-phenyl), 3.53 (3"-OCH3),
3.50 (2"-OCH3), 2.49 /3'-N(CH3)2/, 2.12 (COCH3), 1.73 (H-22), 1.11 (H-21),
0.94 (H-18).
FAB (MH+) 1010.
Example I S
4'-Demycarosyl-3-deoxy-3-oxo-8a-aza-8a-homotylosin 20-dimethylacetal (15)
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
21
The compound 11 (0.40 g, 0.46 mmol) was dissolved in a methanol/conc. NH40H
mixture (4:1, 50 ml) and left to stand for 60 hours at the temperature of
5°C. The
reaction solution was evaporated to an oily residue and then a product was
isolated in
the manner disclosed in Example 9. The obtained crude product (0.25 g) was
purified
by flash chromatography on a silica gel column using the solvent system A to
give a
TLC homogeneous product (15) (0.15 g).
TLC: Rf (A) 0.39.
IR (KBr) cm 1 1739, 1714, 1650, 1620, 1544, 1455, 1375, 1170, 1063.
1H NMR (CDCl3) 8 ppm 6.87 (H-11), 5.77 (H-10), 5.44 (H-13), 5.18 (8a-NH)
exchangeable with DzO, 4.60 (H-20), 4.64 (H-1"), 4.33 (H-1'), 4.17 (H-8),
3.93 (H-5"), 3.89 (H-3"), 3.53 (3"-OCH3), 3.50, 3.26 (H-2), 3.48 (2"-OCH3),
3.30 (20-OCH3), 3.29 (20-OCH3), 2.33 /3'-N(CH3)2/, 1.75 (H-22), 1.25 (H-18).
FAB (MH+) 831.
Example 16
4'-Demycarosyl-3-deoxy-3-oxo-20-deoxo-20-dibenzylamino-8a-aza-8a-
homotylosin (16)
The compound 12 (0.78 g, 0.77 mmol) was dissolved in a methanol/conc. NH40H
mixture (4:1, 50 ml) and left to stand for 24 hours at room temperature. To
the
reaction solution water (80 ml) was added and it was extracted twice with
methylene
chloride at pH 7.5. The combined organic extracts were dried (K2C03) and
evaporated
at reduced pressure and the obtained product (0.66 g) was purified by flash
chromatography on a silica gel column using the solvent system A to give a TLC
homogeneous product (16) (0.32 g).
TLC: Rf (A) 0.55.
IR (KBr) cm 1 1739, 1714, 1650, 1622, 1538, 1454, 1376, 1167, 1082.
1H NMR (CDC13) 8 ppm 7.25 ~ 7.41 (phenyl), 6.90 (H-11), 5.66 (H-13), 5.53 (H-
10),
5.28 (8a-NH) exchangeable with D20, 4.61 (H-1"), 4.16 (H-1'), 4.03 (H-8),
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
72
3.62 (20-N-CH2-phenyl), 3.61 (20-CH2-phenyl, 3"-OCH3), 3.51 (2"-OCH3),
3.78, 3.38 (H-2), 2.5 /3'-N(CH3)~/, 2.38 (H-4), 1.72 (H-22), 1.21 (H-18), 1.08
(H-21).
isC NMR (CDC13) 8 ppm 205.3 (C-3), 172.5 (C-1), 167.2 (9-CONH), 143.9 (C-11),
135.9 (C-12), 135.6 (C-13), 120.0 (C-10), 103.9 (C-1'), 101.0 (C-1'), 72.5
(C-4"), 70.7 (C-4'), 70.4 (C-2'), 61.5 (3"-OCH3), 59.5 (2"-OCH3), 51.7 (C-20),
46.1 (C-2), 44.5 (C-4), 43.3 (C-8), 41.5 /3'-N(CH3)2/, 28.8 (C-19), 22.0 (C-
21),
17.8 (C-18), 12.9 (C-22),
20-N(CH2C6H)2 140.0, 128.8, 128.0, 126.5, 58Ø
FAB (MH+) 967.
Example 17
4'-Demycarosyl-3-deoxy-3-oxo-8a-aza-8a-homotylosin (17)
The compound 15 (0.5 g, 0.60 mmol) was dissolved in an acetonitrile/0.1 N HCl
mixture (1:1, 35 ml) and stirred for 2 hours at room temperature. To the
reaction
solution a saturated NaHC03 solution was added and it was extracted twice with
methylene chloride. The combined organic extracts were dried (K~C03) and
evaporated at reduced pressure and the obtained product (0.42 g) was purified
by flash
chromatography on a silica gel column using the solvent system A to give a TLC
homogeneous product (17) (0.25 g).
TLC: Rf (A) 0.35.
IR (KBr) cm 1 1739, 1719, 1657, 1620, 1545, 1455, 1376, 1169, 1082.
1H NMR (CDC13) b ppm 9.78 (H-20), 7.19 (H-11), 5.72 (H-10), 5.70 (H-13), 5.06
(8a-NH) exchangeable with D20, 4.58 (H-1"), 4.18 (H-1'), 4.23 (H-8), 3.68,
3.32 (H-2), 3.62 (3"-OCH3), 3.49 (2"-OCH3), 2.49 /3'-N(CH3)2/, 1.75 (H-22),
1.25 (H-18), 1.18 (H-21).
i3C NMR (CDCl3) ~ ppm 205.3 (C-3), 203.8 (C-20), 173.5 (C-1), 166.9 (9-CONH),
145.1 (C-11), 138.2 (C-13), 135.1 (C-12), 129.3 (C-10), 103.7 (C-1'), 101.1
(C-1'), 72.8 (C-4"), 71.0 (C-4'), 70.4 (C-2'), 61.5 (3"-OCH3), 59.5 (2"-OCH3),
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
23
46.6 (C-19), 46.1 (C-2), 44.5 (C-4), 43.3 (C-8), 41.5 /3'-N(CH3)2/, 22.4 (C-
21),
17.8 (C-18), 12.9 (C-22).
FAB (MH+) 785.
Example 18
4'-Demycarosyl-2',4'-di-O-acetyl-8a-aza-8a-homotylosin (18)
The compound 1 (0.5 g, 0.55 mmol) was dissolved in an acetonitrile/0.1 N HCl
mixture (1:1, 35 ml) and stirred for 2 hours at room temperature. The
isolation of the
product was carried out in the manner disclosed in Example 17 to give a TLC
homogeneous product (18) (0.34 g).
TLC: Rf (B) 0.35.
IR (KBr) cm 1 1749, 1657, 1620, 1548, 1455, 1375, 1231, 1170, 1059.
1H NMR (CDC13) 8 ppm 9.75 (H-20), 7.21 (H-11), 5.72 (H-10), 5.71 (H-13), 5.08
(8a-NH) exchangeable with D20, 4.89 (H-2'), 4.74 (H-4'), 4.58 (H-1"), 4.26
(H-1'), 3.61 (3"-OCH3), 3.49 (2"-OCH3), 2.33 /3'-N(CH3)2/, 2.05 (COCH3),
2.03 (COCH3), 1.74 (H-22), 1.18 (H-21).
i3C NMR (CDCl3) 8 ppm 203.6 (C-20), 173.3 (C-1), 169.9, 169.5 (2xCOCH3), 166.5
(9-CONH), 145 .2 (C-11 ), 13 8 . 3 (C-13 ), 13 5 . 0 (C-12), 119. 0 (C-10),
101. 6
(C-1'), 100.9 (C-1"), 72.5 (C-4"), 70.6 (C-4'), 70.3 (C-2'), 65.6 (C-3), 61.5
(3"-OCH3), 59.5 (2"-OCH3), 46.3 (C-19), 42.5 (C-8), 41.0 /3'-N(CH3)2/, 38.5
(C-2), 21.6 (C-21), 21.1, 21.0 (2xCOCH3), 12.7 (C-22), 8.1 (C-18).
FAB (MH+) 871.
Example 19
4'-Demycarosyl-2',4',4"-tri-O-acetyl-8a-aza-8a-homotylosin (19)
The compound 3 (0.5 g, 0.52 mmol) was dissolved in an acetonitrile/0.1 N HCl
mixture (1:1, 35 ml) and stirred for 2 hours at room temperature. The
isolation of the
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
24
product was carried out in the manner disclosed in Example 17 to give a TLC
homogeneous product (19) (0.47 g).
TLC: Rf (B) 0.60; Rf (C) o.so.
IR (KBr) cm' 1748, 1659, 1621, 1538, 1455, 1373, 1232, 1171, 1052.
1H NMR (CDCl3) ~ ppm 9.74 (H-20), 7.16 (H-11), 5.69 (H-10), 5.65 (H-13), 4.89
(8a-NH) exchangeable with D20, 4.88 (H-2'), 4.76 (H-4'), 4.64 (H-1"), 4.44
(H-4"), 4.33 (H-1'), 4.18 (H-8), 3.52 (3"-OCH3), 3.46 (2"-OCH3), 2.33
/3'-N(CH3)2/, 2.12 (COCH3), 2.05 (COCH3), 2.03 (COCH3), 1.74 (H-22), 1.16
(H-21).
isC NMR (CDCl3) 8 ppm 203.6 (C-20), 173.1 (C-1), 170.1, 169.8, 169.4
(3xCOCH3),
166.1 (9-CONH), 144.7 (C-11), 138.0 (C-13), 134.9 (C-12), 119.2 (C-10),
103.7 (C-20), 102.1 (C-1'), 100.9 (C-1"), 74.5 (C-4"), 71.4 (C-4'), 70.3 (C-
2'),
65.6 (C-3), 61.3 (3"-OCH3), 59.3 (2"-OCH3), 46.3 (C-19), 42.7 (C-8), 42.6
(C-4), 41.0 /3'-N(CH3)2/, 40.5 (C-2), 34.5 (C-19), 21.9 (C-21), 21.1, 21.0,
20.7
(3xCOCH3), 12.7 (C-22), 8.3 (C-18).
FAB (MH+) 913.
Example 20
4'-Demycarosyl-2',4'-di-O-acetyl-4"-deoxy-4"-oxo-8a-aza-8a-homotylosin (20)
The compound 5 (0.7 g, 0.77 mmol) was dissolved in an acetonitrile/0.1 N HCl
mixture (1:1, 50 ml) and stirred for 1 hour at room temperature. The isolation
of the
product was carried out in the manner disclosed in Example 17 to give a TLC
homogeneous product (20) (0.36 g).
TLC: Rf (B) 0.48.
IR (KBr) cm 1 1749, 1656, 1619, 1543, 1458, 1375, 1230, 1172, 1058.
1H NMR (CDCl3) 8 ppm 9.75 (H-20), 7.21 (H-11), 5.72 (H-10), 5.70 (H-13), 5.08
(8a-NH) exchangeable with D20, 4.88 (H-2'), 4.74 (H-4'), 4.58 (H-1"), 4.30
(H-1'), 4.17 (H-8), 3.98 (H-5"), 3.78 (H-3"), 3.58 (3"-OCH3), 3.48 (2"-OCH3),
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
3.30 (H-2"), 2.33 /3'-N(CH3)2/, 2.05 (COCH3), 2.03 (COCH3), 1.76 (H-22),
1.34 (H-6"), 1.17 (H-21).
i3C NMR (CDC13) 8 ppm 203.0 (C-20), 202.4 (C-4"), 173.1 (C-1), 169.9, 169.5
(2xCOCH3), 166.5 (9-CONH), 145.0 (C-11), 138.1 (C-13), 135.1 (C-12), 119.0
(C-10), 102.1 (C-1"), 100.9 (C-1'), 85.3 (C-3"), 84.2 (C-2"), 73.3 (C-5"),
71.3
(C-4'), 70.3 (C-2'), 65.6 (C-3), 61.5 (3"-OCH3), 59.4 (2"-OCH3), 46.3 (C-19),
42.5 (C-8), 41.0 /3'-N(CH3)2/, 38.5 (C-2), 21.9 (C-21), 21.1, 21.0 (2xCOCH3),
14.0 (C-6"), 12.7 (C-22), 8.3 (C-1).
FAB (MH+) 869.
Example 21
4'-Demycarosyl-4"-O-acetyl-8a-aza-8a-homotylosin (21)
The compound 19 (0.30 g, 0.33 mmol) was dissolved in methanol (20 ml) and left
to
stand for 24 hours at room temperature. The isolation of the product was
carried out in
the manner disclosed in Example 9 and the obtained crude product (0.25 g) was
purified by flash chromatography on a silica gel column using the solvent
system A to
give a TLC homogeneous product (21) (0.19 g).
TLC: Rf (A) 0.28.
IR (KBr) cm 1 1749, 1657, 1620, 1544, 1455, 1375, 1229, 1170, 1063.
' H NMR (CDCl3) 8 ppm 9.78 (H-20), 7.20 (H-11 ), 5.72 (H-10), 5.70 (H-13 ),
5.12
(8a-NH) exchangeable with D20, 4.88 (H-2'), 4.64 (H-1"), 4.44 (H-4"), 4.18
(H-1'), 4.12 (H-8), 3.93 (H-5"), 3.89 (H-3"), 3.53 (3"-OCH3), 3.48 (2"-OCH3),
2.49 /3'-N(CH3)2/, 2.12 (COCH3), 1.75 (H-22).
FAB (MH+) 829.
Example 22
4'-Demycarosyl-4"-deoxy-4"-oxo-8a-aza-8a-hornotylosin (22)
CA 02375812 2001-12-10
WO 00/77016 PCT/HR00/00018
26
The compound 20 (0.23 g, 0.27 mmol) was dissolved in methanol (20 ml) and left
to
stand for 24 hours at room temperature. The isolation of the product was
carried out in
the manner disclosed in Example 9 and the obtained crude product (0.14 g) was
purified by flash chromatography on a silica gel column using the solvent
system A to
give a TLC homogeneous product (22) (0.095 g).
TLC: Rf (A) 0.30.
IR (KBr) cm 1 1717, 1655, 1625, 1542, 1454, 1378, 1170, 1062.
1H NMR (CDC13) 8 ppm 9.76 (H-20), 7.20 (H-11), 5.72 (H-10), 5.70 (H-13), 5.12
(8a-NH) exchangeable with DSO, 4.64 (H-1"), 4.33 (H-1'), 4.18 (H-8), 3.98
(H-5"), 3.78 (H-3"), 3.58 (3"-OCH3), 3.46 (2"-OCH3), 3.30 (H-2"), 3.06
(H-4'), 2.33 /3'-N(CH3)2/, 1.74 (H-22), 1.34 (H-6"), 1.16 (H-21).
isC NMR (CDC13) 8 ppm 203.7 (C-20), 202.5 (C-4"), 173.4 (C-1), 166.6 (9-CONH),
144.9 (C-11), 137.6 (C-13), 135.4 (C-12), 119.4 (C-10), 102.1 (C-1'), 100.9
(C-1"), 71.4 (C-4'), 70.3 (C-2'), 66.3 (C-3), 61.5 (3"-OCH3), 59.7 (2"-OCH3),
46.2 (C-19), 42.7 (C-8), 42.1 (C-4), 41.5 /3'-N(CH3)2/, 39.8 (C-2), 21.7 (C-
21),
14.0 (c-6"), 12.7 (c-22), s.7 (c-18).
FAB (MH+) 785.