Note: Descriptions are shown in the official language in which they were submitted.
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
METHOD AND APPARATUS FOR FORMING
POLYCRYSTALLINE PARTICLES
FIELD OF THE INVENTION
The present invention relates to method and apparatus for the
production of polycrystalline particles, and more specifically, an economical
method and apparatus for forming polycrystalline particles by gas phase
condensation.
BACKGROUND OF THE INVENTION
Polycrystalline particles typically have a crystallite size above about 25
nanometers and a particle size above about 50 nanometers. In the prior art,
others have defined materials having a particle size between about 1-100
nanometers as nanophase, polycrystalline or nanocrystalline materials.
Polycrystalline particles have demonstrated unique chemical and physical
properties, such as high reactivity, enhanced infrared absorption, novel
electronic properties, magnetic properties, and improved hardness and
ductility. From a practical standpoint, polycrystalline materials also have
potential applications in advanced information and energy technologies, as
well as military applications.
Several techniques are known in the art for forming polycrystalline
particles. Two general categories for polycrystalline particle processing are:
1 ) aqueous processing and 2) gas phase processing. Aqueous processing
includes techniques such as spray conversion pyrolysis, sol gel deposition,
and electrodeposition. Gas phase processing may incorporate techniques
such as sputtering, laser ablation, ohmic evaporation, high-energy milling,
chemical vapor condensation, and gas phase condensation. Each of the
aforementioned techniques have their unique characteristics, however, each
is identified by the basic processes of nucleation and growth of a crystalline
structure.
In the field of gas phase condensation for the preparation of
1
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
polycrystalline particles, the basic processes include evaporation of a source
material or materials, nucleation of the material, and growth within a vapor
phase. Typically polycrystalline materials which are produced by gas phase
condensation may be formed within an inert atmosphere or in an atmosphere
consisting of a mixture of inert gases and reactive gases. Within the field of
gas phase condensation, there are various techniques for vaporizing
materials. One such technique is electrical joule heating, also known as
ohmic heating, wherein the material to be vaporized is placed in a refractory
crucible and upon the application of sufficient electrical current the
crucible is
heated and the material is vaporized.
One method of ohmic evaporation is disclosed in U.S. Pat. No.
5,128,081 to Siegel, et al., for "METHOD OF MAKING NANOCRYSTALLINE
ALPHA ALUMINA." However, one disadvantage of using ohmic evaporation,
such as the technique disclosed in the '081 patent, is the temperature
limitation because the source material is heated indirectly. Therefore, high
melting temperature materials such as nickel are difficult to prepare
according to the teachings of Siegel. There are other shortcomings
associated with resistive heating for evaporation, such as a limited heat
conductance rate and poor efficiency. As a result, electrical resist heating
suffers from a low production rate. Furthermore, contamination of the
evaporated species from the heating element and crucible materials of the
heating apparatus is also a problem.
Another method known in the art for evaporation of materials for gas
phase condensation is electron beam evaporation. One such method for
electron beam evaporation used in gas phase condensation processing of
polycrystalline particles is disclosed in U.S. Pat. No. 5,728,195 to Eastman,
et al. for "METHOD FOR PRODUCING NANOCRYSTALLINE
MULTICOMPONENT AND MULTIPHASE MATERIALS," Although the '195
patent discloses a good method for evaporating different sources materials to
form "nanocrystalline" particles, this approach does have its disadvantages.
Electron beam techniques involve sophisticated equipment that requires a
2
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
differential vacuum pumping system and a delicate electron optical system.
Furthermore, electron beam evaporation is not a continuous process, which
prevents it from being a suitable industry technique. Furthermore, the
electron beam itself emits harmful, high-energy radiation. Another application
of electron beam evaporation is disclosed in U.S. Pat. No. 4,448,802 to Buhl,
et al. for "METHOD AND APPARATUS FOR EVAPORATING MATERIAL
UNDER VACUUM USING BOTH AN ARC DISCHARGE AND ELECTRON
BEAM." The '802 patent discloses a technique for evaporating materials by
incorporating energy from an electron gun along with a low-voltage arc
discharge. Although this is an interesting approach, this device suffers from
the complexities discussed regarding the '195 patent along with the additional
complexity of incorporating an electron gun with an arc discharge technique
for evaporation.
Another technique available for evaporation of materials for gas phase
condensation is known as arc discharge, and is also referred to as arc
plasma, or arc evaporation. Arc plasma is a good technique for evaporating
high melting point and low vapor pressure transition metals. One apparatus
for arc evaporation of materials is disclosed in U.S. Pat. No. 4,732,369 to
Araya, et al. for "ARC APPARATUS FOR PRODUCING ULTRAFINE
PARTICLES." Araya discloses an apparatus for forming ultrafine particles by
arc evaporation that is characterized by forming a magnetic blow to an
electric arc by inclining an electrode to the material to be evaporated,
causing
an unbalance in electromagnetic force due to the inclination of the electrode
relative to horizontally disposed source material. Also disclosed in the '369
patent is the step of incorporating a "pinch gas", also commonly known as a
working gas, into the working gas. A working gas is typically, an inert gas
that acts to shield one or more of the electrodes, and more importantly, is
ionized to establish and sustain an arc. Araya discloses using a pinch gas of
Argon mixed with Hydrogen, Nitrogen, or Oxygen in order to increase the
amount of heat produced.
However, U.S. Pat. No. 5,514,349 to Parker, et al. for "A SYSTEM
3
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
FOR MAKING POLYCRYSTALLINED MATERIALS," disclosed a
disadvantage associated with the practice of using Oxygen as a dissociable
gas. Oxygen is not preferably used in a working gas because of the resulting
erosion of the non-consumable electrode. The '349 patent also discloses a
non-consumable electrode inclined at an angle to the source, or evaporative,
material to create an elongated arc plasma tail flame. By including Nitrogen,
Hydrogen or both into the working gas, the plasma tail flame temperature is
increased, which will result in a more complete reaction of the evaporative
material with a reaction gas such as Oxygen, Nitrogen, Helium, Air or a
combination of these gases. The presence of a reaction gas enables the
source material to form nano-sized compounds. For example, if the source
material is Titanium which is evaporated and then exposed to a reaction gas
containing some concentration of Oxygen, Titanium (Ti02) polycrystalline
materials may result.
The above-mentioned patents employ continuous gas injection into a
vaporization chamber, which makes it necessary to include a continuous
vacuum pumping system for gas circulation. Furthermore, as gas is injected
into the vaporization chamber, the chamber pressure will increase. The
dynamic gas injection and gas circulation will require a more sophisticated
system control process for operation, increasing the complexity of this
system. Although productivity has been enhanced by the above-mentioned
techniques, these gas phase condensation processes still involve a great
deal of technical complexity. Furthermore, gas circulation requires the
addition of gas filters and valves which will require maintenance and cleaning
after a period of operation, resulting in system downtime and still more
system complexity. Also, continuous gas injection into the evaporation
chamber, and the subsequent release of the gas from the chamber, will
consume a great quantity of gas, which leads to higher operation costs.
A simplified method for evaporating materials is disclosed in U.S. Pat.
No. 5,096,558 to Ehrich for "METHOD AND APPARATUS FOR
EVAPORATING MATERIAL IN VACUUM." The '558 patent discloses a
4
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
technique for evaporation of materials at very low pressures (10-4 millibars
to
10-2 millibars) for the purpose of coating surfaces. The method and
apparatus disclosed places much emphasis on the benefit of anode
evaporation. The materials disclosed for evaporation by this method are high
vapor pressure, low melting temperature materials. Although this technique
works well for low-melting temperature materials, anode evaporation does not
create sufficiently hot cathode spots to evaporate low vapor pressure, high
temperature materials. Furthermore, the operating pressure range disclosed
is too low to form particles having polycrystalline structures.
An arc plasma is a low resistance electrical conductor consisting of a
high-density mixture of ionized atoms or molecules, electrons, and neutral
species. Because a substantial current passes between the electrodes,
typically tens to hundreds of amperes, a stable arc requires a high density of
conducting molecules. If the chamber pressure is above several tens of Torr,
the working gases, which may be either inert or active, will act as the
current
transfer medium. This type of arc evaporation may be referred to as high-
pressure arc evaporation. High-pressure arc produces greater thermal power
and requires a higher current, and is able to raise a non-consumable
electrode to a high temperature, typically thousands of degrees centigrade.
A common use of high-pressure arc evaporation is in the field of thermal
spray surface coating.
Low-pressure arc evaporation methods operate in a chamber at a
pressure below about 10 Torr. In a low-pressure arc evaporation system, the
arc is sustained by substantial evaporation of electrode materials. The arc is
initiated by some means such as a high frequency ignition or simple contact
ignition. Alternatively, low-pressure arc evaporation may also be referred to
as vacuum arc evaporation. Basically, a vacuum arc is sustained by the
vapor emitted from a consumable electrode which may be either the anode or
cathode. A true vacuum arc uses the evaporated species as the primary
conductor for the arc. The consumable electrode provides the medium for
the current path, which makes vacuum arc technology suitable for a wide
5
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
spectrum of applications for vacuum thin-film coating, however this
technology is not suitable for production of polycrystalline particles.
Accordingly, there exists a need for a simple, efficient technique for forming
polycrystalline particles.
SUMMARY OF THE INVENTION
The present invention overcomes the limitations of the prior art by
providing a method for fabricating a polycrystalline particles by gas phase
condensation, comprising the steps of providing a vacuum chamber;
providing a non-consumable electrode in the vacuum chamber, spatially
disposing a consumable electrode adjacent to the non-consumable electrode,
the consumable electrode comprising evaporative material; evacuating the
vacuum chamber to between about 10 torr and 100 millitorr; creating a
potential between the non-consumable electrode and the consumable
electrode; initiating an arc discharge between the non-consumable electrode
and the consumable electrode to form ionized vapors of the evaporative
material of the consumable electrode, whereby an arc path is provided; and
evaporating the consumable electrode with an arc discharge whereby vapors
from the consumable electrode condense to form polycrystalline particles. In
a preferred embodiment, the chamber is first evacuated to below about 10-5
Torr and then increased between about 10 torr and 100 Millitorr by providing
an inert gas. Furthermore, a reaction gas may be provided to the vacuum
chamber to react with the vaporized materials to form a chemical compound.
The arc discharge may be initiated by contacting the consumable electrode
and the non-consumable electrode. Also, the non-consumable electrode may
be cooled.
Also provided is an apparatus for forming polycrystalline particles by
gas phase condensation by employing arc evaporation without an arc
sustaining gas, comprising a vacuum chamber; means for evacuating the
vacuum chamber to a pressure between about 100 Millitorr and 10 torr; a
non-consumable electrode disposed within the vacuum chamber; a
6
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
consumable electrode spatially disposed adjacent to the non-consumable
electrode, the consumable electrode comprising evaporative material; a
power supply having a positive terminal and a negative terminal, the positive
terminal connected to one of the non-consumable electrode or consumable
electrode, the negative terminal connected to the other of the non-
consumable electrode or consumable electrode; means for initiating an arc
discharge between the non-consumable electrode and the consumable
electrode to form ionized vapors whereby an arc path is provided; and means
for evaporating the consumable electrode by a sustained arc discharge,
whereby vapors from the consumable electrode condense to form
polycrystalline particles.
BRIEF DESCRIPTION OF THE FIGURES
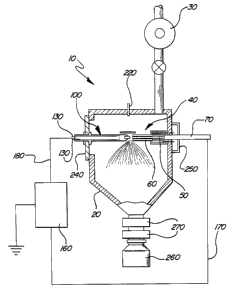
Figure 1 is a schematic cross-sectional view of one embodiment of the
apparatus according to the principles of the present invention;
Figure 2 is an illustration of a non-consumable electrode according to
the principles of the present invention;
Figure 3 is an illustration of a consumable electrode according to the
principles of the present invention;
Figure 4 is an SEM Micrograph of polycrystalline Mg particles
prepared by arc plasma gas plasma condensation at a pressure of 5 torr;
Figure 5 is a graphical representation of an x-ray diffraction pattern for
Mg0 (indicated by "A") and Zr02 (indicated by "B").
DETAILED DESCRIPTION OF THE INVENTION
A vacuum arc is sustained by the vapor emitted from either the
consumable electrode, and is therefore referred to as anode arc evaporation
if the consumable materials evolve from the anode, or cathode arc
evaporation if the vapors evolve from the cathode. Vapor from the cathode is
caused by a well-known phenomenon referred to as "cathode spot", which is
an arc striking spot moving swiftly from one place to another on the cathode
7
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
surface. The actual size of the cathode spot is very small, and in some cases
less than one micrometer. Due to its size, the current density of the cathode
spot is accordingly very large, in some cases a current density of 1 O6 A/cmz
have been estimated. Therefore, the cathode spot has an extremely high
temperature, in some cases the temperature can reach 104 °C or higher.
The
cathode may be a cold cathode or hot cathode depending upon the materials
and objective. A cold cathode is typically formed of a highly thermal
conductive material and is larger in size than the anode. Furthermore, a cold
cathode may be in communication with cooling means to further reduce the
cathode temperature. Alternatively, a hot cathode is one that is not cooled
and is permitted to operate in a non-cooled state. A hot cathode may be
employed during anode evaporation, so long as the vapor pressure of the
cathode is lower than, and the melting temperature is higher than, that of the
anode.
During arc evaporation electrons from the cathode travel across a gap
to the anode. As mentioned above, the transport medium may be an inert
gas, a reactive gas mixed with an inert gas, or as in the case of low-pressure
arc evaporation, evaporated materials from a consumable electrode. The
medium that sustains the arc discharge is a plasma. The plasma is an
ionized vapor that provides an arc path for the current to flow from the
cathode to the anode. Typically, the electrode with a higher vapor pressure
or lower melting point will be the consumed electrode.
Either the cathode or anode may be the consumed electrode. As
stated above, if the anode is consumed, the process is referred to as anode
evaporation. In this case anode vapor can rapidly condense on the surface
of the cathode and may be reevaporated by the cathode spot, which reduces
consumption of the cathode. When the anode is made much smaller in
diameter than the cathode, the anode can be raised to very high temperature,
as high as several thousands of degrees centigrade because of the low
thermal mass and small cross-sectional area for thermal conductance.
The invention disclosed herein is a novel method for forming
8
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
polycrystalline particles by gas phase condensation where evaporation is
achieved by a plasma arc. Referring now to Figure 1, a schematic cross-
sectional illustration of an embodiment of apparatus 10 for forming
polycrystalline materials by gas phase condensation is shown. The
apparatus 10 includes a vacuum chamber 20 in communication with means
for evacuating 30 the chamber 20 to sub-atmospheric pressure. The means
for evacuating 30 may be a mechanical pump or diffusion pump, or any other
suitable means known in the art for evacuating the chamber 20. The
apparatus 10 further comprises a non-consumable electrode 100 disposed
within the chamber 20 adjacent to a consumable electrode 40 also disposed
within the chamber 20. The apparatus 10 further comprises a power supply
160 having a negative terminal 170 and a positive terminal 180, the positive
terminal 180 is connected to one of either the non-consumable electrode 100
or the consumable electrode 40, and the negative terminal 170 is connected
to the other of the non-consumable electrode 100 or consumable electrode
40.
Referring now to Figure 2, a schematic cross-sectional illustration of
the non-consumable electrode 100 is shown. The non-consumable electrode
100 has a refractory tip 110 and may include a cavity 120. The cavity 120
may be provided to circulate a coolant to the non-consumable electrode 100
by cooling lines 130. In the preferred embodiment, the non-consumable
electrode 100 is electrically isolated from the chamber 20 by non-consumable
electrode isolating means 240.
Non-consumable electrode isolating means 240 is provided to prevent
a short between the non-consumable electrode 100 and the chamber 20. In
the exemplary embodiment isolating means 240 is a Pyrex window with an
aperture therein. The non-consumable electrode 100 is disposed within the
aperture of the isolating means 240. The distance between the non-
consumable electrode 100 and the chamber 20, occupied by the isolating
means 240 must be sufficient to avoid an arc short between the non-
9
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
consumable electrode 100 and the chamber wall 20 in order for the isolating
means 240 to be effective. In the preferred embodiment, the chamber wall 20
is grounded to prevent a charge accumulation.
Referring now to Figure 3, an illustration of a consumable electrode 40
according to the principles of the present invention is shown. The
consumable electrode 40 includes a source or evaporative material 50
supported by a support sleeve 60. The evaporative material 50 is electrically
coupled to one of either the negative terminal 170 or the positive terminal
180. In the present example, the support sleeve 60 is disposed within a
consumable electrode body 90. A selectively engageable push rod 70 is
provided to locate the evaporative material 50 within the chamber 20.
Cooling lines 80 may also be provided to be in communication with the
consumable electrode body 90. In the present embodiment, the consumable
electrode 40 is electrically isolated by consumable electrode isolating means
250. In the exemplary embodiment, the consumable electrode isolating
means 250 is Pyrex material with an aperture. The consumable electrode 40
is disposed within the aperture of the isolating means 250. The distance
between the chamber 20 and the consumable electrode 40, occupied by
isolating means 250, must be sufficient to avoid an arc discharge between
the chamber 20 and the consumable electrode 40, in order for the isolating
means 250 to be effective.
The apparatus 10 of the present invention operates at a pressure
between about 10 torr to 100 millitorr to form polycrystalline particles, by
vacuum arc evaporation. Means for evacuating 30 is in communication with
the vacuum chamber 20, and in the preferred embodiment should be capable
of evacuating the chamber 20 to pressure below about 10'5 torr. A gas inlet
manifold 220 is provided to be in communication with the chamber 20,
whereby gases such as inert, reaction, working gases, or any combination
thereof may be provided to the chamber 20.
Referring again to Figure 1, the apparatus 10 of the present invention
CA 02375847 2001-12-06
WO 00/79839 PCTNS00/15878
has negative terminal 170 attached to one of the consumable electrode 40 or
non-consumable electrode 100, thereby forming a cathode. The positive
terminal 180 is attached to the other of the consumable electrode 40 or non-
consumable electrode 100, forming an anode. During evaporation, gap
exists between the consumable electrode 40 and the non-consumable
electrode 100. In order for electrical current to travel from the cathode to
the
anode, a conductor, such as a plasma, must be present between the
electrodes.
Materials evaporated from the source material 50 provide an arc path
between the non-consumable electrode 100 and the consumable electrode
40. Means for initiating an arc discharge between the consumable electrode
40 and the non-consumable electrode 100 form ionized vapors from the
source material 50 to provide an arc path. Any suitable means known in the
art for initiating an arc discharge may be incorporated in the apparatus 10 of
the present invention. Two techniques which are known in the art for
initiating an arc discharge are high-frequency ignition and contact ignition.
In
the preferred embodiment of the present invention the means for initiating an
arc discharge is a simple contact technique.
The push rod 70 is suitably attached to the evaporative material 50. In
the exemplary embodiment, the evaporative material 50 is in the form of a rod
supported by the support sleeve 60 that has inner diameter sufficient to allow
the evaporative material 50 to traverse the sleeve 60. The support sleeve 60
may be formed from any suitable refractory insulator, including Boron Nitride
(BN) and Aluminum Oxide (AIOZ). In the preferred embodiment, the push rod
70 is coupled to either the negative terminal 170 or the positive terminal 180
and is formed of Copper, however, it should become apparent to those skilled
in the art that any suitable alternative material may be substituted for
Copper.
In the exemplary embodiment, the consumable electrode 40 is coupled
to the negative terminal 170 of the power supply 160 through the push rod 70
to achieve cathode arc evaporation. As disclosed above, cathode arc
11
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
evaporation has significant advantages over anode arc evaporation in that a
higher temperature may be achieved with cathode arc evaporation due to the
smaller size of the cathode spot than the anode spot. Although anode arc
evaporation works well for low-melting temperature materials such as
Magnesium, the cathode arc evaporation process of the present invention
may be employed to evaporate high melting temperature transition metals
including, Iron, Molybdenum, Nickel, Tungsten and Zirconium.
In the exemplary embodiment, arc discharge is initiated by contacting
the consumable electrode 40 with the non-consumable electrode 100. The
push rod 70 is engaged to contact the source material 50, disposed within the
consumable electrode 40, with the refractory tip 110, disposed within the non-
consumable electrode 100. The refractory tip 110 may be formed of any
suitable material know in the art, including Tungsten, Hafnium, and Graphite.
Ideally, the evaporative material 50 should have a lower melting point than
the refractory tip 110. Temperature concerns may be compensated by the
addition of non-consumable electrode cooling means or consumable
electrode cooling means, the need for which may be determined by the
source material 50, among other process parameters.
The push rod 70 is engaged whereby the evaporative material 50
contacts, or is brought into sufficiently close proximity to, the refractory
tip
110, to initiate an arc discharge. The arc discharge is formed from an
electrical current traveling from the cathode to the anode, creating a very
high
temperature, whereby a portion of the evaporative material 50 is evaporated.
Ionized vapors are formed by drawing a high current through a very small
spot to achieve a current density sufficient to heat a portion of the
evaporative material 50 to the point of evaporation. Once vapors are formed,
an arc path is provided whereby electrical current may travel from the
cathode to the anode. At this point, the non-consumable electrode 100 and
the consumable electrode 40 may be drawn apart to form a gap. It is within
this gap that the continuous cycle of arc discharge and evaporation occurs. It
is the materials that are evaporated which provide a path for the electrical
12
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
current to sustain evaporation.
In the preferred embodiment the chamber 20 is prepared by
evacuating the chamber 20 to below about 10-5 tort and then releasing a
filling gas, an inert gas, via the gas inlet manifold 220 to the chamber 20 to
increase the chamber pressure to between about 100 Millitorr and 10 tort.
This step may be repeated a number of times in order to remove undesirable
materials from the chamber 20. In the exemplary embodiment, Argon is the
filling gas which is used to provide the correct chamber pressure. Pressure
management means, such as a pressure control valve, is provided to
maintain the chamber pressure throughout production of the polycrystalline
particles.
Pressure management means provides for maintaining the pressure
within the chamber 20 as the temperature within the chamber 20 increases.
Also, as reaction gases are consumed, additional gases must be provided in
order to maintain the chamber pressure. The chamber pressure range has
been found to be an important operating parameter in the formation of
polycrystalline materials.
Also, if the pressure within the chamber 20 becomes too high, the arc
path will be sustained by the inert gas, as opposed to the evaporated
species. If the chamber pressure becomes too low, the evaporated species
will not sufficiently agglomerate, resulting in thin film deposition.
Once the arc discharge is initiated by contact of the electrodes, 40 and
100, and separation, the source material 50 is evaporated to sustain the arc
discharge. In the preferred embodiment, the non-consumable electrode 100
has a larger diameter than the consumable electrode 40. It should be noted
that although the present invention discloses electrodes with a round cross-
section, any suitable electrode geometry may be substituted. The larger
diameter will assist the non-consumable electrode 100 in maintaining a lower
operating temperature because of the larger thermal mass. By reducing the
operating temperature of the non-consumable electrode 100, the likelihood
that the refractory tip 110 will evaporate is reduced. In the exemplary
13
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
embodiment, the non-consumable electrode 100 has a diameter of about 1
inch (1") and is sufficiently cooled by non-consumable electrode cooling
means 140, and the consumable electrode 40 has a diameter of about'/4
inch (1/4") or less. The support sleeve 60 insulates the sides of the rod-
s shaped evaporative material 50, preventing evaporation. Furthermore, the
support sleeve 60 also prevents the evaporative material 50 from collapsing,
as it is not uncommon for the evaporative material 50 to melt since a
substantial vapor is only attainable at a temperature higher than the melting
point of the evaporative material 50.
As stated above, the arc discharge at pressures below about 10 torr
are primarily supported by the vapor from the consumable electrode 40, and
more specifically the evaporative material 50 that actually acts as a tip for
the
consumable electrode 40. Since the arc discharge of the present invention is
primarily supported by the evaporative material, a large portion of the vapor
is
ionized. The plasma which is sustained within the gap between the non-
consumable electrode 100 and the consumable electrode 40 is propelled
away from the arc discharge, or plasma region, due to the high energy of the
ionized species, the high temperature of the plasma region, and the pressure
difference between the plasma region and the surrounding atmosphere. As
the mixture of positively-charged ions and neutral atomic species move
quickly away from the plasma region, electric charge is lost by capturing
electrons, and thermal energy is lost by emitting light. As the expansion
continues, the vaporized species collide with each other or with gases within
the chamber. As stated above, the gases within the chamber may be filling
gases, i.e. inert gases such as Argon or Helium. Also reactive gases such as
Oxygen, Nitrogen, and Hydrogen, may be provided to the chamber 20 to
react with evaporated species to form molecular oxides, nitrides, and
hydrides. Eventually, the vaporized species or reaction products lose most of
their thermal energy and become super-saturated. The super-saturated
vapor nucleates homogeneously to form polycrystalline clusters. The
chamber pressure controls the nucleation rate of the polycrystalline
particles,
14
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
resulting in particle sizes between about 150 nanometers and 200
nanometers. The polycrystalline particles eventually settle within the vacuum
chamber 20 and are guided into a particle collection vessel 260 by any
suitable means known in the art. The particle collection vessel 260 may be
isolated from the vacuum chamber 20 by one or more valves 270.
Referring now to Figure 4, a scanning electron microscope micrograph
of polycrystalline Magnesium particles prepared by the present invention is
shown. Close inspection of the micrograph reveals particle sizes between
about 100 and 200 nanometers in diameter. A number of particles
agglomerated together form particle clusters.
Referring now to Figure 5, a graphical representation of an x-ray
diffraction pattern for Magnesium Oxide, indicated by "A" and Zirconium
Oxide indicated by "B" is shown, where the X-axis is intensity and the Y-axis
is the angle of refraction, also known as 2 theta. The intensity spikes of
line
"A" of Figure 5 show that Magnesium Oxide was indeed produced. The
spikes of line "B" of Figure 5 indicate two separate species of Zirconium
Oxide were formed.
The apparatus 10 and method of the present invention requires a
relatively low power to form polycrystalline particles. Generally, the voltage
required is between about 18-26 volts, and the current is between about 20-
50 amps. The present invention can form 40 grams of Magnesium per hour
at a voltage of 20 volts and current of less than about 50 amps. For very-high
melting temperature materials such as Zirconium, 1 to 2 grams per hour may
be formed at a voltage of 24 volts and current of less than about 50 amps.
The present invention has demonstrated a very high yield with relatively
lower power in the formation of polycrystalline particles. Therefore, it has
been shown that the simplicity of the present invention along with the low
power requirements make the present invention a well received addition to
the art of gas phase condensation by arc plasma.
EXAMPLE 1
CA 02375847 2001-12-06
WO 00/79839 PCTNS00/15878
Magnesium is a material that has a high vapor pressure at
temperatures above 600°C. The high vapor pressure at a relatively low
temperature renders Magnesium a candidate for efficient polycrystalline
particle production by arc plasma gas phase condensation because of the
temperature requirements for evaporation. A Magnesium rod having a
diameter of about one half inch (1/2") was provided as the consumable
electrode, and more specifically as an anode as it is connected to the
positive
terminal of a DC power supply. However, it should be noted that the polarity
of the Magnesium rod may be switched to make it a consumable cathode.
The chamber was first evacuated by mechanical pumping to a
pressure of 20 Millitorr, and then back filled with Argon gas to a pressure of
about 5 Torr. The step of pumping down the pressure of the chamber and
back filling is repeated a number of times to achieve as low an Oxygen
content within the chamber 20 as possible. In order to ignite the arc a direct
touch separation method is used, although a high-frequency plasma can also
be used to start the arc as well. The light emitted from the arc plasma is
green, which is the atomic spectrum of Magnesium, indicating the majority of
ionized species is Magnesium vapor. A specimen of Magnesium particulate
was taken from the vessel and examined by a scanning electron microscope
(SEM).
Magnesium is an active metal and, when in particle form, may be
flammable when exposed to air. The precaution for handling and storing
these Magnesium particles is very important. An effective way to safely
collect and store the materials is in a tightly sealed container filled with
an
atmosphere of Argon gas. The Magnesium sample taken for analysis, which
is only about 1/10 of a milligram, was extracted from an Oxygen-free
container. The surface layer of Magnesium particles is slowly oxidized within
the container to make the surface layer inactive. The details of the particle
morphology and particle size of the Magnesium particulate are shown in
Figure 4. Figure 4 shows that the particles are loosely agglomerated and that
the individual particle has spherical or facet-less morphology. The particle
16
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
size range is between about 100 to 200 nanometers.
EXAMPLE 2
Pure Magnesium Oxide (Mg0) is a refractory ceramic material for high
temperature applications such as a crucible or thermal insulator.
Polycrystalline Magnesium Oxide particles have the advantage of a much
lower sintering temperature than conventional materials. Sintered parts from
polycrystalline Magnesium Oxide particles have a finer microstructure, which
improves the toughness and machinability of the parts. In order to form
Magnesium Oxide polycrystalline particles, an additional step is required
beyond the steps required to form Magnesium polycrystalline particles.
Either pure Oxygen or an Oxygen/inert gas mixture is provided within the
chamber for the preparation of Magnesium Oxide. In the present example, a
mixture of 20% (volumetric percentage) Oxygen with Argon is used. This
mixture is preferred because it was observed that pure Oxygen reacts rapidly
with the tip of the consumable electrode to form an oxide cap. The oxide cap
significantly decreases the vaporization rate of the evaporative material,
therefore the production rate of the polycrystalline oxide particles is
reduced
significantly. Because Magnesium has a very high vapor pressure, the
Magnesium may be adapted to be evaporated from either a consumable
cathode or a consumable anode. The chamber pressure of the present
example for production of Magnesium Oxide is 5 Torr. The x-ray diffraction
pattern of polycrystalline Magnesium Oxide particles is shown in Figure 5 as
graph "A".
EXAMPLE 3
Zirconium (Zr) is a material that has a high melting point and a low
vapor pressure, therefore thermal evaporation of this metal is extremely
difficult. As discussed above, a cathode spot is distinguished from an anode
spot by its spot size which is estimated to be a few micrometers or less. The
temperature of a cathode spot may reach ten thousand (10,000) degrees
17
CA 02375847 2001-12-06
WO 00/79839 PCT/US00/15878
Kelvin or higher. The present invention has demonstrated the capability to
form polycrystalline particles by gas phase condensation from plasma arc
evaporation of refractory materials such as Zr. In the present example, the
source material, Zr, is provided in the form of a rod and electrically coupled
to
the negative lead of a DC power supply, therefore the Zr rod becomes a
cathode. The Zr arc is very stable at a current of about 50 amps and a
potential of 22 volts. In the production of Zirconium Oxide, the concentration
and pressure of the Oxygen/Argon mixture within the chamber 20 is similar to
the concentration and pressure for the production of Magnesium Oxide in the
previous example. The yield efficiency for Zirconium Oxide is estimated to be
a few grams per kilowatt hour (kWh). The x-ray diffraction pattern of
polycrystalline Zirconium Oxide (ZrOz) is shown in Figure 5 as graph "B".
Therefore, it can clearly be seen that the novel teachings of the
present invention show promise for commercial and industrial processing of
polycrystalline particles. While the invention has been described in
connection with preferred embodiments and procedures, it should be
understood that it is not intended to limit the invention to the described
embodiments and procedures. On the contrary, it is intended to cover all
alternatives, modifications, and equivalents which may be included within the
spirit and scope of the claims appended hereto.
18