Note: Descriptions are shown in the official language in which they were submitted.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
METHODS OF OBTAINING 2-METHOXYESTRADIOL OF
HIGH PURITY
RELATED APPLICATIONS
This application claims priority to U.S. Provisional
Application Serial No. 60/150,293 filed August 23, 1999.
FIELD OF THE INVENTION
The invention relates to the estradiol metabolite 2
methoxyestradiol and to methods of obtaining purified 2
methoxyestradiol.
BACKGROUND OF THE INVENTION
2-Methoxyestradiol, 1,3,5(10)-estratrien-2,3,17(3-triol-2-
methyl-ether (2-ME2) is an endogenous metabolite of estradiol, the
major ovarian estrogen. The chemical formula of 2-ME2 is C,9HZ60~,
and the compound has a molecular weight of 302.4. 2-ME2 has low of
estrogenic activity but has been found to have other biological effects.
U.S. Patent Nos. 5,504,074, 5,661,143, and 5,892,069 to
D'Amato et al. disclose methods of treating mammalian diseases
characterized by abnormal cell mitosis using 2-ME2. Undesirable cell
mitosis is characteristic of many diseases, including, but not limited to,
cancer, atherosclerosis, proliferation of solid tumors, vascular
malfunctions, endometriosis, retinopathies, arthropathies, and
abnormal wound healing. In addition, cell mitosis is important in a
wide variety of biological functions, including but not limited to the
normal development of the embryo, formation of the corpus luteum,
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
2
cyclic proliferation of uterine endometrium, wound healing, and
inflammatory and immune responses.
U.S. Patent No. 5,521,168 to Clark discloses using 2-ME2
for lowering intraocular pressure. 2-ME2 also inhibits estrogen-
induced pituitary tumor angiogenesis and suppresses tumor growth in
Fisher 344 rats as reported by Banerjee, S.K. et al., Proc. Amer. Assoc.
Cancer Res. 39, March 1998.
Presently, commercially available preparations of 2-ME2
are either less than 98% pure or contain undesirable steroid
contaminants that are of concern for pharmaceutical uses. Important
contaminants of these preparations are estradiol, 4-hydroxyestradiol, 4
methoxyestradiol, 2-hydroxyestradiol, estrone, and 2-methoxyestrone.
The amounts of these contaminants that are found in presently
available 2-ME2 preparations are unacceptable for pharmaceutical
applications.
Any therapeutic use of 2-ME2 in humans requires 2-ME2
having a high level of purity. In general, therapeutic agents are
required to be substantially pure to avoid negative side effects of
contaminants. In particular, since 2-ME2 has effects that are
counteracted by estradiol and other estrogenic metabolites, it is crucial
to have a 2-ME2 preparation substantially free of such contaminants.
Effects that may be seen from contaminating estradiol, estrone, and 2-
hydroxyestradiol include estrogenic effects such as feminization,
endometrial proliferation, increased risk of uterine and breast cancer,
developmental effects on sexual organs, inhibition of leukopoesis, and
effects on hematopoetic cells. 4-hydroxyestradiol, 4-methoxyestradiol,
and estradiol are known mutagens and carcinogens.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
3
Accordingly, what is needed is a composition of 2-ME2
which is greater than 98% pure and which contains substantially n o
estradiol or other steroids having estrogenic or carcinogenic effects.
What is also needed is a composition containing 2-ME2
that is greater than 99.5% pure.
What is also needed are methods for making 2-MEZ of
greater than 98% purity and containing substantially no estradiol or
other steroids having estrogenic or carcinogenic effects.
Also needed are methods of substantially separating 2
ME2 from estradiol, related molecules, and other contaminants,
resulting in 2-ME2 having a purity of greater than 99.5%.
SUMMARY OF THE INVENTION
The present invention provides 2-ME2 having greater
than 98% purity, more preferably greater than 99% purity, most
preferably greater than 99.5% purity. The 2-ME2 preparations
preferably contain less than 0.03% estradiol, 0.02% or less 2-
hydroxyestradiol, 0.02% or less 4-hydroxyestradiol, 0.02% or less 4-
methoxyestradiol, and less than 0.02% estrone. More preferably, the 2-
ME2 preparations contain 0.01% or less estradiol, 0.02% or less 2-
hydroxyestradiol, 0.01 % or less 4-hydroxyestradiol, 0.01 % or less 4-
methoxyestradiol, and 0.01 % or less estrone.
The present invention also provides methods of
obtaining 2-ME2 of greater than 98% purity, more preferably greater
than 99% purity, most preferably greater than 99.5% purity. In some
embodiments, the methods involve synthetic techniques. In other
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
4
embodiments, the methods involve purification techniques to
separate the 2-ME2 from other compounds. In yet other embodiments,
the methods involve both synthetic techniques and purification
techniques described herein.
The purification methods involve the use of liquid-solid
chromatography (LSC) to separate 2-ME2 from other compounds. The
chromatographic media is preferably silica. The solvent system
comprises a non-polar solvent, such as chloroform, and a polar
solvent, such as methanol.
Accordingly, an object of the present invention is to
provide 2-ME2 having a purity greater than 98%.
Another object of the present invention is to provide 2-
ME2 substantially free of estradiol, related compounds, and other
unwanted impurities.
Still another object of the invention is to provide
methods of obtaining substantially pure 2-ME2 by synthetic techniques.
Another object of the invention is to provide methods of
obtaining substantially pure 2-ME2 by purification techniques.
Other features and advantages of the invention will be
apparent from the following description of preferred embodiments
thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a chromatogram from the reversed phase
HPLC analysis of 2-methoxyestradiol available from Sigma Chemical
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
Company (45H4033). This graph shows that the Sigma product
contains about 0.034% estradiol.
Figure 2 is an expanded view of the chromatogram in
Figure 1 indicating the estradiol impurity.
5 Figure 3 is a chromatogram from the reversed phase
HPLC analysis of 2-methoxyestradiol available from Research Plus
(10699). This graph shows that the Research Plus product contains
about 0.024% estrone and about 0.93% other undesirable estrogens.
Figure 4 is an expanded view of the chromatogram in
Figure 3 indicating the estrone impurity.
Figure 5 is a chromatogram from the reversed phase
HPLC analysis of the unpurified 2-methoxyestradiol employed as the
starting material in Example 2 of the present invention.
Figure 6 is a chromatogram of the 2-ME2 of the present
invention produced in Example 2. The HPLC was run with a non-
overloaded amount of sample, 75.6 ~g (14 ~l at 5.4 ~1/ml).
Figure 7 is an expanded view of the chromatogram in
Figure 6.
Figure 8 is a chromatogram of the 2-ME2 of the present
invention produced in Example 2. The HPLC was run with an
overloaded amount of sample, 270 ~g (50 ~.l at 5.4 ~1/ml).
Figure 9 is an expanded view of the chromatogram in
Figure 8.
WO 01/14405 CA 02376297 2002-O1-23 pCT/US00/23160
6
Figure 10 is a chromatogram of the pooled fractions from
Example 2, assayed using a gradient (20 to 70% acetonitrile over 25
minutes, 1 % acetic acid, and remainder water). 43.2 ~.g (8 ~,1 of the 5.4
~l/ml sample) was injected.
Figure 11 is an expanded view of the chromatogram in
Figure 10.
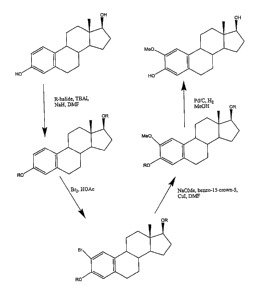
Figure 12 depicts a synthetic reaction scheme for the
production of the 2-methoxyestradiol of the present invention, using
estradiol as a starting material and employing bromine, a crown ether,
and a blocking group on the 3- and 17-position hydroxyl oxygen atoms
of the estradiol.
Figure 13 depicts a synthetic reaction scheme for the
production of the 2-methoxyestradiol of the present invention, using
estradiol as a starting material and employing bromination at the 2
position of the A ring of unblocked estradiol and a crown ether.
Figure 14 depicts a synthetic reaction scheme for the
production of the 2-methoxyestradiol of the present invention, using
estradiol as a starting material and employing a blocking group on the
3- and 17-position hydroxyl oxygen atoms of estradiol, nitration, and a
Sandmeyer reaction.
Figure 15 depicts a synthetic reaction scheme for the
production of the 2-methoxyestradiol of the present invention, using
estrone as a starting material and employing a blocking group on the 3-
position hydroxyl oxygen atom, nitration, and a Sandmeyer reaction.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
7
Figure 16 depicts a synthetic reaction scheme for the
production of the 2-methoxyestradiol of the present invention, using
estradiol as a starting material and employing bromination at the 2-
position of the A ring of unblocked estradiol and reaction with
methanol.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to 2-methoxyestradiol
having a purity of greater than 98.0%, more preferably greater than
99.0%, and most preferably of 99.5% or higher. 2-ME2 can be obtained
through synthesis methods or purification methods described herein
that yield highly pure 2-ME2. The synthesis methods described herein
may also be supplemented with the purification methods described
herein to yield 2-ME2 having even greater purity.
Although the terms "2-methoxyestradiol" and 2-ME2 are
specifically used herein, it should be understood that the methods
disclosed herein can be used for synthesis or purification of other
compounds, such as, but not limited to, estradiol and other
structurally related steroids.
Methods of Synthesis
The present invention provides methods of synthesizing
2-ME2 to a purity of greater than 98.0%, more preferably greater than
99.0%, and most preferably of 99.5% or higher. The synthetic methods
described herein can also be used, with minor modifications, to
synthesize other 2- and 4- derivatives or analogues of estradiol, such
as, for example, 4-methoxyestradiol and 4-hydroxyestradiol.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
g
There are several synthetic approaches that can be taken
to prepare 2-ME2 having a purity greater than 98.0%. Alternatively, 2-
ME2 can be purified according to the following purification methods to
have a purity greater than 98.0%. These different synthetic approaches
utilize different starting materials and intermediates; consequently,
different yields, side reactions and impurities will be obtained.
Two similar approaches employ estradiol as a starting
material and utilize a brominated intermediate, as taught by Rao, P.N.
et al., Synthesis, 1977, 168 and Chen, S-H et al., Steroids, 1986, 47, 63.
The first approach is illustrated in Figure 12. The free hydroxyl groups
of estradiol are protected with a blocking group. A wide range of
blocking groups can be used in the present invention. These blocking
groups include, but are not limited to, alkyl, aryl, aralkyl groups, and
alkyl, aryl, and aralkyl group containing one or more heteroatoms.
For example, protection can be accomplished using an alkyl halide,
such as benzyl bromide, to form an alkyl ether. Appropriate
conditions for hydroxyl protection include reaction of the estradiol and
alkyl halide in the presence of NaH and TBAI, optionally in the
presence of a solvent, such as dimethyl formamide (DMF). The
protected estradiol is then reacted with bromine, for example, in the
presence of acetic acid. Protection of the free hydroxyls during
bromination gives a higher yield of the 2-brominated intermediate
(about 70% vs. about 20% without the protecting groups) (see
Cushman, M. et al., J. Med. Chem. 1997, 40, 2323).
The bromine is then replaced with a methoxide group
using a copper catalyst. For example, the brominated intermediate can
be reacted with NaOMe in the presence of a copper catalyst, such as
WD 01/14405 CA 02376297 2002-O1-23 pCT~S00/23160
9
CuI. The reaction is preferably conducted in a solvent, such as DMF,
optionally in the presence of a promoter. Acceptable promoters,
include, but are not limited to, crown ethers, such as benzo-15-crown-
5.
Removal of the protecting groups, for example, by
catalytic hydrogenation of the alkyl moiety, yields 2-ME2.
Unfortunately, this synthetic route yields about 1-2% impurity of
estradiol from the methoxylation step (a hydride quenches the reactive
copper complex rather than a methoxide). The estradiol can be
removed to undetectable levels by chromatography, such as described
below, or significantly reduced by successive crystallization i n
chloroform.
Another synthetic method utilizing a brominated
intermediate and employing estradiol as the starting material is
illustrated by Figure 13. In this synthetic reaction, the estradiol is ring
brominated without first blocking the hydroxyl groups. The bromine
is then replaced with a methoxide using a copper catalyst in a manner
similar to that described above.
In another approach, estradiol or estrone can be used as a
starting material in a reaction scheme that utilizes nitro/amine
intermediates (see Cushman, M. et al., J. Med. Chem. 1995, 38, 2041).
These synthetic approaches are illustrated in Figure 14 (estradiol
starting material) and Figure 15 (estrone starting material). In these
approaches, the free hydroxyl groups are protected. This protection can
be accomplished, for example, using an alkyl halide, such as benzyl
bromide, to form an alkyl ether. Appropriate conditions for hydroxyl
protection include reaction of the starting material and alkyl halide in
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
the presence of NaH and TBAI, optionally in the presence of a solvent,
such as dimethyl formamide (DMF).
The protected starting material is then nitrated, for
example, with nitric acid and acetic acid or with nitric acid and sulfuric
5 acid, to form the corresponding 2-vitro product. The vitro group is
then reduced. Selective reduction can be accomplished by catalytic
hydrogenation, for example, hydrogenation in the presence of Pd/C to
produce the corresponding 2-amine. The catalytic reduction is
optimally carried out for a period of one hour. Using Sandmeyer
10 conditions (nitrous acid and sodium methoxide), the 2-amino group
can be converted to the 2-methoxy substituent. Catalytic
hydrogenation removes the protecting groups to give 2-ME2 when the
starting material is estradiol and 2-methoxyestrone when the starting
material is estrone. Reduction of the 17-keto group of 2-
methoxyestrone with sodium borohydride yields 2-ME2.
Yet another method employs estradiol as the starting
material and utilizes brominated intermediates. In this synthetic
reaction, the estradiol is ring brominated without first blocking the
hydroxyl groups. Bromination is accomplished, for example, with
bromine and acetic acid in a solvent, such as THF. This reaction
results in bromination at different sites on the ring, including multi-
brominated species. The 2-bromo-estradiol can then be isolated from
the other brominated intermediates, for example, by chromotography
or crystallization, followed by replacement of the bromine with a
methoxide. The bromine can be replaced with a methoxide group, for
example, using sodium methoxide and methanol in the presence of a
copper catalyst, such as CuI, in a manner similar to that described
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
11
above. Alternatively, the intermediates can be reacted to form the
corresponding methoxides, followed by isolation of the 2-
methoxyestradiol by the methods described above.
Methods of Purification
The present invention provides methods of purifying 2-
ME2 to a purity of greater than 98.0%, more preferably greater than
99.0% and most preferably of 99.5% or higher. The 2-ME2 preparations
preferably contain less than 0.03% estradiol, 0.02% or less 2-
hydroxyestradiol, 0.02% or less 4-hydroxyestradiol, 0.02% or less 4-
methoxyestradiol, and less than 0.02% estrone. Most preferably, the 2-
ME2 preparations contain 0.01% or less estradiol, 0.02% or less 2-
hydroxyestradiol, 0.01% or less 4-hydroxyestradiol, 0.01% or less 4-
methoxyestradiol, and 0.01% or less estrone.
The purification methods of the present invention
involve liquid chromatography on an adsorption/partition medium
such as silica, using a solvent system comprising a polar and a non-
polar solvent. The purification methods described herein can also be
used, with minor modifications, to purify compounds similar to 2-
ME2, such as, for example, 4-methoxyestradiol, 4-hydroxyestradiol, 2-
hydroxyestradiol, estradiol, estrone, 2-methoxyestrone, and 4-
methoxyestrone.
The Sample
The sample to be purified can be synthesized, or obtained
from a biological source. The sample may be a commercially available
2-ME2 preparation, such as those sold by Sigma-Aldrich Chemicals of
St. Louis, Missouri, Research Plus, Inc. of Bayonne, NJ, or Calbiochem
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
12
of San Diego, CA. The sample is preferably at least about 50% pure,
more preferably about 75% pure, even more preferably about 90%
pure, and most preferably about 98% pure. The sample can be
subjected to other purification steps prior to the methods described
herein, such as selective crystallization.
The sample is preferably dissolved into or solvent-
exchanged into a loading solvent, as further described below. The
sample is preferably at a concentration in the range of about 0.01 to 2
g/ml, preferably about 0.01 to 1 g/ml, more preferably about 0.05 to 0.2
g/ml.
Chromatographic Media
Silica is preferably used as the chromatographic medium.
Silica gel of about 70-400 mesh is preferred, most preferably about 70-
230 mesh, such as supplied by Merck and other vendors. The medium
can be used loose, in batch chromatography, or packed into a column.
Pre-packed columns, such as those sold by Biotage of Charlottesville,
VA, can also be used. The medium should be equilibrated in an
appropriate solvent before application of the sample to the medium, as
further discussed below.
Column Dimensions
The chromatographic methods described herein can be
achieved using batch or column chromatography. In batch
chromatography, the sample and the chromatographic medium are
combined in a container for a period of time sufficient to allow the 2-
ME2 to be retained by the medium. The medium is then preferably
washed with wash solvent. Elution solvents are then applied to the
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
13
medium. After the loading, wash, and elution steps, the solvent is
removed from the medium, such as by filtration.
For column chromatography, a column having
appropriate dimensions is packed with the chromatography medium.
The column, after equilibration with appropriate solvent, is loaded
with sample by applying the sample to the top, or entrance, of the
column. The ratio of the sample volume to column diameter should
preferably be between about 0.2 to 3 ml/cm, and more preferably
between about 0.5 and 1.5 ml/cm for best results.
Solvents
A solvent system including a polar solvent, such as
methanol (MeOH), and a non-polar solvent, such as chloroform
(CHC13), is used. Other polar solvents that can be used include, but are
not limited to, tetrahydrofuran (THF), ethyl acetate, isopropanol,
ethanol, propanol, and combinations thereof. Other non-polar
solvents that can be used include, but are not limited to, hexane,
dichloromethane, cyclohexane, pentane, and combinations thereof.
More specifically, solvent systems that can be used include
THF/hexane, ethyl acetate/hexane, isopropanol/hexane,
ethanol/CHCI~, propanol/CHCI~, isopropanol/CHCI~, and
combinations thereof.
The sample is soluble in the polar solvent. Some
amount of the polar solvent, generally about 10%, is needed to render
the sample soluble in the loading solvent. The loading solvent thus
will include up to about 10% polar solvent and about 90% non-polar
solvent.
W~ 01/14405 CA 02376297 2002-O1-23 pCT~S00/23160
14
After the sample is loaded onto the medium, the
medium may be washed with a wash solvent that will wash
contaminants off the medium but will not elute the 2-ME2. The wash
solvent comprises mostly non-polar solvent, with enough polar
solvent to prevent the 2-ME2 from precipitating but not enough polar
solvent to elute the 2-ME2.
The sample is eluted with elution solvent that contains
enough polar solvent to elute the 2-ME2 from the medium. The
elution solvent may be applied as a step gradient or as a linear
gradient, as described below.
Column Conditions
The wash and elution solvents can be applied to the
column in a step gradient or in a linear gradient. In a preferred
embodiment, the solvents are applied using a step gradient of
increasing concentration of polar solvent.
The column can be operated using the force of gravity or
can be operated with a pump that forces liquids through the column.
The rate at which the column is operated will depend upon the
volume and dimensions of the column and the silica gel particle size.
In general, the column can be operated at a rate from about 0.5 to 5
ml / min.
The eluant can be monitored visually or, monitored with
a spectrophotometer at a wavelength of about 288 nm, which is the
absorbance maximum of 2-ME2, and collected as the 2-ME2 elutes from
the column.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
The column can optionally be operated under pressure
and can optionally be heated. Preparative high performance liquid
chromatography (HPLC), either normal phase or reversed phase, or
fast performance liquid chromatography (FPLC) techniques can be
5 used. Commercial preparative chromatography apparatus, such as
that sold by Biotage of Charlottesville, VA, can also be used. Other
known methods of improving column efficiency and/or speed can
also be employed.
Sample Collection and Treatment
10 The eluant can be collected as fractions which are then
assayed for 2-ME2 content and purity. These fractions can then be
combined to achieve the desired purity of 2-ME2. The fractions can be
assayed for purity using reverse phase HPLC with a C-18 column
(Waters) and an isocratic solvent system of 30:69:1
15 acetonitrile:water:acetic acid. Other systems can be used for sample
analysis, such those that use solvent gradients instead of the isocratic
solvent system; those that use trifluoroacetic acid or formic acid rather
than acetic acid; and those that use methanol rather than acetonitrile.
Alternatively, or in combination, the eluant can be
monitored in real time and sample collection begun when 2-ME2 of
desired purity elutes from the column.
The solvent is removed from the pooled fractions by use
of a vacuum and/or other solvent removal methods. Lyophilization
and other evaporative methods can be used.
CA 02376297 2002-O1-23
WO 01/14405 PCT/USOO123160
16
Preferred Embodiment
In a preferred embodiment the medium is silica, which
is packed into a column. The sample is dissolved in a mixture oI
CHC13 and MeOH, with enough MeOH to solubilize the 2-ME2,
generally about 90:10 CHCI3:MeOH. The elution conditions are a step
gradient from 99:1 CHCI3:MeOH to 98:2 CHCI3:MeOH.
This invention is further illustrated by the following
examples, which are not to be construed in any way as imposing
limitations upon the scope thereof. On the contrary, it is to be clearly
understood that resort may be had to various other embodiments,
modifications, and equivalents thereof which, after reading the
description herein, may suggest themselves to those skilled in the art
without departing from the spirit of the present invention and/or the
scope of the appended claims.
EXAMPLE 1
Commercially available samples of 2-ME2 were assayed by
analytical HPLC to determine their overall purity and the amounts of
certain contaminants, namely estradiol, 4-hydroxyestradiol, 4-
methoxyestradiol, 2-hydroxyestradiol, estrone, and 2-methoxyestrone.
These analytical HPLC chromatograms were generated
using reverse phase HPLC with a C-18 column (Waters) and a solvent
gradient (20 to 50% acetonitrile over 30 minutes, 50 to 80% acetonitrile
over 5 minutes, 1 % acetic acid, remainder water). The eluant was
monitored at a wavelength of 288 nm. In this system 2-ME2 elutes at
about 21.5 minutes, estradiol elutes at about 20.0 minutes, estrone
WU 01/14405 CA 02376297 2002-0l-23 pCT/US00/23160
17
elutes at about 23.2 minutes, 4-hydroxyestradiol elutes at about 15.0
minutes, 4-methoxyestradiol elutes at about 20.4 minutes, 2-
hydroxyestradiol elutes at about 15.4 minutes, and 2-methoxyestrone
elutes at about 24.4 minutes.
The chromatogram of a sample from Sigma-Aldrich
Chemicals of St. Louis, Missouri is shown in Figure 1. The sample has
an overall purity of 99.2% but has contaminating estradiol of about
0.034%, an unacceptable amount. Figure 2 is an expanded view of the
chromatogram of Figure 1.
Figure 3 is a chromatogram of a sample obtained from
Research Plus, Inc. of Bayonne, NJ that shows that the 2-ME2 has a
purity of 98.6%. The automatic peak calculator and the expanded view
shown in Figure 4 show that the preparation contains 0.024% estrone,
an unacceptable amount of this contaminant. Other samples tested
showed 2-ME2 purity less than 98%, including a second batch obtained
from Research Plus (97.2% 2-ME2) and a sample from CalBiochem of
San Diego, California (91.8% 2-ME2).
Table 1, below, illustrates the purity and contaminants of
these commercially available samples of 2-ME2 and the purified 2-ME2
of the present invention.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
18
TABLE 1
SigmaResearchResearchCalbiochemPharmEcopurified
Plus, Plus,
Lot #1 Lot
#2
2-ME2 99.1898.61 97.17 91.80 97.80 99.98
estradiol 0.03 n.d. n.d. 1.78 2.2 less than
0.01 %
estrone n.d. 0.02 0.43 0.011 n.d.
4-hydroxy-n.d. n.d. n.d. n.d. n.d.
estradiol
4-methoxy-0.49 0.121 0.18 1.99 n.d.
estradiol
2-hydroxy-n.d. n.d. n.d. 0.06 n.d.
estradiol
2-methoxy-n.d. n.d. n.d. 0.20 n.d.
es trone
'~ n.d. means none was detected.
EXAMPLE 2
A 55 cm diameter (60 cm height) glass column was packed
with 600 g silica gel (70-230 mesh from Merck) in 90:10 CHCI3:MeOH.
The column was washed with one liter of CHC13 to remove the MeOH
from the column.
The sample was 3.5 g 2-ME2 in 60 ml 90:10 CHCI3:MeOH.
The 2-ME2 was obtained from PharmEco Laboratories, Inc. of
Lexington, MA, and was 97.8% pure as determined by analytical HPLC
(Figure 5). The peak eluting at 10.917 is estradiol (2.2%).
Analytical HPLC of the starting material, the column
fractions, and the pooled product was performed using reverse phase
HPLC with a C-18 column (Waters) and an isocratic gradient of 30:69:1
WO 01/14405 CA 02376297 2002-O1-23 PCT/US00/23160
19
acetonitrile:water:acetic acid, which provides good separation of 2-ME2
and estradiol. The eluant was monitored at a wavelength of 288 nm.
The sample was applied to the top of the column and
allowed to enter the bed volume. The column was eluted with one
liter of 99:1 CHCI3:MeOH and then 1.5 L of 98:2 CHCI3:MeOH.
Fractions of 50 ml each were collected and 15 fractions containing 2-
ME2 were assayed for 2-ME2 purity using the analytical isocratic HPLC
system described above. Nine to ten fractions that showed no amount
of estradiol were pooled together and solvent was evaporated. After
drying under vacuum for 4 hours, 3.2 g of yellow/white crystals were
collected, for a 91% yield.
Purity of the pooled fractions was determined by
analytical HPLC to be 99.984%, using the isocratic technique described
above. The HPLC chromatograms are shown in Figures 6 through 9.
Figure 6 was generated with a non-overloaded amount of sample, 75.6
~g (14 ~,1 at 5.4 ~1/ml). Figure 7 is an expanded view of the
chromatogram of Figure 6. The automatic peak finder calculated the 2-
ME2 to be 100.0%, although a small, unknown impurity peak is seen
in the expanded view, eluted prior to the 2-ME2. Figure 8 was
generated with an overloaded amount of sample, 270 ~g (50 ~1 at 5.4
~1/ml). Figure 9 is an expanded view of the chromatogram of Figure 8.
The automatic peak finder calculated the 2-ME2 to be 99.984%p pure,
with a small, unknown, impurity that eluted prior to the 2-ME2, and
after estradiol, that was calculated to be 0.016%. The expanded view
shown in Figure 9 shows this impurity peak more clearly and shows
that the 2-ME2 peak is very clean.
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00l23160
The pool was also assayed using a gradient (20 to 70%
acetonitrile over 25 minutes, 1% acetic acid, and remainder water).
43.2 ~,g (8 ~l of the 5.4 ~l/ml sample) was injected. The chromatogram
is shown in Figure 10. The automatic peak finder calculated the 2-ME2
5 to have a purity of 99.825%. However, when an artifact peak, present
in a blank run, at 29.45 minutes is removed from consideration, the
calculated purity is 99.9%. Figure 11 is an expanded view of this
chromatogram. The unknown impurity at 13.43 minutes was
calculated to be 0.012%. If estradiol were present, it would elute
10 between the unknown purity and the 2-ME2 peak. If estradiol is
present, therefore, it can be present at no more than 1/3 to 1/4 of the
amount of the unknown peak. Accordingly, the estradiol amount was
estimated to be no more than 0.004%. The preparation contained
0.02% or less 2-hydroxy-estradiol, 0.01% or less 4-hydroxy-estradiol,
15 0.01 % or less 4-methoxy-estradiol, and 0.01 % or less estrone, as
demonstrated by the lack of any measurable peaks at the expected
retention times.
The purified sample was also subjected to elemental
analysis and the results are shown in Table 2.
20 TABLE 2: Elemental Analysis
Element Theoretical Found
Carbon 75.46 75.21
Hydrogen 8.67 8.65
Oxygen 15.87 16.13 (obtained
by difference)
Chlorine 0.00 0.0
CA 02376297 2002-O1-23
WO 01/14405 PCT/US00/23160
21
The above description is intended to be illustrative and
not restrictive. Many embodiments will be apparent to those of skill
in the art upon reading the above description. The scope of the
invention should, therefore, be determined not with reference to the
above description, but should instead be determined with reference
to the appended claims, along with the full scope of equivalents to
which such claims are entitled. The disclosures of all articles and
references, including patents, patent applications and publications,
are incorporated herein by reference.