Note: Descriptions are shown in the official language in which they were submitted.
05-01-2001 US001583~
CA 02376532 2001-12-05
BIOSENSORS WHICH UTILIZE CHARGE NEUTRAL
CONJUGATED POLYMERS
S This invention claims the priority of provisional application Serial No.
60/138,437 filed
June 10, 1999.
FIELD OF THE INVENTION
The invention is in the field of arrays of sensing electrodes on a chip for
conducting
analysis of biological substances such as DNA.
BACKGROUND OF THE INVENTION
A device for biomolecule detection is generally comprised of supporting matrix
for
probe molecule attachment or entrapment, a sensing probe located on/in the
supporting matrix.
When exposed to a complementary biomolecule target (or analyte), the
biosensing device
produces detectable change in radioactive, optical, or electrical signal to
confirm the existence
of a specific biomolecule target. In general, the biomolecule target to be
detected needs to
be labeled with a marker (or reporter) such as 32P, fluorescent dye, or redox,
depending
on whether the detection means is autoradiography, fluorescent microscope or
electric
tools.
An alternative biosensing device includes a second reporting molecule. The
second
reporting molecule is introduced after the probe molecule has interacted with
its
complementary biomolecule target. Like the probe molecule, the second
reporting molecule
also interacts with the biomolecule target by either binding to the target or
forming a
complex.
Livache, et al Analytical Biochemistry 255, 188-194 (1998) describes an
1
AMENDED SHEET
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
oligonucleotide array constructed on a silicon chip having a matrix of
addressable
microelectrodes. Each electrode is coated with polypyrrole copolymer where
some
of the pyrroles in the copolymer have an oligonucleotide bound to the pyrrole.
The
polymers are made by electrochemical techniques. This copolymer is deposited
on
microelectrodes. Hepatistis C genotypes were detected by hybridization of the
probe
DNA on the electrode to test sample DNA which was PCR amplified to contain a
fluorescent marker group.
WO 95/29199 describes functionalized polypyrrole copolymers where the
functional groups are designed to bind biological molecules such as DNA or
polypeptides.
US Patent 5,837,859 assigned to Cis Bio International describes the
preparation of electrically conductive pyrrole/nucleotide/derivatized/pyrrole
copolymers useful for nucleic acid synthesis, sequencing and hybridization.
The
copolymers are produced electrochemically and coated on microelectrodes for
DNA
analysis.
US Patent 5,202,261 describes conductive sensors and their use in diagnostic
assays.
US Patent 5,403,451 describes the detecting of a target analyte with
conductive
polymer coupled with periodic alternating voltage.
In a typical prior art, the target DNA is usually labeled with a marker (or
reporter)
such as 32P, fluorescent dye, or redox. When the labeled target is exposed to
its
complimentary probe on the conductive polymer or copolymer, a radioactive
signal,
or fluorescence, or electric signal is detected. Generally, fluorescent or
redox labeling
2
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
is preferred due to the stringent experiment conditions required for
radioactive
labeling. However fluorescent dyes in the vicinity of conductive polymers or
copolymers are subject to signal quenching. On the other hand, conductive
polymers
or copolymers contribute to significant background noise when used for redox
labeled
target detection.
SUMMARY OF THE INVENTION
It is an object of the present invention to eliminate the signal quenching
from
conductive polymers when used as supporting matrix for probe attachment or
entrapment for biomolecule detection and a biosensing device to carry-out such
detection.
It is another object of the present invention to reduce the detection noise
from
conductive polymers when used as supporting matrix for probe attachment or
entrapment for biomolecule detection.
It is still another object of the present invention to provide a simplified
method
for biomolecule detection and a biosensing device to carry-out such detection.
The invention is directed to a method of detecting biological molecule
(biomolecule) such as DNA, RNA and polypeptides with the aid of a neutralized
conjugated polymer or copolymer on electrodes. Compared to prior art, the
present
invention makes use of a functionalized polymer or copolymer in its neutral
state,
instead of conductive state as the supporting matrix for biomolecule probe
attachment
or entrapment in a biomolecule detection device.
In one embodiment of the invention, aromatic monomers and functionalized
aromatic monomers are electrochemically polymerized and deposited on an
electrode
surface to generate a functionalized polymer or copolymer. The as-deposited
conjugated polymer or copolymer is in a charged, conductive state. In present
3
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
invention, the charged, functionalized polymer or copolymer is
electrochemically
reduced to a neutral state to form (charge neutral conjugated polymer) before
it is
used in any biomolecule detection.
The charge neutral functionalized polymer or copolymer has low electric
background when used in electric detection of biomolecules. It also does not
quench
I O fluorescent signal when used in fluorescent detection of biomolecules. In
both cases,
the resulting devices have significantly improved signal to noise ratio, thus
enhancing
the sensitivity of biomolecule detection.
Thus, the invention includes a charge neutral conjugated polymer which have
functional groups for binding biomolecule probes to the polmyer. The invention
includes electrodes in electrical communication with such polmyers, arrays of
such
electrodes. The invention includes biosensors which a biomolecule probe is
covalently linked to the functional group of the charge neutral conjugated
polymer on
electrode and a binding of a biomolecule to be detected is measured by an
electrical
detection means, such as AC impedence.
BRIEF DESCRIPTION OF THE DRAWINGS
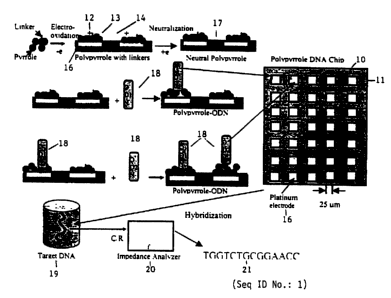
Figure 1 represents a schematic diagram for preparing the array of polypyrrole
coated electrodes and detecting by AC impedance.
Figure 2 illustrates polypyrrole copolymer formulation.
Figure 3 illustrates the electrochemically reduced neutral polypyrrole
copolymer.
Figure 4 illustrates the relationship of capacitance vs. frequency on oxidized
polypyrrole-based electrodes with and without DNA Attachment.
Figure 5 illustrates the relationship of capacitance vs. frequency on neutral
polypyrrole-based electrode with and without DNA attachment.
4
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
Figure 6 illustrates the comparison of response of capacitance vs. frequency
between oxidized and neutralized polypyrrole-based electrodes with DNA
attachment.
Figure 7 AC impedance planes measured in perfect match hybridized DNA
and single stranded DNA system.
Figure 8 is a Frequency Complex diagram obtained from neutralized
polypyrrole Electrodes.
Figure 9 is impedance planes measured in 3-bas mismatch hybridized DNA
and single stranded DNA systems.
Figure 10 is a plot of Resistance vs. u~-''2 for AC impedance measured in 3-
base mismatch hybridized DNA and single stranded DNA systems.
DETAILED DESCRIPTION OF THE INVENTION
The invention is directed to a method of detecting biological molecule with
the
aid of a charge neutral conjugated polymer on electrodes. Charge neutral
conjugated
polymer is meant a polymer with zero charge (negative or positive) on its
backbone,
yet with delocalized pi electron on its backbone. A conjugated polymer is
characterized by its backbone with regular alternation of single and double
chemical
bonds. Examples of conjugated polymers include: polypyrrole, polyphenylene,
polyacetylene, polydiacetylene, polythiophene, polyfuran, polyaniline,
polycarbazole,
poly(phenylene vinylene). More specifically, the invention encompasses a
charge
neutral conjugated polymers containing one or more functional groups capable
of
binding a probe molecule. The charge neutral conjugated polymer deposited on
the
surface of electrodes by electrochemical copolymerization of aromatic monomers
and
functionalized monomers as is known in the art. The as-deposited conjugated
polymer or copolymer is conductive and is usually in its charged state with
its charge
5
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
being balanced by counter ions from the polymerization solution. The charged
state is
the source of signal quenching for nearby fluorescent markers as in the case
of
fluorescence detection. It is also the source of noise for electric detection.
To overcome these potential problems, the polymer or copolymer deposited on
the electrode used in present invention is reduced to its charge-neutral state
from the
as-deposited charged state by reverse biasing right after the polymer or
copolymer is
initially deposited on the surface electrodes. The polymer or copolymer in its
neutral
state is an insulator or semiconductor, which does not quench fluorescence of
nearby
fluorescent markers in fluorescence detection and also give rise to only
limited
background noise in electric detection of biomolecule target.
The functional group used in present invention includes, but not limited to,
amine, hydrazine, ester, amide, carboxylate, halide, hydroxyl, vinyl, vinyl
carboxylate, thiol, phosphate, silicon containing organic compounds, and their
derivatives. The functional group is used to bind biomolecule probes such as
DNA,
RNA, peptides, polypeptides, proteins, antibody, antigen and hormones to the
polymer or copolymer on the electrode. For example, an oligonucleotide which
is in
part complementary to a target DNA is covalently linked to a neutral
polypyrrol
copolymer through an amine functional group.
The electrode used in the present invention is made of at least one of the
following materials: metals such as gold, silver, platinum, copper, and
alloys;
conductive metal oxide such as indium oxide, indium-tin oxide, zinc oxide;
other
conductive materials such carbon black, conductive epoxy and combinations
thereof.
The preferred sensing method in this embodiment is electric or
electrochemical methods. After exposure to a target molecule, the biosensor
senses a
change in electric signal, and reports the change by a readout means such as
display,
6
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
printout. The electric or/and electrochemical methods may be selected from,
but are
not limited to, AC impedance, cyclic voltammetry (CV), pulse voltammetry,
square
wave voltammetry, AC voltammetry (ACV), hydrodynamic modulation voltammetry,
potential step method, potentiometric measurements, amperometric measurements,
current step method, and combinations thereof.
It is more advantageous to detect a biomolecule target without the need of
labeling the target. Present invention provides a highly sensitive method for
detection
of biomolecule target without the need of labeling the target.
Some biomolecules are electrically active and may produce undesired
background noise when a detection is performed by passing charge through those
biomolecules. For example, guanine and adenine can be oxidized around 0.75 V
and
1.05 V, respectively. (Analtica Chimica Acta 319 (1996) 347-352). Thus it is
more
desirable to use impedance methods for labellers biomolecule detection.
The invention includes a method for determining an analyte in a test sample
comprising:
(a) depositing a polymer or copolymer film on an electrode by
electrochemically polymerizing an aromatic monomer and a
monomer with functional group in a solution via a positive bias
with supporting electrolyte;
(b) neutralizing the polymerized polymer by applying a reverse
bias to the electrode;
(c) attaching covalently a biomolecule probe to the neutral
copolymer through the functional monomer;
(d) contacting the electrode with the test sample containing an
electrolyte; and
7
05-01-2001 US001583:
(e) measuring the change of electric or electrochemical properties of
the electrode by electric or/and electrochemical methods when the
analyte is bound to the biomolecule probe on the neutral polymer or
copolymer.
The biosensor may include an array of electrodes in electrical contact with a
matrix of
charge neutral conjugated polymer having different sensing probes for sensing
multiple
biomolecule targets. It is also within the scope of the present invention to
fabricate a high
density biosensor with column and row addressable electrodes coated with
thousands of sensing
probes for screening applications. In the case of a high density array, it is
more practical to
place various biomolecule probes on each electrode with a robotic tool.
The invention is illustrated by neutral polypyrrole conjugated polymer
electrode arrays
used in conjunction with AC impedance detecting methods. The process to make
such arrays is
schematically shown in Fig. 1. The chips 10 are made by microelectric
technology on a silicon
support 11. The probe arrays 15 and electrodes 1 b are made of inert metals
such as gold or
platinum. Polypyrrole 12. with DNA linking group 13 is electrochemically
deposited on the
probe array ~ 4 in 0.1 M pyrrole + S ~M 3-acetate N-hydroxysuaccinimido
pyrrole + 0.1 M
LiCI04/acetonitrile (0/S% water). Then the polypyrrole-film is
electrochemically neutralized
17. Using a nanofluidic-dispensing tool, every probe can be sequentially
attached to a different
oligonucleotide 18 ODN 1 and ODN2. After a hybridization of the probe arrays
in a target
ODN2 solution 19, AC impedance analyzer 20 is used to detect the impedance
change for a
specific DNA sequence 21. (SEQ ID NO.: l )
8
AMENDED SHEET
CA 02376532 2001-12-05
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
In a variation of the aforesaid embodiment, the biomolecule probe can also be
attached to the aromatic functional monomer before it is electrochemically
polymerized with aromatic monomer to yield a conjugated polymer.
This invention will be further described by the following example with
polypyrrole as the conjugated copolymer and DNA as detection target. The
example
is intended to illustrate specific embodiments of the invention but not to
limit this
invention in spirit or scope.
Example 1
In order to demonstrate this invention, platinum electrodes with a diameter of
2 mm were used for electrochemical deposition of polypyrrole. The electrode
surface
was polished by gamma alumina powder (CH Instruments, Inc.) with 0.3 and 0.005
pm in sequence followed by deionized water washing. After polishing, the
electrodes
were immersed in 1 M HSS04 for 20 minutes and then vigorously washed by DI
water. CH 660 potentiostat was used for polypyrrole deposition. Platinum wire
and
Ag/AgCI were used as the counter electrode and reference electrode,
respectively. A
solution containing 0.1 M pyrrole + 5 pM 3-acetate N-hydroxysuaccinimido
pyrrole +
0.1 M LiCI04/acetonitrile (0.5% water) was prepared as the electrolyte. Cyclic
voltammetry (CV) was used for the electrochemical deposition. The potential
range
for the CV was 0.2-1.3 V vs Ag/AgCI for the first cycle and then changed to -
0.1 to
1.0 vs Ag/AgCI for other five cycles. An electrochemical oxidation of the
pyrrole
produced polypyrrole as shown in Figure 2.
The electrolyte was purged by nitrogen gas during whole electrochemical
deposition. The deposited polypyrrole film with the linking function group was
uniform and blue in color. The polypyrrole film is in oxidized form (charged
conductive state). To make a neutralized polypyrrole, the electrode was placed
in the
9
05-01-2001 US001583~
S electrolyte again and cycled over a potential range of -0.2 to 0.3 vs
Ag/AgCI, which is the
reduction zone for this electrochemical system. The neutralization of the
polypyrrole film is
illustrated in Figure 3. The neutralized polypyrrole film coated electrodes
were vigorously
washed for probe oligonucleotide attachment.
Then a 5'-amino-substituted oligonucleotide was attached onto the neutral
polypyrml
film by a direct substitution of the leaving N-hydroxysuaccinimide group in
dimethylformamide containing 10% phosphate buffer at pH = 8.0 at room
temperature for 16
hours. The oligonucleotide CCC TCA AGC AGA (SEQ ID N0.:2) with a terminal
amino
group on it s 5'-phosphorylated position was used. For comparison, the
oxidized polypyrrole
film was modified by oligonucleotide in the same procedure mentioned above.
I S Oxidized and neutralized polypyrrole deposited electrodes with and without
DNA attachment
were tested in deionized water by Soiartro 1260 impedance analyzer. A platinum
sheet with
area of 10 cm2 was used as the counter electrode. Frequency sweeping method
with a bias of
500 mV was conducted over frequency range of 100 mHz to 1 MHz. Since the
double layer
capacitance is proportional to the area of the electrode surface, the
capacitance of the counter
electrode surface, C~»C.~,. Cp represents the probe electrode capacitance.
Thus, the total,
capacitance of the detecting system C, = 1(1/Cp+I/C~~Cp C~(Cp+ C~) = Cp. In
addition, the
solution resistance for a disc-shaped ultramicroelectrode can be expressed as:
R" ~ 1/(4kr) (1)
Where r is the radius or the side length of the electrode and k is the
conductivity of the solution. The R" contributed from the small probe is much
larger
than the counter electrode. The results obtained from the AC impedance can
AMENDED SHEET
CA 02376532 2001-12-05
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
represent the change from the probes, since the surface area of the probe is
much
smaller than that of the counter electrode.
Experimental results are shown in Fig. 4, 5 and 6. Fig. 4 shows the
capacitance changes of the electrode surface vs. frequency, indicating that
the
oxidized polypyrrole-based electrode surface with oligonucleotide attachment
has
larger capacitance response than the surface without oligonucleotide
attachment at the
low frequency range. However, the ratio of signal to noise is not great. Fig.
5
demonstrates that the capacitance of neutralized polypyrrole-based electrode
surface
with oligonucleotide attachment is significantly greater than that of the
surface
without oligonucleotide attachment. Fig. 6 shows that the capacitance on the
neutralized polypyrrol-based electrode surface with oligonucleotide attachment
is
greater than that of oxidized polypyrrole-based surface by about 4 times.
The hybridization of the oligonucleotide probe on neutral polypyrrole with its
complementary strand shows significant improved signal to noise ratio as
compared
to that on charged polypyrrole.
Example 2
The neutralized polypyrrole film coated electrodes were vigorously washed for
DNA attachment. The electrodes coated with polypyrrole were placed in a
mixture of
80 ~L of DMF and 20 qL of 15 nM of Si-amino-3i-fluorescein labeled 15 by
oligonucleotide for 4 hrs at room temperature. At the end the electrode was
washed
with TBE buffer, deionized water thoroughly, and dried at room temperature in
the
air. The condition is not optimized.
The 5'- amino-substituted oligonucleotide of 300 uMconcentration in 25 uL of
dimethylformamide containing 20% phosphate buffer at pH = 8.0 was attached
onto
the neutral polypyrrole film on a microelectrode by a direct substitution of
the leaving
11
05-01-2001 US001583~
CA 02376532 2001-12-05
i . . '
N-hydroxysuaccinimide group at room temperature for 16 hours. The
oiigonucleotide CCC
TCA AGC AGA (SEQ ID N0.:2} with a terminal amino group on its 5'-
phosphorylated
position was used as an example. After the reaction, the microelectrode was
washed with DI
water thoroughly before a baseline AC impedance was measur~l. For
hybridization, the probe
attached to polypyrrole on a microelectrode was exposed to 35 uL of target
molecule of
different concentration (~M to aM) in lx SSC buffer. The hybridization takes
place in a sealed
conical tube at 37 C in a water bath for 24 to 48 hrs. The microelectrode was
then washed with
ample amount of 1 x SSC solution at room temperature before AC impedance
measurement.
A Solarhron Impedance Frequency Analyzer 1260 with Electrochemical Interface
1287 was
used to measure the impedance before and after hybridization of the
polypyrrole
microelectrodes. The counter and reference electrodes were platinum and
Ag/AgCI,
respectively. The measurements were conducted at open circuit voltage (OCR in
1 M LiC104
solution. The measured complex impedance versus frequency is shown in Fig. 8
for single and
hybridized DNA, indicating significant difference of the impedance before and
after
hybridization.
In this experiment, this type of electrodes can detect 0.1 amol of target DNA
in solution due
to the neutralized form of polyrrole film.
Example 3
Experiments for the specificity of the polypyrrole based electrodes were
conducted.
Eight probes attached electrodes were hybridized in buffers containing 2pM and
2 flvi of perfectly matched and three base mismatched target 1 Smer DNAs,
respectively. Results show significant difference between perfect and
mismatched
12
AMENDED SHEET
CA 02376532 2001-12-05
WO 00/77523 PCT/US00/15832
hybridized DNA. Further, the electrodes were placed in 1XSSC buffer for 30
min. of
washing at 37 and 38°C, respectively. AC impedance measurements
demonstrate that
the AC impedance for the mismatched hybridization was getting closer and
closer to
the baseline of the single stranded DNA with the increase of the washing
temperature
while that for the perfectly matched hybridization was almost keeping
constant. The
results are shown in Fig. 7 and 8. Fig. 9 is plotted from Fig. 8, indicating
that the
resistance in the mismatched DNA system continuously decreases with the
increase
of the washing temperature going back to the baseline of the single stranded
DNA.
This invention can be used in any solution containing metal or polymerized
canons, which are ion-conductive and can react with DNA.
The above examples are intended to illustrate the present invention and not to
limit it in spirit or scope.
13